User login
Simulator Tops Standard Training for Laparoscopic Hernia Repair
BOCA RATON, FLA. – A novel simulation-based training curriculum in laparoscopic totally extraperitoneal inguinal herniorrhaphy for general surgery residents led to shorter operating times, better trainee performance, and fewer patient complications in a randomized clinical trial.
The training program has two elements: a cognitive component featuring Web-based PowerPoint presentations and videos along with assigned readings, and psychomotor training on a totally extraperitoneal inguinal herniorrhaphy (TEP) simulator, Dr. Benjamin Zendejas said at the annual meeting of the American Surgical Association.
A key feature is that the skills training – with one instructor per resident – is performed until mastery is attained, however long that takes. Only then does the resident start performing TEPs in the operating room under supervision, explained Dr. Zendejas of the Mayo Clinic, Rochester, Minn.
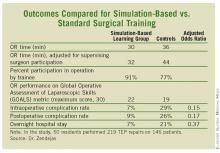
He presented a study in which 50 general surgery residents performed a baseline TEP in the operating room, and then were randomized to the simulation-based training program or standard training.
The simulator was the Limbs & Things Ltd.’s TEP Guildford MATTU Hernia Trainer. Mastery was defined by the average 2 minutes required for five experienced instructors to perform a TEP on the simulator. An average of roughly eight attempts was required for fifth-year residents to achieve mastery on the simulator, compared with 26 for first-year residents.
In the operating room, the 50 residents performed 219 TEP repairs on 146 patients. Each repair was evaluated immediately afterward by two independent raters. The simulation-based training group outperformed residents who were trained in the standard fashion in all outcome measures, including the key end point of operative time adjusted for the impact of supervising surgeon takeover for poorly performing trainees. (See box.)
Intraoperative complications, such as peritoneal tears and procedure conversions, occurred in 7% of procedures performed by simulation-trained residents, and in 29% of controls. Urinary retention, seroma, and other postoperative complications resulted from 9% of the simulation-trained residents’ operations, compared with 26% of those performed by residents trained in TEP in standard fashion. In all, 7% of procedures carried out by simulation-trained residents led to an overnight hospital stay, compared with 21% for controls.
"This will become a seminal paper in simulation-based training education," said discussant Dr. Gary L. Dunnington, who called the study "outstanding."
This work suggests that, on an hour-by-hour basis, training in a psychomotor skills laboratory may be more efficient for residents than time spent in the operating room. These data, "if further substantiated, will be of great value with the increasing constraints of decreasing duty hours," noted Dr. Dunnington, professor and chairman of the department of surgery at Southern Illinois University, Springfield.
He was impressed with the investigators’ documentation of improved clinically relevant patient outcomes in the operating room after simulation-based training – the first study to do so. He also liked the investigators’ use of video recordings rather than crude recall to facilitate the study of operative errors.
"This study sets a new bar for simulation researchers," he concluded.
Dr. Zendejas reported having no financial conflicts.
BOCA RATON, FLA. – A novel simulation-based training curriculum in laparoscopic totally extraperitoneal inguinal herniorrhaphy for general surgery residents led to shorter operating times, better trainee performance, and fewer patient complications in a randomized clinical trial.
The training program has two elements: a cognitive component featuring Web-based PowerPoint presentations and videos along with assigned readings, and psychomotor training on a totally extraperitoneal inguinal herniorrhaphy (TEP) simulator, Dr. Benjamin Zendejas said at the annual meeting of the American Surgical Association.
A key feature is that the skills training – with one instructor per resident – is performed until mastery is attained, however long that takes. Only then does the resident start performing TEPs in the operating room under supervision, explained Dr. Zendejas of the Mayo Clinic, Rochester, Minn.
He presented a study in which 50 general surgery residents performed a baseline TEP in the operating room, and then were randomized to the simulation-based training program or standard training.
The simulator was the Limbs & Things Ltd.’s TEP Guildford MATTU Hernia Trainer. Mastery was defined by the average 2 minutes required for five experienced instructors to perform a TEP on the simulator. An average of roughly eight attempts was required for fifth-year residents to achieve mastery on the simulator, compared with 26 for first-year residents.
In the operating room, the 50 residents performed 219 TEP repairs on 146 patients. Each repair was evaluated immediately afterward by two independent raters. The simulation-based training group outperformed residents who were trained in the standard fashion in all outcome measures, including the key end point of operative time adjusted for the impact of supervising surgeon takeover for poorly performing trainees. (See box.)
Intraoperative complications, such as peritoneal tears and procedure conversions, occurred in 7% of procedures performed by simulation-trained residents, and in 29% of controls. Urinary retention, seroma, and other postoperative complications resulted from 9% of the simulation-trained residents’ operations, compared with 26% of those performed by residents trained in TEP in standard fashion. In all, 7% of procedures carried out by simulation-trained residents led to an overnight hospital stay, compared with 21% for controls.
"This will become a seminal paper in simulation-based training education," said discussant Dr. Gary L. Dunnington, who called the study "outstanding."
This work suggests that, on an hour-by-hour basis, training in a psychomotor skills laboratory may be more efficient for residents than time spent in the operating room. These data, "if further substantiated, will be of great value with the increasing constraints of decreasing duty hours," noted Dr. Dunnington, professor and chairman of the department of surgery at Southern Illinois University, Springfield.
He was impressed with the investigators’ documentation of improved clinically relevant patient outcomes in the operating room after simulation-based training – the first study to do so. He also liked the investigators’ use of video recordings rather than crude recall to facilitate the study of operative errors.
"This study sets a new bar for simulation researchers," he concluded.
Dr. Zendejas reported having no financial conflicts.
BOCA RATON, FLA. – A novel simulation-based training curriculum in laparoscopic totally extraperitoneal inguinal herniorrhaphy for general surgery residents led to shorter operating times, better trainee performance, and fewer patient complications in a randomized clinical trial.
The training program has two elements: a cognitive component featuring Web-based PowerPoint presentations and videos along with assigned readings, and psychomotor training on a totally extraperitoneal inguinal herniorrhaphy (TEP) simulator, Dr. Benjamin Zendejas said at the annual meeting of the American Surgical Association.
A key feature is that the skills training – with one instructor per resident – is performed until mastery is attained, however long that takes. Only then does the resident start performing TEPs in the operating room under supervision, explained Dr. Zendejas of the Mayo Clinic, Rochester, Minn.
He presented a study in which 50 general surgery residents performed a baseline TEP in the operating room, and then were randomized to the simulation-based training program or standard training.
The simulator was the Limbs & Things Ltd.’s TEP Guildford MATTU Hernia Trainer. Mastery was defined by the average 2 minutes required for five experienced instructors to perform a TEP on the simulator. An average of roughly eight attempts was required for fifth-year residents to achieve mastery on the simulator, compared with 26 for first-year residents.
In the operating room, the 50 residents performed 219 TEP repairs on 146 patients. Each repair was evaluated immediately afterward by two independent raters. The simulation-based training group outperformed residents who were trained in the standard fashion in all outcome measures, including the key end point of operative time adjusted for the impact of supervising surgeon takeover for poorly performing trainees. (See box.)
Intraoperative complications, such as peritoneal tears and procedure conversions, occurred in 7% of procedures performed by simulation-trained residents, and in 29% of controls. Urinary retention, seroma, and other postoperative complications resulted from 9% of the simulation-trained residents’ operations, compared with 26% of those performed by residents trained in TEP in standard fashion. In all, 7% of procedures carried out by simulation-trained residents led to an overnight hospital stay, compared with 21% for controls.
"This will become a seminal paper in simulation-based training education," said discussant Dr. Gary L. Dunnington, who called the study "outstanding."
This work suggests that, on an hour-by-hour basis, training in a psychomotor skills laboratory may be more efficient for residents than time spent in the operating room. These data, "if further substantiated, will be of great value with the increasing constraints of decreasing duty hours," noted Dr. Dunnington, professor and chairman of the department of surgery at Southern Illinois University, Springfield.
He was impressed with the investigators’ documentation of improved clinically relevant patient outcomes in the operating room after simulation-based training – the first study to do so. He also liked the investigators’ use of video recordings rather than crude recall to facilitate the study of operative errors.
"This study sets a new bar for simulation researchers," he concluded.
Dr. Zendejas reported having no financial conflicts.
FROM THE ANNUAL MEETING OF THE AMERICAN SURGICAL ASSOCIATION
Major Finding: General surgery residents who learned laparoscopic totally extraperitoneal inguinal herniorrhaphy through a novel simulation-based training program significantly outperformed those trained in standard fashion.
Data Source: Randomized trial with 50 residents.
Disclosures: Dr. Zendejas reported having no financial conflicts.
2011 Kidney Donor Death Highlights Lingering Clip Ligation Problem
PHILADELPHIA – At least five live-kidney donors died worldwide since 2005 from catastrophic hemorrhages attributable to insecure ligation of their renal artery by a locking clip rather than by transfixion.
The most recent of these deaths occurred earlier this year, despite concerns raised during 2004-2006 about the safety of clip ligations and a Food and Drug Administration temporary ban in 2006 on the U.S. sale of polymer locking clips, Dr. Amy L. Friedman said at the American Transplant Congress. Following reintroduction of the polymer locking clips in late 2006, two other deaths attributable to severe renal artery hemorrhages in live kidney donors occurred in 2008, said Dr. Friedman, professor of surgery and director of transplants at Upstate Medical University Hospital in Syracuse, N.Y.
"It’s clear that this is not a frequent event, but even though it’s infrequent it is catastrophic," Dr. Friedman said in an interview. The relative infrequency "does not justify it. We ask surgeons to please respect the privilege of operating on a living kidney donor and not use" a polymer clip to close off the donor’s severed renal artery. Dr. Friedman also noted several other cases since 2003 where patients did not die but had severe hemorrhages because of unreliable artery ligations that produced near-death events.
Dr, Friedman admitted that alternative closure techniques that use transfixion are "challenging." The options are suture ligature, oversewing, or stapling. The most commonly used, safe closure is stapling, which has the drawback of using more of an artery’s length. "If the patient has early branching" of their renal artery, this closure may produce two small arteries instead of one larger one" on the removed kidney, "forcing you to sew them together and making the kidney harder to transplant." But any added inconvenience in transplanting the donated kidney does not outweigh safely closing the donor’s artery, she said. "The stapler is the best alternative to the clip," she said.
The surgeons performing nephrectomies for transplantable kidneys from living donors most commonly are transplant surgeons, urologists, and minimally-invasive surgeons. "There has been extensive pushback" arguing in favor of continued clip use in the urology literature, Dr. Friedman said at the meeting cosponsored by the American Society of Transplant Surgeons.
"The urology community uses clips more frequently, especially for nephrectomies done for other purposes," she said. "In those cases, the length of renal artery that they leave is much longer," experience that seems to have convinced urologists that clipping is safe even when the renal artery is shorter. "What we clearly know is that when the artery stump is left very short to allow a long length of artery to remain with the kidney, clips cannot be used." Some clip proponents also note that clips are less expensive than staples are, and many surgeons also cite personal experience performing hundreds of uneventful renal-artery closures with clips. Dr. Friedman contends that this is not surprising since the severe adverse event rate from clips is very low, but even a handful of deaths is too many.
Many transplant surgeons remain skeptical of the risk because they want to see case reports from deaths and other severe sequelae, data that the FDA, the Centers for Medicare & Medicaid Services, and the United Network for Organ Sharing (UNOS) have generally not shared.
Dr. Friedman contended that these regulatory agencies have balked at releasing case details out of medicolegal concerns about discoverability and confidentiality.
These agencies "make it hard, but these data should be easily available. If surgeons knew that there have been at least five deaths since 2005, it’s hard to imagine that they would not be convinced. I’m doing my best to get the information out," she said.
The five deaths from unstable renal artery closures in kidney donors using locking polymer clips comprised two cases in 2005, two in 2008, and the most recent case reported by UNOS earlier this year. Dr. Friedman said that she had also reviewed a report of a possible sixth death in February 2005, but it remains unclear whether this was the same case as one of the other 2005 deaths she cited. In addition, Dr. Friedman said she was aware of five additional cases of severe hemorrhage complications in living kidney donors treated with polymer clips since 2005.
Following notification by UNOS of the most recent death in February of this year, and a reminder to transplant surgeons not to use polymer clips for artery ligations, Dr. Friedman sent out an electronic survey in March to the members of the American Society of Transplant Surgeons (ASTS). From the 1,095 members she received 217 replies (20%). In reply to a question whether the ASTS members had received the UNOS notification, about two-thirds said they had not. She also asked the ASTS members whether their institutions continued to use hemostatic clips to ligate the renal arteries of live kidney donors. About 20% of all 201 respondents to this question, and more than 10% of the U.S.-based surgeons who responded said that their institutions used clips at least sometimes for these ligations.
Dr. Friedman said that she and her associates have no relevant financial disclosures.
PHILADELPHIA – At least five live-kidney donors died worldwide since 2005 from catastrophic hemorrhages attributable to insecure ligation of their renal artery by a locking clip rather than by transfixion.
The most recent of these deaths occurred earlier this year, despite concerns raised during 2004-2006 about the safety of clip ligations and a Food and Drug Administration temporary ban in 2006 on the U.S. sale of polymer locking clips, Dr. Amy L. Friedman said at the American Transplant Congress. Following reintroduction of the polymer locking clips in late 2006, two other deaths attributable to severe renal artery hemorrhages in live kidney donors occurred in 2008, said Dr. Friedman, professor of surgery and director of transplants at Upstate Medical University Hospital in Syracuse, N.Y.
"It’s clear that this is not a frequent event, but even though it’s infrequent it is catastrophic," Dr. Friedman said in an interview. The relative infrequency "does not justify it. We ask surgeons to please respect the privilege of operating on a living kidney donor and not use" a polymer clip to close off the donor’s severed renal artery. Dr. Friedman also noted several other cases since 2003 where patients did not die but had severe hemorrhages because of unreliable artery ligations that produced near-death events.
Dr, Friedman admitted that alternative closure techniques that use transfixion are "challenging." The options are suture ligature, oversewing, or stapling. The most commonly used, safe closure is stapling, which has the drawback of using more of an artery’s length. "If the patient has early branching" of their renal artery, this closure may produce two small arteries instead of one larger one" on the removed kidney, "forcing you to sew them together and making the kidney harder to transplant." But any added inconvenience in transplanting the donated kidney does not outweigh safely closing the donor’s artery, she said. "The stapler is the best alternative to the clip," she said.
The surgeons performing nephrectomies for transplantable kidneys from living donors most commonly are transplant surgeons, urologists, and minimally-invasive surgeons. "There has been extensive pushback" arguing in favor of continued clip use in the urology literature, Dr. Friedman said at the meeting cosponsored by the American Society of Transplant Surgeons.
"The urology community uses clips more frequently, especially for nephrectomies done for other purposes," she said. "In those cases, the length of renal artery that they leave is much longer," experience that seems to have convinced urologists that clipping is safe even when the renal artery is shorter. "What we clearly know is that when the artery stump is left very short to allow a long length of artery to remain with the kidney, clips cannot be used." Some clip proponents also note that clips are less expensive than staples are, and many surgeons also cite personal experience performing hundreds of uneventful renal-artery closures with clips. Dr. Friedman contends that this is not surprising since the severe adverse event rate from clips is very low, but even a handful of deaths is too many.
Many transplant surgeons remain skeptical of the risk because they want to see case reports from deaths and other severe sequelae, data that the FDA, the Centers for Medicare & Medicaid Services, and the United Network for Organ Sharing (UNOS) have generally not shared.
Dr. Friedman contended that these regulatory agencies have balked at releasing case details out of medicolegal concerns about discoverability and confidentiality.
These agencies "make it hard, but these data should be easily available. If surgeons knew that there have been at least five deaths since 2005, it’s hard to imagine that they would not be convinced. I’m doing my best to get the information out," she said.
The five deaths from unstable renal artery closures in kidney donors using locking polymer clips comprised two cases in 2005, two in 2008, and the most recent case reported by UNOS earlier this year. Dr. Friedman said that she had also reviewed a report of a possible sixth death in February 2005, but it remains unclear whether this was the same case as one of the other 2005 deaths she cited. In addition, Dr. Friedman said she was aware of five additional cases of severe hemorrhage complications in living kidney donors treated with polymer clips since 2005.
