User login
FDA Panel Does Not Recommend Dosing Change for Darbepoetin Alfa
ADELPHI, Md. – The majority of a Food and Drug Administration advisory committee on Oct. 18 did not recommend changes in the way darbepoetin alfa is currently dosed for treating anemia in patients with chronic renal failure who are not on dialysis, based on the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study.
Darbepoetin alfa, an erythropoiesis-stimulating agent (ESA) marketed as Aranesp by Amgen Inc., approved in 2001, is indicated for treating anemia associated with chronic renal failure, in patients who are or are not on dialysis. Darbepoetin is similar to epotin alfa (approved in 1989 and marketed as Epogen and as Procrit), but has a longer half life. (Amgen manufactures Aranesp and Epogen, and Epogen is licensed to Johnson and Johnson.) In 2007, a warning about a greater risk of death and serious CV events associated with targeting higher hemoglobin levels with ESAs, compared with targeting lower levels, was added to the drugs’ labeling.
At the meeting, the FDA’s Cardiovascular and Renal Drugs Advisory Committee was asked to review data from the TREAT study of mostly female patients in their late 60s, with type 2 diabetes and chronic renal failure, who were not on dialysis, to evaluate whether targeting a hemoglobin level of 13 g/dL would reduce the risk of death, cardiovascular and renal outcomes, when compared to the control group of patients. No benefit on these outcomes was seen, although anemia was corrected. Those in the control group were given rescue therapy with darbepoetin once a month when hemoglobin dropped to below 9 g/dL, and treatment was stopped when hemoglobin increased to 9 g/dL or more.
Moreover, the rate of stroke was higher among those in the treatment group overall, compared with those in the control group (4% vs. 2%) and among those with a previous history of stroke, the rate of stroke was threefold higher in the treatment group (12% vs. 4%). Among the patients with a history of malignancy at baseline, the rate of death from malignancies during the study (but not necessarily related to the previous malignancy) was higher among those in the treatment group.
In the control group, the median hemoglobin level achieved was 10.6 g/dL and two-thirds of patients did not receive a blood transfusion, 54% did not require any rescue treatment, and 45% did not need a transfusion or rescue treatment.
But the panel voted 9 to 5 with 3 abstentions, against recommending that the regimen used in the TREAT control group be adopted as the dose for this group of patients. Those voting against recommending a change cited an inadequate amount of data and evidence to support a change, as well as concerns that some patients would suffer with the symptoms of low hemoglobin levels and not meet criteria for treatment.
A warning about the increased risk of stroke was added to the label of all ESAs in 2009. Most of the panel voted against recommending that darbepoetin should be avoided for all patients with chronic kidney disease who have a history of a stroke regardless of whether they were on dialysis. However, several voting no objected to the inclusion of patients with chronic kidney disease who were on dialysis in the FDA’s question, who were not in the TREAT trial. Panelists strongly recommended that patients who are treated with darbepoetin be provided with an adequate informed consent that fully explains the increased risk of stroke.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of possible conflicts of interest related to the topic of the meeting; in some cases, they receive a waiver, but not at this meeting.
ADELPHI, Md. – The majority of a Food and Drug Administration advisory committee on Oct. 18 did not recommend changes in the way darbepoetin alfa is currently dosed for treating anemia in patients with chronic renal failure who are not on dialysis, based on the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study.
Darbepoetin alfa, an erythropoiesis-stimulating agent (ESA) marketed as Aranesp by Amgen Inc., approved in 2001, is indicated for treating anemia associated with chronic renal failure, in patients who are or are not on dialysis. Darbepoetin is similar to epotin alfa (approved in 1989 and marketed as Epogen and as Procrit), but has a longer half life. (Amgen manufactures Aranesp and Epogen, and Epogen is licensed to Johnson and Johnson.) In 2007, a warning about a greater risk of death and serious CV events associated with targeting higher hemoglobin levels with ESAs, compared with targeting lower levels, was added to the drugs’ labeling.
At the meeting, the FDA’s Cardiovascular and Renal Drugs Advisory Committee was asked to review data from the TREAT study of mostly female patients in their late 60s, with type 2 diabetes and chronic renal failure, who were not on dialysis, to evaluate whether targeting a hemoglobin level of 13 g/dL would reduce the risk of death, cardiovascular and renal outcomes, when compared to the control group of patients. No benefit on these outcomes was seen, although anemia was corrected. Those in the control group were given rescue therapy with darbepoetin once a month when hemoglobin dropped to below 9 g/dL, and treatment was stopped when hemoglobin increased to 9 g/dL or more.
Moreover, the rate of stroke was higher among those in the treatment group overall, compared with those in the control group (4% vs. 2%) and among those with a previous history of stroke, the rate of stroke was threefold higher in the treatment group (12% vs. 4%). Among the patients with a history of malignancy at baseline, the rate of death from malignancies during the study (but not necessarily related to the previous malignancy) was higher among those in the treatment group.
In the control group, the median hemoglobin level achieved was 10.6 g/dL and two-thirds of patients did not receive a blood transfusion, 54% did not require any rescue treatment, and 45% did not need a transfusion or rescue treatment.
But the panel voted 9 to 5 with 3 abstentions, against recommending that the regimen used in the TREAT control group be adopted as the dose for this group of patients. Those voting against recommending a change cited an inadequate amount of data and evidence to support a change, as well as concerns that some patients would suffer with the symptoms of low hemoglobin levels and not meet criteria for treatment.
A warning about the increased risk of stroke was added to the label of all ESAs in 2009. Most of the panel voted against recommending that darbepoetin should be avoided for all patients with chronic kidney disease who have a history of a stroke regardless of whether they were on dialysis. However, several voting no objected to the inclusion of patients with chronic kidney disease who were on dialysis in the FDA’s question, who were not in the TREAT trial. Panelists strongly recommended that patients who are treated with darbepoetin be provided with an adequate informed consent that fully explains the increased risk of stroke.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of possible conflicts of interest related to the topic of the meeting; in some cases, they receive a waiver, but not at this meeting.
ADELPHI, Md. – The majority of a Food and Drug Administration advisory committee on Oct. 18 did not recommend changes in the way darbepoetin alfa is currently dosed for treating anemia in patients with chronic renal failure who are not on dialysis, based on the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study.
Darbepoetin alfa, an erythropoiesis-stimulating agent (ESA) marketed as Aranesp by Amgen Inc., approved in 2001, is indicated for treating anemia associated with chronic renal failure, in patients who are or are not on dialysis. Darbepoetin is similar to epotin alfa (approved in 1989 and marketed as Epogen and as Procrit), but has a longer half life. (Amgen manufactures Aranesp and Epogen, and Epogen is licensed to Johnson and Johnson.) In 2007, a warning about a greater risk of death and serious CV events associated with targeting higher hemoglobin levels with ESAs, compared with targeting lower levels, was added to the drugs’ labeling.
At the meeting, the FDA’s Cardiovascular and Renal Drugs Advisory Committee was asked to review data from the TREAT study of mostly female patients in their late 60s, with type 2 diabetes and chronic renal failure, who were not on dialysis, to evaluate whether targeting a hemoglobin level of 13 g/dL would reduce the risk of death, cardiovascular and renal outcomes, when compared to the control group of patients. No benefit on these outcomes was seen, although anemia was corrected. Those in the control group were given rescue therapy with darbepoetin once a month when hemoglobin dropped to below 9 g/dL, and treatment was stopped when hemoglobin increased to 9 g/dL or more.
Moreover, the rate of stroke was higher among those in the treatment group overall, compared with those in the control group (4% vs. 2%) and among those with a previous history of stroke, the rate of stroke was threefold higher in the treatment group (12% vs. 4%). Among the patients with a history of malignancy at baseline, the rate of death from malignancies during the study (but not necessarily related to the previous malignancy) was higher among those in the treatment group.
In the control group, the median hemoglobin level achieved was 10.6 g/dL and two-thirds of patients did not receive a blood transfusion, 54% did not require any rescue treatment, and 45% did not need a transfusion or rescue treatment.
But the panel voted 9 to 5 with 3 abstentions, against recommending that the regimen used in the TREAT control group be adopted as the dose for this group of patients. Those voting against recommending a change cited an inadequate amount of data and evidence to support a change, as well as concerns that some patients would suffer with the symptoms of low hemoglobin levels and not meet criteria for treatment.
A warning about the increased risk of stroke was added to the label of all ESAs in 2009. Most of the panel voted against recommending that darbepoetin should be avoided for all patients with chronic kidney disease who have a history of a stroke regardless of whether they were on dialysis. However, several voting no objected to the inclusion of patients with chronic kidney disease who were on dialysis in the FDA’s question, who were not in the TREAT trial. Panelists strongly recommended that patients who are treated with darbepoetin be provided with an adequate informed consent that fully explains the increased risk of stroke.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of possible conflicts of interest related to the topic of the meeting; in some cases, they receive a waiver, but not at this meeting.
FROM THE FDA’S CARDIOVASCULAR DISEASE AND RENAL DRUGS AVDISORY COMMITTEE
FDA Panel Does Not Recommend Dosing Change for Aranesp
ADELPHI, Md. – The majority of a Food and Drug Administration advisory committee on Oct. 18 did not recommend changes in the way darbepoetin alfa is currently dosed for treating anemia in patients with chronic renal failure who are not on dialysis, based on the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study.
Darbepoetin alfa, an erythropoiesis-stimulating agent (ESA) marketed as Aranesp by Amgen Inc., approved in 2001, is indicated for treating anemia associated with chronic renal failure, in patients who are or are not on dialysis. Darbepoetin is similar to epotin alfa (approved in 1989 and marketed as Epogen and as Procrit), but has a longer half life. (Amgen manufactures Aranesp and Epogen, and Epogen is licensed to Johnson and Johnson.) In 2007, a warning about a greater risk of death and serious CV events associated with targeting higher hemoglobin levels with ESAs, compared with targeting lower levels, was added to the drugs’ labeling.
At the meeting, the FDA’s Cardiovascular and Renal Drugs Advisory Committee was asked to review data from the TREAT study of mostly female patients in their late 60s, with type 2 diabetes and chronic renal failure, who were not on dialysis, to evaluate whether targeting a hemoglobin level of 13 g/dL would reduce the risk of death, cardiovascular and renal outcomes, when compared to the control group of patients. No benefit on these outcomes was seen, although anemia was corrected. Those in the control group were given rescue therapy with darbepoetin once a month when hemoglobin dropped to below 9 g/dL, and treatment was stopped when hemoglobin increased to 9 g/dL or more.
Moreover, the rate of stroke was higher among those in the treatment group overall, compared with those in the control group (4% vs. 2%) and among those with a previous history of stroke, the rate of stroke was threefold higher in the treatment group (12% vs. 4%). Among the patients with a history of malignancy at baseline, the rate of death from malignancies during the study (but not necessarily related to the previous malignancy) was higher among those in the treatment group.
In the control group, the median hemoglobin level achieved was 10.6 g/dL and two-thirds of patients did not receive a blood transfusion, 54% did not require any rescue treatment, and 45% did not need a transfusion or rescue treatment.
But the panel voted 9 to 5 with 3 abstentions, against recommending that the regimen used in the TREAT control group be adopted as the dose for this group of patients. Those voting against recommending a change cited an inadequate amount of data and evidence to support a change, as well as concerns that some patients would suffer with the symptoms of low hemoglobin levels and not meet criteria for treatment.
A warning about the increased risk of stroke was added to the label of all ESAs in 2009. Most of the panel voted against recommending that darbepoetin should be avoided for all patients with chronic kidney disease who have a history of a stroke regardless of whether they were on dialysis. However, several voting no objected to the inclusion of patients with chronic kidney disease who were on dialysis in the FDA’s question, who were not in the TREAT trial. Panelists strongly recommended that patients who are treated with darbepoetin be provided with an adequate informed consent that fully explains the increased risk of stroke.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of possible conflicts of interest related to the topic of the meeting; in some cases, they receive a waiver, but not at this meeting.
ADELPHI, Md. – The majority of a Food and Drug Administration advisory committee on Oct. 18 did not recommend changes in the way darbepoetin alfa is currently dosed for treating anemia in patients with chronic renal failure who are not on dialysis, based on the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study.
Darbepoetin alfa, an erythropoiesis-stimulating agent (ESA) marketed as Aranesp by Amgen Inc., approved in 2001, is indicated for treating anemia associated with chronic renal failure, in patients who are or are not on dialysis. Darbepoetin is similar to epotin alfa (approved in 1989 and marketed as Epogen and as Procrit), but has a longer half life. (Amgen manufactures Aranesp and Epogen, and Epogen is licensed to Johnson and Johnson.) In 2007, a warning about a greater risk of death and serious CV events associated with targeting higher hemoglobin levels with ESAs, compared with targeting lower levels, was added to the drugs’ labeling.
At the meeting, the FDA’s Cardiovascular and Renal Drugs Advisory Committee was asked to review data from the TREAT study of mostly female patients in their late 60s, with type 2 diabetes and chronic renal failure, who were not on dialysis, to evaluate whether targeting a hemoglobin level of 13 g/dL would reduce the risk of death, cardiovascular and renal outcomes, when compared to the control group of patients. No benefit on these outcomes was seen, although anemia was corrected. Those in the control group were given rescue therapy with darbepoetin once a month when hemoglobin dropped to below 9 g/dL, and treatment was stopped when hemoglobin increased to 9 g/dL or more.
Moreover, the rate of stroke was higher among those in the treatment group overall, compared with those in the control group (4% vs. 2%) and among those with a previous history of stroke, the rate of stroke was threefold higher in the treatment group (12% vs. 4%). Among the patients with a history of malignancy at baseline, the rate of death from malignancies during the study (but not necessarily related to the previous malignancy) was higher among those in the treatment group.
In the control group, the median hemoglobin level achieved was 10.6 g/dL and two-thirds of patients did not receive a blood transfusion, 54% did not require any rescue treatment, and 45% did not need a transfusion or rescue treatment.
But the panel voted 9 to 5 with 3 abstentions, against recommending that the regimen used in the TREAT control group be adopted as the dose for this group of patients. Those voting against recommending a change cited an inadequate amount of data and evidence to support a change, as well as concerns that some patients would suffer with the symptoms of low hemoglobin levels and not meet criteria for treatment.
A warning about the increased risk of stroke was added to the label of all ESAs in 2009. Most of the panel voted against recommending that darbepoetin should be avoided for all patients with chronic kidney disease who have a history of a stroke regardless of whether they were on dialysis. However, several voting no objected to the inclusion of patients with chronic kidney disease who were on dialysis in the FDA’s question, who were not in the TREAT trial. Panelists strongly recommended that patients who are treated with darbepoetin be provided with an adequate informed consent that fully explains the increased risk of stroke.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of possible conflicts of interest related to the topic of the meeting; in some cases, they receive a waiver, but not at this meeting.
ADELPHI, Md. – The majority of a Food and Drug Administration advisory committee on Oct. 18 did not recommend changes in the way darbepoetin alfa is currently dosed for treating anemia in patients with chronic renal failure who are not on dialysis, based on the results of the TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study.
Darbepoetin alfa, an erythropoiesis-stimulating agent (ESA) marketed as Aranesp by Amgen Inc., approved in 2001, is indicated for treating anemia associated with chronic renal failure, in patients who are or are not on dialysis. Darbepoetin is similar to epotin alfa (approved in 1989 and marketed as Epogen and as Procrit), but has a longer half life. (Amgen manufactures Aranesp and Epogen, and Epogen is licensed to Johnson and Johnson.) In 2007, a warning about a greater risk of death and serious CV events associated with targeting higher hemoglobin levels with ESAs, compared with targeting lower levels, was added to the drugs’ labeling.
At the meeting, the FDA’s Cardiovascular and Renal Drugs Advisory Committee was asked to review data from the TREAT study of mostly female patients in their late 60s, with type 2 diabetes and chronic renal failure, who were not on dialysis, to evaluate whether targeting a hemoglobin level of 13 g/dL would reduce the risk of death, cardiovascular and renal outcomes, when compared to the control group of patients. No benefit on these outcomes was seen, although anemia was corrected. Those in the control group were given rescue therapy with darbepoetin once a month when hemoglobin dropped to below 9 g/dL, and treatment was stopped when hemoglobin increased to 9 g/dL or more.
Moreover, the rate of stroke was higher among those in the treatment group overall, compared with those in the control group (4% vs. 2%) and among those with a previous history of stroke, the rate of stroke was threefold higher in the treatment group (12% vs. 4%). Among the patients with a history of malignancy at baseline, the rate of death from malignancies during the study (but not necessarily related to the previous malignancy) was higher among those in the treatment group.
In the control group, the median hemoglobin level achieved was 10.6 g/dL and two-thirds of patients did not receive a blood transfusion, 54% did not require any rescue treatment, and 45% did not need a transfusion or rescue treatment.
But the panel voted 9 to 5 with 3 abstentions, against recommending that the regimen used in the TREAT control group be adopted as the dose for this group of patients. Those voting against recommending a change cited an inadequate amount of data and evidence to support a change, as well as concerns that some patients would suffer with the symptoms of low hemoglobin levels and not meet criteria for treatment.
A warning about the increased risk of stroke was added to the label of all ESAs in 2009. Most of the panel voted against recommending that darbepoetin should be avoided for all patients with chronic kidney disease who have a history of a stroke regardless of whether they were on dialysis. However, several voting no objected to the inclusion of patients with chronic kidney disease who were on dialysis in the FDA’s question, who were not in the TREAT trial. Panelists strongly recommended that patients who are treated with darbepoetin be provided with an adequate informed consent that fully explains the increased risk of stroke.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of possible conflicts of interest related to the topic of the meeting; in some cases, they receive a waiver, but not at this meeting.
FROM THE FDA’S CARDIOVASCULAR DISEASE AND RENAL DRUGS ADVISORY COMMITTEE
Study Predicts Large Increase in Surgeries for Stress Incontinence and Pelvic Floor Prolapse
LONG BEACH, CALIF. – If present trends continue, U.S. surgeons will be performing 179,000 more incontinence and prolapse surgeries annually in 2050 than they are today.
The projected increase results primarily from an aging population, Dr. Jennifer Wu said at the annual meeting of the American Urogynecologic Society.
Stress incontinence surgeries are predicted to increase from an estimated 211,000 in 2010 to 310,000 in 2050. Similarly, surgeries for pelvic floor prolapse are predicted to increase from 166,000 this year to 246,000 in 2050.
Dr. Wu of Duke University, Durham, N.C., and her colleagues used three sources of data in making their forecast. The U.S. Census Bureau provided estimates of the female population in various age groups between 2006 and 2050. Data on the number of women undergoing these surgeries, broken down by age group, came from the Nationwide Inpatient Sample of 2007 and the National Survey of Ambulatory Surgery of 2006.
The largest number of surgeries occurred among women aged 40-59 years. During the survey years, 48,050 women in that age group underwent inpatient surgery and 53,790 underwent outpatient surgery for incontinence. Similarly, 49,490 women underwent inpatient surgery and 20,700 underwent outpatient surgery for prolapse.
“One out of every 10 women will undergo surgery for incontinence or prolapse in her lifetime,” Dr. Wu said. “These estimates will provide public health officials and policy makers with important information regarding the future disease burden as well as the economic impact of these procedures.”
Dr. Wu acknowledged that the projections rested on a number of assumptions. For example, the investigators assumed that surgery rates would remain constant. That assumption could be overturned by changes in the incidence of disease, advances in technology and surgical technique, or implementation of successful prevention strategies.
Disclosures: Dr. Wu stated that she had no relevant financial disclosures.
LONG BEACH, CALIF. – If present trends continue, U.S. surgeons will be performing 179,000 more incontinence and prolapse surgeries annually in 2050 than they are today.
The projected increase results primarily from an aging population, Dr. Jennifer Wu said at the annual meeting of the American Urogynecologic Society.
Stress incontinence surgeries are predicted to increase from an estimated 211,000 in 2010 to 310,000 in 2050. Similarly, surgeries for pelvic floor prolapse are predicted to increase from 166,000 this year to 246,000 in 2050.
Dr. Wu of Duke University, Durham, N.C., and her colleagues used three sources of data in making their forecast. The U.S. Census Bureau provided estimates of the female population in various age groups between 2006 and 2050. Data on the number of women undergoing these surgeries, broken down by age group, came from the Nationwide Inpatient Sample of 2007 and the National Survey of Ambulatory Surgery of 2006.
The largest number of surgeries occurred among women aged 40-59 years. During the survey years, 48,050 women in that age group underwent inpatient surgery and 53,790 underwent outpatient surgery for incontinence. Similarly, 49,490 women underwent inpatient surgery and 20,700 underwent outpatient surgery for prolapse.
“One out of every 10 women will undergo surgery for incontinence or prolapse in her lifetime,” Dr. Wu said. “These estimates will provide public health officials and policy makers with important information regarding the future disease burden as well as the economic impact of these procedures.”
Dr. Wu acknowledged that the projections rested on a number of assumptions. For example, the investigators assumed that surgery rates would remain constant. That assumption could be overturned by changes in the incidence of disease, advances in technology and surgical technique, or implementation of successful prevention strategies.
Disclosures: Dr. Wu stated that she had no relevant financial disclosures.
LONG BEACH, CALIF. – If present trends continue, U.S. surgeons will be performing 179,000 more incontinence and prolapse surgeries annually in 2050 than they are today.
The projected increase results primarily from an aging population, Dr. Jennifer Wu said at the annual meeting of the American Urogynecologic Society.
Stress incontinence surgeries are predicted to increase from an estimated 211,000 in 2010 to 310,000 in 2050. Similarly, surgeries for pelvic floor prolapse are predicted to increase from 166,000 this year to 246,000 in 2050.
Dr. Wu of Duke University, Durham, N.C., and her colleagues used three sources of data in making their forecast. The U.S. Census Bureau provided estimates of the female population in various age groups between 2006 and 2050. Data on the number of women undergoing these surgeries, broken down by age group, came from the Nationwide Inpatient Sample of 2007 and the National Survey of Ambulatory Surgery of 2006.
The largest number of surgeries occurred among women aged 40-59 years. During the survey years, 48,050 women in that age group underwent inpatient surgery and 53,790 underwent outpatient surgery for incontinence. Similarly, 49,490 women underwent inpatient surgery and 20,700 underwent outpatient surgery for prolapse.
“One out of every 10 women will undergo surgery for incontinence or prolapse in her lifetime,” Dr. Wu said. “These estimates will provide public health officials and policy makers with important information regarding the future disease burden as well as the economic impact of these procedures.”
Dr. Wu acknowledged that the projections rested on a number of assumptions. For example, the investigators assumed that surgery rates would remain constant. That assumption could be overturned by changes in the incidence of disease, advances in technology and surgical technique, or implementation of successful prevention strategies.
Disclosures: Dr. Wu stated that she had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN UROGYNECOLOGIC SOCIETY
Major Finding: The number of stress incontinence surgeries is predicted to increase from 211,000 to 310,000 between 2010 and 2050. The number of pelvic floor prolapse surgeries is predicted to increase from 166,000 to 246,000.
Data Source: Forecasts from the U.S. Census Bureau, the Nationwide Inpatient Sample conducted in 2007, and the National Survey of Ambulatory Surgery conducted in 2006.
Disclosures: Dr. Wu stated that she had no relevant financial disclosures.
Management of hyponatremia: Providing treatment and avoiding harm
Hyponatremia, defined as a serum sodium concentration below 135 mmol/L, is one of the most frequently encountered electrolyte disorders. In 1981, Flear et al1 reported that 15% of their hospitalized patients had plasma sodium concentrations lower than 134 mmol/L, the cutoff they were using at that time.
Hyponatremia is sometimes merely a laboratory artifact or a result of improper blood collection. If real, it can be due to excessive water intake or, most often, the inability of the kidney to excrete water coupled with continued water intake. Patients with significant underlying cardiac, hepatic, or renal dysfunction are at greatest risk of developing hyponatremia, secondary to the nonosmotic release of antidiuretic hormone (ADH). Others at risk include postoperative patients (especially menstruating women), older patients on thiazide diuretics, patients with malignant or psychiatric illness, and endurance athletes.
In this article, we review the treatment of acute and chronic hyponatremia, emphasizing the importance of basing the therapy on the severity of symptoms and taking care not to raise the serum sodium level too rapidly, which can cause neurologic dysfunction.
Guidelines for managing hyponatremia2 are based mostly on retrospective studies and expert opinion, since few prospective studies have been done. Despite the paucity of evidence-based recommendations, we will attempt to incorporate findings from important human and animal studies and consensus guidelines from expert panels. We will focus initially on the critical diagnostic considerations necessary to initiate treatment.
SYMPTOMATIC VS ASYMPTOMATIC
Subsequent sections will address therapeutic approaches in two clinical settings:
Symptomatic hyponatremia, ie, with severe signs or symptoms of cerebral edema—a medical emergency; and
Asymptomatic hyponatremia, ie, without serious signs or symptoms of cerebral edema.
