User login
Rapid Molecular Diagnosis Deemed a Game Changer for UTI Management
WASHINGTON – Rapid molecular diagnosis of urinary tract infection will soon enable individualized, evidence-based selection of antibiotics "right at the point of care," according to Dr. Joseph C. Liao.
"Currently we rely on urine culture, which takes 2-3 days at a central microbiology laboratory," he said. "What if in the future [you] could obtain molecular diagnosis in less than 1 hour right there in your office? And what if we could tailor the choice of antibiotics for the patient sitting in your office [rather than start broad-spectrum antibiotics empirically]?"
Personalized, evidence-based selection of antibiotics has become an increasingly important goal – for both individual and public health – as the problem of antibiotic resistance has intensified, said Dr. Liao and other speakers at the annual meeting of the American Urological Association.
Over the past several years, Dr. Liao and his colleagues in the urology department at Stanford (Calif.) University have used biosensor technology to develop an assay for rapid pathogen identification, as well as a biosensor-based antimicrobial susceptibility test for urinary tract infection (UTI).
"We’ve been able to achieve pathogen identification within an hour, and antimicrobial susceptibility testing within 3 hours," Dr. Liao reported.
The biosensor (a molecular sensing device that generates a measurable signal in the presence of a target analyte) is already part of everyday clinical practice, he noted. The glucose sensor and the i-STAT portable clinical analyzer, for example, are commonly used biosensor-based devices.
The biosensor being utilized in the "next generation" of UTI diagnostic tools is comprised of a chip about the size of a microscope slide with 16 individual sensors. "Like computer technology, it can be mass produced at a relatively low cost," said Dr. Liao, who is also chief urologist at the Veterans Affairs Palo Alto (Calif.) Health Care System.
The overall strategy for pathogen identification involves lysis of the bacteria present in a urine sample, followed by a hybridization process that enables the sensor to detect bacterial 16S rRNA, a kind of "bacterial molecular fingerprint." This results in a signal output.
"Essentially, we’re converting a molecular hybridization event into an electrical signal," Dr. Liao explained. "And the higher the bacterial concentration, the higher the signal."
Bacterial 16S rRNA is also a marker of bacterial growth, a property that Dr. Liao’s research group has exploited to develop a biosensor-based antimicrobial susceptibility test (AST). By incubating a urine sample in the presence or absence of commonly used antibiotics, and quantifying the 16S rRNA level, "we can follow the differential growth and derive the AST," he said.
Dr. Liao and his colleagues recently completed a clinical validation study in which they compared results from their biosensor platform with results from standard microbiological analysis in more than 200 urine samples collected from patients at the Spinal Cord Injury Service at the Veterans Affairs Palo Alto system.
Pathogen detection sensitivity and specificity were 92% and 97%, respectively, and "in corresponding AST, we found an overall agreement of 94%," said Dr. Liao, whose study was published early this year (J. Urol. 2011;185:148-53).
In the future, Dr. Liao hopes to use biosensor technology to also detect biomarkers that are shown to be indicative of infection in the presence of pathogens, he said. Such an integrated assay would detect both biomarkers and pathogens, and thus address the host immunity response as well as identify the pathogen. This could further improve the now-challenging task of differentiating colonization, simple UTI, and early complicated UTI, "and [could] help us better differentiate and stratify the severity of infection," he said.
Dr. Liao reported that he had no disclosures.
WASHINGTON – Rapid molecular diagnosis of urinary tract infection will soon enable individualized, evidence-based selection of antibiotics "right at the point of care," according to Dr. Joseph C. Liao.
"Currently we rely on urine culture, which takes 2-3 days at a central microbiology laboratory," he said. "What if in the future [you] could obtain molecular diagnosis in less than 1 hour right there in your office? And what if we could tailor the choice of antibiotics for the patient sitting in your office [rather than start broad-spectrum antibiotics empirically]?"
Personalized, evidence-based selection of antibiotics has become an increasingly important goal – for both individual and public health – as the problem of antibiotic resistance has intensified, said Dr. Liao and other speakers at the annual meeting of the American Urological Association.
Over the past several years, Dr. Liao and his colleagues in the urology department at Stanford (Calif.) University have used biosensor technology to develop an assay for rapid pathogen identification, as well as a biosensor-based antimicrobial susceptibility test for urinary tract infection (UTI).
"We’ve been able to achieve pathogen identification within an hour, and antimicrobial susceptibility testing within 3 hours," Dr. Liao reported.
The biosensor (a molecular sensing device that generates a measurable signal in the presence of a target analyte) is already part of everyday clinical practice, he noted. The glucose sensor and the i-STAT portable clinical analyzer, for example, are commonly used biosensor-based devices.
The biosensor being utilized in the "next generation" of UTI diagnostic tools is comprised of a chip about the size of a microscope slide with 16 individual sensors. "Like computer technology, it can be mass produced at a relatively low cost," said Dr. Liao, who is also chief urologist at the Veterans Affairs Palo Alto (Calif.) Health Care System.
The overall strategy for pathogen identification involves lysis of the bacteria present in a urine sample, followed by a hybridization process that enables the sensor to detect bacterial 16S rRNA, a kind of "bacterial molecular fingerprint." This results in a signal output.
"Essentially, we’re converting a molecular hybridization event into an electrical signal," Dr. Liao explained. "And the higher the bacterial concentration, the higher the signal."
Bacterial 16S rRNA is also a marker of bacterial growth, a property that Dr. Liao’s research group has exploited to develop a biosensor-based antimicrobial susceptibility test (AST). By incubating a urine sample in the presence or absence of commonly used antibiotics, and quantifying the 16S rRNA level, "we can follow the differential growth and derive the AST," he said.
Dr. Liao and his colleagues recently completed a clinical validation study in which they compared results from their biosensor platform with results from standard microbiological analysis in more than 200 urine samples collected from patients at the Spinal Cord Injury Service at the Veterans Affairs Palo Alto system.
Pathogen detection sensitivity and specificity were 92% and 97%, respectively, and "in corresponding AST, we found an overall agreement of 94%," said Dr. Liao, whose study was published early this year (J. Urol. 2011;185:148-53).
In the future, Dr. Liao hopes to use biosensor technology to also detect biomarkers that are shown to be indicative of infection in the presence of pathogens, he said. Such an integrated assay would detect both biomarkers and pathogens, and thus address the host immunity response as well as identify the pathogen. This could further improve the now-challenging task of differentiating colonization, simple UTI, and early complicated UTI, "and [could] help us better differentiate and stratify the severity of infection," he said.
Dr. Liao reported that he had no disclosures.
WASHINGTON – Rapid molecular diagnosis of urinary tract infection will soon enable individualized, evidence-based selection of antibiotics "right at the point of care," according to Dr. Joseph C. Liao.
"Currently we rely on urine culture, which takes 2-3 days at a central microbiology laboratory," he said. "What if in the future [you] could obtain molecular diagnosis in less than 1 hour right there in your office? And what if we could tailor the choice of antibiotics for the patient sitting in your office [rather than start broad-spectrum antibiotics empirically]?"
Personalized, evidence-based selection of antibiotics has become an increasingly important goal – for both individual and public health – as the problem of antibiotic resistance has intensified, said Dr. Liao and other speakers at the annual meeting of the American Urological Association.
Over the past several years, Dr. Liao and his colleagues in the urology department at Stanford (Calif.) University have used biosensor technology to develop an assay for rapid pathogen identification, as well as a biosensor-based antimicrobial susceptibility test for urinary tract infection (UTI).
"We’ve been able to achieve pathogen identification within an hour, and antimicrobial susceptibility testing within 3 hours," Dr. Liao reported.
The biosensor (a molecular sensing device that generates a measurable signal in the presence of a target analyte) is already part of everyday clinical practice, he noted. The glucose sensor and the i-STAT portable clinical analyzer, for example, are commonly used biosensor-based devices.
The biosensor being utilized in the "next generation" of UTI diagnostic tools is comprised of a chip about the size of a microscope slide with 16 individual sensors. "Like computer technology, it can be mass produced at a relatively low cost," said Dr. Liao, who is also chief urologist at the Veterans Affairs Palo Alto (Calif.) Health Care System.
The overall strategy for pathogen identification involves lysis of the bacteria present in a urine sample, followed by a hybridization process that enables the sensor to detect bacterial 16S rRNA, a kind of "bacterial molecular fingerprint." This results in a signal output.
"Essentially, we’re converting a molecular hybridization event into an electrical signal," Dr. Liao explained. "And the higher the bacterial concentration, the higher the signal."
Bacterial 16S rRNA is also a marker of bacterial growth, a property that Dr. Liao’s research group has exploited to develop a biosensor-based antimicrobial susceptibility test (AST). By incubating a urine sample in the presence or absence of commonly used antibiotics, and quantifying the 16S rRNA level, "we can follow the differential growth and derive the AST," he said.
Dr. Liao and his colleagues recently completed a clinical validation study in which they compared results from their biosensor platform with results from standard microbiological analysis in more than 200 urine samples collected from patients at the Spinal Cord Injury Service at the Veterans Affairs Palo Alto system.
Pathogen detection sensitivity and specificity were 92% and 97%, respectively, and "in corresponding AST, we found an overall agreement of 94%," said Dr. Liao, whose study was published early this year (J. Urol. 2011;185:148-53).
In the future, Dr. Liao hopes to use biosensor technology to also detect biomarkers that are shown to be indicative of infection in the presence of pathogens, he said. Such an integrated assay would detect both biomarkers and pathogens, and thus address the host immunity response as well as identify the pathogen. This could further improve the now-challenging task of differentiating colonization, simple UTI, and early complicated UTI, "and [could] help us better differentiate and stratify the severity of infection," he said.
Dr. Liao reported that he had no disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN UROLGICAL ASSOCIATION
KEEP Results Show Promise of New Equation
NEW YORK – Boosting patient awareness and use of a new equation for measuring glomerular filtration rate are showing early signs of being the cornerstones to the prevention of chronic kidney disease among people at risk for the disease, according to preliminary results from the National Kidney Foundation’s nationwide screening initiative.
The Kidney Early Evaluation Program (KEEP) has enrolled 150,000 people so far, making it the nation’s largest perpetual chronic kidney disease screening program, according to Dr. Joseph Vassalotti, chief medical officer of the National Kidney Foundation and a faculty member at Mount Sinai School of Medicine, New York.
The point is to identify "individuals in the community with kidney disease along with the risk factors that go with it," said Dr. George Bakris of the University of Chicago Medical Center and a former KEEP principal investigator. All of the participants have some stage of kidney disease or hypertension and are referred to the registry by physicians. Enrollment is voluntary and participants complete a questionnaire and undergo a medical evaluation that includes standard panels plus testing for calcium, phosphorus, and parathyroid hormone. The program does not accept people who have had a kidney transplant or are on dialysis, he said.
Under the program, a new method for estimating glomerular filtration rate (eGFR), known as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, has been used. This equation is more accurate in determining kidney function than is the Modification of Diet in Renal Disease (MDRD) Study equation, said Dr. Lesley Stevens of Tufts Medical Center, Boston. "The use of the CKD-EPI equation more accurately reflects measured GFR and results in a lower prevalence of eGFR below 60 mL/min per 1.73 m2," said Dr. Stevens, a KEEP committee member. As a result, there are fewer false positives and "ultimately, fewer tests and lower costs," she said.
According to Dr. Stevens, the equation has been validated in two clinical studies (Am. J. Kidney Dis. 2010;55:648-59 and Am. J. Kidney Dis. 2010;55:660-70).
One of the goals of KEEP is to determine factors that help slow the slide into chronic kidney disease, as well as the loss of kidney function over time, Dr. Vassalotti said. "Multiple trials on systolic blood pressure show that untreated hypertension results in a loss of 10-12 mL/min per 1.73 m2 eGFR a year," he said. "In individuals with blood pressure in the target range, the annual loss is approximately 2-3 mL/min per 1.73 m2. By educating participants and fostering their engagement with local clinicians, KEEP can impact care."
Managing blood pressure early in kidney disease is critical because as eGFR declines, hypertension becomes more difficult to control, Dr. Vassalotti said. Thus, self-reported hypertension is one of the qualifiers for KEEP enrollment, he said. He cited early data from KEEP that showed prevalence, awareness and treatment of hypertension increased with stage of chronic kidney disease, but that didn’t necessarily translate to control with severe disease. Among those with stage 3 disease, only 82% were undergoing treatment, compared with 92% of those with stage 4 disease. However, 38% of the stage 3 group had systolic blood pressure below 140 mm Hg, while only 35% of the stage 4 group did (Am. J. Med. 2008; 121:332-40).
KEEP data have shown an uptick in overall blood pressure control, from 45% at the initial screening to 49% at rescreening, Dr. Vassalotti said, and even some improvement in control without medication. "But I wouldn’t overstate this."
The project involves merging data with the National Death Index and the United States Renal Data System to track survival and chronic kidney failure outcomes, respectively. Future goals include additional data links with Medicare Parts B and D to gain clarity on morbidity and medication use, Dr. Vassalotti said. "Preliminary longitudinal data show that community screening appears to influence hypertension despite progression of chronic kidney disease," he said.
