User login
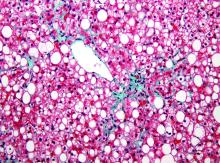
Hemochromatosis variants may confer 10-fold higher risk of liver cancer
Hereditary hemochromatosis is primarily caused by HFE gene variants. Past research suggested that 81% of patients with hereditary hemochromatosis carry the p.C282Y variant and 5% carry the p.C282Y/p.H63D compound heterozygote genotype.
In a new study, the presence of HFE p.C282Y and p.H63D genotypes was associated with a 10-fold greater risk of developing a hepatic malignancy among men of European ancestry aged 40-70 years. In addition, men with HFE variants were 1.2 times more likely to die of any cause, compared with men who had neither pathogenic variant.
Janice L. Atkins, PhD, of the University of Exeter (England), and colleagues reported these findings in JAMA.
For this study, Dr. Atkins and colleagues used follow-up data from a large genotyped community sample to estimate the incidence of primary hepatic carcinomas and deaths by HFE variant status in participants of European descent.
Data for the two linked coprimary endpoints, incident primary liver carcinoma and all-cause mortality, were derived from hospital and death certificate records. Where available, primary care data was also included.
Results: Increased risks for men, not women
The researchers analyzed data from 451,186 men and women, aged 40-70 years, from the UK Biobank. There were 2,890 (0.9%) patients who were p.C282Y homozygous, 1,294 of whom were men.
Among the 1,294 men with HFE p.C282Y homozygosity, 21 were diagnosed with a primary hepatic malignancy. Ten of these patients were not diagnosed with hemochromatosis at baseline.
At a median follow-up of 8.9 years, the risk of primary hepatic malignancy was significantly higher in men with HFE p.C282Y homozygosity, compared with men without HFE pathogenic variants (hazard ratio, 10.5; 95% confidence interval, 6.6-16.7; P < .001).
The risk of all-cause death was significantly higher in men with HFE p.C282Y homozygosity as well (HR, 1.2; 95% CI, 1.0-1.5; P = .046).
In contrast, female HFE p.C282Y homozygotes had no significant increases in the risk of incident primary hepatic malignancy or all-cause mortality.
Life table projections estimated that 7.2% of men with HFE p.C282Y homozygosity will develop a primary hepatic malignancy by age 75, compared with 0.6% of men without p.C282Y or p.H63D variants.
The researchers acknowledged that a key limitation of this study was the ancestral homogeneity of the cohort. Thus, the findings may not be generalizable to all patient populations.
Implications: Earlier diagnosis and treatment
The results of this study underline the importance of early diagnosis and genetic testing, according to the researchers.
“Tragically, men with the hemochromatosis faulty genes have been dying of liver cancer for many years, but this was thought to be rare,” study author David Melzer, MBBCh, PhD, of University of Exeter, said in a press release.
“The large scale of the UK Biobank study allowed us to measure cancer risk accurately. We were shocked to find that more than 7% of men with two faulty genes are likely to develop liver cancer by age 75, particularly considering that the U.K. has the second-highest rate of these faulty genes in the world. Fortunately, most of these cancers could be prevented with early treatment,” Dr. Melzer added.
“Physicians and scientists have long acknowledged that iron overload is an important cofactor fueling the development of many serious diseases, including cancer,” said study author Jeremy Shearman, MBChB, DPhil, of Nuffield Health and South Warwickshire NHS Foundation Trust in the United Kingdom.
“This research is a vital step towards quantifying that risk and should raise awareness of the importance of iron in the minds of both clinicians and patients. Measurement of iron stores and recognition of the genetic risk of iron overload needs to become a routine part of health assessment and monitoring in the U.K.,” Dr. Shearman added.
“The UK Biobank project is a glimpse into the future of medicine where all known genes are tested and then treatable conditions are offered treatment before serious complications develop,” said study author Paul Adams, MD, of the University of Western Ontario in London.
This research was funded by the UK Medical Research Council. Dr. Melzer disclosed financial affiliations with the UK Medical Research Council during the conduct of the study.
SOURCE: Atkins JL et al. JAMA. 2020 Nov 24. doi: 10.1001/jama.2020.21566.
Hereditary hemochromatosis is primarily caused by HFE gene variants. Past research suggested that 81% of patients with hereditary hemochromatosis carry the p.C282Y variant and 5% carry the p.C282Y/p.H63D compound heterozygote genotype.
In a new study, the presence of HFE p.C282Y and p.H63D genotypes was associated with a 10-fold greater risk of developing a hepatic malignancy among men of European ancestry aged 40-70 years. In addition, men with HFE variants were 1.2 times more likely to die of any cause, compared with men who had neither pathogenic variant.
Janice L. Atkins, PhD, of the University of Exeter (England), and colleagues reported these findings in JAMA.
For this study, Dr. Atkins and colleagues used follow-up data from a large genotyped community sample to estimate the incidence of primary hepatic carcinomas and deaths by HFE variant status in participants of European descent.
Data for the two linked coprimary endpoints, incident primary liver carcinoma and all-cause mortality, were derived from hospital and death certificate records. Where available, primary care data was also included.
Results: Increased risks for men, not women
The researchers analyzed data from 451,186 men and women, aged 40-70 years, from the UK Biobank. There were 2,890 (0.9%) patients who were p.C282Y homozygous, 1,294 of whom were men.
Among the 1,294 men with HFE p.C282Y homozygosity, 21 were diagnosed with a primary hepatic malignancy. Ten of these patients were not diagnosed with hemochromatosis at baseline.
At a median follow-up of 8.9 years, the risk of primary hepatic malignancy was significantly higher in men with HFE p.C282Y homozygosity, compared with men without HFE pathogenic variants (hazard ratio, 10.5; 95% confidence interval, 6.6-16.7; P < .001).
The risk of all-cause death was significantly higher in men with HFE p.C282Y homozygosity as well (HR, 1.2; 95% CI, 1.0-1.5; P = .046).
In contrast, female HFE p.C282Y homozygotes had no significant increases in the risk of incident primary hepatic malignancy or all-cause mortality.
Life table projections estimated that 7.2% of men with HFE p.C282Y homozygosity will develop a primary hepatic malignancy by age 75, compared with 0.6% of men without p.C282Y or p.H63D variants.
The researchers acknowledged that a key limitation of this study was the ancestral homogeneity of the cohort. Thus, the findings may not be generalizable to all patient populations.
Implications: Earlier diagnosis and treatment
The results of this study underline the importance of early diagnosis and genetic testing, according to the researchers.
“Tragically, men with the hemochromatosis faulty genes have been dying of liver cancer for many years, but this was thought to be rare,” study author David Melzer, MBBCh, PhD, of University of Exeter, said in a press release.
“The large scale of the UK Biobank study allowed us to measure cancer risk accurately. We were shocked to find that more than 7% of men with two faulty genes are likely to develop liver cancer by age 75, particularly considering that the U.K. has the second-highest rate of these faulty genes in the world. Fortunately, most of these cancers could be prevented with early treatment,” Dr. Melzer added.
“Physicians and scientists have long acknowledged that iron overload is an important cofactor fueling the development of many serious diseases, including cancer,” said study author Jeremy Shearman, MBChB, DPhil, of Nuffield Health and South Warwickshire NHS Foundation Trust in the United Kingdom.
“This research is a vital step towards quantifying that risk and should raise awareness of the importance of iron in the minds of both clinicians and patients. Measurement of iron stores and recognition of the genetic risk of iron overload needs to become a routine part of health assessment and monitoring in the U.K.,” Dr. Shearman added.
“The UK Biobank project is a glimpse into the future of medicine where all known genes are tested and then treatable conditions are offered treatment before serious complications develop,” said study author Paul Adams, MD, of the University of Western Ontario in London.
This research was funded by the UK Medical Research Council. Dr. Melzer disclosed financial affiliations with the UK Medical Research Council during the conduct of the study.
SOURCE: Atkins JL et al. JAMA. 2020 Nov 24. doi: 10.1001/jama.2020.21566.
Hereditary hemochromatosis is primarily caused by HFE gene variants. Past research suggested that 81% of patients with hereditary hemochromatosis carry the p.C282Y variant and 5% carry the p.C282Y/p.H63D compound heterozygote genotype.
In a new study, the presence of HFE p.C282Y and p.H63D genotypes was associated with a 10-fold greater risk of developing a hepatic malignancy among men of European ancestry aged 40-70 years. In addition, men with HFE variants were 1.2 times more likely to die of any cause, compared with men who had neither pathogenic variant.
Janice L. Atkins, PhD, of the University of Exeter (England), and colleagues reported these findings in JAMA.
For this study, Dr. Atkins and colleagues used follow-up data from a large genotyped community sample to estimate the incidence of primary hepatic carcinomas and deaths by HFE variant status in participants of European descent.
Data for the two linked coprimary endpoints, incident primary liver carcinoma and all-cause mortality, were derived from hospital and death certificate records. Where available, primary care data was also included.
Results: Increased risks for men, not women
The researchers analyzed data from 451,186 men and women, aged 40-70 years, from the UK Biobank. There were 2,890 (0.9%) patients who were p.C282Y homozygous, 1,294 of whom were men.
Among the 1,294 men with HFE p.C282Y homozygosity, 21 were diagnosed with a primary hepatic malignancy. Ten of these patients were not diagnosed with hemochromatosis at baseline.
At a median follow-up of 8.9 years, the risk of primary hepatic malignancy was significantly higher in men with HFE p.C282Y homozygosity, compared with men without HFE pathogenic variants (hazard ratio, 10.5; 95% confidence interval, 6.6-16.7; P < .001).
The risk of all-cause death was significantly higher in men with HFE p.C282Y homozygosity as well (HR, 1.2; 95% CI, 1.0-1.5; P = .046).
In contrast, female HFE p.C282Y homozygotes had no significant increases in the risk of incident primary hepatic malignancy or all-cause mortality.
Life table projections estimated that 7.2% of men with HFE p.C282Y homozygosity will develop a primary hepatic malignancy by age 75, compared with 0.6% of men without p.C282Y or p.H63D variants.
The researchers acknowledged that a key limitation of this study was the ancestral homogeneity of the cohort. Thus, the findings may not be generalizable to all patient populations.
Implications: Earlier diagnosis and treatment
The results of this study underline the importance of early diagnosis and genetic testing, according to the researchers.
“Tragically, men with the hemochromatosis faulty genes have been dying of liver cancer for many years, but this was thought to be rare,” study author David Melzer, MBBCh, PhD, of University of Exeter, said in a press release.
“The large scale of the UK Biobank study allowed us to measure cancer risk accurately. We were shocked to find that more than 7% of men with two faulty genes are likely to develop liver cancer by age 75, particularly considering that the U.K. has the second-highest rate of these faulty genes in the world. Fortunately, most of these cancers could be prevented with early treatment,” Dr. Melzer added.
“Physicians and scientists have long acknowledged that iron overload is an important cofactor fueling the development of many serious diseases, including cancer,” said study author Jeremy Shearman, MBChB, DPhil, of Nuffield Health and South Warwickshire NHS Foundation Trust in the United Kingdom.
“This research is a vital step towards quantifying that risk and should raise awareness of the importance of iron in the minds of both clinicians and patients. Measurement of iron stores and recognition of the genetic risk of iron overload needs to become a routine part of health assessment and monitoring in the U.K.,” Dr. Shearman added.
“The UK Biobank project is a glimpse into the future of medicine where all known genes are tested and then treatable conditions are offered treatment before serious complications develop,” said study author Paul Adams, MD, of the University of Western Ontario in London.
This research was funded by the UK Medical Research Council. Dr. Melzer disclosed financial affiliations with the UK Medical Research Council during the conduct of the study.
SOURCE: Atkins JL et al. JAMA. 2020 Nov 24. doi: 10.1001/jama.2020.21566.
FROM JAMA
AASLD 2020: A clinical news roundup
Studies that address fundamental questions in hepatology and have the potential to change or improve clinical practice were the focus of a clinical debrief session from the virtual annual meeting of the American Association for the Study of Liver Diseases.
“We chose papers that had the highest level of evidence, such as randomized controlled trials, controlled studies, and large data sets – and some small data sets too,” said Tamar Taddei, MD, associate professor of medicine in the section of digestive disease at Yale University, New Haven, Conn.
Dr. Taddei and colleagues Silvia Vilarinho, MD, PhD; Simona Jakab, MD; and Ariel Jaffe, MD, all also from Yale, selected the papers from among 197 oral and 1,769 poster abstracts presented at AASLD 2020.
They highlighted the most important findings from presentations on autoimmune and cholestatic disease, transplantation, cirrhosis and portal hypertension, alcoholic liver disease, neoplasia, drug-induced liver injury, and COVID-19. They did not review studies focused primarily on nonalcoholic steatohepatitis or nonalcoholic fatty liver disease, viral hepatitis, or basic science, all of which were covered in separate debriefing sessions.
Cirrhosis and portal hypertension
A study from the Department of Veterans Affairs looked at the prevalence of liver disease risk factors and rates of subsequent testing for and diagnosis of cirrhosis in the Veterans Health Administration system (VHA).
The authors found that, among more than 6.65 million VHA users in 2018 with no prior diagnosis of cirrhosis, approximately half were at risk for cirrhosis, of whom about 75% were screened, and approximately 5% of those who were screened were positive for possible cirrhosis (133,636). Of the patients who screened positive, about 10% (12,566) received a diagnosis of cirrhosis, including 4,120 with liver decompensation.
“This paper underscores the importance of population-level screening in uncovering unrecognized cirrhosis, enabling intervention earlier in the course of disease,” Dr. Taddei said (Abstract #661).
