User login
Baxter issues Class I recall of infusion pumps

of chemotherapy drugs
Credit: Bill Branson
Baxter Healthcare Corporation is recalling some of its infusion pumps after receiving more than 3500 reports of the pumps malfunctioning.
According to the US Food and Drug Administration (FDA), the malfunctioning pumps have resulted in 9 severe adverse events but no deaths.
This Class I recall includes Sigma Spectrum Infusion Pumps with Master Drug Library Model No. 35700BAX and 35700ABB.
The pumps were made between July 1, 2005, and January 15, 2014. They were distributed between February 20, 2013, and January 15, 2014.
The Sigma Spectrum infusion pumps are intended to deliver controlled amounts of medicines, blood, blood products, and other intravenous fluids.
The FDA said there have been more than 3500 reports of these pumps malfunctioning—specifically, reports of System Error 322 “Link Switch Error (low).” This error occurs when the pump detects that the door is open even though it is closed. A System Error 322 may lead to an interruption or delay in therapy.
When this error occurs, the Sigma Spectrum infusion pump stops the infusion, an alarm sounds, and a light flashes (a visual “322” alarm). This requires a clinician to reset the alarm, reprogram the pump, and confirm the infusion is running properly.
The use of affected pumps may cause serious adverse health consequences, including death; hence, the Class I recall.
Customers who encounter a System Error 322 should turn off the pump by pressing the ON/OFF key, then turn the pump back on by pressing the ON/OFF key to clear the alarm.
Clinicians will need to reprogram the infusion after the pump is turned back on. If the alarm cannot be cleared using these instructions, the device should be removed from use and sent to the facility’s biomedical engineering department.
If the System Error 322 reoccurs, the pump may need to be inspected and serviced by Baxter Healthcare. To contact Baxter, call 1-800-356-3454 (choose option 1) Monday through Friday, 7 am to 7 pm, Eastern Time.
Adverse reactions or quality problems related to these pumps can be reported to the FDA’s MedWatch Program. ![]()

of chemotherapy drugs
Credit: Bill Branson
Baxter Healthcare Corporation is recalling some of its infusion pumps after receiving more than 3500 reports of the pumps malfunctioning.
According to the US Food and Drug Administration (FDA), the malfunctioning pumps have resulted in 9 severe adverse events but no deaths.
This Class I recall includes Sigma Spectrum Infusion Pumps with Master Drug Library Model No. 35700BAX and 35700ABB.
The pumps were made between July 1, 2005, and January 15, 2014. They were distributed between February 20, 2013, and January 15, 2014.
The Sigma Spectrum infusion pumps are intended to deliver controlled amounts of medicines, blood, blood products, and other intravenous fluids.
The FDA said there have been more than 3500 reports of these pumps malfunctioning—specifically, reports of System Error 322 “Link Switch Error (low).” This error occurs when the pump detects that the door is open even though it is closed. A System Error 322 may lead to an interruption or delay in therapy.
When this error occurs, the Sigma Spectrum infusion pump stops the infusion, an alarm sounds, and a light flashes (a visual “322” alarm). This requires a clinician to reset the alarm, reprogram the pump, and confirm the infusion is running properly.
The use of affected pumps may cause serious adverse health consequences, including death; hence, the Class I recall.
Customers who encounter a System Error 322 should turn off the pump by pressing the ON/OFF key, then turn the pump back on by pressing the ON/OFF key to clear the alarm.
Clinicians will need to reprogram the infusion after the pump is turned back on. If the alarm cannot be cleared using these instructions, the device should be removed from use and sent to the facility’s biomedical engineering department.
If the System Error 322 reoccurs, the pump may need to be inspected and serviced by Baxter Healthcare. To contact Baxter, call 1-800-356-3454 (choose option 1) Monday through Friday, 7 am to 7 pm, Eastern Time.
Adverse reactions or quality problems related to these pumps can be reported to the FDA’s MedWatch Program. ![]()

of chemotherapy drugs
Credit: Bill Branson
Baxter Healthcare Corporation is recalling some of its infusion pumps after receiving more than 3500 reports of the pumps malfunctioning.
According to the US Food and Drug Administration (FDA), the malfunctioning pumps have resulted in 9 severe adverse events but no deaths.
This Class I recall includes Sigma Spectrum Infusion Pumps with Master Drug Library Model No. 35700BAX and 35700ABB.
The pumps were made between July 1, 2005, and January 15, 2014. They were distributed between February 20, 2013, and January 15, 2014.
The Sigma Spectrum infusion pumps are intended to deliver controlled amounts of medicines, blood, blood products, and other intravenous fluids.
The FDA said there have been more than 3500 reports of these pumps malfunctioning—specifically, reports of System Error 322 “Link Switch Error (low).” This error occurs when the pump detects that the door is open even though it is closed. A System Error 322 may lead to an interruption or delay in therapy.
When this error occurs, the Sigma Spectrum infusion pump stops the infusion, an alarm sounds, and a light flashes (a visual “322” alarm). This requires a clinician to reset the alarm, reprogram the pump, and confirm the infusion is running properly.
The use of affected pumps may cause serious adverse health consequences, including death; hence, the Class I recall.
Customers who encounter a System Error 322 should turn off the pump by pressing the ON/OFF key, then turn the pump back on by pressing the ON/OFF key to clear the alarm.
Clinicians will need to reprogram the infusion after the pump is turned back on. If the alarm cannot be cleared using these instructions, the device should be removed from use and sent to the facility’s biomedical engineering department.
If the System Error 322 reoccurs, the pump may need to be inspected and serviced by Baxter Healthcare. To contact Baxter, call 1-800-356-3454 (choose option 1) Monday through Friday, 7 am to 7 pm, Eastern Time.
Adverse reactions or quality problems related to these pumps can be reported to the FDA’s MedWatch Program. ![]()
Findings could increase use of delayed cord clamping
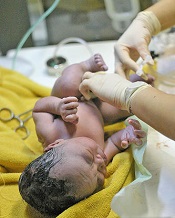
Credit: Meutia Chaerani
and Indradi Soemardjan
A baby’s position prior to delayed umbilical cord clamping does not affect the volume of placental blood transferred, according to a study published in The Lancet.
Researchers found that placing a baby on the mother’s chest or abdomen before clamping does not decrease the amount of blood transferred when compared to holding the child in the recommended introitus position.
As the chest/abdomen position is more desirable, the researchers believe this discovery could help increase the use of delayed cord clamping, which has been shown to reduce the risk of iron deficiency in infancy.
Current recommendations for delayed cord clamping are based on studies conducted 35 years ago. They suggest that, for effective placental transfusion to occur, a baby must be held at the level of the placenta—the introitus position.
The researchers noted that this position can be uncomfortable for the person holding the baby and interferes with immediate contact between the mother and child. These issues could be contributing to low compliance with delayed cord clamping, ultimately resulting in higher-than-necessary levels of iron deficiency in babies.
So the team decided to examine whether the transfer of blood in delayed cord clamping procedures is affected by the position in which the baby is held immediately after birth.
They conducted the study in 3 university-affiliated hospitals in Argentina, evaluating 197 babies who were held in the introitus position and 194 babies who were immediately placed on the mother’s abdomen or chest.
By measuring the babies’ weights at the point of birth and immediately after the delayed cord clamping procedure, the researchers were able to measure the volume of blood that had transferred from the placenta to the child.
They found no statistically significant difference between the 2 groups in the volume of blood transferred. The mean weight change was 56 g for babies in the introitus group and 53 g for babies in the abdomen/chest group (P=0.45).
“Our study suggests that when umbilical cord clamping is delayed for 2 minutes, holding the baby on the mother’s chest or abdomen is no worse than the currently recommended practice of holding the baby below this level,” said study author Nestor Vain, MD, of the Foundation for Maternal and Child Health (FUNDASAMIN) in Buenos Aires, Argentina.
“Because of the potential of enhanced bonding between mother and baby, increased success of breastfeeding, and the compliance with the procedure, holding the infant by the mother immediately after birth should be strongly recommended.”
Writing in a related comment article, Tonse Raju, MD, of the National Institute of Child Health and Human Development in Bethesda, Maryland, noted that introducing delayed cord clamping into practice has not been easy, and logistical issues might be partly responsible.
“Intuitively, to keep the newborn baby’s position below the level of the placenta in situ should maximize the volume of placental transfusion,” Dr Raju wrote. “However, trying to hold on to a wet, vigorously crying, and wriggling infant at the perineum for 2 minutes, in gloved hands, is awkward and can be risky.”
“[This study] should bring a sigh of relief from those trying to incorporate delayed umbilical cord clamping into practice. The results are convincing and show that gravity did not have an effect on volume of placental transfusion.” ![]()

Credit: Meutia Chaerani
and Indradi Soemardjan
A baby’s position prior to delayed umbilical cord clamping does not affect the volume of placental blood transferred, according to a study published in The Lancet.
Researchers found that placing a baby on the mother’s chest or abdomen before clamping does not decrease the amount of blood transferred when compared to holding the child in the recommended introitus position.
As the chest/abdomen position is more desirable, the researchers believe this discovery could help increase the use of delayed cord clamping, which has been shown to reduce the risk of iron deficiency in infancy.
Current recommendations for delayed cord clamping are based on studies conducted 35 years ago. They suggest that, for effective placental transfusion to occur, a baby must be held at the level of the placenta—the introitus position.
The researchers noted that this position can be uncomfortable for the person holding the baby and interferes with immediate contact between the mother and child. These issues could be contributing to low compliance with delayed cord clamping, ultimately resulting in higher-than-necessary levels of iron deficiency in babies.
So the team decided to examine whether the transfer of blood in delayed cord clamping procedures is affected by the position in which the baby is held immediately after birth.
They conducted the study in 3 university-affiliated hospitals in Argentina, evaluating 197 babies who were held in the introitus position and 194 babies who were immediately placed on the mother’s abdomen or chest.
By measuring the babies’ weights at the point of birth and immediately after the delayed cord clamping procedure, the researchers were able to measure the volume of blood that had transferred from the placenta to the child.
They found no statistically significant difference between the 2 groups in the volume of blood transferred. The mean weight change was 56 g for babies in the introitus group and 53 g for babies in the abdomen/chest group (P=0.45).
“Our study suggests that when umbilical cord clamping is delayed for 2 minutes, holding the baby on the mother’s chest or abdomen is no worse than the currently recommended practice of holding the baby below this level,” said study author Nestor Vain, MD, of the Foundation for Maternal and Child Health (FUNDASAMIN) in Buenos Aires, Argentina.
“Because of the potential of enhanced bonding between mother and baby, increased success of breastfeeding, and the compliance with the procedure, holding the infant by the mother immediately after birth should be strongly recommended.”
Writing in a related comment article, Tonse Raju, MD, of the National Institute of Child Health and Human Development in Bethesda, Maryland, noted that introducing delayed cord clamping into practice has not been easy, and logistical issues might be partly responsible.
“Intuitively, to keep the newborn baby’s position below the level of the placenta in situ should maximize the volume of placental transfusion,” Dr Raju wrote. “However, trying to hold on to a wet, vigorously crying, and wriggling infant at the perineum for 2 minutes, in gloved hands, is awkward and can be risky.”
“[This study] should bring a sigh of relief from those trying to incorporate delayed umbilical cord clamping into practice. The results are convincing and show that gravity did not have an effect on volume of placental transfusion.” ![]()

