User login
Chip can remove nanoparticles from blood

Photo from Jacobs School
of Engineering/UC San Diego
Engineers have developed a chip that uses an oscillating electric field to isolate drug-delivery nanoparticles from blood.
They say the device isolated nanoparticles from plasma quickly and easily, without the need to modify the plasma samples or the nanoparticles.
The group believes this technique could be used to separate and recover nanoparticles from other complex fluids for medical, environmental, and industrial applications.
They described the technique in the journal Small.
Previously, nanoparticles proved difficult to separate from plasma due to their small size and low density.
Traditional methods to remove nanoparticles from plasma samples typically involve diluting the plasma, adding a high-concentration sugar solution to the plasma and spinning it in a centrifuge, or attaching a targeting agent to the surface of the nanoparticles.
These methods either alter the normal behavior of the nanoparticles or cannot be applied to some of the most common nanoparticle types.
“This is the first example of isolating a wide range of nanoparticles out of plasma with a minimum amount of manipulation,” said study author Stuart Ibsen, PhD, of the University of California, San Diego.
“We’ve designed a very versatile technique that can be used to recover nanoparticles in a lot of different processes.”
The device used to isolate the drug-delivery nanoparticles was a dime-sized electric chip manufactured by La Jolla-based Biological Dynamics, which licensed the original technology from the University of California, San Diego.
The chip contains hundreds of tiny electrodes that generate a rapidly oscillating electric field that selectively pulls the nanoparticles out of a plasma sample.
The researchers inserted a drop of plasma spiked with nanoparticles into the electric chip and demonstrated nanoparticle recovery within 7 minutes. The technology worked on different types of drug-delivery nanoparticles that are typically studied in various labs.
The researchers said the breakthrough in the technology relies on designing a chip that can work in the high-salt concentration of plasma. The chip’s ability to pull the nanoparticles out of plasma is based on differences in the material properties between the nanoparticles and plasma components.
When the chip’s electrodes apply an oscillating electric field, the positive and negative charges inside the nanoparticles reorient themselves at a different speed than the charges in the surrounding plasma. This momentary imbalance in the charges creates an attractive force between the nanoparticles and the electrodes.
As the electric field oscillates, the nanoparticles are continually pulled toward the electrodes, leaving the rest of the plasma behind. In addition, the electric field is designed to oscillate at just the right frequency: 15,000 times per second.
“It’s amazing that this method works without any modifications to the plasma samples or to the nanoparticles,” Dr Ibsen said.
He and his colleagues believe this technology will enable researchers to better monitor what happens to nanoparticles circulating in a patient’s bloodstream. Researchers could also use this technology in the clinic to determine if the blood chemistry of a particular patient is compatible with the surfaces of certain drug-delivery nanoparticles. ![]()

Photo from Jacobs School
of Engineering/UC San Diego
Engineers have developed a chip that uses an oscillating electric field to isolate drug-delivery nanoparticles from blood.
They say the device isolated nanoparticles from plasma quickly and easily, without the need to modify the plasma samples or the nanoparticles.
The group believes this technique could be used to separate and recover nanoparticles from other complex fluids for medical, environmental, and industrial applications.
They described the technique in the journal Small.
Previously, nanoparticles proved difficult to separate from plasma due to their small size and low density.
Traditional methods to remove nanoparticles from plasma samples typically involve diluting the plasma, adding a high-concentration sugar solution to the plasma and spinning it in a centrifuge, or attaching a targeting agent to the surface of the nanoparticles.
These methods either alter the normal behavior of the nanoparticles or cannot be applied to some of the most common nanoparticle types.
“This is the first example of isolating a wide range of nanoparticles out of plasma with a minimum amount of manipulation,” said study author Stuart Ibsen, PhD, of the University of California, San Diego.
“We’ve designed a very versatile technique that can be used to recover nanoparticles in a lot of different processes.”
The device used to isolate the drug-delivery nanoparticles was a dime-sized electric chip manufactured by La Jolla-based Biological Dynamics, which licensed the original technology from the University of California, San Diego.
The chip contains hundreds of tiny electrodes that generate a rapidly oscillating electric field that selectively pulls the nanoparticles out of a plasma sample.
The researchers inserted a drop of plasma spiked with nanoparticles into the electric chip and demonstrated nanoparticle recovery within 7 minutes. The technology worked on different types of drug-delivery nanoparticles that are typically studied in various labs.
The researchers said the breakthrough in the technology relies on designing a chip that can work in the high-salt concentration of plasma. The chip’s ability to pull the nanoparticles out of plasma is based on differences in the material properties between the nanoparticles and plasma components.
When the chip’s electrodes apply an oscillating electric field, the positive and negative charges inside the nanoparticles reorient themselves at a different speed than the charges in the surrounding plasma. This momentary imbalance in the charges creates an attractive force between the nanoparticles and the electrodes.
As the electric field oscillates, the nanoparticles are continually pulled toward the electrodes, leaving the rest of the plasma behind. In addition, the electric field is designed to oscillate at just the right frequency: 15,000 times per second.
“It’s amazing that this method works without any modifications to the plasma samples or to the nanoparticles,” Dr Ibsen said.
He and his colleagues believe this technology will enable researchers to better monitor what happens to nanoparticles circulating in a patient’s bloodstream. Researchers could also use this technology in the clinic to determine if the blood chemistry of a particular patient is compatible with the surfaces of certain drug-delivery nanoparticles. ![]()

Photo from Jacobs School
of Engineering/UC San Diego
Engineers have developed a chip that uses an oscillating electric field to isolate drug-delivery nanoparticles from blood.
They say the device isolated nanoparticles from plasma quickly and easily, without the need to modify the plasma samples or the nanoparticles.
The group believes this technique could be used to separate and recover nanoparticles from other complex fluids for medical, environmental, and industrial applications.
They described the technique in the journal Small.
Previously, nanoparticles proved difficult to separate from plasma due to their small size and low density.
Traditional methods to remove nanoparticles from plasma samples typically involve diluting the plasma, adding a high-concentration sugar solution to the plasma and spinning it in a centrifuge, or attaching a targeting agent to the surface of the nanoparticles.
These methods either alter the normal behavior of the nanoparticles or cannot be applied to some of the most common nanoparticle types.
“This is the first example of isolating a wide range of nanoparticles out of plasma with a minimum amount of manipulation,” said study author Stuart Ibsen, PhD, of the University of California, San Diego.
“We’ve designed a very versatile technique that can be used to recover nanoparticles in a lot of different processes.”
The device used to isolate the drug-delivery nanoparticles was a dime-sized electric chip manufactured by La Jolla-based Biological Dynamics, which licensed the original technology from the University of California, San Diego.
The chip contains hundreds of tiny electrodes that generate a rapidly oscillating electric field that selectively pulls the nanoparticles out of a plasma sample.
The researchers inserted a drop of plasma spiked with nanoparticles into the electric chip and demonstrated nanoparticle recovery within 7 minutes. The technology worked on different types of drug-delivery nanoparticles that are typically studied in various labs.
The researchers said the breakthrough in the technology relies on designing a chip that can work in the high-salt concentration of plasma. The chip’s ability to pull the nanoparticles out of plasma is based on differences in the material properties between the nanoparticles and plasma components.
When the chip’s electrodes apply an oscillating electric field, the positive and negative charges inside the nanoparticles reorient themselves at a different speed than the charges in the surrounding plasma. This momentary imbalance in the charges creates an attractive force between the nanoparticles and the electrodes.
As the electric field oscillates, the nanoparticles are continually pulled toward the electrodes, leaving the rest of the plasma behind. In addition, the electric field is designed to oscillate at just the right frequency: 15,000 times per second.
“It’s amazing that this method works without any modifications to the plasma samples or to the nanoparticles,” Dr Ibsen said.
He and his colleagues believe this technology will enable researchers to better monitor what happens to nanoparticles circulating in a patient’s bloodstream. Researchers could also use this technology in the clinic to determine if the blood chemistry of a particular patient is compatible with the surfaces of certain drug-delivery nanoparticles. ![]()
Modified mosquitoes may prevent malaria transmission
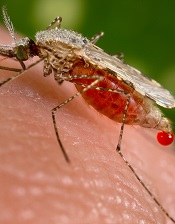
Photo by James Gathany/CDC
Scientists say they have created mosquitoes that can introduce malaria-blocking genes into a mosquito population through their progeny and, ideally, eliminate the insects’ ability to transmit malaria to humans.
The researchers used the CRISPR gene-editing technique to insert a DNA element into the germ line of Anopheles stephensi mosquitoes.
These mosquitoes then passed on genes that may prevent malaria transmission to 99.5% of their offspring.
The scientists said further testing is needed to confirm the efficacy of this approach in preventing malaria transmission, but this is an important first step toward that goal.
“This opens up the real promise that this technique can be adapted for eliminating malaria,” said Anthony James, PhD, of the University of California, Irvine.
Dr James and his colleagues described the technique in PNAS.
In 2012, Dr James and his colleagues showed that antibodies that impair Plasmodium falciparum’s biology could be adapted from the immune systems of mice and introduced into mosquitoes. However, this trait could only be inherited by about half of the progeny.
Earlier this year, Ethan Bier, PhD, and Valentino Gantz, both of the University of California, San Diego, reported a new method for generating mutations in both copies of a gene in fruit flies. This mutagenic chain reaction involved using the CRISPR-associated protein 9 (Cas9) nuclease enzyme and allowed for transmission of mutations through the germ line with an inheritance rate of 95%.
The groups collaborated to fuse Dr Bier and Gantz’s method with Dr James’s mosquitoes. Gantz packaged antimalaria genes with a Cas9 enzyme and a guide RNA to create a genetic “cassette” that, when injected into a mosquito embryo, targeted a highly specific spot on the germ line DNA to insert the antimalaria antibody genes.
To ensure the element carrying the malaria-blocking antibodies had reached the desired DNA site, the researchers included in the cassette a protein that gave the progeny red fluorescence in the eyes. Nearly all offspring—99.5%—exhibited this trait.
Dr James said further testing will be needed to confirm the efficacy of the antibodies, and this could eventually lead to field studies.
“[The current study] is a significant first step,” he said. “We know the gene works. The mosquitoes we created are not the final brand, but we know this technology allows us to efficiently create large populations.” ![]()

