User login
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
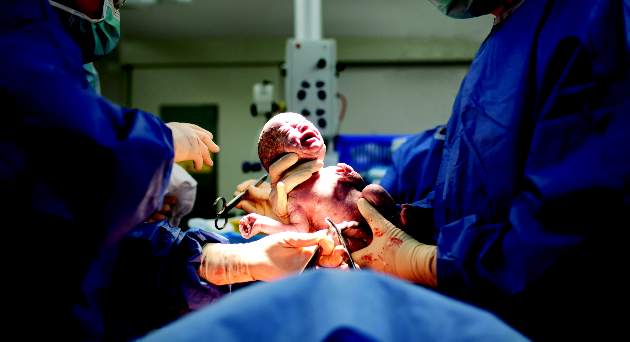
The results of the highly anticipated Antenatal Late Preterm Study recently have become available.1 Data from this randomized controlled trial, conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network, demonstrated that administration of betamethasone to women at risk for preterm delivery between 34 weeks 0 days and 36 weeks 6 days of gestation significantly reduces the rate of neonatal respiratory complications. It may represent the largest study of antenatal corticosteroids (ACS) to date, with 2,827 infants studied, and its results inevitably lead to the logical practical question: Should ACS use be extended beyond the 34 weeks’ gestation limit previously recommended by professional guidelines in the United States2?
There are some issues that bear discussion before such a significant change in standard of care should be promoted.2
Antenatal Late Preterm Study outcomesThe primary outcome in the study was a composite end point describing the need for respiratory support within 72 hours after birth. Based on a pilot study, the investigators had anticipated a 33% decrease in the rate of the primary outcome; however, the reduction was only 20% (relative risk [RR], 0.80; 95% confidence interval [CI], 0.66−0.97). Although the effect size was statistically significant, one could question the clinical relevance of such a small difference.
A 33% reduction effect, more consistent with the preliminary expectations, was noted in the prespecified secondary composite outcome of severe respiratory complications (RR, 0.67; 95% CI, 0.53−0.84). Occurrences included in the secondary composite outcome that also showed significant rate reductions were:
- the use of continuous positive airway pressure (CPAP) or high-flow oxygen via nasal cannula for at least 12 hours (RR, 0.62; 95% CI, 0.48−0.80)
- need for resuscitation at birth (RR, 0.78; 95% CI, 0.66−0.92)
- surfactant use (RR, 0.59; 95% CI, 0.37−0.96)
- transient tachypnea of the newborn (RR, 0.68; 95% CI, 0.53−0.87).
The reported reduction in bronchopulmonary dysplasia (RR, 0.22; 95% CI, 0.02−0.92) cannot plausibly be attributed to ACS. Randomized data aggregated by the Cochrane Database of Systematic Reviews3 do not show improvement in chronic lung disease with ACS use. Moreover, the authors recognize that the assessment for bronchopulmonary dysplasia at only 28 days of life is only partially informative and that longer childhood follow-up is required to confirm the finding.
- Although corticosteroids have been shown to reduce the risk of the baby needing breathing support by 20%, they are associated with a 60% increase in risk for low blood sugar in the newborn (hypoglycemia). Hypoglycemia can place the baby at risk for seizures and even brain damage.
- There is an unknown safety profile for corticosteroid administration at this gestational age. The fetal brain is still developing during this period, and there is some information to suggest that corticosteroids could have an unfavorable effect on brain development.
- Corticosteroids are potent hormones and potentially can have undesired hormonal effects at this gestational age.
- If corticosteroids are given and the mother carries the baby to term (37 weeks or later) there are some studies that suggest the baby is at an increased risk for neurologic, cognitive, metabolic, and/or behavioral abnormalities in later life.
We recommend caution before changing current practiceWe propose prudence with ACS use after 34 weeks’ gestation for the following reasons: the increased risk for neonatal hypoglycemia associated with ACS, the increased risk for ACS-related harm in term-born babies, and safety concerns with ACS in the late preterm period.
Evidence shows an increased risk for neonatal hypoglycemiaThe most profound effect modification observed in the study was an adverse effect—namely, a 60% increase in neonatal hypoglycemia with ACS administration (RR, 1.6; 95% CI, 1.37−1.87). The rate of neonatal hypoglycemia was 24% in the ACS group, compared with 15% in the placebo group.
Results of prior studies have demonstrated either no increased risk of hypoglycemia with ACS use4−7 or a much smaller increase (from 4.2% to 5.7%).8 The higher rate of neonatal hypoglycemia seen in this study suggests the possibility that the late preterm population may be more vulnerable to the negative impact of ACS on neonatal glucose/insulin homeostasis. Presumed mechanisms of action are either maternal hyperglycemia or fetal adrenal suppression or both, with potential for fetal adrenal suppression resulting from betamethasone exposure to affect long-term metabolic outcomes.9
Of note, women with pregestational diabetes were excluded from the study and, in routine practice, inclusion of such patients may further increase the risk of neonatal hypoglycemia.
There are few data on the prognostic significance of neonatal hypoglycemia in preterm infants, with the exception of a single study, the results of which show that it is associated with adverse neurodevelopment at 18 months of age.10
Data reveal increased risk for harm in term-born babiesIn spite of strict protocol specifications to increase the probability of delivery before 37 weeks’ gestation, 16% of women in the trial delivered at term. Investigators of prior randomized studies of ACS, aimed at reducing the risks of prematurity, have reported a rate of term delivery of about one-third,4,11 and in routine practice, administration of ACS after 34 weeks may be associated with even higher rates of term delivery.
This is important because recent evidence shows an unfavorable impact of ACS exposure in term-born children.12 The 5-year follow-up of the largest randomized trial in which multiple ACS courses were used noted that babies born at term had a 4-fold increased odds ratio for neurosensory disability.11 There was no dose−response interaction, with the same adverse odds ratio after 1 or 4 additional ACS courses. This observation was consistent with a previously reported Swedish national cohort, pointing to an unfavorable impact of even a single course of ACS in term-born children, with a greater likelihood of harm than benefit.13
In a UK follow-up of children aged 8 to 15 years who were enrolled in an RCT of ACS before cesarean delivery at term, low academic achievement was significantly more common in the group whose mothers had received ACS.14 In another study of 304 children born at term after exposure to a single course of ACS, investigators noted significantly increased cortisol reactivity to acute psychological stress at ages 6 to 11 years in the ACS-exposed patients, compared with 212 babies of women with threatened preterm labor who did not receive ACS and 372 babies from uncomplicated term pregnancies.15
The relevance of such study findings extends beyond childhood given the fact that elevated hypothalamic-pituitary-adrenal (HPA) axis reactivity has been linked to the pathogenesis of metabolic syndrome and depression in adult life.16 As recently as 2015, investigators of a randomized trial of ACS in 6 low- and middle-income countries highlighted their concern regarding “potentially harmful use of antenatal corticosteroids for infants not delivered preterm.”17
There are safety concerns with ACS in the late preterm periodThe effects of ACS are more pleiotropic than those reflected in a lower incidence of respiratory difficulties. Knowledge of the overall consequences of ACS exposure in infants born late-preterm or at term is still limited. The close-to-term fetus exposed to exogenous corticosteroids is also exposed to the physiologic endogenous surge of cortisol known to occur in the maternal circulation in late pregnancy, which reaches levels 3 times higher than those seen in nonpregnant women.18 Although placental 11 beta-hydroxysteroid dehydrogenase type 2 plays a protective role by allowing no more than 10% to 20% of maternal corticosteroids to cross the placenta, fetal overexposure from concomitant exogenous maternal corticosteroid administration remains a theoretical concern close to term. This is especially worrisome if there is a gestational age−related increase in the sensitivity to corticosteroid-induced in utero fetal programming. It has been reported that fetal overexposure to corticosteroids in late pregnancy can permanently increase the activity of the HPA-axis, with likely consequences in adult life.19
Another concern relates to oligodendrocytes development. Although the neuronal division process in humans usually is completed by 24 weeks’ gestation, the most rapid growth for oligodendrocytes occurs between 34 and 36 weeks’ gestation; these are the cells responsible for the synthesis of myelin. Overexposure to corticosteroids at this vulnerable time in the late preterm fetus potentially may have unanticipated negative neurologic consequences.20
This is the only scenario in which we feel antenatal corticosteroids could be used in a fetus aged 34 weeks to 36 weeks 5 days. In the setting of a scheduled cesarean delivery between 34 weeks and 35 weeks, the concerns relative to term delivery after corticosteroid exposure may not apply, but the concerns in relation to the administration of corticosteroid in the late preterm period—which is a time of possibly increased neurohormonal and neurologic vulnerability—still apply. With regard to the risk of neonatal hypoglycemia, one might argue that close neonatal monitoring of babies so exposed may ensure that any associated neonatal hypoglycemia does not go unnoticed or untreated. However, the prognostic significance of even short periods of neonatal hypoglycemia has not been established.
Where should future studies focus?There is clear neonatal benefit from a single course of ACS given to women who will deliver before 34 weeks’ gestation. It is widely accepted, based on the evidence provided by the 30-year follow-up of the cohort of 534 participants from the Auckland trial (the longest follow-up for any pregnancy trial), that administration of ACS at less than 34 weeks’ gestation is not associated with any obvious major developmental risk.21−23
However, the reassurances provided by the Auckland cohort should be neither directly extrapolated to the administration of ACS in the late preterm period nor applied to term-born babies exposed to ACS, for the simple reason that these subgroups never have been analyzed separately. The risk:benefit ratio of ACS use in the late-preterm period is as yet unknown, and in term-born babies the ratio may be unfavorable.
Follow-up studies are neededWe consider that there is a vital need for long-term follow-up studies. The focus of research on the effects of ACS no longer is on the immediate neonatal outcomes and now is on safety and the long-term outcomes of this exposure.
Bottom lineWe regard the large, high-quality study conducted by the NICHD MFMU Network1 as an opportunity to answer current concerns. It is hoped that the resources necessaryfor in-depth follow-up of the children involved in this study will be provided to the investigators and to the NICHD. It is only with such follow-up that mid- and long-term adverse effects can be assessed. We believe that, at a minimum, mid-term follow-up data should be available before it is wise to make any definitive recommendations for a sweeping change in clinical practice.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery [published online ahead of print February 4, 2016]. N Engl J Med.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117(2 pt 1):422–424.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454.
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525.
- Sann L, Burnod J, Lasne Y, Bethenod M. Antenatal administration of betamethasone: effects upon neonatal blood glucose in premature infants [in French]. Nouv Presse Med. 1979;8(39):3147–3148.
- Rokicki W, Krasnodebski J. Antenatal glucocorticoid administration and neonatal glycemia. Dev Pharmacol Ther. 1987;10(4):307–311.
- Gazquez Serrano IM, Arroyos Plana A, Diaz Morales O, Herraiz Perea C, Holgueras Bragado A. Antenatal corticosteroid therapy and late preterm infant morbidity and mortality [in Spanish]. An Pediatr (Barc). 2014;81(6):374–382.
- Pettit KE, Tran SH, Lee E, Caughey AB. The association of antenatal corticosteroids with neonatal hypoglycemia and hyperbilirubinemia. J Matern Fetal Neonatal Med. 2014;27(7):683–686.
- Aydin M, Derveci U, Hakan N. Neonatal hypoglycemia associated with the antenatal corticosteroids may be secondary to fetal adrenal suppression. J Matern Fetal Neonatal Med. 2015;28(8):892.
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308.
- Asztalos EV, Murphy KE, Willan AR, et al; MACS-5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102–1110.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications [published online ahead of print January 18, 2016]. BJOG. doi:10.1111/1471-0528.13853.
- Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Health consequences of prophylactic exposure to antenatal corticosteroids among children born late preterm or term. Acta Obstet Gynecol Scand. 2012;91(12):1415–1421.
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F195–F200.
- Alexander N, Rosenlocher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381.
- Althabe F, Belizan JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639.
- Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11ß-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdale GR mRNA expression and anxiety-like behavior in the offspring. Eur J Neurosci. 2000;12(3):1047–1054.
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F154–F157.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862.
- Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11Dalziel SR, Walker NK, Parag V, et al. Dalziel SR, Lim VK, Lambert A, et al. Dalziel SR, Rea HH, Walker NK, et al.
The results of the highly anticipated Antenatal Late Preterm Study recently have become available.1 Data from this randomized controlled trial, conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network, demonstrated that administration of betamethasone to women at risk for preterm delivery between 34 weeks 0 days and 36 weeks 6 days of gestation significantly reduces the rate of neonatal respiratory complications. It may represent the largest study of antenatal corticosteroids (ACS) to date, with 2,827 infants studied, and its results inevitably lead to the logical practical question: Should ACS use be extended beyond the 34 weeks’ gestation limit previously recommended by professional guidelines in the United States2?
There are some issues that bear discussion before such a significant change in standard of care should be promoted.2
Antenatal Late Preterm Study outcomesThe primary outcome in the study was a composite end point describing the need for respiratory support within 72 hours after birth. Based on a pilot study, the investigators had anticipated a 33% decrease in the rate of the primary outcome; however, the reduction was only 20% (relative risk [RR], 0.80; 95% confidence interval [CI], 0.66−0.97). Although the effect size was statistically significant, one could question the clinical relevance of such a small difference.
A 33% reduction effect, more consistent with the preliminary expectations, was noted in the prespecified secondary composite outcome of severe respiratory complications (RR, 0.67; 95% CI, 0.53−0.84). Occurrences included in the secondary composite outcome that also showed significant rate reductions were:
- the use of continuous positive airway pressure (CPAP) or high-flow oxygen via nasal cannula for at least 12 hours (RR, 0.62; 95% CI, 0.48−0.80)
- need for resuscitation at birth (RR, 0.78; 95% CI, 0.66−0.92)
- surfactant use (RR, 0.59; 95% CI, 0.37−0.96)
- transient tachypnea of the newborn (RR, 0.68; 95% CI, 0.53−0.87).
The reported reduction in bronchopulmonary dysplasia (RR, 0.22; 95% CI, 0.02−0.92) cannot plausibly be attributed to ACS. Randomized data aggregated by the Cochrane Database of Systematic Reviews3 do not show improvement in chronic lung disease with ACS use. Moreover, the authors recognize that the assessment for bronchopulmonary dysplasia at only 28 days of life is only partially informative and that longer childhood follow-up is required to confirm the finding.
- Although corticosteroids have been shown to reduce the risk of the baby needing breathing support by 20%, they are associated with a 60% increase in risk for low blood sugar in the newborn (hypoglycemia). Hypoglycemia can place the baby at risk for seizures and even brain damage.
- There is an unknown safety profile for corticosteroid administration at this gestational age. The fetal brain is still developing during this period, and there is some information to suggest that corticosteroids could have an unfavorable effect on brain development.
- Corticosteroids are potent hormones and potentially can have undesired hormonal effects at this gestational age.
- If corticosteroids are given and the mother carries the baby to term (37 weeks or later) there are some studies that suggest the baby is at an increased risk for neurologic, cognitive, metabolic, and/or behavioral abnormalities in later life.
We recommend caution before changing current practiceWe propose prudence with ACS use after 34 weeks’ gestation for the following reasons: the increased risk for neonatal hypoglycemia associated with ACS, the increased risk for ACS-related harm in term-born babies, and safety concerns with ACS in the late preterm period.
Evidence shows an increased risk for neonatal hypoglycemiaThe most profound effect modification observed in the study was an adverse effect—namely, a 60% increase in neonatal hypoglycemia with ACS administration (RR, 1.6; 95% CI, 1.37−1.87). The rate of neonatal hypoglycemia was 24% in the ACS group, compared with 15% in the placebo group.
Results of prior studies have demonstrated either no increased risk of hypoglycemia with ACS use4−7 or a much smaller increase (from 4.2% to 5.7%).8 The higher rate of neonatal hypoglycemia seen in this study suggests the possibility that the late preterm population may be more vulnerable to the negative impact of ACS on neonatal glucose/insulin homeostasis. Presumed mechanisms of action are either maternal hyperglycemia or fetal adrenal suppression or both, with potential for fetal adrenal suppression resulting from betamethasone exposure to affect long-term metabolic outcomes.9
Of note, women with pregestational diabetes were excluded from the study and, in routine practice, inclusion of such patients may further increase the risk of neonatal hypoglycemia.
There are few data on the prognostic significance of neonatal hypoglycemia in preterm infants, with the exception of a single study, the results of which show that it is associated with adverse neurodevelopment at 18 months of age.10
Data reveal increased risk for harm in term-born babiesIn spite of strict protocol specifications to increase the probability of delivery before 37 weeks’ gestation, 16% of women in the trial delivered at term. Investigators of prior randomized studies of ACS, aimed at reducing the risks of prematurity, have reported a rate of term delivery of about one-third,4,11 and in routine practice, administration of ACS after 34 weeks may be associated with even higher rates of term delivery.
This is important because recent evidence shows an unfavorable impact of ACS exposure in term-born children.12 The 5-year follow-up of the largest randomized trial in which multiple ACS courses were used noted that babies born at term had a 4-fold increased odds ratio for neurosensory disability.11 There was no dose−response interaction, with the same adverse odds ratio after 1 or 4 additional ACS courses. This observation was consistent with a previously reported Swedish national cohort, pointing to an unfavorable impact of even a single course of ACS in term-born children, with a greater likelihood of harm than benefit.13
In a UK follow-up of children aged 8 to 15 years who were enrolled in an RCT of ACS before cesarean delivery at term, low academic achievement was significantly more common in the group whose mothers had received ACS.14 In another study of 304 children born at term after exposure to a single course of ACS, investigators noted significantly increased cortisol reactivity to acute psychological stress at ages 6 to 11 years in the ACS-exposed patients, compared with 212 babies of women with threatened preterm labor who did not receive ACS and 372 babies from uncomplicated term pregnancies.15
The relevance of such study findings extends beyond childhood given the fact that elevated hypothalamic-pituitary-adrenal (HPA) axis reactivity has been linked to the pathogenesis of metabolic syndrome and depression in adult life.16 As recently as 2015, investigators of a randomized trial of ACS in 6 low- and middle-income countries highlighted their concern regarding “potentially harmful use of antenatal corticosteroids for infants not delivered preterm.”17
There are safety concerns with ACS in the late preterm periodThe effects of ACS are more pleiotropic than those reflected in a lower incidence of respiratory difficulties. Knowledge of the overall consequences of ACS exposure in infants born late-preterm or at term is still limited. The close-to-term fetus exposed to exogenous corticosteroids is also exposed to the physiologic endogenous surge of cortisol known to occur in the maternal circulation in late pregnancy, which reaches levels 3 times higher than those seen in nonpregnant women.18 Although placental 11 beta-hydroxysteroid dehydrogenase type 2 plays a protective role by allowing no more than 10% to 20% of maternal corticosteroids to cross the placenta, fetal overexposure from concomitant exogenous maternal corticosteroid administration remains a theoretical concern close to term. This is especially worrisome if there is a gestational age−related increase in the sensitivity to corticosteroid-induced in utero fetal programming. It has been reported that fetal overexposure to corticosteroids in late pregnancy can permanently increase the activity of the HPA-axis, with likely consequences in adult life.19
Another concern relates to oligodendrocytes development. Although the neuronal division process in humans usually is completed by 24 weeks’ gestation, the most rapid growth for oligodendrocytes occurs between 34 and 36 weeks’ gestation; these are the cells responsible for the synthesis of myelin. Overexposure to corticosteroids at this vulnerable time in the late preterm fetus potentially may have unanticipated negative neurologic consequences.20
This is the only scenario in which we feel antenatal corticosteroids could be used in a fetus aged 34 weeks to 36 weeks 5 days. In the setting of a scheduled cesarean delivery between 34 weeks and 35 weeks, the concerns relative to term delivery after corticosteroid exposure may not apply, but the concerns in relation to the administration of corticosteroid in the late preterm period—which is a time of possibly increased neurohormonal and neurologic vulnerability—still apply. With regard to the risk of neonatal hypoglycemia, one might argue that close neonatal monitoring of babies so exposed may ensure that any associated neonatal hypoglycemia does not go unnoticed or untreated. However, the prognostic significance of even short periods of neonatal hypoglycemia has not been established.
Where should future studies focus?There is clear neonatal benefit from a single course of ACS given to women who will deliver before 34 weeks’ gestation. It is widely accepted, based on the evidence provided by the 30-year follow-up of the cohort of 534 participants from the Auckland trial (the longest follow-up for any pregnancy trial), that administration of ACS at less than 34 weeks’ gestation is not associated with any obvious major developmental risk.21−23
However, the reassurances provided by the Auckland cohort should be neither directly extrapolated to the administration of ACS in the late preterm period nor applied to term-born babies exposed to ACS, for the simple reason that these subgroups never have been analyzed separately. The risk:benefit ratio of ACS use in the late-preterm period is as yet unknown, and in term-born babies the ratio may be unfavorable.
Follow-up studies are neededWe consider that there is a vital need for long-term follow-up studies. The focus of research on the effects of ACS no longer is on the immediate neonatal outcomes and now is on safety and the long-term outcomes of this exposure.
Bottom lineWe regard the large, high-quality study conducted by the NICHD MFMU Network1 as an opportunity to answer current concerns. It is hoped that the resources necessaryfor in-depth follow-up of the children involved in this study will be provided to the investigators and to the NICHD. It is only with such follow-up that mid- and long-term adverse effects can be assessed. We believe that, at a minimum, mid-term follow-up data should be available before it is wise to make any definitive recommendations for a sweeping change in clinical practice.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The results of the highly anticipated Antenatal Late Preterm Study recently have become available.1 Data from this randomized controlled trial, conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network, demonstrated that administration of betamethasone to women at risk for preterm delivery between 34 weeks 0 days and 36 weeks 6 days of gestation significantly reduces the rate of neonatal respiratory complications. It may represent the largest study of antenatal corticosteroids (ACS) to date, with 2,827 infants studied, and its results inevitably lead to the logical practical question: Should ACS use be extended beyond the 34 weeks’ gestation limit previously recommended by professional guidelines in the United States2?
