User login
Aspartame, sweetened drinks don’t increase risk of NHL

beverages at the supermarket
Consuming aspartame and drinking sweetened beverages do not increase a person’s risk of developing non-Hodgkin lymphoma (NHL), according to research published in the Journal of Nutrition.
Investigators analyzed information from more than 100,000 men and women in the US.
The results suggested that neither aspartame intake nor the consumption of sugar-sweetened or artificially sweetened beverages were associated with an increased risk of NHL.
Marjorie L. McCullough, SCD, RD, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this research, analyzing data from the nutrition cohort of the Cancer Prevention Study II, an assessment of cancer incidence
and mortality in the US.
Study subjects first completed a questionnaire in 1992, noting information related to diet and other lifestyle factors. They completed follow-up questionnaires in 1999 and 2003, which included questions related to the consumption of sugar-sweetened and artificially sweetened beverages, as well as tabletop sweeteners containing aspartame.
Among the 100,442 adult men and women who provided information on diet and lifestyle factors in 1999, there were 1196 NHL cases verified during a 10-year follow-up period.
The investigators assessed the risk of NHL associated with sweetened beverage and aspartame consumption, adjusted for the subjects’ smoking status, body mass index, and history of diabetes.
The analysis revealed that, in women and men combined, there was no association between NHL risk and the consumption of 1 or more servings (355 mL) of artificially sweetened beverages. Compared to nondrinkers, subjects who drank artificially sweetened beverages had a risk ratio (RR) of 0.92 (P=0.14).
Similarly, there was no association between NHL risk and sugar-sweetened beverages. Compared to nondrinkers, the RR for sugar-sweetened beverage drinkers was 1.10 (P=0.62).
Furthermore, subjects’ overall aspartame intake, which was estimated from artificially sweetened carbonated beverage consumption and the use of aspartame packets, was not associated with NHL risk.
The RR was 1.02 (P=0.69) for the top quintile (which had a median aspartame intake of 145 mg per day) vs the bottom quintile (which had a median aspartame intake of 0 mg per day).
The investigators also found that associations between disease and sweetened beverage consumption or aspartame intake were generally null for specific NHL subtypes, including multiple myeloma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, follicular lymphoma, and other B-cell lymphomas.
“The study supports the decades of research that have continued to find that aspartame is safe for use in foods and beverages,” said Haley Stevens, PhD, President of the Calorie Control Council. “It also supports the conclusions of the National Cancer Institute, who have determined that aspartame does not increase a person’s risk of developing cancer.” ![]()

beverages at the supermarket
Consuming aspartame and drinking sweetened beverages do not increase a person’s risk of developing non-Hodgkin lymphoma (NHL), according to research published in the Journal of Nutrition.
Investigators analyzed information from more than 100,000 men and women in the US.
The results suggested that neither aspartame intake nor the consumption of sugar-sweetened or artificially sweetened beverages were associated with an increased risk of NHL.
Marjorie L. McCullough, SCD, RD, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this research, analyzing data from the nutrition cohort of the Cancer Prevention Study II, an assessment of cancer incidence
and mortality in the US.
Study subjects first completed a questionnaire in 1992, noting information related to diet and other lifestyle factors. They completed follow-up questionnaires in 1999 and 2003, which included questions related to the consumption of sugar-sweetened and artificially sweetened beverages, as well as tabletop sweeteners containing aspartame.
Among the 100,442 adult men and women who provided information on diet and lifestyle factors in 1999, there were 1196 NHL cases verified during a 10-year follow-up period.
The investigators assessed the risk of NHL associated with sweetened beverage and aspartame consumption, adjusted for the subjects’ smoking status, body mass index, and history of diabetes.
The analysis revealed that, in women and men combined, there was no association between NHL risk and the consumption of 1 or more servings (355 mL) of artificially sweetened beverages. Compared to nondrinkers, subjects who drank artificially sweetened beverages had a risk ratio (RR) of 0.92 (P=0.14).
Similarly, there was no association between NHL risk and sugar-sweetened beverages. Compared to nondrinkers, the RR for sugar-sweetened beverage drinkers was 1.10 (P=0.62).
Furthermore, subjects’ overall aspartame intake, which was estimated from artificially sweetened carbonated beverage consumption and the use of aspartame packets, was not associated with NHL risk.
The RR was 1.02 (P=0.69) for the top quintile (which had a median aspartame intake of 145 mg per day) vs the bottom quintile (which had a median aspartame intake of 0 mg per day).
The investigators also found that associations between disease and sweetened beverage consumption or aspartame intake were generally null for specific NHL subtypes, including multiple myeloma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, follicular lymphoma, and other B-cell lymphomas.
“The study supports the decades of research that have continued to find that aspartame is safe for use in foods and beverages,” said Haley Stevens, PhD, President of the Calorie Control Council. “It also supports the conclusions of the National Cancer Institute, who have determined that aspartame does not increase a person’s risk of developing cancer.” ![]()

beverages at the supermarket
Consuming aspartame and drinking sweetened beverages do not increase a person’s risk of developing non-Hodgkin lymphoma (NHL), according to research published in the Journal of Nutrition.
Investigators analyzed information from more than 100,000 men and women in the US.
The results suggested that neither aspartame intake nor the consumption of sugar-sweetened or artificially sweetened beverages were associated with an increased risk of NHL.
Marjorie L. McCullough, SCD, RD, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this research, analyzing data from the nutrition cohort of the Cancer Prevention Study II, an assessment of cancer incidence
and mortality in the US.
Study subjects first completed a questionnaire in 1992, noting information related to diet and other lifestyle factors. They completed follow-up questionnaires in 1999 and 2003, which included questions related to the consumption of sugar-sweetened and artificially sweetened beverages, as well as tabletop sweeteners containing aspartame.
Among the 100,442 adult men and women who provided information on diet and lifestyle factors in 1999, there were 1196 NHL cases verified during a 10-year follow-up period.
The investigators assessed the risk of NHL associated with sweetened beverage and aspartame consumption, adjusted for the subjects’ smoking status, body mass index, and history of diabetes.
The analysis revealed that, in women and men combined, there was no association between NHL risk and the consumption of 1 or more servings (355 mL) of artificially sweetened beverages. Compared to nondrinkers, subjects who drank artificially sweetened beverages had a risk ratio (RR) of 0.92 (P=0.14).
Similarly, there was no association between NHL risk and sugar-sweetened beverages. Compared to nondrinkers, the RR for sugar-sweetened beverage drinkers was 1.10 (P=0.62).
Furthermore, subjects’ overall aspartame intake, which was estimated from artificially sweetened carbonated beverage consumption and the use of aspartame packets, was not associated with NHL risk.
The RR was 1.02 (P=0.69) for the top quintile (which had a median aspartame intake of 145 mg per day) vs the bottom quintile (which had a median aspartame intake of 0 mg per day).
The investigators also found that associations between disease and sweetened beverage consumption or aspartame intake were generally null for specific NHL subtypes, including multiple myeloma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, follicular lymphoma, and other B-cell lymphomas.
“The study supports the decades of research that have continued to find that aspartame is safe for use in foods and beverages,” said Haley Stevens, PhD, President of the Calorie Control Council. “It also supports the conclusions of the National Cancer Institute, who have determined that aspartame does not increase a person’s risk of developing cancer.” ![]()
NICE recommends ofatumumab in CLL

