User login
FDA restricts obeticholic acid (Ocaliva) over serious liver injury risk
The risk for serious liver injury with obeticholic acid (Ocaliva, Intercept Pharmaceuticals) has prompted the U.S. Food and Drug Administration to restrict its use in patients with primary biliary cholangitis (PBC) and advanced cirrhosis.
The agency has added a new contraindication to the obeticholic acid prescribing information and patient medication guide stating that the drug should not be used in patients with PBC and advanced cirrhosis.
The boxed warning on the label has also been revised to include this information.
For patients with PBC who do not have advanced cirrhosis, the FDA believes the benefits of Ocaliva outweigh the risks, based on the original clinical trials.
Five years ago, the FDA granted accelerated approval to obeticholic acid in combination with ursodeoxycholic acid (UDCA) in adults who fail to respond adequately to UDCA, or as a monotherapy in adults who cannot tolerate UDCA.
Since then, the FDA has identified 25 cases of serious liver injury leading to liver decompensation or liver failure in patients with PBC and cirrhosis who were taking obeticholic acid at recommended doses.
According to the FDA, 18 of the cases happened in patients with PBC and compensated cirrhosis who experienced liver injury that led to decompensation. Ten of these patients had evidence or suspicion of portal hypertension at baseline; in the other eight patients, it was unclear whether portal hypertension was present.
PBC was not expected to progress rapidly in these patients, yet they experienced accelerated clinical deterioration within months of starting obeticholic acid, the FDA said.
The median time to liver decompensation after initiating treatment was 4 months (range, 2 weeks to 10 months). Four patients with PBC and compensated cirrhosis needed a liver transplant within 1.3 years after starting obeticholic acid, and one patient died from liver failure.
The other seven cases of serious liver injury occurred in patients with PBC and decompensated cirrhosis, two of whom died.
Although there was a temporal relationship between starting obeticholic acid and liver injury, it is difficult to distinguish a drug-induced effect from disease progression in the patients with advanced baseline liver disease, the FDA cautioned.
The median time to a new decompensation event after starting the drug was 2.5 months (range, 10 days to 8 months).
Before starting obeticholic acid, clinicians should determine whether a patient with PBC has advanced cirrhosis as the drug is now contraindicated in these patients, the FDA said.
During obeticholic acid treatment, patients should be routinely monitored for progression of PBC with laboratory and clinical assessments to determine whether the drug needs to be discontinued.
The medication should be permanently discontinued in patients with cirrhosis who progress to advanced cirrhosis.
Patients should also be monitored for clinically significant liver-related adverse reactions that may manifest as development of acute-on-chronic liver disease with nausea, vomiting, diarrhea, jaundice, scleral icterus, and/or dark urine.
Obeticholic acid should be stopped permanently in any patient who develops these symptoms, the FDA advised.
Health care professionals are encouraged to report adverse events or side effects related to the use of obeticholic acid to MedWatch, FDA’s adverse event reporting site.
A version of this article first appeared on Medscape.com.
The risk for serious liver injury with obeticholic acid (Ocaliva, Intercept Pharmaceuticals) has prompted the U.S. Food and Drug Administration to restrict its use in patients with primary biliary cholangitis (PBC) and advanced cirrhosis.
The agency has added a new contraindication to the obeticholic acid prescribing information and patient medication guide stating that the drug should not be used in patients with PBC and advanced cirrhosis.
The boxed warning on the label has also been revised to include this information.
For patients with PBC who do not have advanced cirrhosis, the FDA believes the benefits of Ocaliva outweigh the risks, based on the original clinical trials.
Five years ago, the FDA granted accelerated approval to obeticholic acid in combination with ursodeoxycholic acid (UDCA) in adults who fail to respond adequately to UDCA, or as a monotherapy in adults who cannot tolerate UDCA.
Since then, the FDA has identified 25 cases of serious liver injury leading to liver decompensation or liver failure in patients with PBC and cirrhosis who were taking obeticholic acid at recommended doses.
According to the FDA, 18 of the cases happened in patients with PBC and compensated cirrhosis who experienced liver injury that led to decompensation. Ten of these patients had evidence or suspicion of portal hypertension at baseline; in the other eight patients, it was unclear whether portal hypertension was present.
PBC was not expected to progress rapidly in these patients, yet they experienced accelerated clinical deterioration within months of starting obeticholic acid, the FDA said.
The median time to liver decompensation after initiating treatment was 4 months (range, 2 weeks to 10 months). Four patients with PBC and compensated cirrhosis needed a liver transplant within 1.3 years after starting obeticholic acid, and one patient died from liver failure.
The other seven cases of serious liver injury occurred in patients with PBC and decompensated cirrhosis, two of whom died.
Although there was a temporal relationship between starting obeticholic acid and liver injury, it is difficult to distinguish a drug-induced effect from disease progression in the patients with advanced baseline liver disease, the FDA cautioned.
The median time to a new decompensation event after starting the drug was 2.5 months (range, 10 days to 8 months).
Before starting obeticholic acid, clinicians should determine whether a patient with PBC has advanced cirrhosis as the drug is now contraindicated in these patients, the FDA said.
During obeticholic acid treatment, patients should be routinely monitored for progression of PBC with laboratory and clinical assessments to determine whether the drug needs to be discontinued.
The medication should be permanently discontinued in patients with cirrhosis who progress to advanced cirrhosis.
Patients should also be monitored for clinically significant liver-related adverse reactions that may manifest as development of acute-on-chronic liver disease with nausea, vomiting, diarrhea, jaundice, scleral icterus, and/or dark urine.
Obeticholic acid should be stopped permanently in any patient who develops these symptoms, the FDA advised.
Health care professionals are encouraged to report adverse events or side effects related to the use of obeticholic acid to MedWatch, FDA’s adverse event reporting site.
A version of this article first appeared on Medscape.com.
The risk for serious liver injury with obeticholic acid (Ocaliva, Intercept Pharmaceuticals) has prompted the U.S. Food and Drug Administration to restrict its use in patients with primary biliary cholangitis (PBC) and advanced cirrhosis.
The agency has added a new contraindication to the obeticholic acid prescribing information and patient medication guide stating that the drug should not be used in patients with PBC and advanced cirrhosis.
The boxed warning on the label has also been revised to include this information.
For patients with PBC who do not have advanced cirrhosis, the FDA believes the benefits of Ocaliva outweigh the risks, based on the original clinical trials.
Five years ago, the FDA granted accelerated approval to obeticholic acid in combination with ursodeoxycholic acid (UDCA) in adults who fail to respond adequately to UDCA, or as a monotherapy in adults who cannot tolerate UDCA.
Since then, the FDA has identified 25 cases of serious liver injury leading to liver decompensation or liver failure in patients with PBC and cirrhosis who were taking obeticholic acid at recommended doses.
According to the FDA, 18 of the cases happened in patients with PBC and compensated cirrhosis who experienced liver injury that led to decompensation. Ten of these patients had evidence or suspicion of portal hypertension at baseline; in the other eight patients, it was unclear whether portal hypertension was present.
PBC was not expected to progress rapidly in these patients, yet they experienced accelerated clinical deterioration within months of starting obeticholic acid, the FDA said.
The median time to liver decompensation after initiating treatment was 4 months (range, 2 weeks to 10 months). Four patients with PBC and compensated cirrhosis needed a liver transplant within 1.3 years after starting obeticholic acid, and one patient died from liver failure.
The other seven cases of serious liver injury occurred in patients with PBC and decompensated cirrhosis, two of whom died.
Although there was a temporal relationship between starting obeticholic acid and liver injury, it is difficult to distinguish a drug-induced effect from disease progression in the patients with advanced baseline liver disease, the FDA cautioned.
The median time to a new decompensation event after starting the drug was 2.5 months (range, 10 days to 8 months).
Before starting obeticholic acid, clinicians should determine whether a patient with PBC has advanced cirrhosis as the drug is now contraindicated in these patients, the FDA said.
During obeticholic acid treatment, patients should be routinely monitored for progression of PBC with laboratory and clinical assessments to determine whether the drug needs to be discontinued.
The medication should be permanently discontinued in patients with cirrhosis who progress to advanced cirrhosis.
Patients should also be monitored for clinically significant liver-related adverse reactions that may manifest as development of acute-on-chronic liver disease with nausea, vomiting, diarrhea, jaundice, scleral icterus, and/or dark urine.
Obeticholic acid should be stopped permanently in any patient who develops these symptoms, the FDA advised.
Health care professionals are encouraged to report adverse events or side effects related to the use of obeticholic acid to MedWatch, FDA’s adverse event reporting site.
A version of this article first appeared on Medscape.com.
A guide to diagnosing and managing ascites in cirrhosis
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
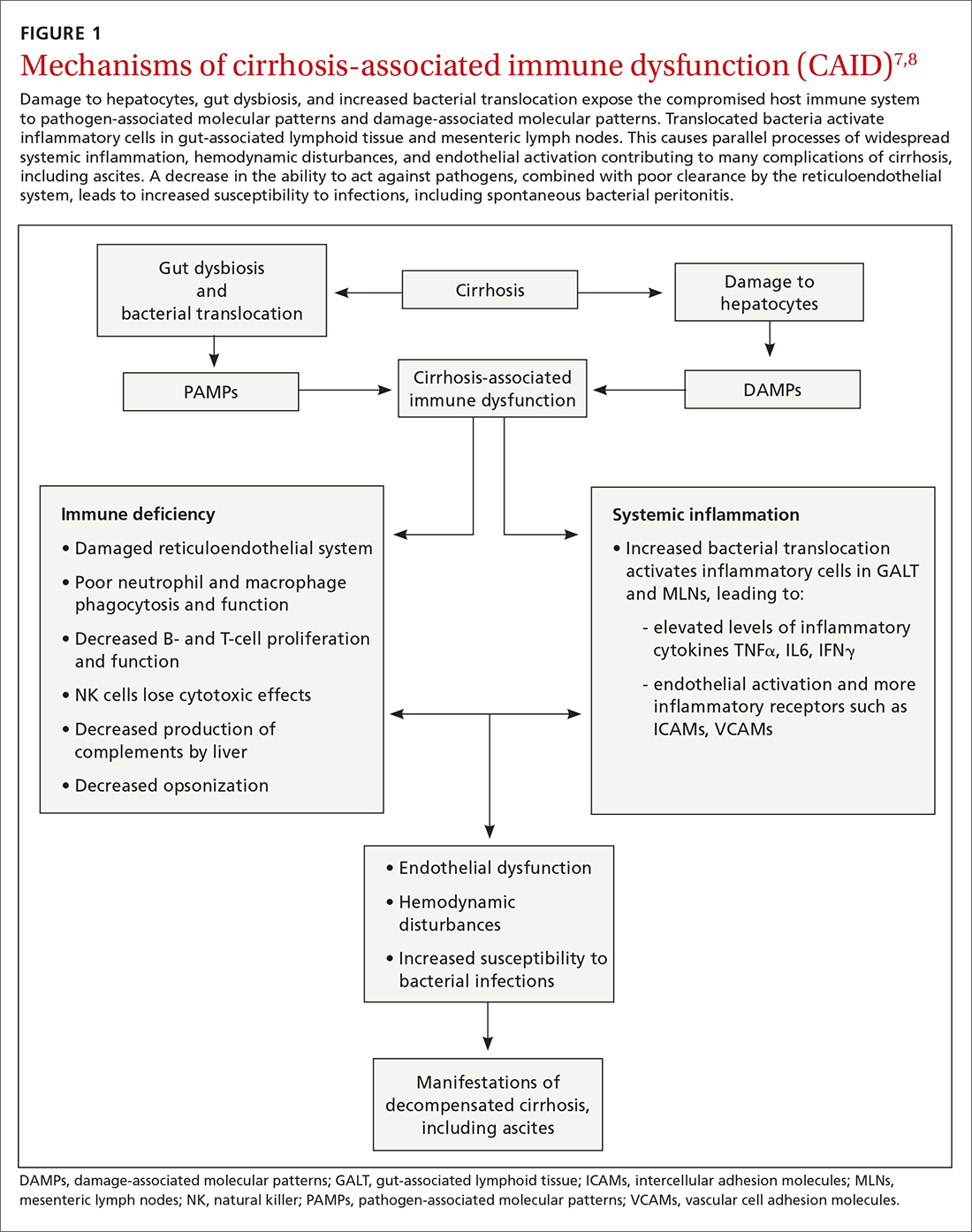
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8
Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).

