User login
FDA approves IV formulation of drug for CINV
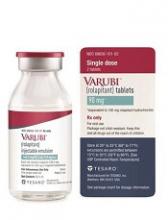
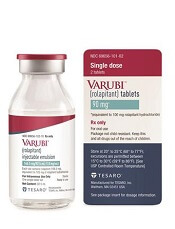
The US Food and Drug Administration (FDA) has approved an intravenous (IV) formulation of rolapitant (VARUBI®) for the same indication as the oral formulation.
This means IV rolapitant is now FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic chemotherapy in adults with cancer.
TESARO Inc., makers of rolapitant, said the US commercial launch of IV rolapitant is planned for November.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 receptors, which play an important role in the delayed phase of chemotherapy-induced nausea and vomiting (CINV).
IV rolapitant features a ready-to-use, single-dose vial for administration. It does not require refrigerated storage or mixing.
The recommended dose of IV rolapitant is 166.5 mg, administered over 30 minutes. The drug is to be administered up to 2 hours before chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone.
The full prescribing information for IV rolapitant is available at www.varubirx.com.
Bioequivalence trial
Results from a bioequivalence trial suggest the IV and oral formulations of rolapitant are comparable.
The study was conducted in healthy volunteers. Subjects were randomized to receive a single dose of IV rolapitant at 166.5 mg administered over 30 minutes (n=61) or oral rolapitant at 180 mg (n=62).
The primary endpoint was bioequivalence, and the 166.5 mg IV infusion of rolapitant met bioequivalence criteria.
Researchers said the safety profile of IV rolapitant was largely consistent with that of oral rolapitant, although infusion-site reactions were observed with the IV formulation. These included the sensation of warmth, abdominal pain, dizziness, and paresthesia.
These results were recently published in The Journal of Clinical Pharmacology.
Oral rolapitant trials
Two phase 3 trials showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after highly emetogenic chemotherapy.
Results from these trials (NCT01499849 and NCT01500213) were published in a single article in The Lancet Oncology.
A third phase 3 trial showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after moderately emetogenic chemotherapy.
Results from this trial (NCT01500226) were also published in The Lancet Oncology. ![]()
The US Food and Drug Administration (FDA) has approved an intravenous (IV) formulation of rolapitant (VARUBI®) for the same indication as the oral formulation.
This means IV rolapitant is now FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic chemotherapy in adults with cancer.
TESARO Inc., makers of rolapitant, said the US commercial launch of IV rolapitant is planned for November.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 receptors, which play an important role in the delayed phase of chemotherapy-induced nausea and vomiting (CINV).
IV rolapitant features a ready-to-use, single-dose vial for administration. It does not require refrigerated storage or mixing.
The recommended dose of IV rolapitant is 166.5 mg, administered over 30 minutes. The drug is to be administered up to 2 hours before chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone.
The full prescribing information for IV rolapitant is available at www.varubirx.com.
Bioequivalence trial
Results from a bioequivalence trial suggest the IV and oral formulations of rolapitant are comparable.
The study was conducted in healthy volunteers. Subjects were randomized to receive a single dose of IV rolapitant at 166.5 mg administered over 30 minutes (n=61) or oral rolapitant at 180 mg (n=62).
The primary endpoint was bioequivalence, and the 166.5 mg IV infusion of rolapitant met bioequivalence criteria.
Researchers said the safety profile of IV rolapitant was largely consistent with that of oral rolapitant, although infusion-site reactions were observed with the IV formulation. These included the sensation of warmth, abdominal pain, dizziness, and paresthesia.
These results were recently published in The Journal of Clinical Pharmacology.
Oral rolapitant trials
Two phase 3 trials showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after highly emetogenic chemotherapy.
Results from these trials (NCT01499849 and NCT01500213) were published in a single article in The Lancet Oncology.
A third phase 3 trial showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after moderately emetogenic chemotherapy.
Results from this trial (NCT01500226) were also published in The Lancet Oncology. ![]()
The US Food and Drug Administration (FDA) has approved an intravenous (IV) formulation of rolapitant (VARUBI®) for the same indication as the oral formulation.
This means IV rolapitant is now FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic chemotherapy in adults with cancer.
TESARO Inc., makers of rolapitant, said the US commercial launch of IV rolapitant is planned for November.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 receptors, which play an important role in the delayed phase of chemotherapy-induced nausea and vomiting (CINV).
IV rolapitant features a ready-to-use, single-dose vial for administration. It does not require refrigerated storage or mixing.
The recommended dose of IV rolapitant is 166.5 mg, administered over 30 minutes. The drug is to be administered up to 2 hours before chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone.
The full prescribing information for IV rolapitant is available at www.varubirx.com.
Bioequivalence trial
Results from a bioequivalence trial suggest the IV and oral formulations of rolapitant are comparable.
The study was conducted in healthy volunteers. Subjects were randomized to receive a single dose of IV rolapitant at 166.5 mg administered over 30 minutes (n=61) or oral rolapitant at 180 mg (n=62).
The primary endpoint was bioequivalence, and the 166.5 mg IV infusion of rolapitant met bioequivalence criteria.
Researchers said the safety profile of IV rolapitant was largely consistent with that of oral rolapitant, although infusion-site reactions were observed with the IV formulation. These included the sensation of warmth, abdominal pain, dizziness, and paresthesia.
These results were recently published in The Journal of Clinical Pharmacology.
Oral rolapitant trials
Two phase 3 trials showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after highly emetogenic chemotherapy.
Results from these trials (NCT01499849 and NCT01500213) were published in a single article in The Lancet Oncology.
A third phase 3 trial showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after moderately emetogenic chemotherapy.
Results from this trial (NCT01500226) were also published in The Lancet Oncology. ![]()
Overcoming resistance to proteasome inhibitors
Preclinical research has revealed a potential method of overcoming resistance to proteasome inhibitors.
By studying a rare genetic disease known as NGLY1 deficiency, researchers have gained new understanding of resistance to proteasome inhibitors.
The team found that treatment with a NGLY1 inhibitor can enhance the activity of the proteasome inhibitor carfilzomib against multiple myeloma (MM) and T-cell acute lymphoblastic leukemia (T-ALL).
Carolyn Bertozzi, PhD, of Stanford University in California, and her colleagues reported these findings in ACS Central Science.
Previous studies have suggested that proteasome inhibitor resistance could be linked to a protein called Nrf1. When proteasome inhibitors swing into action, Nrf1 is spurred into overdrive to restore cancer cells’ normal activities and keep them alive.
Researchers theorized that, if they could block Nrf1, they might be able to overcome proteasome inhibitor resistance.
Through studying NGLY1 deficiency, Dr Bertozzi and her colleagues may have hit upon an approach to do just that.
The researchers were investigating how lacking NGLY1 causes a host of debilitating symptoms, and they found that NGLY1 is responsible for activating Nrf1.
Further testing revealed that inhibiting NGLY1 eliminated interference from Nrf1 and enhanced the cytotoxicity of carfilzomib in MM and T-ALL cell lines.
The researchers treated the MM cell lines U266 and H929 with the NGLY1 inhibitor, known as WRR139, and carfilzomib and observed a significant decrease in cell survival when compared to treatment with carfilzomib alone. The team observed the same results when testing the T-ALL Jurkat cell line.
The addition of WRR139 resulted in a 2.6-fold reduction in carfilzomib’s LD50 for U266, a 2.0-fold reduction for H929, and a 1.5-fold reduction for Jurkat cells.
The researchers said these findings hold promise for the development of combination therapies for hematologic malignancies. ![]()
Preclinical research has revealed a potential method of overcoming resistance to proteasome inhibitors.
By studying a rare genetic disease known as NGLY1 deficiency, researchers have gained new understanding of resistance to proteasome inhibitors.
The team found that treatment with a NGLY1 inhibitor can enhance the activity of the proteasome inhibitor carfilzomib against multiple myeloma (MM) and T-cell acute lymphoblastic leukemia (T-ALL).
Carolyn Bertozzi, PhD, of Stanford University in California, and her colleagues reported these findings in ACS Central Science.
Previous studies have suggested that proteasome inhibitor resistance could be linked to a protein called Nrf1. When proteasome inhibitors swing into action, Nrf1 is spurred into overdrive to restore cancer cells’ normal activities and keep them alive.
Researchers theorized that, if they could block Nrf1, they might be able to overcome proteasome inhibitor resistance.
Through studying NGLY1 deficiency, Dr Bertozzi and her colleagues may have hit upon an approach to do just that.
The researchers were investigating how lacking NGLY1 causes a host of debilitating symptoms, and they found that NGLY1 is responsible for activating Nrf1.
Further testing revealed that inhibiting NGLY1 eliminated interference from Nrf1 and enhanced the cytotoxicity of carfilzomib in MM and T-ALL cell lines.
The researchers treated the MM cell lines U266 and H929 with the NGLY1 inhibitor, known as WRR139, and carfilzomib and observed a significant decrease in cell survival when compared to treatment with carfilzomib alone. The team observed the same results when testing the T-ALL Jurkat cell line.
The addition of WRR139 resulted in a 2.6-fold reduction in carfilzomib’s LD50 for U266, a 2.0-fold reduction for H929, and a 1.5-fold reduction for Jurkat cells.
The researchers said these findings hold promise for the development of combination therapies for hematologic malignancies. ![]()
Preclinical research has revealed a potential method of overcoming resistance to proteasome inhibitors.
By studying a rare genetic disease known as NGLY1 deficiency, researchers have gained new understanding of resistance to proteasome inhibitors.
The team found that treatment with a NGLY1 inhibitor can enhance the activity of the proteasome inhibitor carfilzomib against multiple myeloma (MM) and T-cell acute lymphoblastic leukemia (T-ALL).
Carolyn Bertozzi, PhD, of Stanford University in California, and her colleagues reported these findings in ACS Central Science.
Previous studies have suggested that proteasome inhibitor resistance could be linked to a protein called Nrf1. When proteasome inhibitors swing into action, Nrf1 is spurred into overdrive to restore cancer cells’ normal activities and keep them alive.
Researchers theorized that, if they could block Nrf1, they might be able to overcome proteasome inhibitor resistance.
Through studying NGLY1 deficiency, Dr Bertozzi and her colleagues may have hit upon an approach to do just that.
The researchers were investigating how lacking NGLY1 causes a host of debilitating symptoms, and they found that NGLY1 is responsible for activating Nrf1.
Further testing revealed that inhibiting NGLY1 eliminated interference from Nrf1 and enhanced the cytotoxicity of carfilzomib in MM and T-ALL cell lines.
The researchers treated the MM cell lines U266 and H929 with the NGLY1 inhibitor, known as WRR139, and carfilzomib and observed a significant decrease in cell survival when compared to treatment with carfilzomib alone. The team observed the same results when testing the T-ALL Jurkat cell line.
The addition of WRR139 resulted in a 2.6-fold reduction in carfilzomib’s LD50 for U266, a 2.0-fold reduction for H929, and a 1.5-fold reduction for Jurkat cells.
The researchers said these findings hold promise for the development of combination therapies for hematologic malignancies. ![]()
Optimizing dose of carfilzomib in rel/ref MM
Top-line results from the phase 3 A.R.R.O.W. trial support a 70 mg/m2 weekly dose of carfilzomib in combination with dexamethasone for patients with relapsed and refractory multiple myeloma (MM).
The data suggest a weekly dose of carfilzomib at 70 mg/m2 is more effective than, and just as safe as, a twice-weekly dose of carfilzomib at 27 mg/m2.
These results were recently announced by Amgen, makers of carfilzomib.
The A.R.R.O.W. trial included 478 patients with relapsed and refractory MM who had received 2 to 3 prior therapies, including bortezomib and an immunomodulatory drug.
Patients were randomized to receive once-weekly carfilzomib (20 mg/m2 on day 1 of cycle 1; 70 mg/m2 on days 8 and 15 of cycle 1; and 70 mg/m2 on days 1, 8 and 15 of subsequent cycles) with dexamethasone (40 mg) or twice-weekly carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1; 27 mg/m2 on days 8, 9, 15 and 16 of cycle 1; and 27 mg/m2 on days 1, 2, 8, 9, 15 and 16 of subsequent cycles) with dexamethasone (40 mg).
The study’s primary endpoint is progression-free survival (PFS).
Patients treated with the once-weekly regimen had significantly better PFS than patients who received carfilzomib twice weekly. The median PFS was 11.2 months and 7.6 months, respectively (hazard ratio=0.69, 95% confidence interval, 0.54–0.88).
The overall safety profile of the once-weekly regimen was comparable to that of the twice-weekly regimen.
The most frequently reported treatment-emergent adverse events (occurring in at least 20% of patients) in either treatment arm were anemia, diarrhea, fatigue, hypertension, insomnia, and pyrexia. ![]()
Top-line results from the phase 3 A.R.R.O.W. trial support a 70 mg/m2 weekly dose of carfilzomib in combination with dexamethasone for patients with relapsed and refractory multiple myeloma (MM).
The data suggest a weekly dose of carfilzomib at 70 mg/m2 is more effective than, and just as safe as, a twice-weekly dose of carfilzomib at 27 mg/m2.
These results were recently announced by Amgen, makers of carfilzomib.
The A.R.R.O.W. trial included 478 patients with relapsed and refractory MM who had received 2 to 3 prior therapies, including bortezomib and an immunomodulatory drug.
Patients were randomized to receive once-weekly carfilzomib (20 mg/m2 on day 1 of cycle 1; 70 mg/m2 on days 8 and 15 of cycle 1; and 70 mg/m2 on days 1, 8 and 15 of subsequent cycles) with dexamethasone (40 mg) or twice-weekly carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1; 27 mg/m2 on days 8, 9, 15 and 16 of cycle 1; and 27 mg/m2 on days 1, 2, 8, 9, 15 and 16 of subsequent cycles) with dexamethasone (40 mg).
The study’s primary endpoint is progression-free survival (PFS).
Patients treated with the once-weekly regimen had significantly better PFS than patients who received carfilzomib twice weekly. The median PFS was 11.2 months and 7.6 months, respectively (hazard ratio=0.69, 95% confidence interval, 0.54–0.88).
The overall safety profile of the once-weekly regimen was comparable to that of the twice-weekly regimen.
The most frequently reported treatment-emergent adverse events (occurring in at least 20% of patients) in either treatment arm were anemia, diarrhea, fatigue, hypertension, insomnia, and pyrexia. ![]()
Top-line results from the phase 3 A.R.R.O.W. trial support a 70 mg/m2 weekly dose of carfilzomib in combination with dexamethasone for patients with relapsed and refractory multiple myeloma (MM).
The data suggest a weekly dose of carfilzomib at 70 mg/m2 is more effective than, and just as safe as, a twice-weekly dose of carfilzomib at 27 mg/m2.
These results were recently announced by Amgen, makers of carfilzomib.
The A.R.R.O.W. trial included 478 patients with relapsed and refractory MM who had received 2 to 3 prior therapies, including bortezomib and an immunomodulatory drug.
Patients were randomized to receive once-weekly carfilzomib (20 mg/m2 on day 1 of cycle 1; 70 mg/m2 on days 8 and 15 of cycle 1; and 70 mg/m2 on days 1, 8 and 15 of subsequent cycles) with dexamethasone (40 mg) or twice-weekly carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1; 27 mg/m2 on days 8, 9, 15 and 16 of cycle 1; and 27 mg/m2 on days 1, 2, 8, 9, 15 and 16 of subsequent cycles) with dexamethasone (40 mg).
The study’s primary endpoint is progression-free survival (PFS).
Patients treated with the once-weekly regimen had significantly better PFS than patients who received carfilzomib twice weekly. The median PFS was 11.2 months and 7.6 months, respectively (hazard ratio=0.69, 95% confidence interval, 0.54–0.88).
The overall safety profile of the once-weekly regimen was comparable to that of the twice-weekly regimen.
The most frequently reported treatment-emergent adverse events (occurring in at least 20% of patients) in either treatment arm were anemia, diarrhea, fatigue, hypertension, insomnia, and pyrexia. ![]()
High symptom burden in advanced cancer patients
New research indicates that hospitalized patients with advanced cancer have a high burden of physical and psychological symptoms, and this burden is linked to longer hospital stays and a greater risk for unplanned hospital readmissions and death.
Researchers said these findings highlight the need to develop and test interventions to lessen patients’ symptoms.
Ryan Nipp, MD, of Massachusetts General Hospital in Boston, and his colleagues reported the findings in Cancer.
The researchers noted that patients with advanced cancer often experience frequent and prolonged hospitalizations for reasons that have not been fully explored.
To investigate, the team collected information from 1036 patients with advanced cancer as they were being admitted for an unplanned hospitalization.
The Edmonton Symptom Assessment System (ESAS) was used to assess patients’ physical symptoms, and the Patient Health Questionnaire 4 (PHQ-4) was used to assess their psychological symptoms.
The researchers examined the relationship between patients’ symptom burden and the duration of their hospital stay, risk of readmission, and death.
Many patients reported moderate or severe fatigue (86.7%), poor well-being (74.2%), drowsiness (71.7%), pain (67.7%), and lack of appetite (67.3%). Nearly 30% of patients had clinically significant symptoms of depression (28.8%) and anxiety (28.0%).
The patients’ mean hospital stay was 6.3 days, the readmission rate within 90 days of discharge was 43.1%, and the 90-day mortality rate was 41.6%.
Physical symptoms (P<0.001), total ESAS score (P<0.001), total PHQ-4 score (P=0.040), and depression symptoms (P=0.017) were significantly associated with longer hospital stays, but anxiety symptoms were not (P=0.190).
Physical symptoms (P<0.001), total ESAS score (P<0.001), total PHQ-4 score (P=0.072), and anxiety symptoms (P=0.045) were significantly associated with a higher likelihood of readmission within 90 days, but depression symptoms were not (P=0.219).
Physical symptoms (P<0.001), total ESAS score (P<0.001), total PHQ-4 score (P<0.001), depression symptoms (P<0.001), and anxiety symptoms (P=0.012) were all significantly associated with a higher likelihood of death or readmission within 90 days.
“We demonstrated that many hospitalized patients with advanced cancer experience an immense physical and psychological symptom burden,” Dr Nipp said.
“Interventions to identify and treat symptomatic patients hold great potential for improving patients’ experience with their illness, enhancing their quality of life, and reducing their healthcare utilization.” ![]()
New research indicates that hospitalized patients with advanced cancer have a high burden of physical and psychological symptoms, and this burden is linked to longer hospital stays and a greater risk for unplanned hospital readmissions and death.
Researchers said these findings highlight the need to develop and test interventions to lessen patients’ symptoms.
Ryan Nipp, MD, of Massachusetts General Hospital in Boston, and his colleagues reported the findings in Cancer.
The researchers noted that patients with advanced cancer often experience frequent and prolonged hospitalizations for reasons that have not been fully explored.
To investigate, the team collected information from 1036 patients with advanced cancer as they were being admitted for an unplanned hospitalization.
The Edmonton Symptom Assessment System (ESAS) was used to assess patients’ physical symptoms, and the Patient Health Questionnaire 4 (PHQ-4) was used to assess their psychological symptoms.
The researchers examined the relationship between patients’ symptom burden and the duration of their hospital stay, risk of readmission, and death.
Many patients reported moderate or severe fatigue (86.7%), poor well-being (74.2%), drowsiness (71.7%), pain (67.7%), and lack of appetite (67.3%). Nearly 30% of patients had clinically significant symptoms of depression (28.8%) and anxiety (28.0%).
The patients’ mean hospital stay was 6.3 days, the readmission rate within 90 days of discharge was 43.1%, and the 90-day mortality rate was 41.6%.
Physical symptoms (P<0.001), total ESAS score (P<0.001), total PHQ-4 score (P=0.040), and depression symptoms (P=0.017) were significantly associated with longer hospital stays, but anxiety symptoms were not (P=0.190).
Physical symptoms (P<0.001), total ESAS score (P<0.001), total PHQ-4 score (P=0.072), and anxiety symptoms (P=0.045) were significantly associated with a higher likelihood of readmission within 90 days, but depression symptoms were not (P=0.219).
Physical symptoms (P<0.001), total ESAS score (P<0.001), total PHQ-4 score (P<0.001), depression symptoms (P<0.001), and anxiety symptoms (P=0.012) were all significantly associated with a higher likelihood of death or readmission within 90 days.
“We demonstrated that many hospitalized patients with advanced cancer experience an immense physical and psychological symptom burden,” Dr Nipp said.
“Interventions to identify and treat symptomatic patients hold great potential for improving patients’ experience with their illness, enhancing their quality of life, and reducing their healthcare utilization.” ![]()
New research indicates that hospitalized patients with advanced cancer have a high burden of physical and psychological symptoms, and this burden is linked to longer hospital stays and a greater risk for unplanned hospital readmissions and death.
Researchers said these findings highlight the need to develop and test interventions to lessen patients’ symptoms.
Ryan Nipp, MD, of Massachusetts General Hospital in Boston, and his colleagues reported the findings in Cancer.
The researchers noted that patients with advanced cancer often experience frequent and prolonged hospitalizations for reasons that have not been fully explored.
To investigate, the team collected information from 1036 patients with advanced cancer as they were being admitted for an unplanned hospitalization.
The Edmonton Symptom Assessment System (ESAS) was used to assess patients’ physical symptoms, and the Patient Health Questionnaire 4 (PHQ-4) was used to assess their psychological symptoms.
The researchers examined the relationship between patients’ symptom burden and the duration of their hospital stay, risk of readmission, and death.
Many patients reported moderate or severe fatigue (86.7%), poor well-being (74.2%), drowsiness (71.7%), pain (67.7%), and lack of appetite (67.3%). Nearly 30% of patients had clinically significant symptoms of depression (28.8%) and anxiety (28.0%).
The patients’ mean hospital stay was 6.3 days, the readmission rate within 90 days of discharge was 43.1%, and the 90-day mortality rate was 41.6%.
Physical symptoms (P<0.001), total ESAS score (P<0.001), total PHQ-4 score (P=0.040), and depression symptoms (P=0.017) were significantly associated with longer hospital stays, but anxiety symptoms were not (P=0.190).
Physical symptoms (P<0.001), total ESAS score (P<0.001), total PHQ-4 score (P=0.072), and anxiety symptoms (P=0.045) were significantly associated with a higher likelihood of readmission within 90 days, but depression symptoms were not (P=0.219).
Physical symptoms (P<0.001), total ESAS score (P<0.001), total PHQ-4 score (P<0.001), depression symptoms (P<0.001), and anxiety symptoms (P=0.012) were all significantly associated with a higher likelihood of death or readmission within 90 days.
“We demonstrated that many hospitalized patients with advanced cancer experience an immense physical and psychological symptom burden,” Dr Nipp said.
“Interventions to identify and treat symptomatic patients hold great potential for improving patients’ experience with their illness, enhancing their quality of life, and reducing their healthcare utilization.” ![]()
Cryotherapy can reduce signs of CIPN
A new study suggests cryotherapy can reduce symptoms of chemotherapy-induced peripheral neuropathy (CIPN).
Researchers found that having chemotherapy patients wear frozen gloves and socks for 90-minute periods significantly reduced the incidence of CIPN symptoms.
Hiroshi Ishiguro, MD, PhD, of International University of Health and Welfare Hospital in Tochigi, Japan, and colleagues reported these findings in the Journal of the National Cancer Institute.
The researchers prospectively evaluated the efficacy of cryotherapy for preventing CIPN. Breast cancer patients treated weekly with paclitaxel (80 mg/m2 for 1 hour) wore frozen gloves and socks on one side of their bodies for 90 minutes, including the entire duration of drug infusion.
The researchers then compared symptoms on the treated sides with those on the untreated sides.
The primary endpoint was CIPN incidence assessed by changes in tactile sensitivity from a pretreatment baseline. The researchers also assessed subjective symptoms, as reported in the Patient Neuropathy Questionnaire, and patients' manual dexterity.
Among the 40 patients studied, 4 did not reach the cumulative dose due to the occurrence of pneumonia, severe fatigue, liver dysfunction, and macular edema. Of the 36 remaining patients, none dropped out due to cold intolerance.
The incidence of objective and subjective signs of CIPN was clinically and statistically significantly lower on the intervention side than on the control side for most measurements, which includes (among other measures):
- Hand tactile sensitivity—27.8% and 80.6%, respectively (odds ratio[OR]= 20.00, P<0.001)
- Foot tactile sensitivity—25.0% and 63.9%, respectively (OR=infinite, P<0.001)
- Hand warm sense—8.8% and 32.4%, respectively (OR=9.00, P=0.02)
- Foot warm sense—33.4% and 57.6%, respectively (OR=5.00, P=0.04)
- Hand cold sense—2.8% and 13.9%, respectively (OR=infinite, P=0.13)
- Foot cold sense—12.6% and 18.8%, respectively (OR=2.00, P=0.69)
- Severe CIPN in the hand according to the Patient Neuropathy Questionnaire—2.8% and 41.7%, respectively (OR=infinite, P<0.001)
- Severe CIPN in the foot according to the Patient Neuropathy Questionnaire—2.8% and 36.1%, respectively (OR=infinite, P<0.001).

A new study suggests cryotherapy can reduce symptoms of chemotherapy-induced peripheral neuropathy (CIPN).
Researchers found that having chemotherapy patients wear frozen gloves and socks for 90-minute periods significantly reduced the incidence of CIPN symptoms.
Hiroshi Ishiguro, MD, PhD, of International University of Health and Welfare Hospital in Tochigi, Japan, and colleagues reported these findings in the Journal of the National Cancer Institute.
The researchers prospectively evaluated the efficacy of cryotherapy for preventing CIPN. Breast cancer patients treated weekly with paclitaxel (80 mg/m2 for 1 hour) wore frozen gloves and socks on one side of their bodies for 90 minutes, including the entire duration of drug infusion.
The researchers then compared symptoms on the treated sides with those on the untreated sides.
The primary endpoint was CIPN incidence assessed by changes in tactile sensitivity from a pretreatment baseline. The researchers also assessed subjective symptoms, as reported in the Patient Neuropathy Questionnaire, and patients' manual dexterity.
Among the 40 patients studied, 4 did not reach the cumulative dose due to the occurrence of pneumonia, severe fatigue, liver dysfunction, and macular edema. Of the 36 remaining patients, none dropped out due to cold intolerance.
The incidence of objective and subjective signs of CIPN was clinically and statistically significantly lower on the intervention side than on the control side for most measurements, which includes (among other measures):
- Hand tactile sensitivity—27.8% and 80.6%, respectively (odds ratio[OR]= 20.00, P<0.001)
- Foot tactile sensitivity—25.0% and 63.9%, respectively (OR=infinite, P<0.001)
- Hand warm sense—8.8% and 32.4%, respectively (OR=9.00, P=0.02)
- Foot warm sense—33.4% and 57.6%, respectively (OR=5.00, P=0.04)
- Hand cold sense—2.8% and 13.9%, respectively (OR=infinite, P=0.13)
- Foot cold sense—12.6% and 18.8%, respectively (OR=2.00, P=0.69)
- Severe CIPN in the hand according to the Patient Neuropathy Questionnaire—2.8% and 41.7%, respectively (OR=infinite, P<0.001)
- Severe CIPN in the foot according to the Patient Neuropathy Questionnaire—2.8% and 36.1%, respectively (OR=infinite, P<0.001).

A new study suggests cryotherapy can reduce symptoms of chemotherapy-induced peripheral neuropathy (CIPN).
Researchers found that having chemotherapy patients wear frozen gloves and socks for 90-minute periods significantly reduced the incidence of CIPN symptoms.
Hiroshi Ishiguro, MD, PhD, of International University of Health and Welfare Hospital in Tochigi, Japan, and colleagues reported these findings in the Journal of the National Cancer Institute.
The researchers prospectively evaluated the efficacy of cryotherapy for preventing CIPN. Breast cancer patients treated weekly with paclitaxel (80 mg/m2 for 1 hour) wore frozen gloves and socks on one side of their bodies for 90 minutes, including the entire duration of drug infusion.
The researchers then compared symptoms on the treated sides with those on the untreated sides.
The primary endpoint was CIPN incidence assessed by changes in tactile sensitivity from a pretreatment baseline. The researchers also assessed subjective symptoms, as reported in the Patient Neuropathy Questionnaire, and patients' manual dexterity.
Among the 40 patients studied, 4 did not reach the cumulative dose due to the occurrence of pneumonia, severe fatigue, liver dysfunction, and macular edema. Of the 36 remaining patients, none dropped out due to cold intolerance.
The incidence of objective and subjective signs of CIPN was clinically and statistically significantly lower on the intervention side than on the control side for most measurements, which includes (among other measures):
- Hand tactile sensitivity—27.8% and 80.6%, respectively (odds ratio[OR]= 20.00, P<0.001)
- Foot tactile sensitivity—25.0% and 63.9%, respectively (OR=infinite, P<0.001)
- Hand warm sense—8.8% and 32.4%, respectively (OR=9.00, P=0.02)
- Foot warm sense—33.4% and 57.6%, respectively (OR=5.00, P=0.04)
- Hand cold sense—2.8% and 13.9%, respectively (OR=infinite, P=0.13)
- Foot cold sense—12.6% and 18.8%, respectively (OR=2.00, P=0.69)
- Severe CIPN in the hand according to the Patient Neuropathy Questionnaire—2.8% and 41.7%, respectively (OR=infinite, P<0.001)
- Severe CIPN in the foot according to the Patient Neuropathy Questionnaire—2.8% and 36.1%, respectively (OR=infinite, P<0.001).

Natural selection opportunities tied to cancer rates
Countries with the lowest opportunities for natural selection have higher cancer rates than countries with the highest opportunities for natural selection, according to a study published in Evolutionary Applications.
Researchers said this is because modern medicine is enabling people to survive cancers, and their genetic backgrounds are passing from one generation to the next.
The team said the rate of some cancers has doubled and even quadrupled over the past 100 to 150 years, and human evolution has moved away from “survival of the fittest.”
“Modern medicine has enabled the human species to live much longer than would otherwise be expected in the natural world,” said study author Maciej Henneberg, PhD, DSc, of the University of Adelaide in South Australia.
“Besides the obvious benefits that modern medicine gives, it also brings with it an unexpected side-effect—allowing genetic material to be passed from one generation to the next that predisposes people to have poor health, such as type 1 diabetes or cancer.”
“Because of the quality of our healthcare in western society, we have almost removed natural selection as the ‘janitor of the gene pool.’ Unfortunately, the accumulation of genetic mutations over time and across multiple generations is like a delayed death sentence.”
Country comparison
The researchers studied global cancer data from the World Health Organization as well as other health and socioeconomic data from the United Nations and the World Bank of 173 countries. The team compared the top 10 countries with the highest opportunities for natural selection to the 10 countries with the lowest opportunities for natural selection.
“We looked at countries that offered the greatest opportunity to survive cancer compared with those that didn’t,” said study author Wenpeng You, a PhD student at the University of Adelaide. “This does not only take into account factors such as socioeconomic status, urbanization, and quality of medical services but also low mortality and fertility rates, which are the 2 distinguishing features in the ‘better’ world.”
“Countries with low mortality rates may allow more people with cancer genetic background to reproduce and pass cancer genes/mutations to the next generation. Meanwhile, low fertility rates in these countries may not be able to have diverse biological variations to provide the opportunity for selecting a naturally fit population—for example, people without or with less cancer genetic background. Low mortality rate and low fertility rate in the ‘better’ world may have formed a self-reinforcing cycle which has accumulated cancer genetic background at a greater rate than previously thought.”
Based on the researchers’ analysis, the 20 countries are:
| Lowest opportunities for natural selection | Highest opportunities for natural selection |
| Iceland | Burkina Faso |
| Singapore | Chad |
| Japan | Central African Republic |
| Switzerland | Afghanistan |
| Sweden | Somalia |
| Luxembourg | Sierra Leone |
| Germany | Democratic Republic of the Congo |
| Italy | Guinea-Bissau |
| Cyprus | Burundi |
| Andorra | Cameroon |
Cancer incidence
The researchers found the rates of most cancers were higher in the 10 countries with the lowest opportunities for natural selection. The incidence of all cancers was 2.326 times higher in the low-opportunity countries than the high-opportunity ones.
The increased incidences of hematologic malignancies were as follows:
- Non-Hodgkin lymphoma—2.019 times higher in the low-opportunity countries
- Hodgkin lymphoma—3.314 times higher in the low-opportunity countries
- Leukemia—3.574 times higher in the low-opportunity countries
- Multiple myeloma—4.257 times higher in the low-opportunity countries .
Dr Henneberg said that, having removed natural selection as the “janitor of the gene pool,” our modern society is faced with a controversial issue.
“It may be that the only way humankind can be rid of cancer once and for all is through genetic engineering—to repair our genes and take cancer out of the equation,” he said. ![]()
Countries with the lowest opportunities for natural selection have higher cancer rates than countries with the highest opportunities for natural selection, according to a study published in Evolutionary Applications.
Researchers said this is because modern medicine is enabling people to survive cancers, and their genetic backgrounds are passing from one generation to the next.
The team said the rate of some cancers has doubled and even quadrupled over the past 100 to 150 years, and human evolution has moved away from “survival of the fittest.”
“Modern medicine has enabled the human species to live much longer than would otherwise be expected in the natural world,” said study author Maciej Henneberg, PhD, DSc, of the University of Adelaide in South Australia.
“Besides the obvious benefits that modern medicine gives, it also brings with it an unexpected side-effect—allowing genetic material to be passed from one generation to the next that predisposes people to have poor health, such as type 1 diabetes or cancer.”
“Because of the quality of our healthcare in western society, we have almost removed natural selection as the ‘janitor of the gene pool.’ Unfortunately, the accumulation of genetic mutations over time and across multiple generations is like a delayed death sentence.”
Country comparison
The researchers studied global cancer data from the World Health Organization as well as other health and socioeconomic data from the United Nations and the World Bank of 173 countries. The team compared the top 10 countries with the highest opportunities for natural selection to the 10 countries with the lowest opportunities for natural selection.
“We looked at countries that offered the greatest opportunity to survive cancer compared with those that didn’t,” said study author Wenpeng You, a PhD student at the University of Adelaide. “This does not only take into account factors such as socioeconomic status, urbanization, and quality of medical services but also low mortality and fertility rates, which are the 2 distinguishing features in the ‘better’ world.”
“Countries with low mortality rates may allow more people with cancer genetic background to reproduce and pass cancer genes/mutations to the next generation. Meanwhile, low fertility rates in these countries may not be able to have diverse biological variations to provide the opportunity for selecting a naturally fit population—for example, people without or with less cancer genetic background. Low mortality rate and low fertility rate in the ‘better’ world may have formed a self-reinforcing cycle which has accumulated cancer genetic background at a greater rate than previously thought.”
Based on the researchers’ analysis, the 20 countries are:
| Lowest opportunities for natural selection | Highest opportunities for natural selection |
| Iceland | Burkina Faso |
| Singapore | Chad |
| Japan | Central African Republic |
| Switzerland | Afghanistan |
| Sweden | Somalia |
| Luxembourg | Sierra Leone |
| Germany | Democratic Republic of the Congo |
| Italy | Guinea-Bissau |
| Cyprus | Burundi |
| Andorra | Cameroon |
Cancer incidence
The researchers found the rates of most cancers were higher in the 10 countries with the lowest opportunities for natural selection. The incidence of all cancers was 2.326 times higher in the low-opportunity countries than the high-opportunity ones.
The increased incidences of hematologic malignancies were as follows:
- Non-Hodgkin lymphoma—2.019 times higher in the low-opportunity countries
- Hodgkin lymphoma—3.314 times higher in the low-opportunity countries
- Leukemia—3.574 times higher in the low-opportunity countries
- Multiple myeloma—4.257 times higher in the low-opportunity countries .
Dr Henneberg said that, having removed natural selection as the “janitor of the gene pool,” our modern society is faced with a controversial issue.
“It may be that the only way humankind can be rid of cancer once and for all is through genetic engineering—to repair our genes and take cancer out of the equation,” he said. ![]()
Countries with the lowest opportunities for natural selection have higher cancer rates than countries with the highest opportunities for natural selection, according to a study published in Evolutionary Applications.
Researchers said this is because modern medicine is enabling people to survive cancers, and their genetic backgrounds are passing from one generation to the next.
The team said the rate of some cancers has doubled and even quadrupled over the past 100 to 150 years, and human evolution has moved away from “survival of the fittest.”
“Modern medicine has enabled the human species to live much longer than would otherwise be expected in the natural world,” said study author Maciej Henneberg, PhD, DSc, of the University of Adelaide in South Australia.
“Besides the obvious benefits that modern medicine gives, it also brings with it an unexpected side-effect—allowing genetic material to be passed from one generation to the next that predisposes people to have poor health, such as type 1 diabetes or cancer.”
“Because of the quality of our healthcare in western society, we have almost removed natural selection as the ‘janitor of the gene pool.’ Unfortunately, the accumulation of genetic mutations over time and across multiple generations is like a delayed death sentence.”
Country comparison
The researchers studied global cancer data from the World Health Organization as well as other health and socioeconomic data from the United Nations and the World Bank of 173 countries. The team compared the top 10 countries with the highest opportunities for natural selection to the 10 countries with the lowest opportunities for natural selection.
“We looked at countries that offered the greatest opportunity to survive cancer compared with those that didn’t,” said study author Wenpeng You, a PhD student at the University of Adelaide. “This does not only take into account factors such as socioeconomic status, urbanization, and quality of medical services but also low mortality and fertility rates, which are the 2 distinguishing features in the ‘better’ world.”
“Countries with low mortality rates may allow more people with cancer genetic background to reproduce and pass cancer genes/mutations to the next generation. Meanwhile, low fertility rates in these countries may not be able to have diverse biological variations to provide the opportunity for selecting a naturally fit population—for example, people without or with less cancer genetic background. Low mortality rate and low fertility rate in the ‘better’ world may have formed a self-reinforcing cycle which has accumulated cancer genetic background at a greater rate than previously thought.”
Based on the researchers’ analysis, the 20 countries are:
| Lowest opportunities for natural selection | Highest opportunities for natural selection |
| Iceland | Burkina Faso |
| Singapore | Chad |
| Japan | Central African Republic |
| Switzerland | Afghanistan |
| Sweden | Somalia |
| Luxembourg | Sierra Leone |
| Germany | Democratic Republic of the Congo |
| Italy | Guinea-Bissau |
| Cyprus | Burundi |
| Andorra | Cameroon |
Cancer incidence
The researchers found the rates of most cancers were higher in the 10 countries with the lowest opportunities for natural selection. The incidence of all cancers was 2.326 times higher in the low-opportunity countries than the high-opportunity ones.
The increased incidences of hematologic malignancies were as follows:
- Non-Hodgkin lymphoma—2.019 times higher in the low-opportunity countries
- Hodgkin lymphoma—3.314 times higher in the low-opportunity countries
- Leukemia—3.574 times higher in the low-opportunity countries
- Multiple myeloma—4.257 times higher in the low-opportunity countries .
Dr Henneberg said that, having removed natural selection as the “janitor of the gene pool,” our modern society is faced with a controversial issue.
“It may be that the only way humankind can be rid of cancer once and for all is through genetic engineering—to repair our genes and take cancer out of the equation,” he said. ![]()
NCCN completes resource on radiation therapy
The National Comprehensive Cancer Network® (NCCN) has announced the release of the newly completed NCCN Radiation Therapy Compendium™.
This resource includes information designed to support clinical decision-making regarding the use of radiation therapy in cancer patients.
The content is based on the NCCN Clinical Practice Guidelines in Oncology and includes information from the 41 guidelines that reference radiation therapy.
“By compiling every recommendation for radiation therapy in one place, we’ve made it significantly easier for specialists . . . to stay up-to-date on the very latest recommendations, regardless of how many different cancer types they treat,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“This targeted content provides radiation oncologists with the specific, cutting-edge information they need, without forcing them to sift through any extraneous information. It’s part of our ongoing effort to always provide the most pertinent data on emerging treatment practices in the clearest, most efficient way possible.”
The NCCN Radiation Therapy Compendium includes a full complement of radiation therapy recommendations found in the current NCCN guidelines, including specific treatment modalities such as 2D/3D conformal external beam radiation therapy, intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative body radiotherapy, image-guided radiation therapy, low dose-rate/high dose-rate brachytherapy, radioisotope, and particle therapy.
NCCN first announced the launch of the Radiation Therapy Compendium in March at the NCCN Annual Conference: Improving the Quality, Effectiveness, and Efficiency of Cancer Care.
At the time, the NCCN released a preliminary version of the compendium featuring 24 cancer types. The newly completed version now contains all 41 disease sites that are currently being treated using radiation therapy.
The compendium will be updated on a continual basis in conjunction with the library of clinical guidelines.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. The compendium is available free-of-charge through March 2018. ![]()
The National Comprehensive Cancer Network® (NCCN) has announced the release of the newly completed NCCN Radiation Therapy Compendium™.
This resource includes information designed to support clinical decision-making regarding the use of radiation therapy in cancer patients.
The content is based on the NCCN Clinical Practice Guidelines in Oncology and includes information from the 41 guidelines that reference radiation therapy.
“By compiling every recommendation for radiation therapy in one place, we’ve made it significantly easier for specialists . . . to stay up-to-date on the very latest recommendations, regardless of how many different cancer types they treat,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“This targeted content provides radiation oncologists with the specific, cutting-edge information they need, without forcing them to sift through any extraneous information. It’s part of our ongoing effort to always provide the most pertinent data on emerging treatment practices in the clearest, most efficient way possible.”
The NCCN Radiation Therapy Compendium includes a full complement of radiation therapy recommendations found in the current NCCN guidelines, including specific treatment modalities such as 2D/3D conformal external beam radiation therapy, intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative body radiotherapy, image-guided radiation therapy, low dose-rate/high dose-rate brachytherapy, radioisotope, and particle therapy.
NCCN first announced the launch of the Radiation Therapy Compendium in March at the NCCN Annual Conference: Improving the Quality, Effectiveness, and Efficiency of Cancer Care.
At the time, the NCCN released a preliminary version of the compendium featuring 24 cancer types. The newly completed version now contains all 41 disease sites that are currently being treated using radiation therapy.
The compendium will be updated on a continual basis in conjunction with the library of clinical guidelines.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. The compendium is available free-of-charge through March 2018. ![]()
The National Comprehensive Cancer Network® (NCCN) has announced the release of the newly completed NCCN Radiation Therapy Compendium™.
This resource includes information designed to support clinical decision-making regarding the use of radiation therapy in cancer patients.
The content is based on the NCCN Clinical Practice Guidelines in Oncology and includes information from the 41 guidelines that reference radiation therapy.
“By compiling every recommendation for radiation therapy in one place, we’ve made it significantly easier for specialists . . . to stay up-to-date on the very latest recommendations, regardless of how many different cancer types they treat,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“This targeted content provides radiation oncologists with the specific, cutting-edge information they need, without forcing them to sift through any extraneous information. It’s part of our ongoing effort to always provide the most pertinent data on emerging treatment practices in the clearest, most efficient way possible.”
The NCCN Radiation Therapy Compendium includes a full complement of radiation therapy recommendations found in the current NCCN guidelines, including specific treatment modalities such as 2D/3D conformal external beam radiation therapy, intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative body radiotherapy, image-guided radiation therapy, low dose-rate/high dose-rate brachytherapy, radioisotope, and particle therapy.
NCCN first announced the launch of the Radiation Therapy Compendium in March at the NCCN Annual Conference: Improving the Quality, Effectiveness, and Efficiency of Cancer Care.
At the time, the NCCN released a preliminary version of the compendium featuring 24 cancer types. The newly completed version now contains all 41 disease sites that are currently being treated using radiation therapy.
The compendium will be updated on a continual basis in conjunction with the library of clinical guidelines.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. The compendium is available free-of-charge through March 2018.
Multiple myeloma patients with t(11;14) respond best to venetoclax monotherapy
Phase 1 data on venetoclax monotherapy for relapsed/refractory multiple myeloma, initially presented at the 2016 annual meeting of the American Society of Hematology, have been published in Blood.
Of 66 patients enrolled in the study (NCT01794520), 61% were bortezomib and lenalidomide double refractory, and 46% had t(11;14), said Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minn, and his colleagues.
The overall response rate was 21% (14/66), and 15% achieved very good or better partial responses; 12 of the 14 responses occurred in patients with t(11;14).
(Click here to read the initial report and to view a video of Dr. Kumar discussing the study results at ASH 2016.)
Venetoclax is a selective, orally bioavailable BCL-2 inhibitor that is particularly effective against MM cells harboring t(11;14). Biomarker analysis confirmed that response to venetoclax correlated with higher BCL2:BCL2L1 and BCL2:MCL1 mRNA expression ratios.
The study is sponsored by Abbvie, and Dr. Kumar receives research support from Abbvie.
Phase 1 data on venetoclax monotherapy for relapsed/refractory multiple myeloma, initially presented at the 2016 annual meeting of the American Society of Hematology, have been published in Blood.
Of 66 patients enrolled in the study (NCT01794520), 61% were bortezomib and lenalidomide double refractory, and 46% had t(11;14), said Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minn, and his colleagues.
The overall response rate was 21% (14/66), and 15% achieved very good or better partial responses; 12 of the 14 responses occurred in patients with t(11;14).
(Click here to read the initial report and to view a video of Dr. Kumar discussing the study results at ASH 2016.)
Venetoclax is a selective, orally bioavailable BCL-2 inhibitor that is particularly effective against MM cells harboring t(11;14). Biomarker analysis confirmed that response to venetoclax correlated with higher BCL2:BCL2L1 and BCL2:MCL1 mRNA expression ratios.
The study is sponsored by Abbvie, and Dr. Kumar receives research support from Abbvie.
Phase 1 data on venetoclax monotherapy for relapsed/refractory multiple myeloma, initially presented at the 2016 annual meeting of the American Society of Hematology, have been published in Blood.
Of 66 patients enrolled in the study (NCT01794520), 61% were bortezomib and lenalidomide double refractory, and 46% had t(11;14), said Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minn, and his colleagues.
The overall response rate was 21% (14/66), and 15% achieved very good or better partial responses; 12 of the 14 responses occurred in patients with t(11;14).
(Click here to read the initial report and to view a video of Dr. Kumar discussing the study results at ASH 2016.)
Venetoclax is a selective, orally bioavailable BCL-2 inhibitor that is particularly effective against MM cells harboring t(11;14). Biomarker analysis confirmed that response to venetoclax correlated with higher BCL2:BCL2L1 and BCL2:MCL1 mRNA expression ratios.
The study is sponsored by Abbvie, and Dr. Kumar receives research support from Abbvie.
from blood
Tailored approaches to relapsed/recalcitrant myeloma found within NCCN guidelines
SAN FRANCISCO – The approach to relapsed/refractory multiple myeloma (MM) has to be tailored patient by patient based on the biology of the disease, frailty measures, comorbidities, and prior treatment regimens, Natalie Callander, MD, director of the myeloma clinical program at the University of Wisconsin Carbone Cancer Center, Madison, said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
Despite major treatment breakthroughs in recent years and a wide swath of therapeutic options, multiple myeloma is still an incurable disease that eventually relapses in most patients, Dr. Callander, vice chair of the NCCN Multiple Myeloma Panel, said. “If you are having trouble with your relapsed or refractory patients, you’re not alone.”
As a guide to individualize the treatment approach, the NCCN has put together recommendations for patients with relapsed/recalcitrant MM. “Preferred regimens” include bortezomib-lenalidomide-dexamethasone and other well-established combinations supported by evidence from Phase 1 trials. When relapse occurs more than 6 months after primary induction, one option is simply to repeat the induction therapy.
A long list of “other recommended regimens” includes 21 combination options supported by “very strong Phase 2 data,” she said. The NCCN also has a third list of regimens for the most aggressive disease and certain special circumstances. Two powerful combinations that are used to get the disease under control quickly include high-dose cyclophosphamide or dexamethasone/thalidomide/cisplatin/doxorubicin/cyclophosphamide/etoposide, (DT-PACE) with or without bortezomib.
Dr. Callander underscored the importance of differentiating biochemical relapse, such as increases in serum M protein or other indicators, from clinical relapse, such as new soft tissue plasmacytomas or hypercalcemia.
Patients with clinical relapse typically need a quicker and more vigorous therapeutic response, she said. Studies have found that those in biochemical relapse tend to do better than those in symptomatic relapse.
“This whole concept of biochemical versus symptomatic is really important,” Dr. Callander said.
Among the tips she offered for managing patients with relapse, were to perform a repeat bone marrow biopsy with cytogenetics to gauge the presence of high-risk biology, since this category of patients tends to do far worse and to need a more aggressive treatment approach.
“Getting that kind of information with that follow-up bone marrow biopsy might be very important,” she said.
It’s important to be aware of the phenomenon of “light chain escape,” in which patients with relapsed/refractory disease can stop making intact immunoglobulin G. It’s thought to be due to clonal evolution, in which the clone that was making intact immunoglobulin becomes less dominant. These patients tend to do worse than similar patients who don’t exhibit this phenomenon, Dr. Callander said.
Dr. Callander reported no relevant financial disclosures.
SAN FRANCISCO – The approach to relapsed/refractory multiple myeloma (MM) has to be tailored patient by patient based on the biology of the disease, frailty measures, comorbidities, and prior treatment regimens, Natalie Callander, MD, director of the myeloma clinical program at the University of Wisconsin Carbone Cancer Center, Madison, said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
Despite major treatment breakthroughs in recent years and a wide swath of therapeutic options, multiple myeloma is still an incurable disease that eventually relapses in most patients, Dr. Callander, vice chair of the NCCN Multiple Myeloma Panel, said. “If you are having trouble with your relapsed or refractory patients, you’re not alone.”
As a guide to individualize the treatment approach, the NCCN has put together recommendations for patients with relapsed/recalcitrant MM. “Preferred regimens” include bortezomib-lenalidomide-dexamethasone and other well-established combinations supported by evidence from Phase 1 trials. When relapse occurs more than 6 months after primary induction, one option is simply to repeat the induction therapy.
A long list of “other recommended regimens” includes 21 combination options supported by “very strong Phase 2 data,” she said. The NCCN also has a third list of regimens for the most aggressive disease and certain special circumstances. Two powerful combinations that are used to get the disease under control quickly include high-dose cyclophosphamide or dexamethasone/thalidomide/cisplatin/doxorubicin/cyclophosphamide/etoposide, (DT-PACE) with or without bortezomib.
Dr. Callander underscored the importance of differentiating biochemical relapse, such as increases in serum M protein or other indicators, from clinical relapse, such as new soft tissue plasmacytomas or hypercalcemia.
Patients with clinical relapse typically need a quicker and more vigorous therapeutic response, she said. Studies have found that those in biochemical relapse tend to do better than those in symptomatic relapse.
“This whole concept of biochemical versus symptomatic is really important,” Dr. Callander said.
Among the tips she offered for managing patients with relapse, were to perform a repeat bone marrow biopsy with cytogenetics to gauge the presence of high-risk biology, since this category of patients tends to do far worse and to need a more aggressive treatment approach.
“Getting that kind of information with that follow-up bone marrow biopsy might be very important,” she said.
It’s important to be aware of the phenomenon of “light chain escape,” in which patients with relapsed/refractory disease can stop making intact immunoglobulin G. It’s thought to be due to clonal evolution, in which the clone that was making intact immunoglobulin becomes less dominant. These patients tend to do worse than similar patients who don’t exhibit this phenomenon, Dr. Callander said.
Dr. Callander reported no relevant financial disclosures.
SAN FRANCISCO – The approach to relapsed/refractory multiple myeloma (MM) has to be tailored patient by patient based on the biology of the disease, frailty measures, comorbidities, and prior treatment regimens, Natalie Callander, MD, director of the myeloma clinical program at the University of Wisconsin Carbone Cancer Center, Madison, said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
Despite major treatment breakthroughs in recent years and a wide swath of therapeutic options, multiple myeloma is still an incurable disease that eventually relapses in most patients, Dr. Callander, vice chair of the NCCN Multiple Myeloma Panel, said. “If you are having trouble with your relapsed or refractory patients, you’re not alone.”
As a guide to individualize the treatment approach, the NCCN has put together recommendations for patients with relapsed/recalcitrant MM. “Preferred regimens” include bortezomib-lenalidomide-dexamethasone and other well-established combinations supported by evidence from Phase 1 trials. When relapse occurs more than 6 months after primary induction, one option is simply to repeat the induction therapy.
A long list of “other recommended regimens” includes 21 combination options supported by “very strong Phase 2 data,” she said. The NCCN also has a third list of regimens for the most aggressive disease and certain special circumstances. Two powerful combinations that are used to get the disease under control quickly include high-dose cyclophosphamide or dexamethasone/thalidomide/cisplatin/doxorubicin/cyclophosphamide/etoposide, (DT-PACE) with or without bortezomib.
Dr. Callander underscored the importance of differentiating biochemical relapse, such as increases in serum M protein or other indicators, from clinical relapse, such as new soft tissue plasmacytomas or hypercalcemia.
Patients with clinical relapse typically need a quicker and more vigorous therapeutic response, she said. Studies have found that those in biochemical relapse tend to do better than those in symptomatic relapse.
“This whole concept of biochemical versus symptomatic is really important,” Dr. Callander said.
Among the tips she offered for managing patients with relapse, were to perform a repeat bone marrow biopsy with cytogenetics to gauge the presence of high-risk biology, since this category of patients tends to do far worse and to need a more aggressive treatment approach.
“Getting that kind of information with that follow-up bone marrow biopsy might be very important,” she said.
It’s important to be aware of the phenomenon of “light chain escape,” in which patients with relapsed/refractory disease can stop making intact immunoglobulin G. It’s thought to be due to clonal evolution, in which the clone that was making intact immunoglobulin becomes less dominant. These patients tend to do worse than similar patients who don’t exhibit this phenomenon, Dr. Callander said.
Dr. Callander reported no relevant financial disclosures.
expert analysis AT THE NCCN Hematologic Malignancies Congress
FDA rejects pegfilgrastim biosimilar
The US Food and Drug Administration (FDA) has issued a complete response letter saying the agency cannot approve MYL-1401H, a proposed biosimilar of pegfilgrastim (Neulasta).
Biocon and Mylan are seeking approval of MYL-1401H to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving chemotherapy to treat non-myeloid malignancies.
Biocon and Mylan filed the biologics license application for MYL-1401H in February.
The FDA had planned to issue a decision on the application by October 9.
Biocon and Mylan said the FDA’s complete response letter relates to a pending update to the application. The update involves chemistry manufacturing and control data from facility requalification activities after recent plant modifications.
The complete response letter did not raise any questions on the biosimilarity of MYL-1401H, pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity. (Results of a phase 3 study presented at ESMO 2016 Congress suggested MYL-1401H is equivalent to Neulasta.)
Biocon and Mylan said they do not expect the complete response letter for MYL-1401H to impact the commercial launch timing of the drug in the US. The companies said they are committed to working with the FDA to resolve the issues outlined in the letter.
The US Food and Drug Administration (FDA) has issued a complete response letter saying the agency cannot approve MYL-1401H, a proposed biosimilar of pegfilgrastim (Neulasta).
Biocon and Mylan are seeking approval of MYL-1401H to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving chemotherapy to treat non-myeloid malignancies.
Biocon and Mylan filed the biologics license application for MYL-1401H in February.
The FDA had planned to issue a decision on the application by October 9.
Biocon and Mylan said the FDA’s complete response letter relates to a pending update to the application. The update involves chemistry manufacturing and control data from facility requalification activities after recent plant modifications.
The complete response letter did not raise any questions on the biosimilarity of MYL-1401H, pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity. (Results of a phase 3 study presented at ESMO 2016 Congress suggested MYL-1401H is equivalent to Neulasta.)
Biocon and Mylan said they do not expect the complete response letter for MYL-1401H to impact the commercial launch timing of the drug in the US. The companies said they are committed to working with the FDA to resolve the issues outlined in the letter.
The US Food and Drug Administration (FDA) has issued a complete response letter saying the agency cannot approve MYL-1401H, a proposed biosimilar of pegfilgrastim (Neulasta).
Biocon and Mylan are seeking approval of MYL-1401H to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving chemotherapy to treat non-myeloid malignancies.
Biocon and Mylan filed the biologics license application for MYL-1401H in February.
The FDA had planned to issue a decision on the application by October 9.
Biocon and Mylan said the FDA’s complete response letter relates to a pending update to the application. The update involves chemistry manufacturing and control data from facility requalification activities after recent plant modifications.
The complete response letter did not raise any questions on the biosimilarity of MYL-1401H, pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity. (Results of a phase 3 study presented at ESMO 2016 Congress suggested MYL-1401H is equivalent to Neulasta.)
Biocon and Mylan said they do not expect the complete response letter for MYL-1401H to impact the commercial launch timing of the drug in the US. The companies said they are committed to working with the FDA to resolve the issues outlined in the letter.