Patient awareness is the cornerstone of hypertension management, noted Dr. Adam Whaley-Connell of the University of Missouri. "Awareness has a very important role in not only understanding blood pressure control, especially in the context of the early stages of kidney disease, but in improving kidney related patient outcomes," he said. However, he noted that awareness of kidney disease, particularly in its early stages, "is alarmingly low."
He noted that KEEP data on awareness reflect that of National Health and Nutrition Examination Survey and other CKD cohort studies: Only about 5% of those with stages 1-3 are aware of their disease. "Roughly 95% of individuals entering into the screening program are unaware they have chronic kidney disease," Dr. Whaley-Connell said.
KEEP enrollees with cardiovascular disease had a keener awareness of the risks of kidney disease, he said. "While awareness may be triggered by cardiovascular disease, those unaware in the earlier stages of kidney disease are particularly vulnerable to poor risk factor control and increased risk for mortality and progression to end-stage renal disease," Dr. Whaley-Connell said. "We advocate for targeted education and awareness at earlier stages of kidney disease to improve risk factor control."
"I think any general practitioner seeing patients needs to pay attention to eGFR," Dr. Bakris said. "If the patient has stage 2 disease and an eGFR of 60-80 mL/min per 1.73 m2 now, especially with the CKD-EPI equation, the next question out of his mouth should be, ‘Do you have a family history of kidney disease?’"
However, he cited National Kidney Foundation data that showed only 31% of physicians considered family history an important factor. "If your patient has a family history of kidney disease and an eGFR of 50 mL/min per 1.73 m2 ... you really do need to pay attention to that and do something about it."
KEEP is funded by Amgen, Abbott, Siemens, Astellas, Genzyme, Fresenius Medical Care, Pfizer, Nephroceuticals and the LifeScan unit of Johnson & Johnson. Panel participants disclosed affiliations with a variety of device and pharmaceutical companies.
NEW YORK – Boosting patient awareness and use of a new equation for measuring glomerular filtration rate are showing early signs of being the cornerstones to the prevention of chronic kidney disease among people at risk for the disease, according to preliminary results from the National Kidney Foundation’s nationwide screening initiative.
The Kidney Early Evaluation Program (KEEP) has enrolled 150,000 people so far, making it the nation’s largest perpetual chronic kidney disease screening program, according to Dr. Joseph Vassalotti, chief medical officer of the National Kidney Foundation and a faculty member at Mount Sinai School of Medicine, New York.
The point is to identify "individuals in the community with kidney disease along with the risk factors that go with it," said Dr. George Bakris of the University of Chicago Medical Center and a former KEEP principal investigator. All of the participants have some stage of kidney disease or hypertension and are referred to the registry by physicians. Enrollment is voluntary and participants complete a questionnaire and undergo a medical evaluation that includes standard panels plus testing for calcium, phosphorus, and parathyroid hormone. The program does not accept people who have had a kidney transplant or are on dialysis, he said.
Under the program, a new method for estimating glomerular filtration rate (eGFR), known as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, has been used. This equation is more accurate in determining kidney function than is the Modification of Diet in Renal Disease (MDRD) Study equation, said Dr. Lesley Stevens of Tufts Medical Center, Boston. "The use of the CKD-EPI equation more accurately reflects measured GFR and results in a lower prevalence of eGFR below 60 mL/min per 1.73 m2," said Dr. Stevens, a KEEP committee member. As a result, there are fewer false positives and "ultimately, fewer tests and lower costs," she said.
According to Dr. Stevens, the equation has been validated in two clinical studies (Am. J. Kidney Dis. 2010;55:648-59 and Am. J. Kidney Dis. 2010;55:660-70).
One of the goals of KEEP is to determine factors that help slow the slide into chronic kidney disease, as well as the loss of kidney function over time, Dr. Vassalotti said. "Multiple trials on systolic blood pressure show that untreated hypertension results in a loss of 10-12 mL/min per 1.73 m2 eGFR a year," he said. "In individuals with blood pressure in the target range, the annual loss is approximately 2-3 mL/min per 1.73 m2. By educating participants and fostering their engagement with local clinicians, KEEP can impact care."
Managing blood pressure early in kidney disease is critical because as eGFR declines, hypertension becomes more difficult to control, Dr. Vassalotti said. Thus, self-reported hypertension is one of the qualifiers for KEEP enrollment, he said. He cited early data from KEEP that showed prevalence, awareness and treatment of hypertension increased with stage of chronic kidney disease, but that didn’t necessarily translate to control with severe disease. Among those with stage 3 disease, only 82% were undergoing treatment, compared with 92% of those with stage 4 disease. However, 38% of the stage 3 group had systolic blood pressure below 140 mm Hg, while only 35% of the stage 4 group did (Am. J. Med. 2008; 121:332-40).
KEEP data have shown an uptick in overall blood pressure control, from 45% at the initial screening to 49% at rescreening, Dr. Vassalotti said, and even some improvement in control without medication. "But I wouldn’t overstate this."
The project involves merging data with the National Death Index and the United States Renal Data System to track survival and chronic kidney failure outcomes, respectively. Future goals include additional data links with Medicare Parts B and D to gain clarity on morbidity and medication use, Dr. Vassalotti said. "Preliminary longitudinal data show that community screening appears to influence hypertension despite progression of chronic kidney disease," he said.
Patient awareness is the cornerstone of hypertension management, noted Dr. Adam Whaley-Connell of the University of Missouri. "Awareness has a very important role in not only understanding blood pressure control, especially in the context of the early stages of kidney disease, but in improving kidney related patient outcomes," he said. However, he noted that awareness of kidney disease, particularly in its early stages, "is alarmingly low."
He noted that KEEP data on awareness reflect that of National Health and Nutrition Examination Survey and other CKD cohort studies: Only about 5% of those with stages 1-3 are aware of their disease. "Roughly 95% of individuals entering into the screening program are unaware they have chronic kidney disease," Dr. Whaley-Connell said.
KEEP enrollees with cardiovascular disease had a keener awareness of the risks of kidney disease, he said. "While awareness may be triggered by cardiovascular disease, those unaware in the earlier stages of kidney disease are particularly vulnerable to poor risk factor control and increased risk for mortality and progression to end-stage renal disease," Dr. Whaley-Connell said. "We advocate for targeted education and awareness at earlier stages of kidney disease to improve risk factor control."
"I think any general practitioner seeing patients needs to pay attention to eGFR," Dr. Bakris said. "If the patient has stage 2 disease and an eGFR of 60-80 mL/min per 1.73 m2 now, especially with the CKD-EPI equation, the next question out of his mouth should be, ‘Do you have a family history of kidney disease?’"
However, he cited National Kidney Foundation data that showed only 31% of physicians considered family history an important factor. "If your patient has a family history of kidney disease and an eGFR of 50 mL/min per 1.73 m2 ... you really do need to pay attention to that and do something about it."
KEEP is funded by Amgen, Abbott, Siemens, Astellas, Genzyme, Fresenius Medical Care, Pfizer, Nephroceuticals and the LifeScan unit of Johnson & Johnson. Panel participants disclosed affiliations with a variety of device and pharmaceutical companies.
NEW YORK – Boosting patient awareness and use of a new equation for measuring glomerular filtration rate are showing early signs of being the cornerstones to the prevention of chronic kidney disease among people at risk for the disease, according to preliminary results from the National Kidney Foundation’s nationwide screening initiative.
The Kidney Early Evaluation Program (KEEP) has enrolled 150,000 people so far, making it the nation’s largest perpetual chronic kidney disease screening program, according to Dr. Joseph Vassalotti, chief medical officer of the National Kidney Foundation and a faculty member at Mount Sinai School of Medicine, New York.
The point is to identify "individuals in the community with kidney disease along with the risk factors that go with it," said Dr. George Bakris of the University of Chicago Medical Center and a former KEEP principal investigator. All of the participants have some stage of kidney disease or hypertension and are referred to the registry by physicians. Enrollment is voluntary and participants complete a questionnaire and undergo a medical evaluation that includes standard panels plus testing for calcium, phosphorus, and parathyroid hormone. The program does not accept people who have had a kidney transplant or are on dialysis, he said.
Under the program, a new method for estimating glomerular filtration rate (eGFR), known as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, has been used. This equation is more accurate in determining kidney function than is the Modification of Diet in Renal Disease (MDRD) Study equation, said Dr. Lesley Stevens of Tufts Medical Center, Boston. "The use of the CKD-EPI equation more accurately reflects measured GFR and results in a lower prevalence of eGFR below 60 mL/min per 1.73 m2," said Dr. Stevens, a KEEP committee member. As a result, there are fewer false positives and "ultimately, fewer tests and lower costs," she said.
According to Dr. Stevens, the equation has been validated in two clinical studies (Am. J. Kidney Dis. 2010;55:648-59 and Am. J. Kidney Dis. 2010;55:660-70).
One of the goals of KEEP is to determine factors that help slow the slide into chronic kidney disease, as well as the loss of kidney function over time, Dr. Vassalotti said. "Multiple trials on systolic blood pressure show that untreated hypertension results in a loss of 10-12 mL/min per 1.73 m2 eGFR a year," he said. "In individuals with blood pressure in the target range, the annual loss is approximately 2-3 mL/min per 1.73 m2. By educating participants and fostering their engagement with local clinicians, KEEP can impact care."
Managing blood pressure early in kidney disease is critical because as eGFR declines, hypertension becomes more difficult to control, Dr. Vassalotti said. Thus, self-reported hypertension is one of the qualifiers for KEEP enrollment, he said. He cited early data from KEEP that showed prevalence, awareness and treatment of hypertension increased with stage of chronic kidney disease, but that didn’t necessarily translate to control with severe disease. Among those with stage 3 disease, only 82% were undergoing treatment, compared with 92% of those with stage 4 disease. However, 38% of the stage 3 group had systolic blood pressure below 140 mm Hg, while only 35% of the stage 4 group did (Am. J. Med. 2008; 121:332-40).
KEEP data have shown an uptick in overall blood pressure control, from 45% at the initial screening to 49% at rescreening, Dr. Vassalotti said, and even some improvement in control without medication. "But I wouldn’t overstate this."
The project involves merging data with the National Death Index and the United States Renal Data System to track survival and chronic kidney failure outcomes, respectively. Future goals include additional data links with Medicare Parts B and D to gain clarity on morbidity and medication use, Dr. Vassalotti said. "Preliminary longitudinal data show that community screening appears to influence hypertension despite progression of chronic kidney disease," he said.
Patient awareness is the cornerstone of hypertension management, noted Dr. Adam Whaley-Connell of the University of Missouri. "Awareness has a very important role in not only understanding blood pressure control, especially in the context of the early stages of kidney disease, but in improving kidney related patient outcomes," he said. However, he noted that awareness of kidney disease, particularly in its early stages, "is alarmingly low."
He noted that KEEP data on awareness reflect that of National Health and Nutrition Examination Survey and other CKD cohort studies: Only about 5% of those with stages 1-3 are aware of their disease. "Roughly 95% of individuals entering into the screening program are unaware they have chronic kidney disease," Dr. Whaley-Connell said.
KEEP enrollees with cardiovascular disease had a keener awareness of the risks of kidney disease, he said. "While awareness may be triggered by cardiovascular disease, those unaware in the earlier stages of kidney disease are particularly vulnerable to poor risk factor control and increased risk for mortality and progression to end-stage renal disease," Dr. Whaley-Connell said. "We advocate for targeted education and awareness at earlier stages of kidney disease to improve risk factor control."
"I think any general practitioner seeing patients needs to pay attention to eGFR," Dr. Bakris said. "If the patient has stage 2 disease and an eGFR of 60-80 mL/min per 1.73 m2 now, especially with the CKD-EPI equation, the next question out of his mouth should be, ‘Do you have a family history of kidney disease?’"
However, he cited National Kidney Foundation data that showed only 31% of physicians considered family history an important factor. "If your patient has a family history of kidney disease and an eGFR of 50 mL/min per 1.73 m2 ... you really do need to pay attention to that and do something about it."
KEEP is funded by Amgen, Abbott, Siemens, Astellas, Genzyme, Fresenius Medical Care, Pfizer, Nephroceuticals and the LifeScan unit of Johnson & Johnson. Panel participants disclosed affiliations with a variety of device and pharmaceutical companies.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HYPERTENSION
Major Finding: KEEP data have shown an uptick in overall blood pressure control among patients with early-stage kidney disease or hypertension, from 45% at the initial screening to 49% at rescreening.
Data Source: Preliminary results from the National Kidney Foundation’s screening initiative.
Disclosures: KEEP is funded by Amgen, Abbott, Siemens, Astellas, Genzyme, Fresenius Medical Care, Pfizer, Nephroceuticals and the LifeScan unit of Johnson & Johnson. Panel participants disclosed affiliations with a variety of device and pharmaceutical companies.
Kidney Transplant Survival Benefit Similar for Obese, Lean Patients
PHILADELPHIA (EGMN) – Morbidly obese patients with a body mass index of 40 mg/m2 or greater respond to kidney transplants as well as do ideal-weight patients, based on a review of 120,000 U.S. kidney transplant patients who received their organs during 1996-2009.
