User login
Study aims to define symptoms of Sjögren’s syndrome secondary to SLE
Sjögren’s syndrome secondary to systemic lupus erythematosus rises in frequency with age, affects nearly one-quarter of all people with SLE, and is marked by a systemic inflammatory state with high levels of proinflammatory cytokines.
Those are key findings from a Swedish study that set out to evaluate the subjective and objective symptoms of secondary Sjögren’s syndrome (sSS) from a large cohort of SLE patients and matched controls.
“The diagnosis SS is a clinical entity, based on dryness of eyes and mouth due to destructive inflammation in the exocrine glands, especially tear and salivary glands,” researchers led by Guillermo Ruacho, DMD, and Marika Kvarnström, MD, PhD, of the Karolinska Institute, wrote in a study published in the Journal of Rheumatology (doi: 10.3899/jrheum.190250). “SS can exist [as] isolated, primary SS (pSS) or together with other rheumatic diseases, referred to as secondary SS (sSS). A major difference according to the 2002 Revised American-European Consensus Criteria (AECC) is the classification where the serologic item (SSA/SSB antibodies) is included for pSS, but not for sSS (Ann Rheum Dis. 2002;61:554-8). In SLE, these autoantibodies are common, usually stable over time, and they appear early, even several years before disease onset.”
The researchers evaluated 504 consecutive SLE patients and 319 controls from the general population, who were matched for age and gender to the first 319 SLE patients. They used AECC to define SLE-sSS and conducted a thorough clinical investigation of all patients, including analysis of autoantibodies and 20 selected cytokines.
The researchers found that SLE-sSS occurred in 23% of the SLE patients. In comparison with SLE patients who did not have sSS, those in the SLE-sSS group were an average of 9 years older, more likely to be female (96% vs. 84%, respectively), and more likely to have leukopenia (57% vs. 45%), yet less likely to have nephritis (32% vs. 43%). Of 20 proinflammatory cytokines investigated, 6 were higher in the SLE-sSS group: TNF-alpha, IL-6, MCP-4, MIP-1beta, IL-12/IL-23p40, and IP-10. Other clinical measures higher in the SLE-sSS group were total IgG, anti-SSA/Ro52, anti-SSA/Ro60, anti-SSB/La antibodies, and rheumatoid factor (IgM and IgA; P less than .05 for all comparisons).
“To our knowledge this is the first study to investigate if systemic inflammation, as measured by cytokine levels, differs between SLE-sSS and SLE-nonsSS,” the researchers wrote. “In clinical practice, it is often difficult to delineate pSS from SLE-sSS. Organ manifestations commonly reported in pSS are fever, lymphadenopathy, parotid gland enlargement, Raynaud’s phenomenon, interstitial lung disease, peripheral neuropathy, and vasculitis. All these clinical features, except parotid gland enlargement, were investigated in the present study, but only peripheral neuropathy differed and was more frequent in SLE-sSS than in SLE-nonsSS.”
They acknowledged certain limitations of the study, including the fact that they did not measure saliva and tear production in controls without sicca symptoms.
The study was supported by funds from Swedish local and national governments, medical societies, foundations, and patient advocacy groups, One author is an employee at AstraZeneca, which provided reagents for the cytokine analyses but had no impact on the analyses, the authors said.
SOURCE: Ruacho G et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.190250.
Sjögren’s syndrome secondary to systemic lupus erythematosus rises in frequency with age, affects nearly one-quarter of all people with SLE, and is marked by a systemic inflammatory state with high levels of proinflammatory cytokines.
Those are key findings from a Swedish study that set out to evaluate the subjective and objective symptoms of secondary Sjögren’s syndrome (sSS) from a large cohort of SLE patients and matched controls.
“The diagnosis SS is a clinical entity, based on dryness of eyes and mouth due to destructive inflammation in the exocrine glands, especially tear and salivary glands,” researchers led by Guillermo Ruacho, DMD, and Marika Kvarnström, MD, PhD, of the Karolinska Institute, wrote in a study published in the Journal of Rheumatology (doi: 10.3899/jrheum.190250). “SS can exist [as] isolated, primary SS (pSS) or together with other rheumatic diseases, referred to as secondary SS (sSS). A major difference according to the 2002 Revised American-European Consensus Criteria (AECC) is the classification where the serologic item (SSA/SSB antibodies) is included for pSS, but not for sSS (Ann Rheum Dis. 2002;61:554-8). In SLE, these autoantibodies are common, usually stable over time, and they appear early, even several years before disease onset.”
The researchers evaluated 504 consecutive SLE patients and 319 controls from the general population, who were matched for age and gender to the first 319 SLE patients. They used AECC to define SLE-sSS and conducted a thorough clinical investigation of all patients, including analysis of autoantibodies and 20 selected cytokines.
The researchers found that SLE-sSS occurred in 23% of the SLE patients. In comparison with SLE patients who did not have sSS, those in the SLE-sSS group were an average of 9 years older, more likely to be female (96% vs. 84%, respectively), and more likely to have leukopenia (57% vs. 45%), yet less likely to have nephritis (32% vs. 43%). Of 20 proinflammatory cytokines investigated, 6 were higher in the SLE-sSS group: TNF-alpha, IL-6, MCP-4, MIP-1beta, IL-12/IL-23p40, and IP-10. Other clinical measures higher in the SLE-sSS group were total IgG, anti-SSA/Ro52, anti-SSA/Ro60, anti-SSB/La antibodies, and rheumatoid factor (IgM and IgA; P less than .05 for all comparisons).
“To our knowledge this is the first study to investigate if systemic inflammation, as measured by cytokine levels, differs between SLE-sSS and SLE-nonsSS,” the researchers wrote. “In clinical practice, it is often difficult to delineate pSS from SLE-sSS. Organ manifestations commonly reported in pSS are fever, lymphadenopathy, parotid gland enlargement, Raynaud’s phenomenon, interstitial lung disease, peripheral neuropathy, and vasculitis. All these clinical features, except parotid gland enlargement, were investigated in the present study, but only peripheral neuropathy differed and was more frequent in SLE-sSS than in SLE-nonsSS.”
They acknowledged certain limitations of the study, including the fact that they did not measure saliva and tear production in controls without sicca symptoms.
The study was supported by funds from Swedish local and national governments, medical societies, foundations, and patient advocacy groups, One author is an employee at AstraZeneca, which provided reagents for the cytokine analyses but had no impact on the analyses, the authors said.
SOURCE: Ruacho G et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.190250.
Sjögren’s syndrome secondary to systemic lupus erythematosus rises in frequency with age, affects nearly one-quarter of all people with SLE, and is marked by a systemic inflammatory state with high levels of proinflammatory cytokines.
Those are key findings from a Swedish study that set out to evaluate the subjective and objective symptoms of secondary Sjögren’s syndrome (sSS) from a large cohort of SLE patients and matched controls.
“The diagnosis SS is a clinical entity, based on dryness of eyes and mouth due to destructive inflammation in the exocrine glands, especially tear and salivary glands,” researchers led by Guillermo Ruacho, DMD, and Marika Kvarnström, MD, PhD, of the Karolinska Institute, wrote in a study published in the Journal of Rheumatology (doi: 10.3899/jrheum.190250). “SS can exist [as] isolated, primary SS (pSS) or together with other rheumatic diseases, referred to as secondary SS (sSS). A major difference according to the 2002 Revised American-European Consensus Criteria (AECC) is the classification where the serologic item (SSA/SSB antibodies) is included for pSS, but not for sSS (Ann Rheum Dis. 2002;61:554-8). In SLE, these autoantibodies are common, usually stable over time, and they appear early, even several years before disease onset.”
The researchers evaluated 504 consecutive SLE patients and 319 controls from the general population, who were matched for age and gender to the first 319 SLE patients. They used AECC to define SLE-sSS and conducted a thorough clinical investigation of all patients, including analysis of autoantibodies and 20 selected cytokines.
The researchers found that SLE-sSS occurred in 23% of the SLE patients. In comparison with SLE patients who did not have sSS, those in the SLE-sSS group were an average of 9 years older, more likely to be female (96% vs. 84%, respectively), and more likely to have leukopenia (57% vs. 45%), yet less likely to have nephritis (32% vs. 43%). Of 20 proinflammatory cytokines investigated, 6 were higher in the SLE-sSS group: TNF-alpha, IL-6, MCP-4, MIP-1beta, IL-12/IL-23p40, and IP-10. Other clinical measures higher in the SLE-sSS group were total IgG, anti-SSA/Ro52, anti-SSA/Ro60, anti-SSB/La antibodies, and rheumatoid factor (IgM and IgA; P less than .05 for all comparisons).
“To our knowledge this is the first study to investigate if systemic inflammation, as measured by cytokine levels, differs between SLE-sSS and SLE-nonsSS,” the researchers wrote. “In clinical practice, it is often difficult to delineate pSS from SLE-sSS. Organ manifestations commonly reported in pSS are fever, lymphadenopathy, parotid gland enlargement, Raynaud’s phenomenon, interstitial lung disease, peripheral neuropathy, and vasculitis. All these clinical features, except parotid gland enlargement, were investigated in the present study, but only peripheral neuropathy differed and was more frequent in SLE-sSS than in SLE-nonsSS.”
