User login
Immediate Treatment Best for DVT, Specialist Says
HOLLYWOOD, FLA. — The time to take care of deep vein thrombosis is when it happens, even if it's during pregnancy, Dr. Anthony J. Comerota said.
Women who develop DVT during pregnancy can be safely and effectively treated with thrombolysis while pregnant, thereby avoiding both the acute danger of pulmonary embolism and the risk of long-term complications of postthrombotic syndrome that can make their legs painful and swollen and can eventually disrupt their lives.
“Most physicians shy away from aggressive treatments during pregnancy and avoid using thrombolytic drugs that could increase the risk of bleeding,” Dr. Comerota said at ISET 2008, an international symposium on endovascular therapy.
“These are valid concerns, but it can leave women with an extensive clot burden that interventionalists would ordinarily treat. … If [DVT] progresses to chronic venous disease, it will completely change the patient's life,” he said in an interview.
Dr. Comerota reported his experience using catheter-delivered thrombolytic drugs, with or without additional procedures, to safely treat seven pregnant women with DVT. None of the women had a bleeding complication.
Five women subsequently gave birth to healthy infants by routine vaginal delivery, and one infant was delivered by a cesarean section. The seventh woman lost her fetus during the second trimester because of a very aggressive case of the prothrombotic condition known as antiphospholipid antibody syndrome, which boosted her risk for developing DVT during pregnancy, said Dr. Comerota, director of a vascular treatment and research center in Toledo, Ohio.
Pregnant women are four- to sixfold more likely to develop DVT than are nonpregnant women, and if the DVT persists they are also more likely to have a poor pregnancy outcome. DVT during pregnancy is more likely to occur in ilial veins and to be extensive and cause more sequelae. In nonpregnant women, DVT is usually less extensive and tends to form in the femoral-popliteal veins.
About two-thirds of women who develop DVT during pregnancy go on to have chronic venous insufficiency if their initial treatment during pregnancy is by anticoagulation alone.
Although many physicians are reluctant to use thrombolytic therapy during pregnancy, there is no indication of an elevated risk for major hemorrhage from lytic therapy in pregnant women, Dr. Comerota said. It is known that the placental penetration rates of urokinase (pregnancy category B), streptokinase (category C), and recombinant tissue-type plasminogen activator (category C) are too low to have any fibrinolytic effect in the fetus.
“Thrombolytic therapy appears to be low risk and compatible with pregnancy, but the human pregnancy experience is limited. No cases of fetal hemorrhage or loss secondary to thrombolytics have been reported,” said Gerald Briggs, a clinical pharmacist specializing in obstetrics at Long Beach (Calif.) Memorial Medical Center. He agreed that recombinant tissue-type plasminogen activator (rt-PA), urokinase, and streptokinase are not believed to cross to an embryo or fetus in amounts sufficient to cause hemorrhage.
Of the seven women treated by Dr. Comerota, one received intracatheter urokinase and six were treated with rt-PA. Five women were treated with endovascular devices only. In some cases, the endovascular treatments included Bacchus Vascular Inc.'s Trellis catheter (which uses a pair of balloons to isolate an artery segment, followed by the infusion of a lytic drug, agitation of the drug within the clot, and aspiration of the clot fragments and drug), EKOS Corp.'s EndoWave catheter (which combines lytic infusion with ultrasound), Possis Medical Inc.'s AngioJet system (a clot removal device), and stenting. One patient was treated with both endovascular devices and open surgery, and the seventh patient underwent open surgery only.
Five women were treated only during the antepartum period, and two women were treated both antepartum and postpartum. Five of the women were in the third trimester during their treatment. Two women were in the second trimester, and shielding was used to protect the uterus and fetus during radiologic procedures.
Five women did not have any postthrombotic symptoms. Two patients had mild leg swelling following pregnancy. After pregnancy, all women were placed on chronic warfarin therapy.
Three women later became pregnant again, and during pregnancy they were placed on prophylactic treatment with a low-molecular-weight heparin. None of these three women developed DVT during their subsequent pregnancies, Dr. Comerota said.
Dr. Comerota is a consultant to, on the speakers bureau for, and has received research grants from, Sanofi Aventis, and is also a consultant to and has received research grants from Bacchus Vascular.
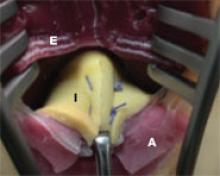
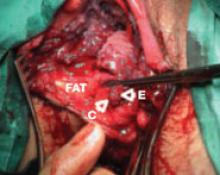
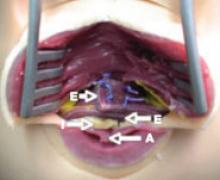
Surgery exposed a large thrombus embedded in a pregnant patient's iliofemoral vein. Photos courtesy Dr. Anthony J. Comerota
The size of the thrombus was better appreciated once it was removed and displayed by itself.
HOLLYWOOD, FLA. — The time to take care of deep vein thrombosis is when it happens, even if it's during pregnancy, Dr. Anthony J. Comerota said.
Women who develop DVT during pregnancy can be safely and effectively treated with thrombolysis while pregnant, thereby avoiding both the acute danger of pulmonary embolism and the risk of long-term complications of postthrombotic syndrome that can make their legs painful and swollen and can eventually disrupt their lives.
“Most physicians shy away from aggressive treatments during pregnancy and avoid using thrombolytic drugs that could increase the risk of bleeding,” Dr. Comerota said at ISET 2008, an international symposium on endovascular therapy.
“These are valid concerns, but it can leave women with an extensive clot burden that interventionalists would ordinarily treat. … If [DVT] progresses to chronic venous disease, it will completely change the patient's life,” he said in an interview.
Dr. Comerota reported his experience using catheter-delivered thrombolytic drugs, with or without additional procedures, to safely treat seven pregnant women with DVT. None of the women had a bleeding complication.
Five women subsequently gave birth to healthy infants by routine vaginal delivery, and one infant was delivered by a cesarean section. The seventh woman lost her fetus during the second trimester because of a very aggressive case of the prothrombotic condition known as antiphospholipid antibody syndrome, which boosted her risk for developing DVT during pregnancy, said Dr. Comerota, director of a vascular treatment and research center in Toledo, Ohio.
Pregnant women are four- to sixfold more likely to develop DVT than are nonpregnant women, and if the DVT persists they are also more likely to have a poor pregnancy outcome. DVT during pregnancy is more likely to occur in ilial veins and to be extensive and cause more sequelae. In nonpregnant women, DVT is usually less extensive and tends to form in the femoral-popliteal veins.
About two-thirds of women who develop DVT during pregnancy go on to have chronic venous insufficiency if their initial treatment during pregnancy is by anticoagulation alone.
Although many physicians are reluctant to use thrombolytic therapy during pregnancy, there is no indication of an elevated risk for major hemorrhage from lytic therapy in pregnant women, Dr. Comerota said. It is known that the placental penetration rates of urokinase (pregnancy category B), streptokinase (category C), and recombinant tissue-type plasminogen activator (category C) are too low to have any fibrinolytic effect in the fetus.
“Thrombolytic therapy appears to be low risk and compatible with pregnancy, but the human pregnancy experience is limited. No cases of fetal hemorrhage or loss secondary to thrombolytics have been reported,” said Gerald Briggs, a clinical pharmacist specializing in obstetrics at Long Beach (Calif.) Memorial Medical Center. He agreed that recombinant tissue-type plasminogen activator (rt-PA), urokinase, and streptokinase are not believed to cross to an embryo or fetus in amounts sufficient to cause hemorrhage.
Of the seven women treated by Dr. Comerota, one received intracatheter urokinase and six were treated with rt-PA. Five women were treated with endovascular devices only. In some cases, the endovascular treatments included Bacchus Vascular Inc.'s Trellis catheter (which uses a pair of balloons to isolate an artery segment, followed by the infusion of a lytic drug, agitation of the drug within the clot, and aspiration of the clot fragments and drug), EKOS Corp.'s EndoWave catheter (which combines lytic infusion with ultrasound), Possis Medical Inc.'s AngioJet system (a clot removal device), and stenting. One patient was treated with both endovascular devices and open surgery, and the seventh patient underwent open surgery only.
Five women were treated only during the antepartum period, and two women were treated both antepartum and postpartum. Five of the women were in the third trimester during their treatment. Two women were in the second trimester, and shielding was used to protect the uterus and fetus during radiologic procedures.
Five women did not have any postthrombotic symptoms. Two patients had mild leg swelling following pregnancy. After pregnancy, all women were placed on chronic warfarin therapy.
Three women later became pregnant again, and during pregnancy they were placed on prophylactic treatment with a low-molecular-weight heparin. None of these three women developed DVT during their subsequent pregnancies, Dr. Comerota said.
Dr. Comerota is a consultant to, on the speakers bureau for, and has received research grants from, Sanofi Aventis, and is also a consultant to and has received research grants from Bacchus Vascular.
Surgery exposed a large thrombus embedded in a pregnant patient's iliofemoral vein. Photos courtesy Dr. Anthony J. Comerota
The size of the thrombus was better appreciated once it was removed and displayed by itself.
HOLLYWOOD, FLA. — The time to take care of deep vein thrombosis is when it happens, even if it's during pregnancy, Dr. Anthony J. Comerota said.
Women who develop DVT during pregnancy can be safely and effectively treated with thrombolysis while pregnant, thereby avoiding both the acute danger of pulmonary embolism and the risk of long-term complications of postthrombotic syndrome that can make their legs painful and swollen and can eventually disrupt their lives.
“Most physicians shy away from aggressive treatments during pregnancy and avoid using thrombolytic drugs that could increase the risk of bleeding,” Dr. Comerota said at ISET 2008, an international symposium on endovascular therapy.
“These are valid concerns, but it can leave women with an extensive clot burden that interventionalists would ordinarily treat. … If [DVT] progresses to chronic venous disease, it will completely change the patient's life,” he said in an interview.
Dr. Comerota reported his experience using catheter-delivered thrombolytic drugs, with or without additional procedures, to safely treat seven pregnant women with DVT. None of the women had a bleeding complication.
Five women subsequently gave birth to healthy infants by routine vaginal delivery, and one infant was delivered by a cesarean section. The seventh woman lost her fetus during the second trimester because of a very aggressive case of the prothrombotic condition known as antiphospholipid antibody syndrome, which boosted her risk for developing DVT during pregnancy, said Dr. Comerota, director of a vascular treatment and research center in Toledo, Ohio.
Pregnant women are four- to sixfold more likely to develop DVT than are nonpregnant women, and if the DVT persists they are also more likely to have a poor pregnancy outcome. DVT during pregnancy is more likely to occur in ilial veins and to be extensive and cause more sequelae. In nonpregnant women, DVT is usually less extensive and tends to form in the femoral-popliteal veins.
About two-thirds of women who develop DVT during pregnancy go on to have chronic venous insufficiency if their initial treatment during pregnancy is by anticoagulation alone.
Although many physicians are reluctant to use thrombolytic therapy during pregnancy, there is no indication of an elevated risk for major hemorrhage from lytic therapy in pregnant women, Dr. Comerota said. It is known that the placental penetration rates of urokinase (pregnancy category B), streptokinase (category C), and recombinant tissue-type plasminogen activator (category C) are too low to have any fibrinolytic effect in the fetus.
“Thrombolytic therapy appears to be low risk and compatible with pregnancy, but the human pregnancy experience is limited. No cases of fetal hemorrhage or loss secondary to thrombolytics have been reported,” said Gerald Briggs, a clinical pharmacist specializing in obstetrics at Long Beach (Calif.) Memorial Medical Center. He agreed that recombinant tissue-type plasminogen activator (rt-PA), urokinase, and streptokinase are not believed to cross to an embryo or fetus in amounts sufficient to cause hemorrhage.
Of the seven women treated by Dr. Comerota, one received intracatheter urokinase and six were treated with rt-PA. Five women were treated with endovascular devices only. In some cases, the endovascular treatments included Bacchus Vascular Inc.'s Trellis catheter (which uses a pair of balloons to isolate an artery segment, followed by the infusion of a lytic drug, agitation of the drug within the clot, and aspiration of the clot fragments and drug), EKOS Corp.'s EndoWave catheter (which combines lytic infusion with ultrasound), Possis Medical Inc.'s AngioJet system (a clot removal device), and stenting. One patient was treated with both endovascular devices and open surgery, and the seventh patient underwent open surgery only.
Five women were treated only during the antepartum period, and two women were treated both antepartum and postpartum. Five of the women were in the third trimester during their treatment. Two women were in the second trimester, and shielding was used to protect the uterus and fetus during radiologic procedures.
Five women did not have any postthrombotic symptoms. Two patients had mild leg swelling following pregnancy. After pregnancy, all women were placed on chronic warfarin therapy.
Three women later became pregnant again, and during pregnancy they were placed on prophylactic treatment with a low-molecular-weight heparin. None of these three women developed DVT during their subsequent pregnancies, Dr. Comerota said.
Dr. Comerota is a consultant to, on the speakers bureau for, and has received research grants from, Sanofi Aventis, and is also a consultant to and has received research grants from Bacchus Vascular.
Surgery exposed a large thrombus embedded in a pregnant patient's iliofemoral vein. Photos courtesy Dr. Anthony J. Comerota
The size of the thrombus was better appreciated once it was removed and displayed by itself.
Postpartum Depression Tied to Prior Obesity
DALLAS — Obese women may be at increased risk for postpartum depression, new research suggests.
In a prospective analysis of 1,282 women who gave birth to singleton infants at term, nearly 30% of women with a prepregnancy body mass index of 30 or more screened positive for postpartum depression 8 weeks after delivery.
The study is the first to use a validated screening tool to evaluate the risk of postpartum depression (PPD) by maternal BMI strata, according to the researchers, who used a score of 12 or more on the Edinburgh Postnatal Depression Screen to define a positive PPD screen.
Women at the extremes of BMI and those with greater weight gains in pregnancy were also at increased risk for PPD, Dr. Yvette LaCoursiere and colleagues at the University of Utah, Salt Lake City, reported in a poster at the annual meeting of the Society for Maternal-Fetal Medicine.
A positive PPD screen was reported in 19% of underweight women (BMI below 18.5), 13% of normal-weight (BMI 18.5–24.9), 16% of overweight women (BMI 25–29.9), 18% with class I obesity (BMI 30–34.9), 28% with class II obesity (BMI 35–39.9), and 29% with class III obesity (BMI greater than or equal to 40). The number of women in each BMI stratum was: 115, 724, 256, 116, 43, and 31, respectively, with incomplete data available on 3 others.
BMI remained a risk factor for PPD even after the researchers controlled for maternal age (mean 27 years), race (86% white, 9% Hispanic), parity (two children), education (mean 14 years), and stressors including financial, traumatic, partner associated, and emotional.
“We're not screening women aggressively for postpartum depression, in general,” Dr. LaCoursiere said in an interview. “When I look at how this changed my practice, if I have women who are obese before delivery I have them come back at a 2-week visit and make sure they get a screening test because they have a very high chance of developing depression.”
Weight gain during pregnancy also influenced a woman's chance of becoming depressed. A positive PPD screen was observed for 10% of normal-weight women who gained 24 pounds or less, 11% of those who gained 25–34 pounds, and 16% of those who gained more than 35 pounds. The rates were similar among overweight women (12%, 14%, and 20%, respectively), but did not increase in a stepwise fashion among the mildly obese (23%, 9%, and 21%, respectively). There were too few women with class II and III obesity to analyze.
Contrary to what one would expect, normal-weight women are more likely than are obese women to exceed the recommended pregnancy weight gain of 25–35 pounds, with obese women typically gaining only about 16–24 pounds during pregnancy, Dr. LaCoursiere said.
The modified Body Shape Questionnaire (BSQ) was also used, and revealed that poor body image was associated with obesity and weight gain during pregnancy. Scores on the BSQ increased significantly with increasing BMI strata (32, 39, 44, 48, 51, and 49; P less than .05).
Surprisingly, only 54% of physicians discussed mood during the postpartum visit and 26% addressed weight, Dr. LaCoursiere said. Fewer than 30 women reported that their evaluation of mood was conducted with a written tool. During pregnancy, 77% of providers addressed weight and 53% discussed mood.
“It might be that we need to make this part of the nursing system so that a woman has to answer a survey when she first comes through the door, so the doctor has the information in hand,” she said. “Another thing that's tough for OBs is what to do with that result when you find it. Most should feel fairly comfortable treating at least mild depression and knowing what resources are available and whom to refer to.”
In all, 50 women (4%) reported using alcohol during pregnancy, 224 (17%) had a history of depression, 109 (9%) had a history of PPD, 23 (2%) had a history of other psychiatric diagnoses, and 175 (14%) had a family history of other psychiatric diagnoses.
At first glance, the percentage of women with a history of depression or PPD seems high. The data may be inflated because they are self-reported and thus do not necessarily reflect those who accessed care and were treated, Dr. LaCoursiere said.
DALLAS — Obese women may be at increased risk for postpartum depression, new research suggests.
In a prospective analysis of 1,282 women who gave birth to singleton infants at term, nearly 30% of women with a prepregnancy body mass index of 30 or more screened positive for postpartum depression 8 weeks after delivery.
The study is the first to use a validated screening tool to evaluate the risk of postpartum depression (PPD) by maternal BMI strata, according to the researchers, who used a score of 12 or more on the Edinburgh Postnatal Depression Screen to define a positive PPD screen.
Women at the extremes of BMI and those with greater weight gains in pregnancy were also at increased risk for PPD, Dr. Yvette LaCoursiere and colleagues at the University of Utah, Salt Lake City, reported in a poster at the annual meeting of the Society for Maternal-Fetal Medicine.
A positive PPD screen was reported in 19% of underweight women (BMI below 18.5), 13% of normal-weight (BMI 18.5–24.9), 16% of overweight women (BMI 25–29.9), 18% with class I obesity (BMI 30–34.9), 28% with class II obesity (BMI 35–39.9), and 29% with class III obesity (BMI greater than or equal to 40). The number of women in each BMI stratum was: 115, 724, 256, 116, 43, and 31, respectively, with incomplete data available on 3 others.
BMI remained a risk factor for PPD even after the researchers controlled for maternal age (mean 27 years), race (86% white, 9% Hispanic), parity (two children), education (mean 14 years), and stressors including financial, traumatic, partner associated, and emotional.
“We're not screening women aggressively for postpartum depression, in general,” Dr. LaCoursiere said in an interview. “When I look at how this changed my practice, if I have women who are obese before delivery I have them come back at a 2-week visit and make sure they get a screening test because they have a very high chance of developing depression.”
Weight gain during pregnancy also influenced a woman's chance of becoming depressed. A positive PPD screen was observed for 10% of normal-weight women who gained 24 pounds or less, 11% of those who gained 25–34 pounds, and 16% of those who gained more than 35 pounds. The rates were similar among overweight women (12%, 14%, and 20%, respectively), but did not increase in a stepwise fashion among the mildly obese (23%, 9%, and 21%, respectively). There were too few women with class II and III obesity to analyze.
Contrary to what one would expect, normal-weight women are more likely than are obese women to exceed the recommended pregnancy weight gain of 25–35 pounds, with obese women typically gaining only about 16–24 pounds during pregnancy, Dr. LaCoursiere said.
The modified Body Shape Questionnaire (BSQ) was also used, and revealed that poor body image was associated with obesity and weight gain during pregnancy. Scores on the BSQ increased significantly with increasing BMI strata (32, 39, 44, 48, 51, and 49; P less than .05).
Surprisingly, only 54% of physicians discussed mood during the postpartum visit and 26% addressed weight, Dr. LaCoursiere said. Fewer than 30 women reported that their evaluation of mood was conducted with a written tool. During pregnancy, 77% of providers addressed weight and 53% discussed mood.
“It might be that we need to make this part of the nursing system so that a woman has to answer a survey when she first comes through the door, so the doctor has the information in hand,” she said. “Another thing that's tough for OBs is what to do with that result when you find it. Most should feel fairly comfortable treating at least mild depression and knowing what resources are available and whom to refer to.”
In all, 50 women (4%) reported using alcohol during pregnancy, 224 (17%) had a history of depression, 109 (9%) had a history of PPD, 23 (2%) had a history of other psychiatric diagnoses, and 175 (14%) had a family history of other psychiatric diagnoses.
At first glance, the percentage of women with a history of depression or PPD seems high. The data may be inflated because they are self-reported and thus do not necessarily reflect those who accessed care and were treated, Dr. LaCoursiere said.
DALLAS — Obese women may be at increased risk for postpartum depression, new research suggests.
In a prospective analysis of 1,282 women who gave birth to singleton infants at term, nearly 30% of women with a prepregnancy body mass index of 30 or more screened positive for postpartum depression 8 weeks after delivery.
The study is the first to use a validated screening tool to evaluate the risk of postpartum depression (PPD) by maternal BMI strata, according to the researchers, who used a score of 12 or more on the Edinburgh Postnatal Depression Screen to define a positive PPD screen.
