User login
Rapid response to chemotherapy in a patient with thymoma with pericardial effusion
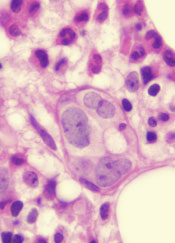
Thymomas are rare tumors involving the anterior mediastinum. Localized thymomas can be surgically resected and have an excellent prognosis, whereas advanced thymomas are treated with induction chemotherapy followed by surgery. We report here the successful use of chemotherapy in a woman with stage IV thymoma with pericardial infiltration and effusion. Our experience suggests that aggressive chemotherapy with a cisplatin- and adriamycin-based regimen can lead to rapid regression of thymoma and significant improvement in symptoms.
*Click on the link to the left for a PDF of the full article.
Thymomas are rare tumors involving the anterior mediastinum. Localized thymomas can be surgically resected and have an excellent prognosis, whereas advanced thymomas are treated with induction chemotherapy followed by surgery. We report here the successful use of chemotherapy in a woman with stage IV thymoma with pericardial infiltration and effusion. Our experience suggests that aggressive chemotherapy with a cisplatin- and adriamycin-based regimen can lead to rapid regression of thymoma and significant improvement in symptoms.
*Click on the link to the left for a PDF of the full article.
Thymomas are rare tumors involving the anterior mediastinum. Localized thymomas can be surgically resected and have an excellent prognosis, whereas advanced thymomas are treated with induction chemotherapy followed by surgery. We report here the successful use of chemotherapy in a woman with stage IV thymoma with pericardial infiltration and effusion. Our experience suggests that aggressive chemotherapy with a cisplatin- and adriamycin-based regimen can lead to rapid regression of thymoma and significant improvement in symptoms.
*Click on the link to the left for a PDF of the full article.
NICE again rejects pixantrone for NHL
The UK’s National Institute for Health and Care Excellence (NICE) has re-examined its draft guidance for pixantrone (Pixuvri) but come to the same conclusion as before.
The organization is still not recommending pixantrone monotherapy to treat multiply relapsed or refractory B-cell non-Hodgkin lymphoma (NHL).
Of course, this recommendation may change, as this is not NICE’s final guidance on pixantrone.
For this second consultation on the draft guidance, an independent appraisal committee re-examined the clinical and cost-effectiveness of pixantrone.
This time, the committee took into consideration a patient access scheme submitted by pixantrone’s manufacturer, Cell Therapeutics. The scheme was designed to make the drug more cost-effective for the National Health Service (NHS).
“Unfortunately, the committee concluded that this scheme . . . does not overcome the uncertainties in the evidence for the drug’s clinical effectiveness over and above current treatments for this disease,” said NICE Chief Executive Sir Andrew Dillon.
In fact, the committee found the scheme did not make pixantrone cost-effective according to the accepted definition—costing £20,000 to £30,000 per quality-adjusted life year (QALY) gained.
Evaluating trial data
When considering the clinical effectiveness of pixantrone, the appraisal committee analyzed data from the EXTEND PIX301 trial, which was submitted by the manufacturer.
The trial enrolled adults with aggressive, de novo, or transformed NHL that had relapsed after 2 or more chemotherapy regimens, including at least 1 standard anthracycline-containing regimen with a response that lasted at least 24 weeks. Seventy patients were randomized to pixantrone, and 70 were randomized to a physician’s choice of single-agent comparators.
The committee pointed out a number of uncertainties associated with the trial. One was that it did not include the planned number of patients (which was 320), so it may not have been sufficiently powered to detect differences between the treatment arms.
Another concern was that the trial’s primary endpoint was complete or unconfirmed complete response, rather than overall survival or progression-free survival. In fact, there was a lack of statistically significant difference in overall survival between treatment arms. And other differences between the treatment arms were not always statistically significant.
These factors led the committee to conclude that there is insufficient evidence to suggest pixantrone is more clinically effective than treatments currently used in clinical practice.
Suitability for the UK
The appraisal committee also heard evidence from clinical experts and patient representatives. This information revealed differences in previous treatment between the PIX301 trial population and UK clinical practice.
Therefore, the committee said it could not determine the clinical effectiveness of pixantrone for a UK population.
In addition, there is doubt regarding the clinical benefit of pixantrone in patients who previously received rituximab. And this applies to virtually all patients with relapsed or refractory aggressive B-cell lymphoma in England and Wales, the committee noted.
(The European Medicines Agency’s conditional approval of pixantrone stipulated that an additional trial must confirm the clinical benefit of the drug in patients who have previously received rituximab.)
Calculating costs
The committee estimated the patient access scheme for pixantrone would most likely result in an incremental cost-effectiveness ratio of £30,700 per QALY gained. This is above the range normally considered to be cost-effective—usually £20,000 to £30,000 per QALY gained.
This factor, along with the lack of clinical effectiveness, prompted the committee to conclude that pixantrone would not be a cost-effective use of NHS resources.
According to Cell Therapeutics, pixantrone costs £553.50 per 20 mL vial. The recommended dosage of pixantrone is 50 mg/m2 on days 1, 8, and 15 of each 28-day cycle, for up to 6 cycles.
The estimated cost of a course of treatment is £19,926. This is based on the median length of treatment in the PIX301 trial—4 cycles, using an average of 3 vials per dose.
About the guidance
Individuals can comment on the pixantrone draft guidance via the NICE website. It is open until November 4, 2013.
This is the third version of the draft guidance published and the second consultation launched. The draft guidance was initially published for consultation in April 2013, followed by a final draft guidance in June 2013. But this document was withdrawn during the appeal stage because the manufacturer submitted the patient access scheme.
Until the final guidance is issued to the NHS, organizations should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

The UK’s National Institute for Health and Care Excellence (NICE) has re-examined its draft guidance for pixantrone (Pixuvri) but come to the same conclusion as before.
The organization is still not recommending pixantrone monotherapy to treat multiply relapsed or refractory B-cell non-Hodgkin lymphoma (NHL).
Of course, this recommendation may change, as this is not NICE’s final guidance on pixantrone.
For this second consultation on the draft guidance, an independent appraisal committee re-examined the clinical and cost-effectiveness of pixantrone.
This time, the committee took into consideration a patient access scheme submitted by pixantrone’s manufacturer, Cell Therapeutics. The scheme was designed to make the drug more cost-effective for the National Health Service (NHS).
“Unfortunately, the committee concluded that this scheme . . . does not overcome the uncertainties in the evidence for the drug’s clinical effectiveness over and above current treatments for this disease,” said NICE Chief Executive Sir Andrew Dillon.
In fact, the committee found the scheme did not make pixantrone cost-effective according to the accepted definition—costing £20,000 to £30,000 per quality-adjusted life year (QALY) gained.
Evaluating trial data
When considering the clinical effectiveness of pixantrone, the appraisal committee analyzed data from the EXTEND PIX301 trial, which was submitted by the manufacturer.
The trial enrolled adults with aggressive, de novo, or transformed NHL that had relapsed after 2 or more chemotherapy regimens, including at least 1 standard anthracycline-containing regimen with a response that lasted at least 24 weeks. Seventy patients were randomized to pixantrone, and 70 were randomized to a physician’s choice of single-agent comparators.
The committee pointed out a number of uncertainties associated with the trial. One was that it did not include the planned number of patients (which was 320), so it may not have been sufficiently powered to detect differences between the treatment arms.
Another concern was that the trial’s primary endpoint was complete or unconfirmed complete response, rather than overall survival or progression-free survival. In fact, there was a lack of statistically significant difference in overall survival between treatment arms. And other differences between the treatment arms were not always statistically significant.
These factors led the committee to conclude that there is insufficient evidence to suggest pixantrone is more clinically effective than treatments currently used in clinical practice.
Suitability for the UK
The appraisal committee also heard evidence from clinical experts and patient representatives. This information revealed differences in previous treatment between the PIX301 trial population and UK clinical practice.
Therefore, the committee said it could not determine the clinical effectiveness of pixantrone for a UK population.
In addition, there is doubt regarding the clinical benefit of pixantrone in patients who previously received rituximab. And this applies to virtually all patients with relapsed or refractory aggressive B-cell lymphoma in England and Wales, the committee noted.
(The European Medicines Agency’s conditional approval of pixantrone stipulated that an additional trial must confirm the clinical benefit of the drug in patients who have previously received rituximab.)
Calculating costs
The committee estimated the patient access scheme for pixantrone would most likely result in an incremental cost-effectiveness ratio of £30,700 per QALY gained. This is above the range normally considered to be cost-effective—usually £20,000 to £30,000 per QALY gained.
This factor, along with the lack of clinical effectiveness, prompted the committee to conclude that pixantrone would not be a cost-effective use of NHS resources.
According to Cell Therapeutics, pixantrone costs £553.50 per 20 mL vial. The recommended dosage of pixantrone is 50 mg/m2 on days 1, 8, and 15 of each 28-day cycle, for up to 6 cycles.
The estimated cost of a course of treatment is £19,926. This is based on the median length of treatment in the PIX301 trial—4 cycles, using an average of 3 vials per dose.
About the guidance
Individuals can comment on the pixantrone draft guidance via the NICE website. It is open until November 4, 2013.
This is the third version of the draft guidance published and the second consultation launched. The draft guidance was initially published for consultation in April 2013, followed by a final draft guidance in June 2013. But this document was withdrawn during the appeal stage because the manufacturer submitted the patient access scheme.
Until the final guidance is issued to the NHS, organizations should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

The UK’s National Institute for Health and Care Excellence (NICE) has re-examined its draft guidance for pixantrone (Pixuvri) but come to the same conclusion as before.
The organization is still not recommending pixantrone monotherapy to treat multiply relapsed or refractory B-cell non-Hodgkin lymphoma (NHL).
Of course, this recommendation may change, as this is not NICE’s final guidance on pixantrone.
For this second consultation on the draft guidance, an independent appraisal committee re-examined the clinical and cost-effectiveness of pixantrone.
This time, the committee took into consideration a patient access scheme submitted by pixantrone’s manufacturer, Cell Therapeutics. The scheme was designed to make the drug more cost-effective for the National Health Service (NHS).
“Unfortunately, the committee concluded that this scheme . . . does not overcome the uncertainties in the evidence for the drug’s clinical effectiveness over and above current treatments for this disease,” said NICE Chief Executive Sir Andrew Dillon.
In fact, the committee found the scheme did not make pixantrone cost-effective according to the accepted definition—costing £20,000 to £30,000 per quality-adjusted life year (QALY) gained.
Evaluating trial data
When considering the clinical effectiveness of pixantrone, the appraisal committee analyzed data from the EXTEND PIX301 trial, which was submitted by the manufacturer.
The trial enrolled adults with aggressive, de novo, or transformed NHL that had relapsed after 2 or more chemotherapy regimens, including at least 1 standard anthracycline-containing regimen with a response that lasted at least 24 weeks. Seventy patients were randomized to pixantrone, and 70 were randomized to a physician’s choice of single-agent comparators.
The committee pointed out a number of uncertainties associated with the trial. One was that it did not include the planned number of patients (which was 320), so it may not have been sufficiently powered to detect differences between the treatment arms.
Another concern was that the trial’s primary endpoint was complete or unconfirmed complete response, rather than overall survival or progression-free survival. In fact, there was a lack of statistically significant difference in overall survival between treatment arms. And other differences between the treatment arms were not always statistically significant.