Credit: Meutia Chaerani
and Indradi Soemardjan
A baby’s position prior to delayed umbilical cord clamping does not affect the volume of placental blood transferred, according to a study published in The Lancet.
Researchers found that placing a baby on the mother’s chest or abdomen before clamping does not decrease the amount of blood transferred when compared to holding the child in the recommended introitus position.
As the chest/abdomen position is more desirable, the researchers believe this discovery could help increase the use of delayed cord clamping, which has been shown to reduce the risk of iron deficiency in infancy.
Current recommendations for delayed cord clamping are based on studies conducted 35 years ago. They suggest that, for effective placental transfusion to occur, a baby must be held at the level of the placenta—the introitus position.
The researchers noted that this position can be uncomfortable for the person holding the baby and interferes with immediate contact between the mother and child. These issues could be contributing to low compliance with delayed cord clamping, ultimately resulting in higher-than-necessary levels of iron deficiency in babies.
So the team decided to examine whether the transfer of blood in delayed cord clamping procedures is affected by the position in which the baby is held immediately after birth.
They conducted the study in 3 university-affiliated hospitals in Argentina, evaluating 197 babies who were held in the introitus position and 194 babies who were immediately placed on the mother’s abdomen or chest.
By measuring the babies’ weights at the point of birth and immediately after the delayed cord clamping procedure, the researchers were able to measure the volume of blood that had transferred from the placenta to the child.
They found no statistically significant difference between the 2 groups in the volume of blood transferred. The mean weight change was 56 g for babies in the introitus group and 53 g for babies in the abdomen/chest group (P=0.45).
“Our study suggests that when umbilical cord clamping is delayed for 2 minutes, holding the baby on the mother’s chest or abdomen is no worse than the currently recommended practice of holding the baby below this level,” said study author Nestor Vain, MD, of the Foundation for Maternal and Child Health (FUNDASAMIN) in Buenos Aires, Argentina.
“Because of the potential of enhanced bonding between mother and baby, increased success of breastfeeding, and the compliance with the procedure, holding the infant by the mother immediately after birth should be strongly recommended.”
Writing in a related comment article, Tonse Raju, MD, of the National Institute of Child Health and Human Development in Bethesda, Maryland, noted that introducing delayed cord clamping into practice has not been easy, and logistical issues might be partly responsible.
“Intuitively, to keep the newborn baby’s position below the level of the placenta in situ should maximize the volume of placental transfusion,” Dr Raju wrote. “However, trying to hold on to a wet, vigorously crying, and wriggling infant at the perineum for 2 minutes, in gloved hands, is awkward and can be risky.”
“[This study] should bring a sigh of relief from those trying to incorporate delayed umbilical cord clamping into practice. The results are convincing and show that gravity did not have an effect on volume of placental transfusion.” ![]()
ASCO releases guidelines for managing cancer survivors

with a cancer patient
NCI/Mathews Media Group
The American Society of Clinical Oncology (ASCO) has issued 3 practice guidelines for preventing and managing symptoms that can affect adult cancer survivors—neuropathy, fatigue, and depression/anxiety.
The guideline on chemotherapy-induced peripheral neuropathy (CIPN) lists a few options for treating the condition but discourages interventions to prevent CIPN, as there is insufficient evidence that these interventions benefit patients.
The guideline on fatigue recommends that healthcare providers start screening cancer patients for the condition at diagnosis and emphasizes the importance of educating patients about fatigue.
The guideline on depression and anxiety recommends periodic evaluations for symptoms of depression and anxiety in all cancer patients. It also suggests that all patients be offered supportive care services.
All 3 of these guidelines are published in the Journal of Clinical Oncology.
Treating and preventing CIPN
ASCO’s guideline on CIPN lists a handful of drugs that may be helpful in diminishing the symptoms of CIPN, but it does not recommend any agents for preventing the condition.
In fact, the guideline provides a list of agents that should not be offered for the prevention of CIPN, including acetyl-L-carnitine, amifostine, amitriptyline, CaMg, diethyldithio-carbamate, glutathione, nimodipine, Org 2766, all-trans retinoic acid, rhuLIF, and vitamin E.
“There is no clear panacea for neuropathy,” said Gary Lyman, MD, MPH, co-chair of the ASCO Survivorship Guidelines Advisory Group.
“Some of the drugs used for prevention or treatment of neuropathy may cause side effects or interfere with other drugs. We want to be clear that if there is no evidence of benefit from those drugs, it’s probably best not to take them.”
As for treatment, the guideline states that data support a “moderate” recommendation for duloxetine.
It also notes that there is no strong evidence of benefit for the use of tricyclic antidepressants, gabapentin, and a topical gel containing baclofen, amitriptyline, and ketamine. However, it may be reasonable to try those agents in select patients.
To develop this guideline, an ASCO panel conducted a systematic review of relevant medical literature. They analyzed data from 48 randomized, clinical trials focused on managing CIPN.
Screening and managing fatigue
ASCO’s guideline on fatigue recommends that all patients be screened for fatigue from the point of diagnosis onward. Healthcare providers should assess fatigue history, disease status, and treatable contributing factors.
All patients should be educated about the differences between normal and cancer-related fatigue, causes of fatigue, and contributing factors.
Healthcare providers should discuss with patients strategies to manage fatigue, including physical activity, psychosocial interventions (such as cognitive and behavioral therapies or psycho-educational therapies), and mind-body interventions (such as yoga or acupuncture).
To develop this guideline, an ASCO panel conducted a systematic review of clinical practice guideline databases and relevant medical literature. The adaptation is based on a Pan-Canadian guideline on fatigue and 2 National Comprehensive Cancer Network guidelines on cancer-related fatigue and survivorship.
Handling anxiety and depression
ASCO’s guideline on anxiety and depression recommends that healthcare providers periodically evaluate all cancer patients for symptoms of depression and anxiety. The assessments should be performed using validated, published measures and procedures.
All patients should have the option of receiving supportive care services, such as education about the normalcy of stress in the context of cancer, signs and symptoms of distress, and stress reduction strategies.
Patients who display moderate or severe symptoms of anxiety and depression should be referred for the appropriate psychological, psychosocial, or psychiatric interventions.
“Doctors sometimes don’t give these symptoms much attention because they think it’s normal that their patients are a little anxious or depressed about their disease,” Dr Lyman said. “But it’s important to keep an eye on the symptoms and step in when they start to interfere with the patients’ quality of life.”
To develop this guideline, an ASCO panel conducted a systematic review of clinical practice guideline databases and relevant medical literature. The adaptation is based on a Pan-Canadian practice guideline on psychological distress in adults with cancer. ![]()

with a cancer patient
NCI/Mathews Media Group
The American Society of Clinical Oncology (ASCO) has issued 3 practice guidelines for preventing and managing symptoms that can affect adult cancer survivors—neuropathy, fatigue, and depression/anxiety.
The guideline on chemotherapy-induced peripheral neuropathy (CIPN) lists a few options for treating the condition but discourages interventions to prevent CIPN, as there is insufficient evidence that these interventions benefit patients.
The guideline on fatigue recommends that healthcare providers start screening cancer patients for the condition at diagnosis and emphasizes the importance of educating patients about fatigue.
The guideline on depression and anxiety recommends periodic evaluations for symptoms of depression and anxiety in all cancer patients. It also suggests that all patients be offered supportive care services.
All 3 of these guidelines are published in the Journal of Clinical Oncology.
Treating and preventing CIPN
ASCO’s guideline on CIPN lists a handful of drugs that may be helpful in diminishing the symptoms of CIPN, but it does not recommend any agents for preventing the condition.
In fact, the guideline provides a list of agents that should not be offered for the prevention of CIPN, including acetyl-L-carnitine, amifostine, amitriptyline, CaMg, diethyldithio-carbamate, glutathione, nimodipine, Org 2766, all-trans retinoic acid, rhuLIF, and vitamin E.
“There is no clear panacea for neuropathy,” said Gary Lyman, MD, MPH, co-chair of the ASCO Survivorship Guidelines Advisory Group.
“Some of the drugs used for prevention or treatment of neuropathy may cause side effects or interfere with other drugs. We want to be clear that if there is no evidence of benefit from those drugs, it’s probably best not to take them.”
As for treatment, the guideline states that data support a “moderate” recommendation for duloxetine.
It also notes that there is no strong evidence of benefit for the use of tricyclic antidepressants, gabapentin, and a topical gel containing baclofen, amitriptyline, and ketamine. However, it may be reasonable to try those agents in select patients.
To develop this guideline, an ASCO panel conducted a systematic review of relevant medical literature. They analyzed data from 48 randomized, clinical trials focused on managing CIPN.
Screening and managing fatigue
ASCO’s guideline on fatigue recommends that all patients be screened for fatigue from the point of diagnosis onward. Healthcare providers should assess fatigue history, disease status, and treatable contributing factors.
All patients should be educated about the differences between normal and cancer-related fatigue, causes of fatigue, and contributing factors.
Healthcare providers should discuss with patients strategies to manage fatigue, including physical activity, psychosocial interventions (such as cognitive and behavioral therapies or psycho-educational therapies), and mind-body interventions (such as yoga or acupuncture).
To develop this guideline, an ASCO panel conducted a systematic review of clinical practice guideline databases and relevant medical literature. The adaptation is based on a Pan-Canadian guideline on fatigue and 2 National Comprehensive Cancer Network guidelines on cancer-related fatigue and survivorship.
Handling anxiety and depression
ASCO’s guideline on anxiety and depression recommends that healthcare providers periodically evaluate all cancer patients for symptoms of depression and anxiety. The assessments should be performed using validated, published measures and procedures.
All patients should have the option of receiving supportive care services, such as education about the normalcy of stress in the context of cancer, signs and symptoms of distress, and stress reduction strategies.
Patients who display moderate or severe symptoms of anxiety and depression should be referred for the appropriate psychological, psychosocial, or psychiatric interventions.
“Doctors sometimes don’t give these symptoms much attention because they think it’s normal that their patients are a little anxious or depressed about their disease,” Dr Lyman said. “But it’s important to keep an eye on the symptoms and step in when they start to interfere with the patients’ quality of life.”
To develop this guideline, an ASCO panel conducted a systematic review of clinical practice guideline databases and relevant medical literature. The adaptation is based on a Pan-Canadian practice guideline on psychological distress in adults with cancer. ![]()