Women at the extremes of BMI and those with greater weight gains in pregnancy were also at increased risk for PPD, Dr. Yvette LaCoursiere and colleagues at the University of Utah, Salt Lake City, reported in a poster at the annual meeting of the Society for Maternal-Fetal Medicine.
A positive PPD screen was reported in 19% of underweight women (BMI below 18.5), 13% of normal-weight (BMI 18.5–24.9), 16% of overweight women (BMI 25–29.9), 18% with class I obesity (BMI 30–34.9), 28% with class II obesity (BMI 35–39.9), and 29% with class III obesity (BMI greater than or equal to 40). The number of women in each BMI stratum was: 115, 724, 256, 116, 43, and 31, respectively, with incomplete data available on 3 others.
BMI remained a risk factor for PPD even after the researchers controlled for maternal age (mean 27 years), race (86% white, 9% Hispanic), parity (two children), education (mean 14 years), and stressors including financial, traumatic, partner associated, and emotional.
“We're not screening women aggressively for postpartum depression, in general,” Dr. LaCoursiere said in an interview. “When I look at how this changed my practice, if I have women who are obese before delivery I have them come back at a 2-week visit and make sure they get a screening test because they have a very high chance of developing depression.”
Weight gain during pregnancy also influenced a woman's chance of becoming depressed. A positive PPD screen was observed for 10% of normal-weight women who gained 24 pounds or less, 11% of those who gained 25–34 pounds, and 16% of those who gained more than 35 pounds. The rates were similar among overweight women (12%, 14%, and 20%, respectively), but did not increase in a stepwise fashion among the mildly obese (23%, 9%, and 21%, respectively). There were too few women with class II and III obesity to analyze.
Contrary to what one would expect, normal-weight women are more likely than are obese women to exceed the recommended pregnancy weight gain of 25–35 pounds, with obese women typically gaining only about 16–24 pounds during pregnancy, Dr. LaCoursiere said.
The modified Body Shape Questionnaire (BSQ) was also used, and revealed that poor body image was associated with obesity and weight gain during pregnancy. Scores on the BSQ increased significantly with increasing BMI strata (32, 39, 44, 48, 51, and 49; P less than .05).
Surprisingly, only 54% of physicians discussed mood during the postpartum visit and 26% addressed weight, Dr. LaCoursiere said. Fewer than 30 women reported that their evaluation of mood was conducted with a written tool. During pregnancy, 77% of providers addressed weight and 53% discussed mood.
“It might be that we need to make this part of the nursing system so that a woman has to answer a survey when she first comes through the door, so the doctor has the information in hand,” she said. “Another thing that's tough for OBs is what to do with that result when you find it. Most should feel fairly comfortable treating at least mild depression and knowing what resources are available and whom to refer to.”
In all, 50 women (4%) reported using alcohol during pregnancy, 224 (17%) had a history of depression, 109 (9%) had a history of PPD, 23 (2%) had a history of other psychiatric diagnoses, and 175 (14%) had a family history of other psychiatric diagnoses.
At first glance, the percentage of women with a history of depression or PPD seems high. The data may be inflated because they are self-reported and thus do not necessarily reflect those who accessed care and were treated, Dr. LaCoursiere said.
Hemangiomas Linked to Placental Abnormalities
Vascular abnormalities of the placenta were strongly correlated with the incidence of infantile hemangiomas in a small group of very-low-birth-weight infants, Dr. Juan Carlos Lopez Gutierrez and his colleagues reported.
Abnormalities such as infarcts and hematomas might create a hypoxic intraplacental environment that stimulates vasculogenesis and predisposes these infants to postnatal hemangioma growth, said Dr. Lopez and his associates of La Paz Children's Hospital, Madrid (Pediatr. Dermatol. 2007;24:353–5).
Although the study is too small to make absolute claims, it does seem to shed additional light on the prevailing theory of hemangioma pathogenesis: embolization of placental endothelial cells. “There are several different factors playing an important role in the pathogenesis of hemangiomas. Just embolization of placental cells as the origin of hemangiomas is too simple a theory,” Dr. Lopez said in an interview.
His case series suggests that placental lesions lead to a hypoxic environment that, in turn, stimulates the unopposed growth of escaped fetal endothelial cells. “Hypoxia is extremely important as a precursor lesion, and placental anomalies provoke a low-oxygen atmosphere, which is one—and probably the most important—of the suggested factors for hemangioma development,” Dr. Lopez said. His study included 26 very-low-birth-weight infants, 13 of whom developed infantile hemangiomas postnatally. The investigators examined each placenta macroscopically and microscopically.
Every placenta in the group of infants who developed hemangiomas was abnormal, they found. Two placentas showed massive retroplacental hematomas; seven showed extensive infarction; and four exhibited large dilated vascular cavitations, severe vasculitis, chorioamnionitis, and funiculitis. Cord insertion was marginal in eight, paracentral in three, and velamentous in two. Among the placentas showing infarcts, the area of ischemia was simple in two and multiple in five, with a mean infarct size of 15 mm. The infarcted areas resulted in decreased growth of peripheral villi in all tissues.
In contrast, all of the placentas of the infants without hemangiomas were free of lesions reflecting disturbed maternal-fetal circulation. Two placentas showed isolated villous immaturity. Cord insertion was central in four and paracentral in nine.
Hypoxia is an important angiogenic stimulator in fetal development. “The relatively low oxygen environment in which the human fetoplacental unit develops during the first trimester is necessary to induce vasculoangiogenesis via embryonic endothelial cells proliferation, as these cells are sensitive to hypoxia and acidosis,” Dr. Lopez and his associates noted.
Prior research suggests that abnormal placentas increase the likelihood of infantile hemangiomas through shearing and embolization of placental tissue. Thus, the hypoxic environment created by placental insufficiency could be the trigger that turns on a vasculogenic response in any endothelial cells that have escaped into a fetus's circulation, they wrote.
Dr. Paula North, chief of pediatric pathology at the Medical College of Wisconsin, Milwaukee, pioneered the embolization theory of hemangioma pathogenesis and said that Dr. Lopez's study raises some valid issues. “The evidence from this study is a little bit circumstantial in supporting the placental origin theory,” Dr. North said in an interview. “What it does suggest is the idea that any kind of placental injury would increase the shedding of vascular precursor cells from the placenta, which then migrate into the baby.”
Once the placental cells enter the fetus, their migratory path and growth are probably influenced by their phenotype. “The cells that make up a hemangioma express a very interesting pattern of molecules that are highly relevant to the immune system,” Dr. North said. “They express indoleamine 2,3-dioxygenase, which helps create a state of maternal immune tolerance to the fetus, and this could help protect the growing hemangioma from attack by activated T cells.”
Hemangioma cells also express chemokine receptor 6. Normally expressed in dendritic cells, it might influence the area where shed placental endothelial cells eventually lodge, working “like a homing mechanism to bring the cells to the skin and liver,” she said.
Vascular abnormalities of the placenta were strongly correlated with the incidence of infantile hemangiomas in a small group of very-low-birth-weight infants, Dr. Juan Carlos Lopez Gutierrez and his colleagues reported.
Abnormalities such as infarcts and hematomas might create a hypoxic intraplacental environment that stimulates vasculogenesis and predisposes these infants to postnatal hemangioma growth, said Dr. Lopez and his associates of La Paz Children's Hospital, Madrid (Pediatr. Dermatol. 2007;24:353–5).
Although the study is too small to make absolute claims, it does seem to shed additional light on the prevailing theory of hemangioma pathogenesis: embolization of placental endothelial cells. “There are several different factors playing an important role in the pathogenesis of hemangiomas. Just embolization of placental cells as the origin of hemangiomas is too simple a theory,” Dr. Lopez said in an interview.
His case series suggests that placental lesions lead to a hypoxic environment that, in turn, stimulates the unopposed growth of escaped fetal endothelial cells. “Hypoxia is extremely important as a precursor lesion, and placental anomalies provoke a low-oxygen atmosphere, which is one—and probably the most important—of the suggested factors for hemangioma development,” Dr. Lopez said. His study included 26 very-low-birth-weight infants, 13 of whom developed infantile hemangiomas postnatally. The investigators examined each placenta macroscopically and microscopically.
Every placenta in the group of infants who developed hemangiomas was abnormal, they found. Two placentas showed massive retroplacental hematomas; seven showed extensive infarction; and four exhibited large dilated vascular cavitations, severe vasculitis, chorioamnionitis, and funiculitis. Cord insertion was marginal in eight, paracentral in three, and velamentous in two. Among the placentas showing infarcts, the area of ischemia was simple in two and multiple in five, with a mean infarct size of 15 mm. The infarcted areas resulted in decreased growth of peripheral villi in all tissues.
In contrast, all of the placentas of the infants without hemangiomas were free of lesions reflecting disturbed maternal-fetal circulation. Two placentas showed isolated villous immaturity. Cord insertion was central in four and paracentral in nine.
Hypoxia is an important angiogenic stimulator in fetal development. “The relatively low oxygen environment in which the human fetoplacental unit develops during the first trimester is necessary to induce vasculoangiogenesis via embryonic endothelial cells proliferation, as these cells are sensitive to hypoxia and acidosis,” Dr. Lopez and his associates noted.
Prior research suggests that abnormal placentas increase the likelihood of infantile hemangiomas through shearing and embolization of placental tissue. Thus, the hypoxic environment created by placental insufficiency could be the trigger that turns on a vasculogenic response in any endothelial cells that have escaped into a fetus's circulation, they wrote.
Dr. Paula North, chief of pediatric pathology at the Medical College of Wisconsin, Milwaukee, pioneered the embolization theory of hemangioma pathogenesis and said that Dr. Lopez's study raises some valid issues. “The evidence from this study is a little bit circumstantial in supporting the placental origin theory,” Dr. North said in an interview. “What it does suggest is the idea that any kind of placental injury would increase the shedding of vascular precursor cells from the placenta, which then migrate into the baby.”
Once the placental cells enter the fetus, their migratory path and growth are probably influenced by their phenotype. “The cells that make up a hemangioma express a very interesting pattern of molecules that are highly relevant to the immune system,” Dr. North said. “They express indoleamine 2,3-dioxygenase, which helps create a state of maternal immune tolerance to the fetus, and this could help protect the growing hemangioma from attack by activated T cells.”
Hemangioma cells also express chemokine receptor 6. Normally expressed in dendritic cells, it might influence the area where shed placental endothelial cells eventually lodge, working “like a homing mechanism to bring the cells to the skin and liver,” she said.
Vascular abnormalities of the placenta were strongly correlated with the incidence of infantile hemangiomas in a small group of very-low-birth-weight infants, Dr. Juan Carlos Lopez Gutierrez and his colleagues reported.
Abnormalities such as infarcts and hematomas might create a hypoxic intraplacental environment that stimulates vasculogenesis and predisposes these infants to postnatal hemangioma growth, said Dr. Lopez and his associates of La Paz Children's Hospital, Madrid (Pediatr. Dermatol. 2007;24:353–5).
Although the study is too small to make absolute claims, it does seem to shed additional light on the prevailing theory of hemangioma pathogenesis: embolization of placental endothelial cells. “There are several different factors playing an important role in the pathogenesis of hemangiomas. Just embolization of placental cells as the origin of hemangiomas is too simple a theory,” Dr. Lopez said in an interview.
His case series suggests that placental lesions lead to a hypoxic environment that, in turn, stimulates the unopposed growth of escaped fetal endothelial cells. “Hypoxia is extremely important as a precursor lesion, and placental anomalies provoke a low-oxygen atmosphere, which is one—and probably the most important—of the suggested factors for hemangioma development,” Dr. Lopez said. His study included 26 very-low-birth-weight infants, 13 of whom developed infantile hemangiomas postnatally. The investigators examined each placenta macroscopically and microscopically.
Every placenta in the group of infants who developed hemangiomas was abnormal, they found. Two placentas showed massive retroplacental hematomas; seven showed extensive infarction; and four exhibited large dilated vascular cavitations, severe vasculitis, chorioamnionitis, and funiculitis. Cord insertion was marginal in eight, paracentral in three, and velamentous in two. Among the placentas showing infarcts, the area of ischemia was simple in two and multiple in five, with a mean infarct size of 15 mm. The infarcted areas resulted in decreased growth of peripheral villi in all tissues.
In contrast, all of the placentas of the infants without hemangiomas were free of lesions reflecting disturbed maternal-fetal circulation. Two placentas showed isolated villous immaturity. Cord insertion was central in four and paracentral in nine.
Hypoxia is an important angiogenic stimulator in fetal development. “The relatively low oxygen environment in which the human fetoplacental unit develops during the first trimester is necessary to induce vasculoangiogenesis via embryonic endothelial cells proliferation, as these cells are sensitive to hypoxia and acidosis,” Dr. Lopez and his associates noted.
Prior research suggests that abnormal placentas increase the likelihood of infantile hemangiomas through shearing and embolization of placental tissue. Thus, the hypoxic environment created by placental insufficiency could be the trigger that turns on a vasculogenic response in any endothelial cells that have escaped into a fetus's circulation, they wrote.
Dr. Paula North, chief of pediatric pathology at the Medical College of Wisconsin, Milwaukee, pioneered the embolization theory of hemangioma pathogenesis and said that Dr. Lopez's study raises some valid issues. “The evidence from this study is a little bit circumstantial in supporting the placental origin theory,” Dr. North said in an interview. “What it does suggest is the idea that any kind of placental injury would increase the shedding of vascular precursor cells from the placenta, which then migrate into the baby.”
Once the placental cells enter the fetus, their migratory path and growth are probably influenced by their phenotype. “The cells that make up a hemangioma express a very interesting pattern of molecules that are highly relevant to the immune system,” Dr. North said. “They express indoleamine 2,3-dioxygenase, which helps create a state of maternal immune tolerance to the fetus, and this could help protect the growing hemangioma from attack by activated T cells.”
Hemangioma cells also express chemokine receptor 6. Normally expressed in dendritic cells, it might influence the area where shed placental endothelial cells eventually lodge, working “like a homing mechanism to bring the cells to the skin and liver,” she said.
IVF Twin Pregnancies Raise Anxiety, but Not Depression
WASHINGTON — Anxiety—but not depression—was higher among women with twin pregnancies than in women with singleton pregnancies after in vitro fertilization, reported Dr. Farnaz Jahangiri of Northwestern University, Chicago.
In this study, presented at the annual meeting of the American Society for Reproductive Medicine, Dr. Jahangiri and colleagues interviewed women at the confirmation of their pregnancies and again at 10–12 weeks' gestation and 21–22 weeks' gestation. The study included 48 singleton pregnancies and 13 sets of twins, and there were no significant demographic differences between the two groups.
The investigators found no differences in depression scores between the women with singleton vs. twin pregnancies at any of the three time points. By contrast, the women with twin pregnancies averaged higher (but not significantly higher) anxiety scores than the singleton group at 10–12 weeks and significantly higher anxiety scores at 22–23 weeks.
Psychological traits in singleton vs. twin IVF pregnancies have not been widely studied. But previous research has shown that women who are pregnant after IVF become less anxious as their pregnancies progress and their self-esteem increases.
The new findings of increased anxiety among women with IVF twin pregnancies during the second trimester can help clinicians discuss the risks associated with multiple gestations when they counsel women who are undergoing infertility treatments, according to the researchers. Depression was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D). Anxiety was assessed using the Spielberger State and Trait Anxiety Inventory.
WASHINGTON — Anxiety—but not depression—was higher among women with twin pregnancies than in women with singleton pregnancies after in vitro fertilization, reported Dr. Farnaz Jahangiri of Northwestern University, Chicago.
In this study, presented at the annual meeting of the American Society for Reproductive Medicine, Dr. Jahangiri and colleagues interviewed women at the confirmation of their pregnancies and again at 10–12 weeks' gestation and 21–22 weeks' gestation. The study included 48 singleton pregnancies and 13 sets of twins, and there were no significant demographic differences between the two groups.
The investigators found no differences in depression scores between the women with singleton vs. twin pregnancies at any of the three time points. By contrast, the women with twin pregnancies averaged higher (but not significantly higher) anxiety scores than the singleton group at 10–12 weeks and significantly higher anxiety scores at 22–23 weeks.
Psychological traits in singleton vs. twin IVF pregnancies have not been widely studied. But previous research has shown that women who are pregnant after IVF become less anxious as their pregnancies progress and their self-esteem increases.
The new findings of increased anxiety among women with IVF twin pregnancies during the second trimester can help clinicians discuss the risks associated with multiple gestations when they counsel women who are undergoing infertility treatments, according to the researchers. Depression was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D). Anxiety was assessed using the Spielberger State and Trait Anxiety Inventory.
WASHINGTON — Anxiety—but not depression—was higher among women with twin pregnancies than in women with singleton pregnancies after in vitro fertilization, reported Dr. Farnaz Jahangiri of Northwestern University, Chicago.
In this study, presented at the annual meeting of the American Society for Reproductive Medicine, Dr. Jahangiri and colleagues interviewed women at the confirmation of their pregnancies and again at 10–12 weeks' gestation and 21–22 weeks' gestation. The study included 48 singleton pregnancies and 13 sets of twins, and there were no significant demographic differences between the two groups.
The investigators found no differences in depression scores between the women with singleton vs. twin pregnancies at any of the three time points. By contrast, the women with twin pregnancies averaged higher (but not significantly higher) anxiety scores than the singleton group at 10–12 weeks and significantly higher anxiety scores at 22–23 weeks.
Psychological traits in singleton vs. twin IVF pregnancies have not been widely studied. But previous research has shown that women who are pregnant after IVF become less anxious as their pregnancies progress and their self-esteem increases.
The new findings of increased anxiety among women with IVF twin pregnancies during the second trimester can help clinicians discuss the risks associated with multiple gestations when they counsel women who are undergoing infertility treatments, according to the researchers. Depression was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D). Anxiety was assessed using the Spielberger State and Trait Anxiety Inventory.
High OGTT in Pregnacy Ups Later Diabetes Risk
Women who have an abnormal glucose tolerance test result during pregnancy but do not develop gestational diabetes still face an increased risk of developing type 2 diabetes later on, investigators reported.
The large retrospective study, published Jan. 25, concluded that even modestly elevated glucose levels double the risk of diabetes within the next 9 years. “The risk of subsequent diabetes … likely occurs since these women have an intermediate form of glucose intolerance” with impaired β-cell functioning, wrote Dr. Darcy B. Carr of the University of Washington, Seattle, and her coauthors (Diabetes Care 2008 Jan. 25 [doi 10.2337/dc07–1957]).
In this retrospective cohort study, the researchers analyzed diabetes risk over a mean 9-year follow-up period in 31,000 women without gestational diabetes who had an oral glucose tolerance test (OGTT) or oral glucose challenge test (OGCT) during their pregnancy. The mean age was 31 years; the median follow-up was 9 years.
The investigators found that the risk of later development of type 2 diabetes rose as the values of the OGCT rose. Compared with women whose levels were normal, women with glucose levels of 5.4–6.2 mmol/L and 6.4–7.3 mmol/L had double the risk of developing the disease, while women with levels greater than 7.3 mmol/L were three times more likely to do so. Women with no abnormal values on the OGTT were at no increased risk of developing type 2 diabetes, but those with one abnormal value were twice as likely to do so.
These associations remained significant even after the researchers controlled for age, primigravidity, and preterm delivery.
The finding is consistent with those from a previous, much smaller longitudinal study that reported higher frequencies of glucose intolerance in women with one abnormal OGTT value.
Dr. Carr and her colleagues noted that their study could not control for race, family history, or body mass index—all important factors to consider when assessing diabetes risk. In addition, subsequent diabetes was not systematically assessed, which may introduce bias in those who were selected for testing, they wrote.
They also said their conclusions are not sufficient for them to make any screening or treatment recommendations: “Whether women who fall within this intermediate range of glucose intolerance during pregnancy may benefit from increased diabetes surveillance as well as lifestyle recommendations proven to reduce the risk of developing diabetes is unknown.”
Women who have an abnormal glucose tolerance test result during pregnancy but do not develop gestational diabetes still face an increased risk of developing type 2 diabetes later on, investigators reported.
The large retrospective study, published Jan. 25, concluded that even modestly elevated glucose levels double the risk of diabetes within the next 9 years. “The risk of subsequent diabetes … likely occurs since these women have an intermediate form of glucose intolerance” with impaired β-cell functioning, wrote Dr. Darcy B. Carr of the University of Washington, Seattle, and her coauthors (Diabetes Care 2008 Jan. 25 [doi 10.2337/dc07–1957]).
In this retrospective cohort study, the researchers analyzed diabetes risk over a mean 9-year follow-up period in 31,000 women without gestational diabetes who had an oral glucose tolerance test (OGTT) or oral glucose challenge test (OGCT) during their pregnancy. The mean age was 31 years; the median follow-up was 9 years.