Photo by James Gathany/CDC
Scientists say they have created mosquitoes that can introduce malaria-blocking genes into a mosquito population through their progeny and, ideally, eliminate the insects’ ability to transmit malaria to humans.
The researchers used the CRISPR gene-editing technique to insert a DNA element into the germ line of Anopheles stephensi mosquitoes.
These mosquitoes then passed on genes that may prevent malaria transmission to 99.5% of their offspring.
The scientists said further testing is needed to confirm the efficacy of this approach in preventing malaria transmission, but this is an important first step toward that goal.
“This opens up the real promise that this technique can be adapted for eliminating malaria,” said Anthony James, PhD, of the University of California, Irvine.
Dr James and his colleagues described the technique in PNAS.
In 2012, Dr James and his colleagues showed that antibodies that impair Plasmodium falciparum’s biology could be adapted from the immune systems of mice and introduced into mosquitoes. However, this trait could only be inherited by about half of the progeny.
Earlier this year, Ethan Bier, PhD, and Valentino Gantz, both of the University of California, San Diego, reported a new method for generating mutations in both copies of a gene in fruit flies. This mutagenic chain reaction involved using the CRISPR-associated protein 9 (Cas9) nuclease enzyme and allowed for transmission of mutations through the germ line with an inheritance rate of 95%.
The groups collaborated to fuse Dr Bier and Gantz’s method with Dr James’s mosquitoes. Gantz packaged antimalaria genes with a Cas9 enzyme and a guide RNA to create a genetic “cassette” that, when injected into a mosquito embryo, targeted a highly specific spot on the germ line DNA to insert the antimalaria antibody genes.
To ensure the element carrying the malaria-blocking antibodies had reached the desired DNA site, the researchers included in the cassette a protein that gave the progeny red fluorescence in the eyes. Nearly all offspring—99.5%—exhibited this trait.
Dr James said further testing will be needed to confirm the efficacy of the antibodies, and this could eventually lead to field studies.
“[The current study] is a significant first step,” he said. “We know the gene works. The mosquitoes we created are not the final brand, but we know this technology allows us to efficiently create large populations.” ![]()

Photo by James Gathany/CDC
Scientists say they have created mosquitoes that can introduce malaria-blocking genes into a mosquito population through their progeny and, ideally, eliminate the insects’ ability to transmit malaria to humans.
The researchers used the CRISPR gene-editing technique to insert a DNA element into the germ line of Anopheles stephensi mosquitoes.
These mosquitoes then passed on genes that may prevent malaria transmission to 99.5% of their offspring.
The scientists said further testing is needed to confirm the efficacy of this approach in preventing malaria transmission, but this is an important first step toward that goal.
“This opens up the real promise that this technique can be adapted for eliminating malaria,” said Anthony James, PhD, of the University of California, Irvine.
Dr James and his colleagues described the technique in PNAS.
In 2012, Dr James and his colleagues showed that antibodies that impair Plasmodium falciparum’s biology could be adapted from the immune systems of mice and introduced into mosquitoes. However, this trait could only be inherited by about half of the progeny.
Earlier this year, Ethan Bier, PhD, and Valentino Gantz, both of the University of California, San Diego, reported a new method for generating mutations in both copies of a gene in fruit flies. This mutagenic chain reaction involved using the CRISPR-associated protein 9 (Cas9) nuclease enzyme and allowed for transmission of mutations through the germ line with an inheritance rate of 95%.
The groups collaborated to fuse Dr Bier and Gantz’s method with Dr James’s mosquitoes. Gantz packaged antimalaria genes with a Cas9 enzyme and a guide RNA to create a genetic “cassette” that, when injected into a mosquito embryo, targeted a highly specific spot on the germ line DNA to insert the antimalaria antibody genes.
To ensure the element carrying the malaria-blocking antibodies had reached the desired DNA site, the researchers included in the cassette a protein that gave the progeny red fluorescence in the eyes. Nearly all offspring—99.5%—exhibited this trait.
Dr James said further testing will be needed to confirm the efficacy of the antibodies, and this could eventually lead to field studies.
“[The current study] is a significant first step,” he said. “We know the gene works. The mosquitoes we created are not the final brand, but we know this technology allows us to efficiently create large populations.” ![]()
MDA may help prevent malaria resurgence

Plasmodium vivax
Image by Mae Melvin
Mass drug administration (MDA) may have helped prevent a resurgence of malaria in Greece, according to research published in PLOS Neglected Tropical Diseases.
Greece was declared malaria-free in 1974 and remained that way until 2011, when there was an outbreak of Plasmodium vivax malaria in Southern Greece.
The outbreak was linked to the presence of agricultural workers from malaria-endemic regions in malaria-receptive areas.
There were 21 P vivax cases from arriving immigrants reported in the Southern agricultural area of Evrotas, along with 36 local cases.
So Greece implemented an integrated control program, with house visits established to screen immigrants from malaria-endemic countries.
Screening included a rapid diagnostic test for those reporting symptoms associated with malaria, along with blood sampling for smear and molecular testing for malaria. Directly observed treatment was provided for all patients who tested positive.
A vector control program was also implemented, with indoor residual spraying and long-lasting insecticide nets provided in areas close to mosquito breeding sites.
Despite these interventions, 20 more cases of malaria were reported in 2012.
Due to fears that the malaria parasite may be re-establishing itself in the area, Greece implemented an MDA program. It consisted of a single course of chloroquine and primaquine, which are the first-line recommended antimalarials for P vivax.
The program was implemented prior to the onset of peak adult mosquito activity, and field teams remained in situ continuing the active case detection until the end of the mosquito season. They recorded and managed adverse events daily.
The researchers identified an immigrant population of 1270 individuals, mostly from Pakistan and Afghanistan. The MDA covered 87% of this population.
No malaria cases were reported for 2013 and 2014, when the MDA was ongoing.
Of the treated individuals, 13% reported gastrointestinal symptoms from primaquine, while 36% reported non-severe side effects from chloroquine, including headaches, dizziness, and gastrointestinal complaints.
One potentially serious adverse event was recorded. It was a case of primaquine-induced hemolysis due to a false-normal G6PD level obtained prior to enrollment. The patient was hospitalized and recovered fully.
The researchers said that, in this case, the MDA program was a suitable and effective response for a small and geographically confined population over a short seasonal transition period. And the combination of 2 drugs minimized the risk of drug resistance.
The team added that, although an observational study of this nature cannot assess the extent to which the MDA program was responsible for eliminating malaria, it indicates that MDA should be considered and can be effective in local settings alongside other malaria control measures. ![]()

Plasmodium vivax
Image by Mae Melvin
Mass drug administration (MDA) may have helped prevent a resurgence of malaria in Greece, according to research published in PLOS Neglected Tropical Diseases.
Greece was declared malaria-free in 1974 and remained that way until 2011, when there was an outbreak of Plasmodium vivax malaria in Southern Greece.
The outbreak was linked to the presence of agricultural workers from malaria-endemic regions in malaria-receptive areas.
There were 21 P vivax cases from arriving immigrants reported in the Southern agricultural area of Evrotas, along with 36 local cases.
So Greece implemented an integrated control program, with house visits established to screen immigrants from malaria-endemic countries.
Screening included a rapid diagnostic test for those reporting symptoms associated with malaria, along with blood sampling for smear and molecular testing for malaria. Directly observed treatment was provided for all patients who tested positive.
A vector control program was also implemented, with indoor residual spraying and long-lasting insecticide nets provided in areas close to mosquito breeding sites.
Despite these interventions, 20 more cases of malaria were reported in 2012.
Due to fears that the malaria parasite may be re-establishing itself in the area, Greece implemented an MDA program. It consisted of a single course of chloroquine and primaquine, which are the first-line recommended antimalarials for P vivax.
The program was implemented prior to the onset of peak adult mosquito activity, and field teams remained in situ continuing the active case detection until the end of the mosquito season. They recorded and managed adverse events daily.
The researchers identified an immigrant population of 1270 individuals, mostly from Pakistan and Afghanistan. The MDA covered 87% of this population.
No malaria cases were reported for 2013 and 2014, when the MDA was ongoing.
Of the treated individuals, 13% reported gastrointestinal symptoms from primaquine, while 36% reported non-severe side effects from chloroquine, including headaches, dizziness, and gastrointestinal complaints.
One potentially serious adverse event was recorded. It was a case of primaquine-induced hemolysis due to a false-normal G6PD level obtained prior to enrollment. The patient was hospitalized and recovered fully.
The researchers said that, in this case, the MDA program was a suitable and effective response for a small and geographically confined population over a short seasonal transition period. And the combination of 2 drugs minimized the risk of drug resistance.
The team added that, although an observational study of this nature cannot assess the extent to which the MDA program was responsible for eliminating malaria, it indicates that MDA should be considered and can be effective in local settings alongside other malaria control measures. ![]()

