User login
Cystoscopy Alone Excels for Bladder Cancer Surveillance
Major Finding: Cystoscopy alone, the least expensive of five surveillance strategies, picked up two invasive bladder cancers that were missed by urine testing.
Data Source: A study in 200 consecutive patients.
Disclosures: Dr. Karam reported having no conflicts of interest related to the study. A coauthor received honoraria from Abbott Molecular.
SAN FRANCISCO — Cystoscopy alone offers the best combination of cost and sensitivity for surveillance after treatment of superficial bladder cancer, new data show.
In a prospective study comparing five surveillance strategies in 200 patients, the addition of various urine tests to cystoscopy increased the cost per cancer detected, but did not find more invasive tumors, researchers reported at a symposium on genitourinary cancers.
“Our data suggest that cystoscopy alone is the most cost-effective strategy, and that the addition of urinary markers adds to cost without improved detection of invasive disease,” said first author Dr. Jose A. Karam, a urologic oncology fellow at the University of Texas M.D. Anderson Cancer Center in Houston.
Evidence-based data are lacking on the outcomes of surveillance strategies among patients who have been treated for superficial (non–muscle invasive) bladder cancer. Many urinary-based markers are now available for use, but cystoscopy remains the standard of care, he said.
In the study, consecutive patients needing surveillance after treatment of superficial bladder cancer underwent these tests at study entry: cystoscopy, urine cytology, NMP22 BladderChek test (a urine test marketed by Inverness Medical Innovations that measures a protein associated with bladder cancer), and FISH (fluorescence in situ hybridization) UroVysion test (a urine test marketed by Abbott Molecular that detects chromosomal abnormalities associated with bladder cancer).
The bladder cancers for which they had been treated were mainly of Ta stage (71%) and low grade (58%). In 25 patients, new tumors were found at study entry or by the first follow-up assessment, a median of 4.1 months later, Dr. Karam reported at the symposium, sponsored by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
Based on 2009 Medicare reimbursement rates, the cost per cancer detected was lowest with cystoscopy alone ($7,692), and highest with cystoscopy plus FISH ($19,111). Values were intermediate for cystoscopy plus cytology ($10,267), cystoscopy plus NMP ($11,143), and cystoscopy plus NMP with FISH confirmation in the event of a positive NMP result ($9,557).
The cancer detection rate was lowest with cystoscopy alone (52%) and highest with cystoscopy plus FISH (72%). Values were intermediate for cystoscopy plus cytology (60%), cystoscopy plus NMP (56%), and cystoscopy plus NMP with FISH confirmation (56%). Subsequent analyses showed that cystoscopy plus FISH picked up more early, noninvasive (carcinoma in situ and Ta) tumors than did cystoscopy alone. But it similarly missed the two more advanced (T1 and T2) tumors that were found, “which arguably are the ones that matter the most,” Dr. Karam commented.
Cystoscopy alone appears to be the most cost-effective approach, he concluded, cautioning that “these [urinary-based] markers should be used carefully and judiciously in patients with bladder cancer.” Because all patients received care from bladder specialists, the findings may not be generalizable to community practices, he added.
Dr. Nicholas J. Vogelzang, one of the developers of the NMP22 assay, chaired a press briefing at which the study was discussed. Dr. Vogelzang, chair and medical director of the developmental therapeutics committee of U.S. Oncology, said he found the results “very chagrining but nonetheless very important.”
Urinary-based markers 'should be used carefully and judiciously in patients with bladder cancer.'
Source Dr. Karam
Major Finding: Cystoscopy alone, the least expensive of five surveillance strategies, picked up two invasive bladder cancers that were missed by urine testing.
Data Source: A study in 200 consecutive patients.
Disclosures: Dr. Karam reported having no conflicts of interest related to the study. A coauthor received honoraria from Abbott Molecular.
SAN FRANCISCO — Cystoscopy alone offers the best combination of cost and sensitivity for surveillance after treatment of superficial bladder cancer, new data show.
In a prospective study comparing five surveillance strategies in 200 patients, the addition of various urine tests to cystoscopy increased the cost per cancer detected, but did not find more invasive tumors, researchers reported at a symposium on genitourinary cancers.
“Our data suggest that cystoscopy alone is the most cost-effective strategy, and that the addition of urinary markers adds to cost without improved detection of invasive disease,” said first author Dr. Jose A. Karam, a urologic oncology fellow at the University of Texas M.D. Anderson Cancer Center in Houston.
Evidence-based data are lacking on the outcomes of surveillance strategies among patients who have been treated for superficial (non–muscle invasive) bladder cancer. Many urinary-based markers are now available for use, but cystoscopy remains the standard of care, he said.
In the study, consecutive patients needing surveillance after treatment of superficial bladder cancer underwent these tests at study entry: cystoscopy, urine cytology, NMP22 BladderChek test (a urine test marketed by Inverness Medical Innovations that measures a protein associated with bladder cancer), and FISH (fluorescence in situ hybridization) UroVysion test (a urine test marketed by Abbott Molecular that detects chromosomal abnormalities associated with bladder cancer).
The bladder cancers for which they had been treated were mainly of Ta stage (71%) and low grade (58%). In 25 patients, new tumors were found at study entry or by the first follow-up assessment, a median of 4.1 months later, Dr. Karam reported at the symposium, sponsored by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
Based on 2009 Medicare reimbursement rates, the cost per cancer detected was lowest with cystoscopy alone ($7,692), and highest with cystoscopy plus FISH ($19,111). Values were intermediate for cystoscopy plus cytology ($10,267), cystoscopy plus NMP ($11,143), and cystoscopy plus NMP with FISH confirmation in the event of a positive NMP result ($9,557).
The cancer detection rate was lowest with cystoscopy alone (52%) and highest with cystoscopy plus FISH (72%). Values were intermediate for cystoscopy plus cytology (60%), cystoscopy plus NMP (56%), and cystoscopy plus NMP with FISH confirmation (56%). Subsequent analyses showed that cystoscopy plus FISH picked up more early, noninvasive (carcinoma in situ and Ta) tumors than did cystoscopy alone. But it similarly missed the two more advanced (T1 and T2) tumors that were found, “which arguably are the ones that matter the most,” Dr. Karam commented.
Cystoscopy alone appears to be the most cost-effective approach, he concluded, cautioning that “these [urinary-based] markers should be used carefully and judiciously in patients with bladder cancer.” Because all patients received care from bladder specialists, the findings may not be generalizable to community practices, he added.
Dr. Nicholas J. Vogelzang, one of the developers of the NMP22 assay, chaired a press briefing at which the study was discussed. Dr. Vogelzang, chair and medical director of the developmental therapeutics committee of U.S. Oncology, said he found the results “very chagrining but nonetheless very important.”
Urinary-based markers 'should be used carefully and judiciously in patients with bladder cancer.'
Source Dr. Karam
Major Finding: Cystoscopy alone, the least expensive of five surveillance strategies, picked up two invasive bladder cancers that were missed by urine testing.
Data Source: A study in 200 consecutive patients.
Disclosures: Dr. Karam reported having no conflicts of interest related to the study. A coauthor received honoraria from Abbott Molecular.
SAN FRANCISCO — Cystoscopy alone offers the best combination of cost and sensitivity for surveillance after treatment of superficial bladder cancer, new data show.
In a prospective study comparing five surveillance strategies in 200 patients, the addition of various urine tests to cystoscopy increased the cost per cancer detected, but did not find more invasive tumors, researchers reported at a symposium on genitourinary cancers.
“Our data suggest that cystoscopy alone is the most cost-effective strategy, and that the addition of urinary markers adds to cost without improved detection of invasive disease,” said first author Dr. Jose A. Karam, a urologic oncology fellow at the University of Texas M.D. Anderson Cancer Center in Houston.
Evidence-based data are lacking on the outcomes of surveillance strategies among patients who have been treated for superficial (non–muscle invasive) bladder cancer. Many urinary-based markers are now available for use, but cystoscopy remains the standard of care, he said.
In the study, consecutive patients needing surveillance after treatment of superficial bladder cancer underwent these tests at study entry: cystoscopy, urine cytology, NMP22 BladderChek test (a urine test marketed by Inverness Medical Innovations that measures a protein associated with bladder cancer), and FISH (fluorescence in situ hybridization) UroVysion test (a urine test marketed by Abbott Molecular that detects chromosomal abnormalities associated with bladder cancer).
The bladder cancers for which they had been treated were mainly of Ta stage (71%) and low grade (58%). In 25 patients, new tumors were found at study entry or by the first follow-up assessment, a median of 4.1 months later, Dr. Karam reported at the symposium, sponsored by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
Based on 2009 Medicare reimbursement rates, the cost per cancer detected was lowest with cystoscopy alone ($7,692), and highest with cystoscopy plus FISH ($19,111). Values were intermediate for cystoscopy plus cytology ($10,267), cystoscopy plus NMP ($11,143), and cystoscopy plus NMP with FISH confirmation in the event of a positive NMP result ($9,557).
The cancer detection rate was lowest with cystoscopy alone (52%) and highest with cystoscopy plus FISH (72%). Values were intermediate for cystoscopy plus cytology (60%), cystoscopy plus NMP (56%), and cystoscopy plus NMP with FISH confirmation (56%). Subsequent analyses showed that cystoscopy plus FISH picked up more early, noninvasive (carcinoma in situ and Ta) tumors than did cystoscopy alone. But it similarly missed the two more advanced (T1 and T2) tumors that were found, “which arguably are the ones that matter the most,” Dr. Karam commented.
Cystoscopy alone appears to be the most cost-effective approach, he concluded, cautioning that “these [urinary-based] markers should be used carefully and judiciously in patients with bladder cancer.” Because all patients received care from bladder specialists, the findings may not be generalizable to community practices, he added.
Dr. Nicholas J. Vogelzang, one of the developers of the NMP22 assay, chaired a press briefing at which the study was discussed. Dr. Vogelzang, chair and medical director of the developmental therapeutics committee of U.S. Oncology, said he found the results “very chagrining but nonetheless very important.”
Urinary-based markers 'should be used carefully and judiciously in patients with bladder cancer.'
Source Dr. Karam
Urinary Assay Improves Prostate Ca Detection
Major Finding: A new urinary assay had high specificity for distinguishing between patients with and without cancer (88%–93%).
Data Source: Two cohort studies in men who were scheduled for prostatectomy or prostate biopsy.
Disclosures: Dr. Wei and Dr. Amberson reported receiving research funding from Gen-Probe Inc. Some coauthors of both studies disclosed employment or leadership roles and stock ownership in Gen-Probe.
SAN FRANCISCO — A new urinary assay for a common gene rearrangement in prostate cancer improves the detection of this disease and the differentiation of its more aggressive forms, according to two cohort studies reported at a symposium on genitourinary cancers.
The studies, conducted among men who were scheduled for prostate biopsy or prostatectomy, found that the assay supplemented conventional risk factors for accurately identifying those having prostate cancer. In addition, higher assay scores correlated with the presence of adverse tumor features.
“This new … urinary assay has the ability to not only detect prostate cancer, but may help move us forward in our paradigm to also detect clinically significant prostate cancer,” said Dr. John T. Wei, who presented results of the first study on behalf of first author Sheila M.J. Aubin, Ph.D., of Gen-Probe Inc., the company that is developing the assay.
Prostate-specific antigen (PSA) level and digital rectal examination both have poor specificity for detecting prostate cancer, Dr. Wei observed. “Because of this, many patients will go on to have false-positive PSA tests and unnecessary prostate biopsies, and suffer a significant amount of distress.”
Moreover, these tests are unable to differentiate indolent from aggressive cancer, which becomes more important given current efforts to tailor management to disease characteristics, according to Dr. Wei, professor of urology at the University of Michigan in Ann Arbor. “Taken together, there is a significant unmet need, and that is to develop a highly specific test that can tell us if a patient has clinically significant cancer or not,” he said.
About half of prostate cancers exhibit fusion of the androgen-regulated TMPRSS2 gene and the ERG oncogene, Dr. Wei told attendees of the symposium, which was sponsored by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Urologic Oncology. Cancers that harbor this fusion gene (abbreviated T2:ERG) have increased cell growth, invasion, and metastasis, and decreased apoptosis.
In the first study, the investigators assessed the performance of the novel assay, which measures levels of T2:ERG messenger RNA in urine, using urine specimens collected after digital rectal examination and before either prostate biopsy (623 men) or prostatectomy (142 men).
Analyses of biopsy-based indicators showed that the T2:ERG score was correlated with the number of cores that were positive, the percentage of cores that were positive, and the greatest percentage involvement of any core by cancer, according to Dr. Wei. Also, the median score was higher among patients with biopsy-significant cancer as defined by Epstein criteria.
Analyses of prostatectomy-based indicators showed that the T2:ERG score was correlated with the maximum tumor dimension. In addition, the median score was higher among patients who had an upgrade of the Gleason score between biopsy and prostatectomy, a prostatectomy Gleason score of greater than 6, and prostatectomy-significant cancer as defined from tumor characteristics.
Compared with the PCPT (Prostate Cancer Prevention Trial) risk score alone, the combination of this score with the T2:ERG score more accurately identified men who had prostate cancer.
Dr. Wei noted that at cutoff scores of 100 and 200, the T2:ERG assay had high specificity for distinguishing between patients with and without cancer (88%–93%), with biopsy-significant and -insignificant cancer (85%–95%), and with prostatectomy-significant and -insignificant cancer (95%–100%).
Independent trials of the assay are needed, Dr. Wei acknowledged. He said that ongoing discussions are looking at the possibility of incorporating the assay into trials of the Early Detection Research Network.
In the second study, investigators tested the same T2:ERG assay using urine specimens that were collected after digital rectal examination from 471 men who were scheduled for prostate cancer biopsy at community clinics, according to presenting author Dr. James B.
Some 44% of patients had positive biopsies, he reported. The median age was 66 years in the patients with cancer and 63 years in the patients without it. The median serum PSA level was 5.0 and 4.3 ng/mL, respectively.
When used alone, the T2:ERG score had a high specificity (87%) for detection of biopsy-proven cancer, reported Dr. Amberson, divisional medical director of Dianon Systems, which performs diagnostic and prognostic testing. Sensitivity was 39%, “in line with the expected gene fusion prevalence in this population of about 50%.”
The median T2:ERG score was higher in patients who had a Gleason score of 7 or greater, involvement of more than 50% of positive cores by cancer, and three or more positive cores.
“The T2:ERG assay significantly improved the diagnostic accuracy of a logistic regression model” for prostate cancer detection, Dr. Amberson said.
The area under the curve was 0.597 for serum PSA level alone, 0.715 for a model using multiple risk factors (serum PSA level, percentage of free PSA, age, prostate volume, family history, race, digital rectal examination result, prior biopsy history, and urine prostate cancer gene 3 level), and 0.754 when the T2:ERG score was added to the model.
Major Finding: A new urinary assay had high specificity for distinguishing between patients with and without cancer (88%–93%).
Data Source: Two cohort studies in men who were scheduled for prostatectomy or prostate biopsy.
Disclosures: Dr. Wei and Dr. Amberson reported receiving research funding from Gen-Probe Inc. Some coauthors of both studies disclosed employment or leadership roles and stock ownership in Gen-Probe.
SAN FRANCISCO — A new urinary assay for a common gene rearrangement in prostate cancer improves the detection of this disease and the differentiation of its more aggressive forms, according to two cohort studies reported at a symposium on genitourinary cancers.
The studies, conducted among men who were scheduled for prostate biopsy or prostatectomy, found that the assay supplemented conventional risk factors for accurately identifying those having prostate cancer. In addition, higher assay scores correlated with the presence of adverse tumor features.
“This new … urinary assay has the ability to not only detect prostate cancer, but may help move us forward in our paradigm to also detect clinically significant prostate cancer,” said Dr. John T. Wei, who presented results of the first study on behalf of first author Sheila M.J. Aubin, Ph.D., of Gen-Probe Inc., the company that is developing the assay.
Prostate-specific antigen (PSA) level and digital rectal examination both have poor specificity for detecting prostate cancer, Dr. Wei observed. “Because of this, many patients will go on to have false-positive PSA tests and unnecessary prostate biopsies, and suffer a significant amount of distress.”
Moreover, these tests are unable to differentiate indolent from aggressive cancer, which becomes more important given current efforts to tailor management to disease characteristics, according to Dr. Wei, professor of urology at the University of Michigan in Ann Arbor. “Taken together, there is a significant unmet need, and that is to develop a highly specific test that can tell us if a patient has clinically significant cancer or not,” he said.
About half of prostate cancers exhibit fusion of the androgen-regulated TMPRSS2 gene and the ERG oncogene, Dr. Wei told attendees of the symposium, which was sponsored by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Urologic Oncology. Cancers that harbor this fusion gene (abbreviated T2:ERG) have increased cell growth, invasion, and metastasis, and decreased apoptosis.
In the first study, the investigators assessed the performance of the novel assay, which measures levels of T2:ERG messenger RNA in urine, using urine specimens collected after digital rectal examination and before either prostate biopsy (623 men) or prostatectomy (142 men).
Analyses of biopsy-based indicators showed that the T2:ERG score was correlated with the number of cores that were positive, the percentage of cores that were positive, and the greatest percentage involvement of any core by cancer, according to Dr. Wei. Also, the median score was higher among patients with biopsy-significant cancer as defined by Epstein criteria.
Analyses of prostatectomy-based indicators showed that the T2:ERG score was correlated with the maximum tumor dimension. In addition, the median score was higher among patients who had an upgrade of the Gleason score between biopsy and prostatectomy, a prostatectomy Gleason score of greater than 6, and prostatectomy-significant cancer as defined from tumor characteristics.
Compared with the PCPT (Prostate Cancer Prevention Trial) risk score alone, the combination of this score with the T2:ERG score more accurately identified men who had prostate cancer.
Dr. Wei noted that at cutoff scores of 100 and 200, the T2:ERG assay had high specificity for distinguishing between patients with and without cancer (88%–93%), with biopsy-significant and -insignificant cancer (85%–95%), and with prostatectomy-significant and -insignificant cancer (95%–100%).
Independent trials of the assay are needed, Dr. Wei acknowledged. He said that ongoing discussions are looking at the possibility of incorporating the assay into trials of the Early Detection Research Network.
In the second study, investigators tested the same T2:ERG assay using urine specimens that were collected after digital rectal examination from 471 men who were scheduled for prostate cancer biopsy at community clinics, according to presenting author Dr. James B.
Some 44% of patients had positive biopsies, he reported. The median age was 66 years in the patients with cancer and 63 years in the patients without it. The median serum PSA level was 5.0 and 4.3 ng/mL, respectively.
When used alone, the T2:ERG score had a high specificity (87%) for detection of biopsy-proven cancer, reported Dr. Amberson, divisional medical director of Dianon Systems, which performs diagnostic and prognostic testing. Sensitivity was 39%, “in line with the expected gene fusion prevalence in this population of about 50%.”
The median T2:ERG score was higher in patients who had a Gleason score of 7 or greater, involvement of more than 50% of positive cores by cancer, and three or more positive cores.
“The T2:ERG assay significantly improved the diagnostic accuracy of a logistic regression model” for prostate cancer detection, Dr. Amberson said.
The area under the curve was 0.597 for serum PSA level alone, 0.715 for a model using multiple risk factors (serum PSA level, percentage of free PSA, age, prostate volume, family history, race, digital rectal examination result, prior biopsy history, and urine prostate cancer gene 3 level), and 0.754 when the T2:ERG score was added to the model.
Major Finding: A new urinary assay had high specificity for distinguishing between patients with and without cancer (88%–93%).
Data Source: Two cohort studies in men who were scheduled for prostatectomy or prostate biopsy.
Disclosures: Dr. Wei and Dr. Amberson reported receiving research funding from Gen-Probe Inc. Some coauthors of both studies disclosed employment or leadership roles and stock ownership in Gen-Probe.
SAN FRANCISCO — A new urinary assay for a common gene rearrangement in prostate cancer improves the detection of this disease and the differentiation of its more aggressive forms, according to two cohort studies reported at a symposium on genitourinary cancers.
The studies, conducted among men who were scheduled for prostate biopsy or prostatectomy, found that the assay supplemented conventional risk factors for accurately identifying those having prostate cancer. In addition, higher assay scores correlated with the presence of adverse tumor features.
“This new … urinary assay has the ability to not only detect prostate cancer, but may help move us forward in our paradigm to also detect clinically significant prostate cancer,” said Dr. John T. Wei, who presented results of the first study on behalf of first author Sheila M.J. Aubin, Ph.D., of Gen-Probe Inc., the company that is developing the assay.
Prostate-specific antigen (PSA) level and digital rectal examination both have poor specificity for detecting prostate cancer, Dr. Wei observed. “Because of this, many patients will go on to have false-positive PSA tests and unnecessary prostate biopsies, and suffer a significant amount of distress.”
Moreover, these tests are unable to differentiate indolent from aggressive cancer, which becomes more important given current efforts to tailor management to disease characteristics, according to Dr. Wei, professor of urology at the University of Michigan in Ann Arbor. “Taken together, there is a significant unmet need, and that is to develop a highly specific test that can tell us if a patient has clinically significant cancer or not,” he said.
About half of prostate cancers exhibit fusion of the androgen-regulated TMPRSS2 gene and the ERG oncogene, Dr. Wei told attendees of the symposium, which was sponsored by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Urologic Oncology. Cancers that harbor this fusion gene (abbreviated T2:ERG) have increased cell growth, invasion, and metastasis, and decreased apoptosis.
In the first study, the investigators assessed the performance of the novel assay, which measures levels of T2:ERG messenger RNA in urine, using urine specimens collected after digital rectal examination and before either prostate biopsy (623 men) or prostatectomy (142 men).
Analyses of biopsy-based indicators showed that the T2:ERG score was correlated with the number of cores that were positive, the percentage of cores that were positive, and the greatest percentage involvement of any core by cancer, according to Dr. Wei. Also, the median score was higher among patients with biopsy-significant cancer as defined by Epstein criteria.
Analyses of prostatectomy-based indicators showed that the T2:ERG score was correlated with the maximum tumor dimension. In addition, the median score was higher among patients who had an upgrade of the Gleason score between biopsy and prostatectomy, a prostatectomy Gleason score of greater than 6, and prostatectomy-significant cancer as defined from tumor characteristics.
Compared with the PCPT (Prostate Cancer Prevention Trial) risk score alone, the combination of this score with the T2:ERG score more accurately identified men who had prostate cancer.
Dr. Wei noted that at cutoff scores of 100 and 200, the T2:ERG assay had high specificity for distinguishing between patients with and without cancer (88%–93%), with biopsy-significant and -insignificant cancer (85%–95%), and with prostatectomy-significant and -insignificant cancer (95%–100%).
Independent trials of the assay are needed, Dr. Wei acknowledged. He said that ongoing discussions are looking at the possibility of incorporating the assay into trials of the Early Detection Research Network.
In the second study, investigators tested the same T2:ERG assay using urine specimens that were collected after digital rectal examination from 471 men who were scheduled for prostate cancer biopsy at community clinics, according to presenting author Dr. James B.
Some 44% of patients had positive biopsies, he reported. The median age was 66 years in the patients with cancer and 63 years in the patients without it. The median serum PSA level was 5.0 and 4.3 ng/mL, respectively.
When used alone, the T2:ERG score had a high specificity (87%) for detection of biopsy-proven cancer, reported Dr. Amberson, divisional medical director of Dianon Systems, which performs diagnostic and prognostic testing. Sensitivity was 39%, “in line with the expected gene fusion prevalence in this population of about 50%.”
The median T2:ERG score was higher in patients who had a Gleason score of 7 or greater, involvement of more than 50% of positive cores by cancer, and three or more positive cores.
“The T2:ERG assay significantly improved the diagnostic accuracy of a logistic regression model” for prostate cancer detection, Dr. Amberson said.
The area under the curve was 0.597 for serum PSA level alone, 0.715 for a model using multiple risk factors (serum PSA level, percentage of free PSA, age, prostate volume, family history, race, digital rectal examination result, prior biopsy history, and urine prostate cancer gene 3 level), and 0.754 when the T2:ERG score was added to the model.
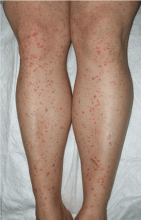
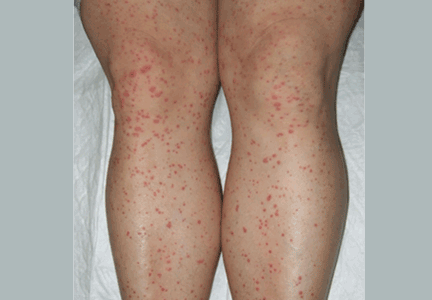
Palpable purpura
Q: Which is the most likely diagnosis?
- Idiopathic thrombocytopenic purpura
- Vitamin C deficiency (scurvy)
- Kaposi sarcoma not related to human immunodeficiency virus (HIV) infection
- Henoch-Schönlein purpura
- Polyarteritis nodosa
A: The correct answer is Henoch-Schönlein purpura.
