User login
CHMP recommends daratumumab for MM

Photo courtesy of Janssen
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended conditional marketing authorization for daratumumab (Darzalex), a first-in-class monoclonal antibody targeting CD38.
The recommended indication for daratumumab is as monotherapy for adults with relapsed and refractory multiple myeloma (MM).
The patients must have progressed on their last therapy and have received treatment with both a proteasome inhibitor and an immunomodulatory agent.
The CHMP’s positive opinion will now be reviewed by the European Commission, which has the authority to grant marketing authorization for medicines in the European Economic Area.
The European Commission’s final decision on daratumumab is anticipated in the coming months.
About conditional authorization
A product may receive conditional marketing authorization if the CHMP finds that, although comprehensive clinical data on the safety and efficacy of the product are not available, all of the following requirements are met:
- The risk-benefit balance of the product is positive
- The company developing the product will likely be in a position to provide comprehensive clinical data in the future
- Unmet medical needs will be fulfilled
- The benefit to public health of the immediate availability of the product outweighs the risk inherent in the fact that additional data are still required.
Conditional marketing authorizations are valid for 1 year, on a renewable basis. The holder will be required to complete ongoing studies or to conduct new studies with a view to confirming that the benefit-risk balance of a product is positive. In addition, specific obligations may be imposed in relation to the collection of pharmacovigilance data.
About daratumumab
Daratumumab is the first CD38-directed monoclonal antibody recommended for approval in Europe. It works by binding to CD38, a signaling molecule highly expressed on the surface of MM cells regardless of stage of disease.
In binding to CD38, daratumumab triggers the patient’s own immune system to attack the cancer cells, resulting in rapid tumor cell death through multiple, immune-mediated mechanisms of action and through immunomodulatory effects, in addition to direct tumor cell death via apoptosis.
The CHMP’s positive opinion of daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies with daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. Janssen licensed daratumumab from Genmab A/S in August 2012. ![]()

Photo courtesy of Janssen
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended conditional marketing authorization for daratumumab (Darzalex), a first-in-class monoclonal antibody targeting CD38.
The recommended indication for daratumumab is as monotherapy for adults with relapsed and refractory multiple myeloma (MM).
The patients must have progressed on their last therapy and have received treatment with both a proteasome inhibitor and an immunomodulatory agent.
The CHMP’s positive opinion will now be reviewed by the European Commission, which has the authority to grant marketing authorization for medicines in the European Economic Area.
The European Commission’s final decision on daratumumab is anticipated in the coming months.
About conditional authorization
A product may receive conditional marketing authorization if the CHMP finds that, although comprehensive clinical data on the safety and efficacy of the product are not available, all of the following requirements are met:
- The risk-benefit balance of the product is positive
- The company developing the product will likely be in a position to provide comprehensive clinical data in the future
- Unmet medical needs will be fulfilled
- The benefit to public health of the immediate availability of the product outweighs the risk inherent in the fact that additional data are still required.
Conditional marketing authorizations are valid for 1 year, on a renewable basis. The holder will be required to complete ongoing studies or to conduct new studies with a view to confirming that the benefit-risk balance of a product is positive. In addition, specific obligations may be imposed in relation to the collection of pharmacovigilance data.
About daratumumab
Daratumumab is the first CD38-directed monoclonal antibody recommended for approval in Europe. It works by binding to CD38, a signaling molecule highly expressed on the surface of MM cells regardless of stage of disease.
In binding to CD38, daratumumab triggers the patient’s own immune system to attack the cancer cells, resulting in rapid tumor cell death through multiple, immune-mediated mechanisms of action and through immunomodulatory effects, in addition to direct tumor cell death via apoptosis.
The CHMP’s positive opinion of daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies with daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. Janssen licensed daratumumab from Genmab A/S in August 2012. ![]()

Photo courtesy of Janssen
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended conditional marketing authorization for daratumumab (Darzalex), a first-in-class monoclonal antibody targeting CD38.
The recommended indication for daratumumab is as monotherapy for adults with relapsed and refractory multiple myeloma (MM).
The patients must have progressed on their last therapy and have received treatment with both a proteasome inhibitor and an immunomodulatory agent.
The CHMP’s positive opinion will now be reviewed by the European Commission, which has the authority to grant marketing authorization for medicines in the European Economic Area.
The European Commission’s final decision on daratumumab is anticipated in the coming months.
About conditional authorization
A product may receive conditional marketing authorization if the CHMP finds that, although comprehensive clinical data on the safety and efficacy of the product are not available, all of the following requirements are met:
- The risk-benefit balance of the product is positive
- The company developing the product will likely be in a position to provide comprehensive clinical data in the future
- Unmet medical needs will be fulfilled
- The benefit to public health of the immediate availability of the product outweighs the risk inherent in the fact that additional data are still required.
Conditional marketing authorizations are valid for 1 year, on a renewable basis. The holder will be required to complete ongoing studies or to conduct new studies with a view to confirming that the benefit-risk balance of a product is positive. In addition, specific obligations may be imposed in relation to the collection of pharmacovigilance data.
About daratumumab
Daratumumab is the first CD38-directed monoclonal antibody recommended for approval in Europe. It works by binding to CD38, a signaling molecule highly expressed on the surface of MM cells regardless of stage of disease.
In binding to CD38, daratumumab triggers the patient’s own immune system to attack the cancer cells, resulting in rapid tumor cell death through multiple, immune-mediated mechanisms of action and through immunomodulatory effects, in addition to direct tumor cell death via apoptosis.
The CHMP’s positive opinion of daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies with daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. Janssen licensed daratumumab from Genmab A/S in August 2012. ![]()
A better method for detecting amyloidosis?

A novel molecular probe can detect amyloidosis at least as well as—and perhaps even better than—traditional methods, according to research published in Amyloid: The Journal of Protein Folding Disorders.
Investigators found that a luminescent conjugated oligothiophene, h-FTAA, allowed them to correctly identify amyloidosis in every sample tested.
But results also suggested h-FTAA may be more sensitive than traditional methods used to diagnose amyloidosis, as h-FTAA detected small amyloid deposits in samples that were previously determined to be amyloid-free.
The investigators said this suggests h-FTAA could be used to detect amyloidosis before symptoms present, leading to faster treatment.
“Given the sensitivity of the probe, we think this would make an excellent complement to traditional methods and could eventually be a replacement,” said study author Per Hammarström, PhD, of Linköping University in Sweden.
Dr Hammarström and his colleagues screened amyloid-containing tissues from 107 patients who had their amyloidosis verified by Congo red staining and/or immunohistochemistry, as well as tissues from 32 negative control cases.
The results showed that h-FTAA could detect amyloidosis with 100% sensitivity, identifying amyloid deposits in all 107 patients.
However, h-FTAA also detected microdeposits of amyloid-like protein aggregates in 5 of the control samples that were negative according to Congo red.
The investigators said they don’t know the clinical significance of these “false-positive” lesions. However, because h-FTAA fluorescence is 1 magnitude brighter than Congo red and because the staining is performed 4 magnitudes lower than the concentration of dye, the team believes these 5 cases may have been beyond detection by Congo red and h-FTAA may be a more sensitive technique.
They therefore concluded that h-FTAA could potentially be used as a complementary technique for accurate detection of amyloid in routine surgical pathology settings, for the detection of prodromal amyloidosis, and for the discovery of new amyloid-like protein aggregates. ![]()

A novel molecular probe can detect amyloidosis at least as well as—and perhaps even better than—traditional methods, according to research published in Amyloid: The Journal of Protein Folding Disorders.
Investigators found that a luminescent conjugated oligothiophene, h-FTAA, allowed them to correctly identify amyloidosis in every sample tested.
But results also suggested h-FTAA may be more sensitive than traditional methods used to diagnose amyloidosis, as h-FTAA detected small amyloid deposits in samples that were previously determined to be amyloid-free.
The investigators said this suggests h-FTAA could be used to detect amyloidosis before symptoms present, leading to faster treatment.
“Given the sensitivity of the probe, we think this would make an excellent complement to traditional methods and could eventually be a replacement,” said study author Per Hammarström, PhD, of Linköping University in Sweden.
Dr Hammarström and his colleagues screened amyloid-containing tissues from 107 patients who had their amyloidosis verified by Congo red staining and/or immunohistochemistry, as well as tissues from 32 negative control cases.
The results showed that h-FTAA could detect amyloidosis with 100% sensitivity, identifying amyloid deposits in all 107 patients.
However, h-FTAA also detected microdeposits of amyloid-like protein aggregates in 5 of the control samples that were negative according to Congo red.
The investigators said they don’t know the clinical significance of these “false-positive” lesions. However, because h-FTAA fluorescence is 1 magnitude brighter than Congo red and because the staining is performed 4 magnitudes lower than the concentration of dye, the team believes these 5 cases may have been beyond detection by Congo red and h-FTAA may be a more sensitive technique.
They therefore concluded that h-FTAA could potentially be used as a complementary technique for accurate detection of amyloid in routine surgical pathology settings, for the detection of prodromal amyloidosis, and for the discovery of new amyloid-like protein aggregates. ![]()

A novel molecular probe can detect amyloidosis at least as well as—and perhaps even better than—traditional methods, according to research published in Amyloid: The Journal of Protein Folding Disorders.
Investigators found that a luminescent conjugated oligothiophene, h-FTAA, allowed them to correctly identify amyloidosis in every sample tested.
But results also suggested h-FTAA may be more sensitive than traditional methods used to diagnose amyloidosis, as h-FTAA detected small amyloid deposits in samples that were previously determined to be amyloid-free.
The investigators said this suggests h-FTAA could be used to detect amyloidosis before symptoms present, leading to faster treatment.
“Given the sensitivity of the probe, we think this would make an excellent complement to traditional methods and could eventually be a replacement,” said study author Per Hammarström, PhD, of Linköping University in Sweden.
Dr Hammarström and his colleagues screened amyloid-containing tissues from 107 patients who had their amyloidosis verified by Congo red staining and/or immunohistochemistry, as well as tissues from 32 negative control cases.
The results showed that h-FTAA could detect amyloidosis with 100% sensitivity, identifying amyloid deposits in all 107 patients.
However, h-FTAA also detected microdeposits of amyloid-like protein aggregates in 5 of the control samples that were negative according to Congo red.
The investigators said they don’t know the clinical significance of these “false-positive” lesions. However, because h-FTAA fluorescence is 1 magnitude brighter than Congo red and because the staining is performed 4 magnitudes lower than the concentration of dye, the team believes these 5 cases may have been beyond detection by Congo red and h-FTAA may be a more sensitive technique.
They therefore concluded that h-FTAA could potentially be used as a complementary technique for accurate detection of amyloid in routine surgical pathology settings, for the detection of prodromal amyloidosis, and for the discovery of new amyloid-like protein aggregates. ![]()
Study: Dying at home doesn’t mean dying sooner
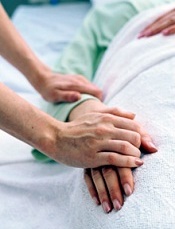
patient’s hand
Choosing to die at home does not hasten death for patients with terminal cancer, according to a study published in Cancer.
The research showed that cancer patients who died at home lived at least as long as patients who spent their last days in hospitals.
Investigators say these results suggest oncologists should not hesitate to refer patients for home-based palliative care simply because less medical treatment may be provided.
“The cancer patient and family tend to be concerned that the quality of medical treatment provided at home will be inferior to that given in a hospital and that survival might be shortened,” said study author Jun Hamano, MD, of the University of Tsukuba in Japan.
“However, our finding—that home death does not actually have a negative influence on the survival of cancer patients at all and, rather, may have a positive influence—could suggest that the patient and family can choose the place of death in terms of their preference and values.”
Dr Hamano and his colleagues conducted this research by prospectively studying 2069 patients—1582 receiving hospital-based palliative care and 487 receiving home-based palliative care.
In all, 1607 patients died in the hospital, and 462 died at home.
Among patients thought to have only days to live, the survival of those who died at home was significantly longer than the survival of those who died in a hospital. The estimated median survival times were 13 days and 9 days, respectively (P<0.006).
Similarly, survival was significantly longer in the home group than the hospital group among patients thought to have weeks to live. The estimated median survival times were 36 days and 29 days, respectively (P<0.007).
There was no significant difference between the home and hospital groups among patients thought to have months to live. The estimated median survival times were 59 days and 62 days, respectively (P=0.925).
Finally, analyses suggested the place of death had a significant influence on the survival time in both unadjusted and adjusted models. The hazard ratios were 0.86 (P<0.01) and 0.87 (P=0.01), respectively.
Based on these findings, Dr Hamano concluded that, “Patients, families, and clinicians should be reassured that good home hospice care does not shorten patient life and even may achieve longer survival.” ![]()

patient’s hand
Choosing to die at home does not hasten death for patients with terminal cancer, according to a study published in Cancer.
The research showed that cancer patients who died at home lived at least as long as patients who spent their last days in hospitals.
Investigators say these results suggest oncologists should not hesitate to refer patients for home-based palliative care simply because less medical treatment may be provided.
“The cancer patient and family tend to be concerned that the quality of medical treatment provided at home will be inferior to that given in a hospital and that survival might be shortened,” said study author Jun Hamano, MD, of the University of Tsukuba in Japan.
“However, our finding—that home death does not actually have a negative influence on the survival of cancer patients at all and, rather, may have a positive influence—could suggest that the patient and family can choose the place of death in terms of their preference and values.”
Dr Hamano and his colleagues conducted this research by prospectively studying 2069 patients—1582 receiving hospital-based palliative care and 487 receiving home-based palliative care.
In all, 1607 patients died in the hospital, and 462 died at home.
Among patients thought to have only days to live, the survival of those who died at home was significantly longer than the survival of those who died in a hospital. The estimated median survival times were 13 days and 9 days, respectively (P<0.006).
Similarly, survival was significantly longer in the home group than the hospital group among patients thought to have weeks to live. The estimated median survival times were 36 days and 29 days, respectively (P<0.007).
There was no significant difference between the home and hospital groups among patients thought to have months to live. The estimated median survival times were 59 days and 62 days, respectively (P=0.925).
Finally, analyses suggested the place of death had a significant influence on the survival time in both unadjusted and adjusted models. The hazard ratios were 0.86 (P<0.01) and 0.87 (P=0.01), respectively.
Based on these findings, Dr Hamano concluded that, “Patients, families, and clinicians should be reassured that good home hospice care does not shorten patient life and even may achieve longer survival.” ![]()

patient’s hand
Choosing to die at home does not hasten death for patients with terminal cancer, according to a study published in Cancer.
The research showed that cancer patients who died at home lived at least as long as patients who spent their last days in hospitals.
Investigators say these results suggest oncologists should not hesitate to refer patients for home-based palliative care simply because less medical treatment may be provided.
“The cancer patient and family tend to be concerned that the quality of medical treatment provided at home will be inferior to that given in a hospital and that survival might be shortened,” said study author Jun Hamano, MD, of the University of Tsukuba in Japan.
“However, our finding—that home death does not actually have a negative influence on the survival of cancer patients at all and, rather, may have a positive influence—could suggest that the patient and family can choose the place of death in terms of their preference and values.”
Dr Hamano and his colleagues conducted this research by prospectively studying 2069 patients—1582 receiving hospital-based palliative care and 487 receiving home-based palliative care.
In all, 1607 patients died in the hospital, and 462 died at home.
Among patients thought to have only days to live, the survival of those who died at home was significantly longer than the survival of those who died in a hospital. The estimated median survival times were 13 days and 9 days, respectively (P<0.006).
Similarly, survival was significantly longer in the home group than the hospital group among patients thought to have weeks to live. The estimated median survival times were 36 days and 29 days, respectively (P<0.007).
There was no significant difference between the home and hospital groups among patients thought to have months to live. The estimated median survival times were 59 days and 62 days, respectively (P=0.925).
Finally, analyses suggested the place of death had a significant influence on the survival time in both unadjusted and adjusted models. The hazard ratios were 0.86 (P<0.01) and 0.87 (P=0.01), respectively.
Based on these findings, Dr Hamano concluded that, “Patients, families, and clinicians should be reassured that good home hospice care does not shorten patient life and even may achieve longer survival.” ![]()
Antibody recognizes human plasma cells

Researchers say they have generated a monoclonal antibody that could have diagnostic and therapeutic applications for multiple myeloma (MM) and other plasma cell disorders.
The team generated this antibody, VLRB MM3, from immunized lampreys, a type of jawless fish.
Experiments with VLRB MM3 showed that it can identify normal plasma cells in samples from healthy donors and malignant plasma cells in samples from patients with MM.
Götz Ehrhardt, PhD, of the University of Toronto in Ontario, Canada, and his colleagues described this work in JCI Insight.
The researchers noted that antibody-secreting plasma cells arise from B-cell precursors and are essential for adaptive immune responses against invading pathogens. Plasma cell dysfunction is associated with autoimmune and neoplastic disorders, including MM.
Surface markers that are specific to plasma cells have not been identified, and antibodies that only recognize these cells have been challenging to generate using conventional systems.
However, Dr Ehrhardt and his colleagues found they could generate a plasma-cell-specific antibody from immunized lampreys.
The researchers injected lamprey larvae with a bone marrow isolate from an MM patient and screened the resulting monoclonal antibodies for those that recognized both malignant and non-malignant plasma cells.
Further characterization of the antibody VLRB MM3 revealed that it is specific to plasma cells and does not recognize other B-cell populations or progenitors.
VLRB MM3 binding was shown to coincide with CD38 dimerization and correlate with and impede the NAD glycohydrolase activity of this glycoprotein.
Considering these findings together, the researchers concluded that VLRB MM3 represents a unique tool that might aid the treatment and diagnosis of plasma cell disorders. ![]()

Researchers say they have generated a monoclonal antibody that could have diagnostic and therapeutic applications for multiple myeloma (MM) and other plasma cell disorders.
The team generated this antibody, VLRB MM3, from immunized lampreys, a type of jawless fish.
Experiments with VLRB MM3 showed that it can identify normal plasma cells in samples from healthy donors and malignant plasma cells in samples from patients with MM.
Götz Ehrhardt, PhD, of the University of Toronto in Ontario, Canada, and his colleagues described this work in JCI Insight.
The researchers noted that antibody-secreting plasma cells arise from B-cell precursors and are essential for adaptive immune responses against invading pathogens. Plasma cell dysfunction is associated with autoimmune and neoplastic disorders, including MM.
Surface markers that are specific to plasma cells have not been identified, and antibodies that only recognize these cells have been challenging to generate using conventional systems.
However, Dr Ehrhardt and his colleagues found they could generate a plasma-cell-specific antibody from immunized lampreys.
The researchers injected lamprey larvae with a bone marrow isolate from an MM patient and screened the resulting monoclonal antibodies for those that recognized both malignant and non-malignant plasma cells.
Further characterization of the antibody VLRB MM3 revealed that it is specific to plasma cells and does not recognize other B-cell populations or progenitors.
VLRB MM3 binding was shown to coincide with CD38 dimerization and correlate with and impede the NAD glycohydrolase activity of this glycoprotein.
Considering these findings together, the researchers concluded that VLRB MM3 represents a unique tool that might aid the treatment and diagnosis of plasma cell disorders. ![]()

Researchers say they have generated a monoclonal antibody that could have diagnostic and therapeutic applications for multiple myeloma (MM) and other plasma cell disorders.
The team generated this antibody, VLRB MM3, from immunized lampreys, a type of jawless fish.
Experiments with VLRB MM3 showed that it can identify normal plasma cells in samples from healthy donors and malignant plasma cells in samples from patients with MM.
Götz Ehrhardt, PhD, of the University of Toronto in Ontario, Canada, and his colleagues described this work in JCI Insight.
The researchers noted that antibody-secreting plasma cells arise from B-cell precursors and are essential for adaptive immune responses against invading pathogens. Plasma cell dysfunction is associated with autoimmune and neoplastic disorders, including MM.
Surface markers that are specific to plasma cells have not been identified, and antibodies that only recognize these cells have been challenging to generate using conventional systems.
However, Dr Ehrhardt and his colleagues found they could generate a plasma-cell-specific antibody from immunized lampreys.
The researchers injected lamprey larvae with a bone marrow isolate from an MM patient and screened the resulting monoclonal antibodies for those that recognized both malignant and non-malignant plasma cells.
Further characterization of the antibody VLRB MM3 revealed that it is specific to plasma cells and does not recognize other B-cell populations or progenitors.
VLRB MM3 binding was shown to coincide with CD38 dimerization and correlate with and impede the NAD glycohydrolase activity of this glycoprotein.
Considering these findings together, the researchers concluded that VLRB MM3 represents a unique tool that might aid the treatment and diagnosis of plasma cell disorders. ![]()
Financial burdens reduce QOL for cancer survivors

receiving treatment
Photo by Rhoda Baer
An analysis of nearly 20 million cancer survivors showed that almost 29% had financial burdens as a result of their cancer diagnosis and/or treatment.
In other words, they borrowed money, declared bankruptcy, worried about paying large medical bills, were unable to cover the cost of medical visits, or made other financial sacrifices.
Furthermore, such hardships could have lasting effects on a cancer survivor’s quality of life (QOL).
Hrishikesh Kale and Norman Carroll, PhD, both of Virginia Commonwealth University School of Pharmacy in Richmond, reported these findings in Cancer.
The pair analyzed 2011 Medical Expenditure Panel Survey data on 19.6 million cancer survivors, assessing financial burden and QOL.
Subjects were considered to have financial burden if they reported 1 of the following problems: borrowed money/declared bankruptcy, worried about paying large medical bills, unable to cover the cost of medical care visits, or other financial sacrifices.
Nearly 29% of the cancer survivors reported at least 1 financial problem resulting from cancer diagnosis, treatment, or lasting effects of that treatment.
Of all the cancer survivors in the analysis, 20.9% worried about paying large medical bills, 11.5% were unable to cover the cost of medical care visits, 7.6% reported borrowing money or going into debt, 1.4% declared bankruptcy, and 8.6% reported other financial sacrifices.
Cancer survivors who faced such financial difficulties had lower physical and mental health-related QOL, higher risk for depressed mood and psychological distress, and were more likely to worry about cancer recurrence, when compared with cancer survivors who did not face financial problems.
In addition, as the number of financial problems reported by cancer survivors increased, their QOL continued to decrease. And their risk for depressed mood, psychological distress, and worries about cancer recurrence continued to increase.
“Our results suggest that policies and practices that minimize cancer patients’ out-of-pocket costs can improve survivors’ health-related quality of life and psychological health,” Dr Carroll said.
“Reducing the financial burden of cancer care requires integrated efforts, and the study findings are useful for survivorship care programs, oncologists, payers, pharmaceutical companies, and patients and their family members.” ![]()

receiving treatment
Photo by Rhoda Baer
An analysis of nearly 20 million cancer survivors showed that almost 29% had financial burdens as a result of their cancer diagnosis and/or treatment.
In other words, they borrowed money, declared bankruptcy, worried about paying large medical bills, were unable to cover the cost of medical visits, or made other financial sacrifices.
Furthermore, such hardships could have lasting effects on a cancer survivor’s quality of life (QOL).
Hrishikesh Kale and Norman Carroll, PhD, both of Virginia Commonwealth University School of Pharmacy in Richmond, reported these findings in Cancer.
The pair analyzed 2011 Medical Expenditure Panel Survey data on 19.6 million cancer survivors, assessing financial burden and QOL.
Subjects were considered to have financial burden if they reported 1 of the following problems: borrowed money/declared bankruptcy, worried about paying large medical bills, unable to cover the cost of medical care visits, or other financial sacrifices.
Nearly 29% of the cancer survivors reported at least 1 financial problem resulting from cancer diagnosis, treatment, or lasting effects of that treatment.
Of all the cancer survivors in the analysis, 20.9% worried about paying large medical bills, 11.5% were unable to cover the cost of medical care visits, 7.6% reported borrowing money or going into debt, 1.4% declared bankruptcy, and 8.6% reported other financial sacrifices.
Cancer survivors who faced such financial difficulties had lower physical and mental health-related QOL, higher risk for depressed mood and psychological distress, and were more likely to worry about cancer recurrence, when compared with cancer survivors who did not face financial problems.
In addition, as the number of financial problems reported by cancer survivors increased, their QOL continued to decrease. And their risk for depressed mood, psychological distress, and worries about cancer recurrence continued to increase.
“Our results suggest that policies and practices that minimize cancer patients’ out-of-pocket costs can improve survivors’ health-related quality of life and psychological health,” Dr Carroll said.
“Reducing the financial burden of cancer care requires integrated efforts, and the study findings are useful for survivorship care programs, oncologists, payers, pharmaceutical companies, and patients and their family members.” ![]()

receiving treatment
Photo by Rhoda Baer
An analysis of nearly 20 million cancer survivors showed that almost 29% had financial burdens as a result of their cancer diagnosis and/or treatment.
In other words, they borrowed money, declared bankruptcy, worried about paying large medical bills, were unable to cover the cost of medical visits, or made other financial sacrifices.
Furthermore, such hardships could have lasting effects on a cancer survivor’s quality of life (QOL).
Hrishikesh Kale and Norman Carroll, PhD, both of Virginia Commonwealth University School of Pharmacy in Richmond, reported these findings in Cancer.
The pair analyzed 2011 Medical Expenditure Panel Survey data on 19.6 million cancer survivors, assessing financial burden and QOL.
Subjects were considered to have financial burden if they reported 1 of the following problems: borrowed money/declared bankruptcy, worried about paying large medical bills, unable to cover the cost of medical care visits, or other financial sacrifices.
Nearly 29% of the cancer survivors reported at least 1 financial problem resulting from cancer diagnosis, treatment, or lasting effects of that treatment.
Of all the cancer survivors in the analysis, 20.9% worried about paying large medical bills, 11.5% were unable to cover the cost of medical care visits, 7.6% reported borrowing money or going into debt, 1.4% declared bankruptcy, and 8.6% reported other financial sacrifices.
Cancer survivors who faced such financial difficulties had lower physical and mental health-related QOL, higher risk for depressed mood and psychological distress, and were more likely to worry about cancer recurrence, when compared with cancer survivors who did not face financial problems.
In addition, as the number of financial problems reported by cancer survivors increased, their QOL continued to decrease. And their risk for depressed mood, psychological distress, and worries about cancer recurrence continued to increase.
“Our results suggest that policies and practices that minimize cancer patients’ out-of-pocket costs can improve survivors’ health-related quality of life and psychological health,” Dr Carroll said.
“Reducing the financial burden of cancer care requires integrated efforts, and the study findings are useful for survivorship care programs, oncologists, payers, pharmaceutical companies, and patients and their family members.” ![]()
FDA approves drug for 2 indications in MM

Photo courtesy of
Spectrum Pharmaceuticals
The US Food and Drug Administration (FDA) has approved a new formulation of melphalan for injection (Evomela) for 2 indications.
The drug is now approved for use as a high-dose conditioning treatment prior to autologous hematopoietic stem cell transplant (HSCT) in patients with multiple myeloma (MM) and for the palliative treatment of patients with MM for whom oral therapy is not appropriate.
Evomela is the first product to be FDA-approved for the high-dose conditioning indication in MM. The FDA previously granted the drug orphan designation for this indication.
About Evomela
Evomela is a Captisol-enabled, propylene glycol-free melphalan formulation. This formulation eliminates the need to use a propylene glycol-containing custom diluent, which is required with other intravenous melphalan formulations and has been reported to cause renal and cardiac side effects.
Captisol is a chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs.
The use of Captisol technology to reformulate melphalan is reported to improve the drug’s stability, extending its use time. The technology allows the admixture solution to be stable for 4 hours at room temperature, in addition to the 1 hour following reconstitution, and for 24 hours at refrigerated temperature (5°C).
This is anticipated to simplify preparation and administration logistics and allow for slower infusion rates and longer administration durations for pre-transplant chemotherapy.
The full prescribing information for Evomela is available at www.evomela.com.
Spectrum Pharmaceuticals gained global development and commercialization rights to Evomela from Ligand Pharmaceuticals Incorporated in March 2013.
Spectrum assumed responsibility for completing the pivotal phase 2 trial of Evomela and was responsible for filing the new drug application. Spectrum filed the application in December 2014, and the FDA accepted it the following March.
Phase 2 study
Evomela was approved by the FDA based on its bioequivalence to the standard melphalan formulation (Alkeran) in a phase 2 study.
Initial results from this trial (phase 2a) were published in Bone Marrow Transplantation in June 2014. Phase 2b results were published in Biology of Blood and Marrow Transplantation in September 2015.
Phase 2b included 61 patients. Fifty-six had newly diagnosed MM, and 5 had relapsed MM following prior HSCT.
The patients received Evomela at 200 mg/m2, given as 2 doses on day -3 and day -2 prior to autologous HSCT (day 0).
All 61 patients achieved myeloablation at a median of 5 days post-HSCT. And all patients had successful neutrophil and platelet engraftment at a median of 12 days and 13 days post-HSCT, respectively.
Efficacy was assessed by clinical response at day 100. According to investigator assessment, the overall response rate was 95%, and the complete response (CR) rate was 31%.
According to independent pathology review, the overall response rate was 100%, and the CR rate was 21%. The lower rate of confirmed CRs in the independent review was due to missing data.
Treatment-related mortality was 0%, and non-hematologic adverse events were mostly grade 1 and 2 in severity. The incidence of grade 3 mucositis and grade 3 stomatitis were 10% and 5%, respectively, with no grade 4 mucositis or stomatitis reported.
Twenty percent of patients experienced treatment-emergent serious adverse events, most of which were grade 3 and consisted of events commonly reported in patients undergoing myeloablative chemotherapy. No new safety signals were identified. ![]()

Photo courtesy of
Spectrum Pharmaceuticals
The US Food and Drug Administration (FDA) has approved a new formulation of melphalan for injection (Evomela) for 2 indications.
The drug is now approved for use as a high-dose conditioning treatment prior to autologous hematopoietic stem cell transplant (HSCT) in patients with multiple myeloma (MM) and for the palliative treatment of patients with MM for whom oral therapy is not appropriate.
Evomela is the first product to be FDA-approved for the high-dose conditioning indication in MM. The FDA previously granted the drug orphan designation for this indication.
About Evomela
Evomela is a Captisol-enabled, propylene glycol-free melphalan formulation. This formulation eliminates the need to use a propylene glycol-containing custom diluent, which is required with other intravenous melphalan formulations and has been reported to cause renal and cardiac side effects.
Captisol is a chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs.
The use of Captisol technology to reformulate melphalan is reported to improve the drug’s stability, extending its use time. The technology allows the admixture solution to be stable for 4 hours at room temperature, in addition to the 1 hour following reconstitution, and for 24 hours at refrigerated temperature (5°C).
This is anticipated to simplify preparation and administration logistics and allow for slower infusion rates and longer administration durations for pre-transplant chemotherapy.
The full prescribing information for Evomela is available at www.evomela.com.
Spectrum Pharmaceuticals gained global development and commercialization rights to Evomela from Ligand Pharmaceuticals Incorporated in March 2013.
Spectrum assumed responsibility for completing the pivotal phase 2 trial of Evomela and was responsible for filing the new drug application. Spectrum filed the application in December 2014, and the FDA accepted it the following March.
Phase 2 study
Evomela was approved by the FDA based on its bioequivalence to the standard melphalan formulation (Alkeran) in a phase 2 study.
Initial results from this trial (phase 2a) were published in Bone Marrow Transplantation in June 2014. Phase 2b results were published in Biology of Blood and Marrow Transplantation in September 2015.
Phase 2b included 61 patients. Fifty-six had newly diagnosed MM, and 5 had relapsed MM following prior HSCT.
The patients received Evomela at 200 mg/m2, given as 2 doses on day -3 and day -2 prior to autologous HSCT (day 0).
All 61 patients achieved myeloablation at a median of 5 days post-HSCT. And all patients had successful neutrophil and platelet engraftment at a median of 12 days and 13 days post-HSCT, respectively.
Efficacy was assessed by clinical response at day 100. According to investigator assessment, the overall response rate was 95%, and the complete response (CR) rate was 31%.
According to independent pathology review, the overall response rate was 100%, and the CR rate was 21%. The lower rate of confirmed CRs in the independent review was due to missing data.
Treatment-related mortality was 0%, and non-hematologic adverse events were mostly grade 1 and 2 in severity. The incidence of grade 3 mucositis and grade 3 stomatitis were 10% and 5%, respectively, with no grade 4 mucositis or stomatitis reported.
Twenty percent of patients experienced treatment-emergent serious adverse events, most of which were grade 3 and consisted of events commonly reported in patients undergoing myeloablative chemotherapy. No new safety signals were identified. ![]()

Photo courtesy of
Spectrum Pharmaceuticals
The US Food and Drug Administration (FDA) has approved a new formulation of melphalan for injection (Evomela) for 2 indications.
The drug is now approved for use as a high-dose conditioning treatment prior to autologous hematopoietic stem cell transplant (HSCT) in patients with multiple myeloma (MM) and for the palliative treatment of patients with MM for whom oral therapy is not appropriate.
Evomela is the first product to be FDA-approved for the high-dose conditioning indication in MM. The FDA previously granted the drug orphan designation for this indication.
About Evomela
Evomela is a Captisol-enabled, propylene glycol-free melphalan formulation. This formulation eliminates the need to use a propylene glycol-containing custom diluent, which is required with other intravenous melphalan formulations and has been reported to cause renal and cardiac side effects.
Captisol is a chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs.
The use of Captisol technology to reformulate melphalan is reported to improve the drug’s stability, extending its use time. The technology allows the admixture solution to be stable for 4 hours at room temperature, in addition to the 1 hour following reconstitution, and for 24 hours at refrigerated temperature (5°C).
This is anticipated to simplify preparation and administration logistics and allow for slower infusion rates and longer administration durations for pre-transplant chemotherapy.
The full prescribing information for Evomela is available at www.evomela.com.
Spectrum Pharmaceuticals gained global development and commercialization rights to Evomela from Ligand Pharmaceuticals Incorporated in March 2013.
Spectrum assumed responsibility for completing the pivotal phase 2 trial of Evomela and was responsible for filing the new drug application. Spectrum filed the application in December 2014, and the FDA accepted it the following March.
Phase 2 study
Evomela was approved by the FDA based on its bioequivalence to the standard melphalan formulation (Alkeran) in a phase 2 study.
Initial results from this trial (phase 2a) were published in Bone Marrow Transplantation in June 2014. Phase 2b results were published in Biology of Blood and Marrow Transplantation in September 2015.
Phase 2b included 61 patients. Fifty-six had newly diagnosed MM, and 5 had relapsed MM following prior HSCT.
The patients received Evomela at 200 mg/m2, given as 2 doses on day -3 and day -2 prior to autologous HSCT (day 0).
All 61 patients achieved myeloablation at a median of 5 days post-HSCT. And all patients had successful neutrophil and platelet engraftment at a median of 12 days and 13 days post-HSCT, respectively.
Efficacy was assessed by clinical response at day 100. According to investigator assessment, the overall response rate was 95%, and the complete response (CR) rate was 31%.
According to independent pathology review, the overall response rate was 100%, and the CR rate was 21%. The lower rate of confirmed CRs in the independent review was due to missing data.
Treatment-related mortality was 0%, and non-hematologic adverse events were mostly grade 1 and 2 in severity. The incidence of grade 3 mucositis and grade 3 stomatitis were 10% and 5%, respectively, with no grade 4 mucositis or stomatitis reported.
Twenty percent of patients experienced treatment-emergent serious adverse events, most of which were grade 3 and consisted of events commonly reported in patients undergoing myeloablative chemotherapy. No new safety signals were identified. ![]()
FDA approves propylene glycol–free melphalan for multiple myeloma
The Food and Drug Administration has approved a new propylene glycol–free formulation of melphalan hydrochloride as a high-dose conditioning treatment for autologous stem cell transplantation, and as a palliative therapy for multiple myeloma.
Evomela (Spectrum Pharmaceuticals) is intended to be reconstituted with normal saline at the time of intravenous administration. The solution is stable for 4 hours using Captisol, a proprietary agent containing modified cyclodextrin. It is also stable for 1 hour after reconstitution.
Extended stability without the need for propylene glycol is its major advantage over the other formulations of melphalan, a chemotherapy agent originally approved in 1964, according to a statement by Spectrum.
Evomela was approved on the basis of a phase IIa pharmacokinetic trial comprising 24 patients undergoing autologous stem cell transplantation. Evomela was bioequivalent with Alkeran, with a 10% higher maximum plasma concentration and area under the plasma concentration-time curve (Bone Marrow Transplant. 2014 Aug;49[8]:1042-5).
According to that, and other studies, adverse reactions included decreased neutrophil count (100%), decreased white blood cell count (100%), decreased lymphocyte count (98%), decreased platelet count (98%), diarrhea (93%), nausea (90%), fatigue (77%), hypokalemia (74%), anemia (66%), and vomiting (64%). About 2% of patients have experienced hypersensitivity reactions.
For palliative treatment, the recommended does is 16 mg/m2 infused over 15-20 minutes at 2-week intervals for four doses, and then, after adequate recovery from toxicity, at 4-week intervals.
For conditioning treatment, the recommended does is 100 mg/m2 per day infused over 30 minutes for 2 consecutive days before stem cell transplant.
The Food and Drug Administration has approved a new propylene glycol–free formulation of melphalan hydrochloride as a high-dose conditioning treatment for autologous stem cell transplantation, and as a palliative therapy for multiple myeloma.
Evomela (Spectrum Pharmaceuticals) is intended to be reconstituted with normal saline at the time of intravenous administration. The solution is stable for 4 hours using Captisol, a proprietary agent containing modified cyclodextrin. It is also stable for 1 hour after reconstitution.
Extended stability without the need for propylene glycol is its major advantage over the other formulations of melphalan, a chemotherapy agent originally approved in 1964, according to a statement by Spectrum.
Evomela was approved on the basis of a phase IIa pharmacokinetic trial comprising 24 patients undergoing autologous stem cell transplantation. Evomela was bioequivalent with Alkeran, with a 10% higher maximum plasma concentration and area under the plasma concentration-time curve (Bone Marrow Transplant. 2014 Aug;49[8]:1042-5).
According to that, and other studies, adverse reactions included decreased neutrophil count (100%), decreased white blood cell count (100%), decreased lymphocyte count (98%), decreased platelet count (98%), diarrhea (93%), nausea (90%), fatigue (77%), hypokalemia (74%), anemia (66%), and vomiting (64%). About 2% of patients have experienced hypersensitivity reactions.
For palliative treatment, the recommended does is 16 mg/m2 infused over 15-20 minutes at 2-week intervals for four doses, and then, after adequate recovery from toxicity, at 4-week intervals.
For conditioning treatment, the recommended does is 100 mg/m2 per day infused over 30 minutes for 2 consecutive days before stem cell transplant.
The Food and Drug Administration has approved a new propylene glycol–free formulation of melphalan hydrochloride as a high-dose conditioning treatment for autologous stem cell transplantation, and as a palliative therapy for multiple myeloma.
Evomela (Spectrum Pharmaceuticals) is intended to be reconstituted with normal saline at the time of intravenous administration. The solution is stable for 4 hours using Captisol, a proprietary agent containing modified cyclodextrin. It is also stable for 1 hour after reconstitution.
Extended stability without the need for propylene glycol is its major advantage over the other formulations of melphalan, a chemotherapy agent originally approved in 1964, according to a statement by Spectrum.
Evomela was approved on the basis of a phase IIa pharmacokinetic trial comprising 24 patients undergoing autologous stem cell transplantation. Evomela was bioequivalent with Alkeran, with a 10% higher maximum plasma concentration and area under the plasma concentration-time curve (Bone Marrow Transplant. 2014 Aug;49[8]:1042-5).
According to that, and other studies, adverse reactions included decreased neutrophil count (100%), decreased white blood cell count (100%), decreased lymphocyte count (98%), decreased platelet count (98%), diarrhea (93%), nausea (90%), fatigue (77%), hypokalemia (74%), anemia (66%), and vomiting (64%). About 2% of patients have experienced hypersensitivity reactions.
For palliative treatment, the recommended does is 16 mg/m2 infused over 15-20 minutes at 2-week intervals for four doses, and then, after adequate recovery from toxicity, at 4-week intervals.
For conditioning treatment, the recommended does is 100 mg/m2 per day infused over 30 minutes for 2 consecutive days before stem cell transplant.
IMWG issues renal impairment recommendations for myeloma patients
The International Myeloma Working Group has issued new recommendations for the diagnosis and management of multiple myeloma–related renal impairment. Depending on whether the condition is defined as elevated serum creatinine or decreased estimated glomerular filtration rate (eGFR), an estimated 20%-50% of patients with multiple myeloma have renal impairment at the time of diagnosis.
The guidelines recommend that all patients with multiple myeloma (MM) at diagnosis and at disease assessment should be tested for serum creatinine, eGFR, electrolytes, and serum free light chain, if available. Additionally, they recommend that all patients have urine electrophoresis of a sample from a 24-hour urine collection. All of the above are grade A recommendations (J Clin Oncol. 2016 Mar 14. doi: 10.1200/JCO.2015.65.0044).
Patients with nonselective proteinuria or significant albuminuria should undergo renal biopsy to determine the cause of the underlying impairment, the IMWG says (grade B recommendation).
For evaluation of eGFR in patients with stabilized serum creatinine, the IMWG favors the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, but also acknowledges that eGFR can be assessed with the Modification of Diet in Renal Disease (MDRD) formula (grade A).
“CKD-EPI seems to more accurately reflect GFR than does MDRD, mostly in higher levels of GFR,” the IMWG wrote.
Because the reversibility of renal dysfunction can affect treatment choice, the recommendations noted that for patients on dialysis, achieving independence from dialysis is “strong indication of improvement. For all other patients, IMWG criteria for renal response to therapy are recommended (grade B).
Management
“Acute renal impairment is a myeloma emergency. Diagnosis should be established as fast as possible, and antimyeloma therapy should be started immediately after confirmation of diagnosis to rapidly restore renal function,” working group members wrote.
Supportive care with increased hydration – at least 3 liters per day – is “mandatory” for all with suspected MM-related renal impairment, they add.
The recommendations also noted that antimyeloma therapy should be initiated immediately to reduce the load of toxic serum free light chains, which can help to improve renal function.
“Bortezomib [Velcade]-based regimens remain the cornerstone of the management of myeloma-related renal impairment (grade A). High-dose dexamethasone should be administered at least for the first month of therapy (grade B),” the working group members wrote.
Lenalidomide (Revlimid) can be given, but because it is excreted through the kidneys, the dose must be adjusted according to the degree of renal impairment. In contrast, thalidomide is not excreted and does not require dose modification in this population.
Patients who are eligible for autologous stem cell transplant could receive bortezomib in a three-drug regimen with thalidomide and dexamethasone, or in combination with a conventional chemotherapeutic agent, either doxorubicin or cyclophosphamide. Patients who are not eligible for transplant can be treated with bortezomib, melphalan, and prednisone, the recommendations said, but add that there are no data on the use of this regimen in patients who are on dialysis.
Regarding newer proteasome inhibitors, the guidelines note that carfilzomib (Kyprolis) can be given safely to patients with creatinine clearance above 15 mL/min, and that the recently approved oral agent, ixazomib (Ninlaro), with lenalidomide and dexamethasone can be administered to patients with clearance rates above 30 mL/min (grade A).
The International Myeloma Working Group has issued new recommendations for the diagnosis and management of multiple myeloma–related renal impairment. Depending on whether the condition is defined as elevated serum creatinine or decreased estimated glomerular filtration rate (eGFR), an estimated 20%-50% of patients with multiple myeloma have renal impairment at the time of diagnosis.
The guidelines recommend that all patients with multiple myeloma (MM) at diagnosis and at disease assessment should be tested for serum creatinine, eGFR, electrolytes, and serum free light chain, if available. Additionally, they recommend that all patients have urine electrophoresis of a sample from a 24-hour urine collection. All of the above are grade A recommendations (J Clin Oncol. 2016 Mar 14. doi: 10.1200/JCO.2015.65.0044).
Patients with nonselective proteinuria or significant albuminuria should undergo renal biopsy to determine the cause of the underlying impairment, the IMWG says (grade B recommendation).
For evaluation of eGFR in patients with stabilized serum creatinine, the IMWG favors the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, but also acknowledges that eGFR can be assessed with the Modification of Diet in Renal Disease (MDRD) formula (grade A).
“CKD-EPI seems to more accurately reflect GFR than does MDRD, mostly in higher levels of GFR,” the IMWG wrote.
Because the reversibility of renal dysfunction can affect treatment choice, the recommendations noted that for patients on dialysis, achieving independence from dialysis is “strong indication of improvement. For all other patients, IMWG criteria for renal response to therapy are recommended (grade B).
Management
“Acute renal impairment is a myeloma emergency. Diagnosis should be established as fast as possible, and antimyeloma therapy should be started immediately after confirmation of diagnosis to rapidly restore renal function,” working group members wrote.
Supportive care with increased hydration – at least 3 liters per day – is “mandatory” for all with suspected MM-related renal impairment, they add.
The recommendations also noted that antimyeloma therapy should be initiated immediately to reduce the load of toxic serum free light chains, which can help to improve renal function.
“Bortezomib [Velcade]-based regimens remain the cornerstone of the management of myeloma-related renal impairment (grade A). High-dose dexamethasone should be administered at least for the first month of therapy (grade B),” the working group members wrote.
Lenalidomide (Revlimid) can be given, but because it is excreted through the kidneys, the dose must be adjusted according to the degree of renal impairment. In contrast, thalidomide is not excreted and does not require dose modification in this population.
Patients who are eligible for autologous stem cell transplant could receive bortezomib in a three-drug regimen with thalidomide and dexamethasone, or in combination with a conventional chemotherapeutic agent, either doxorubicin or cyclophosphamide. Patients who are not eligible for transplant can be treated with bortezomib, melphalan, and prednisone, the recommendations said, but add that there are no data on the use of this regimen in patients who are on dialysis.
Regarding newer proteasome inhibitors, the guidelines note that carfilzomib (Kyprolis) can be given safely to patients with creatinine clearance above 15 mL/min, and that the recently approved oral agent, ixazomib (Ninlaro), with lenalidomide and dexamethasone can be administered to patients with clearance rates above 30 mL/min (grade A).
The International Myeloma Working Group has issued new recommendations for the diagnosis and management of multiple myeloma–related renal impairment. Depending on whether the condition is defined as elevated serum creatinine or decreased estimated glomerular filtration rate (eGFR), an estimated 20%-50% of patients with multiple myeloma have renal impairment at the time of diagnosis.
The guidelines recommend that all patients with multiple myeloma (MM) at diagnosis and at disease assessment should be tested for serum creatinine, eGFR, electrolytes, and serum free light chain, if available. Additionally, they recommend that all patients have urine electrophoresis of a sample from a 24-hour urine collection. All of the above are grade A recommendations (J Clin Oncol. 2016 Mar 14. doi: 10.1200/JCO.2015.65.0044).
Patients with nonselective proteinuria or significant albuminuria should undergo renal biopsy to determine the cause of the underlying impairment, the IMWG says (grade B recommendation).
For evaluation of eGFR in patients with stabilized serum creatinine, the IMWG favors the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, but also acknowledges that eGFR can be assessed with the Modification of Diet in Renal Disease (MDRD) formula (grade A).
“CKD-EPI seems to more accurately reflect GFR than does MDRD, mostly in higher levels of GFR,” the IMWG wrote.
Because the reversibility of renal dysfunction can affect treatment choice, the recommendations noted that for patients on dialysis, achieving independence from dialysis is “strong indication of improvement. For all other patients, IMWG criteria for renal response to therapy are recommended (grade B).
Management
“Acute renal impairment is a myeloma emergency. Diagnosis should be established as fast as possible, and antimyeloma therapy should be started immediately after confirmation of diagnosis to rapidly restore renal function,” working group members wrote.
Supportive care with increased hydration – at least 3 liters per day – is “mandatory” for all with suspected MM-related renal impairment, they add.
The recommendations also noted that antimyeloma therapy should be initiated immediately to reduce the load of toxic serum free light chains, which can help to improve renal function.
“Bortezomib [Velcade]-based regimens remain the cornerstone of the management of myeloma-related renal impairment (grade A). High-dose dexamethasone should be administered at least for the first month of therapy (grade B),” the working group members wrote.
Lenalidomide (Revlimid) can be given, but because it is excreted through the kidneys, the dose must be adjusted according to the degree of renal impairment. In contrast, thalidomide is not excreted and does not require dose modification in this population.
Patients who are eligible for autologous stem cell transplant could receive bortezomib in a three-drug regimen with thalidomide and dexamethasone, or in combination with a conventional chemotherapeutic agent, either doxorubicin or cyclophosphamide. Patients who are not eligible for transplant can be treated with bortezomib, melphalan, and prednisone, the recommendations said, but add that there are no data on the use of this regimen in patients who are on dialysis.
Regarding newer proteasome inhibitors, the guidelines note that carfilzomib (Kyprolis) can be given safely to patients with creatinine clearance above 15 mL/min, and that the recently approved oral agent, ixazomib (Ninlaro), with lenalidomide and dexamethasone can be administered to patients with clearance rates above 30 mL/min (grade A).
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: All patients diagnosed with multiple myeloma should be evaluated for renal impairment.
Major finding: Bortezomib-based regimens are the standard of care for patients with multiple myeloma.
Data source: Evidence-based clinical recommendations.
Disclosures: Many coauthors disclosed multiple relationships with companies that make antimyeloma therapies and other medications.
Lenalidomide, thalidomide in melphalan regimen yields similar progression-free survival
Swapping lenalidomide for thalidomide in a standard regimen for transplant-ineligible patients with untreated multiple myeloma did not improve efficacy, but the toxicity profile may favor the use of lenalidomide in a maintenance regimen, results of a randomized trial suggest.
Among patients with previously untreated multiple myeloma who were not eligible for autologous stem cell transplant, neither median progression-free survival (PFS) nor overall survival (OS) were significantly different for patients treated with either melphalan, prednisone, and thalidomide (MPT-T) followed by thalidomide maintenance, or with the same regimen with lenalidomide (Revlimid) substituted for thalidomide (MPR-R), reported Dr. Sonja Zweegman of Vrije University Medical Center in Amsterdam.
“MPR-R has no advantage over MPT-T with respect to response rate, PFS, and OS. However, the use of thalidomide as maintenance therapy was associated with a high rate of clinically significant neuropathy and is therefore not preferred for maintenance strategies,” they wrote (Blood 2016;127[9];1109-16).
The investigators randomly assigned 637 transplant-ineligible patients with newly-diagnosed multiple myeloma to receive nine 4-week cycles of either MPT-T (318 patients) or MPR-R (319 patients). At 36 months’ median follow-up, median PFS, the primary endpoint, was 20 months for patients treated with MPT-T, compared with 23 months for those treated with MPR-R. This translated into a hazard ratio (HR) of 0.87, P = .12).
The overall response rates were 81% for MPT-T and 84% for MPR-R. Very good partial responses or better were seen in 47% and 45%, of patients, respectively. The complete response rate with MPT-T was 10%, compared with 13% for MPR-R. Median time to response and time to maximum response were similar between the arms.
OS at 2, 3, and 4 years in the MPT-T and MPR-R arms was 73% vs. 84%, 64% vs 69%, and 52% vs. 56%, respectively. These differences were not statistically significant.
The proportion of patients with one or more grade 3 or 4 adverse events was 81% with thalidomide and 86% with lenalidomide.
The investigators noted, however, that there was a high rate of discontinuation during induction therapy in each arm, with 49% of those starting on MPT-T and 41% of those starting on MPR-R halting therapy. Most of the patients who discontinued were older than age 75. Early treatment deaths (within three cycles) occurred in 13 patients on MPT-T, and 8 on MPR-R.
Among patients who started on maintenance therapy, significantly more patients on thalidomide had to discontinue thalidomide than did patients who started on lenalidomide maintenance (60% vs, 17%, P = .017).
The primary reason for the higher rate of discontinuation of MPT-T maintenance was neuropathy, which occurred in 87% of the discontinuations in this study arm, compared with 3% of those in the lenalidomide arm. Neuropathy of at least grade 3 was 16% in the MPT-T arm vs. 2% in MPR-R, resulting in a significantly shorter duration of maintenance therapy (5 vs. 17 months in MPR-R), irrespective of age.
Hematologic toxicities were higher in the MPR-R group, especially grades 3 and 4 neutropenia (64% vs 27%), but this did not translate into a higher clinical infection rate, and the toxicities were manageable in older patients, the investigators reported.
Swapping lenalidomide for thalidomide in a standard regimen for transplant-ineligible patients with untreated multiple myeloma did not improve efficacy, but the toxicity profile may favor the use of lenalidomide in a maintenance regimen, results of a randomized trial suggest.
Among patients with previously untreated multiple myeloma who were not eligible for autologous stem cell transplant, neither median progression-free survival (PFS) nor overall survival (OS) were significantly different for patients treated with either melphalan, prednisone, and thalidomide (MPT-T) followed by thalidomide maintenance, or with the same regimen with lenalidomide (Revlimid) substituted for thalidomide (MPR-R), reported Dr. Sonja Zweegman of Vrije University Medical Center in Amsterdam.
“MPR-R has no advantage over MPT-T with respect to response rate, PFS, and OS. However, the use of thalidomide as maintenance therapy was associated with a high rate of clinically significant neuropathy and is therefore not preferred for maintenance strategies,” they wrote (Blood 2016;127[9];1109-16).
The investigators randomly assigned 637 transplant-ineligible patients with newly-diagnosed multiple myeloma to receive nine 4-week cycles of either MPT-T (318 patients) or MPR-R (319 patients). At 36 months’ median follow-up, median PFS, the primary endpoint, was 20 months for patients treated with MPT-T, compared with 23 months for those treated with MPR-R. This translated into a hazard ratio (HR) of 0.87, P = .12).
The overall response rates were 81% for MPT-T and 84% for MPR-R. Very good partial responses or better were seen in 47% and 45%, of patients, respectively. The complete response rate with MPT-T was 10%, compared with 13% for MPR-R. Median time to response and time to maximum response were similar between the arms.
OS at 2, 3, and 4 years in the MPT-T and MPR-R arms was 73% vs. 84%, 64% vs 69%, and 52% vs. 56%, respectively. These differences were not statistically significant.
The proportion of patients with one or more grade 3 or 4 adverse events was 81% with thalidomide and 86% with lenalidomide.
The investigators noted, however, that there was a high rate of discontinuation during induction therapy in each arm, with 49% of those starting on MPT-T and 41% of those starting on MPR-R halting therapy. Most of the patients who discontinued were older than age 75. Early treatment deaths (within three cycles) occurred in 13 patients on MPT-T, and 8 on MPR-R.
Among patients who started on maintenance therapy, significantly more patients on thalidomide had to discontinue thalidomide than did patients who started on lenalidomide maintenance (60% vs, 17%, P = .017).
The primary reason for the higher rate of discontinuation of MPT-T maintenance was neuropathy, which occurred in 87% of the discontinuations in this study arm, compared with 3% of those in the lenalidomide arm. Neuropathy of at least grade 3 was 16% in the MPT-T arm vs. 2% in MPR-R, resulting in a significantly shorter duration of maintenance therapy (5 vs. 17 months in MPR-R), irrespective of age.
Hematologic toxicities were higher in the MPR-R group, especially grades 3 and 4 neutropenia (64% vs 27%), but this did not translate into a higher clinical infection rate, and the toxicities were manageable in older patients, the investigators reported.
Swapping lenalidomide for thalidomide in a standard regimen for transplant-ineligible patients with untreated multiple myeloma did not improve efficacy, but the toxicity profile may favor the use of lenalidomide in a maintenance regimen, results of a randomized trial suggest.
Among patients with previously untreated multiple myeloma who were not eligible for autologous stem cell transplant, neither median progression-free survival (PFS) nor overall survival (OS) were significantly different for patients treated with either melphalan, prednisone, and thalidomide (MPT-T) followed by thalidomide maintenance, or with the same regimen with lenalidomide (Revlimid) substituted for thalidomide (MPR-R), reported Dr. Sonja Zweegman of Vrije University Medical Center in Amsterdam.
“MPR-R has no advantage over MPT-T with respect to response rate, PFS, and OS. However, the use of thalidomide as maintenance therapy was associated with a high rate of clinically significant neuropathy and is therefore not preferred for maintenance strategies,” they wrote (Blood 2016;127[9];1109-16).
The investigators randomly assigned 637 transplant-ineligible patients with newly-diagnosed multiple myeloma to receive nine 4-week cycles of either MPT-T (318 patients) or MPR-R (319 patients). At 36 months’ median follow-up, median PFS, the primary endpoint, was 20 months for patients treated with MPT-T, compared with 23 months for those treated with MPR-R. This translated into a hazard ratio (HR) of 0.87, P = .12).
The overall response rates were 81% for MPT-T and 84% for MPR-R. Very good partial responses or better were seen in 47% and 45%, of patients, respectively. The complete response rate with MPT-T was 10%, compared with 13% for MPR-R. Median time to response and time to maximum response were similar between the arms.
OS at 2, 3, and 4 years in the MPT-T and MPR-R arms was 73% vs. 84%, 64% vs 69%, and 52% vs. 56%, respectively. These differences were not statistically significant.
The proportion of patients with one or more grade 3 or 4 adverse events was 81% with thalidomide and 86% with lenalidomide.
The investigators noted, however, that there was a high rate of discontinuation during induction therapy in each arm, with 49% of those starting on MPT-T and 41% of those starting on MPR-R halting therapy. Most of the patients who discontinued were older than age 75. Early treatment deaths (within three cycles) occurred in 13 patients on MPT-T, and 8 on MPR-R.
Among patients who started on maintenance therapy, significantly more patients on thalidomide had to discontinue thalidomide than did patients who started on lenalidomide maintenance (60% vs, 17%, P = .017).
The primary reason for the higher rate of discontinuation of MPT-T maintenance was neuropathy, which occurred in 87% of the discontinuations in this study arm, compared with 3% of those in the lenalidomide arm. Neuropathy of at least grade 3 was 16% in the MPT-T arm vs. 2% in MPR-R, resulting in a significantly shorter duration of maintenance therapy (5 vs. 17 months in MPR-R), irrespective of age.
Hematologic toxicities were higher in the MPR-R group, especially grades 3 and 4 neutropenia (64% vs 27%), but this did not translate into a higher clinical infection rate, and the toxicities were manageable in older patients, the investigators reported.
FROM BLOOD
Key clinical point: Lenalidomide or thalidomide added to a melphalan backbone regimen yielded similar outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma.
Major finding: At 36 months median follow-up, median PFS was 20 months for patients treated with thalidomide, compared with 23 months for those treated with lenalidomide.
Data source: Randomized controlled trial of 637 patients with multiple myeloma who were not candidates for autologous stem cell transplantation.
Disclosures: The Dutch Cancer Society, Norwegian Cancer Society, and Celgene supported the study. Dr. Zweegman and other colleagues reported financial relationships with Celgene.
Lenalidomide-dexamethasone yields similar PFS as triplet regimens in elderly multiple myeloma patients
A comparison of lenalidomide-based treatments for multiple myeloma patients who were ineligible for stem cell transplantation showed similar progression-free survival (PFS) for two alkylator-containing triplet regimens and an alkylator-free doublet regimen but a higher risk of hematologic toxicity with a melphalan-prednisone-lenalidomide regimen.
For the triplet regimens, melphalan-prednisone-lenalidomide (MPR) and cyclophosphamide-prednisone-lenalidomide (CPR), the median PFS was 22 months, compared with 21 months for the doublet regimen lenalidomide plus low-dose dexamethasone (Rd). The hazard ratio (HR) was 0.906 (95% CI, 0.739-1.11; P = .344). The 4-year overall survival (OS) was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709) (Blood. 2016;127[9]:1102-8).
The major safety concern, according to the researchers, was the higher toxicity with MPR compared with CPR and Rd. The most frequent toxicities of grade 3 or more were hematologic, with at least one reported event in 68% of the MPR arm, 32% of CPR, and 29% or Rd patients (P less than .0001). In a post hoc analysis of safety according to patient fitness, the incidence of at least one hematologic adverse event ocurred in 75% of fit patients in the MPR arm occurred, 34% in CPR, and 29% in Rd; the incidence of at least one hematologic adverse event in intermediate fitness patients in the MPR arm was 61%, 33% in CPR, and 25% in Rd; in frail patients, 75% in MPR, 28% in CPR, and 3% in Rd (P = .001 for MPR vs. Rd and MPR vs. CPR). Nonhematologic adverse events were similar for the three groups and less than 10%.
Previous studies, such as the FIRST trial, showed the superiority of lenalidomide-containing regimens over standard treatments, but a question remained over the best drug to combine with lenalidomide – an alkylating agent or steroid. Separate analysis of the three arms further illustrated that the addition of an alkylating agent did not lead to better response or outcome. Median PFS for MPR, CPR, and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively; overall response rates were 71%, 68%, and 74%, respectively.
Compared with the previous FIRST study, the less intense regimen in this study (Rd administered for only 9 months as induction treatment, followed by maintenance with lenalidomide at a lower dose) resulted in less hematological toxicity.
“This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability,” wrote Dr. Valeria Magarotto of the myeloma unit in the division of hematology at the University of Torino (Italy), and colleagues. They added, “A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.”
The phase III trial included 654 patients with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation due to advanced age (65 years and older) or comorbidities. Patients were randomized to receive MPR (n = 217), CPR (n = 220), or Rd (n = 217).
A comparison of lenalidomide-based treatments for multiple myeloma patients who were ineligible for stem cell transplantation showed similar progression-free survival (PFS) for two alkylator-containing triplet regimens and an alkylator-free doublet regimen but a higher risk of hematologic toxicity with a melphalan-prednisone-lenalidomide regimen.
For the triplet regimens, melphalan-prednisone-lenalidomide (MPR) and cyclophosphamide-prednisone-lenalidomide (CPR), the median PFS was 22 months, compared with 21 months for the doublet regimen lenalidomide plus low-dose dexamethasone (Rd). The hazard ratio (HR) was 0.906 (95% CI, 0.739-1.11; P = .344). The 4-year overall survival (OS) was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709) (Blood. 2016;127[9]:1102-8).
The major safety concern, according to the researchers, was the higher toxicity with MPR compared with CPR and Rd. The most frequent toxicities of grade 3 or more were hematologic, with at least one reported event in 68% of the MPR arm, 32% of CPR, and 29% or Rd patients (P less than .0001). In a post hoc analysis of safety according to patient fitness, the incidence of at least one hematologic adverse event ocurred in 75% of fit patients in the MPR arm occurred, 34% in CPR, and 29% in Rd; the incidence of at least one hematologic adverse event in intermediate fitness patients in the MPR arm was 61%, 33% in CPR, and 25% in Rd; in frail patients, 75% in MPR, 28% in CPR, and 3% in Rd (P = .001 for MPR vs. Rd and MPR vs. CPR). Nonhematologic adverse events were similar for the three groups and less than 10%.
Previous studies, such as the FIRST trial, showed the superiority of lenalidomide-containing regimens over standard treatments, but a question remained over the best drug to combine with lenalidomide – an alkylating agent or steroid. Separate analysis of the three arms further illustrated that the addition of an alkylating agent did not lead to better response or outcome. Median PFS for MPR, CPR, and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively; overall response rates were 71%, 68%, and 74%, respectively.
Compared with the previous FIRST study, the less intense regimen in this study (Rd administered for only 9 months as induction treatment, followed by maintenance with lenalidomide at a lower dose) resulted in less hematological toxicity.
“This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability,” wrote Dr. Valeria Magarotto of the myeloma unit in the division of hematology at the University of Torino (Italy), and colleagues. They added, “A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.”
The phase III trial included 654 patients with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation due to advanced age (65 years and older) or comorbidities. Patients were randomized to receive MPR (n = 217), CPR (n = 220), or Rd (n = 217).
A comparison of lenalidomide-based treatments for multiple myeloma patients who were ineligible for stem cell transplantation showed similar progression-free survival (PFS) for two alkylator-containing triplet regimens and an alkylator-free doublet regimen but a higher risk of hematologic toxicity with a melphalan-prednisone-lenalidomide regimen.
For the triplet regimens, melphalan-prednisone-lenalidomide (MPR) and cyclophosphamide-prednisone-lenalidomide (CPR), the median PFS was 22 months, compared with 21 months for the doublet regimen lenalidomide plus low-dose dexamethasone (Rd). The hazard ratio (HR) was 0.906 (95% CI, 0.739-1.11; P = .344). The 4-year overall survival (OS) was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709) (Blood. 2016;127[9]:1102-8).
The major safety concern, according to the researchers, was the higher toxicity with MPR compared with CPR and Rd. The most frequent toxicities of grade 3 or more were hematologic, with at least one reported event in 68% of the MPR arm, 32% of CPR, and 29% or Rd patients (P less than .0001). In a post hoc analysis of safety according to patient fitness, the incidence of at least one hematologic adverse event ocurred in 75% of fit patients in the MPR arm occurred, 34% in CPR, and 29% in Rd; the incidence of at least one hematologic adverse event in intermediate fitness patients in the MPR arm was 61%, 33% in CPR, and 25% in Rd; in frail patients, 75% in MPR, 28% in CPR, and 3% in Rd (P = .001 for MPR vs. Rd and MPR vs. CPR). Nonhematologic adverse events were similar for the three groups and less than 10%.
Previous studies, such as the FIRST trial, showed the superiority of lenalidomide-containing regimens over standard treatments, but a question remained over the best drug to combine with lenalidomide – an alkylating agent or steroid. Separate analysis of the three arms further illustrated that the addition of an alkylating agent did not lead to better response or outcome. Median PFS for MPR, CPR, and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively; overall response rates were 71%, 68%, and 74%, respectively.
Compared with the previous FIRST study, the less intense regimen in this study (Rd administered for only 9 months as induction treatment, followed by maintenance with lenalidomide at a lower dose) resulted in less hematological toxicity.
“This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability,” wrote Dr. Valeria Magarotto of the myeloma unit in the division of hematology at the University of Torino (Italy), and colleagues. They added, “A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.”
The phase III trial included 654 patients with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation due to advanced age (65 years and older) or comorbidities. Patients were randomized to receive MPR (n = 217), CPR (n = 220), or Rd (n = 217).
FROM BLOOD
Key clinical point: In elderly patients with newly diagnosed multiple myeloma, progression-free survival was similar for alkylator-containing triplet regimens and an alkylator-free doublet regimen, but the doublet resulted in less hematologic toxicity.
Major finding: Median PFS for MPR, CPR and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively.
Data sources: Phase III trial of 654 patients randomized to receive melphalan-prednisone-lenalidomide (n = 217), cyclophosphamide-prednisone-lenalidomide (n = 220), or lenalidomide plus low-dose dexamethasone (n = 217).
Disclosures: Dr. Magarotto reported having no disclosures. Several of her coauthors reported financial ties to industry sources.