User login
Belimumab for pregnant women with lupus: B-cell concerns remain
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
FROM ANNALS OF THE RHEUMATIC DISEASES
Listeria infection in pregnancy: A potentially serious foodborne illness
CASE Pregnant patient with concerning symptoms of infection
A 28-year-old primigravid woman at 26 weeks’ gestation requests evaluation because of a 3-day history of low-grade fever (38.3 °C), chills, malaise, myalgias, pain in her upper back, nausea, diarrhea, and intermittent uterine contractions. Her symptoms began 2 days after she and her husband dined at a local Mexican restaurant. She specifically recalls eating unpasteurized cheese (queso fresco). Her husband also is experiencing similar symptoms.
- What is the most likely diagnosis?
- What tests should be performed to confirm the diagnosis?
- Does this infection pose a risk to the fetus?
- How should this patient be treated?
Listeriosis, a potentially serious foodborne illness, is an unusual infection in pregnancy. It can cause a number of adverse effects in both the pregnant woman and her fetus, including fetal death in utero. In this article, we review the microbiology and epidemiology of Listeria infection, consider the important steps in diagnosis, and discuss treatment options and prevention measures.
The causative organism in listeriosis
Listeriosis is caused by Listeria monocytogenes, a gram-positive, non–spore-forming bacillus. The organism is catalase positive and oxidase negative, and it exhibits tumbling motility when grown in culture. It can grow at temperatures less than 4 °C, which facilitates foodborne transmission of the bacterium despite adequate refrigeration. Of the 13 serotypes of L monocytogenes, the 1/2a, 1/2b, and 4b are most likely to be associated with human infection. The major virulence factors of L monocytogenes are the internalin surface proteins and the pore-forming listeriolysin O (LLO) cytotoxin. These factors enable the organism to effectively invade host cells.1
The pathogen uses several mechanisms to evade gastrointestinal defenses prior to entry into the bloodstream. It avoids destruction in the stomach by using proton pump inhibitors to elevate the pH of gastric acid. In the duodenum, it survives the antibacterial properties of bile by secreting bile salt hydrolases, which catabolize bile salts. In addition, the cytotoxin listeriolysin S (LLS) disrupts the protective barrier created by the normal gut flora. Once the organism penetrates the gastrointestinal barriers, it disseminates through the blood and lymphatics and then infects other tissues, such as the brain and placenta.1,2
Pathogenesis of infection
The primary reservoir of Listeria is soil and decaying vegetable matter. The organism also has been isolated from animal feed, water, sewage, and many animal species. With rare exceptions, most infections in adults result from inadvertent ingestion of the organism in contaminated food. In certain high-risk occupations, such as veterinary medicine, farming, and laboratory work, infection of the skin or eye can result from direct contact with an infected animal.3
Of note, foodborne illness caused by Listeria has the third highest mortality rate of any foodborne infection, 16% compared with 35% for Vibrio vulnificus and 17% for Clostridium botulinum.2,3 The principal foods that have been linked to listeriosis include:
- soft cheeses, particularly those made from unpasteurized milk
- melon
- hot dogs
- lunch meat, such as bologna
- deli meat, especially chicken
- canned foods, such as smoked seafood, and pâté or meat spreads that are labeled “keep refrigerated”
- unpasteurized milk
- sprouts
- hummus.
In healthy adults, listeriosis is usually a short-lived illness. However, in older adults, immunocompromised patients, and pregnant women, the infection can be devastating. Infection in the pregnant woman also poses major danger to the developing fetus because the organism has a special predilection for placental and fetal tissue.1,3,4
Immunity to Listeria infection depends primarily on T-cell lymphokine activation of macrophages. These latter cells are responsible for clearing the bacterium from the blood. As noted above, the principal virulence factor of L monocytogenes is listeriolysin O, a cholesterol-dependent cytolysin. This substance induces T-cell receptor unresponsiveness, thus interfering with the host immune response to the invading pathogen.1,3-5
Continue to: Clinical manifestations of listeriosis...
Clinical manifestations of listeriosis
Listeria infections may present with various manifestations, depending on the degree of exposure and the underlying immunocompetence of the host (FIGURE). In its most common and simplest form, listeriosis presents as a mild to moderate gastroenteritis following exposure to contaminated food. Symptoms typically develop within 24 hours of exposure and include fever, myalgias, abdominal or back pain, nausea, vomiting, and diarrhea.5
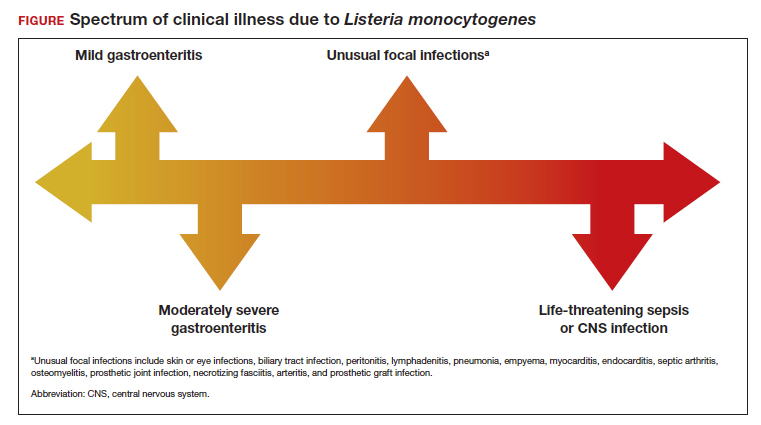
Conversely, in immunocompromised patients, including pregnant women, listeriosis can present as life-threatening sepsis and/or central nervous system (CNS) infection (invasive infection). In this clinical setting, the mean incubation period is 11 days. The manifestations of CNS infection include meningoencephalitis, cerebritis, rhombencephalitis (infection and inflammation of the brain stem), brain abscess, and spinal cord abscess.5
In addition to these 2 clinical presentations, listeriosis can cause unusual focal infections as illustrated in the FIGURE. Some of these infections have unique clinical associations. For example, skin or eye infections may occur as a result of direct inoculation in veterinarians, farmers, and laboratory workers. Listeria peritonitis may occur in patients who are receiving peritoneal dialysis and in those who have cirrhosis. Prosthetic joint and graft infections, of course, may occur in patients who have had invasive procedures for implantation of grafts or prosthetic devices.5
Listeriosis is especially dangerous in pregnancy because it not only can cause serious injury to the mother and even death but it also may pose a major risk to fetal well-being. Possible perinatal complications include fetal death; preterm labor and delivery; and neonatal sepsis, meningitis, and death.5-8
Making the diagnosis
Diagnosis begins with a thorough and focused history to assess for characteristic symptoms and possible Listeria exposure. Exposure should be presumed for patients who report consuming high-risk foods, especially foods recently recalled by the US Food and Drug Administration.
In the asymptomatic pregnant patient, diagnostic testing can be deferred, and the patient should be instructed to return for evaluation if symptoms develop within 2 months of exposure. However, symptomatic, febrile patients require testing. The most valuable testing modality is Gram stain and culture of blood. Gram stain typically will show gram-positive pleomorphic rods with rounded ends. Amniocentesis may be indicated if blood cultures are not definitive. Meconium staining of the amniotic fluid and a positive Gram stain are highly indicative of fetal infection. Cultures of the cerebrospinal fluid are indicated in any individual with focal neurologic findings. Stool cultures are rarely indicated.
When obtaining any of the cultures noted above, the clinician should alert the microbiologist of the concern for listeriosis because L monocytogenes can be confused with common contaminants, such as diphtheroids.5-9
Treatment and follow-up
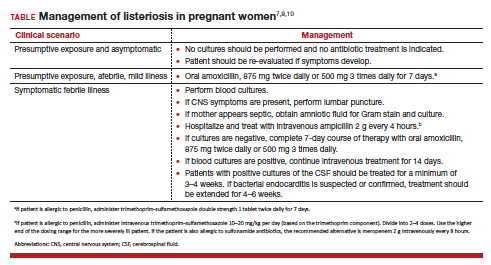
The treatment of listeriosis in pregnancy depends on the severity of the infection and the immune status of the mother. The TABLE offers several different clinical scenarios and the appropriate treatment for each. As noted, several scenarios may require cultures of the blood, cerebrospinal fluid, and amniotic fluid.7,9,10
Following treatment of the mother, serial ultrasound examinations should be performed to monitor fetal growth, CNS anatomy, placental morphology, amniotic fluid volume, and umbilical artery Doppler velocimetry. In the presence of fetal growth restriction, oligohydramnios, or abnormal Doppler velocimetry, biophysical profile testing should be performed. After delivery, the placenta should be examined carefully for histologic evidence of Listeria infection, such as miliary abscesses, and cultured for the bacterium.7-9
Prevention measures
Conservative measures for prevention of Listeria infection in pregnant women include the following7,10-12:
- Refrigerate milk and milk products at 40 °F (4.4 °C).
- Thoroughly cook raw food from animal sources.
- Wash raw vegetables carefully before eating.
- Keep uncooked meats separate from cooked meats and vegetables.
- Do not consume any beverages or foods made from unpasteurized milk.
- After handling uncooked foods, carefully wash all utensils and hands.
- Avoid all soft cheeses, such as Mexican-style feta, Brie, Camembert, and blue cheese, even if they are supposedly made from pasteurized milk.
- Reheat until steaming hot all leftover foods or ready-to-eat foods, such as hot dogs.
- Do not let juice from hot dogs or lunch meat packages drip onto other foods, utensils, or food preparation surfaces.
- Do not store opened hot dog packages in the refrigerator for more than 1 week. Do not store unopened packages for longer than 2 weeks.
- Do not store unopened lunch and deli meat packages in the refrigerator for longer than 2 weeks. Do not store opened packages for longer than 3 to 5 days.
- If other immunosuppressive conditions are present in combination with pregnancy, thoroughly heat cold cuts before eating.
- Do not eat raw or even lightly cooked sprouts of any kind. Cook sprouts thoroughly. Rinsing sprouts will not remove Listeria organisms.
- Do not eat refrigerated pâté or meat spreads from a deli counter or the refrigerated section of a grocery store.
- Canned or shelf-stable pâté and meat spreads are safe to eat, but be sure to refrigerate them after opening the packages.
- Do not eat refrigerated smoked seafood. Canned or shelf-stable seafood, particularly when incorporated into a casserole, is safe to eat.
- Eat cut melon immediately. Refrigerate uneaten melon quickly if not eaten. Discard cut melon that is left at room temperature for more than 4 hours.
CASE Diagnosis made and prompt treatment initiated
The most likely diagnosis in this patient is listeriosis. Because the patient is moderately ill and experiencing uterine contractions, she should be hospitalized and monitored for progressive cervical dilation. Blood cultures should be obtained to identify L monocytogenes. In addition, an amniocentesis should be performed, and the amniotic fluid should be cultured for this microorganism. Stool culture and culture of the cerebrospinal fluid are not indicated. The patient should be treated with intravenous ampicillin, 2 g every 4 hours for 14 days. If she is allergic to penicillin, the alternative drug is trimethoprim-sulfamethoxazole, 8 to 10 mg/kg per day in 2 divided doses, for 14 days. Prompt and effective treatment of the mother should prevent infection in the fetus and newborn. ●
- Listeriosis is primarily a foodborne illness caused by Listeria monocytogenes, a gram-positive bacillus.
- Pregnant women, particularly those who are immunocompromised, are especially susceptible to Listeria infection.
- Foods that pose particular risk of transmitting infection include fresh unpasteurized cheeses, processed meats such as hot dogs, refrigerated pâté and meat spreads, refrigerated smoked seafood, unpasteurized milk, and unwashed raw produce.
- The infection may range from a mild gastroenteritis to life-threatening sepsis and meningitis.
- Listeriosis may cause early and late-onset neonatal infection that presents as either meningitis or sepsis.
- Blood and amniotic fluid cultures are essential to diagnose maternal infection. Stool cultures usually are not indicated.
- Mildly symptomatic but afebrile patients do not require treatment.
- Febrile symptomatic patients should be treated with either intravenous ampicillin or trimethoprim-sulfamethoxazole.
- Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16:32-46. doi:10.1038/nnrmicro.2017.126.
- Johnson JE, Mylonakis E. Listeria monocytogenes. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2020:2543-2549.