KEY DIAGNOSTIC STEPS WHEN STARTING TREATMENT
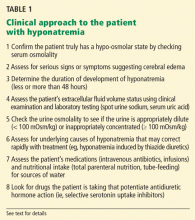
The clinical and laboratory evaluations form the foundation of a proper approach to any patient with hyponatremia. The rationale behind making several important diagnostic distinctions will be discussed here briefly and then expanded on in the remaining text. The reader is referred to another review on the diagnostic evaluation of hyponatremia.3
Confirm that the patient truly has hypo-osmolar hyponatremia
The serum osmolality should be measured to confirm that it is low (ie, < 275 mOsm/kg). In addition, the arterial serum sodium concentration can be measured using a blood gas device if pseudohyponatremia (see below) is suspected. This method uses direct potentiometry and bypasses the dilutional step in the processing of venous samples.4
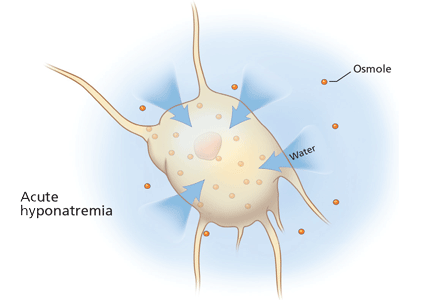
Rationale. The clinical consequences of hyponatremia are due to water moving from hypo-osmolar extracellular fluid into the relatively hyperosmolar interior of the cell. This water movement can cause progressive cerebral edema, resulting in a spectrum of signs and symptoms from headache and ataxia to seizures and coma. But significant fluid shifts and cerebral edema occur only if the extracellular fluid is hypo-osmolar relative to the intracellular fluid.
In fact, hyponatremia can occur in several situations in which the extracellular fluid is not hypo-osmolar. An increase in effective plasma osmoles (substances in the extracellular fluid that do not readily move across the plasma membrane) can cause water to move out of cells, resulting in translocational hyponatremia. This may be seen in hyperglycemia or when mannitol or contrast dye has been given. In these situations, the plasma is either isotonic or even hypertonic to the intracellular fluid, resulting in no movement of water into the cells and therefore no clinical consequences relating to the hyponatremia. Importantly, no therapy is required for the hyponatremia.
Other situations in which hyponatremia is present but not associated with true hypotonicity include states of excess protein or lipid in the blood (pseudohyponatremia). Also, if an infusion of hypotonic fluid is running, clinicians must be sure that blood samples are not drawn proximally in the same vein.
Are there significant signs or symptoms of cerebral edema?
Patients need to be assessed quickly because those with serious neurologic signs or symptoms thought to be related to hyponatremia require urgent treatment with hypertonic saline to increase the serum sodium concentration, regardless of the underlying volume status, the cause of hyponatremia, or the time of onset.
Determine the duration of hyponatremia
One should try to ascertain when the hyponatremia started, as its duration is important in determining the proper pace of correction.
At the onset of hyponatremia, water moves from the extracellular fluid into cells, pulled in by osmosis. The brain can decrease the net amount of water entering into the neurons (and thus regulate its volume) by increasing the flow of water from the interstitium into the cerebrospinal fluid via increased interstitial hydraulic pressure.7
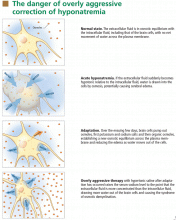
Over the next several days, inorganic solutes (eg, potassium and sodium salts) and various organic solutes are transported out of the cells. In patients in whom this process has had time to occur, treatment of hyponatremia with hypertonic fluids raises the plasma osmolality faster than the cells can recapture the previously transported osmoles. In this situation, overly rapid correction can cause excessive loss of intracellular water, resulting in cell shrinkage and osmotic demyelination syndrome. Osmotic demyelination usually presents during treatment of hyponatremia after an initial improvement in mental status, with worsening neurologic function and various neurologic signs, including paresis and ultimately even death.6
In patients with acute-onset hyponatremia (ie, with onset within the past 48 hours), in whom the above cerebral adaptations have not had time to occur completely, rapid correction is unlikely to result in osmotic demyelination.
In view of the serious risk of osmotic demyelination, if the timing of development of hyponatremia cannot be determined, one should assume it is chronic (> 48 hours) and avoid rapid overcorrection (see discussion below on the rate of correction).
On the other hand, patients who have severe neurologic signs or symptoms initially need their serum sodium increased urgently to safer levels, regardless of the timing of onset (see below for suggested approach). Subsequent treatment of hyponatremia—after the serum sodium level has been raised enough to reverse neurologic symptoms—will be influenced by the duration of the hyponatremia, with careful avoidance of overly rapid correction, especially in patients with chronic hyponatremia.
Assess the patient’s volume status to determine the proper initial treatment
Check urine osmolality to assess for hyponatremic states in which urinary dilution is intact
Measuring urine osmolality is useful in ascertaining whether hyponatremic patients are making appropriately dilute urine (< 100 mOsm/kg). If they are, the cause of the hyponatremia may be excessive water intake, a reset osmostat, or low solute intake. In addition, patients with hypovolemic hyponatremia may have appropriately dilute urine soon after treatment with isotonic intravenous fluids.
The serum sodium concentration often returns to normal if the underlying cause is eliminated (eg, if excessive fluid intake is stopped). If there are no serious signs or symptoms, this can usually be accomplished without additional therapy with intravenous fluids or medications, thereby avoiding the risk of overcorrection.
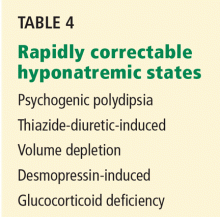
Search for causes of rapidly correctable hyponatremia
TREATING HYPONATREMIC PATIENTS WITH SERIOUS SIGNS OR SYMPTOMS
Patients with hypo-osmolar hyponatremia and serious signs or symptoms of cerebral edema (lethargy, respiratory depression, seizures) need rapid initial correction of the serum sodium level, as this is a true medical emergency.
Certain patients are at greater risk of developing cerebral edema from hyponatremia (Table 2).
On the other hand, patients with chronic hyponatremia are very unlikely to present with signs or symptoms of cerebral edema. In fact, in a patient with chronic hyponatremia, care must be taken to avoid overcorrection beyond that needed to reverse severe signs and symptoms. In the rare case in which a patient with chronic hyponatremia presents with signs or symptoms of cerebral edema, the hypertonic saline infusion must be stopped as soon as the signs or symptoms have resolved. Further rapid changes in serum sodium must be avoided.
During correction of hyponatremia, some patients are at particularly high risk of osmotic demyelination syndrome secondary to underlying abnormalities in cerebral osmotic regulation. These include patients with alcoholism, malnutrition, hypokalemia, and burns, and elderly women on thiazide diuretics.8 These patients should be monitored vigilantly for overly rapid correction during treatment.
INITIAL TREATMENT: REVERSE CEREBRAL EDEMA WITH 3% SALINE
The goal of the initial, rapid phase of correction is to reverse cerebral edema.
Patients with serious signs or symptoms should receive hypertonic (3%) saline at a rate of about 1 mL/kg/hour for the first several hours.8 Those with concomitant hypervolemia (as in congestive heart failure) or underlying cardiovascular disease should also receive a loop diuretic to aid in free-water excretion and to prevent volume overload from the saline infusion. This regimen usually raises the serum sodium concentration enough (usually by about 1 mmol/L/hour) to decrease cerebral edema and improve symptoms.
In patients having active seizures or showing signs of brain herniation, 3% saline can be given initially at a higher rate of about 2 to 3 mL/kg/hour over the first few hours. An alternative approach is an initial 50-mL bolus of 3% saline and an additional 200 mL given over the subsequent 4 to 6 hours.9
No study has compared the efficacy and safety of these approaches, and clinicians should always monitor extracellular fluid volume status, neurologic status, and serum sodium levels closely in any patient treated with hypertonic saline.
After severe signs and symptoms have resolved, 3% saline is promptly discontinued and appropriate therapy is initiated based on the patient’s volume status and underlying cause of hyponatremia (see discussion below).
NEXT, FIND THE APPROPRIATE RATE OF CORRECTION
After the initial serious signs or symptoms have been addressed with hypertonic saline, management should focus on limiting the rate of correction in patients with chronic hyponatremia or hyponatremia of unknown duration.
Animal studies and retrospective human studies have suggested certain guidelines on the appropriate pace and magnitude of correction during treatment of hyponatremia to avoid osmotic demyelination syndrome.2
Clinicians must not attempt to correct the serum sodium to “normal” values. Although patients with acute hyponatremia may tolerate complete correction, there is little evidence that raising the serum sodium concentration acutely by more than 5 to 8 mmol/L is advantageous. Therefore, correction should be judicious in all patients.
Appropriate rates of correction
A recent expert consensus panel suggested that the serum sodium level be raised by no more than 10 to 12 mmol/L during the first 24 hours of treatment, and by less than 18 mmol/L over 48 hours.2
Patients with chronic hyponatremia and signs or symptoms of cerebral edema should have their sodium level raised at an even slower rate—some recommend less than 10 mmol/L in the first 24 hours.10 Aggressive initial correction at the rate of 1.5 to 2 mmol/hour for the first 3 to 4 hours with 3% saline is indicated until serious symptoms (seizure, obtundation) resolve, but correction beyond 10 to 12 mmol/L in the first 24 hours should be avoided. Hypertonic saline therapy should usually be discontinued well before the serum sodium level has risen this much, to avoid a continuing rise in the sodium level after the infusion has stopped.
While hypertonic saline is being infused, serum sodium levels should be checked every 1 to 2 hours. In a study in 56 patients with severe hyponatremia (serum sodium ≤ 105 mmol/L),11 no neurologic complications were observed in patients with chronic hyponatremia whose serum sodium was corrected by less than 12 mmol/L in 24 hours or by less than 18 mmol/L in 48 hours or in whom the average rate of correction to a serum sodium of 120 mmol/L was less than or equal to 0.55 mmol/L per hour.
If the serum sodium concentration has been overcorrected
Desmopressin is effective in preventing and reversing inadvertent overcorrection of hyponatremia. 12 In one study, desmopressin lowered the sodium concentration by 2 to 9 mmol/L in 14 of 20 patients. None of the patients developed any serious adverse consequences.
In addition, intravenous water (dextrose 5%) can be given alone or in combination with desmopressin to prevent or reverse an excessive increase in serum sodium.13 Such therapy may be considered in patients who continue to excrete hypotonic urine and have already reached a serum sodium concentration that meets or exceeds the recommended rate or magnitude of change.
Formulas for estimating the rate of correction
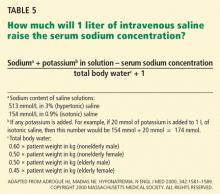
Various formulas have been devised for estimating the change in serum sodium concentration during treatment of hyponatremia.14
An alternative is the Barsoum-Levine equation, which takes into account ongoing urinary losses. Although it is more cumbersome to calculate, it may be more precise.17
Alternatively, in patients without hypovolemia, the clinician can calculate the amount of urinary excretion of free water required to achieve a specific target serum sodium and then measure hourly urinary water excretion during aquaresis induced by furosemide (Lasix).8 Although more physiologic, this method can be clinically cumbersome, requiring timely handling of urine specimens, accurate recording of urine output, and rapid reporting of laboratory results.
Ultimately, these methods serve only as estimates of the change in serum sodium and do not replace careful monitoring of electrolytes (every 1 to 2 hours during acute therapy) and fastidious assessment for clinical signs or symptoms of osmotic demyelination syndrome.
PATIENTS WITH HYPONATREMIA AND NO SERIOUS SIGNS OR SYMPTOMS
General approach
Hyponatremic patients without serious signs or symptoms of cerebral edema do not require urgent therapy to raise the serum sodium.
Patients with chronic asymptomatic hyponatremia are commonly encountered in clinical practice. As a result of cerebral adaptation, they can appear to have no symptoms despite serum sodium levels as low as 115 to 120 mmol/L. However, even if they have no serious signs or symptoms of cerebral edema, some patients may complain of fatigue, lethargy, nausea, gait abnormalities, and muscle cramps and have evidence of milder forms of neurocognitive impairment.18
In a recent case-control study,18 elderly patients with chronic hyponatremia (mean serum sodium concentration 126 ± 5 mmol/L) were more likely to present to the hospital with falls compared with age-matched controls. Further analysis suggested these patients had marked impairments in gait and attention, which improved in some as the serum sodium increased.
Another recent study19 reported that mild hyponatremia (mean serum sodium concentration 132 mmol/L) was independently associated with the risk of fracture, even after adjustment for known osteoporotic risk factors.
Even when there is no need for acute therapy to raise the serum sodium level, the clinician should scrutinize the medical regimen and available clinical data to rule out reversible causes of water excess. These may include ongoing administration of hypotonic fluids (eg, parenteral nutrition or dextrose 5% to “keep the vein open”) or of medications that cause inappropriate release of ADH (eg, selective serotonin reuptake inhibitors) or that impair water excretion (eg, nonsteroidal anti-inflammatory drugs). The clinician should also search for an underlying diagnosis that predisposes to water retention, such as hypothyroidism, adrenal insufficiency, congestive heart failure, or hepatic or renal failure. If hyponatremia is due to endocrine disease, correction of hypothyroidism or adrenal insufficiency should result in water excretion and improvement in the serum sodium.
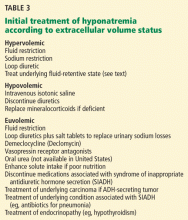
If the cause of the hyponatremia is not immediately apparent, treatment can be started on the basis of assessment of the patient’s extracellular fluid volume status using clinical examination and supplementary laboratory data such as the serum uric acid concentration and urinary sodium concentration.3 Table 3 outlines general treatment options for hypoosmolar hyponatremia according to extracellular fluid volume status.
Of note, physical examination alone has poor sensitivity and specificity in assessing extracellular fluid volume status in patients with hyponatremia.20,21 This highlights the importance of spot measurements of urine sodium and serum uric acid and, when appropriate, isotonic intravenous saline challenge to detect occult hypovolemia.
In general, patients with euvolemia are treated with fluid restriction, and patients with hypovolemia are given isotonic saline. Patients with hypervolemia can be difficult to treat, but in general they are prescribed both sodium and fluid restriction. Loop diuretics can be given to promote excretion of water and sodium. Thiazide diuretics are avoided, as they impair urinary dilution and worsen hyponatremia. Attempts should be made to optimize the treatment of the underlying hypervolemic disorder (congestive heart failure, cirrhosis, advanced renal failure). Vasopressin receptor antagonists can also be used in selected cases of hypervolemic or euvolemic hyponatremia (see discussion below).
How to prescribe fluid restriction rationally
Ideally, patients should not ingest any more fluid than they can excrete in urine and insensible losses—otherwise, the serum sodium can continue to decrease.
Water excretion can be estimated from solute intake and urine osmolarity. In theory, a 70-kg person with a typical daily solute intake of about 10 mOsm/kg and intact urinary dilution to a urine osmolarity of 50 mOsm/L can excrete up to 14 L of urine (700 mOsm/50 mOsm/L) per day. However, a patient with the syndrome of inappropriate ADH secretion (SIADH) and a fixed urine osmolality of 700 mOsm/kg would excrete a similar solute load in only 1 L of urine. Thus, any fluid intake in excess of this volume could worsen hyponatremia.
To excrete free water, urinary sodium plus urinary potassium must be less than the serum sodium concentration. In this regard, the necessary degree of fluid restriction can also be estimated made on the basis of the patient’s urinary electrolytes.22
Increased solute intake to augment water excretion
In patients without hypervolemia, solute intake can be increased to augment water excretion. 22 This can be achieved with salt tablets or oral urea. Although urea can be effective, it is not commonly used because it is not available the United States and it has poor gastrointestinal tolerability. In patients whose nutritional intake is limited and who continue to ingest fluids (such as, for example, an elderly patient subsisting on tea and toast) every effort should be made to increase solute intake with high-protein foods or supplements.
DRUGS TO INHIBIT VASOPRESSIN
Unfortunately, patients often do not adhere to these strategies, as fluid restriction and unpalatable salt tablets or urea can become too burdensome. In such instances, pharmacologic inhibition of vasopressin-mediated water reabsorption can be considered using the following agents.
Demeclocycline (Declomycin) and lithium inhibit the kidney’s response to vasopressin. Because lithium may be nephrotoxic and has unwanted effects on the central nervous system, demeclocycline has become the preferred agent. Given in doses of 300 to 600 mg twice daily, demeclocycline promotes free water excretion, but often takes 1 to 2 weeks of therapy to begin working.
Renal failure due to demeclocycline has been reported in patients with concomitant liver disease.23 Demeclocycline can also cause photosensitivity and is contraindicated in children and pregnant women due to abnormalities in bone and enamel formation. In addition, it can be expensive and may not be covered fully by some prescription plans.
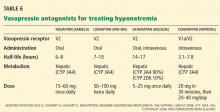
Vasopressin receptor antagonists (‘vaptans’)
ADH, also called vasopressin, interacts with various receptor subtypes, including V1a (causing vasoconstriction, platelet aggregation, inotropic stimulation, myocardial protein synthesis), V1b (causing secretion of adrenocorticotropic hormone), and V2 (causing water reabsorption and release of von Willebrand factor and factor VIII).
Conivaptan (Vaprisol) is a combined V1a-V2 antagonist that has been approved for the treatment of euvolemic and hypervolemic hyponatremia. Conivaptan inhibits the cytochrome P450 3A4 system and thus may interact with other drugs; therefore, its use has been limited to no more than 4 days of intravenous administration in the hospital setting. The recommended dosage is an initial 20-mg infusion over 30 minutes, followed by continuous infusions of 20 to 40 mg/day. Dosing adjustments in renal and hepatic impairment have not been well defined.
Tolvaptan (Samsca) is an oral selective V2 antagonist that has been studied in patients with euvolemic and hypervolemic hyponatremia. 26 Studies have included patients with congestive heart failure, cirrhosis, and SIADH. Although tolvaptan has not been shown to reduce rates of rehospitalization or death in congestive heart failure, it improves serum sodium, overall fluid balance, and congestive symptoms.27 Tolvaptan has recently been approved for the treatment of euvolemic and hypervolemic hyponatremia.
A recent study has confirmed the longterm efficacy of tolvaptan in 111 patients over a mean duration of treatment greater than 700 days.28 While the clinical benefits of chronic tolvaptan therapy have yet to be clearly demonstrated, this study shows that tolvaptan therapy can result in a sustained improvement in serum sodium concentration without an unacceptable increase in adverse events.29
Lixivaptan (VPA-985), another oral selective V2 receptor antagonist, is being studied in patients with euvolemic and hypervolemic hyponatremia.
Current role of vasopressin antagonists
Current studies of vasopressin antagonists in the treatment of hyponatremia are promising, though definite recommendations are needed to ensure slow, careful correction of hyponatremia. Most studies suggest that these agents provide slow, reliable increases in serum sodium. In one large study of patients with congestive heart failure, serum sodium rose by more than 12 mmol/L in 24 hours in fewer than 2% of patients.26
Notably, no cases of osmotic demyelination syndrome have been reported in these studies. However, it should be noted that therapy was started in the hospital with close monitoring of serum sodium levels and discontinuation of fluid restriction; the incidence of overly rapid correction of sodium may be higher outside of carefully done clinical studies. Clinicians should adopt monitoring strategies similar to those used in these rigorous studies.
At present, there is little experience with vasopressin antagonists in hyponatremic patients with serious signs or symptoms of cerebral edema, and most clinicians still view 3% saline as the gold standard for these patients.
Vasopressin antagonists should not be used in patients with hypovolemic hyponatremia, due to concerns about V1a blockade causing hypotension and about V2 blockade producing water excretion and a worsening of the volume-depleted state.
Recent clinical trials have reported that patients often experience increased thirst while taking these agents. This highlights the need to monitor serum sodium during treatment.
These agents are expensive. Tolvaptan costs about $250 per tablet; conivaptan, which is administered intravenously, may cost a little more per treatment course.
THERAPY IN SPECIFIC DISEASE STATES
Patients with hyponatremia and cirrhosis
The focus of treatment remains water and salt restriction and judicious use of loop diuretics and aldosterone antagonists such as spironolactone (Aldactone).
Tolvaptan has been effective at raising the serum sodium level in patients with cirrhosis, 26 while conivaptan should be avoided at present because of vasodilation from V1a receptor antagonism and its potential effects on systemic hemodynamics and risk of variceal bleeding.30
As the severity of cirrhosis increases, the only effective treatment of hyponatremia is liver transplantation.
Patients with SIADH
In most cases, water restriction is the mainstay of therapy. Adequate nutritional intake should also be stressed so that enough solute is available for ongoing water excretion. Although fluid restriction is usually effective, many patients cannot adhere to the level of restriction required.
In cases in which fluid restriction is not effective on its own, demeclocyline can be used to antagonize ADH action and increase water excretion. Sodium tablets and loop diuretics can also be used, taking care to avoid hypovolemia from diuretic-induced sodium losses. The use of tolvaptan in patients with SIADH has resulted in short-term increases in serum sodium.26 A recent study has suggested that this effect can be sustained with longer-term treatment,28 but further studies are needed to show a complementary clinical benefit (eg, improved neurocognition) to guide the use of these costly agents in clinical practice.
Patients with diuretic-induced hyponatremia
Thiazide diuretics should be discontinued and hypovolemia and hypokalemia should be corrected with isotonic saline and potassium supplementation. As the hypokalemia is corrected and the diuretic effect and hypovolemic stimulus to ADH dissipates, water excretion can increase rapidly, resulting in a brisk change in serum sodium.
Serum sodium levels should be closely monitored during therapy to avoid overcorrection. For this reason, use of hypertonic saline should generally be avoided. Hypotonic fluid— eg, half-normal (0.45%) or quarter-normal (0.22%) saline or even desmopressin—may become necessary in the later stages of therapy to avoid overly rapid correction.
Patients with exercise-associated hyponatremia
Patients at highest risk of exercise-associated hyponatremia include those who drink too much fluid during a long-distance race, who have low body weight, who are female, who exercise longer than 4 hours, and who use nonsteroidal anti-inflammatory drugs.31 The cause of hyponatremia is likely multifactorial, with excessive water intake coupled with sodium losses and impaired renal excretion of water due to ADH action and impaired renal dilution. To prevent exercise-associated hyponatremia, fluid intake should be limited to 400 to 800 mL/hour, with the higher end recommended for larger athletes and hotter climates.
Consensus recommendations suggest that most patients with mild hyponatremia (serum sodium 130 to 135 mmol/L) should be treated with fluid restriction and clinical observation, as spontaneous water diuresis leads to improvement in the serum sodium level. Importantly, the reflex to provide isotonic saline infusions should be avoided unless clear signs of volume depletion are present. Intravenous saline has the potential to worsen hyponatremia in the presence of ADH. In addition, some athletes will have retained water in the gastrointestinal tract that may be mobilized after the race, resulting in worsening of hyponatremia.32
In athletes with severe hyponatremia (serum sodium < 120 mmol/L) or symptomatic exercise-associated hyponatremia (lethargy, respiratory depression, seizures), hypertonic saline is the treatment of choice. One protocol suggests giving 100 mL of 3% saline over 10 minutes in the field, followed by prompt transportation to hospital.33
SUMMARY POINTS
- Hyponatremia is a common electrolyte disorder that in its most severe form requires urgent therapy with hypertonic saline to correct cerebral edema.
- In patients without serious signs or symptoms of cerebral edema, recent observations suggest there may be clinically important symptomatology relating to mild neurocognitive dysfunction and an association with risk of bone fracture.
- Multiple treatment strategies are available according to the underlying extracellular fluid volume status and cause of hyponatremia. These include fluid and sodium restriction and augmentation of urinary water excretion with various nutritional and pharmacologic strategies. The most novel therapy includes antagonism of the vasopressin V2 receptor with a class of aquaretic agents known as vaptans.
- There can be serious neurologic injury associated with overly rapid correction of chronic hyponatremia or undercorrection of acute symptomatic hyponatremia.
- Clinicians must be familiar with the details of each of the treatments and have an appreciation of the importance of careful monitoring during treatment.
- Flear CTG, Gill GV, Burn J. Hyponatremia: mechanisms and management. Lancet 1981; 2:26–31.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med 2007; 120(suppl 1):S1–S21.
- Freda BJ, Davidson MB, Hall PM. Evaluation of hyponatremia: a little physiology goes a long way. Cleve Clin J Med 2004; 71:639–650.
- Weisberg LS. Pseudohyponatremia: a reappraisal. Am J Med 1989; 86:315–318.
- Moritz L, Ayus JC. The pathophysiology and treatment of hyponatraemic encephalopathy: an update. Nephrol Dial Transplant 2003; 18:2486–2491.
- Widdess-Walsh P, Sabharwal V, Demirjian S, DeGeorgia M. Neurologic effects of hyponatremia and its treatment. Cleve Clin J Med 2007; 74:377–383.
- Melton JE, Patlak CS, Pettigrew KD, Cserr HF. Volume regulatory loss of Na, Cl, and K from rat brain during acute hyponatremia. Am J Physiol 1987; 252:F661–F669.
- Lauriat SM, Berl T. The hyponatremic patient: practical focus on therapy. J Am Soc Nephrol 1997; 8:1599–1607.
- Kokko JP. Symptomatic hyponatremia with hypoxia is a medical emergency. Kidney Int 2006; 69:1291–1293.
- Ellis SJ. Severe hyponatraemia: complications and treatment. QJM 1995; 88:905–909.
- Sterns RH, Cappuccio JD, Silver SM, Cohen EP. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. J Am Soc Nephrol 1994; 4:1522–1530.
- Perianayagam A, Sterns RH, Silver SM, et al. DDAVP is effective in preventing and reversing inadvertent overcorrection of hyponatremia. Clin J Am Soc Nephrol 2008; 3:331–336.
- Sterns RH, Hix JK. Overcorrection of hyponatremia is a medical emergency. Kidney Int 2009; 76:587–589.
- Nguyen MK, Kurtz I. Analysis of current formulas used for treatment of the dysnatremias. Clin Exp Nephrol 2004; 8:12–16.
- Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med 2000; 342:1581–1589.
- Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH. Hypertonic saline for hyponatremia: risk of inadvertent overcorrection. Clin J Am Soc Nephrol 2007; 2:1110–1117.
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 2007; 356:2064–2072.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 2006; 119:71.e1–e8.
- Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol 2010; 5:275–280.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med 1987; 83:905–988.