History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
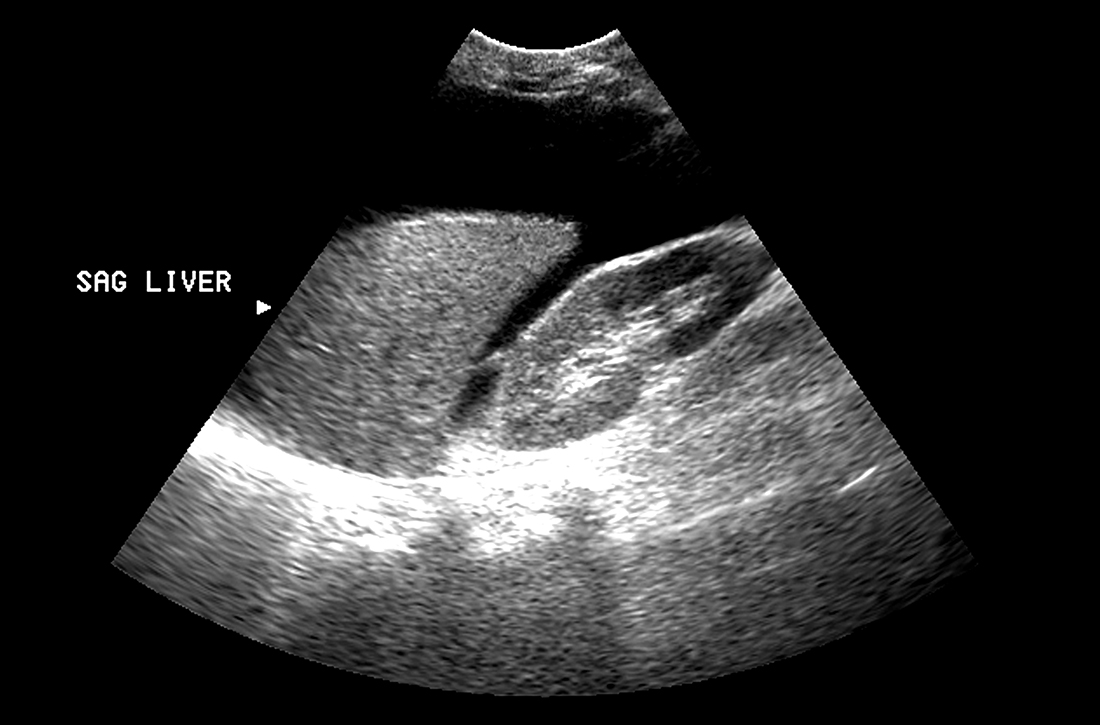
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
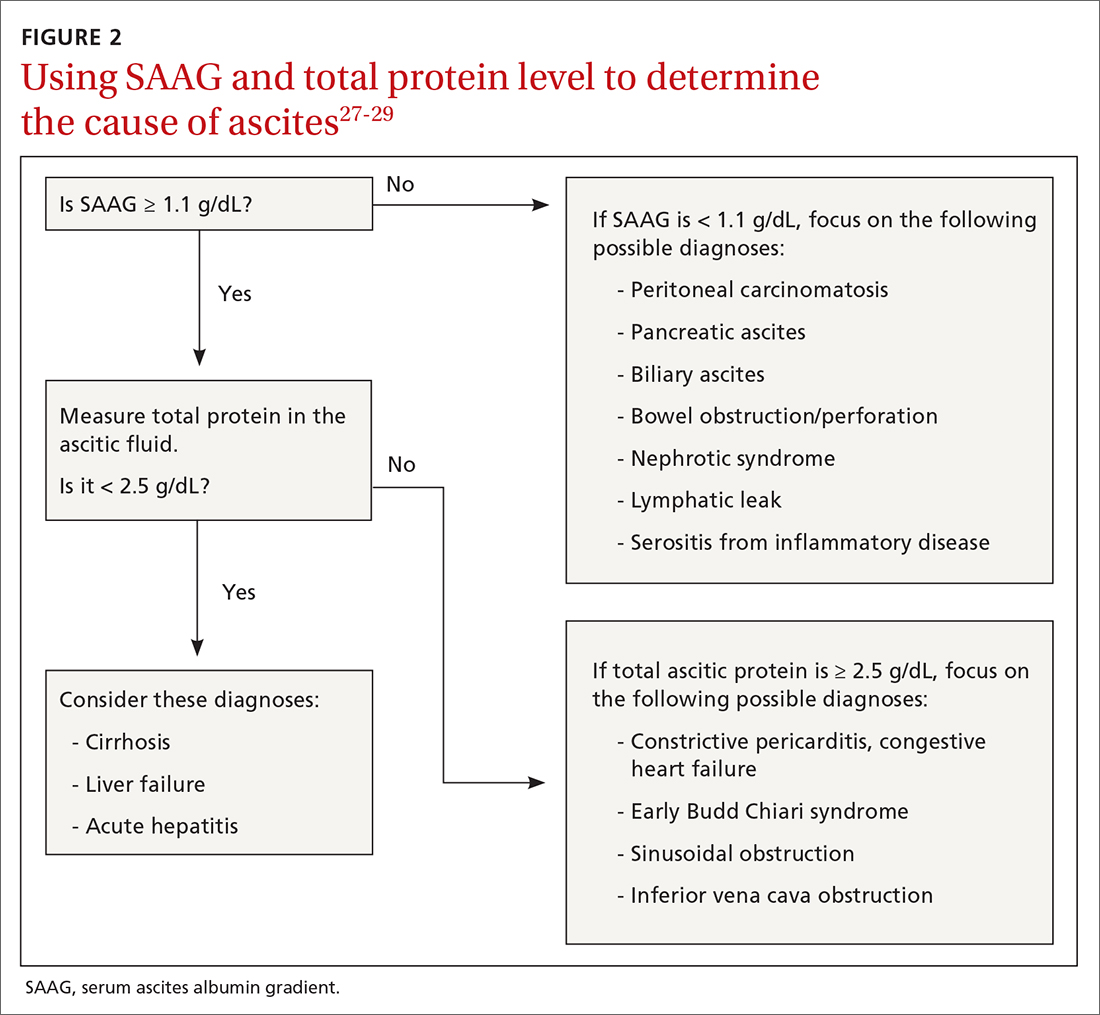
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29
Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8

Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).

History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29

Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8

Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).

History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29

Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
PRACTICE RECOMMENDATIONS
› Calculate the serum ascites albumin gradient and measure the total ascites protein level to distinguish cirrhotic ascites from that caused by heart failure or other disorders. C
› Recommend sodium restriction of 4.9-6.9 g for patients with established ascites secondary to cirrhosis. C
› Avoid giving angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs in cirrhosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Pediatric NAFLD almost always stems from excess body weight, not other etiologies
Nonalcoholic fatty liver disease (NAFLD) in children is almost always caused by excess body weight, not other etiologies, based on a retrospective analysis of 900 patients.
Just 2% of children with overweight or obesity and suspected NAFLD had other causes of liver disease, and none tested positive for autoimmune hepatitis (AIH), reported lead author Toshifumi Yodoshi, MD, PhD, of Cincinnati Children’s Hospital Medical Center, and colleagues.
“Currently, recommended testing of patients with suspected NAFLD includes ruling out the following conditions: AIH, Wilson disease, hemochromatosis, alpha-1 antitrypsin [A1AT] deficiency, viral hepatitis, celiac disease, and thyroid dysfunction,” the investigators wrote in Pediatrics.
Yet evidence supporting this particular battery of tests is scant; just one previous pediatric study has estimated the prevalence of other liver diseases among children with suspected NAFLD. The study showed that the second-most common etiology, after NAFLD, was AIH, at a rate of 4%.
But “the generalizability of these findings is uncertain,” noted Dr. Yodoshi and colleagues, as the study was conducted at one tertiary center in the western United States, among a population that was predominantly Hispanic.
This uncertainty spurred the present study, which was conducted at two pediatric centers: Cincinnati Children’s Hospital Medical Center (2009-2017) and Yale New Haven (Conn.) Children’s Hospital (2012-2017).
The final analysis involved 900 patients aged 18 years or younger with suspected NAFLD based on hepatic steatosis detected via imaging and/or elevated serum aminotransferases. Demographically, a slight majority of the patients were boys (63%), and approximately one-quarter (26%) were Hispanic. Median BMI z score was 2.45, with three out of four patients (76%) exhibiting severe obesity. Out of 900 patients, 358 (40%) underwent liver biopsy, among whom 46% had confirmed nonalcoholic steatohepatitis.
All patients underwent testing to exclude the aforementioned conditions using various diagnostics, revealing that just 2% of the population had etiologies other than NAFLD. Specifically, 11 children had thyroid dysfunction (1.2%), 3 had celiac disease (0.4%), 3 had A1AT deficiency (0.4%), 1 had hemophagocytic lymphohistiocytosis, and 1 had Hodgkin’s lymphoma. None of the children had Wilson disease, hepatitis B or C, or AIH.
Dr. Yodoshi and colleagues highlighted the latter finding, noting that 13% of the patients had autoantibodies for AIH, but “none met composite criteria.” This contrasts with the previous study from 2013, which found an AIH rate of 4%.
“Nonetheless,” the investigators went on, “NAFLD remains a diagnosis of exclusion, and key conditions that require specific treatments must be ruled out in the workup of patients with suspected NAFLD. In the future, the cost-effectiveness of this approach will need to be investigated.”
Interpreting the findings, Francis E. Rushton, MD, of Beaufort (S.C.) Memorial Hospital emphasized the implications for preventive and interventional health care.
“This study showing an absence of etiologies other than obesity in overweight children with NAFLD provides further impetus for pediatricians to work on both preventive and treatment regimens for weight issues,” Dr. Rushton said. “Linking community-based initiatives focused on adequate nutritional support with pediatric clinical support services is critical in solving issues related to overweight in children. Tracking BMI over time and developing healthy habit goals for patients are key parts of clinical interventions.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) in children is almost always caused by excess body weight, not other etiologies, based on a retrospective analysis of 900 patients.
Just 2% of children with overweight or obesity and suspected NAFLD had other causes of liver disease, and none tested positive for autoimmune hepatitis (AIH), reported lead author Toshifumi Yodoshi, MD, PhD, of Cincinnati Children’s Hospital Medical Center, and colleagues.
“Currently, recommended testing of patients with suspected NAFLD includes ruling out the following conditions: AIH, Wilson disease, hemochromatosis, alpha-1 antitrypsin [A1AT] deficiency, viral hepatitis, celiac disease, and thyroid dysfunction,” the investigators wrote in Pediatrics.
Yet evidence supporting this particular battery of tests is scant; just one previous pediatric study has estimated the prevalence of other liver diseases among children with suspected NAFLD. The study showed that the second-most common etiology, after NAFLD, was AIH, at a rate of 4%.
But “the generalizability of these findings is uncertain,” noted Dr. Yodoshi and colleagues, as the study was conducted at one tertiary center in the western United States, among a population that was predominantly Hispanic.
This uncertainty spurred the present study, which was conducted at two pediatric centers: Cincinnati Children’s Hospital Medical Center (2009-2017) and Yale New Haven (Conn.) Children’s Hospital (2012-2017).
The final analysis involved 900 patients aged 18 years or younger with suspected NAFLD based on hepatic steatosis detected via imaging and/or elevated serum aminotransferases. Demographically, a slight majority of the patients were boys (63%), and approximately one-quarter (26%) were Hispanic. Median BMI z score was 2.45, with three out of four patients (76%) exhibiting severe obesity. Out of 900 patients, 358 (40%) underwent liver biopsy, among whom 46% had confirmed nonalcoholic steatohepatitis.
All patients underwent testing to exclude the aforementioned conditions using various diagnostics, revealing that just 2% of the population had etiologies other than NAFLD. Specifically, 11 children had thyroid dysfunction (1.2%), 3 had celiac disease (0.4%), 3 had A1AT deficiency (0.4%), 1 had hemophagocytic lymphohistiocytosis, and 1 had Hodgkin’s lymphoma. None of the children had Wilson disease, hepatitis B or C, or AIH.
Dr. Yodoshi and colleagues highlighted the latter finding, noting that 13% of the patients had autoantibodies for AIH, but “none met composite criteria.” This contrasts with the previous study from 2013, which found an AIH rate of 4%.
“Nonetheless,” the investigators went on, “NAFLD remains a diagnosis of exclusion, and key conditions that require specific treatments must be ruled out in the workup of patients with suspected NAFLD. In the future, the cost-effectiveness of this approach will need to be investigated.”
Interpreting the findings, Francis E. Rushton, MD, of Beaufort (S.C.) Memorial Hospital emphasized the implications for preventive and interventional health care.
“This study showing an absence of etiologies other than obesity in overweight children with NAFLD provides further impetus for pediatricians to work on both preventive and treatment regimens for weight issues,” Dr. Rushton said. “Linking community-based initiatives focused on adequate nutritional support with pediatric clinical support services is critical in solving issues related to overweight in children. Tracking BMI over time and developing healthy habit goals for patients are key parts of clinical interventions.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) in children is almost always caused by excess body weight, not other etiologies, based on a retrospective analysis of 900 patients.
Just 2% of children with overweight or obesity and suspected NAFLD had other causes of liver disease, and none tested positive for autoimmune hepatitis (AIH), reported lead author Toshifumi Yodoshi, MD, PhD, of Cincinnati Children’s Hospital Medical Center, and colleagues.
