User login
Massive Baker Cyst Resulting in Tibial Nerve Compression Neuropathy Secondary to Polyethylene Wear Disease
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
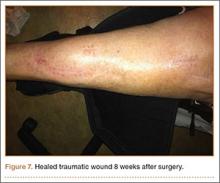
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
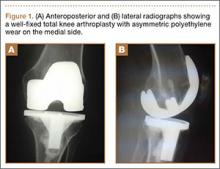
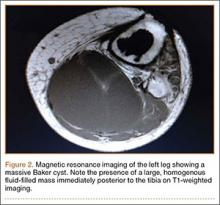
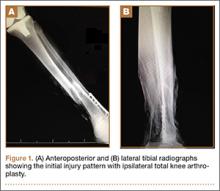
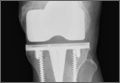
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
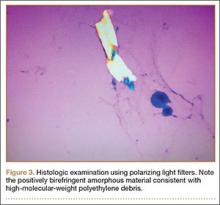
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
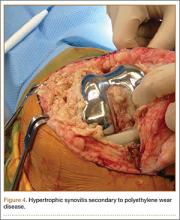
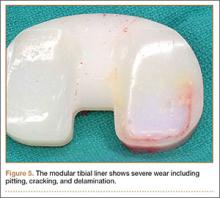
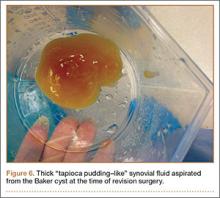
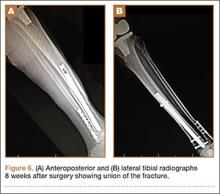
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
Revision Anterior Cruciate Ligament Reconstruction With Bone–Patellar Tendon–Bone Allograft and Extra-Articular Iliotibial Band Tenodesis
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
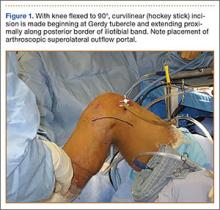
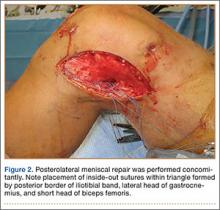
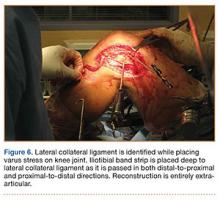
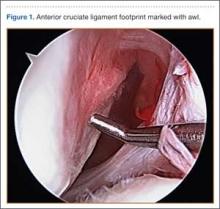
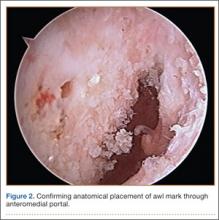
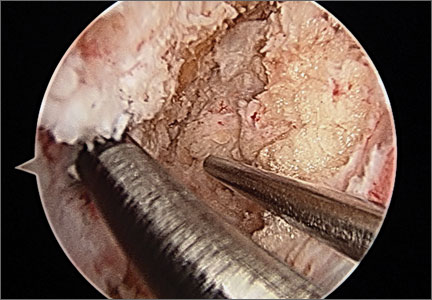
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
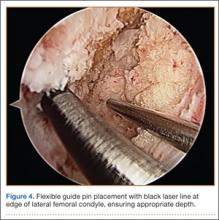
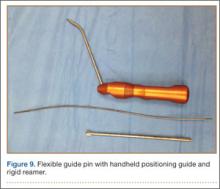
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
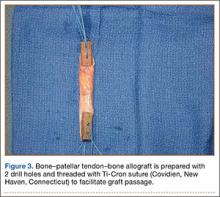
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
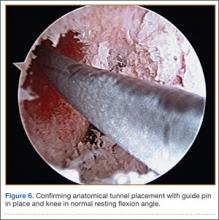
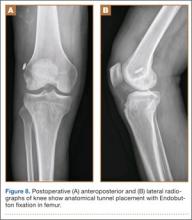
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
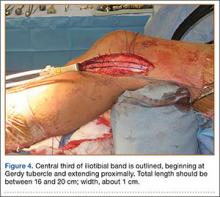
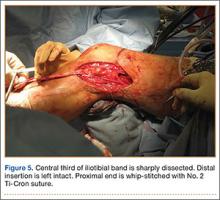
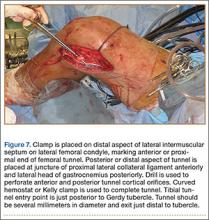
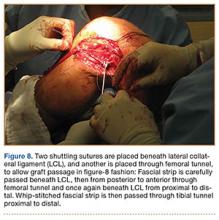
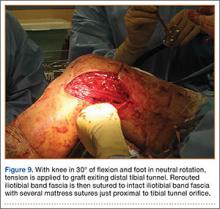
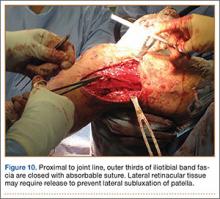
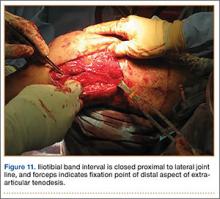
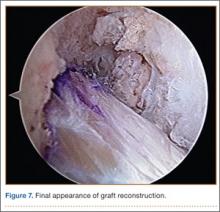
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
Women Fare Better Than Men Following Total Knee, Hip Replacement
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
Black, Hispanic Patients More Likely to Be Readmitted to the Hospital Within 30 Days Following Hip or Knee Replacement Surgery
LAS VEGAS—Black and Hispanic patients were 62% and 50%, respectively, more likely to be readmitted to the hospital within 30 days after total joint replacement (TJR) surgery compared to white patients, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). In addition, Medicaid patients were 40% more likely to be readmitted to the hospital than patients with private insurance. Poorer outcomes, due in part to patient comorbidities, may reflect limited access to primary care, insufficient patient-doctor communication, researchers suggest.
Disparities in the provision of health care services have long been documented, including that black patients utilize hip and total knee replacement at rates nearly 40% less than white patients, despite having comparable or higher rates of osteoarthritis.
In this study, researchers analyzed 5 years of data—demographic (including race/ethnicity), clinical, and billing—on nearly 53,000 patients admitted to Connecticut hospitals for TJR from 2008 to 2012. The average patient age was 67 years, and the vast majority of patients were white (87%), covered by Medicare (56.7%), and female (61%).
The overall 30-day readmission rate for patients was 5.2%. The most common reasons for readmission were postoperative infection (8%), infection and inflammatory reaction due to internal joint prosthesis (6%), hematoma complications during a procedure (3%), and dislocation of a prosthetic joint (3%). Among the other study findings:
• Readmission rates were 83.5 per thousand for black patients, 78.9 for Hispanic patients, and 53.3 for white patients.
• Longer length of hospital stay was significantly associated with increased odds of readmission.
• When controlling for comorbidities and type of insurance coverage, the readmission rate for Hispanic patients dropped 44%, and for black patients, 38%. Black patients remained significantly more likely than white patients to be readmitted following surgery, after controlling for comorbidities.
• Patients covered by Medicare were 30% more likely to be readmitted within 30 days following discharge compared to patients covered by private insurance, and Medicaid patients were 40% more likely.
Recent research using national data on Medicare suggests that community-based factors, such as availability of general practitioners in the area, may be as or more important than hospital factors in determining readmission rates, and that patients may have few options other than hospital care for both urgent and non-urgent conditions related to their surgery or other conditions.
“Using an all-payer database, our study shows that black patients who undergo total knee replacement may have poorer outcomes,” said lead study author and orthopedic surgeon Courtland Lewis, MD. “After controlling for two key variables implicated in race and ethnic disparities in hospital readmission—preoperative comorbidities and type of insurance coverage—black patients still have a 35% higher likelihood of all-cause, 30-day readmission compared to white patients.
“Our ongoing research in this area is focused on other factors, such as the patient’s connection to primary care and patient-provider communication, that may explain this troubling finding,” said Dr. Lewis.
LAS VEGAS—Black and Hispanic patients were 62% and 50%, respectively, more likely to be readmitted to the hospital within 30 days after total joint replacement (TJR) surgery compared to white patients, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). In addition, Medicaid patients were 40% more likely to be readmitted to the hospital than patients with private insurance. Poorer outcomes, due in part to patient comorbidities, may reflect limited access to primary care, insufficient patient-doctor communication, researchers suggest.
Disparities in the provision of health care services have long been documented, including that black patients utilize hip and total knee replacement at rates nearly 40% less than white patients, despite having comparable or higher rates of osteoarthritis.
In this study, researchers analyzed 5 years of data—demographic (including race/ethnicity), clinical, and billing—on nearly 53,000 patients admitted to Connecticut hospitals for TJR from 2008 to 2012. The average patient age was 67 years, and the vast majority of patients were white (87%), covered by Medicare (56.7%), and female (61%).
The overall 30-day readmission rate for patients was 5.2%. The most common reasons for readmission were postoperative infection (8%), infection and inflammatory reaction due to internal joint prosthesis (6%), hematoma complications during a procedure (3%), and dislocation of a prosthetic joint (3%). Among the other study findings:
• Readmission rates were 83.5 per thousand for black patients, 78.9 for Hispanic patients, and 53.3 for white patients.
• Longer length of hospital stay was significantly associated with increased odds of readmission.
• When controlling for comorbidities and type of insurance coverage, the readmission rate for Hispanic patients dropped 44%, and for black patients, 38%. Black patients remained significantly more likely than white patients to be readmitted following surgery, after controlling for comorbidities.
• Patients covered by Medicare were 30% more likely to be readmitted within 30 days following discharge compared to patients covered by private insurance, and Medicaid patients were 40% more likely.
Recent research using national data on Medicare suggests that community-based factors, such as availability of general practitioners in the area, may be as or more important than hospital factors in determining readmission rates, and that patients may have few options other than hospital care for both urgent and non-urgent conditions related to their surgery or other conditions.
“Using an all-payer database, our study shows that black patients who undergo total knee replacement may have poorer outcomes,” said lead study author and orthopedic surgeon Courtland Lewis, MD. “After controlling for two key variables implicated in race and ethnic disparities in hospital readmission—preoperative comorbidities and type of insurance coverage—black patients still have a 35% higher likelihood of all-cause, 30-day readmission compared to white patients.
“Our ongoing research in this area is focused on other factors, such as the patient’s connection to primary care and patient-provider communication, that may explain this troubling finding,” said Dr. Lewis.
LAS VEGAS—Black and Hispanic patients were 62% and 50%, respectively, more likely to be readmitted to the hospital within 30 days after total joint replacement (TJR) surgery compared to white patients, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). In addition, Medicaid patients were 40% more likely to be readmitted to the hospital than patients with private insurance. Poorer outcomes, due in part to patient comorbidities, may reflect limited access to primary care, insufficient patient-doctor communication, researchers suggest.
Disparities in the provision of health care services have long been documented, including that black patients utilize hip and total knee replacement at rates nearly 40% less than white patients, despite having comparable or higher rates of osteoarthritis.
In this study, researchers analyzed 5 years of data—demographic (including race/ethnicity), clinical, and billing—on nearly 53,000 patients admitted to Connecticut hospitals for TJR from 2008 to 2012. The average patient age was 67 years, and the vast majority of patients were white (87%), covered by Medicare (56.7%), and female (61%).
The overall 30-day readmission rate for patients was 5.2%. The most common reasons for readmission were postoperative infection (8%), infection and inflammatory reaction due to internal joint prosthesis (6%), hematoma complications during a procedure (3%), and dislocation of a prosthetic joint (3%). Among the other study findings:
• Readmission rates were 83.5 per thousand for black patients, 78.9 for Hispanic patients, and 53.3 for white patients.
• Longer length of hospital stay was significantly associated with increased odds of readmission.
• When controlling for comorbidities and type of insurance coverage, the readmission rate for Hispanic patients dropped 44%, and for black patients, 38%. Black patients remained significantly more likely than white patients to be readmitted following surgery, after controlling for comorbidities.
• Patients covered by Medicare were 30% more likely to be readmitted within 30 days following discharge compared to patients covered by private insurance, and Medicaid patients were 40% more likely.
Recent research using national data on Medicare suggests that community-based factors, such as availability of general practitioners in the area, may be as or more important than hospital factors in determining readmission rates, and that patients may have few options other than hospital care for both urgent and non-urgent conditions related to their surgery or other conditions.
“Using an all-payer database, our study shows that black patients who undergo total knee replacement may have poorer outcomes,” said lead study author and orthopedic surgeon Courtland Lewis, MD. “After controlling for two key variables implicated in race and ethnic disparities in hospital readmission—preoperative comorbidities and type of insurance coverage—black patients still have a 35% higher likelihood of all-cause, 30-day readmission compared to white patients.
“Our ongoing research in this area is focused on other factors, such as the patient’s connection to primary care and patient-provider communication, that may explain this troubling finding,” said Dr. Lewis.