The investigators found that the risk of later development of type 2 diabetes rose as the values of the OGCT rose. Compared with women whose levels were normal, women with glucose levels of 5.4–6.2 mmol/L and 6.4–7.3 mmol/L had double the risk of developing the disease, while women with levels greater than 7.3 mmol/L were three times more likely to do so. Women with no abnormal values on the OGTT were at no increased risk of developing type 2 diabetes, but those with one abnormal value were twice as likely to do so.
These associations remained significant even after the researchers controlled for age, primigravidity, and preterm delivery.
The finding is consistent with those from a previous, much smaller longitudinal study that reported higher frequencies of glucose intolerance in women with one abnormal OGTT value.
Dr. Carr and her colleagues noted that their study could not control for race, family history, or body mass index—all important factors to consider when assessing diabetes risk. In addition, subsequent diabetes was not systematically assessed, which may introduce bias in those who were selected for testing, they wrote.
They also said their conclusions are not sufficient for them to make any screening or treatment recommendations: “Whether women who fall within this intermediate range of glucose intolerance during pregnancy may benefit from increased diabetes surveillance as well as lifestyle recommendations proven to reduce the risk of developing diabetes is unknown.”
Women who have an abnormal glucose tolerance test result during pregnancy but do not develop gestational diabetes still face an increased risk of developing type 2 diabetes later on, investigators reported.
The large retrospective study, published Jan. 25, concluded that even modestly elevated glucose levels double the risk of diabetes within the next 9 years. “The risk of subsequent diabetes … likely occurs since these women have an intermediate form of glucose intolerance” with impaired β-cell functioning, wrote Dr. Darcy B. Carr of the University of Washington, Seattle, and her coauthors (Diabetes Care 2008 Jan. 25 [doi 10.2337/dc07–1957]).
In this retrospective cohort study, the researchers analyzed diabetes risk over a mean 9-year follow-up period in 31,000 women without gestational diabetes who had an oral glucose tolerance test (OGTT) or oral glucose challenge test (OGCT) during their pregnancy. The mean age was 31 years; the median follow-up was 9 years.
The investigators found that the risk of later development of type 2 diabetes rose as the values of the OGCT rose. Compared with women whose levels were normal, women with glucose levels of 5.4–6.2 mmol/L and 6.4–7.3 mmol/L had double the risk of developing the disease, while women with levels greater than 7.3 mmol/L were three times more likely to do so. Women with no abnormal values on the OGTT were at no increased risk of developing type 2 diabetes, but those with one abnormal value were twice as likely to do so.
These associations remained significant even after the researchers controlled for age, primigravidity, and preterm delivery.
The finding is consistent with those from a previous, much smaller longitudinal study that reported higher frequencies of glucose intolerance in women with one abnormal OGTT value.
Dr. Carr and her colleagues noted that their study could not control for race, family history, or body mass index—all important factors to consider when assessing diabetes risk. In addition, subsequent diabetes was not systematically assessed, which may introduce bias in those who were selected for testing, they wrote.
They also said their conclusions are not sufficient for them to make any screening or treatment recommendations: “Whether women who fall within this intermediate range of glucose intolerance during pregnancy may benefit from increased diabetes surveillance as well as lifestyle recommendations proven to reduce the risk of developing diabetes is unknown.”
Peer Support May Avert Postpartum Depression
MONTREAL — Mother-to-mother support can significantly reduce the development of postpartum depression in women who are at high risk for the condition, Cindy-Lee Dennis, Ph.D., said at the annual conference of the Canadian Psychiatric Association.
“Meta-analyses and predictive studies have clearly suggested the importance of psychosocial variables in the development of postpartum depression,” said Dr. Dennis of the Lawrence S. Bloomberg Faculty of Nursing at the University of Toronto.
“When we look at support variables in particular, it's the lack of a confidante that places the mother at risk for postpartum depression,” she noted.
Her study involved 701 women who were less than 2 weeks post partum and considered to be at high risk for developing postpartum depression based on an Edinburgh Postnatal Depression Scale (EPDS) score of greater than 9.
The women were randomized to a control group (n = 352), which received usual postpartum care, or an intervention group (n = 349) that received usual postpartum care plus telephone peer support. A total of 205 peer-support volunteers, all of whom had recovered from self-reported postpartum depression, were recruited from the community through fliers and advertising. They were given a 4-hour training session and then matched to the new mothers based on health region, and, if the mother desired, on ethnicity.
For the primary outcome measure, an EPDS score of greater than 12, the study found a significant benefit to peer support. “Mothers who received the intervention were two times less likely to develop postpartum depression,” said Dr. Dennis, who reported an incidence of 13.5% in the intervention group and a 26% incidence in the control group.
A secondary outcome measure of anxiety also favored the intervention, with incidences of 20.6% in the intervention group and 26.9% in the control group. “This is bordering on statistical significance, but we think it is clinically relevant and suggests anxiety might be relieved with peer support,” she said.
There were no differences between the groups in their reports of loneliness.
Predictors of a baseline EPDS score of greater than 12 included non-Canadian ethnicity (reported by 19% of participants), not having been born in Canada (reported by 41% of participants), new immigrant status with less than 5 years in Canada (reported by 43% of those not born in Canada), and no family support (reported by 12% of participants). A total of 59% of the participants were primiparous, 31% had a history of depression, 18% had no mother to talk to, and almost 10% were very unhappy with the baby's father.
“Because this was a prevention trial, we felt it was unethical to leave a depressed mother in the community. So we administered the SCID [Structured Clinical Interview for DSM Disorders), and if they were diagnosed with clinical depression or had an EPDS score greater than 20, then we referred them back to the public health department and public health did follow up with them,” said Dr. Dennis.
MONTREAL — Mother-to-mother support can significantly reduce the development of postpartum depression in women who are at high risk for the condition, Cindy-Lee Dennis, Ph.D., said at the annual conference of the Canadian Psychiatric Association.
“Meta-analyses and predictive studies have clearly suggested the importance of psychosocial variables in the development of postpartum depression,” said Dr. Dennis of the Lawrence S. Bloomberg Faculty of Nursing at the University of Toronto.
“When we look at support variables in particular, it's the lack of a confidante that places the mother at risk for postpartum depression,” she noted.
Her study involved 701 women who were less than 2 weeks post partum and considered to be at high risk for developing postpartum depression based on an Edinburgh Postnatal Depression Scale (EPDS) score of greater than 9.
The women were randomized to a control group (n = 352), which received usual postpartum care, or an intervention group (n = 349) that received usual postpartum care plus telephone peer support. A total of 205 peer-support volunteers, all of whom had recovered from self-reported postpartum depression, were recruited from the community through fliers and advertising. They were given a 4-hour training session and then matched to the new mothers based on health region, and, if the mother desired, on ethnicity.
For the primary outcome measure, an EPDS score of greater than 12, the study found a significant benefit to peer support. “Mothers who received the intervention were two times less likely to develop postpartum depression,” said Dr. Dennis, who reported an incidence of 13.5% in the intervention group and a 26% incidence in the control group.
A secondary outcome measure of anxiety also favored the intervention, with incidences of 20.6% in the intervention group and 26.9% in the control group. “This is bordering on statistical significance, but we think it is clinically relevant and suggests anxiety might be relieved with peer support,” she said.
There were no differences between the groups in their reports of loneliness.
Predictors of a baseline EPDS score of greater than 12 included non-Canadian ethnicity (reported by 19% of participants), not having been born in Canada (reported by 41% of participants), new immigrant status with less than 5 years in Canada (reported by 43% of those not born in Canada), and no family support (reported by 12% of participants). A total of 59% of the participants were primiparous, 31% had a history of depression, 18% had no mother to talk to, and almost 10% were very unhappy with the baby's father.
“Because this was a prevention trial, we felt it was unethical to leave a depressed mother in the community. So we administered the SCID [Structured Clinical Interview for DSM Disorders), and if they were diagnosed with clinical depression or had an EPDS score greater than 20, then we referred them back to the public health department and public health did follow up with them,” said Dr. Dennis.
MONTREAL — Mother-to-mother support can significantly reduce the development of postpartum depression in women who are at high risk for the condition, Cindy-Lee Dennis, Ph.D., said at the annual conference of the Canadian Psychiatric Association.
“Meta-analyses and predictive studies have clearly suggested the importance of psychosocial variables in the development of postpartum depression,” said Dr. Dennis of the Lawrence S. Bloomberg Faculty of Nursing at the University of Toronto.
“When we look at support variables in particular, it's the lack of a confidante that places the mother at risk for postpartum depression,” she noted.
Her study involved 701 women who were less than 2 weeks post partum and considered to be at high risk for developing postpartum depression based on an Edinburgh Postnatal Depression Scale (EPDS) score of greater than 9.
The women were randomized to a control group (n = 352), which received usual postpartum care, or an intervention group (n = 349) that received usual postpartum care plus telephone peer support. A total of 205 peer-support volunteers, all of whom had recovered from self-reported postpartum depression, were recruited from the community through fliers and advertising. They were given a 4-hour training session and then matched to the new mothers based on health region, and, if the mother desired, on ethnicity.
For the primary outcome measure, an EPDS score of greater than 12, the study found a significant benefit to peer support. “Mothers who received the intervention were two times less likely to develop postpartum depression,” said Dr. Dennis, who reported an incidence of 13.5% in the intervention group and a 26% incidence in the control group.
A secondary outcome measure of anxiety also favored the intervention, with incidences of 20.6% in the intervention group and 26.9% in the control group. “This is bordering on statistical significance, but we think it is clinically relevant and suggests anxiety might be relieved with peer support,” she said.
There were no differences between the groups in their reports of loneliness.
Predictors of a baseline EPDS score of greater than 12 included non-Canadian ethnicity (reported by 19% of participants), not having been born in Canada (reported by 41% of participants), new immigrant status with less than 5 years in Canada (reported by 43% of those not born in Canada), and no family support (reported by 12% of participants). A total of 59% of the participants were primiparous, 31% had a history of depression, 18% had no mother to talk to, and almost 10% were very unhappy with the baby's father.
“Because this was a prevention trial, we felt it was unethical to leave a depressed mother in the community. So we administered the SCID [Structured Clinical Interview for DSM Disorders), and if they were diagnosed with clinical depression or had an EPDS score greater than 20, then we referred them back to the public health department and public health did follow up with them,” said Dr. Dennis.
Update: Bacterial Vaginosis Screening in Pregnancy
Updated recommendations from the U.S. Preventive Services Task Force advise against screening for bacterial vaginosis in pregnant women who are asymptomatic and at low risk for preterm delivery.
However, the recommendations remain neutral about such screening in high-risk pregnancies because “current evidence is insufficient to assess the balance of benefits and harms,” reported Dr. Ned Calonge, chair of the U.S. Preventive Services Task Force (USPSTF) and his colleagues.
The new recommendations (Ann. Intern. Med. 2008;148:214–9) are an update of those compiled by the task force in 2001 (Am. J. Prev. Med. 2001;20:59–61). They are based on an analysis of new evidence, which was conducted for the task force by Peggy Nygren of the Oregon Health and Science University, Portland, and her associates and funded by the Agency for Healthcare Research and Quality (Ann. Intern. Med. 2008;148:220–33).
The analysis addressed “previously identified gaps, such as the characterization of patients most likely to benefit from screening and the optimal timing of screening and treatment in pregnancy outcomes,” said Dr. Calonge, who is also chief medical officer of the Colorado Department of Public Health and Environment, Denver, and his colleagues.
Ms. Nygren and her associates noted the recent concerns that metronidazole—the antibiotic most commonly used to treat bacterial vaginosis—might increase preterm births in certain populations. “The juxtaposition of these data, along with epidemiologic evidence associating bacterial vaginosis with preterm birth, leads to considerable confusion for clinicians and researchers alike. Whether to screen or treat multiple times, when to start, and at what interval during pregnancy are unanswered questions, as bacterial vaginosis may not necessarily persist throughout pregnancy,” they wrote.
The analysis included studies published after the release of the task force's 2001 recommendations to examine “new evidence on the benefits and harms of screening and treating bacterial vaginosis in asymptomatic pregnant women.”
Asymptomatic patients were defined as those presenting for routine prenatal care and not for evaluation of vaginal discharge, odor, or itching. Low-risk patients were defined as having no history of and no risk factors for preterm delivery, whereas average-risk patients were defined as “the general population,” regardless of risk status. Women with a history of preterm delivery related to spontaneous rupture of membranes or spontaneous preterm labor were categorized as high risk.
The analysis found no benefit in treating women with low- or average-risk pregnancies if they were asymptomatic. For high-risk asymptomatic pregnancies, Ms. Nygren and her colleagues noted that findings from one trial that had been published since the USPSTF 2001 recommendations showed “a significant adverse effect of treatment on delivery before 37 weeks” in 127 women, “indicating that treatment of bacterial vaginosis increased the chance of preterm delivery” significantly (S. Afr. Med. J. 2002;92:231–4).
However, when this study was considered with previous studies that had been included in the 2001 recommendations, the results were “heterogenous and conflicting,” they wrote.
For the outcome of delivery before 37 weeks, three of the trials reported a significant treatment benefit, one showed significant treatment harm, and one showed no benefit.
In keeping with the USPSTF recommendation against screening in low-risk pregnancies, the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG), the Cochrane Pregnancy and Childbirth Group, the British Association for Sexual Health and HIV/Clinical Effectiveness Group (BASHH), and the American Academy of Family Physicians (AAFP) have similar recommendations, according to the authors of the task force's report.
However, although the task force maintains its neutral position regarding high-risk pregnancies, the CDC, ACOG, AAFP and BASHH say there might be high-risk women for whom screening and treatment may be beneficial, the USPSTF authors wrote, noting that optimal treatment for bacterial vaginosis in pregnancy remains unclear.
Updated recommendations from the U.S. Preventive Services Task Force advise against screening for bacterial vaginosis in pregnant women who are asymptomatic and at low risk for preterm delivery.
However, the recommendations remain neutral about such screening in high-risk pregnancies because “current evidence is insufficient to assess the balance of benefits and harms,” reported Dr. Ned Calonge, chair of the U.S. Preventive Services Task Force (USPSTF) and his colleagues.
The new recommendations (Ann. Intern. Med. 2008;148:214–9) are an update of those compiled by the task force in 2001 (Am. J. Prev. Med. 2001;20:59–61). They are based on an analysis of new evidence, which was conducted for the task force by Peggy Nygren of the Oregon Health and Science University, Portland, and her associates and funded by the Agency for Healthcare Research and Quality (Ann. Intern. Med. 2008;148:220–33).
The analysis addressed “previously identified gaps, such as the characterization of patients most likely to benefit from screening and the optimal timing of screening and treatment in pregnancy outcomes,” said Dr. Calonge, who is also chief medical officer of the Colorado Department of Public Health and Environment, Denver, and his colleagues.
Ms. Nygren and her associates noted the recent concerns that metronidazole—the antibiotic most commonly used to treat bacterial vaginosis—might increase preterm births in certain populations. “The juxtaposition of these data, along with epidemiologic evidence associating bacterial vaginosis with preterm birth, leads to considerable confusion for clinicians and researchers alike. Whether to screen or treat multiple times, when to start, and at what interval during pregnancy are unanswered questions, as bacterial vaginosis may not necessarily persist throughout pregnancy,” they wrote.
The analysis included studies published after the release of the task force's 2001 recommendations to examine “new evidence on the benefits and harms of screening and treating bacterial vaginosis in asymptomatic pregnant women.”
Asymptomatic patients were defined as those presenting for routine prenatal care and not for evaluation of vaginal discharge, odor, or itching. Low-risk patients were defined as having no history of and no risk factors for preterm delivery, whereas average-risk patients were defined as “the general population,” regardless of risk status. Women with a history of preterm delivery related to spontaneous rupture of membranes or spontaneous preterm labor were categorized as high risk.
The analysis found no benefit in treating women with low- or average-risk pregnancies if they were asymptomatic. For high-risk asymptomatic pregnancies, Ms. Nygren and her colleagues noted that findings from one trial that had been published since the USPSTF 2001 recommendations showed “a significant adverse effect of treatment on delivery before 37 weeks” in 127 women, “indicating that treatment of bacterial vaginosis increased the chance of preterm delivery” significantly (S. Afr. Med. J. 2002;92:231–4).
However, when this study was considered with previous studies that had been included in the 2001 recommendations, the results were “heterogenous and conflicting,” they wrote.
For the outcome of delivery before 37 weeks, three of the trials reported a significant treatment benefit, one showed significant treatment harm, and one showed no benefit.
In keeping with the USPSTF recommendation against screening in low-risk pregnancies, the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG), the Cochrane Pregnancy and Childbirth Group, the British Association for Sexual Health and HIV/Clinical Effectiveness Group (BASHH), and the American Academy of Family Physicians (AAFP) have similar recommendations, according to the authors of the task force's report.
However, although the task force maintains its neutral position regarding high-risk pregnancies, the CDC, ACOG, AAFP and BASHH say there might be high-risk women for whom screening and treatment may be beneficial, the USPSTF authors wrote, noting that optimal treatment for bacterial vaginosis in pregnancy remains unclear.
Updated recommendations from the U.S. Preventive Services Task Force advise against screening for bacterial vaginosis in pregnant women who are asymptomatic and at low risk for preterm delivery.
However, the recommendations remain neutral about such screening in high-risk pregnancies because “current evidence is insufficient to assess the balance of benefits and harms,” reported Dr. Ned Calonge, chair of the U.S. Preventive Services Task Force (USPSTF) and his colleagues.
The new recommendations (Ann. Intern. Med. 2008;148:214–9) are an update of those compiled by the task force in 2001 (Am. J. Prev. Med. 2001;20:59–61). They are based on an analysis of new evidence, which was conducted for the task force by Peggy Nygren of the Oregon Health and Science University, Portland, and her associates and funded by the Agency for Healthcare Research and Quality (Ann. Intern. Med. 2008;148:220–33).
The analysis addressed “previously identified gaps, such as the characterization of patients most likely to benefit from screening and the optimal timing of screening and treatment in pregnancy outcomes,” said Dr. Calonge, who is also chief medical officer of the Colorado Department of Public Health and Environment, Denver, and his colleagues.
Ms. Nygren and her associates noted the recent concerns that metronidazole—the antibiotic most commonly used to treat bacterial vaginosis—might increase preterm births in certain populations. “The juxtaposition of these data, along with epidemiologic evidence associating bacterial vaginosis with preterm birth, leads to considerable confusion for clinicians and researchers alike. Whether to screen or treat multiple times, when to start, and at what interval during pregnancy are unanswered questions, as bacterial vaginosis may not necessarily persist throughout pregnancy,” they wrote.
The analysis included studies published after the release of the task force's 2001 recommendations to examine “new evidence on the benefits and harms of screening and treating bacterial vaginosis in asymptomatic pregnant women.”
Asymptomatic patients were defined as those presenting for routine prenatal care and not for evaluation of vaginal discharge, odor, or itching. Low-risk patients were defined as having no history of and no risk factors for preterm delivery, whereas average-risk patients were defined as “the general population,” regardless of risk status. Women with a history of preterm delivery related to spontaneous rupture of membranes or spontaneous preterm labor were categorized as high risk.
The analysis found no benefit in treating women with low- or average-risk pregnancies if they were asymptomatic. For high-risk asymptomatic pregnancies, Ms. Nygren and her colleagues noted that findings from one trial that had been published since the USPSTF 2001 recommendations showed “a significant adverse effect of treatment on delivery before 37 weeks” in 127 women, “indicating that treatment of bacterial vaginosis increased the chance of preterm delivery” significantly (S. Afr. Med. J. 2002;92:231–4).
However, when this study was considered with previous studies that had been included in the 2001 recommendations, the results were “heterogenous and conflicting,” they wrote.
For the outcome of delivery before 37 weeks, three of the trials reported a significant treatment benefit, one showed significant treatment harm, and one showed no benefit.
In keeping with the USPSTF recommendation against screening in low-risk pregnancies, the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG), the Cochrane Pregnancy and Childbirth Group, the British Association for Sexual Health and HIV/Clinical Effectiveness Group (BASHH), and the American Academy of Family Physicians (AAFP) have similar recommendations, according to the authors of the task force's report.
However, although the task force maintains its neutral position regarding high-risk pregnancies, the CDC, ACOG, AAFP and BASHH say there might be high-risk women for whom screening and treatment may be beneficial, the USPSTF authors wrote, noting that optimal treatment for bacterial vaginosis in pregnancy remains unclear.
Malpractice Chronicle
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Ethmoid Roof Penetrated During Sinus Surgery
The plaintiff, a 14-year-old boy, was evaluated by his pediatrician, then by a family physician, for pain in his left cheek and significant postnasal drip. He was given several courses of antibiotics, which did not relieve his symptoms. He was then referred to the defendant otolaryngologist, who recommended endoscopic sinus surgery.
