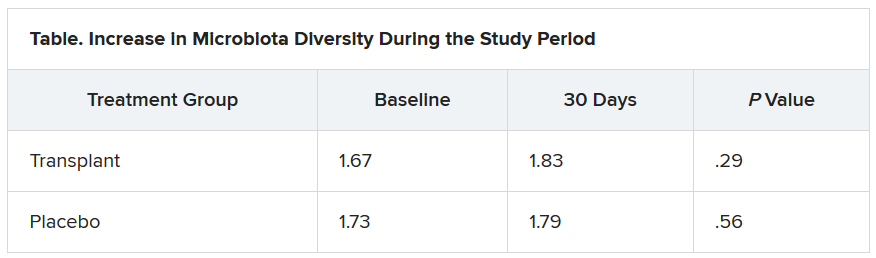User login
Repeat FIB-4 blood tests help predict cirrhosis
Repeat Fibrosis-4 (FIB-4) scores can be used to identify people at greatest risk for cirrhosis of the liver, new research shows.
“Done repeatedly, this test can improve prediction capacity to identify who will develop cirrhosis of the liver later in life,” said lead researcher Hannes Hagström, MD, from the Karolinska University Hospital in Stockholm.
A FIB-4 score that rises from one test to the next indicates that a person is at increased risk for severe liver disease, whereas a score that drops indicates a decreased risk, he told Medscape Medical News. The study results — published online July 1 in the Journal of Hepatology, was presented at the Digital International Liver Congress 2020.
The noninvasive, widely available, cheap FIB-4 test — which is calculated on the basis of age, transaminase level, and platelet count — is commonly used to identify the risk for advanced fibrosis in liver disease, but it has not been used to predict future risk.
To evaluate risk for cirrhosis, Hagström and his colleagues looked at 812,073 blood tests performed from 1985 to 1996 on people enrolled in the Swedish Apolipoprotein Mortality Risk (AMORIS) study.
They excluded people younger than 35 years and older than 79 years and anyone with a diagnosis of any liver disease at baseline.
The 40,729 people who had two FIB-4 measurements taken less than 5 years apart were included in the analysis. Test results were categorized into three risk groups: low (<1.30), intermediate (1.30 - 2.67), and high (>2.67).
After a median of 16.2 years, 11,929 people in the study cohort had died and 581 had a severe liver disease event.
Severe liver disease events were more common in people who had both tests categorized as high risk than in people who had both tests categorized as low risk (13.2% vs 1.0%; aHR, 17.04; 95% CI, 11.67 - 24.88).
The researchers found that a one-unit increase between the two test results was continuously predictive of a severe liver disease event (aHR, 1.81; 95% CI, 1.67 - 1.96).
One test not enough
The absolute risk for severe liver disease in the general population is 2%, but the FIB-4 score is elevated in about one-third of people in the general population.
“A lot of people who have increased levels of this biomarker will never develop cirrhosis,” Hagström told Medscape Medical News.
Although two FIB-4 scores might not identify everyone who will get cirrhosis, comparing scores provides insight into who is at greatest risk, he explained.
This information can be useful, particularly for primary care doctors. If you know that someone is at higher risk, “you can send that patient for a FibroScan, which is a much more sensitive measurement,” but also much more expensive. “Now we can better know who to send,” he said.
However, “the main problem is that these tests are not widely known” or used enough by primary care doctors, Hagström said.
A lack of knowledge about the utility of this test is a problem, agreed Jérôme Boursier, MD, PhD, from Angers University in France.
“The younger doctors are using these tests more often,” he told Medscape Medical News, but “the older doctors are not aware they exist.”
This study supports repeating the tests. “One test offers quite poor prediction,” Boursier said. But “when you have a higher score on a second one, this can help the conversation with the patient.”
Hagström and Boursier have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Repeat Fibrosis-4 (FIB-4) scores can be used to identify people at greatest risk for cirrhosis of the liver, new research shows.
“Done repeatedly, this test can improve prediction capacity to identify who will develop cirrhosis of the liver later in life,” said lead researcher Hannes Hagström, MD, from the Karolinska University Hospital in Stockholm.
A FIB-4 score that rises from one test to the next indicates that a person is at increased risk for severe liver disease, whereas a score that drops indicates a decreased risk, he told Medscape Medical News. The study results — published online July 1 in the Journal of Hepatology, was presented at the Digital International Liver Congress 2020.
The noninvasive, widely available, cheap FIB-4 test — which is calculated on the basis of age, transaminase level, and platelet count — is commonly used to identify the risk for advanced fibrosis in liver disease, but it has not been used to predict future risk.
To evaluate risk for cirrhosis, Hagström and his colleagues looked at 812,073 blood tests performed from 1985 to 1996 on people enrolled in the Swedish Apolipoprotein Mortality Risk (AMORIS) study.
They excluded people younger than 35 years and older than 79 years and anyone with a diagnosis of any liver disease at baseline.
The 40,729 people who had two FIB-4 measurements taken less than 5 years apart were included in the analysis. Test results were categorized into three risk groups: low (<1.30), intermediate (1.30 - 2.67), and high (>2.67).
After a median of 16.2 years, 11,929 people in the study cohort had died and 581 had a severe liver disease event.
Severe liver disease events were more common in people who had both tests categorized as high risk than in people who had both tests categorized as low risk (13.2% vs 1.0%; aHR, 17.04; 95% CI, 11.67 - 24.88).
The researchers found that a one-unit increase between the two test results was continuously predictive of a severe liver disease event (aHR, 1.81; 95% CI, 1.67 - 1.96).
One test not enough
The absolute risk for severe liver disease in the general population is 2%, but the FIB-4 score is elevated in about one-third of people in the general population.
“A lot of people who have increased levels of this biomarker will never develop cirrhosis,” Hagström told Medscape Medical News.
Although two FIB-4 scores might not identify everyone who will get cirrhosis, comparing scores provides insight into who is at greatest risk, he explained.
This information can be useful, particularly for primary care doctors. If you know that someone is at higher risk, “you can send that patient for a FibroScan, which is a much more sensitive measurement,” but also much more expensive. “Now we can better know who to send,” he said.
However, “the main problem is that these tests are not widely known” or used enough by primary care doctors, Hagström said.
A lack of knowledge about the utility of this test is a problem, agreed Jérôme Boursier, MD, PhD, from Angers University in France.
“The younger doctors are using these tests more often,” he told Medscape Medical News, but “the older doctors are not aware they exist.”
This study supports repeating the tests. “One test offers quite poor prediction,” Boursier said. But “when you have a higher score on a second one, this can help the conversation with the patient.”
Hagström and Boursier have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Repeat Fibrosis-4 (FIB-4) scores can be used to identify people at greatest risk for cirrhosis of the liver, new research shows.
“Done repeatedly, this test can improve prediction capacity to identify who will develop cirrhosis of the liver later in life,” said lead researcher Hannes Hagström, MD, from the Karolinska University Hospital in Stockholm.
A FIB-4 score that rises from one test to the next indicates that a person is at increased risk for severe liver disease, whereas a score that drops indicates a decreased risk, he told Medscape Medical News. The study results — published online July 1 in the Journal of Hepatology, was presented at the Digital International Liver Congress 2020.
The noninvasive, widely available, cheap FIB-4 test — which is calculated on the basis of age, transaminase level, and platelet count — is commonly used to identify the risk for advanced fibrosis in liver disease, but it has not been used to predict future risk.
To evaluate risk for cirrhosis, Hagström and his colleagues looked at 812,073 blood tests performed from 1985 to 1996 on people enrolled in the Swedish Apolipoprotein Mortality Risk (AMORIS) study.
They excluded people younger than 35 years and older than 79 years and anyone with a diagnosis of any liver disease at baseline.
The 40,729 people who had two FIB-4 measurements taken less than 5 years apart were included in the analysis. Test results were categorized into three risk groups: low (<1.30), intermediate (1.30 - 2.67), and high (>2.67).
After a median of 16.2 years, 11,929 people in the study cohort had died and 581 had a severe liver disease event.
Severe liver disease events were more common in people who had both tests categorized as high risk than in people who had both tests categorized as low risk (13.2% vs 1.0%; aHR, 17.04; 95% CI, 11.67 - 24.88).
The researchers found that a one-unit increase between the two test results was continuously predictive of a severe liver disease event (aHR, 1.81; 95% CI, 1.67 - 1.96).
One test not enough
The absolute risk for severe liver disease in the general population is 2%, but the FIB-4 score is elevated in about one-third of people in the general population.
“A lot of people who have increased levels of this biomarker will never develop cirrhosis,” Hagström told Medscape Medical News.
Although two FIB-4 scores might not identify everyone who will get cirrhosis, comparing scores provides insight into who is at greatest risk, he explained.
This information can be useful, particularly for primary care doctors. If you know that someone is at higher risk, “you can send that patient for a FibroScan, which is a much more sensitive measurement,” but also much more expensive. “Now we can better know who to send,” he said.
However, “the main problem is that these tests are not widely known” or used enough by primary care doctors, Hagström said.
A lack of knowledge about the utility of this test is a problem, agreed Jérôme Boursier, MD, PhD, from Angers University in France.
“The younger doctors are using these tests more often,” he told Medscape Medical News, but “the older doctors are not aware they exist.”
This study supports repeating the tests. “One test offers quite poor prediction,” Boursier said. But “when you have a higher score on a second one, this can help the conversation with the patient.”
Hagström and Boursier have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Fecal transplant shows promise in reducing alcohol craving
Fecal microbiota transplantation results in a short-term reduction in alcohol craving in patients with alcohol-induced cirrhosis who can’t stop drinking, results from a new study show.
And that reduction could lead to a better psychosocial quality of life for patients with cirrhosis and alcohol use disorder, said investigator Jasmohan Bajaj, MD, from Virginia Commonwealth University, Richmond.
“This is the most common addiction disorder worldwide, but we have nothing to treat these patients with,” he said.
Cirrhosis is associated with an altered gut-brain axis. It leads to organ damage in several parts of the body, including the brain, gut, pancreas, and liver. This makes changing the gut microbes “an attractive target,” Dr. Bajaj said at the Digital International Liver Congress 2020.
For their phase 1, double-blind study, he and his colleagues assessed 20 men from a Virginia veteran’s hospital with untreatable alcohol use disorder who were not eligible for liver transplantation.
All had failed behavioral or pharmacologic therapy and were unwilling to try again. “That’s what made them good candidates to try something new,” Dr. Bajaj said during a press briefing.
Mean age in the study cohort was 65 years, mean Model for End-Stage Liver disease score was 8.9, and demographic characteristics were similar between the 10 men randomly assigned to fecal transplantation and the 10 assigned to placebo. One man in each group dropped out of the study.
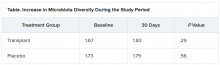
The investigators evaluated cravings, microbiota, and quality of life during the 30-day study period.
At day 15, significantly more men in the transplant group than in the placebo group experienced a reduction in alcohol cravings (90% vs. 30%).
At 30 days, levels of creatinine, serum interleukin-6, and lipopolysaccharide-binding protein were lower in the transplant group than in the placebo group. In addition, levels of butyrate and isobutyrate increased, as did cognition and quality of life scores.
There was also a decrease in urinary ethyl glucuronide in the transplant group, which “is the objective criteria for alcohol intake,” Dr. Bajaj reported, noting that there was no change in ethyl glucuronide in the placebo group.
The increase in microbiota diversity was significant in the transplant group but not in the placebo group. Alistipes, Odoribacter, and Roseburia were more abundant in the transplant group than in the placebo group.
During the 30-day study period, two men in the placebo group required medical attention, one for hyponatremia and the other for atrial fibrillation. However, no adverse events were seen in any men in the transplant group. “This was the No. 1 result,” Dr. Bajaj said.
Liver disease and the microbiome
“Understanding of interactions between the human and microbiome genome [metagenome] in health and disease has represented one of the major areas of progress in the last few years,” said Luca Valenti, MD, from the University of Milan, who is a member of the scientific committee of the European Association the Study of the Liver, which organized the congress.
“These studies lay the groundwork for the exploitation of this new knowledge for the treatment of liver disease,” he said.
“We are [now] diagnosing liver disease and the stages of liver disease based on microbiome changes,” said Jonel Trebicka, MD, PhD, from University Hospital Frankfurt (Germany), who chaired a session at the congress on the role of the microbiome in liver disease.
“This and other studies have shown us that the microbiome itself may influence liver disease,” he added.
Dr. Bajaj is considered one of the world’s experts on cirrhosis and the microbiome, Dr. Trebicka explained. Last year, Dr. Bajaj and his team demonstrated that fecal microbiota transplantation can reduce the incidence of recurrent hepatic encephalopathy, as reported by Medscape Medical News.
The current study also “shows clearly that the microbiome plays a role in craving. FMT reduces the desire for alcohol,” said Dr. Trebicka.
“The way to the brain is through the gut,” Dr. Bajaj said.
Dr. Bajaj, Dr. Trebicka, and Dr. Valenti disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Fecal microbiota transplantation results in a short-term reduction in alcohol craving in patients with alcohol-induced cirrhosis who can’t stop drinking, results from a new study show.
And that reduction could lead to a better psychosocial quality of life for patients with cirrhosis and alcohol use disorder, said investigator Jasmohan Bajaj, MD, from Virginia Commonwealth University, Richmond.
“This is the most common addiction disorder worldwide, but we have nothing to treat these patients with,” he said.
Cirrhosis is associated with an altered gut-brain axis. It leads to organ damage in several parts of the body, including the brain, gut, pancreas, and liver. This makes changing the gut microbes “an attractive target,” Dr. Bajaj said at the Digital International Liver Congress 2020.
