User login
Study provides new insight into malaria transmission
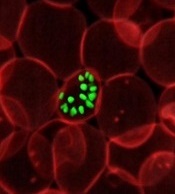
infecting a red blood cell
Image courtesy of St. Jude
Children’s Research Hospital
Research published in PNAS helps explain how the malaria parasite Plasmodium falciparum undergoes the changes that enable transmission of the parasite from humans to mosquitoes.
Investigators determined how the parasite transforms its own structure and the structure of a host red blood cell so the parasite can hide from the body’s normal defenses and later re-enter the bloodstream for transmission via mosquito bite.
The team believes that, by understanding this process, it may be possible to inhibit the blood cell’s transformation.
“Once you understand the molecular mechanisms, it becomes easier to find drugs to target them,” said Sulin Zhang, PhD, of Pennsylvania State University in University Park.
Dr Zhang developed the computational methods used to understand the physical transformations in the infected red blood cells that allow them to avoid removal in the spleen and prepare for transmission to a mosquito host.
He and his colleagues knew that healthy red blood cells are able to squeeze through small slits in the spleen, but damaged and aging red blood cells cannot and are filtered out and removed from the circulation.
To avoid this fate, the sexual stage malaria parasite first makes the red blood cell rigid and hides out in deep tissue. Then, when the parasite is mature, the infected red blood cells become flexible and elastic, ready to be picked up by a mosquito for disease transmission.
To understand these changes, the investigators prepared samples of parasites at each stage and studied the changing microstructure using atomic force microscopy.
This revealed changes in the organization of a meshwork of tiny spring-like proteins in the blood cell membrane. When the parasite is ready for transmission, it reverses the structural changes.
The team then turned to Dr Zhang, who developed a model to explain how subtle changes to the molecular structure of the spring-like proteins were sufficient to make the red blood cell either rigid or flexible.
The investigators are continuing to use Dr Zhang’s model to simulate the overall shapes and the flow dynamics of infected red blood cells in the bloodstream, providing information that could aid researchers looking to inhibit the malaria parasite’s spread. ![]()

infecting a red blood cell
Image courtesy of St. Jude
Children’s Research Hospital
Research published in PNAS helps explain how the malaria parasite Plasmodium falciparum undergoes the changes that enable transmission of the parasite from humans to mosquitoes.
Investigators determined how the parasite transforms its own structure and the structure of a host red blood cell so the parasite can hide from the body’s normal defenses and later re-enter the bloodstream for transmission via mosquito bite.
The team believes that, by understanding this process, it may be possible to inhibit the blood cell’s transformation.
“Once you understand the molecular mechanisms, it becomes easier to find drugs to target them,” said Sulin Zhang, PhD, of Pennsylvania State University in University Park.
Dr Zhang developed the computational methods used to understand the physical transformations in the infected red blood cells that allow them to avoid removal in the spleen and prepare for transmission to a mosquito host.
He and his colleagues knew that healthy red blood cells are able to squeeze through small slits in the spleen, but damaged and aging red blood cells cannot and are filtered out and removed from the circulation.
To avoid this fate, the sexual stage malaria parasite first makes the red blood cell rigid and hides out in deep tissue. Then, when the parasite is mature, the infected red blood cells become flexible and elastic, ready to be picked up by a mosquito for disease transmission.
To understand these changes, the investigators prepared samples of parasites at each stage and studied the changing microstructure using atomic force microscopy.
This revealed changes in the organization of a meshwork of tiny spring-like proteins in the blood cell membrane. When the parasite is ready for transmission, it reverses the structural changes.
The team then turned to Dr Zhang, who developed a model to explain how subtle changes to the molecular structure of the spring-like proteins were sufficient to make the red blood cell either rigid or flexible.
The investigators are continuing to use Dr Zhang’s model to simulate the overall shapes and the flow dynamics of infected red blood cells in the bloodstream, providing information that could aid researchers looking to inhibit the malaria parasite’s spread. ![]()

infecting a red blood cell
Image courtesy of St. Jude
Children’s Research Hospital
Research published in PNAS helps explain how the malaria parasite Plasmodium falciparum undergoes the changes that enable transmission of the parasite from humans to mosquitoes.
Investigators determined how the parasite transforms its own structure and the structure of a host red blood cell so the parasite can hide from the body’s normal defenses and later re-enter the bloodstream for transmission via mosquito bite.
The team believes that, by understanding this process, it may be possible to inhibit the blood cell’s transformation.
“Once you understand the molecular mechanisms, it becomes easier to find drugs to target them,” said Sulin Zhang, PhD, of Pennsylvania State University in University Park.
Dr Zhang developed the computational methods used to understand the physical transformations in the infected red blood cells that allow them to avoid removal in the spleen and prepare for transmission to a mosquito host.
He and his colleagues knew that healthy red blood cells are able to squeeze through small slits in the spleen, but damaged and aging red blood cells cannot and are filtered out and removed from the circulation.
To avoid this fate, the sexual stage malaria parasite first makes the red blood cell rigid and hides out in deep tissue. Then, when the parasite is mature, the infected red blood cells become flexible and elastic, ready to be picked up by a mosquito for disease transmission.
To understand these changes, the investigators prepared samples of parasites at each stage and studied the changing microstructure using atomic force microscopy.
This revealed changes in the organization of a meshwork of tiny spring-like proteins in the blood cell membrane. When the parasite is ready for transmission, it reverses the structural changes.
The team then turned to Dr Zhang, who developed a model to explain how subtle changes to the molecular structure of the spring-like proteins were sufficient to make the red blood cell either rigid or flexible.
The investigators are continuing to use Dr Zhang’s model to simulate the overall shapes and the flow dynamics of infected red blood cells in the bloodstream, providing information that could aid researchers looking to inhibit the malaria parasite’s spread. ![]()
CDC says Zika causes microcephaly, other birth defects

Photo by Nina Matthews
After reviewing existing evidence, scientists from the US Centers for Disease Control and Prevention (CDC) have concluded that Zika virus causes
microcephaly and other severe fetal brain defects.
“This study marks a turning point in the Zika outbreak,” said Tom Frieden, MD, director of the CDC.
“It is now clear that the virus causes microcephaly. We’ve now confirmed what mounting evidence has suggested.”
Details on the CDC’s review were published in NEJM.
The report notes that no single piece of evidence provides conclusive proof that Zika virus infection is a cause of microcephaly and other fetal brain defects. Rather, increasing evidence from a number of recently published studies and a careful evaluation using established scientific criteria support the authors’ conclusions.
The finding that Zika virus infection can cause microcephaly and other severe fetal brain defects means that a woman who is infected with Zika during pregnancy has an increased risk of having a baby with these health problems.
However, as we’ve seen during the current Zika outbreak, some infected women deliver babies that appear to be healthy.
The CDC said establishing the causal relationship between Zika and fetal brain defects is an important step in driving additional prevention efforts, focusing research activities, and reinforcing the need for direct communication about the risks of Zika.
However, the agency noted that many questions about the Zika virus remain. And answering these questions will be the focus of ongoing research.
The CDC also said it is not changing its current recommendations regarding the Zika virus. Pregnant women should continue to avoid travel to areas where Zika is actively spreading.
If a pregnant woman travels to or lives in an area with active Zika virus transmission, she should talk with her healthcare provider and take steps to prevent mosquito bites and sexual transmission of the virus.
The CDC also encourages women and their partners in areas with active Zika transmission to engage in pregnancy planning and counseling with their healthcare providers so they know the risks and the ways to mitigate them. ![]()

