User login
Malawi declares polio outbreak after girl, 3, paralyzed
Health authorities in Malawi have declared an outbreak of wild poliovirus type 1 after a case was confirmed in a 3-year-old girl in the capital, Lilongwe. It was the first case in Africa in 5 years, according to the World Health Organization.
Globally, there were only five cases of wild poliovirus in 2021, the WHO states.
“As long as wild polio exists anywhere in the world all countries remain at risk of importation of the virus,” Matshidiso Moeti, MBBS, WHO regional director for Africa, said in the statement.
Girl paralyzed in November
The Global Polio Eradication Initiative (GPEI) said in a statement that the 3-year-old girl experienced paralysis in November, and stool specimens were collected. Sequencing of the virus was conducted in February, 2022, by the National Institute for Communicable Diseases in South Africa, and the Centers for Disease Control and Prevention confirmed the case as WPV1.
According to the WHO announcement, laboratory analysis shows that the strain identified in Malawi is linked to one circulating in Sindh Province in Pakistan. Polio remains endemic only in Afghanistan and Pakistan.
Kacey C. Ernst, PhD, MPH, professor and infectious disease epidemiologist at the University of Arizona’s Zuckerman College of Public Health in Tucson, pointed out that what is not clear from the press release is whether the girl had traveled to Pakistan or was infected in Malawi.
“This is a very significant detail that would indicate whether or not transmission was actively occurring in Malawi. Until that information is released, it is hard to judge the extent of the possible outbreak,” she said in an interview. “The good news is that this case was in fact detected. The surveillance systems are in place and they were able to identify wild-type cases.”
Dr. Ernst said that although there is cause for concern, it is “not a reason to panic. Malawi has very high polio vaccination rates and it is quite possible that this will be a very small defined outbreak that will be well contained.”
She added that the medical community should be alerted that this case has been identified so travelers who have been to affected areas who have any symptoms can be appropriately screened.
The WHO said it is helping Malawi health authorities in the response, including increasing immunizations.
However, a vaccination campaign comes at a time of health system upheaval in Malawi.
“Malawi, like countries all over the world, has seen an interruption in services due to COVID,” Joia S. Mukherjee, MD, MPH, chief medical officer with Partners in Health and associate professor with the division of global health equity at Brigham and Women’s Hospital and in the department of global health and social medicine at Harvard Medical School, Boston, said in an interview. “In addition, Malawi is currently dealing with the aftermath of a cyclone – where nearly a million people were displaced. Vaccination campaigns work best if there is solid infrastructure. Both COVID and the impact of climate change have shaken the health system.”
UN health agencies warned last year that millions of children who have not received immunizations during the pandemic, especially in Africa, “are now at risk from life-threatening diseases such as measles, polio, yellow fever, and diphtheria,” Reuters reported.
Africa was certified as wild poliovirus free on Aug. 25, 2020. The CDC had served as the lead partner over 3 decades in helping Africa reach the milestone. Africa will retain that status, the WHO stated, because the strain originated in Pakistan.
Five of six WHO regions have been certified polio free. The Americas received eradication certification in 1994.
There is no cure for polio, which can cause irreversible paralysis within hours, but the disease has been largely eradicated globally with an effective vaccine.
GPEI sending teams
The GPEI is sending a team to Malawi to support emergency operations, communications, and surveillance. Partner organizations will also send teams to support operations and innovative vaccination campaign solutions.
GPEI was launched in 1988 with the combined efforts of national governments, WHO, Rotary International, the CDC, and UNICEF. The GPEI partnership has included the Bill & Melinda Gates Foundation and, in recent years, Gavi, the Vaccine Alliance.
The CDC states, “[G]lobal incidence of polio has decreased by 99.9% since GPEI’s foundation. An estimated 16 million people today are walking who would otherwise have been paralyzed by the disease, and more than 1.5 million people are alive, whose lives would otherwise have been lost. Now the task remains to tackle polio in its last few strongholds and get rid of the final 0.1% of polio cases.”
Three wild poliovirus strains
There are three wild poliovirus strains: type 1 (WPV1), type 2 (WPV2), and type 3 (WPV3).
“Symptomatically, all three strains are identical, in that they cause irreversible paralysis or even death. But there are genetic and virologic differences which make these three strains three separate viruses that must each be eradicated individually,” according to WHO.
WPV3 is the second strain to be wiped out, following the certification of the eradication of WPV2 in 2015.
A version of this article first appeared on Medscape.com.
Health authorities in Malawi have declared an outbreak of wild poliovirus type 1 after a case was confirmed in a 3-year-old girl in the capital, Lilongwe. It was the first case in Africa in 5 years, according to the World Health Organization.
Globally, there were only five cases of wild poliovirus in 2021, the WHO states.
“As long as wild polio exists anywhere in the world all countries remain at risk of importation of the virus,” Matshidiso Moeti, MBBS, WHO regional director for Africa, said in the statement.
Girl paralyzed in November
The Global Polio Eradication Initiative (GPEI) said in a statement that the 3-year-old girl experienced paralysis in November, and stool specimens were collected. Sequencing of the virus was conducted in February, 2022, by the National Institute for Communicable Diseases in South Africa, and the Centers for Disease Control and Prevention confirmed the case as WPV1.
According to the WHO announcement, laboratory analysis shows that the strain identified in Malawi is linked to one circulating in Sindh Province in Pakistan. Polio remains endemic only in Afghanistan and Pakistan.
Kacey C. Ernst, PhD, MPH, professor and infectious disease epidemiologist at the University of Arizona’s Zuckerman College of Public Health in Tucson, pointed out that what is not clear from the press release is whether the girl had traveled to Pakistan or was infected in Malawi.
“This is a very significant detail that would indicate whether or not transmission was actively occurring in Malawi. Until that information is released, it is hard to judge the extent of the possible outbreak,” she said in an interview. “The good news is that this case was in fact detected. The surveillance systems are in place and they were able to identify wild-type cases.”
Dr. Ernst said that although there is cause for concern, it is “not a reason to panic. Malawi has very high polio vaccination rates and it is quite possible that this will be a very small defined outbreak that will be well contained.”
She added that the medical community should be alerted that this case has been identified so travelers who have been to affected areas who have any symptoms can be appropriately screened.
The WHO said it is helping Malawi health authorities in the response, including increasing immunizations.
However, a vaccination campaign comes at a time of health system upheaval in Malawi.
“Malawi, like countries all over the world, has seen an interruption in services due to COVID,” Joia S. Mukherjee, MD, MPH, chief medical officer with Partners in Health and associate professor with the division of global health equity at Brigham and Women’s Hospital and in the department of global health and social medicine at Harvard Medical School, Boston, said in an interview. “In addition, Malawi is currently dealing with the aftermath of a cyclone – where nearly a million people were displaced. Vaccination campaigns work best if there is solid infrastructure. Both COVID and the impact of climate change have shaken the health system.”
UN health agencies warned last year that millions of children who have not received immunizations during the pandemic, especially in Africa, “are now at risk from life-threatening diseases such as measles, polio, yellow fever, and diphtheria,” Reuters reported.
Africa was certified as wild poliovirus free on Aug. 25, 2020. The CDC had served as the lead partner over 3 decades in helping Africa reach the milestone. Africa will retain that status, the WHO stated, because the strain originated in Pakistan.
Five of six WHO regions have been certified polio free. The Americas received eradication certification in 1994.
There is no cure for polio, which can cause irreversible paralysis within hours, but the disease has been largely eradicated globally with an effective vaccine.
GPEI sending teams
The GPEI is sending a team to Malawi to support emergency operations, communications, and surveillance. Partner organizations will also send teams to support operations and innovative vaccination campaign solutions.
GPEI was launched in 1988 with the combined efforts of national governments, WHO, Rotary International, the CDC, and UNICEF. The GPEI partnership has included the Bill & Melinda Gates Foundation and, in recent years, Gavi, the Vaccine Alliance.
The CDC states, “[G]lobal incidence of polio has decreased by 99.9% since GPEI’s foundation. An estimated 16 million people today are walking who would otherwise have been paralyzed by the disease, and more than 1.5 million people are alive, whose lives would otherwise have been lost. Now the task remains to tackle polio in its last few strongholds and get rid of the final 0.1% of polio cases.”
Three wild poliovirus strains
There are three wild poliovirus strains: type 1 (WPV1), type 2 (WPV2), and type 3 (WPV3).
“Symptomatically, all three strains are identical, in that they cause irreversible paralysis or even death. But there are genetic and virologic differences which make these three strains three separate viruses that must each be eradicated individually,” according to WHO.
WPV3 is the second strain to be wiped out, following the certification of the eradication of WPV2 in 2015.
A version of this article first appeared on Medscape.com.
Health authorities in Malawi have declared an outbreak of wild poliovirus type 1 after a case was confirmed in a 3-year-old girl in the capital, Lilongwe. It was the first case in Africa in 5 years, according to the World Health Organization.
Globally, there were only five cases of wild poliovirus in 2021, the WHO states.
“As long as wild polio exists anywhere in the world all countries remain at risk of importation of the virus,” Matshidiso Moeti, MBBS, WHO regional director for Africa, said in the statement.
Girl paralyzed in November
The Global Polio Eradication Initiative (GPEI) said in a statement that the 3-year-old girl experienced paralysis in November, and stool specimens were collected. Sequencing of the virus was conducted in February, 2022, by the National Institute for Communicable Diseases in South Africa, and the Centers for Disease Control and Prevention confirmed the case as WPV1.
According to the WHO announcement, laboratory analysis shows that the strain identified in Malawi is linked to one circulating in Sindh Province in Pakistan. Polio remains endemic only in Afghanistan and Pakistan.
Kacey C. Ernst, PhD, MPH, professor and infectious disease epidemiologist at the University of Arizona’s Zuckerman College of Public Health in Tucson, pointed out that what is not clear from the press release is whether the girl had traveled to Pakistan or was infected in Malawi.
“This is a very significant detail that would indicate whether or not transmission was actively occurring in Malawi. Until that information is released, it is hard to judge the extent of the possible outbreak,” she said in an interview. “The good news is that this case was in fact detected. The surveillance systems are in place and they were able to identify wild-type cases.”
Dr. Ernst said that although there is cause for concern, it is “not a reason to panic. Malawi has very high polio vaccination rates and it is quite possible that this will be a very small defined outbreak that will be well contained.”
She added that the medical community should be alerted that this case has been identified so travelers who have been to affected areas who have any symptoms can be appropriately screened.
The WHO said it is helping Malawi health authorities in the response, including increasing immunizations.
However, a vaccination campaign comes at a time of health system upheaval in Malawi.
“Malawi, like countries all over the world, has seen an interruption in services due to COVID,” Joia S. Mukherjee, MD, MPH, chief medical officer with Partners in Health and associate professor with the division of global health equity at Brigham and Women’s Hospital and in the department of global health and social medicine at Harvard Medical School, Boston, said in an interview. “In addition, Malawi is currently dealing with the aftermath of a cyclone – where nearly a million people were displaced. Vaccination campaigns work best if there is solid infrastructure. Both COVID and the impact of climate change have shaken the health system.”
UN health agencies warned last year that millions of children who have not received immunizations during the pandemic, especially in Africa, “are now at risk from life-threatening diseases such as measles, polio, yellow fever, and diphtheria,” Reuters reported.
Africa was certified as wild poliovirus free on Aug. 25, 2020. The CDC had served as the lead partner over 3 decades in helping Africa reach the milestone. Africa will retain that status, the WHO stated, because the strain originated in Pakistan.
Five of six WHO regions have been certified polio free. The Americas received eradication certification in 1994.
There is no cure for polio, which can cause irreversible paralysis within hours, but the disease has been largely eradicated globally with an effective vaccine.
GPEI sending teams
The GPEI is sending a team to Malawi to support emergency operations, communications, and surveillance. Partner organizations will also send teams to support operations and innovative vaccination campaign solutions.
GPEI was launched in 1988 with the combined efforts of national governments, WHO, Rotary International, the CDC, and UNICEF. The GPEI partnership has included the Bill & Melinda Gates Foundation and, in recent years, Gavi, the Vaccine Alliance.
The CDC states, “[G]lobal incidence of polio has decreased by 99.9% since GPEI’s foundation. An estimated 16 million people today are walking who would otherwise have been paralyzed by the disease, and more than 1.5 million people are alive, whose lives would otherwise have been lost. Now the task remains to tackle polio in its last few strongholds and get rid of the final 0.1% of polio cases.”
Three wild poliovirus strains
There are three wild poliovirus strains: type 1 (WPV1), type 2 (WPV2), and type 3 (WPV3).
“Symptomatically, all three strains are identical, in that they cause irreversible paralysis or even death. But there are genetic and virologic differences which make these three strains three separate viruses that must each be eradicated individually,” according to WHO.
WPV3 is the second strain to be wiped out, following the certification of the eradication of WPV2 in 2015.
A version of this article first appeared on Medscape.com.
Mosquito nets do prevent malaria, longitudinal study confirms
It seems obvious that increased use of mosquito bed nets in sub-Saharan Africa would decrease the incidence of malaria, but a lingering question remained:
Malaria from Plasmodium falciparum infection exacts a significant toll in sub-Saharan Africa. According to the World Health Organization, there were about 228 million cases and 602,000 deaths from malaria in 2020 alone. About 80% of those deaths were in children less than 5 years old. In some areas, as many as 5% of children die from malaria by age 5.
Efforts to reduce the burden of malaria have been ongoing for decades. In the 1990s, insecticide-treated nets were shown to reduce illness and deaths from malaria in children.
As a result, the use of bed nets has grown significantly. In 2000, only 5% of households in sub-Saharan Africa had a net in the house. By 2020, that number had risen to 65%. From 2004 to 2019 about 1.9 billion nets were distributed in this region. The nets are estimated to have prevented more than 663 million malaria cases between 2000 and 2015.
As described in the NEJM report, public health researchers conducted a 22-year prospective longitudinal cohort study in rural southern Tanzania following 6,706 children born between 1998 and 2000. Initially, home visits were made every 4 months from May 1998 to April 2003. Remarkably, in 2019, they were able to verify the status of fully 89% of those people by reaching out to families and community/village leaders.
Günther Fink, PhD, associate professor of epidemiology and household economics, University of Basel (Switzerland), explained the approach and primary findings to this news organization. The analysis looked at three main groups – children whose parents said they always slept under treated nets, those who slept protected most of the time, and those who spent less than half the time under bed nets. The hazard ratio for death was 0.57 (95% confidence interval, 0.45-0.72) for the first two groups, compared with the least protected. The corresponding hazard ratio between age 5 and adulthood was 0.93 (95% CI, 0.58-1.49).
The findings confirmed what they had suspected. Dr. Fink summarized simply, “If you always slept under a net, you did much better than if you never slept under the net. If you slept [under a net] more than half of the time, it was much better than if you slept [under a net] less than half the time.” So the more time children slept under bed nets, the less likely they were to acquire malaria. Dr. Fink stressed that the findings showing protective efficacy persisted into adulthood. “It seems just having a healthier early life actually makes you more resilient against other future infections.”
One of the theoretical concerns was that using nets would delay developing functional immunity and that there might be an increase in mortality seen later. This study showed that did not happen.
An accompanying commentary noted that there was some potential that families receiving nets were better off than those that didn’t but concluded that such confounding had been accounted for in other analyses.
Mark Wilson, ScD, professor emeritus of epidemiology, University of Michigan, Ann Arbor, concurred. He told this news organization that the study was “very well designed,” and the researchers “did a fantastic job” in tracking patients 20 years later.
“This is astounding!” he added. “It’s very rare to find this amount of follow-up.”
Dr. Fink’s conclusion? “Bed nets protect you in the short run, and being protected in the short run is also beneficial in the long run. There is no evidence that protecting kids in early childhood is weakening them in any way. So we should keep doing this.”
Dr. Fink and Dr. Wilson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It seems obvious that increased use of mosquito bed nets in sub-Saharan Africa would decrease the incidence of malaria, but a lingering question remained:
Malaria from Plasmodium falciparum infection exacts a significant toll in sub-Saharan Africa. According to the World Health Organization, there were about 228 million cases and 602,000 deaths from malaria in 2020 alone. About 80% of those deaths were in children less than 5 years old. In some areas, as many as 5% of children die from malaria by age 5.
Efforts to reduce the burden of malaria have been ongoing for decades. In the 1990s, insecticide-treated nets were shown to reduce illness and deaths from malaria in children.
As a result, the use of bed nets has grown significantly. In 2000, only 5% of households in sub-Saharan Africa had a net in the house. By 2020, that number had risen to 65%. From 2004 to 2019 about 1.9 billion nets were distributed in this region. The nets are estimated to have prevented more than 663 million malaria cases between 2000 and 2015.
As described in the NEJM report, public health researchers conducted a 22-year prospective longitudinal cohort study in rural southern Tanzania following 6,706 children born between 1998 and 2000. Initially, home visits were made every 4 months from May 1998 to April 2003. Remarkably, in 2019, they were able to verify the status of fully 89% of those people by reaching out to families and community/village leaders.
Günther Fink, PhD, associate professor of epidemiology and household economics, University of Basel (Switzerland), explained the approach and primary findings to this news organization. The analysis looked at three main groups – children whose parents said they always slept under treated nets, those who slept protected most of the time, and those who spent less than half the time under bed nets. The hazard ratio for death was 0.57 (95% confidence interval, 0.45-0.72) for the first two groups, compared with the least protected. The corresponding hazard ratio between age 5 and adulthood was 0.93 (95% CI, 0.58-1.49).