Men Have a Higher Level of Function Before and After Total Knee Replacement Surgery
LAS VEGAS—While men and women have similar levels of improvement following total knee replacement (TKR) surgery, men have higher levels of function before and after TKR, according to new research presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
According to the Agency for Healthcare Research and Quality, more than 600,000 knee replacements are performed in the United States each year. In 2012, 393,345 women and 237,896 men underwent TKR, most often to alleviate the pain and immobility associated with late-stage arthritis. While research has looked at the anatomic differences between the knees of men and women, the higher levels of arthritis in women versus men, and the utilization of TKR among men and women, there has been little study on how gender influences the level of function before and after surgery.
In this study, researchers identified and studied 287 TKR patients at 7 different institutions between 2005 and 2007. All of the patients were between the ages of 21 and 80 years at the time of surgery and had a body mass index less than 40 g/m². All of the patients except 2 had a diagnosis of end-stage arthritis. The patient group included 108 men (112 knees) with a mean age of 67 years, and 170 women with a mean age of 66 years. All of the patients were evaluated preoperatively and at the following 6 points following surgery: 6 weeks, 3 months, 1 year, 2 years, 5 years, and 7 years. A Kaplan-Meier assessment gauged implant survival, and quality-of-life measurements were taken at 3 and 4 years post-surgery. During each evaluation, researchers measured knee function, range of motion, extremity activity, and overall health.
At 5 years post-surgery, implant survival was 100% for men and 99.1% for women. Range of motion also was nearly identical between genders. Functional scores were consistently higher for the men versus women: preoperatively, 57.1 versus 51; postoperatively at 6 weeks, 63.7 versus 51.5; at 3 months, 83.1 versus 74.3; at 2 years, 90 versus 81.6; at 5 years, 90.1 versus 82.9; and at 7 years, 96 versus 79.5. Men also recovered faster within the 6-week recovery time after surgery; however, both genders had almost identical improvement in mean knee score function (improvement from presurgical levels) at 5 years.
“Our data supports that while both genders benefit from TKR, men have higher levels of function and activity both prior to and after TKR compared to women,” said investigators Jeffrey J. Cherian, DO, and Michael A. Mont, MD. “These functional outcome differences are most likely due to many factors, including biologic/genetic, and highlight the need for further research related to the role of gender of both the patient and the surgeon in the decision making process of TKR, sex-based biological differences in functional recovery capacity, and whether sex/gender based pre- and postoperative rehabilitation protocols are warranted.”
LAS VEGAS—While men and women have similar levels of improvement following total knee replacement (TKR) surgery, men have higher levels of function before and after TKR, according to new research presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
According to the Agency for Healthcare Research and Quality, more than 600,000 knee replacements are performed in the United States each year. In 2012, 393,345 women and 237,896 men underwent TKR, most often to alleviate the pain and immobility associated with late-stage arthritis. While research has looked at the anatomic differences between the knees of men and women, the higher levels of arthritis in women versus men, and the utilization of TKR among men and women, there has been little study on how gender influences the level of function before and after surgery.
In this study, researchers identified and studied 287 TKR patients at 7 different institutions between 2005 and 2007. All of the patients were between the ages of 21 and 80 years at the time of surgery and had a body mass index less than 40 g/m². All of the patients except 2 had a diagnosis of end-stage arthritis. The patient group included 108 men (112 knees) with a mean age of 67 years, and 170 women with a mean age of 66 years. All of the patients were evaluated preoperatively and at the following 6 points following surgery: 6 weeks, 3 months, 1 year, 2 years, 5 years, and 7 years. A Kaplan-Meier assessment gauged implant survival, and quality-of-life measurements were taken at 3 and 4 years post-surgery. During each evaluation, researchers measured knee function, range of motion, extremity activity, and overall health.
At 5 years post-surgery, implant survival was 100% for men and 99.1% for women. Range of motion also was nearly identical between genders. Functional scores were consistently higher for the men versus women: preoperatively, 57.1 versus 51; postoperatively at 6 weeks, 63.7 versus 51.5; at 3 months, 83.1 versus 74.3; at 2 years, 90 versus 81.6; at 5 years, 90.1 versus 82.9; and at 7 years, 96 versus 79.5. Men also recovered faster within the 6-week recovery time after surgery; however, both genders had almost identical improvement in mean knee score function (improvement from presurgical levels) at 5 years.
“Our data supports that while both genders benefit from TKR, men have higher levels of function and activity both prior to and after TKR compared to women,” said investigators Jeffrey J. Cherian, DO, and Michael A. Mont, MD. “These functional outcome differences are most likely due to many factors, including biologic/genetic, and highlight the need for further research related to the role of gender of both the patient and the surgeon in the decision making process of TKR, sex-based biological differences in functional recovery capacity, and whether sex/gender based pre- and postoperative rehabilitation protocols are warranted.”
LAS VEGAS—While men and women have similar levels of improvement following total knee replacement (TKR) surgery, men have higher levels of function before and after TKR, according to new research presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
According to the Agency for Healthcare Research and Quality, more than 600,000 knee replacements are performed in the United States each year. In 2012, 393,345 women and 237,896 men underwent TKR, most often to alleviate the pain and immobility associated with late-stage arthritis. While research has looked at the anatomic differences between the knees of men and women, the higher levels of arthritis in women versus men, and the utilization of TKR among men and women, there has been little study on how gender influences the level of function before and after surgery.
In this study, researchers identified and studied 287 TKR patients at 7 different institutions between 2005 and 2007. All of the patients were between the ages of 21 and 80 years at the time of surgery and had a body mass index less than 40 g/m². All of the patients except 2 had a diagnosis of end-stage arthritis. The patient group included 108 men (112 knees) with a mean age of 67 years, and 170 women with a mean age of 66 years. All of the patients were evaluated preoperatively and at the following 6 points following surgery: 6 weeks, 3 months, 1 year, 2 years, 5 years, and 7 years. A Kaplan-Meier assessment gauged implant survival, and quality-of-life measurements were taken at 3 and 4 years post-surgery. During each evaluation, researchers measured knee function, range of motion, extremity activity, and overall health.
At 5 years post-surgery, implant survival was 100% for men and 99.1% for women. Range of motion also was nearly identical between genders. Functional scores were consistently higher for the men versus women: preoperatively, 57.1 versus 51; postoperatively at 6 weeks, 63.7 versus 51.5; at 3 months, 83.1 versus 74.3; at 2 years, 90 versus 81.6; at 5 years, 90.1 versus 82.9; and at 7 years, 96 versus 79.5. Men also recovered faster within the 6-week recovery time after surgery; however, both genders had almost identical improvement in mean knee score function (improvement from presurgical levels) at 5 years.
“Our data supports that while both genders benefit from TKR, men have higher levels of function and activity both prior to and after TKR compared to women,” said investigators Jeffrey J. Cherian, DO, and Michael A. Mont, MD. “These functional outcome differences are most likely due to many factors, including biologic/genetic, and highlight the need for further research related to the role of gender of both the patient and the surgeon in the decision making process of TKR, sex-based biological differences in functional recovery capacity, and whether sex/gender based pre- and postoperative rehabilitation protocols are warranted.”
Arthroscopic Anterior Cruciate Ligament Reconstruction Using a Flexible Guide Pin With a Rigid Reamer
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
Intragrade Intramedullary Nailing of an Open Tibial Shaft Fracture in a Patient With Concomitant Ipsilateral Total Knee Arthroplasty
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
Mycobacterium bovis Infection of Total Knee Arthroplasty After Bacillus Calmette-Guérin Therapy for Bladder Cancer
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
Dilute Betadine Lavage Reduces Implant-Related Bacterial Burden in a Rabbit Knee Prosthetic Infection Model
Surgical site infection after arthroplasty causes substantial morbidity and potential mortality. Prosthetic joint infection (PJI) ranges from simple superficial wound infection and cellulitis to deep subfascial infection that involves the prosthesis. Consistent use of prophylactic antibiotics has reduced postoperative hip and knee arthroplasty infections to rates of 0.25% to 2%.1-4 Treatment of a patient with PJI commonly includes hospitalization, long-term intravenously administered antibiotics, resection arthroplasty, and staged reimplantation. The estimated cost of interventions reaches tens of millions of dollars annually in the United States and does not include the costs of psychosocial effects on patients and their families.5,6
Betadine (povidone-iodine) is a widely used antiseptic for skin and mucous membrane wounds and has been shown to be effective for the prevention of PJI.7 Dilute Betadine solution has been proposed as an aid in treatment of PJI.8 At a minimum concentration of 5%, cytotoxicity has been observed in chicken tibia osteoblasts.9 A balance of the bactericidal and cytotoxic activities of Betadine, while maintaining its efficacy against resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA), is optimized at dilutions between 0.5% and 4%.10-14 We hypothesized that a dilute Betadine lavage of 3.5% would achieve a significant decrease in bacterial counts compared with an isolated saline lavage in an in vivo knee PJI model.
Materials and Methods
Animal Protocol
All surgical procedures were conducted according to the protocol approved by our institutional animal care and use committee. Using a power analysis and data obtained at our institution, we determined that 12 was the minimum number of animals needed to reach significance set at P < .05 and assuming a 50% decrease in colony-forming units (CFU) (SigmaStat Version 2.03; Aspire Software International, Ashburn, Virginia). Eight New Zealand White rabbits were used in our study; because significance was reached early, 12 were not needed. The average weight of the rabbits was 3.5 kg (weight range, 3.2-4.1 kg). All rabbits completed 1 week of acclimation before surgery.
Bacteria Preparation
A broth culture of methicillin-sensitive S aureus (MSSA) (ATCC 25923) was prepared 1 day before surgery. The bacteria were suspended in 5 mL of Trypticase Soy Broth (Becton Dickinson & Co, Franklin Lakes, New Jersey) and incubated at 37°C in a shaking incubator for 16 hours. The next day, the culture was centrifuged and irrigated twice with normal saline to remove the broth and prevent further growth. The bacteria were reconstituted in normal saline, and the concentration was standardized using a turbidity meter (LaMotte 2020e; LaMotte Co, Chestertown, Maryland), which correlated with 106 CFU/100 µl plated on trypticase soy agar plates with 10% sheep blood (Fisher Scientific, Pittsburgh, Pennsylvania).
Surgical and Postoperative Procedures
Our procedure was based on the New Zealand White rabbit knee PJI model.15 General anesthesia was induced with ketamine and xylazine, and maintained with isoflurane inhalation via a nose cone mask. Rabbits were positioned supine, and bilateral knees were shaved, prepped, and draped in a sterile fashion.
A 2-cm longitudinal incision was made over the lateral knee, and arthrotomy was performed, exposing the lateral collateral ligament attachment at the lateral femoral condyle. Using a 4-mm drill bit, a defect was drilled obliquely into the lateral femoral condyle, anterior to the lateral collateral ligament attachment. This produced a defect in the non-weight-bearing, nonarticulating portion of the knee. A fully threaded 4×14-mm stainless steel screw (Synthes, West Chester, Pennsylvania) with a U-shaped ultrahigh-molecular-weight polyethylene washer (Synthes) was inserted into the defect. The joint capsule was closed with a running 3-0 Vicryl suture (Ethicon, Somerville, New Jersey). The knee joint was inoculated with 100 µL of the S aureus preparation using a 22-gauge needle. The skin was closed with a 4-0 Biosyn suture (Ethicon). The procedure was repeated on the contralateral knee (Figures 1A, 1B).
Seven days after the initial surgery, the rabbits were returned to the operating room and were anesthetized, positioned, and prepped for surgery as detailed above. Ceftriaxone (20 mg/kg of body weight) was intravenously administered to all rabbits for the treatment procedure. For each rabbit, a control knee and an experimental knee were randomly assigned. A longitudinal incision was made, exposing the previously placed implants. The screw was loosened slightly to remove the U-shaped polyethylene washer. Each knee then underwent lavage 2 times, for 90 seconds each time, with 3.5% dilute Betadine solution (experimental knee) or with normal saline (control knee). Because Pseudomonas contamination has been reported with povidone-iodine taken from unsterilized bottles,16,17 packets of sterilized povidone-iodine (Aplicare; Clorox, Oakland, California) were used. After the irrigation was complete, a new sterile polyethylene washer was placed and the screw was tightened. The wound closure was repeated as detailed above.
Postoperative analgesia was provided based on a standard institutional animal care and use committee protocol. Rabbits were permitted full cage activity and nutrition ad libitum. Wound healing, body weight, and signs of distress were monitored daily.
