User login
Study reveals incidence of mutations linked to leukemia, lymphoma
At least 2% of people over the age of 40 and 5% over age 70 have mutations linked to leukemia and lymphoma, according to research published in Nature Medicine.
The findings, based on blood samples from nearly 3000 patients, don’t necessarily mean that people with these mutations will develop leukemia or lymphoma.
They may have a higher-than-normal risk of developing these malignancies, but more research is needed to determine the risk.
“We would not want anyone to think they should be screened for these mutations to understand their risk of leukemia or lymphoma,” said Timothy Ley, MD, of the Washington University School of Medicine in St Louis, Missouri.
“The ability to understand how mutations in these genes increase a person’s risk of blood cancers is a long way off, and genetic testing would be of no benefit at this time.”
Dr Ley and his colleagues analyzed blood samples from people enrolled in The Cancer Genome Atlas project. The patients had been diagnosed with cancer but were not known to have leukemia, lymphoma, or a blood disease.
They ranged in age from 10 to 90 at the time of diagnosis and had donated blood and tumor samples before starting cancer treatment. Therefore, any mutations the researchers identified would not have been associated with chemotherapy or radiation.
The team looked closely at 556 known cancer genes. In 341 patients ages 40 to 49, fewer than 1% had mutations in 19 leukemia- or lymphoma-related genes.
But among 475 people ages 70 to 79, more than 5% did. And more than 6% of the 132 people ages 80 to 89 had mutations in these genes.
The researchers noted that 9 of the 19 genes were mutated repeatedly, an indicator that the changes drive or initiate the expansion of blood cells.
This expansion of cells is clearly not leukemia or lymphoma, the researchers said. It may be a precursor to hematologic malignancies in a small subset of patients, but the study was not designed to predict the future risk of developing these diseases.
The researchers also said this study likely underestimates the percentage of people with mutations in leukemia and lymphoma genes because the team was only able to identify small mutations, not large structural variations or the insertions and deletions of chunks of genetic material. ![]()
At least 2% of people over the age of 40 and 5% over age 70 have mutations linked to leukemia and lymphoma, according to research published in Nature Medicine.
The findings, based on blood samples from nearly 3000 patients, don’t necessarily mean that people with these mutations will develop leukemia or lymphoma.
They may have a higher-than-normal risk of developing these malignancies, but more research is needed to determine the risk.
“We would not want anyone to think they should be screened for these mutations to understand their risk of leukemia or lymphoma,” said Timothy Ley, MD, of the Washington University School of Medicine in St Louis, Missouri.
“The ability to understand how mutations in these genes increase a person’s risk of blood cancers is a long way off, and genetic testing would be of no benefit at this time.”
Dr Ley and his colleagues analyzed blood samples from people enrolled in The Cancer Genome Atlas project. The patients had been diagnosed with cancer but were not known to have leukemia, lymphoma, or a blood disease.
They ranged in age from 10 to 90 at the time of diagnosis and had donated blood and tumor samples before starting cancer treatment. Therefore, any mutations the researchers identified would not have been associated with chemotherapy or radiation.
The team looked closely at 556 known cancer genes. In 341 patients ages 40 to 49, fewer than 1% had mutations in 19 leukemia- or lymphoma-related genes.
But among 475 people ages 70 to 79, more than 5% did. And more than 6% of the 132 people ages 80 to 89 had mutations in these genes.
The researchers noted that 9 of the 19 genes were mutated repeatedly, an indicator that the changes drive or initiate the expansion of blood cells.
This expansion of cells is clearly not leukemia or lymphoma, the researchers said. It may be a precursor to hematologic malignancies in a small subset of patients, but the study was not designed to predict the future risk of developing these diseases.
The researchers also said this study likely underestimates the percentage of people with mutations in leukemia and lymphoma genes because the team was only able to identify small mutations, not large structural variations or the insertions and deletions of chunks of genetic material. ![]()
At least 2% of people over the age of 40 and 5% over age 70 have mutations linked to leukemia and lymphoma, according to research published in Nature Medicine.
The findings, based on blood samples from nearly 3000 patients, don’t necessarily mean that people with these mutations will develop leukemia or lymphoma.
They may have a higher-than-normal risk of developing these malignancies, but more research is needed to determine the risk.
“We would not want anyone to think they should be screened for these mutations to understand their risk of leukemia or lymphoma,” said Timothy Ley, MD, of the Washington University School of Medicine in St Louis, Missouri.
“The ability to understand how mutations in these genes increase a person’s risk of blood cancers is a long way off, and genetic testing would be of no benefit at this time.”
Dr Ley and his colleagues analyzed blood samples from people enrolled in The Cancer Genome Atlas project. The patients had been diagnosed with cancer but were not known to have leukemia, lymphoma, or a blood disease.
They ranged in age from 10 to 90 at the time of diagnosis and had donated blood and tumor samples before starting cancer treatment. Therefore, any mutations the researchers identified would not have been associated with chemotherapy or radiation.
The team looked closely at 556 known cancer genes. In 341 patients ages 40 to 49, fewer than 1% had mutations in 19 leukemia- or lymphoma-related genes.
But among 475 people ages 70 to 79, more than 5% did. And more than 6% of the 132 people ages 80 to 89 had mutations in these genes.
The researchers noted that 9 of the 19 genes were mutated repeatedly, an indicator that the changes drive or initiate the expansion of blood cells.
This expansion of cells is clearly not leukemia or lymphoma, the researchers said. It may be a precursor to hematologic malignancies in a small subset of patients, but the study was not designed to predict the future risk of developing these diseases.
The researchers also said this study likely underestimates the percentage of people with mutations in leukemia and lymphoma genes because the team was only able to identify small mutations, not large structural variations or the insertions and deletions of chunks of genetic material. ![]()
Ibrutinib gets EU approval for CLL, MCL

Credit: Steven Harbour
The European Commission has granted marketing approval for the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) in the European Union (EU).
The drug is now approved to treat adult patients with relapsed or refractory mantle cell lymphoma (MCL), adults with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy, and first-line CLL patients who have 17p deletion or TP53 mutation and are unsuitable for chemotherapy.
In the EU and all other countries except the US, ibrutinib is marketed by Janssen Pharmaceutical Companies. In the US, the drug is being jointly developed and commercialized by Pharmacyclics and Janssen Biotech, Inc.
The EU approval of ibrutinib was based on data from a phase 2 study (PCYC-1104) in patients with MCL, the phase 3 RESONATE trial (PCYC-1112-CA) in CLL and small lymphocytic lymphoma (SLL), and a phase 1b/2 study (PCYC-1102) in CLL/SLL.
PCYC-1104: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
RESONATE: Ibrutinib in CLL/SLL
Results of the RESONATE trial were reported at EHA 2014 and published in NEJM in July. This trial was stopped early after an interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
The trial included 391 patients with relapsed or refractory CLL or SLL who were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39% of patients, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia. ![]()

Credit: Steven Harbour
The European Commission has granted marketing approval for the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) in the European Union (EU).
The drug is now approved to treat adult patients with relapsed or refractory mantle cell lymphoma (MCL), adults with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy, and first-line CLL patients who have 17p deletion or TP53 mutation and are unsuitable for chemotherapy.
In the EU and all other countries except the US, ibrutinib is marketed by Janssen Pharmaceutical Companies. In the US, the drug is being jointly developed and commercialized by Pharmacyclics and Janssen Biotech, Inc.
The EU approval of ibrutinib was based on data from a phase 2 study (PCYC-1104) in patients with MCL, the phase 3 RESONATE trial (PCYC-1112-CA) in CLL and small lymphocytic lymphoma (SLL), and a phase 1b/2 study (PCYC-1102) in CLL/SLL.
PCYC-1104: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
RESONATE: Ibrutinib in CLL/SLL
Results of the RESONATE trial were reported at EHA 2014 and published in NEJM in July. This trial was stopped early after an interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
The trial included 391 patients with relapsed or refractory CLL or SLL who were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39% of patients, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia. ![]()

Credit: Steven Harbour
The European Commission has granted marketing approval for the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) in the European Union (EU).
The drug is now approved to treat adult patients with relapsed or refractory mantle cell lymphoma (MCL), adults with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy, and first-line CLL patients who have 17p deletion or TP53 mutation and are unsuitable for chemotherapy.
In the EU and all other countries except the US, ibrutinib is marketed by Janssen Pharmaceutical Companies. In the US, the drug is being jointly developed and commercialized by Pharmacyclics and Janssen Biotech, Inc.
The EU approval of ibrutinib was based on data from a phase 2 study (PCYC-1104) in patients with MCL, the phase 3 RESONATE trial (PCYC-1112-CA) in CLL and small lymphocytic lymphoma (SLL), and a phase 1b/2 study (PCYC-1102) in CLL/SLL.
PCYC-1104: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
RESONATE: Ibrutinib in CLL/SLL
Results of the RESONATE trial were reported at EHA 2014 and published in NEJM in July. This trial was stopped early after an interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
The trial included 391 patients with relapsed or refractory CLL or SLL who were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39% of patients, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia. ![]()
Amoeba could help fight cancers