- Gelfand MS, Swamy GK, Thompson JL. Epidemiology and pathogenesis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-listeria-monocytogenes-infection?sectionName=CLINICAL%20EPIDEMIOLOGY&topicRef=1277&anchor=H4&source=see_link#H4
- Cherubin CE, Appleman MD, Heseltine PN, et al. Epidemiological spectrum and current treatment of listeriosis. Rev Infect Dis. 1991;13:1108-1114.
- Gelfand MS, Swamy GK, Thompson JL. Clinical manifestations and diagnosis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 7, 2022. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-listeriamonocytogenes-infection
- Boucher M, Yonekura ML. Perinatal listeriosis (early-onset): correlation of antenatal manifestations and neonatal outcome. Obstet Gynecol. 1986;68:593-597.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 614: management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol. 2014;124:1241-1244.
- Rouse DJ, Keimig TW, Riley LE, et al. Case 16-2016. A 31-year-old pregnant woman with fever. N Engl J Med. 2016;374:2076-2083.
- Craig AM, Dotters-Katz S, Kuller JA, et al. Listeriosis in pregnancy: a review. Obstet Gynecol Surv. 2019;74: 362-368.
- Gelfand MS, Thompson JL, Swamy GK. Treatment and prevention of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/treatment-and-prevention-of-listeria-monocytogenes-infection?topicRef=1280&source=see_link
- Voetsch AC, Angulo FJ, Jones TF, et al; Centers for Disease Control and Prevention Emerging Infections Program Foodborne Diseases Active Surveillance Networking Group. Reduction in the incidence of invasive listeriosis in Foodborne Diseases Active Surveillance Network sites, 1996-2003. Clin Infect Dis. 2007;44:513-520.
- MacDonald PDM, Whitwan RE, Boggs JD, et al. Outbreak of listeriosis among Mexican immigrants as a result of consumption of illicitly produced Mexican-style cheese. Clin Infect Dis. 2005;40:677-682.
CASE Pregnant patient with concerning symptoms of infection
A 28-year-old primigravid woman at 26 weeks’ gestation requests evaluation because of a 3-day history of low-grade fever (38.3 °C), chills, malaise, myalgias, pain in her upper back, nausea, diarrhea, and intermittent uterine contractions. Her symptoms began 2 days after she and her husband dined at a local Mexican restaurant. She specifically recalls eating unpasteurized cheese (queso fresco). Her husband also is experiencing similar symptoms.
- What is the most likely diagnosis?
- What tests should be performed to confirm the diagnosis?
- Does this infection pose a risk to the fetus?
- How should this patient be treated?
Listeriosis, a potentially serious foodborne illness, is an unusual infection in pregnancy. It can cause a number of adverse effects in both the pregnant woman and her fetus, including fetal death in utero. In this article, we review the microbiology and epidemiology of Listeria infection, consider the important steps in diagnosis, and discuss treatment options and prevention measures.
The causative organism in listeriosis
Listeriosis is caused by Listeria monocytogenes, a gram-positive, non–spore-forming bacillus. The organism is catalase positive and oxidase negative, and it exhibits tumbling motility when grown in culture. It can grow at temperatures less than 4 °C, which facilitates foodborne transmission of the bacterium despite adequate refrigeration. Of the 13 serotypes of L monocytogenes, the 1/2a, 1/2b, and 4b are most likely to be associated with human infection. The major virulence factors of L monocytogenes are the internalin surface proteins and the pore-forming listeriolysin O (LLO) cytotoxin. These factors enable the organism to effectively invade host cells.1
The pathogen uses several mechanisms to evade gastrointestinal defenses prior to entry into the bloodstream. It avoids destruction in the stomach by using proton pump inhibitors to elevate the pH of gastric acid. In the duodenum, it survives the antibacterial properties of bile by secreting bile salt hydrolases, which catabolize bile salts. In addition, the cytotoxin listeriolysin S (LLS) disrupts the protective barrier created by the normal gut flora. Once the organism penetrates the gastrointestinal barriers, it disseminates through the blood and lymphatics and then infects other tissues, such as the brain and placenta.1,2
Pathogenesis of infection
The primary reservoir of Listeria is soil and decaying vegetable matter. The organism also has been isolated from animal feed, water, sewage, and many animal species. With rare exceptions, most infections in adults result from inadvertent ingestion of the organism in contaminated food. In certain high-risk occupations, such as veterinary medicine, farming, and laboratory work, infection of the skin or eye can result from direct contact with an infected animal.3
Of note, foodborne illness caused by Listeria has the third highest mortality rate of any foodborne infection, 16% compared with 35% for Vibrio vulnificus and 17% for Clostridium botulinum.2,3 The principal foods that have been linked to listeriosis include:
- soft cheeses, particularly those made from unpasteurized milk
- melon
- hot dogs
- lunch meat, such as bologna
- deli meat, especially chicken
- canned foods, such as smoked seafood, and pâté or meat spreads that are labeled “keep refrigerated”
- unpasteurized milk
- sprouts
- hummus.
In healthy adults, listeriosis is usually a short-lived illness. However, in older adults, immunocompromised patients, and pregnant women, the infection can be devastating. Infection in the pregnant woman also poses major danger to the developing fetus because the organism has a special predilection for placental and fetal tissue.1,3,4
Immunity to Listeria infection depends primarily on T-cell lymphokine activation of macrophages. These latter cells are responsible for clearing the bacterium from the blood. As noted above, the principal virulence factor of L monocytogenes is listeriolysin O, a cholesterol-dependent cytolysin. This substance induces T-cell receptor unresponsiveness, thus interfering with the host immune response to the invading pathogen.1,3-5
Continue to: Clinical manifestations of listeriosis...
Clinical manifestations of listeriosis
Listeria infections may present with various manifestations, depending on the degree of exposure and the underlying immunocompetence of the host (FIGURE). In its most common and simplest form, listeriosis presents as a mild to moderate gastroenteritis following exposure to contaminated food. Symptoms typically develop within 24 hours of exposure and include fever, myalgias, abdominal or back pain, nausea, vomiting, and diarrhea.5

Conversely, in immunocompromised patients, including pregnant women, listeriosis can present as life-threatening sepsis and/or central nervous system (CNS) infection (invasive infection). In this clinical setting, the mean incubation period is 11 days. The manifestations of CNS infection include meningoencephalitis, cerebritis, rhombencephalitis (infection and inflammation of the brain stem), brain abscess, and spinal cord abscess.5
In addition to these 2 clinical presentations, listeriosis can cause unusual focal infections as illustrated in the FIGURE. Some of these infections have unique clinical associations. For example, skin or eye infections may occur as a result of direct inoculation in veterinarians, farmers, and laboratory workers. Listeria peritonitis may occur in patients who are receiving peritoneal dialysis and in those who have cirrhosis. Prosthetic joint and graft infections, of course, may occur in patients who have had invasive procedures for implantation of grafts or prosthetic devices.5
Listeriosis is especially dangerous in pregnancy because it not only can cause serious injury to the mother and even death but it also may pose a major risk to fetal well-being. Possible perinatal complications include fetal death; preterm labor and delivery; and neonatal sepsis, meningitis, and death.5-8
Making the diagnosis
Diagnosis begins with a thorough and focused history to assess for characteristic symptoms and possible Listeria exposure. Exposure should be presumed for patients who report consuming high-risk foods, especially foods recently recalled by the US Food and Drug Administration.
In the asymptomatic pregnant patient, diagnostic testing can be deferred, and the patient should be instructed to return for evaluation if symptoms develop within 2 months of exposure. However, symptomatic, febrile patients require testing. The most valuable testing modality is Gram stain and culture of blood. Gram stain typically will show gram-positive pleomorphic rods with rounded ends. Amniocentesis may be indicated if blood cultures are not definitive. Meconium staining of the amniotic fluid and a positive Gram stain are highly indicative of fetal infection. Cultures of the cerebrospinal fluid are indicated in any individual with focal neurologic findings. Stool cultures are rarely indicated.
When obtaining any of the cultures noted above, the clinician should alert the microbiologist of the concern for listeriosis because L monocytogenes can be confused with common contaminants, such as diphtheroids.5-9
Treatment and follow-up
The treatment of listeriosis in pregnancy depends on the severity of the infection and the immune status of the mother. The TABLE offers several different clinical scenarios and the appropriate treatment for each. As noted, several scenarios may require cultures of the blood, cerebrospinal fluid, and amniotic fluid.7,9,10

Following treatment of the mother, serial ultrasound examinations should be performed to monitor fetal growth, CNS anatomy, placental morphology, amniotic fluid volume, and umbilical artery Doppler velocimetry. In the presence of fetal growth restriction, oligohydramnios, or abnormal Doppler velocimetry, biophysical profile testing should be performed. After delivery, the placenta should be examined carefully for histologic evidence of Listeria infection, such as miliary abscesses, and cultured for the bacterium.7-9
Prevention measures
Conservative measures for prevention of Listeria infection in pregnant women include the following7,10-12:
- Refrigerate milk and milk products at 40 °F (4.4 °C).
- Thoroughly cook raw food from animal sources.
- Wash raw vegetables carefully before eating.
- Keep uncooked meats separate from cooked meats and vegetables.
- Do not consume any beverages or foods made from unpasteurized milk.
- After handling uncooked foods, carefully wash all utensils and hands.
- Avoid all soft cheeses, such as Mexican-style feta, Brie, Camembert, and blue cheese, even if they are supposedly made from pasteurized milk.
- Reheat until steaming hot all leftover foods or ready-to-eat foods, such as hot dogs.
- Do not let juice from hot dogs or lunch meat packages drip onto other foods, utensils, or food preparation surfaces.
- Do not store opened hot dog packages in the refrigerator for more than 1 week. Do not store unopened packages for longer than 2 weeks.
- Do not store unopened lunch and deli meat packages in the refrigerator for longer than 2 weeks. Do not store opened packages for longer than 3 to 5 days.
- If other immunosuppressive conditions are present in combination with pregnancy, thoroughly heat cold cuts before eating.
- Do not eat raw or even lightly cooked sprouts of any kind. Cook sprouts thoroughly. Rinsing sprouts will not remove Listeria organisms.
- Do not eat refrigerated pâté or meat spreads from a deli counter or the refrigerated section of a grocery store.
- Canned or shelf-stable pâté and meat spreads are safe to eat, but be sure to refrigerate them after opening the packages.
- Do not eat refrigerated smoked seafood. Canned or shelf-stable seafood, particularly when incorporated into a casserole, is safe to eat.
- Eat cut melon immediately. Refrigerate uneaten melon quickly if not eaten. Discard cut melon that is left at room temperature for more than 4 hours.
CASE Diagnosis made and prompt treatment initiated
The most likely diagnosis in this patient is listeriosis. Because the patient is moderately ill and experiencing uterine contractions, she should be hospitalized and monitored for progressive cervical dilation. Blood cultures should be obtained to identify L monocytogenes. In addition, an amniocentesis should be performed, and the amniotic fluid should be cultured for this microorganism. Stool culture and culture of the cerebrospinal fluid are not indicated. The patient should be treated with intravenous ampicillin, 2 g every 4 hours for 14 days. If she is allergic to penicillin, the alternative drug is trimethoprim-sulfamethoxazole, 8 to 10 mg/kg per day in 2 divided doses, for 14 days. Prompt and effective treatment of the mother should prevent infection in the fetus and newborn. ●
- Listeriosis is primarily a foodborne illness caused by Listeria monocytogenes, a gram-positive bacillus.
- Pregnant women, particularly those who are immunocompromised, are especially susceptible to Listeria infection.
- Foods that pose particular risk of transmitting infection include fresh unpasteurized cheeses, processed meats such as hot dogs, refrigerated pâté and meat spreads, refrigerated smoked seafood, unpasteurized milk, and unwashed raw produce.
- The infection may range from a mild gastroenteritis to life-threatening sepsis and meningitis.
- Listeriosis may cause early and late-onset neonatal infection that presents as either meningitis or sepsis.
- Blood and amniotic fluid cultures are essential to diagnose maternal infection. Stool cultures usually are not indicated.
- Mildly symptomatic but afebrile patients do not require treatment.
- Febrile symptomatic patients should be treated with either intravenous ampicillin or trimethoprim-sulfamethoxazole.