Outcome Measures
Seven days after surgery, the rabbits were euthanized with administration of phenobarbital (100 mg/kg of body weight). Arterial blood samples were obtained from the auricular vein to ensure that the rabbits were not systemically infected. Using a sterile technique, the screw, polyethylene washer, lateral femoral condyle bone from the defect, and joint capsule were cultured. Harvested bone and soft tissues were weighed and immediately homogenized (PowerGen Model 35 Handheld Homogenizer; Thermo Fisher Scientific, Inc, Waltham, Massachusetts). Implants were sonicated (UBATH-Y; World Precision Instruments, Inc, Sarasota, Florida) in cold saline to obtain a sensitive culture.18
Bacterial quantification was determined by using trypticase soy agar plates after 24 hours of growth. Final CFU were calculated after serial dilutions and were standardized per gram of biopsied tissues.19 Members of the team were blinded to the treatment type.
Statistical Analysis
Statistical differences in mean bacterial burden were calculated independently for lateral condyle bone, joint capsule, polyethylene, and screws by conducting a Student t test.
Results
Treatment effect was higher than expected, and the study was terminated after 8 animals completed the protocol. All 8 rabbits tolerated the procedures well and were appropriately monitored during the postoperative period. No animals had signs of systemic infection or positive blood culture. All local cultures for screw, polyethylene washer, lateral femoral condyle defect, and joint capsule were positive.
Statistically significant decreases were shown in the bacterial burden of the Betadine-irrigated screws and the Betadine-irrigated polyethylene washers compared with the saline-irrigated controls. Betadine-irrigated screws grew an average of 7.16 × 101 CFU of S aureus/g, whereas screws from control knees grew an average of 1.45 × 103 CFU/g (P = .0003) (Figure 2). Betadine-treated washers grew an average of 1.28 × 103 CFU/g compared with 1.62 × 104 CFU/g for control washers (P =. 04) (Figure 3).
A trend toward decreased bacterial counts was shown in Betadine-treated soft tissues compared with saline-treated soft tissues, but the difference did not reach statistical significance (P = .9). Biopsied joint capsule from knees treated with Betadine grew an average of 2.84 × 104 CFU/g compared with an average of 3.16 × 104 CFU/g in control-rabbit knees (Figure 4). Cultured lateral condyle from Betadine-treated knees had an average bacterial load of 3.22 × 104 CFU/g compared with an average bacterial load of 1 × 105 CFU/g in control knees (Figure 4).
Discussion
Knees irrigated with Betadine showed a significant (P = .0003) decrease in metal implant–related S aureus bacterial counts by 20-fold and a significant (P < .05) decrease in polyethylene implant–related counts by more than 10-fold. This arthroplasty model used Betadine lavage as a treatment adjunct with intravenously administered antibiotics and polyethylene exchange. Our 1-week interval after the index procedure classifies the infection as an acute postoperative arthroplasty infection (occurring less than 4 weeks postoperatively).
The gold standard treatment for these infections is irrigation and débridement with component retention.18 The success rate has been reported to be as high as 71%20 but was closer to 44% in a study by Fridkin and colleagues,21 especially with more virulent bacteria. Staphylococcal species, higher American Society of Anesthesiologists scores, and frank pus around the prosthesis were markers of débridement failure in a recent study by Azzam and colleagues.18
The majority of postoperative joint arthroplasty infections are caused by S aureus, and the incidence of MRSA bacteria continues to rise.22 Community-acquired MRSA is increasing at an alarming rate and is now the predominant organism in skin and soft-tissue infections.23 Organism resistance also occurs at a cellular level by the formation of a glycocalyx layer, or biofilm. This layer assists in changing the phenotypic properties of the organism and decreases the efficacy of antibiotics.24 The self-produced layer of extracellular matrices, deoxyribonucleic acid, and polysaccharides attaches to inert material, preventing phagocytic action by neutrophils. In addition to antibacterial activity, povidone-iodine has antibiofilm activity against Staphylococcal species.25 The active ingredient targets the gene that produces biofilm. This correlates to our study in which the largest decrease in bacterial counts was noted on the implants.
The use of Betadine lavage has shown some promise in vivo as well. A prospective randomized controlled trial26 used 3.5% Betadine irrigation to prevent spine infection. No infections occurred in the Betadine group compared with a deep-infection rate of 2.9% in the control group. Brown and colleagues8 reviewed 1862 hip and knee arthroplasty cases before the use of Betadine lavage and 688 cases after the use of Betadine lavage and found a decrease in infection rate, from 0.97% to 0.15%. S aureus caused 13 of the 18 infections in the control group. These studies8,26 used Betadine lavage for prophylaxis and prevention of deep spine and arthroplasty infection. Betadine lavage as a treatment adjunct for acute arthroplasty infection has not been studied clinically. It has the potential to increase isolated incision and débridement success and to improve component survivorship.
Our arthroplasty model mimics an intra-articular environment and accounts for an implant–polyethylene interface.15 Limitations of our study include the use of MSSA as opposed to MRSA. However, povidone-iodine has the same effects on both MSSA and MRSA.12 We also treated our postoperative infection with 1 dose of antibiotics and not a course, although it should be noted that the single dose of ceftriaxone allowed us to isolate the independent effect of the Betadine lavage. A baseline level of infection severity could have been measured with cultures obtained at the time of irrigation and débridement. Also, a decrease in CFU does not directly correlate to a clinically significant outcome, such as a defined surgical site infection requiring intervention. Nevertheless, it is noteworthy that the decrease in bacterial counts on the stainless steel screws and polyethylene washers were maintained 1 week after the Betadine lavage.
Conclusion
Dilute Betadine lavage is a simple and inexpensive adjunct for the treatment of acute postoperative arthroplasty infection and may increase the rate of component retention. Additionally, the bactericidal and antibiofilm activities of Betadine may improve the effectiveness of systemic antibiotics. Further clinical investigation is warranted.
1. Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72(6):878-883.
2. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
3. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
4. Mahomed NN, Barrett J, Katz JN, Baron JA, Wright J, Losina E. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87(6):1222-1228.
5. Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: treatment options. Orthopedics. 2010;33(9):659.
6. Poultsides LA, Liaropoulos LL, Malizos KN. The socioeconomic impact of musculoskeletal infections. J Bone Joint Surg Am. 2010;92(11):e13.
7. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
8. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
9. Kaysinger KK, Nicholson NC, Ramp WK, Kellam JF. Toxic effects of wound irrigation solutions on cultured tibiae and osteoblasts. J Orthop Trauma. 1995;9(4):303-311.
10. Haley CE, Marling-Cason M, Smith JW, Luby JP, Mackowiak PA. Bactericidal activity of antiseptics against methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;21(6):991-992.
11. Lacey RW, Catto A. Action of povidone-iodine against methicillin-sensitive and -resistant cultures of Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S78-S83.
12. McLure AR, Gordon J. In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1992;21(4):291-299.
13. Suzuki J, Komatsuzawa H, Kozai K, Nagasaka N. In vitro susceptibility of Staphylococcus aureus including MRSA to four disinfectants. ASDC J Dent Child. 1997;64(4):260-263.
14. Yasuda T, Yoshimura S, Katsuno Y, et al. Comparison of bactericidal activities of various disinfectants against methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S66-S69.
15. Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, Brown TE. A novel total knee arthroplasty infection model in rabbits. J Orthop Res. 2005;23(5):1100-1104.
16. Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and debridement. Clin Orthop. 1991;273:113-118.
17. Mont MA, Waldman B, Banerjee C, Pacheco IH, Hungerford DS. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12(4):426-433.
18. Azzam KA, Seeley M, Ghanem E, Austin MS, Purtill JJ, Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25(7):1022-1027.
19. Stall AC, Becker E, Ludwig SC, Gelb D, Poelstra KA. Reduction of postoperative spinal implant infection using gentamicin microspheres. Spine (Phila Pa 1976). 2009;34(5):479-483.
20. Hota B, Ellenbogen C, Hayden MK, Aroutcheva A, Rice TW, Weinstein RA. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch Intern Med. 2007;167(10):1026-1033.
21. Fridkin SK, Hageman JC, Morrison M, et al, Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436-1444.
22. Hosman AH, van der Mei HC, Bulstra SK, Busscher HJ, Neut D. Metal-on-metal bearings in total hip arthroplasties: influence of cobalt and chromium ions on bacterial growth and biofilm formation. J Biomed Mater Res A. 2009;88(3):711-716.
23. Oduwole KO, Glynn AA, Molony DC, et al. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J Orthop Res. 2010;28(9):1252-1256.
24. Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976). 2005;30(15):1689-1693.
25. Anderson RL, Vess RW, Panlilio AL, Favero MS. Prolonged survival of Pseudomonas cepacia in commercially manufactured povidone-iodine. Appl Environ Microbiol. 1990;56(11):3598-3600.
26. Panlilio AL, Beck-Sague CM, Siegel JD, et al. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin Infect Dis. 1992;14(5):1078-1083.
Surgical site infection after arthroplasty causes substantial morbidity and potential mortality. Prosthetic joint infection (PJI) ranges from simple superficial wound infection and cellulitis to deep subfascial infection that involves the prosthesis. Consistent use of prophylactic antibiotics has reduced postoperative hip and knee arthroplasty infections to rates of 0.25% to 2%.1-4 Treatment of a patient with PJI commonly includes hospitalization, long-term intravenously administered antibiotics, resection arthroplasty, and staged reimplantation. The estimated cost of interventions reaches tens of millions of dollars annually in the United States and does not include the costs of psychosocial effects on patients and their families.5,6
Betadine (povidone-iodine) is a widely used antiseptic for skin and mucous membrane wounds and has been shown to be effective for the prevention of PJI.7 Dilute Betadine solution has been proposed as an aid in treatment of PJI.8 At a minimum concentration of 5%, cytotoxicity has been observed in chicken tibia osteoblasts.9 A balance of the bactericidal and cytotoxic activities of Betadine, while maintaining its efficacy against resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA), is optimized at dilutions between 0.5% and 4%.10-14 We hypothesized that a dilute Betadine lavage of 3.5% would achieve a significant decrease in bacterial counts compared with an isolated saline lavage in an in vivo knee PJI model.
Materials and Methods
Animal Protocol
All surgical procedures were conducted according to the protocol approved by our institutional animal care and use committee. Using a power analysis and data obtained at our institution, we determined that 12 was the minimum number of animals needed to reach significance set at P < .05 and assuming a 50% decrease in colony-forming units (CFU) (SigmaStat Version 2.03; Aspire Software International, Ashburn, Virginia). Eight New Zealand White rabbits were used in our study; because significance was reached early, 12 were not needed. The average weight of the rabbits was 3.5 kg (weight range, 3.2-4.1 kg). All rabbits completed 1 week of acclimation before surgery.
Bacteria Preparation
A broth culture of methicillin-sensitive S aureus (MSSA) (ATCC 25923) was prepared 1 day before surgery. The bacteria were suspended in 5 mL of Trypticase Soy Broth (Becton Dickinson & Co, Franklin Lakes, New Jersey) and incubated at 37°C in a shaking incubator for 16 hours. The next day, the culture was centrifuged and irrigated twice with normal saline to remove the broth and prevent further growth. The bacteria were reconstituted in normal saline, and the concentration was standardized using a turbidity meter (LaMotte 2020e; LaMotte Co, Chestertown, Maryland), which correlated with 106 CFU/100 µl plated on trypticase soy agar plates with 10% sheep blood (Fisher Scientific, Pittsburgh, Pennsylvania).
Surgical and Postoperative Procedures
Our procedure was based on the New Zealand White rabbit knee PJI model.15 General anesthesia was induced with ketamine and xylazine, and maintained with isoflurane inhalation via a nose cone mask. Rabbits were positioned supine, and bilateral knees were shaved, prepped, and draped in a sterile fashion.
A 2-cm longitudinal incision was made over the lateral knee, and arthrotomy was performed, exposing the lateral collateral ligament attachment at the lateral femoral condyle. Using a 4-mm drill bit, a defect was drilled obliquely into the lateral femoral condyle, anterior to the lateral collateral ligament attachment. This produced a defect in the non-weight-bearing, nonarticulating portion of the knee. A fully threaded 4×14-mm stainless steel screw (Synthes, West Chester, Pennsylvania) with a U-shaped ultrahigh-molecular-weight polyethylene washer (Synthes) was inserted into the defect. The joint capsule was closed with a running 3-0 Vicryl suture (Ethicon, Somerville, New Jersey). The knee joint was inoculated with 100 µL of the S aureus preparation using a 22-gauge needle. The skin was closed with a 4-0 Biosyn suture (Ethicon). The procedure was repeated on the contralateral knee (Figures 1A, 1B).