Photo by Nina Matthews
After reviewing existing evidence, scientists from the US Centers for Disease Control and Prevention (CDC) have concluded that Zika virus causes
microcephaly and other severe fetal brain defects.
“This study marks a turning point in the Zika outbreak,” said Tom Frieden, MD, director of the CDC.
“It is now clear that the virus causes microcephaly. We’ve now confirmed what mounting evidence has suggested.”
Details on the CDC’s review were published in NEJM.
The report notes that no single piece of evidence provides conclusive proof that Zika virus infection is a cause of microcephaly and other fetal brain defects. Rather, increasing evidence from a number of recently published studies and a careful evaluation using established scientific criteria support the authors’ conclusions.
The finding that Zika virus infection can cause microcephaly and other severe fetal brain defects means that a woman who is infected with Zika during pregnancy has an increased risk of having a baby with these health problems.
However, as we’ve seen during the current Zika outbreak, some infected women deliver babies that appear to be healthy.
The CDC said establishing the causal relationship between Zika and fetal brain defects is an important step in driving additional prevention efforts, focusing research activities, and reinforcing the need for direct communication about the risks of Zika.
However, the agency noted that many questions about the Zika virus remain. And answering these questions will be the focus of ongoing research.
The CDC also said it is not changing its current recommendations regarding the Zika virus. Pregnant women should continue to avoid travel to areas where Zika is actively spreading.
If a pregnant woman travels to or lives in an area with active Zika virus transmission, she should talk with her healthcare provider and take steps to prevent mosquito bites and sexual transmission of the virus.
The CDC also encourages women and their partners in areas with active Zika transmission to engage in pregnancy planning and counseling with their healthcare providers so they know the risks and the ways to mitigate them. ![]()

Photo by Nina Matthews
After reviewing existing evidence, scientists from the US Centers for Disease Control and Prevention (CDC) have concluded that Zika virus causes
microcephaly and other severe fetal brain defects.
“This study marks a turning point in the Zika outbreak,” said Tom Frieden, MD, director of the CDC.
“It is now clear that the virus causes microcephaly. We’ve now confirmed what mounting evidence has suggested.”
Details on the CDC’s review were published in NEJM.
The report notes that no single piece of evidence provides conclusive proof that Zika virus infection is a cause of microcephaly and other fetal brain defects. Rather, increasing evidence from a number of recently published studies and a careful evaluation using established scientific criteria support the authors’ conclusions.
The finding that Zika virus infection can cause microcephaly and other severe fetal brain defects means that a woman who is infected with Zika during pregnancy has an increased risk of having a baby with these health problems.
However, as we’ve seen during the current Zika outbreak, some infected women deliver babies that appear to be healthy.
The CDC said establishing the causal relationship between Zika and fetal brain defects is an important step in driving additional prevention efforts, focusing research activities, and reinforcing the need for direct communication about the risks of Zika.
However, the agency noted that many questions about the Zika virus remain. And answering these questions will be the focus of ongoing research.
The CDC also said it is not changing its current recommendations regarding the Zika virus. Pregnant women should continue to avoid travel to areas where Zika is actively spreading.
If a pregnant woman travels to or lives in an area with active Zika virus transmission, she should talk with her healthcare provider and take steps to prevent mosquito bites and sexual transmission of the virus.
The CDC also encourages women and their partners in areas with active Zika transmission to engage in pregnancy planning and counseling with their healthcare providers so they know the risks and the ways to mitigate them. ![]()
Survey says docs don’t know FDA requirements

A survey of nearly 700 US physicians revealed that many do not know the basic requirements for a drug to receive approval from the US Food and
Drug Administration (FDA).
The results also suggested that most physicians don’t understand the FDA’s “breakthrough therapy” designation.
Since 2012, the FDA has been able to designate a drug as a breakthrough therapy if preliminary clinical evidence suggests it provides an advantage
over existing options.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues wanted to determine how much physicians understood about this designation and if they had a basic understanding of the FDA’s requirements for drug approval.
So the researchers surveyed internists and specialists from the American Board of Internal Medicine’s diplomate list and reported the results of this survey in JAMA.
Of the 1148 physicians contacted, 692 (60%) responded. Participants were asked 3 questions about FDA approval and 5 about breakthrough therapies.
FDA approval
Seventy-three percent of respondents incorrectly believed that, for a drug to gain FDA approval, it must be as effective as drugs that are already approved.
However, 85% of respondents answered correctly that FDA-approved drugs typically have a favorable benefit/harm ratio.
Seventy percent of respondents incorrectly believed that FDA approval requires a drug to have both a statistically significant and a clinically important effect.
Only 6% of respondents knew the correct answer—that neither is required.
Breakthrough designation
Twenty percent of respondents said they were “familiar” (17%) or “very familiar” (3%) with breakthrough therapy designation, while 37% said they were “a little familiar,” and 42% said they were “not familiar at all.”
Fifty-eight percent of respondents said they were “fairly certain” that an FDA-approved breakthrough drug represents a major advance over currently approved treatments for its indication. Thirty-one percent said they were “fairly uncertain,” 5% said they were “very uncertain,” and 6% said they were “very certain.”
Fifty-two percent of respondents incorrectly believed that strong evidence (ie, randomized trials) is needed to earn the breakthrough designation.
However, 45% correctly answered that only preliminary evidence (eg, uncontrolled studies or studies testing surrogate outcomes) is needed. Four percent said very preliminary evidence (eg, in vitro laboratory or animal studies) is needed.
Seventy-seven percent of respondents incorrectly believed that, when the FDA grants breakthrough designation, there is high-quality evidence that the drug is more effective than currently approved treatments.
But 74% of respondents answered correctly that breakthrough designation does not mean there is high-quality evidence that the drug is safer than currently approved treatments.
Dr Kesselheim and his colleagues said the misconceptions identified in this survey may lead physicians to overprescribe newly approved drugs—particularly breakthrough therapies—and inadequately communicate how well these drugs work to patients. ![]()

