User login
Uterine volume, fibroid diameter predict robotic myomectomy duration
LAS VEGAS – It would be nice if surgeons could know beforehand how long robotic laparoscopic myomectomies will take, according to Peter Movilla, MD, a minimally invasive gynecologic surgery fellow at Newton (Mass.) Wellesley Hospital.
Best guesses are sometimes wrong, and it’s not uncommon for robotic cases to go longer than expected, especially when they have to be converted to an open approach.
Among other problems, going long backs up operating room (OR)scheduling and makes families impatient. Also, if it was known beforehand that a robotic case might take 5 hours, patients could be offered a quicker open procedure, especially if they are not good candidates for prolonged pneumoperitoneum.
After a case went past 6 hours at the University of California, San Francisco (UCSF), when Dr. Movilla was an ob.gyn. resident, he wanted to find a better way.
“I saw that we were not the best at guessing how long these surgeries were going to take, and thought maybe we could make prediction a little better by [incorporating] preoperative factors” in a structured way. “I wanted to create something that would give us an answer of how long it will take,” he said at a meeting sponsored by AAGL.
So he and his colleagues reviewed 126 robot-assisted laparoscopic myomectomies at UCSF. The mean operative time from skin incision to closure was 213 minutes, mean specimen weight 264.4 g, mean dominant fibroid diameter 8.5 cm, and mean number of fibroids removed 2.5. Four cases (3%) were converted to open laparotomy.
The team divided the cases by how long they took; 20% were under 3 hours, 70% took 3-5 hours; and 10% went over 5 hours. “Five hours is a long time to be in the OR,” especially when a case could have been done open, Dr. Movilla said.
Length of surgery correlated with 7 of the 21 preoperative factors considered on multivariate logistic regression. Cases tended to be longer in younger women and in women with diabetes, and when surgeons had less experience. There was a trend toward longer cases with higher body mass indices, but it was not statistically significant.
Having three or more fibroids on preoperative imaging and a larger number of fibroids over 3 cm were predictive of operations longer than 3 hours. However, the strongest predictors of long cases were uterine volume and the diameter of the largest fibroid, a mean of 532.4 cm3 and 8.8 cm, respectively, in cases over 5 hours. Posterior and intramural fibroids also increased operative time, but, again, the trends were not statistically significant.
The team put it all together in a risk calculator they tested against their subjects’ actual surgery times. The model tended to underestimate very short and very long cases at either end of the curve, but overall the fit was “not too bad,” and the more cases that are added to the model, the more accurate it will get, Dr. Movilla said.
There was no external funding for the work, and Dr. Movilla had no disclosures.
SOURCE: Movilla P et al. 2018 AAGL Global Congress, Abstract 69.
LAS VEGAS – It would be nice if surgeons could know beforehand how long robotic laparoscopic myomectomies will take, according to Peter Movilla, MD, a minimally invasive gynecologic surgery fellow at Newton (Mass.) Wellesley Hospital.
Best guesses are sometimes wrong, and it’s not uncommon for robotic cases to go longer than expected, especially when they have to be converted to an open approach.
Among other problems, going long backs up operating room (OR)scheduling and makes families impatient. Also, if it was known beforehand that a robotic case might take 5 hours, patients could be offered a quicker open procedure, especially if they are not good candidates for prolonged pneumoperitoneum.
After a case went past 6 hours at the University of California, San Francisco (UCSF), when Dr. Movilla was an ob.gyn. resident, he wanted to find a better way.
“I saw that we were not the best at guessing how long these surgeries were going to take, and thought maybe we could make prediction a little better by [incorporating] preoperative factors” in a structured way. “I wanted to create something that would give us an answer of how long it will take,” he said at a meeting sponsored by AAGL.
So he and his colleagues reviewed 126 robot-assisted laparoscopic myomectomies at UCSF. The mean operative time from skin incision to closure was 213 minutes, mean specimen weight 264.4 g, mean dominant fibroid diameter 8.5 cm, and mean number of fibroids removed 2.5. Four cases (3%) were converted to open laparotomy.
The team divided the cases by how long they took; 20% were under 3 hours, 70% took 3-5 hours; and 10% went over 5 hours. “Five hours is a long time to be in the OR,” especially when a case could have been done open, Dr. Movilla said.
Length of surgery correlated with 7 of the 21 preoperative factors considered on multivariate logistic regression. Cases tended to be longer in younger women and in women with diabetes, and when surgeons had less experience. There was a trend toward longer cases with higher body mass indices, but it was not statistically significant.
Having three or more fibroids on preoperative imaging and a larger number of fibroids over 3 cm were predictive of operations longer than 3 hours. However, the strongest predictors of long cases were uterine volume and the diameter of the largest fibroid, a mean of 532.4 cm3 and 8.8 cm, respectively, in cases over 5 hours. Posterior and intramural fibroids also increased operative time, but, again, the trends were not statistically significant.
The team put it all together in a risk calculator they tested against their subjects’ actual surgery times. The model tended to underestimate very short and very long cases at either end of the curve, but overall the fit was “not too bad,” and the more cases that are added to the model, the more accurate it will get, Dr. Movilla said.
There was no external funding for the work, and Dr. Movilla had no disclosures.
SOURCE: Movilla P et al. 2018 AAGL Global Congress, Abstract 69.
LAS VEGAS – It would be nice if surgeons could know beforehand how long robotic laparoscopic myomectomies will take, according to Peter Movilla, MD, a minimally invasive gynecologic surgery fellow at Newton (Mass.) Wellesley Hospital.
Best guesses are sometimes wrong, and it’s not uncommon for robotic cases to go longer than expected, especially when they have to be converted to an open approach.
Among other problems, going long backs up operating room (OR)scheduling and makes families impatient. Also, if it was known beforehand that a robotic case might take 5 hours, patients could be offered a quicker open procedure, especially if they are not good candidates for prolonged pneumoperitoneum.
After a case went past 6 hours at the University of California, San Francisco (UCSF), when Dr. Movilla was an ob.gyn. resident, he wanted to find a better way.
“I saw that we were not the best at guessing how long these surgeries were going to take, and thought maybe we could make prediction a little better by [incorporating] preoperative factors” in a structured way. “I wanted to create something that would give us an answer of how long it will take,” he said at a meeting sponsored by AAGL.
So he and his colleagues reviewed 126 robot-assisted laparoscopic myomectomies at UCSF. The mean operative time from skin incision to closure was 213 minutes, mean specimen weight 264.4 g, mean dominant fibroid diameter 8.5 cm, and mean number of fibroids removed 2.5. Four cases (3%) were converted to open laparotomy.
The team divided the cases by how long they took; 20% were under 3 hours, 70% took 3-5 hours; and 10% went over 5 hours. “Five hours is a long time to be in the OR,” especially when a case could have been done open, Dr. Movilla said.
Length of surgery correlated with 7 of the 21 preoperative factors considered on multivariate logistic regression. Cases tended to be longer in younger women and in women with diabetes, and when surgeons had less experience. There was a trend toward longer cases with higher body mass indices, but it was not statistically significant.
Having three or more fibroids on preoperative imaging and a larger number of fibroids over 3 cm were predictive of operations longer than 3 hours. However, the strongest predictors of long cases were uterine volume and the diameter of the largest fibroid, a mean of 532.4 cm3 and 8.8 cm, respectively, in cases over 5 hours. Posterior and intramural fibroids also increased operative time, but, again, the trends were not statistically significant.
The team put it all together in a risk calculator they tested against their subjects’ actual surgery times. The model tended to underestimate very short and very long cases at either end of the curve, but overall the fit was “not too bad,” and the more cases that are added to the model, the more accurate it will get, Dr. Movilla said.
There was no external funding for the work, and Dr. Movilla had no disclosures.
SOURCE: Movilla P et al. 2018 AAGL Global Congress, Abstract 69.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: A calculator is in the works to predict exactly how long robotic myomectomies will take.
Major finding: a mean of 532.4 cm3 and 8.8 cm, respectively, in cases over 5 hours.
Study details: Review of 126 cases.
Disclosures: There was no external funding, and Dr. Movilla had no disclosures.
Source: Movilla P et al. 2018 AAGL Global Congress, Abstract 69.
Don’t push women into preterm delivery after myomectomy
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: Vaginal trial of labor is safe after myomectomy, at least if the uterine cavity wasn’t entered.
Major finding: Sixty-five percent of women who didn’t have their uterine cavities entered had vaginal deliveries, a success rate similar to labor after C-section.
Study details: Review of 102 pregnancies with live births after myomectomy at Northwestern University, Chicago
Disclosures: There was no external funding, and the lead investigator didn’t have any disclosures.
Source: King N et al. 2018 AAGL Global Congress, Abstract 162.
Antibiotics backed as standard of care for myomectomies
LAS VEGAS – The surgical site infection rate was 2.9% among women who received perioperative antibiotics for fibroid surgery, but 7.8% among those who did not, in a review of 1,433 cases at Massachusetts General Hospital and Brigham and Women’s Hospital, Boston.
That is despite the fact that antibiotic cases were longer – 155 minutes vs. 89 minutes – and had more blood loss, 200 ml vs. 117 ml. Antibiotic cases also had larger specimen weights – 346 g vs. 176 g – and were more likely to have the uterine cavity entered, 30.2% vs. 14.4%.
“Surgical site infections were more common in the no-antibiotics group despite these being less complex cases.” There was “nearly a fivefold increased odds of surgical site infection or any infectious complication when no antibiotics were given,” after controlling for infection risk factors, including smoking and diabetes, said investigator Nisse V. Clark, MD, a minimally invasive gynecologic surgeon affiliated with Massachusetts General Hospital.
There are no perioperative antibiotic guidelines for myomectomies; maybe there should be. Almost 94% of the women in the review did receive antibiotics at the Harvard-affiliated hospitals, but the nationwide average has been pegged at about two-thirds, she said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
The antibiotic cases usually received a cephalosporin before surgery, and were about evenly about evenly split between abdominal, robotic, and laparoscopic approaches.
About one-third of the 90 women (6.3%) who did not get antibiotics had hysteroscopic procedures in which antibiotics usually are not given because the peritoneal cavity is not breeched. Most of the rest, however, were laparoscopic cases. It’s unknown why they weren’t given antibiotics. In her own practice, Dr. Clark said preop antibiotics are the rule for laparoscopic myomectomies.
The surgical site infection difference was driven largely by higher incidences of pelvic abscesses and other organ space infections in the no-antibiotic group.
The only significant demographic difference between the two groups was that women who received antibiotics were slightly younger (mean 38 versus 39.7 years).
In addition to diabetes and smoking, the team adjusted for age, surgery route, body mass index, uterine entry, intraoperative complications, and myoma weight in their multivariate analysis. Still, women in the no-antibiotic group were 4.59 times more likely to have a surgical site infection, 4.76 more likely to have any infectious complication, and almost 8 times more likely to have a major infectious complication. All of the findings were statistically significant.
The study had no industry funding, and Dr. Clark had no disclosures.
LAS VEGAS – The surgical site infection rate was 2.9% among women who received perioperative antibiotics for fibroid surgery, but 7.8% among those who did not, in a review of 1,433 cases at Massachusetts General Hospital and Brigham and Women’s Hospital, Boston.
That is despite the fact that antibiotic cases were longer – 155 minutes vs. 89 minutes – and had more blood loss, 200 ml vs. 117 ml. Antibiotic cases also had larger specimen weights – 346 g vs. 176 g – and were more likely to have the uterine cavity entered, 30.2% vs. 14.4%.
“Surgical site infections were more common in the no-antibiotics group despite these being less complex cases.” There was “nearly a fivefold increased odds of surgical site infection or any infectious complication when no antibiotics were given,” after controlling for infection risk factors, including smoking and diabetes, said investigator Nisse V. Clark, MD, a minimally invasive gynecologic surgeon affiliated with Massachusetts General Hospital.
There are no perioperative antibiotic guidelines for myomectomies; maybe there should be. Almost 94% of the women in the review did receive antibiotics at the Harvard-affiliated hospitals, but the nationwide average has been pegged at about two-thirds, she said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
The antibiotic cases usually received a cephalosporin before surgery, and were about evenly about evenly split between abdominal, robotic, and laparoscopic approaches.
About one-third of the 90 women (6.3%) who did not get antibiotics had hysteroscopic procedures in which antibiotics usually are not given because the peritoneal cavity is not breeched. Most of the rest, however, were laparoscopic cases. It’s unknown why they weren’t given antibiotics. In her own practice, Dr. Clark said preop antibiotics are the rule for laparoscopic myomectomies.
The surgical site infection difference was driven largely by higher incidences of pelvic abscesses and other organ space infections in the no-antibiotic group.
The only significant demographic difference between the two groups was that women who received antibiotics were slightly younger (mean 38 versus 39.7 years).
In addition to diabetes and smoking, the team adjusted for age, surgery route, body mass index, uterine entry, intraoperative complications, and myoma weight in their multivariate analysis. Still, women in the no-antibiotic group were 4.59 times more likely to have a surgical site infection, 4.76 more likely to have any infectious complication, and almost 8 times more likely to have a major infectious complication. All of the findings were statistically significant.
The study had no industry funding, and Dr. Clark had no disclosures.
LAS VEGAS – The surgical site infection rate was 2.9% among women who received perioperative antibiotics for fibroid surgery, but 7.8% among those who did not, in a review of 1,433 cases at Massachusetts General Hospital and Brigham and Women’s Hospital, Boston.
That is despite the fact that antibiotic cases were longer – 155 minutes vs. 89 minutes – and had more blood loss, 200 ml vs. 117 ml. Antibiotic cases also had larger specimen weights – 346 g vs. 176 g – and were more likely to have the uterine cavity entered, 30.2% vs. 14.4%.
“Surgical site infections were more common in the no-antibiotics group despite these being less complex cases.” There was “nearly a fivefold increased odds of surgical site infection or any infectious complication when no antibiotics were given,” after controlling for infection risk factors, including smoking and diabetes, said investigator Nisse V. Clark, MD, a minimally invasive gynecologic surgeon affiliated with Massachusetts General Hospital.
There are no perioperative antibiotic guidelines for myomectomies; maybe there should be. Almost 94% of the women in the review did receive antibiotics at the Harvard-affiliated hospitals, but the nationwide average has been pegged at about two-thirds, she said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
The antibiotic cases usually received a cephalosporin before surgery, and were about evenly about evenly split between abdominal, robotic, and laparoscopic approaches.
About one-third of the 90 women (6.3%) who did not get antibiotics had hysteroscopic procedures in which antibiotics usually are not given because the peritoneal cavity is not breeched. Most of the rest, however, were laparoscopic cases. It’s unknown why they weren’t given antibiotics. In her own practice, Dr. Clark said preop antibiotics are the rule for laparoscopic myomectomies.
The surgical site infection difference was driven largely by higher incidences of pelvic abscesses and other organ space infections in the no-antibiotic group.
The only significant demographic difference between the two groups was that women who received antibiotics were slightly younger (mean 38 versus 39.7 years).
In addition to diabetes and smoking, the team adjusted for age, surgery route, body mass index, uterine entry, intraoperative complications, and myoma weight in their multivariate analysis. Still, women in the no-antibiotic group were 4.59 times more likely to have a surgical site infection, 4.76 more likely to have any infectious complication, and almost 8 times more likely to have a major infectious complication. All of the findings were statistically significant.
The study had no industry funding, and Dr. Clark had no disclosures.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Key clinical point: A Boston study suggests that even low-risk cases benefit from antibiotics.
Major finding: The surgical site infection rate was 2.9% among women who received perioperative antibiotics for fibroid surgery, but 7.8% among those who did not.
Study details: Review of 1,433 myomectomies at two academic medical centers.
Disclosures: The study had no industry funding, and Dr. Clark had no disclosures.
2018 Update on minimally invasive gynecologic surgery
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel
Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.
Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.
Steps in the procedure

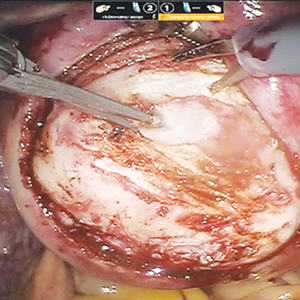
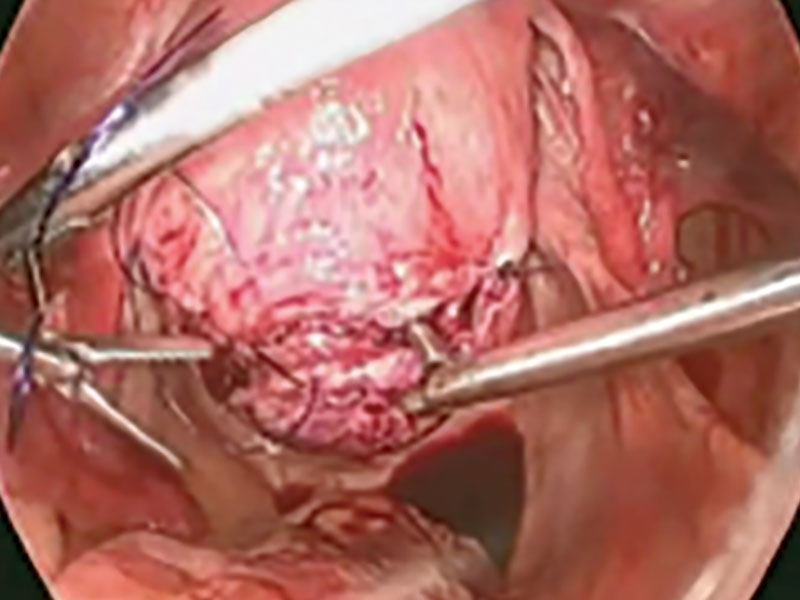
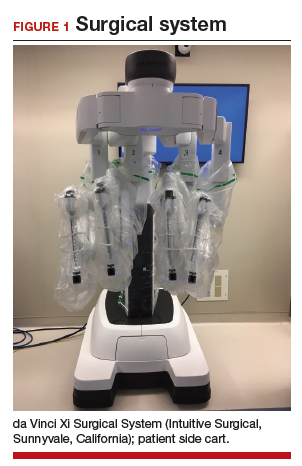
Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
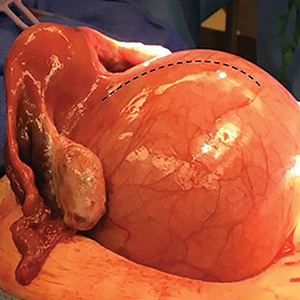
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

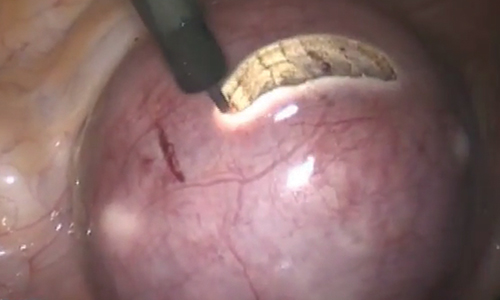
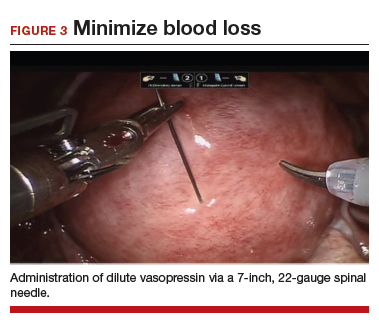
To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28
Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
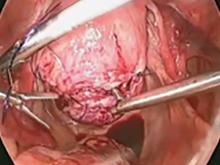
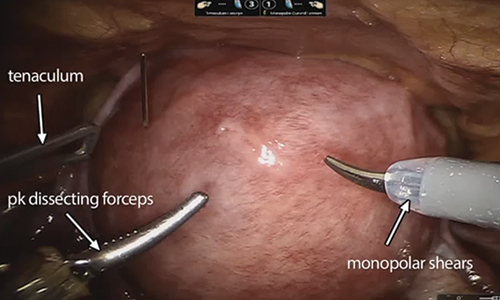
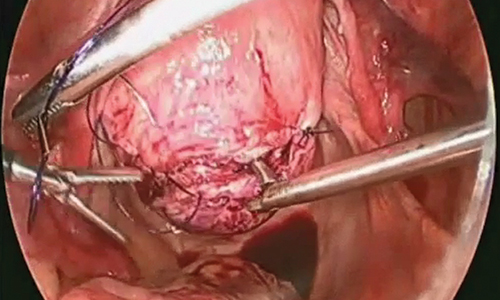
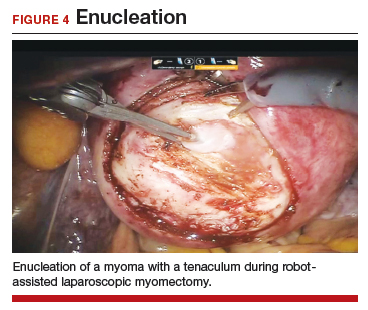
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.
As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
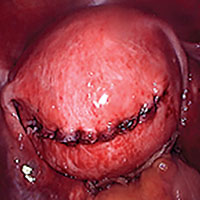
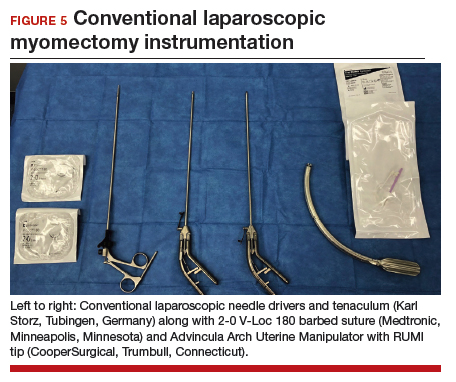
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.
Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel

Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.

Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.

Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28

Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.

As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.

Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel

Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.

Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.

Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28

Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.

As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.

Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
VTE risk after gynecologic surgery lower with laparoscopic procedures
according to a study published in Obstetrics & Gynecology.
The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Laparoscopic gynecologic surgery is associated with a lower risk of postoperative VTE than laparotomy.
Major finding: Laparoscopic hysterectomy was associated with a 78% lower incidence of postoperative VTE than laparotomy.
Study details: Retrospective cohort study of 37,485 patients who underwent 43,751 gynecologic surgical procedures
Disclosures: No conflicts of interest were declared.
Source: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
Myomectomy of a large cervical fibroid in a patient desiring future fertility
Uterine fibroids are the most common tumors of the uterus. Clinically significant fibroids that arise from the cervix are less common.1 Removing large cervical fibroids when a patient desires future fertility is a surgical challenge because of the risks of significant blood loss, bladder and ureteral injury, and unplanned hysterectomy. For women who desire future fertility, myomectomy can improve the chances of pregnancy by restoring normal anatomy.2 In this article, we describe a technique for myomectomy with uterine preservation in a patient with a 20-cm cervical fibroid.
CASE Woman with increasing girth and urinary symptoms is unable to conceive
A 33-year-old white woman with a history of 1 prior vaginal delivery presents with symptoms of increasing abdominal girth, intermittent urinary retention and urgency, and inability to become pregnant. She reports normal monthly menstrual periods. On pelvic examination, the ObGyn notes a large fibroid partially protruding through a dilated cervix. Abdominal examination reveals a fundal height at the level of the umbilicus.
Transvaginal ultrasonography shows a uterus that measures 4.5 x 6.1 x 13.6 cm. Arising from the posterior aspect of the uterine fundus, body, and lower uterine segment is a fibroid that measures 9.7 x 15.5 x 18.9 cm. Magnetic resonance imaging is performed and confirms a fibroid measuring 10 x 16 x 20 cm. The inferior-most aspect of the fibroid appears to be within the endometrial cavity and cervical canal. Most of the fibroid, however, is posterior to the uterus, pressing on and anteriorly displacing the endometrial cavity (FIGURE 1).

What is your surgical approach?
Comprehensive preoperative planning
In this case, the patient should receive extensive preoperative counseling about the significantly increased risk for hysterectomy with an attempted myomectomy. Prior to being scheduled for surgery, she also should have a consultation with a gynecologic oncologist. To optimize visualization during the procedure, we recommend to plan for a midline vertical skin incision. Because of the potential bleeding risks, blood products should be made available in the operating room at the time of surgery.
Techniques for surgery
Intraoperatively, a vertical midline incision exteriorizes the uterus from the peritoneal cavity. Opening of the retroperitoneal spaces allows for identification of the ureters. Perform dissection in the midline away from the ureters. Inject vasopressin (5 U) into the uterine fundus. Incise the uterine serosa over the myoma posteriorly in the midline.
Perform a myomectomy, with gentle “shelling out” of the myoma; in this way the specimen can be removed intact. Reapproximate the fibroid cavity in 3 layers with 0-Vicryl (polyglactin 910) suture in a running fashion (FIGURE 2).
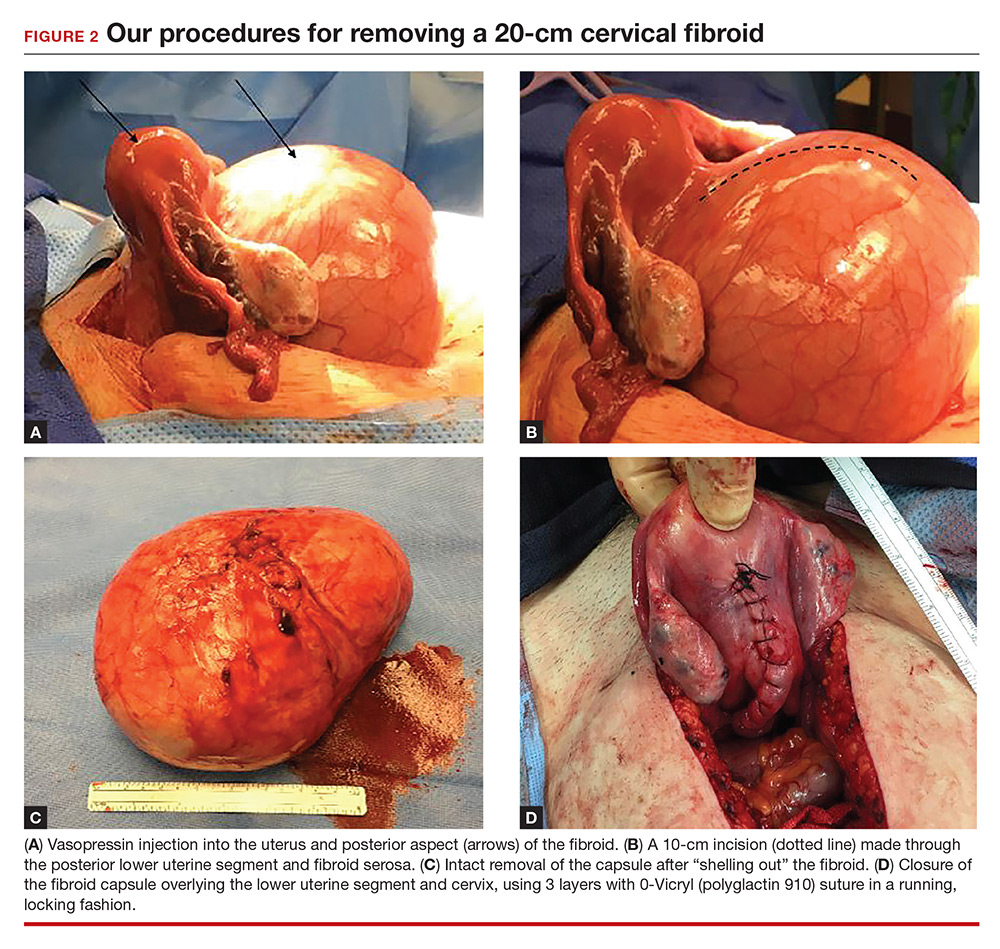
Continue to: CASE Resolved
CASE Resolved
The estimated blood loss during surgery was 50 mL. Final pathology reported a 1,660-g intact myoma. The patient’s postoperative course was uncomplicated and she was discharged home on postoperative day 1.
Her postoperative evaluation was 1 month later. Her abdominal incision was well healed. Her fibroid-related symptoms had resolved, and she planned to attempt pregnancy. Cesarean delivery for future pregnancies was recommended.
Increase the chances of a good outcome
Advanced planning for attempted myomectomy of a large cervical fibroid can increase the probability of a successful outcome. We suggest the following:
Counsel the patient on risks. Our preoperative strategy includes extensive counseling on the significantly increased surgical risks and the possibility of unavoidable hysterectomy. Given the anatomic distortion with respect to the ureters, bladder, and major blood vessels, involving gynecologic oncology is beneficial to the surgery planning process.
Prepare for possible transfusion. Ensure blood products are made available in the operating room in case transfusion is needed.
Control bleeding. Randomized studies have shown that intrauterine injection of vasopressin, through its action as a vasoconstrictor, decreases surgical bleeding.3,4 While little data are available on vasopressin’s most effective dosage and dilution, 5 U at a very dilute concentration (0.1–0.2 U/mL) has been recommended.5 A midline cervical incision away from lateral structures and gentle shelling out of the cervical fibroid help to avoid intraoperative damage to the bladder, ureters, and vascular supply.
Close in multiple layers. This approach can prevent a potential space for hematoma accumulation.6 Further, a multiple-layer closure of a myometrial incision may decrease the risk for uterine rupture in subsequent pregnancies.7
Advise abstinence postsurgery. There are no consistent data to guide patient counseling regarding recommendations for the timing of conception following myomectomy. We counseled our patient to abstain from vaginal intercourse for 4 weeks, after which time she soon should attempt to conceive. Although there are no published data regarding when it is best to resume sexual relations following such a surgery, we advise a 1-month period primarily to allow healing of the skin incision. Any further delay in attempting to become pregnant may allow for the growth of additional fibroids.
Plan for future deliveries. When the myomais extensively involved, such as in this case, we recommend cesarean delivery for future pregnancies to avoid the known risk of uterine rupture.8 In general, we recommend cesarean delivery in future pregnancies if an incision larger than 50% of the myometrial thickness is made in the contractile portion of the uterus.
Final takeaway. Despite increased surgical risks, myomectomy of a large cervical fibroid is possible and can alleviate symptoms and improve future fertility.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312–324.
- Milazzo GN, Catalano A, Badia V, Mallozzi M, Caserta D. Myoma and myomectomy: poor evidence concern in pregnancy. J Obstet Gynaecol Res. 2017;43(12):1789–1804.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97(6):867–872.
- Kongnyuy EJ, van den Broek N, Wiysonge CS. A systematic review of randomized controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. Int J Gynaecol Obstet. 2008;100(1):4–9.
- Barbieri RL. Give vasopressin to reduce bleeding in gynecologic surgery. OBG Manage. 2010;22(3):12–15.
- Tian YC, Long TF, Dai YN. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res. 2015;41(3):350–357.
- Bujold E, Bujold C, Hamilton EF, Harel F, Gauthier RJ. The impact of a single-layer or double-layer closure on uterine rupture. Am J Obstet Gynecol. 2002;186(6):1326–1330.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
Uterine fibroids are the most common tumors of the uterus. Clinically significant fibroids that arise from the cervix are less common.1 Removing large cervical fibroids when a patient desires future fertility is a surgical challenge because of the risks of significant blood loss, bladder and ureteral injury, and unplanned hysterectomy. For women who desire future fertility, myomectomy can improve the chances of pregnancy by restoring normal anatomy.2 In this article, we describe a technique for myomectomy with uterine preservation in a patient with a 20-cm cervical fibroid.
CASE Woman with increasing girth and urinary symptoms is unable to conceive
A 33-year-old white woman with a history of 1 prior vaginal delivery presents with symptoms of increasing abdominal girth, intermittent urinary retention and urgency, and inability to become pregnant. She reports normal monthly menstrual periods. On pelvic examination, the ObGyn notes a large fibroid partially protruding through a dilated cervix. Abdominal examination reveals a fundal height at the level of the umbilicus.
Transvaginal ultrasonography shows a uterus that measures 4.5 x 6.1 x 13.6 cm. Arising from the posterior aspect of the uterine fundus, body, and lower uterine segment is a fibroid that measures 9.7 x 15.5 x 18.9 cm. Magnetic resonance imaging is performed and confirms a fibroid measuring 10 x 16 x 20 cm. The inferior-most aspect of the fibroid appears to be within the endometrial cavity and cervical canal. Most of the fibroid, however, is posterior to the uterus, pressing on and anteriorly displacing the endometrial cavity (FIGURE 1).

What is your surgical approach?
Comprehensive preoperative planning
In this case, the patient should receive extensive preoperative counseling about the significantly increased risk for hysterectomy with an attempted myomectomy. Prior to being scheduled for surgery, she also should have a consultation with a gynecologic oncologist. To optimize visualization during the procedure, we recommend to plan for a midline vertical skin incision. Because of the potential bleeding risks, blood products should be made available in the operating room at the time of surgery.
Techniques for surgery
Intraoperatively, a vertical midline incision exteriorizes the uterus from the peritoneal cavity. Opening of the retroperitoneal spaces allows for identification of the ureters. Perform dissection in the midline away from the ureters. Inject vasopressin (5 U) into the uterine fundus. Incise the uterine serosa over the myoma posteriorly in the midline.
Perform a myomectomy, with gentle “shelling out” of the myoma; in this way the specimen can be removed intact. Reapproximate the fibroid cavity in 3 layers with 0-Vicryl (polyglactin 910) suture in a running fashion (FIGURE 2).

Continue to: CASE Resolved
CASE Resolved
The estimated blood loss during surgery was 50 mL. Final pathology reported a 1,660-g intact myoma. The patient’s postoperative course was uncomplicated and she was discharged home on postoperative day 1.
Her postoperative evaluation was 1 month later. Her abdominal incision was well healed. Her fibroid-related symptoms had resolved, and she planned to attempt pregnancy. Cesarean delivery for future pregnancies was recommended.
Increase the chances of a good outcome
Advanced planning for attempted myomectomy of a large cervical fibroid can increase the probability of a successful outcome. We suggest the following:
Counsel the patient on risks. Our preoperative strategy includes extensive counseling on the significantly increased surgical risks and the possibility of unavoidable hysterectomy. Given the anatomic distortion with respect to the ureters, bladder, and major blood vessels, involving gynecologic oncology is beneficial to the surgery planning process.
Prepare for possible transfusion. Ensure blood products are made available in the operating room in case transfusion is needed.
Control bleeding. Randomized studies have shown that intrauterine injection of vasopressin, through its action as a vasoconstrictor, decreases surgical bleeding.3,4 While little data are available on vasopressin’s most effective dosage and dilution, 5 U at a very dilute concentration (0.1–0.2 U/mL) has been recommended.5 A midline cervical incision away from lateral structures and gentle shelling out of the cervical fibroid help to avoid intraoperative damage to the bladder, ureters, and vascular supply.
Close in multiple layers. This approach can prevent a potential space for hematoma accumulation.6 Further, a multiple-layer closure of a myometrial incision may decrease the risk for uterine rupture in subsequent pregnancies.7
Advise abstinence postsurgery. There are no consistent data to guide patient counseling regarding recommendations for the timing of conception following myomectomy. We counseled our patient to abstain from vaginal intercourse for 4 weeks, after which time she soon should attempt to conceive. Although there are no published data regarding when it is best to resume sexual relations following such a surgery, we advise a 1-month period primarily to allow healing of the skin incision. Any further delay in attempting to become pregnant may allow for the growth of additional fibroids.
Plan for future deliveries. When the myomais extensively involved, such as in this case, we recommend cesarean delivery for future pregnancies to avoid the known risk of uterine rupture.8 In general, we recommend cesarean delivery in future pregnancies if an incision larger than 50% of the myometrial thickness is made in the contractile portion of the uterus.
Final takeaway. Despite increased surgical risks, myomectomy of a large cervical fibroid is possible and can alleviate symptoms and improve future fertility.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Uterine fibroids are the most common tumors of the uterus. Clinically significant fibroids that arise from the cervix are less common.1 Removing large cervical fibroids when a patient desires future fertility is a surgical challenge because of the risks of significant blood loss, bladder and ureteral injury, and unplanned hysterectomy. For women who desire future fertility, myomectomy can improve the chances of pregnancy by restoring normal anatomy.2 In this article, we describe a technique for myomectomy with uterine preservation in a patient with a 20-cm cervical fibroid.
CASE Woman with increasing girth and urinary symptoms is unable to conceive
A 33-year-old white woman with a history of 1 prior vaginal delivery presents with symptoms of increasing abdominal girth, intermittent urinary retention and urgency, and inability to become pregnant. She reports normal monthly menstrual periods. On pelvic examination, the ObGyn notes a large fibroid partially protruding through a dilated cervix. Abdominal examination reveals a fundal height at the level of the umbilicus.
Transvaginal ultrasonography shows a uterus that measures 4.5 x 6.1 x 13.6 cm. Arising from the posterior aspect of the uterine fundus, body, and lower uterine segment is a fibroid that measures 9.7 x 15.5 x 18.9 cm. Magnetic resonance imaging is performed and confirms a fibroid measuring 10 x 16 x 20 cm. The inferior-most aspect of the fibroid appears to be within the endometrial cavity and cervical canal. Most of the fibroid, however, is posterior to the uterus, pressing on and anteriorly displacing the endometrial cavity (FIGURE 1).

What is your surgical approach?
Comprehensive preoperative planning
In this case, the patient should receive extensive preoperative counseling about the significantly increased risk for hysterectomy with an attempted myomectomy. Prior to being scheduled for surgery, she also should have a consultation with a gynecologic oncologist. To optimize visualization during the procedure, we recommend to plan for a midline vertical skin incision. Because of the potential bleeding risks, blood products should be made available in the operating room at the time of surgery.
Techniques for surgery
Intraoperatively, a vertical midline incision exteriorizes the uterus from the peritoneal cavity. Opening of the retroperitoneal spaces allows for identification of the ureters. Perform dissection in the midline away from the ureters. Inject vasopressin (5 U) into the uterine fundus. Incise the uterine serosa over the myoma posteriorly in the midline.
Perform a myomectomy, with gentle “shelling out” of the myoma; in this way the specimen can be removed intact. Reapproximate the fibroid cavity in 3 layers with 0-Vicryl (polyglactin 910) suture in a running fashion (FIGURE 2).

Continue to: CASE Resolved
CASE Resolved
The estimated blood loss during surgery was 50 mL. Final pathology reported a 1,660-g intact myoma. The patient’s postoperative course was uncomplicated and she was discharged home on postoperative day 1.
Her postoperative evaluation was 1 month later. Her abdominal incision was well healed. Her fibroid-related symptoms had resolved, and she planned to attempt pregnancy. Cesarean delivery for future pregnancies was recommended.
Increase the chances of a good outcome
Advanced planning for attempted myomectomy of a large cervical fibroid can increase the probability of a successful outcome. We suggest the following:
Counsel the patient on risks. Our preoperative strategy includes extensive counseling on the significantly increased surgical risks and the possibility of unavoidable hysterectomy. Given the anatomic distortion with respect to the ureters, bladder, and major blood vessels, involving gynecologic oncology is beneficial to the surgery planning process.
Prepare for possible transfusion. Ensure blood products are made available in the operating room in case transfusion is needed.
Control bleeding. Randomized studies have shown that intrauterine injection of vasopressin, through its action as a vasoconstrictor, decreases surgical bleeding.3,4 While little data are available on vasopressin’s most effective dosage and dilution, 5 U at a very dilute concentration (0.1–0.2 U/mL) has been recommended.5 A midline cervical incision away from lateral structures and gentle shelling out of the cervical fibroid help to avoid intraoperative damage to the bladder, ureters, and vascular supply.
Close in multiple layers. This approach can prevent a potential space for hematoma accumulation.6 Further, a multiple-layer closure of a myometrial incision may decrease the risk for uterine rupture in subsequent pregnancies.7
Advise abstinence postsurgery. There are no consistent data to guide patient counseling regarding recommendations for the timing of conception following myomectomy. We counseled our patient to abstain from vaginal intercourse for 4 weeks, after which time she soon should attempt to conceive. Although there are no published data regarding when it is best to resume sexual relations following such a surgery, we advise a 1-month period primarily to allow healing of the skin incision. Any further delay in attempting to become pregnant may allow for the growth of additional fibroids.
Plan for future deliveries. When the myomais extensively involved, such as in this case, we recommend cesarean delivery for future pregnancies to avoid the known risk of uterine rupture.8 In general, we recommend cesarean delivery in future pregnancies if an incision larger than 50% of the myometrial thickness is made in the contractile portion of the uterus.
Final takeaway. Despite increased surgical risks, myomectomy of a large cervical fibroid is possible and can alleviate symptoms and improve future fertility.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312–324.
- Milazzo GN, Catalano A, Badia V, Mallozzi M, Caserta D. Myoma and myomectomy: poor evidence concern in pregnancy. J Obstet Gynaecol Res. 2017;43(12):1789–1804.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97(6):867–872.
- Kongnyuy EJ, van den Broek N, Wiysonge CS. A systematic review of randomized controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. Int J Gynaecol Obstet. 2008;100(1):4–9.
- Barbieri RL. Give vasopressin to reduce bleeding in gynecologic surgery. OBG Manage. 2010;22(3):12–15.
- Tian YC, Long TF, Dai YN. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res. 2015;41(3):350–357.
- Bujold E, Bujold C, Hamilton EF, Harel F, Gauthier RJ. The impact of a single-layer or double-layer closure on uterine rupture. Am J Obstet Gynecol. 2002;186(6):1326–1330.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312–324.
- Milazzo GN, Catalano A, Badia V, Mallozzi M, Caserta D. Myoma and myomectomy: poor evidence concern in pregnancy. J Obstet Gynaecol Res. 2017;43(12):1789–1804.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97(6):867–872.
- Kongnyuy EJ, van den Broek N, Wiysonge CS. A systematic review of randomized controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. Int J Gynaecol Obstet. 2008;100(1):4–9.
- Barbieri RL. Give vasopressin to reduce bleeding in gynecologic surgery. OBG Manage. 2010;22(3):12–15.
- Tian YC, Long TF, Dai YN. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res. 2015;41(3):350–357.
- Bujold E, Bujold C, Hamilton EF, Harel F, Gauthier RJ. The impact of a single-layer or double-layer closure on uterine rupture. Am J Obstet Gynecol. 2002;186(6):1326–1330.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
Laparoscopic myomectomy: Tips for patient selection and technique
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.
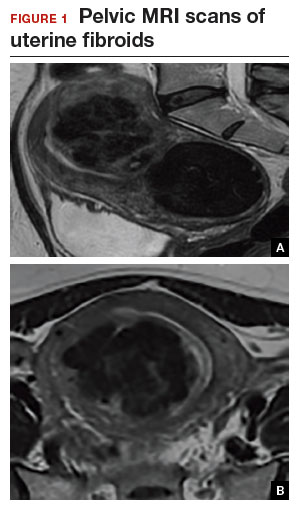
Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.
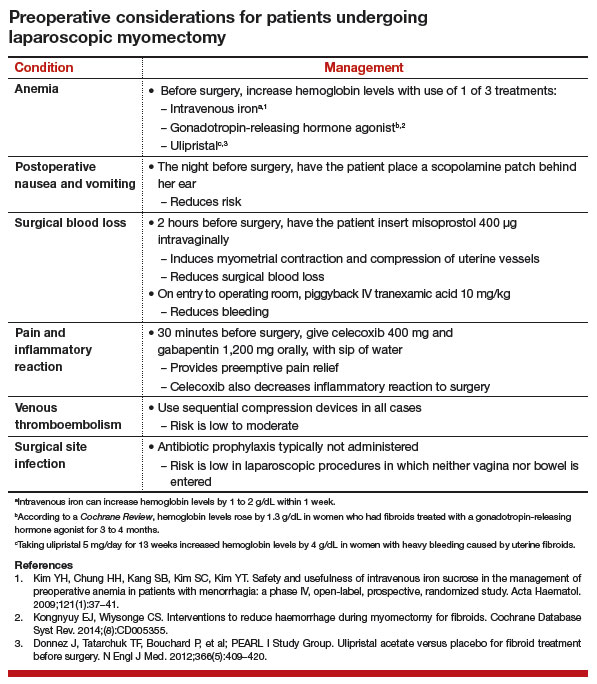
Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.
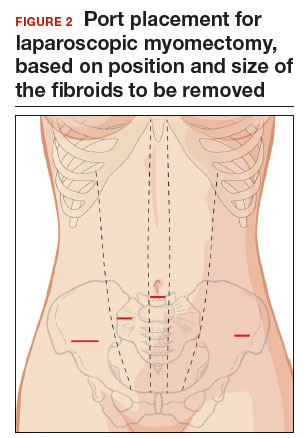
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.
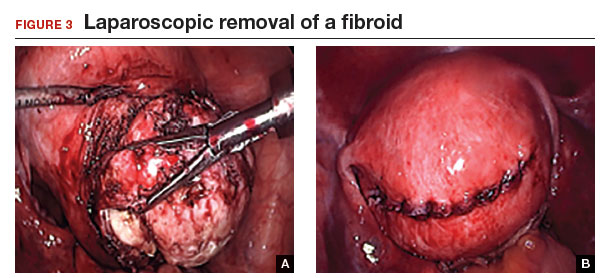
The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.

Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.

Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.

Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.

The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.

Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.

Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.

Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.

The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
Laparoscopic myomectomy technique
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Abdominal myomectomy: Patient and surgical technique considerations
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.
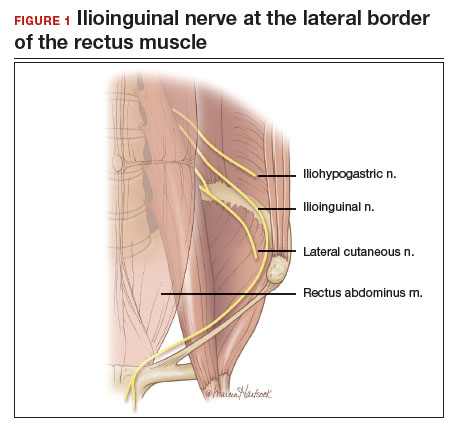
Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.
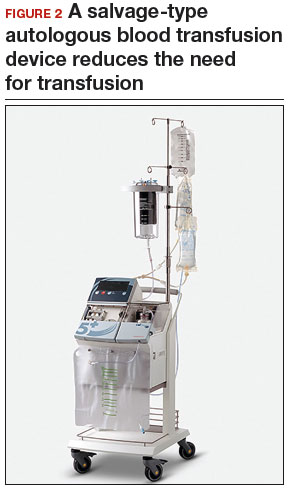
Additional surgical considerations
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17
Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20
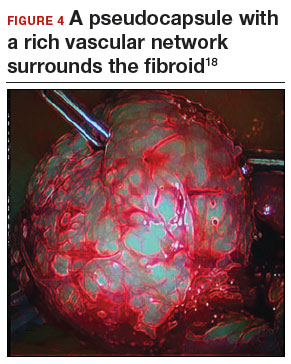
Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.

Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.

Additional surgical considerations
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17

Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20

Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.

Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.

Additional surgical considerations
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17

Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20

Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
7 Myomectomy myths debunked
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6
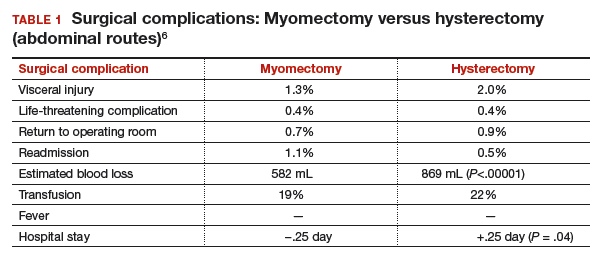
MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.
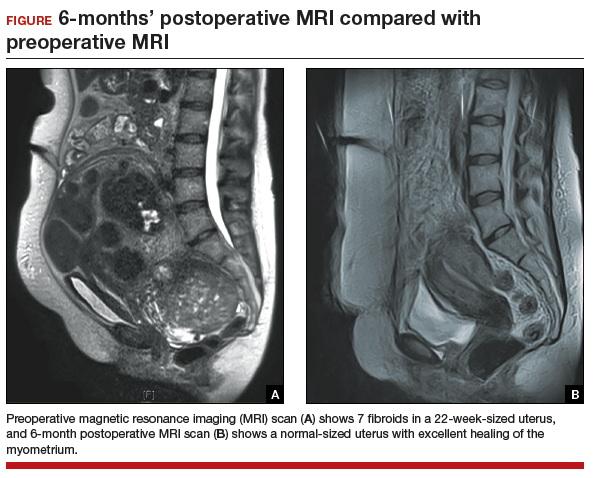
MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15

Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20
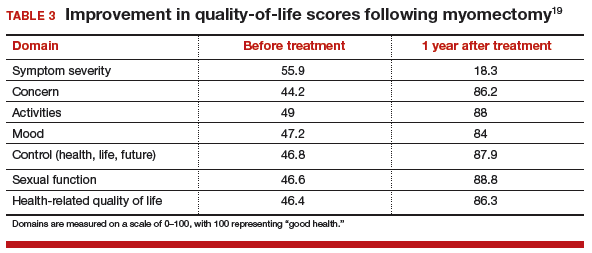
For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6

MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.

MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15

Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20

For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6

MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.

MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15

Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20

For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.