User login
2016-2017 flu season continues to wind down
Influenza activity took another healthy step down as outpatient visits continued to drop, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was down to 3.2% for the week ending March 18, 2017, the CDC reported, compared with 3.6% the week before. (The figure of 3.7% previously reported for last week has been adjusted this week, so the halt in the decline in outpatient visits was actually more of a slowdown.) The national baseline for outpatient ILI visits is 2.2%.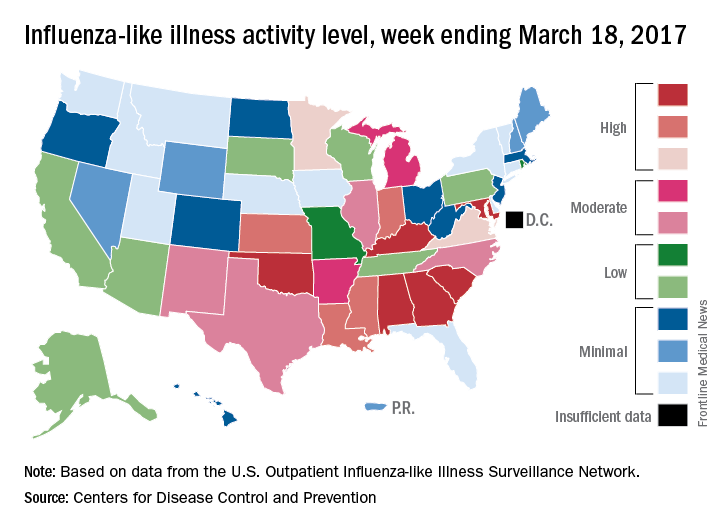
Two flu-related pediatric deaths were reported during the week of March 18, but both occurred earlier: one during the week ending Feb. 18 and the other in the week ending Feb. 25, the CDC reported. The total number of pediatric flu deaths reported is now 55 for the 2016-2017 season.
Influenza activity took another healthy step down as outpatient visits continued to drop, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was down to 3.2% for the week ending March 18, 2017, the CDC reported, compared with 3.6% the week before. (The figure of 3.7% previously reported for last week has been adjusted this week, so the halt in the decline in outpatient visits was actually more of a slowdown.) The national baseline for outpatient ILI visits is 2.2%.
Two flu-related pediatric deaths were reported during the week of March 18, but both occurred earlier: one during the week ending Feb. 18 and the other in the week ending Feb. 25, the CDC reported. The total number of pediatric flu deaths reported is now 55 for the 2016-2017 season.
Influenza activity took another healthy step down as outpatient visits continued to drop, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was down to 3.2% for the week ending March 18, 2017, the CDC reported, compared with 3.6% the week before. (The figure of 3.7% previously reported for last week has been adjusted this week, so the halt in the decline in outpatient visits was actually more of a slowdown.) The national baseline for outpatient ILI visits is 2.2%.
Two flu-related pediatric deaths were reported during the week of March 18, but both occurred earlier: one during the week ending Feb. 18 and the other in the week ending Feb. 25, the CDC reported. The total number of pediatric flu deaths reported is now 55 for the 2016-2017 season.
U.S. influenza activity remains steady
The decline in U.S. influenza activity that started in February paused during the week ending March 11, according to the U.S. Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) stayed at 3.7% for a second consecutive week after declining for 3 weeks in a row. The peak for the season, 5.2%, came during the week ending Feb. 11, CDC data show. The national baseline is 2.2%.
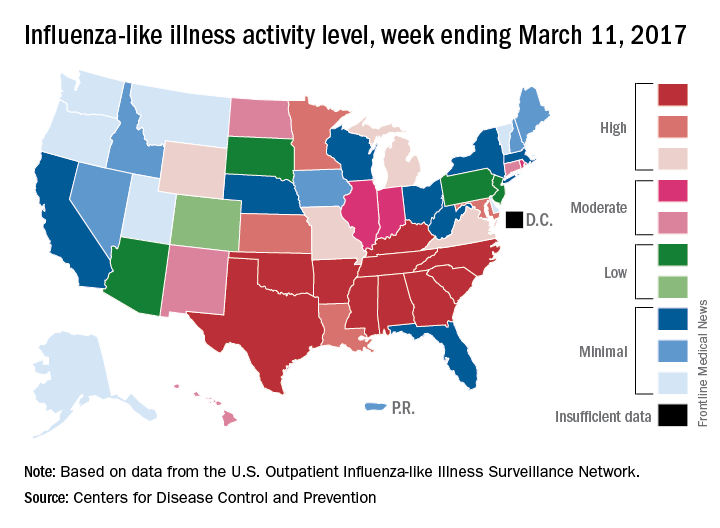
Five ILI-related pediatric deaths were reported to the CDC for the week – all of which occurred during previous weeks – bringing the total to 53 for the 2016-2017 season, the CDC said.
