User login
Styrene exposure linked to myeloid leukemia, HL
A new study links styrene—a chemical used in the manufacture of plastics, rubber, and resins—to certain cancers.
The research showed that, contrary to previous suggestions, employees who have worked with styrene do not have an increased incidence of esophageal, pancreatic, lung, kidney, or bladder cancer.
On the other hand, they may have an increased risk of nasal and paranasal cancer, as well as myeloid leukemia and Hodgkin lymphoma (HL).
The research was published in Epidemiology.
“It is important to know for present and former workers exposed to styrene that they are unlikely to have become ill by doing their job if they have developed cancer of the esophagus, pancreas, lungs, kidneys, bladder, or a wide range of other types of cancer,” said study author Henrik A. Kolstad, MD, PhD, of Aarhus University in Denmark.
“This is also new and important knowledge in the USA, where styrene was added to the list of carcinogenic substances in 2011.”
In relation to the cancers for which the study shows a possible increased risk, Dr Kolstad emphasized that additional research is needed to determine if styrene is the actual cause of the employees’ disease.
For the current study, Dr Kolstad and his colleagues analyzed data on 72,292 employees who worked for 1 of 443 small and medium-sized companies in Denmark that used styrene for the production of wind turbines, pleasure boats, and other products from 1964 to 2007.
There were 8961 incident cases of cancer in this cohort from 1968 to 2012. The standardized incidence rate ratio (SIR) for all cancers was 1.04. When the researchers included a 10-year lag period, the SIR for all cancers was still 1.04.
As for hematologic malignancies, the researchers said they observed increased rate ratios associated with increased duration of employment for HL and myeloid leukemia.
For HL, the SIRs were 1.21 with no lag and 1.22 with a 10-year lag. For myeloid leukemia, the SIRs were 1.06 and 1.13, respectively.
The SIRs for non-Hodgkin lymphoma were 0.97 with no lag and 0.94 with a 10-year lag. The SIRs for multiple myeloma were 0.79 and 0.77, respectively.
For cancers of lymphatic and hematopoietic tissue, the SIRs were 0.97 with no lag and 0.96 with a 10-year lag. For lymphatic leukemia, the SIR was 0.96 for both time points.
The SIRs for monocytic leukemia were 0.77 with no lag and 0.56 with a 10-year lag. The SIRs for other and unspecified leukemias were 1.05 and 1.26, respectively.
The researchers noted that workers first employed in the 1960s had a higher risk of HL than workers first employed in subsequent years.
The SIRs were 2.12 for those first employed in 1964-1969, 0.82 for 1970-1979, 1.07 for 1980-1989, 1.52 for 1990-1999, and 1.10 for those first employed in 2000-2007.
There were no such associations for other cancer sites. ![]()
A new study links styrene—a chemical used in the manufacture of plastics, rubber, and resins—to certain cancers.
The research showed that, contrary to previous suggestions, employees who have worked with styrene do not have an increased incidence of esophageal, pancreatic, lung, kidney, or bladder cancer.
On the other hand, they may have an increased risk of nasal and paranasal cancer, as well as myeloid leukemia and Hodgkin lymphoma (HL).
The research was published in Epidemiology.
“It is important to know for present and former workers exposed to styrene that they are unlikely to have become ill by doing their job if they have developed cancer of the esophagus, pancreas, lungs, kidneys, bladder, or a wide range of other types of cancer,” said study author Henrik A. Kolstad, MD, PhD, of Aarhus University in Denmark.
“This is also new and important knowledge in the USA, where styrene was added to the list of carcinogenic substances in 2011.”
In relation to the cancers for which the study shows a possible increased risk, Dr Kolstad emphasized that additional research is needed to determine if styrene is the actual cause of the employees’ disease.
For the current study, Dr Kolstad and his colleagues analyzed data on 72,292 employees who worked for 1 of 443 small and medium-sized companies in Denmark that used styrene for the production of wind turbines, pleasure boats, and other products from 1964 to 2007.
There were 8961 incident cases of cancer in this cohort from 1968 to 2012. The standardized incidence rate ratio (SIR) for all cancers was 1.04. When the researchers included a 10-year lag period, the SIR for all cancers was still 1.04.
As for hematologic malignancies, the researchers said they observed increased rate ratios associated with increased duration of employment for HL and myeloid leukemia.
For HL, the SIRs were 1.21 with no lag and 1.22 with a 10-year lag. For myeloid leukemia, the SIRs were 1.06 and 1.13, respectively.
The SIRs for non-Hodgkin lymphoma were 0.97 with no lag and 0.94 with a 10-year lag. The SIRs for multiple myeloma were 0.79 and 0.77, respectively.
For cancers of lymphatic and hematopoietic tissue, the SIRs were 0.97 with no lag and 0.96 with a 10-year lag. For lymphatic leukemia, the SIR was 0.96 for both time points.
The SIRs for monocytic leukemia were 0.77 with no lag and 0.56 with a 10-year lag. The SIRs for other and unspecified leukemias were 1.05 and 1.26, respectively.
The researchers noted that workers first employed in the 1960s had a higher risk of HL than workers first employed in subsequent years.
The SIRs were 2.12 for those first employed in 1964-1969, 0.82 for 1970-1979, 1.07 for 1980-1989, 1.52 for 1990-1999, and 1.10 for those first employed in 2000-2007.
There were no such associations for other cancer sites. ![]()
A new study links styrene—a chemical used in the manufacture of plastics, rubber, and resins—to certain cancers.
The research showed that, contrary to previous suggestions, employees who have worked with styrene do not have an increased incidence of esophageal, pancreatic, lung, kidney, or bladder cancer.
On the other hand, they may have an increased risk of nasal and paranasal cancer, as well as myeloid leukemia and Hodgkin lymphoma (HL).
The research was published in Epidemiology.
“It is important to know for present and former workers exposed to styrene that they are unlikely to have become ill by doing their job if they have developed cancer of the esophagus, pancreas, lungs, kidneys, bladder, or a wide range of other types of cancer,” said study author Henrik A. Kolstad, MD, PhD, of Aarhus University in Denmark.
“This is also new and important knowledge in the USA, where styrene was added to the list of carcinogenic substances in 2011.”
In relation to the cancers for which the study shows a possible increased risk, Dr Kolstad emphasized that additional research is needed to determine if styrene is the actual cause of the employees’ disease.
For the current study, Dr Kolstad and his colleagues analyzed data on 72,292 employees who worked for 1 of 443 small and medium-sized companies in Denmark that used styrene for the production of wind turbines, pleasure boats, and other products from 1964 to 2007.
There were 8961 incident cases of cancer in this cohort from 1968 to 2012. The standardized incidence rate ratio (SIR) for all cancers was 1.04. When the researchers included a 10-year lag period, the SIR for all cancers was still 1.04.
As for hematologic malignancies, the researchers said they observed increased rate ratios associated with increased duration of employment for HL and myeloid leukemia.
For HL, the SIRs were 1.21 with no lag and 1.22 with a 10-year lag. For myeloid leukemia, the SIRs were 1.06 and 1.13, respectively.
The SIRs for non-Hodgkin lymphoma were 0.97 with no lag and 0.94 with a 10-year lag. The SIRs for multiple myeloma were 0.79 and 0.77, respectively.
For cancers of lymphatic and hematopoietic tissue, the SIRs were 0.97 with no lag and 0.96 with a 10-year lag. For lymphatic leukemia, the SIR was 0.96 for both time points.
The SIRs for monocytic leukemia were 0.77 with no lag and 0.56 with a 10-year lag. The SIRs for other and unspecified leukemias were 1.05 and 1.26, respectively.
The researchers noted that workers first employed in the 1960s had a higher risk of HL than workers first employed in subsequent years.
The SIRs were 2.12 for those first employed in 1964-1969, 0.82 for 1970-1979, 1.07 for 1980-1989, 1.52 for 1990-1999, and 1.10 for those first employed in 2000-2007.
There were no such associations for other cancer sites. ![]()
How EBV causes lymphoma, other cancers

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
New research published in Nature Communications appears to explain how Epstein-Barr virus (EBV) reprograms cells into cancer cells.
Investigators said they discovered a mechanism by which EBV particles induce chromosomal instability without establishing a chronic infection, thereby conferring a risk for the development of tumors that do not necessarily carry the viral genome.
“The contribution of the viral infection to cancer development in patients with a weakened immune system is well understood,” said study author Henri-Jacques Delecluse, MD, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
“But in the majority of cases, it remains unclear how an EBV infection leads to cancer development.”
With their research, Dr Delecluse and his colleagues found that BNRF1, a protein component of EBV, promotes the development of cancer. They said BNRF1 induces centrosome amplification, which is associated with chromosomal instability.
When a dividing cell comes in contact with EBV, BNRF1 frequently prompts the formation of an excessive number of centrosomes. As a result, chromosomes are no longer divided equally and accurately between daughter cells—a known cancer risk factor.
In contrast, when the investigators studied EBV deficient of BNRF1, they found the virus did not interfere with chromosome distribution to daughter cells.
The team noted that EBV normally remains silent in a few infected cells, but, occasionally, it reactivates to produce viral offspring that infects nearby cells. As a consequence, these cells come in close contact with BNRF1, thus increasing their risk of transforming into cancer cells.
“The novelty of our work is that we have uncovered a component of the viral particle as a cancer driver,” Dr Delecluse said. “All human-tumors viruses that have been studied so far cause cancer in a completely different manner.”
“Usually, the genetic material of the viruses needs to be permanently present in the infected cell, thus causing the activation of one or several viral genes that cause cancer development. However, these gene products are not present in the infectious particle itself.”
Dr Delecluse and his colleagues therefore suspect that EBV could cause cancers other than those that have already been linked to EBV. Certain cancers might not have been linked to the virus because they do not carry the viral genetic material.
“We must push forward with the development of a vaccine against EBV infection,” Dr Delecluse said. “This would be the most direct strategy to prevent an infection with the virus.”
“Our latest results show that the first infection could already be a cancer risk, and this fits with earlier work that showed an increase in the incidence of Hodgkin’s lymphoma in people who underwent an episode of infectious mononucleosis.” ![]()

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
New research published in Nature Communications appears to explain how Epstein-Barr virus (EBV) reprograms cells into cancer cells.
Investigators said they discovered a mechanism by which EBV particles induce chromosomal instability without establishing a chronic infection, thereby conferring a risk for the development of tumors that do not necessarily carry the viral genome.
“The contribution of the viral infection to cancer development in patients with a weakened immune system is well understood,” said study author Henri-Jacques Delecluse, MD, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
“But in the majority of cases, it remains unclear how an EBV infection leads to cancer development.”
With their research, Dr Delecluse and his colleagues found that BNRF1, a protein component of EBV, promotes the development of cancer. They said BNRF1 induces centrosome amplification, which is associated with chromosomal instability.
When a dividing cell comes in contact with EBV, BNRF1 frequently prompts the formation of an excessive number of centrosomes. As a result, chromosomes are no longer divided equally and accurately between daughter cells—a known cancer risk factor.
In contrast, when the investigators studied EBV deficient of BNRF1, they found the virus did not interfere with chromosome distribution to daughter cells.
The team noted that EBV normally remains silent in a few infected cells, but, occasionally, it reactivates to produce viral offspring that infects nearby cells. As a consequence, these cells come in close contact with BNRF1, thus increasing their risk of transforming into cancer cells.
“The novelty of our work is that we have uncovered a component of the viral particle as a cancer driver,” Dr Delecluse said. “All human-tumors viruses that have been studied so far cause cancer in a completely different manner.”
“Usually, the genetic material of the viruses needs to be permanently present in the infected cell, thus causing the activation of one or several viral genes that cause cancer development. However, these gene products are not present in the infectious particle itself.”
Dr Delecluse and his colleagues therefore suspect that EBV could cause cancers other than those that have already been linked to EBV. Certain cancers might not have been linked to the virus because they do not carry the viral genetic material.
“We must push forward with the development of a vaccine against EBV infection,” Dr Delecluse said. “This would be the most direct strategy to prevent an infection with the virus.”
“Our latest results show that the first infection could already be a cancer risk, and this fits with earlier work that showed an increase in the incidence of Hodgkin’s lymphoma in people who underwent an episode of infectious mononucleosis.” ![]()

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
New research published in Nature Communications appears to explain how Epstein-Barr virus (EBV) reprograms cells into cancer cells.
Investigators said they discovered a mechanism by which EBV particles induce chromosomal instability without establishing a chronic infection, thereby conferring a risk for the development of tumors that do not necessarily carry the viral genome.
“The contribution of the viral infection to cancer development in patients with a weakened immune system is well understood,” said study author Henri-Jacques Delecluse, MD, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
“But in the majority of cases, it remains unclear how an EBV infection leads to cancer development.”
With their research, Dr Delecluse and his colleagues found that BNRF1, a protein component of EBV, promotes the development of cancer. They said BNRF1 induces centrosome amplification, which is associated with chromosomal instability.
When a dividing cell comes in contact with EBV, BNRF1 frequently prompts the formation of an excessive number of centrosomes. As a result, chromosomes are no longer divided equally and accurately between daughter cells—a known cancer risk factor.
In contrast, when the investigators studied EBV deficient of BNRF1, they found the virus did not interfere with chromosome distribution to daughter cells.
The team noted that EBV normally remains silent in a few infected cells, but, occasionally, it reactivates to produce viral offspring that infects nearby cells. As a consequence, these cells come in close contact with BNRF1, thus increasing their risk of transforming into cancer cells.
“The novelty of our work is that we have uncovered a component of the viral particle as a cancer driver,” Dr Delecluse said. “All human-tumors viruses that have been studied so far cause cancer in a completely different manner.”
“Usually, the genetic material of the viruses needs to be permanently present in the infected cell, thus causing the activation of one or several viral genes that cause cancer development. However, these gene products are not present in the infectious particle itself.”
Dr Delecluse and his colleagues therefore suspect that EBV could cause cancers other than those that have already been linked to EBV. Certain cancers might not have been linked to the virus because they do not carry the viral genetic material.
“We must push forward with the development of a vaccine against EBV infection,” Dr Delecluse said. “This would be the most direct strategy to prevent an infection with the virus.”
“Our latest results show that the first infection could already be a cancer risk, and this fits with earlier work that showed an increase in the incidence of Hodgkin’s lymphoma in people who underwent an episode of infectious mononucleosis.” ![]()
Long view shows doubling of survival in non-Hodgkin lymphoma
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).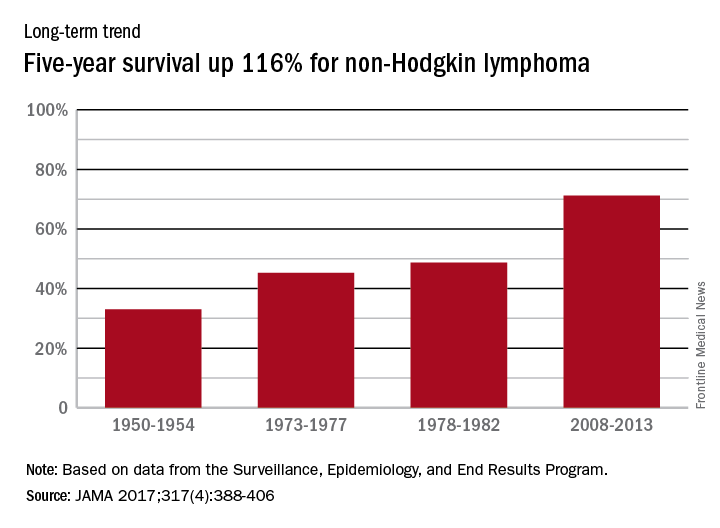
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
FROM JAMA
Hodgkin lymphoma survival has nearly tripled since the 1950s
Five-year relative survival for Hodgkin lymphoma increased 189% over the approximately 60 years from the early 1950s to 2013, according to investigators looking at data from the Surveillance, Epidemiology, and End Results Program.
During 1950-1954, the 5-year relative survival rate for Hodgkin lymphoma was 30%, compared with 86.6% in 2008-2013, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.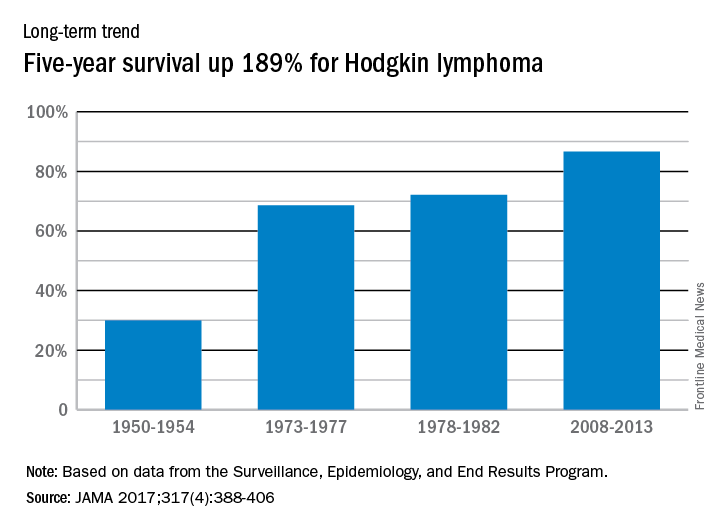
In 2014, mortality for Hodgkin lymphoma was 0.4 per 100,000 population, which put it 27th among the 29 included cancers, with about 36,000 years of life lost, which was 26th of the 29 cancers, Dr. Mokdad and his associates said. This part of their study used deidentified death records from the National Center for Health Statistics and population counts from the Census Bureau, the NCHS, and the Human Mortality Database.
Five-year relative survival for Hodgkin lymphoma increased 189% over the approximately 60 years from the early 1950s to 2013, according to investigators looking at data from the Surveillance, Epidemiology, and End Results Program.
During 1950-1954, the 5-year relative survival rate for Hodgkin lymphoma was 30%, compared with 86.6% in 2008-2013, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
In 2014, mortality for Hodgkin lymphoma was 0.4 per 100,000 population, which put it 27th among the 29 included cancers, with about 36,000 years of life lost, which was 26th of the 29 cancers, Dr. Mokdad and his associates said. This part of their study used deidentified death records from the National Center for Health Statistics and population counts from the Census Bureau, the NCHS, and the Human Mortality Database.
Five-year relative survival for Hodgkin lymphoma increased 189% over the approximately 60 years from the early 1950s to 2013, according to investigators looking at data from the Surveillance, Epidemiology, and End Results Program.
During 1950-1954, the 5-year relative survival rate for Hodgkin lymphoma was 30%, compared with 86.6% in 2008-2013, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
In 2014, mortality for Hodgkin lymphoma was 0.4 per 100,000 population, which put it 27th among the 29 included cancers, with about 36,000 years of life lost, which was 26th of the 29 cancers, Dr. Mokdad and his associates said. This part of their study used deidentified death records from the National Center for Health Statistics and population counts from the Census Bureau, the NCHS, and the Human Mortality Database.
FROM JAMA
Study quantifies 5-year survival rates for blood cancers

chemotherapy
Photo by Rhoda Baer
A new study shows that 5-year survival rates for US patients with hematologic malignancies have increased greatly since the 1950s, but there is still room for improvement, particularly for patients with acute myeloid leukemia (AML).
Researchers found the absolute difference in improvement for 5-year survival from 1950-1954 to 2008-2013 ranged from 38.2% for non-Hodgkin lymphoma (NHL) to 56.6% for Hodgkin lymphoma.
And although the 5-year survival rate for Hodgkin lymphoma patients reached 86.6% for 2008-2013, the 5-year survival rate for patients with AML only reached 27.4%.
This study also revealed large disparities in overall cancer mortality rates between different counties across the country.
Ali H. Mokdad, PhD, of the Institute for Health Metrics and Evaluation in Seattle, Washington, and his colleagues reported these findings in JAMA.
Overall cancer deaths
The researchers found there were 19,511,910 cancer deaths recorded in the US between 1980 and 2014. Cancer mortality decreased by 20.1% between 1980 and 2014, from 240.2 deaths per 100,000 people to 192.0 deaths per 100,000 people.
In 1980, cancer mortality ranged from 130.6 per 100,000 in Summit County, Colorado, to 386.9 per 100,000 in North Slope Borough, Alaska.
In 2014, cancer mortality ranged from 70.7 per 100,000 in Summit County, Colorado, to 503.1 per 100,000 in Union County, Florida.
“Such significant disparities among US counties is unacceptable,” Dr Mokdad said. “Every person should have access to early screenings for cancer, as well as adequate treatment.”
Mortality rates for hematologic malignancies
In 2014, the mortality rates, per 100,000 people, for hematologic malignancies were:
- 0.4 for Hodgkin lymphoma (rank out of all cancers, 27)
- 8.3 for NHL (rank, 7)
- 3.9 for multiple myeloma (rank, 16)
- 9.0 for all leukemias (rank, 6)
- 0.7 for acute lymphoid leukemia (ALL)
- 2.6 for chronic lymphoid leukemia (CLL)
- 5.1 for AML
- 0.6 for chronic myeloid leukemia (CML).
The leukemia subtypes were not assigned a rank.
5-year survival rates for hematologic malignancies
Hodgkin lymphoma
- 30% for 1950-54
- 68.6% for 1973-77
- 72.1% for 1978-82
- 86.6% for 2008-2013
- Absolute difference (between the first and latest year of data), 56.6%.
NHL
- 33% for 1950-54
- 45.3% for 1973-77
- 48.7% for 1978-82
- 71.2% for 2008-2013
- Absolute difference, 38.2%.
Multiple myeloma
- 6% for 1950-54
- 23.4% for 1973-77
- 26.6% for 1978-82
- 49.8% for 2008-2013
- Absolute difference, 43.8%.
Leukemia
- 10% for 1950-54
- 34% for 1973-77
- 36.3% for 1978-82
- 60.1% for 2008-2013
- Absolute difference, 50.1%.
ALL
- 39.2% for 1973-77
- 50.5% for 1978-82
- 68.1% for 2008-2013
- Absolute difference, 28.9%.
CLL
- 67% for 1973-77
- 66.3% for 1978-82
- 82.5% for 2008-2013
- Absolute difference, 15.5%.
AML
- 6.2% for 1973-77
- 7.9% for 1978-82
- 27.4% for 2008-2013
- Absolute difference, 21.2%.
CML
- 21.1% for 1973-77
- 25.8% for 1978-82
- 66.4% for 2008-2013
- Absolute difference, 45.3%.
For the leukemia subtypes, there was no data for 1950 to 1954. ![]()

chemotherapy
Photo by Rhoda Baer
A new study shows that 5-year survival rates for US patients with hematologic malignancies have increased greatly since the 1950s, but there is still room for improvement, particularly for patients with acute myeloid leukemia (AML).
Researchers found the absolute difference in improvement for 5-year survival from 1950-1954 to 2008-2013 ranged from 38.2% for non-Hodgkin lymphoma (NHL) to 56.6% for Hodgkin lymphoma.
And although the 5-year survival rate for Hodgkin lymphoma patients reached 86.6% for 2008-2013, the 5-year survival rate for patients with AML only reached 27.4%.
This study also revealed large disparities in overall cancer mortality rates between different counties across the country.
Ali H. Mokdad, PhD, of the Institute for Health Metrics and Evaluation in Seattle, Washington, and his colleagues reported these findings in JAMA.
Overall cancer deaths
The researchers found there were 19,511,910 cancer deaths recorded in the US between 1980 and 2014. Cancer mortality decreased by 20.1% between 1980 and 2014, from 240.2 deaths per 100,000 people to 192.0 deaths per 100,000 people.
In 1980, cancer mortality ranged from 130.6 per 100,000 in Summit County, Colorado, to 386.9 per 100,000 in North Slope Borough, Alaska.
In 2014, cancer mortality ranged from 70.7 per 100,000 in Summit County, Colorado, to 503.1 per 100,000 in Union County, Florida.
“Such significant disparities among US counties is unacceptable,” Dr Mokdad said. “Every person should have access to early screenings for cancer, as well as adequate treatment.”
Mortality rates for hematologic malignancies
In 2014, the mortality rates, per 100,000 people, for hematologic malignancies were:
- 0.4 for Hodgkin lymphoma (rank out of all cancers, 27)
- 8.3 for NHL (rank, 7)
- 3.9 for multiple myeloma (rank, 16)
- 9.0 for all leukemias (rank, 6)
- 0.7 for acute lymphoid leukemia (ALL)
- 2.6 for chronic lymphoid leukemia (CLL)
- 5.1 for AML
- 0.6 for chronic myeloid leukemia (CML).
The leukemia subtypes were not assigned a rank.
5-year survival rates for hematologic malignancies
Hodgkin lymphoma
- 30% for 1950-54
- 68.6% for 1973-77
- 72.1% for 1978-82
- 86.6% for 2008-2013
- Absolute difference (between the first and latest year of data), 56.6%.
NHL
- 33% for 1950-54
- 45.3% for 1973-77
- 48.7% for 1978-82
- 71.2% for 2008-2013
- Absolute difference, 38.2%.
Multiple myeloma
- 6% for 1950-54
- 23.4% for 1973-77
- 26.6% for 1978-82
- 49.8% for 2008-2013
- Absolute difference, 43.8%.
Leukemia
- 10% for 1950-54
- 34% for 1973-77
- 36.3% for 1978-82
- 60.1% for 2008-2013
- Absolute difference, 50.1%.
ALL
- 39.2% for 1973-77
- 50.5% for 1978-82
- 68.1% for 2008-2013
- Absolute difference, 28.9%.
CLL
- 67% for 1973-77
- 66.3% for 1978-82
- 82.5% for 2008-2013
- Absolute difference, 15.5%.
AML
- 6.2% for 1973-77
- 7.9% for 1978-82
- 27.4% for 2008-2013
- Absolute difference, 21.2%.
CML
- 21.1% for 1973-77
- 25.8% for 1978-82
- 66.4% for 2008-2013
- Absolute difference, 45.3%.
For the leukemia subtypes, there was no data for 1950 to 1954. ![]()

chemotherapy
Photo by Rhoda Baer
A new study shows that 5-year survival rates for US patients with hematologic malignancies have increased greatly since the 1950s, but there is still room for improvement, particularly for patients with acute myeloid leukemia (AML).
Researchers found the absolute difference in improvement for 5-year survival from 1950-1954 to 2008-2013 ranged from 38.2% for non-Hodgkin lymphoma (NHL) to 56.6% for Hodgkin lymphoma.
And although the 5-year survival rate for Hodgkin lymphoma patients reached 86.6% for 2008-2013, the 5-year survival rate for patients with AML only reached 27.4%.
This study also revealed large disparities in overall cancer mortality rates between different counties across the country.
Ali H. Mokdad, PhD, of the Institute for Health Metrics and Evaluation in Seattle, Washington, and his colleagues reported these findings in JAMA.
Overall cancer deaths
The researchers found there were 19,511,910 cancer deaths recorded in the US between 1980 and 2014. Cancer mortality decreased by 20.1% between 1980 and 2014, from 240.2 deaths per 100,000 people to 192.0 deaths per 100,000 people.
In 1980, cancer mortality ranged from 130.6 per 100,000 in Summit County, Colorado, to 386.9 per 100,000 in North Slope Borough, Alaska.
In 2014, cancer mortality ranged from 70.7 per 100,000 in Summit County, Colorado, to 503.1 per 100,000 in Union County, Florida.
“Such significant disparities among US counties is unacceptable,” Dr Mokdad said. “Every person should have access to early screenings for cancer, as well as adequate treatment.”
Mortality rates for hematologic malignancies
In 2014, the mortality rates, per 100,000 people, for hematologic malignancies were:
- 0.4 for Hodgkin lymphoma (rank out of all cancers, 27)
- 8.3 for NHL (rank, 7)
- 3.9 for multiple myeloma (rank, 16)
- 9.0 for all leukemias (rank, 6)
- 0.7 for acute lymphoid leukemia (ALL)
- 2.6 for chronic lymphoid leukemia (CLL)
- 5.1 for AML
- 0.6 for chronic myeloid leukemia (CML).
The leukemia subtypes were not assigned a rank.
5-year survival rates for hematologic malignancies
Hodgkin lymphoma
- 30% for 1950-54
- 68.6% for 1973-77
- 72.1% for 1978-82
- 86.6% for 2008-2013
- Absolute difference (between the first and latest year of data), 56.6%.
NHL
- 33% for 1950-54
- 45.3% for 1973-77
- 48.7% for 1978-82
- 71.2% for 2008-2013
- Absolute difference, 38.2%.
Multiple myeloma
- 6% for 1950-54
- 23.4% for 1973-77
- 26.6% for 1978-82
- 49.8% for 2008-2013
- Absolute difference, 43.8%.
Leukemia
- 10% for 1950-54
- 34% for 1973-77
- 36.3% for 1978-82
- 60.1% for 2008-2013
- Absolute difference, 50.1%.
ALL
- 39.2% for 1973-77
- 50.5% for 1978-82
- 68.1% for 2008-2013
- Absolute difference, 28.9%.
CLL
- 67% for 1973-77
- 66.3% for 1978-82
- 82.5% for 2008-2013
- Absolute difference, 15.5%.
AML
- 6.2% for 1973-77
- 7.9% for 1978-82
- 27.4% for 2008-2013
- Absolute difference, 21.2%.
CML
- 21.1% for 1973-77
- 25.8% for 1978-82
- 66.4% for 2008-2013
- Absolute difference, 45.3%.
For the leukemia subtypes, there was no data for 1950 to 1954. ![]()
Combined checkpoint blockade promising in HL

© Todd Buchanan 2016
SAN DIEGO—Immune checkpoint blockade with nivolumab plus ipilimumab has shown promise in treating hematologic malignancies, particularly classical Hodgkin lymphoma (HL), based on results of the combination cohort of the phase 1 CheckMate 039 study.
Thirty-one heavily pre-treated HL patients achieved an overall response rate (ORR) of 74%, including 6 complete responses.
And in transplant-naïve HL patients, the combination produced an ORR of 67%.
“Most in the room would be familiar with the excellent results that we have seen with monotherapy with nivolumab,” Stephen Ansell, MD, PhD, of the Mayo Clinic in Rochester, Minnesota, said at the 2016 ASH Annual Meeting.
“In classical Hodgkin lymphoma, we’ve seen meaningful and clinically quite stellar results and durable responses.”
“Our plan was, as part of this trial [CheckMate 039], to then move to see whether adding a further checkpoint, ipilimumab, could enhance the results seen with nivolumab.”
Dr Ansell presented the findings for the checkpoint combination as abstract 183. He disclosed research funding from Bristol-Myers Squibb, the company that funded the study.
Checkpoint inhibitors
Nivolumab and ipilimumab are both fully human monoclonal antibodies, but ipilimumab “works in a slightly different fashion from nivolumab,” Dr Ansell said.
Nivolumab targets the programmed death receptor-1 (PD-1) and disrupts PD-1 pathway signaling and restores anti-tumor T-cell function.
Ipilimumab targets cytotoxic T-lymphocyte antigen 4 (CTLA-4) and induces anti-tumor immunity.
The combination has shown superior efficacy, compared to either agent alone, in preclinical studies and a phase 1 trial of patients with advanced melanoma.
So the investigators added a combination cohort to CheckMate 039.
Combination cohort study design
Patients were eligible to enroll if they had relapsed or refractory HL, B-cell non-Hodgkin lymphoma (NHL, including follicular or diffuse large B-cell lymphoma), T-cell NHL (including cutaneous or peripheral T-cell lymphoma), or multiple myeloma (MM).
Patients could not have had prior organ or allogeneic stem cell transplant and no prior immune checkpoint blockade therapy.
Treatment consisted of nivolumab at 3 mg/kg IV plus ipilimumab at 1 mg/kg IV every 3 weeks for 4 doses. The combination phase was followed by nivolumab monotherapy at the same dose every 2 weeks for 2 years.
The primary endpoint was safety and tolerability. Secondary endpoints included investigator-assessed best overall response, duration of response, progression-free survival (PFS), and biomarker analyses.
Patient characteristics
The investigators enrolled 31 HL, 15 B-cell NHL, 11 T-cell NHL, and 7 MM patients. Most patients, Dr Ansell noted, were heavily pretreated.
HL patients were 42% male, 52% had an ECOG status of 1, and they had a median of 4 (range, 2 to 10) prior systemic therapies. Forty-two percent had prior autologous stem cell transplant (ASCT).
“Interestingly, in the Hodgkin cohort, a number of patients had not proceeded to an autologous transplant, but predominantly because these were chemo-refractory or chemo-resistant patients not eligible for a transplant,” Dr Ansell pointed out.
Of the HL patients, 18 were transplant-naïve, 13 were chemo-resistant, 3 were ineligible for ASCT, and 2 declined the procedure.
B-cell NHL patients were 73% male, and 80% had an ECOG status of 1. They had a median of 3 (range, 1 to 16) prior systemic therapies. Seven percent had a prior ASCT.
T-cell NHL patients were 55% male, 73% had an ECOG status of 1, and they had a median of 4 (range, 1 to 11) prior systemic therapies. None had a prior ASCT.
MM patients were 86% male, 71% had an ECOG status of 1, and they had a median of 5 (range, 2 to 20) prior systemic therapies. More than half had a prior ASCT.
Patient disposition
With follow-up approaching a year, more patients with HL are still on treatment (39%) compared with B-cell NHL (13%), T-cell NHL (18%), and MM (0%) patients.
“Of note, however, is that the reasons for going off treatment were predominantly disease progression,” Dr Ansell said.
“The vast majority of patients who came off treatment came off treatment because their disease progressed, and the numbers that came off because of toxicity were relatively low.”
Seven HL patients went off treatment due to disease progression and 2 due to study drug toxicity.
Eleven B-cell NHL patients went off treatment due to disease progression and 2 withdrew due to unrelated adverse events (AEs).
Five T-cell NHL patients went off treatment due to disease progression and 2 due to study drug toxicity.
And 4 MM patients withdrew due to disease progression, 1 due to study drug toxicity, and 1 due to AEs unrelated to the study drug.
About two-thirds of HL patients, over 90% of B-cell NHL patients, about 80% of T-cell NHL patients, and about 70% of MM patients received 90% or more of the intended dose of each drug.
Safety
One patient with primary mediastinal B-cell lymphoma was included in the safety analysis, for a total of 65 patients treated.
“The majority of patients had some degree of adverse event,” Dr Ansell explained. “But if one looks at the grade 3 and 4 adverse events, those were seen in a more modest number of patients, in a minority of patients. And most importantly, if one looks at the adverse events that led to discontinuation, one can see that this was in a significant minority of patients.”
Five patients discontinued due to treatment-related AEs, which were pneumonitis (n=3), pneumonia and pneumonitis (n=1), and diabetic ketoacidosis (n=1).
Overall, 51 patients (78%) experienced an AE; 19 (29%) had a grade 3–4 AE, 14 (22%) had a serious AE, and 5 (8%) discontinued due to an AE.
Of 31 HL patients, 28 (90%) had an AE, 8 (26%) had a grade 3–4 AE, 6 (19%) had a serious AE, and 2 (6%) discontinued due to an AE.
All 11 T-cell NHL patients experienced an AE, 5 patients (45%) a grade 3-4 AE, 4 patients (36%) had a serious AE, and 2 patients (18%) discontinued because of an AE.
About half of B-cell NHL and MM patients experienced an AE, with 1 MM patient discontinuing as a result of it and no B-cell NHL patient discontinuing due to an AE.
“I would highlight that most of the adverse events were, as expected, immunological in nature . . . . ,” Dr Ansell said. “A very modest number of patients had grade 3 and 4 toxicities.”
The most common drug-related AEs of any grade were fatigue (n=17; 26%), pyrexia (n=15; 23%), rash (n=7; 11%), diarrhea (n=12; 18%), and nausea, pneumonitis, cough, and infusion-related reactions, with 9 patients each (14%).
Efficacy
Twenty-three HL patients (74%) achieved an overall response, including 6 patients (19%) with a complete response and 17 (55%) with a partial response. Three patients (10%) had stable disease, and 3 (10%) had relapsed or progressive disease. Response was not reported for 2 patients (6%).
“Most of these responses are durable, and, very encouraging, you can see patients out approaching a year continuing on therapy,” Dr Ansell said.
The ORR in the 18 transplant-naive patients was 67% (n=67).
The median duration of response for HL patients was not reached and ranged from 0.0 to 13.4 months.
B-cell NHL patients had an ORR of 20% (n=3). There were no complete responses and 3 (20%) partial responses. One patient (7%) had stable disease, and 8 (53%) had relapsed or progressive disase. The median duration of partial response was not reached and ranged from 11.0 to 12.7 months.
T-cell NHL patients had an ORR of 9% (n=1). There were no complete responses and 1 (9%) partial response. Four patients (36%) had stable disease, and 3 (27%) had relapsed or progressive disease. The median duration of partial response was not reached and was 3.9 months.
Except for 1 patient with stable disease, MM patients did not respond to therapy.
Biomarker analysis
All 19 HL patients with a known PD-L1 status at baseline saw their tumor burden decrease to below baseline levels. This may be because HL is characterized by high PD-L1 expression and high responsiveness to checkpoint blockade.
Patients with NHL, on the other hand, have a diverse group of tumors characterized by variable PD-L1 expression. Eight of 13 patients with known expression saw their tumor burden decrease with treatment to below baseline.
Encouraged by the results, the investigators believe further investigation of the combination is in order, as the combination, with limited follow-up, achieved a high and durable ORR in HL patients, including those who were transplant-naïve. ![]()

© Todd Buchanan 2016
SAN DIEGO—Immune checkpoint blockade with nivolumab plus ipilimumab has shown promise in treating hematologic malignancies, particularly classical Hodgkin lymphoma (HL), based on results of the combination cohort of the phase 1 CheckMate 039 study.
Thirty-one heavily pre-treated HL patients achieved an overall response rate (ORR) of 74%, including 6 complete responses.
And in transplant-naïve HL patients, the combination produced an ORR of 67%.
“Most in the room would be familiar with the excellent results that we have seen with monotherapy with nivolumab,” Stephen Ansell, MD, PhD, of the Mayo Clinic in Rochester, Minnesota, said at the 2016 ASH Annual Meeting.
“In classical Hodgkin lymphoma, we’ve seen meaningful and clinically quite stellar results and durable responses.”
“Our plan was, as part of this trial [CheckMate 039], to then move to see whether adding a further checkpoint, ipilimumab, could enhance the results seen with nivolumab.”
Dr Ansell presented the findings for the checkpoint combination as abstract 183. He disclosed research funding from Bristol-Myers Squibb, the company that funded the study.
Checkpoint inhibitors
Nivolumab and ipilimumab are both fully human monoclonal antibodies, but ipilimumab “works in a slightly different fashion from nivolumab,” Dr Ansell said.
Nivolumab targets the programmed death receptor-1 (PD-1) and disrupts PD-1 pathway signaling and restores anti-tumor T-cell function.
Ipilimumab targets cytotoxic T-lymphocyte antigen 4 (CTLA-4) and induces anti-tumor immunity.
The combination has shown superior efficacy, compared to either agent alone, in preclinical studies and a phase 1 trial of patients with advanced melanoma.
So the investigators added a combination cohort to CheckMate 039.
Combination cohort study design
Patients were eligible to enroll if they had relapsed or refractory HL, B-cell non-Hodgkin lymphoma (NHL, including follicular or diffuse large B-cell lymphoma), T-cell NHL (including cutaneous or peripheral T-cell lymphoma), or multiple myeloma (MM).
Patients could not have had prior organ or allogeneic stem cell transplant and no prior immune checkpoint blockade therapy.
Treatment consisted of nivolumab at 3 mg/kg IV plus ipilimumab at 1 mg/kg IV every 3 weeks for 4 doses. The combination phase was followed by nivolumab monotherapy at the same dose every 2 weeks for 2 years.
The primary endpoint was safety and tolerability. Secondary endpoints included investigator-assessed best overall response, duration of response, progression-free survival (PFS), and biomarker analyses.
Patient characteristics
The investigators enrolled 31 HL, 15 B-cell NHL, 11 T-cell NHL, and 7 MM patients. Most patients, Dr Ansell noted, were heavily pretreated.
HL patients were 42% male, 52% had an ECOG status of 1, and they had a median of 4 (range, 2 to 10) prior systemic therapies. Forty-two percent had prior autologous stem cell transplant (ASCT).
“Interestingly, in the Hodgkin cohort, a number of patients had not proceeded to an autologous transplant, but predominantly because these were chemo-refractory or chemo-resistant patients not eligible for a transplant,” Dr Ansell pointed out.
Of the HL patients, 18 were transplant-naïve, 13 were chemo-resistant, 3 were ineligible for ASCT, and 2 declined the procedure.
B-cell NHL patients were 73% male, and 80% had an ECOG status of 1. They had a median of 3 (range, 1 to 16) prior systemic therapies. Seven percent had a prior ASCT.
T-cell NHL patients were 55% male, 73% had an ECOG status of 1, and they had a median of 4 (range, 1 to 11) prior systemic therapies. None had a prior ASCT.
MM patients were 86% male, 71% had an ECOG status of 1, and they had a median of 5 (range, 2 to 20) prior systemic therapies. More than half had a prior ASCT.
Patient disposition
With follow-up approaching a year, more patients with HL are still on treatment (39%) compared with B-cell NHL (13%), T-cell NHL (18%), and MM (0%) patients.
“Of note, however, is that the reasons for going off treatment were predominantly disease progression,” Dr Ansell said.
“The vast majority of patients who came off treatment came off treatment because their disease progressed, and the numbers that came off because of toxicity were relatively low.”
Seven HL patients went off treatment due to disease progression and 2 due to study drug toxicity.
Eleven B-cell NHL patients went off treatment due to disease progression and 2 withdrew due to unrelated adverse events (AEs).
Five T-cell NHL patients went off treatment due to disease progression and 2 due to study drug toxicity.
And 4 MM patients withdrew due to disease progression, 1 due to study drug toxicity, and 1 due to AEs unrelated to the study drug.
About two-thirds of HL patients, over 90% of B-cell NHL patients, about 80% of T-cell NHL patients, and about 70% of MM patients received 90% or more of the intended dose of each drug.
Safety
One patient with primary mediastinal B-cell lymphoma was included in the safety analysis, for a total of 65 patients treated.
“The majority of patients had some degree of adverse event,” Dr Ansell explained. “But if one looks at the grade 3 and 4 adverse events, those were seen in a more modest number of patients, in a minority of patients. And most importantly, if one looks at the adverse events that led to discontinuation, one can see that this was in a significant minority of patients.”
Five patients discontinued due to treatment-related AEs, which were pneumonitis (n=3), pneumonia and pneumonitis (n=1), and diabetic ketoacidosis (n=1).
Overall, 51 patients (78%) experienced an AE; 19 (29%) had a grade 3–4 AE, 14 (22%) had a serious AE, and 5 (8%) discontinued due to an AE.
Of 31 HL patients, 28 (90%) had an AE, 8 (26%) had a grade 3–4 AE, 6 (19%) had a serious AE, and 2 (6%) discontinued due to an AE.
All 11 T-cell NHL patients experienced an AE, 5 patients (45%) a grade 3-4 AE, 4 patients (36%) had a serious AE, and 2 patients (18%) discontinued because of an AE.
About half of B-cell NHL and MM patients experienced an AE, with 1 MM patient discontinuing as a result of it and no B-cell NHL patient discontinuing due to an AE.
“I would highlight that most of the adverse events were, as expected, immunological in nature . . . . ,” Dr Ansell said. “A very modest number of patients had grade 3 and 4 toxicities.”
The most common drug-related AEs of any grade were fatigue (n=17; 26%), pyrexia (n=15; 23%), rash (n=7; 11%), diarrhea (n=12; 18%), and nausea, pneumonitis, cough, and infusion-related reactions, with 9 patients each (14%).
Efficacy
Twenty-three HL patients (74%) achieved an overall response, including 6 patients (19%) with a complete response and 17 (55%) with a partial response. Three patients (10%) had stable disease, and 3 (10%) had relapsed or progressive disease. Response was not reported for 2 patients (6%).
“Most of these responses are durable, and, very encouraging, you can see patients out approaching a year continuing on therapy,” Dr Ansell said.
The ORR in the 18 transplant-naive patients was 67% (n=67).
The median duration of response for HL patients was not reached and ranged from 0.0 to 13.4 months.
B-cell NHL patients had an ORR of 20% (n=3). There were no complete responses and 3 (20%) partial responses. One patient (7%) had stable disease, and 8 (53%) had relapsed or progressive disase. The median duration of partial response was not reached and ranged from 11.0 to 12.7 months.
T-cell NHL patients had an ORR of 9% (n=1). There were no complete responses and 1 (9%) partial response. Four patients (36%) had stable disease, and 3 (27%) had relapsed or progressive disease. The median duration of partial response was not reached and was 3.9 months.
Except for 1 patient with stable disease, MM patients did not respond to therapy.
Biomarker analysis
All 19 HL patients with a known PD-L1 status at baseline saw their tumor burden decrease to below baseline levels. This may be because HL is characterized by high PD-L1 expression and high responsiveness to checkpoint blockade.
Patients with NHL, on the other hand, have a diverse group of tumors characterized by variable PD-L1 expression. Eight of 13 patients with known expression saw their tumor burden decrease with treatment to below baseline.
Encouraged by the results, the investigators believe further investigation of the combination is in order, as the combination, with limited follow-up, achieved a high and durable ORR in HL patients, including those who were transplant-naïve. ![]()

© Todd Buchanan 2016
SAN DIEGO—Immune checkpoint blockade with nivolumab plus ipilimumab has shown promise in treating hematologic malignancies, particularly classical Hodgkin lymphoma (HL), based on results of the combination cohort of the phase 1 CheckMate 039 study.
Thirty-one heavily pre-treated HL patients achieved an overall response rate (ORR) of 74%, including 6 complete responses.
And in transplant-naïve HL patients, the combination produced an ORR of 67%.
“Most in the room would be familiar with the excellent results that we have seen with monotherapy with nivolumab,” Stephen Ansell, MD, PhD, of the Mayo Clinic in Rochester, Minnesota, said at the 2016 ASH Annual Meeting.
“In classical Hodgkin lymphoma, we’ve seen meaningful and clinically quite stellar results and durable responses.”
“Our plan was, as part of this trial [CheckMate 039], to then move to see whether adding a further checkpoint, ipilimumab, could enhance the results seen with nivolumab.”
Dr Ansell presented the findings for the checkpoint combination as abstract 183. He disclosed research funding from Bristol-Myers Squibb, the company that funded the study.
Checkpoint inhibitors
Nivolumab and ipilimumab are both fully human monoclonal antibodies, but ipilimumab “works in a slightly different fashion from nivolumab,” Dr Ansell said.
Nivolumab targets the programmed death receptor-1 (PD-1) and disrupts PD-1 pathway signaling and restores anti-tumor T-cell function.
Ipilimumab targets cytotoxic T-lymphocyte antigen 4 (CTLA-4) and induces anti-tumor immunity.
The combination has shown superior efficacy, compared to either agent alone, in preclinical studies and a phase 1 trial of patients with advanced melanoma.
So the investigators added a combination cohort to CheckMate 039.
Combination cohort study design
Patients were eligible to enroll if they had relapsed or refractory HL, B-cell non-Hodgkin lymphoma (NHL, including follicular or diffuse large B-cell lymphoma), T-cell NHL (including cutaneous or peripheral T-cell lymphoma), or multiple myeloma (MM).
Patients could not have had prior organ or allogeneic stem cell transplant and no prior immune checkpoint blockade therapy.
Treatment consisted of nivolumab at 3 mg/kg IV plus ipilimumab at 1 mg/kg IV every 3 weeks for 4 doses. The combination phase was followed by nivolumab monotherapy at the same dose every 2 weeks for 2 years.
The primary endpoint was safety and tolerability. Secondary endpoints included investigator-assessed best overall response, duration of response, progression-free survival (PFS), and biomarker analyses.
Patient characteristics
The investigators enrolled 31 HL, 15 B-cell NHL, 11 T-cell NHL, and 7 MM patients. Most patients, Dr Ansell noted, were heavily pretreated.
HL patients were 42% male, 52% had an ECOG status of 1, and they had a median of 4 (range, 2 to 10) prior systemic therapies. Forty-two percent had prior autologous stem cell transplant (ASCT).
“Interestingly, in the Hodgkin cohort, a number of patients had not proceeded to an autologous transplant, but predominantly because these were chemo-refractory or chemo-resistant patients not eligible for a transplant,” Dr Ansell pointed out.
Of the HL patients, 18 were transplant-naïve, 13 were chemo-resistant, 3 were ineligible for ASCT, and 2 declined the procedure.
B-cell NHL patients were 73% male, and 80% had an ECOG status of 1. They had a median of 3 (range, 1 to 16) prior systemic therapies. Seven percent had a prior ASCT.
T-cell NHL patients were 55% male, 73% had an ECOG status of 1, and they had a median of 4 (range, 1 to 11) prior systemic therapies. None had a prior ASCT.
MM patients were 86% male, 71% had an ECOG status of 1, and they had a median of 5 (range, 2 to 20) prior systemic therapies. More than half had a prior ASCT.
Patient disposition
With follow-up approaching a year, more patients with HL are still on treatment (39%) compared with B-cell NHL (13%), T-cell NHL (18%), and MM (0%) patients.
“Of note, however, is that the reasons for going off treatment were predominantly disease progression,” Dr Ansell said.
“The vast majority of patients who came off treatment came off treatment because their disease progressed, and the numbers that came off because of toxicity were relatively low.”
Seven HL patients went off treatment due to disease progression and 2 due to study drug toxicity.
Eleven B-cell NHL patients went off treatment due to disease progression and 2 withdrew due to unrelated adverse events (AEs).
Five T-cell NHL patients went off treatment due to disease progression and 2 due to study drug toxicity.
And 4 MM patients withdrew due to disease progression, 1 due to study drug toxicity, and 1 due to AEs unrelated to the study drug.
About two-thirds of HL patients, over 90% of B-cell NHL patients, about 80% of T-cell NHL patients, and about 70% of MM patients received 90% or more of the intended dose of each drug.
Safety
One patient with primary mediastinal B-cell lymphoma was included in the safety analysis, for a total of 65 patients treated.
“The majority of patients had some degree of adverse event,” Dr Ansell explained. “But if one looks at the grade 3 and 4 adverse events, those were seen in a more modest number of patients, in a minority of patients. And most importantly, if one looks at the adverse events that led to discontinuation, one can see that this was in a significant minority of patients.”
Five patients discontinued due to treatment-related AEs, which were pneumonitis (n=3), pneumonia and pneumonitis (n=1), and diabetic ketoacidosis (n=1).
Overall, 51 patients (78%) experienced an AE; 19 (29%) had a grade 3–4 AE, 14 (22%) had a serious AE, and 5 (8%) discontinued due to an AE.
Of 31 HL patients, 28 (90%) had an AE, 8 (26%) had a grade 3–4 AE, 6 (19%) had a serious AE, and 2 (6%) discontinued due to an AE.
All 11 T-cell NHL patients experienced an AE, 5 patients (45%) a grade 3-4 AE, 4 patients (36%) had a serious AE, and 2 patients (18%) discontinued because of an AE.
About half of B-cell NHL and MM patients experienced an AE, with 1 MM patient discontinuing as a result of it and no B-cell NHL patient discontinuing due to an AE.
“I would highlight that most of the adverse events were, as expected, immunological in nature . . . . ,” Dr Ansell said. “A very modest number of patients had grade 3 and 4 toxicities.”
The most common drug-related AEs of any grade were fatigue (n=17; 26%), pyrexia (n=15; 23%), rash (n=7; 11%), diarrhea (n=12; 18%), and nausea, pneumonitis, cough, and infusion-related reactions, with 9 patients each (14%).
Efficacy
Twenty-three HL patients (74%) achieved an overall response, including 6 patients (19%) with a complete response and 17 (55%) with a partial response. Three patients (10%) had stable disease, and 3 (10%) had relapsed or progressive disease. Response was not reported for 2 patients (6%).
“Most of these responses are durable, and, very encouraging, you can see patients out approaching a year continuing on therapy,” Dr Ansell said.
The ORR in the 18 transplant-naive patients was 67% (n=67).
The median duration of response for HL patients was not reached and ranged from 0.0 to 13.4 months.
B-cell NHL patients had an ORR of 20% (n=3). There were no complete responses and 3 (20%) partial responses. One patient (7%) had stable disease, and 8 (53%) had relapsed or progressive disase. The median duration of partial response was not reached and ranged from 11.0 to 12.7 months.
T-cell NHL patients had an ORR of 9% (n=1). There were no complete responses and 1 (9%) partial response. Four patients (36%) had stable disease, and 3 (27%) had relapsed or progressive disease. The median duration of partial response was not reached and was 3.9 months.
Except for 1 patient with stable disease, MM patients did not respond to therapy.
Biomarker analysis
All 19 HL patients with a known PD-L1 status at baseline saw their tumor burden decrease to below baseline levels. This may be because HL is characterized by high PD-L1 expression and high responsiveness to checkpoint blockade.
Patients with NHL, on the other hand, have a diverse group of tumors characterized by variable PD-L1 expression. Eight of 13 patients with known expression saw their tumor burden decrease with treatment to below baseline.
Encouraged by the results, the investigators believe further investigation of the combination is in order, as the combination, with limited follow-up, achieved a high and durable ORR in HL patients, including those who were transplant-naïve. ![]()
Why some patients relapse: The case for consolidation therapy in Hodgkin lymphoma

In this editorial, Andreas Engert, MD, makes the case for consolidation therapy in advanced Hodgkin lymphoma.
Dr Engert is a professor of internal medicine, hematology, and oncology at University Hospital of Cologne in Germany. He has received research funding and consultancy fees from Takeda/Millennium Pharmaceuticals and Affimed as well as research funding from Bristol-Myers Squibb.
Historically, Hodgkin lymphoma has been viewed as a cancer with generally favorable outcomes. However, it’s clear that there is an unmet need for patients with advanced stage disease.
Physicians treat newly diagnosed patients with a curative intent, but up to 30% fail to respond to initial therapy or relapse, depending on the treatment regimen used, stage of disease, and risk factors.1-3 Additionally, toxicity from frontline treatment has the potential to impact patients throughout their lives.
In line with the current standard of care, the majority of patients who fail frontline therapy will receive high-dose chemotherapy followed by an autologous stem cell transplant (ASCT).
This path of treatment, similar to frontline regimens, can be effective in eradicating the disease, but approximately half of those who undergo an ASCT subsequently relapse. Outcomes are generally poor for patients whose disease returns post-ASCT, especially if the relapse occurs within the first year.4
Consolidation therapy, used to kill remaining cancer cells after ASCT, may offer a new treatment option to address this problem. Unlike longer-term maintenance therapy, consolidation typically lasts for a short period of time—normally months instead of years—and involves intense treatment to eradicate any remaining disease.
The evidence for consolidation therapy in Hodgkin lymphoma
To understand the rationale for consolidation therapy, first consider why some patients with Hodgkin lymphoma relapse following ASCT. A small number of cancer cells, undetectable using traditional diagnostics, may remain following ASCT. This is known as minimal residual disease, and it may indicate the potential for the cancer to return.
The goal of consolidation therapy is to eliminate minimal residual disease before it progresses and causes a relapse. Unsurprisingly, timing plays a crucial role in the likelihood of achieving that goal.
In order to allow for the best chance for optimal patient outcomes, consolidation treatment should be initiated shortly after ASCT, before regrowth of cancer cells can occur. Tolerability is paramount, though, and timing must be carefully weighed by the treating physician.
Physicians and researchers learned about the impact and use of consolidation therapy from its success in other blood cancers like chronic myeloid leukemia.5,6
To prove the concept of consolidation treatment in Hodgkin lymphoma, a controlled clinical trial was conducted. The AETHERA study evaluated the use of brentuximab vedotin as consolidation therapy in patients with advanced Hodgkin lymphoma who were at increased risk of relapse or progression following ASCT.7
AETHERA was the first completed phase 3 study to explore consolidation treatment immediately following ASCT as a way of extending the effect of transplant in patients with Hodgkin lymphoma.
The results made a strong argument in favor of consolidation therapy, as patients who received brentuximab vedotin plus best supportive care after ASCT lived significantly longer without their disease worsening versus those on the placebo regimen. The safety profile of brentuximab vedotin in the AETHERA trial was generally consistent with the existing prescribing information.
Based on these data, consolidation therapy with brentuximab vedotin has been approved in several countries as a treatment option for patients with Hodgkin lymphoma who are at increased risk for relapse or progression following ASCT.
An important next step: Treating the right patients at the right time
Translating clinical evidence into real-world practice, physicians must look at which patients are most likely to benefit from consolidation therapy following ASCT—namely, those who are at increased risk of relapse. The effort to identify clear risk factors for relapse is still in progress.
Researchers across the world are currently studying patient characteristics and outcomes to determine a definitive set of risk factors that can better illustrate which patients should receive consolidation treatment.
Examples of factors under consideration include the stage of disease at diagnosis, tumor size, time to relapse, and response to previous treatment.8 Experts generally agree, however, that increased risk is cumulative and that it is not clear that any one risk factor is more important than others.
As researchers work to answer outstanding questions about consolidation therapy, there are a number of actions that the Hodgkin lymphoma community can take to help bring the right treatment options to patients.
Existing guidelines need to be evaluated and, if appropriate, adapted to give physicians across the globe the information that will allow them to provide the best care for patients at increased risk of relapse following ASCT.
Hematologists and oncologists then have the responsibility to stay informed of revisions to guidelines and to practically apply the latest research of consolidation therapy into their clinical practices.
The possibility now exists to potentially cure some Hodgkin lymphoma patients within a group that has traditionally experienced poor outcomes. As a result, a new treatment paradigm in this setting is emerging—one that may help solve the challenge of post-ASCT relapse in Hodgkin lymphoma.
Additional information on the use of consolidation therapy in Hodgkin lymphoma is available in the paper, Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? ![]()
1 Diehl, V, Franklin, J, Pfreundschuh, M, et al. Standard and Increased-Dose BEACOPP Chemotherapy Compared with COPP-ABVD for Advanced Hodgkin’s Disease. N Engl J Med 2003;348:2386-95.
2 Duggan, D, Petroni, G, Johnson, J, et al. Randomized Comparison of ABVD and MOPP/ABV Hybrid for the Treatment of Advanced Hodgkin’s Disease: Report of an Intergroup Trial. J Clin Oncol 2003;21:607-614.
3 Federico, M, Luminari, S, Iannitto, E, et al. ABVD Compared With BEACOPP Compared With CEC for the Initial Treatment of Patients With Advanced Hodgkin’s Lymphoma: Results From the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol 2009;27:805-811.
4 Arai S, Fanale M, deVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533.
5 Zonder, J and Schiffer, C. Update on practical aspects of the treatment of chronic myeloid leukemia with imatinib mesylate. Curr Hematol Malig Rep 2006;1:141.
6 Giralt SA, Arora M, Goldman JM, et al. Impact of imatinib therapy on the use of allogeneic haematopoietic progenitor cell transplantation for the treatment of chronic myeloid leukaemia. Br J Haematol 2007;137(5):461-467.
7 Moskowitz CH, Nadamanee A, Masszi T, et al; for the AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853-1862.
8 Bröckelmann PJ, Müller H, Casasnovas O, et al. Risk factors and a prognostic score for progression free survival after treatment with autologous stem cell transplantation (ASCT) in patients with relapsed or refractory Hodgkin lymphoma (rrHL). Poster presented at: 57th American Society of Hematology (ASH) Annual Meeting & Exposition; December 5-8, 2015; Orlando, FL.

In this editorial, Andreas Engert, MD, makes the case for consolidation therapy in advanced Hodgkin lymphoma.
Dr Engert is a professor of internal medicine, hematology, and oncology at University Hospital of Cologne in Germany. He has received research funding and consultancy fees from Takeda/Millennium Pharmaceuticals and Affimed as well as research funding from Bristol-Myers Squibb.
Historically, Hodgkin lymphoma has been viewed as a cancer with generally favorable outcomes. However, it’s clear that there is an unmet need for patients with advanced stage disease.
Physicians treat newly diagnosed patients with a curative intent, but up to 30% fail to respond to initial therapy or relapse, depending on the treatment regimen used, stage of disease, and risk factors.1-3 Additionally, toxicity from frontline treatment has the potential to impact patients throughout their lives.
In line with the current standard of care, the majority of patients who fail frontline therapy will receive high-dose chemotherapy followed by an autologous stem cell transplant (ASCT).
This path of treatment, similar to frontline regimens, can be effective in eradicating the disease, but approximately half of those who undergo an ASCT subsequently relapse. Outcomes are generally poor for patients whose disease returns post-ASCT, especially if the relapse occurs within the first year.4
Consolidation therapy, used to kill remaining cancer cells after ASCT, may offer a new treatment option to address this problem. Unlike longer-term maintenance therapy, consolidation typically lasts for a short period of time—normally months instead of years—and involves intense treatment to eradicate any remaining disease.
The evidence for consolidation therapy in Hodgkin lymphoma
To understand the rationale for consolidation therapy, first consider why some patients with Hodgkin lymphoma relapse following ASCT. A small number of cancer cells, undetectable using traditional diagnostics, may remain following ASCT. This is known as minimal residual disease, and it may indicate the potential for the cancer to return.
The goal of consolidation therapy is to eliminate minimal residual disease before it progresses and causes a relapse. Unsurprisingly, timing plays a crucial role in the likelihood of achieving that goal.
In order to allow for the best chance for optimal patient outcomes, consolidation treatment should be initiated shortly after ASCT, before regrowth of cancer cells can occur. Tolerability is paramount, though, and timing must be carefully weighed by the treating physician.
Physicians and researchers learned about the impact and use of consolidation therapy from its success in other blood cancers like chronic myeloid leukemia.5,6
To prove the concept of consolidation treatment in Hodgkin lymphoma, a controlled clinical trial was conducted. The AETHERA study evaluated the use of brentuximab vedotin as consolidation therapy in patients with advanced Hodgkin lymphoma who were at increased risk of relapse or progression following ASCT.7
AETHERA was the first completed phase 3 study to explore consolidation treatment immediately following ASCT as a way of extending the effect of transplant in patients with Hodgkin lymphoma.
The results made a strong argument in favor of consolidation therapy, as patients who received brentuximab vedotin plus best supportive care after ASCT lived significantly longer without their disease worsening versus those on the placebo regimen. The safety profile of brentuximab vedotin in the AETHERA trial was generally consistent with the existing prescribing information.
Based on these data, consolidation therapy with brentuximab vedotin has been approved in several countries as a treatment option for patients with Hodgkin lymphoma who are at increased risk for relapse or progression following ASCT.
An important next step: Treating the right patients at the right time
Translating clinical evidence into real-world practice, physicians must look at which patients are most likely to benefit from consolidation therapy following ASCT—namely, those who are at increased risk of relapse. The effort to identify clear risk factors for relapse is still in progress.
Researchers across the world are currently studying patient characteristics and outcomes to determine a definitive set of risk factors that can better illustrate which patients should receive consolidation treatment.
Examples of factors under consideration include the stage of disease at diagnosis, tumor size, time to relapse, and response to previous treatment.8 Experts generally agree, however, that increased risk is cumulative and that it is not clear that any one risk factor is more important than others.
As researchers work to answer outstanding questions about consolidation therapy, there are a number of actions that the Hodgkin lymphoma community can take to help bring the right treatment options to patients.
Existing guidelines need to be evaluated and, if appropriate, adapted to give physicians across the globe the information that will allow them to provide the best care for patients at increased risk of relapse following ASCT.
Hematologists and oncologists then have the responsibility to stay informed of revisions to guidelines and to practically apply the latest research of consolidation therapy into their clinical practices.
The possibility now exists to potentially cure some Hodgkin lymphoma patients within a group that has traditionally experienced poor outcomes. As a result, a new treatment paradigm in this setting is emerging—one that may help solve the challenge of post-ASCT relapse in Hodgkin lymphoma.
Additional information on the use of consolidation therapy in Hodgkin lymphoma is available in the paper, Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? ![]()
1 Diehl, V, Franklin, J, Pfreundschuh, M, et al. Standard and Increased-Dose BEACOPP Chemotherapy Compared with COPP-ABVD for Advanced Hodgkin’s Disease. N Engl J Med 2003;348:2386-95.
2 Duggan, D, Petroni, G, Johnson, J, et al. Randomized Comparison of ABVD and MOPP/ABV Hybrid for the Treatment of Advanced Hodgkin’s Disease: Report of an Intergroup Trial. J Clin Oncol 2003;21:607-614.
3 Federico, M, Luminari, S, Iannitto, E, et al. ABVD Compared With BEACOPP Compared With CEC for the Initial Treatment of Patients With Advanced Hodgkin’s Lymphoma: Results From the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol 2009;27:805-811.
4 Arai S, Fanale M, deVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533.
5 Zonder, J and Schiffer, C. Update on practical aspects of the treatment of chronic myeloid leukemia with imatinib mesylate. Curr Hematol Malig Rep 2006;1:141.
6 Giralt SA, Arora M, Goldman JM, et al. Impact of imatinib therapy on the use of allogeneic haematopoietic progenitor cell transplantation for the treatment of chronic myeloid leukaemia. Br J Haematol 2007;137(5):461-467.
7 Moskowitz CH, Nadamanee A, Masszi T, et al; for the AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853-1862.
8 Bröckelmann PJ, Müller H, Casasnovas O, et al. Risk factors and a prognostic score for progression free survival after treatment with autologous stem cell transplantation (ASCT) in patients with relapsed or refractory Hodgkin lymphoma (rrHL). Poster presented at: 57th American Society of Hematology (ASH) Annual Meeting & Exposition; December 5-8, 2015; Orlando, FL.

In this editorial, Andreas Engert, MD, makes the case for consolidation therapy in advanced Hodgkin lymphoma.
Dr Engert is a professor of internal medicine, hematology, and oncology at University Hospital of Cologne in Germany. He has received research funding and consultancy fees from Takeda/Millennium Pharmaceuticals and Affimed as well as research funding from Bristol-Myers Squibb.
Historically, Hodgkin lymphoma has been viewed as a cancer with generally favorable outcomes. However, it’s clear that there is an unmet need for patients with advanced stage disease.
Physicians treat newly diagnosed patients with a curative intent, but up to 30% fail to respond to initial therapy or relapse, depending on the treatment regimen used, stage of disease, and risk factors.1-3 Additionally, toxicity from frontline treatment has the potential to impact patients throughout their lives.
In line with the current standard of care, the majority of patients who fail frontline therapy will receive high-dose chemotherapy followed by an autologous stem cell transplant (ASCT).
This path of treatment, similar to frontline regimens, can be effective in eradicating the disease, but approximately half of those who undergo an ASCT subsequently relapse. Outcomes are generally poor for patients whose disease returns post-ASCT, especially if the relapse occurs within the first year.4
Consolidation therapy, used to kill remaining cancer cells after ASCT, may offer a new treatment option to address this problem. Unlike longer-term maintenance therapy, consolidation typically lasts for a short period of time—normally months instead of years—and involves intense treatment to eradicate any remaining disease.
The evidence for consolidation therapy in Hodgkin lymphoma
To understand the rationale for consolidation therapy, first consider why some patients with Hodgkin lymphoma relapse following ASCT. A small number of cancer cells, undetectable using traditional diagnostics, may remain following ASCT. This is known as minimal residual disease, and it may indicate the potential for the cancer to return.
The goal of consolidation therapy is to eliminate minimal residual disease before it progresses and causes a relapse. Unsurprisingly, timing plays a crucial role in the likelihood of achieving that goal.
In order to allow for the best chance for optimal patient outcomes, consolidation treatment should be initiated shortly after ASCT, before regrowth of cancer cells can occur. Tolerability is paramount, though, and timing must be carefully weighed by the treating physician.
Physicians and researchers learned about the impact and use of consolidation therapy from its success in other blood cancers like chronic myeloid leukemia.5,6
To prove the concept of consolidation treatment in Hodgkin lymphoma, a controlled clinical trial was conducted. The AETHERA study evaluated the use of brentuximab vedotin as consolidation therapy in patients with advanced Hodgkin lymphoma who were at increased risk of relapse or progression following ASCT.7
AETHERA was the first completed phase 3 study to explore consolidation treatment immediately following ASCT as a way of extending the effect of transplant in patients with Hodgkin lymphoma.
The results made a strong argument in favor of consolidation therapy, as patients who received brentuximab vedotin plus best supportive care after ASCT lived significantly longer without their disease worsening versus those on the placebo regimen. The safety profile of brentuximab vedotin in the AETHERA trial was generally consistent with the existing prescribing information.
Based on these data, consolidation therapy with brentuximab vedotin has been approved in several countries as a treatment option for patients with Hodgkin lymphoma who are at increased risk for relapse or progression following ASCT.
An important next step: Treating the right patients at the right time
Translating clinical evidence into real-world practice, physicians must look at which patients are most likely to benefit from consolidation therapy following ASCT—namely, those who are at increased risk of relapse. The effort to identify clear risk factors for relapse is still in progress.
Researchers across the world are currently studying patient characteristics and outcomes to determine a definitive set of risk factors that can better illustrate which patients should receive consolidation treatment.
Examples of factors under consideration include the stage of disease at diagnosis, tumor size, time to relapse, and response to previous treatment.8 Experts generally agree, however, that increased risk is cumulative and that it is not clear that any one risk factor is more important than others.
As researchers work to answer outstanding questions about consolidation therapy, there are a number of actions that the Hodgkin lymphoma community can take to help bring the right treatment options to patients.
Existing guidelines need to be evaluated and, if appropriate, adapted to give physicians across the globe the information that will allow them to provide the best care for patients at increased risk of relapse following ASCT.
Hematologists and oncologists then have the responsibility to stay informed of revisions to guidelines and to practically apply the latest research of consolidation therapy into their clinical practices.
The possibility now exists to potentially cure some Hodgkin lymphoma patients within a group that has traditionally experienced poor outcomes. As a result, a new treatment paradigm in this setting is emerging—one that may help solve the challenge of post-ASCT relapse in Hodgkin lymphoma.
Additional information on the use of consolidation therapy in Hodgkin lymphoma is available in the paper, Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? ![]()
1 Diehl, V, Franklin, J, Pfreundschuh, M, et al. Standard and Increased-Dose BEACOPP Chemotherapy Compared with COPP-ABVD for Advanced Hodgkin’s Disease. N Engl J Med 2003;348:2386-95.
2 Duggan, D, Petroni, G, Johnson, J, et al. Randomized Comparison of ABVD and MOPP/ABV Hybrid for the Treatment of Advanced Hodgkin’s Disease: Report of an Intergroup Trial. J Clin Oncol 2003;21:607-614.
3 Federico, M, Luminari, S, Iannitto, E, et al. ABVD Compared With BEACOPP Compared With CEC for the Initial Treatment of Patients With Advanced Hodgkin’s Lymphoma: Results From the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol 2009;27:805-811.
4 Arai S, Fanale M, deVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533.
5 Zonder, J and Schiffer, C. Update on practical aspects of the treatment of chronic myeloid leukemia with imatinib mesylate. Curr Hematol Malig Rep 2006;1:141.
6 Giralt SA, Arora M, Goldman JM, et al. Impact of imatinib therapy on the use of allogeneic haematopoietic progenitor cell transplantation for the treatment of chronic myeloid leukaemia. Br J Haematol 2007;137(5):461-467.
7 Moskowitz CH, Nadamanee A, Masszi T, et al; for the AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853-1862.
8 Bröckelmann PJ, Müller H, Casasnovas O, et al. Risk factors and a prognostic score for progression free survival after treatment with autologous stem cell transplantation (ASCT) in patients with relapsed or refractory Hodgkin lymphoma (rrHL). Poster presented at: 57th American Society of Hematology (ASH) Annual Meeting & Exposition; December 5-8, 2015; Orlando, FL.
Group estimates global cancer cases, deaths in 2015

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()
FDA grants priority review to sBLA for pembrolizumab

Photo courtesy of Merck
The US Food and Drug Administration (FDA) has granted priority review to the supplemental biologics license application (sBLA) for pembrolizumab (Keytruda®) as a treatment for patients with refractory classical Hodgkin lymphoma (cHL) and for cHL patients who have relapsed after 3 or more prior lines of therapy.
The sBLA will be reviewed under the FDA’s accelerated approval program. The target action date is March 15, 2017.
Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the antitumor immune response.
The drug, which is being developed by Merck, already has FDA approval as a treatment for melanoma, lung cancer, and head and neck cancer.
Pembrolizumab also has breakthrough therapy designation as a treatment for relapsed/refractory cHL.
The current sBLA for pembrolizumab is seeking approval for the drug at a fixed dose of 200 mg, administered intravenously every 3 weeks.
This is the first application for regulatory approval of pembrolizumab in a hematologic malignancy.
The sBLA is supported by data from the phase 1 KEYNOTE-013 trial and the phase 2 KEYNOTE-087 trial.
Results from KEYNOTE-013 (in cHL patients) were presented at the 2014 ASH Annual Meeting, and results from KEYNOTE-087 were presented at the 2016 ASCO Annual Meeting. ![]()

Photo courtesy of Merck
The US Food and Drug Administration (FDA) has granted priority review to the supplemental biologics license application (sBLA) for pembrolizumab (Keytruda®) as a treatment for patients with refractory classical Hodgkin lymphoma (cHL) and for cHL patients who have relapsed after 3 or more prior lines of therapy.
The sBLA will be reviewed under the FDA’s accelerated approval program. The target action date is March 15, 2017.
Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the antitumor immune response.
The drug, which is being developed by Merck, already has FDA approval as a treatment for melanoma, lung cancer, and head and neck cancer.
Pembrolizumab also has breakthrough therapy designation as a treatment for relapsed/refractory cHL.
The current sBLA for pembrolizumab is seeking approval for the drug at a fixed dose of 200 mg, administered intravenously every 3 weeks.
This is the first application for regulatory approval of pembrolizumab in a hematologic malignancy.
The sBLA is supported by data from the phase 1 KEYNOTE-013 trial and the phase 2 KEYNOTE-087 trial.
Results from KEYNOTE-013 (in cHL patients) were presented at the 2014 ASH Annual Meeting, and results from KEYNOTE-087 were presented at the 2016 ASCO Annual Meeting. ![]()

Photo courtesy of Merck
The US Food and Drug Administration (FDA) has granted priority review to the supplemental biologics license application (sBLA) for pembrolizumab (Keytruda®) as a treatment for patients with refractory classical Hodgkin lymphoma (cHL) and for cHL patients who have relapsed after 3 or more prior lines of therapy.
The sBLA will be reviewed under the FDA’s accelerated approval program. The target action date is March 15, 2017.
Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the antitumor immune response.
The drug, which is being developed by Merck, already has FDA approval as a treatment for melanoma, lung cancer, and head and neck cancer.
Pembrolizumab also has breakthrough therapy designation as a treatment for relapsed/refractory cHL.
The current sBLA for pembrolizumab is seeking approval for the drug at a fixed dose of 200 mg, administered intravenously every 3 weeks.
This is the first application for regulatory approval of pembrolizumab in a hematologic malignancy.
The sBLA is supported by data from the phase 1 KEYNOTE-013 trial and the phase 2 KEYNOTE-087 trial.
Results from KEYNOTE-013 (in cHL patients) were presented at the 2014 ASH Annual Meeting, and results from KEYNOTE-087 were presented at the 2016 ASCO Annual Meeting.
EC approves nivolumab for relapsed/refractory cHL

Photo from Business Wire
The European Commission (EC) has approved nivolumab (Opdivo) for the treatment of adults with relapsed or refractory classical Hodgkin lymphoma (cHL) who have already received an autologous hematopoietic stem cell transplant (auto-HSCT) and treatment with brentuximab vedotin (BV).
Nivolumab is the first PD-1 inhibitor approved in the European Economic Area as a treatment for a hematologic malignancy.
The EC previously approved nivolumab to treat advanced melanoma, non-small cell lung cancer, and renal cell carcinoma. In Europe, nivolumab is marketed by Bristol-Myers Squibb.
Trials in cHL
The EC’s approval of nivolumab in cHL is based on an integrated analysis of data from 2 trials—the phase 1 CheckMate -039 trial and the phase 2 CheckMate -205 trial.
In CheckMate -039, researchers evaluated nivolumab in patients with cHL, non-Hodgkin lymphoma, and multiple myeloma. Results from this trial were presented at the 13th International Congress on Malignant Lymphoma in June 2015.
In CheckMate -205, researchers are evaluating nivolumab in 4 cohorts of cHL patients. Cohort A includes patients who previously received auto-HSCT and were BV-naïve at enrollment (n=63). Cohort B includes patients who previously received auto-HSCT followed by BV (n=80).
Cohort C includes patients who previously received BV before and/or after auto-HSCT (n=100). And cohort D, which is currently enrolling, is an evaluation of nivolumab in combination with chemotherapy in newly diagnosed, advanced-stage cHL patients who are treatment-naïve (n=50).
Results from cohort B were presented at the 21st Congress of the European Hematology Association in June 2016. Results from cohort C were presented at the 10th International Symposium on Hodgkin Lymphoma last month.
Integrated analysis
The analysis included cHL patients from CheckMate -205 and -039 who had received auto-HSCT and BV.
In the efficacy population (n=95), the objective response rate was 66%. The percentage of patients with a complete response was 6%. Twenty-three percent of patients had stable disease.
The median time to response was 2.0 months (range, 0.7-11.1), and the median duration of response was 13.1 months (range, 0.0+, 23.1+). At 12 months, the progression-free survival rate was 57%.
The safety of nivolumab in cHL was evaluated in 263 patients from CheckMate -205 (n=240) and CheckMate -039 (n=23). Serious adverse events (AEs) occurred in 21% of these patients.
The most common serious AEs (reported in at least 1% of patients) were infusion-related reactions, pneumonia, pleural effusion, pyrexia, rash, and pneumonitis.
The most common AEs (reported in at least 20% of patients) were fatigue (32%), upper respiratory tract infection (28%), pyrexia (24%), diarrhea (23%), and cough (22%).
Twenty-three percent of patients had a dose delay resulting from an AE, and 4.2% of patients discontinued treatment due to AEs.
Forty patients went on to allogeneic HSCT after nivolumab, and 6 of these patients died from complications of the transplant. The 40 patients had a median follow-up from allogeneic HSCT of 2.9 months (range, 0-22).
Because of these deaths, the US Food and Drug Administration asked Bristol-Myers Squibb to study the safety of allogeneic HSCT after nivolumab.

Photo from Business Wire
The European Commission (EC) has approved nivolumab (Opdivo) for the treatment of adults with relapsed or refractory classical Hodgkin lymphoma (cHL) who have already received an autologous hematopoietic stem cell transplant (auto-HSCT) and treatment with brentuximab vedotin (BV).
Nivolumab is the first PD-1 inhibitor approved in the European Economic Area as a treatment for a hematologic malignancy.
The EC previously approved nivolumab to treat advanced melanoma, non-small cell lung cancer, and renal cell carcinoma. In Europe, nivolumab is marketed by Bristol-Myers Squibb.
Trials in cHL
The EC’s approval of nivolumab in cHL is based on an integrated analysis of data from 2 trials—the phase 1 CheckMate -039 trial and the phase 2 CheckMate -205 trial.
In CheckMate -039, researchers evaluated nivolumab in patients with cHL, non-Hodgkin lymphoma, and multiple myeloma. Results from this trial were presented at the 13th International Congress on Malignant Lymphoma in June 2015.
In CheckMate -205, researchers are evaluating nivolumab in 4 cohorts of cHL patients. Cohort A includes patients who previously received auto-HSCT and were BV-naïve at enrollment (n=63). Cohort B includes patients who previously received auto-HSCT followed by BV (n=80).
Cohort C includes patients who previously received BV before and/or after auto-HSCT (n=100). And cohort D, which is currently enrolling, is an evaluation of nivolumab in combination with chemotherapy in newly diagnosed, advanced-stage cHL patients who are treatment-naïve (n=50).
Results from cohort B were presented at the 21st Congress of the European Hematology Association in June 2016. Results from cohort C were presented at the 10th International Symposium on Hodgkin Lymphoma last month.
Integrated analysis
The analysis included cHL patients from CheckMate -205 and -039 who had received auto-HSCT and BV.
In the efficacy population (n=95), the objective response rate was 66%. The percentage of patients with a complete response was 6%. Twenty-three percent of patients had stable disease.
The median time to response was 2.0 months (range, 0.7-11.1), and the median duration of response was 13.1 months (range, 0.0+, 23.1+). At 12 months, the progression-free survival rate was 57%.
The safety of nivolumab in cHL was evaluated in 263 patients from CheckMate -205 (n=240) and CheckMate -039 (n=23). Serious adverse events (AEs) occurred in 21% of these patients.
The most common serious AEs (reported in at least 1% of patients) were infusion-related reactions, pneumonia, pleural effusion, pyrexia, rash, and pneumonitis.
The most common AEs (reported in at least 20% of patients) were fatigue (32%), upper respiratory tract infection (28%), pyrexia (24%), diarrhea (23%), and cough (22%).
Twenty-three percent of patients had a dose delay resulting from an AE, and 4.2% of patients discontinued treatment due to AEs.
Forty patients went on to allogeneic HSCT after nivolumab, and 6 of these patients died from complications of the transplant. The 40 patients had a median follow-up from allogeneic HSCT of 2.9 months (range, 0-22).
Because of these deaths, the US Food and Drug Administration asked Bristol-Myers Squibb to study the safety of allogeneic HSCT after nivolumab.

Photo from Business Wire
The European Commission (EC) has approved nivolumab (Opdivo) for the treatment of adults with relapsed or refractory classical Hodgkin lymphoma (cHL) who have already received an autologous hematopoietic stem cell transplant (auto-HSCT) and treatment with brentuximab vedotin (BV).
Nivolumab is the first PD-1 inhibitor approved in the European Economic Area as a treatment for a hematologic malignancy.
The EC previously approved nivolumab to treat advanced melanoma, non-small cell lung cancer, and renal cell carcinoma. In Europe, nivolumab is marketed by Bristol-Myers Squibb.
Trials in cHL
The EC’s approval of nivolumab in cHL is based on an integrated analysis of data from 2 trials—the phase 1 CheckMate -039 trial and the phase 2 CheckMate -205 trial.
In CheckMate -039, researchers evaluated nivolumab in patients with cHL, non-Hodgkin lymphoma, and multiple myeloma. Results from this trial were presented at the 13th International Congress on Malignant Lymphoma in June 2015.
In CheckMate -205, researchers are evaluating nivolumab in 4 cohorts of cHL patients. Cohort A includes patients who previously received auto-HSCT and were BV-naïve at enrollment (n=63). Cohort B includes patients who previously received auto-HSCT followed by BV (n=80).
Cohort C includes patients who previously received BV before and/or after auto-HSCT (n=100). And cohort D, which is currently enrolling, is an evaluation of nivolumab in combination with chemotherapy in newly diagnosed, advanced-stage cHL patients who are treatment-naïve (n=50).
Results from cohort B were presented at the 21st Congress of the European Hematology Association in June 2016. Results from cohort C were presented at the 10th International Symposium on Hodgkin Lymphoma last month.
Integrated analysis
The analysis included cHL patients from CheckMate -205 and -039 who had received auto-HSCT and BV.
In the efficacy population (n=95), the objective response rate was 66%. The percentage of patients with a complete response was 6%. Twenty-three percent of patients had stable disease.
The median time to response was 2.0 months (range, 0.7-11.1), and the median duration of response was 13.1 months (range, 0.0+, 23.1+). At 12 months, the progression-free survival rate was 57%.
The safety of nivolumab in cHL was evaluated in 263 patients from CheckMate -205 (n=240) and CheckMate -039 (n=23). Serious adverse events (AEs) occurred in 21% of these patients.
The most common serious AEs (reported in at least 1% of patients) were infusion-related reactions, pneumonia, pleural effusion, pyrexia, rash, and pneumonitis.
The most common AEs (reported in at least 20% of patients) were fatigue (32%), upper respiratory tract infection (28%), pyrexia (24%), diarrhea (23%), and cough (22%).
Twenty-three percent of patients had a dose delay resulting from an AE, and 4.2% of patients discontinued treatment due to AEs.
Forty patients went on to allogeneic HSCT after nivolumab, and 6 of these patients died from complications of the transplant. The 40 patients had a median follow-up from allogeneic HSCT of 2.9 months (range, 0-22).
Because of these deaths, the US Food and Drug Administration asked Bristol-Myers Squibb to study the safety of allogeneic HSCT after nivolumab.

