User login
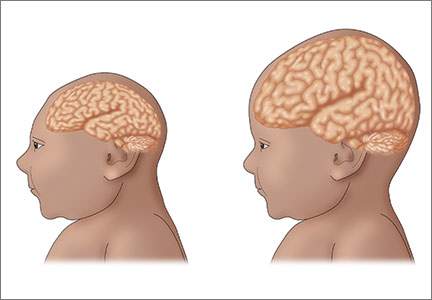
Brazilian study identifies fetal abnormalities linked to Zika virus
Fetal abnormalities were detected among more than a quarter of pregnant women who underwent ultrasound examinations after testing positive for Zika virus infection.
The small study, which included 88 pregnant women enrolled from September 2015 through February 2016 in Rio de Janeiro, was published online March 4 in the New England Journal of Medicine (doi: 10.1056/NEJMoa1602412).
“Our findings provide further support for a link between maternal ZIKV infection and fetal and placental abnormalities that is not unlike that of other viruses that are known to cause congenital infections characterized by intrauterine growth restriction and placental insufficiency,” investigators from Brazil and California reported.
The women in the study had developed a rash within the previous 5 days and were tested for Zika virus infection using reverse transcriptase polymerase chain reaction (RT-PCR) assays. Of the 88 women tested, 72 (82%) tested positive for Zika virus in blood, urine, or both.
Acute Zika infection was found throughout the course of pregnancy, though more than half of the women presented with acute infection during the second trimester. Along with a macular or maculopapular rash with pruritus, other distinctive clinical features of Zika virus infection included conjunctival injection, lymphadenopathy, and an absence of respiratory symptoms.
Two women who were positive for Zika virus had miscarriages during the first trimester. The investigators performed ultrasound for 42 of the remaining 70 women who had tested positive for Zika virus, as well as all women who tested negative for the virus. The other women who tested positive for Zika virus declined the imaging studies.
Fetal abnormalities were detected in 12 (29%) of the 42 women who were Zika virus positive and none of the women who had tested negative.
Among the 12 fetuses with abnormalities, there were two fetal deaths noted on ultrasound after 30 weeks of gestation. There were five fetuses with in utero growth restriction with or without microcephaly on ultrasound. Four fetuses had cerebral calcifications, and other central nervous system alterations were noted in two fetuses. Ultrasound detected abnormal arterial flow in the cerebral or umbilical arteries in four fetuses. Also, oligohydramnios and anhydramnios were seen in two fetuses.
At the time of this report, there had been six live births and two stillbirths among the study cohort and the ultrasound findings had been confirmed. The study was not supported by any research funds. The investigators reported having no financial disclosures.
On Twitter @maryellenny
Fetal abnormalities were detected among more than a quarter of pregnant women who underwent ultrasound examinations after testing positive for Zika virus infection.
The small study, which included 88 pregnant women enrolled from September 2015 through February 2016 in Rio de Janeiro, was published online March 4 in the New England Journal of Medicine (doi: 10.1056/NEJMoa1602412).
“Our findings provide further support for a link between maternal ZIKV infection and fetal and placental abnormalities that is not unlike that of other viruses that are known to cause congenital infections characterized by intrauterine growth restriction and placental insufficiency,” investigators from Brazil and California reported.
The women in the study had developed a rash within the previous 5 days and were tested for Zika virus infection using reverse transcriptase polymerase chain reaction (RT-PCR) assays. Of the 88 women tested, 72 (82%) tested positive for Zika virus in blood, urine, or both.
Acute Zika infection was found throughout the course of pregnancy, though more than half of the women presented with acute infection during the second trimester. Along with a macular or maculopapular rash with pruritus, other distinctive clinical features of Zika virus infection included conjunctival injection, lymphadenopathy, and an absence of respiratory symptoms.
Two women who were positive for Zika virus had miscarriages during the first trimester. The investigators performed ultrasound for 42 of the remaining 70 women who had tested positive for Zika virus, as well as all women who tested negative for the virus. The other women who tested positive for Zika virus declined the imaging studies.
Fetal abnormalities were detected in 12 (29%) of the 42 women who were Zika virus positive and none of the women who had tested negative.
Among the 12 fetuses with abnormalities, there were two fetal deaths noted on ultrasound after 30 weeks of gestation. There were five fetuses with in utero growth restriction with or without microcephaly on ultrasound. Four fetuses had cerebral calcifications, and other central nervous system alterations were noted in two fetuses. Ultrasound detected abnormal arterial flow in the cerebral or umbilical arteries in four fetuses. Also, oligohydramnios and anhydramnios were seen in two fetuses.
At the time of this report, there had been six live births and two stillbirths among the study cohort and the ultrasound findings had been confirmed. The study was not supported by any research funds. The investigators reported having no financial disclosures.
On Twitter @maryellenny
Fetal abnormalities were detected among more than a quarter of pregnant women who underwent ultrasound examinations after testing positive for Zika virus infection.
The small study, which included 88 pregnant women enrolled from September 2015 through February 2016 in Rio de Janeiro, was published online March 4 in the New England Journal of Medicine (doi: 10.1056/NEJMoa1602412).
“Our findings provide further support for a link between maternal ZIKV infection and fetal and placental abnormalities that is not unlike that of other viruses that are known to cause congenital infections characterized by intrauterine growth restriction and placental insufficiency,” investigators from Brazil and California reported.
The women in the study had developed a rash within the previous 5 days and were tested for Zika virus infection using reverse transcriptase polymerase chain reaction (RT-PCR) assays. Of the 88 women tested, 72 (82%) tested positive for Zika virus in blood, urine, or both.
Acute Zika infection was found throughout the course of pregnancy, though more than half of the women presented with acute infection during the second trimester. Along with a macular or maculopapular rash with pruritus, other distinctive clinical features of Zika virus infection included conjunctival injection, lymphadenopathy, and an absence of respiratory symptoms.
Two women who were positive for Zika virus had miscarriages during the first trimester. The investigators performed ultrasound for 42 of the remaining 70 women who had tested positive for Zika virus, as well as all women who tested negative for the virus. The other women who tested positive for Zika virus declined the imaging studies.
Fetal abnormalities were detected in 12 (29%) of the 42 women who were Zika virus positive and none of the women who had tested negative.
Among the 12 fetuses with abnormalities, there were two fetal deaths noted on ultrasound after 30 weeks of gestation. There were five fetuses with in utero growth restriction with or without microcephaly on ultrasound. Four fetuses had cerebral calcifications, and other central nervous system alterations were noted in two fetuses. Ultrasound detected abnormal arterial flow in the cerebral or umbilical arteries in four fetuses. Also, oligohydramnios and anhydramnios were seen in two fetuses.
At the time of this report, there had been six live births and two stillbirths among the study cohort and the ultrasound findings had been confirmed. The study was not supported by any research funds. The investigators reported having no financial disclosures.
On Twitter @maryellenny
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Zika virus infection in pregnancy appears to be associated with in utero growth restriction, central nervous system lesions, and fetal death.
Major finding: Fetal abnormalities were detected by ultrasound in 12 of 42 pregnant women who tested positive for Zika virus infection.
Data source: A prospective study of 88 pregnant women with a rash from September 2015 through February 2016 in Rio de Janeiro.
Disclosures: The study was not supported by any research funds. The investigators reported having no financial disclosures.
Breastfeeding discussions start with listening
The role that health care providers can and should play in promoting breastfeeding has come under scrutiny in recent years, often leaving doctors uncertain about how to discuss infant-feeding intentions with patients.
There’s been a backlash against the public health promotion of breastfeeding and “lactivism,” with critics saying that the efforts lead to shame or guilt in women who do not breastfeed. And often mothers, and their physicians, have been caught in the crossfire.
The American College of Obstetricians and Gynecologists attempted to build a bridge across this divide in their updated Committee Opinion on breastfeeding in February.
In a departure from the language typically included in policy statements or clinical guidelines from other medical organizations, the new ACOG guidelines urged ob.gyns. and other obstetric care providers to “support each woman’s informed decision about whether to initiate or continue breastfeeding, recognizing that she is uniquely qualified to decide whether exclusive breastfeeding, mixed feeding, or formula feeding is optimal for her and her infant.”
At the same time, however, the organization also “recommends exclusive breastfeeding for the first 6 months of life, with continued breastfeeding as complementary foods are introduced through the infant’s first year of life, or longer as mutually desired by the woman and her infant.”
Striking the right balance in providing women with adequate information to make informed choices without inadvertently causing a woman discomfort requires clinicians to start by finding out what their patients already know, according to Dr. Alison Stuebe, lead author of the ACOG opinion and an assistant professor of maternal-fetal medicine at the University of North Carolina at Chapel Hill.
“The clinician’s role is to help that mom make an informed decision, and it’s hard to help her do that if you don’t know where she’s coming from,” Dr. Stuebe said. “It’s important not to assume that a woman knows everything or knows nothing.”
How to start the conversation
Dr. Stuebe, who is also a distinguished scholar of infant and young child feeding in the Gillings School of Global Public Health at UNC, recommended bringing up the subject of breastfeeding early in a woman’s prenatal care with a simple open-ended question: “What have you heard about breastfeeding?” The answer helps tailor the counseling to the mother’s knowledge, feelings, and attitudes.
“We have some moms who have read 47 books on breastfeeding, and then there are women who live in a family where they’ve only seen bottle feeding,” Dr. Stuebe said.
Next, validate what the mother says, and ask for more information from the mom. When the conversation begins early in the physician-patient relationship, there is time to have the conversation over several visits, Dr. Stuebe said.
“I think asking the question in a nonjudgmental, truly open-ended way and listening to what mom says and paraphrasing it back to her hopefully helps her feel comfortable enough to go into a bit more detail,” she said. “I don’t ask people to commit. I take notes and we talk about it at another visit.”
Dr. Stuebe also suggested making sure mothers are aware of the benefits of breastfeeding for their own health – such as a reduced risk of type 2 diabetes and breast cancer – so that the conversation is not framed entirely in terms of benefits for their child.
Fear of upsetting a patient, however, should not dissuade physicians from broaching the subject, she added. “In the fear of making moms feel bad, we sometimes tiptoe and miss an opportunity to provide moms with an opportunity to make an informed choice.”
That would be especially unfortunate given the respect individuals continue to have for advice from their physicians, said Lora Ebert Wallace, Ph.D., professor of sociology at Western Illinois University in Macomb, who has studied the impact of language used in breastfeeding discourse.
“Medical authority is a real thing,” Dr. Wallace said. “People listen to their doctors and respect them, and doctors want to be really thoughtful about how to communicate, starting with questions instead of prescriptions.”
Avoiding ‘risk’ language
In reality, much of the backlash against breastfeeding prescriptivism has not involved the ob.gyn. community, Dr. Wallace noted.
“I think generally ob.gyns. have not been on the forefront of the type of advocacy that people have objected to,” she said. “I think that’s come from other areas of medicine.”
Some of that advocacy has employed “risk language,” in which breastfeeding is presented as the only appropriate choice and the conversation centers around the “risk” of formula feeding instead of the “benefits” of breastfeeding.
“Our research suggests that the use of risk language is premature at this point because it has not been well evaluated, and the evaluations that have been done suggest that it doesn’t increase breastfeeding among people exposed to it,” Dr. Wallace said. “There is some suggestion from qualitative research that you can create a backlash to the information.”
The thinking behind risk language is that using stronger language to encourage breastfeeding will somehow make more women choose to do it, but such a rationale ignores the fact that parents are already trying to do the absolute best they can for their children, Dr. Wallace said.
“I don’t think the research supports the idea that women aren’t breastfeeding because they don’t know it’s good for their babies,” she said. “They’re not breastfeeding because it’s hard because of the way we structure our society and our workplaces.”
Another statement to avoid is “every woman can breastfeed,” said Laura Lallande, the lactation services coordinator at Oregon Health and Science University, Portland.
“There are real, legitimate physical reasons some women cannot or choose not to breastfeed, and we need to stop propagating the myth that formula feeding is equivalent to moral failure,” Ms. Lallande said. “As with anything in health care, our job is to meet clients where they are, not where we want them to be. If we start from a point of judgment, we block progress before it starts.”