There are some issues that bear discussion before such a significant change in standard of care should be promoted.2
Antenatal Late Preterm Study outcomesThe primary outcome in the study was a composite end point describing the need for respiratory support within 72 hours after birth. Based on a pilot study, the investigators had anticipated a 33% decrease in the rate of the primary outcome; however, the reduction was only 20% (relative risk [RR], 0.80; 95% confidence interval [CI], 0.66−0.97). Although the effect size was statistically significant, one could question the clinical relevance of such a small difference.
A 33% reduction effect, more consistent with the preliminary expectations, was noted in the prespecified secondary composite outcome of severe respiratory complications (RR, 0.67; 95% CI, 0.53−0.84). Occurrences included in the secondary composite outcome that also showed significant rate reductions were:
- the use of continuous positive airway pressure (CPAP) or high-flow oxygen via nasal cannula for at least 12 hours (RR, 0.62; 95% CI, 0.48−0.80)
- need for resuscitation at birth (RR, 0.78; 95% CI, 0.66−0.92)
- surfactant use (RR, 0.59; 95% CI, 0.37−0.96)
- transient tachypnea of the newborn (RR, 0.68; 95% CI, 0.53−0.87).
The reported reduction in bronchopulmonary dysplasia (RR, 0.22; 95% CI, 0.02−0.92) cannot plausibly be attributed to ACS. Randomized data aggregated by the Cochrane Database of Systematic Reviews3 do not show improvement in chronic lung disease with ACS use. Moreover, the authors recognize that the assessment for bronchopulmonary dysplasia at only 28 days of life is only partially informative and that longer childhood follow-up is required to confirm the finding.
- Although corticosteroids have been shown to reduce the risk of the baby needing breathing support by 20%, they are associated with a 60% increase in risk for low blood sugar in the newborn (hypoglycemia). Hypoglycemia can place the baby at risk for seizures and even brain damage.
- There is an unknown safety profile for corticosteroid administration at this gestational age. The fetal brain is still developing during this period, and there is some information to suggest that corticosteroids could have an unfavorable effect on brain development.
- Corticosteroids are potent hormones and potentially can have undesired hormonal effects at this gestational age.
- If corticosteroids are given and the mother carries the baby to term (37 weeks or later) there are some studies that suggest the baby is at an increased risk for neurologic, cognitive, metabolic, and/or behavioral abnormalities in later life.
We recommend caution before changing current practiceWe propose prudence with ACS use after 34 weeks’ gestation for the following reasons: the increased risk for neonatal hypoglycemia associated with ACS, the increased risk for ACS-related harm in term-born babies, and safety concerns with ACS in the late preterm period.
Evidence shows an increased risk for neonatal hypoglycemiaThe most profound effect modification observed in the study was an adverse effect—namely, a 60% increase in neonatal hypoglycemia with ACS administration (RR, 1.6; 95% CI, 1.37−1.87). The rate of neonatal hypoglycemia was 24% in the ACS group, compared with 15% in the placebo group.
Results of prior studies have demonstrated either no increased risk of hypoglycemia with ACS use4−7 or a much smaller increase (from 4.2% to 5.7%).8 The higher rate of neonatal hypoglycemia seen in this study suggests the possibility that the late preterm population may be more vulnerable to the negative impact of ACS on neonatal glucose/insulin homeostasis. Presumed mechanisms of action are either maternal hyperglycemia or fetal adrenal suppression or both, with potential for fetal adrenal suppression resulting from betamethasone exposure to affect long-term metabolic outcomes.9
Of note, women with pregestational diabetes were excluded from the study and, in routine practice, inclusion of such patients may further increase the risk of neonatal hypoglycemia.
There are few data on the prognostic significance of neonatal hypoglycemia in preterm infants, with the exception of a single study, the results of which show that it is associated with adverse neurodevelopment at 18 months of age.10
Data reveal increased risk for harm in term-born babiesIn spite of strict protocol specifications to increase the probability of delivery before 37 weeks’ gestation, 16% of women in the trial delivered at term. Investigators of prior randomized studies of ACS, aimed at reducing the risks of prematurity, have reported a rate of term delivery of about one-third,4,11 and in routine practice, administration of ACS after 34 weeks may be associated with even higher rates of term delivery.
This is important because recent evidence shows an unfavorable impact of ACS exposure in term-born children.12 The 5-year follow-up of the largest randomized trial in which multiple ACS courses were used noted that babies born at term had a 4-fold increased odds ratio for neurosensory disability.11 There was no dose−response interaction, with the same adverse odds ratio after 1 or 4 additional ACS courses. This observation was consistent with a previously reported Swedish national cohort, pointing to an unfavorable impact of even a single course of ACS in term-born children, with a greater likelihood of harm than benefit.13
In a UK follow-up of children aged 8 to 15 years who were enrolled in an RCT of ACS before cesarean delivery at term, low academic achievement was significantly more common in the group whose mothers had received ACS.14 In another study of 304 children born at term after exposure to a single course of ACS, investigators noted significantly increased cortisol reactivity to acute psychological stress at ages 6 to 11 years in the ACS-exposed patients, compared with 212 babies of women with threatened preterm labor who did not receive ACS and 372 babies from uncomplicated term pregnancies.15
The relevance of such study findings extends beyond childhood given the fact that elevated hypothalamic-pituitary-adrenal (HPA) axis reactivity has been linked to the pathogenesis of metabolic syndrome and depression in adult life.16 As recently as 2015, investigators of a randomized trial of ACS in 6 low- and middle-income countries highlighted their concern regarding “potentially harmful use of antenatal corticosteroids for infants not delivered preterm.”17
There are safety concerns with ACS in the late preterm periodThe effects of ACS are more pleiotropic than those reflected in a lower incidence of respiratory difficulties. Knowledge of the overall consequences of ACS exposure in infants born late-preterm or at term is still limited. The close-to-term fetus exposed to exogenous corticosteroids is also exposed to the physiologic endogenous surge of cortisol known to occur in the maternal circulation in late pregnancy, which reaches levels 3 times higher than those seen in nonpregnant women.18 Although placental 11 beta-hydroxysteroid dehydrogenase type 2 plays a protective role by allowing no more than 10% to 20% of maternal corticosteroids to cross the placenta, fetal overexposure from concomitant exogenous maternal corticosteroid administration remains a theoretical concern close to term. This is especially worrisome if there is a gestational age−related increase in the sensitivity to corticosteroid-induced in utero fetal programming. It has been reported that fetal overexposure to corticosteroids in late pregnancy can permanently increase the activity of the HPA-axis, with likely consequences in adult life.19
Another concern relates to oligodendrocytes development. Although the neuronal division process in humans usually is completed by 24 weeks’ gestation, the most rapid growth for oligodendrocytes occurs between 34 and 36 weeks’ gestation; these are the cells responsible for the synthesis of myelin. Overexposure to corticosteroids at this vulnerable time in the late preterm fetus potentially may have unanticipated negative neurologic consequences.20
This is the only scenario in which we feel antenatal corticosteroids could be used in a fetus aged 34 weeks to 36 weeks 5 days. In the setting of a scheduled cesarean delivery between 34 weeks and 35 weeks, the concerns relative to term delivery after corticosteroid exposure may not apply, but the concerns in relation to the administration of corticosteroid in the late preterm period—which is a time of possibly increased neurohormonal and neurologic vulnerability—still apply. With regard to the risk of neonatal hypoglycemia, one might argue that close neonatal monitoring of babies so exposed may ensure that any associated neonatal hypoglycemia does not go unnoticed or untreated. However, the prognostic significance of even short periods of neonatal hypoglycemia has not been established.
Where should future studies focus?There is clear neonatal benefit from a single course of ACS given to women who will deliver before 34 weeks’ gestation. It is widely accepted, based on the evidence provided by the 30-year follow-up of the cohort of 534 participants from the Auckland trial (the longest follow-up for any pregnancy trial), that administration of ACS at less than 34 weeks’ gestation is not associated with any obvious major developmental risk.21−23
However, the reassurances provided by the Auckland cohort should be neither directly extrapolated to the administration of ACS in the late preterm period nor applied to term-born babies exposed to ACS, for the simple reason that these subgroups never have been analyzed separately. The risk:benefit ratio of ACS use in the late-preterm period is as yet unknown, and in term-born babies the ratio may be unfavorable.
Follow-up studies are neededWe consider that there is a vital need for long-term follow-up studies. The focus of research on the effects of ACS no longer is on the immediate neonatal outcomes and now is on safety and the long-term outcomes of this exposure.
Bottom lineWe regard the large, high-quality study conducted by the NICHD MFMU Network1 as an opportunity to answer current concerns. It is hoped that the resources necessaryfor in-depth follow-up of the children involved in this study will be provided to the investigators and to the NICHD. It is only with such follow-up that mid- and long-term adverse effects can be assessed. We believe that, at a minimum, mid-term follow-up data should be available before it is wise to make any definitive recommendations for a sweeping change in clinical practice.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery [published online ahead of print February 4, 2016]. N Engl J Med.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117(2 pt 1):422–424.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454.
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525.
- Sann L, Burnod J, Lasne Y, Bethenod M. Antenatal administration of betamethasone: effects upon neonatal blood glucose in premature infants [in French]. Nouv Presse Med. 1979;8(39):3147–3148.
- Rokicki W, Krasnodebski J. Antenatal glucocorticoid administration and neonatal glycemia. Dev Pharmacol Ther. 1987;10(4):307–311.
- Gazquez Serrano IM, Arroyos Plana A, Diaz Morales O, Herraiz Perea C, Holgueras Bragado A. Antenatal corticosteroid therapy and late preterm infant morbidity and mortality [in Spanish]. An Pediatr (Barc). 2014;81(6):374–382.
- Pettit KE, Tran SH, Lee E, Caughey AB. The association of antenatal corticosteroids with neonatal hypoglycemia and hyperbilirubinemia. J Matern Fetal Neonatal Med. 2014;27(7):683–686.
- Aydin M, Derveci U, Hakan N. Neonatal hypoglycemia associated with the antenatal corticosteroids may be secondary to fetal adrenal suppression. J Matern Fetal Neonatal Med. 2015;28(8):892.
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308.
- Asztalos EV, Murphy KE, Willan AR, et al; MACS-5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102–1110.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications [published online ahead of print January 18, 2016]. BJOG. doi:10.1111/1471-0528.13853.
- Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Health consequences of prophylactic exposure to antenatal corticosteroids among children born late preterm or term. Acta Obstet Gynecol Scand. 2012;91(12):1415–1421.
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F195–F200.
- Alexander N, Rosenlocher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381.
- Althabe F, Belizan JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639.
- Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11ß-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdale GR mRNA expression and anxiety-like behavior in the offspring. Eur J Neurosci. 2000;12(3):1047–1054.
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F154–F157.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862.
- Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11Dalziel SR, Walker NK, Parag V, et al. Dalziel SR, Lim VK, Lambert A, et al. Dalziel SR, Rea HH, Walker NK, et al.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery [published online ahead of print February 4, 2016]. N Engl J Med.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117(2 pt 1):422–424.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454.
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525.
- Sann L, Burnod J, Lasne Y, Bethenod M. Antenatal administration of betamethasone: effects upon neonatal blood glucose in premature infants [in French]. Nouv Presse Med. 1979;8(39):3147–3148.
- Rokicki W, Krasnodebski J. Antenatal glucocorticoid administration and neonatal glycemia. Dev Pharmacol Ther. 1987;10(4):307–311.
- Gazquez Serrano IM, Arroyos Plana A, Diaz Morales O, Herraiz Perea C, Holgueras Bragado A. Antenatal corticosteroid therapy and late preterm infant morbidity and mortality [in Spanish]. An Pediatr (Barc). 2014;81(6):374–382.
- Pettit KE, Tran SH, Lee E, Caughey AB. The association of antenatal corticosteroids with neonatal hypoglycemia and hyperbilirubinemia. J Matern Fetal Neonatal Med. 2014;27(7):683–686.
- Aydin M, Derveci U, Hakan N. Neonatal hypoglycemia associated with the antenatal corticosteroids may be secondary to fetal adrenal suppression. J Matern Fetal Neonatal Med. 2015;28(8):892.
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308.
- Asztalos EV, Murphy KE, Willan AR, et al; MACS-5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102–1110.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications [published online ahead of print January 18, 2016]. BJOG. doi:10.1111/1471-0528.13853.
- Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Health consequences of prophylactic exposure to antenatal corticosteroids among children born late preterm or term. Acta Obstet Gynecol Scand. 2012;91(12):1415–1421.
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F195–F200.
- Alexander N, Rosenlocher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381.
- Althabe F, Belizan JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639.
- Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11ß-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdale GR mRNA expression and anxiety-like behavior in the offspring. Eur J Neurosci. 2000;12(3):1047–1054.
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F154–F157.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862.
- Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11Dalziel SR, Walker NK, Parag V, et al. Dalziel SR, Lim VK, Lambert A, et al. Dalziel SR, Rea HH, Walker NK, et al.
Combination Induction Method Speeds Delivery
ATLANTA – Women whose labor was induced by a combination medication and cervical Foley catheter progressed to vaginal delivery almost twice as fast as those induced by a single method.
The combination of misoprostol and a cervical Foley was the most effective, with a mean time to vaginal delivery of 11 hours, compared with 16 hours for the drug and the catheter alone, Dr. Lisa Levine reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
When only vaginal deliveries were considered, the combination was 92% faster than misoprostol alone and 87% faster than Foley alone, said Dr. Levine of the University of Pennsylvania, Philadelphia.
The findings are from a four-armed randomized trial – and the only head-to-head comparison of combination and single-method induction. The findings could be practice-changing, she said.
“More than 20% of pregnant women undergo an induction each year – that’s almost 1 million women,” she said. “If combination methods were used for all these women, there would be more than 3.5 million fewer hours of labor. This would have a large impact on healthcare utilization. If we could find a way to shorten the length of labor without increasing the cesarean delivery rate or neonatal complications, this would have an obvious clinical impact.”
The Foley or Misoprostol for the Management of Induction (FOR MOMI) trial randomized 491 women to four treatment arms: misoprostol alone, Foley cervical catheter alone, misoprostol plus Foley, and oxytocin plus Foley. The primary outcome was time to delivery, regardless of mode. Secondary outcomes were time to vaginal delivery; delivery within 24 hours; time to active labor; maternal and neonatal length of stay; and chorioamnionitis.
There were also composite morbidity outcomes for mother and newborn.
Women in the study were a mean of 27 years old. Most (about 72%) were nulliparous. The mean gestational age at induction was 39 weeks. The mean Bishop score at induction was 3; the mean dilation, 1 cm.
In the primary outcome of time to any delivery, both combination methods led to shorter labor times in both vaginal and cesarean deliveries. Time to delivery was 17 hours in both the misoprostol- and Foley-only groups. It was 13 hours in the misoprostol/Foley combination group, and 14.5 hours in the oxytocin/Foley combination group. Both combination methods led to significantly quicker deliveries in both nulliparous and multiparous women.
The combination methods were also significantly more efficient in the group of only vaginal deliveries. The mean time to vaginal delivery was 11 hours for both combination groups, and 16 hours for both misoprostol- and Foley-only inductions. Again, these differences were seen in both nulliparous and multiparous women.
More women in the combination groups delivered within 24 hours of induction (88% in the misoprostol/Foley group; 84% in the oxytocin/Foley group).
In an analysis that removed all the cesarean deliveries and controlled for other factors, only the combination of misoprostol/Foley retained its significant effect on time to vaginal delivery.
There were no significant between-group differences in the rates of cesarean delivery. The composite measure of maternal morbidity was similar between all the groups. There were no differences in the individual rates of arrest lacerations, endometritis, blood transfusion, infection, readmission, wound dehiscence, or length of stay.
There was no significant difference in any of the neonatal outcomes measures, including Apgar, admission to the neonatal intensive care unit, as well as length of stay, sepsis, or respiratory distress syndrome.
The University of Pennsylvania sponsored the study. Dr. Levine reported having no financial disclosures.
ATLANTA – Women whose labor was induced by a combination medication and cervical Foley catheter progressed to vaginal delivery almost twice as fast as those induced by a single method.
The combination of misoprostol and a cervical Foley was the most effective, with a mean time to vaginal delivery of 11 hours, compared with 16 hours for the drug and the catheter alone, Dr. Lisa Levine reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
When only vaginal deliveries were considered, the combination was 92% faster than misoprostol alone and 87% faster than Foley alone, said Dr. Levine of the University of Pennsylvania, Philadelphia.
The findings are from a four-armed randomized trial – and the only head-to-head comparison of combination and single-method induction. The findings could be practice-changing, she said.
“More than 20% of pregnant women undergo an induction each year – that’s almost 1 million women,” she said. “If combination methods were used for all these women, there would be more than 3.5 million fewer hours of labor. This would have a large impact on healthcare utilization. If we could find a way to shorten the length of labor without increasing the cesarean delivery rate or neonatal complications, this would have an obvious clinical impact.”
The Foley or Misoprostol for the Management of Induction (FOR MOMI) trial randomized 491 women to four treatment arms: misoprostol alone, Foley cervical catheter alone, misoprostol plus Foley, and oxytocin plus Foley. The primary outcome was time to delivery, regardless of mode. Secondary outcomes were time to vaginal delivery; delivery within 24 hours; time to active labor; maternal and neonatal length of stay; and chorioamnionitis.
There were also composite morbidity outcomes for mother and newborn.
Women in the study were a mean of 27 years old. Most (about 72%) were nulliparous. The mean gestational age at induction was 39 weeks. The mean Bishop score at induction was 3; the mean dilation, 1 cm.
In the primary outcome of time to any delivery, both combination methods led to shorter labor times in both vaginal and cesarean deliveries. Time to delivery was 17 hours in both the misoprostol- and Foley-only groups. It was 13 hours in the misoprostol/Foley combination group, and 14.5 hours in the oxytocin/Foley combination group. Both combination methods led to significantly quicker deliveries in both nulliparous and multiparous women.
The combination methods were also significantly more efficient in the group of only vaginal deliveries. The mean time to vaginal delivery was 11 hours for both combination groups, and 16 hours for both misoprostol- and Foley-only inductions. Again, these differences were seen in both nulliparous and multiparous women.
More women in the combination groups delivered within 24 hours of induction (88% in the misoprostol/Foley group; 84% in the oxytocin/Foley group).
In an analysis that removed all the cesarean deliveries and controlled for other factors, only the combination of misoprostol/Foley retained its significant effect on time to vaginal delivery.
There were no significant between-group differences in the rates of cesarean delivery. The composite measure of maternal morbidity was similar between all the groups. There were no differences in the individual rates of arrest lacerations, endometritis, blood transfusion, infection, readmission, wound dehiscence, or length of stay.
There was no significant difference in any of the neonatal outcomes measures, including Apgar, admission to the neonatal intensive care unit, as well as length of stay, sepsis, or respiratory distress syndrome.
The University of Pennsylvania sponsored the study. Dr. Levine reported having no financial disclosures.
ATLANTA – Women whose labor was induced by a combination medication and cervical Foley catheter progressed to vaginal delivery almost twice as fast as those induced by a single method.
The combination of misoprostol and a cervical Foley was the most effective, with a mean time to vaginal delivery of 11 hours, compared with 16 hours for the drug and the catheter alone, Dr. Lisa Levine reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
When only vaginal deliveries were considered, the combination was 92% faster than misoprostol alone and 87% faster than Foley alone, said Dr. Levine of the University of Pennsylvania, Philadelphia.
The findings are from a four-armed randomized trial – and the only head-to-head comparison of combination and single-method induction. The findings could be practice-changing, she said.
“More than 20% of pregnant women undergo an induction each year – that’s almost 1 million women,” she said. “If combination methods were used for all these women, there would be more than 3.5 million fewer hours of labor. This would have a large impact on healthcare utilization. If we could find a way to shorten the length of labor without increasing the cesarean delivery rate or neonatal complications, this would have an obvious clinical impact.”
The Foley or Misoprostol for the Management of Induction (FOR MOMI) trial randomized 491 women to four treatment arms: misoprostol alone, Foley cervical catheter alone, misoprostol plus Foley, and oxytocin plus Foley. The primary outcome was time to delivery, regardless of mode. Secondary outcomes were time to vaginal delivery; delivery within 24 hours; time to active labor; maternal and neonatal length of stay; and chorioamnionitis.
There were also composite morbidity outcomes for mother and newborn.
Women in the study were a mean of 27 years old. Most (about 72%) were nulliparous. The mean gestational age at induction was 39 weeks. The mean Bishop score at induction was 3; the mean dilation, 1 cm.
In the primary outcome of time to any delivery, both combination methods led to shorter labor times in both vaginal and cesarean deliveries. Time to delivery was 17 hours in both the misoprostol- and Foley-only groups. It was 13 hours in the misoprostol/Foley combination group, and 14.5 hours in the oxytocin/Foley combination group. Both combination methods led to significantly quicker deliveries in both nulliparous and multiparous women.
The combination methods were also significantly more efficient in the group of only vaginal deliveries. The mean time to vaginal delivery was 11 hours for both combination groups, and 16 hours for both misoprostol- and Foley-only inductions. Again, these differences were seen in both nulliparous and multiparous women.
More women in the combination groups delivered within 24 hours of induction (88% in the misoprostol/Foley group; 84% in the oxytocin/Foley group).
In an analysis that removed all the cesarean deliveries and controlled for other factors, only the combination of misoprostol/Foley retained its significant effect on time to vaginal delivery.
There were no significant between-group differences in the rates of cesarean delivery. The composite measure of maternal morbidity was similar between all the groups. There were no differences in the individual rates of arrest lacerations, endometritis, blood transfusion, infection, readmission, wound dehiscence, or length of stay.
There was no significant difference in any of the neonatal outcomes measures, including Apgar, admission to the neonatal intensive care unit, as well as length of stay, sepsis, or respiratory distress syndrome.
The University of Pennsylvania sponsored the study. Dr. Levine reported having no financial disclosures.
AT THE PREGNANCY MEETING
Combination induction method speeds delivery
ATLANTA – Women whose labor was induced by a combination medication and cervical Foley catheter progressed to vaginal delivery almost twice as fast as those induced by a single method.