The findings confirmed what they had suspected. Dr. Fink summarized simply, “If you always slept under a net, you did much better than if you never slept under the net. If you slept [under a net] more than half of the time, it was much better than if you slept [under a net] less than half the time.” So the more time children slept under bed nets, the less likely they were to acquire malaria. Dr. Fink stressed that the findings showing protective efficacy persisted into adulthood. “It seems just having a healthier early life actually makes you more resilient against other future infections.”
One of the theoretical concerns was that using nets would delay developing functional immunity and that there might be an increase in mortality seen later. This study showed that did not happen.
An accompanying commentary noted that there was some potential that families receiving nets were better off than those that didn’t but concluded that such confounding had been accounted for in other analyses.
Mark Wilson, ScD, professor emeritus of epidemiology, University of Michigan, Ann Arbor, concurred. He told this news organization that the study was “very well designed,” and the researchers “did a fantastic job” in tracking patients 20 years later.
“This is astounding!” he added. “It’s very rare to find this amount of follow-up.”
Dr. Fink’s conclusion? “Bed nets protect you in the short run, and being protected in the short run is also beneficial in the long run. There is no evidence that protecting kids in early childhood is weakening them in any way. So we should keep doing this.”
Dr. Fink and Dr. Wilson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It seems obvious that increased use of mosquito bed nets in sub-Saharan Africa would decrease the incidence of malaria, but a lingering question remained:
Malaria from Plasmodium falciparum infection exacts a significant toll in sub-Saharan Africa. According to the World Health Organization, there were about 228 million cases and 602,000 deaths from malaria in 2020 alone. About 80% of those deaths were in children less than 5 years old. In some areas, as many as 5% of children die from malaria by age 5.
Efforts to reduce the burden of malaria have been ongoing for decades. In the 1990s, insecticide-treated nets were shown to reduce illness and deaths from malaria in children.
As a result, the use of bed nets has grown significantly. In 2000, only 5% of households in sub-Saharan Africa had a net in the house. By 2020, that number had risen to 65%. From 2004 to 2019 about 1.9 billion nets were distributed in this region. The nets are estimated to have prevented more than 663 million malaria cases between 2000 and 2015.
As described in the NEJM report, public health researchers conducted a 22-year prospective longitudinal cohort study in rural southern Tanzania following 6,706 children born between 1998 and 2000. Initially, home visits were made every 4 months from May 1998 to April 2003. Remarkably, in 2019, they were able to verify the status of fully 89% of those people by reaching out to families and community/village leaders.
Günther Fink, PhD, associate professor of epidemiology and household economics, University of Basel (Switzerland), explained the approach and primary findings to this news organization. The analysis looked at three main groups – children whose parents said they always slept under treated nets, those who slept protected most of the time, and those who spent less than half the time under bed nets. The hazard ratio for death was 0.57 (95% confidence interval, 0.45-0.72) for the first two groups, compared with the least protected. The corresponding hazard ratio between age 5 and adulthood was 0.93 (95% CI, 0.58-1.49).
The findings confirmed what they had suspected. Dr. Fink summarized simply, “If you always slept under a net, you did much better than if you never slept under the net. If you slept [under a net] more than half of the time, it was much better than if you slept [under a net] less than half the time.” So the more time children slept under bed nets, the less likely they were to acquire malaria. Dr. Fink stressed that the findings showing protective efficacy persisted into adulthood. “It seems just having a healthier early life actually makes you more resilient against other future infections.”
One of the theoretical concerns was that using nets would delay developing functional immunity and that there might be an increase in mortality seen later. This study showed that did not happen.
An accompanying commentary noted that there was some potential that families receiving nets were better off than those that didn’t but concluded that such confounding had been accounted for in other analyses.
Mark Wilson, ScD, professor emeritus of epidemiology, University of Michigan, Ann Arbor, concurred. He told this news organization that the study was “very well designed,” and the researchers “did a fantastic job” in tracking patients 20 years later.
“This is astounding!” he added. “It’s very rare to find this amount of follow-up.”
Dr. Fink’s conclusion? “Bed nets protect you in the short run, and being protected in the short run is also beneficial in the long run. There is no evidence that protecting kids in early childhood is weakening them in any way. So we should keep doing this.”
Dr. Fink and Dr. Wilson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Drug-resistant malaria is emerging in Africa. Is the world ready?
In June 2017, Betty Balikagala, MD, PhD, traveled to a hospital in Gulu District, in northern Uganda. It was the rainy season: a peak time for malaria transmission. Dr. Balikagala, a researcher at Juntendo University in Japan, was back in her home country to hunt for mutations in the parasite that causes the disease.
For about 4 weeks, Dr. Balikagala and her colleagues collected blood from infected patients as they were treated with a powerful cocktail of antimalarial drugs. After initial analysis, the team then shipped their samples – glass slides smeared with blood, and filter papers with blood spots – back to Japan.
In their lab at Juntendo University, they looked for traces of malaria in the blood slides, which they had prepared by drawing blood from patients every few hours. In previous years, Dr. Balikagala and her colleagues had observed the drugs efficiently clearing the infection. This time, though, the parasite lingered in some patients. “We were very surprised when we first did the parasite reading for 2017, and we noticed that there were some patients who had delayed clearance,” recalled Dr. Balikagala. “For me, it was a shock.”
Malaria kills more than half a million people per year, most of them small children. Still, between 2000 and 2020, according to the World Health Organization, interventions prevented around 10.6 million malaria deaths, mostly in Africa. Bed nets and insecticides were responsible for most of the progress. But a fairly large number of lives were also saved by a new kind of antimalarial treatment: artemisinin-based combination therapies, or ACTs, that replaced older drugs such as chloroquine.
Used as a first-line treatment, ACTs have averted a significant number of malaria deaths since their introduction in the early 2000s. ACTs pair a derivative of the drug artemisinin with one of five partner drugs or drug combinations. Delivered together, the fast-acting artemisinin component wipes out most of the parasites within a few days, and the longer-acting partner drug clears out the stragglers.
ACTs quickly became a mainstay in malaria treatment. But in 2009, researchers observed signs of resistance to artemisinin along the Thailand-Cambodia border. The artemisinin component failed to clear the parasite quickly, which meant that the partner drug had to pick up that load, creating favorable conditions for partner drug resistance, too. The Greater Mekong Subregion now experiences high rates of multidrug resistance. Scientists have feared that the spread of such resistance to Africa, which accounts for more than 90% of global malaria cases, would be disastrous.
Now, in a pair of reports published last year, scientists have confirmed the emergence of artemisinin resistance in Africa. One study, published in April, reported that ACTs had failed to work quickly for more than 10% of participants at two sites in Rwanda. The prevalence of artemisinin resistance mutations was also higher than detected in previous reports.
In September, Dr. Balikagala’s team published the report from Uganda, which also identified mutations associated with artemisinin resistance. Alarmingly, the resistant malaria parasites had risen from 3.9% of cases in 2015 to nearly 20% in 2019. Genetic analysis shows that the resistance mutations in Rwanda and Uganda have emerged independently.
The latest malaria report from the WHO, published in December, also noted worrying signs of artemisinin resistance in the Horn of Africa, on the eastern side of the continent. No peer-reviewed studies confirming such resistance have been published.
So far, the ACTs still work. But in an experimental setting, as drug resistance sets in, it can lengthen treatment by 3 or 4 days. That may not sound like much, said Timothy Wells, PhD, chief scientific officer of the nonprofit Medicines for Malaria Venture. But “the more days of therapy you need,” he said, “then the more there is the risk that people don’t finish their course of therapy.” Dropping a treatment course midway exposes the parasites to the drug, but doesn’t clear all of them, potentially leaving behind survivors with a higher chance of being drug resistant. “That’s really bad news, because then that sets up a perfect storm for creating more resistance,” said Dr. Wells.
The reports from Uganda and Rwanda have yielded a grim consensus: “We are going to see more and more of such independent emergence,” said Pascal Ringwald, MD, PhD, coordinator at the director’s office for the WHO Global Malaria Program. “This is exactly what we saw in the Greater Mekong.” Luckily, Dr. Wells said, switching to other ACTs helped to combat resistance when it was detected there, avoiding the need for prolonged treatment.
A new malaria vaccine, which recently received the go-ahead from the WHO, may eventually help reduce the number of infections, but its rollout won’t have any significant impact on drug resistance. As for new drugs, even the most promising candidate in the pipeline would take at least 4 years to become widely available.
That leaves public health workers in Africa with only one solid option: Track and surveil resistance to artemisinin and its partner drugs. Effective surveillance systems, experts say, need to ramp up quickly and widely across the continent.
But most experts say that surveillance on the continent is patchy. Indeed, there is considerable uncertainty about how widespread antimalarial resistance already is in sub-Saharan Africa – and disagreement over how to interpret initial reports of emerging partner drug resistance in some countries.
“Our current systems are not as good as they should be,” said Philip Rosenthal, MD, a malaria researcher at the University of California, San Francisco. The new reports of artemisinin resistance, he added, “can be seen as a wake-up call to improve surveillance.”
Malaria drugs have failed before. In the early 20th century, chloroquine helped beat back the pathogen worldwide. Then, about a decade after World War II, resistance to chloroquine surfaced along the Thailand-Cambodia border.
By the 1970s, chloroquine-resistant malaria had spread across India and into Africa, where it killed millions, many of them children. “In retrospect, we know that chloroquine was used for many years after there was a huge resistance problem,” said Dr. Rosenthal. “This probably led to millions of excess deaths that could have been avoided if we were using other drugs.”
The scurry to find new drugs yielded artemisinin. Used by Chinese herbalists some 2,000 years ago to treat malaria-like symptoms, artemisinin was rediscovered in the 1970s by biomedical researchers in China, and its use became widespread in the 2000s.
Haunted by the failure of chloroquine, though, researchers have remained on the lookout for signs that the malaria parasite is evolving to resist artemisinin or its partner drugs. The gold-standard method is a therapeutic efficacy study, which involves closely monitoring infected patients as they are treated with antimalarial drugs, to see how well the drugs perform and if there are any signs of resistance.
The WHO recommends conducting these studies at several sites in a country every 2 years. But “each country interprets that with their capability,” said Philippe Guérin, MD, PhD, director of the WorldWide Antimalarial Resistance Network at the University of Oxford, England. Efficacy studies are slow, costly, and labor intensive. Also, “you don’t get a very good geographical representation,” said Dr. Guérin, because you can do a new clinical trial in only so many places at a time.
To get around the problems associated with efficacy studies, researchers also turn to molecular surveillance. Researchers draw a few drops of blood from an infected individual onto a filter paper, then scan it in the laboratory for certain genetic mutations associated with resistance. The technique is relatively easy and cheap.
With these kinds of surveillance data, policymakers can choose which drugs to use in a particular region. Moreover, early detection of resistance can prompt health authorities to take actions to limit the spread of resistance, including more aggressive screening and treatment campaigns, and expanded efforts to control the mosquitoes that spread malaria.
In practice, though, this warning system is frayed. “There is really no organized surveillance system for the continent,” said Dr. Rosenthal. “Surveillance is haphazard.”
In countries lacking a robust health care system or mired in political instability, experts say, resistance could be spreading undetected. For example, the border of South Sudan is just 60 miles from the site in northern Uganda where Dr. Balikagala and her colleagues confirmed resistance to artemisinin. “Because of the security issues and the refugee-weakened system, there is no surveillance that tells us what is happening in South Sudan,” said Dr. Guérin. The same applies in some parts of the nearby Democratic Republic of the Congo, he added.
In the past, regional antimalarial networks, such as the now defunct East African Network for Monitoring of Antimalarial Treatment, have addressed some surveillance gaps. These networks can help standardize protocols and coordinate surveillance efforts. But such networks have suffered from recent lapses in donor funding. The East African network “will be awakened,” Dr. Balikagala predicted, as concerns about artemisinin-resistant malaria grow.
In southern Africa, eight countries have come together to form the Elimination Eight Initiative, a coalition to facilitate malaria elimination efforts across national borders, which may help jump-start surveillance efforts there.
Dr. Ringwald said drug resistance is a priority for him and his WHO colleagues. At a malaria policy advisory committee meeting last fall, he said, the issue was “high on the agenda.” However, when pressed for answers on how the WHO plans to combat drug resistance in Africa, Dr. Ringwald emailed Undark an excerpt from the organization’s 2021 World Malaria Report. The report states that the WHO will “work with countries to develop a regional plan for a coordinated response,” but does not lay out any specifics on that response plan. The Africa Centers for Disease Control and Prevention, part of the African Union, did not respond to requests for comment on its plans to bolster surveillance.
“There is an ethical obligation to researchers, and to people responsible for surveillance, that if you pick up these problems, share them as quickly as possible, react to them as strongly as possible,” said Karen Barnes, a clinical pharmacologist at the University of Cape Town who cochairs the South African Malaria Elimination Committee. “And try very, very hard” to make sure “that it’s not going to be the same as when we had chloroquine resistance in Africa.”
In absence of more robust surveillance, reports have also identified worrying – but, some scientists say, inconclusive – signs of partner drug resistance.
A series of four studies conducted between 2013 and 2019 at several sites in Angola found the efficacy of artemether-lumefantrine – the most widely used ACT in Africa – had dropped below 90%, the WHO threshold for acceptable malaria treatment. Peer-reviewed studies from Burkina Faso and the Democratic Republic of the Congo have reported similar results.
The studies have not found genes associated with artemisinin resistance, suggesting that the partner drug, lumefantrine, might be faltering. But several malaria researchers told Undark they were skeptical of the studies’ methods and viewed the results as preliminary. “I would have preferred that we look at data with a standardized protocol and exclude any confounding factors like poor microscopy or analytical method,” said Dr. Ringwald.
Mateusz Plucinski, PhD, an epidemiologist at the Centers for Disease Control and Prevention’s Malaria Branch who participated in the Angola research, defended the findings. “The persistence of artemether-lumefantrine efficacy near or under 90% in Angola likely suggests that there is likely a true signal of decreased susceptibility of parasites to this drug,” he wrote in an email to this news organization. In response to the data, Angolan health officials have begun using a different ACT.
For now, it’s unclear how bad the situation is in Africa – or what the years ahead could bring. The research community and the authorities are “at the level of just watching and seeing what happens at this stage,” said Leann Tilley, PhD, a biochemist at the University of Melbourne who researches antimalarial resistance. But experts say that if artemisinin resistance does flare up and starts impinging on the partner drug, policymakers might need to consider changing to a different ACT, or even deploy triple ACTs, with two partner drugs.
Some experts are hopeful that artemisinin resistance will spread more slowly in Africa than it has in southeast Asia. But if high-grade resistance to artemisinin and partner drugs were to arise, it would put Africa in a bind. There are no immediate replacements for ACTs at the moment. The Medicines for Malaria Venture drug pipeline has about 30 molecules that show promise in preliminary testing, and about 15 molecules that are undergoing clinical trials for efficacy and safety, said Dr. Wells. But even the drugs that are at the end of the pipeline will take about 5-6 years from approval by regulatory authorities to be incorporated into WHO guidelines, he noted – if they make it through trials at all.
Dr. Wells cited one promising compound, from the drug maker Novartis, that recently performed well in early clinical trials. Still, Dr. Wells said, the drug won’t be ready to be deployed in Africa until around 2026.
Funds for malaria control and elimination programs remain limited, and scientists worry that, between COVID-19 and the malaria vaccine rollout, attention and resources for conducting surveillance and drug resistance work might dry up. “I really hope that those that do have resources available will understand that investing in Africa’s response to artemisinin resistance today, preferably yesterday, is probably one of the best places that they can put their money,” said Barnes.
The annals of malaria have shown time and again that once resistance emerges, it spreads widely and imperils progress against the deadly disease. For Africa, the writing is on the wall, she said. The bigger question, she asked, is this: “Are we capable of learning from history?”
A version of this article first appeared on Undark.com.
In June 2017, Betty Balikagala, MD, PhD, traveled to a hospital in Gulu District, in northern Uganda. It was the rainy season: a peak time for malaria transmission. Dr. Balikagala, a researcher at Juntendo University in Japan, was back in her home country to hunt for mutations in the parasite that causes the disease.
For about 4 weeks, Dr. Balikagala and her colleagues collected blood from infected patients as they were treated with a powerful cocktail of antimalarial drugs. After initial analysis, the team then shipped their samples – glass slides smeared with blood, and filter papers with blood spots – back to Japan.
In their lab at Juntendo University, they looked for traces of malaria in the blood slides, which they had prepared by drawing blood from patients every few hours. In previous years, Dr. Balikagala and her colleagues had observed the drugs efficiently clearing the infection. This time, though, the parasite lingered in some patients. “We were very surprised when we first did the parasite reading for 2017, and we noticed that there were some patients who had delayed clearance,” recalled Dr. Balikagala. “For me, it was a shock.”
Malaria kills more than half a million people per year, most of them small children. Still, between 2000 and 2020, according to the World Health Organization, interventions prevented around 10.6 million malaria deaths, mostly in Africa. Bed nets and insecticides were responsible for most of the progress. But a fairly large number of lives were also saved by a new kind of antimalarial treatment: artemisinin-based combination therapies, or ACTs, that replaced older drugs such as chloroquine.