with a cancer patient
NCI/Mathews Media Group
The American Society of Clinical Oncology (ASCO) has issued 3 practice guidelines for preventing and managing symptoms that can affect adult cancer survivors—neuropathy, fatigue, and depression/anxiety.
The guideline on chemotherapy-induced peripheral neuropathy (CIPN) lists a few options for treating the condition but discourages interventions to prevent CIPN, as there is insufficient evidence that these interventions benefit patients.
The guideline on fatigue recommends that healthcare providers start screening cancer patients for the condition at diagnosis and emphasizes the importance of educating patients about fatigue.
The guideline on depression and anxiety recommends periodic evaluations for symptoms of depression and anxiety in all cancer patients. It also suggests that all patients be offered supportive care services.
All 3 of these guidelines are published in the Journal of Clinical Oncology.
Treating and preventing CIPN
ASCO’s guideline on CIPN lists a handful of drugs that may be helpful in diminishing the symptoms of CIPN, but it does not recommend any agents for preventing the condition.
In fact, the guideline provides a list of agents that should not be offered for the prevention of CIPN, including acetyl-L-carnitine, amifostine, amitriptyline, CaMg, diethyldithio-carbamate, glutathione, nimodipine, Org 2766, all-trans retinoic acid, rhuLIF, and vitamin E.
“There is no clear panacea for neuropathy,” said Gary Lyman, MD, MPH, co-chair of the ASCO Survivorship Guidelines Advisory Group.
“Some of the drugs used for prevention or treatment of neuropathy may cause side effects or interfere with other drugs. We want to be clear that if there is no evidence of benefit from those drugs, it’s probably best not to take them.”
As for treatment, the guideline states that data support a “moderate” recommendation for duloxetine.
It also notes that there is no strong evidence of benefit for the use of tricyclic antidepressants, gabapentin, and a topical gel containing baclofen, amitriptyline, and ketamine. However, it may be reasonable to try those agents in select patients.
To develop this guideline, an ASCO panel conducted a systematic review of relevant medical literature. They analyzed data from 48 randomized, clinical trials focused on managing CIPN.
Screening and managing fatigue
ASCO’s guideline on fatigue recommends that all patients be screened for fatigue from the point of diagnosis onward. Healthcare providers should assess fatigue history, disease status, and treatable contributing factors.
All patients should be educated about the differences between normal and cancer-related fatigue, causes of fatigue, and contributing factors.
Healthcare providers should discuss with patients strategies to manage fatigue, including physical activity, psychosocial interventions (such as cognitive and behavioral therapies or psycho-educational therapies), and mind-body interventions (such as yoga or acupuncture).
To develop this guideline, an ASCO panel conducted a systematic review of clinical practice guideline databases and relevant medical literature. The adaptation is based on a Pan-Canadian guideline on fatigue and 2 National Comprehensive Cancer Network guidelines on cancer-related fatigue and survivorship.
Handling anxiety and depression
ASCO’s guideline on anxiety and depression recommends that healthcare providers periodically evaluate all cancer patients for symptoms of depression and anxiety. The assessments should be performed using validated, published measures and procedures.
All patients should have the option of receiving supportive care services, such as education about the normalcy of stress in the context of cancer, signs and symptoms of distress, and stress reduction strategies.
Patients who display moderate or severe symptoms of anxiety and depression should be referred for the appropriate psychological, psychosocial, or psychiatric interventions.
“Doctors sometimes don’t give these symptoms much attention because they think it’s normal that their patients are a little anxious or depressed about their disease,” Dr Lyman said. “But it’s important to keep an eye on the symptoms and step in when they start to interfere with the patients’ quality of life.”
To develop this guideline, an ASCO panel conducted a systematic review of clinical practice guideline databases and relevant medical literature. The adaptation is based on a Pan-Canadian practice guideline on psychological distress in adults with cancer. ![]()
Helping SCD patients transition to adult care

A questionnaire may help aid the transition from pediatric to adult care for patients with sickle cell disease (SCD), according to a paper published in the Journal of Pediatric Hematology/Oncology.
Researchers showed that the questionnaire could pinpoint areas in which young SCD patients may need help to transition to an adult clinic.
The questionnaire measured 5 knowledge skill sets—medical, educational/vocational, health benefits, social, and independent living—as well as 3 psychological assessments—feelings, stress, and self-efficacy.
To test how effective the questionnaire can be, Amy Sobota, MD, of Boston Medical Center, and her colleagues looked at the answers provided by 33 patients between the ages of 18 and 22.
Most respondents had good medical knowledge of SCD. Ninety-seven percent said they could explain SCD to another person and understood “how they got” the disease.
Ninety-four percent of patients also understood that SCD might be passed on to their children, and 71% of women said they knew how SCD could affect their pregnancy. However, only 30% of patients reported knowing what their baseline hemoglobin level is.
Likewise, the questionnaire suggested some knowledge gaps with regard to health benefits. Sixty-four percent of patients said they understood the various types of health insurance available to them, and 61% knew how their age could affect their health benefits.
Patients’ educational/vocational knowledge and capabilities were promising overall. Ninety-one percent of patients said they had a specific plan for the future, and 94% said they knew the education or employment required for their job choice. Seventy-six percent said they could identify the type of work that could cause problems related to SCD.
As for independent living, 91% of patients said they could fill their prescriptions on their own, 85% could make doctor’s appointments on their own, and 79% reported going to doctor’s appointments on their own.
With regard to social support, 97% of patients said they had a good social support system. But fewer (70%) had friends they could talk to about SCD, and only 48% knew about community-based SCD programs.
Most patients said they were worried that SCD would hinder them in some ways. Seventy-six percent worried about SCD getting in the way of school or work, and 51% worried it might prevent them from doing things they enjoy.
However, most patients felt sure they could function well. Eighty-eight percent said they could keep doing most of the things they do day-to-day, and 54% said they had ways of managing their pain without medication.
A minority of patients were worried or anxious about transitioning to adult care. Twenty-five percent were “quite a bit” or “extremely” worried, and 9% were similarly anxious about the transition. Sixteen percent said they felt “not at all” or “a little bit” all right to transition to an adult health care setting.
“Our study indicates that this assessment tool—the only one of its kind—provides important information to physicians of patients with sickle cell disease who are transitioning from pediatric to adult care,” Dr Sobota said. “Caregivers can use this information from patients in order to effectively tailor and guide their treatment and education through this transition.” ![]()

A questionnaire may help aid the transition from pediatric to adult care for patients with sickle cell disease (SCD), according to a paper published in the Journal of Pediatric Hematology/Oncology.
Researchers showed that the questionnaire could pinpoint areas in which young SCD patients may need help to transition to an adult clinic.
The questionnaire measured 5 knowledge skill sets—medical, educational/vocational, health benefits, social, and independent living—as well as 3 psychological assessments—feelings, stress, and self-efficacy.
To test how effective the questionnaire can be, Amy Sobota, MD, of Boston Medical Center, and her colleagues looked at the answers provided by 33 patients between the ages of 18 and 22.
Most respondents had good medical knowledge of SCD. Ninety-seven percent said they could explain SCD to another person and understood “how they got” the disease.
Ninety-four percent of patients also understood that SCD might be passed on to their children, and 71% of women said they knew how SCD could affect their pregnancy. However, only 30% of patients reported knowing what their baseline hemoglobin level is.
Likewise, the questionnaire suggested some knowledge gaps with regard to health benefits. Sixty-four percent of patients said they understood the various types of health insurance available to them, and 61% knew how their age could affect their health benefits.
Patients’ educational/vocational knowledge and capabilities were promising overall. Ninety-one percent of patients said they had a specific plan for the future, and 94% said they knew the education or employment required for their job choice. Seventy-six percent said they could identify the type of work that could cause problems related to SCD.
As for independent living, 91% of patients said they could fill their prescriptions on their own, 85% could make doctor’s appointments on their own, and 79% reported going to doctor’s appointments on their own.
With regard to social support, 97% of patients said they had a good social support system. But fewer (70%) had friends they could talk to about SCD, and only 48% knew about community-based SCD programs.
Most patients said they were worried that SCD would hinder them in some ways. Seventy-six percent worried about SCD getting in the way of school or work, and 51% worried it might prevent them from doing things they enjoy.
However, most patients felt sure they could function well. Eighty-eight percent said they could keep doing most of the things they do day-to-day, and 54% said they had ways of managing their pain without medication.
A minority of patients were worried or anxious about transitioning to adult care. Twenty-five percent were “quite a bit” or “extremely” worried, and 9% were similarly anxious about the transition. Sixteen percent said they felt “not at all” or “a little bit” all right to transition to an adult health care setting.
“Our study indicates that this assessment tool—the only one of its kind—provides important information to physicians of patients with sickle cell disease who are transitioning from pediatric to adult care,” Dr Sobota said. “Caregivers can use this information from patients in order to effectively tailor and guide their treatment and education through this transition.” ![]()

A questionnaire may help aid the transition from pediatric to adult care for patients with sickle cell disease (SCD), according to a paper published in the Journal of Pediatric Hematology/Oncology.
Researchers showed that the questionnaire could pinpoint areas in which young SCD patients may need help to transition to an adult clinic.
The questionnaire measured 5 knowledge skill sets—medical, educational/vocational, health benefits, social, and independent living—as well as 3 psychological assessments—feelings, stress, and self-efficacy.
To test how effective the questionnaire can be, Amy Sobota, MD, of Boston Medical Center, and her colleagues looked at the answers provided by 33 patients between the ages of 18 and 22.
Most respondents had good medical knowledge of SCD. Ninety-seven percent said they could explain SCD to another person and understood “how they got” the disease.
Ninety-four percent of patients also understood that SCD might be passed on to their children, and 71% of women said they knew how SCD could affect their pregnancy. However, only 30% of patients reported knowing what their baseline hemoglobin level is.
Likewise, the questionnaire suggested some knowledge gaps with regard to health benefits. Sixty-four percent of patients said they understood the various types of health insurance available to them, and 61% knew how their age could affect their health benefits.
Patients’ educational/vocational knowledge and capabilities were promising overall. Ninety-one percent of patients said they had a specific plan for the future, and 94% said they knew the education or employment required for their job choice. Seventy-six percent said they could identify the type of work that could cause problems related to SCD.
As for independent living, 91% of patients said they could fill their prescriptions on their own, 85% could make doctor’s appointments on their own, and 79% reported going to doctor’s appointments on their own.
With regard to social support, 97% of patients said they had a good social support system. But fewer (70%) had friends they could talk to about SCD, and only 48% knew about community-based SCD programs.
Most patients said they were worried that SCD would hinder them in some ways. Seventy-six percent worried about SCD getting in the way of school or work, and 51% worried it might prevent them from doing things they enjoy.
However, most patients felt sure they could function well. Eighty-eight percent said they could keep doing most of the things they do day-to-day, and 54% said they had ways of managing their pain without medication.
A minority of patients were worried or anxious about transitioning to adult care. Twenty-five percent were “quite a bit” or “extremely” worried, and 9% were similarly anxious about the transition. Sixteen percent said they felt “not at all” or “a little bit” all right to transition to an adult health care setting.
“Our study indicates that this assessment tool—the only one of its kind—provides important information to physicians of patients with sickle cell disease who are transitioning from pediatric to adult care,” Dr Sobota said. “Caregivers can use this information from patients in order to effectively tailor and guide their treatment and education through this transition.” ![]()
Restrictive transfusion approach may cut risk of HAIs