Seven days after the initial surgery, the rabbits were returned to the operating room and were anesthetized, positioned, and prepped for surgery as detailed above. Ceftriaxone (20 mg/kg of body weight) was intravenously administered to all rabbits for the treatment procedure. For each rabbit, a control knee and an experimental knee were randomly assigned. A longitudinal incision was made, exposing the previously placed implants. The screw was loosened slightly to remove the U-shaped polyethylene washer. Each knee then underwent lavage 2 times, for 90 seconds each time, with 3.5% dilute Betadine solution (experimental knee) or with normal saline (control knee). Because Pseudomonas contamination has been reported with povidone-iodine taken from unsterilized bottles,16,17 packets of sterilized povidone-iodine (Aplicare; Clorox, Oakland, California) were used. After the irrigation was complete, a new sterile polyethylene washer was placed and the screw was tightened. The wound closure was repeated as detailed above.
Postoperative analgesia was provided based on a standard institutional animal care and use committee protocol. Rabbits were permitted full cage activity and nutrition ad libitum. Wound healing, body weight, and signs of distress were monitored daily.
Outcome Measures
Seven days after surgery, the rabbits were euthanized with administration of phenobarbital (100 mg/kg of body weight). Arterial blood samples were obtained from the auricular vein to ensure that the rabbits were not systemically infected. Using a sterile technique, the screw, polyethylene washer, lateral femoral condyle bone from the defect, and joint capsule were cultured. Harvested bone and soft tissues were weighed and immediately homogenized (PowerGen Model 35 Handheld Homogenizer; Thermo Fisher Scientific, Inc, Waltham, Massachusetts). Implants were sonicated (UBATH-Y; World Precision Instruments, Inc, Sarasota, Florida) in cold saline to obtain a sensitive culture.18
Bacterial quantification was determined by using trypticase soy agar plates after 24 hours of growth. Final CFU were calculated after serial dilutions and were standardized per gram of biopsied tissues.19 Members of the team were blinded to the treatment type.
Statistical Analysis
Statistical differences in mean bacterial burden were calculated independently for lateral condyle bone, joint capsule, polyethylene, and screws by conducting a Student t test.
Results
Treatment effect was higher than expected, and the study was terminated after 8 animals completed the protocol. All 8 rabbits tolerated the procedures well and were appropriately monitored during the postoperative period. No animals had signs of systemic infection or positive blood culture. All local cultures for screw, polyethylene washer, lateral femoral condyle defect, and joint capsule were positive.
Statistically significant decreases were shown in the bacterial burden of the Betadine-irrigated screws and the Betadine-irrigated polyethylene washers compared with the saline-irrigated controls. Betadine-irrigated screws grew an average of 7.16 × 101 CFU of S aureus/g, whereas screws from control knees grew an average of 1.45 × 103 CFU/g (P = .0003) (Figure 2). Betadine-treated washers grew an average of 1.28 × 103 CFU/g compared with 1.62 × 104 CFU/g for control washers (P =. 04) (Figure 3).
A trend toward decreased bacterial counts was shown in Betadine-treated soft tissues compared with saline-treated soft tissues, but the difference did not reach statistical significance (P = .9). Biopsied joint capsule from knees treated with Betadine grew an average of 2.84 × 104 CFU/g compared with an average of 3.16 × 104 CFU/g in control-rabbit knees (Figure 4). Cultured lateral condyle from Betadine-treated knees had an average bacterial load of 3.22 × 104 CFU/g compared with an average bacterial load of 1 × 105 CFU/g in control knees (Figure 4).
Discussion
Knees irrigated with Betadine showed a significant (P = .0003) decrease in metal implant–related S aureus bacterial counts by 20-fold and a significant (P < .05) decrease in polyethylene implant–related counts by more than 10-fold. This arthroplasty model used Betadine lavage as a treatment adjunct with intravenously administered antibiotics and polyethylene exchange. Our 1-week interval after the index procedure classifies the infection as an acute postoperative arthroplasty infection (occurring less than 4 weeks postoperatively).
The gold standard treatment for these infections is irrigation and débridement with component retention.18 The success rate has been reported to be as high as 71%20 but was closer to 44% in a study by Fridkin and colleagues,21 especially with more virulent bacteria. Staphylococcal species, higher American Society of Anesthesiologists scores, and frank pus around the prosthesis were markers of débridement failure in a recent study by Azzam and colleagues.18
The majority of postoperative joint arthroplasty infections are caused by S aureus, and the incidence of MRSA bacteria continues to rise.22 Community-acquired MRSA is increasing at an alarming rate and is now the predominant organism in skin and soft-tissue infections.23 Organism resistance also occurs at a cellular level by the formation of a glycocalyx layer, or biofilm. This layer assists in changing the phenotypic properties of the organism and decreases the efficacy of antibiotics.24 The self-produced layer of extracellular matrices, deoxyribonucleic acid, and polysaccharides attaches to inert material, preventing phagocytic action by neutrophils. In addition to antibacterial activity, povidone-iodine has antibiofilm activity against Staphylococcal species.25 The active ingredient targets the gene that produces biofilm. This correlates to our study in which the largest decrease in bacterial counts was noted on the implants.
The use of Betadine lavage has shown some promise in vivo as well. A prospective randomized controlled trial26 used 3.5% Betadine irrigation to prevent spine infection. No infections occurred in the Betadine group compared with a deep-infection rate of 2.9% in the control group. Brown and colleagues8 reviewed 1862 hip and knee arthroplasty cases before the use of Betadine lavage and 688 cases after the use of Betadine lavage and found a decrease in infection rate, from 0.97% to 0.15%. S aureus caused 13 of the 18 infections in the control group. These studies8,26 used Betadine lavage for prophylaxis and prevention of deep spine and arthroplasty infection. Betadine lavage as a treatment adjunct for acute arthroplasty infection has not been studied clinically. It has the potential to increase isolated incision and débridement success and to improve component survivorship.
Our arthroplasty model mimics an intra-articular environment and accounts for an implant–polyethylene interface.15 Limitations of our study include the use of MSSA as opposed to MRSA. However, povidone-iodine has the same effects on both MSSA and MRSA.12 We also treated our postoperative infection with 1 dose of antibiotics and not a course, although it should be noted that the single dose of ceftriaxone allowed us to isolate the independent effect of the Betadine lavage. A baseline level of infection severity could have been measured with cultures obtained at the time of irrigation and débridement. Also, a decrease in CFU does not directly correlate to a clinically significant outcome, such as a defined surgical site infection requiring intervention. Nevertheless, it is noteworthy that the decrease in bacterial counts on the stainless steel screws and polyethylene washers were maintained 1 week after the Betadine lavage.
Conclusion
Dilute Betadine lavage is a simple and inexpensive adjunct for the treatment of acute postoperative arthroplasty infection and may increase the rate of component retention. Additionally, the bactericidal and antibiofilm activities of Betadine may improve the effectiveness of systemic antibiotics. Further clinical investigation is warranted.
Surgical site infection after arthroplasty causes substantial morbidity and potential mortality. Prosthetic joint infection (PJI) ranges from simple superficial wound infection and cellulitis to deep subfascial infection that involves the prosthesis. Consistent use of prophylactic antibiotics has reduced postoperative hip and knee arthroplasty infections to rates of 0.25% to 2%.1-4 Treatment of a patient with PJI commonly includes hospitalization, long-term intravenously administered antibiotics, resection arthroplasty, and staged reimplantation. The estimated cost of interventions reaches tens of millions of dollars annually in the United States and does not include the costs of psychosocial effects on patients and their families.5,6
Betadine (povidone-iodine) is a widely used antiseptic for skin and mucous membrane wounds and has been shown to be effective for the prevention of PJI.7 Dilute Betadine solution has been proposed as an aid in treatment of PJI.8 At a minimum concentration of 5%, cytotoxicity has been observed in chicken tibia osteoblasts.9 A balance of the bactericidal and cytotoxic activities of Betadine, while maintaining its efficacy against resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA), is optimized at dilutions between 0.5% and 4%.10-14 We hypothesized that a dilute Betadine lavage of 3.5% would achieve a significant decrease in bacterial counts compared with an isolated saline lavage in an in vivo knee PJI model.
Materials and Methods
Animal Protocol
All surgical procedures were conducted according to the protocol approved by our institutional animal care and use committee. Using a power analysis and data obtained at our institution, we determined that 12 was the minimum number of animals needed to reach significance set at P < .05 and assuming a 50% decrease in colony-forming units (CFU) (SigmaStat Version 2.03; Aspire Software International, Ashburn, Virginia). Eight New Zealand White rabbits were used in our study; because significance was reached early, 12 were not needed. The average weight of the rabbits was 3.5 kg (weight range, 3.2-4.1 kg). All rabbits completed 1 week of acclimation before surgery.
Bacteria Preparation
A broth culture of methicillin-sensitive S aureus (MSSA) (ATCC 25923) was prepared 1 day before surgery. The bacteria were suspended in 5 mL of Trypticase Soy Broth (Becton Dickinson & Co, Franklin Lakes, New Jersey) and incubated at 37°C in a shaking incubator for 16 hours. The next day, the culture was centrifuged and irrigated twice with normal saline to remove the broth and prevent further growth. The bacteria were reconstituted in normal saline, and the concentration was standardized using a turbidity meter (LaMotte 2020e; LaMotte Co, Chestertown, Maryland), which correlated with 106 CFU/100 µl plated on trypticase soy agar plates with 10% sheep blood (Fisher Scientific, Pittsburgh, Pennsylvania).
Surgical and Postoperative Procedures
Our procedure was based on the New Zealand White rabbit knee PJI model.15 General anesthesia was induced with ketamine and xylazine, and maintained with isoflurane inhalation via a nose cone mask. Rabbits were positioned supine, and bilateral knees were shaved, prepped, and draped in a sterile fashion.
A 2-cm longitudinal incision was made over the lateral knee, and arthrotomy was performed, exposing the lateral collateral ligament attachment at the lateral femoral condyle. Using a 4-mm drill bit, a defect was drilled obliquely into the lateral femoral condyle, anterior to the lateral collateral ligament attachment. This produced a defect in the non-weight-bearing, nonarticulating portion of the knee. A fully threaded 4×14-mm stainless steel screw (Synthes, West Chester, Pennsylvania) with a U-shaped ultrahigh-molecular-weight polyethylene washer (Synthes) was inserted into the defect. The joint capsule was closed with a running 3-0 Vicryl suture (Ethicon, Somerville, New Jersey). The knee joint was inoculated with 100 µL of the S aureus preparation using a 22-gauge needle. The skin was closed with a 4-0 Biosyn suture (Ethicon). The procedure was repeated on the contralateral knee (Figures 1A, 1B).
Seven days after the initial surgery, the rabbits were returned to the operating room and were anesthetized, positioned, and prepped for surgery as detailed above. Ceftriaxone (20 mg/kg of body weight) was intravenously administered to all rabbits for the treatment procedure. For each rabbit, a control knee and an experimental knee were randomly assigned. A longitudinal incision was made, exposing the previously placed implants. The screw was loosened slightly to remove the U-shaped polyethylene washer. Each knee then underwent lavage 2 times, for 90 seconds each time, with 3.5% dilute Betadine solution (experimental knee) or with normal saline (control knee). Because Pseudomonas contamination has been reported with povidone-iodine taken from unsterilized bottles,16,17 packets of sterilized povidone-iodine (Aplicare; Clorox, Oakland, California) were used. After the irrigation was complete, a new sterile polyethylene washer was placed and the screw was tightened. The wound closure was repeated as detailed above.
Postoperative analgesia was provided based on a standard institutional animal care and use committee protocol. Rabbits were permitted full cage activity and nutrition ad libitum. Wound healing, body weight, and signs of distress were monitored daily.
Outcome Measures
Seven days after surgery, the rabbits were euthanized with administration of phenobarbital (100 mg/kg of body weight). Arterial blood samples were obtained from the auricular vein to ensure that the rabbits were not systemically infected. Using a sterile technique, the screw, polyethylene washer, lateral femoral condyle bone from the defect, and joint capsule were cultured. Harvested bone and soft tissues were weighed and immediately homogenized (PowerGen Model 35 Handheld Homogenizer; Thermo Fisher Scientific, Inc, Waltham, Massachusetts). Implants were sonicated (UBATH-Y; World Precision Instruments, Inc, Sarasota, Florida) in cold saline to obtain a sensitive culture.18
Bacterial quantification was determined by using trypticase soy agar plates after 24 hours of growth. Final CFU were calculated after serial dilutions and were standardized per gram of biopsied tissues.19 Members of the team were blinded to the treatment type.
Statistical Analysis
Statistical differences in mean bacterial burden were calculated independently for lateral condyle bone, joint capsule, polyethylene, and screws by conducting a Student t test.
Results
Treatment effect was higher than expected, and the study was terminated after 8 animals completed the protocol. All 8 rabbits tolerated the procedures well and were appropriately monitored during the postoperative period. No animals had signs of systemic infection or positive blood culture. All local cultures for screw, polyethylene washer, lateral femoral condyle defect, and joint capsule were positive.