These factors led the committee to conclude that there is insufficient evidence to suggest pixantrone is more clinically effective than treatments currently used in clinical practice.
Suitability for the UK
The appraisal committee also heard evidence from clinical experts and patient representatives. This information revealed differences in previous treatment between the PIX301 trial population and UK clinical practice.
Therefore, the committee said it could not determine the clinical effectiveness of pixantrone for a UK population.
In addition, there is doubt regarding the clinical benefit of pixantrone in patients who previously received rituximab. And this applies to virtually all patients with relapsed or refractory aggressive B-cell lymphoma in England and Wales, the committee noted.
(The European Medicines Agency’s conditional approval of pixantrone stipulated that an additional trial must confirm the clinical benefit of the drug in patients who have previously received rituximab.)
Calculating costs
The committee estimated the patient access scheme for pixantrone would most likely result in an incremental cost-effectiveness ratio of £30,700 per QALY gained. This is above the range normally considered to be cost-effective—usually £20,000 to £30,000 per QALY gained.
This factor, along with the lack of clinical effectiveness, prompted the committee to conclude that pixantrone would not be a cost-effective use of NHS resources.
According to Cell Therapeutics, pixantrone costs £553.50 per 20 mL vial. The recommended dosage of pixantrone is 50 mg/m2 on days 1, 8, and 15 of each 28-day cycle, for up to 6 cycles.
The estimated cost of a course of treatment is £19,926. This is based on the median length of treatment in the PIX301 trial—4 cycles, using an average of 3 vials per dose.
About the guidance
Individuals can comment on the pixantrone draft guidance via the NICE website. It is open until November 4, 2013.
This is the third version of the draft guidance published and the second consultation launched. The draft guidance was initially published for consultation in April 2013, followed by a final draft guidance in June 2013. But this document was withdrawn during the appeal stage because the manufacturer submitted the patient access scheme.
Until the final guidance is issued to the NHS, organizations should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()
Diagnosis and Management of Immunoglobulin Light Chain Amyloidosis
The term amyloidosis refers to a fascinating group of disorders that share a common pathogenesis of extracellular deposition of amyloid material. Fundamentally, it is a disorder of the secondary structure of select proteins whereby the amyloidogenic proteins are misfolded into a β-pleated sheet configuration, resulting in the formation of insoluble extracellular amyloid fibrils. The amyloid fibrils appear as amorphous eosinophilic material when hematoxylin and eosin–stained tissue is examined under light microscope. Electron microscopy reveals remarkable similarity between the amyloid fibrils derived from different precursor proteins in that they range from 7.5 to 10 nm in diameter. This ultrastructural similarity is the underlying basis for the characteristic red-green birefringence with Congo red staining observed under polarized microscopy, the pathological hallmark of the disease.
To read the full article in PDF:
The term amyloidosis refers to a fascinating group of disorders that share a common pathogenesis of extracellular deposition of amyloid material. Fundamentally, it is a disorder of the secondary structure of select proteins whereby the amyloidogenic proteins are misfolded into a β-pleated sheet configuration, resulting in the formation of insoluble extracellular amyloid fibrils. The amyloid fibrils appear as amorphous eosinophilic material when hematoxylin and eosin–stained tissue is examined under light microscope. Electron microscopy reveals remarkable similarity between the amyloid fibrils derived from different precursor proteins in that they range from 7.5 to 10 nm in diameter. This ultrastructural similarity is the underlying basis for the characteristic red-green birefringence with Congo red staining observed under polarized microscopy, the pathological hallmark of the disease.
To read the full article in PDF:
The term amyloidosis refers to a fascinating group of disorders that share a common pathogenesis of extracellular deposition of amyloid material. Fundamentally, it is a disorder of the secondary structure of select proteins whereby the amyloidogenic proteins are misfolded into a β-pleated sheet configuration, resulting in the formation of insoluble extracellular amyloid fibrils. The amyloid fibrils appear as amorphous eosinophilic material when hematoxylin and eosin–stained tissue is examined under light microscope. Electron microscopy reveals remarkable similarity between the amyloid fibrils derived from different precursor proteins in that they range from 7.5 to 10 nm in diameter. This ultrastructural similarity is the underlying basis for the characteristic red-green birefringence with Congo red staining observed under polarized microscopy, the pathological hallmark of the disease.
To read the full article in PDF:
Pixantrone gaining market access in EU

Credit: Bill Branson
The French National Health Authority’s Transparency Commission has granted market access for the antineoplastic drug pixantrone (Pixuvri).
The drug is intended for use as monotherapy to treat adult patients with aggressive B-cell non-Hodgkin lymphoma who have failed 2 or 3 prior lines of therapy.
Pixantrone has already gained conditional marketing authorization for this indication within the European Union.
In the fourth quarter of 2012, the drug was made available to patients in 8 countries—Sweden, Denmark, Finland, Austria, Norway, Germany, the UK, and the Netherlands.
And last month, pixantrone was granted market access in Italy.
Now, the drug’s developers, Cell Therapeutics Inc., announced that pixantrone has been granted market access in France.
The next steps in France’s pharmaceutical reimbursement process are inclusion on the list of medicines approved for hospital use and subsequent publication in the Journal Officiel. And Cell Therapeutics intends to pursue these final goals.
All registered pharmaceuticals in France are subjected to a process known as Evaluation of Therapeutic Benefit, with the resulting evaluation expressed as a classification between 1 and 6.
The Transparency Commission rated pixantrone at level 5, which allows the drug to be included in the reimbursed drugs list for hospital use. The commission will reassess the rating for the drug within 2 years.
France’s authorization and the European Commission’s conditional marketing authorization are based on data from the phase 3 EXTEND PIX301 trial. Although pixantrone prompted positive results in this trial, the US Food and Drug Administration (FDA) has expressed concerns about the number of patients included.
In fact, the FDA rejected a new drug application for pixantrone in 2010. Cell Therapeutics later resubmitted an application for the drug but withdrew it in January of last year. The company has not confirmed plans to refile with the FDA.
As for the European Commission’s conditional approval of pixantrone, it will be renewed on a yearly basis until Cell Therapeutics fullfills its committment to
provide additional data on patients
treated with pixantrone who previously received rituximab. The company said it expects to
have the results of this research by mid-2015.
Pixantrone is a novel aza-anthracenedione that forms stable DNA adducts. The drug was designed so that it cannot bind iron and perpetuate oxygen radical production or form a long-lived hydroxyl metabolite—both of which are the putative mechanisms for anthracycline-induced acute and chronic cardiotoxicity.
For full prescribing information for pixantrone, including the safety and efficacy profile in the approved indication, visit www.pixuvri.eu. ![]()

Credit: Bill Branson
The French National Health Authority’s Transparency Commission has granted market access for the antineoplastic drug pixantrone (Pixuvri).
The drug is intended for use as monotherapy to treat adult patients with aggressive B-cell non-Hodgkin lymphoma who have failed 2 or 3 prior lines of therapy.
Pixantrone has already gained conditional marketing authorization for this indication within the European Union.
In the fourth quarter of 2012, the drug was made available to patients in 8 countries—Sweden, Denmark, Finland, Austria, Norway, Germany, the UK, and the Netherlands.
And last month, pixantrone was granted market access in Italy.
Now, the drug’s developers, Cell Therapeutics Inc., announced that pixantrone has been granted market access in France.
The next steps in France’s pharmaceutical reimbursement process are inclusion on the list of medicines approved for hospital use and subsequent publication in the Journal Officiel. And Cell Therapeutics intends to pursue these final goals.
All registered pharmaceuticals in France are subjected to a process known as Evaluation of Therapeutic Benefit, with the resulting evaluation expressed as a classification between 1 and 6.
The Transparency Commission rated pixantrone at level 5, which allows the drug to be included in the reimbursed drugs list for hospital use. The commission will reassess the rating for the drug within 2 years.
France’s authorization and the European Commission’s conditional marketing authorization are based on data from the phase 3 EXTEND PIX301 trial. Although pixantrone prompted positive results in this trial, the US Food and Drug Administration (FDA) has expressed concerns about the number of patients included.
In fact, the FDA rejected a new drug application for pixantrone in 2010. Cell Therapeutics later resubmitted an application for the drug but withdrew it in January of last year. The company has not confirmed plans to refile with the FDA.
As for the European Commission’s conditional approval of pixantrone, it will be renewed on a yearly basis until Cell Therapeutics fullfills its committment to
provide additional data on patients
treated with pixantrone who previously received rituximab. The company said it expects to
have the results of this research by mid-2015.
Pixantrone is a novel aza-anthracenedione that forms stable DNA adducts. The drug was designed so that it cannot bind iron and perpetuate oxygen radical production or form a long-lived hydroxyl metabolite—both of which are the putative mechanisms for anthracycline-induced acute and chronic cardiotoxicity.
For full prescribing information for pixantrone, including the safety and efficacy profile in the approved indication, visit www.pixuvri.eu. ![]()

Credit: Bill Branson
The French National Health Authority’s Transparency Commission has granted market access for the antineoplastic drug pixantrone (Pixuvri).
The drug is intended for use as monotherapy to treat adult patients with aggressive B-cell non-Hodgkin lymphoma who have failed 2 or 3 prior lines of therapy.
Pixantrone has already gained conditional marketing authorization for this indication within the European Union.
In the fourth quarter of 2012, the drug was made available to patients in 8 countries—Sweden, Denmark, Finland, Austria, Norway, Germany, the UK, and the Netherlands.
And last month, pixantrone was granted market access in Italy.
Now, the drug’s developers, Cell Therapeutics Inc., announced that pixantrone has been granted market access in France.
The next steps in France’s pharmaceutical reimbursement process are inclusion on the list of medicines approved for hospital use and subsequent publication in the Journal Officiel. And Cell Therapeutics intends to pursue these final goals.
All registered pharmaceuticals in France are subjected to a process known as Evaluation of Therapeutic Benefit, with the resulting evaluation expressed as a classification between 1 and 6.
The Transparency Commission rated pixantrone at level 5, which allows the drug to be included in the reimbursed drugs list for hospital use. The commission will reassess the rating for the drug within 2 years.
France’s authorization and the European Commission’s conditional marketing authorization are based on data from the phase 3 EXTEND PIX301 trial. Although pixantrone prompted positive results in this trial, the US Food and Drug Administration (FDA) has expressed concerns about the number of patients included.
In fact, the FDA rejected a new drug application for pixantrone in 2010. Cell Therapeutics later resubmitted an application for the drug but withdrew it in January of last year. The company has not confirmed plans to refile with the FDA.
As for the European Commission’s conditional approval of pixantrone, it will be renewed on a yearly basis until Cell Therapeutics fullfills its committment to
provide additional data on patients
treated with pixantrone who previously received rituximab. The company said it expects to
have the results of this research by mid-2015.
Pixantrone is a novel aza-anthracenedione that forms stable DNA adducts. The drug was designed so that it cannot bind iron and perpetuate oxygen radical production or form a long-lived hydroxyl metabolite—both of which are the putative mechanisms for anthracycline-induced acute and chronic cardiotoxicity.
For full prescribing information for pixantrone, including the safety and efficacy profile in the approved indication, visit www.pixuvri.eu. ![]()
Immune microenvironment linked to prognosis of follicular lymphoma
Tumor-induced genetic changes in the immune cells of lymph nodes predict outcomes in patients with follicular lymphoma, according to a study published in the Journal of Clinical Oncology.
Investigators performed gene expression profiling of tumor-infiltrating T cells (TILs) from lymph node biopsies at diagnosis in 172 treatment-naive patients with follicular lymphoma and of T cells from reactive tonsils and peripheral blood of 12 healthy donors.
Compared with the T cells from healthy donors, the TILs had marked upregulation of several genes and downregulation of others, as well as impaired motility. Moreover, similar changes could be induced in healthy T cells by exposing them to lymphoma cells, said Dr. Shahryar Kiaii of the Institute of Cancer and Barts and the London School of Medicine and Dentistry and his associates.
The numbers and locations within the lymph nodes of the TILs showing altered gene expression predicted both overall survival and the time to transformation to B-cell lymphoma – sometimes dramatically. Certain combinations were associated with 70%-80% reductions in the risks of these outcomes.
"These results contribute to our understanding of the complex interactions of lymphoma cells, TILs, and macrophages in their microenvironment and help us generate hypotheses. But until we have a better understanding of these interactions, it does not yet seem feasible to incorporate [immunohistochemistry] analysis of TILs in [follicular lymphoma] for prognosis," the investigators wrote.
"However, because nonmalignant infiltrating immune cells play a crucial role in outcomes in [follicular lymphoma], understanding the nature and impact of the abnormalities induced in TILs in these patients is vital before any immunotherapeutic strategies can be implemented to alter the immune microenvironment in [follicular lymphoma]," they added.
In the study, the investigators constructed tissue microarrays and used mRNA expression profiles, real-time polymerase chain reaction assays, and immunohistochemistry to assess gene expression in highly purified CD4 and CD8 T cells.
Results showed that the TILs had an abnormal gene expression profile when compared with the healthy T cells, Dr. Kiaii and his associates said (J. Clin. Oncol. 2013;31:2654-61).
The genes showing the greatest upregulation were those for pro-melanin–concentrating hormone (PMCH); ETS translocation variant 1 (ETV1); and tumor necrosis factor receptor superfamily, member 9 (TNFRSF9).
One of the genes showing greatest downregulation was the gene that encodes the cytoskeletal protein actinin (ACTN1), and the TILs indeed showed reduced motility when compared with the healthy T cells (P less than .025).
When cultured alone, healthy T cells did not express PMCH and had normal motility, but when cultured with follicular lymphoma cells, the T cells expressed this protein highly and had reduced motility (P = .0002).
The number of TILs expressing PMCH, ETV1, and NAMPT (nicotinamide phosphoribosyltransferase) and their locations in lymph nodes – in the malignant follicle (intrafollicular area), in the area between follicles (interfollicular area), and overall – as determined immunohistochemically, were significantly associated with both overall survival and time to transformation, the investigators said.
In multivariate analyses, the combination of the interfollicular-to-intrafollicular ratio of PMCH-expressing cells plus a high level of expression of NAMPT and a low level of expression of ETV1 in the intrafollicular area was the strongest predictor of longer time to transformation (hazard ratio, 0.19; P = .003).
Similarly, the combination of the number of PMCH- and NAMPT-expressing cells in the interfollicular area plus the interfollicular-to-intrafollicular ratio of ETV1 cells was the strongest predictor of better overall survival (hazard ratio, 0.32; P = .007).
The study was supported by grants from Cancer Research UK and the National Cancer Institute. One of the investigators disclosed receiving honoraria from Roche/Genentech and Celgene.
The findings of the study by Dr. Kiaii and his colleagues are particularly important as novel T-cell–mediated therapies are being developed for B-cell malignancies.
Lymphoma cells often induce immune tolerance by deleting or inactivating tumor-specific T cells. One attempt at overcoming this phenomenon has been chimeric antigen receptor (CAR) therapy; however, best results have been restricted to patients who had a low tumor burden and received cytotoxic chemotherapy beforehand.

|
|
The chemotherapy administered was likely to have not only depleted malignant cells but also decreased immunosuppressive cells. ... Malignant B cells, and the immunosuppressive tumor microenvironment they promote, may therefore remain a barrier to effective adoptive immunotherapy in B-cell lymphoma, particularly in patients with chemotherapy-resistant, bulky disease.
The data presented confirm that malignant B cells promote a profoundly immunosuppressive microenvironment and thereby protect themselves from being targeted by the immune system.
Future therapies in follicular lymphoma, including immunotherapies such as CAR T cells, will need to not only deplete malignant B cells but also inhibit the immunosuppressive mechanisms by which malignant B cells suppress the antitumor immune response. A dual approach that both depletes malignant cells and promotes immune function may subsequently result in a better clinical outcome for patients with follicular lymphoma.
Dr. Stephen M. Ansell is professor of medicine at the Mayo Clinic in Rochester, Minn. He made his remarks in an accompanying editorial (J. Clin. Oncol. 2013;31:2641-2). Dr. Ansell disclosed having no relevant conflicts of interest.
The findings of the study by Dr. Kiaii and his colleagues are particularly important as novel T-cell–mediated therapies are being developed for B-cell malignancies.
Lymphoma cells often induce immune tolerance by deleting or inactivating tumor-specific T cells. One attempt at overcoming this phenomenon has been chimeric antigen receptor (CAR) therapy; however, best results have been restricted to patients who had a low tumor burden and received cytotoxic chemotherapy beforehand.

|
|
The chemotherapy administered was likely to have not only depleted malignant cells but also decreased immunosuppressive cells. ... Malignant B cells, and the immunosuppressive tumor microenvironment they promote, may therefore remain a barrier to effective adoptive immunotherapy in B-cell lymphoma, particularly in patients with chemotherapy-resistant, bulky disease.
The data presented confirm that malignant B cells promote a profoundly immunosuppressive microenvironment and thereby protect themselves from being targeted by the immune system.
Future therapies in follicular lymphoma, including immunotherapies such as CAR T cells, will need to not only deplete malignant B cells but also inhibit the immunosuppressive mechanisms by which malignant B cells suppress the antitumor immune response. A dual approach that both depletes malignant cells and promotes immune function may subsequently result in a better clinical outcome for patients with follicular lymphoma.
Dr. Stephen M. Ansell is professor of medicine at the Mayo Clinic in Rochester, Minn. He made his remarks in an accompanying editorial (J. Clin. Oncol. 2013;31:2641-2). Dr. Ansell disclosed having no relevant conflicts of interest.
The findings of the study by Dr. Kiaii and his colleagues are particularly important as novel T-cell–mediated therapies are being developed for B-cell malignancies.
Lymphoma cells often induce immune tolerance by deleting or inactivating tumor-specific T cells. One attempt at overcoming this phenomenon has been chimeric antigen receptor (CAR) therapy; however, best results have been restricted to patients who had a low tumor burden and received cytotoxic chemotherapy beforehand.

|
|
The chemotherapy administered was likely to have not only depleted malignant cells but also decreased immunosuppressive cells. ... Malignant B cells, and the immunosuppressive tumor microenvironment they promote, may therefore remain a barrier to effective adoptive immunotherapy in B-cell lymphoma, particularly in patients with chemotherapy-resistant, bulky disease.
The data presented confirm that malignant B cells promote a profoundly immunosuppressive microenvironment and thereby protect themselves from being targeted by the immune system.
Future therapies in follicular lymphoma, including immunotherapies such as CAR T cells, will need to not only deplete malignant B cells but also inhibit the immunosuppressive mechanisms by which malignant B cells suppress the antitumor immune response. A dual approach that both depletes malignant cells and promotes immune function may subsequently result in a better clinical outcome for patients with follicular lymphoma.
Dr. Stephen M. Ansell is professor of medicine at the Mayo Clinic in Rochester, Minn. He made his remarks in an accompanying editorial (J. Clin. Oncol. 2013;31:2641-2). Dr. Ansell disclosed having no relevant conflicts of interest.
Tumor-induced genetic changes in the immune cells of lymph nodes predict outcomes in patients with follicular lymphoma, according to a study published in the Journal of Clinical Oncology.
Investigators performed gene expression profiling of tumor-infiltrating T cells (TILs) from lymph node biopsies at diagnosis in 172 treatment-naive patients with follicular lymphoma and of T cells from reactive tonsils and peripheral blood of 12 healthy donors.
Compared with the T cells from healthy donors, the TILs had marked upregulation of several genes and downregulation of others, as well as impaired motility. Moreover, similar changes could be induced in healthy T cells by exposing them to lymphoma cells, said Dr. Shahryar Kiaii of the Institute of Cancer and Barts and the London School of Medicine and Dentistry and his associates.
The numbers and locations within the lymph nodes of the TILs showing altered gene expression predicted both overall survival and the time to transformation to B-cell lymphoma – sometimes dramatically. Certain combinations were associated with 70%-80% reductions in the risks of these outcomes.
"These results contribute to our understanding of the complex interactions of lymphoma cells, TILs, and macrophages in their microenvironment and help us generate hypotheses. But until we have a better understanding of these interactions, it does not yet seem feasible to incorporate [immunohistochemistry] analysis of TILs in [follicular lymphoma] for prognosis," the investigators wrote.
"However, because nonmalignant infiltrating immune cells play a crucial role in outcomes in [follicular lymphoma], understanding the nature and impact of the abnormalities induced in TILs in these patients is vital before any immunotherapeutic strategies can be implemented to alter the immune microenvironment in [follicular lymphoma]," they added.
In the study, the investigators constructed tissue microarrays and used mRNA expression profiles, real-time polymerase chain reaction assays, and immunohistochemistry to assess gene expression in highly purified CD4 and CD8 T cells.
Results showed that the TILs had an abnormal gene expression profile when compared with the healthy T cells, Dr. Kiaii and his associates said (J. Clin. Oncol. 2013;31:2654-61).
The genes showing the greatest upregulation were those for pro-melanin–concentrating hormone (PMCH); ETS translocation variant 1 (ETV1); and tumor necrosis factor receptor superfamily, member 9 (TNFRSF9).
One of the genes showing greatest downregulation was the gene that encodes the cytoskeletal protein actinin (ACTN1), and the TILs indeed showed reduced motility when compared with the healthy T cells (P less than .025).
When cultured alone, healthy T cells did not express PMCH and had normal motility, but when cultured with follicular lymphoma cells, the T cells expressed this protein highly and had reduced motility (P = .0002).
The number of TILs expressing PMCH, ETV1, and NAMPT (nicotinamide phosphoribosyltransferase) and their locations in lymph nodes – in the malignant follicle (intrafollicular area), in the area between follicles (interfollicular area), and overall – as determined immunohistochemically, were significantly associated with both overall survival and time to transformation, the investigators said.
In multivariate analyses, the combination of the interfollicular-to-intrafollicular ratio of PMCH-expressing cells plus a high level of expression of NAMPT and a low level of expression of ETV1 in the intrafollicular area was the strongest predictor of longer time to transformation (hazard ratio, 0.19; P = .003).
Similarly, the combination of the number of PMCH- and NAMPT-expressing cells in the interfollicular area plus the interfollicular-to-intrafollicular ratio of ETV1 cells was the strongest predictor of better overall survival (hazard ratio, 0.32; P = .007).
The study was supported by grants from Cancer Research UK and the National Cancer Institute. One of the investigators disclosed receiving honoraria from Roche/Genentech and Celgene.
Tumor-induced genetic changes in the immune cells of lymph nodes predict outcomes in patients with follicular lymphoma, according to a study published in the Journal of Clinical Oncology.
Investigators performed gene expression profiling of tumor-infiltrating T cells (TILs) from lymph node biopsies at diagnosis in 172 treatment-naive patients with follicular lymphoma and of T cells from reactive tonsils and peripheral blood of 12 healthy donors.
Compared with the T cells from healthy donors, the TILs had marked upregulation of several genes and downregulation of others, as well as impaired motility. Moreover, similar changes could be induced in healthy T cells by exposing them to lymphoma cells, said Dr. Shahryar Kiaii of the Institute of Cancer and Barts and the London School of Medicine and Dentistry and his associates.
The numbers and locations within the lymph nodes of the TILs showing altered gene expression predicted both overall survival and the time to transformation to B-cell lymphoma – sometimes dramatically. Certain combinations were associated with 70%-80% reductions in the risks of these outcomes.
"These results contribute to our understanding of the complex interactions of lymphoma cells, TILs, and macrophages in their microenvironment and help us generate hypotheses. But until we have a better understanding of these interactions, it does not yet seem feasible to incorporate [immunohistochemistry] analysis of TILs in [follicular lymphoma] for prognosis," the investigators wrote.
"However, because nonmalignant infiltrating immune cells play a crucial role in outcomes in [follicular lymphoma], understanding the nature and impact of the abnormalities induced in TILs in these patients is vital before any immunotherapeutic strategies can be implemented to alter the immune microenvironment in [follicular lymphoma]," they added.
In the study, the investigators constructed tissue microarrays and used mRNA expression profiles, real-time polymerase chain reaction assays, and immunohistochemistry to assess gene expression in highly purified CD4 and CD8 T cells.
Results showed that the TILs had an abnormal gene expression profile when compared with the healthy T cells, Dr. Kiaii and his associates said (J. Clin. Oncol. 2013;31:2654-61).
The genes showing the greatest upregulation were those for pro-melanin–concentrating hormone (PMCH); ETS translocation variant 1 (ETV1); and tumor necrosis factor receptor superfamily, member 9 (TNFRSF9).
One of the genes showing greatest downregulation was the gene that encodes the cytoskeletal protein actinin (ACTN1), and the TILs indeed showed reduced motility when compared with the healthy T cells (P less than .025).
When cultured alone, healthy T cells did not express PMCH and had normal motility, but when cultured with follicular lymphoma cells, the T cells expressed this protein highly and had reduced motility (P = .0002).
The number of TILs expressing PMCH, ETV1, and NAMPT (nicotinamide phosphoribosyltransferase) and their locations in lymph nodes – in the malignant follicle (intrafollicular area), in the area between follicles (interfollicular area), and overall – as determined immunohistochemically, were significantly associated with both overall survival and time to transformation, the investigators said.
In multivariate analyses, the combination of the interfollicular-to-intrafollicular ratio of PMCH-expressing cells plus a high level of expression of NAMPT and a low level of expression of ETV1 in the intrafollicular area was the strongest predictor of longer time to transformation (hazard ratio, 0.19; P = .003).
Similarly, the combination of the number of PMCH- and NAMPT-expressing cells in the interfollicular area plus the interfollicular-to-intrafollicular ratio of ETV1 cells was the strongest predictor of better overall survival (hazard ratio, 0.32; P = .007).
The study was supported by grants from Cancer Research UK and the National Cancer Institute. One of the investigators disclosed receiving honoraria from Roche/Genentech and Celgene.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Major finding: The combination of the number of PMCH- and NAMPT-expressing cells in the interfollicular area plus the interfollicular-to-intrafollicular ratio of ETV1 cells was the strongest predictor of better overall survival (hazard ratio, 0.32; P = .007).
Data source: A gene expression profiling study in 172 patients with follicular lymphoma and 12 healthy individuals.
Disclosures: The study was supported by grants from Cancer Research UK and the National Cancer Institute. One of the investigators disclosed receiving honoraria from Roche/Genentech and Celgene.
Early lenalidomide delays progression of smoldering multiple myeloma
Early oral lenalidomide-based treatment significantly delayed disease progression in an industry-sponsored phase III study of patients who had high-risk smoldering multiple myeloma.
The delay in disease progression with early lenalidomide "translated into a significant overall survival benefit; the proportion of patients who were alive at 3 years was 94% in the treatment group versus 80% in the observation group," reported Dr. María-Victoria Mateos of the Hospital Universitario de Salamanca (Spain) and her associates in the New England Journal of Medicine.
The study findings suggest that patients with smoldering multiple myeloma who are at high risk of progression to active disease should be targeted for early intervention (N. Engl. J. Med. 2013;369:438-47 [doi: 10.1056/NEJMoa1300439]).
Three years after entry into the study, 77% of the patients who received lenalidomide had progression-free survival, compared with only 30% of those who received usual care. Usual care involved simple observation, since there are no other therapeutic options in the early stage of the disease.
The treatment response rate was 79% during the induction phase to reduce the tumor burden, which increased to 90% during the maintenance phase. The regimen’s toxicity was judged to be moderate, and the incidence of second primary tumors was low.
The overall risk of progression with smoldering multiple myeloma is low, at 10% per year. However, a large subgroup of patients has been identified as high risk, with a 50% or more probability of progression to active disease within 2 years of diagnosis. Early intervention rather than observation has been attempted for this subgroup, but, so far, alkylating agents, bisphosphonates, interleukin-1B receptor antagonists, and thalidomide have failed to show clinical benefit.
Dr. Mateos and her colleagues performed the phase III, open-label, randomized trial at 19 medical centers in Spain and at 3 in Portugal, enrolling 119 patients with high-risk smoldering multiple myeloma. This was defined as a plasma-cell bone marrow infiltration of at least 10% and a monoclonal component, or only one of the two criteria plus at least 95% phenotypically aberrant plasma cells in the bone marrow plasma-cell compartment, with reductions in one to two uninvolved immunoglobulins of more than 25% of normal values.
Patients were randomly assigned to receive either usual care (62 patients) or treatment (57 patients). Treatment comprised an induction phase of nine 4-week cycles of oral lenalidomide plus dexamethasone, followed by a maintenance phase of low-dose lenalidomide, for a total duration of no more than 2 years.
For patients in the treatment group who showed asymptomatic biologic progression during the maintenance phase, dexamethasone was permitted. Patients in the observation group received no treatment until they progressed to symptomatic disease. The median follow-up was 40 months (range, 27-57 months).
Myeloma progressed in 13 patients (23%) in the treatment group, compared with 47 patients (76%) in the observation group.
Four patients in the treatment group died during follow-up, for an overall mortality of 7%. There was one death from a treatment-related toxic effect (a fatal respiratory infection), one from surgical complications unrelated to myeloma or its treatment, and two from disease progression. In contrast, 13 patients in the observation group died, for an overall mortality of 21%; all of the deaths were from disease progression, the researchers noted.
Infections were the most common nonhematologic adverse events; the incidence was not significantly different between the treatment and observation groups. Most infections were of low severity, but one patient in the treatment group developed a grade 5 respiratory infection and died. Serious adverse events were more common in the treatment group (12%) than in the observation group (3%).
The cumulative risk of developing a second primary tumor at 5 years was 20% in the treatment group and 25% in the observation group. These included breast cancer in one patient in the treatment group, prostate cancer in two patients in the treatment group, polycythemia vera in one patient in the treatment group, and myelodysplastic syndrome in one patient in the observation group. The most common grade 3 adverse events were infection (6% of patients), asthenia (6%), neutropenia (5%), and rash (3%).
Overall, 88% of the patients in the treatment group completed induction therapy and 70% completed maintenance therapy.
"Future studies should address the effect of early treatment on the quality of life, which we did not assess in this trial," Dr. Mateos and her associates noted.
This trial was funded by Celgene, which also was involved in the data collection and analysis. Dr. Mateos reported ties to Celgene, GenMab, and other companies; her associates reported ties to numerous industry sources.
Early oral lenalidomide-based treatment significantly delayed disease progression in an industry-sponsored phase III study of patients who had high-risk smoldering multiple myeloma.
The delay in disease progression with early lenalidomide "translated into a significant overall survival benefit; the proportion of patients who were alive at 3 years was 94% in the treatment group versus 80% in the observation group," reported Dr. María-Victoria Mateos of the Hospital Universitario de Salamanca (Spain) and her associates in the New England Journal of Medicine.
The study findings suggest that patients with smoldering multiple myeloma who are at high risk of progression to active disease should be targeted for early intervention (N. Engl. J. Med. 2013;369:438-47 [doi: 10.1056/NEJMoa1300439]).
Three years after entry into the study, 77% of the patients who received lenalidomide had progression-free survival, compared with only 30% of those who received usual care. Usual care involved simple observation, since there are no other therapeutic options in the early stage of the disease.
The treatment response rate was 79% during the induction phase to reduce the tumor burden, which increased to 90% during the maintenance phase. The regimen’s toxicity was judged to be moderate, and the incidence of second primary tumors was low.
The overall risk of progression with smoldering multiple myeloma is low, at 10% per year. However, a large subgroup of patients has been identified as high risk, with a 50% or more probability of progression to active disease within 2 years of diagnosis. Early intervention rather than observation has been attempted for this subgroup, but, so far, alkylating agents, bisphosphonates, interleukin-1B receptor antagonists, and thalidomide have failed to show clinical benefit.
Dr. Mateos and her colleagues performed the phase III, open-label, randomized trial at 19 medical centers in Spain and at 3 in Portugal, enrolling 119 patients with high-risk smoldering multiple myeloma. This was defined as a plasma-cell bone marrow infiltration of at least 10% and a monoclonal component, or only one of the two criteria plus at least 95% phenotypically aberrant plasma cells in the bone marrow plasma-cell compartment, with reductions in one to two uninvolved immunoglobulins of more than 25% of normal values.
Patients were randomly assigned to receive either usual care (62 patients) or treatment (57 patients). Treatment comprised an induction phase of nine 4-week cycles of oral lenalidomide plus dexamethasone, followed by a maintenance phase of low-dose lenalidomide, for a total duration of no more than 2 years.
For patients in the treatment group who showed asymptomatic biologic progression during the maintenance phase, dexamethasone was permitted. Patients in the observation group received no treatment until they progressed to symptomatic disease. The median follow-up was 40 months (range, 27-57 months).
Myeloma progressed in 13 patients (23%) in the treatment group, compared with 47 patients (76%) in the observation group.
Four patients in the treatment group died during follow-up, for an overall mortality of 7%. There was one death from a treatment-related toxic effect (a fatal respiratory infection), one from surgical complications unrelated to myeloma or its treatment, and two from disease progression. In contrast, 13 patients in the observation group died, for an overall mortality of 21%; all of the deaths were from disease progression, the researchers noted.
Infections were the most common nonhematologic adverse events; the incidence was not significantly different between the treatment and observation groups. Most infections were of low severity, but one patient in the treatment group developed a grade 5 respiratory infection and died. Serious adverse events were more common in the treatment group (12%) than in the observation group (3%).