Following notification by UNOS of the most recent death in February of this year, and a reminder to transplant surgeons not to use polymer clips for artery ligations, Dr. Friedman sent out an electronic survey in March to the members of the American Society of Transplant Surgeons (ASTS). From the 1,095 members she received 217 replies (20%). In reply to a question whether the ASTS members had received the UNOS notification, about two-thirds said they had not. She also asked the ASTS members whether their institutions continued to use hemostatic clips to ligate the renal arteries of live kidney donors. About 20% of all 201 respondents to this question, and more than 10% of the U.S.-based surgeons who responded said that their institutions used clips at least sometimes for these ligations.
Dr. Friedman said that she and her associates have no relevant financial disclosures.
PHILADELPHIA – At least five live-kidney donors died worldwide since 2005 from catastrophic hemorrhages attributable to insecure ligation of their renal artery by a locking clip rather than by transfixion.
The most recent of these deaths occurred earlier this year, despite concerns raised during 2004-2006 about the safety of clip ligations and a Food and Drug Administration temporary ban in 2006 on the U.S. sale of polymer locking clips, Dr. Amy L. Friedman said at the American Transplant Congress. Following reintroduction of the polymer locking clips in late 2006, two other deaths attributable to severe renal artery hemorrhages in live kidney donors occurred in 2008, said Dr. Friedman, professor of surgery and director of transplants at Upstate Medical University Hospital in Syracuse, N.Y.
"It’s clear that this is not a frequent event, but even though it’s infrequent it is catastrophic," Dr. Friedman said in an interview. The relative infrequency "does not justify it. We ask surgeons to please respect the privilege of operating on a living kidney donor and not use" a polymer clip to close off the donor’s severed renal artery. Dr. Friedman also noted several other cases since 2003 where patients did not die but had severe hemorrhages because of unreliable artery ligations that produced near-death events.
Dr, Friedman admitted that alternative closure techniques that use transfixion are "challenging." The options are suture ligature, oversewing, or stapling. The most commonly used, safe closure is stapling, which has the drawback of using more of an artery’s length. "If the patient has early branching" of their renal artery, this closure may produce two small arteries instead of one larger one" on the removed kidney, "forcing you to sew them together and making the kidney harder to transplant." But any added inconvenience in transplanting the donated kidney does not outweigh safely closing the donor’s artery, she said. "The stapler is the best alternative to the clip," she said.
The surgeons performing nephrectomies for transplantable kidneys from living donors most commonly are transplant surgeons, urologists, and minimally-invasive surgeons. "There has been extensive pushback" arguing in favor of continued clip use in the urology literature, Dr. Friedman said at the meeting cosponsored by the American Society of Transplant Surgeons.
"The urology community uses clips more frequently, especially for nephrectomies done for other purposes," she said. "In those cases, the length of renal artery that they leave is much longer," experience that seems to have convinced urologists that clipping is safe even when the renal artery is shorter. "What we clearly know is that when the artery stump is left very short to allow a long length of artery to remain with the kidney, clips cannot be used." Some clip proponents also note that clips are less expensive than staples are, and many surgeons also cite personal experience performing hundreds of uneventful renal-artery closures with clips. Dr. Friedman contends that this is not surprising since the severe adverse event rate from clips is very low, but even a handful of deaths is too many.
Many transplant surgeons remain skeptical of the risk because they want to see case reports from deaths and other severe sequelae, data that the FDA, the Centers for Medicare & Medicaid Services, and the United Network for Organ Sharing (UNOS) have generally not shared.
Dr. Friedman contended that these regulatory agencies have balked at releasing case details out of medicolegal concerns about discoverability and confidentiality.
These agencies "make it hard, but these data should be easily available. If surgeons knew that there have been at least five deaths since 2005, it’s hard to imagine that they would not be convinced. I’m doing my best to get the information out," she said.
The five deaths from unstable renal artery closures in kidney donors using locking polymer clips comprised two cases in 2005, two in 2008, and the most recent case reported by UNOS earlier this year. Dr. Friedman said that she had also reviewed a report of a possible sixth death in February 2005, but it remains unclear whether this was the same case as one of the other 2005 deaths she cited. In addition, Dr. Friedman said she was aware of five additional cases of severe hemorrhage complications in living kidney donors treated with polymer clips since 2005.
Following notification by UNOS of the most recent death in February of this year, and a reminder to transplant surgeons not to use polymer clips for artery ligations, Dr. Friedman sent out an electronic survey in March to the members of the American Society of Transplant Surgeons (ASTS). From the 1,095 members she received 217 replies (20%). In reply to a question whether the ASTS members had received the UNOS notification, about two-thirds said they had not. She also asked the ASTS members whether their institutions continued to use hemostatic clips to ligate the renal arteries of live kidney donors. About 20% of all 201 respondents to this question, and more than 10% of the U.S.-based surgeons who responded said that their institutions used clips at least sometimes for these ligations.
Dr. Friedman said that she and her associates have no relevant financial disclosures.
FROM THE AMERICAN TRANSPLANT CONGRESS
Major Finding: Since 2005, at least five living kidney donors died from severe hemorrhages secondary to the use of polymer clips for ligating their renal arteries following nephrectomy. One case occurred in early 2011.
Data Source: Review of documented cases.
Disclosures: Dr. Friedman said that she and her associates have no relevant financial disclosures.
2011 Kidney Donor Death Highlights Lingering Clip Ligation Problem
PHILADELPHIA – At least five live-kidney donors died worldwide since 2005 from catastrophic hemorrhages attributable to insecure ligation of their renal artery by a locking clip rather than by transfixion.
The most recent of these deaths occurred earlier this year, despite concerns raised during 2004-2006 about the safety of clip ligations and a Food and Drug Administration temporary ban in 2006 on the U.S. sale of polymer locking clips, Dr. Amy L. Friedman said at the American Transplant Congress. Following reintroduction of the polymer locking clips in late 2006, two other deaths attributable to severe renal artery hemorrhages in live kidney donors occurred in 2008, said Dr. Friedman, professor of surgery and director of transplants at Upstate Medical University Hospital in Syracuse, N.Y.
"It’s clear that this is not a frequent event, but even though it’s infrequent it is catastrophic," Dr. Friedman said in an interview. The relative infrequency "does not justify it. We ask surgeons to please respect the privilege of operating on a living kidney donor and not use" a polymer clip to close off the donor’s severed renal artery. Dr. Friedman also noted several other cases since 2003 where patients did not die but had severe hemorrhages because of unreliable artery ligations that produced near-death events.
Dr, Friedman admitted that alternative closure techniques that use transfixion are "challenging." The options are suture ligature, oversewing, or stapling. The most commonly used, safe closure is stapling, which has the drawback of using more of an artery’s length. "If the patient has early branching" of their renal artery, this closure may produce two small arteries instead of one larger one" on the removed kidney, "forcing you to sew them together and making the kidney harder to transplant." But any added inconvenience in transplanting the donated kidney does not outweigh safely closing the donor’s artery, she said. "The stapler is the best alternative to the clip," she said.
The surgeons performing nephrectomies for transplantable kidneys from living donors most commonly are transplant surgeons, urologists, and minimally-invasive surgeons. "There has been extensive pushback" arguing in favor of continued clip use in the urology literature, Dr. Friedman said at the meeting cosponsored by the American Society of Transplant Surgeons.
"The urology community uses clips more frequently, especially for nephrectomies done for other purposes," she said. "In those cases, the length of renal artery that they leave is much longer," experience that seems to have convinced urologists that clipping is safe even when the renal artery is shorter. "What we clearly know is that when the artery stump is left very short to allow a long length of artery to remain with the kidney, clips cannot be used." Some clip proponents also note that clips are less expensive than staples are, and many surgeons also cite personal experience performing hundreds of uneventful renal-artery closures with clips. Dr. Friedman contends that this is not surprising since the severe adverse event rate from clips is very low, but even a handful of deaths is too many.
Many transplant surgeons remain skeptical of the risk because they want to see case reports from deaths and other severe sequelae, data that the FDA, the Centers for Medicare & Medicaid Services, and the United Network for Organ Sharing (UNOS) have generally not shared.
Dr. Friedman contended that these regulatory agencies have balked at releasing case details out of medicolegal concerns about discoverability and confidentiality.
These agencies "make it hard, but these data should be easily available. If surgeons knew that there have been at least five deaths since 2005, it’s hard to imagine that they would not be convinced. I’m doing my best to get the information out," she said.
The five deaths from unstable renal artery closures in kidney donors using locking polymer clips comprised two cases in 2005, two in 2008, and the most recent case reported by UNOS earlier this year. Dr. Friedman said that she had also reviewed a report of a possible sixth death in February 2005, but it remains unclear whether this was the same case as one of the other 2005 deaths she cited. In addition, Dr. Friedman said she was aware of five additional cases of severe hemorrhage complications in living kidney donors treated with polymer clips since 2005.
Following notification by UNOS of the most recent death in February of this year, and a reminder to transplant surgeons not to use polymer clips for artery ligations, Dr. Friedman sent out an electronic survey in March to the members of the American Society of Transplant Surgeons (ASTS). From the 1,095 members she received 217 replies (20%). In reply to a question whether the ASTS members had received the UNOS notification, about two-thirds said they had not. She also asked the ASTS members whether their institutions continued to use hemostatic clips to ligate the renal arteries of live kidney donors. About 20% of all 201 respondents to this question, and more than 10% of the U.S.-based surgeons who responded said that their institutions used clips at least sometimes for these ligations.
Dr. Friedman said that she and her associates have no relevant financial disclosures.
PHILADELPHIA – At least five live-kidney donors died worldwide since 2005 from catastrophic hemorrhages attributable to insecure ligation of their renal artery by a locking clip rather than by transfixion.
The most recent of these deaths occurred earlier this year, despite concerns raised during 2004-2006 about the safety of clip ligations and a Food and Drug Administration temporary ban in 2006 on the U.S. sale of polymer locking clips, Dr. Amy L. Friedman said at the American Transplant Congress. Following reintroduction of the polymer locking clips in late 2006, two other deaths attributable to severe renal artery hemorrhages in live kidney donors occurred in 2008, said Dr. Friedman, professor of surgery and director of transplants at Upstate Medical University Hospital in Syracuse, N.Y.
"It’s clear that this is not a frequent event, but even though it’s infrequent it is catastrophic," Dr. Friedman said in an interview. The relative infrequency "does not justify it. We ask surgeons to please respect the privilege of operating on a living kidney donor and not use" a polymer clip to close off the donor’s severed renal artery. Dr. Friedman also noted several other cases since 2003 where patients did not die but had severe hemorrhages because of unreliable artery ligations that produced near-death events.
Dr, Friedman admitted that alternative closure techniques that use transfixion are "challenging." The options are suture ligature, oversewing, or stapling. The most commonly used, safe closure is stapling, which has the drawback of using more of an artery’s length. "If the patient has early branching" of their renal artery, this closure may produce two small arteries instead of one larger one" on the removed kidney, "forcing you to sew them together and making the kidney harder to transplant." But any added inconvenience in transplanting the donated kidney does not outweigh safely closing the donor’s artery, she said. "The stapler is the best alternative to the clip," she said.
The surgeons performing nephrectomies for transplantable kidneys from living donors most commonly are transplant surgeons, urologists, and minimally-invasive surgeons. "There has been extensive pushback" arguing in favor of continued clip use in the urology literature, Dr. Friedman said at the meeting cosponsored by the American Society of Transplant Surgeons.
"The urology community uses clips more frequently, especially for nephrectomies done for other purposes," she said. "In those cases, the length of renal artery that they leave is much longer," experience that seems to have convinced urologists that clipping is safe even when the renal artery is shorter. "What we clearly know is that when the artery stump is left very short to allow a long length of artery to remain with the kidney, clips cannot be used." Some clip proponents also note that clips are less expensive than staples are, and many surgeons also cite personal experience performing hundreds of uneventful renal-artery closures with clips. Dr. Friedman contends that this is not surprising since the severe adverse event rate from clips is very low, but even a handful of deaths is too many.
Many transplant surgeons remain skeptical of the risk because they want to see case reports from deaths and other severe sequelae, data that the FDA, the Centers for Medicare & Medicaid Services, and the United Network for Organ Sharing (UNOS) have generally not shared.
Dr. Friedman contended that these regulatory agencies have balked at releasing case details out of medicolegal concerns about discoverability and confidentiality.
These agencies "make it hard, but these data should be easily available. If surgeons knew that there have been at least five deaths since 2005, it’s hard to imagine that they would not be convinced. I’m doing my best to get the information out," she said.
The five deaths from unstable renal artery closures in kidney donors using locking polymer clips comprised two cases in 2005, two in 2008, and the most recent case reported by UNOS earlier this year. Dr. Friedman said that she had also reviewed a report of a possible sixth death in February 2005, but it remains unclear whether this was the same case as one of the other 2005 deaths she cited. In addition, Dr. Friedman said she was aware of five additional cases of severe hemorrhage complications in living kidney donors treated with polymer clips since 2005.
Following notification by UNOS of the most recent death in February of this year, and a reminder to transplant surgeons not to use polymer clips for artery ligations, Dr. Friedman sent out an electronic survey in March to the members of the American Society of Transplant Surgeons (ASTS). From the 1,095 members she received 217 replies (20%). In reply to a question whether the ASTS members had received the UNOS notification, about two-thirds said they had not. She also asked the ASTS members whether their institutions continued to use hemostatic clips to ligate the renal arteries of live kidney donors. About 20% of all 201 respondents to this question, and more than 10% of the U.S.-based surgeons who responded said that their institutions used clips at least sometimes for these ligations.
Dr. Friedman said that she and her associates have no relevant financial disclosures.
PHILADELPHIA – At least five live-kidney donors died worldwide since 2005 from catastrophic hemorrhages attributable to insecure ligation of their renal artery by a locking clip rather than by transfixion.
The most recent of these deaths occurred earlier this year, despite concerns raised during 2004-2006 about the safety of clip ligations and a Food and Drug Administration temporary ban in 2006 on the U.S. sale of polymer locking clips, Dr. Amy L. Friedman said at the American Transplant Congress. Following reintroduction of the polymer locking clips in late 2006, two other deaths attributable to severe renal artery hemorrhages in live kidney donors occurred in 2008, said Dr. Friedman, professor of surgery and director of transplants at Upstate Medical University Hospital in Syracuse, N.Y.
"It’s clear that this is not a frequent event, but even though it’s infrequent it is catastrophic," Dr. Friedman said in an interview. The relative infrequency "does not justify it. We ask surgeons to please respect the privilege of operating on a living kidney donor and not use" a polymer clip to close off the donor’s severed renal artery. Dr. Friedman also noted several other cases since 2003 where patients did not die but had severe hemorrhages because of unreliable artery ligations that produced near-death events.
Dr, Friedman admitted that alternative closure techniques that use transfixion are "challenging." The options are suture ligature, oversewing, or stapling. The most commonly used, safe closure is stapling, which has the drawback of using more of an artery’s length. "If the patient has early branching" of their renal artery, this closure may produce two small arteries instead of one larger one" on the removed kidney, "forcing you to sew them together and making the kidney harder to transplant." But any added inconvenience in transplanting the donated kidney does not outweigh safely closing the donor’s artery, she said. "The stapler is the best alternative to the clip," she said.
The surgeons performing nephrectomies for transplantable kidneys from living donors most commonly are transplant surgeons, urologists, and minimally-invasive surgeons. "There has been extensive pushback" arguing in favor of continued clip use in the urology literature, Dr. Friedman said at the meeting cosponsored by the American Society of Transplant Surgeons.
"The urology community uses clips more frequently, especially for nephrectomies done for other purposes," she said. "In those cases, the length of renal artery that they leave is much longer," experience that seems to have convinced urologists that clipping is safe even when the renal artery is shorter. "What we clearly know is that when the artery stump is left very short to allow a long length of artery to remain with the kidney, clips cannot be used." Some clip proponents also note that clips are less expensive than staples are, and many surgeons also cite personal experience performing hundreds of uneventful renal-artery closures with clips. Dr. Friedman contends that this is not surprising since the severe adverse event rate from clips is very low, but even a handful of deaths is too many.
Many transplant surgeons remain skeptical of the risk because they want to see case reports from deaths and other severe sequelae, data that the FDA, the Centers for Medicare & Medicaid Services, and the United Network for Organ Sharing (UNOS) have generally not shared.
Dr. Friedman contended that these regulatory agencies have balked at releasing case details out of medicolegal concerns about discoverability and confidentiality.
These agencies "make it hard, but these data should be easily available. If surgeons knew that there have been at least five deaths since 2005, it’s hard to imagine that they would not be convinced. I’m doing my best to get the information out," she said.
