User login
Company withdraws MAA for pegfilgrastim biosimilar

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()
Recent price hikes for generic cancer meds exceed 100%

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()
Substantial long-term increase seen in multiple myeloma survival
The 5-year survival rate for multiple myeloma increased eightfold over an approximately 60-year span starting in the early 1950s, said Ali H. Mokdad, PhD, and his associates.
Patients with multiple myeloma had a 5-year relative survival rate of 6% in 1950-1954, compared with 49.8% in 2008-2013, according to data from the Surveillance, Epidemiology, and End Results Program (JAMA 2017;317[4]:388-406).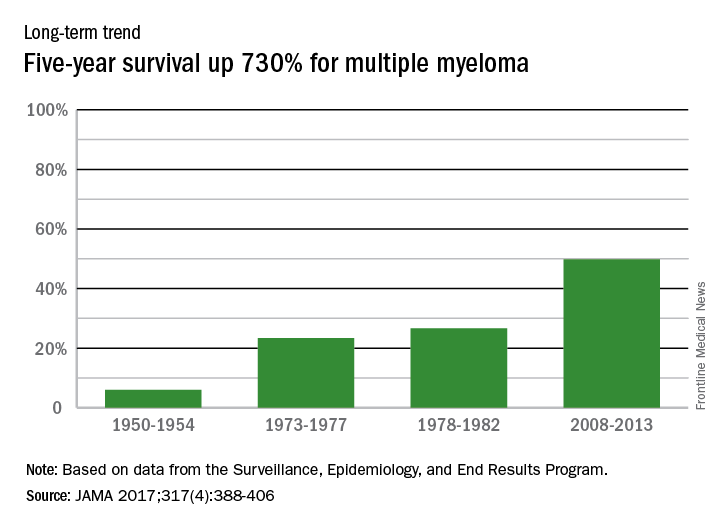
In 2014, there were about 13,000 deaths resulting from multiple myeloma, with 219,000 years of life lost, which ranked 17th among the 29 selected cancers, noted Dr. Mokdad and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
The 5-year survival rate for multiple myeloma increased eightfold over an approximately 60-year span starting in the early 1950s, said Ali H. Mokdad, PhD, and his associates.
Patients with multiple myeloma had a 5-year relative survival rate of 6% in 1950-1954, compared with 49.8% in 2008-2013, according to data from the Surveillance, Epidemiology, and End Results Program (JAMA 2017;317[4]:388-406).
In 2014, there were about 13,000 deaths resulting from multiple myeloma, with 219,000 years of life lost, which ranked 17th among the 29 selected cancers, noted Dr. Mokdad and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
The 5-year survival rate for multiple myeloma increased eightfold over an approximately 60-year span starting in the early 1950s, said Ali H. Mokdad, PhD, and his associates.
Patients with multiple myeloma had a 5-year relative survival rate of 6% in 1950-1954, compared with 49.8% in 2008-2013, according to data from the Surveillance, Epidemiology, and End Results Program (JAMA 2017;317[4]:388-406).
In 2014, there were about 13,000 deaths resulting from multiple myeloma, with 219,000 years of life lost, which ranked 17th among the 29 selected cancers, noted Dr. Mokdad and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
FROM JAMA
CHMP recommends lenalidomide maintenance

Photo courtesy of Celgene
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended a new indication for lenalidomide (Revlimid®).
The CHMP advised the European Commission (EC) to approve the use of lenalidomide as maintenance therapy in adults who had newly diagnosed multiple myeloma (MM) prior to receiving an autologous stem cell transplant (ASCT).
If approved by the EC, lenalidomide will be the first licensed maintenance treatment available to this patient population in the European Union.
The EC, which generally follows the CHMP’s recommendations, is expected to make its final decision on this use of lenalidomide in approximately 2 months.
If approval is granted, detailed conditions for the use of lenalidomide will be described in the Summary of Product Characteristics, which will be published in the revised European Public Assessment Report.
Lenalidomide is a product of Celgene.
The CHMP’s recommendation to approve lenalidomide as maintenance in MM was based on the results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211. Results from both studies were published in NEJM in May 2012.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing ASCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen post-ASCT of lenalidomide monotherapy, followed by continuous daily treatment with lenalidomide or placebo until relapse.
“Studies show that maintenance treatment after ASCT with Revlimid may help control residual malignant cells and delay tumor growth by enhancing immune function,” said Michel Attal, MD, of the Institut Universitaire du Cancer Toulouse Oncopole and Institut Claudius Regaud in France.
“Our primary goal is to delay disease progression for as long as possible, and we have seen in several independent studies that Revlimid maintenance after ASCT can halve the risk of disease progression by sustaining the response.” ![]()

Photo courtesy of Celgene
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended a new indication for lenalidomide (Revlimid®).
The CHMP advised the European Commission (EC) to approve the use of lenalidomide as maintenance therapy in adults who had newly diagnosed multiple myeloma (MM) prior to receiving an autologous stem cell transplant (ASCT).
If approved by the EC, lenalidomide will be the first licensed maintenance treatment available to this patient population in the European Union.
The EC, which generally follows the CHMP’s recommendations, is expected to make its final decision on this use of lenalidomide in approximately 2 months.
If approval is granted, detailed conditions for the use of lenalidomide will be described in the Summary of Product Characteristics, which will be published in the revised European Public Assessment Report.
Lenalidomide is a product of Celgene.
The CHMP’s recommendation to approve lenalidomide as maintenance in MM was based on the results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211. Results from both studies were published in NEJM in May 2012.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing ASCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen post-ASCT of lenalidomide monotherapy, followed by continuous daily treatment with lenalidomide or placebo until relapse.
“Studies show that maintenance treatment after ASCT with Revlimid may help control residual malignant cells and delay tumor growth by enhancing immune function,” said Michel Attal, MD, of the Institut Universitaire du Cancer Toulouse Oncopole and Institut Claudius Regaud in France.
“Our primary goal is to delay disease progression for as long as possible, and we have seen in several independent studies that Revlimid maintenance after ASCT can halve the risk of disease progression by sustaining the response.” ![]()

Photo courtesy of Celgene
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended a new indication for lenalidomide (Revlimid®).
The CHMP advised the European Commission (EC) to approve the use of lenalidomide as maintenance therapy in adults who had newly diagnosed multiple myeloma (MM) prior to receiving an autologous stem cell transplant (ASCT).
If approved by the EC, lenalidomide will be the first licensed maintenance treatment available to this patient population in the European Union.
The EC, which generally follows the CHMP’s recommendations, is expected to make its final decision on this use of lenalidomide in approximately 2 months.
If approval is granted, detailed conditions for the use of lenalidomide will be described in the Summary of Product Characteristics, which will be published in the revised European Public Assessment Report.
Lenalidomide is a product of Celgene.
The CHMP’s recommendation to approve lenalidomide as maintenance in MM was based on the results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211. Results from both studies were published in NEJM in May 2012.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing ASCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen post-ASCT of lenalidomide monotherapy, followed by continuous daily treatment with lenalidomide or placebo until relapse.
“Studies show that maintenance treatment after ASCT with Revlimid may help control residual malignant cells and delay tumor growth by enhancing immune function,” said Michel Attal, MD, of the Institut Universitaire du Cancer Toulouse Oncopole and Institut Claudius Regaud in France.
“Our primary goal is to delay disease progression for as long as possible, and we have seen in several independent studies that Revlimid maintenance after ASCT can halve the risk of disease progression by sustaining the response.” ![]()
Study quantifies 5-year survival rates for blood cancers

chemotherapy
Photo by Rhoda Baer
A new study shows that 5-year survival rates for US patients with hematologic malignancies have increased greatly since the 1950s, but there is still room for improvement, particularly for patients with acute myeloid leukemia (AML).
Researchers found the absolute difference in improvement for 5-year survival from 1950-1954 to 2008-2013 ranged from 38.2% for non-Hodgkin lymphoma (NHL) to 56.6% for Hodgkin lymphoma.
And although the 5-year survival rate for Hodgkin lymphoma patients reached 86.6% for 2008-2013, the 5-year survival rate for patients with AML only reached 27.4%.
This study also revealed large disparities in overall cancer mortality rates between different counties across the country.
Ali H. Mokdad, PhD, of the Institute for Health Metrics and Evaluation in Seattle, Washington, and his colleagues reported these findings in JAMA.
Overall cancer deaths
The researchers found there were 19,511,910 cancer deaths recorded in the US between 1980 and 2014. Cancer mortality decreased by 20.1% between 1980 and 2014, from 240.2 deaths per 100,000 people to 192.0 deaths per 100,000 people.
In 1980, cancer mortality ranged from 130.6 per 100,000 in Summit County, Colorado, to 386.9 per 100,000 in North Slope Borough, Alaska.
In 2014, cancer mortality ranged from 70.7 per 100,000 in Summit County, Colorado, to 503.1 per 100,000 in Union County, Florida.
“Such significant disparities among US counties is unacceptable,” Dr Mokdad said. “Every person should have access to early screenings for cancer, as well as adequate treatment.”
Mortality rates for hematologic malignancies
In 2014, the mortality rates, per 100,000 people, for hematologic malignancies were:
- 0.4 for Hodgkin lymphoma (rank out of all cancers, 27)
- 8.3 for NHL (rank, 7)
- 3.9 for multiple myeloma (rank, 16)
- 9.0 for all leukemias (rank, 6)
- 0.7 for acute lymphoid leukemia (ALL)
- 2.6 for chronic lymphoid leukemia (CLL)
- 5.1 for AML
- 0.6 for chronic myeloid leukemia (CML).
The leukemia subtypes were not assigned a rank.
5-year survival rates for hematologic malignancies
Hodgkin lymphoma
- 30% for 1950-54
- 68.6% for 1973-77
- 72.1% for 1978-82
- 86.6% for 2008-2013
- Absolute difference (between the first and latest year of data), 56.6%.
NHL
- 33% for 1950-54
- 45.3% for 1973-77
- 48.7% for 1978-82
- 71.2% for 2008-2013
- Absolute difference, 38.2%.
Multiple myeloma
- 6% for 1950-54
- 23.4% for 1973-77
- 26.6% for 1978-82
- 49.8% for 2008-2013
- Absolute difference, 43.8%.
Leukemia
- 10% for 1950-54
- 34% for 1973-77
- 36.3% for 1978-82
- 60.1% for 2008-2013
- Absolute difference, 50.1%.
ALL
- 39.2% for 1973-77
- 50.5% for 1978-82
- 68.1% for 2008-2013
- Absolute difference, 28.9%.
CLL
- 67% for 1973-77
- 66.3% for 1978-82
- 82.5% for 2008-2013
- Absolute difference, 15.5%.
AML
- 6.2% for 1973-77
- 7.9% for 1978-82
- 27.4% for 2008-2013
- Absolute difference, 21.2%.
CML
- 21.1% for 1973-77
- 25.8% for 1978-82
- 66.4% for 2008-2013
- Absolute difference, 45.3%.
For the leukemia subtypes, there was no data for 1950 to 1954. ![]()

chemotherapy
Photo by Rhoda Baer
A new study shows that 5-year survival rates for US patients with hematologic malignancies have increased greatly since the 1950s, but there is still room for improvement, particularly for patients with acute myeloid leukemia (AML).
Researchers found the absolute difference in improvement for 5-year survival from 1950-1954 to 2008-2013 ranged from 38.2% for non-Hodgkin lymphoma (NHL) to 56.6% for Hodgkin lymphoma.
And although the 5-year survival rate for Hodgkin lymphoma patients reached 86.6% for 2008-2013, the 5-year survival rate for patients with AML only reached 27.4%.
This study also revealed large disparities in overall cancer mortality rates between different counties across the country.
Ali H. Mokdad, PhD, of the Institute for Health Metrics and Evaluation in Seattle, Washington, and his colleagues reported these findings in JAMA.
Overall cancer deaths
The researchers found there were 19,511,910 cancer deaths recorded in the US between 1980 and 2014. Cancer mortality decreased by 20.1% between 1980 and 2014, from 240.2 deaths per 100,000 people to 192.0 deaths per 100,000 people.
In 1980, cancer mortality ranged from 130.6 per 100,000 in Summit County, Colorado, to 386.9 per 100,000 in North Slope Borough, Alaska.
In 2014, cancer mortality ranged from 70.7 per 100,000 in Summit County, Colorado, to 503.1 per 100,000 in Union County, Florida.
“Such significant disparities among US counties is unacceptable,” Dr Mokdad said. “Every person should have access to early screenings for cancer, as well as adequate treatment.”
Mortality rates for hematologic malignancies
In 2014, the mortality rates, per 100,000 people, for hematologic malignancies were:
- 0.4 for Hodgkin lymphoma (rank out of all cancers, 27)
- 8.3 for NHL (rank, 7)
- 3.9 for multiple myeloma (rank, 16)
- 9.0 for all leukemias (rank, 6)
- 0.7 for acute lymphoid leukemia (ALL)
- 2.6 for chronic lymphoid leukemia (CLL)
- 5.1 for AML
- 0.6 for chronic myeloid leukemia (CML).
The leukemia subtypes were not assigned a rank.
5-year survival rates for hematologic malignancies
Hodgkin lymphoma
- 30% for 1950-54
- 68.6% for 1973-77
- 72.1% for 1978-82
- 86.6% for 2008-2013
- Absolute difference (between the first and latest year of data), 56.6%.
NHL
- 33% for 1950-54
- 45.3% for 1973-77
- 48.7% for 1978-82
- 71.2% for 2008-2013
- Absolute difference, 38.2%.
Multiple myeloma
- 6% for 1950-54
- 23.4% for 1973-77
- 26.6% for 1978-82
- 49.8% for 2008-2013
- Absolute difference, 43.8%.
Leukemia
- 10% for 1950-54
- 34% for 1973-77
- 36.3% for 1978-82
- 60.1% for 2008-2013
- Absolute difference, 50.1%.
ALL
- 39.2% for 1973-77
- 50.5% for 1978-82
- 68.1% for 2008-2013
- Absolute difference, 28.9%.
CLL
- 67% for 1973-77
- 66.3% for 1978-82
- 82.5% for 2008-2013
- Absolute difference, 15.5%.
AML
- 6.2% for 1973-77
- 7.9% for 1978-82
- 27.4% for 2008-2013
- Absolute difference, 21.2%.
CML
- 21.1% for 1973-77
- 25.8% for 1978-82
- 66.4% for 2008-2013
- Absolute difference, 45.3%.
For the leukemia subtypes, there was no data for 1950 to 1954. ![]()

chemotherapy
Photo by Rhoda Baer
A new study shows that 5-year survival rates for US patients with hematologic malignancies have increased greatly since the 1950s, but there is still room for improvement, particularly for patients with acute myeloid leukemia (AML).
Researchers found the absolute difference in improvement for 5-year survival from 1950-1954 to 2008-2013 ranged from 38.2% for non-Hodgkin lymphoma (NHL) to 56.6% for Hodgkin lymphoma.
And although the 5-year survival rate for Hodgkin lymphoma patients reached 86.6% for 2008-2013, the 5-year survival rate for patients with AML only reached 27.4%.
This study also revealed large disparities in overall cancer mortality rates between different counties across the country.
Ali H. Mokdad, PhD, of the Institute for Health Metrics and Evaluation in Seattle, Washington, and his colleagues reported these findings in JAMA.
Overall cancer deaths
The researchers found there were 19,511,910 cancer deaths recorded in the US between 1980 and 2014. Cancer mortality decreased by 20.1% between 1980 and 2014, from 240.2 deaths per 100,000 people to 192.0 deaths per 100,000 people.
In 1980, cancer mortality ranged from 130.6 per 100,000 in Summit County, Colorado, to 386.9 per 100,000 in North Slope Borough, Alaska.
In 2014, cancer mortality ranged from 70.7 per 100,000 in Summit County, Colorado, to 503.1 per 100,000 in Union County, Florida.
“Such significant disparities among US counties is unacceptable,” Dr Mokdad said. “Every person should have access to early screenings for cancer, as well as adequate treatment.”
Mortality rates for hematologic malignancies
In 2014, the mortality rates, per 100,000 people, for hematologic malignancies were:
- 0.4 for Hodgkin lymphoma (rank out of all cancers, 27)
- 8.3 for NHL (rank, 7)
- 3.9 for multiple myeloma (rank, 16)
- 9.0 for all leukemias (rank, 6)
- 0.7 for acute lymphoid leukemia (ALL)
- 2.6 for chronic lymphoid leukemia (CLL)
- 5.1 for AML
- 0.6 for chronic myeloid leukemia (CML).
The leukemia subtypes were not assigned a rank.
5-year survival rates for hematologic malignancies
Hodgkin lymphoma
- 30% for 1950-54
- 68.6% for 1973-77
- 72.1% for 1978-82
- 86.6% for 2008-2013
- Absolute difference (between the first and latest year of data), 56.6%.
NHL
- 33% for 1950-54
- 45.3% for 1973-77
- 48.7% for 1978-82
- 71.2% for 2008-2013
- Absolute difference, 38.2%.
Multiple myeloma
- 6% for 1950-54
- 23.4% for 1973-77
- 26.6% for 1978-82
- 49.8% for 2008-2013
- Absolute difference, 43.8%.
Leukemia
- 10% for 1950-54
- 34% for 1973-77
- 36.3% for 1978-82
- 60.1% for 2008-2013
- Absolute difference, 50.1%.
ALL
- 39.2% for 1973-77
- 50.5% for 1978-82
- 68.1% for 2008-2013
- Absolute difference, 28.9%.
CLL
- 67% for 1973-77
- 66.3% for 1978-82
- 82.5% for 2008-2013
- Absolute difference, 15.5%.
AML
- 6.2% for 1973-77
- 7.9% for 1978-82
- 27.4% for 2008-2013
- Absolute difference, 21.2%.
CML
- 21.1% for 1973-77
- 25.8% for 1978-82
- 66.4% for 2008-2013
- Absolute difference, 45.3%.
For the leukemia subtypes, there was no data for 1950 to 1954. ![]()
Health Canada expands indication for lenalidomide

Photo courtesy of Celgene
Health Canada has expanded the approved indication for lenalidomide (Revlimid®) to include the treatment of patients with multiple myeloma (MM).
Lenalidomide is now approved for use in combination with dexamethasone to treat patients newly diagnosed with MM who are not eligible for stem cell transplant.
Lenalidomide was previously approved in Canada for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q cytogenetic abnormality, with or without additional cytogenetic abnormalities.
Lenalidomide is a product of Celgene Corporation.
“The expanded indication of Revlimid® provides [MM] patients with a treatment much earlier in their disease and offers this patient population an all-oral, melphalan-free option for a disease that continues to be difficult to treat,” said Donna Reece, MD, of Princess Margaret Hospital in Toronto, Ontario, Canada.
The expanded approval of lenalidomide is based on safety and efficacy results from the phase 3 FIRST trial. Updated results from this study were published in the Journal of Clinical Oncology last November.
The trial included 1623 patients with newly diagnosed MM who were not eligible for stem cell transplant.
Patients were randomized to receive:
- Lenalidomide and low-dose dexamethasone (Rd) in 28-day cycles until disease progression (n=535)
- 18 cycles of Rd (Rd18) for 72 weeks (n=541)
- Melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
In the intent-to-treat population, the overall response rate was 81% for the continuous Rd group, 79% for the Rd18 group, and 67% in the MPT group. The complete response rates were 21%, 20%, and 12%, respectively.
The median progression-free survival (PFS) was 26.0 months in the continuous Rd group, 21.0 months in the Rd18 group, and 21.9 months in the MPT group. At 4 years, the PFS rates were 33%, 14%, and 13%, respectively.
The median overall survival (OS) was 58.9 months in the continuous Rd group, 56.7 months in the Rd18 group, and 48.5 months in the MPT group. At 4 years, the OS rates were 60%, 57%, and 51%, respectively.
The most frequent grade 3/4 hematologic treatment-emergent adverse events were neutropenia and anemia. The rate of grade 3/4 neutropenia was higher in the MPT group than the continuous Rd or Rd18 groups.
Infections were the most common grade 3/4 non-hematologic treatment-emergent adverse events. The rate of grade 3/4 infections was higher in the Rd groups than the MPT group.
“With this new clinical evidence, we know that keeping newly diagnosed multiple myeloma patients on Revlimid® may help delay disease progression and reduce the risk of death,” Dr Reece said. “As such, we are looking forward to having Revlimid® as a key option in the first-line setting for the appropriate patients.” ![]()

Photo courtesy of Celgene
Health Canada has expanded the approved indication for lenalidomide (Revlimid®) to include the treatment of patients with multiple myeloma (MM).
Lenalidomide is now approved for use in combination with dexamethasone to treat patients newly diagnosed with MM who are not eligible for stem cell transplant.
Lenalidomide was previously approved in Canada for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q cytogenetic abnormality, with or without additional cytogenetic abnormalities.
Lenalidomide is a product of Celgene Corporation.
“The expanded indication of Revlimid® provides [MM] patients with a treatment much earlier in their disease and offers this patient population an all-oral, melphalan-free option for a disease that continues to be difficult to treat,” said Donna Reece, MD, of Princess Margaret Hospital in Toronto, Ontario, Canada.
The expanded approval of lenalidomide is based on safety and efficacy results from the phase 3 FIRST trial. Updated results from this study were published in the Journal of Clinical Oncology last November.
The trial included 1623 patients with newly diagnosed MM who were not eligible for stem cell transplant.
Patients were randomized to receive:
- Lenalidomide and low-dose dexamethasone (Rd) in 28-day cycles until disease progression (n=535)
- 18 cycles of Rd (Rd18) for 72 weeks (n=541)
- Melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
In the intent-to-treat population, the overall response rate was 81% for the continuous Rd group, 79% for the Rd18 group, and 67% in the MPT group. The complete response rates were 21%, 20%, and 12%, respectively.
The median progression-free survival (PFS) was 26.0 months in the continuous Rd group, 21.0 months in the Rd18 group, and 21.9 months in the MPT group. At 4 years, the PFS rates were 33%, 14%, and 13%, respectively.
The median overall survival (OS) was 58.9 months in the continuous Rd group, 56.7 months in the Rd18 group, and 48.5 months in the MPT group. At 4 years, the OS rates were 60%, 57%, and 51%, respectively.
The most frequent grade 3/4 hematologic treatment-emergent adverse events were neutropenia and anemia. The rate of grade 3/4 neutropenia was higher in the MPT group than the continuous Rd or Rd18 groups.
Infections were the most common grade 3/4 non-hematologic treatment-emergent adverse events. The rate of grade 3/4 infections was higher in the Rd groups than the MPT group.
“With this new clinical evidence, we know that keeping newly diagnosed multiple myeloma patients on Revlimid® may help delay disease progression and reduce the risk of death,” Dr Reece said. “As such, we are looking forward to having Revlimid® as a key option in the first-line setting for the appropriate patients.” ![]()

Photo courtesy of Celgene
Health Canada has expanded the approved indication for lenalidomide (Revlimid®) to include the treatment of patients with multiple myeloma (MM).
Lenalidomide is now approved for use in combination with dexamethasone to treat patients newly diagnosed with MM who are not eligible for stem cell transplant.
Lenalidomide was previously approved in Canada for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q cytogenetic abnormality, with or without additional cytogenetic abnormalities.
Lenalidomide is a product of Celgene Corporation.
“The expanded indication of Revlimid® provides [MM] patients with a treatment much earlier in their disease and offers this patient population an all-oral, melphalan-free option for a disease that continues to be difficult to treat,” said Donna Reece, MD, of Princess Margaret Hospital in Toronto, Ontario, Canada.
The expanded approval of lenalidomide is based on safety and efficacy results from the phase 3 FIRST trial. Updated results from this study were published in the Journal of Clinical Oncology last November.
The trial included 1623 patients with newly diagnosed MM who were not eligible for stem cell transplant.
Patients were randomized to receive:
- Lenalidomide and low-dose dexamethasone (Rd) in 28-day cycles until disease progression (n=535)
- 18 cycles of Rd (Rd18) for 72 weeks (n=541)
- Melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
In the intent-to-treat population, the overall response rate was 81% for the continuous Rd group, 79% for the Rd18 group, and 67% in the MPT group. The complete response rates were 21%, 20%, and 12%, respectively.
The median progression-free survival (PFS) was 26.0 months in the continuous Rd group, 21.0 months in the Rd18 group, and 21.9 months in the MPT group. At 4 years, the PFS rates were 33%, 14%, and 13%, respectively.
The median overall survival (OS) was 58.9 months in the continuous Rd group, 56.7 months in the Rd18 group, and 48.5 months in the MPT group. At 4 years, the OS rates were 60%, 57%, and 51%, respectively.
The most frequent grade 3/4 hematologic treatment-emergent adverse events were neutropenia and anemia. The rate of grade 3/4 neutropenia was higher in the MPT group than the continuous Rd or Rd18 groups.
Infections were the most common grade 3/4 non-hematologic treatment-emergent adverse events. The rate of grade 3/4 infections was higher in the Rd groups than the MPT group.
“With this new clinical evidence, we know that keeping newly diagnosed multiple myeloma patients on Revlimid® may help delay disease progression and reduce the risk of death,” Dr Reece said. “As such, we are looking forward to having Revlimid® as a key option in the first-line setting for the appropriate patients.” ![]()
Bortezomib bolsters hematologic response in AL amyloidosis
SAN DIEGO – Adding bortezomib (B) to melphalan and dexamethasone (MDex) increased the frequency and depth of hematologic responses in a phase III trial of patients with previously untreated immunoglobulin light-chain (AL) amyloidosis.
Rates of hematologic response after three treatment cycles were 79% for BMDex and 52% for MDex (P = .002), Efstathios Kastritis, MD, said at the annual meeting of the American Society of Hematology. Very good partial responses accounted for most of this difference, with rates of 45% and 25%, respectively (P = .02). This first-in-kind trial establishes BMDex “as a novel standard of care in AL amyloidosis,” Dr. Kastritis concluded.
MDex is a standard regimen in intermediate-risk AL amyloidosis, while single-agent therapy with bortezomib yielded a median overall survival time of more than 5 years in one study, noted Dr. Kastritis of the University of Athens. In another matched case-control study, BMDex outperformed MDex based on overall response (69% vs. 51%; P = .01) and complete response (42% vs. 19%; P = .002).
Therefore, Dr. Kastritis and his associates in Europe and Australia randomly assigned 110 patients with newly diagnosed AL amyloidosis to receive either MDex (0.22 mg/kg melphalan plus 40 mg dexamethasone daily for 4 consecutive days every 28 days) or BMDex (MDex plus 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 during cycles one and two, and on days 1, 8, 15, and 22 during subsequent cycles). Treatment continued through nine cycles of MDex or eight cycles of BMDex, or through cycle six if patients had either a complete response or a partial response plus an organ response. Patients stopped treatment after three cycles if they did not have at least a partial response.
After a median of five treatment cycles, BMDex and MDex led to similar rates of complete response (23% vs. 20%), partial response (19% and 17%), cardiac response (38% vs. 29%), and renal response (44% vs. 44%). Twenty-eight patients died, with no significant difference in overall survival between treatment arms. However, overall survival did favor BMDex (P = .03) among the 77 patients who were in cardiac stage II. Also, median time to second-line therapy was not reached for BMDex but was only 12 months with MDex (P less than .001).
The BMDex regimen was associated with higher rates of peripheral neuropathy (19% vs. 4% for MDex; P less than .001). Furthermore, three patients (1%) developed severe peripheral neuropathy on BMDex, while none did so on MDex. Grade 3 or higher adverse events were more common with BMDex than with MDex, but the difference did not reach statistical significance (52% vs. 40%; P = .13). There were four cardiac deaths in the first 100 days of the trial, three in the BMDex arm and one in the MDex arm (P = .31).
The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
SAN DIEGO – Adding bortezomib (B) to melphalan and dexamethasone (MDex) increased the frequency and depth of hematologic responses in a phase III trial of patients with previously untreated immunoglobulin light-chain (AL) amyloidosis.
Rates of hematologic response after three treatment cycles were 79% for BMDex and 52% for MDex (P = .002), Efstathios Kastritis, MD, said at the annual meeting of the American Society of Hematology. Very good partial responses accounted for most of this difference, with rates of 45% and 25%, respectively (P = .02). This first-in-kind trial establishes BMDex “as a novel standard of care in AL amyloidosis,” Dr. Kastritis concluded.
MDex is a standard regimen in intermediate-risk AL amyloidosis, while single-agent therapy with bortezomib yielded a median overall survival time of more than 5 years in one study, noted Dr. Kastritis of the University of Athens. In another matched case-control study, BMDex outperformed MDex based on overall response (69% vs. 51%; P = .01) and complete response (42% vs. 19%; P = .002).
Therefore, Dr. Kastritis and his associates in Europe and Australia randomly assigned 110 patients with newly diagnosed AL amyloidosis to receive either MDex (0.22 mg/kg melphalan plus 40 mg dexamethasone daily for 4 consecutive days every 28 days) or BMDex (MDex plus 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 during cycles one and two, and on days 1, 8, 15, and 22 during subsequent cycles). Treatment continued through nine cycles of MDex or eight cycles of BMDex, or through cycle six if patients had either a complete response or a partial response plus an organ response. Patients stopped treatment after three cycles if they did not have at least a partial response.
After a median of five treatment cycles, BMDex and MDex led to similar rates of complete response (23% vs. 20%), partial response (19% and 17%), cardiac response (38% vs. 29%), and renal response (44% vs. 44%). Twenty-eight patients died, with no significant difference in overall survival between treatment arms. However, overall survival did favor BMDex (P = .03) among the 77 patients who were in cardiac stage II. Also, median time to second-line therapy was not reached for BMDex but was only 12 months with MDex (P less than .001).
The BMDex regimen was associated with higher rates of peripheral neuropathy (19% vs. 4% for MDex; P less than .001). Furthermore, three patients (1%) developed severe peripheral neuropathy on BMDex, while none did so on MDex. Grade 3 or higher adverse events were more common with BMDex than with MDex, but the difference did not reach statistical significance (52% vs. 40%; P = .13). There were four cardiac deaths in the first 100 days of the trial, three in the BMDex arm and one in the MDex arm (P = .31).
The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
SAN DIEGO – Adding bortezomib (B) to melphalan and dexamethasone (MDex) increased the frequency and depth of hematologic responses in a phase III trial of patients with previously untreated immunoglobulin light-chain (AL) amyloidosis.
Rates of hematologic response after three treatment cycles were 79% for BMDex and 52% for MDex (P = .002), Efstathios Kastritis, MD, said at the annual meeting of the American Society of Hematology. Very good partial responses accounted for most of this difference, with rates of 45% and 25%, respectively (P = .02). This first-in-kind trial establishes BMDex “as a novel standard of care in AL amyloidosis,” Dr. Kastritis concluded.
MDex is a standard regimen in intermediate-risk AL amyloidosis, while single-agent therapy with bortezomib yielded a median overall survival time of more than 5 years in one study, noted Dr. Kastritis of the University of Athens. In another matched case-control study, BMDex outperformed MDex based on overall response (69% vs. 51%; P = .01) and complete response (42% vs. 19%; P = .002).
Therefore, Dr. Kastritis and his associates in Europe and Australia randomly assigned 110 patients with newly diagnosed AL amyloidosis to receive either MDex (0.22 mg/kg melphalan plus 40 mg dexamethasone daily for 4 consecutive days every 28 days) or BMDex (MDex plus 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 during cycles one and two, and on days 1, 8, 15, and 22 during subsequent cycles). Treatment continued through nine cycles of MDex or eight cycles of BMDex, or through cycle six if patients had either a complete response or a partial response plus an organ response. Patients stopped treatment after three cycles if they did not have at least a partial response.
After a median of five treatment cycles, BMDex and MDex led to similar rates of complete response (23% vs. 20%), partial response (19% and 17%), cardiac response (38% vs. 29%), and renal response (44% vs. 44%). Twenty-eight patients died, with no significant difference in overall survival between treatment arms. However, overall survival did favor BMDex (P = .03) among the 77 patients who were in cardiac stage II. Also, median time to second-line therapy was not reached for BMDex but was only 12 months with MDex (P less than .001).
The BMDex regimen was associated with higher rates of peripheral neuropathy (19% vs. 4% for MDex; P less than .001). Furthermore, three patients (1%) developed severe peripheral neuropathy on BMDex, while none did so on MDex. Grade 3 or higher adverse events were more common with BMDex than with MDex, but the difference did not reach statistical significance (52% vs. 40%; P = .13). There were four cardiac deaths in the first 100 days of the trial, three in the BMDex arm and one in the MDex arm (P = .31).
The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
AT ASH 2016
Key clinical point: Adding bortezomib (B) to melphalan and dexamethasone (MDex) bolstered hematologic response in immunoglobulin light-chain (AL) amyloidosis.
Major finding: After three treatment cycles, rates of hematologic response were 79% in the BMDex arm and 52% for MDex (P = .002).
Data source: A multicenter randomized trial of 110 patients with newly diagnosed AL amyloidosis.
Disclosures: The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
FDG-PET/CT at maintenance predicts myeloma survival
SAN DIEGO – Having less than three focal lesions on FDG-PET/CT (fluorodeoxyglucose positron emission tomography integrated with computed tomography) when beginning lenalidomide maintenance therapy predicted significantly higher rates of progression-free and overall survival among patients with newly diagnosed multiple myeloma.
Survival in this prospective study of 102 patients also correlated with FDG uptake that did not exceed the level of the liver (Deauville score less than 3), reported Elena Zamagni, MD, PhD, of Bologna (Italy) University. The findings highlight FDG-PET/CT as “a powerful prognostic marker for survival, both in terms of number and score of focal lesions,” she said during an oral presentation at the annual meeting of the American Society of Hematology.
Although FDG-PET/CT is “well recognized” for staging and evaluating prognosis in multiple myeloma, a “major inconsistency in methodology between studies” inspired the current analysis, Dr. Zamagni said. “Different groups have used different interpretation criteria and arbitrary cutoffs with very variable results, especially in terms of posttreatment and borderline cases,” she noted.
Therefore, researchers from eight participating centers evaluated FDG-PET/CT scans from 103 patients with newly diagnosed multiple myeloma who were part of the randomized phase III EMN02 trial. Scans were performed at diagnosis, at the start of induction therapy, and just before patients started maintenance therapy with lenalidomide. Five nuclear medicine experts reviewed the scans in a blinded manner.
About 34% of patients had positive focal lesions on FDG-PET/CT at the start of maintenance, Dr. Zamagni said. After a median follow-up of 2 years, rates of progression-free survival were 84% in those with fewer than three focal lesions and 47% in those with three or more lesions (hazard ratio, 3.5; P = .01). Rates of overall survival followed the same trend at 98% and 68%, respectively (HR, 13.6; P = .0002).
Likewise, among patients whose FDG uptake did not exceed that of the liver, 2-year rates of progression-free and overall survival were 87% and 100%, compared with 69% and 45% in patients with new focal lesions or slightly, moderately, or markedly greater FDG uptake than the liver (Deauville scores of 4 and 5; P less than .001 for each comparison).
Normalization of FDG-PET/CT after induction also predicted improved survival, Dr. Zamagni said. Two-year progression-free survival rates were 85% among patients who had become PET-negative by the time they began maintenance, but were only 66% among patients who remained PET-positive (HR, 1.5; P less than .01). Rates of overall survival at 2 years were 98% among PET-negative patients and 87% among PET-positive patients (HR, 1.6; P = .03).
The study also confirmed the value of performing FDG-PET/CT at de novo myeloma diagnosis. Strikingly, only 20% of patients with baseline FDG-PET/CT evidence of extramedullary disease at this time point were alive and progression free 2 years later, compared with 81% of those without extramedullary disease (HR, 5.0; P = .001). Once again, the same trend emerged for Deauville scores – 99% of patients with scores of 3 or lower at diagnosis were alive 2 years later, compared with 83% of those who scored 4 or 5 (HR, 5.6; P = .03).
The EMN02 trial included 714 patients with newly diagnosed multiple myeloma who underwent induction with bortezomib-cyclophosphamide-dexamethasone (VCD), followed by either standard-dose intensification therapy with bortezomib-melphalan-prednisone (VMP) or high-dose intensification therapy with melphalan followed by single or double autologous stem cell transplantation. After that, patients either underwent consolidation therapy followed by lenalidomide maintenance therapy or proceeded directly to maintenance. Among the study subgroup of 103 patients, median age was 58 years, 25% of patients had high-risk cytogenetics, and 15% were ISS stage III.
“FDG-PET/CT is by now the preferred imaging technique for evaluating and monitoring response to therapy,” Dr. Zamagni concluded. She and her associates will use data from four other trials to further validate prognostic criteria and more precisely define cutoff points, she said.
Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no relevant financial conflicts of interest.
SAN DIEGO – Having less than three focal lesions on FDG-PET/CT (fluorodeoxyglucose positron emission tomography integrated with computed tomography) when beginning lenalidomide maintenance therapy predicted significantly higher rates of progression-free and overall survival among patients with newly diagnosed multiple myeloma.
Survival in this prospective study of 102 patients also correlated with FDG uptake that did not exceed the level of the liver (Deauville score less than 3), reported Elena Zamagni, MD, PhD, of Bologna (Italy) University. The findings highlight FDG-PET/CT as “a powerful prognostic marker for survival, both in terms of number and score of focal lesions,” she said during an oral presentation at the annual meeting of the American Society of Hematology.
Although FDG-PET/CT is “well recognized” for staging and evaluating prognosis in multiple myeloma, a “major inconsistency in methodology between studies” inspired the current analysis, Dr. Zamagni said. “Different groups have used different interpretation criteria and arbitrary cutoffs with very variable results, especially in terms of posttreatment and borderline cases,” she noted.
Therefore, researchers from eight participating centers evaluated FDG-PET/CT scans from 103 patients with newly diagnosed multiple myeloma who were part of the randomized phase III EMN02 trial. Scans were performed at diagnosis, at the start of induction therapy, and just before patients started maintenance therapy with lenalidomide. Five nuclear medicine experts reviewed the scans in a blinded manner.
About 34% of patients had positive focal lesions on FDG-PET/CT at the start of maintenance, Dr. Zamagni said. After a median follow-up of 2 years, rates of progression-free survival were 84% in those with fewer than three focal lesions and 47% in those with three or more lesions (hazard ratio, 3.5; P = .01). Rates of overall survival followed the same trend at 98% and 68%, respectively (HR, 13.6; P = .0002).
Likewise, among patients whose FDG uptake did not exceed that of the liver, 2-year rates of progression-free and overall survival were 87% and 100%, compared with 69% and 45% in patients with new focal lesions or slightly, moderately, or markedly greater FDG uptake than the liver (Deauville scores of 4 and 5; P less than .001 for each comparison).
Normalization of FDG-PET/CT after induction also predicted improved survival, Dr. Zamagni said. Two-year progression-free survival rates were 85% among patients who had become PET-negative by the time they began maintenance, but were only 66% among patients who remained PET-positive (HR, 1.5; P less than .01). Rates of overall survival at 2 years were 98% among PET-negative patients and 87% among PET-positive patients (HR, 1.6; P = .03).
The study also confirmed the value of performing FDG-PET/CT at de novo myeloma diagnosis. Strikingly, only 20% of patients with baseline FDG-PET/CT evidence of extramedullary disease at this time point were alive and progression free 2 years later, compared with 81% of those without extramedullary disease (HR, 5.0; P = .001). Once again, the same trend emerged for Deauville scores – 99% of patients with scores of 3 or lower at diagnosis were alive 2 years later, compared with 83% of those who scored 4 or 5 (HR, 5.6; P = .03).
The EMN02 trial included 714 patients with newly diagnosed multiple myeloma who underwent induction with bortezomib-cyclophosphamide-dexamethasone (VCD), followed by either standard-dose intensification therapy with bortezomib-melphalan-prednisone (VMP) or high-dose intensification therapy with melphalan followed by single or double autologous stem cell transplantation. After that, patients either underwent consolidation therapy followed by lenalidomide maintenance therapy or proceeded directly to maintenance. Among the study subgroup of 103 patients, median age was 58 years, 25% of patients had high-risk cytogenetics, and 15% were ISS stage III.
“FDG-PET/CT is by now the preferred imaging technique for evaluating and monitoring response to therapy,” Dr. Zamagni concluded. She and her associates will use data from four other trials to further validate prognostic criteria and more precisely define cutoff points, she said.
Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no relevant financial conflicts of interest.
SAN DIEGO – Having less than three focal lesions on FDG-PET/CT (fluorodeoxyglucose positron emission tomography integrated with computed tomography) when beginning lenalidomide maintenance therapy predicted significantly higher rates of progression-free and overall survival among patients with newly diagnosed multiple myeloma.
Survival in this prospective study of 102 patients also correlated with FDG uptake that did not exceed the level of the liver (Deauville score less than 3), reported Elena Zamagni, MD, PhD, of Bologna (Italy) University. The findings highlight FDG-PET/CT as “a powerful prognostic marker for survival, both in terms of number and score of focal lesions,” she said during an oral presentation at the annual meeting of the American Society of Hematology.
Although FDG-PET/CT is “well recognized” for staging and evaluating prognosis in multiple myeloma, a “major inconsistency in methodology between studies” inspired the current analysis, Dr. Zamagni said. “Different groups have used different interpretation criteria and arbitrary cutoffs with very variable results, especially in terms of posttreatment and borderline cases,” she noted.
Therefore, researchers from eight participating centers evaluated FDG-PET/CT scans from 103 patients with newly diagnosed multiple myeloma who were part of the randomized phase III EMN02 trial. Scans were performed at diagnosis, at the start of induction therapy, and just before patients started maintenance therapy with lenalidomide. Five nuclear medicine experts reviewed the scans in a blinded manner.
About 34% of patients had positive focal lesions on FDG-PET/CT at the start of maintenance, Dr. Zamagni said. After a median follow-up of 2 years, rates of progression-free survival were 84% in those with fewer than three focal lesions and 47% in those with three or more lesions (hazard ratio, 3.5; P = .01). Rates of overall survival followed the same trend at 98% and 68%, respectively (HR, 13.6; P = .0002).
Likewise, among patients whose FDG uptake did not exceed that of the liver, 2-year rates of progression-free and overall survival were 87% and 100%, compared with 69% and 45% in patients with new focal lesions or slightly, moderately, or markedly greater FDG uptake than the liver (Deauville scores of 4 and 5; P less than .001 for each comparison).
Normalization of FDG-PET/CT after induction also predicted improved survival, Dr. Zamagni said. Two-year progression-free survival rates were 85% among patients who had become PET-negative by the time they began maintenance, but were only 66% among patients who remained PET-positive (HR, 1.5; P less than .01). Rates of overall survival at 2 years were 98% among PET-negative patients and 87% among PET-positive patients (HR, 1.6; P = .03).
The study also confirmed the value of performing FDG-PET/CT at de novo myeloma diagnosis. Strikingly, only 20% of patients with baseline FDG-PET/CT evidence of extramedullary disease at this time point were alive and progression free 2 years later, compared with 81% of those without extramedullary disease (HR, 5.0; P = .001). Once again, the same trend emerged for Deauville scores – 99% of patients with scores of 3 or lower at diagnosis were alive 2 years later, compared with 83% of those who scored 4 or 5 (HR, 5.6; P = .03).
The EMN02 trial included 714 patients with newly diagnosed multiple myeloma who underwent induction with bortezomib-cyclophosphamide-dexamethasone (VCD), followed by either standard-dose intensification therapy with bortezomib-melphalan-prednisone (VMP) or high-dose intensification therapy with melphalan followed by single or double autologous stem cell transplantation. After that, patients either underwent consolidation therapy followed by lenalidomide maintenance therapy or proceeded directly to maintenance. Among the study subgroup of 103 patients, median age was 58 years, 25% of patients had high-risk cytogenetics, and 15% were ISS stage III.
“FDG-PET/CT is by now the preferred imaging technique for evaluating and monitoring response to therapy,” Dr. Zamagni concluded. She and her associates will use data from four other trials to further validate prognostic criteria and more precisely define cutoff points, she said.
Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no relevant financial conflicts of interest.
AT ASH 2016
Key clinical point: FDG-PET/CT helped predict survival in newly diagnosed multiple myeloma, regardless of induction regimen.
Major finding: Progression-free survival at 2 years was 84% when patients had less than three focal lesions at the start of maintenance, vs. 47% when they had three or more lesions (hazard ratio, 3.5; P = .01).
Data source: A prospective study of 103 patients with newly diagnosed, transplant-eligible multiple myeloma from a randomized phase III trial.
Disclosures: Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no conflicts of interest.
EZH2 may be therapeutic target for multiple myeloma

multiple myeloma
Preclinical research published in Oncotarget has provided new insights regarding how the protein EZH2 affects the development of multiple myeloma (MM) and reinforces the idea that EZH2 inhibition may be a way to treat MM.
Previous research by the same group suggested that EZH2 is a potential therapeutic target in MM.
With the current study, the group further investigated the anti-myeloma mechanisms mediated by EZH2 inhibition.
They found that targeting EZH2 with an agent known as UNC1999 reduces the expression of 4 MM-associated oncogenes—IRF-4, XBP-1, PRDM1/BLIMP-1, and c-MYC.
“The role of oncogenes in the development of cancer is to potentiate the survival of the cancer cell, which, instead of undergoing cell death, as is usually the case when the cell is not functioning properly, continues to divide and proliferate,” said study author Helena Jernberg-Wiklund, PhD, of Uppsala University in Uppsala, Sweden.
“In our study, we identified 4 oncogenes that showed lower activity in cells treated with the EZH2 inhibitor as compared to control-treated cells. All 4 genes have previously been shown to be associated with the development of multiple myeloma. This confirms our previous findings that inhibition of EZH2 could be used as a means to treat multiple myeloma.”
However, the researchers were puzzled by the fact that inhibition of EZH2 could decrease the activity of the oncogenes.
The chemical histone modification performed by EZH2 leads to decreased activity of affected genes. Therefore, inhibition of EZH2 should result in a reduced level of chemical modifications, which, in turn, should result in increased gene activity.
“The answer is that there are other genetic factors involved, called microRNAs,” Dr Jernberg-Wiklund said. “In the cells treated with the EZH2 inhibitor, we found 2 microRNA genes with increased activity, and we believe that the oncogenes are regulated by these microRNAs.”
“What happens then is that, when EZH2 is inhibited, there is a reduced histone modification at the microRNA genes. This leads to an increased synthesis of the microRNAs, which, in turn, decreases the activity of the oncogenes. This is a completely new mechanism for EZH2 action.”
The microRNAs the researchers identified are miR-125a-3p and miR-320c. The team found that miR-125a-3p and miR-320c were targets of EZH2 and H3K27me3 in MM cell lines and primary cells.
The researchers said these results support their previous work suggesting EZH2 could be a therapeutic target in MM. ![]()

multiple myeloma
Preclinical research published in Oncotarget has provided new insights regarding how the protein EZH2 affects the development of multiple myeloma (MM) and reinforces the idea that EZH2 inhibition may be a way to treat MM.
Previous research by the same group suggested that EZH2 is a potential therapeutic target in MM.
With the current study, the group further investigated the anti-myeloma mechanisms mediated by EZH2 inhibition.
They found that targeting EZH2 with an agent known as UNC1999 reduces the expression of 4 MM-associated oncogenes—IRF-4, XBP-1, PRDM1/BLIMP-1, and c-MYC.
“The role of oncogenes in the development of cancer is to potentiate the survival of the cancer cell, which, instead of undergoing cell death, as is usually the case when the cell is not functioning properly, continues to divide and proliferate,” said study author Helena Jernberg-Wiklund, PhD, of Uppsala University in Uppsala, Sweden.
“In our study, we identified 4 oncogenes that showed lower activity in cells treated with the EZH2 inhibitor as compared to control-treated cells. All 4 genes have previously been shown to be associated with the development of multiple myeloma. This confirms our previous findings that inhibition of EZH2 could be used as a means to treat multiple myeloma.”
However, the researchers were puzzled by the fact that inhibition of EZH2 could decrease the activity of the oncogenes.
The chemical histone modification performed by EZH2 leads to decreased activity of affected genes. Therefore, inhibition of EZH2 should result in a reduced level of chemical modifications, which, in turn, should result in increased gene activity.
“The answer is that there are other genetic factors involved, called microRNAs,” Dr Jernberg-Wiklund said. “In the cells treated with the EZH2 inhibitor, we found 2 microRNA genes with increased activity, and we believe that the oncogenes are regulated by these microRNAs.”
“What happens then is that, when EZH2 is inhibited, there is a reduced histone modification at the microRNA genes. This leads to an increased synthesis of the microRNAs, which, in turn, decreases the activity of the oncogenes. This is a completely new mechanism for EZH2 action.”
The microRNAs the researchers identified are miR-125a-3p and miR-320c. The team found that miR-125a-3p and miR-320c were targets of EZH2 and H3K27me3 in MM cell lines and primary cells.
The researchers said these results support their previous work suggesting EZH2 could be a therapeutic target in MM. ![]()

multiple myeloma
Preclinical research published in Oncotarget has provided new insights regarding how the protein EZH2 affects the development of multiple myeloma (MM) and reinforces the idea that EZH2 inhibition may be a way to treat MM.
Previous research by the same group suggested that EZH2 is a potential therapeutic target in MM.
With the current study, the group further investigated the anti-myeloma mechanisms mediated by EZH2 inhibition.
They found that targeting EZH2 with an agent known as UNC1999 reduces the expression of 4 MM-associated oncogenes—IRF-4, XBP-1, PRDM1/BLIMP-1, and c-MYC.
“The role of oncogenes in the development of cancer is to potentiate the survival of the cancer cell, which, instead of undergoing cell death, as is usually the case when the cell is not functioning properly, continues to divide and proliferate,” said study author Helena Jernberg-Wiklund, PhD, of Uppsala University in Uppsala, Sweden.
“In our study, we identified 4 oncogenes that showed lower activity in cells treated with the EZH2 inhibitor as compared to control-treated cells. All 4 genes have previously been shown to be associated with the development of multiple myeloma. This confirms our previous findings that inhibition of EZH2 could be used as a means to treat multiple myeloma.”
However, the researchers were puzzled by the fact that inhibition of EZH2 could decrease the activity of the oncogenes.
The chemical histone modification performed by EZH2 leads to decreased activity of affected genes. Therefore, inhibition of EZH2 should result in a reduced level of chemical modifications, which, in turn, should result in increased gene activity.
“The answer is that there are other genetic factors involved, called microRNAs,” Dr Jernberg-Wiklund said. “In the cells treated with the EZH2 inhibitor, we found 2 microRNA genes with increased activity, and we believe that the oncogenes are regulated by these microRNAs.”
“What happens then is that, when EZH2 is inhibited, there is a reduced histone modification at the microRNA genes. This leads to an increased synthesis of the microRNAs, which, in turn, decreases the activity of the oncogenes. This is a completely new mechanism for EZH2 action.”
The microRNAs the researchers identified are miR-125a-3p and miR-320c. The team found that miR-125a-3p and miR-320c were targets of EZH2 and H3K27me3 in MM cell lines and primary cells.
The researchers said these results support their previous work suggesting EZH2 could be a therapeutic target in MM. ![]()
FDA issues CRL for IV formulation of antiemetic agent

chemotherapy
Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has issued a complete response letter (CRL) regarding the new drug application (NDA) for an intravenous (IV) formulation of rolapitant.
An oral formulation of rolapitant, marketed as VARUBI®, is FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy in adults.
The NDA for rolapitant IV is for the same indication.
The FDA requested additional information regarding the in vitro method utilized to demonstrate comparability of drug product produced at the 2 proposed commercial manufacturers for rolapitant IV that were included in the NDA.
TESARO Inc., the company developing rolapitant IV, said it is working to provide the requested information.
The CRL did not identify concerns related to the safety or efficacy of rolapitant IV or request additional clinical studies. No concerns were raised regarding the active pharmaceutical ingredient, which is also used for VARUBI®.
TESARO identified potential deficiencies at the original contract manufacturer for rolapitant IV, secured a second drug product supplier, and included data from this manufacturer in the NDA.
During the NDA review, the FDA requested and TESARO provided in vitro data to demonstrate comparability of drug product made at the 2 manufacturing sites.
“TESARO is committed to bringing this new intravenous formulation of rolapitant to physicians and patients to enable additional flexibility and choice of antiemetic regimens, and we plan to address FDA’s questions expeditiously and complete this application, which we expect to enable approval in the first half of 2017,” said Mary Lynne Hedley, PhD, president and chief operating officer of TESARO. ![]()

chemotherapy
Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has issued a complete response letter (CRL) regarding the new drug application (NDA) for an intravenous (IV) formulation of rolapitant.
An oral formulation of rolapitant, marketed as VARUBI®, is FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy in adults.
The NDA for rolapitant IV is for the same indication.
The FDA requested additional information regarding the in vitro method utilized to demonstrate comparability of drug product produced at the 2 proposed commercial manufacturers for rolapitant IV that were included in the NDA.
TESARO Inc., the company developing rolapitant IV, said it is working to provide the requested information.
The CRL did not identify concerns related to the safety or efficacy of rolapitant IV or request additional clinical studies. No concerns were raised regarding the active pharmaceutical ingredient, which is also used for VARUBI®.
TESARO identified potential deficiencies at the original contract manufacturer for rolapitant IV, secured a second drug product supplier, and included data from this manufacturer in the NDA.
During the NDA review, the FDA requested and TESARO provided in vitro data to demonstrate comparability of drug product made at the 2 manufacturing sites.
“TESARO is committed to bringing this new intravenous formulation of rolapitant to physicians and patients to enable additional flexibility and choice of antiemetic regimens, and we plan to address FDA’s questions expeditiously and complete this application, which we expect to enable approval in the first half of 2017,” said Mary Lynne Hedley, PhD, president and chief operating officer of TESARO. ![]()

chemotherapy
Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has issued a complete response letter (CRL) regarding the new drug application (NDA) for an intravenous (IV) formulation of rolapitant.
An oral formulation of rolapitant, marketed as VARUBI®, is FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy in adults.
The NDA for rolapitant IV is for the same indication.
The FDA requested additional information regarding the in vitro method utilized to demonstrate comparability of drug product produced at the 2 proposed commercial manufacturers for rolapitant IV that were included in the NDA.
TESARO Inc., the company developing rolapitant IV, said it is working to provide the requested information.
The CRL did not identify concerns related to the safety or efficacy of rolapitant IV or request additional clinical studies. No concerns were raised regarding the active pharmaceutical ingredient, which is also used for VARUBI®.
TESARO identified potential deficiencies at the original contract manufacturer for rolapitant IV, secured a second drug product supplier, and included data from this manufacturer in the NDA.
During the NDA review, the FDA requested and TESARO provided in vitro data to demonstrate comparability of drug product made at the 2 manufacturing sites.
“TESARO is committed to bringing this new intravenous formulation of rolapitant to physicians and patients to enable additional flexibility and choice of antiemetic regimens, and we plan to address FDA’s questions expeditiously and complete this application, which we expect to enable approval in the first half of 2017,” said Mary Lynne Hedley, PhD, president and chief operating officer of TESARO.