User login
CSF neurofilament light level could aid in diagnosis
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
FROM JAMA NEUROLOGY
Treatment for hepatitis C reduces risk of Parkinson’s disease
, according to a cohort study published online June 5 in JAMA Neurology. The results provide evidence that hepatitis C virus is a risk factor for Parkinson’s disease.
In the past several years, epidemiologic studies have suggested an association between hepatitis C virus infection and Parkinson’s disease. A study published in 2017, however, found no association between the two. In addition, these investigations did not consider antiviral therapy as a potential modifying factor.
Wey-Yil Lin, MD, a neurologist at Landseed International Hospital in Taoyuan, Taiwan, and colleagues examined claims data from the Taiwan National Health Insurance Research Database to identify the risk of incident Parkinson’s disease in patients with hepatitis C virus infection who received antiviral treatment, compared with those who did not receive treatment.
The investigators selected all patients with a new diagnosis of hepatitis C virus infection with or without hepatitis from January 1, 2003, to December 31, 2013. They excluded patients who were aged 20 years or younger; had Parkinson’s disease, dementia, or stroke; or had had major hepatic diseases on the index date. To ensure that treated patients had had an effective course of therapy, the researchers excluded patients who were lost to follow-up within 6 months of the index date, received antiviral therapy for fewer than 16 weeks, or developed Parkinson’s disease within 6 months of the index date.
The primary outcome was incident Parkinson’s disease. Dr. Lin and colleagues excluded participants with a diagnosis of stroke and dementia before the index date to reduce the possibility of enrolling participants with secondary and atypical parkinsonism.
To minimize the potential selection bias to which observational studies are subject, the investigators performed propensity score matching with sex, age, comorbidities, and medication as covariates. This method was intended to create treated and untreated cohorts with comparable characteristics.
Dr. Lin and colleagues included 188,152 patients in their analysis. After matching, each group included 39,936 participants. In the group that received antiviral treatment, 45.0% of participants were female, and mean age was 52.8 years. In the untreated group, 44.4% of participants were female, and mean age was 52.5 years.
The incidence density of Parkinson’s disease per 1,000 person-years was 1.00 in the treated group and 1.39 in the untreated group. The difference in risk of Parkinson’s disease between the treated and untreated groups was statistically significant at year 5 of follow-up (hazard ratio [HR], 0.75) and at the end of the cohort (HR, 0.71). The risk did not differ significantly at year 1 and year 3, however. A subgroup analysis found a greater benefit of antiviral therapy among patients who concurrently used dihydropyridine calcium channel blockers.
“To our knowledge, this is the first cohort study to investigate the association between antiviral therapy and risk of Parkinson’s disease in patients with chronic hepatitis C viral infection,” said Dr. Lin and colleagues. Although it is possible that interferon-based antiviral therapy directly protected against the development of Parkinson’s disease, the short time of exposure to the antiviral agent “makes protecting against Parkinson’s disease development in 5 years less likely,” they added.
Among the study limitations that the authors acknowledged was the lack of data about hepatic function profile, serum virologic response, viral genotype, and hepatitis C virus RNA-level. The database that the investigators used also lacked data about behavioral factors (e.g., smoking status, coffee consumption, and alcohol consumption) that may have affected the incidence of Parkinson’s disease in the cohort. Investigations with longer follow-up periods will be needed to provide clearer information, they concluded.
The authors reported no conflicts of interest. The study was funded by grants from Chang Gung Medical Research Fund and from Chang Gung Memorial Hospital.
SOURCE: Lin W-Y et al. JAMA Neurol. 2019 Jun 5. doi: 10.1001/jamaneurol.2019.1368.
The findings of Lin et al. suggest a potentially modifiable hepatologic risk factor for Parkinson’s disease, Adolfo Ramirez-Zamora, MD, associate professor of neurology; Christopher W. Hess, MD, assistant professor of neurology; and David R. Nelson, MD, senior vice president for health affairs, all at the University of Florida in Gainesville, wrote in an accompanying editorial. Hepatitis C virus infection might enter the brain through the microvasculature and might induce microglial and macrophage-related inflammatory changes (JAMA Neurol. 2019 June 5. doi: 10.1001/jamaneurol.2019.1377).
Lin et al. estimated high diagnostic accuracy for Parkinson’s disease in their study. Nevertheless, clinical, neuroimaging, and pathological confirmation was unavailable, which is a limitation of their investigation, said Dr. Ramirez-Zamora and colleagues. “The diagnosis of Parkinson’s disease in early stages can be challenging, as other related conditions can mimic Parkinson’s disease, including cirrhosis-related parkinsonism. Moreover, using record-linkage systems excludes patients who did not seek medical advice or those who were misdiagnosed by symptoms alone, which may also underestimate the prevalence of Parkinson’s disease. Using population-based studies would be a more accurate method.”
Because interferon, which was the antiviral therapy used in this study, greatly affects the immune system and has a modest rate of eradicating viral hepatitis C infection, future research should examine the association between Parkinson’s disease and patients who cleared the virus, as well as patients who did not, said Dr. Ramirez-Zamora and colleagues. Such research could shed light on potential mechanisms of treatment response. Lin et al. did not examine the newer direct-acting antiviral therapies for hepatitis C virus infection, which cure more than 90% of patients. Nor did they analyze other well established lifestyle and demographic risk factors for developing the disease. In addition, “the authors could not generalize the results to those aged 75 years or older because of the substantially smaller number of patients in this age group,” said Dr. Ramirez-Zamora and colleagues.
Still, “identification of potentially treatable Parkinson’s disease risk factors presents a unique opportunity for treatment. Additional studies with detailed viral analysis and exposure are needed, including in other geographic and ethnic distributions,” they concluded.
The findings of Lin et al. suggest a potentially modifiable hepatologic risk factor for Parkinson’s disease, Adolfo Ramirez-Zamora, MD, associate professor of neurology; Christopher W. Hess, MD, assistant professor of neurology; and David R. Nelson, MD, senior vice president for health affairs, all at the University of Florida in Gainesville, wrote in an accompanying editorial. Hepatitis C virus infection might enter the brain through the microvasculature and might induce microglial and macrophage-related inflammatory changes (JAMA Neurol. 2019 June 5. doi: 10.1001/jamaneurol.2019.1377).
Lin et al. estimated high diagnostic accuracy for Parkinson’s disease in their study. Nevertheless, clinical, neuroimaging, and pathological confirmation was unavailable, which is a limitation of their investigation, said Dr. Ramirez-Zamora and colleagues. “The diagnosis of Parkinson’s disease in early stages can be challenging, as other related conditions can mimic Parkinson’s disease, including cirrhosis-related parkinsonism. Moreover, using record-linkage systems excludes patients who did not seek medical advice or those who were misdiagnosed by symptoms alone, which may also underestimate the prevalence of Parkinson’s disease. Using population-based studies would be a more accurate method.”
Because interferon, which was the antiviral therapy used in this study, greatly affects the immune system and has a modest rate of eradicating viral hepatitis C infection, future research should examine the association between Parkinson’s disease and patients who cleared the virus, as well as patients who did not, said Dr. Ramirez-Zamora and colleagues. Such research could shed light on potential mechanisms of treatment response. Lin et al. did not examine the newer direct-acting antiviral therapies for hepatitis C virus infection, which cure more than 90% of patients. Nor did they analyze other well established lifestyle and demographic risk factors for developing the disease. In addition, “the authors could not generalize the results to those aged 75 years or older because of the substantially smaller number of patients in this age group,” said Dr. Ramirez-Zamora and colleagues.
Still, “identification of potentially treatable Parkinson’s disease risk factors presents a unique opportunity for treatment. Additional studies with detailed viral analysis and exposure are needed, including in other geographic and ethnic distributions,” they concluded.
The findings of Lin et al. suggest a potentially modifiable hepatologic risk factor for Parkinson’s disease, Adolfo Ramirez-Zamora, MD, associate professor of neurology; Christopher W. Hess, MD, assistant professor of neurology; and David R. Nelson, MD, senior vice president for health affairs, all at the University of Florida in Gainesville, wrote in an accompanying editorial. Hepatitis C virus infection might enter the brain through the microvasculature and might induce microglial and macrophage-related inflammatory changes (JAMA Neurol. 2019 June 5. doi: 10.1001/jamaneurol.2019.1377).
Lin et al. estimated high diagnostic accuracy for Parkinson’s disease in their study. Nevertheless, clinical, neuroimaging, and pathological confirmation was unavailable, which is a limitation of their investigation, said Dr. Ramirez-Zamora and colleagues. “The diagnosis of Parkinson’s disease in early stages can be challenging, as other related conditions can mimic Parkinson’s disease, including cirrhosis-related parkinsonism. Moreover, using record-linkage systems excludes patients who did not seek medical advice or those who were misdiagnosed by symptoms alone, which may also underestimate the prevalence of Parkinson’s disease. Using population-based studies would be a more accurate method.”
Because interferon, which was the antiviral therapy used in this study, greatly affects the immune system and has a modest rate of eradicating viral hepatitis C infection, future research should examine the association between Parkinson’s disease and patients who cleared the virus, as well as patients who did not, said Dr. Ramirez-Zamora and colleagues. Such research could shed light on potential mechanisms of treatment response. Lin et al. did not examine the newer direct-acting antiviral therapies for hepatitis C virus infection, which cure more than 90% of patients. Nor did they analyze other well established lifestyle and demographic risk factors for developing the disease. In addition, “the authors could not generalize the results to those aged 75 years or older because of the substantially smaller number of patients in this age group,” said Dr. Ramirez-Zamora and colleagues.
Still, “identification of potentially treatable Parkinson’s disease risk factors presents a unique opportunity for treatment. Additional studies with detailed viral analysis and exposure are needed, including in other geographic and ethnic distributions,” they concluded.
, according to a cohort study published online June 5 in JAMA Neurology. The results provide evidence that hepatitis C virus is a risk factor for Parkinson’s disease.
In the past several years, epidemiologic studies have suggested an association between hepatitis C virus infection and Parkinson’s disease. A study published in 2017, however, found no association between the two. In addition, these investigations did not consider antiviral therapy as a potential modifying factor.
Wey-Yil Lin, MD, a neurologist at Landseed International Hospital in Taoyuan, Taiwan, and colleagues examined claims data from the Taiwan National Health Insurance Research Database to identify the risk of incident Parkinson’s disease in patients with hepatitis C virus infection who received antiviral treatment, compared with those who did not receive treatment.
