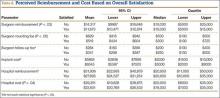
User login
Rationale for Strategic Graft Placement in Anterior Cruciate Ligament Reconstruction: I.D.E.A.L. Femoral Tunnel Position
In the United States, surgeons perform an estimated 200,000 anterior cruciate ligament reconstructions (ACLRs) each year. Over the past decade, there has been a surge in interest in defining anterior cruciate ligament (ACL) anatomy to guide ACLR. With this renewed interest in the anatomical features of the ACL, particularly the insertion site, many authors have advocated an approach for complete or near-complete “footprint restoration” for anatomical ACLR.1,2 Some have recommended a double-bundle (DB) technique that completely “fills” the footprint, but it is seldom used. Others have proposed centralizing the femoral tunnel position within the ACL footprint in the hope of capturing the function of both the anteromedial (AM) and posterolateral (PL) bundles.1,3,4 Indeed, a primary surgical goal of most anatomical ACLR techniques is creation of a femoral tunnel based off the anatomical centrum (center point) of the ACL femoral footprint.3,5 With a single-bundle technique, the femoral socket is localized in the center of the entire footprint; with a DB technique, sockets are created in the centrums of both the AM and PL bundles.
Because of the complex shape of the native ACL, however, the strategy of restoring the femoral footprint with use of either a central tunnel or a DB approach has been challenged. The femoral footprint is 3.5 times larger than the midsubstance of the ACL.6 Detailed anatomical dissections have recently demonstrated that the femoral origin of the ACL has a stout anterior band of fibers with a fanlike extension posteriorly.7 As the ACL fibers extend off the bony footprint, they form a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick.2,8 Within this structure, there is no clear separation of the AM and PL bundles. The presence of this structure makes sense given the anatomical constraints inherent in the notch. Indeed, the space for the native ACL is narrow, as the posterior cruciate ligament (PCL) occupies that largest portion of the notch with the knee in full extension, leaving only a thin, 5-mm slot through which the ACL must pass.9 Therefore, filling the femoral footprint with a tubular ACL graft probably does not reproduce the dynamic 3-dimensional morphology of the ACL.
In light of the discrepancy between the sizes of the femoral footprint and the midsubstance of the native ACL, it seems reasonable that optimizing the position of the ACL femoral tunnel may be more complex than simply centralizing the tunnel within the footprint or attempting to maximize footprint coverage. In this article, we amalgamate the lessons of 4 decades of ACL research into 5 points for strategic femoral tunnel positioning, based on anatomical, histologic, isometric, biomechanical, and clinical data. These points are summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension.
1. Anatomy Considerations
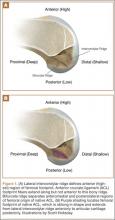
In response to study results demonstrating that some transtibial ACLRs were associated with nonanatomical placement of the femoral tunnel—resulting in vertical graft placement, PCL impingement, and recurrent rotational instability10-16—investigators have reexamined both the anatomy of the femoral origin of the native ACL and the ACL graft. Specifically, a large body of research has been devoted to characterizing the osseous landmarks of the femoral origin of the ACL17 and the dimensions of the femoral footprint.3 In addition, authors have supported the concept that the ACL contains 2 functional bundles, AM and PL.5,17 Several osseous landmarks have been identified as defining the boundaries of the femoral footprint. The lateral intercondylar ridge is the most anterior aspect of the femoral footprint and was first defined by Clancy.18 More recently, the lateral bifurcate ridge, which separates the AM and PL bundle insertion sites, was described19 (Figure 1A).
These osseous ridges delineate the location of the femoral footprint. Studies have shown that ACL fibers attach from the lateral intercondylar ridge on the anterior border of the femoral footprint and extend posteriorly to the cartilage of the lateral femoral condyle (Figure 1B).
ACL fibers from this oblong footprint are organized such that the midsubstance of the ACL is narrower than the femoral footprint. Anatomical dissections have demonstrated that, though the femoral footprint is oval, the native ACL forms a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick as it takes off from the bone.8,20 There is a resulting discrepancy between the femoral footprint size and shape and the morphology of the native ACL, and placing a tunnel in the center of the footprint or “filling the footprint” with ACL graft may not reproduce the morphology or function of the native ACL. Given this size mismatch, strategic decisions need to be made to place the femoral tunnel in a specific region of the femoral footprint to optimize its function.
2. Histologic Findings
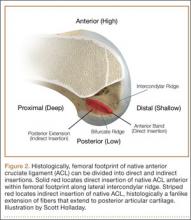
Histologic analysis has further clarified the relationship between the femoral footprint and functional aspects of the native ACL. The femoral origin of the ACL has distinct direct and indirect insertions, as demonstrated by histology and 3-dimensional volume-rendered computed tomography.21 The direct insertion consists of dense collagen fibers anterior in the footprint that is attached to a bony depression immediately posterior to the lateral intercondylar ridge.19 Sasaki and colleagues22 found that these direct fibers extended a mean (SD) of 5.3 (1.1) mm posteriorly but did not continue to the posterior femoral articular cartilage. The indirect insertion consists of more posterior collagen fibers that extend to and blend into the articular cartilage of the posterior aspect of the lateral femoral condyle. Mean (SD) width of this membrane-like tissue, located between the direct insertion and the posterior femoral articular cartilage, was found by Sasaki and colleagues22 to be 4.4 (0.5) mm anteroposteriorly(Figure 2). This anterior band of ACL tissue with the direct insertion histologically corresponds to the fibers in the anterior, more isometric region of the femoral footprint. Conversely, the more posterior band of fibers with its indirect insertion histologically corresponds to the more anisometric region and is seen macroscopically as a fanlike projection extending to the posterior articular cartilage.7
The dense collagen fibers of the direct insertion and the more membrane-like indirect insertion regions of the femoral footprint of the native ACL suggest that these regions have different load-sharing characteristics. The direct fibers of the insertion form a firm, fixed attachment that allows for gradual load distribution into the subchondral bone. From a biomechanical point of view, this attachment is extremely important, a key ligament–bone link transmitting mechanical load to the joint.23 A recent kinematic analysis revealed that the indirect fibers in the posterior region of the footprint, adjacent to the posterior articular cartilage, contribute minimally to restraint of tibial translation and rotations during stability examination.24 This suggests it may be strategically wise to place a tunnel in the direct insertion region of the footprint—eccentrically anterior (high) in the footprint rather than in the centrum.
3. Isometric Considerations
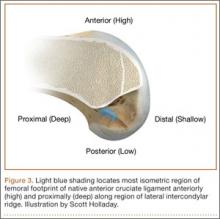
Forty years ago, Artmann and Wirth25 reported that a nearly isometric region existed in the femur such that there is minimal elongation of the native ACL during knee motion. The biomechanical rationale for choosing an isometric region of an ACL graft is that it will maintain function throughout the range of flexion and extension. A nonisometric graft would be expected to slacken during a large portion of the flexion cycle and not restrain anterior translation of the tibia, or, if fixed at the wrong flexion angle, it could capture the knee and cause graft failure by excessive tension. These 2 theoretical undesirable effects from nonisometric graft placement are supported by many experimental and clinical studies demonstrating that nonisometric femoral tunnel placement at time of surgery can cause recurrent anterior laxity of the knee.26-28 Multiple studies have further clarified that the isometric characteristics of an ACL graft are largely determined by femoral positioning. The most isometric region of the femoral footprint is consistently shown to be localized eccentrically within the footprint, in a relatively narrow bandlike region that is proximal (deep) and anterior (along the lateral intercondylar ridge within the footprint)19,29,30 (Figure 3).
A large body of literature has demonstrated that a tunnel placed in the center of the femoral footprint is less isometric than a tunnel in the more anterior region.25,29,31,32 Indeed, the anterior position (high in the footprint) identified by Hefzy and colleagues29 demonstrated minimal anisometry with 1 to 4 mm of length change through the range of motion. In contrast, a central tunnel would be expected to demonstrate 5 to 7 mm of length change, whereas a lower graft (in the PL region of the footprint) would demonstrate about 1 cm of length change through the range of motion.31,32 As such, central grafts, or grafts placed in the PL portion of the femoral footprint, would be expected to see high tension or graft forces as the knee is flexed, or to lose tension completely if the graft is fixed at full extension.32
Importantly, Markolf and colleagues33 reported that the native ACL does not behave exactly in a so-called isometric fashion during the last 30° of extension. They showed that about 3 mm of retraction of a trial wire into the joint during the last 30° of extension (as measured with an isometer) is reasonable to achieve graft length changes approximating those of the intact ACL. Given this important caveat, a primary goal for ACLR is placement of the femoral tunnel within this isometric region so that the length change in the ACL graft is minimized to 3 mm from 30° to full flexion. In addition, results of a time-zero biomechanical study suggested better rotational control with anatomical femoral tunnel position than with an isometric femoral tunnel34 placed outside the femoral footprint. Therefore, maximizing isometry alone is not the goal; placing the graft in the most isometric region within the anatomical femoral footprint is desired. This isometric region in the footprint is in the histologic region that corresponds to the direct fibers. Again, this region is eccentrically located in the anterior (high) and proximal (deep) portion of the footprint.
4. Biomechanical Considerations
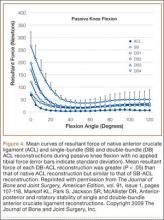
Multiple cadaveric studies have investigated the relationship between femoral tunnel positioning and time-zero stability. These studies often demonstrated superior time-zero control of knee stability, particularly in pivot type maneuvers, with a femoral tunnel placed more centrally in the femoral footprint than with a tunnel placed outside the footprint.34-37 However, an emerging body of literature is finding no significant difference in time-zero stability between an anteriorly placed femoral tunnel within the anatomical footprint (eccentrically located in the footprint) and a centrally placed graft.38,39 Returning to the more isometric tunnel position, still within the femoral footprint, would be expected to confer the benefits of an anatomically based graft position with the advantageous profile of improved isometry, as compared with a centrally placed or PL graft. Biomechanical studies40 have documented that ACL graft fibers placed posteriorly (low) in the footprint cause high graft forces in extension and, in some cases, graft rupture (Figure 4). Accordingly, the importance of reconstructing the posterior region of the footprint to better control time-zero stability is questioned.41
In addition to time-zero control of the stability examination, restoring the low tension-flexion pattern in the ACL graft to replicate the tension-flexion behavior of the native ACL is a fundamental biomechanical principle of ACLR.15,33,42,43 These studies have demonstrated that a femoral tunnel localized anterior (high) and proximal (deep) within the footprint better replicates the tension-flexion behavior of the native ACL, as compared with strategies that attempt to anatomically “fill the footprint.”40 Together, these studies have demonstrated that an eccentric position in the footprint, in the anterior (high) and proximal (deep) region, not only maximizes isometry and restores the direct fibers, but provides favorable time-zero stability and a low tension-flexion pattern biomechanically, particularly as compared with a tunnel in the more central or posterior region of the footprint.
5. Clinical Data
Clinical studies of the traditional transtibial ACLR have shown good results.44,45 However, when the tibial tunnel in the coronal plane was drilled vertical with respect to the medial joint line of the tibia, the transtibially placed femoral tunnel migrated anterior to the anatomical femoral footprint, often on the roof of the notch.10,14 This nonanatomical, vertical placement of the femoral tunnel led to failed normalization of knee kinematics.46-50 Indeed, a higher tension-flexion pattern was found in this nonanatomical “roof” position for the femoral tunnel as compared with the native ACL—a pattern that can result in either loss of flexion or recurrent instability.13,15,51
Clinical results of techniques used to create an anatomical ACLR centrally within the footprint have been mixed. Registry data showed that the revision rate at 4 years was higher with the AM portal technique (5.16%) than with transtibial drilling (3.20%).52 This higher rate may be associated with the more central placement of the femoral tunnel with the AM portal technique than with the transtibial technique, as shown in vivo with high-resolution magnetic resonance imaging.12 Recent reports have documented a higher rate of failure with DB or central ACLR approaches than with traditional transtibial techniques.53 As mentioned, in contrast to a more isometric position, a central femoral tunnel position would be expected to demonstrate 5 to 7 mm of length change, whereas moving the graft more posterior in the footprint (closer to the articular cartilage) would result in more than 1 cm of length change through the range of motion.31,32 As such, these more central grafts, or grafts placed even lower (more posterior) in the footprint, would be expected to see high tension in extension (if fixed in flexion), or to lose tension completely during flexion (if the graft is fixed at full extension).32 This may be a mechanistic cause of the high failure rate in the more posterior bundles of the DB approach.54
Together, these clinical data suggest that the femoral tunnel should be placed within the anatomical footprint of the ACL. However, within the footprint, a more eccentric femoral tunnel position capturing the isometric and direct region of the insertion may be preferable to a more central or posterior (low region) position.
Summary
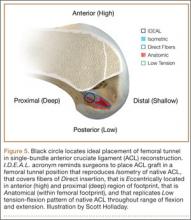
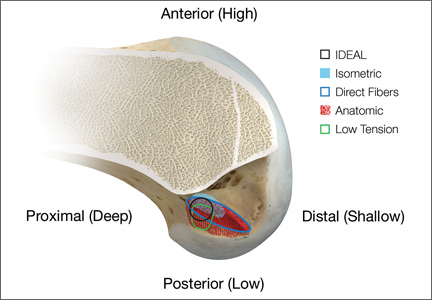
Anatomical, histologic, isometric, biomechanical, and clinical data from more than 4 decades collectively point to an optimal position for the femoral tunnel within the femoral footprint. This position can be summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension (Figure 5).
In vivo and in vitro studies as well as surgical experience suggest a need to avoid both (a) the nonanatomical vertical (roof) femoral tunnel placement that causes PCL impingement, high tension in the ACL graft in flexion, and ultimately graft stretch-out with instability and (b) the femoral tunnel placement in the posterior (lowest) region of the footprint that causes high tension in extension and can result in graft stretch-out with instability.13,15,39,40 The transtibial and AM portal techniques can both be effective in properly placing the femoral tunnel and restoring motion, stability, and function to the knee. Their effectiveness, however, depends on correct placement of the femoral tunnel. We think coming studies will focus on single-bundle ACLR and will be designed to improve the reliability of the transtibial and AM portal techniques for placing a femoral tunnel in keeping with the principles summarized by the I.D.E.A.L. acronym.
1. Siebold R. The concept of complete footprint restoration with guidelines for single- and double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):699-706.
2. Siebold R, Schuhmacher P. Restoration of the tibial ACL footprint area and geometry using the modified insertion site table. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1845-1849.
3. Piefer JW, Pflugner TR, Hwang MD, Lubowitz JH. Anterior cruciate ligament femoral footprint anatomy: systematic review of the 21st century literature. Arthroscopy. 2012;28(6):872-881.
4. Wilson AJ, Yasen SK, Nancoo T, Stannard R, Smith JO, Logan JS. Anatomic all-inside anterior cruciate ligament reconstruction using the translateral technique. Arthrosc Tech. 2013;2(2):e99-e104.
5. Colombet P, Robinson J, Christel P, et al. Morphology of anterior cruciate ligament attachments for anatomic reconstruction: a cadaveric dissection and radiographic study. Arthroscopy. 2006;22(9):984-992.
6. Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15(7):741-749.
7. Mochizuki T, Fujishiro H, Nimura A, et al. Anatomic and histologic analysis of the mid-substance and fan-like extension fibres of the anterior cruciate ligament during knee motion, with special reference to the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):336-344.
8. Siebold R, Schuhmacher P, Fernandez F, et al. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site [published correction appears in Knee Surg Sports Traumatol Arthrosc. 2014 Aug 23. Epub ahead of print]. Knee Surg Sports Traumatol Arthrosc. 2014 May 20. [Epub ahead of print]
9. Triantafyllidi E, Paschos NK, Goussia A, et al. The shape and the thickness of the anterior cruciate ligament along its length in relation to the posterior cruciate ligament: a cadaveric study. Arthroscopy. 2013;29(12):1963-1973.
10. Arnold MP, Kooloos J, van Kampen A. Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):194-199.
11. Ayerza MA, Múscolo DL, Costa-Paz M, Makino A, Rondón L. Comparison of sagittal obliquity of the reconstructed anterior cruciate ligament with native anterior cruciate ligament using magnetic resonance imaging. Arthroscopy. 2003;19(3):257-261.
12. Bowers AL, Bedi A, Lipman JD, et al. Comparison of anterior cruciate ligament tunnel position and graft obliquity with transtibial and anteromedial portal femoral tunnel reaming techniques using high-resolution magnetic resonance imaging. Arthroscopy. 2011;27(11):1511-1522.
13. Howell SM, Gittins ME, Gottlieb JE, Traina SM, Zoellner TM. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29(5):567-574.
14. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
15. Simmons R, Howell SM, Hull ML. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. J Bone Joint Surg Am. 2003;85(6):1018-1029.
16. Stanford FC, Kendoff D, Warren RF, Pearle AD. Native anterior cruciate ligament obliquity versus anterior cruciate ligament graft obliquity: an observational study using navigated measurements. Am J Sports Med. 2009;37(1):114-119.
17. Ferretti M, Ekdahl M, Shen W, Fu FH. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy. 2007;23(11):1218-1225.
18. Hutchinson MR, Ash SA. Resident’s ridge: assessing the cortical thickness of the lateral wall and roof of the intercondylar notch. Arthroscopy. 2003;19(9):931-935.
19. Fu FH, Jordan SS. The lateral intercondylar ridge—a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89(10):2103-2104.
20. Smigielski R, Zdanowicz U, Drwięga M, Ciszek B, Ciszkowska-Łysoń B, Siebold R. Ribbon like appearance of the midsubstance fibres of the anterior cruciate ligament close to its femoral insertion site: a cadaveric study including 111 knees. Knee Surg Sports Traumatol Arthrosc. 2014 Jun 28. [Epub ahead of print]
21. Iwahashi T, Shino K, Nakata K, et al. Direct anterior cruciate ligament insertion to the femur assessed by histology and 3-dimensional volume-rendered computed tomography. Arthroscopy. 2010;26(9 suppl):S13-S20.
22. Sasaki N, Ishibashi Y, Tsuda E, et al. The femoral insertion of the anterior cruciate ligament: discrepancy between macroscopic and histological observations. Arthroscopy. 2012;28(8):1135-1146.
23. Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The “enthesis organ” concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50(10):3306-3313.
24. Pathare NP, Nicholas SJ, Colbrunn R, McHugh MP. Kinematic analysis of the indirect femoral insertion of the anterior cruciate ligament: implications for anatomic femoral tunnel placement. Arthroscopy. 2014;30(11):1430-1438.
25. Artmann M, Wirth CJ. Investigation of the appropriate functional replacement of the anterior cruciate ligament (author’s transl) [in German]. Z Orthop Ihre Grenzgeb. 1974;112(1):160-165.
26. Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;(6 suppl 1):S2-S12.
27. Beynnon BD, Uh BS, Johnson RJ, Fleming BC, Renström PA, Nichols CE. The elongation behavior of the anterior cruciate ligament graft in vivo. A long-term follow-up study. Am J Sports Med. 2001;29(2):161-166.
28. O’Meara PM, O’Brien WR, Henning CE. Anterior cruciate ligament reconstruction stability with continuous passive motion. The role of isometric graft placement. Clin Orthop. 1992;(277):201-209.
29. Hefzy MS, Grood ES, Noyes FR. Factors affecting the region of most isometric femoral attachments. Part II: the anterior cruciate ligament. Am J Sports Med. 1989;17(2):208-216.
30. Zavras TD, Race A, Bull AM, Amis AA. A comparative study of ‘isometric’ points for anterior cruciate ligament graft attachment. Knee Surg Sports Traumatol Arthrosc. 2001;9(1):28-33.
31. Pearle AD, Shannon FJ, Granchi C, Wickiewicz TL, Warren RF. Comparison of 3-dimensional obliquity and anisometric characteristics of anterior cruciate ligament graft positions using surgical navigation. Am J Sports Med. 2008;36(8):1534-1541.
32. Lubowitz JH. Anatomic ACL reconstruction produces greater graft length change during knee range-of-motion than transtibial technique. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1190-1195.
33. Markolf KL, Burchfield DM, Shapiro MM, Davis BR, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part I: insertion of the graft and anterior-posterior testing. J Bone Joint Surg Am. 1996;78(11):1720-1727.
34. Musahl V, Plakseychuk A, VanScyoc A, et al. Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med. 2005;33(5):712-718.
35. Bedi A, Musahl V, Steuber V, et al. Transtibial versus anteromedial portal reaming in anterior cruciate ligament reconstruction: an anatomic and biomechanical evaluation of surgical technique. Arthroscopy. 2011;27(3):380-390.
36. Lim HC, Yoon YC, Wang JH, Bae JH. Anatomical versus non-anatomical single bundle anterior cruciate ligament reconstruction: a cadaveric study of comparison of knee stability. Clin Orthop Surg. 2012;4(4):249-255.
37. Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy. 2003;19(3):297-304.
38. Cross MB, Musahl V, Bedi A, et al. Anteromedial versus central single-bundle graft position: which anatomic graft position to choose? Knee Surg Sports Traumatol Arthrosc. 2012;20(7):1276-1281.
39. Markolf KL, Jackson SR, McAllister DR. A comparison of 11 o’clock versus oblique femoral tunnels in the anterior cruciate ligament–reconstructed knee: knee kinematics during a simulated pivot test. Am J Sports Med. 2010;38(5):912-917.
