User login
For MD-IQ on Family Practice News, but a regular topic for Rheumatology News
Turmeric-, frankincense-derived supplement shows OA benefit
A combination of curcumin extracted from the turmeric rhizome and boswellic acid extracted from Indian frankincense root beat placebo for reducing pain-related symptoms from knee osteoarthritis in a 12-week clinical trial from Armenia with 201 patients 40-70 years old.
The combination (Curamin) also beat a standalone curcumin preparation (CuraMed), according to a report in BMC Complementary and Alternative Medicine.
It appears that combining the two “increases the efficacy of treatment of OA presumably due to synergistic effects of curcumin and boswellic acid”; it’s also possible that boswellic acid increases curcumin bioavailability, said investigators led by Armine Haroyan, PhD, head of rheumatology at Erebuni Medical Center in Yerevan, Armenia.
The subjects were randomized evenly to the combination, curcumin alone, or placebo, all in 500-mg capsules taken three times daily. They had been diagnosed with degenerative hypertrophic OA of knee bone joints.
At the end of 12 weeks, patients on the combination outperformed those on placebo in physical performance tests and joint pain scores; curcumin outperformed placebo in only a few of the physical tests.
For instance, patients on the combination were a mean of 2.03 seconds quicker than baseline in a stair-climbing exercise by the end of the study, versus 0.22 seconds in the placebo group and 1.66 seconds in the curcumin group. Combination patients had a mean 7.38-point improvement on the Western Ontario and McMaster Universities Osteoarthritis Index, versus 2.26 points in the placebo arm and 6.34 points in the curcumin group. The differences versus placebo were statistically significant for the combination, but not for stand-alone curcumin.
The treatments were well tolerated, with only a few patients in each arm reporting nausea, gastroesophageal reflux, and similar problems.
The work was funded in part by EuroPharma USA, maker of the supplements. The authors said they had no competing interests.
SOURCE: Haroyan A et al. BMC Complement Altern Med. 2018 Jan 9;18:7. doi: 10.1186/s12906-017-2062-z
A combination of curcumin extracted from the turmeric rhizome and boswellic acid extracted from Indian frankincense root beat placebo for reducing pain-related symptoms from knee osteoarthritis in a 12-week clinical trial from Armenia with 201 patients 40-70 years old.
The combination (Curamin) also beat a standalone curcumin preparation (CuraMed), according to a report in BMC Complementary and Alternative Medicine.
It appears that combining the two “increases the efficacy of treatment of OA presumably due to synergistic effects of curcumin and boswellic acid”; it’s also possible that boswellic acid increases curcumin bioavailability, said investigators led by Armine Haroyan, PhD, head of rheumatology at Erebuni Medical Center in Yerevan, Armenia.
The subjects were randomized evenly to the combination, curcumin alone, or placebo, all in 500-mg capsules taken three times daily. They had been diagnosed with degenerative hypertrophic OA of knee bone joints.
At the end of 12 weeks, patients on the combination outperformed those on placebo in physical performance tests and joint pain scores; curcumin outperformed placebo in only a few of the physical tests.
For instance, patients on the combination were a mean of 2.03 seconds quicker than baseline in a stair-climbing exercise by the end of the study, versus 0.22 seconds in the placebo group and 1.66 seconds in the curcumin group. Combination patients had a mean 7.38-point improvement on the Western Ontario and McMaster Universities Osteoarthritis Index, versus 2.26 points in the placebo arm and 6.34 points in the curcumin group. The differences versus placebo were statistically significant for the combination, but not for stand-alone curcumin.
The treatments were well tolerated, with only a few patients in each arm reporting nausea, gastroesophageal reflux, and similar problems.
The work was funded in part by EuroPharma USA, maker of the supplements. The authors said they had no competing interests.
SOURCE: Haroyan A et al. BMC Complement Altern Med. 2018 Jan 9;18:7. doi: 10.1186/s12906-017-2062-z
A combination of curcumin extracted from the turmeric rhizome and boswellic acid extracted from Indian frankincense root beat placebo for reducing pain-related symptoms from knee osteoarthritis in a 12-week clinical trial from Armenia with 201 patients 40-70 years old.
The combination (Curamin) also beat a standalone curcumin preparation (CuraMed), according to a report in BMC Complementary and Alternative Medicine.
It appears that combining the two “increases the efficacy of treatment of OA presumably due to synergistic effects of curcumin and boswellic acid”; it’s also possible that boswellic acid increases curcumin bioavailability, said investigators led by Armine Haroyan, PhD, head of rheumatology at Erebuni Medical Center in Yerevan, Armenia.
The subjects were randomized evenly to the combination, curcumin alone, or placebo, all in 500-mg capsules taken three times daily. They had been diagnosed with degenerative hypertrophic OA of knee bone joints.
At the end of 12 weeks, patients on the combination outperformed those on placebo in physical performance tests and joint pain scores; curcumin outperformed placebo in only a few of the physical tests.
For instance, patients on the combination were a mean of 2.03 seconds quicker than baseline in a stair-climbing exercise by the end of the study, versus 0.22 seconds in the placebo group and 1.66 seconds in the curcumin group. Combination patients had a mean 7.38-point improvement on the Western Ontario and McMaster Universities Osteoarthritis Index, versus 2.26 points in the placebo arm and 6.34 points in the curcumin group. The differences versus placebo were statistically significant for the combination, but not for stand-alone curcumin.
The treatments were well tolerated, with only a few patients in each arm reporting nausea, gastroesophageal reflux, and similar problems.
The work was funded in part by EuroPharma USA, maker of the supplements. The authors said they had no competing interests.
SOURCE: Haroyan A et al. BMC Complement Altern Med. 2018 Jan 9;18:7. doi: 10.1186/s12906-017-2062-z
FROM BMC COMPLEMENTARY AND ALTERNATIVE MEDICINE
Arthritis treatment costs per person held steady from 2008 to 2014
but the rise in proportion of the arthritis patients in the U.S. population led to billions more dollars being spent on this population, reported Amit D. Raval, PhD, and Ami Vyas, PhD, of the University of Rhode Island, Kingston.
Aggregate expenditures for patients with arthritis rose from $584.8 billion in 2008 to $645 billion in 2014, accounting for about 4% of the U.S. gross domestic product in these years, the investigators reported in the Journal of Rheumatology. “The increase in aggregate unadjusted total direct health care expenditures was mainly driven through an increase in weighted population of individuals with arthritis,” which rose from 56.1 million adults with arthritis in 2008 to 65.1 million in 2014. Nonetheless, there was a “slowdown in the intensity of adjusted incremental expenditures and [out of pocket expenditures] specifically from 2013, which led to a huge decline in the aggregate direct healthcare expenditures in 2014.”
During this time period, there was an increase in the percentage of Hispanics, poor, obese, and individuals with activity limitations and mental health disorders among both those with and without arthritis. This is consistent with the study findings that incremental health care expenditures in persons with arthritis were largely driven by difference in age, health status, and chronic conditions, such as hypertension, hyperlipidemia, and heart disease, among those with and without arthritis, Dr. Raval and Dr. Vyas said.
Annual total health care expenditures in persons with arthritis fell from $10,424 in 2008 to $9,910 in 2014. The average annual total out-of-pocket expenditures in 2008 was $1,493, which was 14% of total health care expenditures that year; in 2014, this fell to $1,099. There were similar trends in persons without arthritis, Dr. Raval and Dr. Vyas reported.
The top three expenditure categories in 2008 were outpatient (32.6%), inpatient (29.0%), and prescription drug costs (24.7%); in 2014, these changed to outpatient (33.8%), prescription drug (26.8%), and inpatient costs (26.4%). There are a number of ways to explain these trends, the researchers said. It is likely that outpatient services, such as outpatient orthopedic surgeries, are becoming “a common management option for arthritis.” Also, outpatient medication services now may include administration of injectables such as biologics. There also were “relatively stable” inpatient expenditures from 2008 to 2013 and a decline in 2014. “Our findings are consistent with studies assessing the effect of the Affordable Care Act, such as introduction of the Hospital Readmission Reduction Program,” said Dr. Raval and Dr. Vyas.
SOURCE: Amit R et al. J Rheumatol. 2018 Jan 15. doi: 10.3899/jrheum.170368.
but the rise in proportion of the arthritis patients in the U.S. population led to billions more dollars being spent on this population, reported Amit D. Raval, PhD, and Ami Vyas, PhD, of the University of Rhode Island, Kingston.
Aggregate expenditures for patients with arthritis rose from $584.8 billion in 2008 to $645 billion in 2014, accounting for about 4% of the U.S. gross domestic product in these years, the investigators reported in the Journal of Rheumatology. “The increase in aggregate unadjusted total direct health care expenditures was mainly driven through an increase in weighted population of individuals with arthritis,” which rose from 56.1 million adults with arthritis in 2008 to 65.1 million in 2014. Nonetheless, there was a “slowdown in the intensity of adjusted incremental expenditures and [out of pocket expenditures] specifically from 2013, which led to a huge decline in the aggregate direct healthcare expenditures in 2014.”
During this time period, there was an increase in the percentage of Hispanics, poor, obese, and individuals with activity limitations and mental health disorders among both those with and without arthritis. This is consistent with the study findings that incremental health care expenditures in persons with arthritis were largely driven by difference in age, health status, and chronic conditions, such as hypertension, hyperlipidemia, and heart disease, among those with and without arthritis, Dr. Raval and Dr. Vyas said.