Plasmodium vivax
Image by Mae Melvin
Mass drug administration (MDA) may have helped prevent a resurgence of malaria in Greece, according to research published in PLOS Neglected Tropical Diseases.
Greece was declared malaria-free in 1974 and remained that way until 2011, when there was an outbreak of Plasmodium vivax malaria in Southern Greece.
The outbreak was linked to the presence of agricultural workers from malaria-endemic regions in malaria-receptive areas.
There were 21 P vivax cases from arriving immigrants reported in the Southern agricultural area of Evrotas, along with 36 local cases.
So Greece implemented an integrated control program, with house visits established to screen immigrants from malaria-endemic countries.
Screening included a rapid diagnostic test for those reporting symptoms associated with malaria, along with blood sampling for smear and molecular testing for malaria. Directly observed treatment was provided for all patients who tested positive.
A vector control program was also implemented, with indoor residual spraying and long-lasting insecticide nets provided in areas close to mosquito breeding sites.
Despite these interventions, 20 more cases of malaria were reported in 2012.
Due to fears that the malaria parasite may be re-establishing itself in the area, Greece implemented an MDA program. It consisted of a single course of chloroquine and primaquine, which are the first-line recommended antimalarials for P vivax.
The program was implemented prior to the onset of peak adult mosquito activity, and field teams remained in situ continuing the active case detection until the end of the mosquito season. They recorded and managed adverse events daily.
The researchers identified an immigrant population of 1270 individuals, mostly from Pakistan and Afghanistan. The MDA covered 87% of this population.
No malaria cases were reported for 2013 and 2014, when the MDA was ongoing.
Of the treated individuals, 13% reported gastrointestinal symptoms from primaquine, while 36% reported non-severe side effects from chloroquine, including headaches, dizziness, and gastrointestinal complaints.
One potentially serious adverse event was recorded. It was a case of primaquine-induced hemolysis due to a false-normal G6PD level obtained prior to enrollment. The patient was hospitalized and recovered fully.
The researchers said that, in this case, the MDA program was a suitable and effective response for a small and geographically confined population over a short seasonal transition period. And the combination of 2 drugs minimized the risk of drug resistance.
The team added that, although an observational study of this nature cannot assess the extent to which the MDA program was responsible for eliminating malaria, it indicates that MDA should be considered and can be effective in local settings alongside other malaria control measures. ![]()
Template can help guide care for cancer survivors

for radiation therapy
Photo by Rhoda Baer
A new template can help standardize plans for long-term care of cancer survivors who have undergone radiation therapy (RT), according to the American Society for Radiation Oncology (ASTRO).
An ASTRO advisory committee created the template to coordinate post-treatment care for cancer survivors among primary care providers (PCPs) and oncology
specialists (radiation, medical, and surgical), as well as patients themselves.
Details on the template appear in Practical Radiation Oncology.
“Factors such as earlier detection of cancer, increasingly effective treatment options, and an aging population lead to a growing number of cancer survivors and, ultimately, a need to educate and empower these individuals for their ongoing care,” said ASTRO chair Bruce D. Minsky, MD.
“The ASTRO template is designed to foster better coordination of post-treatment care for cancer survivors, including greater clarity in the dialogue between radiation oncologists and PCPs for issues such as less common side effects that may appear well after treatment is complete.”
Many radiation oncologists may already provide their patients with post-treatment materials such as diagnosis and treatment summaries, contacts for ancillary services such as financial or nutritional counselling, and information on potential late treatment effects.
But the ASTRO template coordinates these components in a central, plain-language document.
It also enables practices to meet new accreditation requirements set by the American College of Surgeons Commission on Cancer (CoC).
In response to a 2006 recommendation from the Institutes of Medicine that cancer patients be provided with a survivorship care plan (SCP) following treatment, CoC issued a mandate that cancer programs provide SCPs for all curative cancer patients by 2019 to maintain accreditation.
ASTRO’s template includes both elements required by the CoC in SCPs—namely, a summary of past treatment and directions for future care.
The treatment summary outlines the survivor’s diagnosis and stage information; treatment details such as the site, dosage, and schedule of RT; and contact information for providers who delivered the treatment.
The plan for follow-up care covers anticipated toxicities from RT, expected course of recovery from treatment-related toxicities, possible functional and/or social limitations, recommendations for preventative measures and behaviors, cancer information resources, and referrals to supportive care providers.
“This 2-page template facilitates consistency in SCPs across the discipline and also reduces the time and effort required by providers to complete each individual plan,” said Ronald Chen, MD, of the University of North Carolina at Chapel Hill.
“The field of radiation oncology has a long tradition of creating treatment summaries for each patient, even before the Institute of Medicine recommended survivorship care plans in 2006. This radiation-oncology-specific template will serve a dual purpose as both a traditional radiation oncology treatment summary and a plan for survivorship care that meets CoC requirements, thus reducing the burden on radiation oncologists from having to create 2 documents for each patient.” ![]()

for radiation therapy
Photo by Rhoda Baer
A new template can help standardize plans for long-term care of cancer survivors who have undergone radiation therapy (RT), according to the American Society for Radiation Oncology (ASTRO).
An ASTRO advisory committee created the template to coordinate post-treatment care for cancer survivors among primary care providers (PCPs) and oncology
specialists (radiation, medical, and surgical), as well as patients themselves.
Details on the template appear in Practical Radiation Oncology.
“Factors such as earlier detection of cancer, increasingly effective treatment options, and an aging population lead to a growing number of cancer survivors and, ultimately, a need to educate and empower these individuals for their ongoing care,” said ASTRO chair Bruce D. Minsky, MD.
“The ASTRO template is designed to foster better coordination of post-treatment care for cancer survivors, including greater clarity in the dialogue between radiation oncologists and PCPs for issues such as less common side effects that may appear well after treatment is complete.”
Many radiation oncologists may already provide their patients with post-treatment materials such as diagnosis and treatment summaries, contacts for ancillary services such as financial or nutritional counselling, and information on potential late treatment effects.
But the ASTRO template coordinates these components in a central, plain-language document.
It also enables practices to meet new accreditation requirements set by the American College of Surgeons Commission on Cancer (CoC).
In response to a 2006 recommendation from the Institutes of Medicine that cancer patients be provided with a survivorship care plan (SCP) following treatment, CoC issued a mandate that cancer programs provide SCPs for all curative cancer patients by 2019 to maintain accreditation.
ASTRO’s template includes both elements required by the CoC in SCPs—namely, a summary of past treatment and directions for future care.
The treatment summary outlines the survivor’s diagnosis and stage information; treatment details such as the site, dosage, and schedule of RT; and contact information for providers who delivered the treatment.
The plan for follow-up care covers anticipated toxicities from RT, expected course of recovery from treatment-related toxicities, possible functional and/or social limitations, recommendations for preventative measures and behaviors, cancer information resources, and referrals to supportive care providers.
“This 2-page template facilitates consistency in SCPs across the discipline and also reduces the time and effort required by providers to complete each individual plan,” said Ronald Chen, MD, of the University of North Carolina at Chapel Hill.
“The field of radiation oncology has a long tradition of creating treatment summaries for each patient, even before the Institute of Medicine recommended survivorship care plans in 2006. This radiation-oncology-specific template will serve a dual purpose as both a traditional radiation oncology treatment summary and a plan for survivorship care that meets CoC requirements, thus reducing the burden on radiation oncologists from having to create 2 documents for each patient.” ![]()

for radiation therapy
Photo by Rhoda Baer
A new template can help standardize plans for long-term care of cancer survivors who have undergone radiation therapy (RT), according to the American Society for Radiation Oncology (ASTRO).
An ASTRO advisory committee created the template to coordinate post-treatment care for cancer survivors among primary care providers (PCPs) and oncology
specialists (radiation, medical, and surgical), as well as patients themselves.
Details on the template appear in Practical Radiation Oncology.
“Factors such as earlier detection of cancer, increasingly effective treatment options, and an aging population lead to a growing number of cancer survivors and, ultimately, a need to educate and empower these individuals for their ongoing care,” said ASTRO chair Bruce D. Minsky, MD.
“The ASTRO template is designed to foster better coordination of post-treatment care for cancer survivors, including greater clarity in the dialogue between radiation oncologists and PCPs for issues such as less common side effects that may appear well after treatment is complete.”
Many radiation oncologists may already provide their patients with post-treatment materials such as diagnosis and treatment summaries, contacts for ancillary services such as financial or nutritional counselling, and information on potential late treatment effects.
But the ASTRO template coordinates these components in a central, plain-language document.
It also enables practices to meet new accreditation requirements set by the American College of Surgeons Commission on Cancer (CoC).
In response to a 2006 recommendation from the Institutes of Medicine that cancer patients be provided with a survivorship care plan (SCP) following treatment, CoC issued a mandate that cancer programs provide SCPs for all curative cancer patients by 2019 to maintain accreditation.
ASTRO’s template includes both elements required by the CoC in SCPs—namely, a summary of past treatment and directions for future care.
The treatment summary outlines the survivor’s diagnosis and stage information; treatment details such as the site, dosage, and schedule of RT; and contact information for providers who delivered the treatment.
The plan for follow-up care covers anticipated toxicities from RT, expected course of recovery from treatment-related toxicities, possible functional and/or social limitations, recommendations for preventative measures and behaviors, cancer information resources, and referrals to supportive care providers.
“This 2-page template facilitates consistency in SCPs across the discipline and also reduces the time and effort required by providers to complete each individual plan,” said Ronald Chen, MD, of the University of North Carolina at Chapel Hill.
“The field of radiation oncology has a long tradition of creating treatment summaries for each patient, even before the Institute of Medicine recommended survivorship care plans in 2006. This radiation-oncology-specific template will serve a dual purpose as both a traditional radiation oncology treatment summary and a plan for survivorship care that meets CoC requirements, thus reducing the burden on radiation oncologists from having to create 2 documents for each patient.” ![]()
Companies abuse orphan drug designation, team says