40. Markolf KL, Park S, Jackson SR, McAllister DR. Anterior-posterior and rotatory stability of single and double-bundle anterior cruciate ligament reconstructions. J Bone Joint Surg Am. 2009;91(1):107-118.
41. Markolf KL, Park S, Jackson SR, McAllister DR. Contributions of the posterolateral bundle of the anterior cruciate ligament to anterior-posterior knee laxity and ligament forces. Arthroscopy. 2008;24(7):805-809.
42. Markolf KL, Burchfield DM, Shapiro MM, Cha CW, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part II: forces in the graft compared with forces in the intact ligament. J Bone Joint Surg Am. 1996;78(11):1728-1734.
43. Wallace MP, Howell SM, Hull ML. In vivo tensile behavior of a four-bundle hamstring graft as a replacement for the anterior cruciate ligament. J Orthop Res. 1997;15(4):539-545.
44. Harner CD, Marks PH, Fu FH, Irrgang JJ, Silby MB, Mengato R. Anterior cruciate ligament reconstruction: endoscopic versus two-incision technique. Arthroscopy. 1994;10(5):502-512.
45. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision technique for reconstructing a torn anterior cruciate ligament using hamstring tendons. J Arthroscopy. 1999;15(6):594-606.
46. Chouliaras V, Ristanis S, Moraiti C, Stergiou N, Georgoulis AD. Effectiveness of reconstruction of the anterior cruciate ligament with quadrupled hamstrings and bone–patellar tendon–bone autografts: an in vivo study comparing tibial internal–external rotation. Am J Sports Med. 2007;35(2):189-196.
47. Logan MC, Williams A, Lavelle J, Gedroyc W, Freeman M. Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. Am J Sports Med. 2004;32(4):984-992.
48. Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34(12):2006-2012.
49. Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975-983.
50. Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop. 2007;(454):66-73.
51. Wallace MP, Hull ML, Howell SM. Can an isometer predict the tensile behavior of a double-looped hamstring graft during anterior cruciate ligament reconstruction? J Orthop Res. 1998;16(3):386-393.
52. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind MC. Increased risk of revision after anteromedial compared with transtibial drilling of the femoral tunnel during primary anterior cruciate ligament reconstruction: results from the Danish Knee Ligament Reconstruction Register. Arthroscopy. 2013;29(1):98-105.
53. van Eck CF, Schkrohowsky JG, Working ZM, Irrgang JJ, Fu FH. Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med. 2012;40(4):800-807.
54. Ahn JH, Choi SH, Wang JH, Yoo JC, Yim HS, Chang MJ. Outcomes and second-look arthroscopic evaluation after double-bundle anterior cruciate ligament reconstruction with use of a single tibial tunnel. J Bone Joint Surg Am. 2011;93(20):1865-1872.
In the United States, surgeons perform an estimated 200,000 anterior cruciate ligament reconstructions (ACLRs) each year. Over the past decade, there has been a surge in interest in defining anterior cruciate ligament (ACL) anatomy to guide ACLR. With this renewed interest in the anatomical features of the ACL, particularly the insertion site, many authors have advocated an approach for complete or near-complete “footprint restoration” for anatomical ACLR.1,2 Some have recommended a double-bundle (DB) technique that completely “fills” the footprint, but it is seldom used. Others have proposed centralizing the femoral tunnel position within the ACL footprint in the hope of capturing the function of both the anteromedial (AM) and posterolateral (PL) bundles.1,3,4 Indeed, a primary surgical goal of most anatomical ACLR techniques is creation of a femoral tunnel based off the anatomical centrum (center point) of the ACL femoral footprint.3,5 With a single-bundle technique, the femoral socket is localized in the center of the entire footprint; with a DB technique, sockets are created in the centrums of both the AM and PL bundles.
Because of the complex shape of the native ACL, however, the strategy of restoring the femoral footprint with use of either a central tunnel or a DB approach has been challenged. The femoral footprint is 3.5 times larger than the midsubstance of the ACL.6 Detailed anatomical dissections have recently demonstrated that the femoral origin of the ACL has a stout anterior band of fibers with a fanlike extension posteriorly.7 As the ACL fibers extend off the bony footprint, they form a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick.2,8 Within this structure, there is no clear separation of the AM and PL bundles. The presence of this structure makes sense given the anatomical constraints inherent in the notch. Indeed, the space for the native ACL is narrow, as the posterior cruciate ligament (PCL) occupies that largest portion of the notch with the knee in full extension, leaving only a thin, 5-mm slot through which the ACL must pass.9 Therefore, filling the femoral footprint with a tubular ACL graft probably does not reproduce the dynamic 3-dimensional morphology of the ACL.
In light of the discrepancy between the sizes of the femoral footprint and the midsubstance of the native ACL, it seems reasonable that optimizing the position of the ACL femoral tunnel may be more complex than simply centralizing the tunnel within the footprint or attempting to maximize footprint coverage. In this article, we amalgamate the lessons of 4 decades of ACL research into 5 points for strategic femoral tunnel positioning, based on anatomical, histologic, isometric, biomechanical, and clinical data. These points are summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension.
1. Anatomy Considerations
In response to study results demonstrating that some transtibial ACLRs were associated with nonanatomical placement of the femoral tunnel—resulting in vertical graft placement, PCL impingement, and recurrent rotational instability10-16—investigators have reexamined both the anatomy of the femoral origin of the native ACL and the ACL graft. Specifically, a large body of research has been devoted to characterizing the osseous landmarks of the femoral origin of the ACL17 and the dimensions of the femoral footprint.3 In addition, authors have supported the concept that the ACL contains 2 functional bundles, AM and PL.5,17 Several osseous landmarks have been identified as defining the boundaries of the femoral footprint. The lateral intercondylar ridge is the most anterior aspect of the femoral footprint and was first defined by Clancy.18 More recently, the lateral bifurcate ridge, which separates the AM and PL bundle insertion sites, was described19 (Figure 1A).
These osseous ridges delineate the location of the femoral footprint. Studies have shown that ACL fibers attach from the lateral intercondylar ridge on the anterior border of the femoral footprint and extend posteriorly to the cartilage of the lateral femoral condyle (Figure 1B).
ACL fibers from this oblong footprint are organized such that the midsubstance of the ACL is narrower than the femoral footprint. Anatomical dissections have demonstrated that, though the femoral footprint is oval, the native ACL forms a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick as it takes off from the bone.8,20 There is a resulting discrepancy between the femoral footprint size and shape and the morphology of the native ACL, and placing a tunnel in the center of the footprint or “filling the footprint” with ACL graft may not reproduce the morphology or function of the native ACL. Given this size mismatch, strategic decisions need to be made to place the femoral tunnel in a specific region of the femoral footprint to optimize its function.
2. Histologic Findings
Histologic analysis has further clarified the relationship between the femoral footprint and functional aspects of the native ACL. The femoral origin of the ACL has distinct direct and indirect insertions, as demonstrated by histology and 3-dimensional volume-rendered computed tomography.21 The direct insertion consists of dense collagen fibers anterior in the footprint that is attached to a bony depression immediately posterior to the lateral intercondylar ridge.19 Sasaki and colleagues22 found that these direct fibers extended a mean (SD) of 5.3 (1.1) mm posteriorly but did not continue to the posterior femoral articular cartilage. The indirect insertion consists of more posterior collagen fibers that extend to and blend into the articular cartilage of the posterior aspect of the lateral femoral condyle. Mean (SD) width of this membrane-like tissue, located between the direct insertion and the posterior femoral articular cartilage, was found by Sasaki and colleagues22 to be 4.4 (0.5) mm anteroposteriorly(Figure 2). This anterior band of ACL tissue with the direct insertion histologically corresponds to the fibers in the anterior, more isometric region of the femoral footprint. Conversely, the more posterior band of fibers with its indirect insertion histologically corresponds to the more anisometric region and is seen macroscopically as a fanlike projection extending to the posterior articular cartilage.7
The dense collagen fibers of the direct insertion and the more membrane-like indirect insertion regions of the femoral footprint of the native ACL suggest that these regions have different load-sharing characteristics. The direct fibers of the insertion form a firm, fixed attachment that allows for gradual load distribution into the subchondral bone. From a biomechanical point of view, this attachment is extremely important, a key ligament–bone link transmitting mechanical load to the joint.23 A recent kinematic analysis revealed that the indirect fibers in the posterior region of the footprint, adjacent to the posterior articular cartilage, contribute minimally to restraint of tibial translation and rotations during stability examination.24 This suggests it may be strategically wise to place a tunnel in the direct insertion region of the footprint—eccentrically anterior (high) in the footprint rather than in the centrum.
3. Isometric Considerations
Forty years ago, Artmann and Wirth25 reported that a nearly isometric region existed in the femur such that there is minimal elongation of the native ACL during knee motion. The biomechanical rationale for choosing an isometric region of an ACL graft is that it will maintain function throughout the range of flexion and extension. A nonisometric graft would be expected to slacken during a large portion of the flexion cycle and not restrain anterior translation of the tibia, or, if fixed at the wrong flexion angle, it could capture the knee and cause graft failure by excessive tension. These 2 theoretical undesirable effects from nonisometric graft placement are supported by many experimental and clinical studies demonstrating that nonisometric femoral tunnel placement at time of surgery can cause recurrent anterior laxity of the knee.26-28 Multiple studies have further clarified that the isometric characteristics of an ACL graft are largely determined by femoral positioning. The most isometric region of the femoral footprint is consistently shown to be localized eccentrically within the footprint, in a relatively narrow bandlike region that is proximal (deep) and anterior (along the lateral intercondylar ridge within the footprint)19,29,30 (Figure 3).
A large body of literature has demonstrated that a tunnel placed in the center of the femoral footprint is less isometric than a tunnel in the more anterior region.25,29,31,32 Indeed, the anterior position (high in the footprint) identified by Hefzy and colleagues29 demonstrated minimal anisometry with 1 to 4 mm of length change through the range of motion. In contrast, a central tunnel would be expected to demonstrate 5 to 7 mm of length change, whereas a lower graft (in the PL region of the footprint) would demonstrate about 1 cm of length change through the range of motion.31,32 As such, central grafts, or grafts placed in the PL portion of the femoral footprint, would be expected to see high tension or graft forces as the knee is flexed, or to lose tension completely if the graft is fixed at full extension.32
Importantly, Markolf and colleagues33 reported that the native ACL does not behave exactly in a so-called isometric fashion during the last 30° of extension. They showed that about 3 mm of retraction of a trial wire into the joint during the last 30° of extension (as measured with an isometer) is reasonable to achieve graft length changes approximating those of the intact ACL. Given this important caveat, a primary goal for ACLR is placement of the femoral tunnel within this isometric region so that the length change in the ACL graft is minimized to 3 mm from 30° to full flexion. In addition, results of a time-zero biomechanical study suggested better rotational control with anatomical femoral tunnel position than with an isometric femoral tunnel34 placed outside the femoral footprint. Therefore, maximizing isometry alone is not the goal; placing the graft in the most isometric region within the anatomical femoral footprint is desired. This isometric region in the footprint is in the histologic region that corresponds to the direct fibers. Again, this region is eccentrically located in the anterior (high) and proximal (deep) portion of the footprint.
4. Biomechanical Considerations
Multiple cadaveric studies have investigated the relationship between femoral tunnel positioning and time-zero stability. These studies often demonstrated superior time-zero control of knee stability, particularly in pivot type maneuvers, with a femoral tunnel placed more centrally in the femoral footprint than with a tunnel placed outside the footprint.34-37 However, an emerging body of literature is finding no significant difference in time-zero stability between an anteriorly placed femoral tunnel within the anatomical footprint (eccentrically located in the footprint) and a centrally placed graft.38,39 Returning to the more isometric tunnel position, still within the femoral footprint, would be expected to confer the benefits of an anatomically based graft position with the advantageous profile of improved isometry, as compared with a centrally placed or PL graft. Biomechanical studies40 have documented that ACL graft fibers placed posteriorly (low) in the footprint cause high graft forces in extension and, in some cases, graft rupture (Figure 4). Accordingly, the importance of reconstructing the posterior region of the footprint to better control time-zero stability is questioned.41
In addition to time-zero control of the stability examination, restoring the low tension-flexion pattern in the ACL graft to replicate the tension-flexion behavior of the native ACL is a fundamental biomechanical principle of ACLR.15,33,42,43 These studies have demonstrated that a femoral tunnel localized anterior (high) and proximal (deep) within the footprint better replicates the tension-flexion behavior of the native ACL, as compared with strategies that attempt to anatomically “fill the footprint.”40 Together, these studies have demonstrated that an eccentric position in the footprint, in the anterior (high) and proximal (deep) region, not only maximizes isometry and restores the direct fibers, but provides favorable time-zero stability and a low tension-flexion pattern biomechanically, particularly as compared with a tunnel in the more central or posterior region of the footprint.
5. Clinical Data
Clinical studies of the traditional transtibial ACLR have shown good results.44,45 However, when the tibial tunnel in the coronal plane was drilled vertical with respect to the medial joint line of the tibia, the transtibially placed femoral tunnel migrated anterior to the anatomical femoral footprint, often on the roof of the notch.10,14 This nonanatomical, vertical placement of the femoral tunnel led to failed normalization of knee kinematics.46-50 Indeed, a higher tension-flexion pattern was found in this nonanatomical “roof” position for the femoral tunnel as compared with the native ACL—a pattern that can result in either loss of flexion or recurrent instability.13,15,51
Clinical results of techniques used to create an anatomical ACLR centrally within the footprint have been mixed. Registry data showed that the revision rate at 4 years was higher with the AM portal technique (5.16%) than with transtibial drilling (3.20%).52 This higher rate may be associated with the more central placement of the femoral tunnel with the AM portal technique than with the transtibial technique, as shown in vivo with high-resolution magnetic resonance imaging.12 Recent reports have documented a higher rate of failure with DB or central ACLR approaches than with traditional transtibial techniques.53 As mentioned, in contrast to a more isometric position, a central femoral tunnel position would be expected to demonstrate 5 to 7 mm of length change, whereas moving the graft more posterior in the footprint (closer to the articular cartilage) would result in more than 1 cm of length change through the range of motion.31,32 As such, these more central grafts, or grafts placed even lower (more posterior) in the footprint, would be expected to see high tension in extension (if fixed in flexion), or to lose tension completely during flexion (if the graft is fixed at full extension).32 This may be a mechanistic cause of the high failure rate in the more posterior bundles of the DB approach.54
Together, these clinical data suggest that the femoral tunnel should be placed within the anatomical footprint of the ACL. However, within the footprint, a more eccentric femoral tunnel position capturing the isometric and direct region of the insertion may be preferable to a more central or posterior (low region) position.
Summary
Anatomical, histologic, isometric, biomechanical, and clinical data from more than 4 decades collectively point to an optimal position for the femoral tunnel within the femoral footprint. This position can be summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension (Figure 5).
In vivo and in vitro studies as well as surgical experience suggest a need to avoid both (a) the nonanatomical vertical (roof) femoral tunnel placement that causes PCL impingement, high tension in the ACL graft in flexion, and ultimately graft stretch-out with instability and (b) the femoral tunnel placement in the posterior (lowest) region of the footprint that causes high tension in extension and can result in graft stretch-out with instability.13,15,39,40 The transtibial and AM portal techniques can both be effective in properly placing the femoral tunnel and restoring motion, stability, and function to the knee. Their effectiveness, however, depends on correct placement of the femoral tunnel. We think coming studies will focus on single-bundle ACLR and will be designed to improve the reliability of the transtibial and AM portal techniques for placing a femoral tunnel in keeping with the principles summarized by the I.D.E.A.L. acronym.
In the United States, surgeons perform an estimated 200,000 anterior cruciate ligament reconstructions (ACLRs) each year. Over the past decade, there has been a surge in interest in defining anterior cruciate ligament (ACL) anatomy to guide ACLR. With this renewed interest in the anatomical features of the ACL, particularly the insertion site, many authors have advocated an approach for complete or near-complete “footprint restoration” for anatomical ACLR.1,2 Some have recommended a double-bundle (DB) technique that completely “fills” the footprint, but it is seldom used. Others have proposed centralizing the femoral tunnel position within the ACL footprint in the hope of capturing the function of both the anteromedial (AM) and posterolateral (PL) bundles.1,3,4 Indeed, a primary surgical goal of most anatomical ACLR techniques is creation of a femoral tunnel based off the anatomical centrum (center point) of the ACL femoral footprint.3,5 With a single-bundle technique, the femoral socket is localized in the center of the entire footprint; with a DB technique, sockets are created in the centrums of both the AM and PL bundles.
Because of the complex shape of the native ACL, however, the strategy of restoring the femoral footprint with use of either a central tunnel or a DB approach has been challenged. The femoral footprint is 3.5 times larger than the midsubstance of the ACL.6 Detailed anatomical dissections have recently demonstrated that the femoral origin of the ACL has a stout anterior band of fibers with a fanlike extension posteriorly.7 As the ACL fibers extend off the bony footprint, they form a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick.2,8 Within this structure, there is no clear separation of the AM and PL bundles. The presence of this structure makes sense given the anatomical constraints inherent in the notch. Indeed, the space for the native ACL is narrow, as the posterior cruciate ligament (PCL) occupies that largest portion of the notch with the knee in full extension, leaving only a thin, 5-mm slot through which the ACL must pass.9 Therefore, filling the femoral footprint with a tubular ACL graft probably does not reproduce the dynamic 3-dimensional morphology of the ACL.
In light of the discrepancy between the sizes of the femoral footprint and the midsubstance of the native ACL, it seems reasonable that optimizing the position of the ACL femoral tunnel may be more complex than simply centralizing the tunnel within the footprint or attempting to maximize footprint coverage. In this article, we amalgamate the lessons of 4 decades of ACL research into 5 points for strategic femoral tunnel positioning, based on anatomical, histologic, isometric, biomechanical, and clinical data. These points are summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension.
1. Anatomy Considerations
In response to study results demonstrating that some transtibial ACLRs were associated with nonanatomical placement of the femoral tunnel—resulting in vertical graft placement, PCL impingement, and recurrent rotational instability10-16—investigators have reexamined both the anatomy of the femoral origin of the native ACL and the ACL graft. Specifically, a large body of research has been devoted to characterizing the osseous landmarks of the femoral origin of the ACL17 and the dimensions of the femoral footprint.3 In addition, authors have supported the concept that the ACL contains 2 functional bundles, AM and PL.5,17 Several osseous landmarks have been identified as defining the boundaries of the femoral footprint. The lateral intercondylar ridge is the most anterior aspect of the femoral footprint and was first defined by Clancy.18 More recently, the lateral bifurcate ridge, which separates the AM and PL bundle insertion sites, was described19 (Figure 1A).
These osseous ridges delineate the location of the femoral footprint. Studies have shown that ACL fibers attach from the lateral intercondylar ridge on the anterior border of the femoral footprint and extend posteriorly to the cartilage of the lateral femoral condyle (Figure 1B).
ACL fibers from this oblong footprint are organized such that the midsubstance of the ACL is narrower than the femoral footprint. Anatomical dissections have demonstrated that, though the femoral footprint is oval, the native ACL forms a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick as it takes off from the bone.8,20 There is a resulting discrepancy between the femoral footprint size and shape and the morphology of the native ACL, and placing a tunnel in the center of the footprint or “filling the footprint” with ACL graft may not reproduce the morphology or function of the native ACL. Given this size mismatch, strategic decisions need to be made to place the femoral tunnel in a specific region of the femoral footprint to optimize its function.
2. Histologic Findings
Histologic analysis has further clarified the relationship between the femoral footprint and functional aspects of the native ACL. The femoral origin of the ACL has distinct direct and indirect insertions, as demonstrated by histology and 3-dimensional volume-rendered computed tomography.21 The direct insertion consists of dense collagen fibers anterior in the footprint that is attached to a bony depression immediately posterior to the lateral intercondylar ridge.19 Sasaki and colleagues22 found that these direct fibers extended a mean (SD) of 5.3 (1.1) mm posteriorly but did not continue to the posterior femoral articular cartilage. The indirect insertion consists of more posterior collagen fibers that extend to and blend into the articular cartilage of the posterior aspect of the lateral femoral condyle. Mean (SD) width of this membrane-like tissue, located between the direct insertion and the posterior femoral articular cartilage, was found by Sasaki and colleagues22 to be 4.4 (0.5) mm anteroposteriorly(Figure 2). This anterior band of ACL tissue with the direct insertion histologically corresponds to the fibers in the anterior, more isometric region of the femoral footprint. Conversely, the more posterior band of fibers with its indirect insertion histologically corresponds to the more anisometric region and is seen macroscopically as a fanlike projection extending to the posterior articular cartilage.7
The dense collagen fibers of the direct insertion and the more membrane-like indirect insertion regions of the femoral footprint of the native ACL suggest that these regions have different load-sharing characteristics. The direct fibers of the insertion form a firm, fixed attachment that allows for gradual load distribution into the subchondral bone. From a biomechanical point of view, this attachment is extremely important, a key ligament–bone link transmitting mechanical load to the joint.23 A recent kinematic analysis revealed that the indirect fibers in the posterior region of the footprint, adjacent to the posterior articular cartilage, contribute minimally to restraint of tibial translation and rotations during stability examination.24 This suggests it may be strategically wise to place a tunnel in the direct insertion region of the footprint—eccentrically anterior (high) in the footprint rather than in the centrum.