The decline in U.S. influenza activity that started in February paused during the week ending March 11, according to the U.S. Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) stayed at 3.7% for a second consecutive week after declining for 3 weeks in a row. The peak for the season, 5.2%, came during the week ending Feb. 11, CDC data show. The national baseline is 2.2%.

Five ILI-related pediatric deaths were reported to the CDC for the week – all of which occurred during previous weeks – bringing the total to 53 for the 2016-2017 season, the CDC said.
The decline in U.S. influenza activity that started in February paused during the week ending March 11, according to the U.S. Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) stayed at 3.7% for a second consecutive week after declining for 3 weeks in a row. The peak for the season, 5.2%, came during the week ending Feb. 11, CDC data show. The national baseline is 2.2%.

Five ILI-related pediatric deaths were reported to the CDC for the week – all of which occurred during previous weeks – bringing the total to 53 for the 2016-2017 season, the CDC said.
U.S. flu activity continues to decline
The 2016-2017 U.S. influenza season appears to have peaked, as activity measures dropped for the third consecutive week, the Centers for Disease Control and Prevention reported.
For the week ending March 4, there were 11 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, with another three in the “high” range at levels 8 and 9. The previous week (Feb. 25), there were 22 states at level 10, with a total of 27 in the high range of ILI activity. At the peak of activity during the week of Feb. 11, there were 25 states at level 10, data from the CDC’s Outpatient ILI Surveillance Network show.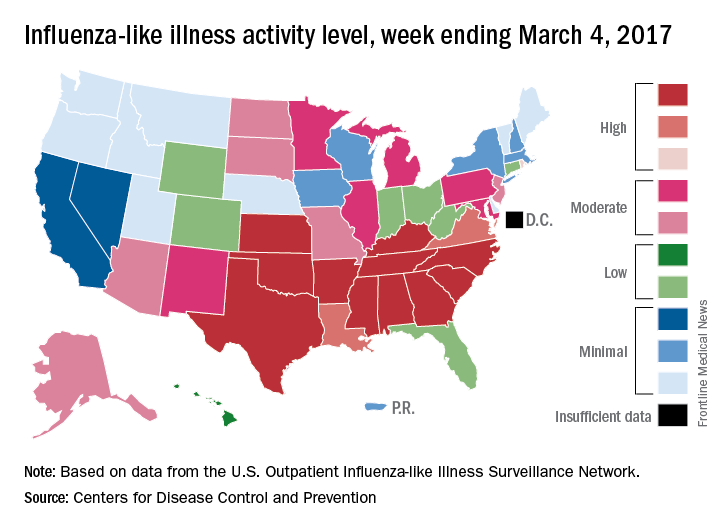
There were eight ILI-related pediatric deaths reported during the week ending March 4, although all occurred in earlier weeks. For the 2016-2017 season so far, 48 ILI-related pediatric deaths have been reported, the CDC said.
For the 70 counties in 13 states that report to the Influenza Hospitalization Surveillance Network, the flu-related hospitalization rate for the season is 43.5 per 100,000 population. The highest rate by age group is for those 65 years and over at 198.8 per 100,000, followed by 50- to 64-year-olds at 42.2 per 100,000 and children aged 0-4 years at 28.8 per 100,000, according to the CDC.
The 2016-2017 U.S. influenza season appears to have peaked, as activity measures dropped for the third consecutive week, the Centers for Disease Control and Prevention reported.
For the week ending March 4, there were 11 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, with another three in the “high” range at levels 8 and 9. The previous week (Feb. 25), there were 22 states at level 10, with a total of 27 in the high range of ILI activity. At the peak of activity during the week of Feb. 11, there were 25 states at level 10, data from the CDC’s Outpatient ILI Surveillance Network show.
There were eight ILI-related pediatric deaths reported during the week ending March 4, although all occurred in earlier weeks. For the 2016-2017 season so far, 48 ILI-related pediatric deaths have been reported, the CDC said.
For the 70 counties in 13 states that report to the Influenza Hospitalization Surveillance Network, the flu-related hospitalization rate for the season is 43.5 per 100,000 population. The highest rate by age group is for those 65 years and over at 198.8 per 100,000, followed by 50- to 64-year-olds at 42.2 per 100,000 and children aged 0-4 years at 28.8 per 100,000, according to the CDC.
The 2016-2017 U.S. influenza season appears to have peaked, as activity measures dropped for the third consecutive week, the Centers for Disease Control and Prevention reported.
For the week ending March 4, there were 11 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, with another three in the “high” range at levels 8 and 9. The previous week (Feb. 25), there were 22 states at level 10, with a total of 27 in the high range of ILI activity. At the peak of activity during the week of Feb. 11, there were 25 states at level 10, data from the CDC’s Outpatient ILI Surveillance Network show.