“Currently, recommended testing of patients with suspected NAFLD includes ruling out the following conditions: AIH, Wilson disease, hemochromatosis, alpha-1 antitrypsin [A1AT] deficiency, viral hepatitis, celiac disease, and thyroid dysfunction,” the investigators wrote in Pediatrics.
Yet evidence supporting this particular battery of tests is scant; just one previous pediatric study has estimated the prevalence of other liver diseases among children with suspected NAFLD. The study showed that the second-most common etiology, after NAFLD, was AIH, at a rate of 4%.
But “the generalizability of these findings is uncertain,” noted Dr. Yodoshi and colleagues, as the study was conducted at one tertiary center in the western United States, among a population that was predominantly Hispanic.
This uncertainty spurred the present study, which was conducted at two pediatric centers: Cincinnati Children’s Hospital Medical Center (2009-2017) and Yale New Haven (Conn.) Children’s Hospital (2012-2017).
The final analysis involved 900 patients aged 18 years or younger with suspected NAFLD based on hepatic steatosis detected via imaging and/or elevated serum aminotransferases. Demographically, a slight majority of the patients were boys (63%), and approximately one-quarter (26%) were Hispanic. Median BMI z score was 2.45, with three out of four patients (76%) exhibiting severe obesity. Out of 900 patients, 358 (40%) underwent liver biopsy, among whom 46% had confirmed nonalcoholic steatohepatitis.
All patients underwent testing to exclude the aforementioned conditions using various diagnostics, revealing that just 2% of the population had etiologies other than NAFLD. Specifically, 11 children had thyroid dysfunction (1.2%), 3 had celiac disease (0.4%), 3 had A1AT deficiency (0.4%), 1 had hemophagocytic lymphohistiocytosis, and 1 had Hodgkin’s lymphoma. None of the children had Wilson disease, hepatitis B or C, or AIH.
Dr. Yodoshi and colleagues highlighted the latter finding, noting that 13% of the patients had autoantibodies for AIH, but “none met composite criteria.” This contrasts with the previous study from 2013, which found an AIH rate of 4%.
“Nonetheless,” the investigators went on, “NAFLD remains a diagnosis of exclusion, and key conditions that require specific treatments must be ruled out in the workup of patients with suspected NAFLD. In the future, the cost-effectiveness of this approach will need to be investigated.”
Interpreting the findings, Francis E. Rushton, MD, of Beaufort (S.C.) Memorial Hospital emphasized the implications for preventive and interventional health care.
“This study showing an absence of etiologies other than obesity in overweight children with NAFLD provides further impetus for pediatricians to work on both preventive and treatment regimens for weight issues,” Dr. Rushton said. “Linking community-based initiatives focused on adequate nutritional support with pediatric clinical support services is critical in solving issues related to overweight in children. Tracking BMI over time and developing healthy habit goals for patients are key parts of clinical interventions.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
FROM PEDIATRICS
Treatment paradigm for chronic HBV in flux
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
FROM GUILD 2021
Liver stiffness predicts hepatic events in NAFLD
Among patients with nonalcoholic fatty liver disease (NAFLD) and compensated advanced chronic liver disease, liver stiffness measurements (LSMs) are associated with risks of hepatic events, according to a retrospective analysis of more than 1,000 patients.
“[N]oninvasive markers that can predict liver disease severity and outcomes in patients with NAFLD and advanced fibrosis are a major unmet need,” wrote lead author Salvatore Petta, MD, of the University of Palermo, Italy, and colleagues. Their report is in Clinical Gastroenterology and Hepatology. “Data about the accuracy of LSM in the prediction of events in NAFLD, and especially in patients with NAFLD and F3-F4 fibrosis, are scarce.”
To address this knowledge gap, the investigators retrospectively analyzed data from 1,039 consecutive patients with NAFLD who had baseline LSMs of more than 10 kPa and/or histologically diagnosed F3-F4 fibrosis. Patients were prospectively recruited at 10 centers in 6 countries, then followed for a median of 35 months, ranging from 19 to 63 months.
All patients had their liver stiffness measured with an M or XL probe at baseline. In addition, approximately half of the patients (n = 533) had a follow-up measurement using the same method, generating a subgroup with changes in liver stiffness. “Improved” liver stiffness was defined as a decrease in LSM greater than 20% from baseline, “impaired” liver stiffness was defined as an increase in LSM greater than 20% from baseline, and “stable” liver stiffness was defined as a change falling between 20% lower and 20% higher than baseline.
At baseline, mean LSM was 17.6 kPa. Cox regression analysis revealed that baseline LSM was independently associated with HCC (hazard ratio, 1.03; 95% confidence interval, 1.00-1.04; P = .003), liver decompensation (HR, 1.03; 95% CI, 1.02-1.04; P < .001), and liver-related death (HR, 1.02; 95% CI, 1.00-1.03; P = .005), but not extrahepatic events.
According to the investigators, the association between LSM at baseline and risk of liver decompensation was maintained after adjustment for the severity of liver disease and for surrogate markers of portal hypertension, they noted. Furthermore, patients with a baseline LSM of at least 21 kPa – which indicates high risk of clinically significant portal hypertension (CSPH) – were at greater risk of liver decompensation than were those with an LSM less than 21 kPa (HR, 3.71; 95% CI, 1.89-6.78; P = .04).
In the subgroup with follow-up measurements, approximately half of the patients had an improved LSM (53.3%), while 27.2% had a stable LSM, and 19.5% had an impaired LSM, a pattern that was significantly associated with diabetes at baseline (P = .01).
“These data agree with the available literature identifying diabetes as a risk factor for liver disease progression and liver-related complications,” the investigators wrote.
Cox regression showed that, among those with follow-up LSM, changes in LSM were independently associated with HCC (HR, 1.72; 95% CI, 1.01-3.02; P = .04), liver decompensation (HR, 1.56; 95% CI, 1.05-2.51; P = . 04), liver-related mortality (HR, 1.96; 95% CI, 1.10-3.38; P = .02), and mortality of any cause (HR, 1.73; 95% CI, 1.11-2.69; P = .01).
These risks could be further stratified by level of change in liver stiffness, with greater impairment predicting greater risk: The crude rate of liver decompensation was 14.4% among those with impaired LSM, compared with 6.2% among those with stable LSM and 3.8% among those with LSM improvement. That said, the categories of changes in LSM were not predictive of decompensation among patients with high risk of CSPH at baseline; however, they remained predictive among those with low risk of CSPH at baseline.
“[T]his study … showed that an integrated assessment of baseline LSM or [changes in LSM] can help in stratifying the risk of development of liver-related complications and of both hepatic and overall mortality,” the investigators concluded. “These data, if further validated, could help personalize prognosis and follow-up in NAFLD with [compensated advanced chronic liver disease].”
The investigators disclosed relationships with AbbVie, Novo Nordisk, Gilead, and others.
Among patients with nonalcoholic fatty liver disease (NAFLD) and compensated advanced chronic liver disease, liver stiffness measurements (LSMs) are associated with risks of hepatic events, according to a retrospective analysis of more than 1,000 patients.
“[N]oninvasive markers that can predict liver disease severity and outcomes in patients with NAFLD and advanced fibrosis are a major unmet need,” wrote lead author Salvatore Petta, MD, of the University of Palermo, Italy, and colleagues. Their report is in Clinical Gastroenterology and Hepatology. “Data about the accuracy of LSM in the prediction of events in NAFLD, and especially in patients with NAFLD and F3-F4 fibrosis, are scarce.”
To address this knowledge gap, the investigators retrospectively analyzed data from 1,039 consecutive patients with NAFLD who had baseline LSMs of more than 10 kPa and/or histologically diagnosed F3-F4 fibrosis. Patients were prospectively recruited at 10 centers in 6 countries, then followed for a median of 35 months, ranging from 19 to 63 months.
All patients had their liver stiffness measured with an M or XL probe at baseline. In addition, approximately half of the patients (n = 533) had a follow-up measurement using the same method, generating a subgroup with changes in liver stiffness. “Improved” liver stiffness was defined as a decrease in LSM greater than 20% from baseline, “impaired” liver stiffness was defined as an increase in LSM greater than 20% from baseline, and “stable” liver stiffness was defined as a change falling between 20% lower and 20% higher than baseline.
At baseline, mean LSM was 17.6 kPa. Cox regression analysis revealed that baseline LSM was independently associated with HCC (hazard ratio, 1.03; 95% confidence interval, 1.00-1.04; P = .003), liver decompensation (HR, 1.03; 95% CI, 1.02-1.04; P < .001), and liver-related death (HR, 1.02; 95% CI, 1.00-1.03; P = .005), but not extrahepatic events.
According to the investigators, the association between LSM at baseline and risk of liver decompensation was maintained after adjustment for the severity of liver disease and for surrogate markers of portal hypertension, they noted. Furthermore, patients with a baseline LSM of at least 21 kPa – which indicates high risk of clinically significant portal hypertension (CSPH) – were at greater risk of liver decompensation than were those with an LSM less than 21 kPa (HR, 3.71; 95% CI, 1.89-6.78; P = .04).
In the subgroup with follow-up measurements, approximately half of the patients had an improved LSM (53.3%), while 27.2% had a stable LSM, and 19.5% had an impaired LSM, a pattern that was significantly associated with diabetes at baseline (P = .01).
“These data agree with the available literature identifying diabetes as a risk factor for liver disease progression and liver-related complications,” the investigators wrote.
Cox regression showed that, among those with follow-up LSM, changes in LSM were independently associated with HCC (HR, 1.72; 95% CI, 1.01-3.02; P = .04), liver decompensation (HR, 1.56; 95% CI, 1.05-2.51; P = . 04), liver-related mortality (HR, 1.96; 95% CI, 1.10-3.38; P = .02), and mortality of any cause (HR, 1.73; 95% CI, 1.11-2.69; P = .01).
These risks could be further stratified by level of change in liver stiffness, with greater impairment predicting greater risk: The crude rate of liver decompensation was 14.4% among those with impaired LSM, compared with 6.2% among those with stable LSM and 3.8% among those with LSM improvement. That said, the categories of changes in LSM were not predictive of decompensation among patients with high risk of CSPH at baseline; however, they remained predictive among those with low risk of CSPH at baseline.
“[T]his study … showed that an integrated assessment of baseline LSM or [changes in LSM] can help in stratifying the risk of development of liver-related complications and of both hepatic and overall mortality,” the investigators concluded. “These data, if further validated, could help personalize prognosis and follow-up in NAFLD with [compensated advanced chronic liver disease].”
The investigators disclosed relationships with AbbVie, Novo Nordisk, Gilead, and others.
Among patients with nonalcoholic fatty liver disease (NAFLD) and compensated advanced chronic liver disease, liver stiffness measurements (LSMs) are associated with risks of hepatic events, according to a retrospective analysis of more than 1,000 patients.
“[N]oninvasive markers that can predict liver disease severity and outcomes in patients with NAFLD and advanced fibrosis are a major unmet need,” wrote lead author Salvatore Petta, MD, of the University of Palermo, Italy, and colleagues. Their report is in Clinical Gastroenterology and Hepatology. “Data about the accuracy of LSM in the prediction of events in NAFLD, and especially in patients with NAFLD and F3-F4 fibrosis, are scarce.”
To address this knowledge gap, the investigators retrospectively analyzed data from 1,039 consecutive patients with NAFLD who had baseline LSMs of more than 10 kPa and/or histologically diagnosed F3-F4 fibrosis. Patients were prospectively recruited at 10 centers in 6 countries, then followed for a median of 35 months, ranging from 19 to 63 months.
All patients had their liver stiffness measured with an M or XL probe at baseline. In addition, approximately half of the patients (n = 533) had a follow-up measurement using the same method, generating a subgroup with changes in liver stiffness. “Improved” liver stiffness was defined as a decrease in LSM greater than 20% from baseline, “impaired” liver stiffness was defined as an increase in LSM greater than 20% from baseline, and “stable” liver stiffness was defined as a change falling between 20% lower and 20% higher than baseline.
At baseline, mean LSM was 17.6 kPa. Cox regression analysis revealed that baseline LSM was independently associated with HCC (hazard ratio, 1.03; 95% confidence interval, 1.00-1.04; P = .003), liver decompensation (HR, 1.03; 95% CI, 1.02-1.04; P < .001), and liver-related death (HR, 1.02; 95% CI, 1.00-1.03; P = .005), but not extrahepatic events.
According to the investigators, the association between LSM at baseline and risk of liver decompensation was maintained after adjustment for the severity of liver disease and for surrogate markers of portal hypertension, they noted. Furthermore, patients with a baseline LSM of at least 21 kPa – which indicates high risk of clinically significant portal hypertension (CSPH) – were at greater risk of liver decompensation than were those with an LSM less than 21 kPa (HR, 3.71; 95% CI, 1.89-6.78; P = .04).
In the subgroup with follow-up measurements, approximately half of the patients had an improved LSM (53.3%), while 27.2% had a stable LSM, and 19.5% had an impaired LSM, a pattern that was significantly associated with diabetes at baseline (P = .01).