A study looking at external validation of novel cirrhosis surgical risk models designed to improve prognostication for a range of common surgeries showed that the VOCAL-Penn score was superior to the Mayo Risk Score, Model for End-stage Liver Disease and MELD-sodium scores for discrimination of 30-day and 90-day postoperative mortality (Abstract #91).
“While these models are not a substitute for clinical acumen, they certainly improve our surgical risk prediction in patients with cirrhosis, a very common question in consultative hepatology,” Dr. Taddei said.
She also cited three abstracts that address the important questions regarding performing studies in patients with varices or ascites, including whether it’s safe to perform transesophageal echocardiography in patients with cirrhosis without first screening for varices, and whether nonselective beta-blockers should be continued in patients with refractory ascites.
A retrospective study of 191 patients with cirrhosis who underwent upper endoscopy within 4 years of transesophageal echocardiography had no overt gastrointestinal bleeding regardless of the presence of esophageal varices, suggesting that routine preprocedure esophagogastroduodenoscopy “is of no utility,” (Abstract #1872).
A study to determine risk of sepsis in 1,198 patients with cirrhosis found that 1-year risk of sepsis was reduced by 50% with the use of nonselective beta-blockers (Abstract #94).
The final abstract in this category touched on the use of an advance care planning video support tool to help transplant-ineligible patients with end-stage liver disease decide whether they want support measures such cardiopulmonary resuscitation or intubation. The authors found that the video decision tool was feasible and acceptable to patients, and improved their knowledge of end-of-life care. More patients randomized to the video arm opted against CPR or intubation, compared with those assigned to a verbal discussion of options (Abstract #712).
Alcohol
The reviewers highlighted two studies of alcohol use: The first was designed to determine the prevalence of early alcohol relapse (resumption within 3 months) in patients who presented with alcoholic hepatitis. The subjects included 478 patients enrolled in the STOPAH trial, and a validation set of 194 patients from the InTeam (Integrated Approaches for identifying Molecular Targets in Alcoholic Hepatitis) Consortium.
“They found that high-risk patients were younger, unemployed, and without a stable relationship. Intermediate risk were middle aged, employed, and in a stable relationship, and low-risk profiles were older, with known cirrhosis; they were mostly retired and in a stable relationship,” Dr. Taddei said.
The identification of nongenetic factors that predict early relapse may aid in personalization of treatment strategies, she said (Abstract #232).
The second study looked at fecal microbial transplant (FMT) for reducing cravings in adults with alcohol use disorder (AUD) and cirrhosis. The investigators saw a nonsignificant trend toward greater total abstinence at 6 months in patients randomized to FMT versus placebo.
“Future trials should be performed to determine the impact of FMT on altering the gut-brain axis in patients with AUD,” she said (Abstract #7).
Transplantation
The prospective controlled QUICKTRANS study by French and Belgian researchers found that patients who underwent early liver transplantation for severe alcoholic hepatitis had numerically but not significantly higher rates of relapse than patients who were transplanted after at least 6 months of abstinence, although heavy drinking was more frequent in patients who underwent early transplant.
The 2-year survival rates for both patients who underwent early transplant and those who underwent transplant after 6 months of sobriety were “identical, and excellent.” In addition, the 2-year survival rate for patients with severe alcoholic hepatitis who underwent transplant was 82.8%, compared with 28.2% for patients who were deemed ineligible for transplant according to a selection algorithm (P < .001).
“Perhaps most important is that studies in this population can be conducted in a controlled fashion across centers with reproducible transplant eligibility algorithms,” Dr. Taddei commented (Abstract #6).
The place of honor – Abstract # 1 – was reserved for a study looking at the effects on liver transplant practice of a new “safety net” policy from the Organ Procurement and Transplantation Network and United Network for Organ Sharing stating that patients awaiting liver transplantation who develop kidney failure may be given priority on the kidney transplant waiting list.
The investigators found that the new policy significantly increased the number of adult primary liver transplant alone candidates who where on dialysis at the time of listing, and did not affect either waiting list mortality or posttransplant outcomes.
The authors also saw a significant increase in kidney transplant listing after liver transplant, especially for patients who were on hemodialysis at the time of list.
In the period after implementation of the policy, there was a significantly higher probability of kidney transplant, and significant reduction in waiting list mortality.
Autoimmune & cholestatic diseases
Investigators performed an analysis of the phase 3 randomized controlled ENHANCE trial of seladelpar in patients with primary biliary cholangitis. The trial was stopped because of an adverse event ultimately deemed to be unrelated to the drug, so the analysis looked at the composite responder rate at month 3.
“The key takeaway from this study is that at the 10-mg dosage of seladelpar, 78% met a composite endpoint, 27% of patients normalized their alkaline phosphatase, and 50% normalized their ALT. There was significant improvement in pruritus,” Dr. Taddei said.
The drug was generally safe and well tolerated. A 52-week phase 3 global registration study will begin enrolling patients in early 2021 (Abstract #LO11).
In a pediatric study, investigators looked at differences in primary sclerosing cholangitis (PSC) among various population, and found that “Black and Hispanic patients have dramatically worse clinical outcomes, compared to White and Asian patients. They are more likely to be diagnosed with PSC at an advanced stage with extensive fibrosis and portal hypertensive manifestations.”
The authors suggested that the differences may be explained in part by socioeconomic disparities leading to delay in diagnosis, to a more aggressive phenotype, or both (Abstract #66).
A meta-analysis of maternal and fetal outcomes in women with autoimmune hepatitis showed that the disease is associated with increased risk of gestational diabetes, premature births, and small-for-gestational age or low-birth-weight babies.
“Pregnant women should be monitored closely before, during and after pregnancy. It’s important to know that, in the prevalence data, flares were most prevalent postpartum at 41%. These finds will help us counsel our patients with autoimmune hepatitis who become pregnant,” Dr. Taddei said (Abstract #97).
Drug-induced liver injury
A study of clinical outcomes following immune checkpoint inhibitor rechallenge in melanoma patients with resolved higher grade 3 or higher checkpoint inhibitor–induced hepatitis showed that 4 of 31 patients (13%) developed recurrence of grade 2 or greater hepatitis, and 15 of 31 (48%) developed an immune-related adverse event after rechallenge.
There was no difference in time to death between patients who were rechallenged and those who were not, and immune-related liver toxicities requiring drug discontinuation after rechallenge were uncommon.
“High-grade immune checkpoint inhibitor hepatitis should be reconsidered as an absolute contraindication for immune checkpoint inhibitor rechallenge,” Dr. Taddei said (Abstract # 116).
Neoplasia
The investigators also highlighted an abstract describing significant urban-rural and racial ethnic differences in hepatocellular carcinoma rates. A fuller description of this study can be found here (Abstract #136).
COVID-19
Finally, the reviewer highlighted a study of the clinical course of COVID-19 in patients with chronic liver disease, and to determine factors associated with adverse outcomes in patients with chronic liver disease who acquire COVID-19.
The investigators found that patients with chronic liver disease and COVID-19 have a 14% morality rate, and that alcohol-related liver disease, decompensated cirrhosis, and hepatocellular carcinoma are all risk factors for increased mortality from COVID-19.
They recommended emphasizing telemedicine, prioritizing patients with chronic liver disease for vaccination, and including these patients in prospective studies and drug trials for COVID-19 therapies.
Dr. Taddei reported having no disclosures.
Studies that address fundamental questions in hepatology and have the potential to change or improve clinical practice were the focus of a clinical debrief session from the virtual annual meeting of the American Association for the Study of Liver Diseases.
“We chose papers that had the highest level of evidence, such as randomized controlled trials, controlled studies, and large data sets – and some small data sets too,” said Tamar Taddei, MD, associate professor of medicine in the section of digestive disease at Yale University, New Haven, Conn.
Dr. Taddei and colleagues Silvia Vilarinho, MD, PhD; Simona Jakab, MD; and Ariel Jaffe, MD, all also from Yale, selected the papers from among 197 oral and 1,769 poster abstracts presented at AASLD 2020.
They highlighted the most important findings from presentations on autoimmune and cholestatic disease, transplantation, cirrhosis and portal hypertension, alcoholic liver disease, neoplasia, drug-induced liver injury, and COVID-19. They did not review studies focused primarily on nonalcoholic steatohepatitis or nonalcoholic fatty liver disease, viral hepatitis, or basic science, all of which were covered in separate debriefing sessions.
Cirrhosis and portal hypertension
A study from the Department of Veterans Affairs looked at the prevalence of liver disease risk factors and rates of subsequent testing for and diagnosis of cirrhosis in the Veterans Health Administration system (VHA).
The authors found that, among more than 6.65 million VHA users in 2018 with no prior diagnosis of cirrhosis, approximately half were at risk for cirrhosis, of whom about 75% were screened, and approximately 5% of those who were screened were positive for possible cirrhosis (133,636). Of the patients who screened positive, about 10% (12,566) received a diagnosis of cirrhosis, including 4,120 with liver decompensation.
“This paper underscores the importance of population-level screening in uncovering unrecognized cirrhosis, enabling intervention earlier in the course of disease,” Dr. Taddei said (Abstract #661).
A study looking at external validation of novel cirrhosis surgical risk models designed to improve prognostication for a range of common surgeries showed that the VOCAL-Penn score was superior to the Mayo Risk Score, Model for End-stage Liver Disease and MELD-sodium scores for discrimination of 30-day and 90-day postoperative mortality (Abstract #91).
“While these models are not a substitute for clinical acumen, they certainly improve our surgical risk prediction in patients with cirrhosis, a very common question in consultative hepatology,” Dr. Taddei said.
She also cited three abstracts that address the important questions regarding performing studies in patients with varices or ascites, including whether it’s safe to perform transesophageal echocardiography in patients with cirrhosis without first screening for varices, and whether nonselective beta-blockers should be continued in patients with refractory ascites.
A retrospective study of 191 patients with cirrhosis who underwent upper endoscopy within 4 years of transesophageal echocardiography had no overt gastrointestinal bleeding regardless of the presence of esophageal varices, suggesting that routine preprocedure esophagogastroduodenoscopy “is of no utility,” (Abstract #1872).
A study to determine risk of sepsis in 1,198 patients with cirrhosis found that 1-year risk of sepsis was reduced by 50% with the use of nonselective beta-blockers (Abstract #94).
The final abstract in this category touched on the use of an advance care planning video support tool to help transplant-ineligible patients with end-stage liver disease decide whether they want support measures such cardiopulmonary resuscitation or intubation. The authors found that the video decision tool was feasible and acceptable to patients, and improved their knowledge of end-of-life care. More patients randomized to the video arm opted against CPR or intubation, compared with those assigned to a verbal discussion of options (Abstract #712).
Alcohol
The reviewers highlighted two studies of alcohol use: The first was designed to determine the prevalence of early alcohol relapse (resumption within 3 months) in patients who presented with alcoholic hepatitis. The subjects included 478 patients enrolled in the STOPAH trial, and a validation set of 194 patients from the InTeam (Integrated Approaches for identifying Molecular Targets in Alcoholic Hepatitis) Consortium.
“They found that high-risk patients were younger, unemployed, and without a stable relationship. Intermediate risk were middle aged, employed, and in a stable relationship, and low-risk profiles were older, with known cirrhosis; they were mostly retired and in a stable relationship,” Dr. Taddei said.
The identification of nongenetic factors that predict early relapse may aid in personalization of treatment strategies, she said (Abstract #232).
The second study looked at fecal microbial transplant (FMT) for reducing cravings in adults with alcohol use disorder (AUD) and cirrhosis. The investigators saw a nonsignificant trend toward greater total abstinence at 6 months in patients randomized to FMT versus placebo.
“Future trials should be performed to determine the impact of FMT on altering the gut-brain axis in patients with AUD,” she said (Abstract #7).
Transplantation
The prospective controlled QUICKTRANS study by French and Belgian researchers found that patients who underwent early liver transplantation for severe alcoholic hepatitis had numerically but not significantly higher rates of relapse than patients who were transplanted after at least 6 months of abstinence, although heavy drinking was more frequent in patients who underwent early transplant.
The 2-year survival rates for both patients who underwent early transplant and those who underwent transplant after 6 months of sobriety were “identical, and excellent.” In addition, the 2-year survival rate for patients with severe alcoholic hepatitis who underwent transplant was 82.8%, compared with 28.2% for patients who were deemed ineligible for transplant according to a selection algorithm (P < .001).
“Perhaps most important is that studies in this population can be conducted in a controlled fashion across centers with reproducible transplant eligibility algorithms,” Dr. Taddei commented (Abstract #6).
The place of honor – Abstract # 1 – was reserved for a study looking at the effects on liver transplant practice of a new “safety net” policy from the Organ Procurement and Transplantation Network and United Network for Organ Sharing stating that patients awaiting liver transplantation who develop kidney failure may be given priority on the kidney transplant waiting list.
The investigators found that the new policy significantly increased the number of adult primary liver transplant alone candidates who where on dialysis at the time of listing, and did not affect either waiting list mortality or posttransplant outcomes.
The authors also saw a significant increase in kidney transplant listing after liver transplant, especially for patients who were on hemodialysis at the time of list.
In the period after implementation of the policy, there was a significantly higher probability of kidney transplant, and significant reduction in waiting list mortality.
Autoimmune & cholestatic diseases
Investigators performed an analysis of the phase 3 randomized controlled ENHANCE trial of seladelpar in patients with primary biliary cholangitis. The trial was stopped because of an adverse event ultimately deemed to be unrelated to the drug, so the analysis looked at the composite responder rate at month 3.
