User login
Intensive BP Control Slows CKD Progression Only in Select Patients
Intensive blood pressure control doesn’t slow the progression of chronic kidney disease any better than standard blood pressure control in most patients, according to a report in the Sept. 2 New England Journal of Medicine.
It appears that the more intensive approach may benefit only patients who have proteinuria with a protein:creatinine ratio greater than 0.22, a value that is compatible with the widely accepted threshold of 300 mg/day for absolute urinary protein excretion, said Dr. Lawrence J. Appel of Johns Hopkins University, Baltimore, and his associates in the AASK (African-American Study of Kidney Disease and Hypertension) Collaborative Research Group.
Until now, “few trials have tested the effects of intensive blood pressure control [compared with conventional control] on the progression of chronic kidney disease, and the findings from such trials have been inconsistent. Despite a lack of compelling evidence, numerous guidelines recommend a reduced blood pressure target in patients with CKD,” they wrote.
Previous studies have rarely followed patients beyond 5 years, even though it typically takes longer than that for end-stage renal disease (ESRD) to develop in patients with CKD.
The AASK study compared outcomes between the two approaches to BP control in 1,094 black adults with mild to moderate hypertensive chronic kidney disease (defined as diastolic BP greater than 95 mm Hg and a glomerular filtration rate of 20-65 mL/min) but without marked proteinuria. Patients with diabetes were excluded from the trial.
In the first phase of the AASK investigation, patients were randomly assigned to either intensive BP control with a target of 92 mm Hg or lower mean arterial pressure (that is, lower than the usual target of 130/80 mm Hg recommended for CKD patients) or to conventional BP control with a target of 102-107 mm Hg mean arterial pressure (which corresponds to the conventional BP target of 140/90 mm Hg).
Throughout this initial phase of the trial, which lasted approximately 4 years, mean blood pressure was significantly lower in the intensive-control group (130/78 mm Hg) than in the standard-control group (141/86 mm Hg), yet there was no significant difference in the primary outcome of progression of kidney disease, development of ESRD, or death. Likewise, there was no significant difference between the two approaches in secondary or clinical outcomes.
In the second phase of the AASK investigation, patients who had not yet developed ESRD were invited to continue in a cohort portion of the trial, in which the BP target was 140/90 mm Hg. In 2004, when national guidelines were changed, this target was amended to lower than 130/80 mm Hg.
After a cumulative follow-up of 8-12 years, there still was no significant difference in primary or secondary outcomes between those who were initially assigned to the intensive-control and the standard-control groups. More intensive BP control did not slow the rate of progression of CKD, Dr. Appel and his associates reported (N. Engl. J. Med. 2010;363:918-29).
However, the intensive-control approach did benefit one subgroup of patients with proteinuria: those who had a protein:creatinine ratio of more than 0.22 at baseline. These patients showed a significant reduction in the primary outcome of progression of kidney disease, development of ESRD, or death, as well as in secondary and clinical outcomes.
The reason for this discrepancy is not known. “Overall, it is hard to develop a coherent, biologically plausible argument for a qualitative interaction between harm in patients without proteinuria and benefit in those with proteinuria,” the researchers said.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the Office of Research in Minority Health, and the National Institutes of Health. King Pharmaceuticals provided financial support and donated antihypertensive medications to each clinical center. Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn also donated antihypertensive drugs. None of these companies had any role in the design of the study, the accrual or analysis of data, or the preparation of the manuscript. Some of the investigators reported being in consultant and/or advisory board roles or receiving funds from numerous companies including Daiichi-Sankyo, Novartis, Amgen, King Pharmaceuticals, Abbott, Boehringer-Ingelheim, Litholink, Eli Lilly, Takeda, Merck, and Watson. Dr. Ingelfinger reported having no conflicts of interest.
This study lends hope to the concept that intensive treatment will improve renal outcomes in at least some patients with hypertension, chronic kidney disease, and microalbuminuria.
Data from other studies also support the conclusion that intensive BP control is beneficial in select patients.
The Modification of Diet in Renal Disease trial showed that intensive BP control, compared with standard control, benefited patients who had more than 1 g of proteinuria at baseline. The ESCAPE (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients) trial also demonstrated that intensive BP control with a fixed dose of an ACE inhibitor significantly slowed the progression of renal disease, with the largest effects seen in children who had substantial proteinuria, hypertension, and a reduced GFR at baseline.
In addition, intensive BP control was beneficial in a recent study of adults in Italy who had idiopathic glomerular diseases associated with hypertension and proteinuria.
Julie R. Ingelfinger, M.D., is chief of pediatric nephrology at Massachusetts General Hospital, Boston, and a deputy editor of the New England Journal of Medicine. These comments were summarized from her editorial accompanying the report (N. Engl. J. Med. 2010;363:974-6). She reported having no relevant conflicts of interest.
This study lends hope to the concept that intensive treatment will improve renal outcomes in at least some patients with hypertension, chronic kidney disease, and microalbuminuria.
Data from other studies also support the conclusion that intensive BP control is beneficial in select patients.
The Modification of Diet in Renal Disease trial showed that intensive BP control, compared with standard control, benefited patients who had more than 1 g of proteinuria at baseline. The ESCAPE (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients) trial also demonstrated that intensive BP control with a fixed dose of an ACE inhibitor significantly slowed the progression of renal disease, with the largest effects seen in children who had substantial proteinuria, hypertension, and a reduced GFR at baseline.
In addition, intensive BP control was beneficial in a recent study of adults in Italy who had idiopathic glomerular diseases associated with hypertension and proteinuria.
Julie R. Ingelfinger, M.D., is chief of pediatric nephrology at Massachusetts General Hospital, Boston, and a deputy editor of the New England Journal of Medicine. These comments were summarized from her editorial accompanying the report (N. Engl. J. Med. 2010;363:974-6). She reported having no relevant conflicts of interest.
This study lends hope to the concept that intensive treatment will improve renal outcomes in at least some patients with hypertension, chronic kidney disease, and microalbuminuria.
Data from other studies also support the conclusion that intensive BP control is beneficial in select patients.
The Modification of Diet in Renal Disease trial showed that intensive BP control, compared with standard control, benefited patients who had more than 1 g of proteinuria at baseline. The ESCAPE (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients) trial also demonstrated that intensive BP control with a fixed dose of an ACE inhibitor significantly slowed the progression of renal disease, with the largest effects seen in children who had substantial proteinuria, hypertension, and a reduced GFR at baseline.
In addition, intensive BP control was beneficial in a recent study of adults in Italy who had idiopathic glomerular diseases associated with hypertension and proteinuria.
Julie R. Ingelfinger, M.D., is chief of pediatric nephrology at Massachusetts General Hospital, Boston, and a deputy editor of the New England Journal of Medicine. These comments were summarized from her editorial accompanying the report (N. Engl. J. Med. 2010;363:974-6). She reported having no relevant conflicts of interest.
Intensive blood pressure control doesn’t slow the progression of chronic kidney disease any better than standard blood pressure control in most patients, according to a report in the Sept. 2 New England Journal of Medicine.
It appears that the more intensive approach may benefit only patients who have proteinuria with a protein:creatinine ratio greater than 0.22, a value that is compatible with the widely accepted threshold of 300 mg/day for absolute urinary protein excretion, said Dr. Lawrence J. Appel of Johns Hopkins University, Baltimore, and his associates in the AASK (African-American Study of Kidney Disease and Hypertension) Collaborative Research Group.
Until now, “few trials have tested the effects of intensive blood pressure control [compared with conventional control] on the progression of chronic kidney disease, and the findings from such trials have been inconsistent. Despite a lack of compelling evidence, numerous guidelines recommend a reduced blood pressure target in patients with CKD,” they wrote.
Previous studies have rarely followed patients beyond 5 years, even though it typically takes longer than that for end-stage renal disease (ESRD) to develop in patients with CKD.
The AASK study compared outcomes between the two approaches to BP control in 1,094 black adults with mild to moderate hypertensive chronic kidney disease (defined as diastolic BP greater than 95 mm Hg and a glomerular filtration rate of 20-65 mL/min) but without marked proteinuria. Patients with diabetes were excluded from the trial.
In the first phase of the AASK investigation, patients were randomly assigned to either intensive BP control with a target of 92 mm Hg or lower mean arterial pressure (that is, lower than the usual target of 130/80 mm Hg recommended for CKD patients) or to conventional BP control with a target of 102-107 mm Hg mean arterial pressure (which corresponds to the conventional BP target of 140/90 mm Hg).
Throughout this initial phase of the trial, which lasted approximately 4 years, mean blood pressure was significantly lower in the intensive-control group (130/78 mm Hg) than in the standard-control group (141/86 mm Hg), yet there was no significant difference in the primary outcome of progression of kidney disease, development of ESRD, or death. Likewise, there was no significant difference between the two approaches in secondary or clinical outcomes.
In the second phase of the AASK investigation, patients who had not yet developed ESRD were invited to continue in a cohort portion of the trial, in which the BP target was 140/90 mm Hg. In 2004, when national guidelines were changed, this target was amended to lower than 130/80 mm Hg.
After a cumulative follow-up of 8-12 years, there still was no significant difference in primary or secondary outcomes between those who were initially assigned to the intensive-control and the standard-control groups. More intensive BP control did not slow the rate of progression of CKD, Dr. Appel and his associates reported (N. Engl. J. Med. 2010;363:918-29).
However, the intensive-control approach did benefit one subgroup of patients with proteinuria: those who had a protein:creatinine ratio of more than 0.22 at baseline. These patients showed a significant reduction in the primary outcome of progression of kidney disease, development of ESRD, or death, as well as in secondary and clinical outcomes.
The reason for this discrepancy is not known. “Overall, it is hard to develop a coherent, biologically plausible argument for a qualitative interaction between harm in patients without proteinuria and benefit in those with proteinuria,” the researchers said.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the Office of Research in Minority Health, and the National Institutes of Health. King Pharmaceuticals provided financial support and donated antihypertensive medications to each clinical center. Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn also donated antihypertensive drugs. None of these companies had any role in the design of the study, the accrual or analysis of data, or the preparation of the manuscript. Some of the investigators reported being in consultant and/or advisory board roles or receiving funds from numerous companies including Daiichi-Sankyo, Novartis, Amgen, King Pharmaceuticals, Abbott, Boehringer-Ingelheim, Litholink, Eli Lilly, Takeda, Merck, and Watson. Dr. Ingelfinger reported having no conflicts of interest.
Intensive blood pressure control doesn’t slow the progression of chronic kidney disease any better than standard blood pressure control in most patients, according to a report in the Sept. 2 New England Journal of Medicine.
It appears that the more intensive approach may benefit only patients who have proteinuria with a protein:creatinine ratio greater than 0.22, a value that is compatible with the widely accepted threshold of 300 mg/day for absolute urinary protein excretion, said Dr. Lawrence J. Appel of Johns Hopkins University, Baltimore, and his associates in the AASK (African-American Study of Kidney Disease and Hypertension) Collaborative Research Group.
Until now, “few trials have tested the effects of intensive blood pressure control [compared with conventional control] on the progression of chronic kidney disease, and the findings from such trials have been inconsistent. Despite a lack of compelling evidence, numerous guidelines recommend a reduced blood pressure target in patients with CKD,” they wrote.
Previous studies have rarely followed patients beyond 5 years, even though it typically takes longer than that for end-stage renal disease (ESRD) to develop in patients with CKD.
The AASK study compared outcomes between the two approaches to BP control in 1,094 black adults with mild to moderate hypertensive chronic kidney disease (defined as diastolic BP greater than 95 mm Hg and a glomerular filtration rate of 20-65 mL/min) but without marked proteinuria. Patients with diabetes were excluded from the trial.
In the first phase of the AASK investigation, patients were randomly assigned to either intensive BP control with a target of 92 mm Hg or lower mean arterial pressure (that is, lower than the usual target of 130/80 mm Hg recommended for CKD patients) or to conventional BP control with a target of 102-107 mm Hg mean arterial pressure (which corresponds to the conventional BP target of 140/90 mm Hg).
Throughout this initial phase of the trial, which lasted approximately 4 years, mean blood pressure was significantly lower in the intensive-control group (130/78 mm Hg) than in the standard-control group (141/86 mm Hg), yet there was no significant difference in the primary outcome of progression of kidney disease, development of ESRD, or death. Likewise, there was no significant difference between the two approaches in secondary or clinical outcomes.
In the second phase of the AASK investigation, patients who had not yet developed ESRD were invited to continue in a cohort portion of the trial, in which the BP target was 140/90 mm Hg. In 2004, when national guidelines were changed, this target was amended to lower than 130/80 mm Hg.
After a cumulative follow-up of 8-12 years, there still was no significant difference in primary or secondary outcomes between those who were initially assigned to the intensive-control and the standard-control groups. More intensive BP control did not slow the rate of progression of CKD, Dr. Appel and his associates reported (N. Engl. J. Med. 2010;363:918-29).
However, the intensive-control approach did benefit one subgroup of patients with proteinuria: those who had a protein:creatinine ratio of more than 0.22 at baseline. These patients showed a significant reduction in the primary outcome of progression of kidney disease, development of ESRD, or death, as well as in secondary and clinical outcomes.
The reason for this discrepancy is not known. “Overall, it is hard to develop a coherent, biologically plausible argument for a qualitative interaction between harm in patients without proteinuria and benefit in those with proteinuria,” the researchers said.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the Office of Research in Minority Health, and the National Institutes of Health. King Pharmaceuticals provided financial support and donated antihypertensive medications to each clinical center. Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn also donated antihypertensive drugs. None of these companies had any role in the design of the study, the accrual or analysis of data, or the preparation of the manuscript. Some of the investigators reported being in consultant and/or advisory board roles or receiving funds from numerous companies including Daiichi-Sankyo, Novartis, Amgen, King Pharmaceuticals, Abbott, Boehringer-Ingelheim, Litholink, Eli Lilly, Takeda, Merck, and Watson. Dr. Ingelfinger reported having no conflicts of interest.
Major Finding: Compared with standard BP control, intensive BP control failed to slow the progression of CKD, prevent the development of end-stage renal disease, or prevent death in most patients who had mild to moderate chronic kidney disease. Intensive BP control was beneficial only in the subgroup of patients who had proteinuria with a protein:creatinine ratio greater than 0.22 at baseline.
Data Source: AASK, a clinical trial with an initial 4-year randomized phase comparing intensive BP control with standard BP control in 1,094 black adults, as well as an observational cohort phase with a further 4-8 years of extended follow-up.
Disclosures: This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the Office of Research in Minority Health, and the National Institutes of Health. King Pharmaceuticals provided financial support and donated antihypertensive medications to each clinical center. Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn also donated antihypertensive drugs. None of these companies had any role in the design of the study, the accrual or analysis of data, or the preparation of the manuscript. Some of the investigators reported being in consultant and/or advisory board roles or receiving funds from numerous companies including Daiichi-Sankyo, Novartis, Amgen, King Pharmaceuticals, Abbott, Boehringer-Ingelheim, Litholink, Eli Lilly, Takeda, Merck, and Watson.
The shrinking woman
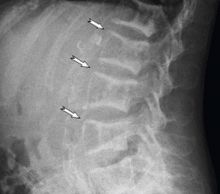
A 45-year-old woman who has been undergoing hemodialysis for 20 years presents with diffuse bone pain, pruritic skin, muscle weakness, and disability. About 8 years ago, she was diagnosed with uremic hyperparathyroidism and underwent two parathyroidectomy procedures and eight sessions of percutaneous alcohol ablation of the parathyroid gland.
A technetium-99m sestamibi radionuclide scan shows bilateral parathyroid hyperplasia with no ectopic parathyroid adenomas. Although a surgeon she consulted 1 month ago declined to perform another parathyroidectomy for technical reasons, another surgeon agreed to do it at this time. Parathyroidectomy was successfully performed, after which the parathyroid hormone level decreased drastically, to 85 pg/mL.
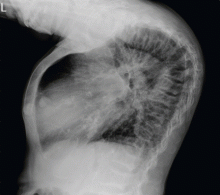
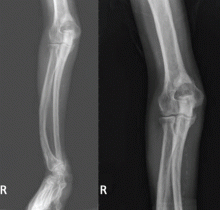
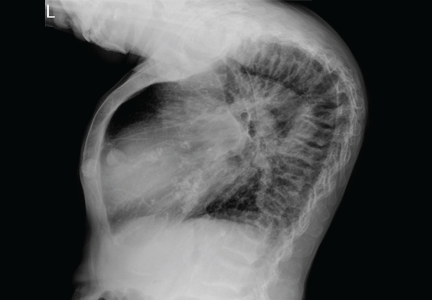
To our surprise, her total body length increased by 6 to 7 cm after surgery, with partial straightening of the back and legs noted about 4 to 5 weeks after surgery. Furthermore, she was able to walk a short distance just a few weeks after surgery. Unfortunately, she died from sepsis the next year.
SEVERE UREMIC HYPERPARATHYROIDISM: A CLINICAL DILEMMA
The clinical appearance of reduced body length and diffuse bony deformity leading to “shrinking” as a consequence of prolonged severe uremic hyperparathyroidism has only rarely been reported.1 However, it is not uncommon for surgeons to decide against parathyroidectomy because of concerns of extensive subcutaneous fibrosis and recurrent laryngeal nerve damage associated with previous operations. The result is that the patient’s uremic hyperparathyroidism goes untreated, increasing the risk of long-term complications, as in this patient.
TYPICAL RADIOGRAPHIC FEATURES
Why the body length increases after parathyroidectomy is not yet known, but plausible mechanisms are the effects of a recovery of muscle strength and the vigorous bony remineralization that strengthens weight-bearing bones after resolution of uremic hyperparathyroidism.
