User login
Hispanics bear brunt of NAFLD disease burden
WASHINGTON — Significant racial and ethnic disparities exist in nonalcoholic fatty liver disease prevalence and severity in the United States, with Hispanics at highest risk and blacks at the lowest, but the risk of death from NAFLD is highest in whites, according to a meta-analysis of 34 studies presented at the annual meeting of the American Association for the Study of Liver Diseases.
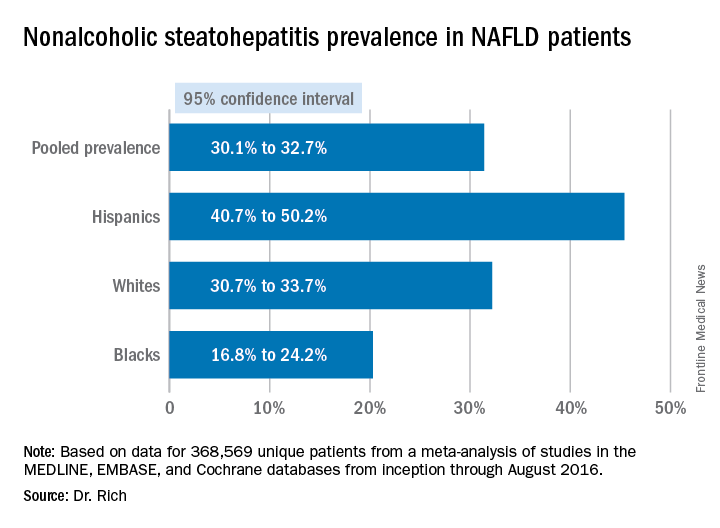
The findings are based on a meta-analysis of studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that included 368,569 unique patients that characterized disparities in NAFLD prevalence, severity, or prognosis, Dr. Rich said.
When the researchers drilled down into the data, they found the disparities dissipated somewhat. “When we looked at the severity of NAFLD we looked at two things: whether there was NASH (nonalcoholic steatohepatitis) present, or if there was the presence of advanced fibrosis,” Dr. Rich said. “We found there was no significant difference in the risk of NASH in Hispanic patients, compared to white patients; however, the proportion of Hispanic NAFLD patients that had NASH was 45.4%, compared to 32.2% in whites.” The pooled relative risk was 1.09.
The meta-analysis tended to attenuate the disparities found in disease severity, compared with the disparities the researchers found in prevalence, Dr. Rich said. “Data are limited and discordant on racial and ethnic differences in NAFLD prognosis and outcomes,” she said. “In the current literature the studies have notable limitations, highlighting the need for future high-quality data in this area, and further studies are needed to determine the need and pathways to reduce NAFLD disparities in the future,” she said.
Dr. Rich had no financial relationships to disclose.
WASHINGTON — Significant racial and ethnic disparities exist in nonalcoholic fatty liver disease prevalence and severity in the United States, with Hispanics at highest risk and blacks at the lowest, but the risk of death from NAFLD is highest in whites, according to a meta-analysis of 34 studies presented at the annual meeting of the American Association for the Study of Liver Diseases.
The findings are based on a meta-analysis of studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that included 368,569 unique patients that characterized disparities in NAFLD prevalence, severity, or prognosis, Dr. Rich said.
When the researchers drilled down into the data, they found the disparities dissipated somewhat. “When we looked at the severity of NAFLD we looked at two things: whether there was NASH (nonalcoholic steatohepatitis) present, or if there was the presence of advanced fibrosis,” Dr. Rich said. “We found there was no significant difference in the risk of NASH in Hispanic patients, compared to white patients; however, the proportion of Hispanic NAFLD patients that had NASH was 45.4%, compared to 32.2% in whites.” The pooled relative risk was 1.09.

The meta-analysis tended to attenuate the disparities found in disease severity, compared with the disparities the researchers found in prevalence, Dr. Rich said. “Data are limited and discordant on racial and ethnic differences in NAFLD prognosis and outcomes,” she said. “In the current literature the studies have notable limitations, highlighting the need for future high-quality data in this area, and further studies are needed to determine the need and pathways to reduce NAFLD disparities in the future,” she said.
Dr. Rich had no financial relationships to disclose.
WASHINGTON — Significant racial and ethnic disparities exist in nonalcoholic fatty liver disease prevalence and severity in the United States, with Hispanics at highest risk and blacks at the lowest, but the risk of death from NAFLD is highest in whites, according to a meta-analysis of 34 studies presented at the annual meeting of the American Association for the Study of Liver Diseases.
The findings are based on a meta-analysis of studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that included 368,569 unique patients that characterized disparities in NAFLD prevalence, severity, or prognosis, Dr. Rich said.
When the researchers drilled down into the data, they found the disparities dissipated somewhat. “When we looked at the severity of NAFLD we looked at two things: whether there was NASH (nonalcoholic steatohepatitis) present, or if there was the presence of advanced fibrosis,” Dr. Rich said. “We found there was no significant difference in the risk of NASH in Hispanic patients, compared to white patients; however, the proportion of Hispanic NAFLD patients that had NASH was 45.4%, compared to 32.2% in whites.” The pooled relative risk was 1.09.

The meta-analysis tended to attenuate the disparities found in disease severity, compared with the disparities the researchers found in prevalence, Dr. Rich said. “Data are limited and discordant on racial and ethnic differences in NAFLD prognosis and outcomes,” she said. “In the current literature the studies have notable limitations, highlighting the need for future high-quality data in this area, and further studies are needed to determine the need and pathways to reduce NAFLD disparities in the future,” she said.
Dr. Rich had no financial relationships to disclose.
AT THE LIVER MEETING 2017
Key clinical point: Hispanics have a much higher incidence of fatty liver disease than other ethnic groups.
Major finding: NAFLD prevalence is highest in Hispanics and lower in blacks, compared with whites, although differences between groups are smaller in high-risk cohorts (range, 47.6%-55.5%) than in population-based cohorts (range, 13.0%-22.9%).
Data source: Meta-analysis of 34 studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that involved 368,569 patients.
Disclosures: Dr. Rich had no financial relationships to disclose.
VIDEO: Metabolic regulator FGF21 improves fibrosis in NASH patients
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
AT THE LIVER MEETING 2017
Middle-aged hepatocellular carcinoma patients increasingly ineligible for transplant
WASHINGTON – Fewer than half of studied hepatocellular carcinoma patients born between 1945 and 1965 were eligible for transplant, despite a 58% increase in HCC rate during the past decade, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases 2017.
This disparity is a cause for concern given that this cohort constitutes nearly 75% of hepatitis C virus (HCV) infections in the United States.
“Understanding hepatocellular carcinoma trends among the 1945-1965 birth cohort is particularly important given the increasing number of chronic liver diseases in that group,” said presenter Ann Robinson, MD, of Highland Hospital, Oakland, Calif.
In a retrospective study, researchers evaluated 38,045 patients born between 1945 and 1965 and who were on the Surveillance, Epidemiology, and End Results (SEER) registry and diagnosed with HCC between 2004 and 2014.
Patients were predominantly male (81.6%), white (50%), insured by Medicare or private insurance (66.2%), and diagnosed with localized tumors (52%).
White and Hispanic patients displayed the largest increase in HCC diagnoses during the study period, growing by 67.6% and 66.1%, respectively, followed by Native American and African American patients, whose HCC diagnoses increased by 61% and 57.2%, respectively.
Overall, 57.2% of patients studied did not meet the Milan criteria, according to Dr. Robinson.
Disparities in patients’ meeting the Milan criteria were apparent once researchers adjusted for patients’ sex, race, insurance status, or cancer subtype.
The largest disparity was seen among patients who were uninsured or on Medicaid, who were half as likely to meet Milan criteria at time of diagnosis, compared with insured patients (odds ratio, less than 0.5; P less than .001).
African Americans also saw lower odds of eligibility for transplantation (OR, less than 0.75; P less than .001), compared with white patients.
While the difference between men and women was statistically significant (OR, 0.875; P = .022), the difference in odds was not as prominent as that of uninsured patients or African American patients was.
These disparities may have to do with a lack of patient knowledge or less frequent screening among these patients, as well as an overall rise in nonalcoholic fatty liver disease, according to Dr. Robinson and her fellow investigators.
“It’s been well documented in prior studies that there is an underutilization of screenings both for one-time hepatitis and baby boomer population, despite recommendations by the CDC [Centers for Disease Control and Prevention]” said Dr. Robinson. Other factors may include whether patients know they should be receive these screenings, whether providers have educated their patients about this, and how much the provider knows about the screening guidelines.
The number of patients who meet the Milan criteria are growing, however, according to investigators. In 2013-2014, 46.3% of baby boomers met the Milan criteria, compared with 36.4% in 2004-2006.
Identifying vulnerabilities within these cohorts and increasing education for both providers and patients will help narrow the gap even further, explained Dr. Robinson.
“Looking at etiology-specific differences to know which populations are not receiving screening, [focusing on] things that can help us communicate this with patients, as well as distribute this information among care providers, and breaking down barriers to treatment,” are all important factors, according to Dr. Robinson.
Investigators were limited by SEER’s exclusion of etiology of HCC and comorbidities. Additionally, the researchers were unaware whether patients were receiving surveillance that was within practice guidelines.
Presenters reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
WASHINGTON – Fewer than half of studied hepatocellular carcinoma patients born between 1945 and 1965 were eligible for transplant, despite a 58% increase in HCC rate during the past decade, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases 2017.
This disparity is a cause for concern given that this cohort constitutes nearly 75% of hepatitis C virus (HCV) infections in the United States.
“Understanding hepatocellular carcinoma trends among the 1945-1965 birth cohort is particularly important given the increasing number of chronic liver diseases in that group,” said presenter Ann Robinson, MD, of Highland Hospital, Oakland, Calif.
In a retrospective study, researchers evaluated 38,045 patients born between 1945 and 1965 and who were on the Surveillance, Epidemiology, and End Results (SEER) registry and diagnosed with HCC between 2004 and 2014.
Patients were predominantly male (81.6%), white (50%), insured by Medicare or private insurance (66.2%), and diagnosed with localized tumors (52%).
White and Hispanic patients displayed the largest increase in HCC diagnoses during the study period, growing by 67.6% and 66.1%, respectively, followed by Native American and African American patients, whose HCC diagnoses increased by 61% and 57.2%, respectively.
Overall, 57.2% of patients studied did not meet the Milan criteria, according to Dr. Robinson.
Disparities in patients’ meeting the Milan criteria were apparent once researchers adjusted for patients’ sex, race, insurance status, or cancer subtype.
The largest disparity was seen among patients who were uninsured or on Medicaid, who were half as likely to meet Milan criteria at time of diagnosis, compared with insured patients (odds ratio, less than 0.5; P less than .001).
African Americans also saw lower odds of eligibility for transplantation (OR, less than 0.75; P less than .001), compared with white patients.
While the difference between men and women was statistically significant (OR, 0.875; P = .022), the difference in odds was not as prominent as that of uninsured patients or African American patients was.
These disparities may have to do with a lack of patient knowledge or less frequent screening among these patients, as well as an overall rise in nonalcoholic fatty liver disease, according to Dr. Robinson and her fellow investigators.
“It’s been well documented in prior studies that there is an underutilization of screenings both for one-time hepatitis and baby boomer population, despite recommendations by the CDC [Centers for Disease Control and Prevention]” said Dr. Robinson. Other factors may include whether patients know they should be receive these screenings, whether providers have educated their patients about this, and how much the provider knows about the screening guidelines.
The number of patients who meet the Milan criteria are growing, however, according to investigators. In 2013-2014, 46.3% of baby boomers met the Milan criteria, compared with 36.4% in 2004-2006.
Identifying vulnerabilities within these cohorts and increasing education for both providers and patients will help narrow the gap even further, explained Dr. Robinson.
“Looking at etiology-specific differences to know which populations are not receiving screening, [focusing on] things that can help us communicate this with patients, as well as distribute this information among care providers, and breaking down barriers to treatment,” are all important factors, according to Dr. Robinson.
Investigators were limited by SEER’s exclusion of etiology of HCC and comorbidities. Additionally, the researchers were unaware whether patients were receiving surveillance that was within practice guidelines.
Presenters reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
WASHINGTON – Fewer than half of studied hepatocellular carcinoma patients born between 1945 and 1965 were eligible for transplant, despite a 58% increase in HCC rate during the past decade, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases 2017.
This disparity is a cause for concern given that this cohort constitutes nearly 75% of hepatitis C virus (HCV) infections in the United States.
“Understanding hepatocellular carcinoma trends among the 1945-1965 birth cohort is particularly important given the increasing number of chronic liver diseases in that group,” said presenter Ann Robinson, MD, of Highland Hospital, Oakland, Calif.
In a retrospective study, researchers evaluated 38,045 patients born between 1945 and 1965 and who were on the Surveillance, Epidemiology, and End Results (SEER) registry and diagnosed with HCC between 2004 and 2014.
Patients were predominantly male (81.6%), white (50%), insured by Medicare or private insurance (66.2%), and diagnosed with localized tumors (52%).
White and Hispanic patients displayed the largest increase in HCC diagnoses during the study period, growing by 67.6% and 66.1%, respectively, followed by Native American and African American patients, whose HCC diagnoses increased by 61% and 57.2%, respectively.
Overall, 57.2% of patients studied did not meet the Milan criteria, according to Dr. Robinson.
Disparities in patients’ meeting the Milan criteria were apparent once researchers adjusted for patients’ sex, race, insurance status, or cancer subtype.
The largest disparity was seen among patients who were uninsured or on Medicaid, who were half as likely to meet Milan criteria at time of diagnosis, compared with insured patients (odds ratio, less than 0.5; P less than .001).
African Americans also saw lower odds of eligibility for transplantation (OR, less than 0.75; P less than .001), compared with white patients.
While the difference between men and women was statistically significant (OR, 0.875; P = .022), the difference in odds was not as prominent as that of uninsured patients or African American patients was.
These disparities may have to do with a lack of patient knowledge or less frequent screening among these patients, as well as an overall rise in nonalcoholic fatty liver disease, according to Dr. Robinson and her fellow investigators.
“It’s been well documented in prior studies that there is an underutilization of screenings both for one-time hepatitis and baby boomer population, despite recommendations by the CDC [Centers for Disease Control and Prevention]” said Dr. Robinson. Other factors may include whether patients know they should be receive these screenings, whether providers have educated their patients about this, and how much the provider knows about the screening guidelines.
The number of patients who meet the Milan criteria are growing, however, according to investigators. In 2013-2014, 46.3% of baby boomers met the Milan criteria, compared with 36.4% in 2004-2006.
Identifying vulnerabilities within these cohorts and increasing education for both providers and patients will help narrow the gap even further, explained Dr. Robinson.
“Looking at etiology-specific differences to know which populations are not receiving screening, [focusing on] things that can help us communicate this with patients, as well as distribute this information among care providers, and breaking down barriers to treatment,” are all important factors, according to Dr. Robinson.
Investigators were limited by SEER’s exclusion of etiology of HCC and comorbidities. Additionally, the researchers were unaware whether patients were receiving surveillance that was within practice guidelines.
Presenters reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: Of HCC patients born between 1945 and 1965, 57.2% did not meet the Milan criteria.
Data source: Retrospective study of 38,045 patients born between 1945 and 1965 who were diagnosed with HCC during 2004-2014 and who were added to the SEER registry.
Disclosures: Presenters reported no relevant financial disclosures.
VIDEO: Liver transplant center competition tied to delisting patients
WASHINGTON – Low market competition among liver transplant centers may affect which patients are considered too sick to transplant, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
With 20% of patients dying while on the transplant wait list, including those who were delisted, understanding the distribution of organs among donor service areas (DSAs) is crucial to lowering mortality during the current organ shortage, according to presenter Yanik Babekov, MD, of Massachusetts General Hospital, Boston.
Investigators studied 3,131 patients who were delisted after being classified as “too sick” from 116 centers in 51 DSAs, between 2002 and 2012.
Researchers used the Herfindahl-Hirschman Index (HHI), which analyzes the market share of each participant to determine the overall level of competition. Measurements on the HHI range between 0 and 1, with 0 being the most competitive and 1 being the least.
Mean delisting Model for End-Stage Liver Disease (MELD) scores considered to be “too sick to transplant” were 26.1, and average HHI among DSAs was 0.46, according to investigators. They found that, for every 1% increase in HHI, the delisting MELD score increased by 0.06, according to a risk-adjustment analysis.
“In other words, more competitive DSAs delist patients for [being] ‘too sick’ at lower MELD scores,” Dr. Babekov explained in a video interview. “Interestingly, race, education, citizenship, and other DSA factors also impacted delisting MELD for ‘too sick.’ ”
While market competition may not be the only factor to explain the phenomenon of patients delisted for being ‘too sick,’ it is important to identify how having more transplant centers in DSAs can help more patients be added to, and stay on, these wait lists, according to investigators.
Dr. Babekov had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
WASHINGTON – Low market competition among liver transplant centers may affect which patients are considered too sick to transplant, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
With 20% of patients dying while on the transplant wait list, including those who were delisted, understanding the distribution of organs among donor service areas (DSAs) is crucial to lowering mortality during the current organ shortage, according to presenter Yanik Babekov, MD, of Massachusetts General Hospital, Boston.
Investigators studied 3,131 patients who were delisted after being classified as “too sick” from 116 centers in 51 DSAs, between 2002 and 2012.
Researchers used the Herfindahl-Hirschman Index (HHI), which analyzes the market share of each participant to determine the overall level of competition. Measurements on the HHI range between 0 and 1, with 0 being the most competitive and 1 being the least.
Mean delisting Model for End-Stage Liver Disease (MELD) scores considered to be “too sick to transplant” were 26.1, and average HHI among DSAs was 0.46, according to investigators. They found that, for every 1% increase in HHI, the delisting MELD score increased by 0.06, according to a risk-adjustment analysis.
“In other words, more competitive DSAs delist patients for [being] ‘too sick’ at lower MELD scores,” Dr. Babekov explained in a video interview. “Interestingly, race, education, citizenship, and other DSA factors also impacted delisting MELD for ‘too sick.’ ”
While market competition may not be the only factor to explain the phenomenon of patients delisted for being ‘too sick,’ it is important to identify how having more transplant centers in DSAs can help more patients be added to, and stay on, these wait lists, according to investigators.
Dr. Babekov had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
WASHINGTON – Low market competition among liver transplant centers may affect which patients are considered too sick to transplant, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
With 20% of patients dying while on the transplant wait list, including those who were delisted, understanding the distribution of organs among donor service areas (DSAs) is crucial to lowering mortality during the current organ shortage, according to presenter Yanik Babekov, MD, of Massachusetts General Hospital, Boston.
Investigators studied 3,131 patients who were delisted after being classified as “too sick” from 116 centers in 51 DSAs, between 2002 and 2012.
Researchers used the Herfindahl-Hirschman Index (HHI), which analyzes the market share of each participant to determine the overall level of competition. Measurements on the HHI range between 0 and 1, with 0 being the most competitive and 1 being the least.
Mean delisting Model for End-Stage Liver Disease (MELD) scores considered to be “too sick to transplant” were 26.1, and average HHI among DSAs was 0.46, according to investigators. They found that, for every 1% increase in HHI, the delisting MELD score increased by 0.06, according to a risk-adjustment analysis.
“In other words, more competitive DSAs delist patients for [being] ‘too sick’ at lower MELD scores,” Dr. Babekov explained in a video interview. “Interestingly, race, education, citizenship, and other DSA factors also impacted delisting MELD for ‘too sick.’ ”
While market competition may not be the only factor to explain the phenomenon of patients delisted for being ‘too sick,’ it is important to identify how having more transplant centers in DSAs can help more patients be added to, and stay on, these wait lists, according to investigators.
Dr. Babekov had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
AT THE LIVER MEETING 2017
VIDEO: Huge database analysis affirms genes associated with NAFLD
WASHINGTON – A genome-wide association study of the Million Veteran Program confirmed three specific genes associated with nonalcoholic fatty liver disease, underscoring the robustness of those loci as well as the clinical phenotyping in the program.
Marina Serper, MD, of the Cpl. Michael J. Crescenz Veterans Affairs Medical Center, and University of Pennsylvania, both in Philadelphia, and her colleagues looked at patients with NAFLD in the Million Veterans Program (MVP), a project of the federal Precision Medicine Initiative designed to leverage the data and experience associated with the Veterans Health Care Administration, Dr. Serper said at the annual meeting of the American Association for the Study of Liver Diseases. Currently, more than 600,000 veterans have been enrolled at over 50 sites across the United States, with a goal of 1 million participants by 2020.
About one-third (108,458) of 352,953 MVP enrollees whose DNA has been analyzed met the study definition of NAFLD. In their study, Dr. Serper and her associates defined the clinical phenotype of NAFLD as patients having abnormal alanine aminotransferase levels (greater than 30 U/L for men and greater than 20 U/L for women) detected twice in a 2-year period, plus at least 1 metabolic risk factor, such as body mass index of 30 kg/m2 or greater, type 2 diabetes or prediabetes, hypertension, or dyslipidemia. Further, included patients did not have alcohol misuse disorders or viral hepatitis.
Most patients were male (90%) and white (72%), with a median age of 64 years. More than half (56%) had a BMI of 30 or greater, 30% were diagnosed with type 2 diabetes, and 71% with dyslipidemia – aligning the cohort closely with rest of the MVP population, Dr. Serper said.
Logistic regression analysis adjusted for age, sex, and principal components stratified by ancestry (European, African American, and Hispanic). On initial analysis, 21 genetic loci met the criteria for genome-wide significant association; specifically, investigators successfully replicated three key variants that have been previously seen associated with NAFLD – PNPLA3, ERLIN1, and TRIB1.
“We were able to use clinical VA data to come up with a robust and clinically relevant definition and validate that definition because the genes we found associated with our definition of NAFLD have previously been shown by others who used biopsy data and imaging data for steatosis,” Dr. Serper said in a video interview. “This is important because the diagnosis of fatty liver disease is really a clinical diagnosis.”
Panel moderator Elizabeth K. Speliotes, MD, of the University of Michigan, Ann Arbor, said, “Really what makes us unique is our genetics and our exposures and the environment, and if we can capture that better, then we can use that to more precisely tailor diagnoses and treatments for patients. That’s really the hope of the next generation.”
The study was supported by the VA Office of Research and Development award 1I01BX003362. Dr. Serper disclosed no relevant conflicts of interest.
Watch this video interview with Dr. Serper and Dr. Chang for more information on the Million Veteran Program.
[email protected]
On Twitter @denisefulton
WASHINGTON – A genome-wide association study of the Million Veteran Program confirmed three specific genes associated with nonalcoholic fatty liver disease, underscoring the robustness of those loci as well as the clinical phenotyping in the program.
Marina Serper, MD, of the Cpl. Michael J. Crescenz Veterans Affairs Medical Center, and University of Pennsylvania, both in Philadelphia, and her colleagues looked at patients with NAFLD in the Million Veterans Program (MVP), a project of the federal Precision Medicine Initiative designed to leverage the data and experience associated with the Veterans Health Care Administration, Dr. Serper said at the annual meeting of the American Association for the Study of Liver Diseases. Currently, more than 600,000 veterans have been enrolled at over 50 sites across the United States, with a goal of 1 million participants by 2020.
About one-third (108,458) of 352,953 MVP enrollees whose DNA has been analyzed met the study definition of NAFLD. In their study, Dr. Serper and her associates defined the clinical phenotype of NAFLD as patients having abnormal alanine aminotransferase levels (greater than 30 U/L for men and greater than 20 U/L for women) detected twice in a 2-year period, plus at least 1 metabolic risk factor, such as body mass index of 30 kg/m2 or greater, type 2 diabetes or prediabetes, hypertension, or dyslipidemia. Further, included patients did not have alcohol misuse disorders or viral hepatitis.
Most patients were male (90%) and white (72%), with a median age of 64 years. More than half (56%) had a BMI of 30 or greater, 30% were diagnosed with type 2 diabetes, and 71% with dyslipidemia – aligning the cohort closely with rest of the MVP population, Dr. Serper said.
Logistic regression analysis adjusted for age, sex, and principal components stratified by ancestry (European, African American, and Hispanic). On initial analysis, 21 genetic loci met the criteria for genome-wide significant association; specifically, investigators successfully replicated three key variants that have been previously seen associated with NAFLD – PNPLA3, ERLIN1, and TRIB1.
“We were able to use clinical VA data to come up with a robust and clinically relevant definition and validate that definition because the genes we found associated with our definition of NAFLD have previously been shown by others who used biopsy data and imaging data for steatosis,” Dr. Serper said in a video interview. “This is important because the diagnosis of fatty liver disease is really a clinical diagnosis.”
Panel moderator Elizabeth K. Speliotes, MD, of the University of Michigan, Ann Arbor, said, “Really what makes us unique is our genetics and our exposures and the environment, and if we can capture that better, then we can use that to more precisely tailor diagnoses and treatments for patients. That’s really the hope of the next generation.”
The study was supported by the VA Office of Research and Development award 1I01BX003362. Dr. Serper disclosed no relevant conflicts of interest.
Watch this video interview with Dr. Serper and Dr. Chang for more information on the Million Veteran Program.
[email protected]
On Twitter @denisefulton
WASHINGTON – A genome-wide association study of the Million Veteran Program confirmed three specific genes associated with nonalcoholic fatty liver disease, underscoring the robustness of those loci as well as the clinical phenotyping in the program.
Marina Serper, MD, of the Cpl. Michael J. Crescenz Veterans Affairs Medical Center, and University of Pennsylvania, both in Philadelphia, and her colleagues looked at patients with NAFLD in the Million Veterans Program (MVP), a project of the federal Precision Medicine Initiative designed to leverage the data and experience associated with the Veterans Health Care Administration, Dr. Serper said at the annual meeting of the American Association for the Study of Liver Diseases. Currently, more than 600,000 veterans have been enrolled at over 50 sites across the United States, with a goal of 1 million participants by 2020.
About one-third (108,458) of 352,953 MVP enrollees whose DNA has been analyzed met the study definition of NAFLD. In their study, Dr. Serper and her associates defined the clinical phenotype of NAFLD as patients having abnormal alanine aminotransferase levels (greater than 30 U/L for men and greater than 20 U/L for women) detected twice in a 2-year period, plus at least 1 metabolic risk factor, such as body mass index of 30 kg/m2 or greater, type 2 diabetes or prediabetes, hypertension, or dyslipidemia. Further, included patients did not have alcohol misuse disorders or viral hepatitis.
Most patients were male (90%) and white (72%), with a median age of 64 years. More than half (56%) had a BMI of 30 or greater, 30% were diagnosed with type 2 diabetes, and 71% with dyslipidemia – aligning the cohort closely with rest of the MVP population, Dr. Serper said.
Logistic regression analysis adjusted for age, sex, and principal components stratified by ancestry (European, African American, and Hispanic). On initial analysis, 21 genetic loci met the criteria for genome-wide significant association; specifically, investigators successfully replicated three key variants that have been previously seen associated with NAFLD – PNPLA3, ERLIN1, and TRIB1.
“We were able to use clinical VA data to come up with a robust and clinically relevant definition and validate that definition because the genes we found associated with our definition of NAFLD have previously been shown by others who used biopsy data and imaging data for steatosis,” Dr. Serper said in a video interview. “This is important because the diagnosis of fatty liver disease is really a clinical diagnosis.”
Panel moderator Elizabeth K. Speliotes, MD, of the University of Michigan, Ann Arbor, said, “Really what makes us unique is our genetics and our exposures and the environment, and if we can capture that better, then we can use that to more precisely tailor diagnoses and treatments for patients. That’s really the hope of the next generation.”
The study was supported by the VA Office of Research and Development award 1I01BX003362. Dr. Serper disclosed no relevant conflicts of interest.
Watch this video interview with Dr. Serper and Dr. Chang for more information on the Million Veteran Program.
[email protected]
On Twitter @denisefulton
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: About one-third (108,458) of 352,953 Million Veteran Program enrollees whose DNA has been analyzed met the study definition of NAFLD.
Data source: Genome-wide association study of more than 100,000 patients.
Disclosures: The study was supported by the Veterans Administration Office of Research and Development award 1I01BX003362. Dr. Serper disclosed no relevant conflicts of interest.
Carvedilol fails to reduce variceal bleeds in acute-on-chronic liver failure
WASHINGTON – Treatment with carvedilol reduced the incidence of sepsis and acute kidney injury and improved survival at 28 days but did not significantly reduce the progression of esophageal varices in patients with acute-on-chronic liver failure.
A total of 136 patients with acute-on-chronic liver failure with small or no esophageal varices and a hepatic venous pressure gradient (HVPG) of 12 mm Hg or greater were enrolled in a single center, prospective, open-label, randomized controlled trial: 66 were randomized to carvedilol and 70 to placebo, according to Sumeet Kainth, MD, of the Institute of Liver and Biliary Sciences in New Delhi.
More than 90% of patients were men with a mean age of 44 years, and composition of the treatment and placebo groups was similar. About 70% in each group had alcoholic hepatitis (the reason for acute liver failure in most). Mean Model for End-Stage Liver Disease (MELD) scores were about 25. Hemodynamic parameters also were comparable, with a mean HVPG of about 19, Dr. Kainth said at the annual meeting of the American Association for the Study of Liver Diseases.
Patients in the treatment group received a median maximum tolerated dose of carvedilol of 12.5 mg, with a range of 3.13 mg to 25 mg.
Morbidity and mortality were high, as is expected with acute-on-chronic liver failure, he noted. A total of 36 patients died before the end of the 90-day study period. Another 23 experienced adverse events and 2 progressed to liver transplant.
HVPG at 90 days decreased significantly in both groups. In the carvedilol group, 90-day HVPG was 16 mm Hg, compared with 19.7 mm Hg at baseline (P less than .01). For placebo patients, 90-day HVPG spontaneously improved to 14.8 mm Hg, compared with a baseline of 17.2 mm Hg (P less than .01).
Carvedilol did not significantly slow the development or growth of varices, however, Dr. Kainth said. At 90 days, varices had progressed in 9 of 40 patients (22.5%) of patients on carvedilol and 8 of 31 (25.8%) of placebo patients.
Significantly fewer patients in the carvedilol group developed acute kidney injury at 28 days (14% vs. 38% on placebo) and sepsis (5% vs. 20%). Mortality also was reduced significantly at 28 days (11% vs. 24%), he reported.
Treatment with carvedilol did not achieve significant reductions in variceal bleeding, “possibly due to the low number of bleeds seen in the study [because of] the exclusion of patients with large varices,” Dr. Kainth said.
The study was sponsored by Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
WASHINGTON – Treatment with carvedilol reduced the incidence of sepsis and acute kidney injury and improved survival at 28 days but did not significantly reduce the progression of esophageal varices in patients with acute-on-chronic liver failure.
A total of 136 patients with acute-on-chronic liver failure with small or no esophageal varices and a hepatic venous pressure gradient (HVPG) of 12 mm Hg or greater were enrolled in a single center, prospective, open-label, randomized controlled trial: 66 were randomized to carvedilol and 70 to placebo, according to Sumeet Kainth, MD, of the Institute of Liver and Biliary Sciences in New Delhi.
More than 90% of patients were men with a mean age of 44 years, and composition of the treatment and placebo groups was similar. About 70% in each group had alcoholic hepatitis (the reason for acute liver failure in most). Mean Model for End-Stage Liver Disease (MELD) scores were about 25. Hemodynamic parameters also were comparable, with a mean HVPG of about 19, Dr. Kainth said at the annual meeting of the American Association for the Study of Liver Diseases.
Patients in the treatment group received a median maximum tolerated dose of carvedilol of 12.5 mg, with a range of 3.13 mg to 25 mg.
Morbidity and mortality were high, as is expected with acute-on-chronic liver failure, he noted. A total of 36 patients died before the end of the 90-day study period. Another 23 experienced adverse events and 2 progressed to liver transplant.
HVPG at 90 days decreased significantly in both groups. In the carvedilol group, 90-day HVPG was 16 mm Hg, compared with 19.7 mm Hg at baseline (P less than .01). For placebo patients, 90-day HVPG spontaneously improved to 14.8 mm Hg, compared with a baseline of 17.2 mm Hg (P less than .01).
Carvedilol did not significantly slow the development or growth of varices, however, Dr. Kainth said. At 90 days, varices had progressed in 9 of 40 patients (22.5%) of patients on carvedilol and 8 of 31 (25.8%) of placebo patients.
Significantly fewer patients in the carvedilol group developed acute kidney injury at 28 days (14% vs. 38% on placebo) and sepsis (5% vs. 20%). Mortality also was reduced significantly at 28 days (11% vs. 24%), he reported.
Treatment with carvedilol did not achieve significant reductions in variceal bleeding, “possibly due to the low number of bleeds seen in the study [because of] the exclusion of patients with large varices,” Dr. Kainth said.
The study was sponsored by Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
WASHINGTON – Treatment with carvedilol reduced the incidence of sepsis and acute kidney injury and improved survival at 28 days but did not significantly reduce the progression of esophageal varices in patients with acute-on-chronic liver failure.
A total of 136 patients with acute-on-chronic liver failure with small or no esophageal varices and a hepatic venous pressure gradient (HVPG) of 12 mm Hg or greater were enrolled in a single center, prospective, open-label, randomized controlled trial: 66 were randomized to carvedilol and 70 to placebo, according to Sumeet Kainth, MD, of the Institute of Liver and Biliary Sciences in New Delhi.
More than 90% of patients were men with a mean age of 44 years, and composition of the treatment and placebo groups was similar. About 70% in each group had alcoholic hepatitis (the reason for acute liver failure in most). Mean Model for End-Stage Liver Disease (MELD) scores were about 25. Hemodynamic parameters also were comparable, with a mean HVPG of about 19, Dr. Kainth said at the annual meeting of the American Association for the Study of Liver Diseases.
Patients in the treatment group received a median maximum tolerated dose of carvedilol of 12.5 mg, with a range of 3.13 mg to 25 mg.
Morbidity and mortality were high, as is expected with acute-on-chronic liver failure, he noted. A total of 36 patients died before the end of the 90-day study period. Another 23 experienced adverse events and 2 progressed to liver transplant.
HVPG at 90 days decreased significantly in both groups. In the carvedilol group, 90-day HVPG was 16 mm Hg, compared with 19.7 mm Hg at baseline (P less than .01). For placebo patients, 90-day HVPG spontaneously improved to 14.8 mm Hg, compared with a baseline of 17.2 mm Hg (P less than .01).
Carvedilol did not significantly slow the development or growth of varices, however, Dr. Kainth said. At 90 days, varices had progressed in 9 of 40 patients (22.5%) of patients on carvedilol and 8 of 31 (25.8%) of placebo patients.
Significantly fewer patients in the carvedilol group developed acute kidney injury at 28 days (14% vs. 38% on placebo) and sepsis (5% vs. 20%). Mortality also was reduced significantly at 28 days (11% vs. 24%), he reported.
Treatment with carvedilol did not achieve significant reductions in variceal bleeding, “possibly due to the low number of bleeds seen in the study [because of] the exclusion of patients with large varices,” Dr. Kainth said.
The study was sponsored by Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: At 90 days, varices had progressed in 9 of 40 (22.5%) patients on carvedilol vs. 8 of 31 (25.8%) of placebo patients.
Data source: A single-center, prospective, open-label, randomized controlled trial of 136 patients with acute-on-chronic liver failure.
Disclosures: The study was sponsored by the Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
New biomarkers improve DILI predictability
WASHINGTON – Researchers have identified six new biomarkers of drug-induced liver injury (DILI) that, when combined with traditional measurements, seemed to better predict the disease course, compared with traditional biomarkers alone, according to a presentation at the annual meeting of the American Association for the Study of Liver Diseases.
In addition, some of these biomarkers may provide a “liquid biopsy” to assess degree of inflammation and mode of hepatocyte death, said Rachel Church, PhD, of the University of North Carolina, Chapel Hill.
“The motivation behind this research is that the standard biomarkers for DILI have several shortcomings,” Dr. Church said. “They’re not entirely liver specific, they’re not mechanistically informative, and they’re not sufficiently predictive of outcome.”
The researchers found that elevated levels of these six candidate biomarkers were predictive for adverse outcome in DILI: total keratin18 (K18); caspase-cleaved K18 (ccK18); alpha-fetoprotein (AFP); osteopontin (OPN); fatty acid–binding protein 1 (FABP1); and macrophage colony-stimulating factor receptor (MCSFR) determined by immunoassay. “We believe that using some of these candidate biomarkers in combination with the standard tests may be the best way to identify individuals at risk for an adverse outcome,” Dr. Church said.
While their analysis found that the traditional international normalized ratio had the overall best predictive value, measured as area under the curve (AUC) of 0.922, the candidate biomarker OPN was second best with an AUC of 0.871, “and actually performed better than total bilirubin,” Dr. Church said.
The study evaluated mechanistic candidate biomarkers by obtaining biopsies in a cohort of 27 patients within 2 weeks of diagnosis, focusing on three physiological reactions: inflammation, necrosis, and apoptosis.
With regard to inflammation, Dr. Church said, “What we found was that MCSFR actually was significantly elevated in patients who had a high score for inflammation; however, there was no significant difference in OPN, although there was a slight elevation.”
They evaluated necrosis using a semiquantitative confluent coagulative necrosis score, and found no difference in the typical biomarkers of cell necrosis, such as alanine transminase, aspartate aminotransferase, and K18. “So we also looked at the regenerative biomarkers, OPN and AFP, and indeed, we observed that both were significantly elevated with high confluent coagulative necrosis scores,” she said.
To evaluate apoptosis, the researchers used the semiquantitative apoptosis score. “We found there was a small but significant elevation in ccK18 in individuals with a high apoptosis score,” she said. They then evaluated the ratio of ccK18 to K18. “The closer the score is to 1, the more apoptosis you have; and the closer the score is to 0, the more necrosis you have,” Dr. Church said.
They also developed a predictive model that combined the traditional biomarkers INR, total bilirubin, and aspartate aminotransferase with the candidate biomarkers OPN and K18, which had an AUC of 0.97. “Some analysis of candidate biomarkers in combination with tests such as MELD score [Model for End-Stage Liver Disease] and ‘Hy’s Law’ saw that incorporating candidate biomarkers was useful,” Dr. Church said.
Dr. Church reported having no financial disclosures.
WASHINGTON – Researchers have identified six new biomarkers of drug-induced liver injury (DILI) that, when combined with traditional measurements, seemed to better predict the disease course, compared with traditional biomarkers alone, according to a presentation at the annual meeting of the American Association for the Study of Liver Diseases.
In addition, some of these biomarkers may provide a “liquid biopsy” to assess degree of inflammation and mode of hepatocyte death, said Rachel Church, PhD, of the University of North Carolina, Chapel Hill.
“The motivation behind this research is that the standard biomarkers for DILI have several shortcomings,” Dr. Church said. “They’re not entirely liver specific, they’re not mechanistically informative, and they’re not sufficiently predictive of outcome.”
The researchers found that elevated levels of these six candidate biomarkers were predictive for adverse outcome in DILI: total keratin18 (K18); caspase-cleaved K18 (ccK18); alpha-fetoprotein (AFP); osteopontin (OPN); fatty acid–binding protein 1 (FABP1); and macrophage colony-stimulating factor receptor (MCSFR) determined by immunoassay. “We believe that using some of these candidate biomarkers in combination with the standard tests may be the best way to identify individuals at risk for an adverse outcome,” Dr. Church said.
While their analysis found that the traditional international normalized ratio had the overall best predictive value, measured as area under the curve (AUC) of 0.922, the candidate biomarker OPN was second best with an AUC of 0.871, “and actually performed better than total bilirubin,” Dr. Church said.
The study evaluated mechanistic candidate biomarkers by obtaining biopsies in a cohort of 27 patients within 2 weeks of diagnosis, focusing on three physiological reactions: inflammation, necrosis, and apoptosis.
With regard to inflammation, Dr. Church said, “What we found was that MCSFR actually was significantly elevated in patients who had a high score for inflammation; however, there was no significant difference in OPN, although there was a slight elevation.”
They evaluated necrosis using a semiquantitative confluent coagulative necrosis score, and found no difference in the typical biomarkers of cell necrosis, such as alanine transminase, aspartate aminotransferase, and K18. “So we also looked at the regenerative biomarkers, OPN and AFP, and indeed, we observed that both were significantly elevated with high confluent coagulative necrosis scores,” she said.
To evaluate apoptosis, the researchers used the semiquantitative apoptosis score. “We found there was a small but significant elevation in ccK18 in individuals with a high apoptosis score,” she said. They then evaluated the ratio of ccK18 to K18. “The closer the score is to 1, the more apoptosis you have; and the closer the score is to 0, the more necrosis you have,” Dr. Church said.
They also developed a predictive model that combined the traditional biomarkers INR, total bilirubin, and aspartate aminotransferase with the candidate biomarkers OPN and K18, which had an AUC of 0.97. “Some analysis of candidate biomarkers in combination with tests such as MELD score [Model for End-Stage Liver Disease] and ‘Hy’s Law’ saw that incorporating candidate biomarkers was useful,” Dr. Church said.
Dr. Church reported having no financial disclosures.
WASHINGTON – Researchers have identified six new biomarkers of drug-induced liver injury (DILI) that, when combined with traditional measurements, seemed to better predict the disease course, compared with traditional biomarkers alone, according to a presentation at the annual meeting of the American Association for the Study of Liver Diseases.
In addition, some of these biomarkers may provide a “liquid biopsy” to assess degree of inflammation and mode of hepatocyte death, said Rachel Church, PhD, of the University of North Carolina, Chapel Hill.
“The motivation behind this research is that the standard biomarkers for DILI have several shortcomings,” Dr. Church said. “They’re not entirely liver specific, they’re not mechanistically informative, and they’re not sufficiently predictive of outcome.”
The researchers found that elevated levels of these six candidate biomarkers were predictive for adverse outcome in DILI: total keratin18 (K18); caspase-cleaved K18 (ccK18); alpha-fetoprotein (AFP); osteopontin (OPN); fatty acid–binding protein 1 (FABP1); and macrophage colony-stimulating factor receptor (MCSFR) determined by immunoassay. “We believe that using some of these candidate biomarkers in combination with the standard tests may be the best way to identify individuals at risk for an adverse outcome,” Dr. Church said.
While their analysis found that the traditional international normalized ratio had the overall best predictive value, measured as area under the curve (AUC) of 0.922, the candidate biomarker OPN was second best with an AUC of 0.871, “and actually performed better than total bilirubin,” Dr. Church said.
The study evaluated mechanistic candidate biomarkers by obtaining biopsies in a cohort of 27 patients within 2 weeks of diagnosis, focusing on three physiological reactions: inflammation, necrosis, and apoptosis.
With regard to inflammation, Dr. Church said, “What we found was that MCSFR actually was significantly elevated in patients who had a high score for inflammation; however, there was no significant difference in OPN, although there was a slight elevation.”
They evaluated necrosis using a semiquantitative confluent coagulative necrosis score, and found no difference in the typical biomarkers of cell necrosis, such as alanine transminase, aspartate aminotransferase, and K18. “So we also looked at the regenerative biomarkers, OPN and AFP, and indeed, we observed that both were significantly elevated with high confluent coagulative necrosis scores,” she said.
To evaluate apoptosis, the researchers used the semiquantitative apoptosis score. “We found there was a small but significant elevation in ccK18 in individuals with a high apoptosis score,” she said. They then evaluated the ratio of ccK18 to K18. “The closer the score is to 1, the more apoptosis you have; and the closer the score is to 0, the more necrosis you have,” Dr. Church said.
They also developed a predictive model that combined the traditional biomarkers INR, total bilirubin, and aspartate aminotransferase with the candidate biomarkers OPN and K18, which had an AUC of 0.97. “Some analysis of candidate biomarkers in combination with tests such as MELD score [Model for End-Stage Liver Disease] and ‘Hy’s Law’ saw that incorporating candidate biomarkers was useful,” Dr. Church said.
Dr. Church reported having no financial disclosures.
AT THE LIVER MEETING 2017
Key clinical point: Combining six candidate biomarkers with traditional biomarkers may improve prediction of adverse outcomes in drug-induced liver injury.
Major finding: Candidate biomarker osteopontin had an area under the cure measure of 0.871, second only to the traditional biomarker international normalized ratio and exceeding that of total bilirubin.
Data source: Analysis of serum samples collected by the DILI Network from 145 patients with a greater than 50% likelihood of having DILI.
Disclosures: Dr. Church reported having no financial disclosures.
Hep C screening falling short in neonatal abstinence syndrome infants
SAN DIEGO – A review of care for neonates born with neonatal abstinence syndrome (NAS) found that screening for hepatitis C virus (HCV) infection is low, based on Medicaid data from the state of Kentucky.
“These children are at high risk for HCV, and the screening rate should really be 100%. We think that it is important to get the message out there,” said Michael Smith, MD, of the department of pediatrics at the Duke University, Durham, N.C.
According to the Kentucky Medicaid data, the rates of NAS are not evenly distributed in the state. Stratifying the incidence rates by eight regions, Dr. Smith reported that 33% of the NAS births in 2016 were in region 8. Although region 8 is a rural Appalachian section on the eastern border of the state, the proportion in this region was more than 50% greater than any other region, including the more populated regions containing Louisville, the largest city, and Lexington, the capital.
Statewide, approximately one in three newborns with NAS were screened for HCV, but the rate was as low as 5% in some areas, and low rates were more common in those counties with the highest rates of opioid use and NAS, Dr. Smith said at an annual scientific meeting on infectious diseases. Although he acknowledged that rates of HCV screening in newborns with NAS appeared to be increasing when 2015 and 2012 data were compared, “there is still a long way to go.”
“Why is this important? There are a couple of reasons. One is that, if you get children into care early, you are more likely to have follow-up,” Dr. Smith said. Follow-up will be important if, as Dr. Smith predicted, HCV therapies become available for children. When providers know which children are infected, treatment can be initiated more efficiently, and this has implications for risk of transmission and, potentially, for outcomes.
At the University of Louisville, children with NAS are typically screened for HCV, HIV, and other transmissible infections that “travel together,” such as syphilis. The evaluation of the Medicaid data suggested that there were no differences in likelihood of HCV testing for sex and race, but Dr. Smith noted that children placed in foster care were significantly more likely to be tested, likely a reflection of processing regulations.
Overall, there are striking differences in the rates of opioid use, rates of NAS, and likelihood of HCV testing in NAS neonates in eastern Appalachian regions of Kentucky and those in regions in the center of the state closer to academic medical centers. The three regions near the University of Louisville, University of Kentucky in Lexington, and the Ohio River border with Cincinnati are known as “the Golden Triangle,” according to Dr. Smith; these regions are where HCV testing rates in neonates with NAS are higher, but testing still is not uniform.
Currently, HCV testing is mandated for adults in several states, but Dr. Smith emphasized that children with NAS are particularly “vulnerable.” He called for policy changes that would require testing in these children and urged HCV screening regardless of whether official policies are established.
SAN DIEGO – A review of care for neonates born with neonatal abstinence syndrome (NAS) found that screening for hepatitis C virus (HCV) infection is low, based on Medicaid data from the state of Kentucky.
“These children are at high risk for HCV, and the screening rate should really be 100%. We think that it is important to get the message out there,” said Michael Smith, MD, of the department of pediatrics at the Duke University, Durham, N.C.
According to the Kentucky Medicaid data, the rates of NAS are not evenly distributed in the state. Stratifying the incidence rates by eight regions, Dr. Smith reported that 33% of the NAS births in 2016 were in region 8. Although region 8 is a rural Appalachian section on the eastern border of the state, the proportion in this region was more than 50% greater than any other region, including the more populated regions containing Louisville, the largest city, and Lexington, the capital.
Statewide, approximately one in three newborns with NAS were screened for HCV, but the rate was as low as 5% in some areas, and low rates were more common in those counties with the highest rates of opioid use and NAS, Dr. Smith said at an annual scientific meeting on infectious diseases. Although he acknowledged that rates of HCV screening in newborns with NAS appeared to be increasing when 2015 and 2012 data were compared, “there is still a long way to go.”
“Why is this important? There are a couple of reasons. One is that, if you get children into care early, you are more likely to have follow-up,” Dr. Smith said. Follow-up will be important if, as Dr. Smith predicted, HCV therapies become available for children. When providers know which children are infected, treatment can be initiated more efficiently, and this has implications for risk of transmission and, potentially, for outcomes.
At the University of Louisville, children with NAS are typically screened for HCV, HIV, and other transmissible infections that “travel together,” such as syphilis. The evaluation of the Medicaid data suggested that there were no differences in likelihood of HCV testing for sex and race, but Dr. Smith noted that children placed in foster care were significantly more likely to be tested, likely a reflection of processing regulations.
Overall, there are striking differences in the rates of opioid use, rates of NAS, and likelihood of HCV testing in NAS neonates in eastern Appalachian regions of Kentucky and those in regions in the center of the state closer to academic medical centers. The three regions near the University of Louisville, University of Kentucky in Lexington, and the Ohio River border with Cincinnati are known as “the Golden Triangle,” according to Dr. Smith; these regions are where HCV testing rates in neonates with NAS are higher, but testing still is not uniform.
Currently, HCV testing is mandated for adults in several states, but Dr. Smith emphasized that children with NAS are particularly “vulnerable.” He called for policy changes that would require testing in these children and urged HCV screening regardless of whether official policies are established.
SAN DIEGO – A review of care for neonates born with neonatal abstinence syndrome (NAS) found that screening for hepatitis C virus (HCV) infection is low, based on Medicaid data from the state of Kentucky.
“These children are at high risk for HCV, and the screening rate should really be 100%. We think that it is important to get the message out there,” said Michael Smith, MD, of the department of pediatrics at the Duke University, Durham, N.C.
According to the Kentucky Medicaid data, the rates of NAS are not evenly distributed in the state. Stratifying the incidence rates by eight regions, Dr. Smith reported that 33% of the NAS births in 2016 were in region 8. Although region 8 is a rural Appalachian section on the eastern border of the state, the proportion in this region was more than 50% greater than any other region, including the more populated regions containing Louisville, the largest city, and Lexington, the capital.
Statewide, approximately one in three newborns with NAS were screened for HCV, but the rate was as low as 5% in some areas, and low rates were more common in those counties with the highest rates of opioid use and NAS, Dr. Smith said at an annual scientific meeting on infectious diseases. Although he acknowledged that rates of HCV screening in newborns with NAS appeared to be increasing when 2015 and 2012 data were compared, “there is still a long way to go.”
“Why is this important? There are a couple of reasons. One is that, if you get children into care early, you are more likely to have follow-up,” Dr. Smith said. Follow-up will be important if, as Dr. Smith predicted, HCV therapies become available for children. When providers know which children are infected, treatment can be initiated more efficiently, and this has implications for risk of transmission and, potentially, for outcomes.
At the University of Louisville, children with NAS are typically screened for HCV, HIV, and other transmissible infections that “travel together,” such as syphilis. The evaluation of the Medicaid data suggested that there were no differences in likelihood of HCV testing for sex and race, but Dr. Smith noted that children placed in foster care were significantly more likely to be tested, likely a reflection of processing regulations.
Overall, there are striking differences in the rates of opioid use, rates of NAS, and likelihood of HCV testing in NAS neonates in eastern Appalachian regions of Kentucky and those in regions in the center of the state closer to academic medical centers. The three regions near the University of Louisville, University of Kentucky in Lexington, and the Ohio River border with Cincinnati are known as “the Golden Triangle,” according to Dr. Smith; these regions are where HCV testing rates in neonates with NAS are higher, but testing still is not uniform.
Currently, HCV testing is mandated for adults in several states, but Dr. Smith emphasized that children with NAS are particularly “vulnerable.” He called for policy changes that would require testing in these children and urged HCV screening regardless of whether official policies are established.
AT ID WEEK 2017
Key clinical point: In Kentucky, which has one of the highest rates of neonates with NAS, screening rates for HCV remain low.
Major finding:
Data source: Retrospective data analysis of Kentucky Medicaid data.
Disclosures: Dr. Smith reported no financial relationships relevant to this study.
Nutrition status predicts outcomes in liver transplant
WASHINGTON – Efforts to improve nutritional status prior to transplant may lead to improved patient outcomes and economic benefits after orthotopic liver transplant.
Clinicians at Austin Health, a tertiary health center in Melbourne, reviewed prospectively acquired data on 390 adult patients who underwent orthotopic liver transplant at their institution between January 2009 and June 2016, according to Brooke Chapman, a dietitian on the center’s transplant team.
“Hand-grip strength test is a functional measure of upper-body strength,” Ms. Chapman said at the annual meeting of the American Association for the Study of Liver Diseases. “It’s quick and cheap and reliable but importantly, it does respond quite readily to changes in nutritional intake and nutrition status.”
Assessments were made as patients were wait listed for liver transplant. Hand-grip strength and subjective global assessment were repeated at the time of transplant.
Patients with fulminant liver failure and those requiring retransplantation were excluded from the final analysis, leaving 321 patients in the cohort. More than two-thirds (69%) were men and the median age was 52 years old. About half of patients had a diagnosis of hepatocellular carcinoma or hepatitis C infection. The median MELD (Model for Endstage Liver Disease) score was 18, with a range of 6-40, and the median time on the wait list was 140 days.
We saw a “high prevalence of malnutrition in patients undergoing liver transplant and the deterioration in nutritional status despite our best efforts while they are on the waiting list,” Ms. Chapman said.
At baseline, two-thirds of patients were malnourished – either mildly to moderately or severely; by transplantation, 77% were malnourished.
“At assessment, we are prescribing and educating patients on a high-calorie, high-protein diet initially, and we give oral nutrition support therapies,” she said. “We really try to get them to improve oral intake, but for patients who do require more aggressive intervention, we will feed them via nasogastric tube.”
Just over half (55%) of patients fell below the cutoff for sarcopenia on the hand-grip test at baseline and at transplant. More than a quarter of patients (27%) were not able to complete the 6-minute walk test.
“On univariate analysis, we saw malnutrition to be strongly associated with increased ICU and hospital length of stay,” Ms. Chapman noted. Severely malnourished patients spent significantly more time in the ICU than did well-nourished patients – a mean 147 hours vs. 89 hours (P = .001). Mean length of stay also was significantly longer at 40 days vs. 16 days (P = .003).
There was also an increased incidence of infection in severely malnourished patients as compared with well-nourished patients – 55.2% vs. 33.8%, she said.
“Aggressive strategies to combat malnutrition and deconditioning in the pretransplant period may lead to improved outcomes after transplant,” Ms. Chapman concluded.
The study was funded by Austin Health. Ms. Chapman declared no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
WASHINGTON – Efforts to improve nutritional status prior to transplant may lead to improved patient outcomes and economic benefits after orthotopic liver transplant.
Clinicians at Austin Health, a tertiary health center in Melbourne, reviewed prospectively acquired data on 390 adult patients who underwent orthotopic liver transplant at their institution between January 2009 and June 2016, according to Brooke Chapman, a dietitian on the center’s transplant team.
“Hand-grip strength test is a functional measure of upper-body strength,” Ms. Chapman said at the annual meeting of the American Association for the Study of Liver Diseases. “It’s quick and cheap and reliable but importantly, it does respond quite readily to changes in nutritional intake and nutrition status.”
Assessments were made as patients were wait listed for liver transplant. Hand-grip strength and subjective global assessment were repeated at the time of transplant.
Patients with fulminant liver failure and those requiring retransplantation were excluded from the final analysis, leaving 321 patients in the cohort. More than two-thirds (69%) were men and the median age was 52 years old. About half of patients had a diagnosis of hepatocellular carcinoma or hepatitis C infection. The median MELD (Model for Endstage Liver Disease) score was 18, with a range of 6-40, and the median time on the wait list was 140 days.
We saw a “high prevalence of malnutrition in patients undergoing liver transplant and the deterioration in nutritional status despite our best efforts while they are on the waiting list,” Ms. Chapman said.
At baseline, two-thirds of patients were malnourished – either mildly to moderately or severely; by transplantation, 77% were malnourished.
“At assessment, we are prescribing and educating patients on a high-calorie, high-protein diet initially, and we give oral nutrition support therapies,” she said. “We really try to get them to improve oral intake, but for patients who do require more aggressive intervention, we will feed them via nasogastric tube.”
Just over half (55%) of patients fell below the cutoff for sarcopenia on the hand-grip test at baseline and at transplant. More than a quarter of patients (27%) were not able to complete the 6-minute walk test.
“On univariate analysis, we saw malnutrition to be strongly associated with increased ICU and hospital length of stay,” Ms. Chapman noted. Severely malnourished patients spent significantly more time in the ICU than did well-nourished patients – a mean 147 hours vs. 89 hours (P = .001). Mean length of stay also was significantly longer at 40 days vs. 16 days (P = .003).
There was also an increased incidence of infection in severely malnourished patients as compared with well-nourished patients – 55.2% vs. 33.8%, she said.
“Aggressive strategies to combat malnutrition and deconditioning in the pretransplant period may lead to improved outcomes after transplant,” Ms. Chapman concluded.
The study was funded by Austin Health. Ms. Chapman declared no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
WASHINGTON – Efforts to improve nutritional status prior to transplant may lead to improved patient outcomes and economic benefits after orthotopic liver transplant.
Clinicians at Austin Health, a tertiary health center in Melbourne, reviewed prospectively acquired data on 390 adult patients who underwent orthotopic liver transplant at their institution between January 2009 and June 2016, according to Brooke Chapman, a dietitian on the center’s transplant team.
“Hand-grip strength test is a functional measure of upper-body strength,” Ms. Chapman said at the annual meeting of the American Association for the Study of Liver Diseases. “It’s quick and cheap and reliable but importantly, it does respond quite readily to changes in nutritional intake and nutrition status.”
Assessments were made as patients were wait listed for liver transplant. Hand-grip strength and subjective global assessment were repeated at the time of transplant.
Patients with fulminant liver failure and those requiring retransplantation were excluded from the final analysis, leaving 321 patients in the cohort. More than two-thirds (69%) were men and the median age was 52 years old. About half of patients had a diagnosis of hepatocellular carcinoma or hepatitis C infection. The median MELD (Model for Endstage Liver Disease) score was 18, with a range of 6-40, and the median time on the wait list was 140 days.
We saw a “high prevalence of malnutrition in patients undergoing liver transplant and the deterioration in nutritional status despite our best efforts while they are on the waiting list,” Ms. Chapman said.
At baseline, two-thirds of patients were malnourished – either mildly to moderately or severely; by transplantation, 77% were malnourished.
“At assessment, we are prescribing and educating patients on a high-calorie, high-protein diet initially, and we give oral nutrition support therapies,” she said. “We really try to get them to improve oral intake, but for patients who do require more aggressive intervention, we will feed them via nasogastric tube.”
Just over half (55%) of patients fell below the cutoff for sarcopenia on the hand-grip test at baseline and at transplant. More than a quarter of patients (27%) were not able to complete the 6-minute walk test.
“On univariate analysis, we saw malnutrition to be strongly associated with increased ICU and hospital length of stay,” Ms. Chapman noted. Severely malnourished patients spent significantly more time in the ICU than did well-nourished patients – a mean 147 hours vs. 89 hours (P = .001). Mean length of stay also was significantly longer at 40 days vs. 16 days (P = .003).
There was also an increased incidence of infection in severely malnourished patients as compared with well-nourished patients – 55.2% vs. 33.8%, she said.
“Aggressive strategies to combat malnutrition and deconditioning in the pretransplant period may lead to improved outcomes after transplant,” Ms. Chapman concluded.
The study was funded by Austin Health. Ms. Chapman declared no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: Severely malnourished patients spent a mean of 147 hours in the ICU vs. 89 hours for well-nourished patients. Mean length of stay also was significantly longer at 40 days vs. 16 days (P = .003).
Data source: Retrospective review of data on 390 adults awaiting liver transplantation between Jan. 2009 and June 2016.
Disclosures: The study was funded by the institutions. The authors reported no relevant conflicts of interest.
Post–liver transplant results similar in acute alcoholic hepatitis, stage 1a
WASHINGTON – Patients with acute alcoholic hepatitis (AAH) have similar early post–liver transplant outcomes to those listed with fulminant hepatic failure, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
Patients with severe AAH have high mortality, but many are unable to survive the 6 months of sobriety required to be accepted as liver transplant candidates, said George Cholankeril, MD, of the gastroenterology and hepatology department at Stanford (Calif.) University.
He and his associates studied wait-list mortality and post–liver transplant survival among 1,912 patients listed for either AAH or fulminant hepatic failure on the United Network for Organ Sharing (UNOS) registry between 2011 and 2016.
A total of 193 patients were listed with AAH, 314 were listed with drug-induced liver injury including acetaminophen (DILI-APAP), and 1,405 were listed as non-DILI patients.
One-year post–liver transplant survival among AAH patients was 93.3%, compared with 87.75% for DILI-APAP patients and 88.4% among non-DILI patients (P less than .001). Survival remained the same among AAH patients 3 years following transplantation, but rates dropped for both the DILI-APAP group (80.8%) and the non-DILI group (81.4%), Dr. Cholankeril reported.
Patients were a median age of 45, 33, and 46 years among the AAH, DILI-APAP, and non-DILI, groups respectively. Patients were majority white among all three groups, with a significantly larger female population among the DILI-APAP group (80.6%) than the AAH (34.7%) or non-DILI (59.4%) groups. Patients in the AAH group had a median Model for End-Stage Liver Disease (MELD) score of 32, compared with 34 for DILI-APAP and 21 for non-DILI.
AAH patients could potentially see significant improvement with a liver transplant, according to investigators; however, the current standards for candidacy have created treatment barriers.
“Patients with AAH have comparable early post-transplant outcomes to those with hepatic liver failure,” said Dr. Cholankeril. “However, there is no consensus or national guidelines for liver transplantation within this patient population.”
Wait-list trends have already started to shift toward more AAH patient acceptance. The number of AAH patients added to the transplant wait lists increased from 14 in 2011 to 58 in 2016. Investigators also found that the number of liver transplant centers accepting AAH patients to their transplant lists increased from 3 to 26.
Investigators were limited by the variations in protocols for each transplant center, as well as by the inconsistency of pre–liver transplant psychosocial metrics. The diagnostic criteria of AAH through UNOS was also a limitation for investigators, according to Dr. Cholankeril.
Although liver transplantation may be able to help some patients, it is only a small fix for a much larger problem. “This is only a solution for a minority of patients with the rising epidemic of alcoholic intoxication in the U.S.,” he said. “As the increasing mortality trends show alcohol-related mortality, and alcoholic liver disease is a contributor to it, we must recognize alcoholic liver disease remains an orphan disease and there is still an unmet need.”
Dr. Cholankeril reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
Transplanting patients with alcohol induced liver disease has been controversial since the earliest days of liver transplantation. Initially some programs were reluctant to transplant patients with a disease that was “self-inflicted” based on an ethical concern about using a scarce resource to save the lives of those whose disease was their own fault, while other patients may die waiting. There was also a concern that transplanting alcoholics would be bad publicity for organ donation and reduce the public’s willingness to donate organs. The highly publicized transplantation of baseball legend and known alcoholic Mickey Mantle in 1995 intensified this debate.
Over time it became clear that the concerns regarding transplanting alcoholics were unfounded. The outcomes were equal or better than for other diseases. Liver transplantation was termed “the ultimate eye opening experience” as serious recidivism turned out to be very uncommon. It was realized that a large percentage of all reasons for seeking medical care can be attributed to self-inflicted harm when one considers cigarette induced malignancy and cardiovascular disease and dietary indiscretion leading to obesity and diabetes. It also became clear that from a practical standpoint prohibiting transplantation of alcoholics simply drove patients to programs that would accept such patients, or caused them and their family to withhold disclosure of alcohol use.
While transplantation of patients with chronic liver disease due to alcohol use has become standard of care, transplanting patients with acute alcoholic hepatitis remains controversial and relatively uncommon. Many programs require a period of abstinence, which is impossible in the setting of acute alcoholic hepatitis. The concern is that it is impossible to discern among actively drinking candidates which ones will be able to achieve sobriety after the transplant. The report by Cholankeril and colleagues documents that the tide is changing. There are increasing numbers of patients being transplanted for acute alcoholic hepatitis, and outcomes are acceptable. However, as the authors point out, the numbers are small and represent a highly selected group of patients. Nevertheless, the pressure on programs to modify rigid abstinence criteria is likely to grow as the evidence accumulates showing selected patients with acute alcoholic hepatitis can do well.
Jeffrey Punch, MD, FACS, is transplant specialist at the University of Michigan in Ann Arbor, and on the Editorial Advisory Board of ACS Surgery News.
Transplanting patients with alcohol induced liver disease has been controversial since the earliest days of liver transplantation. Initially some programs were reluctant to transplant patients with a disease that was “self-inflicted” based on an ethical concern about using a scarce resource to save the lives of those whose disease was their own fault, while other patients may die waiting. There was also a concern that transplanting alcoholics would be bad publicity for organ donation and reduce the public’s willingness to donate organs. The highly publicized transplantation of baseball legend and known alcoholic Mickey Mantle in 1995 intensified this debate.
Over time it became clear that the concerns regarding transplanting alcoholics were unfounded. The outcomes were equal or better than for other diseases. Liver transplantation was termed “the ultimate eye opening experience” as serious recidivism turned out to be very uncommon. It was realized that a large percentage of all reasons for seeking medical care can be attributed to self-inflicted harm when one considers cigarette induced malignancy and cardiovascular disease and dietary indiscretion leading to obesity and diabetes. It also became clear that from a practical standpoint prohibiting transplantation of alcoholics simply drove patients to programs that would accept such patients, or caused them and their family to withhold disclosure of alcohol use.
While transplantation of patients with chronic liver disease due to alcohol use has become standard of care, transplanting patients with acute alcoholic hepatitis remains controversial and relatively uncommon. Many programs require a period of abstinence, which is impossible in the setting of acute alcoholic hepatitis. The concern is that it is impossible to discern among actively drinking candidates which ones will be able to achieve sobriety after the transplant. The report by Cholankeril and colleagues documents that the tide is changing. There are increasing numbers of patients being transplanted for acute alcoholic hepatitis, and outcomes are acceptable. However, as the authors point out, the numbers are small and represent a highly selected group of patients. Nevertheless, the pressure on programs to modify rigid abstinence criteria is likely to grow as the evidence accumulates showing selected patients with acute alcoholic hepatitis can do well.
Jeffrey Punch, MD, FACS, is transplant specialist at the University of Michigan in Ann Arbor, and on the Editorial Advisory Board of ACS Surgery News.
Transplanting patients with alcohol induced liver disease has been controversial since the earliest days of liver transplantation. Initially some programs were reluctant to transplant patients with a disease that was “self-inflicted” based on an ethical concern about using a scarce resource to save the lives of those whose disease was their own fault, while other patients may die waiting. There was also a concern that transplanting alcoholics would be bad publicity for organ donation and reduce the public’s willingness to donate organs. The highly publicized transplantation of baseball legend and known alcoholic Mickey Mantle in 1995 intensified this debate.
Over time it became clear that the concerns regarding transplanting alcoholics were unfounded. The outcomes were equal or better than for other diseases. Liver transplantation was termed “the ultimate eye opening experience” as serious recidivism turned out to be very uncommon. It was realized that a large percentage of all reasons for seeking medical care can be attributed to self-inflicted harm when one considers cigarette induced malignancy and cardiovascular disease and dietary indiscretion leading to obesity and diabetes. It also became clear that from a practical standpoint prohibiting transplantation of alcoholics simply drove patients to programs that would accept such patients, or caused them and their family to withhold disclosure of alcohol use.
While transplantation of patients with chronic liver disease due to alcohol use has become standard of care, transplanting patients with acute alcoholic hepatitis remains controversial and relatively uncommon. Many programs require a period of abstinence, which is impossible in the setting of acute alcoholic hepatitis. The concern is that it is impossible to discern among actively drinking candidates which ones will be able to achieve sobriety after the transplant. The report by Cholankeril and colleagues documents that the tide is changing. There are increasing numbers of patients being transplanted for acute alcoholic hepatitis, and outcomes are acceptable. However, as the authors point out, the numbers are small and represent a highly selected group of patients. Nevertheless, the pressure on programs to modify rigid abstinence criteria is likely to grow as the evidence accumulates showing selected patients with acute alcoholic hepatitis can do well.
Jeffrey Punch, MD, FACS, is transplant specialist at the University of Michigan in Ann Arbor, and on the Editorial Advisory Board of ACS Surgery News.
WASHINGTON – Patients with acute alcoholic hepatitis (AAH) have similar early post–liver transplant outcomes to those listed with fulminant hepatic failure, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
Patients with severe AAH have high mortality, but many are unable to survive the 6 months of sobriety required to be accepted as liver transplant candidates, said George Cholankeril, MD, of the gastroenterology and hepatology department at Stanford (Calif.) University.
He and his associates studied wait-list mortality and post–liver transplant survival among 1,912 patients listed for either AAH or fulminant hepatic failure on the United Network for Organ Sharing (UNOS) registry between 2011 and 2016.
A total of 193 patients were listed with AAH, 314 were listed with drug-induced liver injury including acetaminophen (DILI-APAP), and 1,405 were listed as non-DILI patients.
One-year post–liver transplant survival among AAH patients was 93.3%, compared with 87.75% for DILI-APAP patients and 88.4% among non-DILI patients (P less than .001). Survival remained the same among AAH patients 3 years following transplantation, but rates dropped for both the DILI-APAP group (80.8%) and the non-DILI group (81.4%), Dr. Cholankeril reported.
Patients were a median age of 45, 33, and 46 years among the AAH, DILI-APAP, and non-DILI, groups respectively. Patients were majority white among all three groups, with a significantly larger female population among the DILI-APAP group (80.6%) than the AAH (34.7%) or non-DILI (59.4%) groups. Patients in the AAH group had a median Model for End-Stage Liver Disease (MELD) score of 32, compared with 34 for DILI-APAP and 21 for non-DILI.
AAH patients could potentially see significant improvement with a liver transplant, according to investigators; however, the current standards for candidacy have created treatment barriers.
“Patients with AAH have comparable early post-transplant outcomes to those with hepatic liver failure,” said Dr. Cholankeril. “However, there is no consensus or national guidelines for liver transplantation within this patient population.”
Wait-list trends have already started to shift toward more AAH patient acceptance. The number of AAH patients added to the transplant wait lists increased from 14 in 2011 to 58 in 2016. Investigators also found that the number of liver transplant centers accepting AAH patients to their transplant lists increased from 3 to 26.
Investigators were limited by the variations in protocols for each transplant center, as well as by the inconsistency of pre–liver transplant psychosocial metrics. The diagnostic criteria of AAH through UNOS was also a limitation for investigators, according to Dr. Cholankeril.
Although liver transplantation may be able to help some patients, it is only a small fix for a much larger problem. “This is only a solution for a minority of patients with the rising epidemic of alcoholic intoxication in the U.S.,” he said. “As the increasing mortality trends show alcohol-related mortality, and alcoholic liver disease is a contributor to it, we must recognize alcoholic liver disease remains an orphan disease and there is still an unmet need.”
Dr. Cholankeril reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
WASHINGTON – Patients with acute alcoholic hepatitis (AAH) have similar early post–liver transplant outcomes to those listed with fulminant hepatic failure, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
Patients with severe AAH have high mortality, but many are unable to survive the 6 months of sobriety required to be accepted as liver transplant candidates, said George Cholankeril, MD, of the gastroenterology and hepatology department at Stanford (Calif.) University.
He and his associates studied wait-list mortality and post–liver transplant survival among 1,912 patients listed for either AAH or fulminant hepatic failure on the United Network for Organ Sharing (UNOS) registry between 2011 and 2016.
A total of 193 patients were listed with AAH, 314 were listed with drug-induced liver injury including acetaminophen (DILI-APAP), and 1,405 were listed as non-DILI patients.
One-year post–liver transplant survival among AAH patients was 93.3%, compared with 87.75% for DILI-APAP patients and 88.4% among non-DILI patients (P less than .001). Survival remained the same among AAH patients 3 years following transplantation, but rates dropped for both the DILI-APAP group (80.8%) and the non-DILI group (81.4%), Dr. Cholankeril reported.
Patients were a median age of 45, 33, and 46 years among the AAH, DILI-APAP, and non-DILI, groups respectively. Patients were majority white among all three groups, with a significantly larger female population among the DILI-APAP group (80.6%) than the AAH (34.7%) or non-DILI (59.4%) groups. Patients in the AAH group had a median Model for End-Stage Liver Disease (MELD) score of 32, compared with 34 for DILI-APAP and 21 for non-DILI.
AAH patients could potentially see significant improvement with a liver transplant, according to investigators; however, the current standards for candidacy have created treatment barriers.
“Patients with AAH have comparable early post-transplant outcomes to those with hepatic liver failure,” said Dr. Cholankeril. “However, there is no consensus or national guidelines for liver transplantation within this patient population.”
Wait-list trends have already started to shift toward more AAH patient acceptance. The number of AAH patients added to the transplant wait lists increased from 14 in 2011 to 58 in 2016. Investigators also found that the number of liver transplant centers accepting AAH patients to their transplant lists increased from 3 to 26.
Investigators were limited by the variations in protocols for each transplant center, as well as by the inconsistency of pre–liver transplant psychosocial metrics. The diagnostic criteria of AAH through UNOS was also a limitation for investigators, according to Dr. Cholankeril.
Although liver transplantation may be able to help some patients, it is only a small fix for a much larger problem. “This is only a solution for a minority of patients with the rising epidemic of alcoholic intoxication in the U.S.,” he said. “As the increasing mortality trends show alcohol-related mortality, and alcoholic liver disease is a contributor to it, we must recognize alcoholic liver disease remains an orphan disease and there is still an unmet need.”
Dr. Cholankeril reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: Survival 1 and 3 years after liver transplant was comparable in patients with drug-induced liver injury including acetaminophen (P = .10) and significantly higher than other chronic alcoholic liver disease patients (P less that .001).
Data source: Retrospective study of 1,912 liver transplant patients listed for either AAH or status 1A registered on the UNOS registry between 2011 and 2016.
Disclosures: Presenter reported no relevant financial disclosures.