User login
Kidney Stones Linked to CVD, Metabolic Syndrome
WASHINGTON – Research has documented a strong association between the formation of kidney stones and the presence or development of cardiovascular disease, metabolic syndrome, and a number of components of the metabolic syndrome, said Dr. Dean G. Assimos.
"There is increasing evidence" of this link, he noted at the annual meeting of the American Urological Association. "We need to be cognizant of these associations."
Most recently, an analysis of data from the Coronary Artery Risk Development in Young Adults (CARDIA) study showed that individuals who developed kidney stones had a 1.6-fold increased risk of developing subclinical carotid artery atherosclerosis, even after adjustments for major cardiovascular risk factors were made, said Dr. Assimos, professor of urology at Wake Forest University, Winston-Salem, N.C.
The longitudinal cohort study followed 5,115 white and black men and women who were 18-30 years old at the time of recruitment in 1985-1986. Carotid artery intima-media thickness was measured with serial ultrasound periodically throughout the observation period. By 20 years, almost 4% had reported having kidney stones, and kidney stones were associated with a 60% increased risk of carotid atherosclerosis (J. Urol. 2011;185:920-5). Kidney stones were associated with myocardial infarction (MI) in another recent study aimed specifically at assessing "the risk of a kidney stone former developing an MI," Dr. Assimos said. Investigators of this case-controlled study matched almost 4,600 stone formers on age and sex with almost 11,000 control subjects among residents of Olmstead County, Minn.
During a mean follow-up of 9 years, "despite controlling for other medical comorbidities," investigators found that "stone formers had a 31% increased risk of sustaining an MI," he said.
Chronic kidney disease, which itself is a risk factor for MI, was one of the comorbidities adjusted for (J. Am. Soc. Nephrol. 2010;21:1641-4).
Numerous studies published since 2005 have demonstrated positive associations between kidney stone formation and specific components of the metabolic syndrome, as well as with the full constellation of disorders, Dr. Assimos said.
An analysis of National Health and Nutrition Examination Survey III data published in 2008, for instance, showed that individuals with four traits of the metabolic syndrome had two times the risk of having a history of kidney stones (Am. J. Kidney Dis. 2008;51:741-7). The prevalence of self-reported history of kidney stones, moreover, increased as the number of traits or component disorders of the metabolic syndrome increased, from 3% with no disorders to 7.5% with three disorders, and to almost 10% with five disorders. (An individual must have at least three of the five component disorders to qualify as having the metabolic syndrome.)
Similarly, in an Italian study of hospitalized adults, more than 10% of 725 patients with metabolic syndrome had evidence of nephrolithiasis on renal ultrasound (Nephrol. Dial. Transplant 2009;24:900-6). "This is 10 times higher than [rates reported from] renal ultrasound screening studies done in the general population," Dr. Assimos said.
Data from three large cohorts – the Nurses’ Health Study (NHS) I of older women, the NHS II of younger women, and the Health Professionals Follow-Up Study (HPFS) of men aged 40-75 years – have been crucial in elucidating the associations between kidney stones and specific components of the metabolic syndrome.
In one prospective study of the cohorts that looked at the incidence of symptomatic kidney stones, for instance, investigators documented that the relative risk of an obese individual (body mass index, 30 kg/m2 or greater) for kidney stone formation, compared with individuals with a BMI of 21-23, was 1.9 in the NHS I cohort, 2.09 in the NHS II cohort, and 1.33 in the HPFS cohort (JAMA 2005;293:455-62).
"There was also a positive correlation in all these cohorts with waist circumference," Dr. Assimos said.
Other analyses of these cohorts have documented positive associations between type 2 diabetes or hypertension, and incident kidney stone formation, as well as associations between a history of kidney stone formation and the diagnosis of diabetes or development of hypertension.
The causative factors underlying the associations between stone formation and cardiovascular disease and the metabolic syndrome include low urinary pH levels. At lower urinary pH levels, "more [of the body’s] uric acid is in its undissociated form and is insoluble in urine," for instance, which increases the risk of uric acid stone formation, Dr. Assimos said.
Studies have demonstrated a negative correlation between BMI and urinary pH, he noted. The reasons are not fully known, but "it is hypothesized that individuals [with higher BMI] do not produce ammonium effectively in the proximal tubule," he said.
Individuals with obesity and low urinary pH also excrete greater amounts of calcium and oxalate, and this increases the risk of calcium oxalate stone formation, he said.
Dr. Assimos’s own research team has identified a possible new pathway for the endogenous synthesis of oxalate. It involves the metabolism of glyoxal, which is stimulated by oxidative stress. The glyoxal metabolism "may explain the increased oxalate excretion in those with obesity as well as diabetes," he said.
The associations between kidney stone formation and cardiovascular risk have hit home for Dr. Assimos, he said at the end of his presentation. At age 39, he developed his first kidney stone. By 3 years later, he developed hypertension. "And 3 years ago. I started having symptoms of gastroesophageal reflux when exercising ... I had a stress test ... and here is my coronary arteriogram," he told the audience. The end result, he said, was successful coronary artery bypass grafting.
Dr. Assimos reported that he is an investigator for the National Institutes of Health and a partner at Piedmont Stone, a facility in Winston-Salem that provides lithotripsy procedures.
WASHINGTON – Research has documented a strong association between the formation of kidney stones and the presence or development of cardiovascular disease, metabolic syndrome, and a number of components of the metabolic syndrome, said Dr. Dean G. Assimos.
"There is increasing evidence" of this link, he noted at the annual meeting of the American Urological Association. "We need to be cognizant of these associations."
Most recently, an analysis of data from the Coronary Artery Risk Development in Young Adults (CARDIA) study showed that individuals who developed kidney stones had a 1.6-fold increased risk of developing subclinical carotid artery atherosclerosis, even after adjustments for major cardiovascular risk factors were made, said Dr. Assimos, professor of urology at Wake Forest University, Winston-Salem, N.C.
The longitudinal cohort study followed 5,115 white and black men and women who were 18-30 years old at the time of recruitment in 1985-1986. Carotid artery intima-media thickness was measured with serial ultrasound periodically throughout the observation period. By 20 years, almost 4% had reported having kidney stones, and kidney stones were associated with a 60% increased risk of carotid atherosclerosis (J. Urol. 2011;185:920-5). Kidney stones were associated with myocardial infarction (MI) in another recent study aimed specifically at assessing "the risk of a kidney stone former developing an MI," Dr. Assimos said. Investigators of this case-controlled study matched almost 4,600 stone formers on age and sex with almost 11,000 control subjects among residents of Olmstead County, Minn.
During a mean follow-up of 9 years, "despite controlling for other medical comorbidities," investigators found that "stone formers had a 31% increased risk of sustaining an MI," he said.
Chronic kidney disease, which itself is a risk factor for MI, was one of the comorbidities adjusted for (J. Am. Soc. Nephrol. 2010;21:1641-4).
Numerous studies published since 2005 have demonstrated positive associations between kidney stone formation and specific components of the metabolic syndrome, as well as with the full constellation of disorders, Dr. Assimos said.
An analysis of National Health and Nutrition Examination Survey III data published in 2008, for instance, showed that individuals with four traits of the metabolic syndrome had two times the risk of having a history of kidney stones (Am. J. Kidney Dis. 2008;51:741-7). The prevalence of self-reported history of kidney stones, moreover, increased as the number of traits or component disorders of the metabolic syndrome increased, from 3% with no disorders to 7.5% with three disorders, and to almost 10% with five disorders. (An individual must have at least three of the five component disorders to qualify as having the metabolic syndrome.)
Similarly, in an Italian study of hospitalized adults, more than 10% of 725 patients with metabolic syndrome had evidence of nephrolithiasis on renal ultrasound (Nephrol. Dial. Transplant 2009;24:900-6). "This is 10 times higher than [rates reported from] renal ultrasound screening studies done in the general population," Dr. Assimos said.
Data from three large cohorts – the Nurses’ Health Study (NHS) I of older women, the NHS II of younger women, and the Health Professionals Follow-Up Study (HPFS) of men aged 40-75 years – have been crucial in elucidating the associations between kidney stones and specific components of the metabolic syndrome.
In one prospective study of the cohorts that looked at the incidence of symptomatic kidney stones, for instance, investigators documented that the relative risk of an obese individual (body mass index, 30 kg/m2 or greater) for kidney stone formation, compared with individuals with a BMI of 21-23, was 1.9 in the NHS I cohort, 2.09 in the NHS II cohort, and 1.33 in the HPFS cohort (JAMA 2005;293:455-62).
"There was also a positive correlation in all these cohorts with waist circumference," Dr. Assimos said.
Other analyses of these cohorts have documented positive associations between type 2 diabetes or hypertension, and incident kidney stone formation, as well as associations between a history of kidney stone formation and the diagnosis of diabetes or development of hypertension.
The causative factors underlying the associations between stone formation and cardiovascular disease and the metabolic syndrome include low urinary pH levels. At lower urinary pH levels, "more [of the body’s] uric acid is in its undissociated form and is insoluble in urine," for instance, which increases the risk of uric acid stone formation, Dr. Assimos said.
Studies have demonstrated a negative correlation between BMI and urinary pH, he noted. The reasons are not fully known, but "it is hypothesized that individuals [with higher BMI] do not produce ammonium effectively in the proximal tubule," he said.
Individuals with obesity and low urinary pH also excrete greater amounts of calcium and oxalate, and this increases the risk of calcium oxalate stone formation, he said.
Dr. Assimos’s own research team has identified a possible new pathway for the endogenous synthesis of oxalate. It involves the metabolism of glyoxal, which is stimulated by oxidative stress. The glyoxal metabolism "may explain the increased oxalate excretion in those with obesity as well as diabetes," he said.
The associations between kidney stone formation and cardiovascular risk have hit home for Dr. Assimos, he said at the end of his presentation. At age 39, he developed his first kidney stone. By 3 years later, he developed hypertension. "And 3 years ago. I started having symptoms of gastroesophageal reflux when exercising ... I had a stress test ... and here is my coronary arteriogram," he told the audience. The end result, he said, was successful coronary artery bypass grafting.
Dr. Assimos reported that he is an investigator for the National Institutes of Health and a partner at Piedmont Stone, a facility in Winston-Salem that provides lithotripsy procedures.
WASHINGTON – Research has documented a strong association between the formation of kidney stones and the presence or development of cardiovascular disease, metabolic syndrome, and a number of components of the metabolic syndrome, said Dr. Dean G. Assimos.
"There is increasing evidence" of this link, he noted at the annual meeting of the American Urological Association. "We need to be cognizant of these associations."
Most recently, an analysis of data from the Coronary Artery Risk Development in Young Adults (CARDIA) study showed that individuals who developed kidney stones had a 1.6-fold increased risk of developing subclinical carotid artery atherosclerosis, even after adjustments for major cardiovascular risk factors were made, said Dr. Assimos, professor of urology at Wake Forest University, Winston-Salem, N.C.
The longitudinal cohort study followed 5,115 white and black men and women who were 18-30 years old at the time of recruitment in 1985-1986. Carotid artery intima-media thickness was measured with serial ultrasound periodically throughout the observation period. By 20 years, almost 4% had reported having kidney stones, and kidney stones were associated with a 60% increased risk of carotid atherosclerosis (J. Urol. 2011;185:920-5). Kidney stones were associated with myocardial infarction (MI) in another recent study aimed specifically at assessing "the risk of a kidney stone former developing an MI," Dr. Assimos said. Investigators of this case-controlled study matched almost 4,600 stone formers on age and sex with almost 11,000 control subjects among residents of Olmstead County, Minn.
During a mean follow-up of 9 years, "despite controlling for other medical comorbidities," investigators found that "stone formers had a 31% increased risk of sustaining an MI," he said.
Chronic kidney disease, which itself is a risk factor for MI, was one of the comorbidities adjusted for (J. Am. Soc. Nephrol. 2010;21:1641-4).
Numerous studies published since 2005 have demonstrated positive associations between kidney stone formation and specific components of the metabolic syndrome, as well as with the full constellation of disorders, Dr. Assimos said.
An analysis of National Health and Nutrition Examination Survey III data published in 2008, for instance, showed that individuals with four traits of the metabolic syndrome had two times the risk of having a history of kidney stones (Am. J. Kidney Dis. 2008;51:741-7). The prevalence of self-reported history of kidney stones, moreover, increased as the number of traits or component disorders of the metabolic syndrome increased, from 3% with no disorders to 7.5% with three disorders, and to almost 10% with five disorders. (An individual must have at least three of the five component disorders to qualify as having the metabolic syndrome.)
Similarly, in an Italian study of hospitalized adults, more than 10% of 725 patients with metabolic syndrome had evidence of nephrolithiasis on renal ultrasound (Nephrol. Dial. Transplant 2009;24:900-6). "This is 10 times higher than [rates reported from] renal ultrasound screening studies done in the general population," Dr. Assimos said.
Data from three large cohorts – the Nurses’ Health Study (NHS) I of older women, the NHS II of younger women, and the Health Professionals Follow-Up Study (HPFS) of men aged 40-75 years – have been crucial in elucidating the associations between kidney stones and specific components of the metabolic syndrome.
In one prospective study of the cohorts that looked at the incidence of symptomatic kidney stones, for instance, investigators documented that the relative risk of an obese individual (body mass index, 30 kg/m2 or greater) for kidney stone formation, compared with individuals with a BMI of 21-23, was 1.9 in the NHS I cohort, 2.09 in the NHS II cohort, and 1.33 in the HPFS cohort (JAMA 2005;293:455-62).
"There was also a positive correlation in all these cohorts with waist circumference," Dr. Assimos said.
Other analyses of these cohorts have documented positive associations between type 2 diabetes or hypertension, and incident kidney stone formation, as well as associations between a history of kidney stone formation and the diagnosis of diabetes or development of hypertension.
The causative factors underlying the associations between stone formation and cardiovascular disease and the metabolic syndrome include low urinary pH levels. At lower urinary pH levels, "more [of the body’s] uric acid is in its undissociated form and is insoluble in urine," for instance, which increases the risk of uric acid stone formation, Dr. Assimos said.
Studies have demonstrated a negative correlation between BMI and urinary pH, he noted. The reasons are not fully known, but "it is hypothesized that individuals [with higher BMI] do not produce ammonium effectively in the proximal tubule," he said.
Individuals with obesity and low urinary pH also excrete greater amounts of calcium and oxalate, and this increases the risk of calcium oxalate stone formation, he said.
Dr. Assimos’s own research team has identified a possible new pathway for the endogenous synthesis of oxalate. It involves the metabolism of glyoxal, which is stimulated by oxidative stress. The glyoxal metabolism "may explain the increased oxalate excretion in those with obesity as well as diabetes," he said.
The associations between kidney stone formation and cardiovascular risk have hit home for Dr. Assimos, he said at the end of his presentation. At age 39, he developed his first kidney stone. By 3 years later, he developed hypertension. "And 3 years ago. I started having symptoms of gastroesophageal reflux when exercising ... I had a stress test ... and here is my coronary arteriogram," he told the audience. The end result, he said, was successful coronary artery bypass grafting.
Dr. Assimos reported that he is an investigator for the National Institutes of Health and a partner at Piedmont Stone, a facility in Winston-Salem that provides lithotripsy procedures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN UROLOGICAL ASSOCIATION
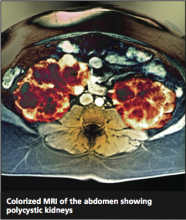
Autosomal Dominant Polycystic Kidney Disease
Twice as common as autism and half as well-known,1 autosomal polycystic kidney disease (ADPKD) occurs in one in 400 to one in 1,000 people.2 It is an inherited progressive genetic disorder that causes hypertension and decreased renal function and, over time, can lead to kidney failure. Two polycstin genes that code for ADPKD, PKD1 and PKD2, were identified in 1994 and 1996, respectively.3,4 Awareness and understanding of the genes responsible for ADPKD have increased clinicians’ ability to identify at-risk patients and to slow or alter the course of the disease.
Case Presentation
A 45-year-old black man presents to your office with severe, nonradiating back pain and new-onset hypertension. Regarding the pain, he stated, “I turned around to see who kicked me, but no one was there.” When the pain began, he went to see the nurse at the school where he is employed, and she found that his blood pressure was high at 162/90 mm Hg. Although the patient’s back pain is resolving, he is very concerned about his blood pressure, since he has never had a high reading before.
He is the baseball coach and physical education teacher at the local high school and is in excellent physical condition as a result of his professional interaction with teenagers every day. He does not smoke or use any illicit drugs but does admit to occasional alcohol consumption. His medical history is significant only for occasional broken fingers and twisted ankles, all occurring while he was engaged in sports.
His family history includes one brother without medical problems, a brother and a sister with hypertension, a sister with diabetes and obesity, and a brother with a congenital abnormality that required a living donor kidney transplant at age 17 (the father served as donor). No family-wide workup has ever been done because no one practitioner has ever made a connection among these conditions and considered a diagnosis of ADPKD.
The patient’s blood pressure in the office is 172/92 mm Hg while sitting and 166/88 mm Hg while standing. He is somewhat sore with a localized spasm in the lumbar-sacral area but no radiation of pain. The patient has trouble touching his toes but reports that he can never touch his toes. His straight leg lift is negative. The rest of his physical exam is noncontributory.
What should be the next step in this patient’s workup?
PATHOPHYSIOLOGY
ADPKD is a progressive expansion of numerous fluid-filled cysts that result in massive enlargement of the kidneys.5 Less than 5% of all nephrons become cystic; however, the average volume of a polycystic kidney is 1,000 mL (normal, 300 mL), that is, the volume of a standard-sized pineapple. Even with this significant enlargement, a decline in the glomerular filtration rate (GFR) is not usually seen initially. Each cyst is derived from a single hyperproliferative epithelial cell. Increased cellular proliferation, followed by fluid secretion and alterations in the extracellular matrix, cause an outpouching from the parent nephron, which eventually detaches from the parent nephron and continues to enlarge and autonomously secrete fluid.6,7
PKD1 and PKD2 are two genes responsible for ADPKD that have been isolated so far. Since there are families carrying neither the PKD1 nor the PKD2 gene that still have an inherited type of ADPKD, there is suspicion that at least one more PKD gene, not yet isolated, exists.8 It is also possible that other genetic or environmental factors may be at play.9,10
In 1994, the PKD1 gene was isolated on chromosome 16,3 and it was found to code for polycystin 1. A lack of polycystin 1 causes an abnormality in the Na+/K(+)-ATPase pumps, leading to abnormal sodium reabsorption.11 How and why this happens is not quite clear. However, the hypertension that is a key objective finding in patients with ADPKD is thought to result from this pump abnormality.
PKD2 is found on the long arm of chromosome 4 and codes for polycystin 2.4 Polycystin 2 is an amino acid that is responsible for voltage-activated cellular calcium channels,5 again explaining the hypertension so commonly seen in the course of ADPKD. ADPKD-associated hypertension may be present as early as the teenage years.12
EPIDEMIOLOGY
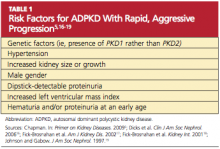
More than 85% of ADPKD cases are associated with PKD1, and this form is called polycystic kidney disease 1 (PKD 1), the more aggressive form of the disease.13,14 PKD 2 (the form associated with the gene PKD2), though less common, is also likely to progress to end-stage renal disease (ESRD), but at a later age (median age of 74 years, compared with 54 in patients with PKD 1).14 ADPKD accounts for about 5% of cases of ESRD in North America,9 but for most patients, presentation and decreased renal function do not occur until the 40s.15 However, patients with the risk factors listed in Table 15,16-19 are likely to experience a more rapid and aggressive form of the disease.
Even with the same germline mutation in a family with this inherited disease, the severity of ADPKD among family members is quite variable; this is true even in the case of twins.9,10,20 Since the age and symptoms at presentation can vary so greatly, a uniform method of identifying patients with ADPKD, along with staging, was needed. Most patients do not undergo genetic testing (ie, DNA linkage or gene-based direct sequencing9) for a diagnosis of ADPKD or to differentiate between the PKD 1 and PKD 2 disease forms unless they are participating in a research study. Diagnostic criteria were needed that were applicable for any type of ADPKD.
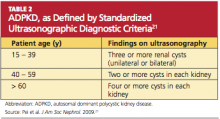
In 2009, the University of Toronto’s Division of Nephrology convened experts in the fields of nephrology and radiology to reach a consensus on standardized ultrasonographic diagnostic criteria.21 They formulated definitions based on a study of 948 individuals who were at risk for either PKD 1 or PKD 2 (see Table 221). The specificity and sensitivity of the resulting criteria range from 82% to 100%, making it possible to standardize the care and classification of renal patients worldwide.
Since family members with the same genotypes can experience very divergent disease manifestations, the two-hit hypothesis has been developed.22 In simple terms, it proposes that after the germline mutation (PKD1 or PKD2), there is a second somatic mutation that leads to progressive cyst formation; when the number and size of cysts increase, the patient starts to experience symptoms of ADPKD.22
Age at presentation can be quite variable, as can the presenting symptoms. Most patients with PKD 1 present in their 50s, with 54 being the average age in US patients.14 The most common presenting symptom is flank or back pain.2,5 The pain is due to the massive enlargement of the kidneys, causing a stretching of the kidney capsule and leading to a chronic, dull and persistent pain in the low back. Severe pain, sharp and cutting, occurs when one of the cysts hemorrhages; to some patients, the pain resembles a quick, powerful “kick in the back.” Hematuria can occur following cyst hemorrhage; depending on the location of the cyst that burst within the kidney (ie, how close it is to the collecting system) and how large it is, the amount and color of the hematuria can be impressive.
ADPKD is more common in men than women, and cyst rupture can be precipitated by trauma or lifting heavy objects. Cyst hemorrhage can turn the urine bright red, which is especially frightening to the male patient. Hematuria is often the key presenting symptom in patients who will be diagnosed with ADPKD-induced hypertension.
Besides hematuria, other common manifestations of ADPKD include:
• Hypertension (60% of affected patients, which increases to 100% by the time ESRD develops)
• Extrarenal cysts (100% of affected patients)
• Urinary tract infections
• Nephrolithiasis (20% of affected patients)
• Proteinuria, occasionally (18% of affected patients).2,5,23
Among these manifestations, those most commonly attributed to a diagnosis of ADPKD are hypertension, kidney stones, and urinary tract or kidney infections. Since isolated proteinuria is unusual in patients with ADPKD, it is recommended that another cause of kidney disease be explored in patients with this presentation.24
Extrarenal manifestations of cyst development are common, eventually occurring in all patients as they age. Hepatic cysts are universal in patients with ADPKD by age 30, although hepatic function is preserved. There may be a mild elevation in the alkaline phosphatase level in patients with ADPKD, resulting from the presence of hepatic cysts. Cysts may also be found in the pancreas, spleen, thyroid, and epididymis.5,25 Some patients may complain of dyspnea, pain, early satiety, or lower extremity edema as a result of enlarged cyst.
The Case Patient
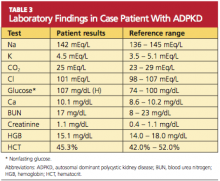
Because you recently attended a lecture about ADPKD, you are aware that flank pain in men with hypertension is indicative of ADPKD until proven otherwise. Believing that this patient’s hypertension is renal in origin, you order an abdominal ultrasound. You also order a comprehensive metabolic panel and a complete blood count. The patient’s GFR is measured at 89 mL/min (indicative of stage 2 kidney disease). Other results are shown in Table 3.
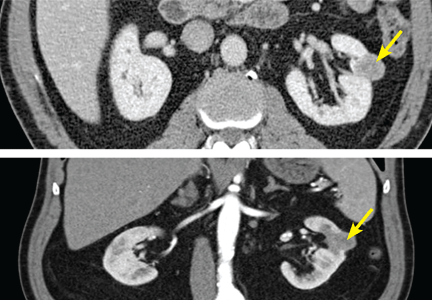
The very broad differential includes essential hypertension, hypertension resulting from intake of “power drinks” or salt in an athlete, illicit use of medications (including steroids), herniated disc leading to transient hypertension, and urinary tract infection or sexually transmitted disease. All of this is moot when the ultrasound shows both kidneys measuring greater than 15 cm, with four distinct cysts on the right kidney and three distinct cysts on the left.
You explain to the patient that ADPKD is a genetic disease and that he and his siblings each had a certain chance of inheriting it. Although different presentations may occur (“congenital” polycystic kidney disease, hypertension, or obesity), they all must undergo ultrasonographic screening for ADPKD. You add that although ADPKD is a genetic disease, it can also be diagnosed in different members of the same family at different ages.
TREATMENT
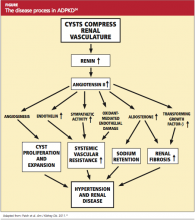
The goal of treatment for the patient with ADPKD is to slow cyst development and the natural course of the disease. If this can be achieved, the need for dialysis or kidney transplantation may be postponed for a number of years. Because cyst growth causes an elevation in renin and activates the angiotensin II renin system26 (see figure,24), an ACE inhibitor is the most effective treatment to lower blood pressure and thus slow the progression of ADPKD. Most patients with ADPKD are started on an ACE inhibitor at an early age to slow the rate of disease progression.27,28 Several studies are under way to determine the best antihypertensive medication and the optimal age for initiating treatment.29,30
Lipid screening and treatment for dyslipidemia are important23 because ADPKD can lead to a reduction in kidney function, resulting in chronic kidney disease (CKD). CKD is considered a coronary heart disease risk equivalent, and most professionals will treat the patient with ADPKD for hyperlipidemia.23,31 While there are no data showing that statin use will reduce the incidence of ESRD or delay the need for dialysis or kidney transplantation in patients with ADPKD, the beneficial effects of good renal blood flow and endothelial function have been noted.32,33
One of the most common and significant complications in ADPKD is intracranial hemorrhage resulting from a ruptured cerebral aneurysm. In the younger adult, the incidence of cerebral aneurysm is 4%, but incidence increases to 10% in patients older than 65.34-36 Family clusters of aneurysms have been reported.37 If an intracranial aneurysm is found in the family history, the risk of an aneurysm in another family member increases to 22%.38
Since rupture of an intracranial hemorrhage is associated with a 30-day mortality rate of 50% and 80% morbidity,5,38 standard of care for patients with ADPKD includes CT or magnetic resonance angiographic (MRA) screening in the asymptomatic patient with a positive family history.34,38 If an aneurysm is found, the lifetime chance of rupture is 50%, although the risk is greater in the case of an aneurysm larger than 10 mm.5
As in all patients with kidney disease, left ventricular hypertrophy is common among patients with ADPKD.23,28,39
The Case Patient
The patient is started on an ACE inhibitor, scheduled for fasting lipid screening, and referred to a nephrology practice for disease management. As research and investigation of possible treatment options for ADPKD are ongoing, the patient may benefit from participating in a new research protocol.
Because the patient’s family has no history of cerebral aneurysm, CT/MRA screening is not required. A discussion of the pros and cons of genetic testing for the entire family, including nieces and nephews, is initiated. The patient and his family are referred to a genetic counselor to decide whether the benefit of early treatment for hypertension outweighs the risk of carrying a diagnosis of ADPKD for his younger relatives, who may later seek health insurance coverage.
NATURAL PROGRESSION OF ADPKD
Hypertension and cyst formation will continue as the patient ages. The natural progression of ADPKD is to renal failure with renal replacement therapy (dialysis or organ transplantation) as treatment options. If the progression of ADPKD can be slowed through pharmacotherapy, the patient may live for many years without needing dialysis. This ideal can be accomplished only through aggressive hypertension control, which should be started in the teenage years.23,30,31
Suggestions to increase fluid consumption and to limit the use of NSAIDs, contrast dye, and MRI with gadolinium are appropriate. It is rare for hypertension to be diagnosed before some organ damage has already occurred.12 Often the patient’s renal function, as determined by measuring the GFR, remains stable until the patient reaches his or her 40s.40 However, kidney damage often begins before any detectable change in GFR. Once the GFR does start to decline, the average decrease is 4.4 to 5.9 mL/min/1.73m2 each year.41
MANAGEMENT CONSIDERATIONS
For ESRD Organ Transplantation
Kidney transplantation—the only curative treatment for ADPKD—can be offered to patients once the GFR falls below 20 mL/min. However, the patient with ADPKD can experience kidney enlargement to such an extent that introducing a third kidney into the limited abdominal space becomes technically difficult. Although nephrectomy is avoided whenever possible, there are cases in which there is no alternative.42
In addition to space concerns, recurrent urinary tract infections, chronic pain, renal cell carcinoma, chronic hematuria, or chronic cyst infections can necessitate a nephrectomy.43,44 A laparoscopic approach with decompression of cysts or removal of only one kidney is preferred.43,45 If removal of both kidneys is required before a transplant, the patient must be maintained on dialysis until after transplantation. Since the transplant waiting list can exceed seven years in some areas, most patients arrange for a willing live donor before agreeing to a bilateral nephrectomy.46,47
Dialysis
Either peritoneal dialysis (PD) or hemodialysis (HD) can be offered to patients with severe ADPKD. Depending on the size of the native kidneys and the history of previous abdominal surgery, PD often offers a better chance of survival in these patients, particularly compared with patients who have ESRD associated with other causes.48
For management of the patient with ADPKD who receives PD, it can be difficult to differentiate between the pain of a cyst and the pain of a peritoneal infection. Generally, cyst rupture is accompanied by hematuria; and peritonitis, by cloudy fluid.5 Management provided by an experienced nephrologist and PD nurse is vital.