- Hoorn EJ, Halperin ML, Zietse R. Diagnostic approach to a patient with hyponatraemia: traditional versus physiology-based options. QJM 2005; 98:529–540.
- Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol 2008; 19:1076–1078.
- Carrilho F, Bosch J, Arroyo V, Mas A, Viver J, Rodes J. Renal failure associated with demeclocycline in cirrhosis. Ann Intern Med 1977; 87:195–197.
- Lehrich RW, Greenberg A. When is it appropriate to use vasopressin receptor antagonists? J Am Soc Nephrol 2008; 19:1054–1058.
- Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 2008; 371:1624–1632.
- Schrier RW, Gross P, Gheorghiade M, et al; SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006; 355:2099–2112.
- Konstam MA, Gheorghiade M, Burnett JC, et al; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007; 297:1319–1331.
- Berl T, Quittnat-Pelletier F, Verbalis JG, et al; SALTWATER Investigators. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol 2010; 21:705–712.
- Greenberg A, Lehrich RW. Treatment of chronic hyponatremia: now we know how, but do we know when or if? J Am Soc Nephrol 2010; 21:552–555.
- Greenberg A, Verbalis JG. Vasopressin receptor antagonists. Kidney Int 2006; 69:2124–2130.
- Rosner MH, Kirven J. Exercise-associated hyponatremia. Clin J Am Soc Nephrol 2007; 2:151–161.
- Halperin ML, Kamel KS, Sterns R. Hyponatremia in marathon runners. N Engl J Med 2005; 353:427–428.
- Hew-Butler T, Almond C, Ayus JC, et al; Exercise-Associated Hyponatremia (EAH) Consensus Panel. Consensus statement of the 1st International Exercise-Associated Hyponatremia Consensus Development Conference, Cape Town, South Africa 2005. Clin J Sport Med 2005; 15:208–213.
Hyponatremia, defined as a serum sodium concentration below 135 mmol/L, is one of the most frequently encountered electrolyte disorders. In 1981, Flear et al1 reported that 15% of their hospitalized patients had plasma sodium concentrations lower than 134 mmol/L, the cutoff they were using at that time.
Hyponatremia is sometimes merely a laboratory artifact or a result of improper blood collection. If real, it can be due to excessive water intake or, most often, the inability of the kidney to excrete water coupled with continued water intake. Patients with significant underlying cardiac, hepatic, or renal dysfunction are at greatest risk of developing hyponatremia, secondary to the nonosmotic release of antidiuretic hormone (ADH). Others at risk include postoperative patients (especially menstruating women), older patients on thiazide diuretics, patients with malignant or psychiatric illness, and endurance athletes.
In this article, we review the treatment of acute and chronic hyponatremia, emphasizing the importance of basing the therapy on the severity of symptoms and taking care not to raise the serum sodium level too rapidly, which can cause neurologic dysfunction.
Guidelines for managing hyponatremia2 are based mostly on retrospective studies and expert opinion, since few prospective studies have been done. Despite the paucity of evidence-based recommendations, we will attempt to incorporate findings from important human and animal studies and consensus guidelines from expert panels. We will focus initially on the critical diagnostic considerations necessary to initiate treatment.
SYMPTOMATIC VS ASYMPTOMATIC
Subsequent sections will address therapeutic approaches in two clinical settings:
Symptomatic hyponatremia, ie, with severe signs or symptoms of cerebral edema—a medical emergency; and
Asymptomatic hyponatremia, ie, without serious signs or symptoms of cerebral edema.
KEY DIAGNOSTIC STEPS WHEN STARTING TREATMENT
The clinical and laboratory evaluations form the foundation of a proper approach to any patient with hyponatremia. The rationale behind making several important diagnostic distinctions will be discussed here briefly and then expanded on in the remaining text. The reader is referred to another review on the diagnostic evaluation of hyponatremia.3
Confirm that the patient truly has hypo-osmolar hyponatremia
The serum osmolality should be measured to confirm that it is low (ie, < 275 mOsm/kg). In addition, the arterial serum sodium concentration can be measured using a blood gas device if pseudohyponatremia (see below) is suspected. This method uses direct potentiometry and bypasses the dilutional step in the processing of venous samples.4
Rationale. The clinical consequences of hyponatremia are due to water moving from hypo-osmolar extracellular fluid into the relatively hyperosmolar interior of the cell. This water movement can cause progressive cerebral edema, resulting in a spectrum of signs and symptoms from headache and ataxia to seizures and coma. But significant fluid shifts and cerebral edema occur only if the extracellular fluid is hypo-osmolar relative to the intracellular fluid.
In fact, hyponatremia can occur in several situations in which the extracellular fluid is not hypo-osmolar. An increase in effective plasma osmoles (substances in the extracellular fluid that do not readily move across the plasma membrane) can cause water to move out of cells, resulting in translocational hyponatremia. This may be seen in hyperglycemia or when mannitol or contrast dye has been given. In these situations, the plasma is either isotonic or even hypertonic to the intracellular fluid, resulting in no movement of water into the cells and therefore no clinical consequences relating to the hyponatremia. Importantly, no therapy is required for the hyponatremia.
Other situations in which hyponatremia is present but not associated with true hypotonicity include states of excess protein or lipid in the blood (pseudohyponatremia). Also, if an infusion of hypotonic fluid is running, clinicians must be sure that blood samples are not drawn proximally in the same vein.
Are there significant signs or symptoms of cerebral edema?
Patients need to be assessed quickly because those with serious neurologic signs or symptoms thought to be related to hyponatremia require urgent treatment with hypertonic saline to increase the serum sodium concentration, regardless of the underlying volume status, the cause of hyponatremia, or the time of onset.
Determine the duration of hyponatremia
One should try to ascertain when the hyponatremia started, as its duration is important in determining the proper pace of correction.
At the onset of hyponatremia, water moves from the extracellular fluid into cells, pulled in by osmosis. The brain can decrease the net amount of water entering into the neurons (and thus regulate its volume) by increasing the flow of water from the interstitium into the cerebrospinal fluid via increased interstitial hydraulic pressure.7
Over the next several days, inorganic solutes (eg, potassium and sodium salts) and various organic solutes are transported out of the cells. In patients in whom this process has had time to occur, treatment of hyponatremia with hypertonic fluids raises the plasma osmolality faster than the cells can recapture the previously transported osmoles. In this situation, overly rapid correction can cause excessive loss of intracellular water, resulting in cell shrinkage and osmotic demyelination syndrome. Osmotic demyelination usually presents during treatment of hyponatremia after an initial improvement in mental status, with worsening neurologic function and various neurologic signs, including paresis and ultimately even death.6
In patients with acute-onset hyponatremia (ie, with onset within the past 48 hours), in whom the above cerebral adaptations have not had time to occur completely, rapid correction is unlikely to result in osmotic demyelination.
In view of the serious risk of osmotic demyelination, if the timing of development of hyponatremia cannot be determined, one should assume it is chronic (> 48 hours) and avoid rapid overcorrection (see discussion below on the rate of correction).
On the other hand, patients who have severe neurologic signs or symptoms initially need their serum sodium increased urgently to safer levels, regardless of the timing of onset (see below for suggested approach). Subsequent treatment of hyponatremia—after the serum sodium level has been raised enough to reverse neurologic symptoms—will be influenced by the duration of the hyponatremia, with careful avoidance of overly rapid correction, especially in patients with chronic hyponatremia.
Assess the patient’s volume status to determine the proper initial treatment
Check urine osmolality to assess for hyponatremic states in which urinary dilution is intact
Measuring urine osmolality is useful in ascertaining whether hyponatremic patients are making appropriately dilute urine (< 100 mOsm/kg). If they are, the cause of the hyponatremia may be excessive water intake, a reset osmostat, or low solute intake. In addition, patients with hypovolemic hyponatremia may have appropriately dilute urine soon after treatment with isotonic intravenous fluids.
The serum sodium concentration often returns to normal if the underlying cause is eliminated (eg, if excessive fluid intake is stopped). If there are no serious signs or symptoms, this can usually be accomplished without additional therapy with intravenous fluids or medications, thereby avoiding the risk of overcorrection.
Search for causes of rapidly correctable hyponatremia
TREATING HYPONATREMIC PATIENTS WITH SERIOUS SIGNS OR SYMPTOMS
Patients with hypo-osmolar hyponatremia and serious signs or symptoms of cerebral edema (lethargy, respiratory depression, seizures) need rapid initial correction of the serum sodium level, as this is a true medical emergency.
Certain patients are at greater risk of developing cerebral edema from hyponatremia (Table 2).
On the other hand, patients with chronic hyponatremia are very unlikely to present with signs or symptoms of cerebral edema. In fact, in a patient with chronic hyponatremia, care must be taken to avoid overcorrection beyond that needed to reverse severe signs and symptoms. In the rare case in which a patient with chronic hyponatremia presents with signs or symptoms of cerebral edema, the hypertonic saline infusion must be stopped as soon as the signs or symptoms have resolved. Further rapid changes in serum sodium must be avoided.
During correction of hyponatremia, some patients are at particularly high risk of osmotic demyelination syndrome secondary to underlying abnormalities in cerebral osmotic regulation. These include patients with alcoholism, malnutrition, hypokalemia, and burns, and elderly women on thiazide diuretics.8 These patients should be monitored vigilantly for overly rapid correction during treatment.
INITIAL TREATMENT: REVERSE CEREBRAL EDEMA WITH 3% SALINE
The goal of the initial, rapid phase of correction is to reverse cerebral edema.
Patients with serious signs or symptoms should receive hypertonic (3%) saline at a rate of about 1 mL/kg/hour for the first several hours.8 Those with concomitant hypervolemia (as in congestive heart failure) or underlying cardiovascular disease should also receive a loop diuretic to aid in free-water excretion and to prevent volume overload from the saline infusion. This regimen usually raises the serum sodium concentration enough (usually by about 1 mmol/L/hour) to decrease cerebral edema and improve symptoms.
In patients having active seizures or showing signs of brain herniation, 3% saline can be given initially at a higher rate of about 2 to 3 mL/kg/hour over the first few hours. An alternative approach is an initial 50-mL bolus of 3% saline and an additional 200 mL given over the subsequent 4 to 6 hours.9
No study has compared the efficacy and safety of these approaches, and clinicians should always monitor extracellular fluid volume status, neurologic status, and serum sodium levels closely in any patient treated with hypertonic saline.
After severe signs and symptoms have resolved, 3% saline is promptly discontinued and appropriate therapy is initiated based on the patient’s volume status and underlying cause of hyponatremia (see discussion below).
NEXT, FIND THE APPROPRIATE RATE OF CORRECTION
After the initial serious signs or symptoms have been addressed with hypertonic saline, management should focus on limiting the rate of correction in patients with chronic hyponatremia or hyponatremia of unknown duration.
Animal studies and retrospective human studies have suggested certain guidelines on the appropriate pace and magnitude of correction during treatment of hyponatremia to avoid osmotic demyelination syndrome.2
Clinicians must not attempt to correct the serum sodium to “normal” values. Although patients with acute hyponatremia may tolerate complete correction, there is little evidence that raising the serum sodium concentration acutely by more than 5 to 8 mmol/L is advantageous. Therefore, correction should be judicious in all patients.
Appropriate rates of correction
A recent expert consensus panel suggested that the serum sodium level be raised by no more than 10 to 12 mmol/L during the first 24 hours of treatment, and by less than 18 mmol/L over 48 hours.2
Patients with chronic hyponatremia and signs or symptoms of cerebral edema should have their sodium level raised at an even slower rate—some recommend less than 10 mmol/L in the first 24 hours.10 Aggressive initial correction at the rate of 1.5 to 2 mmol/hour for the first 3 to 4 hours with 3% saline is indicated until serious symptoms (seizure, obtundation) resolve, but correction beyond 10 to 12 mmol/L in the first 24 hours should be avoided. Hypertonic saline therapy should usually be discontinued well before the serum sodium level has risen this much, to avoid a continuing rise in the sodium level after the infusion has stopped.
While hypertonic saline is being infused, serum sodium levels should be checked every 1 to 2 hours. In a study in 56 patients with severe hyponatremia (serum sodium ≤ 105 mmol/L),11 no neurologic complications were observed in patients with chronic hyponatremia whose serum sodium was corrected by less than 12 mmol/L in 24 hours or by less than 18 mmol/L in 48 hours or in whom the average rate of correction to a serum sodium of 120 mmol/L was less than or equal to 0.55 mmol/L per hour.
If the serum sodium concentration has been overcorrected
Desmopressin is effective in preventing and reversing inadvertent overcorrection of hyponatremia. 12 In one study, desmopressin lowered the sodium concentration by 2 to 9 mmol/L in 14 of 20 patients. None of the patients developed any serious adverse consequences.
In addition, intravenous water (dextrose 5%) can be given alone or in combination with desmopressin to prevent or reverse an excessive increase in serum sodium.13 Such therapy may be considered in patients who continue to excrete hypotonic urine and have already reached a serum sodium concentration that meets or exceeds the recommended rate or magnitude of change.
Formulas for estimating the rate of correction
Various formulas have been devised for estimating the change in serum sodium concentration during treatment of hyponatremia.14
An alternative is the Barsoum-Levine equation, which takes into account ongoing urinary losses. Although it is more cumbersome to calculate, it may be more precise.17
Alternatively, in patients without hypovolemia, the clinician can calculate the amount of urinary excretion of free water required to achieve a specific target serum sodium and then measure hourly urinary water excretion during aquaresis induced by furosemide (Lasix).8 Although more physiologic, this method can be clinically cumbersome, requiring timely handling of urine specimens, accurate recording of urine output, and rapid reporting of laboratory results.
Ultimately, these methods serve only as estimates of the change in serum sodium and do not replace careful monitoring of electrolytes (every 1 to 2 hours during acute therapy) and fastidious assessment for clinical signs or symptoms of osmotic demyelination syndrome.
PATIENTS WITH HYPONATREMIA AND NO SERIOUS SIGNS OR SYMPTOMS
General approach
Hyponatremic patients without serious signs or symptoms of cerebral edema do not require urgent therapy to raise the serum sodium.
Patients with chronic asymptomatic hyponatremia are commonly encountered in clinical practice. As a result of cerebral adaptation, they can appear to have no symptoms despite serum sodium levels as low as 115 to 120 mmol/L. However, even if they have no serious signs or symptoms of cerebral edema, some patients may complain of fatigue, lethargy, nausea, gait abnormalities, and muscle cramps and have evidence of milder forms of neurocognitive impairment.18
In a recent case-control study,18 elderly patients with chronic hyponatremia (mean serum sodium concentration 126 ± 5 mmol/L) were more likely to present to the hospital with falls compared with age-matched controls. Further analysis suggested these patients had marked impairments in gait and attention, which improved in some as the serum sodium increased.
Another recent study19 reported that mild hyponatremia (mean serum sodium concentration 132 mmol/L) was independently associated with the risk of fracture, even after adjustment for known osteoporotic risk factors.
Even when there is no need for acute therapy to raise the serum sodium level, the clinician should scrutinize the medical regimen and available clinical data to rule out reversible causes of water excess. These may include ongoing administration of hypotonic fluids (eg, parenteral nutrition or dextrose 5% to “keep the vein open”) or of medications that cause inappropriate release of ADH (eg, selective serotonin reuptake inhibitors) or that impair water excretion (eg, nonsteroidal anti-inflammatory drugs). The clinician should also search for an underlying diagnosis that predisposes to water retention, such as hypothyroidism, adrenal insufficiency, congestive heart failure, or hepatic or renal failure. If hyponatremia is due to endocrine disease, correction of hypothyroidism or adrenal insufficiency should result in water excretion and improvement in the serum sodium.
If the cause of the hyponatremia is not immediately apparent, treatment can be started on the basis of assessment of the patient’s extracellular fluid volume status using clinical examination and supplementary laboratory data such as the serum uric acid concentration and urinary sodium concentration.3 Table 3 outlines general treatment options for hypoosmolar hyponatremia according to extracellular fluid volume status.
Of note, physical examination alone has poor sensitivity and specificity in assessing extracellular fluid volume status in patients with hyponatremia.20,21 This highlights the importance of spot measurements of urine sodium and serum uric acid and, when appropriate, isotonic intravenous saline challenge to detect occult hypovolemia.
In general, patients with euvolemia are treated with fluid restriction, and patients with hypovolemia are given isotonic saline. Patients with hypervolemia can be difficult to treat, but in general they are prescribed both sodium and fluid restriction. Loop diuretics can be given to promote excretion of water and sodium. Thiazide diuretics are avoided, as they impair urinary dilution and worsen hyponatremia. Attempts should be made to optimize the treatment of the underlying hypervolemic disorder (congestive heart failure, cirrhosis, advanced renal failure). Vasopressin receptor antagonists can also be used in selected cases of hypervolemic or euvolemic hyponatremia (see discussion below).
How to prescribe fluid restriction rationally
Ideally, patients should not ingest any more fluid than they can excrete in urine and insensible losses—otherwise, the serum sodium can continue to decrease.
Water excretion can be estimated from solute intake and urine osmolarity. In theory, a 70-kg person with a typical daily solute intake of about 10 mOsm/kg and intact urinary dilution to a urine osmolarity of 50 mOsm/L can excrete up to 14 L of urine (700 mOsm/50 mOsm/L) per day. However, a patient with the syndrome of inappropriate ADH secretion (SIADH) and a fixed urine osmolality of 700 mOsm/kg would excrete a similar solute load in only 1 L of urine. Thus, any fluid intake in excess of this volume could worsen hyponatremia.
To excrete free water, urinary sodium plus urinary potassium must be less than the serum sodium concentration. In this regard, the necessary degree of fluid restriction can also be estimated made on the basis of the patient’s urinary electrolytes.22
Increased solute intake to augment water excretion
In patients without hypervolemia, solute intake can be increased to augment water excretion. 22 This can be achieved with salt tablets or oral urea. Although urea can be effective, it is not commonly used because it is not available the United States and it has poor gastrointestinal tolerability. In patients whose nutritional intake is limited and who continue to ingest fluids (such as, for example, an elderly patient subsisting on tea and toast) every effort should be made to increase solute intake with high-protein foods or supplements.
DRUGS TO INHIBIT VASOPRESSIN
Unfortunately, patients often do not adhere to these strategies, as fluid restriction and unpalatable salt tablets or urea can become too burdensome. In such instances, pharmacologic inhibition of vasopressin-mediated water reabsorption can be considered using the following agents.
Demeclocycline (Declomycin) and lithium inhibit the kidney’s response to vasopressin. Because lithium may be nephrotoxic and has unwanted effects on the central nervous system, demeclocycline has become the preferred agent. Given in doses of 300 to 600 mg twice daily, demeclocycline promotes free water excretion, but often takes 1 to 2 weeks of therapy to begin working.
Renal failure due to demeclocycline has been reported in patients with concomitant liver disease.23 Demeclocycline can also cause photosensitivity and is contraindicated in children and pregnant women due to abnormalities in bone and enamel formation. In addition, it can be expensive and may not be covered fully by some prescription plans.
Vasopressin receptor antagonists (‘vaptans’)
ADH, also called vasopressin, interacts with various receptor subtypes, including V1a (causing vasoconstriction, platelet aggregation, inotropic stimulation, myocardial protein synthesis), V1b (causing secretion of adrenocorticotropic hormone), and V2 (causing water reabsorption and release of von Willebrand factor and factor VIII).
Conivaptan (Vaprisol) is a combined V1a-V2 antagonist that has been approved for the treatment of euvolemic and hypervolemic hyponatremia. Conivaptan inhibits the cytochrome P450 3A4 system and thus may interact with other drugs; therefore, its use has been limited to no more than 4 days of intravenous administration in the hospital setting. The recommended dosage is an initial 20-mg infusion over 30 minutes, followed by continuous infusions of 20 to 40 mg/day. Dosing adjustments in renal and hepatic impairment have not been well defined.
Tolvaptan (Samsca) is an oral selective V2 antagonist that has been studied in patients with euvolemic and hypervolemic hyponatremia. 26 Studies have included patients with congestive heart failure, cirrhosis, and SIADH. Although tolvaptan has not been shown to reduce rates of rehospitalization or death in congestive heart failure, it improves serum sodium, overall fluid balance, and congestive symptoms.27 Tolvaptan has recently been approved for the treatment of euvolemic and hypervolemic hyponatremia.
A recent study has confirmed the longterm efficacy of tolvaptan in 111 patients over a mean duration of treatment greater than 700 days.28 While the clinical benefits of chronic tolvaptan therapy have yet to be clearly demonstrated, this study shows that tolvaptan therapy can result in a sustained improvement in serum sodium concentration without an unacceptable increase in adverse events.29
Lixivaptan (VPA-985), another oral selective V2 receptor antagonist, is being studied in patients with euvolemic and hypervolemic hyponatremia.
Current role of vasopressin antagonists
Current studies of vasopressin antagonists in the treatment of hyponatremia are promising, though definite recommendations are needed to ensure slow, careful correction of hyponatremia. Most studies suggest that these agents provide slow, reliable increases in serum sodium. In one large study of patients with congestive heart failure, serum sodium rose by more than 12 mmol/L in 24 hours in fewer than 2% of patients.26
Notably, no cases of osmotic demyelination syndrome have been reported in these studies. However, it should be noted that therapy was started in the hospital with close monitoring of serum sodium levels and discontinuation of fluid restriction; the incidence of overly rapid correction of sodium may be higher outside of carefully done clinical studies. Clinicians should adopt monitoring strategies similar to those used in these rigorous studies.
At present, there is little experience with vasopressin antagonists in hyponatremic patients with serious signs or symptoms of cerebral edema, and most clinicians still view 3% saline as the gold standard for these patients.
Vasopressin antagonists should not be used in patients with hypovolemic hyponatremia, due to concerns about V1a blockade causing hypotension and about V2 blockade producing water excretion and a worsening of the volume-depleted state.
Recent clinical trials have reported that patients often experience increased thirst while taking these agents. This highlights the need to monitor serum sodium during treatment.
These agents are expensive. Tolvaptan costs about $250 per tablet; conivaptan, which is administered intravenously, may cost a little more per treatment course.
THERAPY IN SPECIFIC DISEASE STATES
Patients with hyponatremia and cirrhosis
The focus of treatment remains water and salt restriction and judicious use of loop diuretics and aldosterone antagonists such as spironolactone (Aldactone).
Tolvaptan has been effective at raising the serum sodium level in patients with cirrhosis, 26 while conivaptan should be avoided at present because of vasodilation from V1a receptor antagonism and its potential effects on systemic hemodynamics and risk of variceal bleeding.30
As the severity of cirrhosis increases, the only effective treatment of hyponatremia is liver transplantation.
Patients with SIADH
In most cases, water restriction is the mainstay of therapy. Adequate nutritional intake should also be stressed so that enough solute is available for ongoing water excretion. Although fluid restriction is usually effective, many patients cannot adhere to the level of restriction required.
In cases in which fluid restriction is not effective on its own, demeclocyline can be used to antagonize ADH action and increase water excretion. Sodium tablets and loop diuretics can also be used, taking care to avoid hypovolemia from diuretic-induced sodium losses. The use of tolvaptan in patients with SIADH has resulted in short-term increases in serum sodium.26 A recent study has suggested that this effect can be sustained with longer-term treatment,28 but further studies are needed to show a complementary clinical benefit (eg, improved neurocognition) to guide the use of these costly agents in clinical practice.
Patients with diuretic-induced hyponatremia
Thiazide diuretics should be discontinued and hypovolemia and hypokalemia should be corrected with isotonic saline and potassium supplementation. As the hypokalemia is corrected and the diuretic effect and hypovolemic stimulus to ADH dissipates, water excretion can increase rapidly, resulting in a brisk change in serum sodium.
Serum sodium levels should be closely monitored during therapy to avoid overcorrection. For this reason, use of hypertonic saline should generally be avoided. Hypotonic fluid— eg, half-normal (0.45%) or quarter-normal (0.22%) saline or even desmopressin—may become necessary in the later stages of therapy to avoid overly rapid correction.
Patients with exercise-associated hyponatremia
Patients at highest risk of exercise-associated hyponatremia include those who drink too much fluid during a long-distance race, who have low body weight, who are female, who exercise longer than 4 hours, and who use nonsteroidal anti-inflammatory drugs.31 The cause of hyponatremia is likely multifactorial, with excessive water intake coupled with sodium losses and impaired renal excretion of water due to ADH action and impaired renal dilution. To prevent exercise-associated hyponatremia, fluid intake should be limited to 400 to 800 mL/hour, with the higher end recommended for larger athletes and hotter climates.
Consensus recommendations suggest that most patients with mild hyponatremia (serum sodium 130 to 135 mmol/L) should be treated with fluid restriction and clinical observation, as spontaneous water diuresis leads to improvement in the serum sodium level. Importantly, the reflex to provide isotonic saline infusions should be avoided unless clear signs of volume depletion are present. Intravenous saline has the potential to worsen hyponatremia in the presence of ADH. In addition, some athletes will have retained water in the gastrointestinal tract that may be mobilized after the race, resulting in worsening of hyponatremia.32
In athletes with severe hyponatremia (serum sodium < 120 mmol/L) or symptomatic exercise-associated hyponatremia (lethargy, respiratory depression, seizures), hypertonic saline is the treatment of choice. One protocol suggests giving 100 mL of 3% saline over 10 minutes in the field, followed by prompt transportation to hospital.33
SUMMARY POINTS
- Hyponatremia is a common electrolyte disorder that in its most severe form requires urgent therapy with hypertonic saline to correct cerebral edema.
- In patients without serious signs or symptoms of cerebral edema, recent observations suggest there may be clinically important symptomatology relating to mild neurocognitive dysfunction and an association with risk of bone fracture.