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a preliminary draft guidance recommending ofatumumab (Arzerra) for use in patients with chronic lymphocytic leukemia (CLL).
The agency is recommending the anti-CD20 monoclonal antibody in combination with chlorambucil for patients with untreated CLL who are ineligible for treatment with fludarabine combination therapy and for whom bendamustine is unsuitable.
NICE believes ofatumumab is a cost-effective use of National Health Service (NHS) resources for this patient population, as GlaxoSmithKline, the company developing ofatumumab, has agreed to provide the drug at a reduced price.
The company has agreed with the Department of Health that the size of the discount be confidential.
Clinical effectiveness
“The information provided by GlaxoSmithKline, who market the drug, showed that ofatumumab with chlorambucil is a clinically effective treatment option for those people unable to take fludarabine combination therapy or bendamustine,” said Sir Andrew Dillon, NICE chief executive.
In the phase 3 COMPLEMENT 1 trial, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
A NICE advisory committee also considered the use of ofatumumab in combination with bendamustine. But the committee said that, due to limited clinical evidence and the absence of cost-effectiveness estimates, it could not make a recommendation on this combination.
In a phase 2 trial known as OMB115991, ofatumumab plus bendamustine elicited an overall response rate of 95% and a complete response rate of 43%.
Cost-effectiveness
Ofatumumab’s list price is £182 for a 100 mg vial and £1820 for a 1000 mg vial. Assuming 6 cycles and no drug wastage, the mean cost of a treatment course for ofatumumab at its list price is £11,466 for 6300 mg.
GlaxoSmithKline has agreed to a patient access scheme with the Department of Health that makes ofatumumab available with a discount on the list price, though the exact amount is confidential.
The NICE advisory committee said the most plausible cost-effectiveness estimate for ofatumumab plus chlorambucil compared with chlorambucil alone using the
ofatumumab patient access scheme price was £26,000 per quality-adjusted life year gained.
Consultees, including GlaxoSmithKline, healthcare professionals, and members of the public, can now comment on NICE’s preliminary draft guidance. It will be available for public consultation until November 25, 2014.
Until the final guidance is issued, NHS bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a preliminary draft guidance recommending ofatumumab (Arzerra) for use in patients with chronic lymphocytic leukemia (CLL).
The agency is recommending the anti-CD20 monoclonal antibody in combination with chlorambucil for patients with untreated CLL who are ineligible for treatment with fludarabine combination therapy and for whom bendamustine is unsuitable.
NICE believes ofatumumab is a cost-effective use of National Health Service (NHS) resources for this patient population, as GlaxoSmithKline, the company developing ofatumumab, has agreed to provide the drug at a reduced price.
The company has agreed with the Department of Health that the size of the discount be confidential.
Clinical effectiveness
“The information provided by GlaxoSmithKline, who market the drug, showed that ofatumumab with chlorambucil is a clinically effective treatment option for those people unable to take fludarabine combination therapy or bendamustine,” said Sir Andrew Dillon, NICE chief executive.
In the phase 3 COMPLEMENT 1 trial, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
A NICE advisory committee also considered the use of ofatumumab in combination with bendamustine. But the committee said that, due to limited clinical evidence and the absence of cost-effectiveness estimates, it could not make a recommendation on this combination.
In a phase 2 trial known as OMB115991, ofatumumab plus bendamustine elicited an overall response rate of 95% and a complete response rate of 43%.
Cost-effectiveness
Ofatumumab’s list price is £182 for a 100 mg vial and £1820 for a 1000 mg vial. Assuming 6 cycles and no drug wastage, the mean cost of a treatment course for ofatumumab at its list price is £11,466 for 6300 mg.
GlaxoSmithKline has agreed to a patient access scheme with the Department of Health that makes ofatumumab available with a discount on the list price, though the exact amount is confidential.
The NICE advisory committee said the most plausible cost-effectiveness estimate for ofatumumab plus chlorambucil compared with chlorambucil alone using the
ofatumumab patient access scheme price was £26,000 per quality-adjusted life year gained.
Consultees, including GlaxoSmithKline, healthcare professionals, and members of the public, can now comment on NICE’s preliminary draft guidance. It will be available for public consultation until November 25, 2014.
Until the final guidance is issued, NHS bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a preliminary draft guidance recommending ofatumumab (Arzerra) for use in patients with chronic lymphocytic leukemia (CLL).
The agency is recommending the anti-CD20 monoclonal antibody in combination with chlorambucil for patients with untreated CLL who are ineligible for treatment with fludarabine combination therapy and for whom bendamustine is unsuitable.
NICE believes ofatumumab is a cost-effective use of National Health Service (NHS) resources for this patient population, as GlaxoSmithKline, the company developing ofatumumab, has agreed to provide the drug at a reduced price.
The company has agreed with the Department of Health that the size of the discount be confidential.
Clinical effectiveness
“The information provided by GlaxoSmithKline, who market the drug, showed that ofatumumab with chlorambucil is a clinically effective treatment option for those people unable to take fludarabine combination therapy or bendamustine,” said Sir Andrew Dillon, NICE chief executive.
In the phase 3 COMPLEMENT 1 trial, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
A NICE advisory committee also considered the use of ofatumumab in combination with bendamustine. But the committee said that, due to limited clinical evidence and the absence of cost-effectiveness estimates, it could not make a recommendation on this combination.
In a phase 2 trial known as OMB115991, ofatumumab plus bendamustine elicited an overall response rate of 95% and a complete response rate of 43%.
Cost-effectiveness
Ofatumumab’s list price is £182 for a 100 mg vial and £1820 for a 1000 mg vial. Assuming 6 cycles and no drug wastage, the mean cost of a treatment course for ofatumumab at its list price is £11,466 for 6300 mg.
GlaxoSmithKline has agreed to a patient access scheme with the Department of Health that makes ofatumumab available with a discount on the list price, though the exact amount is confidential.
The NICE advisory committee said the most plausible cost-effectiveness estimate for ofatumumab plus chlorambucil compared with chlorambucil alone using the
ofatumumab patient access scheme price was £26,000 per quality-adjusted life year gained.
Consultees, including GlaxoSmithKline, healthcare professionals, and members of the public, can now comment on NICE’s preliminary draft guidance. It will be available for public consultation until November 25, 2014.
Until the final guidance is issued, NHS bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()
Brentuximab tops chemo in HL, doc says

NEW YORK—Brentuximab vendotin—not conventional chemotherapy—is the second-line regimen of choice for recurrent Hodgkin lymphoma (HL) patients prior to stem cell transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Catherine Diefenbach, MD, of New York University’s Langone Medical Center in New York, argued that conventional chemotherapy, the existing paradigm for first salvage therapy, does not maximize a cure or minimize toxicity, is inconvenient, and is not cost-effective.
She noted that a quarter of HL patients relapse or have a primary refractory diagnosis.
“These are young patients,” Dr Diefenbach said. “Autologous stem cell transplant [ASCT] cures only half of them. There is no other established curative salvage therapy.”
She noted that the median time to progression with relapse after transplant is 3.8 months in patients treated with subsequent therapy, with a median survival of 26 months.
After ASCT, the median progression-free survival with relapse is 1.3 years. And about three-quarters of relapses occur within the first year.
“Achieving a complete response [CR] before ASCT is the most important factor in determining long-term disease-free survival and to maximizing transplant-related benefit,” Dr Diefenbach said. “High overall response rates [ORRs] do not equal high CR. Only one-third of patients achieve a CR [with chemotherapy].”
She said studies have shown that chemotherapy with ICE (ifosfamide, carboplatin, and etoposide) leads to a 3-year event-free survival rate of 22%. Post-ASCT, event-free survival increases to more than 52%.
The overall survival post-ASCT is 44%. The median survival of patients who do not get therapy is 3.7 months. Myelosuppression and deaths are common.
“Conventional chemotherapy fails,” Dr Diefenbach continued. “There is inadequate disease control, unacceptable toxicity, it’s not cost-effective, requires patients to be hospitalized, and there is no clear standard of care.”
On the other hand, brentuximab as first salvage is highly active with minimal adverse events in relapsed HL.
“Rash is the only grade 3-4 toxicity,” Dr Diefenbach said. “There are no significant cytopenias [and] no febrile neutropenia. Growth factor support is not required, and it’s administered outpatient.”
Using brentuximab as second-line therapy results in an ORR of 85.7% and a CR of 50%, she added. And ASCT after brentuximab shows similar successes and toxicities.
Brentuximab followed by ICE leads to high rates of PET normalization, allows successful transplantation of virtually all evaluable patients, and poses no issues with stem cell collection.
Furthermore, studies show a 92% disease-free survival, with minimal toxicity at 10 months of follow-up.
In conclusion, Dr Diefenbach said, “Maximizing disease control prior to ASCT will maximize cure from ASCT. Brentuximab vedotin is a novel agent that leads to a high ORR and high CR rate with low toxicity and outpatient administration. In contrast, conventional chemotherapy fails to provide a high CR rate, has unacceptable toxicity, and there is no single standard of care.”
For an opposing opinion on salvage in HL, see “Speaker adovcates chemo-based salvage in HL.” ![]()

NEW YORK—Brentuximab vendotin—not conventional chemotherapy—is the second-line regimen of choice for recurrent Hodgkin lymphoma (HL) patients prior to stem cell transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Catherine Diefenbach, MD, of New York University’s Langone Medical Center in New York, argued that conventional chemotherapy, the existing paradigm for first salvage therapy, does not maximize a cure or minimize toxicity, is inconvenient, and is not cost-effective.
She noted that a quarter of HL patients relapse or have a primary refractory diagnosis.
“These are young patients,” Dr Diefenbach said. “Autologous stem cell transplant [ASCT] cures only half of them. There is no other established curative salvage therapy.”
She noted that the median time to progression with relapse after transplant is 3.8 months in patients treated with subsequent therapy, with a median survival of 26 months.
After ASCT, the median progression-free survival with relapse is 1.3 years. And about three-quarters of relapses occur within the first year.
“Achieving a complete response [CR] before ASCT is the most important factor in determining long-term disease-free survival and to maximizing transplant-related benefit,” Dr Diefenbach said. “High overall response rates [ORRs] do not equal high CR. Only one-third of patients achieve a CR [with chemotherapy].”
She said studies have shown that chemotherapy with ICE (ifosfamide, carboplatin, and etoposide) leads to a 3-year event-free survival rate of 22%. Post-ASCT, event-free survival increases to more than 52%.
The overall survival post-ASCT is 44%. The median survival of patients who do not get therapy is 3.7 months. Myelosuppression and deaths are common.
“Conventional chemotherapy fails,” Dr Diefenbach continued. “There is inadequate disease control, unacceptable toxicity, it’s not cost-effective, requires patients to be hospitalized, and there is no clear standard of care.”
On the other hand, brentuximab as first salvage is highly active with minimal adverse events in relapsed HL.
“Rash is the only grade 3-4 toxicity,” Dr Diefenbach said. “There are no significant cytopenias [and] no febrile neutropenia. Growth factor support is not required, and it’s administered outpatient.”
Using brentuximab as second-line therapy results in an ORR of 85.7% and a CR of 50%, she added. And ASCT after brentuximab shows similar successes and toxicities.
Brentuximab followed by ICE leads to high rates of PET normalization, allows successful transplantation of virtually all evaluable patients, and poses no issues with stem cell collection.
Furthermore, studies show a 92% disease-free survival, with minimal toxicity at 10 months of follow-up.
In conclusion, Dr Diefenbach said, “Maximizing disease control prior to ASCT will maximize cure from ASCT. Brentuximab vedotin is a novel agent that leads to a high ORR and high CR rate with low toxicity and outpatient administration. In contrast, conventional chemotherapy fails to provide a high CR rate, has unacceptable toxicity, and there is no single standard of care.”
For an opposing opinion on salvage in HL, see “Speaker adovcates chemo-based salvage in HL.” ![]()

NEW YORK—Brentuximab vendotin—not conventional chemotherapy—is the second-line regimen of choice for recurrent Hodgkin lymphoma (HL) patients prior to stem cell transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Catherine Diefenbach, MD, of New York University’s Langone Medical Center in New York, argued that conventional chemotherapy, the existing paradigm for first salvage therapy, does not maximize a cure or minimize toxicity, is inconvenient, and is not cost-effective.
She noted that a quarter of HL patients relapse or have a primary refractory diagnosis.
“These are young patients,” Dr Diefenbach said. “Autologous stem cell transplant [ASCT] cures only half of them. There is no other established curative salvage therapy.”
She noted that the median time to progression with relapse after transplant is 3.8 months in patients treated with subsequent therapy, with a median survival of 26 months.
After ASCT, the median progression-free survival with relapse is 1.3 years. And about three-quarters of relapses occur within the first year.
“Achieving a complete response [CR] before ASCT is the most important factor in determining long-term disease-free survival and to maximizing transplant-related benefit,” Dr Diefenbach said. “High overall response rates [ORRs] do not equal high CR. Only one-third of patients achieve a CR [with chemotherapy].”
She said studies have shown that chemotherapy with ICE (ifosfamide, carboplatin, and etoposide) leads to a 3-year event-free survival rate of 22%. Post-ASCT, event-free survival increases to more than 52%.
The overall survival post-ASCT is 44%. The median survival of patients who do not get therapy is 3.7 months. Myelosuppression and deaths are common.
“Conventional chemotherapy fails,” Dr Diefenbach continued. “There is inadequate disease control, unacceptable toxicity, it’s not cost-effective, requires patients to be hospitalized, and there is no clear standard of care.”
On the other hand, brentuximab as first salvage is highly active with minimal adverse events in relapsed HL.
“Rash is the only grade 3-4 toxicity,” Dr Diefenbach said. “There are no significant cytopenias [and] no febrile neutropenia. Growth factor support is not required, and it’s administered outpatient.”
Using brentuximab as second-line therapy results in an ORR of 85.7% and a CR of 50%, she added. And ASCT after brentuximab shows similar successes and toxicities.
Brentuximab followed by ICE leads to high rates of PET normalization, allows successful transplantation of virtually all evaluable patients, and poses no issues with stem cell collection.
Furthermore, studies show a 92% disease-free survival, with minimal toxicity at 10 months of follow-up.
In conclusion, Dr Diefenbach said, “Maximizing disease control prior to ASCT will maximize cure from ASCT. Brentuximab vedotin is a novel agent that leads to a high ORR and high CR rate with low toxicity and outpatient administration. In contrast, conventional chemotherapy fails to provide a high CR rate, has unacceptable toxicity, and there is no single standard of care.”
For an opposing opinion on salvage in HL, see “Speaker adovcates chemo-based salvage in HL.” ![]()
Speaker advocates chemo-based salvage in HL