Experiments in a soil-dwelling amoeba have provided insight that could help us treat cancers characterized by PTEN mutations, researchers have reported in PLOS ONE.
The team discovered that this amoeba has two genes that function like the human tumor suppressor PTEN.
And increasing expression of one of these genes compensated for a mutation in the other gene.
If the same method works in humans with mutated PTEN, this finding could have implications for a range of cancers.
PTEN mutations are thought to be involved in nearly half of all leukemia cases, 40% of breast cancer cases, and up to 70% of prostate cancer cases.
“If you look at tumors across the board . . . , you find that PTEN is the most generally mutated gene, and, when you mutate PTEN in mice, you cause tumors,” said study author David Soll, PhD, of the University of Iowa in Iowa City.
He and his colleagues found that the amoeba Dictyostelium discoideum has the gene ptenA, which mutates similarly to the human PTEN gene and causes behavioral defects in the cell.
They also found a close relative of ptenA in the amoeba, called lpten, that performs the same functions of ptenA but to a lesser degree.
The researchers hypothesized that ramping up the presence of lpten could compensate for the mutated ptenA.
They tested this theory by placing lpten in a plasmid behind a powerful promoter designed to overexpress the gene. They then introduced the super-charged lpten into a cell with the mutated ptenA gene.
The team found that the overexpressed lpten gene fully compensated for all of the defects in the ptenA mutant.
If this method works in human cells, it could lead to a new way to treat cancers, the researchers said. They are now aiming to identify a drug that would activate the promoter for one of PTEN’s close relatives.
Once a patient is diagnosed with cancer caused by a PTEN mutation, the patient could take the drug, overexpress the PTEN replacement gene, and potentially stop cancer in its tracks, Dr Soll said.
This research has also led Dr Soll and his colleagues to study other human genes that may be able to step in for the mutated PTEN gene and perform the same tumor-suppressing role. The team is currently studying 2 close relatives of PTEN.
“And nature might have put them there just for that; that’s the curious thing,” Dr Soll said. “Somewhere, there may be a backup system, what we call ‘redundancy,’ that might be the basis for better identifying tumors and possibly creating cancer-fighting drugs. You have another gene which might be able to step in for the broken gene to keep things normal, and that’s what we’re playing with here. It’s very sophisticated.” ![]()

Experiments in a soil-dwelling amoeba have provided insight that could help us treat cancers characterized by PTEN mutations, researchers have reported in PLOS ONE.
The team discovered that this amoeba has two genes that function like the human tumor suppressor PTEN.
And increasing expression of one of these genes compensated for a mutation in the other gene.
If the same method works in humans with mutated PTEN, this finding could have implications for a range of cancers.
PTEN mutations are thought to be involved in nearly half of all leukemia cases, 40% of breast cancer cases, and up to 70% of prostate cancer cases.
“If you look at tumors across the board . . . , you find that PTEN is the most generally mutated gene, and, when you mutate PTEN in mice, you cause tumors,” said study author David Soll, PhD, of the University of Iowa in Iowa City.
He and his colleagues found that the amoeba Dictyostelium discoideum has the gene ptenA, which mutates similarly to the human PTEN gene and causes behavioral defects in the cell.
They also found a close relative of ptenA in the amoeba, called lpten, that performs the same functions of ptenA but to a lesser degree.
The researchers hypothesized that ramping up the presence of lpten could compensate for the mutated ptenA.
They tested this theory by placing lpten in a plasmid behind a powerful promoter designed to overexpress the gene. They then introduced the super-charged lpten into a cell with the mutated ptenA gene.
The team found that the overexpressed lpten gene fully compensated for all of the defects in the ptenA mutant.
If this method works in human cells, it could lead to a new way to treat cancers, the researchers said. They are now aiming to identify a drug that would activate the promoter for one of PTEN’s close relatives.
Once a patient is diagnosed with cancer caused by a PTEN mutation, the patient could take the drug, overexpress the PTEN replacement gene, and potentially stop cancer in its tracks, Dr Soll said.
This research has also led Dr Soll and his colleagues to study other human genes that may be able to step in for the mutated PTEN gene and perform the same tumor-suppressing role. The team is currently studying 2 close relatives of PTEN.
“And nature might have put them there just for that; that’s the curious thing,” Dr Soll said. “Somewhere, there may be a backup system, what we call ‘redundancy,’ that might be the basis for better identifying tumors and possibly creating cancer-fighting drugs. You have another gene which might be able to step in for the broken gene to keep things normal, and that’s what we’re playing with here. It’s very sophisticated.” ![]()

Experiments in a soil-dwelling amoeba have provided insight that could help us treat cancers characterized by PTEN mutations, researchers have reported in PLOS ONE.
The team discovered that this amoeba has two genes that function like the human tumor suppressor PTEN.
And increasing expression of one of these genes compensated for a mutation in the other gene.
If the same method works in humans with mutated PTEN, this finding could have implications for a range of cancers.
PTEN mutations are thought to be involved in nearly half of all leukemia cases, 40% of breast cancer cases, and up to 70% of prostate cancer cases.
“If you look at tumors across the board . . . , you find that PTEN is the most generally mutated gene, and, when you mutate PTEN in mice, you cause tumors,” said study author David Soll, PhD, of the University of Iowa in Iowa City.
He and his colleagues found that the amoeba Dictyostelium discoideum has the gene ptenA, which mutates similarly to the human PTEN gene and causes behavioral defects in the cell.
They also found a close relative of ptenA in the amoeba, called lpten, that performs the same functions of ptenA but to a lesser degree.
The researchers hypothesized that ramping up the presence of lpten could compensate for the mutated ptenA.
They tested this theory by placing lpten in a plasmid behind a powerful promoter designed to overexpress the gene. They then introduced the super-charged lpten into a cell with the mutated ptenA gene.
The team found that the overexpressed lpten gene fully compensated for all of the defects in the ptenA mutant.
If this method works in human cells, it could lead to a new way to treat cancers, the researchers said. They are now aiming to identify a drug that would activate the promoter for one of PTEN’s close relatives.
Once a patient is diagnosed with cancer caused by a PTEN mutation, the patient could take the drug, overexpress the PTEN replacement gene, and potentially stop cancer in its tracks, Dr Soll said.
This research has also led Dr Soll and his colleagues to study other human genes that may be able to step in for the mutated PTEN gene and perform the same tumor-suppressing role. The team is currently studying 2 close relatives of PTEN.
“And nature might have put them there just for that; that’s the curious thing,” Dr Soll said. “Somewhere, there may be a backup system, what we call ‘redundancy,’ that might be the basis for better identifying tumors and possibly creating cancer-fighting drugs. You have another gene which might be able to step in for the broken gene to keep things normal, and that’s what we’re playing with here. It’s very sophisticated.” ![]()
FDA approves drug for untreated MCL

The US Food and Drug Administration (FDA) has approved bortezomib (Velcade) for use in previously untreated patients with mantle cell
lymphoma (MCL).
This is the first drug to be approved in the US for previously untreated patients with MCL.
The approval extends the utility of bortezomib beyond relapsed or refractory MCL, for which it has been approved since 2006.
The new approval is based on results of a phase 3 trial.
The study was a comparison of VcR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone) and R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in 487 patients newly diagnosed with stage II, III, or IV MCL.
Survival and response
VcR-CAP demonstrated a 59% relative improvement in the study’s primary endpoint of progression-free survival. At a median follow-up of 40 months, the median progression-free survival was 25 months in the VcR-CAP arm and 14 months in the R-CHOP arm (hazard ratio [HR]=0.63, P<0.001).
However, there was no significant improvement in overall survival. The median overall survival was not reached in the VcR-CAP arm and was 56.3 months in the R-CHOP arm (HR=0.80; P=0.173).
Patients in the VcR-CAP arm had a higher rate of complete response/unconfirmed complete response than those in the R-CHOP arm—53% and 42%, respectively (P=0.007). But there was no significant difference in overall response—92% and 90%, respectively (P=0.275).
The time to progression was significantly longer in the VcR-CAP arm—30.5 months, compared to 16.1 months in the R-CHOP arm (HR=0.58; P<0.001). And the median time to subsequent treatment was significantly longer in the VcR-CAP arm—44.5 months vs 24.8 months (HR 0.50; P<0.001).
Adverse events
VcR-CAP was associated with additional but manageable toxicity compared to R-CHOP.
Serious adverse events were reported in 38% of patients in the VcR-CAP arm and 30% in the R-CHOP arm. Grade 3 or higher adverse events were reported in 93% and 85%, respectively.
There were similar rates of all-grade peripheral neuropathy between the VcR-CAP arm and the R-CHOP arm—30% and 29%, respectively. But the rate of grade 3 or higher peripheral neuropathy was significantly higher in the VcR-CAP arm—7.5% vs 4.1%.
The incidence of all-grade thrombocytopenia was substantially higher in the VcR-CAP arm than the R-CHOP arm—72% and 19%, respectively. But there was no significant difference in bleeding events—6% and 5%, respectively.
The incidence of all-grade neutropenia was 88% in the VcR-CAP arm and 74% in the R-CHOP arm. The rate of grade 3 or higher febrile neutropenia was 14% and 15%, respectively, and the rate of infection was 60% and 46%, respectively.
These data were presented at ASCO 2014 as abstract 8500.
Bortezomib is marketed as Velcade by Millennium/Takeda and Janssen Pharmaceutical Companies. Millennium is responsible for commercialization in the US, and Janssen Pharmaceutical Companies are responsible for commercialization in the rest of the world.
For more details on the drug, visit www.velcade.com. ![]()