- Multiple treatment strategies are available according to the underlying extracellular fluid volume status and cause of hyponatremia. These include fluid and sodium restriction and augmentation of urinary water excretion with various nutritional and pharmacologic strategies. The most novel therapy includes antagonism of the vasopressin V2 receptor with a class of aquaretic agents known as vaptans.
- There can be serious neurologic injury associated with overly rapid correction of chronic hyponatremia or undercorrection of acute symptomatic hyponatremia.
- Clinicians must be familiar with the details of each of the treatments and have an appreciation of the importance of careful monitoring during treatment.
Hyponatremia, defined as a serum sodium concentration below 135 mmol/L, is one of the most frequently encountered electrolyte disorders. In 1981, Flear et al1 reported that 15% of their hospitalized patients had plasma sodium concentrations lower than 134 mmol/L, the cutoff they were using at that time.
Hyponatremia is sometimes merely a laboratory artifact or a result of improper blood collection. If real, it can be due to excessive water intake or, most often, the inability of the kidney to excrete water coupled with continued water intake. Patients with significant underlying cardiac, hepatic, or renal dysfunction are at greatest risk of developing hyponatremia, secondary to the nonosmotic release of antidiuretic hormone (ADH). Others at risk include postoperative patients (especially menstruating women), older patients on thiazide diuretics, patients with malignant or psychiatric illness, and endurance athletes.
In this article, we review the treatment of acute and chronic hyponatremia, emphasizing the importance of basing the therapy on the severity of symptoms and taking care not to raise the serum sodium level too rapidly, which can cause neurologic dysfunction.
Guidelines for managing hyponatremia2 are based mostly on retrospective studies and expert opinion, since few prospective studies have been done. Despite the paucity of evidence-based recommendations, we will attempt to incorporate findings from important human and animal studies and consensus guidelines from expert panels. We will focus initially on the critical diagnostic considerations necessary to initiate treatment.
SYMPTOMATIC VS ASYMPTOMATIC
Subsequent sections will address therapeutic approaches in two clinical settings:
Symptomatic hyponatremia, ie, with severe signs or symptoms of cerebral edema—a medical emergency; and
Asymptomatic hyponatremia, ie, without serious signs or symptoms of cerebral edema.
KEY DIAGNOSTIC STEPS WHEN STARTING TREATMENT
The clinical and laboratory evaluations form the foundation of a proper approach to any patient with hyponatremia. The rationale behind making several important diagnostic distinctions will be discussed here briefly and then expanded on in the remaining text. The reader is referred to another review on the diagnostic evaluation of hyponatremia.3
Confirm that the patient truly has hypo-osmolar hyponatremia
The serum osmolality should be measured to confirm that it is low (ie, < 275 mOsm/kg). In addition, the arterial serum sodium concentration can be measured using a blood gas device if pseudohyponatremia (see below) is suspected. This method uses direct potentiometry and bypasses the dilutional step in the processing of venous samples.4
Rationale. The clinical consequences of hyponatremia are due to water moving from hypo-osmolar extracellular fluid into the relatively hyperosmolar interior of the cell. This water movement can cause progressive cerebral edema, resulting in a spectrum of signs and symptoms from headache and ataxia to seizures and coma. But significant fluid shifts and cerebral edema occur only if the extracellular fluid is hypo-osmolar relative to the intracellular fluid.
In fact, hyponatremia can occur in several situations in which the extracellular fluid is not hypo-osmolar. An increase in effective plasma osmoles (substances in the extracellular fluid that do not readily move across the plasma membrane) can cause water to move out of cells, resulting in translocational hyponatremia. This may be seen in hyperglycemia or when mannitol or contrast dye has been given. In these situations, the plasma is either isotonic or even hypertonic to the intracellular fluid, resulting in no movement of water into the cells and therefore no clinical consequences relating to the hyponatremia. Importantly, no therapy is required for the hyponatremia.
Other situations in which hyponatremia is present but not associated with true hypotonicity include states of excess protein or lipid in the blood (pseudohyponatremia). Also, if an infusion of hypotonic fluid is running, clinicians must be sure that blood samples are not drawn proximally in the same vein.
Are there significant signs or symptoms of cerebral edema?
Patients need to be assessed quickly because those with serious neurologic signs or symptoms thought to be related to hyponatremia require urgent treatment with hypertonic saline to increase the serum sodium concentration, regardless of the underlying volume status, the cause of hyponatremia, or the time of onset.
Determine the duration of hyponatremia
One should try to ascertain when the hyponatremia started, as its duration is important in determining the proper pace of correction.
At the onset of hyponatremia, water moves from the extracellular fluid into cells, pulled in by osmosis. The brain can decrease the net amount of water entering into the neurons (and thus regulate its volume) by increasing the flow of water from the interstitium into the cerebrospinal fluid via increased interstitial hydraulic pressure.7
Over the next several days, inorganic solutes (eg, potassium and sodium salts) and various organic solutes are transported out of the cells. In patients in whom this process has had time to occur, treatment of hyponatremia with hypertonic fluids raises the plasma osmolality faster than the cells can recapture the previously transported osmoles. In this situation, overly rapid correction can cause excessive loss of intracellular water, resulting in cell shrinkage and osmotic demyelination syndrome. Osmotic demyelination usually presents during treatment of hyponatremia after an initial improvement in mental status, with worsening neurologic function and various neurologic signs, including paresis and ultimately even death.6
In patients with acute-onset hyponatremia (ie, with onset within the past 48 hours), in whom the above cerebral adaptations have not had time to occur completely, rapid correction is unlikely to result in osmotic demyelination.
In view of the serious risk of osmotic demyelination, if the timing of development of hyponatremia cannot be determined, one should assume it is chronic (> 48 hours) and avoid rapid overcorrection (see discussion below on the rate of correction).
On the other hand, patients who have severe neurologic signs or symptoms initially need their serum sodium increased urgently to safer levels, regardless of the timing of onset (see below for suggested approach). Subsequent treatment of hyponatremia—after the serum sodium level has been raised enough to reverse neurologic symptoms—will be influenced by the duration of the hyponatremia, with careful avoidance of overly rapid correction, especially in patients with chronic hyponatremia.
Assess the patient’s volume status to determine the proper initial treatment
Check urine osmolality to assess for hyponatremic states in which urinary dilution is intact
Measuring urine osmolality is useful in ascertaining whether hyponatremic patients are making appropriately dilute urine (< 100 mOsm/kg). If they are, the cause of the hyponatremia may be excessive water intake, a reset osmostat, or low solute intake. In addition, patients with hypovolemic hyponatremia may have appropriately dilute urine soon after treatment with isotonic intravenous fluids.
The serum sodium concentration often returns to normal if the underlying cause is eliminated (eg, if excessive fluid intake is stopped). If there are no serious signs or symptoms, this can usually be accomplished without additional therapy with intravenous fluids or medications, thereby avoiding the risk of overcorrection.
Search for causes of rapidly correctable hyponatremia
TREATING HYPONATREMIC PATIENTS WITH SERIOUS SIGNS OR SYMPTOMS
Patients with hypo-osmolar hyponatremia and serious signs or symptoms of cerebral edema (lethargy, respiratory depression, seizures) need rapid initial correction of the serum sodium level, as this is a true medical emergency.
Certain patients are at greater risk of developing cerebral edema from hyponatremia (Table 2).
On the other hand, patients with chronic hyponatremia are very unlikely to present with signs or symptoms of cerebral edema. In fact, in a patient with chronic hyponatremia, care must be taken to avoid overcorrection beyond that needed to reverse severe signs and symptoms. In the rare case in which a patient with chronic hyponatremia presents with signs or symptoms of cerebral edema, the hypertonic saline infusion must be stopped as soon as the signs or symptoms have resolved. Further rapid changes in serum sodium must be avoided.
During correction of hyponatremia, some patients are at particularly high risk of osmotic demyelination syndrome secondary to underlying abnormalities in cerebral osmotic regulation. These include patients with alcoholism, malnutrition, hypokalemia, and burns, and elderly women on thiazide diuretics.8 These patients should be monitored vigilantly for overly rapid correction during treatment.
INITIAL TREATMENT: REVERSE CEREBRAL EDEMA WITH 3% SALINE
The goal of the initial, rapid phase of correction is to reverse cerebral edema.
Patients with serious signs or symptoms should receive hypertonic (3%) saline at a rate of about 1 mL/kg/hour for the first several hours.8 Those with concomitant hypervolemia (as in congestive heart failure) or underlying cardiovascular disease should also receive a loop diuretic to aid in free-water excretion and to prevent volume overload from the saline infusion. This regimen usually raises the serum sodium concentration enough (usually by about 1 mmol/L/hour) to decrease cerebral edema and improve symptoms.
In patients having active seizures or showing signs of brain herniation, 3% saline can be given initially at a higher rate of about 2 to 3 mL/kg/hour over the first few hours. An alternative approach is an initial 50-mL bolus of 3% saline and an additional 200 mL given over the subsequent 4 to 6 hours.9
No study has compared the efficacy and safety of these approaches, and clinicians should always monitor extracellular fluid volume status, neurologic status, and serum sodium levels closely in any patient treated with hypertonic saline.
After severe signs and symptoms have resolved, 3% saline is promptly discontinued and appropriate therapy is initiated based on the patient’s volume status and underlying cause of hyponatremia (see discussion below).
NEXT, FIND THE APPROPRIATE RATE OF CORRECTION
After the initial serious signs or symptoms have been addressed with hypertonic saline, management should focus on limiting the rate of correction in patients with chronic hyponatremia or hyponatremia of unknown duration.
Animal studies and retrospective human studies have suggested certain guidelines on the appropriate pace and magnitude of correction during treatment of hyponatremia to avoid osmotic demyelination syndrome.2
Clinicians must not attempt to correct the serum sodium to “normal” values. Although patients with acute hyponatremia may tolerate complete correction, there is little evidence that raising the serum sodium concentration acutely by more than 5 to 8 mmol/L is advantageous. Therefore, correction should be judicious in all patients.
Appropriate rates of correction
A recent expert consensus panel suggested that the serum sodium level be raised by no more than 10 to 12 mmol/L during the first 24 hours of treatment, and by less than 18 mmol/L over 48 hours.2
Patients with chronic hyponatremia and signs or symptoms of cerebral edema should have their sodium level raised at an even slower rate—some recommend less than 10 mmol/L in the first 24 hours.10 Aggressive initial correction at the rate of 1.5 to 2 mmol/hour for the first 3 to 4 hours with 3% saline is indicated until serious symptoms (seizure, obtundation) resolve, but correction beyond 10 to 12 mmol/L in the first 24 hours should be avoided. Hypertonic saline therapy should usually be discontinued well before the serum sodium level has risen this much, to avoid a continuing rise in the sodium level after the infusion has stopped.
While hypertonic saline is being infused, serum sodium levels should be checked every 1 to 2 hours. In a study in 56 patients with severe hyponatremia (serum sodium ≤ 105 mmol/L),11 no neurologic complications were observed in patients with chronic hyponatremia whose serum sodium was corrected by less than 12 mmol/L in 24 hours or by less than 18 mmol/L in 48 hours or in whom the average rate of correction to a serum sodium of 120 mmol/L was less than or equal to 0.55 mmol/L per hour.
If the serum sodium concentration has been overcorrected
Desmopressin is effective in preventing and reversing inadvertent overcorrection of hyponatremia. 12 In one study, desmopressin lowered the sodium concentration by 2 to 9 mmol/L in 14 of 20 patients. None of the patients developed any serious adverse consequences.
In addition, intravenous water (dextrose 5%) can be given alone or in combination with desmopressin to prevent or reverse an excessive increase in serum sodium.13 Such therapy may be considered in patients who continue to excrete hypotonic urine and have already reached a serum sodium concentration that meets or exceeds the recommended rate or magnitude of change.
Formulas for estimating the rate of correction
Various formulas have been devised for estimating the change in serum sodium concentration during treatment of hyponatremia.14
An alternative is the Barsoum-Levine equation, which takes into account ongoing urinary losses. Although it is more cumbersome to calculate, it may be more precise.17
Alternatively, in patients without hypovolemia, the clinician can calculate the amount of urinary excretion of free water required to achieve a specific target serum sodium and then measure hourly urinary water excretion during aquaresis induced by furosemide (Lasix).8 Although more physiologic, this method can be clinically cumbersome, requiring timely handling of urine specimens, accurate recording of urine output, and rapid reporting of laboratory results.
Ultimately, these methods serve only as estimates of the change in serum sodium and do not replace careful monitoring of electrolytes (every 1 to 2 hours during acute therapy) and fastidious assessment for clinical signs or symptoms of osmotic demyelination syndrome.
PATIENTS WITH HYPONATREMIA AND NO SERIOUS SIGNS OR SYMPTOMS
General approach
Hyponatremic patients without serious signs or symptoms of cerebral edema do not require urgent therapy to raise the serum sodium.
Patients with chronic asymptomatic hyponatremia are commonly encountered in clinical practice. As a result of cerebral adaptation, they can appear to have no symptoms despite serum sodium levels as low as 115 to 120 mmol/L. However, even if they have no serious signs or symptoms of cerebral edema, some patients may complain of fatigue, lethargy, nausea, gait abnormalities, and muscle cramps and have evidence of milder forms of neurocognitive impairment.18
In a recent case-control study,18 elderly patients with chronic hyponatremia (mean serum sodium concentration 126 ± 5 mmol/L) were more likely to present to the hospital with falls compared with age-matched controls. Further analysis suggested these patients had marked impairments in gait and attention, which improved in some as the serum sodium increased.
Another recent study19 reported that mild hyponatremia (mean serum sodium concentration 132 mmol/L) was independently associated with the risk of fracture, even after adjustment for known osteoporotic risk factors.
Even when there is no need for acute therapy to raise the serum sodium level, the clinician should scrutinize the medical regimen and available clinical data to rule out reversible causes of water excess. These may include ongoing administration of hypotonic fluids (eg, parenteral nutrition or dextrose 5% to “keep the vein open”) or of medications that cause inappropriate release of ADH (eg, selective serotonin reuptake inhibitors) or that impair water excretion (eg, nonsteroidal anti-inflammatory drugs). The clinician should also search for an underlying diagnosis that predisposes to water retention, such as hypothyroidism, adrenal insufficiency, congestive heart failure, or hepatic or renal failure. If hyponatremia is due to endocrine disease, correction of hypothyroidism or adrenal insufficiency should result in water excretion and improvement in the serum sodium.
If the cause of the hyponatremia is not immediately apparent, treatment can be started on the basis of assessment of the patient’s extracellular fluid volume status using clinical examination and supplementary laboratory data such as the serum uric acid concentration and urinary sodium concentration.3 Table 3 outlines general treatment options for hypoosmolar hyponatremia according to extracellular fluid volume status.
Of note, physical examination alone has poor sensitivity and specificity in assessing extracellular fluid volume status in patients with hyponatremia.20,21 This highlights the importance of spot measurements of urine sodium and serum uric acid and, when appropriate, isotonic intravenous saline challenge to detect occult hypovolemia.
In general, patients with euvolemia are treated with fluid restriction, and patients with hypovolemia are given isotonic saline. Patients with hypervolemia can be difficult to treat, but in general they are prescribed both sodium and fluid restriction. Loop diuretics can be given to promote excretion of water and sodium. Thiazide diuretics are avoided, as they impair urinary dilution and worsen hyponatremia. Attempts should be made to optimize the treatment of the underlying hypervolemic disorder (congestive heart failure, cirrhosis, advanced renal failure). Vasopressin receptor antagonists can also be used in selected cases of hypervolemic or euvolemic hyponatremia (see discussion below).
How to prescribe fluid restriction rationally
Ideally, patients should not ingest any more fluid than they can excrete in urine and insensible losses—otherwise, the serum sodium can continue to decrease.
Water excretion can be estimated from solute intake and urine osmolarity. In theory, a 70-kg person with a typical daily solute intake of about 10 mOsm/kg and intact urinary dilution to a urine osmolarity of 50 mOsm/L can excrete up to 14 L of urine (700 mOsm/50 mOsm/L) per day. However, a patient with the syndrome of inappropriate ADH secretion (SIADH) and a fixed urine osmolality of 700 mOsm/kg would excrete a similar solute load in only 1 L of urine. Thus, any fluid intake in excess of this volume could worsen hyponatremia.
To excrete free water, urinary sodium plus urinary potassium must be less than the serum sodium concentration. In this regard, the necessary degree of fluid restriction can also be estimated made on the basis of the patient’s urinary electrolytes.22
Increased solute intake to augment water excretion
In patients without hypervolemia, solute intake can be increased to augment water excretion. 22 This can be achieved with salt tablets or oral urea. Although urea can be effective, it is not commonly used because it is not available the United States and it has poor gastrointestinal tolerability. In patients whose nutritional intake is limited and who continue to ingest fluids (such as, for example, an elderly patient subsisting on tea and toast) every effort should be made to increase solute intake with high-protein foods or supplements.
DRUGS TO INHIBIT VASOPRESSIN
Unfortunately, patients often do not adhere to these strategies, as fluid restriction and unpalatable salt tablets or urea can become too burdensome. In such instances, pharmacologic inhibition of vasopressin-mediated water reabsorption can be considered using the following agents.
Demeclocycline (Declomycin) and lithium inhibit the kidney’s response to vasopressin. Because lithium may be nephrotoxic and has unwanted effects on the central nervous system, demeclocycline has become the preferred agent. Given in doses of 300 to 600 mg twice daily, demeclocycline promotes free water excretion, but often takes 1 to 2 weeks of therapy to begin working.
Renal failure due to demeclocycline has been reported in patients with concomitant liver disease.23 Demeclocycline can also cause photosensitivity and is contraindicated in children and pregnant women due to abnormalities in bone and enamel formation. In addition, it can be expensive and may not be covered fully by some prescription plans.
Vasopressin receptor antagonists (‘vaptans’)
ADH, also called vasopressin, interacts with various receptor subtypes, including V1a (causing vasoconstriction, platelet aggregation, inotropic stimulation, myocardial protein synthesis), V1b (causing secretion of adrenocorticotropic hormone), and V2 (causing water reabsorption and release of von Willebrand factor and factor VIII).
Conivaptan (Vaprisol) is a combined V1a-V2 antagonist that has been approved for the treatment of euvolemic and hypervolemic hyponatremia. Conivaptan inhibits the cytochrome P450 3A4 system and thus may interact with other drugs; therefore, its use has been limited to no more than 4 days of intravenous administration in the hospital setting. The recommended dosage is an initial 20-mg infusion over 30 minutes, followed by continuous infusions of 20 to 40 mg/day. Dosing adjustments in renal and hepatic impairment have not been well defined.
Tolvaptan (Samsca) is an oral selective V2 antagonist that has been studied in patients with euvolemic and hypervolemic hyponatremia. 26 Studies have included patients with congestive heart failure, cirrhosis, and SIADH. Although tolvaptan has not been shown to reduce rates of rehospitalization or death in congestive heart failure, it improves serum sodium, overall fluid balance, and congestive symptoms.27 Tolvaptan has recently been approved for the treatment of euvolemic and hypervolemic hyponatremia.
A recent study has confirmed the longterm efficacy of tolvaptan in 111 patients over a mean duration of treatment greater than 700 days.28 While the clinical benefits of chronic tolvaptan therapy have yet to be clearly demonstrated, this study shows that tolvaptan therapy can result in a sustained improvement in serum sodium concentration without an unacceptable increase in adverse events.29
Lixivaptan (VPA-985), another oral selective V2 receptor antagonist, is being studied in patients with euvolemic and hypervolemic hyponatremia.
Current role of vasopressin antagonists
Current studies of vasopressin antagonists in the treatment of hyponatremia are promising, though definite recommendations are needed to ensure slow, careful correction of hyponatremia. Most studies suggest that these agents provide slow, reliable increases in serum sodium. In one large study of patients with congestive heart failure, serum sodium rose by more than 12 mmol/L in 24 hours in fewer than 2% of patients.26
Notably, no cases of osmotic demyelination syndrome have been reported in these studies. However, it should be noted that therapy was started in the hospital with close monitoring of serum sodium levels and discontinuation of fluid restriction; the incidence of overly rapid correction of sodium may be higher outside of carefully done clinical studies. Clinicians should adopt monitoring strategies similar to those used in these rigorous studies.
At present, there is little experience with vasopressin antagonists in hyponatremic patients with serious signs or symptoms of cerebral edema, and most clinicians still view 3% saline as the gold standard for these patients.
Vasopressin antagonists should not be used in patients with hypovolemic hyponatremia, due to concerns about V1a blockade causing hypotension and about V2 blockade producing water excretion and a worsening of the volume-depleted state.
Recent clinical trials have reported that patients often experience increased thirst while taking these agents. This highlights the need to monitor serum sodium during treatment.
These agents are expensive. Tolvaptan costs about $250 per tablet; conivaptan, which is administered intravenously, may cost a little more per treatment course.
THERAPY IN SPECIFIC DISEASE STATES
Patients with hyponatremia and cirrhosis
The focus of treatment remains water and salt restriction and judicious use of loop diuretics and aldosterone antagonists such as spironolactone (Aldactone).
Tolvaptan has been effective at raising the serum sodium level in patients with cirrhosis, 26 while conivaptan should be avoided at present because of vasodilation from V1a receptor antagonism and its potential effects on systemic hemodynamics and risk of variceal bleeding.30
As the severity of cirrhosis increases, the only effective treatment of hyponatremia is liver transplantation.
Patients with SIADH
In most cases, water restriction is the mainstay of therapy. Adequate nutritional intake should also be stressed so that enough solute is available for ongoing water excretion. Although fluid restriction is usually effective, many patients cannot adhere to the level of restriction required.
In cases in which fluid restriction is not effective on its own, demeclocyline can be used to antagonize ADH action and increase water excretion. Sodium tablets and loop diuretics can also be used, taking care to avoid hypovolemia from diuretic-induced sodium losses. The use of tolvaptan in patients with SIADH has resulted in short-term increases in serum sodium.26 A recent study has suggested that this effect can be sustained with longer-term treatment,28 but further studies are needed to show a complementary clinical benefit (eg, improved neurocognition) to guide the use of these costly agents in clinical practice.
Patients with diuretic-induced hyponatremia
Thiazide diuretics should be discontinued and hypovolemia and hypokalemia should be corrected with isotonic saline and potassium supplementation. As the hypokalemia is corrected and the diuretic effect and hypovolemic stimulus to ADH dissipates, water excretion can increase rapidly, resulting in a brisk change in serum sodium.
Serum sodium levels should be closely monitored during therapy to avoid overcorrection. For this reason, use of hypertonic saline should generally be avoided. Hypotonic fluid— eg, half-normal (0.45%) or quarter-normal (0.22%) saline or even desmopressin—may become necessary in the later stages of therapy to avoid overly rapid correction.
Patients with exercise-associated hyponatremia
Patients at highest risk of exercise-associated hyponatremia include those who drink too much fluid during a long-distance race, who have low body weight, who are female, who exercise longer than 4 hours, and who use nonsteroidal anti-inflammatory drugs.31 The cause of hyponatremia is likely multifactorial, with excessive water intake coupled with sodium losses and impaired renal excretion of water due to ADH action and impaired renal dilution. To prevent exercise-associated hyponatremia, fluid intake should be limited to 400 to 800 mL/hour, with the higher end recommended for larger athletes and hotter climates.
Consensus recommendations suggest that most patients with mild hyponatremia (serum sodium 130 to 135 mmol/L) should be treated with fluid restriction and clinical observation, as spontaneous water diuresis leads to improvement in the serum sodium level. Importantly, the reflex to provide isotonic saline infusions should be avoided unless clear signs of volume depletion are present. Intravenous saline has the potential to worsen hyponatremia in the presence of ADH. In addition, some athletes will have retained water in the gastrointestinal tract that may be mobilized after the race, resulting in worsening of hyponatremia.32
In athletes with severe hyponatremia (serum sodium < 120 mmol/L) or symptomatic exercise-associated hyponatremia (lethargy, respiratory depression, seizures), hypertonic saline is the treatment of choice. One protocol suggests giving 100 mL of 3% saline over 10 minutes in the field, followed by prompt transportation to hospital.33
SUMMARY POINTS
- Hyponatremia is a common electrolyte disorder that in its most severe form requires urgent therapy with hypertonic saline to correct cerebral edema.
- In patients without serious signs or symptoms of cerebral edema, recent observations suggest there may be clinically important symptomatology relating to mild neurocognitive dysfunction and an association with risk of bone fracture.
- Multiple treatment strategies are available according to the underlying extracellular fluid volume status and cause of hyponatremia. These include fluid and sodium restriction and augmentation of urinary water excretion with various nutritional and pharmacologic strategies. The most novel therapy includes antagonism of the vasopressin V2 receptor with a class of aquaretic agents known as vaptans.
- There can be serious neurologic injury associated with overly rapid correction of chronic hyponatremia or undercorrection of acute symptomatic hyponatremia.
- Clinicians must be familiar with the details of each of the treatments and have an appreciation of the importance of careful monitoring during treatment.
- Flear CTG, Gill GV, Burn J. Hyponatremia: mechanisms and management. Lancet 1981; 2:26–31.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med 2007; 120(suppl 1):S1–S21.
- Freda BJ, Davidson MB, Hall PM. Evaluation of hyponatremia: a little physiology goes a long way. Cleve Clin J Med 2004; 71:639–650.
- Weisberg LS. Pseudohyponatremia: a reappraisal. Am J Med 1989; 86:315–318.
- Moritz L, Ayus JC. The pathophysiology and treatment of hyponatraemic encephalopathy: an update. Nephrol Dial Transplant 2003; 18:2486–2491.
- Widdess-Walsh P, Sabharwal V, Demirjian S, DeGeorgia M. Neurologic effects of hyponatremia and its treatment. Cleve Clin J Med 2007; 74:377–383.