“These data agree with the available literature identifying diabetes as a risk factor for liver disease progression and liver-related complications,” the investigators wrote.
Cox regression showed that, among those with follow-up LSM, changes in LSM were independently associated with HCC (HR, 1.72; 95% CI, 1.01-3.02; P = .04), liver decompensation (HR, 1.56; 95% CI, 1.05-2.51; P = . 04), liver-related mortality (HR, 1.96; 95% CI, 1.10-3.38; P = .02), and mortality of any cause (HR, 1.73; 95% CI, 1.11-2.69; P = .01).
These risks could be further stratified by level of change in liver stiffness, with greater impairment predicting greater risk: The crude rate of liver decompensation was 14.4% among those with impaired LSM, compared with 6.2% among those with stable LSM and 3.8% among those with LSM improvement. That said, the categories of changes in LSM were not predictive of decompensation among patients with high risk of CSPH at baseline; however, they remained predictive among those with low risk of CSPH at baseline.
“[T]his study … showed that an integrated assessment of baseline LSM or [changes in LSM] can help in stratifying the risk of development of liver-related complications and of both hepatic and overall mortality,” the investigators concluded. “These data, if further validated, could help personalize prognosis and follow-up in NAFLD with [compensated advanced chronic liver disease].”
The investigators disclosed relationships with AbbVie, Novo Nordisk, Gilead, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Minorities underrepresented on liver transplant waiting lists
Non-Hispanic Black and Hispanic patients are underrepresented on many liver transplant waiting lists, whereas non-Hispanic White patients are often overrepresented, according to data from 109 centers.
While racial disparities “greatly diminished” after placement on a waiting list, which suggests recent progress in the field, pre–wait-listing disparities may be more challenging to overcome, reported lead author Curtis Warren, MPH, CPH, of the University of Florida, Gainesville, and colleagues.
“In 2020, the Organ Procurement and Transplantation Network implemented a new allocation system for liver transplantation based on concentric circles of geographic proximity rather than somewhat arbitrarily delineated Donor Service Areas (DSAs),” the investigators wrote in Journal of the American College of Surgeons. “Although this was a step toward improving and equalizing access to lifesaving organs for those on the liver transplant wait list, the listing process determining which patients will be considered for transplantation has continued to be a significant hurdle.”
The process is “rife with impediments to equal access to listing,” according to Dr. Warren and colleagues; getting on a waiting list can be affected by factors such as inequitable access to primary care, lack of private health insurance, and subjective selection by transplant centers.
To better characterize these impediments, the investigators gathered center-specific data from the Scientific Registry of Transplant Recipients and the U.S. Census Bureau. The final dataset included 30,353 patients from treated at 109 transplant centers, each of which performed more than 250 transplants between January 2013 and December 2018. The investigators compared waiting list data for each center with demographics from its DSA. Primary variables included race/ethnicity, education level, poverty, and insurance coverage.
Multiple logistic regression analysis was used to compare expected waiting list demographics with observed waiting list demographics with the aid of observed/expected ratios for each race/ethnicity. Univariate and multivariate analyses were used to identify significant predictors, including covariates such as age at listing, distance traveled to transplant center, and center type.
On an adjusted basis, the observed/expected ratios showed that non-Hispanic Black patients were underrepresented on waiting lists at 88 out of 109 centers (81%) and Hispanic patients were underrepresented at 68 centers (62%). In contrast, non-Hispanic White patients were overrepresented on waiting lists at 65 centers (58%). Non-Hispanic White patients were underrepresented on waiting lists at 49 centers, or 45%. Minority underrepresentation was further supported by mean MELD (Model for End-Stage Liver Disease) scores, which were significantly higher among non-Hispanic Black patients (20.2) and Hispanic patients (19.4), compared with non-Hispanic White patients (18.7) (P < .0001 for all) at the time of wait-listing.
Based on the multivariate model, underrepresentation among Black patients was most common in areas with a higher proportion of Black individuals in the population, longer travel distances to transplant centers, and a higher rate of private insurance among transplant recipients. For Hispanic patients, rates of private insurance alone predicted underrepresentation.
Once patients were listed, however, these disparities faded. Non-Hispanic Black patients accounted for 9.8% of all transplants across all hospitals, compared with 7.9% of wait-listed individuals (P < .0001). At approximately two out of three hospitals (65%), the transplanted percentage of Black patients exceeded the wait-listed percentage (P = .002).
“Data from this study show that the wait lists at many transplant centers in the United States underrepresent minority populations, compared with what would be expected based on their service areas,” the investigators concluded. “Future work will need to be devoted to increasing awareness of these trends to promote equitable access to listing for liver transplantation.”
Looking at social determinants of health
According to Lauren D. Nephew, MD, MSc, MAE, of Indiana University, Indianapolis, “The question of access to care is particularly important at this juncture as we examine the inequities that COVID-19 exposed in access to care for racial minorities, and as we prepare for potential changes to health insurance coverage with the new administration.”
Dr. Nephew noted that the reported racial disparities stem from social determinants of health, such as proximity to transplant centers and type of insurance coverage.
“Another striking finding was that the disparity in wait-listing non-Hispanic Black patients increased with the percentage of non-Hispanic Black patients living in the area, further highlighting barriers in access to care in majority Black neighborhoods,” she said. “Inequities such as these are unacceptable, given our mandate to distribute organs in a fair and equitable fashion, and they require prospective studies for further examination.”
Identifying discrimination
Lanla Conteh, MD, MPH, of the Ohio State University Wexner Medical Center, Columbus, described how these inequities are magnified through bias in patient selection.
“Often times two very similar patients may present with the same medical profile and social circumstances; however, one is turned down,” she said. “Often the patient turned down is the non-Hispanic Black patient while the non-Hispanic White patient is given a pass.”
Dr. Conteh suggested that the first step in fixing this bias is recognizing that it is a problem and calling it by its proper name.
“As transplant centers, in order to address and change these significant disparities, we must first be willing to acknowledge that they do exist,” she said. “Only then can we move to the next step of developing awareness and methods to actively combat what we should label as systemic discrimination in medicine. Transplantation is a lifesaving treatment for many patients with decompensated liver disease or liver cancer. Ensuring equitable access for all patients and populations is of paramount importance.”
The study was supported by a Health Resources and Services Administration contract, as well as grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. The investigators and interviewees reported no conflicts of interest.
Non-Hispanic Black and Hispanic patients are underrepresented on many liver transplant waiting lists, whereas non-Hispanic White patients are often overrepresented, according to data from 109 centers.
While racial disparities “greatly diminished” after placement on a waiting list, which suggests recent progress in the field, pre–wait-listing disparities may be more challenging to overcome, reported lead author Curtis Warren, MPH, CPH, of the University of Florida, Gainesville, and colleagues.
“In 2020, the Organ Procurement and Transplantation Network implemented a new allocation system for liver transplantation based on concentric circles of geographic proximity rather than somewhat arbitrarily delineated Donor Service Areas (DSAs),” the investigators wrote in Journal of the American College of Surgeons. “Although this was a step toward improving and equalizing access to lifesaving organs for those on the liver transplant wait list, the listing process determining which patients will be considered for transplantation has continued to be a significant hurdle.”
The process is “rife with impediments to equal access to listing,” according to Dr. Warren and colleagues; getting on a waiting list can be affected by factors such as inequitable access to primary care, lack of private health insurance, and subjective selection by transplant centers.
To better characterize these impediments, the investigators gathered center-specific data from the Scientific Registry of Transplant Recipients and the U.S. Census Bureau. The final dataset included 30,353 patients from treated at 109 transplant centers, each of which performed more than 250 transplants between January 2013 and December 2018. The investigators compared waiting list data for each center with demographics from its DSA. Primary variables included race/ethnicity, education level, poverty, and insurance coverage.
Multiple logistic regression analysis was used to compare expected waiting list demographics with observed waiting list demographics with the aid of observed/expected ratios for each race/ethnicity. Univariate and multivariate analyses were used to identify significant predictors, including covariates such as age at listing, distance traveled to transplant center, and center type.
On an adjusted basis, the observed/expected ratios showed that non-Hispanic Black patients were underrepresented on waiting lists at 88 out of 109 centers (81%) and Hispanic patients were underrepresented at 68 centers (62%). In contrast, non-Hispanic White patients were overrepresented on waiting lists at 65 centers (58%). Non-Hispanic White patients were underrepresented on waiting lists at 49 centers, or 45%. Minority underrepresentation was further supported by mean MELD (Model for End-Stage Liver Disease) scores, which were significantly higher among non-Hispanic Black patients (20.2) and Hispanic patients (19.4), compared with non-Hispanic White patients (18.7) (P < .0001 for all) at the time of wait-listing.
Based on the multivariate model, underrepresentation among Black patients was most common in areas with a higher proportion of Black individuals in the population, longer travel distances to transplant centers, and a higher rate of private insurance among transplant recipients. For Hispanic patients, rates of private insurance alone predicted underrepresentation.
Once patients were listed, however, these disparities faded. Non-Hispanic Black patients accounted for 9.8% of all transplants across all hospitals, compared with 7.9% of wait-listed individuals (P < .0001). At approximately two out of three hospitals (65%), the transplanted percentage of Black patients exceeded the wait-listed percentage (P = .002).
“Data from this study show that the wait lists at many transplant centers in the United States underrepresent minority populations, compared with what would be expected based on their service areas,” the investigators concluded. “Future work will need to be devoted to increasing awareness of these trends to promote equitable access to listing for liver transplantation.”
Looking at social determinants of health
According to Lauren D. Nephew, MD, MSc, MAE, of Indiana University, Indianapolis, “The question of access to care is particularly important at this juncture as we examine the inequities that COVID-19 exposed in access to care for racial minorities, and as we prepare for potential changes to health insurance coverage with the new administration.”
Dr. Nephew noted that the reported racial disparities stem from social determinants of health, such as proximity to transplant centers and type of insurance coverage.
“Another striking finding was that the disparity in wait-listing non-Hispanic Black patients increased with the percentage of non-Hispanic Black patients living in the area, further highlighting barriers in access to care in majority Black neighborhoods,” she said. “Inequities such as these are unacceptable, given our mandate to distribute organs in a fair and equitable fashion, and they require prospective studies for further examination.”
Identifying discrimination
Lanla Conteh, MD, MPH, of the Ohio State University Wexner Medical Center, Columbus, described how these inequities are magnified through bias in patient selection.
“Often times two very similar patients may present with the same medical profile and social circumstances; however, one is turned down,” she said. “Often the patient turned down is the non-Hispanic Black patient while the non-Hispanic White patient is given a pass.”
Dr. Conteh suggested that the first step in fixing this bias is recognizing that it is a problem and calling it by its proper name.
“As transplant centers, in order to address and change these significant disparities, we must first be willing to acknowledge that they do exist,” she said. “Only then can we move to the next step of developing awareness and methods to actively combat what we should label as systemic discrimination in medicine. Transplantation is a lifesaving treatment for many patients with decompensated liver disease or liver cancer. Ensuring equitable access for all patients and populations is of paramount importance.”
The study was supported by a Health Resources and Services Administration contract, as well as grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. The investigators and interviewees reported no conflicts of interest.
Non-Hispanic Black and Hispanic patients are underrepresented on many liver transplant waiting lists, whereas non-Hispanic White patients are often overrepresented, according to data from 109 centers.
While racial disparities “greatly diminished” after placement on a waiting list, which suggests recent progress in the field, pre–wait-listing disparities may be more challenging to overcome, reported lead author Curtis Warren, MPH, CPH, of the University of Florida, Gainesville, and colleagues.
“In 2020, the Organ Procurement and Transplantation Network implemented a new allocation system for liver transplantation based on concentric circles of geographic proximity rather than somewhat arbitrarily delineated Donor Service Areas (DSAs),” the investigators wrote in Journal of the American College of Surgeons. “Although this was a step toward improving and equalizing access to lifesaving organs for those on the liver transplant wait list, the listing process determining which patients will be considered for transplantation has continued to be a significant hurdle.”
The process is “rife with impediments to equal access to listing,” according to Dr. Warren and colleagues; getting on a waiting list can be affected by factors such as inequitable access to primary care, lack of private health insurance, and subjective selection by transplant centers.
To better characterize these impediments, the investigators gathered center-specific data from the Scientific Registry of Transplant Recipients and the U.S. Census Bureau. The final dataset included 30,353 patients from treated at 109 transplant centers, each of which performed more than 250 transplants between January 2013 and December 2018. The investigators compared waiting list data for each center with demographics from its DSA. Primary variables included race/ethnicity, education level, poverty, and insurance coverage.
Multiple logistic regression analysis was used to compare expected waiting list demographics with observed waiting list demographics with the aid of observed/expected ratios for each race/ethnicity. Univariate and multivariate analyses were used to identify significant predictors, including covariates such as age at listing, distance traveled to transplant center, and center type.