The US Food and Drug Administration (FDA) has approved bortezomib (Velcade) for use in previously untreated patients with mantle cell
lymphoma (MCL).
This is the first drug to be approved in the US for previously untreated patients with MCL.
The approval extends the utility of bortezomib beyond relapsed or refractory MCL, for which it has been approved since 2006.
The new approval is based on results of a phase 3 trial.
The study was a comparison of VcR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone) and R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in 487 patients newly diagnosed with stage II, III, or IV MCL.
Survival and response
VcR-CAP demonstrated a 59% relative improvement in the study’s primary endpoint of progression-free survival. At a median follow-up of 40 months, the median progression-free survival was 25 months in the VcR-CAP arm and 14 months in the R-CHOP arm (hazard ratio [HR]=0.63, P<0.001).
However, there was no significant improvement in overall survival. The median overall survival was not reached in the VcR-CAP arm and was 56.3 months in the R-CHOP arm (HR=0.80; P=0.173).
Patients in the VcR-CAP arm had a higher rate of complete response/unconfirmed complete response than those in the R-CHOP arm—53% and 42%, respectively (P=0.007). But there was no significant difference in overall response—92% and 90%, respectively (P=0.275).
The time to progression was significantly longer in the VcR-CAP arm—30.5 months, compared to 16.1 months in the R-CHOP arm (HR=0.58; P<0.001). And the median time to subsequent treatment was significantly longer in the VcR-CAP arm—44.5 months vs 24.8 months (HR 0.50; P<0.001).
Adverse events
VcR-CAP was associated with additional but manageable toxicity compared to R-CHOP.
Serious adverse events were reported in 38% of patients in the VcR-CAP arm and 30% in the R-CHOP arm. Grade 3 or higher adverse events were reported in 93% and 85%, respectively.
There were similar rates of all-grade peripheral neuropathy between the VcR-CAP arm and the R-CHOP arm—30% and 29%, respectively. But the rate of grade 3 or higher peripheral neuropathy was significantly higher in the VcR-CAP arm—7.5% vs 4.1%.
The incidence of all-grade thrombocytopenia was substantially higher in the VcR-CAP arm than the R-CHOP arm—72% and 19%, respectively. But there was no significant difference in bleeding events—6% and 5%, respectively.
The incidence of all-grade neutropenia was 88% in the VcR-CAP arm and 74% in the R-CHOP arm. The rate of grade 3 or higher febrile neutropenia was 14% and 15%, respectively, and the rate of infection was 60% and 46%, respectively.
These data were presented at ASCO 2014 as abstract 8500.
Bortezomib is marketed as Velcade by Millennium/Takeda and Janssen Pharmaceutical Companies. Millennium is responsible for commercialization in the US, and Janssen Pharmaceutical Companies are responsible for commercialization in the rest of the world.
For more details on the drug, visit www.velcade.com. ![]()

The US Food and Drug Administration (FDA) has approved bortezomib (Velcade) for use in previously untreated patients with mantle cell
lymphoma (MCL).
This is the first drug to be approved in the US for previously untreated patients with MCL.
The approval extends the utility of bortezomib beyond relapsed or refractory MCL, for which it has been approved since 2006.
The new approval is based on results of a phase 3 trial.
The study was a comparison of VcR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone) and R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in 487 patients newly diagnosed with stage II, III, or IV MCL.
Survival and response
VcR-CAP demonstrated a 59% relative improvement in the study’s primary endpoint of progression-free survival. At a median follow-up of 40 months, the median progression-free survival was 25 months in the VcR-CAP arm and 14 months in the R-CHOP arm (hazard ratio [HR]=0.63, P<0.001).
However, there was no significant improvement in overall survival. The median overall survival was not reached in the VcR-CAP arm and was 56.3 months in the R-CHOP arm (HR=0.80; P=0.173).
Patients in the VcR-CAP arm had a higher rate of complete response/unconfirmed complete response than those in the R-CHOP arm—53% and 42%, respectively (P=0.007). But there was no significant difference in overall response—92% and 90%, respectively (P=0.275).
The time to progression was significantly longer in the VcR-CAP arm—30.5 months, compared to 16.1 months in the R-CHOP arm (HR=0.58; P<0.001). And the median time to subsequent treatment was significantly longer in the VcR-CAP arm—44.5 months vs 24.8 months (HR 0.50; P<0.001).
Adverse events
VcR-CAP was associated with additional but manageable toxicity compared to R-CHOP.
Serious adverse events were reported in 38% of patients in the VcR-CAP arm and 30% in the R-CHOP arm. Grade 3 or higher adverse events were reported in 93% and 85%, respectively.
There were similar rates of all-grade peripheral neuropathy between the VcR-CAP arm and the R-CHOP arm—30% and 29%, respectively. But the rate of grade 3 or higher peripheral neuropathy was significantly higher in the VcR-CAP arm—7.5% vs 4.1%.
The incidence of all-grade thrombocytopenia was substantially higher in the VcR-CAP arm than the R-CHOP arm—72% and 19%, respectively. But there was no significant difference in bleeding events—6% and 5%, respectively.
The incidence of all-grade neutropenia was 88% in the VcR-CAP arm and 74% in the R-CHOP arm. The rate of grade 3 or higher febrile neutropenia was 14% and 15%, respectively, and the rate of infection was 60% and 46%, respectively.
These data were presented at ASCO 2014 as abstract 8500.
Bortezomib is marketed as Velcade by Millennium/Takeda and Janssen Pharmaceutical Companies. Millennium is responsible for commercialization in the US, and Janssen Pharmaceutical Companies are responsible for commercialization in the rest of the world.
For more details on the drug, visit www.velcade.com. ![]()
Animal studies help explain chemo brain

californica
releasing inkafter being disturbed
Results of preclinical research appear to explain how the anticancer agent doxorubicin can cause chemo brain.
Neuroscientists conducted experiments in cells from rats and Aplysia californica, a marine mollusk that has many of the same memory mechanisms as humans.
This revealed memory mechanisms that are inhibited by doxorubicin, as well as a method of unblocking these mechanisms—administering a drug known as SB203580.
“Our research has implications in the care of people given to cognitive deficits following drug treatment for cancer,” said John H. Byrne, PhD, of the University of Texas Health Medical School.
He added that understanding how drugs like doxorubicin impact the brain is an important first step in alleviating chemo brain, which is characterized by forgetfulness, trouble concentrating, and difficulty multitasking.
Dr Byrne and his colleagues explained this first step in The Journal of Neuroscience.
The researchers knew that, in non-neuronal cells, doxorubicin inhibits the expression of MAPK phosphatases, thereby inhibiting the dephosphorylation of ERK and p38 MAPK, 2 MAPK isoforms that are important for long-term memory.
To evaluate doxorubicin’s effects on levels of phosphorylated ERK and p38 MAPK, the team used cultures of cortical neurons from rats and sensory neurons from Aplysia californica.
Experiments showed that doxorubicin elevated levels of phosphorylated ERK and phosphorylated p38 MAPK in sensory neurons and cortical neurons. In addition, the drug increased phosphorylation of the downstream transcriptional repressor CREB2 in sensory neurons.
The researchers also assessed doxorubicin’s effects on long-term enhanced excitability, long-term synaptic facilitation, and long-term
synaptic depression.
They found that doxorubicin enhanced long-term synaptic depression induced by the neuropeptide Phe-Met-Arg-Phe-NH2. And the drug inhibited long-term synaptic facilitation induced by serotonin.
However, the researchers were able to restore long-term synaptic facilitation with SB203580, an inhibitor of p38 MAPK.
Unfortunately, SB203580 would not be appropriate for human use, Dr Byrne noted, adding that his team would like to identify other drugs that might have the same effect as SB203580.
The researchers also hope to determine if doxorubicin works the same way in humans as it did in these experiments. ![]()

