User login
Low-fat diet upped quality of life in ulcerative colitis
For patients with mild or remitted ulcerative colitis, a catered, low-fat, high-fiber diet improved quality of life and stool markers of dysbiosis and inflammation, according to the findings of a small crossover trial.
Patients with inflammatory bowel disease often ask what they should eat, but few studies have addressed that question, Julia Fritsch, of the University of Miami and her associates wrote in Clinical Gastroenterology and Hepatology. Building on previous findings that a high-fat diet may contribute to inflammatory bowel disease, they randomly assigned 38 adults whose ulcerative colitis was in remission or mild (with a flare within the past 18 months) to receive either a low-fat diet (with 10% of daily calories from fat and high amounts of fruit and vegetables) or an “improved American standard diet” (with 35%-40% of daily calories from fat but more fruit and vegetables than Americans typically eat). Each diet was catered, delivered to patients’ homes, and lasted 4 weeks, followed by a 2-week washout period, after which each participant switched to the other diet.
Of the 38 patients, 17 completed the study. Food recall surveys over 24 hours showed that both diets were healthier than what participants ate at baseline, and daily web-based food diaries (such as www.nutrihand.com/Static/index.html) confirmed that more than 94% of patients adhered to the amount of fat in each diet. Even though participants in both groups ate only about half of the provided fruits and vegetables, the primary outcome of quality of life based on the short inflammatory bowel disease questionnaire (SIBDQ) significantly improved from a median of 4.98 (interquartile range, 4.1-6.0) at baseline to 5.77 (IQR, 5-6.4) with the low-fat diet and 5.55 (IQR, 4.75-6.25) with the improved American standard diet. Both diets also produced significant improvements in quality of life as measured by the 36-Item Short Form Survey and in disease activity as measured by the partial Mayo score.
Notably, however, only the low-fat diet significantly reduced serum amyloid A, which is a marker of mucosal inflammation, and intestinal dysbiosis, which was quantified by 16S RNA ribosomal sequencing. “Of note, there were several variables that were associated with changes in the microbiota composition,” the researchers wrote. These included the SIBDQ, C-reactive protein, interleukin-6, interleukin-1 beta, and 32 dietary components such as protein, potassium, iron, and zinc.
“These data suggest that even patients in remission [from ulcerative colitis] could benefit from a healthier diet,” the investigators concluded. “Just as importantly, neither diet exacerbated symptoms, which is notable given the higher fiber in both catered diets.” They called catering “a feasible way to perform a diet intervention study with high adherence,” noting that “catering a diet for a patient with IBD for a year costs between $19,000 and $21,000 per patient. The cost of a patient on a biologic such as ustekinumab is approximately $130,752 to $261,504.”
The study was supported by the Crohn’s and Colitis Foundation Broad Medical Research Program, Micky and Madeleine Arison Family Foundation Crohn’s and Colitis Discovery Laboratory, and the Martin Kalser Chair. The senior author disclosed ties to Boehringer Ingelheim, Gilead, AbbVie, Seres Therapeutics, Shire, Landos, Pfizer, and several other pharmaceutical companies. The other researchers reported having no conflicts of interest.
Diet plays an important role in Crohn’s disease and ulcerative colitis. Most patients with these diseases look to incorporate dietary modification as part of the treatment plan to achieve and maintain remission. With the development of tools that allow us to sequence the gut microbiome at high resolution, the role of dietary therapy for these diseases is being studied with increasing scientific rigor.
In a crossover study of 17 patients with ulcerative colitis in remission or with only mild disease, Fritsch and colleagues demonstrated that adherence to a low-fat, high-fiber diet was associated with an improvement in the health-related quality of life, a decrease in C-reactive protein, and beneficial changes in the gut bacteria including reduced abundance of Actinobacteria and an increase in organisms with anti-inflammatory potential such as Faecalibacterium prausnitzii. In conjunction with prior experimental studies that suggested an increase in risk of colitis with high fat intake, this study provides some evidence for recommending a lower fat intake in patients with established inflammatory bowel disease (IBD). Furthermore, an increase in fruits, vegetables and fiber intake even in those with a standard American diet was associated with a modest beneficial effect, challenging the longstanding unsupported dogma of broadly limiting all fiber intake in those with established IBD.
The much-needed progress in the scientific study of diet in IBD will provide us with the important answers that our patients are looking for.
Ashwin Ananthakrishnan, MD, MPH , is an associate professor of medicine at Massachusetts General Hospital and Harvard Medical School, both in Boston. He has no conflicts relevant to this commentary to declare.
Diet plays an important role in Crohn’s disease and ulcerative colitis. Most patients with these diseases look to incorporate dietary modification as part of the treatment plan to achieve and maintain remission. With the development of tools that allow us to sequence the gut microbiome at high resolution, the role of dietary therapy for these diseases is being studied with increasing scientific rigor.
In a crossover study of 17 patients with ulcerative colitis in remission or with only mild disease, Fritsch and colleagues demonstrated that adherence to a low-fat, high-fiber diet was associated with an improvement in the health-related quality of life, a decrease in C-reactive protein, and beneficial changes in the gut bacteria including reduced abundance of Actinobacteria and an increase in organisms with anti-inflammatory potential such as Faecalibacterium prausnitzii. In conjunction with prior experimental studies that suggested an increase in risk of colitis with high fat intake, this study provides some evidence for recommending a lower fat intake in patients with established inflammatory bowel disease (IBD). Furthermore, an increase in fruits, vegetables and fiber intake even in those with a standard American diet was associated with a modest beneficial effect, challenging the longstanding unsupported dogma of broadly limiting all fiber intake in those with established IBD.
The much-needed progress in the scientific study of diet in IBD will provide us with the important answers that our patients are looking for.
Ashwin Ananthakrishnan, MD, MPH , is an associate professor of medicine at Massachusetts General Hospital and Harvard Medical School, both in Boston. He has no conflicts relevant to this commentary to declare.
Diet plays an important role in Crohn’s disease and ulcerative colitis. Most patients with these diseases look to incorporate dietary modification as part of the treatment plan to achieve and maintain remission. With the development of tools that allow us to sequence the gut microbiome at high resolution, the role of dietary therapy for these diseases is being studied with increasing scientific rigor.
In a crossover study of 17 patients with ulcerative colitis in remission or with only mild disease, Fritsch and colleagues demonstrated that adherence to a low-fat, high-fiber diet was associated with an improvement in the health-related quality of life, a decrease in C-reactive protein, and beneficial changes in the gut bacteria including reduced abundance of Actinobacteria and an increase in organisms with anti-inflammatory potential such as Faecalibacterium prausnitzii. In conjunction with prior experimental studies that suggested an increase in risk of colitis with high fat intake, this study provides some evidence for recommending a lower fat intake in patients with established inflammatory bowel disease (IBD). Furthermore, an increase in fruits, vegetables and fiber intake even in those with a standard American diet was associated with a modest beneficial effect, challenging the longstanding unsupported dogma of broadly limiting all fiber intake in those with established IBD.
The much-needed progress in the scientific study of diet in IBD will provide us with the important answers that our patients are looking for.
Ashwin Ananthakrishnan, MD, MPH , is an associate professor of medicine at Massachusetts General Hospital and Harvard Medical School, both in Boston. He has no conflicts relevant to this commentary to declare.
For patients with mild or remitted ulcerative colitis, a catered, low-fat, high-fiber diet improved quality of life and stool markers of dysbiosis and inflammation, according to the findings of a small crossover trial.
Patients with inflammatory bowel disease often ask what they should eat, but few studies have addressed that question, Julia Fritsch, of the University of Miami and her associates wrote in Clinical Gastroenterology and Hepatology. Building on previous findings that a high-fat diet may contribute to inflammatory bowel disease, they randomly assigned 38 adults whose ulcerative colitis was in remission or mild (with a flare within the past 18 months) to receive either a low-fat diet (with 10% of daily calories from fat and high amounts of fruit and vegetables) or an “improved American standard diet” (with 35%-40% of daily calories from fat but more fruit and vegetables than Americans typically eat). Each diet was catered, delivered to patients’ homes, and lasted 4 weeks, followed by a 2-week washout period, after which each participant switched to the other diet.
Of the 38 patients, 17 completed the study. Food recall surveys over 24 hours showed that both diets were healthier than what participants ate at baseline, and daily web-based food diaries (such as www.nutrihand.com/Static/index.html) confirmed that more than 94% of patients adhered to the amount of fat in each diet. Even though participants in both groups ate only about half of the provided fruits and vegetables, the primary outcome of quality of life based on the short inflammatory bowel disease questionnaire (SIBDQ) significantly improved from a median of 4.98 (interquartile range, 4.1-6.0) at baseline to 5.77 (IQR, 5-6.4) with the low-fat diet and 5.55 (IQR, 4.75-6.25) with the improved American standard diet. Both diets also produced significant improvements in quality of life as measured by the 36-Item Short Form Survey and in disease activity as measured by the partial Mayo score.
Notably, however, only the low-fat diet significantly reduced serum amyloid A, which is a marker of mucosal inflammation, and intestinal dysbiosis, which was quantified by 16S RNA ribosomal sequencing. “Of note, there were several variables that were associated with changes in the microbiota composition,” the researchers wrote. These included the SIBDQ, C-reactive protein, interleukin-6, interleukin-1 beta, and 32 dietary components such as protein, potassium, iron, and zinc.
“These data suggest that even patients in remission [from ulcerative colitis] could benefit from a healthier diet,” the investigators concluded. “Just as importantly, neither diet exacerbated symptoms, which is notable given the higher fiber in both catered diets.” They called catering “a feasible way to perform a diet intervention study with high adherence,” noting that “catering a diet for a patient with IBD for a year costs between $19,000 and $21,000 per patient. The cost of a patient on a biologic such as ustekinumab is approximately $130,752 to $261,504.”
The study was supported by the Crohn’s and Colitis Foundation Broad Medical Research Program, Micky and Madeleine Arison Family Foundation Crohn’s and Colitis Discovery Laboratory, and the Martin Kalser Chair. The senior author disclosed ties to Boehringer Ingelheim, Gilead, AbbVie, Seres Therapeutics, Shire, Landos, Pfizer, and several other pharmaceutical companies. The other researchers reported having no conflicts of interest.
For patients with mild or remitted ulcerative colitis, a catered, low-fat, high-fiber diet improved quality of life and stool markers of dysbiosis and inflammation, according to the findings of a small crossover trial.
Patients with inflammatory bowel disease often ask what they should eat, but few studies have addressed that question, Julia Fritsch, of the University of Miami and her associates wrote in Clinical Gastroenterology and Hepatology. Building on previous findings that a high-fat diet may contribute to inflammatory bowel disease, they randomly assigned 38 adults whose ulcerative colitis was in remission or mild (with a flare within the past 18 months) to receive either a low-fat diet (with 10% of daily calories from fat and high amounts of fruit and vegetables) or an “improved American standard diet” (with 35%-40% of daily calories from fat but more fruit and vegetables than Americans typically eat). Each diet was catered, delivered to patients’ homes, and lasted 4 weeks, followed by a 2-week washout period, after which each participant switched to the other diet.
Of the 38 patients, 17 completed the study. Food recall surveys over 24 hours showed that both diets were healthier than what participants ate at baseline, and daily web-based food diaries (such as www.nutrihand.com/Static/index.html) confirmed that more than 94% of patients adhered to the amount of fat in each diet. Even though participants in both groups ate only about half of the provided fruits and vegetables, the primary outcome of quality of life based on the short inflammatory bowel disease questionnaire (SIBDQ) significantly improved from a median of 4.98 (interquartile range, 4.1-6.0) at baseline to 5.77 (IQR, 5-6.4) with the low-fat diet and 5.55 (IQR, 4.75-6.25) with the improved American standard diet. Both diets also produced significant improvements in quality of life as measured by the 36-Item Short Form Survey and in disease activity as measured by the partial Mayo score.
Notably, however, only the low-fat diet significantly reduced serum amyloid A, which is a marker of mucosal inflammation, and intestinal dysbiosis, which was quantified by 16S RNA ribosomal sequencing. “Of note, there were several variables that were associated with changes in the microbiota composition,” the researchers wrote. These included the SIBDQ, C-reactive protein, interleukin-6, interleukin-1 beta, and 32 dietary components such as protein, potassium, iron, and zinc.
“These data suggest that even patients in remission [from ulcerative colitis] could benefit from a healthier diet,” the investigators concluded. “Just as importantly, neither diet exacerbated symptoms, which is notable given the higher fiber in both catered diets.” They called catering “a feasible way to perform a diet intervention study with high adherence,” noting that “catering a diet for a patient with IBD for a year costs between $19,000 and $21,000 per patient. The cost of a patient on a biologic such as ustekinumab is approximately $130,752 to $261,504.”
The study was supported by the Crohn’s and Colitis Foundation Broad Medical Research Program, Micky and Madeleine Arison Family Foundation Crohn’s and Colitis Discovery Laboratory, and the Martin Kalser Chair. The senior author disclosed ties to Boehringer Ingelheim, Gilead, AbbVie, Seres Therapeutics, Shire, Landos, Pfizer, and several other pharmaceutical companies. The other researchers reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
IL-6 trans-signaling targeted by olamkicept in IBD
The selective interleukin-6 (IL-6) trans-signaling inhibitor olamkicept was well tolerated and induced clinical remissions in 3 of 16 adults with moderately to severely active inflammatory bowel disease (IBD), and remission was associated with clear alterations in levels of phospho-STAT3 (pSTAT3) in the intestinal mucosa, researchers reported.
In a 12-week, open-label, prospective phase 2a trial, patients received up to seven infusions of 600-mg olamkicept (sgp130Fc) every 2 weeks. Clinical remissions occurred in two of nine patients with ulcerative colitis and one of seven patients with Crohn’s disease. The overall rate of clinical response was 44%, which included five patients with ulcerative colitis and two patients with Crohn’s disease. Transcriptome isolation and high-throughput RNA sequencing of mucosal tissue specimens showed that clinical remitters had a decrease from baseline to week 14 in the expression of TNF, IL-1A, REG1A, IL-8, IL-1B, and LILRA, a known composite molecular surrogate for mucosal inflammation. In addition, exposing whole-blood samples to a recombinant IL-6/IL-6R fusion protein mimicked physiologic IL-6 activity and demonstrated that pSTAT3 levels dropped within 4 hours of the first olamkicept infusion and throughout treatment. “Our overall finding of decreased pSTAT3-positive cells in remission patients indicates that STAT3 is crucially involved in the mechanism of action of olamkicept,” wrote Stefan Schreiber, MD, of University Medical Center Schleswig-Holstein, Campus Kiel (Germany) together with his associates. The study is published in Gastroenterology.
Blocking the IL-6/ILR receptor can induce IBD remissions but causes “profound immunosuppression,” the investigators noted. Building on prior findings that chronic proinflammatory IL-6 activity is primarily mediated by trans-signaling of a complex of IL-6 and soluble IL6R that engages the gp130 receptor, the researchers developed a “decoy protein,” sgp130Fc (now known as olamkicept), which “exclusively blocks” IL-6 proinflammatory trans-signaling. This decoy protein showed promise in preclinical studies, with no evidence of immunosuppression, they wrote. To further evaluate olamkicept, they recruited adults with moderately to severely active ulcerative colitis or Crohn’s disease from two centers in Germany. The primary clinical assessment was remission, defined as a Mayo score under 2, with a bleeding score of 0 and an endoscopy score of less than 1 for patients with ulcerative colitis, and a Crohn’s Disease Activity Index (CDAI) of less than 150 for patients with Crohn’s disease. The primary molecular outcome was change in the composite molecular surrogate score.
Of the 16 patients, 10 completed the trial. At week 14, endoscopic responses were observed in six patients, all of whom also had a clinical response, and all three patients with clinical remissions also had endoscopic remissions. “The drug was well tolerated in all 16 treated individuals, similar to the results of the [two prior] phase 1 trials,” the researchers wrote. Although significant immunosuppression and intestinal perforations were not seen, 13 patients developed adverse events, most commonly seasonal upper respiratory tract infections, recurrence of herpes labialis, and eczema or erythema. There were five serious adverse events, two of which were cardiac in nature. A larger placebo-controlled trial is underway to further evaluate safety. For now, the researchers wrote, it appears that IL-6 trans-signaling inhibition “might open up novel therapeutic avenues for the treatment of IBD.”
University Hospital Schleswig-Holstein sponsored the study. Ferring AG provided funding and donated the olamkicept. Analyses were funded by EU H2020 SYSCID and EU H2020 Innovative Medicines Initiative 2 Joint Undertaking. Dr. Schreiber reported having coinvented IP and having ties to Pfizer, Bristol Myers Squibb, and Roche. Four coinvestigators disclosed ties to Ferring, AbbVie, Chugai, Roche, Regeneron, Pfizer, Sanofi, Conaris, and Genentech Roche. The other researchers reported having no conflicts of interest.
Proinflammatory cytokine inhibition has revolutionized the care of patients with moderate to severe inflammatory bowel disease (IBD). However, some patients don’t respond, never gain remission, or lose response. Therefore, the search continues for more effective therapies. The study by Schreiber and colleagues highlights the importance of continued innovation surrounding inflammatory pathways.
The authors did extensive evaluation of the tissue and molecular effects and discovered possible differential target engagement with interleukin-6 transcriptional inhibition which is encouraging. Notably, however, there were a high number of reported adverse events. Per the authors, these were nonspecific and not indicative of severe immunosuppression. Importantly, there were no intestinal perforations.
Intense optimism for new mechanisms will remain tempered as we have seen other therapies hold promise but fail in larger randomized trials. However, it is encouraging to see how continued work on proinflammatory pathways into more targeted inhibitory approaches can lead to potential new therapies in IBD.
Sara Horst, MD, MPH, FACG, is an associate professor in the division of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center, Nashville, Tenn. She reports having been a consultant for Gilead, Takeda, and Janssen and receiving unrestricted grant funding from UCB.
Proinflammatory cytokine inhibition has revolutionized the care of patients with moderate to severe inflammatory bowel disease (IBD). However, some patients don’t respond, never gain remission, or lose response. Therefore, the search continues for more effective therapies. The study by Schreiber and colleagues highlights the importance of continued innovation surrounding inflammatory pathways.
The authors did extensive evaluation of the tissue and molecular effects and discovered possible differential target engagement with interleukin-6 transcriptional inhibition which is encouraging. Notably, however, there were a high number of reported adverse events. Per the authors, these were nonspecific and not indicative of severe immunosuppression. Importantly, there were no intestinal perforations.
Intense optimism for new mechanisms will remain tempered as we have seen other therapies hold promise but fail in larger randomized trials. However, it is encouraging to see how continued work on proinflammatory pathways into more targeted inhibitory approaches can lead to potential new therapies in IBD.
Sara Horst, MD, MPH, FACG, is an associate professor in the division of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center, Nashville, Tenn. She reports having been a consultant for Gilead, Takeda, and Janssen and receiving unrestricted grant funding from UCB.
Proinflammatory cytokine inhibition has revolutionized the care of patients with moderate to severe inflammatory bowel disease (IBD). However, some patients don’t respond, never gain remission, or lose response. Therefore, the search continues for more effective therapies. The study by Schreiber and colleagues highlights the importance of continued innovation surrounding inflammatory pathways.
The authors did extensive evaluation of the tissue and molecular effects and discovered possible differential target engagement with interleukin-6 transcriptional inhibition which is encouraging. Notably, however, there were a high number of reported adverse events. Per the authors, these were nonspecific and not indicative of severe immunosuppression. Importantly, there were no intestinal perforations.
Intense optimism for new mechanisms will remain tempered as we have seen other therapies hold promise but fail in larger randomized trials. However, it is encouraging to see how continued work on proinflammatory pathways into more targeted inhibitory approaches can lead to potential new therapies in IBD.
Sara Horst, MD, MPH, FACG, is an associate professor in the division of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center, Nashville, Tenn. She reports having been a consultant for Gilead, Takeda, and Janssen and receiving unrestricted grant funding from UCB.
The selective interleukin-6 (IL-6) trans-signaling inhibitor olamkicept was well tolerated and induced clinical remissions in 3 of 16 adults with moderately to severely active inflammatory bowel disease (IBD), and remission was associated with clear alterations in levels of phospho-STAT3 (pSTAT3) in the intestinal mucosa, researchers reported.
In a 12-week, open-label, prospective phase 2a trial, patients received up to seven infusions of 600-mg olamkicept (sgp130Fc) every 2 weeks. Clinical remissions occurred in two of nine patients with ulcerative colitis and one of seven patients with Crohn’s disease. The overall rate of clinical response was 44%, which included five patients with ulcerative colitis and two patients with Crohn’s disease. Transcriptome isolation and high-throughput RNA sequencing of mucosal tissue specimens showed that clinical remitters had a decrease from baseline to week 14 in the expression of TNF, IL-1A, REG1A, IL-8, IL-1B, and LILRA, a known composite molecular surrogate for mucosal inflammation. In addition, exposing whole-blood samples to a recombinant IL-6/IL-6R fusion protein mimicked physiologic IL-6 activity and demonstrated that pSTAT3 levels dropped within 4 hours of the first olamkicept infusion and throughout treatment. “Our overall finding of decreased pSTAT3-positive cells in remission patients indicates that STAT3 is crucially involved in the mechanism of action of olamkicept,” wrote Stefan Schreiber, MD, of University Medical Center Schleswig-Holstein, Campus Kiel (Germany) together with his associates. The study is published in Gastroenterology.
Blocking the IL-6/ILR receptor can induce IBD remissions but causes “profound immunosuppression,” the investigators noted. Building on prior findings that chronic proinflammatory IL-6 activity is primarily mediated by trans-signaling of a complex of IL-6 and soluble IL6R that engages the gp130 receptor, the researchers developed a “decoy protein,” sgp130Fc (now known as olamkicept), which “exclusively blocks” IL-6 proinflammatory trans-signaling. This decoy protein showed promise in preclinical studies, with no evidence of immunosuppression, they wrote. To further evaluate olamkicept, they recruited adults with moderately to severely active ulcerative colitis or Crohn’s disease from two centers in Germany. The primary clinical assessment was remission, defined as a Mayo score under 2, with a bleeding score of 0 and an endoscopy score of less than 1 for patients with ulcerative colitis, and a Crohn’s Disease Activity Index (CDAI) of less than 150 for patients with Crohn’s disease. The primary molecular outcome was change in the composite molecular surrogate score.
Of the 16 patients, 10 completed the trial. At week 14, endoscopic responses were observed in six patients, all of whom also had a clinical response, and all three patients with clinical remissions also had endoscopic remissions. “The drug was well tolerated in all 16 treated individuals, similar to the results of the [two prior] phase 1 trials,” the researchers wrote. Although significant immunosuppression and intestinal perforations were not seen, 13 patients developed adverse events, most commonly seasonal upper respiratory tract infections, recurrence of herpes labialis, and eczema or erythema. There were five serious adverse events, two of which were cardiac in nature. A larger placebo-controlled trial is underway to further evaluate safety. For now, the researchers wrote, it appears that IL-6 trans-signaling inhibition “might open up novel therapeutic avenues for the treatment of IBD.”
University Hospital Schleswig-Holstein sponsored the study. Ferring AG provided funding and donated the olamkicept. Analyses were funded by EU H2020 SYSCID and EU H2020 Innovative Medicines Initiative 2 Joint Undertaking. Dr. Schreiber reported having coinvented IP and having ties to Pfizer, Bristol Myers Squibb, and Roche. Four coinvestigators disclosed ties to Ferring, AbbVie, Chugai, Roche, Regeneron, Pfizer, Sanofi, Conaris, and Genentech Roche. The other researchers reported having no conflicts of interest.
The selective interleukin-6 (IL-6) trans-signaling inhibitor olamkicept was well tolerated and induced clinical remissions in 3 of 16 adults with moderately to severely active inflammatory bowel disease (IBD), and remission was associated with clear alterations in levels of phospho-STAT3 (pSTAT3) in the intestinal mucosa, researchers reported.
In a 12-week, open-label, prospective phase 2a trial, patients received up to seven infusions of 600-mg olamkicept (sgp130Fc) every 2 weeks. Clinical remissions occurred in two of nine patients with ulcerative colitis and one of seven patients with Crohn’s disease. The overall rate of clinical response was 44%, which included five patients with ulcerative colitis and two patients with Crohn’s disease. Transcriptome isolation and high-throughput RNA sequencing of mucosal tissue specimens showed that clinical remitters had a decrease from baseline to week 14 in the expression of TNF, IL-1A, REG1A, IL-8, IL-1B, and LILRA, a known composite molecular surrogate for mucosal inflammation. In addition, exposing whole-blood samples to a recombinant IL-6/IL-6R fusion protein mimicked physiologic IL-6 activity and demonstrated that pSTAT3 levels dropped within 4 hours of the first olamkicept infusion and throughout treatment. “Our overall finding of decreased pSTAT3-positive cells in remission patients indicates that STAT3 is crucially involved in the mechanism of action of olamkicept,” wrote Stefan Schreiber, MD, of University Medical Center Schleswig-Holstein, Campus Kiel (Germany) together with his associates. The study is published in Gastroenterology.
Blocking the IL-6/ILR receptor can induce IBD remissions but causes “profound immunosuppression,” the investigators noted. Building on prior findings that chronic proinflammatory IL-6 activity is primarily mediated by trans-signaling of a complex of IL-6 and soluble IL6R that engages the gp130 receptor, the researchers developed a “decoy protein,” sgp130Fc (now known as olamkicept), which “exclusively blocks” IL-6 proinflammatory trans-signaling. This decoy protein showed promise in preclinical studies, with no evidence of immunosuppression, they wrote. To further evaluate olamkicept, they recruited adults with moderately to severely active ulcerative colitis or Crohn’s disease from two centers in Germany. The primary clinical assessment was remission, defined as a Mayo score under 2, with a bleeding score of 0 and an endoscopy score of less than 1 for patients with ulcerative colitis, and a Crohn’s Disease Activity Index (CDAI) of less than 150 for patients with Crohn’s disease. The primary molecular outcome was change in the composite molecular surrogate score.
Of the 16 patients, 10 completed the trial. At week 14, endoscopic responses were observed in six patients, all of whom also had a clinical response, and all three patients with clinical remissions also had endoscopic remissions. “The drug was well tolerated in all 16 treated individuals, similar to the results of the [two prior] phase 1 trials,” the researchers wrote. Although significant immunosuppression and intestinal perforations were not seen, 13 patients developed adverse events, most commonly seasonal upper respiratory tract infections, recurrence of herpes labialis, and eczema or erythema. There were five serious adverse events, two of which were cardiac in nature. A larger placebo-controlled trial is underway to further evaluate safety. For now, the researchers wrote, it appears that IL-6 trans-signaling inhibition “might open up novel therapeutic avenues for the treatment of IBD.”
University Hospital Schleswig-Holstein sponsored the study. Ferring AG provided funding and donated the olamkicept. Analyses were funded by EU H2020 SYSCID and EU H2020 Innovative Medicines Initiative 2 Joint Undertaking. Dr. Schreiber reported having coinvented IP and having ties to Pfizer, Bristol Myers Squibb, and Roche. Four coinvestigators disclosed ties to Ferring, AbbVie, Chugai, Roche, Regeneron, Pfizer, Sanofi, Conaris, and Genentech Roche. The other researchers reported having no conflicts of interest.
FROM GASTROENTEROLOGY
New oral protein shows promise for ulcerative colitis
A plant-based fusion protein is safe and effective for inducing favorable immune modulation in patients with mild to moderate ulcerative colitis with no immune suppression–side effects reported.
OPRX-106, an orally administered BY2 plant cell–expressing recombinant TNF fusion protein, has demonstrated effectiveness as an anti–TNF-alpha therapy, according to Einat Almon, PhD, of Protalix Biotherapeutics, and colleagues.
“Oral immune therapy is based on the concept of oral administration of nonabsorbable compounds which target the gut immune system to redirect the systemic immune system toward an anti-inflammatory direction, without immunosuppression,” the researchers said.
A phase 1 study of OPRX-106 in healthy human volunteers showed safety and immune modulatory effects at doses of 2, 8, or 16 mg/day.
In this phase 2a clinical trial published in the Journal of Clinical Gastroenterology, the researchers enrolled 24 patients with ulcerative colitis (11 male and 13 female) aged 23-73 years, with an average age of 42.6 years. Patients received either 2 mg or 8 mg of OPRX-106 at least once daily for 8 weeks. All patients were monitored for 6 hours after receiving medication on day 1 and week 8 for pharmacokinetic sampling, and a lower endoscopy was performed at week 8.
After 8 weeks, 67% of the patients demonstrated clinical response and 28% showed clinical remission.
Clinical response and clinical remission were defined by a specific set of criteria including improvement in the Mayo score. Clinical response was a “decrease in the Mayo score of at least 3 points, decrease in the subscore for rectal bleeding of at least 1 point, [and] a rectal bleeding subscore of 0 or 1.” Clinical remission at week 8 was defined as “clinically symptom-free, Mayo score of ≤2 with no individual subscore exceeding 1 point after treatment, histopathological improvement in Geboes histologic grading from baseline to week 8, improvement in high sensitivity C-reactive protein levels from baseline to week 8, improvement in fecal calprotectin levels from baseline to week 8, and changes in systemic immune modulation parameters from baseline to week 8.”
In addition, 89% of the patients experienced some degree of improvement in their Mayo scores, 61% had mucosal improvement, and 33% achieved mucosal healing.
No side effects associated with general immune suppression were reported. No patients discontinued the study because of adverse events, the researchers said. However, overall, 40 adverse events were reported in 15 patients; 95% of these were mild to moderate and 40% were reported as treatment related. No differences appeared in adverse events related to the two doses.
Evidence of a systemic anti-inflammatory effect was seen with a decrease in serum levels of the pro-inflammatory cytokines interleukin-6 and interferon-gamma that correlated with the clinical response, the researchers noted. Similarly, an increase in the CD3+CD4+CD25+Foxp3+ subset of suppressor lymphocytes correlated with clinical response.
The study findings were limited by the small sample size, open-label design, and lack of control subjects. However, by targeting the gut immune system, the drug “may provide an answer to the long-term immune suppression encountered in patients with chronic disorders who use these agents for prolonged periods of time, in addition to loss of response due to neutralizing antibodies,” they concluded.
Findings provide foundation for further research
“Conducting a study of a novel treatment for ulcerative colitis is valuable and timely because the available options are limited,” Atsushi Sakuraba, MD, of the University of Chicago, said in an interview. “The currently available TNF antagonists are administered intravenously or subcutaneously and bear the risk of infectious complications, so the development of an agent that can be administered orally with fewer side effects is of importance.”
Although the data are preliminary, Dr. Sakuraba emphasized that the take-home message for clinicians is that “the present open-label study consisting of a small number of subjects demonstrated that OPRX-106 was effective and safe in active ulcerative colitis, so further investigation is warranted. Larger-powered, randomized, placebo-controlled studies are needed to confirm these findings.”
The study was supported by Protalix Biotherapeutics; Dr. Almon and several coauthors are employed by Protalix Biotherapeutics. Dr. Sakuraba had no financial conflicts to disclose.
A plant-based fusion protein is safe and effective for inducing favorable immune modulation in patients with mild to moderate ulcerative colitis with no immune suppression–side effects reported.
OPRX-106, an orally administered BY2 plant cell–expressing recombinant TNF fusion protein, has demonstrated effectiveness as an anti–TNF-alpha therapy, according to Einat Almon, PhD, of Protalix Biotherapeutics, and colleagues.
“Oral immune therapy is based on the concept of oral administration of nonabsorbable compounds which target the gut immune system to redirect the systemic immune system toward an anti-inflammatory direction, without immunosuppression,” the researchers said.
A phase 1 study of OPRX-106 in healthy human volunteers showed safety and immune modulatory effects at doses of 2, 8, or 16 mg/day.
In this phase 2a clinical trial published in the Journal of Clinical Gastroenterology, the researchers enrolled 24 patients with ulcerative colitis (11 male and 13 female) aged 23-73 years, with an average age of 42.6 years. Patients received either 2 mg or 8 mg of OPRX-106 at least once daily for 8 weeks. All patients were monitored for 6 hours after receiving medication on day 1 and week 8 for pharmacokinetic sampling, and a lower endoscopy was performed at week 8.
After 8 weeks, 67% of the patients demonstrated clinical response and 28% showed clinical remission.
Clinical response and clinical remission were defined by a specific set of criteria including improvement in the Mayo score. Clinical response was a “decrease in the Mayo score of at least 3 points, decrease in the subscore for rectal bleeding of at least 1 point, [and] a rectal bleeding subscore of 0 or 1.” Clinical remission at week 8 was defined as “clinically symptom-free, Mayo score of ≤2 with no individual subscore exceeding 1 point after treatment, histopathological improvement in Geboes histologic grading from baseline to week 8, improvement in high sensitivity C-reactive protein levels from baseline to week 8, improvement in fecal calprotectin levels from baseline to week 8, and changes in systemic immune modulation parameters from baseline to week 8.”
In addition, 89% of the patients experienced some degree of improvement in their Mayo scores, 61% had mucosal improvement, and 33% achieved mucosal healing.
No side effects associated with general immune suppression were reported. No patients discontinued the study because of adverse events, the researchers said. However, overall, 40 adverse events were reported in 15 patients; 95% of these were mild to moderate and 40% were reported as treatment related. No differences appeared in adverse events related to the two doses.
Evidence of a systemic anti-inflammatory effect was seen with a decrease in serum levels of the pro-inflammatory cytokines interleukin-6 and interferon-gamma that correlated with the clinical response, the researchers noted. Similarly, an increase in the CD3+CD4+CD25+Foxp3+ subset of suppressor lymphocytes correlated with clinical response.
The study findings were limited by the small sample size, open-label design, and lack of control subjects. However, by targeting the gut immune system, the drug “may provide an answer to the long-term immune suppression encountered in patients with chronic disorders who use these agents for prolonged periods of time, in addition to loss of response due to neutralizing antibodies,” they concluded.
Findings provide foundation for further research
“Conducting a study of a novel treatment for ulcerative colitis is valuable and timely because the available options are limited,” Atsushi Sakuraba, MD, of the University of Chicago, said in an interview. “The currently available TNF antagonists are administered intravenously or subcutaneously and bear the risk of infectious complications, so the development of an agent that can be administered orally with fewer side effects is of importance.”
Although the data are preliminary, Dr. Sakuraba emphasized that the take-home message for clinicians is that “the present open-label study consisting of a small number of subjects demonstrated that OPRX-106 was effective and safe in active ulcerative colitis, so further investigation is warranted. Larger-powered, randomized, placebo-controlled studies are needed to confirm these findings.”
The study was supported by Protalix Biotherapeutics; Dr. Almon and several coauthors are employed by Protalix Biotherapeutics. Dr. Sakuraba had no financial conflicts to disclose.
A plant-based fusion protein is safe and effective for inducing favorable immune modulation in patients with mild to moderate ulcerative colitis with no immune suppression–side effects reported.
OPRX-106, an orally administered BY2 plant cell–expressing recombinant TNF fusion protein, has demonstrated effectiveness as an anti–TNF-alpha therapy, according to Einat Almon, PhD, of Protalix Biotherapeutics, and colleagues.
“Oral immune therapy is based on the concept of oral administration of nonabsorbable compounds which target the gut immune system to redirect the systemic immune system toward an anti-inflammatory direction, without immunosuppression,” the researchers said.
A phase 1 study of OPRX-106 in healthy human volunteers showed safety and immune modulatory effects at doses of 2, 8, or 16 mg/day.
In this phase 2a clinical trial published in the Journal of Clinical Gastroenterology, the researchers enrolled 24 patients with ulcerative colitis (11 male and 13 female) aged 23-73 years, with an average age of 42.6 years. Patients received either 2 mg or 8 mg of OPRX-106 at least once daily for 8 weeks. All patients were monitored for 6 hours after receiving medication on day 1 and week 8 for pharmacokinetic sampling, and a lower endoscopy was performed at week 8.
After 8 weeks, 67% of the patients demonstrated clinical response and 28% showed clinical remission.
Clinical response and clinical remission were defined by a specific set of criteria including improvement in the Mayo score. Clinical response was a “decrease in the Mayo score of at least 3 points, decrease in the subscore for rectal bleeding of at least 1 point, [and] a rectal bleeding subscore of 0 or 1.” Clinical remission at week 8 was defined as “clinically symptom-free, Mayo score of ≤2 with no individual subscore exceeding 1 point after treatment, histopathological improvement in Geboes histologic grading from baseline to week 8, improvement in high sensitivity C-reactive protein levels from baseline to week 8, improvement in fecal calprotectin levels from baseline to week 8, and changes in systemic immune modulation parameters from baseline to week 8.”
In addition, 89% of the patients experienced some degree of improvement in their Mayo scores, 61% had mucosal improvement, and 33% achieved mucosal healing.
No side effects associated with general immune suppression were reported. No patients discontinued the study because of adverse events, the researchers said. However, overall, 40 adverse events were reported in 15 patients; 95% of these were mild to moderate and 40% were reported as treatment related. No differences appeared in adverse events related to the two doses.
Evidence of a systemic anti-inflammatory effect was seen with a decrease in serum levels of the pro-inflammatory cytokines interleukin-6 and interferon-gamma that correlated with the clinical response, the researchers noted. Similarly, an increase in the CD3+CD4+CD25+Foxp3+ subset of suppressor lymphocytes correlated with clinical response.
The study findings were limited by the small sample size, open-label design, and lack of control subjects. However, by targeting the gut immune system, the drug “may provide an answer to the long-term immune suppression encountered in patients with chronic disorders who use these agents for prolonged periods of time, in addition to loss of response due to neutralizing antibodies,” they concluded.
Findings provide foundation for further research
“Conducting a study of a novel treatment for ulcerative colitis is valuable and timely because the available options are limited,” Atsushi Sakuraba, MD, of the University of Chicago, said in an interview. “The currently available TNF antagonists are administered intravenously or subcutaneously and bear the risk of infectious complications, so the development of an agent that can be administered orally with fewer side effects is of importance.”
Although the data are preliminary, Dr. Sakuraba emphasized that the take-home message for clinicians is that “the present open-label study consisting of a small number of subjects demonstrated that OPRX-106 was effective and safe in active ulcerative colitis, so further investigation is warranted. Larger-powered, randomized, placebo-controlled studies are needed to confirm these findings.”
The study was supported by Protalix Biotherapeutics; Dr. Almon and several coauthors are employed by Protalix Biotherapeutics. Dr. Sakuraba had no financial conflicts to disclose.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
How does fragmented care affect IBD outcomes?
Poor continuity of care may lead to worse outcomes among patients with active inflammatory bowel disease (IBD), according to data from more than 20,000 veterans.
Even in the Veterans Health Administration health care system, which “may provide the ideal environment for care coordination,” patients with active IBD had “substantial variation” in dispersion of care, leading to more frequent surgical interventions, corticosteroid use, and hospitalizations, reported lead author Shirley Cohen-Mekelburg, MD, MS, of the University of Michigan, Ann Arbor, and colleagues.
“Health care in the United States is marked by substantial fragmentation, with patients pursuing and receiving care from multiple clinicians, often at different institutions,” the investigators wrote in JAMA Network Open. “Fragmented care has been associated with poor chronic disease outcomes, higher health care use, duplication in testing, and increased costs of care.”
In the VHA, these issues prompted creation of the Patient Aligned Care Team (PACT), a medical home model in which primary care physicians coordinate clinical teams of specialists and other health care practitioners. But coordination can be challenging with chronic medical conditions like IBD, according to Dr. Cohen-Mekelburg and colleagues.
“High-quality care for IBD includes not only disease-specific management of symptoms but also disease-specific preventive care, such as immunizations and cancer screening, to prevent associated adverse outcomes,” the investigators wrote. “Identifying which physician is responsible for managing each aspect of care requires some degree of coordination and makes patients with IBD vulnerable to care fragmentation.”
Worse outcomes tied to poor first-year continuity
To evaluate care fragmentation within the VHA, the investigators identified 20,079 veterans with IBD who had at least one outpatient encounter with the system between the beginning of 2002 and the end of 2014. Continuity of care (COC) was calculated with the Bice-Boxerman COC index, which measures how much a patient’s care is connected with a distinct physician. The investigators used the first year COC as the primary independent variable.
In the first year of care, the median COC index was 0.24 (interquartile range, 0.13-0.46). The investigators noted that this figure was lower than reported by previous studies involving patients with several other chronic conditions, including IBD.
After controlling for covariates and adjusting for facility-related clustering, the investigators found a lower COC index in the first year was associated with a higher rate of worse outcomes in the subsequent 2 years, including surgical interventions (adjusted hazard ratio, 1.72; 95% confidence interval, 1.43-2.07), hospitalizations (aHR, 1.25; 95% CI, 1.06-1.47), and outpatient flares requiring corticosteroids (aHR, 1.11; 95% CI, 1.01-1.22). Conversely, improving COC index score by 0.1 reduced risk of outpatient flare (aHR, 0.69; 95%CI, 0.58-0.82), hospitalization (aHR, 0.57; 95%CI, 0.41-0.79), and surgical intervention (aHR, 0.25; 95% CI, 0.16-0.38).
Further analyses showed that the relationship between lower COC and worse outcomes carried across measures such as baseline use of an immunomodulator or biological agent, as well as subgroups such as patients with nonsevere IBD and nonsurgical patients.
Among those treated by a VHA gastroenterologist, a lower level of COC was associated with a higher rate of surgical interventions, but not hospitalizations or outpatient flares. Physician-specific COC index scores were highest for primary care providers (0.54), followed by gastroenterologists (0.25) and surgeons (0.17). However, lower physician-specific COC scores did not translate to worse IBD outcomes.
“The level of COC among patients with IBD in the present VHA cohort was ... lower than the values described in previous studies of veterans in the VHA system, including a study of VHA-Medicare dual enrollees who were especially prone to fragmented care because of their ability to seek care both inside and outside of the VHA system,” the investigators wrote, referring to a 2018 study. “The difference in COC among patients with IBD vs. patients without IBD is likely multifactorial and may be associated with confusion about physician accountability and lack of focus on coordination in IBD multidisciplinary care. Patients with IBD require care by primary care providers, gastroenterologists, and surgeons, but the delineation of responsibility by physician is often unclear.”
‘Better care, not just more care,’ is needed
“These outcomes cannot be improved with a more robust treatment armamentarium alone,” according to Jason K. Hou, MD, MS, AGAF, FACG, interim chief of gastroenterology and hepatology at Michael E. DeBakey VA Medical Center and associate professor of medicine at Baylor College of Medicine, Houston, who cowrote a simultaneously published editorial, which was also authored by David I. Fudman, MD.
“Examples exist of improving care coordination and outcomes through patient-aligned care teams in primary care and medical specialty homes for IBD,” Dr. Hou said in an interview. “However, significant barriers to widespread implementation remain.”
Dr. Hou offered several possible approaches to overcome these barriers.
“We need improved methods to identify and follow high-risk patients most likely to have complications and health care utilization,” he said. “We need an investment by payers and health care systems on care coordination so the identified high-risk patients can receive timely testing, referral, and treatment. These changes require reevaluation of how the health care system incentivizes health care to provide better care, not just more care.”
The investigators reported grants from the U.S. Department of Veterans Affairs and the National Institutes of Health and financial relationships with AbbVie, UCB, and Takeda. Dr. Hou reported no conflicts of interest.
Help your patients better understand their IBD treatment options by sharing AGA's patient education, "Living with IBD," in the AGA GI Patient Center at www.gastro.org/IBD.
Poor continuity of care may lead to worse outcomes among patients with active inflammatory bowel disease (IBD), according to data from more than 20,000 veterans.
Even in the Veterans Health Administration health care system, which “may provide the ideal environment for care coordination,” patients with active IBD had “substantial variation” in dispersion of care, leading to more frequent surgical interventions, corticosteroid use, and hospitalizations, reported lead author Shirley Cohen-Mekelburg, MD, MS, of the University of Michigan, Ann Arbor, and colleagues.
“Health care in the United States is marked by substantial fragmentation, with patients pursuing and receiving care from multiple clinicians, often at different institutions,” the investigators wrote in JAMA Network Open. “Fragmented care has been associated with poor chronic disease outcomes, higher health care use, duplication in testing, and increased costs of care.”
In the VHA, these issues prompted creation of the Patient Aligned Care Team (PACT), a medical home model in which primary care physicians coordinate clinical teams of specialists and other health care practitioners. But coordination can be challenging with chronic medical conditions like IBD, according to Dr. Cohen-Mekelburg and colleagues.
“High-quality care for IBD includes not only disease-specific management of symptoms but also disease-specific preventive care, such as immunizations and cancer screening, to prevent associated adverse outcomes,” the investigators wrote. “Identifying which physician is responsible for managing each aspect of care requires some degree of coordination and makes patients with IBD vulnerable to care fragmentation.”
Worse outcomes tied to poor first-year continuity
To evaluate care fragmentation within the VHA, the investigators identified 20,079 veterans with IBD who had at least one outpatient encounter with the system between the beginning of 2002 and the end of 2014. Continuity of care (COC) was calculated with the Bice-Boxerman COC index, which measures how much a patient’s care is connected with a distinct physician. The investigators used the first year COC as the primary independent variable.
In the first year of care, the median COC index was 0.24 (interquartile range, 0.13-0.46). The investigators noted that this figure was lower than reported by previous studies involving patients with several other chronic conditions, including IBD.
After controlling for covariates and adjusting for facility-related clustering, the investigators found a lower COC index in the first year was associated with a higher rate of worse outcomes in the subsequent 2 years, including surgical interventions (adjusted hazard ratio, 1.72; 95% confidence interval, 1.43-2.07), hospitalizations (aHR, 1.25; 95% CI, 1.06-1.47), and outpatient flares requiring corticosteroids (aHR, 1.11; 95% CI, 1.01-1.22). Conversely, improving COC index score by 0.1 reduced risk of outpatient flare (aHR, 0.69; 95%CI, 0.58-0.82), hospitalization (aHR, 0.57; 95%CI, 0.41-0.79), and surgical intervention (aHR, 0.25; 95% CI, 0.16-0.38).
Further analyses showed that the relationship between lower COC and worse outcomes carried across measures such as baseline use of an immunomodulator or biological agent, as well as subgroups such as patients with nonsevere IBD and nonsurgical patients.
Among those treated by a VHA gastroenterologist, a lower level of COC was associated with a higher rate of surgical interventions, but not hospitalizations or outpatient flares. Physician-specific COC index scores were highest for primary care providers (0.54), followed by gastroenterologists (0.25) and surgeons (0.17). However, lower physician-specific COC scores did not translate to worse IBD outcomes.
“The level of COC among patients with IBD in the present VHA cohort was ... lower than the values described in previous studies of veterans in the VHA system, including a study of VHA-Medicare dual enrollees who were especially prone to fragmented care because of their ability to seek care both inside and outside of the VHA system,” the investigators wrote, referring to a 2018 study. “The difference in COC among patients with IBD vs. patients without IBD is likely multifactorial and may be associated with confusion about physician accountability and lack of focus on coordination in IBD multidisciplinary care. Patients with IBD require care by primary care providers, gastroenterologists, and surgeons, but the delineation of responsibility by physician is often unclear.”
‘Better care, not just more care,’ is needed
“These outcomes cannot be improved with a more robust treatment armamentarium alone,” according to Jason K. Hou, MD, MS, AGAF, FACG, interim chief of gastroenterology and hepatology at Michael E. DeBakey VA Medical Center and associate professor of medicine at Baylor College of Medicine, Houston, who cowrote a simultaneously published editorial, which was also authored by David I. Fudman, MD.
“Examples exist of improving care coordination and outcomes through patient-aligned care teams in primary care and medical specialty homes for IBD,” Dr. Hou said in an interview. “However, significant barriers to widespread implementation remain.”
Dr. Hou offered several possible approaches to overcome these barriers.
“We need improved methods to identify and follow high-risk patients most likely to have complications and health care utilization,” he said. “We need an investment by payers and health care systems on care coordination so the identified high-risk patients can receive timely testing, referral, and treatment. These changes require reevaluation of how the health care system incentivizes health care to provide better care, not just more care.”
The investigators reported grants from the U.S. Department of Veterans Affairs and the National Institutes of Health and financial relationships with AbbVie, UCB, and Takeda. Dr. Hou reported no conflicts of interest.
Help your patients better understand their IBD treatment options by sharing AGA's patient education, "Living with IBD," in the AGA GI Patient Center at www.gastro.org/IBD.
Poor continuity of care may lead to worse outcomes among patients with active inflammatory bowel disease (IBD), according to data from more than 20,000 veterans.
Even in the Veterans Health Administration health care system, which “may provide the ideal environment for care coordination,” patients with active IBD had “substantial variation” in dispersion of care, leading to more frequent surgical interventions, corticosteroid use, and hospitalizations, reported lead author Shirley Cohen-Mekelburg, MD, MS, of the University of Michigan, Ann Arbor, and colleagues.
“Health care in the United States is marked by substantial fragmentation, with patients pursuing and receiving care from multiple clinicians, often at different institutions,” the investigators wrote in JAMA Network Open. “Fragmented care has been associated with poor chronic disease outcomes, higher health care use, duplication in testing, and increased costs of care.”
In the VHA, these issues prompted creation of the Patient Aligned Care Team (PACT), a medical home model in which primary care physicians coordinate clinical teams of specialists and other health care practitioners. But coordination can be challenging with chronic medical conditions like IBD, according to Dr. Cohen-Mekelburg and colleagues.
“High-quality care for IBD includes not only disease-specific management of symptoms but also disease-specific preventive care, such as immunizations and cancer screening, to prevent associated adverse outcomes,” the investigators wrote. “Identifying which physician is responsible for managing each aspect of care requires some degree of coordination and makes patients with IBD vulnerable to care fragmentation.”
Worse outcomes tied to poor first-year continuity
To evaluate care fragmentation within the VHA, the investigators identified 20,079 veterans with IBD who had at least one outpatient encounter with the system between the beginning of 2002 and the end of 2014. Continuity of care (COC) was calculated with the Bice-Boxerman COC index, which measures how much a patient’s care is connected with a distinct physician. The investigators used the first year COC as the primary independent variable.
In the first year of care, the median COC index was 0.24 (interquartile range, 0.13-0.46). The investigators noted that this figure was lower than reported by previous studies involving patients with several other chronic conditions, including IBD.
After controlling for covariates and adjusting for facility-related clustering, the investigators found a lower COC index in the first year was associated with a higher rate of worse outcomes in the subsequent 2 years, including surgical interventions (adjusted hazard ratio, 1.72; 95% confidence interval, 1.43-2.07), hospitalizations (aHR, 1.25; 95% CI, 1.06-1.47), and outpatient flares requiring corticosteroids (aHR, 1.11; 95% CI, 1.01-1.22). Conversely, improving COC index score by 0.1 reduced risk of outpatient flare (aHR, 0.69; 95%CI, 0.58-0.82), hospitalization (aHR, 0.57; 95%CI, 0.41-0.79), and surgical intervention (aHR, 0.25; 95% CI, 0.16-0.38).
Further analyses showed that the relationship between lower COC and worse outcomes carried across measures such as baseline use of an immunomodulator or biological agent, as well as subgroups such as patients with nonsevere IBD and nonsurgical patients.
Among those treated by a VHA gastroenterologist, a lower level of COC was associated with a higher rate of surgical interventions, but not hospitalizations or outpatient flares. Physician-specific COC index scores were highest for primary care providers (0.54), followed by gastroenterologists (0.25) and surgeons (0.17). However, lower physician-specific COC scores did not translate to worse IBD outcomes.
“The level of COC among patients with IBD in the present VHA cohort was ... lower than the values described in previous studies of veterans in the VHA system, including a study of VHA-Medicare dual enrollees who were especially prone to fragmented care because of their ability to seek care both inside and outside of the VHA system,” the investigators wrote, referring to a 2018 study. “The difference in COC among patients with IBD vs. patients without IBD is likely multifactorial and may be associated with confusion about physician accountability and lack of focus on coordination in IBD multidisciplinary care. Patients with IBD require care by primary care providers, gastroenterologists, and surgeons, but the delineation of responsibility by physician is often unclear.”
‘Better care, not just more care,’ is needed
“These outcomes cannot be improved with a more robust treatment armamentarium alone,” according to Jason K. Hou, MD, MS, AGAF, FACG, interim chief of gastroenterology and hepatology at Michael E. DeBakey VA Medical Center and associate professor of medicine at Baylor College of Medicine, Houston, who cowrote a simultaneously published editorial, which was also authored by David I. Fudman, MD.
“Examples exist of improving care coordination and outcomes through patient-aligned care teams in primary care and medical specialty homes for IBD,” Dr. Hou said in an interview. “However, significant barriers to widespread implementation remain.”
Dr. Hou offered several possible approaches to overcome these barriers.
“We need improved methods to identify and follow high-risk patients most likely to have complications and health care utilization,” he said. “We need an investment by payers and health care systems on care coordination so the identified high-risk patients can receive timely testing, referral, and treatment. These changes require reevaluation of how the health care system incentivizes health care to provide better care, not just more care.”
The investigators reported grants from the U.S. Department of Veterans Affairs and the National Institutes of Health and financial relationships with AbbVie, UCB, and Takeda. Dr. Hou reported no conflicts of interest.
Help your patients better understand their IBD treatment options by sharing AGA's patient education, "Living with IBD," in the AGA GI Patient Center at www.gastro.org/IBD.
FROM JAMA NETWORK OPEN
Dyssynergic defecation
Introduction
About 40% of the population experiences lower GI symptoms suggestive of gastrointestinal motility disorders.1,2 The global prevalence of chronic constipation is 18%, and the condition includes multiple overlapping subtypes.3 Evacuation disorders affect over half (59%) of patients and include dyssynergic defecation (DD).4 The inability to coordinate the abdominal, rectal, pelvic floor, and anal/puborectalis muscles to evacuate stools causes DD.5 The etiology of DD remains unclear and is often misdiagnosed. Clinically, the symptoms of DD overlap with other lower GI disorders, often leading to unnecessary and invasive procedures.2 We describe the clinical characteristics, diagnostic tools, treatment options, and evidence-based approach for the management of DD.

Clinical presentation
Over two-thirds of patients with DD acquire this disorder during adulthood, and one-third have symptoms from childhood.6 Though there is not usually an inciting event, 29% of patients report that symptoms began after events such as pregnancy or back injury,6 and opioid users have higher prevalence and severity of DD.7
Over 80% of patients report excessive straining, feelings of incomplete evacuation, and hard stools, and 50% report sensation of anal blockage or use of digital maneuvers.2 Other symptoms include infrequent bowel movements, abdominal pain, anal pain, and stool leakage.2 Evaluation of DD includes obtaining a detailed history utilizing the Bristol Stool Form Scale;8 however, patients’ recall of stool habit is often inaccurate, which results in suboptimal care.9,10 Prospective stool diaries can help to provide more objective assessment of patients’ symptoms, eliminate recall bias, and provide more reliable information. Several useful questionnaires are available for clinical and research purposes to characterize lower-GI symptoms, including the Constipation Scoring System,11 Patient Assessment of Constipation Symptoms (PAC-SYM),12 and Patient Assessment of Constipation Quality of Life (PAC-QOL).2,13 The Constipation Stool digital app enhances accuracy of data capture and offers a reliable and user-friendly method for recording bowel symptoms for patients, clinicians, and clinical investigators.14
Diagnosis
The diagnosis of DD requires careful physical and digital rectal examination together with anorectal manometry and a balloon expulsion test. Defecography and colonic transit studies provide additional assessment.
Physical examination
Abdominal examination should include palpation for stool in the colon and identification of abdominal mass or fecal impaction.2A high-quality digital rectal examination can help to identify patients who could benefit from physiological testing to confirm and treat DD.15 Rectal examination is performed by placing examiner’s lubricated gloved right index finger in a patient’s rectum, with the examiner’s left hand on patient’s abdomen, and asking the patient to push and bear down as if defecating.15 The contraction of the abdominal muscles is felt using the left hand, while the anal sphincter relaxation and degree of perineal descent are felt using the right-hand index finger.15 A diagnosis of dyssynergia is suspected if the digital rectal examination reveals two or more of the following abnormalities: inability to contract abdominal muscles (lack of push effort), inability to relax or paradoxical contraction of the anal sphincter and/or puborectalis, or absence of perineal descent.15 Digital rectal examination has good sensitivity (75%), specificity (87%), and positive predictive value (97%) for DD.16
High resolution anorectal manometry
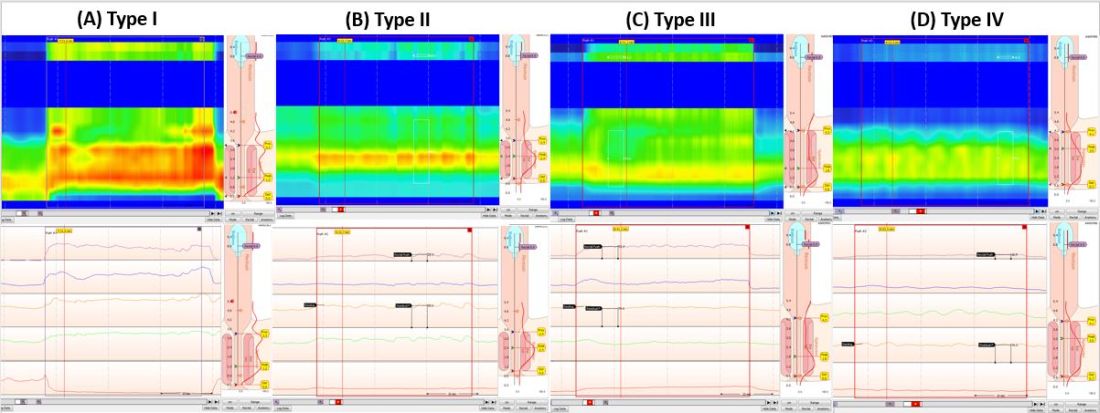
Anorectal manometry (ARM) is the preferred method for the evaluation of defecatory disorders.17,18 ARM is best performed using the high-resolution anorectal manometry (HRAM) systems19 that consist of a flexible probe – 0.5-cm diameter with multiple circumferential sensors along the anal canal – and another two sensors inside a rectal balloon.18 It provides a topographic and waveform display of manometric pressure data (Figure). The 3D high-definition ARM probe is a rigid 1-cm probe that provides 3D topographic profiles.18 ARM is typically performed in both the left lateral position and in a more physiological seated position.20,21 There is considerable variation amongst different institutions on how to perform HRAM, and a recent International Anorectal Physiology Working Group (IAPWG) has provided consensus recommendations for performing this test.22 The procedure for performing HRAM is reviewed elsewhere, but the key elements are summarized below.18
Push maneuver: On HRAM, after the assessment of resting and squeeze anal sphincter pressures, the patient is asked to push or bear down as if to defecate while lying in left lateral decubitus position. The best of two attempts that closely mimics a normal bearing down maneuver is used for categorizing patient’s defecatory pattern.18 In patients with DD, at least four distinct dyssynergia phenotypes have been recognized (Figure),23 though recent studies suggest eight patterns.24 Defecation index (maximum rectal pressure/minimum residual anal pressure when bearing down) greater than 1.2 is considered normal.18
Simulated defecation on commode: The subject is asked to attempt defecation while seated on a commode with intrarectal balloon filled with 60 cc of air, and both the defecation pattern(s) and defecation index are calculated. A lack of coordinated push effort is highly suggestive of DD.21
Rectoanal Inhibitory Reflex (RAIR): RAIR describes the reflex relaxation of the internal anal sphincter after rectal distension. RAIR is dependent on intact autonomic ganglia and myenteric plexus25and is mediated by the release of nitric oxide and vasoactive intestinal peptide.26 The absence of RAIR suggests Hirschsprung disease.22.27.28
Rectal sensory testing: Intermittent balloon distension of the rectum with incremental volumes of air induces a range of rectal sensations that include first sensation, desire to defecate, urgency to defecate, and maximum tolerable volume. Rectal hyposensitivity is diagnosed when two or more sensory thresholds are higher than those seen in normal subjects29.30 and likely results from disruption of afferent gut-brain pathways, cortical perception/rectal wall dysfunction, or both.29 Rectal hyposensitivity affects 40% of patients with constipation30and is associated with DD but not delayed colonic transit.31 Rectal hyposensitivity may also be seen in patients with diabetes or fecal incontinence.18 About two-thirds of patients with rectal hyposensitivity have rectal hypercompliance, and some have megarectum.32 Some patients with DD have coexisting irritable bowel syndrome (IBS) and may have rectal hypersensitivity.18,33 Rectal compliance is measured alongside rectal sensitivity analysis by plotting a graph between the change in intraballoon volume (mL) and change in intrarectal pressures (mm Hg) during incremental balloon distensions.18.34 Rectal hypercompliance may be seen in megarectum and dyssynergic defecation.34,35 Rectal hypocompliance may be seen in patients with inflammatory bowel disease, postpelvic radiation, chronic ischemia, and advanced age.18
Balloon expulsion test: This test is performed by placing a plastic probe with a balloon in the rectum and filling it with 50 cc of warm water. Patients are given 5 minutes to expel the balloon while sitting on a commode. Balloon expulsion time of more than 1 minute suggests a diagnosis of DD,21 although 2 minutes provides a higher level of agreement with manometric findings.36 Balloon type and body position can influence the results.37 Inability to expel the balloon with normal manometric findings is considered an inconclusive finding per the recent London Classification (i.e., it may be associated with generation of anorectal symptoms, but the clinical relevance of this finding is unclear as it may also be seen in healthy subjects).22
Defecography
Defecography is a dynamic fluoroscopic study performed in the sitting position after injecting 150 mL of barium paste into the patient’s rectum. Defecography provides useful information about structural changes (e.g., rectoceles, enteroceles, rectal prolapse, and intussusception), DD, and descending perineum syndrome.38 Methodological differences, radiation exposure, and poor interobserver agreement have limited its wider use; therefore, anorectal manometry and the balloon expulsion test are recommended for the initial evaluation of DD.39 Magnetic resonance defecography may be more useful.17,38
Colonic transit studies
Colonic transit study can be assessed using radiopaque markers, wireless motility capsule, or scintigraphy. Wireless motility capsule and scintigraphy have the advantage of determining gastric, small bowel, and whole gut transit times as well. About two-thirds of patients with DD have slow transit constipation (STC),6 which improves after treatment of DD.40 Hence, in patients with chronic constipation, evaluation and management of DD is recommended first. If symptoms persist, then consider colonic transit assessment.41 Given the overlapping nature of the conditions, documentation of STC at the outset could facilitate treatment of both.
Diagnostic criteria for DD
Patients should fulfill the following criteria for diagnosis of DD:42,43
- Fulfill symptom(s) diagnostic criteria for functional constipation and/or constipation-predominant IBS.
- Demonstrate dyssynergic pattern (Types I-IV; Figure) during attempted defecation on manometry recordings.
- Meet one or more of the following criteria:
- Inability to expel an artificial stool (50 mL water-filled balloon) within 1 minute.
- Inability to evacuate or retention of 50% or more of barium during defecography. (Some institutions use a prolonged colonic transit time: greater than 5 markers or 20% or higher marker retention on a plain abdominal x-Ray at 120 hours after ingestion of one radio-opaque marker capsule containing 24 radio-opaque markers.)
Treatment of DD
The treatment modalities for DD depend on several factors: patient’s age, comorbidities, underlying pathophysiology, and patient expectations. Treatment options include standard management of constipation, but biofeedback therapy is the mainstay.
Standard management
Medications that cause or worsen constipation should be avoided. The patient should consume adequate fluid and exercise regularly. Patients should receive instructions for timed toilet training (twice daily, 30 minutes after meals). Patients should push at about 50%-70% of their ability for no longer than 5 minutes and avoid postponing defecation or use of digital maneuvers to facilitate defecation.42 The patients should take 25 g of soluble fiber (e.g., psyllium) daily. Of note, the benefits of fiber can take days to weeks44 and may be limited in patients with STC and DD.45 Medications including laxatives and intestinal secretagogues (lubiprostone, linaclotide, plecanatide), and enterokinetic agents (prucalopride) can be used as adjunct therapy for management of DD.42 Their use is titrated during and after biofeedback therapy and may decrease after successful treatment.46
Biofeedback therapy
Biofeedback therapy involves operant conditioning techniques using either a solid state anorectal manometry system, electromyography, simulated balloon, or home biofeedback training devices.42,47 The goals of biofeedback therapy are to correct the abdominal pelvic muscle discoordination during defecation and improve rectal sensation to stool if impaired. Biofeedback therapy involves patient education and active training (typically six sessions, 1-2 weeks apart, with each about 30-60 minutes long), followed by a reinforcement stage (three sessions at 3, 6, and 12 months), though there are variations in training protocols.42
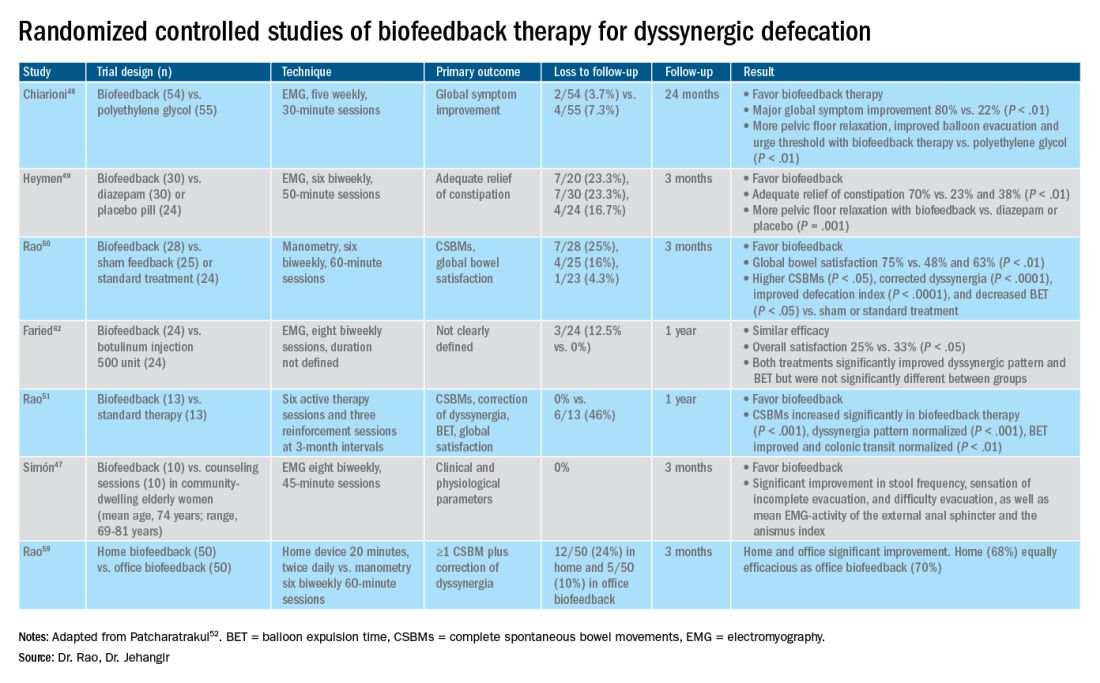
The success of biofeedback therapy depends on the patient’s motivation and the therapist’s skills.42 Compared with standard therapy (diet, exercise, pharmacotherapy), biofeedback therapy provides sustained improvement of bowel symptoms and anorectal function. Up to 70%-80% of DD patients show significant improvement of symptoms in randomized controlled trials (Table).48-52 Biofeedback therapy may also improve dyspeptic symptoms.53 Patients with harder stool consistency, greater willingness to participate, lower baseline bowel satisfaction, lower baseline anal sphincter relaxation, and prolonged balloon expulsion time, as well as patients who used digital maneuvers for defection, more commonly respond to biofeedback therapy.54,55 Longstanding laxative use has been associated with decreased response to biofeedback therapy.56 In patients with rectal hyposensitivity, barostat-assisted sensory training is more effective than a hand-held syringe technique.30 In patients with constipation predominant IBS and rectal hyposensitivity, sensory adaption training is more efficacious and better tolerated than escitalopram.30 Biofeedback therapy was afforded a grade A recommendation for treatment of DD by the American and European Societies of Neurogastroenterology and Motility.57
The access to office-based biofeedback therapy may be limited because of costs and low availability. The time required to attend multiple sessions may be burdensome for some patients, especially if they are taking time off from work. A recent study showed that patients with higher level of education may be less likely to adhere to biofeedback therapy.58 Recently, home biofeedback was shown to be noninferior to office biofeedback and was more cost-effective, which provides an alternative option for treating more patients.59
Endoscopic/surgical options
Other less effective treatment options for DD include botulinum toxin injection and myectomy.60-62 Botulinum toxin injection appears to have mixed effects with less than 50% of patients reporting symptomatic improvement, and it may cause fecal incontinence.60,63
Conclusion
DD is a common yet poorly recognized cause of constipation. Its clinical presentation overlaps with other lower-GI disorders. Its diagnosis requires detailed history, digital rectal examination, prospective stool diaries, anorectal manometry, and balloon expulsion tests. Biofeedback therapy offers excellent and sustained symptomatic improvement; however, access to office-based biofeedback is limited, and there is an urgent need for home-based biofeedback therapy programs.59
Dr. Rao is J. Harold Harrison Distinguished University Chair, professor of medicine, director of neurogastroenterology/motility, and director of digestive health at the Digestive Health Clinical Research Center Augusta (Georgia) University. He is supported by National Institutes of Health grants R01DK121003-02 and U01DK115572. Dr. Jehangir is a gastroenterology and Hepatology Fellow at the Digestive Health Clinical Research Center at Augusta University. They reported having no conflicts of interest.
References
1. Peery AF, et al. Gastroenterology. 2012;143(5):1179-1187.e3 .
2. Curtin B, et al. J Neurogastroenterol Motil. 2020 30;26(4):423-36.
3. Suares NC & Ford AC. Am J Gastroenterol. 2011 Sep;106(9):1582-91.
4. Mertz H, et al. Am J Gastroenterol. 1999;94(3):609-15.
5. Rao SS, et al. Am J Gastroenterol. 1998;93(7):1042-50.
6. Rao SSC, et al. J Clin Gastroenterol. 2004;38(8):680-5.
7. Nojkov B, et al. Am J Gastroenterol. 2019;114(11):1772-7.
8. Heaton KW, et al. Gut. 1992;33(6):818-24.
9. Prichard DO & Bharucha AE. 2018 Oct 15;7:F1000 Faculty Rev-1640.
10. Ashraf W, et al. Am J Gastroenterol. 1996;91(1):26-32.
11. Agachan F, et al.. Dis Colon Rectum. 1996;39(6):681-5.
12. Frank L, et al. Scand J Gastroenterol. 1999;34(9):870-7.
13. Marquis P, et al. Scand J Gastroenterol. 2005;40(5):540-51.
14. Yan Y, et al. Gastroenterology. 2020;158(6):S-400.
15. Rao SSC. Am J Gastroenterol. 2018;113(5):635-8.
16. Tantiphlachiva K, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8(11):955-60.
17. Carrington EV, et al. Nat Rev Gastroenterol Hepatol. 2018;15(5):309-23.
18. Tetangco EP, et al. Performing and analyzing high-resolution anorectal manometry. NeuroGastroLatam Rev. 2018;2:120-32.
19. Lee YY, et al. Curr Gastroenterol Rep. 2013;15(12):360.
20. Sharma M, et al. Neurogastroenterol Motil. 2020;32(10):e13910.
21. Rao SSC, et al.. Am J Gastroenterol. 2006;101(12):2790-6.
22. Carrington EV, et al. Neurogastroenterol Motil. 2020;32(1):e13679.
23. Rao SSC. Gastroenterol Clin North Am. 2008;37(3):569-86, viii.
24. Rao SSC, et al. Gastroenterology. 2016;150(4):S158-9.
25. Guinet A, et al. Int J Colorectal Dis. 2011;26(4):507-13.
26. Rattan S, et al. Gastroenterology. 1992;103(1):43-50.
27. Remes-Troche JM & Rao SSC. 2008;2(3):323-35.
28. Zaafouri H, et al..Int J Surgery. 2015. 2(1):9-17.
29. Remes-Troche JM, et al. Dis Colon Rectum. 2010;53(7):1047-54.
30. Rao SSC, et al. Gastroenterology. 2013;144(5):S-363.
31. Yu T, et al. Medicine (Baltimore). 2016;95(19):e3667.
32. Gladman MA, et al. Neurogastroenterol Motil. 2009;21(5):508-16, e4-5.
33. Lee KJ, et al. Digestion. 2006;73(2-3):133-41 .
34. Rao SSC, et al. Neurogastroenterol Motil. 2002;14(5):553-9.
35. Coss-Adame E, et al.. Clin Gastroenterol Hepatol. 2015;13(6):1143-1150.e1.
36. Chiarioni G, et al. Clin Gastroenterol Hepatol. 2014;12(12):2049-54.
37. Gu G, et al. Gastroenterology. 2018;154(6):S-545–S-546.
38. Savoye-Collet C, et al.. Gastroenterol Clin North Am. 2008;37(3):553-67, viii.
39. Videlock EJ, et al. Neurogastroenterol Motil. 2013;25(6):509-20.
40. Rao SSC, et al. Neurogastroenterol Motil. 2004;16(5):589-96.
41. Wald A, et al. Am J Gastroenterol. 2014;109(8):1141-57 ; (Quiz) 1058.
42. Rao SSC & Patcharatrakul T. J Neurogastroenterol Motil. 2016;22(3):423-35.
43. Rao SS, et al. Functional Anorectal Disorders. Gastroenterology. 2016. S0016-5085(16)00175-X.
44. Bharucha AE, et al.. Gastroenterology. 2013;144(1):218-38.
45. Voderholzer WA, et al. Am J Gastroenterol. 1997;92(1):95-8.
46. Lee HJ, et al. Neurogastroenterol Motil. 2015;27(6):787-95.
47. Simón MA & Bueno AM. J Clin Gastroenterol. 2017;51(10):e90-4.
48. Chiarioni G,et al.. Gastroenterology. 2006;130(3):657-64.
49. Heymen S, et al.. Dis Colon Rectum. 2007;50(4):428-41.
50. Rao SSC, et al. Clin Gastroenterol Hepatol. 2007;5(3):331-8.
51. Rao SSC, et al. Am J Gastroenterol. 2010;105(4):890-6.
52. Patcharatrakul T, et al. Biofeedback therapy. In Clinical and basic neurogastroenterology and motility. India: Stacy Masucci; 2020:517-32.
53. Huaman J-W, et al. Clin Gastroenterol Hepatol. 2020;18(11):2463-2470.e1.
54. Patcharatrakul T, et al. Clin Gastroenterol Hepatol. 2018;16(5):715-21.
55. Chaudhry A, et al. Gastroenterology. 2020;158(6):S-382–S-383.
56. Shim LSE, et al. Aliment Pharmacol Ther. 2011;33(11):1245-51.
57. Rao SSC, et al. Neurogastroenterol Motil. 2015;27(5):594-609.
58. Jangsirikul S, et al. Gastroenterology. 2020;158(6):S-383.
59. Rao SSC, et al. Am J Gastroenterol. 2019;114(6):938-44.
60. Ron Y, et al.. Dis Colon Rectum. 2001;44(12):1821-6.
61. Podzemny V, et al. World J Gastroenterol. 2015;21(4):1053-60.
62. Faried M, et al. J Gastrointest Surg. 2010;14(8):1235-43.
63. Hallan RI, et al. Lancet. 1988;2(8613):714-7.
Introduction
About 40% of the population experiences lower GI symptoms suggestive of gastrointestinal motility disorders.1,2 The global prevalence of chronic constipation is 18%, and the condition includes multiple overlapping subtypes.3 Evacuation disorders affect over half (59%) of patients and include dyssynergic defecation (DD).4 The inability to coordinate the abdominal, rectal, pelvic floor, and anal/puborectalis muscles to evacuate stools causes DD.5 The etiology of DD remains unclear and is often misdiagnosed. Clinically, the symptoms of DD overlap with other lower GI disorders, often leading to unnecessary and invasive procedures.2 We describe the clinical characteristics, diagnostic tools, treatment options, and evidence-based approach for the management of DD.

Clinical presentation
Over two-thirds of patients with DD acquire this disorder during adulthood, and one-third have symptoms from childhood.6 Though there is not usually an inciting event, 29% of patients report that symptoms began after events such as pregnancy or back injury,6 and opioid users have higher prevalence and severity of DD.7
Over 80% of patients report excessive straining, feelings of incomplete evacuation, and hard stools, and 50% report sensation of anal blockage or use of digital maneuvers.2 Other symptoms include infrequent bowel movements, abdominal pain, anal pain, and stool leakage.2 Evaluation of DD includes obtaining a detailed history utilizing the Bristol Stool Form Scale;8 however, patients’ recall of stool habit is often inaccurate, which results in suboptimal care.9,10 Prospective stool diaries can help to provide more objective assessment of patients’ symptoms, eliminate recall bias, and provide more reliable information. Several useful questionnaires are available for clinical and research purposes to characterize lower-GI symptoms, including the Constipation Scoring System,11 Patient Assessment of Constipation Symptoms (PAC-SYM),12 and Patient Assessment of Constipation Quality of Life (PAC-QOL).2,13 The Constipation Stool digital app enhances accuracy of data capture and offers a reliable and user-friendly method for recording bowel symptoms for patients, clinicians, and clinical investigators.14
Diagnosis
The diagnosis of DD requires careful physical and digital rectal examination together with anorectal manometry and a balloon expulsion test. Defecography and colonic transit studies provide additional assessment.