Photo by Steven Harbour
Health experts are calling on US lawmakers and regulators to “close loopholes” in the Orphan Drug Act.
The experts say the loopholes can provide pharmaceutical companies with millions of dollars in unintended subsidies and tax breaks and fuel skyrocketing medication costs.
They argue that companies are exploiting gaps in the law by claiming orphan status for drugs that end up being marketed for more common conditions.
“The industry has been gaming the system by slicing and dicing indications so that drugs qualify for lucrative orphan status benefits,” says Martin Makary, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“As a result, funding support intended for rare disease medicine is diverted to fund the development of blockbuster drugs.”
Dr Makary and his colleagues express this viewpoint in a commentary published in the American Journal of Clinical Oncology.
The US Food and Drug Administration (FDA) grants orphan designation to encourage the development of drugs for diseases that affect fewer than 200,000 people in the US. The Orphan Drug Act was enacted in 1983 to provide incentives for drug companies to develop treatments for so-called orphan diseases that would be unprofitable because of the limited market.
Dr Makary and his colleagues say the legislation has accomplished that mission and sparked the development of life-saving therapies for a range of rare disorders. However, the authors say the law has also invited abuse.
Under the terms of the act, companies can receive federal taxpayer subsidies of up to half a million dollars a year for up to 4 years per drug, large tax credits, and waivers of marketing application fees that can cost more than $2 million. In addition, the FDA can grant companies 7 years of marketing exclusivity for an orphan drug to ensure that companies recoup the costs of research and development.
Dr Makary says companies exploit the law by initially listing only a single indication for a drug’s use—one narrow enough to qualify for orphan disease benefits. After FDA approval, however, some such drugs are marketed and used off-label more broadly, thus turning large profits.
“This is a financially toxic practice that is also unethical,” says study author Michael Daniel, also of Johns Hopkins.
“It’s time to ensure that we also render it illegal. The practice inflates drug prices, and the costs are passed on to consumers in the form of higher health insurance premiums.”
For example, the drug rituximab was originally approved to treat follicular B-cell non-Hodgkin lymphoma, a disease that affects about 14,000 patients a year. Now, rituximab is also used to treat several other types of cancer, organ rejection following kidney transplant, and autoimmune diseases, including rheumatoid arthritis, which affects 1.3 million Americans.
Rituximab, marketed under several trade names, is the top-selling medication approved as an orphan drug, the 12th all-time drug best-seller in the US, and it generated $3.7 billion in domestic sales in 2014.
In fact, 7 of the top 10 best-selling drugs in the US for 2014 came on the market with an orphan designation, according to Dr Makary and his colleagues.
Of the 41 drugs approved by the FDA in 2014, 18 had orphan status designations. The authors predict that, in 2015, orphan drugs will generate sales totaling $107 billion. And that number is expected to reach $176 billion by 2020.
Dr Makary says this projection represents a yearly growth rate of nearly 11%, or double the growth rate of the overall prescription drug market. The authors also cite data showing that, by 2020, orphan drugs are expected to account for 19% of global prescription drug sales, up from 6% in the year 2000.
Although the reasons for this boom in orphan drugs are likely multifactorial, the exploitation of the orphan drug act is an important catalyst behind this trend, the authors say.
Because orphan designation guarantees a 7-year exclusivity deal to market the drug and protects it from generic competition, the price tags for such medications often balloon rapidly.
For example, the drug imatinib was initially priced at $30,000 per year in 2001. By 2012, it cost $92,000 a year.
The drug’s original designation was for chronic myelogenous leukemia, and it would therefore treat 9000 patients a year in the US. Subsequently, imatinib was given 6 additional orphan designations for various conditions, including gastric cancers and immune disorders.
Dr Makary says, in essence, the exclusivity clause guarantees a hyperextended government-sponsored monopoly. So it’s not surprising that the median cost for orphan drugs is more than $98,000 per patient per year, compared with a median cost of just over $5000 per patient per year for drugs without orphan status.
Overall, nearly 15% of already approved orphan drugs subsequently add far more common diseases to their treatment repertoires.
Dr Makary and his colleagues recommend that, once a drug exceeds the basic tenets of the act—to treat fewer than 200,000 people—it should no longer receive government support or marketing exclusivity.
This can be achieved, the authors say, through pricing negotiations, clauses that reduce marketing exclusivity, and leveling of taxes once a medication becomes a blockbuster treatment for conditions not listed in the original FDA approval.
They say such measures would ensure the spirit of the original act is followed while continuing to provide critical economic incentives for truly rare diseases. ![]()

Photo by Steven Harbour
Health experts are calling on US lawmakers and regulators to “close loopholes” in the Orphan Drug Act.
The experts say the loopholes can provide pharmaceutical companies with millions of dollars in unintended subsidies and tax breaks and fuel skyrocketing medication costs.
They argue that companies are exploiting gaps in the law by claiming orphan status for drugs that end up being marketed for more common conditions.
“The industry has been gaming the system by slicing and dicing indications so that drugs qualify for lucrative orphan status benefits,” says Martin Makary, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“As a result, funding support intended for rare disease medicine is diverted to fund the development of blockbuster drugs.”
Dr Makary and his colleagues express this viewpoint in a commentary published in the American Journal of Clinical Oncology.
The US Food and Drug Administration (FDA) grants orphan designation to encourage the development of drugs for diseases that affect fewer than 200,000 people in the US. The Orphan Drug Act was enacted in 1983 to provide incentives for drug companies to develop treatments for so-called orphan diseases that would be unprofitable because of the limited market.
Dr Makary and his colleagues say the legislation has accomplished that mission and sparked the development of life-saving therapies for a range of rare disorders. However, the authors say the law has also invited abuse.
Under the terms of the act, companies can receive federal taxpayer subsidies of up to half a million dollars a year for up to 4 years per drug, large tax credits, and waivers of marketing application fees that can cost more than $2 million. In addition, the FDA can grant companies 7 years of marketing exclusivity for an orphan drug to ensure that companies recoup the costs of research and development.
Dr Makary says companies exploit the law by initially listing only a single indication for a drug’s use—one narrow enough to qualify for orphan disease benefits. After FDA approval, however, some such drugs are marketed and used off-label more broadly, thus turning large profits.
“This is a financially toxic practice that is also unethical,” says study author Michael Daniel, also of Johns Hopkins.
“It’s time to ensure that we also render it illegal. The practice inflates drug prices, and the costs are passed on to consumers in the form of higher health insurance premiums.”
For example, the drug rituximab was originally approved to treat follicular B-cell non-Hodgkin lymphoma, a disease that affects about 14,000 patients a year. Now, rituximab is also used to treat several other types of cancer, organ rejection following kidney transplant, and autoimmune diseases, including rheumatoid arthritis, which affects 1.3 million Americans.
Rituximab, marketed under several trade names, is the top-selling medication approved as an orphan drug, the 12th all-time drug best-seller in the US, and it generated $3.7 billion in domestic sales in 2014.
In fact, 7 of the top 10 best-selling drugs in the US for 2014 came on the market with an orphan designation, according to Dr Makary and his colleagues.
Of the 41 drugs approved by the FDA in 2014, 18 had orphan status designations. The authors predict that, in 2015, orphan drugs will generate sales totaling $107 billion. And that number is expected to reach $176 billion by 2020.
Dr Makary says this projection represents a yearly growth rate of nearly 11%, or double the growth rate of the overall prescription drug market. The authors also cite data showing that, by 2020, orphan drugs are expected to account for 19% of global prescription drug sales, up from 6% in the year 2000.
Although the reasons for this boom in orphan drugs are likely multifactorial, the exploitation of the orphan drug act is an important catalyst behind this trend, the authors say.
Because orphan designation guarantees a 7-year exclusivity deal to market the drug and protects it from generic competition, the price tags for such medications often balloon rapidly.
For example, the drug imatinib was initially priced at $30,000 per year in 2001. By 2012, it cost $92,000 a year.
The drug’s original designation was for chronic myelogenous leukemia, and it would therefore treat 9000 patients a year in the US. Subsequently, imatinib was given 6 additional orphan designations for various conditions, including gastric cancers and immune disorders.
Dr Makary says, in essence, the exclusivity clause guarantees a hyperextended government-sponsored monopoly. So it’s not surprising that the median cost for orphan drugs is more than $98,000 per patient per year, compared with a median cost of just over $5000 per patient per year for drugs without orphan status.
Overall, nearly 15% of already approved orphan drugs subsequently add far more common diseases to their treatment repertoires.
Dr Makary and his colleagues recommend that, once a drug exceeds the basic tenets of the act—to treat fewer than 200,000 people—it should no longer receive government support or marketing exclusivity.
This can be achieved, the authors say, through pricing negotiations, clauses that reduce marketing exclusivity, and leveling of taxes once a medication becomes a blockbuster treatment for conditions not listed in the original FDA approval.
They say such measures would ensure the spirit of the original act is followed while continuing to provide critical economic incentives for truly rare diseases. ![]()