CASE Pregnant patient with concerning symptoms of infection
A 28-year-old primigravid woman at 26 weeks’ gestation requests evaluation because of a 3-day history of low-grade fever (38.3 °C), chills, malaise, myalgias, pain in her upper back, nausea, diarrhea, and intermittent uterine contractions. Her symptoms began 2 days after she and her husband dined at a local Mexican restaurant. She specifically recalls eating unpasteurized cheese (queso fresco). Her husband also is experiencing similar symptoms.
- What is the most likely diagnosis?
- What tests should be performed to confirm the diagnosis?
- Does this infection pose a risk to the fetus?
- How should this patient be treated?
Listeriosis, a potentially serious foodborne illness, is an unusual infection in pregnancy. It can cause a number of adverse effects in both the pregnant woman and her fetus, including fetal death in utero. In this article, we review the microbiology and epidemiology of Listeria infection, consider the important steps in diagnosis, and discuss treatment options and prevention measures.
The causative organism in listeriosis
Listeriosis is caused by Listeria monocytogenes, a gram-positive, non–spore-forming bacillus. The organism is catalase positive and oxidase negative, and it exhibits tumbling motility when grown in culture. It can grow at temperatures less than 4 °C, which facilitates foodborne transmission of the bacterium despite adequate refrigeration. Of the 13 serotypes of L monocytogenes, the 1/2a, 1/2b, and 4b are most likely to be associated with human infection. The major virulence factors of L monocytogenes are the internalin surface proteins and the pore-forming listeriolysin O (LLO) cytotoxin. These factors enable the organism to effectively invade host cells.1
The pathogen uses several mechanisms to evade gastrointestinal defenses prior to entry into the bloodstream. It avoids destruction in the stomach by using proton pump inhibitors to elevate the pH of gastric acid. In the duodenum, it survives the antibacterial properties of bile by secreting bile salt hydrolases, which catabolize bile salts. In addition, the cytotoxin listeriolysin S (LLS) disrupts the protective barrier created by the normal gut flora. Once the organism penetrates the gastrointestinal barriers, it disseminates through the blood and lymphatics and then infects other tissues, such as the brain and placenta.1,2
Pathogenesis of infection
The primary reservoir of Listeria is soil and decaying vegetable matter. The organism also has been isolated from animal feed, water, sewage, and many animal species. With rare exceptions, most infections in adults result from inadvertent ingestion of the organism in contaminated food. In certain high-risk occupations, such as veterinary medicine, farming, and laboratory work, infection of the skin or eye can result from direct contact with an infected animal.3
Of note, foodborne illness caused by Listeria has the third highest mortality rate of any foodborne infection, 16% compared with 35% for Vibrio vulnificus and 17% for Clostridium botulinum.2,3 The principal foods that have been linked to listeriosis include:
- soft cheeses, particularly those made from unpasteurized milk
- melon
- hot dogs
- lunch meat, such as bologna
- deli meat, especially chicken
- canned foods, such as smoked seafood, and pâté or meat spreads that are labeled “keep refrigerated”
- unpasteurized milk
- sprouts
- hummus.
In healthy adults, listeriosis is usually a short-lived illness. However, in older adults, immunocompromised patients, and pregnant women, the infection can be devastating. Infection in the pregnant woman also poses major danger to the developing fetus because the organism has a special predilection for placental and fetal tissue.1,3,4
Immunity to Listeria infection depends primarily on T-cell lymphokine activation of macrophages. These latter cells are responsible for clearing the bacterium from the blood. As noted above, the principal virulence factor of L monocytogenes is listeriolysin O, a cholesterol-dependent cytolysin. This substance induces T-cell receptor unresponsiveness, thus interfering with the host immune response to the invading pathogen.1,3-5
Continue to: Clinical manifestations of listeriosis...
Clinical manifestations of listeriosis
Listeria infections may present with various manifestations, depending on the degree of exposure and the underlying immunocompetence of the host (FIGURE). In its most common and simplest form, listeriosis presents as a mild to moderate gastroenteritis following exposure to contaminated food. Symptoms typically develop within 24 hours of exposure and include fever, myalgias, abdominal or back pain, nausea, vomiting, and diarrhea.5

Conversely, in immunocompromised patients, including pregnant women, listeriosis can present as life-threatening sepsis and/or central nervous system (CNS) infection (invasive infection). In this clinical setting, the mean incubation period is 11 days. The manifestations of CNS infection include meningoencephalitis, cerebritis, rhombencephalitis (infection and inflammation of the brain stem), brain abscess, and spinal cord abscess.5
In addition to these 2 clinical presentations, listeriosis can cause unusual focal infections as illustrated in the FIGURE. Some of these infections have unique clinical associations. For example, skin or eye infections may occur as a result of direct inoculation in veterinarians, farmers, and laboratory workers. Listeria peritonitis may occur in patients who are receiving peritoneal dialysis and in those who have cirrhosis. Prosthetic joint and graft infections, of course, may occur in patients who have had invasive procedures for implantation of grafts or prosthetic devices.5
Listeriosis is especially dangerous in pregnancy because it not only can cause serious injury to the mother and even death but it also may pose a major risk to fetal well-being. Possible perinatal complications include fetal death; preterm labor and delivery; and neonatal sepsis, meningitis, and death.5-8
Making the diagnosis
Diagnosis begins with a thorough and focused history to assess for characteristic symptoms and possible Listeria exposure. Exposure should be presumed for patients who report consuming high-risk foods, especially foods recently recalled by the US Food and Drug Administration.
In the asymptomatic pregnant patient, diagnostic testing can be deferred, and the patient should be instructed to return for evaluation if symptoms develop within 2 months of exposure. However, symptomatic, febrile patients require testing. The most valuable testing modality is Gram stain and culture of blood. Gram stain typically will show gram-positive pleomorphic rods with rounded ends. Amniocentesis may be indicated if blood cultures are not definitive. Meconium staining of the amniotic fluid and a positive Gram stain are highly indicative of fetal infection. Cultures of the cerebrospinal fluid are indicated in any individual with focal neurologic findings. Stool cultures are rarely indicated.
When obtaining any of the cultures noted above, the clinician should alert the microbiologist of the concern for listeriosis because L monocytogenes can be confused with common contaminants, such as diphtheroids.5-9
Treatment and follow-up
The treatment of listeriosis in pregnancy depends on the severity of the infection and the immune status of the mother. The TABLE offers several different clinical scenarios and the appropriate treatment for each. As noted, several scenarios may require cultures of the blood, cerebrospinal fluid, and amniotic fluid.7,9,10

Following treatment of the mother, serial ultrasound examinations should be performed to monitor fetal growth, CNS anatomy, placental morphology, amniotic fluid volume, and umbilical artery Doppler velocimetry. In the presence of fetal growth restriction, oligohydramnios, or abnormal Doppler velocimetry, biophysical profile testing should be performed. After delivery, the placenta should be examined carefully for histologic evidence of Listeria infection, such as miliary abscesses, and cultured for the bacterium.7-9
Prevention measures
Conservative measures for prevention of Listeria infection in pregnant women include the following7,10-12:
- Refrigerate milk and milk products at 40 °F (4.4 °C).
- Thoroughly cook raw food from animal sources.
- Wash raw vegetables carefully before eating.
- Keep uncooked meats separate from cooked meats and vegetables.
- Do not consume any beverages or foods made from unpasteurized milk.
- After handling uncooked foods, carefully wash all utensils and hands.
- Avoid all soft cheeses, such as Mexican-style feta, Brie, Camembert, and blue cheese, even if they are supposedly made from pasteurized milk.
- Reheat until steaming hot all leftover foods or ready-to-eat foods, such as hot dogs.
- Do not let juice from hot dogs or lunch meat packages drip onto other foods, utensils, or food preparation surfaces.
- Do not store opened hot dog packages in the refrigerator for more than 1 week. Do not store unopened packages for longer than 2 weeks.
- Do not store unopened lunch and deli meat packages in the refrigerator for longer than 2 weeks. Do not store opened packages for longer than 3 to 5 days.
- If other immunosuppressive conditions are present in combination with pregnancy, thoroughly heat cold cuts before eating.
- Do not eat raw or even lightly cooked sprouts of any kind. Cook sprouts thoroughly. Rinsing sprouts will not remove Listeria organisms.
- Do not eat refrigerated pâté or meat spreads from a deli counter or the refrigerated section of a grocery store.
- Canned or shelf-stable pâté and meat spreads are safe to eat, but be sure to refrigerate them after opening the packages.
- Do not eat refrigerated smoked seafood. Canned or shelf-stable seafood, particularly when incorporated into a casserole, is safe to eat.
- Eat cut melon immediately. Refrigerate uneaten melon quickly if not eaten. Discard cut melon that is left at room temperature for more than 4 hours.
CASE Diagnosis made and prompt treatment initiated
The most likely diagnosis in this patient is listeriosis. Because the patient is moderately ill and experiencing uterine contractions, she should be hospitalized and monitored for progressive cervical dilation. Blood cultures should be obtained to identify L monocytogenes. In addition, an amniocentesis should be performed, and the amniotic fluid should be cultured for this microorganism. Stool culture and culture of the cerebrospinal fluid are not indicated. The patient should be treated with intravenous ampicillin, 2 g every 4 hours for 14 days. If she is allergic to penicillin, the alternative drug is trimethoprim-sulfamethoxazole, 8 to 10 mg/kg per day in 2 divided doses, for 14 days. Prompt and effective treatment of the mother should prevent infection in the fetus and newborn. ●
- Listeriosis is primarily a foodborne illness caused by Listeria monocytogenes, a gram-positive bacillus.
- Pregnant women, particularly those who are immunocompromised, are especially susceptible to Listeria infection.
- Foods that pose particular risk of transmitting infection include fresh unpasteurized cheeses, processed meats such as hot dogs, refrigerated pâté and meat spreads, refrigerated smoked seafood, unpasteurized milk, and unwashed raw produce.
- The infection may range from a mild gastroenteritis to life-threatening sepsis and meningitis.
- Listeriosis may cause early and late-onset neonatal infection that presents as either meningitis or sepsis.
- Blood and amniotic fluid cultures are essential to diagnose maternal infection. Stool cultures usually are not indicated.
- Mildly symptomatic but afebrile patients do not require treatment.
- Febrile symptomatic patients should be treated with either intravenous ampicillin or trimethoprim-sulfamethoxazole.
- Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16:32-46. doi:10.1038/nnrmicro.2017.126.
- Johnson JE, Mylonakis E. Listeria monocytogenes. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2020:2543-2549.
- Gelfand MS, Swamy GK, Thompson JL. Epidemiology and pathogenesis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-listeria-monocytogenes-infection?sectionName=CLINICAL%20EPIDEMIOLOGY&topicRef=1277&anchor=H4&source=see_link#H4
- Cherubin CE, Appleman MD, Heseltine PN, et al. Epidemiological spectrum and current treatment of listeriosis. Rev Infect Dis. 1991;13:1108-1114.
- Gelfand MS, Swamy GK, Thompson JL. Clinical manifestations and diagnosis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 7, 2022. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-listeriamonocytogenes-infection
- Boucher M, Yonekura ML. Perinatal listeriosis (early-onset): correlation of antenatal manifestations and neonatal outcome. Obstet Gynecol. 1986;68:593-597.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 614: management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol. 2014;124:1241-1244.
- Rouse DJ, Keimig TW, Riley LE, et al. Case 16-2016. A 31-year-old pregnant woman with fever. N Engl J Med. 2016;374:2076-2083.
- Craig AM, Dotters-Katz S, Kuller JA, et al. Listeriosis in pregnancy: a review. Obstet Gynecol Surv. 2019;74: 362-368.
- Gelfand MS, Thompson JL, Swamy GK. Treatment and prevention of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/treatment-and-prevention-of-listeria-monocytogenes-infection?topicRef=1280&source=see_link
- Voetsch AC, Angulo FJ, Jones TF, et al; Centers for Disease Control and Prevention Emerging Infections Program Foodborne Diseases Active Surveillance Networking Group. Reduction in the incidence of invasive listeriosis in Foodborne Diseases Active Surveillance Network sites, 1996-2003. Clin Infect Dis. 2007;44:513-520.
- MacDonald PDM, Whitwan RE, Boggs JD, et al. Outbreak of listeriosis among Mexican immigrants as a result of consumption of illicitly produced Mexican-style cheese. Clin Infect Dis. 2005;40:677-682.
- Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16:32-46. doi:10.1038/nnrmicro.2017.126.
- Johnson JE, Mylonakis E. Listeria monocytogenes. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2020:2543-2549.
- Gelfand MS, Swamy GK, Thompson JL. Epidemiology and pathogenesis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-listeria-monocytogenes-infection?sectionName=CLINICAL%20EPIDEMIOLOGY&topicRef=1277&anchor=H4&source=see_link#H4
- Cherubin CE, Appleman MD, Heseltine PN, et al. Epidemiological spectrum and current treatment of listeriosis. Rev Infect Dis. 1991;13:1108-1114.
- Gelfand MS, Swamy GK, Thompson JL. Clinical manifestations and diagnosis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 7, 2022. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-listeriamonocytogenes-infection
- Boucher M, Yonekura ML. Perinatal listeriosis (early-onset): correlation of antenatal manifestations and neonatal outcome. Obstet Gynecol. 1986;68:593-597.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 614: management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol. 2014;124:1241-1244.
- Rouse DJ, Keimig TW, Riley LE, et al. Case 16-2016. A 31-year-old pregnant woman with fever. N Engl J Med. 2016;374:2076-2083.
- Craig AM, Dotters-Katz S, Kuller JA, et al. Listeriosis in pregnancy: a review. Obstet Gynecol Surv. 2019;74: 362-368.
- Gelfand MS, Thompson JL, Swamy GK. Treatment and prevention of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/treatment-and-prevention-of-listeria-monocytogenes-infection?topicRef=1280&source=see_link
- Voetsch AC, Angulo FJ, Jones TF, et al; Centers for Disease Control and Prevention Emerging Infections Program Foodborne Diseases Active Surveillance Networking Group. Reduction in the incidence of invasive listeriosis in Foodborne Diseases Active Surveillance Network sites, 1996-2003. Clin Infect Dis. 2007;44:513-520.
- MacDonald PDM, Whitwan RE, Boggs JD, et al. Outbreak of listeriosis among Mexican immigrants as a result of consumption of illicitly produced Mexican-style cheese. Clin Infect Dis. 2005;40:677-682.
State quality initiative can reduce postpartum hemorrhage and maternal morbidity
A statewide quality initiative can improve severe maternal morbidity (SMM) and reduce the incidence of maternal morbidity and mortality from postpartum hemorrhage (PPH), a modeling analysis found. Such measures could potentially provide savings to birthing hospitals, according to the California cost-effectiveness study, published in Obstetrics & Gynecology.
A team led by Eric C. Wiesehan, MHA, MBA, a PhD candidate in health policy at Stanford (Calif.) University, examined the effects of the safety initiative of the California Maternal Quality Care Collaborative (CMQCC) in a theoretical cohort of 480,000 births across a mix of hospital settings and sizes. The CMQCC developed a PPH toolkit and quality-improvement protocol to increase recognition, measurement, and timely response to PPH.
Drawing retrospectively on a large 2017 California implementation study, the simulation estimated that collaborative implementation of the CMQCC added 182 quality-adjusted life-years (0.000379 per birth) by averting 913 cases of SMM, 28 emergency hysterectomies, and one maternal mortality. Additionally, it saved $9 million ($17.78 per birth) owing to avoided SMM costs.
According to the Centers for Disease Control and Prevention, pregnancy-related maternal deaths in the United States have increased from 7.2 per 100,000 live births to 16.9 per 100,000 live births over the past 20 years, making it the only country in the Organization for Economic Cooperation and Development with rising rates of maternal mortality. PPH accounts for 11% of maternal deaths.
As to the study’s broader applicability, Dr. Wiesehan said in an interview, “findings of effectiveness in terms of reducing PPH-related SMM are well known outside of California. In terms of costs, however, it is more of an unknown how much is generalizable. It would go a long way if another state quality care collaborative implementing such a project recorded costs prospectively. Prospective costing, particularly microcosting, would be optimal to precisely place where the most, or least, value of this quality improvement project is achieved.”
Studies of PPH safety programs in other U.S. jurisdictions showing reductions in blood transfusions and maternal morbidities suggest the current findings are relevant to a range of hospital settings and regions. “With state perinatal collaboratives already in 47 states, examination of implementation of the PPH-SMM reduction initiative within additional collaboratives would add further robustness to our findings,” the authors wrote.
In 2022, a New York City hospital study reported that learning collaboratives that optimize practice and raise staff awareness could be important tools for improving maternal outcomes.
Still to be answered, said Dr. Wiesehan, are questions about the long-term effectiveness and sustainability of the quality initiative project beyond the early pre/post periods.
The authors indicated no specific funding for the study and had no conflicts of interest to disclose.
A statewide quality initiative can improve severe maternal morbidity (SMM) and reduce the incidence of maternal morbidity and mortality from postpartum hemorrhage (PPH), a modeling analysis found. Such measures could potentially provide savings to birthing hospitals, according to the California cost-effectiveness study, published in Obstetrics & Gynecology.
A team led by Eric C. Wiesehan, MHA, MBA, a PhD candidate in health policy at Stanford (Calif.) University, examined the effects of the safety initiative of the California Maternal Quality Care Collaborative (CMQCC) in a theoretical cohort of 480,000 births across a mix of hospital settings and sizes. The CMQCC developed a PPH toolkit and quality-improvement protocol to increase recognition, measurement, and timely response to PPH.
Drawing retrospectively on a large 2017 California implementation study, the simulation estimated that collaborative implementation of the CMQCC added 182 quality-adjusted life-years (0.000379 per birth) by averting 913 cases of SMM, 28 emergency hysterectomies, and one maternal mortality. Additionally, it saved $9 million ($17.78 per birth) owing to avoided SMM costs.
According to the Centers for Disease Control and Prevention, pregnancy-related maternal deaths in the United States have increased from 7.2 per 100,000 live births to 16.9 per 100,000 live births over the past 20 years, making it the only country in the Organization for Economic Cooperation and Development with rising rates of maternal mortality. PPH accounts for 11% of maternal deaths.
As to the study’s broader applicability, Dr. Wiesehan said in an interview, “findings of effectiveness in terms of reducing PPH-related SMM are well known outside of California. In terms of costs, however, it is more of an unknown how much is generalizable. It would go a long way if another state quality care collaborative implementing such a project recorded costs prospectively. Prospective costing, particularly microcosting, would be optimal to precisely place where the most, or least, value of this quality improvement project is achieved.”
Studies of PPH safety programs in other U.S. jurisdictions showing reductions in blood transfusions and maternal morbidities suggest the current findings are relevant to a range of hospital settings and regions. “With state perinatal collaboratives already in 47 states, examination of implementation of the PPH-SMM reduction initiative within additional collaboratives would add further robustness to our findings,” the authors wrote.
In 2022, a New York City hospital study reported that learning collaboratives that optimize practice and raise staff awareness could be important tools for improving maternal outcomes.
Still to be answered, said Dr. Wiesehan, are questions about the long-term effectiveness and sustainability of the quality initiative project beyond the early pre/post periods.
The authors indicated no specific funding for the study and had no conflicts of interest to disclose.
A statewide quality initiative can improve severe maternal morbidity (SMM) and reduce the incidence of maternal morbidity and mortality from postpartum hemorrhage (PPH), a modeling analysis found. Such measures could potentially provide savings to birthing hospitals, according to the California cost-effectiveness study, published in Obstetrics & Gynecology.
A team led by Eric C. Wiesehan, MHA, MBA, a PhD candidate in health policy at Stanford (Calif.) University, examined the effects of the safety initiative of the California Maternal Quality Care Collaborative (CMQCC) in a theoretical cohort of 480,000 births across a mix of hospital settings and sizes. The CMQCC developed a PPH toolkit and quality-improvement protocol to increase recognition, measurement, and timely response to PPH.
Drawing retrospectively on a large 2017 California implementation study, the simulation estimated that collaborative implementation of the CMQCC added 182 quality-adjusted life-years (0.000379 per birth) by averting 913 cases of SMM, 28 emergency hysterectomies, and one maternal mortality. Additionally, it saved $9 million ($17.78 per birth) owing to avoided SMM costs.
According to the Centers for Disease Control and Prevention, pregnancy-related maternal deaths in the United States have increased from 7.2 per 100,000 live births to 16.9 per 100,000 live births over the past 20 years, making it the only country in the Organization for Economic Cooperation and Development with rising rates of maternal mortality. PPH accounts for 11% of maternal deaths.
As to the study’s broader applicability, Dr. Wiesehan said in an interview, “findings of effectiveness in terms of reducing PPH-related SMM are well known outside of California. In terms of costs, however, it is more of an unknown how much is generalizable. It would go a long way if another state quality care collaborative implementing such a project recorded costs prospectively. Prospective costing, particularly microcosting, would be optimal to precisely place where the most, or least, value of this quality improvement project is achieved.”
Studies of PPH safety programs in other U.S. jurisdictions showing reductions in blood transfusions and maternal morbidities suggest the current findings are relevant to a range of hospital settings and regions. “With state perinatal collaboratives already in 47 states, examination of implementation of the PPH-SMM reduction initiative within additional collaboratives would add further robustness to our findings,” the authors wrote.
In 2022, a New York City hospital study reported that learning collaboratives that optimize practice and raise staff awareness could be important tools for improving maternal outcomes.
Still to be answered, said Dr. Wiesehan, are questions about the long-term effectiveness and sustainability of the quality initiative project beyond the early pre/post periods.
The authors indicated no specific funding for the study and had no conflicts of interest to disclose.
FROM OBSTETRICS & GYNECOLOGY
FDA OKs Tdap shot in pregnancy to protect newborns from pertussis
The Food and Drug Administration has approved another Tdap vaccine option for use during pregnancy to protect newborns from whooping cough.
The agency on Jan. 9 licensed Adacel (Sanofi Pasteur) for immunization during the third trimester to prevent pertussis in infants younger than 2 months old.
The FDA in October approved a different Tdap vaccine, Boostrix (GlaxoSmithKline), for this indication. Boostrix was the first vaccine specifically approved to prevent a disease in newborns whose mothers receive the vaccine while pregnant.
The Centers for Disease Control and Prevention recommend that women receive a dose of Tdap vaccine during each pregnancy, preferably during gestational weeks 27-36 – and ideally toward the earlier end of that window – to help protect babies from whooping cough, the respiratory tract infection caused by Bordetella pertussis.
Providing a Tdap vaccine – tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed – in the third trimester confers passive immunity to the baby, according to the CDC. It also reduces the likelihood that the mother will get pertussis and pass it on to the infant.
One study found that providing Tdap vaccination during gestational weeks 27-36 was 85% more effective at preventing pertussis in infants younger than 2 months old, compared with providing Tdap vaccination to mothers in the hospital postpartum.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” according to a CDC reference page. “Most of these deaths are among infants who are too young to be protected by the childhood pertussis vaccine series that starts when infants are 2 months old.”
The Food and Drug Administration has approved another Tdap vaccine option for use during pregnancy to protect newborns from whooping cough.
The agency on Jan. 9 licensed Adacel (Sanofi Pasteur) for immunization during the third trimester to prevent pertussis in infants younger than 2 months old.
The FDA in October approved a different Tdap vaccine, Boostrix (GlaxoSmithKline), for this indication. Boostrix was the first vaccine specifically approved to prevent a disease in newborns whose mothers receive the vaccine while pregnant.
The Centers for Disease Control and Prevention recommend that women receive a dose of Tdap vaccine during each pregnancy, preferably during gestational weeks 27-36 – and ideally toward the earlier end of that window – to help protect babies from whooping cough, the respiratory tract infection caused by Bordetella pertussis.
Providing a Tdap vaccine – tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed – in the third trimester confers passive immunity to the baby, according to the CDC. It also reduces the likelihood that the mother will get pertussis and pass it on to the infant.
One study found that providing Tdap vaccination during gestational weeks 27-36 was 85% more effective at preventing pertussis in infants younger than 2 months old, compared with providing Tdap vaccination to mothers in the hospital postpartum.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” according to a CDC reference page. “Most of these deaths are among infants who are too young to be protected by the childhood pertussis vaccine series that starts when infants are 2 months old.”
