User login
Method could overcome chemoresistance in lymphoma

Credit: Rhoda Baer
Indirectly targeting the prosurvival protein Mcl-1 can reverse chemoresistance in lymphoma and other cancer cells, investigators have reported in The Journal of Biological Chemistry.
The team found that targeting an enzyme known as protein phosphatase 2A (PP2A) inhibited Mcl-1 dephosphorylation, which prompted the loss of Mcl-1 in chemoresistant Burkitt lymphoma cells.
“These findings may lead to a new target for chemoresistant cancer cells,” said Ruth W. Craig, PhD, of Geisel School of Medicine at Dartmouth in Hanover, New Hampshire.
“These cells are resistant to multiple types of standard chemotherapeutic agents because of [Mcl-1] overexpression. However, Mcl-1 expression plummets when we inhibit [PP2A], and then cancer cells subsequently die.”
Dr Craig and her colleagues found that PP2A can be inhibited to stop the removal of phosphate groups from a regulatory motif in Mcl-1 referred to as the PEST region (enriched with amino acids proline, glutamic acid, serine, and threonine).
And inhibiting the removal of phosphate groups, such as at threonine-163 and serine-159, targets the Mcl-1 protein for rapid destruction.
To reach this conclusion, the investigators studied BL41-3 cells, a Burkitt lymphoma cell line that overexpresses Mcl-1 and has proven resistant to multiple treatments.
The team exposed BL41-3 cells to 2 different PP2A inhibitors, okadaic acid and calyculin A. Both drugs prompted an increase in phosphorylation at threonine-163 and serine-159, as well as a decrease in Mcl-1 expression.
Further investigation confirmed that PP2A interacts with Mcl-1. And, as with therapeutic targeting, shRNA knockdown of PP2A/Aα increased phosphorylation while decreasing Mcl-1 expression.
Finally, the investigators showed the increase in Mcl-1 phosphorylation and decrease in its expression occurred well before markers of cell death appeared—about 2 to 3 hours before.
“PP2A is a complex multi-subunit enzyme, and we hope to identify more specifically which form of PP2A is involved in dephosphorylating Mcl-1,” Dr Craig said. “This could [provide us with] a more specific way of causing Mcl-1 destruction.” ![]()

Credit: Rhoda Baer
Indirectly targeting the prosurvival protein Mcl-1 can reverse chemoresistance in lymphoma and other cancer cells, investigators have reported in The Journal of Biological Chemistry.
The team found that targeting an enzyme known as protein phosphatase 2A (PP2A) inhibited Mcl-1 dephosphorylation, which prompted the loss of Mcl-1 in chemoresistant Burkitt lymphoma cells.
“These findings may lead to a new target for chemoresistant cancer cells,” said Ruth W. Craig, PhD, of Geisel School of Medicine at Dartmouth in Hanover, New Hampshire.
“These cells are resistant to multiple types of standard chemotherapeutic agents because of [Mcl-1] overexpression. However, Mcl-1 expression plummets when we inhibit [PP2A], and then cancer cells subsequently die.”
Dr Craig and her colleagues found that PP2A can be inhibited to stop the removal of phosphate groups from a regulatory motif in Mcl-1 referred to as the PEST region (enriched with amino acids proline, glutamic acid, serine, and threonine).
And inhibiting the removal of phosphate groups, such as at threonine-163 and serine-159, targets the Mcl-1 protein for rapid destruction.
To reach this conclusion, the investigators studied BL41-3 cells, a Burkitt lymphoma cell line that overexpresses Mcl-1 and has proven resistant to multiple treatments.
The team exposed BL41-3 cells to 2 different PP2A inhibitors, okadaic acid and calyculin A. Both drugs prompted an increase in phosphorylation at threonine-163 and serine-159, as well as a decrease in Mcl-1 expression.
Further investigation confirmed that PP2A interacts with Mcl-1. And, as with therapeutic targeting, shRNA knockdown of PP2A/Aα increased phosphorylation while decreasing Mcl-1 expression.
Finally, the investigators showed the increase in Mcl-1 phosphorylation and decrease in its expression occurred well before markers of cell death appeared—about 2 to 3 hours before.
“PP2A is a complex multi-subunit enzyme, and we hope to identify more specifically which form of PP2A is involved in dephosphorylating Mcl-1,” Dr Craig said. “This could [provide us with] a more specific way of causing Mcl-1 destruction.” ![]()

Credit: Rhoda Baer
Indirectly targeting the prosurvival protein Mcl-1 can reverse chemoresistance in lymphoma and other cancer cells, investigators have reported in The Journal of Biological Chemistry.
The team found that targeting an enzyme known as protein phosphatase 2A (PP2A) inhibited Mcl-1 dephosphorylation, which prompted the loss of Mcl-1 in chemoresistant Burkitt lymphoma cells.
“These findings may lead to a new target for chemoresistant cancer cells,” said Ruth W. Craig, PhD, of Geisel School of Medicine at Dartmouth in Hanover, New Hampshire.
“These cells are resistant to multiple types of standard chemotherapeutic agents because of [Mcl-1] overexpression. However, Mcl-1 expression plummets when we inhibit [PP2A], and then cancer cells subsequently die.”
Dr Craig and her colleagues found that PP2A can be inhibited to stop the removal of phosphate groups from a regulatory motif in Mcl-1 referred to as the PEST region (enriched with amino acids proline, glutamic acid, serine, and threonine).
And inhibiting the removal of phosphate groups, such as at threonine-163 and serine-159, targets the Mcl-1 protein for rapid destruction.
To reach this conclusion, the investigators studied BL41-3 cells, a Burkitt lymphoma cell line that overexpresses Mcl-1 and has proven resistant to multiple treatments.
The team exposed BL41-3 cells to 2 different PP2A inhibitors, okadaic acid and calyculin A. Both drugs prompted an increase in phosphorylation at threonine-163 and serine-159, as well as a decrease in Mcl-1 expression.
Further investigation confirmed that PP2A interacts with Mcl-1. And, as with therapeutic targeting, shRNA knockdown of PP2A/Aα increased phosphorylation while decreasing Mcl-1 expression.
Finally, the investigators showed the increase in Mcl-1 phosphorylation and decrease in its expression occurred well before markers of cell death appeared—about 2 to 3 hours before.
“PP2A is a complex multi-subunit enzyme, and we hope to identify more specifically which form of PP2A is involved in dephosphorylating Mcl-1,” Dr Craig said. “This could [provide us with] a more specific way of causing Mcl-1 destruction.” ![]()
Cancer survivors aren’t living healthy, study shows

Credit: Bill Branson
Childhood cancer survivors are no more likely than their cancer-free peers to adhere to healthy living guidelines, according to a study published in the Journal of Cancer Survivorship.
Survivors were less likely to be smokers and had a lower average body mass index (BMI).
But there were no significant differences between survivors and cancer-free control subjects with regard to overall diet, physical activity, or alcohol consumption.
Chloe Berdan, of Promedica in Toledo, Ohio, and her colleagues uncovered these results by examining data from the Chicago Healthy Living Study.
The team assessed adherence to American Cancer Society Guidelines on Nutrition and Physical Activity via interviews with 431 childhood cancer survivors and 361 control subjects who never had cancer. The survivors, ages 18 to 59, were all diagnosed with a malignant cancer before their 21st birthdays.
There were no significant differences in sex or race between survivors and controls. Survivors were younger than controls (28.4±7.8 vs 29.6± 8.3 years, P=0.04) and had less education (14.0±2.0 vs 14.4±2.0 years, P=0.01).
Overall, there was no significant difference between survivors and control subjects in adhering to the American Cancer Society guidelines.
Survivors and controls also had similar scores for several individual measures, including alcohol consumption, overall physical activity, overall diet, the servings of fruits/vegetables consumed, and the consumption of red/processed meat.
However, survivors were significantly less likely than controls to be smokers—11.4% vs 17.5% (P=0.02). Survivors had, on average, a BMI of about 1.2 kg/m² lower than controls (P=0.01). And survivors consumed significantly less fiber than controls—9.2±3.5 vs 9.7±3.8 kcal (P=0.05).
Only about 1 in 10 survivors (10.2%) met fiber recommendations, 17.7% ate 5 fruits or vegetables per day, and 46.2% met the red/processed meat recommendation of less than 18 oz per week. On average, survivors scored under 50% for the quality of their diets.
Survivors were better at meeting the goal of at least 5 hours of moderate activity per week (60.5%) than to sticking to any of the other guidelines.
About 36% of survivors were within a healthy BMI range, 2.9% were underweight, 28.9% were overweight, and 32.4% were obese.
The 0.7% of survivors who adhered fully to the guidelines tended to be women, non-smokers, and people with a good view of their own health.
“There is still much room for improvement in educating and encouraging survivors to follow healthier diets and lifestyles,” Berdan said. “Adopting such behavior during early adulthood may have a lasting impact on their quality of life and overall survival.” ![]()

Credit: Bill Branson
Childhood cancer survivors are no more likely than their cancer-free peers to adhere to healthy living guidelines, according to a study published in the Journal of Cancer Survivorship.
Survivors were less likely to be smokers and had a lower average body mass index (BMI).
But there were no significant differences between survivors and cancer-free control subjects with regard to overall diet, physical activity, or alcohol consumption.
Chloe Berdan, of Promedica in Toledo, Ohio, and her colleagues uncovered these results by examining data from the Chicago Healthy Living Study.
The team assessed adherence to American Cancer Society Guidelines on Nutrition and Physical Activity via interviews with 431 childhood cancer survivors and 361 control subjects who never had cancer. The survivors, ages 18 to 59, were all diagnosed with a malignant cancer before their 21st birthdays.
There were no significant differences in sex or race between survivors and controls. Survivors were younger than controls (28.4±7.8 vs 29.6± 8.3 years, P=0.04) and had less education (14.0±2.0 vs 14.4±2.0 years, P=0.01).
Overall, there was no significant difference between survivors and control subjects in adhering to the American Cancer Society guidelines.
Survivors and controls also had similar scores for several individual measures, including alcohol consumption, overall physical activity, overall diet, the servings of fruits/vegetables consumed, and the consumption of red/processed meat.
However, survivors were significantly less likely than controls to be smokers—11.4% vs 17.5% (P=0.02). Survivors had, on average, a BMI of about 1.2 kg/m² lower than controls (P=0.01). And survivors consumed significantly less fiber than controls—9.2±3.5 vs 9.7±3.8 kcal (P=0.05).
Only about 1 in 10 survivors (10.2%) met fiber recommendations, 17.7% ate 5 fruits or vegetables per day, and 46.2% met the red/processed meat recommendation of less than 18 oz per week. On average, survivors scored under 50% for the quality of their diets.
Survivors were better at meeting the goal of at least 5 hours of moderate activity per week (60.5%) than to sticking to any of the other guidelines.
About 36% of survivors were within a healthy BMI range, 2.9% were underweight, 28.9% were overweight, and 32.4% were obese.
The 0.7% of survivors who adhered fully to the guidelines tended to be women, non-smokers, and people with a good view of their own health.
“There is still much room for improvement in educating and encouraging survivors to follow healthier diets and lifestyles,” Berdan said. “Adopting such behavior during early adulthood may have a lasting impact on their quality of life and overall survival.” ![]()