"The survival benefit of a kidney transplant is no different for morbidly obese and for ... ideal-weight patients," Dr. Roberto Kalil said at the American Transplant Congress, which was sponsored by the American Society of Transplant Surgeons. In both BMI categories, kidney transplants led to an average 45% reduction in the subsequent mortality risk, said Dr. Kalil, a nephrologist and kidney transplant physician at the University of Iowa in Iowa City.
"BMI is not a critical issue," agreed Dr. Lawrence G. Hunsicker, a professor of medicine at Iowa and a coauthor of the study. "You still need to evaluate each morbidly obese patient as a candidate for surgery and as a candidate for kidney transplant, as you would any patient. But should surgeons use BMI as a criterion for surgery? Probably not," Dr. Hunsicker said in an interview.
"BMI is a very imperfect parameter" for judging a patient’s suitability for transplant, added Dr. Kalil.
Despite this finding, many surgeons and transplant programs deny kidney transplants to morbidly obese patients, and many programs have a BMI ceiling for allowing transplants.
"Most centers do not accept patients for kidney transplant with a BMI of 35" or higher, noted Dr. Ignatius Y.S. Tang, a nephrologist and transplant physician at the University of Illinois in Chicago. Even the University of Iowa, where until recently Dr. Hunsicker served as medical director of organ transplantation, has a policy of not placing kidney transplants in patients with a BMI of 40 or higher. "I don’t think that’s right," Dr. Hunsicker said. Transplant centers apply BMI cutoffs individually, and the United Network for Organ Sharing (UNOS) has no blanket U.S. policy, he noted.
The findings reported by Dr. Kalil used data on 237,537 U.S. patients aged 18 years or older who were listed for a first kidney transplant with UNOS during 1996-2009. The group included 8,382 patients with a BMI of 40 or greater (4%). Among these morbidly obese patients, 2,581 actually received a transplant, which accounted for 2% of the more than 120,000 total U.S. patients who received a kidney transplant during the 14-year period examined.
In an analysis that controlled for age, sex, race and ethnicity, diabetes, blood type, listing priority, and type of health insurance, a kidney transplant improved the survival rate among morbidly obese patients by 48%, compared with the survival of morbidly obese patients who did not receive a transplant – similar to the 45% improvement seen in ideal-weight patients, and similar to the survival benefit seen in every other BMI subgroup. Dr. Kalil reported.
The finding carries one important caveat: The analysis could assess the outcomes of only the morbidly obese patients whose physicians decided to add them to the UNOS kidney waiting list. So the findings may represent exceptional, low-risk morbidly obese patients. "I think that’s unlikely, because generally the decision to list these patients or not depends on a center’s policy," said Dr. Hunsicker. "I think some centers listed these patients and others did not. I think the selection was by centers, not by patients," he said.
Additional, BMI-based analyses of the UNOS data showed that the listed morbidly obese patients had a statistically significant (26%) reduced chance of receiving a kidney transplant, compared with ideal-weight patients. Listed patients with a BMI of 40 or greater formed the only BMI subgroup with a significantly different transplantation rate.
The analysis also showed that patients who started with a BMI of 40 or more at the time they first appeared on the UNOS kidney transplant list lost an average index value of 4.7 in the period before they actually received a transplant. However, the change in BMI prior to transplantation had no relationship to the graft survival following transplantation, Dr. Kalil said. Waiting for patients to lose weight before proceeding with a transplant "was not associated with decreased mortality, and it wasted time," Dr. Hunsicker said. The better approach is to proceed with the transplant as soon as possible, he said.
Dr. Kalil, Dr. Hunsicker, and Dr. Tang all said that they had no disclosures.
Roberto Kalil
PHILADELPHIA (EGMN) – Morbidly obese patients with a body mass index of 40 mg/m2 or greater respond to kidney transplants as well as do ideal-weight patients, based on a review of 120,000 U.S. kidney transplant patients who received their organs during 1996-2009.
"The survival benefit of a kidney transplant is no different for morbidly obese and for ... ideal-weight patients," Dr. Roberto Kalil said at the American Transplant Congress, which was sponsored by the American Society of Transplant Surgeons. In both BMI categories, kidney transplants led to an average 45% reduction in the subsequent mortality risk, said Dr. Kalil, a nephrologist and kidney transplant physician at the University of Iowa in Iowa City.
"BMI is not a critical issue," agreed Dr. Lawrence G. Hunsicker, a professor of medicine at Iowa and a coauthor of the study. "You still need to evaluate each morbidly obese patient as a candidate for surgery and as a candidate for kidney transplant, as you would any patient. But should surgeons use BMI as a criterion for surgery? Probably not," Dr. Hunsicker said in an interview.
"BMI is a very imperfect parameter" for judging a patient’s suitability for transplant, added Dr. Kalil.
Despite this finding, many surgeons and transplant programs deny kidney transplants to morbidly obese patients, and many programs have a BMI ceiling for allowing transplants.
"Most centers do not accept patients for kidney transplant with a BMI of 35" or higher, noted Dr. Ignatius Y.S. Tang, a nephrologist and transplant physician at the University of Illinois in Chicago. Even the University of Iowa, where until recently Dr. Hunsicker served as medical director of organ transplantation, has a policy of not placing kidney transplants in patients with a BMI of 40 or higher. "I don’t think that’s right," Dr. Hunsicker said. Transplant centers apply BMI cutoffs individually, and the United Network for Organ Sharing (UNOS) has no blanket U.S. policy, he noted.
The findings reported by Dr. Kalil used data on 237,537 U.S. patients aged 18 years or older who were listed for a first kidney transplant with UNOS during 1996-2009. The group included 8,382 patients with a BMI of 40 or greater (4%). Among these morbidly obese patients, 2,581 actually received a transplant, which accounted for 2% of the more than 120,000 total U.S. patients who received a kidney transplant during the 14-year period examined.
In an analysis that controlled for age, sex, race and ethnicity, diabetes, blood type, listing priority, and type of health insurance, a kidney transplant improved the survival rate among morbidly obese patients by 48%, compared with the survival of morbidly obese patients who did not receive a transplant – similar to the 45% improvement seen in ideal-weight patients, and similar to the survival benefit seen in every other BMI subgroup. Dr. Kalil reported.
The finding carries one important caveat: The analysis could assess the outcomes of only the morbidly obese patients whose physicians decided to add them to the UNOS kidney waiting list. So the findings may represent exceptional, low-risk morbidly obese patients. "I think that’s unlikely, because generally the decision to list these patients or not depends on a center’s policy," said Dr. Hunsicker. "I think some centers listed these patients and others did not. I think the selection was by centers, not by patients," he said.
Additional, BMI-based analyses of the UNOS data showed that the listed morbidly obese patients had a statistically significant (26%) reduced chance of receiving a kidney transplant, compared with ideal-weight patients. Listed patients with a BMI of 40 or greater formed the only BMI subgroup with a significantly different transplantation rate.
The analysis also showed that patients who started with a BMI of 40 or more at the time they first appeared on the UNOS kidney transplant list lost an average index value of 4.7 in the period before they actually received a transplant. However, the change in BMI prior to transplantation had no relationship to the graft survival following transplantation, Dr. Kalil said. Waiting for patients to lose weight before proceeding with a transplant "was not associated with decreased mortality, and it wasted time," Dr. Hunsicker said. The better approach is to proceed with the transplant as soon as possible, he said.
Dr. Kalil, Dr. Hunsicker, and Dr. Tang all said that they had no disclosures.
Roberto Kalil
PHILADELPHIA (EGMN) – Morbidly obese patients with a body mass index of 40 mg/m2 or greater respond to kidney transplants as well as do ideal-weight patients, based on a review of 120,000 U.S. kidney transplant patients who received their organs during 1996-2009.
"The survival benefit of a kidney transplant is no different for morbidly obese and for ... ideal-weight patients," Dr. Roberto Kalil said at the American Transplant Congress, which was sponsored by the American Society of Transplant Surgeons. In both BMI categories, kidney transplants led to an average 45% reduction in the subsequent mortality risk, said Dr. Kalil, a nephrologist and kidney transplant physician at the University of Iowa in Iowa City.
"BMI is not a critical issue," agreed Dr. Lawrence G. Hunsicker, a professor of medicine at Iowa and a coauthor of the study. "You still need to evaluate each morbidly obese patient as a candidate for surgery and as a candidate for kidney transplant, as you would any patient. But should surgeons use BMI as a criterion for surgery? Probably not," Dr. Hunsicker said in an interview.
"BMI is a very imperfect parameter" for judging a patient’s suitability for transplant, added Dr. Kalil.
Despite this finding, many surgeons and transplant programs deny kidney transplants to morbidly obese patients, and many programs have a BMI ceiling for allowing transplants.
"Most centers do not accept patients for kidney transplant with a BMI of 35" or higher, noted Dr. Ignatius Y.S. Tang, a nephrologist and transplant physician at the University of Illinois in Chicago. Even the University of Iowa, where until recently Dr. Hunsicker served as medical director of organ transplantation, has a policy of not placing kidney transplants in patients with a BMI of 40 or higher. "I don’t think that’s right," Dr. Hunsicker said. Transplant centers apply BMI cutoffs individually, and the United Network for Organ Sharing (UNOS) has no blanket U.S. policy, he noted.
The findings reported by Dr. Kalil used data on 237,537 U.S. patients aged 18 years or older who were listed for a first kidney transplant with UNOS during 1996-2009. The group included 8,382 patients with a BMI of 40 or greater (4%). Among these morbidly obese patients, 2,581 actually received a transplant, which accounted for 2% of the more than 120,000 total U.S. patients who received a kidney transplant during the 14-year period examined.
In an analysis that controlled for age, sex, race and ethnicity, diabetes, blood type, listing priority, and type of health insurance, a kidney transplant improved the survival rate among morbidly obese patients by 48%, compared with the survival of morbidly obese patients who did not receive a transplant – similar to the 45% improvement seen in ideal-weight patients, and similar to the survival benefit seen in every other BMI subgroup. Dr. Kalil reported.
The finding carries one important caveat: The analysis could assess the outcomes of only the morbidly obese patients whose physicians decided to add them to the UNOS kidney waiting list. So the findings may represent exceptional, low-risk morbidly obese patients. "I think that’s unlikely, because generally the decision to list these patients or not depends on a center’s policy," said Dr. Hunsicker. "I think some centers listed these patients and others did not. I think the selection was by centers, not by patients," he said.
Additional, BMI-based analyses of the UNOS data showed that the listed morbidly obese patients had a statistically significant (26%) reduced chance of receiving a kidney transplant, compared with ideal-weight patients. Listed patients with a BMI of 40 or greater formed the only BMI subgroup with a significantly different transplantation rate.
The analysis also showed that patients who started with a BMI of 40 or more at the time they first appeared on the UNOS kidney transplant list lost an average index value of 4.7 in the period before they actually received a transplant. However, the change in BMI prior to transplantation had no relationship to the graft survival following transplantation, Dr. Kalil said. Waiting for patients to lose weight before proceeding with a transplant "was not associated with decreased mortality, and it wasted time," Dr. Hunsicker said. The better approach is to proceed with the transplant as soon as possible, he said.
Dr. Kalil, Dr. Hunsicker, and Dr. Tang all said that they had no disclosures.
Roberto Kalil
FROM THE AMERICAN TRANSPLANT CONGRESS
Major Finding: Patients with a BMI of 40 or greater who received a kidney transplant derived a 48% survival benefit, similar to the 45% survival benefit seen in ideal-weight patients who received a kidney transplant.
Data Source: Case data collected by the UNOS on 120,765 U.S. patients who received a kidney transplant during 1996-2009.
Disclosures: Dr. Kalil, Dr. Hunsicker, and Dr. Tang said that they had no disclosures.
Medications and the Renal Patient: TMP-SMX in Kidney Donor
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: I was treating a patient for an uncomplicated urinary tract infection with trimethoprim–sulfamethoxazole (TMP-SMX). The pharmacist called and said the patient could not have any sulfa medications because she was going to donate a kidney to her brother. How do you explain this, and what should be my next step?
There is limited available evidence to support an answer to this question. Instead, I will provide a brief report of the available evidence, followed by the opinions of five experts in kidney transplantation.
TMP-SMX is a combination of two antimicrobial agents that act synergistically against a variety of bacteria.9 TMP decreases urinary excretion of potassium, leading to hyperkalemia—especially in persons with kidney disease or in those also taking other drugs that cause hyperkalemia.10 This scenario is unlikely, because the transplant team would have assessed the potential donor’s kidney function. When dosing the drug, clinicians should consider renal function and adjust the dose in those with a creatinine clearance less than or equal to 30 mL/min. Nephrotoxicity is uncommon in patients who take this drug; however, TMP is known to decrease tubular secretion of creatinine and may interfere with certain creatinine assays, leading to an artificial rise in serum creatinine. This is not reflective of a true reduction in the GFR and often is mild and reversible with discontinuation of the medication.