A survey of nearly 700 US physicians revealed that many do not know the basic requirements for a drug to receive approval from the US Food and
Drug Administration (FDA).
The results also suggested that most physicians don’t understand the FDA’s “breakthrough therapy” designation.
Since 2012, the FDA has been able to designate a drug as a breakthrough therapy if preliminary clinical evidence suggests it provides an advantage
over existing options.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues wanted to determine how much physicians understood about this designation and if they had a basic understanding of the FDA’s requirements for drug approval.
So the researchers surveyed internists and specialists from the American Board of Internal Medicine’s diplomate list and reported the results of this survey in JAMA.
Of the 1148 physicians contacted, 692 (60%) responded. Participants were asked 3 questions about FDA approval and 5 about breakthrough therapies.
FDA approval
Seventy-three percent of respondents incorrectly believed that, for a drug to gain FDA approval, it must be as effective as drugs that are already approved.
However, 85% of respondents answered correctly that FDA-approved drugs typically have a favorable benefit/harm ratio.
Seventy percent of respondents incorrectly believed that FDA approval requires a drug to have both a statistically significant and a clinically important effect.
Only 6% of respondents knew the correct answer—that neither is required.
Breakthrough designation
Twenty percent of respondents said they were “familiar” (17%) or “very familiar” (3%) with breakthrough therapy designation, while 37% said they were “a little familiar,” and 42% said they were “not familiar at all.”
Fifty-eight percent of respondents said they were “fairly certain” that an FDA-approved breakthrough drug represents a major advance over currently approved treatments for its indication. Thirty-one percent said they were “fairly uncertain,” 5% said they were “very uncertain,” and 6% said they were “very certain.”
Fifty-two percent of respondents incorrectly believed that strong evidence (ie, randomized trials) is needed to earn the breakthrough designation.
However, 45% correctly answered that only preliminary evidence (eg, uncontrolled studies or studies testing surrogate outcomes) is needed. Four percent said very preliminary evidence (eg, in vitro laboratory or animal studies) is needed.
Seventy-seven percent of respondents incorrectly believed that, when the FDA grants breakthrough designation, there is high-quality evidence that the drug is more effective than currently approved treatments.
But 74% of respondents answered correctly that breakthrough designation does not mean there is high-quality evidence that the drug is safer than currently approved treatments.
Dr Kesselheim and his colleagues said the misconceptions identified in this survey may lead physicians to overprescribe newly approved drugs—particularly breakthrough therapies—and inadequately communicate how well these drugs work to patients. ![]()

A survey of nearly 700 US physicians revealed that many do not know the basic requirements for a drug to receive approval from the US Food and
Drug Administration (FDA).
The results also suggested that most physicians don’t understand the FDA’s “breakthrough therapy” designation.
Since 2012, the FDA has been able to designate a drug as a breakthrough therapy if preliminary clinical evidence suggests it provides an advantage
over existing options.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues wanted to determine how much physicians understood about this designation and if they had a basic understanding of the FDA’s requirements for drug approval.
So the researchers surveyed internists and specialists from the American Board of Internal Medicine’s diplomate list and reported the results of this survey in JAMA.
Of the 1148 physicians contacted, 692 (60%) responded. Participants were asked 3 questions about FDA approval and 5 about breakthrough therapies.
FDA approval
Seventy-three percent of respondents incorrectly believed that, for a drug to gain FDA approval, it must be as effective as drugs that are already approved.
However, 85% of respondents answered correctly that FDA-approved drugs typically have a favorable benefit/harm ratio.
Seventy percent of respondents incorrectly believed that FDA approval requires a drug to have both a statistically significant and a clinically important effect.
Only 6% of respondents knew the correct answer—that neither is required.
Breakthrough designation
Twenty percent of respondents said they were “familiar” (17%) or “very familiar” (3%) with breakthrough therapy designation, while 37% said they were “a little familiar,” and 42% said they were “not familiar at all.”
Fifty-eight percent of respondents said they were “fairly certain” that an FDA-approved breakthrough drug represents a major advance over currently approved treatments for its indication. Thirty-one percent said they were “fairly uncertain,” 5% said they were “very uncertain,” and 6% said they were “very certain.”
Fifty-two percent of respondents incorrectly believed that strong evidence (ie, randomized trials) is needed to earn the breakthrough designation.
However, 45% correctly answered that only preliminary evidence (eg, uncontrolled studies or studies testing surrogate outcomes) is needed. Four percent said very preliminary evidence (eg, in vitro laboratory or animal studies) is needed.
Seventy-seven percent of respondents incorrectly believed that, when the FDA grants breakthrough designation, there is high-quality evidence that the drug is more effective than currently approved treatments.
But 74% of respondents answered correctly that breakthrough designation does not mean there is high-quality evidence that the drug is safer than currently approved treatments.
Dr Kesselheim and his colleagues said the misconceptions identified in this survey may lead physicians to overprescribe newly approved drugs—particularly breakthrough therapies—and inadequately communicate how well these drugs work to patients. ![]()
Protein distribution impacts T cells’ fate
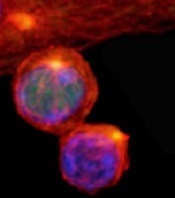
(with c-Myc in green)
Image courtesy of
Katherine Verbist and St. Jude
New research published in Nature helps explain how 2 types of cells arise from activated T cells.
Investigators found that distribution of the regulatory protein c-Myc during asymmetric cell division impacts an activated T cell’s fate, determining whether it will become an effector T cell or a memory T cell.
The team therefore believes that manipulating c-Myc levels could make vaccines more effective or advance immunotherapies for cancer treatment.
“Our work suggests that it may be possible to manipulate the immune response by nudging production of c-Myc in one direction or the other,” said study author Douglas Green, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Potentially, that could mean more effective vaccines or help to advance T-cell immune therapy for cancer treatment.”
Through a series of experiments, Dr Green and his colleagues found that, during asymmetric cell division of activated T cells, high levels of c-Myc accumulated in one daughter cell.
There, c-Myc launched and sustained the rapid proliferation of effector T cells, including those in mice infected with the influenza virus.
In contrast, daughter cells with low levels of c-Myc functioned like memory T cells, proliferating to mount an immune response a month later when mice were again exposed to the virus.
The investigators also identified the metabolic and signaling pathways that serve as a positive feedback loop to sustain the high levels of c-Myc that effector T cells require to maintain their identities and function.
The team showed that disrupting certain components of the system disturbed c-Myc production, which altered the fate of T cells and caused effector T cells to operate like memory T cells.
“While daughter cells of activated T cells seem to have very different fates, we showed their behavior could be altered by manipulating these metabolic and regulatory pathways to increase or decrease c-Myc levels,” Dr Green said. ![]()