There were eight ILI-related pediatric deaths reported during the week ending March 4, although all occurred in earlier weeks. For the 2016-2017 season so far, 48 ILI-related pediatric deaths have been reported, the CDC said.
For the 70 counties in 13 states that report to the Influenza Hospitalization Surveillance Network, the flu-related hospitalization rate for the season is 43.5 per 100,000 population. The highest rate by age group is for those 65 years and over at 198.8 per 100,000, followed by 50- to 64-year-olds at 42.2 per 100,000 and children aged 0-4 years at 28.8 per 100,000, according to the CDC.
FDA committee approves strains for 2017-2018 flu shot
ROCKVILLE, MD. – A committee of Food and Drug Administration advisers backed the World Health Organization’s influenza vaccine recommendations for the 2017-2018 season at a meeting March 9.
In a unanimous vote, members of the Vaccines and Related Biological Products Advisory Committee recommended that trivalent vaccines for the 2017-2018 season should contain the following vaccine strains: A/Michigan/45/2015(H1N1)pdm09-like, A/Hong Kong/4801/2014(H3N2)-like, and B/Brisbane/60/2008-like.
These recommendations echo those from the 2016-2017 season, with the exception of a slight update to the H1N1 strain, which had previously been A/California/7/2009(H1N1)pdm09-like virus.
Regarding vaccine efficacy, the cell propagated A/Hong Kong strain was the strongest candidate, covering 93% of A(H3N2) viruses seen in the 2016-2017 season, according to Jacqueline Katz, PhD, director of the WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza at the Centers for Disease Control and Prevention. In comparison, the egg propagated version of the A/Hong Kong virus covered 59%.
For the influenza B virus, the Yamagata lineage and Victoria lineage strain cycled monthly as the predominant strain in the 2016-2017 season, with a split of “around 50/50,” leaning toward Yamagata in North America, Europe, and Oceana, Dr. Katz explained. The Victoria lineage, in some cases, accounted for nearly 75% of B viruses in Africa and South America.
Committee members expressed concern over the difference between strain prevalence in the United States and abroad and considered recommending a strain that did not coincide with the WHO recommendation, something that has not happened in the history of the advisory committee.
“I’m very aware of influenza vaccinations being a global enterprise, and companies manufacture vaccines for use in multiple countries,” said Committee Chair Kathryn Edwards, MD, professor of pediatrics at Vanderbilt University, Nashville, Tenn. “If we to select a B strain that differed from the WHO recommendation, would that adversely impact vaccine production for the U.S. market?”
Despite these questions, the committee continued to back the WHO recommendations.
Historically, the advisory committee has recommended flu vaccine strains earlier in the year, according to Beverly Taylor, PhD, head of influenza scientific affairs and pandemic readiness at Seqirus Vaccines. Dr. Taylor presented the vaccine manufacturers’ perspective. The delay has put added pressure on manufacturers.
“We haven’t seen impacts yet on start of vaccination dates,” said Dr. Taylor. “But the very clear message from manufacturers is if you keep squashing that manufacturing window, then there will reach a point where we are concerned we will see an impact on vaccine supply time.”
None of the committee members presented waivers of conflict of interest. While the FDA is not obligated to follow the recommendations of the advisory committee, it generally does.
[email protected]
On Twitter @EAZTweets
ROCKVILLE, MD. – A committee of Food and Drug Administration advisers backed the World Health Organization’s influenza vaccine recommendations for the 2017-2018 season at a meeting March 9.
In a unanimous vote, members of the Vaccines and Related Biological Products Advisory Committee recommended that trivalent vaccines for the 2017-2018 season should contain the following vaccine strains: A/Michigan/45/2015(H1N1)pdm09-like, A/Hong Kong/4801/2014(H3N2)-like, and B/Brisbane/60/2008-like.
These recommendations echo those from the 2016-2017 season, with the exception of a slight update to the H1N1 strain, which had previously been A/California/7/2009(H1N1)pdm09-like virus.
Regarding vaccine efficacy, the cell propagated A/Hong Kong strain was the strongest candidate, covering 93% of A(H3N2) viruses seen in the 2016-2017 season, according to Jacqueline Katz, PhD, director of the WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza at the Centers for Disease Control and Prevention. In comparison, the egg propagated version of the A/Hong Kong virus covered 59%.
For the influenza B virus, the Yamagata lineage and Victoria lineage strain cycled monthly as the predominant strain in the 2016-2017 season, with a split of “around 50/50,” leaning toward Yamagata in North America, Europe, and Oceana, Dr. Katz explained. The Victoria lineage, in some cases, accounted for nearly 75% of B viruses in Africa and South America.
Committee members expressed concern over the difference between strain prevalence in the United States and abroad and considered recommending a strain that did not coincide with the WHO recommendation, something that has not happened in the history of the advisory committee.
“I’m very aware of influenza vaccinations being a global enterprise, and companies manufacture vaccines for use in multiple countries,” said Committee Chair Kathryn Edwards, MD, professor of pediatrics at Vanderbilt University, Nashville, Tenn. “If we to select a B strain that differed from the WHO recommendation, would that adversely impact vaccine production for the U.S. market?”
Despite these questions, the committee continued to back the WHO recommendations.
Historically, the advisory committee has recommended flu vaccine strains earlier in the year, according to Beverly Taylor, PhD, head of influenza scientific affairs and pandemic readiness at Seqirus Vaccines. Dr. Taylor presented the vaccine manufacturers’ perspective. The delay has put added pressure on manufacturers.
“We haven’t seen impacts yet on start of vaccination dates,” said Dr. Taylor. “But the very clear message from manufacturers is if you keep squashing that manufacturing window, then there will reach a point where we are concerned we will see an impact on vaccine supply time.”
None of the committee members presented waivers of conflict of interest. While the FDA is not obligated to follow the recommendations of the advisory committee, it generally does.
[email protected]
On Twitter @EAZTweets
ROCKVILLE, MD. – A committee of Food and Drug Administration advisers backed the World Health Organization’s influenza vaccine recommendations for the 2017-2018 season at a meeting March 9.
In a unanimous vote, members of the Vaccines and Related Biological Products Advisory Committee recommended that trivalent vaccines for the 2017-2018 season should contain the following vaccine strains: A/Michigan/45/2015(H1N1)pdm09-like, A/Hong Kong/4801/2014(H3N2)-like, and B/Brisbane/60/2008-like.
These recommendations echo those from the 2016-2017 season, with the exception of a slight update to the H1N1 strain, which had previously been A/California/7/2009(H1N1)pdm09-like virus.
Regarding vaccine efficacy, the cell propagated A/Hong Kong strain was the strongest candidate, covering 93% of A(H3N2) viruses seen in the 2016-2017 season, according to Jacqueline Katz, PhD, director of the WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza at the Centers for Disease Control and Prevention. In comparison, the egg propagated version of the A/Hong Kong virus covered 59%.
For the influenza B virus, the Yamagata lineage and Victoria lineage strain cycled monthly as the predominant strain in the 2016-2017 season, with a split of “around 50/50,” leaning toward Yamagata in North America, Europe, and Oceana, Dr. Katz explained. The Victoria lineage, in some cases, accounted for nearly 75% of B viruses in Africa and South America.
Committee members expressed concern over the difference between strain prevalence in the United States and abroad and considered recommending a strain that did not coincide with the WHO recommendation, something that has not happened in the history of the advisory committee.
“I’m very aware of influenza vaccinations being a global enterprise, and companies manufacture vaccines for use in multiple countries,” said Committee Chair Kathryn Edwards, MD, professor of pediatrics at Vanderbilt University, Nashville, Tenn. “If we to select a B strain that differed from the WHO recommendation, would that adversely impact vaccine production for the U.S. market?”
Despite these questions, the committee continued to back the WHO recommendations.
Historically, the advisory committee has recommended flu vaccine strains earlier in the year, according to Beverly Taylor, PhD, head of influenza scientific affairs and pandemic readiness at Seqirus Vaccines. Dr. Taylor presented the vaccine manufacturers’ perspective. The delay has put added pressure on manufacturers.
“We haven’t seen impacts yet on start of vaccination dates,” said Dr. Taylor. “But the very clear message from manufacturers is if you keep squashing that manufacturing window, then there will reach a point where we are concerned we will see an impact on vaccine supply time.”
None of the committee members presented waivers of conflict of interest. While the FDA is not obligated to follow the recommendations of the advisory committee, it generally does.
[email protected]
On Twitter @EAZTweets
AT AN FDA ADVISORY COMMITTEE MEETING
Outpatient flu visits down slightly
The overall national measure of outpatient flu activity was down for the week ending Feb. 18, and the number of states at the highest level of activity dropped from 25 to 24, according to the Centers for Disease Control and Prevention.
The national proportion of outpatient visits for influenza-like illness (ILI) decreased from 5.2% the previous week to 4.8% for the week ending Feb. 18, the CDC reported.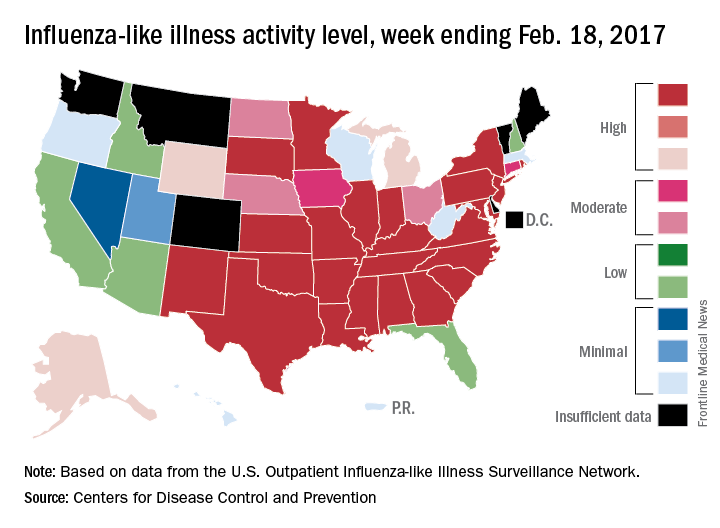
There were 5 ILI-related pediatric deaths reported during the week, bringing the total to 34 for the season so far, but none of the 5 occurred in the current week, the CDC said. There were 89 pediatric deaths reported during the 2015-2016 season, with the peak week occurring in late March/early April (11 deaths). During the 2014-2015 season, there were 148 deaths reported, and 111 were reported in 2013-2014.
The overall national measure of outpatient flu activity was down for the week ending Feb. 18, and the number of states at the highest level of activity dropped from 25 to 24, according to the Centers for Disease Control and Prevention.
The national proportion of outpatient visits for influenza-like illness (ILI) decreased from 5.2% the previous week to 4.8% for the week ending Feb. 18, the CDC reported.
There were 5 ILI-related pediatric deaths reported during the week, bringing the total to 34 for the season so far, but none of the 5 occurred in the current week, the CDC said. There were 89 pediatric deaths reported during the 2015-2016 season, with the peak week occurring in late March/early April (11 deaths). During the 2014-2015 season, there were 148 deaths reported, and 111 were reported in 2013-2014.
The overall national measure of outpatient flu activity was down for the week ending Feb. 18, and the number of states at the highest level of activity dropped from 25 to 24, according to the Centers for Disease Control and Prevention.
The national proportion of outpatient visits for influenza-like illness (ILI) decreased from 5.2% the previous week to 4.8% for the week ending Feb. 18, the CDC reported.
There were 5 ILI-related pediatric deaths reported during the week, bringing the total to 34 for the season so far, but none of the 5 occurred in the current week, the CDC said. There were 89 pediatric deaths reported during the 2015-2016 season, with the peak week occurring in late March/early April (11 deaths). During the 2014-2015 season, there were 148 deaths reported, and 111 were reported in 2013-2014.
Twenty-five states at highest flu activity level
Flu activity in the United States continued to increase as half of the states reached the highest level of influenza-like illness (ILI) activity in the week ending Feb. 11, according to the Centers for Disease Control and Prevention.
For the week, the 25 states at level 10 on the CDC’s 1-10 scale of ILI activity were joined in the high range by Illinois and Kentucky at level 9 and Iowa at level 8, the CDC reported. The previous week, there were 23 states in the high range.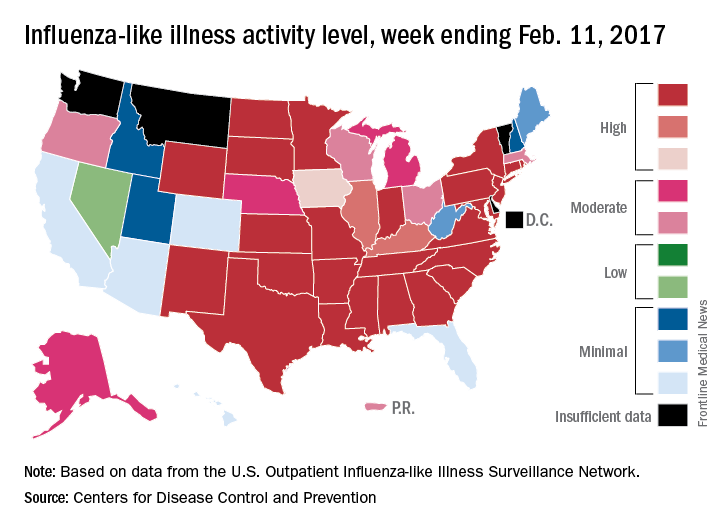
Of the nine flu-related pediatric deaths reported to the CDC during the latest week, eight occurred in earlier weeks. For the 2016-2017 season so far, 29 flu-related pediatric deaths have been reported, the CDC said.
Flu activity in the United States continued to increase as half of the states reached the highest level of influenza-like illness (ILI) activity in the week ending Feb. 11, according to the Centers for Disease Control and Prevention.
For the week, the 25 states at level 10 on the CDC’s 1-10 scale of ILI activity were joined in the high range by Illinois and Kentucky at level 9 and Iowa at level 8, the CDC reported. The previous week, there were 23 states in the high range.
Of the nine flu-related pediatric deaths reported to the CDC during the latest week, eight occurred in earlier weeks. For the 2016-2017 season so far, 29 flu-related pediatric deaths have been reported, the CDC said.
Flu activity in the United States continued to increase as half of the states reached the highest level of influenza-like illness (ILI) activity in the week ending Feb. 11, according to the Centers for Disease Control and Prevention.
For the week, the 25 states at level 10 on the CDC’s 1-10 scale of ILI activity were joined in the high range by Illinois and Kentucky at level 9 and Iowa at level 8, the CDC reported. The previous week, there were 23 states in the high range.
Of the nine flu-related pediatric deaths reported to the CDC during the latest week, eight occurred in earlier weeks. For the 2016-2017 season so far, 29 flu-related pediatric deaths have been reported, the CDC said.
Flu activity levels high in 23 states
The number of states at the highest level of flu activity jumped from 7 to 19 during the week ending Feb. 4, 2017, according to the Centers for Disease Control and Prevention.
The 19 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity were largely concentrated in the South and lower Midwest, with another grouping mainly in the Mid-Atlantic. They were joined in the “high” range of ILI activity by four other states: Indiana and Minnesota at level 9 and New Mexico and Virginia at level 8, the CDC reported.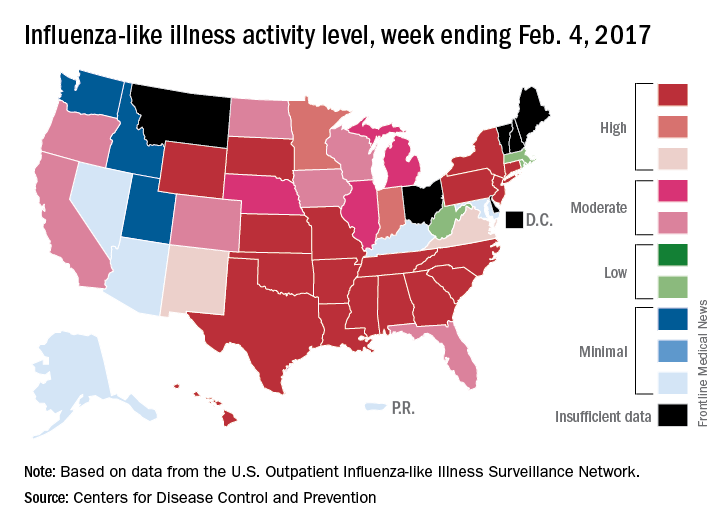
For the 2016-2017 season, 20 flu-related pediatric deaths have been reported: 5 were reported during the week ending Feb. 4, but 4 occurred during the week ending Jan. 28 and 1 occurred during the week ending Jan. 14, according to the CDC.
Since Oct. 1, 2016, there have been 6,804 laboratory-confirmed flu-related hospitalizations reported in the 13 states – including California, Georgia, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 24.3 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
The number of states at the highest level of flu activity jumped from 7 to 19 during the week ending Feb. 4, 2017, according to the Centers for Disease Control and Prevention.
The 19 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity were largely concentrated in the South and lower Midwest, with another grouping mainly in the Mid-Atlantic. They were joined in the “high” range of ILI activity by four other states: Indiana and Minnesota at level 9 and New Mexico and Virginia at level 8, the CDC reported.
For the 2016-2017 season, 20 flu-related pediatric deaths have been reported: 5 were reported during the week ending Feb. 4, but 4 occurred during the week ending Jan. 28 and 1 occurred during the week ending Jan. 14, according to the CDC.
Since Oct. 1, 2016, there have been 6,804 laboratory-confirmed flu-related hospitalizations reported in the 13 states – including California, Georgia, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 24.3 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
The number of states at the highest level of flu activity jumped from 7 to 19 during the week ending Feb. 4, 2017, according to the Centers for Disease Control and Prevention.
The 19 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity were largely concentrated in the South and lower Midwest, with another grouping mainly in the Mid-Atlantic. They were joined in the “high” range of ILI activity by four other states: Indiana and Minnesota at level 9 and New Mexico and Virginia at level 8, the CDC reported.
For the 2016-2017 season, 20 flu-related pediatric deaths have been reported: 5 were reported during the week ending Feb. 4, but 4 occurred during the week ending Jan. 28 and 1 occurred during the week ending Jan. 14, according to the CDC.
Since Oct. 1, 2016, there have been 6,804 laboratory-confirmed flu-related hospitalizations reported in the 13 states – including California, Georgia, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 24.3 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
High levels of flu activity reported in 15 states
Fifteen states experienced high levels of flu activity for the week ending Jan. 28 as the 2016-2017 season moved past last season’s peak, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 3.9% for the week ending Jan. 28, 2017, the CDC reported. That level is higher than the national baseline level of 2.2% and higher than the peak of 3.6% for the 2015-2016 season.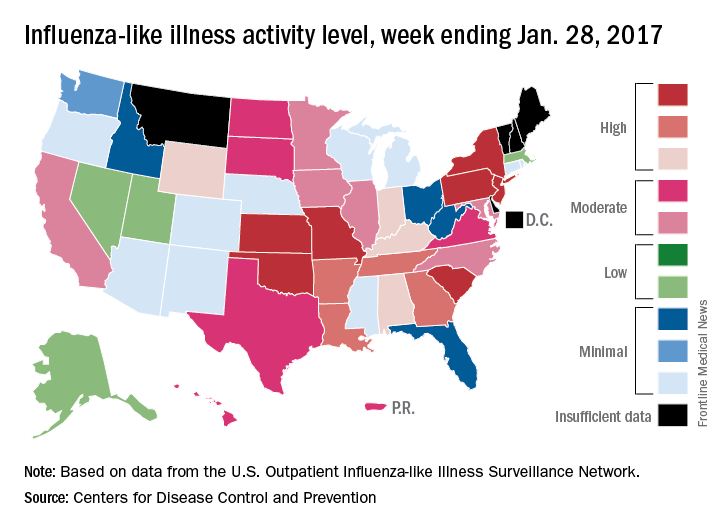
The week also brought reports of seven flu-related pediatric deaths, although five actually occurred during previous weeks. There have now been 15 flu-related pediatric deaths during the 2016-2017 season, the CDC reported.
Fifteen states experienced high levels of flu activity for the week ending Jan. 28 as the 2016-2017 season moved past last season’s peak, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 3.9% for the week ending Jan. 28, 2017, the CDC reported. That level is higher than the national baseline level of 2.2% and higher than the peak of 3.6% for the 2015-2016 season.
The week also brought reports of seven flu-related pediatric deaths, although five actually occurred during previous weeks. There have now been 15 flu-related pediatric deaths during the 2016-2017 season, the CDC reported.
Fifteen states experienced high levels of flu activity for the week ending Jan. 28 as the 2016-2017 season moved past last season’s peak, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 3.9% for the week ending Jan. 28, 2017, the CDC reported. That level is higher than the national baseline level of 2.2% and higher than the peak of 3.6% for the 2015-2016 season.
The week also brought reports of seven flu-related pediatric deaths, although five actually occurred during previous weeks. There have now been 15 flu-related pediatric deaths during the 2016-2017 season, the CDC reported.
Increase brings flu activity back to seasonal high
for the week ending Jan. 21, compared with three states the week before, according to the Centers for Disease Control and Prevention.
Alabama, Kansas, New Jersey, Oklahoma, and South Carolina were at level 10 on the CDC’s 1-10 scale of ILI activity, with Oklahoma reaching that level for the third consecutive week. Georgia (level 9) and Louisiana, Minnesota, Missouri, and Tennessee (level 8) were also in the “high” range, the CDC reported.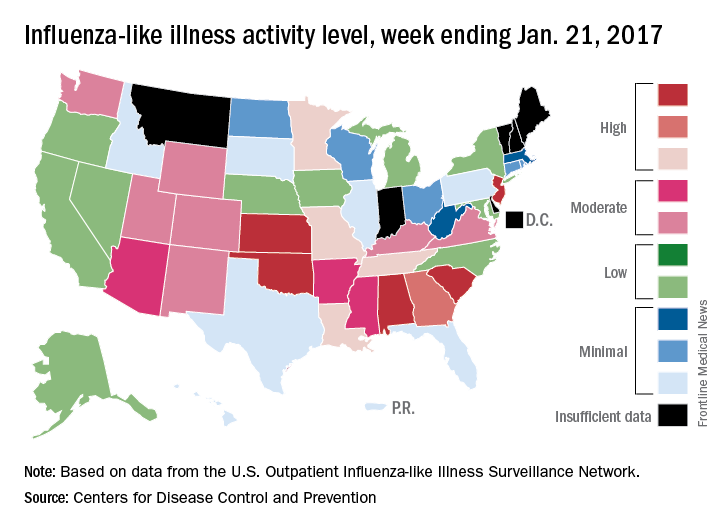
Three flu-related pediatric deaths were reported for the week, although two occurred during the week ending Jan. 14. The two earlier deaths were associated with an influenza A (H3) virus, and the more recent death was associated with an influenza B virus. For the 2016-2017 season so far, there have been a total of eight pediatric deaths, the CDC said.
for the week ending Jan. 21, compared with three states the week before, according to the Centers for Disease Control and Prevention.
Alabama, Kansas, New Jersey, Oklahoma, and South Carolina were at level 10 on the CDC’s 1-10 scale of ILI activity, with Oklahoma reaching that level for the third consecutive week. Georgia (level 9) and Louisiana, Minnesota, Missouri, and Tennessee (level 8) were also in the “high” range, the CDC reported.
Three flu-related pediatric deaths were reported for the week, although two occurred during the week ending Jan. 14. The two earlier deaths were associated with an influenza A (H3) virus, and the more recent death was associated with an influenza B virus. For the 2016-2017 season so far, there have been a total of eight pediatric deaths, the CDC said.
for the week ending Jan. 21, compared with three states the week before, according to the Centers for Disease Control and Prevention.
Alabama, Kansas, New Jersey, Oklahoma, and South Carolina were at level 10 on the CDC’s 1-10 scale of ILI activity, with Oklahoma reaching that level for the third consecutive week. Georgia (level 9) and Louisiana, Minnesota, Missouri, and Tennessee (level 8) were also in the “high” range, the CDC reported.
Three flu-related pediatric deaths were reported for the week, although two occurred during the week ending Jan. 14. The two earlier deaths were associated with an influenza A (H3) virus, and the more recent death was associated with an influenza B virus. For the 2016-2017 season so far, there have been a total of eight pediatric deaths, the CDC said.
Flu activity up slightly, but still down from seasonal peak
After rising to a high point for the season in the last week of 2016, influenza activity dropped a bit in the first week of the new year but then rose again in the second week, according to the Centers for Disease Control and Prevention.
As measured by outpatient visits for influenza-like illness (ILI), activity slipped from 3.4% at the end of 2016 to 3.2% for the week ending Jan. 7 but then ticked up to 3.3% for the week ending Jan. 14, the CDC reported. The national baseline level of outpatient visits is 2.2% for ILI, which is defined as fever (temperature of 100° F or greater) and cough and/or sore throat.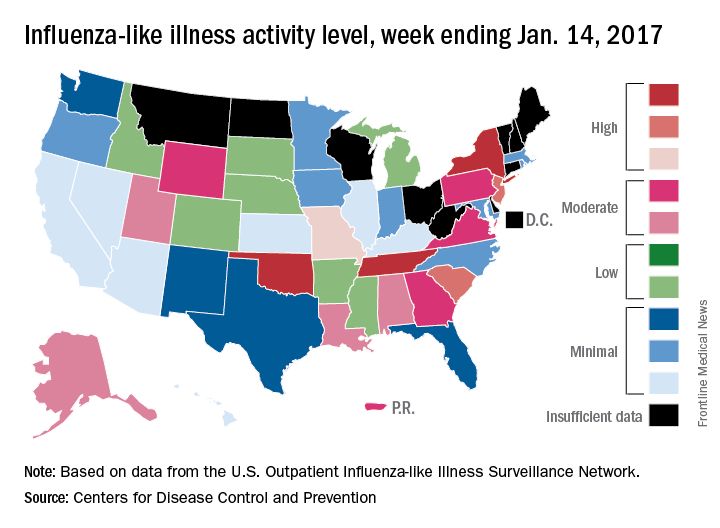
Two influenza-related pediatric deaths were reported for the week ending Jan. 14, although both occurred in earlier weeks: one during the week ending Dec. 10 and one during the week ending Jan. 7. So far for the 2016-2017 season, a total of five flu-related pediatric deaths have been reported, according to the CDC.
After rising to a high point for the season in the last week of 2016, influenza activity dropped a bit in the first week of the new year but then rose again in the second week, according to the Centers for Disease Control and Prevention.
As measured by outpatient visits for influenza-like illness (ILI), activity slipped from 3.4% at the end of 2016 to 3.2% for the week ending Jan. 7 but then ticked up to 3.3% for the week ending Jan. 14, the CDC reported. The national baseline level of outpatient visits is 2.2% for ILI, which is defined as fever (temperature of 100° F or greater) and cough and/or sore throat.
Two influenza-related pediatric deaths were reported for the week ending Jan. 14, although both occurred in earlier weeks: one during the week ending Dec. 10 and one during the week ending Jan. 7. So far for the 2016-2017 season, a total of five flu-related pediatric deaths have been reported, according to the CDC.
After rising to a high point for the season in the last week of 2016, influenza activity dropped a bit in the first week of the new year but then rose again in the second week, according to the Centers for Disease Control and Prevention.
As measured by outpatient visits for influenza-like illness (ILI), activity slipped from 3.4% at the end of 2016 to 3.2% for the week ending Jan. 7 but then ticked up to 3.3% for the week ending Jan. 14, the CDC reported. The national baseline level of outpatient visits is 2.2% for ILI, which is defined as fever (temperature of 100° F or greater) and cough and/or sore throat.
Two influenza-related pediatric deaths were reported for the week ending Jan. 14, although both occurred in earlier weeks: one during the week ending Dec. 10 and one during the week ending Jan. 7. So far for the 2016-2017 season, a total of five flu-related pediatric deaths have been reported, according to the CDC.