User login
Kidney donors at greater risk of preeclampsia, gestational hypertension
Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy, according to a study presented at Kidney Week 2014 and published online simultaneously in the New England Journal of Medicine.
“Information on this potential risk should be included in clinical practice guidelines, shared in the informed-consent processes for potential donors and their recipients when a woman has reproductive potential, and used to guide the care of pregnant donors,” wrote the study authors, led by Dr. Amit X. Garg at the London Kidney Research Unit in London, Ont. (N. Engl. J. Med. 2014 Nov. 14 [doi:10.1056/NEJMoa1408932]).
The Canadian retrospective study matched 85 living kidney donors in a 1:6 ratio with 510 healthy nondonors and followed them for almost 11 years. During this time, 131 pregnancies occurred in the donor group and 788 in the nondonor group.
Gestational hypertension or preeclampsia was diagnosed in 15 donors and 38 nondonors (11% vs. 5%, odds ratio for donors, 2.4; 95% confidence interval, 1.2 to 5.0; P = .01), the investigators reported.
No significant differences were observed between groups for other maternal or fetal outcomes, and there were no maternal or perinatal deaths in the study that was part of the Donor Nephrectomy Outcomes Research Network (DONOR).
However, they noted that the study included limitations, such as not recording body mass index, medication use, or the race of study participants.
Confidence intervals for risk estimates also were wide, and physicians used clinical judgment when applying accepted diagnostic criteria for gestational hypertension and preeclampsia.
“It remains possible that gestational hypertension and preeclampsia were more likely to be diagnosed and recorded among donors than nondonors despite similar clinical presentations in two groups,” the investigators wrote.
“There may be a role for government programs to cover the costs of recommended pregnancy care for donors who lack health insurance, including any costs related to the treatment of hypertension,” they added.
The meeting was sponsored by the American Society of Nephrology. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures.
Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy, according to a study presented at Kidney Week 2014 and published online simultaneously in the New England Journal of Medicine.
“Information on this potential risk should be included in clinical practice guidelines, shared in the informed-consent processes for potential donors and their recipients when a woman has reproductive potential, and used to guide the care of pregnant donors,” wrote the study authors, led by Dr. Amit X. Garg at the London Kidney Research Unit in London, Ont. (N. Engl. J. Med. 2014 Nov. 14 [doi:10.1056/NEJMoa1408932]).
The Canadian retrospective study matched 85 living kidney donors in a 1:6 ratio with 510 healthy nondonors and followed them for almost 11 years. During this time, 131 pregnancies occurred in the donor group and 788 in the nondonor group.
Gestational hypertension or preeclampsia was diagnosed in 15 donors and 38 nondonors (11% vs. 5%, odds ratio for donors, 2.4; 95% confidence interval, 1.2 to 5.0; P = .01), the investigators reported.
No significant differences were observed between groups for other maternal or fetal outcomes, and there were no maternal or perinatal deaths in the study that was part of the Donor Nephrectomy Outcomes Research Network (DONOR).
However, they noted that the study included limitations, such as not recording body mass index, medication use, or the race of study participants.
Confidence intervals for risk estimates also were wide, and physicians used clinical judgment when applying accepted diagnostic criteria for gestational hypertension and preeclampsia.
“It remains possible that gestational hypertension and preeclampsia were more likely to be diagnosed and recorded among donors than nondonors despite similar clinical presentations in two groups,” the investigators wrote.
“There may be a role for government programs to cover the costs of recommended pregnancy care for donors who lack health insurance, including any costs related to the treatment of hypertension,” they added.
The meeting was sponsored by the American Society of Nephrology. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures.
Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy, according to a study presented at Kidney Week 2014 and published online simultaneously in the New England Journal of Medicine.
“Information on this potential risk should be included in clinical practice guidelines, shared in the informed-consent processes for potential donors and their recipients when a woman has reproductive potential, and used to guide the care of pregnant donors,” wrote the study authors, led by Dr. Amit X. Garg at the London Kidney Research Unit in London, Ont. (N. Engl. J. Med. 2014 Nov. 14 [doi:10.1056/NEJMoa1408932]).
The Canadian retrospective study matched 85 living kidney donors in a 1:6 ratio with 510 healthy nondonors and followed them for almost 11 years. During this time, 131 pregnancies occurred in the donor group and 788 in the nondonor group.
Gestational hypertension or preeclampsia was diagnosed in 15 donors and 38 nondonors (11% vs. 5%, odds ratio for donors, 2.4; 95% confidence interval, 1.2 to 5.0; P = .01), the investigators reported.
No significant differences were observed between groups for other maternal or fetal outcomes, and there were no maternal or perinatal deaths in the study that was part of the Donor Nephrectomy Outcomes Research Network (DONOR).
However, they noted that the study included limitations, such as not recording body mass index, medication use, or the race of study participants.
Confidence intervals for risk estimates also were wide, and physicians used clinical judgment when applying accepted diagnostic criteria for gestational hypertension and preeclampsia.
“It remains possible that gestational hypertension and preeclampsia were more likely to be diagnosed and recorded among donors than nondonors despite similar clinical presentations in two groups,” the investigators wrote.
“There may be a role for government programs to cover the costs of recommended pregnancy care for donors who lack health insurance, including any costs related to the treatment of hypertension,” they added.
The meeting was sponsored by the American Society of Nephrology. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures.
FROM KIDNEY WEEK 2014
Key clinical point: Information on an increased risk for preeclampsia and gestational hypertension should be included in clinical practice guidelines and in informed-consent processes for potential kidney donors and their recipients.
Major finding: Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy.
Data source: Retrospective cohort study of 85 kidney donors who were matched on a 1:6 ratio with 510 healthy nondonors and followed for a median of 10.9 years.
Disclosures:Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. The meeting was sponsored by the American Society of Nephrology.
Multitarget induction therapy superior over short term for lupus nephritis
A multitarget therapy of mycophenolate mofetil and tacrolimus for lupus nephritis induction therapy led to almost twice as many complete remissions as did intravenous cyclophosphamide in an open-label, randomized, controlled trial.
“Because immune dysregulation is fundamental to pathogenesis of lupus nephritis, with both B and T cells involved in the development of the disease, it may be necessary to target multiple aspects of the immune response using combined immunosuppressants,” wrote Dr. Zhi-Hong Liu of Nanjing (China) University and her colleagues (Ann. Intern. Med. 2014 Nov. 11 [doi:10.7326/M14-1030]).
Between April 2009 and June 2011, 368 adults aged 18-65 years from 26 renal centers in China were randomized to receive mycophenolate mofetil (MMF) and tacrolimus with a steroid or IV cyclophosphamide with a steroid for 24 weeks. A total of 362 received medication (6 did not receive their assigned cyclophosphamide treatment for unknown reasons), and 310 completed the whole 24-week course of treatment. All participants in the study had biopsy-proven lupus nephritis diagnosed within the previous 6 months and had not been previously treated with MMF, cyclophosphamide, tacrolimus, or high-dose methylprednisolone, nor had they had renal replacement therapy, plasmapheresis, or IV gamma globulin therapy in the previous 12 weeks.
All patients began by receiving IV methylprednisolone pulse therapy (0.5 g/day) for 3 days and then oral prednisone (0.6 mg/kg) daily for 4 weeks, with prednisone tapering to 10 mg/day for the remainder of the study period. Following methylprednisolone pulse therapy, the multitarget group received MMF (0.5 g twice daily) and tacrolimus (2 mg twice daily) while the comparison group received IV cyclophosphamide (initially 0.75 g/m2 body surface area, then adjusted to 0.5-1.0 g/m2).
Nearly half – 45.9% – of patients in the multitarget group achieved full remission, whereas 25.6% of patients receiving IV cyclophosphamide showed complete remission (P < .001). The investigators defined complete remission as a 24-hour urinary protein excretion of 0.4 g or less, the absence of active urine sediments, a serum albumin level of 35 g/L or greater, and normal serum creatinine.
Results on secondary endpoints also favored multitarget therapy. The percentage of patients who had an overall response to treatment (complete and partial remissions) was 83.5% for multitarget therapy and 63% for cyclophosphamide (P < .001). Multitarget therapy patients responded to treatment at a median of 8.9 weeks, compared with cyclophosphamide-treated patients at 13 weeks. The investigators defined partial response as a 50% or greater reduction in proteinuria and urine protein less than 3.5 g/24 hours, a serum albumin level of 30 g/L or higher, and a normal or 25% or lower increase in serum creatinine level from baseline.
After treatment, patients in the multitarget therapy group also had significantly better results on other secondary endpoints, including changes in urine protein, serum albumin, systemic lupus erythematosus disease activity score, and C3 levels.
Adverse events occurred among 50.3% of multitarget recipients and 52.5% of cyclophosphamide recipients. More patients who received multitarget therapy had a serious adverse event (7.2% vs. 2.8%, respectively), and more patients withdrew from the multitarget therapy group (5.5% vs. 1.7%), but there were no statistically significant differences between the groups for either comparison.
The study was supported by the National Basic Research Program of China and the National Key Technology R&D Programs. Information on disclosures was unavailable at the time of publication.
During the last decades, trials in lupus nephritis have been blessed by the publication of large studies involving hundreds of patients. While this is certainly a major achievement, yet it has not been paralleled by long-enough follow-ups. Experience has shown that in lupus nephritis, a large number of patients is “silver” but long-term follow-up is “gold.”
The very good 6-month results of Dr. Liu and colleagues’ study is an example of this aphorism. The importance of this finding is emphasized by data showing that early (within 3-6 months) response to immunosuppressive treatment has good positive predictive value for long-term (10 years) preservation of renal function (Arthritis Rheum. 2004;50:3934-40).
So, should multitarget therapy with combination of tacrolimus with mycophenolate become the new “standard of care” in lupus nephritis?
The authors of this commentary are painfully aware of the unmet needs in the treatment of lupus nephritis and certainly welcome these data. However, our enthusiasm is tempered by the following concerns.
First, there are some methodology issues to be considered such as the fact that six patients randomized to cyclophosphamide for unknown reasons did not receive the treatment and were excluded from the study, or that there were 80 missing visits in the cyclophosphamide group and 38 in the multitarget group. Nonetheless, the researchers handled these issues by data imputation and sensitivity analyses, which confirmed their findings.
Second, the results of this trial may not be generalized to all patients with lupus nephritis. As the authors discuss in the paper, this study was performed in Chinese patients who tend to respond favorably to calcineurin inhibitors. Previous lupus nephritis trials have suggested differences in the efficacy of immunosuppressive agents across patients from different ethnic backgrounds (Rheumatology [Oxford] 2010;49:128-40). Furthermore, included patients had median disease duration of 2 months, only 20.7% had glomerular filtration rate < 60 mL/min per 1.73m2, and most of them did not have high-risk histological features in kidney biopsy (median activity index of 7, chronicity index of 1). Further studies will be required to determine the efficacy of multitarget therapy in patients with adverse prognostic factors such as moderate or severe impairment of renal function, or presence of crescents.