The Food and Drug Administration has approved another Tdap vaccine option for use during pregnancy to protect newborns from whooping cough.
The agency on Jan. 9 licensed Adacel (Sanofi Pasteur) for immunization during the third trimester to prevent pertussis in infants younger than 2 months old.
The FDA in October approved a different Tdap vaccine, Boostrix (GlaxoSmithKline), for this indication. Boostrix was the first vaccine specifically approved to prevent a disease in newborns whose mothers receive the vaccine while pregnant.
The Centers for Disease Control and Prevention recommend that women receive a dose of Tdap vaccine during each pregnancy, preferably during gestational weeks 27-36 – and ideally toward the earlier end of that window – to help protect babies from whooping cough, the respiratory tract infection caused by Bordetella pertussis.
Providing a Tdap vaccine – tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed – in the third trimester confers passive immunity to the baby, according to the CDC. It also reduces the likelihood that the mother will get pertussis and pass it on to the infant.
One study found that providing Tdap vaccination during gestational weeks 27-36 was 85% more effective at preventing pertussis in infants younger than 2 months old, compared with providing Tdap vaccination to mothers in the hospital postpartum.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” according to a CDC reference page. “Most of these deaths are among infants who are too young to be protected by the childhood pertussis vaccine series that starts when infants are 2 months old.”
Racial disparities in cesarean delivery rates
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
Is the limit of viability shifting again?
Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5:e2233331. doi:10.1001/jamanet workopen.2022.33331.
EXPERT COMMENTARY
The single most important intervention available in obstetrics to improve the health outcomes of preterm newborns is the maternal administration of corticosteroids. The 27 randomized controlled trials that formed the basis for this knowledge1 did not include infants delivered at 24 weeks’ gestation or less. This has not dissuaded us, over the last several decades, from using corticosteroids for impending delivery at 24 weeks’ gestation; in the absence of randomized data, this has been based on observational evidence of benefit.
Following the 2011 publication of a retrospective cohort study that analyzed data collected by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network between 1993 and 2009 (the Carlo study),2 ACS started to be used widely even for impending delivery at 23 weeks’ gestation. That study had found that the odds of death and neurodevelopmental impairment at 18 to 22 months of age were significantly lower in cases that received ACS and were born at 23 weeks (n = 1,978). The same benefit could not be verified for infants born at 22 weeks’ gestational age (n = 402).
In a recent study conducted by the same NICHD Neonatal Research Network, antenatal steroid exposure at 21 to 22 weeks of gestation was examined.
Details of the study
Using prospectively collected data from 2016 to 2019, Chawla and colleagues conducted a retrospective cohort study that analyzed data from 431 infants who were born between 22 0/7 and 23 6/7 weeks’ gestation and received neonatal intensive care (179 infants born at 22 weeks’ gestation).3 The infants not exposed to ACS were compared with those who had partial exposure (only 1 dose) and those with complete ACS exposure (2 doses).
Complete ACS exposure proved to be beneficial, increasing survival to discharge from 35.5% in the no-exposure group to 53.9% (adjusted odds ratio [aOR], 1.95; 95% confidence interval [CI], 1.07–3.56). Of the survivors, 26.9% in the complete-exposure group had no major morbidities compared with 10% in the no-exposure group (aOR, 2.74; 95% CI, 1.19–6.30).
Study strengths and limitations
The strengths of this study include the use of a diverse, multicenter cohort, with contemporary delivery data, which increases the generalizability of the findings. The analysis included aspects often overlooked in other similar studies, such as the dose of ACS exposure and the gestational age at the time of exposure.
The observational study design, however, can suggest only associations rather than causal relationships. Observational studies also are apt to be affected by residual confounding. Such limitations can only be overcome by a randomized controlled trial, but such a trial of ACS at periviable gestational ages seems unfeasible due to limited ethical justification.
Another limitation is the reporting on outcomes as a collective group (22–23 weeks’ gestation). It is important to consider each gestational age week separately due to differences in physiology and potential biological limitations. It cannot be assumed that 22 weeks behaves like 23 weeks, just as 21 weeks is not equivalent to 22 weeks.
The study results suggest that the protective effect of ACS was dose dependent. However, the interpretation that only a complete ACS exposure was beneficial should be viewed cautiously because the study had no power to assess the impact of a partial exposure.
A further limitation is the lack of consideration in analysis for maternal comorbidities and fetal growth restriction. In the Carlo study, the beneficial effect of corticosteroids in 23-week gestational age deliveries was not demonstrable in pregnancies affected by fetal growth restriction or maternal hypertension.
Other studies considered
Given all its limitations, can we assume that the study by Chawla and colleagues has reliably refuted the Carlo study’s suggestion of lack of ACS efficacy in infants born at 22 weeks’ gestation? Taken by itself, probably not. In the context of other recent investigations, yes.
A retrospective registry study that used data from the Vermont Oxford Network for the period 2012–2016 on 1,058 infants born at 22 weeks’ gestation found that infants who were exposed to ACS and received postnatal life support were more likely to survive to hospital discharge without major morbidity compared with infants who received postnatal life support alone.4 Overall survival was 38.5% versus 17.7% (adjusted risk ratio [aRR], 2.11; 95% CI, 1.68–2.65), and survival without major morbidity was 4.4% versus 1.0% (aRR, 4.35; 95% CI, 1.84–10.28).
An even larger cohort study that used data from the National Center for Health Statistics concluded that survival at age 1 year for infants born at 22 weeks (n = 2,635) during 2009–2014 was improved in those exposed to ACS followed by postnatal life support compared with postnatal life support alone (45.2% vs 27.8%; aRR, 1.6; 95% CI, 1.2–2.1).5
A meta-analysis of observational studies that reported on infants born between 22 0/7 and 22 6/7 weeks’ gestation (n = 2,226) who received proactive neonatal treatment found that administration of ACS doubled the rate of survival when compared with no ACS administration (39% vs 19.5%; P<.01).6
In September 2021, the recommendations from the American College of Obstetricians and Gynecologists changed, stating that ACS can be considered at 22 weeks’ gestation when active postnatal management is desired.7 This recommendation is largely congruent with those from several other national and international medical organizations, including the World Association of Perinatal Medicine, the Royal Collegeof Obstetricians and Gynaecologists, and the German, Austrian and Swiss societies of gynecology and obstetrics. The implication is that the limit of viability may have shifted again, from 23 to 22 weeks’ gestation, and considering the importance of adequate timing in ACS administration (within 1 week from delivery), Chawla and colleagues posited that ACS administration can be considered as early as 21 weeks’ gestation when birth is anticipated at 22 weeks and active postnatal management is planned (notably, this should be the correct interpretation of the article title, not that ACS may be beneficial in 21-weeks’ gestational age births). ●
In 2001, the Institute of Medicine of the National Academies introduced the concept of shared decision-making as a key component of quality care. In very few other clinical situations is shared decision-making as critical as in the context of planning intervention when delivery is anticipated at 22 weeks’ gestation. The truth remains that even with the coordinated provision of ACS and active postnatal care, survival at this gestational age is still a toss-up, and survivors face a high probability of neurodevelopmental impairment and other long-term adverse health outcomes. In this setting, decision-making is complex, with the need to balance patient autonomy and nonmaleficence. On the other hand, the concept of patient autonomy is blurred because the patient (fetus) is incompetent and the negotiation is conducted between physicians and parents. However, no intervention should be undertaken unless the parents so desire. Since parental wishes are frequently emotional, overwhelmingly driving intervention, thorough and timely interdisciplinary counseling is needed. Evidence indicates that both obstetricians and neonatologists may, at times, underestimate the chance of a favorable health outcome for infants born extremely preterm.8,9 Early involvement of the neonatal and obstetric team is pivotal to put forward a coherent, nonconfusing, nonpaternalistic, balanced message. When outcomes information is shared during prenatal counseling, it should be based on local, not only national, data. Following appropriate consultation with the parents, the physicians will adjust the expectations to the local standards, outcomes data, and availability regarding periviable neonatal support.
Recent data suggest that the rate of cesarean delivery (CD) in the periviable period is increasing.10 There is no clear evidence in favor of CD to improve neonatal outcomes, whereas there is concern that periviable CD is associated with significantly increased maternal risks. Regardless of uterine incision type, periviable CD results in an increased risk of uterine rupture in a subsequent pregnancy.11 Consistent with the principle of nonmaleficence, a discussion of these risks should be included in shared decision-making.
ALEX C. VIDAEFF, MD, MPH, AND NATHAN C. SUNDGREN, MD, PHD
- McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454.
- Carlo WA, McDonald SA, Fanaroff AA, et al; Eunice Kennedy Schriver National Institute for Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22-25 weeks gestation. JAMA. 2011;306:2348-2358.
- Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5(9):e2233331. doi:10.1001/ jamanetworkopen.2022.33331.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi:10.1001/ jamanetworkopen.2018.3235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks. Am J Perinatol. November 28, 2021. doi:10.1055/s-0041-1740062.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Cahill AG, Kaimal AJ, Kuller JA, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Practice advisory: Use of antenatal corticosteroids at 22 weeks of gestation. Accessed December 7, 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation#
- Boland RA, Davis PG, Dawson JA, et al. What are we telling the parents of extremely preterm babies? Aust N Z J Obstet Gynaecol. 2016;56:274-281.
- Blanco F, Suresh G, Howard D, et al. Ensuring accurate knowledge of prematurity outcomes for prenatal counseling. Pediatrics. 2005;115:e478-e487.
- Rossi RM, Hall E, DeFranco EA. Contemporary trends in cesarean delivery utilization for live births between 22 0/7 and 23 6/7 weeks of gestation. Obstet Gynecol. 2019;133:451-458.
- Lannon SMR, Guthrie KA, Vanderhoeven JP, et al. Uterine rupture risk after periviable cesarean delivery. Obstet Gynecol. 2015;125:1095-1100.
Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5:e2233331. doi:10.1001/jamanet workopen.2022.33331.
EXPERT COMMENTARY
The single most important intervention available in obstetrics to improve the health outcomes of preterm newborns is the maternal administration of corticosteroids. The 27 randomized controlled trials that formed the basis for this knowledge1 did not include infants delivered at 24 weeks’ gestation or less. This has not dissuaded us, over the last several decades, from using corticosteroids for impending delivery at 24 weeks’ gestation; in the absence of randomized data, this has been based on observational evidence of benefit.
Following the 2011 publication of a retrospective cohort study that analyzed data collected by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network between 1993 and 2009 (the Carlo study),2 ACS started to be used widely even for impending delivery at 23 weeks’ gestation. That study had found that the odds of death and neurodevelopmental impairment at 18 to 22 months of age were significantly lower in cases that received ACS and were born at 23 weeks (n = 1,978). The same benefit could not be verified for infants born at 22 weeks’ gestational age (n = 402).
In a recent study conducted by the same NICHD Neonatal Research Network, antenatal steroid exposure at 21 to 22 weeks of gestation was examined.

Details of the study
Using prospectively collected data from 2016 to 2019, Chawla and colleagues conducted a retrospective cohort study that analyzed data from 431 infants who were born between 22 0/7 and 23 6/7 weeks’ gestation and received neonatal intensive care (179 infants born at 22 weeks’ gestation).3 The infants not exposed to ACS were compared with those who had partial exposure (only 1 dose) and those with complete ACS exposure (2 doses).
Complete ACS exposure proved to be beneficial, increasing survival to discharge from 35.5% in the no-exposure group to 53.9% (adjusted odds ratio [aOR], 1.95; 95% confidence interval [CI], 1.07–3.56). Of the survivors, 26.9% in the complete-exposure group had no major morbidities compared with 10% in the no-exposure group (aOR, 2.74; 95% CI, 1.19–6.30).
Study strengths and limitations
The strengths of this study include the use of a diverse, multicenter cohort, with contemporary delivery data, which increases the generalizability of the findings. The analysis included aspects often overlooked in other similar studies, such as the dose of ACS exposure and the gestational age at the time of exposure.
The observational study design, however, can suggest only associations rather than causal relationships. Observational studies also are apt to be affected by residual confounding. Such limitations can only be overcome by a randomized controlled trial, but such a trial of ACS at periviable gestational ages seems unfeasible due to limited ethical justification.
