User login
FDA approves first ready-to-use oral solution of methotrexate
The US Food and Drug Administration (FDA) has approved a ready-to-use oral solution of methotrexate (Xatmep) for use in certain pediatric patients.
The drug is approved as part of a multi-phase, combination chemotherapy maintenance regimen to treat pediatric patients with acute lymphoblastic leukemia.
Xatmep is also approved for use in pediatric patients with active polyarticular juvenile idiopathic arthritis who have had an insufficient response to, or cannot tolerate, an adequate trial of first-line therapy, including full-dose non-steroidal anti-inflammatory agents.
Xatmep is the first ready-to-use oral solution of methotrexate to be approved by the FDA.
There was previously no such formulation of the drug approved for use in pediatric patients requiring body surface area dosing (mg/m2), patients who have difficulty swallowing or cannot consume tablets, or those with needle-phobia.
Xatmep (methotrexate) Oral Solution, 2.5 mg/mL, requires no preparation. It eliminates the need for needles, crushing or splitting tablets, or for compounding tablets into a liquid formulation.
Xatmep requires refrigeration but may be stored at room temperature for 60 days after dispensing.
For more on Xatmep, see the prescribing information, which includes a boxed warning detailing the risk of severe toxic reactions, including embryo-fetal toxicity.
Xatmep is available through pharmacies and a qualified mail-order service. For information on how to obtain the drug, call 1-855-379-0382.
Xatmep is a product of Silvergate Pharmaceuticals, Inc. ![]()
The US Food and Drug Administration (FDA) has approved a ready-to-use oral solution of methotrexate (Xatmep) for use in certain pediatric patients.
The drug is approved as part of a multi-phase, combination chemotherapy maintenance regimen to treat pediatric patients with acute lymphoblastic leukemia.
Xatmep is also approved for use in pediatric patients with active polyarticular juvenile idiopathic arthritis who have had an insufficient response to, or cannot tolerate, an adequate trial of first-line therapy, including full-dose non-steroidal anti-inflammatory agents.
Xatmep is the first ready-to-use oral solution of methotrexate to be approved by the FDA.
There was previously no such formulation of the drug approved for use in pediatric patients requiring body surface area dosing (mg/m2), patients who have difficulty swallowing or cannot consume tablets, or those with needle-phobia.
Xatmep (methotrexate) Oral Solution, 2.5 mg/mL, requires no preparation. It eliminates the need for needles, crushing or splitting tablets, or for compounding tablets into a liquid formulation.
Xatmep requires refrigeration but may be stored at room temperature for 60 days after dispensing.
For more on Xatmep, see the prescribing information, which includes a boxed warning detailing the risk of severe toxic reactions, including embryo-fetal toxicity.
Xatmep is available through pharmacies and a qualified mail-order service. For information on how to obtain the drug, call 1-855-379-0382.
Xatmep is a product of Silvergate Pharmaceuticals, Inc. ![]()
The US Food and Drug Administration (FDA) has approved a ready-to-use oral solution of methotrexate (Xatmep) for use in certain pediatric patients.
The drug is approved as part of a multi-phase, combination chemotherapy maintenance regimen to treat pediatric patients with acute lymphoblastic leukemia.
Xatmep is also approved for use in pediatric patients with active polyarticular juvenile idiopathic arthritis who have had an insufficient response to, or cannot tolerate, an adequate trial of first-line therapy, including full-dose non-steroidal anti-inflammatory agents.
Xatmep is the first ready-to-use oral solution of methotrexate to be approved by the FDA.
There was previously no such formulation of the drug approved for use in pediatric patients requiring body surface area dosing (mg/m2), patients who have difficulty swallowing or cannot consume tablets, or those with needle-phobia.
Xatmep (methotrexate) Oral Solution, 2.5 mg/mL, requires no preparation. It eliminates the need for needles, crushing or splitting tablets, or for compounding tablets into a liquid formulation.
Xatmep requires refrigeration but may be stored at room temperature for 60 days after dispensing.
For more on Xatmep, see the prescribing information, which includes a boxed warning detailing the risk of severe toxic reactions, including embryo-fetal toxicity.
Xatmep is available through pharmacies and a qualified mail-order service. For information on how to obtain the drug, call 1-855-379-0382.
Xatmep is a product of Silvergate Pharmaceuticals, Inc. ![]()
Drug receives orphan designation for AML
The US Food and Drug Administration (FDA) has granted orphan drug designation for PTX-200 for the treatment of acute myeloid leukemia (AML).
PTX-200, or triciribine phosphate monohydrate, is a small-molecule Akt inhibitor being developed by Prescient Therapeutics Ltd.
PTX-200 is currently being tested in combination with cytarabine in a phase 1b/2 trial of patients with relapsed or refractory AML.
PTX-200 was previously tested as monotherapy in a phase 1 trial of patients with relapsed or refractory hematologic malignancies.
Results from this trial were published in Leukemia Research in November 2013.
The trial included 41 patients who received at least 1 dose of PTX-200. The patients’ median age was 70 (range, of 23–83), and most (77%) were male.
Thirty-six patients (84%) had AML, 3 had myelodysplastic syndromes/chronic myelomonocytic leukemia (CMML), 2 had chronic lymphocytic leukemia, and 2 had acute lymphoblastic leukemia.
The patients had received a median of 2 prior therapies (range, 0–11), and they received a median of 1 cycle (range, 0–3) of PTX-200 at varying doses.
The maximum-tolerated dose of PTX-200 was 55 mg/m2. Two dose-limiting toxicities (DLTs) occurred in 3 patients in the 65 mg/m2 cohort (2 cases of lipase elevation and 1 case of triglyceride elevation).
One dose-limiting toxicity (mucositis) occurred in the 35 mg/m2 cohort. All DLTs resolved after patients stopped taking PTX-200.
Common treatment-emergent grade 3/4 adverse events included infection/febrile neutropenia (24%), bleeding (2%), mucositis (2%), and elevated lipase (5%).
Thirty-two patients were evaluable for response. There were no responses, but 17 patients had stable disease. The remaining 15 patients progressed.
Three patients with stable disease (all with AML) achieved at least a 50% reduction in bone marrow blasts, and a patient with CMML experienced spleen reduction and resolution of leukocytosis.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for PTX-200 for the treatment of acute myeloid leukemia (AML).
PTX-200, or triciribine phosphate monohydrate, is a small-molecule Akt inhibitor being developed by Prescient Therapeutics Ltd.
PTX-200 is currently being tested in combination with cytarabine in a phase 1b/2 trial of patients with relapsed or refractory AML.
PTX-200 was previously tested as monotherapy in a phase 1 trial of patients with relapsed or refractory hematologic malignancies.
Results from this trial were published in Leukemia Research in November 2013.
The trial included 41 patients who received at least 1 dose of PTX-200. The patients’ median age was 70 (range, of 23–83), and most (77%) were male.
Thirty-six patients (84%) had AML, 3 had myelodysplastic syndromes/chronic myelomonocytic leukemia (CMML), 2 had chronic lymphocytic leukemia, and 2 had acute lymphoblastic leukemia.
The patients had received a median of 2 prior therapies (range, 0–11), and they received a median of 1 cycle (range, 0–3) of PTX-200 at varying doses.