Used as a first-line treatment, ACTs have averted a significant number of malaria deaths since their introduction in the early 2000s. ACTs pair a derivative of the drug artemisinin with one of five partner drugs or drug combinations. Delivered together, the fast-acting artemisinin component wipes out most of the parasites within a few days, and the longer-acting partner drug clears out the stragglers.
ACTs quickly became a mainstay in malaria treatment. But in 2009, researchers observed signs of resistance to artemisinin along the Thailand-Cambodia border. The artemisinin component failed to clear the parasite quickly, which meant that the partner drug had to pick up that load, creating favorable conditions for partner drug resistance, too. The Greater Mekong Subregion now experiences high rates of multidrug resistance. Scientists have feared that the spread of such resistance to Africa, which accounts for more than 90% of global malaria cases, would be disastrous.
Now, in a pair of reports published last year, scientists have confirmed the emergence of artemisinin resistance in Africa. One study, published in April, reported that ACTs had failed to work quickly for more than 10% of participants at two sites in Rwanda. The prevalence of artemisinin resistance mutations was also higher than detected in previous reports.
In September, Dr. Balikagala’s team published the report from Uganda, which also identified mutations associated with artemisinin resistance. Alarmingly, the resistant malaria parasites had risen from 3.9% of cases in 2015 to nearly 20% in 2019. Genetic analysis shows that the resistance mutations in Rwanda and Uganda have emerged independently.
The latest malaria report from the WHO, published in December, also noted worrying signs of artemisinin resistance in the Horn of Africa, on the eastern side of the continent. No peer-reviewed studies confirming such resistance have been published.
So far, the ACTs still work. But in an experimental setting, as drug resistance sets in, it can lengthen treatment by 3 or 4 days. That may not sound like much, said Timothy Wells, PhD, chief scientific officer of the nonprofit Medicines for Malaria Venture. But “the more days of therapy you need,” he said, “then the more there is the risk that people don’t finish their course of therapy.” Dropping a treatment course midway exposes the parasites to the drug, but doesn’t clear all of them, potentially leaving behind survivors with a higher chance of being drug resistant. “That’s really bad news, because then that sets up a perfect storm for creating more resistance,” said Dr. Wells.
The reports from Uganda and Rwanda have yielded a grim consensus: “We are going to see more and more of such independent emergence,” said Pascal Ringwald, MD, PhD, coordinator at the director’s office for the WHO Global Malaria Program. “This is exactly what we saw in the Greater Mekong.” Luckily, Dr. Wells said, switching to other ACTs helped to combat resistance when it was detected there, avoiding the need for prolonged treatment.
A new malaria vaccine, which recently received the go-ahead from the WHO, may eventually help reduce the number of infections, but its rollout won’t have any significant impact on drug resistance. As for new drugs, even the most promising candidate in the pipeline would take at least 4 years to become widely available.
That leaves public health workers in Africa with only one solid option: Track and surveil resistance to artemisinin and its partner drugs. Effective surveillance systems, experts say, need to ramp up quickly and widely across the continent.
But most experts say that surveillance on the continent is patchy. Indeed, there is considerable uncertainty about how widespread antimalarial resistance already is in sub-Saharan Africa – and disagreement over how to interpret initial reports of emerging partner drug resistance in some countries.
“Our current systems are not as good as they should be,” said Philip Rosenthal, MD, a malaria researcher at the University of California, San Francisco. The new reports of artemisinin resistance, he added, “can be seen as a wake-up call to improve surveillance.”
Malaria drugs have failed before. In the early 20th century, chloroquine helped beat back the pathogen worldwide. Then, about a decade after World War II, resistance to chloroquine surfaced along the Thailand-Cambodia border.
By the 1970s, chloroquine-resistant malaria had spread across India and into Africa, where it killed millions, many of them children. “In retrospect, we know that chloroquine was used for many years after there was a huge resistance problem,” said Dr. Rosenthal. “This probably led to millions of excess deaths that could have been avoided if we were using other drugs.”
The scurry to find new drugs yielded artemisinin. Used by Chinese herbalists some 2,000 years ago to treat malaria-like symptoms, artemisinin was rediscovered in the 1970s by biomedical researchers in China, and its use became widespread in the 2000s.
Haunted by the failure of chloroquine, though, researchers have remained on the lookout for signs that the malaria parasite is evolving to resist artemisinin or its partner drugs. The gold-standard method is a therapeutic efficacy study, which involves closely monitoring infected patients as they are treated with antimalarial drugs, to see how well the drugs perform and if there are any signs of resistance.
The WHO recommends conducting these studies at several sites in a country every 2 years. But “each country interprets that with their capability,” said Philippe Guérin, MD, PhD, director of the WorldWide Antimalarial Resistance Network at the University of Oxford, England. Efficacy studies are slow, costly, and labor intensive. Also, “you don’t get a very good geographical representation,” said Dr. Guérin, because you can do a new clinical trial in only so many places at a time.
To get around the problems associated with efficacy studies, researchers also turn to molecular surveillance. Researchers draw a few drops of blood from an infected individual onto a filter paper, then scan it in the laboratory for certain genetic mutations associated with resistance. The technique is relatively easy and cheap.
With these kinds of surveillance data, policymakers can choose which drugs to use in a particular region. Moreover, early detection of resistance can prompt health authorities to take actions to limit the spread of resistance, including more aggressive screening and treatment campaigns, and expanded efforts to control the mosquitoes that spread malaria.
In practice, though, this warning system is frayed. “There is really no organized surveillance system for the continent,” said Dr. Rosenthal. “Surveillance is haphazard.”
In countries lacking a robust health care system or mired in political instability, experts say, resistance could be spreading undetected. For example, the border of South Sudan is just 60 miles from the site in northern Uganda where Dr. Balikagala and her colleagues confirmed resistance to artemisinin. “Because of the security issues and the refugee-weakened system, there is no surveillance that tells us what is happening in South Sudan,” said Dr. Guérin. The same applies in some parts of the nearby Democratic Republic of the Congo, he added.
In the past, regional antimalarial networks, such as the now defunct East African Network for Monitoring of Antimalarial Treatment, have addressed some surveillance gaps. These networks can help standardize protocols and coordinate surveillance efforts. But such networks have suffered from recent lapses in donor funding. The East African network “will be awakened,” Dr. Balikagala predicted, as concerns about artemisinin-resistant malaria grow.
In southern Africa, eight countries have come together to form the Elimination Eight Initiative, a coalition to facilitate malaria elimination efforts across national borders, which may help jump-start surveillance efforts there.
Dr. Ringwald said drug resistance is a priority for him and his WHO colleagues. At a malaria policy advisory committee meeting last fall, he said, the issue was “high on the agenda.” However, when pressed for answers on how the WHO plans to combat drug resistance in Africa, Dr. Ringwald emailed Undark an excerpt from the organization’s 2021 World Malaria Report. The report states that the WHO will “work with countries to develop a regional plan for a coordinated response,” but does not lay out any specifics on that response plan. The Africa Centers for Disease Control and Prevention, part of the African Union, did not respond to requests for comment on its plans to bolster surveillance.
“There is an ethical obligation to researchers, and to people responsible for surveillance, that if you pick up these problems, share them as quickly as possible, react to them as strongly as possible,” said Karen Barnes, a clinical pharmacologist at the University of Cape Town who cochairs the South African Malaria Elimination Committee. “And try very, very hard” to make sure “that it’s not going to be the same as when we had chloroquine resistance in Africa.”
In absence of more robust surveillance, reports have also identified worrying – but, some scientists say, inconclusive – signs of partner drug resistance.
A series of four studies conducted between 2013 and 2019 at several sites in Angola found the efficacy of artemether-lumefantrine – the most widely used ACT in Africa – had dropped below 90%, the WHO threshold for acceptable malaria treatment. Peer-reviewed studies from Burkina Faso and the Democratic Republic of the Congo have reported similar results.
The studies have not found genes associated with artemisinin resistance, suggesting that the partner drug, lumefantrine, might be faltering. But several malaria researchers told Undark they were skeptical of the studies’ methods and viewed the results as preliminary. “I would have preferred that we look at data with a standardized protocol and exclude any confounding factors like poor microscopy or analytical method,” said Dr. Ringwald.
Mateusz Plucinski, PhD, an epidemiologist at the Centers for Disease Control and Prevention’s Malaria Branch who participated in the Angola research, defended the findings. “The persistence of artemether-lumefantrine efficacy near or under 90% in Angola likely suggests that there is likely a true signal of decreased susceptibility of parasites to this drug,” he wrote in an email to this news organization. In response to the data, Angolan health officials have begun using a different ACT.
For now, it’s unclear how bad the situation is in Africa – or what the years ahead could bring. The research community and the authorities are “at the level of just watching and seeing what happens at this stage,” said Leann Tilley, PhD, a biochemist at the University of Melbourne who researches antimalarial resistance. But experts say that if artemisinin resistance does flare up and starts impinging on the partner drug, policymakers might need to consider changing to a different ACT, or even deploy triple ACTs, with two partner drugs.
Some experts are hopeful that artemisinin resistance will spread more slowly in Africa than it has in southeast Asia. But if high-grade resistance to artemisinin and partner drugs were to arise, it would put Africa in a bind. There are no immediate replacements for ACTs at the moment. The Medicines for Malaria Venture drug pipeline has about 30 molecules that show promise in preliminary testing, and about 15 molecules that are undergoing clinical trials for efficacy and safety, said Dr. Wells. But even the drugs that are at the end of the pipeline will take about 5-6 years from approval by regulatory authorities to be incorporated into WHO guidelines, he noted – if they make it through trials at all.
Dr. Wells cited one promising compound, from the drug maker Novartis, that recently performed well in early clinical trials. Still, Dr. Wells said, the drug won’t be ready to be deployed in Africa until around 2026.
Funds for malaria control and elimination programs remain limited, and scientists worry that, between COVID-19 and the malaria vaccine rollout, attention and resources for conducting surveillance and drug resistance work might dry up. “I really hope that those that do have resources available will understand that investing in Africa’s response to artemisinin resistance today, preferably yesterday, is probably one of the best places that they can put their money,” said Barnes.
The annals of malaria have shown time and again that once resistance emerges, it spreads widely and imperils progress against the deadly disease. For Africa, the writing is on the wall, she said. The bigger question, she asked, is this: “Are we capable of learning from history?”
A version of this article first appeared on Undark.com.
In June 2017, Betty Balikagala, MD, PhD, traveled to a hospital in Gulu District, in northern Uganda. It was the rainy season: a peak time for malaria transmission. Dr. Balikagala, a researcher at Juntendo University in Japan, was back in her home country to hunt for mutations in the parasite that causes the disease.
For about 4 weeks, Dr. Balikagala and her colleagues collected blood from infected patients as they were treated with a powerful cocktail of antimalarial drugs. After initial analysis, the team then shipped their samples – glass slides smeared with blood, and filter papers with blood spots – back to Japan.
In their lab at Juntendo University, they looked for traces of malaria in the blood slides, which they had prepared by drawing blood from patients every few hours. In previous years, Dr. Balikagala and her colleagues had observed the drugs efficiently clearing the infection. This time, though, the parasite lingered in some patients. “We were very surprised when we first did the parasite reading for 2017, and we noticed that there were some patients who had delayed clearance,” recalled Dr. Balikagala. “For me, it was a shock.”
Malaria kills more than half a million people per year, most of them small children. Still, between 2000 and 2020, according to the World Health Organization, interventions prevented around 10.6 million malaria deaths, mostly in Africa. Bed nets and insecticides were responsible for most of the progress. But a fairly large number of lives were also saved by a new kind of antimalarial treatment: artemisinin-based combination therapies, or ACTs, that replaced older drugs such as chloroquine.
Used as a first-line treatment, ACTs have averted a significant number of malaria deaths since their introduction in the early 2000s. ACTs pair a derivative of the drug artemisinin with one of five partner drugs or drug combinations. Delivered together, the fast-acting artemisinin component wipes out most of the parasites within a few days, and the longer-acting partner drug clears out the stragglers.
ACTs quickly became a mainstay in malaria treatment. But in 2009, researchers observed signs of resistance to artemisinin along the Thailand-Cambodia border. The artemisinin component failed to clear the parasite quickly, which meant that the partner drug had to pick up that load, creating favorable conditions for partner drug resistance, too. The Greater Mekong Subregion now experiences high rates of multidrug resistance. Scientists have feared that the spread of such resistance to Africa, which accounts for more than 90% of global malaria cases, would be disastrous.
Now, in a pair of reports published last year, scientists have confirmed the emergence of artemisinin resistance in Africa. One study, published in April, reported that ACTs had failed to work quickly for more than 10% of participants at two sites in Rwanda. The prevalence of artemisinin resistance mutations was also higher than detected in previous reports.
In September, Dr. Balikagala’s team published the report from Uganda, which also identified mutations associated with artemisinin resistance. Alarmingly, the resistant malaria parasites had risen from 3.9% of cases in 2015 to nearly 20% in 2019. Genetic analysis shows that the resistance mutations in Rwanda and Uganda have emerged independently.
The latest malaria report from the WHO, published in December, also noted worrying signs of artemisinin resistance in the Horn of Africa, on the eastern side of the continent. No peer-reviewed studies confirming such resistance have been published.
So far, the ACTs still work. But in an experimental setting, as drug resistance sets in, it can lengthen treatment by 3 or 4 days. That may not sound like much, said Timothy Wells, PhD, chief scientific officer of the nonprofit Medicines for Malaria Venture. But “the more days of therapy you need,” he said, “then the more there is the risk that people don’t finish their course of therapy.” Dropping a treatment course midway exposes the parasites to the drug, but doesn’t clear all of them, potentially leaving behind survivors with a higher chance of being drug resistant. “That’s really bad news, because then that sets up a perfect storm for creating more resistance,” said Dr. Wells.
The reports from Uganda and Rwanda have yielded a grim consensus: “We are going to see more and more of such independent emergence,” said Pascal Ringwald, MD, PhD, coordinator at the director’s office for the WHO Global Malaria Program. “This is exactly what we saw in the Greater Mekong.” Luckily, Dr. Wells said, switching to other ACTs helped to combat resistance when it was detected there, avoiding the need for prolonged treatment.
A new malaria vaccine, which recently received the go-ahead from the WHO, may eventually help reduce the number of infections, but its rollout won’t have any significant impact on drug resistance. As for new drugs, even the most promising candidate in the pipeline would take at least 4 years to become widely available.
That leaves public health workers in Africa with only one solid option: Track and surveil resistance to artemisinin and its partner drugs. Effective surveillance systems, experts say, need to ramp up quickly and widely across the continent.
But most experts say that surveillance on the continent is patchy. Indeed, there is considerable uncertainty about how widespread antimalarial resistance already is in sub-Saharan Africa – and disagreement over how to interpret initial reports of emerging partner drug resistance in some countries.
“Our current systems are not as good as they should be,” said Philip Rosenthal, MD, a malaria researcher at the University of California, San Francisco. The new reports of artemisinin resistance, he added, “can be seen as a wake-up call to improve surveillance.”
Malaria drugs have failed before. In the early 20th century, chloroquine helped beat back the pathogen worldwide. Then, about a decade after World War II, resistance to chloroquine surfaced along the Thailand-Cambodia border.
By the 1970s, chloroquine-resistant malaria had spread across India and into Africa, where it killed millions, many of them children. “In retrospect, we know that chloroquine was used for many years after there was a huge resistance problem,” said Dr. Rosenthal. “This probably led to millions of excess deaths that could have been avoided if we were using other drugs.”
The scurry to find new drugs yielded artemisinin. Used by Chinese herbalists some 2,000 years ago to treat malaria-like symptoms, artemisinin was rediscovered in the 1970s by biomedical researchers in China, and its use became widespread in the 2000s.
Haunted by the failure of chloroquine, though, researchers have remained on the lookout for signs that the malaria parasite is evolving to resist artemisinin or its partner drugs. The gold-standard method is a therapeutic efficacy study, which involves closely monitoring infected patients as they are treated with antimalarial drugs, to see how well the drugs perform and if there are any signs of resistance.
The WHO recommends conducting these studies at several sites in a country every 2 years. But “each country interprets that with their capability,” said Philippe Guérin, MD, PhD, director of the WorldWide Antimalarial Resistance Network at the University of Oxford, England. Efficacy studies are slow, costly, and labor intensive. Also, “you don’t get a very good geographical representation,” said Dr. Guérin, because you can do a new clinical trial in only so many places at a time.
To get around the problems associated with efficacy studies, researchers also turn to molecular surveillance. Researchers draw a few drops of blood from an infected individual onto a filter paper, then scan it in the laboratory for certain genetic mutations associated with resistance. The technique is relatively easy and cheap.
With these kinds of surveillance data, policymakers can choose which drugs to use in a particular region. Moreover, early detection of resistance can prompt health authorities to take actions to limit the spread of resistance, including more aggressive screening and treatment campaigns, and expanded efforts to control the mosquitoes that spread malaria.
In practice, though, this warning system is frayed. “There is really no organized surveillance system for the continent,” said Dr. Rosenthal. “Surveillance is haphazard.”