Credit: Elise Amendola
A review of randomized trials indicates that a restrictive approach to blood transfusion can decrease the risk of healthcare-associated infections (HAIs) for some patients.
Investigators found that, overall, restricting red blood cell (RBC) transfusions to patients with hemoglobin concentrations of 7 g/dL or less was associated with a lower incidence of HAIs such as pneumonia, mediastinitis, and sepsis.
However, when they stratified results by patient type, the researchers found that a restrictive transfusion approach significantly decreased the risk of HAIs only for patients who already had sepsis or were undergoing orthopedic surgery.
Jeffrey M. Rohde, MD, of the University of Michigan in Ann Arbor, and his colleagues reported these findings in JAMA.
The investigators set out to compare restrictive and liberal RBC transfusion strategies using data from 21 randomized trials in 9 countries. Eighteen of the trials (n=7593) contained enough information for a meta-analysis.
The pooled risk of all serious HAIs was 11.8% for patients treated with a restrictive transfusion approach and 16.9% for patients treated with a liberal approach. The risk ratio (RR) for the association between transfusion strategies and serious infection was 0.82.
“The fewer the red blood cell transfusions, the less likely hospitalized patients were to develop infections,” Dr Rohde said. “This is most likely due to the patient’s immune system reacting to donor blood [known as transfusion-associated immunomodulation].”
Even when the transfusions were leukoreduced, the risk of infection remained lower with a restrictive transfusion strategy. The RR was 0.80.
The results suggested that, for every 1000 patients in which RBC transfusion is a consideration, 26 could potentially be spared an HAI if restrictive strategies were used.
On the other hand, the investigators found no significant differences in the incidence of HAIs by RBC threshold for patients with cardiac disease, the critically ill, those with acute upper gastrointestinal bleeding, or for infants with low birth weight.
Yet the risk of infection was significantly lower with a restrictive strategy for patients who already had sepsis or were undergoing orthopedic surgery. The RRs were 0.51 and 0.70, respectively.
Dr Rohde and his colleagues said these results support AABB’s 2012 guidelines for transfusing hospitalized patients. The guidelines recommend a restrictive strategy for all hospitalized patients but also list specific hemoglobin-based recommendations for different patient populations. ![]()

Credit: Elise Amendola
A review of randomized trials indicates that a restrictive approach to blood transfusion can decrease the risk of healthcare-associated infections (HAIs) for some patients.
Investigators found that, overall, restricting red blood cell (RBC) transfusions to patients with hemoglobin concentrations of 7 g/dL or less was associated with a lower incidence of HAIs such as pneumonia, mediastinitis, and sepsis.
However, when they stratified results by patient type, the researchers found that a restrictive transfusion approach significantly decreased the risk of HAIs only for patients who already had sepsis or were undergoing orthopedic surgery.
Jeffrey M. Rohde, MD, of the University of Michigan in Ann Arbor, and his colleagues reported these findings in JAMA.
The investigators set out to compare restrictive and liberal RBC transfusion strategies using data from 21 randomized trials in 9 countries. Eighteen of the trials (n=7593) contained enough information for a meta-analysis.
The pooled risk of all serious HAIs was 11.8% for patients treated with a restrictive transfusion approach and 16.9% for patients treated with a liberal approach. The risk ratio (RR) for the association between transfusion strategies and serious infection was 0.82.
“The fewer the red blood cell transfusions, the less likely hospitalized patients were to develop infections,” Dr Rohde said. “This is most likely due to the patient’s immune system reacting to donor blood [known as transfusion-associated immunomodulation].”
Even when the transfusions were leukoreduced, the risk of infection remained lower with a restrictive transfusion strategy. The RR was 0.80.
The results suggested that, for every 1000 patients in which RBC transfusion is a consideration, 26 could potentially be spared an HAI if restrictive strategies were used.
On the other hand, the investigators found no significant differences in the incidence of HAIs by RBC threshold for patients with cardiac disease, the critically ill, those with acute upper gastrointestinal bleeding, or for infants with low birth weight.
Yet the risk of infection was significantly lower with a restrictive strategy for patients who already had sepsis or were undergoing orthopedic surgery. The RRs were 0.51 and 0.70, respectively.
Dr Rohde and his colleagues said these results support AABB’s 2012 guidelines for transfusing hospitalized patients. The guidelines recommend a restrictive strategy for all hospitalized patients but also list specific hemoglobin-based recommendations for different patient populations. ![]()

Credit: Elise Amendola
A review of randomized trials indicates that a restrictive approach to blood transfusion can decrease the risk of healthcare-associated infections (HAIs) for some patients.
Investigators found that, overall, restricting red blood cell (RBC) transfusions to patients with hemoglobin concentrations of 7 g/dL or less was associated with a lower incidence of HAIs such as pneumonia, mediastinitis, and sepsis.
However, when they stratified results by patient type, the researchers found that a restrictive transfusion approach significantly decreased the risk of HAIs only for patients who already had sepsis or were undergoing orthopedic surgery.
Jeffrey M. Rohde, MD, of the University of Michigan in Ann Arbor, and his colleagues reported these findings in JAMA.
The investigators set out to compare restrictive and liberal RBC transfusion strategies using data from 21 randomized trials in 9 countries. Eighteen of the trials (n=7593) contained enough information for a meta-analysis.
The pooled risk of all serious HAIs was 11.8% for patients treated with a restrictive transfusion approach and 16.9% for patients treated with a liberal approach. The risk ratio (RR) for the association between transfusion strategies and serious infection was 0.82.
“The fewer the red blood cell transfusions, the less likely hospitalized patients were to develop infections,” Dr Rohde said. “This is most likely due to the patient’s immune system reacting to donor blood [known as transfusion-associated immunomodulation].”
Even when the transfusions were leukoreduced, the risk of infection remained lower with a restrictive transfusion strategy. The RR was 0.80.
The results suggested that, for every 1000 patients in which RBC transfusion is a consideration, 26 could potentially be spared an HAI if restrictive strategies were used.
On the other hand, the investigators found no significant differences in the incidence of HAIs by RBC threshold for patients with cardiac disease, the critically ill, those with acute upper gastrointestinal bleeding, or for infants with low birth weight.
Yet the risk of infection was significantly lower with a restrictive strategy for patients who already had sepsis or were undergoing orthopedic surgery. The RRs were 0.51 and 0.70, respectively.
Dr Rohde and his colleagues said these results support AABB’s 2012 guidelines for transfusing hospitalized patients. The guidelines recommend a restrictive strategy for all hospitalized patients but also list specific hemoglobin-based recommendations for different patient populations. ![]()
CDC reports update data on HAIs

Staphylococcus infection
Credit: Bill Branson
About 1 in 25 US patients will contract at least 1 healthcare-associated infection (HAI) during the course of hospital care, according to data from the Centers for Disease Control and Prevention (CDC).
The agency has released 2 new reports on the topic.
The first, published in NEJM, is a survey of nearly 200 hospitals, which researchers used to estimate the national burden of HAIs in 2011.
The second is a 2012 annual report on the progress made toward US Health and Human Services HAI
prevention goals.
Together, the reports suggest that US hospitals have made progress in their effort to eliminate HAIs, but more work is needed to improve patient safety.
“Although there has been some progress, today and every day, more than 200 Americans with healthcare-associated infections will die during their hospital stay,” said CDC Director Tom Frieden, MD, MPH.
“The most advanced medical care won’t work if clinicians don’t prevent infections through basic things such as regular hand hygiene. Healthcare workers want the best for their patients. Following standard infection control practices every time will help ensure their patients’ safety.”
Estimating HAI incidence
The CDC Multistate Point-Prevalence Survey of Health Care-Associated Infections, published in NEJM, used 2011 data from 183 US hospitals to estimate the burden of a wide range of infections in hospital patients.
That year, about 721,800 infections occurred in 648,000 hospital patients. About 75,000 patients with HAIs died during their hospitalizations.
The most common infections were pneumonia (22%), surgical-site infections (22%), gastrointestinal infections (17%), urinary tract infections (13%), and bloodstream infections (10%).
The most common causes of HAIs were Clostridium difficile (12%), Staphylococcus aureus (including MRSA; 11%), Klebsiella (10%), Escherichia coli (9%), Enterococcus (9%), and Pseudomonas (7%).
Tracking national progress
The second report, CDC’s National and State Healthcare-associated Infection Progress Report, includes a subset of infection types that are often required to be reported to CDC.
The report revealed a 44% decrease in central line-associated bloodstream infections between 2008 and 2012, as well as a 20% decrease in infections related to 10 surgical procedures between 2008 and 2012.
There was a 4% decrease in hospital-onset MRSA between 2011 and 2012 and a 2% decrease in hospital-onset C difficile infections between 2011 and 2012.
On the other hand, there was a 3% increase in catheter-associated urinary tract infections between 2009 and 2012.
To access both reports and see the updated HAI data, visit the CDC website. ![]()

Staphylococcus infection
Credit: Bill Branson
About 1 in 25 US patients will contract at least 1 healthcare-associated infection (HAI) during the course of hospital care, according to data from the Centers for Disease Control and Prevention (CDC).
The agency has released 2 new reports on the topic.
The first, published in NEJM, is a survey of nearly 200 hospitals, which researchers used to estimate the national burden of HAIs in 2011.
The second is a 2012 annual report on the progress made toward US Health and Human Services HAI
prevention goals.
Together, the reports suggest that US hospitals have made progress in their effort to eliminate HAIs, but more work is needed to improve patient safety.
“Although there has been some progress, today and every day, more than 200 Americans with healthcare-associated infections will die during their hospital stay,” said CDC Director Tom Frieden, MD, MPH.
“The most advanced medical care won’t work if clinicians don’t prevent infections through basic things such as regular hand hygiene. Healthcare workers want the best for their patients. Following standard infection control practices every time will help ensure their patients’ safety.”
Estimating HAI incidence
The CDC Multistate Point-Prevalence Survey of Health Care-Associated Infections, published in NEJM, used 2011 data from 183 US hospitals to estimate the burden of a wide range of infections in hospital patients.
That year, about 721,800 infections occurred in 648,000 hospital patients. About 75,000 patients with HAIs died during their hospitalizations.
The most common infections were pneumonia (22%), surgical-site infections (22%), gastrointestinal infections (17%), urinary tract infections (13%), and bloodstream infections (10%).
The most common causes of HAIs were Clostridium difficile (12%), Staphylococcus aureus (including MRSA; 11%), Klebsiella (10%), Escherichia coli (9%), Enterococcus (9%), and Pseudomonas (7%).
Tracking national progress
The second report, CDC’s National and State Healthcare-associated Infection Progress Report, includes a subset of infection types that are often required to be reported to CDC.
The report revealed a 44% decrease in central line-associated bloodstream infections between 2008 and 2012, as well as a 20% decrease in infections related to 10 surgical procedures between 2008 and 2012.
There was a 4% decrease in hospital-onset MRSA between 2011 and 2012 and a 2% decrease in hospital-onset C difficile infections between 2011 and 2012.
On the other hand, there was a 3% increase in catheter-associated urinary tract infections between 2009 and 2012.
To access both reports and see the updated HAI data, visit the CDC website. ![]()