Credit: Bill Branson
Childhood cancer survivors are no more likely than their cancer-free peers to adhere to healthy living guidelines, according to a study published in the Journal of Cancer Survivorship.
Survivors were less likely to be smokers and had a lower average body mass index (BMI).
But there were no significant differences between survivors and cancer-free control subjects with regard to overall diet, physical activity, or alcohol consumption.
Chloe Berdan, of Promedica in Toledo, Ohio, and her colleagues uncovered these results by examining data from the Chicago Healthy Living Study.
The team assessed adherence to American Cancer Society Guidelines on Nutrition and Physical Activity via interviews with 431 childhood cancer survivors and 361 control subjects who never had cancer. The survivors, ages 18 to 59, were all diagnosed with a malignant cancer before their 21st birthdays.
There were no significant differences in sex or race between survivors and controls. Survivors were younger than controls (28.4±7.8 vs 29.6± 8.3 years, P=0.04) and had less education (14.0±2.0 vs 14.4±2.0 years, P=0.01).
Overall, there was no significant difference between survivors and control subjects in adhering to the American Cancer Society guidelines.
Survivors and controls also had similar scores for several individual measures, including alcohol consumption, overall physical activity, overall diet, the servings of fruits/vegetables consumed, and the consumption of red/processed meat.
However, survivors were significantly less likely than controls to be smokers—11.4% vs 17.5% (P=0.02). Survivors had, on average, a BMI of about 1.2 kg/m² lower than controls (P=0.01). And survivors consumed significantly less fiber than controls—9.2±3.5 vs 9.7±3.8 kcal (P=0.05).
Only about 1 in 10 survivors (10.2%) met fiber recommendations, 17.7% ate 5 fruits or vegetables per day, and 46.2% met the red/processed meat recommendation of less than 18 oz per week. On average, survivors scored under 50% for the quality of their diets.
Survivors were better at meeting the goal of at least 5 hours of moderate activity per week (60.5%) than to sticking to any of the other guidelines.
About 36% of survivors were within a healthy BMI range, 2.9% were underweight, 28.9% were overweight, and 32.4% were obese.
The 0.7% of survivors who adhered fully to the guidelines tended to be women, non-smokers, and people with a good view of their own health.
“There is still much room for improvement in educating and encouraging survivors to follow healthier diets and lifestyles,” Berdan said. “Adopting such behavior during early adulthood may have a lasting impact on their quality of life and overall survival.” ![]()
Radiotherapy plus chemotherapy drive risk of pancreatic cancer in HL patients
Hodgkin’s lymphoma survivors who undergo both radiotherapy and chemotherapy face an increased risk of subsequent pancreatic cancer, an intervention case-control study demonstrated. In fact, the risk was 18-fold among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent.
"Several studies have reported significantly increased risks of pancreatic cancer among long-term HL [Hodgkin’s Lymphoma] survivors, but no prior study of HL survivors has assessed the risk of pancreatic cancer in relation to radiation dose or specific chemotherapeutic agents," researchers led by Dr. Graca M. Dores wrote in the July 25, 2014 issue of the Annals of Oncology. "In the general U.S. population, pancreatic cancer is the fourth most common cause of cancer death, with an overall 5-year relative survival of 5.8%," they noted.
In what the investigators characterized as the first analysis of its kind, Dr. Dores of the division of cancer epidemiology and genetics at the National Cancer Institute and associates drew from six population-based registries and data from main hospitals in the Netherlands to locate HL survivors who received a diagnosis of HL as their primary cancer between 1953 and 2003 and who had survived at least 5 years beyond the initial diagnosis. The cohort was comprised of 19,882 HL survivors, including 36 cases of pancreatic cancer and 70 matched controls. The researchers used logistic regression to estimate odds ratios for pancreatic cancer by comparing the histories of case patients to those of matched controls (Ann. Oncol. July 25 [doi:10.1093/annonc/mdu287]).
The median age at HL diagnosis was 47 years, and 73% had stage I or II disease. Among the 36 patients who developed pancreatic cancer, the median age of pancreatic cancer onset among cases was 61 years, a median of 19 years following the initial HL diagnosis. Dr. Dores and associates found that the risk of pancreatic cancer increased with increasing radiation dose to the location of the pancreatic tumor (P = .005) and increasing number of chemotherapy cycles that contained alkylating agents (P = .008). The risk of prostate cancer was 18-fold higher among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent. "This risk was significantly greater than the OR of 3.8 predicted by an additive model (P = .041) and nonsignificantly greater than the OR of 5.4 predicted by a multiplicative model (P = 0.29)," the researchers wrote. "Analyses based on continuous variables yielded similar results."
In discussing the implications of the findings, Dr. Dores and associates acknowledged that treatment approaches for HL "have changed considerably over the past several decades in an effort to maximize efficacy and minimize toxicity. Although radiotherapy remains an important therapeutic modality, radiation volumes and doses have decreased considerably over time, and subdiaphragmatic radiotherapy is infrequently indicated. While the first-line therapy for many HL patients today includes doxorubicin and dacarbazine, procarbazine and cyclophosphamide continue to be used, although often with lower cumulative doses than used in the past. Our findings for topoisomerase II inhibitors are equivocal, but warrant further investigation."
They went on to note that the study extends the range of solid cancers associated with chemotherapy "and adds to the evidence that the combination of chemotherapy and radiotherapy can increase risks beyond those predicted by a multiplicative model. For HL patients, radiation dose-response relationships have now been demonstrated for second cancers of the lung, female breast, stomach, and pancreas and, with the exception of breast cancer, increased risks of these cancers have been observed after receipt of AA [alkylating agent]-containing therapy. Changes in HL therapy over time should reduce second cancer risks compared to those observed with past treatments. In the interim, health care providers caring for long-term HL survivors should be alert to this treatment sequela and encourage a healthy lifestyle to minimize additional cancer risk factors."
The study was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute contracts to Cancer Care Ontario, Toronto; Danish Cancer Society, Copenhagen; Finnish Cancer Registry, Helsinki; Information Management Services, Inc., Silver Spring, Md.; Karolinska Institute, Stockholm; University of Iowa; The University of Texas MD Anderson Cancer Center; and Westat, Inc., Rockville, Md. The Dutch study also was supported by the Lance Armstrong Foundation and the Dutch Cancer Society.
On Twitter @dougbrunk
Hodgkin’s lymphoma survivors who undergo both radiotherapy and chemotherapy face an increased risk of subsequent pancreatic cancer, an intervention case-control study demonstrated. In fact, the risk was 18-fold among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent.
"Several studies have reported significantly increased risks of pancreatic cancer among long-term HL [Hodgkin’s Lymphoma] survivors, but no prior study of HL survivors has assessed the risk of pancreatic cancer in relation to radiation dose or specific chemotherapeutic agents," researchers led by Dr. Graca M. Dores wrote in the July 25, 2014 issue of the Annals of Oncology. "In the general U.S. population, pancreatic cancer is the fourth most common cause of cancer death, with an overall 5-year relative survival of 5.8%," they noted.
In what the investigators characterized as the first analysis of its kind, Dr. Dores of the division of cancer epidemiology and genetics at the National Cancer Institute and associates drew from six population-based registries and data from main hospitals in the Netherlands to locate HL survivors who received a diagnosis of HL as their primary cancer between 1953 and 2003 and who had survived at least 5 years beyond the initial diagnosis. The cohort was comprised of 19,882 HL survivors, including 36 cases of pancreatic cancer and 70 matched controls. The researchers used logistic regression to estimate odds ratios for pancreatic cancer by comparing the histories of case patients to those of matched controls (Ann. Oncol. July 25 [doi:10.1093/annonc/mdu287]).
The median age at HL diagnosis was 47 years, and 73% had stage I or II disease. Among the 36 patients who developed pancreatic cancer, the median age of pancreatic cancer onset among cases was 61 years, a median of 19 years following the initial HL diagnosis. Dr. Dores and associates found that the risk of pancreatic cancer increased with increasing radiation dose to the location of the pancreatic tumor (P = .005) and increasing number of chemotherapy cycles that contained alkylating agents (P = .008). The risk of prostate cancer was 18-fold higher among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent. "This risk was significantly greater than the OR of 3.8 predicted by an additive model (P = .041) and nonsignificantly greater than the OR of 5.4 predicted by a multiplicative model (P = 0.29)," the researchers wrote. "Analyses based on continuous variables yielded similar results."
In discussing the implications of the findings, Dr. Dores and associates acknowledged that treatment approaches for HL "have changed considerably over the past several decades in an effort to maximize efficacy and minimize toxicity. Although radiotherapy remains an important therapeutic modality, radiation volumes and doses have decreased considerably over time, and subdiaphragmatic radiotherapy is infrequently indicated. While the first-line therapy for many HL patients today includes doxorubicin and dacarbazine, procarbazine and cyclophosphamide continue to be used, although often with lower cumulative doses than used in the past. Our findings for topoisomerase II inhibitors are equivocal, but warrant further investigation."
They went on to note that the study extends the range of solid cancers associated with chemotherapy "and adds to the evidence that the combination of chemotherapy and radiotherapy can increase risks beyond those predicted by a multiplicative model. For HL patients, radiation dose-response relationships have now been demonstrated for second cancers of the lung, female breast, stomach, and pancreas and, with the exception of breast cancer, increased risks of these cancers have been observed after receipt of AA [alkylating agent]-containing therapy. Changes in HL therapy over time should reduce second cancer risks compared to those observed with past treatments. In the interim, health care providers caring for long-term HL survivors should be alert to this treatment sequela and encourage a healthy lifestyle to minimize additional cancer risk factors."
The study was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute contracts to Cancer Care Ontario, Toronto; Danish Cancer Society, Copenhagen; Finnish Cancer Registry, Helsinki; Information Management Services, Inc., Silver Spring, Md.; Karolinska Institute, Stockholm; University of Iowa; The University of Texas MD Anderson Cancer Center; and Westat, Inc., Rockville, Md. The Dutch study also was supported by the Lance Armstrong Foundation and the Dutch Cancer Society.
On Twitter @dougbrunk
Hodgkin’s lymphoma survivors who undergo both radiotherapy and chemotherapy face an increased risk of subsequent pancreatic cancer, an intervention case-control study demonstrated. In fact, the risk was 18-fold among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent.
"Several studies have reported significantly increased risks of pancreatic cancer among long-term HL [Hodgkin’s Lymphoma] survivors, but no prior study of HL survivors has assessed the risk of pancreatic cancer in relation to radiation dose or specific chemotherapeutic agents," researchers led by Dr. Graca M. Dores wrote in the July 25, 2014 issue of the Annals of Oncology. "In the general U.S. population, pancreatic cancer is the fourth most common cause of cancer death, with an overall 5-year relative survival of 5.8%," they noted.
In what the investigators characterized as the first analysis of its kind, Dr. Dores of the division of cancer epidemiology and genetics at the National Cancer Institute and associates drew from six population-based registries and data from main hospitals in the Netherlands to locate HL survivors who received a diagnosis of HL as their primary cancer between 1953 and 2003 and who had survived at least 5 years beyond the initial diagnosis. The cohort was comprised of 19,882 HL survivors, including 36 cases of pancreatic cancer and 70 matched controls. The researchers used logistic regression to estimate odds ratios for pancreatic cancer by comparing the histories of case patients to those of matched controls (Ann. Oncol. July 25 [doi:10.1093/annonc/mdu287]).
The median age at HL diagnosis was 47 years, and 73% had stage I or II disease. Among the 36 patients who developed pancreatic cancer, the median age of pancreatic cancer onset among cases was 61 years, a median of 19 years following the initial HL diagnosis. Dr. Dores and associates found that the risk of pancreatic cancer increased with increasing radiation dose to the location of the pancreatic tumor (P = .005) and increasing number of chemotherapy cycles that contained alkylating agents (P = .008). The risk of prostate cancer was 18-fold higher among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent. "This risk was significantly greater than the OR of 3.8 predicted by an additive model (P = .041) and nonsignificantly greater than the OR of 5.4 predicted by a multiplicative model (P = 0.29)," the researchers wrote. "Analyses based on continuous variables yielded similar results."
In discussing the implications of the findings, Dr. Dores and associates acknowledged that treatment approaches for HL "have changed considerably over the past several decades in an effort to maximize efficacy and minimize toxicity. Although radiotherapy remains an important therapeutic modality, radiation volumes and doses have decreased considerably over time, and subdiaphragmatic radiotherapy is infrequently indicated. While the first-line therapy for many HL patients today includes doxorubicin and dacarbazine, procarbazine and cyclophosphamide continue to be used, although often with lower cumulative doses than used in the past. Our findings for topoisomerase II inhibitors are equivocal, but warrant further investigation."
They went on to note that the study extends the range of solid cancers associated with chemotherapy "and adds to the evidence that the combination of chemotherapy and radiotherapy can increase risks beyond those predicted by a multiplicative model. For HL patients, radiation dose-response relationships have now been demonstrated for second cancers of the lung, female breast, stomach, and pancreas and, with the exception of breast cancer, increased risks of these cancers have been observed after receipt of AA [alkylating agent]-containing therapy. Changes in HL therapy over time should reduce second cancer risks compared to those observed with past treatments. In the interim, health care providers caring for long-term HL survivors should be alert to this treatment sequela and encourage a healthy lifestyle to minimize additional cancer risk factors."
The study was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute contracts to Cancer Care Ontario, Toronto; Danish Cancer Society, Copenhagen; Finnish Cancer Registry, Helsinki; Information Management Services, Inc., Silver Spring, Md.; Karolinska Institute, Stockholm; University of Iowa; The University of Texas MD Anderson Cancer Center; and Westat, Inc., Rockville, Md. The Dutch study also was supported by the Lance Armstrong Foundation and the Dutch Cancer Society.
On Twitter @dougbrunk
FROM ANNALS OF ONCOLOGY
Key clinical point: Both radiotherapy and chemotherapy increase pancreatic cancer risk among Hodgkin’s lymphoma survivors.
Major finding: Survivors of Hodgkin’s lymphoma treated with both subdiaphragmatic radiation and six or more cycles of alkylating agent-containing therapy were 18 times more likely to develop pancreatic cancer, compared with patients who received no such treatment.
Data source: An international case-control study within a cohort of 19,882 HL survivors diagnosed from 1953 to 2003, including 36 cases with pancreatic cancer and 70 matched controls.
Disclosures: The study was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute contracts to Cancer Care Ontario, Toronto; Danish Cancer Society, Copenhagen; Finnish Cancer Registry, Helsinki; Information Management Services, Inc., Silver Spring, Md.; Karolinska Institute, Stockholm; University of Iowa; The University of Texas MD Anderson Cancer Center; and Westat, Inc., Rockville, Md. The Dutch study also was supported by the Lance Armstrong Foundation and the Dutch Cancer Society.
Molecule is active against MYC-driven malignancies