The procedure, performed one month after the boy’s initial presentation, included four endoscopic bilateral procedures (total ethmoidectomy, maxillary sinus antrostomy, frontal sinusotomy, and reduction of inferior turbinates), in addition to partial resection of the left middle turbinate. The surgery left the patient with persistent bitemporal headaches, photophobia, and phonophobia.
The plaintiff claimed that during surgery, the right ethmoid roof was penetrated, causing a bone shard to become dislodged. A review of the materials sent to pathology after surgery, the plaintiff said, revealed the presence of brain matter. The plaintiff claimed negligence in the performance of the procedures and lack of informed consent.
According to a published report, a defense verdict was returned. Posttrial motions were pending.
”He Said, She Said” Over Obstetrics Patient
A 24-year-old woman expecting her second child went to the defendant hospital in labor. The defendant anesthesiologist, Dr. R., administered an epidural anesthetic block.
About 15 minutes later, the patient complained of difficulty breathing, and a nurse responded by raising the head of the bed, administering oxygen by mask, and calling Dr. R. to return to the room. The plaintiff soon complained of not being able to feel her legs and said she felt nauseous. She vomited and again complained of having trouble breathing.
The nurse made an emergency call for Dr. R. to return and began to administer oxygen using a manual ventilator. The anesthesiologist arrived, ordered the ventilation to be stopped, and pronounced the patient fine. The nurse, contesting this determination, placed a pulse oximetry clip on the patient; her oxygen saturation was measured at 62%.
The nurse urged intubation, but when Dr. R. attempted the intervention, he placed the tube into the esophagus rather than the trachea. The nurse then called a “code 99” emergency.
Responding members of the code team testified that Dr. R. had misplaced the intubation tube and that when the team leader attempted to reintubate the patient, Dr. R. shouted an expletive and shoved him away (which Dr. R. denied). Dr. R. then intubated the woman but did not secure the intubation tube. The code team leader also claimed that Dr. R. called for defibrillation, although the patient had a nonshockable rhythm.
An emergency cesarean delivery was performed, after which the code team defibrillated the patient. This maneuver apparently dislodged the intubation tube, necessitating a third intubation. The patient then began spontaneous respirations.
The plaintiff suffered anoxic brain injury. Despite three months of inpatient rehabilitation, she has the mental acuity of a five- to six-year-old and requires constant supervision.
Dr. R. denied that he was called the first time the nurse claimed to have called him. Dr. R. claimed that when he arrived, the plaintiff was turning blue; he argued that he, not the nurse, began to administer supplementary oxygen. He claimed that when he then attempted intubation, the plaintiff became agitated and broke the laryngoscope blade, necessitating reintubation. He also claimed that he, not the nurse, called the code.
The plaintiff claimed that the nurse had been negligent and that numerous late chart entries showed that she had ignored the plaintiff while she was decompensating. The hospital claimed that Dr. R. had placed a high epidural block, leading to the patient’s respiratory distress; this, along with Dr. R.’s failure to properly intubate the patient, resulted in her injuries.
According to a published report, a defense verdict was returned.
Anticoagulation Therapy Times Two
A 45-year-old woman who was taking warfarin underwent a cholecystectomy, with preoperative and postoperative medication adjustment based on her international normalized ratio (INR). IV heparin was administered after the surgery to raise her INR. At the time of the woman’s discharge, the primary surgeon prescribed her usual dose of warfarin. Unknown to the surgeon, a second-year resident also prescribed warfarin, as well as heparin injections.
The patient was instructed to follow up in the anticoagulation therapy clinic every three days. On the way home from her first visit there, she experienced a massive abdominal hemorrhage. Emergency laparotomy was required at a different hospital, where doctors were unable to identify the source of the bleeding.
Two weeks later, the patient was transferred to the original hospital. Shortly thereafter, she died of complications of a massive abdominal hemorrhage, including acute respiratory distress syndrome, sepsis, and multiorgan failure.
The plaintiffs claimed that the defendants were negligent in monitoring the decedent’s INR levels, which should have been done daily. The hemorrhage, the plaintiffs claimed, was caused by the heparin. The order was for heparin to be administered “per pharmacy protocol,” but the hospital pharmacy had no such protocol in place at the time. As a result, too much heparin was given.
The defendants contended that the decedent’s treatment was proper and that abdominal hemorrhaging is a known complication of anticoagulation therapy. Her hemorrhage, they claimed, was triggered by the restriction of her seatbelt when she drove over the railroad tracks on the way home from the anticoagulation therapy clinic. The defendants maintained that the decedent’s INR level was properly monitored and that the use of heparin was within the standard of care.
The matter was arbitrated, resulting in an award of $385,376.
Loss of Vision After Screening Colonoscopy
A 54-year-old man was referred to the defendant gastroenterologist for a screening colonoscopy. The patient had a family history of colon cancer and a long history of multiple medical problems, including four heart attacks, organic heart disease, diabetes, dyslipidemia, and hypertension.
The previous month, when testing revealed the presence of a kidney stone, the man’s blood pressure was 160/88 mm Hg. Before the colonoscopy, his blood pressure measured 93/50 mm Hg. He had no lightheadedness, dizziness, or chest pain.
An IV was started, and the patient was given meperidine and midazolam. After the scope was inserted, his blood pressure declined. The endoscopy nurse reported this to the gastroenterologist, who ordered an increase in IV fluids. The plaintiff’s blood pressure rose, and the procedure was completed.
In recovery, the patient was noted to be alert and oriented. He received a perfect score on discharge criteria and was released from the recovery area with a blood pressure reading of 90/60 mm Hg.
The man went straight to a donut shop, where he ate two donuts. He then experienced nausea that lasted throughout the afternoon, after attempts to eat and drink a number of items.
At about 4 PM, a call was made to the gastroenterologist’s office. The defendant returned the call, instructing the patient to report to the emergency department; the plaintiff later claimed that this was stated only as an option. Instead, the man elected to take an OTC antinausea medication and remain at home. The nausea subsided, and he went to bed and slept through the night.
When the man awoke at 5 AM, he was totally blind. He was taken to the hospital, where he was evaluated by a neuro-ophthalmologist and diagnosed with a posterior ischemic optic neuropathy.
The plaintiff charged that the rare form of blindness he experienced was the result of hypotension during the colonoscopy. He claimed that he was not given sufficient IV fluids to elevate his blood pressure and that he should not have been discharged home.
The defendant claimed that the plaintiff’s blindness was unrelated to the colonoscopy but resulted from hypotension that developed while he was sleeping.
An initial trial ended with a hung jury. At a second trial, a defense verdict was returned.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Ethmoid Roof Penetrated During Sinus Surgery
The plaintiff, a 14-year-old boy, was evaluated by his pediatrician, then by a family physician, for pain in his left cheek and significant postnasal drip. He was given several courses of antibiotics, which did not relieve his symptoms. He was then referred to the defendant otolaryngologist, who recommended endoscopic sinus surgery.
The procedure, performed one month after the boy’s initial presentation, included four endoscopic bilateral procedures (total ethmoidectomy, maxillary sinus antrostomy, frontal sinusotomy, and reduction of inferior turbinates), in addition to partial resection of the left middle turbinate. The surgery left the patient with persistent bitemporal headaches, photophobia, and phonophobia.
The plaintiff claimed that during surgery, the right ethmoid roof was penetrated, causing a bone shard to become dislodged. A review of the materials sent to pathology after surgery, the plaintiff said, revealed the presence of brain matter. The plaintiff claimed negligence in the performance of the procedures and lack of informed consent.
According to a published report, a defense verdict was returned. Posttrial motions were pending.
”He Said, She Said” Over Obstetrics Patient
A 24-year-old woman expecting her second child went to the defendant hospital in labor. The defendant anesthesiologist, Dr. R., administered an epidural anesthetic block.
About 15 minutes later, the patient complained of difficulty breathing, and a nurse responded by raising the head of the bed, administering oxygen by mask, and calling Dr. R. to return to the room. The plaintiff soon complained of not being able to feel her legs and said she felt nauseous. She vomited and again complained of having trouble breathing.
The nurse made an emergency call for Dr. R. to return and began to administer oxygen using a manual ventilator. The anesthesiologist arrived, ordered the ventilation to be stopped, and pronounced the patient fine. The nurse, contesting this determination, placed a pulse oximetry clip on the patient; her oxygen saturation was measured at 62%.
The nurse urged intubation, but when Dr. R. attempted the intervention, he placed the tube into the esophagus rather than the trachea. The nurse then called a “code 99” emergency.
Responding members of the code team testified that Dr. R. had misplaced the intubation tube and that when the team leader attempted to reintubate the patient, Dr. R. shouted an expletive and shoved him away (which Dr. R. denied). Dr. R. then intubated the woman but did not secure the intubation tube. The code team leader also claimed that Dr. R. called for defibrillation, although the patient had a nonshockable rhythm.
An emergency cesarean delivery was performed, after which the code team defibrillated the patient. This maneuver apparently dislodged the intubation tube, necessitating a third intubation. The patient then began spontaneous respirations.
The plaintiff suffered anoxic brain injury. Despite three months of inpatient rehabilitation, she has the mental acuity of a five- to six-year-old and requires constant supervision.
Dr. R. denied that he was called the first time the nurse claimed to have called him. Dr. R. claimed that when he arrived, the plaintiff was turning blue; he argued that he, not the nurse, began to administer supplementary oxygen. He claimed that when he then attempted intubation, the plaintiff became agitated and broke the laryngoscope blade, necessitating reintubation. He also claimed that he, not the nurse, called the code.
The plaintiff claimed that the nurse had been negligent and that numerous late chart entries showed that she had ignored the plaintiff while she was decompensating. The hospital claimed that Dr. R. had placed a high epidural block, leading to the patient’s respiratory distress; this, along with Dr. R.’s failure to properly intubate the patient, resulted in her injuries.
According to a published report, a defense verdict was returned.
Anticoagulation Therapy Times Two
A 45-year-old woman who was taking warfarin underwent a cholecystectomy, with preoperative and postoperative medication adjustment based on her international normalized ratio (INR). IV heparin was administered after the surgery to raise her INR. At the time of the woman’s discharge, the primary surgeon prescribed her usual dose of warfarin. Unknown to the surgeon, a second-year resident also prescribed warfarin, as well as heparin injections.
The patient was instructed to follow up in the anticoagulation therapy clinic every three days. On the way home from her first visit there, she experienced a massive abdominal hemorrhage. Emergency laparotomy was required at a different hospital, where doctors were unable to identify the source of the bleeding.
Two weeks later, the patient was transferred to the original hospital. Shortly thereafter, she died of complications of a massive abdominal hemorrhage, including acute respiratory distress syndrome, sepsis, and multiorgan failure.
The plaintiffs claimed that the defendants were negligent in monitoring the decedent’s INR levels, which should have been done daily. The hemorrhage, the plaintiffs claimed, was caused by the heparin. The order was for heparin to be administered “per pharmacy protocol,” but the hospital pharmacy had no such protocol in place at the time. As a result, too much heparin was given.
The defendants contended that the decedent’s treatment was proper and that abdominal hemorrhaging is a known complication of anticoagulation therapy. Her hemorrhage, they claimed, was triggered by the restriction of her seatbelt when she drove over the railroad tracks on the way home from the anticoagulation therapy clinic. The defendants maintained that the decedent’s INR level was properly monitored and that the use of heparin was within the standard of care.
The matter was arbitrated, resulting in an award of $385,376.
Loss of Vision After Screening Colonoscopy
A 54-year-old man was referred to the defendant gastroenterologist for a screening colonoscopy. The patient had a family history of colon cancer and a long history of multiple medical problems, including four heart attacks, organic heart disease, diabetes, dyslipidemia, and hypertension.
The previous month, when testing revealed the presence of a kidney stone, the man’s blood pressure was 160/88 mm Hg. Before the colonoscopy, his blood pressure measured 93/50 mm Hg. He had no lightheadedness, dizziness, or chest pain.
An IV was started, and the patient was given meperidine and midazolam. After the scope was inserted, his blood pressure declined. The endoscopy nurse reported this to the gastroenterologist, who ordered an increase in IV fluids. The plaintiff’s blood pressure rose, and the procedure was completed.
In recovery, the patient was noted to be alert and oriented. He received a perfect score on discharge criteria and was released from the recovery area with a blood pressure reading of 90/60 mm Hg.
The man went straight to a donut shop, where he ate two donuts. He then experienced nausea that lasted throughout the afternoon, after attempts to eat and drink a number of items.
At about 4 PM, a call was made to the gastroenterologist’s office. The defendant returned the call, instructing the patient to report to the emergency department; the plaintiff later claimed that this was stated only as an option. Instead, the man elected to take an OTC antinausea medication and remain at home. The nausea subsided, and he went to bed and slept through the night.
When the man awoke at 5 AM, he was totally blind. He was taken to the hospital, where he was evaluated by a neuro-ophthalmologist and diagnosed with a posterior ischemic optic neuropathy.
The plaintiff charged that the rare form of blindness he experienced was the result of hypotension during the colonoscopy. He claimed that he was not given sufficient IV fluids to elevate his blood pressure and that he should not have been discharged home.
The defendant claimed that the plaintiff’s blindness was unrelated to the colonoscopy but resulted from hypotension that developed while he was sleeping.
An initial trial ended with a hung jury. At a second trial, a defense verdict was returned.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Ethmoid Roof Penetrated During Sinus Surgery
The plaintiff, a 14-year-old boy, was evaluated by his pediatrician, then by a family physician, for pain in his left cheek and significant postnasal drip. He was given several courses of antibiotics, which did not relieve his symptoms. He was then referred to the defendant otolaryngologist, who recommended endoscopic sinus surgery.
The procedure, performed one month after the boy’s initial presentation, included four endoscopic bilateral procedures (total ethmoidectomy, maxillary sinus antrostomy, frontal sinusotomy, and reduction of inferior turbinates), in addition to partial resection of the left middle turbinate. The surgery left the patient with persistent bitemporal headaches, photophobia, and phonophobia.
The plaintiff claimed that during surgery, the right ethmoid roof was penetrated, causing a bone shard to become dislodged. A review of the materials sent to pathology after surgery, the plaintiff said, revealed the presence of brain matter. The plaintiff claimed negligence in the performance of the procedures and lack of informed consent.
According to a published report, a defense verdict was returned. Posttrial motions were pending.
”He Said, She Said” Over Obstetrics Patient
A 24-year-old woman expecting her second child went to the defendant hospital in labor. The defendant anesthesiologist, Dr. R., administered an epidural anesthetic block.
About 15 minutes later, the patient complained of difficulty breathing, and a nurse responded by raising the head of the bed, administering oxygen by mask, and calling Dr. R. to return to the room. The plaintiff soon complained of not being able to feel her legs and said she felt nauseous. She vomited and again complained of having trouble breathing.
The nurse made an emergency call for Dr. R. to return and began to administer oxygen using a manual ventilator. The anesthesiologist arrived, ordered the ventilation to be stopped, and pronounced the patient fine. The nurse, contesting this determination, placed a pulse oximetry clip on the patient; her oxygen saturation was measured at 62%.
The nurse urged intubation, but when Dr. R. attempted the intervention, he placed the tube into the esophagus rather than the trachea. The nurse then called a “code 99” emergency.
Responding members of the code team testified that Dr. R. had misplaced the intubation tube and that when the team leader attempted to reintubate the patient, Dr. R. shouted an expletive and shoved him away (which Dr. R. denied). Dr. R. then intubated the woman but did not secure the intubation tube. The code team leader also claimed that Dr. R. called for defibrillation, although the patient had a nonshockable rhythm.
An emergency cesarean delivery was performed, after which the code team defibrillated the patient. This maneuver apparently dislodged the intubation tube, necessitating a third intubation. The patient then began spontaneous respirations.
The plaintiff suffered anoxic brain injury. Despite three months of inpatient rehabilitation, she has the mental acuity of a five- to six-year-old and requires constant supervision.
Dr. R. denied that he was called the first time the nurse claimed to have called him. Dr. R. claimed that when he arrived, the plaintiff was turning blue; he argued that he, not the nurse, began to administer supplementary oxygen. He claimed that when he then attempted intubation, the plaintiff became agitated and broke the laryngoscope blade, necessitating reintubation. He also claimed that he, not the nurse, called the code.
The plaintiff claimed that the nurse had been negligent and that numerous late chart entries showed that she had ignored the plaintiff while she was decompensating. The hospital claimed that Dr. R. had placed a high epidural block, leading to the patient’s respiratory distress; this, along with Dr. R.’s failure to properly intubate the patient, resulted in her injuries.
According to a published report, a defense verdict was returned.
Anticoagulation Therapy Times Two
A 45-year-old woman who was taking warfarin underwent a cholecystectomy, with preoperative and postoperative medication adjustment based on her international normalized ratio (INR). IV heparin was administered after the surgery to raise her INR. At the time of the woman’s discharge, the primary surgeon prescribed her usual dose of warfarin. Unknown to the surgeon, a second-year resident also prescribed warfarin, as well as heparin injections.
The patient was instructed to follow up in the anticoagulation therapy clinic every three days. On the way home from her first visit there, she experienced a massive abdominal hemorrhage. Emergency laparotomy was required at a different hospital, where doctors were unable to identify the source of the bleeding.
Two weeks later, the patient was transferred to the original hospital. Shortly thereafter, she died of complications of a massive abdominal hemorrhage, including acute respiratory distress syndrome, sepsis, and multiorgan failure.
The plaintiffs claimed that the defendants were negligent in monitoring the decedent’s INR levels, which should have been done daily. The hemorrhage, the plaintiffs claimed, was caused by the heparin. The order was for heparin to be administered “per pharmacy protocol,” but the hospital pharmacy had no such protocol in place at the time. As a result, too much heparin was given.
The defendants contended that the decedent’s treatment was proper and that abdominal hemorrhaging is a known complication of anticoagulation therapy. Her hemorrhage, they claimed, was triggered by the restriction of her seatbelt when she drove over the railroad tracks on the way home from the anticoagulation therapy clinic. The defendants maintained that the decedent’s INR level was properly monitored and that the use of heparin was within the standard of care.
The matter was arbitrated, resulting in an award of $385,376.
Loss of Vision After Screening Colonoscopy
A 54-year-old man was referred to the defendant gastroenterologist for a screening colonoscopy. The patient had a family history of colon cancer and a long history of multiple medical problems, including four heart attacks, organic heart disease, diabetes, dyslipidemia, and hypertension.
The previous month, when testing revealed the presence of a kidney stone, the man’s blood pressure was 160/88 mm Hg. Before the colonoscopy, his blood pressure measured 93/50 mm Hg. He had no lightheadedness, dizziness, or chest pain.
An IV was started, and the patient was given meperidine and midazolam. After the scope was inserted, his blood pressure declined. The endoscopy nurse reported this to the gastroenterologist, who ordered an increase in IV fluids. The plaintiff’s blood pressure rose, and the procedure was completed.
In recovery, the patient was noted to be alert and oriented. He received a perfect score on discharge criteria and was released from the recovery area with a blood pressure reading of 90/60 mm Hg.
The man went straight to a donut shop, where he ate two donuts. He then experienced nausea that lasted throughout the afternoon, after attempts to eat and drink a number of items.
At about 4 PM, a call was made to the gastroenterologist’s office. The defendant returned the call, instructing the patient to report to the emergency department; the plaintiff later claimed that this was stated only as an option. Instead, the man elected to take an OTC antinausea medication and remain at home. The nausea subsided, and he went to bed and slept through the night.
When the man awoke at 5 AM, he was totally blind. He was taken to the hospital, where he was evaluated by a neuro-ophthalmologist and diagnosed with a posterior ischemic optic neuropathy.
The plaintiff charged that the rare form of blindness he experienced was the result of hypotension during the colonoscopy. He claimed that he was not given sufficient IV fluids to elevate his blood pressure and that he should not have been discharged home.
The defendant claimed that the plaintiff’s blindness was unrelated to the colonoscopy but resulted from hypotension that developed while he was sleeping.
An initial trial ended with a hung jury. At a second trial, a defense verdict was returned.
Obstetric anal sphincter injury: 7 critical questions about care
The authors report no financial relationships relevant to this article.
CASE Large baby, extensive tear
A 28-year-old primigravida undergoes a forceps delivery with a midline episiotomy for failure to progress in the second stage of labor. At birth, the infant weighs 4 kg (8.8 lb), and the episiotomy extends to the anal verge. The resident who delivered the child is uncertain whether the anal sphincter is involved in the injury and asks a consultant to examine the perineum.
What should this examination entail?
The obstetrician is rarely culpable when a third- or fourth-degree obstetric anal sphincter injury (OASIS) occurs—but there is little excuse for letting one go undetected.
To minimize the risk of undiagnosed OASIS, a digital anorectal examination is warranted—before any suturing—in every woman who delivers vaginally. This practice can help you avoid missing isolated tears, such as “buttonhole” of the rectal mucosa, which can occur even when the anal sphincter remains intact (FIGURE 1), or a third- or fourth-degree tear that can sometimes be present behind apparently intact perineal skin (FIGURE 2).1
Clinical training of physicians and midwives also needs to improve.
Every labor room should have a protocol for management of anal sphincter injury2; this article describes detection, diagnosis, and management, focusing on seven critical questions.
Only a physician formally trained in primary anal sphincter repair (or under supervision) should repair OASIS.
FIGURE 1 Buttonhole tear
A buttonhole tear of the rectal mucosa (arrow) with an intact external anal sphincter (EAS) demonstrated during a digital rectal examination. SOURCE: Sultan AH3 (used with permission).
FIGURE 2 Injury obscured by intact skin
(A) Intact perineum on visual examination. (B) Anal sphincter trauma detected after rectal examination. SOURCE: Sultan AH, Kettle C1 (used with permission).
1. When (and how) should the torn perineum be examined?
The first requisite is informed consent for vaginal and rectal examination immediately after delivery. Also vital are adequate exposure of the perineum, good lighting, and, if necessary, sufficient analgesia to prevent pain-related restriction of the evaluation. It may be advisable to place the patient in the lithotomy position to improve exposure.
After visual examination of the perineum, part the labia and examine the vagina to establish the full extent of the tear. Always identify the apex of the vaginal laceration.
Next, perform a rectal examination to exclude injury to the anorectal mucosa and anal sphincter.3
Palpation is necessary to confirm OASIS
Insert the index finger into the anal canal and the thumb into the vagina and perform a pill-rolling motion to palpate the anal sphincter. If this technique is inconclusive, ask the woman to contract her anal sphincter with your fingers still in place. When the sphincter is disrupted, you feel a distinct gap anteriorly. If the perineal skin is intact, there may be an absence of puckering on the perianal skin over any underlying defect that may not be evident under regional or general anesthesia.
Because the external anal sphincter (EAS) is in a state of tonic contraction, the sphincter ends will retract when it is disrupted. These ends need to be grasped and retrieved at the time of repair.
Also identify the internal anal sphincter (IAS). It is a circular smooth muscle (FIGURE 3) that is paler in appearance (similar to the flesh of raw fish) than the striated EAS (similar to raw red meat).4 Under normal circumstances, the distal end of the IAS lies a few millimeters proximal to the distal end of the EAS (FIGURE 4). However, if the EAS is relaxed due to regional or general anesthesia, the distal end of the IAS will appear to be at a lower level. If the IAS or anal epithelium is torn, the EAS is, invariably, torn, too.
General or regional (spinal, epidural, caudal) anesthesia provides analgesia and muscle relaxation and enables proper evaluation of the full extent of the injury.
FIGURE 3 Grade 3b tear
Grade 3b tear with an intact internal anal sphincter (IAS). The external sphincter (EAS) is being grasped with Allis forceps. Note the difference in appearance of the paler IAS and darker EAS. SOURCE: Sultan AH, Kettle C1 (used with permission).
FIGURE 4 Classification of anal sphincter injury
First- and second-degree injuries are described below.
2. Is endoanal US helpful to detect OASIS?
Endoanal ultrasonography (US) to identify OASIS requires specific expertise, particularly in the immediate postpartum period, when the anal canal is lax (especially after an epidural). Ultimately, however, the diagnosis rests on clinical assessment and a rectal examination because, even if a defect is seen on US, it has to be clinically apparent to be repaired.
In a study by Faltin and colleagues, in which routine postpartum endoanal US was used as the gold standard for diagnosis of OASIS, five of 21 women had unnecessary intervention because the sonographic defect was not clinically visible despite exploration of the anal sphincter.5 As a result of this unnecessary exploration based on endoanal US, 20% of these women developed severe fecal incontinence. Therefore, we believe that OASIS is best detected clinically immediately after delivery, provided the physician performs a careful examination with palpation of the anal sphincter.6 In such a scenario, endoanal US is of limited value.
3. How is obstetric anal sphincter trauma classified?
To standardize the classification of perineal trauma, Sultan proposed the following system, which has been adopted by the Royal College of Obstetricians and Gynaecologists and internationally7-9:
First degree: Laceration of the vaginal epithelium or perineal skin only
Second degree: Involvement of the perineal muscles, but not the anal sphincter
Third degree: Disruption of the anal sphincter muscles (FIGURE 4):
- 3a: Less than 50% thickness of the external sphincter is torn
- 3b: More than 50% thickness of the external sphincter is torn
- 3c: Internal sphincter is also torn
Fourth degree: A third-degree anal tear with disruption of the anal epithelium (FIGURE 4).
If there is any ambiguity about grading of the injury, the higher grade should be selected. For example, if there is uncertainty between grades 3a and 3b, the injury should be classified as Grade 3b.
4. Is an operating room necessary?
OASIS should be repaired in the operating theater, where there is access to good lighting, appropriate equipment, and aseptic conditions. In our unit, we have a specially prepared instrument tray containing:
- a Weislander self-retaining retractor
- 4 Allis tissue forceps
- McIndoe scissors
- tooth forceps
- 4 artery forceps
- stitch scissors
- a needle holder.
In addition, deep retractors (e.g., Deavers) are useful when there are associated paravaginal tears.
5. What surgical technique is recommended?
Buttonhole injury
This type of injury can occur in the rectum without disrupting the anal sphincter or perineum. It is best repaired transvaginally using interrupted Vicryl (polyglactin) sutures.
To minimize the risk of persistent rectovaginal fistula, interpose a second layer of tissue between the rectum and vagina by approximating the rectovaginal fascia. A colostomy is rarely indicated unless a large tear extends above the pelvic floor or there is gross fecal contamination of the wound.
Fourth-degree tear
Repair torn anal epithelium with interrupted Vicryl 3-0 sutures, with the knots tied in the anal lumen. Proponents of this widely described technique argue that it reduces the quantity of foreign body (knots) within the tissue and lowers the risk of infection. Concern about a foreign body probably applies to the use of catgut, which dissolves by proteolysis, rather than to newer synthetic material such as Vicryl or Dexon (polyglycolic acid), which dissolves by hydrolysis.
Subcuticular repair of anal epithelium using a transvaginal approach has also been described and could be equally effective if the terminal knots are secure.10
Sphincter muscles
Repair these muscles using 3-0 polydioxanone (PDS) dyed sutures. Compared with braided sutures, monofilament sutures are believed to lessen the risk of infection, although a randomized controlled trial revealed no difference in suture-related morbidity between Vicryl and PDS at 6 weeks postpartum.11 Complete absorption of PDS takes longer than with Vicryl, with 50% tensile strength lasting more than 3 months, compared with 3 weeks for Vicryl.11 To minimize suture migration, cut suture ends short and ensure that they are covered by the overlying superficial perineal muscles.
Internal anal sphincter. Repair the IAS separately from the EAS. Grasp the ends of the torn muscle using Allis forceps and perform an end-to-end repair with interrupted or mattress 3-0 PDS sutures (FIGURE 5). Overlapping repair can be technically difficult.
There is some evidence that repair of an isolated IAS defect benefits patients with established anal incontinence.
External anal sphincter. Because the EAS is normally under tonic contraction, it tends to retract when torn. Therefore, repair requires identification and grasping of the torn ends using Allis tissue forceps (FIGURE 6).
When the EAS is only partially torn (Grade 3a and some cases of Grade 3b), perform an end-to end repair using 2 or 3 mattress sutures, similar to repair of IAS injury, instead of hemostatic “figure of eight” sutures.
For a full-thickness tear (some cases of Grade 3b or 3c, or Grade 4), overlapping repair may be preferable in experienced hands. The EAS may need to be mobilized by dissecting it free of the ischioanal fat laterally using a pair of McIndoe scissors. The torn ends of the EAS can then be overlapped in “double-breasted” fashion (FIGURE 7) using PDS 3-0 sutures. Proper overlap is possible only when the full length of the torn ends is identified.
Overlapping the ends of the sphincter allows for greater surface area of contact between muscle. In contrast, end-to-end repair can be performed without identifying the full length of the EAS and may give rise to incomplete apposition. Fernando and colleagues demonstrated that, in experienced hands, early primary overlap repair carries a lower risk of fecal urgency and anal incontinence than does immediate primary end-to-end repair.12,13
FIGURE 5 End-to-end repair
Internal anal sphincter (I) repair using mattress sutures, demonstrated on the latex Sultan model, used for training (www.perineum.net) (E, external sphincter; A, anal epithelium). SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 6 Locating the external anal sphincter
The external sphincter (E), grasped with Allis forceps, is surrounded by the capsule (C) and lies medial to the ischioanal fat. SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 7 Overlapping sphincter repair
Repair of a fourth degree tear (demonstrated on the Sultan model) using the overlap repair technique on the external sphincter (E). The anal epithelium (A) and the internal sphincter (I) have also been repaired. SOURCE: Sultan AH, Thakar R2 (used with permission).
Perineal muscles
After repair of the sphincter, suture the perineal muscles to reconstruct the perineal body and provide support to the repaired anal sphincter. A short, deficient perineum would leave the anal sphincter more vulnerable to trauma during a subsequent vaginal delivery.
Next, suture the vaginal skin and approximate the perineal skin using Vicryl Rapide 2-0 subcuticular suture.
Examine, and document, the repair
Perform a rectal and vaginal examination to confirm adequate repair and ensure that no other tears have been missed—and that all tampons or swabs have been removed.
Make detailed notes of the findings and repair. A pro forma pictorial representation of the tears proves very useful when notes are reviewed following complications or during audit or litigation.
6. What does postoperative care entail?
Prophylactic antibiotics are common
No randomized trials have substantiated the benefits of intraoperative and postoperative antibiotics after repair of OASIS. Nevertheless, these drugs are commonly prescribed, especially after fourth- degree tears, because infection and wound breakdown could jeopardize the repair and lead to incontinence or fistula.10,14
We prescribe intravenous broad-spectrum antibiotics such as cefuroxime and metronidazole intraoperatively and continue the drugs orally for 5 days.
Bladder catheterization is recommended
Severe perineal discomfort, especially after instrumental delivery, is a known cause of urinary retention. Moreover, after administration of regional anesthesia, it can take up to 12 hours before bladder sensation returns.
We recommend insertion of a Foley catheter for approximately 24 hours, unless medical staff can ensure that spontaneous voiding occurs at least every 3 to 4 hours without bladder overdistension.
Pain may persist after severe injury
The degree of pain following perineal trauma is related to the extent of the injury. OASIS is frequently associated with other more extensive injuries such as paravaginal tears. In one study, 91% of women continued to complain of severe perineal pain 7 days after OASIS.15
In a systematic review, Hedayati and associates found rectal analgesia, such as diclofenac sodium, to be effective at reducing pain from perineal trauma within the first 24 hours after birth; they also found that women used less additional analgesia within the first 48 hours after birth.16 Diclofenac is almost completely bound to protein, so excretion in breast milk is negligible.17
In women who have undergone repair of a fourth-degree tear, administer oral diclofenac; suppositories may be uncomfortable, and there is a theoretical risk of poor healing associated with local anti-inflammatory agents.
Avoid codeine-based preparations because they may cause constipation and lead to excessive straining and disruption of the repair.
Recommend a stool softener
It is vital that constipation be avoided as the patient heals; passage of constipated stool or fecal impaction can disrupt the repair. We prescribe a stool softener (lactulose, 15 mL twice daily) for 10 to 14 days and have encountered no problem with bowel evacuation.18
We recommend that the patient telephone a healthcare provider 24 to 48 hours after hospital discharge to confirm that bowel evacuation has occurred. If it hasn’t, we add mineral oil, magnesium hydroxide, or another oral bowel stimulant to the stool softener and bulking agent.
Mahoney and colleagues conducted a randomized trial (n=105) of constipating versus laxative regimens and found the latter to be associated with earlier and less painful first bowel motion and earlier hospital discharge.19 Nineteen percent of women following the constipating regimen had troublesome constipation (two required hospitalization for fecal impaction), compared with 5% of women receiving a laxative. There were no significant differences in continence scores, anal manometry, and endoanal US findings.
Give the patient adequate information
Before the patient is discharged from the hospital, we give her a booklet that describes the implications of OASIS and explains when and where to seek help if symptoms of infection or incontinence develop. All women also complete a validated bowel-health and quality-of-life questionnaire regarding conditions prior to the delivery. We also recommend pelvic floor and anal sphincter exercises as soon as her discomfort resolves.
Perform a comprehensive follow-up exam
All women who sustain OASIS should be assessed by a senior obstetrician 6 to 8 weeks after delivery. In our practice, these women are seen in a dedicated perineal clinic.20 The clinic provides a supportive environment and increases the patient’s confidence in the team.21
At the clinic, each woman completes the same symptom questionnaire that she was given before hospital discharge. She then undergoes a genital examination in which the physician checks the degree of scarring, residual granulation tissue, and tenderness; ensures that the patient understands the circumstances surrounding the delivery and injury; and addresses any concerns. All women then undergo anal manometry and endoanal US (FIGURE 8). Each patient is encouraged to continue pelvic floor exercises. If she has minimal sphincter contractility, she may need electrical stimulation.
If a dedicated perineal clinic is unavailable, the patient should be given clear instructions, preferably in writing, before leaving the hospital. During the 6 weeks immediately after delivery, she should be instructed to look for signs of infection or wound dehiscence and to telephone the physician to report any increase in pain or swelling, rectal bleeding, or purulent discharge. Any incontinence of stool or flatus also should be reported.
FIGURE 8 Defect visible on US
Endoanal sonogram showing a defect in the external anal sphincter between 11 o’clock and 1 o’clock (between the yellow arrows) (S, subepithelium; E, external anal sphincter). SOURCE: Sultan AH, Thakar R2 (used with permission).
7. Is vaginal delivery advisable after OASIS?
No randomized trials have determined the most appropriate mode of delivery after a third- or fourth-degree tear. We base our counseling of the patient on a completed symptom questionnaire and findings from manometry and endoanal US (FIGURE 8). If vaginal delivery is contemplated, these tests should be performed during the current pregnancy unless they were abnormal at an earlier date. FIGURE 9 is a simple flow diagram from our unit that illustrates management of subsequent delivery after OASIS.
When determining the mode of delivery, thorough counseling and clear documentation of that counseling are extremely important.
FIGURE 9 How do you determine the mode of delivery after OASIS?
Vaginal delivery is possible unless anal sphincter function is impaired
One study found that when a large sonographic defect (more than one quadrant) is present, or the squeeze-pressure increment (above resting pressure) is less than 20 mm Hg, the risk of impaired continence after a subsequent delivery increases dramatically.22
Based on these findings, we conducted a prospective study that found no deterioration of sphincter function or increase in symptoms after vaginal delivery unless the patient had significant compromise of anal sphincter function before the pregnancy.23 Therefore, we encourage asymptomatic women who have minimal compromise of anal sphincter function to undergo vaginal delivery.
Routine episiotomy is not protective
There is no evidence that routine episiotomy prevents recurrent OASIS. If episiotomy is deemed to be necessary—e.g., for a thick inelastic or scarred perineum—mediolateral episiotomy is preferred.
High likelihood of success in some women
Women who have minimal compromise of anal sphincter function should be counseled that they have an 88% (in centers practicing midline episiotomy) to 95% (in centers practicing mediolateral episiotomy) chance of delivering without sustaining another OASIS.24,25 This should reassure them if they have misgivings about vaginal delivery.
Threshold for C-section is lower if additional risk factors are present
If traumatic delivery is anticipated, as in the presence of one or more additional risk factors (macrosomia, shoulder dystocia, prolonged labor, difficult instrumental delivery), cesarean section may be appropriate.
Consider emotional needs
Some women who have sustained OASIS may be scarred emotionally as well as physically and may find it difficult to cope with the thought of another vaginal delivery. These women deserve sympathy, psychological support, and consideration of their request for cesarean section.
When cesarean is a good idea
Women who have a minor degree of incontinence (e.g., fecal urgency or flatus incontinence) may be managed with dietary advice, constipating agents (loperamide or codeine phosphate), and physiotherapy or biofeedback. These women who have some degree of anal sphincter compromise but whose symptoms are controlled should be counseled that cesarean delivery is recommended (FIGURE 9).
Women who have sustained a previous third- or fourth-degree tear with subsequent severe incontinence should be offered secondary sphincter repair by a colorectal surgeon or urogynecologist with expertise in secondary sphincter repair. All subsequent deliveries by these women should be by cesarean section.
Some women with fecal incontinence may choose to complete their family before embarking on anal sphincter surgery. It remains unclear whether these women should be allowed a vaginal delivery, but it is likely that most damage has already occurred and that the risk of further injury is minimal and possibly insignificant. The benefit of cesarean delivery, if any, should be weighed against its risks for all subsequent pregnancies.
Women who have undergone a previous successful secondary sphincter repair for fecal incontinence should be delivered by cesarean delivery.9
Not all women fit neatly into one category
There are going to be women who do not entirely fit any of the categories described—such as those who have isolated internal sphincter defects or irritable bowel syndrome. Management of these women should be individualized, with the mode of delivery determined by mutual agreement after taking into account symptoms and clinical and other findings.
If there are no facilities for anal manometry and US, the physician should base management on symptoms and clinical evaluation. Asymptomatic women who do not have clinical evidence of sphincter compromise during anal tone assessment may be allowed to undergo vaginal delivery. All women who are symptomatic should be referred to a center with facilities for anorectal assessment to establish the ideal management and mode of delivery.
Pay attention to modifiable risk factors
In the case described at the beginning of this article, two risk factors could have been modified to minimize the patient’s risk of OASIS—namely, midline episiotomy and forceps delivery. In a quasirandomized study by Coats, involving 407 nulliparous women, which compared mediolateral and midline episiotomy (when episiotomy was necessary), tears into or through the anal sphincter occurred in 12% of women undergoing midline episiotomy and 2% of those undergoing mediolateral episiotomy.26
If operative vaginal delivery is required, vacuum extraction is preferred. In a meta-analysis of randomized studies, Thakar and Eason found that fewer women have anal sphincter trauma with vacuum delivery than with forceps.27 One anal sphincter tear is avoided for every 18 women delivered by vacuum extraction instead of forceps. A randomized trial conducted in the United Kingdom involving mediolateral episiotomy found severe vaginal laceration in 17% of forceps deliveries and 11% of vacuum deliveries.28 A randomized controlled trial in Canada involving midline episiotomy found third- or fourth-degree tears in 29% of forceps deliveries, versus 12% of vacuum deliveries.29
Q. What is the proper code for reporting an anal sphincter injury incurred in pregnancy?
A. That depends—on when the tear occurred, whether the patient is currently pregnant, and whether there were additional lacerations of the perineum.
ICD-9-CM offers four codes in this setting. Choose one, as follows:
- If you note an anal tear at the time of, or after, delivery but there is no perineal laceration, report 664.6×. This code takes a fifth digit: “1,” for the patient who has just delivered, or “4,” if you are treating the tear after she has been discharged.
- If the tear is noted in addition to a third-degree perineal tear, report 664.2× instead; fifth-digit choices for this code are also “1” and “4.”
- If the patient had an anal tear before delivery, from a prior pregnancy, code 654.8× [congenital or acquired abnormality of the vulva].
- Last, if you are treating the patient for an old anal tear and she is not pregnant at the moment, report 569.43 and add any additional codes that have resulted from the tear, such as fecal incontinence (787.6).
—Melanie Witt, RN, CPC-OGS, MA
1. Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:13-19.
2. Sultan AH, Thakar R. Third and fourth degree tears. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:33-51.
3. Sultan AH. Primary repair of obstetric anal sphincter injury. In: Staskin DR, Cardozo L, ed. Textbook of Female Urology and Urogynaecology. London: ISIS Medical Media; 2006.
4. Thakar R, Fenner DE. Anatomy of the perineum and the anal sphincter. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:1-12.
5. Faltin DL, Boulvain M, Floris LA, Irion O. Diagnosis of anal sphincter tears to prevent fecal incontinence: a randomized controlled trial. Obstet Gynecol. 2005;106:6-13.
6. Andrews V, Thakar R, Sultan AH. Occult anal sphincter injuries—myth or reality. Br J Obstet Gynaecol. 2006;113:195-200.
7. Sultan AH. Obstetric perineal injury and anal incontinence. Clin Risk. 1999;5:193-196.
8. Royal College of Obstetricians and Gynaecologists. Management of third and fourth degree perineal tears following vaginal delivery. Guideline 29. London: RCOG Press; 2001.
9. Norton C, Christensen J, Butler U, et al. Anal Incontinence. 2nd ed. Plymouth: Health Publication Ltd; 2005:985-1044.
10. Sultan AH, Thakar R. Lower genital tract and anal sphincter trauma. Best Pract Res Clin Obstet Gynaecol. 2002;16:99-116.
11. Williams A, Adams EJ, Tincello DG, Alfirevic Z, Walkinshaw SA, Richmond DH. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
12. Fernando RJ, Sultan AH, Kettle C, Radley S, Jones P, O’Brien PMS. Repair techniques for obstetric anal sphincter injuries. A randomized controlled trial. Obstet Gynecol. 2006;107:1261-1268.
13. Fernando R, Sultan AH, Kettle C, Thakar R, Radley S. Methods of repair for obstetric anal sphincter injury. Cochrane Database Syst Rev. 2006;3:CD002866.-
14. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review and national practice survey. BMC Health Serv Res. 2002;2:9.-
15. MacArthur AJ, MacArthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. Am J Obstet Gynecol. 2004;191:1199-1204.
16. Hedayati H, Parsons J, Crowther CA. Rectal analgesia for pain from perineal trauma following childbirth. Cochrane Database Syst Rev. 2003;(3):CD003931.-
17. Kettle C, Hills RK, Jones P, Darby L, Gray R, Johanson R. Continuous versus interrupted perineal repair with standard or rapidly absorbed sutures after spontaneous vaginal birth: a randomised controlled trial. Lancet. 2002;359:2217-2223.
18. Sultan AH, Monga AK, Kumar D, Stanton SL. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
19. Mahony R, Behan M, O’Herlihy C, O’Connell PR. Randomized, clinical trial of bowel confinement vs. laxative use after primary repair of a third-degree obstetric anal sphincter tear. Dis Colon Rectum. 2004;47:12-17.
20. Thakar R, Sultan A. Postpartum problems and the role of a perineal clinic. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:65-79.
21. Williams A, Lavender T, Richmond DH, Tincello DG. Women’s experiences after a third-degree obstetric anal sphincter tear: a qualitative study. Birth. 2005;32:129-136.
22. Fynes M, Donnelly V, Behan M, O’Connell PR, O’Herlihy C. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet. 1999;354:983-986.
23. Scheer I, Thakar R, Sultan A. Should women who sustained obstetric anal sphincter injuries be allowed a vaginal delivery? Neurourol Urodynam. 2006;25:512-513.
24. Peleg D, Kennedy CM, Merrill D, Zlatnik FJ. Risk of repetition of a severe perineal laceration. Obstet Gynecol. 1999;93:1021-1024.
25. Harkin R, Fitzpatrick M, O’Connell PR, O’Herlihy C. Anal sphincter disruption at vaginal delivery: is recurrence predictable? Eur J Obstet Gynaecol Reprod Biol. 2003;109:149-152.
26. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
27. Thakar R, Eason E. Prevention of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:52-64.
28. Johanson RB, Rice C, Doyle M. A randomised prospective study comparing the new vacuum extractor policy with forceps delivery. Br J Obstet Gynaecol. 1993;100:524-530.
29. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol. 1996;175:1325-1330.
The authors report no financial relationships relevant to this article.
CASE Large baby, extensive tear
A 28-year-old primigravida undergoes a forceps delivery with a midline episiotomy for failure to progress in the second stage of labor. At birth, the infant weighs 4 kg (8.8 lb), and the episiotomy extends to the anal verge. The resident who delivered the child is uncertain whether the anal sphincter is involved in the injury and asks a consultant to examine the perineum.
What should this examination entail?
The obstetrician is rarely culpable when a third- or fourth-degree obstetric anal sphincter injury (OASIS) occurs—but there is little excuse for letting one go undetected.
To minimize the risk of undiagnosed OASIS, a digital anorectal examination is warranted—before any suturing—in every woman who delivers vaginally. This practice can help you avoid missing isolated tears, such as “buttonhole” of the rectal mucosa, which can occur even when the anal sphincter remains intact (FIGURE 1), or a third- or fourth-degree tear that can sometimes be present behind apparently intact perineal skin (FIGURE 2).1
Clinical training of physicians and midwives also needs to improve.
Every labor room should have a protocol for management of anal sphincter injury2; this article describes detection, diagnosis, and management, focusing on seven critical questions.
Only a physician formally trained in primary anal sphincter repair (or under supervision) should repair OASIS.
FIGURE 1 Buttonhole tear
A buttonhole tear of the rectal mucosa (arrow) with an intact external anal sphincter (EAS) demonstrated during a digital rectal examination. SOURCE: Sultan AH3 (used with permission).
FIGURE 2 Injury obscured by intact skin
(A) Intact perineum on visual examination. (B) Anal sphincter trauma detected after rectal examination. SOURCE: Sultan AH, Kettle C1 (used with permission).
1. When (and how) should the torn perineum be examined?
The first requisite is informed consent for vaginal and rectal examination immediately after delivery. Also vital are adequate exposure of the perineum, good lighting, and, if necessary, sufficient analgesia to prevent pain-related restriction of the evaluation. It may be advisable to place the patient in the lithotomy position to improve exposure.
After visual examination of the perineum, part the labia and examine the vagina to establish the full extent of the tear. Always identify the apex of the vaginal laceration.
Next, perform a rectal examination to exclude injury to the anorectal mucosa and anal sphincter.3
Palpation is necessary to confirm OASIS
Insert the index finger into the anal canal and the thumb into the vagina and perform a pill-rolling motion to palpate the anal sphincter. If this technique is inconclusive, ask the woman to contract her anal sphincter with your fingers still in place. When the sphincter is disrupted, you feel a distinct gap anteriorly. If the perineal skin is intact, there may be an absence of puckering on the perianal skin over any underlying defect that may not be evident under regional or general anesthesia.
Because the external anal sphincter (EAS) is in a state of tonic contraction, the sphincter ends will retract when it is disrupted. These ends need to be grasped and retrieved at the time of repair.
Also identify the internal anal sphincter (IAS). It is a circular smooth muscle (FIGURE 3) that is paler in appearance (similar to the flesh of raw fish) than the striated EAS (similar to raw red meat).4 Under normal circumstances, the distal end of the IAS lies a few millimeters proximal to the distal end of the EAS (FIGURE 4). However, if the EAS is relaxed due to regional or general anesthesia, the distal end of the IAS will appear to be at a lower level. If the IAS or anal epithelium is torn, the EAS is, invariably, torn, too.
General or regional (spinal, epidural, caudal) anesthesia provides analgesia and muscle relaxation and enables proper evaluation of the full extent of the injury.
FIGURE 3 Grade 3b tear
Grade 3b tear with an intact internal anal sphincter (IAS). The external sphincter (EAS) is being grasped with Allis forceps. Note the difference in appearance of the paler IAS and darker EAS. SOURCE: Sultan AH, Kettle C1 (used with permission).
FIGURE 4 Classification of anal sphincter injury
First- and second-degree injuries are described below.
2. Is endoanal US helpful to detect OASIS?
Endoanal ultrasonography (US) to identify OASIS requires specific expertise, particularly in the immediate postpartum period, when the anal canal is lax (especially after an epidural). Ultimately, however, the diagnosis rests on clinical assessment and a rectal examination because, even if a defect is seen on US, it has to be clinically apparent to be repaired.
In a study by Faltin and colleagues, in which routine postpartum endoanal US was used as the gold standard for diagnosis of OASIS, five of 21 women had unnecessary intervention because the sonographic defect was not clinically visible despite exploration of the anal sphincter.5 As a result of this unnecessary exploration based on endoanal US, 20% of these women developed severe fecal incontinence. Therefore, we believe that OASIS is best detected clinically immediately after delivery, provided the physician performs a careful examination with palpation of the anal sphincter.6 In such a scenario, endoanal US is of limited value.
3. How is obstetric anal sphincter trauma classified?
To standardize the classification of perineal trauma, Sultan proposed the following system, which has been adopted by the Royal College of Obstetricians and Gynaecologists and internationally7-9:
First degree: Laceration of the vaginal epithelium or perineal skin only
Second degree: Involvement of the perineal muscles, but not the anal sphincter
Third degree: Disruption of the anal sphincter muscles (FIGURE 4):
- 3a: Less than 50% thickness of the external sphincter is torn
- 3b: More than 50% thickness of the external sphincter is torn
- 3c: Internal sphincter is also torn
Fourth degree: A third-degree anal tear with disruption of the anal epithelium (FIGURE 4).
If there is any ambiguity about grading of the injury, the higher grade should be selected. For example, if there is uncertainty between grades 3a and 3b, the injury should be classified as Grade 3b.
4. Is an operating room necessary?
OASIS should be repaired in the operating theater, where there is access to good lighting, appropriate equipment, and aseptic conditions. In our unit, we have a specially prepared instrument tray containing:
- a Weislander self-retaining retractor
- 4 Allis tissue forceps
- McIndoe scissors
- tooth forceps
- 4 artery forceps
- stitch scissors
- a needle holder.
In addition, deep retractors (e.g., Deavers) are useful when there are associated paravaginal tears.
5. What surgical technique is recommended?
Buttonhole injury
This type of injury can occur in the rectum without disrupting the anal sphincter or perineum. It is best repaired transvaginally using interrupted Vicryl (polyglactin) sutures.
To minimize the risk of persistent rectovaginal fistula, interpose a second layer of tissue between the rectum and vagina by approximating the rectovaginal fascia. A colostomy is rarely indicated unless a large tear extends above the pelvic floor or there is gross fecal contamination of the wound.
Fourth-degree tear
Repair torn anal epithelium with interrupted Vicryl 3-0 sutures, with the knots tied in the anal lumen. Proponents of this widely described technique argue that it reduces the quantity of foreign body (knots) within the tissue and lowers the risk of infection. Concern about a foreign body probably applies to the use of catgut, which dissolves by proteolysis, rather than to newer synthetic material such as Vicryl or Dexon (polyglycolic acid), which dissolves by hydrolysis.
Subcuticular repair of anal epithelium using a transvaginal approach has also been described and could be equally effective if the terminal knots are secure.10
Sphincter muscles
Repair these muscles using 3-0 polydioxanone (PDS) dyed sutures. Compared with braided sutures, monofilament sutures are believed to lessen the risk of infection, although a randomized controlled trial revealed no difference in suture-related morbidity between Vicryl and PDS at 6 weeks postpartum.11 Complete absorption of PDS takes longer than with Vicryl, with 50% tensile strength lasting more than 3 months, compared with 3 weeks for Vicryl.11 To minimize suture migration, cut suture ends short and ensure that they are covered by the overlying superficial perineal muscles.
Internal anal sphincter. Repair the IAS separately from the EAS. Grasp the ends of the torn muscle using Allis forceps and perform an end-to-end repair with interrupted or mattress 3-0 PDS sutures (FIGURE 5). Overlapping repair can be technically difficult.
There is some evidence that repair of an isolated IAS defect benefits patients with established anal incontinence.
External anal sphincter. Because the EAS is normally under tonic contraction, it tends to retract when torn. Therefore, repair requires identification and grasping of the torn ends using Allis tissue forceps (FIGURE 6).
When the EAS is only partially torn (Grade 3a and some cases of Grade 3b), perform an end-to end repair using 2 or 3 mattress sutures, similar to repair of IAS injury, instead of hemostatic “figure of eight” sutures.
For a full-thickness tear (some cases of Grade 3b or 3c, or Grade 4), overlapping repair may be preferable in experienced hands. The EAS may need to be mobilized by dissecting it free of the ischioanal fat laterally using a pair of McIndoe scissors. The torn ends of the EAS can then be overlapped in “double-breasted” fashion (FIGURE 7) using PDS 3-0 sutures. Proper overlap is possible only when the full length of the torn ends is identified.
Overlapping the ends of the sphincter allows for greater surface area of contact between muscle. In contrast, end-to-end repair can be performed without identifying the full length of the EAS and may give rise to incomplete apposition. Fernando and colleagues demonstrated that, in experienced hands, early primary overlap repair carries a lower risk of fecal urgency and anal incontinence than does immediate primary end-to-end repair.12,13
FIGURE 5 End-to-end repair
Internal anal sphincter (I) repair using mattress sutures, demonstrated on the latex Sultan model, used for training (www.perineum.net) (E, external sphincter; A, anal epithelium). SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 6 Locating the external anal sphincter
The external sphincter (E), grasped with Allis forceps, is surrounded by the capsule (C) and lies medial to the ischioanal fat. SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 7 Overlapping sphincter repair
Repair of a fourth degree tear (demonstrated on the Sultan model) using the overlap repair technique on the external sphincter (E). The anal epithelium (A) and the internal sphincter (I) have also been repaired. SOURCE: Sultan AH, Thakar R2 (used with permission).
Perineal muscles
After repair of the sphincter, suture the perineal muscles to reconstruct the perineal body and provide support to the repaired anal sphincter. A short, deficient perineum would leave the anal sphincter more vulnerable to trauma during a subsequent vaginal delivery.
Next, suture the vaginal skin and approximate the perineal skin using Vicryl Rapide 2-0 subcuticular suture.
Examine, and document, the repair
Perform a rectal and vaginal examination to confirm adequate repair and ensure that no other tears have been missed—and that all tampons or swabs have been removed.
Make detailed notes of the findings and repair. A pro forma pictorial representation of the tears proves very useful when notes are reviewed following complications or during audit or litigation.
6. What does postoperative care entail?
Prophylactic antibiotics are common
No randomized trials have substantiated the benefits of intraoperative and postoperative antibiotics after repair of OASIS. Nevertheless, these drugs are commonly prescribed, especially after fourth- degree tears, because infection and wound breakdown could jeopardize the repair and lead to incontinence or fistula.10,14
We prescribe intravenous broad-spectrum antibiotics such as cefuroxime and metronidazole intraoperatively and continue the drugs orally for 5 days.
Bladder catheterization is recommended
Severe perineal discomfort, especially after instrumental delivery, is a known cause of urinary retention. Moreover, after administration of regional anesthesia, it can take up to 12 hours before bladder sensation returns.
We recommend insertion of a Foley catheter for approximately 24 hours, unless medical staff can ensure that spontaneous voiding occurs at least every 3 to 4 hours without bladder overdistension.
Pain may persist after severe injury
The degree of pain following perineal trauma is related to the extent of the injury. OASIS is frequently associated with other more extensive injuries such as paravaginal tears. In one study, 91% of women continued to complain of severe perineal pain 7 days after OASIS.15
In a systematic review, Hedayati and associates found rectal analgesia, such as diclofenac sodium, to be effective at reducing pain from perineal trauma within the first 24 hours after birth; they also found that women used less additional analgesia within the first 48 hours after birth.16 Diclofenac is almost completely bound to protein, so excretion in breast milk is negligible.17
In women who have undergone repair of a fourth-degree tear, administer oral diclofenac; suppositories may be uncomfortable, and there is a theoretical risk of poor healing associated with local anti-inflammatory agents.
Avoid codeine-based preparations because they may cause constipation and lead to excessive straining and disruption of the repair.
Recommend a stool softener
It is vital that constipation be avoided as the patient heals; passage of constipated stool or fecal impaction can disrupt the repair. We prescribe a stool softener (lactulose, 15 mL twice daily) for 10 to 14 days and have encountered no problem with bowel evacuation.18
We recommend that the patient telephone a healthcare provider 24 to 48 hours after hospital discharge to confirm that bowel evacuation has occurred. If it hasn’t, we add mineral oil, magnesium hydroxide, or another oral bowel stimulant to the stool softener and bulking agent.
Mahoney and colleagues conducted a randomized trial (n=105) of constipating versus laxative regimens and found the latter to be associated with earlier and less painful first bowel motion and earlier hospital discharge.19 Nineteen percent of women following the constipating regimen had troublesome constipation (two required hospitalization for fecal impaction), compared with 5% of women receiving a laxative. There were no significant differences in continence scores, anal manometry, and endoanal US findings.
Give the patient adequate information
Before the patient is discharged from the hospital, we give her a booklet that describes the implications of OASIS and explains when and where to seek help if symptoms of infection or incontinence develop. All women also complete a validated bowel-health and quality-of-life questionnaire regarding conditions prior to the delivery. We also recommend pelvic floor and anal sphincter exercises as soon as her discomfort resolves.
Perform a comprehensive follow-up exam
All women who sustain OASIS should be assessed by a senior obstetrician 6 to 8 weeks after delivery. In our practice, these women are seen in a dedicated perineal clinic.20 The clinic provides a supportive environment and increases the patient’s confidence in the team.21
At the clinic, each woman completes the same symptom questionnaire that she was given before hospital discharge. She then undergoes a genital examination in which the physician checks the degree of scarring, residual granulation tissue, and tenderness; ensures that the patient understands the circumstances surrounding the delivery and injury; and addresses any concerns. All women then undergo anal manometry and endoanal US (FIGURE 8). Each patient is encouraged to continue pelvic floor exercises. If she has minimal sphincter contractility, she may need electrical stimulation.
If a dedicated perineal clinic is unavailable, the patient should be given clear instructions, preferably in writing, before leaving the hospital. During the 6 weeks immediately after delivery, she should be instructed to look for signs of infection or wound dehiscence and to telephone the physician to report any increase in pain or swelling, rectal bleeding, or purulent discharge. Any incontinence of stool or flatus also should be reported.
FIGURE 8 Defect visible on US
Endoanal sonogram showing a defect in the external anal sphincter between 11 o’clock and 1 o’clock (between the yellow arrows) (S, subepithelium; E, external anal sphincter). SOURCE: Sultan AH, Thakar R2 (used with permission).
7. Is vaginal delivery advisable after OASIS?
No randomized trials have determined the most appropriate mode of delivery after a third- or fourth-degree tear. We base our counseling of the patient on a completed symptom questionnaire and findings from manometry and endoanal US (FIGURE 8). If vaginal delivery is contemplated, these tests should be performed during the current pregnancy unless they were abnormal at an earlier date. FIGURE 9 is a simple flow diagram from our unit that illustrates management of subsequent delivery after OASIS.
When determining the mode of delivery, thorough counseling and clear documentation of that counseling are extremely important.
FIGURE 9 How do you determine the mode of delivery after OASIS?
Vaginal delivery is possible unless anal sphincter function is impaired
One study found that when a large sonographic defect (more than one quadrant) is present, or the squeeze-pressure increment (above resting pressure) is less than 20 mm Hg, the risk of impaired continence after a subsequent delivery increases dramatically.22
Based on these findings, we conducted a prospective study that found no deterioration of sphincter function or increase in symptoms after vaginal delivery unless the patient had significant compromise of anal sphincter function before the pregnancy.23 Therefore, we encourage asymptomatic women who have minimal compromise of anal sphincter function to undergo vaginal delivery.
Routine episiotomy is not protective
There is no evidence that routine episiotomy prevents recurrent OASIS. If episiotomy is deemed to be necessary—e.g., for a thick inelastic or scarred perineum—mediolateral episiotomy is preferred.
High likelihood of success in some women
Women who have minimal compromise of anal sphincter function should be counseled that they have an 88% (in centers practicing midline episiotomy) to 95% (in centers practicing mediolateral episiotomy) chance of delivering without sustaining another OASIS.24,25 This should reassure them if they have misgivings about vaginal delivery.
Threshold for C-section is lower if additional risk factors are present
If traumatic delivery is anticipated, as in the presence of one or more additional risk factors (macrosomia, shoulder dystocia, prolonged labor, difficult instrumental delivery), cesarean section may be appropriate.
Consider emotional needs
Some women who have sustained OASIS may be scarred emotionally as well as physically and may find it difficult to cope with the thought of another vaginal delivery. These women deserve sympathy, psychological support, and consideration of their request for cesarean section.
When cesarean is a good idea
Women who have a minor degree of incontinence (e.g., fecal urgency or flatus incontinence) may be managed with dietary advice, constipating agents (loperamide or codeine phosphate), and physiotherapy or biofeedback. These women who have some degree of anal sphincter compromise but whose symptoms are controlled should be counseled that cesarean delivery is recommended (FIGURE 9).
Women who have sustained a previous third- or fourth-degree tear with subsequent severe incontinence should be offered secondary sphincter repair by a colorectal surgeon or urogynecologist with expertise in secondary sphincter repair. All subsequent deliveries by these women should be by cesarean section.
Some women with fecal incontinence may choose to complete their family before embarking on anal sphincter surgery. It remains unclear whether these women should be allowed a vaginal delivery, but it is likely that most damage has already occurred and that the risk of further injury is minimal and possibly insignificant. The benefit of cesarean delivery, if any, should be weighed against its risks for all subsequent pregnancies.
Women who have undergone a previous successful secondary sphincter repair for fecal incontinence should be delivered by cesarean delivery.9
Not all women fit neatly into one category
There are going to be women who do not entirely fit any of the categories described—such as those who have isolated internal sphincter defects or irritable bowel syndrome. Management of these women should be individualized, with the mode of delivery determined by mutual agreement after taking into account symptoms and clinical and other findings.
If there are no facilities for anal manometry and US, the physician should base management on symptoms and clinical evaluation. Asymptomatic women who do not have clinical evidence of sphincter compromise during anal tone assessment may be allowed to undergo vaginal delivery. All women who are symptomatic should be referred to a center with facilities for anorectal assessment to establish the ideal management and mode of delivery.
Pay attention to modifiable risk factors
In the case described at the beginning of this article, two risk factors could have been modified to minimize the patient’s risk of OASIS—namely, midline episiotomy and forceps delivery. In a quasirandomized study by Coats, involving 407 nulliparous women, which compared mediolateral and midline episiotomy (when episiotomy was necessary), tears into or through the anal sphincter occurred in 12% of women undergoing midline episiotomy and 2% of those undergoing mediolateral episiotomy.26
If operative vaginal delivery is required, vacuum extraction is preferred. In a meta-analysis of randomized studies, Thakar and Eason found that fewer women have anal sphincter trauma with vacuum delivery than with forceps.27 One anal sphincter tear is avoided for every 18 women delivered by vacuum extraction instead of forceps. A randomized trial conducted in the United Kingdom involving mediolateral episiotomy found severe vaginal laceration in 17% of forceps deliveries and 11% of vacuum deliveries.28 A randomized controlled trial in Canada involving midline episiotomy found third- or fourth-degree tears in 29% of forceps deliveries, versus 12% of vacuum deliveries.29
Q. What is the proper code for reporting an anal sphincter injury incurred in pregnancy?
A. That depends—on when the tear occurred, whether the patient is currently pregnant, and whether there were additional lacerations of the perineum.
ICD-9-CM offers four codes in this setting. Choose one, as follows:
- If you note an anal tear at the time of, or after, delivery but there is no perineal laceration, report 664.6×. This code takes a fifth digit: “1,” for the patient who has just delivered, or “4,” if you are treating the tear after she has been discharged.
- If the tear is noted in addition to a third-degree perineal tear, report 664.2× instead; fifth-digit choices for this code are also “1” and “4.”
- If the patient had an anal tear before delivery, from a prior pregnancy, code 654.8× [congenital or acquired abnormality of the vulva].
- Last, if you are treating the patient for an old anal tear and she is not pregnant at the moment, report 569.43 and add any additional codes that have resulted from the tear, such as fecal incontinence (787.6).
—Melanie Witt, RN, CPC-OGS, MA
The authors report no financial relationships relevant to this article.
CASE Large baby, extensive tear
A 28-year-old primigravida undergoes a forceps delivery with a midline episiotomy for failure to progress in the second stage of labor. At birth, the infant weighs 4 kg (8.8 lb), and the episiotomy extends to the anal verge. The resident who delivered the child is uncertain whether the anal sphincter is involved in the injury and asks a consultant to examine the perineum.
What should this examination entail?
The obstetrician is rarely culpable when a third- or fourth-degree obstetric anal sphincter injury (OASIS) occurs—but there is little excuse for letting one go undetected.
To minimize the risk of undiagnosed OASIS, a digital anorectal examination is warranted—before any suturing—in every woman who delivers vaginally. This practice can help you avoid missing isolated tears, such as “buttonhole” of the rectal mucosa, which can occur even when the anal sphincter remains intact (FIGURE 1), or a third- or fourth-degree tear that can sometimes be present behind apparently intact perineal skin (FIGURE 2).1
Clinical training of physicians and midwives also needs to improve.
Every labor room should have a protocol for management of anal sphincter injury2; this article describes detection, diagnosis, and management, focusing on seven critical questions.
Only a physician formally trained in primary anal sphincter repair (or under supervision) should repair OASIS.
FIGURE 1 Buttonhole tear
A buttonhole tear of the rectal mucosa (arrow) with an intact external anal sphincter (EAS) demonstrated during a digital rectal examination. SOURCE: Sultan AH3 (used with permission).
FIGURE 2 Injury obscured by intact skin
(A) Intact perineum on visual examination. (B) Anal sphincter trauma detected after rectal examination. SOURCE: Sultan AH, Kettle C1 (used with permission).
1. When (and how) should the torn perineum be examined?
The first requisite is informed consent for vaginal and rectal examination immediately after delivery. Also vital are adequate exposure of the perineum, good lighting, and, if necessary, sufficient analgesia to prevent pain-related restriction of the evaluation. It may be advisable to place the patient in the lithotomy position to improve exposure.
After visual examination of the perineum, part the labia and examine the vagina to establish the full extent of the tear. Always identify the apex of the vaginal laceration.
Next, perform a rectal examination to exclude injury to the anorectal mucosa and anal sphincter.3
Palpation is necessary to confirm OASIS
Insert the index finger into the anal canal and the thumb into the vagina and perform a pill-rolling motion to palpate the anal sphincter. If this technique is inconclusive, ask the woman to contract her anal sphincter with your fingers still in place. When the sphincter is disrupted, you feel a distinct gap anteriorly. If the perineal skin is intact, there may be an absence of puckering on the perianal skin over any underlying defect that may not be evident under regional or general anesthesia.
Because the external anal sphincter (EAS) is in a state of tonic contraction, the sphincter ends will retract when it is disrupted. These ends need to be grasped and retrieved at the time of repair.
Also identify the internal anal sphincter (IAS). It is a circular smooth muscle (FIGURE 3) that is paler in appearance (similar to the flesh of raw fish) than the striated EAS (similar to raw red meat).4 Under normal circumstances, the distal end of the IAS lies a few millimeters proximal to the distal end of the EAS (FIGURE 4). However, if the EAS is relaxed due to regional or general anesthesia, the distal end of the IAS will appear to be at a lower level. If the IAS or anal epithelium is torn, the EAS is, invariably, torn, too.
General or regional (spinal, epidural, caudal) anesthesia provides analgesia and muscle relaxation and enables proper evaluation of the full extent of the injury.
FIGURE 3 Grade 3b tear
Grade 3b tear with an intact internal anal sphincter (IAS). The external sphincter (EAS) is being grasped with Allis forceps. Note the difference in appearance of the paler IAS and darker EAS. SOURCE: Sultan AH, Kettle C1 (used with permission).
FIGURE 4 Classification of anal sphincter injury
First- and second-degree injuries are described below.
2. Is endoanal US helpful to detect OASIS?
Endoanal ultrasonography (US) to identify OASIS requires specific expertise, particularly in the immediate postpartum period, when the anal canal is lax (especially after an epidural). Ultimately, however, the diagnosis rests on clinical assessment and a rectal examination because, even if a defect is seen on US, it has to be clinically apparent to be repaired.
In a study by Faltin and colleagues, in which routine postpartum endoanal US was used as the gold standard for diagnosis of OASIS, five of 21 women had unnecessary intervention because the sonographic defect was not clinically visible despite exploration of the anal sphincter.5 As a result of this unnecessary exploration based on endoanal US, 20% of these women developed severe fecal incontinence. Therefore, we believe that OASIS is best detected clinically immediately after delivery, provided the physician performs a careful examination with palpation of the anal sphincter.6 In such a scenario, endoanal US is of limited value.
3. How is obstetric anal sphincter trauma classified?
To standardize the classification of perineal trauma, Sultan proposed the following system, which has been adopted by the Royal College of Obstetricians and Gynaecologists and internationally7-9:
First degree: Laceration of the vaginal epithelium or perineal skin only
Second degree: Involvement of the perineal muscles, but not the anal sphincter
Third degree: Disruption of the anal sphincter muscles (FIGURE 4):
- 3a: Less than 50% thickness of the external sphincter is torn
- 3b: More than 50% thickness of the external sphincter is torn
- 3c: Internal sphincter is also torn
Fourth degree: A third-degree anal tear with disruption of the anal epithelium (FIGURE 4).
If there is any ambiguity about grading of the injury, the higher grade should be selected. For example, if there is uncertainty between grades 3a and 3b, the injury should be classified as Grade 3b.
4. Is an operating room necessary?
OASIS should be repaired in the operating theater, where there is access to good lighting, appropriate equipment, and aseptic conditions. In our unit, we have a specially prepared instrument tray containing:
- a Weislander self-retaining retractor
- 4 Allis tissue forceps
- McIndoe scissors
- tooth forceps
- 4 artery forceps
- stitch scissors
- a needle holder.
In addition, deep retractors (e.g., Deavers) are useful when there are associated paravaginal tears.
5. What surgical technique is recommended?
Buttonhole injury
This type of injury can occur in the rectum without disrupting the anal sphincter or perineum. It is best repaired transvaginally using interrupted Vicryl (polyglactin) sutures.
To minimize the risk of persistent rectovaginal fistula, interpose a second layer of tissue between the rectum and vagina by approximating the rectovaginal fascia. A colostomy is rarely indicated unless a large tear extends above the pelvic floor or there is gross fecal contamination of the wound.
Fourth-degree tear
Repair torn anal epithelium with interrupted Vicryl 3-0 sutures, with the knots tied in the anal lumen. Proponents of this widely described technique argue that it reduces the quantity of foreign body (knots) within the tissue and lowers the risk of infection. Concern about a foreign body probably applies to the use of catgut, which dissolves by proteolysis, rather than to newer synthetic material such as Vicryl or Dexon (polyglycolic acid), which dissolves by hydrolysis.
Subcuticular repair of anal epithelium using a transvaginal approach has also been described and could be equally effective if the terminal knots are secure.10
Sphincter muscles
Repair these muscles using 3-0 polydioxanone (PDS) dyed sutures. Compared with braided sutures, monofilament sutures are believed to lessen the risk of infection, although a randomized controlled trial revealed no difference in suture-related morbidity between Vicryl and PDS at 6 weeks postpartum.11 Complete absorption of PDS takes longer than with Vicryl, with 50% tensile strength lasting more than 3 months, compared with 3 weeks for Vicryl.11 To minimize suture migration, cut suture ends short and ensure that they are covered by the overlying superficial perineal muscles.
Internal anal sphincter. Repair the IAS separately from the EAS. Grasp the ends of the torn muscle using Allis forceps and perform an end-to-end repair with interrupted or mattress 3-0 PDS sutures (FIGURE 5). Overlapping repair can be technically difficult.
There is some evidence that repair of an isolated IAS defect benefits patients with established anal incontinence.
External anal sphincter. Because the EAS is normally under tonic contraction, it tends to retract when torn. Therefore, repair requires identification and grasping of the torn ends using Allis tissue forceps (FIGURE 6).
When the EAS is only partially torn (Grade 3a and some cases of Grade 3b), perform an end-to end repair using 2 or 3 mattress sutures, similar to repair of IAS injury, instead of hemostatic “figure of eight” sutures.
For a full-thickness tear (some cases of Grade 3b or 3c, or Grade 4), overlapping repair may be preferable in experienced hands. The EAS may need to be mobilized by dissecting it free of the ischioanal fat laterally using a pair of McIndoe scissors. The torn ends of the EAS can then be overlapped in “double-breasted” fashion (FIGURE 7) using PDS 3-0 sutures. Proper overlap is possible only when the full length of the torn ends is identified.
Overlapping the ends of the sphincter allows for greater surface area of contact between muscle. In contrast, end-to-end repair can be performed without identifying the full length of the EAS and may give rise to incomplete apposition. Fernando and colleagues demonstrated that, in experienced hands, early primary overlap repair carries a lower risk of fecal urgency and anal incontinence than does immediate primary end-to-end repair.12,13
FIGURE 5 End-to-end repair
Internal anal sphincter (I) repair using mattress sutures, demonstrated on the latex Sultan model, used for training (www.perineum.net) (E, external sphincter; A, anal epithelium). SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 6 Locating the external anal sphincter
The external sphincter (E), grasped with Allis forceps, is surrounded by the capsule (C) and lies medial to the ischioanal fat. SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 7 Overlapping sphincter repair
Repair of a fourth degree tear (demonstrated on the Sultan model) using the overlap repair technique on the external sphincter (E). The anal epithelium (A) and the internal sphincter (I) have also been repaired. SOURCE: Sultan AH, Thakar R2 (used with permission).
Perineal muscles
After repair of the sphincter, suture the perineal muscles to reconstruct the perineal body and provide support to the repaired anal sphincter. A short, deficient perineum would leave the anal sphincter more vulnerable to trauma during a subsequent vaginal delivery.
Next, suture the vaginal skin and approximate the perineal skin using Vicryl Rapide 2-0 subcuticular suture.
Examine, and document, the repair
Perform a rectal and vaginal examination to confirm adequate repair and ensure that no other tears have been missed—and that all tampons or swabs have been removed.
Make detailed notes of the findings and repair. A pro forma pictorial representation of the tears proves very useful when notes are reviewed following complications or during audit or litigation.
6. What does postoperative care entail?
Prophylactic antibiotics are common
No randomized trials have substantiated the benefits of intraoperative and postoperative antibiotics after repair of OASIS. Nevertheless, these drugs are commonly prescribed, especially after fourth- degree tears, because infection and wound breakdown could jeopardize the repair and lead to incontinence or fistula.10,14
We prescribe intravenous broad-spectrum antibiotics such as cefuroxime and metronidazole intraoperatively and continue the drugs orally for 5 days.
Bladder catheterization is recommended
Severe perineal discomfort, especially after instrumental delivery, is a known cause of urinary retention. Moreover, after administration of regional anesthesia, it can take up to 12 hours before bladder sensation returns.
We recommend insertion of a Foley catheter for approximately 24 hours, unless medical staff can ensure that spontaneous voiding occurs at least every 3 to 4 hours without bladder overdistension.
Pain may persist after severe injury
The degree of pain following perineal trauma is related to the extent of the injury. OASIS is frequently associated with other more extensive injuries such as paravaginal tears. In one study, 91% of women continued to complain of severe perineal pain 7 days after OASIS.15
In a systematic review, Hedayati and associates found rectal analgesia, such as diclofenac sodium, to be effective at reducing pain from perineal trauma within the first 24 hours after birth; they also found that women used less additional analgesia within the first 48 hours after birth.16 Diclofenac is almost completely bound to protein, so excretion in breast milk is negligible.17
In women who have undergone repair of a fourth-degree tear, administer oral diclofenac; suppositories may be uncomfortable, and there is a theoretical risk of poor healing associated with local anti-inflammatory agents.
Avoid codeine-based preparations because they may cause constipation and lead to excessive straining and disruption of the repair.
Recommend a stool softener
It is vital that constipation be avoided as the patient heals; passage of constipated stool or fecal impaction can disrupt the repair. We prescribe a stool softener (lactulose, 15 mL twice daily) for 10 to 14 days and have encountered no problem with bowel evacuation.18
We recommend that the patient telephone a healthcare provider 24 to 48 hours after hospital discharge to confirm that bowel evacuation has occurred. If it hasn’t, we add mineral oil, magnesium hydroxide, or another oral bowel stimulant to the stool softener and bulking agent.
Mahoney and colleagues conducted a randomized trial (n=105) of constipating versus laxative regimens and found the latter to be associated with earlier and less painful first bowel motion and earlier hospital discharge.19 Nineteen percent of women following the constipating regimen had troublesome constipation (two required hospitalization for fecal impaction), compared with 5% of women receiving a laxative. There were no significant differences in continence scores, anal manometry, and endoanal US findings.
Give the patient adequate information
Before the patient is discharged from the hospital, we give her a booklet that describes the implications of OASIS and explains when and where to seek help if symptoms of infection or incontinence develop. All women also complete a validated bowel-health and quality-of-life questionnaire regarding conditions prior to the delivery. We also recommend pelvic floor and anal sphincter exercises as soon as her discomfort resolves.
Perform a comprehensive follow-up exam
All women who sustain OASIS should be assessed by a senior obstetrician 6 to 8 weeks after delivery. In our practice, these women are seen in a dedicated perineal clinic.20 The clinic provides a supportive environment and increases the patient’s confidence in the team.21
At the clinic, each woman completes the same symptom questionnaire that she was given before hospital discharge. She then undergoes a genital examination in which the physician checks the degree of scarring, residual granulation tissue, and tenderness; ensures that the patient understands the circumstances surrounding the delivery and injury; and addresses any concerns. All women then undergo anal manometry and endoanal US (FIGURE 8). Each patient is encouraged to continue pelvic floor exercises. If she has minimal sphincter contractility, she may need electrical stimulation.
If a dedicated perineal clinic is unavailable, the patient should be given clear instructions, preferably in writing, before leaving the hospital. During the 6 weeks immediately after delivery, she should be instructed to look for signs of infection or wound dehiscence and to telephone the physician to report any increase in pain or swelling, rectal bleeding, or purulent discharge. Any incontinence of stool or flatus also should be reported.
FIGURE 8 Defect visible on US
Endoanal sonogram showing a defect in the external anal sphincter between 11 o’clock and 1 o’clock (between the yellow arrows) (S, subepithelium; E, external anal sphincter). SOURCE: Sultan AH, Thakar R2 (used with permission).
7. Is vaginal delivery advisable after OASIS?
No randomized trials have determined the most appropriate mode of delivery after a third- or fourth-degree tear. We base our counseling of the patient on a completed symptom questionnaire and findings from manometry and endoanal US (FIGURE 8). If vaginal delivery is contemplated, these tests should be performed during the current pregnancy unless they were abnormal at an earlier date. FIGURE 9 is a simple flow diagram from our unit that illustrates management of subsequent delivery after OASIS.
When determining the mode of delivery, thorough counseling and clear documentation of that counseling are extremely important.
FIGURE 9 How do you determine the mode of delivery after OASIS?
Vaginal delivery is possible unless anal sphincter function is impaired
One study found that when a large sonographic defect (more than one quadrant) is present, or the squeeze-pressure increment (above resting pressure) is less than 20 mm Hg, the risk of impaired continence after a subsequent delivery increases dramatically.22
Based on these findings, we conducted a prospective study that found no deterioration of sphincter function or increase in symptoms after vaginal delivery unless the patient had significant compromise of anal sphincter function before the pregnancy.23 Therefore, we encourage asymptomatic women who have minimal compromise of anal sphincter function to undergo vaginal delivery.
Routine episiotomy is not protective
There is no evidence that routine episiotomy prevents recurrent OASIS. If episiotomy is deemed to be necessary—e.g., for a thick inelastic or scarred perineum—mediolateral episiotomy is preferred.
High likelihood of success in some women
Women who have minimal compromise of anal sphincter function should be counseled that they have an 88% (in centers practicing midline episiotomy) to 95% (in centers practicing mediolateral episiotomy) chance of delivering without sustaining another OASIS.24,25 This should reassure them if they have misgivings about vaginal delivery.
Threshold for C-section is lower if additional risk factors are present
If traumatic delivery is anticipated, as in the presence of one or more additional risk factors (macrosomia, shoulder dystocia, prolonged labor, difficult instrumental delivery), cesarean section may be appropriate.
Consider emotional needs
Some women who have sustained OASIS may be scarred emotionally as well as physically and may find it difficult to cope with the thought of another vaginal delivery. These women deserve sympathy, psychological support, and consideration of their request for cesarean section.
When cesarean is a good idea
Women who have a minor degree of incontinence (e.g., fecal urgency or flatus incontinence) may be managed with dietary advice, constipating agents (loperamide or codeine phosphate), and physiotherapy or biofeedback. These women who have some degree of anal sphincter compromise but whose symptoms are controlled should be counseled that cesarean delivery is recommended (FIGURE 9).
Women who have sustained a previous third- or fourth-degree tear with subsequent severe incontinence should be offered secondary sphincter repair by a colorectal surgeon or urogynecologist with expertise in secondary sphincter repair. All subsequent deliveries by these women should be by cesarean section.
Some women with fecal incontinence may choose to complete their family before embarking on anal sphincter surgery. It remains unclear whether these women should be allowed a vaginal delivery, but it is likely that most damage has already occurred and that the risk of further injury is minimal and possibly insignificant. The benefit of cesarean delivery, if any, should be weighed against its risks for all subsequent pregnancies.
Women who have undergone a previous successful secondary sphincter repair for fecal incontinence should be delivered by cesarean delivery.9
Not all women fit neatly into one category
There are going to be women who do not entirely fit any of the categories described—such as those who have isolated internal sphincter defects or irritable bowel syndrome. Management of these women should be individualized, with the mode of delivery determined by mutual agreement after taking into account symptoms and clinical and other findings.
If there are no facilities for anal manometry and US, the physician should base management on symptoms and clinical evaluation. Asymptomatic women who do not have clinical evidence of sphincter compromise during anal tone assessment may be allowed to undergo vaginal delivery. All women who are symptomatic should be referred to a center with facilities for anorectal assessment to establish the ideal management and mode of delivery.
Pay attention to modifiable risk factors
In the case described at the beginning of this article, two risk factors could have been modified to minimize the patient’s risk of OASIS—namely, midline episiotomy and forceps delivery. In a quasirandomized study by Coats, involving 407 nulliparous women, which compared mediolateral and midline episiotomy (when episiotomy was necessary), tears into or through the anal sphincter occurred in 12% of women undergoing midline episiotomy and 2% of those undergoing mediolateral episiotomy.26
If operative vaginal delivery is required, vacuum extraction is preferred. In a meta-analysis of randomized studies, Thakar and Eason found that fewer women have anal sphincter trauma with vacuum delivery than with forceps.27 One anal sphincter tear is avoided for every 18 women delivered by vacuum extraction instead of forceps. A randomized trial conducted in the United Kingdom involving mediolateral episiotomy found severe vaginal laceration in 17% of forceps deliveries and 11% of vacuum deliveries.28 A randomized controlled trial in Canada involving midline episiotomy found third- or fourth-degree tears in 29% of forceps deliveries, versus 12% of vacuum deliveries.29
Q. What is the proper code for reporting an anal sphincter injury incurred in pregnancy?
A. That depends—on when the tear occurred, whether the patient is currently pregnant, and whether there were additional lacerations of the perineum.
ICD-9-CM offers four codes in this setting. Choose one, as follows:
- If you note an anal tear at the time of, or after, delivery but there is no perineal laceration, report 664.6×. This code takes a fifth digit: “1,” for the patient who has just delivered, or “4,” if you are treating the tear after she has been discharged.
- If the tear is noted in addition to a third-degree perineal tear, report 664.2× instead; fifth-digit choices for this code are also “1” and “4.”
- If the patient had an anal tear before delivery, from a prior pregnancy, code 654.8× [congenital or acquired abnormality of the vulva].
- Last, if you are treating the patient for an old anal tear and she is not pregnant at the moment, report 569.43 and add any additional codes that have resulted from the tear, such as fecal incontinence (787.6).
—Melanie Witt, RN, CPC-OGS, MA
1. Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:13-19.
2. Sultan AH, Thakar R. Third and fourth degree tears. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:33-51.
3. Sultan AH. Primary repair of obstetric anal sphincter injury. In: Staskin DR, Cardozo L, ed. Textbook of Female Urology and Urogynaecology. London: ISIS Medical Media; 2006.
4. Thakar R, Fenner DE. Anatomy of the perineum and the anal sphincter. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:1-12.
5. Faltin DL, Boulvain M, Floris LA, Irion O. Diagnosis of anal sphincter tears to prevent fecal incontinence: a randomized controlled trial. Obstet Gynecol. 2005;106:6-13.
6. Andrews V, Thakar R, Sultan AH. Occult anal sphincter injuries—myth or reality. Br J Obstet Gynaecol. 2006;113:195-200.
7. Sultan AH. Obstetric perineal injury and anal incontinence. Clin Risk. 1999;5:193-196.
8. Royal College of Obstetricians and Gynaecologists. Management of third and fourth degree perineal tears following vaginal delivery. Guideline 29. London: RCOG Press; 2001.
9. Norton C, Christensen J, Butler U, et al. Anal Incontinence. 2nd ed. Plymouth: Health Publication Ltd; 2005:985-1044.
10. Sultan AH, Thakar R. Lower genital tract and anal sphincter trauma. Best Pract Res Clin Obstet Gynaecol. 2002;16:99-116.
11. Williams A, Adams EJ, Tincello DG, Alfirevic Z, Walkinshaw SA, Richmond DH. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
12. Fernando RJ, Sultan AH, Kettle C, Radley S, Jones P, O’Brien PMS. Repair techniques for obstetric anal sphincter injuries. A randomized controlled trial. Obstet Gynecol. 2006;107:1261-1268.
13. Fernando R, Sultan AH, Kettle C, Thakar R, Radley S. Methods of repair for obstetric anal sphincter injury. Cochrane Database Syst Rev. 2006;3:CD002866.-
14. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review and national practice survey. BMC Health Serv Res. 2002;2:9.-
15. MacArthur AJ, MacArthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. Am J Obstet Gynecol. 2004;191:1199-1204.
16. Hedayati H, Parsons J, Crowther CA. Rectal analgesia for pain from perineal trauma following childbirth. Cochrane Database Syst Rev. 2003;(3):CD003931.-
17. Kettle C, Hills RK, Jones P, Darby L, Gray R, Johanson R. Continuous versus interrupted perineal repair with standard or rapidly absorbed sutures after spontaneous vaginal birth: a randomised controlled trial. Lancet. 2002;359:2217-2223.
18. Sultan AH, Monga AK, Kumar D, Stanton SL. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
19. Mahony R, Behan M, O’Herlihy C, O’Connell PR. Randomized, clinical trial of bowel confinement vs. laxative use after primary repair of a third-degree obstetric anal sphincter tear. Dis Colon Rectum. 2004;47:12-17.
20. Thakar R, Sultan A. Postpartum problems and the role of a perineal clinic. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:65-79.
21. Williams A, Lavender T, Richmond DH, Tincello DG. Women’s experiences after a third-degree obstetric anal sphincter tear: a qualitative study. Birth. 2005;32:129-136.
22. Fynes M, Donnelly V, Behan M, O’Connell PR, O’Herlihy C. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet. 1999;354:983-986.
23. Scheer I, Thakar R, Sultan A. Should women who sustained obstetric anal sphincter injuries be allowed a vaginal delivery? Neurourol Urodynam. 2006;25:512-513.
24. Peleg D, Kennedy CM, Merrill D, Zlatnik FJ. Risk of repetition of a severe perineal laceration. Obstet Gynecol. 1999;93:1021-1024.
25. Harkin R, Fitzpatrick M, O’Connell PR, O’Herlihy C. Anal sphincter disruption at vaginal delivery: is recurrence predictable? Eur J Obstet Gynaecol Reprod Biol. 2003;109:149-152.
26. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
27. Thakar R, Eason E. Prevention of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:52-64.
28. Johanson RB, Rice C, Doyle M. A randomised prospective study comparing the new vacuum extractor policy with forceps delivery. Br J Obstet Gynaecol. 1993;100:524-530.
29. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol. 1996;175:1325-1330.
1. Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:13-19.
2. Sultan AH, Thakar R. Third and fourth degree tears. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:33-51.
3. Sultan AH. Primary repair of obstetric anal sphincter injury. In: Staskin DR, Cardozo L, ed. Textbook of Female Urology and Urogynaecology. London: ISIS Medical Media; 2006.
4. Thakar R, Fenner DE. Anatomy of the perineum and the anal sphincter. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:1-12.
5. Faltin DL, Boulvain M, Floris LA, Irion O. Diagnosis of anal sphincter tears to prevent fecal incontinence: a randomized controlled trial. Obstet Gynecol. 2005;106:6-13.
6. Andrews V, Thakar R, Sultan AH. Occult anal sphincter injuries—myth or reality. Br J Obstet Gynaecol. 2006;113:195-200.
7. Sultan AH. Obstetric perineal injury and anal incontinence. Clin Risk. 1999;5:193-196.
8. Royal College of Obstetricians and Gynaecologists. Management of third and fourth degree perineal tears following vaginal delivery. Guideline 29. London: RCOG Press; 2001.
9. Norton C, Christensen J, Butler U, et al. Anal Incontinence. 2nd ed. Plymouth: Health Publication Ltd; 2005:985-1044.
10. Sultan AH, Thakar R. Lower genital tract and anal sphincter trauma. Best Pract Res Clin Obstet Gynaecol. 2002;16:99-116.
11. Williams A, Adams EJ, Tincello DG, Alfirevic Z, Walkinshaw SA, Richmond DH. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
12. Fernando RJ, Sultan AH, Kettle C, Radley S, Jones P, O’Brien PMS. Repair techniques for obstetric anal sphincter injuries. A randomized controlled trial. Obstet Gynecol. 2006;107:1261-1268.
13. Fernando R, Sultan AH, Kettle C, Thakar R, Radley S. Methods of repair for obstetric anal sphincter injury. Cochrane Database Syst Rev. 2006;3:CD002866.-
14. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review and national practice survey. BMC Health Serv Res. 2002;2:9.-
15. MacArthur AJ, MacArthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. Am J Obstet Gynecol. 2004;191:1199-1204.
16. Hedayati H, Parsons J, Crowther CA. Rectal analgesia for pain from perineal trauma following childbirth. Cochrane Database Syst Rev. 2003;(3):CD003931.-
17. Kettle C, Hills RK, Jones P, Darby L, Gray R, Johanson R. Continuous versus interrupted perineal repair with standard or rapidly absorbed sutures after spontaneous vaginal birth: a randomised controlled trial. Lancet. 2002;359:2217-2223.
18. Sultan AH, Monga AK, Kumar D, Stanton SL. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
19. Mahony R, Behan M, O’Herlihy C, O’Connell PR. Randomized, clinical trial of bowel confinement vs. laxative use after primary repair of a third-degree obstetric anal sphincter tear. Dis Colon Rectum. 2004;47:12-17.
20. Thakar R, Sultan A. Postpartum problems and the role of a perineal clinic. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:65-79.
21. Williams A, Lavender T, Richmond DH, Tincello DG. Women’s experiences after a third-degree obstetric anal sphincter tear: a qualitative study. Birth. 2005;32:129-136.
22. Fynes M, Donnelly V, Behan M, O’Connell PR, O’Herlihy C. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet. 1999;354:983-986.
23. Scheer I, Thakar R, Sultan A. Should women who sustained obstetric anal sphincter injuries be allowed a vaginal delivery? Neurourol Urodynam. 2006;25:512-513.
24. Peleg D, Kennedy CM, Merrill D, Zlatnik FJ. Risk of repetition of a severe perineal laceration. Obstet Gynecol. 1999;93:1021-1024.
25. Harkin R, Fitzpatrick M, O’Connell PR, O’Herlihy C. Anal sphincter disruption at vaginal delivery: is recurrence predictable? Eur J Obstet Gynaecol Reprod Biol. 2003;109:149-152.
26. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
27. Thakar R, Eason E. Prevention of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:52-64.
28. Johanson RB, Rice C, Doyle M. A randomised prospective study comparing the new vacuum extractor policy with forceps delivery. Br J Obstet Gynaecol. 1993;100:524-530.
29. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol. 1996;175:1325-1330.
Genetic Testing for DVT Risk Still Controversial
'[Referring physicians] say, “Does this patient have Factor V Leiden?” I talk them out of it.' DR. WEITZ
ATLANTA — An inherited mutation, Factor V Leiden, puts people at risk for life-threatening blood clots. Carriers can be identified with a simple blood test, so why not use it?
“Genetic testing is highly controversial. This is really not ready for prime time yet,” Dr. David Ginsberg advised during a special session on venous thromboembolism at the annual meeting of the American Society of Hematology.
Factor V Leiden has been associated with risk of miscarriage and possibly other complications, but most women with the mutation have normal pregnancies, he noted. Likewise, while Factor V Leiden has been linked to increased risk of venous thromboembolism in women taking oral contraceptives, they are not contraindicated.
The central issue for Dr. Ginsberg was not whether Factor V Leiden is a risk factor, but what that means and what, if anything, would be done differently when treating patients who test positive.
About 5% of people of European origin have Factor V Leiden, according to Dr. Ginsberg, the James V. Neel Distinguished University Professor of Internal Medicine and Human Genetics at the University of Michigan, Ann Arbor. It “clearly increases” relative risk, compared with no mutation in Factor V, but most people with the mutation do not develop blood clots.
“Nature would not allow this to be in 5% of the population, if it was really all that bad,” he said, speculating that Factor V Leiden might confer a benefit in some patients who develop venous thromboembolism. “Factor V Leiden might not always be 'bad' for you,” he said.
Dr. Ginsberg cited two human studies that found deep venous thrombosis (DVT) was less likely to progress to pulmonary embolism in people with Factor V Leiden. Also, he noted that in the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial evaluating recombinant human activated protein C (rhAPC), or drotrecogin alfa activated, in patients with severe sepsis, 28-day all-cause mortality was lower in 65 patients with Factor V Leiden.
Based on current knowledge, a positive test for Factor V Leiden would not change any treatments, Dr. Ginsberg continued. Patients still would receive heparin or warfarin for acute thrombosis and be given warfarin for 31/2 months as prophylaxis after a first event. If patients have recurrent thromboses, warfarin prophylaxis would be extended, possibly becoming a lifelong intervention.
In the future, he suggested Factor V Leiden testing might be useful when choosing primary therapy and duration of therapy for thrombosis. Likewise, the presence of Factor V Leiden might indicate the need for thrombosis prophylaxis during pregnancy, postoperatively, and after a first thrombotic event. And women may be screened for Factor V Leiden before oral contraceptives are prescribed.
But none of this is done now, and he said more data are needed to support genotype-specific prophylaxis or therapy. Meanwhile, a positive finding could be cause for anxiety and hypervigilance. “My plea is that, until there is clear-cut evidence, testing should be used judiciously,” he said.
In an interview after the session, Dr. Jeffrey Weitz, another speaker at the special session, said he “very much agreed” with Dr. Ginsberg and does not test for Factor V Leiden unless a patient insists. Most patients requesting the test have thrombosis and are referred by primary care physicians, according to Dr. Weitz of Hamilton Civic Hospitals Research Centre in Ontario. “They say, 'Does this patient have Factor V Leiden?' “he said. “I talk them out of it.”
For another speaker, Melanie Bloom, a national patient spokeswoman for the Coalition to Prevent Deep Vein Thrombosis, the test is not so easy to rule out, however. Her husband, David Bloom, died of a DVT that led to pulmonary embolism while covering the Iraq war for NBC news. After his death at the age of 39, the family became aware that he had been at high risk for DVT.
When their three young daughters reach the age where pregnancy and contraception are an issue, Mrs. Bloom said she would want to know whether they have an inherited risk. “David's life could have been saved with awareness and knowledge,” she said. “Less than a quarter of physicians educate high-risk patients about DVT.”
'[Referring physicians] say, “Does this patient have Factor V Leiden?” I talk them out of it.' DR. WEITZ
ATLANTA — An inherited mutation, Factor V Leiden, puts people at risk for life-threatening blood clots. Carriers can be identified with a simple blood test, so why not use it?
“Genetic testing is highly controversial. This is really not ready for prime time yet,” Dr. David Ginsberg advised during a special session on venous thromboembolism at the annual meeting of the American Society of Hematology.
Factor V Leiden has been associated with risk of miscarriage and possibly other complications, but most women with the mutation have normal pregnancies, he noted. Likewise, while Factor V Leiden has been linked to increased risk of venous thromboembolism in women taking oral contraceptives, they are not contraindicated.
The central issue for Dr. Ginsberg was not whether Factor V Leiden is a risk factor, but what that means and what, if anything, would be done differently when treating patients who test positive.
About 5% of people of European origin have Factor V Leiden, according to Dr. Ginsberg, the James V. Neel Distinguished University Professor of Internal Medicine and Human Genetics at the University of Michigan, Ann Arbor. It “clearly increases” relative risk, compared with no mutation in Factor V, but most people with the mutation do not develop blood clots.
“Nature would not allow this to be in 5% of the population, if it was really all that bad,” he said, speculating that Factor V Leiden might confer a benefit in some patients who develop venous thromboembolism. “Factor V Leiden might not always be 'bad' for you,” he said.
Dr. Ginsberg cited two human studies that found deep venous thrombosis (DVT) was less likely to progress to pulmonary embolism in people with Factor V Leiden. Also, he noted that in the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial evaluating recombinant human activated protein C (rhAPC), or drotrecogin alfa activated, in patients with severe sepsis, 28-day all-cause mortality was lower in 65 patients with Factor V Leiden.
Based on current knowledge, a positive test for Factor V Leiden would not change any treatments, Dr. Ginsberg continued. Patients still would receive heparin or warfarin for acute thrombosis and be given warfarin for 31/2 months as prophylaxis after a first event. If patients have recurrent thromboses, warfarin prophylaxis would be extended, possibly becoming a lifelong intervention.
In the future, he suggested Factor V Leiden testing might be useful when choosing primary therapy and duration of therapy for thrombosis. Likewise, the presence of Factor V Leiden might indicate the need for thrombosis prophylaxis during pregnancy, postoperatively, and after a first thrombotic event. And women may be screened for Factor V Leiden before oral contraceptives are prescribed.
But none of this is done now, and he said more data are needed to support genotype-specific prophylaxis or therapy. Meanwhile, a positive finding could be cause for anxiety and hypervigilance. “My plea is that, until there is clear-cut evidence, testing should be used judiciously,” he said.
In an interview after the session, Dr. Jeffrey Weitz, another speaker at the special session, said he “very much agreed” with Dr. Ginsberg and does not test for Factor V Leiden unless a patient insists. Most patients requesting the test have thrombosis and are referred by primary care physicians, according to Dr. Weitz of Hamilton Civic Hospitals Research Centre in Ontario. “They say, 'Does this patient have Factor V Leiden?' “he said. “I talk them out of it.”
For another speaker, Melanie Bloom, a national patient spokeswoman for the Coalition to Prevent Deep Vein Thrombosis, the test is not so easy to rule out, however. Her husband, David Bloom, died of a DVT that led to pulmonary embolism while covering the Iraq war for NBC news. After his death at the age of 39, the family became aware that he had been at high risk for DVT.
When their three young daughters reach the age where pregnancy and contraception are an issue, Mrs. Bloom said she would want to know whether they have an inherited risk. “David's life could have been saved with awareness and knowledge,” she said. “Less than a quarter of physicians educate high-risk patients about DVT.”
'[Referring physicians] say, “Does this patient have Factor V Leiden?” I talk them out of it.' DR. WEITZ
ATLANTA — An inherited mutation, Factor V Leiden, puts people at risk for life-threatening blood clots. Carriers can be identified with a simple blood test, so why not use it?
“Genetic testing is highly controversial. This is really not ready for prime time yet,” Dr. David Ginsberg advised during a special session on venous thromboembolism at the annual meeting of the American Society of Hematology.
Factor V Leiden has been associated with risk of miscarriage and possibly other complications, but most women with the mutation have normal pregnancies, he noted. Likewise, while Factor V Leiden has been linked to increased risk of venous thromboembolism in women taking oral contraceptives, they are not contraindicated.
The central issue for Dr. Ginsberg was not whether Factor V Leiden is a risk factor, but what that means and what, if anything, would be done differently when treating patients who test positive.
About 5% of people of European origin have Factor V Leiden, according to Dr. Ginsberg, the James V. Neel Distinguished University Professor of Internal Medicine and Human Genetics at the University of Michigan, Ann Arbor. It “clearly increases” relative risk, compared with no mutation in Factor V, but most people with the mutation do not develop blood clots.
“Nature would not allow this to be in 5% of the population, if it was really all that bad,” he said, speculating that Factor V Leiden might confer a benefit in some patients who develop venous thromboembolism. “Factor V Leiden might not always be 'bad' for you,” he said.
Dr. Ginsberg cited two human studies that found deep venous thrombosis (DVT) was less likely to progress to pulmonary embolism in people with Factor V Leiden. Also, he noted that in the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial evaluating recombinant human activated protein C (rhAPC), or drotrecogin alfa activated, in patients with severe sepsis, 28-day all-cause mortality was lower in 65 patients with Factor V Leiden.
Based on current knowledge, a positive test for Factor V Leiden would not change any treatments, Dr. Ginsberg continued. Patients still would receive heparin or warfarin for acute thrombosis and be given warfarin for 31/2 months as prophylaxis after a first event. If patients have recurrent thromboses, warfarin prophylaxis would be extended, possibly becoming a lifelong intervention.
In the future, he suggested Factor V Leiden testing might be useful when choosing primary therapy and duration of therapy for thrombosis. Likewise, the presence of Factor V Leiden might indicate the need for thrombosis prophylaxis during pregnancy, postoperatively, and after a first thrombotic event. And women may be screened for Factor V Leiden before oral contraceptives are prescribed.
But none of this is done now, and he said more data are needed to support genotype-specific prophylaxis or therapy. Meanwhile, a positive finding could be cause for anxiety and hypervigilance. “My plea is that, until there is clear-cut evidence, testing should be used judiciously,” he said.
In an interview after the session, Dr. Jeffrey Weitz, another speaker at the special session, said he “very much agreed” with Dr. Ginsberg and does not test for Factor V Leiden unless a patient insists. Most patients requesting the test have thrombosis and are referred by primary care physicians, according to Dr. Weitz of Hamilton Civic Hospitals Research Centre in Ontario. “They say, 'Does this patient have Factor V Leiden?' “he said. “I talk them out of it.”
For another speaker, Melanie Bloom, a national patient spokeswoman for the Coalition to Prevent Deep Vein Thrombosis, the test is not so easy to rule out, however. Her husband, David Bloom, died of a DVT that led to pulmonary embolism while covering the Iraq war for NBC news. After his death at the age of 39, the family became aware that he had been at high risk for DVT.
When their three young daughters reach the age where pregnancy and contraception are an issue, Mrs. Bloom said she would want to know whether they have an inherited risk. “David's life could have been saved with awareness and knowledge,” she said. “Less than a quarter of physicians educate high-risk patients about DVT.”