Staphylococcus infection
Credit: Bill Branson
About 1 in 25 US patients will contract at least 1 healthcare-associated infection (HAI) during the course of hospital care, according to data from the Centers for Disease Control and Prevention (CDC).
The agency has released 2 new reports on the topic.
The first, published in NEJM, is a survey of nearly 200 hospitals, which researchers used to estimate the national burden of HAIs in 2011.
The second is a 2012 annual report on the progress made toward US Health and Human Services HAI
prevention goals.
Together, the reports suggest that US hospitals have made progress in their effort to eliminate HAIs, but more work is needed to improve patient safety.
“Although there has been some progress, today and every day, more than 200 Americans with healthcare-associated infections will die during their hospital stay,” said CDC Director Tom Frieden, MD, MPH.
“The most advanced medical care won’t work if clinicians don’t prevent infections through basic things such as regular hand hygiene. Healthcare workers want the best for their patients. Following standard infection control practices every time will help ensure their patients’ safety.”
Estimating HAI incidence
The CDC Multistate Point-Prevalence Survey of Health Care-Associated Infections, published in NEJM, used 2011 data from 183 US hospitals to estimate the burden of a wide range of infections in hospital patients.
That year, about 721,800 infections occurred in 648,000 hospital patients. About 75,000 patients with HAIs died during their hospitalizations.
The most common infections were pneumonia (22%), surgical-site infections (22%), gastrointestinal infections (17%), urinary tract infections (13%), and bloodstream infections (10%).
The most common causes of HAIs were Clostridium difficile (12%), Staphylococcus aureus (including MRSA; 11%), Klebsiella (10%), Escherichia coli (9%), Enterococcus (9%), and Pseudomonas (7%).
Tracking national progress
The second report, CDC’s National and State Healthcare-associated Infection Progress Report, includes a subset of infection types that are often required to be reported to CDC.
The report revealed a 44% decrease in central line-associated bloodstream infections between 2008 and 2012, as well as a 20% decrease in infections related to 10 surgical procedures between 2008 and 2012.
There was a 4% decrease in hospital-onset MRSA between 2011 and 2012 and a 2% decrease in hospital-onset C difficile infections between 2011 and 2012.
On the other hand, there was a 3% increase in catheter-associated urinary tract infections between 2009 and 2012.
To access both reports and see the updated HAI data, visit the CDC website. ![]()
Video glasses can curb patient anxiety

Credit: CDC
SAN DIEGO—Watching videos through special glasses can calm patients undergoing a biopsy or other minimally invasive treatment, according to research presented at the Society of Interventional Radiology’s 39th Annual Scientific Meeting.
Researchers have explored strategies other than medication to reduce anxiety in these patients, including having patients listen to music or undergo hypnosis. But these methods have had modest benefits.
“Our study—the first of its kind for interventional radiology treatments—puts a spin on using modern technology to provide a safe, potentially cost-effective strategy of reducing anxiety, which can help and improve patient care,” said David L. Waldman, MD, PhD, of the University of Rochester Medical Center in New York.
“Whether they were watching a children’s movie or a nature show, patients wearing video glasses were successful at tuning out their surroundings. It’s an effective distraction technique that helps focus the individual’s attention away from the treatment.”
The study involved 49 patients (33 men and 16 women, ages 18-87) who were undergoing an outpatient interventional radiology treatment, such as a biopsy or placement of a catheter in the arm or chest to receive medication for treating cancer or infection.
Twenty-five of the patients donned video glasses prior to undergoing the treatment, and 24 did not. The video viewers chose from 20 videos, none of which were violent.
All of the patients filled out a standard 20-question test called the State-Trait Anxiety Inventory Form Y before and after the procedure to assess their level of anxiety.
Patients who wore the video glasses were 18.1% less anxious after the treatment than they were before, while those who didn’t wear video glasses were 7.5% less anxious afterward.
And the presence of the video glasses did not bother either the patient or the doctor, Dr Waldman said.
The glasses had no significant effect on blood pressure, heart rate, respiratory rate, pain, procedure time, or the amount of sedation or pain medication used.
“Patients told us the video glasses really helped calm them down and took their mind off the treatment, and we now offer video glasses to help distract patients from medical treatment going on mere inches away,” Dr Waldman said. “It is really comforting for patients, especially the ones who tend to be more nervous.
Dr Waldman and his colleagues presented these results at the meeting as abstract 126. ![]()

Credit: CDC
SAN DIEGO—Watching videos through special glasses can calm patients undergoing a biopsy or other minimally invasive treatment, according to research presented at the Society of Interventional Radiology’s 39th Annual Scientific Meeting.
Researchers have explored strategies other than medication to reduce anxiety in these patients, including having patients listen to music or undergo hypnosis. But these methods have had modest benefits.
“Our study—the first of its kind for interventional radiology treatments—puts a spin on using modern technology to provide a safe, potentially cost-effective strategy of reducing anxiety, which can help and improve patient care,” said David L. Waldman, MD, PhD, of the University of Rochester Medical Center in New York.
“Whether they were watching a children’s movie or a nature show, patients wearing video glasses were successful at tuning out their surroundings. It’s an effective distraction technique that helps focus the individual’s attention away from the treatment.”
The study involved 49 patients (33 men and 16 women, ages 18-87) who were undergoing an outpatient interventional radiology treatment, such as a biopsy or placement of a catheter in the arm or chest to receive medication for treating cancer or infection.
Twenty-five of the patients donned video glasses prior to undergoing the treatment, and 24 did not. The video viewers chose from 20 videos, none of which were violent.
All of the patients filled out a standard 20-question test called the State-Trait Anxiety Inventory Form Y before and after the procedure to assess their level of anxiety.
Patients who wore the video glasses were 18.1% less anxious after the treatment than they were before, while those who didn’t wear video glasses were 7.5% less anxious afterward.
And the presence of the video glasses did not bother either the patient or the doctor, Dr Waldman said.
The glasses had no significant effect on blood pressure, heart rate, respiratory rate, pain, procedure time, or the amount of sedation or pain medication used.
“Patients told us the video glasses really helped calm them down and took their mind off the treatment, and we now offer video glasses to help distract patients from medical treatment going on mere inches away,” Dr Waldman said. “It is really comforting for patients, especially the ones who tend to be more nervous.
Dr Waldman and his colleagues presented these results at the meeting as abstract 126. ![]()

Credit: CDC
SAN DIEGO—Watching videos through special glasses can calm patients undergoing a biopsy or other minimally invasive treatment, according to research presented at the Society of Interventional Radiology’s 39th Annual Scientific Meeting.
Researchers have explored strategies other than medication to reduce anxiety in these patients, including having patients listen to music or undergo hypnosis. But these methods have had modest benefits.
“Our study—the first of its kind for interventional radiology treatments—puts a spin on using modern technology to provide a safe, potentially cost-effective strategy of reducing anxiety, which can help and improve patient care,” said David L. Waldman, MD, PhD, of the University of Rochester Medical Center in New York.
“Whether they were watching a children’s movie or a nature show, patients wearing video glasses were successful at tuning out their surroundings. It’s an effective distraction technique that helps focus the individual’s attention away from the treatment.”
The study involved 49 patients (33 men and 16 women, ages 18-87) who were undergoing an outpatient interventional radiology treatment, such as a biopsy or placement of a catheter in the arm or chest to receive medication for treating cancer or infection.
Twenty-five of the patients donned video glasses prior to undergoing the treatment, and 24 did not. The video viewers chose from 20 videos, none of which were violent.
All of the patients filled out a standard 20-question test called the State-Trait Anxiety Inventory Form Y before and after the procedure to assess their level of anxiety.
Patients who wore the video glasses were 18.1% less anxious after the treatment than they were before, while those who didn’t wear video glasses were 7.5% less anxious afterward.
And the presence of the video glasses did not bother either the patient or the doctor, Dr Waldman said.
The glasses had no significant effect on blood pressure, heart rate, respiratory rate, pain, procedure time, or the amount of sedation or pain medication used.
“Patients told us the video glasses really helped calm them down and took their mind off the treatment, and we now offer video glasses to help distract patients from medical treatment going on mere inches away,” Dr Waldman said. “It is really comforting for patients, especially the ones who tend to be more nervous.
Dr Waldman and his colleagues presented these results at the meeting as abstract 126.
Bloodstream infections treated ‘inappropriately’
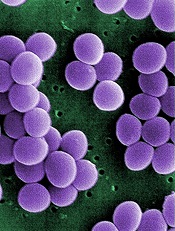
Credit: Janice Haney Carr
An analysis of 9 community hospitals showed that 1 in 3 patients with bloodstream infections received inappropriate therapy.
The study also revealed growing resistance to treatment and a high prevalence of Staphylococcus aureus bacteria in these hospitals.
Investigators said the findings, published in PLOS ONE, provide the most comprehensive look at bloodstream infections in community hospitals to date.
Much of the existing research on bloodstream infections focuses on tertiary care centers.
“Our study provides a much-needed update on what we’re seeing in community hospitals, and ultimately, we’re finding similar types of infections in these hospitals as in tertiary care centers,” said study author Deverick Anderson, MD, of Duke University in Durham North Carolina.
“It’s a challenge to identify bloodstream infections and treat them quickly and appropriately, but this study shows that there is room for improvement in both kinds of hospital settings.”
Types of infection
To better understand the types of bloodstream infections found in community hospitals, Dr Anderson and his colleagues collected information on patients treated at these hospitals in Virginia and North Carolina from 2003 to 2006.
The investigators focused on 1470 patients diagnosed with bloodstream infections. The infections were classified depending on where and when they were contracted.
Infections resulting from prior hospitalization, surgery, invasive devices (such as catheters), or living in long-term care facilities were designated healthcare-associated infections.
Community-acquired infections were contracted outside of medical settings or shortly after being admitted to a hospital. And hospital-onset infections occurred after being in a hospital for several days.
The investigators found that 56% of bloodstream infections were healthcare-associated, but symptoms began prior to hospital admission. Community-acquired infections unrelated to medical care were seen in 29% of patients. And 15% had hospital-onset healthcare-associated infections.
S aureus was the most common pathogen, causing 28% of bloodstream infections. This was closely followed by Escherichia coli, which was found in 24% of patients.
Bloodstream infections due to multidrug-resistant pathogens occurred in 23% of patients—an increase over earlier studies. And methicillin-resistant S aureus (MRSA) was the most common multidrug-resistant pathogen.
“Similar patterns of pathogens and drug resistance have been observed in tertiary care centers, suggesting that bloodstream infections in community hospitals aren’t that different from tertiary care centers,” Dr Anderson said.
“There’s a misconception that community hospitals don’t have to deal with S aureus and MRSA, but our findings dispel that myth, since community hospitals also see these serious infections.”
Inappropriate therapy
The investigators also found that approximately 38% of patients with bloodstream infections received inappropriate empiric antimicrobial therapy or were not initially prescribed an effective antibiotic while the cause of the infection was still unknown.
A multivariate analysis revealed several factors associated with receiving inappropriate therapy, including the hospital where the patient received care (P<0.001), the need for assistance with 3 or more “daily living” activities (P=0.005), and a high Charlson score (P=0.05).
Community-onset healthcare-associated infections (P=0.01) and hospital-onset healthcare-associated infections (P=0.02) were associated with the failure to receive appropriate therapy, when community-acquired infections were used as the reference.
The investigators also incorporated drug resistance into their analysis. And they found that infection due to a multidrug-resistant organism was strongly associated with the failure to receive appropriate therapy (P<0.0001).
But most of the predictors the team initially identified retained their significance. The patient’s hospital (P<0.001), need for assistance with activities (P=0.02), and type of infection remained significant (P=0.04), but the Charlson score did not (P=0.07).
Dr Anderson recommended that clinicians in community hospitals focus on these risk factors when choosing antibiotic therapy for patients with bloodstream infections. He noted that most risk factors for receiving inappropriate therapy are already recorded in electronic health records.
“Developing an intervention where electronic records automatically alert clinicians to these risk factors when they’re choosing antibiotics could help reduce the problem,” he said. “This is just a place to start, but it’s an example of an area where we could improve how we treat patients with bloodstream infections.”