For their phase 1, double-blind study, he and his colleagues assessed 20 men from a Virginia veteran’s hospital with untreatable alcohol use disorder who were not eligible for liver transplantation.
All had failed behavioral or pharmacologic therapy and were unwilling to try again. “That’s what made them good candidates to try something new,” Dr. Bajaj said during a press briefing.
Mean age in the study cohort was 65 years, mean Model for End-Stage Liver disease score was 8.9, and demographic characteristics were similar between the 10 men randomly assigned to fecal transplantation and the 10 assigned to placebo. One man in each group dropped out of the study.
The investigators evaluated cravings, microbiota, and quality of life during the 30-day study period.
At day 15, significantly more men in the transplant group than in the placebo group experienced a reduction in alcohol cravings (90% vs. 30%).
At 30 days, levels of creatinine, serum interleukin-6, and lipopolysaccharide-binding protein were lower in the transplant group than in the placebo group. In addition, levels of butyrate and isobutyrate increased, as did cognition and quality of life scores.
There was also a decrease in urinary ethyl glucuronide in the transplant group, which “is the objective criteria for alcohol intake,” Dr. Bajaj reported, noting that there was no change in ethyl glucuronide in the placebo group.
The increase in microbiota diversity was significant in the transplant group but not in the placebo group. Alistipes, Odoribacter, and Roseburia were more abundant in the transplant group than in the placebo group.
During the 30-day study period, two men in the placebo group required medical attention, one for hyponatremia and the other for atrial fibrillation. However, no adverse events were seen in any men in the transplant group. “This was the No. 1 result,” Dr. Bajaj said.
Liver disease and the microbiome
“Understanding of interactions between the human and microbiome genome [metagenome] in health and disease has represented one of the major areas of progress in the last few years,” said Luca Valenti, MD, from the University of Milan, who is a member of the scientific committee of the European Association the Study of the Liver, which organized the congress.
“These studies lay the groundwork for the exploitation of this new knowledge for the treatment of liver disease,” he said.
“We are [now] diagnosing liver disease and the stages of liver disease based on microbiome changes,” said Jonel Trebicka, MD, PhD, from University Hospital Frankfurt (Germany), who chaired a session at the congress on the role of the microbiome in liver disease.
“This and other studies have shown us that the microbiome itself may influence liver disease,” he added.
Dr. Bajaj is considered one of the world’s experts on cirrhosis and the microbiome, Dr. Trebicka explained. Last year, Dr. Bajaj and his team demonstrated that fecal microbiota transplantation can reduce the incidence of recurrent hepatic encephalopathy, as reported by Medscape Medical News.
The current study also “shows clearly that the microbiome plays a role in craving. FMT reduces the desire for alcohol,” said Dr. Trebicka.
“The way to the brain is through the gut,” Dr. Bajaj said.
Dr. Bajaj, Dr. Trebicka, and Dr. Valenti disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Fecal microbiota transplantation results in a short-term reduction in alcohol craving in patients with alcohol-induced cirrhosis who can’t stop drinking, results from a new study show.
And that reduction could lead to a better psychosocial quality of life for patients with cirrhosis and alcohol use disorder, said investigator Jasmohan Bajaj, MD, from Virginia Commonwealth University, Richmond.
“This is the most common addiction disorder worldwide, but we have nothing to treat these patients with,” he said.
Cirrhosis is associated with an altered gut-brain axis. It leads to organ damage in several parts of the body, including the brain, gut, pancreas, and liver. This makes changing the gut microbes “an attractive target,” Dr. Bajaj said at the Digital International Liver Congress 2020.
For their phase 1, double-blind study, he and his colleagues assessed 20 men from a Virginia veteran’s hospital with untreatable alcohol use disorder who were not eligible for liver transplantation.
All had failed behavioral or pharmacologic therapy and were unwilling to try again. “That’s what made them good candidates to try something new,” Dr. Bajaj said during a press briefing.
Mean age in the study cohort was 65 years, mean Model for End-Stage Liver disease score was 8.9, and demographic characteristics were similar between the 10 men randomly assigned to fecal transplantation and the 10 assigned to placebo. One man in each group dropped out of the study.
The investigators evaluated cravings, microbiota, and quality of life during the 30-day study period.
At day 15, significantly more men in the transplant group than in the placebo group experienced a reduction in alcohol cravings (90% vs. 30%).
At 30 days, levels of creatinine, serum interleukin-6, and lipopolysaccharide-binding protein were lower in the transplant group than in the placebo group. In addition, levels of butyrate and isobutyrate increased, as did cognition and quality of life scores.
There was also a decrease in urinary ethyl glucuronide in the transplant group, which “is the objective criteria for alcohol intake,” Dr. Bajaj reported, noting that there was no change in ethyl glucuronide in the placebo group.
The increase in microbiota diversity was significant in the transplant group but not in the placebo group. Alistipes, Odoribacter, and Roseburia were more abundant in the transplant group than in the placebo group.
During the 30-day study period, two men in the placebo group required medical attention, one for hyponatremia and the other for atrial fibrillation. However, no adverse events were seen in any men in the transplant group. “This was the No. 1 result,” Dr. Bajaj said.
Liver disease and the microbiome
“Understanding of interactions between the human and microbiome genome [metagenome] in health and disease has represented one of the major areas of progress in the last few years,” said Luca Valenti, MD, from the University of Milan, who is a member of the scientific committee of the European Association the Study of the Liver, which organized the congress.
“These studies lay the groundwork for the exploitation of this new knowledge for the treatment of liver disease,” he said.
“We are [now] diagnosing liver disease and the stages of liver disease based on microbiome changes,” said Jonel Trebicka, MD, PhD, from University Hospital Frankfurt (Germany), who chaired a session at the congress on the role of the microbiome in liver disease.
“This and other studies have shown us that the microbiome itself may influence liver disease,” he added.
Dr. Bajaj is considered one of the world’s experts on cirrhosis and the microbiome, Dr. Trebicka explained. Last year, Dr. Bajaj and his team demonstrated that fecal microbiota transplantation can reduce the incidence of recurrent hepatic encephalopathy, as reported by Medscape Medical News.
The current study also “shows clearly that the microbiome plays a role in craving. FMT reduces the desire for alcohol,” said Dr. Trebicka.
“The way to the brain is through the gut,” Dr. Bajaj said.
Dr. Bajaj, Dr. Trebicka, and Dr. Valenti disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
NAFLD may predict arrhythmia recurrence post-AFib ablation
Increasingly recognized as an independent risk factor for new-onset atrial fibrillation (AFib), new research suggests for the first time that nonalcoholic fatty liver disease (NAFLD) also confers a higher risk for arrhythmia recurrence after AFib ablation.
Over 29 months of postablation follow-up, 56% of patients with NAFLD suffered bouts of arrhythmia, compared with 31% of patients without NAFLD, matched on the basis of age, sex, body mass index (BMI), ejection fraction within 5%, and AFib type (P < .0001).
The presence of NAFLD was an independent predictor of arrhythmia recurrence in multivariable analyses adjusted for several confounders, including hemoglobin A1c, BMI, and AFib type (hazard ratio, 3.0; 95% confidence interval, 1.94-4.68).
The association is concerning given that one in four adults in the United States has NAFLD, and up to 6.1 million Americans are estimated to have Afib. Previous studies, such as ARREST-AF and LEGACY, however, have demonstrated the benefits of aggressive preablation cardiometabolic risk factor modification on long-term AFib ablation success.
Indeed, none of the NAFLD patients in the present study who lost at least 10% of their body weight had recurrent arrhythmia, compared with 31% who lost less than 10%, and 91% who gained weight prior to ablation (P < .0001).
All 22 patients whose A1c increased during the 12 months prior to ablation had recurrent arrhythmia, compared with 36% of patients whose A1c improved (P < .0001).
“I don’t think the findings of the study were particularly surprising, given what we know. It’s just further reinforcement of the essential role of risk-factor modification,” lead author Eoin Donnellan, MD, Cleveland Clinic, said in an interview.
The results were published Augus 12 in JACC Clinical Electrophysiology.
For the study, the researchers examined data from 267 consecutive patients with a mean BMI of 32.7 kg/m2 who underwent radiofrequency ablation (98%) or cryoablation (2%) at the Cleveland Clinic between January 2013 and December 2017.
All patients were followed for at least 12 months after ablation and had scheduled clinic visits at 3, 6, and 12 months after pulmonary vein isolation, and annually thereafter.
NAFLD was diagnosed in 89 patients prior to ablation on the basis of CT imaging and abdominal ultrasound or MRI. On the basis of NAFLD-Fibrosis Score (NAFLD-FS), 13 patients had a low probability of liver fibrosis (F0-F2), 54 had an indeterminate probability, and 22 a high probability of fibrosis (F3-F4).
Compared with patients with no or early fibrosis (F0-F2), patients with advanced liver fibrosis (F3-F4) had almost a threefold increase in AFib recurrence (82% vs. 31%; P = .003).
“Cardiologists should make an effort to risk-stratify NAFLD patients either by NAFLD-FS or [an] alternative option, such as transient elastography or MR elastography, given these observations, rather than viewing it as either present or absence [sic] and involve expert multidisciplinary team care early in the clinical course of NAFLD patients with evidence of advanced fibrosis,” Dr. Donnellan and colleagues wrote.
Coauthor Thomas G. Cotter, MD, department of gastroenterology and hepatology, University of Chicago, said in an interview that cardiologists could use just the NAFLD-FS as part of an algorithm for an AFib.
“Because if it shows low risk, then it’s very, very likely the patient will be fine,” he said. “To use more advanced noninvasive testing, there are subtleties in the interpretation that would require referral to a liver doctor or a gastroenterologist and the cost of referring might bulk up the costs. But the NAFLD-FS is freely available and is a validated tool.”
Although it hasn’t specifically been validated in patients with AFib, the NAFLD-FS has been shown to correlate with the development of coronary artery disease (CAD) and was recommended for clinical use in U.S. multisociety guidelines for NAFLD.
The score is calculated using six readily available clinical variables (age, BMI, hyperglycemia or diabetes, AST/ALT, platelets, and albumin). It does not include family history or alcohol consumption, which should be carefully detailed given the large overlap between NAFLD and alcohol-related liver disease, Dr. Cotter observed.
Of note, the study excluded patients with alcohol consumption of more than 30 g/day in men and more than 20 g/day in women, chronic viral hepatitis, Wilson’s disease, and hereditary hemochromatosis.
Finally, CT imaging revealed that epicardial fat volume (EFV) was greater in patients with NAFLD than in those without NAFLD (248 vs. 223 mL; P = .01).
Although increased amounts of epicardial fat have been associated with CAD, there was no significant difference in EFV between patients who did and did not develop recurrent arrhythmia (238 vs. 229 mL; P = .5). Nor was EFV associated with arrhythmia recurrence on Cox proportional hazards analysis (HR, 1.001; P = .17).
“We hypothesized that the increased risk of arrhythmia recurrence may be mediated in part by an increased epicardial fat volume,” Dr. Donnellan said. “The existing literature exploring the link between epicardial fat volume and A[Fib] burden and recurrence is conflicting. But in both this study and our bariatric surgery study, epicardial fat volume was not a significant predictor of arrhythmia recurrence on multivariable analysis.”
It’s likely that the increased recurrence risk is caused by several mechanisms, including NAFLD’s deleterious impact on cardiac structure and function, the bidirectional relationship between NAFLD and sleep apnea, and transcription of proinflammatory cytokines and low-grade systemic inflammation, he suggested.
“Patients with NAFLD represent a particularly high-risk population for arrhythmia recurrence. NAFLD is a reversible disease, and a multidisciplinary approach incorporating dietary and lifestyle interventions should by instituted prior to ablation,” Dr. Donnellan and colleagues concluded.
They noted that serial abdominal imaging to assess for preablation changes in NAFLD was limited in patients and that only 56% of control subjects underwent dedicated abdominal imaging to rule out hepatic steatosis. Also, the heterogeneity of imaging modalities used to diagnose NAFLD may have influenced the results and the study’s single-center, retrospective design limits their generalizability.
The authors reported having no relevant financial relationships.
Help your patients better understand their risk of NASH and NAFLD by sharing AGA patient education content at http://ow.ly/ZKi930r50am.
A version of this article originally appeared on Medscape.com.
Increasingly recognized as an independent risk factor for new-onset atrial fibrillation (AFib), new research suggests for the first time that nonalcoholic fatty liver disease (NAFLD) also confers a higher risk for arrhythmia recurrence after AFib ablation.
Over 29 months of postablation follow-up, 56% of patients with NAFLD suffered bouts of arrhythmia, compared with 31% of patients without NAFLD, matched on the basis of age, sex, body mass index (BMI), ejection fraction within 5%, and AFib type (P < .0001).
The presence of NAFLD was an independent predictor of arrhythmia recurrence in multivariable analyses adjusted for several confounders, including hemoglobin A1c, BMI, and AFib type (hazard ratio, 3.0; 95% confidence interval, 1.94-4.68).
The association is concerning given that one in four adults in the United States has NAFLD, and up to 6.1 million Americans are estimated to have Afib. Previous studies, such as ARREST-AF and LEGACY, however, have demonstrated the benefits of aggressive preablation cardiometabolic risk factor modification on long-term AFib ablation success.
Indeed, none of the NAFLD patients in the present study who lost at least 10% of their body weight had recurrent arrhythmia, compared with 31% who lost less than 10%, and 91% who gained weight prior to ablation (P < .0001).
All 22 patients whose A1c increased during the 12 months prior to ablation had recurrent arrhythmia, compared with 36% of patients whose A1c improved (P < .0001).