The maximum-tolerated dose of PTX-200 was 55 mg/m2. Two dose-limiting toxicities (DLTs) occurred in 3 patients in the 65 mg/m2 cohort (2 cases of lipase elevation and 1 case of triglyceride elevation).
One dose-limiting toxicity (mucositis) occurred in the 35 mg/m2 cohort. All DLTs resolved after patients stopped taking PTX-200.
Common treatment-emergent grade 3/4 adverse events included infection/febrile neutropenia (24%), bleeding (2%), mucositis (2%), and elevated lipase (5%).
Thirty-two patients were evaluable for response. There were no responses, but 17 patients had stable disease. The remaining 15 patients progressed.
Three patients with stable disease (all with AML) achieved at least a 50% reduction in bone marrow blasts, and a patient with CMML experienced spleen reduction and resolution of leukocytosis.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for PTX-200 for the treatment of acute myeloid leukemia (AML).
PTX-200, or triciribine phosphate monohydrate, is a small-molecule Akt inhibitor being developed by Prescient Therapeutics Ltd.
PTX-200 is currently being tested in combination with cytarabine in a phase 1b/2 trial of patients with relapsed or refractory AML.
PTX-200 was previously tested as monotherapy in a phase 1 trial of patients with relapsed or refractory hematologic malignancies.
Results from this trial were published in Leukemia Research in November 2013.
The trial included 41 patients who received at least 1 dose of PTX-200. The patients’ median age was 70 (range, of 23–83), and most (77%) were male.
Thirty-six patients (84%) had AML, 3 had myelodysplastic syndromes/chronic myelomonocytic leukemia (CMML), 2 had chronic lymphocytic leukemia, and 2 had acute lymphoblastic leukemia.
The patients had received a median of 2 prior therapies (range, 0–11), and they received a median of 1 cycle (range, 0–3) of PTX-200 at varying doses.
The maximum-tolerated dose of PTX-200 was 55 mg/m2. Two dose-limiting toxicities (DLTs) occurred in 3 patients in the 65 mg/m2 cohort (2 cases of lipase elevation and 1 case of triglyceride elevation).
One dose-limiting toxicity (mucositis) occurred in the 35 mg/m2 cohort. All DLTs resolved after patients stopped taking PTX-200.
Common treatment-emergent grade 3/4 adverse events included infection/febrile neutropenia (24%), bleeding (2%), mucositis (2%), and elevated lipase (5%).
Thirty-two patients were evaluable for response. There were no responses, but 17 patients had stable disease. The remaining 15 patients progressed.
Three patients with stable disease (all with AML) achieved at least a 50% reduction in bone marrow blasts, and a patient with CMML experienced spleen reduction and resolution of leukocytosis.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
FDA approves midostaurin to treat FLT3+ AML, advanced SM
The US Food and Drug Administration (FDA) has granted approval for the oral, multi-targeted kinase inhibitor midostaurin (Rydapt).
The drug is now approved for the treatment of adults with advanced systemic mastocytosis (SM), including aggressive SM (ASM), SM with associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL).
Midostaurin is also approved for use in combination with standard cytarabine and daunorubicin induction, followed by cytarabine consolidation, in adults with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive, as detected by an FDA-approved test.
The FDA approved a companion diagnostic, the LeukoStrat CDx FLT3 Mutation Assay, for use with midostaurin to test AML patients for the FLT3 mutation.
Midostaurin is a product of Novartis. The companion diagnostic was developed by Novartis and Invivoscribe Technologies, Inc.
Midostaurin in AML
The FDA’s approval of midostaurin in AML is based on results from the phase 3 RATIFY trial, which were presented at the 2015 ASH Annual Meeting.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
Patients in the midostaurin arm experienced a statistically significant improvement in overall survival, with a 23% reduction in risk of death compared to the placebo arm (hazard ratio=0.77, P=0.016).
The median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infections.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis. Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm.
Midostaurin in advanced SM
The FDA’s approval of midostaurin in advanced SM was based on results from a pair of phase 2, single-arm studies, hereafter referred to as Study 2 and Study 3.
Data from Study 2 were published in NEJM in June 2016, and data from Study 3 were presented at the 2010 ASH Annual Meeting.
Study 2 included 116 patients, 115 of whom were evaluable for response.
The overall response rate (ORR) was 17% in the entire cohort, 31% among patients with ASM, 11% among patients with SM-AHN, and 19% among patients with MCL. The complete response rates were 2%, 6%, 0%, and 5%, respectively.
Study 3 included 26 patients with advanced SM. In 3 of the patients, the subtype of SM was unconfirmed.
Among the 17 patients with SM-AHN, there were 10 response (ORR=59%), including 1 partial response and 9 major responses. In the 6 patients with MCL, there were 2 responses (ORR=33%), which included 1 partial response and 1 major response.
In both studies combined, there were 142 adults with ASM, SM-AHN, or MCL.
The most frequent AEs (excluding laboratory abnormalities) that occurred in at least 20% of these patients were nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, constipation, pyrexia, headache, and dyspnea.
The most frequent grade 3 or higher AEs (excluding laboratory abnormalities) that occurred in at least 5% of patients were fatigue, sepsis, gastrointestinal hemorrhage, pneumonia, diarrhea, febrile neutropenia, edema, dyspnea, nausea, vomiting, abdominal pain, and renal insufficiency.
Serious AEs occurred in 68% of patients, most commonly infections and gastrointestinal disorders. Twenty-one percent of patients discontinued treatment due to AEs, the most frequent of which were infection, nausea or vomiting, QT prolongation, and gastrointestinal hemorrhage. ![]()
The US Food and Drug Administration (FDA) has granted approval for the oral, multi-targeted kinase inhibitor midostaurin (Rydapt).
The drug is now approved for the treatment of adults with advanced systemic mastocytosis (SM), including aggressive SM (ASM), SM with associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL).
Midostaurin is also approved for use in combination with standard cytarabine and daunorubicin induction, followed by cytarabine consolidation, in adults with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive, as detected by an FDA-approved test.
The FDA approved a companion diagnostic, the LeukoStrat CDx FLT3 Mutation Assay, for use with midostaurin to test AML patients for the FLT3 mutation.
Midostaurin is a product of Novartis. The companion diagnostic was developed by Novartis and Invivoscribe Technologies, Inc.
Midostaurin in AML
The FDA’s approval of midostaurin in AML is based on results from the phase 3 RATIFY trial, which were presented at the 2015 ASH Annual Meeting.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
Patients in the midostaurin arm experienced a statistically significant improvement in overall survival, with a 23% reduction in risk of death compared to the placebo arm (hazard ratio=0.77, P=0.016).
The median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infections.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis. Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm.
Midostaurin in advanced SM
The FDA’s approval of midostaurin in advanced SM was based on results from a pair of phase 2, single-arm studies, hereafter referred to as Study 2 and Study 3.
Data from Study 2 were published in NEJM in June 2016, and data from Study 3 were presented at the 2010 ASH Annual Meeting.
Study 2 included 116 patients, 115 of whom were evaluable for response.
The overall response rate (ORR) was 17% in the entire cohort, 31% among patients with ASM, 11% among patients with SM-AHN, and 19% among patients with MCL. The complete response rates were 2%, 6%, 0%, and 5%, respectively.
Study 3 included 26 patients with advanced SM. In 3 of the patients, the subtype of SM was unconfirmed.