Statistically significant decreases were shown in the bacterial burden of the Betadine-irrigated screws and the Betadine-irrigated polyethylene washers compared with the saline-irrigated controls. Betadine-irrigated screws grew an average of 7.16 × 101 CFU of S aureus/g, whereas screws from control knees grew an average of 1.45 × 103 CFU/g (P = .0003) (Figure 2). Betadine-treated washers grew an average of 1.28 × 103 CFU/g compared with 1.62 × 104 CFU/g for control washers (P =. 04) (Figure 3).
A trend toward decreased bacterial counts was shown in Betadine-treated soft tissues compared with saline-treated soft tissues, but the difference did not reach statistical significance (P = .9). Biopsied joint capsule from knees treated with Betadine grew an average of 2.84 × 104 CFU/g compared with an average of 3.16 × 104 CFU/g in control-rabbit knees (Figure 4). Cultured lateral condyle from Betadine-treated knees had an average bacterial load of 3.22 × 104 CFU/g compared with an average bacterial load of 1 × 105 CFU/g in control knees (Figure 4).
Discussion
Knees irrigated with Betadine showed a significant (P = .0003) decrease in metal implant–related S aureus bacterial counts by 20-fold and a significant (P < .05) decrease in polyethylene implant–related counts by more than 10-fold. This arthroplasty model used Betadine lavage as a treatment adjunct with intravenously administered antibiotics and polyethylene exchange. Our 1-week interval after the index procedure classifies the infection as an acute postoperative arthroplasty infection (occurring less than 4 weeks postoperatively).
The gold standard treatment for these infections is irrigation and débridement with component retention.18 The success rate has been reported to be as high as 71%20 but was closer to 44% in a study by Fridkin and colleagues,21 especially with more virulent bacteria. Staphylococcal species, higher American Society of Anesthesiologists scores, and frank pus around the prosthesis were markers of débridement failure in a recent study by Azzam and colleagues.18
The majority of postoperative joint arthroplasty infections are caused by S aureus, and the incidence of MRSA bacteria continues to rise.22 Community-acquired MRSA is increasing at an alarming rate and is now the predominant organism in skin and soft-tissue infections.23 Organism resistance also occurs at a cellular level by the formation of a glycocalyx layer, or biofilm. This layer assists in changing the phenotypic properties of the organism and decreases the efficacy of antibiotics.24 The self-produced layer of extracellular matrices, deoxyribonucleic acid, and polysaccharides attaches to inert material, preventing phagocytic action by neutrophils. In addition to antibacterial activity, povidone-iodine has antibiofilm activity against Staphylococcal species.25 The active ingredient targets the gene that produces biofilm. This correlates to our study in which the largest decrease in bacterial counts was noted on the implants.
The use of Betadine lavage has shown some promise in vivo as well. A prospective randomized controlled trial26 used 3.5% Betadine irrigation to prevent spine infection. No infections occurred in the Betadine group compared with a deep-infection rate of 2.9% in the control group. Brown and colleagues8 reviewed 1862 hip and knee arthroplasty cases before the use of Betadine lavage and 688 cases after the use of Betadine lavage and found a decrease in infection rate, from 0.97% to 0.15%. S aureus caused 13 of the 18 infections in the control group. These studies8,26 used Betadine lavage for prophylaxis and prevention of deep spine and arthroplasty infection. Betadine lavage as a treatment adjunct for acute arthroplasty infection has not been studied clinically. It has the potential to increase isolated incision and débridement success and to improve component survivorship.
Our arthroplasty model mimics an intra-articular environment and accounts for an implant–polyethylene interface.15 Limitations of our study include the use of MSSA as opposed to MRSA. However, povidone-iodine has the same effects on both MSSA and MRSA.12 We also treated our postoperative infection with 1 dose of antibiotics and not a course, although it should be noted that the single dose of ceftriaxone allowed us to isolate the independent effect of the Betadine lavage. A baseline level of infection severity could have been measured with cultures obtained at the time of irrigation and débridement. Also, a decrease in CFU does not directly correlate to a clinically significant outcome, such as a defined surgical site infection requiring intervention. Nevertheless, it is noteworthy that the decrease in bacterial counts on the stainless steel screws and polyethylene washers were maintained 1 week after the Betadine lavage.
Conclusion
Dilute Betadine lavage is a simple and inexpensive adjunct for the treatment of acute postoperative arthroplasty infection and may increase the rate of component retention. Additionally, the bactericidal and antibiofilm activities of Betadine may improve the effectiveness of systemic antibiotics. Further clinical investigation is warranted.
1. Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72(6):878-883.
2. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
3. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
4. Mahomed NN, Barrett J, Katz JN, Baron JA, Wright J, Losina E. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87(6):1222-1228.
5. Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: treatment options. Orthopedics. 2010;33(9):659.
6. Poultsides LA, Liaropoulos LL, Malizos KN. The socioeconomic impact of musculoskeletal infections. J Bone Joint Surg Am. 2010;92(11):e13.
7. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
8. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
9. Kaysinger KK, Nicholson NC, Ramp WK, Kellam JF. Toxic effects of wound irrigation solutions on cultured tibiae and osteoblasts. J Orthop Trauma. 1995;9(4):303-311.
10. Haley CE, Marling-Cason M, Smith JW, Luby JP, Mackowiak PA. Bactericidal activity of antiseptics against methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;21(6):991-992.
11. Lacey RW, Catto A. Action of povidone-iodine against methicillin-sensitive and -resistant cultures of Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S78-S83.
12. McLure AR, Gordon J. In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1992;21(4):291-299.
13. Suzuki J, Komatsuzawa H, Kozai K, Nagasaka N. In vitro susceptibility of Staphylococcus aureus including MRSA to four disinfectants. ASDC J Dent Child. 1997;64(4):260-263.
14. Yasuda T, Yoshimura S, Katsuno Y, et al. Comparison of bactericidal activities of various disinfectants against methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S66-S69.
15. Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, Brown TE. A novel total knee arthroplasty infection model in rabbits. J Orthop Res. 2005;23(5):1100-1104.
16. Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and debridement. Clin Orthop. 1991;273:113-118.
17. Mont MA, Waldman B, Banerjee C, Pacheco IH, Hungerford DS. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12(4):426-433.
18. Azzam KA, Seeley M, Ghanem E, Austin MS, Purtill JJ, Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25(7):1022-1027.
19. Stall AC, Becker E, Ludwig SC, Gelb D, Poelstra KA. Reduction of postoperative spinal implant infection using gentamicin microspheres. Spine (Phila Pa 1976). 2009;34(5):479-483.
20. Hota B, Ellenbogen C, Hayden MK, Aroutcheva A, Rice TW, Weinstein RA. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch Intern Med. 2007;167(10):1026-1033.
21. Fridkin SK, Hageman JC, Morrison M, et al, Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436-1444.
22. Hosman AH, van der Mei HC, Bulstra SK, Busscher HJ, Neut D. Metal-on-metal bearings in total hip arthroplasties: influence of cobalt and chromium ions on bacterial growth and biofilm formation. J Biomed Mater Res A. 2009;88(3):711-716.
23. Oduwole KO, Glynn AA, Molony DC, et al. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J Orthop Res. 2010;28(9):1252-1256.
24. Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976). 2005;30(15):1689-1693.
25. Anderson RL, Vess RW, Panlilio AL, Favero MS. Prolonged survival of Pseudomonas cepacia in commercially manufactured povidone-iodine. Appl Environ Microbiol. 1990;56(11):3598-3600.
26. Panlilio AL, Beck-Sague CM, Siegel JD, et al. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin Infect Dis. 1992;14(5):1078-1083.
1. Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72(6):878-883.
2. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
3. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
4. Mahomed NN, Barrett J, Katz JN, Baron JA, Wright J, Losina E. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87(6):1222-1228.
5. Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: treatment options. Orthopedics. 2010;33(9):659.
6. Poultsides LA, Liaropoulos LL, Malizos KN. The socioeconomic impact of musculoskeletal infections. J Bone Joint Surg Am. 2010;92(11):e13.
7. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
8. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
9. Kaysinger KK, Nicholson NC, Ramp WK, Kellam JF. Toxic effects of wound irrigation solutions on cultured tibiae and osteoblasts. J Orthop Trauma. 1995;9(4):303-311.
10. Haley CE, Marling-Cason M, Smith JW, Luby JP, Mackowiak PA. Bactericidal activity of antiseptics against methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;21(6):991-992.
11. Lacey RW, Catto A. Action of povidone-iodine against methicillin-sensitive and -resistant cultures of Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S78-S83.
12. McLure AR, Gordon J. In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1992;21(4):291-299.
13. Suzuki J, Komatsuzawa H, Kozai K, Nagasaka N. In vitro susceptibility of Staphylococcus aureus including MRSA to four disinfectants. ASDC J Dent Child. 1997;64(4):260-263.
14. Yasuda T, Yoshimura S, Katsuno Y, et al. Comparison of bactericidal activities of various disinfectants against methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S66-S69.
15. Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, Brown TE. A novel total knee arthroplasty infection model in rabbits. J Orthop Res. 2005;23(5):1100-1104.
16. Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and debridement. Clin Orthop. 1991;273:113-118.
17. Mont MA, Waldman B, Banerjee C, Pacheco IH, Hungerford DS. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12(4):426-433.
18. Azzam KA, Seeley M, Ghanem E, Austin MS, Purtill JJ, Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25(7):1022-1027.
19. Stall AC, Becker E, Ludwig SC, Gelb D, Poelstra KA. Reduction of postoperative spinal implant infection using gentamicin microspheres. Spine (Phila Pa 1976). 2009;34(5):479-483.
20. Hota B, Ellenbogen C, Hayden MK, Aroutcheva A, Rice TW, Weinstein RA. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch Intern Med. 2007;167(10):1026-1033.
21. Fridkin SK, Hageman JC, Morrison M, et al, Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436-1444.
22. Hosman AH, van der Mei HC, Bulstra SK, Busscher HJ, Neut D. Metal-on-metal bearings in total hip arthroplasties: influence of cobalt and chromium ions on bacterial growth and biofilm formation. J Biomed Mater Res A. 2009;88(3):711-716.
23. Oduwole KO, Glynn AA, Molony DC, et al. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J Orthop Res. 2010;28(9):1252-1256.
24. Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976). 2005;30(15):1689-1693.
25. Anderson RL, Vess RW, Panlilio AL, Favero MS. Prolonged survival of Pseudomonas cepacia in commercially manufactured povidone-iodine. Appl Environ Microbiol. 1990;56(11):3598-3600.
26. Panlilio AL, Beck-Sague CM, Siegel JD, et al. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin Infect Dis. 1992;14(5):1078-1083.
Biomechanical Comparison of Hamstring Tendon Fixation Devices for Anterior Cruciate Ligament Reconstruction: Part 2. Four Tibial Devices
Of the procedures performed by surgeons specializing in sports medicine and by general orthopedists, anterior cruciate ligament (ACL) reconstruction remains one of the most common.1 Recent years have seen a trend toward replacing the “gold standard” of bone–patellar tendon–bone autograft with autograft or allograft hamstring tendon in ACL reconstruction.2 This shift is being made to try to avoid the donor-site morbidity of patellar tendon autografts and decrease the incidence of postoperative anterior knee pain. With increased use of hamstring grafts in ACL reconstruction, it is important to determine the strength of different methods of graft fixation.
Rigid fixation of hamstring grafts is recognized as a crucial factor in the long-term success of ACL reconstruction. Grafts must withstand early rehabilitation forces as high as 500 N.2 There is therefore much concern about the strength of tibial fixation, given the lower bone density of the tibial metaphysis versus the femoral metaphysis. In addition, stability is more a concern in the tibia, as the forces are directly in line with the tibial tunnel.3,4
The challenge has been to engineer devices that provide stable, rigid graft fixation that allows expeditious tendon-to-bone healing and increased construct stiffness. Many new fixation devices are being marketed. There is much interest in determining which devices have the most fixation strength,4-9 but so far several products have not been compared with one another.
We conducted a study to determine if tibial hamstring fixation devices used in ACL reconstruction differ in fixation strength. We hypothesized we would find no differences.
Materials and Methods
Forty porcine tibias were harvested after the animals had been euthanized for other studies at our institution. Our study was approved by the institutional animal care and use committee. Specimens were stored at –25°C and, on day of testing, thawed to room temperature. Gracilis and semitendinosus tendon grafts were donated by a tissue bank (LifeNet Health, Virginia Beach, Virginia). The grafts were stored at –25°C; on day of testing, tendons were thawed to room temperature.
We evaluated 4 different tibial fixation devices (Figure 1): Delta screw and Retroscrew (Arthrex, Naples, Florida), WasherLoc (Arthrotek, Warsaw, Indiana), and Intrafix (Depuy Mitek, Raynham, Massachusetts). For each device, 10 ACL fixation constructs were tested.