Potential sources of shame
It is the “everyone can if you try hard enough” language that can lead to shame, Dr. Stuebe said.
The feelings of shame some women may feel if they don’t breastfeed can arise from the inappropriate conflation of breastfeeding and being a good mother. “Particularly for first-time mothers, the transition from what I want to be as a parent to what I can be as a parent is wrenching for some women,” Dr. Stuebe said.
The social infrastructure in the United States means that breastfeeding is not actually a “choice” for all women, Dr. Stuebe said. This reality is reflected in the ACOG statement, which encourages ob.gyns. to “be in the forefront of policy efforts to enable women to breastfeed, whether through individual patient education, change in hospital practices, community efforts, or supportive legislation” and to promote policies that accommodate milk expression, such as paid maternity leave, on-site child care, break time, and a location other than a bathroom for expressing milk.
Even the way the health care system is set up makes it hard for mothers to get holistic care, Dr. Stuebe said.
“What happens is moms get conflicting advice from [their] provider and the baby’s provider, and sometimes even from a third source such as a lactation consultant, and they’re left trying to triangulate that information,” Dr. Stuebe said. “Nobody is saying, ‘How is this whole mother doing and how can we meet her needs?’ ”
That’s why it’s important to follow up with patients and ask how breastfeeding is going, Dr. Stuebe explained. If it’s not working out, women need to know it’s okay to stop.
“Breast milk is important, but a woman’s well-being is also important and if everything about breastfeeding is awful, that’s not helping her or her baby,” Dr. Stuebe said.
Physicians have a responsibility to tell women that breastfeeding is advantageous, Dr. Wallace said, but they also have a responsibility to listen to patients and be sensitive to what they’re hearing.
“To the parent in the moment, if they’re facing something really important about employment or housing, the breastfeeding decision may not look as important to them,” Dr. Wallace said.
Yet ob.gyns. should never discount how critical their role is in helping mothers successfully breastfeed if they choose to, Ms. Lallande said.
“Even when things are great, breastfeeding is physically and emotionally challenging,” Ms. Lallande said. “Women need support from providers who listen to them and help them navigate the sleep-deprived early weeks of motherhood. Especially with first-time moms, the relationship with the OB is much stronger than the relationship with the pediatrician, so they call the OB for help.”
The role that health care providers can and should play in promoting breastfeeding has come under scrutiny in recent years, often leaving doctors uncertain about how to discuss infant-feeding intentions with patients.
There’s been a backlash against the public health promotion of breastfeeding and “lactivism,” with critics saying that the efforts lead to shame or guilt in women who do not breastfeed. And often mothers, and their physicians, have been caught in the crossfire.
The American College of Obstetricians and Gynecologists attempted to build a bridge across this divide in their updated Committee Opinion on breastfeeding in February.
In a departure from the language typically included in policy statements or clinical guidelines from other medical organizations, the new ACOG guidelines urged ob.gyns. and other obstetric care providers to “support each woman’s informed decision about whether to initiate or continue breastfeeding, recognizing that she is uniquely qualified to decide whether exclusive breastfeeding, mixed feeding, or formula feeding is optimal for her and her infant.”
At the same time, however, the organization also “recommends exclusive breastfeeding for the first 6 months of life, with continued breastfeeding as complementary foods are introduced through the infant’s first year of life, or longer as mutually desired by the woman and her infant.”
Striking the right balance in providing women with adequate information to make informed choices without inadvertently causing a woman discomfort requires clinicians to start by finding out what their patients already know, according to Dr. Alison Stuebe, lead author of the ACOG opinion and an assistant professor of maternal-fetal medicine at the University of North Carolina at Chapel Hill.
“The clinician’s role is to help that mom make an informed decision, and it’s hard to help her do that if you don’t know where she’s coming from,” Dr. Stuebe said. “It’s important not to assume that a woman knows everything or knows nothing.”
How to start the conversation
Dr. Stuebe, who is also a distinguished scholar of infant and young child feeding in the Gillings School of Global Public Health at UNC, recommended bringing up the subject of breastfeeding early in a woman’s prenatal care with a simple open-ended question: “What have you heard about breastfeeding?” The answer helps tailor the counseling to the mother’s knowledge, feelings, and attitudes.
“We have some moms who have read 47 books on breastfeeding, and then there are women who live in a family where they’ve only seen bottle feeding,” Dr. Stuebe said.
Next, validate what the mother says, and ask for more information from the mom. When the conversation begins early in the physician-patient relationship, there is time to have the conversation over several visits, Dr. Stuebe said.
“I think asking the question in a nonjudgmental, truly open-ended way and listening to what mom says and paraphrasing it back to her hopefully helps her feel comfortable enough to go into a bit more detail,” she said. “I don’t ask people to commit. I take notes and we talk about it at another visit.”
Dr. Stuebe also suggested making sure mothers are aware of the benefits of breastfeeding for their own health – such as a reduced risk of type 2 diabetes and breast cancer – so that the conversation is not framed entirely in terms of benefits for their child.
Fear of upsetting a patient, however, should not dissuade physicians from broaching the subject, she added. “In the fear of making moms feel bad, we sometimes tiptoe and miss an opportunity to provide moms with an opportunity to make an informed choice.”
That would be especially unfortunate given the respect individuals continue to have for advice from their physicians, said Lora Ebert Wallace, Ph.D., professor of sociology at Western Illinois University in Macomb, who has studied the impact of language used in breastfeeding discourse.
“Medical authority is a real thing,” Dr. Wallace said. “People listen to their doctors and respect them, and doctors want to be really thoughtful about how to communicate, starting with questions instead of prescriptions.”
Avoiding ‘risk’ language
In reality, much of the backlash against breastfeeding prescriptivism has not involved the ob.gyn. community, Dr. Wallace noted.
“I think generally ob.gyns. have not been on the forefront of the type of advocacy that people have objected to,” she said. “I think that’s come from other areas of medicine.”
Some of that advocacy has employed “risk language,” in which breastfeeding is presented as the only appropriate choice and the conversation centers around the “risk” of formula feeding instead of the “benefits” of breastfeeding.
“Our research suggests that the use of risk language is premature at this point because it has not been well evaluated, and the evaluations that have been done suggest that it doesn’t increase breastfeeding among people exposed to it,” Dr. Wallace said. “There is some suggestion from qualitative research that you can create a backlash to the information.”
The thinking behind risk language is that using stronger language to encourage breastfeeding will somehow make more women choose to do it, but such a rationale ignores the fact that parents are already trying to do the absolute best they can for their children, Dr. Wallace said.
“I don’t think the research supports the idea that women aren’t breastfeeding because they don’t know it’s good for their babies,” she said. “They’re not breastfeeding because it’s hard because of the way we structure our society and our workplaces.”
Another statement to avoid is “every woman can breastfeed,” said Laura Lallande, the lactation services coordinator at Oregon Health and Science University, Portland.
“There are real, legitimate physical reasons some women cannot or choose not to breastfeed, and we need to stop propagating the myth that formula feeding is equivalent to moral failure,” Ms. Lallande said. “As with anything in health care, our job is to meet clients where they are, not where we want them to be. If we start from a point of judgment, we block progress before it starts.”
Potential sources of shame
It is the “everyone can if you try hard enough” language that can lead to shame, Dr. Stuebe said.
The feelings of shame some women may feel if they don’t breastfeed can arise from the inappropriate conflation of breastfeeding and being a good mother. “Particularly for first-time mothers, the transition from what I want to be as a parent to what I can be as a parent is wrenching for some women,” Dr. Stuebe said.
The social infrastructure in the United States means that breastfeeding is not actually a “choice” for all women, Dr. Stuebe said. This reality is reflected in the ACOG statement, which encourages ob.gyns. to “be in the forefront of policy efforts to enable women to breastfeed, whether through individual patient education, change in hospital practices, community efforts, or supportive legislation” and to promote policies that accommodate milk expression, such as paid maternity leave, on-site child care, break time, and a location other than a bathroom for expressing milk.
Even the way the health care system is set up makes it hard for mothers to get holistic care, Dr. Stuebe said.
“What happens is moms get conflicting advice from [their] provider and the baby’s provider, and sometimes even from a third source such as a lactation consultant, and they’re left trying to triangulate that information,” Dr. Stuebe said. “Nobody is saying, ‘How is this whole mother doing and how can we meet her needs?’ ”
That’s why it’s important to follow up with patients and ask how breastfeeding is going, Dr. Stuebe explained. If it’s not working out, women need to know it’s okay to stop.
“Breast milk is important, but a woman’s well-being is also important and if everything about breastfeeding is awful, that’s not helping her or her baby,” Dr. Stuebe said.
Physicians have a responsibility to tell women that breastfeeding is advantageous, Dr. Wallace said, but they also have a responsibility to listen to patients and be sensitive to what they’re hearing.
“To the parent in the moment, if they’re facing something really important about employment or housing, the breastfeeding decision may not look as important to them,” Dr. Wallace said.
Yet ob.gyns. should never discount how critical their role is in helping mothers successfully breastfeed if they choose to, Ms. Lallande said.
“Even when things are great, breastfeeding is physically and emotionally challenging,” Ms. Lallande said. “Women need support from providers who listen to them and help them navigate the sleep-deprived early weeks of motherhood. Especially with first-time moms, the relationship with the OB is much stronger than the relationship with the pediatrician, so they call the OB for help.”
The role that health care providers can and should play in promoting breastfeeding has come under scrutiny in recent years, often leaving doctors uncertain about how to discuss infant-feeding intentions with patients.
There’s been a backlash against the public health promotion of breastfeeding and “lactivism,” with critics saying that the efforts lead to shame or guilt in women who do not breastfeed. And often mothers, and their physicians, have been caught in the crossfire.
The American College of Obstetricians and Gynecologists attempted to build a bridge across this divide in their updated Committee Opinion on breastfeeding in February.
In a departure from the language typically included in policy statements or clinical guidelines from other medical organizations, the new ACOG guidelines urged ob.gyns. and other obstetric care providers to “support each woman’s informed decision about whether to initiate or continue breastfeeding, recognizing that she is uniquely qualified to decide whether exclusive breastfeeding, mixed feeding, or formula feeding is optimal for her and her infant.”
At the same time, however, the organization also “recommends exclusive breastfeeding for the first 6 months of life, with continued breastfeeding as complementary foods are introduced through the infant’s first year of life, or longer as mutually desired by the woman and her infant.”
Striking the right balance in providing women with adequate information to make informed choices without inadvertently causing a woman discomfort requires clinicians to start by finding out what their patients already know, according to Dr. Alison Stuebe, lead author of the ACOG opinion and an assistant professor of maternal-fetal medicine at the University of North Carolina at Chapel Hill.
“The clinician’s role is to help that mom make an informed decision, and it’s hard to help her do that if you don’t know where she’s coming from,” Dr. Stuebe said. “It’s important not to assume that a woman knows everything or knows nothing.”
How to start the conversation
Dr. Stuebe, who is also a distinguished scholar of infant and young child feeding in the Gillings School of Global Public Health at UNC, recommended bringing up the subject of breastfeeding early in a woman’s prenatal care with a simple open-ended question: “What have you heard about breastfeeding?” The answer helps tailor the counseling to the mother’s knowledge, feelings, and attitudes.
“We have some moms who have read 47 books on breastfeeding, and then there are women who live in a family where they’ve only seen bottle feeding,” Dr. Stuebe said.
Next, validate what the mother says, and ask for more information from the mom. When the conversation begins early in the physician-patient relationship, there is time to have the conversation over several visits, Dr. Stuebe said.
“I think asking the question in a nonjudgmental, truly open-ended way and listening to what mom says and paraphrasing it back to her hopefully helps her feel comfortable enough to go into a bit more detail,” she said. “I don’t ask people to commit. I take notes and we talk about it at another visit.”
Dr. Stuebe also suggested making sure mothers are aware of the benefits of breastfeeding for their own health – such as a reduced risk of type 2 diabetes and breast cancer – so that the conversation is not framed entirely in terms of benefits for their child.
Fear of upsetting a patient, however, should not dissuade physicians from broaching the subject, she added. “In the fear of making moms feel bad, we sometimes tiptoe and miss an opportunity to provide moms with an opportunity to make an informed choice.”