Among the 17 patients with SM-AHN, there were 10 response (ORR=59%), including 1 partial response and 9 major responses. In the 6 patients with MCL, there were 2 responses (ORR=33%), which included 1 partial response and 1 major response.
In both studies combined, there were 142 adults with ASM, SM-AHN, or MCL.
The most frequent AEs (excluding laboratory abnormalities) that occurred in at least 20% of these patients were nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, constipation, pyrexia, headache, and dyspnea.
The most frequent grade 3 or higher AEs (excluding laboratory abnormalities) that occurred in at least 5% of patients were fatigue, sepsis, gastrointestinal hemorrhage, pneumonia, diarrhea, febrile neutropenia, edema, dyspnea, nausea, vomiting, abdominal pain, and renal insufficiency.
Serious AEs occurred in 68% of patients, most commonly infections and gastrointestinal disorders. Twenty-one percent of patients discontinued treatment due to AEs, the most frequent of which were infection, nausea or vomiting, QT prolongation, and gastrointestinal hemorrhage. ![]()
The US Food and Drug Administration (FDA) has granted approval for the oral, multi-targeted kinase inhibitor midostaurin (Rydapt).
The drug is now approved for the treatment of adults with advanced systemic mastocytosis (SM), including aggressive SM (ASM), SM with associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL).
Midostaurin is also approved for use in combination with standard cytarabine and daunorubicin induction, followed by cytarabine consolidation, in adults with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive, as detected by an FDA-approved test.
The FDA approved a companion diagnostic, the LeukoStrat CDx FLT3 Mutation Assay, for use with midostaurin to test AML patients for the FLT3 mutation.
Midostaurin is a product of Novartis. The companion diagnostic was developed by Novartis and Invivoscribe Technologies, Inc.
Midostaurin in AML
The FDA’s approval of midostaurin in AML is based on results from the phase 3 RATIFY trial, which were presented at the 2015 ASH Annual Meeting.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
Patients in the midostaurin arm experienced a statistically significant improvement in overall survival, with a 23% reduction in risk of death compared to the placebo arm (hazard ratio=0.77, P=0.016).
The median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infections.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis. Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm.
Midostaurin in advanced SM
The FDA’s approval of midostaurin in advanced SM was based on results from a pair of phase 2, single-arm studies, hereafter referred to as Study 2 and Study 3.
Data from Study 2 were published in NEJM in June 2016, and data from Study 3 were presented at the 2010 ASH Annual Meeting.
Study 2 included 116 patients, 115 of whom were evaluable for response.
The overall response rate (ORR) was 17% in the entire cohort, 31% among patients with ASM, 11% among patients with SM-AHN, and 19% among patients with MCL. The complete response rates were 2%, 6%, 0%, and 5%, respectively.
Study 3 included 26 patients with advanced SM. In 3 of the patients, the subtype of SM was unconfirmed.
Among the 17 patients with SM-AHN, there were 10 response (ORR=59%), including 1 partial response and 9 major responses. In the 6 patients with MCL, there were 2 responses (ORR=33%), which included 1 partial response and 1 major response.
In both studies combined, there were 142 adults with ASM, SM-AHN, or MCL.
The most frequent AEs (excluding laboratory abnormalities) that occurred in at least 20% of these patients were nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, constipation, pyrexia, headache, and dyspnea.
The most frequent grade 3 or higher AEs (excluding laboratory abnormalities) that occurred in at least 5% of patients were fatigue, sepsis, gastrointestinal hemorrhage, pneumonia, diarrhea, febrile neutropenia, edema, dyspnea, nausea, vomiting, abdominal pain, and renal insufficiency.
Serious AEs occurred in 68% of patients, most commonly infections and gastrointestinal disorders. Twenty-one percent of patients discontinued treatment due to AEs, the most frequent of which were infection, nausea or vomiting, QT prolongation, and gastrointestinal hemorrhage. ![]()
FDA approves test to detect FLT3 mutations
The US Food and Drug Administration (FDA) has approved use of the LeukoStrat® CDx FLT3 Mutation Assay as a companion diagnostic test.
The LeukoStrat® CDx FLT3 Mutation Assay is a signal ratio assay that identifies both internal tandem duplication and tyrosine kinase domain mutations.
The assay is the first FDA-approved companion diagnostic for acute myeloid leukemia (AML).
It is approved for use in patients newly diagnosed with AML to determine if they have FLT3 mutations and are therefore eligible to receive treatment with midostaurin (Rydapt).
The FDA granted approval for the LeukoStrat® CDx FLT3 Mutation Assay and midostaurin simultaneously.
The assay was developed by Invivoscribe Technologies, Inc. and Novartis. Midostaurin is a product of Novartis.
Under the current labeling, FLT3 mutation testing with the LeukoStrat® CDx FLT3 Mutation Assay is exclusively performed by The Laboratory for Personalized Molecular Medicine, a subsidiary of Invivoscribe Technologies, Inc.
Under terms of a previously announced agreement with Thermo Fisher, Invivoscribe will also seek FDA approval of the LeukoStrat® CDx FLT3 Mutation Assay that will allow the sale of kits to other laboratories. ![]()
The US Food and Drug Administration (FDA) has approved use of the LeukoStrat® CDx FLT3 Mutation Assay as a companion diagnostic test.
The LeukoStrat® CDx FLT3 Mutation Assay is a signal ratio assay that identifies both internal tandem duplication and tyrosine kinase domain mutations.
The assay is the first FDA-approved companion diagnostic for acute myeloid leukemia (AML).
It is approved for use in patients newly diagnosed with AML to determine if they have FLT3 mutations and are therefore eligible to receive treatment with midostaurin (Rydapt).
The FDA granted approval for the LeukoStrat® CDx FLT3 Mutation Assay and midostaurin simultaneously.
The assay was developed by Invivoscribe Technologies, Inc. and Novartis. Midostaurin is a product of Novartis.
Under the current labeling, FLT3 mutation testing with the LeukoStrat® CDx FLT3 Mutation Assay is exclusively performed by The Laboratory for Personalized Molecular Medicine, a subsidiary of Invivoscribe Technologies, Inc.
Under terms of a previously announced agreement with Thermo Fisher, Invivoscribe will also seek FDA approval of the LeukoStrat® CDx FLT3 Mutation Assay that will allow the sale of kits to other laboratories. ![]()
The US Food and Drug Administration (FDA) has approved use of the LeukoStrat® CDx FLT3 Mutation Assay as a companion diagnostic test.
The LeukoStrat® CDx FLT3 Mutation Assay is a signal ratio assay that identifies both internal tandem duplication and tyrosine kinase domain mutations.
The assay is the first FDA-approved companion diagnostic for acute myeloid leukemia (AML).
It is approved for use in patients newly diagnosed with AML to determine if they have FLT3 mutations and are therefore eligible to receive treatment with midostaurin (Rydapt).
The FDA granted approval for the LeukoStrat® CDx FLT3 Mutation Assay and midostaurin simultaneously.
The assay was developed by Invivoscribe Technologies, Inc. and Novartis. Midostaurin is a product of Novartis.
Under the current labeling, FLT3 mutation testing with the LeukoStrat® CDx FLT3 Mutation Assay is exclusively performed by The Laboratory for Personalized Molecular Medicine, a subsidiary of Invivoscribe Technologies, Inc.