Quadrupled human semitendinosus–gracilis tendon grafts were fixed into the tibias using the 4 tibial fixation devices. All fixations were done according to manufacturer specifications. All interference screws were placed eccentrically. The testing apparatus and procedure are described in an article by Kousa and colleagues.6 The specimens were mounted on the mechanical testing apparatus by threaded bars and custom clamps to secure fixation (Figure 2). Constant tension was maintained on all 4 strands of the hamstring grafts to equalize the tendons. After the looped end of the hamstring graft was secured by clamps, 25 mm of graft was left between the clamp and the intra-articular tunnel.
In the cyclic loading test, the load was applied parallel to the long axis of the tibial tunnel. A 50-N preload was initially applied to each specimen for 10 seconds. Subsequently, 1500 loading cycles between 50 N and 200 N at a rate of 1 cycle per 120 seconds were performed. Standard force-displacement curves were then generated. Each tibial fixation device underwent 10 cyclic loading tests. Specimens surviving the cyclic loading then underwent a single-cycle load-to-failure (LTF) test in which the load was applied parallel to the long axis of the drill hole at a rate of 50 mm per minute.
Residual displacement, stiffness, and ultimate LTF data were recorded from the force-displacement curves. Residual displacement data were generated from the cyclic loading test; residual displacement was determined by subtracting preload displacement from displacement at 1, 10, 50, 100, 250, 500, 1000, and 1500 cycles. Stiffness data were generated from the single-cycle LTF test; stiffness was defined as the linear region slope of the force-displacement curve corresponding to the steepest straight-line tangent to the loading curve. Ultimate LTF (yield load) data were generated from the single-cycle LTF test; ultimate LTF was defined as the load at the point where the slope of the load displacement curve initially decreases.
Statistical analysis generated standard descriptive statistics: means, standard deviations, and proportions. One-way analysis of variance (ANOVA) was used to determine any statistically significant differences in stiffness, yield load, and residual displacement between the different fixation devices. Differences in force (load) between the single cycle and the cyclic loading test were determined by ANOVA. P < .05 was considered statistically significant for all tests.
Results
The modes of failure for the devices were similar. In all 10 tests, Intrafix was pulled through the tunnel with the hamstring allografts. WasherLoc failed in each test, with the tendons eventually being pulled through the washer and thus out through the tunnel. Delta screw and Retroscrew both failed with slippage of the fixation device and the tendons pulled out through the tunnel.
For the cyclic loading tests, 8 of the 10 Delta screws and only 2 of the 10 Retroscrews completed the 1500-cycle loading test before failure. The 2 Delta screws that did not complete the testing failed after about 500 cycles, and the 8 Retroscrews that did not complete the testing failed after about 250 cycles. All 10 WasherLoc and Intrafix devices completed the testing.
Residual displacement data were calculated from the cyclic loading tests (Table). Mean (SS) residual displacement was lowest for Intrafix at 2.9 (1.2) mm, followed by WasherLoc at 5.6 (2.2) mm and Delta at 6.4 (3.3) mm. Retroscrew at 25.5 (11.0) mm had the highest residual displacement, though only 2 completed the cyclic tests. Intrafix, WasherLoc, and Delta were not statistically different, but there was a statistical difference between Retroscrew and the other devices (P < .001).
Stiffness data were calculated from the LTF tests (Table). Mean (SD) stiffness was highest for Intrafix at 129 (32.7) N/mm, followed by WasherLoc at 97 (11.6) N/mm, Delta at 93 (9.5) N/mm, and Retroscrew at 80.2 (8.8) N/mm. Intrafix had statistically higher stiffness compared with WasherLoc (P < .05), Delta (P < .01), and Retroscrew (P < .05). There were no significant differences in stiffness among WasherLoc, Delta, and Retroscrew.
Mean (SD) ultimate LTF was highest for Intrafix at 656 (182.6) N, followed by WasherLoc at 630 (129.3) N, Delta at 430 (90.0) N, and Retroscrew at 285 (33.8) N (Table). There were significant differences between Intrafix and Delta (P < .05) and Retroscrew (P < .05). WasherLoc failed at a significantly higher load compared with Delta (P < .05) and Retroscrew (P < .05). There were no significant differences in mean LTF between Intrafix and WasherLoc.
Discussion
In this biomechanical comparison of 4 different tibial fixation devices, Intrafix had results superior to those of the other implants. Intrafix failed at higher LTF and lower residual displacement and had higher stiffness. WasherLoc performed well and had LTF similar to that of Intrafix. The interference screws performed poorly with respect to LTF, residual displacement, and stiffness, and a large proportion of them failed early into cyclic loading.
Intrafix is a central fixation device that uses a 4-quadrant sleeve and a screw to establish tensioning across all 4 hamstring graft strands. The theory is this configuration increases the contact area between graft and bone for proper integration of graft into bone. Intrafix has performed well in other biomechanical studies. Using a study design similar to ours, Kousa and colleagues7 found the performance of Intrafix to be superior to that of other devices, including interference screws and WasherLoc. Starch and colleagues10 reported that, compared with a standard interference screw, Intrafix required significantly higher load to cause a millimeter of graft laxity. They concluded that this demonstrates superior fixation strength and reduced laxity of the graft after cyclic loading. Coleridge and Amis4 found that, compared with WasherLoc and various interference screws, Intrafix had the lower residual displacement. However, they also found that, compared with Intrafix and interference screws, WasherLoc had the highest ultimate tensile strength. Their findings may be difficult to compare with ours, as they tested fixation of calf extensor tendons, and we tested human hamstring grafts.
An important concern in the present study was the poor performance of the interference screws. Other authors recently expressed concern with using interference screws in soft-tissue ACL grafts—based on biomechanical study results of increased slippage, bone tunnel widening, and less strength.11 Delta screws and Retroscrews have not been specifically evaluated, and their fixation strengths have not been directly compared with those of other devices. In the present study, Delta screws and Retroscrews consistently performed the poorest with respect to ultimate LTF, residual displacement, and stiffness. Twenty percent of the Delta screws and 80% of the Retroscrews did not complete 1500 cycles. The poor performance of the interference screws was echoed in studies by Magen and colleagues12 and Kousa and colleagues,7 in which the only complete failures were in the cyclic loading of the interference screws.
Three possible confounding factors may have affected the performance of the interference screws: bone density of porcine tibia, length of interference screw, and location of screw placement. In addition, in clinical practice these screws may be used with other modes of graft fixation. Combined fixation (interference screws, other devices) was not evaluated in this study.
Porcine models have been used in many biomechanical graft fixation studies.4,6,7,12,13 Some authors have found porcine tibia to be a poor substitute for human cadaver tibia because the volumetric density of porcine bone is higher than that of human bone.14,15 Other authors have demonstrated fairly similar bone density between human and porcine tibia.16 The concern is that interference screw fixation strength correlates with the density of the bone in which screws are fixed.17 Therefore, one limitation of our study is that we did not determine the bone density of the porcine tibias for comparison with that of young human tibias.
Another important variable that could have affected the performance of the interference screws is screw length. One study found no significant difference in screw strength between various lengths, and longer screws failed to protect against graft slippage.18 However, Selby and colleagues19 found that, compared with 28-mm screws, 35-mm bioabsorbable interference screws failed at higher LTF. This is in part why we selected 35-mm Delta screws for our study. Both 35-mm Delta screws and 20-mm Retroscrews performed poorly. However, we could not determine if the poorer performance of Retroscrews was related to their length.
We used an eccentric placement for our interference screws. Although some studies have suggested concentric placement might improve fixation strength by increasing bone–tendon contact,20 Simonian and colleagues21 found no difference in graft slippage or ultimate LTF between eccentrically and concentrically placed screws. Although they were not biomechanically tested in our study, a few grafts were fixed with concentrically placed screws, and these tendons appeared to be more clinically damaged than the eccentrically placed screws.
Combined tibial fixation techniques may be used in clinical practice, but we did not evaluate them in our study. Yoo and colleagues9 compared interference screw, interference screw plus cortical screw and spiked washer, and cortical screw and spiked washer alone. They found that stiffness nearly doubled, residual displacement was less, and ultimate LTF was significantly higher in the group with interference screw plus cortical screw and spiked washer. In a similar study, Walsh and colleagues13 demonstrated improved stiffness and LTF in cyclic testing with the combination of retrograde interference screw and suture button over interference screw alone. Further study may include direct comparisons of additional tibial fixation techniques using more than one device. Cost analysis of use of additional fixation devices would be beneficial as well.
Study results have clearly demonstrated that tibial fixation is the weak point in ACL reconstruction3,17 and that early aggressive rehabilitation can help restore range of motion, strength, and function.22,23 Implants that can withstand early loads during rehabilitation periods are therefore of utmost importance.
Conclusion
Intrafix demonstrated superior strength in the fixation of hamstring grafts in the tibia, followed closely by WasherLoc. When used as the sole tibial fixation device, interference screws had low LTF, decreased stiffness, and high residual displacement, which may have clinical implications for early rehabilitation after ACL reconstruction.
1. Garrett WE Jr, Swiontkowski MF, Weinsten JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
2. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197-207.
3. Brand J Jr, Weiler A, Caborn DN, Brown CH Jr, Johnson DL. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28(5):761-774.
4. Coleridge SD, Amis AA. A comparison of five tibial-fixation systems in hamstring-graft anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):391-397.
5. Fabbriciani C, Mulas PD, Ziranu F, Deriu L, Zarelli D, Milano G. Mechanical analysis of fixation methods for anterior cruciate ligament reconstruction with hamstring tendon graft. An experimental study in sheep knees. Knee. 2005;12(2):135-138.
6. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31(2):174-181.
7. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31(2):182-188.
8. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
9. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455-459.
10. Starch DW, Alexander JW, Noble PC, Reddy S, Lintner DM. Multistranded hamstring tendon graft fixation with a central four-quadrant or a standard tibial interference screw for anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31(3):338-344.
11. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
12. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
13. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
14. Nurmi JT, Järvinen TL, Kannus P, Sievänen H, Toukosalo J, Järvinen M. Compaction versus extraction drilling for fixation of the hamstring tendon graft in anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(2):167-173.
15. Nurmi JT, Sievänen H, Kannus P, Järvinen M, Järvinen TL. Porcine tibia is a poor substitute for human cadaver tibia for evaluating interference screw fixation. Am J Sports Med. 2004;32(3):765-771.
16. Nagarkatti DG, McKeon BP, Donahue BS, Fulkerson JP. Mechanical evaluation of a soft tissue interference screw in free tendon anterior cruciate ligament graft fixation. Am J Sports Med. 2001;29(1):67-71.
17. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
18. Stadelmaier DM, Lowe WR, Ilahi OA, Noble PC, Kohl HW 3rd. Cyclic pull-out strength of hamstring tendon graft fixation with soft tissue interference screws. Influence of screw length. Am J Sports Med. 1999;27(6):778-783.
19. Selby JB, Johnson DL, Hester P, Caborn DN. Effect of screw length on bioabsorbable interference screw fixation in a tibial bone tunnel. Am J Sports Med. 2001;29(5):614-619.
20. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
21. Simonian PT, Sussmann PS, Baldini TH, Crockett HC, Wickiewicz TL. Interference screw position and hamstring graft location for anterior cruciate ligament reconstruction. Arthroscopy. 1998;14(5):459-464.
22. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
23. Shelbourne KD, Wilckens JH. Current concepts in anterior cruciate ligament rehabilitation. Orthop Rev. 1990;19(11):957-964.
Of the procedures performed by surgeons specializing in sports medicine and by general orthopedists, anterior cruciate ligament (ACL) reconstruction remains one of the most common.1 Recent years have seen a trend toward replacing the “gold standard” of bone–patellar tendon–bone autograft with autograft or allograft hamstring tendon in ACL reconstruction.2 This shift is being made to try to avoid the donor-site morbidity of patellar tendon autografts and decrease the incidence of postoperative anterior knee pain. With increased use of hamstring grafts in ACL reconstruction, it is important to determine the strength of different methods of graft fixation.
Rigid fixation of hamstring grafts is recognized as a crucial factor in the long-term success of ACL reconstruction. Grafts must withstand early rehabilitation forces as high as 500 N.2 There is therefore much concern about the strength of tibial fixation, given the lower bone density of the tibial metaphysis versus the femoral metaphysis. In addition, stability is more a concern in the tibia, as the forces are directly in line with the tibial tunnel.3,4
The challenge has been to engineer devices that provide stable, rigid graft fixation that allows expeditious tendon-to-bone healing and increased construct stiffness. Many new fixation devices are being marketed. There is much interest in determining which devices have the most fixation strength,4-9 but so far several products have not been compared with one another.
We conducted a study to determine if tibial hamstring fixation devices used in ACL reconstruction differ in fixation strength. We hypothesized we would find no differences.