Annual total health care expenditures in persons with arthritis fell from $10,424 in 2008 to $9,910 in 2014. The average annual total out-of-pocket expenditures in 2008 was $1,493, which was 14% of total health care expenditures that year; in 2014, this fell to $1,099. There were similar trends in persons without arthritis, Dr. Raval and Dr. Vyas reported.
The top three expenditure categories in 2008 were outpatient (32.6%), inpatient (29.0%), and prescription drug costs (24.7%); in 2014, these changed to outpatient (33.8%), prescription drug (26.8%), and inpatient costs (26.4%). There are a number of ways to explain these trends, the researchers said. It is likely that outpatient services, such as outpatient orthopedic surgeries, are becoming “a common management option for arthritis.” Also, outpatient medication services now may include administration of injectables such as biologics. There also were “relatively stable” inpatient expenditures from 2008 to 2013 and a decline in 2014. “Our findings are consistent with studies assessing the effect of the Affordable Care Act, such as introduction of the Hospital Readmission Reduction Program,” said Dr. Raval and Dr. Vyas.
SOURCE: Amit R et al. J Rheumatol. 2018 Jan 15. doi: 10.3899/jrheum.170368.
but the rise in proportion of the arthritis patients in the U.S. population led to billions more dollars being spent on this population, reported Amit D. Raval, PhD, and Ami Vyas, PhD, of the University of Rhode Island, Kingston.
Aggregate expenditures for patients with arthritis rose from $584.8 billion in 2008 to $645 billion in 2014, accounting for about 4% of the U.S. gross domestic product in these years, the investigators reported in the Journal of Rheumatology. “The increase in aggregate unadjusted total direct health care expenditures was mainly driven through an increase in weighted population of individuals with arthritis,” which rose from 56.1 million adults with arthritis in 2008 to 65.1 million in 2014. Nonetheless, there was a “slowdown in the intensity of adjusted incremental expenditures and [out of pocket expenditures] specifically from 2013, which led to a huge decline in the aggregate direct healthcare expenditures in 2014.”
During this time period, there was an increase in the percentage of Hispanics, poor, obese, and individuals with activity limitations and mental health disorders among both those with and without arthritis. This is consistent with the study findings that incremental health care expenditures in persons with arthritis were largely driven by difference in age, health status, and chronic conditions, such as hypertension, hyperlipidemia, and heart disease, among those with and without arthritis, Dr. Raval and Dr. Vyas said.
Annual total health care expenditures in persons with arthritis fell from $10,424 in 2008 to $9,910 in 2014. The average annual total out-of-pocket expenditures in 2008 was $1,493, which was 14% of total health care expenditures that year; in 2014, this fell to $1,099. There were similar trends in persons without arthritis, Dr. Raval and Dr. Vyas reported.
The top three expenditure categories in 2008 were outpatient (32.6%), inpatient (29.0%), and prescription drug costs (24.7%); in 2014, these changed to outpatient (33.8%), prescription drug (26.8%), and inpatient costs (26.4%). There are a number of ways to explain these trends, the researchers said. It is likely that outpatient services, such as outpatient orthopedic surgeries, are becoming “a common management option for arthritis.” Also, outpatient medication services now may include administration of injectables such as biologics. There also were “relatively stable” inpatient expenditures from 2008 to 2013 and a decline in 2014. “Our findings are consistent with studies assessing the effect of the Affordable Care Act, such as introduction of the Hospital Readmission Reduction Program,” said Dr. Raval and Dr. Vyas.
SOURCE: Amit R et al. J Rheumatol. 2018 Jan 15. doi: 10.3899/jrheum.170368.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: The annual direct health care expenditures per person with arthritis remained relatively stable over the years 2008 to 2014.
Major finding: Aggregate expenditures for patients with arthritis rose from $584.8 billion in 2008 to $645 billion in 2014.
Study details: Medical Expenditure Panel Survey (MEPS) data from approximately 5,000-6,000 persons aged 18 years and older with arthritis each year during 2008-2014 and approximately 17,000-20,000 persons without arthritis each year during that period.
Disclosures: No information on relevant financial disclosures was evident.
Source: Amit R et al. J Rheumatol. 2018 Jan 15. doi: 10.3899/jrheum.170368.
Sprifermin shows cartilage-building potential in knee OA patients
in the initial 2-year results of the ongoing FORWARD trial.
“Sprifermin appears to be the first investigational medicinal product to show dose-dependent prevention of cartilage loss and an increase in cartilage thickness, not only in the total tibiofemoral joint [TFJ] but also in both the medial and lateral compartments, including the central medial femorotibial region,” said Marc H. Hochberg, MD, primary investigator in the trial and division head of rheumatology and clinical immunology at the University of Maryland, Baltimore. “The recommendation is that these findings should be further evaluated in phase 3 clinical trials.”
He and his colleagues randomized 549 osteoarthritis (OA) patients to double-blind treatment with one of four different dosing regimens of sprifermin or placebo. These patients were aged 40-85 with symptomatic radiographic primary femorotibial knee OA measuring grade 2 or 3 on the Kellgren-Lawrence scale and a medial minimum joint space width (mJSW) 2.5 mm or greater.
At 2 years, researchers observed a significant dose-dependent relationship between the amount of sprifermin given and the increase in total TFJ cartilage thickness. Patients who received three 100-mcg intra-articular injections of sprifermin every 6 months (group 1) showed a gain in TFJ cartilage thickness of 0.03 mm as seen on MRI, while those who received three 100-mcg injections of sprifermin every 12 months (group 2) had a gain of 0.02 mm, Dr. Hochberg said during a late-breaking abstract session at the annual meeting of the American College of Rheumatology. By contrast, those who received placebo had a loss in TFJ cartilage thickness of 0.02 mm (P less than .001). The other two groups received 30 mcg of sprifermin in three weekly injections every 6 months (group 3) or every 12 months (group 4), and these had TFJ cartilage thickness losses of about 0.01 mm or less.
Similar dose-dependent relationships were observed for some of the secondary endpoints, which included changes in cartilage thickness seen in the medial and lateral compartments, changes in cartilage thickness in the compartments’ subregions, and changes in mJSW. Significant differences in cartilage thickness were observed between sprifermin treatment groups and placebo in the medial (group 1, gain of 0.02 mm vs. loss of 0.03 mm; P less than .001) and lateral (groups 1 and 2, gain of 0.04 mm vs. loss of 0.01 mm; P less than .001) TFJ compartments, and in central medial and lateral TFJ subregions.
Changes in mJSW as seen on x-ray between those in group 1 and those on placebo were significant for the lateral compartment, with an increase in mJSW at the higher doses and a decline in the placebo group, but not for the medial compartment.
There were no significant differences in Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores among the treatment groups. Dr. Hochberg noted that patients were permitted to take pain medications during the study, which could have affected this result.
The most frequently reported adverse events were musculoskeletal and connective tissue disorders, specifically arthralgias and back pain, Dr. Hochberg said. The incidence of acute inflammatory reactions was higher with sprifermin, compared with placebo, but the increase was only significant after the first injection cycle, he said.
Merck KGaA and the EMD Serono Research Institute funded the study. Dr. Hochberg reported receiving consulting fees from numerous companies that market or are developing OA drugs, including EMD Serono.
in the initial 2-year results of the ongoing FORWARD trial.
“Sprifermin appears to be the first investigational medicinal product to show dose-dependent prevention of cartilage loss and an increase in cartilage thickness, not only in the total tibiofemoral joint [TFJ] but also in both the medial and lateral compartments, including the central medial femorotibial region,” said Marc H. Hochberg, MD, primary investigator in the trial and division head of rheumatology and clinical immunology at the University of Maryland, Baltimore. “The recommendation is that these findings should be further evaluated in phase 3 clinical trials.”
He and his colleagues randomized 549 osteoarthritis (OA) patients to double-blind treatment with one of four different dosing regimens of sprifermin or placebo. These patients were aged 40-85 with symptomatic radiographic primary femorotibial knee OA measuring grade 2 or 3 on the Kellgren-Lawrence scale and a medial minimum joint space width (mJSW) 2.5 mm or greater.
At 2 years, researchers observed a significant dose-dependent relationship between the amount of sprifermin given and the increase in total TFJ cartilage thickness. Patients who received three 100-mcg intra-articular injections of sprifermin every 6 months (group 1) showed a gain in TFJ cartilage thickness of 0.03 mm as seen on MRI, while those who received three 100-mcg injections of sprifermin every 12 months (group 2) had a gain of 0.02 mm, Dr. Hochberg said during a late-breaking abstract session at the annual meeting of the American College of Rheumatology. By contrast, those who received placebo had a loss in TFJ cartilage thickness of 0.02 mm (P less than .001). The other two groups received 30 mcg of sprifermin in three weekly injections every 6 months (group 3) or every 12 months (group 4), and these had TFJ cartilage thickness losses of about 0.01 mm or less.
Similar dose-dependent relationships were observed for some of the secondary endpoints, which included changes in cartilage thickness seen in the medial and lateral compartments, changes in cartilage thickness in the compartments’ subregions, and changes in mJSW. Significant differences in cartilage thickness were observed between sprifermin treatment groups and placebo in the medial (group 1, gain of 0.02 mm vs. loss of 0.03 mm; P less than .001) and lateral (groups 1 and 2, gain of 0.04 mm vs. loss of 0.01 mm; P less than .001) TFJ compartments, and in central medial and lateral TFJ subregions.