Under terms of a previously announced agreement with Thermo Fisher, Invivoscribe will also seek FDA approval of the LeukoStrat® CDx FLT3 Mutation Assay that will allow the sale of kits to other laboratories. ![]()
Longer course of idarubicin consolidation increases leukemia-free survival
In adults with acute myeloid leukemia who achieve complete remission with one to two cycles of induction therapy, a slightly longer course of idarubicin during consolidation therapy increases leukemia-free survival without increasing nonhematologic toxicity, according to new findings.
The optimal dose of anthracyclines, such as idarubicin, during consolidation therapy in this patient population has not been explored to date. Researchers performed a randomized phase III clinical trial, comparing standard (2-day) idarubicin with increased (3-day) idarubicin during consolidation therapy with cytarabine and etoposide.
The 7-year trial involved 293 patients, aged 15-60 years (median age, 45 years), who were treated at 23 Australian hospitals after achieving complete remission with one to two courses of intensive cytarabine-based induction therapy. Study participants were randomly assigned to receive either standard or intensive idarubicin, said Kenneth F. Bradstock, MB, PhD, of the University of Sydney, Australia, and his associates.
More patients who were receiving the higher cumulative dose of idarubicin had leukemia-free survival at 3 years (47%), compared with the standard-dose group (35%), for a hazard ratio of 0.74 favoring intensive idarubicin. The estimated median leukemia-free survival was 2.13 years for intensified idarubicin, compared with 0.93 years for standard idarubicin (J Clin Oncol. 2017 Apr 3. doi: 10.1200/JCO.2016.70.6374).
Additionally, the time to relapse was significantly longer for intensive idarubicin (17 months) than for standard idarubicin (10 months). This study was not powered to detect a difference between the two groups in overall survival.
There was no significant difference between the two study groups in the incidence of serious nonhematologic toxicities or in the rate of treatment-related death, even though the patients receiving intensive idarubicin showed a clear increase in myelotoxicity, as expected.
In exploratory analyses, the researchers could not detect a specific benefit of increased idarubicin dose in any patient subgroups, in particular, younger versus older ages, adverse versus intermediate karyotypes, and mutations in the FLT3 and NPM1 genes. However, they were limited because of the low number of cases in some subgroups.
The trial was supported by the National Health and Medical Research Council of Australia, Pfizer Australia, and Amgen Australia. Dr. Bradstock reported ties to Amgen Australia. His is associates reported ties to numerous industry sources.
In adults with acute myeloid leukemia who achieve complete remission with one to two cycles of induction therapy, a slightly longer course of idarubicin during consolidation therapy increases leukemia-free survival without increasing nonhematologic toxicity, according to new findings.
The optimal dose of anthracyclines, such as idarubicin, during consolidation therapy in this patient population has not been explored to date. Researchers performed a randomized phase III clinical trial, comparing standard (2-day) idarubicin with increased (3-day) idarubicin during consolidation therapy with cytarabine and etoposide.
The 7-year trial involved 293 patients, aged 15-60 years (median age, 45 years), who were treated at 23 Australian hospitals after achieving complete remission with one to two courses of intensive cytarabine-based induction therapy. Study participants were randomly assigned to receive either standard or intensive idarubicin, said Kenneth F. Bradstock, MB, PhD, of the University of Sydney, Australia, and his associates.
More patients who were receiving the higher cumulative dose of idarubicin had leukemia-free survival at 3 years (47%), compared with the standard-dose group (35%), for a hazard ratio of 0.74 favoring intensive idarubicin. The estimated median leukemia-free survival was 2.13 years for intensified idarubicin, compared with 0.93 years for standard idarubicin (J Clin Oncol. 2017 Apr 3. doi: 10.1200/JCO.2016.70.6374).
Additionally, the time to relapse was significantly longer for intensive idarubicin (17 months) than for standard idarubicin (10 months). This study was not powered to detect a difference between the two groups in overall survival.
There was no significant difference between the two study groups in the incidence of serious nonhematologic toxicities or in the rate of treatment-related death, even though the patients receiving intensive idarubicin showed a clear increase in myelotoxicity, as expected.
In exploratory analyses, the researchers could not detect a specific benefit of increased idarubicin dose in any patient subgroups, in particular, younger versus older ages, adverse versus intermediate karyotypes, and mutations in the FLT3 and NPM1 genes. However, they were limited because of the low number of cases in some subgroups.
The trial was supported by the National Health and Medical Research Council of Australia, Pfizer Australia, and Amgen Australia. Dr. Bradstock reported ties to Amgen Australia. His is associates reported ties to numerous industry sources.
In adults with acute myeloid leukemia who achieve complete remission with one to two cycles of induction therapy, a slightly longer course of idarubicin during consolidation therapy increases leukemia-free survival without increasing nonhematologic toxicity, according to new findings.
The optimal dose of anthracyclines, such as idarubicin, during consolidation therapy in this patient population has not been explored to date. Researchers performed a randomized phase III clinical trial, comparing standard (2-day) idarubicin with increased (3-day) idarubicin during consolidation therapy with cytarabine and etoposide.
The 7-year trial involved 293 patients, aged 15-60 years (median age, 45 years), who were treated at 23 Australian hospitals after achieving complete remission with one to two courses of intensive cytarabine-based induction therapy. Study participants were randomly assigned to receive either standard or intensive idarubicin, said Kenneth F. Bradstock, MB, PhD, of the University of Sydney, Australia, and his associates.
More patients who were receiving the higher cumulative dose of idarubicin had leukemia-free survival at 3 years (47%), compared with the standard-dose group (35%), for a hazard ratio of 0.74 favoring intensive idarubicin. The estimated median leukemia-free survival was 2.13 years for intensified idarubicin, compared with 0.93 years for standard idarubicin (J Clin Oncol. 2017 Apr 3. doi: 10.1200/JCO.2016.70.6374).
Additionally, the time to relapse was significantly longer for intensive idarubicin (17 months) than for standard idarubicin (10 months). This study was not powered to detect a difference between the two groups in overall survival.
There was no significant difference between the two study groups in the incidence of serious nonhematologic toxicities or in the rate of treatment-related death, even though the patients receiving intensive idarubicin showed a clear increase in myelotoxicity, as expected.
In exploratory analyses, the researchers could not detect a specific benefit of increased idarubicin dose in any patient subgroups, in particular, younger versus older ages, adverse versus intermediate karyotypes, and mutations in the FLT3 and NPM1 genes. However, they were limited because of the low number of cases in some subgroups.
The trial was supported by the National Health and Medical Research Council of Australia, Pfizer Australia, and Amgen Australia. Dr. Bradstock reported ties to Amgen Australia. His is associates reported ties to numerous industry sources.
Key clinical point:
Major finding: The median leukemia-free survival was 2.13 years for intensified idarubicin, compared with 0.93 years for standard idarubicin.
Data source: A multicenter randomized phase III trial involving 293 patients.
Disclosures: The trial was supported by the National Health and Medical Research Council of Australia, Pfizer Australia, and Amgen Australia. Dr. Bradstock reported ties to Amgen Australis. His associates reported ties to numerous industry sources.
FDA boxed warning leads to drop off in use of ESAs
The Food and Drug Administration’s 2007 “boxed warning” about serious adverse events associated with the use of erythropoietin-stimulating agents (ESAs) was followed by a substantial reduction in their use among patients recovering from colorectal, breast, or lung cancer, according to a new report.