“The key takeaway from this study is that at the 10-mg dosage of seladelpar, 78% met a composite endpoint, 27% of patients normalized their alkaline phosphatase, and 50% normalized their ALT. There was significant improvement in pruritus,” Dr. Taddei said.
The drug was generally safe and well tolerated. A 52-week phase 3 global registration study will begin enrolling patients in early 2021 (Abstract #LO11).
In a pediatric study, investigators looked at differences in primary sclerosing cholangitis (PSC) among various population, and found that “Black and Hispanic patients have dramatically worse clinical outcomes, compared to White and Asian patients. They are more likely to be diagnosed with PSC at an advanced stage with extensive fibrosis and portal hypertensive manifestations.”
The authors suggested that the differences may be explained in part by socioeconomic disparities leading to delay in diagnosis, to a more aggressive phenotype, or both (Abstract #66).
A meta-analysis of maternal and fetal outcomes in women with autoimmune hepatitis showed that the disease is associated with increased risk of gestational diabetes, premature births, and small-for-gestational age or low-birth-weight babies.
“Pregnant women should be monitored closely before, during and after pregnancy. It’s important to know that, in the prevalence data, flares were most prevalent postpartum at 41%. These finds will help us counsel our patients with autoimmune hepatitis who become pregnant,” Dr. Taddei said (Abstract #97).
Drug-induced liver injury
A study of clinical outcomes following immune checkpoint inhibitor rechallenge in melanoma patients with resolved higher grade 3 or higher checkpoint inhibitor–induced hepatitis showed that 4 of 31 patients (13%) developed recurrence of grade 2 or greater hepatitis, and 15 of 31 (48%) developed an immune-related adverse event after rechallenge.
There was no difference in time to death between patients who were rechallenged and those who were not, and immune-related liver toxicities requiring drug discontinuation after rechallenge were uncommon.
“High-grade immune checkpoint inhibitor hepatitis should be reconsidered as an absolute contraindication for immune checkpoint inhibitor rechallenge,” Dr. Taddei said (Abstract # 116).
Neoplasia
The investigators also highlighted an abstract describing significant urban-rural and racial ethnic differences in hepatocellular carcinoma rates. A fuller description of this study can be found here (Abstract #136).
COVID-19
Finally, the reviewer highlighted a study of the clinical course of COVID-19 in patients with chronic liver disease, and to determine factors associated with adverse outcomes in patients with chronic liver disease who acquire COVID-19.
The investigators found that patients with chronic liver disease and COVID-19 have a 14% morality rate, and that alcohol-related liver disease, decompensated cirrhosis, and hepatocellular carcinoma are all risk factors for increased mortality from COVID-19.
They recommended emphasizing telemedicine, prioritizing patients with chronic liver disease for vaccination, and including these patients in prospective studies and drug trials for COVID-19 therapies.
Dr. Taddei reported having no disclosures.
Studies that address fundamental questions in hepatology and have the potential to change or improve clinical practice were the focus of a clinical debrief session from the virtual annual meeting of the American Association for the Study of Liver Diseases.
“We chose papers that had the highest level of evidence, such as randomized controlled trials, controlled studies, and large data sets – and some small data sets too,” said Tamar Taddei, MD, associate professor of medicine in the section of digestive disease at Yale University, New Haven, Conn.
Dr. Taddei and colleagues Silvia Vilarinho, MD, PhD; Simona Jakab, MD; and Ariel Jaffe, MD, all also from Yale, selected the papers from among 197 oral and 1,769 poster abstracts presented at AASLD 2020.
They highlighted the most important findings from presentations on autoimmune and cholestatic disease, transplantation, cirrhosis and portal hypertension, alcoholic liver disease, neoplasia, drug-induced liver injury, and COVID-19. They did not review studies focused primarily on nonalcoholic steatohepatitis or nonalcoholic fatty liver disease, viral hepatitis, or basic science, all of which were covered in separate debriefing sessions.
Cirrhosis and portal hypertension
A study from the Department of Veterans Affairs looked at the prevalence of liver disease risk factors and rates of subsequent testing for and diagnosis of cirrhosis in the Veterans Health Administration system (VHA).
The authors found that, among more than 6.65 million VHA users in 2018 with no prior diagnosis of cirrhosis, approximately half were at risk for cirrhosis, of whom about 75% were screened, and approximately 5% of those who were screened were positive for possible cirrhosis (133,636). Of the patients who screened positive, about 10% (12,566) received a diagnosis of cirrhosis, including 4,120 with liver decompensation.
“This paper underscores the importance of population-level screening in uncovering unrecognized cirrhosis, enabling intervention earlier in the course of disease,” Dr. Taddei said (Abstract #661).
A study looking at external validation of novel cirrhosis surgical risk models designed to improve prognostication for a range of common surgeries showed that the VOCAL-Penn score was superior to the Mayo Risk Score, Model for End-stage Liver Disease and MELD-sodium scores for discrimination of 30-day and 90-day postoperative mortality (Abstract #91).
“While these models are not a substitute for clinical acumen, they certainly improve our surgical risk prediction in patients with cirrhosis, a very common question in consultative hepatology,” Dr. Taddei said.
She also cited three abstracts that address the important questions regarding performing studies in patients with varices or ascites, including whether it’s safe to perform transesophageal echocardiography in patients with cirrhosis without first screening for varices, and whether nonselective beta-blockers should be continued in patients with refractory ascites.
A retrospective study of 191 patients with cirrhosis who underwent upper endoscopy within 4 years of transesophageal echocardiography had no overt gastrointestinal bleeding regardless of the presence of esophageal varices, suggesting that routine preprocedure esophagogastroduodenoscopy “is of no utility,” (Abstract #1872).
A study to determine risk of sepsis in 1,198 patients with cirrhosis found that 1-year risk of sepsis was reduced by 50% with the use of nonselective beta-blockers (Abstract #94).
The final abstract in this category touched on the use of an advance care planning video support tool to help transplant-ineligible patients with end-stage liver disease decide whether they want support measures such cardiopulmonary resuscitation or intubation. The authors found that the video decision tool was feasible and acceptable to patients, and improved their knowledge of end-of-life care. More patients randomized to the video arm opted against CPR or intubation, compared with those assigned to a verbal discussion of options (Abstract #712).
Alcohol
The reviewers highlighted two studies of alcohol use: The first was designed to determine the prevalence of early alcohol relapse (resumption within 3 months) in patients who presented with alcoholic hepatitis. The subjects included 478 patients enrolled in the STOPAH trial, and a validation set of 194 patients from the InTeam (Integrated Approaches for identifying Molecular Targets in Alcoholic Hepatitis) Consortium.
“They found that high-risk patients were younger, unemployed, and without a stable relationship. Intermediate risk were middle aged, employed, and in a stable relationship, and low-risk profiles were older, with known cirrhosis; they were mostly retired and in a stable relationship,” Dr. Taddei said.
The identification of nongenetic factors that predict early relapse may aid in personalization of treatment strategies, she said (Abstract #232).
The second study looked at fecal microbial transplant (FMT) for reducing cravings in adults with alcohol use disorder (AUD) and cirrhosis. The investigators saw a nonsignificant trend toward greater total abstinence at 6 months in patients randomized to FMT versus placebo.
“Future trials should be performed to determine the impact of FMT on altering the gut-brain axis in patients with AUD,” she said (Abstract #7).
Transplantation
The prospective controlled QUICKTRANS study by French and Belgian researchers found that patients who underwent early liver transplantation for severe alcoholic hepatitis had numerically but not significantly higher rates of relapse than patients who were transplanted after at least 6 months of abstinence, although heavy drinking was more frequent in patients who underwent early transplant.
The 2-year survival rates for both patients who underwent early transplant and those who underwent transplant after 6 months of sobriety were “identical, and excellent.” In addition, the 2-year survival rate for patients with severe alcoholic hepatitis who underwent transplant was 82.8%, compared with 28.2% for patients who were deemed ineligible for transplant according to a selection algorithm (P < .001).
“Perhaps most important is that studies in this population can be conducted in a controlled fashion across centers with reproducible transplant eligibility algorithms,” Dr. Taddei commented (Abstract #6).
The place of honor – Abstract # 1 – was reserved for a study looking at the effects on liver transplant practice of a new “safety net” policy from the Organ Procurement and Transplantation Network and United Network for Organ Sharing stating that patients awaiting liver transplantation who develop kidney failure may be given priority on the kidney transplant waiting list.
The investigators found that the new policy significantly increased the number of adult primary liver transplant alone candidates who where on dialysis at the time of listing, and did not affect either waiting list mortality or posttransplant outcomes.
The authors also saw a significant increase in kidney transplant listing after liver transplant, especially for patients who were on hemodialysis at the time of list.
In the period after implementation of the policy, there was a significantly higher probability of kidney transplant, and significant reduction in waiting list mortality.
Autoimmune & cholestatic diseases
Investigators performed an analysis of the phase 3 randomized controlled ENHANCE trial of seladelpar in patients with primary biliary cholangitis. The trial was stopped because of an adverse event ultimately deemed to be unrelated to the drug, so the analysis looked at the composite responder rate at month 3.
“The key takeaway from this study is that at the 10-mg dosage of seladelpar, 78% met a composite endpoint, 27% of patients normalized their alkaline phosphatase, and 50% normalized their ALT. There was significant improvement in pruritus,” Dr. Taddei said.
The drug was generally safe and well tolerated. A 52-week phase 3 global registration study will begin enrolling patients in early 2021 (Abstract #LO11).
In a pediatric study, investigators looked at differences in primary sclerosing cholangitis (PSC) among various population, and found that “Black and Hispanic patients have dramatically worse clinical outcomes, compared to White and Asian patients. They are more likely to be diagnosed with PSC at an advanced stage with extensive fibrosis and portal hypertensive manifestations.”
The authors suggested that the differences may be explained in part by socioeconomic disparities leading to delay in diagnosis, to a more aggressive phenotype, or both (Abstract #66).
A meta-analysis of maternal and fetal outcomes in women with autoimmune hepatitis showed that the disease is associated with increased risk of gestational diabetes, premature births, and small-for-gestational age or low-birth-weight babies.
“Pregnant women should be monitored closely before, during and after pregnancy. It’s important to know that, in the prevalence data, flares were most prevalent postpartum at 41%. These finds will help us counsel our patients with autoimmune hepatitis who become pregnant,” Dr. Taddei said (Abstract #97).
Drug-induced liver injury
A study of clinical outcomes following immune checkpoint inhibitor rechallenge in melanoma patients with resolved higher grade 3 or higher checkpoint inhibitor–induced hepatitis showed that 4 of 31 patients (13%) developed recurrence of grade 2 or greater hepatitis, and 15 of 31 (48%) developed an immune-related adverse event after rechallenge.
There was no difference in time to death between patients who were rechallenged and those who were not, and immune-related liver toxicities requiring drug discontinuation after rechallenge were uncommon.
“High-grade immune checkpoint inhibitor hepatitis should be reconsidered as an absolute contraindication for immune checkpoint inhibitor rechallenge,” Dr. Taddei said (Abstract # 116).
Neoplasia
The investigators also highlighted an abstract describing significant urban-rural and racial ethnic differences in hepatocellular carcinoma rates. A fuller description of this study can be found here (Abstract #136).
COVID-19
Finally, the reviewer highlighted a study of the clinical course of COVID-19 in patients with chronic liver disease, and to determine factors associated with adverse outcomes in patients with chronic liver disease who acquire COVID-19.
The investigators found that patients with chronic liver disease and COVID-19 have a 14% morality rate, and that alcohol-related liver disease, decompensated cirrhosis, and hepatocellular carcinoma are all risk factors for increased mortality from COVID-19.
They recommended emphasizing telemedicine, prioritizing patients with chronic liver disease for vaccination, and including these patients in prospective studies and drug trials for COVID-19 therapies.
Dr. Taddei reported having no disclosures.
FROM THE LIVER MEETING DIGITAL EXPERIENCE
Pronounced racial differences in HBsAg loss after stopping nucleos(t)ide
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
FROM THE LIVER MEETING DIGITAL EXPERIENCE
HCC rates slow in cities, continue to climb in rural areas
The incidence rate of hepatocellular carcinoma in urban areas of the United States began to slow in 2009, but the rate in rural areas of the nation continued to rise at a steady pace, especially among non-Hispanic Whites and Blacks, investigators have found.
Although overall hepatocellular carcinoma (HCC) incidence rates were consistently lower among people living in nonmetro (rural) versus metro (urban) areas, the average annual percentage change in urban areas began to slow from 5.3% for the period of 1995 through 2009 to 2.7% thereafter. In contrast, the average annual percentage change in rural areas remained steady at 5.7%, a disparity that remained even after adjusting for differences among subgroups, reported Christina Gainey, MD, a third-year resident in internal medicine at the University of Southern California Medical Center, Los Angeles.
“We found that there are striking urban-rural disparities in HCC incidence trends that vary by race and ethnicity, and these disparities are growing over time,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
“Our study really highlights a critical public health issue that’s disproportionately affecting rural Americans. They already face considerable health inequities when it comes to access to care, health outcomes, and public health infrastructure and resources, and as of now we still don’t know why cases of HCC continue to rise in these areas,” she said.
Dr. Gainey noted that HCC is the fastest-growing cancer in the United States, according to the 2020 Annual Report to the Nation on the Status of Cancer, issued jointly by the Centers for Disease Control and Prevention, the North American Association of Central Cancer Registries, the American Cancer Society, and the National Cancer Institute.