Idiopathic thrombocytopenic purpura is an autoimmune disease caused by specific antibodies against platelet-membrane glycoproteins. It is characterized by thrombocytopenia not explainable by contact with toxic substances or by other causes. Along with nonpalpable purpura, other common signs are epistaxis, gingival bleeding, menorrhagia, and retinal hemorrhage.
Scurvy is an uncommon deficiency of ascorbic acid (vitamin C). The elderly and alcoholics are at higher risk, as they do not take in enough vitamin C in the diet. Patients usually show perifollicular hemorrhages of the skin and mucous membranes, typically petechial hemorrhage or ecchymosis of the gums around the upper incisors. Other cutaneous signs are follicular hyperkeratosis on the forearms, small corkscrew hairs, and sicca syndrome, which is more common in adults.
Non-HIV Kaposi sarcoma usually affects elderly patients, with pink, red, or brown papules or nodules on the legs and, less commonly, on the head and neck. Histopathologic examination shows newly formed irregular blood vessels with an inflammatory infiltrate of plasma cells and lymphocytes; immunohistochemical human herpes virus staining is usually positive.
Polyarteritis nodosa is a systemic vasculitis that affects medium or small arteries with necrotizing inflammation; renal glomeruli and arterioles, capillaries, and venules are unaffected. Skin manifestations include palpable purpura, livedo reticularis, ulcers, and distal gangrene. The condition also usually affects the kidneys, the heart, and the musculoskeletal and nervous systems.
A SYSTEMIC VASCULITIS
Henoch-Schönlein purpura is a systemic vasculitis affecting the skin, gastrointestinal tract, kidneys, and joints. Palpable purpura and joint pain are the most common and consistent presenting symptoms. The kidneys are affected in about one-third of children and in 60% of adults, and this is the major factor determining the long-term outcome.1
In our patient, laboratory testing that included a complete blood cell count, biochemical testing (including IgA levels), urinary sediment, and coagulation studies showed no abnormalities except elevations of the erythrocyte sedimentation rate and the concentration of C-reactive protein (an acute-phase reactant). These can be normal in some patients. Renal involvement was also not present.
DIAGNOSIS
The diagnosis relies on clinical manifestations. Because Henoch-Schönlein purpura is less common in adults, biopsy plays a more important role in establishing the diagnosis in this age group, and it does this by demonstrating leukocytoclastic vasculitis with a predominance of IgA deposition under immunofluorescence. Recent studies in children showed that an elevated IgA concentration along with reduced IgM levels was associated with a higher rate of severe complications.2 However, depending on the age of the biopsied lesion, IgA may not be detected.
TREATMENT DIRECTED AT SYMPTOMS
Our patient received oral corticosteroids 0.5 mg/kg per day for 20 days, and the lesions resolved by 4 weeks.
Management of Henoch-Schönlein purpura is mainly directed at the symptoms, with oral hydration and nonsteroidal anti-inflammatory drugs. For severe cases, a short course of corticosteroids (0.5–1 mg/kg) may be used.
Although no controlled clinical trial has proven that Henoch-Schönlein purpura responds to corticosteroids, colchicine, or other drugs, corticosteroids are used most often, especially in patients with renal disease. Patients with severe renal insufficiency, abdominal pain, joint involvement, or bleeding should be hospitalized. Plasmapheresis3 has been used in severe cases.
HENOCH-SCHÖNLEIN PURPURA AND MALIGNANCY
During a follow-up evaluation 1 month later, our patient was diagnosed with adenocarcinoma of the breast. This highlights the value of a workup for cancer in adults with cutaneous vasculitis.
Cutaneous vasculitis can represent a paraneoplastic syndrome associated with a malignant tumor. The pathophysiology of this association is unclear, but one proposed mechanism is the exaggerated production of antibodies that react against tumor neoantigens, leading to the formation of immune complexes, or that occasionally recognize endothelial cells because of similarities with tumor antigens. Another theory is that abnormally high levels of inflammatory cytokines are produced by neoplastic cells or in response to decreased immune complex clearance.
Yet another theory is that hyperviscosity of the blood, seen in some cancers, increases the contact time for deposition of immune complexes and causes endothelial damage. Drugs used to treat cancer have also been reported to produce Henoch-Schönlein purpura.4
Although hematologic malignancy is three to five times more common than solid tumors in patients with small-vessel vasculitis, the disease has been associated with solid tumors of the liver, skin, colon, and breast in adults over age 40.5 An evaluation for neoplasm is therefore reasonable in adults with Henoch-Schönlein purpura, as is an evaluation for tumor recurrence or metastasis if the patient has been previously treated for a malignant tumor.
- Rieu P, Noël LH. Henoch-Schönlein nephritis in children and adults. Morphological features and clinicopathological correlations. Ann Med Interne (Paris) 1999; 150:151–159.
- Fretzayas A, Sionti I, Moustaki M, Nicolaidou P. Clinical impact of altered immunoglobulin levels in Henoch-Schönlein purpura. Pediatr Int 2009; 51:381–384.
- Donghi D, Schanz U, Sahrbacher U, et al. Life-threatening or organimpairing Henoch-Schönlein purpura: plasmapheresis may save lives and limit organ damage. Dermatology 2009; 219:167–170.
- Mitsui H, Shibagaki N, Kawamura T, Matsue H, Shimada S. A clinical study of Henoch-Schönlein purpura associated with malignancy. J Eur Acad Dermatol Venereol 2009; 23:394–401.
- Maestri A, Malacarne P, Santini A. Henoch-Schönlein syndrome associated with breast cancer. A case report. Angiology 1995; 46:625–627.
Q: Which is the most likely diagnosis?
- Idiopathic thrombocytopenic purpura
- Vitamin C deficiency (scurvy)
- Kaposi sarcoma not related to human immunodeficiency virus (HIV) infection
- Henoch-Schönlein purpura
- Polyarteritis nodosa
A: The correct answer is Henoch-Schönlein purpura.
Idiopathic thrombocytopenic purpura is an autoimmune disease caused by specific antibodies against platelet-membrane glycoproteins. It is characterized by thrombocytopenia not explainable by contact with toxic substances or by other causes. Along with nonpalpable purpura, other common signs are epistaxis, gingival bleeding, menorrhagia, and retinal hemorrhage.
Scurvy is an uncommon deficiency of ascorbic acid (vitamin C). The elderly and alcoholics are at higher risk, as they do not take in enough vitamin C in the diet. Patients usually show perifollicular hemorrhages of the skin and mucous membranes, typically petechial hemorrhage or ecchymosis of the gums around the upper incisors. Other cutaneous signs are follicular hyperkeratosis on the forearms, small corkscrew hairs, and sicca syndrome, which is more common in adults.
Non-HIV Kaposi sarcoma usually affects elderly patients, with pink, red, or brown papules or nodules on the legs and, less commonly, on the head and neck. Histopathologic examination shows newly formed irregular blood vessels with an inflammatory infiltrate of plasma cells and lymphocytes; immunohistochemical human herpes virus staining is usually positive.
Polyarteritis nodosa is a systemic vasculitis that affects medium or small arteries with necrotizing inflammation; renal glomeruli and arterioles, capillaries, and venules are unaffected. Skin manifestations include palpable purpura, livedo reticularis, ulcers, and distal gangrene. The condition also usually affects the kidneys, the heart, and the musculoskeletal and nervous systems.
A SYSTEMIC VASCULITIS
Henoch-Schönlein purpura is a systemic vasculitis affecting the skin, gastrointestinal tract, kidneys, and joints. Palpable purpura and joint pain are the most common and consistent presenting symptoms. The kidneys are affected in about one-third of children and in 60% of adults, and this is the major factor determining the long-term outcome.1
In our patient, laboratory testing that included a complete blood cell count, biochemical testing (including IgA levels), urinary sediment, and coagulation studies showed no abnormalities except elevations of the erythrocyte sedimentation rate and the concentration of C-reactive protein (an acute-phase reactant). These can be normal in some patients. Renal involvement was also not present.
DIAGNOSIS
The diagnosis relies on clinical manifestations. Because Henoch-Schönlein purpura is less common in adults, biopsy plays a more important role in establishing the diagnosis in this age group, and it does this by demonstrating leukocytoclastic vasculitis with a predominance of IgA deposition under immunofluorescence. Recent studies in children showed that an elevated IgA concentration along with reduced IgM levels was associated with a higher rate of severe complications.2 However, depending on the age of the biopsied lesion, IgA may not be detected.
TREATMENT DIRECTED AT SYMPTOMS
Our patient received oral corticosteroids 0.5 mg/kg per day for 20 days, and the lesions resolved by 4 weeks.
Management of Henoch-Schönlein purpura is mainly directed at the symptoms, with oral hydration and nonsteroidal anti-inflammatory drugs. For severe cases, a short course of corticosteroids (0.5–1 mg/kg) may be used.
Although no controlled clinical trial has proven that Henoch-Schönlein purpura responds to corticosteroids, colchicine, or other drugs, corticosteroids are used most often, especially in patients with renal disease. Patients with severe renal insufficiency, abdominal pain, joint involvement, or bleeding should be hospitalized. Plasmapheresis3 has been used in severe cases.
HENOCH-SCHÖNLEIN PURPURA AND MALIGNANCY
During a follow-up evaluation 1 month later, our patient was diagnosed with adenocarcinoma of the breast. This highlights the value of a workup for cancer in adults with cutaneous vasculitis.
Cutaneous vasculitis can represent a paraneoplastic syndrome associated with a malignant tumor. The pathophysiology of this association is unclear, but one proposed mechanism is the exaggerated production of antibodies that react against tumor neoantigens, leading to the formation of immune complexes, or that occasionally recognize endothelial cells because of similarities with tumor antigens. Another theory is that abnormally high levels of inflammatory cytokines are produced by neoplastic cells or in response to decreased immune complex clearance.
Yet another theory is that hyperviscosity of the blood, seen in some cancers, increases the contact time for deposition of immune complexes and causes endothelial damage. Drugs used to treat cancer have also been reported to produce Henoch-Schönlein purpura.4
Although hematologic malignancy is three to five times more common than solid tumors in patients with small-vessel vasculitis, the disease has been associated with solid tumors of the liver, skin, colon, and breast in adults over age 40.5 An evaluation for neoplasm is therefore reasonable in adults with Henoch-Schönlein purpura, as is an evaluation for tumor recurrence or metastasis if the patient has been previously treated for a malignant tumor.
Q: Which is the most likely diagnosis?
- Idiopathic thrombocytopenic purpura
- Vitamin C deficiency (scurvy)
- Kaposi sarcoma not related to human immunodeficiency virus (HIV) infection
- Henoch-Schönlein purpura
- Polyarteritis nodosa
A: The correct answer is Henoch-Schönlein purpura.
Idiopathic thrombocytopenic purpura is an autoimmune disease caused by specific antibodies against platelet-membrane glycoproteins. It is characterized by thrombocytopenia not explainable by contact with toxic substances or by other causes. Along with nonpalpable purpura, other common signs are epistaxis, gingival bleeding, menorrhagia, and retinal hemorrhage.
Scurvy is an uncommon deficiency of ascorbic acid (vitamin C). The elderly and alcoholics are at higher risk, as they do not take in enough vitamin C in the diet. Patients usually show perifollicular hemorrhages of the skin and mucous membranes, typically petechial hemorrhage or ecchymosis of the gums around the upper incisors. Other cutaneous signs are follicular hyperkeratosis on the forearms, small corkscrew hairs, and sicca syndrome, which is more common in adults.
Non-HIV Kaposi sarcoma usually affects elderly patients, with pink, red, or brown papules or nodules on the legs and, less commonly, on the head and neck. Histopathologic examination shows newly formed irregular blood vessels with an inflammatory infiltrate of plasma cells and lymphocytes; immunohistochemical human herpes virus staining is usually positive.
Polyarteritis nodosa is a systemic vasculitis that affects medium or small arteries with necrotizing inflammation; renal glomeruli and arterioles, capillaries, and venules are unaffected. Skin manifestations include palpable purpura, livedo reticularis, ulcers, and distal gangrene. The condition also usually affects the kidneys, the heart, and the musculoskeletal and nervous systems.
A SYSTEMIC VASCULITIS
Henoch-Schönlein purpura is a systemic vasculitis affecting the skin, gastrointestinal tract, kidneys, and joints. Palpable purpura and joint pain are the most common and consistent presenting symptoms. The kidneys are affected in about one-third of children and in 60% of adults, and this is the major factor determining the long-term outcome.1
In our patient, laboratory testing that included a complete blood cell count, biochemical testing (including IgA levels), urinary sediment, and coagulation studies showed no abnormalities except elevations of the erythrocyte sedimentation rate and the concentration of C-reactive protein (an acute-phase reactant). These can be normal in some patients. Renal involvement was also not present.
DIAGNOSIS
The diagnosis relies on clinical manifestations. Because Henoch-Schönlein purpura is less common in adults, biopsy plays a more important role in establishing the diagnosis in this age group, and it does this by demonstrating leukocytoclastic vasculitis with a predominance of IgA deposition under immunofluorescence. Recent studies in children showed that an elevated IgA concentration along with reduced IgM levels was associated with a higher rate of severe complications.2 However, depending on the age of the biopsied lesion, IgA may not be detected.
TREATMENT DIRECTED AT SYMPTOMS
Our patient received oral corticosteroids 0.5 mg/kg per day for 20 days, and the lesions resolved by 4 weeks.
Management of Henoch-Schönlein purpura is mainly directed at the symptoms, with oral hydration and nonsteroidal anti-inflammatory drugs. For severe cases, a short course of corticosteroids (0.5–1 mg/kg) may be used.
Although no controlled clinical trial has proven that Henoch-Schönlein purpura responds to corticosteroids, colchicine, or other drugs, corticosteroids are used most often, especially in patients with renal disease. Patients with severe renal insufficiency, abdominal pain, joint involvement, or bleeding should be hospitalized. Plasmapheresis3 has been used in severe cases.
HENOCH-SCHÖNLEIN PURPURA AND MALIGNANCY
During a follow-up evaluation 1 month later, our patient was diagnosed with adenocarcinoma of the breast. This highlights the value of a workup for cancer in adults with cutaneous vasculitis.
Cutaneous vasculitis can represent a paraneoplastic syndrome associated with a malignant tumor. The pathophysiology of this association is unclear, but one proposed mechanism is the exaggerated production of antibodies that react against tumor neoantigens, leading to the formation of immune complexes, or that occasionally recognize endothelial cells because of similarities with tumor antigens. Another theory is that abnormally high levels of inflammatory cytokines are produced by neoplastic cells or in response to decreased immune complex clearance.
Yet another theory is that hyperviscosity of the blood, seen in some cancers, increases the contact time for deposition of immune complexes and causes endothelial damage. Drugs used to treat cancer have also been reported to produce Henoch-Schönlein purpura.4
Although hematologic malignancy is three to five times more common than solid tumors in patients with small-vessel vasculitis, the disease has been associated with solid tumors of the liver, skin, colon, and breast in adults over age 40.5 An evaluation for neoplasm is therefore reasonable in adults with Henoch-Schönlein purpura, as is an evaluation for tumor recurrence or metastasis if the patient has been previously treated for a malignant tumor.
- Rieu P, Noël LH. Henoch-Schönlein nephritis in children and adults. Morphological features and clinicopathological correlations. Ann Med Interne (Paris) 1999; 150:151–159.
- Fretzayas A, Sionti I, Moustaki M, Nicolaidou P. Clinical impact of altered immunoglobulin levels in Henoch-Schönlein purpura. Pediatr Int 2009; 51:381–384.
- Donghi D, Schanz U, Sahrbacher U, et al. Life-threatening or organimpairing Henoch-Schönlein purpura: plasmapheresis may save lives and limit organ damage. Dermatology 2009; 219:167–170.
- Mitsui H, Shibagaki N, Kawamura T, Matsue H, Shimada S. A clinical study of Henoch-Schönlein purpura associated with malignancy. J Eur Acad Dermatol Venereol 2009; 23:394–401.
- Maestri A, Malacarne P, Santini A. Henoch-Schönlein syndrome associated with breast cancer. A case report. Angiology 1995; 46:625–627.
- Rieu P, Noël LH. Henoch-Schönlein nephritis in children and adults. Morphological features and clinicopathological correlations. Ann Med Interne (Paris) 1999; 150:151–159.
- Fretzayas A, Sionti I, Moustaki M, Nicolaidou P. Clinical impact of altered immunoglobulin levels in Henoch-Schönlein purpura. Pediatr Int 2009; 51:381–384.
- Donghi D, Schanz U, Sahrbacher U, et al. Life-threatening or organimpairing Henoch-Schönlein purpura: plasmapheresis may save lives and limit organ damage. Dermatology 2009; 219:167–170.
- Mitsui H, Shibagaki N, Kawamura T, Matsue H, Shimada S. A clinical study of Henoch-Schönlein purpura associated with malignancy. J Eur Acad Dermatol Venereol 2009; 23:394–401.
- Maestri A, Malacarne P, Santini A. Henoch-Schönlein syndrome associated with breast cancer. A case report. Angiology 1995; 46:625–627.
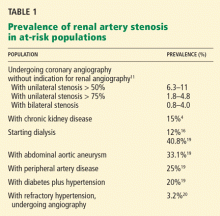
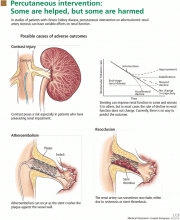
Stenting for atherosclerotic renal artery stenosis: One poorly designed trial after another
The role of stenting for atherosclerotic renal artery stenosis is hotly debated among different specialties.1,2 If we may generalize a bit, interventionalists (cardiologists, interventional radiologists, vascular surgeons, and vascular medicine specialists) have been in favor of liberal use of stenting, and nephrologists often favor medical therapy alone. And as with all controversial issues, each group feels rather strongly about its position.
Because few prospective randomized trials have been completed, the management of atherosclerotic renal artery stenosis has been guided by retrospective studies and case series. 3
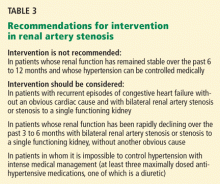
In this issue of the Cleveland Clinic Journal of Medicine, Dr. James Simon4 provides an excellent overview of the prevalence, natural history, and clinical presentation of atherosclerotic renal artery stenosis. In addition, he does an admirable job of reviewing the available prospective randomized trials and providing editorial commentary about the role of the various specialists in the management of renal artery disease. And while the title of his paper says that it is “time to be less aggressive,” Dr. Simon ultimately comes to the same conclusions that we do5 on the indications for renal artery stenting (see Table 3 of Dr. Simon’s article), which are those of the multidisciplinary 2006 American College of Cardiology/American Heart Association guidelines on the management of peripheral artery disease.3
So what then is all the controversy about? We all agree that prospective randomized trials that provide class I, level A evidence impart the only unbiased scientific information on the best treatment strategy for patients with renal artery disease. The basic controversial issue is the interpretation of these trials. We contend that the three randomized trials of stenting vs medical therapy published so far6–8 (see below) are so seriously flawed that it is impossible to make treatment decisions based on their results.
Since these trials were published in wellrespected journals, their results are often taken as gospel. However, careful review of each of these will reveal the flaws in study design and implementation.
THE DRASTIC TRIAL
In the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial,6 106 patients with renal artery stenosis and hypertension (diastolic blood pressure > 95 mm Hg) despite treatment with two antihypertensive medications were randomly assigned to either renal angioplasty (n = 56) or drug therapy (n = 50).
Authors’ conclusions
“In the treatment of patients with hypertension and renal-artery stenosis, angioplasty has little advantage over antihypertensive-drug therapy.”6
Four serious problems
As we discussed in a letter to the editor of the New England Journal of Medicine on August 10, 2000, this study had four serious problems that invalidate its authors’ conclusions.9
The sample size was insufficient to detect a significant difference between treatment groups. In other words, the chance of a type 2 statistical error is high.
Balloon angioplasty without stenting was used as the method of revascularization. Experts now recognize that stenting is required for renal artery intervention to have a durable result.3,5
Renal artery stenosis was defined as greater than 50% stenosis. This allowed a large number of patients to enter the trial who had hemodynamically and clinically insignificant lesions. Most clinicians believe that stenosis of less than 70% is not hemodynamically important.5,10,11
Twenty-two of the 50 patients randomized to medical therapy crossed over to the angioplasty group because their blood pressure became difficult to control. In other words, 44% of the patients in the medical group underwent angioplasty, an astounding percentage in an intention-to-treat analysis comparing one therapy with another.
Despite these serious flaws, the results of DRASTIC influenced therapy for years after its publication.
THE STAR TRIAL
In the Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) trial,7 140 patients with a creatinine clearance of less than 80 mL/min/1.73m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy (n = 64) or medical therapy alone (n = 76). The primary end point was a 20% or greater decrease in creatinine clearance. Secondary end points included measures of safety and cardiovascular morbidity and mortality.
Authors’ conclusions
“Stent placement with medical treatment had no clear effect on progression of impaired renal function but led to a small number of significant procedure-related complications. The study findings favor a conservative approach to patients with [atherosclerotic renal artery stenosis], focused on cardiovascular risk factor management and avoiding stenting.”7
Serious flaws
A number of serious flaws render this study uninterpretable.
Mild renal artery stenosis. At least 33% of the patients in the study had mild renal artery stenosis (50%–70%), and 12 (19%) of the 64 patients in the group randomized to stenting actually had stenosis of less than 50%. How can one expect there to be a benefit to stenting in patients with mild (and hemodynamically insignificant) renal artery stenosis? This is especially true when the primary end point is a change in renal function.
More than half of the patients had unilateral disease. It seems intuitive that if one were to plan a trial with a primary end point of a change in renal function, only patients with bilateral renal artery stenosis of greater than 70% or with stenosis of greater than 70% to a solitary functioning kidney would be included. One would not expect that patients with unilateral disease and a stenosis of less than 70% would derive any benefit from revascularization.
Not all “stent” patients received stents. All of the patients in the medical group received medication and there were no crossovers. However, only 46 (72%) of the 64 patients randomized to stenting actually received a stent, while 18 (28%) did not. There were two technical failures, and 12 patients should not have been randomized because they had less than 50% stenosis on angiography and thus were not stented. Yet all 64 patients were analyzed (by intention to treat) in the stent group. With these numbers, one could predict that the results would be negative.
Like DRASTIC, this trial was underpowered, meaning that the chance of a type 2 statistical error is high. In fact, the editors of the Annals of Internal Medicine, in a note accompanying the article, cautioned that the study “was underpowered to provide a definitive estimate of efficacy.”7 If the study was underpowered to answer the question at hand, why was it deemed worthy of publication?
High complication rates. The periprocedural complication and death rates were much higher than in many other reports on renal artery stenting (see details below).5
THE ASTRAL TRIAL
In the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial,8 the primary outcome measure was the change in renal function over time as assessed by the mean slope of the reciprocal of the serum creatinine. In this trial, 806 patients with atherosclerotic renal artery stenosis were randomized to either stent-based revascularization combined with medical therapy or medical therapy alone.
Authors’ conclusions
“We found substantial risks but no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.”8
Despite size, flaws remain
Unlike the other trials, ASTRAL had a sample size large enough to provide an answer. However, numerous flaws in study design and implementation invalidate its results for the overall population of patients with renal artery stenosis. The major flaws in ASTRAL were:
Selection bias. For a patient to be enrolled, the treating physician had to be undecided on whether the patient should undergo revascularization or medical management alone. However, the treatment of atherosclerotic renal artery stenosis is so controversial that physicians of different specialties cannot agree on the most effective treatment strategy for most patients.1,2 Therefore, to exclude patients when their physicians were sure they needed or did not need renal artery revascularization is incomprehensible and introduces considerable selection bias into the trial design.
Normal renal function at baseline. The primary outcome was a change in renal function over time. Yet 25% of patients had normal renal function at the outset of the trial. In addition, a significant number had unilateral disease, and 41% had a stenosis less than 70%. What made the investigators think that stent implantation could possibly be shown to be beneficial if they entered patients into a renal function study who had near-normal renal function, unilateral disease, and mild renal artery stenosis? These are patients whose condition would not be expected to worsen with medical therapy nor to improve with stenting. Most clinicians would not consider stenting a patient to preserve renal function if the patient has unilateral mild renal artery stenosis.
There was no core laboratory to adjudicate the interpretation of the imaging studies. To determine the degree of stenosis of an artery in an accurate and unbiased fashion, a core laboratory must be used.
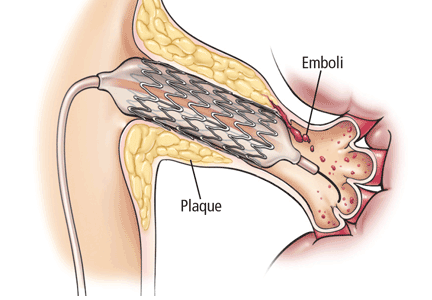
The reason this is so important is that visual assessment of the degree of stenosis on angiography is not accurate and almost always overestimates the degree of stenosis.12,13 In a study assessing the physiologic importance of renal artery lesions, the lesion severity by visual estimation was 74.9% ± 11.5% (range 50%–90%), which exceeded the quantitative vascular angiographic lesion severity of 56.6% ± 10.8% (range 45%–76%).13
Therefore, in ASTRAL, some patients in the 50%–70% stenosis group (about 40% of patients entered) actually had a stenosis of less than 50%. And some patients in the group with stenosis greater than 70% had stenosis of less than 70%. This further illustrates that, for the most part, the patients in ASTRAL had mild to moderate renal artery stenosis.