In countries lacking a robust health care system or mired in political instability, experts say, resistance could be spreading undetected. For example, the border of South Sudan is just 60 miles from the site in northern Uganda where Dr. Balikagala and her colleagues confirmed resistance to artemisinin. “Because of the security issues and the refugee-weakened system, there is no surveillance that tells us what is happening in South Sudan,” said Dr. Guérin. The same applies in some parts of the nearby Democratic Republic of the Congo, he added.
In the past, regional antimalarial networks, such as the now defunct East African Network for Monitoring of Antimalarial Treatment, have addressed some surveillance gaps. These networks can help standardize protocols and coordinate surveillance efforts. But such networks have suffered from recent lapses in donor funding. The East African network “will be awakened,” Dr. Balikagala predicted, as concerns about artemisinin-resistant malaria grow.
In southern Africa, eight countries have come together to form the Elimination Eight Initiative, a coalition to facilitate malaria elimination efforts across national borders, which may help jump-start surveillance efforts there.
Dr. Ringwald said drug resistance is a priority for him and his WHO colleagues. At a malaria policy advisory committee meeting last fall, he said, the issue was “high on the agenda.” However, when pressed for answers on how the WHO plans to combat drug resistance in Africa, Dr. Ringwald emailed Undark an excerpt from the organization’s 2021 World Malaria Report. The report states that the WHO will “work with countries to develop a regional plan for a coordinated response,” but does not lay out any specifics on that response plan. The Africa Centers for Disease Control and Prevention, part of the African Union, did not respond to requests for comment on its plans to bolster surveillance.
“There is an ethical obligation to researchers, and to people responsible for surveillance, that if you pick up these problems, share them as quickly as possible, react to them as strongly as possible,” said Karen Barnes, a clinical pharmacologist at the University of Cape Town who cochairs the South African Malaria Elimination Committee. “And try very, very hard” to make sure “that it’s not going to be the same as when we had chloroquine resistance in Africa.”
In absence of more robust surveillance, reports have also identified worrying – but, some scientists say, inconclusive – signs of partner drug resistance.
A series of four studies conducted between 2013 and 2019 at several sites in Angola found the efficacy of artemether-lumefantrine – the most widely used ACT in Africa – had dropped below 90%, the WHO threshold for acceptable malaria treatment. Peer-reviewed studies from Burkina Faso and the Democratic Republic of the Congo have reported similar results.
The studies have not found genes associated with artemisinin resistance, suggesting that the partner drug, lumefantrine, might be faltering. But several malaria researchers told Undark they were skeptical of the studies’ methods and viewed the results as preliminary. “I would have preferred that we look at data with a standardized protocol and exclude any confounding factors like poor microscopy or analytical method,” said Dr. Ringwald.
Mateusz Plucinski, PhD, an epidemiologist at the Centers for Disease Control and Prevention’s Malaria Branch who participated in the Angola research, defended the findings. “The persistence of artemether-lumefantrine efficacy near or under 90% in Angola likely suggests that there is likely a true signal of decreased susceptibility of parasites to this drug,” he wrote in an email to this news organization. In response to the data, Angolan health officials have begun using a different ACT.
For now, it’s unclear how bad the situation is in Africa – or what the years ahead could bring. The research community and the authorities are “at the level of just watching and seeing what happens at this stage,” said Leann Tilley, PhD, a biochemist at the University of Melbourne who researches antimalarial resistance. But experts say that if artemisinin resistance does flare up and starts impinging on the partner drug, policymakers might need to consider changing to a different ACT, or even deploy triple ACTs, with two partner drugs.
Some experts are hopeful that artemisinin resistance will spread more slowly in Africa than it has in southeast Asia. But if high-grade resistance to artemisinin and partner drugs were to arise, it would put Africa in a bind. There are no immediate replacements for ACTs at the moment. The Medicines for Malaria Venture drug pipeline has about 30 molecules that show promise in preliminary testing, and about 15 molecules that are undergoing clinical trials for efficacy and safety, said Dr. Wells. But even the drugs that are at the end of the pipeline will take about 5-6 years from approval by regulatory authorities to be incorporated into WHO guidelines, he noted – if they make it through trials at all.
Dr. Wells cited one promising compound, from the drug maker Novartis, that recently performed well in early clinical trials. Still, Dr. Wells said, the drug won’t be ready to be deployed in Africa until around 2026.
Funds for malaria control and elimination programs remain limited, and scientists worry that, between COVID-19 and the malaria vaccine rollout, attention and resources for conducting surveillance and drug resistance work might dry up. “I really hope that those that do have resources available will understand that investing in Africa’s response to artemisinin resistance today, preferably yesterday, is probably one of the best places that they can put their money,” said Barnes.
The annals of malaria have shown time and again that once resistance emerges, it spreads widely and imperils progress against the deadly disease. For Africa, the writing is on the wall, she said. The bigger question, she asked, is this: “Are we capable of learning from history?”
A version of this article first appeared on Undark.com.
Antimicrobial resistance linked to 1.2 million global deaths in 2019
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.
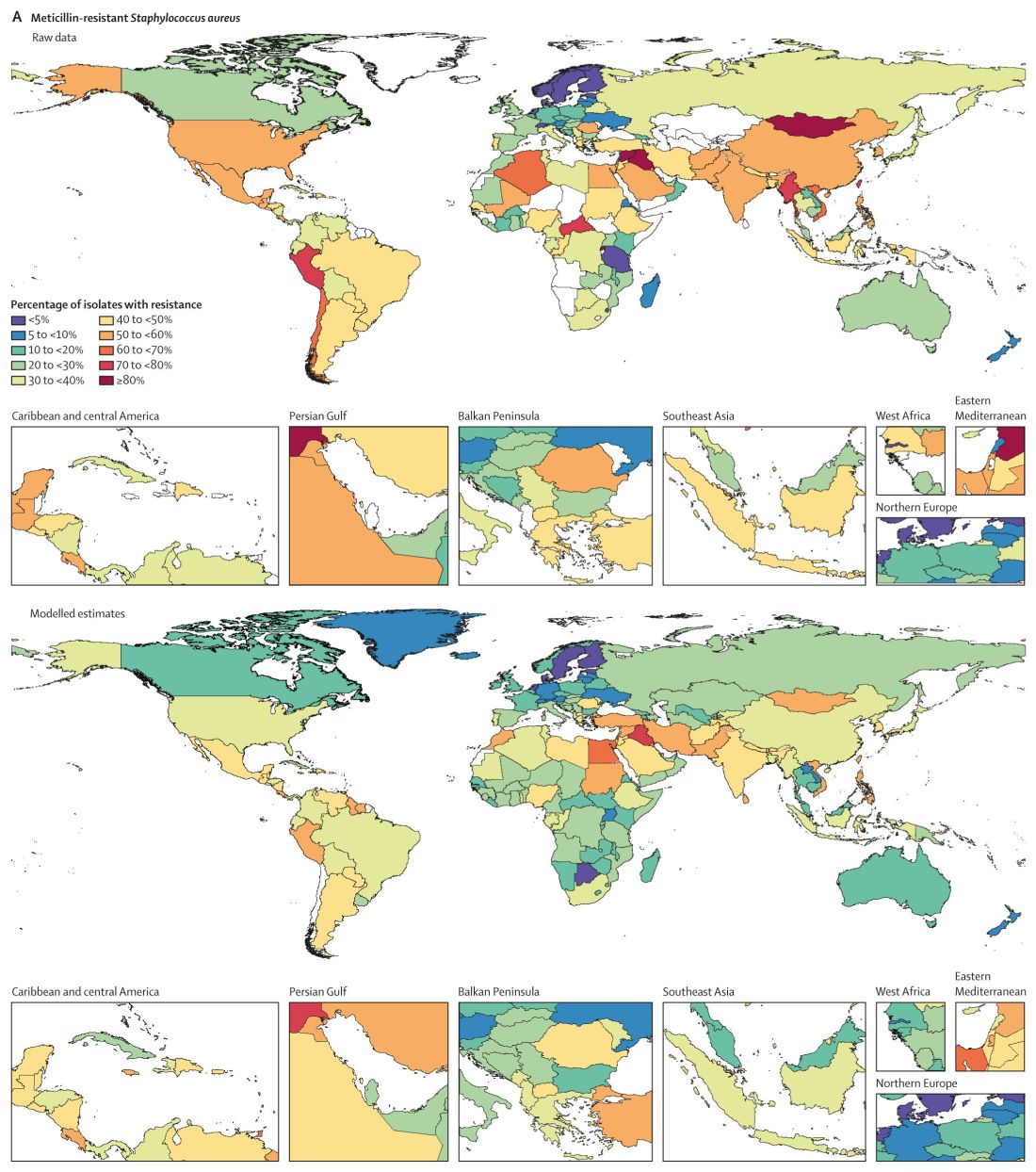
Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.

Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.

Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
ACIP releases new dengue vaccine recommendations
The vaccine is only to be used for children aged 9-16 who live in endemic areas and who have evidence with a specific diagnostic test of prior dengue infection.
Dengue is a mosquito-borne virus found throughout the world, primarily in tropical or subtropical climates. Cases had steadily been increasing to 5.2 million in 2019, and the geographic distribution of cases is broadening with climate change and urbanization. About half of the world’s population is now at risk.
The dengue virus has four serotypes. The first infection may be mild or asymptomatic, but the second one can be life-threatening because of a phenomenon called antibody-dependent enhancement.
The lead author of the new recommendations is Gabriela Paz-Bailey, MD, PhD, division of vector-borne diseases, dengue branch, CDC. She told this news organization that, during the second infection, when there are “low levels of antibodies from that first infection, the antibodies help the virus get inside the cells. There the virus is not killed, and that results in increased viral load, and then that can result in more severe disease and the plasma leakage” syndrome, which can lead to shock, severe bleeding, and organ failure. The death rate for severe dengue is up to 13%.
Previous infection with Zika virus, common in the same areas where dengue is endemic, can also increase the risk for symptomatic and severe dengue for subsequent infections.
In the United States, Puerto Rico is the main focus of control efforts because 95% of domestic dengue cases originate there – almost 30,000 cases between 2010 and 2020, with 11,000 cases and 4,000 hospitalizations occurring in children between the ages of 10 and 19.
Because Aedes aegypti, the primary mosquito vector transmitting dengue, is resistant to all commonly used insecticides in Puerto Rico, preventive efforts have shifted from insecticides to vaccination.
Antibody tests prevaccination
The main concern with the Sanofi’s dengue vaccine is that it could act as an asymptomatic primary dengue infection, in effect priming the body for a severe reaction from antibody-dependent enhancement with a subsequent infection. That is why it’s critical that the vaccine only be given to children with evidence of prior disease.
Dr. Paz-Bailey said: “The CDC came up with recommendations of what the performance of the test used for prevaccination screening should be. And it was 98% specificity and 75% sensitivity. ... But no test by itself was found to have a specificity of 98%, and this is why we’re recommending the two-test algorithm,” in which two different assays are run off the same blood sample, drawn at a prevaccination visit.
If the child has evidence of prior dengue, they can proceed with vaccination to protect against recurrent infection. Dengvaxia is given as a series of three shots over 6 months. Vaccine efficacy is 82% – so not everyone is protected, and additionally, that protection declines over time.
There is concern that it will be difficult to achieve compliance with such a complex regimen. Dr. Paz-Bailey said, “But I think that the trust in vaccines that is highly prevalent for [Puerto] Rico and trusting the health care system, and sort of the importance that is assigned to dengue by providers and by parents because of previous outbreaks and previous experiences is going to help us.” She added, “I think that the COVID experience has been very revealing. And what we have learned is that Puerto Rico has a very strong health care system, a very strong network of vaccine providers. ... Coverage for COVID vaccine is higher than in other parts of the U.S.”
One of the interesting things about dengue is that the first infection can range from asymptomatic to life-threatening. The second infection is generally worse because of this antibody-dependent enhancement phenomenon. Eng Eong Ooi, MD, PhD, professor of microbiology and immunology, National University of Singapore, told this news organization, “After you have two infections, you seem to be protected quite well against the remaining two [serotypes]. The vaccine serves as another episode of infection in those who had prior dengue, so then any natural infections after the vaccination in the seropositive become like the outcome of a third or fourth infection.”
Vaccination alone will not solve dengue. Dr. Ooi said, “There’s not one method that would fully control dengue. You need both vaccines as well as control measures, whether it’s Wolbachia or something else. At the same time, I think we need antiviral drugs, because hitting this virus in just one part of its life cycle wouldn’t make a huge, lasting impact.” Dr. Ooi added that as “the spread of the virus and the population immunity drops, you’re actually now more vulnerable to dengue outbreaks when they do get introduced. So, suppressing transmission alone isn’t the answer. You also have to keep herd immunity levels high. So if we can reduce the virus transmission by controlling either mosquito population or transmission and at the same time vaccinate to keep the immunity levels high, then I think we have a chance of controlling dengue.”
Dr. Paz-Bailey concluded: “I do want to emphasize that we are excited about having these tools, because for years and years, we have had really limited options to prevent and control dengue. It’s an important addition to have the vaccine be approved to be used within the U.S., and it’s going to pave the road for future vaccines.”
Dr. Paz-Bailey and Dr. Ooi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccine is only to be used for children aged 9-16 who live in endemic areas and who have evidence with a specific diagnostic test of prior dengue infection.
Dengue is a mosquito-borne virus found throughout the world, primarily in tropical or subtropical climates. Cases had steadily been increasing to 5.2 million in 2019, and the geographic distribution of cases is broadening with climate change and urbanization. About half of the world’s population is now at risk.
The dengue virus has four serotypes. The first infection may be mild or asymptomatic, but the second one can be life-threatening because of a phenomenon called antibody-dependent enhancement.
The lead author of the new recommendations is Gabriela Paz-Bailey, MD, PhD, division of vector-borne diseases, dengue branch, CDC. She told this news organization that, during the second infection, when there are “low levels of antibodies from that first infection, the antibodies help the virus get inside the cells. There the virus is not killed, and that results in increased viral load, and then that can result in more severe disease and the plasma leakage” syndrome, which can lead to shock, severe bleeding, and organ failure. The death rate for severe dengue is up to 13%.
Previous infection with Zika virus, common in the same areas where dengue is endemic, can also increase the risk for symptomatic and severe dengue for subsequent infections.
In the United States, Puerto Rico is the main focus of control efforts because 95% of domestic dengue cases originate there – almost 30,000 cases between 2010 and 2020, with 11,000 cases and 4,000 hospitalizations occurring in children between the ages of 10 and 19.
Because Aedes aegypti, the primary mosquito vector transmitting dengue, is resistant to all commonly used insecticides in Puerto Rico, preventive efforts have shifted from insecticides to vaccination.
Antibody tests prevaccination
The main concern with the Sanofi’s dengue vaccine is that it could act as an asymptomatic primary dengue infection, in effect priming the body for a severe reaction from antibody-dependent enhancement with a subsequent infection. That is why it’s critical that the vaccine only be given to children with evidence of prior disease.
Dr. Paz-Bailey said: “The CDC came up with recommendations of what the performance of the test used for prevaccination screening should be. And it was 98% specificity and 75% sensitivity. ... But no test by itself was found to have a specificity of 98%, and this is why we’re recommending the two-test algorithm,” in which two different assays are run off the same blood sample, drawn at a prevaccination visit.
If the child has evidence of prior dengue, they can proceed with vaccination to protect against recurrent infection. Dengvaxia is given as a series of three shots over 6 months. Vaccine efficacy is 82% – so not everyone is protected, and additionally, that protection declines over time.
There is concern that it will be difficult to achieve compliance with such a complex regimen. Dr. Paz-Bailey said, “But I think that the trust in vaccines that is highly prevalent for [Puerto] Rico and trusting the health care system, and sort of the importance that is assigned to dengue by providers and by parents because of previous outbreaks and previous experiences is going to help us.” She added, “I think that the COVID experience has been very revealing. And what we have learned is that Puerto Rico has a very strong health care system, a very strong network of vaccine providers. ... Coverage for COVID vaccine is higher than in other parts of the U.S.”
One of the interesting things about dengue is that the first infection can range from asymptomatic to life-threatening. The second infection is generally worse because of this antibody-dependent enhancement phenomenon. Eng Eong Ooi, MD, PhD, professor of microbiology and immunology, National University of Singapore, told this news organization, “After you have two infections, you seem to be protected quite well against the remaining two [serotypes]. The vaccine serves as another episode of infection in those who had prior dengue, so then any natural infections after the vaccination in the seropositive become like the outcome of a third or fourth infection.”
Vaccination alone will not solve dengue. Dr. Ooi said, “There’s not one method that would fully control dengue. You need both vaccines as well as control measures, whether it’s Wolbachia or something else. At the same time, I think we need antiviral drugs, because hitting this virus in just one part of its life cycle wouldn’t make a huge, lasting impact.” Dr. Ooi added that as “the spread of the virus and the population immunity drops, you’re actually now more vulnerable to dengue outbreaks when they do get introduced. So, suppressing transmission alone isn’t the answer. You also have to keep herd immunity levels high. So if we can reduce the virus transmission by controlling either mosquito population or transmission and at the same time vaccinate to keep the immunity levels high, then I think we have a chance of controlling dengue.”
Dr. Paz-Bailey concluded: “I do want to emphasize that we are excited about having these tools, because for years and years, we have had really limited options to prevent and control dengue. It’s an important addition to have the vaccine be approved to be used within the U.S., and it’s going to pave the road for future vaccines.”