californica
releasing inkafter being disturbed
Results of preclinical research appear to explain how the anticancer agent doxorubicin can cause chemo brain.
Neuroscientists conducted experiments in cells from rats and Aplysia californica, a marine mollusk that has many of the same memory mechanisms as humans.
This revealed memory mechanisms that are inhibited by doxorubicin, as well as a method of unblocking these mechanisms—administering a drug known as SB203580.
“Our research has implications in the care of people given to cognitive deficits following drug treatment for cancer,” said John H. Byrne, PhD, of the University of Texas Health Medical School.
He added that understanding how drugs like doxorubicin impact the brain is an important first step in alleviating chemo brain, which is characterized by forgetfulness, trouble concentrating, and difficulty multitasking.
Dr Byrne and his colleagues explained this first step in The Journal of Neuroscience.
The researchers knew that, in non-neuronal cells, doxorubicin inhibits the expression of MAPK phosphatases, thereby inhibiting the dephosphorylation of ERK and p38 MAPK, 2 MAPK isoforms that are important for long-term memory.
To evaluate doxorubicin’s effects on levels of phosphorylated ERK and p38 MAPK, the team used cultures of cortical neurons from rats and sensory neurons from Aplysia californica.
Experiments showed that doxorubicin elevated levels of phosphorylated ERK and phosphorylated p38 MAPK in sensory neurons and cortical neurons. In addition, the drug increased phosphorylation of the downstream transcriptional repressor CREB2 in sensory neurons.
The researchers also assessed doxorubicin’s effects on long-term enhanced excitability, long-term synaptic facilitation, and long-term
synaptic depression.
They found that doxorubicin enhanced long-term synaptic depression induced by the neuropeptide Phe-Met-Arg-Phe-NH2. And the drug inhibited long-term synaptic facilitation induced by serotonin.
However, the researchers were able to restore long-term synaptic facilitation with SB203580, an inhibitor of p38 MAPK.
Unfortunately, SB203580 would not be appropriate for human use, Dr Byrne noted, adding that his team would like to identify other drugs that might have the same effect as SB203580.
The researchers also hope to determine if doxorubicin works the same way in humans as it did in these experiments. ![]()

californica
releasing inkafter being disturbed
Results of preclinical research appear to explain how the anticancer agent doxorubicin can cause chemo brain.
Neuroscientists conducted experiments in cells from rats and Aplysia californica, a marine mollusk that has many of the same memory mechanisms as humans.
This revealed memory mechanisms that are inhibited by doxorubicin, as well as a method of unblocking these mechanisms—administering a drug known as SB203580.
“Our research has implications in the care of people given to cognitive deficits following drug treatment for cancer,” said John H. Byrne, PhD, of the University of Texas Health Medical School.
He added that understanding how drugs like doxorubicin impact the brain is an important first step in alleviating chemo brain, which is characterized by forgetfulness, trouble concentrating, and difficulty multitasking.
Dr Byrne and his colleagues explained this first step in The Journal of Neuroscience.
The researchers knew that, in non-neuronal cells, doxorubicin inhibits the expression of MAPK phosphatases, thereby inhibiting the dephosphorylation of ERK and p38 MAPK, 2 MAPK isoforms that are important for long-term memory.
To evaluate doxorubicin’s effects on levels of phosphorylated ERK and p38 MAPK, the team used cultures of cortical neurons from rats and sensory neurons from Aplysia californica.
Experiments showed that doxorubicin elevated levels of phosphorylated ERK and phosphorylated p38 MAPK in sensory neurons and cortical neurons. In addition, the drug increased phosphorylation of the downstream transcriptional repressor CREB2 in sensory neurons.
The researchers also assessed doxorubicin’s effects on long-term enhanced excitability, long-term synaptic facilitation, and long-term
synaptic depression.
They found that doxorubicin enhanced long-term synaptic depression induced by the neuropeptide Phe-Met-Arg-Phe-NH2. And the drug inhibited long-term synaptic facilitation induced by serotonin.
However, the researchers were able to restore long-term synaptic facilitation with SB203580, an inhibitor of p38 MAPK.
Unfortunately, SB203580 would not be appropriate for human use, Dr Byrne noted, adding that his team would like to identify other drugs that might have the same effect as SB203580.
The researchers also hope to determine if doxorubicin works the same way in humans as it did in these experiments. ![]()
NICE rejects obinutuzumab for CLL

Credit: Linda Bartlett
In a new draft guidance, the UK’s National Institute for Health and Care Excellence (NICE) has said it cannot recommend obinutuzumab (Gazyvaro) to treat chronic lymphocytic leukemia (CLL).
NICE CEO Sir Andrew Dillon said that although data suggest obinutuzumab is effective, there were too many “uncertainties” in the information submitted by Roche, the company developing the drug.
So NICE cannot be sure obinutuzumab would be an effective use of the National Health Service’s resources.
This is despite the fact that Roche offered to discount the drug’s list price of £26,496 per treatment course.
NICE is accepting comments on the draft guidance until 5 pm on October 23.
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells. The drug induces antibody-dependent cellular cytotoxicity and caspase-independent apoptosis.
The European Commission approved obinutuzumab in July for use in combination with chlorambucil to treat patients with previously untreated CLL who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab was approved for this indication in the US in November 2013.
Obinutuzumab is marketed as Gazyvaro in the European Union and Switzerland but as Gazyva in the US and the rest of the world. ![]()

Credit: Linda Bartlett
In a new draft guidance, the UK’s National Institute for Health and Care Excellence (NICE) has said it cannot recommend obinutuzumab (Gazyvaro) to treat chronic lymphocytic leukemia (CLL).
NICE CEO Sir Andrew Dillon said that although data suggest obinutuzumab is effective, there were too many “uncertainties” in the information submitted by Roche, the company developing the drug.
So NICE cannot be sure obinutuzumab would be an effective use of the National Health Service’s resources.
This is despite the fact that Roche offered to discount the drug’s list price of £26,496 per treatment course.
NICE is accepting comments on the draft guidance until 5 pm on October 23.
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells. The drug induces antibody-dependent cellular cytotoxicity and caspase-independent apoptosis.
The European Commission approved obinutuzumab in July for use in combination with chlorambucil to treat patients with previously untreated CLL who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab was approved for this indication in the US in November 2013.
Obinutuzumab is marketed as Gazyvaro in the European Union and Switzerland but as Gazyva in the US and the rest of the world. ![]()

Credit: Linda Bartlett
In a new draft guidance, the UK’s National Institute for Health and Care Excellence (NICE) has said it cannot recommend obinutuzumab (Gazyvaro) to treat chronic lymphocytic leukemia (CLL).
NICE CEO Sir Andrew Dillon said that although data suggest obinutuzumab is effective, there were too many “uncertainties” in the information submitted by Roche, the company developing the drug.
So NICE cannot be sure obinutuzumab would be an effective use of the National Health Service’s resources.
This is despite the fact that Roche offered to discount the drug’s list price of £26,496 per treatment course.
NICE is accepting comments on the draft guidance until 5 pm on October 23.
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells. The drug induces antibody-dependent cellular cytotoxicity and caspase-independent apoptosis.
The European Commission approved obinutuzumab in July for use in combination with chlorambucil to treat patients with previously untreated CLL who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab was approved for this indication in the US in November 2013.
Obinutuzumab is marketed as Gazyvaro in the European Union and Switzerland but as Gazyva in the US and the rest of the world. ![]()
HHS identifies known and likely carcinogens