(with c-Myc in green)
Image courtesy of
Katherine Verbist and St. Jude
New research published in Nature helps explain how 2 types of cells arise from activated T cells.
Investigators found that distribution of the regulatory protein c-Myc during asymmetric cell division impacts an activated T cell’s fate, determining whether it will become an effector T cell or a memory T cell.
The team therefore believes that manipulating c-Myc levels could make vaccines more effective or advance immunotherapies for cancer treatment.
“Our work suggests that it may be possible to manipulate the immune response by nudging production of c-Myc in one direction or the other,” said study author Douglas Green, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Potentially, that could mean more effective vaccines or help to advance T-cell immune therapy for cancer treatment.”
Through a series of experiments, Dr Green and his colleagues found that, during asymmetric cell division of activated T cells, high levels of c-Myc accumulated in one daughter cell.
There, c-Myc launched and sustained the rapid proliferation of effector T cells, including those in mice infected with the influenza virus.
In contrast, daughter cells with low levels of c-Myc functioned like memory T cells, proliferating to mount an immune response a month later when mice were again exposed to the virus.
The investigators also identified the metabolic and signaling pathways that serve as a positive feedback loop to sustain the high levels of c-Myc that effector T cells require to maintain their identities and function.
The team showed that disrupting certain components of the system disturbed c-Myc production, which altered the fate of T cells and caused effector T cells to operate like memory T cells.
“While daughter cells of activated T cells seem to have very different fates, we showed their behavior could be altered by manipulating these metabolic and regulatory pathways to increase or decrease c-Myc levels,” Dr Green said. ![]()

(with c-Myc in green)
Image courtesy of
Katherine Verbist and St. Jude
New research published in Nature helps explain how 2 types of cells arise from activated T cells.
Investigators found that distribution of the regulatory protein c-Myc during asymmetric cell division impacts an activated T cell’s fate, determining whether it will become an effector T cell or a memory T cell.
The team therefore believes that manipulating c-Myc levels could make vaccines more effective or advance immunotherapies for cancer treatment.
“Our work suggests that it may be possible to manipulate the immune response by nudging production of c-Myc in one direction or the other,” said study author Douglas Green, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Potentially, that could mean more effective vaccines or help to advance T-cell immune therapy for cancer treatment.”
Through a series of experiments, Dr Green and his colleagues found that, during asymmetric cell division of activated T cells, high levels of c-Myc accumulated in one daughter cell.
There, c-Myc launched and sustained the rapid proliferation of effector T cells, including those in mice infected with the influenza virus.
In contrast, daughter cells with low levels of c-Myc functioned like memory T cells, proliferating to mount an immune response a month later when mice were again exposed to the virus.
The investigators also identified the metabolic and signaling pathways that serve as a positive feedback loop to sustain the high levels of c-Myc that effector T cells require to maintain their identities and function.
The team showed that disrupting certain components of the system disturbed c-Myc production, which altered the fate of T cells and caused effector T cells to operate like memory T cells.
“While daughter cells of activated T cells seem to have very different fates, we showed their behavior could be altered by manipulating these metabolic and regulatory pathways to increase or decrease c-Myc levels,” Dr Green said. ![]()
Discovery could aid development of new sepsis therapies
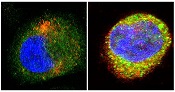
antibodies toward SHARPIN
(red) and caspase 1 (green).
Cell on right showing increase
in caspase 1 upon stimulation
with LPS and ATP and co-
localization with SHARPIN as
visualized by the merged
fluorescence (yellow). Nuclei
are stained blue with DAPI.
Image courtesy of The
American Journal of Pathology
A new study suggests that SHARPIN, a protein involved in regulating inflammation, has anti-septic effects.
Researchers believe this discovery, reported in The American Journal of Pathology, could spur the development of novel treatments for sepsis.
“Sepsis has been linked to enhanced activity of the enzyme caspase 1 and aberrant expression of pro-inflammatory interleukins 1β and 18,” said Liliana Schaefer, MD, of Goethe-Universität in Frankfurt, Germany.
“SHARPIN binds to caspase 1 and inhibits its activation. Our study proposes that the caspase 1/SHARPIN interaction may be a key pharmacological target in sepsis and perhaps in other inflammatory conditions where SHARPIN is involved.”
The researchers found that sepsis in mice bred to be deficient in SHARPIN resulted in enhanced levels of interleukins 1β and 18 and active caspase 1, as well as shortened survival.
Treatment with a caspase 1 inhibitor reversed these effects—reducing levels of interleukins 1β and 18, decreasing cell death in the spleen, and prolonging survival.
The researchers also found evidence to suggest this mechanism may be relevant to human sepsis.
“We found a decline in SHARPIN levels in septic patients correlating with enhanced activation of caspase 1 in circulating mononuclear cells and an increase of interleukin 1β/18 in the plasma,” Dr Schaefer said.
“Our findings suggest that using pharmacological caspase 1 inhibitors could be beneficial in septic patients with low SHARPIN levels, and these therapies may be more efficient than other anti-inflammatory therapies.” ![]()

antibodies toward SHARPIN
(red) and caspase 1 (green).
Cell on right showing increase
in caspase 1 upon stimulation
with LPS and ATP and co-
localization with SHARPIN as
visualized by the merged
fluorescence (yellow). Nuclei
are stained blue with DAPI.
Image courtesy of The
American Journal of Pathology
A new study suggests that SHARPIN, a protein involved in regulating inflammation, has anti-septic effects.
Researchers believe this discovery, reported in The American Journal of Pathology, could spur the development of novel treatments for sepsis.
“Sepsis has been linked to enhanced activity of the enzyme caspase 1 and aberrant expression of pro-inflammatory interleukins 1β and 18,” said Liliana Schaefer, MD, of Goethe-Universität in Frankfurt, Germany.
“SHARPIN binds to caspase 1 and inhibits its activation. Our study proposes that the caspase 1/SHARPIN interaction may be a key pharmacological target in sepsis and perhaps in other inflammatory conditions where SHARPIN is involved.”
The researchers found that sepsis in mice bred to be deficient in SHARPIN resulted in enhanced levels of interleukins 1β and 18 and active caspase 1, as well as shortened survival.
Treatment with a caspase 1 inhibitor reversed these effects—reducing levels of interleukins 1β and 18, decreasing cell death in the spleen, and prolonging survival.
The researchers also found evidence to suggest this mechanism may be relevant to human sepsis.
“We found a decline in SHARPIN levels in septic patients correlating with enhanced activation of caspase 1 in circulating mononuclear cells and an increase of interleukin 1β/18 in the plasma,” Dr Schaefer said.
“Our findings suggest that using pharmacological caspase 1 inhibitors could be beneficial in septic patients with low SHARPIN levels, and these therapies may be more efficient than other anti-inflammatory therapies.” ![]()

