User login
Product updates and reviews
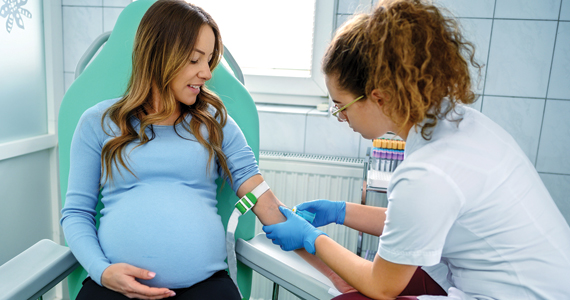
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
COMMENT & CONTROVERSY
Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes?
JAIMEY M. PAULI, MD (JUNE 2022)
Consider this, when it comes to treating chronic hypertension
I welcome the article by Dr. Jaimey Pauli, which focuses on initiating treatment for mild chronic hypertension in pregnancy to reach a goal blood pressure (BP) of <140/90 mm Hg to prevent adverse maternal and fetal outcomes.1 I would like to offer 3 additional thoughts for your consideration. First, it is known that there is a physiological decrease in BP during the second trimester, which results in a normotensive presentation. Thus, it would be beneficial to see if pregnant women with high-normal BP levels before the third trimester be administered a lower dose of antihypertensives. However, there is also a concern that decreased maternal BP may compromise uteroplacental perfusion and fetal circulation, which also could be evaluated.2
Second, I would like to see how comorbidities affect the initiation of antihypertensives for mild chronic hypertension in pregnancy. Research incorporating pregnant women with borderline hypertension and comorbidities such as obesity, hyperlipidemia, and diabetes mellitus type 2 (DM) is likely to yield informative results. This is especially beneficial since, for example, chronic hypertension and DM are independent risk factors for adverse maternal and fetal outcomes; therefore, a mother with both these conditions may have additive effects on obstetric outcomes.3
Lastly, I would suggest you include a brief conversation about prepregnancy ways to manage women with chronic hypertension. Because many women who enter pregnancy with chronic hypertension have hypertension of unknown origin, it would be beneficial to optimize antihypertensive regimens before conception.4 Also, it should be further evaluated whether initiation of lifestyle modifications, such as weight reduction and the DASH diet before pregnancy, for women with chronic hypertension improves pregnancy outcomes.
Cassandra Maafoh, MD
Macon, Georgia
References
- Pauli JM. Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes? OBG Manag. 2022;34:14-15.
- Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014;74:283-296. https://doi.org/10.1007/s40265-014-0187-7.
- Yanit KE, Snowden JM, Cheng YW, et al. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. Am J Obstet Gynecol. 2012;207. https://doi. org/10.1016/j.ajog.2012.06.066.
- Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129:1254-1261. https:// doi.org/10.1161/circulationaha.113.003904.
BARBARA LEVY, MD (AUGUST 2022)
Are these new and rare syndromes’ pathophysiological mechanisms related?
I read with great interest Dr. Barbara Levy’s UPDATE in the August 2022 issue on testosterone therapy for women with hypoactive sexual desire disorder (HSDD), as well as her comments on persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD) that was recently so coined by the International Society for the Study of Women’s Sexual Health (ISSWSH) as a 2-component syndrome.1 The new syndrome, explains Dr. Levy, presents with “the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient (the PGAD component),” combined with “itching, burning, tingling, or pain” (the GPD component).
Although agreeing with ISSWSH that diagnosis and management require a multidisciplinary biopsychosocial approach, in her practical advice, Dr. Levy mentioned: “neuropathic signaling” with “aberrant sensory processing” as the syndrome’s possible main pathophysiology. Interestingly, there are 2 other rare, chronic, and “poorly recognized source(s) of major distress to a small but significant group of patients.” Persistent idiopathic oro-facial pain (PIFP) disorder2 after dental interventions and burning mouth syndrome (BMS),3 defined by the absence of any local or systemic contributing etiology, also present with continuous local burning and pain (as in GPD). Consequently, PGAD/GPD may indeed have the same pathophysiological explanation—as Dr. Levy suggested—of being a (genital) peripheral chronic neuropathic pain condition.
A potentially promising new therapeutic approach for PGAD/GPD would then be to use the same, or similar, antineuropathic medications (Clonazepam, Nortriptyline, Pregabalin, etc.) in the form of topical vaginal swishing solutions similar to the presently recommended antiepileptic and/or antidepressant oral swishing treatment for PIFP and BMS. As the topical approach works well for oral neuropathic pain, vaginal swishing could potentially be the answer for PGAD/GPD peripheral neuropathic pain. Moreover, such a novel topical approach would significantly increase patient motivation for treatment by avoiding the adverse effects of ingested antiepileptic or antidepressant drugs.
This is the first time that anticonvulsant and/or antidepressant vaginal swishing is proposed as topical therapy for GPD peripheral neuropathic pain, still pending scientific/clinical validation. ●
Zwi Hoch, MD
Newton, Massachusetts
- Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) Review of Epidemiology and Pathophysiology, and a Consensus Nomenclature and Process of Care for the Management of Persistent Genital Arousal Disorder/Genito-Pelvic Dysesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
- Baad-Hansen L, Benoliel R. Neuropathic orofacial pain: facts and fiction. Cephalgia. 2017;37:670-679.
- Kuten-Shorer M, Treister NS, Stock S, et al. Safety and tolerability of topical clonazepam solution for management of oral dysesthesia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124: 146-151.
Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes?
JAIMEY M. PAULI, MD (JUNE 2022)
Consider this, when it comes to treating chronic hypertension
I welcome the article by Dr. Jaimey Pauli, which focuses on initiating treatment for mild chronic hypertension in pregnancy to reach a goal blood pressure (BP) of <140/90 mm Hg to prevent adverse maternal and fetal outcomes.1 I would like to offer 3 additional thoughts for your consideration. First, it is known that there is a physiological decrease in BP during the second trimester, which results in a normotensive presentation. Thus, it would be beneficial to see if pregnant women with high-normal BP levels before the third trimester be administered a lower dose of antihypertensives. However, there is also a concern that decreased maternal BP may compromise uteroplacental perfusion and fetal circulation, which also could be evaluated.2
Second, I would like to see how comorbidities affect the initiation of antihypertensives for mild chronic hypertension in pregnancy. Research incorporating pregnant women with borderline hypertension and comorbidities such as obesity, hyperlipidemia, and diabetes mellitus type 2 (DM) is likely to yield informative results. This is especially beneficial since, for example, chronic hypertension and DM are independent risk factors for adverse maternal and fetal outcomes; therefore, a mother with both these conditions may have additive effects on obstetric outcomes.3
Lastly, I would suggest you include a brief conversation about prepregnancy ways to manage women with chronic hypertension. Because many women who enter pregnancy with chronic hypertension have hypertension of unknown origin, it would be beneficial to optimize antihypertensive regimens before conception.4 Also, it should be further evaluated whether initiation of lifestyle modifications, such as weight reduction and the DASH diet before pregnancy, for women with chronic hypertension improves pregnancy outcomes.
Cassandra Maafoh, MD
Macon, Georgia
References
- Pauli JM. Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes? OBG Manag. 2022;34:14-15.
- Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014;74:283-296. https://doi.org/10.1007/s40265-014-0187-7.
- Yanit KE, Snowden JM, Cheng YW, et al. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. Am J Obstet Gynecol. 2012;207. https://doi. org/10.1016/j.ajog.2012.06.066.
- Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129:1254-1261. https:// doi.org/10.1161/circulationaha.113.003904.
BARBARA LEVY, MD (AUGUST 2022)
Are these new and rare syndromes’ pathophysiological mechanisms related?
I read with great interest Dr. Barbara Levy’s UPDATE in the August 2022 issue on testosterone therapy for women with hypoactive sexual desire disorder (HSDD), as well as her comments on persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD) that was recently so coined by the International Society for the Study of Women’s Sexual Health (ISSWSH) as a 2-component syndrome.1 The new syndrome, explains Dr. Levy, presents with “the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient (the PGAD component),” combined with “itching, burning, tingling, or pain” (the GPD component).
Although agreeing with ISSWSH that diagnosis and management require a multidisciplinary biopsychosocial approach, in her practical advice, Dr. Levy mentioned: “neuropathic signaling” with “aberrant sensory processing” as the syndrome’s possible main pathophysiology. Interestingly, there are 2 other rare, chronic, and “poorly recognized source(s) of major distress to a small but significant group of patients.” Persistent idiopathic oro-facial pain (PIFP) disorder2 after dental interventions and burning mouth syndrome (BMS),3 defined by the absence of any local or systemic contributing etiology, also present with continuous local burning and pain (as in GPD). Consequently, PGAD/GPD may indeed have the same pathophysiological explanation—as Dr. Levy suggested—of being a (genital) peripheral chronic neuropathic pain condition.