Credit: Trevor MacInnis
The US Department of Health and Human Services (HHS) has identified 1 chemical substance as a known human carcinogen and 3 additional substances as likely carcinogens.
Ortho-toluidine, which is used to make rubber chemicals, pesticides, and dyes, has been shown to cause urinary bladder cancer and is now listed as a known human carcinogen.
The 3 substances that are likely to be human carcinogens are 1-bromopropane, cumene, and pentachlorophenol.
1-bromopropane is used as a cleaning solvent and spray adhesive. Cumene is used to make phenol and acetone, and it is found in fuel products and tobacco smoke. Pentachlorophenol is a mixture used to preserve wood.
Exposure to pentachlorophenol is associated with an increased risk of non-Hodgkin lymphoma in humans and solid tumor malignancies in mice. Cumene and 1-bromopropane have been linked to solid tumor malignancies in mice as well.
All 4 substances are listed in the HHS’s 13th Report on Carcinogens, a science-based document prepared by the National Toxicology Program (NTP) that identifies chemical, biological, and physical agents considered to be cancer hazards for people living in the US.
The new report has a total of 243 listings, which includes known carcinogens and substances “reasonably anticipated” to be carcinogens.
“Identifying substances in our environment that can make people vulnerable to cancer will help in prevention efforts,” said Linda Birnbaum, PhD, director of the National Institute of Environmental Health Sciences and the NTP.
“This report provides a valuable resource for health regulatory and research agencies, and it empowers the public with information people can use to reduce exposure to cancer-causing substances.”
New known carcinogen
Since 1983, ortho-toluidine has been listed in the HHS’s Report on Carcinogens as reasonably anticipated to be a human carcinogen. However, new cancer studies led the NTP to reevaluate and reclassify ortho-toluidine. It is now classified as a known human carcinogen, based on clinical studies showing it causes urinary bladder cancer.
Ortho-toluidine is a synthetic chemical produced in other countries and imported into the US by several companies in high volumes. It is primarily used to make rubber chemicals, pesticides, and dyes. It is also used in some consumer and medical products.
People are mainly exposed through the workplace, by skin contact and/or inhalation when using ortho-toluidine. They can also be exposed outside the workplace through sources such as tobacco smoke.
Three new substances likely to be carcinogenic
Pentachlorophenol
Pentachlorophenol and byproducts of its synthesis are complex mixtures of chemicals used as wood preservatives. Because virtually everyone exposed to pentachlorophenol is also exposed to its synthesis byproducts, they were evaluated together.
In the US, pentachlorophenol has been regulated since the 1980s as a restricted-use pesticide. It is used industrially for treating utility poles, wood pilings, fence posts, and lumber or timber for construction.
Most exposure has occurred in settings where workers treat lumber or come in contact with treated lumber. People may also be exposed to this mixture from breathing contaminated air or dust, or from contact with contaminated soil.
Exposure to this mixture was associated with an increased risk of non-Hodgkin lymphoma in clinical studies. In mice, it has been shown to cause tumors in the liver and other organs.
1-bromopropane
1-bromopropane is a liquid used as a solvent in many commercial industries. It is used as a cleaner for optics, electronics, and metals, as well as a solvent for aerosol-applied adhesives such as those used in foam cushion manufacturing.
It is also used in dry cleaning and in solvent sprays for aircraft maintenance. Workers in certain occupations may be more exposed to 1-bromopropane than the general population.
The NTP did not identify any clinical studies that evaluated the relationship between human cancer and exposure to 1-bromopropane. However, inhalation exposure to 1-bromopropane in rodents caused tumors in several organs, including the skin, lungs, and large intestine.
Cumene
Cumene is a flammable and volatile liquid with a gasoline-like odor. It is a natural component of coal tar and petroleum, and is found in tobacco smoke. It is used primarily to make acetone and phenol.
People are mainly exposed to cumene through the environment and in workplaces that use or produce cumene. It can be found in emissions from petroleum products.
Inhalation exposure to cumene caused lung tumors in male and female mice, and liver tumors in female mice. The NTP did not identify any clinical studies evaluating the relationship between cancer and exposure to cumene. ![]()

Credit: Trevor MacInnis
The US Department of Health and Human Services (HHS) has identified 1 chemical substance as a known human carcinogen and 3 additional substances as likely carcinogens.
Ortho-toluidine, which is used to make rubber chemicals, pesticides, and dyes, has been shown to cause urinary bladder cancer and is now listed as a known human carcinogen.
The 3 substances that are likely to be human carcinogens are 1-bromopropane, cumene, and pentachlorophenol.
1-bromopropane is used as a cleaning solvent and spray adhesive. Cumene is used to make phenol and acetone, and it is found in fuel products and tobacco smoke. Pentachlorophenol is a mixture used to preserve wood.
Exposure to pentachlorophenol is associated with an increased risk of non-Hodgkin lymphoma in humans and solid tumor malignancies in mice. Cumene and 1-bromopropane have been linked to solid tumor malignancies in mice as well.
All 4 substances are listed in the HHS’s 13th Report on Carcinogens, a science-based document prepared by the National Toxicology Program (NTP) that identifies chemical, biological, and physical agents considered to be cancer hazards for people living in the US.
The new report has a total of 243 listings, which includes known carcinogens and substances “reasonably anticipated” to be carcinogens.
“Identifying substances in our environment that can make people vulnerable to cancer will help in prevention efforts,” said Linda Birnbaum, PhD, director of the National Institute of Environmental Health Sciences and the NTP.
“This report provides a valuable resource for health regulatory and research agencies, and it empowers the public with information people can use to reduce exposure to cancer-causing substances.”
New known carcinogen
Since 1983, ortho-toluidine has been listed in the HHS’s Report on Carcinogens as reasonably anticipated to be a human carcinogen. However, new cancer studies led the NTP to reevaluate and reclassify ortho-toluidine. It is now classified as a known human carcinogen, based on clinical studies showing it causes urinary bladder cancer.
Ortho-toluidine is a synthetic chemical produced in other countries and imported into the US by several companies in high volumes. It is primarily used to make rubber chemicals, pesticides, and dyes. It is also used in some consumer and medical products.
People are mainly exposed through the workplace, by skin contact and/or inhalation when using ortho-toluidine. They can also be exposed outside the workplace through sources such as tobacco smoke.
Three new substances likely to be carcinogenic
Pentachlorophenol
Pentachlorophenol and byproducts of its synthesis are complex mixtures of chemicals used as wood preservatives. Because virtually everyone exposed to pentachlorophenol is also exposed to its synthesis byproducts, they were evaluated together.
In the US, pentachlorophenol has been regulated since the 1980s as a restricted-use pesticide. It is used industrially for treating utility poles, wood pilings, fence posts, and lumber or timber for construction.
Most exposure has occurred in settings where workers treat lumber or come in contact with treated lumber. People may also be exposed to this mixture from breathing contaminated air or dust, or from contact with contaminated soil.
Exposure to this mixture was associated with an increased risk of non-Hodgkin lymphoma in clinical studies. In mice, it has been shown to cause tumors in the liver and other organs.
1-bromopropane
1-bromopropane is a liquid used as a solvent in many commercial industries. It is used as a cleaner for optics, electronics, and metals, as well as a solvent for aerosol-applied adhesives such as those used in foam cushion manufacturing.
It is also used in dry cleaning and in solvent sprays for aircraft maintenance. Workers in certain occupations may be more exposed to 1-bromopropane than the general population.
The NTP did not identify any clinical studies that evaluated the relationship between human cancer and exposure to 1-bromopropane. However, inhalation exposure to 1-bromopropane in rodents caused tumors in several organs, including the skin, lungs, and large intestine.
Cumene
Cumene is a flammable and volatile liquid with a gasoline-like odor. It is a natural component of coal tar and petroleum, and is found in tobacco smoke. It is used primarily to make acetone and phenol.
People are mainly exposed to cumene through the environment and in workplaces that use or produce cumene. It can be found in emissions from petroleum products.
Inhalation exposure to cumene caused lung tumors in male and female mice, and liver tumors in female mice. The NTP did not identify any clinical studies evaluating the relationship between cancer and exposure to cumene. ![]()