The five deaths from unstable renal artery closures in kidney donors using locking polymer clips comprised two cases in 2005, two in 2008, and the most recent case reported by UNOS earlier this year. Dr. Friedman said that she had also reviewed a report of a possible sixth death in February 2005, but it remains unclear whether this was the same case as one of the other 2005 deaths she cited. In addition, Dr. Friedman said she was aware of five additional cases of severe hemorrhage complications in living kidney donors treated with polymer clips since 2005.
Following notification by UNOS of the most recent death in February of this year, and a reminder to transplant surgeons not to use polymer clips for artery ligations, Dr. Friedman sent out an electronic survey in March to the members of the American Society of Transplant Surgeons (ASTS). From the 1,095 members she received 217 replies (20%). In reply to a question whether the ASTS members had received the UNOS notification, about two-thirds said they had not. She also asked the ASTS members whether their institutions continued to use hemostatic clips to ligate the renal arteries of live kidney donors. About 20% of all 201 respondents to this question, and more than 10% of the U.S.-based surgeons who responded said that their institutions used clips at least sometimes for these ligations.
Dr. Friedman said that she and her associates have no relevant financial disclosures.
FROM THE AMERICAN TRANSPLANT CONGRESS
Using Renal Disease to Predict Cardiovascular Risk
New Methods Found Promising for Predicting CKD Progression
Separate research groups offer two new methods for predicting which patients with chronic kidney disease are likely to progress to end-stage renal disease and other complications, according to studies published in the April 20 issue of JAMA.
Ultimately, the methods could help more accurately identify individuals who are at low and high risk for CKD progression, and allow for more appropriate use of health care resources for this population.
In the first report, a "triple-marker" approach using serum creatinine, serum cystatin C, and albuminuria levels yielded more accurate risk prediction than is achieved with current staging systems that are based primarily on creatinine-estimated glomerular filtration rates, said Dr. Carmen A. Peralta of the San Francisco VA Medical Center and her associates.
They assessed the triple-marker technique using a large, population-based cohort of adults (aged 45 years and older) who were participating in a stroke study. A subgroup of 26,643 of these subjects had undergone evaluation of kidney function as part of that study.
Patients in Dr. Peralta’s analysis had a mean age of 65 years. In all, 40% were black, more than half were women, 21% had diabetes, and 59% had hypertension. During a median follow-up of approximately 5 years, 177 subjects developed end-stage renal disease (ESRD) and 1,940 died of all causes.
Adding either the cystatin C or the albuminuria measures to creatinine-based estimated GFR greatly improved the ability to predict the development of ESRD as well as mortality. Using all three measures together improved it even further.
The rate of ESRD was 103-fold higher in subjects who were identified as being at risk via the triple-marker method than in those who were identified via creatinine level alone. Similarly, mortality was nearly eight times higher among those identified as being at risk via the triple-marker method than in those identified via creatinine level alone, the investigators said (JAMA 2011;305:1545-52).
Moreover, one in six subjects who were found to have CKD by the triple-marker method were not identified at all by creatinine level alone, suggesting that people could have occult CKD that would not be diagnosed according to standard methods.
In addition, 25% of subjects who were labeled as having CKD by creatinine-based GFR estimate alone were found not to have CKD when cystatin C and albuminuria levels were added into the risk stratification. This suggests that one-fourth of people screened by standard methods alone may be treated as though they’re at increased risk of ESRD or death when they are not.
Thus, using the triple-marker method "could both reduce unwarranted referrals and unnecessary work-ups for low-risk individuals and would prioritize specialty care and interventions to individuals at highest risk," Dr. Peralta and her colleagues said.
"Several groups are currently advocating new international guidelines that more accurately reflect prognosis of CKD and have proposed adding [albumin:creatinine ratio] to staging of CKD. Our results suggest that a triple-marker approach using both [albuminuria] and cystatin C to confirm CKD more accurately discriminates prognosis for death and progression to ESRD than creatinine and [albuminuria] alone," they added.
In the second report, investigators developed seven possible models for predicting CKD progression, using various combinations of more than 20 candidate variables that are known to affect renal function. To do so, they first assessed these variables in a Canadian cohort of 3,449 adults who were treated at nephrology clinics for stage 3-5 CKD.
These study subjects, who were followed from 2001 through 2008, helped determine which variables were best associated with CKD progression, said Dr. Navdeep Tangri of Tufts Medical Center, Boston, and his associates.
One of the seven models was particularly accurate at predicting CKD progression. It factored in patient age and sex, as well as the estimated GFR, albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin levels. These are basic laboratory measurements "that are obtained routinely in patients with CKD," Dr. Tangri and his colleagues said (JAMA 2011;305:1553-9).
All seven models were tested in a validation cohort of 4,942 patients in a British Columbia registry who had stage 3-5 CKD. The same model was found to be the most accurate in predicting disease progression in that cohort.
"Using our models, lower-risk patients could be managed by the primary care physician without additional testing or treatment of CKD complications," whereas higher-risk patients could receive "more intensive testing, intervention, and early nephrology care," they said.
The models also could be used to triage patients with stage 4 CKD "regarding dialysis education, creation of vascular access, and preemptive transplantation"; to select stage 3 patients for public health interventions; and to select high-risk patients for enrollment in clinical trials, the researchers added.
Dr. Peralta’s study was supported by the National Institute of Neurological Disorders and Stroke, Amgen Corp., the National Institute of Diabetes and Digestive and Kidney Diseases, the Robert Wood Johnson Foundation, and the American Heart Association. Several associates also reported ties to Amgen. Dr. Tangri’s study was supported by a joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research, and the Canadian Society of Nephrology, as well as by the Change Foundation of Ontario. Dr. Tangri and associates reported no financial conflicts of interest.
The findings from both of these studies are "novel and important," but "much additional work is needed before these approaches can be recommended for routine clinical use," said Dr. Marcello Tonelli and Dr. Braden Manns.
Both studies provide proof of concept for two different methods that might enhance prognostic power, compared with the use of creatinine-estimated GFR alone.
However, it would be premature to introduce the triple-marker approach of Dr. Peralta and colleagues into clinical practice for three reasons: First, their study population was highly selected, so the findings cannot be generalized to other settings until further research is done. Second, current practice includes confirmation of estimated GFR with a second measurement to confirm CKD, and this repeat measurement was not addressed in this study. And third, the use of more stringent estimated GFR criteria to define CKD might attenuate the advantage of the triple-marker method.
It also would be premature to implement the prediction model of Dr. Tangri and colleagues because there were missing data in both the cohort used to develop the models and the validation cohort, and values were imputed for subjects who had incomplete information. More importantly, all the study subjects were already in the care of a nephrologist, so they are not representative of the general population.
"Careful consideration and research will be required to determine whether the benefits [of an eight-variable model] would outweigh its added complexity," they concluded.
Dr. Tonelli is at the University of Alberta, Edmonton. Dr. Manns is at the University of Calgary (Alta.). They were supported by the Alberta Heritage Foundation for Medical Research, by Alberta Health and Wellness, and by the University of Alberta. Dr. Tonelli also is supported by a Government of Canada Research Chair in the optimal treatment of CKD. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying the two reports (JAMA 2011:305:1593-5).
The findings from both of these studies are "novel and important," but "much additional work is needed before these approaches can be recommended for routine clinical use," said Dr. Marcello Tonelli and Dr. Braden Manns.
Both studies provide proof of concept for two different methods that might enhance prognostic power, compared with the use of creatinine-estimated GFR alone.
However, it would be premature to introduce the triple-marker approach of Dr. Peralta and colleagues into clinical practice for three reasons: First, their study population was highly selected, so the findings cannot be generalized to other settings until further research is done. Second, current practice includes confirmation of estimated GFR with a second measurement to confirm CKD, and this repeat measurement was not addressed in this study. And third, the use of more stringent estimated GFR criteria to define CKD might attenuate the advantage of the triple-marker method.
It also would be premature to implement the prediction model of Dr. Tangri and colleagues because there were missing data in both the cohort used to develop the models and the validation cohort, and values were imputed for subjects who had incomplete information. More importantly, all the study subjects were already in the care of a nephrologist, so they are not representative of the general population.
"Careful consideration and research will be required to determine whether the benefits [of an eight-variable model] would outweigh its added complexity," they concluded.
Dr. Tonelli is at the University of Alberta, Edmonton. Dr. Manns is at the University of Calgary (Alta.). They were supported by the Alberta Heritage Foundation for Medical Research, by Alberta Health and Wellness, and by the University of Alberta. Dr. Tonelli also is supported by a Government of Canada Research Chair in the optimal treatment of CKD. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying the two reports (JAMA 2011:305:1593-5).
The findings from both of these studies are "novel and important," but "much additional work is needed before these approaches can be recommended for routine clinical use," said Dr. Marcello Tonelli and Dr. Braden Manns.
Both studies provide proof of concept for two different methods that might enhance prognostic power, compared with the use of creatinine-estimated GFR alone.
However, it would be premature to introduce the triple-marker approach of Dr. Peralta and colleagues into clinical practice for three reasons: First, their study population was highly selected, so the findings cannot be generalized to other settings until further research is done. Second, current practice includes confirmation of estimated GFR with a second measurement to confirm CKD, and this repeat measurement was not addressed in this study. And third, the use of more stringent estimated GFR criteria to define CKD might attenuate the advantage of the triple-marker method.
It also would be premature to implement the prediction model of Dr. Tangri and colleagues because there were missing data in both the cohort used to develop the models and the validation cohort, and values were imputed for subjects who had incomplete information. More importantly, all the study subjects were already in the care of a nephrologist, so they are not representative of the general population.
"Careful consideration and research will be required to determine whether the benefits [of an eight-variable model] would outweigh its added complexity," they concluded.
Dr. Tonelli is at the University of Alberta, Edmonton. Dr. Manns is at the University of Calgary (Alta.). They were supported by the Alberta Heritage Foundation for Medical Research, by Alberta Health and Wellness, and by the University of Alberta. Dr. Tonelli also is supported by a Government of Canada Research Chair in the optimal treatment of CKD. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying the two reports (JAMA 2011:305:1593-5).
Separate research groups offer two new methods for predicting which patients with chronic kidney disease are likely to progress to end-stage renal disease and other complications, according to studies published in the April 20 issue of JAMA.
Ultimately, the methods could help more accurately identify individuals who are at low and high risk for CKD progression, and allow for more appropriate use of health care resources for this population.
In the first report, a "triple-marker" approach using serum creatinine, serum cystatin C, and albuminuria levels yielded more accurate risk prediction than is achieved with current staging systems that are based primarily on creatinine-estimated glomerular filtration rates, said Dr. Carmen A. Peralta of the San Francisco VA Medical Center and her associates.
They assessed the triple-marker technique using a large, population-based cohort of adults (aged 45 years and older) who were participating in a stroke study. A subgroup of 26,643 of these subjects had undergone evaluation of kidney function as part of that study.
Patients in Dr. Peralta’s analysis had a mean age of 65 years. In all, 40% were black, more than half were women, 21% had diabetes, and 59% had hypertension. During a median follow-up of approximately 5 years, 177 subjects developed end-stage renal disease (ESRD) and 1,940 died of all causes.
Adding either the cystatin C or the albuminuria measures to creatinine-based estimated GFR greatly improved the ability to predict the development of ESRD as well as mortality. Using all three measures together improved it even further.
The rate of ESRD was 103-fold higher in subjects who were identified as being at risk via the triple-marker method than in those who were identified via creatinine level alone. Similarly, mortality was nearly eight times higher among those identified as being at risk via the triple-marker method than in those identified via creatinine level alone, the investigators said (JAMA 2011;305:1545-52).
Moreover, one in six subjects who were found to have CKD by the triple-marker method were not identified at all by creatinine level alone, suggesting that people could have occult CKD that would not be diagnosed according to standard methods.
In addition, 25% of subjects who were labeled as having CKD by creatinine-based GFR estimate alone were found not to have CKD when cystatin C and albuminuria levels were added into the risk stratification. This suggests that one-fourth of people screened by standard methods alone may be treated as though they’re at increased risk of ESRD or death when they are not.
Thus, using the triple-marker method "could both reduce unwarranted referrals and unnecessary work-ups for low-risk individuals and would prioritize specialty care and interventions to individuals at highest risk," Dr. Peralta and her colleagues said.
"Several groups are currently advocating new international guidelines that more accurately reflect prognosis of CKD and have proposed adding [albumin:creatinine ratio] to staging of CKD. Our results suggest that a triple-marker approach using both [albuminuria] and cystatin C to confirm CKD more accurately discriminates prognosis for death and progression to ESRD than creatinine and [albuminuria] alone," they added.
In the second report, investigators developed seven possible models for predicting CKD progression, using various combinations of more than 20 candidate variables that are known to affect renal function. To do so, they first assessed these variables in a Canadian cohort of 3,449 adults who were treated at nephrology clinics for stage 3-5 CKD.
These study subjects, who were followed from 2001 through 2008, helped determine which variables were best associated with CKD progression, said Dr. Navdeep Tangri of Tufts Medical Center, Boston, and his associates.
One of the seven models was particularly accurate at predicting CKD progression. It factored in patient age and sex, as well as the estimated GFR, albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin levels. These are basic laboratory measurements "that are obtained routinely in patients with CKD," Dr. Tangri and his colleagues said (JAMA 2011;305:1553-9).
All seven models were tested in a validation cohort of 4,942 patients in a British Columbia registry who had stage 3-5 CKD. The same model was found to be the most accurate in predicting disease progression in that cohort.
"Using our models, lower-risk patients could be managed by the primary care physician without additional testing or treatment of CKD complications," whereas higher-risk patients could receive "more intensive testing, intervention, and early nephrology care," they said.
The models also could be used to triage patients with stage 4 CKD "regarding dialysis education, creation of vascular access, and preemptive transplantation"; to select stage 3 patients for public health interventions; and to select high-risk patients for enrollment in clinical trials, the researchers added.
Dr. Peralta’s study was supported by the National Institute of Neurological Disorders and Stroke, Amgen Corp., the National Institute of Diabetes and Digestive and Kidney Diseases, the Robert Wood Johnson Foundation, and the American Heart Association. Several associates also reported ties to Amgen. Dr. Tangri’s study was supported by a joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research, and the Canadian Society of Nephrology, as well as by the Change Foundation of Ontario. Dr. Tangri and associates reported no financial conflicts of interest.
Separate research groups offer two new methods for predicting which patients with chronic kidney disease are likely to progress to end-stage renal disease and other complications, according to studies published in the April 20 issue of JAMA.
Ultimately, the methods could help more accurately identify individuals who are at low and high risk for CKD progression, and allow for more appropriate use of health care resources for this population.
In the first report, a "triple-marker" approach using serum creatinine, serum cystatin C, and albuminuria levels yielded more accurate risk prediction than is achieved with current staging systems that are based primarily on creatinine-estimated glomerular filtration rates, said Dr. Carmen A. Peralta of the San Francisco VA Medical Center and her associates.
They assessed the triple-marker technique using a large, population-based cohort of adults (aged 45 years and older) who were participating in a stroke study. A subgroup of 26,643 of these subjects had undergone evaluation of kidney function as part of that study.
Patients in Dr. Peralta’s analysis had a mean age of 65 years. In all, 40% were black, more than half were women, 21% had diabetes, and 59% had hypertension. During a median follow-up of approximately 5 years, 177 subjects developed end-stage renal disease (ESRD) and 1,940 died of all causes.
Adding either the cystatin C or the albuminuria measures to creatinine-based estimated GFR greatly improved the ability to predict the development of ESRD as well as mortality. Using all three measures together improved it even further.
The rate of ESRD was 103-fold higher in subjects who were identified as being at risk via the triple-marker method than in those who were identified via creatinine level alone. Similarly, mortality was nearly eight times higher among those identified as being at risk via the triple-marker method than in those identified via creatinine level alone, the investigators said (JAMA 2011;305:1545-52).
Moreover, one in six subjects who were found to have CKD by the triple-marker method were not identified at all by creatinine level alone, suggesting that people could have occult CKD that would not be diagnosed according to standard methods.
In addition, 25% of subjects who were labeled as having CKD by creatinine-based GFR estimate alone were found not to have CKD when cystatin C and albuminuria levels were added into the risk stratification. This suggests that one-fourth of people screened by standard methods alone may be treated as though they’re at increased risk of ESRD or death when they are not.
Thus, using the triple-marker method "could both reduce unwarranted referrals and unnecessary work-ups for low-risk individuals and would prioritize specialty care and interventions to individuals at highest risk," Dr. Peralta and her colleagues said.