Boxed warnings are considered one of the strongest mechanisms with which the FDA can communicate concerns about drug safety to the public. However, some critics have questioned the effectiveness of these warnings, and the available evidence “remains inconclusive, largely because almost all of [the data] were drawn from observational studies using pre-post designs without control groups,” said John Bian, PhD, of the University of South Carolina College of Pharmacy and Hollings Cancer Center, Columbia, and his associates.
The investigators analyzed data in the SEER cancer registry for the period immediately before and immediately after the 2007 boxed warning was issued. Their sample comprised 45,319 patients aged 66 years and older who were treated either in the “pre” warning period (January 2004-September 2006) or the “post” period (April 2007-September 2009). This included a control group of 3,375 patients with myelodysplastic syndromes. Use of ESAs in these patients was off-label and was not targeted by the boxed warning (J Clin Oncol. 2017 Apr 25. doi: 10.1200/JCO.2017.72.6273).The use of ESAs declined sharply after the boxed warning was issued, except in the control group. The proportion of breast cancer patients receiving ESAs dropped from 49%-55% before 2007 to 30% in 2007, 16% in 2008, and 9% in 2009.
Similarly, the proportion of colorectal cancer patients receiving ESAs declined from about 35%-40% before 2007 to 18% in 2007, 11% in 2008, and 9% in 2009. The proportion of lung cancer patients receiving ESAs decreased from 56%-58% before 2007 to 40% in 2007, 29% in 2008, and 24% in 2009. In contrast, the proportion of patients with myelodysplastic syndromes receiving ESAs – the control group – remained relatively stable at 39%-42% before 2007, 35% in 2007, and 32% in 2008 and 2009.
This represents a reduction of approximately 40% overall in the use of ESAs among targeted patients after the warning was issued. However, this decrease appeared to have little effect on the incidence of hospitalization for venous thromboembolism in this patient population, Dr. Bian and his associates noted.
The study was supported by the National Institutes of Health. Dr. Bian reported having no relevant financial disclosures. His associates reported ties to Quincy Bioscience, Bristol-Myers Squibb, Taiho Pharmaceutical, Mylan, Eli Lilly, Merck, Amgen, and BDI Pharma.
The Food and Drug Administration’s 2007 “boxed warning” about serious adverse events associated with the use of erythropoietin-stimulating agents (ESAs) was followed by a substantial reduction in their use among patients recovering from colorectal, breast, or lung cancer, according to a new report.
Boxed warnings are considered one of the strongest mechanisms with which the FDA can communicate concerns about drug safety to the public. However, some critics have questioned the effectiveness of these warnings, and the available evidence “remains inconclusive, largely because almost all of [the data] were drawn from observational studies using pre-post designs without control groups,” said John Bian, PhD, of the University of South Carolina College of Pharmacy and Hollings Cancer Center, Columbia, and his associates.
The investigators analyzed data in the SEER cancer registry for the period immediately before and immediately after the 2007 boxed warning was issued. Their sample comprised 45,319 patients aged 66 years and older who were treated either in the “pre” warning period (January 2004-September 2006) or the “post” period (April 2007-September 2009). This included a control group of 3,375 patients with myelodysplastic syndromes. Use of ESAs in these patients was off-label and was not targeted by the boxed warning (J Clin Oncol. 2017 Apr 25. doi: 10.1200/JCO.2017.72.6273).The use of ESAs declined sharply after the boxed warning was issued, except in the control group. The proportion of breast cancer patients receiving ESAs dropped from 49%-55% before 2007 to 30% in 2007, 16% in 2008, and 9% in 2009.
Similarly, the proportion of colorectal cancer patients receiving ESAs declined from about 35%-40% before 2007 to 18% in 2007, 11% in 2008, and 9% in 2009. The proportion of lung cancer patients receiving ESAs decreased from 56%-58% before 2007 to 40% in 2007, 29% in 2008, and 24% in 2009. In contrast, the proportion of patients with myelodysplastic syndromes receiving ESAs – the control group – remained relatively stable at 39%-42% before 2007, 35% in 2007, and 32% in 2008 and 2009.
This represents a reduction of approximately 40% overall in the use of ESAs among targeted patients after the warning was issued. However, this decrease appeared to have little effect on the incidence of hospitalization for venous thromboembolism in this patient population, Dr. Bian and his associates noted.
The study was supported by the National Institutes of Health. Dr. Bian reported having no relevant financial disclosures. His associates reported ties to Quincy Bioscience, Bristol-Myers Squibb, Taiho Pharmaceutical, Mylan, Eli Lilly, Merck, Amgen, and BDI Pharma.
The Food and Drug Administration’s 2007 “boxed warning” about serious adverse events associated with the use of erythropoietin-stimulating agents (ESAs) was followed by a substantial reduction in their use among patients recovering from colorectal, breast, or lung cancer, according to a new report.
Boxed warnings are considered one of the strongest mechanisms with which the FDA can communicate concerns about drug safety to the public. However, some critics have questioned the effectiveness of these warnings, and the available evidence “remains inconclusive, largely because almost all of [the data] were drawn from observational studies using pre-post designs without control groups,” said John Bian, PhD, of the University of South Carolina College of Pharmacy and Hollings Cancer Center, Columbia, and his associates.
The investigators analyzed data in the SEER cancer registry for the period immediately before and immediately after the 2007 boxed warning was issued. Their sample comprised 45,319 patients aged 66 years and older who were treated either in the “pre” warning period (January 2004-September 2006) or the “post” period (April 2007-September 2009). This included a control group of 3,375 patients with myelodysplastic syndromes. Use of ESAs in these patients was off-label and was not targeted by the boxed warning (J Clin Oncol. 2017 Apr 25. doi: 10.1200/JCO.2017.72.6273).The use of ESAs declined sharply after the boxed warning was issued, except in the control group. The proportion of breast cancer patients receiving ESAs dropped from 49%-55% before 2007 to 30% in 2007, 16% in 2008, and 9% in 2009.
Similarly, the proportion of colorectal cancer patients receiving ESAs declined from about 35%-40% before 2007 to 18% in 2007, 11% in 2008, and 9% in 2009. The proportion of lung cancer patients receiving ESAs decreased from 56%-58% before 2007 to 40% in 2007, 29% in 2008, and 24% in 2009. In contrast, the proportion of patients with myelodysplastic syndromes receiving ESAs – the control group – remained relatively stable at 39%-42% before 2007, 35% in 2007, and 32% in 2008 and 2009.
This represents a reduction of approximately 40% overall in the use of ESAs among targeted patients after the warning was issued. However, this decrease appeared to have little effect on the incidence of hospitalization for venous thromboembolism in this patient population, Dr. Bian and his associates noted.
The study was supported by the National Institutes of Health. Dr. Bian reported having no relevant financial disclosures. His associates reported ties to Quincy Bioscience, Bristol-Myers Squibb, Taiho Pharmaceutical, Mylan, Eli Lilly, Merck, Amgen, and BDI Pharma.
Key clinical point:
Major finding: The use of ESAs among cancer patients that were targeted by the boxed warning dropped by about 40% after the warning was issued.
Data source: A retrospective cohort study involving 45,319 cancer patients enrolled in the SEER data registry during 2004-2009.
Disclosures: The study was supported by the National Institutes of Health. Dr. Bian reported having no relevant financial disclosures. His associates reported ties to Quincy Bioscience, Bristol-Myers Squibb, Taiho Pharmaceutical, Mylan, Eli Lilly, Merck, Amgen, and BDI Pharma.
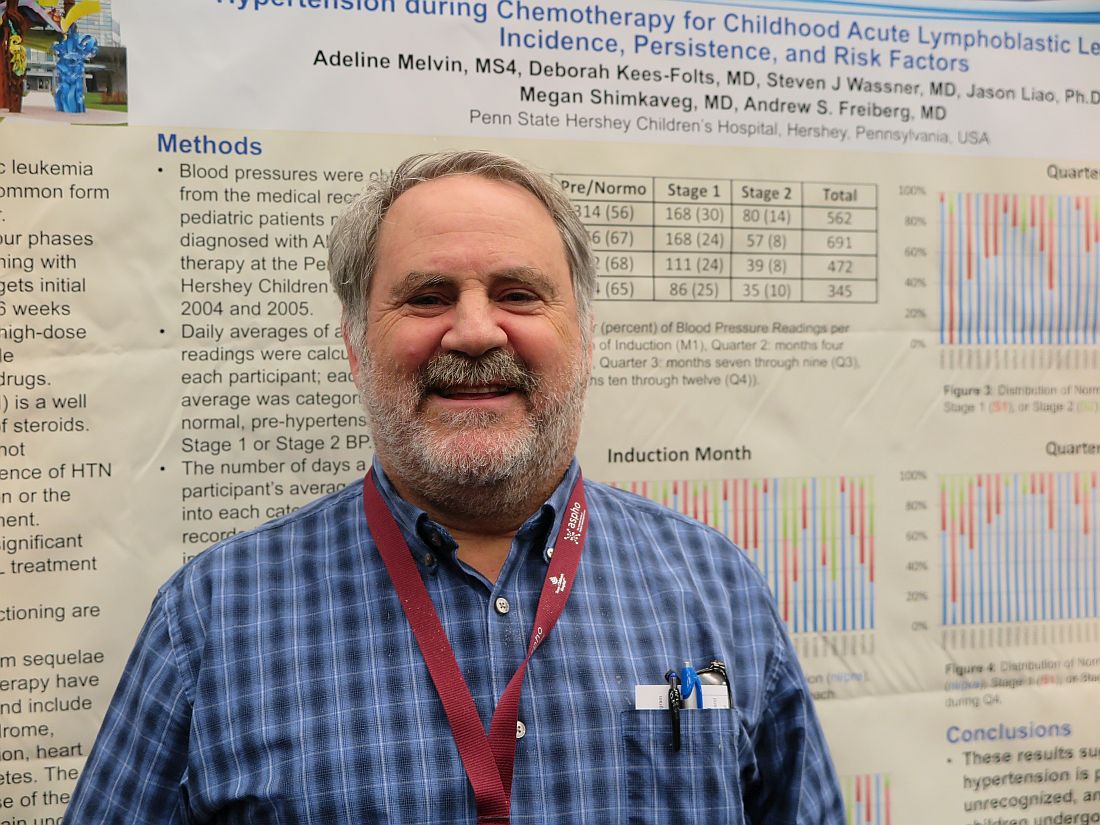
Treatment-related hypertension, kidney injury are undertreated in kids with leukemia
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
At ASPHO 2017
Key clinical point:
Major finding: The combined incidence of stage 1 or 2 hypertension in children during induction therapy for acute lymphoblastic leukemia was 44%.
Data source: Two retrospective institutional studies.
Disclosures: The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
FDA approves midostaurin for adult patients with FLT3+ AML
The Food and Drug Administration has approved midostaurin for the treatment of FLT3 mutation–positive acute myeloid leukemia (FLT3+ AML) in adult patients in combination with standard cytarabine and daunorubicin induction and cytarabine consolidation.
Approval was based on results from a randomized, double-blind, placebo-controlled trial of 717 patients with previously untreated FLT3+ AML. The hazard ratio for overall survival in patients receiving midostaurin, compared with a placebo, was 0.77 (P = .016). A companion diagnostic tool, the LeukoStrat CDx FLT3 Mutation Assay manufactured by Invivoscribe Technologies, was also approved.
Midostaurin was also approved for the treatment of aggressive systemic mastocytosis, SM with associated hematological neoplasm, or mast cell leukemia. This indication approval was based on a single-arm, open-label study of midostaurin 100 mg, taken orally twice daily. Complete plus incomplete remission rates were 38% for ASM and 16% for SM with associated hematological neoplasm. Common adverse events included nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, fever, headache, and dyspnea.
The recommended dose of midostaurin in AML is 50 mg twice daily with food on days 8 to 21 of each cycle of induction and consolidation chemotherapy followed by 50 mg with food as a single agent for up to 12 months. The recommended dose for the treatment of adults with aggressive SM, SM with associated hematological neoplasm, or mast cell leukemia is 100 mg twice daily with food, the FDA said.
Midostaurin will be marketed as Rydapt by Novartis Pharmaceuticals.
The Food and Drug Administration has approved midostaurin for the treatment of FLT3 mutation–positive acute myeloid leukemia (FLT3+ AML) in adult patients in combination with standard cytarabine and daunorubicin induction and cytarabine consolidation.
Approval was based on results from a randomized, double-blind, placebo-controlled trial of 717 patients with previously untreated FLT3+ AML. The hazard ratio for overall survival in patients receiving midostaurin, compared with a placebo, was 0.77 (P = .016). A companion diagnostic tool, the LeukoStrat CDx FLT3 Mutation Assay manufactured by Invivoscribe Technologies, was also approved.
Midostaurin was also approved for the treatment of aggressive systemic mastocytosis, SM with associated hematological neoplasm, or mast cell leukemia. This indication approval was based on a single-arm, open-label study of midostaurin 100 mg, taken orally twice daily. Complete plus incomplete remission rates were 38% for ASM and 16% for SM with associated hematological neoplasm. Common adverse events included nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, fever, headache, and dyspnea.
The recommended dose of midostaurin in AML is 50 mg twice daily with food on days 8 to 21 of each cycle of induction and consolidation chemotherapy followed by 50 mg with food as a single agent for up to 12 months. The recommended dose for the treatment of adults with aggressive SM, SM with associated hematological neoplasm, or mast cell leukemia is 100 mg twice daily with food, the FDA said.
Midostaurin will be marketed as Rydapt by Novartis Pharmaceuticals.
The Food and Drug Administration has approved midostaurin for the treatment of FLT3 mutation–positive acute myeloid leukemia (FLT3+ AML) in adult patients in combination with standard cytarabine and daunorubicin induction and cytarabine consolidation.
Approval was based on results from a randomized, double-blind, placebo-controlled trial of 717 patients with previously untreated FLT3+ AML. The hazard ratio for overall survival in patients receiving midostaurin, compared with a placebo, was 0.77 (P = .016). A companion diagnostic tool, the LeukoStrat CDx FLT3 Mutation Assay manufactured by Invivoscribe Technologies, was also approved.
Midostaurin was also approved for the treatment of aggressive systemic mastocytosis, SM with associated hematological neoplasm, or mast cell leukemia. This indication approval was based on a single-arm, open-label study of midostaurin 100 mg, taken orally twice daily. Complete plus incomplete remission rates were 38% for ASM and 16% for SM with associated hematological neoplasm. Common adverse events included nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, fever, headache, and dyspnea.