Changes in mJSW as seen on x-ray between those in group 1 and those on placebo were significant for the lateral compartment, with an increase in mJSW at the higher doses and a decline in the placebo group, but not for the medial compartment.
There were no significant differences in Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores among the treatment groups. Dr. Hochberg noted that patients were permitted to take pain medications during the study, which could have affected this result.
The most frequently reported adverse events were musculoskeletal and connective tissue disorders, specifically arthralgias and back pain, Dr. Hochberg said. The incidence of acute inflammatory reactions was higher with sprifermin, compared with placebo, but the increase was only significant after the first injection cycle, he said.
Merck KGaA and the EMD Serono Research Institute funded the study. Dr. Hochberg reported receiving consulting fees from numerous companies that market or are developing OA drugs, including EMD Serono.
in the initial 2-year results of the ongoing FORWARD trial.
“Sprifermin appears to be the first investigational medicinal product to show dose-dependent prevention of cartilage loss and an increase in cartilage thickness, not only in the total tibiofemoral joint [TFJ] but also in both the medial and lateral compartments, including the central medial femorotibial region,” said Marc H. Hochberg, MD, primary investigator in the trial and division head of rheumatology and clinical immunology at the University of Maryland, Baltimore. “The recommendation is that these findings should be further evaluated in phase 3 clinical trials.”
He and his colleagues randomized 549 osteoarthritis (OA) patients to double-blind treatment with one of four different dosing regimens of sprifermin or placebo. These patients were aged 40-85 with symptomatic radiographic primary femorotibial knee OA measuring grade 2 or 3 on the Kellgren-Lawrence scale and a medial minimum joint space width (mJSW) 2.5 mm or greater.
At 2 years, researchers observed a significant dose-dependent relationship between the amount of sprifermin given and the increase in total TFJ cartilage thickness. Patients who received three 100-mcg intra-articular injections of sprifermin every 6 months (group 1) showed a gain in TFJ cartilage thickness of 0.03 mm as seen on MRI, while those who received three 100-mcg injections of sprifermin every 12 months (group 2) had a gain of 0.02 mm, Dr. Hochberg said during a late-breaking abstract session at the annual meeting of the American College of Rheumatology. By contrast, those who received placebo had a loss in TFJ cartilage thickness of 0.02 mm (P less than .001). The other two groups received 30 mcg of sprifermin in three weekly injections every 6 months (group 3) or every 12 months (group 4), and these had TFJ cartilage thickness losses of about 0.01 mm or less.
Similar dose-dependent relationships were observed for some of the secondary endpoints, which included changes in cartilage thickness seen in the medial and lateral compartments, changes in cartilage thickness in the compartments’ subregions, and changes in mJSW. Significant differences in cartilage thickness were observed between sprifermin treatment groups and placebo in the medial (group 1, gain of 0.02 mm vs. loss of 0.03 mm; P less than .001) and lateral (groups 1 and 2, gain of 0.04 mm vs. loss of 0.01 mm; P less than .001) TFJ compartments, and in central medial and lateral TFJ subregions.
Changes in mJSW as seen on x-ray between those in group 1 and those on placebo were significant for the lateral compartment, with an increase in mJSW at the higher doses and a decline in the placebo group, but not for the medial compartment.
There were no significant differences in Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores among the treatment groups. Dr. Hochberg noted that patients were permitted to take pain medications during the study, which could have affected this result.
The most frequently reported adverse events were musculoskeletal and connective tissue disorders, specifically arthralgias and back pain, Dr. Hochberg said. The incidence of acute inflammatory reactions was higher with sprifermin, compared with placebo, but the increase was only significant after the first injection cycle, he said.
Merck KGaA and the EMD Serono Research Institute funded the study. Dr. Hochberg reported receiving consulting fees from numerous companies that market or are developing OA drugs, including EMD Serono.
REPORTING FROM ACR 2017
Key clinical point: Sprifermin may help build knee joint cartilage in patients with OA.
Major finding: Patients taking sprifermin 100 mcg three times a week every 6 months or every 12 months had gains in tibiofemoral joint cartilage thickness of 0.03 mm and 0.02 mm, respectively, over a 2-year period.
Study details: A study of 549 patients with symptomatic knee OA randomized to receive either 30 mcg or 100 mcg of sprifermin three times a week every 6 or every 12 months, or placebo.
Disclosures: Merck KGaA and the EMD Serono Research Institute funded the study. The presenter reported receiving consulting fees from numerous companies that market or are developing OA drugs, including EMD Serono.
Source: Hochberg M et al. ACR 2017 abstract 1L.
Evidence builds for long-term ineffectiveness of steroid shots for knee OA
SAN DIEGO – Real-world, nontrial research confirms the findings of a high-profile study released earlier in 2017: Corticosteroid shots are ineffective in the long term for knee osteoarthritis. In fact, researchers found a greater likelihood of a worsening condition in knees treated with the injections.
“Our findings are consistent with the latest randomized, controlled trial,” said study coauthor Jie Wei, MD, of Central South University in Changsha, China. She spoke in a plenary presentation about the study findings at the annual meeting of the American College of Rheumatology.
The use of corticosteroids for knee OA is a controversial topic. As Dr. Wei noted, there has been wide disagreement among medical societies about whether the treatment is useful in the long term for patients with pain flare-ups.
For the randomized, controlled study released in 2017, researchers tracked 140 patients aged 45 and older with inflammation of the synovial membrane. They were randomly assigned to injections of intra-articular triamcinolone or a placebo.
After 2 years of injections every 12 weeks, there was no difference in reported pain between the intervention and control groups. Also, those who received injections lost more cartilage (JAMA. 2017 May 16;317[19]:1967-75).
Researchers launched the new study to seek insight through a real-life cohort. They examined findings from the Osteoarthritis Initiative, a longitudinal study of 4,796 patients aged 45-79 at four U.S. clinics with knee OA or high risk of knee OA. Patients underwent annual examinations at baseline and annually for 4 years.
In an adjusted marginal structural analysis, knee replacement or worsening of Kellgren Lawrence grade at the tibial femoral joint was more likely in 149 injection knees than 2,191 noninjection knees (odds ratio, 5.74; 95% confidence interval, 2.01-16.42).
Knee replacement or joint space width worsening at the tibial femoral joint was also more likely in 120 injection knees than 2,112 noninjection knees (OR, 1.64; 95% CI, 0.91-2.93).
In another analysis, researchers tracked 134 injection knees (58 whose OA progressed) and 498 noninjection knees (132 whose OA progressed) for up to 8 years. After adjustment, the injection knees were more likely to have progressed (hazard ratio, 1.60; 95% CI, 1.21-2.12,).
“Several explanations may account for our study findings,” Dr. Wei said. One possibility, she said, is that corticosteroids may hurt chondrocytes by, among other things, inducing apoptosis and synovial membrane inflammation.
It’s also possible, she said, that patients may feel pain relief after injections and subsequently boost the risk of OA progression by increasing their physical activity.
“We need to know what types of physical activities may increase the OA progression,” she said. “Did patients who received steroid injection indeed increase this type of physical activity compared to subjects without steroid injection?”
Dr. Wei noted the study’s limitations, including the fact that patients who received injections had more pain at baseline, potentially indicating they had worse structural lesions that are more susceptible to progression.
The study authors reported no relevant disclosures. The National Natural Science Foundation of China funded the study. The Osteoarthritis Initiative is a partnership between the National Institutes of Health and Merck, Novartis, GlaxoSmithKline, and Pfizer.
SAN DIEGO – Real-world, nontrial research confirms the findings of a high-profile study released earlier in 2017: Corticosteroid shots are ineffective in the long term for knee osteoarthritis. In fact, researchers found a greater likelihood of a worsening condition in knees treated with the injections.
“Our findings are consistent with the latest randomized, controlled trial,” said study coauthor Jie Wei, MD, of Central South University in Changsha, China. She spoke in a plenary presentation about the study findings at the annual meeting of the American College of Rheumatology.
The use of corticosteroids for knee OA is a controversial topic. As Dr. Wei noted, there has been wide disagreement among medical societies about whether the treatment is useful in the long term for patients with pain flare-ups.
For the randomized, controlled study released in 2017, researchers tracked 140 patients aged 45 and older with inflammation of the synovial membrane. They were randomly assigned to injections of intra-articular triamcinolone or a placebo.
After 2 years of injections every 12 weeks, there was no difference in reported pain between the intervention and control groups. Also, those who received injections lost more cartilage (JAMA. 2017 May 16;317[19]:1967-75).
Researchers launched the new study to seek insight through a real-life cohort. They examined findings from the Osteoarthritis Initiative, a longitudinal study of 4,796 patients aged 45-79 at four U.S. clinics with knee OA or high risk of knee OA. Patients underwent annual examinations at baseline and annually for 4 years.
In an adjusted marginal structural analysis, knee replacement or worsening of Kellgren Lawrence grade at the tibial femoral joint was more likely in 149 injection knees than 2,191 noninjection knees (odds ratio, 5.74; 95% confidence interval, 2.01-16.42).