That would be especially unfortunate given the respect individuals continue to have for advice from their physicians, said Lora Ebert Wallace, Ph.D., professor of sociology at Western Illinois University in Macomb, who has studied the impact of language used in breastfeeding discourse.
“Medical authority is a real thing,” Dr. Wallace said. “People listen to their doctors and respect them, and doctors want to be really thoughtful about how to communicate, starting with questions instead of prescriptions.”
Avoiding ‘risk’ language
In reality, much of the backlash against breastfeeding prescriptivism has not involved the ob.gyn. community, Dr. Wallace noted.
“I think generally ob.gyns. have not been on the forefront of the type of advocacy that people have objected to,” she said. “I think that’s come from other areas of medicine.”
Some of that advocacy has employed “risk language,” in which breastfeeding is presented as the only appropriate choice and the conversation centers around the “risk” of formula feeding instead of the “benefits” of breastfeeding.
“Our research suggests that the use of risk language is premature at this point because it has not been well evaluated, and the evaluations that have been done suggest that it doesn’t increase breastfeeding among people exposed to it,” Dr. Wallace said. “There is some suggestion from qualitative research that you can create a backlash to the information.”
The thinking behind risk language is that using stronger language to encourage breastfeeding will somehow make more women choose to do it, but such a rationale ignores the fact that parents are already trying to do the absolute best they can for their children, Dr. Wallace said.
“I don’t think the research supports the idea that women aren’t breastfeeding because they don’t know it’s good for their babies,” she said. “They’re not breastfeeding because it’s hard because of the way we structure our society and our workplaces.”
Another statement to avoid is “every woman can breastfeed,” said Laura Lallande, the lactation services coordinator at Oregon Health and Science University, Portland.
“There are real, legitimate physical reasons some women cannot or choose not to breastfeed, and we need to stop propagating the myth that formula feeding is equivalent to moral failure,” Ms. Lallande said. “As with anything in health care, our job is to meet clients where they are, not where we want them to be. If we start from a point of judgment, we block progress before it starts.”
Potential sources of shame
It is the “everyone can if you try hard enough” language that can lead to shame, Dr. Stuebe said.
The feelings of shame some women may feel if they don’t breastfeed can arise from the inappropriate conflation of breastfeeding and being a good mother. “Particularly for first-time mothers, the transition from what I want to be as a parent to what I can be as a parent is wrenching for some women,” Dr. Stuebe said.
The social infrastructure in the United States means that breastfeeding is not actually a “choice” for all women, Dr. Stuebe said. This reality is reflected in the ACOG statement, which encourages ob.gyns. to “be in the forefront of policy efforts to enable women to breastfeed, whether through individual patient education, change in hospital practices, community efforts, or supportive legislation” and to promote policies that accommodate milk expression, such as paid maternity leave, on-site child care, break time, and a location other than a bathroom for expressing milk.
Even the way the health care system is set up makes it hard for mothers to get holistic care, Dr. Stuebe said.
“What happens is moms get conflicting advice from [their] provider and the baby’s provider, and sometimes even from a third source such as a lactation consultant, and they’re left trying to triangulate that information,” Dr. Stuebe said. “Nobody is saying, ‘How is this whole mother doing and how can we meet her needs?’ ”
That’s why it’s important to follow up with patients and ask how breastfeeding is going, Dr. Stuebe explained. If it’s not working out, women need to know it’s okay to stop.
“Breast milk is important, but a woman’s well-being is also important and if everything about breastfeeding is awful, that’s not helping her or her baby,” Dr. Stuebe said.
Physicians have a responsibility to tell women that breastfeeding is advantageous, Dr. Wallace said, but they also have a responsibility to listen to patients and be sensitive to what they’re hearing.
“To the parent in the moment, if they’re facing something really important about employment or housing, the breastfeeding decision may not look as important to them,” Dr. Wallace said.
Yet ob.gyns. should never discount how critical their role is in helping mothers successfully breastfeed if they choose to, Ms. Lallande said.
“Even when things are great, breastfeeding is physically and emotionally challenging,” Ms. Lallande said. “Women need support from providers who listen to them and help them navigate the sleep-deprived early weeks of motherhood. Especially with first-time moms, the relationship with the OB is much stronger than the relationship with the pediatrician, so they call the OB for help.”
Is expectant management a safe alternative to immediate delivery in patients with PPROM close to term?
Preterm premature rupture of membranes (PPROM) refers to rupture of membranes prior to the onset of labor before 37 weeks’ gestation. It accounts for one-third of all preterm births.1 Pregnancy complications associated with PPROM include intrauterine infection (chorioamnionitis), preterm labor, and placental abruption. Should such complications develop, immediate delivery is indicated. When to recommend elective delivery in the absence of complications, however, remains controversial.
The American College of Obstetricians and Gynecologists (ACOG) currently recommends elective delivery at or after 34 weeks’ gestation,2 because the prevailing evidence suggests that the risk of pregnancy-related complications (especially ascending infection) exceeds the risks of iatrogenic prematurity at this gestational age. However, ACOG acknowledges that this recommendation is based on “limited and inconsistent scientific evidence.”2 To address deficiencies in the literature, investigators designed the PPROMT (preterm prelabor rupture of the membranes close to term) trial to study women with ruptured membranes before the onset of labor between 34 and 37 weeks’ gestation.
PPROMT study designMorris and colleagues present results of their multicenter, international, randomized controlled trial (RCT) of expectant management versus planned delivery in pregnancies complicated by PPROM at 34 0/7 through 36 6/7 weeks’ gestation carried out in 65 centers across 11 countries. A total of 1,839 women not requiring urgent delivery were randomly assigned to either immediate delivery (n = 924) or expectant management (n = 915).
No difference was noted in the primary outcome of neonatal sepsis between the immediate birth (n = 23 [2%]) and expectant management groups (n = 29 [3%]; relative risk [RR], 0.8; 95% confidence interval [CI], 0.5–1.3). This also was true in the subgroup of women who were colonized with group B streptococcus (RR, 0.9; 95% CI, 0.2–4.5).
There also was no difference in the secondary outcome measure, a composite metric including sepsis, ventilation for 24 or more hours, or death (73 [8%] in the immediate delivery group vs 61 [7%] in the expectant management group; RR, 1.2; 95% CI, 0.9–1.6). However, infants born to women randomly assigned to immediate delivery, versus expectant management, had a significantly higher rate of respiratory distress syndrome (RR, 1.6; 95% CI, 1.1–2.3) and mechanical ventilation (RR, 1.4; 95% CI, 1.0–1.8). In addition, the immediate-delivery infants had a longer median stay in the special care nursery/neonatal intensive care unit (4.0 days, interquartile range [IQR], 0.0–10.0 vs 2.0 days, IQR, 0.0–7.0) and total hospital stay (6.0 days, IQR, 3.0–10.0 vs 4.0 days, IQR, 3.0–8.0). As expected, women in the expectant management group had a significantly longer hospital stay than women in the immediate delivery group, because 75% (688/912) were managed as inpatients. Interestingly, women in the immediate delivery group had a higher cesarean delivery rate than those in the expectant management group (239 [26%] vs 169 [19%], respectively; RR, 1.4; 95% CI, 1.2–1.7), although no explanation was offered.
Strengths and limitationsMajor strengths of this study include the large sample size and superior study design. It is by far the largest RCT to address this question. Because this was a pragmatic RCT, certain practices (such as the choice of latency antibiotic regimen) varied across centers, although randomization would be expected to minimize the effect of such variables on study outcome.
A major limitation is that participant recruitment occurred over a period of more than 10 years, during which time antenatal and neonatal intensive care unit practices likely would have changed.
What this evidence means for practiceFew clinical studies have the potential to significantly change obstetric management. This report by Morris and colleagues is one such study. It was well designed, well executed, and powered to look at the most clinically relevant outcome, namely, neonatal sepsis. While these study results do call into question the current American College of Obstetricians and Gynecologists recommendations to electively deliver patients with PPROM at or after 34 weeks’ gestation, additional discussion is needed at the national level before these recommendations can be changed.
—Denis A. Vaughan, MBBCh, BAO, MRCPI, and Errol R. Norwitz, MD, PhD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med. 1998;339(5):313–320.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 160: premature rupture of membranes. Obstet Gynecol. 2016;127(1):192–194.
Preterm premature rupture of membranes (PPROM) refers to rupture of membranes prior to the onset of labor before 37 weeks’ gestation. It accounts for one-third of all preterm births.1 Pregnancy complications associated with PPROM include intrauterine infection (chorioamnionitis), preterm labor, and placental abruption. Should such complications develop, immediate delivery is indicated. When to recommend elective delivery in the absence of complications, however, remains controversial.
The American College of Obstetricians and Gynecologists (ACOG) currently recommends elective delivery at or after 34 weeks’ gestation,2 because the prevailing evidence suggests that the risk of pregnancy-related complications (especially ascending infection) exceeds the risks of iatrogenic prematurity at this gestational age. However, ACOG acknowledges that this recommendation is based on “limited and inconsistent scientific evidence.”2 To address deficiencies in the literature, investigators designed the PPROMT (preterm prelabor rupture of the membranes close to term) trial to study women with ruptured membranes before the onset of labor between 34 and 37 weeks’ gestation.
PPROMT study designMorris and colleagues present results of their multicenter, international, randomized controlled trial (RCT) of expectant management versus planned delivery in pregnancies complicated by PPROM at 34 0/7 through 36 6/7 weeks’ gestation carried out in 65 centers across 11 countries. A total of 1,839 women not requiring urgent delivery were randomly assigned to either immediate delivery (n = 924) or expectant management (n = 915).
No difference was noted in the primary outcome of neonatal sepsis between the immediate birth (n = 23 [2%]) and expectant management groups (n = 29 [3%]; relative risk [RR], 0.8; 95% confidence interval [CI], 0.5–1.3). This also was true in the subgroup of women who were colonized with group B streptococcus (RR, 0.9; 95% CI, 0.2–4.5).
There also was no difference in the secondary outcome measure, a composite metric including sepsis, ventilation for 24 or more hours, or death (73 [8%] in the immediate delivery group vs 61 [7%] in the expectant management group; RR, 1.2; 95% CI, 0.9–1.6). However, infants born to women randomly assigned to immediate delivery, versus expectant management, had a significantly higher rate of respiratory distress syndrome (RR, 1.6; 95% CI, 1.1–2.3) and mechanical ventilation (RR, 1.4; 95% CI, 1.0–1.8). In addition, the immediate-delivery infants had a longer median stay in the special care nursery/neonatal intensive care unit (4.0 days, interquartile range [IQR], 0.0–10.0 vs 2.0 days, IQR, 0.0–7.0) and total hospital stay (6.0 days, IQR, 3.0–10.0 vs 4.0 days, IQR, 3.0–8.0). As expected, women in the expectant management group had a significantly longer hospital stay than women in the immediate delivery group, because 75% (688/912) were managed as inpatients. Interestingly, women in the immediate delivery group had a higher cesarean delivery rate than those in the expectant management group (239 [26%] vs 169 [19%], respectively; RR, 1.4; 95% CI, 1.2–1.7), although no explanation was offered.
Strengths and limitationsMajor strengths of this study include the large sample size and superior study design. It is by far the largest RCT to address this question. Because this was a pragmatic RCT, certain practices (such as the choice of latency antibiotic regimen) varied across centers, although randomization would be expected to minimize the effect of such variables on study outcome.
A major limitation is that participant recruitment occurred over a period of more than 10 years, during which time antenatal and neonatal intensive care unit practices likely would have changed.
What this evidence means for practiceFew clinical studies have the potential to significantly change obstetric management. This report by Morris and colleagues is one such study. It was well designed, well executed, and powered to look at the most clinically relevant outcome, namely, neonatal sepsis. While these study results do call into question the current American College of Obstetricians and Gynecologists recommendations to electively deliver patients with PPROM at or after 34 weeks’ gestation, additional discussion is needed at the national level before these recommendations can be changed.