Another limitation is the reporting on outcomes as a collective group (22–23 weeks’ gestation). It is important to consider each gestational age week separately due to differences in physiology and potential biological limitations. It cannot be assumed that 22 weeks behaves like 23 weeks, just as 21 weeks is not equivalent to 22 weeks.
The study results suggest that the protective effect of ACS was dose dependent. However, the interpretation that only a complete ACS exposure was beneficial should be viewed cautiously because the study had no power to assess the impact of a partial exposure.
A further limitation is the lack of consideration in analysis for maternal comorbidities and fetal growth restriction. In the Carlo study, the beneficial effect of corticosteroids in 23-week gestational age deliveries was not demonstrable in pregnancies affected by fetal growth restriction or maternal hypertension.
Other studies considered
Given all its limitations, can we assume that the study by Chawla and colleagues has reliably refuted the Carlo study’s suggestion of lack of ACS efficacy in infants born at 22 weeks’ gestation? Taken by itself, probably not. In the context of other recent investigations, yes.
A retrospective registry study that used data from the Vermont Oxford Network for the period 2012–2016 on 1,058 infants born at 22 weeks’ gestation found that infants who were exposed to ACS and received postnatal life support were more likely to survive to hospital discharge without major morbidity compared with infants who received postnatal life support alone.4 Overall survival was 38.5% versus 17.7% (adjusted risk ratio [aRR], 2.11; 95% CI, 1.68–2.65), and survival without major morbidity was 4.4% versus 1.0% (aRR, 4.35; 95% CI, 1.84–10.28).
An even larger cohort study that used data from the National Center for Health Statistics concluded that survival at age 1 year for infants born at 22 weeks (n = 2,635) during 2009–2014 was improved in those exposed to ACS followed by postnatal life support compared with postnatal life support alone (45.2% vs 27.8%; aRR, 1.6; 95% CI, 1.2–2.1).5
A meta-analysis of observational studies that reported on infants born between 22 0/7 and 22 6/7 weeks’ gestation (n = 2,226) who received proactive neonatal treatment found that administration of ACS doubled the rate of survival when compared with no ACS administration (39% vs 19.5%; P<.01).6
In September 2021, the recommendations from the American College of Obstetricians and Gynecologists changed, stating that ACS can be considered at 22 weeks’ gestation when active postnatal management is desired.7 This recommendation is largely congruent with those from several other national and international medical organizations, including the World Association of Perinatal Medicine, the Royal Collegeof Obstetricians and Gynaecologists, and the German, Austrian and Swiss societies of gynecology and obstetrics. The implication is that the limit of viability may have shifted again, from 23 to 22 weeks’ gestation, and considering the importance of adequate timing in ACS administration (within 1 week from delivery), Chawla and colleagues posited that ACS administration can be considered as early as 21 weeks’ gestation when birth is anticipated at 22 weeks and active postnatal management is planned (notably, this should be the correct interpretation of the article title, not that ACS may be beneficial in 21-weeks’ gestational age births). ●
In 2001, the Institute of Medicine of the National Academies introduced the concept of shared decision-making as a key component of quality care. In very few other clinical situations is shared decision-making as critical as in the context of planning intervention when delivery is anticipated at 22 weeks’ gestation. The truth remains that even with the coordinated provision of ACS and active postnatal care, survival at this gestational age is still a toss-up, and survivors face a high probability of neurodevelopmental impairment and other long-term adverse health outcomes. In this setting, decision-making is complex, with the need to balance patient autonomy and nonmaleficence. On the other hand, the concept of patient autonomy is blurred because the patient (fetus) is incompetent and the negotiation is conducted between physicians and parents. However, no intervention should be undertaken unless the parents so desire. Since parental wishes are frequently emotional, overwhelmingly driving intervention, thorough and timely interdisciplinary counseling is needed. Evidence indicates that both obstetricians and neonatologists may, at times, underestimate the chance of a favorable health outcome for infants born extremely preterm.8,9 Early involvement of the neonatal and obstetric team is pivotal to put forward a coherent, nonconfusing, nonpaternalistic, balanced message. When outcomes information is shared during prenatal counseling, it should be based on local, not only national, data. Following appropriate consultation with the parents, the physicians will adjust the expectations to the local standards, outcomes data, and availability regarding periviable neonatal support.
Recent data suggest that the rate of cesarean delivery (CD) in the periviable period is increasing.10 There is no clear evidence in favor of CD to improve neonatal outcomes, whereas there is concern that periviable CD is associated with significantly increased maternal risks. Regardless of uterine incision type, periviable CD results in an increased risk of uterine rupture in a subsequent pregnancy.11 Consistent with the principle of nonmaleficence, a discussion of these risks should be included in shared decision-making.
ALEX C. VIDAEFF, MD, MPH, AND NATHAN C. SUNDGREN, MD, PHD
Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5:e2233331. doi:10.1001/jamanet workopen.2022.33331.
EXPERT COMMENTARY
The single most important intervention available in obstetrics to improve the health outcomes of preterm newborns is the maternal administration of corticosteroids. The 27 randomized controlled trials that formed the basis for this knowledge1 did not include infants delivered at 24 weeks’ gestation or less. This has not dissuaded us, over the last several decades, from using corticosteroids for impending delivery at 24 weeks’ gestation; in the absence of randomized data, this has been based on observational evidence of benefit.
Following the 2011 publication of a retrospective cohort study that analyzed data collected by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network between 1993 and 2009 (the Carlo study),2 ACS started to be used widely even for impending delivery at 23 weeks’ gestation. That study had found that the odds of death and neurodevelopmental impairment at 18 to 22 months of age were significantly lower in cases that received ACS and were born at 23 weeks (n = 1,978). The same benefit could not be verified for infants born at 22 weeks’ gestational age (n = 402).
In a recent study conducted by the same NICHD Neonatal Research Network, antenatal steroid exposure at 21 to 22 weeks of gestation was examined.

Details of the study
Using prospectively collected data from 2016 to 2019, Chawla and colleagues conducted a retrospective cohort study that analyzed data from 431 infants who were born between 22 0/7 and 23 6/7 weeks’ gestation and received neonatal intensive care (179 infants born at 22 weeks’ gestation).3 The infants not exposed to ACS were compared with those who had partial exposure (only 1 dose) and those with complete ACS exposure (2 doses).
Complete ACS exposure proved to be beneficial, increasing survival to discharge from 35.5% in the no-exposure group to 53.9% (adjusted odds ratio [aOR], 1.95; 95% confidence interval [CI], 1.07–3.56). Of the survivors, 26.9% in the complete-exposure group had no major morbidities compared with 10% in the no-exposure group (aOR, 2.74; 95% CI, 1.19–6.30).
Study strengths and limitations
The strengths of this study include the use of a diverse, multicenter cohort, with contemporary delivery data, which increases the generalizability of the findings. The analysis included aspects often overlooked in other similar studies, such as the dose of ACS exposure and the gestational age at the time of exposure.
The observational study design, however, can suggest only associations rather than causal relationships. Observational studies also are apt to be affected by residual confounding. Such limitations can only be overcome by a randomized controlled trial, but such a trial of ACS at periviable gestational ages seems unfeasible due to limited ethical justification.
Another limitation is the reporting on outcomes as a collective group (22–23 weeks’ gestation). It is important to consider each gestational age week separately due to differences in physiology and potential biological limitations. It cannot be assumed that 22 weeks behaves like 23 weeks, just as 21 weeks is not equivalent to 22 weeks.
The study results suggest that the protective effect of ACS was dose dependent. However, the interpretation that only a complete ACS exposure was beneficial should be viewed cautiously because the study had no power to assess the impact of a partial exposure.
A further limitation is the lack of consideration in analysis for maternal comorbidities and fetal growth restriction. In the Carlo study, the beneficial effect of corticosteroids in 23-week gestational age deliveries was not demonstrable in pregnancies affected by fetal growth restriction or maternal hypertension.
Other studies considered
Given all its limitations, can we assume that the study by Chawla and colleagues has reliably refuted the Carlo study’s suggestion of lack of ACS efficacy in infants born at 22 weeks’ gestation? Taken by itself, probably not. In the context of other recent investigations, yes.
A retrospective registry study that used data from the Vermont Oxford Network for the period 2012–2016 on 1,058 infants born at 22 weeks’ gestation found that infants who were exposed to ACS and received postnatal life support were more likely to survive to hospital discharge without major morbidity compared with infants who received postnatal life support alone.4 Overall survival was 38.5% versus 17.7% (adjusted risk ratio [aRR], 2.11; 95% CI, 1.68–2.65), and survival without major morbidity was 4.4% versus 1.0% (aRR, 4.35; 95% CI, 1.84–10.28).
An even larger cohort study that used data from the National Center for Health Statistics concluded that survival at age 1 year for infants born at 22 weeks (n = 2,635) during 2009–2014 was improved in those exposed to ACS followed by postnatal life support compared with postnatal life support alone (45.2% vs 27.8%; aRR, 1.6; 95% CI, 1.2–2.1).5
A meta-analysis of observational studies that reported on infants born between 22 0/7 and 22 6/7 weeks’ gestation (n = 2,226) who received proactive neonatal treatment found that administration of ACS doubled the rate of survival when compared with no ACS administration (39% vs 19.5%; P<.01).6
In September 2021, the recommendations from the American College of Obstetricians and Gynecologists changed, stating that ACS can be considered at 22 weeks’ gestation when active postnatal management is desired.7 This recommendation is largely congruent with those from several other national and international medical organizations, including the World Association of Perinatal Medicine, the Royal Collegeof Obstetricians and Gynaecologists, and the German, Austrian and Swiss societies of gynecology and obstetrics. The implication is that the limit of viability may have shifted again, from 23 to 22 weeks’ gestation, and considering the importance of adequate timing in ACS administration (within 1 week from delivery), Chawla and colleagues posited that ACS administration can be considered as early as 21 weeks’ gestation when birth is anticipated at 22 weeks and active postnatal management is planned (notably, this should be the correct interpretation of the article title, not that ACS may be beneficial in 21-weeks’ gestational age births). ●
In 2001, the Institute of Medicine of the National Academies introduced the concept of shared decision-making as a key component of quality care. In very few other clinical situations is shared decision-making as critical as in the context of planning intervention when delivery is anticipated at 22 weeks’ gestation. The truth remains that even with the coordinated provision of ACS and active postnatal care, survival at this gestational age is still a toss-up, and survivors face a high probability of neurodevelopmental impairment and other long-term adverse health outcomes. In this setting, decision-making is complex, with the need to balance patient autonomy and nonmaleficence. On the other hand, the concept of patient autonomy is blurred because the patient (fetus) is incompetent and the negotiation is conducted between physicians and parents. However, no intervention should be undertaken unless the parents so desire. Since parental wishes are frequently emotional, overwhelmingly driving intervention, thorough and timely interdisciplinary counseling is needed. Evidence indicates that both obstetricians and neonatologists may, at times, underestimate the chance of a favorable health outcome for infants born extremely preterm.8,9 Early involvement of the neonatal and obstetric team is pivotal to put forward a coherent, nonconfusing, nonpaternalistic, balanced message. When outcomes information is shared during prenatal counseling, it should be based on local, not only national, data. Following appropriate consultation with the parents, the physicians will adjust the expectations to the local standards, outcomes data, and availability regarding periviable neonatal support.
Recent data suggest that the rate of cesarean delivery (CD) in the periviable period is increasing.10 There is no clear evidence in favor of CD to improve neonatal outcomes, whereas there is concern that periviable CD is associated with significantly increased maternal risks. Regardless of uterine incision type, periviable CD results in an increased risk of uterine rupture in a subsequent pregnancy.11 Consistent with the principle of nonmaleficence, a discussion of these risks should be included in shared decision-making.
ALEX C. VIDAEFF, MD, MPH, AND NATHAN C. SUNDGREN, MD, PHD
- McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454.
- Carlo WA, McDonald SA, Fanaroff AA, et al; Eunice Kennedy Schriver National Institute for Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22-25 weeks gestation. JAMA. 2011;306:2348-2358.
- Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5(9):e2233331. doi:10.1001/ jamanetworkopen.2022.33331.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi:10.1001/ jamanetworkopen.2018.3235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks. Am J Perinatol. November 28, 2021. doi:10.1055/s-0041-1740062.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Cahill AG, Kaimal AJ, Kuller JA, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Practice advisory: Use of antenatal corticosteroids at 22 weeks of gestation. Accessed December 7, 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation#
- Boland RA, Davis PG, Dawson JA, et al. What are we telling the parents of extremely preterm babies? Aust N Z J Obstet Gynaecol. 2016;56:274-281.