Knee replacement or joint space width worsening at the tibial femoral joint was also more likely in 120 injection knees than 2,112 noninjection knees (OR, 1.64; 95% CI, 0.91-2.93).
In another analysis, researchers tracked 134 injection knees (58 whose OA progressed) and 498 noninjection knees (132 whose OA progressed) for up to 8 years. After adjustment, the injection knees were more likely to have progressed (hazard ratio, 1.60; 95% CI, 1.21-2.12,).
“Several explanations may account for our study findings,” Dr. Wei said. One possibility, she said, is that corticosteroids may hurt chondrocytes by, among other things, inducing apoptosis and synovial membrane inflammation.
It’s also possible, she said, that patients may feel pain relief after injections and subsequently boost the risk of OA progression by increasing their physical activity.
“We need to know what types of physical activities may increase the OA progression,” she said. “Did patients who received steroid injection indeed increase this type of physical activity compared to subjects without steroid injection?”
Dr. Wei noted the study’s limitations, including the fact that patients who received injections had more pain at baseline, potentially indicating they had worse structural lesions that are more susceptible to progression.
The study authors reported no relevant disclosures. The National Natural Science Foundation of China funded the study. The Osteoarthritis Initiative is a partnership between the National Institutes of Health and Merck, Novartis, GlaxoSmithKline, and Pfizer.
SAN DIEGO – Real-world, nontrial research confirms the findings of a high-profile study released earlier in 2017: Corticosteroid shots are ineffective in the long term for knee osteoarthritis. In fact, researchers found a greater likelihood of a worsening condition in knees treated with the injections.
“Our findings are consistent with the latest randomized, controlled trial,” said study coauthor Jie Wei, MD, of Central South University in Changsha, China. She spoke in a plenary presentation about the study findings at the annual meeting of the American College of Rheumatology.
The use of corticosteroids for knee OA is a controversial topic. As Dr. Wei noted, there has been wide disagreement among medical societies about whether the treatment is useful in the long term for patients with pain flare-ups.
For the randomized, controlled study released in 2017, researchers tracked 140 patients aged 45 and older with inflammation of the synovial membrane. They were randomly assigned to injections of intra-articular triamcinolone or a placebo.
After 2 years of injections every 12 weeks, there was no difference in reported pain between the intervention and control groups. Also, those who received injections lost more cartilage (JAMA. 2017 May 16;317[19]:1967-75).
Researchers launched the new study to seek insight through a real-life cohort. They examined findings from the Osteoarthritis Initiative, a longitudinal study of 4,796 patients aged 45-79 at four U.S. clinics with knee OA or high risk of knee OA. Patients underwent annual examinations at baseline and annually for 4 years.
In an adjusted marginal structural analysis, knee replacement or worsening of Kellgren Lawrence grade at the tibial femoral joint was more likely in 149 injection knees than 2,191 noninjection knees (odds ratio, 5.74; 95% confidence interval, 2.01-16.42).
Knee replacement or joint space width worsening at the tibial femoral joint was also more likely in 120 injection knees than 2,112 noninjection knees (OR, 1.64; 95% CI, 0.91-2.93).
In another analysis, researchers tracked 134 injection knees (58 whose OA progressed) and 498 noninjection knees (132 whose OA progressed) for up to 8 years. After adjustment, the injection knees were more likely to have progressed (hazard ratio, 1.60; 95% CI, 1.21-2.12,).
“Several explanations may account for our study findings,” Dr. Wei said. One possibility, she said, is that corticosteroids may hurt chondrocytes by, among other things, inducing apoptosis and synovial membrane inflammation.
It’s also possible, she said, that patients may feel pain relief after injections and subsequently boost the risk of OA progression by increasing their physical activity.
“We need to know what types of physical activities may increase the OA progression,” she said. “Did patients who received steroid injection indeed increase this type of physical activity compared to subjects without steroid injection?”
Dr. Wei noted the study’s limitations, including the fact that patients who received injections had more pain at baseline, potentially indicating they had worse structural lesions that are more susceptible to progression.
The study authors reported no relevant disclosures. The National Natural Science Foundation of China funded the study. The Osteoarthritis Initiative is a partnership between the National Institutes of Health and Merck, Novartis, GlaxoSmithKline, and Pfizer.
REPORTING FROM ACR 2017
Key clinical point:
Major finding: In adjusted analysis of 134 injection knees and 498 noninjection knees tracked for up to 8 years, OA in injection knees was more likely to have progressed (HR, 1.60; 95% CI, 1.21-2.12).
Study details: Cohort analysis of data from the Osteoarthritis Initiative, which tracked patients with (or at high risk of) knee OA at four U.S. clinics.
Disclosures: The study authors reported no relevant disclosures. The National Natural Science Foundation of China funded the study. The Osteoarthritis Initiative is a partnership between the National Institutes of Health and Merck, Novartis, GlaxoSmithKline, and Pfizer.
Source: Lei G et al. ACR 2017 Abstract 1788.
FDA recommends voluntary recall of Limbrel
The Food and Drug Administration announced on Dec. 4 that it recommends the voluntary recall of Limbrel, a medical food product in capsule form that is currently marketed to “manage the metabolic processes associated with osteoarthritis.”
The FDA’s ongoing investigation at this point considers the product to be an unapproved new drug rather than a medical food product. However, the agency does not have mandatory recall authority. It has recommended the recall to the product’s manufacturer, Primus Pharmaceuticals, on the basis of the risk of liver injury and hypersensitivity pneumonitis associated with continued use of the product.
The agency had received 194 adverse event reports as of Nov. 21, of which it found a likely association of the events with Limbrel in at least 30 cases, and continues to evaluate reports, which consumers can submit through MedWatch. The FDA is currently testing samples of the product and has advised consumers to cease taking it, though the manufacturer has declined thus far to recall it.
The safety alert advises that “health care providers who are aware that their patients are taking Limbrel should advise them to immediately stop taking the product.”
The Food and Drug Administration announced on Dec. 4 that it recommends the voluntary recall of Limbrel, a medical food product in capsule form that is currently marketed to “manage the metabolic processes associated with osteoarthritis.”
The FDA’s ongoing investigation at this point considers the product to be an unapproved new drug rather than a medical food product. However, the agency does not have mandatory recall authority. It has recommended the recall to the product’s manufacturer, Primus Pharmaceuticals, on the basis of the risk of liver injury and hypersensitivity pneumonitis associated with continued use of the product.
The agency had received 194 adverse event reports as of Nov. 21, of which it found a likely association of the events with Limbrel in at least 30 cases, and continues to evaluate reports, which consumers can submit through MedWatch. The FDA is currently testing samples of the product and has advised consumers to cease taking it, though the manufacturer has declined thus far to recall it.
The safety alert advises that “health care providers who are aware that their patients are taking Limbrel should advise them to immediately stop taking the product.”
The Food and Drug Administration announced on Dec. 4 that it recommends the voluntary recall of Limbrel, a medical food product in capsule form that is currently marketed to “manage the metabolic processes associated with osteoarthritis.”
The FDA’s ongoing investigation at this point considers the product to be an unapproved new drug rather than a medical food product. However, the agency does not have mandatory recall authority. It has recommended the recall to the product’s manufacturer, Primus Pharmaceuticals, on the basis of the risk of liver injury and hypersensitivity pneumonitis associated with continued use of the product.
The agency had received 194 adverse event reports as of Nov. 21, of which it found a likely association of the events with Limbrel in at least 30 cases, and continues to evaluate reports, which consumers can submit through MedWatch. The FDA is currently testing samples of the product and has advised consumers to cease taking it, though the manufacturer has declined thus far to recall it.
The safety alert advises that “health care providers who are aware that their patients are taking Limbrel should advise them to immediately stop taking the product.”
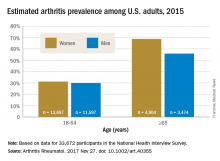
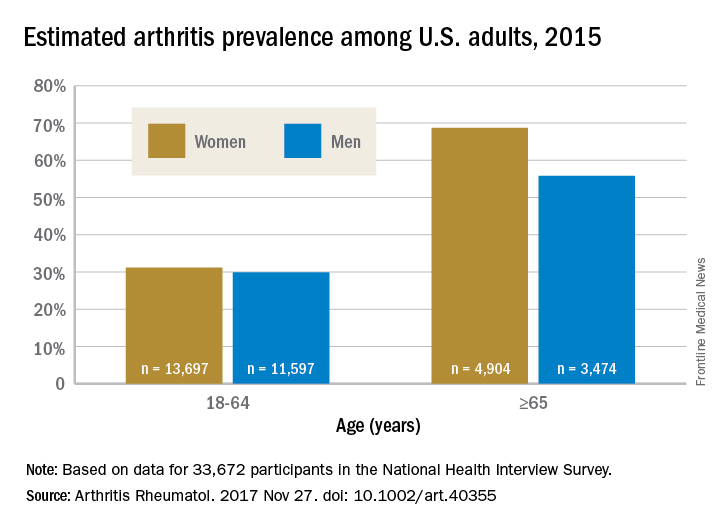
Arthritis prevalence higher than previously thought, especially in adults under 65
The prevalence of arthritis in the United States is much higher than current estimates indicate, especially among adults under 65 years of age, a study showed.