Credit: Janice Haney Carr
An analysis of 9 community hospitals showed that 1 in 3 patients with bloodstream infections received inappropriate therapy.
The study also revealed growing resistance to treatment and a high prevalence of Staphylococcus aureus bacteria in these hospitals.
Investigators said the findings, published in PLOS ONE, provide the most comprehensive look at bloodstream infections in community hospitals to date.
Much of the existing research on bloodstream infections focuses on tertiary care centers.
“Our study provides a much-needed update on what we’re seeing in community hospitals, and ultimately, we’re finding similar types of infections in these hospitals as in tertiary care centers,” said study author Deverick Anderson, MD, of Duke University in Durham North Carolina.
“It’s a challenge to identify bloodstream infections and treat them quickly and appropriately, but this study shows that there is room for improvement in both kinds of hospital settings.”
Types of infection
To better understand the types of bloodstream infections found in community hospitals, Dr Anderson and his colleagues collected information on patients treated at these hospitals in Virginia and North Carolina from 2003 to 2006.
The investigators focused on 1470 patients diagnosed with bloodstream infections. The infections were classified depending on where and when they were contracted.
Infections resulting from prior hospitalization, surgery, invasive devices (such as catheters), or living in long-term care facilities were designated healthcare-associated infections.
Community-acquired infections were contracted outside of medical settings or shortly after being admitted to a hospital. And hospital-onset infections occurred after being in a hospital for several days.
The investigators found that 56% of bloodstream infections were healthcare-associated, but symptoms began prior to hospital admission. Community-acquired infections unrelated to medical care were seen in 29% of patients. And 15% had hospital-onset healthcare-associated infections.
S aureus was the most common pathogen, causing 28% of bloodstream infections. This was closely followed by Escherichia coli, which was found in 24% of patients.
Bloodstream infections due to multidrug-resistant pathogens occurred in 23% of patients—an increase over earlier studies. And methicillin-resistant S aureus (MRSA) was the most common multidrug-resistant pathogen.
“Similar patterns of pathogens and drug resistance have been observed in tertiary care centers, suggesting that bloodstream infections in community hospitals aren’t that different from tertiary care centers,” Dr Anderson said.
“There’s a misconception that community hospitals don’t have to deal with S aureus and MRSA, but our findings dispel that myth, since community hospitals also see these serious infections.”
Inappropriate therapy
The investigators also found that approximately 38% of patients with bloodstream infections received inappropriate empiric antimicrobial therapy or were not initially prescribed an effective antibiotic while the cause of the infection was still unknown.
A multivariate analysis revealed several factors associated with receiving inappropriate therapy, including the hospital where the patient received care (P<0.001), the need for assistance with 3 or more “daily living” activities (P=0.005), and a high Charlson score (P=0.05).
Community-onset healthcare-associated infections (P=0.01) and hospital-onset healthcare-associated infections (P=0.02) were associated with the failure to receive appropriate therapy, when community-acquired infections were used as the reference.
The investigators also incorporated drug resistance into their analysis. And they found that infection due to a multidrug-resistant organism was strongly associated with the failure to receive appropriate therapy (P<0.0001).
But most of the predictors the team initially identified retained their significance. The patient’s hospital (P<0.001), need for assistance with activities (P=0.02), and type of infection remained significant (P=0.04), but the Charlson score did not (P=0.07).
Dr Anderson recommended that clinicians in community hospitals focus on these risk factors when choosing antibiotic therapy for patients with bloodstream infections. He noted that most risk factors for receiving inappropriate therapy are already recorded in electronic health records.
“Developing an intervention where electronic records automatically alert clinicians to these risk factors when they’re choosing antibiotics could help reduce the problem,” he said. “This is just a place to start, but it’s an example of an area where we could improve how we treat patients with bloodstream infections.”

Credit: Janice Haney Carr
An analysis of 9 community hospitals showed that 1 in 3 patients with bloodstream infections received inappropriate therapy.
The study also revealed growing resistance to treatment and a high prevalence of Staphylococcus aureus bacteria in these hospitals.
Investigators said the findings, published in PLOS ONE, provide the most comprehensive look at bloodstream infections in community hospitals to date.
Much of the existing research on bloodstream infections focuses on tertiary care centers.
“Our study provides a much-needed update on what we’re seeing in community hospitals, and ultimately, we’re finding similar types of infections in these hospitals as in tertiary care centers,” said study author Deverick Anderson, MD, of Duke University in Durham North Carolina.
“It’s a challenge to identify bloodstream infections and treat them quickly and appropriately, but this study shows that there is room for improvement in both kinds of hospital settings.”
Types of infection
To better understand the types of bloodstream infections found in community hospitals, Dr Anderson and his colleagues collected information on patients treated at these hospitals in Virginia and North Carolina from 2003 to 2006.
The investigators focused on 1470 patients diagnosed with bloodstream infections. The infections were classified depending on where and when they were contracted.
Infections resulting from prior hospitalization, surgery, invasive devices (such as catheters), or living in long-term care facilities were designated healthcare-associated infections.
Community-acquired infections were contracted outside of medical settings or shortly after being admitted to a hospital. And hospital-onset infections occurred after being in a hospital for several days.
The investigators found that 56% of bloodstream infections were healthcare-associated, but symptoms began prior to hospital admission. Community-acquired infections unrelated to medical care were seen in 29% of patients. And 15% had hospital-onset healthcare-associated infections.
S aureus was the most common pathogen, causing 28% of bloodstream infections. This was closely followed by Escherichia coli, which was found in 24% of patients.
Bloodstream infections due to multidrug-resistant pathogens occurred in 23% of patients—an increase over earlier studies. And methicillin-resistant S aureus (MRSA) was the most common multidrug-resistant pathogen.
“Similar patterns of pathogens and drug resistance have been observed in tertiary care centers, suggesting that bloodstream infections in community hospitals aren’t that different from tertiary care centers,” Dr Anderson said.
“There’s a misconception that community hospitals don’t have to deal with S aureus and MRSA, but our findings dispel that myth, since community hospitals also see these serious infections.”
Inappropriate therapy
The investigators also found that approximately 38% of patients with bloodstream infections received inappropriate empiric antimicrobial therapy or were not initially prescribed an effective antibiotic while the cause of the infection was still unknown.
A multivariate analysis revealed several factors associated with receiving inappropriate therapy, including the hospital where the patient received care (P<0.001), the need for assistance with 3 or more “daily living” activities (P=0.005), and a high Charlson score (P=0.05).
Community-onset healthcare-associated infections (P=0.01) and hospital-onset healthcare-associated infections (P=0.02) were associated with the failure to receive appropriate therapy, when community-acquired infections were used as the reference.
The investigators also incorporated drug resistance into their analysis. And they found that infection due to a multidrug-resistant organism was strongly associated with the failure to receive appropriate therapy (P<0.0001).
But most of the predictors the team initially identified retained their significance. The patient’s hospital (P<0.001), need for assistance with activities (P=0.02), and type of infection remained significant (P=0.04), but the Charlson score did not (P=0.07).
Dr Anderson recommended that clinicians in community hospitals focus on these risk factors when choosing antibiotic therapy for patients with bloodstream infections. He noted that most risk factors for receiving inappropriate therapy are already recorded in electronic health records.
“Developing an intervention where electronic records automatically alert clinicians to these risk factors when they’re choosing antibiotics could help reduce the problem,” he said. “This is just a place to start, but it’s an example of an area where we could improve how we treat patients with bloodstream infections.”
ALL maintenance phase still stressful, studies show

Credit: Bill Branson
The stress and lifestyle changes mothers experience after a child’s leukemia diagnosis do not disappear once the disease is in the maintenance phase, new research suggests.
One study showed that mothers of patients with acute lymphoblastic leukemia (ALL) continued to exhibit signs of stress, anxiety, and depression when their child’s disease was in the maintenance phase.
And another study showed that a family’s daily schedule and sleeping arrangements did not return to the way they were before the child’s diagnosis.
“Even though these mothers were in the maintenance phase of their child’s illness, and the prognosis was good, we heard them say over and over that things could never go back to what they were before,” said study author Madalynn Neu, PhD, RN, of the Colorado University College of Nursing.
Stress, depression, and anxiety
In the Journal of Pediatric Oncology Nursing, Dr Neu and her colleagues reported their analysis of stress, anxiety, and depression among the mothers of patients with ALL. The team evaluated 26 mothers of ALL patients and 26 mothers of healthy children, who were matched according to the child’s age and gender.
To assess anxiety, the researchers collected salivary cortisol from the mothers 4 times a day for 3 consecutive days. The mothers also completed questionnaires—the Hospital Anxiety and Depression Scale and the Perceived Stress Scale.
More mothers in the ALL group had questionnaire scores indicating clinical anxiety (46%) and depressive symptoms (27%). And there was a trend toward increased stress in the mothers of ALL patients.
However, the researchers were surprised to find that the mothers’ anxiety levels—as measured by salivary cortisol—were similar to the mothers of well children.
“This may have been affected by the fact that even the control group wasn’t without anxiety,” said study author Ellen Matthews, PhD, RN, of the Colorado University College of Nursing.
“Financial, marital, social, and career concerns mean that parents of young children experience anxiety even without ALL.”
The sleep-wake experience
In the Journal of Pediatric Nursing, the researchers described their assessment of the sleep-wake experience for mothers of ALL patients. The team conducted interviews with 20 mothers, using open-ended, semi-structured questions, and the answers were transcribed verbatim.
Two main themes emerged during these interviews. The first was dubbed, “It’s a whole new cancer world” and contained 4 subthemes: losing normality, being off-balance/insecure, juggling duties, and making transitions.
“Many [of the mothers] had lost their normal lives—lost jobs, houses, friends,” Dr Matthews said. “Some were juggling their time around their child’s needs, and they had fears about many things—fear of recurrence, fear of making a mistake with medication, fear their kids might get sick with an infection.”
The second theme that emerged in interviews was, “I don’t remember what it’s like to have sleep.” This also contained 4 subthemes: sleeping trouble before and after ALL, the child feeling sick at night, worrying, and coping with exhaustion.
The mothers also noted that once sleep arrangements had changed, they often did not return to their pretreatment “normal.”
“Mothers talked about the difficulty of sleep while giving steroid medication,” Dr Neu said. “And if the ill child got to stay up late watching movies, the siblings wanted to stay up too.”
“The same was true of sleeping in a parent’s room. If an ill child wanted to sleep close to a parent (or if a parent wanted to sleep close to an ill child), siblings tended to move in as well. Sleep can be challenging for parents of well children, and our studies show it’s even more so for parents of children who have experienced ALL.”
The researchers hope these studies increase awareness of maternal concerns after a child’s leukemia diagnosis and that leads to interventions to help mothers manage these lifestyle issues.