THE DANGERS OF DELAYED TREATMENT
Delaying parathyroidectomy may induce prolonged and severe uremic hyperparathyroidism, as in this patient. Nevertheless, despite the delay, surgery was able to partially ameliorate the symptoms of hyperparathyroidism and improve the extreme bone deformity. However, the patient’s informed consent, a detailed preoperative evaluation, and exclusion of ectopic parathyroid adenomas are imperative before surgical treatment.
- Horensten ML, Boner G, Rosenfeld JB. The shrinking man. A manifestation of severe renal osteodystrophy. JAMA 1980; 244:267–268.
- Jevtic V. Imaging of renal osteodystrophy. Eur J Radiol 2003; 46:85–95.
- Ferreira MA. Diagnosis of renal osteodystrophy: when and how to use biochemical markers and non-invasive methods; when bone biopsy is needed. Nephrol Dial Transplant 2000; 15(suppl 5):8–14.
A 45-year-old woman who has been undergoing hemodialysis for 20 years presents with diffuse bone pain, pruritic skin, muscle weakness, and disability. About 8 years ago, she was diagnosed with uremic hyperparathyroidism and underwent two parathyroidectomy procedures and eight sessions of percutaneous alcohol ablation of the parathyroid gland.
A technetium-99m sestamibi radionuclide scan shows bilateral parathyroid hyperplasia with no ectopic parathyroid adenomas. Although a surgeon she consulted 1 month ago declined to perform another parathyroidectomy for technical reasons, another surgeon agreed to do it at this time. Parathyroidectomy was successfully performed, after which the parathyroid hormone level decreased drastically, to 85 pg/mL.
To our surprise, her total body length increased by 6 to 7 cm after surgery, with partial straightening of the back and legs noted about 4 to 5 weeks after surgery. Furthermore, she was able to walk a short distance just a few weeks after surgery. Unfortunately, she died from sepsis the next year.
SEVERE UREMIC HYPERPARATHYROIDISM: A CLINICAL DILEMMA
The clinical appearance of reduced body length and diffuse bony deformity leading to “shrinking” as a consequence of prolonged severe uremic hyperparathyroidism has only rarely been reported.1 However, it is not uncommon for surgeons to decide against parathyroidectomy because of concerns of extensive subcutaneous fibrosis and recurrent laryngeal nerve damage associated with previous operations. The result is that the patient’s uremic hyperparathyroidism goes untreated, increasing the risk of long-term complications, as in this patient.
TYPICAL RADIOGRAPHIC FEATURES
Why the body length increases after parathyroidectomy is not yet known, but plausible mechanisms are the effects of a recovery of muscle strength and the vigorous bony remineralization that strengthens weight-bearing bones after resolution of uremic hyperparathyroidism.
THE DANGERS OF DELAYED TREATMENT
Delaying parathyroidectomy may induce prolonged and severe uremic hyperparathyroidism, as in this patient. Nevertheless, despite the delay, surgery was able to partially ameliorate the symptoms of hyperparathyroidism and improve the extreme bone deformity. However, the patient’s informed consent, a detailed preoperative evaluation, and exclusion of ectopic parathyroid adenomas are imperative before surgical treatment.
A 45-year-old woman who has been undergoing hemodialysis for 20 years presents with diffuse bone pain, pruritic skin, muscle weakness, and disability. About 8 years ago, she was diagnosed with uremic hyperparathyroidism and underwent two parathyroidectomy procedures and eight sessions of percutaneous alcohol ablation of the parathyroid gland.
A technetium-99m sestamibi radionuclide scan shows bilateral parathyroid hyperplasia with no ectopic parathyroid adenomas. Although a surgeon she consulted 1 month ago declined to perform another parathyroidectomy for technical reasons, another surgeon agreed to do it at this time. Parathyroidectomy was successfully performed, after which the parathyroid hormone level decreased drastically, to 85 pg/mL.
To our surprise, her total body length increased by 6 to 7 cm after surgery, with partial straightening of the back and legs noted about 4 to 5 weeks after surgery. Furthermore, she was able to walk a short distance just a few weeks after surgery. Unfortunately, she died from sepsis the next year.
SEVERE UREMIC HYPERPARATHYROIDISM: A CLINICAL DILEMMA
The clinical appearance of reduced body length and diffuse bony deformity leading to “shrinking” as a consequence of prolonged severe uremic hyperparathyroidism has only rarely been reported.1 However, it is not uncommon for surgeons to decide against parathyroidectomy because of concerns of extensive subcutaneous fibrosis and recurrent laryngeal nerve damage associated with previous operations. The result is that the patient’s uremic hyperparathyroidism goes untreated, increasing the risk of long-term complications, as in this patient.
TYPICAL RADIOGRAPHIC FEATURES
Why the body length increases after parathyroidectomy is not yet known, but plausible mechanisms are the effects of a recovery of muscle strength and the vigorous bony remineralization that strengthens weight-bearing bones after resolution of uremic hyperparathyroidism.
THE DANGERS OF DELAYED TREATMENT
Delaying parathyroidectomy may induce prolonged and severe uremic hyperparathyroidism, as in this patient. Nevertheless, despite the delay, surgery was able to partially ameliorate the symptoms of hyperparathyroidism and improve the extreme bone deformity. However, the patient’s informed consent, a detailed preoperative evaluation, and exclusion of ectopic parathyroid adenomas are imperative before surgical treatment.
- Horensten ML, Boner G, Rosenfeld JB. The shrinking man. A manifestation of severe renal osteodystrophy. JAMA 1980; 244:267–268.
- Jevtic V. Imaging of renal osteodystrophy. Eur J Radiol 2003; 46:85–95.
- Ferreira MA. Diagnosis of renal osteodystrophy: when and how to use biochemical markers and non-invasive methods; when bone biopsy is needed. Nephrol Dial Transplant 2000; 15(suppl 5):8–14.
- Horensten ML, Boner G, Rosenfeld JB. The shrinking man. A manifestation of severe renal osteodystrophy. JAMA 1980; 244:267–268.
- Jevtic V. Imaging of renal osteodystrophy. Eur J Radiol 2003; 46:85–95.
- Ferreira MA. Diagnosis of renal osteodystrophy: when and how to use biochemical markers and non-invasive methods; when bone biopsy is needed. Nephrol Dial Transplant 2000; 15(suppl 5):8–14.
Aggressive Local Treatment Likely With Low PSA
Patients newly diagnosed with prostate cancer who have a prostate specific antigen levels of 4.0 ng/mL or below tend to receive aggressive therapy despite having a low risk of disease, according to a retrospective study of 123,934 men in the July 26 issue of the Archives of Internal Medicine.
In a multivariate analysis correcting for age, race, and year of diagnosis, men with screen-detected prostate cancer and prostate specific antigen (PSA) values of 4.0 ng/mL or below were 49% more likely to undergo radical prostatectomy and 39% more likely to undergo radiation therapy than were men with non–screen detected prostate cancer, according to Yu-Hsuan Shao, Ph.D., of the Cancer Institute of New Jersey, New Brunswick, and her colleagues.
Although 54% of men diagnosed with prostate cancer with low PSA levels harbor low-risk disease, 77% of them underwent radical prostatectomy or radiotherapy. Men with low PSA levels and screen-detected prostate cancer were 33% less likely to have high-grade disease than were men with non–screen detected prostate cancer, the investigators said.
“The finding that men in low-risk groups were treated intensively raises the concern of overtreatment, especially among older patients,” the authors wrote.
Investigators used the Surveillance, Epidemiology, and End Results (SEER) database, covering approximately 26% of the U.S. population, to identify men newly diagnosed with prostate cancer between 2004 and 2006. They excluded men under the age of 24 years, those with missing PSA values, or those with missing Gleason scores and clinical stage.
The men in the study were divided into four groups based on PSA values. A total of 14% of the men had PSA values of 4.0 ng/mL or less, 57.6% had values between 4.1-10.0 ng/mL, 15.9% had values between 10.1-20.0 ng/mL, and 12.5% had values above 20 ng/mL (Arch. Int. Med. 2010;170:1256-61).
Of the men with the lowest PSA values, 44% underwent radical prostatectomy, 33% underwent radiation therapy, and 23% were treated with conservative management. In contrast, of the men with the highest PSA levels, 12.5% underwent radical prostatectomy, 31.5% underwent radiation therapy, and 56% were treated with conservative management.
One of the study’s authors acknowledged receiving clinical research funding from the Ohl Foundation, the New Jersey Commission on Cancer Research, and the Agency for Healthcare Research and Quality. Another author acknowledged receiving clinical research funding from Sanofi-Aventis and consultation fees from Blue Cross/Blue Shield. The study was supported by the National Cancer Institute, the Cancer Institute of New Jersey, and the Robert Wood Johnson Foundation.
Patients newly diagnosed with prostate cancer who have a prostate specific antigen levels of 4.0 ng/mL or below tend to receive aggressive therapy despite having a low risk of disease, according to a retrospective study of 123,934 men in the July 26 issue of the Archives of Internal Medicine.
In a multivariate analysis correcting for age, race, and year of diagnosis, men with screen-detected prostate cancer and prostate specific antigen (PSA) values of 4.0 ng/mL or below were 49% more likely to undergo radical prostatectomy and 39% more likely to undergo radiation therapy than were men with non–screen detected prostate cancer, according to Yu-Hsuan Shao, Ph.D., of the Cancer Institute of New Jersey, New Brunswick, and her colleagues.
Although 54% of men diagnosed with prostate cancer with low PSA levels harbor low-risk disease, 77% of them underwent radical prostatectomy or radiotherapy. Men with low PSA levels and screen-detected prostate cancer were 33% less likely to have high-grade disease than were men with non–screen detected prostate cancer, the investigators said.
“The finding that men in low-risk groups were treated intensively raises the concern of overtreatment, especially among older patients,” the authors wrote.
Investigators used the Surveillance, Epidemiology, and End Results (SEER) database, covering approximately 26% of the U.S. population, to identify men newly diagnosed with prostate cancer between 2004 and 2006. They excluded men under the age of 24 years, those with missing PSA values, or those with missing Gleason scores and clinical stage.
The men in the study were divided into four groups based on PSA values. A total of 14% of the men had PSA values of 4.0 ng/mL or less, 57.6% had values between 4.1-10.0 ng/mL, 15.9% had values between 10.1-20.0 ng/mL, and 12.5% had values above 20 ng/mL (Arch. Int. Med. 2010;170:1256-61).
Of the men with the lowest PSA values, 44% underwent radical prostatectomy, 33% underwent radiation therapy, and 23% were treated with conservative management. In contrast, of the men with the highest PSA levels, 12.5% underwent radical prostatectomy, 31.5% underwent radiation therapy, and 56% were treated with conservative management.
One of the study’s authors acknowledged receiving clinical research funding from the Ohl Foundation, the New Jersey Commission on Cancer Research, and the Agency for Healthcare Research and Quality. Another author acknowledged receiving clinical research funding from Sanofi-Aventis and consultation fees from Blue Cross/Blue Shield. The study was supported by the National Cancer Institute, the Cancer Institute of New Jersey, and the Robert Wood Johnson Foundation.
Patients newly diagnosed with prostate cancer who have a prostate specific antigen levels of 4.0 ng/mL or below tend to receive aggressive therapy despite having a low risk of disease, according to a retrospective study of 123,934 men in the July 26 issue of the Archives of Internal Medicine.
In a multivariate analysis correcting for age, race, and year of diagnosis, men with screen-detected prostate cancer and prostate specific antigen (PSA) values of 4.0 ng/mL or below were 49% more likely to undergo radical prostatectomy and 39% more likely to undergo radiation therapy than were men with non–screen detected prostate cancer, according to Yu-Hsuan Shao, Ph.D., of the Cancer Institute of New Jersey, New Brunswick, and her colleagues.
Although 54% of men diagnosed with prostate cancer with low PSA levels harbor low-risk disease, 77% of them underwent radical prostatectomy or radiotherapy. Men with low PSA levels and screen-detected prostate cancer were 33% less likely to have high-grade disease than were men with non–screen detected prostate cancer, the investigators said.
“The finding that men in low-risk groups were treated intensively raises the concern of overtreatment, especially among older patients,” the authors wrote.
Investigators used the Surveillance, Epidemiology, and End Results (SEER) database, covering approximately 26% of the U.S. population, to identify men newly diagnosed with prostate cancer between 2004 and 2006. They excluded men under the age of 24 years, those with missing PSA values, or those with missing Gleason scores and clinical stage.
The men in the study were divided into four groups based on PSA values. A total of 14% of the men had PSA values of 4.0 ng/mL or less, 57.6% had values between 4.1-10.0 ng/mL, 15.9% had values between 10.1-20.0 ng/mL, and 12.5% had values above 20 ng/mL (Arch. Int. Med. 2010;170:1256-61).
Of the men with the lowest PSA values, 44% underwent radical prostatectomy, 33% underwent radiation therapy, and 23% were treated with conservative management. In contrast, of the men with the highest PSA levels, 12.5% underwent radical prostatectomy, 31.5% underwent radiation therapy, and 56% were treated with conservative management.
One of the study’s authors acknowledged receiving clinical research funding from the Ohl Foundation, the New Jersey Commission on Cancer Research, and the Agency for Healthcare Research and Quality. Another author acknowledged receiving clinical research funding from Sanofi-Aventis and consultation fees from Blue Cross/Blue Shield. The study was supported by the National Cancer Institute, the Cancer Institute of New Jersey, and the Robert Wood Johnson Foundation.
Major Finding: Although 54% of men diagnosed with prostate cancer and with PSA levels of 4.0 ng/mL or below have low-risk disease, 77% of them underwent radical prostatectomy or radiation therapy.
Data Source: SEER data on 123,934 men newly diagnosed with prostate cancer in 2004-2006
Disclosures: One of the study’s authors acknowledged receiving clinical research funding from the Ohl Foundation, the New Jersey Commission on Cancer Research, and the Agency for Healthcare Research and Quality. Another author acknowledged receiving clinical research funding from Sanofi-Aventis and consultation fees from Blue Cross/Blue Shield. The study was supported by the National Cancer Institute, the Cancer Institute of New Jersey, and the Robert Wood Johnson Foundation.
Prostate Cancer Risk Increased With High Calcium Intake
Dietary calcium was associated with significantly increased risk of prostate cancer in Chinese men with a below-average body mass index, according to an analysis of a large data set.
Researchers found that among subjects with a BMI below the median 22.9 kg/m
Data from previous studies have suggested a link between calcium and prostate cancer, but these studies have not been able to separate dairy products from calcium, said Lesley M. Butler, Ph.D., of Colorado State University in Fort Collins, and colleagues.
To more accurately assess the link between dietary calcium and prostate cancer, the researchers focused on a population of Chinese men whose dairy intake was relatively low. In general, Asian diets contain few dairy products, compared with Western diets, the researchers noted. Instead, most of the calcium in Asian diets comes from nondairy sources such as broccoli, kale, bok choy, and soy products. The researchers reviewed data from the Singapore Chinese Health Study, focusing on 27,293 men who did not have cancer when they entered the study between April 1993 and December 1998 (Cancer Res. 2010 June 1 [doi:10.1158/0008-5472.CAN-09-4544]).
Overall, dietary calcium was associated with a nonsignificant 25% increase in prostate cancer risk for the highest quartile of calcium intake (median of 659 mg/day) vs. the lowest quartile (median of 211 mg/day).
The study participants completed a 165-item food frequency questionnaire to assess their diets over the past year. Baseline characteristics including age, education, physical activity, and BMI were similar among the four quartiles.
Overall, the median daily intake of dairy products in the study population was 19.3 g. The greatest contributions of different food sources to daily calcium intake were vegetables (19.3%), dairy (17.3%), grain products (14.7%), soy products (11.8%), fruit (7.3%), and fish (6.2%). The variety of food sources suggest that the link between prostate cancer risk and calcium intake is not likely to be related to any particular food group, the researchers noted.
Neither age nor physical activity had an effect on the association between calcium and cancer, the researchers wrote.
“Our study is the first to report a positive association between calcium and prostate cancer risk at such a low calcium level,” the researchers said. Previous studies have shown that calcium is absorbed more efficiently in the Chinese population, compared with the white population, and among thinner people compared with heavier people, which is why a study of relatively thin Chinese men might be more likely to reveal a cancer/calcium connection than a study of heavier white men, the researchers wrote.
The study was limited by an inability to assess any variation of the calcium/cancer connection based on stage of disease, and more research is needed to evaluate the role of calcium in prostate cancer, compared with other components of dairy products, they added.
Disclosures: The study was supported by a grant from the National Cancer Institute. Dr. Butler reported having no financial conflicts.
My Take
Over 1,000 mg May Be Too Much
Previous studies indicated that high intakes of calcium (more than 1,000–1,500 mg/day) may increase prostate cancer risk. As there is no established benefit for men at such high intakes, it makes sense for men to not go much beyond the 1,000-mg/day range until further studies have been done. However, intakes of calcium that are too low (less than 700 mg/day) may increase risk of some conditions, such as hypertension and colorectal cancer. Thus, it is reasonable for men to be in the range of 700–1,000 mg/day, but prudent not to go too much lower.
These results need to be confirmed in other studies where calcium intake is relatively low and there are not many dairy products. Also, since many men take calcium supplements, that might be an informative group to study. Finally, men with prostate cancer need to be studied, as the results would be most pertinent to them.
In addition, the authors theorize that leaner men may absorb calcium better. The evidence for this is limited at this time. This could be a chance finding, so it also needs to be replicated in other populations.
EDWARD GIOVANNUCCI, M.D., is a professor of epidemiology and nutrition at Harvard University, Cambridge, Mass.