“I don’t think the findings of the study were particularly surprising, given what we know. It’s just further reinforcement of the essential role of risk-factor modification,” lead author Eoin Donnellan, MD, Cleveland Clinic, said in an interview.
The results were published Augus 12 in JACC Clinical Electrophysiology.
For the study, the researchers examined data from 267 consecutive patients with a mean BMI of 32.7 kg/m2 who underwent radiofrequency ablation (98%) or cryoablation (2%) at the Cleveland Clinic between January 2013 and December 2017.
All patients were followed for at least 12 months after ablation and had scheduled clinic visits at 3, 6, and 12 months after pulmonary vein isolation, and annually thereafter.
NAFLD was diagnosed in 89 patients prior to ablation on the basis of CT imaging and abdominal ultrasound or MRI. On the basis of NAFLD-Fibrosis Score (NAFLD-FS), 13 patients had a low probability of liver fibrosis (F0-F2), 54 had an indeterminate probability, and 22 a high probability of fibrosis (F3-F4).
Compared with patients with no or early fibrosis (F0-F2), patients with advanced liver fibrosis (F3-F4) had almost a threefold increase in AFib recurrence (82% vs. 31%; P = .003).
“Cardiologists should make an effort to risk-stratify NAFLD patients either by NAFLD-FS or [an] alternative option, such as transient elastography or MR elastography, given these observations, rather than viewing it as either present or absence [sic] and involve expert multidisciplinary team care early in the clinical course of NAFLD patients with evidence of advanced fibrosis,” Dr. Donnellan and colleagues wrote.
Coauthor Thomas G. Cotter, MD, department of gastroenterology and hepatology, University of Chicago, said in an interview that cardiologists could use just the NAFLD-FS as part of an algorithm for an AFib.
“Because if it shows low risk, then it’s very, very likely the patient will be fine,” he said. “To use more advanced noninvasive testing, there are subtleties in the interpretation that would require referral to a liver doctor or a gastroenterologist and the cost of referring might bulk up the costs. But the NAFLD-FS is freely available and is a validated tool.”
Although it hasn’t specifically been validated in patients with AFib, the NAFLD-FS has been shown to correlate with the development of coronary artery disease (CAD) and was recommended for clinical use in U.S. multisociety guidelines for NAFLD.
The score is calculated using six readily available clinical variables (age, BMI, hyperglycemia or diabetes, AST/ALT, platelets, and albumin). It does not include family history or alcohol consumption, which should be carefully detailed given the large overlap between NAFLD and alcohol-related liver disease, Dr. Cotter observed.
Of note, the study excluded patients with alcohol consumption of more than 30 g/day in men and more than 20 g/day in women, chronic viral hepatitis, Wilson’s disease, and hereditary hemochromatosis.
Finally, CT imaging revealed that epicardial fat volume (EFV) was greater in patients with NAFLD than in those without NAFLD (248 vs. 223 mL; P = .01).
Although increased amounts of epicardial fat have been associated with CAD, there was no significant difference in EFV between patients who did and did not develop recurrent arrhythmia (238 vs. 229 mL; P = .5). Nor was EFV associated with arrhythmia recurrence on Cox proportional hazards analysis (HR, 1.001; P = .17).
“We hypothesized that the increased risk of arrhythmia recurrence may be mediated in part by an increased epicardial fat volume,” Dr. Donnellan said. “The existing literature exploring the link between epicardial fat volume and A[Fib] burden and recurrence is conflicting. But in both this study and our bariatric surgery study, epicardial fat volume was not a significant predictor of arrhythmia recurrence on multivariable analysis.”
It’s likely that the increased recurrence risk is caused by several mechanisms, including NAFLD’s deleterious impact on cardiac structure and function, the bidirectional relationship between NAFLD and sleep apnea, and transcription of proinflammatory cytokines and low-grade systemic inflammation, he suggested.
“Patients with NAFLD represent a particularly high-risk population for arrhythmia recurrence. NAFLD is a reversible disease, and a multidisciplinary approach incorporating dietary and lifestyle interventions should by instituted prior to ablation,” Dr. Donnellan and colleagues concluded.
They noted that serial abdominal imaging to assess for preablation changes in NAFLD was limited in patients and that only 56% of control subjects underwent dedicated abdominal imaging to rule out hepatic steatosis. Also, the heterogeneity of imaging modalities used to diagnose NAFLD may have influenced the results and the study’s single-center, retrospective design limits their generalizability.
The authors reported having no relevant financial relationships.
Help your patients better understand their risk of NASH and NAFLD by sharing AGA patient education content at http://ow.ly/ZKi930r50am.
A version of this article originally appeared on Medscape.com.
Increasingly recognized as an independent risk factor for new-onset atrial fibrillation (AFib), new research suggests for the first time that nonalcoholic fatty liver disease (NAFLD) also confers a higher risk for arrhythmia recurrence after AFib ablation.
Over 29 months of postablation follow-up, 56% of patients with NAFLD suffered bouts of arrhythmia, compared with 31% of patients without NAFLD, matched on the basis of age, sex, body mass index (BMI), ejection fraction within 5%, and AFib type (P < .0001).
The presence of NAFLD was an independent predictor of arrhythmia recurrence in multivariable analyses adjusted for several confounders, including hemoglobin A1c, BMI, and AFib type (hazard ratio, 3.0; 95% confidence interval, 1.94-4.68).
The association is concerning given that one in four adults in the United States has NAFLD, and up to 6.1 million Americans are estimated to have Afib. Previous studies, such as ARREST-AF and LEGACY, however, have demonstrated the benefits of aggressive preablation cardiometabolic risk factor modification on long-term AFib ablation success.
Indeed, none of the NAFLD patients in the present study who lost at least 10% of their body weight had recurrent arrhythmia, compared with 31% who lost less than 10%, and 91% who gained weight prior to ablation (P < .0001).
All 22 patients whose A1c increased during the 12 months prior to ablation had recurrent arrhythmia, compared with 36% of patients whose A1c improved (P < .0001).
“I don’t think the findings of the study were particularly surprising, given what we know. It’s just further reinforcement of the essential role of risk-factor modification,” lead author Eoin Donnellan, MD, Cleveland Clinic, said in an interview.
The results were published Augus 12 in JACC Clinical Electrophysiology.
For the study, the researchers examined data from 267 consecutive patients with a mean BMI of 32.7 kg/m2 who underwent radiofrequency ablation (98%) or cryoablation (2%) at the Cleveland Clinic between January 2013 and December 2017.
All patients were followed for at least 12 months after ablation and had scheduled clinic visits at 3, 6, and 12 months after pulmonary vein isolation, and annually thereafter.
NAFLD was diagnosed in 89 patients prior to ablation on the basis of CT imaging and abdominal ultrasound or MRI. On the basis of NAFLD-Fibrosis Score (NAFLD-FS), 13 patients had a low probability of liver fibrosis (F0-F2), 54 had an indeterminate probability, and 22 a high probability of fibrosis (F3-F4).
Compared with patients with no or early fibrosis (F0-F2), patients with advanced liver fibrosis (F3-F4) had almost a threefold increase in AFib recurrence (82% vs. 31%; P = .003).
“Cardiologists should make an effort to risk-stratify NAFLD patients either by NAFLD-FS or [an] alternative option, such as transient elastography or MR elastography, given these observations, rather than viewing it as either present or absence [sic] and involve expert multidisciplinary team care early in the clinical course of NAFLD patients with evidence of advanced fibrosis,” Dr. Donnellan and colleagues wrote.
Coauthor Thomas G. Cotter, MD, department of gastroenterology and hepatology, University of Chicago, said in an interview that cardiologists could use just the NAFLD-FS as part of an algorithm for an AFib.
“Because if it shows low risk, then it’s very, very likely the patient will be fine,” he said. “To use more advanced noninvasive testing, there are subtleties in the interpretation that would require referral to a liver doctor or a gastroenterologist and the cost of referring might bulk up the costs. But the NAFLD-FS is freely available and is a validated tool.”
Although it hasn’t specifically been validated in patients with AFib, the NAFLD-FS has been shown to correlate with the development of coronary artery disease (CAD) and was recommended for clinical use in U.S. multisociety guidelines for NAFLD.
The score is calculated using six readily available clinical variables (age, BMI, hyperglycemia or diabetes, AST/ALT, platelets, and albumin). It does not include family history or alcohol consumption, which should be carefully detailed given the large overlap between NAFLD and alcohol-related liver disease, Dr. Cotter observed.
Of note, the study excluded patients with alcohol consumption of more than 30 g/day in men and more than 20 g/day in women, chronic viral hepatitis, Wilson’s disease, and hereditary hemochromatosis.
Finally, CT imaging revealed that epicardial fat volume (EFV) was greater in patients with NAFLD than in those without NAFLD (248 vs. 223 mL; P = .01).
Although increased amounts of epicardial fat have been associated with CAD, there was no significant difference in EFV between patients who did and did not develop recurrent arrhythmia (238 vs. 229 mL; P = .5). Nor was EFV associated with arrhythmia recurrence on Cox proportional hazards analysis (HR, 1.001; P = .17).
“We hypothesized that the increased risk of arrhythmia recurrence may be mediated in part by an increased epicardial fat volume,” Dr. Donnellan said. “The existing literature exploring the link between epicardial fat volume and A[Fib] burden and recurrence is conflicting. But in both this study and our bariatric surgery study, epicardial fat volume was not a significant predictor of arrhythmia recurrence on multivariable analysis.”
It’s likely that the increased recurrence risk is caused by several mechanisms, including NAFLD’s deleterious impact on cardiac structure and function, the bidirectional relationship between NAFLD and sleep apnea, and transcription of proinflammatory cytokines and low-grade systemic inflammation, he suggested.
“Patients with NAFLD represent a particularly high-risk population for arrhythmia recurrence. NAFLD is a reversible disease, and a multidisciplinary approach incorporating dietary and lifestyle interventions should by instituted prior to ablation,” Dr. Donnellan and colleagues concluded.
They noted that serial abdominal imaging to assess for preablation changes in NAFLD was limited in patients and that only 56% of control subjects underwent dedicated abdominal imaging to rule out hepatic steatosis. Also, the heterogeneity of imaging modalities used to diagnose NAFLD may have influenced the results and the study’s single-center, retrospective design limits their generalizability.
The authors reported having no relevant financial relationships.
Help your patients better understand their risk of NASH and NAFLD by sharing AGA patient education content at http://ow.ly/ZKi930r50am.
A version of this article originally appeared on Medscape.com.
Hepatitis screening now for all patients with cancer on therapy
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
Study finds no link between platelet count, surgery bleed risk in cirrhosis
The findings raise questions about current recommendations that call for transfusing platelet concentrates to reduce bleeding risk during surgery in cirrhosis patients with extremely low platelet counts, Gian Marco Podda, MD, PhD, said at the International Society on Thrombosis and Haemostasis virtual congress.
The overall rate of perioperative bleeding was 8.9% in 996 patients who underwent excision of hepatocellular carcinoma by resection (42%) or radiofrequency ablation (58%) without platelet transfusion between 1998 and 2018. The rates were slightly higher among 65 patients with platelet count of fewer than 50 × 109/L indicating severe thrombocytopenia, and in 292 patients with counts of 50-100 × 109/L, indicating moderate thrombocytopenia (10.8% and 10.2%, respectively), compared with those with a platelet count of higher than 100 × 109/L (8.1%), but the differences were not statistically significant, said Dr. Podda of the University of Milan (Italy).
The corresponding rates among those who underwent radiofrequency ablation were 8.6%, 5.9%, and 5%, and among those who underwent resection, they were 18.8%, 17.7%, and 15.9%.
On multivariate analysis, factors associated with an increased incidence of major bleeding were low hemoglobin level (odds ratio, 0.57), age over 65 years (OR, 1.19), aspartate aminotransferase level greater than twice the upper limit of normal (OR, 2.12), hepatitis B or C cirrhosis versus cryptogenic cirrhosis (OR, 0.08), and resection versus radiofrequency ablation (OR, 3.74), he noted. Logistic regression analysis showed no significant association between platelet count and major bleeding events.
Mortality, a secondary outcome measure, was significantly higher among those with moderate or severe thrombocytopenia (rate of 5.5% for each), compared with those with mild or no thrombocytopenia (2.4%), Dr. Podda said.
Factors associated with mortality on multivariate analysis were severe liver dysfunction as demonstrated by Model for End-Stage Liver Disease score of 10 or greater versus less than 10 (OR, 3.13) and Child-Pugh B and C score versus Child-Pugh A score (OR, 16.72), advanced tumor status as measured by Barcelona-Clínic Liver Cancer staging greater than A4 versus A1 (OR, 5.78), major bleeding (OR, 4.59), and resection versus radiofrequency ablation (OR, 3.31).
“Low platelet count was associated with an increased risk of mortality at 3 months. However, this association disappeared at the multivariate analysis, which took into account markers of severity of liver cirrhosis,” he said.
Dr. Podda and his colleagues conducted the study in light of a recommendation from a consensus conference of the Italian Association for the Study of Liver Disease and the Italian Society of Internal Medicine that called for increasing platelet count by platelet transfusions in patients with cirrhosis who undergo an invasive procedure and who have a platelet count lower than 50 × 109/L.
“This recommendation mostly stemmed from consideration of biological plausibility prospects rather than being based on hard experimental evidence,” he explained, noting that such severe thrombocytopenia affects about 10% of patients with liver cirrhosis.
Based on the findings of this study, the practice is not supported, he concluded.
Dr. Podda reported honoraria from Sanofi, Boehringer Ingelheim.
SOURCE: Ronca V et al. ISTH 2020, Abstract OC 13.4.
The findings raise questions about current recommendations that call for transfusing platelet concentrates to reduce bleeding risk during surgery in cirrhosis patients with extremely low platelet counts, Gian Marco Podda, MD, PhD, said at the International Society on Thrombosis and Haemostasis virtual congress.
The overall rate of perioperative bleeding was 8.9% in 996 patients who underwent excision of hepatocellular carcinoma by resection (42%) or radiofrequency ablation (58%) without platelet transfusion between 1998 and 2018. The rates were slightly higher among 65 patients with platelet count of fewer than 50 × 109/L indicating severe thrombocytopenia, and in 292 patients with counts of 50-100 × 109/L, indicating moderate thrombocytopenia (10.8% and 10.2%, respectively), compared with those with a platelet count of higher than 100 × 109/L (8.1%), but the differences were not statistically significant, said Dr. Podda of the University of Milan (Italy).
The corresponding rates among those who underwent radiofrequency ablation were 8.6%, 5.9%, and 5%, and among those who underwent resection, they were 18.8%, 17.7%, and 15.9%.
On multivariate analysis, factors associated with an increased incidence of major bleeding were low hemoglobin level (odds ratio, 0.57), age over 65 years (OR, 1.19), aspartate aminotransferase level greater than twice the upper limit of normal (OR, 2.12), hepatitis B or C cirrhosis versus cryptogenic cirrhosis (OR, 0.08), and resection versus radiofrequency ablation (OR, 3.74), he noted. Logistic regression analysis showed no significant association between platelet count and major bleeding events.
Mortality, a secondary outcome measure, was significantly higher among those with moderate or severe thrombocytopenia (rate of 5.5% for each), compared with those with mild or no thrombocytopenia (2.4%), Dr. Podda said.
Factors associated with mortality on multivariate analysis were severe liver dysfunction as demonstrated by Model for End-Stage Liver Disease score of 10 or greater versus less than 10 (OR, 3.13) and Child-Pugh B and C score versus Child-Pugh A score (OR, 16.72), advanced tumor status as measured by Barcelona-Clínic Liver Cancer staging greater than A4 versus A1 (OR, 5.78), major bleeding (OR, 4.59), and resection versus radiofrequency ablation (OR, 3.31).
“Low platelet count was associated with an increased risk of mortality at 3 months. However, this association disappeared at the multivariate analysis, which took into account markers of severity of liver cirrhosis,” he said.
Dr. Podda and his colleagues conducted the study in light of a recommendation from a consensus conference of the Italian Association for the Study of Liver Disease and the Italian Society of Internal Medicine that called for increasing platelet count by platelet transfusions in patients with cirrhosis who undergo an invasive procedure and who have a platelet count lower than 50 × 109/L.
“This recommendation mostly stemmed from consideration of biological plausibility prospects rather than being based on hard experimental evidence,” he explained, noting that such severe thrombocytopenia affects about 10% of patients with liver cirrhosis.
Based on the findings of this study, the practice is not supported, he concluded.
Dr. Podda reported honoraria from Sanofi, Boehringer Ingelheim.
SOURCE: Ronca V et al. ISTH 2020, Abstract OC 13.4.
The findings raise questions about current recommendations that call for transfusing platelet concentrates to reduce bleeding risk during surgery in cirrhosis patients with extremely low platelet counts, Gian Marco Podda, MD, PhD, said at the International Society on Thrombosis and Haemostasis virtual congress.
The overall rate of perioperative bleeding was 8.9% in 996 patients who underwent excision of hepatocellular carcinoma by resection (42%) or radiofrequency ablation (58%) without platelet transfusion between 1998 and 2018. The rates were slightly higher among 65 patients with platelet count of fewer than 50 × 109/L indicating severe thrombocytopenia, and in 292 patients with counts of 50-100 × 109/L, indicating moderate thrombocytopenia (10.8% and 10.2%, respectively), compared with those with a platelet count of higher than 100 × 109/L (8.1%), but the differences were not statistically significant, said Dr. Podda of the University of Milan (Italy).
The corresponding rates among those who underwent radiofrequency ablation were 8.6%, 5.9%, and 5%, and among those who underwent resection, they were 18.8%, 17.7%, and 15.9%.
On multivariate analysis, factors associated with an increased incidence of major bleeding were low hemoglobin level (odds ratio, 0.57), age over 65 years (OR, 1.19), aspartate aminotransferase level greater than twice the upper limit of normal (OR, 2.12), hepatitis B or C cirrhosis versus cryptogenic cirrhosis (OR, 0.08), and resection versus radiofrequency ablation (OR, 3.74), he noted. Logistic regression analysis showed no significant association between platelet count and major bleeding events.
Mortality, a secondary outcome measure, was significantly higher among those with moderate or severe thrombocytopenia (rate of 5.5% for each), compared with those with mild or no thrombocytopenia (2.4%), Dr. Podda said.
Factors associated with mortality on multivariate analysis were severe liver dysfunction as demonstrated by Model for End-Stage Liver Disease score of 10 or greater versus less than 10 (OR, 3.13) and Child-Pugh B and C score versus Child-Pugh A score (OR, 16.72), advanced tumor status as measured by Barcelona-Clínic Liver Cancer staging greater than A4 versus A1 (OR, 5.78), major bleeding (OR, 4.59), and resection versus radiofrequency ablation (OR, 3.31).
“Low platelet count was associated with an increased risk of mortality at 3 months. However, this association disappeared at the multivariate analysis, which took into account markers of severity of liver cirrhosis,” he said.
Dr. Podda and his colleagues conducted the study in light of a recommendation from a consensus conference of the Italian Association for the Study of Liver Disease and the Italian Society of Internal Medicine that called for increasing platelet count by platelet transfusions in patients with cirrhosis who undergo an invasive procedure and who have a platelet count lower than 50 × 109/L.
“This recommendation mostly stemmed from consideration of biological plausibility prospects rather than being based on hard experimental evidence,” he explained, noting that such severe thrombocytopenia affects about 10% of patients with liver cirrhosis.
Based on the findings of this study, the practice is not supported, he concluded.
Dr. Podda reported honoraria from Sanofi, Boehringer Ingelheim.
SOURCE: Ronca V et al. ISTH 2020, Abstract OC 13.4.
REPORTING FROM THE 2020 ISTH CONGRESS
Anti-CD8a, anti-IL-17A antibodies improved immune disruption in mice with history of NASH
Changes in a variety of T cells in the liver and visceral adipose tissue play a key role in the pathogenesis of nonalcoholic steatohepatitis, according to the results of a murine study.
Mikhaïl A. Van Herck, of the University of Antwerp (Belgium), and associates fed 8-week old mice a high-fat, high-fructose diet for 20 weeks, and then switched the mice to standard mouse chow for 12 weeks. The high-fat, high-fructose diet induced the metabolic syndrome and nonalcoholic steatohepatitis (NASH), accompanied by shifts in T cells. Interleukin-17–producing (Th17 cells increased in the liver, visceral adipose tissue, and blood, while regulatory T cells decreased in visceral adipose tissue, and cytotoxic T (Tc) cells rose in visceral adipose tissue while dropping in the blood and spleen.
These are “important immune disruptions,” the researchers wrote in Cellular and Molecular Gastroenterology and Hepatology. “In particular, visceral adipose tissue Tc cells are critically involved in NASH pathogenesis, linking adipose tissue inflammation to liver disease.”
After the mice were switched from the high-fat, high-fructose diet to standard mouse chow, their body weight, body fat, and plasma cholesterol significantly decreased and their glucose tolerance and insulin sensitivity improved to resemble the metrics of mice fed standard mouse chow throughout the study. Mice who underwent diet reversal also had significantly decreased liver weight and levels of plasma ALT, compared with mice that remained on the high-fat, high-fructose diet. Diet reversal also improved liver histology (nonalcoholic fatty liver disease activity scores), compared with the high-fat, high-fructose diet, the researchers wrote. “Importantly, the NASH was not significantly different between diet-reversal mice and mice fed the control diet for 32 weeks.”
Genetic tests supported these findings. On multiplex RNA analysis, hepatic expression of Acta2, Col1a1, and Col1a3 reverted to normal with diet reversal, indicating a normalization of hepatic collagen. Hepatic expression of the metabolic genes Ppara, Pparg, and Fgf21 also returned to normal, while visceral adipose tissue showed a decrease in Lep and Fgf21 expression and resolution of adipocyte hypertrophy.
However, diet reversal did not reverse inflammatory changes in T-cell subsets. Administering anti-CD8a antibodies after diet reversal decreased Tc cells in all tissue types that were tested, signifying “a biochemical and histologic attenuation of the high-fat, high-fructose diet-induced NASH,” the investigators wrote. Treating the mice with antibodies targeting IL-17A did not attenuate NASH but did reduce hepatic inflammation.
The fact that “the most pronounced effect” on NASH resulted from correcting immune disruption in visceral adipose tissue underscored “the immense importance of adipose tissue inflammation in [NASH] pathogenesis,” the researchers wrote. The finding that diet reversal alone did not reverse inflammation in hepatic or visceral adipose tissue “challeng[es] our current understanding of the reversibility of NASH and other obesity-related conditions.” They called for studies of underlying mechanisms as part of “the search for a medical treatment for NASH.”
Funders included the University Research Fund, University of Antwerp, and Research Foundation Flanders. The researchers reported having no conflicts of interest except that one coinvestigator is the chief science officer at Biocellvia, which performed some histologic analyses.
SOURCE: Van Herck MA et al. Cell Molec Gastroenterol Hepatol. 2020 Apr 20. doi: 10.1016/j.jcmgh.2020.04.010.
The trajectory of nonalcoholic steatohepatitis (NASH) is a public health watershed moment in gastroenterology and hepatology causing unparalleled morbidity, mortality, and societal costs. This study by Van Herck et al. advances our understanding of just how important a two-pronged environmental and biologic approach is to turn the NASH tide. The authors demonstrate that both dietary environmental exposure and biologic tissue-specific T-cell responses are involved in NASH pathogenesis, and that targeting one part of the equation is insufficient to fully mitigate disease. They observed that mice with more severe diet-induced NASH had more Th17 cells in the liver and visceral adipose tissue and more cytotoxic T cells in VAT. Conversely, there were fewer VAT T-regulatory cells in mice with more liver inflammation. The major novelty of this study is that simply changing the diet to a metabolically healthier and weight-reducing diet failed to correct T-cell dysregulation. Only T cell–directed therapies improved this abnormality.
Rotonya M. Carr, MD, is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia. She is a hepatologist, director of the liver metabolism and fatty liver program, and codirector of the human metabolic tissue resource. Dr. Carr receives research and salary support from Intercept Pharmaceuticals.
The trajectory of nonalcoholic steatohepatitis (NASH) is a public health watershed moment in gastroenterology and hepatology causing unparalleled morbidity, mortality, and societal costs. This study by Van Herck et al. advances our understanding of just how important a two-pronged environmental and biologic approach is to turn the NASH tide. The authors demonstrate that both dietary environmental exposure and biologic tissue-specific T-cell responses are involved in NASH pathogenesis, and that targeting one part of the equation is insufficient to fully mitigate disease. They observed that mice with more severe diet-induced NASH had more Th17 cells in the liver and visceral adipose tissue and more cytotoxic T cells in VAT. Conversely, there were fewer VAT T-regulatory cells in mice with more liver inflammation. The major novelty of this study is that simply changing the diet to a metabolically healthier and weight-reducing diet failed to correct T-cell dysregulation. Only T cell–directed therapies improved this abnormality.
Rotonya M. Carr, MD, is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia. She is a hepatologist, director of the liver metabolism and fatty liver program, and codirector of the human metabolic tissue resource. Dr. Carr receives research and salary support from Intercept Pharmaceuticals.
The trajectory of nonalcoholic steatohepatitis (NASH) is a public health watershed moment in gastroenterology and hepatology causing unparalleled morbidity, mortality, and societal costs. This study by Van Herck et al. advances our understanding of just how important a two-pronged environmental and biologic approach is to turn the NASH tide. The authors demonstrate that both dietary environmental exposure and biologic tissue-specific T-cell responses are involved in NASH pathogenesis, and that targeting one part of the equation is insufficient to fully mitigate disease. They observed that mice with more severe diet-induced NASH had more Th17 cells in the liver and visceral adipose tissue and more cytotoxic T cells in VAT. Conversely, there were fewer VAT T-regulatory cells in mice with more liver inflammation. The major novelty of this study is that simply changing the diet to a metabolically healthier and weight-reducing diet failed to correct T-cell dysregulation. Only T cell–directed therapies improved this abnormality.
Rotonya M. Carr, MD, is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia. She is a hepatologist, director of the liver metabolism and fatty liver program, and codirector of the human metabolic tissue resource. Dr. Carr receives research and salary support from Intercept Pharmaceuticals.
Changes in a variety of T cells in the liver and visceral adipose tissue play a key role in the pathogenesis of nonalcoholic steatohepatitis, according to the results of a murine study.
Mikhaïl A. Van Herck, of the University of Antwerp (Belgium), and associates fed 8-week old mice a high-fat, high-fructose diet for 20 weeks, and then switched the mice to standard mouse chow for 12 weeks. The high-fat, high-fructose diet induced the metabolic syndrome and nonalcoholic steatohepatitis (NASH), accompanied by shifts in T cells. Interleukin-17–producing (Th17 cells increased in the liver, visceral adipose tissue, and blood, while regulatory T cells decreased in visceral adipose tissue, and cytotoxic T (Tc) cells rose in visceral adipose tissue while dropping in the blood and spleen.
These are “important immune disruptions,” the researchers wrote in Cellular and Molecular Gastroenterology and Hepatology. “In particular, visceral adipose tissue Tc cells are critically involved in NASH pathogenesis, linking adipose tissue inflammation to liver disease.”
After the mice were switched from the high-fat, high-fructose diet to standard mouse chow, their body weight, body fat, and plasma cholesterol significantly decreased and their glucose tolerance and insulin sensitivity improved to resemble the metrics of mice fed standard mouse chow throughout the study. Mice who underwent diet reversal also had significantly decreased liver weight and levels of plasma ALT, compared with mice that remained on the high-fat, high-fructose diet. Diet reversal also improved liver histology (nonalcoholic fatty liver disease activity scores), compared with the high-fat, high-fructose diet, the researchers wrote. “Importantly, the NASH was not significantly different between diet-reversal mice and mice fed the control diet for 32 weeks.”
Genetic tests supported these findings. On multiplex RNA analysis, hepatic expression of Acta2, Col1a1, and Col1a3 reverted to normal with diet reversal, indicating a normalization of hepatic collagen. Hepatic expression of the metabolic genes Ppara, Pparg, and Fgf21 also returned to normal, while visceral adipose tissue showed a decrease in Lep and Fgf21 expression and resolution of adipocyte hypertrophy.
However, diet reversal did not reverse inflammatory changes in T-cell subsets. Administering anti-CD8a antibodies after diet reversal decreased Tc cells in all tissue types that were tested, signifying “a biochemical and histologic attenuation of the high-fat, high-fructose diet-induced NASH,” the investigators wrote. Treating the mice with antibodies targeting IL-17A did not attenuate NASH but did reduce hepatic inflammation.
The fact that “the most pronounced effect” on NASH resulted from correcting immune disruption in visceral adipose tissue underscored “the immense importance of adipose tissue inflammation in [NASH] pathogenesis,” the researchers wrote. The finding that diet reversal alone did not reverse inflammation in hepatic or visceral adipose tissue “challeng[es] our current understanding of the reversibility of NASH and other obesity-related conditions.” They called for studies of underlying mechanisms as part of “the search for a medical treatment for NASH.”
Funders included the University Research Fund, University of Antwerp, and Research Foundation Flanders. The researchers reported having no conflicts of interest except that one coinvestigator is the chief science officer at Biocellvia, which performed some histologic analyses.
SOURCE: Van Herck MA et al. Cell Molec Gastroenterol Hepatol. 2020 Apr 20. doi: 10.1016/j.jcmgh.2020.04.010.
Changes in a variety of T cells in the liver and visceral adipose tissue play a key role in the pathogenesis of nonalcoholic steatohepatitis, according to the results of a murine study.
Mikhaïl A. Van Herck, of the University of Antwerp (Belgium), and associates fed 8-week old mice a high-fat, high-fructose diet for 20 weeks, and then switched the mice to standard mouse chow for 12 weeks. The high-fat, high-fructose diet induced the metabolic syndrome and nonalcoholic steatohepatitis (NASH), accompanied by shifts in T cells. Interleukin-17–producing (Th17 cells increased in the liver, visceral adipose tissue, and blood, while regulatory T cells decreased in visceral adipose tissue, and cytotoxic T (Tc) cells rose in visceral adipose tissue while dropping in the blood and spleen.
These are “important immune disruptions,” the researchers wrote in Cellular and Molecular Gastroenterology and Hepatology. “In particular, visceral adipose tissue Tc cells are critically involved in NASH pathogenesis, linking adipose tissue inflammation to liver disease.”
After the mice were switched from the high-fat, high-fructose diet to standard mouse chow, their body weight, body fat, and plasma cholesterol significantly decreased and their glucose tolerance and insulin sensitivity improved to resemble the metrics of mice fed standard mouse chow throughout the study. Mice who underwent diet reversal also had significantly decreased liver weight and levels of plasma ALT, compared with mice that remained on the high-fat, high-fructose diet. Diet reversal also improved liver histology (nonalcoholic fatty liver disease activity scores), compared with the high-fat, high-fructose diet, the researchers wrote. “Importantly, the NASH was not significantly different between diet-reversal mice and mice fed the control diet for 32 weeks.”
Genetic tests supported these findings. On multiplex RNA analysis, hepatic expression of Acta2, Col1a1, and Col1a3 reverted to normal with diet reversal, indicating a normalization of hepatic collagen. Hepatic expression of the metabolic genes Ppara, Pparg, and Fgf21 also returned to normal, while visceral adipose tissue showed a decrease in Lep and Fgf21 expression and resolution of adipocyte hypertrophy.
However, diet reversal did not reverse inflammatory changes in T-cell subsets. Administering anti-CD8a antibodies after diet reversal decreased Tc cells in all tissue types that were tested, signifying “a biochemical and histologic attenuation of the high-fat, high-fructose diet-induced NASH,” the investigators wrote. Treating the mice with antibodies targeting IL-17A did not attenuate NASH but did reduce hepatic inflammation.
The fact that “the most pronounced effect” on NASH resulted from correcting immune disruption in visceral adipose tissue underscored “the immense importance of adipose tissue inflammation in [NASH] pathogenesis,” the researchers wrote. The finding that diet reversal alone did not reverse inflammation in hepatic or visceral adipose tissue “challeng[es] our current understanding of the reversibility of NASH and other obesity-related conditions.” They called for studies of underlying mechanisms as part of “the search for a medical treatment for NASH.”
Funders included the University Research Fund, University of Antwerp, and Research Foundation Flanders. The researchers reported having no conflicts of interest except that one coinvestigator is the chief science officer at Biocellvia, which performed some histologic analyses.
SOURCE: Van Herck MA et al. Cell Molec Gastroenterol Hepatol. 2020 Apr 20. doi: 10.1016/j.jcmgh.2020.04.010.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Studies eyes risks for poor outcomes in primary sclerosing cholangitis
In individuals with inflammatory bowel disease and primary sclerosing cholangitis, younger age at diagnosis, male sex, and Afro-Caribbean heritage were significant risk factors for liver transplantation and disease-related death, based on a 10-year prospective population-based study.
These factors should be incorporated into the design of clinical trials, models for predicting disease, and studies of prognostic biomarkers for primary sclerosing cholangitis, Palak T. Trivedi, MBBS, MRCP, of the Universty of Birmingham (England) wrote with his associates in Gastroenterology.
The researchers identified newly diagnosed cases from a national health care registry in England between 2006 and 2016 (data on outcomes were collected through mid-2019). In all, 284,560 individuals had a new diagnosis of inflammatory bowel disease, among whom 2,588 also had primary sclerosing cholangitis. The investigators tracked deaths, liver transplantation, colonic resection, cholecystectomy, and diagnoses of colorectal cancer, cholangiosarcoma, and cancers of the pancreas, gallbladder, and liver. They evaluated rates of these outcomes among individuals with both primary sclerosing cholangitis and inflammatory bowel disease (PSC-IBD) and those with IBD only.
After controlling for sex, race, socioeconomic level, comorbidities, and older age, the researchers found that both men and women with PSC-IBD had a significantly greater risk for all-cause mortality, compared with individuals with IBD alone (hazard ratio, 3.20; 95% confidence interval, 3.01-3.40; P less than .001). Strikingly, individuals who were diagnosed with PSC when they were younger than 40 years had a more than sevenfold higher rate of all-cause mortality, compared with individuals with IBD only. In contrast, the incidence rate ratio for individuals diagnosed with PSC when they were older than 60 years was less than 1.5, compared with IBD-only individuals.
Having PSC and ulcerative colitis, being younger when diagnosed with PSC, and being of Afro-Carribean heritage all correlated with higher incidence of liver transplantation or death related to PSC. Individuals with PSC-IBD who were of Afro-Caribbean heritage had an approximately twofold greater risk for liver transplantation or PSC-related death compared with Whites (adjusted HR, 2.05; 95% CI, 1.14-3.70; P = .016). In contrast, women with PSC-IBD were at significantly lower risk for liver transplantation or disease-related death than were men (adjusted HR, 0.74; 95% CI, 0.57-0.97; P = .026).
“The onset of PSC confers heightened risks of all hepatobiliary malignancies, although annual imaging surveillance may associate with a reduced risk of cancer-related death,” the investigators found. Among patients with hepatobiliary cancer, annual imaging was associated with a twofold decrease in risk for cancer-related death (HR, 0.43; 95% CI, 0.23-0.80; P = .037).
Colorectal cancer tended to occur at a younger age among individuals with PSC-IBD, compared with those with IBD alone (median ages at diagnosis, 59 vs. 69 years; P less than .001). Notably, individuals with PSC diagnosed under age 50 years had about a fivefold higher incidence of colorectal cancer than did those with IBD alone, while those diagnosed at older ages had only about a twofold increase. With regard to colectomy, men diagnosed with PSC at younger ages were at the greatest risk, compared with women or individuals diagnosed after age 50 years. Individuals with ulcerative colitis and PSC had a 40% greater risk for colectomy risk than did IBD-only individuals (time-dependent adjusted HR, 1.65; 95% CI, 1.45-1.85; P less than .001).
“Whilst all-cause mortality rates increase with age, younger patients [with PSC] show a disproportionately increased incidence of liver transplantation, PSC-related death, and colorectal cancer,” the researchers concluded. “Consideration of age at diagnosis should therefore be applied in the stratification of patients for future clinical trials, disease prediction models, and prognostic biomarker discovery.”
Dr. Trivedi disclosed support from the National Institute for Health Research Birmingham Biomedical Research Centre, at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. No other disclosures were reported.
SOURCE: Trivedi PJ et al. Gastroenterology. 2020 May 19. doi: 10.1053/j.gastro.2020.05.049.
In individuals with inflammatory bowel disease and primary sclerosing cholangitis, younger age at diagnosis, male sex, and Afro-Caribbean heritage were significant risk factors for liver transplantation and disease-related death, based on a 10-year prospective population-based study.
These factors should be incorporated into the design of clinical trials, models for predicting disease, and studies of prognostic biomarkers for primary sclerosing cholangitis, Palak T. Trivedi, MBBS, MRCP, of the Universty of Birmingham (England) wrote with his associates in Gastroenterology.
The researchers identified newly diagnosed cases from a national health care registry in England between 2006 and 2016 (data on outcomes were collected through mid-2019). In all, 284,560 individuals had a new diagnosis of inflammatory bowel disease, among whom 2,588 also had primary sclerosing cholangitis. The investigators tracked deaths, liver transplantation, colonic resection, cholecystectomy, and diagnoses of colorectal cancer, cholangiosarcoma, and cancers of the pancreas, gallbladder, and liver. They evaluated rates of these outcomes among individuals with both primary sclerosing cholangitis and inflammatory bowel disease (PSC-IBD) and those with IBD only.
After controlling for sex, race, socioeconomic level, comorbidities, and older age, the researchers found that both men and women with PSC-IBD had a significantly greater risk for all-cause mortality, compared with individuals with IBD alone (hazard ratio, 3.20; 95% confidence interval, 3.01-3.40; P less than .001). Strikingly, individuals who were diagnosed with PSC when they were younger than 40 years had a more than sevenfold higher rate of all-cause mortality, compared with individuals with IBD only. In contrast, the incidence rate ratio for individuals diagnosed with PSC when they were older than 60 years was less than 1.5, compared with IBD-only individuals.
Having PSC and ulcerative colitis, being younger when diagnosed with PSC, and being of Afro-Carribean heritage all correlated with higher incidence of liver transplantation or death related to PSC. Individuals with PSC-IBD who were of Afro-Caribbean heritage had an approximately twofold greater risk for liver transplantation or PSC-related death compared with Whites (adjusted HR, 2.05; 95% CI, 1.14-3.70; P = .016). In contrast, women with PSC-IBD were at significantly lower risk for liver transplantation or disease-related death than were men (adjusted HR, 0.74; 95% CI, 0.57-0.97; P = .026).
“The onset of PSC confers heightened risks of all hepatobiliary malignancies, although annual imaging surveillance may associate with a reduced risk of cancer-related death,” the investigators found. Among patients with hepatobiliary cancer, annual imaging was associated with a twofold decrease in risk for cancer-related death (HR, 0.43; 95% CI, 0.23-0.80; P = .037).
Colorectal cancer tended to occur at a younger age among individuals with PSC-IBD, compared with those with IBD alone (median ages at diagnosis, 59 vs. 69 years; P less than .001). Notably, individuals with PSC diagnosed under age 50 years had about a fivefold higher incidence of colorectal cancer than did those with IBD alone, while those diagnosed at older ages had only about a twofold increase. With regard to colectomy, men diagnosed with PSC at younger ages were at the greatest risk, compared with women or individuals diagnosed after age 50 years. Individuals with ulcerative colitis and PSC had a 40% greater risk for colectomy risk than did IBD-only individuals (time-dependent adjusted HR, 1.65; 95% CI, 1.45-1.85; P less than .001).
“Whilst all-cause mortality rates increase with age, younger patients [with PSC] show a disproportionately increased incidence of liver transplantation, PSC-related death, and colorectal cancer,” the researchers concluded. “Consideration of age at diagnosis should therefore be applied in the stratification of patients for future clinical trials, disease prediction models, and prognostic biomarker discovery.”
Dr. Trivedi disclosed support from the National Institute for Health Research Birmingham Biomedical Research Centre, at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. No other disclosures were reported.
SOURCE: Trivedi PJ et al. Gastroenterology. 2020 May 19. doi: 10.1053/j.gastro.2020.05.049.
In individuals with inflammatory bowel disease and primary sclerosing cholangitis, younger age at diagnosis, male sex, and Afro-Caribbean heritage were significant risk factors for liver transplantation and disease-related death, based on a 10-year prospective population-based study.
These factors should be incorporated into the design of clinical trials, models for predicting disease, and studies of prognostic biomarkers for primary sclerosing cholangitis, Palak T. Trivedi, MBBS, MRCP, of the Universty of Birmingham (England) wrote with his associates in Gastroenterology.
The researchers identified newly diagnosed cases from a national health care registry in England between 2006 and 2016 (data on outcomes were collected through mid-2019). In all, 284,560 individuals had a new diagnosis of inflammatory bowel disease, among whom 2,588 also had primary sclerosing cholangitis. The investigators tracked deaths, liver transplantation, colonic resection, cholecystectomy, and diagnoses of colorectal cancer, cholangiosarcoma, and cancers of the pancreas, gallbladder, and liver. They evaluated rates of these outcomes among individuals with both primary sclerosing cholangitis and inflammatory bowel disease (PSC-IBD) and those with IBD only.
After controlling for sex, race, socioeconomic level, comorbidities, and older age, the researchers found that both men and women with PSC-IBD had a significantly greater risk for all-cause mortality, compared with individuals with IBD alone (hazard ratio, 3.20; 95% confidence interval, 3.01-3.40; P less than .001). Strikingly, individuals who were diagnosed with PSC when they were younger than 40 years had a more than sevenfold higher rate of all-cause mortality, compared with individuals with IBD only. In contrast, the incidence rate ratio for individuals diagnosed with PSC when they were older than 60 years was less than 1.5, compared with IBD-only individuals.
Having PSC and ulcerative colitis, being younger when diagnosed with PSC, and being of Afro-Carribean heritage all correlated with higher incidence of liver transplantation or death related to PSC. Individuals with PSC-IBD who were of Afro-Caribbean heritage had an approximately twofold greater risk for liver transplantation or PSC-related death compared with Whites (adjusted HR, 2.05; 95% CI, 1.14-3.70; P = .016). In contrast, women with PSC-IBD were at significantly lower risk for liver transplantation or disease-related death than were men (adjusted HR, 0.74; 95% CI, 0.57-0.97; P = .026).
“The onset of PSC confers heightened risks of all hepatobiliary malignancies, although annual imaging surveillance may associate with a reduced risk of cancer-related death,” the investigators found. Among patients with hepatobiliary cancer, annual imaging was associated with a twofold decrease in risk for cancer-related death (HR, 0.43; 95% CI, 0.23-0.80; P = .037).
Colorectal cancer tended to occur at a younger age among individuals with PSC-IBD, compared with those with IBD alone (median ages at diagnosis, 59 vs. 69 years; P less than .001). Notably, individuals with PSC diagnosed under age 50 years had about a fivefold higher incidence of colorectal cancer than did those with IBD alone, while those diagnosed at older ages had only about a twofold increase. With regard to colectomy, men diagnosed with PSC at younger ages were at the greatest risk, compared with women or individuals diagnosed after age 50 years. Individuals with ulcerative colitis and PSC had a 40% greater risk for colectomy risk than did IBD-only individuals (time-dependent adjusted HR, 1.65; 95% CI, 1.45-1.85; P less than .001).
“Whilst all-cause mortality rates increase with age, younger patients [with PSC] show a disproportionately increased incidence of liver transplantation, PSC-related death, and colorectal cancer,” the researchers concluded. “Consideration of age at diagnosis should therefore be applied in the stratification of patients for future clinical trials, disease prediction models, and prognostic biomarker discovery.”
Dr. Trivedi disclosed support from the National Institute for Health Research Birmingham Biomedical Research Centre, at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. No other disclosures were reported.
SOURCE: Trivedi PJ et al. Gastroenterology. 2020 May 19. doi: 10.1053/j.gastro.2020.05.049.
FROM GASTROENTEROLOGY
Model identified heavy drinkers at highest risk of ALD progression
In heavy drinkers with alcohol-related liver disease, a Markov model based on age, sex, body mass index, and duration and extent of alcohol use predicted risk for disease progression, researchers reported in Clinical Gastroenterology and Hepatology.
The study included 2,334 hospitalized adults with consistently abnormal liver test results who had consumed at least 50 grams of alcohol (about 3.5-4 drinks) per day for the previous 5 years. The model was developed using data from 1,599 individuals with baseline liver biopsies and validated in 735 individuals with no baseline liver biopsies but available data on the presence or absence of hepatic decompensation.
For a 40-year-old man with F0-F2 fibrosis who had been drinking alcohol for 15 years, who drank 150 grams of alcohol daily, and who had a body mass index (BMI) of 22 kg/m2, the model predicted a 31.8% likelihood of having a normal liver at baseline, a 61.5% probability of baseline steatosis, and a 6.7% probability of baseline steatohepatitis. In women with the same baseline variables, respective probabilities were 25.1%, 66.5%, and 8.4%. Based on these findings, the 5-year weighted risk for liver complications ranged from 0.2% for men with normal initial liver findings to 10.3% for men with baseline steatohepatitis. Among women, the corresponding risk estimates ranged from 0.5% to 14.7%, wrote PhD student Claire Delacôte of Centre Hospitalier Universitaire de Lille (France), and associates.
“This tool might be used by general practitioners or hepatologists to identify heavy drinkers at high risk for alcohol-related liver disease progression,” the investigators added. “This model might be used to adapt patient care pathways.”
The patients in this study were admitted to the hepatogastroenterology unit of a French hospital between 1982 and 1997. The Markov model incorporated seven stages of alcohol-related liver disease: normal liver (no fibrosis or steatosis), steatosis and F0-F2 fibrosis, alcohol-induced steatohepatitis and F0-F2 fibrosis, steatosis and F3-F4 fibrosis, alcohol-induced steatohepatitis and F3-F4 fibrosis, liver complications without steatohepatitis, and liver complications with alcohol-induced steatohepatitis. Liver complications were defined as hepatocellular carcinoma or liver decompensation (bilirubin >50 mmol/L, gastrointestinal hemorrhage, or ascites). Risk for progressing to liver complications was based on METAVIR score and onset of alcohol-induced steatohepatitis.
The researchers also looked specifically at F3-F4 (severe) fibrosis because of its clinical significance and common use as a study endpoint. Among 40-year-olds with a 15-year history of heavy drinking, the estimated prevalence of alcohol-induced steatohepatitis was 30.0% for men and 33.3% for women. The 5-year risk for liver complications was higher in women (30.1%) than men (24.5%) and was highest among women with baseline alcohol-induced steatohepatitis (41.0%). Overall, women had a 24.8% greater risk for disease progression than men (hazard ratio, 1.248).
Risk for liver complications also increased with age, and each 1-year increase in age at the beginning of heavy drinking heightened the risk for disease progression by 3.8%, regardless of stage of liver disease. “Based on these predictions, 50-year-old women are a high-risk subgroup of [alcohol-related liver] disease progression and should receive close follow-up,” the researchers wrote.
In addition, obese individuals (BMI, 30) had an 11.8% greater risk for progression of alcohol-related liver disease, compared with those with a BMI of 22. Consuming an additional 10 grams of alcohol per day had less impact on risk, the researchers noted.
“If patients are identified as being heavy drinkers by the general practitioner with no evaluation of fibrosis, these patients should be referred to a hepatologist. Nevertheless, we think that the threshold defining the high-risk population, which has been arbitrarily fixed at 5%, should be discussed by experts because it affects the patient’s care pathway. An online application is being developed to help clinicians and general practitioners in their daily practice,” they wrote.
No funding sources were reported. Ms. Delacôte reported having no conflicts of interest. Three coinvestigators disclosed ties to AbbVie, Bayer Healthcare, Eisai, Gilead, MSD, Novartis, Sanofi, and Servier. The others reported having no conflicts.
SOURCE: Delacôte C et al. Clin Gastroenterol Hepatol. 2020 Jan 11. doi: 10.1016/j.cgh.2019.12.041.
In the life of a hepatologist few things are as gratifying as when a patient with alcohol-related liver disease (ALD) quits drinking. Though we wish this were the norm, ALD is both increasingly common and morbid. Tools to connect with and empower real change in our patients with ALD are urgently needed. Unfortunately, our toolbox is somewhat bare.
This is why the Delacôte study is important. The authors create a multistate model with inputs from cohorts of patients with biopsy-proven and staged ALD. The result is a specific 5-year risk of cirrhotic decompensation or hepatocellular carcinoma tailored to the patient’s age, sex, body mass index, alcohol use duration, and liver histology. Although this model’s estimates have confidence intervals and their generalizability would be improved if histology were replaced with noninvasive indices, these data are amongst the most tangible illustrations of risk available for patient-doctor deliberations.
Knowledge, when communicated effectively, is the cornerstone of behavioral change. Translating the abstract concept of progressive ALD into personalized, modifiable risks is a leap forward. We have a new tool, let’s use it.
Elliot B. Tapper, MD, is an assistant professor in gastroenterology and internal medicine at Michigan Medicine, Ann Arbor. He has no conflicts of interest.
In the life of a hepatologist few things are as gratifying as when a patient with alcohol-related liver disease (ALD) quits drinking. Though we wish this were the norm, ALD is both increasingly common and morbid. Tools to connect with and empower real change in our patients with ALD are urgently needed. Unfortunately, our toolbox is somewhat bare.
This is why the Delacôte study is important. The authors create a multistate model with inputs from cohorts of patients with biopsy-proven and staged ALD. The result is a specific 5-year risk of cirrhotic decompensation or hepatocellular carcinoma tailored to the patient’s age, sex, body mass index, alcohol use duration, and liver histology. Although this model’s estimates have confidence intervals and their generalizability would be improved if histology were replaced with noninvasive indices, these data are amongst the most tangible illustrations of risk available for patient-doctor deliberations.
Knowledge, when communicated effectively, is the cornerstone of behavioral change. Translating the abstract concept of progressive ALD into personalized, modifiable risks is a leap forward. We have a new tool, let’s use it.
Elliot B. Tapper, MD, is an assistant professor in gastroenterology and internal medicine at Michigan Medicine, Ann Arbor. He has no conflicts of interest.
In the life of a hepatologist few things are as gratifying as when a patient with alcohol-related liver disease (ALD) quits drinking. Though we wish this were the norm, ALD is both increasingly common and morbid. Tools to connect with and empower real change in our patients with ALD are urgently needed. Unfortunately, our toolbox is somewhat bare.
This is why the Delacôte study is important. The authors create a multistate model with inputs from cohorts of patients with biopsy-proven and staged ALD. The result is a specific 5-year risk of cirrhotic decompensation or hepatocellular carcinoma tailored to the patient’s age, sex, body mass index, alcohol use duration, and liver histology. Although this model’s estimates have confidence intervals and their generalizability would be improved if histology were replaced with noninvasive indices, these data are amongst the most tangible illustrations of risk available for patient-doctor deliberations.
Knowledge, when communicated effectively, is the cornerstone of behavioral change. Translating the abstract concept of progressive ALD into personalized, modifiable risks is a leap forward. We have a new tool, let’s use it.
Elliot B. Tapper, MD, is an assistant professor in gastroenterology and internal medicine at Michigan Medicine, Ann Arbor. He has no conflicts of interest.
In heavy drinkers with alcohol-related liver disease, a Markov model based on age, sex, body mass index, and duration and extent of alcohol use predicted risk for disease progression, researchers reported in Clinical Gastroenterology and Hepatology.
The study included 2,334 hospitalized adults with consistently abnormal liver test results who had consumed at least 50 grams of alcohol (about 3.5-4 drinks) per day for the previous 5 years. The model was developed using data from 1,599 individuals with baseline liver biopsies and validated in 735 individuals with no baseline liver biopsies but available data on the presence or absence of hepatic decompensation.
For a 40-year-old man with F0-F2 fibrosis who had been drinking alcohol for 15 years, who drank 150 grams of alcohol daily, and who had a body mass index (BMI) of 22 kg/m2, the model predicted a 31.8% likelihood of having a normal liver at baseline, a 61.5% probability of baseline steatosis, and a 6.7% probability of baseline steatohepatitis. In women with the same baseline variables, respective probabilities were 25.1%, 66.5%, and 8.4%. Based on these findings, the 5-year weighted risk for liver complications ranged from 0.2% for men with normal initial liver findings to 10.3% for men with baseline steatohepatitis. Among women, the corresponding risk estimates ranged from 0.5% to 14.7%, wrote PhD student Claire Delacôte of Centre Hospitalier Universitaire de Lille (France), and associates.
“This tool might be used by general practitioners or hepatologists to identify heavy drinkers at high risk for alcohol-related liver disease progression,” the investigators added. “This model might be used to adapt patient care pathways.”
The patients in this study were admitted to the hepatogastroenterology unit of a French hospital between 1982 and 1997. The Markov model incorporated seven stages of alcohol-related liver disease: normal liver (no fibrosis or steatosis), steatosis and F0-F2 fibrosis, alcohol-induced steatohepatitis and F0-F2 fibrosis, steatosis and F3-F4 fibrosis, alcohol-induced steatohepatitis and F3-F4 fibrosis, liver complications without steatohepatitis, and liver complications with alcohol-induced steatohepatitis. Liver complications were defined as hepatocellular carcinoma or liver decompensation (bilirubin >50 mmol/L, gastrointestinal hemorrhage, or ascites). Risk for progressing to liver complications was based on METAVIR score and onset of alcohol-induced steatohepatitis.
The researchers also looked specifically at F3-F4 (severe) fibrosis because of its clinical significance and common use as a study endpoint. Among 40-year-olds with a 15-year history of heavy drinking, the estimated prevalence of alcohol-induced steatohepatitis was 30.0% for men and 33.3% for women. The 5-year risk for liver complications was higher in women (30.1%) than men (24.5%) and was highest among women with baseline alcohol-induced steatohepatitis (41.0%). Overall, women had a 24.8% greater risk for disease progression than men (hazard ratio, 1.248).
Risk for liver complications also increased with age, and each 1-year increase in age at the beginning of heavy drinking heightened the risk for disease progression by 3.8%, regardless of stage of liver disease. “Based on these predictions, 50-year-old women are a high-risk subgroup of [alcohol-related liver] disease progression and should receive close follow-up,” the researchers wrote.
In addition, obese individuals (BMI, 30) had an 11.8% greater risk for progression of alcohol-related liver disease, compared with those with a BMI of 22. Consuming an additional 10 grams of alcohol per day had less impact on risk, the researchers noted.
“If patients are identified as being heavy drinkers by the general practitioner with no evaluation of fibrosis, these patients should be referred to a hepatologist. Nevertheless, we think that the threshold defining the high-risk population, which has been arbitrarily fixed at 5%, should be discussed by experts because it affects the patient’s care pathway. An online application is being developed to help clinicians and general practitioners in their daily practice,” they wrote.
No funding sources were reported. Ms. Delacôte reported having no conflicts of interest. Three coinvestigators disclosed ties to AbbVie, Bayer Healthcare, Eisai, Gilead, MSD, Novartis, Sanofi, and Servier. The others reported having no conflicts.
SOURCE: Delacôte C et al. Clin Gastroenterol Hepatol. 2020 Jan 11. doi: 10.1016/j.cgh.2019.12.041.
In heavy drinkers with alcohol-related liver disease, a Markov model based on age, sex, body mass index, and duration and extent of alcohol use predicted risk for disease progression, researchers reported in Clinical Gastroenterology and Hepatology.
The study included 2,334 hospitalized adults with consistently abnormal liver test results who had consumed at least 50 grams of alcohol (about 3.5-4 drinks) per day for the previous 5 years. The model was developed using data from 1,599 individuals with baseline liver biopsies and validated in 735 individuals with no baseline liver biopsies but available data on the presence or absence of hepatic decompensation.
For a 40-year-old man with F0-F2 fibrosis who had been drinking alcohol for 15 years, who drank 150 grams of alcohol daily, and who had a body mass index (BMI) of 22 kg/m2, the model predicted a 31.8% likelihood of having a normal liver at baseline, a 61.5% probability of baseline steatosis, and a 6.7% probability of baseline steatohepatitis. In women with the same baseline variables, respective probabilities were 25.1%, 66.5%, and 8.4%. Based on these findings, the 5-year weighted risk for liver complications ranged from 0.2% for men with normal initial liver findings to 10.3% for men with baseline steatohepatitis. Among women, the corresponding risk estimates ranged from 0.5% to 14.7%, wrote PhD student Claire Delacôte of Centre Hospitalier Universitaire de Lille (France), and associates.
“This tool might be used by general practitioners or hepatologists to identify heavy drinkers at high risk for alcohol-related liver disease progression,” the investigators added. “This model might be used to adapt patient care pathways.”
The patients in this study were admitted to the hepatogastroenterology unit of a French hospital between 1982 and 1997. The Markov model incorporated seven stages of alcohol-related liver disease: normal liver (no fibrosis or steatosis), steatosis and F0-F2 fibrosis, alcohol-induced steatohepatitis and F0-F2 fibrosis, steatosis and F3-F4 fibrosis, alcohol-induced steatohepatitis and F3-F4 fibrosis, liver complications without steatohepatitis, and liver complications with alcohol-induced steatohepatitis. Liver complications were defined as hepatocellular carcinoma or liver decompensation (bilirubin >50 mmol/L, gastrointestinal hemorrhage, or ascites). Risk for progressing to liver complications was based on METAVIR score and onset of alcohol-induced steatohepatitis.
The researchers also looked specifically at F3-F4 (severe) fibrosis because of its clinical significance and common use as a study endpoint. Among 40-year-olds with a 15-year history of heavy drinking, the estimated prevalence of alcohol-induced steatohepatitis was 30.0% for men and 33.3% for women. The 5-year risk for liver complications was higher in women (30.1%) than men (24.5%) and was highest among women with baseline alcohol-induced steatohepatitis (41.0%). Overall, women had a 24.8% greater risk for disease progression than men (hazard ratio, 1.248).
Risk for liver complications also increased with age, and each 1-year increase in age at the beginning of heavy drinking heightened the risk for disease progression by 3.8%, regardless of stage of liver disease. “Based on these predictions, 50-year-old women are a high-risk subgroup of [alcohol-related liver] disease progression and should receive close follow-up,” the researchers wrote.
In addition, obese individuals (BMI, 30) had an 11.8% greater risk for progression of alcohol-related liver disease, compared with those with a BMI of 22. Consuming an additional 10 grams of alcohol per day had less impact on risk, the researchers noted.
“If patients are identified as being heavy drinkers by the general practitioner with no evaluation of fibrosis, these patients should be referred to a hepatologist. Nevertheless, we think that the threshold defining the high-risk population, which has been arbitrarily fixed at 5%, should be discussed by experts because it affects the patient’s care pathway. An online application is being developed to help clinicians and general practitioners in their daily practice,” they wrote.
No funding sources were reported. Ms. Delacôte reported having no conflicts of interest. Three coinvestigators disclosed ties to AbbVie, Bayer Healthcare, Eisai, Gilead, MSD, Novartis, Sanofi, and Servier. The others reported having no conflicts.
SOURCE: Delacôte C et al. Clin Gastroenterol Hepatol. 2020 Jan 11. doi: 10.1016/j.cgh.2019.12.041.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
New CDC guidance for health care personnel exposed to HCV
The new guidance was developed in part as a result of an increase in the incidence of acute HCV infection in the United States, which increases the risk for occupational exposure among HCP. “[I]n certain health care settings, HCP might be exposed to source patients with early HCV infection before those patients develop serologic evidence of infection or symptoms indicative of viral hepatitis,” wrote the authors of the report, published online July 24 in the CDC’s Morbidity and Mortality Weekly Report.
The guidelines, which no longer recommend waiting for spontaneous resolution upon initial diagnosis, include recommendations and algorithms for baseline and follow-up testing, appropriate test type, and recommendations for clinical management. The recommendations were developed on the basis of a current literature review, expert opinion from subject matter experts, and recent guidance from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.
Baseline testing ASAP
Baseline testing of the source patient and the HCP should be performed as soon as possible, preferably within 48 hours of exposure. The source patient should be tested for HCV RNA using a nucleic acid test. Alternatively, screening anti-HCV serology can be performed in patients at low risk for HCV and a nucleic acid test performed if serology is positive.
Baseline testing for the HCP should include anti-HCV testing and, if positive, HCV RNA testing is recommended. HCPs who test positive for HCV RNA at baseline are considered to have a preexisting HCV infection and should be referred for treatment.
Follow-up testing
For HCPs with exposure to blood or body fluids from a patient who is anti-HCV positive but HCV RNA negative, follow-up testing is not required.
If the source patient is HCV RNA positive, or if status of the source patient is unknown, the authors recommend that exposed HCPs have HCV RNA follow-up testing at 3-6 weeks post exposure, in addition to baseline testing. A final anti-HCV test is recommended at 4-6 months post exposure as there can be potential periods of aviremia during acute HCV infection.
Exposed HCPs who develop signs of illness indicative of HCV infection at any time should be tested for HCV RNA.
HCPs with positive HCV RNA test results should be referred for care and curative antiviral therapy.
Postexposure prophylaxis is not recommended
Recent data have shown that the risk for HCV infection from percutaneous exposure is 0.2% and from mucocutaneous exposure is 0%. On the basis of this information, the CDC guidelines no longer recommend routine postexposure prophylaxis for HCPs with occupational exposure to HCV. Rather, curative antiviral regimens should be reserved for instances of documented HCV transmission.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The new guidance was developed in part as a result of an increase in the incidence of acute HCV infection in the United States, which increases the risk for occupational exposure among HCP. “[I]n certain health care settings, HCP might be exposed to source patients with early HCV infection before those patients develop serologic evidence of infection or symptoms indicative of viral hepatitis,” wrote the authors of the report, published online July 24 in the CDC’s Morbidity and Mortality Weekly Report.
The guidelines, which no longer recommend waiting for spontaneous resolution upon initial diagnosis, include recommendations and algorithms for baseline and follow-up testing, appropriate test type, and recommendations for clinical management. The recommendations were developed on the basis of a current literature review, expert opinion from subject matter experts, and recent guidance from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.
Baseline testing ASAP
Baseline testing of the source patient and the HCP should be performed as soon as possible, preferably within 48 hours of exposure. The source patient should be tested for HCV RNA using a nucleic acid test. Alternatively, screening anti-HCV serology can be performed in patients at low risk for HCV and a nucleic acid test performed if serology is positive.
Baseline testing for the HCP should include anti-HCV testing and, if positive, HCV RNA testing is recommended. HCPs who test positive for HCV RNA at baseline are considered to have a preexisting HCV infection and should be referred for treatment.
Follow-up testing
For HCPs with exposure to blood or body fluids from a patient who is anti-HCV positive but HCV RNA negative, follow-up testing is not required.
If the source patient is HCV RNA positive, or if status of the source patient is unknown, the authors recommend that exposed HCPs have HCV RNA follow-up testing at 3-6 weeks post exposure, in addition to baseline testing. A final anti-HCV test is recommended at 4-6 months post exposure as there can be potential periods of aviremia during acute HCV infection.
Exposed HCPs who develop signs of illness indicative of HCV infection at any time should be tested for HCV RNA.
HCPs with positive HCV RNA test results should be referred for care and curative antiviral therapy.
Postexposure prophylaxis is not recommended
Recent data have shown that the risk for HCV infection from percutaneous exposure is 0.2% and from mucocutaneous exposure is 0%. On the basis of this information, the CDC guidelines no longer recommend routine postexposure prophylaxis for HCPs with occupational exposure to HCV. Rather, curative antiviral regimens should be reserved for instances of documented HCV transmission.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The new guidance was developed in part as a result of an increase in the incidence of acute HCV infection in the United States, which increases the risk for occupational exposure among HCP. “[I]n certain health care settings, HCP might be exposed to source patients with early HCV infection before those patients develop serologic evidence of infection or symptoms indicative of viral hepatitis,” wrote the authors of the report, published online July 24 in the CDC’s Morbidity and Mortality Weekly Report.
The guidelines, which no longer recommend waiting for spontaneous resolution upon initial diagnosis, include recommendations and algorithms for baseline and follow-up testing, appropriate test type, and recommendations for clinical management. The recommendations were developed on the basis of a current literature review, expert opinion from subject matter experts, and recent guidance from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.
Baseline testing ASAP
Baseline testing of the source patient and the HCP should be performed as soon as possible, preferably within 48 hours of exposure. The source patient should be tested for HCV RNA using a nucleic acid test. Alternatively, screening anti-HCV serology can be performed in patients at low risk for HCV and a nucleic acid test performed if serology is positive.
Baseline testing for the HCP should include anti-HCV testing and, if positive, HCV RNA testing is recommended. HCPs who test positive for HCV RNA at baseline are considered to have a preexisting HCV infection and should be referred for treatment.
Follow-up testing
For HCPs with exposure to blood or body fluids from a patient who is anti-HCV positive but HCV RNA negative, follow-up testing is not required.
If the source patient is HCV RNA positive, or if status of the source patient is unknown, the authors recommend that exposed HCPs have HCV RNA follow-up testing at 3-6 weeks post exposure, in addition to baseline testing. A final anti-HCV test is recommended at 4-6 months post exposure as there can be potential periods of aviremia during acute HCV infection.
Exposed HCPs who develop signs of illness indicative of HCV infection at any time should be tested for HCV RNA.
HCPs with positive HCV RNA test results should be referred for care and curative antiviral therapy.
Postexposure prophylaxis is not recommended
Recent data have shown that the risk for HCV infection from percutaneous exposure is 0.2% and from mucocutaneous exposure is 0%. On the basis of this information, the CDC guidelines no longer recommend routine postexposure prophylaxis for HCPs with occupational exposure to HCV. Rather, curative antiviral regimens should be reserved for instances of documented HCV transmission.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Terlipressin squeaks by FDA review for hepatorenal syndrome 1
The Food and Drug Administration’s Cardiovascular and Renal Drugs Advisory Committee narrowly recommended, by an 8-7 vote, that the agency grant marketing approval to terlipressin for the treatment of hepatorenal syndrome type 1, a severe, rare, and often rapidly lethal disease. No drugs are currently licensed in the United States for this indication.
The advisory committee’s discussion and vote on July 15 showcased the struggle the 15 members faced parsing data that hinted at efficacy but also featured clear flaws and limitations, with meager evidence showing clinically meaningful patient improvements.
Several advisory committee members voiced their dilemma balancing the desperation of patients and clinicians to have an effective agent to treat a frequently fatal condition against spotty evidence of efficacy.
Their uncertainty over benefit was exacerbated by the substantial rate of serious adverse events, compared with placebo. These events included respiratory failure, which occurred an absolute 9% more often among patients treated with terlipressin than among those who received placebo in the drug’s recent pivotal trial, and sepsis and septic shock, with an absolute 7% excess rate with terlipressin in comparison with placebo.
“This is an important, unmet need, and I want this drug, but the data are not clear that the benefits outweigh the risks,” commented Steven F. Solga, MD, a transplant hepatologist at the University of Pennsylvania, Philadelphia, who is a committee member.
“When you have sick patients with few treatment options, you grope for something to use, but I worry that this won’t help patients,” he said when explaining his vote against approval.
“I look forward to using this medication if I could figure out which patients could benefit from it,” he said.
‘Allow patients to decide if they want this treatment’
Experts estimate that the annual incidence of hepatorenal syndrome type 1 in the United States is about 35,000 patients.
“I would have liked to vote yes, because terlipressin was associated with a short-term increase in renal function, but there was also clear evidence for the risk of sepsis and respiratory failure, and no evidence that it improved survival,” said panel member Patrick H. Nachman, MD.
Dr. Nachman, professor of medicine and director of the division of nephrology and hypertension at the University of Minnesota, Minneapolis, voted against approval.
Several who voted in favor of terlipressin also shared these misgivings.
“The trend for benefit was quite small, I’m very worried about respiratory failure, and I’m uncomfortable with the postrandomization analyses” used by the developer of terlipressin (Mallinckrodt) to buttress the efficacy claims, explained panel member Paul M. Ridker, MD.
“So why did I vote yes? The problem is the enormous unmet need. Patients are in desperate shape, and the standard treatments are used off label, with no data. Here, we have data, and the primary endpoint was met,” said Dr. Ridker, who is professor of medicine at Harvard Medical School and director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, both in Boston.
The effects of terlipressin appear to give clinicians a way to “stabilize renal function and buy time,” making it more feasible to try to rush the patient to liver transplantation or at least to “stop their downward spiral» as patients with decompensated liver failure develop inadequate renal blood flow that produces an acute fall in kidney function,” explained David N. Assis, MD, a hepatologist at Yale University, New Haven, Conn.
“The reality is, nothing else is available, aside from renal replacement therapy and pressors. There is a need for a treatment that buys time,” he said. He voted to recommend approval.
That sentiment was notably echoed in comments from the two nonclinical members of the advisory committee.
“This treatment addresses a major gap in care,” said Jacqueline D. Alikhaani, the panel’s consumer representative. “Allow patients to decide if they want this treatment,” said Daniel Bonner, the committee’s patient representative. Both voted in favor of FDA approval.
Terlipressin has been a long-standing linchpin for treating hepatorenal syndrome type 1 in Europe and other places outside the United States and Canada.
The most recent guidelines for managing patients with decompensated cirrhosis from the European Association for the Study of the Liver say that “[t]erlipressin plus albumin should be considered as the first-line therapeutic option for the treatment of hepatorenal syndrome and acute kidney injury” (J Hepatol. 2018 Aug 1;69).
According to company representatives who presented the case for terlipressin during the meeting, bringing the drug onto the U.S. market has been a 17-year journey, featuring three sequential trials.
- A 112-patient that the FDA accepted as the first of the two supportive trials needed for approval.
- A with 196 patients that tested terlipressin plus albumin against placebo plus albumin and showed a nominal benefit from terlipressin that failed to achieve statistical significance.
- The most recent trial, , which directly led to the advisory committee session. That trial enrolled 300 patients and met its primary endpoint. Data have not yet been published but have been at meetings.
One of the sources of controversy over the benefit from terlipressin centered on the primary endpoint used in CONFIRM, which required that the patient have two consecutive, low readings for serum creatinine, with levels no greater than 1.5 mg/dL while on treatment, and remain alive and free from need for renal replacement therapy for at least 10 days beyond this.
The FDA agreed to accept this as a primary endpoint but nonetheless considered it a surrogate.
According to FDA staffers who presented their take on the application, the agency accepted this primary endpoint “with the understanding that favorable trends in clinical outcomes, thought to be predicted by successful treatment of hepatic renal syndrome type 1, would be expected.”
The lack of many favorable trends in clinical outcomes helped foster the advisory committee’s divided response. The FDA’s staff uses its discretion when considering an advisory committee’s recommendations and making a final determination.
None of the advisory committee members disclosed any relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration’s Cardiovascular and Renal Drugs Advisory Committee narrowly recommended, by an 8-7 vote, that the agency grant marketing approval to terlipressin for the treatment of hepatorenal syndrome type 1, a severe, rare, and often rapidly lethal disease. No drugs are currently licensed in the United States for this indication.
The advisory committee’s discussion and vote on July 15 showcased the struggle the 15 members faced parsing data that hinted at efficacy but also featured clear flaws and limitations, with meager evidence showing clinically meaningful patient improvements.
Several advisory committee members voiced their dilemma balancing the desperation of patients and clinicians to have an effective agent to treat a frequently fatal condition against spotty evidence of efficacy.
Their uncertainty over benefit was exacerbated by the substantial rate of serious adverse events, compared with placebo. These events included respiratory failure, which occurred an absolute 9% more often among patients treated with terlipressin than among those who received placebo in the drug’s recent pivotal trial, and sepsis and septic shock, with an absolute 7% excess rate with terlipressin in comparison with placebo.
“This is an important, unmet need, and I want this drug, but the data are not clear that the benefits outweigh the risks,” commented Steven F. Solga, MD, a transplant hepatologist at the University of Pennsylvania, Philadelphia, who is a committee member.
“When you have sick patients with few treatment options, you grope for something to use, but I worry that this won’t help patients,” he said when explaining his vote against approval.
“I look forward to using this medication if I could figure out which patients could benefit from it,” he said.
‘Allow patients to decide if they want this treatment’
Experts estimate that the annual incidence of hepatorenal syndrome type 1 in the United States is about 35,000 patients.
“I would have liked to vote yes, because terlipressin was associated with a short-term increase in renal function, but there was also clear evidence for the risk of sepsis and respiratory failure, and no evidence that it improved survival,” said panel member Patrick H. Nachman, MD.
Dr. Nachman, professor of medicine and director of the division of nephrology and hypertension at the University of Minnesota, Minneapolis, voted against approval.
Several who voted in favor of terlipressin also shared these misgivings.
“The trend for benefit was quite small, I’m very worried about respiratory failure, and I’m uncomfortable with the postrandomization analyses” used by the developer of terlipressin (Mallinckrodt) to buttress the efficacy claims, explained panel member Paul M. Ridker, MD.
“So why did I vote yes? The problem is the enormous unmet need. Patients are in desperate shape, and the standard treatments are used off label, with no data. Here, we have data, and the primary endpoint was met,” said Dr. Ridker, who is professor of medicine at Harvard Medical School and director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, both in Boston.
The effects of terlipressin appear to give clinicians a way to “stabilize renal function and buy time,” making it more feasible to try to rush the patient to liver transplantation or at least to “stop their downward spiral» as patients with decompensated liver failure develop inadequate renal blood flow that produces an acute fall in kidney function,” explained David N. Assis, MD, a hepatologist at Yale University, New Haven, Conn.
“The reality is, nothing else is available, aside from renal replacement therapy and pressors. There is a need for a treatment that buys time,” he said. He voted to recommend approval.
That sentiment was notably echoed in comments from the two nonclinical members of the advisory committee.
“This treatment addresses a major gap in care,” said Jacqueline D. Alikhaani, the panel’s consumer representative. “Allow patients to decide if they want this treatment,” said Daniel Bonner, the committee’s patient representative. Both voted in favor of FDA approval.
Terlipressin has been a long-standing linchpin for treating hepatorenal syndrome type 1 in Europe and other places outside the United States and Canada.
The most recent guidelines for managing patients with decompensated cirrhosis from the European Association for the Study of the Liver say that “[t]erlipressin plus albumin should be considered as the first-line therapeutic option for the treatment of hepatorenal syndrome and acute kidney injury” (J Hepatol. 2018 Aug 1;69).
According to company representatives who presented the case for terlipressin during the meeting, bringing the drug onto the U.S. market has been a 17-year journey, featuring three sequential trials.
- A 112-patient that the FDA accepted as the first of the two supportive trials needed for approval.
- A with 196 patients that tested terlipressin plus albumin against placebo plus albumin and showed a nominal benefit from terlipressin that failed to achieve statistical significance.
- The most recent trial, , which directly led to the advisory committee session. That trial enrolled 300 patients and met its primary endpoint. Data have not yet been published but have been at meetings.
One of the sources of controversy over the benefit from terlipressin centered on the primary endpoint used in CONFIRM, which required that the patient have two consecutive, low readings for serum creatinine, with levels no greater than 1.5 mg/dL while on treatment, and remain alive and free from need for renal replacement therapy for at least 10 days beyond this.
The FDA agreed to accept this as a primary endpoint but nonetheless considered it a surrogate.
According to FDA staffers who presented their take on the application, the agency accepted this primary endpoint “with the understanding that favorable trends in clinical outcomes, thought to be predicted by successful treatment of hepatic renal syndrome type 1, would be expected.”
The lack of many favorable trends in clinical outcomes helped foster the advisory committee’s divided response. The FDA’s staff uses its discretion when considering an advisory committee’s recommendations and making a final determination.
None of the advisory committee members disclosed any relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration’s Cardiovascular and Renal Drugs Advisory Committee narrowly recommended, by an 8-7 vote, that the agency grant marketing approval to terlipressin for the treatment of hepatorenal syndrome type 1, a severe, rare, and often rapidly lethal disease. No drugs are currently licensed in the United States for this indication.
The advisory committee’s discussion and vote on July 15 showcased the struggle the 15 members faced parsing data that hinted at efficacy but also featured clear flaws and limitations, with meager evidence showing clinically meaningful patient improvements.
Several advisory committee members voiced their dilemma balancing the desperation of patients and clinicians to have an effective agent to treat a frequently fatal condition against spotty evidence of efficacy.
Their uncertainty over benefit was exacerbated by the substantial rate of serious adverse events, compared with placebo. These events included respiratory failure, which occurred an absolute 9% more often among patients treated with terlipressin than among those who received placebo in the drug’s recent pivotal trial, and sepsis and septic shock, with an absolute 7% excess rate with terlipressin in comparison with placebo.
“This is an important, unmet need, and I want this drug, but the data are not clear that the benefits outweigh the risks,” commented Steven F. Solga, MD, a transplant hepatologist at the University of Pennsylvania, Philadelphia, who is a committee member.
“When you have sick patients with few treatment options, you grope for something to use, but I worry that this won’t help patients,” he said when explaining his vote against approval.
“I look forward to using this medication if I could figure out which patients could benefit from it,” he said.
‘Allow patients to decide if they want this treatment’
Experts estimate that the annual incidence of hepatorenal syndrome type 1 in the United States is about 35,000 patients.
“I would have liked to vote yes, because terlipressin was associated with a short-term increase in renal function, but there was also clear evidence for the risk of sepsis and respiratory failure, and no evidence that it improved survival,” said panel member Patrick H. Nachman, MD.
Dr. Nachman, professor of medicine and director of the division of nephrology and hypertension at the University of Minnesota, Minneapolis, voted against approval.
Several who voted in favor of terlipressin also shared these misgivings.
“The trend for benefit was quite small, I’m very worried about respiratory failure, and I’m uncomfortable with the postrandomization analyses” used by the developer of terlipressin (Mallinckrodt) to buttress the efficacy claims, explained panel member Paul M. Ridker, MD.
“So why did I vote yes? The problem is the enormous unmet need. Patients are in desperate shape, and the standard treatments are used off label, with no data. Here, we have data, and the primary endpoint was met,” said Dr. Ridker, who is professor of medicine at Harvard Medical School and director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, both in Boston.
The effects of terlipressin appear to give clinicians a way to “stabilize renal function and buy time,” making it more feasible to try to rush the patient to liver transplantation or at least to “stop their downward spiral» as patients with decompensated liver failure develop inadequate renal blood flow that produces an acute fall in kidney function,” explained David N. Assis, MD, a hepatologist at Yale University, New Haven, Conn.
“The reality is, nothing else is available, aside from renal replacement therapy and pressors. There is a need for a treatment that buys time,” he said. He voted to recommend approval.
That sentiment was notably echoed in comments from the two nonclinical members of the advisory committee.
“This treatment addresses a major gap in care,” said Jacqueline D. Alikhaani, the panel’s consumer representative. “Allow patients to decide if they want this treatment,” said Daniel Bonner, the committee’s patient representative. Both voted in favor of FDA approval.
Terlipressin has been a long-standing linchpin for treating hepatorenal syndrome type 1 in Europe and other places outside the United States and Canada.
The most recent guidelines for managing patients with decompensated cirrhosis from the European Association for the Study of the Liver say that “[t]erlipressin plus albumin should be considered as the first-line therapeutic option for the treatment of hepatorenal syndrome and acute kidney injury” (J Hepatol. 2018 Aug 1;69).
According to company representatives who presented the case for terlipressin during the meeting, bringing the drug onto the U.S. market has been a 17-year journey, featuring three sequential trials.
- A 112-patient that the FDA accepted as the first of the two supportive trials needed for approval.
- A with 196 patients that tested terlipressin plus albumin against placebo plus albumin and showed a nominal benefit from terlipressin that failed to achieve statistical significance.
- The most recent trial, , which directly led to the advisory committee session. That trial enrolled 300 patients and met its primary endpoint. Data have not yet been published but have been at meetings.
One of the sources of controversy over the benefit from terlipressin centered on the primary endpoint used in CONFIRM, which required that the patient have two consecutive, low readings for serum creatinine, with levels no greater than 1.5 mg/dL while on treatment, and remain alive and free from need for renal replacement therapy for at least 10 days beyond this.
The FDA agreed to accept this as a primary endpoint but nonetheless considered it a surrogate.
According to FDA staffers who presented their take on the application, the agency accepted this primary endpoint “with the understanding that favorable trends in clinical outcomes, thought to be predicted by successful treatment of hepatic renal syndrome type 1, would be expected.”
The lack of many favorable trends in clinical outcomes helped foster the advisory committee’s divided response. The FDA’s staff uses its discretion when considering an advisory committee’s recommendations and making a final determination.
None of the advisory committee members disclosed any relevant financial relationships.
A version of this article originally appeared on Medscape.com.