A high adverse event rate. The major adverse event rate in the first 24 hours was 9%, whereas the usual rate is 2% or less.14–18 Of the 280 patients in the revascularization group for whom data on adverse events were available at 1 month, 55 (20%) suffered a serious adverse event (including two patients who died) between 24 hours and 1 month after the procedure. This is in contrast to a major complication rate of 1.99% in five reports involving 727 patients.5
The trial centers were not high-volume centers. During the 7 years of recruitment, 24 centers (42% of all participating centers) randomized between one and five patients, and 32 centers (61% of all participating centers) randomized nine patients or fewer. This means that many participating centers randomized, on average, less than one patient per year! This was not a group of high-volume operators.
WILL CORAL GIVE US THE ANSWER?
The CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial is under way.19 Enrollment was to have ended on January 31, 2010, and it will be several years before the data are available for analysis.
CORAL, a multicenter study funded in 2004 by the National Institutes of Health, will have randomized more than 900 patients with greater than 60% stenosis to optimal medical therapy alone or optimal medical therapy plus renal artery stenting. Inclusion criteria are a documented history of hypertension on two or more antihypertensive drugs or renal dysfunction, defined as stage 3 or greater chronic kidney disease based on the National Kidney Foundation classification (estimated glomerular filtration rate < 60 mL/min/1.73 m2 calculated by the modified Modification of Diet in Renal Disease [MDRD] formula) and stenosis of 60% or greater but less than 100%, as assessed by a core laboratory. The primary end point is survival free of cardiovascular and renal adverse events, defined as a composite of cardiovascular or renal death, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or need for permanent renal replacement therapy.
We hope this trial will give us a clear answer to the question of whether renal artery stenting is beneficial in the patient population studied. One note of caution: recruitment for this trial was difficult and slow. Thus, there were a number of protocol amendments throughout the trial in order to make recruitment easier. Hopefully, this will not be a problem when analyzing the results.
WE ALL AGREE ON THE INDICATIONS FOR STENTING
So, are we really so far apart in our thinking? And is it really “time to be less aggressive” if we follow the precepts below?
All renal arteries with stenosis do not need to be (and should not be) stented.
There must be a good clinical indicationandhemodynamically significant stenosis. This means the degree of stenosis should be more than 70% on angiography or intravascular ultrasonography.
Indications for stenting. Until more data from compelling randomized trials become available, adherence to the American College of Cardiology/American Heart Association guidelines on indications for renal artery stenting is advised3:
- Hypertension: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with hemodynamically significant renal artery stenosis and accelerated hypertension, resistant hypertension, and malignant hypertension.
- Preservation of renal function: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with renal artery stenosis and progressive chronic kidney disease with bilateral renal artery stenosis or a stenosis to a solitary functioning kidney.
- Congestive heart failure: class I, level of evidence B. Percutaneous revascularization is indicated for patients with hemodynamically significant renal artery stenosis (ie, > 70% stenosis on angiography or intravascular ultrasonography) and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema.
- Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis? The case for renal artery stenting for treatment of renal artery stenosis. Circulation 2007; 115:263–269.
- Dworkin LD, Jamerson KA. Is renal artery stenting the correct treatment of renal artery stenosis? Case against angioplasty and stenting of atherosclerotic renal artery stenosis. Circulation 2007; 115:271–276.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association of Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Interventional Radiology, Society for Vascular Medicine and Biology and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006; 113:e463–e654.
- Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve Clin J Med 2010; 77:178–189.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–841.
- Wheatley K, Ives N, Gray R, et al Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Tan WA, Wholey MH, Olin JW. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis [letter]. N Engl J Med 2000; 343:438.
- Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al Atherosclerotic Peripheral Vascular Disease Symposium II: intervention for renal artery disease. Circulation 2008; 118:2873–2878.
- Textor SC, Lerman L, McKusick M. The uncertain value of renal artery interventions: where are we now? JACC Cardiovasc Intervent 2009; 2:175–182.
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92:2333–2342.
- Subramanian R, White CJ, Rosenfield K, et al Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv 2005; 64:480–486.
- Burket MW, Cooper CJ, Kennedy DJ, et al Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 2000; 139:64–71.
- Dorros G, Jaff M, Mathiak L, et al Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 1998; 98:642–647.
- Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol 2005; 46:776–783.
- Tuttle KR, Chouinard RF, Webber JT, et al Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 1998; 32:611–622.
- White CJ, Ramee SR, Collins TJ, Jenkins JS, Escobar A, Shaw D. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J Am Coll Cardiol 1997; 30:1445–1450.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
The role of stenting for atherosclerotic renal artery stenosis is hotly debated among different specialties.1,2 If we may generalize a bit, interventionalists (cardiologists, interventional radiologists, vascular surgeons, and vascular medicine specialists) have been in favor of liberal use of stenting, and nephrologists often favor medical therapy alone. And as with all controversial issues, each group feels rather strongly about its position.
Because few prospective randomized trials have been completed, the management of atherosclerotic renal artery stenosis has been guided by retrospective studies and case series. 3
In this issue of the Cleveland Clinic Journal of Medicine, Dr. James Simon4 provides an excellent overview of the prevalence, natural history, and clinical presentation of atherosclerotic renal artery stenosis. In addition, he does an admirable job of reviewing the available prospective randomized trials and providing editorial commentary about the role of the various specialists in the management of renal artery disease. And while the title of his paper says that it is “time to be less aggressive,” Dr. Simon ultimately comes to the same conclusions that we do5 on the indications for renal artery stenting (see Table 3 of Dr. Simon’s article), which are those of the multidisciplinary 2006 American College of Cardiology/American Heart Association guidelines on the management of peripheral artery disease.3
So what then is all the controversy about? We all agree that prospective randomized trials that provide class I, level A evidence impart the only unbiased scientific information on the best treatment strategy for patients with renal artery disease. The basic controversial issue is the interpretation of these trials. We contend that the three randomized trials of stenting vs medical therapy published so far6–8 (see below) are so seriously flawed that it is impossible to make treatment decisions based on their results.
Since these trials were published in wellrespected journals, their results are often taken as gospel. However, careful review of each of these will reveal the flaws in study design and implementation.
THE DRASTIC TRIAL
In the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial,6 106 patients with renal artery stenosis and hypertension (diastolic blood pressure > 95 mm Hg) despite treatment with two antihypertensive medications were randomly assigned to either renal angioplasty (n = 56) or drug therapy (n = 50).
Authors’ conclusions
“In the treatment of patients with hypertension and renal-artery stenosis, angioplasty has little advantage over antihypertensive-drug therapy.”6
Four serious problems
As we discussed in a letter to the editor of the New England Journal of Medicine on August 10, 2000, this study had four serious problems that invalidate its authors’ conclusions.9
The sample size was insufficient to detect a significant difference between treatment groups. In other words, the chance of a type 2 statistical error is high.
Balloon angioplasty without stenting was used as the method of revascularization. Experts now recognize that stenting is required for renal artery intervention to have a durable result.3,5
Renal artery stenosis was defined as greater than 50% stenosis. This allowed a large number of patients to enter the trial who had hemodynamically and clinically insignificant lesions. Most clinicians believe that stenosis of less than 70% is not hemodynamically important.5,10,11
Twenty-two of the 50 patients randomized to medical therapy crossed over to the angioplasty group because their blood pressure became difficult to control. In other words, 44% of the patients in the medical group underwent angioplasty, an astounding percentage in an intention-to-treat analysis comparing one therapy with another.
Despite these serious flaws, the results of DRASTIC influenced therapy for years after its publication.
THE STAR TRIAL
In the Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) trial,7 140 patients with a creatinine clearance of less than 80 mL/min/1.73m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy (n = 64) or medical therapy alone (n = 76). The primary end point was a 20% or greater decrease in creatinine clearance. Secondary end points included measures of safety and cardiovascular morbidity and mortality.
Authors’ conclusions
“Stent placement with medical treatment had no clear effect on progression of impaired renal function but led to a small number of significant procedure-related complications. The study findings favor a conservative approach to patients with [atherosclerotic renal artery stenosis], focused on cardiovascular risk factor management and avoiding stenting.”7
Serious flaws
A number of serious flaws render this study uninterpretable.
Mild renal artery stenosis. At least 33% of the patients in the study had mild renal artery stenosis (50%–70%), and 12 (19%) of the 64 patients in the group randomized to stenting actually had stenosis of less than 50%. How can one expect there to be a benefit to stenting in patients with mild (and hemodynamically insignificant) renal artery stenosis? This is especially true when the primary end point is a change in renal function.
More than half of the patients had unilateral disease. It seems intuitive that if one were to plan a trial with a primary end point of a change in renal function, only patients with bilateral renal artery stenosis of greater than 70% or with stenosis of greater than 70% to a solitary functioning kidney would be included. One would not expect that patients with unilateral disease and a stenosis of less than 70% would derive any benefit from revascularization.
Not all “stent” patients received stents. All of the patients in the medical group received medication and there were no crossovers. However, only 46 (72%) of the 64 patients randomized to stenting actually received a stent, while 18 (28%) did not. There were two technical failures, and 12 patients should not have been randomized because they had less than 50% stenosis on angiography and thus were not stented. Yet all 64 patients were analyzed (by intention to treat) in the stent group. With these numbers, one could predict that the results would be negative.
Like DRASTIC, this trial was underpowered, meaning that the chance of a type 2 statistical error is high. In fact, the editors of the Annals of Internal Medicine, in a note accompanying the article, cautioned that the study “was underpowered to provide a definitive estimate of efficacy.”7 If the study was underpowered to answer the question at hand, why was it deemed worthy of publication?
High complication rates. The periprocedural complication and death rates were much higher than in many other reports on renal artery stenting (see details below).5
THE ASTRAL TRIAL
In the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial,8 the primary outcome measure was the change in renal function over time as assessed by the mean slope of the reciprocal of the serum creatinine. In this trial, 806 patients with atherosclerotic renal artery stenosis were randomized to either stent-based revascularization combined with medical therapy or medical therapy alone.
Authors’ conclusions
“We found substantial risks but no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.”8
Despite size, flaws remain
Unlike the other trials, ASTRAL had a sample size large enough to provide an answer. However, numerous flaws in study design and implementation invalidate its results for the overall population of patients with renal artery stenosis. The major flaws in ASTRAL were:
Selection bias. For a patient to be enrolled, the treating physician had to be undecided on whether the patient should undergo revascularization or medical management alone. However, the treatment of atherosclerotic renal artery stenosis is so controversial that physicians of different specialties cannot agree on the most effective treatment strategy for most patients.1,2 Therefore, to exclude patients when their physicians were sure they needed or did not need renal artery revascularization is incomprehensible and introduces considerable selection bias into the trial design.
Normal renal function at baseline. The primary outcome was a change in renal function over time. Yet 25% of patients had normal renal function at the outset of the trial. In addition, a significant number had unilateral disease, and 41% had a stenosis less than 70%. What made the investigators think that stent implantation could possibly be shown to be beneficial if they entered patients into a renal function study who had near-normal renal function, unilateral disease, and mild renal artery stenosis? These are patients whose condition would not be expected to worsen with medical therapy nor to improve with stenting. Most clinicians would not consider stenting a patient to preserve renal function if the patient has unilateral mild renal artery stenosis.
There was no core laboratory to adjudicate the interpretation of the imaging studies. To determine the degree of stenosis of an artery in an accurate and unbiased fashion, a core laboratory must be used.
The reason this is so important is that visual assessment of the degree of stenosis on angiography is not accurate and almost always overestimates the degree of stenosis.12,13 In a study assessing the physiologic importance of renal artery lesions, the lesion severity by visual estimation was 74.9% ± 11.5% (range 50%–90%), which exceeded the quantitative vascular angiographic lesion severity of 56.6% ± 10.8% (range 45%–76%).13
Therefore, in ASTRAL, some patients in the 50%–70% stenosis group (about 40% of patients entered) actually had a stenosis of less than 50%. And some patients in the group with stenosis greater than 70% had stenosis of less than 70%. This further illustrates that, for the most part, the patients in ASTRAL had mild to moderate renal artery stenosis.
A high adverse event rate. The major adverse event rate in the first 24 hours was 9%, whereas the usual rate is 2% or less.14–18 Of the 280 patients in the revascularization group for whom data on adverse events were available at 1 month, 55 (20%) suffered a serious adverse event (including two patients who died) between 24 hours and 1 month after the procedure. This is in contrast to a major complication rate of 1.99% in five reports involving 727 patients.5
The trial centers were not high-volume centers. During the 7 years of recruitment, 24 centers (42% of all participating centers) randomized between one and five patients, and 32 centers (61% of all participating centers) randomized nine patients or fewer. This means that many participating centers randomized, on average, less than one patient per year! This was not a group of high-volume operators.
WILL CORAL GIVE US THE ANSWER?
The CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial is under way.19 Enrollment was to have ended on January 31, 2010, and it will be several years before the data are available for analysis.
CORAL, a multicenter study funded in 2004 by the National Institutes of Health, will have randomized more than 900 patients with greater than 60% stenosis to optimal medical therapy alone or optimal medical therapy plus renal artery stenting. Inclusion criteria are a documented history of hypertension on two or more antihypertensive drugs or renal dysfunction, defined as stage 3 or greater chronic kidney disease based on the National Kidney Foundation classification (estimated glomerular filtration rate < 60 mL/min/1.73 m2 calculated by the modified Modification of Diet in Renal Disease [MDRD] formula) and stenosis of 60% or greater but less than 100%, as assessed by a core laboratory. The primary end point is survival free of cardiovascular and renal adverse events, defined as a composite of cardiovascular or renal death, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or need for permanent renal replacement therapy.
We hope this trial will give us a clear answer to the question of whether renal artery stenting is beneficial in the patient population studied. One note of caution: recruitment for this trial was difficult and slow. Thus, there were a number of protocol amendments throughout the trial in order to make recruitment easier. Hopefully, this will not be a problem when analyzing the results.
WE ALL AGREE ON THE INDICATIONS FOR STENTING
So, are we really so far apart in our thinking? And is it really “time to be less aggressive” if we follow the precepts below?
All renal arteries with stenosis do not need to be (and should not be) stented.
There must be a good clinical indicationandhemodynamically significant stenosis. This means the degree of stenosis should be more than 70% on angiography or intravascular ultrasonography.
Indications for stenting. Until more data from compelling randomized trials become available, adherence to the American College of Cardiology/American Heart Association guidelines on indications for renal artery stenting is advised3:
- Hypertension: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with hemodynamically significant renal artery stenosis and accelerated hypertension, resistant hypertension, and malignant hypertension.
- Preservation of renal function: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with renal artery stenosis and progressive chronic kidney disease with bilateral renal artery stenosis or a stenosis to a solitary functioning kidney.
- Congestive heart failure: class I, level of evidence B. Percutaneous revascularization is indicated for patients with hemodynamically significant renal artery stenosis (ie, > 70% stenosis on angiography or intravascular ultrasonography) and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema.
The role of stenting for atherosclerotic renal artery stenosis is hotly debated among different specialties.1,2 If we may generalize a bit, interventionalists (cardiologists, interventional radiologists, vascular surgeons, and vascular medicine specialists) have been in favor of liberal use of stenting, and nephrologists often favor medical therapy alone. And as with all controversial issues, each group feels rather strongly about its position.
Because few prospective randomized trials have been completed, the management of atherosclerotic renal artery stenosis has been guided by retrospective studies and case series. 3
In this issue of the Cleveland Clinic Journal of Medicine, Dr. James Simon4 provides an excellent overview of the prevalence, natural history, and clinical presentation of atherosclerotic renal artery stenosis. In addition, he does an admirable job of reviewing the available prospective randomized trials and providing editorial commentary about the role of the various specialists in the management of renal artery disease. And while the title of his paper says that it is “time to be less aggressive,” Dr. Simon ultimately comes to the same conclusions that we do5 on the indications for renal artery stenting (see Table 3 of Dr. Simon’s article), which are those of the multidisciplinary 2006 American College of Cardiology/American Heart Association guidelines on the management of peripheral artery disease.3
So what then is all the controversy about? We all agree that prospective randomized trials that provide class I, level A evidence impart the only unbiased scientific information on the best treatment strategy for patients with renal artery disease. The basic controversial issue is the interpretation of these trials. We contend that the three randomized trials of stenting vs medical therapy published so far6–8 (see below) are so seriously flawed that it is impossible to make treatment decisions based on their results.
Since these trials were published in wellrespected journals, their results are often taken as gospel. However, careful review of each of these will reveal the flaws in study design and implementation.
THE DRASTIC TRIAL
In the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial,6 106 patients with renal artery stenosis and hypertension (diastolic blood pressure > 95 mm Hg) despite treatment with two antihypertensive medications were randomly assigned to either renal angioplasty (n = 56) or drug therapy (n = 50).
Authors’ conclusions
“In the treatment of patients with hypertension and renal-artery stenosis, angioplasty has little advantage over antihypertensive-drug therapy.”6
Four serious problems
As we discussed in a letter to the editor of the New England Journal of Medicine on August 10, 2000, this study had four serious problems that invalidate its authors’ conclusions.9
The sample size was insufficient to detect a significant difference between treatment groups. In other words, the chance of a type 2 statistical error is high.
Balloon angioplasty without stenting was used as the method of revascularization. Experts now recognize that stenting is required for renal artery intervention to have a durable result.3,5
Renal artery stenosis was defined as greater than 50% stenosis. This allowed a large number of patients to enter the trial who had hemodynamically and clinically insignificant lesions. Most clinicians believe that stenosis of less than 70% is not hemodynamically important.5,10,11
Twenty-two of the 50 patients randomized to medical therapy crossed over to the angioplasty group because their blood pressure became difficult to control. In other words, 44% of the patients in the medical group underwent angioplasty, an astounding percentage in an intention-to-treat analysis comparing one therapy with another.
Despite these serious flaws, the results of DRASTIC influenced therapy for years after its publication.
THE STAR TRIAL
In the Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) trial,7 140 patients with a creatinine clearance of less than 80 mL/min/1.73m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy (n = 64) or medical therapy alone (n = 76). The primary end point was a 20% or greater decrease in creatinine clearance. Secondary end points included measures of safety and cardiovascular morbidity and mortality.
Authors’ conclusions
“Stent placement with medical treatment had no clear effect on progression of impaired renal function but led to a small number of significant procedure-related complications. The study findings favor a conservative approach to patients with [atherosclerotic renal artery stenosis], focused on cardiovascular risk factor management and avoiding stenting.”7
Serious flaws
A number of serious flaws render this study uninterpretable.
Mild renal artery stenosis. At least 33% of the patients in the study had mild renal artery stenosis (50%–70%), and 12 (19%) of the 64 patients in the group randomized to stenting actually had stenosis of less than 50%. How can one expect there to be a benefit to stenting in patients with mild (and hemodynamically insignificant) renal artery stenosis? This is especially true when the primary end point is a change in renal function.
More than half of the patients had unilateral disease. It seems intuitive that if one were to plan a trial with a primary end point of a change in renal function, only patients with bilateral renal artery stenosis of greater than 70% or with stenosis of greater than 70% to a solitary functioning kidney would be included. One would not expect that patients with unilateral disease and a stenosis of less than 70% would derive any benefit from revascularization.
Not all “stent” patients received stents. All of the patients in the medical group received medication and there were no crossovers. However, only 46 (72%) of the 64 patients randomized to stenting actually received a stent, while 18 (28%) did not. There were two technical failures, and 12 patients should not have been randomized because they had less than 50% stenosis on angiography and thus were not stented. Yet all 64 patients were analyzed (by intention to treat) in the stent group. With these numbers, one could predict that the results would be negative.
Like DRASTIC, this trial was underpowered, meaning that the chance of a type 2 statistical error is high. In fact, the editors of the Annals of Internal Medicine, in a note accompanying the article, cautioned that the study “was underpowered to provide a definitive estimate of efficacy.”7 If the study was underpowered to answer the question at hand, why was it deemed worthy of publication?
High complication rates. The periprocedural complication and death rates were much higher than in many other reports on renal artery stenting (see details below).5
THE ASTRAL TRIAL
In the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial,8 the primary outcome measure was the change in renal function over time as assessed by the mean slope of the reciprocal of the serum creatinine. In this trial, 806 patients with atherosclerotic renal artery stenosis were randomized to either stent-based revascularization combined with medical therapy or medical therapy alone.
Authors’ conclusions
“We found substantial risks but no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.”8
Despite size, flaws remain
Unlike the other trials, ASTRAL had a sample size large enough to provide an answer. However, numerous flaws in study design and implementation invalidate its results for the overall population of patients with renal artery stenosis. The major flaws in ASTRAL were:
Selection bias. For a patient to be enrolled, the treating physician had to be undecided on whether the patient should undergo revascularization or medical management alone. However, the treatment of atherosclerotic renal artery stenosis is so controversial that physicians of different specialties cannot agree on the most effective treatment strategy for most patients.1,2 Therefore, to exclude patients when their physicians were sure they needed or did not need renal artery revascularization is incomprehensible and introduces considerable selection bias into the trial design.
Normal renal function at baseline. The primary outcome was a change in renal function over time. Yet 25% of patients had normal renal function at the outset of the trial. In addition, a significant number had unilateral disease, and 41% had a stenosis less than 70%. What made the investigators think that stent implantation could possibly be shown to be beneficial if they entered patients into a renal function study who had near-normal renal function, unilateral disease, and mild renal artery stenosis? These are patients whose condition would not be expected to worsen with medical therapy nor to improve with stenting. Most clinicians would not consider stenting a patient to preserve renal function if the patient has unilateral mild renal artery stenosis.
There was no core laboratory to adjudicate the interpretation of the imaging studies. To determine the degree of stenosis of an artery in an accurate and unbiased fashion, a core laboratory must be used.
The reason this is so important is that visual assessment of the degree of stenosis on angiography is not accurate and almost always overestimates the degree of stenosis.12,13 In a study assessing the physiologic importance of renal artery lesions, the lesion severity by visual estimation was 74.9% ± 11.5% (range 50%–90%), which exceeded the quantitative vascular angiographic lesion severity of 56.6% ± 10.8% (range 45%–76%).13
Therefore, in ASTRAL, some patients in the 50%–70% stenosis group (about 40% of patients entered) actually had a stenosis of less than 50%. And some patients in the group with stenosis greater than 70% had stenosis of less than 70%. This further illustrates that, for the most part, the patients in ASTRAL had mild to moderate renal artery stenosis.
A high adverse event rate. The major adverse event rate in the first 24 hours was 9%, whereas the usual rate is 2% or less.14–18 Of the 280 patients in the revascularization group for whom data on adverse events were available at 1 month, 55 (20%) suffered a serious adverse event (including two patients who died) between 24 hours and 1 month after the procedure. This is in contrast to a major complication rate of 1.99% in five reports involving 727 patients.5
The trial centers were not high-volume centers. During the 7 years of recruitment, 24 centers (42% of all participating centers) randomized between one and five patients, and 32 centers (61% of all participating centers) randomized nine patients or fewer. This means that many participating centers randomized, on average, less than one patient per year! This was not a group of high-volume operators.
WILL CORAL GIVE US THE ANSWER?
The CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial is under way.19 Enrollment was to have ended on January 31, 2010, and it will be several years before the data are available for analysis.
CORAL, a multicenter study funded in 2004 by the National Institutes of Health, will have randomized more than 900 patients with greater than 60% stenosis to optimal medical therapy alone or optimal medical therapy plus renal artery stenting. Inclusion criteria are a documented history of hypertension on two or more antihypertensive drugs or renal dysfunction, defined as stage 3 or greater chronic kidney disease based on the National Kidney Foundation classification (estimated glomerular filtration rate < 60 mL/min/1.73 m2 calculated by the modified Modification of Diet in Renal Disease [MDRD] formula) and stenosis of 60% or greater but less than 100%, as assessed by a core laboratory. The primary end point is survival free of cardiovascular and renal adverse events, defined as a composite of cardiovascular or renal death, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or need for permanent renal replacement therapy.
We hope this trial will give us a clear answer to the question of whether renal artery stenting is beneficial in the patient population studied. One note of caution: recruitment for this trial was difficult and slow. Thus, there were a number of protocol amendments throughout the trial in order to make recruitment easier. Hopefully, this will not be a problem when analyzing the results.
WE ALL AGREE ON THE INDICATIONS FOR STENTING
So, are we really so far apart in our thinking? And is it really “time to be less aggressive” if we follow the precepts below?
All renal arteries with stenosis do not need to be (and should not be) stented.
There must be a good clinical indicationandhemodynamically significant stenosis. This means the degree of stenosis should be more than 70% on angiography or intravascular ultrasonography.
Indications for stenting. Until more data from compelling randomized trials become available, adherence to the American College of Cardiology/American Heart Association guidelines on indications for renal artery stenting is advised3:
- Hypertension: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with hemodynamically significant renal artery stenosis and accelerated hypertension, resistant hypertension, and malignant hypertension.