The recommended dose of midostaurin in AML is 50 mg twice daily with food on days 8 to 21 of each cycle of induction and consolidation chemotherapy followed by 50 mg with food as a single agent for up to 12 months. The recommended dose for the treatment of adults with aggressive SM, SM with associated hematological neoplasm, or mast cell leukemia is 100 mg twice daily with food, the FDA said.
Midostaurin will be marketed as Rydapt by Novartis Pharmaceuticals.
Scientists create online database to aid CML research
A newly launched online database provides researchers with access to data on gene expression in chronic myeloid leukemia (CML).
The LEUKomics database includes datasets relating to clinical parameters in CML, normal and leukemic stem cells, CML disease stages, treatments for the disease, and mouse models of CML.
The database is free for researchers to use and share.
The scientists who developed the database hope it will increase our understanding of CML and lead to new treatments for the disease.
“LEUKomics is a very valuable resource and could help us to reveal new underlying mechanisms that drive CML,” said Jeff Evans, Director of the Institute of Cancer Sciences at the University of Glasgow in Scotland.
“It has the potential to transform CML research on a global level, as the findings can be downloaded and shared with other researchers across the world. We also hope it inspires new research ideas and ultimately fuels a global search into finding cures for CML.”
The LEUKomics database includes datasets with information on gene expression related to:
- Clinical parameters in CML, such as disease aggressiveness and response to treatment
- Stem and progenitor cells from CML patients and healthy individuals
- Chronic, accelerated, and blast phases of CML
- CML treatment (currently only tyrosine kinase inhibitors)
- Stem and progenitor cells from mouse models of CML.
The LEUKomics database was launched by scientists at the University of Glasgow and the University of Melbourne.
The website has been built as part of the stem cell database Stemformatics, with funding from the Scottish Cancer Foundation and Bloodwise.
“Thanks to research, most patients with CML will now live a normal life by taking a single pill,” said Alasdair Rankin, Director of Research at Bloodwise in London, England.
“But treatment is life-long, and not everyone can tolerate the side effects from their treatment or may not respond and see their CML return. There remains a need to develop a permanent cure for all people with this blood cancer. LEUKomics is a highly innovative way to speed up this search for a cure and should be a valuable asset for the global blood cancer research community. We look forward to seeing its impact in the months to come.”
The LEUKomics database can be accessed at: www.stemformatics.org/leukomics. ![]()
A newly launched online database provides researchers with access to data on gene expression in chronic myeloid leukemia (CML).
The LEUKomics database includes datasets relating to clinical parameters in CML, normal and leukemic stem cells, CML disease stages, treatments for the disease, and mouse models of CML.
The database is free for researchers to use and share.
The scientists who developed the database hope it will increase our understanding of CML and lead to new treatments for the disease.
“LEUKomics is a very valuable resource and could help us to reveal new underlying mechanisms that drive CML,” said Jeff Evans, Director of the Institute of Cancer Sciences at the University of Glasgow in Scotland.
“It has the potential to transform CML research on a global level, as the findings can be downloaded and shared with other researchers across the world. We also hope it inspires new research ideas and ultimately fuels a global search into finding cures for CML.”
The LEUKomics database includes datasets with information on gene expression related to:
- Clinical parameters in CML, such as disease aggressiveness and response to treatment
- Stem and progenitor cells from CML patients and healthy individuals
- Chronic, accelerated, and blast phases of CML
- CML treatment (currently only tyrosine kinase inhibitors)
- Stem and progenitor cells from mouse models of CML.
The LEUKomics database was launched by scientists at the University of Glasgow and the University of Melbourne.
The website has been built as part of the stem cell database Stemformatics, with funding from the Scottish Cancer Foundation and Bloodwise.
“Thanks to research, most patients with CML will now live a normal life by taking a single pill,” said Alasdair Rankin, Director of Research at Bloodwise in London, England.
“But treatment is life-long, and not everyone can tolerate the side effects from their treatment or may not respond and see their CML return. There remains a need to develop a permanent cure for all people with this blood cancer. LEUKomics is a highly innovative way to speed up this search for a cure and should be a valuable asset for the global blood cancer research community. We look forward to seeing its impact in the months to come.”
The LEUKomics database can be accessed at: www.stemformatics.org/leukomics. ![]()
A newly launched online database provides researchers with access to data on gene expression in chronic myeloid leukemia (CML).
The LEUKomics database includes datasets relating to clinical parameters in CML, normal and leukemic stem cells, CML disease stages, treatments for the disease, and mouse models of CML.
The database is free for researchers to use and share.
The scientists who developed the database hope it will increase our understanding of CML and lead to new treatments for the disease.
“LEUKomics is a very valuable resource and could help us to reveal new underlying mechanisms that drive CML,” said Jeff Evans, Director of the Institute of Cancer Sciences at the University of Glasgow in Scotland.
“It has the potential to transform CML research on a global level, as the findings can be downloaded and shared with other researchers across the world. We also hope it inspires new research ideas and ultimately fuels a global search into finding cures for CML.”
The LEUKomics database includes datasets with information on gene expression related to:
- Clinical parameters in CML, such as disease aggressiveness and response to treatment
- Stem and progenitor cells from CML patients and healthy individuals
- Chronic, accelerated, and blast phases of CML
- CML treatment (currently only tyrosine kinase inhibitors)
- Stem and progenitor cells from mouse models of CML.
The LEUKomics database was launched by scientists at the University of Glasgow and the University of Melbourne.
The website has been built as part of the stem cell database Stemformatics, with funding from the Scottish Cancer Foundation and Bloodwise.
“Thanks to research, most patients with CML will now live a normal life by taking a single pill,” said Alasdair Rankin, Director of Research at Bloodwise in London, England.
“But treatment is life-long, and not everyone can tolerate the side effects from their treatment or may not respond and see their CML return. There remains a need to develop a permanent cure for all people with this blood cancer. LEUKomics is a highly innovative way to speed up this search for a cure and should be a valuable asset for the global blood cancer research community. We look forward to seeing its impact in the months to come.”
The LEUKomics database can be accessed at: www.stemformatics.org/leukomics. ![]()
Fertility treatments linked to risk of pediatric cancers
Children born to mothers who underwent fertility treatments have an increased risk of developing pediatric neoplasms, according to research published in the American Journal of Obstetrics & Gynecology.
The study showed an increased risk of malignancies and benign tumors among children conceived after fertility treatments.
However, the risk of leukemias and lymphomas among these children was not significantly different from the risk among children who were conceived spontaneously.
The study was a population-based cohort analysis of babies born between 1991 and 2013 at Soroka University Medical Center in Beer-Sheva, Israel, with follow-up to age 18.
Of the 242,187 newborn infants in the study, 237,863 (98.3%) were conceived spontaneously, 2603 (1.1%) were conceived after in vitro fertilization (IVF), and 1721 (0.7%) were conceived after ovulation induction (OI) treatments.
During a median follow-up of 10.55 years, there were 1498 neoplasms reported, including 1074 benign tumors and 429 malignancies.
The rate of neoplasms per 10,000 children was 61.85 for the entire study cohort, 111.41 for the IVF group, 110.40 for the OI group, and 60.96 for the spontaneous conception group (P<0.001 for the comparison between spontaneous conception and both types of fertility treatments).