A potentially promising new therapeutic approach for PGAD/GPD would then be to use the same, or similar, antineuropathic medications (Clonazepam, Nortriptyline, Pregabalin, etc.) in the form of topical vaginal swishing solutions similar to the presently recommended antiepileptic and/or antidepressant oral swishing treatment for PIFP and BMS. As the topical approach works well for oral neuropathic pain, vaginal swishing could potentially be the answer for PGAD/GPD peripheral neuropathic pain. Moreover, such a novel topical approach would significantly increase patient motivation for treatment by avoiding the adverse effects of ingested antiepileptic or antidepressant drugs.
This is the first time that anticonvulsant and/or antidepressant vaginal swishing is proposed as topical therapy for GPD peripheral neuropathic pain, still pending scientific/clinical validation. ●
Zwi Hoch, MD
Newton, Massachusetts
- Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) Review of Epidemiology and Pathophysiology, and a Consensus Nomenclature and Process of Care for the Management of Persistent Genital Arousal Disorder/Genito-Pelvic Dysesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
- Baad-Hansen L, Benoliel R. Neuropathic orofacial pain: facts and fiction. Cephalgia. 2017;37:670-679.
- Kuten-Shorer M, Treister NS, Stock S, et al. Safety and tolerability of topical clonazepam solution for management of oral dysesthesia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124: 146-151.
Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes?
JAIMEY M. PAULI, MD (JUNE 2022)
Consider this, when it comes to treating chronic hypertension
I welcome the article by Dr. Jaimey Pauli, which focuses on initiating treatment for mild chronic hypertension in pregnancy to reach a goal blood pressure (BP) of <140/90 mm Hg to prevent adverse maternal and fetal outcomes.1 I would like to offer 3 additional thoughts for your consideration. First, it is known that there is a physiological decrease in BP during the second trimester, which results in a normotensive presentation. Thus, it would be beneficial to see if pregnant women with high-normal BP levels before the third trimester be administered a lower dose of antihypertensives. However, there is also a concern that decreased maternal BP may compromise uteroplacental perfusion and fetal circulation, which also could be evaluated.2
Second, I would like to see how comorbidities affect the initiation of antihypertensives for mild chronic hypertension in pregnancy. Research incorporating pregnant women with borderline hypertension and comorbidities such as obesity, hyperlipidemia, and diabetes mellitus type 2 (DM) is likely to yield informative results. This is especially beneficial since, for example, chronic hypertension and DM are independent risk factors for adverse maternal and fetal outcomes; therefore, a mother with both these conditions may have additive effects on obstetric outcomes.3
Lastly, I would suggest you include a brief conversation about prepregnancy ways to manage women with chronic hypertension. Because many women who enter pregnancy with chronic hypertension have hypertension of unknown origin, it would be beneficial to optimize antihypertensive regimens before conception.4 Also, it should be further evaluated whether initiation of lifestyle modifications, such as weight reduction and the DASH diet before pregnancy, for women with chronic hypertension improves pregnancy outcomes.
Cassandra Maafoh, MD
Macon, Georgia
References
- Pauli JM. Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes? OBG Manag. 2022;34:14-15.
- Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014;74:283-296. https://doi.org/10.1007/s40265-014-0187-7.
- Yanit KE, Snowden JM, Cheng YW, et al. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. Am J Obstet Gynecol. 2012;207. https://doi. org/10.1016/j.ajog.2012.06.066.
- Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129:1254-1261. https:// doi.org/10.1161/circulationaha.113.003904.
BARBARA LEVY, MD (AUGUST 2022)
Are these new and rare syndromes’ pathophysiological mechanisms related?
I read with great interest Dr. Barbara Levy’s UPDATE in the August 2022 issue on testosterone therapy for women with hypoactive sexual desire disorder (HSDD), as well as her comments on persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD) that was recently so coined by the International Society for the Study of Women’s Sexual Health (ISSWSH) as a 2-component syndrome.1 The new syndrome, explains Dr. Levy, presents with “the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient (the PGAD component),” combined with “itching, burning, tingling, or pain” (the GPD component).
Although agreeing with ISSWSH that diagnosis and management require a multidisciplinary biopsychosocial approach, in her practical advice, Dr. Levy mentioned: “neuropathic signaling” with “aberrant sensory processing” as the syndrome’s possible main pathophysiology. Interestingly, there are 2 other rare, chronic, and “poorly recognized source(s) of major distress to a small but significant group of patients.” Persistent idiopathic oro-facial pain (PIFP) disorder2 after dental interventions and burning mouth syndrome (BMS),3 defined by the absence of any local or systemic contributing etiology, also present with continuous local burning and pain (as in GPD). Consequently, PGAD/GPD may indeed have the same pathophysiological explanation—as Dr. Levy suggested—of being a (genital) peripheral chronic neuropathic pain condition.
A potentially promising new therapeutic approach for PGAD/GPD would then be to use the same, or similar, antineuropathic medications (Clonazepam, Nortriptyline, Pregabalin, etc.) in the form of topical vaginal swishing solutions similar to the presently recommended antiepileptic and/or antidepressant oral swishing treatment for PIFP and BMS. As the topical approach works well for oral neuropathic pain, vaginal swishing could potentially be the answer for PGAD/GPD peripheral neuropathic pain. Moreover, such a novel topical approach would significantly increase patient motivation for treatment by avoiding the adverse effects of ingested antiepileptic or antidepressant drugs.
This is the first time that anticonvulsant and/or antidepressant vaginal swishing is proposed as topical therapy for GPD peripheral neuropathic pain, still pending scientific/clinical validation. ●
Zwi Hoch, MD
Newton, Massachusetts
- Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) Review of Epidemiology and Pathophysiology, and a Consensus Nomenclature and Process of Care for the Management of Persistent Genital Arousal Disorder/Genito-Pelvic Dysesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
- Baad-Hansen L, Benoliel R. Neuropathic orofacial pain: facts and fiction. Cephalgia. 2017;37:670-679.
- Kuten-Shorer M, Treister NS, Stock S, et al. Safety and tolerability of topical clonazepam solution for management of oral dysesthesia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124: 146-151.
Difficulty fitting family into career: Female oncologists
In a survey of just over 1,000 female oncologists, 95% said their career plans were at least somewhat associated with the timing of when to start a family.
The most striking finding was that one third of respondents had miscarried and another one third reported difficulty with infertility that required fertility counseling and/or treatment.
One third reported experiencing discrimination during pregnancy, and another third said they experienced discrimination for taking maternity leave, and having more than one child increased the likelihood of this.
The most common negative factor associated with family planning was long work hours and heavy workload (66.6%),
These findings suggest there are systemic changes needed not only in the healthcare setting but in society as a whole around women in the workplace and their choices of childbearing, say the authors.
The study was published online in JAMA Network Open and led by Anna Lee MD, MPH, from the department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
In an invited commentary, Mona Saleh, MD, and Stephanie Blank, MD, from the department of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai in New York, suggest that cultural changes are needed that go beyond women in medicine.
“These cultural values are so deeply pervasive (one could also say invasive) that they affect even these most educated and wealthy professional women, such as those who participated in this survey,” the editorialists write.
“[The researchers] advocate for early education on assisted reproductive technology (ART) risks, benefits, and success rates, but this is not getting at the underlying issue: Pregnancy discrimination and unfair distribution of childbearing responsibilities are a reflection of a larger problematic culture rather than an issue specific to women in medicine,” they add.
Survey details
The survey comprised a novel 39-item questionnaire distributed to 1,004 U.S. female oncologists from May 7 to June 30, 2020, via email and social media channels.
Most respondents (84.4%) were married, and 71% were currently working full-time.
About one-third (35%) worked in radiation oncology, another third (34.3%) in medical oncology, 18.4% in surgical oncology, and 9.1% in pediatric oncology.
A total of 768 respondents (76.5%) had children, and of these, 415 (41.3%) first gave birth during postgraduate training and 275 (27.4%) gave birth in years 1-5 as an attending physician.
Of all respondents who had been pregnant, approximately two-thirds (65.7%) had some type of pregnancy complication. About one-third of respondents (31.7%) reported having experienced a miscarriage after a confirmed pregnancy; of those, 61.6% reported one miscarriage, while the remainder had two or more miscarriages (38.4%).
Approximately one-third (31.4%) of respondents reported difficulty with infertility that required fertility counseling and/or treatment.
The questionnaire also asked about assisted reproductive technology, and 164 participants (16.3%) reported the use of fertility medications, and 53 (5.3%) reported cryopreservation of eggs. Nearly 13% reported the use of intrauterine insemination and 13.2% reported the use of in vivo fertilization. Among those who experienced fertility concerns, 36.6% (232 of 634) reported facing financial burdens because of fertility or pregnancy that was in some way associated with their career choice.
When asked on the survey if fertility preservation should be discussed with women during medical school and/or residency, 65.7% of respondents stated that it should.