Credit: Ed Uthman
A small molecule can disrupt the interactions between MYC and its binding partner MAX in MYC-driven cancers, according to research published in PNAS.
The molecule, KJ-Pyr-9, inhibited MYC-induced oncogenic transformation in cell culture but had little to no effect on the oncogenic activity of several unrelated oncoproteins.
KJ-Pyr-9 preferentially interfered with proliferation in a range of cells that overexpressed MYC, including leukemia and lymphoma cells.
In vivo, the molecule inhibited the growth of MYC-amplified human cancer cells.
“We finally hit a home run with this—maybe a grand slam,” said study author Kim Janda, PhD, of The Scripps Research Institute in La Jolla, California.
For years, MYC has challenged researchers seeking to disrupt its activity in cancer cells.
“At room temperature or body temperature, MYC without any binding partners is random and constantly shifting,” said study author Jonathan Ross Hart, PhD, also of The Scripps Research Institute. “It’s like a piece of spaghetti.”
So instead of designing a compound to target the structure of MYC, the researchers tested a range of compounds from a library to see if any could disrupt the interactions between MYC and other proteins important in cell proliferation. One did—the small molecule KJ-Pyr-9.
To further investigate, the researchers ran tests in a variety of cell lines, including chronic myeloid leukemia, acute lymphoblastic leukemia, acute myeloid leukemia, Burkitt lymphoma, and solid tumors. And they tested KJ-Pyr-9 in mouse models of breast cancer.
The experiments showed that MYC-dependent cells die if treated with KJ-Pyr-9. In fact, a dose of KJ-Pyr-9 made it seem as if MYC was not present at all.
When mice with MYC-dependent tumors received KJ-Pyr-9, the tumors showed no growth after 31 days, compared with significant tumor growth in untreated mice.
Dr Janda said he hopes further research will reveal exactly how KJ-Pyr-9 interacts with MYC and how the compound can more effectively reach tumor cells. ![]()

Credit: Ed Uthman
A small molecule can disrupt the interactions between MYC and its binding partner MAX in MYC-driven cancers, according to research published in PNAS.
The molecule, KJ-Pyr-9, inhibited MYC-induced oncogenic transformation in cell culture but had little to no effect on the oncogenic activity of several unrelated oncoproteins.
KJ-Pyr-9 preferentially interfered with proliferation in a range of cells that overexpressed MYC, including leukemia and lymphoma cells.
In vivo, the molecule inhibited the growth of MYC-amplified human cancer cells.
“We finally hit a home run with this—maybe a grand slam,” said study author Kim Janda, PhD, of The Scripps Research Institute in La Jolla, California.
For years, MYC has challenged researchers seeking to disrupt its activity in cancer cells.
“At room temperature or body temperature, MYC without any binding partners is random and constantly shifting,” said study author Jonathan Ross Hart, PhD, also of The Scripps Research Institute. “It’s like a piece of spaghetti.”
So instead of designing a compound to target the structure of MYC, the researchers tested a range of compounds from a library to see if any could disrupt the interactions between MYC and other proteins important in cell proliferation. One did—the small molecule KJ-Pyr-9.
To further investigate, the researchers ran tests in a variety of cell lines, including chronic myeloid leukemia, acute lymphoblastic leukemia, acute myeloid leukemia, Burkitt lymphoma, and solid tumors. And they tested KJ-Pyr-9 in mouse models of breast cancer.
The experiments showed that MYC-dependent cells die if treated with KJ-Pyr-9. In fact, a dose of KJ-Pyr-9 made it seem as if MYC was not present at all.
When mice with MYC-dependent tumors received KJ-Pyr-9, the tumors showed no growth after 31 days, compared with significant tumor growth in untreated mice.
Dr Janda said he hopes further research will reveal exactly how KJ-Pyr-9 interacts with MYC and how the compound can more effectively reach tumor cells. ![]()

Credit: Ed Uthman
A small molecule can disrupt the interactions between MYC and its binding partner MAX in MYC-driven cancers, according to research published in PNAS.
The molecule, KJ-Pyr-9, inhibited MYC-induced oncogenic transformation in cell culture but had little to no effect on the oncogenic activity of several unrelated oncoproteins.
KJ-Pyr-9 preferentially interfered with proliferation in a range of cells that overexpressed MYC, including leukemia and lymphoma cells.
In vivo, the molecule inhibited the growth of MYC-amplified human cancer cells.
“We finally hit a home run with this—maybe a grand slam,” said study author Kim Janda, PhD, of The Scripps Research Institute in La Jolla, California.
For years, MYC has challenged researchers seeking to disrupt its activity in cancer cells.
“At room temperature or body temperature, MYC without any binding partners is random and constantly shifting,” said study author Jonathan Ross Hart, PhD, also of The Scripps Research Institute. “It’s like a piece of spaghetti.”
So instead of designing a compound to target the structure of MYC, the researchers tested a range of compounds from a library to see if any could disrupt the interactions between MYC and other proteins important in cell proliferation. One did—the small molecule KJ-Pyr-9.
To further investigate, the researchers ran tests in a variety of cell lines, including chronic myeloid leukemia, acute lymphoblastic leukemia, acute myeloid leukemia, Burkitt lymphoma, and solid tumors. And they tested KJ-Pyr-9 in mouse models of breast cancer.
The experiments showed that MYC-dependent cells die if treated with KJ-Pyr-9. In fact, a dose of KJ-Pyr-9 made it seem as if MYC was not present at all.
When mice with MYC-dependent tumors received KJ-Pyr-9, the tumors showed no growth after 31 days, compared with significant tumor growth in untreated mice.
Dr Janda said he hopes further research will reveal exactly how KJ-Pyr-9 interacts with MYC and how the compound can more effectively reach tumor cells. ![]()
HDAC inhibitor gets orphan status for DLBCL

The US Food and Drug Administration (FDA) has granted orphan designation for the histone deacetylase (HDAC) inhibitor mocetinostat to treat diffuse large B-cell lymphoma (DLBCL). The drug already had orphan designation as a treatment for myelodysplastic syndrome (MDS).
The FDA grants orphan status to support the development of drugs for underserved patient populations or rare disorders affecting fewer than 200,000 people in the US.
Orphan designation provides the drug’s developer, Mirati Therapeutics, Inc., with certain benefits, including market exclusivity upon regulatory approval, exemption of FDA application fees, and tax credits for qualified clinical trials.
Mocetinostat works by reversing aberrant acetylation resulting from mutations in histone acetyltransferases (HATs).
The drug is being developed as a single-agent treatment for patients with DLBCL or bladder cancer characterized by HAT mutations that Mirati believes are critical in the pathogenesis and progression of these cancers.
“We have identified genetic alterations in histone acetylation pathways (CREBBP and EP300) in approximately one-third of DLBCL and bladder tumors,” said Charles Baum, MD, PhD, president and CEO of Mirati.
He added that nonclinical tumor models with these mutations have proven responsive to mocetinostat, so Mirati predicts the HDAC inhibitor will halt tumor progression and reduce tumor burden in patients.
Mocetinostat is also under investigation in phase 2 studies in combination with azacitidine (Vidaza) as a treatment for intermediate- and high-risk MDS.
Mocetinostat previously demonstrated activity, as well as toxicity, in patients with Hodgkin lymphoma. ![]()

The US Food and Drug Administration (FDA) has granted orphan designation for the histone deacetylase (HDAC) inhibitor mocetinostat to treat diffuse large B-cell lymphoma (DLBCL). The drug already had orphan designation as a treatment for myelodysplastic syndrome (MDS).
The FDA grants orphan status to support the development of drugs for underserved patient populations or rare disorders affecting fewer than 200,000 people in the US.
Orphan designation provides the drug’s developer, Mirati Therapeutics, Inc., with certain benefits, including market exclusivity upon regulatory approval, exemption of FDA application fees, and tax credits for qualified clinical trials.
Mocetinostat works by reversing aberrant acetylation resulting from mutations in histone acetyltransferases (HATs).
The drug is being developed as a single-agent treatment for patients with DLBCL or bladder cancer characterized by HAT mutations that Mirati believes are critical in the pathogenesis and progression of these cancers.
“We have identified genetic alterations in histone acetylation pathways (CREBBP and EP300) in approximately one-third of DLBCL and bladder tumors,” said Charles Baum, MD, PhD, president and CEO of Mirati.
He added that nonclinical tumor models with these mutations have proven responsive to mocetinostat, so Mirati predicts the HDAC inhibitor will halt tumor progression and reduce tumor burden in patients.
Mocetinostat is also under investigation in phase 2 studies in combination with azacitidine (Vidaza) as a treatment for intermediate- and high-risk MDS.
Mocetinostat previously demonstrated activity, as well as toxicity, in patients with Hodgkin lymphoma. ![]()

The US Food and Drug Administration (FDA) has granted orphan designation for the histone deacetylase (HDAC) inhibitor mocetinostat to treat diffuse large B-cell lymphoma (DLBCL). The drug already had orphan designation as a treatment for myelodysplastic syndrome (MDS).
The FDA grants orphan status to support the development of drugs for underserved patient populations or rare disorders affecting fewer than 200,000 people in the US.
Orphan designation provides the drug’s developer, Mirati Therapeutics, Inc., with certain benefits, including market exclusivity upon regulatory approval, exemption of FDA application fees, and tax credits for qualified clinical trials.
Mocetinostat works by reversing aberrant acetylation resulting from mutations in histone acetyltransferases (HATs).
The drug is being developed as a single-agent treatment for patients with DLBCL or bladder cancer characterized by HAT mutations that Mirati believes are critical in the pathogenesis and progression of these cancers.
“We have identified genetic alterations in histone acetylation pathways (CREBBP and EP300) in approximately one-third of DLBCL and bladder tumors,” said Charles Baum, MD, PhD, president and CEO of Mirati.
He added that nonclinical tumor models with these mutations have proven responsive to mocetinostat, so Mirati predicts the HDAC inhibitor will halt tumor progression and reduce tumor burden in patients.
Mocetinostat is also under investigation in phase 2 studies in combination with azacitidine (Vidaza) as a treatment for intermediate- and high-risk MDS.
Mocetinostat previously demonstrated activity, as well as toxicity, in patients with Hodgkin lymphoma. ![]()
The Role of Autologous Hematopoietic Stem Cell Transplantation in Mantle Cell Lymphoma
At 5% to 6%, mantle cell lymphoma (MCL) is diagnosed in a relatively small proportion of patients with non-Hodgkin lymphoma. However, MCL is important to recognize because of its relatively poorer prognosis and the important role of autologous hematopoietic stem cell transplantation (HCT) as an adjunct to first-line treatment and, to a lesser extent, in later lines of therapy.
Treatment Options
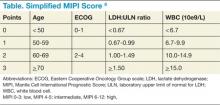
Though pathologic features are beyond the scope of this manuscript, when a definitive diagnosis is made, it is important to differentiate the more aggressive blastoid variant from the more typical pathologic patterns. The indolent form of MCL is diagnosed by clinical presentation as described below. In addition, quantitation of Ki-67 can add prognostic value.1-6 Patients with tumors that express higher levels of Ki-67 have higher relapse rates and shorter overall survivals.2,3,5 The Mantle Cell Lymphoma International Prognostic Index (MIPI) segregates patients into low-, intermediate-, and high-risk groups based on the clinical factors of patient age, performance status, serum lactate dehydrogenase, and total white blood cell count (WBC) (Table). Use of the MIPI at both initial diagnosis and before first-line autologous HCT can also offer significant prognostic value.1-7 Patients with higher MIPI scores have shorter overall survivals.7,8
For patients who present with indolent clinical features such as a stable leukemic phase, splenomegaly without adenopathy, and low tumor burden, watchful waiting can be utilized. However, approximately 80% of patients will require initial treatment with chemotherapy.1-6
For younger patients and those with good performance status and physiologic reserve, randomized trials have not clearly identified a preferred initial regimen, though initial therapy is typically with the hyperCVAD regimen along with the addition of rituximab.5,9 This regimen is fairly aggressive, requires inpatient hospitalization, is associated with cytopenia and risk of infection, and has not been rigorously proven to be superior in prospective randomized studies, but based on select single-arm studies and retrospective controls, this is commonly used as first-line therapy in the U.S.5,9
Of note, the recent SWOG 1106 U.S. Intergroup study comparing initial therapy with R-hyperCVAD to rituximab+bendamustine was closed early due to poor peripheral blood stem cell (PBSC) mobilization in the R-hyperCVAD arm. R-CHOP or R-bendamustine are considered less aggressive alternative regimens for older patients and for those with a poorer overall performance status.
For younger patients, the incorporation of high-dose cytarabine in various combinations during induction has been consistently identified as superior to those regimens without high-dose cytarabine. In general, the comparative studies have rather complex treatment regimens and are not routinely used in the U.S.
Other drugs with proven activity, though currently without a clear therapeutic sequence, include bortezomib, lenalidomide, bendamustine, temsirolimus, and most recently ibrutinib.1-5
Autologous HCT Recommendations
Following initial chemotherapy, autologous HCT is recommended for patients aged < 65 years and with good performance status. Earlier single-arm trials showed that the addition of dose intensification with autologous HCT led to more durable remissions. Both the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) support dose intensification with autologous HCT in first remission.
There are no absolute age restrictions for autologous HCT, though patients must have adequate physiologic reserve and good overall performance status. Prognostic physiologic parameters are not as well characterized for autologous HCT as they are for reduced-intensity allogeneic transplantation and autologous HCT for multiple myeloma. However, risk indices are being developed for patients with non-Hodgkin lymphoma undergoing autologous HCT.
While the addition of rituximab has improved the overall response rate of all chemotherapy regimens in patients with MCL, the most convincing survival plateaus still occur with autologous HCT in first remission. Nonetheless, the best first-line therapy has not been proven in prospective randomized fashion.1-6,9-13
Single-arm studies have shown that the R-HyperCVAD regimen can induce complete responses of 58% to 87% of first-line patients.1-3,5 From a practical perspective, for patients receiving R-hyperCVAD and proceeding with autologous HCT, PBSC are typically harvested after the completion of cycle 1B and patients proceed with autologous HCT after cycle 2B.5,9
The best preparative regimen for autologous HCT has not been clearly identified. Options for dose intensification include the more traditional total body irradiation (TBI)-based regimen as well as chemotherapy only, such as the BEAM (carmustine, etoposide, cytarabine, melphalan) regimen. While there are no comparative studies, a small retrospective analysis suggested benefit for a TBI-based preparative regimen, with a larger and recent European Group for Blood and Marrow Transplantation (EBMT) review suggesting that the benefit of TBI may be limited to those patients who have not achieved complete remission (CR) before autologous HCT.3
Mantle cell lymphoma is known to be a radiosensitive malignancy, and the use of radioimmunotherapy (RIT) along with HCT has shown promising results in single-arm studies when compared with historical control groups. The current unavailability of radioiodine-based RIT (tositumomab) and the unproven benefit of yttrium-based RIT (ibritumomab tiuxetan) makes this approach still of uncertain benefit. Nonetheless, the suggestion of benefit based on retrospective case control studies suggests that the addition of RIT to autologous HCT for MCL is worthy of further investigation.
After remission induction by initial therapy, maintenance rituximab therapy has been evaluated for patients who have received chemotherapy only or those with chemotherapy and autologous HCT.
Currently, the only prospective trial showing overall survival (OS) benefit is in the nontransplant setting following R-CHOP or R-FC (rituximab/fludarabine/cyclophosphamide) chemotherapy performed by the European Mantle Cell Lymphoma Network. This study showed a 4-year OS of 87% for those receiving rituximab maintenance compared with 63% for those receiving interferon alpha maintenance.14
In the autologous HCT setting, support for rituximab maintenance therapy comes from a number of sources. The CALGB 59909 study was a single-arm study showing the efficacy of rituximab along with induction therapy and dose-intensive therapy with autologous HCT followed by a short course of rituximab maintenance. This study showed the feasibility of additional rituximab with 2-year and 5-year PFS of 76% and 56%, respectively.11
Using a preemptive approach, the Nordic MCL-2 study showed both feasibility and a suggestion of delayed time to clinical relapse for intervention with rituximab in those patients who showed molecular relapse. In this study, molecular relapse was defined by increasing PCR-detectable markers following induction and autologous HCT using a BEAM transplant regimen.15 The prospective randomized French GOELAMS LyMa trial compared rituximab maintenance therapy for 3 years compared with no further therapy following first-line autologous HCT. This trial has recently closed and the results have not yet been presented.
While we currently await results of the LyMa trial, it is not possible to uniformly recommend rituximab maintenance to all patients following autologous HCT. Nonetheless, the Nordic MCL-2 study with intervention for molecular relapse and the demonstrated benefit in the nontransplant setting in older patients are compelling, and the generally well-tolerated administration of rituximab, all suggest consideration of rituximab maintenance in select patients until the outcome of the LyMa study is available for review.
Other agents that have demonstrated activity in MCL and have been considered as maintenance following autologous HCT include bortezomib, lenalidomide, and ibrutinib, with lenalidomide being currently studied by the Italian Lymphoma Foundation.
Other Considerations
For those patients who relapse following initial chemotherapy, autologous HCT can be considered following effective debulking chemotherapy. While historically, this group of patients was considered incurable with either chemotherapy or autologous HCT, newer evidence suggests that certain subsets of those patients can be effectively treated with autologous HCT.16 Depending on the number of adverse factors identified, the 5-year progression-free and overall survivals can range from 58% to 15% and from 76% to 32%, respectively.
For those patients who relapse following front-line autologous HCT, select patients with responsive disease, good performance status, and an available donor can be considered for reduced-intensity allogeneic transplantation.17
With the addition of new drugs and potential combinations, it is possible that dose intensification with autologous HCT will come to play a smaller role in the overall therapy of patients with MCL. However, this will require careful assessment in prospective randomized trials, along with better identification of specific patient subsets as well as a more thorough understanding of molecular prognostic and predictive factors.
For patients beyond first remission, autologous HCT can still be of value in those without prior HCT, and in select situations, reduced-intensity allogeneic transplantation also can be considered. Given all these issues, it is strongly encouraged that treating physicians work in concert with HCT programs soon after initial diagnosis so that decisions regarding initial therapy and timing of transplantation can be optimized.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dreyling M, Ferrero S, Hermine O. How I manage mantle cell lymphoma [published online ahead of print May 23, 2014]. Leukemia.
2. Gordon LI, Bernstein SH, Jares P, Kahl BS, Witzig TE, Dreyling M. Recent advances in mantle cell lymphoma: Report of the 2013 Mantle Cell Lymphoma Consortium Workshop [published online ahead of print April 17, 2014]. Leuk Lymphoma.
3. Dreyling M, Kluin-Nelemans HC, Beà S, et al; European MCL Network. Update on the molecular pathogenesis and clinical treatment of mantle cell lymphoma: Report of the 11th annual conference of the European Mantle Cell Lymphoma Network. Leuk Lymphoma. 2013;54(4):699-707.
4. Dreyling M, Thieblemont C, Gallamini A, et al. ESMO Consensus conferences: Guidelines on malignant lymphoma. Part 2: Marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol. 2013;24(4):857-877.
5. Williams ME. Transplantation for mantle cell lymphoma: Is it the right thing to do? Hematology Am Soc Hematol Educ Program. 2013;2013(1):568-574.
6. Dreyling M, Hiddemann W; European MCL Network. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2009;2009(1):542-551.
7. Budde LE, Guthrie KA, Till BG, et al. Mantle cell lymphoma international prognostic index but not pretransplantation induction regimen predicts survival for patients with mantle-cell lymphoma receiving high-dose therapy and autologous stem-cell transplantation. J Clin Oncol. 2011;29(22):3023-3029.
8. Hoster E, Dreyling M, Klapper W, et al; German Low Grade Lymphoma Study Group (GLSG), European MCL Network. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558-565.
9. Geisler CH, Kolstad A, Laurell A, et al; Nordic Lymphoma Group. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687-2693.
10. Delarue R, Haioun C, Ribrag V, et al; Groupe d’Etude des Lymphomes de l’Adulte (GELA). CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: A phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121(1):48-53.
11. Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27(36):6101-6108.
12. Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma:Results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677-2684.
13. Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23(9):1984-1992.
14. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520-531.
15. Andersen NS, Pedersen LB, Laurell A, et al. Pre-emptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J Clin Oncol. 2009;27(26):4365-4370.
16. Cassaday RD, Guthrie KA, Budde EL, et al. Specific features identify patients with relapsed or refractory mantle cell lymphoma benefitting from autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(9):1403-1406.
17. Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104(12):3535-3542.
At 5% to 6%, mantle cell lymphoma (MCL) is diagnosed in a relatively small proportion of patients with non-Hodgkin lymphoma. However, MCL is important to recognize because of its relatively poorer prognosis and the important role of autologous hematopoietic stem cell transplantation (HCT) as an adjunct to first-line treatment and, to a lesser extent, in later lines of therapy.
Treatment Options
Though pathologic features are beyond the scope of this manuscript, when a definitive diagnosis is made, it is important to differentiate the more aggressive blastoid variant from the more typical pathologic patterns. The indolent form of MCL is diagnosed by clinical presentation as described below. In addition, quantitation of Ki-67 can add prognostic value.1-6 Patients with tumors that express higher levels of Ki-67 have higher relapse rates and shorter overall survivals.2,3,5 The Mantle Cell Lymphoma International Prognostic Index (MIPI) segregates patients into low-, intermediate-, and high-risk groups based on the clinical factors of patient age, performance status, serum lactate dehydrogenase, and total white blood cell count (WBC) (Table). Use of the MIPI at both initial diagnosis and before first-line autologous HCT can also offer significant prognostic value.1-7 Patients with higher MIPI scores have shorter overall survivals.7,8
For patients who present with indolent clinical features such as a stable leukemic phase, splenomegaly without adenopathy, and low tumor burden, watchful waiting can be utilized. However, approximately 80% of patients will require initial treatment with chemotherapy.1-6
For younger patients and those with good performance status and physiologic reserve, randomized trials have not clearly identified a preferred initial regimen, though initial therapy is typically with the hyperCVAD regimen along with the addition of rituximab.5,9 This regimen is fairly aggressive, requires inpatient hospitalization, is associated with cytopenia and risk of infection, and has not been rigorously proven to be superior in prospective randomized studies, but based on select single-arm studies and retrospective controls, this is commonly used as first-line therapy in the U.S.5,9
Of note, the recent SWOG 1106 U.S. Intergroup study comparing initial therapy with R-hyperCVAD to rituximab+bendamustine was closed early due to poor peripheral blood stem cell (PBSC) mobilization in the R-hyperCVAD arm. R-CHOP or R-bendamustine are considered less aggressive alternative regimens for older patients and for those with a poorer overall performance status.
For younger patients, the incorporation of high-dose cytarabine in various combinations during induction has been consistently identified as superior to those regimens without high-dose cytarabine. In general, the comparative studies have rather complex treatment regimens and are not routinely used in the U.S.
Other drugs with proven activity, though currently without a clear therapeutic sequence, include bortezomib, lenalidomide, bendamustine, temsirolimus, and most recently ibrutinib.1-5
Autologous HCT Recommendations
Following initial chemotherapy, autologous HCT is recommended for patients aged < 65 years and with good performance status. Earlier single-arm trials showed that the addition of dose intensification with autologous HCT led to more durable remissions. Both the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) support dose intensification with autologous HCT in first remission.
There are no absolute age restrictions for autologous HCT, though patients must have adequate physiologic reserve and good overall performance status. Prognostic physiologic parameters are not as well characterized for autologous HCT as they are for reduced-intensity allogeneic transplantation and autologous HCT for multiple myeloma. However, risk indices are being developed for patients with non-Hodgkin lymphoma undergoing autologous HCT.
While the addition of rituximab has improved the overall response rate of all chemotherapy regimens in patients with MCL, the most convincing survival plateaus still occur with autologous HCT in first remission. Nonetheless, the best first-line therapy has not been proven in prospective randomized fashion.1-6,9-13
Single-arm studies have shown that the R-HyperCVAD regimen can induce complete responses of 58% to 87% of first-line patients.1-3,5 From a practical perspective, for patients receiving R-hyperCVAD and proceeding with autologous HCT, PBSC are typically harvested after the completion of cycle 1B and patients proceed with autologous HCT after cycle 2B.5,9
The best preparative regimen for autologous HCT has not been clearly identified. Options for dose intensification include the more traditional total body irradiation (TBI)-based regimen as well as chemotherapy only, such as the BEAM (carmustine, etoposide, cytarabine, melphalan) regimen. While there are no comparative studies, a small retrospective analysis suggested benefit for a TBI-based preparative regimen, with a larger and recent European Group for Blood and Marrow Transplantation (EBMT) review suggesting that the benefit of TBI may be limited to those patients who have not achieved complete remission (CR) before autologous HCT.3
Mantle cell lymphoma is known to be a radiosensitive malignancy, and the use of radioimmunotherapy (RIT) along with HCT has shown promising results in single-arm studies when compared with historical control groups. The current unavailability of radioiodine-based RIT (tositumomab) and the unproven benefit of yttrium-based RIT (ibritumomab tiuxetan) makes this approach still of uncertain benefit. Nonetheless, the suggestion of benefit based on retrospective case control studies suggests that the addition of RIT to autologous HCT for MCL is worthy of further investigation.
After remission induction by initial therapy, maintenance rituximab therapy has been evaluated for patients who have received chemotherapy only or those with chemotherapy and autologous HCT.
Currently, the only prospective trial showing overall survival (OS) benefit is in the nontransplant setting following R-CHOP or R-FC (rituximab/fludarabine/cyclophosphamide) chemotherapy performed by the European Mantle Cell Lymphoma Network. This study showed a 4-year OS of 87% for those receiving rituximab maintenance compared with 63% for those receiving interferon alpha maintenance.14
In the autologous HCT setting, support for rituximab maintenance therapy comes from a number of sources. The CALGB 59909 study was a single-arm study showing the efficacy of rituximab along with induction therapy and dose-intensive therapy with autologous HCT followed by a short course of rituximab maintenance. This study showed the feasibility of additional rituximab with 2-year and 5-year PFS of 76% and 56%, respectively.11
Using a preemptive approach, the Nordic MCL-2 study showed both feasibility and a suggestion of delayed time to clinical relapse for intervention with rituximab in those patients who showed molecular relapse. In this study, molecular relapse was defined by increasing PCR-detectable markers following induction and autologous HCT using a BEAM transplant regimen.15 The prospective randomized French GOELAMS LyMa trial compared rituximab maintenance therapy for 3 years compared with no further therapy following first-line autologous HCT. This trial has recently closed and the results have not yet been presented.
While we currently await results of the LyMa trial, it is not possible to uniformly recommend rituximab maintenance to all patients following autologous HCT. Nonetheless, the Nordic MCL-2 study with intervention for molecular relapse and the demonstrated benefit in the nontransplant setting in older patients are compelling, and the generally well-tolerated administration of rituximab, all suggest consideration of rituximab maintenance in select patients until the outcome of the LyMa study is available for review.
Other agents that have demonstrated activity in MCL and have been considered as maintenance following autologous HCT include bortezomib, lenalidomide, and ibrutinib, with lenalidomide being currently studied by the Italian Lymphoma Foundation.
Other Considerations
For those patients who relapse following initial chemotherapy, autologous HCT can be considered following effective debulking chemotherapy. While historically, this group of patients was considered incurable with either chemotherapy or autologous HCT, newer evidence suggests that certain subsets of those patients can be effectively treated with autologous HCT.16 Depending on the number of adverse factors identified, the 5-year progression-free and overall survivals can range from 58% to 15% and from 76% to 32%, respectively.
For those patients who relapse following front-line autologous HCT, select patients with responsive disease, good performance status, and an available donor can be considered for reduced-intensity allogeneic transplantation.17
With the addition of new drugs and potential combinations, it is possible that dose intensification with autologous HCT will come to play a smaller role in the overall therapy of patients with MCL. However, this will require careful assessment in prospective randomized trials, along with better identification of specific patient subsets as well as a more thorough understanding of molecular prognostic and predictive factors.
For patients beyond first remission, autologous HCT can still be of value in those without prior HCT, and in select situations, reduced-intensity allogeneic transplantation also can be considered. Given all these issues, it is strongly encouraged that treating physicians work in concert with HCT programs soon after initial diagnosis so that decisions regarding initial therapy and timing of transplantation can be optimized.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
At 5% to 6%, mantle cell lymphoma (MCL) is diagnosed in a relatively small proportion of patients with non-Hodgkin lymphoma. However, MCL is important to recognize because of its relatively poorer prognosis and the important role of autologous hematopoietic stem cell transplantation (HCT) as an adjunct to first-line treatment and, to a lesser extent, in later lines of therapy.
Treatment Options
Though pathologic features are beyond the scope of this manuscript, when a definitive diagnosis is made, it is important to differentiate the more aggressive blastoid variant from the more typical pathologic patterns. The indolent form of MCL is diagnosed by clinical presentation as described below. In addition, quantitation of Ki-67 can add prognostic value.1-6 Patients with tumors that express higher levels of Ki-67 have higher relapse rates and shorter overall survivals.2,3,5 The Mantle Cell Lymphoma International Prognostic Index (MIPI) segregates patients into low-, intermediate-, and high-risk groups based on the clinical factors of patient age, performance status, serum lactate dehydrogenase, and total white blood cell count (WBC) (Table). Use of the MIPI at both initial diagnosis and before first-line autologous HCT can also offer significant prognostic value.1-7 Patients with higher MIPI scores have shorter overall survivals.7,8
For patients who present with indolent clinical features such as a stable leukemic phase, splenomegaly without adenopathy, and low tumor burden, watchful waiting can be utilized. However, approximately 80% of patients will require initial treatment with chemotherapy.1-6
For younger patients and those with good performance status and physiologic reserve, randomized trials have not clearly identified a preferred initial regimen, though initial therapy is typically with the hyperCVAD regimen along with the addition of rituximab.5,9 This regimen is fairly aggressive, requires inpatient hospitalization, is associated with cytopenia and risk of infection, and has not been rigorously proven to be superior in prospective randomized studies, but based on select single-arm studies and retrospective controls, this is commonly used as first-line therapy in the U.S.5,9
Of note, the recent SWOG 1106 U.S. Intergroup study comparing initial therapy with R-hyperCVAD to rituximab+bendamustine was closed early due to poor peripheral blood stem cell (PBSC) mobilization in the R-hyperCVAD arm. R-CHOP or R-bendamustine are considered less aggressive alternative regimens for older patients and for those with a poorer overall performance status.
For younger patients, the incorporation of high-dose cytarabine in various combinations during induction has been consistently identified as superior to those regimens without high-dose cytarabine. In general, the comparative studies have rather complex treatment regimens and are not routinely used in the U.S.
Other drugs with proven activity, though currently without a clear therapeutic sequence, include bortezomib, lenalidomide, bendamustine, temsirolimus, and most recently ibrutinib.1-5
Autologous HCT Recommendations
Following initial chemotherapy, autologous HCT is recommended for patients aged < 65 years and with good performance status. Earlier single-arm trials showed that the addition of dose intensification with autologous HCT led to more durable remissions. Both the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) support dose intensification with autologous HCT in first remission.
There are no absolute age restrictions for autologous HCT, though patients must have adequate physiologic reserve and good overall performance status. Prognostic physiologic parameters are not as well characterized for autologous HCT as they are for reduced-intensity allogeneic transplantation and autologous HCT for multiple myeloma. However, risk indices are being developed for patients with non-Hodgkin lymphoma undergoing autologous HCT.
While the addition of rituximab has improved the overall response rate of all chemotherapy regimens in patients with MCL, the most convincing survival plateaus still occur with autologous HCT in first remission. Nonetheless, the best first-line therapy has not been proven in prospective randomized fashion.1-6,9-13
Single-arm studies have shown that the R-HyperCVAD regimen can induce complete responses of 58% to 87% of first-line patients.1-3,5 From a practical perspective, for patients receiving R-hyperCVAD and proceeding with autologous HCT, PBSC are typically harvested after the completion of cycle 1B and patients proceed with autologous HCT after cycle 2B.5,9
The best preparative regimen for autologous HCT has not been clearly identified. Options for dose intensification include the more traditional total body irradiation (TBI)-based regimen as well as chemotherapy only, such as the BEAM (carmustine, etoposide, cytarabine, melphalan) regimen. While there are no comparative studies, a small retrospective analysis suggested benefit for a TBI-based preparative regimen, with a larger and recent European Group for Blood and Marrow Transplantation (EBMT) review suggesting that the benefit of TBI may be limited to those patients who have not achieved complete remission (CR) before autologous HCT.3
Mantle cell lymphoma is known to be a radiosensitive malignancy, and the use of radioimmunotherapy (RIT) along with HCT has shown promising results in single-arm studies when compared with historical control groups. The current unavailability of radioiodine-based RIT (tositumomab) and the unproven benefit of yttrium-based RIT (ibritumomab tiuxetan) makes this approach still of uncertain benefit. Nonetheless, the suggestion of benefit based on retrospective case control studies suggests that the addition of RIT to autologous HCT for MCL is worthy of further investigation.
After remission induction by initial therapy, maintenance rituximab therapy has been evaluated for patients who have received chemotherapy only or those with chemotherapy and autologous HCT.
Currently, the only prospective trial showing overall survival (OS) benefit is in the nontransplant setting following R-CHOP or R-FC (rituximab/fludarabine/cyclophosphamide) chemotherapy performed by the European Mantle Cell Lymphoma Network. This study showed a 4-year OS of 87% for those receiving rituximab maintenance compared with 63% for those receiving interferon alpha maintenance.14
In the autologous HCT setting, support for rituximab maintenance therapy comes from a number of sources. The CALGB 59909 study was a single-arm study showing the efficacy of rituximab along with induction therapy and dose-intensive therapy with autologous HCT followed by a short course of rituximab maintenance. This study showed the feasibility of additional rituximab with 2-year and 5-year PFS of 76% and 56%, respectively.11
Using a preemptive approach, the Nordic MCL-2 study showed both feasibility and a suggestion of delayed time to clinical relapse for intervention with rituximab in those patients who showed molecular relapse. In this study, molecular relapse was defined by increasing PCR-detectable markers following induction and autologous HCT using a BEAM transplant regimen.15 The prospective randomized French GOELAMS LyMa trial compared rituximab maintenance therapy for 3 years compared with no further therapy following first-line autologous HCT. This trial has recently closed and the results have not yet been presented.
While we currently await results of the LyMa trial, it is not possible to uniformly recommend rituximab maintenance to all patients following autologous HCT. Nonetheless, the Nordic MCL-2 study with intervention for molecular relapse and the demonstrated benefit in the nontransplant setting in older patients are compelling, and the generally well-tolerated administration of rituximab, all suggest consideration of rituximab maintenance in select patients until the outcome of the LyMa study is available for review.
Other agents that have demonstrated activity in MCL and have been considered as maintenance following autologous HCT include bortezomib, lenalidomide, and ibrutinib, with lenalidomide being currently studied by the Italian Lymphoma Foundation.
Other Considerations
For those patients who relapse following initial chemotherapy, autologous HCT can be considered following effective debulking chemotherapy. While historically, this group of patients was considered incurable with either chemotherapy or autologous HCT, newer evidence suggests that certain subsets of those patients can be effectively treated with autologous HCT.16 Depending on the number of adverse factors identified, the 5-year progression-free and overall survivals can range from 58% to 15% and from 76% to 32%, respectively.
For those patients who relapse following front-line autologous HCT, select patients with responsive disease, good performance status, and an available donor can be considered for reduced-intensity allogeneic transplantation.17
With the addition of new drugs and potential combinations, it is possible that dose intensification with autologous HCT will come to play a smaller role in the overall therapy of patients with MCL. However, this will require careful assessment in prospective randomized trials, along with better identification of specific patient subsets as well as a more thorough understanding of molecular prognostic and predictive factors.
For patients beyond first remission, autologous HCT can still be of value in those without prior HCT, and in select situations, reduced-intensity allogeneic transplantation also can be considered. Given all these issues, it is strongly encouraged that treating physicians work in concert with HCT programs soon after initial diagnosis so that decisions regarding initial therapy and timing of transplantation can be optimized.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dreyling M, Ferrero S, Hermine O. How I manage mantle cell lymphoma [published online ahead of print May 23, 2014]. Leukemia.
2. Gordon LI, Bernstein SH, Jares P, Kahl BS, Witzig TE, Dreyling M. Recent advances in mantle cell lymphoma: Report of the 2013 Mantle Cell Lymphoma Consortium Workshop [published online ahead of print April 17, 2014]. Leuk Lymphoma.
3. Dreyling M, Kluin-Nelemans HC, Beà S, et al; European MCL Network. Update on the molecular pathogenesis and clinical treatment of mantle cell lymphoma: Report of the 11th annual conference of the European Mantle Cell Lymphoma Network. Leuk Lymphoma. 2013;54(4):699-707.
4. Dreyling M, Thieblemont C, Gallamini A, et al. ESMO Consensus conferences: Guidelines on malignant lymphoma. Part 2: Marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol. 2013;24(4):857-877.
5. Williams ME. Transplantation for mantle cell lymphoma: Is it the right thing to do? Hematology Am Soc Hematol Educ Program. 2013;2013(1):568-574.
6. Dreyling M, Hiddemann W; European MCL Network. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2009;2009(1):542-551.
7. Budde LE, Guthrie KA, Till BG, et al. Mantle cell lymphoma international prognostic index but not pretransplantation induction regimen predicts survival for patients with mantle-cell lymphoma receiving high-dose therapy and autologous stem-cell transplantation. J Clin Oncol. 2011;29(22):3023-3029.
8. Hoster E, Dreyling M, Klapper W, et al; German Low Grade Lymphoma Study Group (GLSG), European MCL Network. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558-565.
9. Geisler CH, Kolstad A, Laurell A, et al; Nordic Lymphoma Group. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687-2693.
10. Delarue R, Haioun C, Ribrag V, et al; Groupe d’Etude des Lymphomes de l’Adulte (GELA). CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: A phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121(1):48-53.
11. Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27(36):6101-6108.
12. Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma:Results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677-2684.
13. Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23(9):1984-1992.
14. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520-531.
15. Andersen NS, Pedersen LB, Laurell A, et al. Pre-emptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J Clin Oncol. 2009;27(26):4365-4370.
16. Cassaday RD, Guthrie KA, Budde EL, et al. Specific features identify patients with relapsed or refractory mantle cell lymphoma benefitting from autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(9):1403-1406.
17. Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104(12):3535-3542.
1. Dreyling M, Ferrero S, Hermine O. How I manage mantle cell lymphoma [published online ahead of print May 23, 2014]. Leukemia.
2. Gordon LI, Bernstein SH, Jares P, Kahl BS, Witzig TE, Dreyling M. Recent advances in mantle cell lymphoma: Report of the 2013 Mantle Cell Lymphoma Consortium Workshop [published online ahead of print April 17, 2014]. Leuk Lymphoma.
3. Dreyling M, Kluin-Nelemans HC, Beà S, et al; European MCL Network. Update on the molecular pathogenesis and clinical treatment of mantle cell lymphoma: Report of the 11th annual conference of the European Mantle Cell Lymphoma Network. Leuk Lymphoma. 2013;54(4):699-707.
4. Dreyling M, Thieblemont C, Gallamini A, et al. ESMO Consensus conferences: Guidelines on malignant lymphoma. Part 2: Marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol. 2013;24(4):857-877.
5. Williams ME. Transplantation for mantle cell lymphoma: Is it the right thing to do? Hematology Am Soc Hematol Educ Program. 2013;2013(1):568-574.
6. Dreyling M, Hiddemann W; European MCL Network. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2009;2009(1):542-551.
7. Budde LE, Guthrie KA, Till BG, et al. Mantle cell lymphoma international prognostic index but not pretransplantation induction regimen predicts survival for patients with mantle-cell lymphoma receiving high-dose therapy and autologous stem-cell transplantation. J Clin Oncol. 2011;29(22):3023-3029.
8. Hoster E, Dreyling M, Klapper W, et al; German Low Grade Lymphoma Study Group (GLSG), European MCL Network. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558-565.
9. Geisler CH, Kolstad A, Laurell A, et al; Nordic Lymphoma Group. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687-2693.
10. Delarue R, Haioun C, Ribrag V, et al; Groupe d’Etude des Lymphomes de l’Adulte (GELA). CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: A phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121(1):48-53.
11. Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27(36):6101-6108.
12. Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma:Results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677-2684.
13. Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23(9):1984-1992.
14. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520-531.
15. Andersen NS, Pedersen LB, Laurell A, et al. Pre-emptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J Clin Oncol. 2009;27(26):4365-4370.
16. Cassaday RD, Guthrie KA, Budde EL, et al. Specific features identify patients with relapsed or refractory mantle cell lymphoma benefitting from autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(9):1403-1406.
17. Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104(12):3535-3542.
Study reveals potential targets for MYC-dependent cancers

Credit: Juha Klefstrom
New research suggests the MYC protein drives cell growth by inhibiting a handful of genes involved in DNA packaging and cell death.
The study showed that MYC works through a microRNA to suppress the genes’ expression.
This marks the first time that a subset of MYC-controlled genes have been identified as critical players in the protein’s cancer-causing function, and it points to new therapeutic targets for MYC-dependent cancers.
“This is a different way of thinking about the roles of microRNA and chromatin packaging in cancer,” said Dean Felsher, MD, PhD, of the Stanford University School of Medicine in California.
“We were very surprised to learn that the overexpression of one microRNA can mimic the cancerous effect of MYC.”
Dr Felsher and his colleagues reported this discovery in Cancer Cell.
The team noted that MYC overexpression has been known to prompt an increase in the levels of a microRNA family called miR-17-92.
“People have known for several years that MYC regulates the expression of microRNAs,” Dr Felsher said. “But it wasn’t clear how this was related to MYC’s oncogenic function.”
To gain some insight, Dr Felsher and his colleagues analyzed MYC-dependent cancer cells in vitro and in vivo.
The cells in which miR-17-92 expression was locked in the “on” position kept dividing even when MYC expression was blocked. This suggested that MYC works through the microRNA family to exert its cancer-causing effects.
The researchers then looked for an overlap among genes affected by MYC overexpression and those affected by miR-17-92. There were about 401 genes whose expression was either increased or suppressed by both MYC and miR-17-92.
The team chose to focus on genes that were suppressed because these genes exhibited, on average, many more binding sites for the microRNAs. They further narrowed their panel down to 15 genes regulated by more than one miR-17-92 binding site.
Of these genes, 5 stood out. Four of them—Sin3b, Hbp1, Suv420h1, and Btg1—encode proteins known to regulate chromatin packaging.
These 4 proteins affect cell proliferation and senescence by regulating gene accessibility within the chromatin. They had never before been identified as MYC or miR-17-92 targets.
The fifth gene encodes the apoptotic protein Bim. Previous research suggested that Bim expression is affected by miR-17-92.
All 5 of the proteins are known to affect either cellular proliferation, entry into senescence, or apoptosis, in part by granting or prohibiting access to genes in tightly packaged stretches of DNA in the chromatin.
“MYC is still a general amplifier of gene transcription and expression,” Dr Felsher said. “But our study shows that the maintenance of the cancerous state relies on a more focused mechanism.”
Lastly, the researchers showed that suppressing the expression of the 5 target genes, effectively mimicking MYC overexpression, partially mitigates the effect of MYC deactivation.
Up to 30% of MYC-dependent cancer cells in culture continued to grow—compared to 11% of control cells—in the absence of MYC expression. And tumors in mice either failed to regress or recurred within a few weeks.
“One of the biggest unanswered questions in oncology is how oncogenes cause cancer, and whether you can replace an oncogene with another gene product,” Dr Felsher said.
“These experiments begin to reveal how MYC affects the self-renewal decisions of cells. They may also help us target those aspects of MYC overexpression that contribute to the cancer phenotype.” ![]()

