User login
CHMP recommends antifungal agent

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for intravenous (IV) posaconazole (Noxafil), an antifungal agent.
If the European Commission affirms the CHMP opinion, IV posaconazole will be authorized for use in the European Union, Iceland, Liechtenstein, and Norway.
The commission previously granted marketing authorization for posaconazole delayed-release tablets and oral suspension.
Posaconazole is used to prevent invasive fungal infections in severely immunocompromised patients, such as hematopoietic stem cell transplant recipients with graft-vs-host disease or patients with hematologic malignancies and prolonged neutropenia from chemotherapy.
The drug is also used to treat fungal diseases—invasive aspergillosis, fusariosis, chromoblastomycosis, mycetoma, and coccidioidomycosis—when other antifungal agents—amphotericin B, itraconazole, or fluconazole—cannot be tolerated or have failed.
And posaconazole oral suspension is used as a first-line treatment for thrush, a fungal infection of the mouth and throat due to Candida.
Posaconazole injection is administered with a loading dose of 300 mg twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by IV infusion over approximately 90 minutes.
Once combined with a mixture of IV solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2°-8° C (36°-46° F).
The safety and effectiveness of IV posaconazole in patients younger than 18 years has not been established. IV posaconazole should not be used in pediatric patients because of non-clinical safety concerns.
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Patients with known hypersensitivity to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
Hepatic reactions have been reported as well. This includes mild to moderate elevations in ALT, AST, alkaline phosphatase, total bilirubin, and/or clinical hepatitis. Monitoring or discontinuation may be necessary in patients with hepatic reactions to posaconazole.
IV posaconazole should be avoided in patients with moderate or severe renal impairment (estimated glomerular filtration rate <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of posaconazole.
In clinical trials, the adverse events associated with IV posaconazole were generally similar to those in trials of posaconazole oral suspension. The most frequently reported events were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
IV posaconazole is under development by MSD (known as Merck in the US and Canada). ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for intravenous (IV) posaconazole (Noxafil), an antifungal agent.
If the European Commission affirms the CHMP opinion, IV posaconazole will be authorized for use in the European Union, Iceland, Liechtenstein, and Norway.
The commission previously granted marketing authorization for posaconazole delayed-release tablets and oral suspension.
Posaconazole is used to prevent invasive fungal infections in severely immunocompromised patients, such as hematopoietic stem cell transplant recipients with graft-vs-host disease or patients with hematologic malignancies and prolonged neutropenia from chemotherapy.
The drug is also used to treat fungal diseases—invasive aspergillosis, fusariosis, chromoblastomycosis, mycetoma, and coccidioidomycosis—when other antifungal agents—amphotericin B, itraconazole, or fluconazole—cannot be tolerated or have failed.
And posaconazole oral suspension is used as a first-line treatment for thrush, a fungal infection of the mouth and throat due to Candida.
Posaconazole injection is administered with a loading dose of 300 mg twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by IV infusion over approximately 90 minutes.
Once combined with a mixture of IV solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2°-8° C (36°-46° F).
The safety and effectiveness of IV posaconazole in patients younger than 18 years has not been established. IV posaconazole should not be used in pediatric patients because of non-clinical safety concerns.
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Patients with known hypersensitivity to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
Hepatic reactions have been reported as well. This includes mild to moderate elevations in ALT, AST, alkaline phosphatase, total bilirubin, and/or clinical hepatitis. Monitoring or discontinuation may be necessary in patients with hepatic reactions to posaconazole.
IV posaconazole should be avoided in patients with moderate or severe renal impairment (estimated glomerular filtration rate <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of posaconazole.
In clinical trials, the adverse events associated with IV posaconazole were generally similar to those in trials of posaconazole oral suspension. The most frequently reported events were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
IV posaconazole is under development by MSD (known as Merck in the US and Canada). ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for intravenous (IV) posaconazole (Noxafil), an antifungal agent.
If the European Commission affirms the CHMP opinion, IV posaconazole will be authorized for use in the European Union, Iceland, Liechtenstein, and Norway.
The commission previously granted marketing authorization for posaconazole delayed-release tablets and oral suspension.
Posaconazole is used to prevent invasive fungal infections in severely immunocompromised patients, such as hematopoietic stem cell transplant recipients with graft-vs-host disease or patients with hematologic malignancies and prolonged neutropenia from chemotherapy.
The drug is also used to treat fungal diseases—invasive aspergillosis, fusariosis, chromoblastomycosis, mycetoma, and coccidioidomycosis—when other antifungal agents—amphotericin B, itraconazole, or fluconazole—cannot be tolerated or have failed.
And posaconazole oral suspension is used as a first-line treatment for thrush, a fungal infection of the mouth and throat due to Candida.
Posaconazole injection is administered with a loading dose of 300 mg twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by IV infusion over approximately 90 minutes.
Once combined with a mixture of IV solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2°-8° C (36°-46° F).
The safety and effectiveness of IV posaconazole in patients younger than 18 years has not been established. IV posaconazole should not be used in pediatric patients because of non-clinical safety concerns.
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Patients with known hypersensitivity to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
Hepatic reactions have been reported as well. This includes mild to moderate elevations in ALT, AST, alkaline phosphatase, total bilirubin, and/or clinical hepatitis. Monitoring or discontinuation may be necessary in patients with hepatic reactions to posaconazole.
IV posaconazole should be avoided in patients with moderate or severe renal impairment (estimated glomerular filtration rate <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of posaconazole.
In clinical trials, the adverse events associated with IV posaconazole were generally similar to those in trials of posaconazole oral suspension. The most frequently reported events were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
IV posaconazole is under development by MSD (known as Merck in the US and Canada). ![]()
Protein-targeting drug could treat cancers, other diseases

surrounding fused cells
Credit: IRB Barcelona
Experiments in mice suggest the mitochondrial chaperone TRAP-1 is involved in the development of cancers and age-related diseases.
Previous research showed that TRAP-1 is overexpressed in leukemia, lymphoma, and many other cancers.
The new research, published in Cell Reports, clarifies TRAP-1’s role in cancers and age-related conditions. It also suggests gamitrinib, a novel agent targeting TRAP-1, could prove useful in treating these diseases.
TRAP-1 is a member of the heat shock protein 90 (HSP90) family, chaperone proteins that guide the physical formation of other proteins and serve a regulatory function within mitochondria. Tumors use HSP90 proteins like TRAP-1 to help survive therapeutic attack.
To further investigate the effects of TRAP-1, researchers bred TRAP-1 knockout mice. The team found the mice compensate for losing the protein by switching to alternative cellular mechanisms for making energy.
“We see this astounding change in TRAP-1 knockout mice, where they show fewer signs of aging and are less likely to develop cancers,” said Dario C. Altieri, MD, of The Wistar Institute in Philadelphia, Pennsylvania.
“Our findings provide an unexpected explanation for how TRAP-1 and related proteins regulate metabolism within our cells. We usually link the reprogramming of metabolic pathways with human diseases, such as cancer. What we didn’t expect to see were healthier mice with fewer tumors.”
Dr Altieri and his colleagues created the TRAP-1 knockout mice as part of their ongoing investigation into their novel drug, gamitrinib, which targets TRAP-1 in the mitochondria of tumor cells.
“In tumors, the loss of TRAP-1 is devastating, triggering a host of catastrophic defects, including metabolic problems that ultimately result in the death of the tumor cells,” Dr Altieri said. ”Mice that lack TRAP-1 from the start, however, have 3 weeks in the womb to compensate for the loss of the protein.”
The researchers found that, in the knockout mice, the loss of TRAP-1 causes mitochondrial proteins to misfold, which triggers a compensatory response that causes cells to consume more oxygen and metabolize more sugar. This prompts the mitochondria to produce deregulated levels of ATP.
This increased mitochondrial activity actually creates a moderate boost in oxidative stress (free radical damage) and the associated DNA damage. While DNA damage may seem counterproductive to longevity and good health, the low level of DNA damage actually reduces cell proliferation, slowing growth to allow the cell’s natural repair mechanisms to take effect.
According to Dr Altieri, his group’s observations provide a mechanistic foundation for the role of chaperone molecules like HSP90 in the regulation of bioenergetics in mitochondria—how cells produce and use the chemical energy they need to survive and grow.
Their results explain some contradictory findings in the scientific literature regarding the regulation of bioenergetics and show how compensatory mechanisms can arise when these chaperone molecules are taken out of the equation.
“Our findings strengthen the case for targeting HSP90 in tumor cells, but they also open up a fascinating array of questions that may have implications for metabolism and longevity,” Dr Altieri said. “I predict that the TRAP-1 knockout mouse will be a valuable tool for answering these questions.” ![]()