Photo by Steven Harbour
Health experts are calling on US lawmakers and regulators to “close loopholes” in the Orphan Drug Act.
The experts say the loopholes can provide pharmaceutical companies with millions of dollars in unintended subsidies and tax breaks and fuel skyrocketing medication costs.
They argue that companies are exploiting gaps in the law by claiming orphan status for drugs that end up being marketed for more common conditions.
“The industry has been gaming the system by slicing and dicing indications so that drugs qualify for lucrative orphan status benefits,” says Martin Makary, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“As a result, funding support intended for rare disease medicine is diverted to fund the development of blockbuster drugs.”
Dr Makary and his colleagues express this viewpoint in a commentary published in the American Journal of Clinical Oncology.
The US Food and Drug Administration (FDA) grants orphan designation to encourage the development of drugs for diseases that affect fewer than 200,000 people in the US. The Orphan Drug Act was enacted in 1983 to provide incentives for drug companies to develop treatments for so-called orphan diseases that would be unprofitable because of the limited market.
Dr Makary and his colleagues say the legislation has accomplished that mission and sparked the development of life-saving therapies for a range of rare disorders. However, the authors say the law has also invited abuse.
Under the terms of the act, companies can receive federal taxpayer subsidies of up to half a million dollars a year for up to 4 years per drug, large tax credits, and waivers of marketing application fees that can cost more than $2 million. In addition, the FDA can grant companies 7 years of marketing exclusivity for an orphan drug to ensure that companies recoup the costs of research and development.
Dr Makary says companies exploit the law by initially listing only a single indication for a drug’s use—one narrow enough to qualify for orphan disease benefits. After FDA approval, however, some such drugs are marketed and used off-label more broadly, thus turning large profits.
“This is a financially toxic practice that is also unethical,” says study author Michael Daniel, also of Johns Hopkins.
“It’s time to ensure that we also render it illegal. The practice inflates drug prices, and the costs are passed on to consumers in the form of higher health insurance premiums.”
For example, the drug rituximab was originally approved to treat follicular B-cell non-Hodgkin lymphoma, a disease that affects about 14,000 patients a year. Now, rituximab is also used to treat several other types of cancer, organ rejection following kidney transplant, and autoimmune diseases, including rheumatoid arthritis, which affects 1.3 million Americans.
Rituximab, marketed under several trade names, is the top-selling medication approved as an orphan drug, the 12th all-time drug best-seller in the US, and it generated $3.7 billion in domestic sales in 2014.
In fact, 7 of the top 10 best-selling drugs in the US for 2014 came on the market with an orphan designation, according to Dr Makary and his colleagues.
Of the 41 drugs approved by the FDA in 2014, 18 had orphan status designations. The authors predict that, in 2015, orphan drugs will generate sales totaling $107 billion. And that number is expected to reach $176 billion by 2020.
Dr Makary says this projection represents a yearly growth rate of nearly 11%, or double the growth rate of the overall prescription drug market. The authors also cite data showing that, by 2020, orphan drugs are expected to account for 19% of global prescription drug sales, up from 6% in the year 2000.
Although the reasons for this boom in orphan drugs are likely multifactorial, the exploitation of the orphan drug act is an important catalyst behind this trend, the authors say.
Because orphan designation guarantees a 7-year exclusivity deal to market the drug and protects it from generic competition, the price tags for such medications often balloon rapidly.
For example, the drug imatinib was initially priced at $30,000 per year in 2001. By 2012, it cost $92,000 a year.
The drug’s original designation was for chronic myelogenous leukemia, and it would therefore treat 9000 patients a year in the US. Subsequently, imatinib was given 6 additional orphan designations for various conditions, including gastric cancers and immune disorders.
Dr Makary says, in essence, the exclusivity clause guarantees a hyperextended government-sponsored monopoly. So it’s not surprising that the median cost for orphan drugs is more than $98,000 per patient per year, compared with a median cost of just over $5000 per patient per year for drugs without orphan status.
Overall, nearly 15% of already approved orphan drugs subsequently add far more common diseases to their treatment repertoires.
Dr Makary and his colleagues recommend that, once a drug exceeds the basic tenets of the act—to treat fewer than 200,000 people—it should no longer receive government support or marketing exclusivity.
This can be achieved, the authors say, through pricing negotiations, clauses that reduce marketing exclusivity, and leveling of taxes once a medication becomes a blockbuster treatment for conditions not listed in the original FDA approval.
They say such measures would ensure the spirit of the original act is followed while continuing to provide critical economic incentives for truly rare diseases. ![]()
Group uses lettuce to produce clotting factor on large scale

Photo by Daniel Ventura
Investigators have shown they can use lettuce to produce a factor IX product on a large scale.
The product successfully delivered factor IX to mice with hemophilia B while preventing the formation of inhibitors.
“This is a milestone in our field—to make a fully functional drug in plants, produce it at a large scale and in quantities sufficient for human clinical trials,” said Henry Daniell, PhD, of the University of Pennsylvania School of Dental Medicine in Philadelphia.
Dr Daniell and his colleagues described this work in Biomaterials.
This research builds on Dr Daniell’s previous work using genetically modified plants to introduce a protein into the body that would teach the immune system to tolerate clotting factors given as a treatment for hemophilia.
In that study, Dr Daniell and his colleagues successfully stopped and even reversed the production of inhibitors by feeding the plant-based drug to mice with hemophilia A. At that time, the investigators used a tobacco plant platform to “grow” the drug.
To take this approach to humans, Dr Daniell’s team turned to lettuce. They identified the genetic vector to introduce the therapeutic gene into the plant cells’ DNA and grow the drug within the lettuce leaves, which are then freeze-dried and encapsulated.
Two different growing systems were used. One was in Dr Daniell’s greenhouse, a high-tech facility that grows the plants in soil, using natural light.
The second system was used in the Fraunhofer USA facility, which more closely replicates how a commercial pharmaceutical production facility would run, using a hydroponic system and artificial lighting.
The investigators determined they could produce 36,000 doses in just 1000 square feet and harvest a new batch of pharmaceutical-containing lettuce every 4 to 6 weeks.
“This changes the way we think about delivering protein-based drugs and making them affordable to the global population,” Dr Daniell said.
“Over 90% of the global population can’t afford protein drugs, like insulin, due to the expense of production and the required refrigeration for storage or transportation. I am determined to challenge this scenario.” ![]()

Photo by Daniel Ventura
Investigators have shown they can use lettuce to produce a factor IX product on a large scale.
The product successfully delivered factor IX to mice with hemophilia B while preventing the formation of inhibitors.
“This is a milestone in our field—to make a fully functional drug in plants, produce it at a large scale and in quantities sufficient for human clinical trials,” said Henry Daniell, PhD, of the University of Pennsylvania School of Dental Medicine in Philadelphia.
Dr Daniell and his colleagues described this work in Biomaterials.
This research builds on Dr Daniell’s previous work using genetically modified plants to introduce a protein into the body that would teach the immune system to tolerate clotting factors given as a treatment for hemophilia.
In that study, Dr Daniell and his colleagues successfully stopped and even reversed the production of inhibitors by feeding the plant-based drug to mice with hemophilia A. At that time, the investigators used a tobacco plant platform to “grow” the drug.
To take this approach to humans, Dr Daniell’s team turned to lettuce. They identified the genetic vector to introduce the therapeutic gene into the plant cells’ DNA and grow the drug within the lettuce leaves, which are then freeze-dried and encapsulated.
Two different growing systems were used. One was in Dr Daniell’s greenhouse, a high-tech facility that grows the plants in soil, using natural light.
The second system was used in the Fraunhofer USA facility, which more closely replicates how a commercial pharmaceutical production facility would run, using a hydroponic system and artificial lighting.
The investigators determined they could produce 36,000 doses in just 1000 square feet and harvest a new batch of pharmaceutical-containing lettuce every 4 to 6 weeks.
“This changes the way we think about delivering protein-based drugs and making them affordable to the global population,” Dr Daniell said.
“Over 90% of the global population can’t afford protein drugs, like insulin, due to the expense of production and the required refrigeration for storage or transportation. I am determined to challenge this scenario.” ![]()

Photo by Daniel Ventura
Investigators have shown they can use lettuce to produce a factor IX product on a large scale.
The product successfully delivered factor IX to mice with hemophilia B while preventing the formation of inhibitors.
“This is a milestone in our field—to make a fully functional drug in plants, produce it at a large scale and in quantities sufficient for human clinical trials,” said Henry Daniell, PhD, of the University of Pennsylvania School of Dental Medicine in Philadelphia.
Dr Daniell and his colleagues described this work in Biomaterials.
This research builds on Dr Daniell’s previous work using genetically modified plants to introduce a protein into the body that would teach the immune system to tolerate clotting factors given as a treatment for hemophilia.
In that study, Dr Daniell and his colleagues successfully stopped and even reversed the production of inhibitors by feeding the plant-based drug to mice with hemophilia A. At that time, the investigators used a tobacco plant platform to “grow” the drug.
To take this approach to humans, Dr Daniell’s team turned to lettuce. They identified the genetic vector to introduce the therapeutic gene into the plant cells’ DNA and grow the drug within the lettuce leaves, which are then freeze-dried and encapsulated.
Two different growing systems were used. One was in Dr Daniell’s greenhouse, a high-tech facility that grows the plants in soil, using natural light.
The second system was used in the Fraunhofer USA facility, which more closely replicates how a commercial pharmaceutical production facility would run, using a hydroponic system and artificial lighting.
The investigators determined they could produce 36,000 doses in just 1000 square feet and harvest a new batch of pharmaceutical-containing lettuce every 4 to 6 weeks.
“This changes the way we think about delivering protein-based drugs and making them affordable to the global population,” Dr Daniell said.
“Over 90% of the global population can’t afford protein drugs, like insulin, due to the expense of production and the required refrigeration for storage or transportation. I am determined to challenge this scenario.” ![]()
Finger-prick blood tests may need repeating