The rate of benign tumors per 10,000 children was 44.35 for the entire study cohort, 84.51 for the IVF group, 69.73 for the OI group, and 43.72 for the spontaneous conception group (P=0.002).
The rate of malignancies per 10,000 children was 17.71 for the entire study cohort, 26.89 for the IVF group, 40.67 for the OI group, and 17.44 for the spontaneous conception group (P=0.038).
The rate of leukemia per 10,000 children was 3.72 for the entire study cohort (n=90 leukemia cases total), 0 for the IVF group (n=0), 5.81 for the OI group (n=1), and 3.74 for the spontaneous conception group (n=89, P=0.56).
The rate of lymphoma per 10,000 children was 2.27 for the entire study cohort (n=55), 7.68 for the IVF group (n=2), 0 for the OI group (n=0), and 2.23 for the spontaneous conception group (n=53, P=0.15).
The researchers said the association between fertility treatments and total pediatric neoplasms or total malignancies remained significant in analyses controlled for confounders such as gestational diabetes mellitus, hypertensive disorders, preterm birth, and maternal age.
For any fertility treatment, the adjusted hazard ratio (aHR) for all neoplasms was 1.97, and the aHR for all malignancies was 1.96.
For IVF, the aHR was 2.48 for all neoplasms and 1.89 for all malignancies. For OI, the aHR was 1.51 for all neoplasms and 2.03 for all malignancies.
“The research concludes that the association between IVF and total pediatric neoplasms and malignancies is significant,” said study author Eyal Sheiner, MD, PhD, of Ben-Gurion University of the Negev in Beer-Sheva, Israel.
“With increasing numbers of offspring conceived after fertility treatments, it is important to follow up on their health.” ![]()
Children born to mothers who underwent fertility treatments have an increased risk of developing pediatric neoplasms, according to research published in the American Journal of Obstetrics & Gynecology.
The study showed an increased risk of malignancies and benign tumors among children conceived after fertility treatments.
However, the risk of leukemias and lymphomas among these children was not significantly different from the risk among children who were conceived spontaneously.
The study was a population-based cohort analysis of babies born between 1991 and 2013 at Soroka University Medical Center in Beer-Sheva, Israel, with follow-up to age 18.
Of the 242,187 newborn infants in the study, 237,863 (98.3%) were conceived spontaneously, 2603 (1.1%) were conceived after in vitro fertilization (IVF), and 1721 (0.7%) were conceived after ovulation induction (OI) treatments.
During a median follow-up of 10.55 years, there were 1498 neoplasms reported, including 1074 benign tumors and 429 malignancies.
The rate of neoplasms per 10,000 children was 61.85 for the entire study cohort, 111.41 for the IVF group, 110.40 for the OI group, and 60.96 for the spontaneous conception group (P<0.001 for the comparison between spontaneous conception and both types of fertility treatments).
The rate of benign tumors per 10,000 children was 44.35 for the entire study cohort, 84.51 for the IVF group, 69.73 for the OI group, and 43.72 for the spontaneous conception group (P=0.002).
The rate of malignancies per 10,000 children was 17.71 for the entire study cohort, 26.89 for the IVF group, 40.67 for the OI group, and 17.44 for the spontaneous conception group (P=0.038).
The rate of leukemia per 10,000 children was 3.72 for the entire study cohort (n=90 leukemia cases total), 0 for the IVF group (n=0), 5.81 for the OI group (n=1), and 3.74 for the spontaneous conception group (n=89, P=0.56).
The rate of lymphoma per 10,000 children was 2.27 for the entire study cohort (n=55), 7.68 for the IVF group (n=2), 0 for the OI group (n=0), and 2.23 for the spontaneous conception group (n=53, P=0.15).
The researchers said the association between fertility treatments and total pediatric neoplasms or total malignancies remained significant in analyses controlled for confounders such as gestational diabetes mellitus, hypertensive disorders, preterm birth, and maternal age.
For any fertility treatment, the adjusted hazard ratio (aHR) for all neoplasms was 1.97, and the aHR for all malignancies was 1.96.
For IVF, the aHR was 2.48 for all neoplasms and 1.89 for all malignancies. For OI, the aHR was 1.51 for all neoplasms and 2.03 for all malignancies.
“The research concludes that the association between IVF and total pediatric neoplasms and malignancies is significant,” said study author Eyal Sheiner, MD, PhD, of Ben-Gurion University of the Negev in Beer-Sheva, Israel.
“With increasing numbers of offspring conceived after fertility treatments, it is important to follow up on their health.” ![]()
Children born to mothers who underwent fertility treatments have an increased risk of developing pediatric neoplasms, according to research published in the American Journal of Obstetrics & Gynecology.
The study showed an increased risk of malignancies and benign tumors among children conceived after fertility treatments.
However, the risk of leukemias and lymphomas among these children was not significantly different from the risk among children who were conceived spontaneously.
The study was a population-based cohort analysis of babies born between 1991 and 2013 at Soroka University Medical Center in Beer-Sheva, Israel, with follow-up to age 18.
Of the 242,187 newborn infants in the study, 237,863 (98.3%) were conceived spontaneously, 2603 (1.1%) were conceived after in vitro fertilization (IVF), and 1721 (0.7%) were conceived after ovulation induction (OI) treatments.
During a median follow-up of 10.55 years, there were 1498 neoplasms reported, including 1074 benign tumors and 429 malignancies.
The rate of neoplasms per 10,000 children was 61.85 for the entire study cohort, 111.41 for the IVF group, 110.40 for the OI group, and 60.96 for the spontaneous conception group (P<0.001 for the comparison between spontaneous conception and both types of fertility treatments).
The rate of benign tumors per 10,000 children was 44.35 for the entire study cohort, 84.51 for the IVF group, 69.73 for the OI group, and 43.72 for the spontaneous conception group (P=0.002).
The rate of malignancies per 10,000 children was 17.71 for the entire study cohort, 26.89 for the IVF group, 40.67 for the OI group, and 17.44 for the spontaneous conception group (P=0.038).
The rate of leukemia per 10,000 children was 3.72 for the entire study cohort (n=90 leukemia cases total), 0 for the IVF group (n=0), 5.81 for the OI group (n=1), and 3.74 for the spontaneous conception group (n=89, P=0.56).
The rate of lymphoma per 10,000 children was 2.27 for the entire study cohort (n=55), 7.68 for the IVF group (n=2), 0 for the OI group (n=0), and 2.23 for the spontaneous conception group (n=53, P=0.15).
The researchers said the association between fertility treatments and total pediatric neoplasms or total malignancies remained significant in analyses controlled for confounders such as gestational diabetes mellitus, hypertensive disorders, preterm birth, and maternal age.
For any fertility treatment, the adjusted hazard ratio (aHR) for all neoplasms was 1.97, and the aHR for all malignancies was 1.96.
For IVF, the aHR was 2.48 for all neoplasms and 1.89 for all malignancies. For OI, the aHR was 1.51 for all neoplasms and 2.03 for all malignancies.
“The research concludes that the association between IVF and total pediatric neoplasms and malignancies is significant,” said study author Eyal Sheiner, MD, PhD, of Ben-Gurion University of the Negev in Beer-Sheva, Israel.
“With increasing numbers of offspring conceived after fertility treatments, it is important to follow up on their health.” ![]()