surrounding fused cells
Credit: IRB Barcelona
Experiments in mice suggest the mitochondrial chaperone TRAP-1 is involved in the development of cancers and age-related diseases.
Previous research showed that TRAP-1 is overexpressed in leukemia, lymphoma, and many other cancers.
The new research, published in Cell Reports, clarifies TRAP-1’s role in cancers and age-related conditions. It also suggests gamitrinib, a novel agent targeting TRAP-1, could prove useful in treating these diseases.
TRAP-1 is a member of the heat shock protein 90 (HSP90) family, chaperone proteins that guide the physical formation of other proteins and serve a regulatory function within mitochondria. Tumors use HSP90 proteins like TRAP-1 to help survive therapeutic attack.
To further investigate the effects of TRAP-1, researchers bred TRAP-1 knockout mice. The team found the mice compensate for losing the protein by switching to alternative cellular mechanisms for making energy.
“We see this astounding change in TRAP-1 knockout mice, where they show fewer signs of aging and are less likely to develop cancers,” said Dario C. Altieri, MD, of The Wistar Institute in Philadelphia, Pennsylvania.
“Our findings provide an unexpected explanation for how TRAP-1 and related proteins regulate metabolism within our cells. We usually link the reprogramming of metabolic pathways with human diseases, such as cancer. What we didn’t expect to see were healthier mice with fewer tumors.”
Dr Altieri and his colleagues created the TRAP-1 knockout mice as part of their ongoing investigation into their novel drug, gamitrinib, which targets TRAP-1 in the mitochondria of tumor cells.
“In tumors, the loss of TRAP-1 is devastating, triggering a host of catastrophic defects, including metabolic problems that ultimately result in the death of the tumor cells,” Dr Altieri said. ”Mice that lack TRAP-1 from the start, however, have 3 weeks in the womb to compensate for the loss of the protein.”
The researchers found that, in the knockout mice, the loss of TRAP-1 causes mitochondrial proteins to misfold, which triggers a compensatory response that causes cells to consume more oxygen and metabolize more sugar. This prompts the mitochondria to produce deregulated levels of ATP.
This increased mitochondrial activity actually creates a moderate boost in oxidative stress (free radical damage) and the associated DNA damage. While DNA damage may seem counterproductive to longevity and good health, the low level of DNA damage actually reduces cell proliferation, slowing growth to allow the cell’s natural repair mechanisms to take effect.
According to Dr Altieri, his group’s observations provide a mechanistic foundation for the role of chaperone molecules like HSP90 in the regulation of bioenergetics in mitochondria—how cells produce and use the chemical energy they need to survive and grow.
Their results explain some contradictory findings in the scientific literature regarding the regulation of bioenergetics and show how compensatory mechanisms can arise when these chaperone molecules are taken out of the equation.
“Our findings strengthen the case for targeting HSP90 in tumor cells, but they also open up a fascinating array of questions that may have implications for metabolism and longevity,” Dr Altieri said. “I predict that the TRAP-1 knockout mouse will be a valuable tool for answering these questions.” ![]()

surrounding fused cells
Credit: IRB Barcelona
Experiments in mice suggest the mitochondrial chaperone TRAP-1 is involved in the development of cancers and age-related diseases.
Previous research showed that TRAP-1 is overexpressed in leukemia, lymphoma, and many other cancers.
The new research, published in Cell Reports, clarifies TRAP-1’s role in cancers and age-related conditions. It also suggests gamitrinib, a novel agent targeting TRAP-1, could prove useful in treating these diseases.
TRAP-1 is a member of the heat shock protein 90 (HSP90) family, chaperone proteins that guide the physical formation of other proteins and serve a regulatory function within mitochondria. Tumors use HSP90 proteins like TRAP-1 to help survive therapeutic attack.
To further investigate the effects of TRAP-1, researchers bred TRAP-1 knockout mice. The team found the mice compensate for losing the protein by switching to alternative cellular mechanisms for making energy.
“We see this astounding change in TRAP-1 knockout mice, where they show fewer signs of aging and are less likely to develop cancers,” said Dario C. Altieri, MD, of The Wistar Institute in Philadelphia, Pennsylvania.
“Our findings provide an unexpected explanation for how TRAP-1 and related proteins regulate metabolism within our cells. We usually link the reprogramming of metabolic pathways with human diseases, such as cancer. What we didn’t expect to see were healthier mice with fewer tumors.”
Dr Altieri and his colleagues created the TRAP-1 knockout mice as part of their ongoing investigation into their novel drug, gamitrinib, which targets TRAP-1 in the mitochondria of tumor cells.
“In tumors, the loss of TRAP-1 is devastating, triggering a host of catastrophic defects, including metabolic problems that ultimately result in the death of the tumor cells,” Dr Altieri said. ”Mice that lack TRAP-1 from the start, however, have 3 weeks in the womb to compensate for the loss of the protein.”
The researchers found that, in the knockout mice, the loss of TRAP-1 causes mitochondrial proteins to misfold, which triggers a compensatory response that causes cells to consume more oxygen and metabolize more sugar. This prompts the mitochondria to produce deregulated levels of ATP.
This increased mitochondrial activity actually creates a moderate boost in oxidative stress (free radical damage) and the associated DNA damage. While DNA damage may seem counterproductive to longevity and good health, the low level of DNA damage actually reduces cell proliferation, slowing growth to allow the cell’s natural repair mechanisms to take effect.
According to Dr Altieri, his group’s observations provide a mechanistic foundation for the role of chaperone molecules like HSP90 in the regulation of bioenergetics in mitochondria—how cells produce and use the chemical energy they need to survive and grow.
Their results explain some contradictory findings in the scientific literature regarding the regulation of bioenergetics and show how compensatory mechanisms can arise when these chaperone molecules are taken out of the equation.
“Our findings strengthen the case for targeting HSP90 in tumor cells, but they also open up a fascinating array of questions that may have implications for metabolism and longevity,” Dr Altieri said. “I predict that the TRAP-1 knockout mouse will be a valuable tool for answering these questions.” ![]()
Study reveals why HSCs falter with age

in the bone marrow
A new study helps explain how blood production declines with age and why older individuals are not suitable donors for hematopoietic stem cell (HSC) transplant.
The research also reveals a potential approach for mitigating
the negative effects of aging on the blood, which can lead to anemia,
bone marrow failure, and myeloid malignancies.
The study, conducted in mice, suggests HSCs falter with age because they lose the ability to replicate their DNA accurately and efficiently during cell division.
Emmanuelle Passegué, PhD, of the University of California San Francisco, and her colleagues reported this discovery in Nature.
The researchers analyzed old HSCs in mice and found a scarcity of protein components needed to form the mini-chromosome maintenance helicase. This molecular machine unwinds double-stranded DNA so the cell’s genetic material can be duplicated and allocated to daughter cells later in cell division.
The HSCs were stressed by the loss of this machine’s activity. As a result, they had an increased risk for DNA damage and death when forced to divide.
On the other hand, the cells tended to survive unless they were confronted with a “strong replication challenge” like transplantation.
The researchers also discovered that even after the stress associated with DNA replication, old HSCs retained molecular tags on histones, a feature often associated with DNA damage.
However, these old survivors could repair induced DNA damage as efficiently as young stem cells.
“Old stem cells are not just sitting there with damaged DNA ready to develop cancer, as it has long been postulated,” Dr Passegué said.
Of course, not all was well in the old, surviving HSCs. The molecular tags accumulated on genes needed to make ribosomes.
Dr Passegué said she will further explore the consequences of reduced protein production as part of her ongoing research. She hopes it might be possible to prevent declining stem cell populations by developing a drug to prevent the loss of the helicase components needed to unwind and replicate DNA, thereby avoiding immune system failure. ![]()

in the bone marrow
A new study helps explain how blood production declines with age and why older individuals are not suitable donors for hematopoietic stem cell (HSC) transplant.
The research also reveals a potential approach for mitigating
the negative effects of aging on the blood, which can lead to anemia,
bone marrow failure, and myeloid malignancies.
The study, conducted in mice, suggests HSCs falter with age because they lose the ability to replicate their DNA accurately and efficiently during cell division.
Emmanuelle Passegué, PhD, of the University of California San Francisco, and her colleagues reported this discovery in Nature.
The researchers analyzed old HSCs in mice and found a scarcity of protein components needed to form the mini-chromosome maintenance helicase. This molecular machine unwinds double-stranded DNA so the cell’s genetic material can be duplicated and allocated to daughter cells later in cell division.
The HSCs were stressed by the loss of this machine’s activity. As a result, they had an increased risk for DNA damage and death when forced to divide.
On the other hand, the cells tended to survive unless they were confronted with a “strong replication challenge” like transplantation.
The researchers also discovered that even after the stress associated with DNA replication, old HSCs retained molecular tags on histones, a feature often associated with DNA damage.
However, these old survivors could repair induced DNA damage as efficiently as young stem cells.
“Old stem cells are not just sitting there with damaged DNA ready to develop cancer, as it has long been postulated,” Dr Passegué said.
Of course, not all was well in the old, surviving HSCs. The molecular tags accumulated on genes needed to make ribosomes.
Dr Passegué said she will further explore the consequences of reduced protein production as part of her ongoing research. She hopes it might be possible to prevent declining stem cell populations by developing a drug to prevent the loss of the helicase components needed to unwind and replicate DNA, thereby avoiding immune system failure. ![]()

in the bone marrow
A new study helps explain how blood production declines with age and why older individuals are not suitable donors for hematopoietic stem cell (HSC) transplant.
The research also reveals a potential approach for mitigating
the negative effects of aging on the blood, which can lead to anemia,
bone marrow failure, and myeloid malignancies.
The study, conducted in mice, suggests HSCs falter with age because they lose the ability to replicate their DNA accurately and efficiently during cell division.
Emmanuelle Passegué, PhD, of the University of California San Francisco, and her colleagues reported this discovery in Nature.
The researchers analyzed old HSCs in mice and found a scarcity of protein components needed to form the mini-chromosome maintenance helicase. This molecular machine unwinds double-stranded DNA so the cell’s genetic material can be duplicated and allocated to daughter cells later in cell division.
The HSCs were stressed by the loss of this machine’s activity. As a result, they had an increased risk for DNA damage and death when forced to divide.
On the other hand, the cells tended to survive unless they were confronted with a “strong replication challenge” like transplantation.
The researchers also discovered that even after the stress associated with DNA replication, old HSCs retained molecular tags on histones, a feature often associated with DNA damage.
However, these old survivors could repair induced DNA damage as efficiently as young stem cells.
“Old stem cells are not just sitting there with damaged DNA ready to develop cancer, as it has long been postulated,” Dr Passegué said.
Of course, not all was well in the old, surviving HSCs. The molecular tags accumulated on genes needed to make ribosomes.
Dr Passegué said she will further explore the consequences of reduced protein production as part of her ongoing research. She hopes it might be possible to prevent declining stem cell populations by developing a drug to prevent the loss of the helicase components needed to unwind and replicate DNA, thereby avoiding immune system failure. ![]()
Approach could improve treatment of lymphoma, other cancers

Credit: NIH
Targeting a molecule in endothelial cells could make cancer therapies significantly more effective, preclinical research suggests.
The researchers found that a molecule called focal adhesion kinase (FAK) can help protect cancer cells from the damaging effects of chemotherapy and radiotherapy.
But deleting FAK can enhance the effects of treatment directed against melanoma, lung cancer, and lymphoma.
The team recounted these findings in Nature.
“This work shows that sensitivity to cancer treatment is related to our own body mistakenly trying to shield the cancer from cell-killing effects caused by radiotherapy and chemotherapy,” said study author Bernardo Tavora, PhD, of Rockefeller University in New York.
“Although taking out FAK from blood vessels won’t destroy the cancer by itself, it can remove the barrier cancer uses to protect itself from treatment.”
Dr Tavora and his colleagues removed FAK from endothelial cells in mouse models of melanoma, lung cancer, and lymphoma. This had no effect on tumor growth in untreated mice.
However, the loss of endothelial-cell FAK did aid the effects of doxorubicin and radiotherapy. It increased apoptosis and decreased proliferation within perivascular tumor-cell compartments, thereby extending survival in the mice.
The researchers also studied samples from lymphoma patients. And they found that patients with low levels of FAK were more likely to achieve a complete remission after treatment.
Investigation into the mechanism behind these effects revealed that endothelial-cell FAK is required for the production of cytokines and for NF-κB activation induced by DNA damage.
So the loss of endothelial-cell FAK reduces DNA-damage-induced cytokine production, thereby increasing cancer cells’ sensitivity to DNA-damaging therapies in vitro and in vivo.
Taken together, these results suggest that developing drugs to target FAK could help improve the efficacy of cancer treatments and potentially prevent relapse in a number of malignancies. ![]()

Credit: NIH
Targeting a molecule in endothelial cells could make cancer therapies significantly more effective, preclinical research suggests.
The researchers found that a molecule called focal adhesion kinase (FAK) can help protect cancer cells from the damaging effects of chemotherapy and radiotherapy.
But deleting FAK can enhance the effects of treatment directed against melanoma, lung cancer, and lymphoma.
The team recounted these findings in Nature.
“This work shows that sensitivity to cancer treatment is related to our own body mistakenly trying to shield the cancer from cell-killing effects caused by radiotherapy and chemotherapy,” said study author Bernardo Tavora, PhD, of Rockefeller University in New York.
“Although taking out FAK from blood vessels won’t destroy the cancer by itself, it can remove the barrier cancer uses to protect itself from treatment.”
Dr Tavora and his colleagues removed FAK from endothelial cells in mouse models of melanoma, lung cancer, and lymphoma. This had no effect on tumor growth in untreated mice.
However, the loss of endothelial-cell FAK did aid the effects of doxorubicin and radiotherapy. It increased apoptosis and decreased proliferation within perivascular tumor-cell compartments, thereby extending survival in the mice.
The researchers also studied samples from lymphoma patients. And they found that patients with low levels of FAK were more likely to achieve a complete remission after treatment.
Investigation into the mechanism behind these effects revealed that endothelial-cell FAK is required for the production of cytokines and for NF-κB activation induced by DNA damage.
So the loss of endothelial-cell FAK reduces DNA-damage-induced cytokine production, thereby increasing cancer cells’ sensitivity to DNA-damaging therapies in vitro and in vivo.
Taken together, these results suggest that developing drugs to target FAK could help improve the efficacy of cancer treatments and potentially prevent relapse in a number of malignancies. ![]()

Credit: NIH
Targeting a molecule in endothelial cells could make cancer therapies significantly more effective, preclinical research suggests.
The researchers found that a molecule called focal adhesion kinase (FAK) can help protect cancer cells from the damaging effects of chemotherapy and radiotherapy.
But deleting FAK can enhance the effects of treatment directed against melanoma, lung cancer, and lymphoma.
The team recounted these findings in Nature.
“This work shows that sensitivity to cancer treatment is related to our own body mistakenly trying to shield the cancer from cell-killing effects caused by radiotherapy and chemotherapy,” said study author Bernardo Tavora, PhD, of Rockefeller University in New York.
“Although taking out FAK from blood vessels won’t destroy the cancer by itself, it can remove the barrier cancer uses to protect itself from treatment.”
Dr Tavora and his colleagues removed FAK from endothelial cells in mouse models of melanoma, lung cancer, and lymphoma. This had no effect on tumor growth in untreated mice.
However, the loss of endothelial-cell FAK did aid the effects of doxorubicin and radiotherapy. It increased apoptosis and decreased proliferation within perivascular tumor-cell compartments, thereby extending survival in the mice.
The researchers also studied samples from lymphoma patients. And they found that patients with low levels of FAK were more likely to achieve a complete remission after treatment.
Investigation into the mechanism behind these effects revealed that endothelial-cell FAK is required for the production of cytokines and for NF-κB activation induced by DNA damage.
So the loss of endothelial-cell FAK reduces DNA-damage-induced cytokine production, thereby increasing cancer cells’ sensitivity to DNA-damaging therapies in vitro and in vivo.
Taken together, these results suggest that developing drugs to target FAK could help improve the efficacy of cancer treatments and potentially prevent relapse in a number of malignancies. ![]()
Overcoming ibrutinib resistance in MCL

