User login
Second cancers take greater toll on younger patients
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Cancer risk, burden expected to shift in HIV population
WASHINGTON, DC—New research suggests HIV-positive adults in the US will see a shift in cancer risk and burden in the coming years.
The study indicates that, through 2030, people living with HIV will see a decrease in AIDS-defining cancers, such as non-Hodgkin lymphoma (NHL) and Kaposi sarcoma.
But this group will also see an increase in cancers not linked to AIDS, such as prostate and liver cancers.
Researchers made these projections in a presentation at the AACR Annual Meeting 2017 (abstract 5302).
“Declines in cancer incidence rates, particularly for AIDS-defining cancers, are likely driven by widespread treatment with modern antiretroviral therapies, which reduce immune suppression and decrease risk of some cancers,” said Jessica Y. Islam, a doctoral student at the University of North Carolina Gillings School of Global Public Health in Chapel Hill.
She and her collaborators estimated future cancer risk and burden for HIV-positive people using age-specific cancer incidence data from the National Cancer Institute HIV/AIDS Cancer Match (HACM) Study, and projected HIV prevalence data from the Centers for Disease Control and Prevention.
Cancer incidence
From 2000 to 2012, there were 23,907 cancers reported in 463,300 HIV-infected adults in the HACM Study. Based on trends in this study, the researchers made projections for cancer incidence through 2030.
They projected that HIV-positive adults of all ages will see a significant decrease over time in the incidence of NHL, Kaposi sarcoma, cervical cancer, anal cancer among men who have sex with men (MSM), lung cancer, and Hodgkin lymphoma.
Patients age 65 and older will see a significant decrease in colon cancer incidence over time. However, there will be no significant change for patients younger than 65.
HIV-positive adults of all ages will see no significant change over time in the incidence of liver cancer, oral cavity cancer, anal cancer among non-MSMs, and breast cancer.
The incidence of prostate cancer will increase significantly among patients ages 35 to 44 and among patients ages 45 to 64.
Cancer burden
The researchers said the number of adults living with HIV in the US is projected to increase from 1.06 million in 2006 to 1.17 million in 2018, but it is expected to decline to 1.09 million in 2030.
The team noted that, in 2006, there were an estimated 8241 cancers in patients with HIV—3522 AIDS-defining cancers and 4719 malignancies not associated with AIDS.
In 2030, the total number of cancers in the HIV-positive population is projected to be 6692, with decreases in AIDS-defining cancers (n=716) and increases in other cancers (n=5976) from the 2006 data.
In 2010, the most common cancers among HIV-positive patients were estimated to be NHL (n=1488), Kaposi sarcoma (n=1133), and lung cancer (n=815).
But in 2030, the most common cancers are projected to be prostate (n=1587), lung (n=1027), and liver cancers (n=483).
“It is critical to understand both incidence rates and burden over time, as rates capture changes in cancer risk, and burden quantifies the actual number of cancer cases expected to occur,” said study investigator Meredith S. Shiels, PhD, of the National Cancer Institute in Bethesda, Maryland.
“For example, lung cancer rates are expected to decrease in the future, but the burden is expected to increase due to the growing number of older people living with HIV.” ![]()
WASHINGTON, DC—New research suggests HIV-positive adults in the US will see a shift in cancer risk and burden in the coming years.
The study indicates that, through 2030, people living with HIV will see a decrease in AIDS-defining cancers, such as non-Hodgkin lymphoma (NHL) and Kaposi sarcoma.
But this group will also see an increase in cancers not linked to AIDS, such as prostate and liver cancers.
Researchers made these projections in a presentation at the AACR Annual Meeting 2017 (abstract 5302).
“Declines in cancer incidence rates, particularly for AIDS-defining cancers, are likely driven by widespread treatment with modern antiretroviral therapies, which reduce immune suppression and decrease risk of some cancers,” said Jessica Y. Islam, a doctoral student at the University of North Carolina Gillings School of Global Public Health in Chapel Hill.
She and her collaborators estimated future cancer risk and burden for HIV-positive people using age-specific cancer incidence data from the National Cancer Institute HIV/AIDS Cancer Match (HACM) Study, and projected HIV prevalence data from the Centers for Disease Control and Prevention.
Cancer incidence
From 2000 to 2012, there were 23,907 cancers reported in 463,300 HIV-infected adults in the HACM Study. Based on trends in this study, the researchers made projections for cancer incidence through 2030.
They projected that HIV-positive adults of all ages will see a significant decrease over time in the incidence of NHL, Kaposi sarcoma, cervical cancer, anal cancer among men who have sex with men (MSM), lung cancer, and Hodgkin lymphoma.
Patients age 65 and older will see a significant decrease in colon cancer incidence over time. However, there will be no significant change for patients younger than 65.
HIV-positive adults of all ages will see no significant change over time in the incidence of liver cancer, oral cavity cancer, anal cancer among non-MSMs, and breast cancer.
The incidence of prostate cancer will increase significantly among patients ages 35 to 44 and among patients ages 45 to 64.
Cancer burden
The researchers said the number of adults living with HIV in the US is projected to increase from 1.06 million in 2006 to 1.17 million in 2018, but it is expected to decline to 1.09 million in 2030.
The team noted that, in 2006, there were an estimated 8241 cancers in patients with HIV—3522 AIDS-defining cancers and 4719 malignancies not associated with AIDS.
In 2030, the total number of cancers in the HIV-positive population is projected to be 6692, with decreases in AIDS-defining cancers (n=716) and increases in other cancers (n=5976) from the 2006 data.
In 2010, the most common cancers among HIV-positive patients were estimated to be NHL (n=1488), Kaposi sarcoma (n=1133), and lung cancer (n=815).
But in 2030, the most common cancers are projected to be prostate (n=1587), lung (n=1027), and liver cancers (n=483).
“It is critical to understand both incidence rates and burden over time, as rates capture changes in cancer risk, and burden quantifies the actual number of cancer cases expected to occur,” said study investigator Meredith S. Shiels, PhD, of the National Cancer Institute in Bethesda, Maryland.
“For example, lung cancer rates are expected to decrease in the future, but the burden is expected to increase due to the growing number of older people living with HIV.” ![]()
WASHINGTON, DC—New research suggests HIV-positive adults in the US will see a shift in cancer risk and burden in the coming years.
The study indicates that, through 2030, people living with HIV will see a decrease in AIDS-defining cancers, such as non-Hodgkin lymphoma (NHL) and Kaposi sarcoma.
But this group will also see an increase in cancers not linked to AIDS, such as prostate and liver cancers.
Researchers made these projections in a presentation at the AACR Annual Meeting 2017 (abstract 5302).
“Declines in cancer incidence rates, particularly for AIDS-defining cancers, are likely driven by widespread treatment with modern antiretroviral therapies, which reduce immune suppression and decrease risk of some cancers,” said Jessica Y. Islam, a doctoral student at the University of North Carolina Gillings School of Global Public Health in Chapel Hill.
She and her collaborators estimated future cancer risk and burden for HIV-positive people using age-specific cancer incidence data from the National Cancer Institute HIV/AIDS Cancer Match (HACM) Study, and projected HIV prevalence data from the Centers for Disease Control and Prevention.
Cancer incidence
From 2000 to 2012, there were 23,907 cancers reported in 463,300 HIV-infected adults in the HACM Study. Based on trends in this study, the researchers made projections for cancer incidence through 2030.
They projected that HIV-positive adults of all ages will see a significant decrease over time in the incidence of NHL, Kaposi sarcoma, cervical cancer, anal cancer among men who have sex with men (MSM), lung cancer, and Hodgkin lymphoma.
Patients age 65 and older will see a significant decrease in colon cancer incidence over time. However, there will be no significant change for patients younger than 65.
HIV-positive adults of all ages will see no significant change over time in the incidence of liver cancer, oral cavity cancer, anal cancer among non-MSMs, and breast cancer.
The incidence of prostate cancer will increase significantly among patients ages 35 to 44 and among patients ages 45 to 64.
Cancer burden
The researchers said the number of adults living with HIV in the US is projected to increase from 1.06 million in 2006 to 1.17 million in 2018, but it is expected to decline to 1.09 million in 2030.
The team noted that, in 2006, there were an estimated 8241 cancers in patients with HIV—3522 AIDS-defining cancers and 4719 malignancies not associated with AIDS.
In 2030, the total number of cancers in the HIV-positive population is projected to be 6692, with decreases in AIDS-defining cancers (n=716) and increases in other cancers (n=5976) from the 2006 data.
In 2010, the most common cancers among HIV-positive patients were estimated to be NHL (n=1488), Kaposi sarcoma (n=1133), and lung cancer (n=815).
But in 2030, the most common cancers are projected to be prostate (n=1587), lung (n=1027), and liver cancers (n=483).
“It is critical to understand both incidence rates and burden over time, as rates capture changes in cancer risk, and burden quantifies the actual number of cancer cases expected to occur,” said study investigator Meredith S. Shiels, PhD, of the National Cancer Institute in Bethesda, Maryland.
“For example, lung cancer rates are expected to decrease in the future, but the burden is expected to increase due to the growing number of older people living with HIV.” ![]()
CHMP recommends drug for relapsed/refractory cHL
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for the anti-PD-1 therapy pembrolizumab (Keytruda) as a treatment for patients with relapsed or refractory classical Hodgkin lymphoma (cHL).
The recommendation pertains specifically to adults with cHL who have failed autologous hematopoietic stem cell transplant (auto-HSCT) and treatment with brentuximab vedotin (BV) or adults with cHL who are transplant-ineligible and have failed treatment with BV.
The CHMP’s recommendation will be reviewed by the European Commission, which is expected to make a decision about the drug in the second quarter of 2017.
Pembrolizumab is already approved for use in the European Union as a treatment for melanoma and non-small-cell lung cancer.
The CHMP’s positive opinion of pembrolizumab for cHL was based on data from the KEYNOTE-087 and KEYNOTE-013 trials. Results from both trials were presented at ASH 2016 (abstract 1107 and abstract 1108).
KEYNOTE-087
KEYNOTE-087 is a phase 2 trial in which researchers evaluated pembrolizumab (a 200 mg fixed dose every 3 weeks) in patients with relapsed or refractory cHL across 3 cohorts:
- Cohort 1: Patients who progressed after auto-HSCT and subsequent treatment with BV
- Cohort 2: Patients who failed salvage chemotherapy, were ineligible for a transplant, and progressed after BV
- Cohort 3: Patients who progressed after auto-HSCT and did not receive BV after transplant.
Across all 210 enrolled patients, the overall response rate (ORR) was 69.0%, and the complete response (CR) rate was 22.4%.
In Cohort 1 (n=69), the ORR was 73.9%. The CR rate was 21.7%, the partial response (PR) rate was 52.2%, 15.9% of patients had stable disease (SD), and 7.2% progressed. In 82.2% of responders, the response lasted 6 months or more.
In Cohort 2 (n=81), the ORR was 64.2%. The CR rate was 24.7%, the PR rate was 39.5%, 12.3% of patients had SD, and 21.0% progressed. In 70.0% of responders, the response lasted 6 months or more.
In Cohort 3 (n=60), the ORR was 70.0%. Twenty percent of patients had a CR, 50.0% had a PR, 16.7% had SD, and 13.3% progressed. In 75.6% of responders, the response lasted 6 months or more.
Results also included an analysis of patients with primary refractory disease (n=73), which was defined as failure to achieve CR or PR with first-line treatment. In this patient population, the ORR was 79.5%.
An ORR of 67.8% was reported in patients who relapsed after 3 or more lines of prior therapy (99/146).
The most common treatment-related adverse events (AEs) were hypothyroidism (12.4%), pyrexia (10.5%), fatigue (9.0%), rash (7.6%), diarrhea (7.1%), headache (6.2%), nausea (5.7%), cough (5.7%), and neutropenia (5.2%).
The most common grade 3/4 treatment-related AEs were neutropenia (2.4%), diarrhea (1.0%), and dyspnea (1.0%). Immune-mediated AEs included pneumonitis (2.9%), hyperthyroidism (2.9%), colitis (1.0%), and myositis (1.0%).
There were 9 discontinuations because of treatment-related AEs and no treatment-related deaths.
KEYNOTE-013
KEYNOTE-013 is a phase 1b trial that has enrolled 31 patients with relapsed or refractory cHL who failed auto-HSCT and subsequent BV or who were transplant-ineligible.
Patients received pembrolizumab at 10 mg/kg every 2 weeks. The median duration of follow-up was 29 months.
The ORR was 58%. Nineteen percent of patients achieved a CR, 39% had a PR, and 23% had SD.
The median duration of response had not been reached at last follow-up (range, 0.0+ to 26.1+ months), and 70% of responding patients had a response lasting 12 months or more.
The median progression-free survival (PFS) was 11.4 months (range, 4.9-27.8 months). The six-month PFS rate was 66%, and the 12-month PFS rate was 48%.
The median overall survival was not reached. Six-month and 12-month overall survival rates were 100% and 87%, respectively.
The most common treatment-related AEs were diarrhea (19%), hypothyroidism (13%), pneumonitis (13%), nausea (13%), fatigue (10%), and dyspnea (10%).
The most common grade 3/4 treatment-related AEs were colitis (3%), axillary pain (3%), AST increase (3%), joint swelling (3%), nephrotic syndrome back pain (3%), and dyspnea (3%).
AEs leading to discontinuation were nephrotic syndrome (grade 3), interstitial lung disease (grade 2), and pneumonitis (grade 2). There were no treatment-related deaths. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for the anti-PD-1 therapy pembrolizumab (Keytruda) as a treatment for patients with relapsed or refractory classical Hodgkin lymphoma (cHL).
The recommendation pertains specifically to adults with cHL who have failed autologous hematopoietic stem cell transplant (auto-HSCT) and treatment with brentuximab vedotin (BV) or adults with cHL who are transplant-ineligible and have failed treatment with BV.
The CHMP’s recommendation will be reviewed by the European Commission, which is expected to make a decision about the drug in the second quarter of 2017.
Pembrolizumab is already approved for use in the European Union as a treatment for melanoma and non-small-cell lung cancer.
The CHMP’s positive opinion of pembrolizumab for cHL was based on data from the KEYNOTE-087 and KEYNOTE-013 trials. Results from both trials were presented at ASH 2016 (abstract 1107 and abstract 1108).
KEYNOTE-087
KEYNOTE-087 is a phase 2 trial in which researchers evaluated pembrolizumab (a 200 mg fixed dose every 3 weeks) in patients with relapsed or refractory cHL across 3 cohorts:
- Cohort 1: Patients who progressed after auto-HSCT and subsequent treatment with BV
- Cohort 2: Patients who failed salvage chemotherapy, were ineligible for a transplant, and progressed after BV
- Cohort 3: Patients who progressed after auto-HSCT and did not receive BV after transplant.
Across all 210 enrolled patients, the overall response rate (ORR) was 69.0%, and the complete response (CR) rate was 22.4%.
In Cohort 1 (n=69), the ORR was 73.9%. The CR rate was 21.7%, the partial response (PR) rate was 52.2%, 15.9% of patients had stable disease (SD), and 7.2% progressed. In 82.2% of responders, the response lasted 6 months or more.
In Cohort 2 (n=81), the ORR was 64.2%. The CR rate was 24.7%, the PR rate was 39.5%, 12.3% of patients had SD, and 21.0% progressed. In 70.0% of responders, the response lasted 6 months or more.
In Cohort 3 (n=60), the ORR was 70.0%. Twenty percent of patients had a CR, 50.0% had a PR, 16.7% had SD, and 13.3% progressed. In 75.6% of responders, the response lasted 6 months or more.
Results also included an analysis of patients with primary refractory disease (n=73), which was defined as failure to achieve CR or PR with first-line treatment. In this patient population, the ORR was 79.5%.
An ORR of 67.8% was reported in patients who relapsed after 3 or more lines of prior therapy (99/146).
The most common treatment-related adverse events (AEs) were hypothyroidism (12.4%), pyrexia (10.5%), fatigue (9.0%), rash (7.6%), diarrhea (7.1%), headache (6.2%), nausea (5.7%), cough (5.7%), and neutropenia (5.2%).
The most common grade 3/4 treatment-related AEs were neutropenia (2.4%), diarrhea (1.0%), and dyspnea (1.0%). Immune-mediated AEs included pneumonitis (2.9%), hyperthyroidism (2.9%), colitis (1.0%), and myositis (1.0%).
There were 9 discontinuations because of treatment-related AEs and no treatment-related deaths.
KEYNOTE-013
KEYNOTE-013 is a phase 1b trial that has enrolled 31 patients with relapsed or refractory cHL who failed auto-HSCT and subsequent BV or who were transplant-ineligible.
Patients received pembrolizumab at 10 mg/kg every 2 weeks. The median duration of follow-up was 29 months.
The ORR was 58%. Nineteen percent of patients achieved a CR, 39% had a PR, and 23% had SD.
The median duration of response had not been reached at last follow-up (range, 0.0+ to 26.1+ months), and 70% of responding patients had a response lasting 12 months or more.
The median progression-free survival (PFS) was 11.4 months (range, 4.9-27.8 months). The six-month PFS rate was 66%, and the 12-month PFS rate was 48%.
The median overall survival was not reached. Six-month and 12-month overall survival rates were 100% and 87%, respectively.
The most common treatment-related AEs were diarrhea (19%), hypothyroidism (13%), pneumonitis (13%), nausea (13%), fatigue (10%), and dyspnea (10%).
The most common grade 3/4 treatment-related AEs were colitis (3%), axillary pain (3%), AST increase (3%), joint swelling (3%), nephrotic syndrome back pain (3%), and dyspnea (3%).
AEs leading to discontinuation were nephrotic syndrome (grade 3), interstitial lung disease (grade 2), and pneumonitis (grade 2). There were no treatment-related deaths. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for the anti-PD-1 therapy pembrolizumab (Keytruda) as a treatment for patients with relapsed or refractory classical Hodgkin lymphoma (cHL).
The recommendation pertains specifically to adults with cHL who have failed autologous hematopoietic stem cell transplant (auto-HSCT) and treatment with brentuximab vedotin (BV) or adults with cHL who are transplant-ineligible and have failed treatment with BV.
The CHMP’s recommendation will be reviewed by the European Commission, which is expected to make a decision about the drug in the second quarter of 2017.
Pembrolizumab is already approved for use in the European Union as a treatment for melanoma and non-small-cell lung cancer.
The CHMP’s positive opinion of pembrolizumab for cHL was based on data from the KEYNOTE-087 and KEYNOTE-013 trials. Results from both trials were presented at ASH 2016 (abstract 1107 and abstract 1108).