Dr. Paz-Bailey and Dr. Ooi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccine is only to be used for children aged 9-16 who live in endemic areas and who have evidence with a specific diagnostic test of prior dengue infection.
Dengue is a mosquito-borne virus found throughout the world, primarily in tropical or subtropical climates. Cases had steadily been increasing to 5.2 million in 2019, and the geographic distribution of cases is broadening with climate change and urbanization. About half of the world’s population is now at risk.
The dengue virus has four serotypes. The first infection may be mild or asymptomatic, but the second one can be life-threatening because of a phenomenon called antibody-dependent enhancement.
The lead author of the new recommendations is Gabriela Paz-Bailey, MD, PhD, division of vector-borne diseases, dengue branch, CDC. She told this news organization that, during the second infection, when there are “low levels of antibodies from that first infection, the antibodies help the virus get inside the cells. There the virus is not killed, and that results in increased viral load, and then that can result in more severe disease and the plasma leakage” syndrome, which can lead to shock, severe bleeding, and organ failure. The death rate for severe dengue is up to 13%.
Previous infection with Zika virus, common in the same areas where dengue is endemic, can also increase the risk for symptomatic and severe dengue for subsequent infections.
In the United States, Puerto Rico is the main focus of control efforts because 95% of domestic dengue cases originate there – almost 30,000 cases between 2010 and 2020, with 11,000 cases and 4,000 hospitalizations occurring in children between the ages of 10 and 19.
Because Aedes aegypti, the primary mosquito vector transmitting dengue, is resistant to all commonly used insecticides in Puerto Rico, preventive efforts have shifted from insecticides to vaccination.
Antibody tests prevaccination
The main concern with the Sanofi’s dengue vaccine is that it could act as an asymptomatic primary dengue infection, in effect priming the body for a severe reaction from antibody-dependent enhancement with a subsequent infection. That is why it’s critical that the vaccine only be given to children with evidence of prior disease.
Dr. Paz-Bailey said: “The CDC came up with recommendations of what the performance of the test used for prevaccination screening should be. And it was 98% specificity and 75% sensitivity. ... But no test by itself was found to have a specificity of 98%, and this is why we’re recommending the two-test algorithm,” in which two different assays are run off the same blood sample, drawn at a prevaccination visit.
If the child has evidence of prior dengue, they can proceed with vaccination to protect against recurrent infection. Dengvaxia is given as a series of three shots over 6 months. Vaccine efficacy is 82% – so not everyone is protected, and additionally, that protection declines over time.
There is concern that it will be difficult to achieve compliance with such a complex regimen. Dr. Paz-Bailey said, “But I think that the trust in vaccines that is highly prevalent for [Puerto] Rico and trusting the health care system, and sort of the importance that is assigned to dengue by providers and by parents because of previous outbreaks and previous experiences is going to help us.” She added, “I think that the COVID experience has been very revealing. And what we have learned is that Puerto Rico has a very strong health care system, a very strong network of vaccine providers. ... Coverage for COVID vaccine is higher than in other parts of the U.S.”
One of the interesting things about dengue is that the first infection can range from asymptomatic to life-threatening. The second infection is generally worse because of this antibody-dependent enhancement phenomenon. Eng Eong Ooi, MD, PhD, professor of microbiology and immunology, National University of Singapore, told this news organization, “After you have two infections, you seem to be protected quite well against the remaining two [serotypes]. The vaccine serves as another episode of infection in those who had prior dengue, so then any natural infections after the vaccination in the seropositive become like the outcome of a third or fourth infection.”
Vaccination alone will not solve dengue. Dr. Ooi said, “There’s not one method that would fully control dengue. You need both vaccines as well as control measures, whether it’s Wolbachia or something else. At the same time, I think we need antiviral drugs, because hitting this virus in just one part of its life cycle wouldn’t make a huge, lasting impact.” Dr. Ooi added that as “the spread of the virus and the population immunity drops, you’re actually now more vulnerable to dengue outbreaks when they do get introduced. So, suppressing transmission alone isn’t the answer. You also have to keep herd immunity levels high. So if we can reduce the virus transmission by controlling either mosquito population or transmission and at the same time vaccinate to keep the immunity levels high, then I think we have a chance of controlling dengue.”
Dr. Paz-Bailey concluded: “I do want to emphasize that we are excited about having these tools, because for years and years, we have had really limited options to prevent and control dengue. It’s an important addition to have the vaccine be approved to be used within the U.S., and it’s going to pave the road for future vaccines.”
Dr. Paz-Bailey and Dr. Ooi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MMWR RECOMMENDATIONS AND REPORTS
Quebec plans to fine unvaccinated adults
The amount hasn’t been decided yet, but it will be “significant” and more than $100. More details will be released at a later date, The Associated Press reported.
“Those who refuse to get their first doses in the coming weeks will have to pay a new health contribution,” Premier Francois Legault said during a news conference.
Not getting vaccinated burdens the health care system, and not all residents should pay for it, he said. About 10% of adults in Quebec are unvaccinated, but they represent about 50% of intensive care patients.
“I think it’s reasonable a majority of the population is asking that there be consequences,” he said. “It’s a question of fairness for the 90% of the population that have made some sacrifices. We owe them.”
The fine will apply to those who don’t qualify for a medical exemption, Mr. Legault said.
Provinces across Canada have reported a surge in COVID-19 cases due to the Omicron variant, with Quebec being one of the hardest-hit, according to Reuters. The province is regularly recording the highest daily case count across the country.
Quebec also has announced a 10 p.m. to 5 a.m. curfew, the AP reported. Starting Jan. 18, liquor and cannabis stores in the province will require proof of vaccination, and shopping malls and hair salons could soon require them as well.
About a quarter of all Canadians live in Quebec, according to CNN. The province was one of the first in Canada to require proof of vaccination for residents to eat in restaurants, go to the gym, or attend sporting events.
Some European countries have announced fees for unvaccinated residents, the AP reported, but Quebec is the first in Canada to announce a financial penalty for those who don’t get a shot.
In Greece, people older than 60 have until Jan. 16 to receive the first dose, or they will be fined 100 euros for every month they remain unvaccinated, the AP reported.
Austria will impose fines up to 3,600 euros for those who don’t follow the vaccine mandate for ages 14 and older, which is slated to start in February.
In Italy, residents who are 50 and older are required to be vaccinated. In mid-February, those who are unvaccinated could be fined up to 1,600 euros if they enter their workplaces, the AP reported.
A version of this article first appeared on WebMD.com.
The amount hasn’t been decided yet, but it will be “significant” and more than $100. More details will be released at a later date, The Associated Press reported.
“Those who refuse to get their first doses in the coming weeks will have to pay a new health contribution,” Premier Francois Legault said during a news conference.
Not getting vaccinated burdens the health care system, and not all residents should pay for it, he said. About 10% of adults in Quebec are unvaccinated, but they represent about 50% of intensive care patients.
“I think it’s reasonable a majority of the population is asking that there be consequences,” he said. “It’s a question of fairness for the 90% of the population that have made some sacrifices. We owe them.”
The fine will apply to those who don’t qualify for a medical exemption, Mr. Legault said.
Provinces across Canada have reported a surge in COVID-19 cases due to the Omicron variant, with Quebec being one of the hardest-hit, according to Reuters. The province is regularly recording the highest daily case count across the country.
Quebec also has announced a 10 p.m. to 5 a.m. curfew, the AP reported. Starting Jan. 18, liquor and cannabis stores in the province will require proof of vaccination, and shopping malls and hair salons could soon require them as well.
About a quarter of all Canadians live in Quebec, according to CNN. The province was one of the first in Canada to require proof of vaccination for residents to eat in restaurants, go to the gym, or attend sporting events.
Some European countries have announced fees for unvaccinated residents, the AP reported, but Quebec is the first in Canada to announce a financial penalty for those who don’t get a shot.
In Greece, people older than 60 have until Jan. 16 to receive the first dose, or they will be fined 100 euros for every month they remain unvaccinated, the AP reported.
Austria will impose fines up to 3,600 euros for those who don’t follow the vaccine mandate for ages 14 and older, which is slated to start in February.
In Italy, residents who are 50 and older are required to be vaccinated. In mid-February, those who are unvaccinated could be fined up to 1,600 euros if they enter their workplaces, the AP reported.
A version of this article first appeared on WebMD.com.
The amount hasn’t been decided yet, but it will be “significant” and more than $100. More details will be released at a later date, The Associated Press reported.
“Those who refuse to get their first doses in the coming weeks will have to pay a new health contribution,” Premier Francois Legault said during a news conference.
Not getting vaccinated burdens the health care system, and not all residents should pay for it, he said. About 10% of adults in Quebec are unvaccinated, but they represent about 50% of intensive care patients.
“I think it’s reasonable a majority of the population is asking that there be consequences,” he said. “It’s a question of fairness for the 90% of the population that have made some sacrifices. We owe them.”
The fine will apply to those who don’t qualify for a medical exemption, Mr. Legault said.
Provinces across Canada have reported a surge in COVID-19 cases due to the Omicron variant, with Quebec being one of the hardest-hit, according to Reuters. The province is regularly recording the highest daily case count across the country.
Quebec also has announced a 10 p.m. to 5 a.m. curfew, the AP reported. Starting Jan. 18, liquor and cannabis stores in the province will require proof of vaccination, and shopping malls and hair salons could soon require them as well.
About a quarter of all Canadians live in Quebec, according to CNN. The province was one of the first in Canada to require proof of vaccination for residents to eat in restaurants, go to the gym, or attend sporting events.
Some European countries have announced fees for unvaccinated residents, the AP reported, but Quebec is the first in Canada to announce a financial penalty for those who don’t get a shot.
In Greece, people older than 60 have until Jan. 16 to receive the first dose, or they will be fined 100 euros for every month they remain unvaccinated, the AP reported.
Austria will impose fines up to 3,600 euros for those who don’t follow the vaccine mandate for ages 14 and older, which is slated to start in February.
In Italy, residents who are 50 and older are required to be vaccinated. In mid-February, those who are unvaccinated could be fined up to 1,600 euros if they enter their workplaces, the AP reported.
A version of this article first appeared on WebMD.com.
Freshwater aquarium provides source for melioidosis infection
A Maryland woman came down with a severe tropical infection called melioidosis from her freshwater home aquarium, says a report in Emerging Infectious Diseases describing a new route of transmission. Melioidosis is caused by the bacteria Burkholderia pseudomallei in soil or water.
Until last year, almost all U.S. cases of melioidosis were from people who lived or traveled to disease-endemic areas. It has been a rare infection in the United States.
But this is not the first case of melioidosis from an unusual source. Earlier in 2021, CDC and state epidemiologists traced an outbreak of melioidosis in Georgia, Kansas, Minnesota, and Texas to B pseudomallei in a bottle of “Better Homes & Gardens Lavender & Chamomile Essential Oil Infused Aromatherapy Room Spray with Gemstones.”
In the aquarium case, the patient was a 56-year-old woman with diabetes and rheumatologic disease. She had been on immunosuppressives (methotrexate, azathioprine, and prednisone) until 1 month before she became symptomatic. She was hospitalized for fever and pneumonia.
Multiple blood cultures obtained on days 1-4 grew B. pseudomallei, but she had no evidence of endocarditis or intravascular seeding. Despite weeks of meropenem (Merrem), she developed evidence of a lung abscess, and trimethoprim/sulfamethoxazole (Bactrim) was added. Ultimately, the patient required a 12-week course of antibiotics.
CDC epidemiologist Patrick Dawson, PhD, first author of the report, told this news organization that although outbreak investigators always ask about pet ownership, they have not explicitly asked about fish. In this case, the patient did not volunteer exposure to the fish.
When state epidemiologists visited the patient’s home, “one of the first things they saw was a few aquariums,” Dr. Dawson said. Seeing the water and knowing “that most freshwater tropical fish in the U.S. are imported from Southeast Asia” led them to culture specifically for B. pseudomallei, which can be difficult for the microbiology lab to identify.
From there, Dr. Dawson explained, “The Maryland Department of Health sent a team to the local pet store” but did not find any of the bacteria there. (The patient had bought her fish 6 months earlier.) The investigators then worked with the national brand “to identify where they had actually sourced the fish from.”
Two retailers supply almost all of U.S. guppies and plants. While investigators could not find an exact matching isolate after so many months had elapsed, they found a positive PCR for B. pseudomallei in a water sample from imported fish in Los Angeles.
Dr. Dawson said tropical fish are imported from southeast Asia and typically come from small family fish farms. The fish import industry has “certain products that they add to the water to hopefully kill any bacteria.” He was unaware whether this included antibiotics but suggested, “we would have seen many more cases [of antibiotic resistance] by now” if it did.
In general advice for the public, Dr. Dawson said, “I would recommend washing hands before and after contact with the aquarium. If you have cuts or wounds on your hands, it’s really important to wear gloves if you have to go clean or maintain the aquarium and you’re putting your hands in the water, just for that extra layer of protection. It’s probably a strong idea to just avoid that altogether if someone’s immunocompromised. And not letting young children under 5 years old clean aquariums.” These are the “simplest things to do to protect yourself.”
Stephen A. Smith, DVM, PhD, a professor in the Aquatic Medicine Program at Virginia-Maryland College of Veterinary Medicine, Blacksburg, also stressed the importance of careful hand hygiene when caring for aquariums. He said that the filter, filter floss, biofilm, charcoal, and gravel might have exceptionally high concentrations of bacteria. Dr. Smith also recommended gloves when cleaning aquariums and not doing this task if immunocompromised.
Dr. Smith, who was not involved in the CDC study, shared a broader perspective, noting that “the reason why it’s important to federal regulators is that [B. pseudomallei] is a tier 1 select agent. And so, when that was isolated, it sent up all the red flags.” The far more common Mycobacterium marinum, or fish handler’s disease, is not reportable.
Mycobacterium marinum is another pathogen of concern that can be acquired from aquariums. These infections typically occur as nodular lesions on the arms and require months of therapy.
Dr. Smith stressed the importance of physicians eliciting a careful exposure history as the key to diagnosing zoonoses. For most exotic aquarium animals, he noted, “They’re caught in the wild wherever they are. They’re transported to a major hub to transport to the U.S., and a lot of times, we don’t have quarantine for those animals.”
Dr. Smith said.
Many infections also occur in the course of water sports – or even hiking and getting a cut or abrasion wet from a stream or lake. Aeromonas hydrophila can cause life-threatening infections. Vibrio vulnificus infections from salt-water injuries can cause sepsis and characteristic hemorrhagic bullae – large, discolored blisters filled with body fluid – during the summer. And eating contaminated shellfish has a 50%-60% death rate.
Other exposures to water-loving bacteria happen during fishing or cleaning/preparing fish. For example, Streptococcus iniae has caused cellulitis, arthritis, endocarditis, and meningitis following superficial or puncture injuries, notably from cleaning tilapia.
Other infections from contact with fish include Erysipelothrix rhusiopathiae (primarily skin infections) and gastroenteritis from Plesiomonas shigelloides, Campylobacter spp, and Salmonella spp.
Each of these zoonoses illustrates the importance of a careful exposure history when there’s an atypical presentation or an infection that is not responding promptly to empiric treatment. The aquarium case broadens the differential to include melioidosis, a serious disease from southeast Asia.
Dr. Dawson and Dr. Smith have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Maryland woman came down with a severe tropical infection called melioidosis from her freshwater home aquarium, says a report in Emerging Infectious Diseases describing a new route of transmission. Melioidosis is caused by the bacteria Burkholderia pseudomallei in soil or water.
Until last year, almost all U.S. cases of melioidosis were from people who lived or traveled to disease-endemic areas. It has been a rare infection in the United States.
But this is not the first case of melioidosis from an unusual source. Earlier in 2021, CDC and state epidemiologists traced an outbreak of melioidosis in Georgia, Kansas, Minnesota, and Texas to B pseudomallei in a bottle of “Better Homes & Gardens Lavender & Chamomile Essential Oil Infused Aromatherapy Room Spray with Gemstones.”
In the aquarium case, the patient was a 56-year-old woman with diabetes and rheumatologic disease. She had been on immunosuppressives (methotrexate, azathioprine, and prednisone) until 1 month before she became symptomatic. She was hospitalized for fever and pneumonia.
Multiple blood cultures obtained on days 1-4 grew B. pseudomallei, but she had no evidence of endocarditis or intravascular seeding. Despite weeks of meropenem (Merrem), she developed evidence of a lung abscess, and trimethoprim/sulfamethoxazole (Bactrim) was added. Ultimately, the patient required a 12-week course of antibiotics.
CDC epidemiologist Patrick Dawson, PhD, first author of the report, told this news organization that although outbreak investigators always ask about pet ownership, they have not explicitly asked about fish. In this case, the patient did not volunteer exposure to the fish.
When state epidemiologists visited the patient’s home, “one of the first things they saw was a few aquariums,” Dr. Dawson said. Seeing the water and knowing “that most freshwater tropical fish in the U.S. are imported from Southeast Asia” led them to culture specifically for B. pseudomallei, which can be difficult for the microbiology lab to identify.
From there, Dr. Dawson explained, “The Maryland Department of Health sent a team to the local pet store” but did not find any of the bacteria there. (The patient had bought her fish 6 months earlier.) The investigators then worked with the national brand “to identify where they had actually sourced the fish from.”
Two retailers supply almost all of U.S. guppies and plants. While investigators could not find an exact matching isolate after so many months had elapsed, they found a positive PCR for B. pseudomallei in a water sample from imported fish in Los Angeles.
Dr. Dawson said tropical fish are imported from southeast Asia and typically come from small family fish farms. The fish import industry has “certain products that they add to the water to hopefully kill any bacteria.” He was unaware whether this included antibiotics but suggested, “we would have seen many more cases [of antibiotic resistance] by now” if it did.
In general advice for the public, Dr. Dawson said, “I would recommend washing hands before and after contact with the aquarium. If you have cuts or wounds on your hands, it’s really important to wear gloves if you have to go clean or maintain the aquarium and you’re putting your hands in the water, just for that extra layer of protection. It’s probably a strong idea to just avoid that altogether if someone’s immunocompromised. And not letting young children under 5 years old clean aquariums.” These are the “simplest things to do to protect yourself.”
Stephen A. Smith, DVM, PhD, a professor in the Aquatic Medicine Program at Virginia-Maryland College of Veterinary Medicine, Blacksburg, also stressed the importance of careful hand hygiene when caring for aquariums. He said that the filter, filter floss, biofilm, charcoal, and gravel might have exceptionally high concentrations of bacteria. Dr. Smith also recommended gloves when cleaning aquariums and not doing this task if immunocompromised.
Dr. Smith, who was not involved in the CDC study, shared a broader perspective, noting that “the reason why it’s important to federal regulators is that [B. pseudomallei] is a tier 1 select agent. And so, when that was isolated, it sent up all the red flags.” The far more common Mycobacterium marinum, or fish handler’s disease, is not reportable.
Mycobacterium marinum is another pathogen of concern that can be acquired from aquariums. These infections typically occur as nodular lesions on the arms and require months of therapy.
Dr. Smith stressed the importance of physicians eliciting a careful exposure history as the key to diagnosing zoonoses. For most exotic aquarium animals, he noted, “They’re caught in the wild wherever they are. They’re transported to a major hub to transport to the U.S., and a lot of times, we don’t have quarantine for those animals.”
Dr. Smith said.
Many infections also occur in the course of water sports – or even hiking and getting a cut or abrasion wet from a stream or lake. Aeromonas hydrophila can cause life-threatening infections. Vibrio vulnificus infections from salt-water injuries can cause sepsis and characteristic hemorrhagic bullae – large, discolored blisters filled with body fluid – during the summer. And eating contaminated shellfish has a 50%-60% death rate.
Other exposures to water-loving bacteria happen during fishing or cleaning/preparing fish. For example, Streptococcus iniae has caused cellulitis, arthritis, endocarditis, and meningitis following superficial or puncture injuries, notably from cleaning tilapia.
Other infections from contact with fish include Erysipelothrix rhusiopathiae (primarily skin infections) and gastroenteritis from Plesiomonas shigelloides, Campylobacter spp, and Salmonella spp.
Each of these zoonoses illustrates the importance of a careful exposure history when there’s an atypical presentation or an infection that is not responding promptly to empiric treatment. The aquarium case broadens the differential to include melioidosis, a serious disease from southeast Asia.
Dr. Dawson and Dr. Smith have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Maryland woman came down with a severe tropical infection called melioidosis from her freshwater home aquarium, says a report in Emerging Infectious Diseases describing a new route of transmission. Melioidosis is caused by the bacteria Burkholderia pseudomallei in soil or water.
Until last year, almost all U.S. cases of melioidosis were from people who lived or traveled to disease-endemic areas. It has been a rare infection in the United States.
But this is not the first case of melioidosis from an unusual source. Earlier in 2021, CDC and state epidemiologists traced an outbreak of melioidosis in Georgia, Kansas, Minnesota, and Texas to B pseudomallei in a bottle of “Better Homes & Gardens Lavender & Chamomile Essential Oil Infused Aromatherapy Room Spray with Gemstones.”
In the aquarium case, the patient was a 56-year-old woman with diabetes and rheumatologic disease. She had been on immunosuppressives (methotrexate, azathioprine, and prednisone) until 1 month before she became symptomatic. She was hospitalized for fever and pneumonia.
Multiple blood cultures obtained on days 1-4 grew B. pseudomallei, but she had no evidence of endocarditis or intravascular seeding. Despite weeks of meropenem (Merrem), she developed evidence of a lung abscess, and trimethoprim/sulfamethoxazole (Bactrim) was added. Ultimately, the patient required a 12-week course of antibiotics.
CDC epidemiologist Patrick Dawson, PhD, first author of the report, told this news organization that although outbreak investigators always ask about pet ownership, they have not explicitly asked about fish. In this case, the patient did not volunteer exposure to the fish.
When state epidemiologists visited the patient’s home, “one of the first things they saw was a few aquariums,” Dr. Dawson said. Seeing the water and knowing “that most freshwater tropical fish in the U.S. are imported from Southeast Asia” led them to culture specifically for B. pseudomallei, which can be difficult for the microbiology lab to identify.
From there, Dr. Dawson explained, “The Maryland Department of Health sent a team to the local pet store” but did not find any of the bacteria there. (The patient had bought her fish 6 months earlier.) The investigators then worked with the national brand “to identify where they had actually sourced the fish from.”
Two retailers supply almost all of U.S. guppies and plants. While investigators could not find an exact matching isolate after so many months had elapsed, they found a positive PCR for B. pseudomallei in a water sample from imported fish in Los Angeles.
Dr. Dawson said tropical fish are imported from southeast Asia and typically come from small family fish farms. The fish import industry has “certain products that they add to the water to hopefully kill any bacteria.” He was unaware whether this included antibiotics but suggested, “we would have seen many more cases [of antibiotic resistance] by now” if it did.
In general advice for the public, Dr. Dawson said, “I would recommend washing hands before and after contact with the aquarium. If you have cuts or wounds on your hands, it’s really important to wear gloves if you have to go clean or maintain the aquarium and you’re putting your hands in the water, just for that extra layer of protection. It’s probably a strong idea to just avoid that altogether if someone’s immunocompromised. And not letting young children under 5 years old clean aquariums.” These are the “simplest things to do to protect yourself.”
Stephen A. Smith, DVM, PhD, a professor in the Aquatic Medicine Program at Virginia-Maryland College of Veterinary Medicine, Blacksburg, also stressed the importance of careful hand hygiene when caring for aquariums. He said that the filter, filter floss, biofilm, charcoal, and gravel might have exceptionally high concentrations of bacteria. Dr. Smith also recommended gloves when cleaning aquariums and not doing this task if immunocompromised.
Dr. Smith, who was not involved in the CDC study, shared a broader perspective, noting that “the reason why it’s important to federal regulators is that [B. pseudomallei] is a tier 1 select agent. And so, when that was isolated, it sent up all the red flags.” The far more common Mycobacterium marinum, or fish handler’s disease, is not reportable.
Mycobacterium marinum is another pathogen of concern that can be acquired from aquariums. These infections typically occur as nodular lesions on the arms and require months of therapy.
Dr. Smith stressed the importance of physicians eliciting a careful exposure history as the key to diagnosing zoonoses. For most exotic aquarium animals, he noted, “They’re caught in the wild wherever they are. They’re transported to a major hub to transport to the U.S., and a lot of times, we don’t have quarantine for those animals.”
Dr. Smith said.
Many infections also occur in the course of water sports – or even hiking and getting a cut or abrasion wet from a stream or lake. Aeromonas hydrophila can cause life-threatening infections. Vibrio vulnificus infections from salt-water injuries can cause sepsis and characteristic hemorrhagic bullae – large, discolored blisters filled with body fluid – during the summer. And eating contaminated shellfish has a 50%-60% death rate.
Other exposures to water-loving bacteria happen during fishing or cleaning/preparing fish. For example, Streptococcus iniae has caused cellulitis, arthritis, endocarditis, and meningitis following superficial or puncture injuries, notably from cleaning tilapia.
Other infections from contact with fish include Erysipelothrix rhusiopathiae (primarily skin infections) and gastroenteritis from Plesiomonas shigelloides, Campylobacter spp, and Salmonella spp.
Each of these zoonoses illustrates the importance of a careful exposure history when there’s an atypical presentation or an infection that is not responding promptly to empiric treatment. The aquarium case broadens the differential to include melioidosis, a serious disease from southeast Asia.
Dr. Dawson and Dr. Smith have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 interrupted global poliovirus surveillance and immunization
Most (86%) of these outbreaks were caused by cVDPV2 (circulating VDPV type 2 poliovirus, which originated with the vaccine), and most occurred in Africa, according to a new study of vaccine-derived poliovirus outbreaks between Jan. 2020 and June 2021 published in the CDC’s Morbidity and Mortality Weekly Report.
The Global Polio Eradication Initiative (GPEI) was launched in 1988 and used live attenuated oral poliovirus vaccine (OPV). Since then, cases of wild poliovirus have declined more than 99.99%.
The cVDPV2 likely originated among children born in areas with poor vaccine coverage. Jay Wenger, MD, director, Polio, at the Bill and Melinda Gates Foundation, told this news organization that “the inactivated vaccines that we give in most developed countries now are good in that they provide humoral immunity, the antibodies in the bloodstream. They don’t necessarily provide mucosal immunity. They don’t make the kid’s gut immune to getting reinfected or actually immune to reproducing the virus if they get it in their gut. So we could still have a situation where everybody was vaccinated with IPV [inactivated poliovirus], but the virus could still be transmitting around because kids’ guts would still be producing the virus and there will still be transmission in your population, probably without much or any paralysis because of the IPV. As soon as that virus hit a population that was not vaccinated, they would get paralyzed.”
Dr. Wenger added, “The ideal vaccine would be an oral vaccine that didn’t mutate back and couldn’t cause these VDPVs.” Scientists developed such a vaccine, approved by the World Health Organization last year under an Emergency Use Authorization. This nOPV2 (novel oral poliovirus type 2) vaccine has been given since March 2021 in areas with the VDPD2 outbreaks. The nOPV2 should allow them to “basically stamp out the outbreaks.”
The world had almost eradicated the disease, with the last cases of polio from wild virus occurring in Nigeria, Afghanistan, and Pakistan as of 2014. Africa was declared free of wild polio in 2020 after it had been eradicated from Nigeria, which accounted for more than half of the world’s cases only a decade earlier. Now cVDPV outbreaks affect 28 African countries, plus Iran, Yemen, Afghanistan, Pakistan, Tajikistan, Malaysia, the Philippines, and Indonesia. And there was also one case in China. Globally, there were 1,335 cases of cVDPV causing paralysis during the reporting period.
The COVID-19 pandemic has had a significant impact on polio, accounting for much of this year’s increase in cases. Dr. Wenger said, “We couldn’t do any campaigns. We pretty much stopped doing outbreak response campaigns in the middle of the year because of COVID.”
The CDC report notes that many of the supplementary immunizations in response to cVDPV2 outbreaks were of “poor quality,” and prolonged delays enabled geographically expanding cVDPV2 transmission.
Steve Wassilak, MD, chief coauthor of the CDC study, told this news organization that, because of COVID, “what we’ve been lacking is a rapid response for the most part. Some of that is due to laboratory delays and shipment because of COVID’s effect on international travel.” He noted, however, that there has been good recovery in surveillance and immunization activities despite COVID. And, he added, eradication “can be done, and many outbreaks have closed even during the [COVID] outbreak.”
Dr. Wassilak said that in Nigeria, “the face of the campaign became national.” In Pakistan, much of the work is done by national and international partners.
Dr. Wenger said that in Nigeria and other challenging areas, “the approach was essentially to make direct contact with the traditional leaders and the religious leaders and the local actors in each of these populations. So, it’s really getting down to the grassroots level.” Infectious disease officials send teams to speak with individuals in the “local, traditional leader system.”
“Just talking to them actually got us a long way and giving them the information that they need. In most cases, I mean, people want to do things to help their kids,” said Dr. Wenger.
For now, the initial plan, per the CDC, is to “initiate prompt and high coverage outbreak responses with available type 2 OPV to interrupt transmission” until a better supply of nOPV2 is available, then switch to IPVs.
Dr. Wenger and Dr. Wassilak report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most (86%) of these outbreaks were caused by cVDPV2 (circulating VDPV type 2 poliovirus, which originated with the vaccine), and most occurred in Africa, according to a new study of vaccine-derived poliovirus outbreaks between Jan. 2020 and June 2021 published in the CDC’s Morbidity and Mortality Weekly Report.
The Global Polio Eradication Initiative (GPEI) was launched in 1988 and used live attenuated oral poliovirus vaccine (OPV). Since then, cases of wild poliovirus have declined more than 99.99%.
The cVDPV2 likely originated among children born in areas with poor vaccine coverage. Jay Wenger, MD, director, Polio, at the Bill and Melinda Gates Foundation, told this news organization that “the inactivated vaccines that we give in most developed countries now are good in that they provide humoral immunity, the antibodies in the bloodstream. They don’t necessarily provide mucosal immunity. They don’t make the kid’s gut immune to getting reinfected or actually immune to reproducing the virus if they get it in their gut. So we could still have a situation where everybody was vaccinated with IPV [inactivated poliovirus], but the virus could still be transmitting around because kids’ guts would still be producing the virus and there will still be transmission in your population, probably without much or any paralysis because of the IPV. As soon as that virus hit a population that was not vaccinated, they would get paralyzed.”
Dr. Wenger added, “The ideal vaccine would be an oral vaccine that didn’t mutate back and couldn’t cause these VDPVs.” Scientists developed such a vaccine, approved by the World Health Organization last year under an Emergency Use Authorization. This nOPV2 (novel oral poliovirus type 2) vaccine has been given since March 2021 in areas with the VDPD2 outbreaks. The nOPV2 should allow them to “basically stamp out the outbreaks.”
The world had almost eradicated the disease, with the last cases of polio from wild virus occurring in Nigeria, Afghanistan, and Pakistan as of 2014. Africa was declared free of wild polio in 2020 after it had been eradicated from Nigeria, which accounted for more than half of the world’s cases only a decade earlier. Now cVDPV outbreaks affect 28 African countries, plus Iran, Yemen, Afghanistan, Pakistan, Tajikistan, Malaysia, the Philippines, and Indonesia. And there was also one case in China. Globally, there were 1,335 cases of cVDPV causing paralysis during the reporting period.
The COVID-19 pandemic has had a significant impact on polio, accounting for much of this year’s increase in cases. Dr. Wenger said, “We couldn’t do any campaigns. We pretty much stopped doing outbreak response campaigns in the middle of the year because of COVID.”
The CDC report notes that many of the supplementary immunizations in response to cVDPV2 outbreaks were of “poor quality,” and prolonged delays enabled geographically expanding cVDPV2 transmission.
Steve Wassilak, MD, chief coauthor of the CDC study, told this news organization that, because of COVID, “what we’ve been lacking is a rapid response for the most part. Some of that is due to laboratory delays and shipment because of COVID’s effect on international travel.” He noted, however, that there has been good recovery in surveillance and immunization activities despite COVID. And, he added, eradication “can be done, and many outbreaks have closed even during the [COVID] outbreak.”
Dr. Wassilak said that in Nigeria, “the face of the campaign became national.” In Pakistan, much of the work is done by national and international partners.
Dr. Wenger said that in Nigeria and other challenging areas, “the approach was essentially to make direct contact with the traditional leaders and the religious leaders and the local actors in each of these populations. So, it’s really getting down to the grassroots level.” Infectious disease officials send teams to speak with individuals in the “local, traditional leader system.”
“Just talking to them actually got us a long way and giving them the information that they need. In most cases, I mean, people want to do things to help their kids,” said Dr. Wenger.
For now, the initial plan, per the CDC, is to “initiate prompt and high coverage outbreak responses with available type 2 OPV to interrupt transmission” until a better supply of nOPV2 is available, then switch to IPVs.
Dr. Wenger and Dr. Wassilak report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most (86%) of these outbreaks were caused by cVDPV2 (circulating VDPV type 2 poliovirus, which originated with the vaccine), and most occurred in Africa, according to a new study of vaccine-derived poliovirus outbreaks between Jan. 2020 and June 2021 published in the CDC’s Morbidity and Mortality Weekly Report.
The Global Polio Eradication Initiative (GPEI) was launched in 1988 and used live attenuated oral poliovirus vaccine (OPV). Since then, cases of wild poliovirus have declined more than 99.99%.
The cVDPV2 likely originated among children born in areas with poor vaccine coverage. Jay Wenger, MD, director, Polio, at the Bill and Melinda Gates Foundation, told this news organization that “the inactivated vaccines that we give in most developed countries now are good in that they provide humoral immunity, the antibodies in the bloodstream. They don’t necessarily provide mucosal immunity. They don’t make the kid’s gut immune to getting reinfected or actually immune to reproducing the virus if they get it in their gut. So we could still have a situation where everybody was vaccinated with IPV [inactivated poliovirus], but the virus could still be transmitting around because kids’ guts would still be producing the virus and there will still be transmission in your population, probably without much or any paralysis because of the IPV. As soon as that virus hit a population that was not vaccinated, they would get paralyzed.”
Dr. Wenger added, “The ideal vaccine would be an oral vaccine that didn’t mutate back and couldn’t cause these VDPVs.” Scientists developed such a vaccine, approved by the World Health Organization last year under an Emergency Use Authorization. This nOPV2 (novel oral poliovirus type 2) vaccine has been given since March 2021 in areas with the VDPD2 outbreaks. The nOPV2 should allow them to “basically stamp out the outbreaks.”
The world had almost eradicated the disease, with the last cases of polio from wild virus occurring in Nigeria, Afghanistan, and Pakistan as of 2014. Africa was declared free of wild polio in 2020 after it had been eradicated from Nigeria, which accounted for more than half of the world’s cases only a decade earlier. Now cVDPV outbreaks affect 28 African countries, plus Iran, Yemen, Afghanistan, Pakistan, Tajikistan, Malaysia, the Philippines, and Indonesia. And there was also one case in China. Globally, there were 1,335 cases of cVDPV causing paralysis during the reporting period.
The COVID-19 pandemic has had a significant impact on polio, accounting for much of this year’s increase in cases. Dr. Wenger said, “We couldn’t do any campaigns. We pretty much stopped doing outbreak response campaigns in the middle of the year because of COVID.”
The CDC report notes that many of the supplementary immunizations in response to cVDPV2 outbreaks were of “poor quality,” and prolonged delays enabled geographically expanding cVDPV2 transmission.
Steve Wassilak, MD, chief coauthor of the CDC study, told this news organization that, because of COVID, “what we’ve been lacking is a rapid response for the most part. Some of that is due to laboratory delays and shipment because of COVID’s effect on international travel.” He noted, however, that there has been good recovery in surveillance and immunization activities despite COVID. And, he added, eradication “can be done, and many outbreaks have closed even during the [COVID] outbreak.”
Dr. Wassilak said that in Nigeria, “the face of the campaign became national.” In Pakistan, much of the work is done by national and international partners.
Dr. Wenger said that in Nigeria and other challenging areas, “the approach was essentially to make direct contact with the traditional leaders and the religious leaders and the local actors in each of these populations. So, it’s really getting down to the grassroots level.” Infectious disease officials send teams to speak with individuals in the “local, traditional leader system.”
“Just talking to them actually got us a long way and giving them the information that they need. In most cases, I mean, people want to do things to help their kids,” said Dr. Wenger.
For now, the initial plan, per the CDC, is to “initiate prompt and high coverage outbreak responses with available type 2 OPV to interrupt transmission” until a better supply of nOPV2 is available, then switch to IPVs.
Dr. Wenger and Dr. Wassilak report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dracunculiasis – guinea worm disease – is close to eradication. But will we ever reach the finish line?
When in 1988 former U.S. President Jimmy Carter toured Denchira and Elevanyo, two villages near Accra, Ghana, he noticed a young woman who appeared to be cradling a baby. Carter approached her for a chat, but was stopped in his tracks by a disquieting sight.
“It was not a baby. It was her right breast, which was about a foot long, and it had a guinea worm coming out of its nipple,” Mr. Carter later recalled. During his tour of Ghana that year, Mr. Carter saw hundreds of people affected by the guinea worm, an infection known as dracunculiasis – a disease caused by the nematode parasite Dracunculus medinensis. It’s a condition that can cause fever, severe pain, and even permanent damage to affected limbs.
In the late 1980s the country reported as many as 180,000 cases of guinea worm disease per year. Across the globe, that number was a staggering 3.5 million. However, by 2020, the world was down to just 27 cases, all of them in Africa.
This enormous reduction in prevalence is a direct effect of campaigns by endemic countries assisted by organizations such as the Centers for Disease Control and Prevention, the World Health Organization, and the Carter Center (a not-for-profit founded in 1982 by Jimmy Carter), which have strived since the 1980s to eradicate dracunculiasis, hoping to make it the second human disease purposefully wiped off the face of Earth. (Smallpox was the first.)
“That’s an extraordinary public health achievement,” David Molyneux, PhD, parasitologist at the Liverpool School of Tropical Medicine, said in an interview. Yet the eradication goal, currently set for 2030, seems unlikely to be met. What’s more, some experts argue that chasing eradication may be altogether a misguided idea.
Humanity has known dracunculiasis for millennia. Well-preserved specimens of Dracunculus medinensis were discovered in Egyptian mummies, while some researchers claim that the Old Testament’s “fiery serpents” that descended upon the Israelites near the Red Sea were in fact guinea worms, as the parasite was endemic to the area in the past. Even the serpent coiled around the staff of Asclepius, the god of medicine, might have been a guinea worm, according to some historians.
This would make sense considering how the disease is treated. When an adult worm emerges through the skin, a painful and crippling occurrence, it is wound up around a stick or a piece of gauze, a little at a time, to slowly draw it out of the skin. As the worm can be over 3 feet long, this procedure may take weeks. What you end up with is a stick with a long, snake-like animal coiled around it. Asclepius’s staff.
The first step in the infection is when a person drinks water contaminated with copepods, or water fleas, which contain the larvae of Dracunculus medinensis. Next, the larvae are freed in the stomach and start migrating through the body, looking to mate. The fertilized female worm is the one that causes the debilitating symptoms.
About a year after the initial infection, the pregnant female worm looks for exit points from the body, usually through legs or feet, ready to release new larvae. If the unlucky sufferer steps into a pond or a river, the immature larvae escape into the water, where they are eaten by water fleas. “People are fetching water to drink, and they walk into the water thinking they can get cleaner water not along the edge,” Adam Weiss, MPH, director of the Carter Center’s Guinea Worm Eradication Program, said in an interview. The vicious cycle begins anew.
Dracunculiasis may not be a killer disease, but it is painful and disabling. A study on school attendance in Nigeria showed that in 1995 when guinea worm infection prevalence among schoolchildren was as high as 27.7%, it was responsible for almost all school absences. As the result of the infection, children were seen wandering and sitting around the village helplessly. If it was the parents who got infected, children stayed out of school to help around the home. The dracunculiasis’ impact on work and earning capacity is so profound, in fact, that in Mali the infliction is known as “the disease of the empty granary.”
When in 1986 the Carter Center took the reins of the global dracunculiasis eradication campaign, India was the only country with a national program to get rid of the disease. Yet, once other nations joined the struggle, the results rapidly became visible. By 1993, the American Journal of Tropical Medicine and Hygiene published a paper titled, “Dracunculiasis Eradication: Beginning of the End.” The cases plummeted from 3.5 million in 1986 to 221,000 in 1993 and 32,000 in 2003, then to a mere 22 cases in 2015. What worked was a combination of surveillance, education campaigns, safe water provision, and treating potentially contaminated water with a chemical called Abate, a potent larvicide.
Today, many endemic countries, from Chad and Ethiopia to Mali and South Sudan, follow similar procedures. First and foremost is the supply of clean drinking water. However, Mr. Weiss said, this is not a “silver bullet, given how people live.” Those who are seminomadic or otherwise take care of livestock often fetch water outside of the village, from ponds or rivers. This is why dracunculiasis eradication programs include handing out portable water filters, which can be worn around the neck.
But if you don’t know why you should filter water, in all likelihood you won’t do it – cloth filters distributed for home water purification sometimes ended up as decorations or sewn into wedding dresses. That’s why education is key, too. Poster campaigns, comic books, radio broadcasts, instructions by volunteers, even t-shirts with health messages slowly but surely did change behaviors.
Cash rewards for reporting cases of dracunculiasis, which can be as high as $100, also work well to boost surveillance systems. Once a case is identified, patients may be moved to a containment center, both to treat the wound and to prevent patients from spreading the disease. Local water sources, meanwhile, may be sprayed with Abate.
1995 was the first year set as a target date for the eradication of dracunculiasis. Yet the goal wasn’t met – even though the total number of cases did decline by 97%. Next goals followed: 2009, 2020, and now, finally, 2030. For well over a decade now the world has been down to a trickle of cases per year, but the numbers don’t seem to want to budge lower. Mr. Weiss calls it a “limbo period” – we are almost there, but not quite. The final push, it seems, may be the one that’s the most difficult, especially now that we have two further complications: increasing conflicts in some endemic areas and zoonotic transmission.
According to WHO, in places like the Democratic Republic of the Congo, Mali, South Sudan, and Sudan, insecurity “hinders eradication efforts.” Not only does this insecurity make it difficult for health workers to reach endemic areas, but wars and violence also displace people, pushing those infected with guinea worm to walk far distances in search of safety, and spreading the disease during their travels. Case containment and contact tracing become challenging. A recent study by Dr. Molyneux and colleagues showed that, in the 3 years since 2018, conflicts in the endemic areas have increased dramatically.
And then there are the animals. Up until 2012, eradication of guinea worm seemed fairly simple, at least from a biological perspective: Stop infected humans from contaminating drinking water and the parasites won’t be able to continue their life cycle. But in 2012, news came from Chad that a significant number of local dogs were found infected with the Dracunculus medinensis parasite, the very same one that attacks humans. In 2020, close to 1,600 dogs were reported to be infected with guinea worm, most of them in Chad. This left scientists scratching their heads: Dracunculiasis was supposed to be a purely human infliction. How were the dogs getting infected? Did the parasite jump to a new species because we were so efficient at eliminating it from humans?
“I have first seen a guinea worm transmission in dogs back in 2003,” Teshome Gebre, PhD, said in an interview. Dr. Gebre is regional director for Africa at International Trachoma Initiative and has spent more than 40 years fighting to eradicate various diseases, including smallpox and guinea worm. Yet in 2003, Dr. Gebre’s report was dismissed: it couldn’t have been the same species of the parasite, the reasoning went, since Dracunculus medinensis was exclusive to humans.
“I think it’s fair to say that there were infections in dogs before 2012. I find it difficult to believe, logically, that it just came out of nowhere,” Mr. Weiss said. A 2018 genetic study showed that a novel host switch is an unlikely scenario – the parasites must have been infecting dogs in the past, we just haven’t been looking. By 2012, Chad had a very efficient guinea worm surveillance system, with generous cash rewards for human cases, and people started reporting the dogs, too. Soon money was also offered for news on infected animals, and the cases exploded. This was then followed by accounts of afflicted cats and baboons.
To announce the eradication of dracunculiasis in 2030, the requirement will be no more transmission of the parasite for at least 4 years prior anywhere in the world – not only zero human cases, but also no infections in dogs, cats, or baboons. Seven countries remain to be certified as guinea worm free, all of them in Africa. “We have to be a 100% sure that there is no transmission of the parasite in a country,” said Dr. Molyneux, who participated in country certification teams – a rigorous process to validate country reports. He believes that the presence of animal hosts as well as growing insecurities in the region make such certification extremely challenging over the next few years.
“Eradication as it is defined does not seem feasible by 2030 as things stand, [considering] political and resource constraints, the unknowns of the ecology of dogs, and the possible impact of climate change and geopolitical instability and with countries having other health priorities, including COVID,” Dr. Molyneux said.
For Mr. Weiss, dogs are not that much of a problem – since they can be tethered to prevent the spread of the disease. But you can’t tether baboons. “That does raise that more existential threat–related question of: Is this scientifically possible?” he said. Mr. Weiss and colleagues at the Centers for Disease Control and Prevention are currently working on a serologic assay to test whether baboons are important for human transmission.
For some experts, such as Dr. Gebre, the current struggles to bring cases down to zero put a spotlight on a bigger question: is it worthwhile to strive for eradication at all? That last stretch of the eradication campaign can appear a bit like a game of whack-a-mole. “There were times when we’ve achieved zero cases [in Ethiopia]. Zero. And then, it just reemerges,” Dr. Gebre said. Programs aimed at certification are costly, running up to $1.6 million per year in Nigeria. The funds often come from the same donor pockets that pay for the fight against malaria, HIV, polio, as well as other neglected tropical diseases. Dr. Gebre believed it would be more cost and time efficient to switch the effort from total eradication to elimination as a public health care problem.
Of course, there is the risk that the cases would go up again once we ease up on the pressure to eradicate dracunculiasis. “Do we want to be fighting guinea worm in perpetuity?” Mr. Weiss asked. However, Dr. Gebre believed the cases are unlikely to explode anymore.
“The situation in the countries is not the way it was 30 years ago,” Dr. Gebre said, pointing out increased awareness, higher education levels, and better community-based health facilities. “You can cap it around a trickle number of cases a year – 10, 15, 20 maybe.”
The keys, Dr. Gebre and Dr. Molyneux both said, include the provision of safe drinking water and strengthening the healthcare systems of endemic countries in general, so they can deal with whatever cases may come up. “Water, sanitation, surveillance, good public education – and the maintenance of the guinea worm–specific reward system to maintain awareness, as well as continuing research” are all needed, Dr. Molyneux said.
Getting out of the dracunculiasis limbo period won’t be easy. We certainly need more data on animal transmission to better understand what challenges we might be facing. The experts agree that what’s important is to follow the science and stay flexible. “We have made an incredible progress, our investment has been worthwhile,” Dr. Molyneux said. But “you have to adapt to the changing realities.”
Dr. Gebre received no financial support for the review article and has no other conflicts of interest to declare. Dr. Molyneux is a member of the WHO International Commission for the Certification of Dracunculus Eradication, an independent body appointed by the director general of WHO. He acts as a rapporteur for the ICCDE as a paid consultant. He declared he does not receive any financial support for other related activities. Mr. Weiss receives support from the nonprofit Carter Center.
A version of this article first appeared on Medscape.com.
When in 1988 former U.S. President Jimmy Carter toured Denchira and Elevanyo, two villages near Accra, Ghana, he noticed a young woman who appeared to be cradling a baby. Carter approached her for a chat, but was stopped in his tracks by a disquieting sight.
“It was not a baby. It was her right breast, which was about a foot long, and it had a guinea worm coming out of its nipple,” Mr. Carter later recalled. During his tour of Ghana that year, Mr. Carter saw hundreds of people affected by the guinea worm, an infection known as dracunculiasis – a disease caused by the nematode parasite Dracunculus medinensis. It’s a condition that can cause fever, severe pain, and even permanent damage to affected limbs.
In the late 1980s the country reported as many as 180,000 cases of guinea worm disease per year. Across the globe, that number was a staggering 3.5 million. However, by 2020, the world was down to just 27 cases, all of them in Africa.
This enormous reduction in prevalence is a direct effect of campaigns by endemic countries assisted by organizations such as the Centers for Disease Control and Prevention, the World Health Organization, and the Carter Center (a not-for-profit founded in 1982 by Jimmy Carter), which have strived since the 1980s to eradicate dracunculiasis, hoping to make it the second human disease purposefully wiped off the face of Earth. (Smallpox was the first.)
“That’s an extraordinary public health achievement,” David Molyneux, PhD, parasitologist at the Liverpool School of Tropical Medicine, said in an interview. Yet the eradication goal, currently set for 2030, seems unlikely to be met. What’s more, some experts argue that chasing eradication may be altogether a misguided idea.
Humanity has known dracunculiasis for millennia. Well-preserved specimens of Dracunculus medinensis were discovered in Egyptian mummies, while some researchers claim that the Old Testament’s “fiery serpents” that descended upon the Israelites near the Red Sea were in fact guinea worms, as the parasite was endemic to the area in the past. Even the serpent coiled around the staff of Asclepius, the god of medicine, might have been a guinea worm, according to some historians.
This would make sense considering how the disease is treated. When an adult worm emerges through the skin, a painful and crippling occurrence, it is wound up around a stick or a piece of gauze, a little at a time, to slowly draw it out of the skin. As the worm can be over 3 feet long, this procedure may take weeks. What you end up with is a stick with a long, snake-like animal coiled around it. Asclepius’s staff.
The first step in the infection is when a person drinks water contaminated with copepods, or water fleas, which contain the larvae of Dracunculus medinensis. Next, the larvae are freed in the stomach and start migrating through the body, looking to mate. The fertilized female worm is the one that causes the debilitating symptoms.
About a year after the initial infection, the pregnant female worm looks for exit points from the body, usually through legs or feet, ready to release new larvae. If the unlucky sufferer steps into a pond or a river, the immature larvae escape into the water, where they are eaten by water fleas. “People are fetching water to drink, and they walk into the water thinking they can get cleaner water not along the edge,” Adam Weiss, MPH, director of the Carter Center’s Guinea Worm Eradication Program, said in an interview. The vicious cycle begins anew.
Dracunculiasis may not be a killer disease, but it is painful and disabling. A study on school attendance in Nigeria showed that in 1995 when guinea worm infection prevalence among schoolchildren was as high as 27.7%, it was responsible for almost all school absences. As the result of the infection, children were seen wandering and sitting around the village helplessly. If it was the parents who got infected, children stayed out of school to help around the home. The dracunculiasis’ impact on work and earning capacity is so profound, in fact, that in Mali the infliction is known as “the disease of the empty granary.”
When in 1986 the Carter Center took the reins of the global dracunculiasis eradication campaign, India was the only country with a national program to get rid of the disease. Yet, once other nations joined the struggle, the results rapidly became visible. By 1993, the American Journal of Tropical Medicine and Hygiene published a paper titled, “Dracunculiasis Eradication: Beginning of the End.” The cases plummeted from 3.5 million in 1986 to 221,000 in 1993 and 32,000 in 2003, then to a mere 22 cases in 2015. What worked was a combination of surveillance, education campaigns, safe water provision, and treating potentially contaminated water with a chemical called Abate, a potent larvicide.
Today, many endemic countries, from Chad and Ethiopia to Mali and South Sudan, follow similar procedures. First and foremost is the supply of clean drinking water. However, Mr. Weiss said, this is not a “silver bullet, given how people live.” Those who are seminomadic or otherwise take care of livestock often fetch water outside of the village, from ponds or rivers. This is why dracunculiasis eradication programs include handing out portable water filters, which can be worn around the neck.