Materials and Methods
Forty porcine tibias were harvested after the animals had been euthanized for other studies at our institution. Our study was approved by the institutional animal care and use committee. Specimens were stored at –25°C and, on day of testing, thawed to room temperature. Gracilis and semitendinosus tendon grafts were donated by a tissue bank (LifeNet Health, Virginia Beach, Virginia). The grafts were stored at –25°C; on day of testing, tendons were thawed to room temperature.
We evaluated 4 different tibial fixation devices (Figure 1): Delta screw and Retroscrew (Arthrex, Naples, Florida), WasherLoc (Arthrotek, Warsaw, Indiana), and Intrafix (Depuy Mitek, Raynham, Massachusetts). For each device, 10 ACL fixation constructs were tested.
Quadrupled human semitendinosus–gracilis tendon grafts were fixed into the tibias using the 4 tibial fixation devices. All fixations were done according to manufacturer specifications. All interference screws were placed eccentrically. The testing apparatus and procedure are described in an article by Kousa and colleagues.6 The specimens were mounted on the mechanical testing apparatus by threaded bars and custom clamps to secure fixation (Figure 2). Constant tension was maintained on all 4 strands of the hamstring grafts to equalize the tendons. After the looped end of the hamstring graft was secured by clamps, 25 mm of graft was left between the clamp and the intra-articular tunnel.
In the cyclic loading test, the load was applied parallel to the long axis of the tibial tunnel. A 50-N preload was initially applied to each specimen for 10 seconds. Subsequently, 1500 loading cycles between 50 N and 200 N at a rate of 1 cycle per 120 seconds were performed. Standard force-displacement curves were then generated. Each tibial fixation device underwent 10 cyclic loading tests. Specimens surviving the cyclic loading then underwent a single-cycle load-to-failure (LTF) test in which the load was applied parallel to the long axis of the drill hole at a rate of 50 mm per minute.
Residual displacement, stiffness, and ultimate LTF data were recorded from the force-displacement curves. Residual displacement data were generated from the cyclic loading test; residual displacement was determined by subtracting preload displacement from displacement at 1, 10, 50, 100, 250, 500, 1000, and 1500 cycles. Stiffness data were generated from the single-cycle LTF test; stiffness was defined as the linear region slope of the force-displacement curve corresponding to the steepest straight-line tangent to the loading curve. Ultimate LTF (yield load) data were generated from the single-cycle LTF test; ultimate LTF was defined as the load at the point where the slope of the load displacement curve initially decreases.
Statistical analysis generated standard descriptive statistics: means, standard deviations, and proportions. One-way analysis of variance (ANOVA) was used to determine any statistically significant differences in stiffness, yield load, and residual displacement between the different fixation devices. Differences in force (load) between the single cycle and the cyclic loading test were determined by ANOVA. P < .05 was considered statistically significant for all tests.
Results
The modes of failure for the devices were similar. In all 10 tests, Intrafix was pulled through the tunnel with the hamstring allografts. WasherLoc failed in each test, with the tendons eventually being pulled through the washer and thus out through the tunnel. Delta screw and Retroscrew both failed with slippage of the fixation device and the tendons pulled out through the tunnel.
For the cyclic loading tests, 8 of the 10 Delta screws and only 2 of the 10 Retroscrews completed the 1500-cycle loading test before failure. The 2 Delta screws that did not complete the testing failed after about 500 cycles, and the 8 Retroscrews that did not complete the testing failed after about 250 cycles. All 10 WasherLoc and Intrafix devices completed the testing.
Residual displacement data were calculated from the cyclic loading tests (Table). Mean (SS) residual displacement was lowest for Intrafix at 2.9 (1.2) mm, followed by WasherLoc at 5.6 (2.2) mm and Delta at 6.4 (3.3) mm. Retroscrew at 25.5 (11.0) mm had the highest residual displacement, though only 2 completed the cyclic tests. Intrafix, WasherLoc, and Delta were not statistically different, but there was a statistical difference between Retroscrew and the other devices (P < .001).
Stiffness data were calculated from the LTF tests (Table). Mean (SD) stiffness was highest for Intrafix at 129 (32.7) N/mm, followed by WasherLoc at 97 (11.6) N/mm, Delta at 93 (9.5) N/mm, and Retroscrew at 80.2 (8.8) N/mm. Intrafix had statistically higher stiffness compared with WasherLoc (P < .05), Delta (P < .01), and Retroscrew (P < .05). There were no significant differences in stiffness among WasherLoc, Delta, and Retroscrew.
Mean (SD) ultimate LTF was highest for Intrafix at 656 (182.6) N, followed by WasherLoc at 630 (129.3) N, Delta at 430 (90.0) N, and Retroscrew at 285 (33.8) N (Table). There were significant differences between Intrafix and Delta (P < .05) and Retroscrew (P < .05). WasherLoc failed at a significantly higher load compared with Delta (P < .05) and Retroscrew (P < .05). There were no significant differences in mean LTF between Intrafix and WasherLoc.
Discussion
In this biomechanical comparison of 4 different tibial fixation devices, Intrafix had results superior to those of the other implants. Intrafix failed at higher LTF and lower residual displacement and had higher stiffness. WasherLoc performed well and had LTF similar to that of Intrafix. The interference screws performed poorly with respect to LTF, residual displacement, and stiffness, and a large proportion of them failed early into cyclic loading.
Intrafix is a central fixation device that uses a 4-quadrant sleeve and a screw to establish tensioning across all 4 hamstring graft strands. The theory is this configuration increases the contact area between graft and bone for proper integration of graft into bone. Intrafix has performed well in other biomechanical studies. Using a study design similar to ours, Kousa and colleagues7 found the performance of Intrafix to be superior to that of other devices, including interference screws and WasherLoc. Starch and colleagues10 reported that, compared with a standard interference screw, Intrafix required significantly higher load to cause a millimeter of graft laxity. They concluded that this demonstrates superior fixation strength and reduced laxity of the graft after cyclic loading. Coleridge and Amis4 found that, compared with WasherLoc and various interference screws, Intrafix had the lower residual displacement. However, they also found that, compared with Intrafix and interference screws, WasherLoc had the highest ultimate tensile strength. Their findings may be difficult to compare with ours, as they tested fixation of calf extensor tendons, and we tested human hamstring grafts.
An important concern in the present study was the poor performance of the interference screws. Other authors recently expressed concern with using interference screws in soft-tissue ACL grafts—based on biomechanical study results of increased slippage, bone tunnel widening, and less strength.11 Delta screws and Retroscrews have not been specifically evaluated, and their fixation strengths have not been directly compared with those of other devices. In the present study, Delta screws and Retroscrews consistently performed the poorest with respect to ultimate LTF, residual displacement, and stiffness. Twenty percent of the Delta screws and 80% of the Retroscrews did not complete 1500 cycles. The poor performance of the interference screws was echoed in studies by Magen and colleagues12 and Kousa and colleagues,7 in which the only complete failures were in the cyclic loading of the interference screws.
Three possible confounding factors may have affected the performance of the interference screws: bone density of porcine tibia, length of interference screw, and location of screw placement. In addition, in clinical practice these screws may be used with other modes of graft fixation. Combined fixation (interference screws, other devices) was not evaluated in this study.
Porcine models have been used in many biomechanical graft fixation studies.4,6,7,12,13 Some authors have found porcine tibia to be a poor substitute for human cadaver tibia because the volumetric density of porcine bone is higher than that of human bone.14,15 Other authors have demonstrated fairly similar bone density between human and porcine tibia.16 The concern is that interference screw fixation strength correlates with the density of the bone in which screws are fixed.17 Therefore, one limitation of our study is that we did not determine the bone density of the porcine tibias for comparison with that of young human tibias.
Another important variable that could have affected the performance of the interference screws is screw length. One study found no significant difference in screw strength between various lengths, and longer screws failed to protect against graft slippage.18 However, Selby and colleagues19 found that, compared with 28-mm screws, 35-mm bioabsorbable interference screws failed at higher LTF. This is in part why we selected 35-mm Delta screws for our study. Both 35-mm Delta screws and 20-mm Retroscrews performed poorly. However, we could not determine if the poorer performance of Retroscrews was related to their length.
We used an eccentric placement for our interference screws. Although some studies have suggested concentric placement might improve fixation strength by increasing bone–tendon contact,20 Simonian and colleagues21 found no difference in graft slippage or ultimate LTF between eccentrically and concentrically placed screws. Although they were not biomechanically tested in our study, a few grafts were fixed with concentrically placed screws, and these tendons appeared to be more clinically damaged than the eccentrically placed screws.
Combined tibial fixation techniques may be used in clinical practice, but we did not evaluate them in our study. Yoo and colleagues9 compared interference screw, interference screw plus cortical screw and spiked washer, and cortical screw and spiked washer alone. They found that stiffness nearly doubled, residual displacement was less, and ultimate LTF was significantly higher in the group with interference screw plus cortical screw and spiked washer. In a similar study, Walsh and colleagues13 demonstrated improved stiffness and LTF in cyclic testing with the combination of retrograde interference screw and suture button over interference screw alone. Further study may include direct comparisons of additional tibial fixation techniques using more than one device. Cost analysis of use of additional fixation devices would be beneficial as well.
Study results have clearly demonstrated that tibial fixation is the weak point in ACL reconstruction3,17 and that early aggressive rehabilitation can help restore range of motion, strength, and function.22,23 Implants that can withstand early loads during rehabilitation periods are therefore of utmost importance.
Conclusion
Intrafix demonstrated superior strength in the fixation of hamstring grafts in the tibia, followed closely by WasherLoc. When used as the sole tibial fixation device, interference screws had low LTF, decreased stiffness, and high residual displacement, which may have clinical implications for early rehabilitation after ACL reconstruction.
Of the procedures performed by surgeons specializing in sports medicine and by general orthopedists, anterior cruciate ligament (ACL) reconstruction remains one of the most common.1 Recent years have seen a trend toward replacing the “gold standard” of bone–patellar tendon–bone autograft with autograft or allograft hamstring tendon in ACL reconstruction.2 This shift is being made to try to avoid the donor-site morbidity of patellar tendon autografts and decrease the incidence of postoperative anterior knee pain. With increased use of hamstring grafts in ACL reconstruction, it is important to determine the strength of different methods of graft fixation.
Rigid fixation of hamstring grafts is recognized as a crucial factor in the long-term success of ACL reconstruction. Grafts must withstand early rehabilitation forces as high as 500 N.2 There is therefore much concern about the strength of tibial fixation, given the lower bone density of the tibial metaphysis versus the femoral metaphysis. In addition, stability is more a concern in the tibia, as the forces are directly in line with the tibial tunnel.3,4
The challenge has been to engineer devices that provide stable, rigid graft fixation that allows expeditious tendon-to-bone healing and increased construct stiffness. Many new fixation devices are being marketed. There is much interest in determining which devices have the most fixation strength,4-9 but so far several products have not been compared with one another.
We conducted a study to determine if tibial hamstring fixation devices used in ACL reconstruction differ in fixation strength. We hypothesized we would find no differences.
Materials and Methods
Forty porcine tibias were harvested after the animals had been euthanized for other studies at our institution. Our study was approved by the institutional animal care and use committee. Specimens were stored at –25°C and, on day of testing, thawed to room temperature. Gracilis and semitendinosus tendon grafts were donated by a tissue bank (LifeNet Health, Virginia Beach, Virginia). The grafts were stored at –25°C; on day of testing, tendons were thawed to room temperature.
We evaluated 4 different tibial fixation devices (Figure 1): Delta screw and Retroscrew (Arthrex, Naples, Florida), WasherLoc (Arthrotek, Warsaw, Indiana), and Intrafix (Depuy Mitek, Raynham, Massachusetts). For each device, 10 ACL fixation constructs were tested.
Quadrupled human semitendinosus–gracilis tendon grafts were fixed into the tibias using the 4 tibial fixation devices. All fixations were done according to manufacturer specifications. All interference screws were placed eccentrically. The testing apparatus and procedure are described in an article by Kousa and colleagues.6 The specimens were mounted on the mechanical testing apparatus by threaded bars and custom clamps to secure fixation (Figure 2). Constant tension was maintained on all 4 strands of the hamstring grafts to equalize the tendons. After the looped end of the hamstring graft was secured by clamps, 25 mm of graft was left between the clamp and the intra-articular tunnel.
In the cyclic loading test, the load was applied parallel to the long axis of the tibial tunnel. A 50-N preload was initially applied to each specimen for 10 seconds. Subsequently, 1500 loading cycles between 50 N and 200 N at a rate of 1 cycle per 120 seconds were performed. Standard force-displacement curves were then generated. Each tibial fixation device underwent 10 cyclic loading tests. Specimens surviving the cyclic loading then underwent a single-cycle load-to-failure (LTF) test in which the load was applied parallel to the long axis of the drill hole at a rate of 50 mm per minute.