Dietary calcium was associated with significantly increased risk of prostate cancer in Chinese men with a below-average body mass index, according to an analysis of a large data set.
Researchers found that among subjects with a BMI below the median 22.9 kg/m
Data from previous studies have suggested a link between calcium and prostate cancer, but these studies have not been able to separate dairy products from calcium, said Lesley M. Butler, Ph.D., of Colorado State University in Fort Collins, and colleagues.
To more accurately assess the link between dietary calcium and prostate cancer, the researchers focused on a population of Chinese men whose dairy intake was relatively low. In general, Asian diets contain few dairy products, compared with Western diets, the researchers noted. Instead, most of the calcium in Asian diets comes from nondairy sources such as broccoli, kale, bok choy, and soy products. The researchers reviewed data from the Singapore Chinese Health Study, focusing on 27,293 men who did not have cancer when they entered the study between April 1993 and December 1998 (Cancer Res. 2010 June 1 [doi:10.1158/0008-5472.CAN-09-4544]).
Overall, dietary calcium was associated with a nonsignificant 25% increase in prostate cancer risk for the highest quartile of calcium intake (median of 659 mg/day) vs. the lowest quartile (median of 211 mg/day).
The study participants completed a 165-item food frequency questionnaire to assess their diets over the past year. Baseline characteristics including age, education, physical activity, and BMI were similar among the four quartiles.
Overall, the median daily intake of dairy products in the study population was 19.3 g. The greatest contributions of different food sources to daily calcium intake were vegetables (19.3%), dairy (17.3%), grain products (14.7%), soy products (11.8%), fruit (7.3%), and fish (6.2%). The variety of food sources suggest that the link between prostate cancer risk and calcium intake is not likely to be related to any particular food group, the researchers noted.
Neither age nor physical activity had an effect on the association between calcium and cancer, the researchers wrote.
“Our study is the first to report a positive association between calcium and prostate cancer risk at such a low calcium level,” the researchers said. Previous studies have shown that calcium is absorbed more efficiently in the Chinese population, compared with the white population, and among thinner people compared with heavier people, which is why a study of relatively thin Chinese men might be more likely to reveal a cancer/calcium connection than a study of heavier white men, the researchers wrote.
The study was limited by an inability to assess any variation of the calcium/cancer connection based on stage of disease, and more research is needed to evaluate the role of calcium in prostate cancer, compared with other components of dairy products, they added.
Disclosures: The study was supported by a grant from the National Cancer Institute. Dr. Butler reported having no financial conflicts.
My Take
Over 1,000 mg May Be Too Much
Previous studies indicated that high intakes of calcium (more than 1,000–1,500 mg/day) may increase prostate cancer risk. As there is no established benefit for men at such high intakes, it makes sense for men to not go much beyond the 1,000-mg/day range until further studies have been done. However, intakes of calcium that are too low (less than 700 mg/day) may increase risk of some conditions, such as hypertension and colorectal cancer. Thus, it is reasonable for men to be in the range of 700–1,000 mg/day, but prudent not to go too much lower.
These results need to be confirmed in other studies where calcium intake is relatively low and there are not many dairy products. Also, since many men take calcium supplements, that might be an informative group to study. Finally, men with prostate cancer need to be studied, as the results would be most pertinent to them.
In addition, the authors theorize that leaner men may absorb calcium better. The evidence for this is limited at this time. This could be a chance finding, so it also needs to be replicated in other populations.
EDWARD GIOVANNUCCI, M.D., is a professor of epidemiology and nutrition at Harvard University, Cambridge, Mass.
Dietary calcium was associated with significantly increased risk of prostate cancer in Chinese men with a below-average body mass index, according to an analysis of a large data set.
Researchers found that among subjects with a BMI below the median 22.9 kg/m
Data from previous studies have suggested a link between calcium and prostate cancer, but these studies have not been able to separate dairy products from calcium, said Lesley M. Butler, Ph.D., of Colorado State University in Fort Collins, and colleagues.
To more accurately assess the link between dietary calcium and prostate cancer, the researchers focused on a population of Chinese men whose dairy intake was relatively low. In general, Asian diets contain few dairy products, compared with Western diets, the researchers noted. Instead, most of the calcium in Asian diets comes from nondairy sources such as broccoli, kale, bok choy, and soy products. The researchers reviewed data from the Singapore Chinese Health Study, focusing on 27,293 men who did not have cancer when they entered the study between April 1993 and December 1998 (Cancer Res. 2010 June 1 [doi:10.1158/0008-5472.CAN-09-4544]).
Overall, dietary calcium was associated with a nonsignificant 25% increase in prostate cancer risk for the highest quartile of calcium intake (median of 659 mg/day) vs. the lowest quartile (median of 211 mg/day).
The study participants completed a 165-item food frequency questionnaire to assess their diets over the past year. Baseline characteristics including age, education, physical activity, and BMI were similar among the four quartiles.
Overall, the median daily intake of dairy products in the study population was 19.3 g. The greatest contributions of different food sources to daily calcium intake were vegetables (19.3%), dairy (17.3%), grain products (14.7%), soy products (11.8%), fruit (7.3%), and fish (6.2%). The variety of food sources suggest that the link between prostate cancer risk and calcium intake is not likely to be related to any particular food group, the researchers noted.
Neither age nor physical activity had an effect on the association between calcium and cancer, the researchers wrote.
“Our study is the first to report a positive association between calcium and prostate cancer risk at such a low calcium level,” the researchers said. Previous studies have shown that calcium is absorbed more efficiently in the Chinese population, compared with the white population, and among thinner people compared with heavier people, which is why a study of relatively thin Chinese men might be more likely to reveal a cancer/calcium connection than a study of heavier white men, the researchers wrote.
The study was limited by an inability to assess any variation of the calcium/cancer connection based on stage of disease, and more research is needed to evaluate the role of calcium in prostate cancer, compared with other components of dairy products, they added.
Disclosures: The study was supported by a grant from the National Cancer Institute. Dr. Butler reported having no financial conflicts.
My Take
Over 1,000 mg May Be Too Much
Previous studies indicated that high intakes of calcium (more than 1,000–1,500 mg/day) may increase prostate cancer risk. As there is no established benefit for men at such high intakes, it makes sense for men to not go much beyond the 1,000-mg/day range until further studies have been done. However, intakes of calcium that are too low (less than 700 mg/day) may increase risk of some conditions, such as hypertension and colorectal cancer. Thus, it is reasonable for men to be in the range of 700–1,000 mg/day, but prudent not to go too much lower.
These results need to be confirmed in other studies where calcium intake is relatively low and there are not many dairy products. Also, since many men take calcium supplements, that might be an informative group to study. Finally, men with prostate cancer need to be studied, as the results would be most pertinent to them.
In addition, the authors theorize that leaner men may absorb calcium better. The evidence for this is limited at this time. This could be a chance finding, so it also needs to be replicated in other populations.
EDWARD GIOVANNUCCI, M.D., is a professor of epidemiology and nutrition at Harvard University, Cambridge, Mass.
Male Sexual Function Improves With Exercise
Major Finding: Exercise equivalent to 30 minutes of brisk walking per day, 4 days per week, is associated with a 65% decrease in the risk of sexual dysfunction.
Data Source: Study of 178 healthy men.
Disclosures: Dr. McNamara reported that she had no conflicts of interest. The study was supported by the Department of Defense and the Department of Veterans Affairs.
SAN FRANCISCO — Higher levels of exercise are associated with lower levels of sexual dysfunction, according to a study of 178 healthy men.
Men who reported exercise of at least 9 metabolic equivalents (METs) per week were 65% less likely to report sexual dysfunction. Brisk walking for 30 minutes a day for 4 days per week is equivalent to about 9 METs, according to Dr. Erin R. McNamara of Duke University Medical Center, Durham, N.C., who presented the results of her study at the meeting.
“If men won't exercise for the cardiovascular benefits, maybe they'll exercise to have better sex,” Dr. McNamara said at a news briefing.
The men in the study were all enrolled in a prospective case-control study at the Durham Veterans Affairs Medical Center. Their mean age was 62 years, and their mean body mass index was 30.7 kg/m
The sexual function survey consisted of six questions, asking men to evaluate their ability to have an erection, the quality and frequency of their erections, their ability to reach orgasm, their overall sexual ability, and the extent to which they were bothered by their sexual functioning. The investigators converted scores on the survey to a 0–100 scale. Overall, the participants' mean sexual function score was 53.
The men also were asked to assess their duration, intensity, and frequency of exercise. The investigators converted these estimates to MET hours per week. They classified men reporting fewer than 3 MET hours per week as sedentary (53% of the sample), 3–8 MET hours as active (14% of the sample), 9–17 MET hours as moderately active (9% of the sample), and 18 or more MET hours as highly active (24% of the sample).
Mean sexual function scores were 42 for sedentary men, 50 for active men, 72 for moderately active men, and 70 for highly active men. The trend was statistically significant.
In a multivariate analysis, the investigators controlled for age, race, BMI, heart disease, diabetes, medications, and depression. They defined a sexual function score of less than 40 as sexual dysfunction. Compared with sedentary men, those reporting moderate or high levels of physical activity were 65% less likely to have sexual dysfunction.
In an interview, Dr. McNamara emphasized that her study demonstrated only correlation, not causation. Asked to speculate on the reason for the association, she said, “Just as exercise provides cardiovascular benefit by increasing blood flow, we think the same thing probably happens [with sexual function] because the penis is engorged with blood vessels.” She also suggested that exercise may improve sexual function as a psychological byproduct of improved feelings of well-being.
Men with moderate or high levels of physical activity were 65% less likely to have sexual dysfunction.
Source Courtesy Ken Trombatore
Major Finding: Exercise equivalent to 30 minutes of brisk walking per day, 4 days per week, is associated with a 65% decrease in the risk of sexual dysfunction.
Data Source: Study of 178 healthy men.
Disclosures: Dr. McNamara reported that she had no conflicts of interest. The study was supported by the Department of Defense and the Department of Veterans Affairs.
SAN FRANCISCO — Higher levels of exercise are associated with lower levels of sexual dysfunction, according to a study of 178 healthy men.
Men who reported exercise of at least 9 metabolic equivalents (METs) per week were 65% less likely to report sexual dysfunction. Brisk walking for 30 minutes a day for 4 days per week is equivalent to about 9 METs, according to Dr. Erin R. McNamara of Duke University Medical Center, Durham, N.C., who presented the results of her study at the meeting.
“If men won't exercise for the cardiovascular benefits, maybe they'll exercise to have better sex,” Dr. McNamara said at a news briefing.
The men in the study were all enrolled in a prospective case-control study at the Durham Veterans Affairs Medical Center. Their mean age was 62 years, and their mean body mass index was 30.7 kg/m
The sexual function survey consisted of six questions, asking men to evaluate their ability to have an erection, the quality and frequency of their erections, their ability to reach orgasm, their overall sexual ability, and the extent to which they were bothered by their sexual functioning. The investigators converted scores on the survey to a 0–100 scale. Overall, the participants' mean sexual function score was 53.
The men also were asked to assess their duration, intensity, and frequency of exercise. The investigators converted these estimates to MET hours per week. They classified men reporting fewer than 3 MET hours per week as sedentary (53% of the sample), 3–8 MET hours as active (14% of the sample), 9–17 MET hours as moderately active (9% of the sample), and 18 or more MET hours as highly active (24% of the sample).
Mean sexual function scores were 42 for sedentary men, 50 for active men, 72 for moderately active men, and 70 for highly active men. The trend was statistically significant.
In a multivariate analysis, the investigators controlled for age, race, BMI, heart disease, diabetes, medications, and depression. They defined a sexual function score of less than 40 as sexual dysfunction. Compared with sedentary men, those reporting moderate or high levels of physical activity were 65% less likely to have sexual dysfunction.
In an interview, Dr. McNamara emphasized that her study demonstrated only correlation, not causation. Asked to speculate on the reason for the association, she said, “Just as exercise provides cardiovascular benefit by increasing blood flow, we think the same thing probably happens [with sexual function] because the penis is engorged with blood vessels.” She also suggested that exercise may improve sexual function as a psychological byproduct of improved feelings of well-being.
Men with moderate or high levels of physical activity were 65% less likely to have sexual dysfunction.
Source Courtesy Ken Trombatore
Major Finding: Exercise equivalent to 30 minutes of brisk walking per day, 4 days per week, is associated with a 65% decrease in the risk of sexual dysfunction.
Data Source: Study of 178 healthy men.
Disclosures: Dr. McNamara reported that she had no conflicts of interest. The study was supported by the Department of Defense and the Department of Veterans Affairs.
SAN FRANCISCO — Higher levels of exercise are associated with lower levels of sexual dysfunction, according to a study of 178 healthy men.
Men who reported exercise of at least 9 metabolic equivalents (METs) per week were 65% less likely to report sexual dysfunction. Brisk walking for 30 minutes a day for 4 days per week is equivalent to about 9 METs, according to Dr. Erin R. McNamara of Duke University Medical Center, Durham, N.C., who presented the results of her study at the meeting.
“If men won't exercise for the cardiovascular benefits, maybe they'll exercise to have better sex,” Dr. McNamara said at a news briefing.
The men in the study were all enrolled in a prospective case-control study at the Durham Veterans Affairs Medical Center. Their mean age was 62 years, and their mean body mass index was 30.7 kg/m
The sexual function survey consisted of six questions, asking men to evaluate their ability to have an erection, the quality and frequency of their erections, their ability to reach orgasm, their overall sexual ability, and the extent to which they were bothered by their sexual functioning. The investigators converted scores on the survey to a 0–100 scale. Overall, the participants' mean sexual function score was 53.
The men also were asked to assess their duration, intensity, and frequency of exercise. The investigators converted these estimates to MET hours per week. They classified men reporting fewer than 3 MET hours per week as sedentary (53% of the sample), 3–8 MET hours as active (14% of the sample), 9–17 MET hours as moderately active (9% of the sample), and 18 or more MET hours as highly active (24% of the sample).
Mean sexual function scores were 42 for sedentary men, 50 for active men, 72 for moderately active men, and 70 for highly active men. The trend was statistically significant.
In a multivariate analysis, the investigators controlled for age, race, BMI, heart disease, diabetes, medications, and depression. They defined a sexual function score of less than 40 as sexual dysfunction. Compared with sedentary men, those reporting moderate or high levels of physical activity were 65% less likely to have sexual dysfunction.
In an interview, Dr. McNamara emphasized that her study demonstrated only correlation, not causation. Asked to speculate on the reason for the association, she said, “Just as exercise provides cardiovascular benefit by increasing blood flow, we think the same thing probably happens [with sexual function] because the penis is engorged with blood vessels.” She also suggested that exercise may improve sexual function as a psychological byproduct of improved feelings of well-being.
Men with moderate or high levels of physical activity were 65% less likely to have sexual dysfunction.
Source Courtesy Ken Trombatore
Does Vancomycin Deserve the Bad Raps?
Fenofibrate's Effects on Renal Function
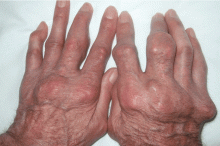
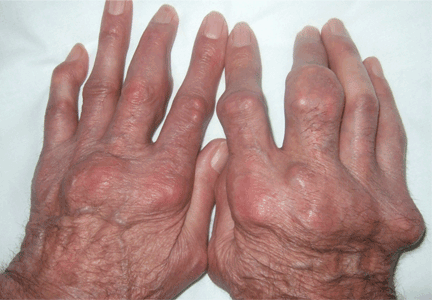
Giant nodules on the hands
Q: Which is the most likely diagnosis?
- Rheumatoid arthritis
- Nodular osteoarthritis
- Tophaceous gout
- Pseudogout
- Xanthoma tuberosum
A: Tophaceous gout is the diagnosis. This patient’s serum urate level was 9 mg/dL (normal range 4.0–8.0) despite allopurinol therapy, with normal levels of lipids, urea, and creatinine. Polarized light microscopy of aspirated synovial fluid showed monosodium urate crystals, thus confirming the diagnosis.
Rheumatoid arthritis is typically polyarticular and symmetrical and spares the distal interphalangeal joints. Subcutaneous rheumatoid nodules may mimic gouty tophi.
Pseudogout shares some of the features of gout. It results from deposits of calcium pyrophosphate crystals in and around the joints. The diagnosis is made by identifying the crystals on microscopy when calcinosis is seen on x-ray. Tophaceous nodules almost never occur.
Xanthoma tuberosum is associated with hypercholesterolemia, particularly with elevated levels of low-density lipoprotein cholesterol. Lesions occur on pressure areas such as the knees or elbows and vary in size and shape from small papules to firm, lobulated tumors. They are yellow or orange, often with an erythematous halo. They are not associated with chronic proliferative arthritis.
CLINICAL PRESENTATION OF GOUT
Gout is a common metabolic disease characterized by an intermittent course of acute inflammatory arthritis initially affecting one or a few joints. Almost all patients have hyperuricemia, but serum urate levels can be normal or low during an acute attack. On the other hand, many hyperuricemic patients never have a clinical event.
If the hyperuricemia is untreated, some patients develop chronic polyarthritis and nephrolithiasis.1 Inadequate treatment of hyperuricemia may result in chronic tophaceous gout. Although tophaceous gout usually is a sign of long-standing hyperuricemia, tophi can in rare cases be a first symptom of the disorder.2
Even though our patient had been on allopurinol therapy, the dose was not high enough to achieve a serum urate level significantly below the saturation point of urate (about 6.7 mg/dL).
- Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis 1997; 56:696–697.
- Thissen CA, Frank J, Lucker GP. Tophi as first clinical sign of gout. Int J Dermatol 2008; 47( suppl 1):49–51.
Q: Which is the most likely diagnosis?