In ADPKD patients who undergo HD, too, survival is better than in patients who have ESRD with other causes49,50; five-year survival can be as high as 10% to 15%.51 This is likely due to the lower incidence of coronary artery disease in the ADPKD population, compared with patients who have ESRD associated with other chronic diseases.49
FUTURE TRENDS AND ONGOING TRIALS
HALT PKD29,30 is an NIH-funded, double-blind study to determine whether adding an angiotensin receptor blocker (ARB) to standard ACE inhibitor therapy results in a more significant decrease in the progression of renal cysts. The rationale for this is that the ARB is expected to block the renin-angiotensin-aldosterone system in the kidney. Use of ACE inhibitor monotherapy versus ARB/ACE inhibitor therapy is being compared in two study arms: patients between ages 15 and 49 with a GFR of 60 mL/min or greater; and patients between ages 18 and 64 with a GFR of 25 to 60 mL/min.29 To date, preliminary results indicate no benefit in adding the second medication.49
The TEMPO Trial52 is a multicenter, double-blind study looking at the effect of tolvaptan on renal cyst growth. Tolvaptan is a potent vasopressin receptor antagonist, and in vitro evidence has shown that intracellular cyclic adenosine monophosphate (cAMP) plays a large role in the development of cysts in patients with ADPKD. If it is possible to block the cAMP that is causing cyst growth, progression of ADPKD should slow.53,54 Only short-term effects of tolvaptan use are currently known.55
High Water Intake to Slow Progression of Polycystic Kidney Disease56 is an open-label, nonrandomized trial in which patients drink a minimum of
3 L of water. Previously, a small study showed that an increase in fluid intake partially suppresses the urine osmolality and the serum antidiuretic hormone (ADH) levels.57 According to this theory, increasing water intake to greater than 3 L/d may result in complete suppression of ADH and cAMP. This is a small study (n = 20),56 since patients with ADPKD are likely to have urinary concentrating defects, and hyponatremia is a concern is in these patients.58
Sirolimus and ADPKD59 is an open-label randomized study to see whether sirolimus (also known as rapamycin) can reduce cyst growth. Originally, it was noted that posttransplant ADPKD patients underwent a regression of both liver and kidney cysts when they were taking sirolimus, and a preliminary crossover study was done.60 However, preliminary results at 18 months showed no difference in renal growth or cyst growth but did show kidney damage as determined by an increase of proteinuria in the treatment group.59 The study is still in progress.
Somatostatin in Polycystic Kidney61 is a long-term (three-year) study following patients who agreed to participate in a randomized, double-blind protocol; in it, an intramuscular injection of either an octreotide (ie, somatastatin) or placebo was administered every four weeks for one year in an effort to reduce the size of kidney and liver cysts.62 At one year, the quality of life in the treatment group was rated better, as measured by pain reduction and improved physical activity. Cyst growth in the treatment group was smaller for both the kidney and liver. However, the GFR decreased to the same degree in both groups.62
CONCLUSION
ADPKD is a common, often overlooked genetic disease that is a cause of hypertension. ADPKD’s presenting symptoms of flank pain, back pain, and/or hematuria often bring the patient to the provider, but a high index of suspicion must be maintained to diagnose these patients at an early age. Due to the variable presentation even within affected families, many patients do not realize that their family carries the PKD gene.
While genetic testing is available, ultrasound is a quick, relatively inexpensive, and easy method to screen for this diagnosis. The progression of ADPKD to ESRD, requiring dialysis or organ transplantation, can be slowed with early and aggressive treatment of hypertension. As with all patients affected by renal impairment, suggestions for patients with ADPKD to avoid use of NSAIDs, contrast dye, and gadolinium-enhanced MRI are appropriate. The primary care PA or NP is in an appropriate position to see to this.
REFERENCES
1. Newschaffer CJ, Falb MD, Gurney JG. National autism prevalence trends from United States special education data. Pediatrics. 2005;115 (3):e277-e282.
2. Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287-1301.
3. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16: European Polycystic Kidney Disease Consortium. Cell. 1994;77(6):881-894.
4. Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996; 272(5266):1339-1342.
5. Chapman AB. Polycystic and other cystic kidney diseases. In: Greenberg A, ed. Primer on Kidney Diseases: Expert Consult. 5th ed. National Kidney Foundation; 2009:345-353.
6. Fischer E, Legue E, Doyen A, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38(1):21-23.
7. Murcia NS, Sweeney WE Jr, Avner ED. New insights into the molecular pathophysiology of polycystic kidney disease. Kidney Int. 1999;55 (4):1187-1197.
8. Paterson AD, Pei Y. Is there a third gene for autosomal dominant polycystic kidney disease? Kidney Int. 1998;54(5):1759-1761.
9. Pei Y. Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2011;118(1):c19-c30.
10. Tan YC, Blumenfeld J, Rennert H. Autosomal dominant polycystic kidney disease: genetics, mutations and microRNAs. Biochim Biophys Acta. 2011 Mar 16 [Epub ahead of print].
11. Avner ED, Sweeney WE Jr, Nelson WJ. Abnormal sodium pump distribution during renal tubulogenesis in congenital murine polycystic kidney disease. Proc Natl Acad Sci U S A. 1992;89(16):7447-7451.
12. Torra R, Badenas C, Darnell A, et al. Linkage, clinical features, and prognosis of autosomal dominant polycystic kidney disease types 1 and 2. J Am Soc Nephrol. 1996;7(10):2142-2151.
13. Davies F, Coles GA, Harper PS, et al. Polycystic kidney disease re-evaluated: a population-based study. Q J Med. 1991;79(290):477-485.
14. Hateboer N, v Dijk MA, Bogdanova N, et al; European PKD1-PKD2 Study Group. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353(9147):103-107.
15. Peters DJ, Breuning MH. Autosomal dominant polycystic kidney disease: modification of disease progression. Lancet. 2001;358(9291):1439-1444.
16. Dicks E, Ravani P, Langman D, et al. Incident renal events and risk factors in autosomal dominant polycystic kidney disease: a population- and family-based cohort followed for 22 years. Clin J Am Soc Nephrol. 2006;1(4):710-717.
17. Fick-Brosnahan GM, Belz MM, McFann KK, etc. Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: a longitudinal study. Am J Kidney Dis. 2002;39(6):1127-1134.
18. Fick-Brosnahan GM, Tran ZV, Johnson AM, et al. Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int. 2001;59(5):1654-1662.
19. Johnson AM, Gabow PA. Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol. 1997;8(10): 1560-1567.
20. Risk D. Autosomal dominant polycystic kidney disease. Presented at: National Kidney Foundation, Spring Clinical Meetings; April 28, 2011; Las Vegas, NV.
21. Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205-212.
22. Watnick T, Germino GG. Molecular basis of autosomal dominant polycystic kidney disease. Semin Nephrol. 1999;19(4):327-343.
23. Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009;5(4):221-228.
24. Patch C, Charlton J, Roderick PJ, Gulliford MC. Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: a population-based study. Am J Kidney Dis. 2011;57(6):856-862.
25. Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17(2):173-180.
26. Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323(16):1091-1096.
27. Jafar TH, Stark PC, Schmid CH, et al. The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int. 2005;67(1):265-271.
28. Schrier RW. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2009;20(9):1888-1893.
29. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH, sponsor. HALT PKD (Halt Progression of Polycystic Kidney Disease): Efficacy of Aggressive Renin-Angiotensin-Aldosterone Axis Blockade in Preventing/Slowing Renal Function Decline in ADPKD. www2.niddk.nih.gov/NR/rdonlyres/175578F6-62B4-429A-9BBF-96CCEC2FFB3A/0/KUHHALT PKDPROTOCOL9107.pdf. Accessed July 22, 2011.
30. Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol. 2010;5(1):102-109.
31. Taylor M, Johnson AM, Tison M, et al. Earlier diagnosis of autosomal dominant polycystic kidney disease: importance of family history and implications for cardiovascular and renal complications. Am J Kidney Dis. 2005;46(3):415-423.
32. Namli S, Oflaz H, Turgut F, et al. Improvement of endothelial dysfunction with simvastatin in patients with autosomal dominant polycystic kidney disease. Ren Fail. 2007;29(1):55-59.
33. Bremmer MS, Jacobs SC. Renal artery embolization for the symptomatic treatment of adult polycystic kidney disease. Nat Clin Pract Nephrol. 2008;4(5):236-237.
34. Chapman AB, Rubinstein D, Hughes R, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Engl J Med. 1992; 327(13):916-920.
35. Schievink WI, Torres VE, Piepgras DG, Wiebers DO. Saccular intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1992;3(1):88-95.
36. Fick GM, Gabow PA. Hereditary and acquired cystic disease of the kidney. Kidney Int. 1994;46(4):951-964.
37. Watson ML. Complications of polycystic kidney disease. Kidney Int. 1997;51(1):353-365.
38. Huston J 3rd, Torres VE, Sulivan PP, et al. Value of magnetic resonance angiography for the detection of intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3(12):1871-1877.
39. Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002;13(7):1918-1927.
40. Meijer E, Rook M, Tent H, et al. Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2010; 5(6):1091-1098.
41. Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76(2):149-168.
42. Tabibi A, Simforoosh N, Abadpour P, et al. Concomitant nephrectomy of massively enlarged kidneys and renal transplantation in autosomal dominant polycystic kidney disease. Transplant Proc. 2005;37(7):2939-2940.
43. Dunn MD, Portis AJ, Elbahnasy AM, et al. Laparoscopic nephrectomy in patients with end-stage renal disease and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35(4):720-725.
44. Sulikowski T, Tejchman K, Zietek Z, et al. Experience with autosomal dominant polycystic kidney disease in patients before and after renal transplantation: a 7-year observation. Transplant Proc. 2009;41(1):177-180.
45. Desai MR, Nandkishore SK, Ganpule A, Thimmegowda M. Pretransplant laparoscopic nephrectomy in adult polycystic kidney disease: a single centre experience. BJU Int. 2008;101 (1):94-97.
46. Glassman DT, Nipkow L, Bartlett ST, Jacobs SC. Bilateral nephrectomy with concomitant renal graft transplantation for autosomal dominant polycystic kidney disease. J Urol. 2000;164 (3 pt 1):661-664.
47. Fuller TF, Brennan TV, Feng S, et al. End stage polycystic kidney disease: indications and timing of native nephrectomy relative to kidney transplantation. J Urol. 2005;174(6):2284-2288.
48. Abbott KC, Agodoa LY. Polycystic kidney disease at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol. 2002;57(3):208-214.
49. Perrone RD, Ruthazer R, Terrin NC. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am J Kidney Dis. 2001;38(4):777-784.
50. Batista PB, Lopes AA, Costa FA. Association between attributed cause of end-stage renal disease and risk of death in Brazilian patients receiving renal replacement therapy. Ren Fail. 2005;27(6):651-656.
51. Pirson Y, Christophe JL, Goffin E. Outcome of renal replacement therapy in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1996;11 suppl 6:24-28.
52. Torres VE, Meijer E, Bae KT, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3-4 Study. Am J Kidney Dis. 2011;57(5):692-699.
53. Calvet JP. Strategies to inhibit cyst formation in ADPKD. Clin J Am Soc Nephrol. 2008;3 (4):1205-1211.
54. Grantham JJ. Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: a systems biology approach. Kidney Int. 2003;64(4):1157-1162.
55. Irazabal MV, Torres VE, Hogan MC, et al. Short-term effects of tolvaptan on renal function and volume in patients with Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2011 May 4 [Epub ahead of print].
56. New York University, sponsor. High Water Intake to Slow Progression of Polycystic Kidney Disease. http://clinicaltrials.gov/ct2/show/NCT00784030. Accessed July 22, 2011.
57. Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol. 2011;6(1):192-197.
58. Grampsas SA, Chandhoke Ps, Fan J, et al. Anatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;36(1):53-57.
59. Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010; 363(9):820-829.
60. Perico N, Antiga L, Caroli A, et al. Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol. 2010;21(6):1031-1040.
61. Mario Negri Institute for Pharmacological Research, sponsor. Somatostatin in Polycystic Kidney: a Long-term Three Year Follow up Study. http://clinicaltrials.gov/ct2/show/NCT00309283. Accessed July 22, 2011.
62. Hogan MC, Masyuk TV, Page LJ, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010; 21(6):1052-1061.
Twice as common as autism and half as well-known,1 autosomal polycystic kidney disease (ADPKD) occurs in one in 400 to one in 1,000 people.2 It is an inherited progressive genetic disorder that causes hypertension and decreased renal function and, over time, can lead to kidney failure. Two polycstin genes that code for ADPKD, PKD1 and PKD2, were identified in 1994 and 1996, respectively.3,4 Awareness and understanding of the genes responsible for ADPKD have increased clinicians’ ability to identify at-risk patients and to slow or alter the course of the disease.
Case Presentation
A 45-year-old black man presents to your office with severe, nonradiating back pain and new-onset hypertension. Regarding the pain, he stated, “I turned around to see who kicked me, but no one was there.” When the pain began, he went to see the nurse at the school where he is employed, and she found that his blood pressure was high at 162/90 mm Hg. Although the patient’s back pain is resolving, he is very concerned about his blood pressure, since he has never had a high reading before.
He is the baseball coach and physical education teacher at the local high school and is in excellent physical condition as a result of his professional interaction with teenagers every day. He does not smoke or use any illicit drugs but does admit to occasional alcohol consumption. His medical history is significant only for occasional broken fingers and twisted ankles, all occurring while he was engaged in sports.
His family history includes one brother without medical problems, a brother and a sister with hypertension, a sister with diabetes and obesity, and a brother with a congenital abnormality that required a living donor kidney transplant at age 17 (the father served as donor). No family-wide workup has ever been done because no one practitioner has ever made a connection among these conditions and considered a diagnosis of ADPKD.
The patient’s blood pressure in the office is 172/92 mm Hg while sitting and 166/88 mm Hg while standing. He is somewhat sore with a localized spasm in the lumbar-sacral area but no radiation of pain. The patient has trouble touching his toes but reports that he can never touch his toes. His straight leg lift is negative. The rest of his physical exam is noncontributory.
What should be the next step in this patient’s workup?
PATHOPHYSIOLOGY
ADPKD is a progressive expansion of numerous fluid-filled cysts that result in massive enlargement of the kidneys.5 Less than 5% of all nephrons become cystic; however, the average volume of a polycystic kidney is 1,000 mL (normal, 300 mL), that is, the volume of a standard-sized pineapple. Even with this significant enlargement, a decline in the glomerular filtration rate (GFR) is not usually seen initially. Each cyst is derived from a single hyperproliferative epithelial cell. Increased cellular proliferation, followed by fluid secretion and alterations in the extracellular matrix, cause an outpouching from the parent nephron, which eventually detaches from the parent nephron and continues to enlarge and autonomously secrete fluid.6,7
PKD1 and PKD2 are two genes responsible for ADPKD that have been isolated so far. Since there are families carrying neither the PKD1 nor the PKD2 gene that still have an inherited type of ADPKD, there is suspicion that at least one more PKD gene, not yet isolated, exists.8 It is also possible that other genetic or environmental factors may be at play.9,10
In 1994, the PKD1 gene was isolated on chromosome 16,3 and it was found to code for polycystin 1. A lack of polycystin 1 causes an abnormality in the Na+/K(+)-ATPase pumps, leading to abnormal sodium reabsorption.11 How and why this happens is not quite clear. However, the hypertension that is a key objective finding in patients with ADPKD is thought to result from this pump abnormality.
PKD2 is found on the long arm of chromosome 4 and codes for polycystin 2.4 Polycystin 2 is an amino acid that is responsible for voltage-activated cellular calcium channels,5 again explaining the hypertension so commonly seen in the course of ADPKD. ADPKD-associated hypertension may be present as early as the teenage years.12
EPIDEMIOLOGY
More than 85% of ADPKD cases are associated with PKD1, and this form is called polycystic kidney disease 1 (PKD 1), the more aggressive form of the disease.13,14 PKD 2 (the form associated with the gene PKD2), though less common, is also likely to progress to end-stage renal disease (ESRD), but at a later age (median age of 74 years, compared with 54 in patients with PKD 1).14 ADPKD accounts for about 5% of cases of ESRD in North America,9 but for most patients, presentation and decreased renal function do not occur until the 40s.15 However, patients with the risk factors listed in Table 15,16-19 are likely to experience a more rapid and aggressive form of the disease.
Even with the same germline mutation in a family with this inherited disease, the severity of ADPKD among family members is quite variable; this is true even in the case of twins.9,10,20 Since the age and symptoms at presentation can vary so greatly, a uniform method of identifying patients with ADPKD, along with staging, was needed. Most patients do not undergo genetic testing (ie, DNA linkage or gene-based direct sequencing9) for a diagnosis of ADPKD or to differentiate between the PKD 1 and PKD 2 disease forms unless they are participating in a research study. Diagnostic criteria were needed that were applicable for any type of ADPKD.
In 2009, the University of Toronto’s Division of Nephrology convened experts in the fields of nephrology and radiology to reach a consensus on standardized ultrasonographic diagnostic criteria.21 They formulated definitions based on a study of 948 individuals who were at risk for either PKD 1 or PKD 2 (see Table 221). The specificity and sensitivity of the resulting criteria range from 82% to 100%, making it possible to standardize the care and classification of renal patients worldwide.
Since family members with the same genotypes can experience very divergent disease manifestations, the two-hit hypothesis has been developed.22 In simple terms, it proposes that after the germline mutation (PKD1 or PKD2), there is a second somatic mutation that leads to progressive cyst formation; when the number and size of cysts increase, the patient starts to experience symptoms of ADPKD.22
Age at presentation can be quite variable, as can the presenting symptoms. Most patients with PKD 1 present in their 50s, with 54 being the average age in US patients.14 The most common presenting symptom is flank or back pain.2,5 The pain is due to the massive enlargement of the kidneys, causing a stretching of the kidney capsule and leading to a chronic, dull and persistent pain in the low back. Severe pain, sharp and cutting, occurs when one of the cysts hemorrhages; to some patients, the pain resembles a quick, powerful “kick in the back.” Hematuria can occur following cyst hemorrhage; depending on the location of the cyst that burst within the kidney (ie, how close it is to the collecting system) and how large it is, the amount and color of the hematuria can be impressive.
ADPKD is more common in men than women, and cyst rupture can be precipitated by trauma or lifting heavy objects. Cyst hemorrhage can turn the urine bright red, which is especially frightening to the male patient. Hematuria is often the key presenting symptom in patients who will be diagnosed with ADPKD-induced hypertension.
Besides hematuria, other common manifestations of ADPKD include:
• Hypertension (60% of affected patients, which increases to 100% by the time ESRD develops)
• Extrarenal cysts (100% of affected patients)
• Urinary tract infections
• Nephrolithiasis (20% of affected patients)
• Proteinuria, occasionally (18% of affected patients).2,5,23
Among these manifestations, those most commonly attributed to a diagnosis of ADPKD are hypertension, kidney stones, and urinary tract or kidney infections. Since isolated proteinuria is unusual in patients with ADPKD, it is recommended that another cause of kidney disease be explored in patients with this presentation.24
Extrarenal manifestations of cyst development are common, eventually occurring in all patients as they age. Hepatic cysts are universal in patients with ADPKD by age 30, although hepatic function is preserved. There may be a mild elevation in the alkaline phosphatase level in patients with ADPKD, resulting from the presence of hepatic cysts. Cysts may also be found in the pancreas, spleen, thyroid, and epididymis.5,25 Some patients may complain of dyspnea, pain, early satiety, or lower extremity edema as a result of enlarged cyst.
The Case Patient
Because you recently attended a lecture about ADPKD, you are aware that flank pain in men with hypertension is indicative of ADPKD until proven otherwise. Believing that this patient’s hypertension is renal in origin, you order an abdominal ultrasound. You also order a comprehensive metabolic panel and a complete blood count. The patient’s GFR is measured at 89 mL/min (indicative of stage 2 kidney disease). Other results are shown in Table 3.
The very broad differential includes essential hypertension, hypertension resulting from intake of “power drinks” or salt in an athlete, illicit use of medications (including steroids), herniated disc leading to transient hypertension, and urinary tract infection or sexually transmitted disease. All of this is moot when the ultrasound shows both kidneys measuring greater than 15 cm, with four distinct cysts on the right kidney and three distinct cysts on the left.
You explain to the patient that ADPKD is a genetic disease and that he and his siblings each had a certain chance of inheriting it. Although different presentations may occur (“congenital” polycystic kidney disease, hypertension, or obesity), they all must undergo ultrasonographic screening for ADPKD. You add that although ADPKD is a genetic disease, it can also be diagnosed in different members of the same family at different ages.
TREATMENT
The goal of treatment for the patient with ADPKD is to slow cyst development and the natural course of the disease. If this can be achieved, the need for dialysis or kidney transplantation may be postponed for a number of years. Because cyst growth causes an elevation in renin and activates the angiotensin II renin system26 (see figure,24), an ACE inhibitor is the most effective treatment to lower blood pressure and thus slow the progression of ADPKD. Most patients with ADPKD are started on an ACE inhibitor at an early age to slow the rate of disease progression.27,28 Several studies are under way to determine the best antihypertensive medication and the optimal age for initiating treatment.29,30
Lipid screening and treatment for dyslipidemia are important23 because ADPKD can lead to a reduction in kidney function, resulting in chronic kidney disease (CKD). CKD is considered a coronary heart disease risk equivalent, and most professionals will treat the patient with ADPKD for hyperlipidemia.23,31 While there are no data showing that statin use will reduce the incidence of ESRD or delay the need for dialysis or kidney transplantation in patients with ADPKD, the beneficial effects of good renal blood flow and endothelial function have been noted.32,33
One of the most common and significant complications in ADPKD is intracranial hemorrhage resulting from a ruptured cerebral aneurysm. In the younger adult, the incidence of cerebral aneurysm is 4%, but incidence increases to 10% in patients older than 65.34-36 Family clusters of aneurysms have been reported.37 If an intracranial aneurysm is found in the family history, the risk of an aneurysm in another family member increases to 22%.38
Since rupture of an intracranial hemorrhage is associated with a 30-day mortality rate of 50% and 80% morbidity,5,38 standard of care for patients with ADPKD includes CT or magnetic resonance angiographic (MRA) screening in the asymptomatic patient with a positive family history.34,38 If an aneurysm is found, the lifetime chance of rupture is 50%, although the risk is greater in the case of an aneurysm larger than 10 mm.5
As in all patients with kidney disease, left ventricular hypertrophy is common among patients with ADPKD.23,28,39
The Case Patient
The patient is started on an ACE inhibitor, scheduled for fasting lipid screening, and referred to a nephrology practice for disease management. As research and investigation of possible treatment options for ADPKD are ongoing, the patient may benefit from participating in a new research protocol.
Because the patient’s family has no history of cerebral aneurysm, CT/MRA screening is not required. A discussion of the pros and cons of genetic testing for the entire family, including nieces and nephews, is initiated. The patient and his family are referred to a genetic counselor to decide whether the benefit of early treatment for hypertension outweighs the risk of carrying a diagnosis of ADPKD for his younger relatives, who may later seek health insurance coverage.
NATURAL PROGRESSION OF ADPKD
Hypertension and cyst formation will continue as the patient ages. The natural progression of ADPKD is to renal failure with renal replacement therapy (dialysis or organ transplantation) as treatment options. If the progression of ADPKD can be slowed through pharmacotherapy, the patient may live for many years without needing dialysis. This ideal can be accomplished only through aggressive hypertension control, which should be started in the teenage years.23,30,31
Suggestions to increase fluid consumption and to limit the use of NSAIDs, contrast dye, and MRI with gadolinium are appropriate. It is rare for hypertension to be diagnosed before some organ damage has already occurred.12 Often the patient’s renal function, as determined by measuring the GFR, remains stable until the patient reaches his or her 40s.40 However, kidney damage often begins before any detectable change in GFR. Once the GFR does start to decline, the average decrease is 4.4 to 5.9 mL/min/1.73m2 each year.41
MANAGEMENT CONSIDERATIONS
For ESRD Organ Transplantation
Kidney transplantation—the only curative treatment for ADPKD—can be offered to patients once the GFR falls below 20 mL/min. However, the patient with ADPKD can experience kidney enlargement to such an extent that introducing a third kidney into the limited abdominal space becomes technically difficult. Although nephrectomy is avoided whenever possible, there are cases in which there is no alternative.42
In addition to space concerns, recurrent urinary tract infections, chronic pain, renal cell carcinoma, chronic hematuria, or chronic cyst infections can necessitate a nephrectomy.43,44 A laparoscopic approach with decompression of cysts or removal of only one kidney is preferred.43,45 If removal of both kidneys is required before a transplant, the patient must be maintained on dialysis until after transplantation. Since the transplant waiting list can exceed seven years in some areas, most patients arrange for a willing live donor before agreeing to a bilateral nephrectomy.46,47
Dialysis
Either peritoneal dialysis (PD) or hemodialysis (HD) can be offered to patients with severe ADPKD. Depending on the size of the native kidneys and the history of previous abdominal surgery, PD often offers a better chance of survival in these patients, particularly compared with patients who have ESRD associated with other causes.48
For management of the patient with ADPKD who receives PD, it can be difficult to differentiate between the pain of a cyst and the pain of a peritoneal infection. Generally, cyst rupture is accompanied by hematuria; and peritonitis, by cloudy fluid.5 Management provided by an experienced nephrologist and PD nurse is vital.
In ADPKD patients who undergo HD, too, survival is better than in patients who have ESRD with other causes49,50; five-year survival can be as high as 10% to 15%.51 This is likely due to the lower incidence of coronary artery disease in the ADPKD population, compared with patients who have ESRD associated with other chronic diseases.49
FUTURE TRENDS AND ONGOING TRIALS
HALT PKD29,30 is an NIH-funded, double-blind study to determine whether adding an angiotensin receptor blocker (ARB) to standard ACE inhibitor therapy results in a more significant decrease in the progression of renal cysts. The rationale for this is that the ARB is expected to block the renin-angiotensin-aldosterone system in the kidney. Use of ACE inhibitor monotherapy versus ARB/ACE inhibitor therapy is being compared in two study arms: patients between ages 15 and 49 with a GFR of 60 mL/min or greater; and patients between ages 18 and 64 with a GFR of 25 to 60 mL/min.29 To date, preliminary results indicate no benefit in adding the second medication.49
The TEMPO Trial52 is a multicenter, double-blind study looking at the effect of tolvaptan on renal cyst growth. Tolvaptan is a potent vasopressin receptor antagonist, and in vitro evidence has shown that intracellular cyclic adenosine monophosphate (cAMP) plays a large role in the development of cysts in patients with ADPKD. If it is possible to block the cAMP that is causing cyst growth, progression of ADPKD should slow.53,54 Only short-term effects of tolvaptan use are currently known.55
High Water Intake to Slow Progression of Polycystic Kidney Disease56 is an open-label, nonrandomized trial in which patients drink a minimum of
3 L of water. Previously, a small study showed that an increase in fluid intake partially suppresses the urine osmolality and the serum antidiuretic hormone (ADH) levels.57 According to this theory, increasing water intake to greater than 3 L/d may result in complete suppression of ADH and cAMP. This is a small study (n = 20),56 since patients with ADPKD are likely to have urinary concentrating defects, and hyponatremia is a concern is in these patients.58
Sirolimus and ADPKD59 is an open-label randomized study to see whether sirolimus (also known as rapamycin) can reduce cyst growth. Originally, it was noted that posttransplant ADPKD patients underwent a regression of both liver and kidney cysts when they were taking sirolimus, and a preliminary crossover study was done.60 However, preliminary results at 18 months showed no difference in renal growth or cyst growth but did show kidney damage as determined by an increase of proteinuria in the treatment group.59 The study is still in progress.