On an adjusted basis, the observed/expected ratios showed that non-Hispanic Black patients were underrepresented on waiting lists at 88 out of 109 centers (81%) and Hispanic patients were underrepresented at 68 centers (62%). In contrast, non-Hispanic White patients were overrepresented on waiting lists at 65 centers (58%). Non-Hispanic White patients were underrepresented on waiting lists at 49 centers, or 45%. Minority underrepresentation was further supported by mean MELD (Model for End-Stage Liver Disease) scores, which were significantly higher among non-Hispanic Black patients (20.2) and Hispanic patients (19.4), compared with non-Hispanic White patients (18.7) (P < .0001 for all) at the time of wait-listing.
Based on the multivariate model, underrepresentation among Black patients was most common in areas with a higher proportion of Black individuals in the population, longer travel distances to transplant centers, and a higher rate of private insurance among transplant recipients. For Hispanic patients, rates of private insurance alone predicted underrepresentation.
Once patients were listed, however, these disparities faded. Non-Hispanic Black patients accounted for 9.8% of all transplants across all hospitals, compared with 7.9% of wait-listed individuals (P < .0001). At approximately two out of three hospitals (65%), the transplanted percentage of Black patients exceeded the wait-listed percentage (P = .002).
“Data from this study show that the wait lists at many transplant centers in the United States underrepresent minority populations, compared with what would be expected based on their service areas,” the investigators concluded. “Future work will need to be devoted to increasing awareness of these trends to promote equitable access to listing for liver transplantation.”
Looking at social determinants of health
According to Lauren D. Nephew, MD, MSc, MAE, of Indiana University, Indianapolis, “The question of access to care is particularly important at this juncture as we examine the inequities that COVID-19 exposed in access to care for racial minorities, and as we prepare for potential changes to health insurance coverage with the new administration.”
Dr. Nephew noted that the reported racial disparities stem from social determinants of health, such as proximity to transplant centers and type of insurance coverage.
“Another striking finding was that the disparity in wait-listing non-Hispanic Black patients increased with the percentage of non-Hispanic Black patients living in the area, further highlighting barriers in access to care in majority Black neighborhoods,” she said. “Inequities such as these are unacceptable, given our mandate to distribute organs in a fair and equitable fashion, and they require prospective studies for further examination.”
Identifying discrimination
Lanla Conteh, MD, MPH, of the Ohio State University Wexner Medical Center, Columbus, described how these inequities are magnified through bias in patient selection.
“Often times two very similar patients may present with the same medical profile and social circumstances; however, one is turned down,” she said. “Often the patient turned down is the non-Hispanic Black patient while the non-Hispanic White patient is given a pass.”
Dr. Conteh suggested that the first step in fixing this bias is recognizing that it is a problem and calling it by its proper name.
“As transplant centers, in order to address and change these significant disparities, we must first be willing to acknowledge that they do exist,” she said. “Only then can we move to the next step of developing awareness and methods to actively combat what we should label as systemic discrimination in medicine. Transplantation is a lifesaving treatment for many patients with decompensated liver disease or liver cancer. Ensuring equitable access for all patients and populations is of paramount importance.”
The study was supported by a Health Resources and Services Administration contract, as well as grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. The investigators and interviewees reported no conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Methotrexate-associated hepatotoxicity risk differs between psoriasis, PsA, and RA patients
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
AGA Clinical Practice Update: Bariatric surgery in patients with cirrhosis
Obesity, a risk factor for nonalcoholic fatty liver disease (NAFLD) and a prevalent comorbidity among people with cirrhosis of all etiologies, is associated with a number of untoward health outcomes, and weight loss is an important goal, according to a clinical practice update from the American Gastroenterological Association. According to one study cited in the update, approximately 30% of patients with cirrhosis have comorbid obesity, and this figure may increase even further as the epidemic of NAFLD progresses.
For obese patients with cirrhosis, weight loss “is an important therapeutic goal” because obesity heightens risks of portal vein thrombosis, portal hypertension, hepatocellular carcinoma, liver failure in acute on chronic liver disease, and other concerns. Despite no longer being an absolute contraindication, obesity can also complicate liver transplantation considerations, Heather Patton, MD, of the Veterans Affairs San Diego Healthcare System and associates wrote in Clinical Gastroenterology and Hepatology. Consideration of individuals with cirrhosis, however, requires careful scrutiny of surgical candidacy, appropriate resources for care of patients with advanced liver disease, and a high-volume bariatric surgical center given the inherent risks of surgical procedures in this patient population.
For patients with cirrhosis and obesity, laparoscopic sleeve gastrectomy is probably the best option for bariatric surgery because it preserves endoscopic access to the biliary tree, facilitates gradual weight loss, and does not cause malabsorption, according to the update.
Clinicians and patients should time bariatric surgery based on liver disease stage – for patients with decompensated disease, surgery should be performed only at the same time as or after liver transplantation, the experts wrote. Clinicians should also evaluate candidacy for liver transplantation before bariatric surgery “so that patients who are ineligible for transplant (and their families) have a clear understanding of this, avoiding the need for the medical team to address this issue urgently if the patient’s condition deteriorates postoperatively.”
One review suggested that bariatric surgery is “the most effective and durable” means of weight loss, according to the authors of the update; however, another review suggested increased surgical risk for bariatric surgery among patients with cirrhosis, so the update’s authors advised individualized risk-benefit assessments. These assessments are made even more complicated by scarcity of relevant randomized trial data, so the experts identified PubMed-indexed, peer-reviewed articles published between 2000 and 2020 and used these to make 10 best practice advice statements for bariatric surgery in obese patients with cirrhosis.
The surgical, anesthesia, and medical teams must be well versed in assessing and operating on patients with portal hypertension and cirrhosis and in managing these patients postoperatively, the experts wrote. The preoperative assessment should include cirrhosis status (compensated versus decompensated), the presence and severity of sarcopenia, ascites, and portal hypertension, and candidacy for liver transplantation. It is vital to check for clinically significant portal hypertension (CSPH) because endoscopic devices should not be used in patients with gastric and/or esophageal varices. To do so, upper endoscopy and cross-sectional imaging are advised, pending better data on noninvasive assessment methods. For patients without CSPH, endoscopic bariatric treatment can be somewhat less effective for weight loss but also might be less likely to lead to postoperative complications. However, head-to-head and long-term safety data are not yet available.
The experts also noted that bariatric surgery increases the effects (blood levels) of alcohol and can increase patients’ risk for developing an alcohol use disorder. Therefore, clinicians should carefully the history of alcohol use and repeatedly educate patients about the risks of consuming alcohol after bariatric surgery. According to a study from 2012 and a review from 2015, male sex, younger age, less social support, and regular or “problematic” alcohol use before bariatric surgery heighten the risk for developing an alcohol use disorder afterward, the experts noted.
Funding sources included the Robert H. Yauk Charitable Trust Gift for Liver Transplant Research 2017-2020 and Regenerative Medicine for Prevention of Post-Transplant Biliary Complications. The authors reported having no conflicts of interest.
This article was updated Feb. 23, 2021.
Obesity, a risk factor for nonalcoholic fatty liver disease (NAFLD) and a prevalent comorbidity among people with cirrhosis of all etiologies, is associated with a number of untoward health outcomes, and weight loss is an important goal, according to a clinical practice update from the American Gastroenterological Association. According to one study cited in the update, approximately 30% of patients with cirrhosis have comorbid obesity, and this figure may increase even further as the epidemic of NAFLD progresses.
For obese patients with cirrhosis, weight loss “is an important therapeutic goal” because obesity heightens risks of portal vein thrombosis, portal hypertension, hepatocellular carcinoma, liver failure in acute on chronic liver disease, and other concerns. Despite no longer being an absolute contraindication, obesity can also complicate liver transplantation considerations, Heather Patton, MD, of the Veterans Affairs San Diego Healthcare System and associates wrote in Clinical Gastroenterology and Hepatology. Consideration of individuals with cirrhosis, however, requires careful scrutiny of surgical candidacy, appropriate resources for care of patients with advanced liver disease, and a high-volume bariatric surgical center given the inherent risks of surgical procedures in this patient population.
For patients with cirrhosis and obesity, laparoscopic sleeve gastrectomy is probably the best option for bariatric surgery because it preserves endoscopic access to the biliary tree, facilitates gradual weight loss, and does not cause malabsorption, according to the update.
Clinicians and patients should time bariatric surgery based on liver disease stage – for patients with decompensated disease, surgery should be performed only at the same time as or after liver transplantation, the experts wrote. Clinicians should also evaluate candidacy for liver transplantation before bariatric surgery “so that patients who are ineligible for transplant (and their families) have a clear understanding of this, avoiding the need for the medical team to address this issue urgently if the patient’s condition deteriorates postoperatively.”
One review suggested that bariatric surgery is “the most effective and durable” means of weight loss, according to the authors of the update; however, another review suggested increased surgical risk for bariatric surgery among patients with cirrhosis, so the update’s authors advised individualized risk-benefit assessments. These assessments are made even more complicated by scarcity of relevant randomized trial data, so the experts identified PubMed-indexed, peer-reviewed articles published between 2000 and 2020 and used these to make 10 best practice advice statements for bariatric surgery in obese patients with cirrhosis.
The surgical, anesthesia, and medical teams must be well versed in assessing and operating on patients with portal hypertension and cirrhosis and in managing these patients postoperatively, the experts wrote. The preoperative assessment should include cirrhosis status (compensated versus decompensated), the presence and severity of sarcopenia, ascites, and portal hypertension, and candidacy for liver transplantation. It is vital to check for clinically significant portal hypertension (CSPH) because endoscopic devices should not be used in patients with gastric and/or esophageal varices. To do so, upper endoscopy and cross-sectional imaging are advised, pending better data on noninvasive assessment methods. For patients without CSPH, endoscopic bariatric treatment can be somewhat less effective for weight loss but also might be less likely to lead to postoperative complications. However, head-to-head and long-term safety data are not yet available.
The experts also noted that bariatric surgery increases the effects (blood levels) of alcohol and can increase patients’ risk for developing an alcohol use disorder. Therefore, clinicians should carefully the history of alcohol use and repeatedly educate patients about the risks of consuming alcohol after bariatric surgery. According to a study from 2012 and a review from 2015, male sex, younger age, less social support, and regular or “problematic” alcohol use before bariatric surgery heighten the risk for developing an alcohol use disorder afterward, the experts noted.
Funding sources included the Robert H. Yauk Charitable Trust Gift for Liver Transplant Research 2017-2020 and Regenerative Medicine for Prevention of Post-Transplant Biliary Complications. The authors reported having no conflicts of interest.
This article was updated Feb. 23, 2021.
Obesity, a risk factor for nonalcoholic fatty liver disease (NAFLD) and a prevalent comorbidity among people with cirrhosis of all etiologies, is associated with a number of untoward health outcomes, and weight loss is an important goal, according to a clinical practice update from the American Gastroenterological Association. According to one study cited in the update, approximately 30% of patients with cirrhosis have comorbid obesity, and this figure may increase even further as the epidemic of NAFLD progresses.
For obese patients with cirrhosis, weight loss “is an important therapeutic goal” because obesity heightens risks of portal vein thrombosis, portal hypertension, hepatocellular carcinoma, liver failure in acute on chronic liver disease, and other concerns. Despite no longer being an absolute contraindication, obesity can also complicate liver transplantation considerations, Heather Patton, MD, of the Veterans Affairs San Diego Healthcare System and associates wrote in Clinical Gastroenterology and Hepatology. Consideration of individuals with cirrhosis, however, requires careful scrutiny of surgical candidacy, appropriate resources for care of patients with advanced liver disease, and a high-volume bariatric surgical center given the inherent risks of surgical procedures in this patient population.
For patients with cirrhosis and obesity, laparoscopic sleeve gastrectomy is probably the best option for bariatric surgery because it preserves endoscopic access to the biliary tree, facilitates gradual weight loss, and does not cause malabsorption, according to the update.
Clinicians and patients should time bariatric surgery based on liver disease stage – for patients with decompensated disease, surgery should be performed only at the same time as or after liver transplantation, the experts wrote. Clinicians should also evaluate candidacy for liver transplantation before bariatric surgery “so that patients who are ineligible for transplant (and their families) have a clear understanding of this, avoiding the need for the medical team to address this issue urgently if the patient’s condition deteriorates postoperatively.”
One review suggested that bariatric surgery is “the most effective and durable” means of weight loss, according to the authors of the update; however, another review suggested increased surgical risk for bariatric surgery among patients with cirrhosis, so the update’s authors advised individualized risk-benefit assessments. These assessments are made even more complicated by scarcity of relevant randomized trial data, so the experts identified PubMed-indexed, peer-reviewed articles published between 2000 and 2020 and used these to make 10 best practice advice statements for bariatric surgery in obese patients with cirrhosis.
The surgical, anesthesia, and medical teams must be well versed in assessing and operating on patients with portal hypertension and cirrhosis and in managing these patients postoperatively, the experts wrote. The preoperative assessment should include cirrhosis status (compensated versus decompensated), the presence and severity of sarcopenia, ascites, and portal hypertension, and candidacy for liver transplantation. It is vital to check for clinically significant portal hypertension (CSPH) because endoscopic devices should not be used in patients with gastric and/or esophageal varices. To do so, upper endoscopy and cross-sectional imaging are advised, pending better data on noninvasive assessment methods. For patients without CSPH, endoscopic bariatric treatment can be somewhat less effective for weight loss but also might be less likely to lead to postoperative complications. However, head-to-head and long-term safety data are not yet available.