Finger-prick blood tests can deliver inaccurate results, according to a study published in the American Journal of Clinical Pathology.
Investigators found that results varied greatly when they performed multiple finger-prick tests on a single subject.
However, averaging the results of multiple droplet tests allowed the investigators to achieve results on par with venous blood tests.
They required 6 to 9 drops of blood to achieve consistent results.
“We began looking at this after we got some surprising results from our controls in an earlier study,” explained lead investigator Rebecca Richards-Kortum, PhD, of Rice University in Houston, Texas.
“Students in my lab are developing novel, low-cost platforms for anemia, platelet, and white blood cell testing in low-resource settings, and one of my students, Meaghan Bond, noticed there was wide variation in some of the benchmark tests that she was performing on hospital-grade blood analyzers.”
The benchmark controls are used to gauge the accuracy of test results from the new technology under study, so the variation among the control data was a sign that something was amiss.
What wasn’t immediately clear was whether the readings resulted from a problem with the current experiments or actual variations in the amount of hemoglobin, platelets, and white blood cells in the different drops of blood.
So Dr Richards-Kortum and Bond designed a simple protocol to test whether there was actual variation and, if so, how much.
They drew 6 successive 20 µL droplets of blood from 11 donors. As an additional test to determine whether minimum droplet size might also affect the results, the pair drew 10 successive 10 µL droplets from 7 additional donors.
All droplets were drawn from the same finger prick, and the investigators followed best practices in obtaining the droplets. The first drop was wiped away to remove contamination from disinfectants, and the finger was not squeezed or “milked,” which can lead to inaccurate results.
For experimental controls, the investigators used venipuncture to draw tubes of blood from an arm vein.
Each 20 µL droplet was analyzed with a hospital-grade blood analyzer for hemoglobin concentration, total white blood cell count, platelet count, and 3-part white blood cell differential. Each 10 µL droplet was tested for hemoglobin concentration with a popular point-of-care blood analyzer.
“A growing number of clinically important tests are performed using finger-prick blood, and this is especially true in low-resource settings,” Bond noted.
“It is important to understand how variations in finger-prick blood collection protocols can affect point-of-care test accuracy as well as how results might vary between different kinds of point-of-care tests that use finger-prick blood from the same patient.”
She and Dr Richards-Kortum found that hemoglobin content, platelet count, and white blood cell count each varied significantly from blood drop to blood drop.
“Some of the differences were surprising,” Bond said. “For example, in some donors, the hemoglobin concentration changed by more than 2 g/dL in the span of 2 successive drops of blood.”
Fortunately, the investigators found that averaging the results of the droplet tests could produce results that were on par with venous blood tests, but tests on 6 to 9 drops of blood were needed to achieve consistent results.
“Finger-prick blood tests can be accurate, and they are an important tool for healthcare providers, particularly in point-of-care and low-resource settings,” Bond said.
“Our results show that people need to take care to administer finger-prick tests in a way that produces accurate results because accuracy in these tests is increasingly important for diagnosing conditions like anemia, infections, and sickle cell anemia, malaria, HIV, and other diseases.” ![]()

Finger-prick blood tests can deliver inaccurate results, according to a study published in the American Journal of Clinical Pathology.
Investigators found that results varied greatly when they performed multiple finger-prick tests on a single subject.
However, averaging the results of multiple droplet tests allowed the investigators to achieve results on par with venous blood tests.
They required 6 to 9 drops of blood to achieve consistent results.
“We began looking at this after we got some surprising results from our controls in an earlier study,” explained lead investigator Rebecca Richards-Kortum, PhD, of Rice University in Houston, Texas.
“Students in my lab are developing novel, low-cost platforms for anemia, platelet, and white blood cell testing in low-resource settings, and one of my students, Meaghan Bond, noticed there was wide variation in some of the benchmark tests that she was performing on hospital-grade blood analyzers.”
The benchmark controls are used to gauge the accuracy of test results from the new technology under study, so the variation among the control data was a sign that something was amiss.
What wasn’t immediately clear was whether the readings resulted from a problem with the current experiments or actual variations in the amount of hemoglobin, platelets, and white blood cells in the different drops of blood.
So Dr Richards-Kortum and Bond designed a simple protocol to test whether there was actual variation and, if so, how much.
They drew 6 successive 20 µL droplets of blood from 11 donors. As an additional test to determine whether minimum droplet size might also affect the results, the pair drew 10 successive 10 µL droplets from 7 additional donors.
All droplets were drawn from the same finger prick, and the investigators followed best practices in obtaining the droplets. The first drop was wiped away to remove contamination from disinfectants, and the finger was not squeezed or “milked,” which can lead to inaccurate results.
For experimental controls, the investigators used venipuncture to draw tubes of blood from an arm vein.
Each 20 µL droplet was analyzed with a hospital-grade blood analyzer for hemoglobin concentration, total white blood cell count, platelet count, and 3-part white blood cell differential. Each 10 µL droplet was tested for hemoglobin concentration with a popular point-of-care blood analyzer.
“A growing number of clinically important tests are performed using finger-prick blood, and this is especially true in low-resource settings,” Bond noted.
“It is important to understand how variations in finger-prick blood collection protocols can affect point-of-care test accuracy as well as how results might vary between different kinds of point-of-care tests that use finger-prick blood from the same patient.”
She and Dr Richards-Kortum found that hemoglobin content, platelet count, and white blood cell count each varied significantly from blood drop to blood drop.
“Some of the differences were surprising,” Bond said. “For example, in some donors, the hemoglobin concentration changed by more than 2 g/dL in the span of 2 successive drops of blood.”
Fortunately, the investigators found that averaging the results of the droplet tests could produce results that were on par with venous blood tests, but tests on 6 to 9 drops of blood were needed to achieve consistent results.
“Finger-prick blood tests can be accurate, and they are an important tool for healthcare providers, particularly in point-of-care and low-resource settings,” Bond said.
“Our results show that people need to take care to administer finger-prick tests in a way that produces accurate results because accuracy in these tests is increasingly important for diagnosing conditions like anemia, infections, and sickle cell anemia, malaria, HIV, and other diseases.” ![]()

Finger-prick blood tests can deliver inaccurate results, according to a study published in the American Journal of Clinical Pathology.
Investigators found that results varied greatly when they performed multiple finger-prick tests on a single subject.
However, averaging the results of multiple droplet tests allowed the investigators to achieve results on par with venous blood tests.
They required 6 to 9 drops of blood to achieve consistent results.
“We began looking at this after we got some surprising results from our controls in an earlier study,” explained lead investigator Rebecca Richards-Kortum, PhD, of Rice University in Houston, Texas.
“Students in my lab are developing novel, low-cost platforms for anemia, platelet, and white blood cell testing in low-resource settings, and one of my students, Meaghan Bond, noticed there was wide variation in some of the benchmark tests that she was performing on hospital-grade blood analyzers.”
The benchmark controls are used to gauge the accuracy of test results from the new technology under study, so the variation among the control data was a sign that something was amiss.
What wasn’t immediately clear was whether the readings resulted from a problem with the current experiments or actual variations in the amount of hemoglobin, platelets, and white blood cells in the different drops of blood.
So Dr Richards-Kortum and Bond designed a simple protocol to test whether there was actual variation and, if so, how much.
They drew 6 successive 20 µL droplets of blood from 11 donors. As an additional test to determine whether minimum droplet size might also affect the results, the pair drew 10 successive 10 µL droplets from 7 additional donors.
All droplets were drawn from the same finger prick, and the investigators followed best practices in obtaining the droplets. The first drop was wiped away to remove contamination from disinfectants, and the finger was not squeezed or “milked,” which can lead to inaccurate results.
For experimental controls, the investigators used venipuncture to draw tubes of blood from an arm vein.
Each 20 µL droplet was analyzed with a hospital-grade blood analyzer for hemoglobin concentration, total white blood cell count, platelet count, and 3-part white blood cell differential. Each 10 µL droplet was tested for hemoglobin concentration with a popular point-of-care blood analyzer.
“A growing number of clinically important tests are performed using finger-prick blood, and this is especially true in low-resource settings,” Bond noted.
“It is important to understand how variations in finger-prick blood collection protocols can affect point-of-care test accuracy as well as how results might vary between different kinds of point-of-care tests that use finger-prick blood from the same patient.”
She and Dr Richards-Kortum found that hemoglobin content, platelet count, and white blood cell count each varied significantly from blood drop to blood drop.
“Some of the differences were surprising,” Bond said. “For example, in some donors, the hemoglobin concentration changed by more than 2 g/dL in the span of 2 successive drops of blood.”
Fortunately, the investigators found that averaging the results of the droplet tests could produce results that were on par with venous blood tests, but tests on 6 to 9 drops of blood were needed to achieve consistent results.
“Finger-prick blood tests can be accurate, and they are an important tool for healthcare providers, particularly in point-of-care and low-resource settings,” Bond said.
“Our results show that people need to take care to administer finger-prick tests in a way that produces accurate results because accuracy in these tests is increasingly important for diagnosing conditions like anemia, infections, and sickle cell anemia, malaria, HIV, and other diseases.”
Coconut oil may prevent bloodstream infection

Coconut oil may combat infection with Candida albicans, according to preclinical research published in mSphere.
Mice on a diet that included coconut oil had significantly lower gastrointestinal colonization by C albicans than mice that were fed high-fat diets without coconut oil or mice that received a standard diet.
“We found that diet can be an effective way to reduce the amount of Candida in the mouse,” said study author Carol Kumamoto, PhD, of Tufts University School of Medicine in Boston, Massachusetts.
“The extension of this finding to the human population is something that needs to be addressed in the future.”
Previous research showed that changes to diet, including changes in the amount and type of fat, can alter gastrointestinal microbiota. And in vitro studies showed that coconut oil has antifungal properties.
So Dr Kumamoto and her colleagues studied the effect of different diets on C albicans colonization in mice.
The mice received standard diets or high-fat diets containing coconut oil, beef tallow, or soybean oil. The mice were fed these diets for 14 days prior to inoculation with C albicans and 21 days after.
At 21 days post-inoculation, gastrointestinal colonization with C albicans was significantly lower in the stomach contents of mice fed the coconut oil than mice fed the beef tallow (P<0.0001), the soybean oil (P<0.0001), or the standard diet (P<0.0001).
“When you compared a mouse on a high-fat diet that contained either beef fat or soybean oil to mice eating coconut oil, there was about a 10-fold drop in colonization,” Dr Kumamoto said.
In another experiment, the researchers switched mice receiving beef tallow to coconut oil.
“Four days after the change in diet, the colonization changed so it looked almost exactly like what you saw in a mouse who had been on coconut oil the entire time,” Dr Kumamoto said.
“There are 2 directions that we would like to take with this research now,” she added. “One of them is finding out the mechanism of how this works. That is a big question we would like to answer. The second question is whether this can have any impact on humans.”
The researchers are in discussion with Joseph Bliss, MD, PhD, of Women and Infants Hospital of Rhode Island, about launching a clinical trial testing coconut oil in hospitalized infants at high risk of developing systemic candidiasis.