Credit: Bill Branson
The stress and lifestyle changes mothers experience after a child’s leukemia diagnosis do not disappear once the disease is in the maintenance phase, new research suggests.
One study showed that mothers of patients with acute lymphoblastic leukemia (ALL) continued to exhibit signs of stress, anxiety, and depression when their child’s disease was in the maintenance phase.
And another study showed that a family’s daily schedule and sleeping arrangements did not return to the way they were before the child’s diagnosis.
“Even though these mothers were in the maintenance phase of their child’s illness, and the prognosis was good, we heard them say over and over that things could never go back to what they were before,” said study author Madalynn Neu, PhD, RN, of the Colorado University College of Nursing.
Stress, depression, and anxiety
In the Journal of Pediatric Oncology Nursing, Dr Neu and her colleagues reported their analysis of stress, anxiety, and depression among the mothers of patients with ALL. The team evaluated 26 mothers of ALL patients and 26 mothers of healthy children, who were matched according to the child’s age and gender.
To assess anxiety, the researchers collected salivary cortisol from the mothers 4 times a day for 3 consecutive days. The mothers also completed questionnaires—the Hospital Anxiety and Depression Scale and the Perceived Stress Scale.
More mothers in the ALL group had questionnaire scores indicating clinical anxiety (46%) and depressive symptoms (27%). And there was a trend toward increased stress in the mothers of ALL patients.
However, the researchers were surprised to find that the mothers’ anxiety levels—as measured by salivary cortisol—were similar to the mothers of well children.
“This may have been affected by the fact that even the control group wasn’t without anxiety,” said study author Ellen Matthews, PhD, RN, of the Colorado University College of Nursing.
“Financial, marital, social, and career concerns mean that parents of young children experience anxiety even without ALL.”
The sleep-wake experience
In the Journal of Pediatric Nursing, the researchers described their assessment of the sleep-wake experience for mothers of ALL patients. The team conducted interviews with 20 mothers, using open-ended, semi-structured questions, and the answers were transcribed verbatim.
Two main themes emerged during these interviews. The first was dubbed, “It’s a whole new cancer world” and contained 4 subthemes: losing normality, being off-balance/insecure, juggling duties, and making transitions.
“Many [of the mothers] had lost their normal lives—lost jobs, houses, friends,” Dr Matthews said. “Some were juggling their time around their child’s needs, and they had fears about many things—fear of recurrence, fear of making a mistake with medication, fear their kids might get sick with an infection.”
The second theme that emerged in interviews was, “I don’t remember what it’s like to have sleep.” This also contained 4 subthemes: sleeping trouble before and after ALL, the child feeling sick at night, worrying, and coping with exhaustion.
The mothers also noted that once sleep arrangements had changed, they often did not return to their pretreatment “normal.”
“Mothers talked about the difficulty of sleep while giving steroid medication,” Dr Neu said. “And if the ill child got to stay up late watching movies, the siblings wanted to stay up too.”
“The same was true of sleeping in a parent’s room. If an ill child wanted to sleep close to a parent (or if a parent wanted to sleep close to an ill child), siblings tended to move in as well. Sleep can be challenging for parents of well children, and our studies show it’s even more so for parents of children who have experienced ALL.”
The researchers hope these studies increase awareness of maternal concerns after a child’s leukemia diagnosis and that leads to interventions to help mothers manage these lifestyle issues.

Credit: Bill Branson
The stress and lifestyle changes mothers experience after a child’s leukemia diagnosis do not disappear once the disease is in the maintenance phase, new research suggests.
One study showed that mothers of patients with acute lymphoblastic leukemia (ALL) continued to exhibit signs of stress, anxiety, and depression when their child’s disease was in the maintenance phase.
And another study showed that a family’s daily schedule and sleeping arrangements did not return to the way they were before the child’s diagnosis.
“Even though these mothers were in the maintenance phase of their child’s illness, and the prognosis was good, we heard them say over and over that things could never go back to what they were before,” said study author Madalynn Neu, PhD, RN, of the Colorado University College of Nursing.
Stress, depression, and anxiety
In the Journal of Pediatric Oncology Nursing, Dr Neu and her colleagues reported their analysis of stress, anxiety, and depression among the mothers of patients with ALL. The team evaluated 26 mothers of ALL patients and 26 mothers of healthy children, who were matched according to the child’s age and gender.
To assess anxiety, the researchers collected salivary cortisol from the mothers 4 times a day for 3 consecutive days. The mothers also completed questionnaires—the Hospital Anxiety and Depression Scale and the Perceived Stress Scale.
More mothers in the ALL group had questionnaire scores indicating clinical anxiety (46%) and depressive symptoms (27%). And there was a trend toward increased stress in the mothers of ALL patients.
However, the researchers were surprised to find that the mothers’ anxiety levels—as measured by salivary cortisol—were similar to the mothers of well children.
“This may have been affected by the fact that even the control group wasn’t without anxiety,” said study author Ellen Matthews, PhD, RN, of the Colorado University College of Nursing.
“Financial, marital, social, and career concerns mean that parents of young children experience anxiety even without ALL.”
The sleep-wake experience
In the Journal of Pediatric Nursing, the researchers described their assessment of the sleep-wake experience for mothers of ALL patients. The team conducted interviews with 20 mothers, using open-ended, semi-structured questions, and the answers were transcribed verbatim.
Two main themes emerged during these interviews. The first was dubbed, “It’s a whole new cancer world” and contained 4 subthemes: losing normality, being off-balance/insecure, juggling duties, and making transitions.
“Many [of the mothers] had lost their normal lives—lost jobs, houses, friends,” Dr Matthews said. “Some were juggling their time around their child’s needs, and they had fears about many things—fear of recurrence, fear of making a mistake with medication, fear their kids might get sick with an infection.”
The second theme that emerged in interviews was, “I don’t remember what it’s like to have sleep.” This also contained 4 subthemes: sleeping trouble before and after ALL, the child feeling sick at night, worrying, and coping with exhaustion.
The mothers also noted that once sleep arrangements had changed, they often did not return to their pretreatment “normal.”
“Mothers talked about the difficulty of sleep while giving steroid medication,” Dr Neu said. “And if the ill child got to stay up late watching movies, the siblings wanted to stay up too.”
“The same was true of sleeping in a parent’s room. If an ill child wanted to sleep close to a parent (or if a parent wanted to sleep close to an ill child), siblings tended to move in as well. Sleep can be challenging for parents of well children, and our studies show it’s even more so for parents of children who have experienced ALL.”
The researchers hope these studies increase awareness of maternal concerns after a child’s leukemia diagnosis and that leads to interventions to help mothers manage these lifestyle issues.
Palliative chemo can have undesired outcomes

Credit: Rhoda Baer
Palliative chemotherapy can negatively impact the end of life for terminally ill cancer patients, according to a paper published in BMJ.
Investigators found that patients who received palliative chemotherapy in their last months of life had an increased risk of requiring intensive medical care, such as resuscitation, and dying in a place they did not choose, such as an intensive care unit.
The researchers therefore suggested that end-of-life discussions may be particularly important for patients who want to receive palliative chemotherapy.
“The results highlight the need for more effective communication by doctors of terminal prognoses and the likely outcomes of chemotherapy for these patients,” said study author Holly Prigerson, PhD, of Weill Cornell Medical College in New York.
“For patients to make informed choices about their care, they need to know if they are incurable and understand what their life expectancy is, that palliative chemotherapy is not intended to cure them, that it may not appreciably prolong their life, and that it may result in the receipt of very aggressive, life-prolonging care at the expense of their quality of life.”
Data have suggested that between 20% and 50% of patients with incurable cancers undergo palliative chemotherapy within 30 days of death. But it has not been clear whether the use of chemotherapy in a patient’s last months is associated with the need for intensive medical care in the last week of life or with the patient’s death.
So Dr Prigerson and her colleagues decided to study the use of palliative chemotherapy in patients with 6 or fewer months to live. The researchers used data from “Coping with Cancer,” a 6-year study of 386 terminally ill patients.
The patients were interviewed around the time of their decision regarding palliative chemotherapy. In the month after each patient died, caregivers were asked to rate their loved ones’ care, quality of life, and place of death as being where the patient would have wanted to die. The investigators then reviewed patients’ medical charts to determine the type of care they actually received in their last week.
Effects of palliative chemo
In all, 56% of patients opted to receive palliative chemotherapy. They were more likely to be younger, married, and better educated than patients not on the treatment.
Patients on chemotherapy also had better performance status, overall quality of life, physical functioning, and psychological well-being at study enrollment.
However, patients who received palliative chemotherapy had a greater risk of requiring cardiopulmonary resuscitation and/or mechanical ventilation (14% vs 2%), and they were more likely to need a feeding tube (11% vs 5%) in their last weeks of life.
Patients on chemotherapy had a greater risk of being admitted to an intensive care unit (14% vs 8%) and of having a late hospice referral (54% vs 37%).
They were also less likely to die where they wanted to (65% vs 80%). They had a greater risk of dying in an intensive care unit (11% vs 2%) and were less likely than their peers to die at home (47% vs 66%).
“It’s hard to see in these data much of a silver lining to palliative chemotherapy for patients in the terminal stage of their cancer,” Dr Prigerson said. “Until now, there hasn’t been evidence of harmful effects of palliative chemotherapy in the last few months of life.”
“This study is a first step in providing evidence that specifically demonstrates what negative outcomes may result. Additional studies are needed to confirm these troubling findings.”
Explaining the negative effects
Dr Prigerson said the harmful effects of palliative chemotherapy may be a result of misunderstanding, a lack of communication, and denial. Patients may not comprehend the purpose and likely consequences of palliative chemotherapy, and they may not fully acknowledge their own prognoses.
In the study, patients receiving palliative chemotherapy were less likely than their peers to talk to their oncologists about end-of-life care (37% vs 48%), to complete Do-Not-Resuscitate orders (36% vs 49%), or to acknowledge that they were terminally ill (35% vs 47%).
“Our finding that patients with terminal cancers were at higher risk of receiving intensive end-of-life care if they were treated with palliative chemotherapy months earlier underscores the importance of oncologists asking patients about their end-of-life wishes,” said Alexi Wright, MD, of the Dana-Farber Cancer Institute in Boston.
“We often wait until patients stop chemotherapy before asking them about where and how they want to die, but this study shows we need to ask patients about their preferences while they are receiving chemotherapy to ensure they receive the kind of care they want near death.”
Moving forward
The investigators stressed that the study results do not suggest patients should be denied palliative chemotherapy.
“The vast majority of patients in this study wanted palliative chemotherapy if it might increase their survival by as little as a week,” Dr Wright said. “This study is a step towards understanding some of the human costs and benefits of palliative chemotherapy.”
The researchers said additional studies should examine whether patients who are aware that chemotherapy is not intended to cure them still want to receive the treatment, confirm the negative outcomes of palliative chemotherapy, and determine if end-of-life discussions promote more informed decision-making and receipt of value-consistent care.
In a related editorial, Mike Rabow, MD, of the University of California, San Francisco, noted that although most patients with metastatic cancer choose to receive chemotherapy, evidence suggests most do not understand its intent.
He said Dr Prigerson’s study suggests the need to “better identify patients who are likely to benefit from chemotherapy near the end of life.” And he encouraged oncologists to discuss with patients the broader implications of chemotherapy when making decisions about treatment.