However, the editorialists suggest that “encouraging formal and directed education regarding the infertility risks specifically toward female physicians (which Lee et al. recommend) could be perceived as a blanket recommendation that it is best for women in medicine to delay childbearing and pursue ART.”
“Medical schools and residency and fellowship training programs should instead focus their energy on creating a framework and culture that normalizes conception during these points in training while also subsidizing and supporting trainees and physicians who prefer to use ART and delay fertility until after training,” they suggest.
The editorialists also emphasized that women may choose to become pregnant at any point during the years that it takes to go from being a medical student to resident/fellow to attending physician, and they should be supported by their workplace on their decisions.
The study was funded by grants from National Institutes of Health/National Cancer Institute Cancer Center.
Dr. Lee and coauthors reported no relevant financial relationships. Dr. Blank reported receiving grants from AstraZeneca, Aravive, Akesobio, GlaxoSmithKline, Merck, and Seattle Genetics outside the submitted work. Dr. Saleh reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of just over 1,000 female oncologists, 95% said their career plans were at least somewhat associated with the timing of when to start a family.
The most striking finding was that one third of respondents had miscarried and another one third reported difficulty with infertility that required fertility counseling and/or treatment.
One third reported experiencing discrimination during pregnancy, and another third said they experienced discrimination for taking maternity leave, and having more than one child increased the likelihood of this.
The most common negative factor associated with family planning was long work hours and heavy workload (66.6%),
These findings suggest there are systemic changes needed not only in the healthcare setting but in society as a whole around women in the workplace and their choices of childbearing, say the authors.
The study was published online in JAMA Network Open and led by Anna Lee MD, MPH, from the department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
In an invited commentary, Mona Saleh, MD, and Stephanie Blank, MD, from the department of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai in New York, suggest that cultural changes are needed that go beyond women in medicine.
“These cultural values are so deeply pervasive (one could also say invasive) that they affect even these most educated and wealthy professional women, such as those who participated in this survey,” the editorialists write.
“[The researchers] advocate for early education on assisted reproductive technology (ART) risks, benefits, and success rates, but this is not getting at the underlying issue: Pregnancy discrimination and unfair distribution of childbearing responsibilities are a reflection of a larger problematic culture rather than an issue specific to women in medicine,” they add.
Survey details
The survey comprised a novel 39-item questionnaire distributed to 1,004 U.S. female oncologists from May 7 to June 30, 2020, via email and social media channels.
Most respondents (84.4%) were married, and 71% were currently working full-time.
About one-third (35%) worked in radiation oncology, another third (34.3%) in medical oncology, 18.4% in surgical oncology, and 9.1% in pediatric oncology.
A total of 768 respondents (76.5%) had children, and of these, 415 (41.3%) first gave birth during postgraduate training and 275 (27.4%) gave birth in years 1-5 as an attending physician.
Of all respondents who had been pregnant, approximately two-thirds (65.7%) had some type of pregnancy complication. About one-third of respondents (31.7%) reported having experienced a miscarriage after a confirmed pregnancy; of those, 61.6% reported one miscarriage, while the remainder had two or more miscarriages (38.4%).
Approximately one-third (31.4%) of respondents reported difficulty with infertility that required fertility counseling and/or treatment.
The questionnaire also asked about assisted reproductive technology, and 164 participants (16.3%) reported the use of fertility medications, and 53 (5.3%) reported cryopreservation of eggs. Nearly 13% reported the use of intrauterine insemination and 13.2% reported the use of in vivo fertilization. Among those who experienced fertility concerns, 36.6% (232 of 634) reported facing financial burdens because of fertility or pregnancy that was in some way associated with their career choice.
When asked on the survey if fertility preservation should be discussed with women during medical school and/or residency, 65.7% of respondents stated that it should.
However, the editorialists suggest that “encouraging formal and directed education regarding the infertility risks specifically toward female physicians (which Lee et al. recommend) could be perceived as a blanket recommendation that it is best for women in medicine to delay childbearing and pursue ART.”
“Medical schools and residency and fellowship training programs should instead focus their energy on creating a framework and culture that normalizes conception during these points in training while also subsidizing and supporting trainees and physicians who prefer to use ART and delay fertility until after training,” they suggest.
The editorialists also emphasized that women may choose to become pregnant at any point during the years that it takes to go from being a medical student to resident/fellow to attending physician, and they should be supported by their workplace on their decisions.
The study was funded by grants from National Institutes of Health/National Cancer Institute Cancer Center.
Dr. Lee and coauthors reported no relevant financial relationships. Dr. Blank reported receiving grants from AstraZeneca, Aravive, Akesobio, GlaxoSmithKline, Merck, and Seattle Genetics outside the submitted work. Dr. Saleh reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of just over 1,000 female oncologists, 95% said their career plans were at least somewhat associated with the timing of when to start a family.
The most striking finding was that one third of respondents had miscarried and another one third reported difficulty with infertility that required fertility counseling and/or treatment.
One third reported experiencing discrimination during pregnancy, and another third said they experienced discrimination for taking maternity leave, and having more than one child increased the likelihood of this.
The most common negative factor associated with family planning was long work hours and heavy workload (66.6%),
These findings suggest there are systemic changes needed not only in the healthcare setting but in society as a whole around women in the workplace and their choices of childbearing, say the authors.
The study was published online in JAMA Network Open and led by Anna Lee MD, MPH, from the department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
In an invited commentary, Mona Saleh, MD, and Stephanie Blank, MD, from the department of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai in New York, suggest that cultural changes are needed that go beyond women in medicine.
“These cultural values are so deeply pervasive (one could also say invasive) that they affect even these most educated and wealthy professional women, such as those who participated in this survey,” the editorialists write.
“[The researchers] advocate for early education on assisted reproductive technology (ART) risks, benefits, and success rates, but this is not getting at the underlying issue: Pregnancy discrimination and unfair distribution of childbearing responsibilities are a reflection of a larger problematic culture rather than an issue specific to women in medicine,” they add.
Survey details
The survey comprised a novel 39-item questionnaire distributed to 1,004 U.S. female oncologists from May 7 to June 30, 2020, via email and social media channels.
Most respondents (84.4%) were married, and 71% were currently working full-time.
About one-third (35%) worked in radiation oncology, another third (34.3%) in medical oncology, 18.4% in surgical oncology, and 9.1% in pediatric oncology.
A total of 768 respondents (76.5%) had children, and of these, 415 (41.3%) first gave birth during postgraduate training and 275 (27.4%) gave birth in years 1-5 as an attending physician.
Of all respondents who had been pregnant, approximately two-thirds (65.7%) had some type of pregnancy complication. About one-third of respondents (31.7%) reported having experienced a miscarriage after a confirmed pregnancy; of those, 61.6% reported one miscarriage, while the remainder had two or more miscarriages (38.4%).
Approximately one-third (31.4%) of respondents reported difficulty with infertility that required fertility counseling and/or treatment.
The questionnaire also asked about assisted reproductive technology, and 164 participants (16.3%) reported the use of fertility medications, and 53 (5.3%) reported cryopreservation of eggs. Nearly 13% reported the use of intrauterine insemination and 13.2% reported the use of in vivo fertilization. Among those who experienced fertility concerns, 36.6% (232 of 634) reported facing financial burdens because of fertility or pregnancy that was in some way associated with their career choice.
When asked on the survey if fertility preservation should be discussed with women during medical school and/or residency, 65.7% of respondents stated that it should.
However, the editorialists suggest that “encouraging formal and directed education regarding the infertility risks specifically toward female physicians (which Lee et al. recommend) could be perceived as a blanket recommendation that it is best for women in medicine to delay childbearing and pursue ART.”
“Medical schools and residency and fellowship training programs should instead focus their energy on creating a framework and culture that normalizes conception during these points in training while also subsidizing and supporting trainees and physicians who prefer to use ART and delay fertility until after training,” they suggest.
The editorialists also emphasized that women may choose to become pregnant at any point during the years that it takes to go from being a medical student to resident/fellow to attending physician, and they should be supported by their workplace on their decisions.
The study was funded by grants from National Institutes of Health/National Cancer Institute Cancer Center.
Dr. Lee and coauthors reported no relevant financial relationships. Dr. Blank reported receiving grants from AstraZeneca, Aravive, Akesobio, GlaxoSmithKline, Merck, and Seattle Genetics outside the submitted work. Dr. Saleh reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Is preeclampsia a cardiovascular time bomb for mothers?
Women who experience preeclampsia during pregnancy are almost twice as likely to have a heart attack or stroke within 20 years of giving birth as pregnant women who did not, according to a new study published in the European Journal of Preventive Cardiology. The risks are especially high in the first decade after giving birth, the researchers found.
Preeclampsia is the onset of high blood pressure after the 20th week of pregnancy combined with signs of organ damage, such as excess protein in the urine. It can occur in up to 8% of pregnancies, and the association between preeclampsia and long-term cardiac risks is well-known. But new research suggests these risks appear much earlier in life than expected – as early as age 30 – at a time when women are often not screened for signs of heart trouble
“Targeted interventions cannot wait until women with preeclampsia become eligible for conventional screening programs in middle age,” Sara Hallum, PhD, a coauthor of the study, told this news organization.