Credit: Rhoda Baer
NEW YORK—Physicians should use chemotherapy-based approaches—not brentuximab vedotin—as second-line therapy for recurrent
Hodgkin lymphoma (HL) prior to transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Nina Wagner-Johnston, MD, of Washington University in St Louis, Missouri, provided the meeting’s audience with a handful of arguments to support chemotherapy-based salvage regimens and a number of reasons why brentuximab is not ideal as salvage therapy in HL.
First, there is data supporting the use of chemotherapy-based salvage regimens in these patients, but the brentuximab data is lacking.
“Other issues include chemosensitivity, stem cell collection, unknown progressive multifocal leukoencephalopathy (PML) risk with brentuximab pre-ASCT [autologous stem cell transplant], cost, and alternative roles for brentuximab to enhance cure upfront post-ASCT maintenance,” Dr Wagner-Johnston said.
She also noted that chemotherapy-based salvage regimens yield high response rates with adequate stem cell collections.
“The vast majority of patients proceed to ASCT, 86% with ICE chemotherapy [ifosfamide, carboplatin, and etoposide],” she said. “Many are cured with a chemotherapy-based approach, with a 5-year PFS [progression-free survival] of 50% to 60%, with a low incidence of febrile neutropenia.”
Dr Wagner-Johnston also raised the possibility that giving patients brentuximab prior to ASCT might increase the risk of PML. The risk is already increased with lymphoma and ASCT. And in cases of PML in which patients are treated with brentuximab, the onset is rapid, with a median of about 2 months after initiation.
Furthermore, the cost of therapy clearly favors chemotherapy over brentuximab, she said. Three cycles of brentuximab at 1.8 mg/kg cost more than $58,000, and 2 cycles of brentuximab (3 weeks on, 1 week off) at 1.2 mg/kg cost more than $78,000.
In comparison, 3 cycles of ICE cost less than $38,000 and 3 cycles of ESHAP (etoposide, methylprednisolone, high-dose cytarabine, and cisplatin) cost $17,000. These costs do not account for the increased risk of febrile neutropenia.
Dr Wagner-Johnston expressed other concerns about brentuximab as well.
“Brentuximab is probably not effective to pursue as a single agent,” she said. “The addition of brentuximab to chemotherapy increases toxicity and cost. Continuous progressions years after ASCT call into question a 2-year benchmark and further highlight the importance of a maintenance approach.”
There are several unanswered pivotal questions about brentuximab, Dr Wagner-Johnston continued.
“Is brentuximab sensitivity equivalent to chemosensitivity?” she asked. “Does improved CR [complete response] rate with brentuximab-based treatment equate to better outcomes? Is the likelihood of cure higher with brentuximab-based approaches?”
She said brentuximab may be best in the upfront setting. There are more upfront cures in the highest-risk group with this novel agent, and it may allow a greater number of patients to avoid the toxicity of ASCT.
Brentuximab has demonstrated safety in phase 1/2 studies, Dr Wagner-Johnston said, adding “we await data from an ongoing phase 3 trial to determine a PFS benefit with brentuximab.”
Furthermore, post-ASCT maintenance with brentuximab may be a better alternative than salvage brentuximab pre-ASCT. In the relapsed setting, the median duration of response for brentuximab is short (6.7 months in a phase 2 study) after a median of 9 cycles.
In the phase 3 placebo-controlled trial of maintenance brentuximab following ASCT, “an interim analysis based on safety and utility recommends continuation,” Dr Wagner-Johnston said.
In conclusion, she said, “Before letting brentuximab take over, we need to confirm its safety, efficacy, and determine the best positioning of brentuximab to enhance outcomes. In the meantime, stick with what we know works.”
For the dissenting opinion on salvage in HL, see “Brentuximab tops chemo in HL, doc says.” ![]()

Credit: Rhoda Baer
NEW YORK—Physicians should use chemotherapy-based approaches—not brentuximab vedotin—as second-line therapy for recurrent
Hodgkin lymphoma (HL) prior to transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Nina Wagner-Johnston, MD, of Washington University in St Louis, Missouri, provided the meeting’s audience with a handful of arguments to support chemotherapy-based salvage regimens and a number of reasons why brentuximab is not ideal as salvage therapy in HL.
First, there is data supporting the use of chemotherapy-based salvage regimens in these patients, but the brentuximab data is lacking.
“Other issues include chemosensitivity, stem cell collection, unknown progressive multifocal leukoencephalopathy (PML) risk with brentuximab pre-ASCT [autologous stem cell transplant], cost, and alternative roles for brentuximab to enhance cure upfront post-ASCT maintenance,” Dr Wagner-Johnston said.
She also noted that chemotherapy-based salvage regimens yield high response rates with adequate stem cell collections.
“The vast majority of patients proceed to ASCT, 86% with ICE chemotherapy [ifosfamide, carboplatin, and etoposide],” she said. “Many are cured with a chemotherapy-based approach, with a 5-year PFS [progression-free survival] of 50% to 60%, with a low incidence of febrile neutropenia.”
Dr Wagner-Johnston also raised the possibility that giving patients brentuximab prior to ASCT might increase the risk of PML. The risk is already increased with lymphoma and ASCT. And in cases of PML in which patients are treated with brentuximab, the onset is rapid, with a median of about 2 months after initiation.
Furthermore, the cost of therapy clearly favors chemotherapy over brentuximab, she said. Three cycles of brentuximab at 1.8 mg/kg cost more than $58,000, and 2 cycles of brentuximab (3 weeks on, 1 week off) at 1.2 mg/kg cost more than $78,000.
In comparison, 3 cycles of ICE cost less than $38,000 and 3 cycles of ESHAP (etoposide, methylprednisolone, high-dose cytarabine, and cisplatin) cost $17,000. These costs do not account for the increased risk of febrile neutropenia.
Dr Wagner-Johnston expressed other concerns about brentuximab as well.
“Brentuximab is probably not effective to pursue as a single agent,” she said. “The addition of brentuximab to chemotherapy increases toxicity and cost. Continuous progressions years after ASCT call into question a 2-year benchmark and further highlight the importance of a maintenance approach.”
There are several unanswered pivotal questions about brentuximab, Dr Wagner-Johnston continued.
“Is brentuximab sensitivity equivalent to chemosensitivity?” she asked. “Does improved CR [complete response] rate with brentuximab-based treatment equate to better outcomes? Is the likelihood of cure higher with brentuximab-based approaches?”
She said brentuximab may be best in the upfront setting. There are more upfront cures in the highest-risk group with this novel agent, and it may allow a greater number of patients to avoid the toxicity of ASCT.
Brentuximab has demonstrated safety in phase 1/2 studies, Dr Wagner-Johnston said, adding “we await data from an ongoing phase 3 trial to determine a PFS benefit with brentuximab.”
Furthermore, post-ASCT maintenance with brentuximab may be a better alternative than salvage brentuximab pre-ASCT. In the relapsed setting, the median duration of response for brentuximab is short (6.7 months in a phase 2 study) after a median of 9 cycles.
In the phase 3 placebo-controlled trial of maintenance brentuximab following ASCT, “an interim analysis based on safety and utility recommends continuation,” Dr Wagner-Johnston said.
In conclusion, she said, “Before letting brentuximab take over, we need to confirm its safety, efficacy, and determine the best positioning of brentuximab to enhance outcomes. In the meantime, stick with what we know works.”
For the dissenting opinion on salvage in HL, see “Brentuximab tops chemo in HL, doc says.” ![]()

Credit: Rhoda Baer
NEW YORK—Physicians should use chemotherapy-based approaches—not brentuximab vedotin—as second-line therapy for recurrent
Hodgkin lymphoma (HL) prior to transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Nina Wagner-Johnston, MD, of Washington University in St Louis, Missouri, provided the meeting’s audience with a handful of arguments to support chemotherapy-based salvage regimens and a number of reasons why brentuximab is not ideal as salvage therapy in HL.
First, there is data supporting the use of chemotherapy-based salvage regimens in these patients, but the brentuximab data is lacking.
“Other issues include chemosensitivity, stem cell collection, unknown progressive multifocal leukoencephalopathy (PML) risk with brentuximab pre-ASCT [autologous stem cell transplant], cost, and alternative roles for brentuximab to enhance cure upfront post-ASCT maintenance,” Dr Wagner-Johnston said.
She also noted that chemotherapy-based salvage regimens yield high response rates with adequate stem cell collections.
“The vast majority of patients proceed to ASCT, 86% with ICE chemotherapy [ifosfamide, carboplatin, and etoposide],” she said. “Many are cured with a chemotherapy-based approach, with a 5-year PFS [progression-free survival] of 50% to 60%, with a low incidence of febrile neutropenia.”
Dr Wagner-Johnston also raised the possibility that giving patients brentuximab prior to ASCT might increase the risk of PML. The risk is already increased with lymphoma and ASCT. And in cases of PML in which patients are treated with brentuximab, the onset is rapid, with a median of about 2 months after initiation.
Furthermore, the cost of therapy clearly favors chemotherapy over brentuximab, she said. Three cycles of brentuximab at 1.8 mg/kg cost more than $58,000, and 2 cycles of brentuximab (3 weeks on, 1 week off) at 1.2 mg/kg cost more than $78,000.
In comparison, 3 cycles of ICE cost less than $38,000 and 3 cycles of ESHAP (etoposide, methylprednisolone, high-dose cytarabine, and cisplatin) cost $17,000. These costs do not account for the increased risk of febrile neutropenia.
Dr Wagner-Johnston expressed other concerns about brentuximab as well.
“Brentuximab is probably not effective to pursue as a single agent,” she said. “The addition of brentuximab to chemotherapy increases toxicity and cost. Continuous progressions years after ASCT call into question a 2-year benchmark and further highlight the importance of a maintenance approach.”
There are several unanswered pivotal questions about brentuximab, Dr Wagner-Johnston continued.
“Is brentuximab sensitivity equivalent to chemosensitivity?” she asked. “Does improved CR [complete response] rate with brentuximab-based treatment equate to better outcomes? Is the likelihood of cure higher with brentuximab-based approaches?”
She said brentuximab may be best in the upfront setting. There are more upfront cures in the highest-risk group with this novel agent, and it may allow a greater number of patients to avoid the toxicity of ASCT.
Brentuximab has demonstrated safety in phase 1/2 studies, Dr Wagner-Johnston said, adding “we await data from an ongoing phase 3 trial to determine a PFS benefit with brentuximab.”
Furthermore, post-ASCT maintenance with brentuximab may be a better alternative than salvage brentuximab pre-ASCT. In the relapsed setting, the median duration of response for brentuximab is short (6.7 months in a phase 2 study) after a median of 9 cycles.
In the phase 3 placebo-controlled trial of maintenance brentuximab following ASCT, “an interim analysis based on safety and utility recommends continuation,” Dr Wagner-Johnston said.
In conclusion, she said, “Before letting brentuximab take over, we need to confirm its safety, efficacy, and determine the best positioning of brentuximab to enhance outcomes. In the meantime, stick with what we know works.”
For the dissenting opinion on salvage in HL, see “Brentuximab tops chemo in HL, doc says.” ![]()
Thumbs-up on laparoscopic splenectomy for malignancies
LAS VEGAS – Laparoscopic splenectomy for hematologic malignancies involving the spleen is an effective surgical strategy, although the operator must expect a higher rate of conversion to laparotomy than when laparoscopic splenectomy is performed for benign hematologic diseases, Dr. Roberta Gelmini said at the annual Minimally Invasive Surgery Week.
The rate of postoperative complications, including wound infection, abdominal abscess, hemiperitoneum, and splenic vein thrombosis, is low and similar to that associated with laparoscopic splenectomy for benign hematologic diseases. The one exception is the complication of pleural effusion, which is seven- to eightfold more frequent following laparoscopic splenectomy for malignancies, probably reflecting affected patients’ larger spleen size and higher conversion rate, observed Dr. Gelmini, professor of surgery at the University of Modena and Reggio Emilia (Italy).
She presented a retrospective analysis of a series of 126 consecutive elective laparoscopic splenectomies performed in adults. Fifty-five were performed for malignant diseases, 71 for benign hematologic conditions. The two groups were well matched except that the patients with benign hematologic diseases were younger and thinner, with a mean age of 39 and a body mass index of 24.4 kg/m2, as compared with 55 years and 25.9 kg/m2 in patients undergoing laparoscopic splenectomy for hematologic malignancies.
The No. 1 indication for splenectomy in patients with benign disease was idiopathic thrombocytopenic purpura, accounting for 61% of cases. In patients with malignancies, non-Hodgkin’s lymphoma was the indication for splenectomy in two-thirds of cases.
As part of their preoperative work-up, all patients underwent ultrasound to establish spleen size and vessel diameter. Bipolar splenic length was significantly greater in the group with malignancies: a mean of 17 cm, compared with 13.4 cm in patients with benign disease.
With regard to key intraoperative findings, the mean 148-minute operative time in patients with malignancies was 22 minutes longer than in patients with benign disease. The conversion rate was 18.2% in patients with malignancies, compared with 5.6% when laparoscopic splenectomy was undertaken for benign disease. But the incidence of intraoperative blood loss greater than 500 mL was similar in the two groups, as was the transfusion rate, Dr. Gelmini reported at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
Four patients underwent conversion to laparotomy or mini-laparotomy for malignancies because of a difficult splenic hilum dissection.
Pleural effusion occurred in 7% of patients operated upon for benign disease, compared with 56% of those with hematologic malignancies. Otherwise the complication rates in the two groups were similar.
Time to refeeding was similar in the two patient groups. Mean postoperative length of hospital stay was 5.3 days in patients who underwent laparoscopic splenectomy for benign disease and similar at 5.8 days for those with malignancies.
“Our data suggest that the nature of the disease does not significantly influence the postoperative outcome,” the surgeon observed.
She added that the results of her series were quite similar to those in an earlier report by surgeons at the University of Genoa (Italy) in terms of conversion rate and complications in 38 patients who underwent laparoscopic splenectomy for benign hematologic diseases and 25 with hematologic malignancies (Tumori 2004;90:229-32).
Dr. Gelmini reported having no financial conflicts regarding her presentation.
LAS VEGAS – Laparoscopic splenectomy for hematologic malignancies involving the spleen is an effective surgical strategy, although the operator must expect a higher rate of conversion to laparotomy than when laparoscopic splenectomy is performed for benign hematologic diseases, Dr. Roberta Gelmini said at the annual Minimally Invasive Surgery Week.
The rate of postoperative complications, including wound infection, abdominal abscess, hemiperitoneum, and splenic vein thrombosis, is low and similar to that associated with laparoscopic splenectomy for benign hematologic diseases. The one exception is the complication of pleural effusion, which is seven- to eightfold more frequent following laparoscopic splenectomy for malignancies, probably reflecting affected patients’ larger spleen size and higher conversion rate, observed Dr. Gelmini, professor of surgery at the University of Modena and Reggio Emilia (Italy).
She presented a retrospective analysis of a series of 126 consecutive elective laparoscopic splenectomies performed in adults. Fifty-five were performed for malignant diseases, 71 for benign hematologic conditions. The two groups were well matched except that the patients with benign hematologic diseases were younger and thinner, with a mean age of 39 and a body mass index of 24.4 kg/m2, as compared with 55 years and 25.9 kg/m2 in patients undergoing laparoscopic splenectomy for hematologic malignancies.
The No. 1 indication for splenectomy in patients with benign disease was idiopathic thrombocytopenic purpura, accounting for 61% of cases. In patients with malignancies, non-Hodgkin’s lymphoma was the indication for splenectomy in two-thirds of cases.
As part of their preoperative work-up, all patients underwent ultrasound to establish spleen size and vessel diameter. Bipolar splenic length was significantly greater in the group with malignancies: a mean of 17 cm, compared with 13.4 cm in patients with benign disease.
With regard to key intraoperative findings, the mean 148-minute operative time in patients with malignancies was 22 minutes longer than in patients with benign disease. The conversion rate was 18.2% in patients with malignancies, compared with 5.6% when laparoscopic splenectomy was undertaken for benign disease. But the incidence of intraoperative blood loss greater than 500 mL was similar in the two groups, as was the transfusion rate, Dr. Gelmini reported at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
Four patients underwent conversion to laparotomy or mini-laparotomy for malignancies because of a difficult splenic hilum dissection.
Pleural effusion occurred in 7% of patients operated upon for benign disease, compared with 56% of those with hematologic malignancies. Otherwise the complication rates in the two groups were similar.
Time to refeeding was similar in the two patient groups. Mean postoperative length of hospital stay was 5.3 days in patients who underwent laparoscopic splenectomy for benign disease and similar at 5.8 days for those with malignancies.
“Our data suggest that the nature of the disease does not significantly influence the postoperative outcome,” the surgeon observed.
She added that the results of her series were quite similar to those in an earlier report by surgeons at the University of Genoa (Italy) in terms of conversion rate and complications in 38 patients who underwent laparoscopic splenectomy for benign hematologic diseases and 25 with hematologic malignancies (Tumori 2004;90:229-32).
Dr. Gelmini reported having no financial conflicts regarding her presentation.
LAS VEGAS – Laparoscopic splenectomy for hematologic malignancies involving the spleen is an effective surgical strategy, although the operator must expect a higher rate of conversion to laparotomy than when laparoscopic splenectomy is performed for benign hematologic diseases, Dr. Roberta Gelmini said at the annual Minimally Invasive Surgery Week.
The rate of postoperative complications, including wound infection, abdominal abscess, hemiperitoneum, and splenic vein thrombosis, is low and similar to that associated with laparoscopic splenectomy for benign hematologic diseases. The one exception is the complication of pleural effusion, which is seven- to eightfold more frequent following laparoscopic splenectomy for malignancies, probably reflecting affected patients’ larger spleen size and higher conversion rate, observed Dr. Gelmini, professor of surgery at the University of Modena and Reggio Emilia (Italy).
She presented a retrospective analysis of a series of 126 consecutive elective laparoscopic splenectomies performed in adults. Fifty-five were performed for malignant diseases, 71 for benign hematologic conditions. The two groups were well matched except that the patients with benign hematologic diseases were younger and thinner, with a mean age of 39 and a body mass index of 24.4 kg/m2, as compared with 55 years and 25.9 kg/m2 in patients undergoing laparoscopic splenectomy for hematologic malignancies.
The No. 1 indication for splenectomy in patients with benign disease was idiopathic thrombocytopenic purpura, accounting for 61% of cases. In patients with malignancies, non-Hodgkin’s lymphoma was the indication for splenectomy in two-thirds of cases.
As part of their preoperative work-up, all patients underwent ultrasound to establish spleen size and vessel diameter. Bipolar splenic length was significantly greater in the group with malignancies: a mean of 17 cm, compared with 13.4 cm in patients with benign disease.
With regard to key intraoperative findings, the mean 148-minute operative time in patients with malignancies was 22 minutes longer than in patients with benign disease. The conversion rate was 18.2% in patients with malignancies, compared with 5.6% when laparoscopic splenectomy was undertaken for benign disease. But the incidence of intraoperative blood loss greater than 500 mL was similar in the two groups, as was the transfusion rate, Dr. Gelmini reported at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
Four patients underwent conversion to laparotomy or mini-laparotomy for malignancies because of a difficult splenic hilum dissection.
Pleural effusion occurred in 7% of patients operated upon for benign disease, compared with 56% of those with hematologic malignancies. Otherwise the complication rates in the two groups were similar.
Time to refeeding was similar in the two patient groups. Mean postoperative length of hospital stay was 5.3 days in patients who underwent laparoscopic splenectomy for benign disease and similar at 5.8 days for those with malignancies.
“Our data suggest that the nature of the disease does not significantly influence the postoperative outcome,” the surgeon observed.
She added that the results of her series were quite similar to those in an earlier report by surgeons at the University of Genoa (Italy) in terms of conversion rate and complications in 38 patients who underwent laparoscopic splenectomy for benign hematologic diseases and 25 with hematologic malignancies (Tumori 2004;90:229-32).
Dr. Gelmini reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM MINIMALLY INVASIVE SURGERY WEEK
Obesity affects toxicity of immunotherapy
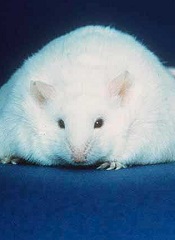
Immunotherapy that can be effective against tumors in young, thin mice can be lethal to obese ones, according to research published in The Journal of Experimental Medicine.
Investigators conducted experiments in mouse models to determine if there is a subset of cancer patients for whom certain immunotherapies might be especially toxic.
The group found a potential link between body fat and the risk of toxicity from some types of immunotherapy.
“Cancer is primarily considered a disease of the aged, and yet preclinical studies generally use young, lean animal models that may not be reflective of the ‘typical’ cancer patient,” said study author Annie Mirsoian, a PhD candidate at the University of California, Davis in Sacramento.
“Aging is a dynamic process that is characterized by increases in inflammatory factors, as well as a shift in body composition where there is a gradual loss of lean muscle mass and an increase in fat accumulation, which effect how the immune system functions.”
Mirsoian and her colleagues sought to determine if, by adjusting the mouse model to more closely reflect the cancer patient phenotype (advanced age and overweight), they could better understand the discrepancies between animal study outcomes and those in patients in the clinic.
So the team examined aged mice on standard diets and compared those to aged mice that were calorie-restricted throughout their lives.
The investigators found that calorie restriction plays a protective role against toxicity. When aged mice ate their standard diet freely throughout life, they became obese and ultimately experienced lethal adverse reactions after receiving a systemic immunotherapy regimen.
“We know that people who are obese in general are at higher risk for complications from surgery, radiation, and chemotherapy,” said study author Arta Monjazeb, MD, PhD, of the University of California, Davis.
“We know that obese people have higher levels of inflammatory markers in their blood, but there is a lack of data examining the effects of obesity on cancer treatment outcomes.”
In follow-up experiments, the investigators found that young mice that are obese also endure similar toxic consequences, demonstrating that fat is a critical factor in toxic responses to stimulatory anticancer immunotherapy regimens.
“It is important to note, however, that the aged mice on standard diets succumbed to lethality at a quicker rate than young obese mice,” Mirsoian said. “Although our data demonstrate that obesity plays a central role in the development of adverse effects, future studies will focus on examining the aged immune system and cellular characteristics that may have enhanced the sensitivity of these mice to inflammation.”
This study follows an earlier paper, which demonstrated that while young, lean mice tolerate immunotherapy regimens without toxicity, the same regimen for an aged cohort resulted in lethal consequences. The new paper takes a deeper look into how fat deposition throughout aging can be critical in determining treatment tolerance and efficacy.
“Obesity has become an epidemic in our society and is now also affecting younger populations,” Mirsoian said. “Therefore, it’s likely that what the ‘typical’ cancer patient looks like will change. Our findings demonstrate the importance of having preclinical animal models that reflect the clinical scenario.”
“Changing the characteristics of our mouse models allowed for a more accurate determination of possible adverse reactions to therapy and more closely modeled what has been reported in the clinic with stimulatory immunotherapies.”
The investigators said factors like age, fat content, and the types of infections experienced throughout life together shape how the immune system reacts, and they will continue to work on improving their modeling system to reflect these changes.
They project that improvements in mouse modeling may help produce data that can better modulate treatment choices for patients, as well as identify early which patients would benefit from the inclusion of drugs to prevent adverse reactions while maintaining anticancer efficacy. ![]()

Immunotherapy that can be effective against tumors in young, thin mice can be lethal to obese ones, according to research published in The Journal of Experimental Medicine.
Investigators conducted experiments in mouse models to determine if there is a subset of cancer patients for whom certain immunotherapies might be especially toxic.
The group found a potential link between body fat and the risk of toxicity from some types of immunotherapy.
“Cancer is primarily considered a disease of the aged, and yet preclinical studies generally use young, lean animal models that may not be reflective of the ‘typical’ cancer patient,” said study author Annie Mirsoian, a PhD candidate at the University of California, Davis in Sacramento.
“Aging is a dynamic process that is characterized by increases in inflammatory factors, as well as a shift in body composition where there is a gradual loss of lean muscle mass and an increase in fat accumulation, which effect how the immune system functions.”
Mirsoian and her colleagues sought to determine if, by adjusting the mouse model to more closely reflect the cancer patient phenotype (advanced age and overweight), they could better understand the discrepancies between animal study outcomes and those in patients in the clinic.
So the team examined aged mice on standard diets and compared those to aged mice that were calorie-restricted throughout their lives.
The investigators found that calorie restriction plays a protective role against toxicity. When aged mice ate their standard diet freely throughout life, they became obese and ultimately experienced lethal adverse reactions after receiving a systemic immunotherapy regimen.
“We know that people who are obese in general are at higher risk for complications from surgery, radiation, and chemotherapy,” said study author Arta Monjazeb, MD, PhD, of the University of California, Davis.
“We know that obese people have higher levels of inflammatory markers in their blood, but there is a lack of data examining the effects of obesity on cancer treatment outcomes.”
In follow-up experiments, the investigators found that young mice that are obese also endure similar toxic consequences, demonstrating that fat is a critical factor in toxic responses to stimulatory anticancer immunotherapy regimens.
“It is important to note, however, that the aged mice on standard diets succumbed to lethality at a quicker rate than young obese mice,” Mirsoian said. “Although our data demonstrate that obesity plays a central role in the development of adverse effects, future studies will focus on examining the aged immune system and cellular characteristics that may have enhanced the sensitivity of these mice to inflammation.”
This study follows an earlier paper, which demonstrated that while young, lean mice tolerate immunotherapy regimens without toxicity, the same regimen for an aged cohort resulted in lethal consequences. The new paper takes a deeper look into how fat deposition throughout aging can be critical in determining treatment tolerance and efficacy.
“Obesity has become an epidemic in our society and is now also affecting younger populations,” Mirsoian said. “Therefore, it’s likely that what the ‘typical’ cancer patient looks like will change. Our findings demonstrate the importance of having preclinical animal models that reflect the clinical scenario.”
“Changing the characteristics of our mouse models allowed for a more accurate determination of possible adverse reactions to therapy and more closely modeled what has been reported in the clinic with stimulatory immunotherapies.”
The investigators said factors like age, fat content, and the types of infections experienced throughout life together shape how the immune system reacts, and they will continue to work on improving their modeling system to reflect these changes.
They project that improvements in mouse modeling may help produce data that can better modulate treatment choices for patients, as well as identify early which patients would benefit from the inclusion of drugs to prevent adverse reactions while maintaining anticancer efficacy. ![]()

Immunotherapy that can be effective against tumors in young, thin mice can be lethal to obese ones, according to research published in The Journal of Experimental Medicine.
Investigators conducted experiments in mouse models to determine if there is a subset of cancer patients for whom certain immunotherapies might be especially toxic.
The group found a potential link between body fat and the risk of toxicity from some types of immunotherapy.
“Cancer is primarily considered a disease of the aged, and yet preclinical studies generally use young, lean animal models that may not be reflective of the ‘typical’ cancer patient,” said study author Annie Mirsoian, a PhD candidate at the University of California, Davis in Sacramento.
“Aging is a dynamic process that is characterized by increases in inflammatory factors, as well as a shift in body composition where there is a gradual loss of lean muscle mass and an increase in fat accumulation, which effect how the immune system functions.”
Mirsoian and her colleagues sought to determine if, by adjusting the mouse model to more closely reflect the cancer patient phenotype (advanced age and overweight), they could better understand the discrepancies between animal study outcomes and those in patients in the clinic.
So the team examined aged mice on standard diets and compared those to aged mice that were calorie-restricted throughout their lives.
The investigators found that calorie restriction plays a protective role against toxicity. When aged mice ate their standard diet freely throughout life, they became obese and ultimately experienced lethal adverse reactions after receiving a systemic immunotherapy regimen.
“We know that people who are obese in general are at higher risk for complications from surgery, radiation, and chemotherapy,” said study author Arta Monjazeb, MD, PhD, of the University of California, Davis.
“We know that obese people have higher levels of inflammatory markers in their blood, but there is a lack of data examining the effects of obesity on cancer treatment outcomes.”
In follow-up experiments, the investigators found that young mice that are obese also endure similar toxic consequences, demonstrating that fat is a critical factor in toxic responses to stimulatory anticancer immunotherapy regimens.
“It is important to note, however, that the aged mice on standard diets succumbed to lethality at a quicker rate than young obese mice,” Mirsoian said. “Although our data demonstrate that obesity plays a central role in the development of adverse effects, future studies will focus on examining the aged immune system and cellular characteristics that may have enhanced the sensitivity of these mice to inflammation.”
This study follows an earlier paper, which demonstrated that while young, lean mice tolerate immunotherapy regimens without toxicity, the same regimen for an aged cohort resulted in lethal consequences. The new paper takes a deeper look into how fat deposition throughout aging can be critical in determining treatment tolerance and efficacy.
“Obesity has become an epidemic in our society and is now also affecting younger populations,” Mirsoian said. “Therefore, it’s likely that what the ‘typical’ cancer patient looks like will change. Our findings demonstrate the importance of having preclinical animal models that reflect the clinical scenario.”
“Changing the characteristics of our mouse models allowed for a more accurate determination of possible adverse reactions to therapy and more closely modeled what has been reported in the clinic with stimulatory immunotherapies.”
The investigators said factors like age, fat content, and the types of infections experienced throughout life together shape how the immune system reacts, and they will continue to work on improving their modeling system to reflect these changes.
They project that improvements in mouse modeling may help produce data that can better modulate treatment choices for patients, as well as identify early which patients would benefit from the inclusion of drugs to prevent adverse reactions while maintaining anticancer efficacy. ![]()
New agents challenge role of transplant in high-risk CLL

Credit: Chad McNeeley
NEW YORK—The role of allogeneic hematopoietic stem cell transplant (HSCT) for patients with high-risk chronic lymphocytic leukemia (CLL) is changing in the age of targeted therapy.
While allogeneic HSCT has been considered standard treatment for these patients, the question arises whether it will maintain its position in the era “of all these wonderful new drugs,” said David Maloney, MD, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
Dr Maloney undertook to convince the audience at the Lymphoma & Myeloma 2014 congress that there is still a role for allogeneic transplant in CLL patients.
He noted that early allogeneic transplant trials used myeloablative conditioning regimens, which were “prohibitively toxic.” They have now given way to reduced-intensity regimens.
“But the breakthrough came about when it was realized that the reason that allogeneic transplant could cure patients with CLL had really nothing to do with their conditioning regimen . . . ,” he said. “[I]t was probably the donor T cells providing immunologic activity and graft-vs-host activity that was actually able to provide graft-vs-tumor activity and cure patients.”
Seattle regimen
Dr Maloney described the reduced-intensity regimen used in Seattle—fludarabine and 2 Gy total body irradiation. The single dose of radiation is typically 1/6 of what a myeloablative regimen would be.
“This is truly an outpatient regimen,” he said. “Most patients, 50%, get through this without ever being in the hospital.”
Follow-up at 5 years showed overall survival to be 43%, progression-free survival 36%, complete responses 52%, and relapse 34%.
“This may not look very good,” Dr Maloney said, but these are fludarabine-refractory CLL patients whose expected median survival is around 12 months.
Dr Maloney noted that approximately the same outcomes were achieved whether the graft was from a matched related or unrelated donor, and cytogenetics really didn’t play a huge role in outcome.
The biggest factor affecting outcome was lymph node size. Patients with nodes 5 cm or larger did very poorly. And patients with lymph nodes smaller than 5 cm, irrespective of white cell count or bone marrow infiltration, actually did quite well in comparison to the group with large lymph nodes.
“So the graft-vs-tumor activity seems to be limited in its ability to get rid of bulky lymphadenopathy in this population,” Dr Maloney said.
Prior alemtuzumab therapy was also associated with the worst outcome in terms of relapse and disease progression.
Patients without comorbidities and without bulky lymphadenopathy have a very good outcome, Dr Maloney noted, saying, “You can cure 60% to 70% with an allogeneic transplant.”
He also pointed out that many groups are now doing this type of transplant with related and unrelated donors.
Transplant vs new agents
In addition to offering a potential cure, allogeneic transplant may provide better-functioning hematopoietic and immune systems after transplant than before, especially in those patients who received FCR (fludarabine, mitoxantrone, and rituximab) or other treatments.
Transplant, while potentially curative with a high complete response rate, has early non-relapse mortality around 15% to 20%.
“So this makes it hard to position in this era of pills that you can take,” Dr Maloney said.
He pointed out that while ibrutinib and idelalisib have excellent outcomes and overall survival, “these studies are very, very early . . . but obviously extremely promising.”
A group of European physicians recently published a position paper proposing a treatment algorithm that includes transplant for high-risk CLL patients. The algorithm indicates that relapsed/refractory patients should try the novel agents first.
Then, if patients respond, they can continue with the novel agent or proceed to transplant. Patients with lower-risk disease or those who are a higher transplant risk should probably continue on the novel agent.
Those who are younger with higher-risk disease, such as a 17p deletion, or who are a low transplant risk may be willing to choose transplant earlier.
Patients who do not respond to the novel agents can consider transplant or an alternative salvage regimen.
“[O]bviously, this is extremely controversial,” Dr Maloney said, “and what everyone is going to do is use these new agents to push transplant further down the road. And I think that’s appropriate.”
At the very least, Dr Maloney believes patients deserve a discussion of options early on.
He added that chimeric antigen receptor (CAR) T cells “will likely bump transplant even down another notch” because patients are likely to be willing to take the risk of CAR T cells before they’ll take the risk of chronic graft-vs-host disease with an unrelated donor.” ![]()

Credit: Chad McNeeley
NEW YORK—The role of allogeneic hematopoietic stem cell transplant (HSCT) for patients with high-risk chronic lymphocytic leukemia (CLL) is changing in the age of targeted therapy.
While allogeneic HSCT has been considered standard treatment for these patients, the question arises whether it will maintain its position in the era “of all these wonderful new drugs,” said David Maloney, MD, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
Dr Maloney undertook to convince the audience at the Lymphoma & Myeloma 2014 congress that there is still a role for allogeneic transplant in CLL patients.
He noted that early allogeneic transplant trials used myeloablative conditioning regimens, which were “prohibitively toxic.” They have now given way to reduced-intensity regimens.
“But the breakthrough came about when it was realized that the reason that allogeneic transplant could cure patients with CLL had really nothing to do with their conditioning regimen . . . ,” he said. “[I]t was probably the donor T cells providing immunologic activity and graft-vs-host activity that was actually able to provide graft-vs-tumor activity and cure patients.”
Seattle regimen
Dr Maloney described the reduced-intensity regimen used in Seattle—fludarabine and 2 Gy total body irradiation. The single dose of radiation is typically 1/6 of what a myeloablative regimen would be.
“This is truly an outpatient regimen,” he said. “Most patients, 50%, get through this without ever being in the hospital.”
Follow-up at 5 years showed overall survival to be 43%, progression-free survival 36%, complete responses 52%, and relapse 34%.
“This may not look very good,” Dr Maloney said, but these are fludarabine-refractory CLL patients whose expected median survival is around 12 months.
Dr Maloney noted that approximately the same outcomes were achieved whether the graft was from a matched related or unrelated donor, and cytogenetics really didn’t play a huge role in outcome.
The biggest factor affecting outcome was lymph node size. Patients with nodes 5 cm or larger did very poorly. And patients with lymph nodes smaller than 5 cm, irrespective of white cell count or bone marrow infiltration, actually did quite well in comparison to the group with large lymph nodes.
“So the graft-vs-tumor activity seems to be limited in its ability to get rid of bulky lymphadenopathy in this population,” Dr Maloney said.
Prior alemtuzumab therapy was also associated with the worst outcome in terms of relapse and disease progression.
Patients without comorbidities and without bulky lymphadenopathy have a very good outcome, Dr Maloney noted, saying, “You can cure 60% to 70% with an allogeneic transplant.”
He also pointed out that many groups are now doing this type of transplant with related and unrelated donors.
Transplant vs new agents
In addition to offering a potential cure, allogeneic transplant may provide better-functioning hematopoietic and immune systems after transplant than before, especially in those patients who received FCR (fludarabine, mitoxantrone, and rituximab) or other treatments.
Transplant, while potentially curative with a high complete response rate, has early non-relapse mortality around 15% to 20%.
“So this makes it hard to position in this era of pills that you can take,” Dr Maloney said.
He pointed out that while ibrutinib and idelalisib have excellent outcomes and overall survival, “these studies are very, very early . . . but obviously extremely promising.”
A group of European physicians recently published a position paper proposing a treatment algorithm that includes transplant for high-risk CLL patients. The algorithm indicates that relapsed/refractory patients should try the novel agents first.
Then, if patients respond, they can continue with the novel agent or proceed to transplant. Patients with lower-risk disease or those who are a higher transplant risk should probably continue on the novel agent.
Those who are younger with higher-risk disease, such as a 17p deletion, or who are a low transplant risk may be willing to choose transplant earlier.
Patients who do not respond to the novel agents can consider transplant or an alternative salvage regimen.
“[O]bviously, this is extremely controversial,” Dr Maloney said, “and what everyone is going to do is use these new agents to push transplant further down the road. And I think that’s appropriate.”
At the very least, Dr Maloney believes patients deserve a discussion of options early on.
He added that chimeric antigen receptor (CAR) T cells “will likely bump transplant even down another notch” because patients are likely to be willing to take the risk of CAR T cells before they’ll take the risk of chronic graft-vs-host disease with an unrelated donor.” ![]()

Credit: Chad McNeeley
NEW YORK—The role of allogeneic hematopoietic stem cell transplant (HSCT) for patients with high-risk chronic lymphocytic leukemia (CLL) is changing in the age of targeted therapy.
While allogeneic HSCT has been considered standard treatment for these patients, the question arises whether it will maintain its position in the era “of all these wonderful new drugs,” said David Maloney, MD, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
Dr Maloney undertook to convince the audience at the Lymphoma & Myeloma 2014 congress that there is still a role for allogeneic transplant in CLL patients.
He noted that early allogeneic transplant trials used myeloablative conditioning regimens, which were “prohibitively toxic.” They have now given way to reduced-intensity regimens.
“But the breakthrough came about when it was realized that the reason that allogeneic transplant could cure patients with CLL had really nothing to do with their conditioning regimen . . . ,” he said. “[I]t was probably the donor T cells providing immunologic activity and graft-vs-host activity that was actually able to provide graft-vs-tumor activity and cure patients.”
Seattle regimen
Dr Maloney described the reduced-intensity regimen used in Seattle—fludarabine and 2 Gy total body irradiation. The single dose of radiation is typically 1/6 of what a myeloablative regimen would be.
“This is truly an outpatient regimen,” he said. “Most patients, 50%, get through this without ever being in the hospital.”
Follow-up at 5 years showed overall survival to be 43%, progression-free survival 36%, complete responses 52%, and relapse 34%.
“This may not look very good,” Dr Maloney said, but these are fludarabine-refractory CLL patients whose expected median survival is around 12 months.
Dr Maloney noted that approximately the same outcomes were achieved whether the graft was from a matched related or unrelated donor, and cytogenetics really didn’t play a huge role in outcome.
The biggest factor affecting outcome was lymph node size. Patients with nodes 5 cm or larger did very poorly. And patients with lymph nodes smaller than 5 cm, irrespective of white cell count or bone marrow infiltration, actually did quite well in comparison to the group with large lymph nodes.
“So the graft-vs-tumor activity seems to be limited in its ability to get rid of bulky lymphadenopathy in this population,” Dr Maloney said.
Prior alemtuzumab therapy was also associated with the worst outcome in terms of relapse and disease progression.
Patients without comorbidities and without bulky lymphadenopathy have a very good outcome, Dr Maloney noted, saying, “You can cure 60% to 70% with an allogeneic transplant.”
He also pointed out that many groups are now doing this type of transplant with related and unrelated donors.
Transplant vs new agents
In addition to offering a potential cure, allogeneic transplant may provide better-functioning hematopoietic and immune systems after transplant than before, especially in those patients who received FCR (fludarabine, mitoxantrone, and rituximab) or other treatments.
Transplant, while potentially curative with a high complete response rate, has early non-relapse mortality around 15% to 20%.
“So this makes it hard to position in this era of pills that you can take,” Dr Maloney said.
He pointed out that while ibrutinib and idelalisib have excellent outcomes and overall survival, “these studies are very, very early . . . but obviously extremely promising.”
A group of European physicians recently published a position paper proposing a treatment algorithm that includes transplant for high-risk CLL patients. The algorithm indicates that relapsed/refractory patients should try the novel agents first.
Then, if patients respond, they can continue with the novel agent or proceed to transplant. Patients with lower-risk disease or those who are a higher transplant risk should probably continue on the novel agent.
Those who are younger with higher-risk disease, such as a 17p deletion, or who are a low transplant risk may be willing to choose transplant earlier.
Patients who do not respond to the novel agents can consider transplant or an alternative salvage regimen.
“[O]bviously, this is extremely controversial,” Dr Maloney said, “and what everyone is going to do is use these new agents to push transplant further down the road. And I think that’s appropriate.”
At the very least, Dr Maloney believes patients deserve a discussion of options early on.
He added that chimeric antigen receptor (CAR) T cells “will likely bump transplant even down another notch” because patients are likely to be willing to take the risk of CAR T cells before they’ll take the risk of chronic graft-vs-host disease with an unrelated donor.” ![]()
Device monitors methotrexate levels faster

Credit: Juan D. Alfonso
A new device can measure methotrexate levels in a patient’s blood in less than a minute, according to research published in Biosensors and Bioelectronics.
Researchers say this nanoscale device is just as accurate and 10 times less expensive than equipment currently used in hospitals.
It has an optical system that can rapidly gauge the optimal dose of methotrexate a patient needs, thereby reducing the risk of adverse effects.
“While effective, methotrexate is also highly toxic and can damage the healthy cells of patients, hence the importance of closely monitoring the drug’s concentration in the serum of treated individuals to adjust the dosage,” said study author Jean François Masson, PhD, of the University of Montreal in Quebec, Canada.
“The operation of the current [methotrexate monitoring] device is based on a cumbersome, expensive platform that requires experienced personnel because of the many samples that need to be manipulated.”
With this in mind, Dr Masson and his colleagues set out to simplify methotrexate monitoring.
In the course of their research, the team developed and manufactured a miniaturized device that works by surface plasmon resonance. It measures the concentration of serum methotrexate through gold nanoparticles on the surface of a receptacle.
In “competing” with methotrexate to block the enzyme dihydrofolate reductase, the gold nanoparticles change the color of the light detected by the instrument. And the color of the light detected reflects the exact concentration of the drug in the blood sample.
The researchers compared the accuracy of measurements taken with the new device to those taken with equipment used at the Maisonneuve-Rosemont Hospital in Montreal.
“Testing was conclusive,” Dr Masson said. “Not only were the measurements as accurate, but our device took less than 60 seconds to produce results, compared to 30 minutes for current devices.”
Moreover, the comparative tests were performed by lab technicians who were not experienced with surface plasmon resonance and did not encounter major difficulties in operating the new equipment or obtaining the same conclusive results as Dr Masson and his research team.
“In the near future, we can foresee the device in doctors’ offices or even at the bedside, where patients would receive individualized and optimal doses while minimizing the risk of complications,” Dr Masson said.
“While traditional equipment requires an investment of around $100,000, the new mobile device would likely cost 10 times less, around $10,000.” ![]()

Credit: Juan D. Alfonso
A new device can measure methotrexate levels in a patient’s blood in less than a minute, according to research published in Biosensors and Bioelectronics.
Researchers say this nanoscale device is just as accurate and 10 times less expensive than equipment currently used in hospitals.
It has an optical system that can rapidly gauge the optimal dose of methotrexate a patient needs, thereby reducing the risk of adverse effects.
“While effective, methotrexate is also highly toxic and can damage the healthy cells of patients, hence the importance of closely monitoring the drug’s concentration in the serum of treated individuals to adjust the dosage,” said study author Jean François Masson, PhD, of the University of Montreal in Quebec, Canada.
“The operation of the current [methotrexate monitoring] device is based on a cumbersome, expensive platform that requires experienced personnel because of the many samples that need to be manipulated.”
With this in mind, Dr Masson and his colleagues set out to simplify methotrexate monitoring.
In the course of their research, the team developed and manufactured a miniaturized device that works by surface plasmon resonance. It measures the concentration of serum methotrexate through gold nanoparticles on the surface of a receptacle.
In “competing” with methotrexate to block the enzyme dihydrofolate reductase, the gold nanoparticles change the color of the light detected by the instrument. And the color of the light detected reflects the exact concentration of the drug in the blood sample.
The researchers compared the accuracy of measurements taken with the new device to those taken with equipment used at the Maisonneuve-Rosemont Hospital in Montreal.
“Testing was conclusive,” Dr Masson said. “Not only were the measurements as accurate, but our device took less than 60 seconds to produce results, compared to 30 minutes for current devices.”
Moreover, the comparative tests were performed by lab technicians who were not experienced with surface plasmon resonance and did not encounter major difficulties in operating the new equipment or obtaining the same conclusive results as Dr Masson and his research team.
“In the near future, we can foresee the device in doctors’ offices or even at the bedside, where patients would receive individualized and optimal doses while minimizing the risk of complications,” Dr Masson said.
“While traditional equipment requires an investment of around $100,000, the new mobile device would likely cost 10 times less, around $10,000.” ![]()

Credit: Juan D. Alfonso
A new device can measure methotrexate levels in a patient’s blood in less than a minute, according to research published in Biosensors and Bioelectronics.
Researchers say this nanoscale device is just as accurate and 10 times less expensive than equipment currently used in hospitals.
It has an optical system that can rapidly gauge the optimal dose of methotrexate a patient needs, thereby reducing the risk of adverse effects.
“While effective, methotrexate is also highly toxic and can damage the healthy cells of patients, hence the importance of closely monitoring the drug’s concentration in the serum of treated individuals to adjust the dosage,” said study author Jean François Masson, PhD, of the University of Montreal in Quebec, Canada.
“The operation of the current [methotrexate monitoring] device is based on a cumbersome, expensive platform that requires experienced personnel because of the many samples that need to be manipulated.”
With this in mind, Dr Masson and his colleagues set out to simplify methotrexate monitoring.
In the course of their research, the team developed and manufactured a miniaturized device that works by surface plasmon resonance. It measures the concentration of serum methotrexate through gold nanoparticles on the surface of a receptacle.
In “competing” with methotrexate to block the enzyme dihydrofolate reductase, the gold nanoparticles change the color of the light detected by the instrument. And the color of the light detected reflects the exact concentration of the drug in the blood sample.
The researchers compared the accuracy of measurements taken with the new device to those taken with equipment used at the Maisonneuve-Rosemont Hospital in Montreal.
“Testing was conclusive,” Dr Masson said. “Not only were the measurements as accurate, but our device took less than 60 seconds to produce results, compared to 30 minutes for current devices.”
Moreover, the comparative tests were performed by lab technicians who were not experienced with surface plasmon resonance and did not encounter major difficulties in operating the new equipment or obtaining the same conclusive results as Dr Masson and his research team.
“In the near future, we can foresee the device in doctors’ offices or even at the bedside, where patients would receive individualized and optimal doses while minimizing the risk of complications,” Dr Masson said.
“While traditional equipment requires an investment of around $100,000, the new mobile device would likely cost 10 times less, around $10,000.”
Managing CNS relapse in cardiac lymphoma

Two cases of central nervous system (CNS) relapse in patients with primary cardiac lymphoma underline the importance of CNS evaluation in these patients, according to researchers.
Cardiac lymphoma is a rare condition, and doctors often overlook the potential of metastasis to the CNS, said study author Niccolò Frungillo, MD, of the European Institute of Oncology in Milan, Italy.
“In my opinion, it is very important to identify prognostic factors that predict the brain relapse of lymphoma,” he said. “It’s a rare—but often fatal—complication.”
In ecancermedicalscience, Dr Frungillo and his colleagues described the diagnosis and treatment of two women with cardiac lymphoma who achieved remission and later presented with isolated CNS relapse.
Doctors diagnosed the patients via endomyocardial biopsy, and results were consistent with diffuse large B-cell lymphoma in both cases. Immunochemotherapy produced complete remissions (CRs) in both women.
The patients then experienced isolated CNS relapses, one 8 weeks after achieving a CR, and one 5 weeks after CR.
In the first patient, MRI and cerebrospinal fluid confirmed her relapse, after she presented with a 3-day history of headache and vomiting.
The patient then received high-dose methotrexate and high-dose cytarabine, which prompted a second CR. She went on to receive an autologous stem cell transplant but died before engraftment due to a pulmonary infection.
The second patient received systemic CNS prophylaxis with high-dose methotrexate prior to her relapse. Nevertheless, one week after she was declared lymphoma-free, she presented with headache and a deterioration of motor skills.
MRI and lumbar puncture confirmed her relapse, and she received induction chemotherapy with high-dose methotrexate. Her disease progressed after two courses of treatment, so she was placed on salvage therapy with cytarabine and high-dose methotrexate.
Whole-brain radiotherapy prompted a CR, and the patient went on to receive an autologous stem cell transplant.
The researchers noted that isolated CNS relapse is very uncommon in cardiac lymphoma, but CNS evaluation should be considered. Additional studies are needed to determine the appropriate management of CNS relapse in cardiac lymphoma.

Two cases of central nervous system (CNS) relapse in patients with primary cardiac lymphoma underline the importance of CNS evaluation in these patients, according to researchers.
Cardiac lymphoma is a rare condition, and doctors often overlook the potential of metastasis to the CNS, said study author Niccolò Frungillo, MD, of the European Institute of Oncology in Milan, Italy.
“In my opinion, it is very important to identify prognostic factors that predict the brain relapse of lymphoma,” he said. “It’s a rare—but often fatal—complication.”
In ecancermedicalscience, Dr Frungillo and his colleagues described the diagnosis and treatment of two women with cardiac lymphoma who achieved remission and later presented with isolated CNS relapse.
Doctors diagnosed the patients via endomyocardial biopsy, and results were consistent with diffuse large B-cell lymphoma in both cases. Immunochemotherapy produced complete remissions (CRs) in both women.
The patients then experienced isolated CNS relapses, one 8 weeks after achieving a CR, and one 5 weeks after CR.
In the first patient, MRI and cerebrospinal fluid confirmed her relapse, after she presented with a 3-day history of headache and vomiting.
The patient then received high-dose methotrexate and high-dose cytarabine, which prompted a second CR. She went on to receive an autologous stem cell transplant but died before engraftment due to a pulmonary infection.
The second patient received systemic CNS prophylaxis with high-dose methotrexate prior to her relapse. Nevertheless, one week after she was declared lymphoma-free, she presented with headache and a deterioration of motor skills.
MRI and lumbar puncture confirmed her relapse, and she received induction chemotherapy with high-dose methotrexate. Her disease progressed after two courses of treatment, so she was placed on salvage therapy with cytarabine and high-dose methotrexate.
Whole-brain radiotherapy prompted a CR, and the patient went on to receive an autologous stem cell transplant.
The researchers noted that isolated CNS relapse is very uncommon in cardiac lymphoma, but CNS evaluation should be considered. Additional studies are needed to determine the appropriate management of CNS relapse in cardiac lymphoma.

Two cases of central nervous system (CNS) relapse in patients with primary cardiac lymphoma underline the importance of CNS evaluation in these patients, according to researchers.
Cardiac lymphoma is a rare condition, and doctors often overlook the potential of metastasis to the CNS, said study author Niccolò Frungillo, MD, of the European Institute of Oncology in Milan, Italy.
“In my opinion, it is very important to identify prognostic factors that predict the brain relapse of lymphoma,” he said. “It’s a rare—but often fatal—complication.”
In ecancermedicalscience, Dr Frungillo and his colleagues described the diagnosis and treatment of two women with cardiac lymphoma who achieved remission and later presented with isolated CNS relapse.
Doctors diagnosed the patients via endomyocardial biopsy, and results were consistent with diffuse large B-cell lymphoma in both cases. Immunochemotherapy produced complete remissions (CRs) in both women.
The patients then experienced isolated CNS relapses, one 8 weeks after achieving a CR, and one 5 weeks after CR.
In the first patient, MRI and cerebrospinal fluid confirmed her relapse, after she presented with a 3-day history of headache and vomiting.
The patient then received high-dose methotrexate and high-dose cytarabine, which prompted a second CR. She went on to receive an autologous stem cell transplant but died before engraftment due to a pulmonary infection.
The second patient received systemic CNS prophylaxis with high-dose methotrexate prior to her relapse. Nevertheless, one week after she was declared lymphoma-free, she presented with headache and a deterioration of motor skills.
MRI and lumbar puncture confirmed her relapse, and she received induction chemotherapy with high-dose methotrexate. Her disease progressed after two courses of treatment, so she was placed on salvage therapy with cytarabine and high-dose methotrexate.
Whole-brain radiotherapy prompted a CR, and the patient went on to receive an autologous stem cell transplant.
The researchers noted that isolated CNS relapse is very uncommon in cardiac lymphoma, but CNS evaluation should be considered. Additional studies are needed to determine the appropriate management of CNS relapse in cardiac lymphoma.
Cancer survivors face financial, work-related issues

chemotherapy
Credit: Rhoda Baer
Many US cancer survivors may be experiencing financial or work-related hardship, a new survey suggests.
Twenty-seven percent of the nearly 1600 survivors surveyed reported at least one financial problem, such as debt or bankruptcy.
And 37% reported having to modify work plans, such as taking extended time off or delaying retirement.
Women, younger survivors, racial/ethnic minorities, and uninsured survivors were all disproportionally burdened by these challenges.
This research (abstract 238*) was presented in a presscast prior to the 2014 Palliative Care in Oncology Symposium, which is scheduled to take place October 24-25 at the Westin Boston Waterfront in Boston.
“We found that many cancer survivors, particularly those who are younger or from underserved populations, experience financial or work-related hardship—even when insured and years out from treatment,” said lead study author Robin Whitney, RN, a cancer survivor and PhD student at the Betty Irene Moore School of Nursing at the University of California, Davis.
“Addressing these challenges is an important aspect of providing quality cancer care, because they can substantially impact quality of life and health outcomes.”
Whitney and her colleagues focused this study on a subset of individuals surveyed in a larger study (2011 Medical Expenditures Panel Survey Experiences with Cancer Survivorship Supplement).
Among the 1592 survivors surveyed, 47% were younger than 65 years of age, 56% were female, 88% were white, and 4% were uninsured. Fourteen percent were in active treatment, 46% were less than 5 years post-treatment, and 39% were 5 years or more post-treatment.
Overall, 27% of those surveyed reported at least one financial difficulty, such as debt, bankruptcy, and worrying about medical bills. Patients in active treatment reported 120% more financial difficulties than survivors who were less than 5 years post-treatment.
Individuals younger than 65 reported 130% more financial difficulties than older survivors. Survivors without insurance had 67% more difficulties than those with insurance. And minorities had 42% more financial difficulties than whites.
In all, 37% of survivors reported making at least one work modification due to their cancer diagnosis, such as changing to a flexible schedule or less demanding job, early or delayed retirement, and extended or unpaid time off.
Women were significantly more likely than men to make at least one work modification. Patients in active treatment made 120% more work modifications than survivors who were less than 5 years post-treatment. And minorities made 57% more modifications than whites.
According to the researchers, these findings are generalizable to the US population and point to the urgent need for screening and support for financial and work challenges across the cancer survivorship trajectory, from diagnosis to long-term survivorship.
*Information presented differs from that in the abstract.

chemotherapy
Credit: Rhoda Baer
Many US cancer survivors may be experiencing financial or work-related hardship, a new survey suggests.
Twenty-seven percent of the nearly 1600 survivors surveyed reported at least one financial problem, such as debt or bankruptcy.
And 37% reported having to modify work plans, such as taking extended time off or delaying retirement.
Women, younger survivors, racial/ethnic minorities, and uninsured survivors were all disproportionally burdened by these challenges.
This research (abstract 238*) was presented in a presscast prior to the 2014 Palliative Care in Oncology Symposium, which is scheduled to take place October 24-25 at the Westin Boston Waterfront in Boston.
“We found that many cancer survivors, particularly those who are younger or from underserved populations, experience financial or work-related hardship—even when insured and years out from treatment,” said lead study author Robin Whitney, RN, a cancer survivor and PhD student at the Betty Irene Moore School of Nursing at the University of California, Davis.
“Addressing these challenges is an important aspect of providing quality cancer care, because they can substantially impact quality of life and health outcomes.”
Whitney and her colleagues focused this study on a subset of individuals surveyed in a larger study (2011 Medical Expenditures Panel Survey Experiences with Cancer Survivorship Supplement).
Among the 1592 survivors surveyed, 47% were younger than 65 years of age, 56% were female, 88% were white, and 4% were uninsured. Fourteen percent were in active treatment, 46% were less than 5 years post-treatment, and 39% were 5 years or more post-treatment.
Overall, 27% of those surveyed reported at least one financial difficulty, such as debt, bankruptcy, and worrying about medical bills. Patients in active treatment reported 120% more financial difficulties than survivors who were less than 5 years post-treatment.
Individuals younger than 65 reported 130% more financial difficulties than older survivors. Survivors without insurance had 67% more difficulties than those with insurance. And minorities had 42% more financial difficulties than whites.
In all, 37% of survivors reported making at least one work modification due to their cancer diagnosis, such as changing to a flexible schedule or less demanding job, early or delayed retirement, and extended or unpaid time off.
Women were significantly more likely than men to make at least one work modification. Patients in active treatment made 120% more work modifications than survivors who were less than 5 years post-treatment. And minorities made 57% more modifications than whites.
According to the researchers, these findings are generalizable to the US population and point to the urgent need for screening and support for financial and work challenges across the cancer survivorship trajectory, from diagnosis to long-term survivorship.
*Information presented differs from that in the abstract.

chemotherapy
Credit: Rhoda Baer
Many US cancer survivors may be experiencing financial or work-related hardship, a new survey suggests.
Twenty-seven percent of the nearly 1600 survivors surveyed reported at least one financial problem, such as debt or bankruptcy.
And 37% reported having to modify work plans, such as taking extended time off or delaying retirement.
Women, younger survivors, racial/ethnic minorities, and uninsured survivors were all disproportionally burdened by these challenges.
This research (abstract 238*) was presented in a presscast prior to the 2014 Palliative Care in Oncology Symposium, which is scheduled to take place October 24-25 at the Westin Boston Waterfront in Boston.
“We found that many cancer survivors, particularly those who are younger or from underserved populations, experience financial or work-related hardship—even when insured and years out from treatment,” said lead study author Robin Whitney, RN, a cancer survivor and PhD student at the Betty Irene Moore School of Nursing at the University of California, Davis.
“Addressing these challenges is an important aspect of providing quality cancer care, because they can substantially impact quality of life and health outcomes.”
Whitney and her colleagues focused this study on a subset of individuals surveyed in a larger study (2011 Medical Expenditures Panel Survey Experiences with Cancer Survivorship Supplement).
Among the 1592 survivors surveyed, 47% were younger than 65 years of age, 56% were female, 88% were white, and 4% were uninsured. Fourteen percent were in active treatment, 46% were less than 5 years post-treatment, and 39% were 5 years or more post-treatment.
Overall, 27% of those surveyed reported at least one financial difficulty, such as debt, bankruptcy, and worrying about medical bills. Patients in active treatment reported 120% more financial difficulties than survivors who were less than 5 years post-treatment.
Individuals younger than 65 reported 130% more financial difficulties than older survivors. Survivors without insurance had 67% more difficulties than those with insurance. And minorities had 42% more financial difficulties than whites.
In all, 37% of survivors reported making at least one work modification due to their cancer diagnosis, such as changing to a flexible schedule or less demanding job, early or delayed retirement, and extended or unpaid time off.
Women were significantly more likely than men to make at least one work modification. Patients in active treatment made 120% more work modifications than survivors who were less than 5 years post-treatment. And minorities made 57% more modifications than whites.
According to the researchers, these findings are generalizable to the US population and point to the urgent need for screening and support for financial and work challenges across the cancer survivorship trajectory, from diagnosis to long-term survivorship.
*Information presented differs from that in the abstract.