The higher prevalence can be largely attributed to “the previous underestimate of arthritis in adults between 18-64 years of age,” according to S. Reza Jafarzadeh, PhD, and David T. Felson , MD, both of Boston University. Using a new surveillance model, they estimated that 91.2 million adults in the United States (36.8%) had arthritis in 2015, compared with a previously reported national estimate of 54.4 million adults (22.7%). Of these, 61.1 million were between 18 and 64 years of age (Arthritis Rheumatol. 2017 Nov 27. doi: 10.1002/art.40355).
Arthritis prevalence was 29.9% in men aged 18-64 years (95% probability interval, 23.4-42.3), 31.2% in women aged 18-64 years (95% PI, 25.8-44.1), 55.8% in men aged 65 years and older (95% PI, 49.9-70.4), and 68.7% in women aged 65 years and older (95% PI, 62.1-79.9), the authors reported.
Among respondents aged 18-64 years, 19.3% of men and 16.7% of women reported that they had chronic joint symptoms but no physician-diagnosed arthritis. Among those 65 years of age or older, 15.7% of men and 13.5% of women responded that they had chronic joint symptoms without physician-diagnosed arthritis.
Current methodology for estimating arthritis prevalence is based on a single survey question asking whether a health care provider has ever told the patient that he or she has arthritis, a method that has previously been shown to have a sensitivity of 68.8% in adults 65 years of age and older and 52.5% for those aged 45-64 years, Dr. Jafarzadeh and Dr. Felson reported. “Such a low sensitivity, especially in a younger population, where almost half of true arthritis cases are missed, results in substantial misclassification and underestimation of prevalence and would have a detrimental effect for planning and needs assessment,” the authors wrote.
The two additional questions on joint pain, aching, and stiffness that the investigators included in the study captured “a substantial (i.e., 65%-80%) fraction of the population with arthritis, who are between 18-64 years of age, but are misclassified as healthy by the doctor-diagnosed arthritis criterion due to low sensitivity,” they said.
The study authors also speculated that younger patients might be more likely to ignore symptoms or visit a doctor less often.
“Further studies are needed to evaluate potential changes in the specific causes of arthritis, especially among adults below the age of 65,” they concluded.
The study was supported by a grant from the National Institutes of Health. The investigators did not disclose any other conflicts of interest.
By including two additional survey criteria in their study of arthritis prevalence, Dr. Jafarzadeh and Dr. Felson introduced a method that “may be considerably more accurate than prior estimates that use the single NHIS [National Health Interview Survey] item on doctor-diagnosed arthritis,” said Jeffrey N. Katz, MD, in an editorial accompanying the study.
The study raises important questions about how “arthritis” is defined, as well as how prevalence estimates could affect policy agendas, and could potentially have “far-reaching consequences” related to investment in research, prevention, and treatment, he added.
Dr. Katz is with the Orthopedic and Arthritis Center for Outcomes Research at Brigham and Women’s Hospital in Boston (Arthritis Rheumatol. 2017 Nov 27. doi: 10.1002/art.40357). No conflicts of interest were disclosed.
By including two additional survey criteria in their study of arthritis prevalence, Dr. Jafarzadeh and Dr. Felson introduced a method that “may be considerably more accurate than prior estimates that use the single NHIS [National Health Interview Survey] item on doctor-diagnosed arthritis,” said Jeffrey N. Katz, MD, in an editorial accompanying the study.
The study raises important questions about how “arthritis” is defined, as well as how prevalence estimates could affect policy agendas, and could potentially have “far-reaching consequences” related to investment in research, prevention, and treatment, he added.
Dr. Katz is with the Orthopedic and Arthritis Center for Outcomes Research at Brigham and Women’s Hospital in Boston (Arthritis Rheumatol. 2017 Nov 27. doi: 10.1002/art.40357). No conflicts of interest were disclosed.
By including two additional survey criteria in their study of arthritis prevalence, Dr. Jafarzadeh and Dr. Felson introduced a method that “may be considerably more accurate than prior estimates that use the single NHIS [National Health Interview Survey] item on doctor-diagnosed arthritis,” said Jeffrey N. Katz, MD, in an editorial accompanying the study.
The study raises important questions about how “arthritis” is defined, as well as how prevalence estimates could affect policy agendas, and could potentially have “far-reaching consequences” related to investment in research, prevention, and treatment, he added.
Dr. Katz is with the Orthopedic and Arthritis Center for Outcomes Research at Brigham and Women’s Hospital in Boston (Arthritis Rheumatol. 2017 Nov 27. doi: 10.1002/art.40357). No conflicts of interest were disclosed.
The prevalence of arthritis in the United States is much higher than current estimates indicate, especially among adults under 65 years of age, a study showed.
The higher prevalence can be largely attributed to “the previous underestimate of arthritis in adults between 18-64 years of age,” according to S. Reza Jafarzadeh, PhD, and David T. Felson , MD, both of Boston University. Using a new surveillance model, they estimated that 91.2 million adults in the United States (36.8%) had arthritis in 2015, compared with a previously reported national estimate of 54.4 million adults (22.7%). Of these, 61.1 million were between 18 and 64 years of age (Arthritis Rheumatol. 2017 Nov 27. doi: 10.1002/art.40355).
Arthritis prevalence was 29.9% in men aged 18-64 years (95% probability interval, 23.4-42.3), 31.2% in women aged 18-64 years (95% PI, 25.8-44.1), 55.8% in men aged 65 years and older (95% PI, 49.9-70.4), and 68.7% in women aged 65 years and older (95% PI, 62.1-79.9), the authors reported.
Among respondents aged 18-64 years, 19.3% of men and 16.7% of women reported that they had chronic joint symptoms but no physician-diagnosed arthritis. Among those 65 years of age or older, 15.7% of men and 13.5% of women responded that they had chronic joint symptoms without physician-diagnosed arthritis.
Current methodology for estimating arthritis prevalence is based on a single survey question asking whether a health care provider has ever told the patient that he or she has arthritis, a method that has previously been shown to have a sensitivity of 68.8% in adults 65 years of age and older and 52.5% for those aged 45-64 years, Dr. Jafarzadeh and Dr. Felson reported. “Such a low sensitivity, especially in a younger population, where almost half of true arthritis cases are missed, results in substantial misclassification and underestimation of prevalence and would have a detrimental effect for planning and needs assessment,” the authors wrote.
The two additional questions on joint pain, aching, and stiffness that the investigators included in the study captured “a substantial (i.e., 65%-80%) fraction of the population with arthritis, who are between 18-64 years of age, but are misclassified as healthy by the doctor-diagnosed arthritis criterion due to low sensitivity,” they said.
The study authors also speculated that younger patients might be more likely to ignore symptoms or visit a doctor less often.
“Further studies are needed to evaluate potential changes in the specific causes of arthritis, especially among adults below the age of 65,” they concluded.
The study was supported by a grant from the National Institutes of Health. The investigators did not disclose any other conflicts of interest.
The prevalence of arthritis in the United States is much higher than current estimates indicate, especially among adults under 65 years of age, a study showed.
The higher prevalence can be largely attributed to “the previous underestimate of arthritis in adults between 18-64 years of age,” according to S. Reza Jafarzadeh, PhD, and David T. Felson , MD, both of Boston University. Using a new surveillance model, they estimated that 91.2 million adults in the United States (36.8%) had arthritis in 2015, compared with a previously reported national estimate of 54.4 million adults (22.7%). Of these, 61.1 million were between 18 and 64 years of age (Arthritis Rheumatol. 2017 Nov 27. doi: 10.1002/art.40355).
Arthritis prevalence was 29.9% in men aged 18-64 years (95% probability interval, 23.4-42.3), 31.2% in women aged 18-64 years (95% PI, 25.8-44.1), 55.8% in men aged 65 years and older (95% PI, 49.9-70.4), and 68.7% in women aged 65 years and older (95% PI, 62.1-79.9), the authors reported.
Among respondents aged 18-64 years, 19.3% of men and 16.7% of women reported that they had chronic joint symptoms but no physician-diagnosed arthritis. Among those 65 years of age or older, 15.7% of men and 13.5% of women responded that they had chronic joint symptoms without physician-diagnosed arthritis.
Current methodology for estimating arthritis prevalence is based on a single survey question asking whether a health care provider has ever told the patient that he or she has arthritis, a method that has previously been shown to have a sensitivity of 68.8% in adults 65 years of age and older and 52.5% for those aged 45-64 years, Dr. Jafarzadeh and Dr. Felson reported. “Such a low sensitivity, especially in a younger population, where almost half of true arthritis cases are missed, results in substantial misclassification and underestimation of prevalence and would have a detrimental effect for planning and needs assessment,” the authors wrote.
The two additional questions on joint pain, aching, and stiffness that the investigators included in the study captured “a substantial (i.e., 65%-80%) fraction of the population with arthritis, who are between 18-64 years of age, but are misclassified as healthy by the doctor-diagnosed arthritis criterion due to low sensitivity,” they said.
The study authors also speculated that younger patients might be more likely to ignore symptoms or visit a doctor less often.
“Further studies are needed to evaluate potential changes in the specific causes of arthritis, especially among adults below the age of 65,” they concluded.
The study was supported by a grant from the National Institutes of Health. The investigators did not disclose any other conflicts of interest.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: An estimated 91.2 million adults in the United States (36.8%) had arthritis in 2015, compared with a previously reported national estimate of 54.4 million adults (22.7%).