Credit: Rhoda Baer
Palliative chemotherapy can negatively impact the end of life for terminally ill cancer patients, according to a paper published in BMJ.
Investigators found that patients who received palliative chemotherapy in their last months of life had an increased risk of requiring intensive medical care, such as resuscitation, and dying in a place they did not choose, such as an intensive care unit.
The researchers therefore suggested that end-of-life discussions may be particularly important for patients who want to receive palliative chemotherapy.
“The results highlight the need for more effective communication by doctors of terminal prognoses and the likely outcomes of chemotherapy for these patients,” said study author Holly Prigerson, PhD, of Weill Cornell Medical College in New York.
“For patients to make informed choices about their care, they need to know if they are incurable and understand what their life expectancy is, that palliative chemotherapy is not intended to cure them, that it may not appreciably prolong their life, and that it may result in the receipt of very aggressive, life-prolonging care at the expense of their quality of life.”
Data have suggested that between 20% and 50% of patients with incurable cancers undergo palliative chemotherapy within 30 days of death. But it has not been clear whether the use of chemotherapy in a patient’s last months is associated with the need for intensive medical care in the last week of life or with the patient’s death.
So Dr Prigerson and her colleagues decided to study the use of palliative chemotherapy in patients with 6 or fewer months to live. The researchers used data from “Coping with Cancer,” a 6-year study of 386 terminally ill patients.
The patients were interviewed around the time of their decision regarding palliative chemotherapy. In the month after each patient died, caregivers were asked to rate their loved ones’ care, quality of life, and place of death as being where the patient would have wanted to die. The investigators then reviewed patients’ medical charts to determine the type of care they actually received in their last week.
Effects of palliative chemo
In all, 56% of patients opted to receive palliative chemotherapy. They were more likely to be younger, married, and better educated than patients not on the treatment.
Patients on chemotherapy also had better performance status, overall quality of life, physical functioning, and psychological well-being at study enrollment.
However, patients who received palliative chemotherapy had a greater risk of requiring cardiopulmonary resuscitation and/or mechanical ventilation (14% vs 2%), and they were more likely to need a feeding tube (11% vs 5%) in their last weeks of life.
Patients on chemotherapy had a greater risk of being admitted to an intensive care unit (14% vs 8%) and of having a late hospice referral (54% vs 37%).
They were also less likely to die where they wanted to (65% vs 80%). They had a greater risk of dying in an intensive care unit (11% vs 2%) and were less likely than their peers to die at home (47% vs 66%).
“It’s hard to see in these data much of a silver lining to palliative chemotherapy for patients in the terminal stage of their cancer,” Dr Prigerson said. “Until now, there hasn’t been evidence of harmful effects of palliative chemotherapy in the last few months of life.”
“This study is a first step in providing evidence that specifically demonstrates what negative outcomes may result. Additional studies are needed to confirm these troubling findings.”
Explaining the negative effects
Dr Prigerson said the harmful effects of palliative chemotherapy may be a result of misunderstanding, a lack of communication, and denial. Patients may not comprehend the purpose and likely consequences of palliative chemotherapy, and they may not fully acknowledge their own prognoses.
In the study, patients receiving palliative chemotherapy were less likely than their peers to talk to their oncologists about end-of-life care (37% vs 48%), to complete Do-Not-Resuscitate orders (36% vs 49%), or to acknowledge that they were terminally ill (35% vs 47%).
“Our finding that patients with terminal cancers were at higher risk of receiving intensive end-of-life care if they were treated with palliative chemotherapy months earlier underscores the importance of oncologists asking patients about their end-of-life wishes,” said Alexi Wright, MD, of the Dana-Farber Cancer Institute in Boston.
“We often wait until patients stop chemotherapy before asking them about where and how they want to die, but this study shows we need to ask patients about their preferences while they are receiving chemotherapy to ensure they receive the kind of care they want near death.”
Moving forward
The investigators stressed that the study results do not suggest patients should be denied palliative chemotherapy.
“The vast majority of patients in this study wanted palliative chemotherapy if it might increase their survival by as little as a week,” Dr Wright said. “This study is a step towards understanding some of the human costs and benefits of palliative chemotherapy.”
The researchers said additional studies should examine whether patients who are aware that chemotherapy is not intended to cure them still want to receive the treatment, confirm the negative outcomes of palliative chemotherapy, and determine if end-of-life discussions promote more informed decision-making and receipt of value-consistent care.
In a related editorial, Mike Rabow, MD, of the University of California, San Francisco, noted that although most patients with metastatic cancer choose to receive chemotherapy, evidence suggests most do not understand its intent.
He said Dr Prigerson’s study suggests the need to “better identify patients who are likely to benefit from chemotherapy near the end of life.” And he encouraged oncologists to discuss with patients the broader implications of chemotherapy when making decisions about treatment.

Credit: Rhoda Baer
Palliative chemotherapy can negatively impact the end of life for terminally ill cancer patients, according to a paper published in BMJ.
Investigators found that patients who received palliative chemotherapy in their last months of life had an increased risk of requiring intensive medical care, such as resuscitation, and dying in a place they did not choose, such as an intensive care unit.
The researchers therefore suggested that end-of-life discussions may be particularly important for patients who want to receive palliative chemotherapy.
“The results highlight the need for more effective communication by doctors of terminal prognoses and the likely outcomes of chemotherapy for these patients,” said study author Holly Prigerson, PhD, of Weill Cornell Medical College in New York.
“For patients to make informed choices about their care, they need to know if they are incurable and understand what their life expectancy is, that palliative chemotherapy is not intended to cure them, that it may not appreciably prolong their life, and that it may result in the receipt of very aggressive, life-prolonging care at the expense of their quality of life.”
Data have suggested that between 20% and 50% of patients with incurable cancers undergo palliative chemotherapy within 30 days of death. But it has not been clear whether the use of chemotherapy in a patient’s last months is associated with the need for intensive medical care in the last week of life or with the patient’s death.
So Dr Prigerson and her colleagues decided to study the use of palliative chemotherapy in patients with 6 or fewer months to live. The researchers used data from “Coping with Cancer,” a 6-year study of 386 terminally ill patients.
The patients were interviewed around the time of their decision regarding palliative chemotherapy. In the month after each patient died, caregivers were asked to rate their loved ones’ care, quality of life, and place of death as being where the patient would have wanted to die. The investigators then reviewed patients’ medical charts to determine the type of care they actually received in their last week.
Effects of palliative chemo
In all, 56% of patients opted to receive palliative chemotherapy. They were more likely to be younger, married, and better educated than patients not on the treatment.
Patients on chemotherapy also had better performance status, overall quality of life, physical functioning, and psychological well-being at study enrollment.
However, patients who received palliative chemotherapy had a greater risk of requiring cardiopulmonary resuscitation and/or mechanical ventilation (14% vs 2%), and they were more likely to need a feeding tube (11% vs 5%) in their last weeks of life.
Patients on chemotherapy had a greater risk of being admitted to an intensive care unit (14% vs 8%) and of having a late hospice referral (54% vs 37%).
They were also less likely to die where they wanted to (65% vs 80%). They had a greater risk of dying in an intensive care unit (11% vs 2%) and were less likely than their peers to die at home (47% vs 66%).
“It’s hard to see in these data much of a silver lining to palliative chemotherapy for patients in the terminal stage of their cancer,” Dr Prigerson said. “Until now, there hasn’t been evidence of harmful effects of palliative chemotherapy in the last few months of life.”
“This study is a first step in providing evidence that specifically demonstrates what negative outcomes may result. Additional studies are needed to confirm these troubling findings.”
Explaining the negative effects
Dr Prigerson said the harmful effects of palliative chemotherapy may be a result of misunderstanding, a lack of communication, and denial. Patients may not comprehend the purpose and likely consequences of palliative chemotherapy, and they may not fully acknowledge their own prognoses.
In the study, patients receiving palliative chemotherapy were less likely than their peers to talk to their oncologists about end-of-life care (37% vs 48%), to complete Do-Not-Resuscitate orders (36% vs 49%), or to acknowledge that they were terminally ill (35% vs 47%).
“Our finding that patients with terminal cancers were at higher risk of receiving intensive end-of-life care if they were treated with palliative chemotherapy months earlier underscores the importance of oncologists asking patients about their end-of-life wishes,” said Alexi Wright, MD, of the Dana-Farber Cancer Institute in Boston.
“We often wait until patients stop chemotherapy before asking them about where and how they want to die, but this study shows we need to ask patients about their preferences while they are receiving chemotherapy to ensure they receive the kind of care they want near death.”
Moving forward
The investigators stressed that the study results do not suggest patients should be denied palliative chemotherapy.
“The vast majority of patients in this study wanted palliative chemotherapy if it might increase their survival by as little as a week,” Dr Wright said. “This study is a step towards understanding some of the human costs and benefits of palliative chemotherapy.”
The researchers said additional studies should examine whether patients who are aware that chemotherapy is not intended to cure them still want to receive the treatment, confirm the negative outcomes of palliative chemotherapy, and determine if end-of-life discussions promote more informed decision-making and receipt of value-consistent care.
In a related editorial, Mike Rabow, MD, of the University of California, San Francisco, noted that although most patients with metastatic cancer choose to receive chemotherapy, evidence suggests most do not understand its intent.
He said Dr Prigerson’s study suggests the need to “better identify patients who are likely to benefit from chemotherapy near the end of life.” And he encouraged oncologists to discuss with patients the broader implications of chemotherapy when making decisions about treatment.