Credit: Rhoda Baer
Investigators have identified drug combinations that may overcome resistance to ibrutinib in patients with mantle cell lymphoma (MCL).
The group discovered a mutation in Bruton’s tyrosine kinase (BTK) that confers resistance to the drug.
They also found that high levels of PI3K-AKT and CDK4 signaling could explain innate resistance to ibrutinib, so combining a CDK4 inhibitor and a PI3K inhibitor might be effective in patients who don’t respond to ibrutinib.
Selina Chen-Kiang, PhD, of Weill Cornell Medical College in New York, and her colleagues detailed these findings in Cancer Discovery. Some of Dr Chen-Kiang’s colleagues reported relationships with Janssen and Pharmacyclics, the companies developing ibrutinib.
“[Ibrutinib] doesn’t work for about 32% of patients, and their lymphomas are said to have primary resistance to ibrutinib,” Dr Chen-Kiang noted. “We are also learning that most patients whose lymphomas respond to ibrutinib eventually relapse because their tumors acquire resistance to the drug.”
“The knowledge that we gained from longitudinal RNA and genomic sequencing of mantle cell lymphomas with primary and acquired resistance to ibrutinib allowed us to identify rational drug combinations that may overcome resistance in these 2 settings.”
Dr Chen-Kiang and her colleagues conducted whole-exome and whole-transcriptome analyses of 5 serial biopsies from a patient with MCL who initially responded to ibrutinib before progressing.
After comparing these data with results from an analysis of healthy tissues from the same patient, the investigators found that a mutation in BTK, the C481S mutation, appeared at relapse.
The researchers found the same mutation at relapse in a second MCL patient with acquired resistance to ibrutinib but not in any patients with primary resistance to the drug.
Further analyses revealed the consequences of the relapse-specific BTK C481S mutation, including activation of the PI3K and CDK4 signaling pathways, which promote cell survival and proliferation.
Inhibiting CDK4 with the investigational anticancer drug palbociclib made ibrutinib-resistant lymphoma cells carrying the BTK C481S mutation sensitive to investigational drugs that inhibit PI3K.
And palbociclib made ibrutinib-resistant lymphoma cells harboring normal BTK sensitive to both ibrutinib and investigational drugs that inhibit PI3K. The researchers recently opened a clinical trial to test ibrutinib and palbociclib in combination (NCT02159755).
“We are very excited to have generated data . . . that may be meaningful for patients,” Dr Chen-Kiang said. “It is also exciting because CDK4 is a new kind of drug target. It controls the cell cycle, which is a central cancer pathway. As such, it is not just important for mantle cell lymphoma but for many forms of cancer.” ![]()

Credit: Rhoda Baer
Investigators have identified drug combinations that may overcome resistance to ibrutinib in patients with mantle cell lymphoma (MCL).
The group discovered a mutation in Bruton’s tyrosine kinase (BTK) that confers resistance to the drug.
They also found that high levels of PI3K-AKT and CDK4 signaling could explain innate resistance to ibrutinib, so combining a CDK4 inhibitor and a PI3K inhibitor might be effective in patients who don’t respond to ibrutinib.
Selina Chen-Kiang, PhD, of Weill Cornell Medical College in New York, and her colleagues detailed these findings in Cancer Discovery. Some of Dr Chen-Kiang’s colleagues reported relationships with Janssen and Pharmacyclics, the companies developing ibrutinib.
“[Ibrutinib] doesn’t work for about 32% of patients, and their lymphomas are said to have primary resistance to ibrutinib,” Dr Chen-Kiang noted. “We are also learning that most patients whose lymphomas respond to ibrutinib eventually relapse because their tumors acquire resistance to the drug.”
“The knowledge that we gained from longitudinal RNA and genomic sequencing of mantle cell lymphomas with primary and acquired resistance to ibrutinib allowed us to identify rational drug combinations that may overcome resistance in these 2 settings.”
Dr Chen-Kiang and her colleagues conducted whole-exome and whole-transcriptome analyses of 5 serial biopsies from a patient with MCL who initially responded to ibrutinib before progressing.
After comparing these data with results from an analysis of healthy tissues from the same patient, the investigators found that a mutation in BTK, the C481S mutation, appeared at relapse.
The researchers found the same mutation at relapse in a second MCL patient with acquired resistance to ibrutinib but not in any patients with primary resistance to the drug.
Further analyses revealed the consequences of the relapse-specific BTK C481S mutation, including activation of the PI3K and CDK4 signaling pathways, which promote cell survival and proliferation.
Inhibiting CDK4 with the investigational anticancer drug palbociclib made ibrutinib-resistant lymphoma cells carrying the BTK C481S mutation sensitive to investigational drugs that inhibit PI3K.
And palbociclib made ibrutinib-resistant lymphoma cells harboring normal BTK sensitive to both ibrutinib and investigational drugs that inhibit PI3K. The researchers recently opened a clinical trial to test ibrutinib and palbociclib in combination (NCT02159755).
“We are very excited to have generated data . . . that may be meaningful for patients,” Dr Chen-Kiang said. “It is also exciting because CDK4 is a new kind of drug target. It controls the cell cycle, which is a central cancer pathway. As such, it is not just important for mantle cell lymphoma but for many forms of cancer.” ![]()

Credit: Rhoda Baer
Investigators have identified drug combinations that may overcome resistance to ibrutinib in patients with mantle cell lymphoma (MCL).
The group discovered a mutation in Bruton’s tyrosine kinase (BTK) that confers resistance to the drug.
They also found that high levels of PI3K-AKT and CDK4 signaling could explain innate resistance to ibrutinib, so combining a CDK4 inhibitor and a PI3K inhibitor might be effective in patients who don’t respond to ibrutinib.
Selina Chen-Kiang, PhD, of Weill Cornell Medical College in New York, and her colleagues detailed these findings in Cancer Discovery. Some of Dr Chen-Kiang’s colleagues reported relationships with Janssen and Pharmacyclics, the companies developing ibrutinib.
“[Ibrutinib] doesn’t work for about 32% of patients, and their lymphomas are said to have primary resistance to ibrutinib,” Dr Chen-Kiang noted. “We are also learning that most patients whose lymphomas respond to ibrutinib eventually relapse because their tumors acquire resistance to the drug.”
“The knowledge that we gained from longitudinal RNA and genomic sequencing of mantle cell lymphomas with primary and acquired resistance to ibrutinib allowed us to identify rational drug combinations that may overcome resistance in these 2 settings.”
Dr Chen-Kiang and her colleagues conducted whole-exome and whole-transcriptome analyses of 5 serial biopsies from a patient with MCL who initially responded to ibrutinib before progressing.
After comparing these data with results from an analysis of healthy tissues from the same patient, the investigators found that a mutation in BTK, the C481S mutation, appeared at relapse.
The researchers found the same mutation at relapse in a second MCL patient with acquired resistance to ibrutinib but not in any patients with primary resistance to the drug.
Further analyses revealed the consequences of the relapse-specific BTK C481S mutation, including activation of the PI3K and CDK4 signaling pathways, which promote cell survival and proliferation.
Inhibiting CDK4 with the investigational anticancer drug palbociclib made ibrutinib-resistant lymphoma cells carrying the BTK C481S mutation sensitive to investigational drugs that inhibit PI3K.
And palbociclib made ibrutinib-resistant lymphoma cells harboring normal BTK sensitive to both ibrutinib and investigational drugs that inhibit PI3K. The researchers recently opened a clinical trial to test ibrutinib and palbociclib in combination (NCT02159755).
“We are very excited to have generated data . . . that may be meaningful for patients,” Dr Chen-Kiang said. “It is also exciting because CDK4 is a new kind of drug target. It controls the cell cycle, which is a central cancer pathway. As such, it is not just important for mantle cell lymphoma but for many forms of cancer.” ![]()
How lymphoma affects male fertility
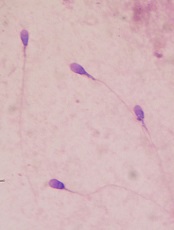
for semen quality testing
New research has shown that lymphoma and its treatment can impact a number of sperm characteristics, thereby reducing fertility in males.
Results also indicated that most patients eventually experience semen recovery, but the degree and timing of that recovery may depend on the patient’s diagnosis and treatment.
In this study, recovery was more likely among patients with Hodgkin lymphoma than those with non-Hodgkin lymphoma.
And recovery was both quicker and more likely among patients who did not receive alkylating chemotherapy.
On the other hand, multivariate analyses suggested that only a patient’s pre-treatment total sperm count was related to recovery.
Louis Bujan, MD, PhD, of Université de Toulouse in France, and colleagues reported these results in Fertility and Sterility.
The study included 75 patients—57 with Hodgkin lymphoma and 18 with non-Hodgkin lymphoma. The researchers collected sperm samples before patients began cancer treatment and again at later intervals: 3 months, 6 months, 12 months, and 24 months post-treatment.
The team compared patients’ sperm characteristics to those of a control group consisting of 257 healthy, fertile men.
Results revealed that lymphoma patients had impaired sperm quality even before they began treatment. Compared to fertile controls, patients had higher levels of sperm chromatin alterations and DNA fragmentation, with the only risk factor being their cancer diagnosis.
However, between 3 months and 6 months post-treatment, patients’ levels of sperm DNA fragmentation and chromatin structure damage improved. The damage level decreased relative to a patient’s own pre-treatment level of damage, while still remaining higher than damage levels in the control group.
After treatment, patients’ sperm density, total count, motility, and vitality decreased, with the lowest values seen at the 3- and 6-month marks.
Alkylating chemotherapy was more detrimental to spermatogenesis than non-alkylating drugs. Patients who received alkylating chemotherapy were more likely to cease sperm production entirely or take longer to resume sperm production than patients receiving non-alkylating chemotherapy.
Twelve months after treatment, mean sperm counts recovered to pre-treatment values for patients who had received doxorubicin, bleomycin, vinblastine, and darcarbacine (ABVD) or ABVD and radiotherapy.
But this was not the case for patients who received doxorubicin, cyclophosphamide, vincristine, and prednisone (CHOP) or mechlorethamine, oncovin, procarbazine, and prednisone (MOPP).
At the 24-month mark, 7% of patients remained azoospermic. Kaplan Meir estimates suggested that, after 24 months, most patients would recover normal sperm counts.
Recovery was projected for 92% of patients who received ABVD and radiotherapy, 90% of patients who received ABVD alone, and 61% of CHOP-treated patients. (There was no estimate for MOPP therapy, perhaps due to a low number of patients.)
A patient’s type of lymphoma appeared to impact sperm count recovery as well. Estimates suggested that, after 24 months, 86% of Hodgkin lymphoma patients would experience recovery, compared to 73% of non-Hodgkin lymphoma patients.
“While many men can look forward to their fertility returning after treatment is over, not all will be so fortunate,” said Rebecca Sokol, MD, MPH, President of the American Society for Reproductive Medicine.
“It is imperative that, prior to the initiation of therapy, counseling and sperm preservation be made available to all lymphoma patients and their partners who may want to have children in the future.” ![]()

for semen quality testing
New research has shown that lymphoma and its treatment can impact a number of sperm characteristics, thereby reducing fertility in males.
Results also indicated that most patients eventually experience semen recovery, but the degree and timing of that recovery may depend on the patient’s diagnosis and treatment.
In this study, recovery was more likely among patients with Hodgkin lymphoma than those with non-Hodgkin lymphoma.
And recovery was both quicker and more likely among patients who did not receive alkylating chemotherapy.
On the other hand, multivariate analyses suggested that only a patient’s pre-treatment total sperm count was related to recovery.
Louis Bujan, MD, PhD, of Université de Toulouse in France, and colleagues reported these results in Fertility and Sterility.
The study included 75 patients—57 with Hodgkin lymphoma and 18 with non-Hodgkin lymphoma. The researchers collected sperm samples before patients began cancer treatment and again at later intervals: 3 months, 6 months, 12 months, and 24 months post-treatment.
The team compared patients’ sperm characteristics to those of a control group consisting of 257 healthy, fertile men.
Results revealed that lymphoma patients had impaired sperm quality even before they began treatment. Compared to fertile controls, patients had higher levels of sperm chromatin alterations and DNA fragmentation, with the only risk factor being their cancer diagnosis.
However, between 3 months and 6 months post-treatment, patients’ levels of sperm DNA fragmentation and chromatin structure damage improved. The damage level decreased relative to a patient’s own pre-treatment level of damage, while still remaining higher than damage levels in the control group.
After treatment, patients’ sperm density, total count, motility, and vitality decreased, with the lowest values seen at the 3- and 6-month marks.
Alkylating chemotherapy was more detrimental to spermatogenesis than non-alkylating drugs. Patients who received alkylating chemotherapy were more likely to cease sperm production entirely or take longer to resume sperm production than patients receiving non-alkylating chemotherapy.
Twelve months after treatment, mean sperm counts recovered to pre-treatment values for patients who had received doxorubicin, bleomycin, vinblastine, and darcarbacine (ABVD) or ABVD and radiotherapy.
But this was not the case for patients who received doxorubicin, cyclophosphamide, vincristine, and prednisone (CHOP) or mechlorethamine, oncovin, procarbazine, and prednisone (MOPP).
At the 24-month mark, 7% of patients remained azoospermic. Kaplan Meir estimates suggested that, after 24 months, most patients would recover normal sperm counts.
Recovery was projected for 92% of patients who received ABVD and radiotherapy, 90% of patients who received ABVD alone, and 61% of CHOP-treated patients. (There was no estimate for MOPP therapy, perhaps due to a low number of patients.)
A patient’s type of lymphoma appeared to impact sperm count recovery as well. Estimates suggested that, after 24 months, 86% of Hodgkin lymphoma patients would experience recovery, compared to 73% of non-Hodgkin lymphoma patients.
“While many men can look forward to their fertility returning after treatment is over, not all will be so fortunate,” said Rebecca Sokol, MD, MPH, President of the American Society for Reproductive Medicine.
“It is imperative that, prior to the initiation of therapy, counseling and sperm preservation be made available to all lymphoma patients and their partners who may want to have children in the future.” ![]()

for semen quality testing
New research has shown that lymphoma and its treatment can impact a number of sperm characteristics, thereby reducing fertility in males.
Results also indicated that most patients eventually experience semen recovery, but the degree and timing of that recovery may depend on the patient’s diagnosis and treatment.
In this study, recovery was more likely among patients with Hodgkin lymphoma than those with non-Hodgkin lymphoma.
And recovery was both quicker and more likely among patients who did not receive alkylating chemotherapy.
On the other hand, multivariate analyses suggested that only a patient’s pre-treatment total sperm count was related to recovery.
Louis Bujan, MD, PhD, of Université de Toulouse in France, and colleagues reported these results in Fertility and Sterility.
The study included 75 patients—57 with Hodgkin lymphoma and 18 with non-Hodgkin lymphoma. The researchers collected sperm samples before patients began cancer treatment and again at later intervals: 3 months, 6 months, 12 months, and 24 months post-treatment.
The team compared patients’ sperm characteristics to those of a control group consisting of 257 healthy, fertile men.
Results revealed that lymphoma patients had impaired sperm quality even before they began treatment. Compared to fertile controls, patients had higher levels of sperm chromatin alterations and DNA fragmentation, with the only risk factor being their cancer diagnosis.
However, between 3 months and 6 months post-treatment, patients’ levels of sperm DNA fragmentation and chromatin structure damage improved. The damage level decreased relative to a patient’s own pre-treatment level of damage, while still remaining higher than damage levels in the control group.
After treatment, patients’ sperm density, total count, motility, and vitality decreased, with the lowest values seen at the 3- and 6-month marks.
Alkylating chemotherapy was more detrimental to spermatogenesis than non-alkylating drugs. Patients who received alkylating chemotherapy were more likely to cease sperm production entirely or take longer to resume sperm production than patients receiving non-alkylating chemotherapy.
Twelve months after treatment, mean sperm counts recovered to pre-treatment values for patients who had received doxorubicin, bleomycin, vinblastine, and darcarbacine (ABVD) or ABVD and radiotherapy.
But this was not the case for patients who received doxorubicin, cyclophosphamide, vincristine, and prednisone (CHOP) or mechlorethamine, oncovin, procarbazine, and prednisone (MOPP).
At the 24-month mark, 7% of patients remained azoospermic. Kaplan Meir estimates suggested that, after 24 months, most patients would recover normal sperm counts.
Recovery was projected for 92% of patients who received ABVD and radiotherapy, 90% of patients who received ABVD alone, and 61% of CHOP-treated patients. (There was no estimate for MOPP therapy, perhaps due to a low number of patients.)
A patient’s type of lymphoma appeared to impact sperm count recovery as well. Estimates suggested that, after 24 months, 86% of Hodgkin lymphoma patients would experience recovery, compared to 73% of non-Hodgkin lymphoma patients.
“While many men can look forward to their fertility returning after treatment is over, not all will be so fortunate,” said Rebecca Sokol, MD, MPH, President of the American Society for Reproductive Medicine.
“It is imperative that, prior to the initiation of therapy, counseling and sperm preservation be made available to all lymphoma patients and their partners who may want to have children in the future.” ![]()
Obinutuzumab approved for CLL in Europe
The European Commission has approved the anti-CD20 monoclonal antibody obinutuzumab for use in the European Union (EU).
Obinutuzumab can now be used in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia (CLL) who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab is already approved for this indication in the US.
Obinutuzumab is marketed as Gazyvaro in the EU and Switzerland but as Gazyva in the US and the rest of the world.
The European Commission’s approval follows a positive opinion granted by The European Medicine Agency’s Committee for Medicinal Products for Human Use in May.
The approval is based on results of the phase 3 CLL11 study, which showed that obinutuzumab plus chlorambucil improved progression-free survival (PFS), when compared to chlorambucil alone or in combination with rituximab.
This 2-stage study included 781 previously untreated CLL patients with comorbidities. In stage 1 (n=589), researchers compared obinutuzumab plus chlorambucil to chlorambucil alone and rituximab plus chlorambucil to chlorambucil alone.
Stage 2 (n=663) was a direct comparison of obinutuzumab plus chlorambucil and rituximab plus chlorambucil.
Stage 1 results were presented at ASCO 2013, stage 2 results were presented at ASH 2013, and the complete results were published in NEJM last March.
Obinutuzumab plus chlorambucil improved PFS when compared to chlorambucil alone. The median PFS was 26.7 months and 11.1 months, respectively (P<0.001).
Obinutuzumab plus chlorambucil also improved PFS when compared to rituximab plus chlorambucil. The median PFS was 26.7 months and 16.3 months, respectively (P<0.001).
Infusion-related reactions and neutropenia were more common in the obinutuzumab arm than in the rituximab arm. But obinutuzumab-treated patients did not have an increased risk of infection.
Obinutuzumab is being developed by Roche. The company said it expects to begin launching the drug in a number of European countries this year. ![]()
The European Commission has approved the anti-CD20 monoclonal antibody obinutuzumab for use in the European Union (EU).
Obinutuzumab can now be used in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia (CLL) who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab is already approved for this indication in the US.
Obinutuzumab is marketed as Gazyvaro in the EU and Switzerland but as Gazyva in the US and the rest of the world.
The European Commission’s approval follows a positive opinion granted by The European Medicine Agency’s Committee for Medicinal Products for Human Use in May.
The approval is based on results of the phase 3 CLL11 study, which showed that obinutuzumab plus chlorambucil improved progression-free survival (PFS), when compared to chlorambucil alone or in combination with rituximab.
This 2-stage study included 781 previously untreated CLL patients with comorbidities. In stage 1 (n=589), researchers compared obinutuzumab plus chlorambucil to chlorambucil alone and rituximab plus chlorambucil to chlorambucil alone.
Stage 2 (n=663) was a direct comparison of obinutuzumab plus chlorambucil and rituximab plus chlorambucil.
Stage 1 results were presented at ASCO 2013, stage 2 results were presented at ASH 2013, and the complete results were published in NEJM last March.
Obinutuzumab plus chlorambucil improved PFS when compared to chlorambucil alone. The median PFS was 26.7 months and 11.1 months, respectively (P<0.001).
Obinutuzumab plus chlorambucil also improved PFS when compared to rituximab plus chlorambucil. The median PFS was 26.7 months and 16.3 months, respectively (P<0.001).
Infusion-related reactions and neutropenia were more common in the obinutuzumab arm than in the rituximab arm. But obinutuzumab-treated patients did not have an increased risk of infection.
Obinutuzumab is being developed by Roche. The company said it expects to begin launching the drug in a number of European countries this year. ![]()
The European Commission has approved the anti-CD20 monoclonal antibody obinutuzumab for use in the European Union (EU).
Obinutuzumab can now be used in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia (CLL) who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab is already approved for this indication in the US.
Obinutuzumab is marketed as Gazyvaro in the EU and Switzerland but as Gazyva in the US and the rest of the world.
The European Commission’s approval follows a positive opinion granted by The European Medicine Agency’s Committee for Medicinal Products for Human Use in May.
The approval is based on results of the phase 3 CLL11 study, which showed that obinutuzumab plus chlorambucil improved progression-free survival (PFS), when compared to chlorambucil alone or in combination with rituximab.
This 2-stage study included 781 previously untreated CLL patients with comorbidities. In stage 1 (n=589), researchers compared obinutuzumab plus chlorambucil to chlorambucil alone and rituximab plus chlorambucil to chlorambucil alone.
Stage 2 (n=663) was a direct comparison of obinutuzumab plus chlorambucil and rituximab plus chlorambucil.
Stage 1 results were presented at ASCO 2013, stage 2 results were presented at ASH 2013, and the complete results were published in NEJM last March.
Obinutuzumab plus chlorambucil improved PFS when compared to chlorambucil alone. The median PFS was 26.7 months and 11.1 months, respectively (P<0.001).
Obinutuzumab plus chlorambucil also improved PFS when compared to rituximab plus chlorambucil. The median PFS was 26.7 months and 16.3 months, respectively (P<0.001).
Infusion-related reactions and neutropenia were more common in the obinutuzumab arm than in the rituximab arm. But obinutuzumab-treated patients did not have an increased risk of infection.
Obinutuzumab is being developed by Roche. The company said it expects to begin launching the drug in a number of European countries this year.
Healthy habits can cut risk of metabolic syndrome in childhood cancer survivors

patient and her father
Credit: Rhoda Baer
Following a healthy lifestyle can decrease the risk of metabolic syndrome in childhood cancer survivors, according to a study published in Cancer.
Unfortunately, only about a quarter of the survivors studied actually practiced healthy lifestyle habits, such as engaging in moderate physical activity; eating the recommended daily serving of fruits, vegetables, and complex carbohydrates; and consuming red meat, alcohol, and sodium in moderation.
Childhood cancer survivors are known to have an increased risk of developing metabolic syndrome.
The syndrome is actually a number of conditions—high blood pressure, increased body fat, and abnormal cholesterol and glucose levels—that, when they occur together, increase a person’s risk of heart disease, stroke, and diabetes.
Kirsten Ness, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and her colleagues wanted to determine if lifestyle habits might affect the risk of metabolic syndrome among childhood cancer survivors.
So the team analyzed 1598 survivors who were cancer-free for at least 10 years. They had a median age of 32.7 years (range, 18.9 to 60).
The analysis showed that failure to follow healthy lifestyle guidelines roughly doubled the survivors’ risk of developing metabolic syndrome. Women had a 2.4-times greater risk, and men had a 2.2-times greater risk of the syndrome if they did not follow the guidelines.
Metabolic syndrome was present in 31.8% of the participants—32.5% of males and 31% of females.
The researchers considered a subject to have metabolic syndrome if he had or received treatment for 3 or more of the following:
- Abdominal obesity (waist circumference of > 102 cm in males and > 88 cm in females)
- Triglycerides ≥ 150 mg/dL
- High-density lipoprotein cholesterol (< 40 mg/dL in males and < 50 mg/dL in females)
- Hypertension (systolic pressure ≥ 130 mm Hg or diastolic pressure ≥ 85 mm Hg)
- Fasting plasma glucose ≥ 100 mg/dL.
Questionnaires and tests helped the researchers assess whether participants followed healthy lifestyle recommendations issued by the World Cancer Research Fund and American Institute for Cancer Research.
The recommendations include:
- Having a body mass index of 25 or lower
- Engaging in moderate physical activity for 150 minutes each week
- Eating 5 or more servings of fruits and vegetables each day
- Consuming 400 g or more of complex carbohydrates daily
- Eating less than 80 g of red meat each day
- Consuming less than 2400 mg of sodium each day
- Low daily alcohol consumption (less than 14 g for females and less than 28 g for males).
Subjects who met at least 4 of these 7 criteria were classified as following the guidelines. And 27% of the participants—25.2% of males and 28.8% of females—were classified as such.
“These findings are important because they indicate that adults who were treated for cancer as children have the opportunity to influence their own health outcomes,” Dr Ness said.
“[A]dopting a lifestyle that includes maintaining a healthy body weight, regular physical activity, and a diet that includes fruits and vegetables and that limits refined sugars, excessive alcohol, red meat, and salt has potential to prevent development of metabolic syndrome.”

patient and her father
Credit: Rhoda Baer
Following a healthy lifestyle can decrease the risk of metabolic syndrome in childhood cancer survivors, according to a study published in Cancer.
Unfortunately, only about a quarter of the survivors studied actually practiced healthy lifestyle habits, such as engaging in moderate physical activity; eating the recommended daily serving of fruits, vegetables, and complex carbohydrates; and consuming red meat, alcohol, and sodium in moderation.
Childhood cancer survivors are known to have an increased risk of developing metabolic syndrome.
The syndrome is actually a number of conditions—high blood pressure, increased body fat, and abnormal cholesterol and glucose levels—that, when they occur together, increase a person’s risk of heart disease, stroke, and diabetes.
Kirsten Ness, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and her colleagues wanted to determine if lifestyle habits might affect the risk of metabolic syndrome among childhood cancer survivors.
So the team analyzed 1598 survivors who were cancer-free for at least 10 years. They had a median age of 32.7 years (range, 18.9 to 60).
The analysis showed that failure to follow healthy lifestyle guidelines roughly doubled the survivors’ risk of developing metabolic syndrome. Women had a 2.4-times greater risk, and men had a 2.2-times greater risk of the syndrome if they did not follow the guidelines.
Metabolic syndrome was present in 31.8% of the participants—32.5% of males and 31% of females.
The researchers considered a subject to have metabolic syndrome if he had or received treatment for 3 or more of the following:
- Abdominal obesity (waist circumference of > 102 cm in males and > 88 cm in females)
- Triglycerides ≥ 150 mg/dL
- High-density lipoprotein cholesterol (< 40 mg/dL in males and < 50 mg/dL in females)
- Hypertension (systolic pressure ≥ 130 mm Hg or diastolic pressure ≥ 85 mm Hg)
- Fasting plasma glucose ≥ 100 mg/dL.
Questionnaires and tests helped the researchers assess whether participants followed healthy lifestyle recommendations issued by the World Cancer Research Fund and American Institute for Cancer Research.
The recommendations include:
- Having a body mass index of 25 or lower
- Engaging in moderate physical activity for 150 minutes each week
- Eating 5 or more servings of fruits and vegetables each day
- Consuming 400 g or more of complex carbohydrates daily
- Eating less than 80 g of red meat each day
- Consuming less than 2400 mg of sodium each day
- Low daily alcohol consumption (less than 14 g for females and less than 28 g for males).
Subjects who met at least 4 of these 7 criteria were classified as following the guidelines. And 27% of the participants—25.2% of males and 28.8% of females—were classified as such.
“These findings are important because they indicate that adults who were treated for cancer as children have the opportunity to influence their own health outcomes,” Dr Ness said.
“[A]dopting a lifestyle that includes maintaining a healthy body weight, regular physical activity, and a diet that includes fruits and vegetables and that limits refined sugars, excessive alcohol, red meat, and salt has potential to prevent development of metabolic syndrome.”

patient and her father
Credit: Rhoda Baer
Following a healthy lifestyle can decrease the risk of metabolic syndrome in childhood cancer survivors, according to a study published in Cancer.
Unfortunately, only about a quarter of the survivors studied actually practiced healthy lifestyle habits, such as engaging in moderate physical activity; eating the recommended daily serving of fruits, vegetables, and complex carbohydrates; and consuming red meat, alcohol, and sodium in moderation.
Childhood cancer survivors are known to have an increased risk of developing metabolic syndrome.
The syndrome is actually a number of conditions—high blood pressure, increased body fat, and abnormal cholesterol and glucose levels—that, when they occur together, increase a person’s risk of heart disease, stroke, and diabetes.
Kirsten Ness, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and her colleagues wanted to determine if lifestyle habits might affect the risk of metabolic syndrome among childhood cancer survivors.
So the team analyzed 1598 survivors who were cancer-free for at least 10 years. They had a median age of 32.7 years (range, 18.9 to 60).
The analysis showed that failure to follow healthy lifestyle guidelines roughly doubled the survivors’ risk of developing metabolic syndrome. Women had a 2.4-times greater risk, and men had a 2.2-times greater risk of the syndrome if they did not follow the guidelines.
Metabolic syndrome was present in 31.8% of the participants—32.5% of males and 31% of females.
The researchers considered a subject to have metabolic syndrome if he had or received treatment for 3 or more of the following:
- Abdominal obesity (waist circumference of > 102 cm in males and > 88 cm in females)
- Triglycerides ≥ 150 mg/dL
- High-density lipoprotein cholesterol (< 40 mg/dL in males and < 50 mg/dL in females)
- Hypertension (systolic pressure ≥ 130 mm Hg or diastolic pressure ≥ 85 mm Hg)
- Fasting plasma glucose ≥ 100 mg/dL.
Questionnaires and tests helped the researchers assess whether participants followed healthy lifestyle recommendations issued by the World Cancer Research Fund and American Institute for Cancer Research.
The recommendations include:
- Having a body mass index of 25 or lower
- Engaging in moderate physical activity for 150 minutes each week
- Eating 5 or more servings of fruits and vegetables each day
- Consuming 400 g or more of complex carbohydrates daily
- Eating less than 80 g of red meat each day
- Consuming less than 2400 mg of sodium each day
- Low daily alcohol consumption (less than 14 g for females and less than 28 g for males).
Subjects who met at least 4 of these 7 criteria were classified as following the guidelines. And 27% of the participants—25.2% of males and 28.8% of females—were classified as such.
“These findings are important because they indicate that adults who were treated for cancer as children have the opportunity to influence their own health outcomes,” Dr Ness said.
“[A]dopting a lifestyle that includes maintaining a healthy body weight, regular physical activity, and a diet that includes fruits and vegetables and that limits refined sugars, excessive alcohol, red meat, and salt has potential to prevent development of metabolic syndrome.”
FDA expands approved use of ibrutinib in CLL

Credit: Steven Harbour
The US Food and Drug Administration (FDA) has expanded the approved use of ibrutinib (Imbruvica) in patients with chronic lymphocytic leukemia (CLL).
The agency previously granted the drug accelerated approval to treat CLL patients who had received at least 1 prior therapy.
Now, the FDA has granted ibrutinib full approval for that indication and expanded the drug’s approved use to include previously treated and untreated CLL patients with 17p deletion.
The FDA’s decision to grant ibrutinib accelerated approval in CLL was based on the drug’s ability to elicit responses in previously treated patients.
Recent trial results have shown the drug can improve survival rates in CLL, which signifies a clinical benefit and allows the FDA to grant ibrutinib full approval.
Ibrutinib in CLL: Trial results
The expanded approval for ibrutinib is based on results of the phase 3 RESONATE trial, which were presented at this year’s ASCO and EHA meetings.
The trial included 391 previously treated patients, 127 of whom had 17p deletion. Patients were randomized to receive ibrutinib or the anti-CD20 monoclonal antibody ofatumumab until disease progression or unacceptable toxicity.
The trial was stopped early after a pre-planned interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
At the time of interim analysis, the patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm.
Ibrutinib significantly prolonged progression-free and overall survival. The median progression-free survival was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Of the 127 patients with 17p deletion, those treated with ibrutinib experienced a 75% reduction in the risk of disease progression or death.
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
Ibrutinib in development
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, mantle cell lymphoma (MCL), Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Before the FDA granted ibrutinib accelerated approval in CLL, the agency granted the drug accelerated approval for use in previously treated MCL patients. Studies to verify ibrutinib’s clinical benefit in MCL are ongoing.
The European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended marketing authorization for ibrutinib to treat adults with relapsed or refractory MCL.
The committee has also recommended the drug for adults with CLL who have received at least 1 prior therapy and CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy.
Ibrutinib is already approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen Biotech and Pharmacyclics, Inc. The companies co-market ibrutinib in the US, but Janssen markets (or will market) ibrutinib in the rest of the world.

Credit: Steven Harbour
The US Food and Drug Administration (FDA) has expanded the approved use of ibrutinib (Imbruvica) in patients with chronic lymphocytic leukemia (CLL).
The agency previously granted the drug accelerated approval to treat CLL patients who had received at least 1 prior therapy.
Now, the FDA has granted ibrutinib full approval for that indication and expanded the drug’s approved use to include previously treated and untreated CLL patients with 17p deletion.
The FDA’s decision to grant ibrutinib accelerated approval in CLL was based on the drug’s ability to elicit responses in previously treated patients.
Recent trial results have shown the drug can improve survival rates in CLL, which signifies a clinical benefit and allows the FDA to grant ibrutinib full approval.
Ibrutinib in CLL: Trial results
The expanded approval for ibrutinib is based on results of the phase 3 RESONATE trial, which were presented at this year’s ASCO and EHA meetings.
The trial included 391 previously treated patients, 127 of whom had 17p deletion. Patients were randomized to receive ibrutinib or the anti-CD20 monoclonal antibody ofatumumab until disease progression or unacceptable toxicity.
The trial was stopped early after a pre-planned interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
At the time of interim analysis, the patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm.
Ibrutinib significantly prolonged progression-free and overall survival. The median progression-free survival was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Of the 127 patients with 17p deletion, those treated with ibrutinib experienced a 75% reduction in the risk of disease progression or death.
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
Ibrutinib in development
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, mantle cell lymphoma (MCL), Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Before the FDA granted ibrutinib accelerated approval in CLL, the agency granted the drug accelerated approval for use in previously treated MCL patients. Studies to verify ibrutinib’s clinical benefit in MCL are ongoing.
The European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended marketing authorization for ibrutinib to treat adults with relapsed or refractory MCL.
The committee has also recommended the drug for adults with CLL who have received at least 1 prior therapy and CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy.
Ibrutinib is already approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen Biotech and Pharmacyclics, Inc. The companies co-market ibrutinib in the US, but Janssen markets (or will market) ibrutinib in the rest of the world.

Credit: Steven Harbour
The US Food and Drug Administration (FDA) has expanded the approved use of ibrutinib (Imbruvica) in patients with chronic lymphocytic leukemia (CLL).
The agency previously granted the drug accelerated approval to treat CLL patients who had received at least 1 prior therapy.
Now, the FDA has granted ibrutinib full approval for that indication and expanded the drug’s approved use to include previously treated and untreated CLL patients with 17p deletion.
The FDA’s decision to grant ibrutinib accelerated approval in CLL was based on the drug’s ability to elicit responses in previously treated patients.
Recent trial results have shown the drug can improve survival rates in CLL, which signifies a clinical benefit and allows the FDA to grant ibrutinib full approval.
Ibrutinib in CLL: Trial results
The expanded approval for ibrutinib is based on results of the phase 3 RESONATE trial, which were presented at this year’s ASCO and EHA meetings.
The trial included 391 previously treated patients, 127 of whom had 17p deletion. Patients were randomized to receive ibrutinib or the anti-CD20 monoclonal antibody ofatumumab until disease progression or unacceptable toxicity.
The trial was stopped early after a pre-planned interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
At the time of interim analysis, the patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm.
Ibrutinib significantly prolonged progression-free and overall survival. The median progression-free survival was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Of the 127 patients with 17p deletion, those treated with ibrutinib experienced a 75% reduction in the risk of disease progression or death.
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
Ibrutinib in development
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, mantle cell lymphoma (MCL), Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Before the FDA granted ibrutinib accelerated approval in CLL, the agency granted the drug accelerated approval for use in previously treated MCL patients. Studies to verify ibrutinib’s clinical benefit in MCL are ongoing.
The European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended marketing authorization for ibrutinib to treat adults with relapsed or refractory MCL.
The committee has also recommended the drug for adults with CLL who have received at least 1 prior therapy and CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy.
Ibrutinib is already approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen Biotech and Pharmacyclics, Inc. The companies co-market ibrutinib in the US, but Janssen markets (or will market) ibrutinib in the rest of the world.
CHMP recommends ibrutinib for CLL, MCL

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for ibrutinib (Imbruvica).
The committee is endorsing the Bruton’s tyrosine kinase (BTK) inhibitor for use in adults with relapsed or refractory mantle cell lymphoma (MCL) and certain adults with chronic lymphocytic leukemia (CLL).
This includes untreated CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy and patients who have received at least 1 prior therapy.
The European Commission will take the CHMP’s opinion into account when deciding whether to authorize the commercialization of ibrutinib in the European Union.
The CHMP based its recommendations on data from 2 CLL studies—the phase 3 RESONATE trial (PCYC-1112) and a phase 1b/2 trial (PCYC-1102)—as well as a phase 2 trial (PCYC-1104) in MCL.
RESONATE trial
Results of RESONATE were recently presented at the 2014 EHA Congress. The trial included 391 patients with relapsed or refractory CLL or small lymphocytic lymphoma (SLL).
Patients were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia.
PCYC-1104 trial: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
About ibrutinib
Ibrutinib works by inhibiting BTK, a protein involved in mediating the cellular signaling pathways that control B-cell maturation and survival. In malignant B cells, there is excessive signaling through the B-cell receptor signaling pathway, which includes BTK.
Ibrutinib forms a strong covalent bond with BTK, which inhibits the excessive transmission of cell survival signals within the malignant B cells and stops their excessive build-up in protected environmental areas such as the lymph nodes.
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, MCL, Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Ibrutinib received accelerated approval from the US Food and Drug Administration in November 2013 to treat MCL. The drug received accelerated approval in February 2014 to treat CLL patients who have received at least 1 prior therapy.
Ibrutinib is also approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen and Pharmacyclics. The companies co-market ibrutinib in the US, but, pending the drug’s approval, Janssen will market ibrutinib in the rest of the world.

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for ibrutinib (Imbruvica).
The committee is endorsing the Bruton’s tyrosine kinase (BTK) inhibitor for use in adults with relapsed or refractory mantle cell lymphoma (MCL) and certain adults with chronic lymphocytic leukemia (CLL).
This includes untreated CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy and patients who have received at least 1 prior therapy.
The European Commission will take the CHMP’s opinion into account when deciding whether to authorize the commercialization of ibrutinib in the European Union.
The CHMP based its recommendations on data from 2 CLL studies—the phase 3 RESONATE trial (PCYC-1112) and a phase 1b/2 trial (PCYC-1102)—as well as a phase 2 trial (PCYC-1104) in MCL.
RESONATE trial
Results of RESONATE were recently presented at the 2014 EHA Congress. The trial included 391 patients with relapsed or refractory CLL or small lymphocytic lymphoma (SLL).
Patients were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia.
PCYC-1104 trial: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
About ibrutinib
Ibrutinib works by inhibiting BTK, a protein involved in mediating the cellular signaling pathways that control B-cell maturation and survival. In malignant B cells, there is excessive signaling through the B-cell receptor signaling pathway, which includes BTK.
Ibrutinib forms a strong covalent bond with BTK, which inhibits the excessive transmission of cell survival signals within the malignant B cells and stops their excessive build-up in protected environmental areas such as the lymph nodes.
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, MCL, Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Ibrutinib received accelerated approval from the US Food and Drug Administration in November 2013 to treat MCL. The drug received accelerated approval in February 2014 to treat CLL patients who have received at least 1 prior therapy.
Ibrutinib is also approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen and Pharmacyclics. The companies co-market ibrutinib in the US, but, pending the drug’s approval, Janssen will market ibrutinib in the rest of the world.

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for ibrutinib (Imbruvica).
The committee is endorsing the Bruton’s tyrosine kinase (BTK) inhibitor for use in adults with relapsed or refractory mantle cell lymphoma (MCL) and certain adults with chronic lymphocytic leukemia (CLL).
This includes untreated CLL patients with 17p deletion or TP53 mutation who cannot receive chemo-immunotherapy and patients who have received at least 1 prior therapy.
The European Commission will take the CHMP’s opinion into account when deciding whether to authorize the commercialization of ibrutinib in the European Union.
The CHMP based its recommendations on data from 2 CLL studies—the phase 3 RESONATE trial (PCYC-1112) and a phase 1b/2 trial (PCYC-1102)—as well as a phase 2 trial (PCYC-1104) in MCL.
RESONATE trial
Results of RESONATE were recently presented at the 2014 EHA Congress. The trial included 391 patients with relapsed or refractory CLL or small lymphocytic lymphoma (SLL).
Patients were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia.
PCYC-1104 trial: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
About ibrutinib
Ibrutinib works by inhibiting BTK, a protein involved in mediating the cellular signaling pathways that control B-cell maturation and survival. In malignant B cells, there is excessive signaling through the B-cell receptor signaling pathway, which includes BTK.
Ibrutinib forms a strong covalent bond with BTK, which inhibits the excessive transmission of cell survival signals within the malignant B cells and stops their excessive build-up in protected environmental areas such as the lymph nodes.
Ibrutinib is being studied alone and in combination with other treatments in several hematologic malignancies, including CLL, MCL, Waldenstrom’s macroglobulinemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma.
Ibrutinib received accelerated approval from the US Food and Drug Administration in November 2013 to treat MCL. The drug received accelerated approval in February 2014 to treat CLL patients who have received at least 1 prior therapy.
Ibrutinib is also approved in Israel for the treatment of adults with MCL who have received at least 1 prior therapy.
Ibrutinib is under development by Janssen and Pharmacyclics. The companies co-market ibrutinib in the US, but, pending the drug’s approval, Janssen will market ibrutinib in the rest of the world.