Data source: Data from 33,672 respondents to the 2015 National Health Interview Survey.
Disclosures: The study was supported by a grant from the National Institutes of Health. The investigators did not disclose any other conflicts of interest.
Injectable agent found to improve knee function in OA patients
SAN DIEGO – Among patients with mild to moderate patellofemoral osteoarthritis, intra-articular administration of the novel agent TPX-100 was safe and associated with functional benefits up to 1 year, a proof-of-concept study showed.
“We don’t yet have a disease-modifying drug for osteoarthritis [OA]; that’s sort of the holy grail for researchers,” lead study author Dawn McGuire, MD, said in an interview at the annual meeting of the American College of Rheumatology. “All of the patient-reported outcome and patient function indices that we studied moved in the same direction, showing a benefit of TPX-100. I think this is very promising.”
The study consisted of two parts. In Part A, four dose cohorts ranging from 20 mg to 200 mg per injection were enrolled. There were no dose-limiting toxicities or safety concerns at any dose, and the 200-mg dose was selected dose for Part B of the study.
The median age of the 118 patients was 60 years and their median body mass index was 29.2 kg/m2. No drug-related serious adverse events and no dose-limiting toxicities occurred across doses ranging from 20 mg to 200 mg per injection. The incidence of common adverse events such as knee pain was similar between placebo- and TPX-100-treated knees.
Quantitative MRI showed no measurable between-knee differences in cartilage thickness or volume at 6 or 12 months. However, statistically significant and clinically meaningful differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, including KOOS activities of daily living (P = .008 at 6 and 12 months), KOOS knee-related quality of life (P = .21 at 6 months and P = .03 at 12 months), and a significant reduction in pain going up or down stairs (P = .004 at 12 months). Subjects’ use of nonsteroidal anti-inflammatory medications declined by 63% during the study.
“In this study, we see some terrific, long-term results in knee-related core activities, which certainly were disease modifying from the patients’ perspective,” said Dr. McGuire, who noted that a phase 3 study is being planned. “In addition, patient’s pain going up and down stairs improved significantly, with a marked reduction in analgesic use. Any of us who are experienced in these tough areas of medicine know that early results can look extremely promising, but we have to do larger confirmatory studies.”
SAN DIEGO – Among patients with mild to moderate patellofemoral osteoarthritis, intra-articular administration of the novel agent TPX-100 was safe and associated with functional benefits up to 1 year, a proof-of-concept study showed.
“We don’t yet have a disease-modifying drug for osteoarthritis [OA]; that’s sort of the holy grail for researchers,” lead study author Dawn McGuire, MD, said in an interview at the annual meeting of the American College of Rheumatology. “All of the patient-reported outcome and patient function indices that we studied moved in the same direction, showing a benefit of TPX-100. I think this is very promising.”
The study consisted of two parts. In Part A, four dose cohorts ranging from 20 mg to 200 mg per injection were enrolled. There were no dose-limiting toxicities or safety concerns at any dose, and the 200-mg dose was selected dose for Part B of the study.
The median age of the 118 patients was 60 years and their median body mass index was 29.2 kg/m2. No drug-related serious adverse events and no dose-limiting toxicities occurred across doses ranging from 20 mg to 200 mg per injection. The incidence of common adverse events such as knee pain was similar between placebo- and TPX-100-treated knees.
Quantitative MRI showed no measurable between-knee differences in cartilage thickness or volume at 6 or 12 months. However, statistically significant and clinically meaningful differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, including KOOS activities of daily living (P = .008 at 6 and 12 months), KOOS knee-related quality of life (P = .21 at 6 months and P = .03 at 12 months), and a significant reduction in pain going up or down stairs (P = .004 at 12 months). Subjects’ use of nonsteroidal anti-inflammatory medications declined by 63% during the study.
“In this study, we see some terrific, long-term results in knee-related core activities, which certainly were disease modifying from the patients’ perspective,” said Dr. McGuire, who noted that a phase 3 study is being planned. “In addition, patient’s pain going up and down stairs improved significantly, with a marked reduction in analgesic use. Any of us who are experienced in these tough areas of medicine know that early results can look extremely promising, but we have to do larger confirmatory studies.”
SAN DIEGO – Among patients with mild to moderate patellofemoral osteoarthritis, intra-articular administration of the novel agent TPX-100 was safe and associated with functional benefits up to 1 year, a proof-of-concept study showed.
“We don’t yet have a disease-modifying drug for osteoarthritis [OA]; that’s sort of the holy grail for researchers,” lead study author Dawn McGuire, MD, said in an interview at the annual meeting of the American College of Rheumatology. “All of the patient-reported outcome and patient function indices that we studied moved in the same direction, showing a benefit of TPX-100. I think this is very promising.”
The study consisted of two parts. In Part A, four dose cohorts ranging from 20 mg to 200 mg per injection were enrolled. There were no dose-limiting toxicities or safety concerns at any dose, and the 200-mg dose was selected dose for Part B of the study.
The median age of the 118 patients was 60 years and their median body mass index was 29.2 kg/m2. No drug-related serious adverse events and no dose-limiting toxicities occurred across doses ranging from 20 mg to 200 mg per injection. The incidence of common adverse events such as knee pain was similar between placebo- and TPX-100-treated knees.
Quantitative MRI showed no measurable between-knee differences in cartilage thickness or volume at 6 or 12 months. However, statistically significant and clinically meaningful differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, including KOOS activities of daily living (P = .008 at 6 and 12 months), KOOS knee-related quality of life (P = .21 at 6 months and P = .03 at 12 months), and a significant reduction in pain going up or down stairs (P = .004 at 12 months). Subjects’ use of nonsteroidal anti-inflammatory medications declined by 63% during the study.
“In this study, we see some terrific, long-term results in knee-related core activities, which certainly were disease modifying from the patients’ perspective,” said Dr. McGuire, who noted that a phase 3 study is being planned. “In addition, patient’s pain going up and down stairs improved significantly, with a marked reduction in analgesic use. Any of us who are experienced in these tough areas of medicine know that early results can look extremely promising, but we have to do larger confirmatory studies.”
AT ACR 2017
Key clinical point:
Major finding: Statistically significant differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, using Knee Injury and Osteoarthritis Outcome Score activities of daily living (P = .008 at 6 and 12 months) and a significant reduction in pain going up or down stairs (P = .004 at 12 months).
Study details: A randomized, proof-of-concept study involving 118 patients with patellofemoral knee OA.
Disclosures: OrthoTrophix sponsored the study. Dr. McGuire is chief medical officer and a cofounder of the company.
FDA approves first extended-release steroid injection for OA knee pain
Flexion Therapeutics announced Oct. 6 the approval of Zilretta (triamcinolone acetonide extended-release injectable suspension) as the first and only extended-release, intra-articular injection for osteoarthritis knee pain.
Zilretta uses a proprietary microsphere-based formulation of triamcinolone acetonide to provide pain relief over 12 weeks by prolonging the release of triamcinolone acetonide inside the synovial fluid. The approval is based on data from a phase 3 clinical trial. The randomized, double-blind study enrolled 484 patients at 37 centers worldwide. The label also includes the results from a double-blind, randomized, parallel-group trial, which examined blood glucose concentrations in patients with type 2 diabetes to show how Zilretta can avoid blood glucose spikes observed with corticosteroid use.
Zilretta is expected to be available in the United States by the end of October.
Flexion Therapeutics announced Oct. 6 the approval of Zilretta (triamcinolone acetonide extended-release injectable suspension) as the first and only extended-release, intra-articular injection for osteoarthritis knee pain.
Zilretta uses a proprietary microsphere-based formulation of triamcinolone acetonide to provide pain relief over 12 weeks by prolonging the release of triamcinolone acetonide inside the synovial fluid. The approval is based on data from a phase 3 clinical trial. The randomized, double-blind study enrolled 484 patients at 37 centers worldwide. The label also includes the results from a double-blind, randomized, parallel-group trial, which examined blood glucose concentrations in patients with type 2 diabetes to show how Zilretta can avoid blood glucose spikes observed with corticosteroid use.
Zilretta is expected to be available in the United States by the end of October.
Flexion Therapeutics announced Oct. 6 the approval of Zilretta (triamcinolone acetonide extended-release injectable suspension) as the first and only extended-release, intra-articular injection for osteoarthritis knee pain.
Zilretta uses a proprietary microsphere-based formulation of triamcinolone acetonide to provide pain relief over 12 weeks by prolonging the release of triamcinolone acetonide inside the synovial fluid. The approval is based on data from a phase 3 clinical trial. The randomized, double-blind study enrolled 484 patients at 37 centers worldwide. The label also includes the results from a double-blind, randomized, parallel-group trial, which examined blood glucose concentrations in patients with type 2 diabetes to show how Zilretta can avoid blood glucose spikes observed with corticosteroid use.
Zilretta is expected to be available in the United States by the end of October.
Dr. Clyde Yancy: CANTOS wows, opens new therapeutic avenues
BARCELONA – For Clyde Yancy, MD, presentation of the bombshell CANTOS trial results at the annual congress of the European Congress of Cardiology made for “a really good day.”
Those results showed that inhibiting the interleukin-1 beta innate immunity pathway with canakinumab reduced recurrent cardiovascular events and lung cancer. But further, they introduced a new way of identifying and treating patients for secondary prevention.