- Melton JE, Patlak CS, Pettigrew KD, Cserr HF. Volume regulatory loss of Na, Cl, and K from rat brain during acute hyponatremia. Am J Physiol 1987; 252:F661–F669.
- Lauriat SM, Berl T. The hyponatremic patient: practical focus on therapy. J Am Soc Nephrol 1997; 8:1599–1607.
- Kokko JP. Symptomatic hyponatremia with hypoxia is a medical emergency. Kidney Int 2006; 69:1291–1293.
- Ellis SJ. Severe hyponatraemia: complications and treatment. QJM 1995; 88:905–909.
- Sterns RH, Cappuccio JD, Silver SM, Cohen EP. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. J Am Soc Nephrol 1994; 4:1522–1530.
- Perianayagam A, Sterns RH, Silver SM, et al. DDAVP is effective in preventing and reversing inadvertent overcorrection of hyponatremia. Clin J Am Soc Nephrol 2008; 3:331–336.
- Sterns RH, Hix JK. Overcorrection of hyponatremia is a medical emergency. Kidney Int 2009; 76:587–589.
- Nguyen MK, Kurtz I. Analysis of current formulas used for treatment of the dysnatremias. Clin Exp Nephrol 2004; 8:12–16.
- Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med 2000; 342:1581–1589.
- Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH. Hypertonic saline for hyponatremia: risk of inadvertent overcorrection. Clin J Am Soc Nephrol 2007; 2:1110–1117.
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 2007; 356:2064–2072.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 2006; 119:71.e1–e8.
- Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol 2010; 5:275–280.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med 1987; 83:905–988.
- Hoorn EJ, Halperin ML, Zietse R. Diagnostic approach to a patient with hyponatraemia: traditional versus physiology-based options. QJM 2005; 98:529–540.
- Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol 2008; 19:1076–1078.
- Carrilho F, Bosch J, Arroyo V, Mas A, Viver J, Rodes J. Renal failure associated with demeclocycline in cirrhosis. Ann Intern Med 1977; 87:195–197.
- Lehrich RW, Greenberg A. When is it appropriate to use vasopressin receptor antagonists? J Am Soc Nephrol 2008; 19:1054–1058.
- Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 2008; 371:1624–1632.
- Schrier RW, Gross P, Gheorghiade M, et al; SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006; 355:2099–2112.
- Konstam MA, Gheorghiade M, Burnett JC, et al; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007; 297:1319–1331.
- Berl T, Quittnat-Pelletier F, Verbalis JG, et al; SALTWATER Investigators. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol 2010; 21:705–712.
- Greenberg A, Lehrich RW. Treatment of chronic hyponatremia: now we know how, but do we know when or if? J Am Soc Nephrol 2010; 21:552–555.
- Greenberg A, Verbalis JG. Vasopressin receptor antagonists. Kidney Int 2006; 69:2124–2130.
- Rosner MH, Kirven J. Exercise-associated hyponatremia. Clin J Am Soc Nephrol 2007; 2:151–161.
- Halperin ML, Kamel KS, Sterns R. Hyponatremia in marathon runners. N Engl J Med 2005; 353:427–428.
- Hew-Butler T, Almond C, Ayus JC, et al; Exercise-Associated Hyponatremia (EAH) Consensus Panel. Consensus statement of the 1st International Exercise-Associated Hyponatremia Consensus Development Conference, Cape Town, South Africa 2005. Clin J Sport Med 2005; 15:208–213.
- Flear CTG, Gill GV, Burn J. Hyponatremia: mechanisms and management. Lancet 1981; 2:26–31.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med 2007; 120(suppl 1):S1–S21.
- Freda BJ, Davidson MB, Hall PM. Evaluation of hyponatremia: a little physiology goes a long way. Cleve Clin J Med 2004; 71:639–650.
- Weisberg LS. Pseudohyponatremia: a reappraisal. Am J Med 1989; 86:315–318.
- Moritz L, Ayus JC. The pathophysiology and treatment of hyponatraemic encephalopathy: an update. Nephrol Dial Transplant 2003; 18:2486–2491.
- Widdess-Walsh P, Sabharwal V, Demirjian S, DeGeorgia M. Neurologic effects of hyponatremia and its treatment. Cleve Clin J Med 2007; 74:377–383.
- Melton JE, Patlak CS, Pettigrew KD, Cserr HF. Volume regulatory loss of Na, Cl, and K from rat brain during acute hyponatremia. Am J Physiol 1987; 252:F661–F669.
- Lauriat SM, Berl T. The hyponatremic patient: practical focus on therapy. J Am Soc Nephrol 1997; 8:1599–1607.
- Kokko JP. Symptomatic hyponatremia with hypoxia is a medical emergency. Kidney Int 2006; 69:1291–1293.
- Ellis SJ. Severe hyponatraemia: complications and treatment. QJM 1995; 88:905–909.
- Sterns RH, Cappuccio JD, Silver SM, Cohen EP. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. J Am Soc Nephrol 1994; 4:1522–1530.
- Perianayagam A, Sterns RH, Silver SM, et al. DDAVP is effective in preventing and reversing inadvertent overcorrection of hyponatremia. Clin J Am Soc Nephrol 2008; 3:331–336.
- Sterns RH, Hix JK. Overcorrection of hyponatremia is a medical emergency. Kidney Int 2009; 76:587–589.
- Nguyen MK, Kurtz I. Analysis of current formulas used for treatment of the dysnatremias. Clin Exp Nephrol 2004; 8:12–16.
- Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med 2000; 342:1581–1589.
- Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH. Hypertonic saline for hyponatremia: risk of inadvertent overcorrection. Clin J Am Soc Nephrol 2007; 2:1110–1117.
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 2007; 356:2064–2072.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 2006; 119:71.e1–e8.
- Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol 2010; 5:275–280.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med 1987; 83:905–988.
- Hoorn EJ, Halperin ML, Zietse R. Diagnostic approach to a patient with hyponatraemia: traditional versus physiology-based options. QJM 2005; 98:529–540.
- Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol 2008; 19:1076–1078.
- Carrilho F, Bosch J, Arroyo V, Mas A, Viver J, Rodes J. Renal failure associated with demeclocycline in cirrhosis. Ann Intern Med 1977; 87:195–197.
- Lehrich RW, Greenberg A. When is it appropriate to use vasopressin receptor antagonists? J Am Soc Nephrol 2008; 19:1054–1058.
- Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 2008; 371:1624–1632.
- Schrier RW, Gross P, Gheorghiade M, et al; SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006; 355:2099–2112.
- Konstam MA, Gheorghiade M, Burnett JC, et al; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007; 297:1319–1331.
- Berl T, Quittnat-Pelletier F, Verbalis JG, et al; SALTWATER Investigators. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol 2010; 21:705–712.
- Greenberg A, Lehrich RW. Treatment of chronic hyponatremia: now we know how, but do we know when or if? J Am Soc Nephrol 2010; 21:552–555.
- Greenberg A, Verbalis JG. Vasopressin receptor antagonists. Kidney Int 2006; 69:2124–2130.
- Rosner MH, Kirven J. Exercise-associated hyponatremia. Clin J Am Soc Nephrol 2007; 2:151–161.
- Halperin ML, Kamel KS, Sterns R. Hyponatremia in marathon runners. N Engl J Med 2005; 353:427–428.
- Hew-Butler T, Almond C, Ayus JC, et al; Exercise-Associated Hyponatremia (EAH) Consensus Panel. Consensus statement of the 1st International Exercise-Associated Hyponatremia Consensus Development Conference, Cape Town, South Africa 2005. Clin J Sport Med 2005; 15:208–213.
KEY POINTS
- Some hyponatremic patients present with acute, life-threatening cerebral edema due to severe hyponatremia. In others, the hyponatremia may be chronic and less severe, causing relatively few symptoms, but representing an important, independent marker of poor prognosis due to an underlying disease (eg, heart failure).
- Even patients with chronic, less severe hyponatremia may have subtle symptoms of neurocognitive dysfunction and a higher risk of bone fractures.
- Overly rapid correction of chronic hyponatremia or undercorrection of acute symptomatic hyponatremia can lead to serious neurologic injury.
- Treatment strategies vary depending on the extracellular fluid volume status and the cause of hyponatremia.
- Vasopressin antagonists (“vaptans”), a new class of aquaretic agents, specifically target the mechanism driving hyponatremia in some patients.
Taking blood pressure: Too important to trust to humans?
The reality of blood pressure measurement is that human beings do not do it very well. The time has come to delegate this job to machines that can do it better.
Several automatic devices are available. Used in physicians’ offices and in patients’ homes, they can eliminate some types of observer error as well as the “white-coat effect,” ie, the tendency of some patients to have higher blood pressure when medical personnel are present than in their natural environment. Since a difference of only a few millimeters of mercury can affect the physician’s decision to start or to modify treatment, measurements that more accurately reflect a person’s average blood pressure are to be desired.
In the following pages, we review the problems that plague blood pressure measurement by human observers, and we describe the advantages of automatic devices.
SHORTCOMINGS OF OFFICE BLOOD PRESSURE MEASUREMENT
For decades, large surveys have provided invaluable information on the prevalence of hypertension, its relationship to cardiovascular disease, and the benefits of treating it.1–3 Unfortunately, the percentage of hypertensive patients whose blood pressure is under control has remained low despite our increased knowledge about hypertension’s diagnosis and therapy.4
In these surveys, blood pressure was measured by auscultation by human observers using mercury or aneroid sphygmomanometers, and most physicians still use this method in clinical practice. But in spite of multiple guidelines for accurate measurement of blood pressure in the office, the overall accuracy and reproducibility of office blood pressure measurements remain poor.5–7
The accuracy of blood pressure measurement with aneroid and mercury manometers is affected by a number of observer errors and patient factors.8,9
Observer errors
Failure to prepare the patient. National guidelines5 state that before having their blood pressure taken, patients should be allowed to sit quietly for at least 5 minutes, which often does not happen. Another error is that clinicians rarely discourage patients from smoking cigarettes or drinking coffee in the 30 minutes prior to measurement.
Equipment and layout problems. Equipment should be properly calibrated and validated. 5 However, even if the sphygmomanometer is periodically calibrated, too often it is mounted on the wall adjacent to the examination table in the examination room, making it difficult to provide a comfortable seat with back and arm support during the reading. The measurement should be done with the patient sitting in a chair (not on an examination table), with feet on the floor and the arm supported at the level of the heart. If the forearm is not supported in the horizontal position and with the cuff at heart level, the blood pressure and heart rate tend to be higher.10 Further, the diastolic blood pressure and heart rate may be misleadingly low with the patient supine rather than seated,11,12 so readings should be taken with the patient sitting.
Miscuffing, ie, the use of a blood pressure cuff that is too large or, more often, too small for the patient’s arm, is a common source of error. The cuff bladder should encircle at least 80% of the arm.5 However, some offices do not have a large blood pressure cuff for overweight patients or a pediatric cuff for children or adults with arms of small circumference. It is recommended that a large blood pressure cuff be used routinely in adults, since a smaller cuff gives falsely high readings in people with large upper arms (circumference > 29 cm).13,14
Digit preference. Many physicians round off the blood pressure to the nearest 5 or 10 mm Hg. This problem may go along with:
Deflating the cuff too rapidly.
Talking to the patient while taking the blood pressure can contribute to higher readings.9
Not taking enough readings. Ideally, at the initial visit, blood pressure should be measured in both arms with the patient seated, and another reading should be taken with the patient standing. The arm with the higher pressure should be used for subsequent readings. Physicians should not make any treatment decisions based on blood pressure during an initial clinic visit, and at least two readings should be taken even on subsequent visits. However, owing to time constraints in busy clinical practices, treatment decisions are often based on single readings or on multiple readings on a single visit.
Discrepancies between observers. The blood pressure readings obtained by the nurse or medical assistant may differ significantly from those obtained by the physician. These differences can be large enough to affect treatment decisions,15,16 and they can be partially corrected by adequate training of all medical personnel who take blood pressure, doctors as well as nurses.
Given that time is tight in busy clinical practices and a trained blood pressure nurse or technician is usually not available, we will probably not see any significant improvement in the accuracy of blood pressure measurement using older technology and current physician practices.
The white-coat effect
Most patients have a higher level of anxiety, and therefore higher blood pressure, in the physician’s office or clinic than in their normal environment (as revealed by ambulatory monitoring or home blood pressure measurements), a phenomenon commonly called the white-coat effect.
Several factors can increase this effect, such as observer-patient interaction during the measurement. The effect tends to be greatest in the initial measurement, but can persist through multiple readings by the doctor or nurse during the same visit.
Whether the white-coat effect is due purely to patient anxiety about an office visit or to a conditioned response has been a point of interest in clinical studies. Regardless, it may result in the misdiagnosis of hypertension or in overestimation of the severity of hypertension and may lead to overly aggressive therapy. Antihypertensive treatment may be unnecessary in the absence of concurrent cardiovascular risk factors.17
“White-coat hypertension” or “isolated office hypertension” is the condition in which a patient who is not on antihypertensive drug therapy has persistently elevated blood pressure in the clinic or office (> 140/90 mm Hg) but normal daytime ambulatory blood pressure (< 135/85 mm Hg).18 Since patients may have an elevated reading when seen for a first office visit, at least several visits are required to establish the diagnosis. Multiple studies have suggested that white-coat hypertension may account for 20% to 25% of the hypertensive population, particularly in older patients, mainly women.19,20
Both white-coat hypertension and the white-coat effect can be avoided by using an automatic and programmable device that can take multiple readings after the clinician leaves the examination room (more about this below).21
MEASURING BLOOD PRESSURE OUTSIDE THE OFFICE
Recent studies have reported that the information obtained by 24-hour ambulatory blood pressure monitoring and by self-measurement of blood pressure in the home more accurately reflects the patient’s risk of future cardiovascular events than do conventional blood pressure measurements taken in the physician’s office. 22–24 Current national guidelines recognize this pattern and the frequent measurement inaccuracies observed in clinical practice, and they are recommending including out-of-office measurements in the diagnosis of hypertension. 25,26
Ambulatory monitoring provides the most accurate measurement of out-of-office blood pressure. With ambulatory monitoring, the normal mean daytime pressure is considered to be lower than 135/85 mm Hg, in contrast to the 140/90 mm Hg cutoff used in the physician’s office with standard aneroid or mercury devices.
Self-monitoring of blood pressure at home has now become widely available with single-measurement oscillometric devices. (Oscillometric means that these devices measure the blood pressure by sensing the oscillations in pressure in the cuff induced by the pulsation of the brachial artery, as opposed to auscultating the Korotkoff sounds.) Blood pressures lower than 135/85 mm Hg outside the clinician’s office are considered normal with these devices.
However, despite its proven value, ambulatory monitoring is neither widely available nor cost-effective for the long-term management of hypertension. Furthermore, few physicians recommend that patients take their blood pressure at home, although the information obtained can be of significant value in the patient’s long-term management.
AUTOMATED MEASUREMENT IN THE OFFICE
In recent years, several automated oscillometric sphygmomanometers have been developed for measuring blood pressure in the office, and more are on the way. These devices can be programmed to take multiple readings without a clinician observer in the examination room, thus reducing the white-coat response.
Omron (Kyoto, Japan) makes several devices, including the HEM-907 and the HEM-705, that have been used in the clinical setting. 21,27–29 They can be programmed to take two or three readings at intervals of 1 to 2 minutes, with up to 5 minutes before the first reading. Unfortunately, data were not recorded with the patient alone in the room in many studies of the Omron devices, even though the devices meet national and international standards for accuracy.
The Microlife Watch BP Office (Microlife, Widnau, Switzerland) is currently undergoing development.30
The BpTRU (BpTRU Medical Devices, Coquitlam, BC, Canada) has enjoyed greater clinical acceptance, since it can take up to five blood pressure readings at intervals of 1 to 5 minutes, and calculates the mean of all five readings, taken with the patient resting comfortably in a quiet room without a clinician present.
The accuracy and durability of the device has been well established. Since the BpTRU self-calibrates between every blood pressure measurement, periodic calibration has not been required. The device can be placed on a table, mounted on the wall, or mounted on a cart if used in several locations in the office.
At Cleveland Clinic, several departments are using the BpTRU on a daily basis. Soon, we will be able to transfer data directly from the BpTRU to our electronic medical record system.
Studies of the BpTRU device
To date, most of the studies of automated office blood pressure measurement have used the BpTRU with the recording interval set at 1 to 2 minutes.
Myers31 used the BpTRU device in 50 hypertensive patients. The physician took the patient’s blood pressure with a mercury sphygmomanometer while the BpTRU device made the first reading, and then he left the room. The next five readings were taken at 2-minute intervals with the patient alone in the room. The mean initial reading by the machine was 162/85 mm Hg; the reading by the physician was 163/86 mm Hg. The third automatic reading was the lowest (averaging 140/84 mm Hg), and the mean of the five automated readings was 142/80 mm Hg, which was significantly lower than the initial reading obtained by the physician (P < .001).
In another study, Myers et al32 compared the measurements obtained by 24-hour ambulatory monitoring and by the BpTRU device (the mean of five readings obtained at 1-minute or 2-minute intervals) in 309 hypertensive patients. The mean blood pressure with the Bp-TRU was 132/75 mm Hg, which correlated well with the mean awake ambulatory blood pressure (134/77 mm Hg; r = 0.62 for the systolic pressure and 0.72 for the diastolic pressure).
We recently reviewed the records of 278 patients seen in our preventive medicine clinic (D.G. Vidt, MD, unpublished data, November 2009). The group included patients with and without established hypertension, and among the hypertensive group, both treated and untreated individuals. We had initially set the device to take readings at 3-minute intervals following the initial nurse-initiated reading. But in view of the recent data on the Bp-TRU using shorter intervals, we also obtained readings in 51 patients with the device set to record at 2-minute intervals, and then in 72 additional patients at 1-minute intervals. In all three groups, blood pressure had stabilized by the third reading after the clinician had left the room. These observations support those reported by Myers et al.31,32 Of particular importance is the observation that the white-coat effect dissipates within 2 to 3 minutes after the clinician leaves the room.33
The shorter measurement intervals can add up in a busy office practice, in which the time relegated to taking blood pressure is often limited.
In fact, waiting 5 minutes between measurements may allow the patient to become too relaxed and the blood pressure to drop too low vis-a-vis the gold standard, ambulatory monitoring. Culleton and colleagues34 compared the blood pressure in 107 hypertensive patients as measured four ways: by the referring physician, by a nurse who was trained to adhere to the protocol of the Canadian Hypertension Education Program, by 24-hour ambulatory monitoring, and by the BpTRU (the mean of five readings obtained at 5-minute intervals). The mean measured values were:
- 150/90 mm Hg by the referring physician
- 139/86 mm Hg by the nurse
- 142/85 mm Hg by ambulatory monitoring
- 132/82 mm Hg by the BpTRU device.
Although the BpTRU reduced the white-coat effect and white-coat hypertension, it underestimated the blood pressure, leading to misclassification of hypertension. Using 140/90 mm Hg as the cutoff for whether the patient was hypertensive and using ambulatory monitoring as the gold standard, the BpTRU misclassified more than half of the patients, agreeing with the classification of hypertensive or not hypertensive by ambulatory monitoring in only 48%. The authors recommended that the BpTRU not be set at 5-minute measurement intervals.34
WHAT ROLE FOR AUTOMATED READINGS IN THE OFFICE?
Although automatic devices, by enabling the physician to leave the room, can eliminate the white-coat effect and white-coat hypertension, physicians must continue to take care to avoid the other potential errors of office blood pressure measurement addressed earlier in this review, for example, by positioning the patient correctly and using a cuff that is large enough. These issues can take on more importance as the clinician leaves the patient alone for brief periods during measurements.
In view of its perennial inaccuracies, some experts have suggested that we abandon routine office measurement of blood pressure.35,36 In its place, ambulatory monitoring would be used for diagnosis and for periodic follow-up. In addition, patients would regularly take their pressure at home with approved, single-measurement oscillometric devices. Unfortunately, in our health care system, periodic ambulatory monitoring for hypertension management would impose a significant financial burden on patients at this time.37
Of particular importance is the observation that the mean of five readings with the BpTRU device, obtained at 1- or 2-minute intervals, closely approximates the mean awake blood pressure obtained in the same patient with an ambulatory monitor.32,38 The ability to obtain readings that correlate exceptionally well with mean daytime ambulatory pressure suggests that this device could well reduce the need for ambulatory monitoring, with its associated cost. The ability to negate the white-coat effect with the use of the BpTRU in the office setting also has particular importance, not only for patient office readings, but for the diagnosis and subsequent treatment of hypertension in individual patients.
Most clinical decisions about the treatment of hypertension are still made on the basis of office determinations of blood pressure. Most office practices still rely on the aneroid manometer or, decreasingly, mercury sphygmomanometers. As noted earlier, although auscultatory blood pressure measurement appears to be simple, it is fraught with a host of observer- or patient-induced errors that not only lead to inaccurate diagnoses, but may also result in the mismanagement of hypertension. Even single-measurement oscillometric devices, now used in a minority of clinical practices, are associated with many of the same measurement issues that lead to overestimation of blood pressure.
We believe the time has come for broader use of oscillometric devices in the outpatient setting. While many available oscillometric devices for use in the home could also be used in the physician’s office, they carry the similar disadvantage of providing only a single measurement. The major disadvantage of all single-measurement devices is the continued presence of the clinician during the reading and the associated white-coat effect observed in most patients.
It is highly likely that the next Joint National Committee Report on Hypertension will further emphasize the role of automated blood pressure devices in the outpatient setting.
Acknowledgment: The authors wish to acknowledge the contributions of Deborah McCoy, RN, and Maria Eckhouse, RN.
- Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995; 25:305–313.
- Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med 1992; 152:56–64.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Grim CM, Grim CE. A curriculum for the training and certification of blood pressure measurement for health care providers. Can J Cardiol 1995; 11(suppl H):38H–42H.
- Pickering TG, Hall JE, Appel LJ, et al; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45:142–161.
- Langlois S. Measuring blood pressure: how competent are we? Perspect Cardiol 1999; 15:29–39.
- Le Pailleur C, Helft G, Landais P, et al. The effects of talking, reading, and silence on the “white coat” phenomenon in hypertensive patients. Am J Hypertens 1998; 11:203–207.
- Webster J, Newnham D, Petrie JC, Lovell HG. Influence of arm position on measurement of blood pressure. Br Med J (Clin Res Ed) 1984; 288:1574–1575.
- Netea RT, Smits P, Lenders JW, Thien T. Does it matter whether blood pressure measurements are taken with subjects sitting or supine? J Hypertens 1998; 16:263–268.
- Silverberg DS, Shemesh E, Iaina A. The unsupported arm: a cause of falsely raised blood pressure readings. Br Med J 1977; 2:1331.
- Manning DM, Kuchirka C, Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation 1983; 68:763–766.
- Iyriboz Y, Hearon CM, Edwards K. Agreement between large and small cuffs in sphygmomanometry: a quantitative assessment. J Clin Monit 1994; 10:127–133.
- Scherwitz LW, Evans LA, Hennrikus DJ, Vallbona C. Procedures and discrepancies of blood pressure measurements in two community health centers. Med Care 1982; 20:727–738.
- La Batide-Alanore A, Chatellier G, Bobrie G, Fofol I, Plouin PF. Comparison of nurse- and physician-determined clinic blood pressure levels in patients referred to a hypertension clinic: implications for subsequent management. J Hypertens 2000; 18:391–398.
- Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000; 35:844–851.
- Ogedegbe G, Pickering TG, Clemow L, et al. The misdiagnosis of hypertension: the role of patient anxiety. Arch Intern Med 2008; 168:2459–2465.
- Pickering TG. Stress, white coat hypertension and masked hypertension. In:Izzo JL, Sica DA, Black HR, editors. Hypertension Primer: The Essentials of High Blood Pressure: Basic Science, Population Science, and Clinical Management. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:289–291.
- Pickering TG, Coats A, Mallion JM, Mancia G, Verdecchia P. Blood Pressure Monitoring. Task force V: White-coat hypertension. Blood Press Monit 1999; 4:333–341.
- Myers MG, Valdivieso MA. Use of an automated blood pressure recording device, the BpTRU, to reduce the “white coat effect” in routine practice. Am J Hypertens 2003; 16:494–497.
- Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension 1998; 31:712–718.
- Ohkubo T, Imai Y, Tsuji I, et al. Prediction of mortality by ambulatory blood pressure monitoring versus screening blood pressure measurements: a pilot study in Ohasama. J Hypertens 1997; 15:357–364.
- Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002; 39:878–885.
- Hemmelgarn BR, McAllister FA, Myers MG, et al; Canadian Hypertension Education Program. The 2005 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1 - blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol 2005; 21:645–656.
- Pickering TG. JNC 7.5. J Clin Hypertens (Greenwich) 2007; 9:901–904.
- White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit 2001; 6:107–110.
- Myers MG, Meglis G, Polemidiotis G. The impact of physician vs automated blood pressure readings on office-induced hypertension. J Hum Hypertens 1997; 11:491–493.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension 2010; 55:195–200.
- Stergiou GS, Tzamouranis D, Protogerou A, Nasothimiou E, Kapralos C. Validation of the Microlife Watch BP Office professional device for office blood pressure measurement according to the International protocol. Blood Press Monit 2008; 13:299–303.
- Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit 2006; 11:59–62.
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit 2008; 13:333–338.
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009; 27:280–286.
- Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit 2006; 11:37–42.
- Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D; American Heart Association. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 2008; 52:1–9.
- Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 2008; 26:1505–1526.
- O’Brien E. Ambulatory blood pressure measurement: the case for implementation in primary care. Hypertension 2008; 51:1435–1441.