Dr. Hallum, who was an epidemiologist at the University of Copenhagen at the time of the study, and colleagues evaluated the medical histories of more than 1.1 million women in Denmark who became pregnant once or twice between 1978 and 2017. Of this group, 3% had experienced preeclampsia. They compared rates of heart attack and stroke between the two groups over time.
While 1.2% of the entire study population had experienced a heart attack or stroke within 20 years of giving birth, 2% of the women with a history of preeclampsia had such an event. Within the first decade after delivery, women with a history of preeclampsia were four times as likely to have a heart attack and three times as likely to have a stroke as other women.
Women aged 30-39 with a history of preeclampsia were nearly five times as likely to have a heart attack and three times as likely to have a stroke as similar-aged women. And if a woman gave birth twice and had preeclampsia only during the second pregnancy, she was at especially high risk for a heart attack, the researchers found.
“Women with a history of preeclampsia should be monitored routinely for modifiable risk factors, particularly for increased blood pressure,” Dr. Hallum said.
The Danish study population is racially homogeneous, so the researchers were not able to distinguish the effects of preeclampsia by racial group. In the United States, strong evidence shows that Black women experience the effects of preeclampsia more than others.
A useful clue to cardiac risk
Ellen Seely, MD, an endocrinologist at Brigham and Women’s Hospital in Boston, who specializes in preeclampsia, said physicians are less likely to ask women who have been pregnant if they had experienced preeclampsia than to ask if they smoke or have a family history of heart attacks. As a result, they may miss a looming cardiovascular event, especially in younger women who appear healthy.
“Emerging high blood pressure shouldn’t be ignored” in a seemingly healthy young woman, Dr. Seely said, particularly if that woman has divulged a history of preeclampsia. The doctor’s first step should be to verify hypertension, Dr. Seely said. If high blood pressure is evident, immediate treatment – such as encouraging more physical activity and a healthier diet – should follow. Watchful waiting in such cases is inappropriate, she added.
Although the experience of having preeclampsia is unpleasant and scary, Dr. Seely noted that in at least one way it can prove advantageous. Some women who did not experience preeclampsia will end up having a heart attack, sometimes with no prior warning that anything was amiss. At least a history of preeclampsia provides a clue that women should take care of their hearts.
“The patient carries their history with them wherever they go,” Dr. Seely said. For now, this reality often requires women to mention their pregnancy history even if a provider doesn’t ask. Someday, Dr. Seely said, asking about that history will become just as routine for providers as asking about family history.
The study was funded by the Danish Heart Foundation. Dr. Hallum and Dr. Seely have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who experience preeclampsia during pregnancy are almost twice as likely to have a heart attack or stroke within 20 years of giving birth as pregnant women who did not, according to a new study published in the European Journal of Preventive Cardiology. The risks are especially high in the first decade after giving birth, the researchers found.
Preeclampsia is the onset of high blood pressure after the 20th week of pregnancy combined with signs of organ damage, such as excess protein in the urine. It can occur in up to 8% of pregnancies, and the association between preeclampsia and long-term cardiac risks is well-known. But new research suggests these risks appear much earlier in life than expected – as early as age 30 – at a time when women are often not screened for signs of heart trouble
“Targeted interventions cannot wait until women with preeclampsia become eligible for conventional screening programs in middle age,” Sara Hallum, PhD, a coauthor of the study, told this news organization.
Dr. Hallum, who was an epidemiologist at the University of Copenhagen at the time of the study, and colleagues evaluated the medical histories of more than 1.1 million women in Denmark who became pregnant once or twice between 1978 and 2017. Of this group, 3% had experienced preeclampsia. They compared rates of heart attack and stroke between the two groups over time.
While 1.2% of the entire study population had experienced a heart attack or stroke within 20 years of giving birth, 2% of the women with a history of preeclampsia had such an event. Within the first decade after delivery, women with a history of preeclampsia were four times as likely to have a heart attack and three times as likely to have a stroke as other women.
Women aged 30-39 with a history of preeclampsia were nearly five times as likely to have a heart attack and three times as likely to have a stroke as similar-aged women. And if a woman gave birth twice and had preeclampsia only during the second pregnancy, she was at especially high risk for a heart attack, the researchers found.
“Women with a history of preeclampsia should be monitored routinely for modifiable risk factors, particularly for increased blood pressure,” Dr. Hallum said.
The Danish study population is racially homogeneous, so the researchers were not able to distinguish the effects of preeclampsia by racial group. In the United States, strong evidence shows that Black women experience the effects of preeclampsia more than others.
A useful clue to cardiac risk
Ellen Seely, MD, an endocrinologist at Brigham and Women’s Hospital in Boston, who specializes in preeclampsia, said physicians are less likely to ask women who have been pregnant if they had experienced preeclampsia than to ask if they smoke or have a family history of heart attacks. As a result, they may miss a looming cardiovascular event, especially in younger women who appear healthy.
“Emerging high blood pressure shouldn’t be ignored” in a seemingly healthy young woman, Dr. Seely said, particularly if that woman has divulged a history of preeclampsia. The doctor’s first step should be to verify hypertension, Dr. Seely said. If high blood pressure is evident, immediate treatment – such as encouraging more physical activity and a healthier diet – should follow. Watchful waiting in such cases is inappropriate, she added.
Although the experience of having preeclampsia is unpleasant and scary, Dr. Seely noted that in at least one way it can prove advantageous. Some women who did not experience preeclampsia will end up having a heart attack, sometimes with no prior warning that anything was amiss. At least a history of preeclampsia provides a clue that women should take care of their hearts.
“The patient carries their history with them wherever they go,” Dr. Seely said. For now, this reality often requires women to mention their pregnancy history even if a provider doesn’t ask. Someday, Dr. Seely said, asking about that history will become just as routine for providers as asking about family history.
The study was funded by the Danish Heart Foundation. Dr. Hallum and Dr. Seely have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who experience preeclampsia during pregnancy are almost twice as likely to have a heart attack or stroke within 20 years of giving birth as pregnant women who did not, according to a new study published in the European Journal of Preventive Cardiology. The risks are especially high in the first decade after giving birth, the researchers found.
Preeclampsia is the onset of high blood pressure after the 20th week of pregnancy combined with signs of organ damage, such as excess protein in the urine. It can occur in up to 8% of pregnancies, and the association between preeclampsia and long-term cardiac risks is well-known. But new research suggests these risks appear much earlier in life than expected – as early as age 30 – at a time when women are often not screened for signs of heart trouble
“Targeted interventions cannot wait until women with preeclampsia become eligible for conventional screening programs in middle age,” Sara Hallum, PhD, a coauthor of the study, told this news organization.
Dr. Hallum, who was an epidemiologist at the University of Copenhagen at the time of the study, and colleagues evaluated the medical histories of more than 1.1 million women in Denmark who became pregnant once or twice between 1978 and 2017. Of this group, 3% had experienced preeclampsia. They compared rates of heart attack and stroke between the two groups over time.
While 1.2% of the entire study population had experienced a heart attack or stroke within 20 years of giving birth, 2% of the women with a history of preeclampsia had such an event. Within the first decade after delivery, women with a history of preeclampsia were four times as likely to have a heart attack and three times as likely to have a stroke as other women.
Women aged 30-39 with a history of preeclampsia were nearly five times as likely to have a heart attack and three times as likely to have a stroke as similar-aged women. And if a woman gave birth twice and had preeclampsia only during the second pregnancy, she was at especially high risk for a heart attack, the researchers found.
“Women with a history of preeclampsia should be monitored routinely for modifiable risk factors, particularly for increased blood pressure,” Dr. Hallum said.
The Danish study population is racially homogeneous, so the researchers were not able to distinguish the effects of preeclampsia by racial group. In the United States, strong evidence shows that Black women experience the effects of preeclampsia more than others.
A useful clue to cardiac risk
Ellen Seely, MD, an endocrinologist at Brigham and Women’s Hospital in Boston, who specializes in preeclampsia, said physicians are less likely to ask women who have been pregnant if they had experienced preeclampsia than to ask if they smoke or have a family history of heart attacks. As a result, they may miss a looming cardiovascular event, especially in younger women who appear healthy.
“Emerging high blood pressure shouldn’t be ignored” in a seemingly healthy young woman, Dr. Seely said, particularly if that woman has divulged a history of preeclampsia. The doctor’s first step should be to verify hypertension, Dr. Seely said. If high blood pressure is evident, immediate treatment – such as encouraging more physical activity and a healthier diet – should follow. Watchful waiting in such cases is inappropriate, she added.
Although the experience of having preeclampsia is unpleasant and scary, Dr. Seely noted that in at least one way it can prove advantageous. Some women who did not experience preeclampsia will end up having a heart attack, sometimes with no prior warning that anything was amiss. At least a history of preeclampsia provides a clue that women should take care of their hearts.