antibodies toward SHARPIN
(red) and caspase 1 (green).
Cell on right showing increase
in caspase 1 upon stimulation
with LPS and ATP and co-
localization with SHARPIN as
visualized by the merged
fluorescence (yellow). Nuclei
are stained blue with DAPI.
Image courtesy of The
American Journal of Pathology
A new study suggests that SHARPIN, a protein involved in regulating inflammation, has anti-septic effects.
Researchers believe this discovery, reported in The American Journal of Pathology, could spur the development of novel treatments for sepsis.
“Sepsis has been linked to enhanced activity of the enzyme caspase 1 and aberrant expression of pro-inflammatory interleukins 1β and 18,” said Liliana Schaefer, MD, of Goethe-Universität in Frankfurt, Germany.
“SHARPIN binds to caspase 1 and inhibits its activation. Our study proposes that the caspase 1/SHARPIN interaction may be a key pharmacological target in sepsis and perhaps in other inflammatory conditions where SHARPIN is involved.”
The researchers found that sepsis in mice bred to be deficient in SHARPIN resulted in enhanced levels of interleukins 1β and 18 and active caspase 1, as well as shortened survival.
Treatment with a caspase 1 inhibitor reversed these effects—reducing levels of interleukins 1β and 18, decreasing cell death in the spleen, and prolonging survival.
The researchers also found evidence to suggest this mechanism may be relevant to human sepsis.
“We found a decline in SHARPIN levels in septic patients correlating with enhanced activation of caspase 1 in circulating mononuclear cells and an increase of interleukin 1β/18 in the plasma,” Dr Schaefer said.
“Our findings suggest that using pharmacological caspase 1 inhibitors could be beneficial in septic patients with low SHARPIN levels, and these therapies may be more efficient than other anti-inflammatory therapies.” ![]()
Study suggests iPSCs pose no cancer risk

Image from the Salk Institute
In tracking the mutational history of somatic cells and induced pluripotent stem cells (iPSCs), researchers found that somatic cells accumulate mutations more frequently than iPSCs.
And none of the mutations found in iPSCs were associated with cancers.
“None of the mutations we found in induced pluripotent stem cells were cancer-driver mutations or mutations in cancer-causing genes,” said Foad Rouhani, of the Wellcome Trust Sanger Institute in the UK.
“We didn’t find anything that would preclude the use of [iPSCs] in therapeutic medicine.”
Rouhani and his colleagues reported these findings in PLOS Genetics.
The researchers generated iPSCs using cells from healthy individuals, then sequenced the genomes of the somatic cells and the derived iPSCs.
They found that somatic cells had a mutation rate of 14 single nucleotide variants per cell per generation, and the mutation rate for iPSCs was 10-fold lower.
The researchers said this is the first time that mutation rates of both types of cells, the donor cell and iPSC, have been calculated and compared.
“Until now, the question of whether generating [iPSCs] and growing them in cell culture creates mutations has not been addressed in detail,” said study author Allan Bradley, PhD, of the Wellcome Trust Sanger Institute.
“If human cells are really to be reprogrammed on a large scale for use in regenerative medicine, then understanding the mutations the donor cells carry will be a crucial step. We now have the tools to do this.”
The researchers also used the iPSCs to trace the history of every mutation that one endothelial progenitor cell had developed from the time it was a fertilized egg to the moment it was taken out of the body.
They said the ability to track the genetic changes in cells over a lifetime could improve scientists’ understanding of how, when, and why mutations lead to cancer. ![]()

Image from the Salk Institute
In tracking the mutational history of somatic cells and induced pluripotent stem cells (iPSCs), researchers found that somatic cells accumulate mutations more frequently than iPSCs.
And none of the mutations found in iPSCs were associated with cancers.
“None of the mutations we found in induced pluripotent stem cells were cancer-driver mutations or mutations in cancer-causing genes,” said Foad Rouhani, of the Wellcome Trust Sanger Institute in the UK.
“We didn’t find anything that would preclude the use of [iPSCs] in therapeutic medicine.”
Rouhani and his colleagues reported these findings in PLOS Genetics.
The researchers generated iPSCs using cells from healthy individuals, then sequenced the genomes of the somatic cells and the derived iPSCs.
They found that somatic cells had a mutation rate of 14 single nucleotide variants per cell per generation, and the mutation rate for iPSCs was 10-fold lower.
The researchers said this is the first time that mutation rates of both types of cells, the donor cell and iPSC, have been calculated and compared.
“Until now, the question of whether generating [iPSCs] and growing them in cell culture creates mutations has not been addressed in detail,” said study author Allan Bradley, PhD, of the Wellcome Trust Sanger Institute.
“If human cells are really to be reprogrammed on a large scale for use in regenerative medicine, then understanding the mutations the donor cells carry will be a crucial step. We now have the tools to do this.”
The researchers also used the iPSCs to trace the history of every mutation that one endothelial progenitor cell had developed from the time it was a fertilized egg to the moment it was taken out of the body.
They said the ability to track the genetic changes in cells over a lifetime could improve scientists’ understanding of how, when, and why mutations lead to cancer. ![]()

Image from the Salk Institute
In tracking the mutational history of somatic cells and induced pluripotent stem cells (iPSCs), researchers found that somatic cells accumulate mutations more frequently than iPSCs.
And none of the mutations found in iPSCs were associated with cancers.
“None of the mutations we found in induced pluripotent stem cells were cancer-driver mutations or mutations in cancer-causing genes,” said Foad Rouhani, of the Wellcome Trust Sanger Institute in the UK.
“We didn’t find anything that would preclude the use of [iPSCs] in therapeutic medicine.”
Rouhani and his colleagues reported these findings in PLOS Genetics.
The researchers generated iPSCs using cells from healthy individuals, then sequenced the genomes of the somatic cells and the derived iPSCs.
They found that somatic cells had a mutation rate of 14 single nucleotide variants per cell per generation, and the mutation rate for iPSCs was 10-fold lower.
The researchers said this is the first time that mutation rates of both types of cells, the donor cell and iPSC, have been calculated and compared.
“Until now, the question of whether generating [iPSCs] and growing them in cell culture creates mutations has not been addressed in detail,” said study author Allan Bradley, PhD, of the Wellcome Trust Sanger Institute.
“If human cells are really to be reprogrammed on a large scale for use in regenerative medicine, then understanding the mutations the donor cells carry will be a crucial step. We now have the tools to do this.”
The researchers also used the iPSCs to trace the history of every mutation that one endothelial progenitor cell had developed from the time it was a fertilized egg to the moment it was taken out of the body.
They said the ability to track the genetic changes in cells over a lifetime could improve scientists’ understanding of how, when, and why mutations lead to cancer. ![]()
Cancer drugs could treat vascular disorder