But if you don’t know why you should filter water, in all likelihood you won’t do it – cloth filters distributed for home water purification sometimes ended up as decorations or sewn into wedding dresses. That’s why education is key, too. Poster campaigns, comic books, radio broadcasts, instructions by volunteers, even t-shirts with health messages slowly but surely did change behaviors.
Cash rewards for reporting cases of dracunculiasis, which can be as high as $100, also work well to boost surveillance systems. Once a case is identified, patients may be moved to a containment center, both to treat the wound and to prevent patients from spreading the disease. Local water sources, meanwhile, may be sprayed with Abate.
1995 was the first year set as a target date for the eradication of dracunculiasis. Yet the goal wasn’t met – even though the total number of cases did decline by 97%. Next goals followed: 2009, 2020, and now, finally, 2030. For well over a decade now the world has been down to a trickle of cases per year, but the numbers don’t seem to want to budge lower. Mr. Weiss calls it a “limbo period” – we are almost there, but not quite. The final push, it seems, may be the one that’s the most difficult, especially now that we have two further complications: increasing conflicts in some endemic areas and zoonotic transmission.
According to WHO, in places like the Democratic Republic of the Congo, Mali, South Sudan, and Sudan, insecurity “hinders eradication efforts.” Not only does this insecurity make it difficult for health workers to reach endemic areas, but wars and violence also displace people, pushing those infected with guinea worm to walk far distances in search of safety, and spreading the disease during their travels. Case containment and contact tracing become challenging. A recent study by Dr. Molyneux and colleagues showed that, in the 3 years since 2018, conflicts in the endemic areas have increased dramatically.
And then there are the animals. Up until 2012, eradication of guinea worm seemed fairly simple, at least from a biological perspective: Stop infected humans from contaminating drinking water and the parasites won’t be able to continue their life cycle. But in 2012, news came from Chad that a significant number of local dogs were found infected with the Dracunculus medinensis parasite, the very same one that attacks humans. In 2020, close to 1,600 dogs were reported to be infected with guinea worm, most of them in Chad. This left scientists scratching their heads: Dracunculiasis was supposed to be a purely human infliction. How were the dogs getting infected? Did the parasite jump to a new species because we were so efficient at eliminating it from humans?
“I have first seen a guinea worm transmission in dogs back in 2003,” Teshome Gebre, PhD, said in an interview. Dr. Gebre is regional director for Africa at International Trachoma Initiative and has spent more than 40 years fighting to eradicate various diseases, including smallpox and guinea worm. Yet in 2003, Dr. Gebre’s report was dismissed: it couldn’t have been the same species of the parasite, the reasoning went, since Dracunculus medinensis was exclusive to humans.
“I think it’s fair to say that there were infections in dogs before 2012. I find it difficult to believe, logically, that it just came out of nowhere,” Mr. Weiss said. A 2018 genetic study showed that a novel host switch is an unlikely scenario – the parasites must have been infecting dogs in the past, we just haven’t been looking. By 2012, Chad had a very efficient guinea worm surveillance system, with generous cash rewards for human cases, and people started reporting the dogs, too. Soon money was also offered for news on infected animals, and the cases exploded. This was then followed by accounts of afflicted cats and baboons.
To announce the eradication of dracunculiasis in 2030, the requirement will be no more transmission of the parasite for at least 4 years prior anywhere in the world – not only zero human cases, but also no infections in dogs, cats, or baboons. Seven countries remain to be certified as guinea worm free, all of them in Africa. “We have to be a 100% sure that there is no transmission of the parasite in a country,” said Dr. Molyneux, who participated in country certification teams – a rigorous process to validate country reports. He believes that the presence of animal hosts as well as growing insecurities in the region make such certification extremely challenging over the next few years.
“Eradication as it is defined does not seem feasible by 2030 as things stand, [considering] political and resource constraints, the unknowns of the ecology of dogs, and the possible impact of climate change and geopolitical instability and with countries having other health priorities, including COVID,” Dr. Molyneux said.
For Mr. Weiss, dogs are not that much of a problem – since they can be tethered to prevent the spread of the disease. But you can’t tether baboons. “That does raise that more existential threat–related question of: Is this scientifically possible?” he said. Mr. Weiss and colleagues at the Centers for Disease Control and Prevention are currently working on a serologic assay to test whether baboons are important for human transmission.
For some experts, such as Dr. Gebre, the current struggles to bring cases down to zero put a spotlight on a bigger question: is it worthwhile to strive for eradication at all? That last stretch of the eradication campaign can appear a bit like a game of whack-a-mole. “There were times when we’ve achieved zero cases [in Ethiopia]. Zero. And then, it just reemerges,” Dr. Gebre said. Programs aimed at certification are costly, running up to $1.6 million per year in Nigeria. The funds often come from the same donor pockets that pay for the fight against malaria, HIV, polio, as well as other neglected tropical diseases. Dr. Gebre believed it would be more cost and time efficient to switch the effort from total eradication to elimination as a public health care problem.
Of course, there is the risk that the cases would go up again once we ease up on the pressure to eradicate dracunculiasis. “Do we want to be fighting guinea worm in perpetuity?” Mr. Weiss asked. However, Dr. Gebre believed the cases are unlikely to explode anymore.
“The situation in the countries is not the way it was 30 years ago,” Dr. Gebre said, pointing out increased awareness, higher education levels, and better community-based health facilities. “You can cap it around a trickle number of cases a year – 10, 15, 20 maybe.”
The keys, Dr. Gebre and Dr. Molyneux both said, include the provision of safe drinking water and strengthening the healthcare systems of endemic countries in general, so they can deal with whatever cases may come up. “Water, sanitation, surveillance, good public education – and the maintenance of the guinea worm–specific reward system to maintain awareness, as well as continuing research” are all needed, Dr. Molyneux said.
Getting out of the dracunculiasis limbo period won’t be easy. We certainly need more data on animal transmission to better understand what challenges we might be facing. The experts agree that what’s important is to follow the science and stay flexible. “We have made an incredible progress, our investment has been worthwhile,” Dr. Molyneux said. But “you have to adapt to the changing realities.”
Dr. Gebre received no financial support for the review article and has no other conflicts of interest to declare. Dr. Molyneux is a member of the WHO International Commission for the Certification of Dracunculus Eradication, an independent body appointed by the director general of WHO. He acts as a rapporteur for the ICCDE as a paid consultant. He declared he does not receive any financial support for other related activities. Mr. Weiss receives support from the nonprofit Carter Center.
A version of this article first appeared on Medscape.com.
When in 1988 former U.S. President Jimmy Carter toured Denchira and Elevanyo, two villages near Accra, Ghana, he noticed a young woman who appeared to be cradling a baby. Carter approached her for a chat, but was stopped in his tracks by a disquieting sight.
“It was not a baby. It was her right breast, which was about a foot long, and it had a guinea worm coming out of its nipple,” Mr. Carter later recalled. During his tour of Ghana that year, Mr. Carter saw hundreds of people affected by the guinea worm, an infection known as dracunculiasis – a disease caused by the nematode parasite Dracunculus medinensis. It’s a condition that can cause fever, severe pain, and even permanent damage to affected limbs.
In the late 1980s the country reported as many as 180,000 cases of guinea worm disease per year. Across the globe, that number was a staggering 3.5 million. However, by 2020, the world was down to just 27 cases, all of them in Africa.
This enormous reduction in prevalence is a direct effect of campaigns by endemic countries assisted by organizations such as the Centers for Disease Control and Prevention, the World Health Organization, and the Carter Center (a not-for-profit founded in 1982 by Jimmy Carter), which have strived since the 1980s to eradicate dracunculiasis, hoping to make it the second human disease purposefully wiped off the face of Earth. (Smallpox was the first.)
“That’s an extraordinary public health achievement,” David Molyneux, PhD, parasitologist at the Liverpool School of Tropical Medicine, said in an interview. Yet the eradication goal, currently set for 2030, seems unlikely to be met. What’s more, some experts argue that chasing eradication may be altogether a misguided idea.
Humanity has known dracunculiasis for millennia. Well-preserved specimens of Dracunculus medinensis were discovered in Egyptian mummies, while some researchers claim that the Old Testament’s “fiery serpents” that descended upon the Israelites near the Red Sea were in fact guinea worms, as the parasite was endemic to the area in the past. Even the serpent coiled around the staff of Asclepius, the god of medicine, might have been a guinea worm, according to some historians.
This would make sense considering how the disease is treated. When an adult worm emerges through the skin, a painful and crippling occurrence, it is wound up around a stick or a piece of gauze, a little at a time, to slowly draw it out of the skin. As the worm can be over 3 feet long, this procedure may take weeks. What you end up with is a stick with a long, snake-like animal coiled around it. Asclepius’s staff.
The first step in the infection is when a person drinks water contaminated with copepods, or water fleas, which contain the larvae of Dracunculus medinensis. Next, the larvae are freed in the stomach and start migrating through the body, looking to mate. The fertilized female worm is the one that causes the debilitating symptoms.
About a year after the initial infection, the pregnant female worm looks for exit points from the body, usually through legs or feet, ready to release new larvae. If the unlucky sufferer steps into a pond or a river, the immature larvae escape into the water, where they are eaten by water fleas. “People are fetching water to drink, and they walk into the water thinking they can get cleaner water not along the edge,” Adam Weiss, MPH, director of the Carter Center’s Guinea Worm Eradication Program, said in an interview. The vicious cycle begins anew.
Dracunculiasis may not be a killer disease, but it is painful and disabling. A study on school attendance in Nigeria showed that in 1995 when guinea worm infection prevalence among schoolchildren was as high as 27.7%, it was responsible for almost all school absences. As the result of the infection, children were seen wandering and sitting around the village helplessly. If it was the parents who got infected, children stayed out of school to help around the home. The dracunculiasis’ impact on work and earning capacity is so profound, in fact, that in Mali the infliction is known as “the disease of the empty granary.”
When in 1986 the Carter Center took the reins of the global dracunculiasis eradication campaign, India was the only country with a national program to get rid of the disease. Yet, once other nations joined the struggle, the results rapidly became visible. By 1993, the American Journal of Tropical Medicine and Hygiene published a paper titled, “Dracunculiasis Eradication: Beginning of the End.” The cases plummeted from 3.5 million in 1986 to 221,000 in 1993 and 32,000 in 2003, then to a mere 22 cases in 2015. What worked was a combination of surveillance, education campaigns, safe water provision, and treating potentially contaminated water with a chemical called Abate, a potent larvicide.
Today, many endemic countries, from Chad and Ethiopia to Mali and South Sudan, follow similar procedures. First and foremost is the supply of clean drinking water. However, Mr. Weiss said, this is not a “silver bullet, given how people live.” Those who are seminomadic or otherwise take care of livestock often fetch water outside of the village, from ponds or rivers. This is why dracunculiasis eradication programs include handing out portable water filters, which can be worn around the neck.
But if you don’t know why you should filter water, in all likelihood you won’t do it – cloth filters distributed for home water purification sometimes ended up as decorations or sewn into wedding dresses. That’s why education is key, too. Poster campaigns, comic books, radio broadcasts, instructions by volunteers, even t-shirts with health messages slowly but surely did change behaviors.
Cash rewards for reporting cases of dracunculiasis, which can be as high as $100, also work well to boost surveillance systems. Once a case is identified, patients may be moved to a containment center, both to treat the wound and to prevent patients from spreading the disease. Local water sources, meanwhile, may be sprayed with Abate.
1995 was the first year set as a target date for the eradication of dracunculiasis. Yet the goal wasn’t met – even though the total number of cases did decline by 97%. Next goals followed: 2009, 2020, and now, finally, 2030. For well over a decade now the world has been down to a trickle of cases per year, but the numbers don’t seem to want to budge lower. Mr. Weiss calls it a “limbo period” – we are almost there, but not quite. The final push, it seems, may be the one that’s the most difficult, especially now that we have two further complications: increasing conflicts in some endemic areas and zoonotic transmission.
According to WHO, in places like the Democratic Republic of the Congo, Mali, South Sudan, and Sudan, insecurity “hinders eradication efforts.” Not only does this insecurity make it difficult for health workers to reach endemic areas, but wars and violence also displace people, pushing those infected with guinea worm to walk far distances in search of safety, and spreading the disease during their travels. Case containment and contact tracing become challenging. A recent study by Dr. Molyneux and colleagues showed that, in the 3 years since 2018, conflicts in the endemic areas have increased dramatically.
And then there are the animals. Up until 2012, eradication of guinea worm seemed fairly simple, at least from a biological perspective: Stop infected humans from contaminating drinking water and the parasites won’t be able to continue their life cycle. But in 2012, news came from Chad that a significant number of local dogs were found infected with the Dracunculus medinensis parasite, the very same one that attacks humans. In 2020, close to 1,600 dogs were reported to be infected with guinea worm, most of them in Chad. This left scientists scratching their heads: Dracunculiasis was supposed to be a purely human infliction. How were the dogs getting infected? Did the parasite jump to a new species because we were so efficient at eliminating it from humans?
“I have first seen a guinea worm transmission in dogs back in 2003,” Teshome Gebre, PhD, said in an interview. Dr. Gebre is regional director for Africa at International Trachoma Initiative and has spent more than 40 years fighting to eradicate various diseases, including smallpox and guinea worm. Yet in 2003, Dr. Gebre’s report was dismissed: it couldn’t have been the same species of the parasite, the reasoning went, since Dracunculus medinensis was exclusive to humans.
“I think it’s fair to say that there were infections in dogs before 2012. I find it difficult to believe, logically, that it just came out of nowhere,” Mr. Weiss said. A 2018 genetic study showed that a novel host switch is an unlikely scenario – the parasites must have been infecting dogs in the past, we just haven’t been looking. By 2012, Chad had a very efficient guinea worm surveillance system, with generous cash rewards for human cases, and people started reporting the dogs, too. Soon money was also offered for news on infected animals, and the cases exploded. This was then followed by accounts of afflicted cats and baboons.
To announce the eradication of dracunculiasis in 2030, the requirement will be no more transmission of the parasite for at least 4 years prior anywhere in the world – not only zero human cases, but also no infections in dogs, cats, or baboons. Seven countries remain to be certified as guinea worm free, all of them in Africa. “We have to be a 100% sure that there is no transmission of the parasite in a country,” said Dr. Molyneux, who participated in country certification teams – a rigorous process to validate country reports. He believes that the presence of animal hosts as well as growing insecurities in the region make such certification extremely challenging over the next few years.
“Eradication as it is defined does not seem feasible by 2030 as things stand, [considering] political and resource constraints, the unknowns of the ecology of dogs, and the possible impact of climate change and geopolitical instability and with countries having other health priorities, including COVID,” Dr. Molyneux said.
For Mr. Weiss, dogs are not that much of a problem – since they can be tethered to prevent the spread of the disease. But you can’t tether baboons. “That does raise that more existential threat–related question of: Is this scientifically possible?” he said. Mr. Weiss and colleagues at the Centers for Disease Control and Prevention are currently working on a serologic assay to test whether baboons are important for human transmission.
For some experts, such as Dr. Gebre, the current struggles to bring cases down to zero put a spotlight on a bigger question: is it worthwhile to strive for eradication at all? That last stretch of the eradication campaign can appear a bit like a game of whack-a-mole. “There were times when we’ve achieved zero cases [in Ethiopia]. Zero. And then, it just reemerges,” Dr. Gebre said. Programs aimed at certification are costly, running up to $1.6 million per year in Nigeria. The funds often come from the same donor pockets that pay for the fight against malaria, HIV, polio, as well as other neglected tropical diseases. Dr. Gebre believed it would be more cost and time efficient to switch the effort from total eradication to elimination as a public health care problem.
Of course, there is the risk that the cases would go up again once we ease up on the pressure to eradicate dracunculiasis. “Do we want to be fighting guinea worm in perpetuity?” Mr. Weiss asked. However, Dr. Gebre believed the cases are unlikely to explode anymore.
“The situation in the countries is not the way it was 30 years ago,” Dr. Gebre said, pointing out increased awareness, higher education levels, and better community-based health facilities. “You can cap it around a trickle number of cases a year – 10, 15, 20 maybe.”
The keys, Dr. Gebre and Dr. Molyneux both said, include the provision of safe drinking water and strengthening the healthcare systems of endemic countries in general, so they can deal with whatever cases may come up. “Water, sanitation, surveillance, good public education – and the maintenance of the guinea worm–specific reward system to maintain awareness, as well as continuing research” are all needed, Dr. Molyneux said.
Getting out of the dracunculiasis limbo period won’t be easy. We certainly need more data on animal transmission to better understand what challenges we might be facing. The experts agree that what’s important is to follow the science and stay flexible. “We have made an incredible progress, our investment has been worthwhile,” Dr. Molyneux said. But “you have to adapt to the changing realities.”
Dr. Gebre received no financial support for the review article and has no other conflicts of interest to declare. Dr. Molyneux is a member of the WHO International Commission for the Certification of Dracunculus Eradication, an independent body appointed by the director general of WHO. He acts as a rapporteur for the ICCDE as a paid consultant. He declared he does not receive any financial support for other related activities. Mr. Weiss receives support from the nonprofit Carter Center.
A version of this article first appeared on Medscape.com.
Surveillance for measles is a victim of the COVID pandemic
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.