Credit: Trevor MacInnis
The US Department of Health and Human Services (HHS) has identified 1 chemical substance as a known human carcinogen and 3 additional substances as likely carcinogens.
Ortho-toluidine, which is used to make rubber chemicals, pesticides, and dyes, has been shown to cause urinary bladder cancer and is now listed as a known human carcinogen.
The 3 substances that are likely to be human carcinogens are 1-bromopropane, cumene, and pentachlorophenol.
1-bromopropane is used as a cleaning solvent and spray adhesive. Cumene is used to make phenol and acetone, and it is found in fuel products and tobacco smoke. Pentachlorophenol is a mixture used to preserve wood.
Exposure to pentachlorophenol is associated with an increased risk of non-Hodgkin lymphoma in humans and solid tumor malignancies in mice. Cumene and 1-bromopropane have been linked to solid tumor malignancies in mice as well.
All 4 substances are listed in the HHS’s 13th Report on Carcinogens, a science-based document prepared by the National Toxicology Program (NTP) that identifies chemical, biological, and physical agents considered to be cancer hazards for people living in the US.
The new report has a total of 243 listings, which includes known carcinogens and substances “reasonably anticipated” to be carcinogens.
“Identifying substances in our environment that can make people vulnerable to cancer will help in prevention efforts,” said Linda Birnbaum, PhD, director of the National Institute of Environmental Health Sciences and the NTP.
“This report provides a valuable resource for health regulatory and research agencies, and it empowers the public with information people can use to reduce exposure to cancer-causing substances.”
New known carcinogen
Since 1983, ortho-toluidine has been listed in the HHS’s Report on Carcinogens as reasonably anticipated to be a human carcinogen. However, new cancer studies led the NTP to reevaluate and reclassify ortho-toluidine. It is now classified as a known human carcinogen, based on clinical studies showing it causes urinary bladder cancer.
Ortho-toluidine is a synthetic chemical produced in other countries and imported into the US by several companies in high volumes. It is primarily used to make rubber chemicals, pesticides, and dyes. It is also used in some consumer and medical products.
People are mainly exposed through the workplace, by skin contact and/or inhalation when using ortho-toluidine. They can also be exposed outside the workplace through sources such as tobacco smoke.
Three new substances likely to be carcinogenic
Pentachlorophenol
Pentachlorophenol and byproducts of its synthesis are complex mixtures of chemicals used as wood preservatives. Because virtually everyone exposed to pentachlorophenol is also exposed to its synthesis byproducts, they were evaluated together.
In the US, pentachlorophenol has been regulated since the 1980s as a restricted-use pesticide. It is used industrially for treating utility poles, wood pilings, fence posts, and lumber or timber for construction.
Most exposure has occurred in settings where workers treat lumber or come in contact with treated lumber. People may also be exposed to this mixture from breathing contaminated air or dust, or from contact with contaminated soil.
Exposure to this mixture was associated with an increased risk of non-Hodgkin lymphoma in clinical studies. In mice, it has been shown to cause tumors in the liver and other organs.
1-bromopropane
1-bromopropane is a liquid used as a solvent in many commercial industries. It is used as a cleaner for optics, electronics, and metals, as well as a solvent for aerosol-applied adhesives such as those used in foam cushion manufacturing.
It is also used in dry cleaning and in solvent sprays for aircraft maintenance. Workers in certain occupations may be more exposed to 1-bromopropane than the general population.
The NTP did not identify any clinical studies that evaluated the relationship between human cancer and exposure to 1-bromopropane. However, inhalation exposure to 1-bromopropane in rodents caused tumors in several organs, including the skin, lungs, and large intestine.
Cumene
Cumene is a flammable and volatile liquid with a gasoline-like odor. It is a natural component of coal tar and petroleum, and is found in tobacco smoke. It is used primarily to make acetone and phenol.
People are mainly exposed to cumene through the environment and in workplaces that use or produce cumene. It can be found in emissions from petroleum products.
Inhalation exposure to cumene caused lung tumors in male and female mice, and liver tumors in female mice. The NTP did not identify any clinical studies evaluating the relationship between cancer and exposure to cumene.
Dendritic cells promote Myc-driven lymphoma
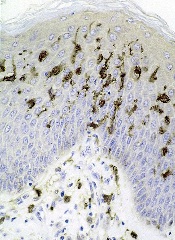
Studies have shown that dendritic cells (DCs) can contribute to tumor growth and help shield the tumor from the immune system in colon, stomach, breast, and prostate cancer.
Now, researchers have found evidence suggesting this phenomenon also occurs in Myc-driven lymphomas.
The team has also identified the molecular mechanism that induces the immune cells to promote tumor growth.
They reported these findings in Nature Communications.
Uta Höpken, PhD, of the Max Delbrück Center for Molecular Medicine in Berlin, Germany, and her colleagues investigated how DCs drive tumor in mouse models of Eµ-Myc lymphoma.
The team began by depleting DCs in these mice and found that tumor growth was delayed—the first clue that DCs are indeed associated with lymphoma growth.
Next, the researchers found that, after contact with lymphoma cells, the DCs increasingly secrete immunomodulatory cytokines and growth factors. The cytokine secretion takes place in the spleen and lymph nodes.
Dr Höpken and her colleagues previously demonstrated that various forms of lymphoma cells settle in the lymph nodes and in the spleen, where they create their own survival niche. This process is regulated by selective cytokines and growth factors the researchers identified a few years ago.
“In these niches, almost everything is already there that the lymphoma cells as malignant B cells need to survive, including blood vessels and connective tissue cells [stromal cells],” Dr Höpken said. “The survival substances secreted by the DCs optimize the niche so that the tumors can grow better.”
This also means the DCs prevent the T lymphocytes from exercising their defensive function. Normally, healthy B or T cells settle in the respective B- or T-cell niches of the spleen and the lymph nodes to be made fit for immune defense.
“What is paradoxical is that the mouse lymphoma cells we studied—malignant B cells—found their survival niche in the T-cell zones of the lymph nodes and the spleen and not in the B-cell zones,” Dr Höpken said.
After making contact with the lymphoma cells, the DCs increasingly upregulate C/EBPβ, a transcription factor that promotes the production of cytokines that mediate inflammation.
The researchers found that C/EBPβ regulates DCs. Without it, the cells could not secrete inflammatory cytokines. C/EBPβ also indirectly blocks apoptosis in the lymphoma cells, allowing the cancer cells to grow unchecked.
The team pointed out that, even if their model of Eµ-Myc lymphoma is not entirely comparable to B-cell lymphomas in humans, it shows that lymphoma cells and DCs interact—a previously unknown molecular mechanism.
Furthermore, these findings may have clinical applications. The researchers noted that the immunomodulatory agent lenalidomide induces downregulation of C/EBPβ, which is secreted by cancer cells.
So Dr Höpken and her colleagues believe it might be appropriate to approve the use of lenalidomide for patients with Myc B-cell lymphoma, as an addition to their existing treatment, to strengthen their immune defense.

Studies have shown that dendritic cells (DCs) can contribute to tumor growth and help shield the tumor from the immune system in colon, stomach, breast, and prostate cancer.
Now, researchers have found evidence suggesting this phenomenon also occurs in Myc-driven lymphomas.
The team has also identified the molecular mechanism that induces the immune cells to promote tumor growth.
They reported these findings in Nature Communications.
Uta Höpken, PhD, of the Max Delbrück Center for Molecular Medicine in Berlin, Germany, and her colleagues investigated how DCs drive tumor in mouse models of Eµ-Myc lymphoma.
The team began by depleting DCs in these mice and found that tumor growth was delayed—the first clue that DCs are indeed associated with lymphoma growth.
Next, the researchers found that, after contact with lymphoma cells, the DCs increasingly secrete immunomodulatory cytokines and growth factors. The cytokine secretion takes place in the spleen and lymph nodes.
Dr Höpken and her colleagues previously demonstrated that various forms of lymphoma cells settle in the lymph nodes and in the spleen, where they create their own survival niche. This process is regulated by selective cytokines and growth factors the researchers identified a few years ago.
“In these niches, almost everything is already there that the lymphoma cells as malignant B cells need to survive, including blood vessels and connective tissue cells [stromal cells],” Dr Höpken said. “The survival substances secreted by the DCs optimize the niche so that the tumors can grow better.”
This also means the DCs prevent the T lymphocytes from exercising their defensive function. Normally, healthy B or T cells settle in the respective B- or T-cell niches of the spleen and the lymph nodes to be made fit for immune defense.
“What is paradoxical is that the mouse lymphoma cells we studied—malignant B cells—found their survival niche in the T-cell zones of the lymph nodes and the spleen and not in the B-cell zones,” Dr Höpken said.
After making contact with the lymphoma cells, the DCs increasingly upregulate C/EBPβ, a transcription factor that promotes the production of cytokines that mediate inflammation.
The researchers found that C/EBPβ regulates DCs. Without it, the cells could not secrete inflammatory cytokines. C/EBPβ also indirectly blocks apoptosis in the lymphoma cells, allowing the cancer cells to grow unchecked.
The team pointed out that, even if their model of Eµ-Myc lymphoma is not entirely comparable to B-cell lymphomas in humans, it shows that lymphoma cells and DCs interact—a previously unknown molecular mechanism.
Furthermore, these findings may have clinical applications. The researchers noted that the immunomodulatory agent lenalidomide induces downregulation of C/EBPβ, which is secreted by cancer cells.
So Dr Höpken and her colleagues believe it might be appropriate to approve the use of lenalidomide for patients with Myc B-cell lymphoma, as an addition to their existing treatment, to strengthen their immune defense.

Studies have shown that dendritic cells (DCs) can contribute to tumor growth and help shield the tumor from the immune system in colon, stomach, breast, and prostate cancer.
Now, researchers have found evidence suggesting this phenomenon also occurs in Myc-driven lymphomas.
The team has also identified the molecular mechanism that induces the immune cells to promote tumor growth.
They reported these findings in Nature Communications.
Uta Höpken, PhD, of the Max Delbrück Center for Molecular Medicine in Berlin, Germany, and her colleagues investigated how DCs drive tumor in mouse models of Eµ-Myc lymphoma.
The team began by depleting DCs in these mice and found that tumor growth was delayed—the first clue that DCs are indeed associated with lymphoma growth.
Next, the researchers found that, after contact with lymphoma cells, the DCs increasingly secrete immunomodulatory cytokines and growth factors. The cytokine secretion takes place in the spleen and lymph nodes.
Dr Höpken and her colleagues previously demonstrated that various forms of lymphoma cells settle in the lymph nodes and in the spleen, where they create their own survival niche. This process is regulated by selective cytokines and growth factors the researchers identified a few years ago.
“In these niches, almost everything is already there that the lymphoma cells as malignant B cells need to survive, including blood vessels and connective tissue cells [stromal cells],” Dr Höpken said. “The survival substances secreted by the DCs optimize the niche so that the tumors can grow better.”
This also means the DCs prevent the T lymphocytes from exercising their defensive function. Normally, healthy B or T cells settle in the respective B- or T-cell niches of the spleen and the lymph nodes to be made fit for immune defense.
“What is paradoxical is that the mouse lymphoma cells we studied—malignant B cells—found their survival niche in the T-cell zones of the lymph nodes and the spleen and not in the B-cell zones,” Dr Höpken said.
After making contact with the lymphoma cells, the DCs increasingly upregulate C/EBPβ, a transcription factor that promotes the production of cytokines that mediate inflammation.
The researchers found that C/EBPβ regulates DCs. Without it, the cells could not secrete inflammatory cytokines. C/EBPβ also indirectly blocks apoptosis in the lymphoma cells, allowing the cancer cells to grow unchecked.
The team pointed out that, even if their model of Eµ-Myc lymphoma is not entirely comparable to B-cell lymphomas in humans, it shows that lymphoma cells and DCs interact—a previously unknown molecular mechanism.
Furthermore, these findings may have clinical applications. The researchers noted that the immunomodulatory agent lenalidomide induces downregulation of C/EBPβ, which is secreted by cancer cells.
So Dr Höpken and her colleagues believe it might be appropriate to approve the use of lenalidomide for patients with Myc B-cell lymphoma, as an addition to their existing treatment, to strengthen their immune defense.
Consolidation can improve PFS in HL
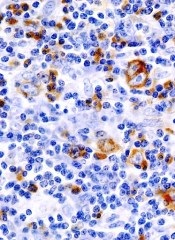
Consolidation therapy with brentuximab vedotin can improve progression-free survival (PFS) for Hodgkin lymphoma (HL) patients who have undergone a transplant, according to a phase 3 study.
The trial, known as AETHERA, is a comparison of single-agent brentuximab vedotin to placebo in patients with HL who were at risk of relapse following autologous stem cell transplant (ASCT).
Brentuximab vedotin conferred a 75% improvement in PFS over placebo.
However, there was no significant difference in overall survival between the 2 treatment arms.
These results were recently announced by Seattle Genetics Inc. and Takeda Pharmaceutical Company Limited, the companies developing brentuximab vedotin (Adcetris).
The companies said more complete results from AETHERA will be presented at the 2014 ASH Annual Meeting in December.
AETHERA is a randomized, double-blind, placebo-controlled study designed to evaluate the potential of brentuximab vedotin to extend PFS post-ASCT in patients with HL who have at least one risk factor for progression. In addition to the primary endpoint of PFS, secondary endpoints included overall survival, safety, and tolerability.
Patients were eligible if they had risk factors for residual HL, defined as a history of refractory HL, those who relapse or progress within a year of receiving frontline chemotherapy, and/or those who have disease outside of the lymph nodes at the time of pre-ASCT relapse.
The study included 329 patients who received brentuximab vedotin or placebo every 3 weeks for up to a year.
The researchers assessed PFS a minimum of 2 years after the initiation of treatment for all patients. There was a significant improvement in PFS with brentuximab vedotin compared to placebo (hazard ratio=0.57; P=0.001).
However, a prespecified interim analysis of overall survival showed no significant difference between the treatment arms.
Patients in both arms who experienced progression received a variety of subsequent therapies. Most patients on the placebo arm received brentuximab vedotin after progression.
A further analysis of overall survival is planned in 2016. The safety profile of brentuximab vedotin in the AETHERA trial was generally consistent with the existing prescribing information.
“We anticipate reporting more complete AETHERA data at the ASH Annual Meeting in December and intend to submit a supplemental Biologics License Application to the FDA in 2015 for approval in this setting,” said Clay B. Siegall, PhD, President and Chief Executive Officer of Seattle Genetics.
The FDA has already granted brentuximab vedotin accelerated approval to treat HL patients after ASCT failure or after the failure of at least 2 prior multiagent chemotherapy regimens in patients who are not ASCT candidates. The FDA also granted the drug accelerated approval to treat systemic anaplastic large cell lymphoma after the failure of at least 1 prior multiagent chemotherapy regimen.
The European Commission granted brentuximab vedotin conditional marketing authorization for the same indications. In both cases, the drug can gain full, traditional approval once studies have shown it confers a clinical benefit.
Brentuximab vedotin has a boxed warning detailing the risk of progressive multifocal leukoencephalopathy associated with use of the drug. The drug has also been shown to pose a risk of pulmonary toxicity when combined with bleomycin.

Consolidation therapy with brentuximab vedotin can improve progression-free survival (PFS) for Hodgkin lymphoma (HL) patients who have undergone a transplant, according to a phase 3 study.
The trial, known as AETHERA, is a comparison of single-agent brentuximab vedotin to placebo in patients with HL who were at risk of relapse following autologous stem cell transplant (ASCT).
Brentuximab vedotin conferred a 75% improvement in PFS over placebo.
However, there was no significant difference in overall survival between the 2 treatment arms.
These results were recently announced by Seattle Genetics Inc. and Takeda Pharmaceutical Company Limited, the companies developing brentuximab vedotin (Adcetris).
The companies said more complete results from AETHERA will be presented at the 2014 ASH Annual Meeting in December.
AETHERA is a randomized, double-blind, placebo-controlled study designed to evaluate the potential of brentuximab vedotin to extend PFS post-ASCT in patients with HL who have at least one risk factor for progression. In addition to the primary endpoint of PFS, secondary endpoints included overall survival, safety, and tolerability.
Patients were eligible if they had risk factors for residual HL, defined as a history of refractory HL, those who relapse or progress within a year of receiving frontline chemotherapy, and/or those who have disease outside of the lymph nodes at the time of pre-ASCT relapse.
The study included 329 patients who received brentuximab vedotin or placebo every 3 weeks for up to a year.
The researchers assessed PFS a minimum of 2 years after the initiation of treatment for all patients. There was a significant improvement in PFS with brentuximab vedotin compared to placebo (hazard ratio=0.57; P=0.001).
However, a prespecified interim analysis of overall survival showed no significant difference between the treatment arms.
Patients in both arms who experienced progression received a variety of subsequent therapies. Most patients on the placebo arm received brentuximab vedotin after progression.
A further analysis of overall survival is planned in 2016. The safety profile of brentuximab vedotin in the AETHERA trial was generally consistent with the existing prescribing information.
“We anticipate reporting more complete AETHERA data at the ASH Annual Meeting in December and intend to submit a supplemental Biologics License Application to the FDA in 2015 for approval in this setting,” said Clay B. Siegall, PhD, President and Chief Executive Officer of Seattle Genetics.
The FDA has already granted brentuximab vedotin accelerated approval to treat HL patients after ASCT failure or after the failure of at least 2 prior multiagent chemotherapy regimens in patients who are not ASCT candidates. The FDA also granted the drug accelerated approval to treat systemic anaplastic large cell lymphoma after the failure of at least 1 prior multiagent chemotherapy regimen.
The European Commission granted brentuximab vedotin conditional marketing authorization for the same indications. In both cases, the drug can gain full, traditional approval once studies have shown it confers a clinical benefit.
Brentuximab vedotin has a boxed warning detailing the risk of progressive multifocal leukoencephalopathy associated with use of the drug. The drug has also been shown to pose a risk of pulmonary toxicity when combined with bleomycin.

