User login
Class of drugs could treat B-cell malignancies
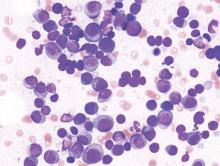
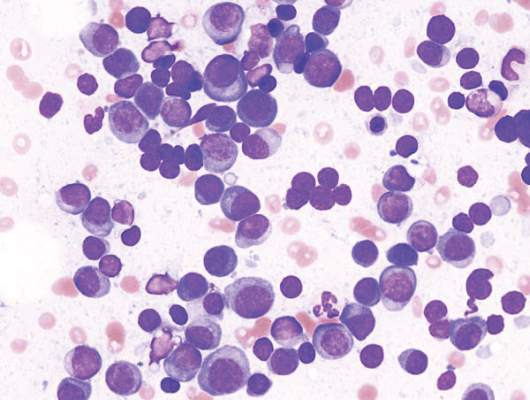
A class of drugs targeting a protein found in the endoplasmic reticulum could be effective against B-cell malignancies, according to a study published in Cancer Research.
The protein, STING, plays a critical role in producing type I interferons that help regulate the immune system.
Previous research suggested that STING agonists can improve immune responses when used in cancer immunotherapy or as vaccine adjuvants.
However, the way B cells respond to STING agonists was not well understood.
Chih-Chi Andrew Hu, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues conducted a study to gain some insight.
The researchers found that normal B cells respond to STING agonists by undergoing mitochondria-mediated apoptosis, and STING agonists induce apoptosis in
malignant B cells through binding to STING.
STING agonists proved cytotoxic to B-cell leukemia, lymphoma, and multiple myeloma in vitro. But the drugs did not induce apoptosis in solid tumor malignancies or normal T cells.
The research also revealed that the IRE-1/XBP-1 stress response pathway is required for normal STING function. And B-cell leukemia, lymphoma, and myeloma require the IRE-1/XBP-1 pathway to be activated for survival.
Stimulation by STING agonists suppressed the IRE-1/XBP-1 pathway, which increased the level of apoptosis in malignant B cells.
The researchers confirmed these results in animal models, as treatment with STING agonists led to regression of chronic lymphocytic leukemia and multiple myeloma in mice.
“This specific cytotoxicity toward B cells strongly supports the use of STING agonists in the treatment of B-cell hematologic malignancies,” said Chih-Hang Anthony Tang, MD, PhD, of The Wistar Institute.
“We also believe that cytotoxicity in normal B cells can be managed with the administration of intravenous immunoglobulin that can help maintain normal levels of antibodies while treatment is being administered. This is something we plan on studying further.”
The Wistar Institute’s business development team is looking for a development partner for the advancement of novel STING agonists in treating B-cell hematologic malignancies. ![]()

A class of drugs targeting a protein found in the endoplasmic reticulum could be effective against B-cell malignancies, according to a study published in Cancer Research.
The protein, STING, plays a critical role in producing type I interferons that help regulate the immune system.
Previous research suggested that STING agonists can improve immune responses when used in cancer immunotherapy or as vaccine adjuvants.
However, the way B cells respond to STING agonists was not well understood.
Chih-Chi Andrew Hu, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues conducted a study to gain some insight.
The researchers found that normal B cells respond to STING agonists by undergoing mitochondria-mediated apoptosis, and STING agonists induce apoptosis in
malignant B cells through binding to STING.
STING agonists proved cytotoxic to B-cell leukemia, lymphoma, and multiple myeloma in vitro. But the drugs did not induce apoptosis in solid tumor malignancies or normal T cells.
The research also revealed that the IRE-1/XBP-1 stress response pathway is required for normal STING function. And B-cell leukemia, lymphoma, and myeloma require the IRE-1/XBP-1 pathway to be activated for survival.
Stimulation by STING agonists suppressed the IRE-1/XBP-1 pathway, which increased the level of apoptosis in malignant B cells.
The researchers confirmed these results in animal models, as treatment with STING agonists led to regression of chronic lymphocytic leukemia and multiple myeloma in mice.
“This specific cytotoxicity toward B cells strongly supports the use of STING agonists in the treatment of B-cell hematologic malignancies,” said Chih-Hang Anthony Tang, MD, PhD, of The Wistar Institute.
“We also believe that cytotoxicity in normal B cells can be managed with the administration of intravenous immunoglobulin that can help maintain normal levels of antibodies while treatment is being administered. This is something we plan on studying further.”
The Wistar Institute’s business development team is looking for a development partner for the advancement of novel STING agonists in treating B-cell hematologic malignancies. ![]()