“Here is an alternative way to get to cardiovascular events; here is bringing inflammation right to the front page of what we do as cardiologists to prevent events; here is a brand-new agent that is a monoclonal antibody against interleukin that may be modifying this risk, and … a remarkable advantage that really needs to be replicated,” said Dr. Yancy, chief of medicine-cardiology at Northwestern University in Chicago, in a video interview.
“This is a really good day” because we’ve got new things to think about, new ways to approach our patients, and [we may soon be] entering the realm where we’ll want personalized therapy based on the unique phenotype a patient represents, and think about the pathways to disease through these brand new schemes” that are helping us understand the burden of disease, he declared.
BARCELONA – For Clyde Yancy, MD, presentation of the bombshell CANTOS trial results at the annual congress of the European Congress of Cardiology made for “a really good day.”
Those results showed that inhibiting the interleukin-1 beta innate immunity pathway with canakinumab reduced recurrent cardiovascular events and lung cancer. But further, they introduced a new way of identifying and treating patients for secondary prevention.
“Here is an alternative way to get to cardiovascular events; here is bringing inflammation right to the front page of what we do as cardiologists to prevent events; here is a brand-new agent that is a monoclonal antibody against interleukin that may be modifying this risk, and … a remarkable advantage that really needs to be replicated,” said Dr. Yancy, chief of medicine-cardiology at Northwestern University in Chicago, in a video interview.
“This is a really good day” because we’ve got new things to think about, new ways to approach our patients, and [we may soon be] entering the realm where we’ll want personalized therapy based on the unique phenotype a patient represents, and think about the pathways to disease through these brand new schemes” that are helping us understand the burden of disease, he declared.
BARCELONA – For Clyde Yancy, MD, presentation of the bombshell CANTOS trial results at the annual congress of the European Congress of Cardiology made for “a really good day.”
Those results showed that inhibiting the interleukin-1 beta innate immunity pathway with canakinumab reduced recurrent cardiovascular events and lung cancer. But further, they introduced a new way of identifying and treating patients for secondary prevention.
“Here is an alternative way to get to cardiovascular events; here is bringing inflammation right to the front page of what we do as cardiologists to prevent events; here is a brand-new agent that is a monoclonal antibody against interleukin that may be modifying this risk, and … a remarkable advantage that really needs to be replicated,” said Dr. Yancy, chief of medicine-cardiology at Northwestern University in Chicago, in a video interview.
“This is a really good day” because we’ve got new things to think about, new ways to approach our patients, and [we may soon be] entering the realm where we’ll want personalized therapy based on the unique phenotype a patient represents, and think about the pathways to disease through these brand new schemes” that are helping us understand the burden of disease, he declared.
AT THE ESC CONGRESS 2017
VIDEO: Prescription-strength ibuprofen worsens blood pressure more than other NSAIDs
BARCELONA – Prescription-strength ibuprofen has a bigger adverse effect on blood pressure than celecoxib or naproxen, a finding that suggests a likely mechanism for the worse cardiovascular event rate documented in ibuprofen-treated arthritis patients in the PRECISION trial, Frank Ruschitzka, MD, said at the annual congress of the European Society of Cardiology.
“Prescription-strength ibuprofen is under pressure – it has a high incidence of new-onset hypertension, particularly when compared to the more selective COX-2 inhibitor celecoxib. Before we did this study, many would have said it’s the other way around,” observed Dr. Ruschitzka, professor of cardiology at the University of Zurich.
He presented the results of PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement).
“These results will have impact on your daily practice when you go home,” the cardiologist promised.
PRECISION-ABPM was a prespecified double-blind, randomized, 60-center substudy of the published PRECISION trial, which included 24,081 U.S. patients who needed daily NSAIDs for arthritis and were also at increased cardiovascular risk. They were randomized to the COX-2 inhibitor celecoxib at 100-200 mg b.i.d. or the nonselective NSAIDs ibuprofen at 600-800 mg three times a day or naproxen at 375-500 mg twice daily. Participants also received a proton pump inhibitor to protect against NSAID-related GI bleeding. In the on-treatment analysis, the ibuprofen group was significantly more likely to experience cardiovascular and all-cause mortality and renal events than were those on celecoxib (N Engl J Med. 2016 Dec 29;375[26]:2519-29).
The PRECISION-ABPM substudy included 444 arthritis patients, 92% of whom had osteoarthritis. During the 4-month study, investigators amassed roughly 60,000 automated blood pressure measurements across the three study arms.
The primary outcome was change from baseline in mean 24-hour systolic blood pressure (SBP). It increased by 3.7 mm Hg in the ibuprofen group and declined by 0.3 mm Hg in the celecoxib group, while the naproxen group occupied the middle ground with a 1.6-mm Hg increase.
The nearly 4-mm Hg increase in mean 24-hour SBP at 4 months in the ibuprofen group is of sufficient magnitude to be clinically important, Dr. Ruschitzka noted. He noted that fully 23.2% of ibuprofen-treated patients who had normal baseline blood pressure developed hypertension as defined by a mean 24-hour SBP of at least 130 and/or a diastolic blood pressure of at least 80 mm Hg. In contrast, incident hypertension occurred in only 10.3% of the celecoxib group and 19% of naproxen-treated patients. Thus, the likelihood of developing hypertension was 61% less with celecoxib than ibuprofen and 51% less with celecoxib than naproxen.
Not treating chronic arthritic pain to avoid the cardiovascular risk of NSAIDs is not a legitimate option.
“Pain is a cardiovascular risk factor,” Dr. Ruschitzka emphasized. “It’s unethical not to treat it. If you don’t treat pain, the patient’s blood pressure goes up, heart rate goes up, and you’re driving patients into inactivity.”
Although he’s convinced there’s no such thing as a safe NSAID from a cardiovascular risk standpoint, the PRECISION and PRECISION-ABPM data show celecoxib is less unsafe than ibuprofen. And as for the oft-heard statement that naproxen is the safest NSAID for the heart, Dr. Ruschitzka snorted, “What an urban legend.”
Discussant Scott Solomon, MD, opined that, while PRECISION-ABPM doesn’t support the notion that conventional NSAIDs such as naproxen or ibuprofen are any safer than celecoxib, it would be wrong to conclude from the study that celecoxib doesn’t affect blood pressure and is safer than the others from a cardiovascular standpoint. That’s because the three study drugs weren’t compared in an equipotent way. Because of safety concerns, the Food and Drug Administration required that the daily dose of celecoxib be capped at the low end of the therapeutic range, while no such constraints were placed on the two nonselective NSAIDS.
“Compared to placebo, all NSAIDs likely raise blood pressure, especially in patients prone to hypertension, those with chronic kidney disease, the elderly – and this is exactly the type of patients who require NSAIDs for arthritis. Whichever NSAID is chosen, clinicians should be aware of this effect and treat hypertension according to guidelines,” said Dr. Solomon, director of noninvasive cardiology at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
Dr. Solomon has been a key figure in the COX-2 inhibitor controversy of the last decade. He was lead author of a 2005 review of data from clinical trials of COX-2 inhibitors for colorectal adenoma prevention, which concluded that the drugs had a cardiovascular safety issue in that setting (N Engl J Med. 2005 Mar 17;352[11]:1071-80).
“Our analysis of celecoxib concluded that a dose-dependent increase in cardiovascular events was there, was real, but notably occurred at doses which were substantially higher than what we typically use for patients with arthritis,” he said.
That report triggered a fevered reaction.
“Amid an enormous amount of hype, hyperbole, and hysteria, the safety of these agents was thrown into question, leading to the withdrawal of all but one of them from the market and a black-box warning around the one remaining agent, celecoxib,” he recalled.
Dr. Ruschitzka discussed his findings in a video interview.
PRECISION-ABPM was sponsored by Pfizer. Dr. Ruschitzka and Dr. Solomon reported having no financial conflicts of interest regarding their presentations.
BARCELONA – Prescription-strength ibuprofen has a bigger adverse effect on blood pressure than celecoxib or naproxen, a finding that suggests a likely mechanism for the worse cardiovascular event rate documented in ibuprofen-treated arthritis patients in the PRECISION trial, Frank Ruschitzka, MD, said at the annual congress of the European Society of Cardiology.
“Prescription-strength ibuprofen is under pressure – it has a high incidence of new-onset hypertension, particularly when compared to the more selective COX-2 inhibitor celecoxib. Before we did this study, many would have said it’s the other way around,” observed Dr. Ruschitzka, professor of cardiology at the University of Zurich.
He presented the results of PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement).
“These results will have impact on your daily practice when you go home,” the cardiologist promised.
PRECISION-ABPM was a prespecified double-blind, randomized, 60-center substudy of the published PRECISION trial, which included 24,081 U.S. patients who needed daily NSAIDs for arthritis and were also at increased cardiovascular risk. They were randomized to the COX-2 inhibitor celecoxib at 100-200 mg b.i.d. or the nonselective NSAIDs ibuprofen at 600-800 mg three times a day or naproxen at 375-500 mg twice daily. Participants also received a proton pump inhibitor to protect against NSAID-related GI bleeding. In the on-treatment analysis, the ibuprofen group was significantly more likely to experience cardiovascular and all-cause mortality and renal events than were those on celecoxib (N Engl J Med. 2016 Dec 29;375[26]:2519-29).