The experts also noted that bariatric surgery increases the effects (blood levels) of alcohol and can increase patients’ risk for developing an alcohol use disorder. Therefore, clinicians should carefully the history of alcohol use and repeatedly educate patients about the risks of consuming alcohol after bariatric surgery. According to a study from 2012 and a review from 2015, male sex, younger age, less social support, and regular or “problematic” alcohol use before bariatric surgery heighten the risk for developing an alcohol use disorder afterward, the experts noted.
Funding sources included the Robert H. Yauk Charitable Trust Gift for Liver Transplant Research 2017-2020 and Regenerative Medicine for Prevention of Post-Transplant Biliary Complications. The authors reported having no conflicts of interest.
This article was updated Feb. 23, 2021.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Widespread liver disease missed in patients with T2D
Among these calls is a pending statement from the Endocrine Society, the American Association of Clinical Endocrinologists, the American Gastroenterology Association, and other groups on what the growing appreciation of highly prevalent liver disease in patients with type 2 diabetes (T2D) means for assessing and managing patients. Publication of the statement is expected by spring 2021, said Christos S. Mantzoros, MD, DSc, PhD, chief of endocrinology for the Veterans Affairs Boston Healthcare System and a representative from the Endocrine Society to the statement-writing panel.
This upcoming “Call to Action” from these groups argues for a “need to collaborate across disciplines, and work together on establishing clinical guidelines, and creating new diagnostics and therapeutics,” said Dr. Mantzoros in an interview.
“Over time, it is becoming clearer that management of NAFLD [nonalcoholic fatty liver disease]/NASH [nonalcoholic steatohepatitis] requires a multidisciplinary panel of doctors ranging from primary care practitioners, to endocrinologists, and hepatologists. Given that the nature of the disease crosses scientific discipline boundaries, and that the number of patients is so large (it is estimated that about one in four U.S. adults have NAFLD), not all patients can be treated at the limited number of hepatology centers.
“However, not all stakeholders have fully realized this fact, and no effort had been undertaken so far by any professional society to develop a coordinated approach and clinical care pathway for NAFLD/NASH. The ‘Call to Action’ meeting can be considered as a starting point for such an important effort,” said Dr. Mantzoros, who is also a professor of medicine at Harvard Medical School and director of the human nutrition unit at Beth Israel Deaconess Medical Center, both in Boston.
Dramatic prevalence rates in patients with T2D
Results from two independent epidemiology reports, published in December 2020, documented steatosis (the fatty liver of NAFLD) in 70%-74% of unselected U.S. patients with T2D, advanced liver fibrosis accompanying this disease in 6%-15%, and previously unrecognized cirrhosis in 3%-8%.
One of these reports analyzed 825 patients with T2D included in the National Health and Nutritional Examination Survey of 2017-2018 run by the Centers for Disease Control and Prevention. All these patients, selected to be representative of the overall U.S. adult population with T2D, underwent transient elastography to identify steatosis and fibrosis, the first U.S. National Health Survey to run this type of population-based survey. The results showed an overall steatosis prevalence of 74% with grade 3 steatosis in 58%, advanced liver fibrosis in 15%, and cirrhosis in 8%, reported the team of Italian researchers who analyzed the data .
The second study focused on a single-center series of 561 patients with T2D who also underwent screening by transient elastography during 2018-2020 and had no history of NAFLD or other liver disease, or alcohol abuse. The imaging results showed a NAFLD prevalence of 70%, with 54% of the entire group diagnosed with severe steatosis, severe fibrosis in 6%, and cirrhosis in 3%. Among the 54% of patients with severe steatosis, 30% also had severe liver fibrosis. About 70% of the 561 patients assessed came from either the family medicine or general internal medicine clinics of the University of Florida, Gainesville, with the remaining 30% enrolled from the center’s endocrinology/diabetes outpatient clinic.
Neither report documented a NASH prevalence, which cannot receive definitive diagnosis by imaging alone. “This is the first study of its kind in the U.S. to establish the magnitude of [liver] disease burden in random patients with T2D seeking regular outpatient care,” wrote the University of Florida research team, led by Kenneth Cusi, MD, professor and chief of the university’s division of endocrinology, diabetes, and metabolism. Their finding that patients with T2D and previously unknown to have NAFLD had a 15% prevalence of moderate or advanced liver fibrosis “should trigger a call to action by all clinicians taking care of patients with T2D. Patient and physician awareness of the hepatic and extrahepatic complications of NASH, and reversing current diagnosis and treatment inertia will be the only way to avert the looming epidemic of cirrhosis in patients with diabetes.”
“Endocrinologists don’t ‘see’ NAFLD and NASH” in their patients with T2D “ because they don’t think about it,” Dr. Mantzoros declared.
“Why is NASH underdiagnosed and undertreated? Because many physicians aren’t aware of it,” agreed Dr. Cusi during a talk in December 2020 at the 18th World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease (WCIRDC). “You never find what you don’t look for.”
“Endocrinologists should do the tests for NASH [in patients with T2D], but we’re all guilty of not doing it enough,” Tracey McLaughlin, MD, an endocrinologist and professor of medicine at Stanford (Calif.) University, commented during the WCIRDC.
These prevalence numbers demand that clinicians suspect liver disease “in any patient with diabetes, especially patients with obesity who are older and have components of metabolic syndrome,” said Dr. Mantzoros. “We need to screen, refer the most advanced cases, and treat the early- and mid-stage cases.”
How to find NASH
Both the American Diabetes Association and the European Association for the Study of Diabetes call for routine screening of patients with T2D, starting with a check of liver enzymes, such as ALT, but no clear consensus exists for the specifics of screening beyond that. Dr. Mantzoros, Dr. Cusi, and other experts agree that the scheme for assessing liver disease in patients with T2D starts with regular monitoring of elevations in liver enzymes including ALT. Next is noninvasive ultrasound assessment of the extent of liver fibrosis inferred from the organ’s stiffness using transient elastography. Another frequently cited initial screening tool is the Fibrosis-4 (FIB-4) score, which incorporates a patient’s age, platelet count, and levels of ALT and a second liver enzyme, AST.
“There is more consensus about FIB-4 and then elastography, but some people use tests other than FIB-4. Unfortunately there is no perfect diagnostic test today. A top priority is to define the best diagnostic test,” said Dr. Mantzoros, who is leading an effort to try to refine screening using artificial intelligence.
“FIB-4 is simple, easy, and well validated,” commented Dr. Cusi during the WCIRDC last December. “FIB-4 and elastography should get you pretty close” to identifying patients with T2D and significant liver disease.
But in a recent editorial, Dr. Cusi agreed on the need for “more reliable tests for the diagnosis of NASH and advanced fibrosis in patients with T2D. Significant work is being done in the field to validate novel and more sophisticated fibrosis biomarkers. Future studies will help us enter a new era of precision medicine where biomarkers will identify and target therapy to those with more active disease at risk for cirrhosis,” he wrote.
“The ultimate goal is to diagnose fibrosis at an early stage to prevent people from developing cirrhosis,” Dr. Cusi said in a recent written statement. “We’re trying to identify these problems before they’re unfixable. Once someone has cirrhosis, there isn’t a whole lot you can do.”
Pioglitazone remains the best-documented treatment
Perhaps some of the inertia in diagnosing NAFLD, NASH, and liver fibrosis in patients with T2D is dissatisfaction with current treatment options, although several proven options exist, notably weight loss and diet, and thiazolidinedione (TZD) pioglitazone. But weight loss and diet pose issues for patient compliance and durability of the intervention, and many clinicians consider pioglitazone flawed by its potential adverse effects.
“When we don’t have an established treatment for something, we tend to not measure it or go after it. That’s been true of liver disease” in patients with T2D, said Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif., during the WCIRDC.
Treatment with pioglitazone has resolved NASH in about a third of patients compared with placebo, prevented fibrosis progression, and cut cardiovascular disease events, noted Dr. Cusi during the WCIRDC.
“Pioglitazone is used in only 8% of patients with T2D, or less, but we need to use it more often because of its proven efficacy in patients with T2D and NASH” said Dr. Mantzoros. “The problem is that pioglitazone has side effects, including weight gain and fluid retention, that makes it less attractive unless one thinks about the diagnosis of NASH.”
Others highlight that the adverse effects of pioglitazone have been either misunderstood, or can be effectively minimized with careful dosing.
“The data with the TZDs are much stronger than the data from anything else. TZDs have gotten a bad name because they also work in the kidney and enhance fluid reabsorption. We use modest dosages of pioglitazone, 15 mg or 30 mg a day, to avoid excess fluid retention,” Ralph A. DeFronzo, MD, chief of the diabetes division and professor of medicine at the University of Texas Health Science Center, San Antonio, said during the WCIRDC. “The best drug for NASH is pioglitazone. No other drug beats it” based on current data, Dr. DeFronzo asserted.
Other strategies include the potential to pair pioglitazone with other interventions that can blunt a weight-gain effect. One intriguing combination would combine pioglitazone with a GLP-1 receptor agonist, a drug class that can produce significant weight loss. Results from a phase 2 study showed promise for semaglutide (Rybelsus) in treating patients with NASH.
Getting the name right
Another factor that may be keeping NAFLD and NASH from achieving a higher profile for patients with T2D are those names, which focus on what the diseases are not – nonalcoholic – rather than what they are.
A series of recent publications in both the endocrinology and hepatology literature have called for renaming these disorders either “metabolic (dysfunction)–associated fatty liver disease (MALFD)”, or “dysmetabolism-associated fatty liver disease (DALFD)”.
“The names NAFLD and NASH indicate absence of alcohol as a cause, but the disease is also characterized by the absence of other causes, such as autoimmune disorders or hepatitis. The names were coined when we did not know much about these diseases. We now know that it is dysmetabolism that causes these conditions, and so we need to adopt a new, more accurate name,” explained Dr. Mantzoros, who has published support for a name change.
While many agree, some have raised concerns as to whether a name change now is premature. A group of hepatologists recently published a rebuttal to an immediate name change , saying that, “although we are in agreement that metabolic fatty liver disease may more accurately and positively reflect the relevant risk factors better than the age-old term nonalcoholic fatty liver disease, the term still leaves a great deal of ambiguity. A name change will be appropriate when informed by a new understanding of the molecular basis of the disease entity, insights that fundamentally change risk stratification, or other important aspects of the disease. We may be on the cusp of this, but we are not there yet.”
Dr. Mantzoros agreed, but for somewhat different reasons.
“We need to be careful and deliberate, because there is a significant body of knowledge and a lot of data from clinical trials collected using the old definitions. We need to find an appropriate time frame for a [name] transition. We need to find a nice and robust way to productively bridge the old to the new,” he said. “We also need new diagnostic criteria, and new therapies. A new name and definition will facilitate progress.”
Dr. Mantzoros been a shareholder of and consultant to Coherus and Pangea, he has been a consultant to AstraZeneca, Eisai, Genfit, Intercept, Novo Nordisk, P.E.S., and Regeneron, and has received travel support from the Metabolic Institute of America and the California Walnut Commission. Dr. Cusi has been a consultant to and has received research funding from numerous drug companies. Dr. McLaughlin is a consultant to January AI. Dr. Handelsman has been a consultant to numerous drug companies. Dr. DeFronzo received research grants from AstraZeneca, Janssen, and Merck; he has been an adviser to AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, and Novo Nordisk; and he has been a speaker on behalf of AstraZeneca and Novo Nordisk.
Among these calls is a pending statement from the Endocrine Society, the American Association of Clinical Endocrinologists, the American Gastroenterology Association, and other groups on what the growing appreciation of highly prevalent liver disease in patients with type 2 diabetes (T2D) means for assessing and managing patients. Publication of the statement is expected by spring 2021, said Christos S. Mantzoros, MD, DSc, PhD, chief of endocrinology for the Veterans Affairs Boston Healthcare System and a representative from the Endocrine Society to the statement-writing panel.
This upcoming “Call to Action” from these groups argues for a “need to collaborate across disciplines, and work together on establishing clinical guidelines, and creating new diagnostics and therapeutics,” said Dr. Mantzoros in an interview.
“Over time, it is becoming clearer that management of NAFLD [nonalcoholic fatty liver disease]/NASH [nonalcoholic steatohepatitis] requires a multidisciplinary panel of doctors ranging from primary care practitioners, to endocrinologists, and hepatologists. Given that the nature of the disease crosses scientific discipline boundaries, and that the number of patients is so large (it is estimated that about one in four U.S. adults have NAFLD), not all patients can be treated at the limited number of hepatology centers.