3. Isometric Considerations
Forty years ago, Artmann and Wirth25 reported that a nearly isometric region existed in the femur such that there is minimal elongation of the native ACL during knee motion. The biomechanical rationale for choosing an isometric region of an ACL graft is that it will maintain function throughout the range of flexion and extension. A nonisometric graft would be expected to slacken during a large portion of the flexion cycle and not restrain anterior translation of the tibia, or, if fixed at the wrong flexion angle, it could capture the knee and cause graft failure by excessive tension. These 2 theoretical undesirable effects from nonisometric graft placement are supported by many experimental and clinical studies demonstrating that nonisometric femoral tunnel placement at time of surgery can cause recurrent anterior laxity of the knee.26-28 Multiple studies have further clarified that the isometric characteristics of an ACL graft are largely determined by femoral positioning. The most isometric region of the femoral footprint is consistently shown to be localized eccentrically within the footprint, in a relatively narrow bandlike region that is proximal (deep) and anterior (along the lateral intercondylar ridge within the footprint)19,29,30 (Figure 3).
A large body of literature has demonstrated that a tunnel placed in the center of the femoral footprint is less isometric than a tunnel in the more anterior region.25,29,31,32 Indeed, the anterior position (high in the footprint) identified by Hefzy and colleagues29 demonstrated minimal anisometry with 1 to 4 mm of length change through the range of motion. In contrast, a central tunnel would be expected to demonstrate 5 to 7 mm of length change, whereas a lower graft (in the PL region of the footprint) would demonstrate about 1 cm of length change through the range of motion.31,32 As such, central grafts, or grafts placed in the PL portion of the femoral footprint, would be expected to see high tension or graft forces as the knee is flexed, or to lose tension completely if the graft is fixed at full extension.32
Importantly, Markolf and colleagues33 reported that the native ACL does not behave exactly in a so-called isometric fashion during the last 30° of extension. They showed that about 3 mm of retraction of a trial wire into the joint during the last 30° of extension (as measured with an isometer) is reasonable to achieve graft length changes approximating those of the intact ACL. Given this important caveat, a primary goal for ACLR is placement of the femoral tunnel within this isometric region so that the length change in the ACL graft is minimized to 3 mm from 30° to full flexion. In addition, results of a time-zero biomechanical study suggested better rotational control with anatomical femoral tunnel position than with an isometric femoral tunnel34 placed outside the femoral footprint. Therefore, maximizing isometry alone is not the goal; placing the graft in the most isometric region within the anatomical femoral footprint is desired. This isometric region in the footprint is in the histologic region that corresponds to the direct fibers. Again, this region is eccentrically located in the anterior (high) and proximal (deep) portion of the footprint.
4. Biomechanical Considerations
Multiple cadaveric studies have investigated the relationship between femoral tunnel positioning and time-zero stability. These studies often demonstrated superior time-zero control of knee stability, particularly in pivot type maneuvers, with a femoral tunnel placed more centrally in the femoral footprint than with a tunnel placed outside the footprint.34-37 However, an emerging body of literature is finding no significant difference in time-zero stability between an anteriorly placed femoral tunnel within the anatomical footprint (eccentrically located in the footprint) and a centrally placed graft.38,39 Returning to the more isometric tunnel position, still within the femoral footprint, would be expected to confer the benefits of an anatomically based graft position with the advantageous profile of improved isometry, as compared with a centrally placed or PL graft. Biomechanical studies40 have documented that ACL graft fibers placed posteriorly (low) in the footprint cause high graft forces in extension and, in some cases, graft rupture (Figure 4). Accordingly, the importance of reconstructing the posterior region of the footprint to better control time-zero stability is questioned.41
In addition to time-zero control of the stability examination, restoring the low tension-flexion pattern in the ACL graft to replicate the tension-flexion behavior of the native ACL is a fundamental biomechanical principle of ACLR.15,33,42,43 These studies have demonstrated that a femoral tunnel localized anterior (high) and proximal (deep) within the footprint better replicates the tension-flexion behavior of the native ACL, as compared with strategies that attempt to anatomically “fill the footprint.”40 Together, these studies have demonstrated that an eccentric position in the footprint, in the anterior (high) and proximal (deep) region, not only maximizes isometry and restores the direct fibers, but provides favorable time-zero stability and a low tension-flexion pattern biomechanically, particularly as compared with a tunnel in the more central or posterior region of the footprint.
5. Clinical Data
Clinical studies of the traditional transtibial ACLR have shown good results.44,45 However, when the tibial tunnel in the coronal plane was drilled vertical with respect to the medial joint line of the tibia, the transtibially placed femoral tunnel migrated anterior to the anatomical femoral footprint, often on the roof of the notch.10,14 This nonanatomical, vertical placement of the femoral tunnel led to failed normalization of knee kinematics.46-50 Indeed, a higher tension-flexion pattern was found in this nonanatomical “roof” position for the femoral tunnel as compared with the native ACL—a pattern that can result in either loss of flexion or recurrent instability.13,15,51
Clinical results of techniques used to create an anatomical ACLR centrally within the footprint have been mixed. Registry data showed that the revision rate at 4 years was higher with the AM portal technique (5.16%) than with transtibial drilling (3.20%).52 This higher rate may be associated with the more central placement of the femoral tunnel with the AM portal technique than with the transtibial technique, as shown in vivo with high-resolution magnetic resonance imaging.12 Recent reports have documented a higher rate of failure with DB or central ACLR approaches than with traditional transtibial techniques.53 As mentioned, in contrast to a more isometric position, a central femoral tunnel position would be expected to demonstrate 5 to 7 mm of length change, whereas moving the graft more posterior in the footprint (closer to the articular cartilage) would result in more than 1 cm of length change through the range of motion.31,32 As such, these more central grafts, or grafts placed even lower (more posterior) in the footprint, would be expected to see high tension in extension (if fixed in flexion), or to lose tension completely during flexion (if the graft is fixed at full extension).32 This may be a mechanistic cause of the high failure rate in the more posterior bundles of the DB approach.54
Together, these clinical data suggest that the femoral tunnel should be placed within the anatomical footprint of the ACL. However, within the footprint, a more eccentric femoral tunnel position capturing the isometric and direct region of the insertion may be preferable to a more central or posterior (low region) position.
Summary
Anatomical, histologic, isometric, biomechanical, and clinical data from more than 4 decades collectively point to an optimal position for the femoral tunnel within the femoral footprint. This position can be summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension (Figure 5).
In vivo and in vitro studies as well as surgical experience suggest a need to avoid both (a) the nonanatomical vertical (roof) femoral tunnel placement that causes PCL impingement, high tension in the ACL graft in flexion, and ultimately graft stretch-out with instability and (b) the femoral tunnel placement in the posterior (lowest) region of the footprint that causes high tension in extension and can result in graft stretch-out with instability.13,15,39,40 The transtibial and AM portal techniques can both be effective in properly placing the femoral tunnel and restoring motion, stability, and function to the knee. Their effectiveness, however, depends on correct placement of the femoral tunnel. We think coming studies will focus on single-bundle ACLR and will be designed to improve the reliability of the transtibial and AM portal techniques for placing a femoral tunnel in keeping with the principles summarized by the I.D.E.A.L. acronym.
1. Siebold R. The concept of complete footprint restoration with guidelines for single- and double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):699-706.
2. Siebold R, Schuhmacher P. Restoration of the tibial ACL footprint area and geometry using the modified insertion site table. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1845-1849.
3. Piefer JW, Pflugner TR, Hwang MD, Lubowitz JH. Anterior cruciate ligament femoral footprint anatomy: systematic review of the 21st century literature. Arthroscopy. 2012;28(6):872-881.
4. Wilson AJ, Yasen SK, Nancoo T, Stannard R, Smith JO, Logan JS. Anatomic all-inside anterior cruciate ligament reconstruction using the translateral technique. Arthrosc Tech. 2013;2(2):e99-e104.
5. Colombet P, Robinson J, Christel P, et al. Morphology of anterior cruciate ligament attachments for anatomic reconstruction: a cadaveric dissection and radiographic study. Arthroscopy. 2006;22(9):984-992.
6. Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15(7):741-749.
7. Mochizuki T, Fujishiro H, Nimura A, et al. Anatomic and histologic analysis of the mid-substance and fan-like extension fibres of the anterior cruciate ligament during knee motion, with special reference to the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):336-344.
8. Siebold R, Schuhmacher P, Fernandez F, et al. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site [published correction appears in Knee Surg Sports Traumatol Arthrosc. 2014 Aug 23. Epub ahead of print]. Knee Surg Sports Traumatol Arthrosc. 2014 May 20. [Epub ahead of print]
9. Triantafyllidi E, Paschos NK, Goussia A, et al. The shape and the thickness of the anterior cruciate ligament along its length in relation to the posterior cruciate ligament: a cadaveric study. Arthroscopy. 2013;29(12):1963-1973.
10. Arnold MP, Kooloos J, van Kampen A. Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):194-199.
11. Ayerza MA, Múscolo DL, Costa-Paz M, Makino A, Rondón L. Comparison of sagittal obliquity of the reconstructed anterior cruciate ligament with native anterior cruciate ligament using magnetic resonance imaging. Arthroscopy. 2003;19(3):257-261.
12. Bowers AL, Bedi A, Lipman JD, et al. Comparison of anterior cruciate ligament tunnel position and graft obliquity with transtibial and anteromedial portal femoral tunnel reaming techniques using high-resolution magnetic resonance imaging. Arthroscopy. 2011;27(11):1511-1522.
13. Howell SM, Gittins ME, Gottlieb JE, Traina SM, Zoellner TM. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29(5):567-574.
14. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
15. Simmons R, Howell SM, Hull ML. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. J Bone Joint Surg Am. 2003;85(6):1018-1029.
16. Stanford FC, Kendoff D, Warren RF, Pearle AD. Native anterior cruciate ligament obliquity versus anterior cruciate ligament graft obliquity: an observational study using navigated measurements. Am J Sports Med. 2009;37(1):114-119.
17. Ferretti M, Ekdahl M, Shen W, Fu FH. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy. 2007;23(11):1218-1225.
18. Hutchinson MR, Ash SA. Resident’s ridge: assessing the cortical thickness of the lateral wall and roof of the intercondylar notch. Arthroscopy. 2003;19(9):931-935.
19. Fu FH, Jordan SS. The lateral intercondylar ridge—a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89(10):2103-2104.
20. Smigielski R, Zdanowicz U, Drwięga M, Ciszek B, Ciszkowska-Łysoń B, Siebold R. Ribbon like appearance of the midsubstance fibres of the anterior cruciate ligament close to its femoral insertion site: a cadaveric study including 111 knees. Knee Surg Sports Traumatol Arthrosc. 2014 Jun 28. [Epub ahead of print]
21. Iwahashi T, Shino K, Nakata K, et al. Direct anterior cruciate ligament insertion to the femur assessed by histology and 3-dimensional volume-rendered computed tomography. Arthroscopy. 2010;26(9 suppl):S13-S20.
22. Sasaki N, Ishibashi Y, Tsuda E, et al. The femoral insertion of the anterior cruciate ligament: discrepancy between macroscopic and histological observations. Arthroscopy. 2012;28(8):1135-1146.
23. Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The “enthesis organ” concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50(10):3306-3313.
24. Pathare NP, Nicholas SJ, Colbrunn R, McHugh MP. Kinematic analysis of the indirect femoral insertion of the anterior cruciate ligament: implications for anatomic femoral tunnel placement. Arthroscopy. 2014;30(11):1430-1438.
25. Artmann M, Wirth CJ. Investigation of the appropriate functional replacement of the anterior cruciate ligament (author’s transl) [in German]. Z Orthop Ihre Grenzgeb. 1974;112(1):160-165.
26. Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;(6 suppl 1):S2-S12.
27. Beynnon BD, Uh BS, Johnson RJ, Fleming BC, Renström PA, Nichols CE. The elongation behavior of the anterior cruciate ligament graft in vivo. A long-term follow-up study. Am J Sports Med. 2001;29(2):161-166.
28. O’Meara PM, O’Brien WR, Henning CE. Anterior cruciate ligament reconstruction stability with continuous passive motion. The role of isometric graft placement. Clin Orthop. 1992;(277):201-209.
29. Hefzy MS, Grood ES, Noyes FR. Factors affecting the region of most isometric femoral attachments. Part II: the anterior cruciate ligament. Am J Sports Med. 1989;17(2):208-216.
30. Zavras TD, Race A, Bull AM, Amis AA. A comparative study of ‘isometric’ points for anterior cruciate ligament graft attachment. Knee Surg Sports Traumatol Arthrosc. 2001;9(1):28-33.
31. Pearle AD, Shannon FJ, Granchi C, Wickiewicz TL, Warren RF. Comparison of 3-dimensional obliquity and anisometric characteristics of anterior cruciate ligament graft positions using surgical navigation. Am J Sports Med. 2008;36(8):1534-1541.
32. Lubowitz JH. Anatomic ACL reconstruction produces greater graft length change during knee range-of-motion than transtibial technique. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1190-1195.
33. Markolf KL, Burchfield DM, Shapiro MM, Davis BR, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part I: insertion of the graft and anterior-posterior testing. J Bone Joint Surg Am. 1996;78(11):1720-1727.
34. Musahl V, Plakseychuk A, VanScyoc A, et al. Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med. 2005;33(5):712-718.
35. Bedi A, Musahl V, Steuber V, et al. Transtibial versus anteromedial portal reaming in anterior cruciate ligament reconstruction: an anatomic and biomechanical evaluation of surgical technique. Arthroscopy. 2011;27(3):380-390.
36. Lim HC, Yoon YC, Wang JH, Bae JH. Anatomical versus non-anatomical single bundle anterior cruciate ligament reconstruction: a cadaveric study of comparison of knee stability. Clin Orthop Surg. 2012;4(4):249-255.
37. Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy. 2003;19(3):297-304.
38. Cross MB, Musahl V, Bedi A, et al. Anteromedial versus central single-bundle graft position: which anatomic graft position to choose? Knee Surg Sports Traumatol Arthrosc. 2012;20(7):1276-1281.
39. Markolf KL, Jackson SR, McAllister DR. A comparison of 11 o’clock versus oblique femoral tunnels in the anterior cruciate ligament–reconstructed knee: knee kinematics during a simulated pivot test. Am J Sports Med. 2010;38(5):912-917.
40. Markolf KL, Park S, Jackson SR, McAllister DR. Anterior-posterior and rotatory stability of single and double-bundle anterior cruciate ligament reconstructions. J Bone Joint Surg Am. 2009;91(1):107-118.
41. Markolf KL, Park S, Jackson SR, McAllister DR. Contributions of the posterolateral bundle of the anterior cruciate ligament to anterior-posterior knee laxity and ligament forces. Arthroscopy. 2008;24(7):805-809.
42. Markolf KL, Burchfield DM, Shapiro MM, Cha CW, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part II: forces in the graft compared with forces in the intact ligament. J Bone Joint Surg Am. 1996;78(11):1728-1734.
43. Wallace MP, Howell SM, Hull ML. In vivo tensile behavior of a four-bundle hamstring graft as a replacement for the anterior cruciate ligament. J Orthop Res. 1997;15(4):539-545.
44. Harner CD, Marks PH, Fu FH, Irrgang JJ, Silby MB, Mengato R. Anterior cruciate ligament reconstruction: endoscopic versus two-incision technique. Arthroscopy. 1994;10(5):502-512.
45. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision technique for reconstructing a torn anterior cruciate ligament using hamstring tendons. J Arthroscopy. 1999;15(6):594-606.
46. Chouliaras V, Ristanis S, Moraiti C, Stergiou N, Georgoulis AD. Effectiveness of reconstruction of the anterior cruciate ligament with quadrupled hamstrings and bone–patellar tendon–bone autografts: an in vivo study comparing tibial internal–external rotation. Am J Sports Med. 2007;35(2):189-196.
47. Logan MC, Williams A, Lavelle J, Gedroyc W, Freeman M. Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. Am J Sports Med. 2004;32(4):984-992.
48. Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34(12):2006-2012.
49. Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975-983.
50. Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop. 2007;(454):66-73.
51. Wallace MP, Hull ML, Howell SM. Can an isometer predict the tensile behavior of a double-looped hamstring graft during anterior cruciate ligament reconstruction? J Orthop Res. 1998;16(3):386-393.
52. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind MC. Increased risk of revision after anteromedial compared with transtibial drilling of the femoral tunnel during primary anterior cruciate ligament reconstruction: results from the Danish Knee Ligament Reconstruction Register. Arthroscopy. 2013;29(1):98-105.
53. van Eck CF, Schkrohowsky JG, Working ZM, Irrgang JJ, Fu FH. Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med. 2012;40(4):800-807.
54. Ahn JH, Choi SH, Wang JH, Yoo JC, Yim HS, Chang MJ. Outcomes and second-look arthroscopic evaluation after double-bundle anterior cruciate ligament reconstruction with use of a single tibial tunnel. J Bone Joint Surg Am. 2011;93(20):1865-1872.
1. Siebold R. The concept of complete footprint restoration with guidelines for single- and double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):699-706.
2. Siebold R, Schuhmacher P. Restoration of the tibial ACL footprint area and geometry using the modified insertion site table. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1845-1849.
3. Piefer JW, Pflugner TR, Hwang MD, Lubowitz JH. Anterior cruciate ligament femoral footprint anatomy: systematic review of the 21st century literature. Arthroscopy. 2012;28(6):872-881.
4. Wilson AJ, Yasen SK, Nancoo T, Stannard R, Smith JO, Logan JS. Anatomic all-inside anterior cruciate ligament reconstruction using the translateral technique. Arthrosc Tech. 2013;2(2):e99-e104.
5. Colombet P, Robinson J, Christel P, et al. Morphology of anterior cruciate ligament attachments for anatomic reconstruction: a cadaveric dissection and radiographic study. Arthroscopy. 2006;22(9):984-992.
6. Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15(7):741-749.
7. Mochizuki T, Fujishiro H, Nimura A, et al. Anatomic and histologic analysis of the mid-substance and fan-like extension fibres of the anterior cruciate ligament during knee motion, with special reference to the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):336-344.
8. Siebold R, Schuhmacher P, Fernandez F, et al. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site [published correction appears in Knee Surg Sports Traumatol Arthrosc. 2014 Aug 23. Epub ahead of print]. Knee Surg Sports Traumatol Arthrosc. 2014 May 20. [Epub ahead of print]
9. Triantafyllidi E, Paschos NK, Goussia A, et al. The shape and the thickness of the anterior cruciate ligament along its length in relation to the posterior cruciate ligament: a cadaveric study. Arthroscopy. 2013;29(12):1963-1973.
10. Arnold MP, Kooloos J, van Kampen A. Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):194-199.
11. Ayerza MA, Múscolo DL, Costa-Paz M, Makino A, Rondón L. Comparison of sagittal obliquity of the reconstructed anterior cruciate ligament with native anterior cruciate ligament using magnetic resonance imaging. Arthroscopy. 2003;19(3):257-261.
12. Bowers AL, Bedi A, Lipman JD, et al. Comparison of anterior cruciate ligament tunnel position and graft obliquity with transtibial and anteromedial portal femoral tunnel reaming techniques using high-resolution magnetic resonance imaging. Arthroscopy. 2011;27(11):1511-1522.
13. Howell SM, Gittins ME, Gottlieb JE, Traina SM, Zoellner TM. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29(5):567-574.
14. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
15. Simmons R, Howell SM, Hull ML. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. J Bone Joint Surg Am. 2003;85(6):1018-1029.
16. Stanford FC, Kendoff D, Warren RF, Pearle AD. Native anterior cruciate ligament obliquity versus anterior cruciate ligament graft obliquity: an observational study using navigated measurements. Am J Sports Med. 2009;37(1):114-119.
17. Ferretti M, Ekdahl M, Shen W, Fu FH. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy. 2007;23(11):1218-1225.
18. Hutchinson MR, Ash SA. Resident’s ridge: assessing the cortical thickness of the lateral wall and roof of the intercondylar notch. Arthroscopy. 2003;19(9):931-935.
19. Fu FH, Jordan SS. The lateral intercondylar ridge—a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89(10):2103-2104.
20. Smigielski R, Zdanowicz U, Drwięga M, Ciszek B, Ciszkowska-Łysoń B, Siebold R. Ribbon like appearance of the midsubstance fibres of the anterior cruciate ligament close to its femoral insertion site: a cadaveric study including 111 knees. Knee Surg Sports Traumatol Arthrosc. 2014 Jun 28. [Epub ahead of print]
21. Iwahashi T, Shino K, Nakata K, et al. Direct anterior cruciate ligament insertion to the femur assessed by histology and 3-dimensional volume-rendered computed tomography. Arthroscopy. 2010;26(9 suppl):S13-S20.
22. Sasaki N, Ishibashi Y, Tsuda E, et al. The femoral insertion of the anterior cruciate ligament: discrepancy between macroscopic and histological observations. Arthroscopy. 2012;28(8):1135-1146.
23. Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The “enthesis organ” concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50(10):3306-3313.
24. Pathare NP, Nicholas SJ, Colbrunn R, McHugh MP. Kinematic analysis of the indirect femoral insertion of the anterior cruciate ligament: implications for anatomic femoral tunnel placement. Arthroscopy. 2014;30(11):1430-1438.
25. Artmann M, Wirth CJ. Investigation of the appropriate functional replacement of the anterior cruciate ligament (author’s transl) [in German]. Z Orthop Ihre Grenzgeb. 1974;112(1):160-165.
26. Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;(6 suppl 1):S2-S12.
27. Beynnon BD, Uh BS, Johnson RJ, Fleming BC, Renström PA, Nichols CE. The elongation behavior of the anterior cruciate ligament graft in vivo. A long-term follow-up study. Am J Sports Med. 2001;29(2):161-166.
28. O’Meara PM, O’Brien WR, Henning CE. Anterior cruciate ligament reconstruction stability with continuous passive motion. The role of isometric graft placement. Clin Orthop. 1992;(277):201-209.
29. Hefzy MS, Grood ES, Noyes FR. Factors affecting the region of most isometric femoral attachments. Part II: the anterior cruciate ligament. Am J Sports Med. 1989;17(2):208-216.
30. Zavras TD, Race A, Bull AM, Amis AA. A comparative study of ‘isometric’ points for anterior cruciate ligament graft attachment. Knee Surg Sports Traumatol Arthrosc. 2001;9(1):28-33.
31. Pearle AD, Shannon FJ, Granchi C, Wickiewicz TL, Warren RF. Comparison of 3-dimensional obliquity and anisometric characteristics of anterior cruciate ligament graft positions using surgical navigation. Am J Sports Med. 2008;36(8):1534-1541.
32. Lubowitz JH. Anatomic ACL reconstruction produces greater graft length change during knee range-of-motion than transtibial technique. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1190-1195.
33. Markolf KL, Burchfield DM, Shapiro MM, Davis BR, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part I: insertion of the graft and anterior-posterior testing. J Bone Joint Surg Am. 1996;78(11):1720-1727.
34. Musahl V, Plakseychuk A, VanScyoc A, et al. Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med. 2005;33(5):712-718.
35. Bedi A, Musahl V, Steuber V, et al. Transtibial versus anteromedial portal reaming in anterior cruciate ligament reconstruction: an anatomic and biomechanical evaluation of surgical technique. Arthroscopy. 2011;27(3):380-390.
36. Lim HC, Yoon YC, Wang JH, Bae JH. Anatomical versus non-anatomical single bundle anterior cruciate ligament reconstruction: a cadaveric study of comparison of knee stability. Clin Orthop Surg. 2012;4(4):249-255.
37. Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy. 2003;19(3):297-304.
38. Cross MB, Musahl V, Bedi A, et al. Anteromedial versus central single-bundle graft position: which anatomic graft position to choose? Knee Surg Sports Traumatol Arthrosc. 2012;20(7):1276-1281.
39. Markolf KL, Jackson SR, McAllister DR. A comparison of 11 o’clock versus oblique femoral tunnels in the anterior cruciate ligament–reconstructed knee: knee kinematics during a simulated pivot test. Am J Sports Med. 2010;38(5):912-917.
40. Markolf KL, Park S, Jackson SR, McAllister DR. Anterior-posterior and rotatory stability of single and double-bundle anterior cruciate ligament reconstructions. J Bone Joint Surg Am. 2009;91(1):107-118.
41. Markolf KL, Park S, Jackson SR, McAllister DR. Contributions of the posterolateral bundle of the anterior cruciate ligament to anterior-posterior knee laxity and ligament forces. Arthroscopy. 2008;24(7):805-809.
42. Markolf KL, Burchfield DM, Shapiro MM, Cha CW, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part II: forces in the graft compared with forces in the intact ligament. J Bone Joint Surg Am. 1996;78(11):1728-1734.
43. Wallace MP, Howell SM, Hull ML. In vivo tensile behavior of a four-bundle hamstring graft as a replacement for the anterior cruciate ligament. J Orthop Res. 1997;15(4):539-545.
44. Harner CD, Marks PH, Fu FH, Irrgang JJ, Silby MB, Mengato R. Anterior cruciate ligament reconstruction: endoscopic versus two-incision technique. Arthroscopy. 1994;10(5):502-512.
45. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision technique for reconstructing a torn anterior cruciate ligament using hamstring tendons. J Arthroscopy. 1999;15(6):594-606.
46. Chouliaras V, Ristanis S, Moraiti C, Stergiou N, Georgoulis AD. Effectiveness of reconstruction of the anterior cruciate ligament with quadrupled hamstrings and bone–patellar tendon–bone autografts: an in vivo study comparing tibial internal–external rotation. Am J Sports Med. 2007;35(2):189-196.
47. Logan MC, Williams A, Lavelle J, Gedroyc W, Freeman M. Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. Am J Sports Med. 2004;32(4):984-992.
48. Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34(12):2006-2012.
49. Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975-983.
50. Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop. 2007;(454):66-73.
51. Wallace MP, Hull ML, Howell SM. Can an isometer predict the tensile behavior of a double-looped hamstring graft during anterior cruciate ligament reconstruction? J Orthop Res. 1998;16(3):386-393.
52. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind MC. Increased risk of revision after anteromedial compared with transtibial drilling of the femoral tunnel during primary anterior cruciate ligament reconstruction: results from the Danish Knee Ligament Reconstruction Register. Arthroscopy. 2013;29(1):98-105.
53. van Eck CF, Schkrohowsky JG, Working ZM, Irrgang JJ, Fu FH. Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med. 2012;40(4):800-807.
54. Ahn JH, Choi SH, Wang JH, Yoo JC, Yim HS, Chang MJ. Outcomes and second-look arthroscopic evaluation after double-bundle anterior cruciate ligament reconstruction with use of a single tibial tunnel. J Bone Joint Surg Am. 2011;93(20):1865-1872.
Alignment Analyses in the Varus Osteoarthritic Knee Using Computer Navigation
Osteoarthritic (OA) knees with varus deformities commonly present with tight, contracted medial collateral ligaments and soft-tissue sleeves.1 More severe varus deformities require more extensive medial releases on the concave side to optimize flexion-extension gaps. Excessive soft-tissue releases in milder varus deformities can result in medial instability in flexion and extension.2-4 Misjudgments in soft-tissue release can therefore lead to knee instability, an important cause of early total knee arthroplasty (TKA) failures.2,5,6 Some authors have reported difficulty in coronal plane balancing in knees with preoperative varus deformity of more than 20°.4,7
Surgeons often refer to varus as a description of coronal malalignment, mainly with the knee in extension. In the surgical setting, however, descriptions are given regarding differential medial soft-tissue tightness in extension and flexion. Balancing the knee in extension may not necessarily balance the knee in flexion. Thus, there is the concept of extension and flexion varus, which has not been well described in the literature. Releases on the anterior medial and posterior medial aspects of the proximal tibia have differential effects on flexion and extension gaps, respectively.2
Intraoperative alignment certainly has a pivotal role in component longevity.8 Since its advent in the 1990s, use of computer navigation in TKA has offered new hope for improving component alignment. Some authors routinely use computer navigation for intraoperative soft-tissue releases.9 A recent meta-analysis found that computer-navigated surgery is associated with fewer outliers in final component alignment compared with conventional TKA.10
Increased use of computer navigation in TKA at our institution in recent years has come with the observation that knees with severe extension varus seem to have correspondingly more severe flexion varus. Before computer navigation, coronal alignment of knees in flexion was almost impossible to measure because of the spatial alignment of the knees in that position.
We conducted a study to evaluate the relationship of extension and flexion varus in OA knees and to determine whether severity of fixed flexion deformity (FFD) in the sagittal plane correlates with severity of coronal plane varus deformity. We hypothesized that there would be differential varus in flexion and extension and that increasing knee extension varus would correlate closely with knee flexion varus beyond a certain tibiofemoral angle. We also hypothesized that severity of sagittal plane deformity will correlate with the severity of coronal plane deformity.
Patients and Methods
Data Collection
After this study was approved by our institution’s ethics review committee, we prospectively collected data from 403 consecutive computer-navigated TKAs performed at our institution between November 2008 and August 2011. Dr. Tan, who was not the primary physician, retrospectively analyzed the radiographic and navigation data.
Each patient’s knee varus-valgus angles were captured by Dr. Teo, an adult reconstruction surgeon, in standard fashion from maximal extension to 0º, 30º, 45º, 60º, 90º, and maximal flexion. An example of standard data capture appears in Table 1. With varus-hyperextension defined as –0.5° or less (more negative), neutral as 0°, and valgus-flexion as 0.5° or more, there were 362 varus knees, 41 valgus knees, and no neutral knees.
Study inclusion criteria were OA and varus deformity. Exclusion criteria were rheumatoid arthritis, other types of inflammatory arthritis, neuromuscular disorders, knees with valgus angulation, and incomplete data (Table 2). Figure 1 summarizes the inclusion/exclusion process, which left 317 knees available for study. Cases of incomplete data were likely due to computer errors or to inadvertent movement when navigation data were being acquired during surgery.
In conventional TKA, the main objective is to equalize flexion-extension gaps with knee at 90° flexion and 0° extension. The ability to achieve this often implies the knee will be balanced throughout its range of motion (ROM). From the data for the 317 study knees, 3 sets of values were extracted: varus angles from maximal knee extension (extension varus), varus angles from 90° knee flexion (flexion varus), and maximal knee extension. All knees were able to achieve 90° flexion.
Power Calculation
Our analysis used a correlation coefficient (r) of at least 0.5 at a 5% level of significance and power of 80%. With 317 knees, the study was more than adequately powered for significance.
Surgical and Navigation Technique
All patients underwent either general or regional anesthesia for their surgeries, which were performed by Dr. Teo. Standard medial parapatellar arthrotomy was performed. Navigation pins were then inserted into the femur and tibia outside the knee wound. Anatomical reference points were digitized per routine navigation requirements. (The reference for varus-valgus alignment of the femur is the mechanical femur axis defined by the digitized hip center and knee center, and the reference for varus-valgus alignment of the tibia is the mechanical tibia axis defined by the digitized tibia center and calculated ankle center. The ankle center is calculated by dividing the digitized transmalleolar axis according to a ratio of 56% lateral to 44% medial with the inherent navigation software.) Our institution uses an imageless navigation system (Navigation System II; Stryker Orthopedics, Mahwah, New Jersey).
The leg was then brought from maximal knee extension to maximal knee flexion to assess preoperative ROM, which indicates inherent flexion contracture or hyperextension. Varus-valgus measurements of the knee were then generated as part of the navigation software protocol. These measurements were obtained without additional varus or valgus stress applied to the knee and before any bony resection. The rest of the operation was completed using navigation to guide bony resection and soft-tissue balancing. The final components used were all cemented cruciate-substituting TKA implants. After component insertion, the knee was again brought through ROM from maximal knee extension to maximal knee flexion to assess postoperative ROM before wound closure.
Extension and Flexion Varus
As none of the patients in the flexion varus dataset (range, –0.5° to –19°) had a varus deformity of more than 20° at 90° flexion, we used a cutoff of 10° to divide these patients into 2 subgroups: less than 10° (237 knees) and 10° or more (80 knees). The extension varus dataset ranged from –0.5° to –24°. Incremental values of –0.5° to –24° in this dataset were then analyzed against the 90° flexion varus subgroups using logistic regression. A scatterplot of the relationship between extension and flexion varus is shown in Figure 2. The probability function was then derived and a probability graph plotted.
FFD and Extension and Flexion Varus
Maximal knee extension, obtained from intraoperative navigation measurements, ranged from –9° (hyperextension) to 33° (FFD) and maximal knee flexion ranged from 90° to 146°. Ninety-two knees had slight hyperextension, and 6 were neutral. Of the 317 OA knees with varus deformity, 219 (69%) had FFD. This sagittal plane alignment parameter was analyzed against coronal plane alignment in maximal knee extension and 90° knee flexion to determine if increasing severity of FFD corresponds with increasing extension or flexion varus.
Statistical Analysis
Statistical analysis was performed with Stata 10.1 (Statacorp, College Station, Texas). Significance was set at P < .05.
Results
Extension and Flexion Varus
Patient demographic data are listed in Table 3. Univariate logistic regression analysis revealed that age (P = .110), body mass index (P = .696), and sex (P = .584) did not affect the association between preoperative extension and flexion varus.
Mean (SD) preoperative extension varus was –9.9° (4.80°), and mean (SD) preoperative flexion 90° varus was –7.02° (3.74°). Linear regression of the data showed a significant positive correlation between preoperative extension varus and flexion varus (Pearson correlation coefficient, 0.57; P < .0001). The probability function was determined as follows: Probability of having flexion varus of more than 10° = 1 / (1 + e–z), where z = –4.014 – 0.265 × extension varus. Plotting the probability graph of flexion varus against varus angles at maximal knee extension from the probability formula yielded a sigmoid graph (Figure 3). The most linear part of the graph corresponds to the 10° to 20° of extension varus (solid line), demonstrating an almost linear increase in the probability of having more than 10° flexion varus with increasing extension varus from 10° to 20°. For extension varus of 20° or more, the probability of having flexion varus of more than 10° approaches 1.
FFD and Extension and Flexion Varus
Mean (SD) preoperative maximal knee extension (analogous to FFD) was 4.41° (7.50°), mean (SD) extension varus was –9.9° (4.80°), and mean (SD) 90° flexion varus was –7.02° (3.74°). We did not find any correlation between preoperative FFD and preoperative flexion varus (r = –0.02; P = .6583) or extension varus (r = –0.11; P = .046) (Figure 4).
Postoperative Alignment
Of the 317 OA knees, 18 had incomplete navigation-acquired postoperative alignment data. The postoperative alignment of the other 299 knees at various degrees of knee flexion is illustrated with a box-and-whisker plot (Figure 5).
Knees With Severe Extension Varus
Fourteen of the 15 knees with severe extension varus (>20°) had flexion varus of more than 9° (range, –9° to –17.5°, with only 1 outlier, at –5°). For the 15 patients, maximal knee extension ranged from –9° hyperextension to 27.5° FFD. Six knees had slight hyperextension, and 9 had FFD demonstrating large variability in sagittal alignment. Despite severe preoperative coronal deformity, all 15 knees had satisfactory deformity correction. Preoperative and postoperative knee alignment data for these 15 knees appear in Table 4 and Figure 6, respectively.
Discussion
OA varus knees represent a majority of the cases being managed by orthopedic surgeons. Soft-tissue contractures involving the medial collateral ligament (MCL), posteromedial capsule, pes anserinus, and semimembranosus muscle are commonly encountered. Bone loss may also occur on the tibial and femoral joint surfaces in knees with severe angular deformity. In an OA varus knee, bone loss tends to be mainly on the medial tibial plateau and usually on the posterior aspect of the tibia because flexion contractures often are concomitant with these marked deformities.11 Therefore, a varus deformity is apparent whether the knee is extended or flexed. Our results showed a correlation between extension and flexion varus in OA varus knees. In contrast, for a valgus deformity, as bone loss can occur on both the tibial and femoral surfaces,11 a similar correlation may not be seen. For that reason, and because there were only 41 valgus knees in this study, they were excluded. For FFD, soft-tissue contractures often involve both the posterior capsule and the posterior cruciate ligament (PCL). Posterior osteophytes often cause tenting of the posterior capsule in knees with FFD. Anteriorly, growth of osteophytes at the tibial spine and intercondylar notch of the femur can result in bony causes of restricted knee extension.12
One would expect increased coronal plane angular deformity to correspond to more severe FFD in the sagittal plane because the same pathology affects soft tissue or bones in an OA knee in both planes. Interestingly, our study results proved otherwise. FFD did not correlate with degree of extension or flexion varus severity. This phenomenon has not been described in the literature likely because clinical measurements of flexion varus and FFD were difficult to perform because of the spatial alignment of the knee in flexion. In recent years, however, computer navigation technology has made such measurements possible.
Mihalko and colleagues2 established that soft-tissue releases on different parts of the proximal tibia have different effects on soft-tissue balancing in flexion and extension. In knees with extension varus, more releases are required on the posterior medial aspect of the tibia (the posterior oblique fibers of the superficial MCL, the posteromedial capsule, and, sometimes, the semimembranosus), whereas knees with flexion varus require more releases on the anterior medial aspect of the tibia (the deep MCL, the anterior fibers of the superficial MCL, and, sometimes, the pes anserinus attachment).13 Consequently, soft-tissue stabilizers seem to have different functions in flexion and extension and cannot reliably be released solely in extension or flexion for optimal gap balancing during TKA.2 Other authors, in cadaveric studies, have found that a larger amount of coronal deformity correction is achieved with more distal soft-tissue releases from the joint line.9,14 Surgical techniques for correcting FFD include removal of prominent anterior and posterior osteophytes, posterior capsular releases, sometimes PCL sacrifices, and even gastrocnemius recession.12
In our study, all 14 patients with severe extension and correspondingly severe flexion varus needed not only modest posterior medial soft-tissue releases for the severe extension varus, but also modest anterior medial releases for the flexion varus. The respective soft-tissue releases were confirmed in real time with computer navigation sequentially after bony resection and osteophyte removal. With this method, we restored final postoperative alignment to within 3° of the mechanical axis (Figure 6). Our experience here led us to believe that, with these patients, modest anterior medial and posterior medial releases could be performed at the start of surgery, as severe extension varus (>20°) almost certainly equates to severe flexion varus (>10°). Therein lies the clinical relevance of our study. However, not all patients with severe coronal plane deformity have correspondingly severe sagittal plane deformity in the form of FFD, as illustrated in our study. Therefore, not all patients with severe varus knee deformity need aggressive posterior capsular release or PCL recession to correct FFD. Some patients have mild hyperextension, which can be attributed partly to the postanesthesia effects of soft-tissue laxity. It is unclear exactly how much anesthesia contributes to this difference in sagittal alignment, though the majority of our patients had FFD. It is not our intent here to discuss the surgical techniques of soft-tissue balancing or to advocate routine use of computer navigation.
Many factors (eg, medial femoral condyle bone loss, medial tibial plateau bone loss, femur or tibia bowing, medial soft-tissue contracture) can contribute to varus malalignment. Current navigation technology cannot isolate the causes of varus alignment, and we did not intend to investigate them in this study. Our primary aim was to assess for a correlation between overall extension varus alignment and expected flexion varus. We also wanted to analyze the correlation between FFD and the coronal plane alignment, in extension and flexion, contributed by the combined bony and soft-tissue components in OA varus knees.
The strengths of this study are that it was a single-surgeon series with knee data from consecutive patients who had computer-navigated TKA. Patient data were prospectively generated from the navigation software and retrospectively analyzed. All navigation alignment was performed by a single surgeon, thereby eliminating examination bias during the time knee alignment data were being obtained. The study was adequately powered and had a large number of patients for data analysis. The authors believe that this is the first study to analyze alignment in both the coronal and sagittal plane in varus OA knees.
We acknowledge a few limitations in our study. Although several investigators have found that navigation can be used to achieve accurate postoperative alignment,10,15,16 subtle errors may be inadvertently introduced at different points of alignment measurement. These error points include identification of visually selected anatomical landmarks; kinematic registration of hip, knee, and ankle; and intraoperative changes in the navigation environment (eg, inadvertent movement of pins or rigid bodies). In addition, different surgeons have different techniques for kinematic registration. However, the surgeries in our study were performed by the same surgeon, so this confounding factor was effectively removed. Another limitation was that navigation alignment was obtained during surgery, when patients were under anesthesia and in a supine, non-weight-bearing position, whereas routine clinical weight-bearing radiographs are taken with nonanesthetized patients and this might overestimate the deformities intraoperatively. However, all parameters were measured in the same patient under the same anesthetic effects, so this should not have affected the analyses. Most surgeons would make an intraoperative assessment of the severity of any deformity before the surgery proper anyway. Nevertheless, some authors have found that knee alignment obtained with intraoperative navigation correlated well with alignment obtained with weight-bearing radiographs.17,18
Conclusion
Our study results showed that, in OA varus knees, extension varus highly correlated with flexion varus. However, there was no correlation between FFD and coronal plane varus deformity.
1. Engh GA. The difficult knee: severe varus and valgus. Clin Orthop. 2003;(416):58-63.
2. Mihalko WM, Saleh KJ, Krackow KA, Whiteside LA. Soft-tissue balancing during total knee arthroplasty in the varus knee. J Am Acad Orthop Surg. 2009;17(12):766-774.
3. Ranawat CS, Flynn WF Jr, Saddler S, Hansraj KK, Maynard MJ. Long-term results of the total condylar knee arthroplasty. A 15-year survivorship study. Clin Orthop. 1993;(286):94-102.
4. Ritter MA, Faris GW, Faris PM, Davis KE. Total knee arthroplasty in patients with angular varus or valgus deformities of > or = 20 degrees. J Arthroplasty. 2004;19(7):862-866.
5. Parratte S, Pagnano MW. Instability after total knee arthroplasty. J Bone Joint Surg Am. 2008;90(1):184-194.
6. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop. 2002;(404):7-13.
7. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Luring C, Hüfner T, Perlick L, Bäthis H, Krettek C, Grifka J. The effectiveness of sequential medial soft tissue release on coronal alignment in total knee arthroplasty: using a computer navigation model. J Arthroplasty. 2006;21(3):428-434.
10. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
11. Insall JN, Easley ME. Surgical techniques and instrumentation in total knee arthroplasty. In: Insall JN, Scott WN, eds. Surgery of the Knee. Vol 2. 3rd ed. New York, NY: Churchill Livingstone; 2001:1553-1620.
12. Scuderi GR, Tria AJ, eds. Surgical Techniques in Total Knee Arthroplasty. New York, NY: Springer-Verlag; 2002.
13. Whiteside LA, Saeki K, Mihalko WM. Functional medial ligament balancing in total knee arthroplasty. Clin Orthop. 2000;(380):45-57.
14. Matsueda M, Gengerke TR, Murphy M, Lew WD, Gustilo RB. Soft tissue release in total knee arthroplasty. Cadaver study using knees without deformities. Clin Orthop. 1999;(366):264-273.
15. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop. 2005;(433):152-159.
16. Mullaji AB, Kanna R, Marawar S, Kohli A, Sharma A. Comparison of limb and component alignment using computer-assisted navigation versus image intensifier–guided conventional total knee arthroplasty: a prospective, randomized, single-surgeon study of 467 knees. J Arthroplasty. 2007;22(7):953-959.
17. Colebatch AN, Hart DJ, Zhai G, Williams FM, Spector TD, Arden NK. Effective measurement of knee alignment using AP knee radiographs. Knee. 2009;16(1):42-45.
18. Yaffe MA, Koo SS, Stulberg SD. Radiographic and navigation measurements of TKA limb alignment do not correlate. Clin Orthop. 2008;466(11):2736-2744.
Osteoarthritic (OA) knees with varus deformities commonly present with tight, contracted medial collateral ligaments and soft-tissue sleeves.1 More severe varus deformities require more extensive medial releases on the concave side to optimize flexion-extension gaps. Excessive soft-tissue releases in milder varus deformities can result in medial instability in flexion and extension.2-4 Misjudgments in soft-tissue release can therefore lead to knee instability, an important cause of early total knee arthroplasty (TKA) failures.2,5,6 Some authors have reported difficulty in coronal plane balancing in knees with preoperative varus deformity of more than 20°.4,7
Surgeons often refer to varus as a description of coronal malalignment, mainly with the knee in extension. In the surgical setting, however, descriptions are given regarding differential medial soft-tissue tightness in extension and flexion. Balancing the knee in extension may not necessarily balance the knee in flexion. Thus, there is the concept of extension and flexion varus, which has not been well described in the literature. Releases on the anterior medial and posterior medial aspects of the proximal tibia have differential effects on flexion and extension gaps, respectively.2
Intraoperative alignment certainly has a pivotal role in component longevity.8 Since its advent in the 1990s, use of computer navigation in TKA has offered new hope for improving component alignment. Some authors routinely use computer navigation for intraoperative soft-tissue releases.9 A recent meta-analysis found that computer-navigated surgery is associated with fewer outliers in final component alignment compared with conventional TKA.10
Increased use of computer navigation in TKA at our institution in recent years has come with the observation that knees with severe extension varus seem to have correspondingly more severe flexion varus. Before computer navigation, coronal alignment of knees in flexion was almost impossible to measure because of the spatial alignment of the knees in that position.
We conducted a study to evaluate the relationship of extension and flexion varus in OA knees and to determine whether severity of fixed flexion deformity (FFD) in the sagittal plane correlates with severity of coronal plane varus deformity. We hypothesized that there would be differential varus in flexion and extension and that increasing knee extension varus would correlate closely with knee flexion varus beyond a certain tibiofemoral angle. We also hypothesized that severity of sagittal plane deformity will correlate with the severity of coronal plane deformity.
Patients and Methods
Data Collection
After this study was approved by our institution’s ethics review committee, we prospectively collected data from 403 consecutive computer-navigated TKAs performed at our institution between November 2008 and August 2011. Dr. Tan, who was not the primary physician, retrospectively analyzed the radiographic and navigation data.
Each patient’s knee varus-valgus angles were captured by Dr. Teo, an adult reconstruction surgeon, in standard fashion from maximal extension to 0º, 30º, 45º, 60º, 90º, and maximal flexion. An example of standard data capture appears in Table 1. With varus-hyperextension defined as –0.5° or less (more negative), neutral as 0°, and valgus-flexion as 0.5° or more, there were 362 varus knees, 41 valgus knees, and no neutral knees.
Study inclusion criteria were OA and varus deformity. Exclusion criteria were rheumatoid arthritis, other types of inflammatory arthritis, neuromuscular disorders, knees with valgus angulation, and incomplete data (Table 2). Figure 1 summarizes the inclusion/exclusion process, which left 317 knees available for study. Cases of incomplete data were likely due to computer errors or to inadvertent movement when navigation data were being acquired during surgery.
In conventional TKA, the main objective is to equalize flexion-extension gaps with knee at 90° flexion and 0° extension. The ability to achieve this often implies the knee will be balanced throughout its range of motion (ROM). From the data for the 317 study knees, 3 sets of values were extracted: varus angles from maximal knee extension (extension varus), varus angles from 90° knee flexion (flexion varus), and maximal knee extension. All knees were able to achieve 90° flexion.
Power Calculation
Our analysis used a correlation coefficient (r) of at least 0.5 at a 5% level of significance and power of 80%. With 317 knees, the study was more than adequately powered for significance.
Surgical and Navigation Technique
All patients underwent either general or regional anesthesia for their surgeries, which were performed by Dr. Teo. Standard medial parapatellar arthrotomy was performed. Navigation pins were then inserted into the femur and tibia outside the knee wound. Anatomical reference points were digitized per routine navigation requirements. (The reference for varus-valgus alignment of the femur is the mechanical femur axis defined by the digitized hip center and knee center, and the reference for varus-valgus alignment of the tibia is the mechanical tibia axis defined by the digitized tibia center and calculated ankle center. The ankle center is calculated by dividing the digitized transmalleolar axis according to a ratio of 56% lateral to 44% medial with the inherent navigation software.) Our institution uses an imageless navigation system (Navigation System II; Stryker Orthopedics, Mahwah, New Jersey).
The leg was then brought from maximal knee extension to maximal knee flexion to assess preoperative ROM, which indicates inherent flexion contracture or hyperextension. Varus-valgus measurements of the knee were then generated as part of the navigation software protocol. These measurements were obtained without additional varus or valgus stress applied to the knee and before any bony resection. The rest of the operation was completed using navigation to guide bony resection and soft-tissue balancing. The final components used were all cemented cruciate-substituting TKA implants. After component insertion, the knee was again brought through ROM from maximal knee extension to maximal knee flexion to assess postoperative ROM before wound closure.
Extension and Flexion Varus
As none of the patients in the flexion varus dataset (range, –0.5° to –19°) had a varus deformity of more than 20° at 90° flexion, we used a cutoff of 10° to divide these patients into 2 subgroups: less than 10° (237 knees) and 10° or more (80 knees). The extension varus dataset ranged from –0.5° to –24°. Incremental values of –0.5° to –24° in this dataset were then analyzed against the 90° flexion varus subgroups using logistic regression. A scatterplot of the relationship between extension and flexion varus is shown in Figure 2. The probability function was then derived and a probability graph plotted.
FFD and Extension and Flexion Varus
Maximal knee extension, obtained from intraoperative navigation measurements, ranged from –9° (hyperextension) to 33° (FFD) and maximal knee flexion ranged from 90° to 146°. Ninety-two knees had slight hyperextension, and 6 were neutral. Of the 317 OA knees with varus deformity, 219 (69%) had FFD. This sagittal plane alignment parameter was analyzed against coronal plane alignment in maximal knee extension and 90° knee flexion to determine if increasing severity of FFD corresponds with increasing extension or flexion varus.
Statistical Analysis
Statistical analysis was performed with Stata 10.1 (Statacorp, College Station, Texas). Significance was set at P < .05.
Results
Extension and Flexion Varus
Patient demographic data are listed in Table 3. Univariate logistic regression analysis revealed that age (P = .110), body mass index (P = .696), and sex (P = .584) did not affect the association between preoperative extension and flexion varus.
Mean (SD) preoperative extension varus was –9.9° (4.80°), and mean (SD) preoperative flexion 90° varus was –7.02° (3.74°). Linear regression of the data showed a significant positive correlation between preoperative extension varus and flexion varus (Pearson correlation coefficient, 0.57; P < .0001). The probability function was determined as follows: Probability of having flexion varus of more than 10° = 1 / (1 + e–z), where z = –4.014 – 0.265 × extension varus. Plotting the probability graph of flexion varus against varus angles at maximal knee extension from the probability formula yielded a sigmoid graph (Figure 3). The most linear part of the graph corresponds to the 10° to 20° of extension varus (solid line), demonstrating an almost linear increase in the probability of having more than 10° flexion varus with increasing extension varus from 10° to 20°. For extension varus of 20° or more, the probability of having flexion varus of more than 10° approaches 1.
FFD and Extension and Flexion Varus
Mean (SD) preoperative maximal knee extension (analogous to FFD) was 4.41° (7.50°), mean (SD) extension varus was –9.9° (4.80°), and mean (SD) 90° flexion varus was –7.02° (3.74°). We did not find any correlation between preoperative FFD and preoperative flexion varus (r = –0.02; P = .6583) or extension varus (r = –0.11; P = .046) (Figure 4).
Postoperative Alignment
Of the 317 OA knees, 18 had incomplete navigation-acquired postoperative alignment data. The postoperative alignment of the other 299 knees at various degrees of knee flexion is illustrated with a box-and-whisker plot (Figure 5).
Knees With Severe Extension Varus
Fourteen of the 15 knees with severe extension varus (>20°) had flexion varus of more than 9° (range, –9° to –17.5°, with only 1 outlier, at –5°). For the 15 patients, maximal knee extension ranged from –9° hyperextension to 27.5° FFD. Six knees had slight hyperextension, and 9 had FFD demonstrating large variability in sagittal alignment. Despite severe preoperative coronal deformity, all 15 knees had satisfactory deformity correction. Preoperative and postoperative knee alignment data for these 15 knees appear in Table 4 and Figure 6, respectively.
Discussion
OA varus knees represent a majority of the cases being managed by orthopedic surgeons. Soft-tissue contractures involving the medial collateral ligament (MCL), posteromedial capsule, pes anserinus, and semimembranosus muscle are commonly encountered. Bone loss may also occur on the tibial and femoral joint surfaces in knees with severe angular deformity. In an OA varus knee, bone loss tends to be mainly on the medial tibial plateau and usually on the posterior aspect of the tibia because flexion contractures often are concomitant with these marked deformities.11 Therefore, a varus deformity is apparent whether the knee is extended or flexed. Our results showed a correlation between extension and flexion varus in OA varus knees. In contrast, for a valgus deformity, as bone loss can occur on both the tibial and femoral surfaces,11 a similar correlation may not be seen. For that reason, and because there were only 41 valgus knees in this study, they were excluded. For FFD, soft-tissue contractures often involve both the posterior capsule and the posterior cruciate ligament (PCL). Posterior osteophytes often cause tenting of the posterior capsule in knees with FFD. Anteriorly, growth of osteophytes at the tibial spine and intercondylar notch of the femur can result in bony causes of restricted knee extension.12
One would expect increased coronal plane angular deformity to correspond to more severe FFD in the sagittal plane because the same pathology affects soft tissue or bones in an OA knee in both planes. Interestingly, our study results proved otherwise. FFD did not correlate with degree of extension or flexion varus severity. This phenomenon has not been described in the literature likely because clinical measurements of flexion varus and FFD were difficult to perform because of the spatial alignment of the knee in flexion. In recent years, however, computer navigation technology has made such measurements possible.
Mihalko and colleagues2 established that soft-tissue releases on different parts of the proximal tibia have different effects on soft-tissue balancing in flexion and extension. In knees with extension varus, more releases are required on the posterior medial aspect of the tibia (the posterior oblique fibers of the superficial MCL, the posteromedial capsule, and, sometimes, the semimembranosus), whereas knees with flexion varus require more releases on the anterior medial aspect of the tibia (the deep MCL, the anterior fibers of the superficial MCL, and, sometimes, the pes anserinus attachment).13 Consequently, soft-tissue stabilizers seem to have different functions in flexion and extension and cannot reliably be released solely in extension or flexion for optimal gap balancing during TKA.2 Other authors, in cadaveric studies, have found that a larger amount of coronal deformity correction is achieved with more distal soft-tissue releases from the joint line.9,14 Surgical techniques for correcting FFD include removal of prominent anterior and posterior osteophytes, posterior capsular releases, sometimes PCL sacrifices, and even gastrocnemius recession.12
In our study, all 14 patients with severe extension and correspondingly severe flexion varus needed not only modest posterior medial soft-tissue releases for the severe extension varus, but also modest anterior medial releases for the flexion varus. The respective soft-tissue releases were confirmed in real time with computer navigation sequentially after bony resection and osteophyte removal. With this method, we restored final postoperative alignment to within 3° of the mechanical axis (Figure 6). Our experience here led us to believe that, with these patients, modest anterior medial and posterior medial releases could be performed at the start of surgery, as severe extension varus (>20°) almost certainly equates to severe flexion varus (>10°). Therein lies the clinical relevance of our study. However, not all patients with severe coronal plane deformity have correspondingly severe sagittal plane deformity in the form of FFD, as illustrated in our study. Therefore, not all patients with severe varus knee deformity need aggressive posterior capsular release or PCL recession to correct FFD. Some patients have mild hyperextension, which can be attributed partly to the postanesthesia effects of soft-tissue laxity. It is unclear exactly how much anesthesia contributes to this difference in sagittal alignment, though the majority of our patients had FFD. It is not our intent here to discuss the surgical techniques of soft-tissue balancing or to advocate routine use of computer navigation.
Many factors (eg, medial femoral condyle bone loss, medial tibial plateau bone loss, femur or tibia bowing, medial soft-tissue contracture) can contribute to varus malalignment. Current navigation technology cannot isolate the causes of varus alignment, and we did not intend to investigate them in this study. Our primary aim was to assess for a correlation between overall extension varus alignment and expected flexion varus. We also wanted to analyze the correlation between FFD and the coronal plane alignment, in extension and flexion, contributed by the combined bony and soft-tissue components in OA varus knees.
The strengths of this study are that it was a single-surgeon series with knee data from consecutive patients who had computer-navigated TKA. Patient data were prospectively generated from the navigation software and retrospectively analyzed. All navigation alignment was performed by a single surgeon, thereby eliminating examination bias during the time knee alignment data were being obtained. The study was adequately powered and had a large number of patients for data analysis. The authors believe that this is the first study to analyze alignment in both the coronal and sagittal plane in varus OA knees.
We acknowledge a few limitations in our study. Although several investigators have found that navigation can be used to achieve accurate postoperative alignment,10,15,16 subtle errors may be inadvertently introduced at different points of alignment measurement. These error points include identification of visually selected anatomical landmarks; kinematic registration of hip, knee, and ankle; and intraoperative changes in the navigation environment (eg, inadvertent movement of pins or rigid bodies). In addition, different surgeons have different techniques for kinematic registration. However, the surgeries in our study were performed by the same surgeon, so this confounding factor was effectively removed. Another limitation was that navigation alignment was obtained during surgery, when patients were under anesthesia and in a supine, non-weight-bearing position, whereas routine clinical weight-bearing radiographs are taken with nonanesthetized patients and this might overestimate the deformities intraoperatively. However, all parameters were measured in the same patient under the same anesthetic effects, so this should not have affected the analyses. Most surgeons would make an intraoperative assessment of the severity of any deformity before the surgery proper anyway. Nevertheless, some authors have found that knee alignment obtained with intraoperative navigation correlated well with alignment obtained with weight-bearing radiographs.17,18
Conclusion
Our study results showed that, in OA varus knees, extension varus highly correlated with flexion varus. However, there was no correlation between FFD and coronal plane varus deformity.
Osteoarthritic (OA) knees with varus deformities commonly present with tight, contracted medial collateral ligaments and soft-tissue sleeves.1 More severe varus deformities require more extensive medial releases on the concave side to optimize flexion-extension gaps. Excessive soft-tissue releases in milder varus deformities can result in medial instability in flexion and extension.2-4 Misjudgments in soft-tissue release can therefore lead to knee instability, an important cause of early total knee arthroplasty (TKA) failures.2,5,6 Some authors have reported difficulty in coronal plane balancing in knees with preoperative varus deformity of more than 20°.4,7
Surgeons often refer to varus as a description of coronal malalignment, mainly with the knee in extension. In the surgical setting, however, descriptions are given regarding differential medial soft-tissue tightness in extension and flexion. Balancing the knee in extension may not necessarily balance the knee in flexion. Thus, there is the concept of extension and flexion varus, which has not been well described in the literature. Releases on the anterior medial and posterior medial aspects of the proximal tibia have differential effects on flexion and extension gaps, respectively.2
Intraoperative alignment certainly has a pivotal role in component longevity.8 Since its advent in the 1990s, use of computer navigation in TKA has offered new hope for improving component alignment. Some authors routinely use computer navigation for intraoperative soft-tissue releases.9 A recent meta-analysis found that computer-navigated surgery is associated with fewer outliers in final component alignment compared with conventional TKA.10
Increased use of computer navigation in TKA at our institution in recent years has come with the observation that knees with severe extension varus seem to have correspondingly more severe flexion varus. Before computer navigation, coronal alignment of knees in flexion was almost impossible to measure because of the spatial alignment of the knees in that position.
We conducted a study to evaluate the relationship of extension and flexion varus in OA knees and to determine whether severity of fixed flexion deformity (FFD) in the sagittal plane correlates with severity of coronal plane varus deformity. We hypothesized that there would be differential varus in flexion and extension and that increasing knee extension varus would correlate closely with knee flexion varus beyond a certain tibiofemoral angle. We also hypothesized that severity of sagittal plane deformity will correlate with the severity of coronal plane deformity.
Patients and Methods
Data Collection
After this study was approved by our institution’s ethics review committee, we prospectively collected data from 403 consecutive computer-navigated TKAs performed at our institution between November 2008 and August 2011. Dr. Tan, who was not the primary physician, retrospectively analyzed the radiographic and navigation data.
Each patient’s knee varus-valgus angles were captured by Dr. Teo, an adult reconstruction surgeon, in standard fashion from maximal extension to 0º, 30º, 45º, 60º, 90º, and maximal flexion. An example of standard data capture appears in Table 1. With varus-hyperextension defined as –0.5° or less (more negative), neutral as 0°, and valgus-flexion as 0.5° or more, there were 362 varus knees, 41 valgus knees, and no neutral knees.
Study inclusion criteria were OA and varus deformity. Exclusion criteria were rheumatoid arthritis, other types of inflammatory arthritis, neuromuscular disorders, knees with valgus angulation, and incomplete data (Table 2). Figure 1 summarizes the inclusion/exclusion process, which left 317 knees available for study. Cases of incomplete data were likely due to computer errors or to inadvertent movement when navigation data were being acquired during surgery.
In conventional TKA, the main objective is to equalize flexion-extension gaps with knee at 90° flexion and 0° extension. The ability to achieve this often implies the knee will be balanced throughout its range of motion (ROM). From the data for the 317 study knees, 3 sets of values were extracted: varus angles from maximal knee extension (extension varus), varus angles from 90° knee flexion (flexion varus), and maximal knee extension. All knees were able to achieve 90° flexion.
Power Calculation
Our analysis used a correlation coefficient (r) of at least 0.5 at a 5% level of significance and power of 80%. With 317 knees, the study was more than adequately powered for significance.
Surgical and Navigation Technique
All patients underwent either general or regional anesthesia for their surgeries, which were performed by Dr. Teo. Standard medial parapatellar arthrotomy was performed. Navigation pins were then inserted into the femur and tibia outside the knee wound. Anatomical reference points were digitized per routine navigation requirements. (The reference for varus-valgus alignment of the femur is the mechanical femur axis defined by the digitized hip center and knee center, and the reference for varus-valgus alignment of the tibia is the mechanical tibia axis defined by the digitized tibia center and calculated ankle center. The ankle center is calculated by dividing the digitized transmalleolar axis according to a ratio of 56% lateral to 44% medial with the inherent navigation software.) Our institution uses an imageless navigation system (Navigation System II; Stryker Orthopedics, Mahwah, New Jersey).
The leg was then brought from maximal knee extension to maximal knee flexion to assess preoperative ROM, which indicates inherent flexion contracture or hyperextension. Varus-valgus measurements of the knee were then generated as part of the navigation software protocol. These measurements were obtained without additional varus or valgus stress applied to the knee and before any bony resection. The rest of the operation was completed using navigation to guide bony resection and soft-tissue balancing. The final components used were all cemented cruciate-substituting TKA implants. After component insertion, the knee was again brought through ROM from maximal knee extension to maximal knee flexion to assess postoperative ROM before wound closure.
Extension and Flexion Varus
As none of the patients in the flexion varus dataset (range, –0.5° to –19°) had a varus deformity of more than 20° at 90° flexion, we used a cutoff of 10° to divide these patients into 2 subgroups: less than 10° (237 knees) and 10° or more (80 knees). The extension varus dataset ranged from –0.5° to –24°. Incremental values of –0.5° to –24° in this dataset were then analyzed against the 90° flexion varus subgroups using logistic regression. A scatterplot of the relationship between extension and flexion varus is shown in Figure 2. The probability function was then derived and a probability graph plotted.
FFD and Extension and Flexion Varus
Maximal knee extension, obtained from intraoperative navigation measurements, ranged from –9° (hyperextension) to 33° (FFD) and maximal knee flexion ranged from 90° to 146°. Ninety-two knees had slight hyperextension, and 6 were neutral. Of the 317 OA knees with varus deformity, 219 (69%) had FFD. This sagittal plane alignment parameter was analyzed against coronal plane alignment in maximal knee extension and 90° knee flexion to determine if increasing severity of FFD corresponds with increasing extension or flexion varus.
Statistical Analysis
Statistical analysis was performed with Stata 10.1 (Statacorp, College Station, Texas). Significance was set at P < .05.
Results
Extension and Flexion Varus
Patient demographic data are listed in Table 3. Univariate logistic regression analysis revealed that age (P = .110), body mass index (P = .696), and sex (P = .584) did not affect the association between preoperative extension and flexion varus.
Mean (SD) preoperative extension varus was –9.9° (4.80°), and mean (SD) preoperative flexion 90° varus was –7.02° (3.74°). Linear regression of the data showed a significant positive correlation between preoperative extension varus and flexion varus (Pearson correlation coefficient, 0.57; P < .0001). The probability function was determined as follows: Probability of having flexion varus of more than 10° = 1 / (1 + e–z), where z = –4.014 – 0.265 × extension varus. Plotting the probability graph of flexion varus against varus angles at maximal knee extension from the probability formula yielded a sigmoid graph (Figure 3). The most linear part of the graph corresponds to the 10° to 20° of extension varus (solid line), demonstrating an almost linear increase in the probability of having more than 10° flexion varus with increasing extension varus from 10° to 20°. For extension varus of 20° or more, the probability of having flexion varus of more than 10° approaches 1.
FFD and Extension and Flexion Varus
Mean (SD) preoperative maximal knee extension (analogous to FFD) was 4.41° (7.50°), mean (SD) extension varus was –9.9° (4.80°), and mean (SD) 90° flexion varus was –7.02° (3.74°). We did not find any correlation between preoperative FFD and preoperative flexion varus (r = –0.02; P = .6583) or extension varus (r = –0.11; P = .046) (Figure 4).
Postoperative Alignment
Of the 317 OA knees, 18 had incomplete navigation-acquired postoperative alignment data. The postoperative alignment of the other 299 knees at various degrees of knee flexion is illustrated with a box-and-whisker plot (Figure 5).
Knees With Severe Extension Varus
Fourteen of the 15 knees with severe extension varus (>20°) had flexion varus of more than 9° (range, –9° to –17.5°, with only 1 outlier, at –5°). For the 15 patients, maximal knee extension ranged from –9° hyperextension to 27.5° FFD. Six knees had slight hyperextension, and 9 had FFD demonstrating large variability in sagittal alignment. Despite severe preoperative coronal deformity, all 15 knees had satisfactory deformity correction. Preoperative and postoperative knee alignment data for these 15 knees appear in Table 4 and Figure 6, respectively.
Discussion
OA varus knees represent a majority of the cases being managed by orthopedic surgeons. Soft-tissue contractures involving the medial collateral ligament (MCL), posteromedial capsule, pes anserinus, and semimembranosus muscle are commonly encountered. Bone loss may also occur on the tibial and femoral joint surfaces in knees with severe angular deformity. In an OA varus knee, bone loss tends to be mainly on the medial tibial plateau and usually on the posterior aspect of the tibia because flexion contractures often are concomitant with these marked deformities.11 Therefore, a varus deformity is apparent whether the knee is extended or flexed. Our results showed a correlation between extension and flexion varus in OA varus knees. In contrast, for a valgus deformity, as bone loss can occur on both the tibial and femoral surfaces,11 a similar correlation may not be seen. For that reason, and because there were only 41 valgus knees in this study, they were excluded. For FFD, soft-tissue contractures often involve both the posterior capsule and the posterior cruciate ligament (PCL). Posterior osteophytes often cause tenting of the posterior capsule in knees with FFD. Anteriorly, growth of osteophytes at the tibial spine and intercondylar notch of the femur can result in bony causes of restricted knee extension.12
One would expect increased coronal plane angular deformity to correspond to more severe FFD in the sagittal plane because the same pathology affects soft tissue or bones in an OA knee in both planes. Interestingly, our study results proved otherwise. FFD did not correlate with degree of extension or flexion varus severity. This phenomenon has not been described in the literature likely because clinical measurements of flexion varus and FFD were difficult to perform because of the spatial alignment of the knee in flexion. In recent years, however, computer navigation technology has made such measurements possible.
Mihalko and colleagues2 established that soft-tissue releases on different parts of the proximal tibia have different effects on soft-tissue balancing in flexion and extension. In knees with extension varus, more releases are required on the posterior medial aspect of the tibia (the posterior oblique fibers of the superficial MCL, the posteromedial capsule, and, sometimes, the semimembranosus), whereas knees with flexion varus require more releases on the anterior medial aspect of the tibia (the deep MCL, the anterior fibers of the superficial MCL, and, sometimes, the pes anserinus attachment).13 Consequently, soft-tissue stabilizers seem to have different functions in flexion and extension and cannot reliably be released solely in extension or flexion for optimal gap balancing during TKA.2 Other authors, in cadaveric studies, have found that a larger amount of coronal deformity correction is achieved with more distal soft-tissue releases from the joint line.9,14 Surgical techniques for correcting FFD include removal of prominent anterior and posterior osteophytes, posterior capsular releases, sometimes PCL sacrifices, and even gastrocnemius recession.12
In our study, all 14 patients with severe extension and correspondingly severe flexion varus needed not only modest posterior medial soft-tissue releases for the severe extension varus, but also modest anterior medial releases for the flexion varus. The respective soft-tissue releases were confirmed in real time with computer navigation sequentially after bony resection and osteophyte removal. With this method, we restored final postoperative alignment to within 3° of the mechanical axis (Figure 6). Our experience here led us to believe that, with these patients, modest anterior medial and posterior medial releases could be performed at the start of surgery, as severe extension varus (>20°) almost certainly equates to severe flexion varus (>10°). Therein lies the clinical relevance of our study. However, not all patients with severe coronal plane deformity have correspondingly severe sagittal plane deformity in the form of FFD, as illustrated in our study. Therefore, not all patients with severe varus knee deformity need aggressive posterior capsular release or PCL recession to correct FFD. Some patients have mild hyperextension, which can be attributed partly to the postanesthesia effects of soft-tissue laxity. It is unclear exactly how much anesthesia contributes to this difference in sagittal alignment, though the majority of our patients had FFD. It is not our intent here to discuss the surgical techniques of soft-tissue balancing or to advocate routine use of computer navigation.
Many factors (eg, medial femoral condyle bone loss, medial tibial plateau bone loss, femur or tibia bowing, medial soft-tissue contracture) can contribute to varus malalignment. Current navigation technology cannot isolate the causes of varus alignment, and we did not intend to investigate them in this study. Our primary aim was to assess for a correlation between overall extension varus alignment and expected flexion varus. We also wanted to analyze the correlation between FFD and the coronal plane alignment, in extension and flexion, contributed by the combined bony and soft-tissue components in OA varus knees.
The strengths of this study are that it was a single-surgeon series with knee data from consecutive patients who had computer-navigated TKA. Patient data were prospectively generated from the navigation software and retrospectively analyzed. All navigation alignment was performed by a single surgeon, thereby eliminating examination bias during the time knee alignment data were being obtained. The study was adequately powered and had a large number of patients for data analysis. The authors believe that this is the first study to analyze alignment in both the coronal and sagittal plane in varus OA knees.
We acknowledge a few limitations in our study. Although several investigators have found that navigation can be used to achieve accurate postoperative alignment,10,15,16 subtle errors may be inadvertently introduced at different points of alignment measurement. These error points include identification of visually selected anatomical landmarks; kinematic registration of hip, knee, and ankle; and intraoperative changes in the navigation environment (eg, inadvertent movement of pins or rigid bodies). In addition, different surgeons have different techniques for kinematic registration. However, the surgeries in our study were performed by the same surgeon, so this confounding factor was effectively removed. Another limitation was that navigation alignment was obtained during surgery, when patients were under anesthesia and in a supine, non-weight-bearing position, whereas routine clinical weight-bearing radiographs are taken with nonanesthetized patients and this might overestimate the deformities intraoperatively. However, all parameters were measured in the same patient under the same anesthetic effects, so this should not have affected the analyses. Most surgeons would make an intraoperative assessment of the severity of any deformity before the surgery proper anyway. Nevertheless, some authors have found that knee alignment obtained with intraoperative navigation correlated well with alignment obtained with weight-bearing radiographs.17,18
Conclusion
Our study results showed that, in OA varus knees, extension varus highly correlated with flexion varus. However, there was no correlation between FFD and coronal plane varus deformity.
1. Engh GA. The difficult knee: severe varus and valgus. Clin Orthop. 2003;(416):58-63.
2. Mihalko WM, Saleh KJ, Krackow KA, Whiteside LA. Soft-tissue balancing during total knee arthroplasty in the varus knee. J Am Acad Orthop Surg. 2009;17(12):766-774.
3. Ranawat CS, Flynn WF Jr, Saddler S, Hansraj KK, Maynard MJ. Long-term results of the total condylar knee arthroplasty. A 15-year survivorship study. Clin Orthop. 1993;(286):94-102.
4. Ritter MA, Faris GW, Faris PM, Davis KE. Total knee arthroplasty in patients with angular varus or valgus deformities of > or = 20 degrees. J Arthroplasty. 2004;19(7):862-866.
5. Parratte S, Pagnano MW. Instability after total knee arthroplasty. J Bone Joint Surg Am. 2008;90(1):184-194.
6. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop. 2002;(404):7-13.
7. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Luring C, Hüfner T, Perlick L, Bäthis H, Krettek C, Grifka J. The effectiveness of sequential medial soft tissue release on coronal alignment in total knee arthroplasty: using a computer navigation model. J Arthroplasty. 2006;21(3):428-434.
10. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
11. Insall JN, Easley ME. Surgical techniques and instrumentation in total knee arthroplasty. In: Insall JN, Scott WN, eds. Surgery of the Knee. Vol 2. 3rd ed. New York, NY: Churchill Livingstone; 2001:1553-1620.
12. Scuderi GR, Tria AJ, eds. Surgical Techniques in Total Knee Arthroplasty. New York, NY: Springer-Verlag; 2002.
13. Whiteside LA, Saeki K, Mihalko WM. Functional medial ligament balancing in total knee arthroplasty. Clin Orthop. 2000;(380):45-57.
14. Matsueda M, Gengerke TR, Murphy M, Lew WD, Gustilo RB. Soft tissue release in total knee arthroplasty. Cadaver study using knees without deformities. Clin Orthop. 1999;(366):264-273.
15. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop. 2005;(433):152-159.
16. Mullaji AB, Kanna R, Marawar S, Kohli A, Sharma A. Comparison of limb and component alignment using computer-assisted navigation versus image intensifier–guided conventional total knee arthroplasty: a prospective, randomized, single-surgeon study of 467 knees. J Arthroplasty. 2007;22(7):953-959.
17. Colebatch AN, Hart DJ, Zhai G, Williams FM, Spector TD, Arden NK. Effective measurement of knee alignment using AP knee radiographs. Knee. 2009;16(1):42-45.
18. Yaffe MA, Koo SS, Stulberg SD. Radiographic and navigation measurements of TKA limb alignment do not correlate. Clin Orthop. 2008;466(11):2736-2744.
1. Engh GA. The difficult knee: severe varus and valgus. Clin Orthop. 2003;(416):58-63.
2. Mihalko WM, Saleh KJ, Krackow KA, Whiteside LA. Soft-tissue balancing during total knee arthroplasty in the varus knee. J Am Acad Orthop Surg. 2009;17(12):766-774.
3. Ranawat CS, Flynn WF Jr, Saddler S, Hansraj KK, Maynard MJ. Long-term results of the total condylar knee arthroplasty. A 15-year survivorship study. Clin Orthop. 1993;(286):94-102.
4. Ritter MA, Faris GW, Faris PM, Davis KE. Total knee arthroplasty in patients with angular varus or valgus deformities of > or = 20 degrees. J Arthroplasty. 2004;19(7):862-866.
5. Parratte S, Pagnano MW. Instability after total knee arthroplasty. J Bone Joint Surg Am. 2008;90(1):184-194.
6. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop. 2002;(404):7-13.
7. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Luring C, Hüfner T, Perlick L, Bäthis H, Krettek C, Grifka J. The effectiveness of sequential medial soft tissue release on coronal alignment in total knee arthroplasty: using a computer navigation model. J Arthroplasty. 2006;21(3):428-434.
10. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
11. Insall JN, Easley ME. Surgical techniques and instrumentation in total knee arthroplasty. In: Insall JN, Scott WN, eds. Surgery of the Knee. Vol 2. 3rd ed. New York, NY: Churchill Livingstone; 2001:1553-1620.
12. Scuderi GR, Tria AJ, eds. Surgical Techniques in Total Knee Arthroplasty. New York, NY: Springer-Verlag; 2002.
13. Whiteside LA, Saeki K, Mihalko WM. Functional medial ligament balancing in total knee arthroplasty. Clin Orthop. 2000;(380):45-57.
14. Matsueda M, Gengerke TR, Murphy M, Lew WD, Gustilo RB. Soft tissue release in total knee arthroplasty. Cadaver study using knees without deformities. Clin Orthop. 1999;(366):264-273.
15. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop. 2005;(433):152-159.
16. Mullaji AB, Kanna R, Marawar S, Kohli A, Sharma A. Comparison of limb and component alignment using computer-assisted navigation versus image intensifier–guided conventional total knee arthroplasty: a prospective, randomized, single-surgeon study of 467 knees. J Arthroplasty. 2007;22(7):953-959.
17. Colebatch AN, Hart DJ, Zhai G, Williams FM, Spector TD, Arden NK. Effective measurement of knee alignment using AP knee radiographs. Knee. 2009;16(1):42-45.
18. Yaffe MA, Koo SS, Stulberg SD. Radiographic and navigation measurements of TKA limb alignment do not correlate. Clin Orthop. 2008;466(11):2736-2744.
Computer Navigation and Robotics for Total Knee Arthroplasty
Total knee arthroplasty (TKA) is a good surgical option to relieve pain and improve function in patients with osteoarthritis. The goal of surgery is to achieve a well-aligned prosthesis with well-balanced ligaments in order to minimize wear and improve implant survival. Overall, 82% to 89% of patients are satisfied with their outcomes after TKA, with good 10- to 15-year implant survivorship; however, there is still a subset of patients that are unsatisfied. In many cases, patient dissatisfaction is attributed to improper component alignment.1-3 Over the past decade, computer navigation and robotics have been introduced to control surgical variables so as to gain greater consistency in implant placement and postoperative component alignment.
Computer-assisted navigation tools were introduced not only to improve implant alignment but, more importantly, to optimize clinical outcomes. Most studies have demonstrated that the use of navigation is associated with fewer radiographic outliers after TKA.4 Various studies have compared radiographic results of navigated TKA with results of TKA using standard instrumentation.4-7 While long-term studies are necessary, short-term follow-up has shown that computer-assisted TKA can improve alignment, especially in patients with severe deformity.8-10 Currently, there is no definitive consensus that computer-assisted TKA leads to significantly better component alignment or postoperative outcomes due to the fact that many studies are limited by study design or small cohorts. However, the currently published articles support better component alignment and clinical outcomes with computer-assisted TKA. While some argue that the use of computer-assisted surgery is dependent on the user’s experience, computer-assisted surgery can assist less-experienced surgeons to reliably achieve good midterm outcomes with a low complication rate.8,11 Various studies have looked at computer-assisted TKA at midterm follow-up, with no significant differences in clinical outcome between navigated and traditional techniques. However, long-term studies showing the benefits of computer navigation are beginning to emerge. For example, de Steiger and colleagues12 recently found that computer-assisted TKA reduced the overall revision rate for loosening after TKA in patients less than 65 years of age.
While surgical navigation helps improve implant planning, robotic tools have emerged as a tool to help refine surgical execution. Coupled with surgical navigation tools, robotic control of surgical gestures may further enhance precision in implant placement and/or enable novel implant design features. At present, robotic techniques are increasingly used in unicompartmental knee arthroplasty (UKA) and TKA.13 Studies have demonstrated that the robotic tool is 3 times more accurate with 3 times less variability than conventional techniques in UKA.14 The utility of robotic tools for TKA remains unclear. Robotic-driven automatic cutting guides have been shown to reduce time and improve accuracy compared with navigation guides in femoral TKA cutting procedures in a cadaveric model.15 However, robotic-enabled TKA procedures are poorly described at present, and the clinical implications of their proposed improved precision remain unclear.
Computer navigation and robotic tools in TKA hold the promise of enhanced control of surgical variables that influence clinical outcome. The variables that may be impacted by these advanced tools include implant positioning, lower limb alignment, soft-tissue balance, and, potentially, implant design and fixation. At present, these tools have primarily been shown to improve lower limb alignment in TKA. The clinical impact of the enhanced control of this single surgical variable (lower limb alignment) has been muted in short-term and midterm studies. Future studies should be directed at understanding which surgical variable, or combination of variables, it is most essential to precisely control so as to positively impact clinical outcomes. ◾
1. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
3. Emmerson KP, Morgan CG, Pinder IM. Survivorship analysis of the Kinematic Stabilizer total knee replacement: a 10- to 14-year follow-up. J Bone Joint Surg Br. 1996;78(3):441-445.
4. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomized study. J Arthroplasty. 2014;29(12):2373-2377.
5. Sparmann M, Wolke B, Czupalla H, Banzer D, Zink A. Positioning of total knee arthroplasty with and without navigation support. A prospective, randomized study. J Bone Joint Surg Br. 2003;85(6):830-835.
6. Hoffart HE, Langenstein E, Vasak N. A prospective study comparing the functional outcome of computer-assisted and conventional total knee replacement. J Bone Joint Surg Br. 2012;94(2):194-199.
7. Cip J, Widemschek M, Luegmair M, Sheinkop MB, Benesch T, Martin A. Conventional versus computer-assisted technique for total knee arthroplasty: a minimum of 5-year follow-up of 200 patients in a prospective randomized comparative trial. J Arthroplasty. 2014;29(9):1795-1802.
8. Huang TW, Peng KT, Huang KC, Lee MS, Hsu RW. Differences in component and limb alignment between computer-assisted and conventional surgery total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):2954-2961.
9. Lee CY, Lin SJ, Kuo LT, et al. The benefits of computer-assisted total knee arthroplasty on coronal alignment with marked femoral bowing in Asian patients. J Orthop Surg Res. 2014;9:122.
10. Hernandez-Vaquero D, Noriega-Fernandez A, Fernandez-Carreira JM, Fernandez-Simon JM, Llorens de los Rios J. Computer-assisted surgery improves rotational positioning of the femoral component but not the tibial component in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3127-3134.
11. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial [published online ahead of print March 14, 2015]. J Arthroplasty.
12. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
13. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
14. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
15. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
Total knee arthroplasty (TKA) is a good surgical option to relieve pain and improve function in patients with osteoarthritis. The goal of surgery is to achieve a well-aligned prosthesis with well-balanced ligaments in order to minimize wear and improve implant survival. Overall, 82% to 89% of patients are satisfied with their outcomes after TKA, with good 10- to 15-year implant survivorship; however, there is still a subset of patients that are unsatisfied. In many cases, patient dissatisfaction is attributed to improper component alignment.1-3 Over the past decade, computer navigation and robotics have been introduced to control surgical variables so as to gain greater consistency in implant placement and postoperative component alignment.
Computer-assisted navigation tools were introduced not only to improve implant alignment but, more importantly, to optimize clinical outcomes. Most studies have demonstrated that the use of navigation is associated with fewer radiographic outliers after TKA.4 Various studies have compared radiographic results of navigated TKA with results of TKA using standard instrumentation.4-7 While long-term studies are necessary, short-term follow-up has shown that computer-assisted TKA can improve alignment, especially in patients with severe deformity.8-10 Currently, there is no definitive consensus that computer-assisted TKA leads to significantly better component alignment or postoperative outcomes due to the fact that many studies are limited by study design or small cohorts. However, the currently published articles support better component alignment and clinical outcomes with computer-assisted TKA. While some argue that the use of computer-assisted surgery is dependent on the user’s experience, computer-assisted surgery can assist less-experienced surgeons to reliably achieve good midterm outcomes with a low complication rate.8,11 Various studies have looked at computer-assisted TKA at midterm follow-up, with no significant differences in clinical outcome between navigated and traditional techniques. However, long-term studies showing the benefits of computer navigation are beginning to emerge. For example, de Steiger and colleagues12 recently found that computer-assisted TKA reduced the overall revision rate for loosening after TKA in patients less than 65 years of age.
While surgical navigation helps improve implant planning, robotic tools have emerged as a tool to help refine surgical execution. Coupled with surgical navigation tools, robotic control of surgical gestures may further enhance precision in implant placement and/or enable novel implant design features. At present, robotic techniques are increasingly used in unicompartmental knee arthroplasty (UKA) and TKA.13 Studies have demonstrated that the robotic tool is 3 times more accurate with 3 times less variability than conventional techniques in UKA.14 The utility of robotic tools for TKA remains unclear. Robotic-driven automatic cutting guides have been shown to reduce time and improve accuracy compared with navigation guides in femoral TKA cutting procedures in a cadaveric model.15 However, robotic-enabled TKA procedures are poorly described at present, and the clinical implications of their proposed improved precision remain unclear.
Computer navigation and robotic tools in TKA hold the promise of enhanced control of surgical variables that influence clinical outcome. The variables that may be impacted by these advanced tools include implant positioning, lower limb alignment, soft-tissue balance, and, potentially, implant design and fixation. At present, these tools have primarily been shown to improve lower limb alignment in TKA. The clinical impact of the enhanced control of this single surgical variable (lower limb alignment) has been muted in short-term and midterm studies. Future studies should be directed at understanding which surgical variable, or combination of variables, it is most essential to precisely control so as to positively impact clinical outcomes. ◾
Total knee arthroplasty (TKA) is a good surgical option to relieve pain and improve function in patients with osteoarthritis. The goal of surgery is to achieve a well-aligned prosthesis with well-balanced ligaments in order to minimize wear and improve implant survival. Overall, 82% to 89% of patients are satisfied with their outcomes after TKA, with good 10- to 15-year implant survivorship; however, there is still a subset of patients that are unsatisfied. In many cases, patient dissatisfaction is attributed to improper component alignment.1-3 Over the past decade, computer navigation and robotics have been introduced to control surgical variables so as to gain greater consistency in implant placement and postoperative component alignment.
Computer-assisted navigation tools were introduced not only to improve implant alignment but, more importantly, to optimize clinical outcomes. Most studies have demonstrated that the use of navigation is associated with fewer radiographic outliers after TKA.4 Various studies have compared radiographic results of navigated TKA with results of TKA using standard instrumentation.4-7 While long-term studies are necessary, short-term follow-up has shown that computer-assisted TKA can improve alignment, especially in patients with severe deformity.8-10 Currently, there is no definitive consensus that computer-assisted TKA leads to significantly better component alignment or postoperative outcomes due to the fact that many studies are limited by study design or small cohorts. However, the currently published articles support better component alignment and clinical outcomes with computer-assisted TKA. While some argue that the use of computer-assisted surgery is dependent on the user’s experience, computer-assisted surgery can assist less-experienced surgeons to reliably achieve good midterm outcomes with a low complication rate.8,11 Various studies have looked at computer-assisted TKA at midterm follow-up, with no significant differences in clinical outcome between navigated and traditional techniques. However, long-term studies showing the benefits of computer navigation are beginning to emerge. For example, de Steiger and colleagues12 recently found that computer-assisted TKA reduced the overall revision rate for loosening after TKA in patients less than 65 years of age.
While surgical navigation helps improve implant planning, robotic tools have emerged as a tool to help refine surgical execution. Coupled with surgical navigation tools, robotic control of surgical gestures may further enhance precision in implant placement and/or enable novel implant design features. At present, robotic techniques are increasingly used in unicompartmental knee arthroplasty (UKA) and TKA.13 Studies have demonstrated that the robotic tool is 3 times more accurate with 3 times less variability than conventional techniques in UKA.14 The utility of robotic tools for TKA remains unclear. Robotic-driven automatic cutting guides have been shown to reduce time and improve accuracy compared with navigation guides in femoral TKA cutting procedures in a cadaveric model.15 However, robotic-enabled TKA procedures are poorly described at present, and the clinical implications of their proposed improved precision remain unclear.
Computer navigation and robotic tools in TKA hold the promise of enhanced control of surgical variables that influence clinical outcome. The variables that may be impacted by these advanced tools include implant positioning, lower limb alignment, soft-tissue balance, and, potentially, implant design and fixation. At present, these tools have primarily been shown to improve lower limb alignment in TKA. The clinical impact of the enhanced control of this single surgical variable (lower limb alignment) has been muted in short-term and midterm studies. Future studies should be directed at understanding which surgical variable, or combination of variables, it is most essential to precisely control so as to positively impact clinical outcomes. ◾
1. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
3. Emmerson KP, Morgan CG, Pinder IM. Survivorship analysis of the Kinematic Stabilizer total knee replacement: a 10- to 14-year follow-up. J Bone Joint Surg Br. 1996;78(3):441-445.
4. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomized study. J Arthroplasty. 2014;29(12):2373-2377.
5. Sparmann M, Wolke B, Czupalla H, Banzer D, Zink A. Positioning of total knee arthroplasty with and without navigation support. A prospective, randomized study. J Bone Joint Surg Br. 2003;85(6):830-835.
6. Hoffart HE, Langenstein E, Vasak N. A prospective study comparing the functional outcome of computer-assisted and conventional total knee replacement. J Bone Joint Surg Br. 2012;94(2):194-199.
7. Cip J, Widemschek M, Luegmair M, Sheinkop MB, Benesch T, Martin A. Conventional versus computer-assisted technique for total knee arthroplasty: a minimum of 5-year follow-up of 200 patients in a prospective randomized comparative trial. J Arthroplasty. 2014;29(9):1795-1802.
8. Huang TW, Peng KT, Huang KC, Lee MS, Hsu RW. Differences in component and limb alignment between computer-assisted and conventional surgery total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):2954-2961.
9. Lee CY, Lin SJ, Kuo LT, et al. The benefits of computer-assisted total knee arthroplasty on coronal alignment with marked femoral bowing in Asian patients. J Orthop Surg Res. 2014;9:122.
10. Hernandez-Vaquero D, Noriega-Fernandez A, Fernandez-Carreira JM, Fernandez-Simon JM, Llorens de los Rios J. Computer-assisted surgery improves rotational positioning of the femoral component but not the tibial component in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3127-3134.
11. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial [published online ahead of print March 14, 2015]. J Arthroplasty.
12. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
13. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
14. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
15. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
1. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
3. Emmerson KP, Morgan CG, Pinder IM. Survivorship analysis of the Kinematic Stabilizer total knee replacement: a 10- to 14-year follow-up. J Bone Joint Surg Br. 1996;78(3):441-445.
4. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomized study. J Arthroplasty. 2014;29(12):2373-2377.
5. Sparmann M, Wolke B, Czupalla H, Banzer D, Zink A. Positioning of total knee arthroplasty with and without navigation support. A prospective, randomized study. J Bone Joint Surg Br. 2003;85(6):830-835.
6. Hoffart HE, Langenstein E, Vasak N. A prospective study comparing the functional outcome of computer-assisted and conventional total knee replacement. J Bone Joint Surg Br. 2012;94(2):194-199.
7. Cip J, Widemschek M, Luegmair M, Sheinkop MB, Benesch T, Martin A. Conventional versus computer-assisted technique for total knee arthroplasty: a minimum of 5-year follow-up of 200 patients in a prospective randomized comparative trial. J Arthroplasty. 2014;29(9):1795-1802.
8. Huang TW, Peng KT, Huang KC, Lee MS, Hsu RW. Differences in component and limb alignment between computer-assisted and conventional surgery total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):2954-2961.
9. Lee CY, Lin SJ, Kuo LT, et al. The benefits of computer-assisted total knee arthroplasty on coronal alignment with marked femoral bowing in Asian patients. J Orthop Surg Res. 2014;9:122.
10. Hernandez-Vaquero D, Noriega-Fernandez A, Fernandez-Carreira JM, Fernandez-Simon JM, Llorens de los Rios J. Computer-assisted surgery improves rotational positioning of the femoral component but not the tibial component in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3127-3134.
11. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial [published online ahead of print March 14, 2015]. J Arthroplasty.
12. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
13. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
14. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
15. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
Arthroscopic Treatment of Tibial Spine Malunion With Resorbable Screws
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
Recurrent Patellar Tendon Rupture in a Patient After Intramedullary Nailing of the Tibia: Reconstruction Using an Achilles Tendon Allograft
Ruptures of the patellar tendon usually occur in patients under age 40 years, with men having a higher incidence than women.1 History of local steroid injection,2,3 total knee arthroplasty,4-8 anterior cruciate ligament reconstruction with central third patellar tendon autograft,9-11 and a variety of systemic diseases are associated with an increased tendency to rupture.12-15 Primary acute ruptures of the patellar tendon can be difficult to repair because of the quality of remaining tissues. In cases of chronic tendon ruptures subject to delayed treatment, additional complications such as tissue contracture and scar-tissue formation are likely to exist.15-17
Complications after intramedullary (IM) nailing of the tibia include infection, compartment syndrome, deep vein thrombosis, thermal necrosis of the bone with alteration of its endosteal architecture, failure of the hardware, malunion, and nonunion.18 The most common complaint after IM nailing of the tibia is chronic anterior knee pain and symptoms similar to tendonitis; incidences as high as 86% have been reported.18-20 Extensive review of the literature found only 2 reports of patellar tendon rupture after IM nailing of the tibia; both cases used a patellar tendon–splitting approach. The first report described patellar tendon rupture 8 years after IM nailing of the tibia during a forced deep-flexion movement.21 Radiographic examination showed the IM nail positioned proud relative to the tibial plateau, impinging upon the patellar tendon. An intraoperative examination confirmed the radiographic findings and found rupture of the patellar tendon to be consistent with the exposed tip of the IM nail. The second report described patellar tendon rupture 2 months postoperatively in a patient with Ehlers-Danlos syndrome, a hereditary disorder characterized by alterations to muscle/tendon tissue and hyperextensible skin.22
Patellar tendon rupture after IM nailing of the tibia is a rare complication. Patellar tendon re-rupture after primary repair in a patient with history of IM tibial nailing has not been reported. This case outlines the progression of such a patient with a recurrent patellar tendon rupture that was successfully reconstructed using an Achilles tendon allograft. The patient’s surgical history of IM tibial nailing through a mid-patellar tendon–splitting approach 4 years prior to initial tendon rupture is noteworthy and potentially predisposed the patient to injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 44-year-old woman, 5 ft, 3 in tall, and weighing 129 lb (body mass index, 22.8), with a history of osteoporosis and transverse myelitis, presented with pain and persistent swelling about the left knee. Her baseline ambulatory status required crutches because of decreased sensation and strength in her lower extremity in conjunction with a foot drop; she had mild quadriceps and hamstring muscle weakness but otherwise normal knee function. The patient had been seen 4 years earlier at our facility for IM fixation of a distal tibia fracture through a patellar tendon–splitting approach. The fracture was well healed and showed no signs of complication or nail migration; the nail was not proud.
Initially, the patient was admitted to another hospital through the emergency department for swelling and pain about the left knee. She was believed to have an infection and was placed on antibiotics by the primary care team. An orthopedic evaluation showed induration, edema, and warmth in the patellar tendon region of the left knee. Magnetic resonance imaging (MRI) showed a full-thickness patellar tendon rupture. Aspiration of the knee was performed and cultures were negative; white blood cell, erythrocyte sedimentation rate, and C-reactive protein values were normal. The risks and benefits of various treatments were discussed, and surgical intervention was elected to repair the patellar tendon.
Intraoperative findings showed a massive midsubstance rupture of the patellar tendon, accompanied by medial and lateral retinacular tears and a quadriceps tendon partial rupture; the central aspect of the quadriceps tendon attaching to the patella remained intact. The patella was retracted proximally; no evidence of active infection was present. Good-quality tissue remained attached to both the tibial tuberosity and the inferior pole of the patella. A No. 2 FiberWire suture (Arthrex, Inc, Naples, Florida) was used to run whip stitches in the distal end of the patellar tendon and a second No. 2 FiberWire suture was used to run whip stitches in the proximal aspect of the patellar tendon rupture. The 4 ends of the sutures were tied together, thus re-approximating the distal and proximal ends of the ruptured patellar tendon. No bone drilling was used because the midsubstance tear was amenable to good repair with reasonable expectation of healing based on tissue quality. The quadriceps tendon, which was partially torn, was repaired with a No. 1 Vicryl suture (Ethicon, Somerville, New Jersey). The medial and lateral retinacula were also repaired with a No. 1 Vicryl suture. The suturing scheme effectively re-approximated the knee extensor mechanism, and the patient was placed in a knee immobilizer that permitted no flexion for 6 weeks postoperatively.
After 3 months of gradual improvement with physical therapy, the patient returned for a follow-up visit, concerned that her knee function was beginning to decline. Physical examination showed patella alta with a thinned and diminutive palpable tendon in the patellar tendon region. She was capable of active flexion to 90º and extension to 50º, but beyond 50º, she was unable to actively extend; she was capable of full passive extension. MRI showed a repeat full-thickness patellar tendon tear with retraction from the inferior pole of the patella; previous tears to the quadriceps tendon were healed. Because of the recurrent nature of the injury, the patient’s physical examination, MRI findings, and anticipated poor quality of remaining tendon tissue, patellar tendon reconstruction using a cadaveric Achilles tendon allograft was recommended. The patient chose surgery for potential improvement in knee range of motion, active extension, and ambulation.
The previous anterior midline incision was used and carried down through the subcutaneous tissues where a complete rupture of the patellar tendon was identified. A limited amount of good-quality tendon tissue remained at the medial aspect of the tibial tuberosity. The remaining tissue located at the patella’s inferior pole was nonviable for use in surgical repair. Retinacular contractures were released to bring the patella distally; the trochlear groove was used as the anatomic landmark for the patella resting position. During reconstruction, the knee was placed into 30° of flexion, with the patella located in the trochlear groove, and the cadaveric Achilles tendon was placed on the midline of the patella, where measurements were done to assess proper length and tension (Figure 1).
The patient’s remaining native tissue on the medial aspect of the tibial tuberosity was used to augment the Achilles tendon graft medially. The cadaveric Achilles tendon graft was primarily used to replace the central and lateral aspects of the patellar tendon. Additionally, the calcaneal bone segment at the end of the Achilles tendon graft was removed prior to use. Cadaveric and host tissues at the medial aspect of the tibial tuberosity were sutured together with a No. 1 Vicryl suture (Figure 2). The distal aspect of the cadaveric Achilles tendon was used to re-approximate the patient’s native patellar tendon insertion at the tibial tuberosity. To supplement the graft anchor, a Richards metallic ligament staple (Smith & Nephew, Memphis, Tennessee) was used to fix the distal aspect of the Achilles tendon graft into the tibial tuberosity.
Proper tensioning of the graft was performed by visualizing patella tracking during the arc-of-knee motion and properly suturing the graft to allow for functional range. The proximal aspect of the cadaveric Achilles tendon was sutured into host tissues surrounding the superior pole of the patellar and quadriceps tendon. The edges of the graft were sutured with supplemental No. 1 Vicryl sutures (Figure 3).
Before surgical closure, knee range of motion was checked and noted to be 0º to 100º. The repaired construct was stable and uncompromised throughout the entire range of motion. Patella tracking was central and significantly improved; knee stability was normal to varus and valgus stress.
The patient was placed in a knee immobilizer for 6 weeks before range of motion was allowed. Seven months postoperatively, the patient returned for a follow-up visit, ambulating with 2 forearm crutches, which was her baseline ambulatory status. Physical examination revealed passive range of motion from 0º to 130º, an extension lag of 10º, and 4/5 quadriceps strength. It was recommended the patient continue physical therapy to improve strength and range of motion.
Conclusion
This is the first report in the literature documenting a recurrent patellar tendon rupture after primary repair in a patient with a history of IM tibial nailing. It is also the first report of a cadaveric Achilles tendon allograft used as a solution to this problem. Complete reconstruction of the patellar tendon using an Achilles tendon allograft is a method commonly used for ruptures after total knee arthroplasty.4-7,23,24 This case report highlights the utility of a cadaveric Achilles tendon in the setting of a recurrent patellar tendon rupture with poor remaining tissue quality.
1. Scott WN, Insall JN. Injuries of the knee. In: Rockwood CA Jr, Green DP, Bucholz RW, eds. Fractures in Adults. 3rd ed. Philadelphia, PA: JB Lippincott; 1991: 1799-1914.
2. Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12(4):300-301.
3. Unverferth LJ, Olix ML. The effect of local steroid injections on tendon. J Sports Med. 1973;1(4):31-37.
4. Cadambi A, Engh GA. Use of a semitendinosus tendon autogenous graft for rupture of the patellar ligament after total knee arthroplasty. A report of seven cases. J Bone Joint Surg Am. 1992;74(7):974-979.
5. Emerson RH Jr, Head WC, Malinin TI. Reconstruction of patellar tendon rupture after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop.1990;(260):154-161.
6. Gustillo RB, Thompson R. Quadriceps and patellar tendon ruptures following total knee arthroplasty. In: Rand JA, Dorr LD, eds. Total Arthroplasty of the Knee: Proceedings of the Knee Society, 1985-1986. Rockville, MD: Aspen; 1987: 41-70.
7. Rand JA, Morrey BF, Bryan RS. Patellar tendon rupture after total knee arthroplasty. Clin Orthop. 1989;(244):233-238.
8. Schoderbek RJ, Brown TE, Mulhall KJ, et al. Extensor mechanism disruption after total knee arthroplasty. Clin Orthop. 2006;446:176-185.
9. Bonamo JJ, Krinik RM, Sporn AA. Rupture of the patellar ligament after use of the central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am. 1984;66(8):1294-1297.
10. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
11. Mickelsen PL, Morgan SJ, Johnson WA, Ferrari JD. Patellar tendon rupture 3 years after anterior cruciate ligament reconstruction with a central one third bone-patellar tendon-bone graft. Arthroscopy. 2001;17(6):648-652.
12. Morgan J, McCarty DJ. Tendon ruptures in patients with systemic lupus erythematosus treated with corticosteroids. Arthritis Rheum. 1974;17(6):1033-1036.
13. Webb LX, Toby EB. Bilateral rupture of the patellar tendon in an otherwise healthy male patient following minor trauma. J Trauma. 1986;26(11):1045-1048.
14. Greis PE, Holmstrom MC, Lahav A. Surgical treatment options for patella tendon rupture, Part I: Acute. Orthopedics. 2005;28(7):672-679.
15. Greis PE, Lahav A, Holstrom MC. Surgical treatment options for patella tendon rupture, part II: chronic. Orthopedics. 2005;28(8):765-769.
16. Lewis PB, Rue JP, Bach BR Jr. Chronic patellar tendon rupture: surgical reconstruction technique using 2 Achilles tendon allografts. J Knee Surg. 2008;21(12):130-135.
17. McNally PD, Marcelli EA. Achilles tendon allograft of a chronic patellar tendon rupture. Arthroscopy. 1998;14(3):340-344.
18. Katsoulis E, Court-Brown C, Giannoudis PV. Incidence and atieology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br. 2006;88(5):576-580.
19. Brumback RJ, Uwagie-Ero S, Lakatos RP, et al. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70(1):1453-1462.
20. Koval KJ, Clapper MF, Brumback RJ, et al. Complications of reamed intramedullary nailing of the tibia. J Orthop Trauma. 1991;5(2):184-189.
21. Kretzler JE, Curtin SL, Wegner DA, Baumgaertner MR, Galloway MT. Patella tendon rupture: a late complication of a tibial nail. Orthopedics. 1995;18(11):1109-1111.
22. Moroney P, McCarthy T, Borton D. Patellar tendon rupture post reamed intra-medullary tibial nail in a patient with Ehlers-Danlos syndrome. A case report. Eur J Orthop Surg Traumatol. 2004;14(1):50-51.
23. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
24. Falconiero RP, Pallis MP. Chronic rupture of a patellar tendon: a technique for reconstruction with Achilles allograft. Arthroscopy. 1996;12(5):623-626.
Ruptures of the patellar tendon usually occur in patients under age 40 years, with men having a higher incidence than women.1 History of local steroid injection,2,3 total knee arthroplasty,4-8 anterior cruciate ligament reconstruction with central third patellar tendon autograft,9-11 and a variety of systemic diseases are associated with an increased tendency to rupture.12-15 Primary acute ruptures of the patellar tendon can be difficult to repair because of the quality of remaining tissues. In cases of chronic tendon ruptures subject to delayed treatment, additional complications such as tissue contracture and scar-tissue formation are likely to exist.15-17
Complications after intramedullary (IM) nailing of the tibia include infection, compartment syndrome, deep vein thrombosis, thermal necrosis of the bone with alteration of its endosteal architecture, failure of the hardware, malunion, and nonunion.18 The most common complaint after IM nailing of the tibia is chronic anterior knee pain and symptoms similar to tendonitis; incidences as high as 86% have been reported.18-20 Extensive review of the literature found only 2 reports of patellar tendon rupture after IM nailing of the tibia; both cases used a patellar tendon–splitting approach. The first report described patellar tendon rupture 8 years after IM nailing of the tibia during a forced deep-flexion movement.21 Radiographic examination showed the IM nail positioned proud relative to the tibial plateau, impinging upon the patellar tendon. An intraoperative examination confirmed the radiographic findings and found rupture of the patellar tendon to be consistent with the exposed tip of the IM nail. The second report described patellar tendon rupture 2 months postoperatively in a patient with Ehlers-Danlos syndrome, a hereditary disorder characterized by alterations to muscle/tendon tissue and hyperextensible skin.22
Patellar tendon rupture after IM nailing of the tibia is a rare complication. Patellar tendon re-rupture after primary repair in a patient with history of IM tibial nailing has not been reported. This case outlines the progression of such a patient with a recurrent patellar tendon rupture that was successfully reconstructed using an Achilles tendon allograft. The patient’s surgical history of IM tibial nailing through a mid-patellar tendon–splitting approach 4 years prior to initial tendon rupture is noteworthy and potentially predisposed the patient to injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 44-year-old woman, 5 ft, 3 in tall, and weighing 129 lb (body mass index, 22.8), with a history of osteoporosis and transverse myelitis, presented with pain and persistent swelling about the left knee. Her baseline ambulatory status required crutches because of decreased sensation and strength in her lower extremity in conjunction with a foot drop; she had mild quadriceps and hamstring muscle weakness but otherwise normal knee function. The patient had been seen 4 years earlier at our facility for IM fixation of a distal tibia fracture through a patellar tendon–splitting approach. The fracture was well healed and showed no signs of complication or nail migration; the nail was not proud.
Initially, the patient was admitted to another hospital through the emergency department for swelling and pain about the left knee. She was believed to have an infection and was placed on antibiotics by the primary care team. An orthopedic evaluation showed induration, edema, and warmth in the patellar tendon region of the left knee. Magnetic resonance imaging (MRI) showed a full-thickness patellar tendon rupture. Aspiration of the knee was performed and cultures were negative; white blood cell, erythrocyte sedimentation rate, and C-reactive protein values were normal. The risks and benefits of various treatments were discussed, and surgical intervention was elected to repair the patellar tendon.
Intraoperative findings showed a massive midsubstance rupture of the patellar tendon, accompanied by medial and lateral retinacular tears and a quadriceps tendon partial rupture; the central aspect of the quadriceps tendon attaching to the patella remained intact. The patella was retracted proximally; no evidence of active infection was present. Good-quality tissue remained attached to both the tibial tuberosity and the inferior pole of the patella. A No. 2 FiberWire suture (Arthrex, Inc, Naples, Florida) was used to run whip stitches in the distal end of the patellar tendon and a second No. 2 FiberWire suture was used to run whip stitches in the proximal aspect of the patellar tendon rupture. The 4 ends of the sutures were tied together, thus re-approximating the distal and proximal ends of the ruptured patellar tendon. No bone drilling was used because the midsubstance tear was amenable to good repair with reasonable expectation of healing based on tissue quality. The quadriceps tendon, which was partially torn, was repaired with a No. 1 Vicryl suture (Ethicon, Somerville, New Jersey). The medial and lateral retinacula were also repaired with a No. 1 Vicryl suture. The suturing scheme effectively re-approximated the knee extensor mechanism, and the patient was placed in a knee immobilizer that permitted no flexion for 6 weeks postoperatively.
After 3 months of gradual improvement with physical therapy, the patient returned for a follow-up visit, concerned that her knee function was beginning to decline. Physical examination showed patella alta with a thinned and diminutive palpable tendon in the patellar tendon region. She was capable of active flexion to 90º and extension to 50º, but beyond 50º, she was unable to actively extend; she was capable of full passive extension. MRI showed a repeat full-thickness patellar tendon tear with retraction from the inferior pole of the patella; previous tears to the quadriceps tendon were healed. Because of the recurrent nature of the injury, the patient’s physical examination, MRI findings, and anticipated poor quality of remaining tendon tissue, patellar tendon reconstruction using a cadaveric Achilles tendon allograft was recommended. The patient chose surgery for potential improvement in knee range of motion, active extension, and ambulation.
The previous anterior midline incision was used and carried down through the subcutaneous tissues where a complete rupture of the patellar tendon was identified. A limited amount of good-quality tendon tissue remained at the medial aspect of the tibial tuberosity. The remaining tissue located at the patella’s inferior pole was nonviable for use in surgical repair. Retinacular contractures were released to bring the patella distally; the trochlear groove was used as the anatomic landmark for the patella resting position. During reconstruction, the knee was placed into 30° of flexion, with the patella located in the trochlear groove, and the cadaveric Achilles tendon was placed on the midline of the patella, where measurements were done to assess proper length and tension (Figure 1).
The patient’s remaining native tissue on the medial aspect of the tibial tuberosity was used to augment the Achilles tendon graft medially. The cadaveric Achilles tendon graft was primarily used to replace the central and lateral aspects of the patellar tendon. Additionally, the calcaneal bone segment at the end of the Achilles tendon graft was removed prior to use. Cadaveric and host tissues at the medial aspect of the tibial tuberosity were sutured together with a No. 1 Vicryl suture (Figure 2). The distal aspect of the cadaveric Achilles tendon was used to re-approximate the patient’s native patellar tendon insertion at the tibial tuberosity. To supplement the graft anchor, a Richards metallic ligament staple (Smith & Nephew, Memphis, Tennessee) was used to fix the distal aspect of the Achilles tendon graft into the tibial tuberosity.
Proper tensioning of the graft was performed by visualizing patella tracking during the arc-of-knee motion and properly suturing the graft to allow for functional range. The proximal aspect of the cadaveric Achilles tendon was sutured into host tissues surrounding the superior pole of the patellar and quadriceps tendon. The edges of the graft were sutured with supplemental No. 1 Vicryl sutures (Figure 3).
Before surgical closure, knee range of motion was checked and noted to be 0º to 100º. The repaired construct was stable and uncompromised throughout the entire range of motion. Patella tracking was central and significantly improved; knee stability was normal to varus and valgus stress.
The patient was placed in a knee immobilizer for 6 weeks before range of motion was allowed. Seven months postoperatively, the patient returned for a follow-up visit, ambulating with 2 forearm crutches, which was her baseline ambulatory status. Physical examination revealed passive range of motion from 0º to 130º, an extension lag of 10º, and 4/5 quadriceps strength. It was recommended the patient continue physical therapy to improve strength and range of motion.
Conclusion
This is the first report in the literature documenting a recurrent patellar tendon rupture after primary repair in a patient with a history of IM tibial nailing. It is also the first report of a cadaveric Achilles tendon allograft used as a solution to this problem. Complete reconstruction of the patellar tendon using an Achilles tendon allograft is a method commonly used for ruptures after total knee arthroplasty.4-7,23,24 This case report highlights the utility of a cadaveric Achilles tendon in the setting of a recurrent patellar tendon rupture with poor remaining tissue quality.
Ruptures of the patellar tendon usually occur in patients under age 40 years, with men having a higher incidence than women.1 History of local steroid injection,2,3 total knee arthroplasty,4-8 anterior cruciate ligament reconstruction with central third patellar tendon autograft,9-11 and a variety of systemic diseases are associated with an increased tendency to rupture.12-15 Primary acute ruptures of the patellar tendon can be difficult to repair because of the quality of remaining tissues. In cases of chronic tendon ruptures subject to delayed treatment, additional complications such as tissue contracture and scar-tissue formation are likely to exist.15-17
Complications after intramedullary (IM) nailing of the tibia include infection, compartment syndrome, deep vein thrombosis, thermal necrosis of the bone with alteration of its endosteal architecture, failure of the hardware, malunion, and nonunion.18 The most common complaint after IM nailing of the tibia is chronic anterior knee pain and symptoms similar to tendonitis; incidences as high as 86% have been reported.18-20 Extensive review of the literature found only 2 reports of patellar tendon rupture after IM nailing of the tibia; both cases used a patellar tendon–splitting approach. The first report described patellar tendon rupture 8 years after IM nailing of the tibia during a forced deep-flexion movement.21 Radiographic examination showed the IM nail positioned proud relative to the tibial plateau, impinging upon the patellar tendon. An intraoperative examination confirmed the radiographic findings and found rupture of the patellar tendon to be consistent with the exposed tip of the IM nail. The second report described patellar tendon rupture 2 months postoperatively in a patient with Ehlers-Danlos syndrome, a hereditary disorder characterized by alterations to muscle/tendon tissue and hyperextensible skin.22
Patellar tendon rupture after IM nailing of the tibia is a rare complication. Patellar tendon re-rupture after primary repair in a patient with history of IM tibial nailing has not been reported. This case outlines the progression of such a patient with a recurrent patellar tendon rupture that was successfully reconstructed using an Achilles tendon allograft. The patient’s surgical history of IM tibial nailing through a mid-patellar tendon–splitting approach 4 years prior to initial tendon rupture is noteworthy and potentially predisposed the patient to injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 44-year-old woman, 5 ft, 3 in tall, and weighing 129 lb (body mass index, 22.8), with a history of osteoporosis and transverse myelitis, presented with pain and persistent swelling about the left knee. Her baseline ambulatory status required crutches because of decreased sensation and strength in her lower extremity in conjunction with a foot drop; she had mild quadriceps and hamstring muscle weakness but otherwise normal knee function. The patient had been seen 4 years earlier at our facility for IM fixation of a distal tibia fracture through a patellar tendon–splitting approach. The fracture was well healed and showed no signs of complication or nail migration; the nail was not proud.
Initially, the patient was admitted to another hospital through the emergency department for swelling and pain about the left knee. She was believed to have an infection and was placed on antibiotics by the primary care team. An orthopedic evaluation showed induration, edema, and warmth in the patellar tendon region of the left knee. Magnetic resonance imaging (MRI) showed a full-thickness patellar tendon rupture. Aspiration of the knee was performed and cultures were negative; white blood cell, erythrocyte sedimentation rate, and C-reactive protein values were normal. The risks and benefits of various treatments were discussed, and surgical intervention was elected to repair the patellar tendon.
Intraoperative findings showed a massive midsubstance rupture of the patellar tendon, accompanied by medial and lateral retinacular tears and a quadriceps tendon partial rupture; the central aspect of the quadriceps tendon attaching to the patella remained intact. The patella was retracted proximally; no evidence of active infection was present. Good-quality tissue remained attached to both the tibial tuberosity and the inferior pole of the patella. A No. 2 FiberWire suture (Arthrex, Inc, Naples, Florida) was used to run whip stitches in the distal end of the patellar tendon and a second No. 2 FiberWire suture was used to run whip stitches in the proximal aspect of the patellar tendon rupture. The 4 ends of the sutures were tied together, thus re-approximating the distal and proximal ends of the ruptured patellar tendon. No bone drilling was used because the midsubstance tear was amenable to good repair with reasonable expectation of healing based on tissue quality. The quadriceps tendon, which was partially torn, was repaired with a No. 1 Vicryl suture (Ethicon, Somerville, New Jersey). The medial and lateral retinacula were also repaired with a No. 1 Vicryl suture. The suturing scheme effectively re-approximated the knee extensor mechanism, and the patient was placed in a knee immobilizer that permitted no flexion for 6 weeks postoperatively.
After 3 months of gradual improvement with physical therapy, the patient returned for a follow-up visit, concerned that her knee function was beginning to decline. Physical examination showed patella alta with a thinned and diminutive palpable tendon in the patellar tendon region. She was capable of active flexion to 90º and extension to 50º, but beyond 50º, she was unable to actively extend; she was capable of full passive extension. MRI showed a repeat full-thickness patellar tendon tear with retraction from the inferior pole of the patella; previous tears to the quadriceps tendon were healed. Because of the recurrent nature of the injury, the patient’s physical examination, MRI findings, and anticipated poor quality of remaining tendon tissue, patellar tendon reconstruction using a cadaveric Achilles tendon allograft was recommended. The patient chose surgery for potential improvement in knee range of motion, active extension, and ambulation.
The previous anterior midline incision was used and carried down through the subcutaneous tissues where a complete rupture of the patellar tendon was identified. A limited amount of good-quality tendon tissue remained at the medial aspect of the tibial tuberosity. The remaining tissue located at the patella’s inferior pole was nonviable for use in surgical repair. Retinacular contractures were released to bring the patella distally; the trochlear groove was used as the anatomic landmark for the patella resting position. During reconstruction, the knee was placed into 30° of flexion, with the patella located in the trochlear groove, and the cadaveric Achilles tendon was placed on the midline of the patella, where measurements were done to assess proper length and tension (Figure 1).
The patient’s remaining native tissue on the medial aspect of the tibial tuberosity was used to augment the Achilles tendon graft medially. The cadaveric Achilles tendon graft was primarily used to replace the central and lateral aspects of the patellar tendon. Additionally, the calcaneal bone segment at the end of the Achilles tendon graft was removed prior to use. Cadaveric and host tissues at the medial aspect of the tibial tuberosity were sutured together with a No. 1 Vicryl suture (Figure 2). The distal aspect of the cadaveric Achilles tendon was used to re-approximate the patient’s native patellar tendon insertion at the tibial tuberosity. To supplement the graft anchor, a Richards metallic ligament staple (Smith & Nephew, Memphis, Tennessee) was used to fix the distal aspect of the Achilles tendon graft into the tibial tuberosity.
Proper tensioning of the graft was performed by visualizing patella tracking during the arc-of-knee motion and properly suturing the graft to allow for functional range. The proximal aspect of the cadaveric Achilles tendon was sutured into host tissues surrounding the superior pole of the patellar and quadriceps tendon. The edges of the graft were sutured with supplemental No. 1 Vicryl sutures (Figure 3).
Before surgical closure, knee range of motion was checked and noted to be 0º to 100º. The repaired construct was stable and uncompromised throughout the entire range of motion. Patella tracking was central and significantly improved; knee stability was normal to varus and valgus stress.
The patient was placed in a knee immobilizer for 6 weeks before range of motion was allowed. Seven months postoperatively, the patient returned for a follow-up visit, ambulating with 2 forearm crutches, which was her baseline ambulatory status. Physical examination revealed passive range of motion from 0º to 130º, an extension lag of 10º, and 4/5 quadriceps strength. It was recommended the patient continue physical therapy to improve strength and range of motion.
Conclusion
This is the first report in the literature documenting a recurrent patellar tendon rupture after primary repair in a patient with a history of IM tibial nailing. It is also the first report of a cadaveric Achilles tendon allograft used as a solution to this problem. Complete reconstruction of the patellar tendon using an Achilles tendon allograft is a method commonly used for ruptures after total knee arthroplasty.4-7,23,24 This case report highlights the utility of a cadaveric Achilles tendon in the setting of a recurrent patellar tendon rupture with poor remaining tissue quality.
1. Scott WN, Insall JN. Injuries of the knee. In: Rockwood CA Jr, Green DP, Bucholz RW, eds. Fractures in Adults. 3rd ed. Philadelphia, PA: JB Lippincott; 1991: 1799-1914.
2. Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12(4):300-301.
3. Unverferth LJ, Olix ML. The effect of local steroid injections on tendon. J Sports Med. 1973;1(4):31-37.
4. Cadambi A, Engh GA. Use of a semitendinosus tendon autogenous graft for rupture of the patellar ligament after total knee arthroplasty. A report of seven cases. J Bone Joint Surg Am. 1992;74(7):974-979.
5. Emerson RH Jr, Head WC, Malinin TI. Reconstruction of patellar tendon rupture after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop.1990;(260):154-161.
6. Gustillo RB, Thompson R. Quadriceps and patellar tendon ruptures following total knee arthroplasty. In: Rand JA, Dorr LD, eds. Total Arthroplasty of the Knee: Proceedings of the Knee Society, 1985-1986. Rockville, MD: Aspen; 1987: 41-70.
7. Rand JA, Morrey BF, Bryan RS. Patellar tendon rupture after total knee arthroplasty. Clin Orthop. 1989;(244):233-238.
8. Schoderbek RJ, Brown TE, Mulhall KJ, et al. Extensor mechanism disruption after total knee arthroplasty. Clin Orthop. 2006;446:176-185.
9. Bonamo JJ, Krinik RM, Sporn AA. Rupture of the patellar ligament after use of the central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am. 1984;66(8):1294-1297.
10. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
11. Mickelsen PL, Morgan SJ, Johnson WA, Ferrari JD. Patellar tendon rupture 3 years after anterior cruciate ligament reconstruction with a central one third bone-patellar tendon-bone graft. Arthroscopy. 2001;17(6):648-652.
12. Morgan J, McCarty DJ. Tendon ruptures in patients with systemic lupus erythematosus treated with corticosteroids. Arthritis Rheum. 1974;17(6):1033-1036.
13. Webb LX, Toby EB. Bilateral rupture of the patellar tendon in an otherwise healthy male patient following minor trauma. J Trauma. 1986;26(11):1045-1048.
14. Greis PE, Holmstrom MC, Lahav A. Surgical treatment options for patella tendon rupture, Part I: Acute. Orthopedics. 2005;28(7):672-679.
15. Greis PE, Lahav A, Holstrom MC. Surgical treatment options for patella tendon rupture, part II: chronic. Orthopedics. 2005;28(8):765-769.
16. Lewis PB, Rue JP, Bach BR Jr. Chronic patellar tendon rupture: surgical reconstruction technique using 2 Achilles tendon allografts. J Knee Surg. 2008;21(12):130-135.
17. McNally PD, Marcelli EA. Achilles tendon allograft of a chronic patellar tendon rupture. Arthroscopy. 1998;14(3):340-344.
18. Katsoulis E, Court-Brown C, Giannoudis PV. Incidence and atieology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br. 2006;88(5):576-580.
19. Brumback RJ, Uwagie-Ero S, Lakatos RP, et al. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70(1):1453-1462.
20. Koval KJ, Clapper MF, Brumback RJ, et al. Complications of reamed intramedullary nailing of the tibia. J Orthop Trauma. 1991;5(2):184-189.
21. Kretzler JE, Curtin SL, Wegner DA, Baumgaertner MR, Galloway MT. Patella tendon rupture: a late complication of a tibial nail. Orthopedics. 1995;18(11):1109-1111.
22. Moroney P, McCarthy T, Borton D. Patellar tendon rupture post reamed intra-medullary tibial nail in a patient with Ehlers-Danlos syndrome. A case report. Eur J Orthop Surg Traumatol. 2004;14(1):50-51.
23. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
24. Falconiero RP, Pallis MP. Chronic rupture of a patellar tendon: a technique for reconstruction with Achilles allograft. Arthroscopy. 1996;12(5):623-626.
1. Scott WN, Insall JN. Injuries of the knee. In: Rockwood CA Jr, Green DP, Bucholz RW, eds. Fractures in Adults. 3rd ed. Philadelphia, PA: JB Lippincott; 1991: 1799-1914.
2. Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12(4):300-301.
3. Unverferth LJ, Olix ML. The effect of local steroid injections on tendon. J Sports Med. 1973;1(4):31-37.
4. Cadambi A, Engh GA. Use of a semitendinosus tendon autogenous graft for rupture of the patellar ligament after total knee arthroplasty. A report of seven cases. J Bone Joint Surg Am. 1992;74(7):974-979.
5. Emerson RH Jr, Head WC, Malinin TI. Reconstruction of patellar tendon rupture after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop.1990;(260):154-161.
6. Gustillo RB, Thompson R. Quadriceps and patellar tendon ruptures following total knee arthroplasty. In: Rand JA, Dorr LD, eds. Total Arthroplasty of the Knee: Proceedings of the Knee Society, 1985-1986. Rockville, MD: Aspen; 1987: 41-70.
7. Rand JA, Morrey BF, Bryan RS. Patellar tendon rupture after total knee arthroplasty. Clin Orthop. 1989;(244):233-238.
8. Schoderbek RJ, Brown TE, Mulhall KJ, et al. Extensor mechanism disruption after total knee arthroplasty. Clin Orthop. 2006;446:176-185.
9. Bonamo JJ, Krinik RM, Sporn AA. Rupture of the patellar ligament after use of the central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am. 1984;66(8):1294-1297.
10. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
11. Mickelsen PL, Morgan SJ, Johnson WA, Ferrari JD. Patellar tendon rupture 3 years after anterior cruciate ligament reconstruction with a central one third bone-patellar tendon-bone graft. Arthroscopy. 2001;17(6):648-652.
12. Morgan J, McCarty DJ. Tendon ruptures in patients with systemic lupus erythematosus treated with corticosteroids. Arthritis Rheum. 1974;17(6):1033-1036.
13. Webb LX, Toby EB. Bilateral rupture of the patellar tendon in an otherwise healthy male patient following minor trauma. J Trauma. 1986;26(11):1045-1048.
14. Greis PE, Holmstrom MC, Lahav A. Surgical treatment options for patella tendon rupture, Part I: Acute. Orthopedics. 2005;28(7):672-679.
15. Greis PE, Lahav A, Holstrom MC. Surgical treatment options for patella tendon rupture, part II: chronic. Orthopedics. 2005;28(8):765-769.
16. Lewis PB, Rue JP, Bach BR Jr. Chronic patellar tendon rupture: surgical reconstruction technique using 2 Achilles tendon allografts. J Knee Surg. 2008;21(12):130-135.
17. McNally PD, Marcelli EA. Achilles tendon allograft of a chronic patellar tendon rupture. Arthroscopy. 1998;14(3):340-344.
18. Katsoulis E, Court-Brown C, Giannoudis PV. Incidence and atieology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br. 2006;88(5):576-580.
19. Brumback RJ, Uwagie-Ero S, Lakatos RP, et al. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70(1):1453-1462.
20. Koval KJ, Clapper MF, Brumback RJ, et al. Complications of reamed intramedullary nailing of the tibia. J Orthop Trauma. 1991;5(2):184-189.
21. Kretzler JE, Curtin SL, Wegner DA, Baumgaertner MR, Galloway MT. Patella tendon rupture: a late complication of a tibial nail. Orthopedics. 1995;18(11):1109-1111.
22. Moroney P, McCarthy T, Borton D. Patellar tendon rupture post reamed intra-medullary tibial nail in a patient with Ehlers-Danlos syndrome. A case report. Eur J Orthop Surg Traumatol. 2004;14(1):50-51.
23. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
24. Falconiero RP, Pallis MP. Chronic rupture of a patellar tendon: a technique for reconstruction with Achilles allograft. Arthroscopy. 1996;12(5):623-626.
Patients’ Perceptions of the Costs of Total Hip and Knee Arthroplasty
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
Total Hip Arthroplasty After Contralateral Hip Disarticulation: A Challenging “Simple Primary”
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
Long-Term Outcomes of Allograft Reconstruction of the Anterior Cruciate Ligament
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
Retrograde Reamer/Irrigator/Aspirator Technique for Autologous Bone Graft Harvesting With the Patient in the Prone Position
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
Successful Surgical Treatment of an Intraneural Ganglion of the Common Peroneal Nerve
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.