They acknowledged certain limitations of the study, including the fact that they did not measure saliva and tear production in controls without sicca symptoms.
The study was supported by funds from Swedish local and national governments, medical societies, foundations, and patient advocacy groups, One author is an employee at AstraZeneca, which provided reagents for the cytokine analyses but had no impact on the analyses, the authors said.
SOURCE: Ruacho G et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.190250.
FROM THE JOURNAL OF RHEUMATOLOGY
ACR looks for continued growth in awareness of rheumatic diseases with latest annual campaign
With the help of a key celebrity spokesperson, the American College of Rheumatology is hoping Rheumatic Disease Awareness Month (RDAM) will continue to raise the profile of the related illnesses and help more patients recognize their symptoms and get on the road to treatment.
This year, the celebrity tapped to lead the now-annual campaign that began in September 2016 is tennis champion Venus Williams, who has been competing while battling Sjögren’s syndrome. She was diagnosed in 2011.
Having a high-profile individual like Venus Williams will “bring more awareness to not a specific illness but just rheumatic diseases in general,” Suleman Bhana, MD, chair of the ACR’s marketing and communications committee, said in an interview, adding that it is a way to help get attention from patients and their family members to be more aware of what they are facing when afflicted with a rheumatic disease.
It also helps to drive traffic to a website (simpletasks.org) set up to help build a community around rheumatic diseases, he noted.
“By signing up on the website, patients and others can get connected with wellness articles and can be informed about any advocacy opportunities in their local area or the national level,” said Dr. Bhana, a rheumatologist with Crystal Run Healthcare in Middletown, N.Y. “It helps us a lot from an ACR point of view just by getting the word out about rheumatic diseases.”
Dr. Bhana said that part of the success of the RDAM campaign is being measured by the number of people who sign up for the website. “Last year we had about 1,600 people sign up, which was more than double from the previous year and we are hoping to do even better this year now that we have Venus Williams as our spokesperson.”
But the campaign involves more than just a celebrity spokesperson and an online community to build awareness for rheumatic diseases and their associated symptoms and treatments. The ACR is working with patients to make sure their voices are heard on Capitol Hill to help ensure proper access to medical care and those treatments.
This year, the ACR – along with more than 100 advocates – met with members of Congress to push for policies that will benefit people with rheumatic diseases. One key item on the agenda targets the use of step therapy.
“This year the ACR’s Government Affairs Committee had a list of several pieces of legislation that they are looking at heavily. [One bill is about] having brakes on what is called step therapy, which is a fairly egregious practice by many insurers that forces patients to use different medications than what the physician and patient decide in their mutual doctor-patient relationship before they can use the medicine that was originally decided upon,” he said. “The reasons for this practice mostly are financial in that insurers get kickbacks from certain pharmaceutical companies, and that is what they term as preferred drugs. It’s a lot more than just cost savings. It’s about the insurers financially benefiting from patients rather than doing the right thing and preserving the doctor-patient relationship.”
One bill in particular that has been highlighted by the ACR is the Safe Step Act of 2019 (H.R. 2279), introduced by Rep. Raul Ruiz, MD (D-Calif.), and Rep. Brad Wenstrup, DPM (R-Ohio), would put limits on step therapy and create a clear process for patients and physicians to seek exemptions.
Other legislative actions that patients and the ACR were advocating for during the visits for RDAM included the EMPOWER for Health Act (H.R. 2781), which would help increase the number of pediatric subspecialists, including pediatric rheumatologists, through loan repayment for health professionals who agree to work at least 2 years in pediatric medicine, and the REDI Act (H.R. 1554), which would defer the accumulation on student loan interest while future doctors serve in a medical internship or residency program.
“The reality of pediatrics is that they don’t get well compensated as a pediatric rheumatologist subspecialist, so there is an ultimate financial disincentive for training pediatricians to go into rheumatology,” Dr. Bhana said. “So we are trying to get support for loan repayment for pediatric rheumatologists or rheumatology fellowship programs, which may help to incentivize young pediatricians to go into rheumatology to at least expand access to care.”
The RDAM campaign also provides useful contacts for an annual survey that the ACR conducts. This year’s survey found that access issues for both physicians and treatments persist, informing the agenda for the advocacy on Capitol Hill.
For example, the 2019 survey found that more than 60% of respondents had to wait at least 31 days from a physician referral to an initial rheumatologist appointment. Dr. Bhana noted that, in his practice, the wait can be 3 months or longer. A little more than 36% of respondents were able to get that initial appointment after referral in 30 days or less.
Additionally, nearly 47% of respondents were subjected to step therapy in the past year.
Treatment pricing was also raised as a concern in the survey, with a little more than 57% of respondents reporting difficulty affording treatments in the past year, and 25% reporting annual out-of-pocket spending at greater than $1,000, with more than 6% reporting annual out-of-pocket spending of greater than $5,000.
Despite all the challenges that lay ahead, Dr. Bhana believes that, as the campaign continues in its fourth year, it is having a positive impact.
“I think there is more awareness among patients to look up their symptoms and prompt their providers to look into, if not test for, rheumatic diseases, direct referrals, and I think because of these campaigns, we’ve gotten some indication that from a social media awareness that more patients are talking about it, about trying to get into seeing a physician.”
With the help of a key celebrity spokesperson, the American College of Rheumatology is hoping Rheumatic Disease Awareness Month (RDAM) will continue to raise the profile of the related illnesses and help more patients recognize their symptoms and get on the road to treatment.
This year, the celebrity tapped to lead the now-annual campaign that began in September 2016 is tennis champion Venus Williams, who has been competing while battling Sjögren’s syndrome. She was diagnosed in 2011.
Having a high-profile individual like Venus Williams will “bring more awareness to not a specific illness but just rheumatic diseases in general,” Suleman Bhana, MD, chair of the ACR’s marketing and communications committee, said in an interview, adding that it is a way to help get attention from patients and their family members to be more aware of what they are facing when afflicted with a rheumatic disease.
It also helps to drive traffic to a website (simpletasks.org) set up to help build a community around rheumatic diseases, he noted.
“By signing up on the website, patients and others can get connected with wellness articles and can be informed about any advocacy opportunities in their local area or the national level,” said Dr. Bhana, a rheumatologist with Crystal Run Healthcare in Middletown, N.Y. “It helps us a lot from an ACR point of view just by getting the word out about rheumatic diseases.”
Dr. Bhana said that part of the success of the RDAM campaign is being measured by the number of people who sign up for the website. “Last year we had about 1,600 people sign up, which was more than double from the previous year and we are hoping to do even better this year now that we have Venus Williams as our spokesperson.”
But the campaign involves more than just a celebrity spokesperson and an online community to build awareness for rheumatic diseases and their associated symptoms and treatments. The ACR is working with patients to make sure their voices are heard on Capitol Hill to help ensure proper access to medical care and those treatments.
This year, the ACR – along with more than 100 advocates – met with members of Congress to push for policies that will benefit people with rheumatic diseases. One key item on the agenda targets the use of step therapy.
“This year the ACR’s Government Affairs Committee had a list of several pieces of legislation that they are looking at heavily. [One bill is about] having brakes on what is called step therapy, which is a fairly egregious practice by many insurers that forces patients to use different medications than what the physician and patient decide in their mutual doctor-patient relationship before they can use the medicine that was originally decided upon,” he said. “The reasons for this practice mostly are financial in that insurers get kickbacks from certain pharmaceutical companies, and that is what they term as preferred drugs. It’s a lot more than just cost savings. It’s about the insurers financially benefiting from patients rather than doing the right thing and preserving the doctor-patient relationship.”
One bill in particular that has been highlighted by the ACR is the Safe Step Act of 2019 (H.R. 2279), introduced by Rep. Raul Ruiz, MD (D-Calif.), and Rep. Brad Wenstrup, DPM (R-Ohio), would put limits on step therapy and create a clear process for patients and physicians to seek exemptions.
Other legislative actions that patients and the ACR were advocating for during the visits for RDAM included the EMPOWER for Health Act (H.R. 2781), which would help increase the number of pediatric subspecialists, including pediatric rheumatologists, through loan repayment for health professionals who agree to work at least 2 years in pediatric medicine, and the REDI Act (H.R. 1554), which would defer the accumulation on student loan interest while future doctors serve in a medical internship or residency program.
“The reality of pediatrics is that they don’t get well compensated as a pediatric rheumatologist subspecialist, so there is an ultimate financial disincentive for training pediatricians to go into rheumatology,” Dr. Bhana said. “So we are trying to get support for loan repayment for pediatric rheumatologists or rheumatology fellowship programs, which may help to incentivize young pediatricians to go into rheumatology to at least expand access to care.”