A class of drugs targeting a protein found in the endoplasmic reticulum could be effective against B-cell malignancies, according to a study published in Cancer Research.
The protein, STING, plays a critical role in producing type I interferons that help regulate the immune system.
Previous research suggested that STING agonists can improve immune responses when used in cancer immunotherapy or as vaccine adjuvants.
However, the way B cells respond to STING agonists was not well understood.
Chih-Chi Andrew Hu, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues conducted a study to gain some insight.
The researchers found that normal B cells respond to STING agonists by undergoing mitochondria-mediated apoptosis, and STING agonists induce apoptosis in
malignant B cells through binding to STING.
STING agonists proved cytotoxic to B-cell leukemia, lymphoma, and multiple myeloma in vitro. But the drugs did not induce apoptosis in solid tumor malignancies or normal T cells.
The research also revealed that the IRE-1/XBP-1 stress response pathway is required for normal STING function. And B-cell leukemia, lymphoma, and myeloma require the IRE-1/XBP-1 pathway to be activated for survival.
Stimulation by STING agonists suppressed the IRE-1/XBP-1 pathway, which increased the level of apoptosis in malignant B cells.
The researchers confirmed these results in animal models, as treatment with STING agonists led to regression of chronic lymphocytic leukemia and multiple myeloma in mice.
“This specific cytotoxicity toward B cells strongly supports the use of STING agonists in the treatment of B-cell hematologic malignancies,” said Chih-Hang Anthony Tang, MD, PhD, of The Wistar Institute.
“We also believe that cytotoxicity in normal B cells can be managed with the administration of intravenous immunoglobulin that can help maintain normal levels of antibodies while treatment is being administered. This is something we plan on studying further.”
The Wistar Institute’s business development team is looking for a development partner for the advancement of novel STING agonists in treating B-cell hematologic malignancies. ![]()
HIV drug may overcome proteasome-inhibitor resistance in multiple myeloma
The protease inhibitor nelfinavir was used successfully to resensitize patients with proteasome inhibitor–refractory multiple myeloma for proteasome-inhibitor treatment, Dr. Christoph Driessen of Kantonsspital St. Gallen (Switzerland) and colleagues reported in Haematologica.
The researchers had hypothesized that nelfinavir would induce the unfolded protein response and would overcome proteasome-inhibitor resistance. The researchers determined a nelfinavir dose of 2,500 mg twice daily based on a dose-finding study in 12 patients with advanced hematologic malignancies.
In an exploratory extension cohort trial that followed, six patients with relapsed, bortezomib-refractory, lenalidomide-resistant myeloma were treated with 2,500 mg of nelfinavir twice daily in combination with bortezomib; three reached a partial response, two had a minor response, and one had progressive disease. All began the investigational therapy less than 2 months after progressing under therapies with bortezomib/bendamustine/dexamethasone, bortezomib/bendamustine, or bortezomib monotherapy, respectively, the researchers said (Haematologica March 2016;101:346-55).
The study protocol did not allow dexamethasone co-administration until completion of cycle three, when patients who did not achieve a minor response were allowed to co-administer 8 mg dexamethasone with bortezomib. Higher doses were excluded because of potential interactions with nelfinavir through the Cyp3A4 system.
Future research should assess whether bortezomib may be replaced by next-generation drugs like carfilzomib to avoid drug interactions and improve activity and whether nelfinavir may likewise be used in combination with novel oral proteasome inhibitors to boost their low single agent activity. “This ultimately suggests exploring the addition of HIV protease inhibitors to established combinations of proteasome inhibitors with immunomodulatory drugs, for example, in the carfilzomib/lenalidomide/dexamethasone regimen, one of the most powerful and tolerable regimens available to date for advanced multiple myeloma,” they concluded.
On Twitter @maryjodales
The protease inhibitor nelfinavir was used successfully to resensitize patients with proteasome inhibitor–refractory multiple myeloma for proteasome-inhibitor treatment, Dr. Christoph Driessen of Kantonsspital St. Gallen (Switzerland) and colleagues reported in Haematologica.
The researchers had hypothesized that nelfinavir would induce the unfolded protein response and would overcome proteasome-inhibitor resistance. The researchers determined a nelfinavir dose of 2,500 mg twice daily based on a dose-finding study in 12 patients with advanced hematologic malignancies.
In an exploratory extension cohort trial that followed, six patients with relapsed, bortezomib-refractory, lenalidomide-resistant myeloma were treated with 2,500 mg of nelfinavir twice daily in combination with bortezomib; three reached a partial response, two had a minor response, and one had progressive disease. All began the investigational therapy less than 2 months after progressing under therapies with bortezomib/bendamustine/dexamethasone, bortezomib/bendamustine, or bortezomib monotherapy, respectively, the researchers said (Haematologica March 2016;101:346-55).
The study protocol did not allow dexamethasone co-administration until completion of cycle three, when patients who did not achieve a minor response were allowed to co-administer 8 mg dexamethasone with bortezomib. Higher doses were excluded because of potential interactions with nelfinavir through the Cyp3A4 system.
Future research should assess whether bortezomib may be replaced by next-generation drugs like carfilzomib to avoid drug interactions and improve activity and whether nelfinavir may likewise be used in combination with novel oral proteasome inhibitors to boost their low single agent activity. “This ultimately suggests exploring the addition of HIV protease inhibitors to established combinations of proteasome inhibitors with immunomodulatory drugs, for example, in the carfilzomib/lenalidomide/dexamethasone regimen, one of the most powerful and tolerable regimens available to date for advanced multiple myeloma,” they concluded.
On Twitter @maryjodales
The protease inhibitor nelfinavir was used successfully to resensitize patients with proteasome inhibitor–refractory multiple myeloma for proteasome-inhibitor treatment, Dr. Christoph Driessen of Kantonsspital St. Gallen (Switzerland) and colleagues reported in Haematologica.
The researchers had hypothesized that nelfinavir would induce the unfolded protein response and would overcome proteasome-inhibitor resistance. The researchers determined a nelfinavir dose of 2,500 mg twice daily based on a dose-finding study in 12 patients with advanced hematologic malignancies.
In an exploratory extension cohort trial that followed, six patients with relapsed, bortezomib-refractory, lenalidomide-resistant myeloma were treated with 2,500 mg of nelfinavir twice daily in combination with bortezomib; three reached a partial response, two had a minor response, and one had progressive disease. All began the investigational therapy less than 2 months after progressing under therapies with bortezomib/bendamustine/dexamethasone, bortezomib/bendamustine, or bortezomib monotherapy, respectively, the researchers said (Haematologica March 2016;101:346-55).
The study protocol did not allow dexamethasone co-administration until completion of cycle three, when patients who did not achieve a minor response were allowed to co-administer 8 mg dexamethasone with bortezomib. Higher doses were excluded because of potential interactions with nelfinavir through the Cyp3A4 system.
Future research should assess whether bortezomib may be replaced by next-generation drugs like carfilzomib to avoid drug interactions and improve activity and whether nelfinavir may likewise be used in combination with novel oral proteasome inhibitors to boost their low single agent activity. “This ultimately suggests exploring the addition of HIV protease inhibitors to established combinations of proteasome inhibitors with immunomodulatory drugs, for example, in the carfilzomib/lenalidomide/dexamethasone regimen, one of the most powerful and tolerable regimens available to date for advanced multiple myeloma,” they concluded.
On Twitter @maryjodales
FROM HAEMATOLOGIA
Key clinical point: Adding HIV protease inhibitors to established combinations of proteasome inhibitors may improve outcomes in patients with multiple myeloma.
Major finding: When six patients with relapsed, bortezomib-refractory, lenalidomide-resistant myeloma were treated with 2,500 mg of nelfinavir twice daily in combination with bortezomib, three reached a partial response, two had a minor response, and disease progressed in one.
Data source: A dose-finding study in 12 patients with advanced hematologic malignancies and an exploratory extension cohort trial that followed.
Disclosures: None of the authors had financial conflicts of interest in relation to the study.
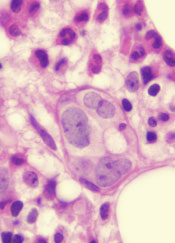
A Mysterious Massive Hemorrhage
Faced with a patient who had a massive retroperitoneal hemorrhage, clinicians from Saint Vincent Hospital in Worcester, Massachusetts, had to decide whether the patient had multiple myeloma or plasma cell leukemia,
The patient, a 64-year-old woman, came to the emergency department (ED) with month-long worsening, debilitating lower back pain. She also had a low-grade fever, shortness of breath for about 3 weeks, and weight loss of 10 pounds.
One month earlier, she had visited the ED for right lower quadrant pain and was discharged with the diagnosis of diverticulitis. An abdominal computed tomography (CT) scan revealed incidental splenomegaly. When she next came to the ED, an abdominal examination revealed rigidity, distention, tender splenomegaly, and Grey Turner’s sign. A neurologic examination revealed weakness and numbness with hyperreflexia in her lower legs. Another CT scan showed that the splenomegaly had worsened, and magneting resonance imaging (MRI) showed a compressed spinal cord.
The patient was in severe hemorrhagic shock and was transferred to the intensive care unit. Based on the clinical and laboratory data, the clinicians excluded plasma cell leukemia, since the peripheral blood showed < 20% plasma cells—the major criterion for diagnosing the condition. In the absence of a bleeding disorder, vascular disease, and anticoagulation medications, the authors say their case illustrates a rare and aggressive form of multiple myeloma. The clinical presentation with major spontaneous bleeding is uncommon. In fact, they say, it’s the first time this type of presentation has been reported in the medical literature.
Source: Alawadhi A, Leb L. Case Rep Hematol. 2016;2016:1-3.
doi:10.1155/2016/8206826.
Faced with a patient who had a massive retroperitoneal hemorrhage, clinicians from Saint Vincent Hospital in Worcester, Massachusetts, had to decide whether the patient had multiple myeloma or plasma cell leukemia,
The patient, a 64-year-old woman, came to the emergency department (ED) with month-long worsening, debilitating lower back pain. She also had a low-grade fever, shortness of breath for about 3 weeks, and weight loss of 10 pounds.
One month earlier, she had visited the ED for right lower quadrant pain and was discharged with the diagnosis of diverticulitis. An abdominal computed tomography (CT) scan revealed incidental splenomegaly. When she next came to the ED, an abdominal examination revealed rigidity, distention, tender splenomegaly, and Grey Turner’s sign. A neurologic examination revealed weakness and numbness with hyperreflexia in her lower legs. Another CT scan showed that the splenomegaly had worsened, and magneting resonance imaging (MRI) showed a compressed spinal cord.
The patient was in severe hemorrhagic shock and was transferred to the intensive care unit. Based on the clinical and laboratory data, the clinicians excluded plasma cell leukemia, since the peripheral blood showed < 20% plasma cells—the major criterion for diagnosing the condition. In the absence of a bleeding disorder, vascular disease, and anticoagulation medications, the authors say their case illustrates a rare and aggressive form of multiple myeloma. The clinical presentation with major spontaneous bleeding is uncommon. In fact, they say, it’s the first time this type of presentation has been reported in the medical literature.
Source: Alawadhi A, Leb L. Case Rep Hematol. 2016;2016:1-3.
doi:10.1155/2016/8206826.
Faced with a patient who had a massive retroperitoneal hemorrhage, clinicians from Saint Vincent Hospital in Worcester, Massachusetts, had to decide whether the patient had multiple myeloma or plasma cell leukemia,
The patient, a 64-year-old woman, came to the emergency department (ED) with month-long worsening, debilitating lower back pain. She also had a low-grade fever, shortness of breath for about 3 weeks, and weight loss of 10 pounds.
One month earlier, she had visited the ED for right lower quadrant pain and was discharged with the diagnosis of diverticulitis. An abdominal computed tomography (CT) scan revealed incidental splenomegaly. When she next came to the ED, an abdominal examination revealed rigidity, distention, tender splenomegaly, and Grey Turner’s sign. A neurologic examination revealed weakness and numbness with hyperreflexia in her lower legs. Another CT scan showed that the splenomegaly had worsened, and magneting resonance imaging (MRI) showed a compressed spinal cord.
The patient was in severe hemorrhagic shock and was transferred to the intensive care unit. Based on the clinical and laboratory data, the clinicians excluded plasma cell leukemia, since the peripheral blood showed < 20% plasma cells—the major criterion for diagnosing the condition. In the absence of a bleeding disorder, vascular disease, and anticoagulation medications, the authors say their case illustrates a rare and aggressive form of multiple myeloma. The clinical presentation with major spontaneous bleeding is uncommon. In fact, they say, it’s the first time this type of presentation has been reported in the medical literature.
Source: Alawadhi A, Leb L. Case Rep Hematol. 2016;2016:1-3.
doi:10.1155/2016/8206826.
Inhibitor exhibits activity against myeloma

A dual kinase inhibitor has shown early promise as a potential treatment for multiple myeloma (MM), according to investigators.
The inhibitor, ON123300, targets both AMPK-related protein kinase 5 (ARK5) and cyclin-dependent kinase 4 (CDK4), proteins responsible for maintaining energy balance within the cell.
Investigators found that ON123300 can induce apoptosis in MM cells in vitro and halt tumor growth in mouse models of MM.
The team reported these findings in Cancer Research.
“ARK5 is critical for myeloma survival, and this study suggests a novel function for ARK5 in bridging the mTOR and MYC pathways,” said study author Deepak Perumal, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York.
“Given that MYC is critically overexpressed in myeloma, we sought to determine whether selective inhibition of ARK5 and CDK4 could be an effective way to target MYC-driven proliferation in myeloma.”
The team at Mount Sinai developed ON123300 in collaboration with Onconova Therapeutics, Inc. And they tested the drug in MM cell lines and primary samples from patients with recurring MM, as well as human HS-5 bone marrow stromal cells.
ON123300 induced rapid cell-cycle arrest and apoptosis in MM cells but was not toxic to normal peripheral blood cells.
In mouse models of MM, ON123300 decreased tumor growth without causing significant toxicity.
The investigators also discovered that MM cells that are sensitive to ON123300 have a unique genomic signature, which could guide the clinical development of the drug.
“Our study results show that ON123300 induces cell death and negatively regulates key oncogenic pathways in multiple myeloma cells,” said Samir Parekh, MD, also of the Icahn School of Medicine at Mount Sinai.
“This is the first report showing potent cytotoxicity of CDK4/ARK5 inhibition in MM and provides the foundation for further clinical trials using CDK4/ARK5 inhibitors to improve outcomes for MM patients.” ![]()

A dual kinase inhibitor has shown early promise as a potential treatment for multiple myeloma (MM), according to investigators.
The inhibitor, ON123300, targets both AMPK-related protein kinase 5 (ARK5) and cyclin-dependent kinase 4 (CDK4), proteins responsible for maintaining energy balance within the cell.
Investigators found that ON123300 can induce apoptosis in MM cells in vitro and halt tumor growth in mouse models of MM.
The team reported these findings in Cancer Research.
“ARK5 is critical for myeloma survival, and this study suggests a novel function for ARK5 in bridging the mTOR and MYC pathways,” said study author Deepak Perumal, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York.
“Given that MYC is critically overexpressed in myeloma, we sought to determine whether selective inhibition of ARK5 and CDK4 could be an effective way to target MYC-driven proliferation in myeloma.”
The team at Mount Sinai developed ON123300 in collaboration with Onconova Therapeutics, Inc. And they tested the drug in MM cell lines and primary samples from patients with recurring MM, as well as human HS-5 bone marrow stromal cells.
ON123300 induced rapid cell-cycle arrest and apoptosis in MM cells but was not toxic to normal peripheral blood cells.
In mouse models of MM, ON123300 decreased tumor growth without causing significant toxicity.
The investigators also discovered that MM cells that are sensitive to ON123300 have a unique genomic signature, which could guide the clinical development of the drug.
“Our study results show that ON123300 induces cell death and negatively regulates key oncogenic pathways in multiple myeloma cells,” said Samir Parekh, MD, also of the Icahn School of Medicine at Mount Sinai.
“This is the first report showing potent cytotoxicity of CDK4/ARK5 inhibition in MM and provides the foundation for further clinical trials using CDK4/ARK5 inhibitors to improve outcomes for MM patients.” ![]()

A dual kinase inhibitor has shown early promise as a potential treatment for multiple myeloma (MM), according to investigators.
The inhibitor, ON123300, targets both AMPK-related protein kinase 5 (ARK5) and cyclin-dependent kinase 4 (CDK4), proteins responsible for maintaining energy balance within the cell.
Investigators found that ON123300 can induce apoptosis in MM cells in vitro and halt tumor growth in mouse models of MM.
The team reported these findings in Cancer Research.
“ARK5 is critical for myeloma survival, and this study suggests a novel function for ARK5 in bridging the mTOR and MYC pathways,” said study author Deepak Perumal, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York.
“Given that MYC is critically overexpressed in myeloma, we sought to determine whether selective inhibition of ARK5 and CDK4 could be an effective way to target MYC-driven proliferation in myeloma.”
The team at Mount Sinai developed ON123300 in collaboration with Onconova Therapeutics, Inc. And they tested the drug in MM cell lines and primary samples from patients with recurring MM, as well as human HS-5 bone marrow stromal cells.
ON123300 induced rapid cell-cycle arrest and apoptosis in MM cells but was not toxic to normal peripheral blood cells.
In mouse models of MM, ON123300 decreased tumor growth without causing significant toxicity.
The investigators also discovered that MM cells that are sensitive to ON123300 have a unique genomic signature, which could guide the clinical development of the drug.
“Our study results show that ON123300 induces cell death and negatively regulates key oncogenic pathways in multiple myeloma cells,” said Samir Parekh, MD, also of the Icahn School of Medicine at Mount Sinai.
“This is the first report showing potent cytotoxicity of CDK4/ARK5 inhibition in MM and provides the foundation for further clinical trials using CDK4/ARK5 inhibitors to improve outcomes for MM patients.” ![]()
AAs have lower rate of most blood cancers than NHWs

receiving treatment
Photo by Rhoda Baer
A new report suggests African Americans (AAs) have significantly lower rates of most hematologic malignancies than non-Hispanic white (NHW) individuals in the US.
AAs of both sexes had significantly lower rates of leukemia, Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL) than NHWs, but the rate of myeloma was significantly higher among AAs.
The death rates for these malignancies followed the same patterns, with the exception of HL. There was no significant difference in HL mortality between AAs and NHWs of either sex.
These findings can be found in the report, “Cancer Statistics for African Americans, 2016,” appearing in CA: A Cancer Journal for Clinicians.
To compile this report, the researchers used data from the Surveillance, Epidemiology, and End Results program and the Centers for Disease Control and Prevention’s National Program of Cancer Registries.
Incidence
For part of the report, the researchers compared the incidence of cancers between AAs and NHWs (divided by gender) for the period from 2008 to 2012.
Among females, the incidence of leukemia was 8.6 per 100,000 in AAs and 10.7 per 100,000 in NHWs (P<0.05). Among males, the incidence was 13.2 per 100,000 in AAs and 17.7 per 100,000 in NHWs (P<0.05).
The incidence of HL in females was 2.4 per 100,000 in AAs and 2.7 per 100,000 in NHWs (P<0.05). The incidence of HL in males was 3.2 per 100,000 in AAs and 3.4 per 100,000 in NHWs (P<0.05).
The incidence of NHL in females was 12.0 per 100,000 in AAs and 16.6 per 100,000 in NHWs (P<0.05). The incidence of NHL in males was 17.2 per 100,000 in AAs and 24.1 per 100,000 in NHWs (P<0.05).
The incidence of myeloma in females was 11.1 per 100,000 in AAs and 4.3 per 100,000 in NHWs (P<0.05). The incidence of myeloma in males was 14.8 per 100,000 in AAs and 7.0 per 100,000 in NHWs (P<0.05).
Mortality
The researchers also compared cancer mortality between AAs and NHWs (divided by gender) for the period from 2008 to 2012.
The death rate for female leukemia patients was 4.8 per 100,000 in AAs and 5.4 per 100,000 in NHWs (P<0.05). The death rate for male leukemia patients was 8.1 per 100,000 in AAs and 9.9 per 100,000 in NHWs (P<0.05).
The death rate for female HL patients was 0.3 per 100,000 for both AAs and NHWs. The death rate for male HL patients was 0.4 per 100,000 for AAs and 0.5 per 100,000 in NHWs (not significant).
The death rate for female NHL patients was 3.6 per 100,000 in AAs and 5.0 per 100,000 in NHWs (P<0.05). The death rate for male NHL patients was 5.9 per 100,000 in AAs and 8.3 per 100,000 in NHWs (P<0.05).
The death rate for female myeloma patients was 5.4 per 100,000 in AAs and 2.4 per 100,000 in NHWs (P<0.05). The death rate for male myeloma patients was 7.8 per 100,000 in AAs and 4.0 per 100,000 in NHWs (P<0.05).
The researchers noted that the reasons for the higher rates of myeloma and myeloma death among AAs are, at present, unknown. ![]()

receiving treatment
Photo by Rhoda Baer
A new report suggests African Americans (AAs) have significantly lower rates of most hematologic malignancies than non-Hispanic white (NHW) individuals in the US.
AAs of both sexes had significantly lower rates of leukemia, Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL) than NHWs, but the rate of myeloma was significantly higher among AAs.
The death rates for these malignancies followed the same patterns, with the exception of HL. There was no significant difference in HL mortality between AAs and NHWs of either sex.
These findings can be found in the report, “Cancer Statistics for African Americans, 2016,” appearing in CA: A Cancer Journal for Clinicians.
To compile this report, the researchers used data from the Surveillance, Epidemiology, and End Results program and the Centers for Disease Control and Prevention’s National Program of Cancer Registries.
Incidence
For part of the report, the researchers compared the incidence of cancers between AAs and NHWs (divided by gender) for the period from 2008 to 2012.
Among females, the incidence of leukemia was 8.6 per 100,000 in AAs and 10.7 per 100,000 in NHWs (P<0.05). Among males, the incidence was 13.2 per 100,000 in AAs and 17.7 per 100,000 in NHWs (P<0.05).
The incidence of HL in females was 2.4 per 100,000 in AAs and 2.7 per 100,000 in NHWs (P<0.05). The incidence of HL in males was 3.2 per 100,000 in AAs and 3.4 per 100,000 in NHWs (P<0.05).
The incidence of NHL in females was 12.0 per 100,000 in AAs and 16.6 per 100,000 in NHWs (P<0.05). The incidence of NHL in males was 17.2 per 100,000 in AAs and 24.1 per 100,000 in NHWs (P<0.05).
The incidence of myeloma in females was 11.1 per 100,000 in AAs and 4.3 per 100,000 in NHWs (P<0.05). The incidence of myeloma in males was 14.8 per 100,000 in AAs and 7.0 per 100,000 in NHWs (P<0.05).
Mortality
The researchers also compared cancer mortality between AAs and NHWs (divided by gender) for the period from 2008 to 2012.
The death rate for female leukemia patients was 4.8 per 100,000 in AAs and 5.4 per 100,000 in NHWs (P<0.05). The death rate for male leukemia patients was 8.1 per 100,000 in AAs and 9.9 per 100,000 in NHWs (P<0.05).
The death rate for female HL patients was 0.3 per 100,000 for both AAs and NHWs. The death rate for male HL patients was 0.4 per 100,000 for AAs and 0.5 per 100,000 in NHWs (not significant).
The death rate for female NHL patients was 3.6 per 100,000 in AAs and 5.0 per 100,000 in NHWs (P<0.05). The death rate for male NHL patients was 5.9 per 100,000 in AAs and 8.3 per 100,000 in NHWs (P<0.05).
The death rate for female myeloma patients was 5.4 per 100,000 in AAs and 2.4 per 100,000 in NHWs (P<0.05). The death rate for male myeloma patients was 7.8 per 100,000 in AAs and 4.0 per 100,000 in NHWs (P<0.05).
The researchers noted that the reasons for the higher rates of myeloma and myeloma death among AAs are, at present, unknown. ![]()

receiving treatment
Photo by Rhoda Baer
A new report suggests African Americans (AAs) have significantly lower rates of most hematologic malignancies than non-Hispanic white (NHW) individuals in the US.
AAs of both sexes had significantly lower rates of leukemia, Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL) than NHWs, but the rate of myeloma was significantly higher among AAs.
The death rates for these malignancies followed the same patterns, with the exception of HL. There was no significant difference in HL mortality between AAs and NHWs of either sex.
These findings can be found in the report, “Cancer Statistics for African Americans, 2016,” appearing in CA: A Cancer Journal for Clinicians.
To compile this report, the researchers used data from the Surveillance, Epidemiology, and End Results program and the Centers for Disease Control and Prevention’s National Program of Cancer Registries.
Incidence
For part of the report, the researchers compared the incidence of cancers between AAs and NHWs (divided by gender) for the period from 2008 to 2012.
Among females, the incidence of leukemia was 8.6 per 100,000 in AAs and 10.7 per 100,000 in NHWs (P<0.05). Among males, the incidence was 13.2 per 100,000 in AAs and 17.7 per 100,000 in NHWs (P<0.05).
The incidence of HL in females was 2.4 per 100,000 in AAs and 2.7 per 100,000 in NHWs (P<0.05). The incidence of HL in males was 3.2 per 100,000 in AAs and 3.4 per 100,000 in NHWs (P<0.05).
The incidence of NHL in females was 12.0 per 100,000 in AAs and 16.6 per 100,000 in NHWs (P<0.05). The incidence of NHL in males was 17.2 per 100,000 in AAs and 24.1 per 100,000 in NHWs (P<0.05).
The incidence of myeloma in females was 11.1 per 100,000 in AAs and 4.3 per 100,000 in NHWs (P<0.05). The incidence of myeloma in males was 14.8 per 100,000 in AAs and 7.0 per 100,000 in NHWs (P<0.05).
Mortality
The researchers also compared cancer mortality between AAs and NHWs (divided by gender) for the period from 2008 to 2012.
The death rate for female leukemia patients was 4.8 per 100,000 in AAs and 5.4 per 100,000 in NHWs (P<0.05). The death rate for male leukemia patients was 8.1 per 100,000 in AAs and 9.9 per 100,000 in NHWs (P<0.05).
The death rate for female HL patients was 0.3 per 100,000 for both AAs and NHWs. The death rate for male HL patients was 0.4 per 100,000 for AAs and 0.5 per 100,000 in NHWs (not significant).
The death rate for female NHL patients was 3.6 per 100,000 in AAs and 5.0 per 100,000 in NHWs (P<0.05). The death rate for male NHL patients was 5.9 per 100,000 in AAs and 8.3 per 100,000 in NHWs (P<0.05).
The death rate for female myeloma patients was 5.4 per 100,000 in AAs and 2.4 per 100,000 in NHWs (P<0.05). The death rate for male myeloma patients was 7.8 per 100,000 in AAs and 4.0 per 100,000 in NHWs (P<0.05).
The researchers noted that the reasons for the higher rates of myeloma and myeloma death among AAs are, at present, unknown. ![]()
How an anticancer drug fights lymphoid malignancies

Photo by Cameron Wells,
Walter and Eliza Hall
Institute of Medical Research
Research published in Cell Reports helps explain how the anticancer agent Nutlin3a fights lymphoma and other hematologic malignancies.
Nutlin3a is known to activate the tumor suppressor p53, but it hasn’t been clear exactly which p53 target genes are essential for the drug’s therapeutic activity.
The new research revealed that PUMA-mediated apoptosis—not p21-mediated cell-cycle arrest or senescence—is responsible for Nutlin3a’s therapeutic activity in lymphoid malignancies.
“By understanding how nutlins are killing cancer cells, we can begin to formulate their best possible use, including choosing the best partner drugs to combine the nutlins with,” said study author Andreas Strasser, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
With this study, Dr Strasser and his colleagues first found that Nutlin3a activates p53 target gene expression and causes cell-cycle arrest and apoptosis in non-transformed mouse lymphoid cells in vitro.
The team then showed that Nutlin3a-mediated killing of these cells requires PUMA but not p21. In vivo, loss of PUMA protected non-transformed mouse lymphoid cells against Nutlin3a-induced killing. Loss of p21 did not provide the same protection.
Next, the researchers found that malignant Eµ-Myc lymphoma cells were much more sensitive to Nutlin3a than were non-transformed lymphoid cells. In vitro experiments with Eµ-Myc lymphoma cells showed that Nutlin3a promotes p53 accumulation and downstream effector pathway activation.
As in previous experiments, PUMA (not p21) proved critical for Nutlin3a-induced killing of Eµ-Myc lymphoma cells in vitro. And loss of PUMA (but not p21) impaired the regression of Eµ-Myc lymphomas induced by Nutlin3a in vivo.
Finally, the researchers found that PUMA contributed to Nutlin3a-induced apoptosis in myeloid leukemia, multiple myeloma, and Burkitt lymphoma cell lines.
The team noted that, because PUMA, a pro-apoptotic BH3-only protein, is critical for the therapeutic impact of Nutlin3a, it may be possible to boost the drug’s efficacy by combining it with BH3 mimetic drugs such as navitoclax or venetoclax. ![]()

Photo by Cameron Wells,
Walter and Eliza Hall
Institute of Medical Research
Research published in Cell Reports helps explain how the anticancer agent Nutlin3a fights lymphoma and other hematologic malignancies.
Nutlin3a is known to activate the tumor suppressor p53, but it hasn’t been clear exactly which p53 target genes are essential for the drug’s therapeutic activity.
The new research revealed that PUMA-mediated apoptosis—not p21-mediated cell-cycle arrest or senescence—is responsible for Nutlin3a’s therapeutic activity in lymphoid malignancies.
“By understanding how nutlins are killing cancer cells, we can begin to formulate their best possible use, including choosing the best partner drugs to combine the nutlins with,” said study author Andreas Strasser, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
With this study, Dr Strasser and his colleagues first found that Nutlin3a activates p53 target gene expression and causes cell-cycle arrest and apoptosis in non-transformed mouse lymphoid cells in vitro.
The team then showed that Nutlin3a-mediated killing of these cells requires PUMA but not p21. In vivo, loss of PUMA protected non-transformed mouse lymphoid cells against Nutlin3a-induced killing. Loss of p21 did not provide the same protection.
Next, the researchers found that malignant Eµ-Myc lymphoma cells were much more sensitive to Nutlin3a than were non-transformed lymphoid cells. In vitro experiments with Eµ-Myc lymphoma cells showed that Nutlin3a promotes p53 accumulation and downstream effector pathway activation.
As in previous experiments, PUMA (not p21) proved critical for Nutlin3a-induced killing of Eµ-Myc lymphoma cells in vitro. And loss of PUMA (but not p21) impaired the regression of Eµ-Myc lymphomas induced by Nutlin3a in vivo.
Finally, the researchers found that PUMA contributed to Nutlin3a-induced apoptosis in myeloid leukemia, multiple myeloma, and Burkitt lymphoma cell lines.
The team noted that, because PUMA, a pro-apoptotic BH3-only protein, is critical for the therapeutic impact of Nutlin3a, it may be possible to boost the drug’s efficacy by combining it with BH3 mimetic drugs such as navitoclax or venetoclax. ![]()

Photo by Cameron Wells,
Walter and Eliza Hall
Institute of Medical Research
Research published in Cell Reports helps explain how the anticancer agent Nutlin3a fights lymphoma and other hematologic malignancies.
Nutlin3a is known to activate the tumor suppressor p53, but it hasn’t been clear exactly which p53 target genes are essential for the drug’s therapeutic activity.
The new research revealed that PUMA-mediated apoptosis—not p21-mediated cell-cycle arrest or senescence—is responsible for Nutlin3a’s therapeutic activity in lymphoid malignancies.
“By understanding how nutlins are killing cancer cells, we can begin to formulate their best possible use, including choosing the best partner drugs to combine the nutlins with,” said study author Andreas Strasser, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
With this study, Dr Strasser and his colleagues first found that Nutlin3a activates p53 target gene expression and causes cell-cycle arrest and apoptosis in non-transformed mouse lymphoid cells in vitro.
The team then showed that Nutlin3a-mediated killing of these cells requires PUMA but not p21. In vivo, loss of PUMA protected non-transformed mouse lymphoid cells against Nutlin3a-induced killing. Loss of p21 did not provide the same protection.
Next, the researchers found that malignant Eµ-Myc lymphoma cells were much more sensitive to Nutlin3a than were non-transformed lymphoid cells. In vitro experiments with Eµ-Myc lymphoma cells showed that Nutlin3a promotes p53 accumulation and downstream effector pathway activation.
As in previous experiments, PUMA (not p21) proved critical for Nutlin3a-induced killing of Eµ-Myc lymphoma cells in vitro. And loss of PUMA (but not p21) impaired the regression of Eµ-Myc lymphomas induced by Nutlin3a in vivo.
Finally, the researchers found that PUMA contributed to Nutlin3a-induced apoptosis in myeloid leukemia, multiple myeloma, and Burkitt lymphoma cell lines.
The team noted that, because PUMA, a pro-apoptotic BH3-only protein, is critical for the therapeutic impact of Nutlin3a, it may be possible to boost the drug’s efficacy by combining it with BH3 mimetic drugs such as navitoclax or venetoclax. ![]()
Panobinostat plus bortezomib and dexamethasone improved outcomes in previously treated multiple myeloma
The addition of panobinostat to bortezomib and dexamethasone (PAN-BTZ-Dex) improved outcomes in patients with multiple myeloma who had received prior treatment with immunomodulatory drugs (IMiDs), prior bortezomib plus an IMiD, and two or more prior regimens including bortezomib and an IMiD.
Subgroup analysis of the PANORAMA phase III trial of patients with relapsed or relapsed and refractory multiple myeloma (MM) reported median progression-free survival (PFS) with PAN-BTZ-Dex versus placebo-BTZ-Dex (Pbo-BTZ-Dex) in groups defined by prior treatment: prior IMiD (12.3 vs. 7.4 months; hazard ratio, 0.54; 95% confidence interval, 0.43-0.68), prior bortezomib plus IMiD (10.6 vs. 5.8 months; HR, 0.52; 95% CI, 0.36-0.76), and two or more prior regimens including bortezomib and an IMiD (12.5 vs. 4.7 months; HR, 0.47; 95% CI, 0.31-0.72) (Blood. 2016 Feb 11. doi: 10.1182/blood-2015-09-665018).
The greatest difference in median PFS between the panobinostat and placebo arms (7.8 months) was observed among patients who had received two or more prior regimens including bortezomib and an IMiD, a population with a poorer prognosis and an urgent unmet need, according to investigators.
“Panobinostat represents a novel addition to the MM treatment armamentarium by introducing an agent with a novel mechanism of action. Novel agents are needed to address the ongoing unmet need in patients who progress on bortezomib and IMiDs as a strategy to overcome therapeutic resistance,” wrote Dr. Paul G. Richardson of the Dana Farber Cancer Institute, Boston, and his colleagues, adding that since the deacetylase inhibitor “acts on distinct epigenetic and protein metabolism pathways, it is uniquely suited to provide benefit in patients previously treated with proteasome inhibitors and/or IMiDs.”
The analysis examined treatment outcomes from the PANORAMA trial, including 485 patients who had received prior IMiD (63% of total population), 193 who received prior bortezomib plus IMiD (25% of total population), and 147 who received two or more prior regimens including bortezomib and an IMiD (19% of total population).
Common adverse events (AEs) by treatment subgroups were similar to those of the overall trial population, which were increased in the PAN-BTZ-Dex compared with the placebo arm. The most common nonhematologic AE was diarrhea and the most common hematologic abnormality was thrombocytopenia.
The PANORAMA 1 study was funded by Novartis Pharmaceuticals. Dr. Richardson reported having no disclosures. Several of his coauthors reported ties to industry.
The addition of panobinostat to bortezomib and dexamethasone (PAN-BTZ-Dex) improved outcomes in patients with multiple myeloma who had received prior treatment with immunomodulatory drugs (IMiDs), prior bortezomib plus an IMiD, and two or more prior regimens including bortezomib and an IMiD.
Subgroup analysis of the PANORAMA phase III trial of patients with relapsed or relapsed and refractory multiple myeloma (MM) reported median progression-free survival (PFS) with PAN-BTZ-Dex versus placebo-BTZ-Dex (Pbo-BTZ-Dex) in groups defined by prior treatment: prior IMiD (12.3 vs. 7.4 months; hazard ratio, 0.54; 95% confidence interval, 0.43-0.68), prior bortezomib plus IMiD (10.6 vs. 5.8 months; HR, 0.52; 95% CI, 0.36-0.76), and two or more prior regimens including bortezomib and an IMiD (12.5 vs. 4.7 months; HR, 0.47; 95% CI, 0.31-0.72) (Blood. 2016 Feb 11. doi: 10.1182/blood-2015-09-665018).
The greatest difference in median PFS between the panobinostat and placebo arms (7.8 months) was observed among patients who had received two or more prior regimens including bortezomib and an IMiD, a population with a poorer prognosis and an urgent unmet need, according to investigators.
“Panobinostat represents a novel addition to the MM treatment armamentarium by introducing an agent with a novel mechanism of action. Novel agents are needed to address the ongoing unmet need in patients who progress on bortezomib and IMiDs as a strategy to overcome therapeutic resistance,” wrote Dr. Paul G. Richardson of the Dana Farber Cancer Institute, Boston, and his colleagues, adding that since the deacetylase inhibitor “acts on distinct epigenetic and protein metabolism pathways, it is uniquely suited to provide benefit in patients previously treated with proteasome inhibitors and/or IMiDs.”
The analysis examined treatment outcomes from the PANORAMA trial, including 485 patients who had received prior IMiD (63% of total population), 193 who received prior bortezomib plus IMiD (25% of total population), and 147 who received two or more prior regimens including bortezomib and an IMiD (19% of total population).
Common adverse events (AEs) by treatment subgroups were similar to those of the overall trial population, which were increased in the PAN-BTZ-Dex compared with the placebo arm. The most common nonhematologic AE was diarrhea and the most common hematologic abnormality was thrombocytopenia.
The PANORAMA 1 study was funded by Novartis Pharmaceuticals. Dr. Richardson reported having no disclosures. Several of his coauthors reported ties to industry.
The addition of panobinostat to bortezomib and dexamethasone (PAN-BTZ-Dex) improved outcomes in patients with multiple myeloma who had received prior treatment with immunomodulatory drugs (IMiDs), prior bortezomib plus an IMiD, and two or more prior regimens including bortezomib and an IMiD.
Subgroup analysis of the PANORAMA phase III trial of patients with relapsed or relapsed and refractory multiple myeloma (MM) reported median progression-free survival (PFS) with PAN-BTZ-Dex versus placebo-BTZ-Dex (Pbo-BTZ-Dex) in groups defined by prior treatment: prior IMiD (12.3 vs. 7.4 months; hazard ratio, 0.54; 95% confidence interval, 0.43-0.68), prior bortezomib plus IMiD (10.6 vs. 5.8 months; HR, 0.52; 95% CI, 0.36-0.76), and two or more prior regimens including bortezomib and an IMiD (12.5 vs. 4.7 months; HR, 0.47; 95% CI, 0.31-0.72) (Blood. 2016 Feb 11. doi: 10.1182/blood-2015-09-665018).
The greatest difference in median PFS between the panobinostat and placebo arms (7.8 months) was observed among patients who had received two or more prior regimens including bortezomib and an IMiD, a population with a poorer prognosis and an urgent unmet need, according to investigators.
“Panobinostat represents a novel addition to the MM treatment armamentarium by introducing an agent with a novel mechanism of action. Novel agents are needed to address the ongoing unmet need in patients who progress on bortezomib and IMiDs as a strategy to overcome therapeutic resistance,” wrote Dr. Paul G. Richardson of the Dana Farber Cancer Institute, Boston, and his colleagues, adding that since the deacetylase inhibitor “acts on distinct epigenetic and protein metabolism pathways, it is uniquely suited to provide benefit in patients previously treated with proteasome inhibitors and/or IMiDs.”
The analysis examined treatment outcomes from the PANORAMA trial, including 485 patients who had received prior IMiD (63% of total population), 193 who received prior bortezomib plus IMiD (25% of total population), and 147 who received two or more prior regimens including bortezomib and an IMiD (19% of total population).
Common adverse events (AEs) by treatment subgroups were similar to those of the overall trial population, which were increased in the PAN-BTZ-Dex compared with the placebo arm. The most common nonhematologic AE was diarrhea and the most common hematologic abnormality was thrombocytopenia.
The PANORAMA 1 study was funded by Novartis Pharmaceuticals. Dr. Richardson reported having no disclosures. Several of his coauthors reported ties to industry.
FROM BLOOD
Key clinical point: Panobinostat plus bortezomib and dexamethasone improved outcomes in patients with previously treated multiple myeloma, particularly those with two or more prior regimens including immunomodulatory drugs (IMiDs) and bortezomib.
Major finding: In patients with two or more prior regimens including IMiDs and bortezomib, progression-free survival for the panobinostat plus bortezomib and dexamethasone arm, compared with placebo plus bortezomib and dexamethasone arm, was 12.5 months (95% CI, 7.3-14.0) vs. 4.7 months (95% CI, 3.7-6.1); hazard ratio, 0.47 (95% CI, 0.31-0.72).
Data source: Subgroup analysis of the phase III PANORAMA trial evaluating panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma.
Disclosures: The PANORAMA 1 study was funded by Novartis Pharmaceuticals. Dr. Richardson reported having no disclosures. Several of his coauthors reported ties to industry.
Drug exhibits activity against myeloma, solid tumors

Image courtesy of PNAS
Researchers say they have determined how the investigational drug ONC201 is active against a range of malignancies.
The team found that ONC201 induced apoptosis and cell cycle arrest in multiple myeloma (MM) and solid tumor cell lines.
The drug triggered an increase in the anticancer protein TRAIL and induced cell death through an integrated stress response (ISR) involving the transcription factor ATF4, the transactivator CHOP, and the TRAIL receptor DR5.
The researchers reported these findings in Science Signaling. Some researchers involved in this study are affiliated with Oncoceutics Inc., the company developing ONC201.
“We have revealed, in unprecedented detail, exactly how ONC201 works across a broad range of tumor types, and this has important clinical implications,” said study author Wafik El-Deiry, MD, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“For example, our findings suggest that patients with various solid tumors, as well as multiple myeloma, may be particularly sensitive to the effects of ONC201. We have identified a potential biomarker that could be used to select which patients are most likely to benefit therapeutically from this drug.”
Dr El-Deiry noted that TRAIL has been shown to induce cell death in a range of cancers while sparing normal cells. However, the therapeutic benefit of stimulating TRAIL is limited because of undesirable drug properties, such as a short half-life, difficult and expensive production, the need to give treatment as an intravenous infusion, and poor penetration into certain tissues like the brain.
“This prompted us to look for better options for therapeutics that can kill tumor cells,” Dr El-Deiry said.
He and his colleagues turned to ONC201, which has been shown to stimulate TRAIL. They tested the drug in 23 cancer cell lines representing 9 tumor types—MM, lymphoma, and glioma, as well as lung, colorectal, thyroid, liver, prostate, and breast cancer.
The team found that ONC201 triggers an increase in TRAIL and TRAIL receptor abundance, leading to tumor cell death through the ISR that tumor cells normally use to survive. ONC201 pushes the ISR too far, causing tumor cells to stop dividing and/or die.
ONC201 boosted expression of the gene encoding ATF4, a central component of the ISR, through a translation initiation factor called eIF2α. This process rapidly arrested the cancer’s cell cycle and resulted in cell death.
In essence, ONC201 delivers a double-whammy to tumor cells, Dr El-Deiry said, which may explain why it has such broad-spectrum anticancer activity.
The researchers believe this study has several clinical implications. For one, it suggests that solid tumors or MM cells that normally create large amounts of protein during growth may be particularly sensitive to ONC201. The ISR is often activated in these cells, and ONC201 may push them over the edge.
“Knowing how ONC201 works helps us look for its effects in patient’s tumor cells that have been treated,” Dr El-Deiry said. “Looking in a tumor or liquid biopsy before and after treatment may help predict who is most likely to benefit.”
“We are optimistic that, through basic and clinical research with ONC201, our findings will lead to improved TRAIL-based therapies for individual cancer patients in the future.”
Another study of ONC201, this one focusing only on hematologic malignancies, has been published in Science Signaling.
Early stage clinical trials for ONC201 are currently underway in patients with brain, colorectal, breast, and lung tumors, as well as leukemia and lymphoma. ![]()

Image courtesy of PNAS
Researchers say they have determined how the investigational drug ONC201 is active against a range of malignancies.
The team found that ONC201 induced apoptosis and cell cycle arrest in multiple myeloma (MM) and solid tumor cell lines.
The drug triggered an increase in the anticancer protein TRAIL and induced cell death through an integrated stress response (ISR) involving the transcription factor ATF4, the transactivator CHOP, and the TRAIL receptor DR5.
The researchers reported these findings in Science Signaling. Some researchers involved in this study are affiliated with Oncoceutics Inc., the company developing ONC201.
“We have revealed, in unprecedented detail, exactly how ONC201 works across a broad range of tumor types, and this has important clinical implications,” said study author Wafik El-Deiry, MD, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“For example, our findings suggest that patients with various solid tumors, as well as multiple myeloma, may be particularly sensitive to the effects of ONC201. We have identified a potential biomarker that could be used to select which patients are most likely to benefit therapeutically from this drug.”
Dr El-Deiry noted that TRAIL has been shown to induce cell death in a range of cancers while sparing normal cells. However, the therapeutic benefit of stimulating TRAIL is limited because of undesirable drug properties, such as a short half-life, difficult and expensive production, the need to give treatment as an intravenous infusion, and poor penetration into certain tissues like the brain.
“This prompted us to look for better options for therapeutics that can kill tumor cells,” Dr El-Deiry said.
He and his colleagues turned to ONC201, which has been shown to stimulate TRAIL. They tested the drug in 23 cancer cell lines representing 9 tumor types—MM, lymphoma, and glioma, as well as lung, colorectal, thyroid, liver, prostate, and breast cancer.
The team found that ONC201 triggers an increase in TRAIL and TRAIL receptor abundance, leading to tumor cell death through the ISR that tumor cells normally use to survive. ONC201 pushes the ISR too far, causing tumor cells to stop dividing and/or die.
ONC201 boosted expression of the gene encoding ATF4, a central component of the ISR, through a translation initiation factor called eIF2α. This process rapidly arrested the cancer’s cell cycle and resulted in cell death.
In essence, ONC201 delivers a double-whammy to tumor cells, Dr El-Deiry said, which may explain why it has such broad-spectrum anticancer activity.
The researchers believe this study has several clinical implications. For one, it suggests that solid tumors or MM cells that normally create large amounts of protein during growth may be particularly sensitive to ONC201. The ISR is often activated in these cells, and ONC201 may push them over the edge.
“Knowing how ONC201 works helps us look for its effects in patient’s tumor cells that have been treated,” Dr El-Deiry said. “Looking in a tumor or liquid biopsy before and after treatment may help predict who is most likely to benefit.”
“We are optimistic that, through basic and clinical research with ONC201, our findings will lead to improved TRAIL-based therapies for individual cancer patients in the future.”
Another study of ONC201, this one focusing only on hematologic malignancies, has been published in Science Signaling.
Early stage clinical trials for ONC201 are currently underway in patients with brain, colorectal, breast, and lung tumors, as well as leukemia and lymphoma. ![]()

Image courtesy of PNAS
Researchers say they have determined how the investigational drug ONC201 is active against a range of malignancies.
The team found that ONC201 induced apoptosis and cell cycle arrest in multiple myeloma (MM) and solid tumor cell lines.
The drug triggered an increase in the anticancer protein TRAIL and induced cell death through an integrated stress response (ISR) involving the transcription factor ATF4, the transactivator CHOP, and the TRAIL receptor DR5.
The researchers reported these findings in Science Signaling. Some researchers involved in this study are affiliated with Oncoceutics Inc., the company developing ONC201.
“We have revealed, in unprecedented detail, exactly how ONC201 works across a broad range of tumor types, and this has important clinical implications,” said study author Wafik El-Deiry, MD, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“For example, our findings suggest that patients with various solid tumors, as well as multiple myeloma, may be particularly sensitive to the effects of ONC201. We have identified a potential biomarker that could be used to select which patients are most likely to benefit therapeutically from this drug.”
Dr El-Deiry noted that TRAIL has been shown to induce cell death in a range of cancers while sparing normal cells. However, the therapeutic benefit of stimulating TRAIL is limited because of undesirable drug properties, such as a short half-life, difficult and expensive production, the need to give treatment as an intravenous infusion, and poor penetration into certain tissues like the brain.
“This prompted us to look for better options for therapeutics that can kill tumor cells,” Dr El-Deiry said.
He and his colleagues turned to ONC201, which has been shown to stimulate TRAIL. They tested the drug in 23 cancer cell lines representing 9 tumor types—MM, lymphoma, and glioma, as well as lung, colorectal, thyroid, liver, prostate, and breast cancer.
The team found that ONC201 triggers an increase in TRAIL and TRAIL receptor abundance, leading to tumor cell death through the ISR that tumor cells normally use to survive. ONC201 pushes the ISR too far, causing tumor cells to stop dividing and/or die.
ONC201 boosted expression of the gene encoding ATF4, a central component of the ISR, through a translation initiation factor called eIF2α. This process rapidly arrested the cancer’s cell cycle and resulted in cell death.
In essence, ONC201 delivers a double-whammy to tumor cells, Dr El-Deiry said, which may explain why it has such broad-spectrum anticancer activity.
The researchers believe this study has several clinical implications. For one, it suggests that solid tumors or MM cells that normally create large amounts of protein during growth may be particularly sensitive to ONC201. The ISR is often activated in these cells, and ONC201 may push them over the edge.
“Knowing how ONC201 works helps us look for its effects in patient’s tumor cells that have been treated,” Dr El-Deiry said. “Looking in a tumor or liquid biopsy before and after treatment may help predict who is most likely to benefit.”
“We are optimistic that, through basic and clinical research with ONC201, our findings will lead to improved TRAIL-based therapies for individual cancer patients in the future.”
Another study of ONC201, this one focusing only on hematologic malignancies, has been published in Science Signaling.
Early stage clinical trials for ONC201 are currently underway in patients with brain, colorectal, breast, and lung tumors, as well as leukemia and lymphoma. ![]()
NICE publishes guideline for multiple myeloma

Photo courtesy of NIH
The National Institute for Health and Care Excellence (NICE) has published a guideline containing recommendations for diagnosing, treating, and monitoring patients with multiple myeloma (MM).
The aim of the guideline is to help the National Health Service provide optimal care for MM patients over the age of 16 in England.
The guideline complements existing NICE guidance on the treatment of MM.
“Although there is no cure for myeloma, several novel drug treatments have been licensed in the past 10 years that have led to substantial improvements in the quality and length of time it is possible to live with the disease,” said Mark Baker, clinical practice director for NICE.
“However, there is still variation across the country in terms of providing a coherent and consistent approach to the management of myeloma. Myeloma is also a difficult condition to diagnose because many of the symptoms are non-specific. Our guideline sets out best-practice care to ensure people live as normal a life as possible for as long as possible.”
Some of the recommendations in the guideline include:
Communication and support: Offer prompt psychological assessment and support to MM patients at diagnosis and, as appropriate, at the beginning and end of each treatment, whenever the disease progresses, and when patients require end-of-life care.
Laboratory investigations to provide prognostic information: Use the same sample for all diagnostic and prognostic tests on bone marrow so patients only have to have one bone marrow aspirate and trephine biopsy.
Imaging for people with suspected MM: Offer imaging to all people with a plasma cell disorder suspected to be myeloma. Doctors should consider whole-body MRI as the first imaging procedure.
Service organizations: Each hospital treating MM patients should provide regional access through its network to facilities for intensive inpatient chemotherapy or transplantation, renal support, spinal disease management, specialized pain management, therapeutic apheresis, radiotherapy, restorative dentistry and oral surgery, and clinical trials, in particular early phase trials.
Managing relapsed MM: Offer a second autologous stem cell transplant to people with relapsed MM who are suitable and who have completed re-induction therapy without disease progression and had a response duration of more than 24 months after their first transplant. A second autologous stem cell transplant should be considered in people who have had a response duration of between 12 and 24 months after their first transplant. ![]()

Photo courtesy of NIH
The National Institute for Health and Care Excellence (NICE) has published a guideline containing recommendations for diagnosing, treating, and monitoring patients with multiple myeloma (MM).
The aim of the guideline is to help the National Health Service provide optimal care for MM patients over the age of 16 in England.
The guideline complements existing NICE guidance on the treatment of MM.
“Although there is no cure for myeloma, several novel drug treatments have been licensed in the past 10 years that have led to substantial improvements in the quality and length of time it is possible to live with the disease,” said Mark Baker, clinical practice director for NICE.
“However, there is still variation across the country in terms of providing a coherent and consistent approach to the management of myeloma. Myeloma is also a difficult condition to diagnose because many of the symptoms are non-specific. Our guideline sets out best-practice care to ensure people live as normal a life as possible for as long as possible.”
Some of the recommendations in the guideline include:
Communication and support: Offer prompt psychological assessment and support to MM patients at diagnosis and, as appropriate, at the beginning and end of each treatment, whenever the disease progresses, and when patients require end-of-life care.
Laboratory investigations to provide prognostic information: Use the same sample for all diagnostic and prognostic tests on bone marrow so patients only have to have one bone marrow aspirate and trephine biopsy.
Imaging for people with suspected MM: Offer imaging to all people with a plasma cell disorder suspected to be myeloma. Doctors should consider whole-body MRI as the first imaging procedure.
Service organizations: Each hospital treating MM patients should provide regional access through its network to facilities for intensive inpatient chemotherapy or transplantation, renal support, spinal disease management, specialized pain management, therapeutic apheresis, radiotherapy, restorative dentistry and oral surgery, and clinical trials, in particular early phase trials.
Managing relapsed MM: Offer a second autologous stem cell transplant to people with relapsed MM who are suitable and who have completed re-induction therapy without disease progression and had a response duration of more than 24 months after their first transplant. A second autologous stem cell transplant should be considered in people who have had a response duration of between 12 and 24 months after their first transplant. ![]()

Photo courtesy of NIH
The National Institute for Health and Care Excellence (NICE) has published a guideline containing recommendations for diagnosing, treating, and monitoring patients with multiple myeloma (MM).
The aim of the guideline is to help the National Health Service provide optimal care for MM patients over the age of 16 in England.
The guideline complements existing NICE guidance on the treatment of MM.
“Although there is no cure for myeloma, several novel drug treatments have been licensed in the past 10 years that have led to substantial improvements in the quality and length of time it is possible to live with the disease,” said Mark Baker, clinical practice director for NICE.
“However, there is still variation across the country in terms of providing a coherent and consistent approach to the management of myeloma. Myeloma is also a difficult condition to diagnose because many of the symptoms are non-specific. Our guideline sets out best-practice care to ensure people live as normal a life as possible for as long as possible.”
Some of the recommendations in the guideline include:
Communication and support: Offer prompt psychological assessment and support to MM patients at diagnosis and, as appropriate, at the beginning and end of each treatment, whenever the disease progresses, and when patients require end-of-life care.
Laboratory investigations to provide prognostic information: Use the same sample for all diagnostic and prognostic tests on bone marrow so patients only have to have one bone marrow aspirate and trephine biopsy.
Imaging for people with suspected MM: Offer imaging to all people with a plasma cell disorder suspected to be myeloma. Doctors should consider whole-body MRI as the first imaging procedure.
Service organizations: Each hospital treating MM patients should provide regional access through its network to facilities for intensive inpatient chemotherapy or transplantation, renal support, spinal disease management, specialized pain management, therapeutic apheresis, radiotherapy, restorative dentistry and oral surgery, and clinical trials, in particular early phase trials.
Managing relapsed MM: Offer a second autologous stem cell transplant to people with relapsed MM who are suitable and who have completed re-induction therapy without disease progression and had a response duration of more than 24 months after their first transplant. A second autologous stem cell transplant should be considered in people who have had a response duration of between 12 and 24 months after their first transplant. ![]()
Lysolipid antigens prominent in MGUS and myeloma
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: In patients with Gaucher disease and in nearly one-third of patients with MGUS or myeloma, clonal immunoglobulins reacted with lysolipid antigens.
Major finding: Clonal immunoglobulin from 17 of 20 patients with Gaucher disease and from 22 of 66 patients with sporadic myeloma were reactive to lyso-glucosylceramide.
Data source: Peripheral blood or bone marrow samples from 25 healthy donors, 20 patients with Gaucher disease, and 66 patients with sporadic monoclonal gammopathy.
Disclosures: Dr. Nair reported having no disclosures. One of her coauthors reported ties to industry.