—Denis A. Vaughan, MBBCh, BAO, MRCPI, and Errol R. Norwitz, MD, PhD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Preterm premature rupture of membranes (PPROM) refers to rupture of membranes prior to the onset of labor before 37 weeks’ gestation. It accounts for one-third of all preterm births.1 Pregnancy complications associated with PPROM include intrauterine infection (chorioamnionitis), preterm labor, and placental abruption. Should such complications develop, immediate delivery is indicated. When to recommend elective delivery in the absence of complications, however, remains controversial.
The American College of Obstetricians and Gynecologists (ACOG) currently recommends elective delivery at or after 34 weeks’ gestation,2 because the prevailing evidence suggests that the risk of pregnancy-related complications (especially ascending infection) exceeds the risks of iatrogenic prematurity at this gestational age. However, ACOG acknowledges that this recommendation is based on “limited and inconsistent scientific evidence.”2 To address deficiencies in the literature, investigators designed the PPROMT (preterm prelabor rupture of the membranes close to term) trial to study women with ruptured membranes before the onset of labor between 34 and 37 weeks’ gestation.
PPROMT study designMorris and colleagues present results of their multicenter, international, randomized controlled trial (RCT) of expectant management versus planned delivery in pregnancies complicated by PPROM at 34 0/7 through 36 6/7 weeks’ gestation carried out in 65 centers across 11 countries. A total of 1,839 women not requiring urgent delivery were randomly assigned to either immediate delivery (n = 924) or expectant management (n = 915).
No difference was noted in the primary outcome of neonatal sepsis between the immediate birth (n = 23 [2%]) and expectant management groups (n = 29 [3%]; relative risk [RR], 0.8; 95% confidence interval [CI], 0.5–1.3). This also was true in the subgroup of women who were colonized with group B streptococcus (RR, 0.9; 95% CI, 0.2–4.5).
There also was no difference in the secondary outcome measure, a composite metric including sepsis, ventilation for 24 or more hours, or death (73 [8%] in the immediate delivery group vs 61 [7%] in the expectant management group; RR, 1.2; 95% CI, 0.9–1.6). However, infants born to women randomly assigned to immediate delivery, versus expectant management, had a significantly higher rate of respiratory distress syndrome (RR, 1.6; 95% CI, 1.1–2.3) and mechanical ventilation (RR, 1.4; 95% CI, 1.0–1.8). In addition, the immediate-delivery infants had a longer median stay in the special care nursery/neonatal intensive care unit (4.0 days, interquartile range [IQR], 0.0–10.0 vs 2.0 days, IQR, 0.0–7.0) and total hospital stay (6.0 days, IQR, 3.0–10.0 vs 4.0 days, IQR, 3.0–8.0). As expected, women in the expectant management group had a significantly longer hospital stay than women in the immediate delivery group, because 75% (688/912) were managed as inpatients. Interestingly, women in the immediate delivery group had a higher cesarean delivery rate than those in the expectant management group (239 [26%] vs 169 [19%], respectively; RR, 1.4; 95% CI, 1.2–1.7), although no explanation was offered.
Strengths and limitationsMajor strengths of this study include the large sample size and superior study design. It is by far the largest RCT to address this question. Because this was a pragmatic RCT, certain practices (such as the choice of latency antibiotic regimen) varied across centers, although randomization would be expected to minimize the effect of such variables on study outcome.
A major limitation is that participant recruitment occurred over a period of more than 10 years, during which time antenatal and neonatal intensive care unit practices likely would have changed.
What this evidence means for practiceFew clinical studies have the potential to significantly change obstetric management. This report by Morris and colleagues is one such study. It was well designed, well executed, and powered to look at the most clinically relevant outcome, namely, neonatal sepsis. While these study results do call into question the current American College of Obstetricians and Gynecologists recommendations to electively deliver patients with PPROM at or after 34 weeks’ gestation, additional discussion is needed at the national level before these recommendations can be changed.
—Denis A. Vaughan, MBBCh, BAO, MRCPI, and Errol R. Norwitz, MD, PhD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med. 1998;339(5):313–320.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 160: premature rupture of membranes. Obstet Gynecol. 2016;127(1):192–194.
- Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med. 1998;339(5):313–320.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 160: premature rupture of membranes. Obstet Gynecol. 2016;127(1):192–194.
WHO guidance for caring for pregnant women in Zika virus areas
The World Health Organization has released guidance for physicians and other healthcare providers on how to care for pregnant women in areas where Zika virus transmission is ongoing.
“The guidance is intended to inform the development of national and local clinical protocols and health policies that relate to pregnancy care in the context of Zika virus transmission,” according to the document, released on March 2.
The WHO does not recommend testing all pregnant women in Zika endemic areas, but suggests that physicians consider offering a first-trimester ultrasound scan to all women presenting for antenatal care to accurately date the pregnancy and perform a basic fetal morphology assessment. Women should also be counseled to present early for treatment and diagnostic work-up if they develop any signs or symptoms of Zika virus infection, including conjunctivitis, joint pain, headache, muscle pain, and fatigue.
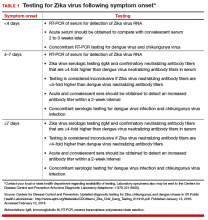
Pregnant women who have signs of infection or a history of Zika virus disease should be tested. The following steps can be taken to diagnose the disease:
• Using reverse transcription polymerase chain reaction in maternal serum within 5 days of onset of symptoms.
• Urine analysis within 3 weeks after the onset of symptoms.
• Saliva analysis.
• Serological tests with immunoglobulin M antibodies from the fifth day following onset of symptoms.
The WHO also recommends routinely performing investigations to exclude syphilis, toxoplasmosis, cytomegalovirus, rubella, and herpes.
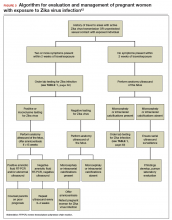
Later in the pregnancy, all women should be offered an 18-20 week anomaly scan to identify, monitor, or exclude fetal brain abnormalities.
Any pregnant women with possible Zika virus and fetal microcephaly and/or brain abnormalities should be referred for specialized care.
The WHO’s recommendations were produced under the agency’s emergency procedures and will remain valid until August, at which time the Department of Reproductive Health and Research at WHO Geneva will renew or update them as appropriate.
The complete guidance is available here.
The World Health Organization has released guidance for physicians and other healthcare providers on how to care for pregnant women in areas where Zika virus transmission is ongoing.
“The guidance is intended to inform the development of national and local clinical protocols and health policies that relate to pregnancy care in the context of Zika virus transmission,” according to the document, released on March 2.
The WHO does not recommend testing all pregnant women in Zika endemic areas, but suggests that physicians consider offering a first-trimester ultrasound scan to all women presenting for antenatal care to accurately date the pregnancy and perform a basic fetal morphology assessment. Women should also be counseled to present early for treatment and diagnostic work-up if they develop any signs or symptoms of Zika virus infection, including conjunctivitis, joint pain, headache, muscle pain, and fatigue.
Pregnant women who have signs of infection or a history of Zika virus disease should be tested. The following steps can be taken to diagnose the disease:
• Using reverse transcription polymerase chain reaction in maternal serum within 5 days of onset of symptoms.
• Urine analysis within 3 weeks after the onset of symptoms.
• Saliva analysis.
• Serological tests with immunoglobulin M antibodies from the fifth day following onset of symptoms.
The WHO also recommends routinely performing investigations to exclude syphilis, toxoplasmosis, cytomegalovirus, rubella, and herpes.
Later in the pregnancy, all women should be offered an 18-20 week anomaly scan to identify, monitor, or exclude fetal brain abnormalities.
Any pregnant women with possible Zika virus and fetal microcephaly and/or brain abnormalities should be referred for specialized care.
The WHO’s recommendations were produced under the agency’s emergency procedures and will remain valid until August, at which time the Department of Reproductive Health and Research at WHO Geneva will renew or update them as appropriate.
The complete guidance is available here.
The World Health Organization has released guidance for physicians and other healthcare providers on how to care for pregnant women in areas where Zika virus transmission is ongoing.
“The guidance is intended to inform the development of national and local clinical protocols and health policies that relate to pregnancy care in the context of Zika virus transmission,” according to the document, released on March 2.
The WHO does not recommend testing all pregnant women in Zika endemic areas, but suggests that physicians consider offering a first-trimester ultrasound scan to all women presenting for antenatal care to accurately date the pregnancy and perform a basic fetal morphology assessment. Women should also be counseled to present early for treatment and diagnostic work-up if they develop any signs or symptoms of Zika virus infection, including conjunctivitis, joint pain, headache, muscle pain, and fatigue.
Pregnant women who have signs of infection or a history of Zika virus disease should be tested. The following steps can be taken to diagnose the disease:
• Using reverse transcription polymerase chain reaction in maternal serum within 5 days of onset of symptoms.
• Urine analysis within 3 weeks after the onset of symptoms.
• Saliva analysis.
• Serological tests with immunoglobulin M antibodies from the fifth day following onset of symptoms.
The WHO also recommends routinely performing investigations to exclude syphilis, toxoplasmosis, cytomegalovirus, rubella, and herpes.
Later in the pregnancy, all women should be offered an 18-20 week anomaly scan to identify, monitor, or exclude fetal brain abnormalities.
Any pregnant women with possible Zika virus and fetal microcephaly and/or brain abnormalities should be referred for specialized care.
The WHO’s recommendations were produced under the agency’s emergency procedures and will remain valid until August, at which time the Department of Reproductive Health and Research at WHO Geneva will renew or update them as appropriate.
The complete guidance is available here.
Postpartum life-threatening strep infection
Postpartum life-threatening strep infection
A pregnant woman received prenatal care from a midwifery practice. A week before her scheduled delivery, the patient became ill with fever and vomiting and visited her midwife. While tests were still pending, the midwife decided to admit the mother to the hospital for induction of labor. The baby was born by vaginal delivery under the midwife’s care. The mother remained in the hospital for observation.
Two days after delivery, the mother began to have nausea, vomiting, and a low-grade fever. The nurse called the midwife, who ordered acetaminophen (Tylenol) but did not come to examine the patient. Two hours later, the nurse notified the midwife that the patient’s condition had worsened and that she was experiencing abdominal pain; the midwife ordered oxycodone. Over the next few hours, the midwife was apprised of the patient’s condition several times by telephone, but she never came to examine the patient nor did she ask her supervising ObGyn to examine the patient.
The next morning, a second midwife noted that the patient was experiencing an itchy rash on her extremities and abdomen. A complete blood count (CBC) showed a “critical lab value” of 44% band neutrophils (normal, 0% to 10% for the hospital laboratory). The second midwife and nurse told the supervising ObGyn that the patient otherwise looked well; he discharged the patient.
At home, the patient’s condition worsened. Her husband called the ObGyn several times and took her to the emergency department (ED) that evening. Her condition deteriorated and she was transferred to another facility where she was diagnosed with a life-threatening Group A Streptococcus (GAS) infection. After weeks of treatment for sepsis, the patient’s foot was amputated.
Patient's claim: The first midwife was negligent in her postpartum treatment of the patient; she should have come to the hospital to examine the patient or have requested that the supervising ObGyn examine the patient. The rash and CBC test results should have initiated further treatment and investigation; the patient should not have been discharged. GAS was not found or treated in a timely manner, resulting in sepsis and amputation.
Defendants' defense: The case was settled during the trial.
Verdict: A $2,500,000 Massachusetts settlement was reached with the midwife, her practice, and the ObGyn.
Failure to follow-up on abnormal Pap
A woman in her 50s reported abnormal bleeding to her gynecologist. Results of an endometrial biopsy were negative for cancer; the gynecologist prescribed hormone therapy. The patient continued to bleed until she entered menopause.
Ten years later, the bleeding returned. Results of a Pap test indicated atypical endometrial cells; an ultrasound showed a markedly abnormal endometrium. The gynecologist recommended a hysteroscopic dilation and curettage (D&C). When he attempted the procedure it ended prematurely because he was unable to enter the patient’s endometrium. The patient’s discharge instructions indicated that she should call the physician for follow up. In a letter to the patient written a month later, the physician discussed the abnormal Pap test results and indicated that the patient had 2 options: another D&C under ultrasound guidance or hysterectomy. He also noted that he would contact the patient’s primary care physician (PCP) for input.