“The patient carries their history with them wherever they go,” Dr. Seely said. For now, this reality often requires women to mention their pregnancy history even if a provider doesn’t ask. Someday, Dr. Seely said, asking about that history will become just as routine for providers as asking about family history.
The study was funded by the Danish Heart Foundation. Dr. Hallum and Dr. Seely have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
Canadian guidance recommends reducing alcohol consumption
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
Have investigators reached the first steps for redefining a diagnostic definition of preeclampsia that includes morbidity?
Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med. 2022;1. DOI: 10.1056/EVIDoa2200161
EXPERT COMMENTARY
The standard core lecture on preeclampsia given to all medical students frequently begins with an epic, if not potentially apocryphal, statement regarding how this disease has been noted in the annals of medical history since the time of the Ancients. Although contemporary diagnostic criteria for preeclampsia are not that far out of date, they are close. The increased urinary protein loss and hypertension preceding eclamptic seizures was first noted at the end of the 19th century. The blood pressure and proteinuria criteria used for diagnosis was codified in its contemporary form in the late 1940s. Since then, “tweak” rather than “overhaul” probably best describes the updates of the obstetrical community to the definition of preeclampsia. This has just changed.
Details of the study
Thadhani and colleagues prospectively recruited a nationally representative observational cohort of patients hospitalized for hypertension during pregnancy, then followed the patients until either the diagnosis of preeclampsia with severe features or for 2 weeks, whichever came first. At enrollment, circulating levels of the soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) were measured. In a 2-phased design, the first 219 participants were used to define a sFlt-1/PlGF ratio that would predict progression to severe preeclampsia within 2 weeks. The next 556 enrollees served to validate the predictive properties of the ratio. The authors found that a sFlt-1/PlGF ratio of ≥40 predicted progression to preeclampsia with severe features with an area under the curve (AUC) of 0.92.
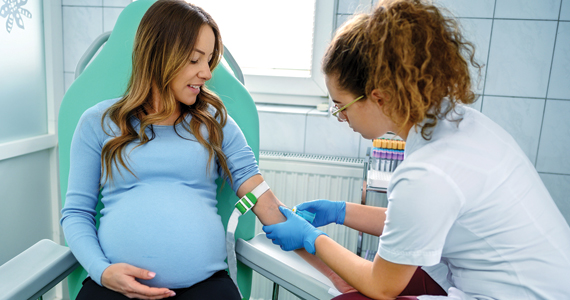
As products of the trophoblasts, both sFlt-1 and PlGF have been mooted for almost 2 decades as potential predictive, if not diagnostic, aids with respect to preeclampsia. Indeed, both analytes are commercially available in Europe for specifically this purpose and many maternal-fetal medicine practitioners working in the European equivalent American tertiary referral centers use an sFlt-1/PlGF ratio as their primary criteria for a diagnosis of preeclampsia. Within the United States, there was an initial flurry of interest in and an infusion of corporate and federal research support for sFlt-1 and PlGF as diagnostic aids for preeclampsia in the mid-2000s. However, at present, the US Food and Drug Administration (FDA) has not sanctioned these (or any) biomarkers to aid in the diagnosis of preeclampsia. As Thermo-Fisher Scientific (Waltham, Massachusetts) is a supporting partner in this study, it is almost certain that these data will be submitted for review by the FDA as part of an application for a preeclampsia diagnostic. At some point in the near future, American practitioners will potentially be able to join their European colleagues in utilizing these biomarkers in the diagnosis of preeclampsia with severe features. ●
Thadhani and colleagues observed that the majority of both maternal and neonatal morbidity in their study, including 8 of the 9 neonatal deaths and both cases of eclampsia, occurred among patients with a ratio ≥40 at admission. There was an inverse relation between the sFlt-1/PlGF ratio and the admission to delivery interval. Where only 17% of patients in the highest quartile of ratios remained pregnant at 14 days post-enrollment, more than 79% of the lowest quartile were still pregnant. If not a causal relationship, sFlt-1 and PlGF are clearly associated with not only the occurrence of preeclampsia with severe features but also the degree of morbidity.
The implication for the disposition of patient care resources is clear. Patients at higher risk for preeclampsia could be seen in specialty high-risk clinics with an emphasis on increased monitoring. In situations where tertiary care is more remote, plans could be developed should they need to be transported to centers able to provide the appropriate level of care. Conversely, patients screening at lower ratios may be more appropriately managed as outpatients, or at least in less clinically involved accommodations.
Thadhani et al do note that there were false negative cases in which the sFlt-1/PlGF ratio at admission was <40 but patients nonetheless progressed to preeclampsia with severe features. The majority of these cases had concurrent pre-pregnancy, chronic hypertension. This observation suggests not only the potential for insights into the pathophysiology of the hypertensive diseases in pregnancy but also that the interpretation of the sFlt/PlGF ratio may eventually need to be stratified by preexisting conditions.
The final implications for the observations of this study are perhaps the most tantalizing. If there is a causal relation between the level of the sFlt-1/PlGF ratio and the morbidity of preeclampsia with severe features, then lowering the circulating concentration of sFlt-1 would ameliorate not only the morbidity but also the risk of preeclampsia. Work with plasma phoresies has suggested that this might be possible, albeit via a clinical intervention demanding more intensive resources. The potential for a targeted pharmacologic moderation of sFlt-1 levels would hold great promise in that those identified as at increased risk could be offered an intervention widely available to all.
Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med. 2022;1. DOI: 10.1056/EVIDoa2200161
EXPERT COMMENTARY
The standard core lecture on preeclampsia given to all medical students frequently begins with an epic, if not potentially apocryphal, statement regarding how this disease has been noted in the annals of medical history since the time of the Ancients. Although contemporary diagnostic criteria for preeclampsia are not that far out of date, they are close. The increased urinary protein loss and hypertension preceding eclamptic seizures was first noted at the end of the 19th century. The blood pressure and proteinuria criteria used for diagnosis was codified in its contemporary form in the late 1940s. Since then, “tweak” rather than “overhaul” probably best describes the updates of the obstetrical community to the definition of preeclampsia. This has just changed.
Details of the study
Thadhani and colleagues prospectively recruited a nationally representative observational cohort of patients hospitalized for hypertension during pregnancy, then followed the patients until either the diagnosis of preeclampsia with severe features or for 2 weeks, whichever came first. At enrollment, circulating levels of the soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) were measured. In a 2-phased design, the first 219 participants were used to define a sFlt-1/PlGF ratio that would predict progression to severe preeclampsia within 2 weeks. The next 556 enrollees served to validate the predictive properties of the ratio. The authors found that a sFlt-1/PlGF ratio of ≥40 predicted progression to preeclampsia with severe features with an area under the curve (AUC) of 0.92.

As products of the trophoblasts, both sFlt-1 and PlGF have been mooted for almost 2 decades as potential predictive, if not diagnostic, aids with respect to preeclampsia. Indeed, both analytes are commercially available in Europe for specifically this purpose and many maternal-fetal medicine practitioners working in the European equivalent American tertiary referral centers use an sFlt-1/PlGF ratio as their primary criteria for a diagnosis of preeclampsia. Within the United States, there was an initial flurry of interest in and an infusion of corporate and federal research support for sFlt-1 and PlGF as diagnostic aids for preeclampsia in the mid-2000s. However, at present, the US Food and Drug Administration (FDA) has not sanctioned these (or any) biomarkers to aid in the diagnosis of preeclampsia. As Thermo-Fisher Scientific (Waltham, Massachusetts) is a supporting partner in this study, it is almost certain that these data will be submitted for review by the FDA as part of an application for a preeclampsia diagnostic. At some point in the near future, American practitioners will potentially be able to join their European colleagues in utilizing these biomarkers in the diagnosis of preeclampsia with severe features. ●
Thadhani and colleagues observed that the majority of both maternal and neonatal morbidity in their study, including 8 of the 9 neonatal deaths and both cases of eclampsia, occurred among patients with a ratio ≥40 at admission. There was an inverse relation between the sFlt-1/PlGF ratio and the admission to delivery interval. Where only 17% of patients in the highest quartile of ratios remained pregnant at 14 days post-enrollment, more than 79% of the lowest quartile were still pregnant. If not a causal relationship, sFlt-1 and PlGF are clearly associated with not only the occurrence of preeclampsia with severe features but also the degree of morbidity.
The implication for the disposition of patient care resources is clear. Patients at higher risk for preeclampsia could be seen in specialty high-risk clinics with an emphasis on increased monitoring. In situations where tertiary care is more remote, plans could be developed should they need to be transported to centers able to provide the appropriate level of care. Conversely, patients screening at lower ratios may be more appropriately managed as outpatients, or at least in less clinically involved accommodations.
Thadhani et al do note that there were false negative cases in which the sFlt-1/PlGF ratio at admission was <40 but patients nonetheless progressed to preeclampsia with severe features. The majority of these cases had concurrent pre-pregnancy, chronic hypertension. This observation suggests not only the potential for insights into the pathophysiology of the hypertensive diseases in pregnancy but also that the interpretation of the sFlt/PlGF ratio may eventually need to be stratified by preexisting conditions.
The final implications for the observations of this study are perhaps the most tantalizing. If there is a causal relation between the level of the sFlt-1/PlGF ratio and the morbidity of preeclampsia with severe features, then lowering the circulating concentration of sFlt-1 would ameliorate not only the morbidity but also the risk of preeclampsia. Work with plasma phoresies has suggested that this might be possible, albeit via a clinical intervention demanding more intensive resources. The potential for a targeted pharmacologic moderation of sFlt-1 levels would hold great promise in that those identified as at increased risk could be offered an intervention widely available to all.
Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med. 2022;1. DOI: 10.1056/EVIDoa2200161
EXPERT COMMENTARY
The standard core lecture on preeclampsia given to all medical students frequently begins with an epic, if not potentially apocryphal, statement regarding how this disease has been noted in the annals of medical history since the time of the Ancients. Although contemporary diagnostic criteria for preeclampsia are not that far out of date, they are close. The increased urinary protein loss and hypertension preceding eclamptic seizures was first noted at the end of the 19th century. The blood pressure and proteinuria criteria used for diagnosis was codified in its contemporary form in the late 1940s. Since then, “tweak” rather than “overhaul” probably best describes the updates of the obstetrical community to the definition of preeclampsia. This has just changed.
Details of the study
Thadhani and colleagues prospectively recruited a nationally representative observational cohort of patients hospitalized for hypertension during pregnancy, then followed the patients until either the diagnosis of preeclampsia with severe features or for 2 weeks, whichever came first. At enrollment, circulating levels of the soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) were measured. In a 2-phased design, the first 219 participants were used to define a sFlt-1/PlGF ratio that would predict progression to severe preeclampsia within 2 weeks. The next 556 enrollees served to validate the predictive properties of the ratio. The authors found that a sFlt-1/PlGF ratio of ≥40 predicted progression to preeclampsia with severe features with an area under the curve (AUC) of 0.92.

As products of the trophoblasts, both sFlt-1 and PlGF have been mooted for almost 2 decades as potential predictive, if not diagnostic, aids with respect to preeclampsia. Indeed, both analytes are commercially available in Europe for specifically this purpose and many maternal-fetal medicine practitioners working in the European equivalent American tertiary referral centers use an sFlt-1/PlGF ratio as their primary criteria for a diagnosis of preeclampsia. Within the United States, there was an initial flurry of interest in and an infusion of corporate and federal research support for sFlt-1 and PlGF as diagnostic aids for preeclampsia in the mid-2000s. However, at present, the US Food and Drug Administration (FDA) has not sanctioned these (or any) biomarkers to aid in the diagnosis of preeclampsia. As Thermo-Fisher Scientific (Waltham, Massachusetts) is a supporting partner in this study, it is almost certain that these data will be submitted for review by the FDA as part of an application for a preeclampsia diagnostic. At some point in the near future, American practitioners will potentially be able to join their European colleagues in utilizing these biomarkers in the diagnosis of preeclampsia with severe features. ●
Thadhani and colleagues observed that the majority of both maternal and neonatal morbidity in their study, including 8 of the 9 neonatal deaths and both cases of eclampsia, occurred among patients with a ratio ≥40 at admission. There was an inverse relation between the sFlt-1/PlGF ratio and the admission to delivery interval. Where only 17% of patients in the highest quartile of ratios remained pregnant at 14 days post-enrollment, more than 79% of the lowest quartile were still pregnant. If not a causal relationship, sFlt-1 and PlGF are clearly associated with not only the occurrence of preeclampsia with severe features but also the degree of morbidity.
The implication for the disposition of patient care resources is clear. Patients at higher risk for preeclampsia could be seen in specialty high-risk clinics with an emphasis on increased monitoring. In situations where tertiary care is more remote, plans could be developed should they need to be transported to centers able to provide the appropriate level of care. Conversely, patients screening at lower ratios may be more appropriately managed as outpatients, or at least in less clinically involved accommodations.
Thadhani et al do note that there were false negative cases in which the sFlt-1/PlGF ratio at admission was <40 but patients nonetheless progressed to preeclampsia with severe features. The majority of these cases had concurrent pre-pregnancy, chronic hypertension. This observation suggests not only the potential for insights into the pathophysiology of the hypertensive diseases in pregnancy but also that the interpretation of the sFlt/PlGF ratio may eventually need to be stratified by preexisting conditions.
The final implications for the observations of this study are perhaps the most tantalizing. If there is a causal relation between the level of the sFlt-1/PlGF ratio and the morbidity of preeclampsia with severe features, then lowering the circulating concentration of sFlt-1 would ameliorate not only the morbidity but also the risk of preeclampsia. Work with plasma phoresies has suggested that this might be possible, albeit via a clinical intervention demanding more intensive resources. The potential for a targeted pharmacologic moderation of sFlt-1 levels would hold great promise in that those identified as at increased risk could be offered an intervention widely available to all.
2023 Update on obstetrics
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country – from small community hospitals to major academic centers – to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women – obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others – are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well?
With that backdrop, it is surprising that the Canadian Task Force on Preventive Health Care has recently recommended against screening with systematic questionnaires, noting that benefits were unclear and not a particular advantage relative to standard practice. The recommendation carries an assumption that standard practice involves queries about mental health. While the task force continues to recommend screening for PPD, their recommendation against screening with a standardized questionnaire represents a bold, sweeping, if not myopic view.
While the Canadian Task Force on Preventive Health Care made their recommendation based on a single randomized controlled trial with the assumption that women were getting mental health counseling, and that women liked getting mental health engagement around their depression, that is not a uniform part of practice. Thus, it is puzzling why the task force would make the recommendation based on such sparse data.
The way to optimize access to care and referral systems for women who are suffering from PPD is not to remove a part of the system that’s already working. Well-validated questionnaires such as the EPDS are easy to administer and are routinely integrated into the electronic health systems records of both small and large centers. These questionnaires are an inexpensive way to increase the likelihood that women get identified and referred for a spectrum of potentially helpful interventions.
PPD is also easy to treat with medications and a wide spectrum of nonpharmacologic interventions. Novel interventions are also being explored to maximize access for women with postpartum mood and anxiety disorders such as peer-delivered behavioral activation and cognitive-behavioral therapy, which could be community based and implemented from urban to rural settings across the United States.
What may need the greatest study is the path to accessing effective treatments and resources for these women and this problem has prompted our group to explore these issues in our more recent investigations. Better understanding of those factors that limit access to mental health providers with expertise in perinatal mental health to the logistical issues of navigating the health care system for sleep-deprived new moms and their families demands greater attention and clearer answers.
The whole field has an obligation to postpartum women to figure out the amalgam of practitioners, resources, and platforms that need to be used to engage women so that they get effective treatment – because we have effective treatments. But the solution to improving perinatal mental health outcomes, unlike the approach of our colleagues in Canada, is not to be found in abandoning questionnaire-based screening, but in identifying the best ways to prevent PPD and to maximize access to care.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country – from small community hospitals to major academic centers – to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women – obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others – are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well?
With that backdrop, it is surprising that the Canadian Task Force on Preventive Health Care has recently recommended against screening with systematic questionnaires, noting that benefits were unclear and not a particular advantage relative to standard practice. The recommendation carries an assumption that standard practice involves queries about mental health. While the task force continues to recommend screening for PPD, their recommendation against screening with a standardized questionnaire represents a bold, sweeping, if not myopic view.
While the Canadian Task Force on Preventive Health Care made their recommendation based on a single randomized controlled trial with the assumption that women were getting mental health counseling, and that women liked getting mental health engagement around their depression, that is not a uniform part of practice. Thus, it is puzzling why the task force would make the recommendation based on such sparse data.
The way to optimize access to care and referral systems for women who are suffering from PPD is not to remove a part of the system that’s already working. Well-validated questionnaires such as the EPDS are easy to administer and are routinely integrated into the electronic health systems records of both small and large centers. These questionnaires are an inexpensive way to increase the likelihood that women get identified and referred for a spectrum of potentially helpful interventions.
PPD is also easy to treat with medications and a wide spectrum of nonpharmacologic interventions. Novel interventions are also being explored to maximize access for women with postpartum mood and anxiety disorders such as peer-delivered behavioral activation and cognitive-behavioral therapy, which could be community based and implemented from urban to rural settings across the United States.
What may need the greatest study is the path to accessing effective treatments and resources for these women and this problem has prompted our group to explore these issues in our more recent investigations. Better understanding of those factors that limit access to mental health providers with expertise in perinatal mental health to the logistical issues of navigating the health care system for sleep-deprived new moms and their families demands greater attention and clearer answers.
The whole field has an obligation to postpartum women to figure out the amalgam of practitioners, resources, and platforms that need to be used to engage women so that they get effective treatment – because we have effective treatments. But the solution to improving perinatal mental health outcomes, unlike the approach of our colleagues in Canada, is not to be found in abandoning questionnaire-based screening, but in identifying the best ways to prevent PPD and to maximize access to care.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country – from small community hospitals to major academic centers – to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women – obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others – are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well?
With that backdrop, it is surprising that the Canadian Task Force on Preventive Health Care has recently recommended against screening with systematic questionnaires, noting that benefits were unclear and not a particular advantage relative to standard practice. The recommendation carries an assumption that standard practice involves queries about mental health. While the task force continues to recommend screening for PPD, their recommendation against screening with a standardized questionnaire represents a bold, sweeping, if not myopic view.
While the Canadian Task Force on Preventive Health Care made their recommendation based on a single randomized controlled trial with the assumption that women were getting mental health counseling, and that women liked getting mental health engagement around their depression, that is not a uniform part of practice. Thus, it is puzzling why the task force would make the recommendation based on such sparse data.
The way to optimize access to care and referral systems for women who are suffering from PPD is not to remove a part of the system that’s already working. Well-validated questionnaires such as the EPDS are easy to administer and are routinely integrated into the electronic health systems records of both small and large centers. These questionnaires are an inexpensive way to increase the likelihood that women get identified and referred for a spectrum of potentially helpful interventions.
PPD is also easy to treat with medications and a wide spectrum of nonpharmacologic interventions. Novel interventions are also being explored to maximize access for women with postpartum mood and anxiety disorders such as peer-delivered behavioral activation and cognitive-behavioral therapy, which could be community based and implemented from urban to rural settings across the United States.
What may need the greatest study is the path to accessing effective treatments and resources for these women and this problem has prompted our group to explore these issues in our more recent investigations. Better understanding of those factors that limit access to mental health providers with expertise in perinatal mental health to the logistical issues of navigating the health care system for sleep-deprived new moms and their families demands greater attention and clearer answers.
The whole field has an obligation to postpartum women to figure out the amalgam of practitioners, resources, and platforms that need to be used to engage women so that they get effective treatment – because we have effective treatments. But the solution to improving perinatal mental health outcomes, unlike the approach of our colleagues in Canada, is not to be found in abandoning questionnaire-based screening, but in identifying the best ways to prevent PPD and to maximize access to care.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
COVID dramatically increases death risk during pregnancy: Study
Women infected with COVID-19 during pregnancy are seven times more likely to die during childbirth or during the pregnancy than uninfected pregnant women, a new study shows. The new report also warns of many other severe complications linked with the virus during pregnancy, as well as risks to the baby after birth.
But the researchers said they did not find that COVID-19 infection during pregnancy impacted the risk of stillbirth or a baby’s growth rate during pregnancy.
The study, which was a meta-analysis of previous research, was published Jan. 16 in the journal BMJ Global Health. Data from 12 studies from 12 countries were combined so researchers could analyze outcomes for 13,136 pregnant women.
Babies born to mothers who were infected with COVID during pregnancy had almost double the risk of needing stays in the neonatal intensive care unit and also were more likely to be born preterm, compared with babies who were born to pregnant women who didn’t get COVID.
The researchers also found that pregnant women who got COVID were more likely to be admitted to intensive care units, need a ventilator to help them survive, develop dangerous blood clots, or develop preeclampsia, which is a high blood pressure disorder that can be fatal for the mother or baby.
One of the strengths of the study was that it included women in different trimesters during pregnancy.
“That’s something new here too is that COVID at any time during pregnancy did bring this extra risk onto mom and babies,” said lead author Emily R. Smith, ScD, MPH, assistant professor of global health at the George Washington University, in a video statement.
The report is prompting calls for improved efforts to convince pregnant women to get vaccinated for COVID-19. The rate among them remains low: About 1 in 5 pregnant women had received the most updated COVID-19 booster as of Jan. 7, according to the CDC.
“The implications here are that it’s really important that if you’re pregnant or if you’re thinking about becoming pregnant, to get vaccinated,” Dr. Smith said. “This can really reduce the risk of having some of these bad outcomes for mom or for baby.”
A version of this article first appeared on WebMD.com.
Women infected with COVID-19 during pregnancy are seven times more likely to die during childbirth or during the pregnancy than uninfected pregnant women, a new study shows. The new report also warns of many other severe complications linked with the virus during pregnancy, as well as risks to the baby after birth.
But the researchers said they did not find that COVID-19 infection during pregnancy impacted the risk of stillbirth or a baby’s growth rate during pregnancy.
The study, which was a meta-analysis of previous research, was published Jan. 16 in the journal BMJ Global Health. Data from 12 studies from 12 countries were combined so researchers could analyze outcomes for 13,136 pregnant women.
Babies born to mothers who were infected with COVID during pregnancy had almost double the risk of needing stays in the neonatal intensive care unit and also were more likely to be born preterm, compared with babies who were born to pregnant women who didn’t get COVID.
The researchers also found that pregnant women who got COVID were more likely to be admitted to intensive care units, need a ventilator to help them survive, develop dangerous blood clots, or develop preeclampsia, which is a high blood pressure disorder that can be fatal for the mother or baby.
One of the strengths of the study was that it included women in different trimesters during pregnancy.
“That’s something new here too is that COVID at any time during pregnancy did bring this extra risk onto mom and babies,” said lead author Emily R. Smith, ScD, MPH, assistant professor of global health at the George Washington University, in a video statement.
The report is prompting calls for improved efforts to convince pregnant women to get vaccinated for COVID-19. The rate among them remains low: About 1 in 5 pregnant women had received the most updated COVID-19 booster as of Jan. 7, according to the CDC.
“The implications here are that it’s really important that if you’re pregnant or if you’re thinking about becoming pregnant, to get vaccinated,” Dr. Smith said. “This can really reduce the risk of having some of these bad outcomes for mom or for baby.”
A version of this article first appeared on WebMD.com.
Women infected with COVID-19 during pregnancy are seven times more likely to die during childbirth or during the pregnancy than uninfected pregnant women, a new study shows. The new report also warns of many other severe complications linked with the virus during pregnancy, as well as risks to the baby after birth.
But the researchers said they did not find that COVID-19 infection during pregnancy impacted the risk of stillbirth or a baby’s growth rate during pregnancy.
The study, which was a meta-analysis of previous research, was published Jan. 16 in the journal BMJ Global Health. Data from 12 studies from 12 countries were combined so researchers could analyze outcomes for 13,136 pregnant women.
Babies born to mothers who were infected with COVID during pregnancy had almost double the risk of needing stays in the neonatal intensive care unit and also were more likely to be born preterm, compared with babies who were born to pregnant women who didn’t get COVID.
The researchers also found that pregnant women who got COVID were more likely to be admitted to intensive care units, need a ventilator to help them survive, develop dangerous blood clots, or develop preeclampsia, which is a high blood pressure disorder that can be fatal for the mother or baby.
One of the strengths of the study was that it included women in different trimesters during pregnancy.
“That’s something new here too is that COVID at any time during pregnancy did bring this extra risk onto mom and babies,” said lead author Emily R. Smith, ScD, MPH, assistant professor of global health at the George Washington University, in a video statement.
The report is prompting calls for improved efforts to convince pregnant women to get vaccinated for COVID-19. The rate among them remains low: About 1 in 5 pregnant women had received the most updated COVID-19 booster as of Jan. 7, according to the CDC.
“The implications here are that it’s really important that if you’re pregnant or if you’re thinking about becoming pregnant, to get vaccinated,” Dr. Smith said. “This can really reduce the risk of having some of these bad outcomes for mom or for baby.”
A version of this article first appeared on WebMD.com.
Post-birth hospitalizations dropped with Medicaid expansion
Women living in states that expanded Medicaid over the past decade were nearly 20% less likely to be hospitalized within 2 months of giving birth, according to a first-of-its-kind study published in Health Affairs.
Researchers analyzed patient records from eight states – four that expanded Medicaid insurance to include a broader swath of residents following the implementation of the Affordable Care Act, and four states that did not.
Hospitalizations in the 60 days after a woman gave birth fell by 17% in states that expanded Medicaid. The analysis also revealed an 8% drop in hospitalizations between 61 days and 6 months post partum.
“This is a very meaningful decline in hospitalization rates,” said Laura Wherry, PhD, a professor of economics and public service at New York University and a co-author of the study.
Women in states that chose not to expand Medicaid experienced a 7% increase in postpartum hospitalizations during that same time frame, the researchers report.
Many states raised income eligibility thresholds to 138% of the federal poverty level in 2014 with the implementation of the Affordable Care Act, which resulted in more coverage for low-income expectant mothers. To date, a dozen states have not implemented Medicaid expansion.
Dr. Wherry and her colleague wanted to take a closer look at outcomes for pregnant women during the postpartum period, both before and after states chose to expand Medicaid.
“A lot of prior work looking at the Medicaid program examined huge expansions to cover pregnant women during pregnancy, but often other periods of a woman’s life have been overlooked,” Dr. Wherry said. “What we were interested in is how that changed with the Affordable Care Act. You no longer needed to be pregnant to qualify.”
The researchers analyzed hospital discharge data between 2010 and 2017 before and after expansion in Iowa, Maryland, New Mexico, and Washington, which expanded coverage under Medicaid, and Florida, Georgia, Mississippi, and Utah, which did not do so.
Prior to 2014, fewer than 2% of births resulted in a postpartum hospitalization during the 60-day period in Medicaid expansion states. But in states that expanded Medicaid, hospitalizations decreased by 0.289 percentage points (P = .052), or 17% during the 60-day post-birth period.
Approximately 75% of the decline was attributed to diagnoses related to complications in pregnancy, childbirth, and the postpartum period.
Dr. Wherry said a variety of factors possibly drove down hospitalizations for new mothers who were able to obtain Medicaid coverage, including access to robust prenatal care, preconception counseling, and improved management of postpartum conditions outside the hospital.
The study provides a strategy for tackling the rising rate of maternal mortality in the United States, an increase largely attributed to postpartum deaths, said Lindsay Admon, MD, an ob.gyn. at the University of Michigan Medical School in Ann Arbor.
“This is one of the first studies showing or suggesting that Medicaid expansion not only led to improvements in Medicaid insurance but health outcomes as well,” said Dr. Admon, who is also researching maternal health and expanded Medicaid coverage.
Federal law has long required states to provide coverage for pregnant women up to 60 days post partum.
The 2021 American Rescue Act allowed states to extend coverage for pregnant women beyond the federal requirement to a year. More than half of states have chosen to do so. Since the study indicates that Medicaid expansion improves outcomes for these enrollees, Dr. Wherry and Dr. Admon said they hope state officials will consider the new findings during discussions to utilize the Rescue Act Coverage for pregnant women.
Dr. Wherry received support for the study from the Robert Wood Johnson Foundation Policies for Action Program and grant funding from the National Institute on Aging and the National Institute of Child Health and Human Development. Another author received grants from the Agency for Healthcare Research and Quality and the National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Women living in states that expanded Medicaid over the past decade were nearly 20% less likely to be hospitalized within 2 months of giving birth, according to a first-of-its-kind study published in Health Affairs.
Researchers analyzed patient records from eight states – four that expanded Medicaid insurance to include a broader swath of residents following the implementation of the Affordable Care Act, and four states that did not.
Hospitalizations in the 60 days after a woman gave birth fell by 17% in states that expanded Medicaid. The analysis also revealed an 8% drop in hospitalizations between 61 days and 6 months post partum.
“This is a very meaningful decline in hospitalization rates,” said Laura Wherry, PhD, a professor of economics and public service at New York University and a co-author of the study.
Women in states that chose not to expand Medicaid experienced a 7% increase in postpartum hospitalizations during that same time frame, the researchers report.
Many states raised income eligibility thresholds to 138% of the federal poverty level in 2014 with the implementation of the Affordable Care Act, which resulted in more coverage for low-income expectant mothers. To date, a dozen states have not implemented Medicaid expansion.
Dr. Wherry and her colleague wanted to take a closer look at outcomes for pregnant women during the postpartum period, both before and after states chose to expand Medicaid.
“A lot of prior work looking at the Medicaid program examined huge expansions to cover pregnant women during pregnancy, but often other periods of a woman’s life have been overlooked,” Dr. Wherry said. “What we were interested in is how that changed with the Affordable Care Act. You no longer needed to be pregnant to qualify.”
The researchers analyzed hospital discharge data between 2010 and 2017 before and after expansion in Iowa, Maryland, New Mexico, and Washington, which expanded coverage under Medicaid, and Florida, Georgia, Mississippi, and Utah, which did not do so.
Prior to 2014, fewer than 2% of births resulted in a postpartum hospitalization during the 60-day period in Medicaid expansion states. But in states that expanded Medicaid, hospitalizations decreased by 0.289 percentage points (P = .052), or 17% during the 60-day post-birth period.
Approximately 75% of the decline was attributed to diagnoses related to complications in pregnancy, childbirth, and the postpartum period.
Dr. Wherry said a variety of factors possibly drove down hospitalizations for new mothers who were able to obtain Medicaid coverage, including access to robust prenatal care, preconception counseling, and improved management of postpartum conditions outside the hospital.
The study provides a strategy for tackling the rising rate of maternal mortality in the United States, an increase largely attributed to postpartum deaths, said Lindsay Admon, MD, an ob.gyn. at the University of Michigan Medical School in Ann Arbor.
“This is one of the first studies showing or suggesting that Medicaid expansion not only led to improvements in Medicaid insurance but health outcomes as well,” said Dr. Admon, who is also researching maternal health and expanded Medicaid coverage.
Federal law has long required states to provide coverage for pregnant women up to 60 days post partum.
The 2021 American Rescue Act allowed states to extend coverage for pregnant women beyond the federal requirement to a year. More than half of states have chosen to do so. Since the study indicates that Medicaid expansion improves outcomes for these enrollees, Dr. Wherry and Dr. Admon said they hope state officials will consider the new findings during discussions to utilize the Rescue Act Coverage for pregnant women.
Dr. Wherry received support for the study from the Robert Wood Johnson Foundation Policies for Action Program and grant funding from the National Institute on Aging and the National Institute of Child Health and Human Development. Another author received grants from the Agency for Healthcare Research and Quality and the National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Women living in states that expanded Medicaid over the past decade were nearly 20% less likely to be hospitalized within 2 months of giving birth, according to a first-of-its-kind study published in Health Affairs.
Researchers analyzed patient records from eight states – four that expanded Medicaid insurance to include a broader swath of residents following the implementation of the Affordable Care Act, and four states that did not.
Hospitalizations in the 60 days after a woman gave birth fell by 17% in states that expanded Medicaid. The analysis also revealed an 8% drop in hospitalizations between 61 days and 6 months post partum.
“This is a very meaningful decline in hospitalization rates,” said Laura Wherry, PhD, a professor of economics and public service at New York University and a co-author of the study.
Women in states that chose not to expand Medicaid experienced a 7% increase in postpartum hospitalizations during that same time frame, the researchers report.
Many states raised income eligibility thresholds to 138% of the federal poverty level in 2014 with the implementation of the Affordable Care Act, which resulted in more coverage for low-income expectant mothers. To date, a dozen states have not implemented Medicaid expansion.
Dr. Wherry and her colleague wanted to take a closer look at outcomes for pregnant women during the postpartum period, both before and after states chose to expand Medicaid.
“A lot of prior work looking at the Medicaid program examined huge expansions to cover pregnant women during pregnancy, but often other periods of a woman’s life have been overlooked,” Dr. Wherry said. “What we were interested in is how that changed with the Affordable Care Act. You no longer needed to be pregnant to qualify.”
The researchers analyzed hospital discharge data between 2010 and 2017 before and after expansion in Iowa, Maryland, New Mexico, and Washington, which expanded coverage under Medicaid, and Florida, Georgia, Mississippi, and Utah, which did not do so.
Prior to 2014, fewer than 2% of births resulted in a postpartum hospitalization during the 60-day period in Medicaid expansion states. But in states that expanded Medicaid, hospitalizations decreased by 0.289 percentage points (P = .052), or 17% during the 60-day post-birth period.
Approximately 75% of the decline was attributed to diagnoses related to complications in pregnancy, childbirth, and the postpartum period.
Dr. Wherry said a variety of factors possibly drove down hospitalizations for new mothers who were able to obtain Medicaid coverage, including access to robust prenatal care, preconception counseling, and improved management of postpartum conditions outside the hospital.
The study provides a strategy for tackling the rising rate of maternal mortality in the United States, an increase largely attributed to postpartum deaths, said Lindsay Admon, MD, an ob.gyn. at the University of Michigan Medical School in Ann Arbor.
“This is one of the first studies showing or suggesting that Medicaid expansion not only led to improvements in Medicaid insurance but health outcomes as well,” said Dr. Admon, who is also researching maternal health and expanded Medicaid coverage.
Federal law has long required states to provide coverage for pregnant women up to 60 days post partum.
The 2021 American Rescue Act allowed states to extend coverage for pregnant women beyond the federal requirement to a year. More than half of states have chosen to do so. Since the study indicates that Medicaid expansion improves outcomes for these enrollees, Dr. Wherry and Dr. Admon said they hope state officials will consider the new findings during discussions to utilize the Rescue Act Coverage for pregnant women.
Dr. Wherry received support for the study from the Robert Wood Johnson Foundation Policies for Action Program and grant funding from the National Institute on Aging and the National Institute of Child Health and Human Development. Another author received grants from the Agency for Healthcare Research and Quality and the National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.