Residual displacement, stiffness, and ultimate LTF data were recorded from the force-displacement curves. Residual displacement data were generated from the cyclic loading test; residual displacement was determined by subtracting preload displacement from displacement at 1, 10, 50, 100, 250, 500, 1000, and 1500 cycles. Stiffness data were generated from the single-cycle LTF test; stiffness was defined as the linear region slope of the force-displacement curve corresponding to the steepest straight-line tangent to the loading curve. Ultimate LTF (yield load) data were generated from the single-cycle LTF test; ultimate LTF was defined as the load at the point where the slope of the load displacement curve initially decreases.
Statistical analysis generated standard descriptive statistics: means, standard deviations, and proportions. One-way analysis of variance (ANOVA) was used to determine any statistically significant differences in stiffness, yield load, and residual displacement between the different fixation devices. Differences in force (load) between the single cycle and the cyclic loading test were determined by ANOVA. P < .05 was considered statistically significant for all tests.
Results
The modes of failure for the devices were similar. In all 10 tests, Intrafix was pulled through the tunnel with the hamstring allografts. WasherLoc failed in each test, with the tendons eventually being pulled through the washer and thus out through the tunnel. Delta screw and Retroscrew both failed with slippage of the fixation device and the tendons pulled out through the tunnel.
For the cyclic loading tests, 8 of the 10 Delta screws and only 2 of the 10 Retroscrews completed the 1500-cycle loading test before failure. The 2 Delta screws that did not complete the testing failed after about 500 cycles, and the 8 Retroscrews that did not complete the testing failed after about 250 cycles. All 10 WasherLoc and Intrafix devices completed the testing.
Residual displacement data were calculated from the cyclic loading tests (Table). Mean (SS) residual displacement was lowest for Intrafix at 2.9 (1.2) mm, followed by WasherLoc at 5.6 (2.2) mm and Delta at 6.4 (3.3) mm. Retroscrew at 25.5 (11.0) mm had the highest residual displacement, though only 2 completed the cyclic tests. Intrafix, WasherLoc, and Delta were not statistically different, but there was a statistical difference between Retroscrew and the other devices (P < .001).
Stiffness data were calculated from the LTF tests (Table). Mean (SD) stiffness was highest for Intrafix at 129 (32.7) N/mm, followed by WasherLoc at 97 (11.6) N/mm, Delta at 93 (9.5) N/mm, and Retroscrew at 80.2 (8.8) N/mm. Intrafix had statistically higher stiffness compared with WasherLoc (P < .05), Delta (P < .01), and Retroscrew (P < .05). There were no significant differences in stiffness among WasherLoc, Delta, and Retroscrew.
Mean (SD) ultimate LTF was highest for Intrafix at 656 (182.6) N, followed by WasherLoc at 630 (129.3) N, Delta at 430 (90.0) N, and Retroscrew at 285 (33.8) N (Table). There were significant differences between Intrafix and Delta (P < .05) and Retroscrew (P < .05). WasherLoc failed at a significantly higher load compared with Delta (P < .05) and Retroscrew (P < .05). There were no significant differences in mean LTF between Intrafix and WasherLoc.
Discussion
In this biomechanical comparison of 4 different tibial fixation devices, Intrafix had results superior to those of the other implants. Intrafix failed at higher LTF and lower residual displacement and had higher stiffness. WasherLoc performed well and had LTF similar to that of Intrafix. The interference screws performed poorly with respect to LTF, residual displacement, and stiffness, and a large proportion of them failed early into cyclic loading.
Intrafix is a central fixation device that uses a 4-quadrant sleeve and a screw to establish tensioning across all 4 hamstring graft strands. The theory is this configuration increases the contact area between graft and bone for proper integration of graft into bone. Intrafix has performed well in other biomechanical studies. Using a study design similar to ours, Kousa and colleagues7 found the performance of Intrafix to be superior to that of other devices, including interference screws and WasherLoc. Starch and colleagues10 reported that, compared with a standard interference screw, Intrafix required significantly higher load to cause a millimeter of graft laxity. They concluded that this demonstrates superior fixation strength and reduced laxity of the graft after cyclic loading. Coleridge and Amis4 found that, compared with WasherLoc and various interference screws, Intrafix had the lower residual displacement. However, they also found that, compared with Intrafix and interference screws, WasherLoc had the highest ultimate tensile strength. Their findings may be difficult to compare with ours, as they tested fixation of calf extensor tendons, and we tested human hamstring grafts.
An important concern in the present study was the poor performance of the interference screws. Other authors recently expressed concern with using interference screws in soft-tissue ACL grafts—based on biomechanical study results of increased slippage, bone tunnel widening, and less strength.11 Delta screws and Retroscrews have not been specifically evaluated, and their fixation strengths have not been directly compared with those of other devices. In the present study, Delta screws and Retroscrews consistently performed the poorest with respect to ultimate LTF, residual displacement, and stiffness. Twenty percent of the Delta screws and 80% of the Retroscrews did not complete 1500 cycles. The poor performance of the interference screws was echoed in studies by Magen and colleagues12 and Kousa and colleagues,7 in which the only complete failures were in the cyclic loading of the interference screws.
Three possible confounding factors may have affected the performance of the interference screws: bone density of porcine tibia, length of interference screw, and location of screw placement. In addition, in clinical practice these screws may be used with other modes of graft fixation. Combined fixation (interference screws, other devices) was not evaluated in this study.
Porcine models have been used in many biomechanical graft fixation studies.4,6,7,12,13 Some authors have found porcine tibia to be a poor substitute for human cadaver tibia because the volumetric density of porcine bone is higher than that of human bone.14,15 Other authors have demonstrated fairly similar bone density between human and porcine tibia.16 The concern is that interference screw fixation strength correlates with the density of the bone in which screws are fixed.17 Therefore, one limitation of our study is that we did not determine the bone density of the porcine tibias for comparison with that of young human tibias.
Another important variable that could have affected the performance of the interference screws is screw length. One study found no significant difference in screw strength between various lengths, and longer screws failed to protect against graft slippage.18 However, Selby and colleagues19 found that, compared with 28-mm screws, 35-mm bioabsorbable interference screws failed at higher LTF. This is in part why we selected 35-mm Delta screws for our study. Both 35-mm Delta screws and 20-mm Retroscrews performed poorly. However, we could not determine if the poorer performance of Retroscrews was related to their length.
We used an eccentric placement for our interference screws. Although some studies have suggested concentric placement might improve fixation strength by increasing bone–tendon contact,20 Simonian and colleagues21 found no difference in graft slippage or ultimate LTF between eccentrically and concentrically placed screws. Although they were not biomechanically tested in our study, a few grafts were fixed with concentrically placed screws, and these tendons appeared to be more clinically damaged than the eccentrically placed screws.
Combined tibial fixation techniques may be used in clinical practice, but we did not evaluate them in our study. Yoo and colleagues9 compared interference screw, interference screw plus cortical screw and spiked washer, and cortical screw and spiked washer alone. They found that stiffness nearly doubled, residual displacement was less, and ultimate LTF was significantly higher in the group with interference screw plus cortical screw and spiked washer. In a similar study, Walsh and colleagues13 demonstrated improved stiffness and LTF in cyclic testing with the combination of retrograde interference screw and suture button over interference screw alone. Further study may include direct comparisons of additional tibial fixation techniques using more than one device. Cost analysis of use of additional fixation devices would be beneficial as well.
Study results have clearly demonstrated that tibial fixation is the weak point in ACL reconstruction3,17 and that early aggressive rehabilitation can help restore range of motion, strength, and function.22,23 Implants that can withstand early loads during rehabilitation periods are therefore of utmost importance.
Conclusion
Intrafix demonstrated superior strength in the fixation of hamstring grafts in the tibia, followed closely by WasherLoc. When used as the sole tibial fixation device, interference screws had low LTF, decreased stiffness, and high residual displacement, which may have clinical implications for early rehabilitation after ACL reconstruction.
1. Garrett WE Jr, Swiontkowski MF, Weinsten JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
2. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197-207.
3. Brand J Jr, Weiler A, Caborn DN, Brown CH Jr, Johnson DL. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28(5):761-774.
4. Coleridge SD, Amis AA. A comparison of five tibial-fixation systems in hamstring-graft anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):391-397.
5. Fabbriciani C, Mulas PD, Ziranu F, Deriu L, Zarelli D, Milano G. Mechanical analysis of fixation methods for anterior cruciate ligament reconstruction with hamstring tendon graft. An experimental study in sheep knees. Knee. 2005;12(2):135-138.
6. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31(2):174-181.
7. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31(2):182-188.
8. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
9. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455-459.
10. Starch DW, Alexander JW, Noble PC, Reddy S, Lintner DM. Multistranded hamstring tendon graft fixation with a central four-quadrant or a standard tibial interference screw for anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31(3):338-344.
11. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
12. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
13. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
14. Nurmi JT, Järvinen TL, Kannus P, Sievänen H, Toukosalo J, Järvinen M. Compaction versus extraction drilling for fixation of the hamstring tendon graft in anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(2):167-173.
15. Nurmi JT, Sievänen H, Kannus P, Järvinen M, Järvinen TL. Porcine tibia is a poor substitute for human cadaver tibia for evaluating interference screw fixation. Am J Sports Med. 2004;32(3):765-771.
16. Nagarkatti DG, McKeon BP, Donahue BS, Fulkerson JP. Mechanical evaluation of a soft tissue interference screw in free tendon anterior cruciate ligament graft fixation. Am J Sports Med. 2001;29(1):67-71.
17. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
18. Stadelmaier DM, Lowe WR, Ilahi OA, Noble PC, Kohl HW 3rd. Cyclic pull-out strength of hamstring tendon graft fixation with soft tissue interference screws. Influence of screw length. Am J Sports Med. 1999;27(6):778-783.
19. Selby JB, Johnson DL, Hester P, Caborn DN. Effect of screw length on bioabsorbable interference screw fixation in a tibial bone tunnel. Am J Sports Med. 2001;29(5):614-619.
20. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
21. Simonian PT, Sussmann PS, Baldini TH, Crockett HC, Wickiewicz TL. Interference screw position and hamstring graft location for anterior cruciate ligament reconstruction. Arthroscopy. 1998;14(5):459-464.
22. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
23. Shelbourne KD, Wilckens JH. Current concepts in anterior cruciate ligament rehabilitation. Orthop Rev. 1990;19(11):957-964.
1. Garrett WE Jr, Swiontkowski MF, Weinsten JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
2. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197-207.
3. Brand J Jr, Weiler A, Caborn DN, Brown CH Jr, Johnson DL. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28(5):761-774.
4. Coleridge SD, Amis AA. A comparison of five tibial-fixation systems in hamstring-graft anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):391-397.
5. Fabbriciani C, Mulas PD, Ziranu F, Deriu L, Zarelli D, Milano G. Mechanical analysis of fixation methods for anterior cruciate ligament reconstruction with hamstring tendon graft. An experimental study in sheep knees. Knee. 2005;12(2):135-138.
6. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31(2):174-181.
7. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31(2):182-188.
8. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
9. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455-459.
10. Starch DW, Alexander JW, Noble PC, Reddy S, Lintner DM. Multistranded hamstring tendon graft fixation with a central four-quadrant or a standard tibial interference screw for anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31(3):338-344.
11. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
12. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
13. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
14. Nurmi JT, Järvinen TL, Kannus P, Sievänen H, Toukosalo J, Järvinen M. Compaction versus extraction drilling for fixation of the hamstring tendon graft in anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(2):167-173.
15. Nurmi JT, Sievänen H, Kannus P, Järvinen M, Järvinen TL. Porcine tibia is a poor substitute for human cadaver tibia for evaluating interference screw fixation. Am J Sports Med. 2004;32(3):765-771.
16. Nagarkatti DG, McKeon BP, Donahue BS, Fulkerson JP. Mechanical evaluation of a soft tissue interference screw in free tendon anterior cruciate ligament graft fixation. Am J Sports Med. 2001;29(1):67-71.
17. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
18. Stadelmaier DM, Lowe WR, Ilahi OA, Noble PC, Kohl HW 3rd. Cyclic pull-out strength of hamstring tendon graft fixation with soft tissue interference screws. Influence of screw length. Am J Sports Med. 1999;27(6):778-783.
19. Selby JB, Johnson DL, Hester P, Caborn DN. Effect of screw length on bioabsorbable interference screw fixation in a tibial bone tunnel. Am J Sports Med. 2001;29(5):614-619.
20. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
21. Simonian PT, Sussmann PS, Baldini TH, Crockett HC, Wickiewicz TL. Interference screw position and hamstring graft location for anterior cruciate ligament reconstruction. Arthroscopy. 1998;14(5):459-464.
22. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
23. Shelbourne KD, Wilckens JH. Current concepts in anterior cruciate ligament rehabilitation. Orthop Rev. 1990;19(11):957-964.