The current study was an induction-only trial. The podocyte-preserving and anti-proteinuric effects of calcineurin inhibitors may account for the higher and faster rates of resolution of proteinuria and complete renal remission in the multitarget group. The clinical significance of this finding, especially in view of the slow-mode of action of cyclophosphamide, remains to be seen. In fact, several studies have shown that in spite of the lower rates of renal remission with cyclophosphamide, the majority of patients maintain their renal function if proteinuria is less than 2 g/day (Ann. Intern. Med. 2001;135:248-57). Whether there is a benefit of the multitarget therapy in terms of renal flares and long-term stabilization of the renal function remains to be seen. To this end, the authors do not indicate how the renal remission in these patients will be maintained and the optimal dosage and duration of immunosuppressive treatment. This is of particular importance considering also the potential nephrotoxic effects of calcineurin inhibitors when used for long time periods.
Thus, at this point, while the use of multitarget therapy from the beginning may increase the rates of complete renal remission, it increases the cost and the complexity of lupus nephritis treatment. One may consider adding calcineurin inhibitors if the patient does not reach partial renal response after 3-6 months. Meanwhile, we are eagerly awaiting longer follow-up of this most valuable cohort of patients and information of how this remission can be maintained, how durable it is, and more importantly, whether after 5 years of therapy there is a difference in the preservation of renal function.
Dr. George Bertsias is with the department of rheumatology, clinical immunology, and allergy at the University of Crete Medical School, Heraklion, Greece. Dr. John Boletis is with the nephrology department and renal transplant unit at Laiko General Hospital, University of Athens. Dr. Dimitrios T. Boumpas is with the fourth department of internal medicine, Attikon Hospital, University of Athens. They had nothing to disclose.
During the last decades, trials in lupus nephritis have been blessed by the publication of large studies involving hundreds of patients. While this is certainly a major achievement, yet it has not been paralleled by long-enough follow-ups. Experience has shown that in lupus nephritis, a large number of patients is “silver” but long-term follow-up is “gold.”
The very good 6-month results of Dr. Liu and colleagues’ study is an example of this aphorism. The importance of this finding is emphasized by data showing that early (within 3-6 months) response to immunosuppressive treatment has good positive predictive value for long-term (10 years) preservation of renal function (Arthritis Rheum. 2004;50:3934-40).
So, should multitarget therapy with combination of tacrolimus with mycophenolate become the new “standard of care” in lupus nephritis?
The authors of this commentary are painfully aware of the unmet needs in the treatment of lupus nephritis and certainly welcome these data. However, our enthusiasm is tempered by the following concerns.
First, there are some methodology issues to be considered such as the fact that six patients randomized to cyclophosphamide for unknown reasons did not receive the treatment and were excluded from the study, or that there were 80 missing visits in the cyclophosphamide group and 38 in the multitarget group. Nonetheless, the researchers handled these issues by data imputation and sensitivity analyses, which confirmed their findings.
Second, the results of this trial may not be generalized to all patients with lupus nephritis. As the authors discuss in the paper, this study was performed in Chinese patients who tend to respond favorably to calcineurin inhibitors. Previous lupus nephritis trials have suggested differences in the efficacy of immunosuppressive agents across patients from different ethnic backgrounds (Rheumatology [Oxford] 2010;49:128-40). Furthermore, included patients had median disease duration of 2 months, only 20.7% had glomerular filtration rate < 60 mL/min per 1.73m2, and most of them did not have high-risk histological features in kidney biopsy (median activity index of 7, chronicity index of 1). Further studies will be required to determine the efficacy of multitarget therapy in patients with adverse prognostic factors such as moderate or severe impairment of renal function, or presence of crescents.
The current study was an induction-only trial. The podocyte-preserving and anti-proteinuric effects of calcineurin inhibitors may account for the higher and faster rates of resolution of proteinuria and complete renal remission in the multitarget group. The clinical significance of this finding, especially in view of the slow-mode of action of cyclophosphamide, remains to be seen. In fact, several studies have shown that in spite of the lower rates of renal remission with cyclophosphamide, the majority of patients maintain their renal function if proteinuria is less than 2 g/day (Ann. Intern. Med. 2001;135:248-57). Whether there is a benefit of the multitarget therapy in terms of renal flares and long-term stabilization of the renal function remains to be seen. To this end, the authors do not indicate how the renal remission in these patients will be maintained and the optimal dosage and duration of immunosuppressive treatment. This is of particular importance considering also the potential nephrotoxic effects of calcineurin inhibitors when used for long time periods.
Thus, at this point, while the use of multitarget therapy from the beginning may increase the rates of complete renal remission, it increases the cost and the complexity of lupus nephritis treatment. One may consider adding calcineurin inhibitors if the patient does not reach partial renal response after 3-6 months. Meanwhile, we are eagerly awaiting longer follow-up of this most valuable cohort of patients and information of how this remission can be maintained, how durable it is, and more importantly, whether after 5 years of therapy there is a difference in the preservation of renal function.
Dr. George Bertsias is with the department of rheumatology, clinical immunology, and allergy at the University of Crete Medical School, Heraklion, Greece. Dr. John Boletis is with the nephrology department and renal transplant unit at Laiko General Hospital, University of Athens. Dr. Dimitrios T. Boumpas is with the fourth department of internal medicine, Attikon Hospital, University of Athens. They had nothing to disclose.
During the last decades, trials in lupus nephritis have been blessed by the publication of large studies involving hundreds of patients. While this is certainly a major achievement, yet it has not been paralleled by long-enough follow-ups. Experience has shown that in lupus nephritis, a large number of patients is “silver” but long-term follow-up is “gold.”
The very good 6-month results of Dr. Liu and colleagues’ study is an example of this aphorism. The importance of this finding is emphasized by data showing that early (within 3-6 months) response to immunosuppressive treatment has good positive predictive value for long-term (10 years) preservation of renal function (Arthritis Rheum. 2004;50:3934-40).
So, should multitarget therapy with combination of tacrolimus with mycophenolate become the new “standard of care” in lupus nephritis?
The authors of this commentary are painfully aware of the unmet needs in the treatment of lupus nephritis and certainly welcome these data. However, our enthusiasm is tempered by the following concerns.
First, there are some methodology issues to be considered such as the fact that six patients randomized to cyclophosphamide for unknown reasons did not receive the treatment and were excluded from the study, or that there were 80 missing visits in the cyclophosphamide group and 38 in the multitarget group. Nonetheless, the researchers handled these issues by data imputation and sensitivity analyses, which confirmed their findings.
Second, the results of this trial may not be generalized to all patients with lupus nephritis. As the authors discuss in the paper, this study was performed in Chinese patients who tend to respond favorably to calcineurin inhibitors. Previous lupus nephritis trials have suggested differences in the efficacy of immunosuppressive agents across patients from different ethnic backgrounds (Rheumatology [Oxford] 2010;49:128-40). Furthermore, included patients had median disease duration of 2 months, only 20.7% had glomerular filtration rate < 60 mL/min per 1.73m2, and most of them did not have high-risk histological features in kidney biopsy (median activity index of 7, chronicity index of 1). Further studies will be required to determine the efficacy of multitarget therapy in patients with adverse prognostic factors such as moderate or severe impairment of renal function, or presence of crescents.
The current study was an induction-only trial. The podocyte-preserving and anti-proteinuric effects of calcineurin inhibitors may account for the higher and faster rates of resolution of proteinuria and complete renal remission in the multitarget group. The clinical significance of this finding, especially in view of the slow-mode of action of cyclophosphamide, remains to be seen. In fact, several studies have shown that in spite of the lower rates of renal remission with cyclophosphamide, the majority of patients maintain their renal function if proteinuria is less than 2 g/day (Ann. Intern. Med. 2001;135:248-57). Whether there is a benefit of the multitarget therapy in terms of renal flares and long-term stabilization of the renal function remains to be seen. To this end, the authors do not indicate how the renal remission in these patients will be maintained and the optimal dosage and duration of immunosuppressive treatment. This is of particular importance considering also the potential nephrotoxic effects of calcineurin inhibitors when used for long time periods.
Thus, at this point, while the use of multitarget therapy from the beginning may increase the rates of complete renal remission, it increases the cost and the complexity of lupus nephritis treatment. One may consider adding calcineurin inhibitors if the patient does not reach partial renal response after 3-6 months. Meanwhile, we are eagerly awaiting longer follow-up of this most valuable cohort of patients and information of how this remission can be maintained, how durable it is, and more importantly, whether after 5 years of therapy there is a difference in the preservation of renal function.
Dr. George Bertsias is with the department of rheumatology, clinical immunology, and allergy at the University of Crete Medical School, Heraklion, Greece. Dr. John Boletis is with the nephrology department and renal transplant unit at Laiko General Hospital, University of Athens. Dr. Dimitrios T. Boumpas is with the fourth department of internal medicine, Attikon Hospital, University of Athens. They had nothing to disclose.
A multitarget therapy of mycophenolate mofetil and tacrolimus for lupus nephritis induction therapy led to almost twice as many complete remissions as did intravenous cyclophosphamide in an open-label, randomized, controlled trial.
“Because immune dysregulation is fundamental to pathogenesis of lupus nephritis, with both B and T cells involved in the development of the disease, it may be necessary to target multiple aspects of the immune response using combined immunosuppressants,” wrote Dr. Zhi-Hong Liu of Nanjing (China) University and her colleagues (Ann. Intern. Med. 2014 Nov. 11 [doi:10.7326/M14-1030]).
Between April 2009 and June 2011, 368 adults aged 18-65 years from 26 renal centers in China were randomized to receive mycophenolate mofetil (MMF) and tacrolimus with a steroid or IV cyclophosphamide with a steroid for 24 weeks. A total of 362 received medication (6 did not receive their assigned cyclophosphamide treatment for unknown reasons), and 310 completed the whole 24-week course of treatment. All participants in the study had biopsy-proven lupus nephritis diagnosed within the previous 6 months and had not been previously treated with MMF, cyclophosphamide, tacrolimus, or high-dose methylprednisolone, nor had they had renal replacement therapy, plasmapheresis, or IV gamma globulin therapy in the previous 12 weeks.
All patients began by receiving IV methylprednisolone pulse therapy (0.5 g/day) for 3 days and then oral prednisone (0.6 mg/kg) daily for 4 weeks, with prednisone tapering to 10 mg/day for the remainder of the study period. Following methylprednisolone pulse therapy, the multitarget group received MMF (0.5 g twice daily) and tacrolimus (2 mg twice daily) while the comparison group received IV cyclophosphamide (initially 0.75 g/m2 body surface area, then adjusted to 0.5-1.0 g/m2).
Nearly half – 45.9% – of patients in the multitarget group achieved full remission, whereas 25.6% of patients receiving IV cyclophosphamide showed complete remission (P < .001). The investigators defined complete remission as a 24-hour urinary protein excretion of 0.4 g or less, the absence of active urine sediments, a serum albumin level of 35 g/L or greater, and normal serum creatinine.
Results on secondary endpoints also favored multitarget therapy. The percentage of patients who had an overall response to treatment (complete and partial remissions) was 83.5% for multitarget therapy and 63% for cyclophosphamide (P < .001). Multitarget therapy patients responded to treatment at a median of 8.9 weeks, compared with cyclophosphamide-treated patients at 13 weeks. The investigators defined partial response as a 50% or greater reduction in proteinuria and urine protein less than 3.5 g/24 hours, a serum albumin level of 30 g/L or higher, and a normal or 25% or lower increase in serum creatinine level from baseline.
After treatment, patients in the multitarget therapy group also had significantly better results on other secondary endpoints, including changes in urine protein, serum albumin, systemic lupus erythematosus disease activity score, and C3 levels.
Adverse events occurred among 50.3% of multitarget recipients and 52.5% of cyclophosphamide recipients. More patients who received multitarget therapy had a serious adverse event (7.2% vs. 2.8%, respectively), and more patients withdrew from the multitarget therapy group (5.5% vs. 1.7%), but there were no statistically significant differences between the groups for either comparison.
The study was supported by the National Basic Research Program of China and the National Key Technology R&D Programs. Information on disclosures was unavailable at the time of publication.
A multitarget therapy of mycophenolate mofetil and tacrolimus for lupus nephritis induction therapy led to almost twice as many complete remissions as did intravenous cyclophosphamide in an open-label, randomized, controlled trial.
“Because immune dysregulation is fundamental to pathogenesis of lupus nephritis, with both B and T cells involved in the development of the disease, it may be necessary to target multiple aspects of the immune response using combined immunosuppressants,” wrote Dr. Zhi-Hong Liu of Nanjing (China) University and her colleagues (Ann. Intern. Med. 2014 Nov. 11 [doi:10.7326/M14-1030]).
Between April 2009 and June 2011, 368 adults aged 18-65 years from 26 renal centers in China were randomized to receive mycophenolate mofetil (MMF) and tacrolimus with a steroid or IV cyclophosphamide with a steroid for 24 weeks. A total of 362 received medication (6 did not receive their assigned cyclophosphamide treatment for unknown reasons), and 310 completed the whole 24-week course of treatment. All participants in the study had biopsy-proven lupus nephritis diagnosed within the previous 6 months and had not been previously treated with MMF, cyclophosphamide, tacrolimus, or high-dose methylprednisolone, nor had they had renal replacement therapy, plasmapheresis, or IV gamma globulin therapy in the previous 12 weeks.
All patients began by receiving IV methylprednisolone pulse therapy (0.5 g/day) for 3 days and then oral prednisone (0.6 mg/kg) daily for 4 weeks, with prednisone tapering to 10 mg/day for the remainder of the study period. Following methylprednisolone pulse therapy, the multitarget group received MMF (0.5 g twice daily) and tacrolimus (2 mg twice daily) while the comparison group received IV cyclophosphamide (initially 0.75 g/m2 body surface area, then adjusted to 0.5-1.0 g/m2).
Nearly half – 45.9% – of patients in the multitarget group achieved full remission, whereas 25.6% of patients receiving IV cyclophosphamide showed complete remission (P < .001). The investigators defined complete remission as a 24-hour urinary protein excretion of 0.4 g or less, the absence of active urine sediments, a serum albumin level of 35 g/L or greater, and normal serum creatinine.
Results on secondary endpoints also favored multitarget therapy. The percentage of patients who had an overall response to treatment (complete and partial remissions) was 83.5% for multitarget therapy and 63% for cyclophosphamide (P < .001). Multitarget therapy patients responded to treatment at a median of 8.9 weeks, compared with cyclophosphamide-treated patients at 13 weeks. The investigators defined partial response as a 50% or greater reduction in proteinuria and urine protein less than 3.5 g/24 hours, a serum albumin level of 30 g/L or higher, and a normal or 25% or lower increase in serum creatinine level from baseline.
After treatment, patients in the multitarget therapy group also had significantly better results on other secondary endpoints, including changes in urine protein, serum albumin, systemic lupus erythematosus disease activity score, and C3 levels.
Adverse events occurred among 50.3% of multitarget recipients and 52.5% of cyclophosphamide recipients. More patients who received multitarget therapy had a serious adverse event (7.2% vs. 2.8%, respectively), and more patients withdrew from the multitarget therapy group (5.5% vs. 1.7%), but there were no statistically significant differences between the groups for either comparison.
The study was supported by the National Basic Research Program of China and the National Key Technology R&D Programs. Information on disclosures was unavailable at the time of publication.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Multitarget treatment is superior to intravenous cyclophosphamide as induction therapy for lupus nephritis.
Major finding: Among of patients receiving mycophenolate mofetil and tacrolimus, 45.9% achieved complete remission, compared with 25.6% receiving IV cyclophosphamide (P < .001).
Data source: A 24-week, open-label, randomized, controlled trial of 368 adults from 26 centers in China between April 2009 and June 2011.
Disclosures: The National Basic Research Program of China and the National Key Technology R&D Programs funded the study. Information on disclosures was unavailable at the time of publication.
Guidelines: Urinate 2 liters daily to stop kidney stones’ return
Patients who have had kidney stones at least once should increase their fluid consumption to produce at least 2 L of urine per day to prevent the condition from recurring, according to new guidelines published by the American College of Physicians.
If increased fluid intake doesn’t prevent recurrent nephrolithiasis, the updated ACP guidelines recommend adding treatment with a thiazide diuretic, citrate, or allopurinol. “Increased fluid intake spread throughout the day can decrease stone recurrence by at least half with virtually no side effects,” said Dr. David A. Fleming, ACP president, in a statement. “However, people who already drink the recommended amount of liquids, or when increased fluid intake is contraindicated, should not increase their fluid intake.”
The guidelines are based on a review of nephrolithiasis studies, in which the ACP’s director of clinical policy, Dr. Amir Qaseem, and his associates examined baseline stone composition, blood and urine chemistries, and final health outcomes, among other factors. Only English-language trials were used, and research was collected through searches of MEDLINE, the Cochrane Database of Systematic Reviews, Google Scholar, ClinicalTrials.gov, and Web of Science (Ann. Intern. Med. 2014;161:659-67).
Thiazide diuretics, citrates, or allopurinol significantly reduced recurrence of calcium-based stones (the most common type of kidney stone) in patients who had nephrolithiasis at least twice before, according to the analysis.
The ACP guidelines also caution against consuming non-fruit–flavored sodas and carbonated beverages, because they are acidified by phosphoric acid, and dietary animal protein and purines.
However, the guidelines encourage consumption of dietary oxalate – commonly found in chocolate, beets, nuts, rhubarb, spinach, strawberries, tea, and wheat bran – and support maintaining normal dietary calcium. There was no evidence to suggest a risk reduction advantage in drinking tap water rather than mineral water.
The recommendations do not apply to “patients with suspected hyperparathyroidism or other rare cases,” the guidelines authors noted.
Financial support for the guidelines came from the ACP operating budget. The authors had no other relevant financial disclosures.
Patients who have had kidney stones at least once should increase their fluid consumption to produce at least 2 L of urine per day to prevent the condition from recurring, according to new guidelines published by the American College of Physicians.
If increased fluid intake doesn’t prevent recurrent nephrolithiasis, the updated ACP guidelines recommend adding treatment with a thiazide diuretic, citrate, or allopurinol. “Increased fluid intake spread throughout the day can decrease stone recurrence by at least half with virtually no side effects,” said Dr. David A. Fleming, ACP president, in a statement. “However, people who already drink the recommended amount of liquids, or when increased fluid intake is contraindicated, should not increase their fluid intake.”
The guidelines are based on a review of nephrolithiasis studies, in which the ACP’s director of clinical policy, Dr. Amir Qaseem, and his associates examined baseline stone composition, blood and urine chemistries, and final health outcomes, among other factors. Only English-language trials were used, and research was collected through searches of MEDLINE, the Cochrane Database of Systematic Reviews, Google Scholar, ClinicalTrials.gov, and Web of Science (Ann. Intern. Med. 2014;161:659-67).
Thiazide diuretics, citrates, or allopurinol significantly reduced recurrence of calcium-based stones (the most common type of kidney stone) in patients who had nephrolithiasis at least twice before, according to the analysis.
The ACP guidelines also caution against consuming non-fruit–flavored sodas and carbonated beverages, because they are acidified by phosphoric acid, and dietary animal protein and purines.
However, the guidelines encourage consumption of dietary oxalate – commonly found in chocolate, beets, nuts, rhubarb, spinach, strawberries, tea, and wheat bran – and support maintaining normal dietary calcium. There was no evidence to suggest a risk reduction advantage in drinking tap water rather than mineral water.
The recommendations do not apply to “patients with suspected hyperparathyroidism or other rare cases,” the guidelines authors noted.
Financial support for the guidelines came from the ACP operating budget. The authors had no other relevant financial disclosures.
Patients who have had kidney stones at least once should increase their fluid consumption to produce at least 2 L of urine per day to prevent the condition from recurring, according to new guidelines published by the American College of Physicians.
If increased fluid intake doesn’t prevent recurrent nephrolithiasis, the updated ACP guidelines recommend adding treatment with a thiazide diuretic, citrate, or allopurinol. “Increased fluid intake spread throughout the day can decrease stone recurrence by at least half with virtually no side effects,” said Dr. David A. Fleming, ACP president, in a statement. “However, people who already drink the recommended amount of liquids, or when increased fluid intake is contraindicated, should not increase their fluid intake.”
The guidelines are based on a review of nephrolithiasis studies, in which the ACP’s director of clinical policy, Dr. Amir Qaseem, and his associates examined baseline stone composition, blood and urine chemistries, and final health outcomes, among other factors. Only English-language trials were used, and research was collected through searches of MEDLINE, the Cochrane Database of Systematic Reviews, Google Scholar, ClinicalTrials.gov, and Web of Science (Ann. Intern. Med. 2014;161:659-67).
Thiazide diuretics, citrates, or allopurinol significantly reduced recurrence of calcium-based stones (the most common type of kidney stone) in patients who had nephrolithiasis at least twice before, according to the analysis.
The ACP guidelines also caution against consuming non-fruit–flavored sodas and carbonated beverages, because they are acidified by phosphoric acid, and dietary animal protein and purines.
However, the guidelines encourage consumption of dietary oxalate – commonly found in chocolate, beets, nuts, rhubarb, spinach, strawberries, tea, and wheat bran – and support maintaining normal dietary calcium. There was no evidence to suggest a risk reduction advantage in drinking tap water rather than mineral water.
The recommendations do not apply to “patients with suspected hyperparathyroidism or other rare cases,” the guidelines authors noted.
Financial support for the guidelines came from the ACP operating budget. The authors had no other relevant financial disclosures.
FROM THE ANNALS OF INTERNAL MEDICINE
Genetics of Renal Disease: APOL1 Variations
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
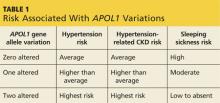
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
The Risk and Treatment for Wilms Tumors
Q) In school, they always emphasized the abdominal exam to rule out Wilms tumors. Are Wilms tumors still with us? Has treatment and evaluation changed?
Wilms tumor is a renal cancer found most commonly in children younger than 9 and represents approximately 7% of all malignancies in children.8,9 It can occur in one or both kidneys, with earlier diagnosis noted with bilateral involvement. Risk is highest among non-Hispanic white persons and African-Americans and lowest among Asians.8
Wilms tumor develops due to a genetic mutation in the WT1 gene located on the 11p13 chromosome. Defects are also noted on the 11p15 chromosome and the p53 tumor suppressor gene.10 Urbach et al recently identified a relationship between the LIN28 gene and Wilms tumor.11 Tumors develop when embryonic renal cells that should cease growing at the time of birth continue to grow in the postnatal period. Wilms tumor can be familial or sporadic. It can also be associated with various congenital anomalies manifested within various syndromes (see Table 2), as well as isolated genitourinary abnormalities, especially in boys.10
Most children present with a palpable, smooth, firm, generally painless mass in the abdomen; those who have bilateral renal involvement usually present earlier than those with unilateral involvement. Palpation of the abdomen during examination, if vigorous, can result in rupture of the renal capsule and tumor spillage. Additional symptoms include hematuria, fever, and hypertension. Referral to pediatric oncology is imperative.12
Definitive diagnosis is made by histologic evaluation following biopsy or surgical excision.13 Other possible diagnostic tests include but are not limited to abdominal ultrasound or CT; chest CT (to rule out metastatic lung disease); urinalysis (to evaluate for hematuria and proteinuria); liver function studies (to evaluate for hepatic involvement); and laboratory studies to measure coagulation, serum calcium, blood urea nitrogen, creatinine, and complete blood count.
Histologic examination for staging (I-V) occurs following surgical excision of the tumor. There are two staging systems available: the National Wilms Tumor Study, based on postoperative tumor evaluation, and the International Society of Pediatric Oncology, based on postchemotherapy evaluation.13
Treatment options include surgical excision (including complete nephrectomy of the affected kidney), chemotherapy based on tumor staging, and internal and/or external radiation therapy.13
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personal izedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Dis ease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) In school, they always emphasized the abdominal exam to rule out Wilms tumors. Are Wilms tumors still with us? Has treatment and evaluation changed?
Wilms tumor is a renal cancer found most commonly in children younger than 9 and represents approximately 7% of all malignancies in children.8,9 It can occur in one or both kidneys, with earlier diagnosis noted with bilateral involvement. Risk is highest among non-Hispanic white persons and African-Americans and lowest among Asians.8
Wilms tumor develops due to a genetic mutation in the WT1 gene located on the 11p13 chromosome. Defects are also noted on the 11p15 chromosome and the p53 tumor suppressor gene.10 Urbach et al recently identified a relationship between the LIN28 gene and Wilms tumor.11 Tumors develop when embryonic renal cells that should cease growing at the time of birth continue to grow in the postnatal period. Wilms tumor can be familial or sporadic. It can also be associated with various congenital anomalies manifested within various syndromes (see Table 2), as well as isolated genitourinary abnormalities, especially in boys.10
Most children present with a palpable, smooth, firm, generally painless mass in the abdomen; those who have bilateral renal involvement usually present earlier than those with unilateral involvement. Palpation of the abdomen during examination, if vigorous, can result in rupture of the renal capsule and tumor spillage. Additional symptoms include hematuria, fever, and hypertension. Referral to pediatric oncology is imperative.12
Definitive diagnosis is made by histologic evaluation following biopsy or surgical excision.13 Other possible diagnostic tests include but are not limited to abdominal ultrasound or CT; chest CT (to rule out metastatic lung disease); urinalysis (to evaluate for hematuria and proteinuria); liver function studies (to evaluate for hepatic involvement); and laboratory studies to measure coagulation, serum calcium, blood urea nitrogen, creatinine, and complete blood count.
Histologic examination for staging (I-V) occurs following surgical excision of the tumor. There are two staging systems available: the National Wilms Tumor Study, based on postoperative tumor evaluation, and the International Society of Pediatric Oncology, based on postchemotherapy evaluation.13
Treatment options include surgical excision (including complete nephrectomy of the affected kidney), chemotherapy based on tumor staging, and internal and/or external radiation therapy.13
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personal izedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Dis ease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) In school, they always emphasized the abdominal exam to rule out Wilms tumors. Are Wilms tumors still with us? Has treatment and evaluation changed?
Wilms tumor is a renal cancer found most commonly in children younger than 9 and represents approximately 7% of all malignancies in children.8,9 It can occur in one or both kidneys, with earlier diagnosis noted with bilateral involvement. Risk is highest among non-Hispanic white persons and African-Americans and lowest among Asians.8
Wilms tumor develops due to a genetic mutation in the WT1 gene located on the 11p13 chromosome. Defects are also noted on the 11p15 chromosome and the p53 tumor suppressor gene.10 Urbach et al recently identified a relationship between the LIN28 gene and Wilms tumor.11 Tumors develop when embryonic renal cells that should cease growing at the time of birth continue to grow in the postnatal period. Wilms tumor can be familial or sporadic. It can also be associated with various congenital anomalies manifested within various syndromes (see Table 2), as well as isolated genitourinary abnormalities, especially in boys.10
Most children present with a palpable, smooth, firm, generally painless mass in the abdomen; those who have bilateral renal involvement usually present earlier than those with unilateral involvement. Palpation of the abdomen during examination, if vigorous, can result in rupture of the renal capsule and tumor spillage. Additional symptoms include hematuria, fever, and hypertension. Referral to pediatric oncology is imperative.12
Definitive diagnosis is made by histologic evaluation following biopsy or surgical excision.13 Other possible diagnostic tests include but are not limited to abdominal ultrasound or CT; chest CT (to rule out metastatic lung disease); urinalysis (to evaluate for hematuria and proteinuria); liver function studies (to evaluate for hepatic involvement); and laboratory studies to measure coagulation, serum calcium, blood urea nitrogen, creatinine, and complete blood count.
Histologic examination for staging (I-V) occurs following surgical excision of the tumor. There are two staging systems available: the National Wilms Tumor Study, based on postoperative tumor evaluation, and the International Society of Pediatric Oncology, based on postchemotherapy evaluation.13
Treatment options include surgical excision (including complete nephrectomy of the affected kidney), chemotherapy based on tumor staging, and internal and/or external radiation therapy.13
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personal izedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Dis ease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Intraoperative evaluation may be best for predicting AVF success
CORONADO, CALIF. – Intraoperative vessel assessment, not preoperative vein mapping, was more accurately associated with maturation and cumulative functional patency rates of arteriovenous fistulas, results from a 5-year, single-center retrospective study showed.
“The prevalence of chronic kidney disease has increased over the last 3 decades, and efforts by the Centers for Medicare & Medicaid Services and the National Kidney Foundation have sought to increase the use of fistulas,” Dr. Khanh P. Nguyen said at the annual meeting of the Western Vascular Society.
“Since 2003, the incidence and prevalence of fistulas have increased. More recently, even higher goals have been set. Arteriovenous fistulas are the preferred procedures due to higher patency rates, reduced rates of reintervention, and lower costs, compared with central venous catheters or grafts.”
Dr. Nguyen, formerly of Loma Linda (Calif.) Medical Center who is now a vascular surgery fellow at Oregon Health and Science University, noted that while many studies as well as the Society for Vascular Surgery have advocated the use of routine preoperative ultrasound in predicting the success of arteriovenous fistulas (AVFs), its use varies among vascular surgeons. “Given the additional costs and time of preoperative ultrasound, this study was undertaken to examine the use of this technique and compare it to intraoperative assessment,” she said.
The researchers examined all autologous AVFs created for patients with end-stage-renal disease at the Veterans Affairs Loma Linda Health System between February 2007 and July 2012. Preoperative ultrasound mapping of upper-extremity veins occurred, and patients were divided into two groups: those with veins less than 3 mm in size and those with veins 3 mm or greater in size. Subjective intraoperative evaluation was conducted by the operative surgeon, who rated the vein as either “good” or “poor” because of factors such as inadequate diameter, sclerosis, and calcification. Kaplan-Meier analysis was used to calculate maturation and patency rates.
Over the 5-year period, 387 fistulas were created in 361 patients. Of these, 198 had preoperative vein mapping; 36% were less than 3 mm in size, and 64% were 3 mm or greater in size.
By intraoperative assessment, 14% of patients were determined to have had poor vessels, and 86% were found to have good vessels. About half of the fistulas (51%) were created at the wrist. The average age of patients was 65 years, their mean body mass index was 28 kg/m2, and their mean time on dialysis was 84 years. The majority (97%) were male.
Among patients with preoperative veins less than 3 mm in size or 3 mm in size or greater, the maturation and overall failure rates were similar at 71% vs. 75% (P = .61) and 68% vs. 58% (P = .15). However, among patients with assessments of poor or good veins at the time of operation, the maturation and overall failure rates were 42% vs. 82% (P < .001) and 86% vs. 54% (P < .001).
Subgroup analysis revealed that patients with good intraoperative evaluation, regardless of preoperative ultrasound findings, had higher maturation rates. “Likewise, patients with good intraoperative assessment, regardless of preoperative ultrasound findings, had higher cumulative functional patency rates. Of note, no patient who had both poor preoperative ultrasound and intraoperative assessments had a functional fistula at the time of last follow-up.”
Dr. Nguyen and her associates concluded that intraoperative vessel assessment, and not preoperative ultrasound, was more accurately associated with maturation and cumulative functional patency rates. “Even in patients with inadequate preoperative ultrasound vein mapping, intraoperative assessment may still be warranted,” she said. “If both preoperative and intraoperative assessments conclude that vessels are inadequate, do not create an AVF at that site.”
Dr. Nguyen reported having no relevant financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – Intraoperative vessel assessment, not preoperative vein mapping, was more accurately associated with maturation and cumulative functional patency rates of arteriovenous fistulas, results from a 5-year, single-center retrospective study showed.
“The prevalence of chronic kidney disease has increased over the last 3 decades, and efforts by the Centers for Medicare & Medicaid Services and the National Kidney Foundation have sought to increase the use of fistulas,” Dr. Khanh P. Nguyen said at the annual meeting of the Western Vascular Society.
“Since 2003, the incidence and prevalence of fistulas have increased. More recently, even higher goals have been set. Arteriovenous fistulas are the preferred procedures due to higher patency rates, reduced rates of reintervention, and lower costs, compared with central venous catheters or grafts.”
Dr. Nguyen, formerly of Loma Linda (Calif.) Medical Center who is now a vascular surgery fellow at Oregon Health and Science University, noted that while many studies as well as the Society for Vascular Surgery have advocated the use of routine preoperative ultrasound in predicting the success of arteriovenous fistulas (AVFs), its use varies among vascular surgeons. “Given the additional costs and time of preoperative ultrasound, this study was undertaken to examine the use of this technique and compare it to intraoperative assessment,” she said.
The researchers examined all autologous AVFs created for patients with end-stage-renal disease at the Veterans Affairs Loma Linda Health System between February 2007 and July 2012. Preoperative ultrasound mapping of upper-extremity veins occurred, and patients were divided into two groups: those with veins less than 3 mm in size and those with veins 3 mm or greater in size. Subjective intraoperative evaluation was conducted by the operative surgeon, who rated the vein as either “good” or “poor” because of factors such as inadequate diameter, sclerosis, and calcification. Kaplan-Meier analysis was used to calculate maturation and patency rates.
Over the 5-year period, 387 fistulas were created in 361 patients. Of these, 198 had preoperative vein mapping; 36% were less than 3 mm in size, and 64% were 3 mm or greater in size.
By intraoperative assessment, 14% of patients were determined to have had poor vessels, and 86% were found to have good vessels. About half of the fistulas (51%) were created at the wrist. The average age of patients was 65 years, their mean body mass index was 28 kg/m2, and their mean time on dialysis was 84 years. The majority (97%) were male.
Among patients with preoperative veins less than 3 mm in size or 3 mm in size or greater, the maturation and overall failure rates were similar at 71% vs. 75% (P = .61) and 68% vs. 58% (P = .15). However, among patients with assessments of poor or good veins at the time of operation, the maturation and overall failure rates were 42% vs. 82% (P < .001) and 86% vs. 54% (P < .001).
Subgroup analysis revealed that patients with good intraoperative evaluation, regardless of preoperative ultrasound findings, had higher maturation rates. “Likewise, patients with good intraoperative assessment, regardless of preoperative ultrasound findings, had higher cumulative functional patency rates. Of note, no patient who had both poor preoperative ultrasound and intraoperative assessments had a functional fistula at the time of last follow-up.”
Dr. Nguyen and her associates concluded that intraoperative vessel assessment, and not preoperative ultrasound, was more accurately associated with maturation and cumulative functional patency rates. “Even in patients with inadequate preoperative ultrasound vein mapping, intraoperative assessment may still be warranted,” she said. “If both preoperative and intraoperative assessments conclude that vessels are inadequate, do not create an AVF at that site.”
Dr. Nguyen reported having no relevant financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – Intraoperative vessel assessment, not preoperative vein mapping, was more accurately associated with maturation and cumulative functional patency rates of arteriovenous fistulas, results from a 5-year, single-center retrospective study showed.
“The prevalence of chronic kidney disease has increased over the last 3 decades, and efforts by the Centers for Medicare & Medicaid Services and the National Kidney Foundation have sought to increase the use of fistulas,” Dr. Khanh P. Nguyen said at the annual meeting of the Western Vascular Society.
“Since 2003, the incidence and prevalence of fistulas have increased. More recently, even higher goals have been set. Arteriovenous fistulas are the preferred procedures due to higher patency rates, reduced rates of reintervention, and lower costs, compared with central venous catheters or grafts.”
Dr. Nguyen, formerly of Loma Linda (Calif.) Medical Center who is now a vascular surgery fellow at Oregon Health and Science University, noted that while many studies as well as the Society for Vascular Surgery have advocated the use of routine preoperative ultrasound in predicting the success of arteriovenous fistulas (AVFs), its use varies among vascular surgeons. “Given the additional costs and time of preoperative ultrasound, this study was undertaken to examine the use of this technique and compare it to intraoperative assessment,” she said.
The researchers examined all autologous AVFs created for patients with end-stage-renal disease at the Veterans Affairs Loma Linda Health System between February 2007 and July 2012. Preoperative ultrasound mapping of upper-extremity veins occurred, and patients were divided into two groups: those with veins less than 3 mm in size and those with veins 3 mm or greater in size. Subjective intraoperative evaluation was conducted by the operative surgeon, who rated the vein as either “good” or “poor” because of factors such as inadequate diameter, sclerosis, and calcification. Kaplan-Meier analysis was used to calculate maturation and patency rates.
Over the 5-year period, 387 fistulas were created in 361 patients. Of these, 198 had preoperative vein mapping; 36% were less than 3 mm in size, and 64% were 3 mm or greater in size.
By intraoperative assessment, 14% of patients were determined to have had poor vessels, and 86% were found to have good vessels. About half of the fistulas (51%) were created at the wrist. The average age of patients was 65 years, their mean body mass index was 28 kg/m2, and their mean time on dialysis was 84 years. The majority (97%) were male.
Among patients with preoperative veins less than 3 mm in size or 3 mm in size or greater, the maturation and overall failure rates were similar at 71% vs. 75% (P = .61) and 68% vs. 58% (P = .15). However, among patients with assessments of poor or good veins at the time of operation, the maturation and overall failure rates were 42% vs. 82% (P < .001) and 86% vs. 54% (P < .001).
Subgroup analysis revealed that patients with good intraoperative evaluation, regardless of preoperative ultrasound findings, had higher maturation rates. “Likewise, patients with good intraoperative assessment, regardless of preoperative ultrasound findings, had higher cumulative functional patency rates. Of note, no patient who had both poor preoperative ultrasound and intraoperative assessments had a functional fistula at the time of last follow-up.”
Dr. Nguyen and her associates concluded that intraoperative vessel assessment, and not preoperative ultrasound, was more accurately associated with maturation and cumulative functional patency rates. “Even in patients with inadequate preoperative ultrasound vein mapping, intraoperative assessment may still be warranted,” she said. “If both preoperative and intraoperative assessments conclude that vessels are inadequate, do not create an AVF at that site.”
Dr. Nguyen reported having no relevant financial disclosures.
On Twitter @dougbrunk
AT THE WESTERN VASCULAR SOCIETY ANNUAL MEETING
Key clinical point: Routine preoperative ultrasound is not reliable for predicting the success of arteriovenous fistulas.
Major finding: Among patients with assessments of poor or good veins at the time of operation, the maturation and overall failure rates were 42% vs. 82% (P < .001) and 86% vs. 54% (P < .001).
Data source: A retrospective study of 387 AVFs created in 361 patients at the VA Loma Linda Health System between February 2007 and July 2012.
Disclosures:Dr. Nguyen reported having no relevant financial disclosures.
Why is metformin contraindicated in chronic kidney disease?
To the Editor: In their article about the care of patients with advanced chronic kidney disease, Sakhuja et al1 mentioned that metformin is contraindicated in chronic kidney disease.
Metformin is a good and useful drug. Not only is it one of the cheapest antidiabetic medications, it is the only one shown to reduce cardiovascular mortality rates in type 2 diabetes mellitus.
Although metformin is thought to increase the risk of lactic acidosis, a Cochrane review2 found that the incidence of lactic acidosis was only 4.3 cases per 100,000 patient-years in patients taking metformin, compared with 5.4 cases per 100,000 patient-years in patients not taking metformin. Furthermore, in a large registry of patients with type 2 diabetes and atherothrombosis,3 the rate of all-cause mortality was 24% lower in metformin users than in nonusers, and in those who had moderate renal impairment (creatinine clearance 30–59 mL/min/1.73 m2) the difference was 36%.3
A trial by Rachmani et al4 raised questions about the standard contraindications to metformin. The authors reviewed 393 patients who had at least one contraindication to metformin but who were receiving it anyway. Their serum creatinine levels ranged from 1.5 to 2.5 mg/dL. There were no cases of lactic acidosis reported. The patients were then randomized either to continue taking metformin or to stop taking it. At 2 years, the group that had stopped taking it had gained more weight, and their glycemic control was worse.
In the Cochrane analysis,2 although individual creatinine levels were not available, 53% of the studies reviewed did not exclude patients with serum creatinine levels higher than 1.5 mg/dL. This equated to 37,360 patient-years of metformin use in studies that included patients with chronic kidney disease, and did not lead to lactic acidosis.
Even though metformin’s US package insert says that it is contraindicated if the serum creatinine level is 1.5 mg/dL or higher in men or 1.4 mg/dL or higher in women or if the creatinine clearance is “abnormal,” in view of the available evidence, many countries (eg, the United Kingdom, Australia, the Netherlands) now allow metformin to be used in patients with glomerular filtration rates as low as 30 mL/min/1.73m2, with lower doses if the glomerular filtration rate is lower than 45.5
The current contraindication to metformin in chronic kidney disease needs to be reviewed. In poor countries like India, this cheap medicine may be the only option available for treating type 2 diabetes mellitus, and it remains the first-line therapy for type 2 diabetes mellitus as recommended by the International Diabetes Federation, the American Diabetes Association, and the European Association for the Study of Diabetes.5
- Sakhuja A, Hyland J, Simon JF. Managing advanced chronic kidney disease: a primary care guide. Cleve Clin J Med 2014; 81:289–299.
- Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitis. Cochrane Database Syst Rev 2010; 4:CD002967.
- Roussel R, Travert F, Pasquet B, et al; Reduction of Atherothrombosis for Continued Health (REACH) Registry Investigators. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 2010; 170:1892–1899.
- Rachmani R, Slavachevski I, Levi Z, Zadok B, Kedar Y, Ravid M. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. Eur J Intern Med 2002; 13:428–433.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
To the Editor: In their article about the care of patients with advanced chronic kidney disease, Sakhuja et al1 mentioned that metformin is contraindicated in chronic kidney disease.
Metformin is a good and useful drug. Not only is it one of the cheapest antidiabetic medications, it is the only one shown to reduce cardiovascular mortality rates in type 2 diabetes mellitus.
Although metformin is thought to increase the risk of lactic acidosis, a Cochrane review2 found that the incidence of lactic acidosis was only 4.3 cases per 100,000 patient-years in patients taking metformin, compared with 5.4 cases per 100,000 patient-years in patients not taking metformin. Furthermore, in a large registry of patients with type 2 diabetes and atherothrombosis,3 the rate of all-cause mortality was 24% lower in metformin users than in nonusers, and in those who had moderate renal impairment (creatinine clearance 30–59 mL/min/1.73 m2) the difference was 36%.3
A trial by Rachmani et al4 raised questions about the standard contraindications to metformin. The authors reviewed 393 patients who had at least one contraindication to metformin but who were receiving it anyway. Their serum creatinine levels ranged from 1.5 to 2.5 mg/dL. There were no cases of lactic acidosis reported. The patients were then randomized either to continue taking metformin or to stop taking it. At 2 years, the group that had stopped taking it had gained more weight, and their glycemic control was worse.
In the Cochrane analysis,2 although individual creatinine levels were not available, 53% of the studies reviewed did not exclude patients with serum creatinine levels higher than 1.5 mg/dL. This equated to 37,360 patient-years of metformin use in studies that included patients with chronic kidney disease, and did not lead to lactic acidosis.
Even though metformin’s US package insert says that it is contraindicated if the serum creatinine level is 1.5 mg/dL or higher in men or 1.4 mg/dL or higher in women or if the creatinine clearance is “abnormal,” in view of the available evidence, many countries (eg, the United Kingdom, Australia, the Netherlands) now allow metformin to be used in patients with glomerular filtration rates as low as 30 mL/min/1.73m2, with lower doses if the glomerular filtration rate is lower than 45.5
The current contraindication to metformin in chronic kidney disease needs to be reviewed. In poor countries like India, this cheap medicine may be the only option available for treating type 2 diabetes mellitus, and it remains the first-line therapy for type 2 diabetes mellitus as recommended by the International Diabetes Federation, the American Diabetes Association, and the European Association for the Study of Diabetes.5
To the Editor: In their article about the care of patients with advanced chronic kidney disease, Sakhuja et al1 mentioned that metformin is contraindicated in chronic kidney disease.
Metformin is a good and useful drug. Not only is it one of the cheapest antidiabetic medications, it is the only one shown to reduce cardiovascular mortality rates in type 2 diabetes mellitus.
Although metformin is thought to increase the risk of lactic acidosis, a Cochrane review2 found that the incidence of lactic acidosis was only 4.3 cases per 100,000 patient-years in patients taking metformin, compared with 5.4 cases per 100,000 patient-years in patients not taking metformin. Furthermore, in a large registry of patients with type 2 diabetes and atherothrombosis,3 the rate of all-cause mortality was 24% lower in metformin users than in nonusers, and in those who had moderate renal impairment (creatinine clearance 30–59 mL/min/1.73 m2) the difference was 36%.3
A trial by Rachmani et al4 raised questions about the standard contraindications to metformin. The authors reviewed 393 patients who had at least one contraindication to metformin but who were receiving it anyway. Their serum creatinine levels ranged from 1.5 to 2.5 mg/dL. There were no cases of lactic acidosis reported. The patients were then randomized either to continue taking metformin or to stop taking it. At 2 years, the group that had stopped taking it had gained more weight, and their glycemic control was worse.
In the Cochrane analysis,2 although individual creatinine levels were not available, 53% of the studies reviewed did not exclude patients with serum creatinine levels higher than 1.5 mg/dL. This equated to 37,360 patient-years of metformin use in studies that included patients with chronic kidney disease, and did not lead to lactic acidosis.
Even though metformin’s US package insert says that it is contraindicated if the serum creatinine level is 1.5 mg/dL or higher in men or 1.4 mg/dL or higher in women or if the creatinine clearance is “abnormal,” in view of the available evidence, many countries (eg, the United Kingdom, Australia, the Netherlands) now allow metformin to be used in patients with glomerular filtration rates as low as 30 mL/min/1.73m2, with lower doses if the glomerular filtration rate is lower than 45.5
The current contraindication to metformin in chronic kidney disease needs to be reviewed. In poor countries like India, this cheap medicine may be the only option available for treating type 2 diabetes mellitus, and it remains the first-line therapy for type 2 diabetes mellitus as recommended by the International Diabetes Federation, the American Diabetes Association, and the European Association for the Study of Diabetes.5
- Sakhuja A, Hyland J, Simon JF. Managing advanced chronic kidney disease: a primary care guide. Cleve Clin J Med 2014; 81:289–299.
- Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitis. Cochrane Database Syst Rev 2010; 4:CD002967.
- Roussel R, Travert F, Pasquet B, et al; Reduction of Atherothrombosis for Continued Health (REACH) Registry Investigators. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 2010; 170:1892–1899.
- Rachmani R, Slavachevski I, Levi Z, Zadok B, Kedar Y, Ravid M. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. Eur J Intern Med 2002; 13:428–433.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Sakhuja A, Hyland J, Simon JF. Managing advanced chronic kidney disease: a primary care guide. Cleve Clin J Med 2014; 81:289–299.
- Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitis. Cochrane Database Syst Rev 2010; 4:CD002967.
- Roussel R, Travert F, Pasquet B, et al; Reduction of Atherothrombosis for Continued Health (REACH) Registry Investigators. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 2010; 170:1892–1899.
- Rachmani R, Slavachevski I, Levi Z, Zadok B, Kedar Y, Ravid M. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. Eur J Intern Med 2002; 13:428–433.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
In reply: Why is metformin contraindicated in chronic kidney disease?
In Reply: We appreciate Dr. Imam’s comments regarding using metformin in those with chronic kidney disease.
The US Food and Drug Administration currently lists metformin as contraindicated in those with mild to moderate renal insufficiency, with serum creatinine levels greater than or equal to 1.5 mg/dL in males and greater than or equal to 1.4 mg/dL in females. This contraindication is based on the pharmacokinetics of the medication and, likely, the association of a similar medication, phenformin, with lactic acidosis, which eventually led to its withdrawal from the market. However, lactic acidosis is much less frequent with metformin than with phenformin.1
We agree that metformin is an invaluable medication for diabetes mellitus not requiring insulin. We also agree that lactic acidosis is rare, especially in those with mild renal insufficiency. However, lactic acidosis does occur in patients with chronic kidney disease while on metformin and, however rare, when it does occur it is a life-threatening event.2
The clearance of metformin is strongly dependent on kidney function,3 and therefore guidelines still recommend reducing the dose in those with moderate renal insufficiency and recommend considering stopping the medication in those with severe renal insufficiency—the population we were talking about in our article.4 We are aware of changes to the guidelines that have been made by various groups, and in many circumstances we ourselves take an individualized approach, weighing the risks and benefits of continued therapy with the patient and his or her primary care provider. That being said, we did not believe that such nuanced recommendations were appropriate for our article, especially since they are contrary to marketing restrictions for the drug.
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334:574–579.
- Lalau JD, Race JM. Lactic acidosis in metformin-treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf 1999; 20:377–384.
- Sambol NC, Chiang J, Lin ET, et al. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol 1995; 35:1094–1102.
- Sakhuja A, Hyland J, Simon JF. Managing advanced chronic kidney disease: a primary care guide. Cleve Clin J Med 2014; 81:289–299.
In Reply: We appreciate Dr. Imam’s comments regarding using metformin in those with chronic kidney disease.
The US Food and Drug Administration currently lists metformin as contraindicated in those with mild to moderate renal insufficiency, with serum creatinine levels greater than or equal to 1.5 mg/dL in males and greater than or equal to 1.4 mg/dL in females. This contraindication is based on the pharmacokinetics of the medication and, likely, the association of a similar medication, phenformin, with lactic acidosis, which eventually led to its withdrawal from the market. However, lactic acidosis is much less frequent with metformin than with phenformin.1
We agree that metformin is an invaluable medication for diabetes mellitus not requiring insulin. We also agree that lactic acidosis is rare, especially in those with mild renal insufficiency. However, lactic acidosis does occur in patients with chronic kidney disease while on metformin and, however rare, when it does occur it is a life-threatening event.2
The clearance of metformin is strongly dependent on kidney function,3 and therefore guidelines still recommend reducing the dose in those with moderate renal insufficiency and recommend considering stopping the medication in those with severe renal insufficiency—the population we were talking about in our article.4 We are aware of changes to the guidelines that have been made by various groups, and in many circumstances we ourselves take an individualized approach, weighing the risks and benefits of continued therapy with the patient and his or her primary care provider. That being said, we did not believe that such nuanced recommendations were appropriate for our article, especially since they are contrary to marketing restrictions for the drug.
In Reply: We appreciate Dr. Imam’s comments regarding using metformin in those with chronic kidney disease.
The US Food and Drug Administration currently lists metformin as contraindicated in those with mild to moderate renal insufficiency, with serum creatinine levels greater than or equal to 1.5 mg/dL in males and greater than or equal to 1.4 mg/dL in females. This contraindication is based on the pharmacokinetics of the medication and, likely, the association of a similar medication, phenformin, with lactic acidosis, which eventually led to its withdrawal from the market. However, lactic acidosis is much less frequent with metformin than with phenformin.1
We agree that metformin is an invaluable medication for diabetes mellitus not requiring insulin. We also agree that lactic acidosis is rare, especially in those with mild renal insufficiency. However, lactic acidosis does occur in patients with chronic kidney disease while on metformin and, however rare, when it does occur it is a life-threatening event.2
The clearance of metformin is strongly dependent on kidney function,3 and therefore guidelines still recommend reducing the dose in those with moderate renal insufficiency and recommend considering stopping the medication in those with severe renal insufficiency—the population we were talking about in our article.4 We are aware of changes to the guidelines that have been made by various groups, and in many circumstances we ourselves take an individualized approach, weighing the risks and benefits of continued therapy with the patient and his or her primary care provider. That being said, we did not believe that such nuanced recommendations were appropriate for our article, especially since they are contrary to marketing restrictions for the drug.
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334:574–579.
- Lalau JD, Race JM. Lactic acidosis in metformin-treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf 1999; 20:377–384.
- Sambol NC, Chiang J, Lin ET, et al. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol 1995; 35:1094–1102.
- Sakhuja A, Hyland J, Simon JF. Managing advanced chronic kidney disease: a primary care guide. Cleve Clin J Med 2014; 81:289–299.
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334:574–579.
- Lalau JD, Race JM. Lactic acidosis in metformin-treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf 1999; 20:377–384.
- Sambol NC, Chiang J, Lin ET, et al. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol 1995; 35:1094–1102.
- Sakhuja A, Hyland J, Simon JF. Managing advanced chronic kidney disease: a primary care guide. Cleve Clin J Med 2014; 81:289–299.
Intensive glycemic control safely cut end-stage renal disease
VIENNA – Five years of intensive glycemic control in patients with type 2 diabetes safely halved long-term rate of end-stage renal disease, compared with placebo, in a multicenter study with about 8,000 patients, a finding that refutes prior suggestions that more intensive glycemic control can harm patients.
Intensive glucose control that produced an average hemoglobin A1c of 6.5% “is important for preventing serious renal complications and does not cause harm in patients with established type 2 diabetes,” Dr. Sophia Zoungas said at the annual meeting of the European Association for the Study of Diabetes.
Following the report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial in 2008 (N. Engl. J. Med. 2008;358;2545-59), which showed increased mortality with intensive glycemic control, compared with standard treatment, many physicians became leery of treating patients to a HbA1c level below 7%. But 5-year follow-up results of patients originally enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial (N. Engl. J. Med. 2008;358:2560-72) has now shown that a prolonged period maintaining patients at a HbA1c of roughly 6.5% produced no excess rates of total mortality, major macrovascular events, or major clinical microvascular events when compared with patients on standard care with an average HbA1c of 7.2%, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
“Whether the excess mortality seen in ACCORD was a true effect or not, there has been concern [about tight glycemic control] that has translated into where HbA1c targets have been set,” Dr. Zoungas said in an interview. “We have been blinded by this signal of increased fatal myocardial infarctions [seen in ACCORD] that many have been a chance effect.”
She suggested that the safety that intense glycemic control showed during 10-year follow-up of patients in the ADVANCE posttrial Observational Study (ADVANCE ON) may be explained by the more gradual HbA1c reductions achieved in the ADVANCE patients, compared with patients in ACCORD. In ADVANCE, patients’ HbA1c levels came down to about 6.5% over the course of a year, compared with a 3-6 month period in ACCORD, Dr. Zoungas noted.
In addition to establishing safety, the new results reported by Dr. Zoungas showed a statistically significant, 46% reduction in the incidence of end-stage renal disease (defined as progression to dialysis or need for kidney transplant) during the entire, 10-year follow-up. Simultaneous with Dr. Zoungas’s report at the meeting the ADVANCE ON results were published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407963]).
“This is the first time study results have shown that maintaining a HbA1cof less than 7% is associated with a long-term reduction in end-stage kidney disease,” she said. “End-stage kidney disease has been hard to study [as an endpoint] because the overall incidence is low, so patients need to be followed for a long time to see enough events.”
ADVANCE ON initially included 8,494 of the roughly 10,000 patients who completed the 5-year ADVANCE intervention trial at 215 centers in 20 countries. ADVANCE tested the impact of an antihypertensive intervention as well as the effect of intensified glycemic control in patients aged 55 years or older with type 2 diabetes and at least one other cardiovascular risk factor. At the time ADVANCE ended, average HbA1c levels were 7.2% in the control patients and 6.5% in those who had been on a more intensive hypoglycemic regimen. However, at the time patients began ADVANCE ON, their average HbA1c was 7.5%, regardless of which arm of ADVANCE they had been in, and the average level remained there through a median of 5 more years of follow-up. A total of 5,131 patients remained under study for the entire 10 years of follow-up. Analysis of the ADVANCE ON findings also showed a significant long-term effect from 5 years of added antihypertensive treatment, results reported in early September at the annual congress of the European Society of Cardiology.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
On Twitter @mitchelzoler
The ADVANCE ON results change our perspective on the medical treatment of patients with type 2 diabetes.
The results provide more evidence for the safety of metformin and sulfonylureas, two of the major drugs used on patients in the study during the intensive-management phase. This is not level I evidence, but it is evidence of safety. The results from ADVANCE ON as well as from other trials also suggest that these drugs reduce microvascular and macrovascular complications. The long-term legacy effect of intensive glycemic control to reduce the incidence of end-stage kidney disease was impressive. The hemoglobin A1c target established by existing management guidelines can remain in place.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Joachim Spranger |
The results also highlight our desperate need for glucose-lowering drugs that can substantially reduce fatal cardiovascular disease.
New drugs being considered for treatment of type 2 diabetes need to outperform metformin, sulfonylureas, and insulin for reducing clinically relevant endpoints. It is not enough for trials to show that new drugs can reduce glucose while causing fewer adverse effects than the older agents. Proven impact on clinical endpoints is also needed. We also must remember that the safety of treatment with three oral agents in a single regimen remains unproven.
Dr. Joachim Spranger is a professor of endocrinology at Charité Medical University in Berlin. He had no disclosures. He made these comments as the invited discussant for ADVANCE ON.
The ADVANCE ON results change our perspective on the medical treatment of patients with type 2 diabetes.
The results provide more evidence for the safety of metformin and sulfonylureas, two of the major drugs used on patients in the study during the intensive-management phase. This is not level I evidence, but it is evidence of safety. The results from ADVANCE ON as well as from other trials also suggest that these drugs reduce microvascular and macrovascular complications. The long-term legacy effect of intensive glycemic control to reduce the incidence of end-stage kidney disease was impressive. The hemoglobin A1c target established by existing management guidelines can remain in place.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Joachim Spranger |
The results also highlight our desperate need for glucose-lowering drugs that can substantially reduce fatal cardiovascular disease.
New drugs being considered for treatment of type 2 diabetes need to outperform metformin, sulfonylureas, and insulin for reducing clinically relevant endpoints. It is not enough for trials to show that new drugs can reduce glucose while causing fewer adverse effects than the older agents. Proven impact on clinical endpoints is also needed. We also must remember that the safety of treatment with three oral agents in a single regimen remains unproven.
Dr. Joachim Spranger is a professor of endocrinology at Charité Medical University in Berlin. He had no disclosures. He made these comments as the invited discussant for ADVANCE ON.
The ADVANCE ON results change our perspective on the medical treatment of patients with type 2 diabetes.
The results provide more evidence for the safety of metformin and sulfonylureas, two of the major drugs used on patients in the study during the intensive-management phase. This is not level I evidence, but it is evidence of safety. The results from ADVANCE ON as well as from other trials also suggest that these drugs reduce microvascular and macrovascular complications. The long-term legacy effect of intensive glycemic control to reduce the incidence of end-stage kidney disease was impressive. The hemoglobin A1c target established by existing management guidelines can remain in place.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Joachim Spranger |
The results also highlight our desperate need for glucose-lowering drugs that can substantially reduce fatal cardiovascular disease.
New drugs being considered for treatment of type 2 diabetes need to outperform metformin, sulfonylureas, and insulin for reducing clinically relevant endpoints. It is not enough for trials to show that new drugs can reduce glucose while causing fewer adverse effects than the older agents. Proven impact on clinical endpoints is also needed. We also must remember that the safety of treatment with three oral agents in a single regimen remains unproven.
Dr. Joachim Spranger is a professor of endocrinology at Charité Medical University in Berlin. He had no disclosures. He made these comments as the invited discussant for ADVANCE ON.
VIENNA – Five years of intensive glycemic control in patients with type 2 diabetes safely halved long-term rate of end-stage renal disease, compared with placebo, in a multicenter study with about 8,000 patients, a finding that refutes prior suggestions that more intensive glycemic control can harm patients.
Intensive glucose control that produced an average hemoglobin A1c of 6.5% “is important for preventing serious renal complications and does not cause harm in patients with established type 2 diabetes,” Dr. Sophia Zoungas said at the annual meeting of the European Association for the Study of Diabetes.
Following the report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial in 2008 (N. Engl. J. Med. 2008;358;2545-59), which showed increased mortality with intensive glycemic control, compared with standard treatment, many physicians became leery of treating patients to a HbA1c level below 7%. But 5-year follow-up results of patients originally enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial (N. Engl. J. Med. 2008;358:2560-72) has now shown that a prolonged period maintaining patients at a HbA1c of roughly 6.5% produced no excess rates of total mortality, major macrovascular events, or major clinical microvascular events when compared with patients on standard care with an average HbA1c of 7.2%, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
“Whether the excess mortality seen in ACCORD was a true effect or not, there has been concern [about tight glycemic control] that has translated into where HbA1c targets have been set,” Dr. Zoungas said in an interview. “We have been blinded by this signal of increased fatal myocardial infarctions [seen in ACCORD] that many have been a chance effect.”
She suggested that the safety that intense glycemic control showed during 10-year follow-up of patients in the ADVANCE posttrial Observational Study (ADVANCE ON) may be explained by the more gradual HbA1c reductions achieved in the ADVANCE patients, compared with patients in ACCORD. In ADVANCE, patients’ HbA1c levels came down to about 6.5% over the course of a year, compared with a 3-6 month period in ACCORD, Dr. Zoungas noted.
In addition to establishing safety, the new results reported by Dr. Zoungas showed a statistically significant, 46% reduction in the incidence of end-stage renal disease (defined as progression to dialysis or need for kidney transplant) during the entire, 10-year follow-up. Simultaneous with Dr. Zoungas’s report at the meeting the ADVANCE ON results were published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407963]).
“This is the first time study results have shown that maintaining a HbA1cof less than 7% is associated with a long-term reduction in end-stage kidney disease,” she said. “End-stage kidney disease has been hard to study [as an endpoint] because the overall incidence is low, so patients need to be followed for a long time to see enough events.”
ADVANCE ON initially included 8,494 of the roughly 10,000 patients who completed the 5-year ADVANCE intervention trial at 215 centers in 20 countries. ADVANCE tested the impact of an antihypertensive intervention as well as the effect of intensified glycemic control in patients aged 55 years or older with type 2 diabetes and at least one other cardiovascular risk factor. At the time ADVANCE ended, average HbA1c levels were 7.2% in the control patients and 6.5% in those who had been on a more intensive hypoglycemic regimen. However, at the time patients began ADVANCE ON, their average HbA1c was 7.5%, regardless of which arm of ADVANCE they had been in, and the average level remained there through a median of 5 more years of follow-up. A total of 5,131 patients remained under study for the entire 10 years of follow-up. Analysis of the ADVANCE ON findings also showed a significant long-term effect from 5 years of added antihypertensive treatment, results reported in early September at the annual congress of the European Society of Cardiology.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
On Twitter @mitchelzoler
VIENNA – Five years of intensive glycemic control in patients with type 2 diabetes safely halved long-term rate of end-stage renal disease, compared with placebo, in a multicenter study with about 8,000 patients, a finding that refutes prior suggestions that more intensive glycemic control can harm patients.
Intensive glucose control that produced an average hemoglobin A1c of 6.5% “is important for preventing serious renal complications and does not cause harm in patients with established type 2 diabetes,” Dr. Sophia Zoungas said at the annual meeting of the European Association for the Study of Diabetes.
Following the report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial in 2008 (N. Engl. J. Med. 2008;358;2545-59), which showed increased mortality with intensive glycemic control, compared with standard treatment, many physicians became leery of treating patients to a HbA1c level below 7%. But 5-year follow-up results of patients originally enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial (N. Engl. J. Med. 2008;358:2560-72) has now shown that a prolonged period maintaining patients at a HbA1c of roughly 6.5% produced no excess rates of total mortality, major macrovascular events, or major clinical microvascular events when compared with patients on standard care with an average HbA1c of 7.2%, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
“Whether the excess mortality seen in ACCORD was a true effect or not, there has been concern [about tight glycemic control] that has translated into where HbA1c targets have been set,” Dr. Zoungas said in an interview. “We have been blinded by this signal of increased fatal myocardial infarctions [seen in ACCORD] that many have been a chance effect.”
She suggested that the safety that intense glycemic control showed during 10-year follow-up of patients in the ADVANCE posttrial Observational Study (ADVANCE ON) may be explained by the more gradual HbA1c reductions achieved in the ADVANCE patients, compared with patients in ACCORD. In ADVANCE, patients’ HbA1c levels came down to about 6.5% over the course of a year, compared with a 3-6 month period in ACCORD, Dr. Zoungas noted.
In addition to establishing safety, the new results reported by Dr. Zoungas showed a statistically significant, 46% reduction in the incidence of end-stage renal disease (defined as progression to dialysis or need for kidney transplant) during the entire, 10-year follow-up. Simultaneous with Dr. Zoungas’s report at the meeting the ADVANCE ON results were published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407963]).
“This is the first time study results have shown that maintaining a HbA1cof less than 7% is associated with a long-term reduction in end-stage kidney disease,” she said. “End-stage kidney disease has been hard to study [as an endpoint] because the overall incidence is low, so patients need to be followed for a long time to see enough events.”
ADVANCE ON initially included 8,494 of the roughly 10,000 patients who completed the 5-year ADVANCE intervention trial at 215 centers in 20 countries. ADVANCE tested the impact of an antihypertensive intervention as well as the effect of intensified glycemic control in patients aged 55 years or older with type 2 diabetes and at least one other cardiovascular risk factor. At the time ADVANCE ended, average HbA1c levels were 7.2% in the control patients and 6.5% in those who had been on a more intensive hypoglycemic regimen. However, at the time patients began ADVANCE ON, their average HbA1c was 7.5%, regardless of which arm of ADVANCE they had been in, and the average level remained there through a median of 5 more years of follow-up. A total of 5,131 patients remained under study for the entire 10 years of follow-up. Analysis of the ADVANCE ON findings also showed a significant long-term effect from 5 years of added antihypertensive treatment, results reported in early September at the annual congress of the European Society of Cardiology.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
On Twitter @mitchelzoler
AT EASD 2014
Key clinical point: Intensive glycemic control in patients with type 2 diabetes safely led to a long-term reduction in end-stage kidney disease, compared with standard management.
Major finding: Intensive glycemic-control patients had a 46% reduced rate of end-stage kidney disease, compared with standard-care patients.
Data source: The ADVANCE ON study, which followed 8,494 patients after their participation in an international glycemia-control trial.
Disclosures: ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
Saxagliptin reverses proteinuria in type 2 diabetes
VIENNA – Patients with type 2 diabetes treated with the oral hypoglycemic saxagliptin (Onglyza) benefited from stabilization and, in some cases, reversal of urinary protein levels in a prespecified, secondary analysis of data collected from more than 16,000 patients in a randomized, international trial.
Progression of patients with diabetes from normoalbuminuria to microalbuminuria and then proteinuria tracks along with their deteriorating renal function. Until now, no agent has been identified that could stop or reverse this process, although a similar effect had been documented in results from a few prior, much smaller studies using other drugs from the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which includes saxagliptin.
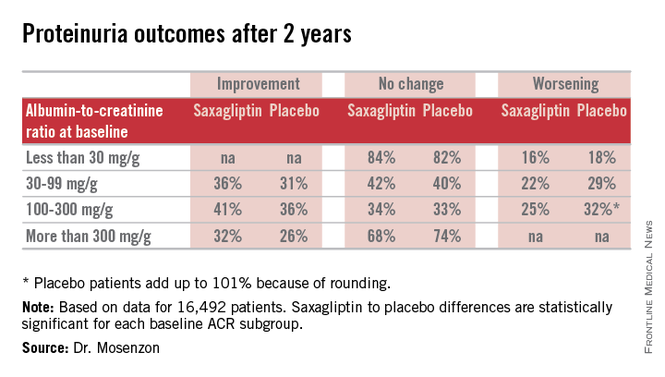
“It’s a very important finding that needs further investigation in longer-term studies so we can see whether the improvement in the [albumin to creatinine ratio (ACR)] will also mean less deterioration of the eGFR [estimated glomerular filtration rate] and thereby prevent end-stage renal disease,” Dr. Ofri Mosenzon said following her report at the annual meeting of the European Association for the Study of Diabetes.
The new analysis also showed that saxagliptin did this safely, without causing any renal damage, and that the effect on microalbuminuria and proteinuria was completely independent of the drug’s glycemic control effect measured by hemoglobin A1c levels. Dr. Mosenzon had no simple explanation for the mechanism by which saxagliptin and other DPP-4 inhibitors might exert this effect, though she suggested that an anti-inflammatory pleiotropic effect of the drug class might be involved.
“We have this clinical result. Now we need to look for an explanation,” said Dr. Mosenzon, a diabetologist at Hadassah Hospital in Jerusalem.
Her analysis used data collected in the the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which had the primary purpose of assessing the cardiovascular safety of saxagliptin in 16,492 patients with type 2 diabetes and a high risk for cardiovascular events (N. Engl. J. Med. 2013;369:1317-26). The study randomized patients to treatment with either 5 mg saxagliptin daily (2.5 mg daily in patients with impaired renal function at entry) or placebo in addition to whatever other standard medications their physicians prescribed. The secondary analysis of the impact of saxagliptin on proteinuria and renal function used prespecified definitions for evaluating the renal safety and renal-protective efficacy of treatment.
On the safety side, treatment with saxagliptin, compared with placebo linked with no statistically significant differences in the rate or magnitude of serum creatinine increases or in the rates of progression to dialysis or renal transplant.
For efficacy, treatment with saxagliptin, compared with placebo led to a consistent pattern of stabilization and reversal of the severity of microalbuminuria and proteinuria that cut across patients at all baseline levels of urinary protein (see table). For example, among 1,638 categorized as having proteinuria at entry into the study, based on an ACR of more than 300 mg/g, 32% of the patients treated with saxagliptin had improved to daily urinary protein excretions of less than 300 mg/g after an average 2.1 years on treatment, compared with a 26% rate in the placebo-treated control patients, a statistically significant difference. The extent of reversals and progressions prevented were also statistically significant among the 9,696 patients who entered the study with normoalbuminuria and urinary protein levels of less than 30 mg/g and those who entered with microalbuminuria and daily urinary protein levels of 30-300 mg/g.
Expressed another way, when compared with placebo-treated patients those patients treated with saxagliptin for 2 years who entered the study with an eGFR of more than 50 mL/min/1.73 m2 had on average 19 mg less daily protein in their urine, those who entered with an eGFR of 30-50 mL/min/1.73 m2 averaged 105 mg less daily urine protein, and those who entered with an eGFR of less than 30 mL/min/1.73 m2 averaged 245 mg less daily urine protein.
SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.
VIENNA – Patients with type 2 diabetes treated with the oral hypoglycemic saxagliptin (Onglyza) benefited from stabilization and, in some cases, reversal of urinary protein levels in a prespecified, secondary analysis of data collected from more than 16,000 patients in a randomized, international trial.
Progression of patients with diabetes from normoalbuminuria to microalbuminuria and then proteinuria tracks along with their deteriorating renal function. Until now, no agent has been identified that could stop or reverse this process, although a similar effect had been documented in results from a few prior, much smaller studies using other drugs from the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which includes saxagliptin.

“It’s a very important finding that needs further investigation in longer-term studies so we can see whether the improvement in the [albumin to creatinine ratio (ACR)] will also mean less deterioration of the eGFR [estimated glomerular filtration rate] and thereby prevent end-stage renal disease,” Dr. Ofri Mosenzon said following her report at the annual meeting of the European Association for the Study of Diabetes.
The new analysis also showed that saxagliptin did this safely, without causing any renal damage, and that the effect on microalbuminuria and proteinuria was completely independent of the drug’s glycemic control effect measured by hemoglobin A1c levels. Dr. Mosenzon had no simple explanation for the mechanism by which saxagliptin and other DPP-4 inhibitors might exert this effect, though she suggested that an anti-inflammatory pleiotropic effect of the drug class might be involved.
“We have this clinical result. Now we need to look for an explanation,” said Dr. Mosenzon, a diabetologist at Hadassah Hospital in Jerusalem.
Her analysis used data collected in the the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which had the primary purpose of assessing the cardiovascular safety of saxagliptin in 16,492 patients with type 2 diabetes and a high risk for cardiovascular events (N. Engl. J. Med. 2013;369:1317-26). The study randomized patients to treatment with either 5 mg saxagliptin daily (2.5 mg daily in patients with impaired renal function at entry) or placebo in addition to whatever other standard medications their physicians prescribed. The secondary analysis of the impact of saxagliptin on proteinuria and renal function used prespecified definitions for evaluating the renal safety and renal-protective efficacy of treatment.
On the safety side, treatment with saxagliptin, compared with placebo linked with no statistically significant differences in the rate or magnitude of serum creatinine increases or in the rates of progression to dialysis or renal transplant.
For efficacy, treatment with saxagliptin, compared with placebo led to a consistent pattern of stabilization and reversal of the severity of microalbuminuria and proteinuria that cut across patients at all baseline levels of urinary protein (see table). For example, among 1,638 categorized as having proteinuria at entry into the study, based on an ACR of more than 300 mg/g, 32% of the patients treated with saxagliptin had improved to daily urinary protein excretions of less than 300 mg/g after an average 2.1 years on treatment, compared with a 26% rate in the placebo-treated control patients, a statistically significant difference. The extent of reversals and progressions prevented were also statistically significant among the 9,696 patients who entered the study with normoalbuminuria and urinary protein levels of less than 30 mg/g and those who entered with microalbuminuria and daily urinary protein levels of 30-300 mg/g.
Expressed another way, when compared with placebo-treated patients those patients treated with saxagliptin for 2 years who entered the study with an eGFR of more than 50 mL/min/1.73 m2 had on average 19 mg less daily protein in their urine, those who entered with an eGFR of 30-50 mL/min/1.73 m2 averaged 105 mg less daily urine protein, and those who entered with an eGFR of less than 30 mL/min/1.73 m2 averaged 245 mg less daily urine protein.
SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.
VIENNA – Patients with type 2 diabetes treated with the oral hypoglycemic saxagliptin (Onglyza) benefited from stabilization and, in some cases, reversal of urinary protein levels in a prespecified, secondary analysis of data collected from more than 16,000 patients in a randomized, international trial.
Progression of patients with diabetes from normoalbuminuria to microalbuminuria and then proteinuria tracks along with their deteriorating renal function. Until now, no agent has been identified that could stop or reverse this process, although a similar effect had been documented in results from a few prior, much smaller studies using other drugs from the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which includes saxagliptin.

“It’s a very important finding that needs further investigation in longer-term studies so we can see whether the improvement in the [albumin to creatinine ratio (ACR)] will also mean less deterioration of the eGFR [estimated glomerular filtration rate] and thereby prevent end-stage renal disease,” Dr. Ofri Mosenzon said following her report at the annual meeting of the European Association for the Study of Diabetes.
The new analysis also showed that saxagliptin did this safely, without causing any renal damage, and that the effect on microalbuminuria and proteinuria was completely independent of the drug’s glycemic control effect measured by hemoglobin A1c levels. Dr. Mosenzon had no simple explanation for the mechanism by which saxagliptin and other DPP-4 inhibitors might exert this effect, though she suggested that an anti-inflammatory pleiotropic effect of the drug class might be involved.
“We have this clinical result. Now we need to look for an explanation,” said Dr. Mosenzon, a diabetologist at Hadassah Hospital in Jerusalem.
Her analysis used data collected in the the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which had the primary purpose of assessing the cardiovascular safety of saxagliptin in 16,492 patients with type 2 diabetes and a high risk for cardiovascular events (N. Engl. J. Med. 2013;369:1317-26). The study randomized patients to treatment with either 5 mg saxagliptin daily (2.5 mg daily in patients with impaired renal function at entry) or placebo in addition to whatever other standard medications their physicians prescribed. The secondary analysis of the impact of saxagliptin on proteinuria and renal function used prespecified definitions for evaluating the renal safety and renal-protective efficacy of treatment.
On the safety side, treatment with saxagliptin, compared with placebo linked with no statistically significant differences in the rate or magnitude of serum creatinine increases or in the rates of progression to dialysis or renal transplant.
For efficacy, treatment with saxagliptin, compared with placebo led to a consistent pattern of stabilization and reversal of the severity of microalbuminuria and proteinuria that cut across patients at all baseline levels of urinary protein (see table). For example, among 1,638 categorized as having proteinuria at entry into the study, based on an ACR of more than 300 mg/g, 32% of the patients treated with saxagliptin had improved to daily urinary protein excretions of less than 300 mg/g after an average 2.1 years on treatment, compared with a 26% rate in the placebo-treated control patients, a statistically significant difference. The extent of reversals and progressions prevented were also statistically significant among the 9,696 patients who entered the study with normoalbuminuria and urinary protein levels of less than 30 mg/g and those who entered with microalbuminuria and daily urinary protein levels of 30-300 mg/g.
Expressed another way, when compared with placebo-treated patients those patients treated with saxagliptin for 2 years who entered the study with an eGFR of more than 50 mL/min/1.73 m2 had on average 19 mg less daily protein in their urine, those who entered with an eGFR of 30-50 mL/min/1.73 m2 averaged 105 mg less daily urine protein, and those who entered with an eGFR of less than 30 mL/min/1.73 m2 averaged 245 mg less daily urine protein.
SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.
AT EASD 2014
Key clinical point: Treatment with saxagliptin led to stabilization and in some patients reversal of microalbuminuria and proteinuria in patients with type 2 diabetes.
Major finding: Proteinuria reversed in 32% of patients treated with saxagliptin during 2.1 years, compared with a 26% rate in controls.
Data source: Prespecified, secondary analysis of data from SAVOR-TIMI 53, an international, randomized trial with 16,492 patients with type 2 diabetes..
Disclosures: SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.