The PRECISION-ABPM substudy included 444 arthritis patients, 92% of whom had osteoarthritis. During the 4-month study, investigators amassed roughly 60,000 automated blood pressure measurements across the three study arms.
The primary outcome was change from baseline in mean 24-hour systolic blood pressure (SBP). It increased by 3.7 mm Hg in the ibuprofen group and declined by 0.3 mm Hg in the celecoxib group, while the naproxen group occupied the middle ground with a 1.6-mm Hg increase.
The nearly 4-mm Hg increase in mean 24-hour SBP at 4 months in the ibuprofen group is of sufficient magnitude to be clinically important, Dr. Ruschitzka noted. He noted that fully 23.2% of ibuprofen-treated patients who had normal baseline blood pressure developed hypertension as defined by a mean 24-hour SBP of at least 130 and/or a diastolic blood pressure of at least 80 mm Hg. In contrast, incident hypertension occurred in only 10.3% of the celecoxib group and 19% of naproxen-treated patients. Thus, the likelihood of developing hypertension was 61% less with celecoxib than ibuprofen and 51% less with celecoxib than naproxen.
Not treating chronic arthritic pain to avoid the cardiovascular risk of NSAIDs is not a legitimate option.
“Pain is a cardiovascular risk factor,” Dr. Ruschitzka emphasized. “It’s unethical not to treat it. If you don’t treat pain, the patient’s blood pressure goes up, heart rate goes up, and you’re driving patients into inactivity.”
Although he’s convinced there’s no such thing as a safe NSAID from a cardiovascular risk standpoint, the PRECISION and PRECISION-ABPM data show celecoxib is less unsafe than ibuprofen. And as for the oft-heard statement that naproxen is the safest NSAID for the heart, Dr. Ruschitzka snorted, “What an urban legend.”
Discussant Scott Solomon, MD, opined that, while PRECISION-ABPM doesn’t support the notion that conventional NSAIDs such as naproxen or ibuprofen are any safer than celecoxib, it would be wrong to conclude from the study that celecoxib doesn’t affect blood pressure and is safer than the others from a cardiovascular standpoint. That’s because the three study drugs weren’t compared in an equipotent way. Because of safety concerns, the Food and Drug Administration required that the daily dose of celecoxib be capped at the low end of the therapeutic range, while no such constraints were placed on the two nonselective NSAIDS.
“Compared to placebo, all NSAIDs likely raise blood pressure, especially in patients prone to hypertension, those with chronic kidney disease, the elderly – and this is exactly the type of patients who require NSAIDs for arthritis. Whichever NSAID is chosen, clinicians should be aware of this effect and treat hypertension according to guidelines,” said Dr. Solomon, director of noninvasive cardiology at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
Dr. Solomon has been a key figure in the COX-2 inhibitor controversy of the last decade. He was lead author of a 2005 review of data from clinical trials of COX-2 inhibitors for colorectal adenoma prevention, which concluded that the drugs had a cardiovascular safety issue in that setting (N Engl J Med. 2005 Mar 17;352[11]:1071-80).
“Our analysis of celecoxib concluded that a dose-dependent increase in cardiovascular events was there, was real, but notably occurred at doses which were substantially higher than what we typically use for patients with arthritis,” he said.
That report triggered a fevered reaction.
“Amid an enormous amount of hype, hyperbole, and hysteria, the safety of these agents was thrown into question, leading to the withdrawal of all but one of them from the market and a black-box warning around the one remaining agent, celecoxib,” he recalled.
Dr. Ruschitzka discussed his findings in a video interview.
PRECISION-ABPM was sponsored by Pfizer. Dr. Ruschitzka and Dr. Solomon reported having no financial conflicts of interest regarding their presentations.
BARCELONA – Prescription-strength ibuprofen has a bigger adverse effect on blood pressure than celecoxib or naproxen, a finding that suggests a likely mechanism for the worse cardiovascular event rate documented in ibuprofen-treated arthritis patients in the PRECISION trial, Frank Ruschitzka, MD, said at the annual congress of the European Society of Cardiology.
“Prescription-strength ibuprofen is under pressure – it has a high incidence of new-onset hypertension, particularly when compared to the more selective COX-2 inhibitor celecoxib. Before we did this study, many would have said it’s the other way around,” observed Dr. Ruschitzka, professor of cardiology at the University of Zurich.
He presented the results of PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement).
“These results will have impact on your daily practice when you go home,” the cardiologist promised.
PRECISION-ABPM was a prespecified double-blind, randomized, 60-center substudy of the published PRECISION trial, which included 24,081 U.S. patients who needed daily NSAIDs for arthritis and were also at increased cardiovascular risk. They were randomized to the COX-2 inhibitor celecoxib at 100-200 mg b.i.d. or the nonselective NSAIDs ibuprofen at 600-800 mg three times a day or naproxen at 375-500 mg twice daily. Participants also received a proton pump inhibitor to protect against NSAID-related GI bleeding. In the on-treatment analysis, the ibuprofen group was significantly more likely to experience cardiovascular and all-cause mortality and renal events than were those on celecoxib (N Engl J Med. 2016 Dec 29;375[26]:2519-29).
The PRECISION-ABPM substudy included 444 arthritis patients, 92% of whom had osteoarthritis. During the 4-month study, investigators amassed roughly 60,000 automated blood pressure measurements across the three study arms.
The primary outcome was change from baseline in mean 24-hour systolic blood pressure (SBP). It increased by 3.7 mm Hg in the ibuprofen group and declined by 0.3 mm Hg in the celecoxib group, while the naproxen group occupied the middle ground with a 1.6-mm Hg increase.
The nearly 4-mm Hg increase in mean 24-hour SBP at 4 months in the ibuprofen group is of sufficient magnitude to be clinically important, Dr. Ruschitzka noted. He noted that fully 23.2% of ibuprofen-treated patients who had normal baseline blood pressure developed hypertension as defined by a mean 24-hour SBP of at least 130 and/or a diastolic blood pressure of at least 80 mm Hg. In contrast, incident hypertension occurred in only 10.3% of the celecoxib group and 19% of naproxen-treated patients. Thus, the likelihood of developing hypertension was 61% less with celecoxib than ibuprofen and 51% less with celecoxib than naproxen.
Not treating chronic arthritic pain to avoid the cardiovascular risk of NSAIDs is not a legitimate option.
“Pain is a cardiovascular risk factor,” Dr. Ruschitzka emphasized. “It’s unethical not to treat it. If you don’t treat pain, the patient’s blood pressure goes up, heart rate goes up, and you’re driving patients into inactivity.”
Although he’s convinced there’s no such thing as a safe NSAID from a cardiovascular risk standpoint, the PRECISION and PRECISION-ABPM data show celecoxib is less unsafe than ibuprofen. And as for the oft-heard statement that naproxen is the safest NSAID for the heart, Dr. Ruschitzka snorted, “What an urban legend.”
Discussant Scott Solomon, MD, opined that, while PRECISION-ABPM doesn’t support the notion that conventional NSAIDs such as naproxen or ibuprofen are any safer than celecoxib, it would be wrong to conclude from the study that celecoxib doesn’t affect blood pressure and is safer than the others from a cardiovascular standpoint. That’s because the three study drugs weren’t compared in an equipotent way. Because of safety concerns, the Food and Drug Administration required that the daily dose of celecoxib be capped at the low end of the therapeutic range, while no such constraints were placed on the two nonselective NSAIDS.
“Compared to placebo, all NSAIDs likely raise blood pressure, especially in patients prone to hypertension, those with chronic kidney disease, the elderly – and this is exactly the type of patients who require NSAIDs for arthritis. Whichever NSAID is chosen, clinicians should be aware of this effect and treat hypertension according to guidelines,” said Dr. Solomon, director of noninvasive cardiology at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
Dr. Solomon has been a key figure in the COX-2 inhibitor controversy of the last decade. He was lead author of a 2005 review of data from clinical trials of COX-2 inhibitors for colorectal adenoma prevention, which concluded that the drugs had a cardiovascular safety issue in that setting (N Engl J Med. 2005 Mar 17;352[11]:1071-80).
“Our analysis of celecoxib concluded that a dose-dependent increase in cardiovascular events was there, was real, but notably occurred at doses which were substantially higher than what we typically use for patients with arthritis,” he said.
That report triggered a fevered reaction.
“Amid an enormous amount of hype, hyperbole, and hysteria, the safety of these agents was thrown into question, leading to the withdrawal of all but one of them from the market and a black-box warning around the one remaining agent, celecoxib,” he recalled.
Dr. Ruschitzka discussed his findings in a video interview.
PRECISION-ABPM was sponsored by Pfizer. Dr. Ruschitzka and Dr. Solomon reported having no financial conflicts of interest regarding their presentations.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: Incident hypertension occurred within 4 months in 23.2% of arthritis patients on ibuprofen, compared with 10.3% taking celecoxib and 19% on naproxen.
Data source: This was a randomized, double-blind, multicenter, prospective trial including 444 arthritis patients at increased cardiovascular risk who underwent 4 months of ambulatory blood pressure monitoring after being assigned to prescription-strength ibuprofen, naproxen, or celecoxib.
Disclosures: The PRECISION-ABPM trial was sponsored by Pfizer. The presenter reported having no financial conflicts of interest.