Photo by Aaron Logan
Two research teams have found evidence to suggest that mutations in PIK3CA, a gene linked to cancer, may drive venous malformations (VMs) in some patients.
Both groups of researchers showed that PIK3CA mutations give rise to VMs in mice, and PIK3CA mutations are present in humans with VMs.
Subsequent experiments with the mice suggested that PIK3CA inhibitors could be used to treat VMs.
Both groups reported their findings in Science Translational Medicine.
Pau Castel, of Memorial Sloan Kettering Cancer Center in New York, New York, and his colleagues were originally studying the role of PIK3CA in uterine cancer when they noticed that mice harboring PIK3CA mutations developed defective blood vessels that closely resembled VMs.
Sandra Castillo, PhD, of University College London in the UK, and her colleagues generated mice with PIK3CA-activating mutations that also mimicked the human disease, including during mouse embryonic development.
Both teams found the mutations spurred uncontrolled growth of endothelial cells, which formed abnormal clusters and faulty blood vessels.
To verify their mouse models, the researchers analyzed samples from patients with VMs. Dr Castillo and her colleagues looked at samples from 13 children, while Castel and his colleagues evaluated samples from 32 patients (both adults and children).
Dr Castillo and her colleagues found PIK3CA mutations in 25% of patients, and Castel and his colleagues found mutations in PIK3CA and related genes of the PI3K/AKT pathway in about 30% of patients.
Both groups of researchers then tested PI3K inhibitors in their mouse models and found these drugs could stunt blood vessel overgrowth.
“Rapamycin is a drug that blocks a signaling process that happens downstream of PIK3CA, so it stops one of PIK3CA’s effects but does not block it at the source,” Dr Castillo said. “When we gave rapamycin to the mice, it showed clinical benefit, but, in patients, it can have serious side effects and compromise the immune system.”
“Our colleagues at MSK [Memorial Sloan Kettering] then tested drugs on the mice that directly inhibit PIK3CA, developed to treat cancer. These drugs worked well and significantly reduced the size of the malformations, not only when given through the bloodstream but also when applied directly to the skin as a cream.” ![]()

Photo by Aaron Logan
Two research teams have found evidence to suggest that mutations in PIK3CA, a gene linked to cancer, may drive venous malformations (VMs) in some patients.
Both groups of researchers showed that PIK3CA mutations give rise to VMs in mice, and PIK3CA mutations are present in humans with VMs.
Subsequent experiments with the mice suggested that PIK3CA inhibitors could be used to treat VMs.
Both groups reported their findings in Science Translational Medicine.
Pau Castel, of Memorial Sloan Kettering Cancer Center in New York, New York, and his colleagues were originally studying the role of PIK3CA in uterine cancer when they noticed that mice harboring PIK3CA mutations developed defective blood vessels that closely resembled VMs.
Sandra Castillo, PhD, of University College London in the UK, and her colleagues generated mice with PIK3CA-activating mutations that also mimicked the human disease, including during mouse embryonic development.
Both teams found the mutations spurred uncontrolled growth of endothelial cells, which formed abnormal clusters and faulty blood vessels.
To verify their mouse models, the researchers analyzed samples from patients with VMs. Dr Castillo and her colleagues looked at samples from 13 children, while Castel and his colleagues evaluated samples from 32 patients (both adults and children).
Dr Castillo and her colleagues found PIK3CA mutations in 25% of patients, and Castel and his colleagues found mutations in PIK3CA and related genes of the PI3K/AKT pathway in about 30% of patients.
Both groups of researchers then tested PI3K inhibitors in their mouse models and found these drugs could stunt blood vessel overgrowth.
“Rapamycin is a drug that blocks a signaling process that happens downstream of PIK3CA, so it stops one of PIK3CA’s effects but does not block it at the source,” Dr Castillo said. “When we gave rapamycin to the mice, it showed clinical benefit, but, in patients, it can have serious side effects and compromise the immune system.”
“Our colleagues at MSK [Memorial Sloan Kettering] then tested drugs on the mice that directly inhibit PIK3CA, developed to treat cancer. These drugs worked well and significantly reduced the size of the malformations, not only when given through the bloodstream but also when applied directly to the skin as a cream.” ![]()

Photo by Aaron Logan
Two research teams have found evidence to suggest that mutations in PIK3CA, a gene linked to cancer, may drive venous malformations (VMs) in some patients.
Both groups of researchers showed that PIK3CA mutations give rise to VMs in mice, and PIK3CA mutations are present in humans with VMs.
Subsequent experiments with the mice suggested that PIK3CA inhibitors could be used to treat VMs.
Both groups reported their findings in Science Translational Medicine.
Pau Castel, of Memorial Sloan Kettering Cancer Center in New York, New York, and his colleagues were originally studying the role of PIK3CA in uterine cancer when they noticed that mice harboring PIK3CA mutations developed defective blood vessels that closely resembled VMs.
Sandra Castillo, PhD, of University College London in the UK, and her colleagues generated mice with PIK3CA-activating mutations that also mimicked the human disease, including during mouse embryonic development.
Both teams found the mutations spurred uncontrolled growth of endothelial cells, which formed abnormal clusters and faulty blood vessels.
To verify their mouse models, the researchers analyzed samples from patients with VMs. Dr Castillo and her colleagues looked at samples from 13 children, while Castel and his colleagues evaluated samples from 32 patients (both adults and children).
Dr Castillo and her colleagues found PIK3CA mutations in 25% of patients, and Castel and his colleagues found mutations in PIK3CA and related genes of the PI3K/AKT pathway in about 30% of patients.
Both groups of researchers then tested PI3K inhibitors in their mouse models and found these drugs could stunt blood vessel overgrowth.
“Rapamycin is a drug that blocks a signaling process that happens downstream of PIK3CA, so it stops one of PIK3CA’s effects but does not block it at the source,” Dr Castillo said. “When we gave rapamycin to the mice, it showed clinical benefit, but, in patients, it can have serious side effects and compromise the immune system.”
“Our colleagues at MSK [Memorial Sloan Kettering] then tested drugs on the mice that directly inhibit PIK3CA, developed to treat cancer. These drugs worked well and significantly reduced the size of the malformations, not only when given through the bloodstream but also when applied directly to the skin as a cream.”
Macaque-to-human transmission of malaria

Photo by Sakurai Midori
The parasite responsible for a form of malaria now spreading from macaques to humans in South Asia could evolve to infect humans more efficiently, according to a study published in Nature Communications.
Researchers identified a sugar variant on the surface of human red blood cells (RBCs) that currently limits the ability of the parasite Plasmodium knowlesi to invade.
But the team also found the parasite can evolve to get around this barrier and pass into the human population in a more virulent form.
“With increasing concern about the spread of P knowlesi into human populations, it is great to be able to gain insight into what the molecular stumbling blocks are for P knowlesi infection of humans and how the parasite can potentially overcome them,” said study author Selasi Dankwa, PhD, of Harvard T.H. Chan School of Public Health in Boston, Massachusetts.
The macaque malaria parasite P knowlesi has emerged as a major source of human infections in Southeast Asia. While most human infections are mild, increasing numbers of severe infections are being reported, leading to concerns that the parasite is adapting to infect humans more efficiently.
With this in mind, Dr Dankwa and her colleagues decided to explore the parasite’s ability to invade and adapt.
The team introduced the macaque sugar variant onto the human RBC surface and demonstrated that the parasite normally dependent on the macaque variant for invasion was unable to use the human version.
Specifically, macaques synthesize the sialic acid variant N-glycolylneuraminic acid (Neu5Gc), but humans lack Neu5Gc because of an Alu-mediated exon deletion in the gene encoding CMAH, which converts N-acetylneuraminic acid (Neu5Ac) to Neu5Gc.
So the absence of Neu5Gc on human RBCs limits P knowlesi invasion, but the researchers found that parasites can evolve to invade human RBCs via sialic acid-independent pathways.
Following prolonged adaptation to growth on human RBCs, P knowlesi invaded human RBCs independently of Neu5Gc. This occurred via duplication of the region containing the sialic acid-independent gene PkDBPα and complete deletion of the sialic acid-dependent gene PkDBPγ.
Based on these findings, the researchers are calling for continued monitoring of the P knowlesi parasite to ensure that it has not switched to using a sialic acid-independent pathway to invade human RBCs.