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord 2005; 5:18.
The reality of blood pressure measurement is that human beings do not do it very well. The time has come to delegate this job to machines that can do it better.
Several automatic devices are available. Used in physicians’ offices and in patients’ homes, they can eliminate some types of observer error as well as the “white-coat effect,” ie, the tendency of some patients to have higher blood pressure when medical personnel are present than in their natural environment. Since a difference of only a few millimeters of mercury can affect the physician’s decision to start or to modify treatment, measurements that more accurately reflect a person’s average blood pressure are to be desired.
In the following pages, we review the problems that plague blood pressure measurement by human observers, and we describe the advantages of automatic devices.
SHORTCOMINGS OF OFFICE BLOOD PRESSURE MEASUREMENT
For decades, large surveys have provided invaluable information on the prevalence of hypertension, its relationship to cardiovascular disease, and the benefits of treating it.1–3 Unfortunately, the percentage of hypertensive patients whose blood pressure is under control has remained low despite our increased knowledge about hypertension’s diagnosis and therapy.4
In these surveys, blood pressure was measured by auscultation by human observers using mercury or aneroid sphygmomanometers, and most physicians still use this method in clinical practice. But in spite of multiple guidelines for accurate measurement of blood pressure in the office, the overall accuracy and reproducibility of office blood pressure measurements remain poor.5–7
The accuracy of blood pressure measurement with aneroid and mercury manometers is affected by a number of observer errors and patient factors.8,9
Observer errors
Failure to prepare the patient. National guidelines5 state that before having their blood pressure taken, patients should be allowed to sit quietly for at least 5 minutes, which often does not happen. Another error is that clinicians rarely discourage patients from smoking cigarettes or drinking coffee in the 30 minutes prior to measurement.
Equipment and layout problems. Equipment should be properly calibrated and validated. 5 However, even if the sphygmomanometer is periodically calibrated, too often it is mounted on the wall adjacent to the examination table in the examination room, making it difficult to provide a comfortable seat with back and arm support during the reading. The measurement should be done with the patient sitting in a chair (not on an examination table), with feet on the floor and the arm supported at the level of the heart. If the forearm is not supported in the horizontal position and with the cuff at heart level, the blood pressure and heart rate tend to be higher.10 Further, the diastolic blood pressure and heart rate may be misleadingly low with the patient supine rather than seated,11,12 so readings should be taken with the patient sitting.
Miscuffing, ie, the use of a blood pressure cuff that is too large or, more often, too small for the patient’s arm, is a common source of error. The cuff bladder should encircle at least 80% of the arm.5 However, some offices do not have a large blood pressure cuff for overweight patients or a pediatric cuff for children or adults with arms of small circumference. It is recommended that a large blood pressure cuff be used routinely in adults, since a smaller cuff gives falsely high readings in people with large upper arms (circumference > 29 cm).13,14
Digit preference. Many physicians round off the blood pressure to the nearest 5 or 10 mm Hg. This problem may go along with:
Deflating the cuff too rapidly.
Talking to the patient while taking the blood pressure can contribute to higher readings.9
Not taking enough readings. Ideally, at the initial visit, blood pressure should be measured in both arms with the patient seated, and another reading should be taken with the patient standing. The arm with the higher pressure should be used for subsequent readings. Physicians should not make any treatment decisions based on blood pressure during an initial clinic visit, and at least two readings should be taken even on subsequent visits. However, owing to time constraints in busy clinical practices, treatment decisions are often based on single readings or on multiple readings on a single visit.
Discrepancies between observers. The blood pressure readings obtained by the nurse or medical assistant may differ significantly from those obtained by the physician. These differences can be large enough to affect treatment decisions,15,16 and they can be partially corrected by adequate training of all medical personnel who take blood pressure, doctors as well as nurses.
Given that time is tight in busy clinical practices and a trained blood pressure nurse or technician is usually not available, we will probably not see any significant improvement in the accuracy of blood pressure measurement using older technology and current physician practices.
The white-coat effect
Most patients have a higher level of anxiety, and therefore higher blood pressure, in the physician’s office or clinic than in their normal environment (as revealed by ambulatory monitoring or home blood pressure measurements), a phenomenon commonly called the white-coat effect.
Several factors can increase this effect, such as observer-patient interaction during the measurement. The effect tends to be greatest in the initial measurement, but can persist through multiple readings by the doctor or nurse during the same visit.
Whether the white-coat effect is due purely to patient anxiety about an office visit or to a conditioned response has been a point of interest in clinical studies. Regardless, it may result in the misdiagnosis of hypertension or in overestimation of the severity of hypertension and may lead to overly aggressive therapy. Antihypertensive treatment may be unnecessary in the absence of concurrent cardiovascular risk factors.17
“White-coat hypertension” or “isolated office hypertension” is the condition in which a patient who is not on antihypertensive drug therapy has persistently elevated blood pressure in the clinic or office (> 140/90 mm Hg) but normal daytime ambulatory blood pressure (< 135/85 mm Hg).18 Since patients may have an elevated reading when seen for a first office visit, at least several visits are required to establish the diagnosis. Multiple studies have suggested that white-coat hypertension may account for 20% to 25% of the hypertensive population, particularly in older patients, mainly women.19,20
Both white-coat hypertension and the white-coat effect can be avoided by using an automatic and programmable device that can take multiple readings after the clinician leaves the examination room (more about this below).21
MEASURING BLOOD PRESSURE OUTSIDE THE OFFICE
Recent studies have reported that the information obtained by 24-hour ambulatory blood pressure monitoring and by self-measurement of blood pressure in the home more accurately reflects the patient’s risk of future cardiovascular events than do conventional blood pressure measurements taken in the physician’s office. 22–24 Current national guidelines recognize this pattern and the frequent measurement inaccuracies observed in clinical practice, and they are recommending including out-of-office measurements in the diagnosis of hypertension. 25,26
Ambulatory monitoring provides the most accurate measurement of out-of-office blood pressure. With ambulatory monitoring, the normal mean daytime pressure is considered to be lower than 135/85 mm Hg, in contrast to the 140/90 mm Hg cutoff used in the physician’s office with standard aneroid or mercury devices.
Self-monitoring of blood pressure at home has now become widely available with single-measurement oscillometric devices. (Oscillometric means that these devices measure the blood pressure by sensing the oscillations in pressure in the cuff induced by the pulsation of the brachial artery, as opposed to auscultating the Korotkoff sounds.) Blood pressures lower than 135/85 mm Hg outside the clinician’s office are considered normal with these devices.
However, despite its proven value, ambulatory monitoring is neither widely available nor cost-effective for the long-term management of hypertension. Furthermore, few physicians recommend that patients take their blood pressure at home, although the information obtained can be of significant value in the patient’s long-term management.
AUTOMATED MEASUREMENT IN THE OFFICE
In recent years, several automated oscillometric sphygmomanometers have been developed for measuring blood pressure in the office, and more are on the way. These devices can be programmed to take multiple readings without a clinician observer in the examination room, thus reducing the white-coat response.
Omron (Kyoto, Japan) makes several devices, including the HEM-907 and the HEM-705, that have been used in the clinical setting. 21,27–29 They can be programmed to take two or three readings at intervals of 1 to 2 minutes, with up to 5 minutes before the first reading. Unfortunately, data were not recorded with the patient alone in the room in many studies of the Omron devices, even though the devices meet national and international standards for accuracy.
The Microlife Watch BP Office (Microlife, Widnau, Switzerland) is currently undergoing development.30
The BpTRU (BpTRU Medical Devices, Coquitlam, BC, Canada) has enjoyed greater clinical acceptance, since it can take up to five blood pressure readings at intervals of 1 to 5 minutes, and calculates the mean of all five readings, taken with the patient resting comfortably in a quiet room without a clinician present.
The accuracy and durability of the device has been well established. Since the BpTRU self-calibrates between every blood pressure measurement, periodic calibration has not been required. The device can be placed on a table, mounted on the wall, or mounted on a cart if used in several locations in the office.
At Cleveland Clinic, several departments are using the BpTRU on a daily basis. Soon, we will be able to transfer data directly from the BpTRU to our electronic medical record system.
Studies of the BpTRU device
To date, most of the studies of automated office blood pressure measurement have used the BpTRU with the recording interval set at 1 to 2 minutes.
Myers31 used the BpTRU device in 50 hypertensive patients. The physician took the patient’s blood pressure with a mercury sphygmomanometer while the BpTRU device made the first reading, and then he left the room. The next five readings were taken at 2-minute intervals with the patient alone in the room. The mean initial reading by the machine was 162/85 mm Hg; the reading by the physician was 163/86 mm Hg. The third automatic reading was the lowest (averaging 140/84 mm Hg), and the mean of the five automated readings was 142/80 mm Hg, which was significantly lower than the initial reading obtained by the physician (P < .001).
In another study, Myers et al32 compared the measurements obtained by 24-hour ambulatory monitoring and by the BpTRU device (the mean of five readings obtained at 1-minute or 2-minute intervals) in 309 hypertensive patients. The mean blood pressure with the Bp-TRU was 132/75 mm Hg, which correlated well with the mean awake ambulatory blood pressure (134/77 mm Hg; r = 0.62 for the systolic pressure and 0.72 for the diastolic pressure).
We recently reviewed the records of 278 patients seen in our preventive medicine clinic (D.G. Vidt, MD, unpublished data, November 2009). The group included patients with and without established hypertension, and among the hypertensive group, both treated and untreated individuals. We had initially set the device to take readings at 3-minute intervals following the initial nurse-initiated reading. But in view of the recent data on the Bp-TRU using shorter intervals, we also obtained readings in 51 patients with the device set to record at 2-minute intervals, and then in 72 additional patients at 1-minute intervals. In all three groups, blood pressure had stabilized by the third reading after the clinician had left the room. These observations support those reported by Myers et al.31,32 Of particular importance is the observation that the white-coat effect dissipates within 2 to 3 minutes after the clinician leaves the room.33
The shorter measurement intervals can add up in a busy office practice, in which the time relegated to taking blood pressure is often limited.
In fact, waiting 5 minutes between measurements may allow the patient to become too relaxed and the blood pressure to drop too low vis-a-vis the gold standard, ambulatory monitoring. Culleton and colleagues34 compared the blood pressure in 107 hypertensive patients as measured four ways: by the referring physician, by a nurse who was trained to adhere to the protocol of the Canadian Hypertension Education Program, by 24-hour ambulatory monitoring, and by the BpTRU (the mean of five readings obtained at 5-minute intervals). The mean measured values were:
- 150/90 mm Hg by the referring physician
- 139/86 mm Hg by the nurse
- 142/85 mm Hg by ambulatory monitoring
- 132/82 mm Hg by the BpTRU device.
Although the BpTRU reduced the white-coat effect and white-coat hypertension, it underestimated the blood pressure, leading to misclassification of hypertension. Using 140/90 mm Hg as the cutoff for whether the patient was hypertensive and using ambulatory monitoring as the gold standard, the BpTRU misclassified more than half of the patients, agreeing with the classification of hypertensive or not hypertensive by ambulatory monitoring in only 48%. The authors recommended that the BpTRU not be set at 5-minute measurement intervals.34
WHAT ROLE FOR AUTOMATED READINGS IN THE OFFICE?
Although automatic devices, by enabling the physician to leave the room, can eliminate the white-coat effect and white-coat hypertension, physicians must continue to take care to avoid the other potential errors of office blood pressure measurement addressed earlier in this review, for example, by positioning the patient correctly and using a cuff that is large enough. These issues can take on more importance as the clinician leaves the patient alone for brief periods during measurements.
In view of its perennial inaccuracies, some experts have suggested that we abandon routine office measurement of blood pressure.35,36 In its place, ambulatory monitoring would be used for diagnosis and for periodic follow-up. In addition, patients would regularly take their pressure at home with approved, single-measurement oscillometric devices. Unfortunately, in our health care system, periodic ambulatory monitoring for hypertension management would impose a significant financial burden on patients at this time.37
Of particular importance is the observation that the mean of five readings with the BpTRU device, obtained at 1- or 2-minute intervals, closely approximates the mean awake blood pressure obtained in the same patient with an ambulatory monitor.32,38 The ability to obtain readings that correlate exceptionally well with mean daytime ambulatory pressure suggests that this device could well reduce the need for ambulatory monitoring, with its associated cost. The ability to negate the white-coat effect with the use of the BpTRU in the office setting also has particular importance, not only for patient office readings, but for the diagnosis and subsequent treatment of hypertension in individual patients.
Most clinical decisions about the treatment of hypertension are still made on the basis of office determinations of blood pressure. Most office practices still rely on the aneroid manometer or, decreasingly, mercury sphygmomanometers. As noted earlier, although auscultatory blood pressure measurement appears to be simple, it is fraught with a host of observer- or patient-induced errors that not only lead to inaccurate diagnoses, but may also result in the mismanagement of hypertension. Even single-measurement oscillometric devices, now used in a minority of clinical practices, are associated with many of the same measurement issues that lead to overestimation of blood pressure.
We believe the time has come for broader use of oscillometric devices in the outpatient setting. While many available oscillometric devices for use in the home could also be used in the physician’s office, they carry the similar disadvantage of providing only a single measurement. The major disadvantage of all single-measurement devices is the continued presence of the clinician during the reading and the associated white-coat effect observed in most patients.
It is highly likely that the next Joint National Committee Report on Hypertension will further emphasize the role of automated blood pressure devices in the outpatient setting.
Acknowledgment: The authors wish to acknowledge the contributions of Deborah McCoy, RN, and Maria Eckhouse, RN.
The reality of blood pressure measurement is that human beings do not do it very well. The time has come to delegate this job to machines that can do it better.
Several automatic devices are available. Used in physicians’ offices and in patients’ homes, they can eliminate some types of observer error as well as the “white-coat effect,” ie, the tendency of some patients to have higher blood pressure when medical personnel are present than in their natural environment. Since a difference of only a few millimeters of mercury can affect the physician’s decision to start or to modify treatment, measurements that more accurately reflect a person’s average blood pressure are to be desired.
In the following pages, we review the problems that plague blood pressure measurement by human observers, and we describe the advantages of automatic devices.
SHORTCOMINGS OF OFFICE BLOOD PRESSURE MEASUREMENT
For decades, large surveys have provided invaluable information on the prevalence of hypertension, its relationship to cardiovascular disease, and the benefits of treating it.1–3 Unfortunately, the percentage of hypertensive patients whose blood pressure is under control has remained low despite our increased knowledge about hypertension’s diagnosis and therapy.4
In these surveys, blood pressure was measured by auscultation by human observers using mercury or aneroid sphygmomanometers, and most physicians still use this method in clinical practice. But in spite of multiple guidelines for accurate measurement of blood pressure in the office, the overall accuracy and reproducibility of office blood pressure measurements remain poor.5–7
The accuracy of blood pressure measurement with aneroid and mercury manometers is affected by a number of observer errors and patient factors.8,9
Observer errors
Failure to prepare the patient. National guidelines5 state that before having their blood pressure taken, patients should be allowed to sit quietly for at least 5 minutes, which often does not happen. Another error is that clinicians rarely discourage patients from smoking cigarettes or drinking coffee in the 30 minutes prior to measurement.
Equipment and layout problems. Equipment should be properly calibrated and validated. 5 However, even if the sphygmomanometer is periodically calibrated, too often it is mounted on the wall adjacent to the examination table in the examination room, making it difficult to provide a comfortable seat with back and arm support during the reading. The measurement should be done with the patient sitting in a chair (not on an examination table), with feet on the floor and the arm supported at the level of the heart. If the forearm is not supported in the horizontal position and with the cuff at heart level, the blood pressure and heart rate tend to be higher.10 Further, the diastolic blood pressure and heart rate may be misleadingly low with the patient supine rather than seated,11,12 so readings should be taken with the patient sitting.
Miscuffing, ie, the use of a blood pressure cuff that is too large or, more often, too small for the patient’s arm, is a common source of error. The cuff bladder should encircle at least 80% of the arm.5 However, some offices do not have a large blood pressure cuff for overweight patients or a pediatric cuff for children or adults with arms of small circumference. It is recommended that a large blood pressure cuff be used routinely in adults, since a smaller cuff gives falsely high readings in people with large upper arms (circumference > 29 cm).13,14
Digit preference. Many physicians round off the blood pressure to the nearest 5 or 10 mm Hg. This problem may go along with:
Deflating the cuff too rapidly.
Talking to the patient while taking the blood pressure can contribute to higher readings.9
Not taking enough readings. Ideally, at the initial visit, blood pressure should be measured in both arms with the patient seated, and another reading should be taken with the patient standing. The arm with the higher pressure should be used for subsequent readings. Physicians should not make any treatment decisions based on blood pressure during an initial clinic visit, and at least two readings should be taken even on subsequent visits. However, owing to time constraints in busy clinical practices, treatment decisions are often based on single readings or on multiple readings on a single visit.
Discrepancies between observers. The blood pressure readings obtained by the nurse or medical assistant may differ significantly from those obtained by the physician. These differences can be large enough to affect treatment decisions,15,16 and they can be partially corrected by adequate training of all medical personnel who take blood pressure, doctors as well as nurses.
Given that time is tight in busy clinical practices and a trained blood pressure nurse or technician is usually not available, we will probably not see any significant improvement in the accuracy of blood pressure measurement using older technology and current physician practices.
The white-coat effect
Most patients have a higher level of anxiety, and therefore higher blood pressure, in the physician’s office or clinic than in their normal environment (as revealed by ambulatory monitoring or home blood pressure measurements), a phenomenon commonly called the white-coat effect.
Several factors can increase this effect, such as observer-patient interaction during the measurement. The effect tends to be greatest in the initial measurement, but can persist through multiple readings by the doctor or nurse during the same visit.
Whether the white-coat effect is due purely to patient anxiety about an office visit or to a conditioned response has been a point of interest in clinical studies. Regardless, it may result in the misdiagnosis of hypertension or in overestimation of the severity of hypertension and may lead to overly aggressive therapy. Antihypertensive treatment may be unnecessary in the absence of concurrent cardiovascular risk factors.17
“White-coat hypertension” or “isolated office hypertension” is the condition in which a patient who is not on antihypertensive drug therapy has persistently elevated blood pressure in the clinic or office (> 140/90 mm Hg) but normal daytime ambulatory blood pressure (< 135/85 mm Hg).18 Since patients may have an elevated reading when seen for a first office visit, at least several visits are required to establish the diagnosis. Multiple studies have suggested that white-coat hypertension may account for 20% to 25% of the hypertensive population, particularly in older patients, mainly women.19,20
Both white-coat hypertension and the white-coat effect can be avoided by using an automatic and programmable device that can take multiple readings after the clinician leaves the examination room (more about this below).21
MEASURING BLOOD PRESSURE OUTSIDE THE OFFICE
Recent studies have reported that the information obtained by 24-hour ambulatory blood pressure monitoring and by self-measurement of blood pressure in the home more accurately reflects the patient’s risk of future cardiovascular events than do conventional blood pressure measurements taken in the physician’s office. 22–24 Current national guidelines recognize this pattern and the frequent measurement inaccuracies observed in clinical practice, and they are recommending including out-of-office measurements in the diagnosis of hypertension. 25,26
Ambulatory monitoring provides the most accurate measurement of out-of-office blood pressure. With ambulatory monitoring, the normal mean daytime pressure is considered to be lower than 135/85 mm Hg, in contrast to the 140/90 mm Hg cutoff used in the physician’s office with standard aneroid or mercury devices.
Self-monitoring of blood pressure at home has now become widely available with single-measurement oscillometric devices. (Oscillometric means that these devices measure the blood pressure by sensing the oscillations in pressure in the cuff induced by the pulsation of the brachial artery, as opposed to auscultating the Korotkoff sounds.) Blood pressures lower than 135/85 mm Hg outside the clinician’s office are considered normal with these devices.
However, despite its proven value, ambulatory monitoring is neither widely available nor cost-effective for the long-term management of hypertension. Furthermore, few physicians recommend that patients take their blood pressure at home, although the information obtained can be of significant value in the patient’s long-term management.
AUTOMATED MEASUREMENT IN THE OFFICE
In recent years, several automated oscillometric sphygmomanometers have been developed for measuring blood pressure in the office, and more are on the way. These devices can be programmed to take multiple readings without a clinician observer in the examination room, thus reducing the white-coat response.
Omron (Kyoto, Japan) makes several devices, including the HEM-907 and the HEM-705, that have been used in the clinical setting. 21,27–29 They can be programmed to take two or three readings at intervals of 1 to 2 minutes, with up to 5 minutes before the first reading. Unfortunately, data were not recorded with the patient alone in the room in many studies of the Omron devices, even though the devices meet national and international standards for accuracy.
The Microlife Watch BP Office (Microlife, Widnau, Switzerland) is currently undergoing development.30
The BpTRU (BpTRU Medical Devices, Coquitlam, BC, Canada) has enjoyed greater clinical acceptance, since it can take up to five blood pressure readings at intervals of 1 to 5 minutes, and calculates the mean of all five readings, taken with the patient resting comfortably in a quiet room without a clinician present.
The accuracy and durability of the device has been well established. Since the BpTRU self-calibrates between every blood pressure measurement, periodic calibration has not been required. The device can be placed on a table, mounted on the wall, or mounted on a cart if used in several locations in the office.
At Cleveland Clinic, several departments are using the BpTRU on a daily basis. Soon, we will be able to transfer data directly from the BpTRU to our electronic medical record system.
Studies of the BpTRU device
To date, most of the studies of automated office blood pressure measurement have used the BpTRU with the recording interval set at 1 to 2 minutes.
Myers31 used the BpTRU device in 50 hypertensive patients. The physician took the patient’s blood pressure with a mercury sphygmomanometer while the BpTRU device made the first reading, and then he left the room. The next five readings were taken at 2-minute intervals with the patient alone in the room. The mean initial reading by the machine was 162/85 mm Hg; the reading by the physician was 163/86 mm Hg. The third automatic reading was the lowest (averaging 140/84 mm Hg), and the mean of the five automated readings was 142/80 mm Hg, which was significantly lower than the initial reading obtained by the physician (P < .001).
In another study, Myers et al32 compared the measurements obtained by 24-hour ambulatory monitoring and by the BpTRU device (the mean of five readings obtained at 1-minute or 2-minute intervals) in 309 hypertensive patients. The mean blood pressure with the Bp-TRU was 132/75 mm Hg, which correlated well with the mean awake ambulatory blood pressure (134/77 mm Hg; r = 0.62 for the systolic pressure and 0.72 for the diastolic pressure).
We recently reviewed the records of 278 patients seen in our preventive medicine clinic (D.G. Vidt, MD, unpublished data, November 2009). The group included patients with and without established hypertension, and among the hypertensive group, both treated and untreated individuals. We had initially set the device to take readings at 3-minute intervals following the initial nurse-initiated reading. But in view of the recent data on the Bp-TRU using shorter intervals, we also obtained readings in 51 patients with the device set to record at 2-minute intervals, and then in 72 additional patients at 1-minute intervals. In all three groups, blood pressure had stabilized by the third reading after the clinician had left the room. These observations support those reported by Myers et al.31,32 Of particular importance is the observation that the white-coat effect dissipates within 2 to 3 minutes after the clinician leaves the room.33
The shorter measurement intervals can add up in a busy office practice, in which the time relegated to taking blood pressure is often limited.
In fact, waiting 5 minutes between measurements may allow the patient to become too relaxed and the blood pressure to drop too low vis-a-vis the gold standard, ambulatory monitoring. Culleton and colleagues34 compared the blood pressure in 107 hypertensive patients as measured four ways: by the referring physician, by a nurse who was trained to adhere to the protocol of the Canadian Hypertension Education Program, by 24-hour ambulatory monitoring, and by the BpTRU (the mean of five readings obtained at 5-minute intervals). The mean measured values were:
- 150/90 mm Hg by the referring physician
- 139/86 mm Hg by the nurse
- 142/85 mm Hg by ambulatory monitoring
- 132/82 mm Hg by the BpTRU device.
Although the BpTRU reduced the white-coat effect and white-coat hypertension, it underestimated the blood pressure, leading to misclassification of hypertension. Using 140/90 mm Hg as the cutoff for whether the patient was hypertensive and using ambulatory monitoring as the gold standard, the BpTRU misclassified more than half of the patients, agreeing with the classification of hypertensive or not hypertensive by ambulatory monitoring in only 48%. The authors recommended that the BpTRU not be set at 5-minute measurement intervals.34
WHAT ROLE FOR AUTOMATED READINGS IN THE OFFICE?
Although automatic devices, by enabling the physician to leave the room, can eliminate the white-coat effect and white-coat hypertension, physicians must continue to take care to avoid the other potential errors of office blood pressure measurement addressed earlier in this review, for example, by positioning the patient correctly and using a cuff that is large enough. These issues can take on more importance as the clinician leaves the patient alone for brief periods during measurements.
In view of its perennial inaccuracies, some experts have suggested that we abandon routine office measurement of blood pressure.35,36 In its place, ambulatory monitoring would be used for diagnosis and for periodic follow-up. In addition, patients would regularly take their pressure at home with approved, single-measurement oscillometric devices. Unfortunately, in our health care system, periodic ambulatory monitoring for hypertension management would impose a significant financial burden on patients at this time.37
Of particular importance is the observation that the mean of five readings with the BpTRU device, obtained at 1- or 2-minute intervals, closely approximates the mean awake blood pressure obtained in the same patient with an ambulatory monitor.32,38 The ability to obtain readings that correlate exceptionally well with mean daytime ambulatory pressure suggests that this device could well reduce the need for ambulatory monitoring, with its associated cost. The ability to negate the white-coat effect with the use of the BpTRU in the office setting also has particular importance, not only for patient office readings, but for the diagnosis and subsequent treatment of hypertension in individual patients.