Querying experienced transplant professionals (two nephrologists, two transplant coordinators, and one doctoral-prepared pharmacist) yielded similar results. They all agreed that the only plausible reason to withhold TMP-SMX from this potential kidney donor was the risk of a transient rise in creatinine due to impaired secretion associated with TMP use. Many transplant teams recommend avoiding any medications that may affect the kidneys. So considering the lack of available evidence, I would recommend that you consult with the transplant team where your patient’s brother is receiving care before you prescribe TMP-SMX.
Debra Hain, PhD, APRN, GNP-BC
Florida Atlantic University, Boca Raton; Cleveland Clinic Florida; Department of Nephrology, Weston
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: I was treating a patient for an uncomplicated urinary tract infection with trimethoprim–sulfamethoxazole (TMP-SMX). The pharmacist called and said the patient could not have any sulfa medications because she was going to donate a kidney to her brother. How do you explain this, and what should be my next step?
There is limited available evidence to support an answer to this question. Instead, I will provide a brief report of the available evidence, followed by the opinions of five experts in kidney transplantation.
TMP-SMX is a combination of two antimicrobial agents that act synergistically against a variety of bacteria.9 TMP decreases urinary excretion of potassium, leading to hyperkalemia—especially in persons with kidney disease or in those also taking other drugs that cause hyperkalemia.10 This scenario is unlikely, because the transplant team would have assessed the potential donor’s kidney function. When dosing the drug, clinicians should consider renal function and adjust the dose in those with a creatinine clearance less than or equal to 30 mL/min. Nephrotoxicity is uncommon in patients who take this drug; however, TMP is known to decrease tubular secretion of creatinine and may interfere with certain creatinine assays, leading to an artificial rise in serum creatinine. This is not reflective of a true reduction in the GFR and often is mild and reversible with discontinuation of the medication.
Querying experienced transplant professionals (two nephrologists, two transplant coordinators, and one doctoral-prepared pharmacist) yielded similar results. They all agreed that the only plausible reason to withhold TMP-SMX from this potential kidney donor was the risk of a transient rise in creatinine due to impaired secretion associated with TMP use. Many transplant teams recommend avoiding any medications that may affect the kidneys. So considering the lack of available evidence, I would recommend that you consult with the transplant team where your patient’s brother is receiving care before you prescribe TMP-SMX.
Debra Hain, PhD, APRN, GNP-BC
Florida Atlantic University, Boca Raton; Cleveland Clinic Florida; Department of Nephrology, Weston
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: I was treating a patient for an uncomplicated urinary tract infection with trimethoprim–sulfamethoxazole (TMP-SMX). The pharmacist called and said the patient could not have any sulfa medications because she was going to donate a kidney to her brother. How do you explain this, and what should be my next step?
There is limited available evidence to support an answer to this question. Instead, I will provide a brief report of the available evidence, followed by the opinions of five experts in kidney transplantation.
TMP-SMX is a combination of two antimicrobial agents that act synergistically against a variety of bacteria.9 TMP decreases urinary excretion of potassium, leading to hyperkalemia—especially in persons with kidney disease or in those also taking other drugs that cause hyperkalemia.10 This scenario is unlikely, because the transplant team would have assessed the potential donor’s kidney function. When dosing the drug, clinicians should consider renal function and adjust the dose in those with a creatinine clearance less than or equal to 30 mL/min. Nephrotoxicity is uncommon in patients who take this drug; however, TMP is known to decrease tubular secretion of creatinine and may interfere with certain creatinine assays, leading to an artificial rise in serum creatinine. This is not reflective of a true reduction in the GFR and often is mild and reversible with discontinuation of the medication.
Querying experienced transplant professionals (two nephrologists, two transplant coordinators, and one doctoral-prepared pharmacist) yielded similar results. They all agreed that the only plausible reason to withhold TMP-SMX from this potential kidney donor was the risk of a transient rise in creatinine due to impaired secretion associated with TMP use. Many transplant teams recommend avoiding any medications that may affect the kidneys. So considering the lack of available evidence, I would recommend that you consult with the transplant team where your patient’s brother is receiving care before you prescribe TMP-SMX.
Debra Hain, PhD, APRN, GNP-BC
Florida Atlantic University, Boca Raton; Cleveland Clinic Florida; Department of Nephrology, Weston
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
Medications and the Renal Patient: CKD and Thiazide Diuretics
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: I have a patient with CKD stage 4 (GFR, 30 mL/min/1.73m2). My supervising physician said to take him off his hydrochlorothiazide (50 mg qd) and start furosemide. Why do I do this, and at what dose do I start?
Thiazide diuretics work by blocking approximately 40% of sodium chloride reabsorption in the distal convoluting tubule of the nephron. This process increases the fractional excretion of sodium and provides a natriuresis with reduced blood pressure (BP). Thiazides also have a second mode of action in lowering BP by reducing peripheral vascular resistance.5
It is generally thought that thiazides are ineffective in patients with more advanced CKD because of more proximal sodium reabsorption in the nephron. This results in less sodium being delivered to the distal tubule and therefore less thiazide diuretic action in the distal tubule.5
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (K/DOQI)6 recommends that hypertension in CKD be treated with combination therapy: renin-angiotensin system blockade (ACE inhibitor/angiotensin receptor blocker) and thiazide diuretics in patients with CKD stages 1 to 3; and loop diuretics in those with CKD stages 4 and 5. No large study has evaluated thiazide diuretic use in patients with CKD stages 4 and 5. In one small study (n = 7) in which thiazides were compared with loop diuretics, there was similar BP control between the groups, but patients in the thiazide group had superior urinary fractional sodium excretion.5
There is new concern that thiazides may partially contribute to the increased risk for metabolic syndrome or diabetes. Reungjui et al7 have used animal models to demonstrate that the hypokalemic and hyperuricemic effects of thiazides are the culprits for this risk. They recommend managing the potassium and uric acid levels to manage or prevent this risk.
When a change is made from thiazides to loop diuretics, K/DOQI recommends starting furosemide at 40 mg/d and titrating upward as needed for BP and edema control. In my experience, counseling patients to follow a low-sodium diet (the American Heart Association recommends restricting sodium intake to 1500 mg/d) allows for a lower dose of furosemide (20 mg) to be effective.8
Elizabeth Evans, DNP
Renal Medicine Associates, Albuquerque, NM
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: I have a patient with CKD stage 4 (GFR, 30 mL/min/1.73m2). My supervising physician said to take him off his hydrochlorothiazide (50 mg qd) and start furosemide. Why do I do this, and at what dose do I start?
Thiazide diuretics work by blocking approximately 40% of sodium chloride reabsorption in the distal convoluting tubule of the nephron. This process increases the fractional excretion of sodium and provides a natriuresis with reduced blood pressure (BP). Thiazides also have a second mode of action in lowering BP by reducing peripheral vascular resistance.5
It is generally thought that thiazides are ineffective in patients with more advanced CKD because of more proximal sodium reabsorption in the nephron. This results in less sodium being delivered to the distal tubule and therefore less thiazide diuretic action in the distal tubule.5
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (K/DOQI)6 recommends that hypertension in CKD be treated with combination therapy: renin-angiotensin system blockade (ACE inhibitor/angiotensin receptor blocker) and thiazide diuretics in patients with CKD stages 1 to 3; and loop diuretics in those with CKD stages 4 and 5. No large study has evaluated thiazide diuretic use in patients with CKD stages 4 and 5. In one small study (n = 7) in which thiazides were compared with loop diuretics, there was similar BP control between the groups, but patients in the thiazide group had superior urinary fractional sodium excretion.5
There is new concern that thiazides may partially contribute to the increased risk for metabolic syndrome or diabetes. Reungjui et al7 have used animal models to demonstrate that the hypokalemic and hyperuricemic effects of thiazides are the culprits for this risk. They recommend managing the potassium and uric acid levels to manage or prevent this risk.
When a change is made from thiazides to loop diuretics, K/DOQI recommends starting furosemide at 40 mg/d and titrating upward as needed for BP and edema control. In my experience, counseling patients to follow a low-sodium diet (the American Heart Association recommends restricting sodium intake to 1500 mg/d) allows for a lower dose of furosemide (20 mg) to be effective.8
Elizabeth Evans, DNP
Renal Medicine Associates, Albuquerque, NM
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: I have a patient with CKD stage 4 (GFR, 30 mL/min/1.73m2). My supervising physician said to take him off his hydrochlorothiazide (50 mg qd) and start furosemide. Why do I do this, and at what dose do I start?
Thiazide diuretics work by blocking approximately 40% of sodium chloride reabsorption in the distal convoluting tubule of the nephron. This process increases the fractional excretion of sodium and provides a natriuresis with reduced blood pressure (BP). Thiazides also have a second mode of action in lowering BP by reducing peripheral vascular resistance.5
It is generally thought that thiazides are ineffective in patients with more advanced CKD because of more proximal sodium reabsorption in the nephron. This results in less sodium being delivered to the distal tubule and therefore less thiazide diuretic action in the distal tubule.5
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (K/DOQI)6 recommends that hypertension in CKD be treated with combination therapy: renin-angiotensin system blockade (ACE inhibitor/angiotensin receptor blocker) and thiazide diuretics in patients with CKD stages 1 to 3; and loop diuretics in those with CKD stages 4 and 5. No large study has evaluated thiazide diuretic use in patients with CKD stages 4 and 5. In one small study (n = 7) in which thiazides were compared with loop diuretics, there was similar BP control between the groups, but patients in the thiazide group had superior urinary fractional sodium excretion.5
There is new concern that thiazides may partially contribute to the increased risk for metabolic syndrome or diabetes. Reungjui et al7 have used animal models to demonstrate that the hypokalemic and hyperuricemic effects of thiazides are the culprits for this risk. They recommend managing the potassium and uric acid levels to manage or prevent this risk.
When a change is made from thiazides to loop diuretics, K/DOQI recommends starting furosemide at 40 mg/d and titrating upward as needed for BP and edema control. In my experience, counseling patients to follow a low-sodium diet (the American Heart Association recommends restricting sodium intake to 1500 mg/d) allows for a lower dose of furosemide (20 mg) to be effective.8
Elizabeth Evans, DNP
Renal Medicine Associates, Albuquerque, NM
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
Medications and the Renal Patient: NSAIDs and Acute Kidney Injury
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: In my orthopedic practice, we have a woman we are treating conservatively for low back pain (NSAIDs, muscle relaxants, and physical therapy). Her primary care provider told her that she cannot take the NSAIDs because of her kidney disease (she has chronic kidney disease [CKD] stage 3). Is there a safe dose of NSAIDs that she can use, or do I need to start narcotics? I would rather not do that!
Unfortunately, all NSAIDs increase the risk for acute kidney injury (AKI) and may exacerbate progression to chronic renal failure, particularly when large doses are taken chronically.3,4 Increased incidence of renal injury with NSAIDs has been seen in patients with existing CKD, hypertension, diabetes, and frequent hospitalizations.3 Most renal damage associated with NSAIDs is related to inhibition of prostaglandin synthesis (discussed below), but NSAIDs can also cause other types of kidney injury, such as interstitial nephritis, analgesic nephropathy, and membranous nephropathy. NSAID use is also associated with hyperkalemia, hyponatremia, and edema (sodium retention).
The primary mechanism of NSAID nephrotoxicity is inhibition of prostaglandin synthesis in the setting of decreased renal perfusion.3 Prostaglandins induce vasodilation of the afferent arterioles to maintain renal perfusion (and consequently GFR). If renal perfusion or effective fluid volume decreases (as in dehydration, diuretic therapy, heart failure, and cirrhosis), renal perfusion is maintained by increasing prostaglandin synthesis. NSAIDs block prostaglandin synthesis, therefore blunting this protective mechanism. Blocking prostaglandin with NSAIDs when renal perfusion is decreased can cause an AKI.
AKI can occur in patients with or without CKD, and it is associated with increased morbidity and mortality. AKI due to NSAID use may progress to end-stage renal disease if the NSAID is not stopped promptly. When NSAIDs are discontinued, renal function most often stabilizes, but residual renal insufficiency is likely to be permanent. In some patients, even after discontinuing the NSAID, the AKI progresses to more advanced kidney disease.
All NSAIDs carry the same risk for acute and chronic kidney disease, with little evidence to suggest that some are safer than others. Higher doses are more likely to cause renal damage, but dosing to prevent renal damage has not been defined. Indomethacin and ketorolac have most frequently been associated with AKI. If appropriate for the patient, acetaminophen 650 mg taken three times daily (scheduled, not “as needed”) can provide pain relief with less risk for kidney injury.
Additionally, narcotic medications are often necessary when treating severe pain in patients with high risk for NSAID-associated kidney injury.
Catherine Wells, DNP, ACNP, CNN-NP
University of Mississippi Health Care, Division of Nephrology, Jackson
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: In my orthopedic practice, we have a woman we are treating conservatively for low back pain (NSAIDs, muscle relaxants, and physical therapy). Her primary care provider told her that she cannot take the NSAIDs because of her kidney disease (she has chronic kidney disease [CKD] stage 3). Is there a safe dose of NSAIDs that she can use, or do I need to start narcotics? I would rather not do that!
Unfortunately, all NSAIDs increase the risk for acute kidney injury (AKI) and may exacerbate progression to chronic renal failure, particularly when large doses are taken chronically.3,4 Increased incidence of renal injury with NSAIDs has been seen in patients with existing CKD, hypertension, diabetes, and frequent hospitalizations.3 Most renal damage associated with NSAIDs is related to inhibition of prostaglandin synthesis (discussed below), but NSAIDs can also cause other types of kidney injury, such as interstitial nephritis, analgesic nephropathy, and membranous nephropathy. NSAID use is also associated with hyperkalemia, hyponatremia, and edema (sodium retention).
The primary mechanism of NSAID nephrotoxicity is inhibition of prostaglandin synthesis in the setting of decreased renal perfusion.3 Prostaglandins induce vasodilation of the afferent arterioles to maintain renal perfusion (and consequently GFR). If renal perfusion or effective fluid volume decreases (as in dehydration, diuretic therapy, heart failure, and cirrhosis), renal perfusion is maintained by increasing prostaglandin synthesis. NSAIDs block prostaglandin synthesis, therefore blunting this protective mechanism. Blocking prostaglandin with NSAIDs when renal perfusion is decreased can cause an AKI.
AKI can occur in patients with or without CKD, and it is associated with increased morbidity and mortality. AKI due to NSAID use may progress to end-stage renal disease if the NSAID is not stopped promptly. When NSAIDs are discontinued, renal function most often stabilizes, but residual renal insufficiency is likely to be permanent. In some patients, even after discontinuing the NSAID, the AKI progresses to more advanced kidney disease.
All NSAIDs carry the same risk for acute and chronic kidney disease, with little evidence to suggest that some are safer than others. Higher doses are more likely to cause renal damage, but dosing to prevent renal damage has not been defined. Indomethacin and ketorolac have most frequently been associated with AKI. If appropriate for the patient, acetaminophen 650 mg taken three times daily (scheduled, not “as needed”) can provide pain relief with less risk for kidney injury.
Additionally, narcotic medications are often necessary when treating severe pain in patients with high risk for NSAID-associated kidney injury.
Catherine Wells, DNP, ACNP, CNN-NP
University of Mississippi Health Care, Division of Nephrology, Jackson
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: In my orthopedic practice, we have a woman we are treating conservatively for low back pain (NSAIDs, muscle relaxants, and physical therapy). Her primary care provider told her that she cannot take the NSAIDs because of her kidney disease (she has chronic kidney disease [CKD] stage 3). Is there a safe dose of NSAIDs that she can use, or do I need to start narcotics? I would rather not do that!
Unfortunately, all NSAIDs increase the risk for acute kidney injury (AKI) and may exacerbate progression to chronic renal failure, particularly when large doses are taken chronically.3,4 Increased incidence of renal injury with NSAIDs has been seen in patients with existing CKD, hypertension, diabetes, and frequent hospitalizations.3 Most renal damage associated with NSAIDs is related to inhibition of prostaglandin synthesis (discussed below), but NSAIDs can also cause other types of kidney injury, such as interstitial nephritis, analgesic nephropathy, and membranous nephropathy. NSAID use is also associated with hyperkalemia, hyponatremia, and edema (sodium retention).
The primary mechanism of NSAID nephrotoxicity is inhibition of prostaglandin synthesis in the setting of decreased renal perfusion.3 Prostaglandins induce vasodilation of the afferent arterioles to maintain renal perfusion (and consequently GFR). If renal perfusion or effective fluid volume decreases (as in dehydration, diuretic therapy, heart failure, and cirrhosis), renal perfusion is maintained by increasing prostaglandin synthesis. NSAIDs block prostaglandin synthesis, therefore blunting this protective mechanism. Blocking prostaglandin with NSAIDs when renal perfusion is decreased can cause an AKI.
AKI can occur in patients with or without CKD, and it is associated with increased morbidity and mortality. AKI due to NSAID use may progress to end-stage renal disease if the NSAID is not stopped promptly. When NSAIDs are discontinued, renal function most often stabilizes, but residual renal insufficiency is likely to be permanent. In some patients, even after discontinuing the NSAID, the AKI progresses to more advanced kidney disease.
All NSAIDs carry the same risk for acute and chronic kidney disease, with little evidence to suggest that some are safer than others. Higher doses are more likely to cause renal damage, but dosing to prevent renal damage has not been defined. Indomethacin and ketorolac have most frequently been associated with AKI. If appropriate for the patient, acetaminophen 650 mg taken three times daily (scheduled, not “as needed”) can provide pain relief with less risk for kidney injury.
Additionally, narcotic medications are often necessary when treating severe pain in patients with high risk for NSAID-associated kidney injury.
Catherine Wells, DNP, ACNP, CNN-NP
University of Mississippi Health Care, Division of Nephrology, Jackson
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
Medications and the Renal Patient: Dialysis and BP
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: I work in a cardiology practice. We received a note from the dialysis center telling us that one of our patients is hypotensive (systole < 100 mm Hg) during his dialysis treatment. His BP is usually 140/86 mm Hg in the office. Why the difference?
When considering BP values within this population, it is important to keep in mind that BP in dialysis patients can vary widely, with lower values in the period immediately following dialysis, then slowly increasing as patients’ fluid levels rise.
There are a few reasons why hypotension typically occurs during treatment. Taking sedating medication just before arriving for dialysis can dramatically lower BP during dialysis and should generally be avoided; advise the patient to take the medication after dialysis or at night instead.11 Many antihypertensive drugs that are removed by dialysis are often prescribed to be taken at night.
Another common reason for hypotension during dialysis is large-volume fluid removal. Patients are advised to limit fluids between treatments to avoid fluid overload, thereby limiting the volume of removal needed. Incorrect dry weight calculations can also cause hypotension during dialysis; if a patient gains weight that is not fluid related and attempts are made to dialyze the patient to the dry weight, hypotension can occur.11 The patient who sees another practitioner right before dialysis may appear volume-overloaded—or immediately after dialysis, may appear volume-depleted; neither impression is correct. Also, a 2- to 4-kg weight gain between dialysis treatments is acceptable.
It has been learned through observational research that hemodialysis patients tend to have higher mortality rates with a predialysis systolic BP (SBP) below 110 mm Hg, a postdialysis SBP greater than 180 mm Hg, or a postdialysis diastolic BP exceeding 110 mm Hg.12 According to the National Kidney Foundation’s K/DOQI practice guidelines,13 a predialysis BP of 140/90 mm Hg and a postdialysis BP of 130/80 mm Hg are reasonable targets. However, as with all guidelines, goals must be individualized to fit the patient’s age, comorbidities, and symptoms.14 This is a delicate balance, and safe management requires ongoing communication between providers.
Of interest, researchers for the Dialysis Outcomes and Practice Patterns Study suggested that patients with a predialysis SBP of 110 to 130 mm Hg had a higher risk for mortality than those with an SBP of 130 to 140 mm Hg. The same study showed an increased risk for death in patients with predialysis SBP greater than 160 mm Hg.14
Kristina Unterseher, CNN-NP
Idaho Nephrology Associates, Boise
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: I work in a cardiology practice. We received a note from the dialysis center telling us that one of our patients is hypotensive (systole < 100 mm Hg) during his dialysis treatment. His BP is usually 140/86 mm Hg in the office. Why the difference?
When considering BP values within this population, it is important to keep in mind that BP in dialysis patients can vary widely, with lower values in the period immediately following dialysis, then slowly increasing as patients’ fluid levels rise.
There are a few reasons why hypotension typically occurs during treatment. Taking sedating medication just before arriving for dialysis can dramatically lower BP during dialysis and should generally be avoided; advise the patient to take the medication after dialysis or at night instead.11 Many antihypertensive drugs that are removed by dialysis are often prescribed to be taken at night.
Another common reason for hypotension during dialysis is large-volume fluid removal. Patients are advised to limit fluids between treatments to avoid fluid overload, thereby limiting the volume of removal needed. Incorrect dry weight calculations can also cause hypotension during dialysis; if a patient gains weight that is not fluid related and attempts are made to dialyze the patient to the dry weight, hypotension can occur.11 The patient who sees another practitioner right before dialysis may appear volume-overloaded—or immediately after dialysis, may appear volume-depleted; neither impression is correct. Also, a 2- to 4-kg weight gain between dialysis treatments is acceptable.
It has been learned through observational research that hemodialysis patients tend to have higher mortality rates with a predialysis systolic BP (SBP) below 110 mm Hg, a postdialysis SBP greater than 180 mm Hg, or a postdialysis diastolic BP exceeding 110 mm Hg.12 According to the National Kidney Foundation’s K/DOQI practice guidelines,13 a predialysis BP of 140/90 mm Hg and a postdialysis BP of 130/80 mm Hg are reasonable targets. However, as with all guidelines, goals must be individualized to fit the patient’s age, comorbidities, and symptoms.14 This is a delicate balance, and safe management requires ongoing communication between providers.
Of interest, researchers for the Dialysis Outcomes and Practice Patterns Study suggested that patients with a predialysis SBP of 110 to 130 mm Hg had a higher risk for mortality than those with an SBP of 130 to 140 mm Hg. The same study showed an increased risk for death in patients with predialysis SBP greater than 160 mm Hg.14
Kristina Unterseher, CNN-NP
Idaho Nephrology Associates, Boise
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
When prescribing medications for patients, it is always advisable to know their estimated glomerular filtration rate (eGFR). The creatinine and blood urea nitrogen (BUN) by themselves are not always good indicators of renal function. If you have doubts, any reliable pharmacy source can guide you to dosing adjustments. Most medications do not require adjustments for eGFR greater than 60 mL/min/1.73m2.
Patients with an eGFR of less than 60 should never be prescribed NSAIDs, and extreme caution is advised with use of aminoglycosides and contrast dyes.
With medications such as ACE inhibitors, which can affect renal function (particularly levels of creatinine and potassium), eGFR should be monitored initially and within two weeks of each dosing adjustment. Other commonly prescribed drugs requiring dosing adjustment in patients with eGFR below 60 include gabapentin, metoclopramide, and ranitidine.1,2
As always, inquire about your patient’s use of complementary and alternative therapies, including herbal remedies, as these often are contraindicated in this population.
Jane S. Davis, CRNP, DNP
Q: I work in a cardiology practice. We received a note from the dialysis center telling us that one of our patients is hypotensive (systole < 100 mm Hg) during his dialysis treatment. His BP is usually 140/86 mm Hg in the office. Why the difference?
When considering BP values within this population, it is important to keep in mind that BP in dialysis patients can vary widely, with lower values in the period immediately following dialysis, then slowly increasing as patients’ fluid levels rise.
There are a few reasons why hypotension typically occurs during treatment. Taking sedating medication just before arriving for dialysis can dramatically lower BP during dialysis and should generally be avoided; advise the patient to take the medication after dialysis or at night instead.11 Many antihypertensive drugs that are removed by dialysis are often prescribed to be taken at night.
Another common reason for hypotension during dialysis is large-volume fluid removal. Patients are advised to limit fluids between treatments to avoid fluid overload, thereby limiting the volume of removal needed. Incorrect dry weight calculations can also cause hypotension during dialysis; if a patient gains weight that is not fluid related and attempts are made to dialyze the patient to the dry weight, hypotension can occur.11 The patient who sees another practitioner right before dialysis may appear volume-overloaded—or immediately after dialysis, may appear volume-depleted; neither impression is correct. Also, a 2- to 4-kg weight gain between dialysis treatments is acceptable.
It has been learned through observational research that hemodialysis patients tend to have higher mortality rates with a predialysis systolic BP (SBP) below 110 mm Hg, a postdialysis SBP greater than 180 mm Hg, or a postdialysis diastolic BP exceeding 110 mm Hg.12 According to the National Kidney Foundation’s K/DOQI practice guidelines,13 a predialysis BP of 140/90 mm Hg and a postdialysis BP of 130/80 mm Hg are reasonable targets. However, as with all guidelines, goals must be individualized to fit the patient’s age, comorbidities, and symptoms.14 This is a delicate balance, and safe management requires ongoing communication between providers.
Of interest, researchers for the Dialysis Outcomes and Practice Patterns Study suggested that patients with a predialysis SBP of 110 to 130 mm Hg had a higher risk for mortality than those with an SBP of 130 to 140 mm Hg. The same study showed an increased risk for death in patients with predialysis SBP greater than 160 mm Hg.14
Kristina Unterseher, CNN-NP
Idaho Nephrology Associates, Boise
REFERENCES
1. Gabardi S, Abramson S. Drug dosing in chronic kidney disease. Med Clin North Am. 2005;89(3):649-687.
2. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
3. Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3): 531-539.
4. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006; 164(9):881-889.
5. Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008;101(5):84-85.
6. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
7. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470-476.
8. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82-96.
9. Pharmacokinetics. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, eds. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:31-48.
10. Masters PA, O’Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402-410.
11. Singapuri MS, Lea JP. Management of hypertension in the end-stage renal disease patient. J Clin Outcomes Manage. 2010;17(2):87-95.
12. Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3): 507-517.
13. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153.
14. Schieszer J. BP guidelines may be inappropriate for HD patients. Renal Urol News. 2010 May 21. www.renalandurologynews.com/bp-guidelines-may-be-inappropriate-for-hd-patients/article/170707. Accessed May 19, 2011.
Unexpected skin necrosis of the thighs
A 62-YEAR-OLD WOMAN sought care at our clinic for painful skin lesions that had developed on her thighs 5 days earlier. She had received ongoing treatment at our clinic over the past few years for diabetes, hyperlipidemia, hypertension, and sarcoidosis. In the last 2 years, she’d had 2 hospitalizations for acute renal failure, with a creatinine value as high as 3.8 mg/dL and a persistent glomerular filtration rate consistent with stage 3 chronic kidney disease.
The medications she was taking included glyburide, pravastatin, and lisinopril. During the 2 years prior to her recent clinic visit, she’d had some intermittently elevated calcium readings. Repeat calcium levels each time were normal. In addition, her parathyroid hormone levels fluctuated between low, high, and normal. Her technetium sestamibi scan was negative for hyperparathyroidism. The patient was unemployed and gave no history of recent travel, injuries, or exposure to animals.
On examination, we noted large, poorly demarcated, warm, indurated erythematous lesions on her lateral thighs. She was given a diagnosis of cellulitis and treated with trimethoprim/sulfa-methoxazole 160/800 mg twice daily for 10 days. During follow-up visits 3 and 7 days later, she indicated that the lesions were less painful and they appeared to be less swollen.
Three weeks later, the patient returned to the clinic with skin sloughing that had produced necrotic lesions with black es-char on the bases (FIGURE 1). In addition, new lesions appeared on her anterior thighs. An initial punch biopsy of the lesions revealed no significant pathologic abnormality.
FIGURE 1
What started as indurated plaques…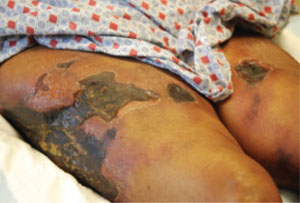
The patient initially came in for the treatment of indurated plaques, which developed into ulcerative skin lesions with erythematous edges and eschar on the bases.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Calciphylaxis
Calciphylaxis is an uncommon disorder of vascular calcification and thrombosis resulting in skin necrosis.1 It most commonly occurs in people with end-stage renal disease (ESRD) on hemodialysis, but in nonuremic patients the most frequent cause is primary hyperparathyroidism.2,3 Similar vascular calcifications may be observed in milk alkali syndrome, rickets, collagen diseases, and hypervitaminosis D. Progression to necrosis in these cases is extremely rare.1 There are only a few documented cases of calciphylaxis associated with sarcoidosis, hypercalcemia, and non-ESRD.4
Female sex and diabetes appear to be risk factors.2 The presence of autoimmune disorders is a major feature in patients without ESRD.2,5 Although this patient did not have a previously diagnosed autoimmune disorder, an antinuclear antibody (ANA) test and lupus anticoagulant values were later found to be positive. In patients with autoimmune disorders, prednisone administration is associated with an increased risk of calciphylaxis.5 A hypercoagulable state can also underlie development of calciphylaxis. Our patient did have a mild protein C and S deficiency.
The prognosis of patients diagnosed with calciphylaxis is very poor. The mortality rate is reported to be as high as 60% to 80%.6
4 other possibilities comprise the differential diagnosis
Several conditions may present with erythema or necrosis similar to that of calciphylaxis (TABLE).
Warfarin-induced skin necrosis may produce hemorrhagic bullae and necrotic eschar, but generally presents within 3 to 10 days of initiating warfarin therapy.7 Severe dermatologic manifestations tend to affect the breasts, buttocks, and thighs.
Cutaneous anthrax causes painless necrotic lesions with black eschar, but is linked to bioterrorism or contact with infected animals. Constitutional symptoms such as fever, chills, and malaise are often present. Skin lesions are located primarily on the face, neck, and upper extremities.
Cholesterol embolization results from cholesterol crystals detaching and obstructing smaller arteries. Skin involvement includes livedo reticularis, petechiae, purpura, and ulcerations.
Vasculitis can affect all sizes of blood vessels. It can occur as a complication of connective tissue disorders, viral infections such as hepatitis B and C, or hypersensitivity reactions to medications such as penicillins and cephalosporins. Systemic symptoms are common, as is palpable purpura. Tissue biopsy is important for diagnosis and reveals blood vessel inflammation, not vessel wall calcification.
TABLE
Is it calciphylaxis or something else?1,3,7-9
| Condition | Characteristics |
|---|---|
| Warfarin-induced skin necrosis | Painful, erythematous, edematous lesions; rapidly progressive; petechiae, hemorrhagic bullae, then necrotic eschar |
| Cutaneous anthrax | Small painless, pruritic papules; advances to bullae; finally erodes to painless necrotic lesions with black eschar |
| Cholesterol embolization | Majority with livedo reticularis, cyanosis, or gangrene; smaller percentage with cutaneous ulceration, purpura, petechiae, or painful, firm erythematous nodules |
| Vasculitis | Palpable purpura; biopsy of most affected area is necessary for diagnosis |
| Calciphylaxis | Painful erythematous papules, plaques, nodules, or ulcerations in areas with high adiposity; may progress to necrosis |
What to do when the biopsy isn’t helpful
This case points out an important pathologic rule: If the biopsy doesn’t correlate with the observed disease, additional biopsies are indicated. Calciphylaxis is diagnosed on tissue microscopy, but the initial punch biopsy of the lesion revealed no significant pathologic abnormality. However, a subsequent deep-tissue biopsy showed extensive vascular wall calcification and septal fibrosis with subcutaneous fat necrosis.
Repeating abnormal laboratory testing is often appropriate, too. However, in this patient’s case, it probably would not have been helpful because she had intermittently elevated calcium levels over the years.
Wound cultures are often inaccurate in identifying a causative agent and this patient did not appear to have acute infection.
Management is mainly supportive
If you have a patient with calciphylaxis, address predisposing conditions such as hyperparathyroidism, hypercalcemia, and renal dysfunction5 (strength of recommendation [SOR]: C). In addition, discontinue calcium and vitamin D supplementation6 (SOR: C).
Finally, the patient will need meticulous wound care with adequate pain control; special attention to prevention of secondary infection is essential1,6 (SOR: C).
Our patient was one of the lucky ones
We treated this patient’s hypercalcemia, which was noted on admission to the hospital, with zoledronate and corrected her hypophosphatemia. Her renal function significantly improved with aggressive hydration.
With correction of electrolytes and normalization of kidney function, lesion progression was arrested. Granulation tissue developed in the lesions and split-thickness expanded skin grafts were performed on the large lesions (FIGURE 2). Fortunately, this patient survived despite the usual high rate of mortality. JFP
FIGURE 2
Good granulation beds, followed by closure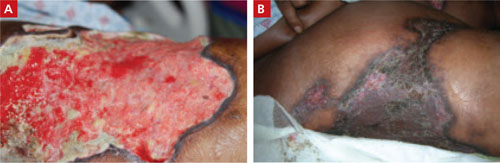
After aggressive treatment of renal dysfunction, correction of electrolyte abnormalities, and meticulous wound care, the patient’s lesions developed good granulation beds and showed signs of healing (A). The second image (B), taken 9 months after the patient first sought treatment for the lesions, shows the wounds after skin grafting.
CORRESPONDENCE
E.J. Mayeaux, Jr, MD, DABFP, FAAFP, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130; [email protected]
1. Kent RB 3rd, Lylerly RT. Systemic calciphylaxis. South Med J. 1994;87:278-281.
2. Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes. Clin J Am Soc Nephrol. 2008;3:1139-1143.
3. Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating. Kidney Int. 2002;61:2210-2217.
4. Swanson AM, Desai SR, Jackson JD, et al. Calciphylaxis associated with chronic inflammatory conditions, immunosuppression therapy, and normal renal function: a report of 2 cases. Arch Dermatol. 2009;145:723-725.
5. Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
6. Al-Hwiesh AK. Calciphylaxis of both proximal and distal distribution. Saudi J Kidney Dis Transpl. 2008;19:82-86.
7. Renick AM Jr. Anticoagulant-induced necrosis of skin and sub-cutaneous tissues. South Med J. 1976;69:775-778, 804.
8. Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
9. Falanga V, Fine MJ, Kapoor WN. The cutaneous manifestations of cholesterol crystal embolization. Arch Dermatol. 1986;122:1194-1198.
A 62-YEAR-OLD WOMAN sought care at our clinic for painful skin lesions that had developed on her thighs 5 days earlier. She had received ongoing treatment at our clinic over the past few years for diabetes, hyperlipidemia, hypertension, and sarcoidosis. In the last 2 years, she’d had 2 hospitalizations for acute renal failure, with a creatinine value as high as 3.8 mg/dL and a persistent glomerular filtration rate consistent with stage 3 chronic kidney disease.
The medications she was taking included glyburide, pravastatin, and lisinopril. During the 2 years prior to her recent clinic visit, she’d had some intermittently elevated calcium readings. Repeat calcium levels each time were normal. In addition, her parathyroid hormone levels fluctuated between low, high, and normal. Her technetium sestamibi scan was negative for hyperparathyroidism. The patient was unemployed and gave no history of recent travel, injuries, or exposure to animals.
On examination, we noted large, poorly demarcated, warm, indurated erythematous lesions on her lateral thighs. She was given a diagnosis of cellulitis and treated with trimethoprim/sulfa-methoxazole 160/800 mg twice daily for 10 days. During follow-up visits 3 and 7 days later, she indicated that the lesions were less painful and they appeared to be less swollen.
Three weeks later, the patient returned to the clinic with skin sloughing that had produced necrotic lesions with black es-char on the bases (FIGURE 1). In addition, new lesions appeared on her anterior thighs. An initial punch biopsy of the lesions revealed no significant pathologic abnormality.
FIGURE 1
What started as indurated plaques…
The patient initially came in for the treatment of indurated plaques, which developed into ulcerative skin lesions with erythematous edges and eschar on the bases.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Calciphylaxis
Calciphylaxis is an uncommon disorder of vascular calcification and thrombosis resulting in skin necrosis.1 It most commonly occurs in people with end-stage renal disease (ESRD) on hemodialysis, but in nonuremic patients the most frequent cause is primary hyperparathyroidism.2,3 Similar vascular calcifications may be observed in milk alkali syndrome, rickets, collagen diseases, and hypervitaminosis D. Progression to necrosis in these cases is extremely rare.1 There are only a few documented cases of calciphylaxis associated with sarcoidosis, hypercalcemia, and non-ESRD.4
Female sex and diabetes appear to be risk factors.2 The presence of autoimmune disorders is a major feature in patients without ESRD.2,5 Although this patient did not have a previously diagnosed autoimmune disorder, an antinuclear antibody (ANA) test and lupus anticoagulant values were later found to be positive. In patients with autoimmune disorders, prednisone administration is associated with an increased risk of calciphylaxis.5 A hypercoagulable state can also underlie development of calciphylaxis. Our patient did have a mild protein C and S deficiency.
The prognosis of patients diagnosed with calciphylaxis is very poor. The mortality rate is reported to be as high as 60% to 80%.6
4 other possibilities comprise the differential diagnosis
Several conditions may present with erythema or necrosis similar to that of calciphylaxis (TABLE).
Warfarin-induced skin necrosis may produce hemorrhagic bullae and necrotic eschar, but generally presents within 3 to 10 days of initiating warfarin therapy.7 Severe dermatologic manifestations tend to affect the breasts, buttocks, and thighs.
Cutaneous anthrax causes painless necrotic lesions with black eschar, but is linked to bioterrorism or contact with infected animals. Constitutional symptoms such as fever, chills, and malaise are often present. Skin lesions are located primarily on the face, neck, and upper extremities.
Cholesterol embolization results from cholesterol crystals detaching and obstructing smaller arteries. Skin involvement includes livedo reticularis, petechiae, purpura, and ulcerations.
Vasculitis can affect all sizes of blood vessels. It can occur as a complication of connective tissue disorders, viral infections such as hepatitis B and C, or hypersensitivity reactions to medications such as penicillins and cephalosporins. Systemic symptoms are common, as is palpable purpura. Tissue biopsy is important for diagnosis and reveals blood vessel inflammation, not vessel wall calcification.
TABLE
Is it calciphylaxis or something else?1,3,7-9
| Condition | Characteristics |
|---|---|
| Warfarin-induced skin necrosis | Painful, erythematous, edematous lesions; rapidly progressive; petechiae, hemorrhagic bullae, then necrotic eschar |
| Cutaneous anthrax | Small painless, pruritic papules; advances to bullae; finally erodes to painless necrotic lesions with black eschar |
| Cholesterol embolization | Majority with livedo reticularis, cyanosis, or gangrene; smaller percentage with cutaneous ulceration, purpura, petechiae, or painful, firm erythematous nodules |
| Vasculitis | Palpable purpura; biopsy of most affected area is necessary for diagnosis |
| Calciphylaxis | Painful erythematous papules, plaques, nodules, or ulcerations in areas with high adiposity; may progress to necrosis |
What to do when the biopsy isn’t helpful
This case points out an important pathologic rule: If the biopsy doesn’t correlate with the observed disease, additional biopsies are indicated. Calciphylaxis is diagnosed on tissue microscopy, but the initial punch biopsy of the lesion revealed no significant pathologic abnormality. However, a subsequent deep-tissue biopsy showed extensive vascular wall calcification and septal fibrosis with subcutaneous fat necrosis.
Repeating abnormal laboratory testing is often appropriate, too. However, in this patient’s case, it probably would not have been helpful because she had intermittently elevated calcium levels over the years.
Wound cultures are often inaccurate in identifying a causative agent and this patient did not appear to have acute infection.
Management is mainly supportive
If you have a patient with calciphylaxis, address predisposing conditions such as hyperparathyroidism, hypercalcemia, and renal dysfunction5 (strength of recommendation [SOR]: C). In addition, discontinue calcium and vitamin D supplementation6 (SOR: C).
Finally, the patient will need meticulous wound care with adequate pain control; special attention to prevention of secondary infection is essential1,6 (SOR: C).
Our patient was one of the lucky ones
We treated this patient’s hypercalcemia, which was noted on admission to the hospital, with zoledronate and corrected her hypophosphatemia. Her renal function significantly improved with aggressive hydration.
With correction of electrolytes and normalization of kidney function, lesion progression was arrested. Granulation tissue developed in the lesions and split-thickness expanded skin grafts were performed on the large lesions (FIGURE 2). Fortunately, this patient survived despite the usual high rate of mortality. JFP
FIGURE 2
Good granulation beds, followed by closure
After aggressive treatment of renal dysfunction, correction of electrolyte abnormalities, and meticulous wound care, the patient’s lesions developed good granulation beds and showed signs of healing (A). The second image (B), taken 9 months after the patient first sought treatment for the lesions, shows the wounds after skin grafting.
CORRESPONDENCE
E.J. Mayeaux, Jr, MD, DABFP, FAAFP, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130; [email protected]
A 62-YEAR-OLD WOMAN sought care at our clinic for painful skin lesions that had developed on her thighs 5 days earlier. She had received ongoing treatment at our clinic over the past few years for diabetes, hyperlipidemia, hypertension, and sarcoidosis. In the last 2 years, she’d had 2 hospitalizations for acute renal failure, with a creatinine value as high as 3.8 mg/dL and a persistent glomerular filtration rate consistent with stage 3 chronic kidney disease.
The medications she was taking included glyburide, pravastatin, and lisinopril. During the 2 years prior to her recent clinic visit, she’d had some intermittently elevated calcium readings. Repeat calcium levels each time were normal. In addition, her parathyroid hormone levels fluctuated between low, high, and normal. Her technetium sestamibi scan was negative for hyperparathyroidism. The patient was unemployed and gave no history of recent travel, injuries, or exposure to animals.
On examination, we noted large, poorly demarcated, warm, indurated erythematous lesions on her lateral thighs. She was given a diagnosis of cellulitis and treated with trimethoprim/sulfa-methoxazole 160/800 mg twice daily for 10 days. During follow-up visits 3 and 7 days later, she indicated that the lesions were less painful and they appeared to be less swollen.
Three weeks later, the patient returned to the clinic with skin sloughing that had produced necrotic lesions with black es-char on the bases (FIGURE 1). In addition, new lesions appeared on her anterior thighs. An initial punch biopsy of the lesions revealed no significant pathologic abnormality.
FIGURE 1
What started as indurated plaques…
The patient initially came in for the treatment of indurated plaques, which developed into ulcerative skin lesions with erythematous edges and eschar on the bases.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Calciphylaxis
Calciphylaxis is an uncommon disorder of vascular calcification and thrombosis resulting in skin necrosis.1 It most commonly occurs in people with end-stage renal disease (ESRD) on hemodialysis, but in nonuremic patients the most frequent cause is primary hyperparathyroidism.2,3 Similar vascular calcifications may be observed in milk alkali syndrome, rickets, collagen diseases, and hypervitaminosis D. Progression to necrosis in these cases is extremely rare.1 There are only a few documented cases of calciphylaxis associated with sarcoidosis, hypercalcemia, and non-ESRD.4
Female sex and diabetes appear to be risk factors.2 The presence of autoimmune disorders is a major feature in patients without ESRD.2,5 Although this patient did not have a previously diagnosed autoimmune disorder, an antinuclear antibody (ANA) test and lupus anticoagulant values were later found to be positive. In patients with autoimmune disorders, prednisone administration is associated with an increased risk of calciphylaxis.5 A hypercoagulable state can also underlie development of calciphylaxis. Our patient did have a mild protein C and S deficiency.
The prognosis of patients diagnosed with calciphylaxis is very poor. The mortality rate is reported to be as high as 60% to 80%.6
4 other possibilities comprise the differential diagnosis
Several conditions may present with erythema or necrosis similar to that of calciphylaxis (TABLE).
Warfarin-induced skin necrosis may produce hemorrhagic bullae and necrotic eschar, but generally presents within 3 to 10 days of initiating warfarin therapy.7 Severe dermatologic manifestations tend to affect the breasts, buttocks, and thighs.
Cutaneous anthrax causes painless necrotic lesions with black eschar, but is linked to bioterrorism or contact with infected animals. Constitutional symptoms such as fever, chills, and malaise are often present. Skin lesions are located primarily on the face, neck, and upper extremities.
Cholesterol embolization results from cholesterol crystals detaching and obstructing smaller arteries. Skin involvement includes livedo reticularis, petechiae, purpura, and ulcerations.
Vasculitis can affect all sizes of blood vessels. It can occur as a complication of connective tissue disorders, viral infections such as hepatitis B and C, or hypersensitivity reactions to medications such as penicillins and cephalosporins. Systemic symptoms are common, as is palpable purpura. Tissue biopsy is important for diagnosis and reveals blood vessel inflammation, not vessel wall calcification.
TABLE
Is it calciphylaxis or something else?1,3,7-9
| Condition | Characteristics |
|---|---|
| Warfarin-induced skin necrosis | Painful, erythematous, edematous lesions; rapidly progressive; petechiae, hemorrhagic bullae, then necrotic eschar |
| Cutaneous anthrax | Small painless, pruritic papules; advances to bullae; finally erodes to painless necrotic lesions with black eschar |
| Cholesterol embolization | Majority with livedo reticularis, cyanosis, or gangrene; smaller percentage with cutaneous ulceration, purpura, petechiae, or painful, firm erythematous nodules |
| Vasculitis | Palpable purpura; biopsy of most affected area is necessary for diagnosis |
| Calciphylaxis | Painful erythematous papules, plaques, nodules, or ulcerations in areas with high adiposity; may progress to necrosis |
What to do when the biopsy isn’t helpful
This case points out an important pathologic rule: If the biopsy doesn’t correlate with the observed disease, additional biopsies are indicated. Calciphylaxis is diagnosed on tissue microscopy, but the initial punch biopsy of the lesion revealed no significant pathologic abnormality. However, a subsequent deep-tissue biopsy showed extensive vascular wall calcification and septal fibrosis with subcutaneous fat necrosis.
Repeating abnormal laboratory testing is often appropriate, too. However, in this patient’s case, it probably would not have been helpful because she had intermittently elevated calcium levels over the years.
Wound cultures are often inaccurate in identifying a causative agent and this patient did not appear to have acute infection.
Management is mainly supportive
If you have a patient with calciphylaxis, address predisposing conditions such as hyperparathyroidism, hypercalcemia, and renal dysfunction5 (strength of recommendation [SOR]: C). In addition, discontinue calcium and vitamin D supplementation6 (SOR: C).
Finally, the patient will need meticulous wound care with adequate pain control; special attention to prevention of secondary infection is essential1,6 (SOR: C).
Our patient was one of the lucky ones
We treated this patient’s hypercalcemia, which was noted on admission to the hospital, with zoledronate and corrected her hypophosphatemia. Her renal function significantly improved with aggressive hydration.
With correction of electrolytes and normalization of kidney function, lesion progression was arrested. Granulation tissue developed in the lesions and split-thickness expanded skin grafts were performed on the large lesions (FIGURE 2). Fortunately, this patient survived despite the usual high rate of mortality. JFP
FIGURE 2
Good granulation beds, followed by closure
After aggressive treatment of renal dysfunction, correction of electrolyte abnormalities, and meticulous wound care, the patient’s lesions developed good granulation beds and showed signs of healing (A). The second image (B), taken 9 months after the patient first sought treatment for the lesions, shows the wounds after skin grafting.
CORRESPONDENCE
E.J. Mayeaux, Jr, MD, DABFP, FAAFP, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130; [email protected]
1. Kent RB 3rd, Lylerly RT. Systemic calciphylaxis. South Med J. 1994;87:278-281.
2. Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes. Clin J Am Soc Nephrol. 2008;3:1139-1143.
3. Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating. Kidney Int. 2002;61:2210-2217.
4. Swanson AM, Desai SR, Jackson JD, et al. Calciphylaxis associated with chronic inflammatory conditions, immunosuppression therapy, and normal renal function: a report of 2 cases. Arch Dermatol. 2009;145:723-725.
5. Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
6. Al-Hwiesh AK. Calciphylaxis of both proximal and distal distribution. Saudi J Kidney Dis Transpl. 2008;19:82-86.
7. Renick AM Jr. Anticoagulant-induced necrosis of skin and sub-cutaneous tissues. South Med J. 1976;69:775-778, 804.
8. Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
9. Falanga V, Fine MJ, Kapoor WN. The cutaneous manifestations of cholesterol crystal embolization. Arch Dermatol. 1986;122:1194-1198.
1. Kent RB 3rd, Lylerly RT. Systemic calciphylaxis. South Med J. 1994;87:278-281.
2. Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes. Clin J Am Soc Nephrol. 2008;3:1139-1143.
3. Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating. Kidney Int. 2002;61:2210-2217.
4. Swanson AM, Desai SR, Jackson JD, et al. Calciphylaxis associated with chronic inflammatory conditions, immunosuppression therapy, and normal renal function: a report of 2 cases. Arch Dermatol. 2009;145:723-725.
5. Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
6. Al-Hwiesh AK. Calciphylaxis of both proximal and distal distribution. Saudi J Kidney Dis Transpl. 2008;19:82-86.
7. Renick AM Jr. Anticoagulant-induced necrosis of skin and sub-cutaneous tissues. South Med J. 1976;69:775-778, 804.
8. Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
9. Falanga V, Fine MJ, Kapoor WN. The cutaneous manifestations of cholesterol crystal embolization. Arch Dermatol. 1986;122:1194-1198.
Kidney Transplants to Black Patients Remain Low Despite HLA-B Elimination
PHILADELPHIA – Eliminating HLA-B matching from the U.S. priority-points formula that was used for allocating deceased-donor kidneys partially eliminated the allocation disparity against black recipients, but a residual disparity remained, based on an analysis of more than 57,000 U.S. kidney transplants during 2000-2009.
Removal of HLA-B matching from the allocation criteria cut the amount of disparity in organs received by black patients, compared with white patients, by a relative 23%, but during 2006-2009 blacks received deceased-donor kidneys at a rate that was 19% below the rate of kidneys that went to white patients, Dr. Erin C. Hall said at the American Transplant Congress, which was sponsored by the American Society of Transplant Surgeons.
In May 2003, the U.S. Organ Procurement and Transplantation Network – the deceased-donor organ distribution network established by the federal government and administered by the United Network for Organ Sharing (UNOS) – eliminated HLA-B matching as an allocation criterion.
Factors that may explain the residual disparity remain unidentified. Future studies could focus on an examination of center-by-center allocation differences by race in an effort to identify other sources for the disparity, said Dr. Hall, a researcher in the transplant surgery division at Johns Hopkins Hospital in Baltimore.
Before May 2003, U.S. kidney allocation procedures gave priority to recipients who matched for more of the six HLA loci, a policy that improved transplantation outcomes but decreased the number of nonwhite recipients who received organs (N. Engl. J. Med. 2004;350:545-51).
To investigate the impact of this change, Dr. Hall and her associated reviewed UNOS records for the 12,956 kidney transplants that were done during 2000–May 2003 using deceased-donor organs, and for the 44,704 transplants done during May 2003–2009. During the two periods, the level of black patients who were registered with UNOS to await kidney transplants held very steady, accounting for 44% all of listed patients before the criterion change, and for 45% after the change.
During 2000-2003, black patients received deceased-donor kidney transplants at 38% below the rate of white patients, according to a multivariate analysis that adjusted for patient differences in age, sex, primary renal disease cause, dialysis modality, use of preemptive transplant, insurance status, education, panel-reactive antibody level over time, and blood type. During 2003-2009, the transplantation rate among black patients was 23% less than it was in white patients. To allow for equilibration in transplant rates following the 2003 criterion change, Dr. Hall also analyzed transplantation rates during 2006-2009. During this period, the rate in black patients lagged 19% behind the rate in white patients, she reported. All three between-group differences were statistically significant. The amount of change between the 38% disparity rate during 2000-2003 and the 23% disparity rate during 2003-2009 constituted a 23% relative decline in the disparity rate.
Dr. Hall said that she had no relevant financial disclosures.
PHILADELPHIA – Eliminating HLA-B matching from the U.S. priority-points formula that was used for allocating deceased-donor kidneys partially eliminated the allocation disparity against black recipients, but a residual disparity remained, based on an analysis of more than 57,000 U.S. kidney transplants during 2000-2009.
Removal of HLA-B matching from the allocation criteria cut the amount of disparity in organs received by black patients, compared with white patients, by a relative 23%, but during 2006-2009 blacks received deceased-donor kidneys at a rate that was 19% below the rate of kidneys that went to white patients, Dr. Erin C. Hall said at the American Transplant Congress, which was sponsored by the American Society of Transplant Surgeons.
In May 2003, the U.S. Organ Procurement and Transplantation Network – the deceased-donor organ distribution network established by the federal government and administered by the United Network for Organ Sharing (UNOS) – eliminated HLA-B matching as an allocation criterion.
Factors that may explain the residual disparity remain unidentified. Future studies could focus on an examination of center-by-center allocation differences by race in an effort to identify other sources for the disparity, said Dr. Hall, a researcher in the transplant surgery division at Johns Hopkins Hospital in Baltimore.
Before May 2003, U.S. kidney allocation procedures gave priority to recipients who matched for more of the six HLA loci, a policy that improved transplantation outcomes but decreased the number of nonwhite recipients who received organs (N. Engl. J. Med. 2004;350:545-51).
To investigate the impact of this change, Dr. Hall and her associated reviewed UNOS records for the 12,956 kidney transplants that were done during 2000–May 2003 using deceased-donor organs, and for the 44,704 transplants done during May 2003–2009. During the two periods, the level of black patients who were registered with UNOS to await kidney transplants held very steady, accounting for 44% all of listed patients before the criterion change, and for 45% after the change.
During 2000-2003, black patients received deceased-donor kidney transplants at 38% below the rate of white patients, according to a multivariate analysis that adjusted for patient differences in age, sex, primary renal disease cause, dialysis modality, use of preemptive transplant, insurance status, education, panel-reactive antibody level over time, and blood type. During 2003-2009, the transplantation rate among black patients was 23% less than it was in white patients. To allow for equilibration in transplant rates following the 2003 criterion change, Dr. Hall also analyzed transplantation rates during 2006-2009. During this period, the rate in black patients lagged 19% behind the rate in white patients, she reported. All three between-group differences were statistically significant. The amount of change between the 38% disparity rate during 2000-2003 and the 23% disparity rate during 2003-2009 constituted a 23% relative decline in the disparity rate.
Dr. Hall said that she had no relevant financial disclosures.
PHILADELPHIA – Eliminating HLA-B matching from the U.S. priority-points formula that was used for allocating deceased-donor kidneys partially eliminated the allocation disparity against black recipients, but a residual disparity remained, based on an analysis of more than 57,000 U.S. kidney transplants during 2000-2009.
Removal of HLA-B matching from the allocation criteria cut the amount of disparity in organs received by black patients, compared with white patients, by a relative 23%, but during 2006-2009 blacks received deceased-donor kidneys at a rate that was 19% below the rate of kidneys that went to white patients, Dr. Erin C. Hall said at the American Transplant Congress, which was sponsored by the American Society of Transplant Surgeons.
In May 2003, the U.S. Organ Procurement and Transplantation Network – the deceased-donor organ distribution network established by the federal government and administered by the United Network for Organ Sharing (UNOS) – eliminated HLA-B matching as an allocation criterion.
Factors that may explain the residual disparity remain unidentified. Future studies could focus on an examination of center-by-center allocation differences by race in an effort to identify other sources for the disparity, said Dr. Hall, a researcher in the transplant surgery division at Johns Hopkins Hospital in Baltimore.
Before May 2003, U.S. kidney allocation procedures gave priority to recipients who matched for more of the six HLA loci, a policy that improved transplantation outcomes but decreased the number of nonwhite recipients who received organs (N. Engl. J. Med. 2004;350:545-51).
To investigate the impact of this change, Dr. Hall and her associated reviewed UNOS records for the 12,956 kidney transplants that were done during 2000–May 2003 using deceased-donor organs, and for the 44,704 transplants done during May 2003–2009. During the two periods, the level of black patients who were registered with UNOS to await kidney transplants held very steady, accounting for 44% all of listed patients before the criterion change, and for 45% after the change.
During 2000-2003, black patients received deceased-donor kidney transplants at 38% below the rate of white patients, according to a multivariate analysis that adjusted for patient differences in age, sex, primary renal disease cause, dialysis modality, use of preemptive transplant, insurance status, education, panel-reactive antibody level over time, and blood type. During 2003-2009, the transplantation rate among black patients was 23% less than it was in white patients. To allow for equilibration in transplant rates following the 2003 criterion change, Dr. Hall also analyzed transplantation rates during 2006-2009. During this period, the rate in black patients lagged 19% behind the rate in white patients, she reported. All three between-group differences were statistically significant. The amount of change between the 38% disparity rate during 2000-2003 and the 23% disparity rate during 2003-2009 constituted a 23% relative decline in the disparity rate.
Dr. Hall said that she had no relevant financial disclosures.
FROM THE AMERICAN TRANSPLANT CONGRESS
Kidney Transplants to Black Patients Remain Low Despite HLA-B Elimination
PHILADELPHIA – Eliminating HLA-B matching from the U.S. priority-points formula that was used for allocating deceased-donor kidneys partially eliminated the allocation disparity against black recipients, but a residual disparity remained, based on an analysis of more than 57,000 U.S. kidney transplants during 2000-2009.
Removal of HLA-B matching from the allocation criteria cut the amount of disparity in organs received by black patients, compared with white patients, by a relative 23%, but during 2006-2009 blacks received deceased-donor kidneys at a rate that was 19% below the rate of kidneys that went to white patients, Dr. Erin C. Hall said at the American Transplant Congress, which was sponsored by the American Society of Transplant Surgeons.
In May 2003, the U.S. Organ Procurement and Transplantation Network – the deceased-donor organ distribution network established by the federal government and administered by the United Network for Organ Sharing (UNOS) – eliminated HLA-B matching as an allocation criterion.
Factors that may explain the residual disparity remain unidentified. Future studies could focus on an examination of center-by-center allocation differences by race in an effort to identify other sources for the disparity, said Dr. Hall, a researcher in the transplant surgery division at Johns Hopkins Hospital in Baltimore.
Before May 2003, U.S. kidney allocation procedures gave priority to recipients who matched for more of the six HLA loci, a policy that improved transplantation outcomes but decreased the number of nonwhite recipients who received organs (N. Engl. J. Med. 2004;350:545-51).
To investigate the impact of this change, Dr. Hall and her associated reviewed UNOS records for the 12,956 kidney transplants that were done during 2000–May 2003 using deceased-donor organs, and for the 44,704 transplants done during May 2003–2009. During the two periods, the level of black patients who were registered with UNOS to await kidney transplants held very steady, accounting for 44% all of listed patients before the criterion change, and for 45% after the change.
During 2000-2003, black patients received deceased-donor kidney transplants at 38% below the rate of white patients, according to a multivariate analysis that adjusted for patient differences in age, sex, primary renal disease cause, dialysis modality, use of preemptive transplant, insurance status, education, panel-reactive antibody level over time, and blood type. During 2003-2009, the transplantation rate among black patients was 23% less than it was in white patients. To allow for equilibration in transplant rates following the 2003 criterion change, Dr. Hall also analyzed transplantation rates during 2006-2009. During this period, the rate in black patients lagged 19% behind the rate in white patients, she reported. All three between-group differences were statistically significant. The amount of change between the 38% disparity rate during 2000-2003 and the 23% disparity rate during 2003-2009 constituted a 23% relative decline in the disparity rate.
Dr. Hall said that she had no relevant financial disclosures.
PHILADELPHIA – Eliminating HLA-B matching from the U.S. priority-points formula that was used for allocating deceased-donor kidneys partially eliminated the allocation disparity against black recipients, but a residual disparity remained, based on an analysis of more than 57,000 U.S. kidney transplants during 2000-2009.
Removal of HLA-B matching from the allocation criteria cut the amount of disparity in organs received by black patients, compared with white patients, by a relative 23%, but during 2006-2009 blacks received deceased-donor kidneys at a rate that was 19% below the rate of kidneys that went to white patients, Dr. Erin C. Hall said at the American Transplant Congress, which was sponsored by the American Society of Transplant Surgeons.
In May 2003, the U.S. Organ Procurement and Transplantation Network – the deceased-donor organ distribution network established by the federal government and administered by the United Network for Organ Sharing (UNOS) – eliminated HLA-B matching as an allocation criterion.
Factors that may explain the residual disparity remain unidentified. Future studies could focus on an examination of center-by-center allocation differences by race in an effort to identify other sources for the disparity, said Dr. Hall, a researcher in the transplant surgery division at Johns Hopkins Hospital in Baltimore.
Before May 2003, U.S. kidney allocation procedures gave priority to recipients who matched for more of the six HLA loci, a policy that improved transplantation outcomes but decreased the number of nonwhite recipients who received organs (N. Engl. J. Med. 2004;350:545-51).
To investigate the impact of this change, Dr. Hall and her associated reviewed UNOS records for the 12,956 kidney transplants that were done during 2000–May 2003 using deceased-donor organs, and for the 44,704 transplants done during May 2003–2009. During the two periods, the level of black patients who were registered with UNOS to await kidney transplants held very steady, accounting for 44% all of listed patients before the criterion change, and for 45% after the change.
During 2000-2003, black patients received deceased-donor kidney transplants at 38% below the rate of white patients, according to a multivariate analysis that adjusted for patient differences in age, sex, primary renal disease cause, dialysis modality, use of preemptive transplant, insurance status, education, panel-reactive antibody level over time, and blood type. During 2003-2009, the transplantation rate among black patients was 23% less than it was in white patients. To allow for equilibration in transplant rates following the 2003 criterion change, Dr. Hall also analyzed transplantation rates during 2006-2009. During this period, the rate in black patients lagged 19% behind the rate in white patients, she reported. All three between-group differences were statistically significant. The amount of change between the 38% disparity rate during 2000-2003 and the 23% disparity rate during 2003-2009 constituted a 23% relative decline in the disparity rate.
Dr. Hall said that she had no relevant financial disclosures.
PHILADELPHIA – Eliminating HLA-B matching from the U.S. priority-points formula that was used for allocating deceased-donor kidneys partially eliminated the allocation disparity against black recipients, but a residual disparity remained, based on an analysis of more than 57,000 U.S. kidney transplants during 2000-2009.
Removal of HLA-B matching from the allocation criteria cut the amount of disparity in organs received by black patients, compared with white patients, by a relative 23%, but during 2006-2009 blacks received deceased-donor kidneys at a rate that was 19% below the rate of kidneys that went to white patients, Dr. Erin C. Hall said at the American Transplant Congress, which was sponsored by the American Society of Transplant Surgeons.
In May 2003, the U.S. Organ Procurement and Transplantation Network – the deceased-donor organ distribution network established by the federal government and administered by the United Network for Organ Sharing (UNOS) – eliminated HLA-B matching as an allocation criterion.
Factors that may explain the residual disparity remain unidentified. Future studies could focus on an examination of center-by-center allocation differences by race in an effort to identify other sources for the disparity, said Dr. Hall, a researcher in the transplant surgery division at Johns Hopkins Hospital in Baltimore.
Before May 2003, U.S. kidney allocation procedures gave priority to recipients who matched for more of the six HLA loci, a policy that improved transplantation outcomes but decreased the number of nonwhite recipients who received organs (N. Engl. J. Med. 2004;350:545-51).
To investigate the impact of this change, Dr. Hall and her associated reviewed UNOS records for the 12,956 kidney transplants that were done during 2000–May 2003 using deceased-donor organs, and for the 44,704 transplants done during May 2003–2009. During the two periods, the level of black patients who were registered with UNOS to await kidney transplants held very steady, accounting for 44% all of listed patients before the criterion change, and for 45% after the change.
During 2000-2003, black patients received deceased-donor kidney transplants at 38% below the rate of white patients, according to a multivariate analysis that adjusted for patient differences in age, sex, primary renal disease cause, dialysis modality, use of preemptive transplant, insurance status, education, panel-reactive antibody level over time, and blood type. During 2003-2009, the transplantation rate among black patients was 23% less than it was in white patients. To allow for equilibration in transplant rates following the 2003 criterion change, Dr. Hall also analyzed transplantation rates during 2006-2009. During this period, the rate in black patients lagged 19% behind the rate in white patients, she reported. All three between-group differences were statistically significant. The amount of change between the 38% disparity rate during 2000-2003 and the 23% disparity rate during 2003-2009 constituted a 23% relative decline in the disparity rate.
Dr. Hall said that she had no relevant financial disclosures.
FROM THE AMERICAN TRANSPLANT CONGRESS
Major Finding: Elimination of HLA-B matching as an allocation criterion for U.S. kidney transplants in 2003 cut the allocation bias against blacks by a relative 23%, but a significant allocation bias remained through 2009.
Data Source: Review of 57,660 deceased-donor U.S. kidney transplants done during 2000-2009 using data collected by the U.S. Organ Procurement and Transplantation Network.
Disclosures: Dr. Hall said that she had no relevant financial disclosures.