The investigators selected all patients with a new diagnosis of hepatitis C virus infection with or without hepatitis from January 1, 2003, to December 31, 2013. They excluded patients who were aged 20 years or younger; had Parkinson’s disease, dementia, or stroke; or had had major hepatic diseases on the index date. To ensure that treated patients had had an effective course of therapy, the researchers excluded patients who were lost to follow-up within 6 months of the index date, received antiviral therapy for fewer than 16 weeks, or developed Parkinson’s disease within 6 months of the index date.
The primary outcome was incident Parkinson’s disease. Dr. Lin and colleagues excluded participants with a diagnosis of stroke and dementia before the index date to reduce the possibility of enrolling participants with secondary and atypical parkinsonism.
To minimize the potential selection bias to which observational studies are subject, the investigators performed propensity score matching with sex, age, comorbidities, and medication as covariates. This method was intended to create treated and untreated cohorts with comparable characteristics.
Dr. Lin and colleagues included 188,152 patients in their analysis. After matching, each group included 39,936 participants. In the group that received antiviral treatment, 45.0% of participants were female, and mean age was 52.8 years. In the untreated group, 44.4% of participants were female, and mean age was 52.5 years.
The incidence density of Parkinson’s disease per 1,000 person-years was 1.00 in the treated group and 1.39 in the untreated group. The difference in risk of Parkinson’s disease between the treated and untreated groups was statistically significant at year 5 of follow-up (hazard ratio [HR], 0.75) and at the end of the cohort (HR, 0.71). The risk did not differ significantly at year 1 and year 3, however. A subgroup analysis found a greater benefit of antiviral therapy among patients who concurrently used dihydropyridine calcium channel blockers.
“To our knowledge, this is the first cohort study to investigate the association between antiviral therapy and risk of Parkinson’s disease in patients with chronic hepatitis C viral infection,” said Dr. Lin and colleagues. Although it is possible that interferon-based antiviral therapy directly protected against the development of Parkinson’s disease, the short time of exposure to the antiviral agent “makes protecting against Parkinson’s disease development in 5 years less likely,” they added.
Among the study limitations that the authors acknowledged was the lack of data about hepatic function profile, serum virologic response, viral genotype, and hepatitis C virus RNA-level. The database that the investigators used also lacked data about behavioral factors (e.g., smoking status, coffee consumption, and alcohol consumption) that may have affected the incidence of Parkinson’s disease in the cohort. Investigations with longer follow-up periods will be needed to provide clearer information, they concluded.
The authors reported no conflicts of interest. The study was funded by grants from Chang Gung Medical Research Fund and from Chang Gung Memorial Hospital.
SOURCE: Lin W-Y et al. JAMA Neurol. 2019 Jun 5. doi: 10.1001/jamaneurol.2019.1368.
, according to a cohort study published online June 5 in JAMA Neurology. The results provide evidence that hepatitis C virus is a risk factor for Parkinson’s disease.
In the past several years, epidemiologic studies have suggested an association between hepatitis C virus infection and Parkinson’s disease. A study published in 2017, however, found no association between the two. In addition, these investigations did not consider antiviral therapy as a potential modifying factor.
Wey-Yil Lin, MD, a neurologist at Landseed International Hospital in Taoyuan, Taiwan, and colleagues examined claims data from the Taiwan National Health Insurance Research Database to identify the risk of incident Parkinson’s disease in patients with hepatitis C virus infection who received antiviral treatment, compared with those who did not receive treatment.
The investigators selected all patients with a new diagnosis of hepatitis C virus infection with or without hepatitis from January 1, 2003, to December 31, 2013. They excluded patients who were aged 20 years or younger; had Parkinson’s disease, dementia, or stroke; or had had major hepatic diseases on the index date. To ensure that treated patients had had an effective course of therapy, the researchers excluded patients who were lost to follow-up within 6 months of the index date, received antiviral therapy for fewer than 16 weeks, or developed Parkinson’s disease within 6 months of the index date.
The primary outcome was incident Parkinson’s disease. Dr. Lin and colleagues excluded participants with a diagnosis of stroke and dementia before the index date to reduce the possibility of enrolling participants with secondary and atypical parkinsonism.
To minimize the potential selection bias to which observational studies are subject, the investigators performed propensity score matching with sex, age, comorbidities, and medication as covariates. This method was intended to create treated and untreated cohorts with comparable characteristics.
Dr. Lin and colleagues included 188,152 patients in their analysis. After matching, each group included 39,936 participants. In the group that received antiviral treatment, 45.0% of participants were female, and mean age was 52.8 years. In the untreated group, 44.4% of participants were female, and mean age was 52.5 years.
The incidence density of Parkinson’s disease per 1,000 person-years was 1.00 in the treated group and 1.39 in the untreated group. The difference in risk of Parkinson’s disease between the treated and untreated groups was statistically significant at year 5 of follow-up (hazard ratio [HR], 0.75) and at the end of the cohort (HR, 0.71). The risk did not differ significantly at year 1 and year 3, however. A subgroup analysis found a greater benefit of antiviral therapy among patients who concurrently used dihydropyridine calcium channel blockers.
“To our knowledge, this is the first cohort study to investigate the association between antiviral therapy and risk of Parkinson’s disease in patients with chronic hepatitis C viral infection,” said Dr. Lin and colleagues. Although it is possible that interferon-based antiviral therapy directly protected against the development of Parkinson’s disease, the short time of exposure to the antiviral agent “makes protecting against Parkinson’s disease development in 5 years less likely,” they added.
Among the study limitations that the authors acknowledged was the lack of data about hepatic function profile, serum virologic response, viral genotype, and hepatitis C virus RNA-level. The database that the investigators used also lacked data about behavioral factors (e.g., smoking status, coffee consumption, and alcohol consumption) that may have affected the incidence of Parkinson’s disease in the cohort. Investigations with longer follow-up periods will be needed to provide clearer information, they concluded.
The authors reported no conflicts of interest. The study was funded by grants from Chang Gung Medical Research Fund and from Chang Gung Memorial Hospital.
SOURCE: Lin W-Y et al. JAMA Neurol. 2019 Jun 5. doi: 10.1001/jamaneurol.2019.1368.
FROM JAMA NEUROLOGY
Between a rock and a hard place
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
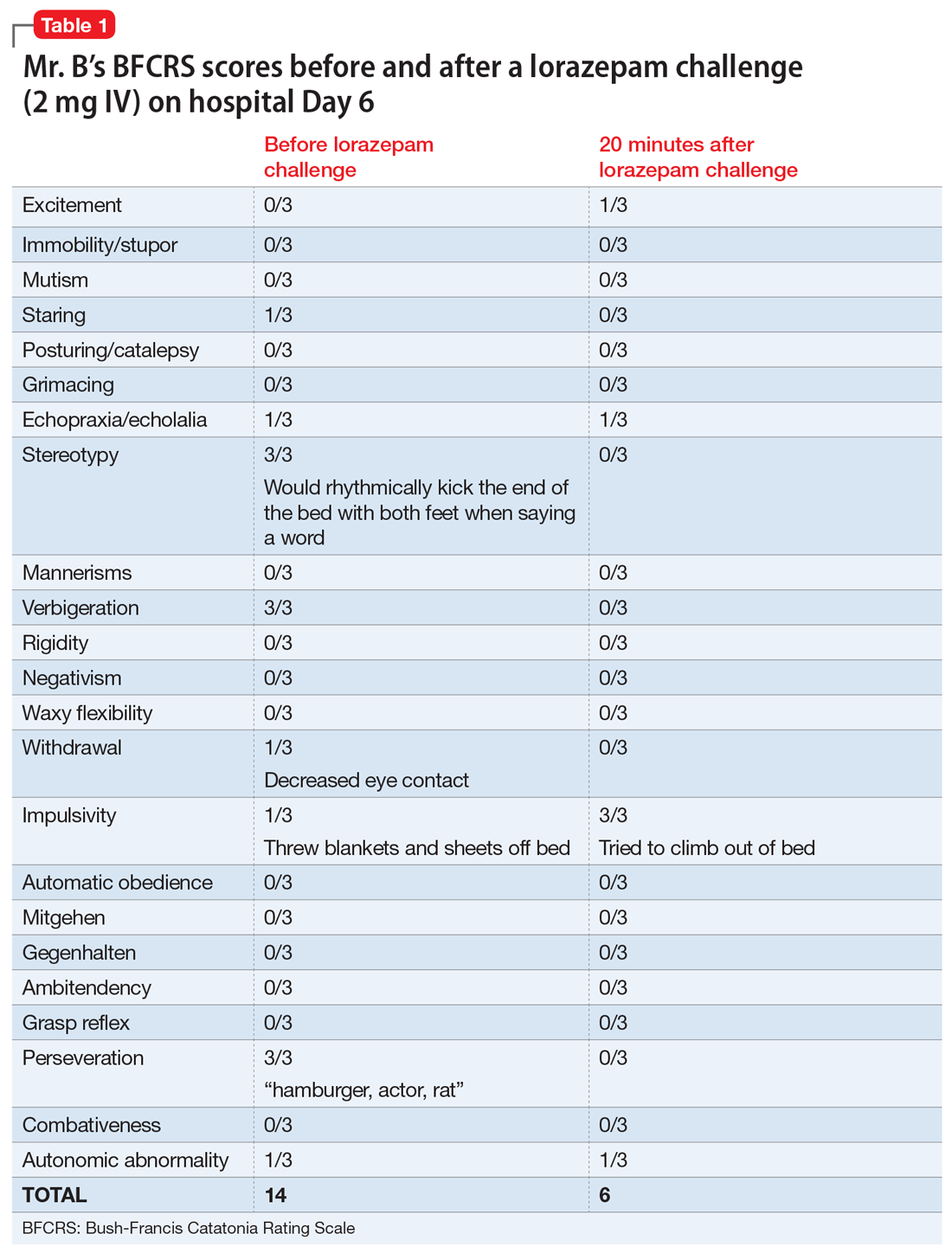
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.
Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.

Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.

Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
Deutetrabenazine benefit may increase over time in patients with tardive dyskinesia
PHILADELPHIA –
The mean Abnormal Involuntary Movement Scale (AIMS) score in the study continued to increase over 3 years of treatment with this VMAT2 inhibitor, which was safe and well tolerated over the course of the study, said investigator Robert A. Hauser, MD, MBA, director of the Parkinson’s and Movement Disorder Center and professor in the department of neurology at the University of South Florida in Tampa.
The apparent improvement over time was “fascinating” to observe, Dr. Hauser said. The finding deserves further study to identify potential confounders, such as rater bias over time or placebo effects, and if those “trivial” causes can be ruled out to determine a potential mechanism of action.
“I will also say that the mechanism may not be that important if we can really show this important clinical effect,” he said. “So I think this needs more work.”
The FDA approved deutetrabenazine (Austedo, Teva) for tardive dyskinesia treatment based on ARM-TD and AIM-TD, two randomized, double-blind, placebo-controlled trials. Those studies demonstrated improvements in AIMS scores for the VMAT2 inhibitor versus placebo, with low rates of adverse events and discontinuations, Dr. Hauser said.
Dr. Hauser presented results up to week 145 from C-20, an ongoing, 3-year, open-label extension study designed to evaluate the agent’s long-term safety and efficacy.
A total of 343 patients from ARM-TD and AIM-TD rolled over directly into C-20, started at 12 mg/day of deutetrabenazine, and titrated until adequate tardive dyskinesia control was achieved, up to 48 mg/day. Sixty percent of the patients had psychotic disorders as the background comorbid illness, while 40% had mood disorders, according to the interim report.
The mean AIMS score was 10.9 at baseline, 6.0 at 54 weeks, 5.8 at 106 weeks, and 4.1 at 145 weeks, the report showed. The corresponding change in AIMS score decreased from baseline for patients who remained in the study, from –4.8 at 54 weeks to –5.6 at 106 weeks and –7.0 at 145 weeks.*
A subsequent completer analysis showed that the apparent improvement in efficacy over the long term was not due to poor responders dropping out over time, Dr. Hauser said.
“I think the data clearly show the benefit is maintained, and intriguingly, I think they suggest that there may be increasing benefit over time,” he added.
The treatment was safe and well tolerated in long-term use. The most common adverse events were anxiety, somnolence, fatigue, insomnia, and headache. Adverse events did not increase in frequency from the parent studies to the open-label study, he noted.
The study was sponsored by Teva Pharmaceuticals. Dr. Hauser reported disclosures related to Teva, AbbVie, AstraZeneca, Biotie Thrapies, Cynapsus Therapeutics, Neurocrine Biosciences, Sunovion Pharmaceuticals, and Pfizer, among others.
SOURCE: Hauser RA et al. AAN 2019. Abstract S4.009.
*Correction, 6/26/19 An earlier version of this article mischaracterized changes in the mean Abnormal Involuntary Movement Scale scores during treatment.
PHILADELPHIA –
The mean Abnormal Involuntary Movement Scale (AIMS) score in the study continued to increase over 3 years of treatment with this VMAT2 inhibitor, which was safe and well tolerated over the course of the study, said investigator Robert A. Hauser, MD, MBA, director of the Parkinson’s and Movement Disorder Center and professor in the department of neurology at the University of South Florida in Tampa.
The apparent improvement over time was “fascinating” to observe, Dr. Hauser said. The finding deserves further study to identify potential confounders, such as rater bias over time or placebo effects, and if those “trivial” causes can be ruled out to determine a potential mechanism of action.
“I will also say that the mechanism may not be that important if we can really show this important clinical effect,” he said. “So I think this needs more work.”
The FDA approved deutetrabenazine (Austedo, Teva) for tardive dyskinesia treatment based on ARM-TD and AIM-TD, two randomized, double-blind, placebo-controlled trials. Those studies demonstrated improvements in AIMS scores for the VMAT2 inhibitor versus placebo, with low rates of adverse events and discontinuations, Dr. Hauser said.
Dr. Hauser presented results up to week 145 from C-20, an ongoing, 3-year, open-label extension study designed to evaluate the agent’s long-term safety and efficacy.
A total of 343 patients from ARM-TD and AIM-TD rolled over directly into C-20, started at 12 mg/day of deutetrabenazine, and titrated until adequate tardive dyskinesia control was achieved, up to 48 mg/day. Sixty percent of the patients had psychotic disorders as the background comorbid illness, while 40% had mood disorders, according to the interim report.
The mean AIMS score was 10.9 at baseline, 6.0 at 54 weeks, 5.8 at 106 weeks, and 4.1 at 145 weeks, the report showed. The corresponding change in AIMS score decreased from baseline for patients who remained in the study, from –4.8 at 54 weeks to –5.6 at 106 weeks and –7.0 at 145 weeks.*
A subsequent completer analysis showed that the apparent improvement in efficacy over the long term was not due to poor responders dropping out over time, Dr. Hauser said.
“I think the data clearly show the benefit is maintained, and intriguingly, I think they suggest that there may be increasing benefit over time,” he added.
The treatment was safe and well tolerated in long-term use. The most common adverse events were anxiety, somnolence, fatigue, insomnia, and headache. Adverse events did not increase in frequency from the parent studies to the open-label study, he noted.
The study was sponsored by Teva Pharmaceuticals. Dr. Hauser reported disclosures related to Teva, AbbVie, AstraZeneca, Biotie Thrapies, Cynapsus Therapeutics, Neurocrine Biosciences, Sunovion Pharmaceuticals, and Pfizer, among others.
SOURCE: Hauser RA et al. AAN 2019. Abstract S4.009.
*Correction, 6/26/19 An earlier version of this article mischaracterized changes in the mean Abnormal Involuntary Movement Scale scores during treatment.
PHILADELPHIA –
The mean Abnormal Involuntary Movement Scale (AIMS) score in the study continued to increase over 3 years of treatment with this VMAT2 inhibitor, which was safe and well tolerated over the course of the study, said investigator Robert A. Hauser, MD, MBA, director of the Parkinson’s and Movement Disorder Center and professor in the department of neurology at the University of South Florida in Tampa.
The apparent improvement over time was “fascinating” to observe, Dr. Hauser said. The finding deserves further study to identify potential confounders, such as rater bias over time or placebo effects, and if those “trivial” causes can be ruled out to determine a potential mechanism of action.
“I will also say that the mechanism may not be that important if we can really show this important clinical effect,” he said. “So I think this needs more work.”
The FDA approved deutetrabenazine (Austedo, Teva) for tardive dyskinesia treatment based on ARM-TD and AIM-TD, two randomized, double-blind, placebo-controlled trials. Those studies demonstrated improvements in AIMS scores for the VMAT2 inhibitor versus placebo, with low rates of adverse events and discontinuations, Dr. Hauser said.
Dr. Hauser presented results up to week 145 from C-20, an ongoing, 3-year, open-label extension study designed to evaluate the agent’s long-term safety and efficacy.
A total of 343 patients from ARM-TD and AIM-TD rolled over directly into C-20, started at 12 mg/day of deutetrabenazine, and titrated until adequate tardive dyskinesia control was achieved, up to 48 mg/day. Sixty percent of the patients had psychotic disorders as the background comorbid illness, while 40% had mood disorders, according to the interim report.
The mean AIMS score was 10.9 at baseline, 6.0 at 54 weeks, 5.8 at 106 weeks, and 4.1 at 145 weeks, the report showed. The corresponding change in AIMS score decreased from baseline for patients who remained in the study, from –4.8 at 54 weeks to –5.6 at 106 weeks and –7.0 at 145 weeks.*
A subsequent completer analysis showed that the apparent improvement in efficacy over the long term was not due to poor responders dropping out over time, Dr. Hauser said.
“I think the data clearly show the benefit is maintained, and intriguingly, I think they suggest that there may be increasing benefit over time,” he added.
The treatment was safe and well tolerated in long-term use. The most common adverse events were anxiety, somnolence, fatigue, insomnia, and headache. Adverse events did not increase in frequency from the parent studies to the open-label study, he noted.
The study was sponsored by Teva Pharmaceuticals. Dr. Hauser reported disclosures related to Teva, AbbVie, AstraZeneca, Biotie Thrapies, Cynapsus Therapeutics, Neurocrine Biosciences, Sunovion Pharmaceuticals, and Pfizer, among others.
SOURCE: Hauser RA et al. AAN 2019. Abstract S4.009.
*Correction, 6/26/19 An earlier version of this article mischaracterized changes in the mean Abnormal Involuntary Movement Scale scores during treatment.
REPORTING FROM AAN 2019
Key clinical point: The benefit of deutetrabenazine in patients with tardive dyskinesia is maintained in the long term and may actually increase over time, though further study is needed.
Major finding: Change from baseline in Abnormal Involuntary Movement Scale (AIMS) score decreased from –4.8 at 54 weeks to –5.6 at 106 weeks and –7.0 at 154 weeks.
Study details: Interim analysis of C-20, an open-label extension study including 343 patients initially enrolled in one of two pivotal randomized phase 3 studies.
Disclosures: The study was sponsored by Teva Pharmaceuticals. Dr. Hauser reported disclosures related to Teva, AbbVie, AstraZeneca, Biotie Thrapies, Cynapsus Therapeutics, Neurocrine Biosciences, Sunovion Pharmaceuticals, and Pfizer, among others.
Source: Hauser RA et al. AAN 2019. Abstract S4.009.
Appendectomy linked to increased risk of subsequent Parkinson’s
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
REPORTING FROM DDW 2019
Key clinical point: Appendectomy appears to increase the risk of Parkinson’s disease.
Major finding: The overall relative risk of developing Parkinson’s disease in patients after appendectomy was 3.19 (95% CI, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure.
Study details: A population-based study of more than 62 million medical records from a national database.
Disclosures: The researchers reported having no financial disclosures.
Source: Sheriff MZ et al. DDW 2019, Abstract 739.
Patients describe significant impact of epilepsy on their lives
PHILADELPHIA – said Jacqueline French, MD, a professor at the Comprehensive Epilepsy Center at New York University.
“This underscores the need to consider these experiences, and potentially the stage of disease, when developing patient-reported outcome measures,” she said at the annual meeting of the American Academy of Neurology.
To describe the patient’s experience of living with epilepsy, including the occurrence of disease-related signs and symptoms and impact on daily life at different disease stages, Dr. French conducted qualitative, semistructured interviews with adults with focal epilepsy at the following stages: early (1 year or less since diagnosis), middle (1-5 years since diagnosis), and late (more than 5 years since diagnosis). The patients had varying seizure frequency and treatment experiences. They were asked to describe the symptoms and functional impact they had experienced related to epilepsy, and then to rate the degree to which each symptom and impact “bothered” them, using a disturbance rating scale from 0 (not at all) to 10 (extremely).
A total of 62 patients who were aged 18-60 years (mean age, 37 years; 73% female) were interviewed. In all, 19 of the patients had early-stage disease, 17 had middle-stage, and 26 had late-stage disease. Symptoms reported with the highest frequency and highest average disturbance (AD) ratings across all cohorts included twitching/tremors (80% of patients; AD, 5.3), confusion (78%; AD, 7.8), difficulty talking (75%; AD, 8.1), impaired/loss of consciousness (70%; AD, 6.8), stiffening (65%; AD, 5.4), déjà vu (62%; AD, 5.1), difficulty remembering (60%; AD, 8.5), and dizziness/light-headedness (58%; AD, 6.4).
The high-frequency/high-disturbance daily impact of epilepsy included the inability to drive (74%; AD, 7.1), limited ability to work and/or go to school (61%; AD, 6.7), limitations on leisure and social activities (58%; AD, 6.3), and memory loss (47%; AD, 8.4).
Dr French noted that, although disease experiences were similar among the cohorts, some heterogeneity across patient subgroups was observed.
Eisai sponsored the study.
PHILADELPHIA – said Jacqueline French, MD, a professor at the Comprehensive Epilepsy Center at New York University.
“This underscores the need to consider these experiences, and potentially the stage of disease, when developing patient-reported outcome measures,” she said at the annual meeting of the American Academy of Neurology.
To describe the patient’s experience of living with epilepsy, including the occurrence of disease-related signs and symptoms and impact on daily life at different disease stages, Dr. French conducted qualitative, semistructured interviews with adults with focal epilepsy at the following stages: early (1 year or less since diagnosis), middle (1-5 years since diagnosis), and late (more than 5 years since diagnosis). The patients had varying seizure frequency and treatment experiences. They were asked to describe the symptoms and functional impact they had experienced related to epilepsy, and then to rate the degree to which each symptom and impact “bothered” them, using a disturbance rating scale from 0 (not at all) to 10 (extremely).
A total of 62 patients who were aged 18-60 years (mean age, 37 years; 73% female) were interviewed. In all, 19 of the patients had early-stage disease, 17 had middle-stage, and 26 had late-stage disease. Symptoms reported with the highest frequency and highest average disturbance (AD) ratings across all cohorts included twitching/tremors (80% of patients; AD, 5.3), confusion (78%; AD, 7.8), difficulty talking (75%; AD, 8.1), impaired/loss of consciousness (70%; AD, 6.8), stiffening (65%; AD, 5.4), déjà vu (62%; AD, 5.1), difficulty remembering (60%; AD, 8.5), and dizziness/light-headedness (58%; AD, 6.4).
The high-frequency/high-disturbance daily impact of epilepsy included the inability to drive (74%; AD, 7.1), limited ability to work and/or go to school (61%; AD, 6.7), limitations on leisure and social activities (58%; AD, 6.3), and memory loss (47%; AD, 8.4).
Dr French noted that, although disease experiences were similar among the cohorts, some heterogeneity across patient subgroups was observed.
Eisai sponsored the study.
PHILADELPHIA – said Jacqueline French, MD, a professor at the Comprehensive Epilepsy Center at New York University.
“This underscores the need to consider these experiences, and potentially the stage of disease, when developing patient-reported outcome measures,” she said at the annual meeting of the American Academy of Neurology.
To describe the patient’s experience of living with epilepsy, including the occurrence of disease-related signs and symptoms and impact on daily life at different disease stages, Dr. French conducted qualitative, semistructured interviews with adults with focal epilepsy at the following stages: early (1 year or less since diagnosis), middle (1-5 years since diagnosis), and late (more than 5 years since diagnosis). The patients had varying seizure frequency and treatment experiences. They were asked to describe the symptoms and functional impact they had experienced related to epilepsy, and then to rate the degree to which each symptom and impact “bothered” them, using a disturbance rating scale from 0 (not at all) to 10 (extremely).
A total of 62 patients who were aged 18-60 years (mean age, 37 years; 73% female) were interviewed. In all, 19 of the patients had early-stage disease, 17 had middle-stage, and 26 had late-stage disease. Symptoms reported with the highest frequency and highest average disturbance (AD) ratings across all cohorts included twitching/tremors (80% of patients; AD, 5.3), confusion (78%; AD, 7.8), difficulty talking (75%; AD, 8.1), impaired/loss of consciousness (70%; AD, 6.8), stiffening (65%; AD, 5.4), déjà vu (62%; AD, 5.1), difficulty remembering (60%; AD, 8.5), and dizziness/light-headedness (58%; AD, 6.4).
The high-frequency/high-disturbance daily impact of epilepsy included the inability to drive (74%; AD, 7.1), limited ability to work and/or go to school (61%; AD, 6.7), limitations on leisure and social activities (58%; AD, 6.3), and memory loss (47%; AD, 8.4).
Dr French noted that, although disease experiences were similar among the cohorts, some heterogeneity across patient subgroups was observed.
Eisai sponsored the study.
REPORTING FROM AAN 2019
Key clinical point: Adults with focal epilepsy report a range of high-disturbance symptoms and impacts on daily life.
Major finding: The high-frequency/high-disturbance daily impact of epilepsy included the inability to drive (reported by 74% of respondents), limited ability to work and/or go to school (61%), limitations on leisure and social activities (58%), and memory loss (47%).
Study details: Qualitative, semistructured interviews with 62 adults with focal epilepsy at different stages of illness: early, middle, and late.
Disclosures: Eisai sponsored the study.
Cannabidiol reduces seizures in Dravet syndrome
Philadelphia – according to research presented at the annual meeting of the American Academy of Neurology. The two dosages in the study appear to have comparable efficacy.
“It’s exciting to be able to offer another alternative for children with this debilitating form of epilepsy and their families,” said Ian Miller, MD, director of the epilepsy and neurophysiology program at Nicklaus Children’s Hospital in Miami, in a press release. “The children in this study had already tried an average of four epilepsy drugs with no success and at the time were taking an average of three additional drugs, so to have this measure of success with CBD is a major victory.”
Dravet syndrome is a rare developmental and epileptic encephalopathy. Onset occurs during infancy, and the syndrome is associated with drug-resistant seizures. Dr. Miller and colleagues designed a trial to evaluate the efficacy of two dosages of CBD as adjunctive anticonvulsant therapy in patients with Dravet syndrome and drug-resistant seizures.
The study population included 199 patients whose seizures were recorded for 4 weeks at baseline. The investigators randomized participants in approximately equal groups to receive placebo or highly purified CBD (the formulation approved under the name Epidiolex) at 20 mg/kg per day or 10 mg/kg per day. The study’s primary outcome was the change from baseline in frequency of convulsive seizures over 14 weeks of treatment.
Participants’ mean age was 9 years. Patients were taking a median of three concomitant antiepileptic drugs and had discontinued a median of four such drugs previously.
The reduction in the frequency of convulsive seizures was 46% for the high dose of CBD, 49% for the low dose of CBD, and 27% for placebo. The proportion of participants with a 50% or greater reduction in seizures was 49% in the high-dose group, 44% in the low-dose group, and 26% among controls. In addition, the reduction in the rate of total seizures was 47% for the high-dose group, 56% for the low-dose group, and 30% among controls.
The rate of adverse events was similar in all groups (90% for the high-dose group, 88% for the low-dose group, and 89% for controls). The five most common adverse events were diarrhea, somnolence, pyrexia, fatigue, and decreased appetite. The rate of serious adverse events was 25% for the high-dose group, 20% for the low-dose group, and 15% for controls. Discontinuations because of adverse events were limited to the high-dose group (7%). The rate of transaminases that exceeded three times the upper limit of normal was 19% in the high-dose group and 5% in the low-dose group. All of these elevations resolved. No patients died.
“Based on these results, dose increases above 10 mg/kg per day should be carefully considered based on the effectiveness and safety for each individual,” said Dr. Miller.
GW Research, the developer of cannabidiol, supported the study. GW operates through its affiliate Greenwich Biosciences in the United States. Dr. Miller has received compensation and research support from several companies, including GW Pharma.
SOURCE: Miller I et al. AAN 2019, Abstract P3.6-0.76.
Philadelphia – according to research presented at the annual meeting of the American Academy of Neurology. The two dosages in the study appear to have comparable efficacy.
“It’s exciting to be able to offer another alternative for children with this debilitating form of epilepsy and their families,” said Ian Miller, MD, director of the epilepsy and neurophysiology program at Nicklaus Children’s Hospital in Miami, in a press release. “The children in this study had already tried an average of four epilepsy drugs with no success and at the time were taking an average of three additional drugs, so to have this measure of success with CBD is a major victory.”
Dravet syndrome is a rare developmental and epileptic encephalopathy. Onset occurs during infancy, and the syndrome is associated with drug-resistant seizures. Dr. Miller and colleagues designed a trial to evaluate the efficacy of two dosages of CBD as adjunctive anticonvulsant therapy in patients with Dravet syndrome and drug-resistant seizures.
The study population included 199 patients whose seizures were recorded for 4 weeks at baseline. The investigators randomized participants in approximately equal groups to receive placebo or highly purified CBD (the formulation approved under the name Epidiolex) at 20 mg/kg per day or 10 mg/kg per day. The study’s primary outcome was the change from baseline in frequency of convulsive seizures over 14 weeks of treatment.
Participants’ mean age was 9 years. Patients were taking a median of three concomitant antiepileptic drugs and had discontinued a median of four such drugs previously.
The reduction in the frequency of convulsive seizures was 46% for the high dose of CBD, 49% for the low dose of CBD, and 27% for placebo. The proportion of participants with a 50% or greater reduction in seizures was 49% in the high-dose group, 44% in the low-dose group, and 26% among controls. In addition, the reduction in the rate of total seizures was 47% for the high-dose group, 56% for the low-dose group, and 30% among controls.
The rate of adverse events was similar in all groups (90% for the high-dose group, 88% for the low-dose group, and 89% for controls). The five most common adverse events were diarrhea, somnolence, pyrexia, fatigue, and decreased appetite. The rate of serious adverse events was 25% for the high-dose group, 20% for the low-dose group, and 15% for controls. Discontinuations because of adverse events were limited to the high-dose group (7%). The rate of transaminases that exceeded three times the upper limit of normal was 19% in the high-dose group and 5% in the low-dose group. All of these elevations resolved. No patients died.
“Based on these results, dose increases above 10 mg/kg per day should be carefully considered based on the effectiveness and safety for each individual,” said Dr. Miller.
GW Research, the developer of cannabidiol, supported the study. GW operates through its affiliate Greenwich Biosciences in the United States. Dr. Miller has received compensation and research support from several companies, including GW Pharma.
SOURCE: Miller I et al. AAN 2019, Abstract P3.6-0.76.
Philadelphia – according to research presented at the annual meeting of the American Academy of Neurology. The two dosages in the study appear to have comparable efficacy.
“It’s exciting to be able to offer another alternative for children with this debilitating form of epilepsy and their families,” said Ian Miller, MD, director of the epilepsy and neurophysiology program at Nicklaus Children’s Hospital in Miami, in a press release. “The children in this study had already tried an average of four epilepsy drugs with no success and at the time were taking an average of three additional drugs, so to have this measure of success with CBD is a major victory.”
Dravet syndrome is a rare developmental and epileptic encephalopathy. Onset occurs during infancy, and the syndrome is associated with drug-resistant seizures. Dr. Miller and colleagues designed a trial to evaluate the efficacy of two dosages of CBD as adjunctive anticonvulsant therapy in patients with Dravet syndrome and drug-resistant seizures.
The study population included 199 patients whose seizures were recorded for 4 weeks at baseline. The investigators randomized participants in approximately equal groups to receive placebo or highly purified CBD (the formulation approved under the name Epidiolex) at 20 mg/kg per day or 10 mg/kg per day. The study’s primary outcome was the change from baseline in frequency of convulsive seizures over 14 weeks of treatment.
Participants’ mean age was 9 years. Patients were taking a median of three concomitant antiepileptic drugs and had discontinued a median of four such drugs previously.
The reduction in the frequency of convulsive seizures was 46% for the high dose of CBD, 49% for the low dose of CBD, and 27% for placebo. The proportion of participants with a 50% or greater reduction in seizures was 49% in the high-dose group, 44% in the low-dose group, and 26% among controls. In addition, the reduction in the rate of total seizures was 47% for the high-dose group, 56% for the low-dose group, and 30% among controls.
The rate of adverse events was similar in all groups (90% for the high-dose group, 88% for the low-dose group, and 89% for controls). The five most common adverse events were diarrhea, somnolence, pyrexia, fatigue, and decreased appetite. The rate of serious adverse events was 25% for the high-dose group, 20% for the low-dose group, and 15% for controls. Discontinuations because of adverse events were limited to the high-dose group (7%). The rate of transaminases that exceeded three times the upper limit of normal was 19% in the high-dose group and 5% in the low-dose group. All of these elevations resolved. No patients died.
“Based on these results, dose increases above 10 mg/kg per day should be carefully considered based on the effectiveness and safety for each individual,” said Dr. Miller.
GW Research, the developer of cannabidiol, supported the study. GW operates through its affiliate Greenwich Biosciences in the United States. Dr. Miller has received compensation and research support from several companies, including GW Pharma.
SOURCE: Miller I et al. AAN 2019, Abstract P3.6-0.76.
REPORTING FROM AAN 2019
Guideline issued for treating Tourette syndrome and chronic tics
PHILADELPHIA – Approaches to managing tics in patients with Tourette syndrome or chronic tic disorders “should be individualized, and the choice should be the result of a collaborative decision among patient, caregiver, and clinician, during which the benefits and harms of individual treatments as well as the presence of comorbid disorders are considered,” according to Tamara Pringsheim, MD, lead author of a practice guideline published May 6, 2019, by the American Academy of Neurology, and her collaborators.
The panel of nine physicians, two psychologists, and two patient representatives developed the recommendations based on a comprehensive systematic literature review. They concluded that treatments may decrease the frequency and severity of tics but rarely eliminate them.
The guideline was endorsed by the Child Neurology Society and the European Academy of Neurology and is the first such guideline for American neurologists, said Dr. Pringsheim of the University of Calgary (Alta.). Like recent Canadian and European guidelines, it strongly supports the Comprehensive Behavioral Intervention for Tics (CBIT) as a treatment option for tics.
After examining which medical, behavioral, and neurostimulation interventions, compared with placebo or other active interventions, improve tic severity and tic-related impairment in children and adults with Tourette syndrome or a chronic tic disorder, the guideline writers recommended that the evidence was strongest for CBIT as a first-line treatment, relative to other behavioral treatments and medications.
If symptoms affect a patient’s daily life, doctors should consider CBIT, said guideline author John Piacentini, PhD, of the University of California, Los Angeles, at the annual meeting of the American Academy of Neurology. “This treatment combines habit-reversal training, which teaches patients how to control their urges to tic, with other behavioral strategies to reduce stress and other factors that often make tics worse.”
Patients typically see results from CBIT in 8-12 weeks. More CBIT providers are needed, however, to make the treatment readily available to all patients, he said.
The guideline panel members said that there was moderate confidence in the evidence for reduced tic severity for the following therapeutic approaches, compared with placebo: haloperidol, risperidone, aripiprazole (children only), tiapride (children only), clonidine, onabotulinumtoxinA injections, ningdong granule (as formulated by Zhao), (children only), and ling granule (children only). There was low or very low confidence in the evidence for all other therapies for reducing tic severity.
Comorbid conditions
Many people with tic disorders have neurodevelopmental or psychiatric conditions such as ADHD, obsessive-compulsive disorder, and mood and anxiety disorders. The guideline recommends that people with tics be evaluated for these conditions.
Alpha2-adrenergic agonists may improve symptoms of tic disorders and ADHD, the authors said. There was moderate confidence in the evidence for reduced tic severity for people with a comorbid diagnosis of ADHD with clonidine plus methylphenidate (children only) and methylphenidate alone (children only), compared with placebo.
Adults with severe Tourette syndrome who are resistant to medical and behavioral therapy may benefit from deep brain stimulation (DBS), the guideline states. There was moderate confidence in the evidence for reduced tic severity for DBS of the globus pallidus, compared with sham DBS, as an option for adults with severe tics who have failed CBIT and drugs. These patients first must be screened by a mental health professional and continue to be monitored throughout DBS treatment.
Adults with Tourette syndrome who self-treat their tics with cannabis in states where cannabis is legal should see a doctor who can monitor the use of cannabis for efficacy and adverse effects, the guideline says.
Adverse effects of therapy
The panel also examined the risks of harm, including weight gain, elevated prolactin levels, sedation, drug-induced movement disorders, hypotension, bradycardia, and ECG changes with medical treatments, compared with placebo or other active interventions. In regard to weight gain, the panel concluded with moderate confidence that people with tics receiving risperidone or aripiprazole (children only) are probably more likely to gain weight than people receiving placebo. There was low confidence for associations between specific therapies and elevated prolactin levels.
Compared with people receiving placebo, there was moderate confidence that tiapride is probably associated with higher rates of physical tiredness and sleep disturbances (children only), that clonidine is probably associated with sedation, and that guanfacine is probably associated with drowsiness (children only). There was moderate evidence that pimozide is probably associated with extrapyramidal symptoms. There was low confidence that any specific treatment led to hypotension, bradycardia, or ECG changes.
Additional guideline specifics
The guideline’s practice recommendations include explaining the natural history of tic disorders to patients and caregivers and evaluating patients for functional impairment. Watchful waiting is an acceptable approach in people who do not experience functional impairment, and patients receiving medications for tics must have periodic reevaluations for the need for ongoing medical treatment. People with Tourette syndrome should be referred to resources for psychoeducation for teachers and peers, such as the Tourette Association of America.
Comorbid ADHD occurs in 30%-50% of patients with tics. If screening for ADHD is positive, the burden of ADHD symptoms should be assessed and those with functionally impairing ADHD should be treated for the disorder. Similarly, obsessive-compulsive behaviors occur in 10%-50% of those with Tourette syndrome. If an assessment finds comorbid obsessive-compulsive disorder, it should be treated.
Other psychiatric comorbidities with Tourette syndrome include anxiety disorders, oppositional defiant disorder, and mood disorders. When screening for these conditions, one must inquire about suicidal thoughts and suicide attempts and refer to appropriate resources if present, according to the guidelines.
Individuals with tics and comorbid ADHD should be advised that alpha2-adrenergic agonists may provide benefit for both conditions. Alpha2-adrenergic agonists should be prescribed for the treatment of tics when the benefits of treatment outweigh the risks and patients must be counseled regarding common side effects of alpha2-adrenergic agonists, including sedation. Heart rate and blood pressure must be monitored in patients with tics treated with alpha2-adrenergic agonists. If prescribing extended-release guanfacine, one must monitor the QTc interval in patients with a history of cardiac conditions, patients taking other QT-prolonging agents, or patients with a family history of long QT syndrome. If discontinuing alpha2-adrenergic agonists, they must gradually be tapered to avoid rebound hypertension.
If considering antipsychotic therapies, patients must be counseled on the relative risk for extrapyramidal, hormonal, and metabolic adverse effects to inform decision making on which antipsychotic should be prescribed. Before prescribing antipsychotics for tics, ECGs must be performed. The QTc interval must be measured before and after starting pimozide or ziprasidone, or if antipsychotics are coadministered with other drugs that can prolong the QT interval. The lowest effective dose should be prescribed to decrease the risk of adverse effects, and patients should be monitored for drug-induced movement disorders and for metabolic and hormonal adverse effects of antipsychotics. When attempting to discontinue antipsychotics for tics, the medications should be gradually tapered over weeks to months to avoid withdrawal dyskinesias.
If topiramate is prescribed, patients must be counseled regarding common adverse effects, including cognitive and language problems, somnolence, weight loss, and an increased risk of renal stones.
Some patients with Tourette syndrome use cannabis as a self-medication for tics and comorbidities. Because of the risks associated with cannabis use and widespread self-medication with cannabis for tics, where regional legislation and resources allow, physicians must offer to direct patients to appropriate medical supervision when cannabis is used as self-medication for tics. Appropriate medical supervision would entail education and monitoring for efficacy and adverse effects, according to the guidelines.
Where regional legislation allows, physicians prescribing cannabis-based medication must prescribe the lowest effective dose to decrease the risk of adverse effects. Physicians prescribing cannabis-based medication must inform patients that medication may impair driving ability. Physicians prescribing cannabis-based medication to patients with Tourette syndrome must periodically reevaluate the need for ongoing treatment.
A multidisciplinary evaluation is needed to establish when the benefits of treatment outweigh the risks for prescribing DBS for medication-resistant motor and phonic tics. The DSM-5 diagnosis of Tourette syndrome must be confirmed and exclude secondary and functional tic-like movements when considering DBS for medication-resistant tics. A mental health professional must screen patients preoperatively and follow patients postoperatively for psychiatric disorders that may impede the long-term success of the therapy. Physicians must confirm that multiple classes of medication (antipsychotics, dopamine depleters, alpha1 agonists) and behavioral therapy have been administered (or are contraindicated) before prescribing DBS for tics.
The practice guideline was developed with financial support from AAN. Dr. Pringsheim reported no disclosures. Dr. Piacentini reported receiving funding for travel and speaking from foundations and universities and has received royalties from publishers. In addition, he has performed behavior therapy for tics for approximately 50% of his clinical time and has received financial or material support from Pfizer, the National Institute of Mental Health, and foundations.
SOURCE: Pringsheim T et al. Neurology. 2019 May 6. doi: 10.1212/WNL.0000000000007466.
PHILADELPHIA – Approaches to managing tics in patients with Tourette syndrome or chronic tic disorders “should be individualized, and the choice should be the result of a collaborative decision among patient, caregiver, and clinician, during which the benefits and harms of individual treatments as well as the presence of comorbid disorders are considered,” according to Tamara Pringsheim, MD, lead author of a practice guideline published May 6, 2019, by the American Academy of Neurology, and her collaborators.
The panel of nine physicians, two psychologists, and two patient representatives developed the recommendations based on a comprehensive systematic literature review. They concluded that treatments may decrease the frequency and severity of tics but rarely eliminate them.
The guideline was endorsed by the Child Neurology Society and the European Academy of Neurology and is the first such guideline for American neurologists, said Dr. Pringsheim of the University of Calgary (Alta.). Like recent Canadian and European guidelines, it strongly supports the Comprehensive Behavioral Intervention for Tics (CBIT) as a treatment option for tics.
After examining which medical, behavioral, and neurostimulation interventions, compared with placebo or other active interventions, improve tic severity and tic-related impairment in children and adults with Tourette syndrome or a chronic tic disorder, the guideline writers recommended that the evidence was strongest for CBIT as a first-line treatment, relative to other behavioral treatments and medications.
If symptoms affect a patient’s daily life, doctors should consider CBIT, said guideline author John Piacentini, PhD, of the University of California, Los Angeles, at the annual meeting of the American Academy of Neurology. “This treatment combines habit-reversal training, which teaches patients how to control their urges to tic, with other behavioral strategies to reduce stress and other factors that often make tics worse.”
Patients typically see results from CBIT in 8-12 weeks. More CBIT providers are needed, however, to make the treatment readily available to all patients, he said.
The guideline panel members said that there was moderate confidence in the evidence for reduced tic severity for the following therapeutic approaches, compared with placebo: haloperidol, risperidone, aripiprazole (children only), tiapride (children only), clonidine, onabotulinumtoxinA injections, ningdong granule (as formulated by Zhao), (children only), and ling granule (children only). There was low or very low confidence in the evidence for all other therapies for reducing tic severity.
Comorbid conditions
Many people with tic disorders have neurodevelopmental or psychiatric conditions such as ADHD, obsessive-compulsive disorder, and mood and anxiety disorders. The guideline recommends that people with tics be evaluated for these conditions.
Alpha2-adrenergic agonists may improve symptoms of tic disorders and ADHD, the authors said. There was moderate confidence in the evidence for reduced tic severity for people with a comorbid diagnosis of ADHD with clonidine plus methylphenidate (children only) and methylphenidate alone (children only), compared with placebo.
Adults with severe Tourette syndrome who are resistant to medical and behavioral therapy may benefit from deep brain stimulation (DBS), the guideline states. There was moderate confidence in the evidence for reduced tic severity for DBS of the globus pallidus, compared with sham DBS, as an option for adults with severe tics who have failed CBIT and drugs. These patients first must be screened by a mental health professional and continue to be monitored throughout DBS treatment.
Adults with Tourette syndrome who self-treat their tics with cannabis in states where cannabis is legal should see a doctor who can monitor the use of cannabis for efficacy and adverse effects, the guideline says.
Adverse effects of therapy
The panel also examined the risks of harm, including weight gain, elevated prolactin levels, sedation, drug-induced movement disorders, hypotension, bradycardia, and ECG changes with medical treatments, compared with placebo or other active interventions. In regard to weight gain, the panel concluded with moderate confidence that people with tics receiving risperidone or aripiprazole (children only) are probably more likely to gain weight than people receiving placebo. There was low confidence for associations between specific therapies and elevated prolactin levels.
Compared with people receiving placebo, there was moderate confidence that tiapride is probably associated with higher rates of physical tiredness and sleep disturbances (children only), that clonidine is probably associated with sedation, and that guanfacine is probably associated with drowsiness (children only). There was moderate evidence that pimozide is probably associated with extrapyramidal symptoms. There was low confidence that any specific treatment led to hypotension, bradycardia, or ECG changes.
Additional guideline specifics
The guideline’s practice recommendations include explaining the natural history of tic disorders to patients and caregivers and evaluating patients for functional impairment. Watchful waiting is an acceptable approach in people who do not experience functional impairment, and patients receiving medications for tics must have periodic reevaluations for the need for ongoing medical treatment. People with Tourette syndrome should be referred to resources for psychoeducation for teachers and peers, such as the Tourette Association of America.
Comorbid ADHD occurs in 30%-50% of patients with tics. If screening for ADHD is positive, the burden of ADHD symptoms should be assessed and those with functionally impairing ADHD should be treated for the disorder. Similarly, obsessive-compulsive behaviors occur in 10%-50% of those with Tourette syndrome. If an assessment finds comorbid obsessive-compulsive disorder, it should be treated.
Other psychiatric comorbidities with Tourette syndrome include anxiety disorders, oppositional defiant disorder, and mood disorders. When screening for these conditions, one must inquire about suicidal thoughts and suicide attempts and refer to appropriate resources if present, according to the guidelines.
Individuals with tics and comorbid ADHD should be advised that alpha2-adrenergic agonists may provide benefit for both conditions. Alpha2-adrenergic agonists should be prescribed for the treatment of tics when the benefits of treatment outweigh the risks and patients must be counseled regarding common side effects of alpha2-adrenergic agonists, including sedation. Heart rate and blood pressure must be monitored in patients with tics treated with alpha2-adrenergic agonists. If prescribing extended-release guanfacine, one must monitor the QTc interval in patients with a history of cardiac conditions, patients taking other QT-prolonging agents, or patients with a family history of long QT syndrome. If discontinuing alpha2-adrenergic agonists, they must gradually be tapered to avoid rebound hypertension.
If considering antipsychotic therapies, patients must be counseled on the relative risk for extrapyramidal, hormonal, and metabolic adverse effects to inform decision making on which antipsychotic should be prescribed. Before prescribing antipsychotics for tics, ECGs must be performed. The QTc interval must be measured before and after starting pimozide or ziprasidone, or if antipsychotics are coadministered with other drugs that can prolong the QT interval. The lowest effective dose should be prescribed to decrease the risk of adverse effects, and patients should be monitored for drug-induced movement disorders and for metabolic and hormonal adverse effects of antipsychotics. When attempting to discontinue antipsychotics for tics, the medications should be gradually tapered over weeks to months to avoid withdrawal dyskinesias.
If topiramate is prescribed, patients must be counseled regarding common adverse effects, including cognitive and language problems, somnolence, weight loss, and an increased risk of renal stones.
Some patients with Tourette syndrome use cannabis as a self-medication for tics and comorbidities. Because of the risks associated with cannabis use and widespread self-medication with cannabis for tics, where regional legislation and resources allow, physicians must offer to direct patients to appropriate medical supervision when cannabis is used as self-medication for tics. Appropriate medical supervision would entail education and monitoring for efficacy and adverse effects, according to the guidelines.
Where regional legislation allows, physicians prescribing cannabis-based medication must prescribe the lowest effective dose to decrease the risk of adverse effects. Physicians prescribing cannabis-based medication must inform patients that medication may impair driving ability. Physicians prescribing cannabis-based medication to patients with Tourette syndrome must periodically reevaluate the need for ongoing treatment.
A multidisciplinary evaluation is needed to establish when the benefits of treatment outweigh the risks for prescribing DBS for medication-resistant motor and phonic tics. The DSM-5 diagnosis of Tourette syndrome must be confirmed and exclude secondary and functional tic-like movements when considering DBS for medication-resistant tics. A mental health professional must screen patients preoperatively and follow patients postoperatively for psychiatric disorders that may impede the long-term success of the therapy. Physicians must confirm that multiple classes of medication (antipsychotics, dopamine depleters, alpha1 agonists) and behavioral therapy have been administered (or are contraindicated) before prescribing DBS for tics.
The practice guideline was developed with financial support from AAN. Dr. Pringsheim reported no disclosures. Dr. Piacentini reported receiving funding for travel and speaking from foundations and universities and has received royalties from publishers. In addition, he has performed behavior therapy for tics for approximately 50% of his clinical time and has received financial or material support from Pfizer, the National Institute of Mental Health, and foundations.
SOURCE: Pringsheim T et al. Neurology. 2019 May 6. doi: 10.1212/WNL.0000000000007466.
PHILADELPHIA – Approaches to managing tics in patients with Tourette syndrome or chronic tic disorders “should be individualized, and the choice should be the result of a collaborative decision among patient, caregiver, and clinician, during which the benefits and harms of individual treatments as well as the presence of comorbid disorders are considered,” according to Tamara Pringsheim, MD, lead author of a practice guideline published May 6, 2019, by the American Academy of Neurology, and her collaborators.
The panel of nine physicians, two psychologists, and two patient representatives developed the recommendations based on a comprehensive systematic literature review. They concluded that treatments may decrease the frequency and severity of tics but rarely eliminate them.
The guideline was endorsed by the Child Neurology Society and the European Academy of Neurology and is the first such guideline for American neurologists, said Dr. Pringsheim of the University of Calgary (Alta.). Like recent Canadian and European guidelines, it strongly supports the Comprehensive Behavioral Intervention for Tics (CBIT) as a treatment option for tics.
After examining which medical, behavioral, and neurostimulation interventions, compared with placebo or other active interventions, improve tic severity and tic-related impairment in children and adults with Tourette syndrome or a chronic tic disorder, the guideline writers recommended that the evidence was strongest for CBIT as a first-line treatment, relative to other behavioral treatments and medications.
If symptoms affect a patient’s daily life, doctors should consider CBIT, said guideline author John Piacentini, PhD, of the University of California, Los Angeles, at the annual meeting of the American Academy of Neurology. “This treatment combines habit-reversal training, which teaches patients how to control their urges to tic, with other behavioral strategies to reduce stress and other factors that often make tics worse.”
Patients typically see results from CBIT in 8-12 weeks. More CBIT providers are needed, however, to make the treatment readily available to all patients, he said.
The guideline panel members said that there was moderate confidence in the evidence for reduced tic severity for the following therapeutic approaches, compared with placebo: haloperidol, risperidone, aripiprazole (children only), tiapride (children only), clonidine, onabotulinumtoxinA injections, ningdong granule (as formulated by Zhao), (children only), and ling granule (children only). There was low or very low confidence in the evidence for all other therapies for reducing tic severity.
Comorbid conditions
Many people with tic disorders have neurodevelopmental or psychiatric conditions such as ADHD, obsessive-compulsive disorder, and mood and anxiety disorders. The guideline recommends that people with tics be evaluated for these conditions.
Alpha2-adrenergic agonists may improve symptoms of tic disorders and ADHD, the authors said. There was moderate confidence in the evidence for reduced tic severity for people with a comorbid diagnosis of ADHD with clonidine plus methylphenidate (children only) and methylphenidate alone (children only), compared with placebo.
Adults with severe Tourette syndrome who are resistant to medical and behavioral therapy may benefit from deep brain stimulation (DBS), the guideline states. There was moderate confidence in the evidence for reduced tic severity for DBS of the globus pallidus, compared with sham DBS, as an option for adults with severe tics who have failed CBIT and drugs. These patients first must be screened by a mental health professional and continue to be monitored throughout DBS treatment.
Adults with Tourette syndrome who self-treat their tics with cannabis in states where cannabis is legal should see a doctor who can monitor the use of cannabis for efficacy and adverse effects, the guideline says.
Adverse effects of therapy
The panel also examined the risks of harm, including weight gain, elevated prolactin levels, sedation, drug-induced movement disorders, hypotension, bradycardia, and ECG changes with medical treatments, compared with placebo or other active interventions. In regard to weight gain, the panel concluded with moderate confidence that people with tics receiving risperidone or aripiprazole (children only) are probably more likely to gain weight than people receiving placebo. There was low confidence for associations between specific therapies and elevated prolactin levels.
Compared with people receiving placebo, there was moderate confidence that tiapride is probably associated with higher rates of physical tiredness and sleep disturbances (children only), that clonidine is probably associated with sedation, and that guanfacine is probably associated with drowsiness (children only). There was moderate evidence that pimozide is probably associated with extrapyramidal symptoms. There was low confidence that any specific treatment led to hypotension, bradycardia, or ECG changes.
Additional guideline specifics
The guideline’s practice recommendations include explaining the natural history of tic disorders to patients and caregivers and evaluating patients for functional impairment. Watchful waiting is an acceptable approach in people who do not experience functional impairment, and patients receiving medications for tics must have periodic reevaluations for the need for ongoing medical treatment. People with Tourette syndrome should be referred to resources for psychoeducation for teachers and peers, such as the Tourette Association of America.
Comorbid ADHD occurs in 30%-50% of patients with tics. If screening for ADHD is positive, the burden of ADHD symptoms should be assessed and those with functionally impairing ADHD should be treated for the disorder. Similarly, obsessive-compulsive behaviors occur in 10%-50% of those with Tourette syndrome. If an assessment finds comorbid obsessive-compulsive disorder, it should be treated.
Other psychiatric comorbidities with Tourette syndrome include anxiety disorders, oppositional defiant disorder, and mood disorders. When screening for these conditions, one must inquire about suicidal thoughts and suicide attempts and refer to appropriate resources if present, according to the guidelines.
Individuals with tics and comorbid ADHD should be advised that alpha2-adrenergic agonists may provide benefit for both conditions. Alpha2-adrenergic agonists should be prescribed for the treatment of tics when the benefits of treatment outweigh the risks and patients must be counseled regarding common side effects of alpha2-adrenergic agonists, including sedation. Heart rate and blood pressure must be monitored in patients with tics treated with alpha2-adrenergic agonists. If prescribing extended-release guanfacine, one must monitor the QTc interval in patients with a history of cardiac conditions, patients taking other QT-prolonging agents, or patients with a family history of long QT syndrome. If discontinuing alpha2-adrenergic agonists, they must gradually be tapered to avoid rebound hypertension.
If considering antipsychotic therapies, patients must be counseled on the relative risk for extrapyramidal, hormonal, and metabolic adverse effects to inform decision making on which antipsychotic should be prescribed. Before prescribing antipsychotics for tics, ECGs must be performed. The QTc interval must be measured before and after starting pimozide or ziprasidone, or if antipsychotics are coadministered with other drugs that can prolong the QT interval. The lowest effective dose should be prescribed to decrease the risk of adverse effects, and patients should be monitored for drug-induced movement disorders and for metabolic and hormonal adverse effects of antipsychotics. When attempting to discontinue antipsychotics for tics, the medications should be gradually tapered over weeks to months to avoid withdrawal dyskinesias.
If topiramate is prescribed, patients must be counseled regarding common adverse effects, including cognitive and language problems, somnolence, weight loss, and an increased risk of renal stones.
Some patients with Tourette syndrome use cannabis as a self-medication for tics and comorbidities. Because of the risks associated with cannabis use and widespread self-medication with cannabis for tics, where regional legislation and resources allow, physicians must offer to direct patients to appropriate medical supervision when cannabis is used as self-medication for tics. Appropriate medical supervision would entail education and monitoring for efficacy and adverse effects, according to the guidelines.
Where regional legislation allows, physicians prescribing cannabis-based medication must prescribe the lowest effective dose to decrease the risk of adverse effects. Physicians prescribing cannabis-based medication must inform patients that medication may impair driving ability. Physicians prescribing cannabis-based medication to patients with Tourette syndrome must periodically reevaluate the need for ongoing treatment.
A multidisciplinary evaluation is needed to establish when the benefits of treatment outweigh the risks for prescribing DBS for medication-resistant motor and phonic tics. The DSM-5 diagnosis of Tourette syndrome must be confirmed and exclude secondary and functional tic-like movements when considering DBS for medication-resistant tics. A mental health professional must screen patients preoperatively and follow patients postoperatively for psychiatric disorders that may impede the long-term success of the therapy. Physicians must confirm that multiple classes of medication (antipsychotics, dopamine depleters, alpha1 agonists) and behavioral therapy have been administered (or are contraindicated) before prescribing DBS for tics.
The practice guideline was developed with financial support from AAN. Dr. Pringsheim reported no disclosures. Dr. Piacentini reported receiving funding for travel and speaking from foundations and universities and has received royalties from publishers. In addition, he has performed behavior therapy for tics for approximately 50% of his clinical time and has received financial or material support from Pfizer, the National Institute of Mental Health, and foundations.
SOURCE: Pringsheim T et al. Neurology. 2019 May 6. doi: 10.1212/WNL.0000000000007466.
REPORTING FROM AAN 2019
Laquinimod may not improve motor function in Huntington’s disease
PHILADELPHIA – Laquinimod appears not to improve motor function or clinical outcomes in patients with Huntington’s disease, according to a study presented at the annual meeting of the American Academy of Neurology. However, the drug reduced brain volume loss in the caudate and other regions.
Laquinimod is an investigational immunomodulatory drug that prevents inflammation and neurodegeneration in the CNS. The treatment was studied as a therapy for multiple sclerosis (MS), but development for this indication has been stopped. Researchers have observed that laquinimod modulates inflammatory pathways that are involved in Huntington’s disease pathology.
Ralf Reilmann, MD, PhD, founder of the George Huntington Institute and chair of the Huntington unit at the University of Münster (Germany), and colleagues conducted the phase 2 LEGATO-HD study at 48 sites in 10 countries to examine the efficacy and safety of laquinimod in patients with early Huntington’s disease. Participants were randomized in double-blind fashion to daily placebo or 0.5-mg, 1.0-mg, or 1.5-mg doses of laquinimod for 52 weeks. After the initiation of this trial, studies of the drug in MS indicated that the 1.5-mg dose was associated with cardiovascular risks, and Dr. Reilmann and his colleagues discontinued the 1.5-mg arm of their trial as a precaution.
The primary endpoint of LEGATO-HD was the change from baseline in the Unified Huntington’s Disease Rating Scale (UHDRS)–Total Motor Score (TMS). The secondary endpoint was the percent change in caudate volume at week 52 for the 1.0-mg dose group, compared with controls. The investigators also examined exploratory endpoints such as changes in MRI volume measures and Quantitative Motor, Clinician Interview-Based Impression of Change plus caregiver input, UHDRS–Total Functional Capacity and UHDRS–Functional Assessment scores. Adverse event reporting and clinical and laboratory examinations constituted the safety measures.
Dr. Reilmann and colleagues found no difference between the treated patients and controls in UHDRS-TMS. However, they did observe less caudate volume loss in the laquinimod group, compared with controls. All MRI exploratory measures also favored laquinimod. The researchers found no treatment effects of laquinimod in rater-dependent clinical outcome measures. Laquinimod was well tolerated, and the study yielded no new safety findings.
Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018 sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
SOURCE: Reilmann R et al. AAN 2019, Abstract S16.007.
PHILADELPHIA – Laquinimod appears not to improve motor function or clinical outcomes in patients with Huntington’s disease, according to a study presented at the annual meeting of the American Academy of Neurology. However, the drug reduced brain volume loss in the caudate and other regions.
Laquinimod is an investigational immunomodulatory drug that prevents inflammation and neurodegeneration in the CNS. The treatment was studied as a therapy for multiple sclerosis (MS), but development for this indication has been stopped. Researchers have observed that laquinimod modulates inflammatory pathways that are involved in Huntington’s disease pathology.
Ralf Reilmann, MD, PhD, founder of the George Huntington Institute and chair of the Huntington unit at the University of Münster (Germany), and colleagues conducted the phase 2 LEGATO-HD study at 48 sites in 10 countries to examine the efficacy and safety of laquinimod in patients with early Huntington’s disease. Participants were randomized in double-blind fashion to daily placebo or 0.5-mg, 1.0-mg, or 1.5-mg doses of laquinimod for 52 weeks. After the initiation of this trial, studies of the drug in MS indicated that the 1.5-mg dose was associated with cardiovascular risks, and Dr. Reilmann and his colleagues discontinued the 1.5-mg arm of their trial as a precaution.
The primary endpoint of LEGATO-HD was the change from baseline in the Unified Huntington’s Disease Rating Scale (UHDRS)–Total Motor Score (TMS). The secondary endpoint was the percent change in caudate volume at week 52 for the 1.0-mg dose group, compared with controls. The investigators also examined exploratory endpoints such as changes in MRI volume measures and Quantitative Motor, Clinician Interview-Based Impression of Change plus caregiver input, UHDRS–Total Functional Capacity and UHDRS–Functional Assessment scores. Adverse event reporting and clinical and laboratory examinations constituted the safety measures.
Dr. Reilmann and colleagues found no difference between the treated patients and controls in UHDRS-TMS. However, they did observe less caudate volume loss in the laquinimod group, compared with controls. All MRI exploratory measures also favored laquinimod. The researchers found no treatment effects of laquinimod in rater-dependent clinical outcome measures. Laquinimod was well tolerated, and the study yielded no new safety findings.
Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018 sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
SOURCE: Reilmann R et al. AAN 2019, Abstract S16.007.
PHILADELPHIA – Laquinimod appears not to improve motor function or clinical outcomes in patients with Huntington’s disease, according to a study presented at the annual meeting of the American Academy of Neurology. However, the drug reduced brain volume loss in the caudate and other regions.
Laquinimod is an investigational immunomodulatory drug that prevents inflammation and neurodegeneration in the CNS. The treatment was studied as a therapy for multiple sclerosis (MS), but development for this indication has been stopped. Researchers have observed that laquinimod modulates inflammatory pathways that are involved in Huntington’s disease pathology.
Ralf Reilmann, MD, PhD, founder of the George Huntington Institute and chair of the Huntington unit at the University of Münster (Germany), and colleagues conducted the phase 2 LEGATO-HD study at 48 sites in 10 countries to examine the efficacy and safety of laquinimod in patients with early Huntington’s disease. Participants were randomized in double-blind fashion to daily placebo or 0.5-mg, 1.0-mg, or 1.5-mg doses of laquinimod for 52 weeks. After the initiation of this trial, studies of the drug in MS indicated that the 1.5-mg dose was associated with cardiovascular risks, and Dr. Reilmann and his colleagues discontinued the 1.5-mg arm of their trial as a precaution.
The primary endpoint of LEGATO-HD was the change from baseline in the Unified Huntington’s Disease Rating Scale (UHDRS)–Total Motor Score (TMS). The secondary endpoint was the percent change in caudate volume at week 52 for the 1.0-mg dose group, compared with controls. The investigators also examined exploratory endpoints such as changes in MRI volume measures and Quantitative Motor, Clinician Interview-Based Impression of Change plus caregiver input, UHDRS–Total Functional Capacity and UHDRS–Functional Assessment scores. Adverse event reporting and clinical and laboratory examinations constituted the safety measures.
Dr. Reilmann and colleagues found no difference between the treated patients and controls in UHDRS-TMS. However, they did observe less caudate volume loss in the laquinimod group, compared with controls. All MRI exploratory measures also favored laquinimod. The researchers found no treatment effects of laquinimod in rater-dependent clinical outcome measures. Laquinimod was well tolerated, and the study yielded no new safety findings.
Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018 sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
SOURCE: Reilmann R et al. AAN 2019, Abstract S16.007.
REPORTING FROM AAN 2019
Key clinical point: Investigators found no difference between laquinimod and placebo on motor function in Huntington’s disease.
Major finding: The study examined 0.5-mg, 1.0-mg, and 1.5-mg doses of laquinimod.
Study details: The phase 2 LEGATO-HD trial included 352 patients with Huntington’s disease who underwent a 52-week treatment period.
Disclosures: Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018, sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
Source: Reilmann R et al. AAN 2019, Abstract S16.007.
Biomarkers in tears may help identify patients with Parkinson’s disease
PHILADELPHIA – according to research presented at the annual meeting of the American Academy of Neurology. Chemokine (C-C motif) ligand 2 (CCL2), a cytokine, may be used for the same purpose, according to researchers.
Lacrimal glands have high numbers of cholinergic and other neurons. Parasympathetic and sympathetic neural pathways stimulate the tears that lacrimal grands secrete. It is possible that the production, packaging, and secretion of proteins into tears may alter when nerve function in the lacrimal glands and cornea changes. This idea may be tested by collecting reflex tears (i.e., stimulated tears provoked by an unanesthetized Schirmer’s test).
Mark Lew, MD, professor of clinical neurology at University of Southern California, Los Angeles, and colleagues previously studied patients using basal tears collected from an anesthetized Schirmer’s test. To examine whether the protein composition of reflex tears differs in patients with Parkinson’s disease, compared with healthy controls, they collected reflex tears from 85 patients with Parkinson’s disease and 80 age- and sex-matched healthy controls using an unanesthetized Schirmer’s test. The researchers pooled samples from both eyes to analyze alpha synuclein, CCL2, and total protein using enzyme-linked immunosorbent assays or multiplex ELISA.
Eligible participants were aged 30-85 years and had a Montreal Cognitive Assessment (MoCA) score of 21 or higher. Patients with Parkinson’s disease had a lower MoCA score than controls did, although it was still in the normal range. Tear flow was significantly decreased in patients with Parkinson’s disease, compared with controls.
Dr. Lew and colleagues found that the amount of oligomeric alpha synuclein was increased nearly 400% in the tears of patients with Parkinson’s disease, compared with those of healthy controls (4.21 ng/mg tear protein vs. 0.90 ng/mg tear protein). This difference was statistically significant. Similarly, CCL2 was significantly increased in the tears of patients with Parkinson’s disease, compared with those of healthy controls (165.8 pg/mg tear protein vs. 116.3 pg/mg tear protein). Among men, Parkinson’s disease was associated with greater rises in oligomeric alpha synuclein (4.95 ng/mg tear protein vs. 0.89 ng/mg tear protein in healthy controls) and CCL2 (201.5 pg/mg tear protein vs. 117.9 pg/mg tear protein in healthy controls) than in women. The origin of sex differences in these biomarker values requires further study, the investigators said.
Dr. Lew reported receiving research support from the Parkinson’s Study Group, the Michael J. Fox Foundation, Biotie Therapies, NeuroDerm, Enterin, Pharm2B Fellowship Grants, Allergan, and Medtronic.
SOURCE: Lew M et al. AAN 2019, Abstract S10.001
PHILADELPHIA – according to research presented at the annual meeting of the American Academy of Neurology. Chemokine (C-C motif) ligand 2 (CCL2), a cytokine, may be used for the same purpose, according to researchers.
Lacrimal glands have high numbers of cholinergic and other neurons. Parasympathetic and sympathetic neural pathways stimulate the tears that lacrimal grands secrete. It is possible that the production, packaging, and secretion of proteins into tears may alter when nerve function in the lacrimal glands and cornea changes. This idea may be tested by collecting reflex tears (i.e., stimulated tears provoked by an unanesthetized Schirmer’s test).
Mark Lew, MD, professor of clinical neurology at University of Southern California, Los Angeles, and colleagues previously studied patients using basal tears collected from an anesthetized Schirmer’s test. To examine whether the protein composition of reflex tears differs in patients with Parkinson’s disease, compared with healthy controls, they collected reflex tears from 85 patients with Parkinson’s disease and 80 age- and sex-matched healthy controls using an unanesthetized Schirmer’s test. The researchers pooled samples from both eyes to analyze alpha synuclein, CCL2, and total protein using enzyme-linked immunosorbent assays or multiplex ELISA.
Eligible participants were aged 30-85 years and had a Montreal Cognitive Assessment (MoCA) score of 21 or higher. Patients with Parkinson’s disease had a lower MoCA score than controls did, although it was still in the normal range. Tear flow was significantly decreased in patients with Parkinson’s disease, compared with controls.
Dr. Lew and colleagues found that the amount of oligomeric alpha synuclein was increased nearly 400% in the tears of patients with Parkinson’s disease, compared with those of healthy controls (4.21 ng/mg tear protein vs. 0.90 ng/mg tear protein). This difference was statistically significant. Similarly, CCL2 was significantly increased in the tears of patients with Parkinson’s disease, compared with those of healthy controls (165.8 pg/mg tear protein vs. 116.3 pg/mg tear protein). Among men, Parkinson’s disease was associated with greater rises in oligomeric alpha synuclein (4.95 ng/mg tear protein vs. 0.89 ng/mg tear protein in healthy controls) and CCL2 (201.5 pg/mg tear protein vs. 117.9 pg/mg tear protein in healthy controls) than in women. The origin of sex differences in these biomarker values requires further study, the investigators said.
Dr. Lew reported receiving research support from the Parkinson’s Study Group, the Michael J. Fox Foundation, Biotie Therapies, NeuroDerm, Enterin, Pharm2B Fellowship Grants, Allergan, and Medtronic.
SOURCE: Lew M et al. AAN 2019, Abstract S10.001
PHILADELPHIA – according to research presented at the annual meeting of the American Academy of Neurology. Chemokine (C-C motif) ligand 2 (CCL2), a cytokine, may be used for the same purpose, according to researchers.
Lacrimal glands have high numbers of cholinergic and other neurons. Parasympathetic and sympathetic neural pathways stimulate the tears that lacrimal grands secrete. It is possible that the production, packaging, and secretion of proteins into tears may alter when nerve function in the lacrimal glands and cornea changes. This idea may be tested by collecting reflex tears (i.e., stimulated tears provoked by an unanesthetized Schirmer’s test).
Mark Lew, MD, professor of clinical neurology at University of Southern California, Los Angeles, and colleagues previously studied patients using basal tears collected from an anesthetized Schirmer’s test. To examine whether the protein composition of reflex tears differs in patients with Parkinson’s disease, compared with healthy controls, they collected reflex tears from 85 patients with Parkinson’s disease and 80 age- and sex-matched healthy controls using an unanesthetized Schirmer’s test. The researchers pooled samples from both eyes to analyze alpha synuclein, CCL2, and total protein using enzyme-linked immunosorbent assays or multiplex ELISA.
Eligible participants were aged 30-85 years and had a Montreal Cognitive Assessment (MoCA) score of 21 or higher. Patients with Parkinson’s disease had a lower MoCA score than controls did, although it was still in the normal range. Tear flow was significantly decreased in patients with Parkinson’s disease, compared with controls.
Dr. Lew and colleagues found that the amount of oligomeric alpha synuclein was increased nearly 400% in the tears of patients with Parkinson’s disease, compared with those of healthy controls (4.21 ng/mg tear protein vs. 0.90 ng/mg tear protein). This difference was statistically significant. Similarly, CCL2 was significantly increased in the tears of patients with Parkinson’s disease, compared with those of healthy controls (165.8 pg/mg tear protein vs. 116.3 pg/mg tear protein). Among men, Parkinson’s disease was associated with greater rises in oligomeric alpha synuclein (4.95 ng/mg tear protein vs. 0.89 ng/mg tear protein in healthy controls) and CCL2 (201.5 pg/mg tear protein vs. 117.9 pg/mg tear protein in healthy controls) than in women. The origin of sex differences in these biomarker values requires further study, the investigators said.
Dr. Lew reported receiving research support from the Parkinson’s Study Group, the Michael J. Fox Foundation, Biotie Therapies, NeuroDerm, Enterin, Pharm2B Fellowship Grants, Allergan, and Medtronic.
SOURCE: Lew M et al. AAN 2019, Abstract S10.001
REPORTING FROM AAN 2019