- Rheumatoid arthritis
- Nodular osteoarthritis
- Tophaceous gout
- Pseudogout
- Xanthoma tuberosum
A: Tophaceous gout is the diagnosis. This patient’s serum urate level was 9 mg/dL (normal range 4.0–8.0) despite allopurinol therapy, with normal levels of lipids, urea, and creatinine. Polarized light microscopy of aspirated synovial fluid showed monosodium urate crystals, thus confirming the diagnosis.
Rheumatoid arthritis is typically polyarticular and symmetrical and spares the distal interphalangeal joints. Subcutaneous rheumatoid nodules may mimic gouty tophi.
Pseudogout shares some of the features of gout. It results from deposits of calcium pyrophosphate crystals in and around the joints. The diagnosis is made by identifying the crystals on microscopy when calcinosis is seen on x-ray. Tophaceous nodules almost never occur.
Xanthoma tuberosum is associated with hypercholesterolemia, particularly with elevated levels of low-density lipoprotein cholesterol. Lesions occur on pressure areas such as the knees or elbows and vary in size and shape from small papules to firm, lobulated tumors. They are yellow or orange, often with an erythematous halo. They are not associated with chronic proliferative arthritis.
CLINICAL PRESENTATION OF GOUT
Gout is a common metabolic disease characterized by an intermittent course of acute inflammatory arthritis initially affecting one or a few joints. Almost all patients have hyperuricemia, but serum urate levels can be normal or low during an acute attack. On the other hand, many hyperuricemic patients never have a clinical event.
If the hyperuricemia is untreated, some patients develop chronic polyarthritis and nephrolithiasis.1 Inadequate treatment of hyperuricemia may result in chronic tophaceous gout. Although tophaceous gout usually is a sign of long-standing hyperuricemia, tophi can in rare cases be a first symptom of the disorder.2
Even though our patient had been on allopurinol therapy, the dose was not high enough to achieve a serum urate level significantly below the saturation point of urate (about 6.7 mg/dL).
Q: Which is the most likely diagnosis?
- Rheumatoid arthritis
- Nodular osteoarthritis
- Tophaceous gout
- Pseudogout
- Xanthoma tuberosum
A: Tophaceous gout is the diagnosis. This patient’s serum urate level was 9 mg/dL (normal range 4.0–8.0) despite allopurinol therapy, with normal levels of lipids, urea, and creatinine. Polarized light microscopy of aspirated synovial fluid showed monosodium urate crystals, thus confirming the diagnosis.
Rheumatoid arthritis is typically polyarticular and symmetrical and spares the distal interphalangeal joints. Subcutaneous rheumatoid nodules may mimic gouty tophi.
Pseudogout shares some of the features of gout. It results from deposits of calcium pyrophosphate crystals in and around the joints. The diagnosis is made by identifying the crystals on microscopy when calcinosis is seen on x-ray. Tophaceous nodules almost never occur.
Xanthoma tuberosum is associated with hypercholesterolemia, particularly with elevated levels of low-density lipoprotein cholesterol. Lesions occur on pressure areas such as the knees or elbows and vary in size and shape from small papules to firm, lobulated tumors. They are yellow or orange, often with an erythematous halo. They are not associated with chronic proliferative arthritis.
CLINICAL PRESENTATION OF GOUT
Gout is a common metabolic disease characterized by an intermittent course of acute inflammatory arthritis initially affecting one or a few joints. Almost all patients have hyperuricemia, but serum urate levels can be normal or low during an acute attack. On the other hand, many hyperuricemic patients never have a clinical event.
If the hyperuricemia is untreated, some patients develop chronic polyarthritis and nephrolithiasis.1 Inadequate treatment of hyperuricemia may result in chronic tophaceous gout. Although tophaceous gout usually is a sign of long-standing hyperuricemia, tophi can in rare cases be a first symptom of the disorder.2
Even though our patient had been on allopurinol therapy, the dose was not high enough to achieve a serum urate level significantly below the saturation point of urate (about 6.7 mg/dL).
- Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis 1997; 56:696–697.
- Thissen CA, Frank J, Lucker GP. Tophi as first clinical sign of gout. Int J Dermatol 2008; 47( suppl 1):49–51.
- Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis 1997; 56:696–697.
- Thissen CA, Frank J, Lucker GP. Tophi as first clinical sign of gout. Int J Dermatol 2008; 47( suppl 1):49–51.
Help patients with chronic kidney disease stave off dialysis
• Screen all patients for chronic kidney disease (CKD) by estimated glomerular filtration rate and persistent proteinuria. A
• Treat all CKD patients with angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors, unless there is a contraindication. A
• Recommend a heart-healthy diet and refer patients with CKD to a registered dietitian for more intensive dietary modifications. A
• Integrate motivational interviewing into your care of CKD patients. This health coaching technique has been shown to be causally and independently associated with positive behavioral outcomes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the last decade, the prevalence of chronic kidney disease (CKD) has grown approximately 20% to 25%, and current estimates are that the disease affects about 15% of the general population.1 All-cause hospitalization rates are almost 3 times higher among CKD patients than in those without the disease, and costs associated with CKD account for as much as 28% of the Medicare budget.1 Most disturbingly, the incidence at which patients diagnosed with CKD progress to end-stage renal disease (ESRD) continues to increase annually, reaching 354 cases per million population in 2007.1 By 2020, estimates are that more than 750,000 people in the United States will need dialysis for kidney failure.1
Guidelines exist, but awareness falls short
Several initiatives to increase awareness of CKD have been publicized. They include the Kidney Disease Outcome Quality Initiative (KDOQI) of the National Kidney Foundation (NKF), which issued clinical practice guidelines for treating chronic kidney disease in 2002, and Healthy People 2010, which includes specific measures to reduce the number of new cases and the complications, disability, economic costs, and mortality associated with the disease.2,3 Despite these efforts, studies show that many primary care providers are still unaware of these guidelines.4,5
Patients go undiagnosed until they reach the later stages of the disease, and many receive suboptimal care—even when they are identified—including lack of timely referral to a nephrologist and inadequate management of CKD comorbidities.6-13 (More on comorbidities, in a bit.)
Plus, there’s a lack of support … Care for these conditions is complex and difficult, and consultation or referral to a nephrologist may not be readily available, as the current pool of specialists is barely adequate to meet the needs of a growing population of CKD patients and the number of physicians-in-training entering the specialty is not adequate to meet the need.14 In this situation, primary care providers will have to assume an ever-enlarging share of the responsibility for care of CKD patients, including some clinical activities that are currently performed by specialists.
The first step: Screen all patients for CKD
Incorporating CKD screening into routine blood work for all patients facilitates earlier detection, evaluation, and treatment of the disease. Screening tests include the estimated glomerular filtration rate (GFR) based on serum creatinine as well as measurements of urine albumin and proteinuria. The persistence of proteinuria must be confirmed by 2 of 3 abnormal readings over a minimum of 3 months, because factors such as fever or exercise may affect test results. Measurement of albumin or total protein concentration in a spot sample avoids the need for timed collections. Factoring the concentration of total protein or albumin by urine creatinine concentration and using age/sex-specific thresholds eliminates most variations in measurement.15
Keep these comorbidities on your radar screen
Diabetes and hypertension are 2 of the most common causes of CKD in the United States, and the number of kidney failure cases due to these problems is increasing. The most important adverse outcomes of CKD are not only progression to ESRD, but also increased risk for cardiovascular disease (CVD). Studies show that the presence of albuminuria and a decreased estimated GFR consistently and incrementally increase the risk for CVD.16 Decreased GFR is an independent risk factor for CVD outcomes and for all-cause mortality, including sudden death in patients with existing coronary artery disease. Moreover, patients with CKD are 100 times more likely to die from CVD than to develop kidney failure.16
Depression is another prevalent, yet commonly overlooked, comorbid condition. Patients with any chronic disease are at risk for depression, with the incidence rising with the severity of the medical condition.17 CKD is no exception. Rates of depression as high as 29%, as well as rates of anxiety disorders as high as 46%, have been documented in patients on dialysis.18 Patients with depression are impaired in overall functioning and less able to follow medical regimens.17 In addition, low quality of life and depression predict higher morbidity and mortality rates in patients with ESRD.19 Because the diagnosis of depression is frequently missed in primary care settings, screening for depression should be a basic element in CKD management.20
Clinical management of kidney disease
The treatment goal for early-stage kidney disease is to address the risk factors that contribute to the progression of kidney disease: hypertension, heart disease, stroke, diabetes, and dyslipidemia. The TABLE reviews clinical management areas by disease stage.
Prescribe angiotensin antagonists. Angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors antagonize the toxic effects of increases in circulating angiotensin II and are therefore a key component of a therapeutic strategy to halt progressive kidney disease.2,21
Review medications, promote a healthier lifestyle. In addition to prescribing ARBs or ACE inhibitors, the family physician should review the CKD patient’s current medications to eliminate nephrotoxic drugs and adjust other medications on the basis of the patient’s creatinine clearance. Other measures include making sure vaccinations for influenza, pneumococcal pneumonia, and hepatitis B are up to date and emphasizing the importance of smoking cessation and exercise.
Treat comorbid conditions. Hypertension and diabetes must be treated aggressively. Patients with dyslipidemia should be managed with statins.2 Certain complications of progressive kidney disease, such as anemia, bone/mineral metabolic disease, and metabolic disorders, are typically treated by a nephrologist. Nevertheless, primary care providers need to understand these conditions in order to work together with the nephrologist in managing the CKD patient.
Check thyroid hormone and vitamin D levels. Understanding which factors predict disease progression or poor outcome is particularly useful. Most patients with CKD have low T3 syndrome, that is, low serum triiodothyronine levels in the absence of a thyroidal illness. In a recent paper, Song and colleagues showed that low T3 syndrome was common in early CKD and that estimated GFR was positively related with T3, independent of age and serum albumin.22
In another recent study, Ravani et al showed that plasma 25-hydroxyvitamin D is an independent, inverse predictor of disease progression and death in patients with stage 2 to 5 CKD.23 Vitamin D deficiency has been linked to CVD and early mortality in patients on hemodialysis.23 Checking for these 2 markers—low T3 syndrome and vitamin D deficiency—should therefore be part of your screening process for early stage CKD.
Refer to a dietitian. Dietary modification is another important component of the treatment plan. Dietary modifications are often needed to protect against CVD, help control blood pressure, reduce proteinuria, and improve metabolic control in patients with diabetes.2,24 Dietary modifications for CKD patients may go well beyond standard recommendations for a heart-healthy diet.2,24 Calcium, sodium, phosphorus, and potassium may need to be restricted according to laboratory values and stage of the disease.25 The KDOQI guidelines recommend referring CKD patients to a registered dietitian with experience in CKD for a complete nutritional assessment, comprehensive education on dietary restrictions and guidelines, and detailed dietary instruction.2
Manage CKD-associated anemia. Current guidelines do not propose normalizing hemoglobin in patients with renal disease, because lower levels of hemoglobin probably represent an adaptive response and correction to a “normal” level may disturb that response and lead to worse outcomes.26 For a discussion of management of anemia associated with CKD, see “Anemia and chronic kidney disease: What’s the connection?” in the January 2010 issue of this journal.27
Refer to a nephrologist early. A recent study by Chan et al demonstrates the beneficial effects of early referral to a nephrologist.28 There is no clear definition of early vs late referral and, at times, the only criterion is how much time elapsed before the patient was put on dialysis. Referral is considered “late” when management could have been improved by earlier contact with a specialist. It is probably prudent to refer stage 3 and 4 patients, at least for initial consultation. Chan’s meta-analysis found that patients referred late had nearly a 2-fold risk of death compared with those with early referrals. This risk persists at least up to 1 year after the initiation of renal replacement therapy.
Prepare patients for dialysis. It is very important that new hemodialysis patients present for initial treatment with an arteriovenous fistula in place, as first access for hemodialysis. Fistula placement is one of the most important reasons for timely referral to a nephrologist. Later referral is associated with a significantly prolonged hospital stay for initial renal replacement therapy. Late-referred patients are sicker, and many of the complications discussed here have not been optimally treated.
The optimal time to start preparing your patient for dialysis is when GFR measures between 15 and 29 mL/min/1.73 m2. Preparation includes counseling on nutrition and exercise, hepatitis B vaccination if needed, and scheduling for fistula placement.29
The hardest part: Changing habits
Effective CKD treatment must emphasize lifestyle management. You need to persuade smokers to quit and “couch potatoes” to start exercising regularly. Eating habits need to change, as well: This means fewer calories and restrictions on intake of salt and certain minerals. Medications for high blood pressure, diabetes, and kidney disease need to be taken consistently, as prescribed. The TABLE reviews the lifestyle issues that are particularly salient at each stage of CKD.
TABLE
Keying interventions to CKD stages
| Stage | Description | GFR (mL/min/1.73 m2) | Clinical action | Lifestyle management |
|---|---|---|---|---|
| At increased risk | ≥60 (with CKD risk factors) | Screening; CKD risk reduction | Healthy habits according to public health recommendations | |
| 1 | Kidney damage with normal or increased GFR | ≥90 | Diagnosis and treatment; treatment of comorbid conditions; slowing of progression; CVD risk reduction | Emphasis on heart health: physical activity, healthy diet, weight management, and stress management. Restricted sodium, potassium, calcium, phosphorus, and protein, with emphasis on plant vs animal food sources. Treatment adherence to medications and CV/diabetes/hypertension treatment plan if applicable. Assessment of depression and referral to treatment if appropriate. |
| 2 | Kidney damage with mildly decreased GFR | 60-89 | Same, plus estimation of progression | Same recommendations as stage 1 |
| 3 | Moderately decreased GFR | 30-59 | Same, plus evaluation and treatment of complications | Same recommendations as stage 1 |
| 4 | Severely decreased GFR | 15-29 | Preparation for kidney replacement therapy | Same as above, plus assessment of social support to prepare for dialysis treatment if appropriate |
| 5 | Kidney failure | <15 or dialysis | Replacement (if uremia present) | Same as above, plus restricted fluid intake and additional protein intake |
| Note: Shaded area identifies patients who have CKD; unshaded area designates individuals who are at increased risk for developing CKD. CKD is defined as either kidney damage or GFR <60 ml/min/1.73 m2 for ≥3 months. Kidney damage is defined as pathologic abnormalities or markers of damage, including abnormalities in blood or urine tests or imaging studies. | ||||
| CKD, chronic kidney disease; CVD, cardiovascular disease; GFR, glomerular filtration rate. | ||||
| Adapted from: Table 3: chronic kidney disease: a clinical action plan. National Kidney foundation. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. 2002. | ||||
Lifestyle modifications like these are very difficult, and helping patients make them involves much more than simple patient education. In 1 study, Durose et al found that patients on hemodialysis failed to stay on their diets even when they knew which dietary restrictions they should follow and what the consequences of going off their diets would be.30
Update your persuasive techniques: take on the role of coach
Newer theories of behavior change no longer rely on simply providing information and advice, but rather address the complex interaction of motivations involved in attempts to change. These include cues to action, perception of benefits and consequences, environmental and cultural influences, sense of self-efficacy, ambivalence, and the intention to change.31
Unfortunately, health care providers are rarely trained in motivational techniques. Often, their approach to inducing change is authoritarian, confrontational, overly forceful, or guilt inducing. Such attitudes not only limit progress, but are actually correlated with negative behavioral and clinical outcomes.32,33 Recent research has verified the power of the patient–provider interaction in influencing treatment adherence and lifestyle change.33
To be successful in getting patients to adopt new behaviors, physicians need to move away from authoritarian modes and take on some of the attributes of a coach urging on the team.
How this coaching technique works
Motivational Interviewing is a health coaching technique that has been shown to be causally and independently associated with positive behavioral outcomes.34 The techniques used in the motivational interviewing approach are summarized in “The motivational interviewing tool kit”. Motivational interviewing is a goal-oriented, patient-centered counseling style for helping patients explore and resolve their ambivalence about behavior change.35 The approach has been used in diverse populations, settings, and medical conditions. Its efficacy was first demonstrated in the treatment of addictions to illegal drugs and alcohol.36 Continued research and 2 recent meta-analyses using rigorous methodology have validated the usefulness of this approach.37,38
Express empathy
Objective: To establish rapport and avoid resistance by demonstrating your understanding of the patient’s situation.
Example: “It’s not easy making all these changes.”
Follow-up: “But you also say you know these numbers put you at risk for more serious disease.”
Roll with resistance
Objective: To avoid magnifying resistance by allowing patients to explore their barriers in a nonjudgmental, supportive manner.
Example: “You really don’t want to take the medication anymore. It’s hard to remember and you don’t feel sick, so you don’t see why you need it.”
Follow-up: “I’m wondering where you see yourself in 6 months if you stop taking the medication?”
Elicit/provide reminder/elicit
Objective: To find out what the patient already knows, fill in the gaps or correct misconceptions, and explore how the change you suggest will fit into the patient’s life. This is a time-saving strategy that both validates patient knowledge and allows time to address barriers.
Example:
Elicit: “Mrs. Roberts, can you tell me what you know about managing your chronic kidney disease?”
Provide reminder: “That’s great. You’ve pretty much got it nailed. I’d just like to remind you about taking your statin medications and keeping close track of your blood pressure.”
Elicit: “What do you think the biggest barrier is for you right now in managing this condition?”
Support autonomy
Objective: To reduce resistance by assuring patients you know you can’t make them do anything—it’s their choice.
Example: “Of course, it’s your choice, but as your doctor, I’d be concerned if you decided not to try this medication.”
Follow-up: “Nobody can make you do anything that you don’t want to do. You need to consider all your options and make the choice that’s right for you at this time. If you do decide to try this medication, I assure you that we will monitor any side effects closely and adjust the dosage to minimize any problems.”