Most clinical decisions about the treatment of hypertension are still made on the basis of office determinations of blood pressure. Most office practices still rely on the aneroid manometer or, decreasingly, mercury sphygmomanometers. As noted earlier, although auscultatory blood pressure measurement appears to be simple, it is fraught with a host of observer- or patient-induced errors that not only lead to inaccurate diagnoses, but may also result in the mismanagement of hypertension. Even single-measurement oscillometric devices, now used in a minority of clinical practices, are associated with many of the same measurement issues that lead to overestimation of blood pressure.
We believe the time has come for broader use of oscillometric devices in the outpatient setting. While many available oscillometric devices for use in the home could also be used in the physician’s office, they carry the similar disadvantage of providing only a single measurement. The major disadvantage of all single-measurement devices is the continued presence of the clinician during the reading and the associated white-coat effect observed in most patients.
It is highly likely that the next Joint National Committee Report on Hypertension will further emphasize the role of automated blood pressure devices in the outpatient setting.
Acknowledgment: The authors wish to acknowledge the contributions of Deborah McCoy, RN, and Maria Eckhouse, RN.
- Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995; 25:305–313.
- Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med 1992; 152:56–64.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Grim CM, Grim CE. A curriculum for the training and certification of blood pressure measurement for health care providers. Can J Cardiol 1995; 11(suppl H):38H–42H.
- Pickering TG, Hall JE, Appel LJ, et al; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45:142–161.
- Langlois S. Measuring blood pressure: how competent are we? Perspect Cardiol 1999; 15:29–39.
- Le Pailleur C, Helft G, Landais P, et al. The effects of talking, reading, and silence on the “white coat” phenomenon in hypertensive patients. Am J Hypertens 1998; 11:203–207.
- Webster J, Newnham D, Petrie JC, Lovell HG. Influence of arm position on measurement of blood pressure. Br Med J (Clin Res Ed) 1984; 288:1574–1575.
- Netea RT, Smits P, Lenders JW, Thien T. Does it matter whether blood pressure measurements are taken with subjects sitting or supine? J Hypertens 1998; 16:263–268.
- Silverberg DS, Shemesh E, Iaina A. The unsupported arm: a cause of falsely raised blood pressure readings. Br Med J 1977; 2:1331.
- Manning DM, Kuchirka C, Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation 1983; 68:763–766.
- Iyriboz Y, Hearon CM, Edwards K. Agreement between large and small cuffs in sphygmomanometry: a quantitative assessment. J Clin Monit 1994; 10:127–133.
- Scherwitz LW, Evans LA, Hennrikus DJ, Vallbona C. Procedures and discrepancies of blood pressure measurements in two community health centers. Med Care 1982; 20:727–738.
- La Batide-Alanore A, Chatellier G, Bobrie G, Fofol I, Plouin PF. Comparison of nurse- and physician-determined clinic blood pressure levels in patients referred to a hypertension clinic: implications for subsequent management. J Hypertens 2000; 18:391–398.
- Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000; 35:844–851.
- Ogedegbe G, Pickering TG, Clemow L, et al. The misdiagnosis of hypertension: the role of patient anxiety. Arch Intern Med 2008; 168:2459–2465.
- Pickering TG. Stress, white coat hypertension and masked hypertension. In:Izzo JL, Sica DA, Black HR, editors. Hypertension Primer: The Essentials of High Blood Pressure: Basic Science, Population Science, and Clinical Management. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:289–291.
- Pickering TG, Coats A, Mallion JM, Mancia G, Verdecchia P. Blood Pressure Monitoring. Task force V: White-coat hypertension. Blood Press Monit 1999; 4:333–341.
- Myers MG, Valdivieso MA. Use of an automated blood pressure recording device, the BpTRU, to reduce the “white coat effect” in routine practice. Am J Hypertens 2003; 16:494–497.
- Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension 1998; 31:712–718.
- Ohkubo T, Imai Y, Tsuji I, et al. Prediction of mortality by ambulatory blood pressure monitoring versus screening blood pressure measurements: a pilot study in Ohasama. J Hypertens 1997; 15:357–364.
- Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002; 39:878–885.
- Hemmelgarn BR, McAllister FA, Myers MG, et al; Canadian Hypertension Education Program. The 2005 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1 - blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol 2005; 21:645–656.
- Pickering TG. JNC 7.5. J Clin Hypertens (Greenwich) 2007; 9:901–904.
- White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit 2001; 6:107–110.
- Myers MG, Meglis G, Polemidiotis G. The impact of physician vs automated blood pressure readings on office-induced hypertension. J Hum Hypertens 1997; 11:491–493.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension 2010; 55:195–200.
- Stergiou GS, Tzamouranis D, Protogerou A, Nasothimiou E, Kapralos C. Validation of the Microlife Watch BP Office professional device for office blood pressure measurement according to the International protocol. Blood Press Monit 2008; 13:299–303.
- Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit 2006; 11:59–62.
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit 2008; 13:333–338.
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009; 27:280–286.
- Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit 2006; 11:37–42.
- Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D; American Heart Association. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 2008; 52:1–9.
- Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 2008; 26:1505–1526.
- O’Brien E. Ambulatory blood pressure measurement: the case for implementation in primary care. Hypertension 2008; 51:1435–1441.
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord 2005; 5:18.
- Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995; 25:305–313.
- Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med 1992; 152:56–64.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Grim CM, Grim CE. A curriculum for the training and certification of blood pressure measurement for health care providers. Can J Cardiol 1995; 11(suppl H):38H–42H.
- Pickering TG, Hall JE, Appel LJ, et al; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45:142–161.
- Langlois S. Measuring blood pressure: how competent are we? Perspect Cardiol 1999; 15:29–39.
- Le Pailleur C, Helft G, Landais P, et al. The effects of talking, reading, and silence on the “white coat” phenomenon in hypertensive patients. Am J Hypertens 1998; 11:203–207.
- Webster J, Newnham D, Petrie JC, Lovell HG. Influence of arm position on measurement of blood pressure. Br Med J (Clin Res Ed) 1984; 288:1574–1575.
- Netea RT, Smits P, Lenders JW, Thien T. Does it matter whether blood pressure measurements are taken with subjects sitting or supine? J Hypertens 1998; 16:263–268.
- Silverberg DS, Shemesh E, Iaina A. The unsupported arm: a cause of falsely raised blood pressure readings. Br Med J 1977; 2:1331.
- Manning DM, Kuchirka C, Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation 1983; 68:763–766.
- Iyriboz Y, Hearon CM, Edwards K. Agreement between large and small cuffs in sphygmomanometry: a quantitative assessment. J Clin Monit 1994; 10:127–133.
- Scherwitz LW, Evans LA, Hennrikus DJ, Vallbona C. Procedures and discrepancies of blood pressure measurements in two community health centers. Med Care 1982; 20:727–738.
- La Batide-Alanore A, Chatellier G, Bobrie G, Fofol I, Plouin PF. Comparison of nurse- and physician-determined clinic blood pressure levels in patients referred to a hypertension clinic: implications for subsequent management. J Hypertens 2000; 18:391–398.
- Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000; 35:844–851.
- Ogedegbe G, Pickering TG, Clemow L, et al. The misdiagnosis of hypertension: the role of patient anxiety. Arch Intern Med 2008; 168:2459–2465.
- Pickering TG. Stress, white coat hypertension and masked hypertension. In:Izzo JL, Sica DA, Black HR, editors. Hypertension Primer: The Essentials of High Blood Pressure: Basic Science, Population Science, and Clinical Management. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:289–291.
- Pickering TG, Coats A, Mallion JM, Mancia G, Verdecchia P. Blood Pressure Monitoring. Task force V: White-coat hypertension. Blood Press Monit 1999; 4:333–341.
- Myers MG, Valdivieso MA. Use of an automated blood pressure recording device, the BpTRU, to reduce the “white coat effect” in routine practice. Am J Hypertens 2003; 16:494–497.
- Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension 1998; 31:712–718.
- Ohkubo T, Imai Y, Tsuji I, et al. Prediction of mortality by ambulatory blood pressure monitoring versus screening blood pressure measurements: a pilot study in Ohasama. J Hypertens 1997; 15:357–364.
- Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002; 39:878–885.
- Hemmelgarn BR, McAllister FA, Myers MG, et al; Canadian Hypertension Education Program. The 2005 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1 - blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol 2005; 21:645–656.
- Pickering TG. JNC 7.5. J Clin Hypertens (Greenwich) 2007; 9:901–904.
- White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit 2001; 6:107–110.
- Myers MG, Meglis G, Polemidiotis G. The impact of physician vs automated blood pressure readings on office-induced hypertension. J Hum Hypertens 1997; 11:491–493.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension 2010; 55:195–200.
- Stergiou GS, Tzamouranis D, Protogerou A, Nasothimiou E, Kapralos C. Validation of the Microlife Watch BP Office professional device for office blood pressure measurement according to the International protocol. Blood Press Monit 2008; 13:299–303.
- Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit 2006; 11:59–62.
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit 2008; 13:333–338.
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009; 27:280–286.
- Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit 2006; 11:37–42.
- Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D; American Heart Association. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 2008; 52:1–9.
- Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 2008; 26:1505–1526.
- O’Brien E. Ambulatory blood pressure measurement: the case for implementation in primary care. Hypertension 2008; 51:1435–1441.
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord 2005; 5:18.
KEY POINTS
- The white-coat effect, ie, the tendency of many patients to have higher blood pressure in the presence of medical personnel than in their own environment, can lead to inappropriate diagnosis of hypertension and unnecessary treatment.
- Out-of-office blood pressure correlates better with cardiovascular risk than does the blood pressure in the physician’s office, but ambulatory monitoring is costly and not widely available, and few physicians recommend self-measurement at home.
- Several available devices can take a series of blood pressure measurements at preset intervals while the patient sits alone in the examination room, eliminating the white-coat effect.
- The mean of five automatic readings taken at intervals of 1 or 2 minutes correlates well with the mean value while awake on ambulatory monitoring.
Program to Improve Prescribing of Primarily Renally Cleared Oral Medications in Older Veteran Community Living Center Patients
Study: Pubic Bone Stabilization Slings Don't Cause Osteomyelitis
ST. LOUIS – Treatment of urinary incontinence by means of a pubic bone stabilization sling – a suburethral sling that is anchored to the pubic bone using titanium screws – is highly effective and is not associated with an increased risk of osteomyelitis, according to findings from the largest prospective observational study to date.
Although concerns that the procedure could cause osseous complications have been circulating for years and have discouraged some surgeons from using the pubic bone stabilization sling, only 1 case occurred in the 2,331 patients in the study, for an incidence of 0.000043%, Dr. S. Robert Kovac reported at the 19th International Pelvic Reconstructive and Vaginal Surgery Conference, which was sponsored by the Society of Pelvic Reconstructive Surgeons.
Patients were treated for intrinsic sphincter dysfunction (ISD) and/or stress urinary incontinence (SUI), and were followed for a mean of 13 years, and up to 17 years at four different institutions, said Dr. Kovac, the John D. Thompson Distinguished Professor of Gynecologic Surgery and director of the center for pelvic reconstructive surgery and urogynecology at Emory University, Atlanta. His associates are Dr. P.D. Dietz, Dr. M. Muniz, and Dr. S.H. Cruikshank.
Follow-up was done by exams, questionnaires, and telephone conversations.
The cure rate for those with ISD and/or SUI who had total dryness was 92%.
In a prospective study published in 2004, the incidence of osteomyelitis was 0.08% in 1,228 patients who underwent transvaginal bone anchor fixation in female pelvic reconstructive surgery, Dr. Kovac noted (Urology 2004; 64:669-74).
By comparison, abdominal sacrocolpopexy is associated with an osteomyelitis incidence of 11%, according to reports in the literature, Dr. Kovac said.
“I think we got off on the wrong track,” he said of the unfounded fears regarding osteomyelitis in patients undergoing pubic bone stabilization (PBS) sling procedures.
Dr. Kovac, who developed the PBS sling procedure more than 20 years ago, said that more than 350,000 have been performed worldwide, and that in all that time he hasn’t seen a single case of osteomyelitis or osteitis pubis in any of his patients who underwent the procedure.
Furthermore, the PBS sling has the lowest complication rate and the best long-term outcomes of the various suburethral slings currently used for urinary incontinence, he said.
Currently, the procedure is performed transvaginally by placing a suburethral sling of Biodesign Surgisis over the midurethra, and securing it with titanium bone screws to the posterior-inferior pubis to restore proper anatomy for continence.
In addition to the high cure rate and low complication rate, the approach has several other advantages, Dr. Kovac said, including the following:
• It is a totally vaginal, unified approach; all defects can be treated during one procedure.
• There is efficacy for both urethral hypermobility and ISD-related SUI.
• It functions as a retropubic procedure, a vaginal Marshall-Marchetti-Krantz (MMK) operation without the need for an abdominal incision.
• It is easy to learn and teach.
• There is low or no pain.
• There is rapid return to normal voiding postoperatively.
• There is no need for blindly placed trocars.
• Surgical time is less than 30 minutes.
• There is no need for mesh, so there are no mesh-related complications.
• There is no voiding dysfunction; it is truly tension free.
The procedure also has little reliance on cystoscopy, although Dr. Kovac said that he recommends cystoscoping all patients to avoid potential bladder-related hazards.
Dr. Kovac disclosed that he is a consultant for Cook Medical Inc. and Ethicon-Endo Surgery Inc., but he sold his patent on the PBS sling to American Medical Systems and has no financial interest in the procedure.
ST. LOUIS – Treatment of urinary incontinence by means of a pubic bone stabilization sling – a suburethral sling that is anchored to the pubic bone using titanium screws – is highly effective and is not associated with an increased risk of osteomyelitis, according to findings from the largest prospective observational study to date.
Although concerns that the procedure could cause osseous complications have been circulating for years and have discouraged some surgeons from using the pubic bone stabilization sling, only 1 case occurred in the 2,331 patients in the study, for an incidence of 0.000043%, Dr. S. Robert Kovac reported at the 19th International Pelvic Reconstructive and Vaginal Surgery Conference, which was sponsored by the Society of Pelvic Reconstructive Surgeons.
Patients were treated for intrinsic sphincter dysfunction (ISD) and/or stress urinary incontinence (SUI), and were followed for a mean of 13 years, and up to 17 years at four different institutions, said Dr. Kovac, the John D. Thompson Distinguished Professor of Gynecologic Surgery and director of the center for pelvic reconstructive surgery and urogynecology at Emory University, Atlanta. His associates are Dr. P.D. Dietz, Dr. M. Muniz, and Dr. S.H. Cruikshank.
Follow-up was done by exams, questionnaires, and telephone conversations.
The cure rate for those with ISD and/or SUI who had total dryness was 92%.
In a prospective study published in 2004, the incidence of osteomyelitis was 0.08% in 1,228 patients who underwent transvaginal bone anchor fixation in female pelvic reconstructive surgery, Dr. Kovac noted (Urology 2004; 64:669-74).
By comparison, abdominal sacrocolpopexy is associated with an osteomyelitis incidence of 11%, according to reports in the literature, Dr. Kovac said.
“I think we got off on the wrong track,” he said of the unfounded fears regarding osteomyelitis in patients undergoing pubic bone stabilization (PBS) sling procedures.
Dr. Kovac, who developed the PBS sling procedure more than 20 years ago, said that more than 350,000 have been performed worldwide, and that in all that time he hasn’t seen a single case of osteomyelitis or osteitis pubis in any of his patients who underwent the procedure.
Furthermore, the PBS sling has the lowest complication rate and the best long-term outcomes of the various suburethral slings currently used for urinary incontinence, he said.
Currently, the procedure is performed transvaginally by placing a suburethral sling of Biodesign Surgisis over the midurethra, and securing it with titanium bone screws to the posterior-inferior pubis to restore proper anatomy for continence.
In addition to the high cure rate and low complication rate, the approach has several other advantages, Dr. Kovac said, including the following:
• It is a totally vaginal, unified approach; all defects can be treated during one procedure.
• There is efficacy for both urethral hypermobility and ISD-related SUI.
• It functions as a retropubic procedure, a vaginal Marshall-Marchetti-Krantz (MMK) operation without the need for an abdominal incision.
• It is easy to learn and teach.
• There is low or no pain.
• There is rapid return to normal voiding postoperatively.
• There is no need for blindly placed trocars.
• Surgical time is less than 30 minutes.
• There is no need for mesh, so there are no mesh-related complications.
• There is no voiding dysfunction; it is truly tension free.
The procedure also has little reliance on cystoscopy, although Dr. Kovac said that he recommends cystoscoping all patients to avoid potential bladder-related hazards.
Dr. Kovac disclosed that he is a consultant for Cook Medical Inc. and Ethicon-Endo Surgery Inc., but he sold his patent on the PBS sling to American Medical Systems and has no financial interest in the procedure.
ST. LOUIS – Treatment of urinary incontinence by means of a pubic bone stabilization sling – a suburethral sling that is anchored to the pubic bone using titanium screws – is highly effective and is not associated with an increased risk of osteomyelitis, according to findings from the largest prospective observational study to date.
Although concerns that the procedure could cause osseous complications have been circulating for years and have discouraged some surgeons from using the pubic bone stabilization sling, only 1 case occurred in the 2,331 patients in the study, for an incidence of 0.000043%, Dr. S. Robert Kovac reported at the 19th International Pelvic Reconstructive and Vaginal Surgery Conference, which was sponsored by the Society of Pelvic Reconstructive Surgeons.
Patients were treated for intrinsic sphincter dysfunction (ISD) and/or stress urinary incontinence (SUI), and were followed for a mean of 13 years, and up to 17 years at four different institutions, said Dr. Kovac, the John D. Thompson Distinguished Professor of Gynecologic Surgery and director of the center for pelvic reconstructive surgery and urogynecology at Emory University, Atlanta. His associates are Dr. P.D. Dietz, Dr. M. Muniz, and Dr. S.H. Cruikshank.
Follow-up was done by exams, questionnaires, and telephone conversations.
The cure rate for those with ISD and/or SUI who had total dryness was 92%.
In a prospective study published in 2004, the incidence of osteomyelitis was 0.08% in 1,228 patients who underwent transvaginal bone anchor fixation in female pelvic reconstructive surgery, Dr. Kovac noted (Urology 2004; 64:669-74).
By comparison, abdominal sacrocolpopexy is associated with an osteomyelitis incidence of 11%, according to reports in the literature, Dr. Kovac said.
“I think we got off on the wrong track,” he said of the unfounded fears regarding osteomyelitis in patients undergoing pubic bone stabilization (PBS) sling procedures.
Dr. Kovac, who developed the PBS sling procedure more than 20 years ago, said that more than 350,000 have been performed worldwide, and that in all that time he hasn’t seen a single case of osteomyelitis or osteitis pubis in any of his patients who underwent the procedure.
Furthermore, the PBS sling has the lowest complication rate and the best long-term outcomes of the various suburethral slings currently used for urinary incontinence, he said.
Currently, the procedure is performed transvaginally by placing a suburethral sling of Biodesign Surgisis over the midurethra, and securing it with titanium bone screws to the posterior-inferior pubis to restore proper anatomy for continence.
In addition to the high cure rate and low complication rate, the approach has several other advantages, Dr. Kovac said, including the following:
• It is a totally vaginal, unified approach; all defects can be treated during one procedure.
• There is efficacy for both urethral hypermobility and ISD-related SUI.
• It functions as a retropubic procedure, a vaginal Marshall-Marchetti-Krantz (MMK) operation without the need for an abdominal incision.
• It is easy to learn and teach.
• There is low or no pain.
• There is rapid return to normal voiding postoperatively.
• There is no need for blindly placed trocars.
• Surgical time is less than 30 minutes.
• There is no need for mesh, so there are no mesh-related complications.
• There is no voiding dysfunction; it is truly tension free.
The procedure also has little reliance on cystoscopy, although Dr. Kovac said that he recommends cystoscoping all patients to avoid potential bladder-related hazards.
Dr. Kovac disclosed that he is a consultant for Cook Medical Inc. and Ethicon-Endo Surgery Inc., but he sold his patent on the PBS sling to American Medical Systems and has no financial interest in the procedure.
Major Finding: Although concerns that the procedure could cause osseous complications have been circulating for years and have discouraged some surgeons from using the procedure, only 1 case occurred in the 2,331 patients in the study, for an incidence of 0.000043%.
Data Source: A prospective observational study of 2,331 patients.
Disclosures: Dr. Kovac disclosed that he is a consultant for Cook Medical Inc. and Ethicon-Endo Surgery Inc. He developed the PBS sling, but sold the procedure’s patent to American Medical Systems and has no financial interest in it.
FDA Strengthens Gadolinium Warnings
The Food and Drug Administration on Sept. 9 required manufacturers of five gadolinium contrast agents to strengthen boxed warnings on their products to minimize the risk of nephrogenic systemic fibrosis in some patients with kidney dysfunction.
Three of the agents – Magnevist (gadopentetate dimeglumine), Omniscan (gadodiamide), and Optimark (gadoversetamide) – are now contraindicated in patients with acute kidney injury (AKI) or chronic, severe kidney disease. Severe kidney disease is marked by a glomerular filtration rate of less than 30 mL/min/1.73m2.
In addition, the revised labeling recommends that healthcare professionals screen all patients being considered for any gadolinium-based contrast agent (GBCA) for AKI or chronic, severe kidney disease. GBCAs also should be avoided in patients suspected or known to have impaired drug elimination unless the diagnostic information obtained is essential and cannot be acquired without the use of a contrast agent.
It’s been known since at least 2006 that patients with AKI or chronic, severe kidney disease are at increased risk of developing nephrogenic systemic fibrosis (NSF). In 2007 the FDA issued its first boxed warning about the risk of NSF associated with GBCA use. That warning stated only that healthcare professionals should be aware of the possibility that some patients might develop NSF. The new warning adds the absolute contraindication for the use of the three agents in patients with AKI and chronic, severe kidney disease.
The earlier boxed warning appears to have had the effect of reducing the incidence of NSF, according to one FDA official. Late last year, at a joint meeting of the FDA’s Cardiovascular and Renal Drugs and Drug Safety and Risk Management advisory committees, Dr. James Kaiser of the agency’s office of surveillance and epidemiology noted that in 2006 there were 194 cases of NSF associated with GBCAs. That number decreased to 128 cases in 2007, 55 in 2008, and 6 in 2009 (through September).
While three GBCAs had been associated with numerous cases of NSF, the other two – MultiHance (gadobenate dimeglumine) and ProHance (gadoteridol) ‑ have not. As of September 2009, a total of 382 reports of NSF had been associated with Omniscan (GE HealthCare), 195 with Magnevist (Bayer HealthCare), 35 with OptiMARK (Covidien), 1 with MultiHance (Bracco Diagnostics), and 0 with ProHance (Bracco Diagnostics). At last year’s meeting a majority of advisory committee members recommended that Omniscan and OptiMARK be contraindicated in patients with serious kidney dysfunction. There was less agreement on whether Magnevist should also be contraindicated.
The Food and Drug Administration on Sept. 9 required manufacturers of five gadolinium contrast agents to strengthen boxed warnings on their products to minimize the risk of nephrogenic systemic fibrosis in some patients with kidney dysfunction.
Three of the agents – Magnevist (gadopentetate dimeglumine), Omniscan (gadodiamide), and Optimark (gadoversetamide) – are now contraindicated in patients with acute kidney injury (AKI) or chronic, severe kidney disease. Severe kidney disease is marked by a glomerular filtration rate of less than 30 mL/min/1.73m2.
In addition, the revised labeling recommends that healthcare professionals screen all patients being considered for any gadolinium-based contrast agent (GBCA) for AKI or chronic, severe kidney disease. GBCAs also should be avoided in patients suspected or known to have impaired drug elimination unless the diagnostic information obtained is essential and cannot be acquired without the use of a contrast agent.
It’s been known since at least 2006 that patients with AKI or chronic, severe kidney disease are at increased risk of developing nephrogenic systemic fibrosis (NSF). In 2007 the FDA issued its first boxed warning about the risk of NSF associated with GBCA use. That warning stated only that healthcare professionals should be aware of the possibility that some patients might develop NSF. The new warning adds the absolute contraindication for the use of the three agents in patients with AKI and chronic, severe kidney disease.
The earlier boxed warning appears to have had the effect of reducing the incidence of NSF, according to one FDA official. Late last year, at a joint meeting of the FDA’s Cardiovascular and Renal Drugs and Drug Safety and Risk Management advisory committees, Dr. James Kaiser of the agency’s office of surveillance and epidemiology noted that in 2006 there were 194 cases of NSF associated with GBCAs. That number decreased to 128 cases in 2007, 55 in 2008, and 6 in 2009 (through September).
While three GBCAs had been associated with numerous cases of NSF, the other two – MultiHance (gadobenate dimeglumine) and ProHance (gadoteridol) ‑ have not. As of September 2009, a total of 382 reports of NSF had been associated with Omniscan (GE HealthCare), 195 with Magnevist (Bayer HealthCare), 35 with OptiMARK (Covidien), 1 with MultiHance (Bracco Diagnostics), and 0 with ProHance (Bracco Diagnostics). At last year’s meeting a majority of advisory committee members recommended that Omniscan and OptiMARK be contraindicated in patients with serious kidney dysfunction. There was less agreement on whether Magnevist should also be contraindicated.
The Food and Drug Administration on Sept. 9 required manufacturers of five gadolinium contrast agents to strengthen boxed warnings on their products to minimize the risk of nephrogenic systemic fibrosis in some patients with kidney dysfunction.