Photo by Sakurai Midori
The parasite responsible for a form of malaria now spreading from macaques to humans in South Asia could evolve to infect humans more efficiently, according to a study published in Nature Communications.
Researchers identified a sugar variant on the surface of human red blood cells (RBCs) that currently limits the ability of the parasite Plasmodium knowlesi to invade.
But the team also found the parasite can evolve to get around this barrier and pass into the human population in a more virulent form.
“With increasing concern about the spread of P knowlesi into human populations, it is great to be able to gain insight into what the molecular stumbling blocks are for P knowlesi infection of humans and how the parasite can potentially overcome them,” said study author Selasi Dankwa, PhD, of Harvard T.H. Chan School of Public Health in Boston, Massachusetts.
The macaque malaria parasite P knowlesi has emerged as a major source of human infections in Southeast Asia. While most human infections are mild, increasing numbers of severe infections are being reported, leading to concerns that the parasite is adapting to infect humans more efficiently.
With this in mind, Dr Dankwa and her colleagues decided to explore the parasite’s ability to invade and adapt.
The team introduced the macaque sugar variant onto the human RBC surface and demonstrated that the parasite normally dependent on the macaque variant for invasion was unable to use the human version.
Specifically, macaques synthesize the sialic acid variant N-glycolylneuraminic acid (Neu5Gc), but humans lack Neu5Gc because of an Alu-mediated exon deletion in the gene encoding CMAH, which converts N-acetylneuraminic acid (Neu5Ac) to Neu5Gc.
So the absence of Neu5Gc on human RBCs limits P knowlesi invasion, but the researchers found that parasites can evolve to invade human RBCs via sialic acid-independent pathways.
Following prolonged adaptation to growth on human RBCs, P knowlesi invaded human RBCs independently of Neu5Gc. This occurred via duplication of the region containing the sialic acid-independent gene PkDBPα and complete deletion of the sialic acid-dependent gene PkDBPγ.
Based on these findings, the researchers are calling for continued monitoring of the P knowlesi parasite to ensure that it has not switched to using a sialic acid-independent pathway to invade human RBCs.

Photo by Sakurai Midori
The parasite responsible for a form of malaria now spreading from macaques to humans in South Asia could evolve to infect humans more efficiently, according to a study published in Nature Communications.
Researchers identified a sugar variant on the surface of human red blood cells (RBCs) that currently limits the ability of the parasite Plasmodium knowlesi to invade.
But the team also found the parasite can evolve to get around this barrier and pass into the human population in a more virulent form.
“With increasing concern about the spread of P knowlesi into human populations, it is great to be able to gain insight into what the molecular stumbling blocks are for P knowlesi infection of humans and how the parasite can potentially overcome them,” said study author Selasi Dankwa, PhD, of Harvard T.H. Chan School of Public Health in Boston, Massachusetts.
The macaque malaria parasite P knowlesi has emerged as a major source of human infections in Southeast Asia. While most human infections are mild, increasing numbers of severe infections are being reported, leading to concerns that the parasite is adapting to infect humans more efficiently.
With this in mind, Dr Dankwa and her colleagues decided to explore the parasite’s ability to invade and adapt.
The team introduced the macaque sugar variant onto the human RBC surface and demonstrated that the parasite normally dependent on the macaque variant for invasion was unable to use the human version.
Specifically, macaques synthesize the sialic acid variant N-glycolylneuraminic acid (Neu5Gc), but humans lack Neu5Gc because of an Alu-mediated exon deletion in the gene encoding CMAH, which converts N-acetylneuraminic acid (Neu5Ac) to Neu5Gc.
So the absence of Neu5Gc on human RBCs limits P knowlesi invasion, but the researchers found that parasites can evolve to invade human RBCs via sialic acid-independent pathways.
Following prolonged adaptation to growth on human RBCs, P knowlesi invaded human RBCs independently of Neu5Gc. This occurred via duplication of the region containing the sialic acid-independent gene PkDBPα and complete deletion of the sialic acid-dependent gene PkDBPγ.
Based on these findings, the researchers are calling for continued monitoring of the P knowlesi parasite to ensure that it has not switched to using a sialic acid-independent pathway to invade human RBCs.
Study provides new insight into blood vessel formation
A study published in Nature Cell Biology helps explain how hemodynamic forces contribute to the formation of new vascular lumens during blood vessel morphogenesis.
Investigators found that blood flow drives lumen expansion during sprouting angiogenesis in vivo by inducing spherical deformations of the apical
membrane of endothelial cells, in a process dubbed “inverse blebbing.”
“This work, combined with previous studies, highlights the importance of balanced endothelial cell contractility in allowing the expansion and maintenance of endothelial lumens during blood vessel development,” said study author Holger Gerhardt, PhD, of the Max Delbrück Center for Molecular Medicine in Berlin, Germany.
These results challenge the previous idea that sprouting cells expand lumens independently of blood flow during angiogenesis through the generation and fusion of intracellular vacuoles.
The investigators showed that hemodynamic forces dynamically shape the apical membrane of single or groups of endothelial cells during angiogenesis to form and expand new lumenized vascular tubes.
“We find that this process relies on a tight balance between the forces applied on the membrane and the local contractile responses from the endothelial cells, as impairing this balance either way leads to lumen defects,” Dr Gerhardt said.
These findings suggest the process of blebbing does not require a specific polarity but is likely to be generally applicable to situations in which external versus internal pressure differences challenge the stability and elasticity of the actin cortex.
It more generally raises the question of the role of apical membrane contractility in the adaptation to varying hemodynamic environments, both during blood vessel morphogenesis, as connections form or remodel, and in pathological settings.
“Understanding whether and how this plasticity of the apical membrane and its underlying cortex is challenged in pathological conditions, where vessels exhibit altered perfusion and lack organized structure, has the potential to provide deeper insight into mechanisms of vascular adaptation and maladaptation,” Dr Gerhardt said. “We will definitely further investigate this.”