The combination of misoprostol and a cervical Foley was the most effective, with a mean time to vaginal delivery of 11 hours, compared with 16 hours for the drug and the catheter alone, Dr. Lisa Levine reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
When only vaginal deliveries were considered, the combination was 92% faster than misoprostol alone and 87% faster than Foley alone, said Dr. Levine of the University of Pennsylvania, Philadelphia.
The findings are from a four-armed randomized trial – and the only head-to-head comparison of combination and single-method induction. The findings could be practice-changing, she said.
“More than 20% of pregnant women undergo an induction each year – that’s almost 1 million women,” she said. “If combination methods were used for all these women, there would be more than 3.5 million fewer hours of labor. This would have a large impact on healthcare utilization. If we could find a way to shorten the length of labor without increasing the cesarean delivery rate or neonatal complications, this would have an obvious clinical impact.”
The Foley or Misoprostol for the Management of Induction (FOR MOMI) trial randomized 491 women to four treatment arms: misoprostol alone, Foley cervical catheter alone, misoprostol plus Foley, and oxytocin plus Foley. The primary outcome was time to delivery, regardless of mode. Secondary outcomes were time to vaginal delivery; delivery within 24 hours; time to active labor; maternal and neonatal length of stay; and chorioamnionitis.
There were also composite morbidity outcomes for mother and newborn.
Women in the study were a mean of 27 years old. Most (about 72%) were nulliparous. The mean gestational age at induction was 39 weeks. The mean Bishop score at induction was 3; the mean dilation, 1 cm.
In the primary outcome of time to any delivery, both combination methods led to shorter labor times in both vaginal and cesarean deliveries. Time to delivery was 17 hours in both the misoprostol- and Foley-only groups. It was 13 hours in the misoprostol/Foley combination group, and 14.5 hours in the oxytocin/Foley combination group. Both combination methods led to significantly quicker deliveries in both nulliparous and multiparous women.
The combination methods were also significantly more efficient in the group of only vaginal deliveries. The mean time to vaginal delivery was 11 hours for both combination groups, and 16 hours for both misoprostol- and Foley-only inductions. Again, these differences were seen in both nulliparous and multiparous women.
More women in the combination groups delivered within 24 hours of induction (88% in the misoprostol/Foley group; 84% in the oxytocin/Foley group).
In an analysis that removed all the cesarean deliveries and controlled for other factors, only the combination of misoprostol/Foley retained its significant effect on time to vaginal delivery.
There were no significant between-group differences in the rates of cesarean delivery. The composite measure of maternal morbidity was similar between all the groups. There were no differences in the individual rates of arrest lacerations, endometritis, blood transfusion, infection, readmission, wound dehiscence, or length of stay.
There was no significant difference in any of the neonatal outcomes measures, including Apgar, admission to the neonatal intensive care unit, as well as length of stay, sepsis, or respiratory distress syndrome.
The University of Pennsylvania sponsored the study. Dr. Levine reported having no financial disclosures.
ATLANTA – Women whose labor was induced by a combination medication and cervical Foley catheter progressed to vaginal delivery almost twice as fast as those induced by a single method.
The combination of misoprostol and a cervical Foley was the most effective, with a mean time to vaginal delivery of 11 hours, compared with 16 hours for the drug and the catheter alone, Dr. Lisa Levine reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
When only vaginal deliveries were considered, the combination was 92% faster than misoprostol alone and 87% faster than Foley alone, said Dr. Levine of the University of Pennsylvania, Philadelphia.
The findings are from a four-armed randomized trial – and the only head-to-head comparison of combination and single-method induction. The findings could be practice-changing, she said.
“More than 20% of pregnant women undergo an induction each year – that’s almost 1 million women,” she said. “If combination methods were used for all these women, there would be more than 3.5 million fewer hours of labor. This would have a large impact on healthcare utilization. If we could find a way to shorten the length of labor without increasing the cesarean delivery rate or neonatal complications, this would have an obvious clinical impact.”
The Foley or Misoprostol for the Management of Induction (FOR MOMI) trial randomized 491 women to four treatment arms: misoprostol alone, Foley cervical catheter alone, misoprostol plus Foley, and oxytocin plus Foley. The primary outcome was time to delivery, regardless of mode. Secondary outcomes were time to vaginal delivery; delivery within 24 hours; time to active labor; maternal and neonatal length of stay; and chorioamnionitis.
There were also composite morbidity outcomes for mother and newborn.
Women in the study were a mean of 27 years old. Most (about 72%) were nulliparous. The mean gestational age at induction was 39 weeks. The mean Bishop score at induction was 3; the mean dilation, 1 cm.
In the primary outcome of time to any delivery, both combination methods led to shorter labor times in both vaginal and cesarean deliveries. Time to delivery was 17 hours in both the misoprostol- and Foley-only groups. It was 13 hours in the misoprostol/Foley combination group, and 14.5 hours in the oxytocin/Foley combination group. Both combination methods led to significantly quicker deliveries in both nulliparous and multiparous women.
The combination methods were also significantly more efficient in the group of only vaginal deliveries. The mean time to vaginal delivery was 11 hours for both combination groups, and 16 hours for both misoprostol- and Foley-only inductions. Again, these differences were seen in both nulliparous and multiparous women.
More women in the combination groups delivered within 24 hours of induction (88% in the misoprostol/Foley group; 84% in the oxytocin/Foley group).
In an analysis that removed all the cesarean deliveries and controlled for other factors, only the combination of misoprostol/Foley retained its significant effect on time to vaginal delivery.
There were no significant between-group differences in the rates of cesarean delivery. The composite measure of maternal morbidity was similar between all the groups. There were no differences in the individual rates of arrest lacerations, endometritis, blood transfusion, infection, readmission, wound dehiscence, or length of stay.
There was no significant difference in any of the neonatal outcomes measures, including Apgar, admission to the neonatal intensive care unit, as well as length of stay, sepsis, or respiratory distress syndrome.
The University of Pennsylvania sponsored the study. Dr. Levine reported having no financial disclosures.
ATLANTA – Women whose labor was induced by a combination medication and cervical Foley catheter progressed to vaginal delivery almost twice as fast as those induced by a single method.
The combination of misoprostol and a cervical Foley was the most effective, with a mean time to vaginal delivery of 11 hours, compared with 16 hours for the drug and the catheter alone, Dr. Lisa Levine reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
When only vaginal deliveries were considered, the combination was 92% faster than misoprostol alone and 87% faster than Foley alone, said Dr. Levine of the University of Pennsylvania, Philadelphia.
The findings are from a four-armed randomized trial – and the only head-to-head comparison of combination and single-method induction. The findings could be practice-changing, she said.
“More than 20% of pregnant women undergo an induction each year – that’s almost 1 million women,” she said. “If combination methods were used for all these women, there would be more than 3.5 million fewer hours of labor. This would have a large impact on healthcare utilization. If we could find a way to shorten the length of labor without increasing the cesarean delivery rate or neonatal complications, this would have an obvious clinical impact.”
The Foley or Misoprostol for the Management of Induction (FOR MOMI) trial randomized 491 women to four treatment arms: misoprostol alone, Foley cervical catheter alone, misoprostol plus Foley, and oxytocin plus Foley. The primary outcome was time to delivery, regardless of mode. Secondary outcomes were time to vaginal delivery; delivery within 24 hours; time to active labor; maternal and neonatal length of stay; and chorioamnionitis.
There were also composite morbidity outcomes for mother and newborn.
Women in the study were a mean of 27 years old. Most (about 72%) were nulliparous. The mean gestational age at induction was 39 weeks. The mean Bishop score at induction was 3; the mean dilation, 1 cm.
In the primary outcome of time to any delivery, both combination methods led to shorter labor times in both vaginal and cesarean deliveries. Time to delivery was 17 hours in both the misoprostol- and Foley-only groups. It was 13 hours in the misoprostol/Foley combination group, and 14.5 hours in the oxytocin/Foley combination group. Both combination methods led to significantly quicker deliveries in both nulliparous and multiparous women.
The combination methods were also significantly more efficient in the group of only vaginal deliveries. The mean time to vaginal delivery was 11 hours for both combination groups, and 16 hours for both misoprostol- and Foley-only inductions. Again, these differences were seen in both nulliparous and multiparous women.
More women in the combination groups delivered within 24 hours of induction (88% in the misoprostol/Foley group; 84% in the oxytocin/Foley group).
In an analysis that removed all the cesarean deliveries and controlled for other factors, only the combination of misoprostol/Foley retained its significant effect on time to vaginal delivery.
There were no significant between-group differences in the rates of cesarean delivery. The composite measure of maternal morbidity was similar between all the groups. There were no differences in the individual rates of arrest lacerations, endometritis, blood transfusion, infection, readmission, wound dehiscence, or length of stay.
There was no significant difference in any of the neonatal outcomes measures, including Apgar, admission to the neonatal intensive care unit, as well as length of stay, sepsis, or respiratory distress syndrome.
The University of Pennsylvania sponsored the study. Dr. Levine reported having no financial disclosures.
AT THE PREGNANCY MEETING
Key clinical point: The combination of misoprostol and cervical Foley significantly sped time to vaginal delivery, compared with either method alone.
Major finding: The combination was 92% faster than only misoprostol and 82% faster than only Foley for vaginal deliveries.
Data source: The four-armed trial randomized 491 women.
Disclosures: The University of Pennsylvania sponsored the study. Dr. Levine reported having no financial disclosures.
WHO’s psychosocial guidelines for Zika are a useful tool
As the Zika virus spreads across 31 countries in the Americas, bringing with it the threat of microcephaly seen in Brazil, local and international public health experts are scrambling to assess the extent of the threat. Systems for epidemiologic surveillance are emerging, as are guidelines for pregnant women and those of childbearing age.
Amid all of this is the World Health Organization’s recently released guidelines, “Psychosocial Support for Pregnant Women and for Families With Microcephaly and Other Neurological Complications in the Context of Zika Virus” (http://who.int/csr/resources/publications/zika/psychosocial-support/en). These guidelines, an adaptation of previous interventions used in disasters, are a helpful resource for physicians.
The guidelines emphasize eight areas: having accurate information, what information is conveyed, how that information is conveyed, understanding common distress reactions, providing basic support, strengthening social support, teaching stress reduction, and educating mothers about parenting children with microcephaly. Readers familiar with psychological first aid (PFA), used in disaster response to provide basic psychological support, will recognize these elements as distillations of PFA specifically for Zika.
PFA focuses on addressing peoples’ basic physiologic, safety, and social needs as a means of addressing their overall psychological needs. It has been promulgated as a best practice by the National Institute of Mental Health since the Sept. 11, 2001, terrorist attacks.
Another useful aspect of the guidelines is that they are aimed at health professionals in general, rather than mental health professionals in particular. This approach makes sense, because women concerned about potential infection with Zika, of course, are not going to go to a mental health professional to address their anxiety but to an internist, family physician, ob.gyn., or possibly a pediatrician. They are understandably focused on the distressing problem rather than on the distress itself.
Health care professionals now on the frontlines of the Zika public health response may naturally be following many of the principles in the psychosocial guidelines. Nevertheless, they probably would benefit from reviewing them in order to lend some more structure to the psychosocial soundness of their practice. In addition, becoming aware of the guidelines might help those health professionals deal with their own risk for burnout.
In discussing common distress reactions, the guidelines note that severely distressed individuals should be referred for “specialized care,” which means a mental health professional when psychosocial care is discussed in low-resource settings around the world. The overwhelming majority of countries in the Americas where Zika transmission has been reported are low- and middle-income countries, according to the World Bank’s ranking system. As such, they are surely the places where governments are most likely to devote the least amount of their health care budgets to mental health services.
And, even in high-income countries among the list of Zika-affected countries, it is not clear from consulting the World Health Organization’s 2014 Mental Health Atlas that even those countries fund anything more than inpatient psychiatric care (for example, information from Barbados lists only inpatient psychiatric resources). My point? The ranks of mental health professionals in Zika-affected countries who might benefit from the WHO’s Zika guidelines probably are few. Those who are there probably are overwhelmed tending to the preexisting (mostly inpatient) psychiatric needs of their countries.
Fortunately, the WHO’s Mental Health Atlas shows Brazil to be an exception with a comparatively robust outpatient as well as inpatient public mental health system. This is fortuitous given that Brazil for the moment is the center of the surge in microcephaly. Hopefully, the Northeastern region of Brazil, where that surge is highest, has a proportionate share of Brazil’s mental health resources. For mental health professionals there and potentially elsewhere in Zika-affected countries, the guidelines for psychosocial support can prove to be an essential tool.
To the extent that mental health professionals can and should provide support to their Zika-affected communities, these guidelines will help them to “stand down” from a traditional psychiatric model of care to a more normalizing one, where diagnoses and treatment are not the focus of attention. This was certainly the case in the comparable setting of post-Ebola Liberia, where mental health clinicians trained by the Carter Center found PFA central to what they could offer their devastated communities and to gaining unprecedented acceptance from those communities (unpublished observations).
As of this writing, the Zika virus has not spared the continental United States. Florida has been hit the hardest, followed by Texas, according to data from the Centers for Disease Control and Prevention. Those of us looking to help patients deal with the possibility of coming in contact with the Zika virus should remember the WHO psychosocial guidelines. They can help health professionals integrate mental health into their practices, and help mental health professionals transfer their skills and knowledge to their communities.
Dr. Katz is associate clinical professor of psychiatry and medical education, and director of the program in global mental health, at the Icahn School of Medicine at Mount Sinai, New York.
As the Zika virus spreads across 31 countries in the Americas, bringing with it the threat of microcephaly seen in Brazil, local and international public health experts are scrambling to assess the extent of the threat. Systems for epidemiologic surveillance are emerging, as are guidelines for pregnant women and those of childbearing age.
Amid all of this is the World Health Organization’s recently released guidelines, “Psychosocial Support for Pregnant Women and for Families With Microcephaly and Other Neurological Complications in the Context of Zika Virus” (http://who.int/csr/resources/publications/zika/psychosocial-support/en). These guidelines, an adaptation of previous interventions used in disasters, are a helpful resource for physicians.
The guidelines emphasize eight areas: having accurate information, what information is conveyed, how that information is conveyed, understanding common distress reactions, providing basic support, strengthening social support, teaching stress reduction, and educating mothers about parenting children with microcephaly. Readers familiar with psychological first aid (PFA), used in disaster response to provide basic psychological support, will recognize these elements as distillations of PFA specifically for Zika.
PFA focuses on addressing peoples’ basic physiologic, safety, and social needs as a means of addressing their overall psychological needs. It has been promulgated as a best practice by the National Institute of Mental Health since the Sept. 11, 2001, terrorist attacks.
Another useful aspect of the guidelines is that they are aimed at health professionals in general, rather than mental health professionals in particular. This approach makes sense, because women concerned about potential infection with Zika, of course, are not going to go to a mental health professional to address their anxiety but to an internist, family physician, ob.gyn., or possibly a pediatrician. They are understandably focused on the distressing problem rather than on the distress itself.
Health care professionals now on the frontlines of the Zika public health response may naturally be following many of the principles in the psychosocial guidelines. Nevertheless, they probably would benefit from reviewing them in order to lend some more structure to the psychosocial soundness of their practice. In addition, becoming aware of the guidelines might help those health professionals deal with their own risk for burnout.
In discussing common distress reactions, the guidelines note that severely distressed individuals should be referred for “specialized care,” which means a mental health professional when psychosocial care is discussed in low-resource settings around the world. The overwhelming majority of countries in the Americas where Zika transmission has been reported are low- and middle-income countries, according to the World Bank’s ranking system. As such, they are surely the places where governments are most likely to devote the least amount of their health care budgets to mental health services.
And, even in high-income countries among the list of Zika-affected countries, it is not clear from consulting the World Health Organization’s 2014 Mental Health Atlas that even those countries fund anything more than inpatient psychiatric care (for example, information from Barbados lists only inpatient psychiatric resources). My point? The ranks of mental health professionals in Zika-affected countries who might benefit from the WHO’s Zika guidelines probably are few. Those who are there probably are overwhelmed tending to the preexisting (mostly inpatient) psychiatric needs of their countries.
Fortunately, the WHO’s Mental Health Atlas shows Brazil to be an exception with a comparatively robust outpatient as well as inpatient public mental health system. This is fortuitous given that Brazil for the moment is the center of the surge in microcephaly. Hopefully, the Northeastern region of Brazil, where that surge is highest, has a proportionate share of Brazil’s mental health resources. For mental health professionals there and potentially elsewhere in Zika-affected countries, the guidelines for psychosocial support can prove to be an essential tool.
To the extent that mental health professionals can and should provide support to their Zika-affected communities, these guidelines will help them to “stand down” from a traditional psychiatric model of care to a more normalizing one, where diagnoses and treatment are not the focus of attention. This was certainly the case in the comparable setting of post-Ebola Liberia, where mental health clinicians trained by the Carter Center found PFA central to what they could offer their devastated communities and to gaining unprecedented acceptance from those communities (unpublished observations).
As of this writing, the Zika virus has not spared the continental United States. Florida has been hit the hardest, followed by Texas, according to data from the Centers for Disease Control and Prevention. Those of us looking to help patients deal with the possibility of coming in contact with the Zika virus should remember the WHO psychosocial guidelines. They can help health professionals integrate mental health into their practices, and help mental health professionals transfer their skills and knowledge to their communities.
Dr. Katz is associate clinical professor of psychiatry and medical education, and director of the program in global mental health, at the Icahn School of Medicine at Mount Sinai, New York.
As the Zika virus spreads across 31 countries in the Americas, bringing with it the threat of microcephaly seen in Brazil, local and international public health experts are scrambling to assess the extent of the threat. Systems for epidemiologic surveillance are emerging, as are guidelines for pregnant women and those of childbearing age.
Amid all of this is the World Health Organization’s recently released guidelines, “Psychosocial Support for Pregnant Women and for Families With Microcephaly and Other Neurological Complications in the Context of Zika Virus” (http://who.int/csr/resources/publications/zika/psychosocial-support/en). These guidelines, an adaptation of previous interventions used in disasters, are a helpful resource for physicians.
The guidelines emphasize eight areas: having accurate information, what information is conveyed, how that information is conveyed, understanding common distress reactions, providing basic support, strengthening social support, teaching stress reduction, and educating mothers about parenting children with microcephaly. Readers familiar with psychological first aid (PFA), used in disaster response to provide basic psychological support, will recognize these elements as distillations of PFA specifically for Zika.
PFA focuses on addressing peoples’ basic physiologic, safety, and social needs as a means of addressing their overall psychological needs. It has been promulgated as a best practice by the National Institute of Mental Health since the Sept. 11, 2001, terrorist attacks.
Another useful aspect of the guidelines is that they are aimed at health professionals in general, rather than mental health professionals in particular. This approach makes sense, because women concerned about potential infection with Zika, of course, are not going to go to a mental health professional to address their anxiety but to an internist, family physician, ob.gyn., or possibly a pediatrician. They are understandably focused on the distressing problem rather than on the distress itself.
Health care professionals now on the frontlines of the Zika public health response may naturally be following many of the principles in the psychosocial guidelines. Nevertheless, they probably would benefit from reviewing them in order to lend some more structure to the psychosocial soundness of their practice. In addition, becoming aware of the guidelines might help those health professionals deal with their own risk for burnout.
In discussing common distress reactions, the guidelines note that severely distressed individuals should be referred for “specialized care,” which means a mental health professional when psychosocial care is discussed in low-resource settings around the world. The overwhelming majority of countries in the Americas where Zika transmission has been reported are low- and middle-income countries, according to the World Bank’s ranking system. As such, they are surely the places where governments are most likely to devote the least amount of their health care budgets to mental health services.
And, even in high-income countries among the list of Zika-affected countries, it is not clear from consulting the World Health Organization’s 2014 Mental Health Atlas that even those countries fund anything more than inpatient psychiatric care (for example, information from Barbados lists only inpatient psychiatric resources). My point? The ranks of mental health professionals in Zika-affected countries who might benefit from the WHO’s Zika guidelines probably are few. Those who are there probably are overwhelmed tending to the preexisting (mostly inpatient) psychiatric needs of their countries.
Fortunately, the WHO’s Mental Health Atlas shows Brazil to be an exception with a comparatively robust outpatient as well as inpatient public mental health system. This is fortuitous given that Brazil for the moment is the center of the surge in microcephaly. Hopefully, the Northeastern region of Brazil, where that surge is highest, has a proportionate share of Brazil’s mental health resources. For mental health professionals there and potentially elsewhere in Zika-affected countries, the guidelines for psychosocial support can prove to be an essential tool.
To the extent that mental health professionals can and should provide support to their Zika-affected communities, these guidelines will help them to “stand down” from a traditional psychiatric model of care to a more normalizing one, where diagnoses and treatment are not the focus of attention. This was certainly the case in the comparable setting of post-Ebola Liberia, where mental health clinicians trained by the Carter Center found PFA central to what they could offer their devastated communities and to gaining unprecedented acceptance from those communities (unpublished observations).
As of this writing, the Zika virus has not spared the continental United States. Florida has been hit the hardest, followed by Texas, according to data from the Centers for Disease Control and Prevention. Those of us looking to help patients deal with the possibility of coming in contact with the Zika virus should remember the WHO psychosocial guidelines. They can help health professionals integrate mental health into their practices, and help mental health professionals transfer their skills and knowledge to their communities.
Dr. Katz is associate clinical professor of psychiatry and medical education, and director of the program in global mental health, at the Icahn School of Medicine at Mount Sinai, New York.
Zika more complex public health challenge than Ebola
The vector-borne and often asymptomatic nature of Zika virus infection makes it a more complex public health challenge than Ebola, researchers say.
The Zika virus was first isolated from a macaque in Uganda in 1947, and has historically been restricted to Africa and Asia. However since its introduction to Brazil in 2014 or early 2015, possibly via Polynesia, it has spread rapidly and estimates now point to between 440,000 and 1,300,000 cases of Zika virus infection in Brazil during 2015.
In the past, Zika virus infection in adults has presented with non-life-threatening symptoms including mild fever, maculopapular rash, arthralgia, myalgia, headache, retro-orbital pain and vomiting.
Writing in the March 1 online edition of PLoS Neglected Tropical Diseases, researchers say the viral variant currently associated with the outbreak in Brazil is presenting a new and more challenging public health problem.