"Several groups are currently advocating new international guidelines that more accurately reflect prognosis of CKD and have proposed adding [albumin:creatinine ratio] to staging of CKD. Our results suggest that a triple-marker approach using both [albuminuria] and cystatin C to confirm CKD more accurately discriminates prognosis for death and progression to ESRD than creatinine and [albuminuria] alone," they added.
In the second report, investigators developed seven possible models for predicting CKD progression, using various combinations of more than 20 candidate variables that are known to affect renal function. To do so, they first assessed these variables in a Canadian cohort of 3,449 adults who were treated at nephrology clinics for stage 3-5 CKD.
These study subjects, who were followed from 2001 through 2008, helped determine which variables were best associated with CKD progression, said Dr. Navdeep Tangri of Tufts Medical Center, Boston, and his associates.
One of the seven models was particularly accurate at predicting CKD progression. It factored in patient age and sex, as well as the estimated GFR, albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin levels. These are basic laboratory measurements "that are obtained routinely in patients with CKD," Dr. Tangri and his colleagues said (JAMA 2011;305:1553-9).
All seven models were tested in a validation cohort of 4,942 patients in a British Columbia registry who had stage 3-5 CKD. The same model was found to be the most accurate in predicting disease progression in that cohort.
"Using our models, lower-risk patients could be managed by the primary care physician without additional testing or treatment of CKD complications," whereas higher-risk patients could receive "more intensive testing, intervention, and early nephrology care," they said.
The models also could be used to triage patients with stage 4 CKD "regarding dialysis education, creation of vascular access, and preemptive transplantation"; to select stage 3 patients for public health interventions; and to select high-risk patients for enrollment in clinical trials, the researchers added.
Dr. Peralta’s study was supported by the National Institute of Neurological Disorders and Stroke, Amgen Corp., the National Institute of Diabetes and Digestive and Kidney Diseases, the Robert Wood Johnson Foundation, and the American Heart Association. Several associates also reported ties to Amgen. Dr. Tangri’s study was supported by a joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research, and the Canadian Society of Nephrology, as well as by the Change Foundation of Ontario. Dr. Tangri and associates reported no financial conflicts of interest.
FROM JAMA
Major Finding: A triple-marker method incorporating serum creatinine, serum cystatin C, and albuminuria levels predicted CKD progression more accurately than did standard creatinine-estimated GFR alone. Another model incorporating seven patient variables also was more accurate at predicting disease progression than was standard creatinine-estimated GFR alone.
Data Source: The first study was a secondary analysis of a population-based cohort involving 26,643 adults. The second study was an analysis of CKD outcomes following the development and validation of seven models for predicting disease progression in adults with stage 3-5 CKD.
Disclosures: Dr. Peralta’s study was supported by the National Institute of Neurological Disorders and Stroke, Amgen Corp., the National Institute of Diabetes and Digestive and Kidney Diseases, the Robert Wood Johnson Foundation, and the American Heart Association. Several associates also reported ties to Amgen. Dr. Tangri’s study was supported by a joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research, and the Canadian Society of Nephrology, as well as by the Change Foundation of Ontario. Dr. Tangri and associates reported no financial conflicts of interest.
New Methods Found Promising for Predicting CKD Progression
Separate research groups offer two new methods for predicting which patients with chronic kidney disease are likely to progress to end-stage renal disease and other complications, according to studies published in the April 20 issue of JAMA.
Ultimately, the methods could help more accurately identify individuals who are at low and high risk for CKD progression, and allow for more appropriate use of health care resources for this population.
In the first report, a "triple-marker" approach using serum creatinine, serum cystatin C, and albuminuria levels yielded more accurate risk prediction than is achieved with current staging systems that are based primarily on creatinine-estimated glomerular filtration rates, said Dr. Carmen A. Peralta of the San Francisco VA Medical Center and her associates.
They assessed the triple-marker technique using a large, population-based cohort of adults (aged 45 years and older) who were participating in a stroke study. A subgroup of 26,643 of these subjects had undergone evaluation of kidney function as part of that study.
Patients in Dr. Peralta’s analysis had a mean age of 65 years. In all, 40% were black, more than half were women, 21% had diabetes, and 59% had hypertension. During a median follow-up of approximately 5 years, 177 subjects developed end-stage renal disease (ESRD) and 1,940 died of all causes.
Adding either the cystatin C or the albuminuria measures to creatinine-based estimated GFR greatly improved the ability to predict the development of ESRD as well as mortality. Using all three measures together improved it even further.
The rate of ESRD was 103-fold higher in subjects who were identified as being at risk via the triple-marker method than in those who were identified via creatinine level alone. Similarly, mortality was nearly eight times higher among those identified as being at risk via the triple-marker method than in those identified via creatinine level alone, the investigators said (JAMA 2011;305:1545-52).
Moreover, one in six subjects who were found to have CKD by the triple-marker method were not identified at all by creatinine level alone, suggesting that people could have occult CKD that would not be diagnosed according to standard methods.
In addition, 25% of subjects who were labeled as having CKD by creatinine-based GFR estimate alone were found not to have CKD when cystatin C and albuminuria levels were added into the risk stratification. This suggests that one-fourth of people screened by standard methods alone may be treated as though they’re at increased risk of ESRD or death when they are not.
Thus, using the triple-marker method "could both reduce unwarranted referrals and unnecessary work-ups for low-risk individuals and would prioritize specialty care and interventions to individuals at highest risk," Dr. Peralta and her colleagues said.
"Several groups are currently advocating new international guidelines that more accurately reflect prognosis of CKD and have proposed adding [albumin:creatinine ratio] to staging of CKD. Our results suggest that a triple-marker approach using both [albuminuria] and cystatin C to confirm CKD more accurately discriminates prognosis for death and progression to ESRD than creatinine and [albuminuria] alone," they added.
In the second report, investigators developed seven possible models for predicting CKD progression, using various combinations of more than 20 candidate variables that are known to affect renal function. To do so, they first assessed these variables in a Canadian cohort of 3,449 adults who were treated at nephrology clinics for stage 3-5 CKD.
These study subjects, who were followed from 2001 through 2008, helped determine which variables were best associated with CKD progression, said Dr. Navdeep Tangri of Tufts Medical Center, Boston, and his associates.
One of the seven models was particularly accurate at predicting CKD progression. It factored in patient age and sex, as well as the estimated GFR, albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin levels. These are basic laboratory measurements "that are obtained routinely in patients with CKD," Dr. Tangri and his colleagues said (JAMA 2011;305:1553-9).
All seven models were tested in a validation cohort of 4,942 patients in a British Columbia registry who had stage 3-5 CKD. The same model was found to be the most accurate in predicting disease progression in that cohort.
"Using our models, lower-risk patients could be managed by the primary care physician without additional testing or treatment of CKD complications," whereas higher-risk patients could receive "more intensive testing, intervention, and early nephrology care," they said.
The models also could be used to triage patients with stage 4 CKD "regarding dialysis education, creation of vascular access, and preemptive transplantation"; to select stage 3 patients for public health interventions; and to select high-risk patients for enrollment in clinical trials, the researchers added.
Dr. Peralta’s study was supported by the National Institute of Neurological Disorders and Stroke, Amgen Corp., the National Institute of Diabetes and Digestive and Kidney Diseases, the Robert Wood Johnson Foundation, and the American Heart Association. Several associates also reported ties to Amgen. Dr. Tangri’s study was supported by a joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research, and the Canadian Society of Nephrology, as well as by the Change Foundation of Ontario. Dr. Tangri and associates reported no financial conflicts of interest.
The findings from both of these studies are "novel and important," but "much additional work is needed before these approaches can be recommended for routine clinical use," said Dr. Marcello Tonelli and Dr. Braden Manns.
Both studies provide proof of concept for two different methods that might enhance prognostic power, compared with the use of creatinine-estimated GFR alone.
However, it would be premature to introduce the triple-marker approach of Dr. Peralta and colleagues into clinical practice for three reasons: First, their study population was highly selected, so the findings cannot be generalized to other settings until further research is done. Second, current practice includes confirmation of estimated GFR with a second measurement to confirm CKD, and this repeat measurement was not addressed in this study. And third, the use of more stringent estimated GFR criteria to define CKD might attenuate the advantage of the triple-marker method.
It also would be premature to implement the prediction model of Dr. Tangri and colleagues because there were missing data in both the cohort used to develop the models and the validation cohort, and values were imputed for subjects who had incomplete information. More importantly, all the study subjects were already in the care of a nephrologist, so they are not representative of the general population.
"Careful consideration and research will be required to determine whether the benefits [of an eight-variable model] would outweigh its added complexity," they concluded.
Dr. Tonelli is at the University of Alberta, Edmonton. Dr. Manns is at the University of Calgary (Alta.). They were supported by the Alberta Heritage Foundation for Medical Research, by Alberta Health and Wellness, and by the University of Alberta. Dr. Tonelli also is supported by a Government of Canada Research Chair in the optimal treatment of CKD. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying the two reports (JAMA 2011:305:1593-5).
The findings from both of these studies are "novel and important," but "much additional work is needed before these approaches can be recommended for routine clinical use," said Dr. Marcello Tonelli and Dr. Braden Manns.
Both studies provide proof of concept for two different methods that might enhance prognostic power, compared with the use of creatinine-estimated GFR alone.
However, it would be premature to introduce the triple-marker approach of Dr. Peralta and colleagues into clinical practice for three reasons: First, their study population was highly selected, so the findings cannot be generalized to other settings until further research is done. Second, current practice includes confirmation of estimated GFR with a second measurement to confirm CKD, and this repeat measurement was not addressed in this study. And third, the use of more stringent estimated GFR criteria to define CKD might attenuate the advantage of the triple-marker method.
It also would be premature to implement the prediction model of Dr. Tangri and colleagues because there were missing data in both the cohort used to develop the models and the validation cohort, and values were imputed for subjects who had incomplete information. More importantly, all the study subjects were already in the care of a nephrologist, so they are not representative of the general population.
"Careful consideration and research will be required to determine whether the benefits [of an eight-variable model] would outweigh its added complexity," they concluded.
Dr. Tonelli is at the University of Alberta, Edmonton. Dr. Manns is at the University of Calgary (Alta.). They were supported by the Alberta Heritage Foundation for Medical Research, by Alberta Health and Wellness, and by the University of Alberta. Dr. Tonelli also is supported by a Government of Canada Research Chair in the optimal treatment of CKD. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying the two reports (JAMA 2011:305:1593-5).
The findings from both of these studies are "novel and important," but "much additional work is needed before these approaches can be recommended for routine clinical use," said Dr. Marcello Tonelli and Dr. Braden Manns.
Both studies provide proof of concept for two different methods that might enhance prognostic power, compared with the use of creatinine-estimated GFR alone.
However, it would be premature to introduce the triple-marker approach of Dr. Peralta and colleagues into clinical practice for three reasons: First, their study population was highly selected, so the findings cannot be generalized to other settings until further research is done. Second, current practice includes confirmation of estimated GFR with a second measurement to confirm CKD, and this repeat measurement was not addressed in this study. And third, the use of more stringent estimated GFR criteria to define CKD might attenuate the advantage of the triple-marker method.
It also would be premature to implement the prediction model of Dr. Tangri and colleagues because there were missing data in both the cohort used to develop the models and the validation cohort, and values were imputed for subjects who had incomplete information. More importantly, all the study subjects were already in the care of a nephrologist, so they are not representative of the general population.
"Careful consideration and research will be required to determine whether the benefits [of an eight-variable model] would outweigh its added complexity," they concluded.
Dr. Tonelli is at the University of Alberta, Edmonton. Dr. Manns is at the University of Calgary (Alta.). They were supported by the Alberta Heritage Foundation for Medical Research, by Alberta Health and Wellness, and by the University of Alberta. Dr. Tonelli also is supported by a Government of Canada Research Chair in the optimal treatment of CKD. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying the two reports (JAMA 2011:305:1593-5).
Separate research groups offer two new methods for predicting which patients with chronic kidney disease are likely to progress to end-stage renal disease and other complications, according to studies published in the April 20 issue of JAMA.
Ultimately, the methods could help more accurately identify individuals who are at low and high risk for CKD progression, and allow for more appropriate use of health care resources for this population.
In the first report, a "triple-marker" approach using serum creatinine, serum cystatin C, and albuminuria levels yielded more accurate risk prediction than is achieved with current staging systems that are based primarily on creatinine-estimated glomerular filtration rates, said Dr. Carmen A. Peralta of the San Francisco VA Medical Center and her associates.
They assessed the triple-marker technique using a large, population-based cohort of adults (aged 45 years and older) who were participating in a stroke study. A subgroup of 26,643 of these subjects had undergone evaluation of kidney function as part of that study.
Patients in Dr. Peralta’s analysis had a mean age of 65 years. In all, 40% were black, more than half were women, 21% had diabetes, and 59% had hypertension. During a median follow-up of approximately 5 years, 177 subjects developed end-stage renal disease (ESRD) and 1,940 died of all causes.
Adding either the cystatin C or the albuminuria measures to creatinine-based estimated GFR greatly improved the ability to predict the development of ESRD as well as mortality. Using all three measures together improved it even further.
The rate of ESRD was 103-fold higher in subjects who were identified as being at risk via the triple-marker method than in those who were identified via creatinine level alone. Similarly, mortality was nearly eight times higher among those identified as being at risk via the triple-marker method than in those identified via creatinine level alone, the investigators said (JAMA 2011;305:1545-52).
Moreover, one in six subjects who were found to have CKD by the triple-marker method were not identified at all by creatinine level alone, suggesting that people could have occult CKD that would not be diagnosed according to standard methods.
In addition, 25% of subjects who were labeled as having CKD by creatinine-based GFR estimate alone were found not to have CKD when cystatin C and albuminuria levels were added into the risk stratification. This suggests that one-fourth of people screened by standard methods alone may be treated as though they’re at increased risk of ESRD or death when they are not.
Thus, using the triple-marker method "could both reduce unwarranted referrals and unnecessary work-ups for low-risk individuals and would prioritize specialty care and interventions to individuals at highest risk," Dr. Peralta and her colleagues said.
"Several groups are currently advocating new international guidelines that more accurately reflect prognosis of CKD and have proposed adding [albumin:creatinine ratio] to staging of CKD. Our results suggest that a triple-marker approach using both [albuminuria] and cystatin C to confirm CKD more accurately discriminates prognosis for death and progression to ESRD than creatinine and [albuminuria] alone," they added.
In the second report, investigators developed seven possible models for predicting CKD progression, using various combinations of more than 20 candidate variables that are known to affect renal function. To do so, they first assessed these variables in a Canadian cohort of 3,449 adults who were treated at nephrology clinics for stage 3-5 CKD.
These study subjects, who were followed from 2001 through 2008, helped determine which variables were best associated with CKD progression, said Dr. Navdeep Tangri of Tufts Medical Center, Boston, and his associates.
One of the seven models was particularly accurate at predicting CKD progression. It factored in patient age and sex, as well as the estimated GFR, albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin levels. These are basic laboratory measurements "that are obtained routinely in patients with CKD," Dr. Tangri and his colleagues said (JAMA 2011;305:1553-9).
All seven models were tested in a validation cohort of 4,942 patients in a British Columbia registry who had stage 3-5 CKD. The same model was found to be the most accurate in predicting disease progression in that cohort.
"Using our models, lower-risk patients could be managed by the primary care physician without additional testing or treatment of CKD complications," whereas higher-risk patients could receive "more intensive testing, intervention, and early nephrology care," they said.
The models also could be used to triage patients with stage 4 CKD "regarding dialysis education, creation of vascular access, and preemptive transplantation"; to select stage 3 patients for public health interventions; and to select high-risk patients for enrollment in clinical trials, the researchers added.
Dr. Peralta’s study was supported by the National Institute of Neurological Disorders and Stroke, Amgen Corp., the National Institute of Diabetes and Digestive and Kidney Diseases, the Robert Wood Johnson Foundation, and the American Heart Association. Several associates also reported ties to Amgen. Dr. Tangri’s study was supported by a joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research, and the Canadian Society of Nephrology, as well as by the Change Foundation of Ontario. Dr. Tangri and associates reported no financial conflicts of interest.
Separate research groups offer two new methods for predicting which patients with chronic kidney disease are likely to progress to end-stage renal disease and other complications, according to studies published in the April 20 issue of JAMA.
Ultimately, the methods could help more accurately identify individuals who are at low and high risk for CKD progression, and allow for more appropriate use of health care resources for this population.
In the first report, a "triple-marker" approach using serum creatinine, serum cystatin C, and albuminuria levels yielded more accurate risk prediction than is achieved with current staging systems that are based primarily on creatinine-estimated glomerular filtration rates, said Dr. Carmen A. Peralta of the San Francisco VA Medical Center and her associates.
They assessed the triple-marker technique using a large, population-based cohort of adults (aged 45 years and older) who were participating in a stroke study. A subgroup of 26,643 of these subjects had undergone evaluation of kidney function as part of that study.
Patients in Dr. Peralta’s analysis had a mean age of 65 years. In all, 40% were black, more than half were women, 21% had diabetes, and 59% had hypertension. During a median follow-up of approximately 5 years, 177 subjects developed end-stage renal disease (ESRD) and 1,940 died of all causes.
Adding either the cystatin C or the albuminuria measures to creatinine-based estimated GFR greatly improved the ability to predict the development of ESRD as well as mortality. Using all three measures together improved it even further.
The rate of ESRD was 103-fold higher in subjects who were identified as being at risk via the triple-marker method than in those who were identified via creatinine level alone. Similarly, mortality was nearly eight times higher among those identified as being at risk via the triple-marker method than in those identified via creatinine level alone, the investigators said (JAMA 2011;305:1545-52).
Moreover, one in six subjects who were found to have CKD by the triple-marker method were not identified at all by creatinine level alone, suggesting that people could have occult CKD that would not be diagnosed according to standard methods.
In addition, 25% of subjects who were labeled as having CKD by creatinine-based GFR estimate alone were found not to have CKD when cystatin C and albuminuria levels were added into the risk stratification. This suggests that one-fourth of people screened by standard methods alone may be treated as though they’re at increased risk of ESRD or death when they are not.
Thus, using the triple-marker method "could both reduce unwarranted referrals and unnecessary work-ups for low-risk individuals and would prioritize specialty care and interventions to individuals at highest risk," Dr. Peralta and her colleagues said.
"Several groups are currently advocating new international guidelines that more accurately reflect prognosis of CKD and have proposed adding [albumin:creatinine ratio] to staging of CKD. Our results suggest that a triple-marker approach using both [albuminuria] and cystatin C to confirm CKD more accurately discriminates prognosis for death and progression to ESRD than creatinine and [albuminuria] alone," they added.
In the second report, investigators developed seven possible models for predicting CKD progression, using various combinations of more than 20 candidate variables that are known to affect renal function. To do so, they first assessed these variables in a Canadian cohort of 3,449 adults who were treated at nephrology clinics for stage 3-5 CKD.
These study subjects, who were followed from 2001 through 2008, helped determine which variables were best associated with CKD progression, said Dr. Navdeep Tangri of Tufts Medical Center, Boston, and his associates.
One of the seven models was particularly accurate at predicting CKD progression. It factored in patient age and sex, as well as the estimated GFR, albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin levels. These are basic laboratory measurements "that are obtained routinely in patients with CKD," Dr. Tangri and his colleagues said (JAMA 2011;305:1553-9).
All seven models were tested in a validation cohort of 4,942 patients in a British Columbia registry who had stage 3-5 CKD. The same model was found to be the most accurate in predicting disease progression in that cohort.
"Using our models, lower-risk patients could be managed by the primary care physician without additional testing or treatment of CKD complications," whereas higher-risk patients could receive "more intensive testing, intervention, and early nephrology care," they said.
The models also could be used to triage patients with stage 4 CKD "regarding dialysis education, creation of vascular access, and preemptive transplantation"; to select stage 3 patients for public health interventions; and to select high-risk patients for enrollment in clinical trials, the researchers added.
Dr. Peralta’s study was supported by the National Institute of Neurological Disorders and Stroke, Amgen Corp., the National Institute of Diabetes and Digestive and Kidney Diseases, the Robert Wood Johnson Foundation, and the American Heart Association. Several associates also reported ties to Amgen. Dr. Tangri’s study was supported by a joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research, and the Canadian Society of Nephrology, as well as by the Change Foundation of Ontario. Dr. Tangri and associates reported no financial conflicts of interest.
FROM JAMA
Transplant/Nontransplant Outcomes Similar After Endovascular Intervention
LAKE BUENA VISTA, FLA. – Primary, primary-assisted, and secondary patency and limb salvage rates are similar in transplant and nontransplant populations, but renal transplant patients have slightly – though not significantly – worse outcomes than do heart transplant patients, according to findings from a study of endovascular peripheral interventions in these populations.
A total of 122 lesions in 58 renal or cardiac transplant patients were identified using information from a prospective lower-extremity database encompassing more than 1,500 interventions performed from 2004 through 2010 at a single, high-volume vascular and transplant center. The data were cross-referenced with heart failure and renal transplant registries from the same center, Dr. Katherine A. Gallagher said at the annual meeting of the Society for Clinical Vascular Surgery.
The transplant patients – 44 men and 14 women with a mean age of 63.5 years – all were on active immunosuppressive treatment and were followed clinically and with noninvasive laboratory testing for 30 months. Indications for lower-extremity interventions were claudication in 48% of cases and critical limb ischemia in 52% of cases. Mean lesion length was 108.6 mm, said Dr. Gallagher of Weill Cornell/Columbia University, New York.
Primary, primary-assisted, and secondary patency and limb salvage rates were "essentially the same" at 50 months in the transplant patients and in 1,162 nontransplant control patents, at about 40% vs. 45%, 55% vs. 60%, 60% vs. 65%, and 70% vs. 65% cumulative survival, respectively, she said.
Subgroup analyses showed that heart transplant patients showed a trend for better primary patency rates than did renal transplant patients with similar TASC classification and comorbid conditions (about 75% vs. 55%) , although the heart recipients had less critical limb ischemia.
After controlling for potential confounding factors, the researchers found in subgroup analyses that male transplant patients had significantly less severe disease and much better outcomes, Dr. Gallagher said in an interview.
Mean follow-up in these patients was 18.6 months, Dr. Gallagher noted.
Percutaneous intervention has become the first-line treatment for many patient groups, but outcomes in transplant patients prior to this study were relatively unknown, she said.
"We do know that the incidence of peripheral artery disease [PAD] is high in patients with renal insufficiency, and we know that both open and endovascular outcomes in this patient cohort portend poor patency and limb salvage rates," she said, adding that there is evidence that renal transplant patients have a high incidence of PAD, but outcomes following endovascular intervention in these populations are unknown.
"We can theorize that they might have improved outcomes because they have improved renal function, but they might also have poorer outcomes because of the deleterious effects of immunosuppression as it relates to hyperlipidemia and hyperglycemia," she said.
Furthermore, it is known that cardiac transplant patients are at risk for PAD, but the effects of immunosuppression in this population are unknown.
"Specifically these patients are on a lot of tacrolimus and sirolimus, which have been well studied in drug-eluting stents, but we don’t know the effects of these drugs on endovascular interventions," she said.
In this study, transplant patients had higher rates of diabetes and renal insufficiency – two factors shown to be independent predictors of poor outcome – so they would be expected to have poorer outcomes than would nontransplant patients, yet outcomes between the two groups were very similar.
"We believe this is potentially due to protective effects from systemic immunosuppression, although this requires further investigation, she concluded.
Dr. Gallagher said that she had no relevant financial disclosures.
LAKE BUENA VISTA, FLA. – Primary, primary-assisted, and secondary patency and limb salvage rates are similar in transplant and nontransplant populations, but renal transplant patients have slightly – though not significantly – worse outcomes than do heart transplant patients, according to findings from a study of endovascular peripheral interventions in these populations.
A total of 122 lesions in 58 renal or cardiac transplant patients were identified using information from a prospective lower-extremity database encompassing more than 1,500 interventions performed from 2004 through 2010 at a single, high-volume vascular and transplant center. The data were cross-referenced with heart failure and renal transplant registries from the same center, Dr. Katherine A. Gallagher said at the annual meeting of the Society for Clinical Vascular Surgery.
The transplant patients – 44 men and 14 women with a mean age of 63.5 years – all were on active immunosuppressive treatment and were followed clinically and with noninvasive laboratory testing for 30 months. Indications for lower-extremity interventions were claudication in 48% of cases and critical limb ischemia in 52% of cases. Mean lesion length was 108.6 mm, said Dr. Gallagher of Weill Cornell/Columbia University, New York.
Primary, primary-assisted, and secondary patency and limb salvage rates were "essentially the same" at 50 months in the transplant patients and in 1,162 nontransplant control patents, at about 40% vs. 45%, 55% vs. 60%, 60% vs. 65%, and 70% vs. 65% cumulative survival, respectively, she said.
Subgroup analyses showed that heart transplant patients showed a trend for better primary patency rates than did renal transplant patients with similar TASC classification and comorbid conditions (about 75% vs. 55%) , although the heart recipients had less critical limb ischemia.
After controlling for potential confounding factors, the researchers found in subgroup analyses that male transplant patients had significantly less severe disease and much better outcomes, Dr. Gallagher said in an interview.
Mean follow-up in these patients was 18.6 months, Dr. Gallagher noted.
Percutaneous intervention has become the first-line treatment for many patient groups, but outcomes in transplant patients prior to this study were relatively unknown, she said.
"We do know that the incidence of peripheral artery disease [PAD] is high in patients with renal insufficiency, and we know that both open and endovascular outcomes in this patient cohort portend poor patency and limb salvage rates," she said, adding that there is evidence that renal transplant patients have a high incidence of PAD, but outcomes following endovascular intervention in these populations are unknown.
"We can theorize that they might have improved outcomes because they have improved renal function, but they might also have poorer outcomes because of the deleterious effects of immunosuppression as it relates to hyperlipidemia and hyperglycemia," she said.
Furthermore, it is known that cardiac transplant patients are at risk for PAD, but the effects of immunosuppression in this population are unknown.
"Specifically these patients are on a lot of tacrolimus and sirolimus, which have been well studied in drug-eluting stents, but we don’t know the effects of these drugs on endovascular interventions," she said.
In this study, transplant patients had higher rates of diabetes and renal insufficiency – two factors shown to be independent predictors of poor outcome – so they would be expected to have poorer outcomes than would nontransplant patients, yet outcomes between the two groups were very similar.
"We believe this is potentially due to protective effects from systemic immunosuppression, although this requires further investigation, she concluded.
Dr. Gallagher said that she had no relevant financial disclosures.
LAKE BUENA VISTA, FLA. – Primary, primary-assisted, and secondary patency and limb salvage rates are similar in transplant and nontransplant populations, but renal transplant patients have slightly – though not significantly – worse outcomes than do heart transplant patients, according to findings from a study of endovascular peripheral interventions in these populations.
A total of 122 lesions in 58 renal or cardiac transplant patients were identified using information from a prospective lower-extremity database encompassing more than 1,500 interventions performed from 2004 through 2010 at a single, high-volume vascular and transplant center. The data were cross-referenced with heart failure and renal transplant registries from the same center, Dr. Katherine A. Gallagher said at the annual meeting of the Society for Clinical Vascular Surgery.
The transplant patients – 44 men and 14 women with a mean age of 63.5 years – all were on active immunosuppressive treatment and were followed clinically and with noninvasive laboratory testing for 30 months. Indications for lower-extremity interventions were claudication in 48% of cases and critical limb ischemia in 52% of cases. Mean lesion length was 108.6 mm, said Dr. Gallagher of Weill Cornell/Columbia University, New York.
Primary, primary-assisted, and secondary patency and limb salvage rates were "essentially the same" at 50 months in the transplant patients and in 1,162 nontransplant control patents, at about 40% vs. 45%, 55% vs. 60%, 60% vs. 65%, and 70% vs. 65% cumulative survival, respectively, she said.
Subgroup analyses showed that heart transplant patients showed a trend for better primary patency rates than did renal transplant patients with similar TASC classification and comorbid conditions (about 75% vs. 55%) , although the heart recipients had less critical limb ischemia.
After controlling for potential confounding factors, the researchers found in subgroup analyses that male transplant patients had significantly less severe disease and much better outcomes, Dr. Gallagher said in an interview.
Mean follow-up in these patients was 18.6 months, Dr. Gallagher noted.
Percutaneous intervention has become the first-line treatment for many patient groups, but outcomes in transplant patients prior to this study were relatively unknown, she said.
"We do know that the incidence of peripheral artery disease [PAD] is high in patients with renal insufficiency, and we know that both open and endovascular outcomes in this patient cohort portend poor patency and limb salvage rates," she said, adding that there is evidence that renal transplant patients have a high incidence of PAD, but outcomes following endovascular intervention in these populations are unknown.
"We can theorize that they might have improved outcomes because they have improved renal function, but they might also have poorer outcomes because of the deleterious effects of immunosuppression as it relates to hyperlipidemia and hyperglycemia," she said.
Furthermore, it is known that cardiac transplant patients are at risk for PAD, but the effects of immunosuppression in this population are unknown.
"Specifically these patients are on a lot of tacrolimus and sirolimus, which have been well studied in drug-eluting stents, but we don’t know the effects of these drugs on endovascular interventions," she said.
In this study, transplant patients had higher rates of diabetes and renal insufficiency – two factors shown to be independent predictors of poor outcome – so they would be expected to have poorer outcomes than would nontransplant patients, yet outcomes between the two groups were very similar.
"We believe this is potentially due to protective effects from systemic immunosuppression, although this requires further investigation, she concluded.
Dr. Gallagher said that she had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR CLINICAL VASCULAR SURGERY
Major Finding: Primary, primary-assisted, and secondary patency and limb salvage rates were "essentially the same" in the transplant and nontransplant control patents.
Data Source: Database study of more than 1,500 lower-extremity interventions with cross-referencing of heart failure and renal transplant registries.
Disclosures: Dr. Gallagher said that she had no disclosures.
Transplant/Nontransplant Outcomes Similar After Endovascular Intervention
LAKE BUENA VISTA, FLA. – Primary, primary-assisted, and secondary patency and limb salvage rates are similar in transplant and nontransplant populations, but renal transplant patients have slightly – though not significantly – worse outcomes than do heart transplant patients, according to findings from a study of endovascular peripheral interventions in these populations.
A total of 122 lesions in 58 renal or cardiac transplant patients were identified using information from a prospective lower-extremity database encompassing more than 1,500 interventions performed from 2004 through 2010 at a single, high-volume vascular and transplant center. The data were cross-referenced with heart failure and renal transplant registries from the same center, Dr. Katherine A. Gallagher said at the annual meeting of the Society for Clinical Vascular Surgery.
The transplant patients – 44 men and 14 women with a mean age of 63.5 years – all were on active immunosuppressive treatment and were followed clinically and with noninvasive laboratory testing for 30 months. Indications for lower-extremity interventions were claudication in 48% of cases and critical limb ischemia in 52% of cases. Mean lesion length was 108.6 mm, said Dr. Gallagher of Weill Cornell/Columbia University, New York.
Primary, primary-assisted, and secondary patency and limb salvage rates were "essentially the same" at 50 months in the transplant patients and in 1,162 nontransplant control patents, at about 40% vs. 45%, 55% vs. 60%, 60% vs. 65%, and 70% vs. 65% cumulative survival, respectively, she said.
Subgroup analyses showed that heart transplant patients showed a trend for better primary patency rates than did renal transplant patients with similar TASC classification and comorbid conditions (about 75% vs. 55%) , although the heart recipients had less critical limb ischemia.
After controlling for potential confounding factors, the researchers found in subgroup analyses that male transplant patients had significantly less severe disease and much better outcomes, Dr. Gallagher said in an interview.
Mean follow-up in these patients was 18.6 months, Dr. Gallagher noted.
Percutaneous intervention has become the first-line treatment for many patient groups, but outcomes in transplant patients prior to this study were relatively unknown, she said.
"We do know that the incidence of peripheral artery disease [PAD] is high in patients with renal insufficiency, and we know that both open and endovascular outcomes in this patient cohort portend poor patency and limb salvage rates," she said, adding that there is evidence that renal transplant patients have a high incidence of PAD, but outcomes following endovascular intervention in these populations are unknown.
"We can theorize that they might have improved outcomes because they have improved renal function, but they might also have poorer outcomes because of the deleterious effects of immunosuppression as it relates to hyperlipidemia and hyperglycemia," she said.
Furthermore, it is known that cardiac transplant patients are at risk for PAD, but the effects of immunosuppression in this population are unknown.
"Specifically these patients are on a lot of tacrolimus and sirolimus, which have been well studied in drug-eluting stents, but we don’t know the effects of these drugs on endovascular interventions," she said.
In this study, transplant patients had higher rates of diabetes and renal insufficiency – two factors shown to be independent predictors of poor outcome – so they would be expected to have poorer outcomes than would nontransplant patients, yet outcomes between the two groups were very similar.
"We believe this is potentially due to protective effects from systemic immunosuppression, although this requires further investigation, she concluded.
Dr. Gallagher said that she had no relevant financial disclosures.
LAKE BUENA VISTA, FLA. – Primary, primary-assisted, and secondary patency and limb salvage rates are similar in transplant and nontransplant populations, but renal transplant patients have slightly – though not significantly – worse outcomes than do heart transplant patients, according to findings from a study of endovascular peripheral interventions in these populations.
A total of 122 lesions in 58 renal or cardiac transplant patients were identified using information from a prospective lower-extremity database encompassing more than 1,500 interventions performed from 2004 through 2010 at a single, high-volume vascular and transplant center. The data were cross-referenced with heart failure and renal transplant registries from the same center, Dr. Katherine A. Gallagher said at the annual meeting of the Society for Clinical Vascular Surgery.
The transplant patients – 44 men and 14 women with a mean age of 63.5 years – all were on active immunosuppressive treatment and were followed clinically and with noninvasive laboratory testing for 30 months. Indications for lower-extremity interventions were claudication in 48% of cases and critical limb ischemia in 52% of cases. Mean lesion length was 108.6 mm, said Dr. Gallagher of Weill Cornell/Columbia University, New York.
Primary, primary-assisted, and secondary patency and limb salvage rates were "essentially the same" at 50 months in the transplant patients and in 1,162 nontransplant control patents, at about 40% vs. 45%, 55% vs. 60%, 60% vs. 65%, and 70% vs. 65% cumulative survival, respectively, she said.
Subgroup analyses showed that heart transplant patients showed a trend for better primary patency rates than did renal transplant patients with similar TASC classification and comorbid conditions (about 75% vs. 55%) , although the heart recipients had less critical limb ischemia.
After controlling for potential confounding factors, the researchers found in subgroup analyses that male transplant patients had significantly less severe disease and much better outcomes, Dr. Gallagher said in an interview.
Mean follow-up in these patients was 18.6 months, Dr. Gallagher noted.
Percutaneous intervention has become the first-line treatment for many patient groups, but outcomes in transplant patients prior to this study were relatively unknown, she said.
"We do know that the incidence of peripheral artery disease [PAD] is high in patients with renal insufficiency, and we know that both open and endovascular outcomes in this patient cohort portend poor patency and limb salvage rates," she said, adding that there is evidence that renal transplant patients have a high incidence of PAD, but outcomes following endovascular intervention in these populations are unknown.
"We can theorize that they might have improved outcomes because they have improved renal function, but they might also have poorer outcomes because of the deleterious effects of immunosuppression as it relates to hyperlipidemia and hyperglycemia," she said.
Furthermore, it is known that cardiac transplant patients are at risk for PAD, but the effects of immunosuppression in this population are unknown.
"Specifically these patients are on a lot of tacrolimus and sirolimus, which have been well studied in drug-eluting stents, but we don’t know the effects of these drugs on endovascular interventions," she said.
In this study, transplant patients had higher rates of diabetes and renal insufficiency – two factors shown to be independent predictors of poor outcome – so they would be expected to have poorer outcomes than would nontransplant patients, yet outcomes between the two groups were very similar.
"We believe this is potentially due to protective effects from systemic immunosuppression, although this requires further investigation, she concluded.
Dr. Gallagher said that she had no relevant financial disclosures.
LAKE BUENA VISTA, FLA. – Primary, primary-assisted, and secondary patency and limb salvage rates are similar in transplant and nontransplant populations, but renal transplant patients have slightly – though not significantly – worse outcomes than do heart transplant patients, according to findings from a study of endovascular peripheral interventions in these populations.
A total of 122 lesions in 58 renal or cardiac transplant patients were identified using information from a prospective lower-extremity database encompassing more than 1,500 interventions performed from 2004 through 2010 at a single, high-volume vascular and transplant center. The data were cross-referenced with heart failure and renal transplant registries from the same center, Dr. Katherine A. Gallagher said at the annual meeting of the Society for Clinical Vascular Surgery.
The transplant patients – 44 men and 14 women with a mean age of 63.5 years – all were on active immunosuppressive treatment and were followed clinically and with noninvasive laboratory testing for 30 months. Indications for lower-extremity interventions were claudication in 48% of cases and critical limb ischemia in 52% of cases. Mean lesion length was 108.6 mm, said Dr. Gallagher of Weill Cornell/Columbia University, New York.
Primary, primary-assisted, and secondary patency and limb salvage rates were "essentially the same" at 50 months in the transplant patients and in 1,162 nontransplant control patents, at about 40% vs. 45%, 55% vs. 60%, 60% vs. 65%, and 70% vs. 65% cumulative survival, respectively, she said.
Subgroup analyses showed that heart transplant patients showed a trend for better primary patency rates than did renal transplant patients with similar TASC classification and comorbid conditions (about 75% vs. 55%) , although the heart recipients had less critical limb ischemia.
After controlling for potential confounding factors, the researchers found in subgroup analyses that male transplant patients had significantly less severe disease and much better outcomes, Dr. Gallagher said in an interview.
Mean follow-up in these patients was 18.6 months, Dr. Gallagher noted.
Percutaneous intervention has become the first-line treatment for many patient groups, but outcomes in transplant patients prior to this study were relatively unknown, she said.
"We do know that the incidence of peripheral artery disease [PAD] is high in patients with renal insufficiency, and we know that both open and endovascular outcomes in this patient cohort portend poor patency and limb salvage rates," she said, adding that there is evidence that renal transplant patients have a high incidence of PAD, but outcomes following endovascular intervention in these populations are unknown.
"We can theorize that they might have improved outcomes because they have improved renal function, but they might also have poorer outcomes because of the deleterious effects of immunosuppression as it relates to hyperlipidemia and hyperglycemia," she said.
Furthermore, it is known that cardiac transplant patients are at risk for PAD, but the effects of immunosuppression in this population are unknown.
"Specifically these patients are on a lot of tacrolimus and sirolimus, which have been well studied in drug-eluting stents, but we don’t know the effects of these drugs on endovascular interventions," she said.
In this study, transplant patients had higher rates of diabetes and renal insufficiency – two factors shown to be independent predictors of poor outcome – so they would be expected to have poorer outcomes than would nontransplant patients, yet outcomes between the two groups were very similar.
"We believe this is potentially due to protective effects from systemic immunosuppression, although this requires further investigation, she concluded.
Dr. Gallagher said that she had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR CLINICAL VASCULAR SURGERY
Probiotic Treatment Halves Recurrent UTI Risk
A probiotic medication has been shown to reduce the risk of recurrent urinary tract infections by nearly half – making it comparable to a prophylactic course of antibiotics, the current standard treatment for recurrent urinary tract infections.
Concerns about increasing resistance to commonly used antimicrobials such as trimethoprim-sulfamethoxazole and fluoroquinolones has led to a search for alternative means to prevent urinary tract infections (UTIs), with recent investigations focusing on hydrogen peroxide-producing strains of lactobacilli.
These lactobacilli comprise the dominant vaginal flora, competing with and regulating other urogenital microbes, including the harmful Escherichia coli. Women with recurrent UTIs have been shown to typically have depletion of vaginal lactobacilli at the time of their E. coli infections (J. Infect. Dis. 1998;178:446-50).
The new findings, from a randomized, placebo-controlled trial of a vaginal suppository containing Lactobacillus crispatus in 100 young women, were published online April 15 in Clinical Infectious Diseases (Clin. Infect. Dis. 2011;52:1212-17).
For their research, Dr. Ann E. Stapleton of the University of Washington in Seattle, and her colleagues, recruited women with current cystitis and histories of recurrent UTI to be randomized to Lactin-V (Osel Inc., Mountain View, Calif.), a product containing L. crispatus, or placebo. The women (median age 21, median number of UTIs before study 4.5) applied either Lactin-V or placebo daily for 5 days, then once weekly for 10 weeks. Participants were seen in scheduled follow-up visits at 1 week (visit 3) and 10 weeks (visit 4) after beginning Lactin-V or placebo and for symptomatic UTIs. Investigators collected urine samples for culture and vaginal swabs to assess the level of colonization of L. crispatus. Of the 50 women randomized in each group, 48 were entered into analysis.
Recurrent culture-confirmed UTI occurred in 7 of 48 women (15%) receiving Lactin-V, compared with 13 of 48 women (27%) receiving placebo (RR 0.5; 95% confidence interval, 0.2-1.2).
The investigators named as one of the strengths of the study the quantitative molecular method used to assess vaginal flora following UTI, which afforded them a more precise picture of changes in the balance of vaginal microbiota than seen in previous trials.
The investigators also noted that high-level vaginal colonization with L. crispatus throughout follow-up was associated with a significant reduction in recurrent UTI only for women using the suppositories. In their analysis, Dr. Stapleton and colleagues called this finding "striking" and hypothesized that the use of Lactin-V "confers a significant advantage over repopulation of the vaginal microbiota with endogenous L. crispatus," and that the L. crispatus isolate used in the intervention offered "unique properties for protection" over the natural recolonization process.
The investigators did not compare Lactin-V to prophylactic antibiotics in their study. However, they noted that a meta-analysis of 10 randomized controlled trials evaluating continuous antimicrobial prophylaxis (Clin. Evid. [Online] 2008 Jul 17;2008. pii:0801) found rates of rates of recurrent UTI reduced to 12% (24 of 195 participants) compared with 65% (116 of 177 participants) among placebo recipients, "with a [relative risk] of rUTI that is very comparable to our findings (RR, 0.21; 95% CI, 0.13-0.33)."
Dr. Stapleton and colleagues concluded that "[t]his antimicrobial-sparing, well-tolerated intervention compares favorably with historical data regarding antimicrobial prophylaxis, the current standard of care for the prevention of rUTI."
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and the Office of Research in Women’s Health, National Institutes of Health; Dr. Stapleton and her coauthors said they had no relevant financial disclosures.
A probiotic medication has been shown to reduce the risk of recurrent urinary tract infections by nearly half – making it comparable to a prophylactic course of antibiotics, the current standard treatment for recurrent urinary tract infections.
Concerns about increasing resistance to commonly used antimicrobials such as trimethoprim-sulfamethoxazole and fluoroquinolones has led to a search for alternative means to prevent urinary tract infections (UTIs), with recent investigations focusing on hydrogen peroxide-producing strains of lactobacilli.
These lactobacilli comprise the dominant vaginal flora, competing with and regulating other urogenital microbes, including the harmful Escherichia coli. Women with recurrent UTIs have been shown to typically have depletion of vaginal lactobacilli at the time of their E. coli infections (J. Infect. Dis. 1998;178:446-50).
The new findings, from a randomized, placebo-controlled trial of a vaginal suppository containing Lactobacillus crispatus in 100 young women, were published online April 15 in Clinical Infectious Diseases (Clin. Infect. Dis. 2011;52:1212-17).
For their research, Dr. Ann E. Stapleton of the University of Washington in Seattle, and her colleagues, recruited women with current cystitis and histories of recurrent UTI to be randomized to Lactin-V (Osel Inc., Mountain View, Calif.), a product containing L. crispatus, or placebo. The women (median age 21, median number of UTIs before study 4.5) applied either Lactin-V or placebo daily for 5 days, then once weekly for 10 weeks. Participants were seen in scheduled follow-up visits at 1 week (visit 3) and 10 weeks (visit 4) after beginning Lactin-V or placebo and for symptomatic UTIs. Investigators collected urine samples for culture and vaginal swabs to assess the level of colonization of L. crispatus. Of the 50 women randomized in each group, 48 were entered into analysis.
Recurrent culture-confirmed UTI occurred in 7 of 48 women (15%) receiving Lactin-V, compared with 13 of 48 women (27%) receiving placebo (RR 0.5; 95% confidence interval, 0.2-1.2).
The investigators named as one of the strengths of the study the quantitative molecular method used to assess vaginal flora following UTI, which afforded them a more precise picture of changes in the balance of vaginal microbiota than seen in previous trials.
The investigators also noted that high-level vaginal colonization with L. crispatus throughout follow-up was associated with a significant reduction in recurrent UTI only for women using the suppositories. In their analysis, Dr. Stapleton and colleagues called this finding "striking" and hypothesized that the use of Lactin-V "confers a significant advantage over repopulation of the vaginal microbiota with endogenous L. crispatus," and that the L. crispatus isolate used in the intervention offered "unique properties for protection" over the natural recolonization process.
The investigators did not compare Lactin-V to prophylactic antibiotics in their study. However, they noted that a meta-analysis of 10 randomized controlled trials evaluating continuous antimicrobial prophylaxis (Clin. Evid. [Online] 2008 Jul 17;2008. pii:0801) found rates of rates of recurrent UTI reduced to 12% (24 of 195 participants) compared with 65% (116 of 177 participants) among placebo recipients, "with a [relative risk] of rUTI that is very comparable to our findings (RR, 0.21; 95% CI, 0.13-0.33)."
Dr. Stapleton and colleagues concluded that "[t]his antimicrobial-sparing, well-tolerated intervention compares favorably with historical data regarding antimicrobial prophylaxis, the current standard of care for the prevention of rUTI."
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and the Office of Research in Women’s Health, National Institutes of Health; Dr. Stapleton and her coauthors said they had no relevant financial disclosures.
A probiotic medication has been shown to reduce the risk of recurrent urinary tract infections by nearly half – making it comparable to a prophylactic course of antibiotics, the current standard treatment for recurrent urinary tract infections.
Concerns about increasing resistance to commonly used antimicrobials such as trimethoprim-sulfamethoxazole and fluoroquinolones has led to a search for alternative means to prevent urinary tract infections (UTIs), with recent investigations focusing on hydrogen peroxide-producing strains of lactobacilli.
These lactobacilli comprise the dominant vaginal flora, competing with and regulating other urogenital microbes, including the harmful Escherichia coli. Women with recurrent UTIs have been shown to typically have depletion of vaginal lactobacilli at the time of their E. coli infections (J. Infect. Dis. 1998;178:446-50).
The new findings, from a randomized, placebo-controlled trial of a vaginal suppository containing Lactobacillus crispatus in 100 young women, were published online April 15 in Clinical Infectious Diseases (Clin. Infect. Dis. 2011;52:1212-17).
For their research, Dr. Ann E. Stapleton of the University of Washington in Seattle, and her colleagues, recruited women with current cystitis and histories of recurrent UTI to be randomized to Lactin-V (Osel Inc., Mountain View, Calif.), a product containing L. crispatus, or placebo. The women (median age 21, median number of UTIs before study 4.5) applied either Lactin-V or placebo daily for 5 days, then once weekly for 10 weeks. Participants were seen in scheduled follow-up visits at 1 week (visit 3) and 10 weeks (visit 4) after beginning Lactin-V or placebo and for symptomatic UTIs. Investigators collected urine samples for culture and vaginal swabs to assess the level of colonization of L. crispatus. Of the 50 women randomized in each group, 48 were entered into analysis.
Recurrent culture-confirmed UTI occurred in 7 of 48 women (15%) receiving Lactin-V, compared with 13 of 48 women (27%) receiving placebo (RR 0.5; 95% confidence interval, 0.2-1.2).
The investigators named as one of the strengths of the study the quantitative molecular method used to assess vaginal flora following UTI, which afforded them a more precise picture of changes in the balance of vaginal microbiota than seen in previous trials.
The investigators also noted that high-level vaginal colonization with L. crispatus throughout follow-up was associated with a significant reduction in recurrent UTI only for women using the suppositories. In their analysis, Dr. Stapleton and colleagues called this finding "striking" and hypothesized that the use of Lactin-V "confers a significant advantage over repopulation of the vaginal microbiota with endogenous L. crispatus," and that the L. crispatus isolate used in the intervention offered "unique properties for protection" over the natural recolonization process.
The investigators did not compare Lactin-V to prophylactic antibiotics in their study. However, they noted that a meta-analysis of 10 randomized controlled trials evaluating continuous antimicrobial prophylaxis (Clin. Evid. [Online] 2008 Jul 17;2008. pii:0801) found rates of rates of recurrent UTI reduced to 12% (24 of 195 participants) compared with 65% (116 of 177 participants) among placebo recipients, "with a [relative risk] of rUTI that is very comparable to our findings (RR, 0.21; 95% CI, 0.13-0.33)."
Dr. Stapleton and colleagues concluded that "[t]his antimicrobial-sparing, well-tolerated intervention compares favorably with historical data regarding antimicrobial prophylaxis, the current standard of care for the prevention of rUTI."
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and the Office of Research in Women’s Health, National Institutes of Health; Dr. Stapleton and her coauthors said they had no relevant financial disclosures.
FROM CLINICAL INFECTIOUS DISEASES
Major Finding: Recurrent culture-confirmed UTI occurred in 7 of 48 women (15%) receiving Lactin-V, compared with 13 of 48 (27%) receiving placebo.
Data Source: A randomized, placebo-controlled trial involving 100 women in a university clinic setting who used vaginal suppositories containing Lactobacillus crispatus.
Disclosures: The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and the Office of Research in Women’s Health, National Institutes of Health; Dr. Stapleton and her coauthors said they had no relevant financial disclosures.
The Perfect Match: Dispelling the Myths About New Kidney Allocation Concept
By now, you have most likely heard about the United Network for Organ Sharing’s Concepts for Kidney Allocation, which outlines potential changes to the way deceased-donor kidneys are, so to speak, “distributed” to any of the 85,000 people currently waiting for one. Depending on your source, however, what you have heard about the concept document probably varies from “It is an effort to make the best use of a limited resource” (true) to “No one over the age of 60 will get a kidney!” (erroneous and also, frankly, outrageous).
The simple fact is that there are not enough available kidneys in the United States to make a dent in the waiting list. UNOS’s concept document does not address the issue of how to increase donations (one manufactured controversy is sufficient for a single document). What it does is provide a rationale for ensuring that each available kidney finds its best possible match.
“We’re not going to create any new kidneys with this, unfortunately,” says John J. Friedewald, MD, Vice Chair of UNOS’s Kidney Transplantation Committee and Assistant Professor in Medicine–Nephrology and Surgery at Northwestern University. “What we are going to do, the idea of this, is to give the right kidney to the right person.”
Tremendous Shift in Transplant Recipients
Just who is the right person for each kidney? And why, after years of the traditionally accepted “first come, first served” method, would the US even need a change in plan?
For one thing, statistics from the US Renal Data System paint a picture of a changing kidney transplantation system. In 1991, three in 10 patients were older than 50; by 2008, that proportion had doubled to six in 10, and one in six was older than 65. Since 2000, the transplantation rate has decreased 30% for persons ages 29 to 50 and increased 49% for those older than 65.
“All of a sudden, we’ve had this tremendous shift, which has only just begun, to transplanting an elderly population,” says Barbara Weis Malone, CFNP, Senior Instructor in the Division of Renal Disease and Hypertension at the University of Colorado Health Sciences Center. “I remember when I started in the transplant community nine years ago, we would barely look at a 60-year-old, and now, regularly, we are transplanting 64-year-olds and occasionally even putting people at 70 on the transplant list.”
At the same time, the average deceased donor is still from among the younger portion of the population, which can lead to serious disparities between graft longevity and recipient longevity. No one is saying older persons don’t deserve a kidney as much as younger persons—but, realistically, does a 68-year-old man need a 6-year-old’s kidney?
“Currently, our system can give a very long-lived kidney to a person who is not expected to live very long,” Friedewald points out. “And vice versa, which is sometimes worse—give a short-lived kidney to someone who is expected to live really long. What that means is that person, usually a younger person, may need a second or even third transplant in his or her lifetime.”
Almost 15% of the waiting list represents people waiting for their second, third, fourth, or even fifth transplant, Friedewald says. Part of the return on investment if the concept document becomes policy could be a reduction in that number. “If we give organs to younger people who are going to live a long time with them, then eventually—not tomorrow; maybe in five, 10, or 15 years—there will be fewer people returning to the wait list,” according to Friedewald. (But we’re getting ahead of ourselves, because the document cannot even be accurately termed a proposal at this point.)
For clinicians in nephrology, seeing the current allocation system in action can be distressing, even though they want to provide the best care to all patients. A sweet, 66-year-old grandmother may end up with an 11-year-old’s kidney, while a 20-year-old, otherwise healthy man with glomerular nephritis may spend years waiting and not get the kidney he ideally needs, one that will last 30 years or longer.
“When I first came into kidney transplant, I thought, ‘It’s just the first person in line; that’s wonderful,’” says Weis Malone. “But now it kind of breaks my heart. As a medical professional, it’s hard to see your younger population who could really use a kidney tomorrow.”
In essence, along with the disparities comes a sense of wasted opportunity. “One in three people dies with a functioning kidney,” says Kim Zuber, PA-C, MSPS, DFAAPA, Chair of the National Kidney Foundation Council of Advanced Practitioners, who practices at Metropolitan Nephrology in Alexandria, Virginia, and Clinton, Maryland. “As far as I’m concerned, that’s a waste, because the kidney should fail before you die; then you’ve used it all up.”
And for those who insist that “first come, first served” is fair, Friedewald has a different opinion, calling it “a complete fallacy. The first come aren’t the first served. Some people won’t live long enough to wait for a kidney, if the line is too long. Everyone believes that a line is ‘first come, first served,’ because we’re used to delis and things like that, where you actually get served. But a lot of people aren’t getting served.”
That may never change, but the concepts outlined in the UNOS document aim to achieve an additional 15,223 life-years lived with a transplanted kidney for every year’s worth of deceased-donor transplants. How? Let’s explore.
How the System Would Change
Early on, Concepts for Kidney Allocation (available at optn.transplant.hrsa.gov/SharedContentDocuments/KidneyConceptDocument.pdf) outlines the flaws of the current system: “[I]t does not strive to minimize death on the waiting list nor maximize survival following transplant. It does not recognize that all candidates do not have the same ability to survive the wait. It does not attempt to match the characteristics of a donor’s kidney to the candidate’s characteristics to promote a long and healthy survival post-transplant. The system can be better, and it can be designed to achieve more in the way of health and longevity than it currently does.”
Aware of the problems, and with input from hundreds of individuals—including transplant professionals, recipients, and candidates, as well as donor families, living donors, and the general public—UNOS’s Kidney Transplantation Committee has spent six years (so far) developing goals for a new allocation system. The concept document delineates three key factors in achieving those goals:
• Utilizing a kidney donor profile index (KDPI), a continuous measure used to estimate the potential function of a donated kidney if it were transplanted into the average recipient, to better characterize donor kidneys.
• Allocating some kidneys (20%) by a combination of the KDPI and a candidate’s estimated post-transplant survival (EPTS).
• Allocating the majority of organs (80%) by age-matching so that candidates within 15 years older or younger than the donor are prioritized.
The KDPI is based on the characteristics of the donor, including age, race/ethnicity, height, and weight, and whether he/she had any of the following factors: hypertension, diabetes, hepatitis C, elevated creatinine level, a cerebrovascular cause of death, or cardiac death. It is important to remember that a score below 20% indicates a kidney with the predicted longest function; it’s like being in the top 20% of your graduating class.
A candidate’s EPTS is based on age, length of time on dialysis, diabetes status, and history of prior organ transplant. “While no calculation will be able to predict life expectancy with 100% certainty, these four factors provide a reasonable estimate for identifying those candidates who have the longest possible EPTS,” the committee writes.
Once a KDPI has been established, there are two scenarios for how a candidate might be selected. Kidneys with a KDPI of 20% or lower are allocated based on EPTS. Those with a KDPI of more than 20% are allocated based on age-matching within 15 years (older or younger) of the recipient’s age.
To use the examples given in the report: Let’s say there are three candidates for a kidney. Mary is 30, Sophia is 21, and David is 60. Along comes a kidney with a KDPI of 10%. Since Mary and Sophia have better estimated survival than David (EPTS, 19%, 12%, and 75% respectively; again, it’s the percentile, so lower is better), they would have priority for that kidney.
However, if the available kidney has a KDPI of, say, 40%, age becomes a bigger factor. If the donor is 34, Mary and Sophia continue to have priority over David, since recipients ages 19 to 49 would be considered first. If the donor is 55, however, David would have precedence over Mary and Sophia, since the age range for that kidney recipient would be 40 to 70.
All of this can be confusing, and it leads one to suspect that some of the news entities who misspoke either did not understand what they were reading or perhaps did not even finish reading the report. But these components do not even tell the entire story; they are exactly that, components. The final proposal, which will be written only when all the feedback from a public comment period has been compiled and analyzed, “will include all the details of who gets the kidney,” as Friedewald says.
What is important to know now is that “waiting time will continue to be a major part of who gets a kidney” if the revised allocation concepts become policy, Friedewald emphasizes. “What this document says is that we’re generally going to group people based on the quality of the donor kidney. But once we do that—let’s say it’s a top 20% kidney—well, then the top 20% of candidates who are expected to live the longest will be up for that kidney. But that may be 10,000 people. Which of the 10,000 people gets the kidney? The person who waits the longest.”
Besides making better use of the available kidneys, the revised allocation system would also make use of more kidneys, by eliminating the previous designation of “expanded criteria donor,” or ECD, kidneys. Some patients turn down ECD kidneys when they are offered, because they may consider the designation a kind of stigma.
“People think ‘expanded criteria’ is this horrible kidney that came from someone who was very old or who probably drank his whole life or had HIV and hepatitis B,” Weis Malone says. “But it could be a 55-year-old person who was taking one blood pressure medication. Right now, there is something like a 12% waste of kidneys that are just being thrown away. As the transplant list grows, it would be nice to utilize those kidneys.”
Obviously, no one would object to someone turning down a kidney from, say, a known IV drug user who was testing negative at the time of death. But does it make sense for a 70-year-old man to turn down an ECD kidney with a creatine level of 1.3, indicative of slight kidney damage? “When you’re talking about someone who has all kidney damage,” Zuber says, “you want to say, ‘What are you thinking? You don’t need a perfect kidney; you need a kidney.’”
Two other provisos: The revised allocation criteria do not affect persons waiting for a multiple-organ transplant (such as kidney/pancreas, kidney/heart, or kidney/liver). They also would apply to the adult (18 and older) population; pediatric candidates would continue to have priority, because as Zuber says, “If you don’t transplant a child, they will lose growth. It makes a huge difference when you’re a child, whereas when you’re an adult, it doesn’t matter if you’re 20 or 50.”
Focus on Prevention, Solutions
As indicated above, Concepts for Kidney Allocation is the latest step in the process of revising how kidneys are allocated. The Kidney Transplantation Committee will assess the feedback it receives, determine if there is a consensus, then issue a final proposal. UNOS and the Health Resources and Services Administration will be the groups that vote on whether to adopt the recommendations as policy.
In the meantime, other aspects of kidney allocation and transplantation will continue to be discussed and debated, whether officially or among the general public. Among the questions: How can the pool of living donors be increased? Should the US adopt a “presumed consent” policy that would require people to expressly opt out of organ donation? What other methods might increase donations—better education, payment of funeral expenses for the deceased donor, or something as yet undetermined?
For nephrology clinicians, the goal remains keeping people alive while they await transplantation. “Even when I’m doing a perfect job—that’s a perfect job, and at few times in my life am I perfect—I am no better than 15% of a kidney,” Zuber says of her dialysis work. At the same time, it must be accepted that even transplantation is “not a cure,” as Weis Malone points out. “It’s a treatment, just like dialysis is a treatment. Not everyone does well with it.”
For Weis Malone, “the focus always needs to be on the prevention of kidney disease, especially as diabetes continues to grow massively in this country. Probably 30% to 40% of people who start dialysis had no idea they had kidney disease. So that’s where it has to start, with the education of the medical community that the only way you can tell kidney function is through a blood test or a urine dip.”
And the search for solutions to the growing problem of kidney disease, and subsequent kidney failure, will continue. “This is what the public debate is about: what is acceptable,” Friedewald says. “There is no right or wrong here. But what is acceptable, and what kind of trade-offs are we willing to make to get more out of a scarce resource?”
By now, you have most likely heard about the United Network for Organ Sharing’s Concepts for Kidney Allocation, which outlines potential changes to the way deceased-donor kidneys are, so to speak, “distributed” to any of the 85,000 people currently waiting for one. Depending on your source, however, what you have heard about the concept document probably varies from “It is an effort to make the best use of a limited resource” (true) to “No one over the age of 60 will get a kidney!” (erroneous and also, frankly, outrageous).
The simple fact is that there are not enough available kidneys in the United States to make a dent in the waiting list. UNOS’s concept document does not address the issue of how to increase donations (one manufactured controversy is sufficient for a single document). What it does is provide a rationale for ensuring that each available kidney finds its best possible match.
“We’re not going to create any new kidneys with this, unfortunately,” says John J. Friedewald, MD, Vice Chair of UNOS’s Kidney Transplantation Committee and Assistant Professor in Medicine–Nephrology and Surgery at Northwestern University. “What we are going to do, the idea of this, is to give the right kidney to the right person.”
Tremendous Shift in Transplant Recipients
Just who is the right person for each kidney? And why, after years of the traditionally accepted “first come, first served” method, would the US even need a change in plan?
For one thing, statistics from the US Renal Data System paint a picture of a changing kidney transplantation system. In 1991, three in 10 patients were older than 50; by 2008, that proportion had doubled to six in 10, and one in six was older than 65. Since 2000, the transplantation rate has decreased 30% for persons ages 29 to 50 and increased 49% for those older than 65.
“All of a sudden, we’ve had this tremendous shift, which has only just begun, to transplanting an elderly population,” says Barbara Weis Malone, CFNP, Senior Instructor in the Division of Renal Disease and Hypertension at the University of Colorado Health Sciences Center. “I remember when I started in the transplant community nine years ago, we would barely look at a 60-year-old, and now, regularly, we are transplanting 64-year-olds and occasionally even putting people at 70 on the transplant list.”
At the same time, the average deceased donor is still from among the younger portion of the population, which can lead to serious disparities between graft longevity and recipient longevity. No one is saying older persons don’t deserve a kidney as much as younger persons—but, realistically, does a 68-year-old man need a 6-year-old’s kidney?
“Currently, our system can give a very long-lived kidney to a person who is not expected to live very long,” Friedewald points out. “And vice versa, which is sometimes worse—give a short-lived kidney to someone who is expected to live really long. What that means is that person, usually a younger person, may need a second or even third transplant in his or her lifetime.”
Almost 15% of the waiting list represents people waiting for their second, third, fourth, or even fifth transplant, Friedewald says. Part of the return on investment if the concept document becomes policy could be a reduction in that number. “If we give organs to younger people who are going to live a long time with them, then eventually—not tomorrow; maybe in five, 10, or 15 years—there will be fewer people returning to the wait list,” according to Friedewald. (But we’re getting ahead of ourselves, because the document cannot even be accurately termed a proposal at this point.)
For clinicians in nephrology, seeing the current allocation system in action can be distressing, even though they want to provide the best care to all patients. A sweet, 66-year-old grandmother may end up with an 11-year-old’s kidney, while a 20-year-old, otherwise healthy man with glomerular nephritis may spend years waiting and not get the kidney he ideally needs, one that will last 30 years or longer.
“When I first came into kidney transplant, I thought, ‘It’s just the first person in line; that’s wonderful,’” says Weis Malone. “But now it kind of breaks my heart. As a medical professional, it’s hard to see your younger population who could really use a kidney tomorrow.”
In essence, along with the disparities comes a sense of wasted opportunity. “One in three people dies with a functioning kidney,” says Kim Zuber, PA-C, MSPS, DFAAPA, Chair of the National Kidney Foundation Council of Advanced Practitioners, who practices at Metropolitan Nephrology in Alexandria, Virginia, and Clinton, Maryland. “As far as I’m concerned, that’s a waste, because the kidney should fail before you die; then you’ve used it all up.”
And for those who insist that “first come, first served” is fair, Friedewald has a different opinion, calling it “a complete fallacy. The first come aren’t the first served. Some people won’t live long enough to wait for a kidney, if the line is too long. Everyone believes that a line is ‘first come, first served,’ because we’re used to delis and things like that, where you actually get served. But a lot of people aren’t getting served.”
That may never change, but the concepts outlined in the UNOS document aim to achieve an additional 15,223 life-years lived with a transplanted kidney for every year’s worth of deceased-donor transplants. How? Let’s explore.
How the System Would Change
Early on, Concepts for Kidney Allocation (available at optn.transplant.hrsa.gov/SharedContentDocuments/KidneyConceptDocument.pdf) outlines the flaws of the current system: “[I]t does not strive to minimize death on the waiting list nor maximize survival following transplant. It does not recognize that all candidates do not have the same ability to survive the wait. It does not attempt to match the characteristics of a donor’s kidney to the candidate’s characteristics to promote a long and healthy survival post-transplant. The system can be better, and it can be designed to achieve more in the way of health and longevity than it currently does.”
Aware of the problems, and with input from hundreds of individuals—including transplant professionals, recipients, and candidates, as well as donor families, living donors, and the general public—UNOS’s Kidney Transplantation Committee has spent six years (so far) developing goals for a new allocation system. The concept document delineates three key factors in achieving those goals:
• Utilizing a kidney donor profile index (KDPI), a continuous measure used to estimate the potential function of a donated kidney if it were transplanted into the average recipient, to better characterize donor kidneys.
• Allocating some kidneys (20%) by a combination of the KDPI and a candidate’s estimated post-transplant survival (EPTS).
• Allocating the majority of organs (80%) by age-matching so that candidates within 15 years older or younger than the donor are prioritized.
The KDPI is based on the characteristics of the donor, including age, race/ethnicity, height, and weight, and whether he/she had any of the following factors: hypertension, diabetes, hepatitis C, elevated creatinine level, a cerebrovascular cause of death, or cardiac death. It is important to remember that a score below 20% indicates a kidney with the predicted longest function; it’s like being in the top 20% of your graduating class.
A candidate’s EPTS is based on age, length of time on dialysis, diabetes status, and history of prior organ transplant. “While no calculation will be able to predict life expectancy with 100% certainty, these four factors provide a reasonable estimate for identifying those candidates who have the longest possible EPTS,” the committee writes.
Once a KDPI has been established, there are two scenarios for how a candidate might be selected. Kidneys with a KDPI of 20% or lower are allocated based on EPTS. Those with a KDPI of more than 20% are allocated based on age-matching within 15 years (older or younger) of the recipient’s age.
To use the examples given in the report: Let’s say there are three candidates for a kidney. Mary is 30, Sophia is 21, and David is 60. Along comes a kidney with a KDPI of 10%. Since Mary and Sophia have better estimated survival than David (EPTS, 19%, 12%, and 75% respectively; again, it’s the percentile, so lower is better), they would have priority for that kidney.
However, if the available kidney has a KDPI of, say, 40%, age becomes a bigger factor. If the donor is 34, Mary and Sophia continue to have priority over David, since recipients ages 19 to 49 would be considered first. If the donor is 55, however, David would have precedence over Mary and Sophia, since the age range for that kidney recipient would be 40 to 70.
All of this can be confusing, and it leads one to suspect that some of the news entities who misspoke either did not understand what they were reading or perhaps did not even finish reading the report. But these components do not even tell the entire story; they are exactly that, components. The final proposal, which will be written only when all the feedback from a public comment period has been compiled and analyzed, “will include all the details of who gets the kidney,” as Friedewald says.
What is important to know now is that “waiting time will continue to be a major part of who gets a kidney” if the revised allocation concepts become policy, Friedewald emphasizes. “What this document says is that we’re generally going to group people based on the quality of the donor kidney. But once we do that—let’s say it’s a top 20% kidney—well, then the top 20% of candidates who are expected to live the longest will be up for that kidney. But that may be 10,000 people. Which of the 10,000 people gets the kidney? The person who waits the longest.”
Besides making better use of the available kidneys, the revised allocation system would also make use of more kidneys, by eliminating the previous designation of “expanded criteria donor,” or ECD, kidneys. Some patients turn down ECD kidneys when they are offered, because they may consider the designation a kind of stigma.
“People think ‘expanded criteria’ is this horrible kidney that came from someone who was very old or who probably drank his whole life or had HIV and hepatitis B,” Weis Malone says. “But it could be a 55-year-old person who was taking one blood pressure medication. Right now, there is something like a 12% waste of kidneys that are just being thrown away. As the transplant list grows, it would be nice to utilize those kidneys.”
Obviously, no one would object to someone turning down a kidney from, say, a known IV drug user who was testing negative at the time of death. But does it make sense for a 70-year-old man to turn down an ECD kidney with a creatine level of 1.3, indicative of slight kidney damage? “When you’re talking about someone who has all kidney damage,” Zuber says, “you want to say, ‘What are you thinking? You don’t need a perfect kidney; you need a kidney.’”
Two other provisos: The revised allocation criteria do not affect persons waiting for a multiple-organ transplant (such as kidney/pancreas, kidney/heart, or kidney/liver). They also would apply to the adult (18 and older) population; pediatric candidates would continue to have priority, because as Zuber says, “If you don’t transplant a child, they will lose growth. It makes a huge difference when you’re a child, whereas when you’re an adult, it doesn’t matter if you’re 20 or 50.”
Focus on Prevention, Solutions
As indicated above, Concepts for Kidney Allocation is the latest step in the process of revising how kidneys are allocated. The Kidney Transplantation Committee will assess the feedback it receives, determine if there is a consensus, then issue a final proposal. UNOS and the Health Resources and Services Administration will be the groups that vote on whether to adopt the recommendations as policy.
In the meantime, other aspects of kidney allocation and transplantation will continue to be discussed and debated, whether officially or among the general public. Among the questions: How can the pool of living donors be increased? Should the US adopt a “presumed consent” policy that would require people to expressly opt out of organ donation? What other methods might increase donations—better education, payment of funeral expenses for the deceased donor, or something as yet undetermined?
For nephrology clinicians, the goal remains keeping people alive while they await transplantation. “Even when I’m doing a perfect job—that’s a perfect job, and at few times in my life am I perfect—I am no better than 15% of a kidney,” Zuber says of her dialysis work. At the same time, it must be accepted that even transplantation is “not a cure,” as Weis Malone points out. “It’s a treatment, just like dialysis is a treatment. Not everyone does well with it.”
For Weis Malone, “the focus always needs to be on the prevention of kidney disease, especially as diabetes continues to grow massively in this country. Probably 30% to 40% of people who start dialysis had no idea they had kidney disease. So that’s where it has to start, with the education of the medical community that the only way you can tell kidney function is through a blood test or a urine dip.”
And the search for solutions to the growing problem of kidney disease, and subsequent kidney failure, will continue. “This is what the public debate is about: what is acceptable,” Friedewald says. “There is no right or wrong here. But what is acceptable, and what kind of trade-offs are we willing to make to get more out of a scarce resource?”
By now, you have most likely heard about the United Network for Organ Sharing’s Concepts for Kidney Allocation, which outlines potential changes to the way deceased-donor kidneys are, so to speak, “distributed” to any of the 85,000 people currently waiting for one. Depending on your source, however, what you have heard about the concept document probably varies from “It is an effort to make the best use of a limited resource” (true) to “No one over the age of 60 will get a kidney!” (erroneous and also, frankly, outrageous).
The simple fact is that there are not enough available kidneys in the United States to make a dent in the waiting list. UNOS’s concept document does not address the issue of how to increase donations (one manufactured controversy is sufficient for a single document). What it does is provide a rationale for ensuring that each available kidney finds its best possible match.
“We’re not going to create any new kidneys with this, unfortunately,” says John J. Friedewald, MD, Vice Chair of UNOS’s Kidney Transplantation Committee and Assistant Professor in Medicine–Nephrology and Surgery at Northwestern University. “What we are going to do, the idea of this, is to give the right kidney to the right person.”
Tremendous Shift in Transplant Recipients
Just who is the right person for each kidney? And why, after years of the traditionally accepted “first come, first served” method, would the US even need a change in plan?
For one thing, statistics from the US Renal Data System paint a picture of a changing kidney transplantation system. In 1991, three in 10 patients were older than 50; by 2008, that proportion had doubled to six in 10, and one in six was older than 65. Since 2000, the transplantation rate has decreased 30% for persons ages 29 to 50 and increased 49% for those older than 65.
“All of a sudden, we’ve had this tremendous shift, which has only just begun, to transplanting an elderly population,” says Barbara Weis Malone, CFNP, Senior Instructor in the Division of Renal Disease and Hypertension at the University of Colorado Health Sciences Center. “I remember when I started in the transplant community nine years ago, we would barely look at a 60-year-old, and now, regularly, we are transplanting 64-year-olds and occasionally even putting people at 70 on the transplant list.”
At the same time, the average deceased donor is still from among the younger portion of the population, which can lead to serious disparities between graft longevity and recipient longevity. No one is saying older persons don’t deserve a kidney as much as younger persons—but, realistically, does a 68-year-old man need a 6-year-old’s kidney?
“Currently, our system can give a very long-lived kidney to a person who is not expected to live very long,” Friedewald points out. “And vice versa, which is sometimes worse—give a short-lived kidney to someone who is expected to live really long. What that means is that person, usually a younger person, may need a second or even third transplant in his or her lifetime.”
Almost 15% of the waiting list represents people waiting for their second, third, fourth, or even fifth transplant, Friedewald says. Part of the return on investment if the concept document becomes policy could be a reduction in that number. “If we give organs to younger people who are going to live a long time with them, then eventually—not tomorrow; maybe in five, 10, or 15 years—there will be fewer people returning to the wait list,” according to Friedewald. (But we’re getting ahead of ourselves, because the document cannot even be accurately termed a proposal at this point.)
For clinicians in nephrology, seeing the current allocation system in action can be distressing, even though they want to provide the best care to all patients. A sweet, 66-year-old grandmother may end up with an 11-year-old’s kidney, while a 20-year-old, otherwise healthy man with glomerular nephritis may spend years waiting and not get the kidney he ideally needs, one that will last 30 years or longer.
“When I first came into kidney transplant, I thought, ‘It’s just the first person in line; that’s wonderful,’” says Weis Malone. “But now it kind of breaks my heart. As a medical professional, it’s hard to see your younger population who could really use a kidney tomorrow.”
In essence, along with the disparities comes a sense of wasted opportunity. “One in three people dies with a functioning kidney,” says Kim Zuber, PA-C, MSPS, DFAAPA, Chair of the National Kidney Foundation Council of Advanced Practitioners, who practices at Metropolitan Nephrology in Alexandria, Virginia, and Clinton, Maryland. “As far as I’m concerned, that’s a waste, because the kidney should fail before you die; then you’ve used it all up.”
And for those who insist that “first come, first served” is fair, Friedewald has a different opinion, calling it “a complete fallacy. The first come aren’t the first served. Some people won’t live long enough to wait for a kidney, if the line is too long. Everyone believes that a line is ‘first come, first served,’ because we’re used to delis and things like that, where you actually get served. But a lot of people aren’t getting served.”
That may never change, but the concepts outlined in the UNOS document aim to achieve an additional 15,223 life-years lived with a transplanted kidney for every year’s worth of deceased-donor transplants. How? Let’s explore.
How the System Would Change
Early on, Concepts for Kidney Allocation (available at optn.transplant.hrsa.gov/SharedContentDocuments/KidneyConceptDocument.pdf) outlines the flaws of the current system: “[I]t does not strive to minimize death on the waiting list nor maximize survival following transplant. It does not recognize that all candidates do not have the same ability to survive the wait. It does not attempt to match the characteristics of a donor’s kidney to the candidate’s characteristics to promote a long and healthy survival post-transplant. The system can be better, and it can be designed to achieve more in the way of health and longevity than it currently does.”
Aware of the problems, and with input from hundreds of individuals—including transplant professionals, recipients, and candidates, as well as donor families, living donors, and the general public—UNOS’s Kidney Transplantation Committee has spent six years (so far) developing goals for a new allocation system. The concept document delineates three key factors in achieving those goals:
• Utilizing a kidney donor profile index (KDPI), a continuous measure used to estimate the potential function of a donated kidney if it were transplanted into the average recipient, to better characterize donor kidneys.
• Allocating some kidneys (20%) by a combination of the KDPI and a candidate’s estimated post-transplant survival (EPTS).
• Allocating the majority of organs (80%) by age-matching so that candidates within 15 years older or younger than the donor are prioritized.
The KDPI is based on the characteristics of the donor, including age, race/ethnicity, height, and weight, and whether he/she had any of the following factors: hypertension, diabetes, hepatitis C, elevated creatinine level, a cerebrovascular cause of death, or cardiac death. It is important to remember that a score below 20% indicates a kidney with the predicted longest function; it’s like being in the top 20% of your graduating class.
A candidate’s EPTS is based on age, length of time on dialysis, diabetes status, and history of prior organ transplant. “While no calculation will be able to predict life expectancy with 100% certainty, these four factors provide a reasonable estimate for identifying those candidates who have the longest possible EPTS,” the committee writes.
Once a KDPI has been established, there are two scenarios for how a candidate might be selected. Kidneys with a KDPI of 20% or lower are allocated based on EPTS. Those with a KDPI of more than 20% are allocated based on age-matching within 15 years (older or younger) of the recipient’s age.
To use the examples given in the report: Let’s say there are three candidates for a kidney. Mary is 30, Sophia is 21, and David is 60. Along comes a kidney with a KDPI of 10%. Since Mary and Sophia have better estimated survival than David (EPTS, 19%, 12%, and 75% respectively; again, it’s the percentile, so lower is better), they would have priority for that kidney.
However, if the available kidney has a KDPI of, say, 40%, age becomes a bigger factor. If the donor is 34, Mary and Sophia continue to have priority over David, since recipients ages 19 to 49 would be considered first. If the donor is 55, however, David would have precedence over Mary and Sophia, since the age range for that kidney recipient would be 40 to 70.
All of this can be confusing, and it leads one to suspect that some of the news entities who misspoke either did not understand what they were reading or perhaps did not even finish reading the report. But these components do not even tell the entire story; they are exactly that, components. The final proposal, which will be written only when all the feedback from a public comment period has been compiled and analyzed, “will include all the details of who gets the kidney,” as Friedewald says.
What is important to know now is that “waiting time will continue to be a major part of who gets a kidney” if the revised allocation concepts become policy, Friedewald emphasizes. “What this document says is that we’re generally going to group people based on the quality of the donor kidney. But once we do that—let’s say it’s a top 20% kidney—well, then the top 20% of candidates who are expected to live the longest will be up for that kidney. But that may be 10,000 people. Which of the 10,000 people gets the kidney? The person who waits the longest.”
Besides making better use of the available kidneys, the revised allocation system would also make use of more kidneys, by eliminating the previous designation of “expanded criteria donor,” or ECD, kidneys. Some patients turn down ECD kidneys when they are offered, because they may consider the designation a kind of stigma.
“People think ‘expanded criteria’ is this horrible kidney that came from someone who was very old or who probably drank his whole life or had HIV and hepatitis B,” Weis Malone says. “But it could be a 55-year-old person who was taking one blood pressure medication. Right now, there is something like a 12% waste of kidneys that are just being thrown away. As the transplant list grows, it would be nice to utilize those kidneys.”
Obviously, no one would object to someone turning down a kidney from, say, a known IV drug user who was testing negative at the time of death. But does it make sense for a 70-year-old man to turn down an ECD kidney with a creatine level of 1.3, indicative of slight kidney damage? “When you’re talking about someone who has all kidney damage,” Zuber says, “you want to say, ‘What are you thinking? You don’t need a perfect kidney; you need a kidney.’”
Two other provisos: The revised allocation criteria do not affect persons waiting for a multiple-organ transplant (such as kidney/pancreas, kidney/heart, or kidney/liver). They also would apply to the adult (18 and older) population; pediatric candidates would continue to have priority, because as Zuber says, “If you don’t transplant a child, they will lose growth. It makes a huge difference when you’re a child, whereas when you’re an adult, it doesn’t matter if you’re 20 or 50.”
Focus on Prevention, Solutions
As indicated above, Concepts for Kidney Allocation is the latest step in the process of revising how kidneys are allocated. The Kidney Transplantation Committee will assess the feedback it receives, determine if there is a consensus, then issue a final proposal. UNOS and the Health Resources and Services Administration will be the groups that vote on whether to adopt the recommendations as policy.
In the meantime, other aspects of kidney allocation and transplantation will continue to be discussed and debated, whether officially or among the general public. Among the questions: How can the pool of living donors be increased? Should the US adopt a “presumed consent” policy that would require people to expressly opt out of organ donation? What other methods might increase donations—better education, payment of funeral expenses for the deceased donor, or something as yet undetermined?
For nephrology clinicians, the goal remains keeping people alive while they await transplantation. “Even when I’m doing a perfect job—that’s a perfect job, and at few times in my life am I perfect—I am no better than 15% of a kidney,” Zuber says of her dialysis work. At the same time, it must be accepted that even transplantation is “not a cure,” as Weis Malone points out. “It’s a treatment, just like dialysis is a treatment. Not everyone does well with it.”
For Weis Malone, “the focus always needs to be on the prevention of kidney disease, especially as diabetes continues to grow massively in this country. Probably 30% to 40% of people who start dialysis had no idea they had kidney disease. So that’s where it has to start, with the education of the medical community that the only way you can tell kidney function is through a blood test or a urine dip.”
And the search for solutions to the growing problem of kidney disease, and subsequent kidney failure, will continue. “This is what the public debate is about: what is acceptable,” Friedewald says. “There is no right or wrong here. But what is acceptable, and what kind of trade-offs are we willing to make to get more out of a scarce resource?”