Previous studies have identified disparities between urban and rural regions in care of patients with cervical cancer, colorectal cancer, and other malignancies, but there are very few data on urban-rural differences in HCC incidence, she said.
Incidence trends
To better understand whether such differences exists, the investigators compared trends in age-adjusted incidence rates of HCC in both rural and urban areas of the United States from 1995 to 2016, with stratification of trends by race/ethnicity and other demographic factors.
They drew from the NAACR database, which captures 93% of the U.S. population, in contrast to the CDC’s Surveillance, Epidemiology, and End Results (SEER) database which samples just 18% of the population.
Patients with HCC were defined by diagnostic codes, with diagnoses of intrahepatic bile duct cancers excluded.
They used 2013 U.S. Department of Agriculture Rural-Urban Continuum Codes to identify rural areas (regions of open countryside with town populations fewer than 2,500 people) and urban areas (populations ranging from 2,500 to 49,999, but not part of a larger labor market area).
The investigators identified a total of 310,635 HCC cases, 85% in urban areas and 15% in rural areas. Three-fourths of the patients (77%) were male. The median age ranged from 55-59 years.
There were notable demographic differences between the regions with non-Hispanic Whites comprising only 57% of the urban sample, but 82% of the rural sample. The urban sample included 16% non-Hispanic Blacks, 10% Asian/Pacific Islanders, and 17% Hispanics. The respective proportions in the rural areas were 8%, 2%, and 8%.
As noted before, age-adjusted incidence rates (adjusted to the year 2000 U.S. population) were lower in rural areas, at 4.9 per 100,000 population, compared with 6.9/100,000 in urban areas.
But when they looked at the average annual percentage changes using jointpoint regression, they saw that beginning in 2009 the AAPC in urban areas began to slow, from 5.3% for the period prior to 2009 to 2.7% thereafter, while the average annual percentage change in urban areas remained steady at 5.7%.
The largest increase in incidence over the course of the study was among rural non-Hispanic Whites, with an AAPC of 5.7%. Among urban non-Hispanic Blacks, the AAPC rose by 6.6% from 1995 to 2009, but slowed thereafter.
In contrast, among rural non-Hispanic Blacks the AAPC remained steady, at 5.4%.
The only group to see a decline in incidence was urban Asians/Pacific Islanders, who had an overall decline of 1%.
Among all groups, rural Hispanics had the highest age-adjusted incidence rates, at 14.9 per 100,000 in 2016.
Awareness gap?
Lewis R. Roberts, MB, ChB, PhD, a hepatobiliary cancer researcher at the Mayo Clinic in Rochester, Minn., who was not involved in the study, said in an interview that the difference in incidence rates between cities and the country may be attributable to a number of factors, including the opioid crisis, which can lead to an increase in injectable drug use or sexual behaviors resulting in increases in chronic hepatitis C infections and cirrhosis, known risk factors for HCC, as well as a lack of awareness of infections as a risk factor.
“In order for people to find these diseases, they have to be looking, and many of these are hidden diseases in our community,” he said. “What the study made me wonder was whether it just happens to be that they are in some ways more hidden in a rural community than they are in an urban community.”
He noted that clinicians in urban communities are more accustomed to treating more diverse populations who may have higher susceptibility to viral hepatitis, for example, and that screening and treatment for hepatitis C may be more common in urban areas than rural areas, he said.
No funding source for the study was reported. Dr. Gainey and Dr. Roberts reported having no conflicts of interest to disclose.
SOURCE: Gainey C et al. Liver Meeting 2020, Abstract 136.
The incidence rate of hepatocellular carcinoma in urban areas of the United States began to slow in 2009, but the rate in rural areas of the nation continued to rise at a steady pace, especially among non-Hispanic Whites and Blacks, investigators have found.
Although overall hepatocellular carcinoma (HCC) incidence rates were consistently lower among people living in nonmetro (rural) versus metro (urban) areas, the average annual percentage change in urban areas began to slow from 5.3% for the period of 1995 through 2009 to 2.7% thereafter. In contrast, the average annual percentage change in rural areas remained steady at 5.7%, a disparity that remained even after adjusting for differences among subgroups, reported Christina Gainey, MD, a third-year resident in internal medicine at the University of Southern California Medical Center, Los Angeles.
“We found that there are striking urban-rural disparities in HCC incidence trends that vary by race and ethnicity, and these disparities are growing over time,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
“Our study really highlights a critical public health issue that’s disproportionately affecting rural Americans. They already face considerable health inequities when it comes to access to care, health outcomes, and public health infrastructure and resources, and as of now we still don’t know why cases of HCC continue to rise in these areas,” she said.
Dr. Gainey noted that HCC is the fastest-growing cancer in the United States, according to the 2020 Annual Report to the Nation on the Status of Cancer, issued jointly by the Centers for Disease Control and Prevention, the North American Association of Central Cancer Registries, the American Cancer Society, and the National Cancer Institute.
Previous studies have identified disparities between urban and rural regions in care of patients with cervical cancer, colorectal cancer, and other malignancies, but there are very few data on urban-rural differences in HCC incidence, she said.
Incidence trends
To better understand whether such differences exists, the investigators compared trends in age-adjusted incidence rates of HCC in both rural and urban areas of the United States from 1995 to 2016, with stratification of trends by race/ethnicity and other demographic factors.
They drew from the NAACR database, which captures 93% of the U.S. population, in contrast to the CDC’s Surveillance, Epidemiology, and End Results (SEER) database which samples just 18% of the population.
Patients with HCC were defined by diagnostic codes, with diagnoses of intrahepatic bile duct cancers excluded.
They used 2013 U.S. Department of Agriculture Rural-Urban Continuum Codes to identify rural areas (regions of open countryside with town populations fewer than 2,500 people) and urban areas (populations ranging from 2,500 to 49,999, but not part of a larger labor market area).
The investigators identified a total of 310,635 HCC cases, 85% in urban areas and 15% in rural areas. Three-fourths of the patients (77%) were male. The median age ranged from 55-59 years.
There were notable demographic differences between the regions with non-Hispanic Whites comprising only 57% of the urban sample, but 82% of the rural sample. The urban sample included 16% non-Hispanic Blacks, 10% Asian/Pacific Islanders, and 17% Hispanics. The respective proportions in the rural areas were 8%, 2%, and 8%.
As noted before, age-adjusted incidence rates (adjusted to the year 2000 U.S. population) were lower in rural areas, at 4.9 per 100,000 population, compared with 6.9/100,000 in urban areas.
But when they looked at the average annual percentage changes using jointpoint regression, they saw that beginning in 2009 the AAPC in urban areas began to slow, from 5.3% for the period prior to 2009 to 2.7% thereafter, while the average annual percentage change in urban areas remained steady at 5.7%.
The largest increase in incidence over the course of the study was among rural non-Hispanic Whites, with an AAPC of 5.7%. Among urban non-Hispanic Blacks, the AAPC rose by 6.6% from 1995 to 2009, but slowed thereafter.
In contrast, among rural non-Hispanic Blacks the AAPC remained steady, at 5.4%.
The only group to see a decline in incidence was urban Asians/Pacific Islanders, who had an overall decline of 1%.
Among all groups, rural Hispanics had the highest age-adjusted incidence rates, at 14.9 per 100,000 in 2016.
Awareness gap?
Lewis R. Roberts, MB, ChB, PhD, a hepatobiliary cancer researcher at the Mayo Clinic in Rochester, Minn., who was not involved in the study, said in an interview that the difference in incidence rates between cities and the country may be attributable to a number of factors, including the opioid crisis, which can lead to an increase in injectable drug use or sexual behaviors resulting in increases in chronic hepatitis C infections and cirrhosis, known risk factors for HCC, as well as a lack of awareness of infections as a risk factor.
“In order for people to find these diseases, they have to be looking, and many of these are hidden diseases in our community,” he said. “What the study made me wonder was whether it just happens to be that they are in some ways more hidden in a rural community than they are in an urban community.”
He noted that clinicians in urban communities are more accustomed to treating more diverse populations who may have higher susceptibility to viral hepatitis, for example, and that screening and treatment for hepatitis C may be more common in urban areas than rural areas, he said.
No funding source for the study was reported. Dr. Gainey and Dr. Roberts reported having no conflicts of interest to disclose.
SOURCE: Gainey C et al. Liver Meeting 2020, Abstract 136.
The incidence rate of hepatocellular carcinoma in urban areas of the United States began to slow in 2009, but the rate in rural areas of the nation continued to rise at a steady pace, especially among non-Hispanic Whites and Blacks, investigators have found.
Although overall hepatocellular carcinoma (HCC) incidence rates were consistently lower among people living in nonmetro (rural) versus metro (urban) areas, the average annual percentage change in urban areas began to slow from 5.3% for the period of 1995 through 2009 to 2.7% thereafter. In contrast, the average annual percentage change in rural areas remained steady at 5.7%, a disparity that remained even after adjusting for differences among subgroups, reported Christina Gainey, MD, a third-year resident in internal medicine at the University of Southern California Medical Center, Los Angeles.
“We found that there are striking urban-rural disparities in HCC incidence trends that vary by race and ethnicity, and these disparities are growing over time,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
“Our study really highlights a critical public health issue that’s disproportionately affecting rural Americans. They already face considerable health inequities when it comes to access to care, health outcomes, and public health infrastructure and resources, and as of now we still don’t know why cases of HCC continue to rise in these areas,” she said.
Dr. Gainey noted that HCC is the fastest-growing cancer in the United States, according to the 2020 Annual Report to the Nation on the Status of Cancer, issued jointly by the Centers for Disease Control and Prevention, the North American Association of Central Cancer Registries, the American Cancer Society, and the National Cancer Institute.
Previous studies have identified disparities between urban and rural regions in care of patients with cervical cancer, colorectal cancer, and other malignancies, but there are very few data on urban-rural differences in HCC incidence, she said.
Incidence trends
To better understand whether such differences exists, the investigators compared trends in age-adjusted incidence rates of HCC in both rural and urban areas of the United States from 1995 to 2016, with stratification of trends by race/ethnicity and other demographic factors.
They drew from the NAACR database, which captures 93% of the U.S. population, in contrast to the CDC’s Surveillance, Epidemiology, and End Results (SEER) database which samples just 18% of the population.
Patients with HCC were defined by diagnostic codes, with diagnoses of intrahepatic bile duct cancers excluded.
They used 2013 U.S. Department of Agriculture Rural-Urban Continuum Codes to identify rural areas (regions of open countryside with town populations fewer than 2,500 people) and urban areas (populations ranging from 2,500 to 49,999, but not part of a larger labor market area).
The investigators identified a total of 310,635 HCC cases, 85% in urban areas and 15% in rural areas. Three-fourths of the patients (77%) were male. The median age ranged from 55-59 years.
There were notable demographic differences between the regions with non-Hispanic Whites comprising only 57% of the urban sample, but 82% of the rural sample. The urban sample included 16% non-Hispanic Blacks, 10% Asian/Pacific Islanders, and 17% Hispanics. The respective proportions in the rural areas were 8%, 2%, and 8%.
As noted before, age-adjusted incidence rates (adjusted to the year 2000 U.S. population) were lower in rural areas, at 4.9 per 100,000 population, compared with 6.9/100,000 in urban areas.
But when they looked at the average annual percentage changes using jointpoint regression, they saw that beginning in 2009 the AAPC in urban areas began to slow, from 5.3% for the period prior to 2009 to 2.7% thereafter, while the average annual percentage change in urban areas remained steady at 5.7%.
The largest increase in incidence over the course of the study was among rural non-Hispanic Whites, with an AAPC of 5.7%. Among urban non-Hispanic Blacks, the AAPC rose by 6.6% from 1995 to 2009, but slowed thereafter.
In contrast, among rural non-Hispanic Blacks the AAPC remained steady, at 5.4%.
The only group to see a decline in incidence was urban Asians/Pacific Islanders, who had an overall decline of 1%.
Among all groups, rural Hispanics had the highest age-adjusted incidence rates, at 14.9 per 100,000 in 2016.
Awareness gap?
Lewis R. Roberts, MB, ChB, PhD, a hepatobiliary cancer researcher at the Mayo Clinic in Rochester, Minn., who was not involved in the study, said in an interview that the difference in incidence rates between cities and the country may be attributable to a number of factors, including the opioid crisis, which can lead to an increase in injectable drug use or sexual behaviors resulting in increases in chronic hepatitis C infections and cirrhosis, known risk factors for HCC, as well as a lack of awareness of infections as a risk factor.
“In order for people to find these diseases, they have to be looking, and many of these are hidden diseases in our community,” he said. “What the study made me wonder was whether it just happens to be that they are in some ways more hidden in a rural community than they are in an urban community.”
He noted that clinicians in urban communities are more accustomed to treating more diverse populations who may have higher susceptibility to viral hepatitis, for example, and that screening and treatment for hepatitis C may be more common in urban areas than rural areas, he said.
No funding source for the study was reported. Dr. Gainey and Dr. Roberts reported having no conflicts of interest to disclose.
SOURCE: Gainey C et al. Liver Meeting 2020, Abstract 136.
FROM THE LIVER MEETING DIGITAL EXPERIENCE
GLIMMER of hope for itch in primary biliary cholangitis
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Liver-related deaths decline after Medicaid expansion under ACA
Liver-related deaths declined and liver transplant waitlist inequities decreased in states that implemented Medicaid expansion under the Affordable Care Act (ACA), results of an innovative study showed.
About 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality in 18 states that took advantage of expanded coverage began to decline, whereas the rate of liver-related deaths in 14 states that did not expand Medicaid continued to climb, reported Nabeel Wahid, MD, a resident at Weill Cornell Medicine, New York.
The differences in liver-related mortality between Medicaid expansion and nonexpansion states was particularly pronounced in people of Asian background, in Whites, and in African Americans, he said in an oral abstract presentation during the virtual annual meeting of the American Association for the Study of Liver Diseases.
“The expansion of government health care programs such as Medicaid may improve liver-related mortality and liver transplant waitlist placement,” he said.
The implementation of the ACA, also known as Obamacare, resulted in “a pretty dramatic increase in the number of Americans with health insurance today, and there’s a lot of literature out there looking at a variety of domains throughout health care that have found that Medicaid expansion increased access and decreased disparities in care,” he added.
For example, a report published in 2019 by the nonpartisan National Bureau of Economic Research Priorities showed a significant reduction in disease-related deaths in Americans aged 55-64 in Medicaid expansion states compared with nonexpansion states.
In addition as previously reported, before Medicaid expansion African Americans were 4.8% less likely than were Whites to receive timely cancer treatment, defined as treatment starting within 30 days of diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups had dwindled to just 0.8% and was no longer statistically significant.
“However, specifically in the realm of liver transplantation and liver disease, there’s very limited literature showing any sort of significant impact on care resulting from Medicaid expansion,” Dr. Wahid said.
Listing-to-death ratio
To test their hypothesis that Medicaid expansion decreased racial disparities and improved liver-related deaths and transplant waitlist placement, Dr. Wahid and colleagues compared liver-related deaths and liver transplants listings between Medicaid expansion and nonexpansion states in the 5 years before expansion (2009-2013) and in the 5 years after expansion (2014-2018). They excluded all states without transplant centers as well as patients younger than 25 or older than 64, who were likely to be covered by other types of insurance.
They obtained data for listing from the United Network for Organ Sharing (UNOS) and on end-stage liver disease from the Centers for Disease Control and Prevention’s WONDER database.
They also used a novel measure called the listing-to-death ratio (LDR), a surrogate endpoint for waitlist placement calculated as the ratio of listings for liver transplantation relative to the number of deaths from liver disease, with a higher LDR score corresponding to improved waitlist placement.
They found that throughout the entire study period, Medicaid expansion states had lower liver-related deaths, higher liver transplant listings, and higher LDR.
Using joinpoint regression to examine changes at a specific time point, the investigators determined that the annual percentage change in liver-related deaths increased in both nonexpansion states (mean 4.3%) and expansion states (3.0%) before 2014. However, beginning around 2015, liver-related deaths began to decline in expansion states by a mean of –0.6%, while they continued on an upward trajectory in the nonexpansion states, albeit at a somewhat slower pace (mean APC 2% from 2014 through 2018).
Among all racial and ethnic groups (Whites, African Americans, Hispanics, and Asians) liver-related deaths increased from 2014 to 2018 in nonexpansion states, with the highest annual percentage change in Asians, at slightly more than 8%.
In contrast, among Asians in the expansion states, liver-related deaths over the same period increased by less than 1%, and in both Whites and African Americans liver-related deaths declined.
In addition, starting in 2015, the annual percent change in LDR increased only in expansion states primarily because of fewer end-stage liver disease deaths (the denominator in the LDR equation) rather than increased listings (the numerator).
‘No-brainer’
A gastroenterologist who was not involved in the study said that there are two key points in favor of the study.
“The obvious message is that transplant is costly, and patients need to be insured to get a liver transplant, because nobody is paying for this out of pocket. So if more candidates are insured that will reduce disparities and improve access to liver transplant for those who need it,” said Elliot Benjamin Tapper, MD, of the University of Michigan, Ann Arbor.
“It’s a no-brainer that the lack of insurance accessibility for the most vulnerable people in the United States meant that they were dying of cirrhosis instead of being transplanted,” he said in an interview.
He also commended the authors on their use of population-based data to identify outcomes.
“We know how many people are listed for liver transplant, but we don’t know how many people could have been listed. We know how many people are transplanted, but we don’t know how many people with decompensated cirrhosis should have been given that chance. We lacked that denominator,” he said.
“The innovation that makes this particular paper worthwhile is that in the absence of that denominator, they were able to construct it, so we can know from other data sources distinct from the waitlist rolls how many people are dying of cirrhosis,” he added.
No study funding source was reported. Dr. Wahid and Dr. Tapper reported no conflicts of interest.
SOURCE: Wahid N et al. AASLD 2020. Abstract 153.
Liver-related deaths declined and liver transplant waitlist inequities decreased in states that implemented Medicaid expansion under the Affordable Care Act (ACA), results of an innovative study showed.
About 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality in 18 states that took advantage of expanded coverage began to decline, whereas the rate of liver-related deaths in 14 states that did not expand Medicaid continued to climb, reported Nabeel Wahid, MD, a resident at Weill Cornell Medicine, New York.
The differences in liver-related mortality between Medicaid expansion and nonexpansion states was particularly pronounced in people of Asian background, in Whites, and in African Americans, he said in an oral abstract presentation during the virtual annual meeting of the American Association for the Study of Liver Diseases.
“The expansion of government health care programs such as Medicaid may improve liver-related mortality and liver transplant waitlist placement,” he said.
The implementation of the ACA, also known as Obamacare, resulted in “a pretty dramatic increase in the number of Americans with health insurance today, and there’s a lot of literature out there looking at a variety of domains throughout health care that have found that Medicaid expansion increased access and decreased disparities in care,” he added.
For example, a report published in 2019 by the nonpartisan National Bureau of Economic Research Priorities showed a significant reduction in disease-related deaths in Americans aged 55-64 in Medicaid expansion states compared with nonexpansion states.
In addition as previously reported, before Medicaid expansion African Americans were 4.8% less likely than were Whites to receive timely cancer treatment, defined as treatment starting within 30 days of diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups had dwindled to just 0.8% and was no longer statistically significant.
“However, specifically in the realm of liver transplantation and liver disease, there’s very limited literature showing any sort of significant impact on care resulting from Medicaid expansion,” Dr. Wahid said.
Listing-to-death ratio
To test their hypothesis that Medicaid expansion decreased racial disparities and improved liver-related deaths and transplant waitlist placement, Dr. Wahid and colleagues compared liver-related deaths and liver transplants listings between Medicaid expansion and nonexpansion states in the 5 years before expansion (2009-2013) and in the 5 years after expansion (2014-2018). They excluded all states without transplant centers as well as patients younger than 25 or older than 64, who were likely to be covered by other types of insurance.
They obtained data for listing from the United Network for Organ Sharing (UNOS) and on end-stage liver disease from the Centers for Disease Control and Prevention’s WONDER database.
They also used a novel measure called the listing-to-death ratio (LDR), a surrogate endpoint for waitlist placement calculated as the ratio of listings for liver transplantation relative to the number of deaths from liver disease, with a higher LDR score corresponding to improved waitlist placement.
They found that throughout the entire study period, Medicaid expansion states had lower liver-related deaths, higher liver transplant listings, and higher LDR.
Using joinpoint regression to examine changes at a specific time point, the investigators determined that the annual percentage change in liver-related deaths increased in both nonexpansion states (mean 4.3%) and expansion states (3.0%) before 2014. However, beginning around 2015, liver-related deaths began to decline in expansion states by a mean of –0.6%, while they continued on an upward trajectory in the nonexpansion states, albeit at a somewhat slower pace (mean APC 2% from 2014 through 2018).
Among all racial and ethnic groups (Whites, African Americans, Hispanics, and Asians) liver-related deaths increased from 2014 to 2018 in nonexpansion states, with the highest annual percentage change in Asians, at slightly more than 8%.
In contrast, among Asians in the expansion states, liver-related deaths over the same period increased by less than 1%, and in both Whites and African Americans liver-related deaths declined.
In addition, starting in 2015, the annual percent change in LDR increased only in expansion states primarily because of fewer end-stage liver disease deaths (the denominator in the LDR equation) rather than increased listings (the numerator).
‘No-brainer’
A gastroenterologist who was not involved in the study said that there are two key points in favor of the study.
“The obvious message is that transplant is costly, and patients need to be insured to get a liver transplant, because nobody is paying for this out of pocket. So if more candidates are insured that will reduce disparities and improve access to liver transplant for those who need it,” said Elliot Benjamin Tapper, MD, of the University of Michigan, Ann Arbor.
“It’s a no-brainer that the lack of insurance accessibility for the most vulnerable people in the United States meant that they were dying of cirrhosis instead of being transplanted,” he said in an interview.
He also commended the authors on their use of population-based data to identify outcomes.
“We know how many people are listed for liver transplant, but we don’t know how many people could have been listed. We know how many people are transplanted, but we don’t know how many people with decompensated cirrhosis should have been given that chance. We lacked that denominator,” he said.
“The innovation that makes this particular paper worthwhile is that in the absence of that denominator, they were able to construct it, so we can know from other data sources distinct from the waitlist rolls how many people are dying of cirrhosis,” he added.
No study funding source was reported. Dr. Wahid and Dr. Tapper reported no conflicts of interest.
SOURCE: Wahid N et al. AASLD 2020. Abstract 153.
Liver-related deaths declined and liver transplant waitlist inequities decreased in states that implemented Medicaid expansion under the Affordable Care Act (ACA), results of an innovative study showed.
About 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality in 18 states that took advantage of expanded coverage began to decline, whereas the rate of liver-related deaths in 14 states that did not expand Medicaid continued to climb, reported Nabeel Wahid, MD, a resident at Weill Cornell Medicine, New York.
The differences in liver-related mortality between Medicaid expansion and nonexpansion states was particularly pronounced in people of Asian background, in Whites, and in African Americans, he said in an oral abstract presentation during the virtual annual meeting of the American Association for the Study of Liver Diseases.
“The expansion of government health care programs such as Medicaid may improve liver-related mortality and liver transplant waitlist placement,” he said.
The implementation of the ACA, also known as Obamacare, resulted in “a pretty dramatic increase in the number of Americans with health insurance today, and there’s a lot of literature out there looking at a variety of domains throughout health care that have found that Medicaid expansion increased access and decreased disparities in care,” he added.
For example, a report published in 2019 by the nonpartisan National Bureau of Economic Research Priorities showed a significant reduction in disease-related deaths in Americans aged 55-64 in Medicaid expansion states compared with nonexpansion states.
In addition as previously reported, before Medicaid expansion African Americans were 4.8% less likely than were Whites to receive timely cancer treatment, defined as treatment starting within 30 days of diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups had dwindled to just 0.8% and was no longer statistically significant.
“However, specifically in the realm of liver transplantation and liver disease, there’s very limited literature showing any sort of significant impact on care resulting from Medicaid expansion,” Dr. Wahid said.
Listing-to-death ratio
To test their hypothesis that Medicaid expansion decreased racial disparities and improved liver-related deaths and transplant waitlist placement, Dr. Wahid and colleagues compared liver-related deaths and liver transplants listings between Medicaid expansion and nonexpansion states in the 5 years before expansion (2009-2013) and in the 5 years after expansion (2014-2018). They excluded all states without transplant centers as well as patients younger than 25 or older than 64, who were likely to be covered by other types of insurance.
They obtained data for listing from the United Network for Organ Sharing (UNOS) and on end-stage liver disease from the Centers for Disease Control and Prevention’s WONDER database.
They also used a novel measure called the listing-to-death ratio (LDR), a surrogate endpoint for waitlist placement calculated as the ratio of listings for liver transplantation relative to the number of deaths from liver disease, with a higher LDR score corresponding to improved waitlist placement.
They found that throughout the entire study period, Medicaid expansion states had lower liver-related deaths, higher liver transplant listings, and higher LDR.
Using joinpoint regression to examine changes at a specific time point, the investigators determined that the annual percentage change in liver-related deaths increased in both nonexpansion states (mean 4.3%) and expansion states (3.0%) before 2014. However, beginning around 2015, liver-related deaths began to decline in expansion states by a mean of –0.6%, while they continued on an upward trajectory in the nonexpansion states, albeit at a somewhat slower pace (mean APC 2% from 2014 through 2018).
Among all racial and ethnic groups (Whites, African Americans, Hispanics, and Asians) liver-related deaths increased from 2014 to 2018 in nonexpansion states, with the highest annual percentage change in Asians, at slightly more than 8%.
In contrast, among Asians in the expansion states, liver-related deaths over the same period increased by less than 1%, and in both Whites and African Americans liver-related deaths declined.
In addition, starting in 2015, the annual percent change in LDR increased only in expansion states primarily because of fewer end-stage liver disease deaths (the denominator in the LDR equation) rather than increased listings (the numerator).
‘No-brainer’
A gastroenterologist who was not involved in the study said that there are two key points in favor of the study.
“The obvious message is that transplant is costly, and patients need to be insured to get a liver transplant, because nobody is paying for this out of pocket. So if more candidates are insured that will reduce disparities and improve access to liver transplant for those who need it,” said Elliot Benjamin Tapper, MD, of the University of Michigan, Ann Arbor.
“It’s a no-brainer that the lack of insurance accessibility for the most vulnerable people in the United States meant that they were dying of cirrhosis instead of being transplanted,” he said in an interview.
He also commended the authors on their use of population-based data to identify outcomes.
“We know how many people are listed for liver transplant, but we don’t know how many people could have been listed. We know how many people are transplanted, but we don’t know how many people with decompensated cirrhosis should have been given that chance. We lacked that denominator,” he said.
“The innovation that makes this particular paper worthwhile is that in the absence of that denominator, they were able to construct it, so we can know from other data sources distinct from the waitlist rolls how many people are dying of cirrhosis,” he added.
No study funding source was reported. Dr. Wahid and Dr. Tapper reported no conflicts of interest.
SOURCE: Wahid N et al. AASLD 2020. Abstract 153.
FROM THE LIVER MEETING DIGITAL EXPERIENCE
Liquid biopsy captures key NASH pathology hallmarks
Large-scale scanning of serum proteins offers a potential method for noninvasive screening and monitoring of patients with nonalcoholic steatohepatitis (NASH), the technique’s developers claim.
By scanning for about 5,000 proteins in nearly 3,000 samples from patients enrolled in studies from the Clinical Research Network in NASH (NASH CRN), Rachel Ostroff, PhD, and colleagues from SomaLogic in Boulder, Colo., created four protein models that mimic results of the major pathologic findings in liver tissue biopsy.
“Concurrent positive results from the protein models had performance characteristics of ‘rule-out’ tests for pathologists’ diagnosis of NASH. These tests may assist in new drug development and medical intervention decisions,” they wrote in a late-breaking poster presented at the virtual annual meeting of the American Association for the Study of Liver Diseases.
“There is no single noninvasive method that can accurately and simultaneously capture steatosis, inflammation, hepatocyte ballooning and fibrosis, the four major pathologic components assessed by biopsy. Each of these is relevant to the multiple mechanisms targeted in drug development for NASH,” they wrote.
To see whether large-scale protemoics could serve as an alternative to invasive liver biopsy for use in clinical trials or in longitudinal studies of NASH, they used a modified aptamer proteomics platform to scan for liver-related proteins. Aptamers are olignonucleotide or peptide molecules designed to home in on a specific target.
They scanned for approximately 5,000 proteins in 2,852 serum samples from 638 patients in a NASH CRN natural history cohort, and in patients enrolled in two NASH treatment trials: the PIVENS trial, which is evaluating pioglitazone versus vitamin E and placebo in nondiabetic patients, and the FLINT trial, which is comparing obeticholic acid with placebo. All of the patients in the natural history cohort and half of all patients in the clinical trial cohorts were included in the training sets, with the remaining half included in the validation set.
The accuracy of the models, as measured by the area under the curve (AUC) of receiver operating characteristics in the training and validation sets, respectively, were as follows:
- Fibrosis: AUC 0.92/0.85.
- Steatosis: AUC 0.95/0.79.
- Inflammation: AUC 0.83/0.72.
- Hepatocyte Ballooning: AUC 0.87/0.83.
“A concurrent positive score for steatosis, inflammation and ballooning predicted the biopsy diagnosis of NASH with an accuracy of 73%,” Ostroff and colleagues wrote.
They also found that model scores applied over time showed improvements in symptoms in the patients on active therapies in the clinical trials, compared with patients on placebo.
A specialist in liver pathology and nonalcoholic fatty liver disease who was not involved in the study said in an interview that she finds the results highly promising.
Impressive results
Elizabeth M. Brunt, MD, emeritus professor of pathology and immunology at Washington University in St. Louis, was a member of the NASH CRN when SomaLogic first proposed using the groups’ data for this study.
“I was impressed with them then, and I am very impressed with what they’re presenting here, and I can’t say that about all the noninvasive tests,” she said. “I think a lot of noninvasive tests are way over-simplifying what NASH is.”
She acknowledged that, although she spent much of her career performing liver biopsies, “you can’t biopsy every single patients who you suspect of having NASH, or certainly if you want to follow them over time – it’s unrealistic,” she said in an interview.
Although the protein scanning method cannot – and is not intended to – replace a well-conducted biopsy with the interpretation of a skilled pathologist, the proteins the company investigators identified can reflect the dynamic nature of liver disease and the liver’s ability to heal itself with a high degree of accuracy and hold promise for both screening patients and for monitoring responses to therapy, Dr. Brunt said.
The study was sponsored by SomaLogic. The authors are employees of the company. Dr. Brunt had no relevant disclosures.
SOURCE: Ostroff R et al. The Liver Disease Meeting Digital Experience, Abstract LP11
Large-scale scanning of serum proteins offers a potential method for noninvasive screening and monitoring of patients with nonalcoholic steatohepatitis (NASH), the technique’s developers claim.
By scanning for about 5,000 proteins in nearly 3,000 samples from patients enrolled in studies from the Clinical Research Network in NASH (NASH CRN), Rachel Ostroff, PhD, and colleagues from SomaLogic in Boulder, Colo., created four protein models that mimic results of the major pathologic findings in liver tissue biopsy.
“Concurrent positive results from the protein models had performance characteristics of ‘rule-out’ tests for pathologists’ diagnosis of NASH. These tests may assist in new drug development and medical intervention decisions,” they wrote in a late-breaking poster presented at the virtual annual meeting of the American Association for the Study of Liver Diseases.
“There is no single noninvasive method that can accurately and simultaneously capture steatosis, inflammation, hepatocyte ballooning and fibrosis, the four major pathologic components assessed by biopsy. Each of these is relevant to the multiple mechanisms targeted in drug development for NASH,” they wrote.
To see whether large-scale protemoics could serve as an alternative to invasive liver biopsy for use in clinical trials or in longitudinal studies of NASH, they used a modified aptamer proteomics platform to scan for liver-related proteins. Aptamers are olignonucleotide or peptide molecules designed to home in on a specific target.
They scanned for approximately 5,000 proteins in 2,852 serum samples from 638 patients in a NASH CRN natural history cohort, and in patients enrolled in two NASH treatment trials: the PIVENS trial, which is evaluating pioglitazone versus vitamin E and placebo in nondiabetic patients, and the FLINT trial, which is comparing obeticholic acid with placebo. All of the patients in the natural history cohort and half of all patients in the clinical trial cohorts were included in the training sets, with the remaining half included in the validation set.
The accuracy of the models, as measured by the area under the curve (AUC) of receiver operating characteristics in the training and validation sets, respectively, were as follows:
- Fibrosis: AUC 0.92/0.85.
- Steatosis: AUC 0.95/0.79.
- Inflammation: AUC 0.83/0.72.
- Hepatocyte Ballooning: AUC 0.87/0.83.
“A concurrent positive score for steatosis, inflammation and ballooning predicted the biopsy diagnosis of NASH with an accuracy of 73%,” Ostroff and colleagues wrote.
They also found that model scores applied over time showed improvements in symptoms in the patients on active therapies in the clinical trials, compared with patients on placebo.
A specialist in liver pathology and nonalcoholic fatty liver disease who was not involved in the study said in an interview that she finds the results highly promising.
Impressive results
Elizabeth M. Brunt, MD, emeritus professor of pathology and immunology at Washington University in St. Louis, was a member of the NASH CRN when SomaLogic first proposed using the groups’ data for this study.
“I was impressed with them then, and I am very impressed with what they’re presenting here, and I can’t say that about all the noninvasive tests,” she said. “I think a lot of noninvasive tests are way over-simplifying what NASH is.”
She acknowledged that, although she spent much of her career performing liver biopsies, “you can’t biopsy every single patients who you suspect of having NASH, or certainly if you want to follow them over time – it’s unrealistic,” she said in an interview.
Although the protein scanning method cannot – and is not intended to – replace a well-conducted biopsy with the interpretation of a skilled pathologist, the proteins the company investigators identified can reflect the dynamic nature of liver disease and the liver’s ability to heal itself with a high degree of accuracy and hold promise for both screening patients and for monitoring responses to therapy, Dr. Brunt said.
The study was sponsored by SomaLogic. The authors are employees of the company. Dr. Brunt had no relevant disclosures.
SOURCE: Ostroff R et al. The Liver Disease Meeting Digital Experience, Abstract LP11
Large-scale scanning of serum proteins offers a potential method for noninvasive screening and monitoring of patients with nonalcoholic steatohepatitis (NASH), the technique’s developers claim.
By scanning for about 5,000 proteins in nearly 3,000 samples from patients enrolled in studies from the Clinical Research Network in NASH (NASH CRN), Rachel Ostroff, PhD, and colleagues from SomaLogic in Boulder, Colo., created four protein models that mimic results of the major pathologic findings in liver tissue biopsy.
“Concurrent positive results from the protein models had performance characteristics of ‘rule-out’ tests for pathologists’ diagnosis of NASH. These tests may assist in new drug development and medical intervention decisions,” they wrote in a late-breaking poster presented at the virtual annual meeting of the American Association for the Study of Liver Diseases.
“There is no single noninvasive method that can accurately and simultaneously capture steatosis, inflammation, hepatocyte ballooning and fibrosis, the four major pathologic components assessed by biopsy. Each of these is relevant to the multiple mechanisms targeted in drug development for NASH,” they wrote.
To see whether large-scale protemoics could serve as an alternative to invasive liver biopsy for use in clinical trials or in longitudinal studies of NASH, they used a modified aptamer proteomics platform to scan for liver-related proteins. Aptamers are olignonucleotide or peptide molecules designed to home in on a specific target.
They scanned for approximately 5,000 proteins in 2,852 serum samples from 638 patients in a NASH CRN natural history cohort, and in patients enrolled in two NASH treatment trials: the PIVENS trial, which is evaluating pioglitazone versus vitamin E and placebo in nondiabetic patients, and the FLINT trial, which is comparing obeticholic acid with placebo. All of the patients in the natural history cohort and half of all patients in the clinical trial cohorts were included in the training sets, with the remaining half included in the validation set.
The accuracy of the models, as measured by the area under the curve (AUC) of receiver operating characteristics in the training and validation sets, respectively, were as follows:
- Fibrosis: AUC 0.92/0.85.
- Steatosis: AUC 0.95/0.79.
- Inflammation: AUC 0.83/0.72.
- Hepatocyte Ballooning: AUC 0.87/0.83.
“A concurrent positive score for steatosis, inflammation and ballooning predicted the biopsy diagnosis of NASH with an accuracy of 73%,” Ostroff and colleagues wrote.
They also found that model scores applied over time showed improvements in symptoms in the patients on active therapies in the clinical trials, compared with patients on placebo.
A specialist in liver pathology and nonalcoholic fatty liver disease who was not involved in the study said in an interview that she finds the results highly promising.
Impressive results
Elizabeth M. Brunt, MD, emeritus professor of pathology and immunology at Washington University in St. Louis, was a member of the NASH CRN when SomaLogic first proposed using the groups’ data for this study.
“I was impressed with them then, and I am very impressed with what they’re presenting here, and I can’t say that about all the noninvasive tests,” she said. “I think a lot of noninvasive tests are way over-simplifying what NASH is.”
She acknowledged that, although she spent much of her career performing liver biopsies, “you can’t biopsy every single patients who you suspect of having NASH, or certainly if you want to follow them over time – it’s unrealistic,” she said in an interview.
Although the protein scanning method cannot – and is not intended to – replace a well-conducted biopsy with the interpretation of a skilled pathologist, the proteins the company investigators identified can reflect the dynamic nature of liver disease and the liver’s ability to heal itself with a high degree of accuracy and hold promise for both screening patients and for monitoring responses to therapy, Dr. Brunt said.
The study was sponsored by SomaLogic. The authors are employees of the company. Dr. Brunt had no relevant disclosures.
SOURCE: Ostroff R et al. The Liver Disease Meeting Digital Experience, Abstract LP11
FROM THE LIVER DISEASE MEETING DIGITAL EXPERIENCE
Semaglutide shows promise in NASH phase 2 study
according to a phase 2, double-blind, randomized, placebo-controlled trial published in the New England Journal of Medicine and presented at the 2020 American Association for the Study of Liver Diseases (AASLD) meeting.
“This bodes well for further study of semaglutide and is supported further by marked improvements in weight, glycemic control and lipid profile,” commented the study’s senior author Philip N. Newsome, PhD, FRCPE, of the University of Birmingham (England), in an interview.
The highest daily dose (0.4 mg) of the glucagonlike peptide-1 (GLP-1) receptor agonist, semaglutide, which is approved for the treatment of type 2 diabetes, led to levels of NASH resolution “which are higher than any previously demonstrated,” noted Dr. Newsome. “This was also accompanied by improvement in noninvasive markers of liver fibrosis and also less fibrosis progression, compared to placebo.”
“I think this represents an exciting advance and will, if confirmed in further studies, mark a step-change in our management of patients with NASH,” he added.
The multicenter study, conducted at 143 sites in 16 countries, included 320 patients, aged 18-75 years, with or without type 2 diabetes, who had histologic evidence of NASH and stage 1-3 liver fibrosis.
They were randomized in a 3:3:3:1:1:1 ratio to receive once-daily subcutaneous semaglutide at a dose of 0.1, 0.2, or 0.4 mg, or placebo for 72 weeks.
The primary endpoint was resolution of NASH and no worsening of fibrosis, with a secondary endpoint being improvement of fibrosis by at least one stage without worsening of NASH.
The study found 40% of patients in the 0.1-mg semaglutide group, 36% in the 0.2-mg group, and 59% in the 0.4-mg group achieved NASH resolution with no worsening of fibrosis, compared with 17% of the placebo group (odds ratio, 6.87; P < .001 for the highest semaglutide dose). However, the treatment did not lead to significant between-group differences in the secondary endpoint, which occurred in 43% of patients on the highest semaglutide dose compared to 33% in the placebo group (OR, 1.42; P = .48).
Treatment with semaglutide also resulted in dose-dependent reductions in body weight, as well as in glycated hemoglobin levels. Bodyweight was reduced by a mean of 5% in the 0.1-mg semaglutide group, followed by mean reductions of 9% and 13% in the 0.2-mg and 0.4-mg groups respectively. This compared to a mean reduction of 1% in the placebo group.
Similarly, glycated hemoglobin levels among patients with type 2 diabetes dropped by 0.63, 1.07, and 1.15 percentage points in the 0.1-mg, 0.2-mg, and 0.4-mg semaglutide groups respectively, compared with a drop of 0.01 percentage point in the placebo group.
“The fact that the percentage of patients who had an improvement in fibrosis stage was not significantly higher with semaglutide than with placebo – despite a greater benefit with respect to NASH resolution and dose-dependent weight loss – was unexpected, given that previous studies have suggested that resolution of NASH and improvements in activity scores for the components of nonalcoholic fatty liver disease are associated with regression of fibrosis,” wrote the authors. “However, the temporal association among NASH resolution, weight loss, and improvement in fibrosis stage is not fully understood. It is possible that the current trial was not of sufficient duration for improvements in fibrosis stage to become apparent.”
The authors also noted that the safety profile of semaglutide was “consistent with that observed in patients with type 2 diabetes in other trials and with the known effects of GLP-1 receptor agonists,” with gastrointestinal disorders being the most commonly reported.
Nausea, constipation, and vomiting were reported more often in the 0.4-mg semaglutide group than in the placebo group (nausea, 42% vs. 11%; constipation, 22% vs. 12%; and vomiting, 15% vs. 2%).
The overall incidence of benign, malignant, or unspecified neoplasms was 15% in the treatment groups versus 8% in the placebo group.
Rowen K. Zetterman, MD, who was not involved with the study, noted that “treatment of NASH is currently limited, and no therapies have yet been approved by the Food and Drug Administration.”
The findings are “important but not yet exciting,” added Dr. Zetterman, who is professor emeritus of internal medicine and associate vice chancellor for strategic planning for the University of Nebraska Medical Center, Omaha.
“Though reversal of liver fibrosis was not noted, the resolution of hepatic inflammation and liver cell injury by semaglutide suggests it may be slowing disease progression,” said Dr. Zetterman, who also serves on the editorial advisory board of Internal Medicine News. This “warrants additional studies where longer treatment with semaglutide may prove reversal of fibrosis and/or prevention of progression to cirrhosis.”
The study was sponsored by Novo Nordisk. Dr. Newsome reported disclosures related to Novo Nordisk during the conduct of the study, and to Boehringer Ingelheim, Bristol-Myers Squibb, Echosens, Gilead, Pfizer, Pharmaxis, and Poxel. Several of the other study authors reported receiving fees and grants from various pharmaceutical companies, including Novo Nordisk One author reported pending patents for the use of semaglutide. Dr. Zetterman had no relevant disclosures.
SOURCE: Newsome PN et al. N Engl J Med. 2020 Nov 13. doi: 10.1056/NEJMoa2028395.
according to a phase 2, double-blind, randomized, placebo-controlled trial published in the New England Journal of Medicine and presented at the 2020 American Association for the Study of Liver Diseases (AASLD) meeting.
“This bodes well for further study of semaglutide and is supported further by marked improvements in weight, glycemic control and lipid profile,” commented the study’s senior author Philip N. Newsome, PhD, FRCPE, of the University of Birmingham (England), in an interview.
The highest daily dose (0.4 mg) of the glucagonlike peptide-1 (GLP-1) receptor agonist, semaglutide, which is approved for the treatment of type 2 diabetes, led to levels of NASH resolution “which are higher than any previously demonstrated,” noted Dr. Newsome. “This was also accompanied by improvement in noninvasive markers of liver fibrosis and also less fibrosis progression, compared to placebo.”
“I think this represents an exciting advance and will, if confirmed in further studies, mark a step-change in our management of patients with NASH,” he added.
The multicenter study, conducted at 143 sites in 16 countries, included 320 patients, aged 18-75 years, with or without type 2 diabetes, who had histologic evidence of NASH and stage 1-3 liver fibrosis.
They were randomized in a 3:3:3:1:1:1 ratio to receive once-daily subcutaneous semaglutide at a dose of 0.1, 0.2, or 0.4 mg, or placebo for 72 weeks.
The primary endpoint was resolution of NASH and no worsening of fibrosis, with a secondary endpoint being improvement of fibrosis by at least one stage without worsening of NASH.
The study found 40% of patients in the 0.1-mg semaglutide group, 36% in the 0.2-mg group, and 59% in the 0.4-mg group achieved NASH resolution with no worsening of fibrosis, compared with 17% of the placebo group (odds ratio, 6.87; P < .001 for the highest semaglutide dose). However, the treatment did not lead to significant between-group differences in the secondary endpoint, which occurred in 43% of patients on the highest semaglutide dose compared to 33% in the placebo group (OR, 1.42; P = .48).
Treatment with semaglutide also resulted in dose-dependent reductions in body weight, as well as in glycated hemoglobin levels. Bodyweight was reduced by a mean of 5% in the 0.1-mg semaglutide group, followed by mean reductions of 9% and 13% in the 0.2-mg and 0.4-mg groups respectively. This compared to a mean reduction of 1% in the placebo group.
Similarly, glycated hemoglobin levels among patients with type 2 diabetes dropped by 0.63, 1.07, and 1.15 percentage points in the 0.1-mg, 0.2-mg, and 0.4-mg semaglutide groups respectively, compared with a drop of 0.01 percentage point in the placebo group.
“The fact that the percentage of patients who had an improvement in fibrosis stage was not significantly higher with semaglutide than with placebo – despite a greater benefit with respect to NASH resolution and dose-dependent weight loss – was unexpected, given that previous studies have suggested that resolution of NASH and improvements in activity scores for the components of nonalcoholic fatty liver disease are associated with regression of fibrosis,” wrote the authors. “However, the temporal association among NASH resolution, weight loss, and improvement in fibrosis stage is not fully understood. It is possible that the current trial was not of sufficient duration for improvements in fibrosis stage to become apparent.”
The authors also noted that the safety profile of semaglutide was “consistent with that observed in patients with type 2 diabetes in other trials and with the known effects of GLP-1 receptor agonists,” with gastrointestinal disorders being the most commonly reported.
Nausea, constipation, and vomiting were reported more often in the 0.4-mg semaglutide group than in the placebo group (nausea, 42% vs. 11%; constipation, 22% vs. 12%; and vomiting, 15% vs. 2%).
The overall incidence of benign, malignant, or unspecified neoplasms was 15% in the treatment groups versus 8% in the placebo group.
Rowen K. Zetterman, MD, who was not involved with the study, noted that “treatment of NASH is currently limited, and no therapies have yet been approved by the Food and Drug Administration.”
The findings are “important but not yet exciting,” added Dr. Zetterman, who is professor emeritus of internal medicine and associate vice chancellor for strategic planning for the University of Nebraska Medical Center, Omaha.
“Though reversal of liver fibrosis was not noted, the resolution of hepatic inflammation and liver cell injury by semaglutide suggests it may be slowing disease progression,” said Dr. Zetterman, who also serves on the editorial advisory board of Internal Medicine News. This “warrants additional studies where longer treatment with semaglutide may prove reversal of fibrosis and/or prevention of progression to cirrhosis.”
The study was sponsored by Novo Nordisk. Dr. Newsome reported disclosures related to Novo Nordisk during the conduct of the study, and to Boehringer Ingelheim, Bristol-Myers Squibb, Echosens, Gilead, Pfizer, Pharmaxis, and Poxel. Several of the other study authors reported receiving fees and grants from various pharmaceutical companies, including Novo Nordisk One author reported pending patents for the use of semaglutide. Dr. Zetterman had no relevant disclosures.
SOURCE: Newsome PN et al. N Engl J Med. 2020 Nov 13. doi: 10.1056/NEJMoa2028395.
according to a phase 2, double-blind, randomized, placebo-controlled trial published in the New England Journal of Medicine and presented at the 2020 American Association for the Study of Liver Diseases (AASLD) meeting.
“This bodes well for further study of semaglutide and is supported further by marked improvements in weight, glycemic control and lipid profile,” commented the study’s senior author Philip N. Newsome, PhD, FRCPE, of the University of Birmingham (England), in an interview.
The highest daily dose (0.4 mg) of the glucagonlike peptide-1 (GLP-1) receptor agonist, semaglutide, which is approved for the treatment of type 2 diabetes, led to levels of NASH resolution “which are higher than any previously demonstrated,” noted Dr. Newsome. “This was also accompanied by improvement in noninvasive markers of liver fibrosis and also less fibrosis progression, compared to placebo.”
“I think this represents an exciting advance and will, if confirmed in further studies, mark a step-change in our management of patients with NASH,” he added.
The multicenter study, conducted at 143 sites in 16 countries, included 320 patients, aged 18-75 years, with or without type 2 diabetes, who had histologic evidence of NASH and stage 1-3 liver fibrosis.
They were randomized in a 3:3:3:1:1:1 ratio to receive once-daily subcutaneous semaglutide at a dose of 0.1, 0.2, or 0.4 mg, or placebo for 72 weeks.
The primary endpoint was resolution of NASH and no worsening of fibrosis, with a secondary endpoint being improvement of fibrosis by at least one stage without worsening of NASH.
The study found 40% of patients in the 0.1-mg semaglutide group, 36% in the 0.2-mg group, and 59% in the 0.4-mg group achieved NASH resolution with no worsening of fibrosis, compared with 17% of the placebo group (odds ratio, 6.87; P < .001 for the highest semaglutide dose). However, the treatment did not lead to significant between-group differences in the secondary endpoint, which occurred in 43% of patients on the highest semaglutide dose compared to 33% in the placebo group (OR, 1.42; P = .48).
Treatment with semaglutide also resulted in dose-dependent reductions in body weight, as well as in glycated hemoglobin levels. Bodyweight was reduced by a mean of 5% in the 0.1-mg semaglutide group, followed by mean reductions of 9% and 13% in the 0.2-mg and 0.4-mg groups respectively. This compared to a mean reduction of 1% in the placebo group.
Similarly, glycated hemoglobin levels among patients with type 2 diabetes dropped by 0.63, 1.07, and 1.15 percentage points in the 0.1-mg, 0.2-mg, and 0.4-mg semaglutide groups respectively, compared with a drop of 0.01 percentage point in the placebo group.
“The fact that the percentage of patients who had an improvement in fibrosis stage was not significantly higher with semaglutide than with placebo – despite a greater benefit with respect to NASH resolution and dose-dependent weight loss – was unexpected, given that previous studies have suggested that resolution of NASH and improvements in activity scores for the components of nonalcoholic fatty liver disease are associated with regression of fibrosis,” wrote the authors. “However, the temporal association among NASH resolution, weight loss, and improvement in fibrosis stage is not fully understood. It is possible that the current trial was not of sufficient duration for improvements in fibrosis stage to become apparent.”
The authors also noted that the safety profile of semaglutide was “consistent with that observed in patients with type 2 diabetes in other trials and with the known effects of GLP-1 receptor agonists,” with gastrointestinal disorders being the most commonly reported.
Nausea, constipation, and vomiting were reported more often in the 0.4-mg semaglutide group than in the placebo group (nausea, 42% vs. 11%; constipation, 22% vs. 12%; and vomiting, 15% vs. 2%).
The overall incidence of benign, malignant, or unspecified neoplasms was 15% in the treatment groups versus 8% in the placebo group.
Rowen K. Zetterman, MD, who was not involved with the study, noted that “treatment of NASH is currently limited, and no therapies have yet been approved by the Food and Drug Administration.”
The findings are “important but not yet exciting,” added Dr. Zetterman, who is professor emeritus of internal medicine and associate vice chancellor for strategic planning for the University of Nebraska Medical Center, Omaha.
“Though reversal of liver fibrosis was not noted, the resolution of hepatic inflammation and liver cell injury by semaglutide suggests it may be slowing disease progression,” said Dr. Zetterman, who also serves on the editorial advisory board of Internal Medicine News. This “warrants additional studies where longer treatment with semaglutide may prove reversal of fibrosis and/or prevention of progression to cirrhosis.”
The study was sponsored by Novo Nordisk. Dr. Newsome reported disclosures related to Novo Nordisk during the conduct of the study, and to Boehringer Ingelheim, Bristol-Myers Squibb, Echosens, Gilead, Pfizer, Pharmaxis, and Poxel. Several of the other study authors reported receiving fees and grants from various pharmaceutical companies, including Novo Nordisk One author reported pending patents for the use of semaglutide. Dr. Zetterman had no relevant disclosures.
SOURCE: Newsome PN et al. N Engl J Med. 2020 Nov 13. doi: 10.1056/NEJMoa2028395.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Lipid profiles distinguish obese and nonobese NAFLD patients
Both obese and nonobese individuals can develop nonalcoholic fatty liver disease (NAFLD), and lipid profiles were effective predictors in both groups, based on data from a cross-sectional study of 361 individuals.
Given the strong association between obesity and NAFLD, previous research on lipidomic profiles have focused on obese White patients, wrote Youngae Jung, MD, of the Korea Basic Science Institute, Seoul, and colleagues.
“However, there are insufficient data on circulating lipidomics of nonobese NAFLD patients,” they added.
In a study published in Alimentary Pharmacology and Therapeutics, the researchers identified 295 adults with NAFLD and 66 controls. Overall, 130 participants were nonobese (body mass index <25 kg/m2) and 231 were obese (BMI, 25 or higher). The nonobese group included 51 patients with NAFL, 31 with nonalcoholic steatohepatitis , and 48 controls; the obese group included 106 patients with NAFL, 107 with NASH, and 18 controls.
Lipid profiles show predictive promise
Overall, changes in diacylglycerol (DAG) and triacylglycerol (TAG) appeared in both obese and nonobese patients with increases from NAFL to NASH, the researchers noted.
“Levels of DAGs with relatively short chains and a low degree of desaturation significantly increased in NAFL versus no NAFLD, regardless of obesity,” the researchers said. “In contrast, levels of DAGs with relatively long chains and a high degree of desaturation significantly decreased in NASH versus NAFL in the obese group, which was not observed in the nonobese group,” they noted.
In addition, saturated sphingomyelin (SM) species were significantly associated with visceral adiposity in nonobese NAFLD patients, but not in obese NAFLD patients, and SM levels were significantly associated with both systemic and adipose tissue insulin resistance.
The researchers identified five potential lipid metabolites for nonobese subjects and seven potential lipids for obese subjects that included DAGs, TAGs, and SMs that were distinct between NAFL and NASH patients in order to predict the histologic severity of NAFLD. Overall, these metabolite combinations were effective predictors of NAFLD/NASH in nonobese and obese patients. The areas under the receiver operator characteristic curve were 0.916 versus 0.813 for NAFLD versus non-NAFLD in nonobese patients, and 0.967 versus 0.812 for NAFLD versus non-NAFLD in the obese patients.
More BMI groups may yield more information
The key study limitation was the cross-sectional study design, the researchers noted. In addition, dividing patients into only two groups based on BMI may not reveal any distinct biology among lean NAFLD patients, who were included with overweight patients in the nonobese group.
However, the results were strengthened by the large amount of data and the confirmed diagnoses of NASH and fibrosis by an expert liver pathologist, they added.
“Therefore, our findings provide new insights that aid in the understanding of pathophysiological mechanisms responsible for the development and severity of nonobese NAFLD, which are relevant to precision medicine and personalized therapy based on various phenotypes of NAFLD,” they concluded.
Validation needed in other populations
“Liver biopsy remains the gold standard for diagnosing NAFLD/NASH but has its own limitations and risks, so many researchers in this field are looking for a noninvasive alternative to help with diagnosis,” Zachary Henry, MD, of the University of Virginia, Charlottesville, said in an interview. “Imaging methods such as transient elastography and MR-elastography have been introduced and many biochemical markers have been evaluated, yet all have their limitations. In the current study, the authors report a high diagnostic accuracy for evaluating NAFLD using lipidomic profiles, which could introduce a new noninvasive measurement of NAFLD.”
Dr. Henry said that he was not surprised by the study findings. However, “I believe they are important as they define a lipid profile that shows differences between patients with NASH versus patients with NAFLD versus patients without NAFLD,” he said. “NAFLD is a disease of disordered lipid metabolism in hepatocytes, and although it stands to reason there would be differences in lipid profiles, it is interesting to see the changes between DAGs and TAGs especially as disease progresses from NAFLD to NASH.
“Clinically, I do not think this really changes practice right now since it needs to be validated in other populations of NAFLD. However, it certainly adds to a growing armamentarium of noninvasive testing. Hopefully, we are able to combine some of these noninvasive tests in the future to better predict NAFLD and NASH as well as outcomes such as cirrhosis and hepatocellular carcinoma,” he said.
“I think these lipidomic profiles need to be validated in non-Korean populations of patients with NAFLD to determine if these changes are ubiquitous to everyone or if a different profile exists based upon different genetics and environment,” Dr. Henry added. “As the authors note in their paper, there have been previously published differences between populations of NAFLD in Asia as compared to Western countries and it is unclear how, if at all, these lipidomic profiles relate to those differences.”
The study was funded by the Korea Basic Science Institute, the Korea Health Industry Department Institute, and the National Research Foundation of Korea. The researchers had no financial conflicts to disclose. Dr. Henry had no financial conflicts to disclose.
SOURCE: Jung Y et al. Aliment Pharmacol Ther. 2020 Sep 6. doi: 10.1111/apt.16066.
Both obese and nonobese individuals can develop nonalcoholic fatty liver disease (NAFLD), and lipid profiles were effective predictors in both groups, based on data from a cross-sectional study of 361 individuals.
Given the strong association between obesity and NAFLD, previous research on lipidomic profiles have focused on obese White patients, wrote Youngae Jung, MD, of the Korea Basic Science Institute, Seoul, and colleagues.
“However, there are insufficient data on circulating lipidomics of nonobese NAFLD patients,” they added.
In a study published in Alimentary Pharmacology and Therapeutics, the researchers identified 295 adults with NAFLD and 66 controls. Overall, 130 participants were nonobese (body mass index <25 kg/m2) and 231 were obese (BMI, 25 or higher). The nonobese group included 51 patients with NAFL, 31 with nonalcoholic steatohepatitis , and 48 controls; the obese group included 106 patients with NAFL, 107 with NASH, and 18 controls.
Lipid profiles show predictive promise
Overall, changes in diacylglycerol (DAG) and triacylglycerol (TAG) appeared in both obese and nonobese patients with increases from NAFL to NASH, the researchers noted.
“Levels of DAGs with relatively short chains and a low degree of desaturation significantly increased in NAFL versus no NAFLD, regardless of obesity,” the researchers said. “In contrast, levels of DAGs with relatively long chains and a high degree of desaturation significantly decreased in NASH versus NAFL in the obese group, which was not observed in the nonobese group,” they noted.
In addition, saturated sphingomyelin (SM) species were significantly associated with visceral adiposity in nonobese NAFLD patients, but not in obese NAFLD patients, and SM levels were significantly associated with both systemic and adipose tissue insulin resistance.
The researchers identified five potential lipid metabolites for nonobese subjects and seven potential lipids for obese subjects that included DAGs, TAGs, and SMs that were distinct between NAFL and NASH patients in order to predict the histologic severity of NAFLD. Overall, these metabolite combinations were effective predictors of NAFLD/NASH in nonobese and obese patients. The areas under the receiver operator characteristic curve were 0.916 versus 0.813 for NAFLD versus non-NAFLD in nonobese patients, and 0.967 versus 0.812 for NAFLD versus non-NAFLD in the obese patients.
More BMI groups may yield more information
The key study limitation was the cross-sectional study design, the researchers noted. In addition, dividing patients into only two groups based on BMI may not reveal any distinct biology among lean NAFLD patients, who were included with overweight patients in the nonobese group.
However, the results were strengthened by the large amount of data and the confirmed diagnoses of NASH and fibrosis by an expert liver pathologist, they added.
“Therefore, our findings provide new insights that aid in the understanding of pathophysiological mechanisms responsible for the development and severity of nonobese NAFLD, which are relevant to precision medicine and personalized therapy based on various phenotypes of NAFLD,” they concluded.
Validation needed in other populations
“Liver biopsy remains the gold standard for diagnosing NAFLD/NASH but has its own limitations and risks, so many researchers in this field are looking for a noninvasive alternative to help with diagnosis,” Zachary Henry, MD, of the University of Virginia, Charlottesville, said in an interview. “Imaging methods such as transient elastography and MR-elastography have been introduced and many biochemical markers have been evaluated, yet all have their limitations. In the current study, the authors report a high diagnostic accuracy for evaluating NAFLD using lipidomic profiles, which could introduce a new noninvasive measurement of NAFLD.”
Dr. Henry said that he was not surprised by the study findings. However, “I believe they are important as they define a lipid profile that shows differences between patients with NASH versus patients with NAFLD versus patients without NAFLD,” he said. “NAFLD is a disease of disordered lipid metabolism in hepatocytes, and although it stands to reason there would be differences in lipid profiles, it is interesting to see the changes between DAGs and TAGs especially as disease progresses from NAFLD to NASH.
“Clinically, I do not think this really changes practice right now since it needs to be validated in other populations of NAFLD. However, it certainly adds to a growing armamentarium of noninvasive testing. Hopefully, we are able to combine some of these noninvasive tests in the future to better predict NAFLD and NASH as well as outcomes such as cirrhosis and hepatocellular carcinoma,” he said.
“I think these lipidomic profiles need to be validated in non-Korean populations of patients with NAFLD to determine if these changes are ubiquitous to everyone or if a different profile exists based upon different genetics and environment,” Dr. Henry added. “As the authors note in their paper, there have been previously published differences between populations of NAFLD in Asia as compared to Western countries and it is unclear how, if at all, these lipidomic profiles relate to those differences.”
The study was funded by the Korea Basic Science Institute, the Korea Health Industry Department Institute, and the National Research Foundation of Korea. The researchers had no financial conflicts to disclose. Dr. Henry had no financial conflicts to disclose.
SOURCE: Jung Y et al. Aliment Pharmacol Ther. 2020 Sep 6. doi: 10.1111/apt.16066.
Both obese and nonobese individuals can develop nonalcoholic fatty liver disease (NAFLD), and lipid profiles were effective predictors in both groups, based on data from a cross-sectional study of 361 individuals.
Given the strong association between obesity and NAFLD, previous research on lipidomic profiles have focused on obese White patients, wrote Youngae Jung, MD, of the Korea Basic Science Institute, Seoul, and colleagues.
“However, there are insufficient data on circulating lipidomics of nonobese NAFLD patients,” they added.
In a study published in Alimentary Pharmacology and Therapeutics, the researchers identified 295 adults with NAFLD and 66 controls. Overall, 130 participants were nonobese (body mass index <25 kg/m2) and 231 were obese (BMI, 25 or higher). The nonobese group included 51 patients with NAFL, 31 with nonalcoholic steatohepatitis , and 48 controls; the obese group included 106 patients with NAFL, 107 with NASH, and 18 controls.
Lipid profiles show predictive promise
Overall, changes in diacylglycerol (DAG) and triacylglycerol (TAG) appeared in both obese and nonobese patients with increases from NAFL to NASH, the researchers noted.
“Levels of DAGs with relatively short chains and a low degree of desaturation significantly increased in NAFL versus no NAFLD, regardless of obesity,” the researchers said. “In contrast, levels of DAGs with relatively long chains and a high degree of desaturation significantly decreased in NASH versus NAFL in the obese group, which was not observed in the nonobese group,” they noted.
In addition, saturated sphingomyelin (SM) species were significantly associated with visceral adiposity in nonobese NAFLD patients, but not in obese NAFLD patients, and SM levels were significantly associated with both systemic and adipose tissue insulin resistance.
The researchers identified five potential lipid metabolites for nonobese subjects and seven potential lipids for obese subjects that included DAGs, TAGs, and SMs that were distinct between NAFL and NASH patients in order to predict the histologic severity of NAFLD. Overall, these metabolite combinations were effective predictors of NAFLD/NASH in nonobese and obese patients. The areas under the receiver operator characteristic curve were 0.916 versus 0.813 for NAFLD versus non-NAFLD in nonobese patients, and 0.967 versus 0.812 for NAFLD versus non-NAFLD in the obese patients.
More BMI groups may yield more information
The key study limitation was the cross-sectional study design, the researchers noted. In addition, dividing patients into only two groups based on BMI may not reveal any distinct biology among lean NAFLD patients, who were included with overweight patients in the nonobese group.
However, the results were strengthened by the large amount of data and the confirmed diagnoses of NASH and fibrosis by an expert liver pathologist, they added.
“Therefore, our findings provide new insights that aid in the understanding of pathophysiological mechanisms responsible for the development and severity of nonobese NAFLD, which are relevant to precision medicine and personalized therapy based on various phenotypes of NAFLD,” they concluded.
Validation needed in other populations
“Liver biopsy remains the gold standard for diagnosing NAFLD/NASH but has its own limitations and risks, so many researchers in this field are looking for a noninvasive alternative to help with diagnosis,” Zachary Henry, MD, of the University of Virginia, Charlottesville, said in an interview. “Imaging methods such as transient elastography and MR-elastography have been introduced and many biochemical markers have been evaluated, yet all have their limitations. In the current study, the authors report a high diagnostic accuracy for evaluating NAFLD using lipidomic profiles, which could introduce a new noninvasive measurement of NAFLD.”
Dr. Henry said that he was not surprised by the study findings. However, “I believe they are important as they define a lipid profile that shows differences between patients with NASH versus patients with NAFLD versus patients without NAFLD,” he said. “NAFLD is a disease of disordered lipid metabolism in hepatocytes, and although it stands to reason there would be differences in lipid profiles, it is interesting to see the changes between DAGs and TAGs especially as disease progresses from NAFLD to NASH.
“Clinically, I do not think this really changes practice right now since it needs to be validated in other populations of NAFLD. However, it certainly adds to a growing armamentarium of noninvasive testing. Hopefully, we are able to combine some of these noninvasive tests in the future to better predict NAFLD and NASH as well as outcomes such as cirrhosis and hepatocellular carcinoma,” he said.
“I think these lipidomic profiles need to be validated in non-Korean populations of patients with NAFLD to determine if these changes are ubiquitous to everyone or if a different profile exists based upon different genetics and environment,” Dr. Henry added. “As the authors note in their paper, there have been previously published differences between populations of NAFLD in Asia as compared to Western countries and it is unclear how, if at all, these lipidomic profiles relate to those differences.”
The study was funded by the Korea Basic Science Institute, the Korea Health Industry Department Institute, and the National Research Foundation of Korea. The researchers had no financial conflicts to disclose. Dr. Henry had no financial conflicts to disclose.
SOURCE: Jung Y et al. Aliment Pharmacol Ther. 2020 Sep 6. doi: 10.1111/apt.16066.
FROM ALIMENTARY PHARMACOLOGY & THERAPEUTICS
Cirrhosis, Child-Pugh score predict ERCP complications
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
FROM ACG 2020