Two years later, the patient returned to the gynecologist because the bleeding, which had never stopped, had increased in intensity. Endometrial cancer was diagnosed.
Patient's claim: The gynecologist never followed up with the patient or her PCP after the incomplete D&C. There is no record that communication ever occurred between the gynecologist and PCP. Lack of follow-up and treatment resulted in progression of the cancer from stage 1 to stage 3C, with a 5-year survivability of 47% (stage 1 survivability is 83%).
Physician's defense: The gynecologist was surprised that no one had ever followed up with the patient. The patient was comparatively negligent for failing to seek medical care for the 2-year period.
Verdict: A $430,000 Minnesota settlement was reached at mediation.
LIVER DISEASE LED TO STILLBIRTH
A 37-year-old woman reported nausea, vomiting, headaches, heartburn, and upper abdominal pain to her ObGyn several times during her third trimester. She had been pregnant before and knew that this pregnancy “felt” different. She went to the ED 1 week before the birth of her child, but she was discharged. The child was stillborn.
Parent's claim: Neither the ObGyn who provided prenatal care nor the on-call ED ObGyn ordered laboratory testing, which would have revealed a rare disease: acute fatty liver of pregnancy. Action could have saved the life of her child.
The patient’s ObGyn disregarded the patient’s reported symptoms; no blood work or liver testing was done. The ObGyn should have recognized the symptoms of liver disease that presented during the third trimester. A diagnosis of liver disease would have initiated induction of labor.
The patient’s expert witness noted that the severity of the third trimester symptoms warranted follow-up testing; the patient should not have had all of those symptoms so late in pregnancy. Testing would have revealed that, by not functioning properly, the liver was creating a toxic environment for the fetus. Labor should have been induced at 36 weeks when the fetal heart testing was still normal.
The ED nurses contacted the on-call ObGyn by telephone to discuss the patient’s symptoms; the ObGyn did not come to the ED to examine the patient or order testing.
The patient suffered emotional distress as a result of the loss of her child.
Defendants' defense: The medical center and the on-call ObGyn settled prior to trial.
The ObGyn claimed that the patient’s symptoms were common for pregnancy and that the disease could not be diagnosed based on the presented symptoms. It was not a violation of the standard of care for the extremely rare liver disease to not be diagnosed. The defense’s expert claimed that the symptoms reported by the patient did not warrant follow-up blood work. There was no way to determine whether or not the fetus died as a result of the mother’s liver disease or nuchal cord involvement.
A placental pathologist noted that the placenta was injured by thrombosis; the fetus’ death was most likely idiopathic. He later acknowledged that thrombosis can be related to liver disease.
Verdict: Jurors were instructed to consider this a personal injury case for the mother due to an unborn fetus’ lacks standing for injury or death under California law. A $160,090 California verdict was returned against the ObGyn who provided prenatal care.
Postpartum life-threatening strep infection
A pregnant woman received prenatal care from a midwifery practice. A week before her scheduled delivery, the patient became ill with fever and vomiting and visited her midwife. While tests were still pending, the midwife decided to admit the mother to the hospital for induction of labor. The baby was born by vaginal delivery under the midwife’s care. The mother remained in the hospital for observation.
Two days after delivery, the mother began to have nausea, vomiting, and a low-grade fever. The nurse called the midwife, who ordered acetaminophen (Tylenol) but did not come to examine the patient. Two hours later, the nurse notified the midwife that the patient’s condition had worsened and that she was experiencing abdominal pain; the midwife ordered oxycodone. Over the next few hours, the midwife was apprised of the patient’s condition several times by telephone, but she never came to examine the patient nor did she ask her supervising ObGyn to examine the patient.
The next morning, a second midwife noted that the patient was experiencing an itchy rash on her extremities and abdomen. A complete blood count (CBC) showed a “critical lab value” of 44% band neutrophils (normal, 0% to 10% for the hospital laboratory). The second midwife and nurse told the supervising ObGyn that the patient otherwise looked well; he discharged the patient.
At home, the patient’s condition worsened. Her husband called the ObGyn several times and took her to the emergency department (ED) that evening. Her condition deteriorated and she was transferred to another facility where she was diagnosed with a life-threatening Group A Streptococcus (GAS) infection. After weeks of treatment for sepsis, the patient’s foot was amputated.
Patient's claim: The first midwife was negligent in her postpartum treatment of the patient; she should have come to the hospital to examine the patient or have requested that the supervising ObGyn examine the patient. The rash and CBC test results should have initiated further treatment and investigation; the patient should not have been discharged. GAS was not found or treated in a timely manner, resulting in sepsis and amputation.
Defendants' defense: The case was settled during the trial.
Verdict: A $2,500,000 Massachusetts settlement was reached with the midwife, her practice, and the ObGyn.
Failure to follow-up on abnormal Pap
A woman in her 50s reported abnormal bleeding to her gynecologist. Results of an endometrial biopsy were negative for cancer; the gynecologist prescribed hormone therapy. The patient continued to bleed until she entered menopause.
Ten years later, the bleeding returned. Results of a Pap test indicated atypical endometrial cells; an ultrasound showed a markedly abnormal endometrium. The gynecologist recommended a hysteroscopic dilation and curettage (D&C). When he attempted the procedure it ended prematurely because he was unable to enter the patient’s endometrium. The patient’s discharge instructions indicated that she should call the physician for follow up. In a letter to the patient written a month later, the physician discussed the abnormal Pap test results and indicated that the patient had 2 options: another D&C under ultrasound guidance or hysterectomy. He also noted that he would contact the patient’s primary care physician (PCP) for input.
Two years later, the patient returned to the gynecologist because the bleeding, which had never stopped, had increased in intensity. Endometrial cancer was diagnosed.
Patient's claim: The gynecologist never followed up with the patient or her PCP after the incomplete D&C. There is no record that communication ever occurred between the gynecologist and PCP. Lack of follow-up and treatment resulted in progression of the cancer from stage 1 to stage 3C, with a 5-year survivability of 47% (stage 1 survivability is 83%).
Physician's defense: The gynecologist was surprised that no one had ever followed up with the patient. The patient was comparatively negligent for failing to seek medical care for the 2-year period.
Verdict: A $430,000 Minnesota settlement was reached at mediation.
LIVER DISEASE LED TO STILLBIRTH
A 37-year-old woman reported nausea, vomiting, headaches, heartburn, and upper abdominal pain to her ObGyn several times during her third trimester. She had been pregnant before and knew that this pregnancy “felt” different. She went to the ED 1 week before the birth of her child, but she was discharged. The child was stillborn.
Parent's claim: Neither the ObGyn who provided prenatal care nor the on-call ED ObGyn ordered laboratory testing, which would have revealed a rare disease: acute fatty liver of pregnancy. Action could have saved the life of her child.
The patient’s ObGyn disregarded the patient’s reported symptoms; no blood work or liver testing was done. The ObGyn should have recognized the symptoms of liver disease that presented during the third trimester. A diagnosis of liver disease would have initiated induction of labor.
The patient’s expert witness noted that the severity of the third trimester symptoms warranted follow-up testing; the patient should not have had all of those symptoms so late in pregnancy. Testing would have revealed that, by not functioning properly, the liver was creating a toxic environment for the fetus. Labor should have been induced at 36 weeks when the fetal heart testing was still normal.
The ED nurses contacted the on-call ObGyn by telephone to discuss the patient’s symptoms; the ObGyn did not come to the ED to examine the patient or order testing.
The patient suffered emotional distress as a result of the loss of her child.
Defendants' defense: The medical center and the on-call ObGyn settled prior to trial.
The ObGyn claimed that the patient’s symptoms were common for pregnancy and that the disease could not be diagnosed based on the presented symptoms. It was not a violation of the standard of care for the extremely rare liver disease to not be diagnosed. The defense’s expert claimed that the symptoms reported by the patient did not warrant follow-up blood work. There was no way to determine whether or not the fetus died as a result of the mother’s liver disease or nuchal cord involvement.
A placental pathologist noted that the placenta was injured by thrombosis; the fetus’ death was most likely idiopathic. He later acknowledged that thrombosis can be related to liver disease.
Verdict: Jurors were instructed to consider this a personal injury case for the mother due to an unborn fetus’ lacks standing for injury or death under California law. A $160,090 California verdict was returned against the ObGyn who provided prenatal care.
Postpartum life-threatening strep infection
A pregnant woman received prenatal care from a midwifery practice. A week before her scheduled delivery, the patient became ill with fever and vomiting and visited her midwife. While tests were still pending, the midwife decided to admit the mother to the hospital for induction of labor. The baby was born by vaginal delivery under the midwife’s care. The mother remained in the hospital for observation.
Two days after delivery, the mother began to have nausea, vomiting, and a low-grade fever. The nurse called the midwife, who ordered acetaminophen (Tylenol) but did not come to examine the patient. Two hours later, the nurse notified the midwife that the patient’s condition had worsened and that she was experiencing abdominal pain; the midwife ordered oxycodone. Over the next few hours, the midwife was apprised of the patient’s condition several times by telephone, but she never came to examine the patient nor did she ask her supervising ObGyn to examine the patient.
The next morning, a second midwife noted that the patient was experiencing an itchy rash on her extremities and abdomen. A complete blood count (CBC) showed a “critical lab value” of 44% band neutrophils (normal, 0% to 10% for the hospital laboratory). The second midwife and nurse told the supervising ObGyn that the patient otherwise looked well; he discharged the patient.
At home, the patient’s condition worsened. Her husband called the ObGyn several times and took her to the emergency department (ED) that evening. Her condition deteriorated and she was transferred to another facility where she was diagnosed with a life-threatening Group A Streptococcus (GAS) infection. After weeks of treatment for sepsis, the patient’s foot was amputated.
Patient's claim: The first midwife was negligent in her postpartum treatment of the patient; she should have come to the hospital to examine the patient or have requested that the supervising ObGyn examine the patient. The rash and CBC test results should have initiated further treatment and investigation; the patient should not have been discharged. GAS was not found or treated in a timely manner, resulting in sepsis and amputation.
Defendants' defense: The case was settled during the trial.
Verdict: A $2,500,000 Massachusetts settlement was reached with the midwife, her practice, and the ObGyn.
Failure to follow-up on abnormal Pap
A woman in her 50s reported abnormal bleeding to her gynecologist. Results of an endometrial biopsy were negative for cancer; the gynecologist prescribed hormone therapy. The patient continued to bleed until she entered menopause.
Ten years later, the bleeding returned. Results of a Pap test indicated atypical endometrial cells; an ultrasound showed a markedly abnormal endometrium. The gynecologist recommended a hysteroscopic dilation and curettage (D&C). When he attempted the procedure it ended prematurely because he was unable to enter the patient’s endometrium. The patient’s discharge instructions indicated that she should call the physician for follow up. In a letter to the patient written a month later, the physician discussed the abnormal Pap test results and indicated that the patient had 2 options: another D&C under ultrasound guidance or hysterectomy. He also noted that he would contact the patient’s primary care physician (PCP) for input.
Two years later, the patient returned to the gynecologist because the bleeding, which had never stopped, had increased in intensity. Endometrial cancer was diagnosed.
Patient's claim: The gynecologist never followed up with the patient or her PCP after the incomplete D&C. There is no record that communication ever occurred between the gynecologist and PCP. Lack of follow-up and treatment resulted in progression of the cancer from stage 1 to stage 3C, with a 5-year survivability of 47% (stage 1 survivability is 83%).
Physician's defense: The gynecologist was surprised that no one had ever followed up with the patient. The patient was comparatively negligent for failing to seek medical care for the 2-year period.
Verdict: A $430,000 Minnesota settlement was reached at mediation.
LIVER DISEASE LED TO STILLBIRTH
A 37-year-old woman reported nausea, vomiting, headaches, heartburn, and upper abdominal pain to her ObGyn several times during her third trimester. She had been pregnant before and knew that this pregnancy “felt” different. She went to the ED 1 week before the birth of her child, but she was discharged. The child was stillborn.
Parent's claim: Neither the ObGyn who provided prenatal care nor the on-call ED ObGyn ordered laboratory testing, which would have revealed a rare disease: acute fatty liver of pregnancy. Action could have saved the life of her child.
The patient’s ObGyn disregarded the patient’s reported symptoms; no blood work or liver testing was done. The ObGyn should have recognized the symptoms of liver disease that presented during the third trimester. A diagnosis of liver disease would have initiated induction of labor.
The patient’s expert witness noted that the severity of the third trimester symptoms warranted follow-up testing; the patient should not have had all of those symptoms so late in pregnancy. Testing would have revealed that, by not functioning properly, the liver was creating a toxic environment for the fetus. Labor should have been induced at 36 weeks when the fetal heart testing was still normal.
The ED nurses contacted the on-call ObGyn by telephone to discuss the patient’s symptoms; the ObGyn did not come to the ED to examine the patient or order testing.
The patient suffered emotional distress as a result of the loss of her child.
Defendants' defense: The medical center and the on-call ObGyn settled prior to trial.
The ObGyn claimed that the patient’s symptoms were common for pregnancy and that the disease could not be diagnosed based on the presented symptoms. It was not a violation of the standard of care for the extremely rare liver disease to not be diagnosed. The defense’s expert claimed that the symptoms reported by the patient did not warrant follow-up blood work. There was no way to determine whether or not the fetus died as a result of the mother’s liver disease or nuchal cord involvement.
A placental pathologist noted that the placenta was injured by thrombosis; the fetus’ death was most likely idiopathic. He later acknowledged that thrombosis can be related to liver disease.
Verdict: Jurors were instructed to consider this a personal injury case for the mother due to an unborn fetus’ lacks standing for injury or death under California law. A $160,090 California verdict was returned against the ObGyn who provided prenatal care.
Additional Medical Verdicts
• Failure to follow-up on abnormal Pap
• Liver disease led to stillbirth
Induction at 39 Weeks Doesn’t Affect C-section Rate in Older Women
For women aged 35 years and older, induction of labor at 39 weeks neither raised nor lowered the rate of cesarean delivery, compared with expectant management, in a randomized clinical trial in the United Kingdom.
Labor induction at or before the due date may be beneficial in older women “because the gestational age at delivery that is associated with the lowest cumulative risk of perinatal death is 38 weeks.” However, induction in general is associated with an increased rate of cesarean delivery.
Previous studies of the relative benefits and harms of labor induction in this age group haven’t shed much light on the issue; they were small, observational in design, and were performed in the 1970s, so their findings may not be applicable to modern obstetric practice, wrote Dr. Kate F. Walker of University of Nottingham, England, and her associates.
They tested the hypothesis that labor induction at 39 weeks would reduce the rate of cesarean delivery in nulliparous women of advanced maternal age in the 35/39 Trial. It involved 619 women aged 35 years and older with singleton, uncomplicated pregnancies who were treated during a 2.5-year period at 39 secondary- and tertiary-care medical centers across the United Kingdom. The study participants were randomly assigned late in their pregnancies to either labor induction at 39 weeks (304 women) or expectant management (314 women).
The study hypothesis was not proven: there was no significant difference in the frequency of cesarean section between the induction group (32%) and the expectant management group (33%), for a relative risk of 0.99.
There also were no significant differences between the two study groups in maternal outcomes or neonatal outcomes (N Engl J Med. 2016 Mar 2;374:813-22. doi: 10.1056/NEJMoa1509117).
The trial did not address whether induction at 39 weeks’ gestation could prevent stillbirth, the researchers reported, but it provides support for the safety of performing a larger trial to test the effects of induction on stillbirths and other adverse neonatal outcomes.
The women assigned to labor induction did not differ from those assigned to expectant management in their subjective assessments of satisfaction with the childbirth experience. However, all the study participants had agreed to randomization. Women who had strong preferences about induction or expectant management, or strong feelings about their involvement in treatment decisions – and who may therefore have had a different take on their childbirth experience if labor were induced – were excluded from the study.
The study was supported by the National Institute for Health Research in the United Kingdom. Dr. Walker reported having no relevant financial disclosures; one of her associates reported ties to Roche Diagnostics, GlaxoSmithKline, General Electric, Chiesi, and Action Medical Research.
The findings of Walker et al. suggest that a fundamental belief that guides our decisions about the timing of delivery – namely, that induction of labor at term increases the risk of cesarean delivery – may not be true after all.

|
Dr. William A, Grobman |
However, the authors note that larger studies of the issue are needed to confirm these findings and to further explore the effects of induction on maternal and neonatal outcomes, especially stillbirth and other uncommon adverse outcomes. I am the principal investigator of such a trial (NCT01990612), which is currently underway within the Maternal–Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
This trial, which has a targeted enrollment of 6,000 women, is designed to identify differences in perinatal outcomes among nulliparous women with uncomplicated singleton pregnancies who are randomly assigned to induction between 39 weeks 0 days and 39 weeks 4 days of gestation or to expectant management. The trial is more than halfway complete.
Dr. William A. Grobman is at Northwestern University, Chicago. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 March 2. doi: 10.1056/NEJMe1516461).
The findings of Walker et al. suggest that a fundamental belief that guides our decisions about the timing of delivery – namely, that induction of labor at term increases the risk of cesarean delivery – may not be true after all.

|
Dr. William A, Grobman |
However, the authors note that larger studies of the issue are needed to confirm these findings and to further explore the effects of induction on maternal and neonatal outcomes, especially stillbirth and other uncommon adverse outcomes. I am the principal investigator of such a trial (NCT01990612), which is currently underway within the Maternal–Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
This trial, which has a targeted enrollment of 6,000 women, is designed to identify differences in perinatal outcomes among nulliparous women with uncomplicated singleton pregnancies who are randomly assigned to induction between 39 weeks 0 days and 39 weeks 4 days of gestation or to expectant management. The trial is more than halfway complete.
Dr. William A. Grobman is at Northwestern University, Chicago. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 March 2. doi: 10.1056/NEJMe1516461).
The findings of Walker et al. suggest that a fundamental belief that guides our decisions about the timing of delivery – namely, that induction of labor at term increases the risk of cesarean delivery – may not be true after all.

|
Dr. William A, Grobman |
However, the authors note that larger studies of the issue are needed to confirm these findings and to further explore the effects of induction on maternal and neonatal outcomes, especially stillbirth and other uncommon adverse outcomes. I am the principal investigator of such a trial (NCT01990612), which is currently underway within the Maternal–Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
This trial, which has a targeted enrollment of 6,000 women, is designed to identify differences in perinatal outcomes among nulliparous women with uncomplicated singleton pregnancies who are randomly assigned to induction between 39 weeks 0 days and 39 weeks 4 days of gestation or to expectant management. The trial is more than halfway complete.
Dr. William A. Grobman is at Northwestern University, Chicago. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 March 2. doi: 10.1056/NEJMe1516461).
For women aged 35 years and older, induction of labor at 39 weeks neither raised nor lowered the rate of cesarean delivery, compared with expectant management, in a randomized clinical trial in the United Kingdom.
Labor induction at or before the due date may be beneficial in older women “because the gestational age at delivery that is associated with the lowest cumulative risk of perinatal death is 38 weeks.” However, induction in general is associated with an increased rate of cesarean delivery.
Previous studies of the relative benefits and harms of labor induction in this age group haven’t shed much light on the issue; they were small, observational in design, and were performed in the 1970s, so their findings may not be applicable to modern obstetric practice, wrote Dr. Kate F. Walker of University of Nottingham, England, and her associates.
They tested the hypothesis that labor induction at 39 weeks would reduce the rate of cesarean delivery in nulliparous women of advanced maternal age in the 35/39 Trial. It involved 619 women aged 35 years and older with singleton, uncomplicated pregnancies who were treated during a 2.5-year period at 39 secondary- and tertiary-care medical centers across the United Kingdom. The study participants were randomly assigned late in their pregnancies to either labor induction at 39 weeks (304 women) or expectant management (314 women).
The study hypothesis was not proven: there was no significant difference in the frequency of cesarean section between the induction group (32%) and the expectant management group (33%), for a relative risk of 0.99.
There also were no significant differences between the two study groups in maternal outcomes or neonatal outcomes (N Engl J Med. 2016 Mar 2;374:813-22. doi: 10.1056/NEJMoa1509117).
The trial did not address whether induction at 39 weeks’ gestation could prevent stillbirth, the researchers reported, but it provides support for the safety of performing a larger trial to test the effects of induction on stillbirths and other adverse neonatal outcomes.
The women assigned to labor induction did not differ from those assigned to expectant management in their subjective assessments of satisfaction with the childbirth experience. However, all the study participants had agreed to randomization. Women who had strong preferences about induction or expectant management, or strong feelings about their involvement in treatment decisions – and who may therefore have had a different take on their childbirth experience if labor were induced – were excluded from the study.
The study was supported by the National Institute for Health Research in the United Kingdom. Dr. Walker reported having no relevant financial disclosures; one of her associates reported ties to Roche Diagnostics, GlaxoSmithKline, General Electric, Chiesi, and Action Medical Research.
For women aged 35 years and older, induction of labor at 39 weeks neither raised nor lowered the rate of cesarean delivery, compared with expectant management, in a randomized clinical trial in the United Kingdom.
Labor induction at or before the due date may be beneficial in older women “because the gestational age at delivery that is associated with the lowest cumulative risk of perinatal death is 38 weeks.” However, induction in general is associated with an increased rate of cesarean delivery.
Previous studies of the relative benefits and harms of labor induction in this age group haven’t shed much light on the issue; they were small, observational in design, and were performed in the 1970s, so their findings may not be applicable to modern obstetric practice, wrote Dr. Kate F. Walker of University of Nottingham, England, and her associates.
They tested the hypothesis that labor induction at 39 weeks would reduce the rate of cesarean delivery in nulliparous women of advanced maternal age in the 35/39 Trial. It involved 619 women aged 35 years and older with singleton, uncomplicated pregnancies who were treated during a 2.5-year period at 39 secondary- and tertiary-care medical centers across the United Kingdom. The study participants were randomly assigned late in their pregnancies to either labor induction at 39 weeks (304 women) or expectant management (314 women).
The study hypothesis was not proven: there was no significant difference in the frequency of cesarean section between the induction group (32%) and the expectant management group (33%), for a relative risk of 0.99.
There also were no significant differences between the two study groups in maternal outcomes or neonatal outcomes (N Engl J Med. 2016 Mar 2;374:813-22. doi: 10.1056/NEJMoa1509117).
The trial did not address whether induction at 39 weeks’ gestation could prevent stillbirth, the researchers reported, but it provides support for the safety of performing a larger trial to test the effects of induction on stillbirths and other adverse neonatal outcomes.
The women assigned to labor induction did not differ from those assigned to expectant management in their subjective assessments of satisfaction with the childbirth experience. However, all the study participants had agreed to randomization. Women who had strong preferences about induction or expectant management, or strong feelings about their involvement in treatment decisions – and who may therefore have had a different take on their childbirth experience if labor were induced – were excluded from the study.
The study was supported by the National Institute for Health Research in the United Kingdom. Dr. Walker reported having no relevant financial disclosures; one of her associates reported ties to Roche Diagnostics, GlaxoSmithKline, General Electric, Chiesi, and Action Medical Research.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Induction at 39 weeks doesn’t affect C-section rate in older women
For women aged 35 years and older, induction of labor at 39 weeks neither raised nor lowered the rate of cesarean delivery, compared with expectant management, in a randomized clinical trial in the United Kingdom.
Labor induction at or before the due date may be beneficial in older women “because the gestational age at delivery that is associated with the lowest cumulative risk of perinatal death is 38 weeks.” However, induction in general is associated with an increased rate of cesarean delivery.
Previous studies of the relative benefits and harms of labor induction in this age group haven’t shed much light on the issue; they were small, observational in design, and were performed in the 1970s, so their findings may not be applicable to modern obstetric practice, wrote Dr. Kate F. Walker of University of Nottingham, England, and her associates.
They tested the hypothesis that labor induction at 39 weeks would reduce the rate of cesarean delivery in nulliparous women of advanced maternal age in the 35/39 Trial. It involved 619 women aged 35 years and older with singleton, uncomplicated pregnancies who were treated during a 2.5-year period at 39 secondary- and tertiary-care medical centers across the United Kingdom. The study participants were randomly assigned late in their pregnancies to either labor induction at 39 weeks (304 women) or expectant management (314 women).
The study hypothesis was not proven: there was no significant difference in the frequency of cesarean section between the induction group (32%) and the expectant management group (33%), for a relative risk of 0.99.
There also were no significant differences between the two study groups in maternal outcomes or neonatal outcomes (N Engl J Med. 2016 Mar 2;374:813-22. doi: 10.1056/NEJMoa1509117).
The trial did not address whether induction at 39 weeks’ gestation could prevent stillbirth, the researchers reported, but it provides support for the safety of performing a larger trial to test the effects of induction on stillbirths and other adverse neonatal outcomes.
The women assigned to labor induction did not differ from those assigned to expectant management in their subjective assessments of satisfaction with the childbirth experience. However, all the study participants had agreed to randomization. Women who had strong preferences about induction or expectant management, or strong feelings about their involvement in treatment decisions – and who may therefore have had a different take on their childbirth experience if labor were induced – were excluded from the study.
The study was supported by the National Institute for Health Research in the United Kingdom. Dr. Walker reported having no relevant financial disclosures; one of her associates reported ties to Roche Diagnostics, GlaxoSmithKline, General Electric, Chiesi, and Action Medical Research.
The findings of Walker et al. suggest that a fundamental belief that guides our decisions about the timing of delivery – namely, that induction of labor at term increases the risk of cesarean delivery – may not be true after all.

|
Dr. William A, Grobman |
However, the authors note that larger studies of the issue are needed to confirm these findings and to further explore the effects of induction on maternal and neonatal outcomes, especially stillbirth and other uncommon adverse outcomes. I am the principal investigator of such a trial (NCT01990612), which is currently underway within the Maternal–Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
This trial, which has a targeted enrollment of 6,000 women, is designed to identify differences in perinatal outcomes among nulliparous women with uncomplicated singleton pregnancies who are randomly assigned to induction between 39 weeks 0 days and 39 weeks 4 days of gestation or to expectant management. The trial is more than halfway complete.
Dr. William A. Grobman is at Northwestern University, Chicago. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 March 2. doi: 10.1056/NEJMe1516461).
The findings of Walker et al. suggest that a fundamental belief that guides our decisions about the timing of delivery – namely, that induction of labor at term increases the risk of cesarean delivery – may not be true after all.

|
Dr. William A, Grobman |
However, the authors note that larger studies of the issue are needed to confirm these findings and to further explore the effects of induction on maternal and neonatal outcomes, especially stillbirth and other uncommon adverse outcomes. I am the principal investigator of such a trial (NCT01990612), which is currently underway within the Maternal–Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
This trial, which has a targeted enrollment of 6,000 women, is designed to identify differences in perinatal outcomes among nulliparous women with uncomplicated singleton pregnancies who are randomly assigned to induction between 39 weeks 0 days and 39 weeks 4 days of gestation or to expectant management. The trial is more than halfway complete.
Dr. William A. Grobman is at Northwestern University, Chicago. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 March 2. doi: 10.1056/NEJMe1516461).
The findings of Walker et al. suggest that a fundamental belief that guides our decisions about the timing of delivery – namely, that induction of labor at term increases the risk of cesarean delivery – may not be true after all.

|
Dr. William A, Grobman |
However, the authors note that larger studies of the issue are needed to confirm these findings and to further explore the effects of induction on maternal and neonatal outcomes, especially stillbirth and other uncommon adverse outcomes. I am the principal investigator of such a trial (NCT01990612), which is currently underway within the Maternal–Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
This trial, which has a targeted enrollment of 6,000 women, is designed to identify differences in perinatal outcomes among nulliparous women with uncomplicated singleton pregnancies who are randomly assigned to induction between 39 weeks 0 days and 39 weeks 4 days of gestation or to expectant management. The trial is more than halfway complete.
Dr. William A. Grobman is at Northwestern University, Chicago. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 March 2. doi: 10.1056/NEJMe1516461).
For women aged 35 years and older, induction of labor at 39 weeks neither raised nor lowered the rate of cesarean delivery, compared with expectant management, in a randomized clinical trial in the United Kingdom.
Labor induction at or before the due date may be beneficial in older women “because the gestational age at delivery that is associated with the lowest cumulative risk of perinatal death is 38 weeks.” However, induction in general is associated with an increased rate of cesarean delivery.
Previous studies of the relative benefits and harms of labor induction in this age group haven’t shed much light on the issue; they were small, observational in design, and were performed in the 1970s, so their findings may not be applicable to modern obstetric practice, wrote Dr. Kate F. Walker of University of Nottingham, England, and her associates.
They tested the hypothesis that labor induction at 39 weeks would reduce the rate of cesarean delivery in nulliparous women of advanced maternal age in the 35/39 Trial. It involved 619 women aged 35 years and older with singleton, uncomplicated pregnancies who were treated during a 2.5-year period at 39 secondary- and tertiary-care medical centers across the United Kingdom. The study participants were randomly assigned late in their pregnancies to either labor induction at 39 weeks (304 women) or expectant management (314 women).
The study hypothesis was not proven: there was no significant difference in the frequency of cesarean section between the induction group (32%) and the expectant management group (33%), for a relative risk of 0.99.
There also were no significant differences between the two study groups in maternal outcomes or neonatal outcomes (N Engl J Med. 2016 Mar 2;374:813-22. doi: 10.1056/NEJMoa1509117).
The trial did not address whether induction at 39 weeks’ gestation could prevent stillbirth, the researchers reported, but it provides support for the safety of performing a larger trial to test the effects of induction on stillbirths and other adverse neonatal outcomes.
The women assigned to labor induction did not differ from those assigned to expectant management in their subjective assessments of satisfaction with the childbirth experience. However, all the study participants had agreed to randomization. Women who had strong preferences about induction or expectant management, or strong feelings about their involvement in treatment decisions – and who may therefore have had a different take on their childbirth experience if labor were induced – were excluded from the study.
The study was supported by the National Institute for Health Research in the United Kingdom. Dr. Walker reported having no relevant financial disclosures; one of her associates reported ties to Roche Diagnostics, GlaxoSmithKline, General Electric, Chiesi, and Action Medical Research.
For women aged 35 years and older, induction of labor at 39 weeks neither raised nor lowered the rate of cesarean delivery, compared with expectant management, in a randomized clinical trial in the United Kingdom.
Labor induction at or before the due date may be beneficial in older women “because the gestational age at delivery that is associated with the lowest cumulative risk of perinatal death is 38 weeks.” However, induction in general is associated with an increased rate of cesarean delivery.
Previous studies of the relative benefits and harms of labor induction in this age group haven’t shed much light on the issue; they were small, observational in design, and were performed in the 1970s, so their findings may not be applicable to modern obstetric practice, wrote Dr. Kate F. Walker of University of Nottingham, England, and her associates.
They tested the hypothesis that labor induction at 39 weeks would reduce the rate of cesarean delivery in nulliparous women of advanced maternal age in the 35/39 Trial. It involved 619 women aged 35 years and older with singleton, uncomplicated pregnancies who were treated during a 2.5-year period at 39 secondary- and tertiary-care medical centers across the United Kingdom. The study participants were randomly assigned late in their pregnancies to either labor induction at 39 weeks (304 women) or expectant management (314 women).
The study hypothesis was not proven: there was no significant difference in the frequency of cesarean section between the induction group (32%) and the expectant management group (33%), for a relative risk of 0.99.
There also were no significant differences between the two study groups in maternal outcomes or neonatal outcomes (N Engl J Med. 2016 Mar 2;374:813-22. doi: 10.1056/NEJMoa1509117).
The trial did not address whether induction at 39 weeks’ gestation could prevent stillbirth, the researchers reported, but it provides support for the safety of performing a larger trial to test the effects of induction on stillbirths and other adverse neonatal outcomes.
The women assigned to labor induction did not differ from those assigned to expectant management in their subjective assessments of satisfaction with the childbirth experience. However, all the study participants had agreed to randomization. Women who had strong preferences about induction or expectant management, or strong feelings about their involvement in treatment decisions – and who may therefore have had a different take on their childbirth experience if labor were induced – were excluded from the study.
The study was supported by the National Institute for Health Research in the United Kingdom. Dr. Walker reported having no relevant financial disclosures; one of her associates reported ties to Roche Diagnostics, GlaxoSmithKline, General Electric, Chiesi, and Action Medical Research.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Among women aged 35 years and older, labor induction at 39 weeks didn’t affect the rate of cesarean delivery.
Major finding: There was no significant difference in the frequency of C-section between the induction group (32%) and the expectant management group (33%).
Data source: A multicenter, randomized trial involving 619 older pregnant women in the United Kingdom.
Disclosures: The study was supported by the National Institute for Health Research in the United Kingdom. Dr. Walker reported having no relevant financial disclosures; one of her associates reported ties to Roche Diagnostics, GlaxoSmithKline, General Electric, Chiesi, and Action Medical Research.
Unintended pregnancies fall to 30-year low
The rate of unintended pregnancies in the United States declined 18% between 2008 and 2011 after a long interval of minimal change, and it is now at the lowest level in 30 years, according to a report published online March 2 in the New England Journal of Medicine.
This “substantial” reduction occurred across all ages, ethnicities, income levels, education levels, and religious affiliations. However, large disparities among demographic groups were still present in 2011, the most recent year for which national data are available for analysis.
“In particular, poor, black, and Hispanic women and girls continued to have much higher rates of unintended pregnancy than did whites and those with higher incomes. Much more progress can be made in eliminating these disparities,” wrote Lawrence B. Finer, Ph.D., and Mia R. Zolna of the Guttmacher Institute, New York.
National rates of unintended pregnancies haven’t been examined since 2008. To update these figures, the investigators analyzed information from the National Center for Health Statistics, the National Survey of Family Growth, and others.
They calculated that there were 6.1 million pregnancies in the United States in 2011, of which 45% (2.8 million) were unintended. The rate was 45 unintended pregnancies for every 1,000 women of childbearing age in 2011, compared with 54/1,000 in 2008, which corresponds to an 18% decline (N Engl J Med.2016;374:843-52. doi: 10.1056/NEJMsa1506575).
“This was the first substantial decline since at least 1981,” the investigators wrote.
Abortion rates remained steady during the study period. In 2011, the percentage of unintended pregnancies that ended in abortion was 42%, compared with 40% in 2008.
Although the study was not designed to determine the reasons for the decline, several possible factors deserve consideration, according to the investigators. Changes in sexual behavior are unlikely to have driven this reduction, since the incidence of sexual activity tends to remain static over time; changes in the composition of the population also aren’t likely causes, since the demographic subgroups who have higher rates of unintended pregnancy actually increased during this period, the investigators said.
Changes in the desire for pregnancy may have contributed a small amount to the decline, as surveys showed that many women said they would reduce or delay their childbearing during the recent economic recession.
The most likely explanation for the decline, the investigators wrote, is a change in the frequency and type of contraceptive use. Several studies have reported that women at high risk of unintended pregnancy increased both their use of any contraception and their use of highly effective long-acting methods, particularly IUDs.
This study was supported chiefly by the Susan Thompson Buffet Foundation, with additional support from the Guttmacher Center through a grant from the National Institutes of Health. The investigators reported having no other relevant financial disclosures.
The rate of unintended pregnancies in the United States declined 18% between 2008 and 2011 after a long interval of minimal change, and it is now at the lowest level in 30 years, according to a report published online March 2 in the New England Journal of Medicine.
This “substantial” reduction occurred across all ages, ethnicities, income levels, education levels, and religious affiliations. However, large disparities among demographic groups were still present in 2011, the most recent year for which national data are available for analysis.
“In particular, poor, black, and Hispanic women and girls continued to have much higher rates of unintended pregnancy than did whites and those with higher incomes. Much more progress can be made in eliminating these disparities,” wrote Lawrence B. Finer, Ph.D., and Mia R. Zolna of the Guttmacher Institute, New York.
National rates of unintended pregnancies haven’t been examined since 2008. To update these figures, the investigators analyzed information from the National Center for Health Statistics, the National Survey of Family Growth, and others.
They calculated that there were 6.1 million pregnancies in the United States in 2011, of which 45% (2.8 million) were unintended. The rate was 45 unintended pregnancies for every 1,000 women of childbearing age in 2011, compared with 54/1,000 in 2008, which corresponds to an 18% decline (N Engl J Med.2016;374:843-52. doi: 10.1056/NEJMsa1506575).
“This was the first substantial decline since at least 1981,” the investigators wrote.
Abortion rates remained steady during the study period. In 2011, the percentage of unintended pregnancies that ended in abortion was 42%, compared with 40% in 2008.
Although the study was not designed to determine the reasons for the decline, several possible factors deserve consideration, according to the investigators. Changes in sexual behavior are unlikely to have driven this reduction, since the incidence of sexual activity tends to remain static over time; changes in the composition of the population also aren’t likely causes, since the demographic subgroups who have higher rates of unintended pregnancy actually increased during this period, the investigators said.
Changes in the desire for pregnancy may have contributed a small amount to the decline, as surveys showed that many women said they would reduce or delay their childbearing during the recent economic recession.
The most likely explanation for the decline, the investigators wrote, is a change in the frequency and type of contraceptive use. Several studies have reported that women at high risk of unintended pregnancy increased both their use of any contraception and their use of highly effective long-acting methods, particularly IUDs.
This study was supported chiefly by the Susan Thompson Buffet Foundation, with additional support from the Guttmacher Center through a grant from the National Institutes of Health. The investigators reported having no other relevant financial disclosures.
The rate of unintended pregnancies in the United States declined 18% between 2008 and 2011 after a long interval of minimal change, and it is now at the lowest level in 30 years, according to a report published online March 2 in the New England Journal of Medicine.
This “substantial” reduction occurred across all ages, ethnicities, income levels, education levels, and religious affiliations. However, large disparities among demographic groups were still present in 2011, the most recent year for which national data are available for analysis.
“In particular, poor, black, and Hispanic women and girls continued to have much higher rates of unintended pregnancy than did whites and those with higher incomes. Much more progress can be made in eliminating these disparities,” wrote Lawrence B. Finer, Ph.D., and Mia R. Zolna of the Guttmacher Institute, New York.
National rates of unintended pregnancies haven’t been examined since 2008. To update these figures, the investigators analyzed information from the National Center for Health Statistics, the National Survey of Family Growth, and others.
They calculated that there were 6.1 million pregnancies in the United States in 2011, of which 45% (2.8 million) were unintended. The rate was 45 unintended pregnancies for every 1,000 women of childbearing age in 2011, compared with 54/1,000 in 2008, which corresponds to an 18% decline (N Engl J Med.2016;374:843-52. doi: 10.1056/NEJMsa1506575).
“This was the first substantial decline since at least 1981,” the investigators wrote.
Abortion rates remained steady during the study period. In 2011, the percentage of unintended pregnancies that ended in abortion was 42%, compared with 40% in 2008.
Although the study was not designed to determine the reasons for the decline, several possible factors deserve consideration, according to the investigators. Changes in sexual behavior are unlikely to have driven this reduction, since the incidence of sexual activity tends to remain static over time; changes in the composition of the population also aren’t likely causes, since the demographic subgroups who have higher rates of unintended pregnancy actually increased during this period, the investigators said.
Changes in the desire for pregnancy may have contributed a small amount to the decline, as surveys showed that many women said they would reduce or delay their childbearing during the recent economic recession.
The most likely explanation for the decline, the investigators wrote, is a change in the frequency and type of contraceptive use. Several studies have reported that women at high risk of unintended pregnancy increased both their use of any contraception and their use of highly effective long-acting methods, particularly IUDs.
This study was supported chiefly by the Susan Thompson Buffet Foundation, with additional support from the Guttmacher Center through a grant from the National Institutes of Health. The investigators reported having no other relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Unintended pregnancies fell by 18% between 2008 and 2011.
Major finding: The rate of unintended pregnancies was 45 for every 1,000 women of childbearing age in 2011, compared with 54/1,000 in 2008.
Data source: An analysis of data from numerous nationally representative surveys and databases regarding pregnancy intentions and outcomes in 2011.
Disclosures: The study was supported chiefly by the Susan Thompson Buffet Foundation, with additional support from the Guttmacher Center through a grant from the National Institutes of Health. The investigators reported having no other relevant financial disclosures.
Zika virus: Counseling considerations for this emerging perinatal threat
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||

| 
| |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |

|
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||

| 
| |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |

|
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||

| 
| |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |

|
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
In this Article
- Management strategies for pregnant patients with Zika virus exposure
- Fetal surveillance
- Perinatal counseling on exposure prevention
- Algorithm for evaluation and management
Twin Birth Study: No benefit with planned C-section
ATLANTA – A policy of planned cesarean delivery provides no significant benefit, compared with a policy of planned vaginal delivery in cases involving uncomplicated twin pregnancies between 32 and 38 weeks gestation where the first twin is in cephalic presentation, according to 2-year findings from the Twin Birth Study.
Prior findings from the randomized trial demonstrated that a policy of planned cesarean delivery benefited neither the mother nor the baby, compared with a policy of planned vaginal delivery; there was no difference between the groups with respect to the primary outcome of fetal or neonatal death or serious neonatal morbidity. The current analysis looked at the secondary outcome – a composite outcome of death or neurodevelopmental delay in offspring at age 2 years.
This composite outcome, corrected for gestational age at birth, occurred in 5.99% of 2,320 planned cesarean deliveries and in 5.83% of 2,283 planned vaginal deliveries (odds ratio, 1.04), Dr. Elizabeth Asztalos of the University of Toronto reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
There also was no significant difference between the groups in the individual components of the composite outcome. Death occurred in 1.51% and 1.01% of deliveries in the planned cesarean and planned vaginal delivery groups, respectively (OR, 1.48). In the surviving children, neurodevelopmental delay occurred in 4.55% and 4.87% (OR, 0.95), Dr. Asztalos said.
The Twin Birth study enrolled only women with uncomplicated twin pregnancies between 32 and 38 weeks’ gestation with the first twin in cephalic presentation. Subjects were randomized to either a planned cesarean or planned vaginal delivery group, and for the current analysis, children were assessed, using the Ages and Stages Questionnaire, at a mean age of 25-26 months in both groups.
Abnormal findings were validated by a clinical neurodevelopmental assessment.
The rate of cerebral palsy was extremely low in the study population and was not amenable to analysis, Dr. Asztalos said.
A post hoc analysis to determine if there were any differences between twin A and twin B with respect to the secondary outcome measure, showed no significant difference based on birth order, she added.
Notably, the incidence of the primary outcome in the study was almost threefold higher than the 2% originally anticipated in the planned vaginal delivery group. This may be due to the fact that for both approaches to delivery management, just under half of the infants were born preterm, she said.
The 2-year follow-up of the Twin Birth Study reinforces the initial findings of the international study, she said.
The Twin Birth Study was sponsored by Sunnybrook Health Sciences Centre in Toronto.
ATLANTA – A policy of planned cesarean delivery provides no significant benefit, compared with a policy of planned vaginal delivery in cases involving uncomplicated twin pregnancies between 32 and 38 weeks gestation where the first twin is in cephalic presentation, according to 2-year findings from the Twin Birth Study.
Prior findings from the randomized trial demonstrated that a policy of planned cesarean delivery benefited neither the mother nor the baby, compared with a policy of planned vaginal delivery; there was no difference between the groups with respect to the primary outcome of fetal or neonatal death or serious neonatal morbidity. The current analysis looked at the secondary outcome – a composite outcome of death or neurodevelopmental delay in offspring at age 2 years.
This composite outcome, corrected for gestational age at birth, occurred in 5.99% of 2,320 planned cesarean deliveries and in 5.83% of 2,283 planned vaginal deliveries (odds ratio, 1.04), Dr. Elizabeth Asztalos of the University of Toronto reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
There also was no significant difference between the groups in the individual components of the composite outcome. Death occurred in 1.51% and 1.01% of deliveries in the planned cesarean and planned vaginal delivery groups, respectively (OR, 1.48). In the surviving children, neurodevelopmental delay occurred in 4.55% and 4.87% (OR, 0.95), Dr. Asztalos said.
The Twin Birth study enrolled only women with uncomplicated twin pregnancies between 32 and 38 weeks’ gestation with the first twin in cephalic presentation. Subjects were randomized to either a planned cesarean or planned vaginal delivery group, and for the current analysis, children were assessed, using the Ages and Stages Questionnaire, at a mean age of 25-26 months in both groups.
Abnormal findings were validated by a clinical neurodevelopmental assessment.
The rate of cerebral palsy was extremely low in the study population and was not amenable to analysis, Dr. Asztalos said.
A post hoc analysis to determine if there were any differences between twin A and twin B with respect to the secondary outcome measure, showed no significant difference based on birth order, she added.
Notably, the incidence of the primary outcome in the study was almost threefold higher than the 2% originally anticipated in the planned vaginal delivery group. This may be due to the fact that for both approaches to delivery management, just under half of the infants were born preterm, she said.
The 2-year follow-up of the Twin Birth Study reinforces the initial findings of the international study, she said.
The Twin Birth Study was sponsored by Sunnybrook Health Sciences Centre in Toronto.
ATLANTA – A policy of planned cesarean delivery provides no significant benefit, compared with a policy of planned vaginal delivery in cases involving uncomplicated twin pregnancies between 32 and 38 weeks gestation where the first twin is in cephalic presentation, according to 2-year findings from the Twin Birth Study.
Prior findings from the randomized trial demonstrated that a policy of planned cesarean delivery benefited neither the mother nor the baby, compared with a policy of planned vaginal delivery; there was no difference between the groups with respect to the primary outcome of fetal or neonatal death or serious neonatal morbidity. The current analysis looked at the secondary outcome – a composite outcome of death or neurodevelopmental delay in offspring at age 2 years.
This composite outcome, corrected for gestational age at birth, occurred in 5.99% of 2,320 planned cesarean deliveries and in 5.83% of 2,283 planned vaginal deliveries (odds ratio, 1.04), Dr. Elizabeth Asztalos of the University of Toronto reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
There also was no significant difference between the groups in the individual components of the composite outcome. Death occurred in 1.51% and 1.01% of deliveries in the planned cesarean and planned vaginal delivery groups, respectively (OR, 1.48). In the surviving children, neurodevelopmental delay occurred in 4.55% and 4.87% (OR, 0.95), Dr. Asztalos said.
The Twin Birth study enrolled only women with uncomplicated twin pregnancies between 32 and 38 weeks’ gestation with the first twin in cephalic presentation. Subjects were randomized to either a planned cesarean or planned vaginal delivery group, and for the current analysis, children were assessed, using the Ages and Stages Questionnaire, at a mean age of 25-26 months in both groups.
Abnormal findings were validated by a clinical neurodevelopmental assessment.
The rate of cerebral palsy was extremely low in the study population and was not amenable to analysis, Dr. Asztalos said.
A post hoc analysis to determine if there were any differences between twin A and twin B with respect to the secondary outcome measure, showed no significant difference based on birth order, she added.
Notably, the incidence of the primary outcome in the study was almost threefold higher than the 2% originally anticipated in the planned vaginal delivery group. This may be due to the fact that for both approaches to delivery management, just under half of the infants were born preterm, she said.
The 2-year follow-up of the Twin Birth Study reinforces the initial findings of the international study, she said.
The Twin Birth Study was sponsored by Sunnybrook Health Sciences Centre in Toronto.
AT THE PREGNANCY MEETING
Key clinical point: Planned cesarean delivery provides no significant benefit in uncomplicated twin pregnancies, compared with planned vaginal delivery.
Major finding: The composite outcome occurred in 5.99% of 2,320 planned cesarean deliveries and in 5.83% of 2,283 planned vaginal deliveries (odds ratio, 1.04).
Data source: The randomized Twin Birth Study involving 4,603 children.
Disclosures: The Twin Birth Study was sponsored by Sunnybrook Health Sciences Centre in Toronto.