- Preservation of renal function: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with renal artery stenosis and progressive chronic kidney disease with bilateral renal artery stenosis or a stenosis to a solitary functioning kidney.
- Congestive heart failure: class I, level of evidence B. Percutaneous revascularization is indicated for patients with hemodynamically significant renal artery stenosis (ie, > 70% stenosis on angiography or intravascular ultrasonography) and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema.
- Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis? The case for renal artery stenting for treatment of renal artery stenosis. Circulation 2007; 115:263–269.
- Dworkin LD, Jamerson KA. Is renal artery stenting the correct treatment of renal artery stenosis? Case against angioplasty and stenting of atherosclerotic renal artery stenosis. Circulation 2007; 115:271–276.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association of Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Interventional Radiology, Society for Vascular Medicine and Biology and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006; 113:e463–e654.
- Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve Clin J Med 2010; 77:178–189.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–841.
- Wheatley K, Ives N, Gray R, et al Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Tan WA, Wholey MH, Olin JW. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis [letter]. N Engl J Med 2000; 343:438.
- Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al Atherosclerotic Peripheral Vascular Disease Symposium II: intervention for renal artery disease. Circulation 2008; 118:2873–2878.
- Textor SC, Lerman L, McKusick M. The uncertain value of renal artery interventions: where are we now? JACC Cardiovasc Intervent 2009; 2:175–182.
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92:2333–2342.
- Subramanian R, White CJ, Rosenfield K, et al Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv 2005; 64:480–486.
- Burket MW, Cooper CJ, Kennedy DJ, et al Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 2000; 139:64–71.
- Dorros G, Jaff M, Mathiak L, et al Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 1998; 98:642–647.
- Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol 2005; 46:776–783.
- Tuttle KR, Chouinard RF, Webber JT, et al Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 1998; 32:611–622.
- White CJ, Ramee SR, Collins TJ, Jenkins JS, Escobar A, Shaw D. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J Am Coll Cardiol 1997; 30:1445–1450.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
- Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis? The case for renal artery stenting for treatment of renal artery stenosis. Circulation 2007; 115:263–269.
- Dworkin LD, Jamerson KA. Is renal artery stenting the correct treatment of renal artery stenosis? Case against angioplasty and stenting of atherosclerotic renal artery stenosis. Circulation 2007; 115:271–276.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association of Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Interventional Radiology, Society for Vascular Medicine and Biology and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006; 113:e463–e654.
- Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve Clin J Med 2010; 77:178–189.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–841.
- Wheatley K, Ives N, Gray R, et al Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Tan WA, Wholey MH, Olin JW. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis [letter]. N Engl J Med 2000; 343:438.
- Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al Atherosclerotic Peripheral Vascular Disease Symposium II: intervention for renal artery disease. Circulation 2008; 118:2873–2878.
- Textor SC, Lerman L, McKusick M. The uncertain value of renal artery interventions: where are we now? JACC Cardiovasc Intervent 2009; 2:175–182.
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92:2333–2342.
- Subramanian R, White CJ, Rosenfield K, et al Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv 2005; 64:480–486.
- Burket MW, Cooper CJ, Kennedy DJ, et al Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 2000; 139:64–71.
- Dorros G, Jaff M, Mathiak L, et al Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 1998; 98:642–647.
- Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol 2005; 46:776–783.
- Tuttle KR, Chouinard RF, Webber JT, et al Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 1998; 32:611–622.
- White CJ, Ramee SR, Collins TJ, Jenkins JS, Escobar A, Shaw D. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J Am Coll Cardiol 1997; 30:1445–1450.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
Stenting atherosclerotic renal arteries: Time to be less aggressive
Author’s note: Atherosclerosis accounts for about 90% of cases of renal artery stenosis in people over age 40.1 Fibromuscular dysplasia, the other major cause, is a separate topic; in this paper “renal artery stenosis” refers to atherosclerotic disease only.
Renal artery stenosis is very common, and the number of angioplasty-stenting procedures performed every year is on the rise. Yet there is no overwhelming evidence that intervention yields clinical benefits—ie, better blood pressure control or renal function— than does medical therapy.
Earlier randomized controlled trials comparing angioplasty without stents and medical management showed no impressive difference in blood pressure.2,3 The data on renal function were even more questionable, with some studies suggesting that, with stenting, the chance of worsening renal function is equal to that of improvement.4
Two large randomized trials comparing renal intervention with medical therapy failed to show any benefit of intervention.5–7 A third study is under way.8
It is time to strongly reconsider the current aggressive approach to revascularization of stenotic renal arteries and take a more coordinated, critical approach.
RENAL INTERVENTIONS ON THE RISE
Renal angioplasty began replacing surgical revascularization in the 1990s, as this less-invasive procedure became more readily available and was shown to have similar clinical outcomes.9 In the last decade, stent placement during angioplasty has become standard, improving the rates of technical success.
As these procedures have become easier to perform and their radiographic outcomes have become more consistent, interventionalists have become more likely, if they see stenosis in a renal artery, to intervene and insert a stent, regardless of proven benefit. In addition, interventionalists from at least three different specialties now compete for these procedures, often by looking at the renal arteries during angiography of other vascular beds (the “drive-by”).
As a result, the number of renal interventions has been rising. Medicare received 21,660 claims for renal artery interventions (surgery or angioplasty) in 2000, compared with 13,380 in 1996—an increase of 62%. However, the number of surgeries actually decreased by 45% during this time, while the number of percutaneous procedures increased by 240%. The number of endovascular claims for renal artery stenosis by cardiologists alone rose 390%.10 Since then, the reports on intervention have been mixed, with one report citing a continued increase in 2005 to 35,000 claims,11 and another suggesting a decrease back to 1997 levels.12
HOW COMMON IS RENAL ARTERY STENOSIS?
The prevalence of renal artery stenosis depends on the definition used and the population screened. It is more common in older patients who have risk factors for other vascular diseases than in the general population.
Renal Doppler ultrasonography can detect stenosis only if the artery is narrowed by more than 60%. Hansen et al13 used ultrasonography to screen 870 people over age 65 and found a lesion (a narrowing of more than 60%) in 6.8%.
Angiography (direct, computed tomographic, or magnetic resonance) can detect less-severe stenosis. Thus, most angiographic studies define renal artery stenosis as a narrowing of more than 50%, and severe disease as a narrowing of more than 70%. Many experts believe that unilateral stenosis needs to be more than 70% to pose a risk to the kidney.14,15
Angiographic prevalence studies have been performed only in patients who were undergoing angiography for another reason such as coronary or peripheral arterial disease that inherently places them at higher risk of renal artery stenosis. For instance, renal artery stenosis is found in 11% to 28% of patients undergoing diagnostic cardiac catheterization. 16
No studies of the prevalence of renal artery stenosis have been performed in the general population. Medicare data indicate that from 1999 to 2001 the incidence of diagnosed renal artery stenosis was 3.7 per 1,000 patientyears. 17 Holley et al,18 in an autopsy series, found renal artery stenosis of greater than 50% in 27% of patients over age 50 and in 56.4% of hypertensive patients. The prevalence was 10% in normotensive patients.
WHO IS AT RISK?
Factors associated with a higher risk of finding renal artery stenosis on a radiographic study include14:
- Older age
- Female sex
- Hypertension
- Three-vessel coronary artery disease
- Peripheral artery disease
- Chronic kidney disease
- Diabetes
- A low level of high-density lipoprotein cholesterol
- The use of at least two cardiovascular drugs.
The prevalence of renal artery stenosis in at-risk populations ranges from 3% to 75% (Table 1).2,4,6,19,20
HOW OFTEN DOES STENOSIS PROGRESS?
The reported rates of progression of atherosclerotic renal artery lesions vary depending on the type of imaging test used and the reason for doing it.
In studies that used duplex ultrasonography, roughly half of lesions smaller than 60% grew to greater than 60% over 3 years.21,22 The risk of total occlusion of an artery was relatively low and depended on the severity of stenosis: 0.7% if the baseline stenosis was less than 60% and 2.3% to 7% if it was greater.21,22
In a seminal study in 1984, Schreiber and colleagues23 compared serial angiograms obtained a mean of 52 months apart in 85 patients who did not undergo intervention. Stenosis had progressed in 37 (44%), and to the point of total occlusion in 14 (16%). In contrast, a 1998 study found progression in 11.1% over 2.6 years, with older patients, women, and those with baseline coronary artery disease at higher risk.24
The the rates of progression differed in these two studies probably because the indications for screening were different (clinical suspicion23 vs routine screening during coronary angiography24), as was the severity of stenosis at the time of diagnosis. Also, when these studies were done, fewer people were taking statins. Thus, similar studies, if repeated, might show even lower rates of progression.
Finally, progression of renal artery stenosis has not been correlated with worsening renal function.
FOUR CLINICAL PRESENTATIONS OF RENAL ARTERY STENOSIS
Renal artery stenosis can present in one of four ways:
Clinically silent stenosis. Because renal artery stenosis is most often found in older patients, who are more likely to have essential hypertension and chronic kidney disease due to other causes, it can be an incidental finding that is completely clinically silent.16,25
Renovascular hypertension is defined as high blood pressure due to up-regulation of neurohormonal activity in response to decreased perfusion from renal artery stenosis. Renal artery stenosis is estimated to be the cause of hypertension in only 0.5% to 4.0% of hypertensive patients, or in 26% of patients with secondary hypertension.3
Ischemic nephropathy is more difficult to define because ischemia alone rarely explains the damage done to the kidneys. Activation of neurohormonal pathways and microvascular injury are thought to contribute to oxidative stress and fibrosis.26 These phenomena may explain why similar degrees of stenosis lead to varying degrees of kidney damage in different patients and why the severity of stenosis does not correlate with the degree of renal dysfunction.27
Furthermore, stenosis may lead to irreversible but stable kidney damage. It is therefore not surprising that, in studies in unselected populations (ie, studies that included patients with all presentations of renal artery stenosis, not just those more likely to benefit), up to two-thirds of renal interventions yielded no clinical benefit.25
As a result, if we define ischemic nephropathy as renal artery stenosis with renal dysfunction not attributable to another cause, we probably will overestimate the prevalence of ischemic nephropathy, leading to overly optimistic expectations about the response to revascularization.
Recurrent “flash” pulmonary edema is a less common presentation, usually occurring in patients with critical bilateral renal artery stenosis or unilateral stenosis in an artery supplying a solitary functioning kidney. Most have severe hypertension (average systolic blood pressure 174–207 mm Hg) and poor renal function.28–30
The association between pulmonary edema and bilateral renal artery stenosis was first noted in 1998 by Pickering et al,31 who in several case series showed that 82% to 92% of patients with recurrent pulmonary edema and renal artery stenosis had bilateral stenosis, compared with 20% to 65% of those with other presentations. Later case series corroborated this finding: 85% to 100% of patients with renal artery stenosis and pulmonary edema had bilateral stenosis.28–30
STENTING IS NOW STANDARD
Stenting has become standard in the endovascular treatment of renal artery stenosis.
Most atherosclerotic renal artery lesions are located in the ostium (ie, where the artery branches off from the aorta), and many are extensions of calcified aortic plaque.26,32 These hard lesions tend to rebound to their original shape more often with balloon angioplasty alone. Stenting provides the additional force needed to permanently disrupt the lesion, leading to a longer-lasting result.
Rates of technical success (dilating the artery during the intervention) are higher with stents than without them (98% vs 46%– 77%).33,34 If the lesion is ostial, this difference is even more impressive (75% vs 29%). In addition, restenosis rates at 6 months are lower with stents (14% vs 26%–48%).34
GOALS: LOWER THE BLOOD PRESSURE, SAVE THE KIDNEY
Because endovascular procedures pose some risk to the patient, it is critical to intervene only in patients most likely to respond clinically. The decision to intervene depends largely on the clinical goal, which should depend on the clinical presentation.
However, if renal artery stenosis is clinically silent, most of the evidence suggests that intervention has no benefit. Furthermore, although retrospective studies have indicated that intervention may improve survival rates,35,36 prospective studies have not. Similarly, studies have not shown that intervention generally improves cardiovascular outcomes, even though renal artery stenosis is associated with cardiovascular risk.
Hypertension plus stenosis is not necessarily renovascular hypertension
Essential hypertension and clinically silent renal artery stenosis often coexist, which is why blood pressure control often does not improve after stenting. Also, essential hypertension often coexists with renovascular hypertension.37 In this situation, stenting may not eliminate the need for antihypertensive drugs, although it may improve blood pressure control and decrease the drug burden.
Before stents came into use, several randomized controlled trials found that blood pressure was no better controlled after angioplasty, 2,3,38 except in cases of bilateral stenosis.2 This may be because stenosis tended to recur after angioplasty without stents.
The 2000 Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) study was the first randomized controlled trial to examine the effect of angioplasty on blood pressure control in renal artery stenosis.38 It had significant design flaws. For example, many patients crossed over from the medical management group to the intervention group because their hypertension was resistant to medical therapy. Overall, intervention (balloon angioplasty without stents in 54 of 56 patients, with stents in the other 2) carried no benefit. However, in subgroup analysis, the patients who crossed over because of resistant hypertension (failure of a three-drug regimen) were more likely to benefit from angioplasty. This suggested that risk stratification should take place early on, before proceeding with revascularization.
With stents, Zeller,39 in a prospective nonrandomized study, found that the mean arterial pressure decreased by 10 mm Hg. Randomized trials (see below) have failed to demonstrate such a benefit.
Stenting may not improve renal function
Coincidental renal artery stenosis in a patient with unrelated chronic kidney disease is very hard to differentiate from true ischemic nephropathy. Furthermore, most patients with ischemic nephropathy do not benefit from revascularization, making it challenging to identify those few whose renal function may respond.
Given that patients with chronic kidney disease tend to have a higher risk of cardiovascular disease, it is not surprising that 15% of them may have renal artery stenosis,4 most often incidental.
Chábová40 examined the outcomes of 68 patients who had chronic kidney disease and a renal artery lesion larger than 70% who did not undergo angioplasty. In only 10 (15%) of the patients did the glomerular filtration rate (GFR) decline by more than 50% of its baseline value during the study period of 3 years. Given the retrospective nature of the study, it cannot be determined (and is rather unlikely) that ischemic nephropathy was the cause of the decline in kidney function in all 10 patients.
In a prospective cohort study in 304 patients with chronic kidney disease and renal artery stenosis who underwent surgical revascularization, Textor4 reported that the serum creatinine level showed a meaningful improvement afterward in 28%, worsened in 19.7%, and remained unchanged in 160 52.6%. (A “meaningful” change was defined as > 1.0 mg/dL.) Findings were similar in a cohort that underwent stenting.33
Davies et al41 found that 20% of patients who underwent renal stenting had a persistent increase in serum creatinine of 0.5 mg/dL or more. These patients were nearly three times more likely (19% vs 7%) to eventually require dialysis, and they had a lower 5-year survival rate (41% vs 71%).
Zeller et al39 found that renal function improved slightly in 52% of patients who received stents. The mean decrease in serum creatinine in this group was 0.22 mg/dL. However, the other 48% had a mean increase in serum creatinine of 1.1 mg/dL.
From these data we can conclude that, in an unselected population with renal artery stenosis, stenting provides no benefit to renal function, and that the risk of a worsening of renal function after intervention is roughly equal to the likelihood of achieving any benefit.
Other predictors of improvement in renal function have been proposed, but the evidence supporting them has not been consistent. For example, although Radermacher et al42 reported that a renal resistive index (which reflects arterial stiffness downstream of the stenosis) lower than 0.8 predicted a response in renal function, this finding has not been reliably reproduced.43,44 Similarly, while several studies suggest that patients with milder renal dysfunction have a higher likelihood of a renal response,45,46 other studies suggest either that the opposite is true39 or that baseline renal function alone has no impact on outcome.47
In addition, once significant renal atrophy occurs, revascularization may not help much, since irreversible sclerosis has developed. Thus, the goal is to identify kidneys being harmed by renal artery stenosis during the ischemic phase, when the tissue is still viable.
Unfortunately, we still lack a good renal stress test—eg, analogous to the cardiac stress test—to diagnose reversible ischemia in the kidney. The captopril renal scan has that capability but is not accurate in patients with bilateral stenosis or a GFR less than 50 mL/min, severely limiting its applicability.26 Newer technologies such as blood-oxygen-level-dependent (BOLD) magnetic resonance imaging are being investigated for such a role.48
Cohort studies in patients with declining renal function
In several case series, patients whose renal function had been declining before intervention had impressive rates of better renal function afterward.33,39,47,49–54 In a prospective cohort study by Muray et al,47 a rise in serum creatinine of more than 0.1 mg/mL/month before intervention seemed to predict an improvement in renal function afterward.
One would expect that, for renal function to respond to intervention, severe bilateral stenosis or unilateral stenosis to a solitary functioning kidney would need to be present. However, this was an inconsistent finding in these case series.33,39,47,52,53 The Angioplasty and Stent for Renal Artery Lesions (ASTRAL) trial,6,7 discussed later, sheds a bit more light on this.
Stenting usually improves flash pulmonary edema
Acute pulmonary edema in the setting of bilateral renal artery stenosis seems to be a unique case in which improvement in clinical status can be expected in most patients after intervention. Blood pressure improves in 94% to 100% of patients,28,31 renal function either improves or stabilizes in 77% to 91%,28–31 and pulmonary edema resolves without recurrence in 77% to 100%.28–30
NEW RANDOMIZED TRIALS: STAR, ASTRAL, AND CORAL
Despite the lack of evidence supporting revascularization of renal artery stenosis, many interventionalists practice under the assumption that the radiographic finding of renal artery stenosis alone is an indication for renal revascularization. Only three randomized controlled trials in the modern era attempt to examine this hypothesis: STAR, ASTRAL, and CORAL.
STAR: No clear benefit
The Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial5 was a European multicenter trial that enrolled 140 patients with ostial renal artery stenosis greater than 50%, blood pressure controlled to less than 140/90 mm Hg, and creatinine clearance 15 to 80 mL/min.
Patients were randomized to undergo stenting or medical therapy alone. High blood pressure was treated according to a protocol in which angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers were relegated to second-line use. All patients received a statin, regardless of lipid levels.
At 2 years, the primary end point (a decline in creatinine clearance of 20% or greater) had been reached in 10 (16%) of the 64 patients in the stent group and 16 (22%) of the 76 patients in the medication group; the difference was not statistically significant (hazard ratio 0.73, 95% confidence interval 0.33–1.61). No difference was seen in the secondary end points of the degree of blood pressure control or the rates of cardiovascular morbidity and death.5
ASTRAL: Also no clear benefit
In the international, multicenter ASTRAL trial,6,7 806 patients with at least one stenotic renal artery considered suitable for balloon angioplasty, stenting, or both7 were randomized to undergo intervention or medical management. Hypertension treatment was not specified by a protocol. The mean estimated GFR was 40 mL/min. Most patients (95%–96%) were on statin therapy. The primary outcome was the rate of decline of renal function over time. Secondary outcomes included blood pressure control, renal events, cardiovascular events, and death.
Results. At a mean follow-up of 33.6 months (range 1–4 years), no difference was noted between treatment groups in decline in renal function or blood pressure control at 1 year. Renal function worsened slightly in both groups.
The decline in renal function over time, calculated as the mean slope of the reciprocal of the serum creatinine level over time, was slightly slower in the revascularization group, but the difference was not statistically significant (−0.07 × 10−3 vs −0.13 × 10−3 L/μmol/year, P = .06). This difference did not appear until the last year of the study. There was no difference in the number of patients whose renal function improved or declined during the study.
There was no difference in the rate of any secondary outcome. The medical management group required a slightly higher number of antihypertensive drugs, reaching statistical but not clinical significance (2.97 vs 2.77 drugs, P = .03). More people in the revascularization group were taking ACE inhibitors or angiotensin receptor blockers. There was no difference in the number of patients on any antihypertensive therapy (97% vs 99%). Interestingly, amputations were more common in the revascularization group, occurring in 42 (12%) of the 386 patients in the revascularization group vs 29 (7%) of the 395 patients in the medical group (P = .04).
Seventeen percent of patients randomized to intervention did not have the procedure done. An as-treated analysis of the 317 (83%) patients randomized to revascularization who did receive it showed no differences in outcomes.
There were no differences in outcomes among specific, predefined subgroups based on severity of stenosis at baseline, renal length, baseline estimated GFR, baseline serum creatinine, and rate of progression of renal dysfunction before randomization.7
Comments. ASTRAL contradicts previous nonrandomized studies that suggested that rapidly declining renal function (loss of 20% in 1 year) predicts response to intervention. Considering the large number of patients with unilateral disease in the study, it would be interesting to see what effect stenting had on patients with both severe disease and declining renal function.
ASTRAL has been criticized because it lacked a central laboratory to interpret the severity of stenosis, it did not use a standardized intervention technique (5% of patients underwent angioplasty without stents, although this did not affect outcomes7), and patients were enrolled only if the clinician involved in the case was uncertain of the appropriate management.
This last issue raises the concern for selection bias toward inclusion of more difficult cases that may not respond to intervention. But these shortcomings are not serious enough to negate the fact that preliminary results from the largest randomized controlled trial to date confirm conclusions of other randomized trials, ie, that intervention in renal artery stenosis yields no benefits over medical management in most patients.
Based on the results of STAR and ASTRAL, the practice of indiscriminately revascularizing stenosed renal arteries without strong evidence that the procedure will provide a clinical benefit is no longer tenable. The challenge is to identify those few patients who will respond, and to intervene only on them. Unfortunately, none of the subgroups from ASTRAL helped characterize this population.
CORAL: A large trial is ongoing
The Cardiovascular Outcomes in Renal Artherosclerotic Lesions (CORAL) trial,8 an ongoing multicenter randomized controlled trial in the United States, may be of additional help.
Unlike ASTRAL, CORAL is studying patients who have difficult-to-control hypertension (systolic blood pressure ≥ 155 mm Hg on two or more drugs).8 Chronic kidney disease is not an exclusion criterion unless the serum creatinine concentration is greater than 3.0 mg/dL.
CORAL is using a standardized medical protocol to control blood pressure. In addition, use of embolic protection devices during stenting is encouraged. Hopefully, the large size (a goal of 1,080 patients) and the inclusion of patients with more marked hypertension will address the utility of intervention in higher-risk populations with renal artery stenosis.
RECOMMENDED APPROACH TO INTERVENTION IN RENAL ARTERY STENOSIS
As we wait for CORAL to be completed, we have two modern-era randomized controlled trials that leave us with fewer indications for renal intervention. Table 2 lists commonly cited indications for intervention in renal artery stenosis and the evidence to support them. As most of these are based on retrospective data or have conflicting support in the literature, their utility remains in question. At this point we can safely recommend:
- Patients with preserved or even decreased but stable renal function will not likely have a benefit in renal function after intervention.
- Patients with resistant hypertension may benefit.
- The best evidence supporting intervention is for bilateral stenosis with flash pulmonary edema, but the evidence is from retrospective studies.
- Stenting in bilateral disease without another indication has no apparent benefit.
- Declining renal function is not a guarantee of success.
- It is unclear if patients with severe bilateral stenosis or severe stenosis to a solitary functioning kidney with declining renal function will benefit. Anecdotally, they do respond more often, but as with many other indications for intervention that have gone by the wayside, this may not bear out when studied properly.
As the utility of intervention narrows, the scope of practice for such interventions should narrow accordingly. Attention should now be focusing on clinical, rather than radiographic, indications for intervening on renal artery stenosis.
Therefore, the decision to intervene must not be made solely by the interventionalist. A multidisciplinary approach should be adopted that at the very least includes the input of a nephrologist well versed in renal artery stenosis. In this way, the clinical risks and benefits of renal intervention can be discussed with the patient by providers who are likely to be involved in their care should renal function or hypertension fail to improve afterward.
RISK OF ATHEROEMBOLISM
While renal stenting yields improved technical success in the treatment of renal artery stenosis, it carries with it an increasingly common risk to kidney function: atheroembolism as the stent crushes the plaque against the vessel wall. This may lead to obstruction of the renal microvasculature, increasing the risk of irreversible damage to renal function.
Atheroembolic kidney disease can manifest as progressive renal failure occurring over weeks to months, commonly misdiagnosed as permanent damage from contrast nephropathy.55
Embolic protection devices, inserted downstream of the lesion before stenting to catch any debris that may break loose, have been developed to help address this problem.
Holden et al 57 prospectively studied 63 patients with renal artery stenosis and deteriorating renal function (undefined) who underwent stenting with an embolic protection device. At 6 months after the intervention, renal function had either improved or stabilized in 97% of patients, suggesting that many of the deleterious effects of stenting on renal function are related to atheroembolism.
The Prospective Randomized Study Comparing Renal Artery Stenting With or Without Distal Protection (RESIST) trial, in which renal dysfunction was mild and the GFR was not declining (average estimated GFR 59.3 mL/min), found contrary results.57 In a two-by-two factorial study, patients were randomized to undergo stenting alone, stenting with the antiplatelet agent abciximab (ReoPro), stenting with an embolic protection device, or stenting with both abciximab and an embolic protection device. Interestingly, renal function declined in the first three groups, but remained stable in the group that received both abciximab and an embolic protection device.
ANTIPLATELET THERAPY AFTER RENAL STENTING: HOW LONG?
We have no data on the optimal duration of antiplatelet therapy after renal stenting, and guidelines from professional societies do not comment on it.58 As a result, practice patterns vary significantly among practitioners.
While in-stent restenosis rates are acceptably low after renal stenting, the risks and side effects of antiplatelet therapy often lead to arbitrary withdrawal of these drugs. The effect on stent patency is yet to be determined.
FUTURE DEVELOPMENTS
Results of STAR and ASTRAL confirm the growing suspicion that the surge seen in the last decade in renal artery stenting should be coming to an end. We await results either from CORAL or possibly a post hoc analysis of ASTRAL that might identify potential high-risk groups that will benefit from renal intervention. And as embolic protection devices become more agile and suitable to different renal lesions, there remains the possibility that, due to lower rates of unidentified atheroembolic kidney disease, CORAL may demonstrate improved renal outcomes after stenting. If not, the search for the best means to predict who should have renal intervention will continue.
We know through experience that stenting does provide great benefits for some patients with renal artery stenosis. Furthermore, the clinical problem is too intriguing, and too profitable, to die altogether.
- Choncol M, Linas S. Diagnosis and management of ischemic nephropathy. Clin J Am Soc Nephrol 2006; 1:172–181.
- Webster J, Marshall F, Abdalla M, et al Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens 1998; 12:329–335.
- Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998; 31:823–829.
- Textor SC. Revascularization in atherosclerotic renal artery disease. Kidney Int 1998; 53:799–811.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848.
- Mistry S, Ives N, Harding J, et al Angioplasty and STent for Renal Artery Lesions (ASTRAL trial): rationale, methods and results so far. J Hum Hypertens 2007; 21:511–515.
- Wheatley K, Ives N, Kalra P, Moss J. Revascularization versus medical therapy for renal-artery stenosis (ASTRAL). N Engl J Med 2009; 361:1953–1962.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
- Galaria II, Surowiec SM, Rhodes JM, et al Percutaneous and open renal revascularizations have equivalent long-term functional outcomes. Ann Vasc Surg 2005; 19:218–228.
- Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery interventions by Medicare beneficiaries 1996–2000. AJR Am J Roentgenol 2004; 183:561–568.
- Textor SC. Atherosclerotic renal artery stenosis: overtreated but underrated? J Am Soc Nephrol 2008; 19:656–659.
- Kalra PA, Guo H, Gilbertson DT, et al Atherosclerotic renovascular disease in the United States. Kidney Int 2010; 77:37–43.
- Hansen KJ, Edwards MS, Craven TE, et al Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Cohen MG, Pascua JA, Garcia-Ben M, et al A simple prediction rule for significant renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 2005; 150:1204–1211.
- Buller CE, Nogareda JG, Ramanathan K, et al The profile of cardiac patients with renal artery stenosis. J Am Coll Cardiol 2004; 43:1606–1613.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- Kalra PA, Guo H, Kausz AT, et al Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 69:293–301.
- Holley KE, Hunt JC, Brown AL, Kincaid OW, Sheps SG. Renal artery stenosis. A clinical-pathologic study in normotensive and hypertensive patients. Am J Med 1964; 37:14–22.
- de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systemic literature review. J Hypertens 2009; 27:1333–1340.
- Kuczera P, Włoszczynska E, Adamczak M, Pencak P, Chudek J, Wiecek A. Frequency of renal artery stenosis and variants of renal vascularization in hypertensive patients: analysis of 1550 angiographies in one centre. J Hum Hypertens 2009; 23:396–401.
- Caps MT, Perissinotto C, Zierler RE, et al Prospective study of atherosclerotic disease progression in the renal artery. Circulation 1998; 98:2866–2872.
- Zierler RE, Bergelin RO, Davidson RC, Cantwell-Gab K, Polissar NL, Strandness DE. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens 1996; 9:1055–1061.
- Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urol Clin North Am 1984; 11:383–392.
- Crowley JJ, Santos RM, Peter RH, et al Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 1998; 136:913–918.
- Textor SC. Renovascular hypertension update. Curr Hypertens Rep 2006; 8:521–527.
- Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol 2004; 15:1974–1982.
- Wright JR, Shurrab AE, Cheung C, et al A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis 2002; 39:1153–1161.
- Messina LM, Zelenock GB, Yao KA, Stanley JC. Renal revascularization for recurrent pulmonary edema in patients with poorly controlled hypertension and renal insufficiency: a distinct subgroup of patients with arteriosclerotic renal artery occlusive disease. J Vasc Surg 1992; 15:73–80.
- Bloch MJ, Trost DW, Pickering TG, Sos TA, August P. Prevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placement. Am J Hypertens 1999; 12:1–7.
- Gray BH, Olin JW, Childs MB, Sullivan TM, Bacharach JM. Clinical benefit of renal artery angioplasty with stenting for the control of recurrent and refractory congestive heart failure. Vasc Med 2002; 7:275–279.
- Pickering TG, Herman L, Devereux RB, et al Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet 1988; 2:551–552.
- Kennedy DJ, Colyer WR, Brewster PS, et al Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am J Kidney Dis 2003; 14:926–935.
- Beutler JJ, Van Ampting JM, Van De Ven PJ, et al Long-term effects of arterial stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol 2001; 12:1475–1481.
- Van de Ven PJ, Kaatee R, Beutler JJ, et al Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomized trial. Lancet 1999; 353:282–286.
- Isles C, Main J, O’Connell J, et al Survival associated with renovascular disease in Glasgow and Newcastle: a collaborative study. Scott Med J 1990; 35:70–73.
- Hunt JC, Sheps SG, Harrison EG, Strong CG, Bernatz PE. Renal and renovascular hypertension. A reasoned approach to diagnosis and management. Arch Intern Med 1974; 133:988–999.
- Textor SC. Atherosclerotic renal artery stenosis: how big is the problem, and what happens if nothing is done? J Hypertens Suppl 2005; 23:S5–S13.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Zeller T, Frank U, Müller C, et al Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation 2003; 108;2244–2249.
- Chábová V, Schirger A, Stanson AW, McKusick MA, Textor SC. Outcomes of atherosclerotic renal artery stenosis managed without revascularization. Mayo Clin Proc 2000; 75:437–444.
- Davies MG, Saad WE, Peden EK, Mohiuddin IT, Naoum JJ, Lumsden AB. Implications of acute functional injury following percutaneous renal artery intervention. Ann Vasc Surg 2008; 22:783–789.
- Radermacher J, Chavin A, Bleck J, et al Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Eng J Med 2001; 344:410–417.
- García-Criado A, Gilabert R, Nicolau C, et al Value of Doppler sonography for predicting clinical outcome after renal artery revascularization in atherosclerotic renal artery stenosis. J Ultrasound Med 2005; 24:1641–1647.
- Zeller T, Müller C, Frank U, et al Stent angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosis. Catheter Cardiovasc Interv 2003; 58:510–515.
- Harden PN, MacLeod MJ, Rodger RSC, et al Effect of renal-artery stenting on progression of renovascular renal failure. Lancet 1997; 349:1133–1136.
- Isles CG, Robertson S, Hill D. Management of renovascular disease: a review of renal artery stenting in ten studies. QJM 1999; 92:159–167.
- Muray S, Martın M, Amoedo ML, et al Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis 2002; 39:60–66.
- Textor SC, Glockner JF, Lerman LO, et al The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 2008; 19:780–788.
- Paraskevas KI, Perrea D, Briana DD, Liapis CD. Management of atherosclerotic renovascular disease: the effect of renal artery stenting on renal function and blood pressure. Int Urol Nephrol 2006; 38:683–691.
- Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation 2000; 102:1671–1677.
- Dean RH, Kieffer RW, Smith BM, et al Renovascular hypertension: anatomic and renal function changes during drug therapy. Arch Surg 1981; 116:1408–1415.
- Zhang Q, Shen W, Zhang R, Zhang J, Hu J, Zhang X. Effects of renal artery stenting on renal function and blood pressure in patients with atherosclerotic renovascular disease. Chin Med J (Engl) 2003; 116:1451–1454.
- Ramos F, Kotliar C, Alvarez D, et al Renal function and outcome of PTRA and stenting for atherosclerotic renal artery stenosis. Kidney Int 2003; 63:276–282.
- Rocha-Singh KJ, Ahuja RK, Sung CH, Rutherford J. Long-term renal function preservation after renal artery stenting in patients with progressive ischemic nephropathy. Catheter Cardiovasc Interv 2002; 57:135–141.
- Thadhani RI, Camargo CA, Xavier RJ, Fang LS, Bazari H. Atheroembolic renal failure after invasive procedures. Natural history based on 52 histologically proven cases. Medicine (Baltimore) 1995; 74:350–358.
- Holden A, Hill A, Jaff MR, Pilmore H. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int 2006; 70:948–955.
- Cooper CJ, Haller ST, Colyer W, et al Embolic protection and platelet inhibition during renal artery stenting. Circulation 2008; 117:2752–2760.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
Author’s note: Atherosclerosis accounts for about 90% of cases of renal artery stenosis in people over age 40.1 Fibromuscular dysplasia, the other major cause, is a separate topic; in this paper “renal artery stenosis” refers to atherosclerotic disease only.
Renal artery stenosis is very common, and the number of angioplasty-stenting procedures performed every year is on the rise. Yet there is no overwhelming evidence that intervention yields clinical benefits—ie, better blood pressure control or renal function— than does medical therapy.
Earlier randomized controlled trials comparing angioplasty without stents and medical management showed no impressive difference in blood pressure.2,3 The data on renal function were even more questionable, with some studies suggesting that, with stenting, the chance of worsening renal function is equal to that of improvement.4
Two large randomized trials comparing renal intervention with medical therapy failed to show any benefit of intervention.5–7 A third study is under way.8
It is time to strongly reconsider the current aggressive approach to revascularization of stenotic renal arteries and take a more coordinated, critical approach.
RENAL INTERVENTIONS ON THE RISE
Renal angioplasty began replacing surgical revascularization in the 1990s, as this less-invasive procedure became more readily available and was shown to have similar clinical outcomes.9 In the last decade, stent placement during angioplasty has become standard, improving the rates of technical success.
As these procedures have become easier to perform and their radiographic outcomes have become more consistent, interventionalists have become more likely, if they see stenosis in a renal artery, to intervene and insert a stent, regardless of proven benefit. In addition, interventionalists from at least three different specialties now compete for these procedures, often by looking at the renal arteries during angiography of other vascular beds (the “drive-by”).
As a result, the number of renal interventions has been rising. Medicare received 21,660 claims for renal artery interventions (surgery or angioplasty) in 2000, compared with 13,380 in 1996—an increase of 62%. However, the number of surgeries actually decreased by 45% during this time, while the number of percutaneous procedures increased by 240%. The number of endovascular claims for renal artery stenosis by cardiologists alone rose 390%.10 Since then, the reports on intervention have been mixed, with one report citing a continued increase in 2005 to 35,000 claims,11 and another suggesting a decrease back to 1997 levels.12
HOW COMMON IS RENAL ARTERY STENOSIS?
The prevalence of renal artery stenosis depends on the definition used and the population screened. It is more common in older patients who have risk factors for other vascular diseases than in the general population.
Renal Doppler ultrasonography can detect stenosis only if the artery is narrowed by more than 60%. Hansen et al13 used ultrasonography to screen 870 people over age 65 and found a lesion (a narrowing of more than 60%) in 6.8%.
Angiography (direct, computed tomographic, or magnetic resonance) can detect less-severe stenosis. Thus, most angiographic studies define renal artery stenosis as a narrowing of more than 50%, and severe disease as a narrowing of more than 70%. Many experts believe that unilateral stenosis needs to be more than 70% to pose a risk to the kidney.14,15
Angiographic prevalence studies have been performed only in patients who were undergoing angiography for another reason such as coronary or peripheral arterial disease that inherently places them at higher risk of renal artery stenosis. For instance, renal artery stenosis is found in 11% to 28% of patients undergoing diagnostic cardiac catheterization. 16
No studies of the prevalence of renal artery stenosis have been performed in the general population. Medicare data indicate that from 1999 to 2001 the incidence of diagnosed renal artery stenosis was 3.7 per 1,000 patientyears. 17 Holley et al,18 in an autopsy series, found renal artery stenosis of greater than 50% in 27% of patients over age 50 and in 56.4% of hypertensive patients. The prevalence was 10% in normotensive patients.
WHO IS AT RISK?
Factors associated with a higher risk of finding renal artery stenosis on a radiographic study include14:
- Older age
- Female sex
- Hypertension
- Three-vessel coronary artery disease
- Peripheral artery disease
- Chronic kidney disease
- Diabetes
- A low level of high-density lipoprotein cholesterol
- The use of at least two cardiovascular drugs.
The prevalence of renal artery stenosis in at-risk populations ranges from 3% to 75% (Table 1).2,4,6,19,20
HOW OFTEN DOES STENOSIS PROGRESS?
The reported rates of progression of atherosclerotic renal artery lesions vary depending on the type of imaging test used and the reason for doing it.
In studies that used duplex ultrasonography, roughly half of lesions smaller than 60% grew to greater than 60% over 3 years.21,22 The risk of total occlusion of an artery was relatively low and depended on the severity of stenosis: 0.7% if the baseline stenosis was less than 60% and 2.3% to 7% if it was greater.21,22
In a seminal study in 1984, Schreiber and colleagues23 compared serial angiograms obtained a mean of 52 months apart in 85 patients who did not undergo intervention. Stenosis had progressed in 37 (44%), and to the point of total occlusion in 14 (16%). In contrast, a 1998 study found progression in 11.1% over 2.6 years, with older patients, women, and those with baseline coronary artery disease at higher risk.24
The the rates of progression differed in these two studies probably because the indications for screening were different (clinical suspicion23 vs routine screening during coronary angiography24), as was the severity of stenosis at the time of diagnosis. Also, when these studies were done, fewer people were taking statins. Thus, similar studies, if repeated, might show even lower rates of progression.
Finally, progression of renal artery stenosis has not been correlated with worsening renal function.
FOUR CLINICAL PRESENTATIONS OF RENAL ARTERY STENOSIS
Renal artery stenosis can present in one of four ways:
Clinically silent stenosis. Because renal artery stenosis is most often found in older patients, who are more likely to have essential hypertension and chronic kidney disease due to other causes, it can be an incidental finding that is completely clinically silent.16,25
Renovascular hypertension is defined as high blood pressure due to up-regulation of neurohormonal activity in response to decreased perfusion from renal artery stenosis. Renal artery stenosis is estimated to be the cause of hypertension in only 0.5% to 4.0% of hypertensive patients, or in 26% of patients with secondary hypertension.3
Ischemic nephropathy is more difficult to define because ischemia alone rarely explains the damage done to the kidneys. Activation of neurohormonal pathways and microvascular injury are thought to contribute to oxidative stress and fibrosis.26 These phenomena may explain why similar degrees of stenosis lead to varying degrees of kidney damage in different patients and why the severity of stenosis does not correlate with the degree of renal dysfunction.27
Furthermore, stenosis may lead to irreversible but stable kidney damage. It is therefore not surprising that, in studies in unselected populations (ie, studies that included patients with all presentations of renal artery stenosis, not just those more likely to benefit), up to two-thirds of renal interventions yielded no clinical benefit.25
As a result, if we define ischemic nephropathy as renal artery stenosis with renal dysfunction not attributable to another cause, we probably will overestimate the prevalence of ischemic nephropathy, leading to overly optimistic expectations about the response to revascularization.
Recurrent “flash” pulmonary edema is a less common presentation, usually occurring in patients with critical bilateral renal artery stenosis or unilateral stenosis in an artery supplying a solitary functioning kidney. Most have severe hypertension (average systolic blood pressure 174–207 mm Hg) and poor renal function.28–30
The association between pulmonary edema and bilateral renal artery stenosis was first noted in 1998 by Pickering et al,31 who in several case series showed that 82% to 92% of patients with recurrent pulmonary edema and renal artery stenosis had bilateral stenosis, compared with 20% to 65% of those with other presentations. Later case series corroborated this finding: 85% to 100% of patients with renal artery stenosis and pulmonary edema had bilateral stenosis.28–30
STENTING IS NOW STANDARD
Stenting has become standard in the endovascular treatment of renal artery stenosis.
Most atherosclerotic renal artery lesions are located in the ostium (ie, where the artery branches off from the aorta), and many are extensions of calcified aortic plaque.26,32 These hard lesions tend to rebound to their original shape more often with balloon angioplasty alone. Stenting provides the additional force needed to permanently disrupt the lesion, leading to a longer-lasting result.
Rates of technical success (dilating the artery during the intervention) are higher with stents than without them (98% vs 46%– 77%).33,34 If the lesion is ostial, this difference is even more impressive (75% vs 29%). In addition, restenosis rates at 6 months are lower with stents (14% vs 26%–48%).34
GOALS: LOWER THE BLOOD PRESSURE, SAVE THE KIDNEY
Because endovascular procedures pose some risk to the patient, it is critical to intervene only in patients most likely to respond clinically. The decision to intervene depends largely on the clinical goal, which should depend on the clinical presentation.
However, if renal artery stenosis is clinically silent, most of the evidence suggests that intervention has no benefit. Furthermore, although retrospective studies have indicated that intervention may improve survival rates,35,36 prospective studies have not. Similarly, studies have not shown that intervention generally improves cardiovascular outcomes, even though renal artery stenosis is associated with cardiovascular risk.
Hypertension plus stenosis is not necessarily renovascular hypertension
Essential hypertension and clinically silent renal artery stenosis often coexist, which is why blood pressure control often does not improve after stenting. Also, essential hypertension often coexists with renovascular hypertension.37 In this situation, stenting may not eliminate the need for antihypertensive drugs, although it may improve blood pressure control and decrease the drug burden.
Before stents came into use, several randomized controlled trials found that blood pressure was no better controlled after angioplasty, 2,3,38 except in cases of bilateral stenosis.2 This may be because stenosis tended to recur after angioplasty without stents.
The 2000 Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) study was the first randomized controlled trial to examine the effect of angioplasty on blood pressure control in renal artery stenosis.38 It had significant design flaws. For example, many patients crossed over from the medical management group to the intervention group because their hypertension was resistant to medical therapy. Overall, intervention (balloon angioplasty without stents in 54 of 56 patients, with stents in the other 2) carried no benefit. However, in subgroup analysis, the patients who crossed over because of resistant hypertension (failure of a three-drug regimen) were more likely to benefit from angioplasty. This suggested that risk stratification should take place early on, before proceeding with revascularization.
With stents, Zeller,39 in a prospective nonrandomized study, found that the mean arterial pressure decreased by 10 mm Hg. Randomized trials (see below) have failed to demonstrate such a benefit.
Stenting may not improve renal function
Coincidental renal artery stenosis in a patient with unrelated chronic kidney disease is very hard to differentiate from true ischemic nephropathy. Furthermore, most patients with ischemic nephropathy do not benefit from revascularization, making it challenging to identify those few whose renal function may respond.
Given that patients with chronic kidney disease tend to have a higher risk of cardiovascular disease, it is not surprising that 15% of them may have renal artery stenosis,4 most often incidental.
Chábová40 examined the outcomes of 68 patients who had chronic kidney disease and a renal artery lesion larger than 70% who did not undergo angioplasty. In only 10 (15%) of the patients did the glomerular filtration rate (GFR) decline by more than 50% of its baseline value during the study period of 3 years. Given the retrospective nature of the study, it cannot be determined (and is rather unlikely) that ischemic nephropathy was the cause of the decline in kidney function in all 10 patients.
In a prospective cohort study in 304 patients with chronic kidney disease and renal artery stenosis who underwent surgical revascularization, Textor4 reported that the serum creatinine level showed a meaningful improvement afterward in 28%, worsened in 19.7%, and remained unchanged in 160 52.6%. (A “meaningful” change was defined as > 1.0 mg/dL.) Findings were similar in a cohort that underwent stenting.33
Davies et al41 found that 20% of patients who underwent renal stenting had a persistent increase in serum creatinine of 0.5 mg/dL or more. These patients were nearly three times more likely (19% vs 7%) to eventually require dialysis, and they had a lower 5-year survival rate (41% vs 71%).
Zeller et al39 found that renal function improved slightly in 52% of patients who received stents. The mean decrease in serum creatinine in this group was 0.22 mg/dL. However, the other 48% had a mean increase in serum creatinine of 1.1 mg/dL.
From these data we can conclude that, in an unselected population with renal artery stenosis, stenting provides no benefit to renal function, and that the risk of a worsening of renal function after intervention is roughly equal to the likelihood of achieving any benefit.
Other predictors of improvement in renal function have been proposed, but the evidence supporting them has not been consistent. For example, although Radermacher et al42 reported that a renal resistive index (which reflects arterial stiffness downstream of the stenosis) lower than 0.8 predicted a response in renal function, this finding has not been reliably reproduced.43,44 Similarly, while several studies suggest that patients with milder renal dysfunction have a higher likelihood of a renal response,45,46 other studies suggest either that the opposite is true39 or that baseline renal function alone has no impact on outcome.47
In addition, once significant renal atrophy occurs, revascularization may not help much, since irreversible sclerosis has developed. Thus, the goal is to identify kidneys being harmed by renal artery stenosis during the ischemic phase, when the tissue is still viable.
Unfortunately, we still lack a good renal stress test—eg, analogous to the cardiac stress test—to diagnose reversible ischemia in the kidney. The captopril renal scan has that capability but is not accurate in patients with bilateral stenosis or a GFR less than 50 mL/min, severely limiting its applicability.26 Newer technologies such as blood-oxygen-level-dependent (BOLD) magnetic resonance imaging are being investigated for such a role.48
Cohort studies in patients with declining renal function
In several case series, patients whose renal function had been declining before intervention had impressive rates of better renal function afterward.33,39,47,49–54 In a prospective cohort study by Muray et al,47 a rise in serum creatinine of more than 0.1 mg/mL/month before intervention seemed to predict an improvement in renal function afterward.
One would expect that, for renal function to respond to intervention, severe bilateral stenosis or unilateral stenosis to a solitary functioning kidney would need to be present. However, this was an inconsistent finding in these case series.33,39,47,52,53 The Angioplasty and Stent for Renal Artery Lesions (ASTRAL) trial,6,7 discussed later, sheds a bit more light on this.
Stenting usually improves flash pulmonary edema
Acute pulmonary edema in the setting of bilateral renal artery stenosis seems to be a unique case in which improvement in clinical status can be expected in most patients after intervention. Blood pressure improves in 94% to 100% of patients,28,31 renal function either improves or stabilizes in 77% to 91%,28–31 and pulmonary edema resolves without recurrence in 77% to 100%.28–30
NEW RANDOMIZED TRIALS: STAR, ASTRAL, AND CORAL
Despite the lack of evidence supporting revascularization of renal artery stenosis, many interventionalists practice under the assumption that the radiographic finding of renal artery stenosis alone is an indication for renal revascularization. Only three randomized controlled trials in the modern era attempt to examine this hypothesis: STAR, ASTRAL, and CORAL.
STAR: No clear benefit
The Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial5 was a European multicenter trial that enrolled 140 patients with ostial renal artery stenosis greater than 50%, blood pressure controlled to less than 140/90 mm Hg, and creatinine clearance 15 to 80 mL/min.
Patients were randomized to undergo stenting or medical therapy alone. High blood pressure was treated according to a protocol in which angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers were relegated to second-line use. All patients received a statin, regardless of lipid levels.
At 2 years, the primary end point (a decline in creatinine clearance of 20% or greater) had been reached in 10 (16%) of the 64 patients in the stent group and 16 (22%) of the 76 patients in the medication group; the difference was not statistically significant (hazard ratio 0.73, 95% confidence interval 0.33–1.61). No difference was seen in the secondary end points of the degree of blood pressure control or the rates of cardiovascular morbidity and death.5
ASTRAL: Also no clear benefit
In the international, multicenter ASTRAL trial,6,7 806 patients with at least one stenotic renal artery considered suitable for balloon angioplasty, stenting, or both7 were randomized to undergo intervention or medical management. Hypertension treatment was not specified by a protocol. The mean estimated GFR was 40 mL/min. Most patients (95%–96%) were on statin therapy. The primary outcome was the rate of decline of renal function over time. Secondary outcomes included blood pressure control, renal events, cardiovascular events, and death.
Results. At a mean follow-up of 33.6 months (range 1–4 years), no difference was noted between treatment groups in decline in renal function or blood pressure control at 1 year. Renal function worsened slightly in both groups.
The decline in renal function over time, calculated as the mean slope of the reciprocal of the serum creatinine level over time, was slightly slower in the revascularization group, but the difference was not statistically significant (−0.07 × 10−3 vs −0.13 × 10−3 L/μmol/year, P = .06). This difference did not appear until the last year of the study. There was no difference in the number of patients whose renal function improved or declined during the study.
There was no difference in the rate of any secondary outcome. The medical management group required a slightly higher number of antihypertensive drugs, reaching statistical but not clinical significance (2.97 vs 2.77 drugs, P = .03). More people in the revascularization group were taking ACE inhibitors or angiotensin receptor blockers. There was no difference in the number of patients on any antihypertensive therapy (97% vs 99%). Interestingly, amputations were more common in the revascularization group, occurring in 42 (12%) of the 386 patients in the revascularization group vs 29 (7%) of the 395 patients in the medical group (P = .04).
Seventeen percent of patients randomized to intervention did not have the procedure done. An as-treated analysis of the 317 (83%) patients randomized to revascularization who did receive it showed no differences in outcomes.
There were no differences in outcomes among specific, predefined subgroups based on severity of stenosis at baseline, renal length, baseline estimated GFR, baseline serum creatinine, and rate of progression of renal dysfunction before randomization.7
Comments. ASTRAL contradicts previous nonrandomized studies that suggested that rapidly declining renal function (loss of 20% in 1 year) predicts response to intervention. Considering the large number of patients with unilateral disease in the study, it would be interesting to see what effect stenting had on patients with both severe disease and declining renal function.
ASTRAL has been criticized because it lacked a central laboratory to interpret the severity of stenosis, it did not use a standardized intervention technique (5% of patients underwent angioplasty without stents, although this did not affect outcomes7), and patients were enrolled only if the clinician involved in the case was uncertain of the appropriate management.
This last issue raises the concern for selection bias toward inclusion of more difficult cases that may not respond to intervention. But these shortcomings are not serious enough to negate the fact that preliminary results from the largest randomized controlled trial to date confirm conclusions of other randomized trials, ie, that intervention in renal artery stenosis yields no benefits over medical management in most patients.
Based on the results of STAR and ASTRAL, the practice of indiscriminately revascularizing stenosed renal arteries without strong evidence that the procedure will provide a clinical benefit is no longer tenable. The challenge is to identify those few patients who will respond, and to intervene only on them. Unfortunately, none of the subgroups from ASTRAL helped characterize this population.
CORAL: A large trial is ongoing
The Cardiovascular Outcomes in Renal Artherosclerotic Lesions (CORAL) trial,8 an ongoing multicenter randomized controlled trial in the United States, may be of additional help.
Unlike ASTRAL, CORAL is studying patients who have difficult-to-control hypertension (systolic blood pressure ≥ 155 mm Hg on two or more drugs).8 Chronic kidney disease is not an exclusion criterion unless the serum creatinine concentration is greater than 3.0 mg/dL.
CORAL is using a standardized medical protocol to control blood pressure. In addition, use of embolic protection devices during stenting is encouraged. Hopefully, the large size (a goal of 1,080 patients) and the inclusion of patients with more marked hypertension will address the utility of intervention in higher-risk populations with renal artery stenosis.
RECOMMENDED APPROACH TO INTERVENTION IN RENAL ARTERY STENOSIS
As we wait for CORAL to be completed, we have two modern-era randomized controlled trials that leave us with fewer indications for renal intervention. Table 2 lists commonly cited indications for intervention in renal artery stenosis and the evidence to support them. As most of these are based on retrospective data or have conflicting support in the literature, their utility remains in question. At this point we can safely recommend:
- Patients with preserved or even decreased but stable renal function will not likely have a benefit in renal function after intervention.
- Patients with resistant hypertension may benefit.
- The best evidence supporting intervention is for bilateral stenosis with flash pulmonary edema, but the evidence is from retrospective studies.
- Stenting in bilateral disease without another indication has no apparent benefit.
- Declining renal function is not a guarantee of success.
- It is unclear if patients with severe bilateral stenosis or severe stenosis to a solitary functioning kidney with declining renal function will benefit. Anecdotally, they do respond more often, but as with many other indications for intervention that have gone by the wayside, this may not bear out when studied properly.
As the utility of intervention narrows, the scope of practice for such interventions should narrow accordingly. Attention should now be focusing on clinical, rather than radiographic, indications for intervening on renal artery stenosis.
Therefore, the decision to intervene must not be made solely by the interventionalist. A multidisciplinary approach should be adopted that at the very least includes the input of a nephrologist well versed in renal artery stenosis. In this way, the clinical risks and benefits of renal intervention can be discussed with the patient by providers who are likely to be involved in their care should renal function or hypertension fail to improve afterward.
RISK OF ATHEROEMBOLISM
While renal stenting yields improved technical success in the treatment of renal artery stenosis, it carries with it an increasingly common risk to kidney function: atheroembolism as the stent crushes the plaque against the vessel wall. This may lead to obstruction of the renal microvasculature, increasing the risk of irreversible damage to renal function.
Atheroembolic kidney disease can manifest as progressive renal failure occurring over weeks to months, commonly misdiagnosed as permanent damage from contrast nephropathy.55
Embolic protection devices, inserted downstream of the lesion before stenting to catch any debris that may break loose, have been developed to help address this problem.
Holden et al 57 prospectively studied 63 patients with renal artery stenosis and deteriorating renal function (undefined) who underwent stenting with an embolic protection device. At 6 months after the intervention, renal function had either improved or stabilized in 97% of patients, suggesting that many of the deleterious effects of stenting on renal function are related to atheroembolism.
The Prospective Randomized Study Comparing Renal Artery Stenting With or Without Distal Protection (RESIST) trial, in which renal dysfunction was mild and the GFR was not declining (average estimated GFR 59.3 mL/min), found contrary results.57 In a two-by-two factorial study, patients were randomized to undergo stenting alone, stenting with the antiplatelet agent abciximab (ReoPro), stenting with an embolic protection device, or stenting with both abciximab and an embolic protection device. Interestingly, renal function declined in the first three groups, but remained stable in the group that received both abciximab and an embolic protection device.
ANTIPLATELET THERAPY AFTER RENAL STENTING: HOW LONG?
We have no data on the optimal duration of antiplatelet therapy after renal stenting, and guidelines from professional societies do not comment on it.58 As a result, practice patterns vary significantly among practitioners.
While in-stent restenosis rates are acceptably low after renal stenting, the risks and side effects of antiplatelet therapy often lead to arbitrary withdrawal of these drugs. The effect on stent patency is yet to be determined.
FUTURE DEVELOPMENTS
Results of STAR and ASTRAL confirm the growing suspicion that the surge seen in the last decade in renal artery stenting should be coming to an end. We await results either from CORAL or possibly a post hoc analysis of ASTRAL that might identify potential high-risk groups that will benefit from renal intervention. And as embolic protection devices become more agile and suitable to different renal lesions, there remains the possibility that, due to lower rates of unidentified atheroembolic kidney disease, CORAL may demonstrate improved renal outcomes after stenting. If not, the search for the best means to predict who should have renal intervention will continue.
We know through experience that stenting does provide great benefits for some patients with renal artery stenosis. Furthermore, the clinical problem is too intriguing, and too profitable, to die altogether.
Author’s note: Atherosclerosis accounts for about 90% of cases of renal artery stenosis in people over age 40.1 Fibromuscular dysplasia, the other major cause, is a separate topic; in this paper “renal artery stenosis” refers to atherosclerotic disease only.
Renal artery stenosis is very common, and the number of angioplasty-stenting procedures performed every year is on the rise. Yet there is no overwhelming evidence that intervention yields clinical benefits—ie, better blood pressure control or renal function— than does medical therapy.
Earlier randomized controlled trials comparing angioplasty without stents and medical management showed no impressive difference in blood pressure.2,3 The data on renal function were even more questionable, with some studies suggesting that, with stenting, the chance of worsening renal function is equal to that of improvement.4
Two large randomized trials comparing renal intervention with medical therapy failed to show any benefit of intervention.5–7 A third study is under way.8
It is time to strongly reconsider the current aggressive approach to revascularization of stenotic renal arteries and take a more coordinated, critical approach.
RENAL INTERVENTIONS ON THE RISE
Renal angioplasty began replacing surgical revascularization in the 1990s, as this less-invasive procedure became more readily available and was shown to have similar clinical outcomes.9 In the last decade, stent placement during angioplasty has become standard, improving the rates of technical success.
As these procedures have become easier to perform and their radiographic outcomes have become more consistent, interventionalists have become more likely, if they see stenosis in a renal artery, to intervene and insert a stent, regardless of proven benefit. In addition, interventionalists from at least three different specialties now compete for these procedures, often by looking at the renal arteries during angiography of other vascular beds (the “drive-by”).
As a result, the number of renal interventions has been rising. Medicare received 21,660 claims for renal artery interventions (surgery or angioplasty) in 2000, compared with 13,380 in 1996—an increase of 62%. However, the number of surgeries actually decreased by 45% during this time, while the number of percutaneous procedures increased by 240%. The number of endovascular claims for renal artery stenosis by cardiologists alone rose 390%.10 Since then, the reports on intervention have been mixed, with one report citing a continued increase in 2005 to 35,000 claims,11 and another suggesting a decrease back to 1997 levels.12
HOW COMMON IS RENAL ARTERY STENOSIS?
The prevalence of renal artery stenosis depends on the definition used and the population screened. It is more common in older patients who have risk factors for other vascular diseases than in the general population.
Renal Doppler ultrasonography can detect stenosis only if the artery is narrowed by more than 60%. Hansen et al13 used ultrasonography to screen 870 people over age 65 and found a lesion (a narrowing of more than 60%) in 6.8%.
Angiography (direct, computed tomographic, or magnetic resonance) can detect less-severe stenosis. Thus, most angiographic studies define renal artery stenosis as a narrowing of more than 50%, and severe disease as a narrowing of more than 70%. Many experts believe that unilateral stenosis needs to be more than 70% to pose a risk to the kidney.14,15
Angiographic prevalence studies have been performed only in patients who were undergoing angiography for another reason such as coronary or peripheral arterial disease that inherently places them at higher risk of renal artery stenosis. For instance, renal artery stenosis is found in 11% to 28% of patients undergoing diagnostic cardiac catheterization. 16
No studies of the prevalence of renal artery stenosis have been performed in the general population. Medicare data indicate that from 1999 to 2001 the incidence of diagnosed renal artery stenosis was 3.7 per 1,000 patientyears. 17 Holley et al,18 in an autopsy series, found renal artery stenosis of greater than 50% in 27% of patients over age 50 and in 56.4% of hypertensive patients. The prevalence was 10% in normotensive patients.
WHO IS AT RISK?
Factors associated with a higher risk of finding renal artery stenosis on a radiographic study include14:
- Older age
- Female sex
- Hypertension
- Three-vessel coronary artery disease
- Peripheral artery disease
- Chronic kidney disease
- Diabetes
- A low level of high-density lipoprotein cholesterol
- The use of at least two cardiovascular drugs.
The prevalence of renal artery stenosis in at-risk populations ranges from 3% to 75% (Table 1).2,4,6,19,20
HOW OFTEN DOES STENOSIS PROGRESS?
The reported rates of progression of atherosclerotic renal artery lesions vary depending on the type of imaging test used and the reason for doing it.
In studies that used duplex ultrasonography, roughly half of lesions smaller than 60% grew to greater than 60% over 3 years.21,22 The risk of total occlusion of an artery was relatively low and depended on the severity of stenosis: 0.7% if the baseline stenosis was less than 60% and 2.3% to 7% if it was greater.21,22
In a seminal study in 1984, Schreiber and colleagues23 compared serial angiograms obtained a mean of 52 months apart in 85 patients who did not undergo intervention. Stenosis had progressed in 37 (44%), and to the point of total occlusion in 14 (16%). In contrast, a 1998 study found progression in 11.1% over 2.6 years, with older patients, women, and those with baseline coronary artery disease at higher risk.24
The the rates of progression differed in these two studies probably because the indications for screening were different (clinical suspicion23 vs routine screening during coronary angiography24), as was the severity of stenosis at the time of diagnosis. Also, when these studies were done, fewer people were taking statins. Thus, similar studies, if repeated, might show even lower rates of progression.
Finally, progression of renal artery stenosis has not been correlated with worsening renal function.
FOUR CLINICAL PRESENTATIONS OF RENAL ARTERY STENOSIS
Renal artery stenosis can present in one of four ways:
Clinically silent stenosis. Because renal artery stenosis is most often found in older patients, who are more likely to have essential hypertension and chronic kidney disease due to other causes, it can be an incidental finding that is completely clinically silent.16,25
Renovascular hypertension is defined as high blood pressure due to up-regulation of neurohormonal activity in response to decreased perfusion from renal artery stenosis. Renal artery stenosis is estimated to be the cause of hypertension in only 0.5% to 4.0% of hypertensive patients, or in 26% of patients with secondary hypertension.3
Ischemic nephropathy is more difficult to define because ischemia alone rarely explains the damage done to the kidneys. Activation of neurohormonal pathways and microvascular injury are thought to contribute to oxidative stress and fibrosis.26 These phenomena may explain why similar degrees of stenosis lead to varying degrees of kidney damage in different patients and why the severity of stenosis does not correlate with the degree of renal dysfunction.27
Furthermore, stenosis may lead to irreversible but stable kidney damage. It is therefore not surprising that, in studies in unselected populations (ie, studies that included patients with all presentations of renal artery stenosis, not just those more likely to benefit), up to two-thirds of renal interventions yielded no clinical benefit.25
As a result, if we define ischemic nephropathy as renal artery stenosis with renal dysfunction not attributable to another cause, we probably will overestimate the prevalence of ischemic nephropathy, leading to overly optimistic expectations about the response to revascularization.
Recurrent “flash” pulmonary edema is a less common presentation, usually occurring in patients with critical bilateral renal artery stenosis or unilateral stenosis in an artery supplying a solitary functioning kidney. Most have severe hypertension (average systolic blood pressure 174–207 mm Hg) and poor renal function.28–30
The association between pulmonary edema and bilateral renal artery stenosis was first noted in 1998 by Pickering et al,31 who in several case series showed that 82% to 92% of patients with recurrent pulmonary edema and renal artery stenosis had bilateral stenosis, compared with 20% to 65% of those with other presentations. Later case series corroborated this finding: 85% to 100% of patients with renal artery stenosis and pulmonary edema had bilateral stenosis.28–30
STENTING IS NOW STANDARD
Stenting has become standard in the endovascular treatment of renal artery stenosis.
Most atherosclerotic renal artery lesions are located in the ostium (ie, where the artery branches off from the aorta), and many are extensions of calcified aortic plaque.26,32 These hard lesions tend to rebound to their original shape more often with balloon angioplasty alone. Stenting provides the additional force needed to permanently disrupt the lesion, leading to a longer-lasting result.
Rates of technical success (dilating the artery during the intervention) are higher with stents than without them (98% vs 46%– 77%).33,34 If the lesion is ostial, this difference is even more impressive (75% vs 29%). In addition, restenosis rates at 6 months are lower with stents (14% vs 26%–48%).34
GOALS: LOWER THE BLOOD PRESSURE, SAVE THE KIDNEY
Because endovascular procedures pose some risk to the patient, it is critical to intervene only in patients most likely to respond clinically. The decision to intervene depends largely on the clinical goal, which should depend on the clinical presentation.
However, if renal artery stenosis is clinically silent, most of the evidence suggests that intervention has no benefit. Furthermore, although retrospective studies have indicated that intervention may improve survival rates,35,36 prospective studies have not. Similarly, studies have not shown that intervention generally improves cardiovascular outcomes, even though renal artery stenosis is associated with cardiovascular risk.
Hypertension plus stenosis is not necessarily renovascular hypertension
Essential hypertension and clinically silent renal artery stenosis often coexist, which is why blood pressure control often does not improve after stenting. Also, essential hypertension often coexists with renovascular hypertension.37 In this situation, stenting may not eliminate the need for antihypertensive drugs, although it may improve blood pressure control and decrease the drug burden.
Before stents came into use, several randomized controlled trials found that blood pressure was no better controlled after angioplasty, 2,3,38 except in cases of bilateral stenosis.2 This may be because stenosis tended to recur after angioplasty without stents.
The 2000 Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) study was the first randomized controlled trial to examine the effect of angioplasty on blood pressure control in renal artery stenosis.38 It had significant design flaws. For example, many patients crossed over from the medical management group to the intervention group because their hypertension was resistant to medical therapy. Overall, intervention (balloon angioplasty without stents in 54 of 56 patients, with stents in the other 2) carried no benefit. However, in subgroup analysis, the patients who crossed over because of resistant hypertension (failure of a three-drug regimen) were more likely to benefit from angioplasty. This suggested that risk stratification should take place early on, before proceeding with revascularization.
With stents, Zeller,39 in a prospective nonrandomized study, found that the mean arterial pressure decreased by 10 mm Hg. Randomized trials (see below) have failed to demonstrate such a benefit.
Stenting may not improve renal function
Coincidental renal artery stenosis in a patient with unrelated chronic kidney disease is very hard to differentiate from true ischemic nephropathy. Furthermore, most patients with ischemic nephropathy do not benefit from revascularization, making it challenging to identify those few whose renal function may respond.
Given that patients with chronic kidney disease tend to have a higher risk of cardiovascular disease, it is not surprising that 15% of them may have renal artery stenosis,4 most often incidental.
Chábová40 examined the outcomes of 68 patients who had chronic kidney disease and a renal artery lesion larger than 70% who did not undergo angioplasty. In only 10 (15%) of the patients did the glomerular filtration rate (GFR) decline by more than 50% of its baseline value during the study period of 3 years. Given the retrospective nature of the study, it cannot be determined (and is rather unlikely) that ischemic nephropathy was the cause of the decline in kidney function in all 10 patients.
In a prospective cohort study in 304 patients with chronic kidney disease and renal artery stenosis who underwent surgical revascularization, Textor4 reported that the serum creatinine level showed a meaningful improvement afterward in 28%, worsened in 19.7%, and remained unchanged in 160 52.6%. (A “meaningful” change was defined as > 1.0 mg/dL.) Findings were similar in a cohort that underwent stenting.33
Davies et al41 found that 20% of patients who underwent renal stenting had a persistent increase in serum creatinine of 0.5 mg/dL or more. These patients were nearly three times more likely (19% vs 7%) to eventually require dialysis, and they had a lower 5-year survival rate (41% vs 71%).
Zeller et al39 found that renal function improved slightly in 52% of patients who received stents. The mean decrease in serum creatinine in this group was 0.22 mg/dL. However, the other 48% had a mean increase in serum creatinine of 1.1 mg/dL.
From these data we can conclude that, in an unselected population with renal artery stenosis, stenting provides no benefit to renal function, and that the risk of a worsening of renal function after intervention is roughly equal to the likelihood of achieving any benefit.
Other predictors of improvement in renal function have been proposed, but the evidence supporting them has not been consistent. For example, although Radermacher et al42 reported that a renal resistive index (which reflects arterial stiffness downstream of the stenosis) lower than 0.8 predicted a response in renal function, this finding has not been reliably reproduced.43,44 Similarly, while several studies suggest that patients with milder renal dysfunction have a higher likelihood of a renal response,45,46 other studies suggest either that the opposite is true39 or that baseline renal function alone has no impact on outcome.47
In addition, once significant renal atrophy occurs, revascularization may not help much, since irreversible sclerosis has developed. Thus, the goal is to identify kidneys being harmed by renal artery stenosis during the ischemic phase, when the tissue is still viable.
Unfortunately, we still lack a good renal stress test—eg, analogous to the cardiac stress test—to diagnose reversible ischemia in the kidney. The captopril renal scan has that capability but is not accurate in patients with bilateral stenosis or a GFR less than 50 mL/min, severely limiting its applicability.26 Newer technologies such as blood-oxygen-level-dependent (BOLD) magnetic resonance imaging are being investigated for such a role.48
Cohort studies in patients with declining renal function
In several case series, patients whose renal function had been declining before intervention had impressive rates of better renal function afterward.33,39,47,49–54 In a prospective cohort study by Muray et al,47 a rise in serum creatinine of more than 0.1 mg/mL/month before intervention seemed to predict an improvement in renal function afterward.
One would expect that, for renal function to respond to intervention, severe bilateral stenosis or unilateral stenosis to a solitary functioning kidney would need to be present. However, this was an inconsistent finding in these case series.33,39,47,52,53 The Angioplasty and Stent for Renal Artery Lesions (ASTRAL) trial,6,7 discussed later, sheds a bit more light on this.
Stenting usually improves flash pulmonary edema
Acute pulmonary edema in the setting of bilateral renal artery stenosis seems to be a unique case in which improvement in clinical status can be expected in most patients after intervention. Blood pressure improves in 94% to 100% of patients,28,31 renal function either improves or stabilizes in 77% to 91%,28–31 and pulmonary edema resolves without recurrence in 77% to 100%.28–30
NEW RANDOMIZED TRIALS: STAR, ASTRAL, AND CORAL
Despite the lack of evidence supporting revascularization of renal artery stenosis, many interventionalists practice under the assumption that the radiographic finding of renal artery stenosis alone is an indication for renal revascularization. Only three randomized controlled trials in the modern era attempt to examine this hypothesis: STAR, ASTRAL, and CORAL.
STAR: No clear benefit
The Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial5 was a European multicenter trial that enrolled 140 patients with ostial renal artery stenosis greater than 50%, blood pressure controlled to less than 140/90 mm Hg, and creatinine clearance 15 to 80 mL/min.
Patients were randomized to undergo stenting or medical therapy alone. High blood pressure was treated according to a protocol in which angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers were relegated to second-line use. All patients received a statin, regardless of lipid levels.
At 2 years, the primary end point (a decline in creatinine clearance of 20% or greater) had been reached in 10 (16%) of the 64 patients in the stent group and 16 (22%) of the 76 patients in the medication group; the difference was not statistically significant (hazard ratio 0.73, 95% confidence interval 0.33–1.61). No difference was seen in the secondary end points of the degree of blood pressure control or the rates of cardiovascular morbidity and death.5
ASTRAL: Also no clear benefit
In the international, multicenter ASTRAL trial,6,7 806 patients with at least one stenotic renal artery considered suitable for balloon angioplasty, stenting, or both7 were randomized to undergo intervention or medical management. Hypertension treatment was not specified by a protocol. The mean estimated GFR was 40 mL/min. Most patients (95%–96%) were on statin therapy. The primary outcome was the rate of decline of renal function over time. Secondary outcomes included blood pressure control, renal events, cardiovascular events, and death.
Results. At a mean follow-up of 33.6 months (range 1–4 years), no difference was noted between treatment groups in decline in renal function or blood pressure control at 1 year. Renal function worsened slightly in both groups.
The decline in renal function over time, calculated as the mean slope of the reciprocal of the serum creatinine level over time, was slightly slower in the revascularization group, but the difference was not statistically significant (−0.07 × 10−3 vs −0.13 × 10−3 L/μmol/year, P = .06). This difference did not appear until the last year of the study. There was no difference in the number of patients whose renal function improved or declined during the study.
There was no difference in the rate of any secondary outcome. The medical management group required a slightly higher number of antihypertensive drugs, reaching statistical but not clinical significance (2.97 vs 2.77 drugs, P = .03). More people in the revascularization group were taking ACE inhibitors or angiotensin receptor blockers. There was no difference in the number of patients on any antihypertensive therapy (97% vs 99%). Interestingly, amputations were more common in the revascularization group, occurring in 42 (12%) of the 386 patients in the revascularization group vs 29 (7%) of the 395 patients in the medical group (P = .04).
Seventeen percent of patients randomized to intervention did not have the procedure done. An as-treated analysis of the 317 (83%) patients randomized to revascularization who did receive it showed no differences in outcomes.
There were no differences in outcomes among specific, predefined subgroups based on severity of stenosis at baseline, renal length, baseline estimated GFR, baseline serum creatinine, and rate of progression of renal dysfunction before randomization.7
Comments. ASTRAL contradicts previous nonrandomized studies that suggested that rapidly declining renal function (loss of 20% in 1 year) predicts response to intervention. Considering the large number of patients with unilateral disease in the study, it would be interesting to see what effect stenting had on patients with both severe disease and declining renal function.
ASTRAL has been criticized because it lacked a central laboratory to interpret the severity of stenosis, it did not use a standardized intervention technique (5% of patients underwent angioplasty without stents, although this did not affect outcomes7), and patients were enrolled only if the clinician involved in the case was uncertain of the appropriate management.
This last issue raises the concern for selection bias toward inclusion of more difficult cases that may not respond to intervention. But these shortcomings are not serious enough to negate the fact that preliminary results from the largest randomized controlled trial to date confirm conclusions of other randomized trials, ie, that intervention in renal artery stenosis yields no benefits over medical management in most patients.
Based on the results of STAR and ASTRAL, the practice of indiscriminately revascularizing stenosed renal arteries without strong evidence that the procedure will provide a clinical benefit is no longer tenable. The challenge is to identify those few patients who will respond, and to intervene only on them. Unfortunately, none of the subgroups from ASTRAL helped characterize this population.
CORAL: A large trial is ongoing
The Cardiovascular Outcomes in Renal Artherosclerotic Lesions (CORAL) trial,8 an ongoing multicenter randomized controlled trial in the United States, may be of additional help.
Unlike ASTRAL, CORAL is studying patients who have difficult-to-control hypertension (systolic blood pressure ≥ 155 mm Hg on two or more drugs).8 Chronic kidney disease is not an exclusion criterion unless the serum creatinine concentration is greater than 3.0 mg/dL.
CORAL is using a standardized medical protocol to control blood pressure. In addition, use of embolic protection devices during stenting is encouraged. Hopefully, the large size (a goal of 1,080 patients) and the inclusion of patients with more marked hypertension will address the utility of intervention in higher-risk populations with renal artery stenosis.
RECOMMENDED APPROACH TO INTERVENTION IN RENAL ARTERY STENOSIS
As we wait for CORAL to be completed, we have two modern-era randomized controlled trials that leave us with fewer indications for renal intervention. Table 2 lists commonly cited indications for intervention in renal artery stenosis and the evidence to support them. As most of these are based on retrospective data or have conflicting support in the literature, their utility remains in question. At this point we can safely recommend:
- Patients with preserved or even decreased but stable renal function will not likely have a benefit in renal function after intervention.
- Patients with resistant hypertension may benefit.
- The best evidence supporting intervention is for bilateral stenosis with flash pulmonary edema, but the evidence is from retrospective studies.
- Stenting in bilateral disease without another indication has no apparent benefit.
- Declining renal function is not a guarantee of success.
- It is unclear if patients with severe bilateral stenosis or severe stenosis to a solitary functioning kidney with declining renal function will benefit. Anecdotally, they do respond more often, but as with many other indications for intervention that have gone by the wayside, this may not bear out when studied properly.
As the utility of intervention narrows, the scope of practice for such interventions should narrow accordingly. Attention should now be focusing on clinical, rather than radiographic, indications for intervening on renal artery stenosis.
Therefore, the decision to intervene must not be made solely by the interventionalist. A multidisciplinary approach should be adopted that at the very least includes the input of a nephrologist well versed in renal artery stenosis. In this way, the clinical risks and benefits of renal intervention can be discussed with the patient by providers who are likely to be involved in their care should renal function or hypertension fail to improve afterward.
RISK OF ATHEROEMBOLISM
While renal stenting yields improved technical success in the treatment of renal artery stenosis, it carries with it an increasingly common risk to kidney function: atheroembolism as the stent crushes the plaque against the vessel wall. This may lead to obstruction of the renal microvasculature, increasing the risk of irreversible damage to renal function.
Atheroembolic kidney disease can manifest as progressive renal failure occurring over weeks to months, commonly misdiagnosed as permanent damage from contrast nephropathy.55
Embolic protection devices, inserted downstream of the lesion before stenting to catch any debris that may break loose, have been developed to help address this problem.
Holden et al 57 prospectively studied 63 patients with renal artery stenosis and deteriorating renal function (undefined) who underwent stenting with an embolic protection device. At 6 months after the intervention, renal function had either improved or stabilized in 97% of patients, suggesting that many of the deleterious effects of stenting on renal function are related to atheroembolism.
The Prospective Randomized Study Comparing Renal Artery Stenting With or Without Distal Protection (RESIST) trial, in which renal dysfunction was mild and the GFR was not declining (average estimated GFR 59.3 mL/min), found contrary results.57 In a two-by-two factorial study, patients were randomized to undergo stenting alone, stenting with the antiplatelet agent abciximab (ReoPro), stenting with an embolic protection device, or stenting with both abciximab and an embolic protection device. Interestingly, renal function declined in the first three groups, but remained stable in the group that received both abciximab and an embolic protection device.
ANTIPLATELET THERAPY AFTER RENAL STENTING: HOW LONG?
We have no data on the optimal duration of antiplatelet therapy after renal stenting, and guidelines from professional societies do not comment on it.58 As a result, practice patterns vary significantly among practitioners.
While in-stent restenosis rates are acceptably low after renal stenting, the risks and side effects of antiplatelet therapy often lead to arbitrary withdrawal of these drugs. The effect on stent patency is yet to be determined.
FUTURE DEVELOPMENTS
Results of STAR and ASTRAL confirm the growing suspicion that the surge seen in the last decade in renal artery stenting should be coming to an end. We await results either from CORAL or possibly a post hoc analysis of ASTRAL that might identify potential high-risk groups that will benefit from renal intervention. And as embolic protection devices become more agile and suitable to different renal lesions, there remains the possibility that, due to lower rates of unidentified atheroembolic kidney disease, CORAL may demonstrate improved renal outcomes after stenting. If not, the search for the best means to predict who should have renal intervention will continue.
We know through experience that stenting does provide great benefits for some patients with renal artery stenosis. Furthermore, the clinical problem is too intriguing, and too profitable, to die altogether.
- Choncol M, Linas S. Diagnosis and management of ischemic nephropathy. Clin J Am Soc Nephrol 2006; 1:172–181.
- Webster J, Marshall F, Abdalla M, et al Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens 1998; 12:329–335.
- Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998; 31:823–829.
- Textor SC. Revascularization in atherosclerotic renal artery disease. Kidney Int 1998; 53:799–811.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848.
- Mistry S, Ives N, Harding J, et al Angioplasty and STent for Renal Artery Lesions (ASTRAL trial): rationale, methods and results so far. J Hum Hypertens 2007; 21:511–515.
- Wheatley K, Ives N, Kalra P, Moss J. Revascularization versus medical therapy for renal-artery stenosis (ASTRAL). N Engl J Med 2009; 361:1953–1962.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
- Galaria II, Surowiec SM, Rhodes JM, et al Percutaneous and open renal revascularizations have equivalent long-term functional outcomes. Ann Vasc Surg 2005; 19:218–228.
- Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery interventions by Medicare beneficiaries 1996–2000. AJR Am J Roentgenol 2004; 183:561–568.
- Textor SC. Atherosclerotic renal artery stenosis: overtreated but underrated? J Am Soc Nephrol 2008; 19:656–659.
- Kalra PA, Guo H, Gilbertson DT, et al Atherosclerotic renovascular disease in the United States. Kidney Int 2010; 77:37–43.
- Hansen KJ, Edwards MS, Craven TE, et al Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Cohen MG, Pascua JA, Garcia-Ben M, et al A simple prediction rule for significant renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 2005; 150:1204–1211.
- Buller CE, Nogareda JG, Ramanathan K, et al The profile of cardiac patients with renal artery stenosis. J Am Coll Cardiol 2004; 43:1606–1613.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- Kalra PA, Guo H, Kausz AT, et al Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 69:293–301.
- Holley KE, Hunt JC, Brown AL, Kincaid OW, Sheps SG. Renal artery stenosis. A clinical-pathologic study in normotensive and hypertensive patients. Am J Med 1964; 37:14–22.
- de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systemic literature review. J Hypertens 2009; 27:1333–1340.
- Kuczera P, Włoszczynska E, Adamczak M, Pencak P, Chudek J, Wiecek A. Frequency of renal artery stenosis and variants of renal vascularization in hypertensive patients: analysis of 1550 angiographies in one centre. J Hum Hypertens 2009; 23:396–401.
- Caps MT, Perissinotto C, Zierler RE, et al Prospective study of atherosclerotic disease progression in the renal artery. Circulation 1998; 98:2866–2872.
- Zierler RE, Bergelin RO, Davidson RC, Cantwell-Gab K, Polissar NL, Strandness DE. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens 1996; 9:1055–1061.
- Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urol Clin North Am 1984; 11:383–392.
- Crowley JJ, Santos RM, Peter RH, et al Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 1998; 136:913–918.
- Textor SC. Renovascular hypertension update. Curr Hypertens Rep 2006; 8:521–527.
- Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol 2004; 15:1974–1982.
- Wright JR, Shurrab AE, Cheung C, et al A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis 2002; 39:1153–1161.
- Messina LM, Zelenock GB, Yao KA, Stanley JC. Renal revascularization for recurrent pulmonary edema in patients with poorly controlled hypertension and renal insufficiency: a distinct subgroup of patients with arteriosclerotic renal artery occlusive disease. J Vasc Surg 1992; 15:73–80.
- Bloch MJ, Trost DW, Pickering TG, Sos TA, August P. Prevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placement. Am J Hypertens 1999; 12:1–7.
- Gray BH, Olin JW, Childs MB, Sullivan TM, Bacharach JM. Clinical benefit of renal artery angioplasty with stenting for the control of recurrent and refractory congestive heart failure. Vasc Med 2002; 7:275–279.
- Pickering TG, Herman L, Devereux RB, et al Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet 1988; 2:551–552.
- Kennedy DJ, Colyer WR, Brewster PS, et al Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am J Kidney Dis 2003; 14:926–935.
- Beutler JJ, Van Ampting JM, Van De Ven PJ, et al Long-term effects of arterial stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol 2001; 12:1475–1481.
- Van de Ven PJ, Kaatee R, Beutler JJ, et al Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomized trial. Lancet 1999; 353:282–286.
- Isles C, Main J, O’Connell J, et al Survival associated with renovascular disease in Glasgow and Newcastle: a collaborative study. Scott Med J 1990; 35:70–73.
- Hunt JC, Sheps SG, Harrison EG, Strong CG, Bernatz PE. Renal and renovascular hypertension. A reasoned approach to diagnosis and management. Arch Intern Med 1974; 133:988–999.
- Textor SC. Atherosclerotic renal artery stenosis: how big is the problem, and what happens if nothing is done? J Hypertens Suppl 2005; 23:S5–S13.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Zeller T, Frank U, Müller C, et al Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation 2003; 108;2244–2249.
- Chábová V, Schirger A, Stanson AW, McKusick MA, Textor SC. Outcomes of atherosclerotic renal artery stenosis managed without revascularization. Mayo Clin Proc 2000; 75:437–444.
- Davies MG, Saad WE, Peden EK, Mohiuddin IT, Naoum JJ, Lumsden AB. Implications of acute functional injury following percutaneous renal artery intervention. Ann Vasc Surg 2008; 22:783–789.
- Radermacher J, Chavin A, Bleck J, et al Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Eng J Med 2001; 344:410–417.
- García-Criado A, Gilabert R, Nicolau C, et al Value of Doppler sonography for predicting clinical outcome after renal artery revascularization in atherosclerotic renal artery stenosis. J Ultrasound Med 2005; 24:1641–1647.
- Zeller T, Müller C, Frank U, et al Stent angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosis. Catheter Cardiovasc Interv 2003; 58:510–515.
- Harden PN, MacLeod MJ, Rodger RSC, et al Effect of renal-artery stenting on progression of renovascular renal failure. Lancet 1997; 349:1133–1136.
- Isles CG, Robertson S, Hill D. Management of renovascular disease: a review of renal artery stenting in ten studies. QJM 1999; 92:159–167.
- Muray S, Martın M, Amoedo ML, et al Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis 2002; 39:60–66.
- Textor SC, Glockner JF, Lerman LO, et al The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 2008; 19:780–788.
- Paraskevas KI, Perrea D, Briana DD, Liapis CD. Management of atherosclerotic renovascular disease: the effect of renal artery stenting on renal function and blood pressure. Int Urol Nephrol 2006; 38:683–691.
- Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation 2000; 102:1671–1677.
- Dean RH, Kieffer RW, Smith BM, et al Renovascular hypertension: anatomic and renal function changes during drug therapy. Arch Surg 1981; 116:1408–1415.
- Zhang Q, Shen W, Zhang R, Zhang J, Hu J, Zhang X. Effects of renal artery stenting on renal function and blood pressure in patients with atherosclerotic renovascular disease. Chin Med J (Engl) 2003; 116:1451–1454.
- Ramos F, Kotliar C, Alvarez D, et al Renal function and outcome of PTRA and stenting for atherosclerotic renal artery stenosis. Kidney Int 2003; 63:276–282.
- Rocha-Singh KJ, Ahuja RK, Sung CH, Rutherford J. Long-term renal function preservation after renal artery stenting in patients with progressive ischemic nephropathy. Catheter Cardiovasc Interv 2002; 57:135–141.
- Thadhani RI, Camargo CA, Xavier RJ, Fang LS, Bazari H. Atheroembolic renal failure after invasive procedures. Natural history based on 52 histologically proven cases. Medicine (Baltimore) 1995; 74:350–358.
- Holden A, Hill A, Jaff MR, Pilmore H. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int 2006; 70:948–955.
- Cooper CJ, Haller ST, Colyer W, et al Embolic protection and platelet inhibition during renal artery stenting. Circulation 2008; 117:2752–2760.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Choncol M, Linas S. Diagnosis and management of ischemic nephropathy. Clin J Am Soc Nephrol 2006; 1:172–181.
- Webster J, Marshall F, Abdalla M, et al Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens 1998; 12:329–335.
- Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998; 31:823–829.
- Textor SC. Revascularization in atherosclerotic renal artery disease. Kidney Int 1998; 53:799–811.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848.
- Mistry S, Ives N, Harding J, et al Angioplasty and STent for Renal Artery Lesions (ASTRAL trial): rationale, methods and results so far. J Hum Hypertens 2007; 21:511–515.
- Wheatley K, Ives N, Kalra P, Moss J. Revascularization versus medical therapy for renal-artery stenosis (ASTRAL). N Engl J Med 2009; 361:1953–1962.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
- Galaria II, Surowiec SM, Rhodes JM, et al Percutaneous and open renal revascularizations have equivalent long-term functional outcomes. Ann Vasc Surg 2005; 19:218–228.
- Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery interventions by Medicare beneficiaries 1996–2000. AJR Am J Roentgenol 2004; 183:561–568.
- Textor SC. Atherosclerotic renal artery stenosis: overtreated but underrated? J Am Soc Nephrol 2008; 19:656–659.
- Kalra PA, Guo H, Gilbertson DT, et al Atherosclerotic renovascular disease in the United States. Kidney Int 2010; 77:37–43.
- Hansen KJ, Edwards MS, Craven TE, et al Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Cohen MG, Pascua JA, Garcia-Ben M, et al A simple prediction rule for significant renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 2005; 150:1204–1211.
- Buller CE, Nogareda JG, Ramanathan K, et al The profile of cardiac patients with renal artery stenosis. J Am Coll Cardiol 2004; 43:1606–1613.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- Kalra PA, Guo H, Kausz AT, et al Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 69:293–301.
- Holley KE, Hunt JC, Brown AL, Kincaid OW, Sheps SG. Renal artery stenosis. A clinical-pathologic study in normotensive and hypertensive patients. Am J Med 1964; 37:14–22.
- de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systemic literature review. J Hypertens 2009; 27:1333–1340.
- Kuczera P, Włoszczynska E, Adamczak M, Pencak P, Chudek J, Wiecek A. Frequency of renal artery stenosis and variants of renal vascularization in hypertensive patients: analysis of 1550 angiographies in one centre. J Hum Hypertens 2009; 23:396–401.
- Caps MT, Perissinotto C, Zierler RE, et al Prospective study of atherosclerotic disease progression in the renal artery. Circulation 1998; 98:2866–2872.
- Zierler RE, Bergelin RO, Davidson RC, Cantwell-Gab K, Polissar NL, Strandness DE. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens 1996; 9:1055–1061.
- Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urol Clin North Am 1984; 11:383–392.
- Crowley JJ, Santos RM, Peter RH, et al Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 1998; 136:913–918.
- Textor SC. Renovascular hypertension update. Curr Hypertens Rep 2006; 8:521–527.
- Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol 2004; 15:1974–1982.
- Wright JR, Shurrab AE, Cheung C, et al A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis 2002; 39:1153–1161.
- Messina LM, Zelenock GB, Yao KA, Stanley JC. Renal revascularization for recurrent pulmonary edema in patients with poorly controlled hypertension and renal insufficiency: a distinct subgroup of patients with arteriosclerotic renal artery occlusive disease. J Vasc Surg 1992; 15:73–80.
- Bloch MJ, Trost DW, Pickering TG, Sos TA, August P. Prevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placement. Am J Hypertens 1999; 12:1–7.
- Gray BH, Olin JW, Childs MB, Sullivan TM, Bacharach JM. Clinical benefit of renal artery angioplasty with stenting for the control of recurrent and refractory congestive heart failure. Vasc Med 2002; 7:275–279.
- Pickering TG, Herman L, Devereux RB, et al Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet 1988; 2:551–552.
- Kennedy DJ, Colyer WR, Brewster PS, et al Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am J Kidney Dis 2003; 14:926–935.
- Beutler JJ, Van Ampting JM, Van De Ven PJ, et al Long-term effects of arterial stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol 2001; 12:1475–1481.
- Van de Ven PJ, Kaatee R, Beutler JJ, et al Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomized trial. Lancet 1999; 353:282–286.
- Isles C, Main J, O’Connell J, et al Survival associated with renovascular disease in Glasgow and Newcastle: a collaborative study. Scott Med J 1990; 35:70–73.
- Hunt JC, Sheps SG, Harrison EG, Strong CG, Bernatz PE. Renal and renovascular hypertension. A reasoned approach to diagnosis and management. Arch Intern Med 1974; 133:988–999.
- Textor SC. Atherosclerotic renal artery stenosis: how big is the problem, and what happens if nothing is done? J Hypertens Suppl 2005; 23:S5–S13.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Zeller T, Frank U, Müller C, et al Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation 2003; 108;2244–2249.
- Chábová V, Schirger A, Stanson AW, McKusick MA, Textor SC. Outcomes of atherosclerotic renal artery stenosis managed without revascularization. Mayo Clin Proc 2000; 75:437–444.
- Davies MG, Saad WE, Peden EK, Mohiuddin IT, Naoum JJ, Lumsden AB. Implications of acute functional injury following percutaneous renal artery intervention. Ann Vasc Surg 2008; 22:783–789.
- Radermacher J, Chavin A, Bleck J, et al Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Eng J Med 2001; 344:410–417.
- García-Criado A, Gilabert R, Nicolau C, et al Value of Doppler sonography for predicting clinical outcome after renal artery revascularization in atherosclerotic renal artery stenosis. J Ultrasound Med 2005; 24:1641–1647.
- Zeller T, Müller C, Frank U, et al Stent angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosis. Catheter Cardiovasc Interv 2003; 58:510–515.
- Harden PN, MacLeod MJ, Rodger RSC, et al Effect of renal-artery stenting on progression of renovascular renal failure. Lancet 1997; 349:1133–1136.
- Isles CG, Robertson S, Hill D. Management of renovascular disease: a review of renal artery stenting in ten studies. QJM 1999; 92:159–167.
- Muray S, Martın M, Amoedo ML, et al Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis 2002; 39:60–66.
- Textor SC, Glockner JF, Lerman LO, et al The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 2008; 19:780–788.
- Paraskevas KI, Perrea D, Briana DD, Liapis CD. Management of atherosclerotic renovascular disease: the effect of renal artery stenting on renal function and blood pressure. Int Urol Nephrol 2006; 38:683–691.
- Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation 2000; 102:1671–1677.
- Dean RH, Kieffer RW, Smith BM, et al Renovascular hypertension: anatomic and renal function changes during drug therapy. Arch Surg 1981; 116:1408–1415.
- Zhang Q, Shen W, Zhang R, Zhang J, Hu J, Zhang X. Effects of renal artery stenting on renal function and blood pressure in patients with atherosclerotic renovascular disease. Chin Med J (Engl) 2003; 116:1451–1454.
- Ramos F, Kotliar C, Alvarez D, et al Renal function and outcome of PTRA and stenting for atherosclerotic renal artery stenosis. Kidney Int 2003; 63:276–282.
- Rocha-Singh KJ, Ahuja RK, Sung CH, Rutherford J. Long-term renal function preservation after renal artery stenting in patients with progressive ischemic nephropathy. Catheter Cardiovasc Interv 2002; 57:135–141.
- Thadhani RI, Camargo CA, Xavier RJ, Fang LS, Bazari H. Atheroembolic renal failure after invasive procedures. Natural history based on 52 histologically proven cases. Medicine (Baltimore) 1995; 74:350–358.
- Holden A, Hill A, Jaff MR, Pilmore H. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int 2006; 70:948–955.
- Cooper CJ, Haller ST, Colyer W, et al Embolic protection and platelet inhibition during renal artery stenting. Circulation 2008; 117:2752–2760.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
KEY POINTS
- Two large randomized trials of intervention vs medical therapy showed negative results for intervention. A third trial is under way.
- Intervention is not recommended if renal function has remained stable over the past 6 to 12 months and if hypertension can be controlled medically.
- The best evidence supporting intervention is for bilateral stenosis with “flash” pulmonary edema, but the evidence is from retrospective studies.
- Stenosis by itself, even if bilateral, is not an indication for renal artery stenting.
Low Body Fat Tied to High Mortality in Dialysis
SAN DIEGO — A low percentage of total body fat is associated with higher 5-year mortality in hemodialysis patients, even after adjustment for demographics, comorbid conditions, and other surrogates of nutritional status, results from a large study showed.
“Hemodialysis patients do exhibit an obesity paradox,” Debbie Benner said during a press briefing at the annual meeting of the American Society of Nephrology.
“Low body mass index is associated with greater mortality, whereas higher BMI appears to be protective,” Ms. Benner said.
In a study led by Dr. Kamyar Kalantar-Zadeh of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, researchers used near-infrared interactance technology to measure body fat percentage in the upper arm of 671 hemodialysis patients from eight centers in California operated by DaVita Inc., and investigated their survival between 2001 and 2006.
Ms. Benner, a registered dietitian who serves as vice president of clinical support for DaVita, a nationwide provider of dialysis services, described near-infrared interactance as a “noninvasive, simple, and rapid method of assessing percent body fat based on light absorption and reflection using near-infrared light emission.”
The study was conducted because protein energy wasting “is a common problem in chronic kidney disease patients and is associated with a reduction in muscle and body fat stores. Measuring body composition including total body fat in dialysis patients may provide important information about nutritional status and outcomes in dialysis patients,” she said.
The mean age of patients was 54 years: 52% were men, 30% were African American, 54% were diabetic, and their mean total body fat percentage was 27%.
The researchers divided the patients into five groups based on body fat percentage: less than 10% (34 patients); 10% to less than 20% (156 patients); 20% to less than 30% (210 patients), 30% to less than 40% (182 patients), and 40% or higher (89 patients).
Using patients with 20%–30% body fat as the referent group, Ms. Benner and her associates performed a survival analysis adjusted for age, gender, presence of diabetes, Charlson index score, and race.
They also controlled these associations for the surrogates of the malnutrition-inflammation complex syndrome (MICS), including serum albumin, hemoglobin, normalized protein catabolic rate, phosphorus, total iron-binding capacity, ferritin, calcium, and creatinine.
The association between body fat and mortality was then assessed.
Case-mix analysis revealed that dialysis patients with less than 10% body fat were 2.54 times more likely to die than those in the referent group, while the MICS analysis revealed a 2.96-fold increased risk of death.
Analysis of the other groups confirmed a direct relationship between body fat and mortality risk. “When the body fat percentage increased, the mortality risk declined, and vice versa,” Ms. Benner said.
She acknowledged certain limitations of the study, including the potential for selection bias “and the fact that other measures of nutritional status were not tested.”
Disclosures: The study was funded by the National Institutes of Health and by DaVita. Ms. Benner disclosed no other conflicts other than her employment with DaVita.
'Hemodialysis patients do exhibit an obesity paradox.'
Source MS. BENNER
SAN DIEGO — A low percentage of total body fat is associated with higher 5-year mortality in hemodialysis patients, even after adjustment for demographics, comorbid conditions, and other surrogates of nutritional status, results from a large study showed.
“Hemodialysis patients do exhibit an obesity paradox,” Debbie Benner said during a press briefing at the annual meeting of the American Society of Nephrology.
“Low body mass index is associated with greater mortality, whereas higher BMI appears to be protective,” Ms. Benner said.
In a study led by Dr. Kamyar Kalantar-Zadeh of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, researchers used near-infrared interactance technology to measure body fat percentage in the upper arm of 671 hemodialysis patients from eight centers in California operated by DaVita Inc., and investigated their survival between 2001 and 2006.
Ms. Benner, a registered dietitian who serves as vice president of clinical support for DaVita, a nationwide provider of dialysis services, described near-infrared interactance as a “noninvasive, simple, and rapid method of assessing percent body fat based on light absorption and reflection using near-infrared light emission.”
The study was conducted because protein energy wasting “is a common problem in chronic kidney disease patients and is associated with a reduction in muscle and body fat stores. Measuring body composition including total body fat in dialysis patients may provide important information about nutritional status and outcomes in dialysis patients,” she said.
The mean age of patients was 54 years: 52% were men, 30% were African American, 54% were diabetic, and their mean total body fat percentage was 27%.
The researchers divided the patients into five groups based on body fat percentage: less than 10% (34 patients); 10% to less than 20% (156 patients); 20% to less than 30% (210 patients), 30% to less than 40% (182 patients), and 40% or higher (89 patients).
Using patients with 20%–30% body fat as the referent group, Ms. Benner and her associates performed a survival analysis adjusted for age, gender, presence of diabetes, Charlson index score, and race.
They also controlled these associations for the surrogates of the malnutrition-inflammation complex syndrome (MICS), including serum albumin, hemoglobin, normalized protein catabolic rate, phosphorus, total iron-binding capacity, ferritin, calcium, and creatinine.
The association between body fat and mortality was then assessed.
Case-mix analysis revealed that dialysis patients with less than 10% body fat were 2.54 times more likely to die than those in the referent group, while the MICS analysis revealed a 2.96-fold increased risk of death.
Analysis of the other groups confirmed a direct relationship between body fat and mortality risk. “When the body fat percentage increased, the mortality risk declined, and vice versa,” Ms. Benner said.
She acknowledged certain limitations of the study, including the potential for selection bias “and the fact that other measures of nutritional status were not tested.”
Disclosures: The study was funded by the National Institutes of Health and by DaVita. Ms. Benner disclosed no other conflicts other than her employment with DaVita.
'Hemodialysis patients do exhibit an obesity paradox.'
Source MS. BENNER
SAN DIEGO — A low percentage of total body fat is associated with higher 5-year mortality in hemodialysis patients, even after adjustment for demographics, comorbid conditions, and other surrogates of nutritional status, results from a large study showed.
“Hemodialysis patients do exhibit an obesity paradox,” Debbie Benner said during a press briefing at the annual meeting of the American Society of Nephrology.
“Low body mass index is associated with greater mortality, whereas higher BMI appears to be protective,” Ms. Benner said.
In a study led by Dr. Kamyar Kalantar-Zadeh of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, researchers used near-infrared interactance technology to measure body fat percentage in the upper arm of 671 hemodialysis patients from eight centers in California operated by DaVita Inc., and investigated their survival between 2001 and 2006.
Ms. Benner, a registered dietitian who serves as vice president of clinical support for DaVita, a nationwide provider of dialysis services, described near-infrared interactance as a “noninvasive, simple, and rapid method of assessing percent body fat based on light absorption and reflection using near-infrared light emission.”
The study was conducted because protein energy wasting “is a common problem in chronic kidney disease patients and is associated with a reduction in muscle and body fat stores. Measuring body composition including total body fat in dialysis patients may provide important information about nutritional status and outcomes in dialysis patients,” she said.
The mean age of patients was 54 years: 52% were men, 30% were African American, 54% were diabetic, and their mean total body fat percentage was 27%.
The researchers divided the patients into five groups based on body fat percentage: less than 10% (34 patients); 10% to less than 20% (156 patients); 20% to less than 30% (210 patients), 30% to less than 40% (182 patients), and 40% or higher (89 patients).
Using patients with 20%–30% body fat as the referent group, Ms. Benner and her associates performed a survival analysis adjusted for age, gender, presence of diabetes, Charlson index score, and race.
They also controlled these associations for the surrogates of the malnutrition-inflammation complex syndrome (MICS), including serum albumin, hemoglobin, normalized protein catabolic rate, phosphorus, total iron-binding capacity, ferritin, calcium, and creatinine.
The association between body fat and mortality was then assessed.
Case-mix analysis revealed that dialysis patients with less than 10% body fat were 2.54 times more likely to die than those in the referent group, while the MICS analysis revealed a 2.96-fold increased risk of death.
Analysis of the other groups confirmed a direct relationship between body fat and mortality risk. “When the body fat percentage increased, the mortality risk declined, and vice versa,” Ms. Benner said.
She acknowledged certain limitations of the study, including the potential for selection bias “and the fact that other measures of nutritional status were not tested.”
Disclosures: The study was funded by the National Institutes of Health and by DaVita. Ms. Benner disclosed no other conflicts other than her employment with DaVita.
'Hemodialysis patients do exhibit an obesity paradox.'
Source MS. BENNER
Stage of Kidney Disease Affects Heart Failure Risk
SAN DIEGO — The more advanced the stage of chronic kidney disease, the greater the risk of developing heart failure and the higher the subsequent risk of death, results from a large analysis of Medicare patients showed.
“Even a modest degree of chronic kidney disease is a very strong predictor of having cardiovascular morbidity and mortality,” Dr. Charles A. Herzog said in an interview during a poster session at the annual meeting of the American Society of Nephrology.
“Chronic kidney disease is something that primary care physicians can easily detect, because it's very easy to do a serum creatinine in an office setting,” he said.
Dr. Herzog, director of the Minneapolis-based cardiovascular special studies center of the United States Renal Data System Coordinating Center, and his associate, Shuling Li, identified 1,089,716 patients aged 66 years and older from the general Medicare database and followed them between Jan. 1, 2006, and Dec. 31, 2007.
Patients with heart failure and end-stage renal disease at baseline were excluded from the analysis.
The researchers used a Cox proportional hazard model to assess the risk of developing incident heart failure, adjusting for demographics, comorbidities, and stage of chronic kidney disease based on ICD-9 codes 585.1-585.5 and 585.9.
They used the Kaplan-Meier method to estimate the age-adjusted survival of patients after the development of incident congestive heart failure.
At baseline, 59% of the patients were female and 88% were white; 23% were aged 66-69, 25% were aged 70-74, 22% were aged 75-79, 16% were aged 80-84, and 14% were aged 85 or older.
The majority (95.8%) had no chronic kidney disease, 0.4% had stage I-II disease, 1.4% had stage III-IV disease, and the remainder (2.4%) had an unknown stage of disease.
Dr. Herzog reported that after the 1 year of follow-up, heart failure occurred in 5.3% of patients with no chronic kidney disease at baseline, 12.7% of those with stage I-II disease, 15% of those with stage III-IV disease, and 12.3% of those whose disease stage was unknown.
Independent predictors of heart failure were as follows: age 70-74 years (hazard ratio, 1.30), age 75-79 years (HR, 1.75), age 80-84 years (HR, 2.42), and age 85 years and older (HR, 3.82).
Other independent predictors included black race (HR, 1.21), stage I-II chronic kidney disease (HR, 1.45), stage III-IV disease (HR, 1.68), and unknown stage of chronic kidney disease (HR, 1.27).
The researchers also found that the presence of certain comorbid conditions predicted heart failure, including anemia (HR, 1.22), diabetes (HR, 1.57), atherosclerotic heart disease (HR, 1.67), and dysrhythmia (HR, 1.94).
Over the 1-year period, 83% of patients with no chronic kidney disease survived, compared with 77% of those with stage I-II disease, 75% of those with stage II-IV disease, and 67% of those whose disease stage was unknown.
Dr. Herzog acknowledged that the study's reliance on Medicare claims data is a limitation.
“There can be some inaccuracy in claims,” he said. “But the large size of the sample probably helps deal with some of the deficiencies in the accuracy of the coding.”
Disclosures: Dr. Herzog disclosed that he is a consultant for Amgen Inc. and is a scientific adviser for CorMedix Inc. He also is a member of the Roche Foundation for Anemia Research board of trustees.
'Even a modest degree of chronic kidney disease is a very strong predictor' of cardiovascular morbidity.
Source DR. HERZOG
SAN DIEGO — The more advanced the stage of chronic kidney disease, the greater the risk of developing heart failure and the higher the subsequent risk of death, results from a large analysis of Medicare patients showed.
“Even a modest degree of chronic kidney disease is a very strong predictor of having cardiovascular morbidity and mortality,” Dr. Charles A. Herzog said in an interview during a poster session at the annual meeting of the American Society of Nephrology.
“Chronic kidney disease is something that primary care physicians can easily detect, because it's very easy to do a serum creatinine in an office setting,” he said.
Dr. Herzog, director of the Minneapolis-based cardiovascular special studies center of the United States Renal Data System Coordinating Center, and his associate, Shuling Li, identified 1,089,716 patients aged 66 years and older from the general Medicare database and followed them between Jan. 1, 2006, and Dec. 31, 2007.
Patients with heart failure and end-stage renal disease at baseline were excluded from the analysis.
The researchers used a Cox proportional hazard model to assess the risk of developing incident heart failure, adjusting for demographics, comorbidities, and stage of chronic kidney disease based on ICD-9 codes 585.1-585.5 and 585.9.
They used the Kaplan-Meier method to estimate the age-adjusted survival of patients after the development of incident congestive heart failure.
At baseline, 59% of the patients were female and 88% were white; 23% were aged 66-69, 25% were aged 70-74, 22% were aged 75-79, 16% were aged 80-84, and 14% were aged 85 or older.
The majority (95.8%) had no chronic kidney disease, 0.4% had stage I-II disease, 1.4% had stage III-IV disease, and the remainder (2.4%) had an unknown stage of disease.
Dr. Herzog reported that after the 1 year of follow-up, heart failure occurred in 5.3% of patients with no chronic kidney disease at baseline, 12.7% of those with stage I-II disease, 15% of those with stage III-IV disease, and 12.3% of those whose disease stage was unknown.
Independent predictors of heart failure were as follows: age 70-74 years (hazard ratio, 1.30), age 75-79 years (HR, 1.75), age 80-84 years (HR, 2.42), and age 85 years and older (HR, 3.82).
Other independent predictors included black race (HR, 1.21), stage I-II chronic kidney disease (HR, 1.45), stage III-IV disease (HR, 1.68), and unknown stage of chronic kidney disease (HR, 1.27).
The researchers also found that the presence of certain comorbid conditions predicted heart failure, including anemia (HR, 1.22), diabetes (HR, 1.57), atherosclerotic heart disease (HR, 1.67), and dysrhythmia (HR, 1.94).
Over the 1-year period, 83% of patients with no chronic kidney disease survived, compared with 77% of those with stage I-II disease, 75% of those with stage II-IV disease, and 67% of those whose disease stage was unknown.
Dr. Herzog acknowledged that the study's reliance on Medicare claims data is a limitation.
“There can be some inaccuracy in claims,” he said. “But the large size of the sample probably helps deal with some of the deficiencies in the accuracy of the coding.”
Disclosures: Dr. Herzog disclosed that he is a consultant for Amgen Inc. and is a scientific adviser for CorMedix Inc. He also is a member of the Roche Foundation for Anemia Research board of trustees.
'Even a modest degree of chronic kidney disease is a very strong predictor' of cardiovascular morbidity.
Source DR. HERZOG
SAN DIEGO — The more advanced the stage of chronic kidney disease, the greater the risk of developing heart failure and the higher the subsequent risk of death, results from a large analysis of Medicare patients showed.
“Even a modest degree of chronic kidney disease is a very strong predictor of having cardiovascular morbidity and mortality,” Dr. Charles A. Herzog said in an interview during a poster session at the annual meeting of the American Society of Nephrology.
“Chronic kidney disease is something that primary care physicians can easily detect, because it's very easy to do a serum creatinine in an office setting,” he said.
Dr. Herzog, director of the Minneapolis-based cardiovascular special studies center of the United States Renal Data System Coordinating Center, and his associate, Shuling Li, identified 1,089,716 patients aged 66 years and older from the general Medicare database and followed them between Jan. 1, 2006, and Dec. 31, 2007.
Patients with heart failure and end-stage renal disease at baseline were excluded from the analysis.
The researchers used a Cox proportional hazard model to assess the risk of developing incident heart failure, adjusting for demographics, comorbidities, and stage of chronic kidney disease based on ICD-9 codes 585.1-585.5 and 585.9.
They used the Kaplan-Meier method to estimate the age-adjusted survival of patients after the development of incident congestive heart failure.
At baseline, 59% of the patients were female and 88% were white; 23% were aged 66-69, 25% were aged 70-74, 22% were aged 75-79, 16% were aged 80-84, and 14% were aged 85 or older.
The majority (95.8%) had no chronic kidney disease, 0.4% had stage I-II disease, 1.4% had stage III-IV disease, and the remainder (2.4%) had an unknown stage of disease.
Dr. Herzog reported that after the 1 year of follow-up, heart failure occurred in 5.3% of patients with no chronic kidney disease at baseline, 12.7% of those with stage I-II disease, 15% of those with stage III-IV disease, and 12.3% of those whose disease stage was unknown.
Independent predictors of heart failure were as follows: age 70-74 years (hazard ratio, 1.30), age 75-79 years (HR, 1.75), age 80-84 years (HR, 2.42), and age 85 years and older (HR, 3.82).
Other independent predictors included black race (HR, 1.21), stage I-II chronic kidney disease (HR, 1.45), stage III-IV disease (HR, 1.68), and unknown stage of chronic kidney disease (HR, 1.27).
The researchers also found that the presence of certain comorbid conditions predicted heart failure, including anemia (HR, 1.22), diabetes (HR, 1.57), atherosclerotic heart disease (HR, 1.67), and dysrhythmia (HR, 1.94).
Over the 1-year period, 83% of patients with no chronic kidney disease survived, compared with 77% of those with stage I-II disease, 75% of those with stage II-IV disease, and 67% of those whose disease stage was unknown.
Dr. Herzog acknowledged that the study's reliance on Medicare claims data is a limitation.
“There can be some inaccuracy in claims,” he said. “But the large size of the sample probably helps deal with some of the deficiencies in the accuracy of the coding.”
Disclosures: Dr. Herzog disclosed that he is a consultant for Amgen Inc. and is a scientific adviser for CorMedix Inc. He also is a member of the Roche Foundation for Anemia Research board of trustees.
'Even a modest degree of chronic kidney disease is a very strong predictor' of cardiovascular morbidity.
Source DR. HERZOG
Kidney stones
To the Editor: Thanks for the excellent review articles on nephrolithiasis in your October 2009 issue.1,2
Dr. Hall1 cites studies in which patients given the alpha blocker tamsulosin (Flomax) or the calcium channel blocker nifedipine (Procardia) had improved rates of kidney stone passage compared with placebo. As a primary care physician, I am often confronted with the challenge of managing a patient who is waiting for a kidney stone to pass while taking tamsulosin. Is Dr. Hall aware of any clinical studies, or at least theoretical reasons, which would support adding nifedipine in such cases?
Secondly, Dr. Hall cites studies that demonstrated that a higher intake of dietary calcium is actually associated with fewer calcium stone events in both men and women. An unanswered question is whether patients taking calcium supplements for osteoporosis or osteopenia can safely continue to do so after a calcium stone event, or indeed, whether calcium supplementation might actually be helpful in preventing a recurrent calcum stone.
If there is an absence of randomized studies to answer these questions, Dr. Hall’s recommendations based on his expert experience would be most welcome.
- Hall PM. Nephrolithiasis: treatment, causes, and prevention. Cleve Clin J Med 2009; 76:583–591.
- Samplaski MK, Irwin BH, Desai M. Less-invasive ways to remove stones from the kidneys and ureters. Cleve Clin J Med 2009; 76:592–598.
To the Editor: Thanks for the excellent review articles on nephrolithiasis in your October 2009 issue.1,2
Dr. Hall1 cites studies in which patients given the alpha blocker tamsulosin (Flomax) or the calcium channel blocker nifedipine (Procardia) had improved rates of kidney stone passage compared with placebo. As a primary care physician, I am often confronted with the challenge of managing a patient who is waiting for a kidney stone to pass while taking tamsulosin. Is Dr. Hall aware of any clinical studies, or at least theoretical reasons, which would support adding nifedipine in such cases?
Secondly, Dr. Hall cites studies that demonstrated that a higher intake of dietary calcium is actually associated with fewer calcium stone events in both men and women. An unanswered question is whether patients taking calcium supplements for osteoporosis or osteopenia can safely continue to do so after a calcium stone event, or indeed, whether calcium supplementation might actually be helpful in preventing a recurrent calcum stone.
If there is an absence of randomized studies to answer these questions, Dr. Hall’s recommendations based on his expert experience would be most welcome.
To the Editor: Thanks for the excellent review articles on nephrolithiasis in your October 2009 issue.1,2
Dr. Hall1 cites studies in which patients given the alpha blocker tamsulosin (Flomax) or the calcium channel blocker nifedipine (Procardia) had improved rates of kidney stone passage compared with placebo. As a primary care physician, I am often confronted with the challenge of managing a patient who is waiting for a kidney stone to pass while taking tamsulosin. Is Dr. Hall aware of any clinical studies, or at least theoretical reasons, which would support adding nifedipine in such cases?
Secondly, Dr. Hall cites studies that demonstrated that a higher intake of dietary calcium is actually associated with fewer calcium stone events in both men and women. An unanswered question is whether patients taking calcium supplements for osteoporosis or osteopenia can safely continue to do so after a calcium stone event, or indeed, whether calcium supplementation might actually be helpful in preventing a recurrent calcum stone.
If there is an absence of randomized studies to answer these questions, Dr. Hall’s recommendations based on his expert experience would be most welcome.
- Hall PM. Nephrolithiasis: treatment, causes, and prevention. Cleve Clin J Med 2009; 76:583–591.
- Samplaski MK, Irwin BH, Desai M. Less-invasive ways to remove stones from the kidneys and ureters. Cleve Clin J Med 2009; 76:592–598.
- Hall PM. Nephrolithiasis: treatment, causes, and prevention. Cleve Clin J Med 2009; 76:583–591.
- Samplaski MK, Irwin BH, Desai M. Less-invasive ways to remove stones from the kidneys and ureters. Cleve Clin J Med 2009; 76:592–598.
Fragility fractures in chronic kidney disease: A clarification of views
To the Editor: I was pleased to see my article on fragility fractures in patients with chronic kidney disease (CKD) in the Cleveland Clinic Journal of Medicine1 and your preamble Letter from the Editor.2
However, Dr. Coco’s accompanying editorial3 misquoted a particular point I cautiously and consistently make—not only in the CCJM article, but in other invited papers on the topic of fractures in CKD. I specifically state that bisphosphonates should only be considered in stage 4–5 CKD in fracturing patients, not just those with “low bone mineral density,” who have clear-cut osteoporosis by exclusion of other causes of fractures in this population. Hence, Dr. Coco’s statement that “… the author advocates the use of bisphosphonate therapy in patients with chronic kidney disease who have low bone mineral density” is inaccurate.
If one carefully reads the last four paragraphs of my paper on page 721, one will see that I emphasize this caution repeatedly and even specifically state: “Treating only on the basis of low bone mineral density and other risk factors seems to be associated with greater risk than benefit.”
Thank you for your consideration.
1. Miller PD. Fragility fractures in chronic kidney disease: an opinion-based approach. Cleve Clin J Med 2009; 76:715–723.
2. Mandell BF. Low bone density is not always bisphosphonate deficiency (From the Editor). Cleve Clin J Med 2009; 76:683.
3. Coco M. Treating the renal patient who has a fracture: opinion vs evidence. Cleve Clin J Med 2009; 76:684–688.
To the Editor: I was pleased to see my article on fragility fractures in patients with chronic kidney disease (CKD) in the Cleveland Clinic Journal of Medicine1 and your preamble Letter from the Editor.2
However, Dr. Coco’s accompanying editorial3 misquoted a particular point I cautiously and consistently make—not only in the CCJM article, but in other invited papers on the topic of fractures in CKD. I specifically state that bisphosphonates should only be considered in stage 4–5 CKD in fracturing patients, not just those with “low bone mineral density,” who have clear-cut osteoporosis by exclusion of other causes of fractures in this population. Hence, Dr. Coco’s statement that “… the author advocates the use of bisphosphonate therapy in patients with chronic kidney disease who have low bone mineral density” is inaccurate.
If one carefully reads the last four paragraphs of my paper on page 721, one will see that I emphasize this caution repeatedly and even specifically state: “Treating only on the basis of low bone mineral density and other risk factors seems to be associated with greater risk than benefit.”
Thank you for your consideration.
To the Editor: I was pleased to see my article on fragility fractures in patients with chronic kidney disease (CKD) in the Cleveland Clinic Journal of Medicine1 and your preamble Letter from the Editor.2
However, Dr. Coco’s accompanying editorial3 misquoted a particular point I cautiously and consistently make—not only in the CCJM article, but in other invited papers on the topic of fractures in CKD. I specifically state that bisphosphonates should only be considered in stage 4–5 CKD in fracturing patients, not just those with “low bone mineral density,” who have clear-cut osteoporosis by exclusion of other causes of fractures in this population. Hence, Dr. Coco’s statement that “… the author advocates the use of bisphosphonate therapy in patients with chronic kidney disease who have low bone mineral density” is inaccurate.
If one carefully reads the last four paragraphs of my paper on page 721, one will see that I emphasize this caution repeatedly and even specifically state: “Treating only on the basis of low bone mineral density and other risk factors seems to be associated with greater risk than benefit.”
Thank you for your consideration.
1. Miller PD. Fragility fractures in chronic kidney disease: an opinion-based approach. Cleve Clin J Med 2009; 76:715–723.
2. Mandell BF. Low bone density is not always bisphosphonate deficiency (From the Editor). Cleve Clin J Med 2009; 76:683.
3. Coco M. Treating the renal patient who has a fracture: opinion vs evidence. Cleve Clin J Med 2009; 76:684–688.
1. Miller PD. Fragility fractures in chronic kidney disease: an opinion-based approach. Cleve Clin J Med 2009; 76:715–723.
2. Mandell BF. Low bone density is not always bisphosphonate deficiency (From the Editor). Cleve Clin J Med 2009; 76:683.
3. Coco M. Treating the renal patient who has a fracture: opinion vs evidence. Cleve Clin J Med 2009; 76:684–688.
In reply: Fragility fractures in chronic kidney disease: A clarification of views
In Reply: Bone disease in the patient with chronic kidney disease (CKD), especially in the presence of a fracture, is indeed a vexing problem. Clinically, it is very difficult to differentiate between low bone turnover—not uncommon in patients with CKD—and patients who have osteoporosis. Clinically, these patients present similarly: both can have abnormal bone density measurements (usually low bone mineral density with T scores less than −2.5 standard deviation), and both can have fractures. But both should not be treated the same without further evidence.
In Dr. Miller’s article, bisphosphonate and other therapies are named as possible treatments for “osteoporosis” in patients with CKD stages 1 through 3. “Treatment decisions are more difficult … in stage 4 and especially stage 5 chronic kidney disease with fragility fractures…."
Dr. Miller indeed states that “patients without fractures with stage 5 … should not be given bisphosphonates …” He also states, “Treating only on the basis of low bone mineral density … seems to be associated with greater risk than benefit.” In Dr. Miller’s opinion, the latter group of patients may be treated with a bisphosphonate if there has been a fracture. However, many of these patients may have fractured because of low turnover bone disease; unfortunately, they cannot have “clear-cut osteoporosis by exclusions of other causes.” Bisphosphonate therapy may further suppress bone activity (if there is any activity left) and may predispose to extraosseous and cardiovascular calcifications and further non-bone pathology.
Dr. Miller does caution regarding unknown risks in these patients with advanced kidney disease.
Treating metabolic bone disease is certainly not straightforward, especially when present in the fracturing renal patient. We need more evidence before making treatment paradigms.
In Reply: Bone disease in the patient with chronic kidney disease (CKD), especially in the presence of a fracture, is indeed a vexing problem. Clinically, it is very difficult to differentiate between low bone turnover—not uncommon in patients with CKD—and patients who have osteoporosis. Clinically, these patients present similarly: both can have abnormal bone density measurements (usually low bone mineral density with T scores less than −2.5 standard deviation), and both can have fractures. But both should not be treated the same without further evidence.
In Dr. Miller’s article, bisphosphonate and other therapies are named as possible treatments for “osteoporosis” in patients with CKD stages 1 through 3. “Treatment decisions are more difficult … in stage 4 and especially stage 5 chronic kidney disease with fragility fractures…."
Dr. Miller indeed states that “patients without fractures with stage 5 … should not be given bisphosphonates …” He also states, “Treating only on the basis of low bone mineral density … seems to be associated with greater risk than benefit.” In Dr. Miller’s opinion, the latter group of patients may be treated with a bisphosphonate if there has been a fracture. However, many of these patients may have fractured because of low turnover bone disease; unfortunately, they cannot have “clear-cut osteoporosis by exclusions of other causes.” Bisphosphonate therapy may further suppress bone activity (if there is any activity left) and may predispose to extraosseous and cardiovascular calcifications and further non-bone pathology.
Dr. Miller does caution regarding unknown risks in these patients with advanced kidney disease.
Treating metabolic bone disease is certainly not straightforward, especially when present in the fracturing renal patient. We need more evidence before making treatment paradigms.
In Reply: Bone disease in the patient with chronic kidney disease (CKD), especially in the presence of a fracture, is indeed a vexing problem. Clinically, it is very difficult to differentiate between low bone turnover—not uncommon in patients with CKD—and patients who have osteoporosis. Clinically, these patients present similarly: both can have abnormal bone density measurements (usually low bone mineral density with T scores less than −2.5 standard deviation), and both can have fractures. But both should not be treated the same without further evidence.
In Dr. Miller’s article, bisphosphonate and other therapies are named as possible treatments for “osteoporosis” in patients with CKD stages 1 through 3. “Treatment decisions are more difficult … in stage 4 and especially stage 5 chronic kidney disease with fragility fractures…."
Dr. Miller indeed states that “patients without fractures with stage 5 … should not be given bisphosphonates …” He also states, “Treating only on the basis of low bone mineral density … seems to be associated with greater risk than benefit.” In Dr. Miller’s opinion, the latter group of patients may be treated with a bisphosphonate if there has been a fracture. However, many of these patients may have fractured because of low turnover bone disease; unfortunately, they cannot have “clear-cut osteoporosis by exclusions of other causes.” Bisphosphonate therapy may further suppress bone activity (if there is any activity left) and may predispose to extraosseous and cardiovascular calcifications and further non-bone pathology.
Dr. Miller does caution regarding unknown risks in these patients with advanced kidney disease.
Treating metabolic bone disease is certainly not straightforward, especially when present in the fracturing renal patient. We need more evidence before making treatment paradigms.