Physical examination
Abdominal examination should include palpation for stool in the colon and identification of abdominal mass or fecal impaction.2A high-quality digital rectal examination can help to identify patients who could benefit from physiological testing to confirm and treat DD.15 Rectal examination is performed by placing examiner’s lubricated gloved right index finger in a patient’s rectum, with the examiner’s left hand on patient’s abdomen, and asking the patient to push and bear down as if defecating.15 The contraction of the abdominal muscles is felt using the left hand, while the anal sphincter relaxation and degree of perineal descent are felt using the right-hand index finger.15 A diagnosis of dyssynergia is suspected if the digital rectal examination reveals two or more of the following abnormalities: inability to contract abdominal muscles (lack of push effort), inability to relax or paradoxical contraction of the anal sphincter and/or puborectalis, or absence of perineal descent.15 Digital rectal examination has good sensitivity (75%), specificity (87%), and positive predictive value (97%) for DD.16
High resolution anorectal manometry

Anorectal manometry (ARM) is the preferred method for the evaluation of defecatory disorders.17,18 ARM is best performed using the high-resolution anorectal manometry (HRAM) systems19 that consist of a flexible probe – 0.5-cm diameter with multiple circumferential sensors along the anal canal – and another two sensors inside a rectal balloon.18 It provides a topographic and waveform display of manometric pressure data (Figure). The 3D high-definition ARM probe is a rigid 1-cm probe that provides 3D topographic profiles.18 ARM is typically performed in both the left lateral position and in a more physiological seated position.20,21 There is considerable variation amongst different institutions on how to perform HRAM, and a recent International Anorectal Physiology Working Group (IAPWG) has provided consensus recommendations for performing this test.22 The procedure for performing HRAM is reviewed elsewhere, but the key elements are summarized below.18
Push maneuver: On HRAM, after the assessment of resting and squeeze anal sphincter pressures, the patient is asked to push or bear down as if to defecate while lying in left lateral decubitus position. The best of two attempts that closely mimics a normal bearing down maneuver is used for categorizing patient’s defecatory pattern.18 In patients with DD, at least four distinct dyssynergia phenotypes have been recognized (Figure),23 though recent studies suggest eight patterns.24 Defecation index (maximum rectal pressure/minimum residual anal pressure when bearing down) greater than 1.2 is considered normal.18
Simulated defecation on commode: The subject is asked to attempt defecation while seated on a commode with intrarectal balloon filled with 60 cc of air, and both the defecation pattern(s) and defecation index are calculated. A lack of coordinated push effort is highly suggestive of DD.21
Rectoanal Inhibitory Reflex (RAIR): RAIR describes the reflex relaxation of the internal anal sphincter after rectal distension. RAIR is dependent on intact autonomic ganglia and myenteric plexus25and is mediated by the release of nitric oxide and vasoactive intestinal peptide.26 The absence of RAIR suggests Hirschsprung disease.22.27.28
Rectal sensory testing: Intermittent balloon distension of the rectum with incremental volumes of air induces a range of rectal sensations that include first sensation, desire to defecate, urgency to defecate, and maximum tolerable volume. Rectal hyposensitivity is diagnosed when two or more sensory thresholds are higher than those seen in normal subjects29.30 and likely results from disruption of afferent gut-brain pathways, cortical perception/rectal wall dysfunction, or both.29 Rectal hyposensitivity affects 40% of patients with constipation30and is associated with DD but not delayed colonic transit.31 Rectal hyposensitivity may also be seen in patients with diabetes or fecal incontinence.18 About two-thirds of patients with rectal hyposensitivity have rectal hypercompliance, and some have megarectum.32 Some patients with DD have coexisting irritable bowel syndrome (IBS) and may have rectal hypersensitivity.18,33 Rectal compliance is measured alongside rectal sensitivity analysis by plotting a graph between the change in intraballoon volume (mL) and change in intrarectal pressures (mm Hg) during incremental balloon distensions.18.34 Rectal hypercompliance may be seen in megarectum and dyssynergic defecation.34,35 Rectal hypocompliance may be seen in patients with inflammatory bowel disease, postpelvic radiation, chronic ischemia, and advanced age.18
Balloon expulsion test: This test is performed by placing a plastic probe with a balloon in the rectum and filling it with 50 cc of warm water. Patients are given 5 minutes to expel the balloon while sitting on a commode. Balloon expulsion time of more than 1 minute suggests a diagnosis of DD,21 although 2 minutes provides a higher level of agreement with manometric findings.36 Balloon type and body position can influence the results.37 Inability to expel the balloon with normal manometric findings is considered an inconclusive finding per the recent London Classification (i.e., it may be associated with generation of anorectal symptoms, but the clinical relevance of this finding is unclear as it may also be seen in healthy subjects).22
Defecography
Defecography is a dynamic fluoroscopic study performed in the sitting position after injecting 150 mL of barium paste into the patient’s rectum. Defecography provides useful information about structural changes (e.g., rectoceles, enteroceles, rectal prolapse, and intussusception), DD, and descending perineum syndrome.38 Methodological differences, radiation exposure, and poor interobserver agreement have limited its wider use; therefore, anorectal manometry and the balloon expulsion test are recommended for the initial evaluation of DD.39 Magnetic resonance defecography may be more useful.17,38
Colonic transit studies
Colonic transit study can be assessed using radiopaque markers, wireless motility capsule, or scintigraphy. Wireless motility capsule and scintigraphy have the advantage of determining gastric, small bowel, and whole gut transit times as well. About two-thirds of patients with DD have slow transit constipation (STC),6 which improves after treatment of DD.40 Hence, in patients with chronic constipation, evaluation and management of DD is recommended first. If symptoms persist, then consider colonic transit assessment.41 Given the overlapping nature of the conditions, documentation of STC at the outset could facilitate treatment of both.
Diagnostic criteria for DD
Patients should fulfill the following criteria for diagnosis of DD:42,43
- Fulfill symptom(s) diagnostic criteria for functional constipation and/or constipation-predominant IBS.
- Demonstrate dyssynergic pattern (Types I-IV; Figure) during attempted defecation on manometry recordings.
- Meet one or more of the following criteria:
- Inability to expel an artificial stool (50 mL water-filled balloon) within 1 minute.
- Inability to evacuate or retention of 50% or more of barium during defecography. (Some institutions use a prolonged colonic transit time: greater than 5 markers or 20% or higher marker retention on a plain abdominal x-Ray at 120 hours after ingestion of one radio-opaque marker capsule containing 24 radio-opaque markers.)
Treatment of DD
The treatment modalities for DD depend on several factors: patient’s age, comorbidities, underlying pathophysiology, and patient expectations. Treatment options include standard management of constipation, but biofeedback therapy is the mainstay.
Standard management
Medications that cause or worsen constipation should be avoided. The patient should consume adequate fluid and exercise regularly. Patients should receive instructions for timed toilet training (twice daily, 30 minutes after meals). Patients should push at about 50%-70% of their ability for no longer than 5 minutes and avoid postponing defecation or use of digital maneuvers to facilitate defecation.42 The patients should take 25 g of soluble fiber (e.g., psyllium) daily. Of note, the benefits of fiber can take days to weeks44 and may be limited in patients with STC and DD.45 Medications including laxatives and intestinal secretagogues (lubiprostone, linaclotide, plecanatide), and enterokinetic agents (prucalopride) can be used as adjunct therapy for management of DD.42 Their use is titrated during and after biofeedback therapy and may decrease after successful treatment.46
Biofeedback therapy
Biofeedback therapy involves operant conditioning techniques using either a solid state anorectal manometry system, electromyography, simulated balloon, or home biofeedback training devices.42,47 The goals of biofeedback therapy are to correct the abdominal pelvic muscle discoordination during defecation and improve rectal sensation to stool if impaired. Biofeedback therapy involves patient education and active training (typically six sessions, 1-2 weeks apart, with each about 30-60 minutes long), followed by a reinforcement stage (three sessions at 3, 6, and 12 months), though there are variations in training protocols.42

The success of biofeedback therapy depends on the patient’s motivation and the therapist’s skills.42 Compared with standard therapy (diet, exercise, pharmacotherapy), biofeedback therapy provides sustained improvement of bowel symptoms and anorectal function. Up to 70%-80% of DD patients show significant improvement of symptoms in randomized controlled trials (Table).48-52 Biofeedback therapy may also improve dyspeptic symptoms.53 Patients with harder stool consistency, greater willingness to participate, lower baseline bowel satisfaction, lower baseline anal sphincter relaxation, and prolonged balloon expulsion time, as well as patients who used digital maneuvers for defection, more commonly respond to biofeedback therapy.54,55 Longstanding laxative use has been associated with decreased response to biofeedback therapy.56 In patients with rectal hyposensitivity, barostat-assisted sensory training is more effective than a hand-held syringe technique.30 In patients with constipation predominant IBS and rectal hyposensitivity, sensory adaption training is more efficacious and better tolerated than escitalopram.30 Biofeedback therapy was afforded a grade A recommendation for treatment of DD by the American and European Societies of Neurogastroenterology and Motility.57
The access to office-based biofeedback therapy may be limited because of costs and low availability. The time required to attend multiple sessions may be burdensome for some patients, especially if they are taking time off from work. A recent study showed that patients with higher level of education may be less likely to adhere to biofeedback therapy.58 Recently, home biofeedback was shown to be noninferior to office biofeedback and was more cost-effective, which provides an alternative option for treating more patients.59
Endoscopic/surgical options
Other less effective treatment options for DD include botulinum toxin injection and myectomy.60-62 Botulinum toxin injection appears to have mixed effects with less than 50% of patients reporting symptomatic improvement, and it may cause fecal incontinence.60,63
Conclusion
DD is a common yet poorly recognized cause of constipation. Its clinical presentation overlaps with other lower-GI disorders. Its diagnosis requires detailed history, digital rectal examination, prospective stool diaries, anorectal manometry, and balloon expulsion tests. Biofeedback therapy offers excellent and sustained symptomatic improvement; however, access to office-based biofeedback is limited, and there is an urgent need for home-based biofeedback therapy programs.59
Dr. Rao is J. Harold Harrison Distinguished University Chair, professor of medicine, director of neurogastroenterology/motility, and director of digestive health at the Digestive Health Clinical Research Center Augusta (Georgia) University. He is supported by National Institutes of Health grants R01DK121003-02 and U01DK115572. Dr. Jehangir is a gastroenterology and Hepatology Fellow at the Digestive Health Clinical Research Center at Augusta University. They reported having no conflicts of interest.
References
1. Peery AF, et al. Gastroenterology. 2012;143(5):1179-1187.e3 .
2. Curtin B, et al. J Neurogastroenterol Motil. 2020 30;26(4):423-36.
3. Suares NC & Ford AC. Am J Gastroenterol. 2011 Sep;106(9):1582-91.
4. Mertz H, et al. Am J Gastroenterol. 1999;94(3):609-15.
5. Rao SS, et al. Am J Gastroenterol. 1998;93(7):1042-50.
6. Rao SSC, et al. J Clin Gastroenterol. 2004;38(8):680-5.
7. Nojkov B, et al. Am J Gastroenterol. 2019;114(11):1772-7.
8. Heaton KW, et al. Gut. 1992;33(6):818-24.
9. Prichard DO & Bharucha AE. 2018 Oct 15;7:F1000 Faculty Rev-1640.
10. Ashraf W, et al. Am J Gastroenterol. 1996;91(1):26-32.
11. Agachan F, et al.. Dis Colon Rectum. 1996;39(6):681-5.
12. Frank L, et al. Scand J Gastroenterol. 1999;34(9):870-7.
13. Marquis P, et al. Scand J Gastroenterol. 2005;40(5):540-51.
14. Yan Y, et al. Gastroenterology. 2020;158(6):S-400.
15. Rao SSC. Am J Gastroenterol. 2018;113(5):635-8.
16. Tantiphlachiva K, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8(11):955-60.
17. Carrington EV, et al. Nat Rev Gastroenterol Hepatol. 2018;15(5):309-23.
18. Tetangco EP, et al. Performing and analyzing high-resolution anorectal manometry. NeuroGastroLatam Rev. 2018;2:120-32.
19. Lee YY, et al. Curr Gastroenterol Rep. 2013;15(12):360.
20. Sharma M, et al. Neurogastroenterol Motil. 2020;32(10):e13910.
21. Rao SSC, et al.. Am J Gastroenterol. 2006;101(12):2790-6.
22. Carrington EV, et al. Neurogastroenterol Motil. 2020;32(1):e13679.
23. Rao SSC. Gastroenterol Clin North Am. 2008;37(3):569-86, viii.
24. Rao SSC, et al. Gastroenterology. 2016;150(4):S158-9.
25. Guinet A, et al. Int J Colorectal Dis. 2011;26(4):507-13.
26. Rattan S, et al. Gastroenterology. 1992;103(1):43-50.
27. Remes-Troche JM & Rao SSC. 2008;2(3):323-35.
28. Zaafouri H, et al..Int J Surgery. 2015. 2(1):9-17.
29. Remes-Troche JM, et al. Dis Colon Rectum. 2010;53(7):1047-54.
30. Rao SSC, et al. Gastroenterology. 2013;144(5):S-363.
31. Yu T, et al. Medicine (Baltimore). 2016;95(19):e3667.
32. Gladman MA, et al. Neurogastroenterol Motil. 2009;21(5):508-16, e4-5.
33. Lee KJ, et al. Digestion. 2006;73(2-3):133-41 .
34. Rao SSC, et al. Neurogastroenterol Motil. 2002;14(5):553-9.
35. Coss-Adame E, et al.. Clin Gastroenterol Hepatol. 2015;13(6):1143-1150.e1.
36. Chiarioni G, et al. Clin Gastroenterol Hepatol. 2014;12(12):2049-54.
37. Gu G, et al. Gastroenterology. 2018;154(6):S-545–S-546.
38. Savoye-Collet C, et al.. Gastroenterol Clin North Am. 2008;37(3):553-67, viii.
39. Videlock EJ, et al. Neurogastroenterol Motil. 2013;25(6):509-20.
40. Rao SSC, et al. Neurogastroenterol Motil. 2004;16(5):589-96.
41. Wald A, et al. Am J Gastroenterol. 2014;109(8):1141-57 ; (Quiz) 1058.
42. Rao SSC & Patcharatrakul T. J Neurogastroenterol Motil. 2016;22(3):423-35.
43. Rao SS, et al. Functional Anorectal Disorders. Gastroenterology. 2016. S0016-5085(16)00175-X.
44. Bharucha AE, et al.. Gastroenterology. 2013;144(1):218-38.
45. Voderholzer WA, et al. Am J Gastroenterol. 1997;92(1):95-8.
46. Lee HJ, et al. Neurogastroenterol Motil. 2015;27(6):787-95.
47. Simón MA & Bueno AM. J Clin Gastroenterol. 2017;51(10):e90-4.
48. Chiarioni G,et al.. Gastroenterology. 2006;130(3):657-64.
49. Heymen S, et al.. Dis Colon Rectum. 2007;50(4):428-41.
50. Rao SSC, et al. Clin Gastroenterol Hepatol. 2007;5(3):331-8.
51. Rao SSC, et al. Am J Gastroenterol. 2010;105(4):890-6.
52. Patcharatrakul T, et al. Biofeedback therapy. In Clinical and basic neurogastroenterology and motility. India: Stacy Masucci; 2020:517-32.
53. Huaman J-W, et al. Clin Gastroenterol Hepatol. 2020;18(11):2463-2470.e1.
54. Patcharatrakul T, et al. Clin Gastroenterol Hepatol. 2018;16(5):715-21.
55. Chaudhry A, et al. Gastroenterology. 2020;158(6):S-382–S-383.
56. Shim LSE, et al. Aliment Pharmacol Ther. 2011;33(11):1245-51.
57. Rao SSC, et al. Neurogastroenterol Motil. 2015;27(5):594-609.
58. Jangsirikul S, et al. Gastroenterology. 2020;158(6):S-383.
59. Rao SSC, et al. Am J Gastroenterol. 2019;114(6):938-44.
60. Ron Y, et al.. Dis Colon Rectum. 2001;44(12):1821-6.
61. Podzemny V, et al. World J Gastroenterol. 2015;21(4):1053-60.
62. Faried M, et al. J Gastrointest Surg. 2010;14(8):1235-43.
63. Hallan RI, et al. Lancet. 1988;2(8613):714-7.
Introduction
About 40% of the population experiences lower GI symptoms suggestive of gastrointestinal motility disorders.1,2 The global prevalence of chronic constipation is 18%, and the condition includes multiple overlapping subtypes.3 Evacuation disorders affect over half (59%) of patients and include dyssynergic defecation (DD).4 The inability to coordinate the abdominal, rectal, pelvic floor, and anal/puborectalis muscles to evacuate stools causes DD.5 The etiology of DD remains unclear and is often misdiagnosed. Clinically, the symptoms of DD overlap with other lower GI disorders, often leading to unnecessary and invasive procedures.2 We describe the clinical characteristics, diagnostic tools, treatment options, and evidence-based approach for the management of DD.

Clinical presentation
Over two-thirds of patients with DD acquire this disorder during adulthood, and one-third have symptoms from childhood.6 Though there is not usually an inciting event, 29% of patients report that symptoms began after events such as pregnancy or back injury,6 and opioid users have higher prevalence and severity of DD.7
Over 80% of patients report excessive straining, feelings of incomplete evacuation, and hard stools, and 50% report sensation of anal blockage or use of digital maneuvers.2 Other symptoms include infrequent bowel movements, abdominal pain, anal pain, and stool leakage.2 Evaluation of DD includes obtaining a detailed history utilizing the Bristol Stool Form Scale;8 however, patients’ recall of stool habit is often inaccurate, which results in suboptimal care.9,10 Prospective stool diaries can help to provide more objective assessment of patients’ symptoms, eliminate recall bias, and provide more reliable information. Several useful questionnaires are available for clinical and research purposes to characterize lower-GI symptoms, including the Constipation Scoring System,11 Patient Assessment of Constipation Symptoms (PAC-SYM),12 and Patient Assessment of Constipation Quality of Life (PAC-QOL).2,13 The Constipation Stool digital app enhances accuracy of data capture and offers a reliable and user-friendly method for recording bowel symptoms for patients, clinicians, and clinical investigators.14
Diagnosis
The diagnosis of DD requires careful physical and digital rectal examination together with anorectal manometry and a balloon expulsion test. Defecography and colonic transit studies provide additional assessment.
Physical examination
Abdominal examination should include palpation for stool in the colon and identification of abdominal mass or fecal impaction.2A high-quality digital rectal examination can help to identify patients who could benefit from physiological testing to confirm and treat DD.15 Rectal examination is performed by placing examiner’s lubricated gloved right index finger in a patient’s rectum, with the examiner’s left hand on patient’s abdomen, and asking the patient to push and bear down as if defecating.15 The contraction of the abdominal muscles is felt using the left hand, while the anal sphincter relaxation and degree of perineal descent are felt using the right-hand index finger.15 A diagnosis of dyssynergia is suspected if the digital rectal examination reveals two or more of the following abnormalities: inability to contract abdominal muscles (lack of push effort), inability to relax or paradoxical contraction of the anal sphincter and/or puborectalis, or absence of perineal descent.15 Digital rectal examination has good sensitivity (75%), specificity (87%), and positive predictive value (97%) for DD.16
High resolution anorectal manometry

Anorectal manometry (ARM) is the preferred method for the evaluation of defecatory disorders.17,18 ARM is best performed using the high-resolution anorectal manometry (HRAM) systems19 that consist of a flexible probe – 0.5-cm diameter with multiple circumferential sensors along the anal canal – and another two sensors inside a rectal balloon.18 It provides a topographic and waveform display of manometric pressure data (Figure). The 3D high-definition ARM probe is a rigid 1-cm probe that provides 3D topographic profiles.18 ARM is typically performed in both the left lateral position and in a more physiological seated position.20,21 There is considerable variation amongst different institutions on how to perform HRAM, and a recent International Anorectal Physiology Working Group (IAPWG) has provided consensus recommendations for performing this test.22 The procedure for performing HRAM is reviewed elsewhere, but the key elements are summarized below.18
Push maneuver: On HRAM, after the assessment of resting and squeeze anal sphincter pressures, the patient is asked to push or bear down as if to defecate while lying in left lateral decubitus position. The best of two attempts that closely mimics a normal bearing down maneuver is used for categorizing patient’s defecatory pattern.18 In patients with DD, at least four distinct dyssynergia phenotypes have been recognized (Figure),23 though recent studies suggest eight patterns.24 Defecation index (maximum rectal pressure/minimum residual anal pressure when bearing down) greater than 1.2 is considered normal.18
Simulated defecation on commode: The subject is asked to attempt defecation while seated on a commode with intrarectal balloon filled with 60 cc of air, and both the defecation pattern(s) and defecation index are calculated. A lack of coordinated push effort is highly suggestive of DD.21
Rectoanal Inhibitory Reflex (RAIR): RAIR describes the reflex relaxation of the internal anal sphincter after rectal distension. RAIR is dependent on intact autonomic ganglia and myenteric plexus25and is mediated by the release of nitric oxide and vasoactive intestinal peptide.26 The absence of RAIR suggests Hirschsprung disease.22.27.28
Rectal sensory testing: Intermittent balloon distension of the rectum with incremental volumes of air induces a range of rectal sensations that include first sensation, desire to defecate, urgency to defecate, and maximum tolerable volume. Rectal hyposensitivity is diagnosed when two or more sensory thresholds are higher than those seen in normal subjects29.30 and likely results from disruption of afferent gut-brain pathways, cortical perception/rectal wall dysfunction, or both.29 Rectal hyposensitivity affects 40% of patients with constipation30and is associated with DD but not delayed colonic transit.31 Rectal hyposensitivity may also be seen in patients with diabetes or fecal incontinence.18 About two-thirds of patients with rectal hyposensitivity have rectal hypercompliance, and some have megarectum.32 Some patients with DD have coexisting irritable bowel syndrome (IBS) and may have rectal hypersensitivity.18,33 Rectal compliance is measured alongside rectal sensitivity analysis by plotting a graph between the change in intraballoon volume (mL) and change in intrarectal pressures (mm Hg) during incremental balloon distensions.18.34 Rectal hypercompliance may be seen in megarectum and dyssynergic defecation.34,35 Rectal hypocompliance may be seen in patients with inflammatory bowel disease, postpelvic radiation, chronic ischemia, and advanced age.18
Balloon expulsion test: This test is performed by placing a plastic probe with a balloon in the rectum and filling it with 50 cc of warm water. Patients are given 5 minutes to expel the balloon while sitting on a commode. Balloon expulsion time of more than 1 minute suggests a diagnosis of DD,21 although 2 minutes provides a higher level of agreement with manometric findings.36 Balloon type and body position can influence the results.37 Inability to expel the balloon with normal manometric findings is considered an inconclusive finding per the recent London Classification (i.e., it may be associated with generation of anorectal symptoms, but the clinical relevance of this finding is unclear as it may also be seen in healthy subjects).22
Defecography
Defecography is a dynamic fluoroscopic study performed in the sitting position after injecting 150 mL of barium paste into the patient’s rectum. Defecography provides useful information about structural changes (e.g., rectoceles, enteroceles, rectal prolapse, and intussusception), DD, and descending perineum syndrome.38 Methodological differences, radiation exposure, and poor interobserver agreement have limited its wider use; therefore, anorectal manometry and the balloon expulsion test are recommended for the initial evaluation of DD.39 Magnetic resonance defecography may be more useful.17,38
Colonic transit studies
Colonic transit study can be assessed using radiopaque markers, wireless motility capsule, or scintigraphy. Wireless motility capsule and scintigraphy have the advantage of determining gastric, small bowel, and whole gut transit times as well. About two-thirds of patients with DD have slow transit constipation (STC),6 which improves after treatment of DD.40 Hence, in patients with chronic constipation, evaluation and management of DD is recommended first. If symptoms persist, then consider colonic transit assessment.41 Given the overlapping nature of the conditions, documentation of STC at the outset could facilitate treatment of both.
Diagnostic criteria for DD
Patients should fulfill the following criteria for diagnosis of DD:42,43
- Fulfill symptom(s) diagnostic criteria for functional constipation and/or constipation-predominant IBS.
- Demonstrate dyssynergic pattern (Types I-IV; Figure) during attempted defecation on manometry recordings.
- Meet one or more of the following criteria:
- Inability to expel an artificial stool (50 mL water-filled balloon) within 1 minute.
- Inability to evacuate or retention of 50% or more of barium during defecography. (Some institutions use a prolonged colonic transit time: greater than 5 markers or 20% or higher marker retention on a plain abdominal x-Ray at 120 hours after ingestion of one radio-opaque marker capsule containing 24 radio-opaque markers.)
Treatment of DD
The treatment modalities for DD depend on several factors: patient’s age, comorbidities, underlying pathophysiology, and patient expectations. Treatment options include standard management of constipation, but biofeedback therapy is the mainstay.
Standard management
Medications that cause or worsen constipation should be avoided. The patient should consume adequate fluid and exercise regularly. Patients should receive instructions for timed toilet training (twice daily, 30 minutes after meals). Patients should push at about 50%-70% of their ability for no longer than 5 minutes and avoid postponing defecation or use of digital maneuvers to facilitate defecation.42 The patients should take 25 g of soluble fiber (e.g., psyllium) daily. Of note, the benefits of fiber can take days to weeks44 and may be limited in patients with STC and DD.45 Medications including laxatives and intestinal secretagogues (lubiprostone, linaclotide, plecanatide), and enterokinetic agents (prucalopride) can be used as adjunct therapy for management of DD.42 Their use is titrated during and after biofeedback therapy and may decrease after successful treatment.46
Biofeedback therapy
Biofeedback therapy involves operant conditioning techniques using either a solid state anorectal manometry system, electromyography, simulated balloon, or home biofeedback training devices.42,47 The goals of biofeedback therapy are to correct the abdominal pelvic muscle discoordination during defecation and improve rectal sensation to stool if impaired. Biofeedback therapy involves patient education and active training (typically six sessions, 1-2 weeks apart, with each about 30-60 minutes long), followed by a reinforcement stage (three sessions at 3, 6, and 12 months), though there are variations in training protocols.42

The success of biofeedback therapy depends on the patient’s motivation and the therapist’s skills.42 Compared with standard therapy (diet, exercise, pharmacotherapy), biofeedback therapy provides sustained improvement of bowel symptoms and anorectal function. Up to 70%-80% of DD patients show significant improvement of symptoms in randomized controlled trials (Table).48-52 Biofeedback therapy may also improve dyspeptic symptoms.53 Patients with harder stool consistency, greater willingness to participate, lower baseline bowel satisfaction, lower baseline anal sphincter relaxation, and prolonged balloon expulsion time, as well as patients who used digital maneuvers for defection, more commonly respond to biofeedback therapy.54,55 Longstanding laxative use has been associated with decreased response to biofeedback therapy.56 In patients with rectal hyposensitivity, barostat-assisted sensory training is more effective than a hand-held syringe technique.30 In patients with constipation predominant IBS and rectal hyposensitivity, sensory adaption training is more efficacious and better tolerated than escitalopram.30 Biofeedback therapy was afforded a grade A recommendation for treatment of DD by the American and European Societies of Neurogastroenterology and Motility.57
The access to office-based biofeedback therapy may be limited because of costs and low availability. The time required to attend multiple sessions may be burdensome for some patients, especially if they are taking time off from work. A recent study showed that patients with higher level of education may be less likely to adhere to biofeedback therapy.58 Recently, home biofeedback was shown to be noninferior to office biofeedback and was more cost-effective, which provides an alternative option for treating more patients.59
Endoscopic/surgical options
Other less effective treatment options for DD include botulinum toxin injection and myectomy.60-62 Botulinum toxin injection appears to have mixed effects with less than 50% of patients reporting symptomatic improvement, and it may cause fecal incontinence.60,63
Conclusion
DD is a common yet poorly recognized cause of constipation. Its clinical presentation overlaps with other lower-GI disorders. Its diagnosis requires detailed history, digital rectal examination, prospective stool diaries, anorectal manometry, and balloon expulsion tests. Biofeedback therapy offers excellent and sustained symptomatic improvement; however, access to office-based biofeedback is limited, and there is an urgent need for home-based biofeedback therapy programs.59
Dr. Rao is J. Harold Harrison Distinguished University Chair, professor of medicine, director of neurogastroenterology/motility, and director of digestive health at the Digestive Health Clinical Research Center Augusta (Georgia) University. He is supported by National Institutes of Health grants R01DK121003-02 and U01DK115572. Dr. Jehangir is a gastroenterology and Hepatology Fellow at the Digestive Health Clinical Research Center at Augusta University. They reported having no conflicts of interest.
References
1. Peery AF, et al. Gastroenterology. 2012;143(5):1179-1187.e3 .
2. Curtin B, et al. J Neurogastroenterol Motil. 2020 30;26(4):423-36.
3. Suares NC & Ford AC. Am J Gastroenterol. 2011 Sep;106(9):1582-91.
4. Mertz H, et al. Am J Gastroenterol. 1999;94(3):609-15.
5. Rao SS, et al. Am J Gastroenterol. 1998;93(7):1042-50.
6. Rao SSC, et al. J Clin Gastroenterol. 2004;38(8):680-5.
7. Nojkov B, et al. Am J Gastroenterol. 2019;114(11):1772-7.
8. Heaton KW, et al. Gut. 1992;33(6):818-24.
9. Prichard DO & Bharucha AE. 2018 Oct 15;7:F1000 Faculty Rev-1640.
10. Ashraf W, et al. Am J Gastroenterol. 1996;91(1):26-32.
11. Agachan F, et al.. Dis Colon Rectum. 1996;39(6):681-5.
12. Frank L, et al. Scand J Gastroenterol. 1999;34(9):870-7.
13. Marquis P, et al. Scand J Gastroenterol. 2005;40(5):540-51.
14. Yan Y, et al. Gastroenterology. 2020;158(6):S-400.
15. Rao SSC. Am J Gastroenterol. 2018;113(5):635-8.
16. Tantiphlachiva K, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8(11):955-60.
17. Carrington EV, et al. Nat Rev Gastroenterol Hepatol. 2018;15(5):309-23.
18. Tetangco EP, et al. Performing and analyzing high-resolution anorectal manometry. NeuroGastroLatam Rev. 2018;2:120-32.
19. Lee YY, et al. Curr Gastroenterol Rep. 2013;15(12):360.
20. Sharma M, et al. Neurogastroenterol Motil. 2020;32(10):e13910.
21. Rao SSC, et al.. Am J Gastroenterol. 2006;101(12):2790-6.
22. Carrington EV, et al. Neurogastroenterol Motil. 2020;32(1):e13679.
23. Rao SSC. Gastroenterol Clin North Am. 2008;37(3):569-86, viii.
24. Rao SSC, et al. Gastroenterology. 2016;150(4):S158-9.
25. Guinet A, et al. Int J Colorectal Dis. 2011;26(4):507-13.
26. Rattan S, et al. Gastroenterology. 1992;103(1):43-50.
27. Remes-Troche JM & Rao SSC. 2008;2(3):323-35.
28. Zaafouri H, et al..Int J Surgery. 2015. 2(1):9-17.
29. Remes-Troche JM, et al. Dis Colon Rectum. 2010;53(7):1047-54.
30. Rao SSC, et al. Gastroenterology. 2013;144(5):S-363.
31. Yu T, et al. Medicine (Baltimore). 2016;95(19):e3667.
32. Gladman MA, et al. Neurogastroenterol Motil. 2009;21(5):508-16, e4-5.
33. Lee KJ, et al. Digestion. 2006;73(2-3):133-41 .
34. Rao SSC, et al. Neurogastroenterol Motil. 2002;14(5):553-9.
35. Coss-Adame E, et al.. Clin Gastroenterol Hepatol. 2015;13(6):1143-1150.e1.
36. Chiarioni G, et al. Clin Gastroenterol Hepatol. 2014;12(12):2049-54.
37. Gu G, et al. Gastroenterology. 2018;154(6):S-545–S-546.
38. Savoye-Collet C, et al.. Gastroenterol Clin North Am. 2008;37(3):553-67, viii.
39. Videlock EJ, et al. Neurogastroenterol Motil. 2013;25(6):509-20.
40. Rao SSC, et al. Neurogastroenterol Motil. 2004;16(5):589-96.
41. Wald A, et al. Am J Gastroenterol. 2014;109(8):1141-57 ; (Quiz) 1058.
42. Rao SSC & Patcharatrakul T. J Neurogastroenterol Motil. 2016;22(3):423-35.
43. Rao SS, et al. Functional Anorectal Disorders. Gastroenterology. 2016. S0016-5085(16)00175-X.
44. Bharucha AE, et al.. Gastroenterology. 2013;144(1):218-38.
45. Voderholzer WA, et al. Am J Gastroenterol. 1997;92(1):95-8.
46. Lee HJ, et al. Neurogastroenterol Motil. 2015;27(6):787-95.
47. Simón MA & Bueno AM. J Clin Gastroenterol. 2017;51(10):e90-4.
48. Chiarioni G,et al.. Gastroenterology. 2006;130(3):657-64.
49. Heymen S, et al.. Dis Colon Rectum. 2007;50(4):428-41.
50. Rao SSC, et al. Clin Gastroenterol Hepatol. 2007;5(3):331-8.
51. Rao SSC, et al. Am J Gastroenterol. 2010;105(4):890-6.
52. Patcharatrakul T, et al. Biofeedback therapy. In Clinical and basic neurogastroenterology and motility. India: Stacy Masucci; 2020:517-32.
53. Huaman J-W, et al. Clin Gastroenterol Hepatol. 2020;18(11):2463-2470.e1.
54. Patcharatrakul T, et al. Clin Gastroenterol Hepatol. 2018;16(5):715-21.
55. Chaudhry A, et al. Gastroenterology. 2020;158(6):S-382–S-383.
56. Shim LSE, et al. Aliment Pharmacol Ther. 2011;33(11):1245-51.
57. Rao SSC, et al. Neurogastroenterol Motil. 2015;27(5):594-609.
58. Jangsirikul S, et al. Gastroenterology. 2020;158(6):S-383.
59. Rao SSC, et al. Am J Gastroenterol. 2019;114(6):938-44.
60. Ron Y, et al.. Dis Colon Rectum. 2001;44(12):1821-6.
61. Podzemny V, et al. World J Gastroenterol. 2015;21(4):1053-60.
62. Faried M, et al. J Gastrointest Surg. 2010;14(8):1235-43.
63. Hallan RI, et al. Lancet. 1988;2(8613):714-7.
When should antibiotics be used in acute uncomplicated diverticulitis?
Dear colleagues and friends,
The Perspectives series returns, this time with an exciting discussion about antibiotic use in acute uncomplicated diverticulitis. It has been fascinating to witness this field evolve from an era where not using antibiotics was inconceivable! Dr. Anne F. Peery and Dr. Neil Stollman, both recognized experts in the matter, provide arguments to both sides of the debate, as well as much-needed nuance. As always, I welcome your comments and suggestions for future topics at [email protected]. Thank you for your support, and I hope you will enjoy the reading and learning from this as much as I did.
Charles J. Kahi, MD, MS, AGAF, is a professor of medicine at Indiana University, Indianapolis. He is also an associate editor for GI & Hepatology News.
Think carefully about when to withhold
For decades, it was standard practice to give antibiotics to all patients with acute uncomplicated diverticulitis (AUD). While most patients with a first diagnosis of AUD recover within a few weeks, a small proportion will develop a complication.1 Among generally healthy patients with an initial diagnosis of AUD, about 3% will progress to complicated diverticulitis, and about 1% will require emergency surgery within 6 months. Around another 6% of cases will develop chronic diverticulitis with ongoing diverticular inflammation that persists for weeks to months.
Because the complications are uncommon, we don’t know if antibiotics reduce the risk of progression to complicated diverticulitis, emergency surgery, or the development of chronic diverticulitis. Investigating these patient-centered and morbid outcomes would require trials enrolling thousands of patients and to following these patients for months. This trial hasn’t happened yet.
To date, only small studies have compared the use of antibiotics with no antibiotics in patients with AUD. A review sponsored by the Agency for Healthcare Research and Quality published last year concluded that the current evidence was too sparse or too inconsistent to make strong conclusions about the use of antibiotics for patients with uncomplicated diverticulitis.2
With little evidence for or against antibiotics, recent guidelines have begun to recommend that antibiotics be used selectively, rather than routinely, in patients with diverticulitis.3 “Selectively” clearly means that there are some patients who should receive antibiotics, but the guidelines are vague about who those patients are. To this end, it is safest to refer to those small, underpowered trials to identify which patients are at the greatest risk of developing a complication.1,4 The authors of those trials considered a number of groups high risk and therefore excluded them from those trials. In the absence of further definitive research, it seems clear that those groups, listed below, should therefore be selected for antibiotic treatment:
- Patients with complicated diverticulitis including paracolic extraluminal air on CT scan.
- Patients who are immunocompromised.
- Patients with a high fever, affected general condition, or clinical suspicion of sepsis.
- Patients with inflammatory bowel disease.
- Patients who are pregnant or breast feeding.
As with most clinical trials, participants in these smaller trials were younger (median age, late 50s) and healthier (63% normal, healthy patient; 34% mild systemic disease; 4% severe systemic disease) than the general population. In secondary analyses, however, several factors were independently associated with a complicated disease course after an initial diagnosis of acute uncomplicated diverticulitis. As with the first list above, the following high-risk patients should also be treated with antibiotics at diagnosis:
- Patients with American Society of Anesthesiologists scores III or IV were 4.4 times more likely to have a poor outcome, compared with those with ASA score I.
- Patients with ASA score II were 2.0 times more likely to have a poor outcome, compared with ASA score I.
- Patients with symptoms for more than 5 days at diagnosis were 3.3 times more likely to have a poor outcome, compared with those with symptoms for 5 days or less.
- Patients with vomiting at diagnosis were 3.9 times more likely to have a poor outcome, compared with those who were not vomiting.
- Patients with C-reactive protein levels higher than 140 mg/L at diagnosis were 2.9 times more likely to have a poor outcome, compared with C-reactive protein level of 140 mg/L or less.
- Patients with white blood cell count greater than 15 x 109 cells/L at diagnosis were 3.7 times more likely to have a poor outcome, compared with those with 15 x 109 cells/L.
- Patients with a longer segment (>86mm) of inflamed colon on CT scan were more likely to have a poor outcome, compared those who had a shorter segment (<65mm).
To help clinicians think about antibiotic treatment in patients with AUD, a recent American Gastroenterological Association clinical practice update provided the following advice: First, antibiotic treatment is advised in patients with uncomplicated diverticulitis who have comorbidities or are frail, who present with refractory symptoms or vomiting, or who have a C-reactive protein level greater than 140 mg/L, or baseline white blood cell count greater than 15 x 109 cells/L.5 Also, antibiotic treatment is advised in patients with complicated diverticulitis or uncomplicated diverticulitis with a fluid collection or longer segment of inflammation on CT scan. Finally, patients with uncomplicated diverticulitis who are immunosuppressed are high risk for progression to complicated diverticulitis or sepsis and should be treated with antibiotics.
The lists above clearly leave some patients with AUD who may be managed without antibiotics. These patients are otherwise healthy, have good social support, access to health care, and are experiencing a mild, self-limited episode. Avoiding antibiotics requires shared decision-making with a well-informed patient. I have patients who have embraced this approach, while others found this unacceptable. Given the current level of uncertainty in the literature, I make it my practice to offer antibiotics to any patient who feels strongly about receiving them.
As with many issues in modern medicine, the use of antibiotics in AUD is an unsettled question. Given the known harms of progression of diverticulitis, it is clearly safest to treat patients who were excluded from the small studies we have or flagged by those same studies as being at increased risk of progression. Our uncertainty also demands a shared decision-making model, filling in our patients on what we can and cannot say with confidence. As is often the case, further research is desperately needed. Until that happens, antibiotics for AUD will remain a regular part of my practice.
Anne F. Peery, MD, MSCR, is with the center for gastrointestinal biology and disease at the University of North Carolina at Chapel Hill. She has no conflicts to disclose.
References
1. Daniels L et al. Br J Surg. 2017 Jan;104(1):52-61.
2. Balk EM et al. Management of Colonic Diverticulitis. Comparative Effectiveness Review No. 233. Agency for Healthcare Research and Quality. 2020 Oct. doi: 10.23970/AHRQEPCCER233.
3. Stollman N et al. Gastroenterology. 2015 Dec;149(7):1944-9.
4. Chabok A et al. Br J Surg. 2012 Jan 30;99(4):532-9.
5. Peery AF et al. Gastroenterology. 2021 Feb;160(3):906-11.e1.
The data are robust for withholding more often
That we are engaged in a legitimate debate about the role of antibiotics in acute uncomplicated diverticulitis (AUD) is itself quite notable. In the 1999 American College of Gastroenterology Practice Guidelines,1 we did not even entertain the concept of withholding antibiotics; the only discussion points were intravenous versus oral. Fast forward 15 years, and in the 2015 American Gastroenterological Association practice guidelines (which the other contributor for this installment of Perspectives, Anne F. Peery, MD, and I worked on together) our first recommendation was that antibiotics should be used “selectively,” rather than routinely.2 This did generate some raised eyebrows and hand-wringing in the community, but our position was the result of a rigorous data analysis process and we stood by it.
In fact, Dr. Peery and I also coauthored an accompanying editorial that concluded with an important endorsement “allowing the clinician to consider withholding antibiotics from select uncomplicated patients with mild disease.” I suspect, then, that Dr. Peery and I are very much coincident in our overall thoughts here, and I’m pretty sure that neither of us would defend an “always” or “never” stance on this issue, so for this educational debate, we’re really talking about where in the middle to draw the line (that is, how to define “selectively”). To that end, I will defend the supposition that the subsequent data in support of withholding antibiotics remains robust and even more supportive of this practice in many (but certainly not all) patients with acute, uncomplicated diverticulitis.
The logic underlying selective use was based on two drivers over the past decade, one being an emerging concept that diverticular pathology was often inflammatory rather than solely infectious, coupled with a timely and very important worldwide push toward restraint of antibiotic use. A recent retrospective claims analysis of outpatient antibiotic use for immunocompetent patients with AUD did reported that the dominant antibiotic combination used, metronidazole plus a fluoroquinolone, was associated with an increased risk of Clostridioides difficile infection, compared with the far less frequently used amoxicillin/clavulanate regimen, which highlights that routine antibiotics are not without risk.3
Recently, the U.S. Agency for Healthcare Research and Quality performed a rigorous meta-analysis of this body of evidence, and summarized that “antibiotic treatment may not affect pain symptoms, length of hospital stay, recurrence risk, quality of life, or need for surgery, compared to no antibiotic treatment,” with an admitted low strength of evidence (based on four randomized, controlled trials).4
Finally, I’ll close my evidence-based case with a quote from a Clinical Practice Update just published in February 2021 in Gastroenterology: “Antibiotic treatment can be used selectively, rather than routinely, in immunocompetent patients with mild uncomplicated diverticulitis. ... Antibiotic treatment is advised in patients with uncomplicated diverticulitis who have comorbidities or are frail, who present with refractory symptoms or vomiting, or who have a [C-reactive protein] >140 mg/L or baseline [white blood cell count] > 15 x 109. Antibiotic treatment is advised in patients with complicated diverticulitis or uncomplicated diverticulitis with a fluid collection or longer segment of inflammation on CT scan.”5 I think this is most excellent advice, providing a practical and clinically useful framework for those in whom antibiotics absolutely should be used, but permitting a fairly large group to avoid antibiotics, which may carry significant harm, based on strong consistent data that does not support a benefit.
So, practically speaking, things are a bit harder for us now when our patient shows up in the ED and if found to have AUD on CT. In the “old days” (that is, the early 2000s), a patient with either clinically suspected or CT-confirmed AUD was simply given antibiotics, mostly a fluoroquinolone, and this was community standard. The only real decision tree was inpatient or outpatient. But now, I would respectfully suggest, we need to invest a bit more time on a nuanced discussion with the potential “no antibiotics” patient (for example, one without immunosuppression, severe comorbidities, or lab or imaging markers of aggressive disease). It is now appropriate to inform such a patient that the data suggest that a conservative approach will not increase their risk of complications and may well spare them antibiotic-related morbidity.
In the context of that informed discussion, factually framed by the practitioner, many patients should (and will, in my experience, although the San Francisco Bay Area may not entirely reflect the country as a whole) choose a strategy of observation. Of course, close follow-up with such patients is required, as is clear reassurance that if things “turn bad” that we’re available and antibiotics remain a salvage option. The “write a script and be done” days are over, and while withholding antibiotics may still feel dangerous or uncomfortable to the patient (or to us), the data is the data, and our patients deserve to at least be offered that option.
Neil Stollman, MD, AGAF, FACG, is chairman of the division of gastroenterology at Alta Bates Summit Medical Center in Oakland, Calif., and an associate clinical professor of medicine in the division of gastroenterology at the University of California, San Francisco. He discloses being a consultant for Cosmo Pharmaceuticals, which has a potential future diverticulitis study of a rifampin-class antibiotic.
References
1. Stollman N and Raskin JB. Am J Gastroenterol. 1999 Nov;94(11):3110-21.
2. Peery AF and Stollman N. Gastroenterology. 2015 Dec;149(7):1944-9.
3. Gaber CE et al. Ann Intern Med. 2021 Feb 23. doi: 10.7326/M20-6315.
4. Balk EM et al. Management of Colonic Diverticulitis. Comparative Effectiveness Review No. 233. Agency for Healthcare Research and Quality. October 2020. doi: 10.23970/AHRQEPCCER233.
5. Peery AF et al. Gastroenterology. 2021 Feb;160(3):906-11.e1.
Dear colleagues and friends,
The Perspectives series returns, this time with an exciting discussion about antibiotic use in acute uncomplicated diverticulitis. It has been fascinating to witness this field evolve from an era where not using antibiotics was inconceivable! Dr. Anne F. Peery and Dr. Neil Stollman, both recognized experts in the matter, provide arguments to both sides of the debate, as well as much-needed nuance. As always, I welcome your comments and suggestions for future topics at [email protected]. Thank you for your support, and I hope you will enjoy the reading and learning from this as much as I did.
Charles J. Kahi, MD, MS, AGAF, is a professor of medicine at Indiana University, Indianapolis. He is also an associate editor for GI & Hepatology News.
Think carefully about when to withhold
For decades, it was standard practice to give antibiotics to all patients with acute uncomplicated diverticulitis (AUD). While most patients with a first diagnosis of AUD recover within a few weeks, a small proportion will develop a complication.1 Among generally healthy patients with an initial diagnosis of AUD, about 3% will progress to complicated diverticulitis, and about 1% will require emergency surgery within 6 months. Around another 6% of cases will develop chronic diverticulitis with ongoing diverticular inflammation that persists for weeks to months.
Because the complications are uncommon, we don’t know if antibiotics reduce the risk of progression to complicated diverticulitis, emergency surgery, or the development of chronic diverticulitis. Investigating these patient-centered and morbid outcomes would require trials enrolling thousands of patients and to following these patients for months. This trial hasn’t happened yet.
To date, only small studies have compared the use of antibiotics with no antibiotics in patients with AUD. A review sponsored by the Agency for Healthcare Research and Quality published last year concluded that the current evidence was too sparse or too inconsistent to make strong conclusions about the use of antibiotics for patients with uncomplicated diverticulitis.2
With little evidence for or against antibiotics, recent guidelines have begun to recommend that antibiotics be used selectively, rather than routinely, in patients with diverticulitis.3 “Selectively” clearly means that there are some patients who should receive antibiotics, but the guidelines are vague about who those patients are. To this end, it is safest to refer to those small, underpowered trials to identify which patients are at the greatest risk of developing a complication.1,4 The authors of those trials considered a number of groups high risk and therefore excluded them from those trials. In the absence of further definitive research, it seems clear that those groups, listed below, should therefore be selected for antibiotic treatment:
- Patients with complicated diverticulitis including paracolic extraluminal air on CT scan.
- Patients who are immunocompromised.
- Patients with a high fever, affected general condition, or clinical suspicion of sepsis.
- Patients with inflammatory bowel disease.
- Patients who are pregnant or breast feeding.
As with most clinical trials, participants in these smaller trials were younger (median age, late 50s) and healthier (63% normal, healthy patient; 34% mild systemic disease; 4% severe systemic disease) than the general population. In secondary analyses, however, several factors were independently associated with a complicated disease course after an initial diagnosis of acute uncomplicated diverticulitis. As with the first list above, the following high-risk patients should also be treated with antibiotics at diagnosis:
- Patients with American Society of Anesthesiologists scores III or IV were 4.4 times more likely to have a poor outcome, compared with those with ASA score I.
- Patients with ASA score II were 2.0 times more likely to have a poor outcome, compared with ASA score I.
- Patients with symptoms for more than 5 days at diagnosis were 3.3 times more likely to have a poor outcome, compared with those with symptoms for 5 days or less.
- Patients with vomiting at diagnosis were 3.9 times more likely to have a poor outcome, compared with those who were not vomiting.
- Patients with C-reactive protein levels higher than 140 mg/L at diagnosis were 2.9 times more likely to have a poor outcome, compared with C-reactive protein level of 140 mg/L or less.
- Patients with white blood cell count greater than 15 x 109 cells/L at diagnosis were 3.7 times more likely to have a poor outcome, compared with those with 15 x 109 cells/L.
- Patients with a longer segment (>86mm) of inflamed colon on CT scan were more likely to have a poor outcome, compared those who had a shorter segment (<65mm).
To help clinicians think about antibiotic treatment in patients with AUD, a recent American Gastroenterological Association clinical practice update provided the following advice: First, antibiotic treatment is advised in patients with uncomplicated diverticulitis who have comorbidities or are frail, who present with refractory symptoms or vomiting, or who have a C-reactive protein level greater than 140 mg/L, or baseline white blood cell count greater than 15 x 109 cells/L.5 Also, antibiotic treatment is advised in patients with complicated diverticulitis or uncomplicated diverticulitis with a fluid collection or longer segment of inflammation on CT scan. Finally, patients with uncomplicated diverticulitis who are immunosuppressed are high risk for progression to complicated diverticulitis or sepsis and should be treated with antibiotics.
The lists above clearly leave some patients with AUD who may be managed without antibiotics. These patients are otherwise healthy, have good social support, access to health care, and are experiencing a mild, self-limited episode. Avoiding antibiotics requires shared decision-making with a well-informed patient. I have patients who have embraced this approach, while others found this unacceptable. Given the current level of uncertainty in the literature, I make it my practice to offer antibiotics to any patient who feels strongly about receiving them.
As with many issues in modern medicine, the use of antibiotics in AUD is an unsettled question. Given the known harms of progression of diverticulitis, it is clearly safest to treat patients who were excluded from the small studies we have or flagged by those same studies as being at increased risk of progression. Our uncertainty also demands a shared decision-making model, filling in our patients on what we can and cannot say with confidence. As is often the case, further research is desperately needed. Until that happens, antibiotics for AUD will remain a regular part of my practice.
Anne F. Peery, MD, MSCR, is with the center for gastrointestinal biology and disease at the University of North Carolina at Chapel Hill. She has no conflicts to disclose.
References
1. Daniels L et al. Br J Surg. 2017 Jan;104(1):52-61.
2. Balk EM et al. Management of Colonic Diverticulitis. Comparative Effectiveness Review No. 233. Agency for Healthcare Research and Quality. 2020 Oct. doi: 10.23970/AHRQEPCCER233.
3. Stollman N et al. Gastroenterology. 2015 Dec;149(7):1944-9.
4. Chabok A et al. Br J Surg. 2012 Jan 30;99(4):532-9.
5. Peery AF et al. Gastroenterology. 2021 Feb;160(3):906-11.e1.
The data are robust for withholding more often
That we are engaged in a legitimate debate about the role of antibiotics in acute uncomplicated diverticulitis (AUD) is itself quite notable. In the 1999 American College of Gastroenterology Practice Guidelines,1 we did not even entertain the concept of withholding antibiotics; the only discussion points were intravenous versus oral. Fast forward 15 years, and in the 2015 American Gastroenterological Association practice guidelines (which the other contributor for this installment of Perspectives, Anne F. Peery, MD, and I worked on together) our first recommendation was that antibiotics should be used “selectively,” rather than routinely.2 This did generate some raised eyebrows and hand-wringing in the community, but our position was the result of a rigorous data analysis process and we stood by it.
In fact, Dr. Peery and I also coauthored an accompanying editorial that concluded with an important endorsement “allowing the clinician to consider withholding antibiotics from select uncomplicated patients with mild disease.” I suspect, then, that Dr. Peery and I are very much coincident in our overall thoughts here, and I’m pretty sure that neither of us would defend an “always” or “never” stance on this issue, so for this educational debate, we’re really talking about where in the middle to draw the line (that is, how to define “selectively”). To that end, I will defend the supposition that the subsequent data in support of withholding antibiotics remains robust and even more supportive of this practice in many (but certainly not all) patients with acute, uncomplicated diverticulitis.
The logic underlying selective use was based on two drivers over the past decade, one being an emerging concept that diverticular pathology was often inflammatory rather than solely infectious, coupled with a timely and very important worldwide push toward restraint of antibiotic use. A recent retrospective claims analysis of outpatient antibiotic use for immunocompetent patients with AUD did reported that the dominant antibiotic combination used, metronidazole plus a fluoroquinolone, was associated with an increased risk of Clostridioides difficile infection, compared with the far less frequently used amoxicillin/clavulanate regimen, which highlights that routine antibiotics are not without risk.3
Recently, the U.S. Agency for Healthcare Research and Quality performed a rigorous meta-analysis of this body of evidence, and summarized that “antibiotic treatment may not affect pain symptoms, length of hospital stay, recurrence risk, quality of life, or need for surgery, compared to no antibiotic treatment,” with an admitted low strength of evidence (based on four randomized, controlled trials).4
Finally, I’ll close my evidence-based case with a quote from a Clinical Practice Update just published in February 2021 in Gastroenterology: “Antibiotic treatment can be used selectively, rather than routinely, in immunocompetent patients with mild uncomplicated diverticulitis. ... Antibiotic treatment is advised in patients with uncomplicated diverticulitis who have comorbidities or are frail, who present with refractory symptoms or vomiting, or who have a [C-reactive protein] >140 mg/L or baseline [white blood cell count] > 15 x 109. Antibiotic treatment is advised in patients with complicated diverticulitis or uncomplicated diverticulitis with a fluid collection or longer segment of inflammation on CT scan.”5 I think this is most excellent advice, providing a practical and clinically useful framework for those in whom antibiotics absolutely should be used, but permitting a fairly large group to avoid antibiotics, which may carry significant harm, based on strong consistent data that does not support a benefit.
So, practically speaking, things are a bit harder for us now when our patient shows up in the ED and if found to have AUD on CT. In the “old days” (that is, the early 2000s), a patient with either clinically suspected or CT-confirmed AUD was simply given antibiotics, mostly a fluoroquinolone, and this was community standard. The only real decision tree was inpatient or outpatient. But now, I would respectfully suggest, we need to invest a bit more time on a nuanced discussion with the potential “no antibiotics” patient (for example, one without immunosuppression, severe comorbidities, or lab or imaging markers of aggressive disease). It is now appropriate to inform such a patient that the data suggest that a conservative approach will not increase their risk of complications and may well spare them antibiotic-related morbidity.
In the context of that informed discussion, factually framed by the practitioner, many patients should (and will, in my experience, although the San Francisco Bay Area may not entirely reflect the country as a whole) choose a strategy of observation. Of course, close follow-up with such patients is required, as is clear reassurance that if things “turn bad” that we’re available and antibiotics remain a salvage option. The “write a script and be done” days are over, and while withholding antibiotics may still feel dangerous or uncomfortable to the patient (or to us), the data is the data, and our patients deserve to at least be offered that option.
Neil Stollman, MD, AGAF, FACG, is chairman of the division of gastroenterology at Alta Bates Summit Medical Center in Oakland, Calif., and an associate clinical professor of medicine in the division of gastroenterology at the University of California, San Francisco. He discloses being a consultant for Cosmo Pharmaceuticals, which has a potential future diverticulitis study of a rifampin-class antibiotic.
References
1. Stollman N and Raskin JB. Am J Gastroenterol. 1999 Nov;94(11):3110-21.
2. Peery AF and Stollman N. Gastroenterology. 2015 Dec;149(7):1944-9.
3. Gaber CE et al. Ann Intern Med. 2021 Feb 23. doi: 10.7326/M20-6315.
4. Balk EM et al. Management of Colonic Diverticulitis. Comparative Effectiveness Review No. 233. Agency for Healthcare Research and Quality. October 2020. doi: 10.23970/AHRQEPCCER233.
5. Peery AF et al. Gastroenterology. 2021 Feb;160(3):906-11.e1.
Dear colleagues and friends,
The Perspectives series returns, this time with an exciting discussion about antibiotic use in acute uncomplicated diverticulitis. It has been fascinating to witness this field evolve from an era where not using antibiotics was inconceivable! Dr. Anne F. Peery and Dr. Neil Stollman, both recognized experts in the matter, provide arguments to both sides of the debate, as well as much-needed nuance. As always, I welcome your comments and suggestions for future topics at [email protected]. Thank you for your support, and I hope you will enjoy the reading and learning from this as much as I did.
Charles J. Kahi, MD, MS, AGAF, is a professor of medicine at Indiana University, Indianapolis. He is also an associate editor for GI & Hepatology News.
Think carefully about when to withhold
For decades, it was standard practice to give antibiotics to all patients with acute uncomplicated diverticulitis (AUD). While most patients with a first diagnosis of AUD recover within a few weeks, a small proportion will develop a complication.1 Among generally healthy patients with an initial diagnosis of AUD, about 3% will progress to complicated diverticulitis, and about 1% will require emergency surgery within 6 months. Around another 6% of cases will develop chronic diverticulitis with ongoing diverticular inflammation that persists for weeks to months.
Because the complications are uncommon, we don’t know if antibiotics reduce the risk of progression to complicated diverticulitis, emergency surgery, or the development of chronic diverticulitis. Investigating these patient-centered and morbid outcomes would require trials enrolling thousands of patients and to following these patients for months. This trial hasn’t happened yet.
To date, only small studies have compared the use of antibiotics with no antibiotics in patients with AUD. A review sponsored by the Agency for Healthcare Research and Quality published last year concluded that the current evidence was too sparse or too inconsistent to make strong conclusions about the use of antibiotics for patients with uncomplicated diverticulitis.2
With little evidence for or against antibiotics, recent guidelines have begun to recommend that antibiotics be used selectively, rather than routinely, in patients with diverticulitis.3 “Selectively” clearly means that there are some patients who should receive antibiotics, but the guidelines are vague about who those patients are. To this end, it is safest to refer to those small, underpowered trials to identify which patients are at the greatest risk of developing a complication.1,4 The authors of those trials considered a number of groups high risk and therefore excluded them from those trials. In the absence of further definitive research, it seems clear that those groups, listed below, should therefore be selected for antibiotic treatment:
- Patients with complicated diverticulitis including paracolic extraluminal air on CT scan.
- Patients who are immunocompromised.
- Patients with a high fever, affected general condition, or clinical suspicion of sepsis.
- Patients with inflammatory bowel disease.
- Patients who are pregnant or breast feeding.
As with most clinical trials, participants in these smaller trials were younger (median age, late 50s) and healthier (63% normal, healthy patient; 34% mild systemic disease; 4% severe systemic disease) than the general population. In secondary analyses, however, several factors were independently associated with a complicated disease course after an initial diagnosis of acute uncomplicated diverticulitis. As with the first list above, the following high-risk patients should also be treated with antibiotics at diagnosis:
- Patients with American Society of Anesthesiologists scores III or IV were 4.4 times more likely to have a poor outcome, compared with those with ASA score I.
- Patients with ASA score II were 2.0 times more likely to have a poor outcome, compared with ASA score I.
- Patients with symptoms for more than 5 days at diagnosis were 3.3 times more likely to have a poor outcome, compared with those with symptoms for 5 days or less.
- Patients with vomiting at diagnosis were 3.9 times more likely to have a poor outcome, compared with those who were not vomiting.
- Patients with C-reactive protein levels higher than 140 mg/L at diagnosis were 2.9 times more likely to have a poor outcome, compared with C-reactive protein level of 140 mg/L or less.
- Patients with white blood cell count greater than 15 x 109 cells/L at diagnosis were 3.7 times more likely to have a poor outcome, compared with those with 15 x 109 cells/L.
- Patients with a longer segment (>86mm) of inflamed colon on CT scan were more likely to have a poor outcome, compared those who had a shorter segment (<65mm).
To help clinicians think about antibiotic treatment in patients with AUD, a recent American Gastroenterological Association clinical practice update provided the following advice: First, antibiotic treatment is advised in patients with uncomplicated diverticulitis who have comorbidities or are frail, who present with refractory symptoms or vomiting, or who have a C-reactive protein level greater than 140 mg/L, or baseline white blood cell count greater than 15 x 109 cells/L.5 Also, antibiotic treatment is advised in patients with complicated diverticulitis or uncomplicated diverticulitis with a fluid collection or longer segment of inflammation on CT scan. Finally, patients with uncomplicated diverticulitis who are immunosuppressed are high risk for progression to complicated diverticulitis or sepsis and should be treated with antibiotics.
The lists above clearly leave some patients with AUD who may be managed without antibiotics. These patients are otherwise healthy, have good social support, access to health care, and are experiencing a mild, self-limited episode. Avoiding antibiotics requires shared decision-making with a well-informed patient. I have patients who have embraced this approach, while others found this unacceptable. Given the current level of uncertainty in the literature, I make it my practice to offer antibiotics to any patient who feels strongly about receiving them.
As with many issues in modern medicine, the use of antibiotics in AUD is an unsettled question. Given the known harms of progression of diverticulitis, it is clearly safest to treat patients who were excluded from the small studies we have or flagged by those same studies as being at increased risk of progression. Our uncertainty also demands a shared decision-making model, filling in our patients on what we can and cannot say with confidence. As is often the case, further research is desperately needed. Until that happens, antibiotics for AUD will remain a regular part of my practice.
Anne F. Peery, MD, MSCR, is with the center for gastrointestinal biology and disease at the University of North Carolina at Chapel Hill. She has no conflicts to disclose.
References
1. Daniels L et al. Br J Surg. 2017 Jan;104(1):52-61.
2. Balk EM et al. Management of Colonic Diverticulitis. Comparative Effectiveness Review No. 233. Agency for Healthcare Research and Quality. 2020 Oct. doi: 10.23970/AHRQEPCCER233.
3. Stollman N et al. Gastroenterology. 2015 Dec;149(7):1944-9.
4. Chabok A et al. Br J Surg. 2012 Jan 30;99(4):532-9.
5. Peery AF et al. Gastroenterology. 2021 Feb;160(3):906-11.e1.
The data are robust for withholding more often
That we are engaged in a legitimate debate about the role of antibiotics in acute uncomplicated diverticulitis (AUD) is itself quite notable. In the 1999 American College of Gastroenterology Practice Guidelines,1 we did not even entertain the concept of withholding antibiotics; the only discussion points were intravenous versus oral. Fast forward 15 years, and in the 2015 American Gastroenterological Association practice guidelines (which the other contributor for this installment of Perspectives, Anne F. Peery, MD, and I worked on together) our first recommendation was that antibiotics should be used “selectively,” rather than routinely.2 This did generate some raised eyebrows and hand-wringing in the community, but our position was the result of a rigorous data analysis process and we stood by it.
In fact, Dr. Peery and I also coauthored an accompanying editorial that concluded with an important endorsement “allowing the clinician to consider withholding antibiotics from select uncomplicated patients with mild disease.” I suspect, then, that Dr. Peery and I are very much coincident in our overall thoughts here, and I’m pretty sure that neither of us would defend an “always” or “never” stance on this issue, so for this educational debate, we’re really talking about where in the middle to draw the line (that is, how to define “selectively”). To that end, I will defend the supposition that the subsequent data in support of withholding antibiotics remains robust and even more supportive of this practice in many (but certainly not all) patients with acute, uncomplicated diverticulitis.
The logic underlying selective use was based on two drivers over the past decade, one being an emerging concept that diverticular pathology was often inflammatory rather than solely infectious, coupled with a timely and very important worldwide push toward restraint of antibiotic use. A recent retrospective claims analysis of outpatient antibiotic use for immunocompetent patients with AUD did reported that the dominant antibiotic combination used, metronidazole plus a fluoroquinolone, was associated with an increased risk of Clostridioides difficile infection, compared with the far less frequently used amoxicillin/clavulanate regimen, which highlights that routine antibiotics are not without risk.3
Recently, the U.S. Agency for Healthcare Research and Quality performed a rigorous meta-analysis of this body of evidence, and summarized that “antibiotic treatment may not affect pain symptoms, length of hospital stay, recurrence risk, quality of life, or need for surgery, compared to no antibiotic treatment,” with an admitted low strength of evidence (based on four randomized, controlled trials).4
Finally, I’ll close my evidence-based case with a quote from a Clinical Practice Update just published in February 2021 in Gastroenterology: “Antibiotic treatment can be used selectively, rather than routinely, in immunocompetent patients with mild uncomplicated diverticulitis. ... Antibiotic treatment is advised in patients with uncomplicated diverticulitis who have comorbidities or are frail, who present with refractory symptoms or vomiting, or who have a [C-reactive protein] >140 mg/L or baseline [white blood cell count] > 15 x 109. Antibiotic treatment is advised in patients with complicated diverticulitis or uncomplicated diverticulitis with a fluid collection or longer segment of inflammation on CT scan.”5 I think this is most excellent advice, providing a practical and clinically useful framework for those in whom antibiotics absolutely should be used, but permitting a fairly large group to avoid antibiotics, which may carry significant harm, based on strong consistent data that does not support a benefit.
So, practically speaking, things are a bit harder for us now when our patient shows up in the ED and if found to have AUD on CT. In the “old days” (that is, the early 2000s), a patient with either clinically suspected or CT-confirmed AUD was simply given antibiotics, mostly a fluoroquinolone, and this was community standard. The only real decision tree was inpatient or outpatient. But now, I would respectfully suggest, we need to invest a bit more time on a nuanced discussion with the potential “no antibiotics” patient (for example, one without immunosuppression, severe comorbidities, or lab or imaging markers of aggressive disease). It is now appropriate to inform such a patient that the data suggest that a conservative approach will not increase their risk of complications and may well spare them antibiotic-related morbidity.
In the context of that informed discussion, factually framed by the practitioner, many patients should (and will, in my experience, although the San Francisco Bay Area may not entirely reflect the country as a whole) choose a strategy of observation. Of course, close follow-up with such patients is required, as is clear reassurance that if things “turn bad” that we’re available and antibiotics remain a salvage option. The “write a script and be done” days are over, and while withholding antibiotics may still feel dangerous or uncomfortable to the patient (or to us), the data is the data, and our patients deserve to at least be offered that option.
Neil Stollman, MD, AGAF, FACG, is chairman of the division of gastroenterology at Alta Bates Summit Medical Center in Oakland, Calif., and an associate clinical professor of medicine in the division of gastroenterology at the University of California, San Francisco. He discloses being a consultant for Cosmo Pharmaceuticals, which has a potential future diverticulitis study of a rifampin-class antibiotic.
References
1. Stollman N and Raskin JB. Am J Gastroenterol. 1999 Nov;94(11):3110-21.
2. Peery AF and Stollman N. Gastroenterology. 2015 Dec;149(7):1944-9.
3. Gaber CE et al. Ann Intern Med. 2021 Feb 23. doi: 10.7326/M20-6315.
4. Balk EM et al. Management of Colonic Diverticulitis. Comparative Effectiveness Review No. 233. Agency for Healthcare Research and Quality. October 2020. doi: 10.23970/AHRQEPCCER233.
5. Peery AF et al. Gastroenterology. 2021 Feb;160(3):906-11.e1.
Most patients with chronic inflammatory diseases have sufficient response to COVID-19 vaccination
Glucocorticoids and B-cell–depleting therapies are trouble spots
Although most patients with chronic inflammatory diseases mounted immune responses after two doses of mRNA-based COVID-19 vaccines, glucocorticoids and B-cell–depleting therapies markedly reduced the response, according to a recently published preprint of a new study.
The study, published on MedRxiv and not yet peer reviewed, involved a prospective look at 133 patients with chronic inflammatory disease (CID) and 53 patients with healthy immune systems at Washington University, St. Louis, and the University of California, San Francisco. It is regarded as the largest and most detailed study yet in how vaccines perform in people with immune-mediated inflammatory disease. The patients were enrolled between December 2020 and March 2021, and the most common diseases were inflammatory bowel disease (32%), rheumatoid arthritis (29%), spondyloarthritis (15%), and systemic lupus erythematosus (11%).
A ‘modest’ reduction in antibody response
Senior author Alfred Kim, MD, PhD, of the department of medicine at Washington University, said the overall results so far are encouraging.
“Most patients with an autoimmune disease that are on immunosuppression can mount antibody responses,” he said. “We’re seeing the majority of our subjects respond.”
The immune-healthy controls and most of the patients with CID had a robust immune response against the spike protein, although the CID group had a mean reduction in antibody titers that was three times lower than the controls (P = .0092). The CID group similarly had a 2.7-fold reduction in preventing neutralization, or halting the virus’ ability to infect (P < .0001), researchers reported.
This reduction in response is “modest,” he said.
“Is the level of reduction going to be detrimental for protection? Time will tell,” he said, adding that researchers anticipate that it won’t have a critical effect on protection because responses tended to be within the range of the immunocompetent controls, who themselves had wildly varied antibody titers across a 20-fold range. “ ‘Optimal’ isn’t necessarily the same as ‘sufficient.’ ”
Type of medication has big impact on antibody titers
But there was a wide variety of effects on the immune response depending on the medication. Glucorticoids resulted in a response that was 10 times lower than the immune-healthy controls, as well as fewer circulating plasmablasts after vaccination. Researchers found that 98% of controls were seropositive for antibody, compared with 92% of those with CID who were not taking prednisone, and 65% of CID patients on prednisone (P = .0006 and .0115, respectively). Prevention of neutralization of the virus was similarly reduced in those groups, compared with the controls. Dr. Kim noted this was a small sample size, with about 15 patients. These effects were seen regardless of the dose.
“We would’ve anticipated this would have been dose dependent, so this was a little bit surprising,” Dr. Kim said.
B-cell–depleting therapies, such as rituximab (Rituxan) and ocrelizumab (Ocrevus), reduced antibody titers by 36 times, compared with controls (P < .0001), with a similar reduction in preventing infection (P = .0066), the researchers found. The reduction in antibody titers was the most pronounced among those who had received B-cell–depleting therapies within the previous 6 months. Dr. Kim noted this was a small sample size, with about 10 patients.
CID study subjects taking an antimetabolite, including methotrexate, had an average of a two- to threefold reduction in antibody titers and in neutralization (P = .0006). This reduction was greatest with methotrexate, researchers found (P = .0027).
JAK inhibitors also significantly reduced antibody titers (P = .0066), but the reduction in neutralization of the virus was not significant. In addition, researchers found a reduction in antibody titers, the prevention of viral infection, and circulating plasmablasts among those on tumor necrosis factor (TNF) inhibitors, compared with controls, but these were insignificant statistically except for virus neutralization.
Dr. Kim said he hopes the glucocorticoid data spur physicians to try harder to wean patients off the drugs, when possible, in keeping with recommendations already in place.
“The general culture in rheumatology has been very lax about the need to reduce glucocorticoids,” he said. “This reinvigorates that call.” Questions about possible drug holidays from glucocorticoids remain, regarding how long a holiday would be needed, he said. He noted that many patients on glucocorticoids nonetheless mounted responses.
Those on B-cell–depleting therapies present a “much more difficult” question, he said. Some patients possibly could wait a bit longer than their normal, every-6-month schedule, but it’s an individual decision, he said. Since a booster of influenza vaccine has been found to enhance the response even within the 6-month window among ocrelizumab patients, a booster of COVID-19 vaccine might also help, although this remains to be studied.
The study group has already increased its sample size and is looking at adverse reactions and long-term immune responses, Dr. Kim said.
Encouraging, rather than discouraging, results
Leonard Calabrese, DO, professor of medicine at the Cleveland Clinic in Ohio, said the findings shouldn’t discourage clinicians from encouraging vaccination.
“There’s still a preponderance of people who will develop a robust antibody vaccine response,” he said.
He cautioned that the findings look only at antibodies to the spike protein and at plasmablasts. The reduction in these titers is “of concern,” he said, but “we don’t really know with certainty what are the effects of these drugs, and these data are on the overall biologic protective effect of the vaccine. There’s much more to a vaccine response than anti–spike protein and plasmablasts,” including cell-mediated immune response.
For an individual patient, the findings “mean a lot,” he said.
“I think that people who are on significant prednisone and B-cell–depleting agents, I think you have to share with them that there’s a reasonable chance that you’re not going to be making a response similar to healthy people,” he said. “Thus, even with your vaccine, we’re not going to cut you loose to do things that are violating social distancing and group settings. … Should you be hugging your grandchildren if you’re a rituximab vaccine recipient? I think I would wait until we have a little bit more data.”
Kevin Winthrop, MD, MPH, professor of ophthalmology at Oregon Health & Science University, Portland, where he studies vaccinations in the immunocompromised, said that glucocorticoids tend to have little effect on vaccinations generally at low doses.
When effects are seen they can be difficult to interpret, he said.
“It’s hard to extricate that from the effect of the underlying disease,” he said. The drug can be a proxy for worse disease control.
Although it’s a small study, it’s reassuring that overall the responses were similar to healthy controls.
For B-cell–depleting therapies, his usual guidance is to not give vaccine until a patient is at least 3 months out from their last dose, and not to restart until at least 2 weeks after vaccination.
“It’s not surprising that some of these DMARDs [disease-modifying antirheumatic drugs] do negatively affect vaccine response, particularly B-cell–depletion therapy. We need to do some studies to find a way to overcome that, or optimize delivery of the vaccine.”
Dr. Kim reported participating in consulting, advisory board, or speaker’s bureau for Alexion, Aurinia, Annexon Biosciences, Exagen Diagnostics, and GlaxoSmithKline, and receiving funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline. Dr. Winthrop reported receiving consulting fees from Pfizer, AbbVie, UCB, Eli Lilly, Galapagos, GlaxoSmithKline, Roche, Gilead, Bristol-Myers Squibb, Regeneron, Sanofi, AstraZeneca, Novartis, and research grants from Bristol-Myers Squibb and Pfizer. Dr. Calabrese reported no relevant disclosures.
Glucocorticoids and B-cell–depleting therapies are trouble spots
Glucocorticoids and B-cell–depleting therapies are trouble spots
Although most patients with chronic inflammatory diseases mounted immune responses after two doses of mRNA-based COVID-19 vaccines, glucocorticoids and B-cell–depleting therapies markedly reduced the response, according to a recently published preprint of a new study.
The study, published on MedRxiv and not yet peer reviewed, involved a prospective look at 133 patients with chronic inflammatory disease (CID) and 53 patients with healthy immune systems at Washington University, St. Louis, and the University of California, San Francisco. It is regarded as the largest and most detailed study yet in how vaccines perform in people with immune-mediated inflammatory disease. The patients were enrolled between December 2020 and March 2021, and the most common diseases were inflammatory bowel disease (32%), rheumatoid arthritis (29%), spondyloarthritis (15%), and systemic lupus erythematosus (11%).
A ‘modest’ reduction in antibody response
Senior author Alfred Kim, MD, PhD, of the department of medicine at Washington University, said the overall results so far are encouraging.
“Most patients with an autoimmune disease that are on immunosuppression can mount antibody responses,” he said. “We’re seeing the majority of our subjects respond.”
The immune-healthy controls and most of the patients with CID had a robust immune response against the spike protein, although the CID group had a mean reduction in antibody titers that was three times lower than the controls (P = .0092). The CID group similarly had a 2.7-fold reduction in preventing neutralization, or halting the virus’ ability to infect (P < .0001), researchers reported.
This reduction in response is “modest,” he said.
“Is the level of reduction going to be detrimental for protection? Time will tell,” he said, adding that researchers anticipate that it won’t have a critical effect on protection because responses tended to be within the range of the immunocompetent controls, who themselves had wildly varied antibody titers across a 20-fold range. “ ‘Optimal’ isn’t necessarily the same as ‘sufficient.’ ”
Type of medication has big impact on antibody titers
But there was a wide variety of effects on the immune response depending on the medication. Glucorticoids resulted in a response that was 10 times lower than the immune-healthy controls, as well as fewer circulating plasmablasts after vaccination. Researchers found that 98% of controls were seropositive for antibody, compared with 92% of those with CID who were not taking prednisone, and 65% of CID patients on prednisone (P = .0006 and .0115, respectively). Prevention of neutralization of the virus was similarly reduced in those groups, compared with the controls. Dr. Kim noted this was a small sample size, with about 15 patients. These effects were seen regardless of the dose.
“We would’ve anticipated this would have been dose dependent, so this was a little bit surprising,” Dr. Kim said.
B-cell–depleting therapies, such as rituximab (Rituxan) and ocrelizumab (Ocrevus), reduced antibody titers by 36 times, compared with controls (P < .0001), with a similar reduction in preventing infection (P = .0066), the researchers found. The reduction in antibody titers was the most pronounced among those who had received B-cell–depleting therapies within the previous 6 months. Dr. Kim noted this was a small sample size, with about 10 patients.
CID study subjects taking an antimetabolite, including methotrexate, had an average of a two- to threefold reduction in antibody titers and in neutralization (P = .0006). This reduction was greatest with methotrexate, researchers found (P = .0027).
JAK inhibitors also significantly reduced antibody titers (P = .0066), but the reduction in neutralization of the virus was not significant. In addition, researchers found a reduction in antibody titers, the prevention of viral infection, and circulating plasmablasts among those on tumor necrosis factor (TNF) inhibitors, compared with controls, but these were insignificant statistically except for virus neutralization.
Dr. Kim said he hopes the glucocorticoid data spur physicians to try harder to wean patients off the drugs, when possible, in keeping with recommendations already in place.
“The general culture in rheumatology has been very lax about the need to reduce glucocorticoids,” he said. “This reinvigorates that call.” Questions about possible drug holidays from glucocorticoids remain, regarding how long a holiday would be needed, he said. He noted that many patients on glucocorticoids nonetheless mounted responses.
Those on B-cell–depleting therapies present a “much more difficult” question, he said. Some patients possibly could wait a bit longer than their normal, every-6-month schedule, but it’s an individual decision, he said. Since a booster of influenza vaccine has been found to enhance the response even within the 6-month window among ocrelizumab patients, a booster of COVID-19 vaccine might also help, although this remains to be studied.
The study group has already increased its sample size and is looking at adverse reactions and long-term immune responses, Dr. Kim said.
Encouraging, rather than discouraging, results
Leonard Calabrese, DO, professor of medicine at the Cleveland Clinic in Ohio, said the findings shouldn’t discourage clinicians from encouraging vaccination.
“There’s still a preponderance of people who will develop a robust antibody vaccine response,” he said.
He cautioned that the findings look only at antibodies to the spike protein and at plasmablasts. The reduction in these titers is “of concern,” he said, but “we don’t really know with certainty what are the effects of these drugs, and these data are on the overall biologic protective effect of the vaccine. There’s much more to a vaccine response than anti–spike protein and plasmablasts,” including cell-mediated immune response.
For an individual patient, the findings “mean a lot,” he said.
“I think that people who are on significant prednisone and B-cell–depleting agents, I think you have to share with them that there’s a reasonable chance that you’re not going to be making a response similar to healthy people,” he said. “Thus, even with your vaccine, we’re not going to cut you loose to do things that are violating social distancing and group settings. … Should you be hugging your grandchildren if you’re a rituximab vaccine recipient? I think I would wait until we have a little bit more data.”
Kevin Winthrop, MD, MPH, professor of ophthalmology at Oregon Health & Science University, Portland, where he studies vaccinations in the immunocompromised, said that glucocorticoids tend to have little effect on vaccinations generally at low doses.
When effects are seen they can be difficult to interpret, he said.
“It’s hard to extricate that from the effect of the underlying disease,” he said. The drug can be a proxy for worse disease control.
Although it’s a small study, it’s reassuring that overall the responses were similar to healthy controls.
For B-cell–depleting therapies, his usual guidance is to not give vaccine until a patient is at least 3 months out from their last dose, and not to restart until at least 2 weeks after vaccination.
“It’s not surprising that some of these DMARDs [disease-modifying antirheumatic drugs] do negatively affect vaccine response, particularly B-cell–depletion therapy. We need to do some studies to find a way to overcome that, or optimize delivery of the vaccine.”
Dr. Kim reported participating in consulting, advisory board, or speaker’s bureau for Alexion, Aurinia, Annexon Biosciences, Exagen Diagnostics, and GlaxoSmithKline, and receiving funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline. Dr. Winthrop reported receiving consulting fees from Pfizer, AbbVie, UCB, Eli Lilly, Galapagos, GlaxoSmithKline, Roche, Gilead, Bristol-Myers Squibb, Regeneron, Sanofi, AstraZeneca, Novartis, and research grants from Bristol-Myers Squibb and Pfizer. Dr. Calabrese reported no relevant disclosures.
Although most patients with chronic inflammatory diseases mounted immune responses after two doses of mRNA-based COVID-19 vaccines, glucocorticoids and B-cell–depleting therapies markedly reduced the response, according to a recently published preprint of a new study.
The study, published on MedRxiv and not yet peer reviewed, involved a prospective look at 133 patients with chronic inflammatory disease (CID) and 53 patients with healthy immune systems at Washington University, St. Louis, and the University of California, San Francisco. It is regarded as the largest and most detailed study yet in how vaccines perform in people with immune-mediated inflammatory disease. The patients were enrolled between December 2020 and March 2021, and the most common diseases were inflammatory bowel disease (32%), rheumatoid arthritis (29%), spondyloarthritis (15%), and systemic lupus erythematosus (11%).
A ‘modest’ reduction in antibody response
Senior author Alfred Kim, MD, PhD, of the department of medicine at Washington University, said the overall results so far are encouraging.
“Most patients with an autoimmune disease that are on immunosuppression can mount antibody responses,” he said. “We’re seeing the majority of our subjects respond.”
The immune-healthy controls and most of the patients with CID had a robust immune response against the spike protein, although the CID group had a mean reduction in antibody titers that was three times lower than the controls (P = .0092). The CID group similarly had a 2.7-fold reduction in preventing neutralization, or halting the virus’ ability to infect (P < .0001), researchers reported.
This reduction in response is “modest,” he said.
“Is the level of reduction going to be detrimental for protection? Time will tell,” he said, adding that researchers anticipate that it won’t have a critical effect on protection because responses tended to be within the range of the immunocompetent controls, who themselves had wildly varied antibody titers across a 20-fold range. “ ‘Optimal’ isn’t necessarily the same as ‘sufficient.’ ”
Type of medication has big impact on antibody titers
But there was a wide variety of effects on the immune response depending on the medication. Glucorticoids resulted in a response that was 10 times lower than the immune-healthy controls, as well as fewer circulating plasmablasts after vaccination. Researchers found that 98% of controls were seropositive for antibody, compared with 92% of those with CID who were not taking prednisone, and 65% of CID patients on prednisone (P = .0006 and .0115, respectively). Prevention of neutralization of the virus was similarly reduced in those groups, compared with the controls. Dr. Kim noted this was a small sample size, with about 15 patients. These effects were seen regardless of the dose.
“We would’ve anticipated this would have been dose dependent, so this was a little bit surprising,” Dr. Kim said.
B-cell–depleting therapies, such as rituximab (Rituxan) and ocrelizumab (Ocrevus), reduced antibody titers by 36 times, compared with controls (P < .0001), with a similar reduction in preventing infection (P = .0066), the researchers found. The reduction in antibody titers was the most pronounced among those who had received B-cell–depleting therapies within the previous 6 months. Dr. Kim noted this was a small sample size, with about 10 patients.
CID study subjects taking an antimetabolite, including methotrexate, had an average of a two- to threefold reduction in antibody titers and in neutralization (P = .0006). This reduction was greatest with methotrexate, researchers found (P = .0027).
JAK inhibitors also significantly reduced antibody titers (P = .0066), but the reduction in neutralization of the virus was not significant. In addition, researchers found a reduction in antibody titers, the prevention of viral infection, and circulating plasmablasts among those on tumor necrosis factor (TNF) inhibitors, compared with controls, but these were insignificant statistically except for virus neutralization.
Dr. Kim said he hopes the glucocorticoid data spur physicians to try harder to wean patients off the drugs, when possible, in keeping with recommendations already in place.
“The general culture in rheumatology has been very lax about the need to reduce glucocorticoids,” he said. “This reinvigorates that call.” Questions about possible drug holidays from glucocorticoids remain, regarding how long a holiday would be needed, he said. He noted that many patients on glucocorticoids nonetheless mounted responses.
Those on B-cell–depleting therapies present a “much more difficult” question, he said. Some patients possibly could wait a bit longer than their normal, every-6-month schedule, but it’s an individual decision, he said. Since a booster of influenza vaccine has been found to enhance the response even within the 6-month window among ocrelizumab patients, a booster of COVID-19 vaccine might also help, although this remains to be studied.
The study group has already increased its sample size and is looking at adverse reactions and long-term immune responses, Dr. Kim said.
Encouraging, rather than discouraging, results
Leonard Calabrese, DO, professor of medicine at the Cleveland Clinic in Ohio, said the findings shouldn’t discourage clinicians from encouraging vaccination.
“There’s still a preponderance of people who will develop a robust antibody vaccine response,” he said.
He cautioned that the findings look only at antibodies to the spike protein and at plasmablasts. The reduction in these titers is “of concern,” he said, but “we don’t really know with certainty what are the effects of these drugs, and these data are on the overall biologic protective effect of the vaccine. There’s much more to a vaccine response than anti–spike protein and plasmablasts,” including cell-mediated immune response.
For an individual patient, the findings “mean a lot,” he said.
“I think that people who are on significant prednisone and B-cell–depleting agents, I think you have to share with them that there’s a reasonable chance that you’re not going to be making a response similar to healthy people,” he said. “Thus, even with your vaccine, we’re not going to cut you loose to do things that are violating social distancing and group settings. … Should you be hugging your grandchildren if you’re a rituximab vaccine recipient? I think I would wait until we have a little bit more data.”
Kevin Winthrop, MD, MPH, professor of ophthalmology at Oregon Health & Science University, Portland, where he studies vaccinations in the immunocompromised, said that glucocorticoids tend to have little effect on vaccinations generally at low doses.
When effects are seen they can be difficult to interpret, he said.
“It’s hard to extricate that from the effect of the underlying disease,” he said. The drug can be a proxy for worse disease control.
Although it’s a small study, it’s reassuring that overall the responses were similar to healthy controls.
For B-cell–depleting therapies, his usual guidance is to not give vaccine until a patient is at least 3 months out from their last dose, and not to restart until at least 2 weeks after vaccination.
“It’s not surprising that some of these DMARDs [disease-modifying antirheumatic drugs] do negatively affect vaccine response, particularly B-cell–depletion therapy. We need to do some studies to find a way to overcome that, or optimize delivery of the vaccine.”
Dr. Kim reported participating in consulting, advisory board, or speaker’s bureau for Alexion, Aurinia, Annexon Biosciences, Exagen Diagnostics, and GlaxoSmithKline, and receiving funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline. Dr. Winthrop reported receiving consulting fees from Pfizer, AbbVie, UCB, Eli Lilly, Galapagos, GlaxoSmithKline, Roche, Gilead, Bristol-Myers Squibb, Regeneron, Sanofi, AstraZeneca, Novartis, and research grants from Bristol-Myers Squibb and Pfizer. Dr. Calabrese reported no relevant disclosures.
FROM MEDRXIV
Verification bias casts doubt on IgA tTG in celiac disease
Immunoglobulin A tissue transglutaminase offers a noninvasive way to detect celiac disease, but new research suggests that its sensitivity may be overestimated and that it may not be an effective screening test, at least in asymptomatic individuals. The reason comes down to verification bias, wherein a technique appears to have higher sensitivity and lower specificity because individuals who screen positive are more likely to have their disease confirmed by a follow-up small-bowel biopsy while those who screen negative are unlikely to have a follow-up biopsy that could reveal missed celiac disease.
“The issue with verification bias is that only the patients that screen positive on that index test are going to be getting the reference test, so there’s probably a good chance that if they screen positive when they go to that reference test they’ll also be positive. What you’re missing from when you’re calculating sensitivity is, what about the ones that are negative on the index test? Would they have been positive on that reference test? That’s not even coming into your calculation because they’re not getting that reference test,” said Marisa Stahl, MD, a physician and researcher at the Children’s Hospital Colorado Center of Celiac Disease in Aurora. Dr. Stahl was not involved in the meta-analysis, but commented on it in an interview.
The only way to fully correct for this bias is to conduct both IgA tissue transglutaminase (tTG) testing and small bowel biopsy on a complete or random sample of patients and compare the sensitivity and specificity of IgA tTG with the preferred method small-bowel biopsy. However, this is rarely done. Instead, when the U.S. Preventive Services Task Force concluded that evidence was insufficient for IgA tTG testing for celiac disease, it relied on a 2016 comparative effectiveness review of nine studies that estimated sensitivity at 92.6% and specificity at 97.6%. USPSTF remained noncommittal because of inadequate evidence surrounding the balance of benefit and harms of screening for celiac disease in asymptomatic individuals.
In the current meta-analysis, Isabel Hujoel, MD, of the Mayo Clinic, Rochester, Minn., and colleagues tested whether the studies used by USPSTF may have overestimated sensitivity because of verification bias. In a report in the Journal of Clinical Gastroenterology, they reviewed those same nine studies to see the potential impact of verification bias. They rated each individual study as being at high, low, or unclear risk of verification bias and found five they considered to be high risk.
To reveal the impact of small-bowel biopsy referral rates on sensitivity and specificity, the researchers reviewed a separate set of nine retrospective and prospective studies to determine the frequency of referral for both IgA tTG–positive patients (positive referral rate) and IgA tTG–negative patients (negative referral rate), which were 79.2% and 3.6%, respectively.
The researchers then used these values to recalculate the sensitivities and specificities in the five original studies considered high risk for verification bias, then pooled those adjusted values with the remaining, unadjusted values from the studies considered low or unclear risk of bias. The new overall values were 57.1% sensitivity (95% confidence interval, 35.4%-76.4%) and 99.6% specificity (95% CI, 98.4%-99.9%).
“The reported sensitivity and specificity of IgA tTG ... are substantially biased due to a lack of adjustment for verification bias. Specifically, adjusting for verification bias decreases the sensitivity of IgA tTG from 92.5% to 57.1%, with a drop in the lower limit of the 95% CI to 35.4%, and an increase in the specificity from 97.9% to 99.6%, The low estimated sensitivity of IgA tTG raises concern on the accuracy of this test and supports performing a systematic review that accounts for verification bias. ... After adjusting for verification bias, the estimated sensitivity of IgA tTG falls to the point where the serologic marker may no longer be clinically useful as a screening test,” the authors wrote.
The numbers came as a bit of a shock to Dr. Stahl because the sensitivity was so much lower than has been traditionally accepted. “But the more important concept from the paper is that the sensitivity is probably lower than what we oftentimes reference, and we should think more about the population of patients that could potentially screen negative and still have celiac disease,” she said. Although there is no literature to back this up at this time, Dr. Stahl also believes that this may be more common in adults, who have a higher incidence of seronegative Celiac disease.
The issue isn’t restricted to celiac disease. Verification bias can also affect the sensitivity and specificity values from other index screens that are followed by invasive reference tests, like occult blood and colonoscopy or hepatitis C serology and liver biopsy. “A lot of times you ethically cannot put everyone through the [more invasive] reference test, so it definitely applies to other tests we screen for in GI. When we’re quoting numbers and doing systematic reviews and meta-analyses, we should be accounting for those biases,” said Dr. Stahl.
No source of funding was disclosed. The authors declared that they have nothing to disclose. Dr. Stahl consults for Evo-Endo.
Immunoglobulin A tissue transglutaminase offers a noninvasive way to detect celiac disease, but new research suggests that its sensitivity may be overestimated and that it may not be an effective screening test, at least in asymptomatic individuals. The reason comes down to verification bias, wherein a technique appears to have higher sensitivity and lower specificity because individuals who screen positive are more likely to have their disease confirmed by a follow-up small-bowel biopsy while those who screen negative are unlikely to have a follow-up biopsy that could reveal missed celiac disease.
“The issue with verification bias is that only the patients that screen positive on that index test are going to be getting the reference test, so there’s probably a good chance that if they screen positive when they go to that reference test they’ll also be positive. What you’re missing from when you’re calculating sensitivity is, what about the ones that are negative on the index test? Would they have been positive on that reference test? That’s not even coming into your calculation because they’re not getting that reference test,” said Marisa Stahl, MD, a physician and researcher at the Children’s Hospital Colorado Center of Celiac Disease in Aurora. Dr. Stahl was not involved in the meta-analysis, but commented on it in an interview.
The only way to fully correct for this bias is to conduct both IgA tissue transglutaminase (tTG) testing and small bowel biopsy on a complete or random sample of patients and compare the sensitivity and specificity of IgA tTG with the preferred method small-bowel biopsy. However, this is rarely done. Instead, when the U.S. Preventive Services Task Force concluded that evidence was insufficient for IgA tTG testing for celiac disease, it relied on a 2016 comparative effectiveness review of nine studies that estimated sensitivity at 92.6% and specificity at 97.6%. USPSTF remained noncommittal because of inadequate evidence surrounding the balance of benefit and harms of screening for celiac disease in asymptomatic individuals.
In the current meta-analysis, Isabel Hujoel, MD, of the Mayo Clinic, Rochester, Minn., and colleagues tested whether the studies used by USPSTF may have overestimated sensitivity because of verification bias. In a report in the Journal of Clinical Gastroenterology, they reviewed those same nine studies to see the potential impact of verification bias. They rated each individual study as being at high, low, or unclear risk of verification bias and found five they considered to be high risk.
To reveal the impact of small-bowel biopsy referral rates on sensitivity and specificity, the researchers reviewed a separate set of nine retrospective and prospective studies to determine the frequency of referral for both IgA tTG–positive patients (positive referral rate) and IgA tTG–negative patients (negative referral rate), which were 79.2% and 3.6%, respectively.
The researchers then used these values to recalculate the sensitivities and specificities in the five original studies considered high risk for verification bias, then pooled those adjusted values with the remaining, unadjusted values from the studies considered low or unclear risk of bias. The new overall values were 57.1% sensitivity (95% confidence interval, 35.4%-76.4%) and 99.6% specificity (95% CI, 98.4%-99.9%).
“The reported sensitivity and specificity of IgA tTG ... are substantially biased due to a lack of adjustment for verification bias. Specifically, adjusting for verification bias decreases the sensitivity of IgA tTG from 92.5% to 57.1%, with a drop in the lower limit of the 95% CI to 35.4%, and an increase in the specificity from 97.9% to 99.6%, The low estimated sensitivity of IgA tTG raises concern on the accuracy of this test and supports performing a systematic review that accounts for verification bias. ... After adjusting for verification bias, the estimated sensitivity of IgA tTG falls to the point where the serologic marker may no longer be clinically useful as a screening test,” the authors wrote.
The numbers came as a bit of a shock to Dr. Stahl because the sensitivity was so much lower than has been traditionally accepted. “But the more important concept from the paper is that the sensitivity is probably lower than what we oftentimes reference, and we should think more about the population of patients that could potentially screen negative and still have celiac disease,” she said. Although there is no literature to back this up at this time, Dr. Stahl also believes that this may be more common in adults, who have a higher incidence of seronegative Celiac disease.
The issue isn’t restricted to celiac disease. Verification bias can also affect the sensitivity and specificity values from other index screens that are followed by invasive reference tests, like occult blood and colonoscopy or hepatitis C serology and liver biopsy. “A lot of times you ethically cannot put everyone through the [more invasive] reference test, so it definitely applies to other tests we screen for in GI. When we’re quoting numbers and doing systematic reviews and meta-analyses, we should be accounting for those biases,” said Dr. Stahl.
No source of funding was disclosed. The authors declared that they have nothing to disclose. Dr. Stahl consults for Evo-Endo.
Immunoglobulin A tissue transglutaminase offers a noninvasive way to detect celiac disease, but new research suggests that its sensitivity may be overestimated and that it may not be an effective screening test, at least in asymptomatic individuals. The reason comes down to verification bias, wherein a technique appears to have higher sensitivity and lower specificity because individuals who screen positive are more likely to have their disease confirmed by a follow-up small-bowel biopsy while those who screen negative are unlikely to have a follow-up biopsy that could reveal missed celiac disease.
“The issue with verification bias is that only the patients that screen positive on that index test are going to be getting the reference test, so there’s probably a good chance that if they screen positive when they go to that reference test they’ll also be positive. What you’re missing from when you’re calculating sensitivity is, what about the ones that are negative on the index test? Would they have been positive on that reference test? That’s not even coming into your calculation because they’re not getting that reference test,” said Marisa Stahl, MD, a physician and researcher at the Children’s Hospital Colorado Center of Celiac Disease in Aurora. Dr. Stahl was not involved in the meta-analysis, but commented on it in an interview.
The only way to fully correct for this bias is to conduct both IgA tissue transglutaminase (tTG) testing and small bowel biopsy on a complete or random sample of patients and compare the sensitivity and specificity of IgA tTG with the preferred method small-bowel biopsy. However, this is rarely done. Instead, when the U.S. Preventive Services Task Force concluded that evidence was insufficient for IgA tTG testing for celiac disease, it relied on a 2016 comparative effectiveness review of nine studies that estimated sensitivity at 92.6% and specificity at 97.6%. USPSTF remained noncommittal because of inadequate evidence surrounding the balance of benefit and harms of screening for celiac disease in asymptomatic individuals.
In the current meta-analysis, Isabel Hujoel, MD, of the Mayo Clinic, Rochester, Minn., and colleagues tested whether the studies used by USPSTF may have overestimated sensitivity because of verification bias. In a report in the Journal of Clinical Gastroenterology, they reviewed those same nine studies to see the potential impact of verification bias. They rated each individual study as being at high, low, or unclear risk of verification bias and found five they considered to be high risk.
To reveal the impact of small-bowel biopsy referral rates on sensitivity and specificity, the researchers reviewed a separate set of nine retrospective and prospective studies to determine the frequency of referral for both IgA tTG–positive patients (positive referral rate) and IgA tTG–negative patients (negative referral rate), which were 79.2% and 3.6%, respectively.
The researchers then used these values to recalculate the sensitivities and specificities in the five original studies considered high risk for verification bias, then pooled those adjusted values with the remaining, unadjusted values from the studies considered low or unclear risk of bias. The new overall values were 57.1% sensitivity (95% confidence interval, 35.4%-76.4%) and 99.6% specificity (95% CI, 98.4%-99.9%).
“The reported sensitivity and specificity of IgA tTG ... are substantially biased due to a lack of adjustment for verification bias. Specifically, adjusting for verification bias decreases the sensitivity of IgA tTG from 92.5% to 57.1%, with a drop in the lower limit of the 95% CI to 35.4%, and an increase in the specificity from 97.9% to 99.6%, The low estimated sensitivity of IgA tTG raises concern on the accuracy of this test and supports performing a systematic review that accounts for verification bias. ... After adjusting for verification bias, the estimated sensitivity of IgA tTG falls to the point where the serologic marker may no longer be clinically useful as a screening test,” the authors wrote.
The numbers came as a bit of a shock to Dr. Stahl because the sensitivity was so much lower than has been traditionally accepted. “But the more important concept from the paper is that the sensitivity is probably lower than what we oftentimes reference, and we should think more about the population of patients that could potentially screen negative and still have celiac disease,” she said. Although there is no literature to back this up at this time, Dr. Stahl also believes that this may be more common in adults, who have a higher incidence of seronegative Celiac disease.
The issue isn’t restricted to celiac disease. Verification bias can also affect the sensitivity and specificity values from other index screens that are followed by invasive reference tests, like occult blood and colonoscopy or hepatitis C serology and liver biopsy. “A lot of times you ethically cannot put everyone through the [more invasive] reference test, so it definitely applies to other tests we screen for in GI. When we’re quoting numbers and doing systematic reviews and meta-analyses, we should be accounting for those biases,” said Dr. Stahl.
No source of funding was disclosed. The authors declared that they have nothing to disclose. Dr. Stahl consults for Evo-Endo.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Lasting norovirus immunity may depend on T cells
Protection against norovirus gastroenteritis is supported in part by norovirus-specific CD8+ T cells that reside in peripheral, intestinal, and lymphoid tissues, according to investigators.
These findings, and the molecular tools used to discover them, could guide development of a norovirus vaccine and novel cellular therapies, according to lead author Ajinkya Pattekar, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
“Currently, there are no approved pharmacologic therapies against norovirus, and despite several promising clinical trials, an effective vaccine is not available,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, which may stem from an incomplete understanding of norovirus immunity, according to Dr. Pattekar and colleagues.
They noted that most previous research has focused on humoral immunity, which appears variable between individuals, with some people exhibiting a strong humoral response, while others mount only partial humoral protection. The investigators also noted that, depending on which studies were examined, this type of defense could last years or fade within weeks to months and that “immune mechanisms other than antibodies may be important for protection against noroviruses.”
Specifically, cellular immunity may be at work. A 2020 study involving volunteers showed that T cells were cross-reactive to a type of norovirus the participants had never been exposed to.
“These findings suggest that T cells may target conserved epitopes and could offer cross-protection against a broad range of noroviruses,” Dr. Pattekar and colleagues wrote.
To test this hypothesis, they first collected peripheral blood mononuclear cells (PBMCs) from three healthy volunteers with unknown norovirus exposure history. Then serum samples were screened for norovirus functional antibodies via the binding between virus-like particles (VLPs) and histo–blood group antigens (HBGAs). This revealed disparate profiles of blocking antibodies against various norovirus strains. While donor 1 and donor 2 had antibodies against multiple strains, donor 3 lacked norovirus antibodies. Further testing showed that this latter individual was a nonsecretor with limited exposure history.
Next, the investigators tested donor PBMCs for norovirus-specific T-cell responses with use of overlapping libraries of peptides for each of the three norovirus open reading frames (ORF1, ORF2, and ORF3). T-cell responses, predominantly involving CD8+ T cells, were observed in all donors. While donor 1 had the greatest response to ORF1, donors 2 and 3 had responses that focused on ORF2.
“Thus, norovirus-specific T cells targeting ORF1 and ORF2 epitopes are present in peripheral blood from healthy donors regardless of secretor status,” the investigators wrote.
To better characterize T-cell epitopes, the investigators subdivided the overlapping peptide libraries into groups of shorter peptides, then exposed serum to these smaller component pools. This revealed eight HLA class I restricted epitopes that were derived from a genogroup II.4 pandemic norovirus strain; this group of variants has been responsible for all six of the norovirus pandemics since 1996.
Closer examination of the epitopes showed that they were “broadly conserved beyond GII.4.” Only one epitope exhibited variation in the C-terminal aromatic anchor, and it was nondominant. The investigators therefore identified seven immunodominant CD8+ epitopes, which they considered “valuable targets for vaccine and cell-based therapies.
“These data further confirm that epitope-specific CD8+ T cells are a universal feature of the overall norovirus immune response and could be an attractive target for future vaccines,” the investigators wrote.
Additional testing involving samples of spleen, mesenteric lymph nodes, and duodenum from deceased individuals showed presence of norovirus-specific CD8+ T cells, with particular abundance in intestinal tissue, and distinct phenotypes and functional properties in different tissue types.
“Future studies using tetramers and intestinal samples should build on these observations and fully define the location and microenvironment of norovirus-specific T cells,” the investigators wrote. “If carried out in the context of a vaccine trial, such studies could be highly valuable in elucidating tissue-resident memory correlates of norovirus immunity.”
The study was funded by the National Institutes of Health, the Wellcome Trust, and Deutsche Forschungsgemeinschaft. The investigators reported no conflicts of interest.
Understanding the immune correlates of protection for norovirus is important for the development and evaluation of candidate vaccines and to better clarify the variation in host susceptibility to infection.
Prior research on the human immune response to norovirus infection has largely focused on the antibody response. There is less known about the antinorovirus T cell response, which can target and clear virus-infected cells. Notably, anti-viral CD8+ T cells are critical for control of norovirus infection in mouse models, which suggests a similarly important role in humans. In this study by Dr. Pattekar and colleagues, the authors generated human norovirus-specific peptides covering the entire viral proteome, and then they used these peptides to identify and characterize norovirus-specific CD8+ T cells from the blood, spleen, lymph nodes, and intestinal lamina propria of human donors who were not actively infected by norovirus. The authors identified virus-specific memory T cells in the blood and intestines. Further, they found several HLA class I restricted virus epitopes that are highly conserved amongst the most commonly circulating GII.4 noroviruses. These norovirus-specific T cells represented about 0.5% of all cells and reveal that norovirus induces a durable population of memory T cells.
Further research is needed to determine whether norovirus-specific CD8+ T cells are necessary or sufficient for preventing norovirus infection and disease in people. This important study provides novel tools and increases our understanding of cell-mediated immunity to human norovirus infection that will influence future vaccine design and evaluation for this important human pathogen.
Craig B. Wilen, MD, PhD, is assistant professor of laboratory medicine and immunobiology at Yale University, New Haven, Conn. He does not have any conflicts to disclose.
Understanding the immune correlates of protection for norovirus is important for the development and evaluation of candidate vaccines and to better clarify the variation in host susceptibility to infection.
Prior research on the human immune response to norovirus infection has largely focused on the antibody response. There is less known about the antinorovirus T cell response, which can target and clear virus-infected cells. Notably, anti-viral CD8+ T cells are critical for control of norovirus infection in mouse models, which suggests a similarly important role in humans. In this study by Dr. Pattekar and colleagues, the authors generated human norovirus-specific peptides covering the entire viral proteome, and then they used these peptides to identify and characterize norovirus-specific CD8+ T cells from the blood, spleen, lymph nodes, and intestinal lamina propria of human donors who were not actively infected by norovirus. The authors identified virus-specific memory T cells in the blood and intestines. Further, they found several HLA class I restricted virus epitopes that are highly conserved amongst the most commonly circulating GII.4 noroviruses. These norovirus-specific T cells represented about 0.5% of all cells and reveal that norovirus induces a durable population of memory T cells.
Further research is needed to determine whether norovirus-specific CD8+ T cells are necessary or sufficient for preventing norovirus infection and disease in people. This important study provides novel tools and increases our understanding of cell-mediated immunity to human norovirus infection that will influence future vaccine design and evaluation for this important human pathogen.
Craig B. Wilen, MD, PhD, is assistant professor of laboratory medicine and immunobiology at Yale University, New Haven, Conn. He does not have any conflicts to disclose.
Understanding the immune correlates of protection for norovirus is important for the development and evaluation of candidate vaccines and to better clarify the variation in host susceptibility to infection.
Prior research on the human immune response to norovirus infection has largely focused on the antibody response. There is less known about the antinorovirus T cell response, which can target and clear virus-infected cells. Notably, anti-viral CD8+ T cells are critical for control of norovirus infection in mouse models, which suggests a similarly important role in humans. In this study by Dr. Pattekar and colleagues, the authors generated human norovirus-specific peptides covering the entire viral proteome, and then they used these peptides to identify and characterize norovirus-specific CD8+ T cells from the blood, spleen, lymph nodes, and intestinal lamina propria of human donors who were not actively infected by norovirus. The authors identified virus-specific memory T cells in the blood and intestines. Further, they found several HLA class I restricted virus epitopes that are highly conserved amongst the most commonly circulating GII.4 noroviruses. These norovirus-specific T cells represented about 0.5% of all cells and reveal that norovirus induces a durable population of memory T cells.
Further research is needed to determine whether norovirus-specific CD8+ T cells are necessary or sufficient for preventing norovirus infection and disease in people. This important study provides novel tools and increases our understanding of cell-mediated immunity to human norovirus infection that will influence future vaccine design and evaluation for this important human pathogen.
Craig B. Wilen, MD, PhD, is assistant professor of laboratory medicine and immunobiology at Yale University, New Haven, Conn. He does not have any conflicts to disclose.
Protection against norovirus gastroenteritis is supported in part by norovirus-specific CD8+ T cells that reside in peripheral, intestinal, and lymphoid tissues, according to investigators.
These findings, and the molecular tools used to discover them, could guide development of a norovirus vaccine and novel cellular therapies, according to lead author Ajinkya Pattekar, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
“Currently, there are no approved pharmacologic therapies against norovirus, and despite several promising clinical trials, an effective vaccine is not available,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, which may stem from an incomplete understanding of norovirus immunity, according to Dr. Pattekar and colleagues.
They noted that most previous research has focused on humoral immunity, which appears variable between individuals, with some people exhibiting a strong humoral response, while others mount only partial humoral protection. The investigators also noted that, depending on which studies were examined, this type of defense could last years or fade within weeks to months and that “immune mechanisms other than antibodies may be important for protection against noroviruses.”
Specifically, cellular immunity may be at work. A 2020 study involving volunteers showed that T cells were cross-reactive to a type of norovirus the participants had never been exposed to.
“These findings suggest that T cells may target conserved epitopes and could offer cross-protection against a broad range of noroviruses,” Dr. Pattekar and colleagues wrote.
To test this hypothesis, they first collected peripheral blood mononuclear cells (PBMCs) from three healthy volunteers with unknown norovirus exposure history. Then serum samples were screened for norovirus functional antibodies via the binding between virus-like particles (VLPs) and histo–blood group antigens (HBGAs). This revealed disparate profiles of blocking antibodies against various norovirus strains. While donor 1 and donor 2 had antibodies against multiple strains, donor 3 lacked norovirus antibodies. Further testing showed that this latter individual was a nonsecretor with limited exposure history.
Next, the investigators tested donor PBMCs for norovirus-specific T-cell responses with use of overlapping libraries of peptides for each of the three norovirus open reading frames (ORF1, ORF2, and ORF3). T-cell responses, predominantly involving CD8+ T cells, were observed in all donors. While donor 1 had the greatest response to ORF1, donors 2 and 3 had responses that focused on ORF2.
“Thus, norovirus-specific T cells targeting ORF1 and ORF2 epitopes are present in peripheral blood from healthy donors regardless of secretor status,” the investigators wrote.
To better characterize T-cell epitopes, the investigators subdivided the overlapping peptide libraries into groups of shorter peptides, then exposed serum to these smaller component pools. This revealed eight HLA class I restricted epitopes that were derived from a genogroup II.4 pandemic norovirus strain; this group of variants has been responsible for all six of the norovirus pandemics since 1996.
Closer examination of the epitopes showed that they were “broadly conserved beyond GII.4.” Only one epitope exhibited variation in the C-terminal aromatic anchor, and it was nondominant. The investigators therefore identified seven immunodominant CD8+ epitopes, which they considered “valuable targets for vaccine and cell-based therapies.
“These data further confirm that epitope-specific CD8+ T cells are a universal feature of the overall norovirus immune response and could be an attractive target for future vaccines,” the investigators wrote.
Additional testing involving samples of spleen, mesenteric lymph nodes, and duodenum from deceased individuals showed presence of norovirus-specific CD8+ T cells, with particular abundance in intestinal tissue, and distinct phenotypes and functional properties in different tissue types.
“Future studies using tetramers and intestinal samples should build on these observations and fully define the location and microenvironment of norovirus-specific T cells,” the investigators wrote. “If carried out in the context of a vaccine trial, such studies could be highly valuable in elucidating tissue-resident memory correlates of norovirus immunity.”
The study was funded by the National Institutes of Health, the Wellcome Trust, and Deutsche Forschungsgemeinschaft. The investigators reported no conflicts of interest.
Protection against norovirus gastroenteritis is supported in part by norovirus-specific CD8+ T cells that reside in peripheral, intestinal, and lymphoid tissues, according to investigators.
These findings, and the molecular tools used to discover them, could guide development of a norovirus vaccine and novel cellular therapies, according to lead author Ajinkya Pattekar, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
“Currently, there are no approved pharmacologic therapies against norovirus, and despite several promising clinical trials, an effective vaccine is not available,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, which may stem from an incomplete understanding of norovirus immunity, according to Dr. Pattekar and colleagues.
They noted that most previous research has focused on humoral immunity, which appears variable between individuals, with some people exhibiting a strong humoral response, while others mount only partial humoral protection. The investigators also noted that, depending on which studies were examined, this type of defense could last years or fade within weeks to months and that “immune mechanisms other than antibodies may be important for protection against noroviruses.”
Specifically, cellular immunity may be at work. A 2020 study involving volunteers showed that T cells were cross-reactive to a type of norovirus the participants had never been exposed to.
“These findings suggest that T cells may target conserved epitopes and could offer cross-protection against a broad range of noroviruses,” Dr. Pattekar and colleagues wrote.
To test this hypothesis, they first collected peripheral blood mononuclear cells (PBMCs) from three healthy volunteers with unknown norovirus exposure history. Then serum samples were screened for norovirus functional antibodies via the binding between virus-like particles (VLPs) and histo–blood group antigens (HBGAs). This revealed disparate profiles of blocking antibodies against various norovirus strains. While donor 1 and donor 2 had antibodies against multiple strains, donor 3 lacked norovirus antibodies. Further testing showed that this latter individual was a nonsecretor with limited exposure history.
Next, the investigators tested donor PBMCs for norovirus-specific T-cell responses with use of overlapping libraries of peptides for each of the three norovirus open reading frames (ORF1, ORF2, and ORF3). T-cell responses, predominantly involving CD8+ T cells, were observed in all donors. While donor 1 had the greatest response to ORF1, donors 2 and 3 had responses that focused on ORF2.
“Thus, norovirus-specific T cells targeting ORF1 and ORF2 epitopes are present in peripheral blood from healthy donors regardless of secretor status,” the investigators wrote.
To better characterize T-cell epitopes, the investigators subdivided the overlapping peptide libraries into groups of shorter peptides, then exposed serum to these smaller component pools. This revealed eight HLA class I restricted epitopes that were derived from a genogroup II.4 pandemic norovirus strain; this group of variants has been responsible for all six of the norovirus pandemics since 1996.
Closer examination of the epitopes showed that they were “broadly conserved beyond GII.4.” Only one epitope exhibited variation in the C-terminal aromatic anchor, and it was nondominant. The investigators therefore identified seven immunodominant CD8+ epitopes, which they considered “valuable targets for vaccine and cell-based therapies.
“These data further confirm that epitope-specific CD8+ T cells are a universal feature of the overall norovirus immune response and could be an attractive target for future vaccines,” the investigators wrote.
Additional testing involving samples of spleen, mesenteric lymph nodes, and duodenum from deceased individuals showed presence of norovirus-specific CD8+ T cells, with particular abundance in intestinal tissue, and distinct phenotypes and functional properties in different tissue types.
“Future studies using tetramers and intestinal samples should build on these observations and fully define the location and microenvironment of norovirus-specific T cells,” the investigators wrote. “If carried out in the context of a vaccine trial, such studies could be highly valuable in elucidating tissue-resident memory correlates of norovirus immunity.”
The study was funded by the National Institutes of Health, the Wellcome Trust, and Deutsche Forschungsgemeinschaft. The investigators reported no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Infliximab weakens COVID-19 antibody response for IBD patients
Patients treated with infliximab for inflammatory bowel disease (IBD) showed significantly reduced response to COVID-19 antibodies, compared with those treated with vedolizumab, according to data from nearly 7,000 patients.
Although anti–tumor necrosis factor (anti-TNF) drugs are routinely used for patients with IBD, the impact of their immune-suppressing properties on protective immunity to COVID-19 is unknown, wrote Nicholas A. Kennedy, MD, of the University of Exeter (England) and colleagues. These drugs have been reported to impair protective immunity following vaccines for other diseases, such as those for influenza and viral hepatitis.
“By suppressing immune responses, biological and immunosuppression therapies may lead to chronic SARS-CoV-2 infection and have recently been implicated in the evolution and emergence of novel variants,” they noted, citing a study published in Cell.
In the current study, published in Gut, the researchers used data from the CLARITY IBD study to identify 6,935 patients with IBD aged 5 years and older seen at 92 hospitals in the United Kingdom between Sept. 22, 2020, and Dec. 23, 2020. Of these, 4,685 were treated with infliximab, and 2,250 received vedolizumab. The proportion of study participants with a positive anti–SARS-CoV-2 antibody test was the primary outcome, with secondary outcomes including proportion with positive antibodies following positive polymerase chain reaction test for SARS-CoV-2 and the magnitude of antibody reactivity.
Substantial seroprevalence differences seen
Overall, rates of symptomatic and proven SARS-CoV-2 infection and hospitalization were similar between infliximab-treated and vedolizumab-treated patients with IBD. However, seroprevalence was significantly lower in the infliximab group, compared with the vedolizumab group (3.4% vs. 6.0%; P < .0001). In addition, infliximab and immunomodulator use were each independently associated with lower seropositivity, compared with vedolizumab (odds ratio, 0.66 for infliximab and OR, 0.70 for immunomodulators) in a multivariate analysis.
In a sensitivity analysis, 39 of 81 infliximab-treated patients with polymerase chain reaction–confirmed COVID-19 infection seroconverted (48%), compared with 30 of 36 vedolizumab-treated patients (83%) (P < .00044). Infliximab-treated patients with confirmed infections also showed a lower magnitude of anti–SARS-CoV-2 reactivity, compared with vedolizumab-treated patients (P < .0001).
From a clinical perspective, the lower seroconversion rates and reduced levels of anti–SARS-CoV-2 antibody reactivity might increase susceptibility to recurrent COVID-19 infections in infliximab-treated IBD patients, the researchers noted. In addition, the impaired serological responses might promote chronic nasopharyngeal colonization and consequently promote the development of COVID-19 variants and drive persistent transmission, the researchers said.
The study findings were limited by several factors including lack of knowledge on the impact of attenuated immune response on infection risk, the potential for recall bias associated with patient reports, and the focus on infliximab only, the researchers pointed out. However, the key findings are likely apply to other anti-TNF monoclonal antibodies including adalimumab, certolizumab and golimumab, they suggested.
The study was strengthened by the recruitment of a large number of patients in a narrow time frame and comprehensive collection of data on patient-reported outcomes, COVID-19 testing, and serological assay results, the researchers said. Overall, the findings support the public health value of serological testing and virus surveillance to identify suboptimal vaccine response and to consider implications for practice, they added. “If attenuated serological responses following vaccination are also observed, then modified immunization strategies will need to be designed for millions of patients worldwide,” they emphasized.
Findings inform clinical practice and public health
The study is very important for many reasons, said Kim L. Isaacs, MD, PhD, AGAF, of the University of North Carolina at Chapel Hill in an interview. “It is known that there is decreased responsiveness to a number of routine vaccinations in IBD patients on immune active therapy. In terms of SARS-CoV-2, development of an immune response with infection is important in terms of severity of infection, reinfection, and possibly limiting spread of infection in this patient population,” she said. “Looking at both serum seroconversion and reactivity of immune response in patients with known SARS-CoV-2 infection will help to define clinical and public health guidance, and also may be predictive as to what might happen with SARS-CoV-2 immunization based on background biologic or immunosuppressant therapy,” she noted.
Dr. Isaacs said that she was not surprised by the study findings. “Anti-TNF, thiopurine, and methotrexate therapy are all thought to be systemically active and likely to suppress the immune response to infection and vaccination,” she said. Vedolizumab, on the other hand, is thought to be less systemically active and clinically is associated with fewer serious infections.
Data will drive patient counseling
“These results affect counseling of IBD patients on immune active therapy who have had a SARS-CoV-2 infection,” said Dr. Isaacs. “They should be made aware that infection does not indicate protection for further infection. Although the issues that are raised in this study are of concern, patients should not have clinically beneficial therapy discontinued or switched based on these results,” she said.
“Additional research is needed to determine what the seroconversion rate is with the currently available immunizations for SARS-CoV-2,” said Dr. Isaacs. More questions to address include whether there are differences in the different products available, whether immunization after SARS-CoV-2 infection improves both seroconversion and immune reactivity, and whether there is any benefit to transiently stopping dual immune active therapy during the time of immunization, she said.
Further studies can fill knowledge gaps
“There is a knowledge gap in our understanding of susceptibility to SARS-CoV-2 infections among patients with IBD who have previously been infected,” Shirley Cohen-Mekelburg, MD, MS, staff physician and research scientist in the inflammatory bowel disease program at the Veterans Affairs Ann Arbor (Mich.) Healthcare System, said in an interview. ”This is a first step in beginning to narrow this gap – to provide patients and providers with data to drive recommendations during this COVID-19 pandemic.”
She added that, while further work needs to be done, the study findings do support potential benefit for ongoing vigilance among patients receiving infliximab for IBD. “The study findings also drive us to seek answers to more questions: For example, should we consider serological testing for patients on infliximab? How does the presence or absence of anti–SARS-CoV-2 antibodies associate with susceptibility to infection for patients with infliximab?
“Further studies examining anti–SARS-CoV-2 reactivity are necessary to better understand antibody responses between patients with IBD to the general population, or between patients on immunosuppressive therapy and the general population,” she said. “Observational studies are also not designed to examine the causal relationship between infections, medications, and antibody responses. There may be some inherent differences to patients who receive infliximab as compared to vedolizumab for IBD.”
The study was supported by Biogen (Switzerland), Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, Hull University Teaching Hospital NHS Trust, and the Royal Devon and Exeter NHS Foundation Trust. The study authors disclosed financial and nonfinancial relationships with numerous companies, including AbbVie, Biogen, Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, and Immundiagnostik, as well as Janssen, who markets infliximab, and Takeda, who markets vedolizumab. Dr. Isaacs and Dr. Cohen-Mekelburg had no relevant financial conflicts to disclose.
This article was updated 3/31/21.
Patients treated with infliximab for inflammatory bowel disease (IBD) showed significantly reduced response to COVID-19 antibodies, compared with those treated with vedolizumab, according to data from nearly 7,000 patients.
Although anti–tumor necrosis factor (anti-TNF) drugs are routinely used for patients with IBD, the impact of their immune-suppressing properties on protective immunity to COVID-19 is unknown, wrote Nicholas A. Kennedy, MD, of the University of Exeter (England) and colleagues. These drugs have been reported to impair protective immunity following vaccines for other diseases, such as those for influenza and viral hepatitis.
“By suppressing immune responses, biological and immunosuppression therapies may lead to chronic SARS-CoV-2 infection and have recently been implicated in the evolution and emergence of novel variants,” they noted, citing a study published in Cell.
In the current study, published in Gut, the researchers used data from the CLARITY IBD study to identify 6,935 patients with IBD aged 5 years and older seen at 92 hospitals in the United Kingdom between Sept. 22, 2020, and Dec. 23, 2020. Of these, 4,685 were treated with infliximab, and 2,250 received vedolizumab. The proportion of study participants with a positive anti–SARS-CoV-2 antibody test was the primary outcome, with secondary outcomes including proportion with positive antibodies following positive polymerase chain reaction test for SARS-CoV-2 and the magnitude of antibody reactivity.
Substantial seroprevalence differences seen
Overall, rates of symptomatic and proven SARS-CoV-2 infection and hospitalization were similar between infliximab-treated and vedolizumab-treated patients with IBD. However, seroprevalence was significantly lower in the infliximab group, compared with the vedolizumab group (3.4% vs. 6.0%; P < .0001). In addition, infliximab and immunomodulator use were each independently associated with lower seropositivity, compared with vedolizumab (odds ratio, 0.66 for infliximab and OR, 0.70 for immunomodulators) in a multivariate analysis.
In a sensitivity analysis, 39 of 81 infliximab-treated patients with polymerase chain reaction–confirmed COVID-19 infection seroconverted (48%), compared with 30 of 36 vedolizumab-treated patients (83%) (P < .00044). Infliximab-treated patients with confirmed infections also showed a lower magnitude of anti–SARS-CoV-2 reactivity, compared with vedolizumab-treated patients (P < .0001).
From a clinical perspective, the lower seroconversion rates and reduced levels of anti–SARS-CoV-2 antibody reactivity might increase susceptibility to recurrent COVID-19 infections in infliximab-treated IBD patients, the researchers noted. In addition, the impaired serological responses might promote chronic nasopharyngeal colonization and consequently promote the development of COVID-19 variants and drive persistent transmission, the researchers said.
The study findings were limited by several factors including lack of knowledge on the impact of attenuated immune response on infection risk, the potential for recall bias associated with patient reports, and the focus on infliximab only, the researchers pointed out. However, the key findings are likely apply to other anti-TNF monoclonal antibodies including adalimumab, certolizumab and golimumab, they suggested.
The study was strengthened by the recruitment of a large number of patients in a narrow time frame and comprehensive collection of data on patient-reported outcomes, COVID-19 testing, and serological assay results, the researchers said. Overall, the findings support the public health value of serological testing and virus surveillance to identify suboptimal vaccine response and to consider implications for practice, they added. “If attenuated serological responses following vaccination are also observed, then modified immunization strategies will need to be designed for millions of patients worldwide,” they emphasized.
Findings inform clinical practice and public health
The study is very important for many reasons, said Kim L. Isaacs, MD, PhD, AGAF, of the University of North Carolina at Chapel Hill in an interview. “It is known that there is decreased responsiveness to a number of routine vaccinations in IBD patients on immune active therapy. In terms of SARS-CoV-2, development of an immune response with infection is important in terms of severity of infection, reinfection, and possibly limiting spread of infection in this patient population,” she said. “Looking at both serum seroconversion and reactivity of immune response in patients with known SARS-CoV-2 infection will help to define clinical and public health guidance, and also may be predictive as to what might happen with SARS-CoV-2 immunization based on background biologic or immunosuppressant therapy,” she noted.
Dr. Isaacs said that she was not surprised by the study findings. “Anti-TNF, thiopurine, and methotrexate therapy are all thought to be systemically active and likely to suppress the immune response to infection and vaccination,” she said. Vedolizumab, on the other hand, is thought to be less systemically active and clinically is associated with fewer serious infections.
Data will drive patient counseling
“These results affect counseling of IBD patients on immune active therapy who have had a SARS-CoV-2 infection,” said Dr. Isaacs. “They should be made aware that infection does not indicate protection for further infection. Although the issues that are raised in this study are of concern, patients should not have clinically beneficial therapy discontinued or switched based on these results,” she said.
“Additional research is needed to determine what the seroconversion rate is with the currently available immunizations for SARS-CoV-2,” said Dr. Isaacs. More questions to address include whether there are differences in the different products available, whether immunization after SARS-CoV-2 infection improves both seroconversion and immune reactivity, and whether there is any benefit to transiently stopping dual immune active therapy during the time of immunization, she said.
Further studies can fill knowledge gaps
“There is a knowledge gap in our understanding of susceptibility to SARS-CoV-2 infections among patients with IBD who have previously been infected,” Shirley Cohen-Mekelburg, MD, MS, staff physician and research scientist in the inflammatory bowel disease program at the Veterans Affairs Ann Arbor (Mich.) Healthcare System, said in an interview. ”This is a first step in beginning to narrow this gap – to provide patients and providers with data to drive recommendations during this COVID-19 pandemic.”
She added that, while further work needs to be done, the study findings do support potential benefit for ongoing vigilance among patients receiving infliximab for IBD. “The study findings also drive us to seek answers to more questions: For example, should we consider serological testing for patients on infliximab? How does the presence or absence of anti–SARS-CoV-2 antibodies associate with susceptibility to infection for patients with infliximab?
“Further studies examining anti–SARS-CoV-2 reactivity are necessary to better understand antibody responses between patients with IBD to the general population, or between patients on immunosuppressive therapy and the general population,” she said. “Observational studies are also not designed to examine the causal relationship between infections, medications, and antibody responses. There may be some inherent differences to patients who receive infliximab as compared to vedolizumab for IBD.”
The study was supported by Biogen (Switzerland), Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, Hull University Teaching Hospital NHS Trust, and the Royal Devon and Exeter NHS Foundation Trust. The study authors disclosed financial and nonfinancial relationships with numerous companies, including AbbVie, Biogen, Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, and Immundiagnostik, as well as Janssen, who markets infliximab, and Takeda, who markets vedolizumab. Dr. Isaacs and Dr. Cohen-Mekelburg had no relevant financial conflicts to disclose.
This article was updated 3/31/21.
Patients treated with infliximab for inflammatory bowel disease (IBD) showed significantly reduced response to COVID-19 antibodies, compared with those treated with vedolizumab, according to data from nearly 7,000 patients.
Although anti–tumor necrosis factor (anti-TNF) drugs are routinely used for patients with IBD, the impact of their immune-suppressing properties on protective immunity to COVID-19 is unknown, wrote Nicholas A. Kennedy, MD, of the University of Exeter (England) and colleagues. These drugs have been reported to impair protective immunity following vaccines for other diseases, such as those for influenza and viral hepatitis.
“By suppressing immune responses, biological and immunosuppression therapies may lead to chronic SARS-CoV-2 infection and have recently been implicated in the evolution and emergence of novel variants,” they noted, citing a study published in Cell.
In the current study, published in Gut, the researchers used data from the CLARITY IBD study to identify 6,935 patients with IBD aged 5 years and older seen at 92 hospitals in the United Kingdom between Sept. 22, 2020, and Dec. 23, 2020. Of these, 4,685 were treated with infliximab, and 2,250 received vedolizumab. The proportion of study participants with a positive anti–SARS-CoV-2 antibody test was the primary outcome, with secondary outcomes including proportion with positive antibodies following positive polymerase chain reaction test for SARS-CoV-2 and the magnitude of antibody reactivity.
Substantial seroprevalence differences seen
Overall, rates of symptomatic and proven SARS-CoV-2 infection and hospitalization were similar between infliximab-treated and vedolizumab-treated patients with IBD. However, seroprevalence was significantly lower in the infliximab group, compared with the vedolizumab group (3.4% vs. 6.0%; P < .0001). In addition, infliximab and immunomodulator use were each independently associated with lower seropositivity, compared with vedolizumab (odds ratio, 0.66 for infliximab and OR, 0.70 for immunomodulators) in a multivariate analysis.
In a sensitivity analysis, 39 of 81 infliximab-treated patients with polymerase chain reaction–confirmed COVID-19 infection seroconverted (48%), compared with 30 of 36 vedolizumab-treated patients (83%) (P < .00044). Infliximab-treated patients with confirmed infections also showed a lower magnitude of anti–SARS-CoV-2 reactivity, compared with vedolizumab-treated patients (P < .0001).
From a clinical perspective, the lower seroconversion rates and reduced levels of anti–SARS-CoV-2 antibody reactivity might increase susceptibility to recurrent COVID-19 infections in infliximab-treated IBD patients, the researchers noted. In addition, the impaired serological responses might promote chronic nasopharyngeal colonization and consequently promote the development of COVID-19 variants and drive persistent transmission, the researchers said.
The study findings were limited by several factors including lack of knowledge on the impact of attenuated immune response on infection risk, the potential for recall bias associated with patient reports, and the focus on infliximab only, the researchers pointed out. However, the key findings are likely apply to other anti-TNF monoclonal antibodies including adalimumab, certolizumab and golimumab, they suggested.
The study was strengthened by the recruitment of a large number of patients in a narrow time frame and comprehensive collection of data on patient-reported outcomes, COVID-19 testing, and serological assay results, the researchers said. Overall, the findings support the public health value of serological testing and virus surveillance to identify suboptimal vaccine response and to consider implications for practice, they added. “If attenuated serological responses following vaccination are also observed, then modified immunization strategies will need to be designed for millions of patients worldwide,” they emphasized.
Findings inform clinical practice and public health
The study is very important for many reasons, said Kim L. Isaacs, MD, PhD, AGAF, of the University of North Carolina at Chapel Hill in an interview. “It is known that there is decreased responsiveness to a number of routine vaccinations in IBD patients on immune active therapy. In terms of SARS-CoV-2, development of an immune response with infection is important in terms of severity of infection, reinfection, and possibly limiting spread of infection in this patient population,” she said. “Looking at both serum seroconversion and reactivity of immune response in patients with known SARS-CoV-2 infection will help to define clinical and public health guidance, and also may be predictive as to what might happen with SARS-CoV-2 immunization based on background biologic or immunosuppressant therapy,” she noted.
Dr. Isaacs said that she was not surprised by the study findings. “Anti-TNF, thiopurine, and methotrexate therapy are all thought to be systemically active and likely to suppress the immune response to infection and vaccination,” she said. Vedolizumab, on the other hand, is thought to be less systemically active and clinically is associated with fewer serious infections.
Data will drive patient counseling
“These results affect counseling of IBD patients on immune active therapy who have had a SARS-CoV-2 infection,” said Dr. Isaacs. “They should be made aware that infection does not indicate protection for further infection. Although the issues that are raised in this study are of concern, patients should not have clinically beneficial therapy discontinued or switched based on these results,” she said.
“Additional research is needed to determine what the seroconversion rate is with the currently available immunizations for SARS-CoV-2,” said Dr. Isaacs. More questions to address include whether there are differences in the different products available, whether immunization after SARS-CoV-2 infection improves both seroconversion and immune reactivity, and whether there is any benefit to transiently stopping dual immune active therapy during the time of immunization, she said.
Further studies can fill knowledge gaps
“There is a knowledge gap in our understanding of susceptibility to SARS-CoV-2 infections among patients with IBD who have previously been infected,” Shirley Cohen-Mekelburg, MD, MS, staff physician and research scientist in the inflammatory bowel disease program at the Veterans Affairs Ann Arbor (Mich.) Healthcare System, said in an interview. ”This is a first step in beginning to narrow this gap – to provide patients and providers with data to drive recommendations during this COVID-19 pandemic.”
She added that, while further work needs to be done, the study findings do support potential benefit for ongoing vigilance among patients receiving infliximab for IBD. “The study findings also drive us to seek answers to more questions: For example, should we consider serological testing for patients on infliximab? How does the presence or absence of anti–SARS-CoV-2 antibodies associate with susceptibility to infection for patients with infliximab?
“Further studies examining anti–SARS-CoV-2 reactivity are necessary to better understand antibody responses between patients with IBD to the general population, or between patients on immunosuppressive therapy and the general population,” she said. “Observational studies are also not designed to examine the causal relationship between infections, medications, and antibody responses. There may be some inherent differences to patients who receive infliximab as compared to vedolizumab for IBD.”
The study was supported by Biogen (Switzerland), Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, Hull University Teaching Hospital NHS Trust, and the Royal Devon and Exeter NHS Foundation Trust. The study authors disclosed financial and nonfinancial relationships with numerous companies, including AbbVie, Biogen, Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, and Immundiagnostik, as well as Janssen, who markets infliximab, and Takeda, who markets vedolizumab. Dr. Isaacs and Dr. Cohen-Mekelburg had no relevant financial conflicts to disclose.
This article was updated 3/31/21.
FROM GUT