“What makes this outbreak a high priority global public health concern is the association with incidence of birth defects involving the central nervous system and the apparent increased incidence of Guillain-Barré syndrome,” wrote Dr. Robert W. Malone, from RW Malone MD, and his coauthors (PLoS Negl Trop Dis. 2016 Mar 1. doi: 10.1371/journal.pntd.0004530).
They cited one retrospective study in French Polynesia that suggested there was a ratio of one case of Zika-associated Guillain-Barré syndrome for every 208 suspected cases of Zika virus infection.
The outbreak has also been linked to an unusually high incidence of the otherwise rare microcephaly, with Brazil recording a 20-fold increase in incidence during 2015. The connection with Zika virus is supported by a case study in which large numbers of viral particles were found in the central nervous system tissue of a microcephalic Zika-infected fetus.
Based on estimates of the overall incidence of Zika virus infection, researchers have calculated that Brazilian mothers infected with the virus are 3,700-11,000 times more likely to deliver infants with primary microcephaly, compared with those who are not infected.
There are still some key uncertainties around the transmission of Zika virus, the authors said.
“The degree to which humans, nonhuman primates, or other animals can amplify and transmit the virus to insect vectors is poorly understood,” they wrote. “The typical range and types of insect vectors observed in the past may not be predictive for the virus now circulating in the Americas [and] infectivity of the circulating strain, viremia levels, duration, and risk of occult persistence are not yet understood.”
The virus is transmitted primarily by mosquito vectors such as Aedes aegypti and Aedes albopictus, with primates – including humans – the best documented animal reservoir.
“Recent reports indicate the potential for both human blood-borne and sexual transmission of Zika virus, including prolonged presence of virus in semen,” the authors wrote.
The virus has also been found in the saliva of infected individuals, and viral sequences have been identified in breast milk.Commenting on possible medical countermeasures to combat the spread and impact of the Zika virus, the paper’s authors noted that due to the absence of an existing vaccine and long potential development times for candidate vaccines, other prophylactics and therapeutics need to be explored.
In particular, they called for development and deployment of Zika diagnostics to regional clinical health laboratories, discussions between obstetricians and patients about the risks to ongoing or planned pregnancies, and resources for neurologists dealing with “unprecedented” Guillain-Barré syndrome outbreaks.
“Perhaps the biggest challenge with Zika will be to recognize it for what it is: a new disease which does not fit the epidemiology or response paradigm of Ebola or dengue and which will demand effort, resources, unparalleled collaboration, and above all, open mindedness in formulating responses.”
Two authors declared employment with and equity holdings in RW Malone MD, and two authors declared employment with – and one of these also declared equity holdings in – Nanotherapeutics.
The vector-borne and often asymptomatic nature of Zika virus infection makes it a more complex public health challenge than Ebola, researchers say.
The Zika virus was first isolated from a macaque in Uganda in 1947, and has historically been restricted to Africa and Asia. However since its introduction to Brazil in 2014 or early 2015, possibly via Polynesia, it has spread rapidly and estimates now point to between 440,000 and 1,300,000 cases of Zika virus infection in Brazil during 2015.
In the past, Zika virus infection in adults has presented with non-life-threatening symptoms including mild fever, maculopapular rash, arthralgia, myalgia, headache, retro-orbital pain and vomiting.
Writing in the March 1 online edition of PLoS Neglected Tropical Diseases, researchers say the viral variant currently associated with the outbreak in Brazil is presenting a new and more challenging public health problem.
“What makes this outbreak a high priority global public health concern is the association with incidence of birth defects involving the central nervous system and the apparent increased incidence of Guillain-Barré syndrome,” wrote Dr. Robert W. Malone, from RW Malone MD, and his coauthors (PLoS Negl Trop Dis. 2016 Mar 1. doi: 10.1371/journal.pntd.0004530).
They cited one retrospective study in French Polynesia that suggested there was a ratio of one case of Zika-associated Guillain-Barré syndrome for every 208 suspected cases of Zika virus infection.
The outbreak has also been linked to an unusually high incidence of the otherwise rare microcephaly, with Brazil recording a 20-fold increase in incidence during 2015. The connection with Zika virus is supported by a case study in which large numbers of viral particles were found in the central nervous system tissue of a microcephalic Zika-infected fetus.
Based on estimates of the overall incidence of Zika virus infection, researchers have calculated that Brazilian mothers infected with the virus are 3,700-11,000 times more likely to deliver infants with primary microcephaly, compared with those who are not infected.
There are still some key uncertainties around the transmission of Zika virus, the authors said.
“The degree to which humans, nonhuman primates, or other animals can amplify and transmit the virus to insect vectors is poorly understood,” they wrote. “The typical range and types of insect vectors observed in the past may not be predictive for the virus now circulating in the Americas [and] infectivity of the circulating strain, viremia levels, duration, and risk of occult persistence are not yet understood.”
The virus is transmitted primarily by mosquito vectors such as Aedes aegypti and Aedes albopictus, with primates – including humans – the best documented animal reservoir.
“Recent reports indicate the potential for both human blood-borne and sexual transmission of Zika virus, including prolonged presence of virus in semen,” the authors wrote.
The virus has also been found in the saliva of infected individuals, and viral sequences have been identified in breast milk.Commenting on possible medical countermeasures to combat the spread and impact of the Zika virus, the paper’s authors noted that due to the absence of an existing vaccine and long potential development times for candidate vaccines, other prophylactics and therapeutics need to be explored.
In particular, they called for development and deployment of Zika diagnostics to regional clinical health laboratories, discussions between obstetricians and patients about the risks to ongoing or planned pregnancies, and resources for neurologists dealing with “unprecedented” Guillain-Barré syndrome outbreaks.
“Perhaps the biggest challenge with Zika will be to recognize it for what it is: a new disease which does not fit the epidemiology or response paradigm of Ebola or dengue and which will demand effort, resources, unparalleled collaboration, and above all, open mindedness in formulating responses.”
Two authors declared employment with and equity holdings in RW Malone MD, and two authors declared employment with – and one of these also declared equity holdings in – Nanotherapeutics.
The vector-borne and often asymptomatic nature of Zika virus infection makes it a more complex public health challenge than Ebola, researchers say.
The Zika virus was first isolated from a macaque in Uganda in 1947, and has historically been restricted to Africa and Asia. However since its introduction to Brazil in 2014 or early 2015, possibly via Polynesia, it has spread rapidly and estimates now point to between 440,000 and 1,300,000 cases of Zika virus infection in Brazil during 2015.
In the past, Zika virus infection in adults has presented with non-life-threatening symptoms including mild fever, maculopapular rash, arthralgia, myalgia, headache, retro-orbital pain and vomiting.
Writing in the March 1 online edition of PLoS Neglected Tropical Diseases, researchers say the viral variant currently associated with the outbreak in Brazil is presenting a new and more challenging public health problem.
“What makes this outbreak a high priority global public health concern is the association with incidence of birth defects involving the central nervous system and the apparent increased incidence of Guillain-Barré syndrome,” wrote Dr. Robert W. Malone, from RW Malone MD, and his coauthors (PLoS Negl Trop Dis. 2016 Mar 1. doi: 10.1371/journal.pntd.0004530).
They cited one retrospective study in French Polynesia that suggested there was a ratio of one case of Zika-associated Guillain-Barré syndrome for every 208 suspected cases of Zika virus infection.
The outbreak has also been linked to an unusually high incidence of the otherwise rare microcephaly, with Brazil recording a 20-fold increase in incidence during 2015. The connection with Zika virus is supported by a case study in which large numbers of viral particles were found in the central nervous system tissue of a microcephalic Zika-infected fetus.
Based on estimates of the overall incidence of Zika virus infection, researchers have calculated that Brazilian mothers infected with the virus are 3,700-11,000 times more likely to deliver infants with primary microcephaly, compared with those who are not infected.
There are still some key uncertainties around the transmission of Zika virus, the authors said.
“The degree to which humans, nonhuman primates, or other animals can amplify and transmit the virus to insect vectors is poorly understood,” they wrote. “The typical range and types of insect vectors observed in the past may not be predictive for the virus now circulating in the Americas [and] infectivity of the circulating strain, viremia levels, duration, and risk of occult persistence are not yet understood.”
The virus is transmitted primarily by mosquito vectors such as Aedes aegypti and Aedes albopictus, with primates – including humans – the best documented animal reservoir.
“Recent reports indicate the potential for both human blood-borne and sexual transmission of Zika virus, including prolonged presence of virus in semen,” the authors wrote.
The virus has also been found in the saliva of infected individuals, and viral sequences have been identified in breast milk.Commenting on possible medical countermeasures to combat the spread and impact of the Zika virus, the paper’s authors noted that due to the absence of an existing vaccine and long potential development times for candidate vaccines, other prophylactics and therapeutics need to be explored.
In particular, they called for development and deployment of Zika diagnostics to regional clinical health laboratories, discussions between obstetricians and patients about the risks to ongoing or planned pregnancies, and resources for neurologists dealing with “unprecedented” Guillain-Barré syndrome outbreaks.
“Perhaps the biggest challenge with Zika will be to recognize it for what it is: a new disease which does not fit the epidemiology or response paradigm of Ebola or dengue and which will demand effort, resources, unparalleled collaboration, and above all, open mindedness in formulating responses.”
Two authors declared employment with and equity holdings in RW Malone MD, and two authors declared employment with – and one of these also declared equity holdings in – Nanotherapeutics.
FROM PLOS NEGLECTED TROPICAL DISEASES
Readers weigh in on vaginal cleansing prior to cesarean delivery
“SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
In his January 2016 Editorial, Editor in Chief Robert L. Barbieri, MD, presented evidence supporting the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery (CD) to prevent postoperative endometritis. He then asked readers if they would consider adopting such a practice. More than 250 readers weighed in through the Quick Poll at obgmanagement.com, and many readers sent in letters with follow-up questions and comments on controlling bacterial contamination, vaginal seeding, etc. Here are some of the letters, along with Dr. Barbieri’s response and the Quick Poll results.
A contradiction in definitions?
There seems to be a contradiction in definitions. The second sentence of the article defines endometritis as the presence of fever plus low abdominal tenderness. However, the studies presented state that vaginal cleansing pre-CD decreased endometritis but did not decrease postpartum fever. Is this not a discrepancy?
Nancy Kerr, MD, MPH
Albuquerque, New Mexico
A question about povidone-iodine
Have any studies been done on newborn iodine levels after vaginal cleansing with povidone-iodine prior to CD?
G. Millard Simmons Jr, MD
Hilton Head, Bluffton, South Carolina
Additional tips for controlling bacterial contamination
Dr. Barbieri’s editorial on vaginal cleansing prior to CD is eye opening. I have a few additional suggestions to control bacterial contamination.
First, I examine my patients in labor as few times as necessary, and I ask the nurses (RNs) not to place their fingers in the patient’s vagina while she is pushing. I remove the Foley catheter when I feel progress (descent of fetal head) is being achieved. In addition, physicians as well as RNs should consider changing their scrubs between deliveries, as I believe that bacterial contamination is splattered all over the place, especially into the birth canal. These methods have worked for me in my over-20 years of practice.
I also firmly remind the RN circulator to perform a generous vaginal cleanse with povidone-iodine, in addition to the usual intravenous prophylaxis, before hysterectomy.
Luis Leyva Jr, MD
Miami, Florida
Mixed feelings
My first reaction to this Editorial was: Is this a solution in search of a problem? That is to say, how much of a clinical problem is endometritis after CD? Are we really treating the proposed problem, and does treatment affect long-term outcomes?
Upon reflection, I have concluded that vaginal cleansing pre-CD does intuitively make sense. What sways me in this direction is that the practice is simple, easy, and inexpensive. Since we typically have the patient positioned for Foley catheter insertion, performing vaginal cleansing as we put in the Foley would be easy. If vaginal cleansing were to be done, I definitely would be in favor of doing such practice liberally—for all CDs to make vaginal cleansing part of the “routine.”
Keep in mind that we are still chasing a problem of little clinical significance.
The biggest accomplishment has been to get everyone to give antibiotics preoperatively rather than after cutting the umbilical cord. We knew that this was best practice as early as the late 1980s/early 1990s, and I have been fighting this battle ever since. Believe it or not, there are still a few holdouts.
George H. Davis, DO
Johnson City, Tennessee
Would vaginal cleansing benefit all women in labor?
Vaginal cleansing before CD reminds me of my residency days when all women having hysterectomies were admitted early and given povidone-iodine (Betadine) douches the evening before surgery (unless an iodine allergy was present).
While reading your Editorial, I had several thoughts and questions. 1) Since vaginal cleansing seems to benefit CD patients, might it not benefit all laboring patients? 2) Is the timing of vaginal cleansing critical? 3) Should we do vaginal cleansing on all laboring patients if timing is not critical?
I plan to bring up the topic of vaginal cleansing for CD with my colleagues at our next department meeting, since it seems like such a simple, logical, inexpensive, and beneficial thing to do.
Douglas G. Tolley, MD
Yuba City, California
An early study on using povidone-iodine gel before CD
When I was a chief resident at Kings County Hospital in 1973, we had a very high rate of post-CD endometritis. I conducted a small study on the use of povidone-iodine gel in the last month of pregnancy. Before commencing, we confirmed that the gel did not interfere with diagnosing ruptured membranes.
Obstetric service patients were randomly divided into “A” and “B” groups. The A patients were asked to use povidone-iodine gel at night for the last 2 weeks before their estimated due date. When admitted in labor, they were asked to confirm its use. When a resident diagnosed post-CD endometritis, we kept track of which group the patient was in and whether or not that patient had used povidone-iodine. Approximately 100 infected patients were evaluated from each group.
As it turned out, there were about 3 times the number of infections among the patients who did not use povidone-iodine than among those who said they used it. It did not seem to matter how many times povidone-iodine was used. The “As” who did not use povidone-iodine had results similar to the “Bs.”
It was many years ago, and the study design was crude. However, it does seem to support the suggestion for vaginal cleansing.
Steve Ross, MD
Port Jefferson, New York
Two different ideas about the vaginal biome
This Editorial is timely in that Dr. Dominguez-Bello and colleagues recently published an article in Nature Medicine titled, “Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer.”1 Dr. Dominguez-Bello is one of the founders of the idea of “vaginal seeding,” or using the natural biome of the vagina on a newborn immediately after CD by swabbing the baby with the bacteria from the vagina.
I find it interesting that there are two very different ideas about the biome at this time. Vaginal seeding is a new trend that a few patients have asked about during prenatal care. The jury is still out on seeding, but a larger study is currently underway at New York University. Of course, infection is one of the risks of seeding. I appreciate hearing both sides of the issue.
Deborah Herchelroath, DO
Harrisburg, Pennsylvania
Reference
- Dominguez-Bello MG, De Jesus-Labor KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer [published online ahead of print February 1, 2016]. Nat Med. doi:10.1038/nm.4039.
Dr. Barbieri responds
I would like to thank our readers for taking the time from their busy schedules to write about their clinical experiences and current practices for reducing infectious complications following CD.
Dr. Kerr raises the important issue of the apparent contradictory finding of the beneficial impact of vaginal cleansing on endometritis without a beneficial effect on the overall rate of fever. In the trial reported by Starr,1 fever was defined as a temperature above 38˚C at any time after CD and endometritis was defined as a temperature above 38.4˚C PLUS uterine tenderness occurring more than 24 hours after CD. Given these 2 definitions one can understand the differential effect of vaginal cleansing on fever versus endometritis.
Dr. Simmons raises the intriguing question of the impact of an iodine-containing surgical preparation on newborn thyroid function. There are few studies addressing this issue. One study reports a transient increase in thyroid-stimulating hormone (TSH) levels in a small percentage of newborns whose mothers received an iodine preparation.2 Another study reports no effect of an iodine surgical preparation on newborn thyroid function indices.3
I agree with the guidance of Drs. Leyva and Davis that we can help prevent postcesarean endometritis by minimizing the number of cervical examinations, changing scrubs between deliveries, and by ensuring that an intravenous anti‑ biotic is given before skin incision.
Dr. Tolley wonders if all women should receive vaginal cleansing, regardless of delivery route. It is possible that such an approach would be effective and it deserves study. Given the lower rate of endometritis following vaginal delivery compared with CD, many more women having a vaginal delivery would need to be treated to prevent one case of endometritis. Dr. Ross mentions his experience with the benefit of outpatient vaginal cleansing in the 2 weeks prior to delivery. Many general surgeons are recommending that their patients shower with chlorhexidine the day before surgery in order to reduce the rate of postoperative infection. Short-term and long-term outpatient vaginal cleansing prior to delivery deserves additional study.
Dr. Herchelroath raises the possibility that vaginal cleansing will decrease the ability of the newborn to develop a normal microbiome because it may not be exposed to sufficient vaginal bacteria. This possibility certainly deserves additional study.
The questions and guidance of our readers were incredibly helpful and stimulating. Thank you for sharing your perspective.
References
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Nili F, Hantoushzadeh S, Alimohamadi A, et al. Iodine-containing disinfectants in preparation for cesarean section: impact on thyroid profile in cord blood. Postgrad Med J. 2015;91(1082):681–684.
- Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. The effect of type of delivery and povidone-iodine application at delivery on cord dried-blood-specimen thyrotropin level and the rate of hyperthyrotropinemia in mature and normal-birth-weight neonates residing in an iodine-replete area. Thyroid. 2007;17(11):1097–1102.
“CELL-FREE DNA SCREENING FOR WOMEN AT LOW RISK FOR FETAL ANEUPLOIDY” MARY E. NORTON, MD (JANUARY 2016)
The price of cfDNA screening is dropping
I found Dr. Norton’s article on cell-free DNA (cfDNA) screening for women at low risk for fetal abnormalities to be enlightening and educational. The section addressing cost-effectiveness, however, was somewhat obsolete. The referenced study by Cuckle and colleagues,1 which estimated the cost of cfDNA per case of Down syndrome in low-risk patients at $3.6 million, was published in 2013. With 4 major companies in the market, the cost/benefit ratio has been changing rapidly. At least one company has dropped the cost of the cfDNA test nearly 80% from 2015 to 2016, making the above reference irrelevant. Recently, Ariosa dropped the price of their Harmony cfDNA test to just $119 in our area, regardless of a patient’s insurance or poverty level. This is significantly less than the cost of performing an early screen and is being welcomed by my patients even after substantial counseling on the test’s limitations in the low-risk population. Natera, another laboratory with a similar test, offers a low-cost option. However, patients must provide proof that their income is below a specified level.
Guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) likely will have a hard time keeping up with the cost-effectiveness of noninvasive prenatal testing, as the price continues to be dynamic.
Samuel Wolf, DO
Panama City, Florida
Reference
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
“DOES THE DISCONTINUATION OF MENOPAUSAL HORMONE THERAPY AFFECT A WOMAN’S CARDIOVASCULAR RISK?”
ANDREW M. KAUNITZ, MD; JOANN E. MANSON, MD, DRPH; AND CYNTHIA A. STUENKEL, MD(EXAMINING THE EVIDENCE; DECEMBER 2015)
Disagrees with conclusion
In their expert commentary, Drs. Kaunitz, Manson, and Stuenkel state:
They support this claim by citing Heiss 2008.1 In fact, however, the Women’s Health Initiative (WHI) data show opposite to their statement: In the WHI, all-cause mortality was increased among the women who were assigned to estrogen-progestin therapy (EPT) relative to those who were assigned to placebo within the 3 years of EPT cessation (hazard ratio [HR], 1.15; 95% confidence interval [CI], 0.95–1.39). More importantly, mortality was significantly increased among women who were originally assigned to EPT relative to those who were assigned to placebo and were at least 80% adherent with intervention (HR, 1.53; 95% CI, 1.04–2.24). Thus, the statement by Drs. Kaunitz, Manson, and Stuenkel is incorrect.
In addition to the WHI studies, data are available from at least 2 other randomized controlled trials addressing the issue of HT withdrawal. In the Heart and Estrogen/progestin Replacement Study (HERS) II,2 the unblinded 2.7-year follow-up to the HERS trial, women originally assigned to EPT had a 3.3-fold higher rate of ventricular arrhythmia requiring resuscitation than women assigned to placebo (HR, 3.30; 95% CI, 1.08–10.10). During the first 6 months of posttrial follow-up of the Women’s Estrogen for Stroke Trial (WEST),3 there were 3 fatal strokes and 18 nonfatal strokes among the women originally randomized to estradiol therapy; there were 9 strokes (1 fatal and 8 nonfatal) among the women originally assigned to placebo (HR, 2.3; 95% CI, 1.1–5.0; P = .03).
In our study we detected that women who stopped HT, compared with women who continued HT, had a 2.3-fold (95% CI, 2.12–2.50) greater risk of cardiac death within the first post-HT year and a 1.3-fold (95% CI, 1.21–1.31) greater risk of cardiac death more than 1 year after stopping HT.4 In addition, women who stopped HT, compared with women who continuedHT, had a 2.5-fold (95% CI, 2.28–2.77) greater risk of dying from stroke within the first post-HT year and a 1.3-fold (95% CI, 1.19–1.31) greater risk of dying from stroke more than 1 year after stopping HT. We believe that these data substantially further our understanding of the posttrial data from WHI, as well as HERS and WEST. Thus, cumulative data support that HT withdrawal potentially has detrimental implications for women. In total, the data are highly informative when counseling women regarding use or discontinuation of HT.
Tomi Mikkola, MD
Helsinki, Finland
References
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- Grady D, Herrington D, Bittner V, et al; HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002;288(9):1064]. JAMA. 2002;288(1):49–57.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–4594.
Drs. Kaunitz, Manson, and Stuenkel respond
We thank Dr. Mikkola for his response to our commentary, but we do not agree with his interpretation of the WHI reports or our conclusions. As we originally stated, the WHI trial of estrogen-only therapy (ET) and EPT provides an opportunity to observe outcomes in the largest randomized controlled trial of HT in healthy postmenopausal women. Our commentary was based on the most recent, 13-year follow-up of the WHI trials,1 and we are confident in the accuracy of our presentation of the results.
As the debate apparently focuses on the safety of stopping HT, we wish to reiterate, for those who may not be familiar with the data, that, in the ET trial, all-cause mortality declined (although not significantly) after stopping ET, as summarized here:
HR (95% CI) | |
Intervention phase | 1.03 (0.88–1.21) |
Postintervention phase (after stopping study medication) | 0.96 (0.84–1.10) |
Cumulative 13 years of follow-up | 0.99 (0.90–1.10) |
Similarly, in the EPT trial, as the following findings indicate, stopping HT did not increase all-cause mortality:
HR (95% CI) | |
Intervention phase | 0.97 (0.81–1.16) |
Postintervention phase (afterstopping study medication) | 1.01 (0.91–1.11) |
Cumulative 13 years of follow-up | 0.99 (0.91–1.08) |
Again, these findings from the largest randomized trial of HT in healthy postmenopausal women are adequate for us to conclude that stopping HT does not elevate risk of mortality. Among all women participating in the WHI HT trials, HRs for coronary heart disease, pulmonary embolism, stroke, and cardiovascular disease mortality likewise were lower (better) after stopping treatment than during the intervention phase. The results for these outcomes in younger women followed similar patterns but, due to smaller numbers of events, could not be tested formally for differences in time trends.
Moreover, the data Dr. Mikkola cites from analyses conducted 3 years postcessation2 reflected a borderline increased risk of cancer mortality that emerged in the EPT trial after stopping treatment. This clearly was related to the prolonged effects of EPT on breast cancer and other cancers, given the known latency period for cancer, and was not observed in the ET trial postcessation. The risk elevation in the EPT trial became attenuated with longer follow-up and, as of 13 years, the HRs for cancer mortality were 1.07 (0.93–1.23) in the EPT trial and 0.95 (0.81–1.13) in the ET trial.
It is interesting that Dr. Mikkola now inculcates his interpretation of his findings3 with those from secondary prevention trials such as the Heart and Estrogen/progestin Replacement Study and the Women’s Estrogen for Stroke Trial, neither of which was included as corroborative evidence in the discussion section of his originally published manuscript, and neither of which is considered applicable to healthy postmenopausal women taking HT for treatment of menopausal symptoms. Based on these findings, we do not recommend that clinicians counsel women that stopping HT increases their risk of cardiovascular or overall mortality. Thank you for the opportunity to clarify the evidence and our position.
References
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
“SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
In his January 2016 Editorial, Editor in Chief Robert L. Barbieri, MD, presented evidence supporting the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery (CD) to prevent postoperative endometritis. He then asked readers if they would consider adopting such a practice. More than 250 readers weighed in through the Quick Poll at obgmanagement.com, and many readers sent in letters with follow-up questions and comments on controlling bacterial contamination, vaginal seeding, etc. Here are some of the letters, along with Dr. Barbieri’s response and the Quick Poll results.
A contradiction in definitions?
There seems to be a contradiction in definitions. The second sentence of the article defines endometritis as the presence of fever plus low abdominal tenderness. However, the studies presented state that vaginal cleansing pre-CD decreased endometritis but did not decrease postpartum fever. Is this not a discrepancy?
Nancy Kerr, MD, MPH
Albuquerque, New Mexico
A question about povidone-iodine
Have any studies been done on newborn iodine levels after vaginal cleansing with povidone-iodine prior to CD?
G. Millard Simmons Jr, MD
Hilton Head, Bluffton, South Carolina
Additional tips for controlling bacterial contamination
Dr. Barbieri’s editorial on vaginal cleansing prior to CD is eye opening. I have a few additional suggestions to control bacterial contamination.
First, I examine my patients in labor as few times as necessary, and I ask the nurses (RNs) not to place their fingers in the patient’s vagina while she is pushing. I remove the Foley catheter when I feel progress (descent of fetal head) is being achieved. In addition, physicians as well as RNs should consider changing their scrubs between deliveries, as I believe that bacterial contamination is splattered all over the place, especially into the birth canal. These methods have worked for me in my over-20 years of practice.
I also firmly remind the RN circulator to perform a generous vaginal cleanse with povidone-iodine, in addition to the usual intravenous prophylaxis, before hysterectomy.
Luis Leyva Jr, MD
Miami, Florida
Mixed feelings
My first reaction to this Editorial was: Is this a solution in search of a problem? That is to say, how much of a clinical problem is endometritis after CD? Are we really treating the proposed problem, and does treatment affect long-term outcomes?
Upon reflection, I have concluded that vaginal cleansing pre-CD does intuitively make sense. What sways me in this direction is that the practice is simple, easy, and inexpensive. Since we typically have the patient positioned for Foley catheter insertion, performing vaginal cleansing as we put in the Foley would be easy. If vaginal cleansing were to be done, I definitely would be in favor of doing such practice liberally—for all CDs to make vaginal cleansing part of the “routine.”
Keep in mind that we are still chasing a problem of little clinical significance.
The biggest accomplishment has been to get everyone to give antibiotics preoperatively rather than after cutting the umbilical cord. We knew that this was best practice as early as the late 1980s/early 1990s, and I have been fighting this battle ever since. Believe it or not, there are still a few holdouts.
George H. Davis, DO
Johnson City, Tennessee
Would vaginal cleansing benefit all women in labor?
Vaginal cleansing before CD reminds me of my residency days when all women having hysterectomies were admitted early and given povidone-iodine (Betadine) douches the evening before surgery (unless an iodine allergy was present).
While reading your Editorial, I had several thoughts and questions. 1) Since vaginal cleansing seems to benefit CD patients, might it not benefit all laboring patients? 2) Is the timing of vaginal cleansing critical? 3) Should we do vaginal cleansing on all laboring patients if timing is not critical?
I plan to bring up the topic of vaginal cleansing for CD with my colleagues at our next department meeting, since it seems like such a simple, logical, inexpensive, and beneficial thing to do.
Douglas G. Tolley, MD
Yuba City, California
An early study on using povidone-iodine gel before CD
When I was a chief resident at Kings County Hospital in 1973, we had a very high rate of post-CD endometritis. I conducted a small study on the use of povidone-iodine gel in the last month of pregnancy. Before commencing, we confirmed that the gel did not interfere with diagnosing ruptured membranes.
Obstetric service patients were randomly divided into “A” and “B” groups. The A patients were asked to use povidone-iodine gel at night for the last 2 weeks before their estimated due date. When admitted in labor, they were asked to confirm its use. When a resident diagnosed post-CD endometritis, we kept track of which group the patient was in and whether or not that patient had used povidone-iodine. Approximately 100 infected patients were evaluated from each group.
As it turned out, there were about 3 times the number of infections among the patients who did not use povidone-iodine than among those who said they used it. It did not seem to matter how many times povidone-iodine was used. The “As” who did not use povidone-iodine had results similar to the “Bs.”
It was many years ago, and the study design was crude. However, it does seem to support the suggestion for vaginal cleansing.
Steve Ross, MD
Port Jefferson, New York
Two different ideas about the vaginal biome
This Editorial is timely in that Dr. Dominguez-Bello and colleagues recently published an article in Nature Medicine titled, “Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer.”1 Dr. Dominguez-Bello is one of the founders of the idea of “vaginal seeding,” or using the natural biome of the vagina on a newborn immediately after CD by swabbing the baby with the bacteria from the vagina.
I find it interesting that there are two very different ideas about the biome at this time. Vaginal seeding is a new trend that a few patients have asked about during prenatal care. The jury is still out on seeding, but a larger study is currently underway at New York University. Of course, infection is one of the risks of seeding. I appreciate hearing both sides of the issue.
Deborah Herchelroath, DO
Harrisburg, Pennsylvania
Reference
- Dominguez-Bello MG, De Jesus-Labor KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer [published online ahead of print February 1, 2016]. Nat Med. doi:10.1038/nm.4039.
Dr. Barbieri responds
I would like to thank our readers for taking the time from their busy schedules to write about their clinical experiences and current practices for reducing infectious complications following CD.
Dr. Kerr raises the important issue of the apparent contradictory finding of the beneficial impact of vaginal cleansing on endometritis without a beneficial effect on the overall rate of fever. In the trial reported by Starr,1 fever was defined as a temperature above 38˚C at any time after CD and endometritis was defined as a temperature above 38.4˚C PLUS uterine tenderness occurring more than 24 hours after CD. Given these 2 definitions one can understand the differential effect of vaginal cleansing on fever versus endometritis.
Dr. Simmons raises the intriguing question of the impact of an iodine-containing surgical preparation on newborn thyroid function. There are few studies addressing this issue. One study reports a transient increase in thyroid-stimulating hormone (TSH) levels in a small percentage of newborns whose mothers received an iodine preparation.2 Another study reports no effect of an iodine surgical preparation on newborn thyroid function indices.3
I agree with the guidance of Drs. Leyva and Davis that we can help prevent postcesarean endometritis by minimizing the number of cervical examinations, changing scrubs between deliveries, and by ensuring that an intravenous anti‑ biotic is given before skin incision.
Dr. Tolley wonders if all women should receive vaginal cleansing, regardless of delivery route. It is possible that such an approach would be effective and it deserves study. Given the lower rate of endometritis following vaginal delivery compared with CD, many more women having a vaginal delivery would need to be treated to prevent one case of endometritis. Dr. Ross mentions his experience with the benefit of outpatient vaginal cleansing in the 2 weeks prior to delivery. Many general surgeons are recommending that their patients shower with chlorhexidine the day before surgery in order to reduce the rate of postoperative infection. Short-term and long-term outpatient vaginal cleansing prior to delivery deserves additional study.
Dr. Herchelroath raises the possibility that vaginal cleansing will decrease the ability of the newborn to develop a normal microbiome because it may not be exposed to sufficient vaginal bacteria. This possibility certainly deserves additional study.
The questions and guidance of our readers were incredibly helpful and stimulating. Thank you for sharing your perspective.
References
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Nili F, Hantoushzadeh S, Alimohamadi A, et al. Iodine-containing disinfectants in preparation for cesarean section: impact on thyroid profile in cord blood. Postgrad Med J. 2015;91(1082):681–684.
- Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. The effect of type of delivery and povidone-iodine application at delivery on cord dried-blood-specimen thyrotropin level and the rate of hyperthyrotropinemia in mature and normal-birth-weight neonates residing in an iodine-replete area. Thyroid. 2007;17(11):1097–1102.
“CELL-FREE DNA SCREENING FOR WOMEN AT LOW RISK FOR FETAL ANEUPLOIDY” MARY E. NORTON, MD (JANUARY 2016)
The price of cfDNA screening is dropping
I found Dr. Norton’s article on cell-free DNA (cfDNA) screening for women at low risk for fetal abnormalities to be enlightening and educational. The section addressing cost-effectiveness, however, was somewhat obsolete. The referenced study by Cuckle and colleagues,1 which estimated the cost of cfDNA per case of Down syndrome in low-risk patients at $3.6 million, was published in 2013. With 4 major companies in the market, the cost/benefit ratio has been changing rapidly. At least one company has dropped the cost of the cfDNA test nearly 80% from 2015 to 2016, making the above reference irrelevant. Recently, Ariosa dropped the price of their Harmony cfDNA test to just $119 in our area, regardless of a patient’s insurance or poverty level. This is significantly less than the cost of performing an early screen and is being welcomed by my patients even after substantial counseling on the test’s limitations in the low-risk population. Natera, another laboratory with a similar test, offers a low-cost option. However, patients must provide proof that their income is below a specified level.
Guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) likely will have a hard time keeping up with the cost-effectiveness of noninvasive prenatal testing, as the price continues to be dynamic.
Samuel Wolf, DO
Panama City, Florida
Reference
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
“DOES THE DISCONTINUATION OF MENOPAUSAL HORMONE THERAPY AFFECT A WOMAN’S CARDIOVASCULAR RISK?”
ANDREW M. KAUNITZ, MD; JOANN E. MANSON, MD, DRPH; AND CYNTHIA A. STUENKEL, MD(EXAMINING THE EVIDENCE; DECEMBER 2015)
Disagrees with conclusion
In their expert commentary, Drs. Kaunitz, Manson, and Stuenkel state:
They support this claim by citing Heiss 2008.1 In fact, however, the Women’s Health Initiative (WHI) data show opposite to their statement: In the WHI, all-cause mortality was increased among the women who were assigned to estrogen-progestin therapy (EPT) relative to those who were assigned to placebo within the 3 years of EPT cessation (hazard ratio [HR], 1.15; 95% confidence interval [CI], 0.95–1.39). More importantly, mortality was significantly increased among women who were originally assigned to EPT relative to those who were assigned to placebo and were at least 80% adherent with intervention (HR, 1.53; 95% CI, 1.04–2.24). Thus, the statement by Drs. Kaunitz, Manson, and Stuenkel is incorrect.
In addition to the WHI studies, data are available from at least 2 other randomized controlled trials addressing the issue of HT withdrawal. In the Heart and Estrogen/progestin Replacement Study (HERS) II,2 the unblinded 2.7-year follow-up to the HERS trial, women originally assigned to EPT had a 3.3-fold higher rate of ventricular arrhythmia requiring resuscitation than women assigned to placebo (HR, 3.30; 95% CI, 1.08–10.10). During the first 6 months of posttrial follow-up of the Women’s Estrogen for Stroke Trial (WEST),3 there were 3 fatal strokes and 18 nonfatal strokes among the women originally randomized to estradiol therapy; there were 9 strokes (1 fatal and 8 nonfatal) among the women originally assigned to placebo (HR, 2.3; 95% CI, 1.1–5.0; P = .03).
In our study we detected that women who stopped HT, compared with women who continued HT, had a 2.3-fold (95% CI, 2.12–2.50) greater risk of cardiac death within the first post-HT year and a 1.3-fold (95% CI, 1.21–1.31) greater risk of cardiac death more than 1 year after stopping HT.4 In addition, women who stopped HT, compared with women who continuedHT, had a 2.5-fold (95% CI, 2.28–2.77) greater risk of dying from stroke within the first post-HT year and a 1.3-fold (95% CI, 1.19–1.31) greater risk of dying from stroke more than 1 year after stopping HT. We believe that these data substantially further our understanding of the posttrial data from WHI, as well as HERS and WEST. Thus, cumulative data support that HT withdrawal potentially has detrimental implications for women. In total, the data are highly informative when counseling women regarding use or discontinuation of HT.
Tomi Mikkola, MD
Helsinki, Finland
References
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- Grady D, Herrington D, Bittner V, et al; HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002;288(9):1064]. JAMA. 2002;288(1):49–57.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–4594.
Drs. Kaunitz, Manson, and Stuenkel respond
We thank Dr. Mikkola for his response to our commentary, but we do not agree with his interpretation of the WHI reports or our conclusions. As we originally stated, the WHI trial of estrogen-only therapy (ET) and EPT provides an opportunity to observe outcomes in the largest randomized controlled trial of HT in healthy postmenopausal women. Our commentary was based on the most recent, 13-year follow-up of the WHI trials,1 and we are confident in the accuracy of our presentation of the results.
As the debate apparently focuses on the safety of stopping HT, we wish to reiterate, for those who may not be familiar with the data, that, in the ET trial, all-cause mortality declined (although not significantly) after stopping ET, as summarized here:
HR (95% CI) | |
Intervention phase | 1.03 (0.88–1.21) |
Postintervention phase (after stopping study medication) | 0.96 (0.84–1.10) |
Cumulative 13 years of follow-up | 0.99 (0.90–1.10) |
Similarly, in the EPT trial, as the following findings indicate, stopping HT did not increase all-cause mortality:
HR (95% CI) | |
Intervention phase | 0.97 (0.81–1.16) |
Postintervention phase (afterstopping study medication) | 1.01 (0.91–1.11) |
Cumulative 13 years of follow-up | 0.99 (0.91–1.08) |
Again, these findings from the largest randomized trial of HT in healthy postmenopausal women are adequate for us to conclude that stopping HT does not elevate risk of mortality. Among all women participating in the WHI HT trials, HRs for coronary heart disease, pulmonary embolism, stroke, and cardiovascular disease mortality likewise were lower (better) after stopping treatment than during the intervention phase. The results for these outcomes in younger women followed similar patterns but, due to smaller numbers of events, could not be tested formally for differences in time trends.
Moreover, the data Dr. Mikkola cites from analyses conducted 3 years postcessation2 reflected a borderline increased risk of cancer mortality that emerged in the EPT trial after stopping treatment. This clearly was related to the prolonged effects of EPT on breast cancer and other cancers, given the known latency period for cancer, and was not observed in the ET trial postcessation. The risk elevation in the EPT trial became attenuated with longer follow-up and, as of 13 years, the HRs for cancer mortality were 1.07 (0.93–1.23) in the EPT trial and 0.95 (0.81–1.13) in the ET trial.
It is interesting that Dr. Mikkola now inculcates his interpretation of his findings3 with those from secondary prevention trials such as the Heart and Estrogen/progestin Replacement Study and the Women’s Estrogen for Stroke Trial, neither of which was included as corroborative evidence in the discussion section of his originally published manuscript, and neither of which is considered applicable to healthy postmenopausal women taking HT for treatment of menopausal symptoms. Based on these findings, we do not recommend that clinicians counsel women that stopping HT increases their risk of cardiovascular or overall mortality. Thank you for the opportunity to clarify the evidence and our position.
References
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
“SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
In his January 2016 Editorial, Editor in Chief Robert L. Barbieri, MD, presented evidence supporting the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery (CD) to prevent postoperative endometritis. He then asked readers if they would consider adopting such a practice. More than 250 readers weighed in through the Quick Poll at obgmanagement.com, and many readers sent in letters with follow-up questions and comments on controlling bacterial contamination, vaginal seeding, etc. Here are some of the letters, along with Dr. Barbieri’s response and the Quick Poll results.
A contradiction in definitions?
There seems to be a contradiction in definitions. The second sentence of the article defines endometritis as the presence of fever plus low abdominal tenderness. However, the studies presented state that vaginal cleansing pre-CD decreased endometritis but did not decrease postpartum fever. Is this not a discrepancy?
Nancy Kerr, MD, MPH
Albuquerque, New Mexico
A question about povidone-iodine
Have any studies been done on newborn iodine levels after vaginal cleansing with povidone-iodine prior to CD?
G. Millard Simmons Jr, MD
Hilton Head, Bluffton, South Carolina
Additional tips for controlling bacterial contamination
Dr. Barbieri’s editorial on vaginal cleansing prior to CD is eye opening. I have a few additional suggestions to control bacterial contamination.
First, I examine my patients in labor as few times as necessary, and I ask the nurses (RNs) not to place their fingers in the patient’s vagina while she is pushing. I remove the Foley catheter when I feel progress (descent of fetal head) is being achieved. In addition, physicians as well as RNs should consider changing their scrubs between deliveries, as I believe that bacterial contamination is splattered all over the place, especially into the birth canal. These methods have worked for me in my over-20 years of practice.
I also firmly remind the RN circulator to perform a generous vaginal cleanse with povidone-iodine, in addition to the usual intravenous prophylaxis, before hysterectomy.
Luis Leyva Jr, MD
Miami, Florida
Mixed feelings
My first reaction to this Editorial was: Is this a solution in search of a problem? That is to say, how much of a clinical problem is endometritis after CD? Are we really treating the proposed problem, and does treatment affect long-term outcomes?
Upon reflection, I have concluded that vaginal cleansing pre-CD does intuitively make sense. What sways me in this direction is that the practice is simple, easy, and inexpensive. Since we typically have the patient positioned for Foley catheter insertion, performing vaginal cleansing as we put in the Foley would be easy. If vaginal cleansing were to be done, I definitely would be in favor of doing such practice liberally—for all CDs to make vaginal cleansing part of the “routine.”
Keep in mind that we are still chasing a problem of little clinical significance.
The biggest accomplishment has been to get everyone to give antibiotics preoperatively rather than after cutting the umbilical cord. We knew that this was best practice as early as the late 1980s/early 1990s, and I have been fighting this battle ever since. Believe it or not, there are still a few holdouts.
George H. Davis, DO
Johnson City, Tennessee
Would vaginal cleansing benefit all women in labor?
Vaginal cleansing before CD reminds me of my residency days when all women having hysterectomies were admitted early and given povidone-iodine (Betadine) douches the evening before surgery (unless an iodine allergy was present).
While reading your Editorial, I had several thoughts and questions. 1) Since vaginal cleansing seems to benefit CD patients, might it not benefit all laboring patients? 2) Is the timing of vaginal cleansing critical? 3) Should we do vaginal cleansing on all laboring patients if timing is not critical?
I plan to bring up the topic of vaginal cleansing for CD with my colleagues at our next department meeting, since it seems like such a simple, logical, inexpensive, and beneficial thing to do.
Douglas G. Tolley, MD
Yuba City, California
An early study on using povidone-iodine gel before CD
When I was a chief resident at Kings County Hospital in 1973, we had a very high rate of post-CD endometritis. I conducted a small study on the use of povidone-iodine gel in the last month of pregnancy. Before commencing, we confirmed that the gel did not interfere with diagnosing ruptured membranes.
Obstetric service patients were randomly divided into “A” and “B” groups. The A patients were asked to use povidone-iodine gel at night for the last 2 weeks before their estimated due date. When admitted in labor, they were asked to confirm its use. When a resident diagnosed post-CD endometritis, we kept track of which group the patient was in and whether or not that patient had used povidone-iodine. Approximately 100 infected patients were evaluated from each group.
As it turned out, there were about 3 times the number of infections among the patients who did not use povidone-iodine than among those who said they used it. It did not seem to matter how many times povidone-iodine was used. The “As” who did not use povidone-iodine had results similar to the “Bs.”
It was many years ago, and the study design was crude. However, it does seem to support the suggestion for vaginal cleansing.
Steve Ross, MD
Port Jefferson, New York
Two different ideas about the vaginal biome
This Editorial is timely in that Dr. Dominguez-Bello and colleagues recently published an article in Nature Medicine titled, “Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer.”1 Dr. Dominguez-Bello is one of the founders of the idea of “vaginal seeding,” or using the natural biome of the vagina on a newborn immediately after CD by swabbing the baby with the bacteria from the vagina.
I find it interesting that there are two very different ideas about the biome at this time. Vaginal seeding is a new trend that a few patients have asked about during prenatal care. The jury is still out on seeding, but a larger study is currently underway at New York University. Of course, infection is one of the risks of seeding. I appreciate hearing both sides of the issue.
Deborah Herchelroath, DO
Harrisburg, Pennsylvania
Reference
- Dominguez-Bello MG, De Jesus-Labor KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer [published online ahead of print February 1, 2016]. Nat Med. doi:10.1038/nm.4039.
Dr. Barbieri responds
I would like to thank our readers for taking the time from their busy schedules to write about their clinical experiences and current practices for reducing infectious complications following CD.
Dr. Kerr raises the important issue of the apparent contradictory finding of the beneficial impact of vaginal cleansing on endometritis without a beneficial effect on the overall rate of fever. In the trial reported by Starr,1 fever was defined as a temperature above 38˚C at any time after CD and endometritis was defined as a temperature above 38.4˚C PLUS uterine tenderness occurring more than 24 hours after CD. Given these 2 definitions one can understand the differential effect of vaginal cleansing on fever versus endometritis.
Dr. Simmons raises the intriguing question of the impact of an iodine-containing surgical preparation on newborn thyroid function. There are few studies addressing this issue. One study reports a transient increase in thyroid-stimulating hormone (TSH) levels in a small percentage of newborns whose mothers received an iodine preparation.2 Another study reports no effect of an iodine surgical preparation on newborn thyroid function indices.3
I agree with the guidance of Drs. Leyva and Davis that we can help prevent postcesarean endometritis by minimizing the number of cervical examinations, changing scrubs between deliveries, and by ensuring that an intravenous anti‑ biotic is given before skin incision.
Dr. Tolley wonders if all women should receive vaginal cleansing, regardless of delivery route. It is possible that such an approach would be effective and it deserves study. Given the lower rate of endometritis following vaginal delivery compared with CD, many more women having a vaginal delivery would need to be treated to prevent one case of endometritis. Dr. Ross mentions his experience with the benefit of outpatient vaginal cleansing in the 2 weeks prior to delivery. Many general surgeons are recommending that their patients shower with chlorhexidine the day before surgery in order to reduce the rate of postoperative infection. Short-term and long-term outpatient vaginal cleansing prior to delivery deserves additional study.
Dr. Herchelroath raises the possibility that vaginal cleansing will decrease the ability of the newborn to develop a normal microbiome because it may not be exposed to sufficient vaginal bacteria. This possibility certainly deserves additional study.
The questions and guidance of our readers were incredibly helpful and stimulating. Thank you for sharing your perspective.
References
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Nili F, Hantoushzadeh S, Alimohamadi A, et al. Iodine-containing disinfectants in preparation for cesarean section: impact on thyroid profile in cord blood. Postgrad Med J. 2015;91(1082):681–684.
- Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. The effect of type of delivery and povidone-iodine application at delivery on cord dried-blood-specimen thyrotropin level and the rate of hyperthyrotropinemia in mature and normal-birth-weight neonates residing in an iodine-replete area. Thyroid. 2007;17(11):1097–1102.
“CELL-FREE DNA SCREENING FOR WOMEN AT LOW RISK FOR FETAL ANEUPLOIDY” MARY E. NORTON, MD (JANUARY 2016)
The price of cfDNA screening is dropping
I found Dr. Norton’s article on cell-free DNA (cfDNA) screening for women at low risk for fetal abnormalities to be enlightening and educational. The section addressing cost-effectiveness, however, was somewhat obsolete. The referenced study by Cuckle and colleagues,1 which estimated the cost of cfDNA per case of Down syndrome in low-risk patients at $3.6 million, was published in 2013. With 4 major companies in the market, the cost/benefit ratio has been changing rapidly. At least one company has dropped the cost of the cfDNA test nearly 80% from 2015 to 2016, making the above reference irrelevant. Recently, Ariosa dropped the price of their Harmony cfDNA test to just $119 in our area, regardless of a patient’s insurance or poverty level. This is significantly less than the cost of performing an early screen and is being welcomed by my patients even after substantial counseling on the test’s limitations in the low-risk population. Natera, another laboratory with a similar test, offers a low-cost option. However, patients must provide proof that their income is below a specified level.
Guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) likely will have a hard time keeping up with the cost-effectiveness of noninvasive prenatal testing, as the price continues to be dynamic.
Samuel Wolf, DO
Panama City, Florida
Reference
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
“DOES THE DISCONTINUATION OF MENOPAUSAL HORMONE THERAPY AFFECT A WOMAN’S CARDIOVASCULAR RISK?”
ANDREW M. KAUNITZ, MD; JOANN E. MANSON, MD, DRPH; AND CYNTHIA A. STUENKEL, MD(EXAMINING THE EVIDENCE; DECEMBER 2015)
Disagrees with conclusion
In their expert commentary, Drs. Kaunitz, Manson, and Stuenkel state:
They support this claim by citing Heiss 2008.1 In fact, however, the Women’s Health Initiative (WHI) data show opposite to their statement: In the WHI, all-cause mortality was increased among the women who were assigned to estrogen-progestin therapy (EPT) relative to those who were assigned to placebo within the 3 years of EPT cessation (hazard ratio [HR], 1.15; 95% confidence interval [CI], 0.95–1.39). More importantly, mortality was significantly increased among women who were originally assigned to EPT relative to those who were assigned to placebo and were at least 80% adherent with intervention (HR, 1.53; 95% CI, 1.04–2.24). Thus, the statement by Drs. Kaunitz, Manson, and Stuenkel is incorrect.
In addition to the WHI studies, data are available from at least 2 other randomized controlled trials addressing the issue of HT withdrawal. In the Heart and Estrogen/progestin Replacement Study (HERS) II,2 the unblinded 2.7-year follow-up to the HERS trial, women originally assigned to EPT had a 3.3-fold higher rate of ventricular arrhythmia requiring resuscitation than women assigned to placebo (HR, 3.30; 95% CI, 1.08–10.10). During the first 6 months of posttrial follow-up of the Women’s Estrogen for Stroke Trial (WEST),3 there were 3 fatal strokes and 18 nonfatal strokes among the women originally randomized to estradiol therapy; there were 9 strokes (1 fatal and 8 nonfatal) among the women originally assigned to placebo (HR, 2.3; 95% CI, 1.1–5.0; P = .03).
In our study we detected that women who stopped HT, compared with women who continued HT, had a 2.3-fold (95% CI, 2.12–2.50) greater risk of cardiac death within the first post-HT year and a 1.3-fold (95% CI, 1.21–1.31) greater risk of cardiac death more than 1 year after stopping HT.4 In addition, women who stopped HT, compared with women who continuedHT, had a 2.5-fold (95% CI, 2.28–2.77) greater risk of dying from stroke within the first post-HT year and a 1.3-fold (95% CI, 1.19–1.31) greater risk of dying from stroke more than 1 year after stopping HT. We believe that these data substantially further our understanding of the posttrial data from WHI, as well as HERS and WEST. Thus, cumulative data support that HT withdrawal potentially has detrimental implications for women. In total, the data are highly informative when counseling women regarding use or discontinuation of HT.
Tomi Mikkola, MD
Helsinki, Finland
References
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- Grady D, Herrington D, Bittner V, et al; HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002;288(9):1064]. JAMA. 2002;288(1):49–57.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–4594.
Drs. Kaunitz, Manson, and Stuenkel respond
We thank Dr. Mikkola for his response to our commentary, but we do not agree with his interpretation of the WHI reports or our conclusions. As we originally stated, the WHI trial of estrogen-only therapy (ET) and EPT provides an opportunity to observe outcomes in the largest randomized controlled trial of HT in healthy postmenopausal women. Our commentary was based on the most recent, 13-year follow-up of the WHI trials,1 and we are confident in the accuracy of our presentation of the results.
As the debate apparently focuses on the safety of stopping HT, we wish to reiterate, for those who may not be familiar with the data, that, in the ET trial, all-cause mortality declined (although not significantly) after stopping ET, as summarized here:
HR (95% CI) | |
Intervention phase | 1.03 (0.88–1.21) |
Postintervention phase (after stopping study medication) | 0.96 (0.84–1.10) |
Cumulative 13 years of follow-up | 0.99 (0.90–1.10) |
Similarly, in the EPT trial, as the following findings indicate, stopping HT did not increase all-cause mortality:
HR (95% CI) | |
Intervention phase | 0.97 (0.81–1.16) |
Postintervention phase (afterstopping study medication) | 1.01 (0.91–1.11) |
Cumulative 13 years of follow-up | 0.99 (0.91–1.08) |
Again, these findings from the largest randomized trial of HT in healthy postmenopausal women are adequate for us to conclude that stopping HT does not elevate risk of mortality. Among all women participating in the WHI HT trials, HRs for coronary heart disease, pulmonary embolism, stroke, and cardiovascular disease mortality likewise were lower (better) after stopping treatment than during the intervention phase. The results for these outcomes in younger women followed similar patterns but, due to smaller numbers of events, could not be tested formally for differences in time trends.
Moreover, the data Dr. Mikkola cites from analyses conducted 3 years postcessation2 reflected a borderline increased risk of cancer mortality that emerged in the EPT trial after stopping treatment. This clearly was related to the prolonged effects of EPT on breast cancer and other cancers, given the known latency period for cancer, and was not observed in the ET trial postcessation. The risk elevation in the EPT trial became attenuated with longer follow-up and, as of 13 years, the HRs for cancer mortality were 1.07 (0.93–1.23) in the EPT trial and 0.95 (0.81–1.13) in the ET trial.
It is interesting that Dr. Mikkola now inculcates his interpretation of his findings3 with those from secondary prevention trials such as the Heart and Estrogen/progestin Replacement Study and the Women’s Estrogen for Stroke Trial, neither of which was included as corroborative evidence in the discussion section of his originally published manuscript, and neither of which is considered applicable to healthy postmenopausal women taking HT for treatment of menopausal symptoms. Based on these findings, we do not recommend that clinicians counsel women that stopping HT increases their risk of cardiovascular or overall mortality. Thank you for the opportunity to clarify the evidence and our position.
References
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
‘Vaginal seeding’ practice comes with risks
Newborns delivered by cesarean section have different microbial colonization patterns than babies delivered vaginally, and several studies have suggested that their immunologic and metabolic development may be disadvantaged as a result.
Preliminary results from a recent proof-of concept study in 18 babies, published online in Nature Medicine, showed that transferring vaginal fluid to newborns shortly after delivery by C-section resulted in microbiome profiles resembling those of babies delivered vaginally (doi: 10.1038/nm.4039).
But it is still unknown whether promoting colonization in this way approximates the microbial transfer that occurs during labor, or whether it affects later health outcomes in these infants. The researchers, led by Maria G. Dominguez-Bello, Ph.D., of New York University in New York City, stressed in their study that considerably more time and research are needed to answer these questions.
Still, as the practice gains more attention in the press and in parenting blogs, patients are asking clinicians to perform vaginal fluid transfer or “vaginal seeding” following C-sections.
BMJ highlights concerns
A Feb. 23 editorial in the BMJ stressed that clinicians not recommend vaginal seeding, because of insufficient evidence and because of the potential for unrecognized infections to be transmitted from mother to newborn (BMJ. 2016;352:i227. doi: 10.1136/bmj.i227).
“It might seem reasonable to perform this simple and cheap procedure, even without clear evidence of benefit, but only if we can be sure that it is safe,” wrote a group of clinicians led by Dr. Aubrey J. Cunnington of Imperial College London. For now, they wrote, breastfeeding and avoidance of unnecessary antibiotics should be stressed to promote early microbiome development.
But even if clinicians and hospitals refuse to perform seeding – which can be done by placing gauze in the vagina prior to delivery and then using it to swab the newborn – “mothers can easily do it themselves,” Dr. Cunnington and colleagues said in their editorial.
The potential demand for vaginal seeding raises a host of issues, according to three U.S.-based ob.gyns., all of whom said they agree with the caution expressed in the BMJ editorial, but have varying approaches to how to advise their patients.
“From a clinician’s point of view, I think it’s really important to not wait for a woman to say, ‘Should I do this?’ but instead for doctors to proactively say, ‘You may have read about this and we don’t recommend it,’ ” Dr. Lauren Streicher,of Northwestern University, Chicago, said in an interview.
“The real concern is that since you don’t need a doctor to do it, mothers are going to take it upon themselves,” Dr. Streicher said, particularly as the practice gains more attention in the lay press, or if vaginal seeding gets endorsed or promoted by someone famous, something she said is all but inevitable.
Patients need to be aware that there are risks inherent in either mode of delivery, Dr. Streicher noted, and vaginal delivery comes with the risk of transmitting undiagnosed group B streptococcus, chlamydia, gonorrhea, or herpes viruses.
Dr. Layne Kumetz, an ob.gyn. in Beverly Hills, Calif., said in an interview that her patients tend to be educated and “early adopters” when it comes to medical trends. She said she has already received patient queries about vaginal seeding and counseled patients on the potential risks. As a mother herself, she has also noticed active discussion about vaginal seeding on parenting blogs.
But Dr. Kumetz said that pending much more study, “routine practicing of the procedure is simply not appropriate.”
She added, “The long term implications about whether this will alter the health of newborns have not been determined, whether this is a safe procedure has not been determined, [and] what the proper protocol should be in terms of women wishing to have this procedure performed has not been determined.”
The women in the pilot study who had used vaginal seeding, Dr. Kumetz noted, had been carefully selected and screened, had been treated with antibiotics prior to their C-sections, and were group B strep negative. In the general population, meanwhile, nearly a third of patients are group B positive, she said.
Dr. Kumetz said she would not bring up the seeding with patients who don’t ask, nor was it something that the hospitals she’s working with are willing to perform.
Emphasize alternatives
Dr. Eliza Bennett of the University of Wisconsin–Madison said that requests for vaginal seeding had become more frequent, even months before the pilot study was published. The procedure is allowed at her hospital, but is available only by request.
Dr. Bennett stressed that she, like Dr. Kumetz, does not discuss the practice with patients unless they bring it up themselves. For women who do request it, nurses have been trained in the practice, she said, and “we have established really strict exclusion criteria,” including repeat screening for group B strep, HIV, genital herpes, and sexually transmitted infections during pregnancy.
Dr. Bennett said she would much rather emphasize the safer, evidence-based practices already in place at her hospital to promote microbiome development in C-section newborns. These include delaying the first bath until after 12 hours, placing babies directly on mothers’ skin in the operating room in the first minutes post delivery, and “offering the ability for moms to breastfeed right in the OR,” she said.
Like Dr. Streicher and Dr. Kumetz, Dr. Bennett said she’s not expecting to see data resolving the questions about the risks and benefits of vaginal seeding anytime soon.
“Instead of focusing on vaginal seeding, we should continue to focus our efforts on decreasing cesarean delivery rates and ensuring and encouraging appropriate candidates to have the opportunity to have a trial of labor after cesarean delivery,” she said.
Avoiding C-section, she added, “confers multiple maternal and fetal benefits beyond the microbiome.”
Newborns delivered by cesarean section have different microbial colonization patterns than babies delivered vaginally, and several studies have suggested that their immunologic and metabolic development may be disadvantaged as a result.
Preliminary results from a recent proof-of concept study in 18 babies, published online in Nature Medicine, showed that transferring vaginal fluid to newborns shortly after delivery by C-section resulted in microbiome profiles resembling those of babies delivered vaginally (doi: 10.1038/nm.4039).
But it is still unknown whether promoting colonization in this way approximates the microbial transfer that occurs during labor, or whether it affects later health outcomes in these infants. The researchers, led by Maria G. Dominguez-Bello, Ph.D., of New York University in New York City, stressed in their study that considerably more time and research are needed to answer these questions.
Still, as the practice gains more attention in the press and in parenting blogs, patients are asking clinicians to perform vaginal fluid transfer or “vaginal seeding” following C-sections.
BMJ highlights concerns
A Feb. 23 editorial in the BMJ stressed that clinicians not recommend vaginal seeding, because of insufficient evidence and because of the potential for unrecognized infections to be transmitted from mother to newborn (BMJ. 2016;352:i227. doi: 10.1136/bmj.i227).
“It might seem reasonable to perform this simple and cheap procedure, even without clear evidence of benefit, but only if we can be sure that it is safe,” wrote a group of clinicians led by Dr. Aubrey J. Cunnington of Imperial College London. For now, they wrote, breastfeeding and avoidance of unnecessary antibiotics should be stressed to promote early microbiome development.
But even if clinicians and hospitals refuse to perform seeding – which can be done by placing gauze in the vagina prior to delivery and then using it to swab the newborn – “mothers can easily do it themselves,” Dr. Cunnington and colleagues said in their editorial.
The potential demand for vaginal seeding raises a host of issues, according to three U.S.-based ob.gyns., all of whom said they agree with the caution expressed in the BMJ editorial, but have varying approaches to how to advise their patients.
“From a clinician’s point of view, I think it’s really important to not wait for a woman to say, ‘Should I do this?’ but instead for doctors to proactively say, ‘You may have read about this and we don’t recommend it,’ ” Dr. Lauren Streicher,of Northwestern University, Chicago, said in an interview.
“The real concern is that since you don’t need a doctor to do it, mothers are going to take it upon themselves,” Dr. Streicher said, particularly as the practice gains more attention in the lay press, or if vaginal seeding gets endorsed or promoted by someone famous, something she said is all but inevitable.
Patients need to be aware that there are risks inherent in either mode of delivery, Dr. Streicher noted, and vaginal delivery comes with the risk of transmitting undiagnosed group B streptococcus, chlamydia, gonorrhea, or herpes viruses.
Dr. Layne Kumetz, an ob.gyn. in Beverly Hills, Calif., said in an interview that her patients tend to be educated and “early adopters” when it comes to medical trends. She said she has already received patient queries about vaginal seeding and counseled patients on the potential risks. As a mother herself, she has also noticed active discussion about vaginal seeding on parenting blogs.
But Dr. Kumetz said that pending much more study, “routine practicing of the procedure is simply not appropriate.”
She added, “The long term implications about whether this will alter the health of newborns have not been determined, whether this is a safe procedure has not been determined, [and] what the proper protocol should be in terms of women wishing to have this procedure performed has not been determined.”
The women in the pilot study who had used vaginal seeding, Dr. Kumetz noted, had been carefully selected and screened, had been treated with antibiotics prior to their C-sections, and were group B strep negative. In the general population, meanwhile, nearly a third of patients are group B positive, she said.
Dr. Kumetz said she would not bring up the seeding with patients who don’t ask, nor was it something that the hospitals she’s working with are willing to perform.
Emphasize alternatives
Dr. Eliza Bennett of the University of Wisconsin–Madison said that requests for vaginal seeding had become more frequent, even months before the pilot study was published. The procedure is allowed at her hospital, but is available only by request.
Dr. Bennett stressed that she, like Dr. Kumetz, does not discuss the practice with patients unless they bring it up themselves. For women who do request it, nurses have been trained in the practice, she said, and “we have established really strict exclusion criteria,” including repeat screening for group B strep, HIV, genital herpes, and sexually transmitted infections during pregnancy.
Dr. Bennett said she would much rather emphasize the safer, evidence-based practices already in place at her hospital to promote microbiome development in C-section newborns. These include delaying the first bath until after 12 hours, placing babies directly on mothers’ skin in the operating room in the first minutes post delivery, and “offering the ability for moms to breastfeed right in the OR,” she said.
Like Dr. Streicher and Dr. Kumetz, Dr. Bennett said she’s not expecting to see data resolving the questions about the risks and benefits of vaginal seeding anytime soon.
“Instead of focusing on vaginal seeding, we should continue to focus our efforts on decreasing cesarean delivery rates and ensuring and encouraging appropriate candidates to have the opportunity to have a trial of labor after cesarean delivery,” she said.
Avoiding C-section, she added, “confers multiple maternal and fetal benefits beyond the microbiome.”
Newborns delivered by cesarean section have different microbial colonization patterns than babies delivered vaginally, and several studies have suggested that their immunologic and metabolic development may be disadvantaged as a result.
Preliminary results from a recent proof-of concept study in 18 babies, published online in Nature Medicine, showed that transferring vaginal fluid to newborns shortly after delivery by C-section resulted in microbiome profiles resembling those of babies delivered vaginally (doi: 10.1038/nm.4039).
But it is still unknown whether promoting colonization in this way approximates the microbial transfer that occurs during labor, or whether it affects later health outcomes in these infants. The researchers, led by Maria G. Dominguez-Bello, Ph.D., of New York University in New York City, stressed in their study that considerably more time and research are needed to answer these questions.
Still, as the practice gains more attention in the press and in parenting blogs, patients are asking clinicians to perform vaginal fluid transfer or “vaginal seeding” following C-sections.
BMJ highlights concerns
A Feb. 23 editorial in the BMJ stressed that clinicians not recommend vaginal seeding, because of insufficient evidence and because of the potential for unrecognized infections to be transmitted from mother to newborn (BMJ. 2016;352:i227. doi: 10.1136/bmj.i227).
“It might seem reasonable to perform this simple and cheap procedure, even without clear evidence of benefit, but only if we can be sure that it is safe,” wrote a group of clinicians led by Dr. Aubrey J. Cunnington of Imperial College London. For now, they wrote, breastfeeding and avoidance of unnecessary antibiotics should be stressed to promote early microbiome development.
But even if clinicians and hospitals refuse to perform seeding – which can be done by placing gauze in the vagina prior to delivery and then using it to swab the newborn – “mothers can easily do it themselves,” Dr. Cunnington and colleagues said in their editorial.
The potential demand for vaginal seeding raises a host of issues, according to three U.S.-based ob.gyns., all of whom said they agree with the caution expressed in the BMJ editorial, but have varying approaches to how to advise their patients.
“From a clinician’s point of view, I think it’s really important to not wait for a woman to say, ‘Should I do this?’ but instead for doctors to proactively say, ‘You may have read about this and we don’t recommend it,’ ” Dr. Lauren Streicher,of Northwestern University, Chicago, said in an interview.
“The real concern is that since you don’t need a doctor to do it, mothers are going to take it upon themselves,” Dr. Streicher said, particularly as the practice gains more attention in the lay press, or if vaginal seeding gets endorsed or promoted by someone famous, something she said is all but inevitable.
Patients need to be aware that there are risks inherent in either mode of delivery, Dr. Streicher noted, and vaginal delivery comes with the risk of transmitting undiagnosed group B streptococcus, chlamydia, gonorrhea, or herpes viruses.
Dr. Layne Kumetz, an ob.gyn. in Beverly Hills, Calif., said in an interview that her patients tend to be educated and “early adopters” when it comes to medical trends. She said she has already received patient queries about vaginal seeding and counseled patients on the potential risks. As a mother herself, she has also noticed active discussion about vaginal seeding on parenting blogs.
But Dr. Kumetz said that pending much more study, “routine practicing of the procedure is simply not appropriate.”
She added, “The long term implications about whether this will alter the health of newborns have not been determined, whether this is a safe procedure has not been determined, [and] what the proper protocol should be in terms of women wishing to have this procedure performed has not been determined.”
The women in the pilot study who had used vaginal seeding, Dr. Kumetz noted, had been carefully selected and screened, had been treated with antibiotics prior to their C-sections, and were group B strep negative. In the general population, meanwhile, nearly a third of patients are group B positive, she said.
Dr. Kumetz said she would not bring up the seeding with patients who don’t ask, nor was it something that the hospitals she’s working with are willing to perform.
Emphasize alternatives
Dr. Eliza Bennett of the University of Wisconsin–Madison said that requests for vaginal seeding had become more frequent, even months before the pilot study was published. The procedure is allowed at her hospital, but is available only by request.
Dr. Bennett stressed that she, like Dr. Kumetz, does not discuss the practice with patients unless they bring it up themselves. For women who do request it, nurses have been trained in the practice, she said, and “we have established really strict exclusion criteria,” including repeat screening for group B strep, HIV, genital herpes, and sexually transmitted infections during pregnancy.
Dr. Bennett said she would much rather emphasize the safer, evidence-based practices already in place at her hospital to promote microbiome development in C-section newborns. These include delaying the first bath until after 12 hours, placing babies directly on mothers’ skin in the operating room in the first minutes post delivery, and “offering the ability for moms to breastfeed right in the OR,” she said.
Like Dr. Streicher and Dr. Kumetz, Dr. Bennett said she’s not expecting to see data resolving the questions about the risks and benefits of vaginal seeding anytime soon.
“Instead of focusing on vaginal seeding, we should continue to focus our efforts on decreasing cesarean delivery rates and ensuring and encouraging appropriate candidates to have the opportunity to have a trial of labor after cesarean delivery,” she said.
Avoiding C-section, she added, “confers multiple maternal and fetal benefits beyond the microbiome.”
CDC reports nine U.S. Zika cases among pregnant women
Officials at the Centers for Disease Control and Prevention reported that they are aware of at least nine cases of laboratory-confirmed Zika virus infection in pregnant travelers, and that in four of these cases, fetuses were either spontaneously lost or aborted.
Confirmed cases of Zika virus infection were reported among women who had traveled to one or more of the following nine areas with ongoing local transmission of Zika virus: American Samoa, Brazil, El Salvador, Guatemala, Haiti, Honduras, Mexico, Puerto Rico, and Samoa, according to the CDC’s latest Morbidity and Mortality Weekly Report (2016 Feb 26. doi: http://dx.doi.org/10.15585/mmwr.mm6508e1er).
The agency is also investigating reports of 10 other cases of pregnant women with possible Zika virus infection.
Of the nine confirmed cases, six women were infected with Zika virus in their first trimester. In two cases, the pregnancies were terminated and two of the pregnancies resulted in stillbirths. Another woman gave birth to an infant with microcephaly, and one woman is still pregnant and has not experienced any complications so far.
Two of the nine women became infected with Zika virus during their second trimesters, one of whom has delivered a healthy infant and the other of whom is still pregnant with no known complications thus far. The last of the nine women, who became infected during her third trimester, gave birth to a healthy infant with no known complications.
There were no Zika virus–related hospitalizations or deaths among the nine women with Zika virus.
“To better understand the effects of Zika virus infection during pregnancy, CDC has established the U.S. Pregnancy Registry for Zika Virus Infection,” announced Dr. Denise J. Jamieson, colead of the Pregnancy and Birth Defects Team at the CDC. “This registry will provide information about the effects of Zika virus on pregnant women and their children.”
Participation in the registry is voluntary and information will be available on the CDC website soon, Dr. Jamieson added. Until then, the CDC maintains a 24/7 consultation hotline for both pregnant women and health care providers concerned about Zika virus infections, at 1-800-CDC-INFO. Patients and providers can also email [email protected].
Additionally, CDC officials stated that they have received reports of at least 14 instances in which Zika virus may have been transmitted between individuals through sexual contact. Two of these cases are confirmed to have been transmitted to women from men who visited a Zika-endemic area, while another four are “probable cases” of sexual transmission, and another six are under investigation. Two reported cases were excluded after receiving additional information.
“Men who reside in or have traveled to an area of ongoing Zika virus transmission and have a pregnant partner should abstain from sexual activity or consistently and correctly use condoms during sex with their pregnant partner for the duration of the pregnancy,” said the CDC in a statement (MMWR. 2016 Feb 26. doi: http://dx.doi.org/10.15585/mmwr.mm6508e2er).
The World Health Organization also released a new situation report on Zika virus, microcephaly, and Guillain-Barré syndrome, saying that although Zika virus has been spreading to more geographic areas since the beginning of the crisis, cases of microcephaly and neonatal malformations are increasing only in Brazil and French Polynesia.
Additionally, the WHO released interim guidelines on psychosocial support for pregnant women who may be infected with Zika virus and families dealing with an infant born with either microcephaly or another neurologic disorder.
At a press briefing on Feb. 26, CDC Director Dr. Tom Frieden, noted that it has been 6 weeks since the CDC issued its first travel warning regarding Zika virus, and that the agency is “learning more about Zika everyday.” He added that the current state of the Zika virus outbreak is an “unprecedented situation.”
But Dr. Frieden also stressed that the exact link between Zika virus infection and microcephaly is still unknown, and it is not clear at what stage in the pregnancy Zika virus affects the fetus.
It’s also unknown whether infants born to mothers with Zika virus infection who don’t develop microcephaly will have any other health problems in the future. “Unfortunately, this is something we may not know for many years,” Dr. Frieden said.
Officials at the Centers for Disease Control and Prevention reported that they are aware of at least nine cases of laboratory-confirmed Zika virus infection in pregnant travelers, and that in four of these cases, fetuses were either spontaneously lost or aborted.
Confirmed cases of Zika virus infection were reported among women who had traveled to one or more of the following nine areas with ongoing local transmission of Zika virus: American Samoa, Brazil, El Salvador, Guatemala, Haiti, Honduras, Mexico, Puerto Rico, and Samoa, according to the CDC’s latest Morbidity and Mortality Weekly Report (2016 Feb 26. doi: http://dx.doi.org/10.15585/mmwr.mm6508e1er).
The agency is also investigating reports of 10 other cases of pregnant women with possible Zika virus infection.
Of the nine confirmed cases, six women were infected with Zika virus in their first trimester. In two cases, the pregnancies were terminated and two of the pregnancies resulted in stillbirths. Another woman gave birth to an infant with microcephaly, and one woman is still pregnant and has not experienced any complications so far.
Two of the nine women became infected with Zika virus during their second trimesters, one of whom has delivered a healthy infant and the other of whom is still pregnant with no known complications thus far. The last of the nine women, who became infected during her third trimester, gave birth to a healthy infant with no known complications.
There were no Zika virus–related hospitalizations or deaths among the nine women with Zika virus.
“To better understand the effects of Zika virus infection during pregnancy, CDC has established the U.S. Pregnancy Registry for Zika Virus Infection,” announced Dr. Denise J. Jamieson, colead of the Pregnancy and Birth Defects Team at the CDC. “This registry will provide information about the effects of Zika virus on pregnant women and their children.”
Participation in the registry is voluntary and information will be available on the CDC website soon, Dr. Jamieson added. Until then, the CDC maintains a 24/7 consultation hotline for both pregnant women and health care providers concerned about Zika virus infections, at 1-800-CDC-INFO. Patients and providers can also email [email protected].
Additionally, CDC officials stated that they have received reports of at least 14 instances in which Zika virus may have been transmitted between individuals through sexual contact. Two of these cases are confirmed to have been transmitted to women from men who visited a Zika-endemic area, while another four are “probable cases” of sexual transmission, and another six are under investigation. Two reported cases were excluded after receiving additional information.
“Men who reside in or have traveled to an area of ongoing Zika virus transmission and have a pregnant partner should abstain from sexual activity or consistently and correctly use condoms during sex with their pregnant partner for the duration of the pregnancy,” said the CDC in a statement (MMWR. 2016 Feb 26. doi: http://dx.doi.org/10.15585/mmwr.mm6508e2er).
The World Health Organization also released a new situation report on Zika virus, microcephaly, and Guillain-Barré syndrome, saying that although Zika virus has been spreading to more geographic areas since the beginning of the crisis, cases of microcephaly and neonatal malformations are increasing only in Brazil and French Polynesia.
Additionally, the WHO released interim guidelines on psychosocial support for pregnant women who may be infected with Zika virus and families dealing with an infant born with either microcephaly or another neurologic disorder.
At a press briefing on Feb. 26, CDC Director Dr. Tom Frieden, noted that it has been 6 weeks since the CDC issued its first travel warning regarding Zika virus, and that the agency is “learning more about Zika everyday.” He added that the current state of the Zika virus outbreak is an “unprecedented situation.”
But Dr. Frieden also stressed that the exact link between Zika virus infection and microcephaly is still unknown, and it is not clear at what stage in the pregnancy Zika virus affects the fetus.
It’s also unknown whether infants born to mothers with Zika virus infection who don’t develop microcephaly will have any other health problems in the future. “Unfortunately, this is something we may not know for many years,” Dr. Frieden said.
Officials at the Centers for Disease Control and Prevention reported that they are aware of at least nine cases of laboratory-confirmed Zika virus infection in pregnant travelers, and that in four of these cases, fetuses were either spontaneously lost or aborted.
Confirmed cases of Zika virus infection were reported among women who had traveled to one or more of the following nine areas with ongoing local transmission of Zika virus: American Samoa, Brazil, El Salvador, Guatemala, Haiti, Honduras, Mexico, Puerto Rico, and Samoa, according to the CDC’s latest Morbidity and Mortality Weekly Report (2016 Feb 26. doi: http://dx.doi.org/10.15585/mmwr.mm6508e1er).
The agency is also investigating reports of 10 other cases of pregnant women with possible Zika virus infection.
Of the nine confirmed cases, six women were infected with Zika virus in their first trimester. In two cases, the pregnancies were terminated and two of the pregnancies resulted in stillbirths. Another woman gave birth to an infant with microcephaly, and one woman is still pregnant and has not experienced any complications so far.
Two of the nine women became infected with Zika virus during their second trimesters, one of whom has delivered a healthy infant and the other of whom is still pregnant with no known complications thus far. The last of the nine women, who became infected during her third trimester, gave birth to a healthy infant with no known complications.
There were no Zika virus–related hospitalizations or deaths among the nine women with Zika virus.
“To better understand the effects of Zika virus infection during pregnancy, CDC has established the U.S. Pregnancy Registry for Zika Virus Infection,” announced Dr. Denise J. Jamieson, colead of the Pregnancy and Birth Defects Team at the CDC. “This registry will provide information about the effects of Zika virus on pregnant women and their children.”
Participation in the registry is voluntary and information will be available on the CDC website soon, Dr. Jamieson added. Until then, the CDC maintains a 24/7 consultation hotline for both pregnant women and health care providers concerned about Zika virus infections, at 1-800-CDC-INFO. Patients and providers can also email [email protected].
Additionally, CDC officials stated that they have received reports of at least 14 instances in which Zika virus may have been transmitted between individuals through sexual contact. Two of these cases are confirmed to have been transmitted to women from men who visited a Zika-endemic area, while another four are “probable cases” of sexual transmission, and another six are under investigation. Two reported cases were excluded after receiving additional information.
“Men who reside in or have traveled to an area of ongoing Zika virus transmission and have a pregnant partner should abstain from sexual activity or consistently and correctly use condoms during sex with their pregnant partner for the duration of the pregnancy,” said the CDC in a statement (MMWR. 2016 Feb 26. doi: http://dx.doi.org/10.15585/mmwr.mm6508e2er).
The World Health Organization also released a new situation report on Zika virus, microcephaly, and Guillain-Barré syndrome, saying that although Zika virus has been spreading to more geographic areas since the beginning of the crisis, cases of microcephaly and neonatal malformations are increasing only in Brazil and French Polynesia.
Additionally, the WHO released interim guidelines on psychosocial support for pregnant women who may be infected with Zika virus and families dealing with an infant born with either microcephaly or another neurologic disorder.
At a press briefing on Feb. 26, CDC Director Dr. Tom Frieden, noted that it has been 6 weeks since the CDC issued its first travel warning regarding Zika virus, and that the agency is “learning more about Zika everyday.” He added that the current state of the Zika virus outbreak is an “unprecedented situation.”
But Dr. Frieden also stressed that the exact link between Zika virus infection and microcephaly is still unknown, and it is not clear at what stage in the pregnancy Zika virus affects the fetus.
It’s also unknown whether infants born to mothers with Zika virus infection who don’t develop microcephaly will have any other health problems in the future. “Unfortunately, this is something we may not know for many years,” Dr. Frieden said.
FROM MMWR
New data points to slower course of labor
Only recently has evidence emerged that challenges our long-held understanding of “normal” and “abnormal” labor. We now know there is a much wider range of normal labor progress in women who go on to have good labor outcomes. We have a new labor curve to guide us – one that shows us, for example, that active labor occurs most commonly after 6 cm dilation rather than 4 cm as we’d previously thought.
By appreciating this new labor paradigm, we can potentially have a significant impact on the cesarean rate in the United States. While our use of the older labor curve is not the only reason for the rise in cesarean deliveries over the last 30 years, it very likely has played a role. A study published in 2011 of more than 32,000 live births at a major academic hospital demonstrated that one of the most common reasons for primary cesarean is abnormal labor or arrest (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Another study by the Consortium on Safe Labor – an analysis of labor and delivery information from more than 228,000 women across the United States – showed that half of the cesarean deliveries performed for dystocia in women undergoing labor induction were performed before 6 cm of cervical dilation and relatively soon after the previous cervical examination (Am J Obstet Gynecol. 2010 Oct; 203[4]: 326.e1–326.e10).
Our new labor paradigm brings to the forefront a host of new issues and questions about how we can best manage labor to optimize outcomes. In a way, recent discoveries about labor progress have highlighted a dearth of evidence and made “old” issues in labor management seem new and urgent.
As we strive to learn more, however, we are challenged to change our practices and behavior at the bedside with the evidence we currently have. By appreciating both the new labor curve and our current understanding of how labor induction, obesity, and other patient characteristics and clinical conditions can affect labor progress, we can expect that many women will simply progress much more slowly than was historically expected.
As long as we have indications of the well-being of the baby and the well-being of the mother, a slower but progressive labor in the first stage should not prompt us to intervene. We should no longer apply the standards of active-phase progress – standards that have traditionally driven our diagnoses of labor dystocia – until the patient has achieved 6 cm of dilation.
The labor curve that had shaped our thinking about normal and abnormal labor progress until recently was developed by Dr. Emanuel Friedman. Based on findings from a prospective cohort study of 500 nulliparous women, Dr. Friedman plotted labor progress with centimeters of cervical dilation on the Y-axis and time on the X-axis, and divided labor into several stages and phases. In this curve, the rate of change of cervical dilation over time started increasing significantly at 4 cm; this period of increasing slope defined the active phase of labor.
Abnormal labor progress in the active phase was then defined, based on the 95th percentile, as cervical dilation of less than 1.2 cm per hour for nulliparous women and less than 1.5 cm per hour for multiparous women. Based on Dr. Friedman’s work, a woman was deemed to be in active-phase arrest when she had no cervical changes for 2 hours or more while having adequate uterine contractions and cervical dilation of at least 4 cm. These concepts came to govern labor management.
The paradigm shifted when the Consortium on Safe Labor reported in 2010 on a retrospective cohort study of more than 62,000 women at 19 U.S. hospitals. The women had a singleton term gestation, spontaneous labor, vertex presentation, vaginal delivery, and a normal perinatal outcome. In their analysis of labor and delivery information, Dr. Jun Zhang of the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and his colleagues accounted for the fact that the exact times of cervical change are unknown.
They used modern statistical methods and analytical tools that took into account the specific nature of cervical dilation data – that cervical measurements are interval-censored (we never know the exact time when a woman’s cervix changes) and that multiple exams of the cervix in the same patient are not independent (Obstet Gynecol. 2010 Dec;116[6]:1281-7).
The methodology used in the Consortium study accounted for both the interval-censored and repeated-measures nature of cervical dilation data. It thus addressed analytical flaws in the previous approach to labor data, which was purely descriptive of the exam findings and did not consider the nature of the data itself.
Under the new analysis and in the larger, contemporary population of patients, the period of increasing slope was found to occur most commonly after 6 cm, not 4 cm. The slowest 5% of nulliparous women had cervical dilation of 0.4 cm per hour (with the median at 1.9 cm per hour), compared with 1.2 cm per hour (with a median of 3.0 cm per hour) as in the Friedman data.
Dr. Zhang’s study showed us that labor may take more than 6 hours to progress from 4 to 5 cm dilation, and more than 3 hours to progress from 5 to 6 cm dilation – a rate of progress that is significantly slower than what Dr. Friedman had described. The new data showed us, moreover, that from 4 cm-6 cm dilation, nulliparous and multiparous women progressed similarly slowly. Beyond 6 cm, multiparous women dilated more rapidly, with a steeper acceleration phase than previously described.
A consensus statement published in 2014 by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) on “Safe Prevention of the Primary Cesarean Delivery” encourages use of the Consortium data to revisit the definition of labor dystocia. While the data “do not directly address an optimal duration for the diagnosis of active-phase protraction or labor arrest, [they] do suggest that neither should be diagnosed before 6 cm dilation” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
The ACOG-SMFM statement makes a series of recommendations for managing the first and second stages of labor, based not only on the Consortium data but on a broader literature review. It recommends that if mother and fetus appear well, cesarean delivery for active-phase arrest in the first stage of labor be reserved for women of at least 6 cm of dilation with ruptured membranes who fail to progress despite 4 hours of adequate uterine activity, or at least 6 hours of oxytocin administration with inadequate uterine activity and no cervical change.
Regarding the latent phase of labor, the statement says that most women with a prolonged latent phase ultimately will enter the active phase with expectant management. It advises that a prolonged latent phase (for example, greater than 20 hours in nulliparous women and greater than 14 hours in multiparous women) should not be an isolated indication for cesarean delivery.
The consensus statement also recognizes recent data showing that women who undergo labor induction have an even slower “normal” course of labor, particularly a longer latent phase, than women who labor spontaneously. A retrospective cohort study of more than 5,000 women, for instance, found that before 6 cm, women whose labor is induced can spend up to 10 hours to achieve each 1 cm of dilation (Obstet Gynecol. 2012 Jun;119[6]:1113-8).
As long as maternal and fetal status are reassuring, the statement says, cesarean deliveries for failed induction of labor in the latent phase can be avoided by allowing longer durations of the latent phase (up to 24 hours) and by requiring that oxytocin be administered for 12-18 hours after membrane rupture before deeming induction a failure.
Each of these described recommendations were graded in the ACOG-SMFM consensus document as “strong” recommendations with “moderate quality evidence.”
Examining our standards
Moving forward, we must further develop and define our thresholds for identifying who will most benefit from a cesarean delivery. We have many specific aspects of labor management to address as well, such as the optimal timing of artificial membrane rupture and the safety and efficacy of different oxytocin protocols. We may also want to revisit recommendations for serial cervical assessment, possibly adjusting the intervals given our understanding of the new labor curve.
Under the new labor paradigm, moreover, we must think not only about the clinical decisions we make at the bedside, but about the decisions we make early in the labor management process.
The timing of admission is one such decision. A statement published in 2012 on “Preventing the First Cesarean Delivery” by ACOG, SMFM, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development advises us to avoid admittance of women during the early latent phase of labor (Obstet Gynecol. 2012 Nov;120[5]:1181-93).
It may even be advisable that we consider admittance at higher cervical dilation. A study published this year shows that women admitted at less than 6 cm of dilation had an increased risk of cesarean delivery, compared with women admitted at higher cervical dilation (Am J Perinatol. 2016 Jan;33[2]:188-94). We have more to learn, but certainly, given what we know now about labor progress and the start of active labor, the timing of admission is an important factor to consider.
The second stage of labor, defined as the interval from complete cervical dilation through delivery of the fetus, presents many questions as well. There is a paucity of quality published data concerning what is normal, how long the stage should last, and how we should manage it. Historically, we have been taught to allow 2 hours of pushing for nulliparous women and 1 hour for multiparous women, when epidural anesthesia has not been administered, and to add an additional hour when epidural is used.
The 2014 ACOG-SMFM consensus statement recommends extending each of these limits by an hour, if maternal and fetal conditions permit, so that we allow at least 3 hours of pushing for nulliparous women and at least 2 hours for multiparous women before diagnosing arrest of labor in the second stage. Longer durations may be appropriate with the use of epidural anesthesia and on an individualized basis.
At this time, it is unclear whether there is any absolute maximum length of time beyond which all women in the second stage of labor should undergo cesarean delivery. We also still do not know the optimal technique for managing maternal pushing during the second stage. Should women with an epidural push right away or should they allow for a period of spontaneous descent? Many of the high-quality studies reported thus far that compare delayed and immediate pushing have limited applicability to current practice because they involved now-obsolete midpelvic forceps deliveries. A large multicenter randomized trial currently underway should provide us with some answers.
Dr. Cahill is an associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University School of Medicine in St. Louis. She reported having no relevant financial disclosures.
Only recently has evidence emerged that challenges our long-held understanding of “normal” and “abnormal” labor. We now know there is a much wider range of normal labor progress in women who go on to have good labor outcomes. We have a new labor curve to guide us – one that shows us, for example, that active labor occurs most commonly after 6 cm dilation rather than 4 cm as we’d previously thought.
By appreciating this new labor paradigm, we can potentially have a significant impact on the cesarean rate in the United States. While our use of the older labor curve is not the only reason for the rise in cesarean deliveries over the last 30 years, it very likely has played a role. A study published in 2011 of more than 32,000 live births at a major academic hospital demonstrated that one of the most common reasons for primary cesarean is abnormal labor or arrest (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Another study by the Consortium on Safe Labor – an analysis of labor and delivery information from more than 228,000 women across the United States – showed that half of the cesarean deliveries performed for dystocia in women undergoing labor induction were performed before 6 cm of cervical dilation and relatively soon after the previous cervical examination (Am J Obstet Gynecol. 2010 Oct; 203[4]: 326.e1–326.e10).
Our new labor paradigm brings to the forefront a host of new issues and questions about how we can best manage labor to optimize outcomes. In a way, recent discoveries about labor progress have highlighted a dearth of evidence and made “old” issues in labor management seem new and urgent.
As we strive to learn more, however, we are challenged to change our practices and behavior at the bedside with the evidence we currently have. By appreciating both the new labor curve and our current understanding of how labor induction, obesity, and other patient characteristics and clinical conditions can affect labor progress, we can expect that many women will simply progress much more slowly than was historically expected.
As long as we have indications of the well-being of the baby and the well-being of the mother, a slower but progressive labor in the first stage should not prompt us to intervene. We should no longer apply the standards of active-phase progress – standards that have traditionally driven our diagnoses of labor dystocia – until the patient has achieved 6 cm of dilation.
The labor curve that had shaped our thinking about normal and abnormal labor progress until recently was developed by Dr. Emanuel Friedman. Based on findings from a prospective cohort study of 500 nulliparous women, Dr. Friedman plotted labor progress with centimeters of cervical dilation on the Y-axis and time on the X-axis, and divided labor into several stages and phases. In this curve, the rate of change of cervical dilation over time started increasing significantly at 4 cm; this period of increasing slope defined the active phase of labor.
Abnormal labor progress in the active phase was then defined, based on the 95th percentile, as cervical dilation of less than 1.2 cm per hour for nulliparous women and less than 1.5 cm per hour for multiparous women. Based on Dr. Friedman’s work, a woman was deemed to be in active-phase arrest when she had no cervical changes for 2 hours or more while having adequate uterine contractions and cervical dilation of at least 4 cm. These concepts came to govern labor management.
The paradigm shifted when the Consortium on Safe Labor reported in 2010 on a retrospective cohort study of more than 62,000 women at 19 U.S. hospitals. The women had a singleton term gestation, spontaneous labor, vertex presentation, vaginal delivery, and a normal perinatal outcome. In their analysis of labor and delivery information, Dr. Jun Zhang of the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and his colleagues accounted for the fact that the exact times of cervical change are unknown.
They used modern statistical methods and analytical tools that took into account the specific nature of cervical dilation data – that cervical measurements are interval-censored (we never know the exact time when a woman’s cervix changes) and that multiple exams of the cervix in the same patient are not independent (Obstet Gynecol. 2010 Dec;116[6]:1281-7).
The methodology used in the Consortium study accounted for both the interval-censored and repeated-measures nature of cervical dilation data. It thus addressed analytical flaws in the previous approach to labor data, which was purely descriptive of the exam findings and did not consider the nature of the data itself.
Under the new analysis and in the larger, contemporary population of patients, the period of increasing slope was found to occur most commonly after 6 cm, not 4 cm. The slowest 5% of nulliparous women had cervical dilation of 0.4 cm per hour (with the median at 1.9 cm per hour), compared with 1.2 cm per hour (with a median of 3.0 cm per hour) as in the Friedman data.
Dr. Zhang’s study showed us that labor may take more than 6 hours to progress from 4 to 5 cm dilation, and more than 3 hours to progress from 5 to 6 cm dilation – a rate of progress that is significantly slower than what Dr. Friedman had described. The new data showed us, moreover, that from 4 cm-6 cm dilation, nulliparous and multiparous women progressed similarly slowly. Beyond 6 cm, multiparous women dilated more rapidly, with a steeper acceleration phase than previously described.
A consensus statement published in 2014 by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) on “Safe Prevention of the Primary Cesarean Delivery” encourages use of the Consortium data to revisit the definition of labor dystocia. While the data “do not directly address an optimal duration for the diagnosis of active-phase protraction or labor arrest, [they] do suggest that neither should be diagnosed before 6 cm dilation” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
The ACOG-SMFM statement makes a series of recommendations for managing the first and second stages of labor, based not only on the Consortium data but on a broader literature review. It recommends that if mother and fetus appear well, cesarean delivery for active-phase arrest in the first stage of labor be reserved for women of at least 6 cm of dilation with ruptured membranes who fail to progress despite 4 hours of adequate uterine activity, or at least 6 hours of oxytocin administration with inadequate uterine activity and no cervical change.
Regarding the latent phase of labor, the statement says that most women with a prolonged latent phase ultimately will enter the active phase with expectant management. It advises that a prolonged latent phase (for example, greater than 20 hours in nulliparous women and greater than 14 hours in multiparous women) should not be an isolated indication for cesarean delivery.
The consensus statement also recognizes recent data showing that women who undergo labor induction have an even slower “normal” course of labor, particularly a longer latent phase, than women who labor spontaneously. A retrospective cohort study of more than 5,000 women, for instance, found that before 6 cm, women whose labor is induced can spend up to 10 hours to achieve each 1 cm of dilation (Obstet Gynecol. 2012 Jun;119[6]:1113-8).
As long as maternal and fetal status are reassuring, the statement says, cesarean deliveries for failed induction of labor in the latent phase can be avoided by allowing longer durations of the latent phase (up to 24 hours) and by requiring that oxytocin be administered for 12-18 hours after membrane rupture before deeming induction a failure.
Each of these described recommendations were graded in the ACOG-SMFM consensus document as “strong” recommendations with “moderate quality evidence.”
Examining our standards
Moving forward, we must further develop and define our thresholds for identifying who will most benefit from a cesarean delivery. We have many specific aspects of labor management to address as well, such as the optimal timing of artificial membrane rupture and the safety and efficacy of different oxytocin protocols. We may also want to revisit recommendations for serial cervical assessment, possibly adjusting the intervals given our understanding of the new labor curve.
Under the new labor paradigm, moreover, we must think not only about the clinical decisions we make at the bedside, but about the decisions we make early in the labor management process.
The timing of admission is one such decision. A statement published in 2012 on “Preventing the First Cesarean Delivery” by ACOG, SMFM, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development advises us to avoid admittance of women during the early latent phase of labor (Obstet Gynecol. 2012 Nov;120[5]:1181-93).
It may even be advisable that we consider admittance at higher cervical dilation. A study published this year shows that women admitted at less than 6 cm of dilation had an increased risk of cesarean delivery, compared with women admitted at higher cervical dilation (Am J Perinatol. 2016 Jan;33[2]:188-94). We have more to learn, but certainly, given what we know now about labor progress and the start of active labor, the timing of admission is an important factor to consider.
The second stage of labor, defined as the interval from complete cervical dilation through delivery of the fetus, presents many questions as well. There is a paucity of quality published data concerning what is normal, how long the stage should last, and how we should manage it. Historically, we have been taught to allow 2 hours of pushing for nulliparous women and 1 hour for multiparous women, when epidural anesthesia has not been administered, and to add an additional hour when epidural is used.
The 2014 ACOG-SMFM consensus statement recommends extending each of these limits by an hour, if maternal and fetal conditions permit, so that we allow at least 3 hours of pushing for nulliparous women and at least 2 hours for multiparous women before diagnosing arrest of labor in the second stage. Longer durations may be appropriate with the use of epidural anesthesia and on an individualized basis.
At this time, it is unclear whether there is any absolute maximum length of time beyond which all women in the second stage of labor should undergo cesarean delivery. We also still do not know the optimal technique for managing maternal pushing during the second stage. Should women with an epidural push right away or should they allow for a period of spontaneous descent? Many of the high-quality studies reported thus far that compare delayed and immediate pushing have limited applicability to current practice because they involved now-obsolete midpelvic forceps deliveries. A large multicenter randomized trial currently underway should provide us with some answers.
Dr. Cahill is an associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University School of Medicine in St. Louis. She reported having no relevant financial disclosures.
Only recently has evidence emerged that challenges our long-held understanding of “normal” and “abnormal” labor. We now know there is a much wider range of normal labor progress in women who go on to have good labor outcomes. We have a new labor curve to guide us – one that shows us, for example, that active labor occurs most commonly after 6 cm dilation rather than 4 cm as we’d previously thought.
By appreciating this new labor paradigm, we can potentially have a significant impact on the cesarean rate in the United States. While our use of the older labor curve is not the only reason for the rise in cesarean deliveries over the last 30 years, it very likely has played a role. A study published in 2011 of more than 32,000 live births at a major academic hospital demonstrated that one of the most common reasons for primary cesarean is abnormal labor or arrest (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Another study by the Consortium on Safe Labor – an analysis of labor and delivery information from more than 228,000 women across the United States – showed that half of the cesarean deliveries performed for dystocia in women undergoing labor induction were performed before 6 cm of cervical dilation and relatively soon after the previous cervical examination (Am J Obstet Gynecol. 2010 Oct; 203[4]: 326.e1–326.e10).
Our new labor paradigm brings to the forefront a host of new issues and questions about how we can best manage labor to optimize outcomes. In a way, recent discoveries about labor progress have highlighted a dearth of evidence and made “old” issues in labor management seem new and urgent.
As we strive to learn more, however, we are challenged to change our practices and behavior at the bedside with the evidence we currently have. By appreciating both the new labor curve and our current understanding of how labor induction, obesity, and other patient characteristics and clinical conditions can affect labor progress, we can expect that many women will simply progress much more slowly than was historically expected.
As long as we have indications of the well-being of the baby and the well-being of the mother, a slower but progressive labor in the first stage should not prompt us to intervene. We should no longer apply the standards of active-phase progress – standards that have traditionally driven our diagnoses of labor dystocia – until the patient has achieved 6 cm of dilation.
The labor curve that had shaped our thinking about normal and abnormal labor progress until recently was developed by Dr. Emanuel Friedman. Based on findings from a prospective cohort study of 500 nulliparous women, Dr. Friedman plotted labor progress with centimeters of cervical dilation on the Y-axis and time on the X-axis, and divided labor into several stages and phases. In this curve, the rate of change of cervical dilation over time started increasing significantly at 4 cm; this period of increasing slope defined the active phase of labor.
Abnormal labor progress in the active phase was then defined, based on the 95th percentile, as cervical dilation of less than 1.2 cm per hour for nulliparous women and less than 1.5 cm per hour for multiparous women. Based on Dr. Friedman’s work, a woman was deemed to be in active-phase arrest when she had no cervical changes for 2 hours or more while having adequate uterine contractions and cervical dilation of at least 4 cm. These concepts came to govern labor management.
The paradigm shifted when the Consortium on Safe Labor reported in 2010 on a retrospective cohort study of more than 62,000 women at 19 U.S. hospitals. The women had a singleton term gestation, spontaneous labor, vertex presentation, vaginal delivery, and a normal perinatal outcome. In their analysis of labor and delivery information, Dr. Jun Zhang of the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and his colleagues accounted for the fact that the exact times of cervical change are unknown.
They used modern statistical methods and analytical tools that took into account the specific nature of cervical dilation data – that cervical measurements are interval-censored (we never know the exact time when a woman’s cervix changes) and that multiple exams of the cervix in the same patient are not independent (Obstet Gynecol. 2010 Dec;116[6]:1281-7).
The methodology used in the Consortium study accounted for both the interval-censored and repeated-measures nature of cervical dilation data. It thus addressed analytical flaws in the previous approach to labor data, which was purely descriptive of the exam findings and did not consider the nature of the data itself.
Under the new analysis and in the larger, contemporary population of patients, the period of increasing slope was found to occur most commonly after 6 cm, not 4 cm. The slowest 5% of nulliparous women had cervical dilation of 0.4 cm per hour (with the median at 1.9 cm per hour), compared with 1.2 cm per hour (with a median of 3.0 cm per hour) as in the Friedman data.
Dr. Zhang’s study showed us that labor may take more than 6 hours to progress from 4 to 5 cm dilation, and more than 3 hours to progress from 5 to 6 cm dilation – a rate of progress that is significantly slower than what Dr. Friedman had described. The new data showed us, moreover, that from 4 cm-6 cm dilation, nulliparous and multiparous women progressed similarly slowly. Beyond 6 cm, multiparous women dilated more rapidly, with a steeper acceleration phase than previously described.
A consensus statement published in 2014 by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) on “Safe Prevention of the Primary Cesarean Delivery” encourages use of the Consortium data to revisit the definition of labor dystocia. While the data “do not directly address an optimal duration for the diagnosis of active-phase protraction or labor arrest, [they] do suggest that neither should be diagnosed before 6 cm dilation” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
The ACOG-SMFM statement makes a series of recommendations for managing the first and second stages of labor, based not only on the Consortium data but on a broader literature review. It recommends that if mother and fetus appear well, cesarean delivery for active-phase arrest in the first stage of labor be reserved for women of at least 6 cm of dilation with ruptured membranes who fail to progress despite 4 hours of adequate uterine activity, or at least 6 hours of oxytocin administration with inadequate uterine activity and no cervical change.
Regarding the latent phase of labor, the statement says that most women with a prolonged latent phase ultimately will enter the active phase with expectant management. It advises that a prolonged latent phase (for example, greater than 20 hours in nulliparous women and greater than 14 hours in multiparous women) should not be an isolated indication for cesarean delivery.
The consensus statement also recognizes recent data showing that women who undergo labor induction have an even slower “normal” course of labor, particularly a longer latent phase, than women who labor spontaneously. A retrospective cohort study of more than 5,000 women, for instance, found that before 6 cm, women whose labor is induced can spend up to 10 hours to achieve each 1 cm of dilation (Obstet Gynecol. 2012 Jun;119[6]:1113-8).
As long as maternal and fetal status are reassuring, the statement says, cesarean deliveries for failed induction of labor in the latent phase can be avoided by allowing longer durations of the latent phase (up to 24 hours) and by requiring that oxytocin be administered for 12-18 hours after membrane rupture before deeming induction a failure.
Each of these described recommendations were graded in the ACOG-SMFM consensus document as “strong” recommendations with “moderate quality evidence.”
Examining our standards
Moving forward, we must further develop and define our thresholds for identifying who will most benefit from a cesarean delivery. We have many specific aspects of labor management to address as well, such as the optimal timing of artificial membrane rupture and the safety and efficacy of different oxytocin protocols. We may also want to revisit recommendations for serial cervical assessment, possibly adjusting the intervals given our understanding of the new labor curve.
Under the new labor paradigm, moreover, we must think not only about the clinical decisions we make at the bedside, but about the decisions we make early in the labor management process.
The timing of admission is one such decision. A statement published in 2012 on “Preventing the First Cesarean Delivery” by ACOG, SMFM, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development advises us to avoid admittance of women during the early latent phase of labor (Obstet Gynecol. 2012 Nov;120[5]:1181-93).
It may even be advisable that we consider admittance at higher cervical dilation. A study published this year shows that women admitted at less than 6 cm of dilation had an increased risk of cesarean delivery, compared with women admitted at higher cervical dilation (Am J Perinatol. 2016 Jan;33[2]:188-94). We have more to learn, but certainly, given what we know now about labor progress and the start of active labor, the timing of admission is an important factor to consider.
The second stage of labor, defined as the interval from complete cervical dilation through delivery of the fetus, presents many questions as well. There is a paucity of quality published data concerning what is normal, how long the stage should last, and how we should manage it. Historically, we have been taught to allow 2 hours of pushing for nulliparous women and 1 hour for multiparous women, when epidural anesthesia has not been administered, and to add an additional hour when epidural is used.
The 2014 ACOG-SMFM consensus statement recommends extending each of these limits by an hour, if maternal and fetal conditions permit, so that we allow at least 3 hours of pushing for nulliparous women and at least 2 hours for multiparous women before diagnosing arrest of labor in the second stage. Longer durations may be appropriate with the use of epidural anesthesia and on an individualized basis.
At this time, it is unclear whether there is any absolute maximum length of time beyond which all women in the second stage of labor should undergo cesarean delivery. We also still do not know the optimal technique for managing maternal pushing during the second stage. Should women with an epidural push right away or should they allow for a period of spontaneous descent? Many of the high-quality studies reported thus far that compare delayed and immediate pushing have limited applicability to current practice because they involved now-obsolete midpelvic forceps deliveries. A large multicenter randomized trial currently underway should provide us with some answers.
Dr. Cahill is an associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University School of Medicine in St. Louis. She reported having no relevant financial disclosures.
Rethinking the management of labor
Over the last 50 years, we have witnessed some incredible advancements that have vastly improved maternal and fetal outcomes, even in the face of the most complex obstetrical dilemmas. As our practice and the research continues to evolve, it is increasingly important that we carefully review our practice standards to ensure that every woman and her baby receives the most up-to-date medical care.
This month’s Master Class highlights a critical area of obstetrics where the convergence of technology, clinical observation, and research stimulated a change in practice guidelines: the use of the labor curve to monitor normal versus abnormal labor. Until quite recently, ob.gyns. had based labor criteria on the “Friedman Curve,” first established in the mid-1950s, and supported by other smaller and less comprehensive studies. This work was adopted by the American College of Obstetricians and Gynecologists.
For more than half a century, we used these parameters to determine if a woman had entered active-phase arrest, and to make the very important decision of whether to perform a cesarean section. However, work in the early 2000s strongly suggested that the old criteria no longer applied to the full course of labor in contemporary patients (Am J Obstet Gynecol. 2002 Oct;187[4]:824-8). A 2010 comprehensive study showed that we needed to consider a new approach to labor management (Am J Obstet Gynecol. 2010 Oct;203[4]:326.e1-326.e10).
It may seem incredible that it took such a long time to update our thinking about what constitutes normal versus abnormal labor progression. However, we must keep in mind that many studies supported the original labor curve, and advanced tools to assess fetal health during labor were just being developed. The first commercially available fetal heart rate monitor would not be produced until 1968, and debates about the utility of these devices would continue into the early 1990s.
Additionally, our patient population has changed. As we have discussed in previous columns, the incidence and severity of other chronic conditions, such as diabetes and obesity, has increased significantly and deeply impacted labor progression.
Just as technology has advanced and our patients’ needs have changed, so, too, must our practice standards. We have invited Dr. Alison G. Cahill, associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University, St. Louis, to discuss the importance and implications of the new labor curve.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Over the last 50 years, we have witnessed some incredible advancements that have vastly improved maternal and fetal outcomes, even in the face of the most complex obstetrical dilemmas. As our practice and the research continues to evolve, it is increasingly important that we carefully review our practice standards to ensure that every woman and her baby receives the most up-to-date medical care.
This month’s Master Class highlights a critical area of obstetrics where the convergence of technology, clinical observation, and research stimulated a change in practice guidelines: the use of the labor curve to monitor normal versus abnormal labor. Until quite recently, ob.gyns. had based labor criteria on the “Friedman Curve,” first established in the mid-1950s, and supported by other smaller and less comprehensive studies. This work was adopted by the American College of Obstetricians and Gynecologists.
For more than half a century, we used these parameters to determine if a woman had entered active-phase arrest, and to make the very important decision of whether to perform a cesarean section. However, work in the early 2000s strongly suggested that the old criteria no longer applied to the full course of labor in contemporary patients (Am J Obstet Gynecol. 2002 Oct;187[4]:824-8). A 2010 comprehensive study showed that we needed to consider a new approach to labor management (Am J Obstet Gynecol. 2010 Oct;203[4]:326.e1-326.e10).
It may seem incredible that it took such a long time to update our thinking about what constitutes normal versus abnormal labor progression. However, we must keep in mind that many studies supported the original labor curve, and advanced tools to assess fetal health during labor were just being developed. The first commercially available fetal heart rate monitor would not be produced until 1968, and debates about the utility of these devices would continue into the early 1990s.
Additionally, our patient population has changed. As we have discussed in previous columns, the incidence and severity of other chronic conditions, such as diabetes and obesity, has increased significantly and deeply impacted labor progression.
Just as technology has advanced and our patients’ needs have changed, so, too, must our practice standards. We have invited Dr. Alison G. Cahill, associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University, St. Louis, to discuss the importance and implications of the new labor curve.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Over the last 50 years, we have witnessed some incredible advancements that have vastly improved maternal and fetal outcomes, even in the face of the most complex obstetrical dilemmas. As our practice and the research continues to evolve, it is increasingly important that we carefully review our practice standards to ensure that every woman and her baby receives the most up-to-date medical care.
This month’s Master Class highlights a critical area of obstetrics where the convergence of technology, clinical observation, and research stimulated a change in practice guidelines: the use of the labor curve to monitor normal versus abnormal labor. Until quite recently, ob.gyns. had based labor criteria on the “Friedman Curve,” first established in the mid-1950s, and supported by other smaller and less comprehensive studies. This work was adopted by the American College of Obstetricians and Gynecologists.
For more than half a century, we used these parameters to determine if a woman had entered active-phase arrest, and to make the very important decision of whether to perform a cesarean section. However, work in the early 2000s strongly suggested that the old criteria no longer applied to the full course of labor in contemporary patients (Am J Obstet Gynecol. 2002 Oct;187[4]:824-8). A 2010 comprehensive study showed that we needed to consider a new approach to labor management (Am J Obstet Gynecol. 2010 Oct;203[4]:326.e1-326.e10).
It may seem incredible that it took such a long time to update our thinking about what constitutes normal versus abnormal labor progression. However, we must keep in mind that many studies supported the original labor curve, and advanced tools to assess fetal health during labor were just being developed. The first commercially available fetal heart rate monitor would not be produced until 1968, and debates about the utility of these devices would continue into the early 1990s.
Additionally, our patient population has changed. As we have discussed in previous columns, the incidence and severity of other chronic conditions, such as diabetes and obesity, has increased significantly and deeply impacted labor progression.
Just as technology has advanced and our patients’ needs have changed, so, too, must our practice standards. We have invited Dr. Alison G. Cahill, associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University, St. Louis, to discuss the importance and implications of the new labor curve.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].