“However, not all stakeholders have fully realized this fact, and no effort had been undertaken so far by any professional society to develop a coordinated approach and clinical care pathway for NAFLD/NASH. The ‘Call to Action’ meeting can be considered as a starting point for such an important effort,” said Dr. Mantzoros, who is also a professor of medicine at Harvard Medical School and director of the human nutrition unit at Beth Israel Deaconess Medical Center, both in Boston.
Dramatic prevalence rates in patients with T2D
Results from two independent epidemiology reports, published in December 2020, documented steatosis (the fatty liver of NAFLD) in 70%-74% of unselected U.S. patients with T2D, advanced liver fibrosis accompanying this disease in 6%-15%, and previously unrecognized cirrhosis in 3%-8%.
One of these reports analyzed 825 patients with T2D included in the National Health and Nutritional Examination Survey of 2017-2018 run by the Centers for Disease Control and Prevention. All these patients, selected to be representative of the overall U.S. adult population with T2D, underwent transient elastography to identify steatosis and fibrosis, the first U.S. National Health Survey to run this type of population-based survey. The results showed an overall steatosis prevalence of 74% with grade 3 steatosis in 58%, advanced liver fibrosis in 15%, and cirrhosis in 8%, reported the team of Italian researchers who analyzed the data .
The second study focused on a single-center series of 561 patients with T2D who also underwent screening by transient elastography during 2018-2020 and had no history of NAFLD or other liver disease, or alcohol abuse. The imaging results showed a NAFLD prevalence of 70%, with 54% of the entire group diagnosed with severe steatosis, severe fibrosis in 6%, and cirrhosis in 3%. Among the 54% of patients with severe steatosis, 30% also had severe liver fibrosis. About 70% of the 561 patients assessed came from either the family medicine or general internal medicine clinics of the University of Florida, Gainesville, with the remaining 30% enrolled from the center’s endocrinology/diabetes outpatient clinic.
Neither report documented a NASH prevalence, which cannot receive definitive diagnosis by imaging alone. “This is the first study of its kind in the U.S. to establish the magnitude of [liver] disease burden in random patients with T2D seeking regular outpatient care,” wrote the University of Florida research team, led by Kenneth Cusi, MD, professor and chief of the university’s division of endocrinology, diabetes, and metabolism. Their finding that patients with T2D and previously unknown to have NAFLD had a 15% prevalence of moderate or advanced liver fibrosis “should trigger a call to action by all clinicians taking care of patients with T2D. Patient and physician awareness of the hepatic and extrahepatic complications of NASH, and reversing current diagnosis and treatment inertia will be the only way to avert the looming epidemic of cirrhosis in patients with diabetes.”
“Endocrinologists don’t ‘see’ NAFLD and NASH” in their patients with T2D “ because they don’t think about it,” Dr. Mantzoros declared.
“Why is NASH underdiagnosed and undertreated? Because many physicians aren’t aware of it,” agreed Dr. Cusi during a talk in December 2020 at the 18th World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease (WCIRDC). “You never find what you don’t look for.”
“Endocrinologists should do the tests for NASH [in patients with T2D], but we’re all guilty of not doing it enough,” Tracey McLaughlin, MD, an endocrinologist and professor of medicine at Stanford (Calif.) University, commented during the WCIRDC.
These prevalence numbers demand that clinicians suspect liver disease “in any patient with diabetes, especially patients with obesity who are older and have components of metabolic syndrome,” said Dr. Mantzoros. “We need to screen, refer the most advanced cases, and treat the early- and mid-stage cases.”
How to find NASH
Both the American Diabetes Association and the European Association for the Study of Diabetes call for routine screening of patients with T2D, starting with a check of liver enzymes, such as ALT, but no clear consensus exists for the specifics of screening beyond that. Dr. Mantzoros, Dr. Cusi, and other experts agree that the scheme for assessing liver disease in patients with T2D starts with regular monitoring of elevations in liver enzymes including ALT. Next is noninvasive ultrasound assessment of the extent of liver fibrosis inferred from the organ’s stiffness using transient elastography. Another frequently cited initial screening tool is the Fibrosis-4 (FIB-4) score, which incorporates a patient’s age, platelet count, and levels of ALT and a second liver enzyme, AST.
“There is more consensus about FIB-4 and then elastography, but some people use tests other than FIB-4. Unfortunately there is no perfect diagnostic test today. A top priority is to define the best diagnostic test,” said Dr. Mantzoros, who is leading an effort to try to refine screening using artificial intelligence.
“FIB-4 is simple, easy, and well validated,” commented Dr. Cusi during the WCIRDC last December. “FIB-4 and elastography should get you pretty close” to identifying patients with T2D and significant liver disease.
But in a recent editorial, Dr. Cusi agreed on the need for “more reliable tests for the diagnosis of NASH and advanced fibrosis in patients with T2D. Significant work is being done in the field to validate novel and more sophisticated fibrosis biomarkers. Future studies will help us enter a new era of precision medicine where biomarkers will identify and target therapy to those with more active disease at risk for cirrhosis,” he wrote.
“The ultimate goal is to diagnose fibrosis at an early stage to prevent people from developing cirrhosis,” Dr. Cusi said in a recent written statement. “We’re trying to identify these problems before they’re unfixable. Once someone has cirrhosis, there isn’t a whole lot you can do.”
Pioglitazone remains the best-documented treatment
Perhaps some of the inertia in diagnosing NAFLD, NASH, and liver fibrosis in patients with T2D is dissatisfaction with current treatment options, although several proven options exist, notably weight loss and diet, and thiazolidinedione (TZD) pioglitazone. But weight loss and diet pose issues for patient compliance and durability of the intervention, and many clinicians consider pioglitazone flawed by its potential adverse effects.
“When we don’t have an established treatment for something, we tend to not measure it or go after it. That’s been true of liver disease” in patients with T2D, said Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif., during the WCIRDC.
Treatment with pioglitazone has resolved NASH in about a third of patients compared with placebo, prevented fibrosis progression, and cut cardiovascular disease events, noted Dr. Cusi during the WCIRDC.
“Pioglitazone is used in only 8% of patients with T2D, or less, but we need to use it more often because of its proven efficacy in patients with T2D and NASH” said Dr. Mantzoros. “The problem is that pioglitazone has side effects, including weight gain and fluid retention, that makes it less attractive unless one thinks about the diagnosis of NASH.”
Others highlight that the adverse effects of pioglitazone have been either misunderstood, or can be effectively minimized with careful dosing.
“The data with the TZDs are much stronger than the data from anything else. TZDs have gotten a bad name because they also work in the kidney and enhance fluid reabsorption. We use modest dosages of pioglitazone, 15 mg or 30 mg a day, to avoid excess fluid retention,” Ralph A. DeFronzo, MD, chief of the diabetes division and professor of medicine at the University of Texas Health Science Center, San Antonio, said during the WCIRDC. “The best drug for NASH is pioglitazone. No other drug beats it” based on current data, Dr. DeFronzo asserted.
Other strategies include the potential to pair pioglitazone with other interventions that can blunt a weight-gain effect. One intriguing combination would combine pioglitazone with a GLP-1 receptor agonist, a drug class that can produce significant weight loss. Results from a phase 2 study showed promise for semaglutide (Rybelsus) in treating patients with NASH.
Getting the name right
Another factor that may be keeping NAFLD and NASH from achieving a higher profile for patients with T2D are those names, which focus on what the diseases are not – nonalcoholic – rather than what they are.
A series of recent publications in both the endocrinology and hepatology literature have called for renaming these disorders either “metabolic (dysfunction)–associated fatty liver disease (MALFD)”, or “dysmetabolism-associated fatty liver disease (DALFD)”.
“The names NAFLD and NASH indicate absence of alcohol as a cause, but the disease is also characterized by the absence of other causes, such as autoimmune disorders or hepatitis. The names were coined when we did not know much about these diseases. We now know that it is dysmetabolism that causes these conditions, and so we need to adopt a new, more accurate name,” explained Dr. Mantzoros, who has published support for a name change.
While many agree, some have raised concerns as to whether a name change now is premature. A group of hepatologists recently published a rebuttal to an immediate name change , saying that, “although we are in agreement that metabolic fatty liver disease may more accurately and positively reflect the relevant risk factors better than the age-old term nonalcoholic fatty liver disease, the term still leaves a great deal of ambiguity. A name change will be appropriate when informed by a new understanding of the molecular basis of the disease entity, insights that fundamentally change risk stratification, or other important aspects of the disease. We may be on the cusp of this, but we are not there yet.”
Dr. Mantzoros agreed, but for somewhat different reasons.
“We need to be careful and deliberate, because there is a significant body of knowledge and a lot of data from clinical trials collected using the old definitions. We need to find an appropriate time frame for a [name] transition. We need to find a nice and robust way to productively bridge the old to the new,” he said. “We also need new diagnostic criteria, and new therapies. A new name and definition will facilitate progress.”
Dr. Mantzoros been a shareholder of and consultant to Coherus and Pangea, he has been a consultant to AstraZeneca, Eisai, Genfit, Intercept, Novo Nordisk, P.E.S., and Regeneron, and has received travel support from the Metabolic Institute of America and the California Walnut Commission. Dr. Cusi has been a consultant to and has received research funding from numerous drug companies. Dr. McLaughlin is a consultant to January AI. Dr. Handelsman has been a consultant to numerous drug companies. Dr. DeFronzo received research grants from AstraZeneca, Janssen, and Merck; he has been an adviser to AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, and Novo Nordisk; and he has been a speaker on behalf of AstraZeneca and Novo Nordisk.
Among these calls is a pending statement from the Endocrine Society, the American Association of Clinical Endocrinologists, the American Gastroenterology Association, and other groups on what the growing appreciation of highly prevalent liver disease in patients with type 2 diabetes (T2D) means for assessing and managing patients. Publication of the statement is expected by spring 2021, said Christos S. Mantzoros, MD, DSc, PhD, chief of endocrinology for the Veterans Affairs Boston Healthcare System and a representative from the Endocrine Society to the statement-writing panel.
This upcoming “Call to Action” from these groups argues for a “need to collaborate across disciplines, and work together on establishing clinical guidelines, and creating new diagnostics and therapeutics,” said Dr. Mantzoros in an interview.
“Over time, it is becoming clearer that management of NAFLD [nonalcoholic fatty liver disease]/NASH [nonalcoholic steatohepatitis] requires a multidisciplinary panel of doctors ranging from primary care practitioners, to endocrinologists, and hepatologists. Given that the nature of the disease crosses scientific discipline boundaries, and that the number of patients is so large (it is estimated that about one in four U.S. adults have NAFLD), not all patients can be treated at the limited number of hepatology centers.
“However, not all stakeholders have fully realized this fact, and no effort had been undertaken so far by any professional society to develop a coordinated approach and clinical care pathway for NAFLD/NASH. The ‘Call to Action’ meeting can be considered as a starting point for such an important effort,” said Dr. Mantzoros, who is also a professor of medicine at Harvard Medical School and director of the human nutrition unit at Beth Israel Deaconess Medical Center, both in Boston.
Dramatic prevalence rates in patients with T2D
Results from two independent epidemiology reports, published in December 2020, documented steatosis (the fatty liver of NAFLD) in 70%-74% of unselected U.S. patients with T2D, advanced liver fibrosis accompanying this disease in 6%-15%, and previously unrecognized cirrhosis in 3%-8%.
One of these reports analyzed 825 patients with T2D included in the National Health and Nutritional Examination Survey of 2017-2018 run by the Centers for Disease Control and Prevention. All these patients, selected to be representative of the overall U.S. adult population with T2D, underwent transient elastography to identify steatosis and fibrosis, the first U.S. National Health Survey to run this type of population-based survey. The results showed an overall steatosis prevalence of 74% with grade 3 steatosis in 58%, advanced liver fibrosis in 15%, and cirrhosis in 8%, reported the team of Italian researchers who analyzed the data .
The second study focused on a single-center series of 561 patients with T2D who also underwent screening by transient elastography during 2018-2020 and had no history of NAFLD or other liver disease, or alcohol abuse. The imaging results showed a NAFLD prevalence of 70%, with 54% of the entire group diagnosed with severe steatosis, severe fibrosis in 6%, and cirrhosis in 3%. Among the 54% of patients with severe steatosis, 30% also had severe liver fibrosis. About 70% of the 561 patients assessed came from either the family medicine or general internal medicine clinics of the University of Florida, Gainesville, with the remaining 30% enrolled from the center’s endocrinology/diabetes outpatient clinic.
Neither report documented a NASH prevalence, which cannot receive definitive diagnosis by imaging alone. “This is the first study of its kind in the U.S. to establish the magnitude of [liver] disease burden in random patients with T2D seeking regular outpatient care,” wrote the University of Florida research team, led by Kenneth Cusi, MD, professor and chief of the university’s division of endocrinology, diabetes, and metabolism. Their finding that patients with T2D and previously unknown to have NAFLD had a 15% prevalence of moderate or advanced liver fibrosis “should trigger a call to action by all clinicians taking care of patients with T2D. Patient and physician awareness of the hepatic and extrahepatic complications of NASH, and reversing current diagnosis and treatment inertia will be the only way to avert the looming epidemic of cirrhosis in patients with diabetes.”