Explore ambivalence
Objective: To help the patient consider the pros and cons of change in a relaxed yet systematic manner.
Example: “So let’s talk about the pros and cons of trying to quit smoking at this time.”
Follow-up: “Let me see if I can summarize where you are. On the one hand, it’s pretty stressful for you right now and smoking helps you cope. You’ve tried to quit before and you couldn’t keep it up for very long. On the other hand, you really do understand the damage it’s doing to your body and how it is making it more difficult for us to treat your heart disease. Your wife is willing to quit with you and you’ve heard about this new quit medication that can help curb cravings. Did I get it all? What are you thinking you are going to do?”
Elicit change talk
Objective: To evoke the patient’s reasons, desire, ability, and need for change. This “change talk” predicts increased commitment to the lifestyle change, which, in turn, is correlated to a good clinical outcome.
Examples:
“What makes it important to you to start an exercise program?”
“What benefits would come from losing weight?”
“Why do you want to quit smoking?”
Follow-up: “You know that exercise will help you manage your stress, lose some weight, and lower your cholesterol levels. Plus, when you did it before, you had more energy and slept better. You also want to be a good role model for the kids and be able to play sports with them.”
Develop an action plan
Objective: To help the patient develop a plan that is realistic and fits into his or her life. When a patient “owns” the plan, he or she is more likely to follow through.
Examples:
“So what’s the next step for you?”
“What do you think you could do (and would be willing to do) for your health right now that would make the most difference?”
“What do you think your best option is?”
Follow-up: “You’ve outlined a great plan. You’re going to try to eat more vegetables and less meat, plus cut back on portion sizes. You’re also going to try and walk more. Lastly, you’re willing to try the pill box to see if it makes it easier to take your medications correctly.” (Pause). “So, are you going to do this?”
Motivational interviewing has been shown to be effective in improving general health status and sense of well-being, promoting physical activity, improving nutritional habits, encouraging medication adherence, and managing chronic conditions such as hypertension, hypercholesterolemia, obesity, and diabetes.35 A review of the literature on health behavior change demonstrates that motivational interviewing outperforms traditional advice-giving in the treatment of a broad range of behavioral problems and diseases.38
Motivational interviewing is focused on helping patients explore their ambivalence and identify individual barriers that are preventing change. The skill set that motivational interviewing provides can be modified for use in the brief patient encounters typically found in the primary care setting. For an example of how you might use motivational interviewing techniques with your CKD patients, see “Talking about change: A motivational interviewing conversation”.
Physician: Now that we’ve gone over your lab values and you don’t have any more questions, I’d like to take a few minutes to talk about how you’re doing with your treatment plan. Would that be okay with you?
Patient: Sure, doc.
Physician: You’re dealing with a lot of things all together—trying to change your diet, watching your weight, monitoring your blood sugar, and taking your medications.
Patient: It is a lot. Guess it’s obvious from my labs that I’m not doing so well. I feel like I get a handle on one thing but something else blows up.
Physician: Sounds like it feels a bit overwhelming right now.
Patient: Yeah, it really is…but I think I could do better.
Physician: Why don’t we start with reviewing what you’re doing well? you are getting your prescriptions filled, and it seems like you’re taking your medications regularly.
Patient: I really do, nearly all of the time.
Physician: What else are you doing well?
Patient: I’ve cut down on my salt intake. We’re using that salt substitute and it’s okay. Ummm…but I guess by the labs I’m not watching my potassium and phosphorus like I should.
Physician: What else are you doing well?
Patient: Well, my blood pressure is down from what it was. But my sugars are still out of whack and I can’t seem to lose weight.
Physician: Okay, so you’ve done a great job taking your medications and you’ve started to change your diet with the salt—both of which have really helped your blood pressure. As you say, there are some things we still need to tackle. But let’s break it down into small steps—forget the whole list. Can you think of just 1 or 2 more small things that you think you could do that would make a difference right now?
Patient: Well, my wife walks every evening after dinner. She’s been nagging me to walk with her. I guess I wouldn’t mind that so much as long as she doesn’t drag me too far. That would help me drop a few pounds and that might motivate me to be more careful with my diet. Plus, I know that exercise is also supposed to help my blood sugar.
Physician: So, a walk after dinner. Do you think you can do this?
Patient: Yes, I do.
Physician: When would you be willing to start?
Patient: Heck, I could start tomorrow. That’s something that wouldn’t be that big of a deal.
Physician: Great! Seems like a plan then. I’m confident that by taking these small steps like a walk every evening, you can get this under control. You have already improved in some important areas.
Patient: Thanks doc! I’ll see you next visit and hopefully my numbers will be better.
Your crucial role
CKD is well on its way to becoming a full-blown epidemic in the United States. Primary care providers carry the brunt of responsibility for the care of these patients, and with an increasing shortage of nephrologists, the scope of those activities will likely grow. Physicians in solo or small group practice must be prepared to deliver both the clinical and behavioral/lifestyle components of care themselves. While this is a challenging endeavor, we believe the framework outlined here will improve your ability to meet the complex needs of CKD patients.
CORRESPONDENCE Ariel Linden, DrPH, MS, Linden Consulting Group, 6208 NE Chestnut Street, Hillsboro, OR 97124; [email protected]
1. US Renal Data Systems. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
2. National Kidney Foundation. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. 2002. Available at: http://www.kidney.org/professionals/kdoqi/guidelines_ckd/toc.htm. Accessed January 12, 2009.
3. U.S. Department of Health and Human Services. Healthy People 2010. (“Understanding and Improving Health” and “Objectives for Improving Health,” 2 volumes.) 2nd ed. Washington, DC: U.S. Government Printing Office, November 2000.
4. Fox CH, Brooks A, Zayas LE, et al. Primary care physicians’ knowledge and practice patterns in the treatment of chronic kidney disease: an Upstate New York Practice-based Research Network (UNYNET) study. J Am Board Fam Med. 2006;19:54-61.
5. Lea JP, McClellan WM, Melcher C, et al. CKD risk factors reported by primary care physicians: do guidelines make a difference? Am J Kidney Dis. 2006;47:72-77.
6. Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489-495.
7. Nissenson AR, Collins AJ, Hurley J, et al. Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol. 2001;12:1713-1720.
8. McClellan WM, Knight DF, Karp H, et al. Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: important differences between practice and published guidelines. Am J Kidney Dis. 1997;29:368-375.
9. Obrador GT, Ruthazer R, Arora P, et al. Prevalence of and factors associated with suboptimal care before initiation of dialysis in the United States. J Am Soc Nephrol. 1999;10:1793-1800.
10. Boulware LE, Troll MU, Jaar BG, et al. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis. 2006;48:192-204.
11. John R, Webb M, Young A, et al. Unreferred chronic kidney disease: a longitudinal study. Am J Kidney Dis. 2004;43:825-835.
12. Wauters JP, Lameire N, Davison A, et al. Why patients with progressing kidney disease are referred late to the nephrologists: on causes and proposals for improvement. Nephrol Dial Transplant. 2005;20:490-496.
13. Ouseph R, Hendricks P, Hollon JA, et al. Under-recognition of chronic kidney disease in elderly outpatients. Clin Nephrol. 2007;68:373-378.
14. Himmelfarb J, Berns A, Szczech L, et al. Cost, quality, and value: the changing political economy of dialysis care. J Am Soc Nephrol. 2007;18:2021-2027.
15. Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50:169-180.
16. Saran AM, DuBose TD, Jr. Cardiovascular disease in chronic kidney disease. Ther Adv Cardiovasc Dis. 2008;2:425-434.
17. Mental Health America. Fact sheet: co-occurring disorders and depression. Available at: http://www.nmha.org/index.cfm?objectid=C7DF94C1-1372-4D20-C8FE4E509C20471B. Accessed January 25, 2009.
18. Cukor D, Coplan J, Brown C, et al. Course of depression and anxiety diagnosis in patients treated with hemodialysis: a 16-month follow-up. Am Soc Nephrol. 2008;3:1752-1758.
19. Lopez Revuelta K, Garcia Lopez FJ, de Alvaro Moreno F, et al. Perceived mental health at the start of dialysis as a predictor of morbidity and mortality in patients with end stage renal disease (CALVIDIA Study). Nephrol Dial Transplant. 2004;19:2347-2353.
20. Ford DE. A primary care approach: Managing depression in the face of chronic medical conditions. Am J Med. 2008;121(suppl 2):S38-S44.
21. Ferrari P. Prescribing angiotensin converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Nephrol. 2007;12:81-89.
22. Song SH, Kwak IS, Lee DW, et al. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant. 2009;24:1534-1538.
23. Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88-95.
24. Cupisti A, Aparicio M, Barsotti G. Potential benefits of renal diets on cardiovascular risk factors in chronic kidney disease patients. Ren Fail. 2007;29:529-534.
25. Beto JA, Bansal VK. Medical nutrition therapy in chronic kidney failure: Integrating clinical practice guidelines. J Am Diet Assoc. 2004;104:404-409.
26. Al-Aly Z. The new role of calcimimetics as vasculotropic agents. Kidney Int. 2009;75:9-12.
27. Taliercio JJ. Anemia and chronic kidney disease: what’s the connection? J Fam Pract. 2010;59:14-18.
28. Chan MR, Dall AT, Fletcher KE, et al. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med. 2007;120:1063-1070.
29. Almaguer M, Herrera R, Alfonso J, et al. Primary health care strategies for the prevention of end-stage renal disease in Cuba. Kidney Int. 2005;68(suppl 97s):S4-S10.
30. Durose CL, Holdsworth M, Watson V, et al. Knowledge of dietary restrictions and the medical consequences of noncompliance by patients on hemodialysis are not predictive of dietary compliance. Am Diet Assoc. 2004;104:35-41.
31. Linden A, Butterworth SW, Roberts N. Disease management interventions II: what else is in the black box? Dis Manage. 2006;9:73-85.
32. Moyers TB, Martin T. Therapist influence on client language during motivational interviewing sessions. J Subst Abuse Treat. 2006;30:245-251.
33. Moyers TB, Martin T, Christopher PJ, et al. Client language as a mediator of motivational interviewing efficacy: where is the evidence? Alcohol Clin Exp Res. 2007;31(10 suppl):40s-47s.
34. Butterworth S, Linden A, McClay W. Health coaching as an intervention in health management programs. Dis Manage Health Outcomes. 2007;15:299-307.
35. Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008.
36. Miller WR. Motivational interviewing with problem drinkers. Behav Psychother. 1983;11:147-172.
37. Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psych. 2005;1:91-111.
38. Rubak S, Sandbaek A, Lauritzen T, et al. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55:305-312.
• Screen all patients for chronic kidney disease (CKD) by estimated glomerular filtration rate and persistent proteinuria. A
• Treat all CKD patients with angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors, unless there is a contraindication. A
• Recommend a heart-healthy diet and refer patients with CKD to a registered dietitian for more intensive dietary modifications. A
• Integrate motivational interviewing into your care of CKD patients. This health coaching technique has been shown to be causally and independently associated with positive behavioral outcomes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the last decade, the prevalence of chronic kidney disease (CKD) has grown approximately 20% to 25%, and current estimates are that the disease affects about 15% of the general population.1 All-cause hospitalization rates are almost 3 times higher among CKD patients than in those without the disease, and costs associated with CKD account for as much as 28% of the Medicare budget.1 Most disturbingly, the incidence at which patients diagnosed with CKD progress to end-stage renal disease (ESRD) continues to increase annually, reaching 354 cases per million population in 2007.1 By 2020, estimates are that more than 750,000 people in the United States will need dialysis for kidney failure.1
Guidelines exist, but awareness falls short
Several initiatives to increase awareness of CKD have been publicized. They include the Kidney Disease Outcome Quality Initiative (KDOQI) of the National Kidney Foundation (NKF), which issued clinical practice guidelines for treating chronic kidney disease in 2002, and Healthy People 2010, which includes specific measures to reduce the number of new cases and the complications, disability, economic costs, and mortality associated with the disease.2,3 Despite these efforts, studies show that many primary care providers are still unaware of these guidelines.4,5
Patients go undiagnosed until they reach the later stages of the disease, and many receive suboptimal care—even when they are identified—including lack of timely referral to a nephrologist and inadequate management of CKD comorbidities.6-13 (More on comorbidities, in a bit.)
Plus, there’s a lack of support … Care for these conditions is complex and difficult, and consultation or referral to a nephrologist may not be readily available, as the current pool of specialists is barely adequate to meet the needs of a growing population of CKD patients and the number of physicians-in-training entering the specialty is not adequate to meet the need.14 In this situation, primary care providers will have to assume an ever-enlarging share of the responsibility for care of CKD patients, including some clinical activities that are currently performed by specialists.
The first step: Screen all patients for CKD
Incorporating CKD screening into routine blood work for all patients facilitates earlier detection, evaluation, and treatment of the disease. Screening tests include the estimated glomerular filtration rate (GFR) based on serum creatinine as well as measurements of urine albumin and proteinuria. The persistence of proteinuria must be confirmed by 2 of 3 abnormal readings over a minimum of 3 months, because factors such as fever or exercise may affect test results. Measurement of albumin or total protein concentration in a spot sample avoids the need for timed collections. Factoring the concentration of total protein or albumin by urine creatinine concentration and using age/sex-specific thresholds eliminates most variations in measurement.15
Keep these comorbidities on your radar screen
Diabetes and hypertension are 2 of the most common causes of CKD in the United States, and the number of kidney failure cases due to these problems is increasing. The most important adverse outcomes of CKD are not only progression to ESRD, but also increased risk for cardiovascular disease (CVD). Studies show that the presence of albuminuria and a decreased estimated GFR consistently and incrementally increase the risk for CVD.16 Decreased GFR is an independent risk factor for CVD outcomes and for all-cause mortality, including sudden death in patients with existing coronary artery disease. Moreover, patients with CKD are 100 times more likely to die from CVD than to develop kidney failure.16
Depression is another prevalent, yet commonly overlooked, comorbid condition. Patients with any chronic disease are at risk for depression, with the incidence rising with the severity of the medical condition.17 CKD is no exception. Rates of depression as high as 29%, as well as rates of anxiety disorders as high as 46%, have been documented in patients on dialysis.18 Patients with depression are impaired in overall functioning and less able to follow medical regimens.17 In addition, low quality of life and depression predict higher morbidity and mortality rates in patients with ESRD.19 Because the diagnosis of depression is frequently missed in primary care settings, screening for depression should be a basic element in CKD management.20
Clinical management of kidney disease
The treatment goal for early-stage kidney disease is to address the risk factors that contribute to the progression of kidney disease: hypertension, heart disease, stroke, diabetes, and dyslipidemia. The TABLE reviews clinical management areas by disease stage.
Prescribe angiotensin antagonists. Angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors antagonize the toxic effects of increases in circulating angiotensin II and are therefore a key component of a therapeutic strategy to halt progressive kidney disease.2,21
Review medications, promote a healthier lifestyle. In addition to prescribing ARBs or ACE inhibitors, the family physician should review the CKD patient’s current medications to eliminate nephrotoxic drugs and adjust other medications on the basis of the patient’s creatinine clearance. Other measures include making sure vaccinations for influenza, pneumococcal pneumonia, and hepatitis B are up to date and emphasizing the importance of smoking cessation and exercise.
Treat comorbid conditions. Hypertension and diabetes must be treated aggressively. Patients with dyslipidemia should be managed with statins.2 Certain complications of progressive kidney disease, such as anemia, bone/mineral metabolic disease, and metabolic disorders, are typically treated by a nephrologist. Nevertheless, primary care providers need to understand these conditions in order to work together with the nephrologist in managing the CKD patient.
Check thyroid hormone and vitamin D levels. Understanding which factors predict disease progression or poor outcome is particularly useful. Most patients with CKD have low T3 syndrome, that is, low serum triiodothyronine levels in the absence of a thyroidal illness. In a recent paper, Song and colleagues showed that low T3 syndrome was common in early CKD and that estimated GFR was positively related with T3, independent of age and serum albumin.22
In another recent study, Ravani et al showed that plasma 25-hydroxyvitamin D is an independent, inverse predictor of disease progression and death in patients with stage 2 to 5 CKD.23 Vitamin D deficiency has been linked to CVD and early mortality in patients on hemodialysis.23 Checking for these 2 markers—low T3 syndrome and vitamin D deficiency—should therefore be part of your screening process for early stage CKD.
Refer to a dietitian. Dietary modification is another important component of the treatment plan. Dietary modifications are often needed to protect against CVD, help control blood pressure, reduce proteinuria, and improve metabolic control in patients with diabetes.2,24 Dietary modifications for CKD patients may go well beyond standard recommendations for a heart-healthy diet.2,24 Calcium, sodium, phosphorus, and potassium may need to be restricted according to laboratory values and stage of the disease.25 The KDOQI guidelines recommend referring CKD patients to a registered dietitian with experience in CKD for a complete nutritional assessment, comprehensive education on dietary restrictions and guidelines, and detailed dietary instruction.2
Manage CKD-associated anemia. Current guidelines do not propose normalizing hemoglobin in patients with renal disease, because lower levels of hemoglobin probably represent an adaptive response and correction to a “normal” level may disturb that response and lead to worse outcomes.26 For a discussion of management of anemia associated with CKD, see “Anemia and chronic kidney disease: What’s the connection?” in the January 2010 issue of this journal.27
Refer to a nephrologist early. A recent study by Chan et al demonstrates the beneficial effects of early referral to a nephrologist.28 There is no clear definition of early vs late referral and, at times, the only criterion is how much time elapsed before the patient was put on dialysis. Referral is considered “late” when management could have been improved by earlier contact with a specialist. It is probably prudent to refer stage 3 and 4 patients, at least for initial consultation. Chan’s meta-analysis found that patients referred late had nearly a 2-fold risk of death compared with those with early referrals. This risk persists at least up to 1 year after the initiation of renal replacement therapy.
Prepare patients for dialysis. It is very important that new hemodialysis patients present for initial treatment with an arteriovenous fistula in place, as first access for hemodialysis. Fistula placement is one of the most important reasons for timely referral to a nephrologist. Later referral is associated with a significantly prolonged hospital stay for initial renal replacement therapy. Late-referred patients are sicker, and many of the complications discussed here have not been optimally treated.
The optimal time to start preparing your patient for dialysis is when GFR measures between 15 and 29 mL/min/1.73 m2. Preparation includes counseling on nutrition and exercise, hepatitis B vaccination if needed, and scheduling for fistula placement.29
The hardest part: Changing habits
Effective CKD treatment must emphasize lifestyle management. You need to persuade smokers to quit and “couch potatoes” to start exercising regularly. Eating habits need to change, as well: This means fewer calories and restrictions on intake of salt and certain minerals. Medications for high blood pressure, diabetes, and kidney disease need to be taken consistently, as prescribed. The TABLE reviews the lifestyle issues that are particularly salient at each stage of CKD.
TABLE
Keying interventions to CKD stages
| Stage | Description | GFR (mL/min/1.73 m2) | Clinical action | Lifestyle management |
|---|---|---|---|---|
| At increased risk | ≥60 (with CKD risk factors) | Screening; CKD risk reduction | Healthy habits according to public health recommendations | |
| 1 | Kidney damage with normal or increased GFR | ≥90 | Diagnosis and treatment; treatment of comorbid conditions; slowing of progression; CVD risk reduction | Emphasis on heart health: physical activity, healthy diet, weight management, and stress management. Restricted sodium, potassium, calcium, phosphorus, and protein, with emphasis on plant vs animal food sources. Treatment adherence to medications and CV/diabetes/hypertension treatment plan if applicable. Assessment of depression and referral to treatment if appropriate. |
| 2 | Kidney damage with mildly decreased GFR | 60-89 | Same, plus estimation of progression | Same recommendations as stage 1 |
| 3 | Moderately decreased GFR | 30-59 | Same, plus evaluation and treatment of complications | Same recommendations as stage 1 |
| 4 | Severely decreased GFR | 15-29 | Preparation for kidney replacement therapy | Same as above, plus assessment of social support to prepare for dialysis treatment if appropriate |
| 5 | Kidney failure | <15 or dialysis | Replacement (if uremia present) | Same as above, plus restricted fluid intake and additional protein intake |
| Note: Shaded area identifies patients who have CKD; unshaded area designates individuals who are at increased risk for developing CKD. CKD is defined as either kidney damage or GFR <60 ml/min/1.73 m2 for ≥3 months. Kidney damage is defined as pathologic abnormalities or markers of damage, including abnormalities in blood or urine tests or imaging studies. | ||||
| CKD, chronic kidney disease; CVD, cardiovascular disease; GFR, glomerular filtration rate. | ||||
| Adapted from: Table 3: chronic kidney disease: a clinical action plan. National Kidney foundation. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. 2002. | ||||
Lifestyle modifications like these are very difficult, and helping patients make them involves much more than simple patient education. In 1 study, Durose et al found that patients on hemodialysis failed to stay on their diets even when they knew which dietary restrictions they should follow and what the consequences of going off their diets would be.30
Update your persuasive techniques: take on the role of coach
Newer theories of behavior change no longer rely on simply providing information and advice, but rather address the complex interaction of motivations involved in attempts to change. These include cues to action, perception of benefits and consequences, environmental and cultural influences, sense of self-efficacy, ambivalence, and the intention to change.31
Unfortunately, health care providers are rarely trained in motivational techniques. Often, their approach to inducing change is authoritarian, confrontational, overly forceful, or guilt inducing. Such attitudes not only limit progress, but are actually correlated with negative behavioral and clinical outcomes.32,33 Recent research has verified the power of the patient–provider interaction in influencing treatment adherence and lifestyle change.33
To be successful in getting patients to adopt new behaviors, physicians need to move away from authoritarian modes and take on some of the attributes of a coach urging on the team.
How this coaching technique works
Motivational Interviewing is a health coaching technique that has been shown to be causally and independently associated with positive behavioral outcomes.34 The techniques used in the motivational interviewing approach are summarized in “The motivational interviewing tool kit”. Motivational interviewing is a goal-oriented, patient-centered counseling style for helping patients explore and resolve their ambivalence about behavior change.35 The approach has been used in diverse populations, settings, and medical conditions. Its efficacy was first demonstrated in the treatment of addictions to illegal drugs and alcohol.36 Continued research and 2 recent meta-analyses using rigorous methodology have validated the usefulness of this approach.37,38
Express empathy
Objective: To establish rapport and avoid resistance by demonstrating your understanding of the patient’s situation.
Example: “It’s not easy making all these changes.”
Follow-up: “But you also say you know these numbers put you at risk for more serious disease.”
Roll with resistance
Objective: To avoid magnifying resistance by allowing patients to explore their barriers in a nonjudgmental, supportive manner.
Example: “You really don’t want to take the medication anymore. It’s hard to remember and you don’t feel sick, so you don’t see why you need it.”
Follow-up: “I’m wondering where you see yourself in 6 months if you stop taking the medication?”
Elicit/provide reminder/elicit
Objective: To find out what the patient already knows, fill in the gaps or correct misconceptions, and explore how the change you suggest will fit into the patient’s life. This is a time-saving strategy that both validates patient knowledge and allows time to address barriers.
Example:
Elicit: “Mrs. Roberts, can you tell me what you know about managing your chronic kidney disease?”
Provide reminder: “That’s great. You’ve pretty much got it nailed. I’d just like to remind you about taking your statin medications and keeping close track of your blood pressure.”
Elicit: “What do you think the biggest barrier is for you right now in managing this condition?”
Support autonomy
Objective: To reduce resistance by assuring patients you know you can’t make them do anything—it’s their choice.
Example: “Of course, it’s your choice, but as your doctor, I’d be concerned if you decided not to try this medication.”
Follow-up: “Nobody can make you do anything that you don’t want to do. You need to consider all your options and make the choice that’s right for you at this time. If you do decide to try this medication, I assure you that we will monitor any side effects closely and adjust the dosage to minimize any problems.”
Explore ambivalence
Objective: To help the patient consider the pros and cons of change in a relaxed yet systematic manner.
Example: “So let’s talk about the pros and cons of trying to quit smoking at this time.”
Follow-up: “Let me see if I can summarize where you are. On the one hand, it’s pretty stressful for you right now and smoking helps you cope. You’ve tried to quit before and you couldn’t keep it up for very long. On the other hand, you really do understand the damage it’s doing to your body and how it is making it more difficult for us to treat your heart disease. Your wife is willing to quit with you and you’ve heard about this new quit medication that can help curb cravings. Did I get it all? What are you thinking you are going to do?”
Elicit change talk
Objective: To evoke the patient’s reasons, desire, ability, and need for change. This “change talk” predicts increased commitment to the lifestyle change, which, in turn, is correlated to a good clinical outcome.
Examples:
“What makes it important to you to start an exercise program?”
“What benefits would come from losing weight?”
“Why do you want to quit smoking?”
Follow-up: “You know that exercise will help you manage your stress, lose some weight, and lower your cholesterol levels. Plus, when you did it before, you had more energy and slept better. You also want to be a good role model for the kids and be able to play sports with them.”
Develop an action plan
Objective: To help the patient develop a plan that is realistic and fits into his or her life. When a patient “owns” the plan, he or she is more likely to follow through.
Examples:
“So what’s the next step for you?”
“What do you think you could do (and would be willing to do) for your health right now that would make the most difference?”
“What do you think your best option is?”
Follow-up: “You’ve outlined a great plan. You’re going to try to eat more vegetables and less meat, plus cut back on portion sizes. You’re also going to try and walk more. Lastly, you’re willing to try the pill box to see if it makes it easier to take your medications correctly.” (Pause). “So, are you going to do this?”
Motivational interviewing has been shown to be effective in improving general health status and sense of well-being, promoting physical activity, improving nutritional habits, encouraging medication adherence, and managing chronic conditions such as hypertension, hypercholesterolemia, obesity, and diabetes.35 A review of the literature on health behavior change demonstrates that motivational interviewing outperforms traditional advice-giving in the treatment of a broad range of behavioral problems and diseases.38
Motivational interviewing is focused on helping patients explore their ambivalence and identify individual barriers that are preventing change. The skill set that motivational interviewing provides can be modified for use in the brief patient encounters typically found in the primary care setting. For an example of how you might use motivational interviewing techniques with your CKD patients, see “Talking about change: A motivational interviewing conversation”.
Physician: Now that we’ve gone over your lab values and you don’t have any more questions, I’d like to take a few minutes to talk about how you’re doing with your treatment plan. Would that be okay with you?
Patient: Sure, doc.
Physician: You’re dealing with a lot of things all together—trying to change your diet, watching your weight, monitoring your blood sugar, and taking your medications.
Patient: It is a lot. Guess it’s obvious from my labs that I’m not doing so well. I feel like I get a handle on one thing but something else blows up.
Physician: Sounds like it feels a bit overwhelming right now.
Patient: Yeah, it really is…but I think I could do better.
Physician: Why don’t we start with reviewing what you’re doing well? you are getting your prescriptions filled, and it seems like you’re taking your medications regularly.
Patient: I really do, nearly all of the time.
Physician: What else are you doing well?
Patient: I’ve cut down on my salt intake. We’re using that salt substitute and it’s okay. Ummm…but I guess by the labs I’m not watching my potassium and phosphorus like I should.
Physician: What else are you doing well?
Patient: Well, my blood pressure is down from what it was. But my sugars are still out of whack and I can’t seem to lose weight.
Physician: Okay, so you’ve done a great job taking your medications and you’ve started to change your diet with the salt—both of which have really helped your blood pressure. As you say, there are some things we still need to tackle. But let’s break it down into small steps—forget the whole list. Can you think of just 1 or 2 more small things that you think you could do that would make a difference right now?
Patient: Well, my wife walks every evening after dinner. She’s been nagging me to walk with her. I guess I wouldn’t mind that so much as long as she doesn’t drag me too far. That would help me drop a few pounds and that might motivate me to be more careful with my diet. Plus, I know that exercise is also supposed to help my blood sugar.
Physician: So, a walk after dinner. Do you think you can do this?
Patient: Yes, I do.
Physician: When would you be willing to start?
Patient: Heck, I could start tomorrow. That’s something that wouldn’t be that big of a deal.
Physician: Great! Seems like a plan then. I’m confident that by taking these small steps like a walk every evening, you can get this under control. You have already improved in some important areas.
Patient: Thanks doc! I’ll see you next visit and hopefully my numbers will be better.
Your crucial role
CKD is well on its way to becoming a full-blown epidemic in the United States. Primary care providers carry the brunt of responsibility for the care of these patients, and with an increasing shortage of nephrologists, the scope of those activities will likely grow. Physicians in solo or small group practice must be prepared to deliver both the clinical and behavioral/lifestyle components of care themselves. While this is a challenging endeavor, we believe the framework outlined here will improve your ability to meet the complex needs of CKD patients.
CORRESPONDENCE Ariel Linden, DrPH, MS, Linden Consulting Group, 6208 NE Chestnut Street, Hillsboro, OR 97124; [email protected]
• Screen all patients for chronic kidney disease (CKD) by estimated glomerular filtration rate and persistent proteinuria. A
• Treat all CKD patients with angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors, unless there is a contraindication. A
• Recommend a heart-healthy diet and refer patients with CKD to a registered dietitian for more intensive dietary modifications. A
• Integrate motivational interviewing into your care of CKD patients. This health coaching technique has been shown to be causally and independently associated with positive behavioral outcomes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the last decade, the prevalence of chronic kidney disease (CKD) has grown approximately 20% to 25%, and current estimates are that the disease affects about 15% of the general population.1 All-cause hospitalization rates are almost 3 times higher among CKD patients than in those without the disease, and costs associated with CKD account for as much as 28% of the Medicare budget.1 Most disturbingly, the incidence at which patients diagnosed with CKD progress to end-stage renal disease (ESRD) continues to increase annually, reaching 354 cases per million population in 2007.1 By 2020, estimates are that more than 750,000 people in the United States will need dialysis for kidney failure.1
Guidelines exist, but awareness falls short
Several initiatives to increase awareness of CKD have been publicized. They include the Kidney Disease Outcome Quality Initiative (KDOQI) of the National Kidney Foundation (NKF), which issued clinical practice guidelines for treating chronic kidney disease in 2002, and Healthy People 2010, which includes specific measures to reduce the number of new cases and the complications, disability, economic costs, and mortality associated with the disease.2,3 Despite these efforts, studies show that many primary care providers are still unaware of these guidelines.4,5
Patients go undiagnosed until they reach the later stages of the disease, and many receive suboptimal care—even when they are identified—including lack of timely referral to a nephrologist and inadequate management of CKD comorbidities.6-13 (More on comorbidities, in a bit.)
Plus, there’s a lack of support … Care for these conditions is complex and difficult, and consultation or referral to a nephrologist may not be readily available, as the current pool of specialists is barely adequate to meet the needs of a growing population of CKD patients and the number of physicians-in-training entering the specialty is not adequate to meet the need.14 In this situation, primary care providers will have to assume an ever-enlarging share of the responsibility for care of CKD patients, including some clinical activities that are currently performed by specialists.
The first step: Screen all patients for CKD
Incorporating CKD screening into routine blood work for all patients facilitates earlier detection, evaluation, and treatment of the disease. Screening tests include the estimated glomerular filtration rate (GFR) based on serum creatinine as well as measurements of urine albumin and proteinuria. The persistence of proteinuria must be confirmed by 2 of 3 abnormal readings over a minimum of 3 months, because factors such as fever or exercise may affect test results. Measurement of albumin or total protein concentration in a spot sample avoids the need for timed collections. Factoring the concentration of total protein or albumin by urine creatinine concentration and using age/sex-specific thresholds eliminates most variations in measurement.15
Keep these comorbidities on your radar screen
Diabetes and hypertension are 2 of the most common causes of CKD in the United States, and the number of kidney failure cases due to these problems is increasing. The most important adverse outcomes of CKD are not only progression to ESRD, but also increased risk for cardiovascular disease (CVD). Studies show that the presence of albuminuria and a decreased estimated GFR consistently and incrementally increase the risk for CVD.16 Decreased GFR is an independent risk factor for CVD outcomes and for all-cause mortality, including sudden death in patients with existing coronary artery disease. Moreover, patients with CKD are 100 times more likely to die from CVD than to develop kidney failure.16
Depression is another prevalent, yet commonly overlooked, comorbid condition. Patients with any chronic disease are at risk for depression, with the incidence rising with the severity of the medical condition.17 CKD is no exception. Rates of depression as high as 29%, as well as rates of anxiety disorders as high as 46%, have been documented in patients on dialysis.18 Patients with depression are impaired in overall functioning and less able to follow medical regimens.17 In addition, low quality of life and depression predict higher morbidity and mortality rates in patients with ESRD.19 Because the diagnosis of depression is frequently missed in primary care settings, screening for depression should be a basic element in CKD management.20
Clinical management of kidney disease
The treatment goal for early-stage kidney disease is to address the risk factors that contribute to the progression of kidney disease: hypertension, heart disease, stroke, diabetes, and dyslipidemia. The TABLE reviews clinical management areas by disease stage.
Prescribe angiotensin antagonists. Angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors antagonize the toxic effects of increases in circulating angiotensin II and are therefore a key component of a therapeutic strategy to halt progressive kidney disease.2,21
Review medications, promote a healthier lifestyle. In addition to prescribing ARBs or ACE inhibitors, the family physician should review the CKD patient’s current medications to eliminate nephrotoxic drugs and adjust other medications on the basis of the patient’s creatinine clearance. Other measures include making sure vaccinations for influenza, pneumococcal pneumonia, and hepatitis B are up to date and emphasizing the importance of smoking cessation and exercise.
Treat comorbid conditions. Hypertension and diabetes must be treated aggressively. Patients with dyslipidemia should be managed with statins.2 Certain complications of progressive kidney disease, such as anemia, bone/mineral metabolic disease, and metabolic disorders, are typically treated by a nephrologist. Nevertheless, primary care providers need to understand these conditions in order to work together with the nephrologist in managing the CKD patient.
Check thyroid hormone and vitamin D levels. Understanding which factors predict disease progression or poor outcome is particularly useful. Most patients with CKD have low T3 syndrome, that is, low serum triiodothyronine levels in the absence of a thyroidal illness. In a recent paper, Song and colleagues showed that low T3 syndrome was common in early CKD and that estimated GFR was positively related with T3, independent of age and serum albumin.22
In another recent study, Ravani et al showed that plasma 25-hydroxyvitamin D is an independent, inverse predictor of disease progression and death in patients with stage 2 to 5 CKD.23 Vitamin D deficiency has been linked to CVD and early mortality in patients on hemodialysis.23 Checking for these 2 markers—low T3 syndrome and vitamin D deficiency—should therefore be part of your screening process for early stage CKD.
Refer to a dietitian. Dietary modification is another important component of the treatment plan. Dietary modifications are often needed to protect against CVD, help control blood pressure, reduce proteinuria, and improve metabolic control in patients with diabetes.2,24 Dietary modifications for CKD patients may go well beyond standard recommendations for a heart-healthy diet.2,24 Calcium, sodium, phosphorus, and potassium may need to be restricted according to laboratory values and stage of the disease.25 The KDOQI guidelines recommend referring CKD patients to a registered dietitian with experience in CKD for a complete nutritional assessment, comprehensive education on dietary restrictions and guidelines, and detailed dietary instruction.2
Manage CKD-associated anemia. Current guidelines do not propose normalizing hemoglobin in patients with renal disease, because lower levels of hemoglobin probably represent an adaptive response and correction to a “normal” level may disturb that response and lead to worse outcomes.26 For a discussion of management of anemia associated with CKD, see “Anemia and chronic kidney disease: What’s the connection?” in the January 2010 issue of this journal.27
Refer to a nephrologist early. A recent study by Chan et al demonstrates the beneficial effects of early referral to a nephrologist.28 There is no clear definition of early vs late referral and, at times, the only criterion is how much time elapsed before the patient was put on dialysis. Referral is considered “late” when management could have been improved by earlier contact with a specialist. It is probably prudent to refer stage 3 and 4 patients, at least for initial consultation. Chan’s meta-analysis found that patients referred late had nearly a 2-fold risk of death compared with those with early referrals. This risk persists at least up to 1 year after the initiation of renal replacement therapy.
Prepare patients for dialysis. It is very important that new hemodialysis patients present for initial treatment with an arteriovenous fistula in place, as first access for hemodialysis. Fistula placement is one of the most important reasons for timely referral to a nephrologist. Later referral is associated with a significantly prolonged hospital stay for initial renal replacement therapy. Late-referred patients are sicker, and many of the complications discussed here have not been optimally treated.
The optimal time to start preparing your patient for dialysis is when GFR measures between 15 and 29 mL/min/1.73 m2. Preparation includes counseling on nutrition and exercise, hepatitis B vaccination if needed, and scheduling for fistula placement.29
The hardest part: Changing habits
Effective CKD treatment must emphasize lifestyle management. You need to persuade smokers to quit and “couch potatoes” to start exercising regularly. Eating habits need to change, as well: This means fewer calories and restrictions on intake of salt and certain minerals. Medications for high blood pressure, diabetes, and kidney disease need to be taken consistently, as prescribed. The TABLE reviews the lifestyle issues that are particularly salient at each stage of CKD.
TABLE
Keying interventions to CKD stages
| Stage | Description | GFR (mL/min/1.73 m2) | Clinical action | Lifestyle management |
|---|---|---|---|---|
| At increased risk | ≥60 (with CKD risk factors) | Screening; CKD risk reduction | Healthy habits according to public health recommendations | |
| 1 | Kidney damage with normal or increased GFR | ≥90 | Diagnosis and treatment; treatment of comorbid conditions; slowing of progression; CVD risk reduction | Emphasis on heart health: physical activity, healthy diet, weight management, and stress management. Restricted sodium, potassium, calcium, phosphorus, and protein, with emphasis on plant vs animal food sources. Treatment adherence to medications and CV/diabetes/hypertension treatment plan if applicable. Assessment of depression and referral to treatment if appropriate. |
| 2 | Kidney damage with mildly decreased GFR | 60-89 | Same, plus estimation of progression | Same recommendations as stage 1 |
| 3 | Moderately decreased GFR | 30-59 | Same, plus evaluation and treatment of complications | Same recommendations as stage 1 |
| 4 | Severely decreased GFR | 15-29 | Preparation for kidney replacement therapy | Same as above, plus assessment of social support to prepare for dialysis treatment if appropriate |
| 5 | Kidney failure | <15 or dialysis | Replacement (if uremia present) | Same as above, plus restricted fluid intake and additional protein intake |
| Note: Shaded area identifies patients who have CKD; unshaded area designates individuals who are at increased risk for developing CKD. CKD is defined as either kidney damage or GFR <60 ml/min/1.73 m2 for ≥3 months. Kidney damage is defined as pathologic abnormalities or markers of damage, including abnormalities in blood or urine tests or imaging studies. | ||||
| CKD, chronic kidney disease; CVD, cardiovascular disease; GFR, glomerular filtration rate. | ||||
| Adapted from: Table 3: chronic kidney disease: a clinical action plan. National Kidney foundation. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. 2002. | ||||
Lifestyle modifications like these are very difficult, and helping patients make them involves much more than simple patient education. In 1 study, Durose et al found that patients on hemodialysis failed to stay on their diets even when they knew which dietary restrictions they should follow and what the consequences of going off their diets would be.30
Update your persuasive techniques: take on the role of coach
Newer theories of behavior change no longer rely on simply providing information and advice, but rather address the complex interaction of motivations involved in attempts to change. These include cues to action, perception of benefits and consequences, environmental and cultural influences, sense of self-efficacy, ambivalence, and the intention to change.31
Unfortunately, health care providers are rarely trained in motivational techniques. Often, their approach to inducing change is authoritarian, confrontational, overly forceful, or guilt inducing. Such attitudes not only limit progress, but are actually correlated with negative behavioral and clinical outcomes.32,33 Recent research has verified the power of the patient–provider interaction in influencing treatment adherence and lifestyle change.33
To be successful in getting patients to adopt new behaviors, physicians need to move away from authoritarian modes and take on some of the attributes of a coach urging on the team.
How this coaching technique works
Motivational Interviewing is a health coaching technique that has been shown to be causally and independently associated with positive behavioral outcomes.34 The techniques used in the motivational interviewing approach are summarized in “The motivational interviewing tool kit”. Motivational interviewing is a goal-oriented, patient-centered counseling style for helping patients explore and resolve their ambivalence about behavior change.35 The approach has been used in diverse populations, settings, and medical conditions. Its efficacy was first demonstrated in the treatment of addictions to illegal drugs and alcohol.36 Continued research and 2 recent meta-analyses using rigorous methodology have validated the usefulness of this approach.37,38
Express empathy
Objective: To establish rapport and avoid resistance by demonstrating your understanding of the patient’s situation.
Example: “It’s not easy making all these changes.”
Follow-up: “But you also say you know these numbers put you at risk for more serious disease.”
Roll with resistance
Objective: To avoid magnifying resistance by allowing patients to explore their barriers in a nonjudgmental, supportive manner.
Example: “You really don’t want to take the medication anymore. It’s hard to remember and you don’t feel sick, so you don’t see why you need it.”
Follow-up: “I’m wondering where you see yourself in 6 months if you stop taking the medication?”
Elicit/provide reminder/elicit
Objective: To find out what the patient already knows, fill in the gaps or correct misconceptions, and explore how the change you suggest will fit into the patient’s life. This is a time-saving strategy that both validates patient knowledge and allows time to address barriers.
Example:
Elicit: “Mrs. Roberts, can you tell me what you know about managing your chronic kidney disease?”
Provide reminder: “That’s great. You’ve pretty much got it nailed. I’d just like to remind you about taking your statin medications and keeping close track of your blood pressure.”
Elicit: “What do you think the biggest barrier is for you right now in managing this condition?”
Support autonomy
Objective: To reduce resistance by assuring patients you know you can’t make them do anything—it’s their choice.
Example: “Of course, it’s your choice, but as your doctor, I’d be concerned if you decided not to try this medication.”
Follow-up: “Nobody can make you do anything that you don’t want to do. You need to consider all your options and make the choice that’s right for you at this time. If you do decide to try this medication, I assure you that we will monitor any side effects closely and adjust the dosage to minimize any problems.”
Explore ambivalence
Objective: To help the patient consider the pros and cons of change in a relaxed yet systematic manner.
Example: “So let’s talk about the pros and cons of trying to quit smoking at this time.”
Follow-up: “Let me see if I can summarize where you are. On the one hand, it’s pretty stressful for you right now and smoking helps you cope. You’ve tried to quit before and you couldn’t keep it up for very long. On the other hand, you really do understand the damage it’s doing to your body and how it is making it more difficult for us to treat your heart disease. Your wife is willing to quit with you and you’ve heard about this new quit medication that can help curb cravings. Did I get it all? What are you thinking you are going to do?”
Elicit change talk
Objective: To evoke the patient’s reasons, desire, ability, and need for change. This “change talk” predicts increased commitment to the lifestyle change, which, in turn, is correlated to a good clinical outcome.
Examples:
“What makes it important to you to start an exercise program?”
“What benefits would come from losing weight?”
“Why do you want to quit smoking?”
Follow-up: “You know that exercise will help you manage your stress, lose some weight, and lower your cholesterol levels. Plus, when you did it before, you had more energy and slept better. You also want to be a good role model for the kids and be able to play sports with them.”
Develop an action plan
Objective: To help the patient develop a plan that is realistic and fits into his or her life. When a patient “owns” the plan, he or she is more likely to follow through.
Examples:
“So what’s the next step for you?”
“What do you think you could do (and would be willing to do) for your health right now that would make the most difference?”
“What do you think your best option is?”
Follow-up: “You’ve outlined a great plan. You’re going to try to eat more vegetables and less meat, plus cut back on portion sizes. You’re also going to try and walk more. Lastly, you’re willing to try the pill box to see if it makes it easier to take your medications correctly.” (Pause). “So, are you going to do this?”
Motivational interviewing has been shown to be effective in improving general health status and sense of well-being, promoting physical activity, improving nutritional habits, encouraging medication adherence, and managing chronic conditions such as hypertension, hypercholesterolemia, obesity, and diabetes.35 A review of the literature on health behavior change demonstrates that motivational interviewing outperforms traditional advice-giving in the treatment of a broad range of behavioral problems and diseases.38
Motivational interviewing is focused on helping patients explore their ambivalence and identify individual barriers that are preventing change. The skill set that motivational interviewing provides can be modified for use in the brief patient encounters typically found in the primary care setting. For an example of how you might use motivational interviewing techniques with your CKD patients, see “Talking about change: A motivational interviewing conversation”.
Physician: Now that we’ve gone over your lab values and you don’t have any more questions, I’d like to take a few minutes to talk about how you’re doing with your treatment plan. Would that be okay with you?
Patient: Sure, doc.
Physician: You’re dealing with a lot of things all together—trying to change your diet, watching your weight, monitoring your blood sugar, and taking your medications.
Patient: It is a lot. Guess it’s obvious from my labs that I’m not doing so well. I feel like I get a handle on one thing but something else blows up.
Physician: Sounds like it feels a bit overwhelming right now.
Patient: Yeah, it really is…but I think I could do better.
Physician: Why don’t we start with reviewing what you’re doing well? you are getting your prescriptions filled, and it seems like you’re taking your medications regularly.
Patient: I really do, nearly all of the time.
Physician: What else are you doing well?
Patient: I’ve cut down on my salt intake. We’re using that salt substitute and it’s okay. Ummm…but I guess by the labs I’m not watching my potassium and phosphorus like I should.
Physician: What else are you doing well?
Patient: Well, my blood pressure is down from what it was. But my sugars are still out of whack and I can’t seem to lose weight.
Physician: Okay, so you’ve done a great job taking your medications and you’ve started to change your diet with the salt—both of which have really helped your blood pressure. As you say, there are some things we still need to tackle. But let’s break it down into small steps—forget the whole list. Can you think of just 1 or 2 more small things that you think you could do that would make a difference right now?
Patient: Well, my wife walks every evening after dinner. She’s been nagging me to walk with her. I guess I wouldn’t mind that so much as long as she doesn’t drag me too far. That would help me drop a few pounds and that might motivate me to be more careful with my diet. Plus, I know that exercise is also supposed to help my blood sugar.
Physician: So, a walk after dinner. Do you think you can do this?
Patient: Yes, I do.
Physician: When would you be willing to start?
Patient: Heck, I could start tomorrow. That’s something that wouldn’t be that big of a deal.
Physician: Great! Seems like a plan then. I’m confident that by taking these small steps like a walk every evening, you can get this under control. You have already improved in some important areas.
Patient: Thanks doc! I’ll see you next visit and hopefully my numbers will be better.
Your crucial role
CKD is well on its way to becoming a full-blown epidemic in the United States. Primary care providers carry the brunt of responsibility for the care of these patients, and with an increasing shortage of nephrologists, the scope of those activities will likely grow. Physicians in solo or small group practice must be prepared to deliver both the clinical and behavioral/lifestyle components of care themselves. While this is a challenging endeavor, we believe the framework outlined here will improve your ability to meet the complex needs of CKD patients.
CORRESPONDENCE Ariel Linden, DrPH, MS, Linden Consulting Group, 6208 NE Chestnut Street, Hillsboro, OR 97124; [email protected]
1. US Renal Data Systems. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
2. National Kidney Foundation. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. 2002. Available at: http://www.kidney.org/professionals/kdoqi/guidelines_ckd/toc.htm. Accessed January 12, 2009.
3. U.S. Department of Health and Human Services. Healthy People 2010. (“Understanding and Improving Health” and “Objectives for Improving Health,” 2 volumes.) 2nd ed. Washington, DC: U.S. Government Printing Office, November 2000.
4. Fox CH, Brooks A, Zayas LE, et al. Primary care physicians’ knowledge and practice patterns in the treatment of chronic kidney disease: an Upstate New York Practice-based Research Network (UNYNET) study. J Am Board Fam Med. 2006;19:54-61.
5. Lea JP, McClellan WM, Melcher C, et al. CKD risk factors reported by primary care physicians: do guidelines make a difference? Am J Kidney Dis. 2006;47:72-77.
6. Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489-495.
7. Nissenson AR, Collins AJ, Hurley J, et al. Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol. 2001;12:1713-1720.
8. McClellan WM, Knight DF, Karp H, et al. Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: important differences between practice and published guidelines. Am J Kidney Dis. 1997;29:368-375.
9. Obrador GT, Ruthazer R, Arora P, et al. Prevalence of and factors associated with suboptimal care before initiation of dialysis in the United States. J Am Soc Nephrol. 1999;10:1793-1800.
10. Boulware LE, Troll MU, Jaar BG, et al. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis. 2006;48:192-204.
11. John R, Webb M, Young A, et al. Unreferred chronic kidney disease: a longitudinal study. Am J Kidney Dis. 2004;43:825-835.
12. Wauters JP, Lameire N, Davison A, et al. Why patients with progressing kidney disease are referred late to the nephrologists: on causes and proposals for improvement. Nephrol Dial Transplant. 2005;20:490-496.
13. Ouseph R, Hendricks P, Hollon JA, et al. Under-recognition of chronic kidney disease in elderly outpatients. Clin Nephrol. 2007;68:373-378.
14. Himmelfarb J, Berns A, Szczech L, et al. Cost, quality, and value: the changing political economy of dialysis care. J Am Soc Nephrol. 2007;18:2021-2027.
15. Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50:169-180.
16. Saran AM, DuBose TD, Jr. Cardiovascular disease in chronic kidney disease. Ther Adv Cardiovasc Dis. 2008;2:425-434.
17. Mental Health America. Fact sheet: co-occurring disorders and depression. Available at: http://www.nmha.org/index.cfm?objectid=C7DF94C1-1372-4D20-C8FE4E509C20471B. Accessed January 25, 2009.
18. Cukor D, Coplan J, Brown C, et al. Course of depression and anxiety diagnosis in patients treated with hemodialysis: a 16-month follow-up. Am Soc Nephrol. 2008;3:1752-1758.
19. Lopez Revuelta K, Garcia Lopez FJ, de Alvaro Moreno F, et al. Perceived mental health at the start of dialysis as a predictor of morbidity and mortality in patients with end stage renal disease (CALVIDIA Study). Nephrol Dial Transplant. 2004;19:2347-2353.
20. Ford DE. A primary care approach: Managing depression in the face of chronic medical conditions. Am J Med. 2008;121(suppl 2):S38-S44.
21. Ferrari P. Prescribing angiotensin converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Nephrol. 2007;12:81-89.
22. Song SH, Kwak IS, Lee DW, et al. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant. 2009;24:1534-1538.
23. Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88-95.
24. Cupisti A, Aparicio M, Barsotti G. Potential benefits of renal diets on cardiovascular risk factors in chronic kidney disease patients. Ren Fail. 2007;29:529-534.
25. Beto JA, Bansal VK. Medical nutrition therapy in chronic kidney failure: Integrating clinical practice guidelines. J Am Diet Assoc. 2004;104:404-409.
26. Al-Aly Z. The new role of calcimimetics as vasculotropic agents. Kidney Int. 2009;75:9-12.
27. Taliercio JJ. Anemia and chronic kidney disease: what’s the connection? J Fam Pract. 2010;59:14-18.
28. Chan MR, Dall AT, Fletcher KE, et al. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med. 2007;120:1063-1070.
29. Almaguer M, Herrera R, Alfonso J, et al. Primary health care strategies for the prevention of end-stage renal disease in Cuba. Kidney Int. 2005;68(suppl 97s):S4-S10.
30. Durose CL, Holdsworth M, Watson V, et al. Knowledge of dietary restrictions and the medical consequences of noncompliance by patients on hemodialysis are not predictive of dietary compliance. Am Diet Assoc. 2004;104:35-41.
31. Linden A, Butterworth SW, Roberts N. Disease management interventions II: what else is in the black box? Dis Manage. 2006;9:73-85.
32. Moyers TB, Martin T. Therapist influence on client language during motivational interviewing sessions. J Subst Abuse Treat. 2006;30:245-251.
33. Moyers TB, Martin T, Christopher PJ, et al. Client language as a mediator of motivational interviewing efficacy: where is the evidence? Alcohol Clin Exp Res. 2007;31(10 suppl):40s-47s.
34. Butterworth S, Linden A, McClay W. Health coaching as an intervention in health management programs. Dis Manage Health Outcomes. 2007;15:299-307.
35. Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008.
36. Miller WR. Motivational interviewing with problem drinkers. Behav Psychother. 1983;11:147-172.
37. Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psych. 2005;1:91-111.
38. Rubak S, Sandbaek A, Lauritzen T, et al. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55:305-312.
1. US Renal Data Systems. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
2. National Kidney Foundation. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. 2002. Available at: http://www.kidney.org/professionals/kdoqi/guidelines_ckd/toc.htm. Accessed January 12, 2009.
3. U.S. Department of Health and Human Services. Healthy People 2010. (“Understanding and Improving Health” and “Objectives for Improving Health,” 2 volumes.) 2nd ed. Washington, DC: U.S. Government Printing Office, November 2000.
4. Fox CH, Brooks A, Zayas LE, et al. Primary care physicians’ knowledge and practice patterns in the treatment of chronic kidney disease: an Upstate New York Practice-based Research Network (UNYNET) study. J Am Board Fam Med. 2006;19:54-61.
5. Lea JP, McClellan WM, Melcher C, et al. CKD risk factors reported by primary care physicians: do guidelines make a difference? Am J Kidney Dis. 2006;47:72-77.
6. Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489-495.
7. Nissenson AR, Collins AJ, Hurley J, et al. Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol. 2001;12:1713-1720.
8. McClellan WM, Knight DF, Karp H, et al. Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: important differences between practice and published guidelines. Am J Kidney Dis. 1997;29:368-375.
9. Obrador GT, Ruthazer R, Arora P, et al. Prevalence of and factors associated with suboptimal care before initiation of dialysis in the United States. J Am Soc Nephrol. 1999;10:1793-1800.
10. Boulware LE, Troll MU, Jaar BG, et al. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis. 2006;48:192-204.
11. John R, Webb M, Young A, et al. Unreferred chronic kidney disease: a longitudinal study. Am J Kidney Dis. 2004;43:825-835.
12. Wauters JP, Lameire N, Davison A, et al. Why patients with progressing kidney disease are referred late to the nephrologists: on causes and proposals for improvement. Nephrol Dial Transplant. 2005;20:490-496.
13. Ouseph R, Hendricks P, Hollon JA, et al. Under-recognition of chronic kidney disease in elderly outpatients. Clin Nephrol. 2007;68:373-378.
14. Himmelfarb J, Berns A, Szczech L, et al. Cost, quality, and value: the changing political economy of dialysis care. J Am Soc Nephrol. 2007;18:2021-2027.
15. Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50:169-180.
16. Saran AM, DuBose TD, Jr. Cardiovascular disease in chronic kidney disease. Ther Adv Cardiovasc Dis. 2008;2:425-434.
17. Mental Health America. Fact sheet: co-occurring disorders and depression. Available at: http://www.nmha.org/index.cfm?objectid=C7DF94C1-1372-4D20-C8FE4E509C20471B. Accessed January 25, 2009.
18. Cukor D, Coplan J, Brown C, et al. Course of depression and anxiety diagnosis in patients treated with hemodialysis: a 16-month follow-up. Am Soc Nephrol. 2008;3:1752-1758.
19. Lopez Revuelta K, Garcia Lopez FJ, de Alvaro Moreno F, et al. Perceived mental health at the start of dialysis as a predictor of morbidity and mortality in patients with end stage renal disease (CALVIDIA Study). Nephrol Dial Transplant. 2004;19:2347-2353.
20. Ford DE. A primary care approach: Managing depression in the face of chronic medical conditions. Am J Med. 2008;121(suppl 2):S38-S44.
21. Ferrari P. Prescribing angiotensin converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Nephrol. 2007;12:81-89.
22. Song SH, Kwak IS, Lee DW, et al. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant. 2009;24:1534-1538.
23. Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88-95.
24. Cupisti A, Aparicio M, Barsotti G. Potential benefits of renal diets on cardiovascular risk factors in chronic kidney disease patients. Ren Fail. 2007;29:529-534.
25. Beto JA, Bansal VK. Medical nutrition therapy in chronic kidney failure: Integrating clinical practice guidelines. J Am Diet Assoc. 2004;104:404-409.
26. Al-Aly Z. The new role of calcimimetics as vasculotropic agents. Kidney Int. 2009;75:9-12.
27. Taliercio JJ. Anemia and chronic kidney disease: what’s the connection? J Fam Pract. 2010;59:14-18.
28. Chan MR, Dall AT, Fletcher KE, et al. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med. 2007;120:1063-1070.
29. Almaguer M, Herrera R, Alfonso J, et al. Primary health care strategies for the prevention of end-stage renal disease in Cuba. Kidney Int. 2005;68(suppl 97s):S4-S10.
30. Durose CL, Holdsworth M, Watson V, et al. Knowledge of dietary restrictions and the medical consequences of noncompliance by patients on hemodialysis are not predictive of dietary compliance. Am Diet Assoc. 2004;104:35-41.
31. Linden A, Butterworth SW, Roberts N. Disease management interventions II: what else is in the black box? Dis Manage. 2006;9:73-85.
32. Moyers TB, Martin T. Therapist influence on client language during motivational interviewing sessions. J Subst Abuse Treat. 2006;30:245-251.
33. Moyers TB, Martin T, Christopher PJ, et al. Client language as a mediator of motivational interviewing efficacy: where is the evidence? Alcohol Clin Exp Res. 2007;31(10 suppl):40s-47s.
34. Butterworth S, Linden A, McClay W. Health coaching as an intervention in health management programs. Dis Manage Health Outcomes. 2007;15:299-307.
35. Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008.
36. Miller WR. Motivational interviewing with problem drinkers. Behav Psychother. 1983;11:147-172.
37. Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psych. 2005;1:91-111.
38. Rubak S, Sandbaek A, Lauritzen T, et al. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55:305-312.
The Journal of Family Practice ©2010 Dowden Health Media
PSA May Trigger Overtreatment of Prostate Ca
Major Finding: Common PSA triggers would have led to overtreatment of prostate cancer in 14%–84% of men on active surveillance.
Data Source: Prospective study of 315 men with localized prostate cancer and PSA levels less than 15 ng/mL at enrollment in monitoring program.
Disclosures: Dr. Loblaw reported having no conflicts of interest related to the study.
SAN FRANCISCO — Men on active surveillance for prostate cancer may be overtreated if clinicians rely strictly on certain commonly used prostate-specific antigen triggers for starting treatment, Dr. Andrew Loblaw said at a symposium on genitourinary cancers.
In a cohort of 315 such men who had no evidence of disease progression, the percentage in whom treatment would have been falsely triggered ranged widely—from 14% to 84%—depending on which of nine PSA measures was used for monitoring. The lowest value seen was with a PSA threshold of 20 ng/mL.
“For patients managed on active surveillance, this raises what we believe is a cautionary tale that strict follow-up with the definitions of either linear regression, threshold, or velocities may lead to a false rate of treatment for patients on surveillance who don't need it,” said Dr. Loblaw, a radiation oncologist at the Sunnybrook Health Sciences Centre in Toronto.
Research shows that active surveillance can achieve good outcomes in men with low-risk prostate cancer, he said. This surveillance typically entails PSA monitoring, with initiatation of treatment often based on a PSA trigger.
The researchers tested the performance of various PSA triggers in 315 men with localized prostate cancer who declined radical treatment, were enrolled in an active surveillance program, and did not have any evidence of disease progression after a median follow-up of 6.8 years (7.2 years for survivors).
At enrollment, the patients' PSA levels were all less than 15 ng/mL. Their monitoring had consisted of periodic physical examination, digital rectal examination, blood work, transrectal ultrasound, bone scans, and repeated prostate biopsies.
“All of the triggers or definitions that we looked at had a false or high trigger for treatment,” Dr. Loblaw reported at the symposium, which was sponsored by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Urologic Oncology.
The false trigger rate was lowest for a PSA threshold of 20 ng/mL (according to which 14% of the men would have been treated) and highest for a successive PSA velocity of greater than 2 ng/mL (84%).
The rate was intermediate for a PSA threshold of 10 ng/mL (38%), first-last PSA doubling times of less than 2 and less than 3 years (39% and 50%), linear regression PSA doubling times of less than 2 and less than 3 years (37% and 48%), overall PSA velocity of greater than 2 ng/mL per year (42%), and a 1-year PSA velocity of greater than 2 ng/mL (51%).
The findings suggest that only a PSA threshold of 20 ng/mL has a low false trigger rate and that men on active surveillance may be overtreated when clinicians rely on the other PSA triggers, Dr. Loblaw said.
To assess PSA changes, he said, clinicians at his center have started using and are validating a general linear mixed model that is freely available online (www.asure.ca
“It allows us to smooth out these PSA variations and helps us interpret the PSA undulations over time,” Dr. Loblaw said. “We are hoping that this will be a beneficial tool … to use in terms of managing patients on active surveillance.”
'All of the triggers or definitions that we looked at had a false or high trigger for treatment.'
Source Dr. Loblaw
My Take
Many Patients and Clinicians May Not Accept 20 ng/mL as Threshold
Active surveillance is a hot topic. Many men with localized prostate cancer may be candidates, but questions remain, including these:
▸ Is it prudent to offer active surveillance to young, healthy men who have a very long life expectancy?
▸ Does a patient on active surveillance need repeat prostate biopsies every year or two, or can we rely on PSA?
▸ As Dr. Loblaw and his colleagues asked, what is the best PSA change determinant to suggest a switch from active surveillance to active treatment?
The researchers found that a PSA above 20 ng/mL may be the most appropriate marker for switching to active treatment. But many patients would be very uncomfortable letting their PSA go as high as 20 before considering a repeat biopsy or treatment. With that strategy, we will be treating only high-risk patients who have progressed on active surveillance. This may be unacceptable to many clinicians and patients.
We need more randomized trials—such as the multicenter START trial—that address active surveillance. I congratulate the researchers on excellent work, and look forward to further studies of and new information about active surveillance.
Major Finding: Common PSA triggers would have led to overtreatment of prostate cancer in 14%–84% of men on active surveillance.
Data Source: Prospective study of 315 men with localized prostate cancer and PSA levels less than 15 ng/mL at enrollment in monitoring program.
Disclosures: Dr. Loblaw reported having no conflicts of interest related to the study.
SAN FRANCISCO — Men on active surveillance for prostate cancer may be overtreated if clinicians rely strictly on certain commonly used prostate-specific antigen triggers for starting treatment, Dr. Andrew Loblaw said at a symposium on genitourinary cancers.
In a cohort of 315 such men who had no evidence of disease progression, the percentage in whom treatment would have been falsely triggered ranged widely—from 14% to 84%—depending on which of nine PSA measures was used for monitoring. The lowest value seen was with a PSA threshold of 20 ng/mL.
“For patients managed on active surveillance, this raises what we believe is a cautionary tale that strict follow-up with the definitions of either linear regression, threshold, or velocities may lead to a false rate of treatment for patients on surveillance who don't need it,” said Dr. Loblaw, a radiation oncologist at the Sunnybrook Health Sciences Centre in Toronto.
Research shows that active surveillance can achieve good outcomes in men with low-risk prostate cancer, he said. This surveillance typically entails PSA monitoring, with initiatation of treatment often based on a PSA trigger.
The researchers tested the performance of various PSA triggers in 315 men with localized prostate cancer who declined radical treatment, were enrolled in an active surveillance program, and did not have any evidence of disease progression after a median follow-up of 6.8 years (7.2 years for survivors).
At enrollment, the patients' PSA levels were all less than 15 ng/mL. Their monitoring had consisted of periodic physical examination, digital rectal examination, blood work, transrectal ultrasound, bone scans, and repeated prostate biopsies.
“All of the triggers or definitions that we looked at had a false or high trigger for treatment,” Dr. Loblaw reported at the symposium, which was sponsored by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Urologic Oncology.
The false trigger rate was lowest for a PSA threshold of 20 ng/mL (according to which 14% of the men would have been treated) and highest for a successive PSA velocity of greater than 2 ng/mL (84%).
The rate was intermediate for a PSA threshold of 10 ng/mL (38%), first-last PSA doubling times of less than 2 and less than 3 years (39% and 50%), linear regression PSA doubling times of less than 2 and less than 3 years (37% and 48%), overall PSA velocity of greater than 2 ng/mL per year (42%), and a 1-year PSA velocity of greater than 2 ng/mL (51%).
The findings suggest that only a PSA threshold of 20 ng/mL has a low false trigger rate and that men on active surveillance may be overtreated when clinicians rely on the other PSA triggers, Dr. Loblaw said.
To assess PSA changes, he said, clinicians at his center have started using and are validating a general linear mixed model that is freely available online (www.asure.ca
“It allows us to smooth out these PSA variations and helps us interpret the PSA undulations over time,” Dr. Loblaw said. “We are hoping that this will be a beneficial tool … to use in terms of managing patients on active surveillance.”
'All of the triggers or definitions that we looked at had a false or high trigger for treatment.'
Source Dr. Loblaw
My Take
Many Patients and Clinicians May Not Accept 20 ng/mL as Threshold
Active surveillance is a hot topic. Many men with localized prostate cancer may be candidates, but questions remain, including these:
▸ Is it prudent to offer active surveillance to young, healthy men who have a very long life expectancy?
▸ Does a patient on active surveillance need repeat prostate biopsies every year or two, or can we rely on PSA?
▸ As Dr. Loblaw and his colleagues asked, what is the best PSA change determinant to suggest a switch from active surveillance to active treatment?
The researchers found that a PSA above 20 ng/mL may be the most appropriate marker for switching to active treatment. But many patients would be very uncomfortable letting their PSA go as high as 20 before considering a repeat biopsy or treatment. With that strategy, we will be treating only high-risk patients who have progressed on active surveillance. This may be unacceptable to many clinicians and patients.
We need more randomized trials—such as the multicenter START trial—that address active surveillance. I congratulate the researchers on excellent work, and look forward to further studies of and new information about active surveillance.
Major Finding: Common PSA triggers would have led to overtreatment of prostate cancer in 14%–84% of men on active surveillance.
Data Source: Prospective study of 315 men with localized prostate cancer and PSA levels less than 15 ng/mL at enrollment in monitoring program.
Disclosures: Dr. Loblaw reported having no conflicts of interest related to the study.
SAN FRANCISCO — Men on active surveillance for prostate cancer may be overtreated if clinicians rely strictly on certain commonly used prostate-specific antigen triggers for starting treatment, Dr. Andrew Loblaw said at a symposium on genitourinary cancers.
In a cohort of 315 such men who had no evidence of disease progression, the percentage in whom treatment would have been falsely triggered ranged widely—from 14% to 84%—depending on which of nine PSA measures was used for monitoring. The lowest value seen was with a PSA threshold of 20 ng/mL.
“For patients managed on active surveillance, this raises what we believe is a cautionary tale that strict follow-up with the definitions of either linear regression, threshold, or velocities may lead to a false rate of treatment for patients on surveillance who don't need it,” said Dr. Loblaw, a radiation oncologist at the Sunnybrook Health Sciences Centre in Toronto.
Research shows that active surveillance can achieve good outcomes in men with low-risk prostate cancer, he said. This surveillance typically entails PSA monitoring, with initiatation of treatment often based on a PSA trigger.
The researchers tested the performance of various PSA triggers in 315 men with localized prostate cancer who declined radical treatment, were enrolled in an active surveillance program, and did not have any evidence of disease progression after a median follow-up of 6.8 years (7.2 years for survivors).
At enrollment, the patients' PSA levels were all less than 15 ng/mL. Their monitoring had consisted of periodic physical examination, digital rectal examination, blood work, transrectal ultrasound, bone scans, and repeated prostate biopsies.
“All of the triggers or definitions that we looked at had a false or high trigger for treatment,” Dr. Loblaw reported at the symposium, which was sponsored by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Urologic Oncology.
The false trigger rate was lowest for a PSA threshold of 20 ng/mL (according to which 14% of the men would have been treated) and highest for a successive PSA velocity of greater than 2 ng/mL (84%).
The rate was intermediate for a PSA threshold of 10 ng/mL (38%), first-last PSA doubling times of less than 2 and less than 3 years (39% and 50%), linear regression PSA doubling times of less than 2 and less than 3 years (37% and 48%), overall PSA velocity of greater than 2 ng/mL per year (42%), and a 1-year PSA velocity of greater than 2 ng/mL (51%).
The findings suggest that only a PSA threshold of 20 ng/mL has a low false trigger rate and that men on active surveillance may be overtreated when clinicians rely on the other PSA triggers, Dr. Loblaw said.
To assess PSA changes, he said, clinicians at his center have started using and are validating a general linear mixed model that is freely available online (www.asure.ca
“It allows us to smooth out these PSA variations and helps us interpret the PSA undulations over time,” Dr. Loblaw said. “We are hoping that this will be a beneficial tool … to use in terms of managing patients on active surveillance.”
'All of the triggers or definitions that we looked at had a false or high trigger for treatment.'
Source Dr. Loblaw
My Take
Many Patients and Clinicians May Not Accept 20 ng/mL as Threshold
Active surveillance is a hot topic. Many men with localized prostate cancer may be candidates, but questions remain, including these:
▸ Is it prudent to offer active surveillance to young, healthy men who have a very long life expectancy?
▸ Does a patient on active surveillance need repeat prostate biopsies every year or two, or can we rely on PSA?
▸ As Dr. Loblaw and his colleagues asked, what is the best PSA change determinant to suggest a switch from active surveillance to active treatment?
The researchers found that a PSA above 20 ng/mL may be the most appropriate marker for switching to active treatment. But many patients would be very uncomfortable letting their PSA go as high as 20 before considering a repeat biopsy or treatment. With that strategy, we will be treating only high-risk patients who have progressed on active surveillance. This may be unacceptable to many clinicians and patients.
We need more randomized trials—such as the multicenter START trial—that address active surveillance. I congratulate the researchers on excellent work, and look forward to further studies of and new information about active surveillance.