Somatostatin in Polycystic Kidney61 is a long-term (three-year) study following patients who agreed to participate in a randomized, double-blind protocol; in it, an intramuscular injection of either an octreotide (ie, somatastatin) or placebo was administered every four weeks for one year in an effort to reduce the size of kidney and liver cysts.62 At one year, the quality of life in the treatment group was rated better, as measured by pain reduction and improved physical activity. Cyst growth in the treatment group was smaller for both the kidney and liver. However, the GFR decreased to the same degree in both groups.62
CONCLUSION
ADPKD is a common, often overlooked genetic disease that is a cause of hypertension. ADPKD’s presenting symptoms of flank pain, back pain, and/or hematuria often bring the patient to the provider, but a high index of suspicion must be maintained to diagnose these patients at an early age. Due to the variable presentation even within affected families, many patients do not realize that their family carries the PKD gene.
While genetic testing is available, ultrasound is a quick, relatively inexpensive, and easy method to screen for this diagnosis. The progression of ADPKD to ESRD, requiring dialysis or organ transplantation, can be slowed with early and aggressive treatment of hypertension. As with all patients affected by renal impairment, suggestions for patients with ADPKD to avoid use of NSAIDs, contrast dye, and gadolinium-enhanced MRI are appropriate. The primary care PA or NP is in an appropriate position to see to this.
REFERENCES
1. Newschaffer CJ, Falb MD, Gurney JG. National autism prevalence trends from United States special education data. Pediatrics. 2005;115 (3):e277-e282.
2. Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287-1301.
3. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16: European Polycystic Kidney Disease Consortium. Cell. 1994;77(6):881-894.
4. Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996; 272(5266):1339-1342.
5. Chapman AB. Polycystic and other cystic kidney diseases. In: Greenberg A, ed. Primer on Kidney Diseases: Expert Consult. 5th ed. National Kidney Foundation; 2009:345-353.
6. Fischer E, Legue E, Doyen A, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38(1):21-23.
7. Murcia NS, Sweeney WE Jr, Avner ED. New insights into the molecular pathophysiology of polycystic kidney disease. Kidney Int. 1999;55 (4):1187-1197.
8. Paterson AD, Pei Y. Is there a third gene for autosomal dominant polycystic kidney disease? Kidney Int. 1998;54(5):1759-1761.
9. Pei Y. Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2011;118(1):c19-c30.
10. Tan YC, Blumenfeld J, Rennert H. Autosomal dominant polycystic kidney disease: genetics, mutations and microRNAs. Biochim Biophys Acta. 2011 Mar 16 [Epub ahead of print].
11. Avner ED, Sweeney WE Jr, Nelson WJ. Abnormal sodium pump distribution during renal tubulogenesis in congenital murine polycystic kidney disease. Proc Natl Acad Sci U S A. 1992;89(16):7447-7451.
12. Torra R, Badenas C, Darnell A, et al. Linkage, clinical features, and prognosis of autosomal dominant polycystic kidney disease types 1 and 2. J Am Soc Nephrol. 1996;7(10):2142-2151.
13. Davies F, Coles GA, Harper PS, et al. Polycystic kidney disease re-evaluated: a population-based study. Q J Med. 1991;79(290):477-485.
14. Hateboer N, v Dijk MA, Bogdanova N, et al; European PKD1-PKD2 Study Group. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353(9147):103-107.
15. Peters DJ, Breuning MH. Autosomal dominant polycystic kidney disease: modification of disease progression. Lancet. 2001;358(9291):1439-1444.
16. Dicks E, Ravani P, Langman D, et al. Incident renal events and risk factors in autosomal dominant polycystic kidney disease: a population- and family-based cohort followed for 22 years. Clin J Am Soc Nephrol. 2006;1(4):710-717.
17. Fick-Brosnahan GM, Belz MM, McFann KK, etc. Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: a longitudinal study. Am J Kidney Dis. 2002;39(6):1127-1134.
18. Fick-Brosnahan GM, Tran ZV, Johnson AM, et al. Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int. 2001;59(5):1654-1662.
19. Johnson AM, Gabow PA. Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol. 1997;8(10): 1560-1567.
20. Risk D. Autosomal dominant polycystic kidney disease. Presented at: National Kidney Foundation, Spring Clinical Meetings; April 28, 2011; Las Vegas, NV.
21. Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205-212.
22. Watnick T, Germino GG. Molecular basis of autosomal dominant polycystic kidney disease. Semin Nephrol. 1999;19(4):327-343.
23. Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009;5(4):221-228.
24. Patch C, Charlton J, Roderick PJ, Gulliford MC. Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: a population-based study. Am J Kidney Dis. 2011;57(6):856-862.
25. Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17(2):173-180.
26. Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323(16):1091-1096.
27. Jafar TH, Stark PC, Schmid CH, et al. The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int. 2005;67(1):265-271.
28. Schrier RW. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2009;20(9):1888-1893.
29. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH, sponsor. HALT PKD (Halt Progression of Polycystic Kidney Disease): Efficacy of Aggressive Renin-Angiotensin-Aldosterone Axis Blockade in Preventing/Slowing Renal Function Decline in ADPKD. www2.niddk.nih.gov/NR/rdonlyres/175578F6-62B4-429A-9BBF-96CCEC2FFB3A/0/KUHHALT PKDPROTOCOL9107.pdf. Accessed July 22, 2011.
30. Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol. 2010;5(1):102-109.
31. Taylor M, Johnson AM, Tison M, et al. Earlier diagnosis of autosomal dominant polycystic kidney disease: importance of family history and implications for cardiovascular and renal complications. Am J Kidney Dis. 2005;46(3):415-423.
32. Namli S, Oflaz H, Turgut F, et al. Improvement of endothelial dysfunction with simvastatin in patients with autosomal dominant polycystic kidney disease. Ren Fail. 2007;29(1):55-59.
33. Bremmer MS, Jacobs SC. Renal artery embolization for the symptomatic treatment of adult polycystic kidney disease. Nat Clin Pract Nephrol. 2008;4(5):236-237.
34. Chapman AB, Rubinstein D, Hughes R, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Engl J Med. 1992; 327(13):916-920.
35. Schievink WI, Torres VE, Piepgras DG, Wiebers DO. Saccular intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1992;3(1):88-95.
36. Fick GM, Gabow PA. Hereditary and acquired cystic disease of the kidney. Kidney Int. 1994;46(4):951-964.
37. Watson ML. Complications of polycystic kidney disease. Kidney Int. 1997;51(1):353-365.
38. Huston J 3rd, Torres VE, Sulivan PP, et al. Value of magnetic resonance angiography for the detection of intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3(12):1871-1877.
39. Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002;13(7):1918-1927.
40. Meijer E, Rook M, Tent H, et al. Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2010; 5(6):1091-1098.
41. Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76(2):149-168.
42. Tabibi A, Simforoosh N, Abadpour P, et al. Concomitant nephrectomy of massively enlarged kidneys and renal transplantation in autosomal dominant polycystic kidney disease. Transplant Proc. 2005;37(7):2939-2940.
43. Dunn MD, Portis AJ, Elbahnasy AM, et al. Laparoscopic nephrectomy in patients with end-stage renal disease and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35(4):720-725.
44. Sulikowski T, Tejchman K, Zietek Z, et al. Experience with autosomal dominant polycystic kidney disease in patients before and after renal transplantation: a 7-year observation. Transplant Proc. 2009;41(1):177-180.
45. Desai MR, Nandkishore SK, Ganpule A, Thimmegowda M. Pretransplant laparoscopic nephrectomy in adult polycystic kidney disease: a single centre experience. BJU Int. 2008;101 (1):94-97.
46. Glassman DT, Nipkow L, Bartlett ST, Jacobs SC. Bilateral nephrectomy with concomitant renal graft transplantation for autosomal dominant polycystic kidney disease. J Urol. 2000;164 (3 pt 1):661-664.
47. Fuller TF, Brennan TV, Feng S, et al. End stage polycystic kidney disease: indications and timing of native nephrectomy relative to kidney transplantation. J Urol. 2005;174(6):2284-2288.
48. Abbott KC, Agodoa LY. Polycystic kidney disease at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol. 2002;57(3):208-214.
49. Perrone RD, Ruthazer R, Terrin NC. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am J Kidney Dis. 2001;38(4):777-784.
50. Batista PB, Lopes AA, Costa FA. Association between attributed cause of end-stage renal disease and risk of death in Brazilian patients receiving renal replacement therapy. Ren Fail. 2005;27(6):651-656.
51. Pirson Y, Christophe JL, Goffin E. Outcome of renal replacement therapy in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1996;11 suppl 6:24-28.
52. Torres VE, Meijer E, Bae KT, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3-4 Study. Am J Kidney Dis. 2011;57(5):692-699.
53. Calvet JP. Strategies to inhibit cyst formation in ADPKD. Clin J Am Soc Nephrol. 2008;3 (4):1205-1211.
54. Grantham JJ. Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: a systems biology approach. Kidney Int. 2003;64(4):1157-1162.
55. Irazabal MV, Torres VE, Hogan MC, et al. Short-term effects of tolvaptan on renal function and volume in patients with Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2011 May 4 [Epub ahead of print].
56. New York University, sponsor. High Water Intake to Slow Progression of Polycystic Kidney Disease. http://clinicaltrials.gov/ct2/show/NCT00784030. Accessed July 22, 2011.
57. Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol. 2011;6(1):192-197.
58. Grampsas SA, Chandhoke Ps, Fan J, et al. Anatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;36(1):53-57.
59. Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010; 363(9):820-829.
60. Perico N, Antiga L, Caroli A, et al. Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol. 2010;21(6):1031-1040.
61. Mario Negri Institute for Pharmacological Research, sponsor. Somatostatin in Polycystic Kidney: a Long-term Three Year Follow up Study. http://clinicaltrials.gov/ct2/show/NCT00309283. Accessed July 22, 2011.
62. Hogan MC, Masyuk TV, Page LJ, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010; 21(6):1052-1061.
Twice as common as autism and half as well-known,1 autosomal polycystic kidney disease (ADPKD) occurs in one in 400 to one in 1,000 people.2 It is an inherited progressive genetic disorder that causes hypertension and decreased renal function and, over time, can lead to kidney failure. Two polycstin genes that code for ADPKD, PKD1 and PKD2, were identified in 1994 and 1996, respectively.3,4 Awareness and understanding of the genes responsible for ADPKD have increased clinicians’ ability to identify at-risk patients and to slow or alter the course of the disease.
Case Presentation
A 45-year-old black man presents to your office with severe, nonradiating back pain and new-onset hypertension. Regarding the pain, he stated, “I turned around to see who kicked me, but no one was there.” When the pain began, he went to see the nurse at the school where he is employed, and she found that his blood pressure was high at 162/90 mm Hg. Although the patient’s back pain is resolving, he is very concerned about his blood pressure, since he has never had a high reading before.
He is the baseball coach and physical education teacher at the local high school and is in excellent physical condition as a result of his professional interaction with teenagers every day. He does not smoke or use any illicit drugs but does admit to occasional alcohol consumption. His medical history is significant only for occasional broken fingers and twisted ankles, all occurring while he was engaged in sports.
His family history includes one brother without medical problems, a brother and a sister with hypertension, a sister with diabetes and obesity, and a brother with a congenital abnormality that required a living donor kidney transplant at age 17 (the father served as donor). No family-wide workup has ever been done because no one practitioner has ever made a connection among these conditions and considered a diagnosis of ADPKD.
The patient’s blood pressure in the office is 172/92 mm Hg while sitting and 166/88 mm Hg while standing. He is somewhat sore with a localized spasm in the lumbar-sacral area but no radiation of pain. The patient has trouble touching his toes but reports that he can never touch his toes. His straight leg lift is negative. The rest of his physical exam is noncontributory.
What should be the next step in this patient’s workup?
PATHOPHYSIOLOGY
ADPKD is a progressive expansion of numerous fluid-filled cysts that result in massive enlargement of the kidneys.5 Less than 5% of all nephrons become cystic; however, the average volume of a polycystic kidney is 1,000 mL (normal, 300 mL), that is, the volume of a standard-sized pineapple. Even with this significant enlargement, a decline in the glomerular filtration rate (GFR) is not usually seen initially. Each cyst is derived from a single hyperproliferative epithelial cell. Increased cellular proliferation, followed by fluid secretion and alterations in the extracellular matrix, cause an outpouching from the parent nephron, which eventually detaches from the parent nephron and continues to enlarge and autonomously secrete fluid.6,7
PKD1 and PKD2 are two genes responsible for ADPKD that have been isolated so far. Since there are families carrying neither the PKD1 nor the PKD2 gene that still have an inherited type of ADPKD, there is suspicion that at least one more PKD gene, not yet isolated, exists.8 It is also possible that other genetic or environmental factors may be at play.9,10
In 1994, the PKD1 gene was isolated on chromosome 16,3 and it was found to code for polycystin 1. A lack of polycystin 1 causes an abnormality in the Na+/K(+)-ATPase pumps, leading to abnormal sodium reabsorption.11 How and why this happens is not quite clear. However, the hypertension that is a key objective finding in patients with ADPKD is thought to result from this pump abnormality.
PKD2 is found on the long arm of chromosome 4 and codes for polycystin 2.4 Polycystin 2 is an amino acid that is responsible for voltage-activated cellular calcium channels,5 again explaining the hypertension so commonly seen in the course of ADPKD. ADPKD-associated hypertension may be present as early as the teenage years.12
EPIDEMIOLOGY
More than 85% of ADPKD cases are associated with PKD1, and this form is called polycystic kidney disease 1 (PKD 1), the more aggressive form of the disease.13,14 PKD 2 (the form associated with the gene PKD2), though less common, is also likely to progress to end-stage renal disease (ESRD), but at a later age (median age of 74 years, compared with 54 in patients with PKD 1).14 ADPKD accounts for about 5% of cases of ESRD in North America,9 but for most patients, presentation and decreased renal function do not occur until the 40s.15 However, patients with the risk factors listed in Table 15,16-19 are likely to experience a more rapid and aggressive form of the disease.
Even with the same germline mutation in a family with this inherited disease, the severity of ADPKD among family members is quite variable; this is true even in the case of twins.9,10,20 Since the age and symptoms at presentation can vary so greatly, a uniform method of identifying patients with ADPKD, along with staging, was needed. Most patients do not undergo genetic testing (ie, DNA linkage or gene-based direct sequencing9) for a diagnosis of ADPKD or to differentiate between the PKD 1 and PKD 2 disease forms unless they are participating in a research study. Diagnostic criteria were needed that were applicable for any type of ADPKD.
In 2009, the University of Toronto’s Division of Nephrology convened experts in the fields of nephrology and radiology to reach a consensus on standardized ultrasonographic diagnostic criteria.21 They formulated definitions based on a study of 948 individuals who were at risk for either PKD 1 or PKD 2 (see Table 221). The specificity and sensitivity of the resulting criteria range from 82% to 100%, making it possible to standardize the care and classification of renal patients worldwide.
Since family members with the same genotypes can experience very divergent disease manifestations, the two-hit hypothesis has been developed.22 In simple terms, it proposes that after the germline mutation (PKD1 or PKD2), there is a second somatic mutation that leads to progressive cyst formation; when the number and size of cysts increase, the patient starts to experience symptoms of ADPKD.22
Age at presentation can be quite variable, as can the presenting symptoms. Most patients with PKD 1 present in their 50s, with 54 being the average age in US patients.14 The most common presenting symptom is flank or back pain.2,5 The pain is due to the massive enlargement of the kidneys, causing a stretching of the kidney capsule and leading to a chronic, dull and persistent pain in the low back. Severe pain, sharp and cutting, occurs when one of the cysts hemorrhages; to some patients, the pain resembles a quick, powerful “kick in the back.” Hematuria can occur following cyst hemorrhage; depending on the location of the cyst that burst within the kidney (ie, how close it is to the collecting system) and how large it is, the amount and color of the hematuria can be impressive.
ADPKD is more common in men than women, and cyst rupture can be precipitated by trauma or lifting heavy objects. Cyst hemorrhage can turn the urine bright red, which is especially frightening to the male patient. Hematuria is often the key presenting symptom in patients who will be diagnosed with ADPKD-induced hypertension.
Besides hematuria, other common manifestations of ADPKD include:
• Hypertension (60% of affected patients, which increases to 100% by the time ESRD develops)
• Extrarenal cysts (100% of affected patients)
• Urinary tract infections
• Nephrolithiasis (20% of affected patients)
• Proteinuria, occasionally (18% of affected patients).2,5,23
Among these manifestations, those most commonly attributed to a diagnosis of ADPKD are hypertension, kidney stones, and urinary tract or kidney infections. Since isolated proteinuria is unusual in patients with ADPKD, it is recommended that another cause of kidney disease be explored in patients with this presentation.24
Extrarenal manifestations of cyst development are common, eventually occurring in all patients as they age. Hepatic cysts are universal in patients with ADPKD by age 30, although hepatic function is preserved. There may be a mild elevation in the alkaline phosphatase level in patients with ADPKD, resulting from the presence of hepatic cysts. Cysts may also be found in the pancreas, spleen, thyroid, and epididymis.5,25 Some patients may complain of dyspnea, pain, early satiety, or lower extremity edema as a result of enlarged cyst.
The Case Patient
Because you recently attended a lecture about ADPKD, you are aware that flank pain in men with hypertension is indicative of ADPKD until proven otherwise. Believing that this patient’s hypertension is renal in origin, you order an abdominal ultrasound. You also order a comprehensive metabolic panel and a complete blood count. The patient’s GFR is measured at 89 mL/min (indicative of stage 2 kidney disease). Other results are shown in Table 3.
The very broad differential includes essential hypertension, hypertension resulting from intake of “power drinks” or salt in an athlete, illicit use of medications (including steroids), herniated disc leading to transient hypertension, and urinary tract infection or sexually transmitted disease. All of this is moot when the ultrasound shows both kidneys measuring greater than 15 cm, with four distinct cysts on the right kidney and three distinct cysts on the left.
You explain to the patient that ADPKD is a genetic disease and that he and his siblings each had a certain chance of inheriting it. Although different presentations may occur (“congenital” polycystic kidney disease, hypertension, or obesity), they all must undergo ultrasonographic screening for ADPKD. You add that although ADPKD is a genetic disease, it can also be diagnosed in different members of the same family at different ages.
TREATMENT
The goal of treatment for the patient with ADPKD is to slow cyst development and the natural course of the disease. If this can be achieved, the need for dialysis or kidney transplantation may be postponed for a number of years. Because cyst growth causes an elevation in renin and activates the angiotensin II renin system26 (see figure,24), an ACE inhibitor is the most effective treatment to lower blood pressure and thus slow the progression of ADPKD. Most patients with ADPKD are started on an ACE inhibitor at an early age to slow the rate of disease progression.27,28 Several studies are under way to determine the best antihypertensive medication and the optimal age for initiating treatment.29,30
Lipid screening and treatment for dyslipidemia are important23 because ADPKD can lead to a reduction in kidney function, resulting in chronic kidney disease (CKD). CKD is considered a coronary heart disease risk equivalent, and most professionals will treat the patient with ADPKD for hyperlipidemia.23,31 While there are no data showing that statin use will reduce the incidence of ESRD or delay the need for dialysis or kidney transplantation in patients with ADPKD, the beneficial effects of good renal blood flow and endothelial function have been noted.32,33
One of the most common and significant complications in ADPKD is intracranial hemorrhage resulting from a ruptured cerebral aneurysm. In the younger adult, the incidence of cerebral aneurysm is 4%, but incidence increases to 10% in patients older than 65.34-36 Family clusters of aneurysms have been reported.37 If an intracranial aneurysm is found in the family history, the risk of an aneurysm in another family member increases to 22%.38
Since rupture of an intracranial hemorrhage is associated with a 30-day mortality rate of 50% and 80% morbidity,5,38 standard of care for patients with ADPKD includes CT or magnetic resonance angiographic (MRA) screening in the asymptomatic patient with a positive family history.34,38 If an aneurysm is found, the lifetime chance of rupture is 50%, although the risk is greater in the case of an aneurysm larger than 10 mm.5
As in all patients with kidney disease, left ventricular hypertrophy is common among patients with ADPKD.23,28,39
The Case Patient
The patient is started on an ACE inhibitor, scheduled for fasting lipid screening, and referred to a nephrology practice for disease management. As research and investigation of possible treatment options for ADPKD are ongoing, the patient may benefit from participating in a new research protocol.
Because the patient’s family has no history of cerebral aneurysm, CT/MRA screening is not required. A discussion of the pros and cons of genetic testing for the entire family, including nieces and nephews, is initiated. The patient and his family are referred to a genetic counselor to decide whether the benefit of early treatment for hypertension outweighs the risk of carrying a diagnosis of ADPKD for his younger relatives, who may later seek health insurance coverage.
NATURAL PROGRESSION OF ADPKD
Hypertension and cyst formation will continue as the patient ages. The natural progression of ADPKD is to renal failure with renal replacement therapy (dialysis or organ transplantation) as treatment options. If the progression of ADPKD can be slowed through pharmacotherapy, the patient may live for many years without needing dialysis. This ideal can be accomplished only through aggressive hypertension control, which should be started in the teenage years.23,30,31
Suggestions to increase fluid consumption and to limit the use of NSAIDs, contrast dye, and MRI with gadolinium are appropriate. It is rare for hypertension to be diagnosed before some organ damage has already occurred.12 Often the patient’s renal function, as determined by measuring the GFR, remains stable until the patient reaches his or her 40s.40 However, kidney damage often begins before any detectable change in GFR. Once the GFR does start to decline, the average decrease is 4.4 to 5.9 mL/min/1.73m2 each year.41
MANAGEMENT CONSIDERATIONS
For ESRD Organ Transplantation
Kidney transplantation—the only curative treatment for ADPKD—can be offered to patients once the GFR falls below 20 mL/min. However, the patient with ADPKD can experience kidney enlargement to such an extent that introducing a third kidney into the limited abdominal space becomes technically difficult. Although nephrectomy is avoided whenever possible, there are cases in which there is no alternative.42
In addition to space concerns, recurrent urinary tract infections, chronic pain, renal cell carcinoma, chronic hematuria, or chronic cyst infections can necessitate a nephrectomy.43,44 A laparoscopic approach with decompression of cysts or removal of only one kidney is preferred.43,45 If removal of both kidneys is required before a transplant, the patient must be maintained on dialysis until after transplantation. Since the transplant waiting list can exceed seven years in some areas, most patients arrange for a willing live donor before agreeing to a bilateral nephrectomy.46,47
Dialysis
Either peritoneal dialysis (PD) or hemodialysis (HD) can be offered to patients with severe ADPKD. Depending on the size of the native kidneys and the history of previous abdominal surgery, PD often offers a better chance of survival in these patients, particularly compared with patients who have ESRD associated with other causes.48
For management of the patient with ADPKD who receives PD, it can be difficult to differentiate between the pain of a cyst and the pain of a peritoneal infection. Generally, cyst rupture is accompanied by hematuria; and peritonitis, by cloudy fluid.5 Management provided by an experienced nephrologist and PD nurse is vital.
In ADPKD patients who undergo HD, too, survival is better than in patients who have ESRD with other causes49,50; five-year survival can be as high as 10% to 15%.51 This is likely due to the lower incidence of coronary artery disease in the ADPKD population, compared with patients who have ESRD associated with other chronic diseases.49
FUTURE TRENDS AND ONGOING TRIALS
HALT PKD29,30 is an NIH-funded, double-blind study to determine whether adding an angiotensin receptor blocker (ARB) to standard ACE inhibitor therapy results in a more significant decrease in the progression of renal cysts. The rationale for this is that the ARB is expected to block the renin-angiotensin-aldosterone system in the kidney. Use of ACE inhibitor monotherapy versus ARB/ACE inhibitor therapy is being compared in two study arms: patients between ages 15 and 49 with a GFR of 60 mL/min or greater; and patients between ages 18 and 64 with a GFR of 25 to 60 mL/min.29 To date, preliminary results indicate no benefit in adding the second medication.49
The TEMPO Trial52 is a multicenter, double-blind study looking at the effect of tolvaptan on renal cyst growth. Tolvaptan is a potent vasopressin receptor antagonist, and in vitro evidence has shown that intracellular cyclic adenosine monophosphate (cAMP) plays a large role in the development of cysts in patients with ADPKD. If it is possible to block the cAMP that is causing cyst growth, progression of ADPKD should slow.53,54 Only short-term effects of tolvaptan use are currently known.55
High Water Intake to Slow Progression of Polycystic Kidney Disease56 is an open-label, nonrandomized trial in which patients drink a minimum of
3 L of water. Previously, a small study showed that an increase in fluid intake partially suppresses the urine osmolality and the serum antidiuretic hormone (ADH) levels.57 According to this theory, increasing water intake to greater than 3 L/d may result in complete suppression of ADH and cAMP. This is a small study (n = 20),56 since patients with ADPKD are likely to have urinary concentrating defects, and hyponatremia is a concern is in these patients.58
Sirolimus and ADPKD59 is an open-label randomized study to see whether sirolimus (also known as rapamycin) can reduce cyst growth. Originally, it was noted that posttransplant ADPKD patients underwent a regression of both liver and kidney cysts when they were taking sirolimus, and a preliminary crossover study was done.60 However, preliminary results at 18 months showed no difference in renal growth or cyst growth but did show kidney damage as determined by an increase of proteinuria in the treatment group.59 The study is still in progress.
Somatostatin in Polycystic Kidney61 is a long-term (three-year) study following patients who agreed to participate in a randomized, double-blind protocol; in it, an intramuscular injection of either an octreotide (ie, somatastatin) or placebo was administered every four weeks for one year in an effort to reduce the size of kidney and liver cysts.62 At one year, the quality of life in the treatment group was rated better, as measured by pain reduction and improved physical activity. Cyst growth in the treatment group was smaller for both the kidney and liver. However, the GFR decreased to the same degree in both groups.62
CONCLUSION
ADPKD is a common, often overlooked genetic disease that is a cause of hypertension. ADPKD’s presenting symptoms of flank pain, back pain, and/or hematuria often bring the patient to the provider, but a high index of suspicion must be maintained to diagnose these patients at an early age. Due to the variable presentation even within affected families, many patients do not realize that their family carries the PKD gene.
While genetic testing is available, ultrasound is a quick, relatively inexpensive, and easy method to screen for this diagnosis. The progression of ADPKD to ESRD, requiring dialysis or organ transplantation, can be slowed with early and aggressive treatment of hypertension. As with all patients affected by renal impairment, suggestions for patients with ADPKD to avoid use of NSAIDs, contrast dye, and gadolinium-enhanced MRI are appropriate. The primary care PA or NP is in an appropriate position to see to this.
REFERENCES
1. Newschaffer CJ, Falb MD, Gurney JG. National autism prevalence trends from United States special education data. Pediatrics. 2005;115 (3):e277-e282.
2. Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287-1301.
3. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16: European Polycystic Kidney Disease Consortium. Cell. 1994;77(6):881-894.
4. Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996; 272(5266):1339-1342.
5. Chapman AB. Polycystic and other cystic kidney diseases. In: Greenberg A, ed. Primer on Kidney Diseases: Expert Consult. 5th ed. National Kidney Foundation; 2009:345-353.
6. Fischer E, Legue E, Doyen A, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38(1):21-23.
7. Murcia NS, Sweeney WE Jr, Avner ED. New insights into the molecular pathophysiology of polycystic kidney disease. Kidney Int. 1999;55 (4):1187-1197.
8. Paterson AD, Pei Y. Is there a third gene for autosomal dominant polycystic kidney disease? Kidney Int. 1998;54(5):1759-1761.
9. Pei Y. Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2011;118(1):c19-c30.
10. Tan YC, Blumenfeld J, Rennert H. Autosomal dominant polycystic kidney disease: genetics, mutations and microRNAs. Biochim Biophys Acta. 2011 Mar 16 [Epub ahead of print].
11. Avner ED, Sweeney WE Jr, Nelson WJ. Abnormal sodium pump distribution during renal tubulogenesis in congenital murine polycystic kidney disease. Proc Natl Acad Sci U S A. 1992;89(16):7447-7451.
12. Torra R, Badenas C, Darnell A, et al. Linkage, clinical features, and prognosis of autosomal dominant polycystic kidney disease types 1 and 2. J Am Soc Nephrol. 1996;7(10):2142-2151.
13. Davies F, Coles GA, Harper PS, et al. Polycystic kidney disease re-evaluated: a population-based study. Q J Med. 1991;79(290):477-485.
14. Hateboer N, v Dijk MA, Bogdanova N, et al; European PKD1-PKD2 Study Group. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353(9147):103-107.
15. Peters DJ, Breuning MH. Autosomal dominant polycystic kidney disease: modification of disease progression. Lancet. 2001;358(9291):1439-1444.
16. Dicks E, Ravani P, Langman D, et al. Incident renal events and risk factors in autosomal dominant polycystic kidney disease: a population- and family-based cohort followed for 22 years. Clin J Am Soc Nephrol. 2006;1(4):710-717.
17. Fick-Brosnahan GM, Belz MM, McFann KK, etc. Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: a longitudinal study. Am J Kidney Dis. 2002;39(6):1127-1134.
18. Fick-Brosnahan GM, Tran ZV, Johnson AM, et al. Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int. 2001;59(5):1654-1662.
19. Johnson AM, Gabow PA. Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol. 1997;8(10): 1560-1567.
20. Risk D. Autosomal dominant polycystic kidney disease. Presented at: National Kidney Foundation, Spring Clinical Meetings; April 28, 2011; Las Vegas, NV.
21. Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205-212.
22. Watnick T, Germino GG. Molecular basis of autosomal dominant polycystic kidney disease. Semin Nephrol. 1999;19(4):327-343.
23. Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009;5(4):221-228.
24. Patch C, Charlton J, Roderick PJ, Gulliford MC. Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: a population-based study. Am J Kidney Dis. 2011;57(6):856-862.
25. Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17(2):173-180.
26. Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323(16):1091-1096.
27. Jafar TH, Stark PC, Schmid CH, et al. The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int. 2005;67(1):265-271.
28. Schrier RW. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2009;20(9):1888-1893.
29. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH, sponsor. HALT PKD (Halt Progression of Polycystic Kidney Disease): Efficacy of Aggressive Renin-Angiotensin-Aldosterone Axis Blockade in Preventing/Slowing Renal Function Decline in ADPKD. www2.niddk.nih.gov/NR/rdonlyres/175578F6-62B4-429A-9BBF-96CCEC2FFB3A/0/KUHHALT PKDPROTOCOL9107.pdf. Accessed July 22, 2011.
30. Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol. 2010;5(1):102-109.
31. Taylor M, Johnson AM, Tison M, et al. Earlier diagnosis of autosomal dominant polycystic kidney disease: importance of family history and implications for cardiovascular and renal complications. Am J Kidney Dis. 2005;46(3):415-423.
32. Namli S, Oflaz H, Turgut F, et al. Improvement of endothelial dysfunction with simvastatin in patients with autosomal dominant polycystic kidney disease. Ren Fail. 2007;29(1):55-59.
33. Bremmer MS, Jacobs SC. Renal artery embolization for the symptomatic treatment of adult polycystic kidney disease. Nat Clin Pract Nephrol. 2008;4(5):236-237.
34. Chapman AB, Rubinstein D, Hughes R, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Engl J Med. 1992; 327(13):916-920.
35. Schievink WI, Torres VE, Piepgras DG, Wiebers DO. Saccular intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1992;3(1):88-95.
36. Fick GM, Gabow PA. Hereditary and acquired cystic disease of the kidney. Kidney Int. 1994;46(4):951-964.
37. Watson ML. Complications of polycystic kidney disease. Kidney Int. 1997;51(1):353-365.
38. Huston J 3rd, Torres VE, Sulivan PP, et al. Value of magnetic resonance angiography for the detection of intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3(12):1871-1877.
39. Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002;13(7):1918-1927.
40. Meijer E, Rook M, Tent H, et al. Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2010; 5(6):1091-1098.
41. Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76(2):149-168.
42. Tabibi A, Simforoosh N, Abadpour P, et al. Concomitant nephrectomy of massively enlarged kidneys and renal transplantation in autosomal dominant polycystic kidney disease. Transplant Proc. 2005;37(7):2939-2940.
43. Dunn MD, Portis AJ, Elbahnasy AM, et al. Laparoscopic nephrectomy in patients with end-stage renal disease and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35(4):720-725.
44. Sulikowski T, Tejchman K, Zietek Z, et al. Experience with autosomal dominant polycystic kidney disease in patients before and after renal transplantation: a 7-year observation. Transplant Proc. 2009;41(1):177-180.
45. Desai MR, Nandkishore SK, Ganpule A, Thimmegowda M. Pretransplant laparoscopic nephrectomy in adult polycystic kidney disease: a single centre experience. BJU Int. 2008;101 (1):94-97.
46. Glassman DT, Nipkow L, Bartlett ST, Jacobs SC. Bilateral nephrectomy with concomitant renal graft transplantation for autosomal dominant polycystic kidney disease. J Urol. 2000;164 (3 pt 1):661-664.
47. Fuller TF, Brennan TV, Feng S, et al. End stage polycystic kidney disease: indications and timing of native nephrectomy relative to kidney transplantation. J Urol. 2005;174(6):2284-2288.
48. Abbott KC, Agodoa LY. Polycystic kidney disease at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol. 2002;57(3):208-214.
49. Perrone RD, Ruthazer R, Terrin NC. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am J Kidney Dis. 2001;38(4):777-784.
50. Batista PB, Lopes AA, Costa FA. Association between attributed cause of end-stage renal disease and risk of death in Brazilian patients receiving renal replacement therapy. Ren Fail. 2005;27(6):651-656.
51. Pirson Y, Christophe JL, Goffin E. Outcome of renal replacement therapy in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1996;11 suppl 6:24-28.
52. Torres VE, Meijer E, Bae KT, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3-4 Study. Am J Kidney Dis. 2011;57(5):692-699.
53. Calvet JP. Strategies to inhibit cyst formation in ADPKD. Clin J Am Soc Nephrol. 2008;3 (4):1205-1211.
54. Grantham JJ. Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: a systems biology approach. Kidney Int. 2003;64(4):1157-1162.
55. Irazabal MV, Torres VE, Hogan MC, et al. Short-term effects of tolvaptan on renal function and volume in patients with Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2011 May 4 [Epub ahead of print].
56. New York University, sponsor. High Water Intake to Slow Progression of Polycystic Kidney Disease. http://clinicaltrials.gov/ct2/show/NCT00784030. Accessed July 22, 2011.
57. Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol. 2011;6(1):192-197.
58. Grampsas SA, Chandhoke Ps, Fan J, et al. Anatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;36(1):53-57.
59. Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010; 363(9):820-829.
60. Perico N, Antiga L, Caroli A, et al. Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol. 2010;21(6):1031-1040.
61. Mario Negri Institute for Pharmacological Research, sponsor. Somatostatin in Polycystic Kidney: a Long-term Three Year Follow up Study. http://clinicaltrials.gov/ct2/show/NCT00309283. Accessed July 22, 2011.
62. Hogan MC, Masyuk TV, Page LJ, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010; 21(6):1052-1061.
Small renal masses: Toward more rational treatment
Opinion about treatment of mall renal masses has changed considerably in the past 2 decades.
Traditionally, the most common treatment was surgical removal of the whole kidney, ie, radical nephrectomy. However, recent studies have shown that many patients who undergo radical nephrectomy develop chronic kidney disease. Furthermore, radical nephrectomy often constitutes over-treatment, as many of these lesions are benign or, if malignant, would follow an indolent course if left alone.
Now that we better understand the biology of small renal masses and are more aware of the morbidity and mortality related to chronic kidney disease, we try to avoid radical nephrectomy whenever possible, favoring nephron-sparing approaches instead.
In this article, we review the current clinical management of small renal masses.
SMALL RENAL MASSES ARE A HETEROGENEOUS GROUP
Small renal masses are defined as solid renal tumors that enhance on computed tomography (CT) and magnetic resonance imaging (MRI) and are suspected of being renal cell carcinomas. They are generally low-stage and relatively small (< 4 cm in diameter) at presentation. Most are now discovered incidentally on CT or MRI done for various abdominal symptoms. From 20,000 to 30,000 new cases are diagnosed each year in the United States, and the rate is increasing by 3% to 4% per year as the use of CT and MRI increases.1,2
With more small renal masses being detected incidentally, renal cell carcinoma has been going through a stage and size migration—ie, more of these tumors are being discovered in clinical stage T1 (ie, confined to the kidney and measuring less than 7 cm) than in the past. Currently, clinical T1 renal tumors account for 48% to 66% of cases.3
This indicates that the disease is being detected and treated earlier in its course than in the past. However, cancer-specific deaths from renal cell carcinoma have not declined, suggesting that for many of these patients, our traditional practice of aggressive surgical management with radical nephrectomy may not be warranted.4
Small renal masses vary in biologic aggressiveness
Recent large surgical series indicate that up to 20% of small renal masses are benign, 55% to 60% are indolent renal cell carcinomas, and only 20% to 25% have potentially aggressive features, defined by high nuclear grade or locally invasive characteristics.5–7
A relatively strong predictor of the aggressiveness of renal tumors is their size, which directly correlates with the risk of malignant pathology. Of lesions smaller than 1.0 cm, 38% to 46% are benign, dramatically decreasing to 6.3% to 7.1% for lesions larger than 7.0 cm.5 Each 1.0-cm increase in tumor diameter correlates with a 16% increase in the risk of malignancy.8
Our knowledge of the natural history of small renal masses is limited, being based on small, retrospective series. In these studies, when small renal masses were followed over time, relatively few progressed (ie, metastasized), and there have been no documented reports of disease progression in the absence of demonstrable tumor growth, suggesting a predominance of nonaggressive phenotypes.9
In light of these observations, patients with small renal masses should be carefully evaluated to determine if they are candidates for active surveillance as opposed to more aggressive treatment, ie, surgery or thermal ablation.
CT AND MRI ARE THE PREFERRED DIAGNOSTIC STUDIES
In the past, most patients with renal tumors presented with gross hematuria, flank pain, or a palpable abdominal mass. These presentations are now uncommon, as most cases are asymptomatic and are diagnosed incidentally. In a series of 349 small renal masses, microhematuria was found in only 8 cases.10
Systemic manifestations or paraneoplastic syndromes such as hypercalcemia or hypertension are more common in patients with metastatic renal cell carcinoma than in those with localized tumors. It was because of these varied clinical presentations that renal cell carcinoma was previously known as the “internist’s tumor”; however, small renal masses are better termed the “radiologist’s tumor.”11
High-quality axial imaging with CT or MRI is preferred for evaluating renal cortical neoplasms. Enhancement on CT or MRI is the characteristic finding of a renal lesion that should be suspected of being renal cell carcinoma (Figure 1). Triple-phase CT is ideal, with images taken before contrast is given, immediately after contrast (the early vascular phase), and later (the delayed phase). Alternatively, MRI can be used in patients who are allergic to intravenous contrast or who have moderate renal dysfunction.
Renal tumors with enhancement of more than 15 Hounsfield units (HU) on CT imaging are considered suggestive of renal cell carcinoma, whereas those with less than 10 HU of enhancement are more likely to be benign. Enhancement in the range of 10 to 15 HU is considered equivocal.
Differential diagnosis
By far, most small renal masses are renal cell carcinomas. However, other possibilities include oncocytoma, atypical or fat-poor angiomyolipoma, metanephric adenoma, urothelial carcinoma, metastatic lesions, lymphoma, renal abscess or infarction, mixed epithelial or stromal tumor, pseudotumor, and vascular malformations.
With rare exceptions, dense fat within a renal mass reliably indicates benign angiomyolipoma, and all renal tumors should be reviewed carefully for this feature. Beyond this, no clinical or radiologic feature ensures that a small renal mass is benign.
Imaging’s inability to accurately classify these enhancing renal lesions has led to a renewed interest in renal mass sampling to aid in the evaluation of small renal masses.
RENAL MASS SAMPLING: SAFER, MORE ACCURATE THAN THOUGHT
Renal mass sampling (ie, biopsy) has traditionally had a restricted role in the management of small renal masses, limited specifically to patients with a clinical history suggesting renal lymphoma, carcinoma that had metastasized to the kidney, or primary renal abscess. However, this may be changing, with more interest in it as a way to subtype and stratify select patients with small renal masses, especially potential candidates for active surveillance.
Our thinking about renal mass sampling has changed substantially over the last 2 decades. Previously, it was not routinely performed, because of concern over high false-negative rates (commonly quoted as being as high as 18%) and its potential associated morbidity. A common perception was that a negative biopsy could not be trusted and, therefore, renal mass sampling would not ultimately change patient management. However, many of these false-negative results were actually “noninformative,” ie, cases in which the renal tumor could not be adequately sampled or the pathologist lacked a sufficient specimen to allow for a definitive diagnosis.
Recent evidence suggests that these concerns were exaggerated and that renal mass sampling is more accurate and safer than previously thought. A meta-analysis of studies done before 2001 found that the diagnostic accuracy of renal mass sampling averaged 82%, whereas contemporary series indicate that its accuracy in differentiating benign from malignant tumors is actually greater than 95%.12 In addition, false-negative rates are now consistently less than 1%.13
Furthermore, serious complications requiring clinical intervention or hospitalization occur in fewer than 1% of cases. Seeding of the needle tract with tumor cells, which was another concern, is also exceedingly rare for these small, well-circumscribed renal masses.12
Overall morbidity is low with renal mass sampling, which is routinely performed as an outpatient procedure using CT or ultrasono-graphic guidance and local anesthesia.
However, 10% of biopsy results are still noninformative. In this situation, biopsy can be repeated, or the mass can be surgically excised, or the patient can undergo conservative management if he or she is unfit or unwilling to undergo surgery.
The encouraging results with renal mass sampling have led to greater use of it at many centers in the evaluation and risk-stratification of patients with small renal masses. It may be especially useful in patients considering several treatment options.
For example, a 75-year-old patient with modest comorbidities and a 2.0-cm enhancing renal mass could be a candidate for partial nephrectomy, thermal ablation, or active surveillance, and a reasonable argument could be made for each of these options. Renal mass sampling in this instance could be instrumental in guiding this decision, as a tissue diagnosis of high-grade renal cell carcinoma would favor partial nephrectomy, whereas a diagnosis of “oncocytoma neoplasm” would support a more conservative approach.
Older, frail patients with significant comorbidities who are unlikely to be candidates for aggressive surgical management would not need renal mass sampling, as they will ultimately be managed with active surveillance or thermal ablation.
Recent studies have also indicated that molecular profiling through gene expression analysis or proteomic analysis can further improve the accuracy of renal mass sampling.14 This will likely be the holy grail for this field, allowing for truly rational management (Figure 2).
TREATMENT OPTIONS
Radical nephrectomy: Still the most common treatment
In the past, complete removal of the kidney was standard for nearly all renal masses suspected of being renal cell carcinomas. Partial nephrectomy was generally reserved for patients who had a solitary kidney, bilateral tumors, or preexisting chronic kidney disease.
Although the two procedures provide equivalent oncologic outcomes for clinical T1 lesions, Miller et al15 reported that, before 2001, only 20% of small renal masses in the United States were managed with partial nephrectomy. That percentage has increased modestly, but radical nephrectomy still predominates.
One explanation for why the radical procedure is done more frequently is that partial nephrectomy is more technically difficult, as it involves renal reconstruction. Furthermore, radical nephrectomy can almost always be performed via a minimally invasive approach, which is inherently appealing to patients and surgeons alike. Laparoscopic radical nephrectomy has been called “the great seductress” because of these considerations.16 However, total removal of the kidney comes at a great price—loss of renal function.
Over the last decade, various studies have highlighted the association between radical nephrectomy and the subsequent clinical onset of chronic kidney disease, and the potential correlations between chronic kidney disease and cardiovascular events and elevated mortality rates.17
In a landmark study, Huang et al18 evaluated the outcomes of 662 patients who had small renal masses, a “normal” serum creatinine concentration (≤ 124 μmol/L [1.4 mg/dL]), and a normal-appearing contralateral kidney who underwent radical or partial nephrectomy. Of these, 26% were found to have preexisting stage 3 chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated using the Modification of Diet in Renal Disease equation). Additionally, 65% of patients treated with radical nephrectomy were found to have stage 3 chronic kidney disease after surgery vs 20% of patients managed with partial nephrectomy.
The misconception remains that the risk of chronic kidney disease after radical nephrectomy is insignificant, since the risk is low in renal transplant donors.19 However, renal transplant donors undergo stringent screening to ensure that their general health is good and that their renal function is robust, both of which are not true in many patients with small renal masses, particularly if they are elderly.
The overuse of radical nephrectomy is worrisome in light of the potential implications of chronic kidney disease, such as increased risk of morbid cardiovascular events and elevated mortality rates. Many experts believe that over-treatment of small renal masses may have contributed to the paradoxical increase in overall mortality rates observed with radical nephrectomy in some studies.4
Although radical nephrectomy remains an important treatment for locally advanced renal cell carcinoma, it should be performed for small renal masses only if nephron-sparing surgery is not feasible (Table 2).
Partial nephrectomy: The new gold standard for most patients
Over the last 5 years, greater emphasis has been placed on lessening the risk of chronic kidney disease in the management of all urologic conditions, including small renal masses.
The overuse of radical nephrectomy prompted the American Urological Association to commission a panel to provide guidelines for the management of clinical stage T1 renal masses.17 After an extensive review and rigorous meta-analysis, the panel concluded that partial nephrectomy is the gold standard for most patients (Table 1, Table 2).
Partial nephrectomy involves excision of the tumor with a small margin of normal tissue, preserving as much functional renal parenchyma as possible, followed by closure of the collecting system, suture ligation of any transected vessels, and reapproximation of the capsule. Tumor excision is usually performed during temporary occlusion of the renal vasculature, allowing for a bloodless field. Regional hypothermia (cold ischemia) can also be used to minimize ischemic injury.
Contemporary series have documented that partial and radical nephrectomy have comparable oncologic efficacy for patients with small renal masses.20,21 Local recurrence rates are only 1% to 2% with partial nephrectomy, and 5- and 10-year cancer-specific survival rates of 96% and 90% have been reported.22
Furthermore, some studies have shown that patients undergoing partial nephrectomy have higher overall survival rates than those managed with radical nephrectomy—perhaps in part due to greater preservation of renal function and a lower incidence of subsequent chronic kidney disease.23,24 At Cleveland Clinic, we are now studying the determinants of ultimate renal function after partial nephrectomy in an effort to minimize ischemic injury and optimize this technique.25
Complications. Partial nephrectomy does have a potential downside in that it carries a higher risk of urologic complications such as urine leak and postoperative hemorrhage, which is not surprising because it requires a reconstruction that must heal. In a recent meta-analysis, urologic complications occurred in 6.3% patients who underwent open partial nephrectomy and in 9.0% of patients who underwent laparoscopic partial nephrectomy.17 Fortunately, most complications associated with partial nephrectomy can be managed with conservative measures.
Postoperative bleeding occurs in about 1% to 2% of patients and is the most serious complication. However, it is typically managed with superselective embolization, which has a high success rate and facilitates renal preservation.
Urine leak occurs in about 3% to 5% of cases and almost always resolves with prolonged drainage, occasionally complemented with a ureteral stent to promote antegrade drainage.
A new refinement, robotic-assisted partial nephrectomy promises to reduce the morbidity of this procedure. This approach takes less time to learn than standard laparoscopic surgery and has expanded the indications for minimally invasive partial nephrectomy, although more-difficult cases are still better done through a traditional, open surgical approach.
Thermal ablation: Another minimally invasive option
Cryoablation and radiofrequency ablation (collectively called thermal ablation) have recently emerged as alternate minimally invasive treatments for small renal masses. They are appealing options for patients with small renal tumors (< 3.5 cm) who have significant comorbidities but still prefer a proactive approach. They can also be considered as salvage procedures in patients with local recurrence after partial nephrectomy or in select patients with multifocal disease.
Both procedures can be performed percutaneously or laparoscopically, offering the potential for rapid convalescence and reduced morbidity.26,27 A laparoscopic approach is necessary to mobilize the tumor from adjacent organs if they are juxtaposed, whereas a percutaneous approach is less invasive and is better suited for posterior renal masses.28 Renal mass sampling should be performed in these patients before treatment to define the histology and to guide surveillance and should be repeated postoperatively if there is suspicion of local recurrence based on imaging.
Cryoablation destroys tumor cells through rapid cycles of freezing to less than −20°C (−4°F) and thawing, which can be monitored in real time via thermocoupling (ie, a thermometer microprobe strategically placed outside the tumor to ensure that lethal temperatures are extended beyond the edge of the tumor) or via ultrasonography, or both. Treatment is continued until the “ice ball” extends about 1 cm beyond the edge of the tumor.
Initial series reported local tumor control rates in the range of 90% to 95%; however, follow-up was very limited.29 In a more robust single-institution experience,30 renal cryoablation demonstrated 5-year cancer-specific and recurrence-free survival rates of 93% and 83%, respectively, substantially lower than what would be expected with surgical excision in a similar patient population.
Another concern with cryoablation is that options are limited for surgical salvage if the initial treatment fails. Nguyen and Campbell31 reported that partial nephrectomy and minimally invasive surgery were often precluded in this situation because of the extensive fibrotic reaction caused by the prior treatment. If cryoablation fails, surgical salvage thus often requires open, radical surgery.
Radiofrequency ablation produces tumor coagulation via protein denaturation and disruption of cell membranes after heating tissues to temperatures above 50°C (122°F) for 4 to 6 minutes.32 One of its disadvantages is that one cannot monitor treatment progress in real time, as there is no identifiable change in tissue appearance analagous to the ice ball that is seen with cryoablation.
Although the outcomes of radiofrequency ablation are less robust than those of cryoablation, most studies suggest that local control is achieved in 80% to 90% of cases based on radiographic loss of enhancement after treatment.17,30,33 A recent meta-analysis comparing these treatments found that lesions treated with radiofrequency ablation had a significantly higher rate of local tumor progression than those treated with cryoablation (12.3% vs 4.7%, P < .0001).34 Both of these local recurrence rates are substantially higher than that seen after surgical excision, despite much shorter follow-up after thermal ablation.
Tempered enthusiasm. Because thermal ablation has been developed relatively recently, its long-term outcomes and treatment efficacy have not been well established, and current studies have confirmed higher local recurrence rates with thermal ablation than with surgical excision (Table 1). Furthermore, there are significant deficiencies in the literature about thermal ablation, including limited follow-up, lack of pathologic confirmation, and controversies regarding histologic or radiologic definitions of success (Table 2).
Although current enthusiasm for thermal ablation has been tempered by suboptimal results, further refinement in technique and acknowledgment of its limitations will help to define appropriate candidates for these treatments.
Active surveillance for select patients
In select patients with extensive medical comorbidities or short life expectancy, the risks associated with proactive management may outweigh the benefits, especially considering the indolent nature of many small renal masses. In such patients, active surveillance is reasonable.
A recent meta-analysis found that most small enhancing renal masses grew relatively slowly (median 0.28 cm/year) and posed a low risk of metastasis (1%–2%).17,22 Furthermore, almost all renal lesions that progressed to metastatic disease demonstrated rapid radiographic growth, suggesting that the radiographic growth of a renal mass under active surveillance may serve as an indicator for aggressive behavior.35
Unfortunately, the growth rates of small renal masses do not reliably predict malignancy, and one study reported that 83% of tumors without demonstrable growth were malignant.36
Studies of active surveillance to date have had several other important limitations. Many did not incorporate pathologic confirmation, so that about 20% of the tumors were actually benign, thus artificially reducing the risk of adverse outcomes.5,22,37 Furthermore, the follow-up has been short, with most studies including data for only 2 to 3 years, which is clearly inadequate for this type of malignancy.37,38 Finally, most series had significant selection bias towards small, homogenous masses. In general, small renal masses that appear to be more aggressive are treated and thus excluded from these surveillance populations (Table 2).
Another concern about active surveillance is the small but real risk of tumor progression to metastatic disease, rendering these patients incurable even with new, targeted molecular therapies. Additionally, some patients may lose their window of opportunity for nephron-sparing surgery if significant tumor growth occurs during observation, rendering partial nephrectomy unfeasible. Therefore, active surveillance is not advisable for young, otherwise healthy patients (Table 2).
In the future, advances in renal mass sampling with molecular profiling may help determine which renal lesions are less biologically aggressive and, thereby, help identify appropriate candidates for observation (Figure 2).
- Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J 2008; 14:288–301.
- Lane BR, Campbell SC. Management of small renal masses. AUA Update Series 2009; 28:313–324.
- Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer 2004; 100:738–745.
- Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 2006; 98:1331–1334.
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003; 170:2217–2220.
- Russo P. Should elective partial nephrectomy be performed for renal cell carcinoma >4 cm in size? Nat Clin Pract Urol 2008; 5:482–483.
- Thomas AA, Aron M, Hernandez AV, Lane BR, Gill IS. Laparoscopic partial nephrectomy in octogenarians. Urology 2009; 74:1042–1046.
- Thompson RH, Kurta JM, Kaag M, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol 2009; 181:2033–2036.
- Mues AC, Landman J. Small renal masses: current concepts regarding the natural history and reflections on the American Urological Association guidelines. Curr Opin Urol 2010; 20:105–110.
- Patard JJ, Bensalah K, Vincendeau S, Rioux-Leclerq N, Guillé F, Lobel B. [Correlation between the mode of presentation of renal tumors and patient survival]. Prog Urol 2003; 13:23–28.
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009; 373:1119–1132.
- Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy—a renaissance? J Urol 2008; 179:20–27.
- Samplaski MK, Zhou M, Lane BR, Herts B, Campbell SC. Renal mass sampling: an enlightened perspective. Int J Urol 2011; 18:5–19.
- Tan MH, Rogers CG, Cooper JT, et al. Gene expression profiling of renal cell carcinoma. Clin Cancer Res 2004; 10:6315S–6321S.
- Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol 2006; 175:853–857.
- Lane BR, Poggio ED, Herts BR, Novick AC, Campbell SC. Renal function assessment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol 2009; 182:435–444.
- Campbell SC, Novick AC, Belldegrun A, et al; Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182:1271–1279.
- Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006; 7:735–740.
- Boorjian SA, Uzzo RG. The evolving management of small renal masses. Curr Oncol Rep 2009; 11:211–217.
- Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol 1999; 162:1930–1933.
- Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol 2000; 163:730–736.
- Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 2006; 175:425–431.
- Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol 2009; 181:55–61.
- Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol 2008; 179:468–471.
- Thomas AA, Demirjian S, Lane BR, et al. Acute kidney injury: novel biomarkers and potential utility for patient care in urology. Urology 2011; 77:5–11.
- Hinshaw JL, Shadid AM, Nakada SY, Hedican SP, Winter TC, Lee FT. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol 2008; 191:1159–1168.
- Sterrett SP, Nakada SY, Wingo MS, Williams SK, Leveillee RJ. Renal thermal ablative therapy. Urol Clin North Am 2008; 35:397–414.
- Hafron J, Kaouk JH. Ablative techniques for the management of kidney cancer. Nat Clin Pract Urol 2007; 4:261–269.
- Matin SF, Ahrar K. Nephron-sparing probe ablative therapy: longterm outcomes. Curr Opin Urol 2008; 18:150–156.
- Berger A, Kamoi K, Gill IS, Aron M. Cryoablation for renal tumors: current status. Curr Opin Urol 2009; 19:138–142.
- Nguyen CT, Campbell SC. Salvage of local recurrence after primary thermal ablation for small renal masses. Expert Rev Anticancer Ther 2008; 8:1899–1905.
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000; 174:323–331.
- Carraway WA, Raman JD, Cadeddu JA. Current status of renal radiofrequency ablation. Curr Opin Urol 2009; 19:143–147.
- Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 2008; 113:2671–2680.
- Kunkle DA, Kutikov A, Uzzo RG. Management of small renal masses. Semin Ultrasound CT MR 2009; 30:352–358.
- Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol 2007; 177:849–853.
- Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review. J Urol 2008; 179:1227–1233.
- Jewett MA, Zuniga A. Renal tumor natural history: the rationale and role for active surveillance. Urol Clin North Am 2008; 35:627–634.
Opinion about treatment of mall renal masses has changed considerably in the past 2 decades.
Traditionally, the most common treatment was surgical removal of the whole kidney, ie, radical nephrectomy. However, recent studies have shown that many patients who undergo radical nephrectomy develop chronic kidney disease. Furthermore, radical nephrectomy often constitutes over-treatment, as many of these lesions are benign or, if malignant, would follow an indolent course if left alone.
Now that we better understand the biology of small renal masses and are more aware of the morbidity and mortality related to chronic kidney disease, we try to avoid radical nephrectomy whenever possible, favoring nephron-sparing approaches instead.
In this article, we review the current clinical management of small renal masses.
SMALL RENAL MASSES ARE A HETEROGENEOUS GROUP
Small renal masses are defined as solid renal tumors that enhance on computed tomography (CT) and magnetic resonance imaging (MRI) and are suspected of being renal cell carcinomas. They are generally low-stage and relatively small (< 4 cm in diameter) at presentation. Most are now discovered incidentally on CT or MRI done for various abdominal symptoms. From 20,000 to 30,000 new cases are diagnosed each year in the United States, and the rate is increasing by 3% to 4% per year as the use of CT and MRI increases.1,2
With more small renal masses being detected incidentally, renal cell carcinoma has been going through a stage and size migration—ie, more of these tumors are being discovered in clinical stage T1 (ie, confined to the kidney and measuring less than 7 cm) than in the past. Currently, clinical T1 renal tumors account for 48% to 66% of cases.3
This indicates that the disease is being detected and treated earlier in its course than in the past. However, cancer-specific deaths from renal cell carcinoma have not declined, suggesting that for many of these patients, our traditional practice of aggressive surgical management with radical nephrectomy may not be warranted.4
Small renal masses vary in biologic aggressiveness
Recent large surgical series indicate that up to 20% of small renal masses are benign, 55% to 60% are indolent renal cell carcinomas, and only 20% to 25% have potentially aggressive features, defined by high nuclear grade or locally invasive characteristics.5–7
A relatively strong predictor of the aggressiveness of renal tumors is their size, which directly correlates with the risk of malignant pathology. Of lesions smaller than 1.0 cm, 38% to 46% are benign, dramatically decreasing to 6.3% to 7.1% for lesions larger than 7.0 cm.5 Each 1.0-cm increase in tumor diameter correlates with a 16% increase in the risk of malignancy.8
Our knowledge of the natural history of small renal masses is limited, being based on small, retrospective series. In these studies, when small renal masses were followed over time, relatively few progressed (ie, metastasized), and there have been no documented reports of disease progression in the absence of demonstrable tumor growth, suggesting a predominance of nonaggressive phenotypes.9
In light of these observations, patients with small renal masses should be carefully evaluated to determine if they are candidates for active surveillance as opposed to more aggressive treatment, ie, surgery or thermal ablation.
CT AND MRI ARE THE PREFERRED DIAGNOSTIC STUDIES
In the past, most patients with renal tumors presented with gross hematuria, flank pain, or a palpable abdominal mass. These presentations are now uncommon, as most cases are asymptomatic and are diagnosed incidentally. In a series of 349 small renal masses, microhematuria was found in only 8 cases.10
Systemic manifestations or paraneoplastic syndromes such as hypercalcemia or hypertension are more common in patients with metastatic renal cell carcinoma than in those with localized tumors. It was because of these varied clinical presentations that renal cell carcinoma was previously known as the “internist’s tumor”; however, small renal masses are better termed the “radiologist’s tumor.”11
High-quality axial imaging with CT or MRI is preferred for evaluating renal cortical neoplasms. Enhancement on CT or MRI is the characteristic finding of a renal lesion that should be suspected of being renal cell carcinoma (Figure 1). Triple-phase CT is ideal, with images taken before contrast is given, immediately after contrast (the early vascular phase), and later (the delayed phase). Alternatively, MRI can be used in patients who are allergic to intravenous contrast or who have moderate renal dysfunction.
Renal tumors with enhancement of more than 15 Hounsfield units (HU) on CT imaging are considered suggestive of renal cell carcinoma, whereas those with less than 10 HU of enhancement are more likely to be benign. Enhancement in the range of 10 to 15 HU is considered equivocal.
Differential diagnosis
By far, most small renal masses are renal cell carcinomas. However, other possibilities include oncocytoma, atypical or fat-poor angiomyolipoma, metanephric adenoma, urothelial carcinoma, metastatic lesions, lymphoma, renal abscess or infarction, mixed epithelial or stromal tumor, pseudotumor, and vascular malformations.
With rare exceptions, dense fat within a renal mass reliably indicates benign angiomyolipoma, and all renal tumors should be reviewed carefully for this feature. Beyond this, no clinical or radiologic feature ensures that a small renal mass is benign.
Imaging’s inability to accurately classify these enhancing renal lesions has led to a renewed interest in renal mass sampling to aid in the evaluation of small renal masses.
RENAL MASS SAMPLING: SAFER, MORE ACCURATE THAN THOUGHT
Renal mass sampling (ie, biopsy) has traditionally had a restricted role in the management of small renal masses, limited specifically to patients with a clinical history suggesting renal lymphoma, carcinoma that had metastasized to the kidney, or primary renal abscess. However, this may be changing, with more interest in it as a way to subtype and stratify select patients with small renal masses, especially potential candidates for active surveillance.
Our thinking about renal mass sampling has changed substantially over the last 2 decades. Previously, it was not routinely performed, because of concern over high false-negative rates (commonly quoted as being as high as 18%) and its potential associated morbidity. A common perception was that a negative biopsy could not be trusted and, therefore, renal mass sampling would not ultimately change patient management. However, many of these false-negative results were actually “noninformative,” ie, cases in which the renal tumor could not be adequately sampled or the pathologist lacked a sufficient specimen to allow for a definitive diagnosis.
Recent evidence suggests that these concerns were exaggerated and that renal mass sampling is more accurate and safer than previously thought. A meta-analysis of studies done before 2001 found that the diagnostic accuracy of renal mass sampling averaged 82%, whereas contemporary series indicate that its accuracy in differentiating benign from malignant tumors is actually greater than 95%.12 In addition, false-negative rates are now consistently less than 1%.13
Furthermore, serious complications requiring clinical intervention or hospitalization occur in fewer than 1% of cases. Seeding of the needle tract with tumor cells, which was another concern, is also exceedingly rare for these small, well-circumscribed renal masses.12
Overall morbidity is low with renal mass sampling, which is routinely performed as an outpatient procedure using CT or ultrasono-graphic guidance and local anesthesia.
However, 10% of biopsy results are still noninformative. In this situation, biopsy can be repeated, or the mass can be surgically excised, or the patient can undergo conservative management if he or she is unfit or unwilling to undergo surgery.
The encouraging results with renal mass sampling have led to greater use of it at many centers in the evaluation and risk-stratification of patients with small renal masses. It may be especially useful in patients considering several treatment options.
For example, a 75-year-old patient with modest comorbidities and a 2.0-cm enhancing renal mass could be a candidate for partial nephrectomy, thermal ablation, or active surveillance, and a reasonable argument could be made for each of these options. Renal mass sampling in this instance could be instrumental in guiding this decision, as a tissue diagnosis of high-grade renal cell carcinoma would favor partial nephrectomy, whereas a diagnosis of “oncocytoma neoplasm” would support a more conservative approach.
Older, frail patients with significant comorbidities who are unlikely to be candidates for aggressive surgical management would not need renal mass sampling, as they will ultimately be managed with active surveillance or thermal ablation.
Recent studies have also indicated that molecular profiling through gene expression analysis or proteomic analysis can further improve the accuracy of renal mass sampling.14 This will likely be the holy grail for this field, allowing for truly rational management (Figure 2).
TREATMENT OPTIONS
Radical nephrectomy: Still the most common treatment
In the past, complete removal of the kidney was standard for nearly all renal masses suspected of being renal cell carcinomas. Partial nephrectomy was generally reserved for patients who had a solitary kidney, bilateral tumors, or preexisting chronic kidney disease.
Although the two procedures provide equivalent oncologic outcomes for clinical T1 lesions, Miller et al15 reported that, before 2001, only 20% of small renal masses in the United States were managed with partial nephrectomy. That percentage has increased modestly, but radical nephrectomy still predominates.
One explanation for why the radical procedure is done more frequently is that partial nephrectomy is more technically difficult, as it involves renal reconstruction. Furthermore, radical nephrectomy can almost always be performed via a minimally invasive approach, which is inherently appealing to patients and surgeons alike. Laparoscopic radical nephrectomy has been called “the great seductress” because of these considerations.16 However, total removal of the kidney comes at a great price—loss of renal function.
Over the last decade, various studies have highlighted the association between radical nephrectomy and the subsequent clinical onset of chronic kidney disease, and the potential correlations between chronic kidney disease and cardiovascular events and elevated mortality rates.17
In a landmark study, Huang et al18 evaluated the outcomes of 662 patients who had small renal masses, a “normal” serum creatinine concentration (≤ 124 μmol/L [1.4 mg/dL]), and a normal-appearing contralateral kidney who underwent radical or partial nephrectomy. Of these, 26% were found to have preexisting stage 3 chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated using the Modification of Diet in Renal Disease equation). Additionally, 65% of patients treated with radical nephrectomy were found to have stage 3 chronic kidney disease after surgery vs 20% of patients managed with partial nephrectomy.
The misconception remains that the risk of chronic kidney disease after radical nephrectomy is insignificant, since the risk is low in renal transplant donors.19 However, renal transplant donors undergo stringent screening to ensure that their general health is good and that their renal function is robust, both of which are not true in many patients with small renal masses, particularly if they are elderly.
The overuse of radical nephrectomy is worrisome in light of the potential implications of chronic kidney disease, such as increased risk of morbid cardiovascular events and elevated mortality rates. Many experts believe that over-treatment of small renal masses may have contributed to the paradoxical increase in overall mortality rates observed with radical nephrectomy in some studies.4
Although radical nephrectomy remains an important treatment for locally advanced renal cell carcinoma, it should be performed for small renal masses only if nephron-sparing surgery is not feasible (Table 2).
Partial nephrectomy: The new gold standard for most patients
Over the last 5 years, greater emphasis has been placed on lessening the risk of chronic kidney disease in the management of all urologic conditions, including small renal masses.
The overuse of radical nephrectomy prompted the American Urological Association to commission a panel to provide guidelines for the management of clinical stage T1 renal masses.17 After an extensive review and rigorous meta-analysis, the panel concluded that partial nephrectomy is the gold standard for most patients (Table 1, Table 2).
Partial nephrectomy involves excision of the tumor with a small margin of normal tissue, preserving as much functional renal parenchyma as possible, followed by closure of the collecting system, suture ligation of any transected vessels, and reapproximation of the capsule. Tumor excision is usually performed during temporary occlusion of the renal vasculature, allowing for a bloodless field. Regional hypothermia (cold ischemia) can also be used to minimize ischemic injury.
Contemporary series have documented that partial and radical nephrectomy have comparable oncologic efficacy for patients with small renal masses.20,21 Local recurrence rates are only 1% to 2% with partial nephrectomy, and 5- and 10-year cancer-specific survival rates of 96% and 90% have been reported.22
Furthermore, some studies have shown that patients undergoing partial nephrectomy have higher overall survival rates than those managed with radical nephrectomy—perhaps in part due to greater preservation of renal function and a lower incidence of subsequent chronic kidney disease.23,24 At Cleveland Clinic, we are now studying the determinants of ultimate renal function after partial nephrectomy in an effort to minimize ischemic injury and optimize this technique.25
Complications. Partial nephrectomy does have a potential downside in that it carries a higher risk of urologic complications such as urine leak and postoperative hemorrhage, which is not surprising because it requires a reconstruction that must heal. In a recent meta-analysis, urologic complications occurred in 6.3% patients who underwent open partial nephrectomy and in 9.0% of patients who underwent laparoscopic partial nephrectomy.17 Fortunately, most complications associated with partial nephrectomy can be managed with conservative measures.
Postoperative bleeding occurs in about 1% to 2% of patients and is the most serious complication. However, it is typically managed with superselective embolization, which has a high success rate and facilitates renal preservation.
Urine leak occurs in about 3% to 5% of cases and almost always resolves with prolonged drainage, occasionally complemented with a ureteral stent to promote antegrade drainage.
A new refinement, robotic-assisted partial nephrectomy promises to reduce the morbidity of this procedure. This approach takes less time to learn than standard laparoscopic surgery and has expanded the indications for minimally invasive partial nephrectomy, although more-difficult cases are still better done through a traditional, open surgical approach.
Thermal ablation: Another minimally invasive option
Cryoablation and radiofrequency ablation (collectively called thermal ablation) have recently emerged as alternate minimally invasive treatments for small renal masses. They are appealing options for patients with small renal tumors (< 3.5 cm) who have significant comorbidities but still prefer a proactive approach. They can also be considered as salvage procedures in patients with local recurrence after partial nephrectomy or in select patients with multifocal disease.
Both procedures can be performed percutaneously or laparoscopically, offering the potential for rapid convalescence and reduced morbidity.26,27 A laparoscopic approach is necessary to mobilize the tumor from adjacent organs if they are juxtaposed, whereas a percutaneous approach is less invasive and is better suited for posterior renal masses.28 Renal mass sampling should be performed in these patients before treatment to define the histology and to guide surveillance and should be repeated postoperatively if there is suspicion of local recurrence based on imaging.
Cryoablation destroys tumor cells through rapid cycles of freezing to less than −20°C (−4°F) and thawing, which can be monitored in real time via thermocoupling (ie, a thermometer microprobe strategically placed outside the tumor to ensure that lethal temperatures are extended beyond the edge of the tumor) or via ultrasonography, or both. Treatment is continued until the “ice ball” extends about 1 cm beyond the edge of the tumor.
Initial series reported local tumor control rates in the range of 90% to 95%; however, follow-up was very limited.29 In a more robust single-institution experience,30 renal cryoablation demonstrated 5-year cancer-specific and recurrence-free survival rates of 93% and 83%, respectively, substantially lower than what would be expected with surgical excision in a similar patient population.
Another concern with cryoablation is that options are limited for surgical salvage if the initial treatment fails. Nguyen and Campbell31 reported that partial nephrectomy and minimally invasive surgery were often precluded in this situation because of the extensive fibrotic reaction caused by the prior treatment. If cryoablation fails, surgical salvage thus often requires open, radical surgery.
Radiofrequency ablation produces tumor coagulation via protein denaturation and disruption of cell membranes after heating tissues to temperatures above 50°C (122°F) for 4 to 6 minutes.32 One of its disadvantages is that one cannot monitor treatment progress in real time, as there is no identifiable change in tissue appearance analagous to the ice ball that is seen with cryoablation.
Although the outcomes of radiofrequency ablation are less robust than those of cryoablation, most studies suggest that local control is achieved in 80% to 90% of cases based on radiographic loss of enhancement after treatment.17,30,33 A recent meta-analysis comparing these treatments found that lesions treated with radiofrequency ablation had a significantly higher rate of local tumor progression than those treated with cryoablation (12.3% vs 4.7%, P < .0001).34 Both of these local recurrence rates are substantially higher than that seen after surgical excision, despite much shorter follow-up after thermal ablation.
Tempered enthusiasm. Because thermal ablation has been developed relatively recently, its long-term outcomes and treatment efficacy have not been well established, and current studies have confirmed higher local recurrence rates with thermal ablation than with surgical excision (Table 1). Furthermore, there are significant deficiencies in the literature about thermal ablation, including limited follow-up, lack of pathologic confirmation, and controversies regarding histologic or radiologic definitions of success (Table 2).
Although current enthusiasm for thermal ablation has been tempered by suboptimal results, further refinement in technique and acknowledgment of its limitations will help to define appropriate candidates for these treatments.
Active surveillance for select patients
In select patients with extensive medical comorbidities or short life expectancy, the risks associated with proactive management may outweigh the benefits, especially considering the indolent nature of many small renal masses. In such patients, active surveillance is reasonable.
A recent meta-analysis found that most small enhancing renal masses grew relatively slowly (median 0.28 cm/year) and posed a low risk of metastasis (1%–2%).17,22 Furthermore, almost all renal lesions that progressed to metastatic disease demonstrated rapid radiographic growth, suggesting that the radiographic growth of a renal mass under active surveillance may serve as an indicator for aggressive behavior.35
Unfortunately, the growth rates of small renal masses do not reliably predict malignancy, and one study reported that 83% of tumors without demonstrable growth were malignant.36
Studies of active surveillance to date have had several other important limitations. Many did not incorporate pathologic confirmation, so that about 20% of the tumors were actually benign, thus artificially reducing the risk of adverse outcomes.5,22,37 Furthermore, the follow-up has been short, with most studies including data for only 2 to 3 years, which is clearly inadequate for this type of malignancy.37,38 Finally, most series had significant selection bias towards small, homogenous masses. In general, small renal masses that appear to be more aggressive are treated and thus excluded from these surveillance populations (Table 2).
Another concern about active surveillance is the small but real risk of tumor progression to metastatic disease, rendering these patients incurable even with new, targeted molecular therapies. Additionally, some patients may lose their window of opportunity for nephron-sparing surgery if significant tumor growth occurs during observation, rendering partial nephrectomy unfeasible. Therefore, active surveillance is not advisable for young, otherwise healthy patients (Table 2).
In the future, advances in renal mass sampling with molecular profiling may help determine which renal lesions are less biologically aggressive and, thereby, help identify appropriate candidates for observation (Figure 2).
Opinion about treatment of mall renal masses has changed considerably in the past 2 decades.
Traditionally, the most common treatment was surgical removal of the whole kidney, ie, radical nephrectomy. However, recent studies have shown that many patients who undergo radical nephrectomy develop chronic kidney disease. Furthermore, radical nephrectomy often constitutes over-treatment, as many of these lesions are benign or, if malignant, would follow an indolent course if left alone.
Now that we better understand the biology of small renal masses and are more aware of the morbidity and mortality related to chronic kidney disease, we try to avoid radical nephrectomy whenever possible, favoring nephron-sparing approaches instead.
In this article, we review the current clinical management of small renal masses.
SMALL RENAL MASSES ARE A HETEROGENEOUS GROUP
Small renal masses are defined as solid renal tumors that enhance on computed tomography (CT) and magnetic resonance imaging (MRI) and are suspected of being renal cell carcinomas. They are generally low-stage and relatively small (< 4 cm in diameter) at presentation. Most are now discovered incidentally on CT or MRI done for various abdominal symptoms. From 20,000 to 30,000 new cases are diagnosed each year in the United States, and the rate is increasing by 3% to 4% per year as the use of CT and MRI increases.1,2
With more small renal masses being detected incidentally, renal cell carcinoma has been going through a stage and size migration—ie, more of these tumors are being discovered in clinical stage T1 (ie, confined to the kidney and measuring less than 7 cm) than in the past. Currently, clinical T1 renal tumors account for 48% to 66% of cases.3
This indicates that the disease is being detected and treated earlier in its course than in the past. However, cancer-specific deaths from renal cell carcinoma have not declined, suggesting that for many of these patients, our traditional practice of aggressive surgical management with radical nephrectomy may not be warranted.4
Small renal masses vary in biologic aggressiveness
Recent large surgical series indicate that up to 20% of small renal masses are benign, 55% to 60% are indolent renal cell carcinomas, and only 20% to 25% have potentially aggressive features, defined by high nuclear grade or locally invasive characteristics.5–7
A relatively strong predictor of the aggressiveness of renal tumors is their size, which directly correlates with the risk of malignant pathology. Of lesions smaller than 1.0 cm, 38% to 46% are benign, dramatically decreasing to 6.3% to 7.1% for lesions larger than 7.0 cm.5 Each 1.0-cm increase in tumor diameter correlates with a 16% increase in the risk of malignancy.8
Our knowledge of the natural history of small renal masses is limited, being based on small, retrospective series. In these studies, when small renal masses were followed over time, relatively few progressed (ie, metastasized), and there have been no documented reports of disease progression in the absence of demonstrable tumor growth, suggesting a predominance of nonaggressive phenotypes.9
In light of these observations, patients with small renal masses should be carefully evaluated to determine if they are candidates for active surveillance as opposed to more aggressive treatment, ie, surgery or thermal ablation.
CT AND MRI ARE THE PREFERRED DIAGNOSTIC STUDIES
In the past, most patients with renal tumors presented with gross hematuria, flank pain, or a palpable abdominal mass. These presentations are now uncommon, as most cases are asymptomatic and are diagnosed incidentally. In a series of 349 small renal masses, microhematuria was found in only 8 cases.10
Systemic manifestations or paraneoplastic syndromes such as hypercalcemia or hypertension are more common in patients with metastatic renal cell carcinoma than in those with localized tumors. It was because of these varied clinical presentations that renal cell carcinoma was previously known as the “internist’s tumor”; however, small renal masses are better termed the “radiologist’s tumor.”11
High-quality axial imaging with CT or MRI is preferred for evaluating renal cortical neoplasms. Enhancement on CT or MRI is the characteristic finding of a renal lesion that should be suspected of being renal cell carcinoma (Figure 1). Triple-phase CT is ideal, with images taken before contrast is given, immediately after contrast (the early vascular phase), and later (the delayed phase). Alternatively, MRI can be used in patients who are allergic to intravenous contrast or who have moderate renal dysfunction.
Renal tumors with enhancement of more than 15 Hounsfield units (HU) on CT imaging are considered suggestive of renal cell carcinoma, whereas those with less than 10 HU of enhancement are more likely to be benign. Enhancement in the range of 10 to 15 HU is considered equivocal.
Differential diagnosis
By far, most small renal masses are renal cell carcinomas. However, other possibilities include oncocytoma, atypical or fat-poor angiomyolipoma, metanephric adenoma, urothelial carcinoma, metastatic lesions, lymphoma, renal abscess or infarction, mixed epithelial or stromal tumor, pseudotumor, and vascular malformations.
With rare exceptions, dense fat within a renal mass reliably indicates benign angiomyolipoma, and all renal tumors should be reviewed carefully for this feature. Beyond this, no clinical or radiologic feature ensures that a small renal mass is benign.
Imaging’s inability to accurately classify these enhancing renal lesions has led to a renewed interest in renal mass sampling to aid in the evaluation of small renal masses.
RENAL MASS SAMPLING: SAFER, MORE ACCURATE THAN THOUGHT
Renal mass sampling (ie, biopsy) has traditionally had a restricted role in the management of small renal masses, limited specifically to patients with a clinical history suggesting renal lymphoma, carcinoma that had metastasized to the kidney, or primary renal abscess. However, this may be changing, with more interest in it as a way to subtype and stratify select patients with small renal masses, especially potential candidates for active surveillance.
Our thinking about renal mass sampling has changed substantially over the last 2 decades. Previously, it was not routinely performed, because of concern over high false-negative rates (commonly quoted as being as high as 18%) and its potential associated morbidity. A common perception was that a negative biopsy could not be trusted and, therefore, renal mass sampling would not ultimately change patient management. However, many of these false-negative results were actually “noninformative,” ie, cases in which the renal tumor could not be adequately sampled or the pathologist lacked a sufficient specimen to allow for a definitive diagnosis.
Recent evidence suggests that these concerns were exaggerated and that renal mass sampling is more accurate and safer than previously thought. A meta-analysis of studies done before 2001 found that the diagnostic accuracy of renal mass sampling averaged 82%, whereas contemporary series indicate that its accuracy in differentiating benign from malignant tumors is actually greater than 95%.12 In addition, false-negative rates are now consistently less than 1%.13
Furthermore, serious complications requiring clinical intervention or hospitalization occur in fewer than 1% of cases. Seeding of the needle tract with tumor cells, which was another concern, is also exceedingly rare for these small, well-circumscribed renal masses.12
Overall morbidity is low with renal mass sampling, which is routinely performed as an outpatient procedure using CT or ultrasono-graphic guidance and local anesthesia.
However, 10% of biopsy results are still noninformative. In this situation, biopsy can be repeated, or the mass can be surgically excised, or the patient can undergo conservative management if he or she is unfit or unwilling to undergo surgery.
The encouraging results with renal mass sampling have led to greater use of it at many centers in the evaluation and risk-stratification of patients with small renal masses. It may be especially useful in patients considering several treatment options.
For example, a 75-year-old patient with modest comorbidities and a 2.0-cm enhancing renal mass could be a candidate for partial nephrectomy, thermal ablation, or active surveillance, and a reasonable argument could be made for each of these options. Renal mass sampling in this instance could be instrumental in guiding this decision, as a tissue diagnosis of high-grade renal cell carcinoma would favor partial nephrectomy, whereas a diagnosis of “oncocytoma neoplasm” would support a more conservative approach.
Older, frail patients with significant comorbidities who are unlikely to be candidates for aggressive surgical management would not need renal mass sampling, as they will ultimately be managed with active surveillance or thermal ablation.
Recent studies have also indicated that molecular profiling through gene expression analysis or proteomic analysis can further improve the accuracy of renal mass sampling.14 This will likely be the holy grail for this field, allowing for truly rational management (Figure 2).
TREATMENT OPTIONS
Radical nephrectomy: Still the most common treatment
In the past, complete removal of the kidney was standard for nearly all renal masses suspected of being renal cell carcinomas. Partial nephrectomy was generally reserved for patients who had a solitary kidney, bilateral tumors, or preexisting chronic kidney disease.
Although the two procedures provide equivalent oncologic outcomes for clinical T1 lesions, Miller et al15 reported that, before 2001, only 20% of small renal masses in the United States were managed with partial nephrectomy. That percentage has increased modestly, but radical nephrectomy still predominates.
One explanation for why the radical procedure is done more frequently is that partial nephrectomy is more technically difficult, as it involves renal reconstruction. Furthermore, radical nephrectomy can almost always be performed via a minimally invasive approach, which is inherently appealing to patients and surgeons alike. Laparoscopic radical nephrectomy has been called “the great seductress” because of these considerations.16 However, total removal of the kidney comes at a great price—loss of renal function.
Over the last decade, various studies have highlighted the association between radical nephrectomy and the subsequent clinical onset of chronic kidney disease, and the potential correlations between chronic kidney disease and cardiovascular events and elevated mortality rates.17
In a landmark study, Huang et al18 evaluated the outcomes of 662 patients who had small renal masses, a “normal” serum creatinine concentration (≤ 124 μmol/L [1.4 mg/dL]), and a normal-appearing contralateral kidney who underwent radical or partial nephrectomy. Of these, 26% were found to have preexisting stage 3 chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated using the Modification of Diet in Renal Disease equation). Additionally, 65% of patients treated with radical nephrectomy were found to have stage 3 chronic kidney disease after surgery vs 20% of patients managed with partial nephrectomy.
The misconception remains that the risk of chronic kidney disease after radical nephrectomy is insignificant, since the risk is low in renal transplant donors.19 However, renal transplant donors undergo stringent screening to ensure that their general health is good and that their renal function is robust, both of which are not true in many patients with small renal masses, particularly if they are elderly.
The overuse of radical nephrectomy is worrisome in light of the potential implications of chronic kidney disease, such as increased risk of morbid cardiovascular events and elevated mortality rates. Many experts believe that over-treatment of small renal masses may have contributed to the paradoxical increase in overall mortality rates observed with radical nephrectomy in some studies.4
Although radical nephrectomy remains an important treatment for locally advanced renal cell carcinoma, it should be performed for small renal masses only if nephron-sparing surgery is not feasible (Table 2).
Partial nephrectomy: The new gold standard for most patients
Over the last 5 years, greater emphasis has been placed on lessening the risk of chronic kidney disease in the management of all urologic conditions, including small renal masses.
The overuse of radical nephrectomy prompted the American Urological Association to commission a panel to provide guidelines for the management of clinical stage T1 renal masses.17 After an extensive review and rigorous meta-analysis, the panel concluded that partial nephrectomy is the gold standard for most patients (Table 1, Table 2).
Partial nephrectomy involves excision of the tumor with a small margin of normal tissue, preserving as much functional renal parenchyma as possible, followed by closure of the collecting system, suture ligation of any transected vessels, and reapproximation of the capsule. Tumor excision is usually performed during temporary occlusion of the renal vasculature, allowing for a bloodless field. Regional hypothermia (cold ischemia) can also be used to minimize ischemic injury.
Contemporary series have documented that partial and radical nephrectomy have comparable oncologic efficacy for patients with small renal masses.20,21 Local recurrence rates are only 1% to 2% with partial nephrectomy, and 5- and 10-year cancer-specific survival rates of 96% and 90% have been reported.22
Furthermore, some studies have shown that patients undergoing partial nephrectomy have higher overall survival rates than those managed with radical nephrectomy—perhaps in part due to greater preservation of renal function and a lower incidence of subsequent chronic kidney disease.23,24 At Cleveland Clinic, we are now studying the determinants of ultimate renal function after partial nephrectomy in an effort to minimize ischemic injury and optimize this technique.25
Complications. Partial nephrectomy does have a potential downside in that it carries a higher risk of urologic complications such as urine leak and postoperative hemorrhage, which is not surprising because it requires a reconstruction that must heal. In a recent meta-analysis, urologic complications occurred in 6.3% patients who underwent open partial nephrectomy and in 9.0% of patients who underwent laparoscopic partial nephrectomy.17 Fortunately, most complications associated with partial nephrectomy can be managed with conservative measures.
Postoperative bleeding occurs in about 1% to 2% of patients and is the most serious complication. However, it is typically managed with superselective embolization, which has a high success rate and facilitates renal preservation.
Urine leak occurs in about 3% to 5% of cases and almost always resolves with prolonged drainage, occasionally complemented with a ureteral stent to promote antegrade drainage.
A new refinement, robotic-assisted partial nephrectomy promises to reduce the morbidity of this procedure. This approach takes less time to learn than standard laparoscopic surgery and has expanded the indications for minimally invasive partial nephrectomy, although more-difficult cases are still better done through a traditional, open surgical approach.
Thermal ablation: Another minimally invasive option
Cryoablation and radiofrequency ablation (collectively called thermal ablation) have recently emerged as alternate minimally invasive treatments for small renal masses. They are appealing options for patients with small renal tumors (< 3.5 cm) who have significant comorbidities but still prefer a proactive approach. They can also be considered as salvage procedures in patients with local recurrence after partial nephrectomy or in select patients with multifocal disease.
Both procedures can be performed percutaneously or laparoscopically, offering the potential for rapid convalescence and reduced morbidity.26,27 A laparoscopic approach is necessary to mobilize the tumor from adjacent organs if they are juxtaposed, whereas a percutaneous approach is less invasive and is better suited for posterior renal masses.28 Renal mass sampling should be performed in these patients before treatment to define the histology and to guide surveillance and should be repeated postoperatively if there is suspicion of local recurrence based on imaging.
Cryoablation destroys tumor cells through rapid cycles of freezing to less than −20°C (−4°F) and thawing, which can be monitored in real time via thermocoupling (ie, a thermometer microprobe strategically placed outside the tumor to ensure that lethal temperatures are extended beyond the edge of the tumor) or via ultrasonography, or both. Treatment is continued until the “ice ball” extends about 1 cm beyond the edge of the tumor.
Initial series reported local tumor control rates in the range of 90% to 95%; however, follow-up was very limited.29 In a more robust single-institution experience,30 renal cryoablation demonstrated 5-year cancer-specific and recurrence-free survival rates of 93% and 83%, respectively, substantially lower than what would be expected with surgical excision in a similar patient population.
Another concern with cryoablation is that options are limited for surgical salvage if the initial treatment fails. Nguyen and Campbell31 reported that partial nephrectomy and minimally invasive surgery were often precluded in this situation because of the extensive fibrotic reaction caused by the prior treatment. If cryoablation fails, surgical salvage thus often requires open, radical surgery.
Radiofrequency ablation produces tumor coagulation via protein denaturation and disruption of cell membranes after heating tissues to temperatures above 50°C (122°F) for 4 to 6 minutes.32 One of its disadvantages is that one cannot monitor treatment progress in real time, as there is no identifiable change in tissue appearance analagous to the ice ball that is seen with cryoablation.
Although the outcomes of radiofrequency ablation are less robust than those of cryoablation, most studies suggest that local control is achieved in 80% to 90% of cases based on radiographic loss of enhancement after treatment.17,30,33 A recent meta-analysis comparing these treatments found that lesions treated with radiofrequency ablation had a significantly higher rate of local tumor progression than those treated with cryoablation (12.3% vs 4.7%, P < .0001).34 Both of these local recurrence rates are substantially higher than that seen after surgical excision, despite much shorter follow-up after thermal ablation.
Tempered enthusiasm. Because thermal ablation has been developed relatively recently, its long-term outcomes and treatment efficacy have not been well established, and current studies have confirmed higher local recurrence rates with thermal ablation than with surgical excision (Table 1). Furthermore, there are significant deficiencies in the literature about thermal ablation, including limited follow-up, lack of pathologic confirmation, and controversies regarding histologic or radiologic definitions of success (Table 2).
Although current enthusiasm for thermal ablation has been tempered by suboptimal results, further refinement in technique and acknowledgment of its limitations will help to define appropriate candidates for these treatments.
Active surveillance for select patients
In select patients with extensive medical comorbidities or short life expectancy, the risks associated with proactive management may outweigh the benefits, especially considering the indolent nature of many small renal masses. In such patients, active surveillance is reasonable.
A recent meta-analysis found that most small enhancing renal masses grew relatively slowly (median 0.28 cm/year) and posed a low risk of metastasis (1%–2%).17,22 Furthermore, almost all renal lesions that progressed to metastatic disease demonstrated rapid radiographic growth, suggesting that the radiographic growth of a renal mass under active surveillance may serve as an indicator for aggressive behavior.35
Unfortunately, the growth rates of small renal masses do not reliably predict malignancy, and one study reported that 83% of tumors without demonstrable growth were malignant.36
Studies of active surveillance to date have had several other important limitations. Many did not incorporate pathologic confirmation, so that about 20% of the tumors were actually benign, thus artificially reducing the risk of adverse outcomes.5,22,37 Furthermore, the follow-up has been short, with most studies including data for only 2 to 3 years, which is clearly inadequate for this type of malignancy.37,38 Finally, most series had significant selection bias towards small, homogenous masses. In general, small renal masses that appear to be more aggressive are treated and thus excluded from these surveillance populations (Table 2).
Another concern about active surveillance is the small but real risk of tumor progression to metastatic disease, rendering these patients incurable even with new, targeted molecular therapies. Additionally, some patients may lose their window of opportunity for nephron-sparing surgery if significant tumor growth occurs during observation, rendering partial nephrectomy unfeasible. Therefore, active surveillance is not advisable for young, otherwise healthy patients (Table 2).
In the future, advances in renal mass sampling with molecular profiling may help determine which renal lesions are less biologically aggressive and, thereby, help identify appropriate candidates for observation (Figure 2).
- Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J 2008; 14:288–301.
- Lane BR, Campbell SC. Management of small renal masses. AUA Update Series 2009; 28:313–324.
- Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer 2004; 100:738–745.
- Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 2006; 98:1331–1334.
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003; 170:2217–2220.
- Russo P. Should elective partial nephrectomy be performed for renal cell carcinoma >4 cm in size? Nat Clin Pract Urol 2008; 5:482–483.
- Thomas AA, Aron M, Hernandez AV, Lane BR, Gill IS. Laparoscopic partial nephrectomy in octogenarians. Urology 2009; 74:1042–1046.
- Thompson RH, Kurta JM, Kaag M, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol 2009; 181:2033–2036.
- Mues AC, Landman J. Small renal masses: current concepts regarding the natural history and reflections on the American Urological Association guidelines. Curr Opin Urol 2010; 20:105–110.
- Patard JJ, Bensalah K, Vincendeau S, Rioux-Leclerq N, Guillé F, Lobel B. [Correlation between the mode of presentation of renal tumors and patient survival]. Prog Urol 2003; 13:23–28.
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009; 373:1119–1132.
- Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy—a renaissance? J Urol 2008; 179:20–27.
- Samplaski MK, Zhou M, Lane BR, Herts B, Campbell SC. Renal mass sampling: an enlightened perspective. Int J Urol 2011; 18:5–19.
- Tan MH, Rogers CG, Cooper JT, et al. Gene expression profiling of renal cell carcinoma. Clin Cancer Res 2004; 10:6315S–6321S.
- Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol 2006; 175:853–857.
- Lane BR, Poggio ED, Herts BR, Novick AC, Campbell SC. Renal function assessment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol 2009; 182:435–444.
- Campbell SC, Novick AC, Belldegrun A, et al; Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182:1271–1279.
- Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006; 7:735–740.
- Boorjian SA, Uzzo RG. The evolving management of small renal masses. Curr Oncol Rep 2009; 11:211–217.
- Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol 1999; 162:1930–1933.
- Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol 2000; 163:730–736.
- Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 2006; 175:425–431.
- Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol 2009; 181:55–61.
- Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol 2008; 179:468–471.
- Thomas AA, Demirjian S, Lane BR, et al. Acute kidney injury: novel biomarkers and potential utility for patient care in urology. Urology 2011; 77:5–11.
- Hinshaw JL, Shadid AM, Nakada SY, Hedican SP, Winter TC, Lee FT. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol 2008; 191:1159–1168.
- Sterrett SP, Nakada SY, Wingo MS, Williams SK, Leveillee RJ. Renal thermal ablative therapy. Urol Clin North Am 2008; 35:397–414.
- Hafron J, Kaouk JH. Ablative techniques for the management of kidney cancer. Nat Clin Pract Urol 2007; 4:261–269.
- Matin SF, Ahrar K. Nephron-sparing probe ablative therapy: longterm outcomes. Curr Opin Urol 2008; 18:150–156.
- Berger A, Kamoi K, Gill IS, Aron M. Cryoablation for renal tumors: current status. Curr Opin Urol 2009; 19:138–142.
- Nguyen CT, Campbell SC. Salvage of local recurrence after primary thermal ablation for small renal masses. Expert Rev Anticancer Ther 2008; 8:1899–1905.
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000; 174:323–331.
- Carraway WA, Raman JD, Cadeddu JA. Current status of renal radiofrequency ablation. Curr Opin Urol 2009; 19:143–147.
- Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 2008; 113:2671–2680.
- Kunkle DA, Kutikov A, Uzzo RG. Management of small renal masses. Semin Ultrasound CT MR 2009; 30:352–358.
- Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol 2007; 177:849–853.
- Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review. J Urol 2008; 179:1227–1233.
- Jewett MA, Zuniga A. Renal tumor natural history: the rationale and role for active surveillance. Urol Clin North Am 2008; 35:627–634.
- Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J 2008; 14:288–301.
- Lane BR, Campbell SC. Management of small renal masses. AUA Update Series 2009; 28:313–324.
- Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer 2004; 100:738–745.
- Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 2006; 98:1331–1334.
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003; 170:2217–2220.
- Russo P. Should elective partial nephrectomy be performed for renal cell carcinoma >4 cm in size? Nat Clin Pract Urol 2008; 5:482–483.
- Thomas AA, Aron M, Hernandez AV, Lane BR, Gill IS. Laparoscopic partial nephrectomy in octogenarians. Urology 2009; 74:1042–1046.
- Thompson RH, Kurta JM, Kaag M, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol 2009; 181:2033–2036.
- Mues AC, Landman J. Small renal masses: current concepts regarding the natural history and reflections on the American Urological Association guidelines. Curr Opin Urol 2010; 20:105–110.
- Patard JJ, Bensalah K, Vincendeau S, Rioux-Leclerq N, Guillé F, Lobel B. [Correlation between the mode of presentation of renal tumors and patient survival]. Prog Urol 2003; 13:23–28.
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009; 373:1119–1132.
- Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy—a renaissance? J Urol 2008; 179:20–27.
- Samplaski MK, Zhou M, Lane BR, Herts B, Campbell SC. Renal mass sampling: an enlightened perspective. Int J Urol 2011; 18:5–19.
- Tan MH, Rogers CG, Cooper JT, et al. Gene expression profiling of renal cell carcinoma. Clin Cancer Res 2004; 10:6315S–6321S.
- Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol 2006; 175:853–857.
- Lane BR, Poggio ED, Herts BR, Novick AC, Campbell SC. Renal function assessment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol 2009; 182:435–444.
- Campbell SC, Novick AC, Belldegrun A, et al; Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182:1271–1279.
- Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006; 7:735–740.
- Boorjian SA, Uzzo RG. The evolving management of small renal masses. Curr Oncol Rep 2009; 11:211–217.
- Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol 1999; 162:1930–1933.
- Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol 2000; 163:730–736.
- Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 2006; 175:425–431.
- Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol 2009; 181:55–61.
- Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol 2008; 179:468–471.
- Thomas AA, Demirjian S, Lane BR, et al. Acute kidney injury: novel biomarkers and potential utility for patient care in urology. Urology 2011; 77:5–11.
- Hinshaw JL, Shadid AM, Nakada SY, Hedican SP, Winter TC, Lee FT. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol 2008; 191:1159–1168.
- Sterrett SP, Nakada SY, Wingo MS, Williams SK, Leveillee RJ. Renal thermal ablative therapy. Urol Clin North Am 2008; 35:397–414.
- Hafron J, Kaouk JH. Ablative techniques for the management of kidney cancer. Nat Clin Pract Urol 2007; 4:261–269.
- Matin SF, Ahrar K. Nephron-sparing probe ablative therapy: longterm outcomes. Curr Opin Urol 2008; 18:150–156.
- Berger A, Kamoi K, Gill IS, Aron M. Cryoablation for renal tumors: current status. Curr Opin Urol 2009; 19:138–142.
- Nguyen CT, Campbell SC. Salvage of local recurrence after primary thermal ablation for small renal masses. Expert Rev Anticancer Ther 2008; 8:1899–1905.
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000; 174:323–331.
- Carraway WA, Raman JD, Cadeddu JA. Current status of renal radiofrequency ablation. Curr Opin Urol 2009; 19:143–147.
- Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 2008; 113:2671–2680.
- Kunkle DA, Kutikov A, Uzzo RG. Management of small renal masses. Semin Ultrasound CT MR 2009; 30:352–358.
- Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol 2007; 177:849–853.
- Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review. J Urol 2008; 179:1227–1233.
- Jewett MA, Zuniga A. Renal tumor natural history: the rationale and role for active surveillance. Urol Clin North Am 2008; 35:627–634.
KEY POINTS
- Small renal masses are a heterogeneous group of tumors, and only 20% are aggressive renal cell carcinoma.
- In general, nephron-sparing treatments are preferred to avoid chronic kidney disease, which often occurs after radical nephrectomy.
- Thermal ablation and active surveillance are valid treatment strategies in select patients who are not optimal surgical candidates or who have limited life expectancy.
Short-Term ADT with Radiotherapy Ups Prostate Cancer Survival
Adding short-term androgen-deprivation therapy before and during conventional radiotherapy for early localized prostate cancer confers a modest but significant increase in 10-year survival, according to a new report in the July 14 issue of the New England Journal of Medicine.
The strategy also halves disease-specific 10-year mortality, reduces the rate of biochemical failure (recurrence of elevated PSA), cuts the incidence of distant metastases, and decreases the rate of positive findings on repeat prostate biopsy after 2 years, said Dr. Christopher U. Jones of Radiological Associates of Sacramento and his associates in this Radiation Therapy Oncology Group (RTOG) phase III clinical trial.
These benefits were most pronounced for men deemed to be at intermediate risk at diagnosis, they noted.
The RTOG initiated the international randomized trial because little is known about the role of short-term androgen-deprivation therapy (ADT) for nonbulky localized tumors. Study subjects comprised 1,979 men with stage T1b, T1c, T2a, or T2b prostate adenocarcinomas and an initial PSA level of 20 ng per milliliter or lower.
Patients were randomly assigned to standard radiotherapy alone (992 subjects) or radiotherapy plus three-times-daily flutamide and either subcutaneous goserelin or intramuscular leuprolide beginning 2 months before initiation of radiotherapy and continuing through 2 months of radiotherapy (987 subjects).
The primary end point was overall survival after a sufficiently long interval had passed to allow for recurrence of this often indolent cancer. The 10-year overall survival was 62% with added ADT, compared with 57% with radiotherapy alone.
The 10-year disease-specific mortality was 4% with combined therapy and 8% with radiotherapy alone. The 10-year rate of biochemical failure was 26% with combined therapy and 41% with radiotherapy alone. The 10-year cumulative incidence of distant metastases was 6% with combined therapy and 8% with radiotherapy alone.
Approximately 45% of the study population underwent repeat prostate biopsy after 2 years. Persistent cancer was detected in 20% of the specimens from men who had received radiotherapy plus ADT, compared with 39% of the men who had received radiotherapy alone.
A subgroup analysis showed that adding ADT to radiotherapy was most beneficial for men who had been considered to be at intermediate risk at baseline; they comprised slightly more than half of the study subjects. In this subgroup of 524 men who received combination therapy, 10-year overall survival was 61% (vs. 54% in the 544 intermediate-risk men who received radiotherapy alone) and 10-year disease-specific mortality was 3% (vs. 10%).
Low-risk men did not show these benefits when ADT was added to radiotherapy, but ADT did significantly decrease the incidence of biochemical failure and the rate of positive results on repeat biopsy in low-risk men. "It is conceivable that in patients with indolent disease, longer follow-up is required to show a benefit with respect to the disease-specific mortality and overall survival rates," Dr. Jones and his colleagues said (N. Engl. J. Med. 2011;365:107-18).
For the small number of subjects (11% of both study groups) considered to be at high risk at baseline, adding short-term ADT to radiotherapy did not appear to be beneficial. This comparison, however, may have been underpowered. It also may be that, as previous clinical trials have suggested, more than 4 months of ADT is required for maximum benefit in this population, the investigators added.
In all, 395 of the study subjects were black, and black men showed similar benefits from short-term ADT as did white men. Adding ADT decreased the 10-year disease-specific mortality from 7% to 5% and cut the 10-year rate of biochemical failure from 40% to 19% in black men. Overall survival was worse among black men compared with white, but disease-specific mortality was similar.
Acute and late radiation-induced toxic effects were similar between subjects who received radiotherapy alone and those who received radiotherapy plus ADT. The rate of grade 3 or higher toxic effects related to ADT was less than 5%.
Given that the addition of ADT may not be as beneficial for low-risk patients, the treatment’s toxic effects, which do affect quality of life, may tip the balance against using this approach in low-risk men. Hot flashes and erectile dysfunction were more common with ADT, and previous studies have suggested that such erectile dysfunction may be less responsive to interventions than after radiotherapy alone. Moreover, other studies have reported that even short-term ADT can cause measurable muscle loss, fat accumulation, decreased insulin sensitivity, and increases in cholesterol and triglyceride levels, Dr. Jones and his associates said.
They noted that radiotherapy techniques have changed somewhat since this study was initiated, and intensity-modulated radiotherapy, low-dose-rate brachytherapy, and high-dose-rate brachytherapy now allow "the safe delivery of higher doses of radiation than was possible when this study was conducted." The value of adding short-term ADT to these techniques is not yet known but is currently being studied in another RTOG clinical trial, they said.
The study was supported by the National Cancer Institute and no commercial support was provided. Dr. Jones’ associates reported ties to Amgen, Ferring, GlaxoSmithKline, Eli Lilly, Calypso Medical, and Varian.
The findings by Dr. Christopher U. Jones and his colleagues make it seem reasonable to conclude that hormonal therapy is not indicated in men with low-risk prostate cancer, said Dr. Anthony V. D’Amico.
However, further study is needed to determine whether it may be worthwhile in men deemed low risk who have one adverse factor such as less than 50% positive findings on prostate biopsy, perineural invasion, or a PSA velocity of more than 2 ng/mL per year.
The combined regimen must be evaluated more closely in this patient subgroup and the subgroup of men who are considered to be high risk at baseline, whose numbers may have been too small in this study to allow definitive conclusions to be reached, he wrote.
Dr. D’Amico is with the department of radiation oncology at Brigham and Women’s Hospital and the Dana-Farber Cancer Institute, Boston. He reported no financial conflicts of interest. These remarks were adapted from his editorial accompanying Dr. Jones’ report (N. Engl. J. Med. 2011;365:169-70)..
The findings by Dr. Christopher U. Jones and his colleagues make it seem reasonable to conclude that hormonal therapy is not indicated in men with low-risk prostate cancer, said Dr. Anthony V. D’Amico.
However, further study is needed to determine whether it may be worthwhile in men deemed low risk who have one adverse factor such as less than 50% positive findings on prostate biopsy, perineural invasion, or a PSA velocity of more than 2 ng/mL per year.
The combined regimen must be evaluated more closely in this patient subgroup and the subgroup of men who are considered to be high risk at baseline, whose numbers may have been too small in this study to allow definitive conclusions to be reached, he wrote.
Dr. D’Amico is with the department of radiation oncology at Brigham and Women’s Hospital and the Dana-Farber Cancer Institute, Boston. He reported no financial conflicts of interest. These remarks were adapted from his editorial accompanying Dr. Jones’ report (N. Engl. J. Med. 2011;365:169-70)..
The findings by Dr. Christopher U. Jones and his colleagues make it seem reasonable to conclude that hormonal therapy is not indicated in men with low-risk prostate cancer, said Dr. Anthony V. D’Amico.
However, further study is needed to determine whether it may be worthwhile in men deemed low risk who have one adverse factor such as less than 50% positive findings on prostate biopsy, perineural invasion, or a PSA velocity of more than 2 ng/mL per year.
The combined regimen must be evaluated more closely in this patient subgroup and the subgroup of men who are considered to be high risk at baseline, whose numbers may have been too small in this study to allow definitive conclusions to be reached, he wrote.
Dr. D’Amico is with the department of radiation oncology at Brigham and Women’s Hospital and the Dana-Farber Cancer Institute, Boston. He reported no financial conflicts of interest. These remarks were adapted from his editorial accompanying Dr. Jones’ report (N. Engl. J. Med. 2011;365:169-70)..
Adding short-term androgen-deprivation therapy before and during conventional radiotherapy for early localized prostate cancer confers a modest but significant increase in 10-year survival, according to a new report in the July 14 issue of the New England Journal of Medicine.
The strategy also halves disease-specific 10-year mortality, reduces the rate of biochemical failure (recurrence of elevated PSA), cuts the incidence of distant metastases, and decreases the rate of positive findings on repeat prostate biopsy after 2 years, said Dr. Christopher U. Jones of Radiological Associates of Sacramento and his associates in this Radiation Therapy Oncology Group (RTOG) phase III clinical trial.
These benefits were most pronounced for men deemed to be at intermediate risk at diagnosis, they noted.
The RTOG initiated the international randomized trial because little is known about the role of short-term androgen-deprivation therapy (ADT) for nonbulky localized tumors. Study subjects comprised 1,979 men with stage T1b, T1c, T2a, or T2b prostate adenocarcinomas and an initial PSA level of 20 ng per milliliter or lower.
Patients were randomly assigned to standard radiotherapy alone (992 subjects) or radiotherapy plus three-times-daily flutamide and either subcutaneous goserelin or intramuscular leuprolide beginning 2 months before initiation of radiotherapy and continuing through 2 months of radiotherapy (987 subjects).
The primary end point was overall survival after a sufficiently long interval had passed to allow for recurrence of this often indolent cancer. The 10-year overall survival was 62% with added ADT, compared with 57% with radiotherapy alone.
The 10-year disease-specific mortality was 4% with combined therapy and 8% with radiotherapy alone. The 10-year rate of biochemical failure was 26% with combined therapy and 41% with radiotherapy alone. The 10-year cumulative incidence of distant metastases was 6% with combined therapy and 8% with radiotherapy alone.
Approximately 45% of the study population underwent repeat prostate biopsy after 2 years. Persistent cancer was detected in 20% of the specimens from men who had received radiotherapy plus ADT, compared with 39% of the men who had received radiotherapy alone.
A subgroup analysis showed that adding ADT to radiotherapy was most beneficial for men who had been considered to be at intermediate risk at baseline; they comprised slightly more than half of the study subjects. In this subgroup of 524 men who received combination therapy, 10-year overall survival was 61% (vs. 54% in the 544 intermediate-risk men who received radiotherapy alone) and 10-year disease-specific mortality was 3% (vs. 10%).
Low-risk men did not show these benefits when ADT was added to radiotherapy, but ADT did significantly decrease the incidence of biochemical failure and the rate of positive results on repeat biopsy in low-risk men. "It is conceivable that in patients with indolent disease, longer follow-up is required to show a benefit with respect to the disease-specific mortality and overall survival rates," Dr. Jones and his colleagues said (N. Engl. J. Med. 2011;365:107-18).
For the small number of subjects (11% of both study groups) considered to be at high risk at baseline, adding short-term ADT to radiotherapy did not appear to be beneficial. This comparison, however, may have been underpowered. It also may be that, as previous clinical trials have suggested, more than 4 months of ADT is required for maximum benefit in this population, the investigators added.
In all, 395 of the study subjects were black, and black men showed similar benefits from short-term ADT as did white men. Adding ADT decreased the 10-year disease-specific mortality from 7% to 5% and cut the 10-year rate of biochemical failure from 40% to 19% in black men. Overall survival was worse among black men compared with white, but disease-specific mortality was similar.
Acute and late radiation-induced toxic effects were similar between subjects who received radiotherapy alone and those who received radiotherapy plus ADT. The rate of grade 3 or higher toxic effects related to ADT was less than 5%.
Given that the addition of ADT may not be as beneficial for low-risk patients, the treatment’s toxic effects, which do affect quality of life, may tip the balance against using this approach in low-risk men. Hot flashes and erectile dysfunction were more common with ADT, and previous studies have suggested that such erectile dysfunction may be less responsive to interventions than after radiotherapy alone. Moreover, other studies have reported that even short-term ADT can cause measurable muscle loss, fat accumulation, decreased insulin sensitivity, and increases in cholesterol and triglyceride levels, Dr. Jones and his associates said.
They noted that radiotherapy techniques have changed somewhat since this study was initiated, and intensity-modulated radiotherapy, low-dose-rate brachytherapy, and high-dose-rate brachytherapy now allow "the safe delivery of higher doses of radiation than was possible when this study was conducted." The value of adding short-term ADT to these techniques is not yet known but is currently being studied in another RTOG clinical trial, they said.
The study was supported by the National Cancer Institute and no commercial support was provided. Dr. Jones’ associates reported ties to Amgen, Ferring, GlaxoSmithKline, Eli Lilly, Calypso Medical, and Varian.
Adding short-term androgen-deprivation therapy before and during conventional radiotherapy for early localized prostate cancer confers a modest but significant increase in 10-year survival, according to a new report in the July 14 issue of the New England Journal of Medicine.
The strategy also halves disease-specific 10-year mortality, reduces the rate of biochemical failure (recurrence of elevated PSA), cuts the incidence of distant metastases, and decreases the rate of positive findings on repeat prostate biopsy after 2 years, said Dr. Christopher U. Jones of Radiological Associates of Sacramento and his associates in this Radiation Therapy Oncology Group (RTOG) phase III clinical trial.
These benefits were most pronounced for men deemed to be at intermediate risk at diagnosis, they noted.
The RTOG initiated the international randomized trial because little is known about the role of short-term androgen-deprivation therapy (ADT) for nonbulky localized tumors. Study subjects comprised 1,979 men with stage T1b, T1c, T2a, or T2b prostate adenocarcinomas and an initial PSA level of 20 ng per milliliter or lower.
Patients were randomly assigned to standard radiotherapy alone (992 subjects) or radiotherapy plus three-times-daily flutamide and either subcutaneous goserelin or intramuscular leuprolide beginning 2 months before initiation of radiotherapy and continuing through 2 months of radiotherapy (987 subjects).
The primary end point was overall survival after a sufficiently long interval had passed to allow for recurrence of this often indolent cancer. The 10-year overall survival was 62% with added ADT, compared with 57% with radiotherapy alone.
The 10-year disease-specific mortality was 4% with combined therapy and 8% with radiotherapy alone. The 10-year rate of biochemical failure was 26% with combined therapy and 41% with radiotherapy alone. The 10-year cumulative incidence of distant metastases was 6% with combined therapy and 8% with radiotherapy alone.
Approximately 45% of the study population underwent repeat prostate biopsy after 2 years. Persistent cancer was detected in 20% of the specimens from men who had received radiotherapy plus ADT, compared with 39% of the men who had received radiotherapy alone.
A subgroup analysis showed that adding ADT to radiotherapy was most beneficial for men who had been considered to be at intermediate risk at baseline; they comprised slightly more than half of the study subjects. In this subgroup of 524 men who received combination therapy, 10-year overall survival was 61% (vs. 54% in the 544 intermediate-risk men who received radiotherapy alone) and 10-year disease-specific mortality was 3% (vs. 10%).
Low-risk men did not show these benefits when ADT was added to radiotherapy, but ADT did significantly decrease the incidence of biochemical failure and the rate of positive results on repeat biopsy in low-risk men. "It is conceivable that in patients with indolent disease, longer follow-up is required to show a benefit with respect to the disease-specific mortality and overall survival rates," Dr. Jones and his colleagues said (N. Engl. J. Med. 2011;365:107-18).
For the small number of subjects (11% of both study groups) considered to be at high risk at baseline, adding short-term ADT to radiotherapy did not appear to be beneficial. This comparison, however, may have been underpowered. It also may be that, as previous clinical trials have suggested, more than 4 months of ADT is required for maximum benefit in this population, the investigators added.
In all, 395 of the study subjects were black, and black men showed similar benefits from short-term ADT as did white men. Adding ADT decreased the 10-year disease-specific mortality from 7% to 5% and cut the 10-year rate of biochemical failure from 40% to 19% in black men. Overall survival was worse among black men compared with white, but disease-specific mortality was similar.
Acute and late radiation-induced toxic effects were similar between subjects who received radiotherapy alone and those who received radiotherapy plus ADT. The rate of grade 3 or higher toxic effects related to ADT was less than 5%.
Given that the addition of ADT may not be as beneficial for low-risk patients, the treatment’s toxic effects, which do affect quality of life, may tip the balance against using this approach in low-risk men. Hot flashes and erectile dysfunction were more common with ADT, and previous studies have suggested that such erectile dysfunction may be less responsive to interventions than after radiotherapy alone. Moreover, other studies have reported that even short-term ADT can cause measurable muscle loss, fat accumulation, decreased insulin sensitivity, and increases in cholesterol and triglyceride levels, Dr. Jones and his associates said.
They noted that radiotherapy techniques have changed somewhat since this study was initiated, and intensity-modulated radiotherapy, low-dose-rate brachytherapy, and high-dose-rate brachytherapy now allow "the safe delivery of higher doses of radiation than was possible when this study was conducted." The value of adding short-term ADT to these techniques is not yet known but is currently being studied in another RTOG clinical trial, they said.
The study was supported by the National Cancer Institute and no commercial support was provided. Dr. Jones’ associates reported ties to Amgen, Ferring, GlaxoSmithKline, Eli Lilly, Calypso Medical, and Varian.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: Adding short-term androgen-deprivation therapy to standard radiotherapy raised overall survival at 10 years from 57% to 62%, cut disease-specific mortality from 8% to 4%, lowered biochemical failure from 41% to 26%, and decreased the incidence of distant metastases from 8% to 6%.
Data Source: An international phase III randomized controlled trial comparing conventional radiotherapy with radiotherapy plus short-term ADT in 1,979 men with early localized prostate cancer who were followed for a minimum of 9 years.
Disclosures: The study was supported by the National Cancer Institute and no commercial support was provided. Dr. Jones’ associates reported ties to Amgen, Ferring, GlaxoSmithKline, Eli Lilly, Calypso Medical, and Varian.
Discordant Antibiotic Therapy for UTI Stretches Hospital Stays
DENVER – Discordant antibiotic therapy occurred in 11% of a large series of children admitted for urinary tract infection at five major freestanding children’s hospitals.
Antibiotic discordance occurs when the causative organism demonstrates in vitro nonsusceptibility to the empiric antibiotic therapy that was administered before the urine culture results became available. In this five-hospital study, discordant antibiotic therapy was associated with a significantly increased length of stay, Dr. Karen E. Jerardi reported at the annual meeting of the Pediatric Academic Societies.
"For most of the cases of antibiotic discordance, the antibiotics were clinically appropriate in the setting of presumed [urinary tract infection], but the bacteria themselves were more resistant," said Dr. Jerardi of Cincinnati Children’s Hospital.
The implication is: Know your local uropathogen resistance patterns and align the initial empiric antibiotic therapy accordingly, she said.
The study involved 192 patients aged 3 days to 18 years who were hospitalized for urinary tract infection (UTI), with a median 3-day length of stay. The major uropathogens identified were Escherichia coli in 66% of cases, Klebsiella species in 11%, Enterococcus species in 6%, and Pseudomonas species in 5%. Mixed-organism UTIs accounted for 5% of the total.
The most common initial antibiotics were third-generation cephalosporins in 39% of cases, followed by ampicillin plus a third- or fourth-generation cephalosporin in 16%, and ampicillin plus gentamicin in 11%, with other agents being employed in the low single digits.
Antibiotic discordance was most frequent in UTIs caused by Klebsiella species, with a 7% rate. The other causative organisms where antibiotic discordance was common were mixed-organism infections, with a 5% antibiotic discordance rate, and E. coli and enterococcus, each with a 3% rate.
There were no significant differences between the concordant and discordant groups in terms of patient age, sex, chronic care conditions, presence of vesicoureteral reflux, or the use of prophylactic antibiotics. In a multivariate linear regression analysis adjusted for these factors, length of stay was a median 1.8 days shorter for patients treated initially with a concordant antibiotic. For the two-thirds of patients with an E. coli UTI, concordant antibiotic therapy was associated with a 3.1-day shorter stay.
As UTI is a common condition – accounting for 2% of all pediatric hospitalizations – selecting an initial antibiotic based on local uropathogen resistance patterns could result in significant cost savings as well as reduced exposure to unnecessary antibiotics, Dr. Jerardi observed.
She said she had no relevant financial disclosures.
Antibiotic discordance, UTI, Dr. Karen E. Jerardi, the Pediatric Academic Societies, bacteria, uropathogen resistance patterns, empiric antibiotic therapy, uropathogens, Escherichia coli, Klebsiella, Enterococcus, Pseudomonas, cephalosporins, ampicillin,
DENVER – Discordant antibiotic therapy occurred in 11% of a large series of children admitted for urinary tract infection at five major freestanding children’s hospitals.
Antibiotic discordance occurs when the causative organism demonstrates in vitro nonsusceptibility to the empiric antibiotic therapy that was administered before the urine culture results became available. In this five-hospital study, discordant antibiotic therapy was associated with a significantly increased length of stay, Dr. Karen E. Jerardi reported at the annual meeting of the Pediatric Academic Societies.
"For most of the cases of antibiotic discordance, the antibiotics were clinically appropriate in the setting of presumed [urinary tract infection], but the bacteria themselves were more resistant," said Dr. Jerardi of Cincinnati Children’s Hospital.
The implication is: Know your local uropathogen resistance patterns and align the initial empiric antibiotic therapy accordingly, she said.
The study involved 192 patients aged 3 days to 18 years who were hospitalized for urinary tract infection (UTI), with a median 3-day length of stay. The major uropathogens identified were Escherichia coli in 66% of cases, Klebsiella species in 11%, Enterococcus species in 6%, and Pseudomonas species in 5%. Mixed-organism UTIs accounted for 5% of the total.
The most common initial antibiotics were third-generation cephalosporins in 39% of cases, followed by ampicillin plus a third- or fourth-generation cephalosporin in 16%, and ampicillin plus gentamicin in 11%, with other agents being employed in the low single digits.
Antibiotic discordance was most frequent in UTIs caused by Klebsiella species, with a 7% rate. The other causative organisms where antibiotic discordance was common were mixed-organism infections, with a 5% antibiotic discordance rate, and E. coli and enterococcus, each with a 3% rate.
There were no significant differences between the concordant and discordant groups in terms of patient age, sex, chronic care conditions, presence of vesicoureteral reflux, or the use of prophylactic antibiotics. In a multivariate linear regression analysis adjusted for these factors, length of stay was a median 1.8 days shorter for patients treated initially with a concordant antibiotic. For the two-thirds of patients with an E. coli UTI, concordant antibiotic therapy was associated with a 3.1-day shorter stay.
As UTI is a common condition – accounting for 2% of all pediatric hospitalizations – selecting an initial antibiotic based on local uropathogen resistance patterns could result in significant cost savings as well as reduced exposure to unnecessary antibiotics, Dr. Jerardi observed.
She said she had no relevant financial disclosures.
DENVER – Discordant antibiotic therapy occurred in 11% of a large series of children admitted for urinary tract infection at five major freestanding children’s hospitals.
Antibiotic discordance occurs when the causative organism demonstrates in vitro nonsusceptibility to the empiric antibiotic therapy that was administered before the urine culture results became available. In this five-hospital study, discordant antibiotic therapy was associated with a significantly increased length of stay, Dr. Karen E. Jerardi reported at the annual meeting of the Pediatric Academic Societies.
"For most of the cases of antibiotic discordance, the antibiotics were clinically appropriate in the setting of presumed [urinary tract infection], but the bacteria themselves were more resistant," said Dr. Jerardi of Cincinnati Children’s Hospital.
The implication is: Know your local uropathogen resistance patterns and align the initial empiric antibiotic therapy accordingly, she said.
The study involved 192 patients aged 3 days to 18 years who were hospitalized for urinary tract infection (UTI), with a median 3-day length of stay. The major uropathogens identified were Escherichia coli in 66% of cases, Klebsiella species in 11%, Enterococcus species in 6%, and Pseudomonas species in 5%. Mixed-organism UTIs accounted for 5% of the total.
The most common initial antibiotics were third-generation cephalosporins in 39% of cases, followed by ampicillin plus a third- or fourth-generation cephalosporin in 16%, and ampicillin plus gentamicin in 11%, with other agents being employed in the low single digits.
Antibiotic discordance was most frequent in UTIs caused by Klebsiella species, with a 7% rate. The other causative organisms where antibiotic discordance was common were mixed-organism infections, with a 5% antibiotic discordance rate, and E. coli and enterococcus, each with a 3% rate.
There were no significant differences between the concordant and discordant groups in terms of patient age, sex, chronic care conditions, presence of vesicoureteral reflux, or the use of prophylactic antibiotics. In a multivariate linear regression analysis adjusted for these factors, length of stay was a median 1.8 days shorter for patients treated initially with a concordant antibiotic. For the two-thirds of patients with an E. coli UTI, concordant antibiotic therapy was associated with a 3.1-day shorter stay.
As UTI is a common condition – accounting for 2% of all pediatric hospitalizations – selecting an initial antibiotic based on local uropathogen resistance patterns could result in significant cost savings as well as reduced exposure to unnecessary antibiotics, Dr. Jerardi observed.
She said she had no relevant financial disclosures.
Antibiotic discordance, UTI, Dr. Karen E. Jerardi, the Pediatric Academic Societies, bacteria, uropathogen resistance patterns, empiric antibiotic therapy, uropathogens, Escherichia coli, Klebsiella, Enterococcus, Pseudomonas, cephalosporins, ampicillin,
Antibiotic discordance, UTI, Dr. Karen E. Jerardi, the Pediatric Academic Societies, bacteria, uropathogen resistance patterns, empiric antibiotic therapy, uropathogens, Escherichia coli, Klebsiella, Enterococcus, Pseudomonas, cephalosporins, ampicillin,
FROM THE ANNUAL MEETING OF THE PEDIATRIC ACADEMIC SOCIETIES
Discordant Antibiotic Therapy for UTI Stretches Hospital Stays
DENVER – Discordant antibiotic therapy occurred in 11% of a large series of children admitted for urinary tract infection at five major freestanding children’s hospitals.
Antibiotic discordance occurs when the causative organism demonstrates in vitro nonsusceptibility to the empiric antibiotic therapy that was administered before the urine culture results became available. In this five-hospital study, discordant antibiotic therapy was associated with a significantly increased length of stay, Dr. Karen E. Jerardi reported at the annual meeting of the Pediatric Academic Societies.
"For most of the cases of antibiotic discordance, the antibiotics were clinically appropriate in the setting of presumed [urinary tract infection], but the bacteria themselves were more resistant," said Dr. Jerardi of Cincinnati Children’s Hospital.
The implication is: Know your local uropathogen resistance patterns and align the initial empiric antibiotic therapy accordingly, she said.
The study involved 192 patients aged 3 days to 18 years who were hospitalized for urinary tract infection (UTI), with a median 3-day length of stay. The major uropathogens identified were Escherichia coli in 66% of cases, Klebsiella species in 11%, Enterococcus species in 6%, and Pseudomonas species in 5%. Mixed-organism UTIs accounted for 5% of the total.
The most common initial antibiotics were third-generation cephalosporins in 39% of cases, followed by ampicillin plus a third- or fourth-generation cephalosporin in 16%, and ampicillin plus gentamicin in 11%, with other agents being employed in the low single digits.
Antibiotic discordance was most frequent in UTIs caused by Klebsiella species, with a 7% rate. The other causative organisms where antibiotic discordance was common were mixed-organism infections, with a 5% antibiotic discordance rate, and E. coli and enterococcus, each with a 3% rate.
There were no significant differences between the concordant and discordant groups in terms of patient age, sex, chronic care conditions, presence of vesicoureteral reflux, or the use of prophylactic antibiotics. In a multivariate linear regression analysis adjusted for these factors, length of stay was a median 1.8 days shorter for patients treated initially with a concordant antibiotic. For the two-thirds of patients with an E. coli UTI, concordant antibiotic therapy was associated with a 3.1-day shorter stay.
As UTI is a common condition – accounting for 2% of all pediatric hospitalizations – selecting an initial antibiotic based on local uropathogen resistance patterns could result in significant cost savings as well as reduced exposure to unnecessary antibiotics, Dr. Jerardi observed.
She said she had no relevant financial disclosures.
Antibiotic discordance, UTI, Dr. Karen E. Jerardi, the Pediatric Academic Societies, bacteria, uropathogen resistance patterns, empiric antibiotic therapy, uropathogens, Escherichia coli, Klebsiella, Enterococcus, Pseudomonas, cephalosporins, ampicillin,
DENVER – Discordant antibiotic therapy occurred in 11% of a large series of children admitted for urinary tract infection at five major freestanding children’s hospitals.
Antibiotic discordance occurs when the causative organism demonstrates in vitro nonsusceptibility to the empiric antibiotic therapy that was administered before the urine culture results became available. In this five-hospital study, discordant antibiotic therapy was associated with a significantly increased length of stay, Dr. Karen E. Jerardi reported at the annual meeting of the Pediatric Academic Societies.
"For most of the cases of antibiotic discordance, the antibiotics were clinically appropriate in the setting of presumed [urinary tract infection], but the bacteria themselves were more resistant," said Dr. Jerardi of Cincinnati Children’s Hospital.
The implication is: Know your local uropathogen resistance patterns and align the initial empiric antibiotic therapy accordingly, she said.
The study involved 192 patients aged 3 days to 18 years who were hospitalized for urinary tract infection (UTI), with a median 3-day length of stay. The major uropathogens identified were Escherichia coli in 66% of cases, Klebsiella species in 11%, Enterococcus species in 6%, and Pseudomonas species in 5%. Mixed-organism UTIs accounted for 5% of the total.
The most common initial antibiotics were third-generation cephalosporins in 39% of cases, followed by ampicillin plus a third- or fourth-generation cephalosporin in 16%, and ampicillin plus gentamicin in 11%, with other agents being employed in the low single digits.
Antibiotic discordance was most frequent in UTIs caused by Klebsiella species, with a 7% rate. The other causative organisms where antibiotic discordance was common were mixed-organism infections, with a 5% antibiotic discordance rate, and E. coli and enterococcus, each with a 3% rate.
There were no significant differences between the concordant and discordant groups in terms of patient age, sex, chronic care conditions, presence of vesicoureteral reflux, or the use of prophylactic antibiotics. In a multivariate linear regression analysis adjusted for these factors, length of stay was a median 1.8 days shorter for patients treated initially with a concordant antibiotic. For the two-thirds of patients with an E. coli UTI, concordant antibiotic therapy was associated with a 3.1-day shorter stay.
As UTI is a common condition – accounting for 2% of all pediatric hospitalizations – selecting an initial antibiotic based on local uropathogen resistance patterns could result in significant cost savings as well as reduced exposure to unnecessary antibiotics, Dr. Jerardi observed.
She said she had no relevant financial disclosures.
DENVER – Discordant antibiotic therapy occurred in 11% of a large series of children admitted for urinary tract infection at five major freestanding children’s hospitals.
Antibiotic discordance occurs when the causative organism demonstrates in vitro nonsusceptibility to the empiric antibiotic therapy that was administered before the urine culture results became available. In this five-hospital study, discordant antibiotic therapy was associated with a significantly increased length of stay, Dr. Karen E. Jerardi reported at the annual meeting of the Pediatric Academic Societies.
"For most of the cases of antibiotic discordance, the antibiotics were clinically appropriate in the setting of presumed [urinary tract infection], but the bacteria themselves were more resistant," said Dr. Jerardi of Cincinnati Children’s Hospital.
The implication is: Know your local uropathogen resistance patterns and align the initial empiric antibiotic therapy accordingly, she said.
The study involved 192 patients aged 3 days to 18 years who were hospitalized for urinary tract infection (UTI), with a median 3-day length of stay. The major uropathogens identified were Escherichia coli in 66% of cases, Klebsiella species in 11%, Enterococcus species in 6%, and Pseudomonas species in 5%. Mixed-organism UTIs accounted for 5% of the total.
The most common initial antibiotics were third-generation cephalosporins in 39% of cases, followed by ampicillin plus a third- or fourth-generation cephalosporin in 16%, and ampicillin plus gentamicin in 11%, with other agents being employed in the low single digits.
Antibiotic discordance was most frequent in UTIs caused by Klebsiella species, with a 7% rate. The other causative organisms where antibiotic discordance was common were mixed-organism infections, with a 5% antibiotic discordance rate, and E. coli and enterococcus, each with a 3% rate.
There were no significant differences between the concordant and discordant groups in terms of patient age, sex, chronic care conditions, presence of vesicoureteral reflux, or the use of prophylactic antibiotics. In a multivariate linear regression analysis adjusted for these factors, length of stay was a median 1.8 days shorter for patients treated initially with a concordant antibiotic. For the two-thirds of patients with an E. coli UTI, concordant antibiotic therapy was associated with a 3.1-day shorter stay.
As UTI is a common condition – accounting for 2% of all pediatric hospitalizations – selecting an initial antibiotic based on local uropathogen resistance patterns could result in significant cost savings as well as reduced exposure to unnecessary antibiotics, Dr. Jerardi observed.
She said she had no relevant financial disclosures.
Antibiotic discordance, UTI, Dr. Karen E. Jerardi, the Pediatric Academic Societies, bacteria, uropathogen resistance patterns, empiric antibiotic therapy, uropathogens, Escherichia coli, Klebsiella, Enterococcus, Pseudomonas, cephalosporins, ampicillin,
Antibiotic discordance, UTI, Dr. Karen E. Jerardi, the Pediatric Academic Societies, bacteria, uropathogen resistance patterns, empiric antibiotic therapy, uropathogens, Escherichia coli, Klebsiella, Enterococcus, Pseudomonas, cephalosporins, ampicillin,
FROM THE ANNUAL MEETING OF THE PEDIATRIC ACADEMIC SOCIETIES
Major Finding: Discordant antibiotic therapy occurred in 11% of children admitted for urinary tract infection.
Data Source: A series of 192 patients aged 3 days to 18 years who were hospitalized for UTI at five children’s hospitals.
Disclosures: Dr. Jerardi reported having no relevant financial disclosures.
FDA Tightens ESA Dosing Recommendations for CKD Anemia
The Food and Drug Administration on June 24 issued more conservative dosing guidelines for the use of erythropoiesis-stimulating agents to treat anemia in chronic kidney disease patients who face increased risks of cardiovascular events associated with these drugs.
The new recommendations do away with a targeted range of 10-12 g/dL in hemoglobin levels in patients with chronic kidney disease (CKD). Instead, they set ceilings of 10 to 11 g/dL, depending on whether or not the anemic patient is on dialysis.
Clinicians should "individualize dosing and use the lowest dose of ESA [erythropoiesis-stimulating agent] sufficient to reduce the need for red blood cell transfusions," the agency said. The label recommends that dosing be adjusted "as appropriate."
Based on dialysis status, the new recommendations are as follows:
• If the patient is not on dialysis, the FDA advises that clinicians "consider" starting treatment with an ESA when a patient’s hemoglobin level drops below 10 g/dL "and when certain other considerations apply," for instance, if the rate of hemoglobin decline indicates that a transfusion will likely be needed.
The recommendation "does not define how far below 10 g/dL is appropriate for an individual to initiate," the agency said. It calls on clinicians to "reduce or interrupt the dose of ESA" if the patient’s level goes above 10 g/dL.
• If the patient is on dialysis, the FDA says to start ESA treatment when the patient’s hemoglobin level goes below 10 g/dL. In this case, the threshold for reducing or interrupting the ESA dose is when the hemoglobin level "approaches or exceeds 11 g/dL."
Previously, the recommendation was to dose ESAs to achieve and maintain hemoglobin levels in the target range of 10-12 g/dL; this has been removed from ESA labels. Targeting the hemoglobin level to above 11 g/dL has been found to increase the risk of myocardial infarction, stroke, and other serious adverse cardiovascular events in patients with CKD, and "has not been shown to provide additional patient benefit," the FDA statement said.
ESAs currently available in the United States are epoetin alfa (Epogen and Procrit), and darbepoetin alfa (Aranesp). All are manufactured by Amgen; Procrit is marketed by Centecor Ortho Biotech. These agents also are approved to treat anemia associated with cancer chemotherapy.
The intention of the new dosing guidelines and revised label is to "encourage flexibility" of dosing and to reinforce the message that "serious risks have been demonstrated" when the target is above 11 g/dL, Dr. Robert Kane, acting deputy director for safety in the Division of Hematology Products, FDA Center for Drug Evaluation and Research (CDER), said during a briefing.
He pointed out that the recommendations are different for patients with CKD who are on dialysis and those not on dialysis, because the risk-benefit profile is different for these groups.
The risks of ESAs in patients with CKD have been discussed at two FDA advisory panel meetings, most recently in October 2010, when an expert panel reviewed the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of darbepoetin alfa, which found an increased risk for stroke associated with a hemoglobin target of 13.0 g/dL in CKD patients not on dialysis. The new dosing recommendations have been added to the boxed warning and other sections of the label for ESAs.
Dr. Richard Pazdur, director of the FDA’s Office of Oncology Drug Products in CDER, said that the new dosing recommendations do not directly affect the use of ESAs in people with cancer, but statements included in the warnings and precautions section of the label apply to all patients who are treated with ESAs.
Amgen issued a statement that it supports the changes and is in discussion with the FDA on what additional postmarketing studies are needed.
The FDA statement and additional information are available at http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Adverse events associated with ESAs should be reported to the FDA’s MedWatch program at 800-332-1088 or http://www.fda.gov/medwatch/.
The Food and Drug Administration on June 24 issued more conservative dosing guidelines for the use of erythropoiesis-stimulating agents to treat anemia in chronic kidney disease patients who face increased risks of cardiovascular events associated with these drugs.
The new recommendations do away with a targeted range of 10-12 g/dL in hemoglobin levels in patients with chronic kidney disease (CKD). Instead, they set ceilings of 10 to 11 g/dL, depending on whether or not the anemic patient is on dialysis.
Clinicians should "individualize dosing and use the lowest dose of ESA [erythropoiesis-stimulating agent] sufficient to reduce the need for red blood cell transfusions," the agency said. The label recommends that dosing be adjusted "as appropriate."
Based on dialysis status, the new recommendations are as follows:
• If the patient is not on dialysis, the FDA advises that clinicians "consider" starting treatment with an ESA when a patient’s hemoglobin level drops below 10 g/dL "and when certain other considerations apply," for instance, if the rate of hemoglobin decline indicates that a transfusion will likely be needed.
The recommendation "does not define how far below 10 g/dL is appropriate for an individual to initiate," the agency said. It calls on clinicians to "reduce or interrupt the dose of ESA" if the patient’s level goes above 10 g/dL.
• If the patient is on dialysis, the FDA says to start ESA treatment when the patient’s hemoglobin level goes below 10 g/dL. In this case, the threshold for reducing or interrupting the ESA dose is when the hemoglobin level "approaches or exceeds 11 g/dL."
Previously, the recommendation was to dose ESAs to achieve and maintain hemoglobin levels in the target range of 10-12 g/dL; this has been removed from ESA labels. Targeting the hemoglobin level to above 11 g/dL has been found to increase the risk of myocardial infarction, stroke, and other serious adverse cardiovascular events in patients with CKD, and "has not been shown to provide additional patient benefit," the FDA statement said.
ESAs currently available in the United States are epoetin alfa (Epogen and Procrit), and darbepoetin alfa (Aranesp). All are manufactured by Amgen; Procrit is marketed by Centecor Ortho Biotech. These agents also are approved to treat anemia associated with cancer chemotherapy.
The intention of the new dosing guidelines and revised label is to "encourage flexibility" of dosing and to reinforce the message that "serious risks have been demonstrated" when the target is above 11 g/dL, Dr. Robert Kane, acting deputy director for safety in the Division of Hematology Products, FDA Center for Drug Evaluation and Research (CDER), said during a briefing.
He pointed out that the recommendations are different for patients with CKD who are on dialysis and those not on dialysis, because the risk-benefit profile is different for these groups.
The risks of ESAs in patients with CKD have been discussed at two FDA advisory panel meetings, most recently in October 2010, when an expert panel reviewed the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of darbepoetin alfa, which found an increased risk for stroke associated with a hemoglobin target of 13.0 g/dL in CKD patients not on dialysis. The new dosing recommendations have been added to the boxed warning and other sections of the label for ESAs.
Dr. Richard Pazdur, director of the FDA’s Office of Oncology Drug Products in CDER, said that the new dosing recommendations do not directly affect the use of ESAs in people with cancer, but statements included in the warnings and precautions section of the label apply to all patients who are treated with ESAs.
Amgen issued a statement that it supports the changes and is in discussion with the FDA on what additional postmarketing studies are needed.
The FDA statement and additional information are available at http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Adverse events associated with ESAs should be reported to the FDA’s MedWatch program at 800-332-1088 or http://www.fda.gov/medwatch/.
The Food and Drug Administration on June 24 issued more conservative dosing guidelines for the use of erythropoiesis-stimulating agents to treat anemia in chronic kidney disease patients who face increased risks of cardiovascular events associated with these drugs.
The new recommendations do away with a targeted range of 10-12 g/dL in hemoglobin levels in patients with chronic kidney disease (CKD). Instead, they set ceilings of 10 to 11 g/dL, depending on whether or not the anemic patient is on dialysis.
Clinicians should "individualize dosing and use the lowest dose of ESA [erythropoiesis-stimulating agent] sufficient to reduce the need for red blood cell transfusions," the agency said. The label recommends that dosing be adjusted "as appropriate."
Based on dialysis status, the new recommendations are as follows:
• If the patient is not on dialysis, the FDA advises that clinicians "consider" starting treatment with an ESA when a patient’s hemoglobin level drops below 10 g/dL "and when certain other considerations apply," for instance, if the rate of hemoglobin decline indicates that a transfusion will likely be needed.
The recommendation "does not define how far below 10 g/dL is appropriate for an individual to initiate," the agency said. It calls on clinicians to "reduce or interrupt the dose of ESA" if the patient’s level goes above 10 g/dL.
• If the patient is on dialysis, the FDA says to start ESA treatment when the patient’s hemoglobin level goes below 10 g/dL. In this case, the threshold for reducing or interrupting the ESA dose is when the hemoglobin level "approaches or exceeds 11 g/dL."
Previously, the recommendation was to dose ESAs to achieve and maintain hemoglobin levels in the target range of 10-12 g/dL; this has been removed from ESA labels. Targeting the hemoglobin level to above 11 g/dL has been found to increase the risk of myocardial infarction, stroke, and other serious adverse cardiovascular events in patients with CKD, and "has not been shown to provide additional patient benefit," the FDA statement said.
ESAs currently available in the United States are epoetin alfa (Epogen and Procrit), and darbepoetin alfa (Aranesp). All are manufactured by Amgen; Procrit is marketed by Centecor Ortho Biotech. These agents also are approved to treat anemia associated with cancer chemotherapy.
The intention of the new dosing guidelines and revised label is to "encourage flexibility" of dosing and to reinforce the message that "serious risks have been demonstrated" when the target is above 11 g/dL, Dr. Robert Kane, acting deputy director for safety in the Division of Hematology Products, FDA Center for Drug Evaluation and Research (CDER), said during a briefing.
He pointed out that the recommendations are different for patients with CKD who are on dialysis and those not on dialysis, because the risk-benefit profile is different for these groups.
The risks of ESAs in patients with CKD have been discussed at two FDA advisory panel meetings, most recently in October 2010, when an expert panel reviewed the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of darbepoetin alfa, which found an increased risk for stroke associated with a hemoglobin target of 13.0 g/dL in CKD patients not on dialysis. The new dosing recommendations have been added to the boxed warning and other sections of the label for ESAs.
Dr. Richard Pazdur, director of the FDA’s Office of Oncology Drug Products in CDER, said that the new dosing recommendations do not directly affect the use of ESAs in people with cancer, but statements included in the warnings and precautions section of the label apply to all patients who are treated with ESAs.
Amgen issued a statement that it supports the changes and is in discussion with the FDA on what additional postmarketing studies are needed.
The FDA statement and additional information are available at http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Adverse events associated with ESAs should be reported to the FDA’s MedWatch program at 800-332-1088 or http://www.fda.gov/medwatch/.
FROM THE FOOD AND DRUG ADMINISTRATION
FDA Tightens ESA Dosing Recommendations for CKD Anemia
The Food and Drug Administration on June 24 issued more conservative dosing guidelines for the use of erythropoiesis-stimulating agents to treat anemia in chronic kidney disease patients who face increased risks of cardiovascular events associated with these drugs.
The new recommendations do away with a targeted range of 10-12 g/dL in hemoglobin levels in patients with chronic kidney disease (CKD). Instead, they set ceilings of 10 to 11 g/dL, depending on whether or not the anemic patient is on dialysis.
Clinicians should "individualize dosing and use the lowest dose of ESA [erythropoiesis-stimulating agent] sufficient to reduce the need for red blood cell transfusions," the agency said. The label recommends that dosing be adjusted "as appropriate."
Based on dialysis status, the new recommendations are as follows:
• If the patient is not on dialysis, the FDA advises that clinicians "consider" starting treatment with an ESA when a patient’s hemoglobin level drops below 10 g/dL "and when certain other considerations apply," for instance, if the rate of hemoglobin decline indicates that a transfusion will likely be needed.
The recommendation "does not define how far below 10 g/dL is appropriate for an individual to initiate," the agency said. It calls on clinicians to "reduce or interrupt the dose of ESA" if the patient’s level goes above 10 g/dL.
• If the patient is on dialysis, the FDA says to start ESA treatment when the patient’s hemoglobin level goes below 10 g/dL. In this case, the threshold for reducing or interrupting the ESA dose is when the hemoglobin level "approaches or exceeds 11 g/dL."
Previously, the recommendation was to dose ESAs to achieve and maintain hemoglobin levels in the target range of 10-12 g/dL; this has been removed from ESA labels. Targeting the hemoglobin level to above 11 g/dL has been found to increase the risk of myocardial infarction, stroke, and other serious adverse cardiovascular events in patients with CKD, and "has not been shown to provide additional patient benefit," the FDA statement said.
ESAs currently available in the United States are epoetin alfa (Epogen and Procrit), and darbepoetin alfa (Aranesp). All are manufactured by Amgen; Procrit is marketed by Centecor Ortho Biotech. These agents also are approved to treat anemia associated with cancer chemotherapy.
The intention of the new dosing guidelines and revised label is to "encourage flexibility" of dosing and to reinforce the message that "serious risks have been demonstrated" when the target is above 11 g/dL, Dr. Robert Kane, acting deputy director for safety in the Division of Hematology Products, FDA Center for Drug Evaluation and Research (CDER), said during a briefing.
He pointed out that the recommendations are different for patients with CKD who are on dialysis and those not on dialysis, because the risk-benefit profile is different for these groups.
The risks of ESAs in patients with CKD have been discussed at two FDA advisory panel meetings, most recently in October 2010, when an expert panel reviewed the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of darbepoetin alfa, which found an increased risk for stroke associated with a hemoglobin target of 13.0 g/dL in CKD patients not on dialysis. The new dosing recommendations have been added to the boxed warning and other sections of the label for ESAs.
Dr. Richard Pazdur, director of the FDA’s Office of Oncology Drug Products in CDER, said that the new dosing recommendations do not directly affect the use of ESAs in people with cancer, but statements included in the warnings and precautions section of the label apply to all patients who are treated with ESAs.
Amgen issued a statement that it supports the changes and is in discussion with the FDA on what additional postmarketing studies are needed.
The FDA statement and additional information are available at http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Adverse events associated with ESAs should be reported to the FDA’s MedWatch program at 800-332-1088 or http://www.fda.gov/medwatch/.
The Food and Drug Administration on June 24 issued more conservative dosing guidelines for the use of erythropoiesis-stimulating agents to treat anemia in chronic kidney disease patients who face increased risks of cardiovascular events associated with these drugs.
The new recommendations do away with a targeted range of 10-12 g/dL in hemoglobin levels in patients with chronic kidney disease (CKD). Instead, they set ceilings of 10 to 11 g/dL, depending on whether or not the anemic patient is on dialysis.
Clinicians should "individualize dosing and use the lowest dose of ESA [erythropoiesis-stimulating agent] sufficient to reduce the need for red blood cell transfusions," the agency said. The label recommends that dosing be adjusted "as appropriate."
Based on dialysis status, the new recommendations are as follows:
• If the patient is not on dialysis, the FDA advises that clinicians "consider" starting treatment with an ESA when a patient’s hemoglobin level drops below 10 g/dL "and when certain other considerations apply," for instance, if the rate of hemoglobin decline indicates that a transfusion will likely be needed.
The recommendation "does not define how far below 10 g/dL is appropriate for an individual to initiate," the agency said. It calls on clinicians to "reduce or interrupt the dose of ESA" if the patient’s level goes above 10 g/dL.
• If the patient is on dialysis, the FDA says to start ESA treatment when the patient’s hemoglobin level goes below 10 g/dL. In this case, the threshold for reducing or interrupting the ESA dose is when the hemoglobin level "approaches or exceeds 11 g/dL."
Previously, the recommendation was to dose ESAs to achieve and maintain hemoglobin levels in the target range of 10-12 g/dL; this has been removed from ESA labels. Targeting the hemoglobin level to above 11 g/dL has been found to increase the risk of myocardial infarction, stroke, and other serious adverse cardiovascular events in patients with CKD, and "has not been shown to provide additional patient benefit," the FDA statement said.
ESAs currently available in the United States are epoetin alfa (Epogen and Procrit), and darbepoetin alfa (Aranesp). All are manufactured by Amgen; Procrit is marketed by Centecor Ortho Biotech. These agents also are approved to treat anemia associated with cancer chemotherapy.
The intention of the new dosing guidelines and revised label is to "encourage flexibility" of dosing and to reinforce the message that "serious risks have been demonstrated" when the target is above 11 g/dL, Dr. Robert Kane, acting deputy director for safety in the Division of Hematology Products, FDA Center for Drug Evaluation and Research (CDER), said during a briefing.
He pointed out that the recommendations are different for patients with CKD who are on dialysis and those not on dialysis, because the risk-benefit profile is different for these groups.
The risks of ESAs in patients with CKD have been discussed at two FDA advisory panel meetings, most recently in October 2010, when an expert panel reviewed the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of darbepoetin alfa, which found an increased risk for stroke associated with a hemoglobin target of 13.0 g/dL in CKD patients not on dialysis. The new dosing recommendations have been added to the boxed warning and other sections of the label for ESAs.
Dr. Richard Pazdur, director of the FDA’s Office of Oncology Drug Products in CDER, said that the new dosing recommendations do not directly affect the use of ESAs in people with cancer, but statements included in the warnings and precautions section of the label apply to all patients who are treated with ESAs.
Amgen issued a statement that it supports the changes and is in discussion with the FDA on what additional postmarketing studies are needed.
The FDA statement and additional information are available at http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Adverse events associated with ESAs should be reported to the FDA’s MedWatch program at 800-332-1088 or http://www.fda.gov/medwatch/.
The Food and Drug Administration on June 24 issued more conservative dosing guidelines for the use of erythropoiesis-stimulating agents to treat anemia in chronic kidney disease patients who face increased risks of cardiovascular events associated with these drugs.
The new recommendations do away with a targeted range of 10-12 g/dL in hemoglobin levels in patients with chronic kidney disease (CKD). Instead, they set ceilings of 10 to 11 g/dL, depending on whether or not the anemic patient is on dialysis.
Clinicians should "individualize dosing and use the lowest dose of ESA [erythropoiesis-stimulating agent] sufficient to reduce the need for red blood cell transfusions," the agency said. The label recommends that dosing be adjusted "as appropriate."
Based on dialysis status, the new recommendations are as follows:
• If the patient is not on dialysis, the FDA advises that clinicians "consider" starting treatment with an ESA when a patient’s hemoglobin level drops below 10 g/dL "and when certain other considerations apply," for instance, if the rate of hemoglobin decline indicates that a transfusion will likely be needed.
The recommendation "does not define how far below 10 g/dL is appropriate for an individual to initiate," the agency said. It calls on clinicians to "reduce or interrupt the dose of ESA" if the patient’s level goes above 10 g/dL.
• If the patient is on dialysis, the FDA says to start ESA treatment when the patient’s hemoglobin level goes below 10 g/dL. In this case, the threshold for reducing or interrupting the ESA dose is when the hemoglobin level "approaches or exceeds 11 g/dL."
Previously, the recommendation was to dose ESAs to achieve and maintain hemoglobin levels in the target range of 10-12 g/dL; this has been removed from ESA labels. Targeting the hemoglobin level to above 11 g/dL has been found to increase the risk of myocardial infarction, stroke, and other serious adverse cardiovascular events in patients with CKD, and "has not been shown to provide additional patient benefit," the FDA statement said.
ESAs currently available in the United States are epoetin alfa (Epogen and Procrit), and darbepoetin alfa (Aranesp). All are manufactured by Amgen; Procrit is marketed by Centecor Ortho Biotech. These agents also are approved to treat anemia associated with cancer chemotherapy.
The intention of the new dosing guidelines and revised label is to "encourage flexibility" of dosing and to reinforce the message that "serious risks have been demonstrated" when the target is above 11 g/dL, Dr. Robert Kane, acting deputy director for safety in the Division of Hematology Products, FDA Center for Drug Evaluation and Research (CDER), said during a briefing.
He pointed out that the recommendations are different for patients with CKD who are on dialysis and those not on dialysis, because the risk-benefit profile is different for these groups.
The risks of ESAs in patients with CKD have been discussed at two FDA advisory panel meetings, most recently in October 2010, when an expert panel reviewed the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of darbepoetin alfa, which found an increased risk for stroke associated with a hemoglobin target of 13.0 g/dL in CKD patients not on dialysis. The new dosing recommendations have been added to the boxed warning and other sections of the label for ESAs.
Dr. Richard Pazdur, director of the FDA’s Office of Oncology Drug Products in CDER, said that the new dosing recommendations do not directly affect the use of ESAs in people with cancer, but statements included in the warnings and precautions section of the label apply to all patients who are treated with ESAs.
Amgen issued a statement that it supports the changes and is in discussion with the FDA on what additional postmarketing studies are needed.
The FDA statement and additional information are available at http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Adverse events associated with ESAs should be reported to the FDA’s MedWatch program at 800-332-1088 or http://www.fda.gov/medwatch/.
FROM THE FOOD AND DRUG ADMINISTRATION
Managing Recurrent UTIs in the Patient With Neurogenic Bladder
WASHINGTON – How should one manage a 35-year-old woman with multiple sclerosis on self-clean intermittent catheterization who complains of pelvic pain and cloudy urine?
Such a patient with "neurogenic bladder" and possible urinary tract infection needs careful diagnosis, catheterization review, and possibly other management considerations, said Dr. Stephen R. Kraus during a panel discussion of recurrent UTIs at the annual meeting of the American Urological Association.
Patients with neurogenic bladder commonly have chronic bacteriuria and recurrent UTIs, and thus generally require a combination of bacteriuria and leukocyturia – as well as clinical symptoms or an increase in autonomic dysreflexia – for the initiation of empirical UTI therapy. Such criteria will help avoid unnecessary use of antibiotics.
"Original criteria were based on bacterial colonization counts but were criticized for being highly insensitive," said Dr. Kraus, professor and vice chairman of the department of urology at the University of Texas, San Antonio.
Assuming the patient has already had a video urodynamic test, Dr. Kraus said, he would obtain a catheterized specimen for urinalysis, culture, and a sensitivity test; treat as needed; and then consider increasing her catheterization frequency. A trial of a hydrophilic catheter could also be considered in the context of recurrent UTIs, he said.
Various catheter modifications – from silver alloy catheters to antibiotic-impregnated catheters – have been used with some success in reducing the risk of UTIs, but "they carry their own problems such as cost, development of resistance, and even, as one study suggested, the possibility of silver toxicity," Dr. Kraus said.
Two randomized, controlled trials have shown that hydrophilic catheters will reduce the risk of UTIs, compared with regular polyvinyl chloride catheters, he noted. Although the choice of single-use vs. reusable catheters is "always a point of contention," several studies have "clearly" shown that clean intermittent catheterization (CIC) poses no greater risk of recurrent UTIs than do single-use catheters, he added.
Frequent changing of intermittent catheters can prevent biofilm development, and one study showed that UTI was five times less likely when CIC was performed six times per day rather than three times per day, he noted.
Routine chronic antibiotic prophylaxis should be avoided in patients with neurogenic bladder, he said, but a short course of antibiotics could be useful during the initial CIC period, and is certainly prudent before any invasive genitourinary procedures are performed.
Dr. Kraus said he is intrigued by the concept of a weekly oral cyclic antibiotic (WOCA) program that uses weekly alternating antibiotics as a prophylactic measure. In one 2-year trial of WOCA, investigators "saw dramatic reductions in UTIs (from 9.4 to 1.8 per patient year) ... and most importantly, they did not see any change in the number of multidrug-resistant infections," he said.
As a final management option for the above-described patient, Dr. Kraus said he would consider injections of botulinum toxin (Botox). This approach "has exploded in the market for neurogenic bladder management, and it has been associated with a significant reduction in UTI at 6 months ... presumably because the neurogenic bladder management is that much better," he said.
The term "neurogenic bladder," Dr. Kraus noted, is one that’s "not very precise." For the purposes of his discussion, he defined it as a condition in which the bladder is affected by a neurologic process and has an impaired ability to store and empty urine.
Dr. Kraus disclosed that he is an investigator for the National Institute of Diabetes and Digestive and Kidney Diseases, a course director for Laborie (which manufactures catheters and other products for urinary and pelvic disorders), and a consultant/adviser for Pfizer.
WASHINGTON – How should one manage a 35-year-old woman with multiple sclerosis on self-clean intermittent catheterization who complains of pelvic pain and cloudy urine?
Such a patient with "neurogenic bladder" and possible urinary tract infection needs careful diagnosis, catheterization review, and possibly other management considerations, said Dr. Stephen R. Kraus during a panel discussion of recurrent UTIs at the annual meeting of the American Urological Association.
Patients with neurogenic bladder commonly have chronic bacteriuria and recurrent UTIs, and thus generally require a combination of bacteriuria and leukocyturia – as well as clinical symptoms or an increase in autonomic dysreflexia – for the initiation of empirical UTI therapy. Such criteria will help avoid unnecessary use of antibiotics.
"Original criteria were based on bacterial colonization counts but were criticized for being highly insensitive," said Dr. Kraus, professor and vice chairman of the department of urology at the University of Texas, San Antonio.
Assuming the patient has already had a video urodynamic test, Dr. Kraus said, he would obtain a catheterized specimen for urinalysis, culture, and a sensitivity test; treat as needed; and then consider increasing her catheterization frequency. A trial of a hydrophilic catheter could also be considered in the context of recurrent UTIs, he said.
Various catheter modifications – from silver alloy catheters to antibiotic-impregnated catheters – have been used with some success in reducing the risk of UTIs, but "they carry their own problems such as cost, development of resistance, and even, as one study suggested, the possibility of silver toxicity," Dr. Kraus said.
Two randomized, controlled trials have shown that hydrophilic catheters will reduce the risk of UTIs, compared with regular polyvinyl chloride catheters, he noted. Although the choice of single-use vs. reusable catheters is "always a point of contention," several studies have "clearly" shown that clean intermittent catheterization (CIC) poses no greater risk of recurrent UTIs than do single-use catheters, he added.
Frequent changing of intermittent catheters can prevent biofilm development, and one study showed that UTI was five times less likely when CIC was performed six times per day rather than three times per day, he noted.
Routine chronic antibiotic prophylaxis should be avoided in patients with neurogenic bladder, he said, but a short course of antibiotics could be useful during the initial CIC period, and is certainly prudent before any invasive genitourinary procedures are performed.
Dr. Kraus said he is intrigued by the concept of a weekly oral cyclic antibiotic (WOCA) program that uses weekly alternating antibiotics as a prophylactic measure. In one 2-year trial of WOCA, investigators "saw dramatic reductions in UTIs (from 9.4 to 1.8 per patient year) ... and most importantly, they did not see any change in the number of multidrug-resistant infections," he said.
As a final management option for the above-described patient, Dr. Kraus said he would consider injections of botulinum toxin (Botox). This approach "has exploded in the market for neurogenic bladder management, and it has been associated with a significant reduction in UTI at 6 months ... presumably because the neurogenic bladder management is that much better," he said.
The term "neurogenic bladder," Dr. Kraus noted, is one that’s "not very precise." For the purposes of his discussion, he defined it as a condition in which the bladder is affected by a neurologic process and has an impaired ability to store and empty urine.
Dr. Kraus disclosed that he is an investigator for the National Institute of Diabetes and Digestive and Kidney Diseases, a course director for Laborie (which manufactures catheters and other products for urinary and pelvic disorders), and a consultant/adviser for Pfizer.
WASHINGTON – How should one manage a 35-year-old woman with multiple sclerosis on self-clean intermittent catheterization who complains of pelvic pain and cloudy urine?
Such a patient with "neurogenic bladder" and possible urinary tract infection needs careful diagnosis, catheterization review, and possibly other management considerations, said Dr. Stephen R. Kraus during a panel discussion of recurrent UTIs at the annual meeting of the American Urological Association.
Patients with neurogenic bladder commonly have chronic bacteriuria and recurrent UTIs, and thus generally require a combination of bacteriuria and leukocyturia – as well as clinical symptoms or an increase in autonomic dysreflexia – for the initiation of empirical UTI therapy. Such criteria will help avoid unnecessary use of antibiotics.
"Original criteria were based on bacterial colonization counts but were criticized for being highly insensitive," said Dr. Kraus, professor and vice chairman of the department of urology at the University of Texas, San Antonio.
Assuming the patient has already had a video urodynamic test, Dr. Kraus said, he would obtain a catheterized specimen for urinalysis, culture, and a sensitivity test; treat as needed; and then consider increasing her catheterization frequency. A trial of a hydrophilic catheter could also be considered in the context of recurrent UTIs, he said.
Various catheter modifications – from silver alloy catheters to antibiotic-impregnated catheters – have been used with some success in reducing the risk of UTIs, but "they carry their own problems such as cost, development of resistance, and even, as one study suggested, the possibility of silver toxicity," Dr. Kraus said.
Two randomized, controlled trials have shown that hydrophilic catheters will reduce the risk of UTIs, compared with regular polyvinyl chloride catheters, he noted. Although the choice of single-use vs. reusable catheters is "always a point of contention," several studies have "clearly" shown that clean intermittent catheterization (CIC) poses no greater risk of recurrent UTIs than do single-use catheters, he added.
Frequent changing of intermittent catheters can prevent biofilm development, and one study showed that UTI was five times less likely when CIC was performed six times per day rather than three times per day, he noted.
Routine chronic antibiotic prophylaxis should be avoided in patients with neurogenic bladder, he said, but a short course of antibiotics could be useful during the initial CIC period, and is certainly prudent before any invasive genitourinary procedures are performed.
Dr. Kraus said he is intrigued by the concept of a weekly oral cyclic antibiotic (WOCA) program that uses weekly alternating antibiotics as a prophylactic measure. In one 2-year trial of WOCA, investigators "saw dramatic reductions in UTIs (from 9.4 to 1.8 per patient year) ... and most importantly, they did not see any change in the number of multidrug-resistant infections," he said.
As a final management option for the above-described patient, Dr. Kraus said he would consider injections of botulinum toxin (Botox). This approach "has exploded in the market for neurogenic bladder management, and it has been associated with a significant reduction in UTI at 6 months ... presumably because the neurogenic bladder management is that much better," he said.
The term "neurogenic bladder," Dr. Kraus noted, is one that’s "not very precise." For the purposes of his discussion, he defined it as a condition in which the bladder is affected by a neurologic process and has an impaired ability to store and empty urine.
Dr. Kraus disclosed that he is an investigator for the National Institute of Diabetes and Digestive and Kidney Diseases, a course director for Laborie (which manufactures catheters and other products for urinary and pelvic disorders), and a consultant/adviser for Pfizer.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN UROLOGICAL ASSOCIATION
Managing Recurrent UTIs in the Patient With Neurogenic Bladder
WASHINGTON – How should one manage a 35-year-old woman with multiple sclerosis on self-clean intermittent catheterization who complains of pelvic pain and cloudy urine?
Such a patient with "neurogenic bladder" and possible urinary tract infection needs careful diagnosis, catheterization review, and possibly other management considerations, said Dr. Stephen R. Kraus during a panel discussion of recurrent UTIs at the annual meeting of the American Urological Association.
Patients with neurogenic bladder commonly have chronic bacteriuria and recurrent UTIs, and thus generally require a combination of bacteriuria and leukocyturia – as well as clinical symptoms or an increase in autonomic dysreflexia – for the initiation of empirical UTI therapy. Such criteria will help avoid unnecessary use of antibiotics.
"Original criteria were based on bacterial colonization counts but were criticized for being highly insensitive," said Dr. Kraus, professor and vice chairman of the department of urology at the University of Texas, San Antonio.
Assuming the patient has already had a video urodynamic test, Dr. Kraus said, he would obtain a catheterized specimen for urinalysis, culture, and a sensitivity test; treat as needed; and then consider increasing her catheterization frequency. A trial of a hydrophilic catheter could also be considered in the context of recurrent UTIs, he said.
Various catheter modifications – from silver alloy catheters to antibiotic-impregnated catheters – have been used with some success in reducing the risk of UTIs, but "they carry their own problems such as cost, development of resistance, and even, as one study suggested, the possibility of silver toxicity," Dr. Kraus said.
Two randomized, controlled trials have shown that hydrophilic catheters will reduce the risk of UTIs, compared with regular polyvinyl chloride catheters, he noted. Although the choice of single-use vs. reusable catheters is "always a point of contention," several studies have "clearly" shown that clean intermittent catheterization (CIC) poses no greater risk of recurrent UTIs than do single-use catheters, he added.
Frequent changing of intermittent catheters can prevent biofilm development, and one study showed that UTI was five times less likely when CIC was performed six times per day rather than three times per day, he noted.
Routine chronic antibiotic prophylaxis should be avoided in patients with neurogenic bladder, he said, but a short course of antibiotics could be useful during the initial CIC period, and is certainly prudent before any invasive genitourinary procedures are performed.
Dr. Kraus said he is intrigued by the concept of a weekly oral cyclic antibiotic (WOCA) program that uses weekly alternating antibiotics as a prophylactic measure. In one 2-year trial of WOCA, investigators "saw dramatic reductions in UTIs (from 9.4 to 1.8 per patient year) ... and most importantly, they did not see any change in the number of multidrug-resistant infections," he said.
As a final management option for the above-described patient, Dr. Kraus said he would consider injections of botulinum toxin (Botox). This approach "has exploded in the market for neurogenic bladder management, and it has been associated with a significant reduction in UTI at 6 months ... presumably because the neurogenic bladder management is that much better," he said.
The term "neurogenic bladder," Dr. Kraus noted, is one that’s "not very precise." For the purposes of his discussion, he defined it as a condition in which the bladder is affected by a neurologic process and has an impaired ability to store and empty urine.
Dr. Kraus disclosed that he is an investigator for the National Institute of Diabetes and Digestive and Kidney Diseases, a course director for Laborie (which manufactures catheters and other products for urinary and pelvic disorders), and a consultant/adviser for Pfizer.
WASHINGTON – How should one manage a 35-year-old woman with multiple sclerosis on self-clean intermittent catheterization who complains of pelvic pain and cloudy urine?
Such a patient with "neurogenic bladder" and possible urinary tract infection needs careful diagnosis, catheterization review, and possibly other management considerations, said Dr. Stephen R. Kraus during a panel discussion of recurrent UTIs at the annual meeting of the American Urological Association.
Patients with neurogenic bladder commonly have chronic bacteriuria and recurrent UTIs, and thus generally require a combination of bacteriuria and leukocyturia – as well as clinical symptoms or an increase in autonomic dysreflexia – for the initiation of empirical UTI therapy. Such criteria will help avoid unnecessary use of antibiotics.
"Original criteria were based on bacterial colonization counts but were criticized for being highly insensitive," said Dr. Kraus, professor and vice chairman of the department of urology at the University of Texas, San Antonio.
Assuming the patient has already had a video urodynamic test, Dr. Kraus said, he would obtain a catheterized specimen for urinalysis, culture, and a sensitivity test; treat as needed; and then consider increasing her catheterization frequency. A trial of a hydrophilic catheter could also be considered in the context of recurrent UTIs, he said.
Various catheter modifications – from silver alloy catheters to antibiotic-impregnated catheters – have been used with some success in reducing the risk of UTIs, but "they carry their own problems such as cost, development of resistance, and even, as one study suggested, the possibility of silver toxicity," Dr. Kraus said.
Two randomized, controlled trials have shown that hydrophilic catheters will reduce the risk of UTIs, compared with regular polyvinyl chloride catheters, he noted. Although the choice of single-use vs. reusable catheters is "always a point of contention," several studies have "clearly" shown that clean intermittent catheterization (CIC) poses no greater risk of recurrent UTIs than do single-use catheters, he added.
Frequent changing of intermittent catheters can prevent biofilm development, and one study showed that UTI was five times less likely when CIC was performed six times per day rather than three times per day, he noted.
Routine chronic antibiotic prophylaxis should be avoided in patients with neurogenic bladder, he said, but a short course of antibiotics could be useful during the initial CIC period, and is certainly prudent before any invasive genitourinary procedures are performed.
Dr. Kraus said he is intrigued by the concept of a weekly oral cyclic antibiotic (WOCA) program that uses weekly alternating antibiotics as a prophylactic measure. In one 2-year trial of WOCA, investigators "saw dramatic reductions in UTIs (from 9.4 to 1.8 per patient year) ... and most importantly, they did not see any change in the number of multidrug-resistant infections," he said.
As a final management option for the above-described patient, Dr. Kraus said he would consider injections of botulinum toxin (Botox). This approach "has exploded in the market for neurogenic bladder management, and it has been associated with a significant reduction in UTI at 6 months ... presumably because the neurogenic bladder management is that much better," he said.
The term "neurogenic bladder," Dr. Kraus noted, is one that’s "not very precise." For the purposes of his discussion, he defined it as a condition in which the bladder is affected by a neurologic process and has an impaired ability to store and empty urine.
Dr. Kraus disclosed that he is an investigator for the National Institute of Diabetes and Digestive and Kidney Diseases, a course director for Laborie (which manufactures catheters and other products for urinary and pelvic disorders), and a consultant/adviser for Pfizer.
WASHINGTON – How should one manage a 35-year-old woman with multiple sclerosis on self-clean intermittent catheterization who complains of pelvic pain and cloudy urine?
Such a patient with "neurogenic bladder" and possible urinary tract infection needs careful diagnosis, catheterization review, and possibly other management considerations, said Dr. Stephen R. Kraus during a panel discussion of recurrent UTIs at the annual meeting of the American Urological Association.
Patients with neurogenic bladder commonly have chronic bacteriuria and recurrent UTIs, and thus generally require a combination of bacteriuria and leukocyturia – as well as clinical symptoms or an increase in autonomic dysreflexia – for the initiation of empirical UTI therapy. Such criteria will help avoid unnecessary use of antibiotics.
"Original criteria were based on bacterial colonization counts but were criticized for being highly insensitive," said Dr. Kraus, professor and vice chairman of the department of urology at the University of Texas, San Antonio.
Assuming the patient has already had a video urodynamic test, Dr. Kraus said, he would obtain a catheterized specimen for urinalysis, culture, and a sensitivity test; treat as needed; and then consider increasing her catheterization frequency. A trial of a hydrophilic catheter could also be considered in the context of recurrent UTIs, he said.
Various catheter modifications – from silver alloy catheters to antibiotic-impregnated catheters – have been used with some success in reducing the risk of UTIs, but "they carry their own problems such as cost, development of resistance, and even, as one study suggested, the possibility of silver toxicity," Dr. Kraus said.
Two randomized, controlled trials have shown that hydrophilic catheters will reduce the risk of UTIs, compared with regular polyvinyl chloride catheters, he noted. Although the choice of single-use vs. reusable catheters is "always a point of contention," several studies have "clearly" shown that clean intermittent catheterization (CIC) poses no greater risk of recurrent UTIs than do single-use catheters, he added.
Frequent changing of intermittent catheters can prevent biofilm development, and one study showed that UTI was five times less likely when CIC was performed six times per day rather than three times per day, he noted.
Routine chronic antibiotic prophylaxis should be avoided in patients with neurogenic bladder, he said, but a short course of antibiotics could be useful during the initial CIC period, and is certainly prudent before any invasive genitourinary procedures are performed.
Dr. Kraus said he is intrigued by the concept of a weekly oral cyclic antibiotic (WOCA) program that uses weekly alternating antibiotics as a prophylactic measure. In one 2-year trial of WOCA, investigators "saw dramatic reductions in UTIs (from 9.4 to 1.8 per patient year) ... and most importantly, they did not see any change in the number of multidrug-resistant infections," he said.
As a final management option for the above-described patient, Dr. Kraus said he would consider injections of botulinum toxin (Botox). This approach "has exploded in the market for neurogenic bladder management, and it has been associated with a significant reduction in UTI at 6 months ... presumably because the neurogenic bladder management is that much better," he said.
The term "neurogenic bladder," Dr. Kraus noted, is one that’s "not very precise." For the purposes of his discussion, he defined it as a condition in which the bladder is affected by a neurologic process and has an impaired ability to store and empty urine.
Dr. Kraus disclosed that he is an investigator for the National Institute of Diabetes and Digestive and Kidney Diseases, a course director for Laborie (which manufactures catheters and other products for urinary and pelvic disorders), and a consultant/adviser for Pfizer.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN UROLOGICAL ASSOCIATION