“Endocrinologists don’t ‘see’ NAFLD and NASH” in their patients with T2D “ because they don’t think about it,” Dr. Mantzoros declared.
“Why is NASH underdiagnosed and undertreated? Because many physicians aren’t aware of it,” agreed Dr. Cusi during a talk in December 2020 at the 18th World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease (WCIRDC). “You never find what you don’t look for.”
“Endocrinologists should do the tests for NASH [in patients with T2D], but we’re all guilty of not doing it enough,” Tracey McLaughlin, MD, an endocrinologist and professor of medicine at Stanford (Calif.) University, commented during the WCIRDC.
These prevalence numbers demand that clinicians suspect liver disease “in any patient with diabetes, especially patients with obesity who are older and have components of metabolic syndrome,” said Dr. Mantzoros. “We need to screen, refer the most advanced cases, and treat the early- and mid-stage cases.”
How to find NASH
Both the American Diabetes Association and the European Association for the Study of Diabetes call for routine screening of patients with T2D, starting with a check of liver enzymes, such as ALT, but no clear consensus exists for the specifics of screening beyond that. Dr. Mantzoros, Dr. Cusi, and other experts agree that the scheme for assessing liver disease in patients with T2D starts with regular monitoring of elevations in liver enzymes including ALT. Next is noninvasive ultrasound assessment of the extent of liver fibrosis inferred from the organ’s stiffness using transient elastography. Another frequently cited initial screening tool is the Fibrosis-4 (FIB-4) score, which incorporates a patient’s age, platelet count, and levels of ALT and a second liver enzyme, AST.
“There is more consensus about FIB-4 and then elastography, but some people use tests other than FIB-4. Unfortunately there is no perfect diagnostic test today. A top priority is to define the best diagnostic test,” said Dr. Mantzoros, who is leading an effort to try to refine screening using artificial intelligence.
“FIB-4 is simple, easy, and well validated,” commented Dr. Cusi during the WCIRDC last December. “FIB-4 and elastography should get you pretty close” to identifying patients with T2D and significant liver disease.
But in a recent editorial, Dr. Cusi agreed on the need for “more reliable tests for the diagnosis of NASH and advanced fibrosis in patients with T2D. Significant work is being done in the field to validate novel and more sophisticated fibrosis biomarkers. Future studies will help us enter a new era of precision medicine where biomarkers will identify and target therapy to those with more active disease at risk for cirrhosis,” he wrote.
“The ultimate goal is to diagnose fibrosis at an early stage to prevent people from developing cirrhosis,” Dr. Cusi said in a recent written statement. “We’re trying to identify these problems before they’re unfixable. Once someone has cirrhosis, there isn’t a whole lot you can do.”
Pioglitazone remains the best-documented treatment
Perhaps some of the inertia in diagnosing NAFLD, NASH, and liver fibrosis in patients with T2D is dissatisfaction with current treatment options, although several proven options exist, notably weight loss and diet, and thiazolidinedione (TZD) pioglitazone. But weight loss and diet pose issues for patient compliance and durability of the intervention, and many clinicians consider pioglitazone flawed by its potential adverse effects.
“When we don’t have an established treatment for something, we tend to not measure it or go after it. That’s been true of liver disease” in patients with T2D, said Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif., during the WCIRDC.
Treatment with pioglitazone has resolved NASH in about a third of patients compared with placebo, prevented fibrosis progression, and cut cardiovascular disease events, noted Dr. Cusi during the WCIRDC.
“Pioglitazone is used in only 8% of patients with T2D, or less, but we need to use it more often because of its proven efficacy in patients with T2D and NASH” said Dr. Mantzoros. “The problem is that pioglitazone has side effects, including weight gain and fluid retention, that makes it less attractive unless one thinks about the diagnosis of NASH.”
Others highlight that the adverse effects of pioglitazone have been either misunderstood, or can be effectively minimized with careful dosing.
“The data with the TZDs are much stronger than the data from anything else. TZDs have gotten a bad name because they also work in the kidney and enhance fluid reabsorption. We use modest dosages of pioglitazone, 15 mg or 30 mg a day, to avoid excess fluid retention,” Ralph A. DeFronzo, MD, chief of the diabetes division and professor of medicine at the University of Texas Health Science Center, San Antonio, said during the WCIRDC. “The best drug for NASH is pioglitazone. No other drug beats it” based on current data, Dr. DeFronzo asserted.
Other strategies include the potential to pair pioglitazone with other interventions that can blunt a weight-gain effect. One intriguing combination would combine pioglitazone with a GLP-1 receptor agonist, a drug class that can produce significant weight loss. Results from a phase 2 study showed promise for semaglutide (Rybelsus) in treating patients with NASH.
Getting the name right
Another factor that may be keeping NAFLD and NASH from achieving a higher profile for patients with T2D are those names, which focus on what the diseases are not – nonalcoholic – rather than what they are.
A series of recent publications in both the endocrinology and hepatology literature have called for renaming these disorders either “metabolic (dysfunction)–associated fatty liver disease (MALFD)”, or “dysmetabolism-associated fatty liver disease (DALFD)”.
“The names NAFLD and NASH indicate absence of alcohol as a cause, but the disease is also characterized by the absence of other causes, such as autoimmune disorders or hepatitis. The names were coined when we did not know much about these diseases. We now know that it is dysmetabolism that causes these conditions, and so we need to adopt a new, more accurate name,” explained Dr. Mantzoros, who has published support for a name change.
While many agree, some have raised concerns as to whether a name change now is premature. A group of hepatologists recently published a rebuttal to an immediate name change , saying that, “although we are in agreement that metabolic fatty liver disease may more accurately and positively reflect the relevant risk factors better than the age-old term nonalcoholic fatty liver disease, the term still leaves a great deal of ambiguity. A name change will be appropriate when informed by a new understanding of the molecular basis of the disease entity, insights that fundamentally change risk stratification, or other important aspects of the disease. We may be on the cusp of this, but we are not there yet.”
Dr. Mantzoros agreed, but for somewhat different reasons.
“We need to be careful and deliberate, because there is a significant body of knowledge and a lot of data from clinical trials collected using the old definitions. We need to find an appropriate time frame for a [name] transition. We need to find a nice and robust way to productively bridge the old to the new,” he said. “We also need new diagnostic criteria, and new therapies. A new name and definition will facilitate progress.”
Dr. Mantzoros been a shareholder of and consultant to Coherus and Pangea, he has been a consultant to AstraZeneca, Eisai, Genfit, Intercept, Novo Nordisk, P.E.S., and Regeneron, and has received travel support from the Metabolic Institute of America and the California Walnut Commission. Dr. Cusi has been a consultant to and has received research funding from numerous drug companies. Dr. McLaughlin is a consultant to January AI. Dr. Handelsman has been a consultant to numerous drug companies. Dr. DeFronzo received research grants from AstraZeneca, Janssen, and Merck; he has been an adviser to AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, and Novo Nordisk; and he has been a speaker on behalf of AstraZeneca and Novo Nordisk.
Eliminating hepatitis by 2030: HHS releases new strategic plan
In an effort to counteract alarming trends in rising hepatitis infections, the U.S. Department of Health and Human Services has developed and released its Viral Hepatitis National Strategic Plan 2021-2025, which aims to eliminate viral hepatitis infection in the United States by 2030.
An estimated 3.3 million people in the United States were chronically infected with hepatitis B (HBV) and hepatitis C (HCV) as of 2016. In addition, the country “is currently facing unprecedented hepatitis A (HAV) outbreaks, while progress in preventing hepatitis B has stalled, and hepatitis C rates nearly tripled from 2011 to 2018,” according to the HHS.
The new plan, “A Roadmap to Elimination for the United States,” builds upon previous initiatives the HHS has made to tackle the diseases and was coordinated by the Office of the Assistant Secretary for Health through the Office of Infectious Disease and HIV/AIDS Policy.
The plan focuses on HAV, HBV, and HCV, which have the largest impact on the health of the nation, according to the HHS. The plan addresses populations with the highest burden of viral hepatitis based on nationwide data so that resources can be focused there to achieve the greatest impact. Persons who inject drugs are a priority population for all three hepatitis viruses. HAV efforts will also include a focus on the homeless population. HBV efforts will also focus on Asian and Pacific Islander and the Black, non-Hispanic populations, while HCV efforts will include a focus on Black, non-Hispanic people, people born during 1945-1965, people with HIV, and the American Indian/Alaska Native population.
Goal-setting
There are five main goals outlined in the plan, according to the HHS:
- Prevent new hepatitis infections.
- Improve hepatitis-related health outcomes of people with viral hepatitis.
- Reduce hepatitis-related disparities and health inequities.
- Improve hepatitis surveillance and data use.
- Achieve integrated, coordinated efforts that address the viral hepatitis epidemics among all partners and stakeholders.
“The United States will be a place where new viral hepatitis infections are prevented, every person knows their status, and every person with viral hepatitis has high-quality health care and treatment and lives free from stigma and discrimination. This vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” according to the HHS vision statement.
In an effort to counteract alarming trends in rising hepatitis infections, the U.S. Department of Health and Human Services has developed and released its Viral Hepatitis National Strategic Plan 2021-2025, which aims to eliminate viral hepatitis infection in the United States by 2030.
An estimated 3.3 million people in the United States were chronically infected with hepatitis B (HBV) and hepatitis C (HCV) as of 2016. In addition, the country “is currently facing unprecedented hepatitis A (HAV) outbreaks, while progress in preventing hepatitis B has stalled, and hepatitis C rates nearly tripled from 2011 to 2018,” according to the HHS.
The new plan, “A Roadmap to Elimination for the United States,” builds upon previous initiatives the HHS has made to tackle the diseases and was coordinated by the Office of the Assistant Secretary for Health through the Office of Infectious Disease and HIV/AIDS Policy.
The plan focuses on HAV, HBV, and HCV, which have the largest impact on the health of the nation, according to the HHS. The plan addresses populations with the highest burden of viral hepatitis based on nationwide data so that resources can be focused there to achieve the greatest impact. Persons who inject drugs are a priority population for all three hepatitis viruses. HAV efforts will also include a focus on the homeless population. HBV efforts will also focus on Asian and Pacific Islander and the Black, non-Hispanic populations, while HCV efforts will include a focus on Black, non-Hispanic people, people born during 1945-1965, people with HIV, and the American Indian/Alaska Native population.
Goal-setting
There are five main goals outlined in the plan, according to the HHS:
- Prevent new hepatitis infections.
- Improve hepatitis-related health outcomes of people with viral hepatitis.
- Reduce hepatitis-related disparities and health inequities.
- Improve hepatitis surveillance and data use.
- Achieve integrated, coordinated efforts that address the viral hepatitis epidemics among all partners and stakeholders.
“The United States will be a place where new viral hepatitis infections are prevented, every person knows their status, and every person with viral hepatitis has high-quality health care and treatment and lives free from stigma and discrimination. This vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” according to the HHS vision statement.
In an effort to counteract alarming trends in rising hepatitis infections, the U.S. Department of Health and Human Services has developed and released its Viral Hepatitis National Strategic Plan 2021-2025, which aims to eliminate viral hepatitis infection in the United States by 2030.
An estimated 3.3 million people in the United States were chronically infected with hepatitis B (HBV) and hepatitis C (HCV) as of 2016. In addition, the country “is currently facing unprecedented hepatitis A (HAV) outbreaks, while progress in preventing hepatitis B has stalled, and hepatitis C rates nearly tripled from 2011 to 2018,” according to the HHS.
The new plan, “A Roadmap to Elimination for the United States,” builds upon previous initiatives the HHS has made to tackle the diseases and was coordinated by the Office of the Assistant Secretary for Health through the Office of Infectious Disease and HIV/AIDS Policy.
The plan focuses on HAV, HBV, and HCV, which have the largest impact on the health of the nation, according to the HHS. The plan addresses populations with the highest burden of viral hepatitis based on nationwide data so that resources can be focused there to achieve the greatest impact. Persons who inject drugs are a priority population for all three hepatitis viruses. HAV efforts will also include a focus on the homeless population. HBV efforts will also focus on Asian and Pacific Islander and the Black, non-Hispanic populations, while HCV efforts will include a focus on Black, non-Hispanic people, people born during 1945-1965, people with HIV, and the American Indian/Alaska Native population.
Goal-setting
There are five main goals outlined in the plan, according to the HHS:
- Prevent new hepatitis infections.
- Improve hepatitis-related health outcomes of people with viral hepatitis.
- Reduce hepatitis-related disparities and health inequities.
- Improve hepatitis surveillance and data use.
- Achieve integrated, coordinated efforts that address the viral hepatitis epidemics among all partners and stakeholders.
“The United States will be a place where new viral hepatitis infections are prevented, every person knows their status, and every person with viral hepatitis has high-quality health care and treatment and lives free from stigma and discrimination. This vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” according to the HHS vision statement.
NEWS FROM HHS