Credit: Juha Klefstrom
New research suggests the MYC protein drives cell growth by inhibiting a handful of genes involved in DNA packaging and cell death.
The study showed that MYC works through a microRNA to suppress the genes’ expression.
This marks the first time that a subset of MYC-controlled genes have been identified as critical players in the protein’s cancer-causing function, and it points to new therapeutic targets for MYC-dependent cancers.
“This is a different way of thinking about the roles of microRNA and chromatin packaging in cancer,” said Dean Felsher, MD, PhD, of the Stanford University School of Medicine in California.
“We were very surprised to learn that the overexpression of one microRNA can mimic the cancerous effect of MYC.”
Dr Felsher and his colleagues reported this discovery in Cancer Cell.
The team noted that MYC overexpression has been known to prompt an increase in the levels of a microRNA family called miR-17-92.
“People have known for several years that MYC regulates the expression of microRNAs,” Dr Felsher said. “But it wasn’t clear how this was related to MYC’s oncogenic function.”
To gain some insight, Dr Felsher and his colleagues analyzed MYC-dependent cancer cells in vitro and in vivo.
The cells in which miR-17-92 expression was locked in the “on” position kept dividing even when MYC expression was blocked. This suggested that MYC works through the microRNA family to exert its cancer-causing effects.
The researchers then looked for an overlap among genes affected by MYC overexpression and those affected by miR-17-92. There were about 401 genes whose expression was either increased or suppressed by both MYC and miR-17-92.
The team chose to focus on genes that were suppressed because these genes exhibited, on average, many more binding sites for the microRNAs. They further narrowed their panel down to 15 genes regulated by more than one miR-17-92 binding site.
Of these genes, 5 stood out. Four of them—Sin3b, Hbp1, Suv420h1, and Btg1—encode proteins known to regulate chromatin packaging.
These 4 proteins affect cell proliferation and senescence by regulating gene accessibility within the chromatin. They had never before been identified as MYC or miR-17-92 targets.
The fifth gene encodes the apoptotic protein Bim. Previous research suggested that Bim expression is affected by miR-17-92.
All 5 of the proteins are known to affect either cellular proliferation, entry into senescence, or apoptosis, in part by granting or prohibiting access to genes in tightly packaged stretches of DNA in the chromatin.
“MYC is still a general amplifier of gene transcription and expression,” Dr Felsher said. “But our study shows that the maintenance of the cancerous state relies on a more focused mechanism.”
Lastly, the researchers showed that suppressing the expression of the 5 target genes, effectively mimicking MYC overexpression, partially mitigates the effect of MYC deactivation.
Up to 30% of MYC-dependent cancer cells in culture continued to grow—compared to 11% of control cells—in the absence of MYC expression. And tumors in mice either failed to regress or recurred within a few weeks.
“One of the biggest unanswered questions in oncology is how oncogenes cause cancer, and whether you can replace an oncogene with another gene product,” Dr Felsher said.
“These experiments begin to reveal how MYC affects the self-renewal decisions of cells. They may also help us target those aspects of MYC overexpression that contribute to the cancer phenotype.” ![]()

Credit: Juha Klefstrom
New research suggests the MYC protein drives cell growth by inhibiting a handful of genes involved in DNA packaging and cell death.
The study showed that MYC works through a microRNA to suppress the genes’ expression.
This marks the first time that a subset of MYC-controlled genes have been identified as critical players in the protein’s cancer-causing function, and it points to new therapeutic targets for MYC-dependent cancers.
“This is a different way of thinking about the roles of microRNA and chromatin packaging in cancer,” said Dean Felsher, MD, PhD, of the Stanford University School of Medicine in California.
“We were very surprised to learn that the overexpression of one microRNA can mimic the cancerous effect of MYC.”
Dr Felsher and his colleagues reported this discovery in Cancer Cell.
The team noted that MYC overexpression has been known to prompt an increase in the levels of a microRNA family called miR-17-92.
“People have known for several years that MYC regulates the expression of microRNAs,” Dr Felsher said. “But it wasn’t clear how this was related to MYC’s oncogenic function.”
To gain some insight, Dr Felsher and his colleagues analyzed MYC-dependent cancer cells in vitro and in vivo.
The cells in which miR-17-92 expression was locked in the “on” position kept dividing even when MYC expression was blocked. This suggested that MYC works through the microRNA family to exert its cancer-causing effects.
The researchers then looked for an overlap among genes affected by MYC overexpression and those affected by miR-17-92. There were about 401 genes whose expression was either increased or suppressed by both MYC and miR-17-92.
The team chose to focus on genes that were suppressed because these genes exhibited, on average, many more binding sites for the microRNAs. They further narrowed their panel down to 15 genes regulated by more than one miR-17-92 binding site.
Of these genes, 5 stood out. Four of them—Sin3b, Hbp1, Suv420h1, and Btg1—encode proteins known to regulate chromatin packaging.
These 4 proteins affect cell proliferation and senescence by regulating gene accessibility within the chromatin. They had never before been identified as MYC or miR-17-92 targets.
The fifth gene encodes the apoptotic protein Bim. Previous research suggested that Bim expression is affected by miR-17-92.
All 5 of the proteins are known to affect either cellular proliferation, entry into senescence, or apoptosis, in part by granting or prohibiting access to genes in tightly packaged stretches of DNA in the chromatin.
“MYC is still a general amplifier of gene transcription and expression,” Dr Felsher said. “But our study shows that the maintenance of the cancerous state relies on a more focused mechanism.”
Lastly, the researchers showed that suppressing the expression of the 5 target genes, effectively mimicking MYC overexpression, partially mitigates the effect of MYC deactivation.
Up to 30% of MYC-dependent cancer cells in culture continued to grow—compared to 11% of control cells—in the absence of MYC expression. And tumors in mice either failed to regress or recurred within a few weeks.
“One of the biggest unanswered questions in oncology is how oncogenes cause cancer, and whether you can replace an oncogene with another gene product,” Dr Felsher said.
“These experiments begin to reveal how MYC affects the self-renewal decisions of cells. They may also help us target those aspects of MYC overexpression that contribute to the cancer phenotype.” ![]()
Method could speed up cancer diagnosis

Credit: NIGMS
A new technique could enable faster diagnosis of cancer and various prenatal conditions, according to a paper published in Proceedings of the National Academy of Sciences.
The method, known as convex lens-induced confinement (CLIC), allows researchers to load long strands of DNA into a tunable, nanoscale imaging chamber in ways that maintain their structural identity and under conditions that are similar to those in the human body.
CLIC lets researchers map large genomes rapidly and identify specific gene sequences from single cells with single-molecule resolution, a process that is critical to diagnosing diseases like cancer.
“Current practices of genomic analysis typically require tens of thousands of cells worth of genomic material to obtain the information we need, but this new approach works with single cells,” said study author Rob Sladek, MD, of McGill University in Montreal, Canada.
“CLIC will allow researchers to avoid having to spend time stitching together maps of entire genomes, as we do under current techniques, and promises to make genomic analysis a much simpler and more efficient process.”
The CLIC imaging chamber can sit on top of a standard inverted fluorescence microscope, and strands of DNA can be loaded into the chamber from above, which allows the strands to maintain their integrity.
Existing tools used for genomic analysis rely on side-loading DNA under pressure into nanochannels in the imaging chamber. This breaks the DNA molecules into small pieces, making it a challenge to reconstruct the genome.
CLIC, on the other hand, is “like squeezing many soft spaghetti noodles into long, narrow tubes without breaking them,” according to study author Sabrina Leslie, PhD, also of McGill University.
“Once these long strands of DNA are gently squeezed down into nanochannels from a nanoscale bath above, they become effectively rigid, which means that we can map positions along uniformly stretched strands of DNA, while holding them still,” she said.
“This means diagnostics can be performed quickly, one cell at a time, which is critical for diagnosing many prenatal conditions and the onset of cancer.” ![]()

Credit: NIGMS
A new technique could enable faster diagnosis of cancer and various prenatal conditions, according to a paper published in Proceedings of the National Academy of Sciences.
The method, known as convex lens-induced confinement (CLIC), allows researchers to load long strands of DNA into a tunable, nanoscale imaging chamber in ways that maintain their structural identity and under conditions that are similar to those in the human body.
CLIC lets researchers map large genomes rapidly and identify specific gene sequences from single cells with single-molecule resolution, a process that is critical to diagnosing diseases like cancer.
“Current practices of genomic analysis typically require tens of thousands of cells worth of genomic material to obtain the information we need, but this new approach works with single cells,” said study author Rob Sladek, MD, of McGill University in Montreal, Canada.
“CLIC will allow researchers to avoid having to spend time stitching together maps of entire genomes, as we do under current techniques, and promises to make genomic analysis a much simpler and more efficient process.”
The CLIC imaging chamber can sit on top of a standard inverted fluorescence microscope, and strands of DNA can be loaded into the chamber from above, which allows the strands to maintain their integrity.
Existing tools used for genomic analysis rely on side-loading DNA under pressure into nanochannels in the imaging chamber. This breaks the DNA molecules into small pieces, making it a challenge to reconstruct the genome.
CLIC, on the other hand, is “like squeezing many soft spaghetti noodles into long, narrow tubes without breaking them,” according to study author Sabrina Leslie, PhD, also of McGill University.
“Once these long strands of DNA are gently squeezed down into nanochannels from a nanoscale bath above, they become effectively rigid, which means that we can map positions along uniformly stretched strands of DNA, while holding them still,” she said.
“This means diagnostics can be performed quickly, one cell at a time, which is critical for diagnosing many prenatal conditions and the onset of cancer.” ![]()

Credit: NIGMS
A new technique could enable faster diagnosis of cancer and various prenatal conditions, according to a paper published in Proceedings of the National Academy of Sciences.
The method, known as convex lens-induced confinement (CLIC), allows researchers to load long strands of DNA into a tunable, nanoscale imaging chamber in ways that maintain their structural identity and under conditions that are similar to those in the human body.
CLIC lets researchers map large genomes rapidly and identify specific gene sequences from single cells with single-molecule resolution, a process that is critical to diagnosing diseases like cancer.
“Current practices of genomic analysis typically require tens of thousands of cells worth of genomic material to obtain the information we need, but this new approach works with single cells,” said study author Rob Sladek, MD, of McGill University in Montreal, Canada.
“CLIC will allow researchers to avoid having to spend time stitching together maps of entire genomes, as we do under current techniques, and promises to make genomic analysis a much simpler and more efficient process.”
The CLIC imaging chamber can sit on top of a standard inverted fluorescence microscope, and strands of DNA can be loaded into the chamber from above, which allows the strands to maintain their integrity.
Existing tools used for genomic analysis rely on side-loading DNA under pressure into nanochannels in the imaging chamber. This breaks the DNA molecules into small pieces, making it a challenge to reconstruct the genome.
CLIC, on the other hand, is “like squeezing many soft spaghetti noodles into long, narrow tubes without breaking them,” according to study author Sabrina Leslie, PhD, also of McGill University.
“Once these long strands of DNA are gently squeezed down into nanochannels from a nanoscale bath above, they become effectively rigid, which means that we can map positions along uniformly stretched strands of DNA, while holding them still,” she said.
“This means diagnostics can be performed quickly, one cell at a time, which is critical for diagnosing many prenatal conditions and the onset of cancer.” ![]()
Gene plays crucial role in cancer development, team says
Credit: Beth A. Sullivan
New research suggests DNA ligase 3 is crucial for the evolutionary processes that drive cancer.
“We have identified a gene that, as cells age, seems to regulate whether the cells become cancerous or not,” said Eric A. Hendrickson, PhD, of the University of Minnesota in Minneapolis.
“This gene has never been identified before in this role, so this makes it a potentially very important therapeutic target.”
Dr Hendrickson and his colleagues recounted this discovery in Cell Reports.
The researchers noted that short, dysfunctional telomeres can fuse, thereby generating dicentric chromosomes and initiating breakage-fusion-bridge cycles. The cells that manage to escape the subsequent crisis have genomic rearrangements that drive clonal evolution and malignant progression.
The team wanted to determine exactly what allows these malignant cells to escape telomere-driven crisis and avoid death.
To find out, the group disabled certain genes in human cells and then studied the impact this had on telomere fusion.
They found that cells escaped death when ligase 3 was active but not when its action, which appears to promote fusion within like chromosomes rather than between different chromosomes, was inhibited.
“Telomere dysfunction has been identified in many human cancers,” said study author Duncan Baird, PhD, of Cardiff University in the UK.
“And, as we have shown previously, short telomeres can predict the outcome of patients with [chronic lymphocytic leukemia] and probably many other tumor types. Thus, the discovery that ligase 3 is required for this process is fundamentally important.”
This research was made possible by a chance meeting between Dr Baird and Dr Hendrickson at an international conference. The pair discovered they were both looking at the role of ligase 3 in cancer and decided to collaborate.
“The collaboration paid off, as we were able to uncover something that neither one of us could have done on our own,” Dr Hendrickson said.
Additional studies are already underway. The researchers are investigating the discovery that the reliance on ligase 3 appears to be dependent upon the activity of another key DNA repair gene, p53.
“Since p53 is the most commonly mutated gene in human cancer, it now behooves us to discover how these two genes are interacting and to see if we can’t use that information to develop synergistic treatment modalities,” Dr Hendrickson concluded. ![]()

Credit: Beth A. Sullivan
New research suggests DNA ligase 3 is crucial for the evolutionary processes that drive cancer.
“We have identified a gene that, as cells age, seems to regulate whether the cells become cancerous or not,” said Eric A. Hendrickson, PhD, of the University of Minnesota in Minneapolis.
“This gene has never been identified before in this role, so this makes it a potentially very important therapeutic target.”
Dr Hendrickson and his colleagues recounted this discovery in Cell Reports.
The researchers noted that short, dysfunctional telomeres can fuse, thereby generating dicentric chromosomes and initiating breakage-fusion-bridge cycles. The cells that manage to escape the subsequent crisis have genomic rearrangements that drive clonal evolution and malignant progression.
The team wanted to determine exactly what allows these malignant cells to escape telomere-driven crisis and avoid death.
To find out, the group disabled certain genes in human cells and then studied the impact this had on telomere fusion.
They found that cells escaped death when ligase 3 was active but not when its action, which appears to promote fusion within like chromosomes rather than between different chromosomes, was inhibited.
“Telomere dysfunction has been identified in many human cancers,” said study author Duncan Baird, PhD, of Cardiff University in the UK.
“And, as we have shown previously, short telomeres can predict the outcome of patients with [chronic lymphocytic leukemia] and probably many other tumor types. Thus, the discovery that ligase 3 is required for this process is fundamentally important.”
This research was made possible by a chance meeting between Dr Baird and Dr Hendrickson at an international conference. The pair discovered they were both looking at the role of ligase 3 in cancer and decided to collaborate.
“The collaboration paid off, as we were able to uncover something that neither one of us could have done on our own,” Dr Hendrickson said.
Additional studies are already underway. The researchers are investigating the discovery that the reliance on ligase 3 appears to be dependent upon the activity of another key DNA repair gene, p53.
“Since p53 is the most commonly mutated gene in human cancer, it now behooves us to discover how these two genes are interacting and to see if we can’t use that information to develop synergistic treatment modalities,” Dr Hendrickson concluded. ![]()

Credit: Beth A. Sullivan
New research suggests DNA ligase 3 is crucial for the evolutionary processes that drive cancer.
“We have identified a gene that, as cells age, seems to regulate whether the cells become cancerous or not,” said Eric A. Hendrickson, PhD, of the University of Minnesota in Minneapolis.
“This gene has never been identified before in this role, so this makes it a potentially very important therapeutic target.”
Dr Hendrickson and his colleagues recounted this discovery in Cell Reports.
The researchers noted that short, dysfunctional telomeres can fuse, thereby generating dicentric chromosomes and initiating breakage-fusion-bridge cycles. The cells that manage to escape the subsequent crisis have genomic rearrangements that drive clonal evolution and malignant progression.
The team wanted to determine exactly what allows these malignant cells to escape telomere-driven crisis and avoid death.
To find out, the group disabled certain genes in human cells and then studied the impact this had on telomere fusion.
They found that cells escaped death when ligase 3 was active but not when its action, which appears to promote fusion within like chromosomes rather than between different chromosomes, was inhibited.
“Telomere dysfunction has been identified in many human cancers,” said study author Duncan Baird, PhD, of Cardiff University in the UK.
“And, as we have shown previously, short telomeres can predict the outcome of patients with [chronic lymphocytic leukemia] and probably many other tumor types. Thus, the discovery that ligase 3 is required for this process is fundamentally important.”
This research was made possible by a chance meeting between Dr Baird and Dr Hendrickson at an international conference. The pair discovered they were both looking at the role of ligase 3 in cancer and decided to collaborate.
“The collaboration paid off, as we were able to uncover something that neither one of us could have done on our own,” Dr Hendrickson said.
Additional studies are already underway. The researchers are investigating the discovery that the reliance on ligase 3 appears to be dependent upon the activity of another key DNA repair gene, p53.
“Since p53 is the most commonly mutated gene in human cancer, it now behooves us to discover how these two genes are interacting and to see if we can’t use that information to develop synergistic treatment modalities,” Dr Hendrickson concluded.
Ofatumumab prompts fatal reaction in CLL patient

Credit: Bill Branson
Health Canada and GlaxoSmithKline (GSK) have reported a fatal infusion reaction in a patient receiving the monoclonal antibody ofatumumab (Arzerra) to treat
chronic lymphocytic leukemia (CLL).
The patient had no known history of cardiac disease.
Ofatumumab’s product monograph is being updated to include a warning about the potential for fatal infusion reactions.
In Canada, ofatumumab is approved to treat patients with CLL that is refractory to fludarabine and alemtuzumab.
The drug received this marketing authorization with conditions, pending the results of trials to verify its clinical benefit.
In light of the fatal infusion reaction, GSK and Health Canada are reminding healthcare professionals that ofatumumab should be administered under the supervision of a physician experienced in the use of cancer therapy. The drug should be given in an environment where facilities to adequately monitor and treat infusion reactions are available.
Prior to infusion, patients should always receive the appropriate premedication, as outlined in the product label. However, serious infusion reactions may occur despite premedication.
If you suspect a severe infusion reaction, stop the infusion immediately and provide symptomatic treatment. Signs and symptoms of an infusion reaction may include swelling of the face or mouth, fever, chills, difficulty breathing, tightness of the chest and/or throat, light headedness, nausea, diarrhea, and rash.
These symptoms can occur during or shortly after the infusion, predominantly with the first 2 infusions. So ensure patients are closely monitored, especially those with heart conditions. And inform patients about the risk of fatal infusion reactions associated with ofatumumab.
GSK has sent a letter to healthcare professionals detailing the risk of fatal infusion reactions. The information is also available on the Canadian website of GSK and the Health Canada website.
Any case of serious hypersensitivity, infusion reactions, or other serious or unexpected side effects in patients receiving ofatumumab should be reported to GSK or Health Canada.
Ofatumumab is also known to pose a risk of hepatitis B virus reactivation, progressive multifocal leukoencephalopathy, serious and/or fatal cardiovascular events, and serious and/or fatal infections (bacterial, fungal, and viral).
Ofatumumab recently received approval in the European Union to be used in combination with chlorambucil or bendamustine for untreated CLL patients who are not eligible for fludarabine-based therapy. The drug previously received conditional approval in Europe as monotherapy to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab is approved for both of these indications in the US as well.

Credit: Bill Branson
Health Canada and GlaxoSmithKline (GSK) have reported a fatal infusion reaction in a patient receiving the monoclonal antibody ofatumumab (Arzerra) to treat
chronic lymphocytic leukemia (CLL).
The patient had no known history of cardiac disease.
Ofatumumab’s product monograph is being updated to include a warning about the potential for fatal infusion reactions.
In Canada, ofatumumab is approved to treat patients with CLL that is refractory to fludarabine and alemtuzumab.
The drug received this marketing authorization with conditions, pending the results of trials to verify its clinical benefit.
In light of the fatal infusion reaction, GSK and Health Canada are reminding healthcare professionals that ofatumumab should be administered under the supervision of a physician experienced in the use of cancer therapy. The drug should be given in an environment where facilities to adequately monitor and treat infusion reactions are available.
Prior to infusion, patients should always receive the appropriate premedication, as outlined in the product label. However, serious infusion reactions may occur despite premedication.
If you suspect a severe infusion reaction, stop the infusion immediately and provide symptomatic treatment. Signs and symptoms of an infusion reaction may include swelling of the face or mouth, fever, chills, difficulty breathing, tightness of the chest and/or throat, light headedness, nausea, diarrhea, and rash.
These symptoms can occur during or shortly after the infusion, predominantly with the first 2 infusions. So ensure patients are closely monitored, especially those with heart conditions. And inform patients about the risk of fatal infusion reactions associated with ofatumumab.
GSK has sent a letter to healthcare professionals detailing the risk of fatal infusion reactions. The information is also available on the Canadian website of GSK and the Health Canada website.
Any case of serious hypersensitivity, infusion reactions, or other serious or unexpected side effects in patients receiving ofatumumab should be reported to GSK or Health Canada.
Ofatumumab is also known to pose a risk of hepatitis B virus reactivation, progressive multifocal leukoencephalopathy, serious and/or fatal cardiovascular events, and serious and/or fatal infections (bacterial, fungal, and viral).
Ofatumumab recently received approval in the European Union to be used in combination with chlorambucil or bendamustine for untreated CLL patients who are not eligible for fludarabine-based therapy. The drug previously received conditional approval in Europe as monotherapy to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab is approved for both of these indications in the US as well.

Credit: Bill Branson
Health Canada and GlaxoSmithKline (GSK) have reported a fatal infusion reaction in a patient receiving the monoclonal antibody ofatumumab (Arzerra) to treat
chronic lymphocytic leukemia (CLL).
The patient had no known history of cardiac disease.
Ofatumumab’s product monograph is being updated to include a warning about the potential for fatal infusion reactions.
In Canada, ofatumumab is approved to treat patients with CLL that is refractory to fludarabine and alemtuzumab.
The drug received this marketing authorization with conditions, pending the results of trials to verify its clinical benefit.
In light of the fatal infusion reaction, GSK and Health Canada are reminding healthcare professionals that ofatumumab should be administered under the supervision of a physician experienced in the use of cancer therapy. The drug should be given in an environment where facilities to adequately monitor and treat infusion reactions are available.
Prior to infusion, patients should always receive the appropriate premedication, as outlined in the product label. However, serious infusion reactions may occur despite premedication.
If you suspect a severe infusion reaction, stop the infusion immediately and provide symptomatic treatment. Signs and symptoms of an infusion reaction may include swelling of the face or mouth, fever, chills, difficulty breathing, tightness of the chest and/or throat, light headedness, nausea, diarrhea, and rash.
These symptoms can occur during or shortly after the infusion, predominantly with the first 2 infusions. So ensure patients are closely monitored, especially those with heart conditions. And inform patients about the risk of fatal infusion reactions associated with ofatumumab.
GSK has sent a letter to healthcare professionals detailing the risk of fatal infusion reactions. The information is also available on the Canadian website of GSK and the Health Canada website.
Any case of serious hypersensitivity, infusion reactions, or other serious or unexpected side effects in patients receiving ofatumumab should be reported to GSK or Health Canada.
Ofatumumab is also known to pose a risk of hepatitis B virus reactivation, progressive multifocal leukoencephalopathy, serious and/or fatal cardiovascular events, and serious and/or fatal infections (bacterial, fungal, and viral).
Ofatumumab recently received approval in the European Union to be used in combination with chlorambucil or bendamustine for untreated CLL patients who are not eligible for fludarabine-based therapy. The drug previously received conditional approval in Europe as monotherapy to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab is approved for both of these indications in the US as well.