The RDAM campaign also provides useful contacts for an annual survey that the ACR conducts. This year’s survey found that access issues for both physicians and treatments persist, informing the agenda for the advocacy on Capitol Hill.
For example, the 2019 survey found that more than 60% of respondents had to wait at least 31 days from a physician referral to an initial rheumatologist appointment. Dr. Bhana noted that, in his practice, the wait can be 3 months or longer. A little more than 36% of respondents were able to get that initial appointment after referral in 30 days or less.
Additionally, nearly 47% of respondents were subjected to step therapy in the past year.
Treatment pricing was also raised as a concern in the survey, with a little more than 57% of respondents reporting difficulty affording treatments in the past year, and 25% reporting annual out-of-pocket spending at greater than $1,000, with more than 6% reporting annual out-of-pocket spending of greater than $5,000.
Despite all the challenges that lay ahead, Dr. Bhana believes that, as the campaign continues in its fourth year, it is having a positive impact.
“I think there is more awareness among patients to look up their symptoms and prompt their providers to look into, if not test for, rheumatic diseases, direct referrals, and I think because of these campaigns, we’ve gotten some indication that from a social media awareness that more patients are talking about it, about trying to get into seeing a physician.”
With the help of a key celebrity spokesperson, the American College of Rheumatology is hoping Rheumatic Disease Awareness Month (RDAM) will continue to raise the profile of the related illnesses and help more patients recognize their symptoms and get on the road to treatment.
This year, the celebrity tapped to lead the now-annual campaign that began in September 2016 is tennis champion Venus Williams, who has been competing while battling Sjögren’s syndrome. She was diagnosed in 2011.
Having a high-profile individual like Venus Williams will “bring more awareness to not a specific illness but just rheumatic diseases in general,” Suleman Bhana, MD, chair of the ACR’s marketing and communications committee, said in an interview, adding that it is a way to help get attention from patients and their family members to be more aware of what they are facing when afflicted with a rheumatic disease.
It also helps to drive traffic to a website (simpletasks.org) set up to help build a community around rheumatic diseases, he noted.
“By signing up on the website, patients and others can get connected with wellness articles and can be informed about any advocacy opportunities in their local area or the national level,” said Dr. Bhana, a rheumatologist with Crystal Run Healthcare in Middletown, N.Y. “It helps us a lot from an ACR point of view just by getting the word out about rheumatic diseases.”
Dr. Bhana said that part of the success of the RDAM campaign is being measured by the number of people who sign up for the website. “Last year we had about 1,600 people sign up, which was more than double from the previous year and we are hoping to do even better this year now that we have Venus Williams as our spokesperson.”
But the campaign involves more than just a celebrity spokesperson and an online community to build awareness for rheumatic diseases and their associated symptoms and treatments. The ACR is working with patients to make sure their voices are heard on Capitol Hill to help ensure proper access to medical care and those treatments.
This year, the ACR – along with more than 100 advocates – met with members of Congress to push for policies that will benefit people with rheumatic diseases. One key item on the agenda targets the use of step therapy.
“This year the ACR’s Government Affairs Committee had a list of several pieces of legislation that they are looking at heavily. [One bill is about] having brakes on what is called step therapy, which is a fairly egregious practice by many insurers that forces patients to use different medications than what the physician and patient decide in their mutual doctor-patient relationship before they can use the medicine that was originally decided upon,” he said. “The reasons for this practice mostly are financial in that insurers get kickbacks from certain pharmaceutical companies, and that is what they term as preferred drugs. It’s a lot more than just cost savings. It’s about the insurers financially benefiting from patients rather than doing the right thing and preserving the doctor-patient relationship.”
One bill in particular that has been highlighted by the ACR is the Safe Step Act of 2019 (H.R. 2279), introduced by Rep. Raul Ruiz, MD (D-Calif.), and Rep. Brad Wenstrup, DPM (R-Ohio), would put limits on step therapy and create a clear process for patients and physicians to seek exemptions.
Other legislative actions that patients and the ACR were advocating for during the visits for RDAM included the EMPOWER for Health Act (H.R. 2781), which would help increase the number of pediatric subspecialists, including pediatric rheumatologists, through loan repayment for health professionals who agree to work at least 2 years in pediatric medicine, and the REDI Act (H.R. 1554), which would defer the accumulation on student loan interest while future doctors serve in a medical internship or residency program.
“The reality of pediatrics is that they don’t get well compensated as a pediatric rheumatologist subspecialist, so there is an ultimate financial disincentive for training pediatricians to go into rheumatology,” Dr. Bhana said. “So we are trying to get support for loan repayment for pediatric rheumatologists or rheumatology fellowship programs, which may help to incentivize young pediatricians to go into rheumatology to at least expand access to care.”
The RDAM campaign also provides useful contacts for an annual survey that the ACR conducts. This year’s survey found that access issues for both physicians and treatments persist, informing the agenda for the advocacy on Capitol Hill.
For example, the 2019 survey found that more than 60% of respondents had to wait at least 31 days from a physician referral to an initial rheumatologist appointment. Dr. Bhana noted that, in his practice, the wait can be 3 months or longer. A little more than 36% of respondents were able to get that initial appointment after referral in 30 days or less.
Additionally, nearly 47% of respondents were subjected to step therapy in the past year.
Treatment pricing was also raised as a concern in the survey, with a little more than 57% of respondents reporting difficulty affording treatments in the past year, and 25% reporting annual out-of-pocket spending at greater than $1,000, with more than 6% reporting annual out-of-pocket spending of greater than $5,000.
Despite all the challenges that lay ahead, Dr. Bhana believes that, as the campaign continues in its fourth year, it is having a positive impact.
“I think there is more awareness among patients to look up their symptoms and prompt their providers to look into, if not test for, rheumatic diseases, direct referrals, and I think because of these campaigns, we’ve gotten some indication that from a social media awareness that more patients are talking about it, about trying to get into seeing a physician.”
Antimalarial exposure above recommended doses in lupus contributes most retinopathy risk
Antimalarial retinal complications occurred in about 5% of patients with systemic lupus erythematosus (SLE) exposed to hydroxychloroquine or chloroquine during an average of nearly 13 years of follow-up, without any cases of retinal toxicity occurring within the first 5 years of use, according to findings from a case-control study.
The rate of retinal complications observed in the study is slightly lower or within the range reported in other studies of antimalarial use by patients with SLE, first author Elvis-Raymond Mukwikwi of the University of Montreal and coauthors from McGill University, Montreal, reported in the Journal of Rheumatology.
Hydroxychloroquine (HCQ) and chloroquine (CQ) are commonly used to treat SLE and rheumatoid arthritis, and they are being investigated for use in diabetes, cancer, and cardiovascular disease. However, in rare cases, the drugs can lead to irreversible retinopathy.
To better understand the factors associated with retinopathy, the researchers analyzed data from 326 records obtained from the McGill University Health Center Lupus Clinic Registry. A total of 18 patients (5.5%) had confirmed retinal toxicity, and the investigators matched each of these patients to 3 control patients with SLE and exposure to HCQ/CQ who did not develop retinopathy and had the same duration of SLE and age at SLE diagnosis.
The minimum number of years of HCQ/CQ exposure before retinopathy developed was 8 years, and the maximum number was 33 years. Overall, 17 retinopathy cases had exposure to HCQ, and 12 of those cases (71%) received average doses higher than current recommendations. Eight patients were exposed to average daily doses of HCQ higher than 5 mg/kg and four to average daily doses of CQ above 2.3 mg/kg. Exposure to an average dose higher than currently recommended occurred in 49% of controls. The exposure to doses higher than current recommendations was “not surprising given that these were issued in 2016 and most of our patients had been taking HCQ for longer than this,” the authors wrote.
High-dose exposure was common, with 83% of controls and all retinopathy cases having been exposed to HCQ/CQ doses above recommendations, as determined during at least one annual assessment.
Among patients with retinopathy, exposure to CQ was three times more frequent than it was for those without the condition (39% vs. 13%; 95% confidence interval, 1.8%-52%), although all of the patients exposed to CQ also were exposed to long periods of HCQ, making it impossible to determine retinopathy risk to CQ exposure alone.
The researchers also found gaps in monitoring. In the 5 years before discontinuation of medication, 53% of cases had missed one or more annual ophthalmologic assessments to screen for retinal damage, compared with 75% of controls.
Concomitant renal damage, believed to be a risk factor for retinal toxicity, occurred more often in the retinopathy group, though the difference failed to meet statistical significance (23% versus 15%). Patients with retinopathy were less likely to be Caucasian (61% versus 74%), but this also did not reach statistical significance.
The authors did not disclose information on funding or conflicts of interest.
SOURCE: Mukwikwi ER et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.181102
Antimalarial retinal complications occurred in about 5% of patients with systemic lupus erythematosus (SLE) exposed to hydroxychloroquine or chloroquine during an average of nearly 13 years of follow-up, without any cases of retinal toxicity occurring within the first 5 years of use, according to findings from a case-control study.
The rate of retinal complications observed in the study is slightly lower or within the range reported in other studies of antimalarial use by patients with SLE, first author Elvis-Raymond Mukwikwi of the University of Montreal and coauthors from McGill University, Montreal, reported in the Journal of Rheumatology.
Hydroxychloroquine (HCQ) and chloroquine (CQ) are commonly used to treat SLE and rheumatoid arthritis, and they are being investigated for use in diabetes, cancer, and cardiovascular disease. However, in rare cases, the drugs can lead to irreversible retinopathy.
To better understand the factors associated with retinopathy, the researchers analyzed data from 326 records obtained from the McGill University Health Center Lupus Clinic Registry. A total of 18 patients (5.5%) had confirmed retinal toxicity, and the investigators matched each of these patients to 3 control patients with SLE and exposure to HCQ/CQ who did not develop retinopathy and had the same duration of SLE and age at SLE diagnosis.
The minimum number of years of HCQ/CQ exposure before retinopathy developed was 8 years, and the maximum number was 33 years. Overall, 17 retinopathy cases had exposure to HCQ, and 12 of those cases (71%) received average doses higher than current recommendations. Eight patients were exposed to average daily doses of HCQ higher than 5 mg/kg and four to average daily doses of CQ above 2.3 mg/kg. Exposure to an average dose higher than currently recommended occurred in 49% of controls. The exposure to doses higher than current recommendations was “not surprising given that these were issued in 2016 and most of our patients had been taking HCQ for longer than this,” the authors wrote.
High-dose exposure was common, with 83% of controls and all retinopathy cases having been exposed to HCQ/CQ doses above recommendations, as determined during at least one annual assessment.
Among patients with retinopathy, exposure to CQ was three times more frequent than it was for those without the condition (39% vs. 13%; 95% confidence interval, 1.8%-52%), although all of the patients exposed to CQ also were exposed to long periods of HCQ, making it impossible to determine retinopathy risk to CQ exposure alone.
The researchers also found gaps in monitoring. In the 5 years before discontinuation of medication, 53% of cases had missed one or more annual ophthalmologic assessments to screen for retinal damage, compared with 75% of controls.
Concomitant renal damage, believed to be a risk factor for retinal toxicity, occurred more often in the retinopathy group, though the difference failed to meet statistical significance (23% versus 15%). Patients with retinopathy were less likely to be Caucasian (61% versus 74%), but this also did not reach statistical significance.
The authors did not disclose information on funding or conflicts of interest.
SOURCE: Mukwikwi ER et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.181102
Antimalarial retinal complications occurred in about 5% of patients with systemic lupus erythematosus (SLE) exposed to hydroxychloroquine or chloroquine during an average of nearly 13 years of follow-up, without any cases of retinal toxicity occurring within the first 5 years of use, according to findings from a case-control study.
The rate of retinal complications observed in the study is slightly lower or within the range reported in other studies of antimalarial use by patients with SLE, first author Elvis-Raymond Mukwikwi of the University of Montreal and coauthors from McGill University, Montreal, reported in the Journal of Rheumatology.
Hydroxychloroquine (HCQ) and chloroquine (CQ) are commonly used to treat SLE and rheumatoid arthritis, and they are being investigated for use in diabetes, cancer, and cardiovascular disease. However, in rare cases, the drugs can lead to irreversible retinopathy.
To better understand the factors associated with retinopathy, the researchers analyzed data from 326 records obtained from the McGill University Health Center Lupus Clinic Registry. A total of 18 patients (5.5%) had confirmed retinal toxicity, and the investigators matched each of these patients to 3 control patients with SLE and exposure to HCQ/CQ who did not develop retinopathy and had the same duration of SLE and age at SLE diagnosis.
The minimum number of years of HCQ/CQ exposure before retinopathy developed was 8 years, and the maximum number was 33 years. Overall, 17 retinopathy cases had exposure to HCQ, and 12 of those cases (71%) received average doses higher than current recommendations. Eight patients were exposed to average daily doses of HCQ higher than 5 mg/kg and four to average daily doses of CQ above 2.3 mg/kg. Exposure to an average dose higher than currently recommended occurred in 49% of controls. The exposure to doses higher than current recommendations was “not surprising given that these were issued in 2016 and most of our patients had been taking HCQ for longer than this,” the authors wrote.
High-dose exposure was common, with 83% of controls and all retinopathy cases having been exposed to HCQ/CQ doses above recommendations, as determined during at least one annual assessment.
Among patients with retinopathy, exposure to CQ was three times more frequent than it was for those without the condition (39% vs. 13%; 95% confidence interval, 1.8%-52%), although all of the patients exposed to CQ also were exposed to long periods of HCQ, making it impossible to determine retinopathy risk to CQ exposure alone.
The researchers also found gaps in monitoring. In the 5 years before discontinuation of medication, 53% of cases had missed one or more annual ophthalmologic assessments to screen for retinal damage, compared with 75% of controls.
Concomitant renal damage, believed to be a risk factor for retinal toxicity, occurred more often in the retinopathy group, though the difference failed to meet statistical significance (23% versus 15%). Patients with retinopathy were less likely to be Caucasian (61% versus 74%), but this also did not reach statistical significance.
The authors did not disclose information on funding or conflicts of interest.
SOURCE: Mukwikwi ER et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.181102
FROM THE JOURNAL OF RHEUMATOLOGY
FDA approves nintedanib for scleroderma interstitial lung disease
The Food and Drug Administration has approved nintedanib (Ofev) for the rare but sometimes deadly form of interstitial lung disease that’s caused by systemic sclerosis, or scleroderma.
Although scleroderma itself is rare, half of those patients present with scleroderma-related interstitial lung disease (SSc-ILD), and it remains the leading cause of death in scleroderma patients because it can lead to loss of pulmonary function. Nintedanib appears to slow the progress of SSc-ILD and is the first treatment approved for it, according to a news release from the FDA.
The approval is based on a randomized, double-blind, placebo-controlled trial of 576 patients aged 20-79 years with SSc-ILD. The primary efficacy endpoint was forced vital capacity, and patients on nintedanib showed less decline than did those on placebo.
The most frequent serious adverse event reported in this trial was pneumonia (2.8% with nintedanib vs. 0.3% with placebo). Adverse reactions that led to permanent dose reductions occurred in 34% of nintedanib patients and 4% of placebo-treated patients; the most common of these was diarrhea.
The full prescribing information, which is available on the FDA website, includes warnings for patients with moderate to severe hepatic impairment, elevated liver enzymes, and drug-induced liver injury, as well as those with gastrointestinal disorders. Nintedanib may cause embryo-fetal toxicity, so women of childbearing age should be counseled to avoid pregnancy while taking this drug.
Nintedanib received both Priority Review and Orphan Drug designation. The former meant the FDA intends to take action on the application within 6 months because the agency has determined that, if approved, it would have important effects on treatment of a serious condition. The latter provides incentives to assist and encourage development of drugs for rare diseases. The drug was approved in 2014 for adult patients with idiopathic pulmonary fibrosis, another interstitial lung disease.
The full release is available on the FDA website.
The Food and Drug Administration has approved nintedanib (Ofev) for the rare but sometimes deadly form of interstitial lung disease that’s caused by systemic sclerosis, or scleroderma.
Although scleroderma itself is rare, half of those patients present with scleroderma-related interstitial lung disease (SSc-ILD), and it remains the leading cause of death in scleroderma patients because it can lead to loss of pulmonary function. Nintedanib appears to slow the progress of SSc-ILD and is the first treatment approved for it, according to a news release from the FDA.
The approval is based on a randomized, double-blind, placebo-controlled trial of 576 patients aged 20-79 years with SSc-ILD. The primary efficacy endpoint was forced vital capacity, and patients on nintedanib showed less decline than did those on placebo.
The most frequent serious adverse event reported in this trial was pneumonia (2.8% with nintedanib vs. 0.3% with placebo). Adverse reactions that led to permanent dose reductions occurred in 34% of nintedanib patients and 4% of placebo-treated patients; the most common of these was diarrhea.
The full prescribing information, which is available on the FDA website, includes warnings for patients with moderate to severe hepatic impairment, elevated liver enzymes, and drug-induced liver injury, as well as those with gastrointestinal disorders. Nintedanib may cause embryo-fetal toxicity, so women of childbearing age should be counseled to avoid pregnancy while taking this drug.
Nintedanib received both Priority Review and Orphan Drug designation. The former meant the FDA intends to take action on the application within 6 months because the agency has determined that, if approved, it would have important effects on treatment of a serious condition. The latter provides incentives to assist and encourage development of drugs for rare diseases. The drug was approved in 2014 for adult patients with idiopathic pulmonary fibrosis, another interstitial lung disease.
The full release is available on the FDA website.
The Food and Drug Administration has approved nintedanib (Ofev) for the rare but sometimes deadly form of interstitial lung disease that’s caused by systemic sclerosis, or scleroderma.
Although scleroderma itself is rare, half of those patients present with scleroderma-related interstitial lung disease (SSc-ILD), and it remains the leading cause of death in scleroderma patients because it can lead to loss of pulmonary function. Nintedanib appears to slow the progress of SSc-ILD and is the first treatment approved for it, according to a news release from the FDA.
The approval is based on a randomized, double-blind, placebo-controlled trial of 576 patients aged 20-79 years with SSc-ILD. The primary efficacy endpoint was forced vital capacity, and patients on nintedanib showed less decline than did those on placebo.
The most frequent serious adverse event reported in this trial was pneumonia (2.8% with nintedanib vs. 0.3% with placebo). Adverse reactions that led to permanent dose reductions occurred in 34% of nintedanib patients and 4% of placebo-treated patients; the most common of these was diarrhea.
The full prescribing information, which is available on the FDA website, includes warnings for patients with moderate to severe hepatic impairment, elevated liver enzymes, and drug-induced liver injury, as well as those with gastrointestinal disorders. Nintedanib may cause embryo-fetal toxicity, so women of childbearing age should be counseled to avoid pregnancy while taking this drug.
Nintedanib received both Priority Review and Orphan Drug designation. The former meant the FDA intends to take action on the application within 6 months because the agency has determined that, if approved, it would have important effects on treatment of a serious condition. The latter provides incentives to assist and encourage development of drugs for rare diseases. The drug was approved in 2014 for adult patients with idiopathic pulmonary fibrosis, another interstitial lung disease.
The full release is available on the FDA website.
Cannabidiol may interact with rheumatologic drugs
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
FROM RHEUMATOLOGY
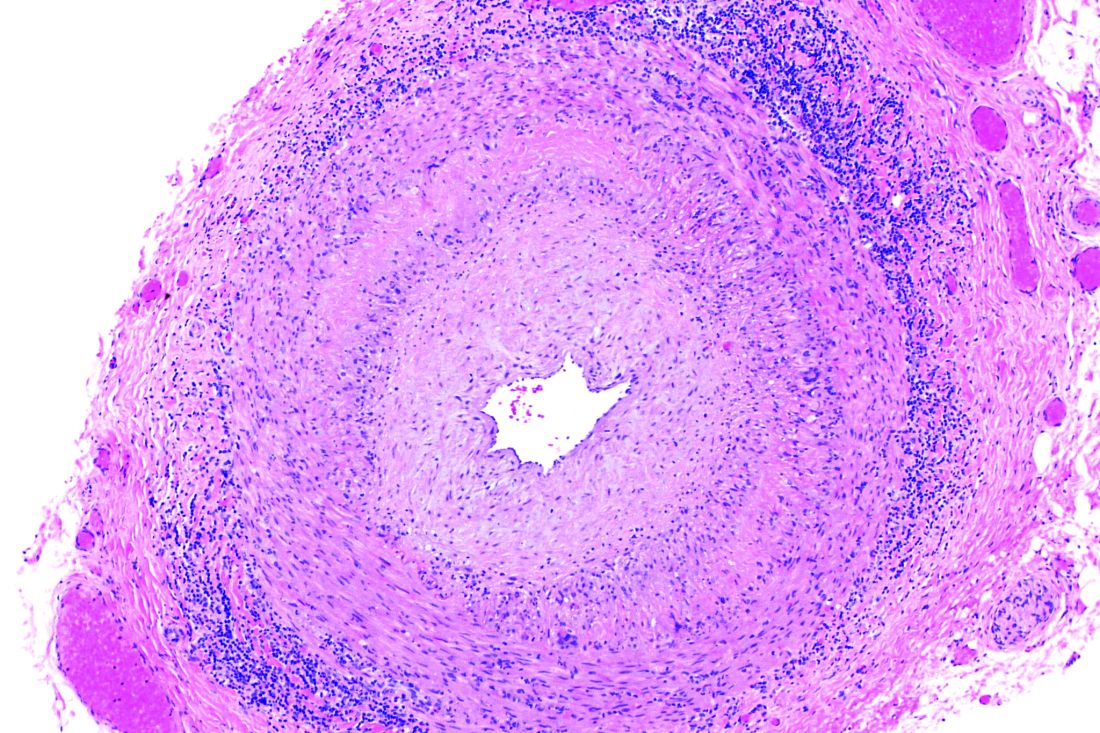
In vasculitis, the skin tells the story
MILAN – , Robert Micheletti, MD, said at the World Congress of Dermatology.
In granulomatous vasculitis, histiocytes and giant cells can play a significant role, explained Dr. Micheletti, director of the cutaneous vasculitis clinic at the University of Pennsylvania, Philadelphia. The condition may be secondary to an autoimmune disease such as lupus erythematosus or RA; a granulomatous disease such as Crohn’s disease or sarcoidosis; infections such as tuberculosis, a fungal disease, or herpes or zoster viruses, or lymphoma, Dr. Micheletti said.
However, a primary systemic vasculitis such as granulomatosis with polyangiitis (GPA; formerly known as Wegener’s polyangiitis) or eosinophilic granulomatosis with polyangiitis (EGPA; also known as Churg-Strauss vasculitis), giant cell arteritis, or Takayasu arteritis may also be responsible, he said. Occasionally, the culprit can also be a drug-induced vasculitis.
The physical examination gives clues to the size of involved vessels, which in turn helps to classify the vasculitis, Dr. Micheletti said.
When vasculitis affects small vessels, the skin findings will be palpable purpura, urticarial papules, vesicles, and petechiae, he said, adding that “The small vessel involvement accounts for the small size of the lesions, and complement cascade and inflammation account for the palpability of the lesions and the symptomatology.” As red blood cells extravasate from the affected vessels, nonblanching purpura develop, and gravity’s effect on the deposition of immune complex material dictates how lesions are distributed.
“Manifestations more typical of medium vessel vasculitis include subcutaneous nodules, livedo reticularis, retiform purpura, larger hemorrhagic bullae, and more significant ulceration and necrosis,” he said. “If such lesions are seen, suspect medium-vessel vasculitis or vasculitis overlapping small and medium vessels.” Cutaneous or systemic polyarteritis nodosa, antineutrophilic cytoplasmic autoantibody (ANCA)–associated vasculitis, and cryoglobulinemic vasculitis are examples, he added.
The particularities of renal manifestations of vasculitis also offer clues to the vessels involved. When a vasculitis patient has glomerulonephritis, suspect small-vessel involvement, Dr. Micheletti said. However, vasculitis affecting medium-sized vessels will cause renovascular hypertension and, potentially renal arterial aneurysms.
Nerves are typically spared in small-vessel vasculitis, while wrist or foot drop can be seen in mononeuritis multiplex.
Recently, the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS) looked at more than 6,800 patients at over 130 sites around the world, proposing new classification criteria for ANCA-associated vasculitis (AAV) and large-vessel vasculitis. The study found that skin findings are common in AAV, with 30%-50% of cases presenting initially with skin lesions. Petechiae and/or purpura are the most common of the skin manifestations, he said. By contrast, for EGPA, allergic and nonspecific findings were the most common findings.
Although skin biopsy can confirm the diagnosis in up to 94% of AAV cases, it’s underutilized and performed in less than half (24%-44%) of cases, Dr. Micheletti said. The study’s findings “demonstrate the importance of a good skin exam, as well as its utility for diagnosis” of vasculitis, he said.
An additional finding form the DCVAS study was that skin lesions can give clues to severity of vasculitis: “Among 1,184 patients with ANCA-associated vasculitis, those with cutaneous involvement were more likely to have systemic manifestations of disease, more likely to have such severe manifestations as glomerulonephritis, alveolar hemorrhage, and mononeuritis,” said Dr. Micheletti, with a hazard ratio of 2.0 among those individuals who had EGPA or GPA.
“Skin findings have diagnostic and, potentially, prognostic importance,” he said. “Use the physician exam and your clinical acumen to your advantage,” but always confirm vasculitis with a biopsy. “Clinicopathologic correlation is key.” A simple urinalysis will screen for renal involvement, and is of “paramount importance,” he added.
Dr. Micheletti reported that he had no relevant disclosures.
MILAN – , Robert Micheletti, MD, said at the World Congress of Dermatology.
In granulomatous vasculitis, histiocytes and giant cells can play a significant role, explained Dr. Micheletti, director of the cutaneous vasculitis clinic at the University of Pennsylvania, Philadelphia. The condition may be secondary to an autoimmune disease such as lupus erythematosus or RA; a granulomatous disease such as Crohn’s disease or sarcoidosis; infections such as tuberculosis, a fungal disease, or herpes or zoster viruses, or lymphoma, Dr. Micheletti said.
However, a primary systemic vasculitis such as granulomatosis with polyangiitis (GPA; formerly known as Wegener’s polyangiitis) or eosinophilic granulomatosis with polyangiitis (EGPA; also known as Churg-Strauss vasculitis), giant cell arteritis, or Takayasu arteritis may also be responsible, he said. Occasionally, the culprit can also be a drug-induced vasculitis.
The physical examination gives clues to the size of involved vessels, which in turn helps to classify the vasculitis, Dr. Micheletti said.
When vasculitis affects small vessels, the skin findings will be palpable purpura, urticarial papules, vesicles, and petechiae, he said, adding that “The small vessel involvement accounts for the small size of the lesions, and complement cascade and inflammation account for the palpability of the lesions and the symptomatology.” As red blood cells extravasate from the affected vessels, nonblanching purpura develop, and gravity’s effect on the deposition of immune complex material dictates how lesions are distributed.
“Manifestations more typical of medium vessel vasculitis include subcutaneous nodules, livedo reticularis, retiform purpura, larger hemorrhagic bullae, and more significant ulceration and necrosis,” he said. “If such lesions are seen, suspect medium-vessel vasculitis or vasculitis overlapping small and medium vessels.” Cutaneous or systemic polyarteritis nodosa, antineutrophilic cytoplasmic autoantibody (ANCA)–associated vasculitis, and cryoglobulinemic vasculitis are examples, he added.
The particularities of renal manifestations of vasculitis also offer clues to the vessels involved. When a vasculitis patient has glomerulonephritis, suspect small-vessel involvement, Dr. Micheletti said. However, vasculitis affecting medium-sized vessels will cause renovascular hypertension and, potentially renal arterial aneurysms.
Nerves are typically spared in small-vessel vasculitis, while wrist or foot drop can be seen in mononeuritis multiplex.
Recently, the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS) looked at more than 6,800 patients at over 130 sites around the world, proposing new classification criteria for ANCA-associated vasculitis (AAV) and large-vessel vasculitis. The study found that skin findings are common in AAV, with 30%-50% of cases presenting initially with skin lesions. Petechiae and/or purpura are the most common of the skin manifestations, he said. By contrast, for EGPA, allergic and nonspecific findings were the most common findings.
Although skin biopsy can confirm the diagnosis in up to 94% of AAV cases, it’s underutilized and performed in less than half (24%-44%) of cases, Dr. Micheletti said. The study’s findings “demonstrate the importance of a good skin exam, as well as its utility for diagnosis” of vasculitis, he said.
An additional finding form the DCVAS study was that skin lesions can give clues to severity of vasculitis: “Among 1,184 patients with ANCA-associated vasculitis, those with cutaneous involvement were more likely to have systemic manifestations of disease, more likely to have such severe manifestations as glomerulonephritis, alveolar hemorrhage, and mononeuritis,” said Dr. Micheletti, with a hazard ratio of 2.0 among those individuals who had EGPA or GPA.
“Skin findings have diagnostic and, potentially, prognostic importance,” he said. “Use the physician exam and your clinical acumen to your advantage,” but always confirm vasculitis with a biopsy. “Clinicopathologic correlation is key.” A simple urinalysis will screen for renal involvement, and is of “paramount importance,” he added.
Dr. Micheletti reported that he had no relevant disclosures.
MILAN – , Robert Micheletti, MD, said at the World Congress of Dermatology.
In granulomatous vasculitis, histiocytes and giant cells can play a significant role, explained Dr. Micheletti, director of the cutaneous vasculitis clinic at the University of Pennsylvania, Philadelphia. The condition may be secondary to an autoimmune disease such as lupus erythematosus or RA; a granulomatous disease such as Crohn’s disease or sarcoidosis; infections such as tuberculosis, a fungal disease, or herpes or zoster viruses, or lymphoma, Dr. Micheletti said.
However, a primary systemic vasculitis such as granulomatosis with polyangiitis (GPA; formerly known as Wegener’s polyangiitis) or eosinophilic granulomatosis with polyangiitis (EGPA; also known as Churg-Strauss vasculitis), giant cell arteritis, or Takayasu arteritis may also be responsible, he said. Occasionally, the culprit can also be a drug-induced vasculitis.
The physical examination gives clues to the size of involved vessels, which in turn helps to classify the vasculitis, Dr. Micheletti said.
When vasculitis affects small vessels, the skin findings will be palpable purpura, urticarial papules, vesicles, and petechiae, he said, adding that “The small vessel involvement accounts for the small size of the lesions, and complement cascade and inflammation account for the palpability of the lesions and the symptomatology.” As red blood cells extravasate from the affected vessels, nonblanching purpura develop, and gravity’s effect on the deposition of immune complex material dictates how lesions are distributed.
“Manifestations more typical of medium vessel vasculitis include subcutaneous nodules, livedo reticularis, retiform purpura, larger hemorrhagic bullae, and more significant ulceration and necrosis,” he said. “If such lesions are seen, suspect medium-vessel vasculitis or vasculitis overlapping small and medium vessels.” Cutaneous or systemic polyarteritis nodosa, antineutrophilic cytoplasmic autoantibody (ANCA)–associated vasculitis, and cryoglobulinemic vasculitis are examples, he added.
The particularities of renal manifestations of vasculitis also offer clues to the vessels involved. When a vasculitis patient has glomerulonephritis, suspect small-vessel involvement, Dr. Micheletti said. However, vasculitis affecting medium-sized vessels will cause renovascular hypertension and, potentially renal arterial aneurysms.
Nerves are typically spared in small-vessel vasculitis, while wrist or foot drop can be seen in mononeuritis multiplex.
Recently, the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS) looked at more than 6,800 patients at over 130 sites around the world, proposing new classification criteria for ANCA-associated vasculitis (AAV) and large-vessel vasculitis. The study found that skin findings are common in AAV, with 30%-50% of cases presenting initially with skin lesions. Petechiae and/or purpura are the most common of the skin manifestations, he said. By contrast, for EGPA, allergic and nonspecific findings were the most common findings.
Although skin biopsy can confirm the diagnosis in up to 94% of AAV cases, it’s underutilized and performed in less than half (24%-44%) of cases, Dr. Micheletti said. The study’s findings “demonstrate the importance of a good skin exam, as well as its utility for diagnosis” of vasculitis, he said.
An additional finding form the DCVAS study was that skin lesions can give clues to severity of vasculitis: “Among 1,184 patients with ANCA-associated vasculitis, those with cutaneous involvement were more likely to have systemic manifestations of disease, more likely to have such severe manifestations as glomerulonephritis, alveolar hemorrhage, and mononeuritis,” said Dr. Micheletti, with a hazard ratio of 2.0 among those individuals who had EGPA or GPA.
“Skin findings have diagnostic and, potentially, prognostic importance,” he said. “Use the physician exam and your clinical acumen to your advantage,” but always confirm vasculitis with a biopsy. “Clinicopathologic correlation is key.” A simple urinalysis will screen for renal involvement, and is of “paramount importance,” he added.
Dr. Micheletti reported that he had no relevant disclosures.
AT WCD2019
EULAR updates vaccination recommendations for autoimmune inflammatory rheumatic disease patients
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
FROM ANNALS OF THE RHEUMATIC DISEASES
Peripheral nervous system events have lasting impact on SLE patients
Peripheral nervous system disease, predominantly neuropathies, constitutes a substantial proportion of the manifestations of neuropsychiatric systemic lupus erythematosus (SLE) and has a lasting negative impact on health-related quality of life, John G. Hanly, MD, of Queen Elizabeth II Health Sciences Center and Dalhousie University, Halifax, N.S., and associates reported in Arthritis & Rheumatology.
According to the study of 1,827 SLE patients who had been recently diagnosed and enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network at sites in Europe, Asia, and North America during 1999-2011, 161 peripheral nervous system (PNS) events occurred in 139 of the patients (8%) over a mean 7.6 years of follow-up.
Using the seven American College of Rheumatology case definitions for PNS disease in neuropsychiatric SLE, most of the events were peripheral neuropathy (41%), mononeuropathy (27%), and cranial neuropathy (24%). For 110 with peripheral neuropathy or mononeuropathy who underwent electrophysiologic testing, axonal damage was often present (42%), followed by demyelination (22%).
The PNS events were attributed to SLE in about 58%-75% of the patients. Based on these data the investigators estimated that after 10 years the cumulative incidence of any PNS event regardless of its attribution was about 9%, and it was nearly 7% for events attributed to SLE.
The probability that the neuropathies would not resolve over time was estimated at about 43% for peripheral neuropathy, 29% for mononeuropathy, and 30% for cranial neuropathy. Resolution of neuropathy was most rapid for cranial neuropathy, followed by mononeuropathy and peripheral neuropathy.
Patients with PNS events had significantly lower physical and mental health component scores on the 36-item Short Form Health Survey than did patients without a neuropsychiatric event up to the study assessment, and these differences persisted for 10 years of follow-up.
These “findings provide a benchmark for the assessment of future treatment modalities,” the investigators concluded.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41070.
Peripheral nervous system disease, predominantly neuropathies, constitutes a substantial proportion of the manifestations of neuropsychiatric systemic lupus erythematosus (SLE) and has a lasting negative impact on health-related quality of life, John G. Hanly, MD, of Queen Elizabeth II Health Sciences Center and Dalhousie University, Halifax, N.S., and associates reported in Arthritis & Rheumatology.
According to the study of 1,827 SLE patients who had been recently diagnosed and enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network at sites in Europe, Asia, and North America during 1999-2011, 161 peripheral nervous system (PNS) events occurred in 139 of the patients (8%) over a mean 7.6 years of follow-up.
Using the seven American College of Rheumatology case definitions for PNS disease in neuropsychiatric SLE, most of the events were peripheral neuropathy (41%), mononeuropathy (27%), and cranial neuropathy (24%). For 110 with peripheral neuropathy or mononeuropathy who underwent electrophysiologic testing, axonal damage was often present (42%), followed by demyelination (22%).
The PNS events were attributed to SLE in about 58%-75% of the patients. Based on these data the investigators estimated that after 10 years the cumulative incidence of any PNS event regardless of its attribution was about 9%, and it was nearly 7% for events attributed to SLE.
The probability that the neuropathies would not resolve over time was estimated at about 43% for peripheral neuropathy, 29% for mononeuropathy, and 30% for cranial neuropathy. Resolution of neuropathy was most rapid for cranial neuropathy, followed by mononeuropathy and peripheral neuropathy.
Patients with PNS events had significantly lower physical and mental health component scores on the 36-item Short Form Health Survey than did patients without a neuropsychiatric event up to the study assessment, and these differences persisted for 10 years of follow-up.
These “findings provide a benchmark for the assessment of future treatment modalities,” the investigators concluded.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41070.
Peripheral nervous system disease, predominantly neuropathies, constitutes a substantial proportion of the manifestations of neuropsychiatric systemic lupus erythematosus (SLE) and has a lasting negative impact on health-related quality of life, John G. Hanly, MD, of Queen Elizabeth II Health Sciences Center and Dalhousie University, Halifax, N.S., and associates reported in Arthritis & Rheumatology.
According to the study of 1,827 SLE patients who had been recently diagnosed and enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network at sites in Europe, Asia, and North America during 1999-2011, 161 peripheral nervous system (PNS) events occurred in 139 of the patients (8%) over a mean 7.6 years of follow-up.
Using the seven American College of Rheumatology case definitions for PNS disease in neuropsychiatric SLE, most of the events were peripheral neuropathy (41%), mononeuropathy (27%), and cranial neuropathy (24%). For 110 with peripheral neuropathy or mononeuropathy who underwent electrophysiologic testing, axonal damage was often present (42%), followed by demyelination (22%).
The PNS events were attributed to SLE in about 58%-75% of the patients. Based on these data the investigators estimated that after 10 years the cumulative incidence of any PNS event regardless of its attribution was about 9%, and it was nearly 7% for events attributed to SLE.
The probability that the neuropathies would not resolve over time was estimated at about 43% for peripheral neuropathy, 29% for mononeuropathy, and 30% for cranial neuropathy. Resolution of neuropathy was most rapid for cranial neuropathy, followed by mononeuropathy and peripheral neuropathy.
Patients with PNS events had significantly lower physical and mental health component scores on the 36-item Short Form Health Survey than did patients without a neuropsychiatric event up to the study assessment, and these differences persisted for 10 years of follow-up.
These “findings provide a benchmark for the assessment of future treatment modalities,” the investigators concluded.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41070.
REPORTING FROM ARTHRITIS & RHEUMATOLOGY
PROMIS tools provide useful data for managing rheumatology patients
LAKE BUENA VISTA, FLA. –
The PROMIS tools – which like most patient-reported outcome (PRO) measurement tools are designed to evaluate and monitor physical, mental, and social health – can be used both for the general population and for individuals living with chronic conditions, Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham (UAB), said at the annual meeting of the Florida Society of Rheumatology.
The tools take a deeper dive into various symptoms and their effects; for instance, with respect to physical health, they measure fatigue, physical function, sleep disturbance, pain intensity, and pain interference – the extent to which pain “messes your patient’s life up,” explained Dr. Curtis, who also is codirector of the UAB Pharmacoepidemiology and Pharmacoeconomics Unit.
Additional physical health domains that PROs measure include dyspnea, gastrointestinal symptoms, pain behavior, pain quality, sexual function, and sleep-related impairment.
These are “things that, honestly, we don’t talk about much as a field, but absolutely affect patients with autoimmune diseases,” he said. “You know, sexual function – that doesn’t come up in my practice spontaneously very often, but there are ways you can quantify that, and for many patients that’s actually a big deal.”
The domains measured by PROMIS tools for mental health look at anxiety and depression, but also delve into alcohol use, anger, cognitive function, life satisfaction, self-efficacy for managing chronic conditions, substance use, and more. The domains for social health address ability to participate in social roles and activities, as well as companionship, satisfaction with social roles and activity, social isolation, and social support.
“You can’t go on a hike with friends [and] be far from a bathroom, because you have bad arthritis and you have Crohn’s disease. Well, that’s kind of an important thing that may or may not come up in your discussions about inflammatory arthritis associated with [inflammatory bowel disease],” he said.
Another example is a patient who is embarrassed attending social functions or wearing a swimsuit because of really bad psoriasis.
“These are the kinds of things that I’m suggesting you and I probably want to measure if we’re providing holistic care to rheumatology patients,” Dr. Curtis said.
The PROMIS tools provide a simple, user-friendly means for doing so in English, Spanish, and many other languages, he noted.
All the scales use the same 1-100 scoring range, which simplifies measurements. They are available for free by download and can be printed or used electronically for use in the office, at home, on the web, and via smartphone.
The NIH developed the PROMIS tools several years ago and validated them for multiple chronic disease populations, Dr. Curtis said, adding that the tools include multiple individual domains and overall “profiles” of varying lengths.
Most are fixed-length scales that are between 4 and 10 questions and can be completed within 30-60 seconds per scale, so several scales can be completed within 5-10 minutes.
However, some scales are longer and provide greater detail.
“The nice thing is that if you ask a few more questions you can get more precise information – there’s more of a floor and ceiling. You can detect people who do really well. You can distinguish between the marathon runners and the 5K-ers and the people who can walk 2 miles but aren’t going to run a race,” he explained.
Further, the PROMIS tools, like the 36-item Short Form Health Survey (SF-36), are benchmarked against the U.S. adult population, allowing for assessment of how a specific drug or treatment “impacts your arthritis patient on a scale that would also be relevant for somebody who doesn’t have arthritis, they have diabetes.”
The metrics and scales are the same, and that can be helpful when trying to get a payer to pay for a particular drug, he said.
“None of these are rheumatology specific; this puts PROs into a language that can help rheumatology contend for the value of the care that we provide on a scale that would be relevant for any other chronic illness, even for nonrheumatology patients,” he explained.
In addition, minimally important differences (group mean change of about 2-3 units) and minimally clinical important differences for individuals (5 units) have been established.
“So we know what the numbers mean, and this is true for all of the scales,” he said.
PROMIS tools also include computer-adaptive testing (CAT) versions, which helps to personalize the scales to provide more precise information for a given patient and eliminate irrelevant information.
Of note, PROMIS health measures are among the data that can be tracked on a smartphone using Arthritis Power, an arthritis research registry developed with the help of a recent infrastructure grant awarded to the Center for Education and Research and Therapeutics of Musculoskeletal Disorders at UAB, Dr. Curtis said.
The measures were also shown in the AWARE study to track closely with other measures, including the Clinical Disease Activity Index (CDAI), and with patient improvement on therapy.
“So these PROMIS scores are tracking with things that you and I are familiar with ... and it looks like these scores are faithfully tracking, over time, patients getting better on therapies that we would expect them to,” he said. “I think this is additional validation – not just from the National Institutes of Health and a decade of research by lots of different groups, but in our own field – that these actually correlate with disease activity ... and that when you start an effective therapy like a [tumor necrosis factor inhibitor] they’re going to improve as you would anticipate.”
Dr. Curtis reported funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Patient-Centered Outcomes Research Institute. He has also consulted for or received research grants from Amgen, AbbVie, Bristol-Myers Squibb, CORRONA, Lilly, Janssen, Myriad, Novartis, Roche, Pfizer, and Sanofi/Regeneron.
LAKE BUENA VISTA, FLA. –
The PROMIS tools – which like most patient-reported outcome (PRO) measurement tools are designed to evaluate and monitor physical, mental, and social health – can be used both for the general population and for individuals living with chronic conditions, Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham (UAB), said at the annual meeting of the Florida Society of Rheumatology.
The tools take a deeper dive into various symptoms and their effects; for instance, with respect to physical health, they measure fatigue, physical function, sleep disturbance, pain intensity, and pain interference – the extent to which pain “messes your patient’s life up,” explained Dr. Curtis, who also is codirector of the UAB Pharmacoepidemiology and Pharmacoeconomics Unit.
Additional physical health domains that PROs measure include dyspnea, gastrointestinal symptoms, pain behavior, pain quality, sexual function, and sleep-related impairment.
These are “things that, honestly, we don’t talk about much as a field, but absolutely affect patients with autoimmune diseases,” he said. “You know, sexual function – that doesn’t come up in my practice spontaneously very often, but there are ways you can quantify that, and for many patients that’s actually a big deal.”
The domains measured by PROMIS tools for mental health look at anxiety and depression, but also delve into alcohol use, anger, cognitive function, life satisfaction, self-efficacy for managing chronic conditions, substance use, and more. The domains for social health address ability to participate in social roles and activities, as well as companionship, satisfaction with social roles and activity, social isolation, and social support.
“You can’t go on a hike with friends [and] be far from a bathroom, because you have bad arthritis and you have Crohn’s disease. Well, that’s kind of an important thing that may or may not come up in your discussions about inflammatory arthritis associated with [inflammatory bowel disease],” he said.
Another example is a patient who is embarrassed attending social functions or wearing a swimsuit because of really bad psoriasis.
“These are the kinds of things that I’m suggesting you and I probably want to measure if we’re providing holistic care to rheumatology patients,” Dr. Curtis said.
The PROMIS tools provide a simple, user-friendly means for doing so in English, Spanish, and many other languages, he noted.
All the scales use the same 1-100 scoring range, which simplifies measurements. They are available for free by download and can be printed or used electronically for use in the office, at home, on the web, and via smartphone.
The NIH developed the PROMIS tools several years ago and validated them for multiple chronic disease populations, Dr. Curtis said, adding that the tools include multiple individual domains and overall “profiles” of varying lengths.
Most are fixed-length scales that are between 4 and 10 questions and can be completed within 30-60 seconds per scale, so several scales can be completed within 5-10 minutes.
However, some scales are longer and provide greater detail.
“The nice thing is that if you ask a few more questions you can get more precise information – there’s more of a floor and ceiling. You can detect people who do really well. You can distinguish between the marathon runners and the 5K-ers and the people who can walk 2 miles but aren’t going to run a race,” he explained.
Further, the PROMIS tools, like the 36-item Short Form Health Survey (SF-36), are benchmarked against the U.S. adult population, allowing for assessment of how a specific drug or treatment “impacts your arthritis patient on a scale that would also be relevant for somebody who doesn’t have arthritis, they have diabetes.”
The metrics and scales are the same, and that can be helpful when trying to get a payer to pay for a particular drug, he said.
“None of these are rheumatology specific; this puts PROs into a language that can help rheumatology contend for the value of the care that we provide on a scale that would be relevant for any other chronic illness, even for nonrheumatology patients,” he explained.
In addition, minimally important differences (group mean change of about 2-3 units) and minimally clinical important differences for individuals (5 units) have been established.
“So we know what the numbers mean, and this is true for all of the scales,” he said.
PROMIS tools also include computer-adaptive testing (CAT) versions, which helps to personalize the scales to provide more precise information for a given patient and eliminate irrelevant information.
Of note, PROMIS health measures are among the data that can be tracked on a smartphone using Arthritis Power, an arthritis research registry developed with the help of a recent infrastructure grant awarded to the Center for Education and Research and Therapeutics of Musculoskeletal Disorders at UAB, Dr. Curtis said.
The measures were also shown in the AWARE study to track closely with other measures, including the Clinical Disease Activity Index (CDAI), and with patient improvement on therapy.
“So these PROMIS scores are tracking with things that you and I are familiar with ... and it looks like these scores are faithfully tracking, over time, patients getting better on therapies that we would expect them to,” he said. “I think this is additional validation – not just from the National Institutes of Health and a decade of research by lots of different groups, but in our own field – that these actually correlate with disease activity ... and that when you start an effective therapy like a [tumor necrosis factor inhibitor] they’re going to improve as you would anticipate.”
Dr. Curtis reported funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Patient-Centered Outcomes Research Institute. He has also consulted for or received research grants from Amgen, AbbVie, Bristol-Myers Squibb, CORRONA, Lilly, Janssen, Myriad, Novartis, Roche, Pfizer, and Sanofi/Regeneron.
LAKE BUENA VISTA, FLA. –
The PROMIS tools – which like most patient-reported outcome (PRO) measurement tools are designed to evaluate and monitor physical, mental, and social health – can be used both for the general population and for individuals living with chronic conditions, Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham (UAB), said at the annual meeting of the Florida Society of Rheumatology.
The tools take a deeper dive into various symptoms and their effects; for instance, with respect to physical health, they measure fatigue, physical function, sleep disturbance, pain intensity, and pain interference – the extent to which pain “messes your patient’s life up,” explained Dr. Curtis, who also is codirector of the UAB Pharmacoepidemiology and Pharmacoeconomics Unit.
Additional physical health domains that PROs measure include dyspnea, gastrointestinal symptoms, pain behavior, pain quality, sexual function, and sleep-related impairment.
These are “things that, honestly, we don’t talk about much as a field, but absolutely affect patients with autoimmune diseases,” he said. “You know, sexual function – that doesn’t come up in my practice spontaneously very often, but there are ways you can quantify that, and for many patients that’s actually a big deal.”
The domains measured by PROMIS tools for mental health look at anxiety and depression, but also delve into alcohol use, anger, cognitive function, life satisfaction, self-efficacy for managing chronic conditions, substance use, and more. The domains for social health address ability to participate in social roles and activities, as well as companionship, satisfaction with social roles and activity, social isolation, and social support.
“You can’t go on a hike with friends [and] be far from a bathroom, because you have bad arthritis and you have Crohn’s disease. Well, that’s kind of an important thing that may or may not come up in your discussions about inflammatory arthritis associated with [inflammatory bowel disease],” he said.
Another example is a patient who is embarrassed attending social functions or wearing a swimsuit because of really bad psoriasis.
“These are the kinds of things that I’m suggesting you and I probably want to measure if we’re providing holistic care to rheumatology patients,” Dr. Curtis said.
The PROMIS tools provide a simple, user-friendly means for doing so in English, Spanish, and many other languages, he noted.
All the scales use the same 1-100 scoring range, which simplifies measurements. They are available for free by download and can be printed or used electronically for use in the office, at home, on the web, and via smartphone.
The NIH developed the PROMIS tools several years ago and validated them for multiple chronic disease populations, Dr. Curtis said, adding that the tools include multiple individual domains and overall “profiles” of varying lengths.
Most are fixed-length scales that are between 4 and 10 questions and can be completed within 30-60 seconds per scale, so several scales can be completed within 5-10 minutes.
However, some scales are longer and provide greater detail.
“The nice thing is that if you ask a few more questions you can get more precise information – there’s more of a floor and ceiling. You can detect people who do really well. You can distinguish between the marathon runners and the 5K-ers and the people who can walk 2 miles but aren’t going to run a race,” he explained.
Further, the PROMIS tools, like the 36-item Short Form Health Survey (SF-36), are benchmarked against the U.S. adult population, allowing for assessment of how a specific drug or treatment “impacts your arthritis patient on a scale that would also be relevant for somebody who doesn’t have arthritis, they have diabetes.”
The metrics and scales are the same, and that can be helpful when trying to get a payer to pay for a particular drug, he said.
“None of these are rheumatology specific; this puts PROs into a language that can help rheumatology contend for the value of the care that we provide on a scale that would be relevant for any other chronic illness, even for nonrheumatology patients,” he explained.
In addition, minimally important differences (group mean change of about 2-3 units) and minimally clinical important differences for individuals (5 units) have been established.
“So we know what the numbers mean, and this is true for all of the scales,” he said.
PROMIS tools also include computer-adaptive testing (CAT) versions, which helps to personalize the scales to provide more precise information for a given patient and eliminate irrelevant information.
Of note, PROMIS health measures are among the data that can be tracked on a smartphone using Arthritis Power, an arthritis research registry developed with the help of a recent infrastructure grant awarded to the Center for Education and Research and Therapeutics of Musculoskeletal Disorders at UAB, Dr. Curtis said.
The measures were also shown in the AWARE study to track closely with other measures, including the Clinical Disease Activity Index (CDAI), and with patient improvement on therapy.
“So these PROMIS scores are tracking with things that you and I are familiar with ... and it looks like these scores are faithfully tracking, over time, patients getting better on therapies that we would expect them to,” he said. “I think this is additional validation – not just from the National Institutes of Health and a decade of research by lots of different groups, but in our own field – that these actually correlate with disease activity ... and that when you start an effective therapy like a [tumor necrosis factor inhibitor] they’re going to improve as you would anticipate.”
Dr. Curtis reported funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Patient-Centered Outcomes Research Institute. He has also consulted for or received research grants from Amgen, AbbVie, Bristol-Myers Squibb, CORRONA, Lilly, Janssen, Myriad, Novartis, Roche, Pfizer, and Sanofi/Regeneron.
EXPERT ANALYSIS FROM FSR 2019
White and black patients have similar rates of giant cell arteritis
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
FROM JAMA OPHTHALMOLOGY