A study published in Nature Cell Biology helps explain how hemodynamic forces contribute to the formation of new vascular lumens during blood vessel morphogenesis.
Investigators found that blood flow drives lumen expansion during sprouting angiogenesis in vivo by inducing spherical deformations of the apical
membrane of endothelial cells, in a process dubbed “inverse blebbing.”
“This work, combined with previous studies, highlights the importance of balanced endothelial cell contractility in allowing the expansion and maintenance of endothelial lumens during blood vessel development,” said study author Holger Gerhardt, PhD, of the Max Delbrück Center for Molecular Medicine in Berlin, Germany.
These results challenge the previous idea that sprouting cells expand lumens independently of blood flow during angiogenesis through the generation and fusion of intracellular vacuoles.
The investigators showed that hemodynamic forces dynamically shape the apical membrane of single or groups of endothelial cells during angiogenesis to form and expand new lumenized vascular tubes.
“We find that this process relies on a tight balance between the forces applied on the membrane and the local contractile responses from the endothelial cells, as impairing this balance either way leads to lumen defects,” Dr Gerhardt said.
These findings suggest the process of blebbing does not require a specific polarity but is likely to be generally applicable to situations in which external versus internal pressure differences challenge the stability and elasticity of the actin cortex.
It more generally raises the question of the role of apical membrane contractility in the adaptation to varying hemodynamic environments, both during blood vessel morphogenesis, as connections form or remodel, and in pathological settings.
“Understanding whether and how this plasticity of the apical membrane and its underlying cortex is challenged in pathological conditions, where vessels exhibit altered perfusion and lack organized structure, has the potential to provide deeper insight into mechanisms of vascular adaptation and maladaptation,” Dr Gerhardt said. “We will definitely further investigate this.”

A study published in Nature Cell Biology helps explain how hemodynamic forces contribute to the formation of new vascular lumens during blood vessel morphogenesis.
Investigators found that blood flow drives lumen expansion during sprouting angiogenesis in vivo by inducing spherical deformations of the apical
membrane of endothelial cells, in a process dubbed “inverse blebbing.”
“This work, combined with previous studies, highlights the importance of balanced endothelial cell contractility in allowing the expansion and maintenance of endothelial lumens during blood vessel development,” said study author Holger Gerhardt, PhD, of the Max Delbrück Center for Molecular Medicine in Berlin, Germany.
These results challenge the previous idea that sprouting cells expand lumens independently of blood flow during angiogenesis through the generation and fusion of intracellular vacuoles.
The investigators showed that hemodynamic forces dynamically shape the apical membrane of single or groups of endothelial cells during angiogenesis to form and expand new lumenized vascular tubes.
“We find that this process relies on a tight balance between the forces applied on the membrane and the local contractile responses from the endothelial cells, as impairing this balance either way leads to lumen defects,” Dr Gerhardt said.
These findings suggest the process of blebbing does not require a specific polarity but is likely to be generally applicable to situations in which external versus internal pressure differences challenge the stability and elasticity of the actin cortex.
It more generally raises the question of the role of apical membrane contractility in the adaptation to varying hemodynamic environments, both during blood vessel morphogenesis, as connections form or remodel, and in pathological settings.
“Understanding whether and how this plasticity of the apical membrane and its underlying cortex is challenged in pathological conditions, where vessels exhibit altered perfusion and lack organized structure, has the potential to provide deeper insight into mechanisms of vascular adaptation and maladaptation,” Dr Gerhardt said. “We will definitely further investigate this.”
Gene discovery could help fight malaria

Photo by James Gathany
Researchers believe they may have discovered a male-determining gene in the malaria-carrying mosquito species Anopheles gambiae.
The discovery of this gene provides scientists with a foundation for studying male mosquito biology.
And this is significant because male mosquitoes offer the potential to develop novel vector control strategies to combat malaria because males do not feed on blood or transmit disease.
One vector control method under development involves genetic modification of the mosquito to bias the population sex ratio toward males.
Modeling has shown the most efficient means for genetic modification of mosquitoes is engineering a driving Y chromosome.
A molecular-level understanding of the Y chromosome of the malaria-carrying mosquito is important to inform and optimize this type of a strategy.
So Omar Akbari, PhD, of the University of California, Riverside, and his colleagues set out to gain such an understanding.
The team used multiple genome sequencing techniques to identify an extensive dataset of Y chromosome sequences and explore their organization and evolution in the Anopheles gambiae complex, a group of at least 7 morphologically indistinguishable species of mosquitoes in the genus Anopheles.
This revealed that only 1 gene, YG2, is exclusive to the Y chromosome across the species complex and may therefore be a male-determining gene.
Dr Akbari and his colleagues described this discovery in PNAS.

Photo by James Gathany
Researchers believe they may have discovered a male-determining gene in the malaria-carrying mosquito species Anopheles gambiae.
The discovery of this gene provides scientists with a foundation for studying male mosquito biology.
And this is significant because male mosquitoes offer the potential to develop novel vector control strategies to combat malaria because males do not feed on blood or transmit disease.
One vector control method under development involves genetic modification of the mosquito to bias the population sex ratio toward males.
Modeling has shown the most efficient means for genetic modification of mosquitoes is engineering a driving Y chromosome.
A molecular-level understanding of the Y chromosome of the malaria-carrying mosquito is important to inform and optimize this type of a strategy.
So Omar Akbari, PhD, of the University of California, Riverside, and his colleagues set out to gain such an understanding.
The team used multiple genome sequencing techniques to identify an extensive dataset of Y chromosome sequences and explore their organization and evolution in the Anopheles gambiae complex, a group of at least 7 morphologically indistinguishable species of mosquitoes in the genus Anopheles.
This revealed that only 1 gene, YG2, is exclusive to the Y chromosome across the species complex and may therefore be a male-determining gene.
Dr Akbari and his colleagues described this discovery in PNAS.

Photo by James Gathany
Researchers believe they may have discovered a male-determining gene in the malaria-carrying mosquito species Anopheles gambiae.
The discovery of this gene provides scientists with a foundation for studying male mosquito biology.
And this is significant because male mosquitoes offer the potential to develop novel vector control strategies to combat malaria because males do not feed on blood or transmit disease.
One vector control method under development involves genetic modification of the mosquito to bias the population sex ratio toward males.
Modeling has shown the most efficient means for genetic modification of mosquitoes is engineering a driving Y chromosome.
A molecular-level understanding of the Y chromosome of the malaria-carrying mosquito is important to inform and optimize this type of a strategy.
So Omar Akbari, PhD, of the University of California, Riverside, and his colleagues set out to gain such an understanding.
The team used multiple genome sequencing techniques to identify an extensive dataset of Y chromosome sequences and explore their organization and evolution in the Anopheles gambiae complex, a group of at least 7 morphologically indistinguishable species of mosquitoes in the genus Anopheles.
This revealed that only 1 gene, YG2, is exclusive to the Y chromosome across the species complex and may therefore be a male-determining gene.
Dr Akbari and his colleagues described this discovery in PNAS.