Consolidation therapy with brentuximab vedotin can improve progression-free survival (PFS) for Hodgkin lymphoma (HL) patients who have undergone a transplant, according to a phase 3 study.
The trial, known as AETHERA, is a comparison of single-agent brentuximab vedotin to placebo in patients with HL who were at risk of relapse following autologous stem cell transplant (ASCT).
Brentuximab vedotin conferred a 75% improvement in PFS over placebo.
However, there was no significant difference in overall survival between the 2 treatment arms.
These results were recently announced by Seattle Genetics Inc. and Takeda Pharmaceutical Company Limited, the companies developing brentuximab vedotin (Adcetris).
The companies said more complete results from AETHERA will be presented at the 2014 ASH Annual Meeting in December.
AETHERA is a randomized, double-blind, placebo-controlled study designed to evaluate the potential of brentuximab vedotin to extend PFS post-ASCT in patients with HL who have at least one risk factor for progression. In addition to the primary endpoint of PFS, secondary endpoints included overall survival, safety, and tolerability.
Patients were eligible if they had risk factors for residual HL, defined as a history of refractory HL, those who relapse or progress within a year of receiving frontline chemotherapy, and/or those who have disease outside of the lymph nodes at the time of pre-ASCT relapse.
The study included 329 patients who received brentuximab vedotin or placebo every 3 weeks for up to a year.
The researchers assessed PFS a minimum of 2 years after the initiation of treatment for all patients. There was a significant improvement in PFS with brentuximab vedotin compared to placebo (hazard ratio=0.57; P=0.001).
However, a prespecified interim analysis of overall survival showed no significant difference between the treatment arms.
Patients in both arms who experienced progression received a variety of subsequent therapies. Most patients on the placebo arm received brentuximab vedotin after progression.
A further analysis of overall survival is planned in 2016. The safety profile of brentuximab vedotin in the AETHERA trial was generally consistent with the existing prescribing information.
“We anticipate reporting more complete AETHERA data at the ASH Annual Meeting in December and intend to submit a supplemental Biologics License Application to the FDA in 2015 for approval in this setting,” said Clay B. Siegall, PhD, President and Chief Executive Officer of Seattle Genetics.
The FDA has already granted brentuximab vedotin accelerated approval to treat HL patients after ASCT failure or after the failure of at least 2 prior multiagent chemotherapy regimens in patients who are not ASCT candidates. The FDA also granted the drug accelerated approval to treat systemic anaplastic large cell lymphoma after the failure of at least 1 prior multiagent chemotherapy regimen.
The European Commission granted brentuximab vedotin conditional marketing authorization for the same indications. In both cases, the drug can gain full, traditional approval once studies have shown it confers a clinical benefit.
Brentuximab vedotin has a boxed warning detailing the risk of progressive multifocal leukoencephalopathy associated with use of the drug. The drug has also been shown to pose a risk of pulmonary toxicity when combined with bleomycin.
Maintenance may be unnecessary in FL

Credit: Bill Branson
New research suggests maintenance therapy may not be necessary for patients with follicular lymphoma (FL) who have a low tumor burden.
Investigators compared rituximab re-treatment with rituximab maintenance in nearly 300 FL patients, and results showed no significant difference between the treatment groups in the time to disease recurrence.
The researchers also noted that the re-treatment strategy was more cost-effective.
“For those 2 reasons, we recommend a retreatment strategy over a maintenance strategy in this patient population,” said Brad S. Kahl, MD, of the University of Wisconsin in Madison.
Dr Kahl and his colleagues described this research—the RESORT trial—in the Journal of Clinical Oncology. Early results from this trial were previously presented at the 2011 ASH Annual Meeting.
The team evaluated 289 patients with previously untreated, low-tumor-burden FL. All patients responded to initial treatment with rituximab (4 doses).
Patients were then randomized to receive maintenance therapy—a single dose of rituximab every 3 months until treatment failure—or rituximab re-treatment upon disease recurrence. Patients receiving re-treatment could receive rituximab every time they experienced progression, until treatment failure.
The median number of rituximab doses was 4 in the re-treatment arm and 18 in the maintenance arm. Three-year freedom from cytotoxic therapy was 84% in the re-treatment arm and 95% in the maintenance arm (P=0.03).
There was no significant difference between the arms in the time to disease recurrence. With a median follow-up of 4.5 years, the estimated median time to treatment failure was 3.9 years in the re-treatment arm and 4.3 years in the maintenance arm (P=0.54).
The researchers found no difference in health-related quality of life or anxiety between the treatment arms.
They also said grade 3 to 5 adverse events were infrequent in both arms. One patient developed progressive multifocal leukoencephalopathy after the 15th maintenance dose of rituximab and died.
Second malignancies were reported in 16 patients receiving re-treatment and 14 patients on maintenance therapy, but there were no obvious trends toward specific cancers.
“The study shows that a rituximab re-treatment strategy provides comparable disease control to a maintenance strategy in low-tumor-burden follicular lymphoma,” Dr Kahl said. “In addition, a re-treatment strategy is more cost-effective, as it requires about a quarter as much drug utilization.”
The study was accompanied by an editorial saying these results should change clinical practice.

Credit: Bill Branson
New research suggests maintenance therapy may not be necessary for patients with follicular lymphoma (FL) who have a low tumor burden.
Investigators compared rituximab re-treatment with rituximab maintenance in nearly 300 FL patients, and results showed no significant difference between the treatment groups in the time to disease recurrence.
The researchers also noted that the re-treatment strategy was more cost-effective.
“For those 2 reasons, we recommend a retreatment strategy over a maintenance strategy in this patient population,” said Brad S. Kahl, MD, of the University of Wisconsin in Madison.
Dr Kahl and his colleagues described this research—the RESORT trial—in the Journal of Clinical Oncology. Early results from this trial were previously presented at the 2011 ASH Annual Meeting.
The team evaluated 289 patients with previously untreated, low-tumor-burden FL. All patients responded to initial treatment with rituximab (4 doses).
Patients were then randomized to receive maintenance therapy—a single dose of rituximab every 3 months until treatment failure—or rituximab re-treatment upon disease recurrence. Patients receiving re-treatment could receive rituximab every time they experienced progression, until treatment failure.
The median number of rituximab doses was 4 in the re-treatment arm and 18 in the maintenance arm. Three-year freedom from cytotoxic therapy was 84% in the re-treatment arm and 95% in the maintenance arm (P=0.03).
There was no significant difference between the arms in the time to disease recurrence. With a median follow-up of 4.5 years, the estimated median time to treatment failure was 3.9 years in the re-treatment arm and 4.3 years in the maintenance arm (P=0.54).
The researchers found no difference in health-related quality of life or anxiety between the treatment arms.
They also said grade 3 to 5 adverse events were infrequent in both arms. One patient developed progressive multifocal leukoencephalopathy after the 15th maintenance dose of rituximab and died.
Second malignancies were reported in 16 patients receiving re-treatment and 14 patients on maintenance therapy, but there were no obvious trends toward specific cancers.
“The study shows that a rituximab re-treatment strategy provides comparable disease control to a maintenance strategy in low-tumor-burden follicular lymphoma,” Dr Kahl said. “In addition, a re-treatment strategy is more cost-effective, as it requires about a quarter as much drug utilization.”
The study was accompanied by an editorial saying these results should change clinical practice.

Credit: Bill Branson
New research suggests maintenance therapy may not be necessary for patients with follicular lymphoma (FL) who have a low tumor burden.
Investigators compared rituximab re-treatment with rituximab maintenance in nearly 300 FL patients, and results showed no significant difference between the treatment groups in the time to disease recurrence.
The researchers also noted that the re-treatment strategy was more cost-effective.
“For those 2 reasons, we recommend a retreatment strategy over a maintenance strategy in this patient population,” said Brad S. Kahl, MD, of the University of Wisconsin in Madison.
Dr Kahl and his colleagues described this research—the RESORT trial—in the Journal of Clinical Oncology. Early results from this trial were previously presented at the 2011 ASH Annual Meeting.
The team evaluated 289 patients with previously untreated, low-tumor-burden FL. All patients responded to initial treatment with rituximab (4 doses).
Patients were then randomized to receive maintenance therapy—a single dose of rituximab every 3 months until treatment failure—or rituximab re-treatment upon disease recurrence. Patients receiving re-treatment could receive rituximab every time they experienced progression, until treatment failure.
The median number of rituximab doses was 4 in the re-treatment arm and 18 in the maintenance arm. Three-year freedom from cytotoxic therapy was 84% in the re-treatment arm and 95% in the maintenance arm (P=0.03).
There was no significant difference between the arms in the time to disease recurrence. With a median follow-up of 4.5 years, the estimated median time to treatment failure was 3.9 years in the re-treatment arm and 4.3 years in the maintenance arm (P=0.54).
The researchers found no difference in health-related quality of life or anxiety between the treatment arms.
They also said grade 3 to 5 adverse events were infrequent in both arms. One patient developed progressive multifocal leukoencephalopathy after the 15th maintenance dose of rituximab and died.
Second malignancies were reported in 16 patients receiving re-treatment and 14 patients on maintenance therapy, but there were no obvious trends toward specific cancers.
“The study shows that a rituximab re-treatment strategy provides comparable disease control to a maintenance strategy in low-tumor-burden follicular lymphoma,” Dr Kahl said. “In addition, a re-treatment strategy is more cost-effective, as it requires about a quarter as much drug utilization.”
The study was accompanied by an editorial saying these results should change clinical practice.