- Blanco F, Suresh G, Howard D, et al. Ensuring accurate knowledge of prematurity outcomes for prenatal counseling. Pediatrics. 2005;115:e478-e487.
- Rossi RM, Hall E, DeFranco EA. Contemporary trends in cesarean delivery utilization for live births between 22 0/7 and 23 6/7 weeks of gestation. Obstet Gynecol. 2019;133:451-458.
- Lannon SMR, Guthrie KA, Vanderhoeven JP, et al. Uterine rupture risk after periviable cesarean delivery. Obstet Gynecol. 2015;125:1095-1100.
- McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454.
- Carlo WA, McDonald SA, Fanaroff AA, et al; Eunice Kennedy Schriver National Institute for Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22-25 weeks gestation. JAMA. 2011;306:2348-2358.
- Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5(9):e2233331. doi:10.1001/ jamanetworkopen.2022.33331.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi:10.1001/ jamanetworkopen.2018.3235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks. Am J Perinatol. November 28, 2021. doi:10.1055/s-0041-1740062.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Cahill AG, Kaimal AJ, Kuller JA, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Practice advisory: Use of antenatal corticosteroids at 22 weeks of gestation. Accessed December 7, 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation#
- Boland RA, Davis PG, Dawson JA, et al. What are we telling the parents of extremely preterm babies? Aust N Z J Obstet Gynaecol. 2016;56:274-281.
- Blanco F, Suresh G, Howard D, et al. Ensuring accurate knowledge of prematurity outcomes for prenatal counseling. Pediatrics. 2005;115:e478-e487.
- Rossi RM, Hall E, DeFranco EA. Contemporary trends in cesarean delivery utilization for live births between 22 0/7 and 23 6/7 weeks of gestation. Obstet Gynecol. 2019;133:451-458.
- Lannon SMR, Guthrie KA, Vanderhoeven JP, et al. Uterine rupture risk after periviable cesarean delivery. Obstet Gynecol. 2015;125:1095-1100.
Abortion laws link to state-level mortality
States that have moderate state abortion laws had lower rates of maternal, fetal, and infant mortality, but not lower all-cause mortality, in reproductive-aged females compared with states that have restrictive laws, new research using 2000-2019 data indicates.
Additionally, having a higher number of laws that restrict abortion in a state was linked with higher maternal and infant mortality in the state, the study states.
Mortality increases with number of laws
“Each additional abortion regulation was associated with an increase in maternal mortality (1.09/100,000 live births; 95% confidence interval, 0.36-1.82) and infant mortality (0.20/1,000 live births; 95% CI, 0.12-0.26),” the authors write.
Lorie M. Harper, MD, MSCI, with the department of women’s health, University of Texas at Austin, led a team that simultaneously studied four categories of mortality: all-cause mortality in females ages 15-49 years; maternal mortality; infant mortality; and fetal mortality.
Her team did a retrospective cohort study using the Centers for Disease Control and Prevention’s WONDER (Wide-ranging ONline Data for Epidemiologic Research) database. WONDER collects publicly available data with information on mortality by state.
Data were available from 2000 to 2019 for all-cause, maternal, and infant mortality. Fetal mortality data were available from 2005 to 2019.
Findings were published ahead of print in February’s Obstetrics & Gynecology.
Though the study was done before the Supreme Court decision overturning Roe v. Wade in June 2022, its findings may shed light on potential trends depending on states’ decisions.
In the wake of the Dobbs v. Jackson Women’s Health Organization, an estimated 33 million U.S. women will live in states without available abortion services, the authors note.
State abortion laws changed dramatically in the study period
The authors chose the 2-decade time frame because data could be accessed for both maternal and infant mortality for all the years and because state level abortion laws changed dramatically in that time.
The Guttmacher Institute has analyzed each state’s abortion policy landscape and scored states as restrictive, moderate, or supportive. Certain types of abortion laws were associated with higher rates of all-cause mortality in reproductive-age females.
Those laws included trigger laws (the laws that automatically outlaw abortion in a state when federal law is overturned), laws that limit access to medication abortion, and parental consent laws, according to the paper.
Additionally, states that restrict access to abortion have higher rates of maternal and infant death, even after accounting for the general health of the population.
Trigger-law states have strong mortality association
Blair G. Darney, PhD, MPH, with the department of obstetrics and gynecology, Oregon Health & Science University and the OHSU-PSU School of Public Health in Portland, and two OHSU coauthors note in an accompanying editorial that having a trigger law in place was associated with elevations in all four mortality measures. However, trigger laws had not yet been enacted during the study.
The existence of trigger laws is likely a proxy for other factors, they write.
“[T]he relationship between trigger laws and increased mortality may more likely be explained by other factors that were not considered in the analysis than by laws that were not enacted at the time of the study,” the editorialists write.
Those factors may include lack of Medicaid expansion, socioeconomic inequities, and other policies.
The editorialists also caution that just because some types of abortion restrictions don’t appear to increase mortality, it doesn’t mean they aren’t harmful.
“For example, laws prohibiting private or Medicaid insurance coverage for abortions were not associated with maternal mortality, but these laws may simply shift the burden of payment to the individual seeking an abortion or be mitigated by funds that support an individual’s out-of-pocket cost for care. The lack of an association may therefore reflect women’s resilience and their ability to obtain needed health care in the face of financial challenges,” they write.
They point out that, conversely, Targeted Regulation of Abortion Providers (TRAP) laws, which supporters say protect women, have been associated instead with increases in maternal mortality.
Dr. Harper and study coauthors, noting that the relationship between abortion laws and mortality is complex, urge states with restrictive abortion laws to consider proven countermeasures to offset the rates, including Medicaid expansion.
One coauthor served on a medical advisory board, was a site primary investigator for several clinical trials, and received royalties from UpToDate for two topics related to trial of labor after cesarean. The other authors did not report any potential conflicts of interest. Dr. Darney’s institution receives research funding from Organon and the Office of Population Affairs on which she is the primary investigator, and she is a member of the board of directors of the Society of Family Planning and a deputy editor at Contraception. She has received an honorarium from ACOG for committee work. The other editorialists did not report any potential conflicts of interest.
States that have moderate state abortion laws had lower rates of maternal, fetal, and infant mortality, but not lower all-cause mortality, in reproductive-aged females compared with states that have restrictive laws, new research using 2000-2019 data indicates.
Additionally, having a higher number of laws that restrict abortion in a state was linked with higher maternal and infant mortality in the state, the study states.
Mortality increases with number of laws
“Each additional abortion regulation was associated with an increase in maternal mortality (1.09/100,000 live births; 95% confidence interval, 0.36-1.82) and infant mortality (0.20/1,000 live births; 95% CI, 0.12-0.26),” the authors write.
Lorie M. Harper, MD, MSCI, with the department of women’s health, University of Texas at Austin, led a team that simultaneously studied four categories of mortality: all-cause mortality in females ages 15-49 years; maternal mortality; infant mortality; and fetal mortality.
Her team did a retrospective cohort study using the Centers for Disease Control and Prevention’s WONDER (Wide-ranging ONline Data for Epidemiologic Research) database. WONDER collects publicly available data with information on mortality by state.
Data were available from 2000 to 2019 for all-cause, maternal, and infant mortality. Fetal mortality data were available from 2005 to 2019.
Findings were published ahead of print in February’s Obstetrics & Gynecology.
Though the study was done before the Supreme Court decision overturning Roe v. Wade in June 2022, its findings may shed light on potential trends depending on states’ decisions.
In the wake of the Dobbs v. Jackson Women’s Health Organization, an estimated 33 million U.S. women will live in states without available abortion services, the authors note.
State abortion laws changed dramatically in the study period
The authors chose the 2-decade time frame because data could be accessed for both maternal and infant mortality for all the years and because state level abortion laws changed dramatically in that time.
The Guttmacher Institute has analyzed each state’s abortion policy landscape and scored states as restrictive, moderate, or supportive. Certain types of abortion laws were associated with higher rates of all-cause mortality in reproductive-age females.
Those laws included trigger laws (the laws that automatically outlaw abortion in a state when federal law is overturned), laws that limit access to medication abortion, and parental consent laws, according to the paper.
Additionally, states that restrict access to abortion have higher rates of maternal and infant death, even after accounting for the general health of the population.
Trigger-law states have strong mortality association
Blair G. Darney, PhD, MPH, with the department of obstetrics and gynecology, Oregon Health & Science University and the OHSU-PSU School of Public Health in Portland, and two OHSU coauthors note in an accompanying editorial that having a trigger law in place was associated with elevations in all four mortality measures. However, trigger laws had not yet been enacted during the study.
The existence of trigger laws is likely a proxy for other factors, they write.
“[T]he relationship between trigger laws and increased mortality may more likely be explained by other factors that were not considered in the analysis than by laws that were not enacted at the time of the study,” the editorialists write.
Those factors may include lack of Medicaid expansion, socioeconomic inequities, and other policies.
The editorialists also caution that just because some types of abortion restrictions don’t appear to increase mortality, it doesn’t mean they aren’t harmful.
“For example, laws prohibiting private or Medicaid insurance coverage for abortions were not associated with maternal mortality, but these laws may simply shift the burden of payment to the individual seeking an abortion or be mitigated by funds that support an individual’s out-of-pocket cost for care. The lack of an association may therefore reflect women’s resilience and their ability to obtain needed health care in the face of financial challenges,” they write.
They point out that, conversely, Targeted Regulation of Abortion Providers (TRAP) laws, which supporters say protect women, have been associated instead with increases in maternal mortality.
Dr. Harper and study coauthors, noting that the relationship between abortion laws and mortality is complex, urge states with restrictive abortion laws to consider proven countermeasures to offset the rates, including Medicaid expansion.
One coauthor served on a medical advisory board, was a site primary investigator for several clinical trials, and received royalties from UpToDate for two topics related to trial of labor after cesarean. The other authors did not report any potential conflicts of interest. Dr. Darney’s institution receives research funding from Organon and the Office of Population Affairs on which she is the primary investigator, and she is a member of the board of directors of the Society of Family Planning and a deputy editor at Contraception. She has received an honorarium from ACOG for committee work. The other editorialists did not report any potential conflicts of interest.
States that have moderate state abortion laws had lower rates of maternal, fetal, and infant mortality, but not lower all-cause mortality, in reproductive-aged females compared with states that have restrictive laws, new research using 2000-2019 data indicates.
Additionally, having a higher number of laws that restrict abortion in a state was linked with higher maternal and infant mortality in the state, the study states.
Mortality increases with number of laws
“Each additional abortion regulation was associated with an increase in maternal mortality (1.09/100,000 live births; 95% confidence interval, 0.36-1.82) and infant mortality (0.20/1,000 live births; 95% CI, 0.12-0.26),” the authors write.
Lorie M. Harper, MD, MSCI, with the department of women’s health, University of Texas at Austin, led a team that simultaneously studied four categories of mortality: all-cause mortality in females ages 15-49 years; maternal mortality; infant mortality; and fetal mortality.
Her team did a retrospective cohort study using the Centers for Disease Control and Prevention’s WONDER (Wide-ranging ONline Data for Epidemiologic Research) database. WONDER collects publicly available data with information on mortality by state.
Data were available from 2000 to 2019 for all-cause, maternal, and infant mortality. Fetal mortality data were available from 2005 to 2019.
Findings were published ahead of print in February’s Obstetrics & Gynecology.
Though the study was done before the Supreme Court decision overturning Roe v. Wade in June 2022, its findings may shed light on potential trends depending on states’ decisions.
In the wake of the Dobbs v. Jackson Women’s Health Organization, an estimated 33 million U.S. women will live in states without available abortion services, the authors note.
State abortion laws changed dramatically in the study period
The authors chose the 2-decade time frame because data could be accessed for both maternal and infant mortality for all the years and because state level abortion laws changed dramatically in that time.
The Guttmacher Institute has analyzed each state’s abortion policy landscape and scored states as restrictive, moderate, or supportive. Certain types of abortion laws were associated with higher rates of all-cause mortality in reproductive-age females.