Coconut oil may combat infection with Candida albicans, according to preclinical research published in mSphere.
Mice on a diet that included coconut oil had significantly lower gastrointestinal colonization by C albicans than mice that were fed high-fat diets without coconut oil or mice that received a standard diet.
“We found that diet can be an effective way to reduce the amount of Candida in the mouse,” said study author Carol Kumamoto, PhD, of Tufts University School of Medicine in Boston, Massachusetts.
“The extension of this finding to the human population is something that needs to be addressed in the future.”
Previous research showed that changes to diet, including changes in the amount and type of fat, can alter gastrointestinal microbiota. And in vitro studies showed that coconut oil has antifungal properties.
So Dr Kumamoto and her colleagues studied the effect of different diets on C albicans colonization in mice.
The mice received standard diets or high-fat diets containing coconut oil, beef tallow, or soybean oil. The mice were fed these diets for 14 days prior to inoculation with C albicans and 21 days after.
At 21 days post-inoculation, gastrointestinal colonization with C albicans was significantly lower in the stomach contents of mice fed the coconut oil than mice fed the beef tallow (P<0.0001), the soybean oil (P<0.0001), or the standard diet (P<0.0001).
“When you compared a mouse on a high-fat diet that contained either beef fat or soybean oil to mice eating coconut oil, there was about a 10-fold drop in colonization,” Dr Kumamoto said.
In another experiment, the researchers switched mice receiving beef tallow to coconut oil.
“Four days after the change in diet, the colonization changed so it looked almost exactly like what you saw in a mouse who had been on coconut oil the entire time,” Dr Kumamoto said.
“There are 2 directions that we would like to take with this research now,” she added. “One of them is finding out the mechanism of how this works. That is a big question we would like to answer. The second question is whether this can have any impact on humans.”
The researchers are in discussion with Joseph Bliss, MD, PhD, of Women and Infants Hospital of Rhode Island, about launching a clinical trial testing coconut oil in hospitalized infants at high risk of developing systemic candidiasis.

Coconut oil may combat infection with Candida albicans, according to preclinical research published in mSphere.
Mice on a diet that included coconut oil had significantly lower gastrointestinal colonization by C albicans than mice that were fed high-fat diets without coconut oil or mice that received a standard diet.
“We found that diet can be an effective way to reduce the amount of Candida in the mouse,” said study author Carol Kumamoto, PhD, of Tufts University School of Medicine in Boston, Massachusetts.
“The extension of this finding to the human population is something that needs to be addressed in the future.”
Previous research showed that changes to diet, including changes in the amount and type of fat, can alter gastrointestinal microbiota. And in vitro studies showed that coconut oil has antifungal properties.
So Dr Kumamoto and her colleagues studied the effect of different diets on C albicans colonization in mice.
The mice received standard diets or high-fat diets containing coconut oil, beef tallow, or soybean oil. The mice were fed these diets for 14 days prior to inoculation with C albicans and 21 days after.
At 21 days post-inoculation, gastrointestinal colonization with C albicans was significantly lower in the stomach contents of mice fed the coconut oil than mice fed the beef tallow (P<0.0001), the soybean oil (P<0.0001), or the standard diet (P<0.0001).
“When you compared a mouse on a high-fat diet that contained either beef fat or soybean oil to mice eating coconut oil, there was about a 10-fold drop in colonization,” Dr Kumamoto said.
In another experiment, the researchers switched mice receiving beef tallow to coconut oil.
“Four days after the change in diet, the colonization changed so it looked almost exactly like what you saw in a mouse who had been on coconut oil the entire time,” Dr Kumamoto said.
“There are 2 directions that we would like to take with this research now,” she added. “One of them is finding out the mechanism of how this works. That is a big question we would like to answer. The second question is whether this can have any impact on humans.”
The researchers are in discussion with Joseph Bliss, MD, PhD, of Women and Infants Hospital of Rhode Island, about launching a clinical trial testing coconut oil in hospitalized infants at high risk of developing systemic candidiasis.
High-fat diet linked to RBC dysfunction

Preclinical research suggests a high-fat diet is associated with red blood cell (RBC) dysfunction and helps explain how these dysfunctional cells may mediate atherosclerosis.
The researchers believe their findings may have implications for the pathogenesis of atherosclerosis in obesity, but the work may aid the study of other health conditions as well, such as thrombosis in the context of cancer.
The team detailed their findings in Circulation.
“Obesity caused by chronic consumption of a high-calorie, high-fat diet is a worldwide epidemic, representing one of the greatest threats to global health,” said principal investigator Vladimir Bogdanov, PhD, of the University of Cincinnati in Ohio.
“White blood cells play a key role in fueling adipose tissue inflammation and insulin resistance in obesity and also promote the clogging of arteries, or atherosclerosis, setting the stage for heart attack and stroke. While these outcomes linked with a high-fat diet and fat in the blood on white blood cells have been shown in animal models and humans, the impact of high-fat diets on other bone marrow-derived cells, like red blood cells, is not well-defined.”
“Evidence is emerging that red blood cells play an important regulatory role in the development of atherosclerosis, binding pro-inflammatory proteins that cause dysfunction in the inner lining of the blood vessel wall—the endothelium. We explored how a high fat-diet causes red blood cell dysfunction in this study.”
Dr Bogdanov and his team fed a 60% high-fat diet to mice for 12 weeks and compared these animals to control mice that received a normal diet.
There was an increase in the level of chemokines bound to the RBCs of mice that received the high-fat diet. These chemokines were bound to RBCs via the Duffy antigen receptor for chemokines.
The researchers exposed RBCs from mice on the high-fat diet to an endothelial monolayer in vitro, and they observed “significantly enhanced” macrophage transendothelial migration. They said this confirms the functional importance of RBC-bound chemokines in the setting of a high-fat diet.
“In red blood cells from animal models fed a high-fat diet, there was an increase in cholesterol found in the cell membrane and phosphatidylserine levels, promoting inflammatory reactions,” Dr Bogdanov added.
“Phosphatidylserine is a phospholipid membrane component which plays a key role in the cycle of cells. When red blood cells from the animals being fed the high-fat diet were injected into a control group, eating a normal diet, there was a 3-fold increase in their spleens’ uptake of red blood cells. The spleen is involved in the removal of blood cells, as well as systemic inflammation.”
“All of these findings show that the dysfunction of red blood cells, corresponding with dysfunction of the lining of blood vessels, occurs very early in diet-induced obesity and may play a part in the formation of atherosclerosis. Diets high in saturated fat have long been associated with endothelial dysfunction, the precursor to atherosclerosis, but, to our knowledge, the effects of high-fat diet on red blood cells have not been rigorously examined.”
Dr Bogdanov noted that, in humans, high cholesterol is associated with alterations in RBCs that are improved by treatment with statins. But the majority of obese humans do not have severe high cholesterol, as was the case with the animals in this study.
The researchers are now working on translating their findings to humans.

Preclinical research suggests a high-fat diet is associated with red blood cell (RBC) dysfunction and helps explain how these dysfunctional cells may mediate atherosclerosis.
The researchers believe their findings may have implications for the pathogenesis of atherosclerosis in obesity, but the work may aid the study of other health conditions as well, such as thrombosis in the context of cancer.
The team detailed their findings in Circulation.
“Obesity caused by chronic consumption of a high-calorie, high-fat diet is a worldwide epidemic, representing one of the greatest threats to global health,” said principal investigator Vladimir Bogdanov, PhD, of the University of Cincinnati in Ohio.
“White blood cells play a key role in fueling adipose tissue inflammation and insulin resistance in obesity and also promote the clogging of arteries, or atherosclerosis, setting the stage for heart attack and stroke. While these outcomes linked with a high-fat diet and fat in the blood on white blood cells have been shown in animal models and humans, the impact of high-fat diets on other bone marrow-derived cells, like red blood cells, is not well-defined.”
“Evidence is emerging that red blood cells play an important regulatory role in the development of atherosclerosis, binding pro-inflammatory proteins that cause dysfunction in the inner lining of the blood vessel wall—the endothelium. We explored how a high fat-diet causes red blood cell dysfunction in this study.”
Dr Bogdanov and his team fed a 60% high-fat diet to mice for 12 weeks and compared these animals to control mice that received a normal diet.
There was an increase in the level of chemokines bound to the RBCs of mice that received the high-fat diet. These chemokines were bound to RBCs via the Duffy antigen receptor for chemokines.
The researchers exposed RBCs from mice on the high-fat diet to an endothelial monolayer in vitro, and they observed “significantly enhanced” macrophage transendothelial migration. They said this confirms the functional importance of RBC-bound chemokines in the setting of a high-fat diet.
“In red blood cells from animal models fed a high-fat diet, there was an increase in cholesterol found in the cell membrane and phosphatidylserine levels, promoting inflammatory reactions,” Dr Bogdanov added.
“Phosphatidylserine is a phospholipid membrane component which plays a key role in the cycle of cells. When red blood cells from the animals being fed the high-fat diet were injected into a control group, eating a normal diet, there was a 3-fold increase in their spleens’ uptake of red blood cells. The spleen is involved in the removal of blood cells, as well as systemic inflammation.”
“All of these findings show that the dysfunction of red blood cells, corresponding with dysfunction of the lining of blood vessels, occurs very early in diet-induced obesity and may play a part in the formation of atherosclerosis. Diets high in saturated fat have long been associated with endothelial dysfunction, the precursor to atherosclerosis, but, to our knowledge, the effects of high-fat diet on red blood cells have not been rigorously examined.”
Dr Bogdanov noted that, in humans, high cholesterol is associated with alterations in RBCs that are improved by treatment with statins. But the majority of obese humans do not have severe high cholesterol, as was the case with the animals in this study.
The researchers are now working on translating their findings to humans.

Preclinical research suggests a high-fat diet is associated with red blood cell (RBC) dysfunction and helps explain how these dysfunctional cells may mediate atherosclerosis.
The researchers believe their findings may have implications for the pathogenesis of atherosclerosis in obesity, but the work may aid the study of other health conditions as well, such as thrombosis in the context of cancer.
The team detailed their findings in Circulation.
“Obesity caused by chronic consumption of a high-calorie, high-fat diet is a worldwide epidemic, representing one of the greatest threats to global health,” said principal investigator Vladimir Bogdanov, PhD, of the University of Cincinnati in Ohio.
“White blood cells play a key role in fueling adipose tissue inflammation and insulin resistance in obesity and also promote the clogging of arteries, or atherosclerosis, setting the stage for heart attack and stroke. While these outcomes linked with a high-fat diet and fat in the blood on white blood cells have been shown in animal models and humans, the impact of high-fat diets on other bone marrow-derived cells, like red blood cells, is not well-defined.”
“Evidence is emerging that red blood cells play an important regulatory role in the development of atherosclerosis, binding pro-inflammatory proteins that cause dysfunction in the inner lining of the blood vessel wall—the endothelium. We explored how a high fat-diet causes red blood cell dysfunction in this study.”
Dr Bogdanov and his team fed a 60% high-fat diet to mice for 12 weeks and compared these animals to control mice that received a normal diet.
There was an increase in the level of chemokines bound to the RBCs of mice that received the high-fat diet. These chemokines were bound to RBCs via the Duffy antigen receptor for chemokines.
The researchers exposed RBCs from mice on the high-fat diet to an endothelial monolayer in vitro, and they observed “significantly enhanced” macrophage transendothelial migration. They said this confirms the functional importance of RBC-bound chemokines in the setting of a high-fat diet.
“In red blood cells from animal models fed a high-fat diet, there was an increase in cholesterol found in the cell membrane and phosphatidylserine levels, promoting inflammatory reactions,” Dr Bogdanov added.
“Phosphatidylserine is a phospholipid membrane component which plays a key role in the cycle of cells. When red blood cells from the animals being fed the high-fat diet were injected into a control group, eating a normal diet, there was a 3-fold increase in their spleens’ uptake of red blood cells. The spleen is involved in the removal of blood cells, as well as systemic inflammation.”
“All of these findings show that the dysfunction of red blood cells, corresponding with dysfunction of the lining of blood vessels, occurs very early in diet-induced obesity and may play a part in the formation of atherosclerosis. Diets high in saturated fat have long been associated with endothelial dysfunction, the precursor to atherosclerosis, but, to our knowledge, the effects of high-fat diet on red blood cells have not been rigorously examined.”
Dr Bogdanov noted that, in humans, high cholesterol is associated with alterations in RBCs that are improved by treatment with statins. But the majority of obese humans do not have severe high cholesterol, as was the case with the animals in this study.
The researchers are now working on translating their findings to humans.
Strategy could slow spread of resistant malaria

Plasmodium falciparum
Image by Mae Melvin
A varied treatment approach could slow the spread of artemisinin-resistant malaria, according to research published in The Lancet Global Health.
Computer simulations suggested that giving a population multiple artemisinin-based combination therapies simultaneously, along with a non-artemisinin therapy, is the best way to combat malaria and reduce the spread of resistant disease.
Investigators found this approach worked best even when the non-artemisinin drug was only effective in treating malaria 85% of the time.
The team ran their computer simulations to determine if there was an optimal strategy that could stop the spread of drug-resistant Plasmodium falciparum parasites across populations while still effectively treating malaria in individual patients.
The simulations showed that simultaneously dosing a population with several artemisinin-combination therapies—for example, by prescribing artemisinin in combination with different partner drugs on different days of the week—was more effective than either cycling between different artemisinin combination therapies or sticking to one specific combination until that combination started failing.
The simulations also showed that if this simultaneous dosing included a combination without artemisinin, malaria parasites that were resistant to artemisinin were slower to emerge and slower to spread.
Including this potentially less effective treatment option didn’t necessarily mean that many more people would not recover from malaria.
In the worst-case scenario of the non-artemisinin treatment being only 75% as effective as artemisinin-based combination therapy, fewer than 7% of patients would still have post-treatment malaria parasites in their blood as a result of not receiving an artemisinin-based therapy.
“For this subgroup of patients, second-line treatment with an artemisinin combination therapy would be recommended,” said study author Maciej Boni, PhD, of the Hospital for Tropical Diseases in Ho Chi Minh City, Vietnam.
“The ethical implications of such a treatment policy will need to be weighed against the benefit of delaying and slowing down the spread of artemisinin resistance. But the nightmare we all want to avoid is the establishment of artemisinin resistance in Africa, where hundreds of millions of individuals rely on artemisinin-based therapies as their first-line antimalarial treatment.”
“By deploying different antimalarial therapies simultaneously—including non-artemisinin-based therapies—national malaria control programs in Africa should be able to slow down the spread of artemisinin-resistant parasites when they are imported into the continent.”

Plasmodium falciparum
Image by Mae Melvin
A varied treatment approach could slow the spread of artemisinin-resistant malaria, according to research published in The Lancet Global Health.
Computer simulations suggested that giving a population multiple artemisinin-based combination therapies simultaneously, along with a non-artemisinin therapy, is the best way to combat malaria and reduce the spread of resistant disease.
Investigators found this approach worked best even when the non-artemisinin drug was only effective in treating malaria 85% of the time.
The team ran their computer simulations to determine if there was an optimal strategy that could stop the spread of drug-resistant Plasmodium falciparum parasites across populations while still effectively treating malaria in individual patients.
The simulations showed that simultaneously dosing a population with several artemisinin-combination therapies—for example, by prescribing artemisinin in combination with different partner drugs on different days of the week—was more effective than either cycling between different artemisinin combination therapies or sticking to one specific combination until that combination started failing.
The simulations also showed that if this simultaneous dosing included a combination without artemisinin, malaria parasites that were resistant to artemisinin were slower to emerge and slower to spread.
Including this potentially less effective treatment option didn’t necessarily mean that many more people would not recover from malaria.
In the worst-case scenario of the non-artemisinin treatment being only 75% as effective as artemisinin-based combination therapy, fewer than 7% of patients would still have post-treatment malaria parasites in their blood as a result of not receiving an artemisinin-based therapy.
“For this subgroup of patients, second-line treatment with an artemisinin combination therapy would be recommended,” said study author Maciej Boni, PhD, of the Hospital for Tropical Diseases in Ho Chi Minh City, Vietnam.
“The ethical implications of such a treatment policy will need to be weighed against the benefit of delaying and slowing down the spread of artemisinin resistance. But the nightmare we all want to avoid is the establishment of artemisinin resistance in Africa, where hundreds of millions of individuals rely on artemisinin-based therapies as their first-line antimalarial treatment.”
“By deploying different antimalarial therapies simultaneously—including non-artemisinin-based therapies—national malaria control programs in Africa should be able to slow down the spread of artemisinin-resistant parasites when they are imported into the continent.”

Plasmodium falciparum
Image by Mae Melvin
A varied treatment approach could slow the spread of artemisinin-resistant malaria, according to research published in The Lancet Global Health.
Computer simulations suggested that giving a population multiple artemisinin-based combination therapies simultaneously, along with a non-artemisinin therapy, is the best way to combat malaria and reduce the spread of resistant disease.
Investigators found this approach worked best even when the non-artemisinin drug was only effective in treating malaria 85% of the time.
The team ran their computer simulations to determine if there was an optimal strategy that could stop the spread of drug-resistant Plasmodium falciparum parasites across populations while still effectively treating malaria in individual patients.
The simulations showed that simultaneously dosing a population with several artemisinin-combination therapies—for example, by prescribing artemisinin in combination with different partner drugs on different days of the week—was more effective than either cycling between different artemisinin combination therapies or sticking to one specific combination until that combination started failing.
The simulations also showed that if this simultaneous dosing included a combination without artemisinin, malaria parasites that were resistant to artemisinin were slower to emerge and slower to spread.
Including this potentially less effective treatment option didn’t necessarily mean that many more people would not recover from malaria.
In the worst-case scenario of the non-artemisinin treatment being only 75% as effective as artemisinin-based combination therapy, fewer than 7% of patients would still have post-treatment malaria parasites in their blood as a result of not receiving an artemisinin-based therapy.
“For this subgroup of patients, second-line treatment with an artemisinin combination therapy would be recommended,” said study author Maciej Boni, PhD, of the Hospital for Tropical Diseases in Ho Chi Minh City, Vietnam.
“The ethical implications of such a treatment policy will need to be weighed against the benefit of delaying and slowing down the spread of artemisinin resistance. But the nightmare we all want to avoid is the establishment of artemisinin resistance in Africa, where hundreds of millions of individuals rely on artemisinin-based therapies as their first-line antimalarial treatment.”
“By deploying different antimalarial therapies simultaneously—including non-artemisinin-based therapies—national malaria control programs in Africa should be able to slow down the spread of artemisinin-resistant parasites when they are imported into the continent.”