KEYNOTE-087
KEYNOTE-087 is a phase 2 trial in which researchers evaluated pembrolizumab (a 200 mg fixed dose every 3 weeks) in patients with relapsed or refractory cHL across 3 cohorts:
- Cohort 1: Patients who progressed after auto-HSCT and subsequent treatment with BV
- Cohort 2: Patients who failed salvage chemotherapy, were ineligible for a transplant, and progressed after BV
- Cohort 3: Patients who progressed after auto-HSCT and did not receive BV after transplant.
Across all 210 enrolled patients, the overall response rate (ORR) was 69.0%, and the complete response (CR) rate was 22.4%.
In Cohort 1 (n=69), the ORR was 73.9%. The CR rate was 21.7%, the partial response (PR) rate was 52.2%, 15.9% of patients had stable disease (SD), and 7.2% progressed. In 82.2% of responders, the response lasted 6 months or more.
In Cohort 2 (n=81), the ORR was 64.2%. The CR rate was 24.7%, the PR rate was 39.5%, 12.3% of patients had SD, and 21.0% progressed. In 70.0% of responders, the response lasted 6 months or more.
In Cohort 3 (n=60), the ORR was 70.0%. Twenty percent of patients had a CR, 50.0% had a PR, 16.7% had SD, and 13.3% progressed. In 75.6% of responders, the response lasted 6 months or more.
Results also included an analysis of patients with primary refractory disease (n=73), which was defined as failure to achieve CR or PR with first-line treatment. In this patient population, the ORR was 79.5%.
An ORR of 67.8% was reported in patients who relapsed after 3 or more lines of prior therapy (99/146).
The most common treatment-related adverse events (AEs) were hypothyroidism (12.4%), pyrexia (10.5%), fatigue (9.0%), rash (7.6%), diarrhea (7.1%), headache (6.2%), nausea (5.7%), cough (5.7%), and neutropenia (5.2%).
The most common grade 3/4 treatment-related AEs were neutropenia (2.4%), diarrhea (1.0%), and dyspnea (1.0%). Immune-mediated AEs included pneumonitis (2.9%), hyperthyroidism (2.9%), colitis (1.0%), and myositis (1.0%).
There were 9 discontinuations because of treatment-related AEs and no treatment-related deaths.
KEYNOTE-013
KEYNOTE-013 is a phase 1b trial that has enrolled 31 patients with relapsed or refractory cHL who failed auto-HSCT and subsequent BV or who were transplant-ineligible.
Patients received pembrolizumab at 10 mg/kg every 2 weeks. The median duration of follow-up was 29 months.
The ORR was 58%. Nineteen percent of patients achieved a CR, 39% had a PR, and 23% had SD.
The median duration of response had not been reached at last follow-up (range, 0.0+ to 26.1+ months), and 70% of responding patients had a response lasting 12 months or more.
The median progression-free survival (PFS) was 11.4 months (range, 4.9-27.8 months). The six-month PFS rate was 66%, and the 12-month PFS rate was 48%.
The median overall survival was not reached. Six-month and 12-month overall survival rates were 100% and 87%, respectively.
The most common treatment-related AEs were diarrhea (19%), hypothyroidism (13%), pneumonitis (13%), nausea (13%), fatigue (10%), and dyspnea (10%).
The most common grade 3/4 treatment-related AEs were colitis (3%), axillary pain (3%), AST increase (3%), joint swelling (3%), nephrotic syndrome back pain (3%), and dyspnea (3%).
AEs leading to discontinuation were nephrotic syndrome (grade 3), interstitial lung disease (grade 2), and pneumonitis (grade 2). There were no treatment-related deaths. ![]()
Most blood cancer mutations due to DNA replication errors
A new study supports the idea that most cancer-driving mutations are a result of DNA replication errors, not heredity or lifestyle/environmental factors.
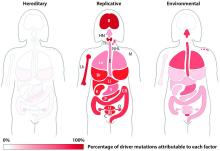
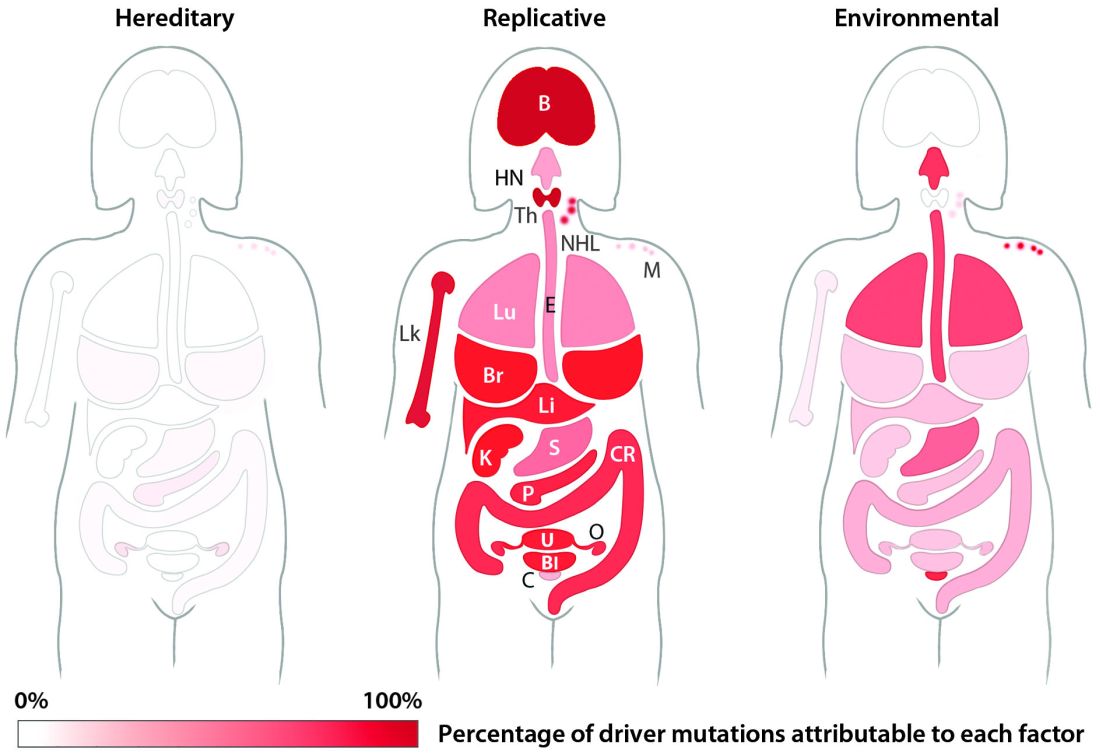
For all 32 cancer types studied, researchers found that 66% of driver mutations resulted from DNA replication errors, 29% could be attributed to lifestyle or environmental factors, and the remaining 5% were inherited.
In hematologic malignancies, the percentage of mutations caused by DNA replication errors was even higher—70% in Hodgkin lymphoma, 85% in leukemias, 96% in non-Hodgkin lymphomas, and 99% in myeloma.
Cristian Tomasetti, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these findings in Science.
“It is well-known that we must avoid environmental factors such as smoking to decrease our risk of getting cancer, but it is not as well-known that each time a normal cell divides and copies its DNA to produce 2 new cells, it makes multiple mistakes,” Dr Tomasetti said.
“These copying mistakes are a potent source of cancer mutations that, historically, have been scientifically undervalued, and this new work provides the first estimate of the fraction of mutations caused by these mistakes.”
In 2015, Dr Tomasetti and his colleagues reported that DNA replication errors could explain why certain cancers occur more often than others in the US.
The current study builds upon that research but includes additional cancers and encompasses an international population.
The researchers first studied the relationship between the number of normal stem cell divisions and the risk of 17 cancer types in 69 countries representing 4.8 billion people, or more than half of the world’s population.
The team said they observed a strong correlation between cancer incidence and normal stem cell divisions in all countries, regardless of their environment.
Next, the researchers set out to determine the percentage of driver mutations caused by DNA replication errors in 32 cancer types. The team developed a mathematical model using DNA sequencing data from The Cancer Genome Atlas and epidemiologic data from the Cancer Research UK database.
According to the researchers, it generally takes 2 or more critical mutations for cancer to occur. In an individual, these mutations can be due to random DNA replication errors, the environment, or inherited genes.
Knowing this, the researchers used their mathematical model to show, for example, that when critical mutations in leukemia are added together, 85.2% of them are due to random DNA replication errors, 14.3% to environmental factors, and 0.5% to heredity.
In Hodgkin lymphoma, 69.5% are due to DNA replication errors, 30% to environmental factors, and 0.5% to heredity. In non-Hodgkin lymphoma, 95.6% are due to random DNA replication errors, 3.9% to environmental factors, and 0.5% to heredity.
In myeloma, 99.3% are due to DNA replication errors, 0.2% to environmental factors, and 0.5% to heredity.
Dr Tomasetti said these random DNA replication errors will only get more important as aging populations continue to grow, prolonging the opportunity for cells to make more and more errors.
“We need to continue to encourage people to avoid environmental agents and lifestyles that increase their risk of developing cancer mutations,” said study author Bert Vogelstein, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
“However, many people will still develop cancers due to these random DNA copying errors, and better methods to detect all cancers earlier, while they are still curable, are urgently needed.” ![]()
A new study supports the idea that most cancer-driving mutations are a result of DNA replication errors, not heredity or lifestyle/environmental factors.
For all 32 cancer types studied, researchers found that 66% of driver mutations resulted from DNA replication errors, 29% could be attributed to lifestyle or environmental factors, and the remaining 5% were inherited.
In hematologic malignancies, the percentage of mutations caused by DNA replication errors was even higher—70% in Hodgkin lymphoma, 85% in leukemias, 96% in non-Hodgkin lymphomas, and 99% in myeloma.
Cristian Tomasetti, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these findings in Science.
“It is well-known that we must avoid environmental factors such as smoking to decrease our risk of getting cancer, but it is not as well-known that each time a normal cell divides and copies its DNA to produce 2 new cells, it makes multiple mistakes,” Dr Tomasetti said.
“These copying mistakes are a potent source of cancer mutations that, historically, have been scientifically undervalued, and this new work provides the first estimate of the fraction of mutations caused by these mistakes.”
In 2015, Dr Tomasetti and his colleagues reported that DNA replication errors could explain why certain cancers occur more often than others in the US.
The current study builds upon that research but includes additional cancers and encompasses an international population.
The researchers first studied the relationship between the number of normal stem cell divisions and the risk of 17 cancer types in 69 countries representing 4.8 billion people, or more than half of the world’s population.
The team said they observed a strong correlation between cancer incidence and normal stem cell divisions in all countries, regardless of their environment.
Next, the researchers set out to determine the percentage of driver mutations caused by DNA replication errors in 32 cancer types. The team developed a mathematical model using DNA sequencing data from The Cancer Genome Atlas and epidemiologic data from the Cancer Research UK database.
According to the researchers, it generally takes 2 or more critical mutations for cancer to occur. In an individual, these mutations can be due to random DNA replication errors, the environment, or inherited genes.
Knowing this, the researchers used their mathematical model to show, for example, that when critical mutations in leukemia are added together, 85.2% of them are due to random DNA replication errors, 14.3% to environmental factors, and 0.5% to heredity.
In Hodgkin lymphoma, 69.5% are due to DNA replication errors, 30% to environmental factors, and 0.5% to heredity. In non-Hodgkin lymphoma, 95.6% are due to random DNA replication errors, 3.9% to environmental factors, and 0.5% to heredity.
In myeloma, 99.3% are due to DNA replication errors, 0.2% to environmental factors, and 0.5% to heredity.
Dr Tomasetti said these random DNA replication errors will only get more important as aging populations continue to grow, prolonging the opportunity for cells to make more and more errors.
“We need to continue to encourage people to avoid environmental agents and lifestyles that increase their risk of developing cancer mutations,” said study author Bert Vogelstein, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
“However, many people will still develop cancers due to these random DNA copying errors, and better methods to detect all cancers earlier, while they are still curable, are urgently needed.” ![]()
A new study supports the idea that most cancer-driving mutations are a result of DNA replication errors, not heredity or lifestyle/environmental factors.
For all 32 cancer types studied, researchers found that 66% of driver mutations resulted from DNA replication errors, 29% could be attributed to lifestyle or environmental factors, and the remaining 5% were inherited.
In hematologic malignancies, the percentage of mutations caused by DNA replication errors was even higher—70% in Hodgkin lymphoma, 85% in leukemias, 96% in non-Hodgkin lymphomas, and 99% in myeloma.
Cristian Tomasetti, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these findings in Science.
“It is well-known that we must avoid environmental factors such as smoking to decrease our risk of getting cancer, but it is not as well-known that each time a normal cell divides and copies its DNA to produce 2 new cells, it makes multiple mistakes,” Dr Tomasetti said.
“These copying mistakes are a potent source of cancer mutations that, historically, have been scientifically undervalued, and this new work provides the first estimate of the fraction of mutations caused by these mistakes.”
In 2015, Dr Tomasetti and his colleagues reported that DNA replication errors could explain why certain cancers occur more often than others in the US.
The current study builds upon that research but includes additional cancers and encompasses an international population.
The researchers first studied the relationship between the number of normal stem cell divisions and the risk of 17 cancer types in 69 countries representing 4.8 billion people, or more than half of the world’s population.
The team said they observed a strong correlation between cancer incidence and normal stem cell divisions in all countries, regardless of their environment.
Next, the researchers set out to determine the percentage of driver mutations caused by DNA replication errors in 32 cancer types. The team developed a mathematical model using DNA sequencing data from The Cancer Genome Atlas and epidemiologic data from the Cancer Research UK database.
According to the researchers, it generally takes 2 or more critical mutations for cancer to occur. In an individual, these mutations can be due to random DNA replication errors, the environment, or inherited genes.
Knowing this, the researchers used their mathematical model to show, for example, that when critical mutations in leukemia are added together, 85.2% of them are due to random DNA replication errors, 14.3% to environmental factors, and 0.5% to heredity.
In Hodgkin lymphoma, 69.5% are due to DNA replication errors, 30% to environmental factors, and 0.5% to heredity. In non-Hodgkin lymphoma, 95.6% are due to random DNA replication errors, 3.9% to environmental factors, and 0.5% to heredity.
In myeloma, 99.3% are due to DNA replication errors, 0.2% to environmental factors, and 0.5% to heredity.
Dr Tomasetti said these random DNA replication errors will only get more important as aging populations continue to grow, prolonging the opportunity for cells to make more and more errors.
“We need to continue to encourage people to avoid environmental agents and lifestyles that increase their risk of developing cancer mutations,” said study author Bert Vogelstein, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
“However, many people will still develop cancers due to these random DNA copying errors, and better methods to detect all cancers earlier, while they are still curable, are urgently needed.” ![]()
Preterm births more common in cancer survivors
Women diagnosed with cancer during their childbearing years have an increased risk of preterm births, according to research published in JAMA Oncology.
The study showed that cancer survivors were more likely than women who never had cancer to give birth prematurely, have underweight babies, and undergo cesarean section deliveries.
The researchers said women diagnosed with cancer during pregnancy may be delivering early in order to start their cancer treatment, but that does not fully explain these findings.
The team also detected an increased risk of preterm delivery in women who had already received cancer treatment.
“We found that women were more likely to deliver preterm if they’ve been treated for cancer overall, with greater risks for women who had chemotherapy,” said study author Hazel B. Nichols, PhD, of University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill.
“While we believe these findings are something women should be aware of, we still have a lot of work to do to understand why this risk is becoming apparent and whether or not the children who are born preterm to these women go on to develop any health concerns.”
Dr Nichols and her colleagues analyzed data on 2598 births to female adolescent and young adult cancer survivors (ages 15 to 39) and 12,990 births to women without a cancer diagnosis.
Among cancer survivors, there was a significantly increased prevalence of preterm birth (prevalence ratio [PR]=1.52), low birth weight (PR=1.59), and cesarean delivery (PR=1.08), compared to women without a cancer diagnosis.
Timing of diagnosis and cancer type
When the researchers broke the data down by cancer diagnosis, they found a higher risk of preterm birth and low birth weight for women with lymphoma as well as breast and gynecologic cancers.
The PR for preterm birth was 1.59 for Hodgkin lymphoma, 1.98 for breast cancer, 2.11 for non-Hodgkin lymphoma, and 2.58 for gynecologic cancer. The PR for low birth weight was 1.59 for breast cancer, 2.41 for non-Hodgkin lymphoma, and 2.74 for gynecologic cancer.
The researchers found an increased risk of adverse birth outcomes among women who were diagnosed with cancer while pregnant and before pregnancy.
Among women diagnosed while pregnant, the PR was 2.97 for preterm birth, 2.82 for low birth weight, 1.21 for cesarean delivery, and 1.90 for low Apgar score. Among women diagnosed before pregnancy, the PR was 1.23 for preterm birth and 1.36 for low birth weight.
Role of treatment
Compared to women without a cancer diagnosis, cancer survivors who received chemotherapy but no radiation were more likely to have preterm births (PR=2.11), infants with low birth weight (PR=2.36), and cesarean deliveries (PR=1.16).
There was no significant increase in adverse birth outcomes among cancer survivors who received radiation but not chemotherapy.
Among the cancer survivors, women who received chemotherapy without radiation were more likely to have preterm births (PR=2.12), infants with low birth weight (PR=2.13), and infants who were small for their gestational age (PR=1.43) when compared to women treated with surgery only.
Dr Nichols said the role of treatment is an area of possible future research.
“We’d like to get better information about the types of chemotherapy women receive,” she said. “Chemotherapy is a very broad category, and the agents have very different effects on the body. In the future, we’d like to get more detailed information on the types of drugs that were involved in treatment.” ![]()
Women diagnosed with cancer during their childbearing years have an increased risk of preterm births, according to research published in JAMA Oncology.
The study showed that cancer survivors were more likely than women who never had cancer to give birth prematurely, have underweight babies, and undergo cesarean section deliveries.
The researchers said women diagnosed with cancer during pregnancy may be delivering early in order to start their cancer treatment, but that does not fully explain these findings.
The team also detected an increased risk of preterm delivery in women who had already received cancer treatment.
“We found that women were more likely to deliver preterm if they’ve been treated for cancer overall, with greater risks for women who had chemotherapy,” said study author Hazel B. Nichols, PhD, of University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill.
“While we believe these findings are something women should be aware of, we still have a lot of work to do to understand why this risk is becoming apparent and whether or not the children who are born preterm to these women go on to develop any health concerns.”
Dr Nichols and her colleagues analyzed data on 2598 births to female adolescent and young adult cancer survivors (ages 15 to 39) and 12,990 births to women without a cancer diagnosis.
Among cancer survivors, there was a significantly increased prevalence of preterm birth (prevalence ratio [PR]=1.52), low birth weight (PR=1.59), and cesarean delivery (PR=1.08), compared to women without a cancer diagnosis.
Timing of diagnosis and cancer type
When the researchers broke the data down by cancer diagnosis, they found a higher risk of preterm birth and low birth weight for women with lymphoma as well as breast and gynecologic cancers.
The PR for preterm birth was 1.59 for Hodgkin lymphoma, 1.98 for breast cancer, 2.11 for non-Hodgkin lymphoma, and 2.58 for gynecologic cancer. The PR for low birth weight was 1.59 for breast cancer, 2.41 for non-Hodgkin lymphoma, and 2.74 for gynecologic cancer.
The researchers found an increased risk of adverse birth outcomes among women who were diagnosed with cancer while pregnant and before pregnancy.
Among women diagnosed while pregnant, the PR was 2.97 for preterm birth, 2.82 for low birth weight, 1.21 for cesarean delivery, and 1.90 for low Apgar score. Among women diagnosed before pregnancy, the PR was 1.23 for preterm birth and 1.36 for low birth weight.
Role of treatment
Compared to women without a cancer diagnosis, cancer survivors who received chemotherapy but no radiation were more likely to have preterm births (PR=2.11), infants with low birth weight (PR=2.36), and cesarean deliveries (PR=1.16).
There was no significant increase in adverse birth outcomes among cancer survivors who received radiation but not chemotherapy.
Among the cancer survivors, women who received chemotherapy without radiation were more likely to have preterm births (PR=2.12), infants with low birth weight (PR=2.13), and infants who were small for their gestational age (PR=1.43) when compared to women treated with surgery only.
Dr Nichols said the role of treatment is an area of possible future research.
“We’d like to get better information about the types of chemotherapy women receive,” she said. “Chemotherapy is a very broad category, and the agents have very different effects on the body. In the future, we’d like to get more detailed information on the types of drugs that were involved in treatment.” ![]()
Women diagnosed with cancer during their childbearing years have an increased risk of preterm births, according to research published in JAMA Oncology.
The study showed that cancer survivors were more likely than women who never had cancer to give birth prematurely, have underweight babies, and undergo cesarean section deliveries.
The researchers said women diagnosed with cancer during pregnancy may be delivering early in order to start their cancer treatment, but that does not fully explain these findings.
The team also detected an increased risk of preterm delivery in women who had already received cancer treatment.
“We found that women were more likely to deliver preterm if they’ve been treated for cancer overall, with greater risks for women who had chemotherapy,” said study author Hazel B. Nichols, PhD, of University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill.
“While we believe these findings are something women should be aware of, we still have a lot of work to do to understand why this risk is becoming apparent and whether or not the children who are born preterm to these women go on to develop any health concerns.”
Dr Nichols and her colleagues analyzed data on 2598 births to female adolescent and young adult cancer survivors (ages 15 to 39) and 12,990 births to women without a cancer diagnosis.
Among cancer survivors, there was a significantly increased prevalence of preterm birth (prevalence ratio [PR]=1.52), low birth weight (PR=1.59), and cesarean delivery (PR=1.08), compared to women without a cancer diagnosis.
Timing of diagnosis and cancer type
When the researchers broke the data down by cancer diagnosis, they found a higher risk of preterm birth and low birth weight for women with lymphoma as well as breast and gynecologic cancers.
The PR for preterm birth was 1.59 for Hodgkin lymphoma, 1.98 for breast cancer, 2.11 for non-Hodgkin lymphoma, and 2.58 for gynecologic cancer. The PR for low birth weight was 1.59 for breast cancer, 2.41 for non-Hodgkin lymphoma, and 2.74 for gynecologic cancer.
The researchers found an increased risk of adverse birth outcomes among women who were diagnosed with cancer while pregnant and before pregnancy.
Among women diagnosed while pregnant, the PR was 2.97 for preterm birth, 2.82 for low birth weight, 1.21 for cesarean delivery, and 1.90 for low Apgar score. Among women diagnosed before pregnancy, the PR was 1.23 for preterm birth and 1.36 for low birth weight.
Role of treatment
Compared to women without a cancer diagnosis, cancer survivors who received chemotherapy but no radiation were more likely to have preterm births (PR=2.11), infants with low birth weight (PR=2.36), and cesarean deliveries (PR=1.16).
There was no significant increase in adverse birth outcomes among cancer survivors who received radiation but not chemotherapy.
Among the cancer survivors, women who received chemotherapy without radiation were more likely to have preterm births (PR=2.12), infants with low birth weight (PR=2.13), and infants who were small for their gestational age (PR=1.43) when compared to women treated with surgery only.
Dr Nichols said the role of treatment is an area of possible future research.
“We’d like to get better information about the types of chemotherapy women receive,” she said. “Chemotherapy is a very broad category, and the agents have very different effects on the body. In the future, we’d like to get more detailed information on the types of drugs that were involved in treatment.” ![]()
Unavoidable, random DNA replication errors are the most common cancer drivers
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Key clinical point:
Major finding: Two-thirds (66%) of cancer drivers are replication errors, 29% are environmentally induced, and 5% are hereditary.
Data source: The researchers examined cancer mutation drivers in two cohorts that spanned 69 countries.
Disclosures: Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
Veterans don’t have higher risk of leukemia, lymphoma
People who have served in the Armed Forces do not have an increased risk of leukemia or lymphoma, according to research published in Cancer Epidemiology.
Researchers analyzed the long-term risks of developing leukemia, Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL) in veterans living in Scotland.
At a mean 30 years of follow-up, there were no significant differences in the risk of the aforementioned malignancies between veterans and non-veterans in Scotland.
This retrospective study included 56,205 veterans and 172,741 non-veterans.
The veterans’ earliest date of entering service was January 1960, and the latest date of leaving service was December 2012.
At a mean follow-up of 29.3 years, 294 (0.52%) veterans and 974 (0.56%) non-veterans were diagnosed with leukemia, HL, or NHL.
There were 125 (0.22%) cases of leukemia in veterans and 365 (0.21%) in non-veterans. There were 59 (0.10%) cases of HL in veterans and 182 (0.11%) in non-veterans. And there were 144 (0.26%) cases of NHL in veterans and 538 (0.31%) in non-veterans.
There was no significant difference in the risk of all 3 cancer types between the veterans and non-veterans. The unadjusted hazard ratio (HR) was 0.96 (P=0.541).
There were no significant differences in an adjusted analysis either. (The analysis was adjusted for regional deprivation, which takes into account information on income, employment, health, education, housing, crime, and access to services.)
The adjusted HR was 1.03 (P=0.773) for leukemias, 1.19 (P=0.272) for HL, and 0.86 (P=0.110) for NHL.
“This is an important study which provides reassurance that military service in the last 50 years does not increase people’s risk of leukemia overall,” said study author Beverly Bergman, PhD, of the University of Glasgow in the UK.
“The Armed Forces comply with all relevant health and safety legislation and regulations, and we can now see that their risk is no different from the general population.” ![]()
People who have served in the Armed Forces do not have an increased risk of leukemia or lymphoma, according to research published in Cancer Epidemiology.
Researchers analyzed the long-term risks of developing leukemia, Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL) in veterans living in Scotland.
At a mean 30 years of follow-up, there were no significant differences in the risk of the aforementioned malignancies between veterans and non-veterans in Scotland.
This retrospective study included 56,205 veterans and 172,741 non-veterans.
The veterans’ earliest date of entering service was January 1960, and the latest date of leaving service was December 2012.
At a mean follow-up of 29.3 years, 294 (0.52%) veterans and 974 (0.56%) non-veterans were diagnosed with leukemia, HL, or NHL.
There were 125 (0.22%) cases of leukemia in veterans and 365 (0.21%) in non-veterans. There were 59 (0.10%) cases of HL in veterans and 182 (0.11%) in non-veterans. And there were 144 (0.26%) cases of NHL in veterans and 538 (0.31%) in non-veterans.
There was no significant difference in the risk of all 3 cancer types between the veterans and non-veterans. The unadjusted hazard ratio (HR) was 0.96 (P=0.541).
There were no significant differences in an adjusted analysis either. (The analysis was adjusted for regional deprivation, which takes into account information on income, employment, health, education, housing, crime, and access to services.)
The adjusted HR was 1.03 (P=0.773) for leukemias, 1.19 (P=0.272) for HL, and 0.86 (P=0.110) for NHL.
“This is an important study which provides reassurance that military service in the last 50 years does not increase people’s risk of leukemia overall,” said study author Beverly Bergman, PhD, of the University of Glasgow in the UK.
“The Armed Forces comply with all relevant health and safety legislation and regulations, and we can now see that their risk is no different from the general population.” ![]()
People who have served in the Armed Forces do not have an increased risk of leukemia or lymphoma, according to research published in Cancer Epidemiology.
Researchers analyzed the long-term risks of developing leukemia, Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL) in veterans living in Scotland.
At a mean 30 years of follow-up, there were no significant differences in the risk of the aforementioned malignancies between veterans and non-veterans in Scotland.
This retrospective study included 56,205 veterans and 172,741 non-veterans.
The veterans’ earliest date of entering service was January 1960, and the latest date of leaving service was December 2012.
At a mean follow-up of 29.3 years, 294 (0.52%) veterans and 974 (0.56%) non-veterans were diagnosed with leukemia, HL, or NHL.
There were 125 (0.22%) cases of leukemia in veterans and 365 (0.21%) in non-veterans. There were 59 (0.10%) cases of HL in veterans and 182 (0.11%) in non-veterans. And there were 144 (0.26%) cases of NHL in veterans and 538 (0.31%) in non-veterans.
There was no significant difference in the risk of all 3 cancer types between the veterans and non-veterans. The unadjusted hazard ratio (HR) was 0.96 (P=0.541).
There were no significant differences in an adjusted analysis either. (The analysis was adjusted for regional deprivation, which takes into account information on income, employment, health, education, housing, crime, and access to services.)
The adjusted HR was 1.03 (P=0.773) for leukemias, 1.19 (P=0.272) for HL, and 0.86 (P=0.110) for NHL.
“This is an important study which provides reassurance that military service in the last 50 years does not increase people’s risk of leukemia overall,” said study author Beverly Bergman, PhD, of the University of Glasgow in the UK.
“The Armed Forces comply with all relevant health and safety legislation and regulations, and we can now see that their risk is no different from the general population.” ![]()
FDA approves pembrolizumab to treat cHL
The US Food and Drug Administration (FDA) has granted accelerated approval for pembrolizumab (Keytruda) as a treatment for adult and pediatric patients with relapsed or refractory classical Hodgkin lymphoma (cHL).
Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the antitumor immune response.
The drug, which is being developed by Merck, previously received FDA approval as a treatment for melanoma, lung cancer, and head and neck cancer.
Now, pembrolizumab has received accelerated approval to treat adult and pediatric patients with refractory cHL or those with cHL who have relapsed after 3 or more prior lines of therapy.
The accelerated approval was based on tumor response rate and durability of response. Continued approval of pembrolizumab for cHL patients may be contingent upon the verification and description of clinical benefit in confirmatory trials.
In adults with cHL, pembrolizumab is administered at a fixed dose of 200 mg every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
In pediatric patients with cHL, pembrolizumab is administered at a dose of 2 mg/kg (up to a maximum of 200 mg) every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab trials
The FDA’s approval of pembrolizumab in adults with cHL is based on data from the phase 2 KEYNOTE-087 trial. (The following data were provided by Merck.)
The trial enrolled 210 patients who received pembrolizumab at a dose of 200 mg every 3 weeks until unacceptable toxicity or documented disease progression, or for up to 24 months in patients who did not progress.
Fifty-eight percent of patients were refractory to their last prior therapy, including 35% with primary refractory disease and 14% whose disease was refractory to all prior regimens.
Sixty-one percent of patients had undergone prior autologous hematopoietic stem cell transplant, 83% had prior brentuximab use, and 36% had prior radiation therapy.
At a median follow-up of 9.4 months, the overall response rate was 69%, and the complete response rate was 22%. The median duration of response was 11.1 months (range, 0.0+ to 11.1 months).
Five percent of patients discontinued pembrolizumab due to adverse events (AEs), and 26% had dose interruptions due to AEs. Fifteen percent of patients had an AE requiring systemic corticosteroid therapy.
The most common AEs (occurring in ≥20% of patients) were fatigue (26%), pyrexia (24%), cough (24%), musculoskeletal pain (21%), diarrhea (20%), and rash (20%).
Serious AEs occurred in 16% of patients. The most frequent serious AEs (≥1%) were pneumonia, pneumonitis, pyrexia, dyspnea, graft-vs-host disease, and herpes zoster.
Two patients died from causes other than disease progression. One death was a result of graft-vs-host disease after subsequent allogeneic transplant, and the other was from septic shock.
There is limited experience with pembrolizumab in pediatric patients. The efficacy of the drug for pediatric patients was extrapolated from the results in the adult cHL population.
However, there is safety data on pembrolizumab in pediatric patients enrolled in the phase 1/2 KEYNOTE-051 trial. (These data were also provided by Merck.)
The trial included 40 pediatric patients with advanced melanoma or PD-L1–positive advanced, relapsed, or refractory solid tumors or lymphoma. Patients in this trial received pembrolizumab for a median of 43 days (range, 1-414 days).
The safety profile in these patients was similar to the profile in adults. Toxicities that occurred at a higher rate (≥15% difference) in pediatric patients than in adults under age 65 were fatigue (45%), vomiting (38%), abdominal pain (28%), hypertransaminasemia (28%), and hyponatremia (18%). ![]()
The US Food and Drug Administration (FDA) has granted accelerated approval for pembrolizumab (Keytruda) as a treatment for adult and pediatric patients with relapsed or refractory classical Hodgkin lymphoma (cHL).
Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the antitumor immune response.
The drug, which is being developed by Merck, previously received FDA approval as a treatment for melanoma, lung cancer, and head and neck cancer.
Now, pembrolizumab has received accelerated approval to treat adult and pediatric patients with refractory cHL or those with cHL who have relapsed after 3 or more prior lines of therapy.
The accelerated approval was based on tumor response rate and durability of response. Continued approval of pembrolizumab for cHL patients may be contingent upon the verification and description of clinical benefit in confirmatory trials.
In adults with cHL, pembrolizumab is administered at a fixed dose of 200 mg every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
In pediatric patients with cHL, pembrolizumab is administered at a dose of 2 mg/kg (up to a maximum of 200 mg) every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab trials
The FDA’s approval of pembrolizumab in adults with cHL is based on data from the phase 2 KEYNOTE-087 trial. (The following data were provided by Merck.)
The trial enrolled 210 patients who received pembrolizumab at a dose of 200 mg every 3 weeks until unacceptable toxicity or documented disease progression, or for up to 24 months in patients who did not progress.
Fifty-eight percent of patients were refractory to their last prior therapy, including 35% with primary refractory disease and 14% whose disease was refractory to all prior regimens.
Sixty-one percent of patients had undergone prior autologous hematopoietic stem cell transplant, 83% had prior brentuximab use, and 36% had prior radiation therapy.
At a median follow-up of 9.4 months, the overall response rate was 69%, and the complete response rate was 22%. The median duration of response was 11.1 months (range, 0.0+ to 11.1 months).
Five percent of patients discontinued pembrolizumab due to adverse events (AEs), and 26% had dose interruptions due to AEs. Fifteen percent of patients had an AE requiring systemic corticosteroid therapy.
The most common AEs (occurring in ≥20% of patients) were fatigue (26%), pyrexia (24%), cough (24%), musculoskeletal pain (21%), diarrhea (20%), and rash (20%).
Serious AEs occurred in 16% of patients. The most frequent serious AEs (≥1%) were pneumonia, pneumonitis, pyrexia, dyspnea, graft-vs-host disease, and herpes zoster.
Two patients died from causes other than disease progression. One death was a result of graft-vs-host disease after subsequent allogeneic transplant, and the other was from septic shock.
There is limited experience with pembrolizumab in pediatric patients. The efficacy of the drug for pediatric patients was extrapolated from the results in the adult cHL population.
However, there is safety data on pembrolizumab in pediatric patients enrolled in the phase 1/2 KEYNOTE-051 trial. (These data were also provided by Merck.)
The trial included 40 pediatric patients with advanced melanoma or PD-L1–positive advanced, relapsed, or refractory solid tumors or lymphoma. Patients in this trial received pembrolizumab for a median of 43 days (range, 1-414 days).
The safety profile in these patients was similar to the profile in adults. Toxicities that occurred at a higher rate (≥15% difference) in pediatric patients than in adults under age 65 were fatigue (45%), vomiting (38%), abdominal pain (28%), hypertransaminasemia (28%), and hyponatremia (18%). ![]()
The US Food and Drug Administration (FDA) has granted accelerated approval for pembrolizumab (Keytruda) as a treatment for adult and pediatric patients with relapsed or refractory classical Hodgkin lymphoma (cHL).
Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the antitumor immune response.
The drug, which is being developed by Merck, previously received FDA approval as a treatment for melanoma, lung cancer, and head and neck cancer.
Now, pembrolizumab has received accelerated approval to treat adult and pediatric patients with refractory cHL or those with cHL who have relapsed after 3 or more prior lines of therapy.
The accelerated approval was based on tumor response rate and durability of response. Continued approval of pembrolizumab for cHL patients may be contingent upon the verification and description of clinical benefit in confirmatory trials.
In adults with cHL, pembrolizumab is administered at a fixed dose of 200 mg every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
In pediatric patients with cHL, pembrolizumab is administered at a dose of 2 mg/kg (up to a maximum of 200 mg) every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab trials
The FDA’s approval of pembrolizumab in adults with cHL is based on data from the phase 2 KEYNOTE-087 trial. (The following data were provided by Merck.)
The trial enrolled 210 patients who received pembrolizumab at a dose of 200 mg every 3 weeks until unacceptable toxicity or documented disease progression, or for up to 24 months in patients who did not progress.
Fifty-eight percent of patients were refractory to their last prior therapy, including 35% with primary refractory disease and 14% whose disease was refractory to all prior regimens.
Sixty-one percent of patients had undergone prior autologous hematopoietic stem cell transplant, 83% had prior brentuximab use, and 36% had prior radiation therapy.
At a median follow-up of 9.4 months, the overall response rate was 69%, and the complete response rate was 22%. The median duration of response was 11.1 months (range, 0.0+ to 11.1 months).
Five percent of patients discontinued pembrolizumab due to adverse events (AEs), and 26% had dose interruptions due to AEs. Fifteen percent of patients had an AE requiring systemic corticosteroid therapy.
The most common AEs (occurring in ≥20% of patients) were fatigue (26%), pyrexia (24%), cough (24%), musculoskeletal pain (21%), diarrhea (20%), and rash (20%).
Serious AEs occurred in 16% of patients. The most frequent serious AEs (≥1%) were pneumonia, pneumonitis, pyrexia, dyspnea, graft-vs-host disease, and herpes zoster.
Two patients died from causes other than disease progression. One death was a result of graft-vs-host disease after subsequent allogeneic transplant, and the other was from septic shock.
There is limited experience with pembrolizumab in pediatric patients. The efficacy of the drug for pediatric patients was extrapolated from the results in the adult cHL population.
However, there is safety data on pembrolizumab in pediatric patients enrolled in the phase 1/2 KEYNOTE-051 trial. (These data were also provided by Merck.)
The trial included 40 pediatric patients with advanced melanoma or PD-L1–positive advanced, relapsed, or refractory solid tumors or lymphoma. Patients in this trial received pembrolizumab for a median of 43 days (range, 1-414 days).
The safety profile in these patients was similar to the profile in adults. Toxicities that occurred at a higher rate (≥15% difference) in pediatric patients than in adults under age 65 were fatigue (45%), vomiting (38%), abdominal pain (28%), hypertransaminasemia (28%), and hyponatremia (18%).
Family history impacts risk of second cancer after HL
A new study suggests Hodgkin lymphoma (HL) survivors have a high risk of developing a second malignancy, particularly if they have a family history of that malignancy.
The research showed that HL survivors in Sweden were roughly 2.4 times more likely than individuals in the country’s general population to develop a second cancer.
The risk for HL survivors remained high 30 years after treatment, and the risk was even greater in HL survivors who had a family history of specific cancers.
“The vast majority of patients with Hodgkin lymphoma are cured with a combination of chemotherapy and radiotherapy,” said study author Amit Sud, MBChB, of The Institute of Cancer Research, London in the UK.
“Our research has shown that these patients are at substantially increased risk of a second cancer later in life and particularly if they have a family history of cancer.”
Dr Sud and his colleagues described this research in the Journal of Clinical Oncology.
The team analyzed data from the Swedish Family-Cancer Project Database. They identified 9522 HL patients diagnosed between 1965 and 2013. During a median follow-up of 12.6 years, there were 1215 second cancers in 1121 HL patients (12%).
Compared to the general population, the HL patients had a significantly higher risk of all second malignancies, with a standardized incident ratio (SIR) of 2.39 and an absolute excess risk of 71.2 cases per 10,000 person-years.
Cancer types
HL patients had a significantly increased risk of several malignancies. The overall SIRs were as follows:
- NHL—7.99
- Leukemia—6.46
- Connective tissue cancer—5.73
- Thyroid cancer—5.13
- Squamous cell carcinoma—4.44
- Lung cancer—3.61
- Pharyngeal cancer—3.52
- Esophageal cancer—2.62
- Brain cancer—2.58
- Breast cancer—2.52
- Colon cancer—2.21
- Pancreatic cancer—2.09
- Melanoma—2.08
- Colorectal cancer—1.85
- Stomach cancer—1.78
- Bladder cancer—1.57
- Prostate cancer—1.21.
The researchers calculated SIRs over time and found the risk for many of the cancers remained high over 30 years following HL treatment.
Family history
The researchers identified 28,277 first-degree relatives of the HL survivors. Thirty percent of HL survivors (n=2785) had 1 or more first-degree relatives with a family history of cancer.
The SIR for cancers was 1.02 in the relatives. The SIR for second cancers was 2.83 for HL survivors who had first-degree relatives with cancer and 2.16 for HL survivors who did not have any first-degree relatives with cancer.
The researchers said the increased risk of second malignancy was correlated with the number of first-degree relatives with cancer.
The SIR was 2.67 for HL patients who had a single first-degree relative with cancer and 3.40 for HL patients who had 2 or more first-degree relatives with cancer.
The SIRs for different cancer types (for HL patients with at least 1 first-degree relative with cancer and no first-degree relatives with cancer, respectively) were as follows:
- NHL—14.43 vs 7.83
- Leukemia—14.31 vs 6.37
- Squamous cell carcinoma—10.85 vs 4.30
- Lung cancer—11.24 vs 3.39
- Breast cancer—4.36 vs 2.36
- Colorectal cancer—3.71 vs 1.76.
Sex and age
The researchers found significant differences in the SIRs for second cancers between HL patients diagnosed before the age of 35 and those diagnosed after age 35.
For men, the SIRs were:
- All cancers—4.26 for <35, 2.08 for ≥ 35
- Colorectal cancer—4.07 for < 35, 1.73 for ≥35
- Lung cancer—6.16 for < 35, 3.20 for ≥35
- Breast cancer—12.60 for < 35, 4.58 for ≥35
- Squamous cell carcinoma—5.89 for < 35, 3.96 for ≥35
- NHL—15.9 for < 35, 6.93 for ≥35
- Leukemia—12.15 for < 35, 5.57 for ≥35.
For women, the SIRs were:
- All cancers—4.61 for <35, 1.73 for ≥ 35
- Colorectal cancer—1.31 for < 35, 1.65 for ≥35
- Lung cancer—8.84 for < 35, 2.50 for ≥35
- Breast cancer—6.00 for < 35, 1.14 for ≥35
- Squamous cell carcinoma—6.37 for < 35, 4.87 for ≥35
- NHL—6.23 for < 35, 6.55 for ≥35
- Leukemia—10.36 for < 35, 4.51 for ≥35.
“Younger women who have been treated with radiotherapy to the chest for Hodgkin lymphoma are already screened for breast cancer, but our study suggests that we should be looking at ways of monitoring survivors for other forms of cancer too, and potentially offering preventative interventions,” Dr Sud said.
“After patients are cured, they no longer encounter oncologists, so it’s important that other healthcare providers are aware of the increased risk to Hodgkin lymphoma survivors to improve early diagnosis of second cancers.” ![]()
A new study suggests Hodgkin lymphoma (HL) survivors have a high risk of developing a second malignancy, particularly if they have a family history of that malignancy.
The research showed that HL survivors in Sweden were roughly 2.4 times more likely than individuals in the country’s general population to develop a second cancer.
The risk for HL survivors remained high 30 years after treatment, and the risk was even greater in HL survivors who had a family history of specific cancers.
“The vast majority of patients with Hodgkin lymphoma are cured with a combination of chemotherapy and radiotherapy,” said study author Amit Sud, MBChB, of The Institute of Cancer Research, London in the UK.
“Our research has shown that these patients are at substantially increased risk of a second cancer later in life and particularly if they have a family history of cancer.”
Dr Sud and his colleagues described this research in the Journal of Clinical Oncology.
The team analyzed data from the Swedish Family-Cancer Project Database. They identified 9522 HL patients diagnosed between 1965 and 2013. During a median follow-up of 12.6 years, there were 1215 second cancers in 1121 HL patients (12%).
Compared to the general population, the HL patients had a significantly higher risk of all second malignancies, with a standardized incident ratio (SIR) of 2.39 and an absolute excess risk of 71.2 cases per 10,000 person-years.
Cancer types
HL patients had a significantly increased risk of several malignancies. The overall SIRs were as follows:
- NHL—7.99
- Leukemia—6.46
- Connective tissue cancer—5.73
- Thyroid cancer—5.13
- Squamous cell carcinoma—4.44
- Lung cancer—3.61
- Pharyngeal cancer—3.52
- Esophageal cancer—2.62
- Brain cancer—2.58
- Breast cancer—2.52
- Colon cancer—2.21
- Pancreatic cancer—2.09
- Melanoma—2.08
- Colorectal cancer—1.85
- Stomach cancer—1.78
- Bladder cancer—1.57
- Prostate cancer—1.21.
The researchers calculated SIRs over time and found the risk for many of the cancers remained high over 30 years following HL treatment.
Family history
The researchers identified 28,277 first-degree relatives of the HL survivors. Thirty percent of HL survivors (n=2785) had 1 or more first-degree relatives with a family history of cancer.
The SIR for cancers was 1.02 in the relatives. The SIR for second cancers was 2.83 for HL survivors who had first-degree relatives with cancer and 2.16 for HL survivors who did not have any first-degree relatives with cancer.
The researchers said the increased risk of second malignancy was correlated with the number of first-degree relatives with cancer.
The SIR was 2.67 for HL patients who had a single first-degree relative with cancer and 3.40 for HL patients who had 2 or more first-degree relatives with cancer.
The SIRs for different cancer types (for HL patients with at least 1 first-degree relative with cancer and no first-degree relatives with cancer, respectively) were as follows:
- NHL—14.43 vs 7.83
- Leukemia—14.31 vs 6.37
- Squamous cell carcinoma—10.85 vs 4.30
- Lung cancer—11.24 vs 3.39
- Breast cancer—4.36 vs 2.36
- Colorectal cancer—3.71 vs 1.76.
Sex and age
The researchers found significant differences in the SIRs for second cancers between HL patients diagnosed before the age of 35 and those diagnosed after age 35.
For men, the SIRs were:
- All cancers—4.26 for <35, 2.08 for ≥ 35
- Colorectal cancer—4.07 for < 35, 1.73 for ≥35
- Lung cancer—6.16 for < 35, 3.20 for ≥35
- Breast cancer—12.60 for < 35, 4.58 for ≥35
- Squamous cell carcinoma—5.89 for < 35, 3.96 for ≥35
- NHL—15.9 for < 35, 6.93 for ≥35
- Leukemia—12.15 for < 35, 5.57 for ≥35.
For women, the SIRs were:
- All cancers—4.61 for <35, 1.73 for ≥ 35
- Colorectal cancer—1.31 for < 35, 1.65 for ≥35
- Lung cancer—8.84 for < 35, 2.50 for ≥35
- Breast cancer—6.00 for < 35, 1.14 for ≥35
- Squamous cell carcinoma—6.37 for < 35, 4.87 for ≥35
- NHL—6.23 for < 35, 6.55 for ≥35
- Leukemia—10.36 for < 35, 4.51 for ≥35.
“Younger women who have been treated with radiotherapy to the chest for Hodgkin lymphoma are already screened for breast cancer, but our study suggests that we should be looking at ways of monitoring survivors for other forms of cancer too, and potentially offering preventative interventions,” Dr Sud said.
“After patients are cured, they no longer encounter oncologists, so it’s important that other healthcare providers are aware of the increased risk to Hodgkin lymphoma survivors to improve early diagnosis of second cancers.” ![]()
A new study suggests Hodgkin lymphoma (HL) survivors have a high risk of developing a second malignancy, particularly if they have a family history of that malignancy.
The research showed that HL survivors in Sweden were roughly 2.4 times more likely than individuals in the country’s general population to develop a second cancer.
The risk for HL survivors remained high 30 years after treatment, and the risk was even greater in HL survivors who had a family history of specific cancers.
“The vast majority of patients with Hodgkin lymphoma are cured with a combination of chemotherapy and radiotherapy,” said study author Amit Sud, MBChB, of The Institute of Cancer Research, London in the UK.
“Our research has shown that these patients are at substantially increased risk of a second cancer later in life and particularly if they have a family history of cancer.”
Dr Sud and his colleagues described this research in the Journal of Clinical Oncology.
The team analyzed data from the Swedish Family-Cancer Project Database. They identified 9522 HL patients diagnosed between 1965 and 2013. During a median follow-up of 12.6 years, there were 1215 second cancers in 1121 HL patients (12%).
Compared to the general population, the HL patients had a significantly higher risk of all second malignancies, with a standardized incident ratio (SIR) of 2.39 and an absolute excess risk of 71.2 cases per 10,000 person-years.
Cancer types
HL patients had a significantly increased risk of several malignancies. The overall SIRs were as follows:
- NHL—7.99
- Leukemia—6.46
- Connective tissue cancer—5.73
- Thyroid cancer—5.13
- Squamous cell carcinoma—4.44
- Lung cancer—3.61
- Pharyngeal cancer—3.52
- Esophageal cancer—2.62
- Brain cancer—2.58
- Breast cancer—2.52
- Colon cancer—2.21
- Pancreatic cancer—2.09
- Melanoma—2.08
- Colorectal cancer—1.85
- Stomach cancer—1.78
- Bladder cancer—1.57
- Prostate cancer—1.21.
The researchers calculated SIRs over time and found the risk for many of the cancers remained high over 30 years following HL treatment.
Family history
The researchers identified 28,277 first-degree relatives of the HL survivors. Thirty percent of HL survivors (n=2785) had 1 or more first-degree relatives with a family history of cancer.
The SIR for cancers was 1.02 in the relatives. The SIR for second cancers was 2.83 for HL survivors who had first-degree relatives with cancer and 2.16 for HL survivors who did not have any first-degree relatives with cancer.
The researchers said the increased risk of second malignancy was correlated with the number of first-degree relatives with cancer.
The SIR was 2.67 for HL patients who had a single first-degree relative with cancer and 3.40 for HL patients who had 2 or more first-degree relatives with cancer.
The SIRs for different cancer types (for HL patients with at least 1 first-degree relative with cancer and no first-degree relatives with cancer, respectively) were as follows:
- NHL—14.43 vs 7.83
- Leukemia—14.31 vs 6.37
- Squamous cell carcinoma—10.85 vs 4.30
- Lung cancer—11.24 vs 3.39
- Breast cancer—4.36 vs 2.36
- Colorectal cancer—3.71 vs 1.76.
Sex and age
The researchers found significant differences in the SIRs for second cancers between HL patients diagnosed before the age of 35 and those diagnosed after age 35.
For men, the SIRs were:
- All cancers—4.26 for <35, 2.08 for ≥ 35
- Colorectal cancer—4.07 for < 35, 1.73 for ≥35
- Lung cancer—6.16 for < 35, 3.20 for ≥35
- Breast cancer—12.60 for < 35, 4.58 for ≥35
- Squamous cell carcinoma—5.89 for < 35, 3.96 for ≥35
- NHL—15.9 for < 35, 6.93 for ≥35
- Leukemia—12.15 for < 35, 5.57 for ≥35.
For women, the SIRs were:
- All cancers—4.61 for <35, 1.73 for ≥ 35
- Colorectal cancer—1.31 for < 35, 1.65 for ≥35
- Lung cancer—8.84 for < 35, 2.50 for ≥35
- Breast cancer—6.00 for < 35, 1.14 for ≥35
- Squamous cell carcinoma—6.37 for < 35, 4.87 for ≥35
- NHL—6.23 for < 35, 6.55 for ≥35
- Leukemia—10.36 for < 35, 4.51 for ≥35.
“Younger women who have been treated with radiotherapy to the chest for Hodgkin lymphoma are already screened for breast cancer, but our study suggests that we should be looking at ways of monitoring survivors for other forms of cancer too, and potentially offering preventative interventions,” Dr Sud said.
“After patients are cured, they no longer encounter oncologists, so it’s important that other healthcare providers are aware of the increased risk to Hodgkin lymphoma survivors to improve early diagnosis of second cancers.” ![]()
Immunotherapy receives fast track designation
The US Food and Drug Administration (FDA) has granted fast track designation to CMD-003 (baltaleucel-T) for patients with relapsed/refractory lymphoma and post-transplant lymphoproliferative disease associated with Epstein-Barr virus (EBV).
CMD-003 consists of patient-derived T cells that have been activated to kill malignant cells expressing antigens associated with EBV.
The T cells specifically target 4 EBV epitopes—LMP1, LMP2, EBNA, and BARF1.
CMD-003 is being developed by Cell Medica and the Baylor College of Medicine with funding provided, in part, by the Cancer Prevention and Research Institute of Texas.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
CMD-003-related research
CMD-003 is currently under investigation in the phase 2 CITADEL trial for patients with extranodal natural killer T-cell lymphoma and the phase 2 CIVIC trial for patients with EBV-associated diffuse large B-cell lymphoma, Hodgkin lymphoma, and post-transplant lymphoproliferative disease.
Researchers have not published results from any trials of CMD-003, but they have published results with EBV-specific T-cell products related to CMD-003.
In one study, published in the Journal of Clinical Oncology in 2014, researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of the patients died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery.
The US Food and Drug Administration (FDA) has granted fast track designation to CMD-003 (baltaleucel-T) for patients with relapsed/refractory lymphoma and post-transplant lymphoproliferative disease associated with Epstein-Barr virus (EBV).
CMD-003 consists of patient-derived T cells that have been activated to kill malignant cells expressing antigens associated with EBV.
The T cells specifically target 4 EBV epitopes—LMP1, LMP2, EBNA, and BARF1.
CMD-003 is being developed by Cell Medica and the Baylor College of Medicine with funding provided, in part, by the Cancer Prevention and Research Institute of Texas.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
CMD-003-related research
CMD-003 is currently under investigation in the phase 2 CITADEL trial for patients with extranodal natural killer T-cell lymphoma and the phase 2 CIVIC trial for patients with EBV-associated diffuse large B-cell lymphoma, Hodgkin lymphoma, and post-transplant lymphoproliferative disease.
Researchers have not published results from any trials of CMD-003, but they have published results with EBV-specific T-cell products related to CMD-003.
In one study, published in the Journal of Clinical Oncology in 2014, researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of the patients died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery.
The US Food and Drug Administration (FDA) has granted fast track designation to CMD-003 (baltaleucel-T) for patients with relapsed/refractory lymphoma and post-transplant lymphoproliferative disease associated with Epstein-Barr virus (EBV).
CMD-003 consists of patient-derived T cells that have been activated to kill malignant cells expressing antigens associated with EBV.
The T cells specifically target 4 EBV epitopes—LMP1, LMP2, EBNA, and BARF1.
CMD-003 is being developed by Cell Medica and the Baylor College of Medicine with funding provided, in part, by the Cancer Prevention and Research Institute of Texas.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
CMD-003-related research
CMD-003 is currently under investigation in the phase 2 CITADEL trial for patients with extranodal natural killer T-cell lymphoma and the phase 2 CIVIC trial for patients with EBV-associated diffuse large B-cell lymphoma, Hodgkin lymphoma, and post-transplant lymphoproliferative disease.
Researchers have not published results from any trials of CMD-003, but they have published results with EBV-specific T-cell products related to CMD-003.
In one study, published in the Journal of Clinical Oncology in 2014, researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of the patients died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery.