User login
Isradipine for Parkinson’s disease fails in phase 3 study
PHILADELPHIA - There was no significant difference in Unified Parkinson’s Disease Rating Scale (UPDRS) scores between patients who received the calcium channel blocker isradipine and those who received placebo, according to the final results of the STEADY-PD III study, which will be presented at the annual meeting of the American Academy of Neurology.
Use of the drug to treat high blood pressure has been linked to lower risk of developing Parkinson’s disease, said study author Tanya Simuni, MD, a professor of neurology at Northwestern University, Chicago, in a news release.
“Unfortunately, the people who were taking isradipine did not have any difference in their Parkinson’s symptoms over the 3 years of the study, compared with the people who took a placebo,” Dr. Simuni said in the press release.
Hopes were high that isradipine might be the first drug to slow progression of Parkinson’s disease after promising animal studies and a phase 2 study showing no safety concerns, according to the news release.
The STEADY-PD III study, which was conducted at 54 Parkinson Study Group sites in the United States and Canada, included 336 participants with early Parkinson’s disease randomized to isradipine 10 mg daily or placebo. The median age of patients in the study was 62 years, and 68% were male. The median time from diagnosis was 0.9 years, and the mean UPDRS I-III score at baseline was 23.1, according to an abstract describing the study results.
The primary endpoint was change in UPDRS Part I-III score measured in the ON state from baseline to month 36 of treatment. That change over 36 months was 2.99 points in the isradipine arm and 3.26 points in the placebo arm, for a treatment effect of 0.27 points (95% confidence interval, –2.5 to 3.0; P = 0.85), investigators reported in the abstract. Adjustment for use of symptomatic therapy did not affect the comparison, the researchers noted.
Isradipine had no effect on secondary outcomes, including change in UPDRS-III in the OFF state, use of dopaminergic therapy, motor complications, or quality of life, investigators said in the abstract. Edema was the most notable side effect of isradipine treatment, investigators said.
These findings are “disappointing” but will not deter researchers in their work to find a treatment that will slow Parkinson’s disease progression, Dr. Simuni said in the news release. “Negative results are important because they provide a clear answer, especially for a drug that is commercially available,” she added.
Secondary analyses in progress will explore “biological and clinical correlates of disease progression” among the study participants, researchers said in their study abstract.
The study was supported by the National Institute of Neurological Disorders and Stroke (NINDS) and also received some funding from The Michael J. Fox Foundation for Parkinson’s Research. Dr. Simuni reported disclosures related to AbbVie, Acadia, Accorda, Adamas, Allergan, Anavex, Biogen, Denali, the Michael J. Fox Foundation, Neurocrine, NeuroDerm, NINDS, the Parkinson Foundation, PhotoPharmics, Revance, Roche, Sanofi, Sunovion, Sun Pharma, Takeda, Teva, Voyager, and US World Meds.
PHILADELPHIA - There was no significant difference in Unified Parkinson’s Disease Rating Scale (UPDRS) scores between patients who received the calcium channel blocker isradipine and those who received placebo, according to the final results of the STEADY-PD III study, which will be presented at the annual meeting of the American Academy of Neurology.
Use of the drug to treat high blood pressure has been linked to lower risk of developing Parkinson’s disease, said study author Tanya Simuni, MD, a professor of neurology at Northwestern University, Chicago, in a news release.
“Unfortunately, the people who were taking isradipine did not have any difference in their Parkinson’s symptoms over the 3 years of the study, compared with the people who took a placebo,” Dr. Simuni said in the press release.
Hopes were high that isradipine might be the first drug to slow progression of Parkinson’s disease after promising animal studies and a phase 2 study showing no safety concerns, according to the news release.
The STEADY-PD III study, which was conducted at 54 Parkinson Study Group sites in the United States and Canada, included 336 participants with early Parkinson’s disease randomized to isradipine 10 mg daily or placebo. The median age of patients in the study was 62 years, and 68% were male. The median time from diagnosis was 0.9 years, and the mean UPDRS I-III score at baseline was 23.1, according to an abstract describing the study results.
The primary endpoint was change in UPDRS Part I-III score measured in the ON state from baseline to month 36 of treatment. That change over 36 months was 2.99 points in the isradipine arm and 3.26 points in the placebo arm, for a treatment effect of 0.27 points (95% confidence interval, –2.5 to 3.0; P = 0.85), investigators reported in the abstract. Adjustment for use of symptomatic therapy did not affect the comparison, the researchers noted.
Isradipine had no effect on secondary outcomes, including change in UPDRS-III in the OFF state, use of dopaminergic therapy, motor complications, or quality of life, investigators said in the abstract. Edema was the most notable side effect of isradipine treatment, investigators said.
These findings are “disappointing” but will not deter researchers in their work to find a treatment that will slow Parkinson’s disease progression, Dr. Simuni said in the news release. “Negative results are important because they provide a clear answer, especially for a drug that is commercially available,” she added.
Secondary analyses in progress will explore “biological and clinical correlates of disease progression” among the study participants, researchers said in their study abstract.
The study was supported by the National Institute of Neurological Disorders and Stroke (NINDS) and also received some funding from The Michael J. Fox Foundation for Parkinson’s Research. Dr. Simuni reported disclosures related to AbbVie, Acadia, Accorda, Adamas, Allergan, Anavex, Biogen, Denali, the Michael J. Fox Foundation, Neurocrine, NeuroDerm, NINDS, the Parkinson Foundation, PhotoPharmics, Revance, Roche, Sanofi, Sunovion, Sun Pharma, Takeda, Teva, Voyager, and US World Meds.
PHILADELPHIA - There was no significant difference in Unified Parkinson’s Disease Rating Scale (UPDRS) scores between patients who received the calcium channel blocker isradipine and those who received placebo, according to the final results of the STEADY-PD III study, which will be presented at the annual meeting of the American Academy of Neurology.
Use of the drug to treat high blood pressure has been linked to lower risk of developing Parkinson’s disease, said study author Tanya Simuni, MD, a professor of neurology at Northwestern University, Chicago, in a news release.
“Unfortunately, the people who were taking isradipine did not have any difference in their Parkinson’s symptoms over the 3 years of the study, compared with the people who took a placebo,” Dr. Simuni said in the press release.
Hopes were high that isradipine might be the first drug to slow progression of Parkinson’s disease after promising animal studies and a phase 2 study showing no safety concerns, according to the news release.
The STEADY-PD III study, which was conducted at 54 Parkinson Study Group sites in the United States and Canada, included 336 participants with early Parkinson’s disease randomized to isradipine 10 mg daily or placebo. The median age of patients in the study was 62 years, and 68% were male. The median time from diagnosis was 0.9 years, and the mean UPDRS I-III score at baseline was 23.1, according to an abstract describing the study results.
The primary endpoint was change in UPDRS Part I-III score measured in the ON state from baseline to month 36 of treatment. That change over 36 months was 2.99 points in the isradipine arm and 3.26 points in the placebo arm, for a treatment effect of 0.27 points (95% confidence interval, –2.5 to 3.0; P = 0.85), investigators reported in the abstract. Adjustment for use of symptomatic therapy did not affect the comparison, the researchers noted.
Isradipine had no effect on secondary outcomes, including change in UPDRS-III in the OFF state, use of dopaminergic therapy, motor complications, or quality of life, investigators said in the abstract. Edema was the most notable side effect of isradipine treatment, investigators said.
These findings are “disappointing” but will not deter researchers in their work to find a treatment that will slow Parkinson’s disease progression, Dr. Simuni said in the news release. “Negative results are important because they provide a clear answer, especially for a drug that is commercially available,” she added.
Secondary analyses in progress will explore “biological and clinical correlates of disease progression” among the study participants, researchers said in their study abstract.
The study was supported by the National Institute of Neurological Disorders and Stroke (NINDS) and also received some funding from The Michael J. Fox Foundation for Parkinson’s Research. Dr. Simuni reported disclosures related to AbbVie, Acadia, Accorda, Adamas, Allergan, Anavex, Biogen, Denali, the Michael J. Fox Foundation, Neurocrine, NeuroDerm, NINDS, the Parkinson Foundation, PhotoPharmics, Revance, Roche, Sanofi, Sunovion, Sun Pharma, Takeda, Teva, Voyager, and US World Meds.
FROM AAN 2019
Opicapone increased on-time without dyskinesia in patients with Parkinson’s disease
PHILADELPHIA -
The 2-hour improvement was considered clinically meaningful, although the average patient in the studies had about 6 hours of off-time, said investigator Peter LeWitt, MD, of Henry Ford Hospital in West Bloomfield, Mich., and the department of neurology at Wayne State University, Detroit. Dr. LeWitt and colleagues will present the data at the annual meeting of the American Academy of Neurology.
“While this is a substantial improvement, it is 2 hours improvement over a total of 6 hours of off-time, which is not perfect,” Dr. LeWitt said in an interview. “So how could we do better is the challenge for all of us who are doing research.”
Opicapone is under development in the United States; it is currently approved in the European Union as adjunctive therapy to preparations of levodopa/DOPA decarboxylase inhibitors for patients with Parkinson’s disease and end-of-dose motor fluctuations.
The ability of opicapone to prolong the clinical actions of levodopa has been evaluated in BIPARK-1 and BIPARK-2. These two international phase 3 studies evaluated the third-generation COMT inhibitor against placebo and, in the case of BIPARK-1, against the COMT inhibitor entacapone as an active control. Each study was 14-15 weeks in duration and included a 1-year open-label phase.
In BIPARK-1, on-time without troublesome dyskinesia was significantly increased for opicapone 50 mg versus placebo, with an absolute increase of 1.9 versus 0.9 hours, respectively, from baseline to week 14 or 15 (P = .002), investigators said. Similarly, BIPARK-2 data showed an increase in this endpoint, at 1.7 versus 0.9 hours for opicapone and placebo, respectively (P = .025).
The 50-mg dose of opicapone was received by 115 patients in BIPARK-1 and 147 patients in BIPARK-2, while placebo was received by 120 and 135 patients in those two studies, respectively.
In the long-term extension studies, the mean change in on-time without dyskinesia from baseline to the end of the open-label endpoint was 2.0 hours for all 494 opicapone-treated patients in BIPARK-1 and 1.8 hours for all 339 opicapone-treated patients in BIPARK-2.
Dyskinesia was reported as a treatment-emergent adverse effect for 17.4% of opicapone-treated patients and 6.2% of placebo-treated patients, according to results of a pooled safety analysis of BIPARK-1 and BIPARK-2. However, only 1.9% of opicapone-treated patients and 0.4% of placebo-treated patients had treatment-emergent dyskinesia leading to discontinuation, and the dyskinesia was considered serious in 0.3% of the opicapone group and 0.0% of the placebo group, investigators added.
Neurocrine Biosciences has announced plans to file a New Drug Application for opicapone for Parkinson’s disease in the United States. That filing is expected to take place in the second quarter of 2019, according to an April 29 press release.
Dr. LeWitt disclosed that he has served as an advisor to Neurocrine Biosciences. He also provided disclosures related to Acadia, Acorda, Adamas, BioElectron Technology, Biotie, Britannia, Intec, Jazz Pharmaceuticals, Lundbeck, the Michael J. Fox Foundation for Parkinson’s Research, Merz, NeuroDerm, the Parkinson Study Group, Pfizer, Prexton, Sage, Scion, Sunovion, SynAgile, and US WorldMeds.
SOURCE: LeWitt P et al. AAN 2019, Abstract S4.003.
PHILADELPHIA -
The 2-hour improvement was considered clinically meaningful, although the average patient in the studies had about 6 hours of off-time, said investigator Peter LeWitt, MD, of Henry Ford Hospital in West Bloomfield, Mich., and the department of neurology at Wayne State University, Detroit. Dr. LeWitt and colleagues will present the data at the annual meeting of the American Academy of Neurology.
“While this is a substantial improvement, it is 2 hours improvement over a total of 6 hours of off-time, which is not perfect,” Dr. LeWitt said in an interview. “So how could we do better is the challenge for all of us who are doing research.”
Opicapone is under development in the United States; it is currently approved in the European Union as adjunctive therapy to preparations of levodopa/DOPA decarboxylase inhibitors for patients with Parkinson’s disease and end-of-dose motor fluctuations.
The ability of opicapone to prolong the clinical actions of levodopa has been evaluated in BIPARK-1 and BIPARK-2. These two international phase 3 studies evaluated the third-generation COMT inhibitor against placebo and, in the case of BIPARK-1, against the COMT inhibitor entacapone as an active control. Each study was 14-15 weeks in duration and included a 1-year open-label phase.
In BIPARK-1, on-time without troublesome dyskinesia was significantly increased for opicapone 50 mg versus placebo, with an absolute increase of 1.9 versus 0.9 hours, respectively, from baseline to week 14 or 15 (P = .002), investigators said. Similarly, BIPARK-2 data showed an increase in this endpoint, at 1.7 versus 0.9 hours for opicapone and placebo, respectively (P = .025).
The 50-mg dose of opicapone was received by 115 patients in BIPARK-1 and 147 patients in BIPARK-2, while placebo was received by 120 and 135 patients in those two studies, respectively.
In the long-term extension studies, the mean change in on-time without dyskinesia from baseline to the end of the open-label endpoint was 2.0 hours for all 494 opicapone-treated patients in BIPARK-1 and 1.8 hours for all 339 opicapone-treated patients in BIPARK-2.
Dyskinesia was reported as a treatment-emergent adverse effect for 17.4% of opicapone-treated patients and 6.2% of placebo-treated patients, according to results of a pooled safety analysis of BIPARK-1 and BIPARK-2. However, only 1.9% of opicapone-treated patients and 0.4% of placebo-treated patients had treatment-emergent dyskinesia leading to discontinuation, and the dyskinesia was considered serious in 0.3% of the opicapone group and 0.0% of the placebo group, investigators added.
Neurocrine Biosciences has announced plans to file a New Drug Application for opicapone for Parkinson’s disease in the United States. That filing is expected to take place in the second quarter of 2019, according to an April 29 press release.
Dr. LeWitt disclosed that he has served as an advisor to Neurocrine Biosciences. He also provided disclosures related to Acadia, Acorda, Adamas, BioElectron Technology, Biotie, Britannia, Intec, Jazz Pharmaceuticals, Lundbeck, the Michael J. Fox Foundation for Parkinson’s Research, Merz, NeuroDerm, the Parkinson Study Group, Pfizer, Prexton, Sage, Scion, Sunovion, SynAgile, and US WorldMeds.
SOURCE: LeWitt P et al. AAN 2019, Abstract S4.003.
PHILADELPHIA -
The 2-hour improvement was considered clinically meaningful, although the average patient in the studies had about 6 hours of off-time, said investigator Peter LeWitt, MD, of Henry Ford Hospital in West Bloomfield, Mich., and the department of neurology at Wayne State University, Detroit. Dr. LeWitt and colleagues will present the data at the annual meeting of the American Academy of Neurology.
“While this is a substantial improvement, it is 2 hours improvement over a total of 6 hours of off-time, which is not perfect,” Dr. LeWitt said in an interview. “So how could we do better is the challenge for all of us who are doing research.”
Opicapone is under development in the United States; it is currently approved in the European Union as adjunctive therapy to preparations of levodopa/DOPA decarboxylase inhibitors for patients with Parkinson’s disease and end-of-dose motor fluctuations.
The ability of opicapone to prolong the clinical actions of levodopa has been evaluated in BIPARK-1 and BIPARK-2. These two international phase 3 studies evaluated the third-generation COMT inhibitor against placebo and, in the case of BIPARK-1, against the COMT inhibitor entacapone as an active control. Each study was 14-15 weeks in duration and included a 1-year open-label phase.
In BIPARK-1, on-time without troublesome dyskinesia was significantly increased for opicapone 50 mg versus placebo, with an absolute increase of 1.9 versus 0.9 hours, respectively, from baseline to week 14 or 15 (P = .002), investigators said. Similarly, BIPARK-2 data showed an increase in this endpoint, at 1.7 versus 0.9 hours for opicapone and placebo, respectively (P = .025).
The 50-mg dose of opicapone was received by 115 patients in BIPARK-1 and 147 patients in BIPARK-2, while placebo was received by 120 and 135 patients in those two studies, respectively.
In the long-term extension studies, the mean change in on-time without dyskinesia from baseline to the end of the open-label endpoint was 2.0 hours for all 494 opicapone-treated patients in BIPARK-1 and 1.8 hours for all 339 opicapone-treated patients in BIPARK-2.
Dyskinesia was reported as a treatment-emergent adverse effect for 17.4% of opicapone-treated patients and 6.2% of placebo-treated patients, according to results of a pooled safety analysis of BIPARK-1 and BIPARK-2. However, only 1.9% of opicapone-treated patients and 0.4% of placebo-treated patients had treatment-emergent dyskinesia leading to discontinuation, and the dyskinesia was considered serious in 0.3% of the opicapone group and 0.0% of the placebo group, investigators added.
Neurocrine Biosciences has announced plans to file a New Drug Application for opicapone for Parkinson’s disease in the United States. That filing is expected to take place in the second quarter of 2019, according to an April 29 press release.
Dr. LeWitt disclosed that he has served as an advisor to Neurocrine Biosciences. He also provided disclosures related to Acadia, Acorda, Adamas, BioElectron Technology, Biotie, Britannia, Intec, Jazz Pharmaceuticals, Lundbeck, the Michael J. Fox Foundation for Parkinson’s Research, Merz, NeuroDerm, the Parkinson Study Group, Pfizer, Prexton, Sage, Scion, Sunovion, SynAgile, and US WorldMeds.
SOURCE: LeWitt P et al. AAN 2019, Abstract S4.003.
FROM AAN 2019
Can intraputamenal infusions of GDNF treat Parkinson’s disease?
researchers reported. The investigational therapy, delivered through a skull-mounted port, was well tolerated in a 40-week, randomized, controlled trial and a 40-week, open-label extension.
Neither study met its primary endpoint, but post hoc analyses suggest possible clinical benefits. In addition, PET imaging after the 40-week, randomized trial found significantly increased 18F-DOPA uptake in patients who received GDNF. The randomized trial was published in the March 2019 issue of Brain; data from the open-label extension were published online ahead of print Feb. 26, 2019, in the Journal of Parkinson’s Disease.
“The spatial and relative magnitude of the improvement in the brain scans is beyond anything seen previously in trials of surgically delivered growth-factor treatments for Parkinson’s [disease],” said principal investigator Alan L. Whone, MBChB, PhD, of the University of Bristol (England) and North Bristol National Health Service Trust. “This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s [disease].”
Nevertheless, the trial did not confirm clinical benefits. The hypothesis that growth factors can benefit patients with Parkinson’s disease may be incorrect, the researchers acknowledged. It also is possible that the hypothesis is valid and that a trial with a higher GDNF dose, longer treatment duration, patients with an earlier disease stage, or different outcome measures would yield positive results. GDNF warrants further study, they wrote.
The findings could have implications for other neurologic disorders as well.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patients’ brains over months or years. This is a significant breakthrough in our ability to treat neurologic conditions ... because most drugs that might work cannot cross from the bloodstream into the brain,” said Steven Gill, MB, MS. Mr. Gill, of the North Bristol NHS Trust and the U.K.-based engineering firm Renishaw, designed the convection-enhanced delivery system used in the studies.
A neurotrophic protein
GDNF has neurorestorative and neuroprotective effects in animal models of Parkinson’s disease. In open-label studies, continuous, low-rate intraputamenal administration of GDNF has shown signs of potential efficacy, but a placebo-controlled trial did not replicate clinical benefits. In the present studies, the researchers assessed intermittent GDNF administration using convection-enhanced delivery, which can achieve wider and more even distribution of GDNF, compared with the previous approach.
The researchers conducted a single-center, randomized, double-blind, placebo-controlled trial to study this novel administration approach. Patients were aged 35-75 years, had motor symptoms for at least 5 years, and had moderate disease severity in the off state (that is, Hoehn and Yahr stage 2-3 and Unified Parkinson’s Disease Rating Scale motor score–part III [UPDRS-III] of 25-45).
In a pilot stage of the trial, six patients were randomized 2:1 to receive GDNF (120 mcg per putamen) or placebo. In the primary stage, another 35 patients were randomized 1:1 to GDNF or placebo. The primary outcome was the percentage change from baseline to week 40 in the off-state UPDRS-III among patients from the primary stage of the trial. Further analyses included all 41 patients from the pilot and primary stages.
Patients in the primary analysis had a mean age of 56.4 years and mean disease duration of 10.9 years. About half were female.
Results on primary and secondary clinical endpoints did not significantly differ between the groups. Average off state UPDRS motor score decreased by 17.3 in the active treatment group, compared with 11.8 in the placebo group.
A post hoc analysis, however, found that nine patients (43%) in the active-treatment group had a large, clinically important motor improvement of 10 or more points in the off state, whereas no placebo patients did. These “10-point responders in the GDNF group are a potential focus of interest; however, as this is a post hoc finding we would not wish to overinterpret its meaning,” Dr. Whone and his colleagues wrote. Among patients who received GDNF, PET imaging demonstrated significantly increased 18F-DOPA uptake throughout the putamen, ranging from a 25% increase in the left anterior putamen to a 100% increase in both posterior putamena, whereas patients who received placebo did not have significantly increased uptake.
No drug-related serious adverse events were reported. “The majority of device-related adverse events were port site associated, most commonly local hypertrophic scarring or infections, amenable to antibiotics,” the investigators wrote. “The frequency of these declined during the trial as surgical and device handling experience improved.”
Open-label extension
By week 80, when all participants had received GDNF, both groups showed moderate to large improvement in symptoms, compared with baseline. From baseline to week 80, percentage change in UPDRS motor score in the off state did not significantly differ between patients who received GDNF for 80 weeks and patients who received placebo followed by GDNF (26.7% vs. 27.6%). Secondary endpoints also did not differ between the groups. Treatment compliance was 97.8%; no patients discontinued the study.
The trials were funded by Parkinson’s UK with support from the Cure Parkinson’s Trust and in association with the North Bristol NHS Trust. GDNF and additional resources and funding were provided by MedGenesis Therapeutix, which owns the license for GDNF and received funding from the Michael J. Fox Foundation for Parkinson’s Research. Renishaw manufactured the convection-enhanced delivery device on behalf of North Bristol NHS Trust. The Gatsby Foundation provided a 3T MRI scanner. Some study authors are employed by and have shares or share options with MedGenesis Therapeutix. Other authors are employees of Renishaw. Dr. Gill is Renishaw’s medical director and may have a future royalty share from the drug delivery system that he invented.
SOURCES: Whone AL et al. Brain. 2019 Feb 26. doi: 10.1093/brain/awz023; Whone AL et al. J Parkinsons Dis. 2019 Feb 26. doi: 10.3233/JPD-191576.
researchers reported. The investigational therapy, delivered through a skull-mounted port, was well tolerated in a 40-week, randomized, controlled trial and a 40-week, open-label extension.
Neither study met its primary endpoint, but post hoc analyses suggest possible clinical benefits. In addition, PET imaging after the 40-week, randomized trial found significantly increased 18F-DOPA uptake in patients who received GDNF. The randomized trial was published in the March 2019 issue of Brain; data from the open-label extension were published online ahead of print Feb. 26, 2019, in the Journal of Parkinson’s Disease.
“The spatial and relative magnitude of the improvement in the brain scans is beyond anything seen previously in trials of surgically delivered growth-factor treatments for Parkinson’s [disease],” said principal investigator Alan L. Whone, MBChB, PhD, of the University of Bristol (England) and North Bristol National Health Service Trust. “This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s [disease].”
Nevertheless, the trial did not confirm clinical benefits. The hypothesis that growth factors can benefit patients with Parkinson’s disease may be incorrect, the researchers acknowledged. It also is possible that the hypothesis is valid and that a trial with a higher GDNF dose, longer treatment duration, patients with an earlier disease stage, or different outcome measures would yield positive results. GDNF warrants further study, they wrote.
The findings could have implications for other neurologic disorders as well.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patients’ brains over months or years. This is a significant breakthrough in our ability to treat neurologic conditions ... because most drugs that might work cannot cross from the bloodstream into the brain,” said Steven Gill, MB, MS. Mr. Gill, of the North Bristol NHS Trust and the U.K.-based engineering firm Renishaw, designed the convection-enhanced delivery system used in the studies.
A neurotrophic protein
GDNF has neurorestorative and neuroprotective effects in animal models of Parkinson’s disease. In open-label studies, continuous, low-rate intraputamenal administration of GDNF has shown signs of potential efficacy, but a placebo-controlled trial did not replicate clinical benefits. In the present studies, the researchers assessed intermittent GDNF administration using convection-enhanced delivery, which can achieve wider and more even distribution of GDNF, compared with the previous approach.
The researchers conducted a single-center, randomized, double-blind, placebo-controlled trial to study this novel administration approach. Patients were aged 35-75 years, had motor symptoms for at least 5 years, and had moderate disease severity in the off state (that is, Hoehn and Yahr stage 2-3 and Unified Parkinson’s Disease Rating Scale motor score–part III [UPDRS-III] of 25-45).
In a pilot stage of the trial, six patients were randomized 2:1 to receive GDNF (120 mcg per putamen) or placebo. In the primary stage, another 35 patients were randomized 1:1 to GDNF or placebo. The primary outcome was the percentage change from baseline to week 40 in the off-state UPDRS-III among patients from the primary stage of the trial. Further analyses included all 41 patients from the pilot and primary stages.
Patients in the primary analysis had a mean age of 56.4 years and mean disease duration of 10.9 years. About half were female.
Results on primary and secondary clinical endpoints did not significantly differ between the groups. Average off state UPDRS motor score decreased by 17.3 in the active treatment group, compared with 11.8 in the placebo group.
A post hoc analysis, however, found that nine patients (43%) in the active-treatment group had a large, clinically important motor improvement of 10 or more points in the off state, whereas no placebo patients did. These “10-point responders in the GDNF group are a potential focus of interest; however, as this is a post hoc finding we would not wish to overinterpret its meaning,” Dr. Whone and his colleagues wrote. Among patients who received GDNF, PET imaging demonstrated significantly increased 18F-DOPA uptake throughout the putamen, ranging from a 25% increase in the left anterior putamen to a 100% increase in both posterior putamena, whereas patients who received placebo did not have significantly increased uptake.
No drug-related serious adverse events were reported. “The majority of device-related adverse events were port site associated, most commonly local hypertrophic scarring or infections, amenable to antibiotics,” the investigators wrote. “The frequency of these declined during the trial as surgical and device handling experience improved.”
Open-label extension
By week 80, when all participants had received GDNF, both groups showed moderate to large improvement in symptoms, compared with baseline. From baseline to week 80, percentage change in UPDRS motor score in the off state did not significantly differ between patients who received GDNF for 80 weeks and patients who received placebo followed by GDNF (26.7% vs. 27.6%). Secondary endpoints also did not differ between the groups. Treatment compliance was 97.8%; no patients discontinued the study.
The trials were funded by Parkinson’s UK with support from the Cure Parkinson’s Trust and in association with the North Bristol NHS Trust. GDNF and additional resources and funding were provided by MedGenesis Therapeutix, which owns the license for GDNF and received funding from the Michael J. Fox Foundation for Parkinson’s Research. Renishaw manufactured the convection-enhanced delivery device on behalf of North Bristol NHS Trust. The Gatsby Foundation provided a 3T MRI scanner. Some study authors are employed by and have shares or share options with MedGenesis Therapeutix. Other authors are employees of Renishaw. Dr. Gill is Renishaw’s medical director and may have a future royalty share from the drug delivery system that he invented.
SOURCES: Whone AL et al. Brain. 2019 Feb 26. doi: 10.1093/brain/awz023; Whone AL et al. J Parkinsons Dis. 2019 Feb 26. doi: 10.3233/JPD-191576.
researchers reported. The investigational therapy, delivered through a skull-mounted port, was well tolerated in a 40-week, randomized, controlled trial and a 40-week, open-label extension.
Neither study met its primary endpoint, but post hoc analyses suggest possible clinical benefits. In addition, PET imaging after the 40-week, randomized trial found significantly increased 18F-DOPA uptake in patients who received GDNF. The randomized trial was published in the March 2019 issue of Brain; data from the open-label extension were published online ahead of print Feb. 26, 2019, in the Journal of Parkinson’s Disease.
“The spatial and relative magnitude of the improvement in the brain scans is beyond anything seen previously in trials of surgically delivered growth-factor treatments for Parkinson’s [disease],” said principal investigator Alan L. Whone, MBChB, PhD, of the University of Bristol (England) and North Bristol National Health Service Trust. “This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s [disease].”
Nevertheless, the trial did not confirm clinical benefits. The hypothesis that growth factors can benefit patients with Parkinson’s disease may be incorrect, the researchers acknowledged. It also is possible that the hypothesis is valid and that a trial with a higher GDNF dose, longer treatment duration, patients with an earlier disease stage, or different outcome measures would yield positive results. GDNF warrants further study, they wrote.
The findings could have implications for other neurologic disorders as well.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patients’ brains over months or years. This is a significant breakthrough in our ability to treat neurologic conditions ... because most drugs that might work cannot cross from the bloodstream into the brain,” said Steven Gill, MB, MS. Mr. Gill, of the North Bristol NHS Trust and the U.K.-based engineering firm Renishaw, designed the convection-enhanced delivery system used in the studies.
A neurotrophic protein
GDNF has neurorestorative and neuroprotective effects in animal models of Parkinson’s disease. In open-label studies, continuous, low-rate intraputamenal administration of GDNF has shown signs of potential efficacy, but a placebo-controlled trial did not replicate clinical benefits. In the present studies, the researchers assessed intermittent GDNF administration using convection-enhanced delivery, which can achieve wider and more even distribution of GDNF, compared with the previous approach.
The researchers conducted a single-center, randomized, double-blind, placebo-controlled trial to study this novel administration approach. Patients were aged 35-75 years, had motor symptoms for at least 5 years, and had moderate disease severity in the off state (that is, Hoehn and Yahr stage 2-3 and Unified Parkinson’s Disease Rating Scale motor score–part III [UPDRS-III] of 25-45).
In a pilot stage of the trial, six patients were randomized 2:1 to receive GDNF (120 mcg per putamen) or placebo. In the primary stage, another 35 patients were randomized 1:1 to GDNF or placebo. The primary outcome was the percentage change from baseline to week 40 in the off-state UPDRS-III among patients from the primary stage of the trial. Further analyses included all 41 patients from the pilot and primary stages.
Patients in the primary analysis had a mean age of 56.4 years and mean disease duration of 10.9 years. About half were female.
Results on primary and secondary clinical endpoints did not significantly differ between the groups. Average off state UPDRS motor score decreased by 17.3 in the active treatment group, compared with 11.8 in the placebo group.
A post hoc analysis, however, found that nine patients (43%) in the active-treatment group had a large, clinically important motor improvement of 10 or more points in the off state, whereas no placebo patients did. These “10-point responders in the GDNF group are a potential focus of interest; however, as this is a post hoc finding we would not wish to overinterpret its meaning,” Dr. Whone and his colleagues wrote. Among patients who received GDNF, PET imaging demonstrated significantly increased 18F-DOPA uptake throughout the putamen, ranging from a 25% increase in the left anterior putamen to a 100% increase in both posterior putamena, whereas patients who received placebo did not have significantly increased uptake.
No drug-related serious adverse events were reported. “The majority of device-related adverse events were port site associated, most commonly local hypertrophic scarring or infections, amenable to antibiotics,” the investigators wrote. “The frequency of these declined during the trial as surgical and device handling experience improved.”
Open-label extension
By week 80, when all participants had received GDNF, both groups showed moderate to large improvement in symptoms, compared with baseline. From baseline to week 80, percentage change in UPDRS motor score in the off state did not significantly differ between patients who received GDNF for 80 weeks and patients who received placebo followed by GDNF (26.7% vs. 27.6%). Secondary endpoints also did not differ between the groups. Treatment compliance was 97.8%; no patients discontinued the study.
The trials were funded by Parkinson’s UK with support from the Cure Parkinson’s Trust and in association with the North Bristol NHS Trust. GDNF and additional resources and funding were provided by MedGenesis Therapeutix, which owns the license for GDNF and received funding from the Michael J. Fox Foundation for Parkinson’s Research. Renishaw manufactured the convection-enhanced delivery device on behalf of North Bristol NHS Trust. The Gatsby Foundation provided a 3T MRI scanner. Some study authors are employed by and have shares or share options with MedGenesis Therapeutix. Other authors are employees of Renishaw. Dr. Gill is Renishaw’s medical director and may have a future royalty share from the drug delivery system that he invented.
SOURCES: Whone AL et al. Brain. 2019 Feb 26. doi: 10.1093/brain/awz023; Whone AL et al. J Parkinsons Dis. 2019 Feb 26. doi: 10.3233/JPD-191576.
Does adherence to a Mediterranean diet reduce the risk of Parkinson’s disease?
Among older adults, adherence to a Mediterranean diet is associated with lower probability of prodromal Parkinson’s disease, according to research published in Movement Disorders.
“Recommending the Mediterranean diet pattern, either to reduce the risk or lessen the effects ... of prodromal Parkinson’s disease, needs to be considered and further explored,” said lead author Maria I. Maraki, PhD, of the department of nutrition and dietetics at Harokopio University in Athens, Greece, and her research colleagues.
Evidence regarding the effect of a Mediterranean diet on Parkinson’s disease risk remains limited, however, and physicians should be cautious in interpreting the data, researchers noted in accompanying editorials.
“There is a puzzling constellation of information and data that cannot be reconciled with a simple model accounting for the role of diet, vascular risk factors, and the neurodegenerative process and mechanisms underlying Parkinson’s disease,” Connie Marras, MD, PhD, and Jose A. Obeso, MD, PhD, said in an editorial. Given Maraki et al.’s findings, “most of us would be glad to accept that such a causal inverse association exists and can therefore be strongly recommended to our patients,” but “further work is needed before definitive conclusions can be reached,” Dr. Marras and Dr. Obeso wrote. Dr. Marras is affiliated with the University Health Network and the University of Toronto. Dr. Obeso is affiliated with University Hospital HM Puerta del Sur, CEU San Pablo University, Móstoles, Spain.
The role of diet
Prior research has suggested that adherence to the Mediterranean diet – characterized by consumption of nonrefined cereals, fruits, vegetables, legumes, potatoes, fish, and olive oil – may be associated with reduced risk of Parkinson’s disease. In addition, studies have found that adherence to the Mediterranean diet may be protective in other diseases, including dementia and cardiovascular disease. Dr. Maraki and her colleagues sought to assess whether adherence to the Mediterranean diet is associated with the likelihood of prodromal Parkinson’s disease or its manifestations. To calculate the probability of prodromal Parkinson’s disease, the investigators used a tool created by the International Parkinson and Movement Disorder Society (MDS) that takes into account baseline risk factors as well as prodromal markers such as constipation and motor slowing.
They analyzed data from 1,731 participants in the population-based Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) cohort in Greece. Participants, 41% of whom were male, were aged 65 years or older and did not have Parkinson’s disease. They completed a detailed food frequency questionnaire, and the researchers calculated how closely each participant’s diet adhered to the Mediterranean diet. Diet adherence scores ranged from 0 to 55, with higher scores indicating greater adherence.
The median probability of prodromal Parkinson’s disease was 1.9% (range, 0.2%-96.7%), and the probability was lower among those with greater adherence to the Mediterranean diet. This difference was “driven mostly by nonmotor markers of prodromal Parkinson’s disease,” including depression, constipation, urinary dysfunction, and daytime somnolence, the researchers said. “Each unit increase in Mediterranean diet score was associated with a 2% decreased probability for prodromal Parkinson’s disease.” Compared with participants in the lowest quartile of Mediterranean diet adherence, those in the highest quartile had an approximately 21% lower probability for prodromal Parkinson’s disease.
Potential confounding
“This study pushes the prodromal criteria into performing a job they were never designed to do,” which presents potential pitfalls, Ronald B. Postuma, MD, of the department of neurology at Montreal General Hospital in Quebec, said in an accompanying editorial.
While the MDS criteria were designed to assess the likelihood that any person over age 50 years is in a state of prodromal Parkinson’s disease, the present study aimed to evaluate whether a single putative risk factor for Parkinson’s disease is associated with the likelihood of its prodromal state.
In addition, the analysis did not include some of the prodromal markers that are part of the MDS criteria, including olfaction, polysomnographic-proven REM sleep behavior disorder, and dopaminergic functional neuroimaging.
“As pointed out by the researchers, many of the risk factors in the prodromal criteria are potentially confounded by factors other than Parkinson’s disease; for example, one could imagine that older people, men, or farmers (with their higher pesticide exposure) are less likely to follow the Mediterranean diet simply because of different cultural lifestyle patterns,” Dr. Postuma said.
It is also possible that the Mediterranean diet affects prodromal markers such as constipation, sleep, or depression without affecting underlying neurodegenerative disease. In any case, the effect sizes observed in the study were small, and there was no evidence that participants who adhered most closely to a Mediterranean diet had less parkinsonism, Dr. Postuma said.
These limitations do not preclude physicians from recommending the diet for other reasons. “Numerous studies, reviews, meta-analyses, and randomized controlled trials consistently rank the Mediterranean diet as among the healthiest diets available,” Dr. Postuma said. “So, one can clearly recommend diets such as these, even if not necessarily for Parkinson’s disease prevention.”
Adding insights
The researchers used a Mediterranean diet score that was developed in a population of adults from metropolitan Athens, “an area not unlike the one in which the score is being applied in the HELIAD study,” Christy C. Tangney, PhD, professor of clinical nutrition and preventive medicine and associate dean for research at Rush University Medical Center, Chicago, said in a separate editorial. As expected, the average Mediterranean diet adherence score in this study was higher than that in the Chicago Health and Aging Project (33.2 vs. 28.2).
“If we can identify differences in diet or lifestyle patterns and risk of this latent phase of Parkinson’s disease neurodegeneration, we may be one step closer to identifying preventive measures,” she said. Follow-up reports from HELIAD and other cohorts may allow researchers to assess how changes in dietary patterns relate to changes in Parkinson’s disease markers, the probability of prodromal Parkinson’s disease, and incident Parkinson’s disease, Dr. Tangney said.
The study authors had no conflicts of interest or financial disclosures. The study was supported by a grant from the Alzheimer’s Association, an ESPA‐EU grant cofunded by the European Social Fund and Greek National resources, and a grant from the Ministry for Health and Social Solidarity (Greece). Dr. Maraki and a coauthor have received financial support from the Greek State Scholarships Foundation. Dr. Tangney and Dr. Postuma had no conflicts of interest.
SOURCE: Maraki MI et al. Mov Disord. 2018 Oct 10. doi: 10.1002/mds.27489.
Among older adults, adherence to a Mediterranean diet is associated with lower probability of prodromal Parkinson’s disease, according to research published in Movement Disorders.
“Recommending the Mediterranean diet pattern, either to reduce the risk or lessen the effects ... of prodromal Parkinson’s disease, needs to be considered and further explored,” said lead author Maria I. Maraki, PhD, of the department of nutrition and dietetics at Harokopio University in Athens, Greece, and her research colleagues.
Evidence regarding the effect of a Mediterranean diet on Parkinson’s disease risk remains limited, however, and physicians should be cautious in interpreting the data, researchers noted in accompanying editorials.
“There is a puzzling constellation of information and data that cannot be reconciled with a simple model accounting for the role of diet, vascular risk factors, and the neurodegenerative process and mechanisms underlying Parkinson’s disease,” Connie Marras, MD, PhD, and Jose A. Obeso, MD, PhD, said in an editorial. Given Maraki et al.’s findings, “most of us would be glad to accept that such a causal inverse association exists and can therefore be strongly recommended to our patients,” but “further work is needed before definitive conclusions can be reached,” Dr. Marras and Dr. Obeso wrote. Dr. Marras is affiliated with the University Health Network and the University of Toronto. Dr. Obeso is affiliated with University Hospital HM Puerta del Sur, CEU San Pablo University, Móstoles, Spain.
The role of diet
Prior research has suggested that adherence to the Mediterranean diet – characterized by consumption of nonrefined cereals, fruits, vegetables, legumes, potatoes, fish, and olive oil – may be associated with reduced risk of Parkinson’s disease. In addition, studies have found that adherence to the Mediterranean diet may be protective in other diseases, including dementia and cardiovascular disease. Dr. Maraki and her colleagues sought to assess whether adherence to the Mediterranean diet is associated with the likelihood of prodromal Parkinson’s disease or its manifestations. To calculate the probability of prodromal Parkinson’s disease, the investigators used a tool created by the International Parkinson and Movement Disorder Society (MDS) that takes into account baseline risk factors as well as prodromal markers such as constipation and motor slowing.
They analyzed data from 1,731 participants in the population-based Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) cohort in Greece. Participants, 41% of whom were male, were aged 65 years or older and did not have Parkinson’s disease. They completed a detailed food frequency questionnaire, and the researchers calculated how closely each participant’s diet adhered to the Mediterranean diet. Diet adherence scores ranged from 0 to 55, with higher scores indicating greater adherence.
The median probability of prodromal Parkinson’s disease was 1.9% (range, 0.2%-96.7%), and the probability was lower among those with greater adherence to the Mediterranean diet. This difference was “driven mostly by nonmotor markers of prodromal Parkinson’s disease,” including depression, constipation, urinary dysfunction, and daytime somnolence, the researchers said. “Each unit increase in Mediterranean diet score was associated with a 2% decreased probability for prodromal Parkinson’s disease.” Compared with participants in the lowest quartile of Mediterranean diet adherence, those in the highest quartile had an approximately 21% lower probability for prodromal Parkinson’s disease.
Potential confounding
“This study pushes the prodromal criteria into performing a job they were never designed to do,” which presents potential pitfalls, Ronald B. Postuma, MD, of the department of neurology at Montreal General Hospital in Quebec, said in an accompanying editorial.
While the MDS criteria were designed to assess the likelihood that any person over age 50 years is in a state of prodromal Parkinson’s disease, the present study aimed to evaluate whether a single putative risk factor for Parkinson’s disease is associated with the likelihood of its prodromal state.
In addition, the analysis did not include some of the prodromal markers that are part of the MDS criteria, including olfaction, polysomnographic-proven REM sleep behavior disorder, and dopaminergic functional neuroimaging.
“As pointed out by the researchers, many of the risk factors in the prodromal criteria are potentially confounded by factors other than Parkinson’s disease; for example, one could imagine that older people, men, or farmers (with their higher pesticide exposure) are less likely to follow the Mediterranean diet simply because of different cultural lifestyle patterns,” Dr. Postuma said.
It is also possible that the Mediterranean diet affects prodromal markers such as constipation, sleep, or depression without affecting underlying neurodegenerative disease. In any case, the effect sizes observed in the study were small, and there was no evidence that participants who adhered most closely to a Mediterranean diet had less parkinsonism, Dr. Postuma said.
These limitations do not preclude physicians from recommending the diet for other reasons. “Numerous studies, reviews, meta-analyses, and randomized controlled trials consistently rank the Mediterranean diet as among the healthiest diets available,” Dr. Postuma said. “So, one can clearly recommend diets such as these, even if not necessarily for Parkinson’s disease prevention.”
Adding insights
The researchers used a Mediterranean diet score that was developed in a population of adults from metropolitan Athens, “an area not unlike the one in which the score is being applied in the HELIAD study,” Christy C. Tangney, PhD, professor of clinical nutrition and preventive medicine and associate dean for research at Rush University Medical Center, Chicago, said in a separate editorial. As expected, the average Mediterranean diet adherence score in this study was higher than that in the Chicago Health and Aging Project (33.2 vs. 28.2).
“If we can identify differences in diet or lifestyle patterns and risk of this latent phase of Parkinson’s disease neurodegeneration, we may be one step closer to identifying preventive measures,” she said. Follow-up reports from HELIAD and other cohorts may allow researchers to assess how changes in dietary patterns relate to changes in Parkinson’s disease markers, the probability of prodromal Parkinson’s disease, and incident Parkinson’s disease, Dr. Tangney said.
The study authors had no conflicts of interest or financial disclosures. The study was supported by a grant from the Alzheimer’s Association, an ESPA‐EU grant cofunded by the European Social Fund and Greek National resources, and a grant from the Ministry for Health and Social Solidarity (Greece). Dr. Maraki and a coauthor have received financial support from the Greek State Scholarships Foundation. Dr. Tangney and Dr. Postuma had no conflicts of interest.
SOURCE: Maraki MI et al. Mov Disord. 2018 Oct 10. doi: 10.1002/mds.27489.
Among older adults, adherence to a Mediterranean diet is associated with lower probability of prodromal Parkinson’s disease, according to research published in Movement Disorders.
“Recommending the Mediterranean diet pattern, either to reduce the risk or lessen the effects ... of prodromal Parkinson’s disease, needs to be considered and further explored,” said lead author Maria I. Maraki, PhD, of the department of nutrition and dietetics at Harokopio University in Athens, Greece, and her research colleagues.
Evidence regarding the effect of a Mediterranean diet on Parkinson’s disease risk remains limited, however, and physicians should be cautious in interpreting the data, researchers noted in accompanying editorials.
“There is a puzzling constellation of information and data that cannot be reconciled with a simple model accounting for the role of diet, vascular risk factors, and the neurodegenerative process and mechanisms underlying Parkinson’s disease,” Connie Marras, MD, PhD, and Jose A. Obeso, MD, PhD, said in an editorial. Given Maraki et al.’s findings, “most of us would be glad to accept that such a causal inverse association exists and can therefore be strongly recommended to our patients,” but “further work is needed before definitive conclusions can be reached,” Dr. Marras and Dr. Obeso wrote. Dr. Marras is affiliated with the University Health Network and the University of Toronto. Dr. Obeso is affiliated with University Hospital HM Puerta del Sur, CEU San Pablo University, Móstoles, Spain.
The role of diet
Prior research has suggested that adherence to the Mediterranean diet – characterized by consumption of nonrefined cereals, fruits, vegetables, legumes, potatoes, fish, and olive oil – may be associated with reduced risk of Parkinson’s disease. In addition, studies have found that adherence to the Mediterranean diet may be protective in other diseases, including dementia and cardiovascular disease. Dr. Maraki and her colleagues sought to assess whether adherence to the Mediterranean diet is associated with the likelihood of prodromal Parkinson’s disease or its manifestations. To calculate the probability of prodromal Parkinson’s disease, the investigators used a tool created by the International Parkinson and Movement Disorder Society (MDS) that takes into account baseline risk factors as well as prodromal markers such as constipation and motor slowing.
They analyzed data from 1,731 participants in the population-based Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) cohort in Greece. Participants, 41% of whom were male, were aged 65 years or older and did not have Parkinson’s disease. They completed a detailed food frequency questionnaire, and the researchers calculated how closely each participant’s diet adhered to the Mediterranean diet. Diet adherence scores ranged from 0 to 55, with higher scores indicating greater adherence.
The median probability of prodromal Parkinson’s disease was 1.9% (range, 0.2%-96.7%), and the probability was lower among those with greater adherence to the Mediterranean diet. This difference was “driven mostly by nonmotor markers of prodromal Parkinson’s disease,” including depression, constipation, urinary dysfunction, and daytime somnolence, the researchers said. “Each unit increase in Mediterranean diet score was associated with a 2% decreased probability for prodromal Parkinson’s disease.” Compared with participants in the lowest quartile of Mediterranean diet adherence, those in the highest quartile had an approximately 21% lower probability for prodromal Parkinson’s disease.
Potential confounding
“This study pushes the prodromal criteria into performing a job they were never designed to do,” which presents potential pitfalls, Ronald B. Postuma, MD, of the department of neurology at Montreal General Hospital in Quebec, said in an accompanying editorial.
While the MDS criteria were designed to assess the likelihood that any person over age 50 years is in a state of prodromal Parkinson’s disease, the present study aimed to evaluate whether a single putative risk factor for Parkinson’s disease is associated with the likelihood of its prodromal state.
In addition, the analysis did not include some of the prodromal markers that are part of the MDS criteria, including olfaction, polysomnographic-proven REM sleep behavior disorder, and dopaminergic functional neuroimaging.
“As pointed out by the researchers, many of the risk factors in the prodromal criteria are potentially confounded by factors other than Parkinson’s disease; for example, one could imagine that older people, men, or farmers (with their higher pesticide exposure) are less likely to follow the Mediterranean diet simply because of different cultural lifestyle patterns,” Dr. Postuma said.
It is also possible that the Mediterranean diet affects prodromal markers such as constipation, sleep, or depression without affecting underlying neurodegenerative disease. In any case, the effect sizes observed in the study were small, and there was no evidence that participants who adhered most closely to a Mediterranean diet had less parkinsonism, Dr. Postuma said.
These limitations do not preclude physicians from recommending the diet for other reasons. “Numerous studies, reviews, meta-analyses, and randomized controlled trials consistently rank the Mediterranean diet as among the healthiest diets available,” Dr. Postuma said. “So, one can clearly recommend diets such as these, even if not necessarily for Parkinson’s disease prevention.”
Adding insights
The researchers used a Mediterranean diet score that was developed in a population of adults from metropolitan Athens, “an area not unlike the one in which the score is being applied in the HELIAD study,” Christy C. Tangney, PhD, professor of clinical nutrition and preventive medicine and associate dean for research at Rush University Medical Center, Chicago, said in a separate editorial. As expected, the average Mediterranean diet adherence score in this study was higher than that in the Chicago Health and Aging Project (33.2 vs. 28.2).
“If we can identify differences in diet or lifestyle patterns and risk of this latent phase of Parkinson’s disease neurodegeneration, we may be one step closer to identifying preventive measures,” she said. Follow-up reports from HELIAD and other cohorts may allow researchers to assess how changes in dietary patterns relate to changes in Parkinson’s disease markers, the probability of prodromal Parkinson’s disease, and incident Parkinson’s disease, Dr. Tangney said.
The study authors had no conflicts of interest or financial disclosures. The study was supported by a grant from the Alzheimer’s Association, an ESPA‐EU grant cofunded by the European Social Fund and Greek National resources, and a grant from the Ministry for Health and Social Solidarity (Greece). Dr. Maraki and a coauthor have received financial support from the Greek State Scholarships Foundation. Dr. Tangney and Dr. Postuma had no conflicts of interest.
SOURCE: Maraki MI et al. Mov Disord. 2018 Oct 10. doi: 10.1002/mds.27489.
FROM MOVEMENT DISORDERS
Key clinical point: Adherence to a Mediterranean diet is associated with lower probability of prodromal Parkinson’s disease.
Major finding: Each 1-unit increase in Mediterranean diet score was associated with a 2% decreased probability for prodromal Parkinson’s disease.
Study details: A study of 1,731 older adults in the population-based Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) cohort in Greece.
Disclosures: The study authors had no conflicts of interest or financial disclosures. The study was supported by a grant from the Alzheimer’s Association, an ESPA‐EU grant cofunded by the European Social Fund and Greek National resources, and a grant from the Ministry for Health and Social Solidarity (Greece). Dr. Maraki and a coauthor have received financial support from the Greek State Scholarships Foundation.
Source: Maraki MI et al. Mov Disord. 2018 Oct 10. doi:10.1002/mds.27489.
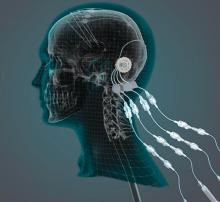
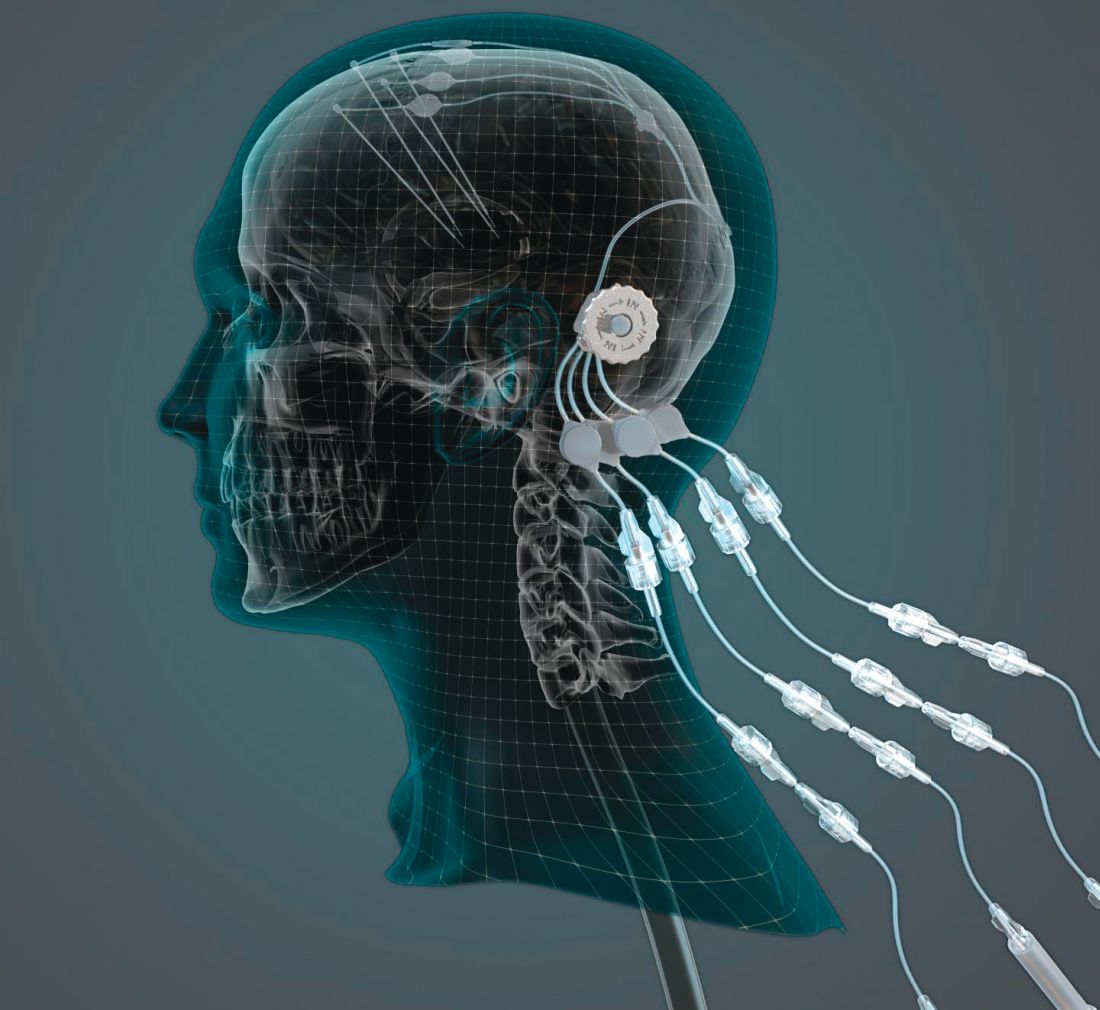
Researchers compare focused ultrasound and DBS for essential tremor
LAS VEGAS – according to two presentations delivered at the annual meeting of the North American Neuromodulation Society. The techniques’ surgical procedures, associated risks, and adverse event profiles may influence neurologists and patients in their choice of treatment.
FUS allows neurosurgeons to apply thermal ablation to create a lesion on the thalamus. MRI guidance enables precise control of the lesion location (within approximately 1 mm) and of the treatment intensity. The surgery can be performed with high-resolution stereotactic framing.
DBS entails the surgical implantation of a neurostimulator and attached leads and electrodes. The neurosurgeon drills a hole of approximately 14 mm in diameter into the skull so that the electrode can be inserted stereotactically while the patient is awake or asleep. The neurostimulator is installed separately.
Both treatments provide functional benefits
In 2016, W. Jeff Elias, MD, director of stereotactic and functional neurosurgery at the University of Virginia in Charlottesville, and his colleagues published the results of a randomized controlled trial that compared FUS with sham treatment in 76 patients with essential tremor. At three months, hand tremor had improved by approximately 50% among treated patients, but controls had no significant benefit(N Engl J Med. 2016 Aug 25;375[8]:730-9). The improvement among treated patients was maintained for 12 months. Disability and quality of life also improved after FUS.
A study by Schuurman et al. published in 2000 (N Engl J Med. 2000 Feb 17;342[7]:461-8) showed that DBS and FUS had similar efficacy at 1 year, said Kathryn L. Holloway, MD, professor of neurosurgery at Virginia Commonwealth University in Richmond. It included 45 patients with Parkinson’s disease, 13 with essential tremor, and 10 with multiple sclerosis who were randomized 1:1 to FUS or DBS. The primary outcome was activities of daily living, and blinded physicians assessed patient videos. Most of the patients who improved had received DBS, and most of the ones who worsened had received FUS, said Dr. Holloway. Among patients with essential tremor, tremor improved by between 94% and 100% with either treatment.
To find more recent data about these treatments, Dr. Holloway searched the literature for studies of FUS or DBS for essential tremor. She analyzed only studies that included unselected populations, blinded evaluations within 1 or 2 years of surgery, and tremor scores for the treated side. She found two studies of FUS, including Dr. Elias’s 2016 trial and a 2018 follow-up (Ann Neurol. 2018 Jan;83[1]:107-14). Dr. Holloway also identified three trials of DBS.
In these studies, reduction of hand tremor was 55% with FUS and between 63% and 69% with DBS. Reduction of postural tremor was approximately 72% with FUS and approximately 67% with DBS. Reduction of action tremor was about 52% with FUS and between 65% and 71% with DBS. Overall, DBS appears to be more effective, said Dr. Holloway.
A 2015 study (Mov Disord. 2015 Dec;30[14]:1937-43) that compared bilateral DBS, unilateral DBS, and unilateral FUS for essential tremor indicated that the treatments provide similar benefits on hand tremor, disability, and quality of life, said Dr. Elias. FUS is inferior to DBS, however, for total tremor and axial tremor.
Furthermore, the efficacy of FUS wanes over time, said Dr. Elias. He and his colleagues conducted a pilot study of 15 patients with essential tremor who received FUS (N Engl J Med. 2013 Aug 15;369[7]:640-8). At 6 years, 6 of 13 patients whose data were available still had a 50% improvement in tremor. “Some went on to [receive] DBS,” said Dr. Elias. “Functional improvements persisted more than the tremor improvement.”
Adverse events
In their 2016 trial of FUS, Dr. Elias and his colleagues observed 210 adverse events, which is approximately “what you would expect with a modern day, FDA-monitored clinical trial.” Sensory effects and gait disturbance accounted for most of the thalamotomy-related adverse events. Sensory problems such as numbness or parestheisa persisted at 1 year in 14% of treated patients, and gait disturbance persisted at 1 year in 9%. The investigators did not observe any hemorrhages, infections, or cavitation-related effects from FUS.
In a 2018 analysis of five clinical trials of FUS for essential tremor, Fishman et al. found that 79% of adverse events were mild and 1% were severe (Mov Disord. 2018 May;33[5]:843-7). The risk of a severe adverse event therefore can be considered low, and it may decrease as neurosurgeons gain experience with the procedure, said Dr. Elias.
In the 2000 Schuurman et al. study, the researchers observed significantly fewer adverse events overall among patients with Parkinson’s disease or essential tremor who received DBS, compared with patients who received FUS. Cognitive deterioration, severe dysarthria, and severe ataxia were more common in the FUS group than in the DBS group. Dr. Holloway’s analysis of adverse events in the five more recent trials that she identified yielded similar results.
Although MRI-guided FUS is a precise way to make lesions, functional areas in the thalamus overlap, which makes it more difficult to target only the intended region, said Dr. Holloway. The functional overlap thus increases the risk of adverse events (e.g., sensory impairments, dysarthria, or ataxia). The adverse events that result from FUS may last as long as a year. “Patients will put up anything for about a month after surgery, and then they start to get annoyed,” said Dr. Holloway.
In addition, Schuurman et al. found that FUS entailed a greater risk of permanent side effects, compared with DBS. “That’s the key point here,” said Dr. Holloway. Most of the adverse effects in the DBS group were resolved by adjusting or turning off the stimulator. Hardware issues resulting from DBS are frustrating, but reversible, but a patient with an adverse event after FUS often is “stuck with it,” said Dr. Holloway. The Schuurman et al. data indicated that, in terms of adverse events, “thalamotomy was inferior to DBS,” she added.
Implantation of DBS entails the risks inherent to surgeries that open the skull (such as seizures, air embolism, and hemorrhage). DBS entails a 2% risk of hemorrhage or infection, said Dr. Elias. Furthermore, as much as 15% of patients who undergo DBS implantation require additional surgery.
“FUS is not going to cause a life-threatening hemorrhage, but DBS certainly can,” said Dr. Holloway.
Managing disease progression
Essential tremor is a progressive disease, and older patients are more likely to have exponential progression than linear progression. Data, such as those published by Zhang et al. (J Neurosurg. 2010 Jun;112[6]:1271-6), indicate that DBS can “keep up with the progression of the disease,” said Dr. Holloway. The authors found that tremor scores did not change significantly over approximately 5 years when patients with essential tremor who had received DBS implantation had periodic assessments and increases in stimulation parameters when appropriate.
If a patient with essential tremor undergoes FUS thalamotomy and has subsequent disease progression, DBS may be considered for reducing tremor, said Dr. Holloway. Most adverse events resulting from DBS implantation are reversible with adjustment of the stimulation parameters. A second thalamotomy, however, could cause severe dysarthria and other irreversible adverse events. “Only DBS can safely address tremor progression,” said Dr. Holloway.
LAS VEGAS – according to two presentations delivered at the annual meeting of the North American Neuromodulation Society. The techniques’ surgical procedures, associated risks, and adverse event profiles may influence neurologists and patients in their choice of treatment.
FUS allows neurosurgeons to apply thermal ablation to create a lesion on the thalamus. MRI guidance enables precise control of the lesion location (within approximately 1 mm) and of the treatment intensity. The surgery can be performed with high-resolution stereotactic framing.
DBS entails the surgical implantation of a neurostimulator and attached leads and electrodes. The neurosurgeon drills a hole of approximately 14 mm in diameter into the skull so that the electrode can be inserted stereotactically while the patient is awake or asleep. The neurostimulator is installed separately.
Both treatments provide functional benefits
In 2016, W. Jeff Elias, MD, director of stereotactic and functional neurosurgery at the University of Virginia in Charlottesville, and his colleagues published the results of a randomized controlled trial that compared FUS with sham treatment in 76 patients with essential tremor. At three months, hand tremor had improved by approximately 50% among treated patients, but controls had no significant benefit(N Engl J Med. 2016 Aug 25;375[8]:730-9). The improvement among treated patients was maintained for 12 months. Disability and quality of life also improved after FUS.
A study by Schuurman et al. published in 2000 (N Engl J Med. 2000 Feb 17;342[7]:461-8) showed that DBS and FUS had similar efficacy at 1 year, said Kathryn L. Holloway, MD, professor of neurosurgery at Virginia Commonwealth University in Richmond. It included 45 patients with Parkinson’s disease, 13 with essential tremor, and 10 with multiple sclerosis who were randomized 1:1 to FUS or DBS. The primary outcome was activities of daily living, and blinded physicians assessed patient videos. Most of the patients who improved had received DBS, and most of the ones who worsened had received FUS, said Dr. Holloway. Among patients with essential tremor, tremor improved by between 94% and 100% with either treatment.
To find more recent data about these treatments, Dr. Holloway searched the literature for studies of FUS or DBS for essential tremor. She analyzed only studies that included unselected populations, blinded evaluations within 1 or 2 years of surgery, and tremor scores for the treated side. She found two studies of FUS, including Dr. Elias’s 2016 trial and a 2018 follow-up (Ann Neurol. 2018 Jan;83[1]:107-14). Dr. Holloway also identified three trials of DBS.
In these studies, reduction of hand tremor was 55% with FUS and between 63% and 69% with DBS. Reduction of postural tremor was approximately 72% with FUS and approximately 67% with DBS. Reduction of action tremor was about 52% with FUS and between 65% and 71% with DBS. Overall, DBS appears to be more effective, said Dr. Holloway.
A 2015 study (Mov Disord. 2015 Dec;30[14]:1937-43) that compared bilateral DBS, unilateral DBS, and unilateral FUS for essential tremor indicated that the treatments provide similar benefits on hand tremor, disability, and quality of life, said Dr. Elias. FUS is inferior to DBS, however, for total tremor and axial tremor.
Furthermore, the efficacy of FUS wanes over time, said Dr. Elias. He and his colleagues conducted a pilot study of 15 patients with essential tremor who received FUS (N Engl J Med. 2013 Aug 15;369[7]:640-8). At 6 years, 6 of 13 patients whose data were available still had a 50% improvement in tremor. “Some went on to [receive] DBS,” said Dr. Elias. “Functional improvements persisted more than the tremor improvement.”
Adverse events
In their 2016 trial of FUS, Dr. Elias and his colleagues observed 210 adverse events, which is approximately “what you would expect with a modern day, FDA-monitored clinical trial.” Sensory effects and gait disturbance accounted for most of the thalamotomy-related adverse events. Sensory problems such as numbness or parestheisa persisted at 1 year in 14% of treated patients, and gait disturbance persisted at 1 year in 9%. The investigators did not observe any hemorrhages, infections, or cavitation-related effects from FUS.
In a 2018 analysis of five clinical trials of FUS for essential tremor, Fishman et al. found that 79% of adverse events were mild and 1% were severe (Mov Disord. 2018 May;33[5]:843-7). The risk of a severe adverse event therefore can be considered low, and it may decrease as neurosurgeons gain experience with the procedure, said Dr. Elias.
In the 2000 Schuurman et al. study, the researchers observed significantly fewer adverse events overall among patients with Parkinson’s disease or essential tremor who received DBS, compared with patients who received FUS. Cognitive deterioration, severe dysarthria, and severe ataxia were more common in the FUS group than in the DBS group. Dr. Holloway’s analysis of adverse events in the five more recent trials that she identified yielded similar results.
Although MRI-guided FUS is a precise way to make lesions, functional areas in the thalamus overlap, which makes it more difficult to target only the intended region, said Dr. Holloway. The functional overlap thus increases the risk of adverse events (e.g., sensory impairments, dysarthria, or ataxia). The adverse events that result from FUS may last as long as a year. “Patients will put up anything for about a month after surgery, and then they start to get annoyed,” said Dr. Holloway.
In addition, Schuurman et al. found that FUS entailed a greater risk of permanent side effects, compared with DBS. “That’s the key point here,” said Dr. Holloway. Most of the adverse effects in the DBS group were resolved by adjusting or turning off the stimulator. Hardware issues resulting from DBS are frustrating, but reversible, but a patient with an adverse event after FUS often is “stuck with it,” said Dr. Holloway. The Schuurman et al. data indicated that, in terms of adverse events, “thalamotomy was inferior to DBS,” she added.
Implantation of DBS entails the risks inherent to surgeries that open the skull (such as seizures, air embolism, and hemorrhage). DBS entails a 2% risk of hemorrhage or infection, said Dr. Elias. Furthermore, as much as 15% of patients who undergo DBS implantation require additional surgery.
“FUS is not going to cause a life-threatening hemorrhage, but DBS certainly can,” said Dr. Holloway.
Managing disease progression
Essential tremor is a progressive disease, and older patients are more likely to have exponential progression than linear progression. Data, such as those published by Zhang et al. (J Neurosurg. 2010 Jun;112[6]:1271-6), indicate that DBS can “keep up with the progression of the disease,” said Dr. Holloway. The authors found that tremor scores did not change significantly over approximately 5 years when patients with essential tremor who had received DBS implantation had periodic assessments and increases in stimulation parameters when appropriate.
If a patient with essential tremor undergoes FUS thalamotomy and has subsequent disease progression, DBS may be considered for reducing tremor, said Dr. Holloway. Most adverse events resulting from DBS implantation are reversible with adjustment of the stimulation parameters. A second thalamotomy, however, could cause severe dysarthria and other irreversible adverse events. “Only DBS can safely address tremor progression,” said Dr. Holloway.
LAS VEGAS – according to two presentations delivered at the annual meeting of the North American Neuromodulation Society. The techniques’ surgical procedures, associated risks, and adverse event profiles may influence neurologists and patients in their choice of treatment.
FUS allows neurosurgeons to apply thermal ablation to create a lesion on the thalamus. MRI guidance enables precise control of the lesion location (within approximately 1 mm) and of the treatment intensity. The surgery can be performed with high-resolution stereotactic framing.
DBS entails the surgical implantation of a neurostimulator and attached leads and electrodes. The neurosurgeon drills a hole of approximately 14 mm in diameter into the skull so that the electrode can be inserted stereotactically while the patient is awake or asleep. The neurostimulator is installed separately.
Both treatments provide functional benefits
In 2016, W. Jeff Elias, MD, director of stereotactic and functional neurosurgery at the University of Virginia in Charlottesville, and his colleagues published the results of a randomized controlled trial that compared FUS with sham treatment in 76 patients with essential tremor. At three months, hand tremor had improved by approximately 50% among treated patients, but controls had no significant benefit(N Engl J Med. 2016 Aug 25;375[8]:730-9). The improvement among treated patients was maintained for 12 months. Disability and quality of life also improved after FUS.
A study by Schuurman et al. published in 2000 (N Engl J Med. 2000 Feb 17;342[7]:461-8) showed that DBS and FUS had similar efficacy at 1 year, said Kathryn L. Holloway, MD, professor of neurosurgery at Virginia Commonwealth University in Richmond. It included 45 patients with Parkinson’s disease, 13 with essential tremor, and 10 with multiple sclerosis who were randomized 1:1 to FUS or DBS. The primary outcome was activities of daily living, and blinded physicians assessed patient videos. Most of the patients who improved had received DBS, and most of the ones who worsened had received FUS, said Dr. Holloway. Among patients with essential tremor, tremor improved by between 94% and 100% with either treatment.
To find more recent data about these treatments, Dr. Holloway searched the literature for studies of FUS or DBS for essential tremor. She analyzed only studies that included unselected populations, blinded evaluations within 1 or 2 years of surgery, and tremor scores for the treated side. She found two studies of FUS, including Dr. Elias’s 2016 trial and a 2018 follow-up (Ann Neurol. 2018 Jan;83[1]:107-14). Dr. Holloway also identified three trials of DBS.
In these studies, reduction of hand tremor was 55% with FUS and between 63% and 69% with DBS. Reduction of postural tremor was approximately 72% with FUS and approximately 67% with DBS. Reduction of action tremor was about 52% with FUS and between 65% and 71% with DBS. Overall, DBS appears to be more effective, said Dr. Holloway.
A 2015 study (Mov Disord. 2015 Dec;30[14]:1937-43) that compared bilateral DBS, unilateral DBS, and unilateral FUS for essential tremor indicated that the treatments provide similar benefits on hand tremor, disability, and quality of life, said Dr. Elias. FUS is inferior to DBS, however, for total tremor and axial tremor.
Furthermore, the efficacy of FUS wanes over time, said Dr. Elias. He and his colleagues conducted a pilot study of 15 patients with essential tremor who received FUS (N Engl J Med. 2013 Aug 15;369[7]:640-8). At 6 years, 6 of 13 patients whose data were available still had a 50% improvement in tremor. “Some went on to [receive] DBS,” said Dr. Elias. “Functional improvements persisted more than the tremor improvement.”
Adverse events
In their 2016 trial of FUS, Dr. Elias and his colleagues observed 210 adverse events, which is approximately “what you would expect with a modern day, FDA-monitored clinical trial.” Sensory effects and gait disturbance accounted for most of the thalamotomy-related adverse events. Sensory problems such as numbness or parestheisa persisted at 1 year in 14% of treated patients, and gait disturbance persisted at 1 year in 9%. The investigators did not observe any hemorrhages, infections, or cavitation-related effects from FUS.
In a 2018 analysis of five clinical trials of FUS for essential tremor, Fishman et al. found that 79% of adverse events were mild and 1% were severe (Mov Disord. 2018 May;33[5]:843-7). The risk of a severe adverse event therefore can be considered low, and it may decrease as neurosurgeons gain experience with the procedure, said Dr. Elias.
In the 2000 Schuurman et al. study, the researchers observed significantly fewer adverse events overall among patients with Parkinson’s disease or essential tremor who received DBS, compared with patients who received FUS. Cognitive deterioration, severe dysarthria, and severe ataxia were more common in the FUS group than in the DBS group. Dr. Holloway’s analysis of adverse events in the five more recent trials that she identified yielded similar results.
Although MRI-guided FUS is a precise way to make lesions, functional areas in the thalamus overlap, which makes it more difficult to target only the intended region, said Dr. Holloway. The functional overlap thus increases the risk of adverse events (e.g., sensory impairments, dysarthria, or ataxia). The adverse events that result from FUS may last as long as a year. “Patients will put up anything for about a month after surgery, and then they start to get annoyed,” said Dr. Holloway.
In addition, Schuurman et al. found that FUS entailed a greater risk of permanent side effects, compared with DBS. “That’s the key point here,” said Dr. Holloway. Most of the adverse effects in the DBS group were resolved by adjusting or turning off the stimulator. Hardware issues resulting from DBS are frustrating, but reversible, but a patient with an adverse event after FUS often is “stuck with it,” said Dr. Holloway. The Schuurman et al. data indicated that, in terms of adverse events, “thalamotomy was inferior to DBS,” she added.
Implantation of DBS entails the risks inherent to surgeries that open the skull (such as seizures, air embolism, and hemorrhage). DBS entails a 2% risk of hemorrhage or infection, said Dr. Elias. Furthermore, as much as 15% of patients who undergo DBS implantation require additional surgery.
“FUS is not going to cause a life-threatening hemorrhage, but DBS certainly can,” said Dr. Holloway.
Managing disease progression
Essential tremor is a progressive disease, and older patients are more likely to have exponential progression than linear progression. Data, such as those published by Zhang et al. (J Neurosurg. 2010 Jun;112[6]:1271-6), indicate that DBS can “keep up with the progression of the disease,” said Dr. Holloway. The authors found that tremor scores did not change significantly over approximately 5 years when patients with essential tremor who had received DBS implantation had periodic assessments and increases in stimulation parameters when appropriate.
If a patient with essential tremor undergoes FUS thalamotomy and has subsequent disease progression, DBS may be considered for reducing tremor, said Dr. Holloway. Most adverse events resulting from DBS implantation are reversible with adjustment of the stimulation parameters. A second thalamotomy, however, could cause severe dysarthria and other irreversible adverse events. “Only DBS can safely address tremor progression,” said Dr. Holloway.
REPORTING FROM NANS 2019
DBS may improve nonmotor symptoms in Parkinson’s disease
LAS VEGAS – , according to a small study presented at the annual meeting of the North American Neuromodulation Society. DBS of the subthalamic nucleus (STN), however, does not significantly improve these symptoms.
“Further work will be needed to confirm whether DBS needs to be bilateral ... and whether demographic differences are significant,” said Michael Gillogly, RN, clinical research nurse in the department of neurosurgery at Albany (New York) Medical Center. “The pilot data suggest that, if all else is equal, and the patient has significant urinary dysfunction as a major complaint, GPI DBS may be preferentially considered.”
The benefits of DBS on motor symptoms in Parkinson’s disease are well documented in the literature, but the technique’s effects on nonmotor symptoms are less clear. Nonmotor symptoms – such as cognitive deficits, gastrointestinal dysfunction, genitourinary dysfunction, and sleep disturbance – are common in all stages of Parkinson’s disease and significantly impair quality of life. Data indicate that speech and neuropsychological symptoms worsen with DBS of the STN, but research into the effect of DBS of the GPI on nonmotor symptoms is limited.
Mr. Gillogly and his colleagues considered all surgical candidates at their facility for enrollment into a study evaluating nonmotor outcomes in Parkinson’s disease at baseline, before implantation, and at 6 months after DBS. Study outcomes were patient perception of urinary, swallowing, and gastrointestinal function at 6 months after DBS of the GPI, compared with DBS of the STN.
The researchers chose two tools each to measure sialorrhea, dysphagia, and genitourinary dysfunction. These tools included the Drooling Severity and Frequency Scale (DSFS), the Swallowing Disturbance Questionnaire, and the International Prostate Symptom Score (IPSS). The investigators also collected demographic information, including sex, age at the time of surgery, duration of illness, neuropsychological profile, and medication inventory.
In all, 34 patients (12 women) were enrolled in the study and completed each outcome measure preoperatively and at 6 months postoperatively. The mean age of our subjects at the time of surgery was 64 years. Eight received DBS of the GPI, and 26 received DBS of the STN. Mr. Gillogly and his colleagues observed a significant 31% improvement in DSFS score and a significant 24% improvement on the IPSS among GPI-targeted patients. They found no significant improvements among patients who had STN targeting. When the investigators compared patients with unilateral lead placement and those with bilateral lead placement, they observed that all of the significant improvement among patients with GPI targeting occurred when treatment was bilateral.
The small sample size is a notable limitation of the study, and subset analyses were limited, said Mr. Gillogly. In addition, it was difficult to determine whether the symptoms studied were directly related to Parkinson’s disease, because they often arise as part of the natural aging process. “Other limitations of the study include lack of objective measurements, as these are all patient perception, and the innate limitations of self-reported questionnaires,” said Mr. Gillogly.
Two of the researchers reported having consulted for Medtronic, which markets a DBS system. One author received grant funding and consulting fees from Boston Scientific, Medtronic, and Abbott, all of which make DBS devices.
LAS VEGAS – , according to a small study presented at the annual meeting of the North American Neuromodulation Society. DBS of the subthalamic nucleus (STN), however, does not significantly improve these symptoms.
“Further work will be needed to confirm whether DBS needs to be bilateral ... and whether demographic differences are significant,” said Michael Gillogly, RN, clinical research nurse in the department of neurosurgery at Albany (New York) Medical Center. “The pilot data suggest that, if all else is equal, and the patient has significant urinary dysfunction as a major complaint, GPI DBS may be preferentially considered.”
The benefits of DBS on motor symptoms in Parkinson’s disease are well documented in the literature, but the technique’s effects on nonmotor symptoms are less clear. Nonmotor symptoms – such as cognitive deficits, gastrointestinal dysfunction, genitourinary dysfunction, and sleep disturbance – are common in all stages of Parkinson’s disease and significantly impair quality of life. Data indicate that speech and neuropsychological symptoms worsen with DBS of the STN, but research into the effect of DBS of the GPI on nonmotor symptoms is limited.
Mr. Gillogly and his colleagues considered all surgical candidates at their facility for enrollment into a study evaluating nonmotor outcomes in Parkinson’s disease at baseline, before implantation, and at 6 months after DBS. Study outcomes were patient perception of urinary, swallowing, and gastrointestinal function at 6 months after DBS of the GPI, compared with DBS of the STN.
The researchers chose two tools each to measure sialorrhea, dysphagia, and genitourinary dysfunction. These tools included the Drooling Severity and Frequency Scale (DSFS), the Swallowing Disturbance Questionnaire, and the International Prostate Symptom Score (IPSS). The investigators also collected demographic information, including sex, age at the time of surgery, duration of illness, neuropsychological profile, and medication inventory.
In all, 34 patients (12 women) were enrolled in the study and completed each outcome measure preoperatively and at 6 months postoperatively. The mean age of our subjects at the time of surgery was 64 years. Eight received DBS of the GPI, and 26 received DBS of the STN. Mr. Gillogly and his colleagues observed a significant 31% improvement in DSFS score and a significant 24% improvement on the IPSS among GPI-targeted patients. They found no significant improvements among patients who had STN targeting. When the investigators compared patients with unilateral lead placement and those with bilateral lead placement, they observed that all of the significant improvement among patients with GPI targeting occurred when treatment was bilateral.
The small sample size is a notable limitation of the study, and subset analyses were limited, said Mr. Gillogly. In addition, it was difficult to determine whether the symptoms studied were directly related to Parkinson’s disease, because they often arise as part of the natural aging process. “Other limitations of the study include lack of objective measurements, as these are all patient perception, and the innate limitations of self-reported questionnaires,” said Mr. Gillogly.
Two of the researchers reported having consulted for Medtronic, which markets a DBS system. One author received grant funding and consulting fees from Boston Scientific, Medtronic, and Abbott, all of which make DBS devices.
LAS VEGAS – , according to a small study presented at the annual meeting of the North American Neuromodulation Society. DBS of the subthalamic nucleus (STN), however, does not significantly improve these symptoms.
“Further work will be needed to confirm whether DBS needs to be bilateral ... and whether demographic differences are significant,” said Michael Gillogly, RN, clinical research nurse in the department of neurosurgery at Albany (New York) Medical Center. “The pilot data suggest that, if all else is equal, and the patient has significant urinary dysfunction as a major complaint, GPI DBS may be preferentially considered.”
The benefits of DBS on motor symptoms in Parkinson’s disease are well documented in the literature, but the technique’s effects on nonmotor symptoms are less clear. Nonmotor symptoms – such as cognitive deficits, gastrointestinal dysfunction, genitourinary dysfunction, and sleep disturbance – are common in all stages of Parkinson’s disease and significantly impair quality of life. Data indicate that speech and neuropsychological symptoms worsen with DBS of the STN, but research into the effect of DBS of the GPI on nonmotor symptoms is limited.
Mr. Gillogly and his colleagues considered all surgical candidates at their facility for enrollment into a study evaluating nonmotor outcomes in Parkinson’s disease at baseline, before implantation, and at 6 months after DBS. Study outcomes were patient perception of urinary, swallowing, and gastrointestinal function at 6 months after DBS of the GPI, compared with DBS of the STN.
The researchers chose two tools each to measure sialorrhea, dysphagia, and genitourinary dysfunction. These tools included the Drooling Severity and Frequency Scale (DSFS), the Swallowing Disturbance Questionnaire, and the International Prostate Symptom Score (IPSS). The investigators also collected demographic information, including sex, age at the time of surgery, duration of illness, neuropsychological profile, and medication inventory.
In all, 34 patients (12 women) were enrolled in the study and completed each outcome measure preoperatively and at 6 months postoperatively. The mean age of our subjects at the time of surgery was 64 years. Eight received DBS of the GPI, and 26 received DBS of the STN. Mr. Gillogly and his colleagues observed a significant 31% improvement in DSFS score and a significant 24% improvement on the IPSS among GPI-targeted patients. They found no significant improvements among patients who had STN targeting. When the investigators compared patients with unilateral lead placement and those with bilateral lead placement, they observed that all of the significant improvement among patients with GPI targeting occurred when treatment was bilateral.
The small sample size is a notable limitation of the study, and subset analyses were limited, said Mr. Gillogly. In addition, it was difficult to determine whether the symptoms studied were directly related to Parkinson’s disease, because they often arise as part of the natural aging process. “Other limitations of the study include lack of objective measurements, as these are all patient perception, and the innate limitations of self-reported questionnaires,” said Mr. Gillogly.
Two of the researchers reported having consulted for Medtronic, which markets a DBS system. One author received grant funding and consulting fees from Boston Scientific, Medtronic, and Abbott, all of which make DBS devices.
REPORTING FROM NANS 2019
Key clinical point: Bilateral stimulation of the globus pallidus internus reduces sialorrhea and improves genitourinary symptoms.
Major finding: Patients reported 31% improvement in sialorrhea and 24% improvement in urinary function.
Study details: A prospective study of 34 patients receiving DBS of the STN or GPI.
Disclosures: No funding was reported.
Peripheral nerve stimulation reduces hand tremor
LAS VEGAS – In addition, sensors worn on the wrist can provide objective measures of tremor in the home environment, said the investigators, who presented their research at the annual meeting of the North American Neuromodulation Society.
The current hypothesis is that tremulous activity within a central tremor network causes essential tremor, but the specific mechanisms are unknown. Research suggests that invasive neuromodulation of deep brain structures within this tremor network provides clinical benefit. The question of whether noninvasive neuromodulation of the peripheral nerve inputs connected to this network is beneficial has received comparatively little attention, however.
Rajesh Pahwa, MD, movement disorders division chief at the University of Kansas Medical Center in Kansas City, and his colleagues examined the safety and efficacy of noninvasive neuromodulation for hand tremor in patients with essential tremor. To provide treatment, they used a wristband with three electrodes that targeted the median and radial nerves. The stimulation pattern was adjusted to interrupt each patient’s tremulous signal in the clinical setting and at home. Participants were asked to hold a certain posture for 20 seconds while the device recorded tremor frequency. After determining the peak tremor frequency, the device was able to adapt stimulation parameters to each patient.
Dr. Pahwa and his colleagues conducted an acute in-office study and an at-home study. In the in-office study, 77 participants were randomized to peripheral nerve treatment or sham stimulation of the tremor-dominant hand. The researchers evaluated tremor before and after one stimulation session using the Essential Tremor Rating Assessment Scale (TETRAS) Upper Limb Tremor Scale and the TETRAS Archimedes Spiral Rating Scale. In the at-home study, 61 participants were randomized to stimulation, sham, or standard of care for 2 weeks. After that point, all participants underwent two to five 40-minute stimulation sessions daily for 2 weeks. Patients in the treatment and sham groups had at least two sessions per day.
In the in-office study, the researchers randomized 40 patients to stimulation and 37 patients to sham. Dr. Pahwa and his colleagues determined that participants had been blinded successfully. The investigators observed a mean improvement in forward posture of approximately 0.75 points among treated patients, compared with a mean improvement of 0.3 points in the sham group. The difference between groups was statistically significant. Treated patients had significant improvements in upper limb tremor score and total performance score, compared with the sham group. The investigators also observed greater mean improvements in spiral drawing, lateral posture, and movement among treated patients, compared with the sham group, but the differences were not statistically significant.
Participants in the in-office study rated their improvement on activities of daily living. Average improvement across tasks was significantly greater for the treated group, compared to the sham group. Improvement on each individual task also was greater for the treated group than the sham group. The differences in improvement were significant for holding a cup of tea, dialing a telephone, picking up change, and unlocking a door with a key.
In the at-home study, the decrease in tremor amplitude, as measured by the wrist-worn device, was significantly greater among participants who received stimulation than in the sham group. “These randomized, controlled studies suggest that noninvasive peripheral neuromodulation may offer meaningful symptomatic relief from hand tremor in essential tremor with a favorable safety profile, compared to other available therapies,” noted Dr. Pahwa and his colleagues. “At-home monitoring may provide key insights into evaluating and treating tremor,” they added.
The studies were supported by Cala Health.
LAS VEGAS – In addition, sensors worn on the wrist can provide objective measures of tremor in the home environment, said the investigators, who presented their research at the annual meeting of the North American Neuromodulation Society.
The current hypothesis is that tremulous activity within a central tremor network causes essential tremor, but the specific mechanisms are unknown. Research suggests that invasive neuromodulation of deep brain structures within this tremor network provides clinical benefit. The question of whether noninvasive neuromodulation of the peripheral nerve inputs connected to this network is beneficial has received comparatively little attention, however.
Rajesh Pahwa, MD, movement disorders division chief at the University of Kansas Medical Center in Kansas City, and his colleagues examined the safety and efficacy of noninvasive neuromodulation for hand tremor in patients with essential tremor. To provide treatment, they used a wristband with three electrodes that targeted the median and radial nerves. The stimulation pattern was adjusted to interrupt each patient’s tremulous signal in the clinical setting and at home. Participants were asked to hold a certain posture for 20 seconds while the device recorded tremor frequency. After determining the peak tremor frequency, the device was able to adapt stimulation parameters to each patient.
Dr. Pahwa and his colleagues conducted an acute in-office study and an at-home study. In the in-office study, 77 participants were randomized to peripheral nerve treatment or sham stimulation of the tremor-dominant hand. The researchers evaluated tremor before and after one stimulation session using the Essential Tremor Rating Assessment Scale (TETRAS) Upper Limb Tremor Scale and the TETRAS Archimedes Spiral Rating Scale. In the at-home study, 61 participants were randomized to stimulation, sham, or standard of care for 2 weeks. After that point, all participants underwent two to five 40-minute stimulation sessions daily for 2 weeks. Patients in the treatment and sham groups had at least two sessions per day.
In the in-office study, the researchers randomized 40 patients to stimulation and 37 patients to sham. Dr. Pahwa and his colleagues determined that participants had been blinded successfully. The investigators observed a mean improvement in forward posture of approximately 0.75 points among treated patients, compared with a mean improvement of 0.3 points in the sham group. The difference between groups was statistically significant. Treated patients had significant improvements in upper limb tremor score and total performance score, compared with the sham group. The investigators also observed greater mean improvements in spiral drawing, lateral posture, and movement among treated patients, compared with the sham group, but the differences were not statistically significant.
Participants in the in-office study rated their improvement on activities of daily living. Average improvement across tasks was significantly greater for the treated group, compared to the sham group. Improvement on each individual task also was greater for the treated group than the sham group. The differences in improvement were significant for holding a cup of tea, dialing a telephone, picking up change, and unlocking a door with a key.
In the at-home study, the decrease in tremor amplitude, as measured by the wrist-worn device, was significantly greater among participants who received stimulation than in the sham group. “These randomized, controlled studies suggest that noninvasive peripheral neuromodulation may offer meaningful symptomatic relief from hand tremor in essential tremor with a favorable safety profile, compared to other available therapies,” noted Dr. Pahwa and his colleagues. “At-home monitoring may provide key insights into evaluating and treating tremor,” they added.
The studies were supported by Cala Health.
LAS VEGAS – In addition, sensors worn on the wrist can provide objective measures of tremor in the home environment, said the investigators, who presented their research at the annual meeting of the North American Neuromodulation Society.
The current hypothesis is that tremulous activity within a central tremor network causes essential tremor, but the specific mechanisms are unknown. Research suggests that invasive neuromodulation of deep brain structures within this tremor network provides clinical benefit. The question of whether noninvasive neuromodulation of the peripheral nerve inputs connected to this network is beneficial has received comparatively little attention, however.
Rajesh Pahwa, MD, movement disorders division chief at the University of Kansas Medical Center in Kansas City, and his colleagues examined the safety and efficacy of noninvasive neuromodulation for hand tremor in patients with essential tremor. To provide treatment, they used a wristband with three electrodes that targeted the median and radial nerves. The stimulation pattern was adjusted to interrupt each patient’s tremulous signal in the clinical setting and at home. Participants were asked to hold a certain posture for 20 seconds while the device recorded tremor frequency. After determining the peak tremor frequency, the device was able to adapt stimulation parameters to each patient.
Dr. Pahwa and his colleagues conducted an acute in-office study and an at-home study. In the in-office study, 77 participants were randomized to peripheral nerve treatment or sham stimulation of the tremor-dominant hand. The researchers evaluated tremor before and after one stimulation session using the Essential Tremor Rating Assessment Scale (TETRAS) Upper Limb Tremor Scale and the TETRAS Archimedes Spiral Rating Scale. In the at-home study, 61 participants were randomized to stimulation, sham, or standard of care for 2 weeks. After that point, all participants underwent two to five 40-minute stimulation sessions daily for 2 weeks. Patients in the treatment and sham groups had at least two sessions per day.
In the in-office study, the researchers randomized 40 patients to stimulation and 37 patients to sham. Dr. Pahwa and his colleagues determined that participants had been blinded successfully. The investigators observed a mean improvement in forward posture of approximately 0.75 points among treated patients, compared with a mean improvement of 0.3 points in the sham group. The difference between groups was statistically significant. Treated patients had significant improvements in upper limb tremor score and total performance score, compared with the sham group. The investigators also observed greater mean improvements in spiral drawing, lateral posture, and movement among treated patients, compared with the sham group, but the differences were not statistically significant.
Participants in the in-office study rated their improvement on activities of daily living. Average improvement across tasks was significantly greater for the treated group, compared to the sham group. Improvement on each individual task also was greater for the treated group than the sham group. The differences in improvement were significant for holding a cup of tea, dialing a telephone, picking up change, and unlocking a door with a key.
In the at-home study, the decrease in tremor amplitude, as measured by the wrist-worn device, was significantly greater among participants who received stimulation than in the sham group. “These randomized, controlled studies suggest that noninvasive peripheral neuromodulation may offer meaningful symptomatic relief from hand tremor in essential tremor with a favorable safety profile, compared to other available therapies,” noted Dr. Pahwa and his colleagues. “At-home monitoring may provide key insights into evaluating and treating tremor,” they added.
The studies were supported by Cala Health.
REPORTING FROM NANS 2019
Key clinical point: Two randomized trials indicate that peripheral nerve stimulation provides clinical benefits in essential tremor.
Major finding: Peripheral nerve stimulation reduced hand tremor and improved activities of daily living.
Study details: Two randomized, controlled studies including 138 patients with essential tremor and hand tremor.
Disclosures: Cala Health supported the studies.
No evidence for disease-modifying effect of levodopa in Parkinson’s disease
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Levodopa did not have any significant disease-modifying effects in patients with early Parkinson’s disease.
Major finding: Change in the Unified Parkinson’s Disease Rating Scale (UPDRS) was –1.0 after 80 weeks of levodopa/carbodopa versus –2.0 for 40 weeks of placebo followed by 40 weeks of treatment (P = .44).
Study details: A delayed-start trial including 445 patients with early Parkinson’s disease randomized to 80 weeks of treatment or 40 weeks of placebo plus 40 weeks of treatment.
Disclosures: Study authors reported disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
Source: Verschuur CVM et al. N Engl J Med. 2019;380:315-324.
Tic disorders are associated with obesity and diabetes
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
FROM JAMA NEUROLOGY
Key clinical point: Monitor cardiometabolic health in patients with Tourette syndrome or chronic tic disorder.
Major finding: Patients with Tourette syndrome or chronic tic disorder have a higher risk of metabolic or cardiovascular disorders, compared with the general population (adjusted hazard ratio, 1.99) and sibling controls (adjusted hazard ratio, 1.37).
Study details: A Swedish longitudinal, population-based cohort study of 7,804 individuals with Tourette syndrome or chronic tic disorder.
Disclosures: The study was supported by a research grant from Tourettes Action. Authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
Source: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
Nuedexta mainly prescribed for dementia, Parkinson’s
Only 15% of patients prescribed dextromethorphan hydrobromide plus quinidine sulfate had pseudobulbar affect due to multiple sclerosis or amyotrophic lateral sclerosis, the condition for which this drug is labeled, according to an analysis of two national commercial insurance claims databases published online Jan. 7 in JAMA Internal Medicine.
Conversely, 57% of patients prescribed dextromethorphan-quinidine (Nuedexta) had a diagnosis of Parkinson’s disease or dementia. Furthermore, according to Medicare Part D data, prescriptions for dextromethorphan-quinidine rose 15-fold during a recent 6-year period, with a concurrent 50-fold rise in reimbursement. “In response to findings such as ours, further attention should be paid to educating prescribers about the actual benefits and risks of this costly drug combination,” Michael Fralick, MD, and his associates at Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote in their paper.
The Food and Drug Administration approved Nuedexta in 2010 for the treatment of pseudobulbar affect after it produced modest improvements in laughing or crying episodes in a 12-week, placebo-controlled trial of patients with multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS). The initial FDA label noted: “Nuedexta has not been shown to be safe or effective in other types of emotional lability that can commonly occur, for example, in Alzheimer’s disease and other dementias.” Then, in 2015, patients with Alzheimer’s disease showed modest improvements in agitation scores when they received dextromethorphan-quinidine in a 10-week, placebo-controlled, industry-designed and sponsored trial. Although the dextromethorphan-quinidine arm also had higher rates of falls, urinary tract infections, and serious adverse events, the prescribing information was updated in 2015 to remove the statement about patients with dementia.
To assess real-world prescribing patterns for dextromethorphan-quinidine, Dr. Fralick and his associates analyzed data from 12,858 patients who filled a prescription for this medication between 2010 and 2017 and were recorded in the Optum Clinformatics Data Mart or Truven Health MarketScan databases. Only 8.4% of patients had a diagnosis of MS and only 6.8% had ALS, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis. The number of patients prescribed dextromethorphan-quinidine rose from nearly 3,300 in 2011 to more than 50,000 in 2016, while spending on this medication by the Centers for Medicare & Medicaid Services increased from $3.9 million to $200.4 million during the same time period.
Current treatments for behavioral symptoms of dementia “are largely ineffective, and thus clinicians may want to prescribe dextromethorphan-quinidine to see if it helps their patients,” the researchers wrote. “Yet the absence of data showing efficacy, coupled with the demonstrated risks of falls and possible cardiac effects, calls this strategy into question.
“Further studies should be required to evaluate the safety and effectiveness of this medication as it is currently being used,” the authors suggested.
Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
SOURCE: Fralick M et al. JAMA Inter Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112
Only 15% of patients prescribed dextromethorphan hydrobromide plus quinidine sulfate had pseudobulbar affect due to multiple sclerosis or amyotrophic lateral sclerosis, the condition for which this drug is labeled, according to an analysis of two national commercial insurance claims databases published online Jan. 7 in JAMA Internal Medicine.
Conversely, 57% of patients prescribed dextromethorphan-quinidine (Nuedexta) had a diagnosis of Parkinson’s disease or dementia. Furthermore, according to Medicare Part D data, prescriptions for dextromethorphan-quinidine rose 15-fold during a recent 6-year period, with a concurrent 50-fold rise in reimbursement. “In response to findings such as ours, further attention should be paid to educating prescribers about the actual benefits and risks of this costly drug combination,” Michael Fralick, MD, and his associates at Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote in their paper.
The Food and Drug Administration approved Nuedexta in 2010 for the treatment of pseudobulbar affect after it produced modest improvements in laughing or crying episodes in a 12-week, placebo-controlled trial of patients with multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS). The initial FDA label noted: “Nuedexta has not been shown to be safe or effective in other types of emotional lability that can commonly occur, for example, in Alzheimer’s disease and other dementias.” Then, in 2015, patients with Alzheimer’s disease showed modest improvements in agitation scores when they received dextromethorphan-quinidine in a 10-week, placebo-controlled, industry-designed and sponsored trial. Although the dextromethorphan-quinidine arm also had higher rates of falls, urinary tract infections, and serious adverse events, the prescribing information was updated in 2015 to remove the statement about patients with dementia.
To assess real-world prescribing patterns for dextromethorphan-quinidine, Dr. Fralick and his associates analyzed data from 12,858 patients who filled a prescription for this medication between 2010 and 2017 and were recorded in the Optum Clinformatics Data Mart or Truven Health MarketScan databases. Only 8.4% of patients had a diagnosis of MS and only 6.8% had ALS, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis. The number of patients prescribed dextromethorphan-quinidine rose from nearly 3,300 in 2011 to more than 50,000 in 2016, while spending on this medication by the Centers for Medicare & Medicaid Services increased from $3.9 million to $200.4 million during the same time period.
Current treatments for behavioral symptoms of dementia “are largely ineffective, and thus clinicians may want to prescribe dextromethorphan-quinidine to see if it helps their patients,” the researchers wrote. “Yet the absence of data showing efficacy, coupled with the demonstrated risks of falls and possible cardiac effects, calls this strategy into question.
“Further studies should be required to evaluate the safety and effectiveness of this medication as it is currently being used,” the authors suggested.
Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
SOURCE: Fralick M et al. JAMA Inter Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112
Only 15% of patients prescribed dextromethorphan hydrobromide plus quinidine sulfate had pseudobulbar affect due to multiple sclerosis or amyotrophic lateral sclerosis, the condition for which this drug is labeled, according to an analysis of two national commercial insurance claims databases published online Jan. 7 in JAMA Internal Medicine.
Conversely, 57% of patients prescribed dextromethorphan-quinidine (Nuedexta) had a diagnosis of Parkinson’s disease or dementia. Furthermore, according to Medicare Part D data, prescriptions for dextromethorphan-quinidine rose 15-fold during a recent 6-year period, with a concurrent 50-fold rise in reimbursement. “In response to findings such as ours, further attention should be paid to educating prescribers about the actual benefits and risks of this costly drug combination,” Michael Fralick, MD, and his associates at Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote in their paper.
The Food and Drug Administration approved Nuedexta in 2010 for the treatment of pseudobulbar affect after it produced modest improvements in laughing or crying episodes in a 12-week, placebo-controlled trial of patients with multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS). The initial FDA label noted: “Nuedexta has not been shown to be safe or effective in other types of emotional lability that can commonly occur, for example, in Alzheimer’s disease and other dementias.” Then, in 2015, patients with Alzheimer’s disease showed modest improvements in agitation scores when they received dextromethorphan-quinidine in a 10-week, placebo-controlled, industry-designed and sponsored trial. Although the dextromethorphan-quinidine arm also had higher rates of falls, urinary tract infections, and serious adverse events, the prescribing information was updated in 2015 to remove the statement about patients with dementia.
To assess real-world prescribing patterns for dextromethorphan-quinidine, Dr. Fralick and his associates analyzed data from 12,858 patients who filled a prescription for this medication between 2010 and 2017 and were recorded in the Optum Clinformatics Data Mart or Truven Health MarketScan databases. Only 8.4% of patients had a diagnosis of MS and only 6.8% had ALS, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis. The number of patients prescribed dextromethorphan-quinidine rose from nearly 3,300 in 2011 to more than 50,000 in 2016, while spending on this medication by the Centers for Medicare & Medicaid Services increased from $3.9 million to $200.4 million during the same time period.
Current treatments for behavioral symptoms of dementia “are largely ineffective, and thus clinicians may want to prescribe dextromethorphan-quinidine to see if it helps their patients,” the researchers wrote. “Yet the absence of data showing efficacy, coupled with the demonstrated risks of falls and possible cardiac effects, calls this strategy into question.
“Further studies should be required to evaluate the safety and effectiveness of this medication as it is currently being used,” the authors suggested.
Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
SOURCE: Fralick M et al. JAMA Inter Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Only 8.4% of patients had a diagnosis of multiple sclerosis and only 6.8% had amyotrophic lateral sclerosis, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis.
Study details: Population-based cohort study of 12,858 patients prescribed dextromethorphan-quinidine between 2010 and 2017.
Disclosures: Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
Source: Fralick M et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112.