The cumulative risk of developing a second primary tumor at 5 years was 20% in the treatment group and 25% in the observation group. These included breast cancer in one patient in the treatment group, prostate cancer in two patients in the treatment group, polycythemia vera in one patient in the treatment group, and myelodysplastic syndrome in one patient in the observation group. The most common grade 3 adverse events were infection (6% of patients), asthenia (6%), neutropenia (5%), and rash (3%).
Overall, 88% of the patients in the treatment group completed induction therapy and 70% completed maintenance therapy.
"Future studies should address the effect of early treatment on the quality of life, which we did not assess in this trial," Dr. Mateos and her associates noted.
This trial was funded by Celgene, which also was involved in the data collection and analysis. Dr. Mateos reported ties to Celgene, GenMab, and other companies; her associates reported ties to numerous industry sources.
Early oral lenalidomide-based treatment significantly delayed disease progression in an industry-sponsored phase III study of patients who had high-risk smoldering multiple myeloma.
The delay in disease progression with early lenalidomide "translated into a significant overall survival benefit; the proportion of patients who were alive at 3 years was 94% in the treatment group versus 80% in the observation group," reported Dr. María-Victoria Mateos of the Hospital Universitario de Salamanca (Spain) and her associates in the New England Journal of Medicine.
The study findings suggest that patients with smoldering multiple myeloma who are at high risk of progression to active disease should be targeted for early intervention (N. Engl. J. Med. 2013;369:438-47 [doi: 10.1056/NEJMoa1300439]).
Three years after entry into the study, 77% of the patients who received lenalidomide had progression-free survival, compared with only 30% of those who received usual care. Usual care involved simple observation, since there are no other therapeutic options in the early stage of the disease.
The treatment response rate was 79% during the induction phase to reduce the tumor burden, which increased to 90% during the maintenance phase. The regimen’s toxicity was judged to be moderate, and the incidence of second primary tumors was low.
The overall risk of progression with smoldering multiple myeloma is low, at 10% per year. However, a large subgroup of patients has been identified as high risk, with a 50% or more probability of progression to active disease within 2 years of diagnosis. Early intervention rather than observation has been attempted for this subgroup, but, so far, alkylating agents, bisphosphonates, interleukin-1B receptor antagonists, and thalidomide have failed to show clinical benefit.
Dr. Mateos and her colleagues performed the phase III, open-label, randomized trial at 19 medical centers in Spain and at 3 in Portugal, enrolling 119 patients with high-risk smoldering multiple myeloma. This was defined as a plasma-cell bone marrow infiltration of at least 10% and a monoclonal component, or only one of the two criteria plus at least 95% phenotypically aberrant plasma cells in the bone marrow plasma-cell compartment, with reductions in one to two uninvolved immunoglobulins of more than 25% of normal values.
Patients were randomly assigned to receive either usual care (62 patients) or treatment (57 patients). Treatment comprised an induction phase of nine 4-week cycles of oral lenalidomide plus dexamethasone, followed by a maintenance phase of low-dose lenalidomide, for a total duration of no more than 2 years.
For patients in the treatment group who showed asymptomatic biologic progression during the maintenance phase, dexamethasone was permitted. Patients in the observation group received no treatment until they progressed to symptomatic disease. The median follow-up was 40 months (range, 27-57 months).
Myeloma progressed in 13 patients (23%) in the treatment group, compared with 47 patients (76%) in the observation group.
Four patients in the treatment group died during follow-up, for an overall mortality of 7%. There was one death from a treatment-related toxic effect (a fatal respiratory infection), one from surgical complications unrelated to myeloma or its treatment, and two from disease progression. In contrast, 13 patients in the observation group died, for an overall mortality of 21%; all of the deaths were from disease progression, the researchers noted.
Infections were the most common nonhematologic adverse events; the incidence was not significantly different between the treatment and observation groups. Most infections were of low severity, but one patient in the treatment group developed a grade 5 respiratory infection and died. Serious adverse events were more common in the treatment group (12%) than in the observation group (3%).
The cumulative risk of developing a second primary tumor at 5 years was 20% in the treatment group and 25% in the observation group. These included breast cancer in one patient in the treatment group, prostate cancer in two patients in the treatment group, polycythemia vera in one patient in the treatment group, and myelodysplastic syndrome in one patient in the observation group. The most common grade 3 adverse events were infection (6% of patients), asthenia (6%), neutropenia (5%), and rash (3%).
Overall, 88% of the patients in the treatment group completed induction therapy and 70% completed maintenance therapy.
"Future studies should address the effect of early treatment on the quality of life, which we did not assess in this trial," Dr. Mateos and her associates noted.
This trial was funded by Celgene, which also was involved in the data collection and analysis. Dr. Mateos reported ties to Celgene, GenMab, and other companies; her associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major finding: Three-year survival was 94% among patients who received early oral lenalidomide, compared with 80% of patients who received usual care.
Data source: An open-label, phase III, randomized clinical trial involving 119 patients with high-risk smoldering multiple myeloma who were followed for a median of 40 months.
Disclosures: This trial was funded by Celgene, which also was involved in the data collection and analysis. Dr. Mateos reported ties to Celgene, GenMab, and other companies; her associates reported ties to numerous industry sources.
Pomalidomide plus low-dose dexamethasone may be new standard for MM
CHICAGO – The combination of pomalidomide and low-dose dexamethasone is superior to high-dose dexamethasone monotherapy for treating patients with relapsed and refractory multiple myeloma, based on updated results from the multicenter, randomized MM-003 trial.
Among the 455 patients studied in the trial, those assigned to the combination therapy had a 52% lower risk of progression or death and a 26% lower risk of death alone when compared with peers assigned to single-agent high-dose dexamethasone.
The two regimens had much the same toxicity profile, although the combination was associated with a higher rate of grade 3/4 hematologic toxicity.
"Pomalidomide in combination with low-dose dexamethasone should be considered as a new standard of care for treatment of relapsed and refractory multiple myeloma patients after treatment with lenalidomide and bortezomib," presenting author Dr. Katja C. Weisel commented at the annual meeting of the American Society of Clinical Oncology.
All of the patients enrolled in the MM-003 trial had received at least two prior therapies and had disease refractory to their last therapy, according to Dr. Weisel, a hematologist-oncologist with the University Hospital Tübingen, Germany. All had experienced a failure of both Millennium’s bortezomib (Velcade) and Celgene’s lenalidomide (Revlimid).
The patients were randomized 2:1 to receive low-dose dexamethasone plus Celgene’s pomalidomide (Pomalyst), an antiangiogenic and immune-modulating agent, or high-dose dexamethasone alone. Patients given pomalidomide or who had a history of deep vein thrombosis were given thromboprophylaxis.
Patients who experienced progression on high-dose dexamethasone entered the companion MM-003C trial, in which they were given pomalidomide.
Initial trial results after a median follow-up of 4 months, which were previously reported, showed there were significantly better progression-free survival and overall survival with the combination. These results led to a recommendation by the trial’s monitoring committee that all patients in the high-dose dexamethasone group be given access to pomalidomide regardless of whether they had progression. In all, half of the patients in that group received pomalidomide after high-dose dexamethasone due to either this recommendation or entry into the companion trial.
In the updated analysis, now with a median follow-up of 10 months, progression-free survival was still significantly better with pomalidomide plus low-dose dexamethasone than with high-dose dexamethasone (4.0 vs. 1.9 months; hazard ratio, 0.48; P less than .001).
Overall survival was also still significantly better with the combination (12.7 vs. 8.1 months; HR, 0.74; P = .028).
"This overall survival benefit was maintained despite a high crossover rate, in 50% of the patients. ... In addition, all patients who were still alive at this time in the high-dose dexamethasone group had received pomalidomide as a salvage treatment," Dr. Weisel noted.
The progression-free survival and overall survival benefits were generally similar across subgroups of patients whose disease was refractory to both lenalidomide and bortezomib, who had received lenalidomide as their last prior therapy, and who had received bortezomib as their last prior therapy.
"The safety profile of pomalidomide is predictable and manageable, and the drug with its oral application is generally well tolerated in this heavily pretreated patient group," Dr. Weisel commented.
The main toxicity with the combination was hematologic toxicity: The rate of grade 3/4 neutropenia was 48% with the combination, compared with 16% with high-dose dexamethasone. The combination group and the high-dose dexamethasone group had essentially the same rates of grade 3/4 deep vein thrombosis and pulmonary embolism (1% and 0%, respectively), peripheral neuropathy (1% and 1%), and discontinuation due to adverse events (9% and 10%).
Dr. Weisel disclosed that she is a consultant to and receives honoraria from Celgene and Janssen.
The median progression-free survival in patients who achieved a minimal response (MR) of 8 months was about the same as the 7 months seen in patients who achieved a partial response or better.
When you don’t have many options left, even something like an MR can carry significant clinical benefit. And the important message in my mind here is that if you don’t get a PR (partial response) or you don’t get a CR (complete response), don’t throw the therapy away. These minor benefits may actually translate into significant long-term clinical benefits, and it’s a different situation than we are discussing in the context of a newly diagnosed, treatment-naive patient, where our goal ultimately should be a CR.
Also, despite the clear survival benefit of the combination, there was a late crossing of the overall survival curves in favor of high-dose dexamethasone. If you look at the number of patients who stayed on high-dose dexamethasone, it was vanishingly small. So I think that late improvement in survival was a consequence of getting the better therapy as part of the crossover design.
Dr. Sagar Lonial, of the Winship Cancer Institute, Emory University, Atlanta, was the invited discussant of the study. Dr. Lonial disclosed that he is a consultant to and receives research funding from Bristol-Myers Squibb, Celgene, Millennium, and Novartis, and also is a consultant to Onyx.
The median progression-free survival in patients who achieved a minimal response (MR) of 8 months was about the same as the 7 months seen in patients who achieved a partial response or better.
When you don’t have many options left, even something like an MR can carry significant clinical benefit. And the important message in my mind here is that if you don’t get a PR (partial response) or you don’t get a CR (complete response), don’t throw the therapy away. These minor benefits may actually translate into significant long-term clinical benefits, and it’s a different situation than we are discussing in the context of a newly diagnosed, treatment-naive patient, where our goal ultimately should be a CR.
Also, despite the clear survival benefit of the combination, there was a late crossing of the overall survival curves in favor of high-dose dexamethasone. If you look at the number of patients who stayed on high-dose dexamethasone, it was vanishingly small. So I think that late improvement in survival was a consequence of getting the better therapy as part of the crossover design.
Dr. Sagar Lonial, of the Winship Cancer Institute, Emory University, Atlanta, was the invited discussant of the study. Dr. Lonial disclosed that he is a consultant to and receives research funding from Bristol-Myers Squibb, Celgene, Millennium, and Novartis, and also is a consultant to Onyx.
The median progression-free survival in patients who achieved a minimal response (MR) of 8 months was about the same as the 7 months seen in patients who achieved a partial response or better.
When you don’t have many options left, even something like an MR can carry significant clinical benefit. And the important message in my mind here is that if you don’t get a PR (partial response) or you don’t get a CR (complete response), don’t throw the therapy away. These minor benefits may actually translate into significant long-term clinical benefits, and it’s a different situation than we are discussing in the context of a newly diagnosed, treatment-naive patient, where our goal ultimately should be a CR.
Also, despite the clear survival benefit of the combination, there was a late crossing of the overall survival curves in favor of high-dose dexamethasone. If you look at the number of patients who stayed on high-dose dexamethasone, it was vanishingly small. So I think that late improvement in survival was a consequence of getting the better therapy as part of the crossover design.
Dr. Sagar Lonial, of the Winship Cancer Institute, Emory University, Atlanta, was the invited discussant of the study. Dr. Lonial disclosed that he is a consultant to and receives research funding from Bristol-Myers Squibb, Celgene, Millennium, and Novartis, and also is a consultant to Onyx.
CHICAGO – The combination of pomalidomide and low-dose dexamethasone is superior to high-dose dexamethasone monotherapy for treating patients with relapsed and refractory multiple myeloma, based on updated results from the multicenter, randomized MM-003 trial.
Among the 455 patients studied in the trial, those assigned to the combination therapy had a 52% lower risk of progression or death and a 26% lower risk of death alone when compared with peers assigned to single-agent high-dose dexamethasone.
The two regimens had much the same toxicity profile, although the combination was associated with a higher rate of grade 3/4 hematologic toxicity.
"Pomalidomide in combination with low-dose dexamethasone should be considered as a new standard of care for treatment of relapsed and refractory multiple myeloma patients after treatment with lenalidomide and bortezomib," presenting author Dr. Katja C. Weisel commented at the annual meeting of the American Society of Clinical Oncology.
All of the patients enrolled in the MM-003 trial had received at least two prior therapies and had disease refractory to their last therapy, according to Dr. Weisel, a hematologist-oncologist with the University Hospital Tübingen, Germany. All had experienced a failure of both Millennium’s bortezomib (Velcade) and Celgene’s lenalidomide (Revlimid).
The patients were randomized 2:1 to receive low-dose dexamethasone plus Celgene’s pomalidomide (Pomalyst), an antiangiogenic and immune-modulating agent, or high-dose dexamethasone alone. Patients given pomalidomide or who had a history of deep vein thrombosis were given thromboprophylaxis.
Patients who experienced progression on high-dose dexamethasone entered the companion MM-003C trial, in which they were given pomalidomide.
Initial trial results after a median follow-up of 4 months, which were previously reported, showed there were significantly better progression-free survival and overall survival with the combination. These results led to a recommendation by the trial’s monitoring committee that all patients in the high-dose dexamethasone group be given access to pomalidomide regardless of whether they had progression. In all, half of the patients in that group received pomalidomide after high-dose dexamethasone due to either this recommendation or entry into the companion trial.
In the updated analysis, now with a median follow-up of 10 months, progression-free survival was still significantly better with pomalidomide plus low-dose dexamethasone than with high-dose dexamethasone (4.0 vs. 1.9 months; hazard ratio, 0.48; P less than .001).
Overall survival was also still significantly better with the combination (12.7 vs. 8.1 months; HR, 0.74; P = .028).
"This overall survival benefit was maintained despite a high crossover rate, in 50% of the patients. ... In addition, all patients who were still alive at this time in the high-dose dexamethasone group had received pomalidomide as a salvage treatment," Dr. Weisel noted.
The progression-free survival and overall survival benefits were generally similar across subgroups of patients whose disease was refractory to both lenalidomide and bortezomib, who had received lenalidomide as their last prior therapy, and who had received bortezomib as their last prior therapy.
"The safety profile of pomalidomide is predictable and manageable, and the drug with its oral application is generally well tolerated in this heavily pretreated patient group," Dr. Weisel commented.
The main toxicity with the combination was hematologic toxicity: The rate of grade 3/4 neutropenia was 48% with the combination, compared with 16% with high-dose dexamethasone. The combination group and the high-dose dexamethasone group had essentially the same rates of grade 3/4 deep vein thrombosis and pulmonary embolism (1% and 0%, respectively), peripheral neuropathy (1% and 1%), and discontinuation due to adverse events (9% and 10%).
Dr. Weisel disclosed that she is a consultant to and receives honoraria from Celgene and Janssen.
CHICAGO – The combination of pomalidomide and low-dose dexamethasone is superior to high-dose dexamethasone monotherapy for treating patients with relapsed and refractory multiple myeloma, based on updated results from the multicenter, randomized MM-003 trial.
Among the 455 patients studied in the trial, those assigned to the combination therapy had a 52% lower risk of progression or death and a 26% lower risk of death alone when compared with peers assigned to single-agent high-dose dexamethasone.
The two regimens had much the same toxicity profile, although the combination was associated with a higher rate of grade 3/4 hematologic toxicity.
"Pomalidomide in combination with low-dose dexamethasone should be considered as a new standard of care for treatment of relapsed and refractory multiple myeloma patients after treatment with lenalidomide and bortezomib," presenting author Dr. Katja C. Weisel commented at the annual meeting of the American Society of Clinical Oncology.
All of the patients enrolled in the MM-003 trial had received at least two prior therapies and had disease refractory to their last therapy, according to Dr. Weisel, a hematologist-oncologist with the University Hospital Tübingen, Germany. All had experienced a failure of both Millennium’s bortezomib (Velcade) and Celgene’s lenalidomide (Revlimid).
The patients were randomized 2:1 to receive low-dose dexamethasone plus Celgene’s pomalidomide (Pomalyst), an antiangiogenic and immune-modulating agent, or high-dose dexamethasone alone. Patients given pomalidomide or who had a history of deep vein thrombosis were given thromboprophylaxis.
Patients who experienced progression on high-dose dexamethasone entered the companion MM-003C trial, in which they were given pomalidomide.
Initial trial results after a median follow-up of 4 months, which were previously reported, showed there were significantly better progression-free survival and overall survival with the combination. These results led to a recommendation by the trial’s monitoring committee that all patients in the high-dose dexamethasone group be given access to pomalidomide regardless of whether they had progression. In all, half of the patients in that group received pomalidomide after high-dose dexamethasone due to either this recommendation or entry into the companion trial.
In the updated analysis, now with a median follow-up of 10 months, progression-free survival was still significantly better with pomalidomide plus low-dose dexamethasone than with high-dose dexamethasone (4.0 vs. 1.9 months; hazard ratio, 0.48; P less than .001).
Overall survival was also still significantly better with the combination (12.7 vs. 8.1 months; HR, 0.74; P = .028).
"This overall survival benefit was maintained despite a high crossover rate, in 50% of the patients. ... In addition, all patients who were still alive at this time in the high-dose dexamethasone group had received pomalidomide as a salvage treatment," Dr. Weisel noted.
The progression-free survival and overall survival benefits were generally similar across subgroups of patients whose disease was refractory to both lenalidomide and bortezomib, who had received lenalidomide as their last prior therapy, and who had received bortezomib as their last prior therapy.
"The safety profile of pomalidomide is predictable and manageable, and the drug with its oral application is generally well tolerated in this heavily pretreated patient group," Dr. Weisel commented.
The main toxicity with the combination was hematologic toxicity: The rate of grade 3/4 neutropenia was 48% with the combination, compared with 16% with high-dose dexamethasone. The combination group and the high-dose dexamethasone group had essentially the same rates of grade 3/4 deep vein thrombosis and pulmonary embolism (1% and 0%, respectively), peripheral neuropathy (1% and 1%), and discontinuation due to adverse events (9% and 10%).
Dr. Weisel disclosed that she is a consultant to and receives honoraria from Celgene and Janssen.
AT THE ASCO ANNUAL MEETING 2013
Major finding: Compared with high-dose dexamethasone, pomalidomide plus low-dose dexamethasone yielded better median progression-free survival (4.0 vs. 1.9 months) and overall survival (12.7 vs. 8.1 months).
Data source: A phase III, multicenter, randomized open-label trial of 455 patients with relapsed and refractory multiple myeloma (MM-003 trial).
Disclosures: Dr. Weisel disclosed that she is a consultant to and receives honoraria from Janssen and Celgene, the maker of pomalidomide.
CD30 expression in EBV+ DLBCL confers poor prognosis
Epstein-Barr virus-positive diffuse large B-cell lymphoma, or EBV+ DLBCL, represents only a small proportion of DLBCLs, and has an activated B-cell type immunophenotype and a unique gene expression profile and genetic signature that distinguish the neoplasm from EBV-negative DLBCL, according to a report from the International DLBCL Rituximab-CHOP Consortium Program Study.
Further, CD30 expression is more common in EBV+ DLBCL than in EBV-negative DLBCL and confers an adverse outcome, Dr. Ken He Young reported at the annual International Conference on Malignant Lymphoma in Lugano, Switzerland.
The findings are based on a study of 451 HIV-negative de novo DLBCL patients who had tissue microarrays from primary biopsy specimens available. All were uniformly treated with rituximab plus chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), said Dr. Young of the department of hematopathology at the University of Texas MD Anderson Cancer Center, Houston (Hematol. Oncol. 2013;31[(Suppl 1]:96-150[abstract 9]).
Of the 451 patients, 27 (6%) had positive EBV-encoded RNA (EBER) test results, defined as reactivity in more than 10% of malignant cells. The median age of the EBV+ and EBV- patients was similar at 61 and 64 years, respectively, as were the presenting clinical characteristics, Dr. Young noted.
However, an activated B-cell type signature occurred in 70% of EBV+ patients, compared with 48% or 49% (based on gene expression profiling and immunohistochemistry using the Visco-Young algorithm, respectively) in the EBV- patients, and CD30 expression occurred in 48% of EBV+ patients, compared with 15% of EBV- patients, he said.
No difference was seen in the expression of BCL2, MYC or both proteins between the EBV+ and EBV- groups, but the EBV+ patients had no detrimental TP53 mutation and only 18% presented rearrangements involving BCL2, C-MYC, or BCL6 genes.
Five-year overall survival appeared inferior in the EBV+ DLBCL patients at 50%, compared with 62% for the EBV- DLBCL patients, even after stratification by age, but the difference between the groups did not reach statistical significance. The same was true for progression-free survival, Dr. Young noted.
Among EBV+ DLBCL patients with CD30 expression, however, the prognosis was dismal, with median overall and progression-free survival of 37% and 35%, respectively, compared with 74% and 56%, respectively, in those with CD30 negative EBV+ DLBCL. These differences were statistically significant, Dr. Young said, adding that gene expression profiling revealed a unique expression signature in EBV+ DLBCL with NF-kB pathway activation.
In a separate report from the International DLBCL Rituximab-CHOP Consortium Program Study published online in May in Blood, Dr. Young and his colleagues explained that EBV+ DLBCL of the elderly, which was initially described 10 years ago, is a provisional entity within the World Health Organization classification system, and is defined as "an EBV-positive monoclonal large B-cell proliferation that occurs in patients greater than 50 years of age and in whom there is no known immunodeficiency or history of lymphoma," (2013;121: 2715-24 [doi:10.1182/blood-2012-10-461848]).
"It is hoped that the improved understanding of these tumors will lead to development of novel therapeutic approaches, enhance the effective clinical trials, and improve the prognosis," the investigators said.
Epstein-Barr virus-positive diffuse large B-cell lymphoma, or EBV+ DLBCL, represents only a small proportion of DLBCLs, and has an activated B-cell type immunophenotype and a unique gene expression profile and genetic signature that distinguish the neoplasm from EBV-negative DLBCL, according to a report from the International DLBCL Rituximab-CHOP Consortium Program Study.
Further, CD30 expression is more common in EBV+ DLBCL than in EBV-negative DLBCL and confers an adverse outcome, Dr. Ken He Young reported at the annual International Conference on Malignant Lymphoma in Lugano, Switzerland.
The findings are based on a study of 451 HIV-negative de novo DLBCL patients who had tissue microarrays from primary biopsy specimens available. All were uniformly treated with rituximab plus chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), said Dr. Young of the department of hematopathology at the University of Texas MD Anderson Cancer Center, Houston (Hematol. Oncol. 2013;31[(Suppl 1]:96-150[abstract 9]).
Of the 451 patients, 27 (6%) had positive EBV-encoded RNA (EBER) test results, defined as reactivity in more than 10% of malignant cells. The median age of the EBV+ and EBV- patients was similar at 61 and 64 years, respectively, as were the presenting clinical characteristics, Dr. Young noted.
However, an activated B-cell type signature occurred in 70% of EBV+ patients, compared with 48% or 49% (based on gene expression profiling and immunohistochemistry using the Visco-Young algorithm, respectively) in the EBV- patients, and CD30 expression occurred in 48% of EBV+ patients, compared with 15% of EBV- patients, he said.
No difference was seen in the expression of BCL2, MYC or both proteins between the EBV+ and EBV- groups, but the EBV+ patients had no detrimental TP53 mutation and only 18% presented rearrangements involving BCL2, C-MYC, or BCL6 genes.
Five-year overall survival appeared inferior in the EBV+ DLBCL patients at 50%, compared with 62% for the EBV- DLBCL patients, even after stratification by age, but the difference between the groups did not reach statistical significance. The same was true for progression-free survival, Dr. Young noted.
Among EBV+ DLBCL patients with CD30 expression, however, the prognosis was dismal, with median overall and progression-free survival of 37% and 35%, respectively, compared with 74% and 56%, respectively, in those with CD30 negative EBV+ DLBCL. These differences were statistically significant, Dr. Young said, adding that gene expression profiling revealed a unique expression signature in EBV+ DLBCL with NF-kB pathway activation.
In a separate report from the International DLBCL Rituximab-CHOP Consortium Program Study published online in May in Blood, Dr. Young and his colleagues explained that EBV+ DLBCL of the elderly, which was initially described 10 years ago, is a provisional entity within the World Health Organization classification system, and is defined as "an EBV-positive monoclonal large B-cell proliferation that occurs in patients greater than 50 years of age and in whom there is no known immunodeficiency or history of lymphoma," (2013;121: 2715-24 [doi:10.1182/blood-2012-10-461848]).
"It is hoped that the improved understanding of these tumors will lead to development of novel therapeutic approaches, enhance the effective clinical trials, and improve the prognosis," the investigators said.
Epstein-Barr virus-positive diffuse large B-cell lymphoma, or EBV+ DLBCL, represents only a small proportion of DLBCLs, and has an activated B-cell type immunophenotype and a unique gene expression profile and genetic signature that distinguish the neoplasm from EBV-negative DLBCL, according to a report from the International DLBCL Rituximab-CHOP Consortium Program Study.
Further, CD30 expression is more common in EBV+ DLBCL than in EBV-negative DLBCL and confers an adverse outcome, Dr. Ken He Young reported at the annual International Conference on Malignant Lymphoma in Lugano, Switzerland.
The findings are based on a study of 451 HIV-negative de novo DLBCL patients who had tissue microarrays from primary biopsy specimens available. All were uniformly treated with rituximab plus chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), said Dr. Young of the department of hematopathology at the University of Texas MD Anderson Cancer Center, Houston (Hematol. Oncol. 2013;31[(Suppl 1]:96-150[abstract 9]).
Of the 451 patients, 27 (6%) had positive EBV-encoded RNA (EBER) test results, defined as reactivity in more than 10% of malignant cells. The median age of the EBV+ and EBV- patients was similar at 61 and 64 years, respectively, as were the presenting clinical characteristics, Dr. Young noted.
However, an activated B-cell type signature occurred in 70% of EBV+ patients, compared with 48% or 49% (based on gene expression profiling and immunohistochemistry using the Visco-Young algorithm, respectively) in the EBV- patients, and CD30 expression occurred in 48% of EBV+ patients, compared with 15% of EBV- patients, he said.
No difference was seen in the expression of BCL2, MYC or both proteins between the EBV+ and EBV- groups, but the EBV+ patients had no detrimental TP53 mutation and only 18% presented rearrangements involving BCL2, C-MYC, or BCL6 genes.
Five-year overall survival appeared inferior in the EBV+ DLBCL patients at 50%, compared with 62% for the EBV- DLBCL patients, even after stratification by age, but the difference between the groups did not reach statistical significance. The same was true for progression-free survival, Dr. Young noted.
Among EBV+ DLBCL patients with CD30 expression, however, the prognosis was dismal, with median overall and progression-free survival of 37% and 35%, respectively, compared with 74% and 56%, respectively, in those with CD30 negative EBV+ DLBCL. These differences were statistically significant, Dr. Young said, adding that gene expression profiling revealed a unique expression signature in EBV+ DLBCL with NF-kB pathway activation.
In a separate report from the International DLBCL Rituximab-CHOP Consortium Program Study published online in May in Blood, Dr. Young and his colleagues explained that EBV+ DLBCL of the elderly, which was initially described 10 years ago, is a provisional entity within the World Health Organization classification system, and is defined as "an EBV-positive monoclonal large B-cell proliferation that occurs in patients greater than 50 years of age and in whom there is no known immunodeficiency or history of lymphoma," (2013;121: 2715-24 [doi:10.1182/blood-2012-10-461848]).
"It is hoped that the improved understanding of these tumors will lead to development of novel therapeutic approaches, enhance the effective clinical trials, and improve the prognosis," the investigators said.
FROM THE ANNUAL ICML
Major finding: EBV+ DLBCL has unique gene expression profile and genetic signature.
Data source: An analysis of data from 451 patients from the International DLBCL Rituximab-CHOP Consortium Program Study.
Disclosures: Not listed.
Ibrutinib shows promise for CLL del 17p
Single-agent ibrutinib therapy was highly effective and well tolerated in 29 patients with chronic lymphocytic leukemia and deletion of part of the short arm of chromosome 17 (del 17p) who were part of a phase II study.
Treatment with the investigational selective Bruton’s tyrosine kinase inhibitor was associated with rapid control of disease in the nodes, spleen, marrow, and blood in both treatment-naive and relapsed/refractory patients, Dr. Adrian Wiestner reported at the annual International Conference on Malignant Lymphoma in Lugano, Switzerland.
These findings are notable because patients with chronic lymphocytic leukemia (CLL) and del 17p typically have poor progression-free and overall survival when treated with standard chemoimmunotherapy, Dr. Wiestner said, adding that the potential role of ibrutinib for the treatment of these difficult-to-treat patients will be further investigated.
At a median follow-up of 9 months, 88% of 25 evaluable patients had a nodal response with a median 70% reduction in lymph node size, 48% had a partial response by International Workshop on CLL (IWCLL) criteria, and 40% had a partial response with lymphocytosis. One patient had progressive disease, said Dr. Wiestner of the National Heart, Lung, and Blood Institute, National Institutes of Health.
Of 15 treatment-naïve patients aged 33-82 years, 82% achieved a nodal response, and of 14 relapsed/refractory patients aged 56-79 years, 93% achieved a nodal response. The estimated 12-month event-free survival among the patients, who were the first in the study to reach 9-month median follow-up, was 90%, Dr. Wiestner said.
Also, 22 evaluable patients had a median 46% reduction in splenomegaly (median volume reduction of 446 mL), and tumor burden in bone marrow biopsies of 23 patients decreased by a median of 76% as assessed by immunohistochemistry for CD79a.
The percentage of tumor cells with del 17p decreased by a median of 55% in 15 patients, remained unchanged in 1 patient, and increased in 3 patients.
Treatment-naive patients included adults aged 33-82 years, and relapsed/refractory patients were adults aged 56-79 years. All were treated with 420 mg of oral ibrutinib daily until disease progression; treatment response was assessed every 6 months.
Ibrutinib, which has been granted multiple Breakthrough Therapy designations by the Food and Drug Administration – including a recent designation for the treatment of CLL or small lymphocytic lymphoma with del 17p – was well tolerated; 14% of patients experienced grade 3 or higher nonhematologic toxicities. Two deaths occurred among patients in the study, but were not deemed treatment related.
This study was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute.
Single-agent ibrutinib therapy was highly effective and well tolerated in 29 patients with chronic lymphocytic leukemia and deletion of part of the short arm of chromosome 17 (del 17p) who were part of a phase II study.
Treatment with the investigational selective Bruton’s tyrosine kinase inhibitor was associated with rapid control of disease in the nodes, spleen, marrow, and blood in both treatment-naive and relapsed/refractory patients, Dr. Adrian Wiestner reported at the annual International Conference on Malignant Lymphoma in Lugano, Switzerland.
These findings are notable because patients with chronic lymphocytic leukemia (CLL) and del 17p typically have poor progression-free and overall survival when treated with standard chemoimmunotherapy, Dr. Wiestner said, adding that the potential role of ibrutinib for the treatment of these difficult-to-treat patients will be further investigated.
At a median follow-up of 9 months, 88% of 25 evaluable patients had a nodal response with a median 70% reduction in lymph node size, 48% had a partial response by International Workshop on CLL (IWCLL) criteria, and 40% had a partial response with lymphocytosis. One patient had progressive disease, said Dr. Wiestner of the National Heart, Lung, and Blood Institute, National Institutes of Health.
Of 15 treatment-naïve patients aged 33-82 years, 82% achieved a nodal response, and of 14 relapsed/refractory patients aged 56-79 years, 93% achieved a nodal response. The estimated 12-month event-free survival among the patients, who were the first in the study to reach 9-month median follow-up, was 90%, Dr. Wiestner said.
Also, 22 evaluable patients had a median 46% reduction in splenomegaly (median volume reduction of 446 mL), and tumor burden in bone marrow biopsies of 23 patients decreased by a median of 76% as assessed by immunohistochemistry for CD79a.
The percentage of tumor cells with del 17p decreased by a median of 55% in 15 patients, remained unchanged in 1 patient, and increased in 3 patients.
Treatment-naive patients included adults aged 33-82 years, and relapsed/refractory patients were adults aged 56-79 years. All were treated with 420 mg of oral ibrutinib daily until disease progression; treatment response was assessed every 6 months.
Ibrutinib, which has been granted multiple Breakthrough Therapy designations by the Food and Drug Administration – including a recent designation for the treatment of CLL or small lymphocytic lymphoma with del 17p – was well tolerated; 14% of patients experienced grade 3 or higher nonhematologic toxicities. Two deaths occurred among patients in the study, but were not deemed treatment related.
This study was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute.
Single-agent ibrutinib therapy was highly effective and well tolerated in 29 patients with chronic lymphocytic leukemia and deletion of part of the short arm of chromosome 17 (del 17p) who were part of a phase II study.
Treatment with the investigational selective Bruton’s tyrosine kinase inhibitor was associated with rapid control of disease in the nodes, spleen, marrow, and blood in both treatment-naive and relapsed/refractory patients, Dr. Adrian Wiestner reported at the annual International Conference on Malignant Lymphoma in Lugano, Switzerland.
These findings are notable because patients with chronic lymphocytic leukemia (CLL) and del 17p typically have poor progression-free and overall survival when treated with standard chemoimmunotherapy, Dr. Wiestner said, adding that the potential role of ibrutinib for the treatment of these difficult-to-treat patients will be further investigated.
At a median follow-up of 9 months, 88% of 25 evaluable patients had a nodal response with a median 70% reduction in lymph node size, 48% had a partial response by International Workshop on CLL (IWCLL) criteria, and 40% had a partial response with lymphocytosis. One patient had progressive disease, said Dr. Wiestner of the National Heart, Lung, and Blood Institute, National Institutes of Health.
Of 15 treatment-naïve patients aged 33-82 years, 82% achieved a nodal response, and of 14 relapsed/refractory patients aged 56-79 years, 93% achieved a nodal response. The estimated 12-month event-free survival among the patients, who were the first in the study to reach 9-month median follow-up, was 90%, Dr. Wiestner said.
Also, 22 evaluable patients had a median 46% reduction in splenomegaly (median volume reduction of 446 mL), and tumor burden in bone marrow biopsies of 23 patients decreased by a median of 76% as assessed by immunohistochemistry for CD79a.
The percentage of tumor cells with del 17p decreased by a median of 55% in 15 patients, remained unchanged in 1 patient, and increased in 3 patients.
Treatment-naive patients included adults aged 33-82 years, and relapsed/refractory patients were adults aged 56-79 years. All were treated with 420 mg of oral ibrutinib daily until disease progression; treatment response was assessed every 6 months.
Ibrutinib, which has been granted multiple Breakthrough Therapy designations by the Food and Drug Administration – including a recent designation for the treatment of CLL or small lymphocytic lymphoma with del 17p – was well tolerated; 14% of patients experienced grade 3 or higher nonhematologic toxicities. Two deaths occurred among patients in the study, but were not deemed treatment related.
This study was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute.
FROM THE ANNUAL ICML
Major finding: A nodal response with a median 70% reduction in lymph node size was seen in 88% of evaluable patients.
Data source: A phase II study involving 29 CLL del 17p patients.
Disclosures: This study was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute.
Rituximab plus chlorambucil extend event-free survival in MALT lymphoma
Patients who have mucosa-associated lymphoid tissue lymphoma and are treated with chlorambucil plus rituximab have better event-free survival and progression-free survival than do comparable patients treated with either drug alone, according to findings from a randomized phase III study.
In the International Extranodal Lymphoma Study Group study (IELSG-19), 5-year event-free survival was 70% in 131 patients treated with the combination therapy, 52% in 130 patients treated with chlorambucil alone, and 51% in 132 patients treated with rituximab alone. Progression-free survival also was significantly improved in the combination therapy arm compared with the two single-agent arms, Dr. Emanuele Zucca reported at the annual International Conference on Malignant Lymphoma in Lugano, Switzerland.
Overall survival at 5 years, however, was similar at about 90% for all three treatment arms, according to Dr. Zucca of the Oncology Institute of Southern Switzerland, Ospedale San Giovanni, Bellinzona.
Hematologic toxicity was more pronounced in the combination therapy arm than in the single-agent arms, but there were no unexpected severe side effects, Dr. Zucca reported.
IELSG-19 is the largest randomized study to date in MALT lymphoma, and is the first to compare combination chlorambucil and rituximab with both agents alone, he said.
The primary lymphoma site was the stomach in 43% of the393 study participants, and 34% had lymph node involvement. The international prognostic index score was low or low-intermediate in 81%. Just 8% of the patients had prior local therapy, Dr. Zucca said.
Study participants were adults with disseminated extranodal marginal zone B-cell lymphoma or localized disease not amenable to local therapy. Those randomized to the chlorambucil therapy arm received 6 mg/m2 of oral chlorambucil daily for 6 weeks, and those who responded or who had stable disease received the same dose daily for 14 consecutive days every 28 days for four cycles. Those in the combination therapy arm received the chlorambucil dosing, plus 375 mg/m2 of rituximab intravenously on days 1, 8, 15, 22, 56, 84, 112, and 140. Those in the rituximab-only group received the same rituximab dosing without chlorambucil.
In a published report of the outcomes in patients enrolled in the first two protocol arms (combination therapy and chlorambucil-only therapy), Dr. Zucca and his colleagues noted that survival rates in patients with MALT lymphoma are typically high. The significant differences in event-free survival have not yet translated into improved overall survival (J. Clin. Oncol 2013 Jan. 7 [doi:10.1200/JCO.2011.40.6272]).
Nonetheless, the findings – among the first to demonstrate the activity of chlorambucil with and without rituximab in MALT lymphoma – suggest the combination is a safe and effective approach that could improve outcomes, Dr. Zucca said.
The findings he presented expand upon the initial published analysis by including data from the rituximab-only arm, which was added following a more-rapid-than-expected initial recruitment into the combination and chlorambucil-only arms.
These findings are of note, because aside from H. pylori eradication for localized gastric disease, no consensus exists on the standard therapy for MALT lymphoma, he said.
Several study authors reported serving as consultants for or receiving honoraria from Roche International, which sponsored the IELSG-19 study.
Patients who have mucosa-associated lymphoid tissue lymphoma and are treated with chlorambucil plus rituximab have better event-free survival and progression-free survival than do comparable patients treated with either drug alone, according to findings from a randomized phase III study.
In the International Extranodal Lymphoma Study Group study (IELSG-19), 5-year event-free survival was 70% in 131 patients treated with the combination therapy, 52% in 130 patients treated with chlorambucil alone, and 51% in 132 patients treated with rituximab alone. Progression-free survival also was significantly improved in the combination therapy arm compared with the two single-agent arms, Dr. Emanuele Zucca reported at the annual International Conference on Malignant Lymphoma in Lugano, Switzerland.
Overall survival at 5 years, however, was similar at about 90% for all three treatment arms, according to Dr. Zucca of the Oncology Institute of Southern Switzerland, Ospedale San Giovanni, Bellinzona.
Hematologic toxicity was more pronounced in the combination therapy arm than in the single-agent arms, but there were no unexpected severe side effects, Dr. Zucca reported.
IELSG-19 is the largest randomized study to date in MALT lymphoma, and is the first to compare combination chlorambucil and rituximab with both agents alone, he said.
The primary lymphoma site was the stomach in 43% of the393 study participants, and 34% had lymph node involvement. The international prognostic index score was low or low-intermediate in 81%. Just 8% of the patients had prior local therapy, Dr. Zucca said.
Study participants were adults with disseminated extranodal marginal zone B-cell lymphoma or localized disease not amenable to local therapy. Those randomized to the chlorambucil therapy arm received 6 mg/m2 of oral chlorambucil daily for 6 weeks, and those who responded or who had stable disease received the same dose daily for 14 consecutive days every 28 days for four cycles. Those in the combination therapy arm received the chlorambucil dosing, plus 375 mg/m2 of rituximab intravenously on days 1, 8, 15, 22, 56, 84, 112, and 140. Those in the rituximab-only group received the same rituximab dosing without chlorambucil.
In a published report of the outcomes in patients enrolled in the first two protocol arms (combination therapy and chlorambucil-only therapy), Dr. Zucca and his colleagues noted that survival rates in patients with MALT lymphoma are typically high. The significant differences in event-free survival have not yet translated into improved overall survival (J. Clin. Oncol 2013 Jan. 7 [doi:10.1200/JCO.2011.40.6272]).
Nonetheless, the findings – among the first to demonstrate the activity of chlorambucil with and without rituximab in MALT lymphoma – suggest the combination is a safe and effective approach that could improve outcomes, Dr. Zucca said.
The findings he presented expand upon the initial published analysis by including data from the rituximab-only arm, which was added following a more-rapid-than-expected initial recruitment into the combination and chlorambucil-only arms.
These findings are of note, because aside from H. pylori eradication for localized gastric disease, no consensus exists on the standard therapy for MALT lymphoma, he said.
Several study authors reported serving as consultants for or receiving honoraria from Roche International, which sponsored the IELSG-19 study.
Patients who have mucosa-associated lymphoid tissue lymphoma and are treated with chlorambucil plus rituximab have better event-free survival and progression-free survival than do comparable patients treated with either drug alone, according to findings from a randomized phase III study.
In the International Extranodal Lymphoma Study Group study (IELSG-19), 5-year event-free survival was 70% in 131 patients treated with the combination therapy, 52% in 130 patients treated with chlorambucil alone, and 51% in 132 patients treated with rituximab alone. Progression-free survival also was significantly improved in the combination therapy arm compared with the two single-agent arms, Dr. Emanuele Zucca reported at the annual International Conference on Malignant Lymphoma in Lugano, Switzerland.
Overall survival at 5 years, however, was similar at about 90% for all three treatment arms, according to Dr. Zucca of the Oncology Institute of Southern Switzerland, Ospedale San Giovanni, Bellinzona.
Hematologic toxicity was more pronounced in the combination therapy arm than in the single-agent arms, but there were no unexpected severe side effects, Dr. Zucca reported.
IELSG-19 is the largest randomized study to date in MALT lymphoma, and is the first to compare combination chlorambucil and rituximab with both agents alone, he said.
The primary lymphoma site was the stomach in 43% of the393 study participants, and 34% had lymph node involvement. The international prognostic index score was low or low-intermediate in 81%. Just 8% of the patients had prior local therapy, Dr. Zucca said.
Study participants were adults with disseminated extranodal marginal zone B-cell lymphoma or localized disease not amenable to local therapy. Those randomized to the chlorambucil therapy arm received 6 mg/m2 of oral chlorambucil daily for 6 weeks, and those who responded or who had stable disease received the same dose daily for 14 consecutive days every 28 days for four cycles. Those in the combination therapy arm received the chlorambucil dosing, plus 375 mg/m2 of rituximab intravenously on days 1, 8, 15, 22, 56, 84, 112, and 140. Those in the rituximab-only group received the same rituximab dosing without chlorambucil.
In a published report of the outcomes in patients enrolled in the first two protocol arms (combination therapy and chlorambucil-only therapy), Dr. Zucca and his colleagues noted that survival rates in patients with MALT lymphoma are typically high. The significant differences in event-free survival have not yet translated into improved overall survival (J. Clin. Oncol 2013 Jan. 7 [doi:10.1200/JCO.2011.40.6272]).
Nonetheless, the findings – among the first to demonstrate the activity of chlorambucil with and without rituximab in MALT lymphoma – suggest the combination is a safe and effective approach that could improve outcomes, Dr. Zucca said.
The findings he presented expand upon the initial published analysis by including data from the rituximab-only arm, which was added following a more-rapid-than-expected initial recruitment into the combination and chlorambucil-only arms.
These findings are of note, because aside from H. pylori eradication for localized gastric disease, no consensus exists on the standard therapy for MALT lymphoma, he said.
Several study authors reported serving as consultants for or receiving honoraria from Roche International, which sponsored the IELSG-19 study.
FROM THE ANNUAL ICML
Major finding: Five-year event-free survival was 70% with chlorambucil plus rituximab, 52% for chlorambucil and 51% for rituximab.
Data source: A preliminary analysis of data from 393 study participants in the International Extranodal Lymphoma Study Group study (IELSG-19).
Disclosures: Several of the study authors reported serving as consultants for or receiving honoraria from Roche International, which sponsored the IELSG-19 study.