Three of the agents – Magnevist (gadopentetate dimeglumine), Omniscan (gadodiamide), and Optimark (gadoversetamide) – are now contraindicated in patients with acute kidney injury (AKI) or chronic, severe kidney disease. Severe kidney disease is marked by a glomerular filtration rate of less than 30 mL/min/1.73m2.
In addition, the revised labeling recommends that healthcare professionals screen all patients being considered for any gadolinium-based contrast agent (GBCA) for AKI or chronic, severe kidney disease. GBCAs also should be avoided in patients suspected or known to have impaired drug elimination unless the diagnostic information obtained is essential and cannot be acquired without the use of a contrast agent.
It’s been known since at least 2006 that patients with AKI or chronic, severe kidney disease are at increased risk of developing nephrogenic systemic fibrosis (NSF). In 2007 the FDA issued its first boxed warning about the risk of NSF associated with GBCA use. That warning stated only that healthcare professionals should be aware of the possibility that some patients might develop NSF. The new warning adds the absolute contraindication for the use of the three agents in patients with AKI and chronic, severe kidney disease.
The earlier boxed warning appears to have had the effect of reducing the incidence of NSF, according to one FDA official. Late last year, at a joint meeting of the FDA’s Cardiovascular and Renal Drugs and Drug Safety and Risk Management advisory committees, Dr. James Kaiser of the agency’s office of surveillance and epidemiology noted that in 2006 there were 194 cases of NSF associated with GBCAs. That number decreased to 128 cases in 2007, 55 in 2008, and 6 in 2009 (through September).
While three GBCAs had been associated with numerous cases of NSF, the other two – MultiHance (gadobenate dimeglumine) and ProHance (gadoteridol) ‑ have not. As of September 2009, a total of 382 reports of NSF had been associated with Omniscan (GE HealthCare), 195 with Magnevist (Bayer HealthCare), 35 with OptiMARK (Covidien), 1 with MultiHance (Bracco Diagnostics), and 0 with ProHance (Bracco Diagnostics). At last year’s meeting a majority of advisory committee members recommended that Omniscan and OptiMARK be contraindicated in patients with serious kidney dysfunction. There was less agreement on whether Magnevist should also be contraindicated.
FDA Strengthens Gadolinium Warnings
The Food and Drug Administration on Sept. 9 required manufacturers of five gadolinium contrast agents to strengthen boxed warnings on their products to minimize the risk of nephrogenic systemic fibrosis in some patients with kidney dysfunction.
Three of the agents – Magnevist (gadopentetate dimeglumine), Omniscan (gadodiamide), and Optimark (gadoversetamide) – are now contraindicated in patients with acute kidney injury (AKI) or chronic, severe kidney disease. Severe kidney disease is marked by a glomerular filtration rate of less than 30 mL/min/1.73m2.
In addition, the revised labeling recommends that healthcare professionals screen all patients being considered for any gadolinium-based contrast agent (GBCA) for AKI or chronic, severe kidney disease. GBCAs also should be avoided in patients suspected or known to have impaired drug elimination unless the diagnostic information obtained is essential and cannot be acquired without the use of a contrast agent.
It’s been known since at least 2006 that patients with AKI or chronic, severe kidney disease are at increased risk of developing nephrogenic systemic fibrosis (NSF). In 2007 the FDA issued its first boxed warning about the risk of NSF associated with GBCA use. That warning stated only that healthcare professionals should be aware of the possibility that some patients might develop NSF. The new warning adds the absolute contraindication for the use of the three agents in patients with AKI and chronic, severe kidney disease.
The earlier boxed warning appears to have had the effect of reducing the incidence of NSF, according to one FDA official. Late last year, at a joint meeting of the FDA’s Cardiovascular and Renal Drugs and Drug Safety and Risk Management advisory committees, Dr. James Kaiser of the agency’s office of surveillance and epidemiology noted that in 2006 there were 194 cases of NSF associated with GBCAs. That number decreased to 128 cases in 2007, 55 in 2008, and 6 in 2009 (through September).
While three GBCAs had been associated with numerous cases of NSF, the other two – MultiHance (gadobenate dimeglumine) and ProHance (gadoteridol) ‑ have not. As of September 2009, a total of 382 reports of NSF had been associated with Omniscan (GE HealthCare), 195 with Magnevist (Bayer HealthCare), 35 with OptiMARK (Covidien), 1 with MultiHance (Bracco Diagnostics), and 0 with ProHance (Bracco Diagnostics). At last year’s meeting a majority of advisory committee members recommended that Omniscan and OptiMARK be contraindicated in patients with serious kidney dysfunction. There was less agreement on whether Magnevist should also be contraindicated.
The Food and Drug Administration on Sept. 9 required manufacturers of five gadolinium contrast agents to strengthen boxed warnings on their products to minimize the risk of nephrogenic systemic fibrosis in some patients with kidney dysfunction.
Three of the agents – Magnevist (gadopentetate dimeglumine), Omniscan (gadodiamide), and Optimark (gadoversetamide) – are now contraindicated in patients with acute kidney injury (AKI) or chronic, severe kidney disease. Severe kidney disease is marked by a glomerular filtration rate of less than 30 mL/min/1.73m2.
In addition, the revised labeling recommends that healthcare professionals screen all patients being considered for any gadolinium-based contrast agent (GBCA) for AKI or chronic, severe kidney disease. GBCAs also should be avoided in patients suspected or known to have impaired drug elimination unless the diagnostic information obtained is essential and cannot be acquired without the use of a contrast agent.
It’s been known since at least 2006 that patients with AKI or chronic, severe kidney disease are at increased risk of developing nephrogenic systemic fibrosis (NSF). In 2007 the FDA issued its first boxed warning about the risk of NSF associated with GBCA use. That warning stated only that healthcare professionals should be aware of the possibility that some patients might develop NSF. The new warning adds the absolute contraindication for the use of the three agents in patients with AKI and chronic, severe kidney disease.
The earlier boxed warning appears to have had the effect of reducing the incidence of NSF, according to one FDA official. Late last year, at a joint meeting of the FDA’s Cardiovascular and Renal Drugs and Drug Safety and Risk Management advisory committees, Dr. James Kaiser of the agency’s office of surveillance and epidemiology noted that in 2006 there were 194 cases of NSF associated with GBCAs. That number decreased to 128 cases in 2007, 55 in 2008, and 6 in 2009 (through September).
While three GBCAs had been associated with numerous cases of NSF, the other two – MultiHance (gadobenate dimeglumine) and ProHance (gadoteridol) ‑ have not. As of September 2009, a total of 382 reports of NSF had been associated with Omniscan (GE HealthCare), 195 with Magnevist (Bayer HealthCare), 35 with OptiMARK (Covidien), 1 with MultiHance (Bracco Diagnostics), and 0 with ProHance (Bracco Diagnostics). At last year’s meeting a majority of advisory committee members recommended that Omniscan and OptiMARK be contraindicated in patients with serious kidney dysfunction. There was less agreement on whether Magnevist should also be contraindicated.
The Food and Drug Administration on Sept. 9 required manufacturers of five gadolinium contrast agents to strengthen boxed warnings on their products to minimize the risk of nephrogenic systemic fibrosis in some patients with kidney dysfunction.
Three of the agents – Magnevist (gadopentetate dimeglumine), Omniscan (gadodiamide), and Optimark (gadoversetamide) – are now contraindicated in patients with acute kidney injury (AKI) or chronic, severe kidney disease. Severe kidney disease is marked by a glomerular filtration rate of less than 30 mL/min/1.73m2.
In addition, the revised labeling recommends that healthcare professionals screen all patients being considered for any gadolinium-based contrast agent (GBCA) for AKI or chronic, severe kidney disease. GBCAs also should be avoided in patients suspected or known to have impaired drug elimination unless the diagnostic information obtained is essential and cannot be acquired without the use of a contrast agent.
It’s been known since at least 2006 that patients with AKI or chronic, severe kidney disease are at increased risk of developing nephrogenic systemic fibrosis (NSF). In 2007 the FDA issued its first boxed warning about the risk of NSF associated with GBCA use. That warning stated only that healthcare professionals should be aware of the possibility that some patients might develop NSF. The new warning adds the absolute contraindication for the use of the three agents in patients with AKI and chronic, severe kidney disease.
The earlier boxed warning appears to have had the effect of reducing the incidence of NSF, according to one FDA official. Late last year, at a joint meeting of the FDA’s Cardiovascular and Renal Drugs and Drug Safety and Risk Management advisory committees, Dr. James Kaiser of the agency’s office of surveillance and epidemiology noted that in 2006 there were 194 cases of NSF associated with GBCAs. That number decreased to 128 cases in 2007, 55 in 2008, and 6 in 2009 (through September).
While three GBCAs had been associated with numerous cases of NSF, the other two – MultiHance (gadobenate dimeglumine) and ProHance (gadoteridol) ‑ have not. As of September 2009, a total of 382 reports of NSF had been associated with Omniscan (GE HealthCare), 195 with Magnevist (Bayer HealthCare), 35 with OptiMARK (Covidien), 1 with MultiHance (Bracco Diagnostics), and 0 with ProHance (Bracco Diagnostics). At last year’s meeting a majority of advisory committee members recommended that Omniscan and OptiMARK be contraindicated in patients with serious kidney dysfunction. There was less agreement on whether Magnevist should also be contraindicated.
Intensive BP Control Slows CKD Progression Only in Select Patients
Intensive blood pressure control doesn’t slow the progression of chronic kidney disease any better than standard blood pressure control in most patients, according to a report in the Sept. 2 New England Journal of Medicine.
It appears that the more intensive approach may benefit only patients who have proteinuria with a protein:creatinine ratio greater than 0.22, a value that is compatible with the widely accepted threshold of 300 mg/day for absolute urinary protein excretion, said Dr. Lawrence J. Appel of Johns Hopkins University, Baltimore, and his associates in the AASK (African-American Study of Kidney Disease and Hypertension) Collaborative Research Group.
Until now, “few trials have tested the effects of intensive blood pressure control [compared with conventional control] on the progression of chronic kidney disease, and the findings from such trials have been inconsistent. Despite a lack of compelling evidence, numerous guidelines recommend a reduced blood pressure target in patients with CKD,” they wrote.
Previous studies have rarely followed patients beyond 5 years, even though it typically takes longer than that for end-stage renal disease (ESRD) to develop in patients with CKD.
The AASK study compared outcomes between the two approaches to BP control in 1,094 black adults with mild to moderate hypertensive chronic kidney disease (defined as diastolic BP greater than 95 mm Hg and a glomerular filtration rate of 20-65 mL/min) but without marked proteinuria. Patients with diabetes were excluded from the trial.
In the first phase of the AASK investigation, patients were randomly assigned to either intensive BP control with a target of 92 mm Hg or lower mean arterial pressure (that is, lower than the usual target of 130/80 mm Hg recommended for CKD patients) or to conventional BP control with a target of 102-107 mm Hg mean arterial pressure (which corresponds to the conventional BP target of 140/90 mm Hg).
Throughout this initial phase of the trial, which lasted approximately 4 years, mean blood pressure was significantly lower in the intensive-control group (130/78 mm Hg) than in the standard-control group (141/86 mm Hg), yet there was no significant difference in the primary outcome of progression of kidney disease, development of ESRD, or death. Likewise, there was no significant difference between the two approaches in secondary or clinical outcomes.
In the second phase of the AASK investigation, patients who had not yet developed ESRD were invited to continue in a cohort portion of the trial, in which the BP target was 140/90 mm Hg. In 2004, when national guidelines were changed, this target was amended to lower than 130/80 mm Hg.
After a cumulative follow-up of 8-12 years, there still was no significant difference in primary or secondary outcomes between those who were initially assigned to the intensive-control and the standard-control groups. More intensive BP control did not slow the rate of progression of CKD, Dr. Appel and his associates reported (N. Engl. J. Med. 2010;363:918-29).
However, the intensive-control approach did benefit one subgroup of patients with proteinuria: those who had a protein:creatinine ratio of more than 0.22 at baseline. These patients showed a significant reduction in the primary outcome of progression of kidney disease, development of ESRD, or death, as well as in secondary and clinical outcomes.
The reason for this discrepancy is not known. “Overall, it is hard to develop a coherent, biologically plausible argument for a qualitative interaction between harm in patients without proteinuria and benefit in those with proteinuria,” the researchers said.
In an accompanying editorial, Dr. Julie R. Ingelfinger, chief of pediatric nephrology at Massachusetts General Hospital, Boston, and a deputy editor of the New England Journal of Medicine, wrote that the study lends hope to the concept that intensive treatment will improve renal outcomes in at least some patients with hypertension, chronic kidney disease, and microalbuminuria (N. Engl. J. Med. 2010;363:974-6). She noted that the Modification of Diet in Renal Disease trial showed that intensive BP control, compared with standard control, benefited patients who had more than 1 g of proteinuria at baseline. The ESCAPE trial (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients) also demonstrated that intensive BP control with a fixed dose of an ACE inhibitor significantly slowed the progression of renal disease, with the largest effects seen in children who had substantial proteinuria, hypertension, and a reduced GFR at baseline. And intensive BP control was beneficial in a recent study of adults in Italy who had idiopathic glomerular diseases associated with hypertension and proteinuria, Dr. Ingelfinger wrote.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the Office of Research in Minority Health, and the National Institutes of Health. King Pharmaceuticals provided financial support and donated antihypertensive medications to each clinical center. Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn also donated antihypertensive drugs. None of these companies had any role in the design of the study, the accrual or analysis of data, or the preparation of the manuscript. Some of the investigators reported being in consultant and/or advisory board roles or receiving funds from numerous companies including Daiichi-Sankyo, Novartis, Amgen, King Pharmaceuticals, Abbott, Boehringer-Ingelheim, Litholink, Eli Lilly, Takeda, Merck, and Watson. Dr. Ingelfinger reported having no conflicts of interest.
This study lends hope to the concept that intensive treatment will improve renal outcomes in at least some patients with hypertension, chronic kidney disease, and microalbuminuria.
Data from other studies also support the conclusion that intensive BP control is beneficial in select patients.
The Modification of Diet in Renal Disease trial showed that intensive BP control, compared with standard control, benefited patients who had more than 1 g of proteinuria at baseline. The ESCAPE (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients) trial also demonstrated that intensive BP control with a fixed dose of an ACE inhibitor significantly slowed the progression of renal disease, with the largest effects seen in children who had substantial proteinuria, hypertension, and a reduced GFR at baseline.
In addition, intensive BP control was beneficial in a recent study of adults in Italy who had idiopathic glomerular diseases associated with hypertension and proteinuria.
Julie R. Ingelfinger, M.D., is chief of pediatric nephrology at Massachusetts General Hospital, Boston, and a deputy editor of the New England Journal of Medicine. These comments were summarized from her editorial accompanying the report (N. Engl. J. Med. 2010;363:974-6). She reported having no relevant conflicts of interest.
This study lends hope to the concept that intensive treatment will improve renal outcomes in at least some patients with hypertension, chronic kidney disease, and microalbuminuria.
Data from other studies also support the conclusion that intensive BP control is beneficial in select patients.
The Modification of Diet in Renal Disease trial showed that intensive BP control, compared with standard control, benefited patients who had more than 1 g of proteinuria at baseline. The ESCAPE (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients) trial also demonstrated that intensive BP control with a fixed dose of an ACE inhibitor significantly slowed the progression of renal disease, with the largest effects seen in children who had substantial proteinuria, hypertension, and a reduced GFR at baseline.
In addition, intensive BP control was beneficial in a recent study of adults in Italy who had idiopathic glomerular diseases associated with hypertension and proteinuria.
Julie R. Ingelfinger, M.D., is chief of pediatric nephrology at Massachusetts General Hospital, Boston, and a deputy editor of the New England Journal of Medicine. These comments were summarized from her editorial accompanying the report (N. Engl. J. Med. 2010;363:974-6). She reported having no relevant conflicts of interest.
This study lends hope to the concept that intensive treatment will improve renal outcomes in at least some patients with hypertension, chronic kidney disease, and microalbuminuria.
Data from other studies also support the conclusion that intensive BP control is beneficial in select patients.
The Modification of Diet in Renal Disease trial showed that intensive BP control, compared with standard control, benefited patients who had more than 1 g of proteinuria at baseline. The ESCAPE (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients) trial also demonstrated that intensive BP control with a fixed dose of an ACE inhibitor significantly slowed the progression of renal disease, with the largest effects seen in children who had substantial proteinuria, hypertension, and a reduced GFR at baseline.
In addition, intensive BP control was beneficial in a recent study of adults in Italy who had idiopathic glomerular diseases associated with hypertension and proteinuria.
Julie R. Ingelfinger, M.D., is chief of pediatric nephrology at Massachusetts General Hospital, Boston, and a deputy editor of the New England Journal of Medicine. These comments were summarized from her editorial accompanying the report (N. Engl. J. Med. 2010;363:974-6). She reported having no relevant conflicts of interest.
Intensive blood pressure control doesn’t slow the progression of chronic kidney disease any better than standard blood pressure control in most patients, according to a report in the Sept. 2 New England Journal of Medicine.
It appears that the more intensive approach may benefit only patients who have proteinuria with a protein:creatinine ratio greater than 0.22, a value that is compatible with the widely accepted threshold of 300 mg/day for absolute urinary protein excretion, said Dr. Lawrence J. Appel of Johns Hopkins University, Baltimore, and his associates in the AASK (African-American Study of Kidney Disease and Hypertension) Collaborative Research Group.
Until now, “few trials have tested the effects of intensive blood pressure control [compared with conventional control] on the progression of chronic kidney disease, and the findings from such trials have been inconsistent. Despite a lack of compelling evidence, numerous guidelines recommend a reduced blood pressure target in patients with CKD,” they wrote.
Previous studies have rarely followed patients beyond 5 years, even though it typically takes longer than that for end-stage renal disease (ESRD) to develop in patients with CKD.
The AASK study compared outcomes between the two approaches to BP control in 1,094 black adults with mild to moderate hypertensive chronic kidney disease (defined as diastolic BP greater than 95 mm Hg and a glomerular filtration rate of 20-65 mL/min) but without marked proteinuria. Patients with diabetes were excluded from the trial.
In the first phase of the AASK investigation, patients were randomly assigned to either intensive BP control with a target of 92 mm Hg or lower mean arterial pressure (that is, lower than the usual target of 130/80 mm Hg recommended for CKD patients) or to conventional BP control with a target of 102-107 mm Hg mean arterial pressure (which corresponds to the conventional BP target of 140/90 mm Hg).
Throughout this initial phase of the trial, which lasted approximately 4 years, mean blood pressure was significantly lower in the intensive-control group (130/78 mm Hg) than in the standard-control group (141/86 mm Hg), yet there was no significant difference in the primary outcome of progression of kidney disease, development of ESRD, or death. Likewise, there was no significant difference between the two approaches in secondary or clinical outcomes.
In the second phase of the AASK investigation, patients who had not yet developed ESRD were invited to continue in a cohort portion of the trial, in which the BP target was 140/90 mm Hg. In 2004, when national guidelines were changed, this target was amended to lower than 130/80 mm Hg.
After a cumulative follow-up of 8-12 years, there still was no significant difference in primary or secondary outcomes between those who were initially assigned to the intensive-control and the standard-control groups. More intensive BP control did not slow the rate of progression of CKD, Dr. Appel and his associates reported (N. Engl. J. Med. 2010;363:918-29).
However, the intensive-control approach did benefit one subgroup of patients with proteinuria: those who had a protein:creatinine ratio of more than 0.22 at baseline. These patients showed a significant reduction in the primary outcome of progression of kidney disease, development of ESRD, or death, as well as in secondary and clinical outcomes.
The reason for this discrepancy is not known. “Overall, it is hard to develop a coherent, biologically plausible argument for a qualitative interaction between harm in patients without proteinuria and benefit in those with proteinuria,” the researchers said.
In an accompanying editorial, Dr. Julie R. Ingelfinger, chief of pediatric nephrology at Massachusetts General Hospital, Boston, and a deputy editor of the New England Journal of Medicine, wrote that the study lends hope to the concept that intensive treatment will improve renal outcomes in at least some patients with hypertension, chronic kidney disease, and microalbuminuria (N. Engl. J. Med. 2010;363:974-6). She noted that the Modification of Diet in Renal Disease trial showed that intensive BP control, compared with standard control, benefited patients who had more than 1 g of proteinuria at baseline. The ESCAPE trial (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients) also demonstrated that intensive BP control with a fixed dose of an ACE inhibitor significantly slowed the progression of renal disease, with the largest effects seen in children who had substantial proteinuria, hypertension, and a reduced GFR at baseline. And intensive BP control was beneficial in a recent study of adults in Italy who had idiopathic glomerular diseases associated with hypertension and proteinuria, Dr. Ingelfinger wrote.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the Office of Research in Minority Health, and the National Institutes of Health. King Pharmaceuticals provided financial support and donated antihypertensive medications to each clinical center. Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn also donated antihypertensive drugs. None of these companies had any role in the design of the study, the accrual or analysis of data, or the preparation of the manuscript. Some of the investigators reported being in consultant and/or advisory board roles or receiving funds from numerous companies including Daiichi-Sankyo, Novartis, Amgen, King Pharmaceuticals, Abbott, Boehringer-Ingelheim, Litholink, Eli Lilly, Takeda, Merck, and Watson. Dr. Ingelfinger reported having no conflicts of interest.
Intensive blood pressure control doesn’t slow the progression of chronic kidney disease any better than standard blood pressure control in most patients, according to a report in the Sept. 2 New England Journal of Medicine.
It appears that the more intensive approach may benefit only patients who have proteinuria with a protein:creatinine ratio greater than 0.22, a value that is compatible with the widely accepted threshold of 300 mg/day for absolute urinary protein excretion, said Dr. Lawrence J. Appel of Johns Hopkins University, Baltimore, and his associates in the AASK (African-American Study of Kidney Disease and Hypertension) Collaborative Research Group.
Until now, “few trials have tested the effects of intensive blood pressure control [compared with conventional control] on the progression of chronic kidney disease, and the findings from such trials have been inconsistent. Despite a lack of compelling evidence, numerous guidelines recommend a reduced blood pressure target in patients with CKD,” they wrote.
Previous studies have rarely followed patients beyond 5 years, even though it typically takes longer than that for end-stage renal disease (ESRD) to develop in patients with CKD.
The AASK study compared outcomes between the two approaches to BP control in 1,094 black adults with mild to moderate hypertensive chronic kidney disease (defined as diastolic BP greater than 95 mm Hg and a glomerular filtration rate of 20-65 mL/min) but without marked proteinuria. Patients with diabetes were excluded from the trial.
In the first phase of the AASK investigation, patients were randomly assigned to either intensive BP control with a target of 92 mm Hg or lower mean arterial pressure (that is, lower than the usual target of 130/80 mm Hg recommended for CKD patients) or to conventional BP control with a target of 102-107 mm Hg mean arterial pressure (which corresponds to the conventional BP target of 140/90 mm Hg).
Throughout this initial phase of the trial, which lasted approximately 4 years, mean blood pressure was significantly lower in the intensive-control group (130/78 mm Hg) than in the standard-control group (141/86 mm Hg), yet there was no significant difference in the primary outcome of progression of kidney disease, development of ESRD, or death. Likewise, there was no significant difference between the two approaches in secondary or clinical outcomes.
In the second phase of the AASK investigation, patients who had not yet developed ESRD were invited to continue in a cohort portion of the trial, in which the BP target was 140/90 mm Hg. In 2004, when national guidelines were changed, this target was amended to lower than 130/80 mm Hg.
After a cumulative follow-up of 8-12 years, there still was no significant difference in primary or secondary outcomes between those who were initially assigned to the intensive-control and the standard-control groups. More intensive BP control did not slow the rate of progression of CKD, Dr. Appel and his associates reported (N. Engl. J. Med. 2010;363:918-29).
However, the intensive-control approach did benefit one subgroup of patients with proteinuria: those who had a protein:creatinine ratio of more than 0.22 at baseline. These patients showed a significant reduction in the primary outcome of progression of kidney disease, development of ESRD, or death, as well as in secondary and clinical outcomes.
The reason for this discrepancy is not known. “Overall, it is hard to develop a coherent, biologically plausible argument for a qualitative interaction between harm in patients without proteinuria and benefit in those with proteinuria,” the researchers said.
In an accompanying editorial, Dr. Julie R. Ingelfinger, chief of pediatric nephrology at Massachusetts General Hospital, Boston, and a deputy editor of the New England Journal of Medicine, wrote that the study lends hope to the concept that intensive treatment will improve renal outcomes in at least some patients with hypertension, chronic kidney disease, and microalbuminuria (N. Engl. J. Med. 2010;363:974-6). She noted that the Modification of Diet in Renal Disease trial showed that intensive BP control, compared with standard control, benefited patients who had more than 1 g of proteinuria at baseline. The ESCAPE trial (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients) also demonstrated that intensive BP control with a fixed dose of an ACE inhibitor significantly slowed the progression of renal disease, with the largest effects seen in children who had substantial proteinuria, hypertension, and a reduced GFR at baseline. And intensive BP control was beneficial in a recent study of adults in Italy who had idiopathic glomerular diseases associated with hypertension and proteinuria, Dr. Ingelfinger wrote.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the Office of Research in Minority Health, and the National Institutes of Health. King Pharmaceuticals provided financial support and donated antihypertensive medications to each clinical center. Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn also donated antihypertensive drugs. None of these companies had any role in the design of the study, the accrual or analysis of data, or the preparation of the manuscript. Some of the investigators reported being in consultant and/or advisory board roles or receiving funds from numerous companies including Daiichi-Sankyo, Novartis, Amgen, King Pharmaceuticals, Abbott, Boehringer-Ingelheim, Litholink, Eli Lilly, Takeda, Merck, and Watson. Dr. Ingelfinger reported having no conflicts of interest.