Those laws included trigger laws (the laws that automatically outlaw abortion in a state when federal law is overturned), laws that limit access to medication abortion, and parental consent laws, according to the paper.
Additionally, states that restrict access to abortion have higher rates of maternal and infant death, even after accounting for the general health of the population.
Trigger-law states have strong mortality association
Blair G. Darney, PhD, MPH, with the department of obstetrics and gynecology, Oregon Health & Science University and the OHSU-PSU School of Public Health in Portland, and two OHSU coauthors note in an accompanying editorial that having a trigger law in place was associated with elevations in all four mortality measures. However, trigger laws had not yet been enacted during the study.
The existence of trigger laws is likely a proxy for other factors, they write.
“[T]he relationship between trigger laws and increased mortality may more likely be explained by other factors that were not considered in the analysis than by laws that were not enacted at the time of the study,” the editorialists write.
Those factors may include lack of Medicaid expansion, socioeconomic inequities, and other policies.
The editorialists also caution that just because some types of abortion restrictions don’t appear to increase mortality, it doesn’t mean they aren’t harmful.
“For example, laws prohibiting private or Medicaid insurance coverage for abortions were not associated with maternal mortality, but these laws may simply shift the burden of payment to the individual seeking an abortion or be mitigated by funds that support an individual’s out-of-pocket cost for care. The lack of an association may therefore reflect women’s resilience and their ability to obtain needed health care in the face of financial challenges,” they write.
They point out that, conversely, Targeted Regulation of Abortion Providers (TRAP) laws, which supporters say protect women, have been associated instead with increases in maternal mortality.
Dr. Harper and study coauthors, noting that the relationship between abortion laws and mortality is complex, urge states with restrictive abortion laws to consider proven countermeasures to offset the rates, including Medicaid expansion.
One coauthor served on a medical advisory board, was a site primary investigator for several clinical trials, and received royalties from UpToDate for two topics related to trial of labor after cesarean. The other authors did not report any potential conflicts of interest. Dr. Darney’s institution receives research funding from Organon and the Office of Population Affairs on which she is the primary investigator, and she is a member of the board of directors of the Society of Family Planning and a deputy editor at Contraception. She has received an honorarium from ACOG for committee work. The other editorialists did not report any potential conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
Mediterranean diet linked with fewer pregnancy complications
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
FROM JAMA NETWORK OPEN
Home births in the United States, 2019—2021
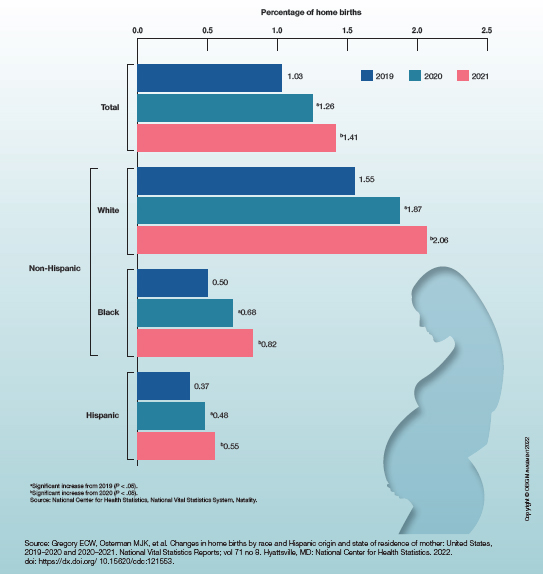


Sleep-disordered breathing promotes elevated arterial stiffness and preeclampsia
, based on data from 181 individuals.
The intermittent hypoxia resulting from sleep-disordered breathing (SDB) has been linked to cardiovascular disease and hypertension, wrote Kim Phan, PhD, of McGill University, Montreal, and colleagues.
SDB has been associated with increased preeclampsia risk, and women with preeclampsia show increased arterial stiffness, but an association between SDB and arterial stiffness in pregnancy has not been explored, they said.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 181 women with high-risk singleton pregnancies recruited from two tertiary obstetrics clinics in Montreal. High-risk pregnancy was defined as meeting at least one of the following criteria: age 35 years and older, body mass index 25 kg/m2 or higher, chronic hypertension, preexisting diabetes mellitus, preexisting renal disease, or personal or first-degree relative with a history of preeclampsia.
Participants were assessed at each trimester via the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, and Restless Legs Syndrome questionnaire. Sleep-disordered breathing was defined as loud snoring or witnessed sleep apneas at least three times a week. Arterial stiffness was assessed via applanation tonometry every 4 weeks from baseline throughout pregnancy.
Overall, 23% of the study population met the criteria for SDB; SDB in the first or second trimester was associated with a significantly increased risk of preeclampsia (odds ratio 3.4). The effect of SDB on preeclampsia was increased in women who reported excessive daytime sleepiness, defined as scores higher than 10 on the Epworth Sleepiness Scale. The odds ratio for preeclampsia in the first or second trimester increased to 5.7 in women with hypersomnolence in addition to SDB. The risk of preeclampsia was even higher (OR 8.2) in the third trimester.
Self-reported total sleep time decreased in the second and third trimesters compared with the first, but reports of excessive daytime sleepiness remained consistent throughout the pregnancies, the researchers noted.
The results highlight the need to screen pregnant women for SDB in all three trimesters; however, “future studies will need to assess the incremental benefit of integrating SDB into risk assessment calculators in pregnancy,” the researchers wrote in their discussion. Randomized trials are needed to determine the value of interventions such as continuous positive airway pressure to reduce arterial stiffness and the risks of hypertensive disorders of pregnancy, they said. More data also are needed to examine the role of excessive daytime sleepiness as a modifier of arterial stiffness and preeclampsia risk, they noted.
The findings were limited by the prospective design, which prevents conclusions of causality, the researchers noted. Other limitations included the focus on high-risk pregnancy, which may limit generalizability, and the use of symptoms, not sleep recordings, to identify SDB, they said.
However, the results show an independent association between SDB and arterial stiffness during pregnancy, and offer potentially useful insights into the mechanisms of SDB-associated cardiovascular conditions, they noted.
“This work may inform future studies exploring the value of using arterial stiffness, as an early noninvasive indicator of subclinical vascular dysfunction in pregnant women with SDB,” they concluded.
The study was supported by the Fonds de recherche du Quebec – Sante (FRQS), Heart and Stroke Foundation of Canada, McGill University’s academic enrichment fund, and the Canadian Foundation for Women’s Health. The researchers had no financial conflicts to disclose.
, based on data from 181 individuals.
The intermittent hypoxia resulting from sleep-disordered breathing (SDB) has been linked to cardiovascular disease and hypertension, wrote Kim Phan, PhD, of McGill University, Montreal, and colleagues.
SDB has been associated with increased preeclampsia risk, and women with preeclampsia show increased arterial stiffness, but an association between SDB and arterial stiffness in pregnancy has not been explored, they said.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 181 women with high-risk singleton pregnancies recruited from two tertiary obstetrics clinics in Montreal. High-risk pregnancy was defined as meeting at least one of the following criteria: age 35 years and older, body mass index 25 kg/m2 or higher, chronic hypertension, preexisting diabetes mellitus, preexisting renal disease, or personal or first-degree relative with a history of preeclampsia.
Participants were assessed at each trimester via the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, and Restless Legs Syndrome questionnaire. Sleep-disordered breathing was defined as loud snoring or witnessed sleep apneas at least three times a week. Arterial stiffness was assessed via applanation tonometry every 4 weeks from baseline throughout pregnancy.
Overall, 23% of the study population met the criteria for SDB; SDB in the first or second trimester was associated with a significantly increased risk of preeclampsia (odds ratio 3.4). The effect of SDB on preeclampsia was increased in women who reported excessive daytime sleepiness, defined as scores higher than 10 on the Epworth Sleepiness Scale. The odds ratio for preeclampsia in the first or second trimester increased to 5.7 in women with hypersomnolence in addition to SDB. The risk of preeclampsia was even higher (OR 8.2) in the third trimester.
Self-reported total sleep time decreased in the second and third trimesters compared with the first, but reports of excessive daytime sleepiness remained consistent throughout the pregnancies, the researchers noted.
The results highlight the need to screen pregnant women for SDB in all three trimesters; however, “future studies will need to assess the incremental benefit of integrating SDB into risk assessment calculators in pregnancy,” the researchers wrote in their discussion. Randomized trials are needed to determine the value of interventions such as continuous positive airway pressure to reduce arterial stiffness and the risks of hypertensive disorders of pregnancy, they said. More data also are needed to examine the role of excessive daytime sleepiness as a modifier of arterial stiffness and preeclampsia risk, they noted.
The findings were limited by the prospective design, which prevents conclusions of causality, the researchers noted. Other limitations included the focus on high-risk pregnancy, which may limit generalizability, and the use of symptoms, not sleep recordings, to identify SDB, they said.
However, the results show an independent association between SDB and arterial stiffness during pregnancy, and offer potentially useful insights into the mechanisms of SDB-associated cardiovascular conditions, they noted.
“This work may inform future studies exploring the value of using arterial stiffness, as an early noninvasive indicator of subclinical vascular dysfunction in pregnant women with SDB,” they concluded.
The study was supported by the Fonds de recherche du Quebec – Sante (FRQS), Heart and Stroke Foundation of Canada, McGill University’s academic enrichment fund, and the Canadian Foundation for Women’s Health. The researchers had no financial conflicts to disclose.
, based on data from 181 individuals.
The intermittent hypoxia resulting from sleep-disordered breathing (SDB) has been linked to cardiovascular disease and hypertension, wrote Kim Phan, PhD, of McGill University, Montreal, and colleagues.
SDB has been associated with increased preeclampsia risk, and women with preeclampsia show increased arterial stiffness, but an association between SDB and arterial stiffness in pregnancy has not been explored, they said.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 181 women with high-risk singleton pregnancies recruited from two tertiary obstetrics clinics in Montreal. High-risk pregnancy was defined as meeting at least one of the following criteria: age 35 years and older, body mass index 25 kg/m2 or higher, chronic hypertension, preexisting diabetes mellitus, preexisting renal disease, or personal or first-degree relative with a history of preeclampsia.
Participants were assessed at each trimester via the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, and Restless Legs Syndrome questionnaire. Sleep-disordered breathing was defined as loud snoring or witnessed sleep apneas at least three times a week. Arterial stiffness was assessed via applanation tonometry every 4 weeks from baseline throughout pregnancy.
Overall, 23% of the study population met the criteria for SDB; SDB in the first or second trimester was associated with a significantly increased risk of preeclampsia (odds ratio 3.4). The effect of SDB on preeclampsia was increased in women who reported excessive daytime sleepiness, defined as scores higher than 10 on the Epworth Sleepiness Scale. The odds ratio for preeclampsia in the first or second trimester increased to 5.7 in women with hypersomnolence in addition to SDB. The risk of preeclampsia was even higher (OR 8.2) in the third trimester.
Self-reported total sleep time decreased in the second and third trimesters compared with the first, but reports of excessive daytime sleepiness remained consistent throughout the pregnancies, the researchers noted.
The results highlight the need to screen pregnant women for SDB in all three trimesters; however, “future studies will need to assess the incremental benefit of integrating SDB into risk assessment calculators in pregnancy,” the researchers wrote in their discussion. Randomized trials are needed to determine the value of interventions such as continuous positive airway pressure to reduce arterial stiffness and the risks of hypertensive disorders of pregnancy, they said. More data also are needed to examine the role of excessive daytime sleepiness as a modifier of arterial stiffness and preeclampsia risk, they noted.
The findings were limited by the prospective design, which prevents conclusions of causality, the researchers noted. Other limitations included the focus on high-risk pregnancy, which may limit generalizability, and the use of symptoms, not sleep recordings, to identify SDB, they said.
However, the results show an independent association between SDB and arterial stiffness during pregnancy, and offer potentially useful insights into the mechanisms of SDB-associated cardiovascular conditions, they noted.
“This work may inform future studies exploring the value of using arterial stiffness, as an early noninvasive indicator of subclinical vascular dysfunction in pregnant women with SDB,” they concluded.
The study was supported by the Fonds de recherche du Quebec – Sante (FRQS), Heart and Stroke Foundation of Canada, McGill University’s academic enrichment fund, and the Canadian Foundation for Women’s Health. The researchers had no financial conflicts to disclose.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY