User login
CPPD nomenclature is sore subject for gout group
LA JOLLA, CALIF. – Twelve years ago, an international task force of the European Alliance of Associations for Rheumatology (EULAR) released recommendations regarding nomenclature in calcium pyrophosphate deposition disease (CPPD), aiming to standardize the way the condition is described. “Pseudogout” was out, and “acute CPP crystal arthritis” was in, and a confusing array of multi-named phenotypes gained specific labels.
Since 2011, the nomenclature guidelines have been cited hundreds of times, but a new report finds that the medical literature mostly hasn’t followed the recommendations. The findings were released at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN), which is taking another stab at overhauling CPPD nomenclature.
“The objective is to uniform and standardize the labels of the disease, the disease elements, and the clinical states,” rheumatologist Charlotte Jauffret, MD, of the Catholic University of Lille (France), said in a presentation. CPPD is a widely underdiagnosed disease that’s worth the same efforts to standardize nomenclature as occurred in gout, she said.
As Dr. Jauffret explained, CPPD has a diversity of phenotypes in asymptomatic, acute, and chronic forms that pose challenges to diagnosis. “The same terms are used to depict different concepts, and some disease elements are depicted through different names,” she said.
Among other suggestions, the 2011 EULAR recommendations suggested that rheumatologists use the terms chronic CPP crystal inflammatory arthritis instead of “pseudo-rheumatoid arthritis,” osteoarthritis with CPPD, instead of “pseudo-osteoarthritis,” and severe joint degeneration instead of “pseudo-neuropathic joint disease.”
Later reports noted that terms such as CPPD and chondrocalcinosis are still wrongly used interchangeably and called for an international consensus on nomenclature, Dr. Jauffret said.
For the new report, Dr. Jauffret and colleagues examined 985 articles from 2000-2022. The guidelines were often not followed even after the release of the recommendations.
For example, 49% of relevant papers used the label “pseudogout” before 2011, and 43% did afterward. A total of 34% of relevant papers described CPPD as chondrocalcinosis prior to 2011, and 22% did afterward.
G-CAN’s next steps are to reach consensus on terminology through online and in-person meetings in 2024, Dr. Jauffret said.
Use of correct gout nomenclature labels improved
In a related presentation, rheumatologist Ellen Prendergast, MBChB, of Dunedin Hospital in New Zealand, reported the results of a newly published review of gout studies before and after G-CAN released consensus recommendations for gout nomenclature in 2019. “There has been improvement in the agreed labels in some areas, but there remains quite significant variability,” she said.
The review examined American College of Rheumatology and EULAR annual meeting abstracts: 596 from 2016-2017 and 392 from 2020-2021. Dr. Prendergast said researchers focused on abstracts instead of published studies in order to gain the most up-to-date understanding of nomenclature.
“Use of the agreed labels ‘urate’ and ‘gout flare’ increased between the two periods. There were 219 of 383 (57.2%) abstracts with the agreed label ‘urate’ in 2016-2017, compared with 164 of 232 (70.7%) in 2020-2021 (P = .001),” the researchers reported. “There were 60 of 175 (34.3%) abstracts with the agreed label ‘gout flare’ in 2016-2017, compared with 57 of 109 (52.3%) in 2020-2021 (P = .003).”
And the use of the term “chronic gout,” which the guidelines recommend against, fell from 29 of 596 (4.9%) abstracts in 2016-2017 to 8 of 392 (2.0%) abstracts in 2020-2021 (P = .02).
One author of the gout nomenclature study reports various consulting fees, speaker fees, or grants outside the submitted work. The other authors of the two studies report no disclosures.
LA JOLLA, CALIF. – Twelve years ago, an international task force of the European Alliance of Associations for Rheumatology (EULAR) released recommendations regarding nomenclature in calcium pyrophosphate deposition disease (CPPD), aiming to standardize the way the condition is described. “Pseudogout” was out, and “acute CPP crystal arthritis” was in, and a confusing array of multi-named phenotypes gained specific labels.
Since 2011, the nomenclature guidelines have been cited hundreds of times, but a new report finds that the medical literature mostly hasn’t followed the recommendations. The findings were released at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN), which is taking another stab at overhauling CPPD nomenclature.
“The objective is to uniform and standardize the labels of the disease, the disease elements, and the clinical states,” rheumatologist Charlotte Jauffret, MD, of the Catholic University of Lille (France), said in a presentation. CPPD is a widely underdiagnosed disease that’s worth the same efforts to standardize nomenclature as occurred in gout, she said.
As Dr. Jauffret explained, CPPD has a diversity of phenotypes in asymptomatic, acute, and chronic forms that pose challenges to diagnosis. “The same terms are used to depict different concepts, and some disease elements are depicted through different names,” she said.
Among other suggestions, the 2011 EULAR recommendations suggested that rheumatologists use the terms chronic CPP crystal inflammatory arthritis instead of “pseudo-rheumatoid arthritis,” osteoarthritis with CPPD, instead of “pseudo-osteoarthritis,” and severe joint degeneration instead of “pseudo-neuropathic joint disease.”
Later reports noted that terms such as CPPD and chondrocalcinosis are still wrongly used interchangeably and called for an international consensus on nomenclature, Dr. Jauffret said.
For the new report, Dr. Jauffret and colleagues examined 985 articles from 2000-2022. The guidelines were often not followed even after the release of the recommendations.
For example, 49% of relevant papers used the label “pseudogout” before 2011, and 43% did afterward. A total of 34% of relevant papers described CPPD as chondrocalcinosis prior to 2011, and 22% did afterward.
G-CAN’s next steps are to reach consensus on terminology through online and in-person meetings in 2024, Dr. Jauffret said.
Use of correct gout nomenclature labels improved
In a related presentation, rheumatologist Ellen Prendergast, MBChB, of Dunedin Hospital in New Zealand, reported the results of a newly published review of gout studies before and after G-CAN released consensus recommendations for gout nomenclature in 2019. “There has been improvement in the agreed labels in some areas, but there remains quite significant variability,” she said.
The review examined American College of Rheumatology and EULAR annual meeting abstracts: 596 from 2016-2017 and 392 from 2020-2021. Dr. Prendergast said researchers focused on abstracts instead of published studies in order to gain the most up-to-date understanding of nomenclature.
“Use of the agreed labels ‘urate’ and ‘gout flare’ increased between the two periods. There were 219 of 383 (57.2%) abstracts with the agreed label ‘urate’ in 2016-2017, compared with 164 of 232 (70.7%) in 2020-2021 (P = .001),” the researchers reported. “There were 60 of 175 (34.3%) abstracts with the agreed label ‘gout flare’ in 2016-2017, compared with 57 of 109 (52.3%) in 2020-2021 (P = .003).”
And the use of the term “chronic gout,” which the guidelines recommend against, fell from 29 of 596 (4.9%) abstracts in 2016-2017 to 8 of 392 (2.0%) abstracts in 2020-2021 (P = .02).
One author of the gout nomenclature study reports various consulting fees, speaker fees, or grants outside the submitted work. The other authors of the two studies report no disclosures.
LA JOLLA, CALIF. – Twelve years ago, an international task force of the European Alliance of Associations for Rheumatology (EULAR) released recommendations regarding nomenclature in calcium pyrophosphate deposition disease (CPPD), aiming to standardize the way the condition is described. “Pseudogout” was out, and “acute CPP crystal arthritis” was in, and a confusing array of multi-named phenotypes gained specific labels.
Since 2011, the nomenclature guidelines have been cited hundreds of times, but a new report finds that the medical literature mostly hasn’t followed the recommendations. The findings were released at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN), which is taking another stab at overhauling CPPD nomenclature.
“The objective is to uniform and standardize the labels of the disease, the disease elements, and the clinical states,” rheumatologist Charlotte Jauffret, MD, of the Catholic University of Lille (France), said in a presentation. CPPD is a widely underdiagnosed disease that’s worth the same efforts to standardize nomenclature as occurred in gout, she said.
As Dr. Jauffret explained, CPPD has a diversity of phenotypes in asymptomatic, acute, and chronic forms that pose challenges to diagnosis. “The same terms are used to depict different concepts, and some disease elements are depicted through different names,” she said.
Among other suggestions, the 2011 EULAR recommendations suggested that rheumatologists use the terms chronic CPP crystal inflammatory arthritis instead of “pseudo-rheumatoid arthritis,” osteoarthritis with CPPD, instead of “pseudo-osteoarthritis,” and severe joint degeneration instead of “pseudo-neuropathic joint disease.”
Later reports noted that terms such as CPPD and chondrocalcinosis are still wrongly used interchangeably and called for an international consensus on nomenclature, Dr. Jauffret said.
For the new report, Dr. Jauffret and colleagues examined 985 articles from 2000-2022. The guidelines were often not followed even after the release of the recommendations.
For example, 49% of relevant papers used the label “pseudogout” before 2011, and 43% did afterward. A total of 34% of relevant papers described CPPD as chondrocalcinosis prior to 2011, and 22% did afterward.
G-CAN’s next steps are to reach consensus on terminology through online and in-person meetings in 2024, Dr. Jauffret said.
Use of correct gout nomenclature labels improved
In a related presentation, rheumatologist Ellen Prendergast, MBChB, of Dunedin Hospital in New Zealand, reported the results of a newly published review of gout studies before and after G-CAN released consensus recommendations for gout nomenclature in 2019. “There has been improvement in the agreed labels in some areas, but there remains quite significant variability,” she said.
The review examined American College of Rheumatology and EULAR annual meeting abstracts: 596 from 2016-2017 and 392 from 2020-2021. Dr. Prendergast said researchers focused on abstracts instead of published studies in order to gain the most up-to-date understanding of nomenclature.
“Use of the agreed labels ‘urate’ and ‘gout flare’ increased between the two periods. There were 219 of 383 (57.2%) abstracts with the agreed label ‘urate’ in 2016-2017, compared with 164 of 232 (70.7%) in 2020-2021 (P = .001),” the researchers reported. “There were 60 of 175 (34.3%) abstracts with the agreed label ‘gout flare’ in 2016-2017, compared with 57 of 109 (52.3%) in 2020-2021 (P = .003).”
And the use of the term “chronic gout,” which the guidelines recommend against, fell from 29 of 596 (4.9%) abstracts in 2016-2017 to 8 of 392 (2.0%) abstracts in 2020-2021 (P = .02).
One author of the gout nomenclature study reports various consulting fees, speaker fees, or grants outside the submitted work. The other authors of the two studies report no disclosures.
At G-CAN 2023
55-year-old woman • myalgias and progressive symmetrical proximal weakness • history of type 2 diabetes and hyperlipidemia • Dx?
THE CASE
A 55-year-old woman developed subacute progression of myalgias and subjective weakness in her proximal extremities after starting a new exercise regimen. The patient had a history of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia, for which she had taken atorvastatin 40 mg/d for several years before discontinuing it 2 years earlier for unknown reasons. She had been evaluated multiple times in the primary care clinic and emergency department over the previous month. Each time, her strength was minimally reduced in the upper extremities on examination, her renal function and electrolytes were normal, and her creatine kinase (CK) level was elevated (16,000-20,000 U/L; normal range, 26-192 U/L). She was managed conservatively with fluids and given return precautions each time.
After her myalgias and weakness increased in severity, she presented to the emergency department with a muscle strength score of 4/5 in both shoulders, triceps, hip flexors, hip extensors, abductors, and adductors. Her laboratory results were significant for the presence of blood without red blood cells on her urine dipstick test and a CK level of 25,070 U/L. She was admitted for further evaluation of progressive myopathy and given aggressive IV fluid hydration to prevent renal injury based on her history of unilateral renal agenesis.
Infectious disease testing, which included a respiratory virus panel, acute hepatitis panel, HIV screening, Lyme antibody testing, cytomegalovirus DNA detection by polymerase chain reaction, Epstein-Barr virus capsid immunoglobulin M, and anti-streptolysin O, were negative. Electrolytes, inflammatory markers, and kidney function were normal. However, high-sensitivity troponin-T levels were elevated, with a peak value of 216.3 ng/L (normal range, 0-19 ng/L). The patient denied having any chest pain, and her electrocardiogram and transthoracic echocardiogram were normal. By hospital Day 4, her myalgias and weakness had improved, CK had stabilized (19,000-21,000 U/L), cardiac enzymes had improved, and urinalysis had normalized. She was discharged with a referral to a rheumatologist.
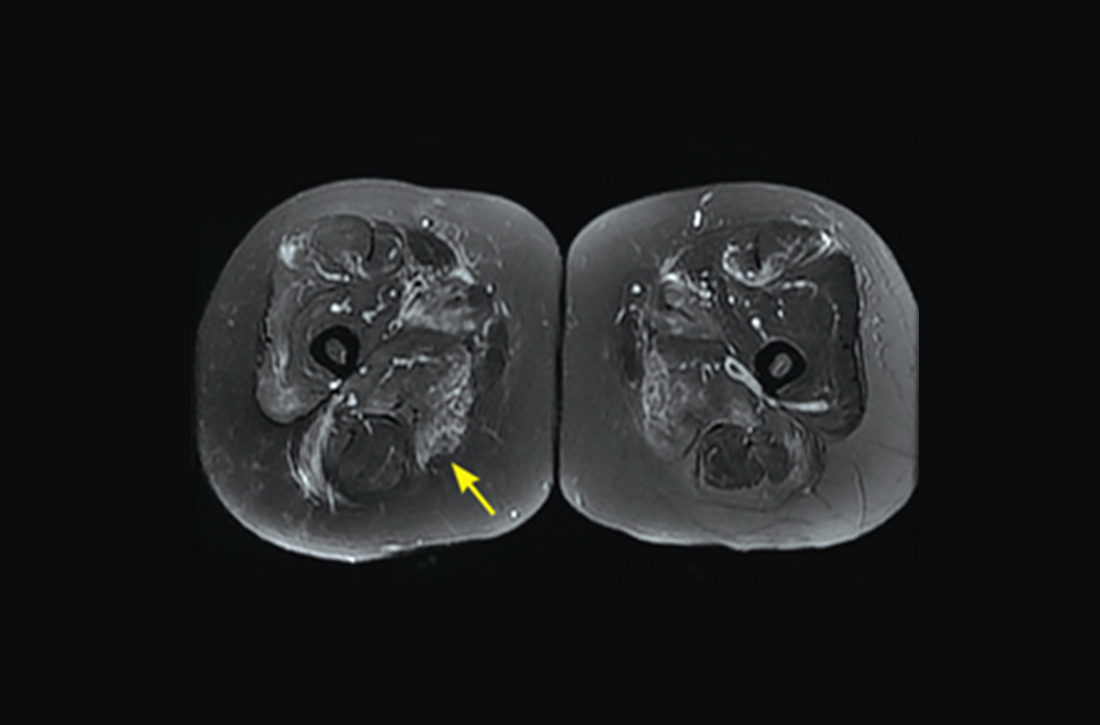
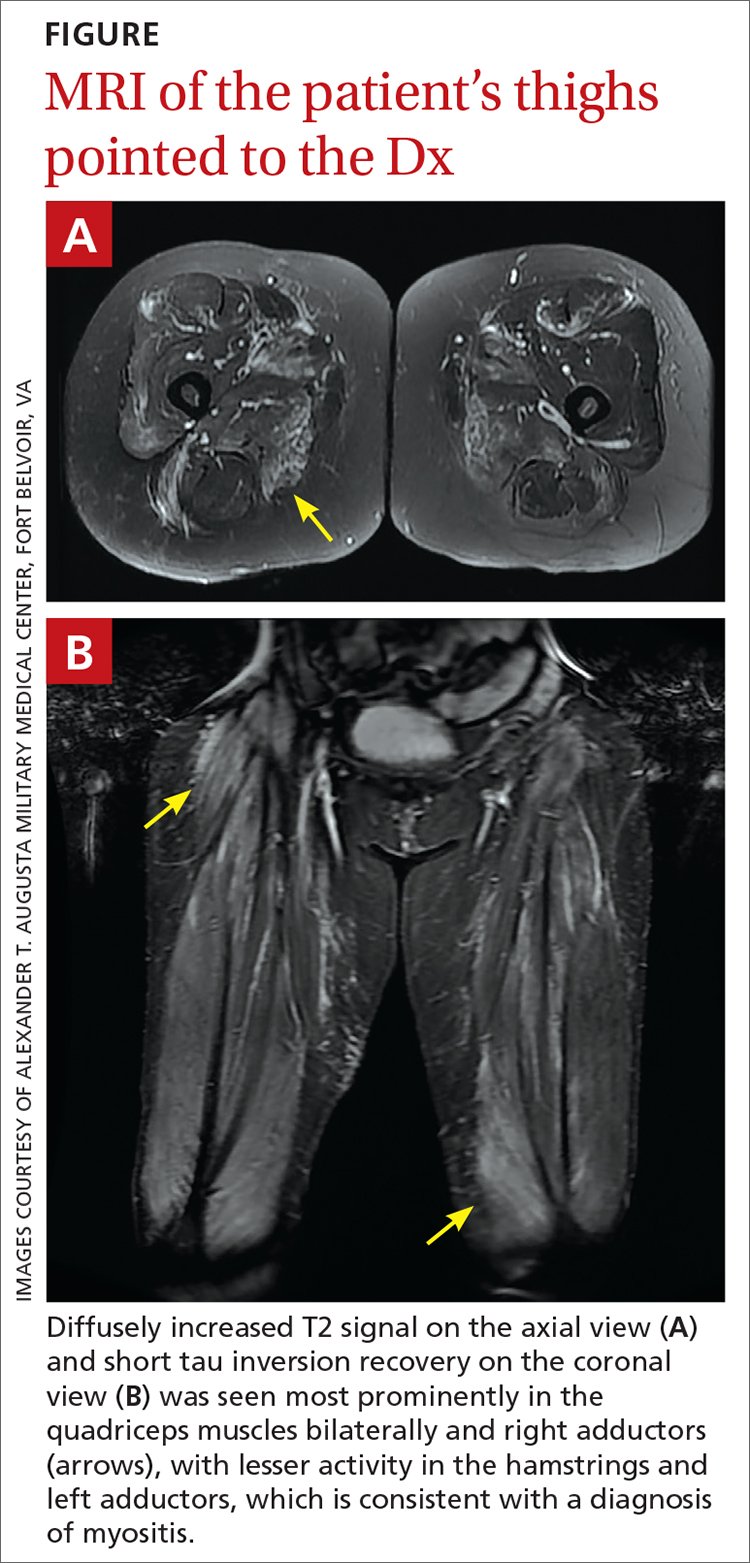
However, 10 days later—before she could see a rheumatologist—she was readmitted to a community hospital for recurrence of severe myalgias, progressive weakness, positive blood on urine dipstick testing, and a rising CK level (to 24,580 U/L) found during a follow-up appointment with her primary care physician. At this point, Neurology and Rheumatology were consulted and myositis-specific and myositis-associated autoantibody tests were sent out. Magnetic resonance imaging (MRI) of her thighs was performed and showed diffusely increased T2 signal and short tau inversion recovery in multiple proximal muscles (FIGURE).
DIAGNOSIS
Given her symmetrical proximal muscle weakness (which was refractory to IV fluid resuscitation), MRI findings, and the exclusion of infection and metabolic derangements, the patient was given a working diagnosis of myositis and treated with 1-g IV methylprednisolone followed by a 4-month steroid taper, methotrexate 20 mg weekly, and physical therapy. This working diagnosis was later confirmed with the results of her autoantibody tests.
At her 1-month follow-up visit, the patient reported minimal improvement in her strength, new neck weakness, and dysphagia with solids. Testing revealed anti–3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibody levels of more than 200 U/L (negative < 20 U/L; positive > 59 U/L), which pointed to a more refined diagnosis of anti-HMGCR immune-mediated necrotizing myositis.
DISCUSSION
Myositis should be in the differential diagnosis for patients with symmetrical proximal muscle weakness. Bohan and Peter devised a 5-part set of criteria to help diagnose myositis, shown in the TABLE.1,2 This simple framework broadens the differential and guides diagnostic testing. Our patient’s presentation was fairly typical for anti-HMGCR myositis, a subset of immune-mediated necrotizing myositis,3 with a pretest probability of 62% per the European League Against Rheumatism/American College of Rheumatology classification criteria.2 Probability of this diagnosis was further increased by the high-titer anti-HMGCR, so biopsy and electromyography (EMG), as noted by Bohan and Peter, were not pursued.
Continue to: Autoimmune myopathies...
Autoimmune myopathies occur in 9 to 14 per 100,000 people,4 with 6% of patients having anti-HMGCR auto-antibodies.5 Anti-HMGCR myositis is more prevalent in older women, patients with type 2 diabetes, and those with a history of atorvastatin use.3,6 Two-thirds of patients with anti-HMGCR myositis report current or prior statin use, and this increases to more than 90% in those age 50 years or older.5 Anti-HMGCR myositis causes significant muscle weakness that does not resolve with discontinuation of the statin and can occur years after the initiation or discontinuation of statin treatment.6 Cardiac involvement is rare4 but dysphagia is relatively common.7,8 Anti-HMGCR myositis also has a weak association with cancer, most commonly gastrointestinal and lung cancers.4,7
Distinguishing statin-induced myalgias from statin-induced myositis guides management. Statin-induced myalgias are associated with normal or slightly increased CK levels (typically < 1000 U/L) and resolve with discontinuation of the statin; the patient can often tolerate re-challenge with a statin.6 In contrast, CK elevation in patients with statin-induced myositis is typically more than 10,000 U/L6 and requires aggressive treatment with immunomodulatory medications to prevent permanent muscle damage.
Treatment recommendations are supported only by case series, observational studies, and expert opinion. Typical first-line treatment includes induction with high-dose corticosteroids followed by prolonged taper plus a conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, azathioprine, or mycophenolate.4 Maintenance therapy often is achieved with csDMARD therapy for 2 years.4 Severe cases frequently are treated with combination csDMARD therapy (eg, methotrexate and azathioprine or methotrexate and mycophenolate).4 Rituximab and IV immunoglobulin (IVIG) are typically reserved for refractory cases.6 Usual monitoring for relapse includes muscle strength testing on examination and evaluation of trending CK levels.8
Our patient received monthly 2-g/kg IVIG infusions, which led to slow, consistent improvement in her strength and normalization of her CK levels to 181 U/L after 6 months.
THE TAKEAWAY
Anti-HMGCR myositis should be suspected in any patient currently or previously treated with a statin who presents with proximal muscle weakness, myalgias, or an elevated CK level. We suggest early subspecialty consultation to discuss whether antibody testing, EMG, or muscle biopsy are warranted. If anti-HMGCR myositis is confirmed, it is advisable to rule out comorbid malignancy and initiate early combination treatment to minimize relapses and permanent muscle damage.
CORRESPONDENCE
Daniel T. Schoenherr, MD, Family Medicine Residency, National Capital Consortium–Alexander T. Augusta Military Medical Center, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. doi: 10.1056/NEJM197502132920706
2. Bottai M, Tjärnlund A, Santoni G, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3:e000507. doi: 10.1136/rmdopen-2017-000507
3. Basharat P, Lahouti AH, Paik JJ, et al. Statin-induced anti-HMGCR-associated myopathy. J Am Coll Cardiol. 2016;68:234-235. doi: 10.1016/j.jacc.2016.04.037
4. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20:21. doi: 10.1007/s11926-018-0732-6
5. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713-721. doi: 10.1002/art.30156
6. Irvine NJ. Anti-HMGCR myopathy: a rare and serious side effect of statins. J Am Board Fam Med. 2020;33:785-788. doi: 10.3122/jabfm.2020.05.190450
7. Basharat P, Christopher-Stine L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep. 2015;17:72. doi: 10.1007/s11926-015-0548-6
8. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Int Med. 2016;280:8-23. doi: 10.1111/joim.12451
THE CASE
A 55-year-old woman developed subacute progression of myalgias and subjective weakness in her proximal extremities after starting a new exercise regimen. The patient had a history of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia, for which she had taken atorvastatin 40 mg/d for several years before discontinuing it 2 years earlier for unknown reasons. She had been evaluated multiple times in the primary care clinic and emergency department over the previous month. Each time, her strength was minimally reduced in the upper extremities on examination, her renal function and electrolytes were normal, and her creatine kinase (CK) level was elevated (16,000-20,000 U/L; normal range, 26-192 U/L). She was managed conservatively with fluids and given return precautions each time.
After her myalgias and weakness increased in severity, she presented to the emergency department with a muscle strength score of 4/5 in both shoulders, triceps, hip flexors, hip extensors, abductors, and adductors. Her laboratory results were significant for the presence of blood without red blood cells on her urine dipstick test and a CK level of 25,070 U/L. She was admitted for further evaluation of progressive myopathy and given aggressive IV fluid hydration to prevent renal injury based on her history of unilateral renal agenesis.
Infectious disease testing, which included a respiratory virus panel, acute hepatitis panel, HIV screening, Lyme antibody testing, cytomegalovirus DNA detection by polymerase chain reaction, Epstein-Barr virus capsid immunoglobulin M, and anti-streptolysin O, were negative. Electrolytes, inflammatory markers, and kidney function were normal. However, high-sensitivity troponin-T levels were elevated, with a peak value of 216.3 ng/L (normal range, 0-19 ng/L). The patient denied having any chest pain, and her electrocardiogram and transthoracic echocardiogram were normal. By hospital Day 4, her myalgias and weakness had improved, CK had stabilized (19,000-21,000 U/L), cardiac enzymes had improved, and urinalysis had normalized. She was discharged with a referral to a rheumatologist.
However, 10 days later—before she could see a rheumatologist—she was readmitted to a community hospital for recurrence of severe myalgias, progressive weakness, positive blood on urine dipstick testing, and a rising CK level (to 24,580 U/L) found during a follow-up appointment with her primary care physician. At this point, Neurology and Rheumatology were consulted and myositis-specific and myositis-associated autoantibody tests were sent out. Magnetic resonance imaging (MRI) of her thighs was performed and showed diffusely increased T2 signal and short tau inversion recovery in multiple proximal muscles (FIGURE).

DIAGNOSIS
Given her symmetrical proximal muscle weakness (which was refractory to IV fluid resuscitation), MRI findings, and the exclusion of infection and metabolic derangements, the patient was given a working diagnosis of myositis and treated with 1-g IV methylprednisolone followed by a 4-month steroid taper, methotrexate 20 mg weekly, and physical therapy. This working diagnosis was later confirmed with the results of her autoantibody tests.
At her 1-month follow-up visit, the patient reported minimal improvement in her strength, new neck weakness, and dysphagia with solids. Testing revealed anti–3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibody levels of more than 200 U/L (negative < 20 U/L; positive > 59 U/L), which pointed to a more refined diagnosis of anti-HMGCR immune-mediated necrotizing myositis.
DISCUSSION
Myositis should be in the differential diagnosis for patients with symmetrical proximal muscle weakness. Bohan and Peter devised a 5-part set of criteria to help diagnose myositis, shown in the TABLE.1,2 This simple framework broadens the differential and guides diagnostic testing. Our patient’s presentation was fairly typical for anti-HMGCR myositis, a subset of immune-mediated necrotizing myositis,3 with a pretest probability of 62% per the European League Against Rheumatism/American College of Rheumatology classification criteria.2 Probability of this diagnosis was further increased by the high-titer anti-HMGCR, so biopsy and electromyography (EMG), as noted by Bohan and Peter, were not pursued.

Continue to: Autoimmune myopathies...
Autoimmune myopathies occur in 9 to 14 per 100,000 people,4 with 6% of patients having anti-HMGCR auto-antibodies.5 Anti-HMGCR myositis is more prevalent in older women, patients with type 2 diabetes, and those with a history of atorvastatin use.3,6 Two-thirds of patients with anti-HMGCR myositis report current or prior statin use, and this increases to more than 90% in those age 50 years or older.5 Anti-HMGCR myositis causes significant muscle weakness that does not resolve with discontinuation of the statin and can occur years after the initiation or discontinuation of statin treatment.6 Cardiac involvement is rare4 but dysphagia is relatively common.7,8 Anti-HMGCR myositis also has a weak association with cancer, most commonly gastrointestinal and lung cancers.4,7
Distinguishing statin-induced myalgias from statin-induced myositis guides management. Statin-induced myalgias are associated with normal or slightly increased CK levels (typically < 1000 U/L) and resolve with discontinuation of the statin; the patient can often tolerate re-challenge with a statin.6 In contrast, CK elevation in patients with statin-induced myositis is typically more than 10,000 U/L6 and requires aggressive treatment with immunomodulatory medications to prevent permanent muscle damage.
Treatment recommendations are supported only by case series, observational studies, and expert opinion. Typical first-line treatment includes induction with high-dose corticosteroids followed by prolonged taper plus a conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, azathioprine, or mycophenolate.4 Maintenance therapy often is achieved with csDMARD therapy for 2 years.4 Severe cases frequently are treated with combination csDMARD therapy (eg, methotrexate and azathioprine or methotrexate and mycophenolate).4 Rituximab and IV immunoglobulin (IVIG) are typically reserved for refractory cases.6 Usual monitoring for relapse includes muscle strength testing on examination and evaluation of trending CK levels.8
Our patient received monthly 2-g/kg IVIG infusions, which led to slow, consistent improvement in her strength and normalization of her CK levels to 181 U/L after 6 months.
THE TAKEAWAY
Anti-HMGCR myositis should be suspected in any patient currently or previously treated with a statin who presents with proximal muscle weakness, myalgias, or an elevated CK level. We suggest early subspecialty consultation to discuss whether antibody testing, EMG, or muscle biopsy are warranted. If anti-HMGCR myositis is confirmed, it is advisable to rule out comorbid malignancy and initiate early combination treatment to minimize relapses and permanent muscle damage.
CORRESPONDENCE
Daniel T. Schoenherr, MD, Family Medicine Residency, National Capital Consortium–Alexander T. Augusta Military Medical Center, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
THE CASE
A 55-year-old woman developed subacute progression of myalgias and subjective weakness in her proximal extremities after starting a new exercise regimen. The patient had a history of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia, for which she had taken atorvastatin 40 mg/d for several years before discontinuing it 2 years earlier for unknown reasons. She had been evaluated multiple times in the primary care clinic and emergency department over the previous month. Each time, her strength was minimally reduced in the upper extremities on examination, her renal function and electrolytes were normal, and her creatine kinase (CK) level was elevated (16,000-20,000 U/L; normal range, 26-192 U/L). She was managed conservatively with fluids and given return precautions each time.
After her myalgias and weakness increased in severity, she presented to the emergency department with a muscle strength score of 4/5 in both shoulders, triceps, hip flexors, hip extensors, abductors, and adductors. Her laboratory results were significant for the presence of blood without red blood cells on her urine dipstick test and a CK level of 25,070 U/L. She was admitted for further evaluation of progressive myopathy and given aggressive IV fluid hydration to prevent renal injury based on her history of unilateral renal agenesis.
Infectious disease testing, which included a respiratory virus panel, acute hepatitis panel, HIV screening, Lyme antibody testing, cytomegalovirus DNA detection by polymerase chain reaction, Epstein-Barr virus capsid immunoglobulin M, and anti-streptolysin O, were negative. Electrolytes, inflammatory markers, and kidney function were normal. However, high-sensitivity troponin-T levels were elevated, with a peak value of 216.3 ng/L (normal range, 0-19 ng/L). The patient denied having any chest pain, and her electrocardiogram and transthoracic echocardiogram were normal. By hospital Day 4, her myalgias and weakness had improved, CK had stabilized (19,000-21,000 U/L), cardiac enzymes had improved, and urinalysis had normalized. She was discharged with a referral to a rheumatologist.
However, 10 days later—before she could see a rheumatologist—she was readmitted to a community hospital for recurrence of severe myalgias, progressive weakness, positive blood on urine dipstick testing, and a rising CK level (to 24,580 U/L) found during a follow-up appointment with her primary care physician. At this point, Neurology and Rheumatology were consulted and myositis-specific and myositis-associated autoantibody tests were sent out. Magnetic resonance imaging (MRI) of her thighs was performed and showed diffusely increased T2 signal and short tau inversion recovery in multiple proximal muscles (FIGURE).

DIAGNOSIS
Given her symmetrical proximal muscle weakness (which was refractory to IV fluid resuscitation), MRI findings, and the exclusion of infection and metabolic derangements, the patient was given a working diagnosis of myositis and treated with 1-g IV methylprednisolone followed by a 4-month steroid taper, methotrexate 20 mg weekly, and physical therapy. This working diagnosis was later confirmed with the results of her autoantibody tests.
At her 1-month follow-up visit, the patient reported minimal improvement in her strength, new neck weakness, and dysphagia with solids. Testing revealed anti–3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibody levels of more than 200 U/L (negative < 20 U/L; positive > 59 U/L), which pointed to a more refined diagnosis of anti-HMGCR immune-mediated necrotizing myositis.
DISCUSSION
Myositis should be in the differential diagnosis for patients with symmetrical proximal muscle weakness. Bohan and Peter devised a 5-part set of criteria to help diagnose myositis, shown in the TABLE.1,2 This simple framework broadens the differential and guides diagnostic testing. Our patient’s presentation was fairly typical for anti-HMGCR myositis, a subset of immune-mediated necrotizing myositis,3 with a pretest probability of 62% per the European League Against Rheumatism/American College of Rheumatology classification criteria.2 Probability of this diagnosis was further increased by the high-titer anti-HMGCR, so biopsy and electromyography (EMG), as noted by Bohan and Peter, were not pursued.

Continue to: Autoimmune myopathies...
Autoimmune myopathies occur in 9 to 14 per 100,000 people,4 with 6% of patients having anti-HMGCR auto-antibodies.5 Anti-HMGCR myositis is more prevalent in older women, patients with type 2 diabetes, and those with a history of atorvastatin use.3,6 Two-thirds of patients with anti-HMGCR myositis report current or prior statin use, and this increases to more than 90% in those age 50 years or older.5 Anti-HMGCR myositis causes significant muscle weakness that does not resolve with discontinuation of the statin and can occur years after the initiation or discontinuation of statin treatment.6 Cardiac involvement is rare4 but dysphagia is relatively common.7,8 Anti-HMGCR myositis also has a weak association with cancer, most commonly gastrointestinal and lung cancers.4,7
Distinguishing statin-induced myalgias from statin-induced myositis guides management. Statin-induced myalgias are associated with normal or slightly increased CK levels (typically < 1000 U/L) and resolve with discontinuation of the statin; the patient can often tolerate re-challenge with a statin.6 In contrast, CK elevation in patients with statin-induced myositis is typically more than 10,000 U/L6 and requires aggressive treatment with immunomodulatory medications to prevent permanent muscle damage.
Treatment recommendations are supported only by case series, observational studies, and expert opinion. Typical first-line treatment includes induction with high-dose corticosteroids followed by prolonged taper plus a conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, azathioprine, or mycophenolate.4 Maintenance therapy often is achieved with csDMARD therapy for 2 years.4 Severe cases frequently are treated with combination csDMARD therapy (eg, methotrexate and azathioprine or methotrexate and mycophenolate).4 Rituximab and IV immunoglobulin (IVIG) are typically reserved for refractory cases.6 Usual monitoring for relapse includes muscle strength testing on examination and evaluation of trending CK levels.8
Our patient received monthly 2-g/kg IVIG infusions, which led to slow, consistent improvement in her strength and normalization of her CK levels to 181 U/L after 6 months.
THE TAKEAWAY
Anti-HMGCR myositis should be suspected in any patient currently or previously treated with a statin who presents with proximal muscle weakness, myalgias, or an elevated CK level. We suggest early subspecialty consultation to discuss whether antibody testing, EMG, or muscle biopsy are warranted. If anti-HMGCR myositis is confirmed, it is advisable to rule out comorbid malignancy and initiate early combination treatment to minimize relapses and permanent muscle damage.
CORRESPONDENCE
Daniel T. Schoenherr, MD, Family Medicine Residency, National Capital Consortium–Alexander T. Augusta Military Medical Center, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. doi: 10.1056/NEJM197502132920706
2. Bottai M, Tjärnlund A, Santoni G, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3:e000507. doi: 10.1136/rmdopen-2017-000507
3. Basharat P, Lahouti AH, Paik JJ, et al. Statin-induced anti-HMGCR-associated myopathy. J Am Coll Cardiol. 2016;68:234-235. doi: 10.1016/j.jacc.2016.04.037
4. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20:21. doi: 10.1007/s11926-018-0732-6
5. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713-721. doi: 10.1002/art.30156
6. Irvine NJ. Anti-HMGCR myopathy: a rare and serious side effect of statins. J Am Board Fam Med. 2020;33:785-788. doi: 10.3122/jabfm.2020.05.190450
7. Basharat P, Christopher-Stine L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep. 2015;17:72. doi: 10.1007/s11926-015-0548-6
8. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Int Med. 2016;280:8-23. doi: 10.1111/joim.12451
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. doi: 10.1056/NEJM197502132920706
2. Bottai M, Tjärnlund A, Santoni G, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3:e000507. doi: 10.1136/rmdopen-2017-000507
3. Basharat P, Lahouti AH, Paik JJ, et al. Statin-induced anti-HMGCR-associated myopathy. J Am Coll Cardiol. 2016;68:234-235. doi: 10.1016/j.jacc.2016.04.037
4. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20:21. doi: 10.1007/s11926-018-0732-6
5. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713-721. doi: 10.1002/art.30156
6. Irvine NJ. Anti-HMGCR myopathy: a rare and serious side effect of statins. J Am Board Fam Med. 2020;33:785-788. doi: 10.3122/jabfm.2020.05.190450
7. Basharat P, Christopher-Stine L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep. 2015;17:72. doi: 10.1007/s11926-015-0548-6
8. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Int Med. 2016;280:8-23. doi: 10.1111/joim.12451
► Myalgias and progressive symmetrical proximal weakness
► History of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia
Gout: Studies support early use of urate-lowering therapy, warn of peripheral arterial disease
LA JOLLA, Calif. – A new analysis suggests that it may not be necessary to delay urate-lowering therapy (ULT) in gout flares, and a study warns of the potential heightened risk of peripheral arterial disease (PAD) in gout.
The reports were released at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
The urate-lowering report, a systematic review and meta-analysis, suggests that the use of ULT during a gout flare “does not affect flare severity nor the duration of the flare or risk of recurrence in the subsequent month,” lead author Vicky Tai, MBChB, of the University of Auckland (New Zealand), said in a presentation.
She noted that there’s ongoing debate about whether ULT should be delayed until a week or two after gout flares subside to avoid their return. “This is reflected in guidelines on gout management, which have provided inconsistent recommendations on the issue,” Dr. Tai said.
As she noted, the American College of Rheumatology’s 2020 gout guidelines conditionally recommended starting ULT during gout flares – and not afterward – if it’s indicated. (The guidelines also conditionally recommend against ULT in a first gout flare, however, with a few exceptions.)
The British Society for Rheumatology’s 2017 gout guidelines suggested waiting until flares have settled down and “the patient was no longer in pain,” although ULT may be started in patients with frequent attacks. The European Alliance of Associations for Rheumatology’s gout guidelines from 2016 didn’t address timing, Dr. Tai said.
For the new analysis, Dr. Tai and colleagues examined six randomized studies from the United States (two), China (two), Taiwan (one), and Thailand (one) that examined the use of allopurinol (three studies), febuxostat (two studies), and probenecid (one). The studies, dated from 2012 to 2023, randomized 226 subjects with gout to early initiation of ULT vs. 219 who received placebo or delayed ULT. Subjects were tracked for a median of 28 days (15 days to 12 weeks).
Three of the studies were deemed to have high risk of bias.
There were no differences in patient-rated pain scores at various time points, duration of gout flares (examined in three studies), or recurrence of gout flares (examined in four studies).
“Other outcomes of interest, including long-term adherence, time to achieve target serum urate, and patient satisfaction with treatment, were not examined,” Dr. Tai said. “Adverse events were similar between groups.”
She cautioned that the sample sizes are small, and the findings may not be applicable to patients with tophaceous gout or comorbid renal disease.
A similar meta-analysis published in 2022 examined five studies (including three of those in the new analysis); among the five was one study from 1987 that examined azapropazone and indomethacin plus allopurinol. The review found “that initiation of ULT during an acute gout flare did not prolong the duration of acute flares.”
Risk for PAD
In the other study, researchers raised an alarm after finding a high rate of PAD in patients with gout regardless of whether they also had diabetes, which is a known risk factor for PAD. “Our data suggest that gout is an underrecognized risk factor for PAD and indicates the importance of assessing for PAD in gout patients,” lead author Nicole Leung, MD, of NYU Langone Health, said in a presentation.
According to Dr. Leung, there’s little known about links between PAD and gout, although she highlighted a 2018 study that found that patients with obstructive coronary artery disease were more likely to have poor outcomes if they also developed gout after catheterization. She highlighted a 2022 study that found higher rates of lower-extremity amputations in patients with gout independent of cardiovascular disease and diabetes. However, she noted that a link to PAD is unclear, and the study found a link between gout and amputations that was independent of PAD.
Patients with gout, she added, are not routinely screened for PAD.
For the new retrospective, cross-sectional analysis, Dr. Leung and colleagues examined Veterans Administration data from 2014 to 2018 for 7.2 million patients. The population was largely male.
Of those, 140,862 (2.52%) – the control group – had no gout or diabetes. In comparison, 11,449 (5.56%) of 205,904 with gout but not diabetes had PAD, for a rate 2.2 times greater than the control group). PAD occurred in 101,582 (8.70%) of 1,168,138 with diabetes but not gout, giving a rate 3.2 times greater than the control group. The rate was highest among people with both gout and diabetes, at 9.97% (9,905 of 99,377), which is about four times greater than the control group.
The link between gout and PAD remained after adjustment for creatinine levels, age, gender, and body mass index. Diabetes was linked to a higher risk for PAD than was gout, and the effect of both conditions combined was “less than additive.” This “may suggest an overlap and pathophysiology between the two,” she said.
Disclosure information was not provided. The Rheumatology Research Foundation funded the PAD study; funding information for the ULT/gout flare analysis was not provided.
LA JOLLA, Calif. – A new analysis suggests that it may not be necessary to delay urate-lowering therapy (ULT) in gout flares, and a study warns of the potential heightened risk of peripheral arterial disease (PAD) in gout.
The reports were released at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
The urate-lowering report, a systematic review and meta-analysis, suggests that the use of ULT during a gout flare “does not affect flare severity nor the duration of the flare or risk of recurrence in the subsequent month,” lead author Vicky Tai, MBChB, of the University of Auckland (New Zealand), said in a presentation.
She noted that there’s ongoing debate about whether ULT should be delayed until a week or two after gout flares subside to avoid their return. “This is reflected in guidelines on gout management, which have provided inconsistent recommendations on the issue,” Dr. Tai said.
As she noted, the American College of Rheumatology’s 2020 gout guidelines conditionally recommended starting ULT during gout flares – and not afterward – if it’s indicated. (The guidelines also conditionally recommend against ULT in a first gout flare, however, with a few exceptions.)
The British Society for Rheumatology’s 2017 gout guidelines suggested waiting until flares have settled down and “the patient was no longer in pain,” although ULT may be started in patients with frequent attacks. The European Alliance of Associations for Rheumatology’s gout guidelines from 2016 didn’t address timing, Dr. Tai said.
For the new analysis, Dr. Tai and colleagues examined six randomized studies from the United States (two), China (two), Taiwan (one), and Thailand (one) that examined the use of allopurinol (three studies), febuxostat (two studies), and probenecid (one). The studies, dated from 2012 to 2023, randomized 226 subjects with gout to early initiation of ULT vs. 219 who received placebo or delayed ULT. Subjects were tracked for a median of 28 days (15 days to 12 weeks).
Three of the studies were deemed to have high risk of bias.
There were no differences in patient-rated pain scores at various time points, duration of gout flares (examined in three studies), or recurrence of gout flares (examined in four studies).
“Other outcomes of interest, including long-term adherence, time to achieve target serum urate, and patient satisfaction with treatment, were not examined,” Dr. Tai said. “Adverse events were similar between groups.”
She cautioned that the sample sizes are small, and the findings may not be applicable to patients with tophaceous gout or comorbid renal disease.
A similar meta-analysis published in 2022 examined five studies (including three of those in the new analysis); among the five was one study from 1987 that examined azapropazone and indomethacin plus allopurinol. The review found “that initiation of ULT during an acute gout flare did not prolong the duration of acute flares.”
Risk for PAD
In the other study, researchers raised an alarm after finding a high rate of PAD in patients with gout regardless of whether they also had diabetes, which is a known risk factor for PAD. “Our data suggest that gout is an underrecognized risk factor for PAD and indicates the importance of assessing for PAD in gout patients,” lead author Nicole Leung, MD, of NYU Langone Health, said in a presentation.
According to Dr. Leung, there’s little known about links between PAD and gout, although she highlighted a 2018 study that found that patients with obstructive coronary artery disease were more likely to have poor outcomes if they also developed gout after catheterization. She highlighted a 2022 study that found higher rates of lower-extremity amputations in patients with gout independent of cardiovascular disease and diabetes. However, she noted that a link to PAD is unclear, and the study found a link between gout and amputations that was independent of PAD.
Patients with gout, she added, are not routinely screened for PAD.
For the new retrospective, cross-sectional analysis, Dr. Leung and colleagues examined Veterans Administration data from 2014 to 2018 for 7.2 million patients. The population was largely male.
Of those, 140,862 (2.52%) – the control group – had no gout or diabetes. In comparison, 11,449 (5.56%) of 205,904 with gout but not diabetes had PAD, for a rate 2.2 times greater than the control group). PAD occurred in 101,582 (8.70%) of 1,168,138 with diabetes but not gout, giving a rate 3.2 times greater than the control group. The rate was highest among people with both gout and diabetes, at 9.97% (9,905 of 99,377), which is about four times greater than the control group.
The link between gout and PAD remained after adjustment for creatinine levels, age, gender, and body mass index. Diabetes was linked to a higher risk for PAD than was gout, and the effect of both conditions combined was “less than additive.” This “may suggest an overlap and pathophysiology between the two,” she said.
Disclosure information was not provided. The Rheumatology Research Foundation funded the PAD study; funding information for the ULT/gout flare analysis was not provided.
LA JOLLA, Calif. – A new analysis suggests that it may not be necessary to delay urate-lowering therapy (ULT) in gout flares, and a study warns of the potential heightened risk of peripheral arterial disease (PAD) in gout.
The reports were released at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
The urate-lowering report, a systematic review and meta-analysis, suggests that the use of ULT during a gout flare “does not affect flare severity nor the duration of the flare or risk of recurrence in the subsequent month,” lead author Vicky Tai, MBChB, of the University of Auckland (New Zealand), said in a presentation.
She noted that there’s ongoing debate about whether ULT should be delayed until a week or two after gout flares subside to avoid their return. “This is reflected in guidelines on gout management, which have provided inconsistent recommendations on the issue,” Dr. Tai said.
As she noted, the American College of Rheumatology’s 2020 gout guidelines conditionally recommended starting ULT during gout flares – and not afterward – if it’s indicated. (The guidelines also conditionally recommend against ULT in a first gout flare, however, with a few exceptions.)
The British Society for Rheumatology’s 2017 gout guidelines suggested waiting until flares have settled down and “the patient was no longer in pain,” although ULT may be started in patients with frequent attacks. The European Alliance of Associations for Rheumatology’s gout guidelines from 2016 didn’t address timing, Dr. Tai said.
For the new analysis, Dr. Tai and colleagues examined six randomized studies from the United States (two), China (two), Taiwan (one), and Thailand (one) that examined the use of allopurinol (three studies), febuxostat (two studies), and probenecid (one). The studies, dated from 2012 to 2023, randomized 226 subjects with gout to early initiation of ULT vs. 219 who received placebo or delayed ULT. Subjects were tracked for a median of 28 days (15 days to 12 weeks).
Three of the studies were deemed to have high risk of bias.
There were no differences in patient-rated pain scores at various time points, duration of gout flares (examined in three studies), or recurrence of gout flares (examined in four studies).
“Other outcomes of interest, including long-term adherence, time to achieve target serum urate, and patient satisfaction with treatment, were not examined,” Dr. Tai said. “Adverse events were similar between groups.”
She cautioned that the sample sizes are small, and the findings may not be applicable to patients with tophaceous gout or comorbid renal disease.
A similar meta-analysis published in 2022 examined five studies (including three of those in the new analysis); among the five was one study from 1987 that examined azapropazone and indomethacin plus allopurinol. The review found “that initiation of ULT during an acute gout flare did not prolong the duration of acute flares.”
Risk for PAD
In the other study, researchers raised an alarm after finding a high rate of PAD in patients with gout regardless of whether they also had diabetes, which is a known risk factor for PAD. “Our data suggest that gout is an underrecognized risk factor for PAD and indicates the importance of assessing for PAD in gout patients,” lead author Nicole Leung, MD, of NYU Langone Health, said in a presentation.
According to Dr. Leung, there’s little known about links between PAD and gout, although she highlighted a 2018 study that found that patients with obstructive coronary artery disease were more likely to have poor outcomes if they also developed gout after catheterization. She highlighted a 2022 study that found higher rates of lower-extremity amputations in patients with gout independent of cardiovascular disease and diabetes. However, she noted that a link to PAD is unclear, and the study found a link between gout and amputations that was independent of PAD.
Patients with gout, she added, are not routinely screened for PAD.
For the new retrospective, cross-sectional analysis, Dr. Leung and colleagues examined Veterans Administration data from 2014 to 2018 for 7.2 million patients. The population was largely male.
Of those, 140,862 (2.52%) – the control group – had no gout or diabetes. In comparison, 11,449 (5.56%) of 205,904 with gout but not diabetes had PAD, for a rate 2.2 times greater than the control group). PAD occurred in 101,582 (8.70%) of 1,168,138 with diabetes but not gout, giving a rate 3.2 times greater than the control group. The rate was highest among people with both gout and diabetes, at 9.97% (9,905 of 99,377), which is about four times greater than the control group.
The link between gout and PAD remained after adjustment for creatinine levels, age, gender, and body mass index. Diabetes was linked to a higher risk for PAD than was gout, and the effect of both conditions combined was “less than additive.” This “may suggest an overlap and pathophysiology between the two,” she said.
Disclosure information was not provided. The Rheumatology Research Foundation funded the PAD study; funding information for the ULT/gout flare analysis was not provided.
AT G-CAN 2023
Review estimates acne risk with JAK inhibitor therapy
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
Researchers tease apart multiple biologic failure in psoriasis, PsA
WASHINGTON – Multiple biologic failure in a minority of patients with psoriasis may have several causes, from genetic endotypes and immunologic factors to lower serum drug levels, the presence of anti-drug antibody levels, female sex, and certain comorbidities, Wilson Liao, MD, said at the annual research symposium of the National Psoriasis Foundation.
“Tough-to-treat psoriasis remains a challenge despite newer therapies ... Why do we still have this sub-population of patients who seem to be refractory?” said Dr. Liao, professor and associate vice chair of research in the department of dermatology at the University of California, San Francisco, who coauthored a 2015-2022 prospective cohort analysis that documented about 6% of patients failing two or more biologic agents of different mechanistic classes.
“These patients are really suffering,” he said. “We need to have better guidelines and treatment algorithms for these patients.”
A significant number of patients with psoriatic arthritis (PsA), meanwhile, are inadequate responders to tumor necrosis factor (TNF) inhibition, Christopher T. Ritchlin, MD, PhD, professor of medicine in the division of allergy/immunology and rheumatology and the Center of Musculoskeletal Research at the University of Rochester (N.Y.), said during another session at the meeting.
The long-term “persistence,” or usage, of first-line biologics in patients with PsA – and of second-line biologics in patients who failed one TNF-inhibitor – is low, but the literature offers little information on the reasons for TNF-inhibitor discontinuation, said Dr. Ritchlin, who coauthored a perspective piece in Arthritis & Rheumatology on managing the patient with PsA who fails one TNF inhibitor.
Dr. Ritchlin and his coauthors were asked to provide evidence-informed advice and algorithms, but the task was difficult. “It’s hard to know what to recommend for the next step if we don’t know why patients failed the first,” he said. “The point is, we need more data. [Clinical trials] are not recording the kind of information we need.”
Anti-drug antibodies, genetics, other factors in psoriasis
Research shows that in large cohorts, “all the biologics do seem to lose efficacy over time,” said Dr. Liao, who directs the UCSF Psoriasis and Skin Treatment Center. “Some are better than others, but we do see a loss of effectiveness over time.”
A cohort study published in 2022 in JAMA Dermatology, for instance, documented declining “drug survival” associated with ineffectiveness during 2 years of treatment for each of five biologics studied (adalimumab [Humira], ustekinumab [Stelara], secukinumab [Cosentyx], guselkumab [Tremfya], and ixekizumab [Taltz]).
“There have been a number of theories put forward” as to why that’s the case, including lower serum drug levels, “which of course can be related to anti-drug antibody production,” he said.
He pointed to two studies of ustekinumab: One prospective observational cohort study that reported an association of lower early drug levels of the IL-12/23 receptor antagonist with lower Psoriasis Area and Severity Index (PASI) scores, and another observational study that documented an association between anti-drug antibody positivity with lower ustekinumab levels and impaired clinical response.
“We also now know ... that there are genetic endotypes in psoriasis, and that patients who are [HLA-C*06:02]-positive tend to respond a little better to drugs like ustekinumab, and those who are [HLA-C*06:02]-negative tend to do a little better with the TNF inhibitors,” Dr. Liao said. The human leukocyte antigen (HLA) allele HLA-C*06:02 is associated with susceptibility to psoriasis.
In a study using a national psoriasis registry, HLA-C*06:02-negative patients were 3 times more likely to achieve PASI90 status in response to adalimumab, a TNF-alpha inhibitor, than with ustekinumab treatment. And in a meta-analysis covering eight studies with more than 1,000 patients with psoriasis, the median PASI75 response rate after 6 months of ustekinumab therapy was 92% in the HLA-C*06:02-positive group and 67% in HLA-C*06:02-negative patients.
The recently published cohort study showing a 6% rate of multiple biologic failure evaluated patients in the multicenter CorEvitas Psoriasis Registry who initiated their first biologic between 2015 and 2020 and were followed for 2 or more years. Investigators looked for sociodemographic and clinical differences between the patients who continued use of their first biologic for at least 2 years (“good response”), and those who discontinued two or more biologics of different classes, each used for at least 90 days, because of inadequate efficacy.
Of 1,039 evaluated patients, 490 (47.2%) had good clinical response to their first biologic and 65 (6.3%) had multiple biologic failure. All biologic classes were represented among those who failed multiple biologics. The first and second biologic classes used were attempted for a mean duration of 10 months – “an adequate trial” of each, Dr. Liao said.
In multivariable regression analysis, six variables were significantly associated with multiple biologic failure: female sex at birth, shorter disease duration, earlier year of biologic initiation, prior nonbiologic systemic therapy, having Medicaid insurance, and a history of hyperlipidemia. The latter is “interesting because other studies have shown that metabolic syndrome, of which hyperlipidemia is a component, can also relate to poor response to biologics,” Dr. Liao said.
The most common sequences of first-to-second biologics among those with multiple biologic failure were TNF inhibitor to IL-17 inhibitor (30.8%); IL-12/23 inhibitor to IL-17 inhibitor (21.5%); TNF inhibitor to IL-12/23 inhibitor (12.3%); and IL-17 inhibitor to IL-23 inhibitor (10.8%).
The vast majority of patients failed more than two biologics, however, and “more than 20% had five or more biologics tried over a relatively short period,” Dr. Liao said.
Comorbidities and biologic failure in psoriasis, PsA
In practice, it was said during a discussion period, biologic failures in psoriasis can be of two types: a primary inadequate response or initial failure, or a secondary failure with initial improvement followed by declining or no response. “I agree 100% that these probably represent two different endotypes,” Dr. Liao said. “There’s research emerging that psoriasis isn’t necessarily a clean phenotype.”
The option of focusing on comorbidities in the face of biologic failure was another point of discussion. “Maybe the next biologic is not the answer,” a meeting participant said. “Maybe we should focus on metabolic syndrome.”
“I agree,” Dr. Liao said. “In clinic, there are people who may not respond to therapies but have other comorbidities and factors that make it difficult to manage [their psoriasis] ... that may be causative for psoriasis. Maybe if we treat the comorbidities, it will make it easier to treat the psoriasis.”
Addressing comorbidities and “extra-articular traits” such as poorly controlled diabetes, centralized pain, anxiety and depression, and obesity is something Dr. Ritchlin advocates for PsA. “Centralized pain, I believe, is a major driver of nonresponse,” he said at the meeting. “We have to be careful about blaming nonresponse and lack of efficacy of biologics when it could be a wholly different mechanism the biologic won’t treat ... for example, centralized pain.”
As with psoriasis, the emergence of antidrug antibodies may be one reason for the secondary failure of biologic agents for PsA, Dr. Ritchlin and his coauthors wrote in their paper on management of PsA after failure of one TNF inhibitor. Other areas to consider in evaluating failure, they wrote, are compliance and time of dosing, and financial barriers.
Low long-term persistence of second-line biologics for patients with PsA was demonstrated in a national cohort study utilizing the French health insurance database, Dr. Ritchlin noted at the research meeting.
The French study covered almost 3,000 patients who started a second biologic after discontinuing a TNF inhibitor during 2015-2020. Overall, 1-year and 3-year persistence rates were 42% and 17%, respectively.
Dr. Liao disclosed research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and Trex Bio. Dr. Ritchlin reported no disclosures.
WASHINGTON – Multiple biologic failure in a minority of patients with psoriasis may have several causes, from genetic endotypes and immunologic factors to lower serum drug levels, the presence of anti-drug antibody levels, female sex, and certain comorbidities, Wilson Liao, MD, said at the annual research symposium of the National Psoriasis Foundation.
“Tough-to-treat psoriasis remains a challenge despite newer therapies ... Why do we still have this sub-population of patients who seem to be refractory?” said Dr. Liao, professor and associate vice chair of research in the department of dermatology at the University of California, San Francisco, who coauthored a 2015-2022 prospective cohort analysis that documented about 6% of patients failing two or more biologic agents of different mechanistic classes.
“These patients are really suffering,” he said. “We need to have better guidelines and treatment algorithms for these patients.”
A significant number of patients with psoriatic arthritis (PsA), meanwhile, are inadequate responders to tumor necrosis factor (TNF) inhibition, Christopher T. Ritchlin, MD, PhD, professor of medicine in the division of allergy/immunology and rheumatology and the Center of Musculoskeletal Research at the University of Rochester (N.Y.), said during another session at the meeting.
The long-term “persistence,” or usage, of first-line biologics in patients with PsA – and of second-line biologics in patients who failed one TNF-inhibitor – is low, but the literature offers little information on the reasons for TNF-inhibitor discontinuation, said Dr. Ritchlin, who coauthored a perspective piece in Arthritis & Rheumatology on managing the patient with PsA who fails one TNF inhibitor.
Dr. Ritchlin and his coauthors were asked to provide evidence-informed advice and algorithms, but the task was difficult. “It’s hard to know what to recommend for the next step if we don’t know why patients failed the first,” he said. “The point is, we need more data. [Clinical trials] are not recording the kind of information we need.”
Anti-drug antibodies, genetics, other factors in psoriasis
Research shows that in large cohorts, “all the biologics do seem to lose efficacy over time,” said Dr. Liao, who directs the UCSF Psoriasis and Skin Treatment Center. “Some are better than others, but we do see a loss of effectiveness over time.”
A cohort study published in 2022 in JAMA Dermatology, for instance, documented declining “drug survival” associated with ineffectiveness during 2 years of treatment for each of five biologics studied (adalimumab [Humira], ustekinumab [Stelara], secukinumab [Cosentyx], guselkumab [Tremfya], and ixekizumab [Taltz]).
“There have been a number of theories put forward” as to why that’s the case, including lower serum drug levels, “which of course can be related to anti-drug antibody production,” he said.
He pointed to two studies of ustekinumab: One prospective observational cohort study that reported an association of lower early drug levels of the IL-12/23 receptor antagonist with lower Psoriasis Area and Severity Index (PASI) scores, and another observational study that documented an association between anti-drug antibody positivity with lower ustekinumab levels and impaired clinical response.
“We also now know ... that there are genetic endotypes in psoriasis, and that patients who are [HLA-C*06:02]-positive tend to respond a little better to drugs like ustekinumab, and those who are [HLA-C*06:02]-negative tend to do a little better with the TNF inhibitors,” Dr. Liao said. The human leukocyte antigen (HLA) allele HLA-C*06:02 is associated with susceptibility to psoriasis.
In a study using a national psoriasis registry, HLA-C*06:02-negative patients were 3 times more likely to achieve PASI90 status in response to adalimumab, a TNF-alpha inhibitor, than with ustekinumab treatment. And in a meta-analysis covering eight studies with more than 1,000 patients with psoriasis, the median PASI75 response rate after 6 months of ustekinumab therapy was 92% in the HLA-C*06:02-positive group and 67% in HLA-C*06:02-negative patients.
The recently published cohort study showing a 6% rate of multiple biologic failure evaluated patients in the multicenter CorEvitas Psoriasis Registry who initiated their first biologic between 2015 and 2020 and were followed for 2 or more years. Investigators looked for sociodemographic and clinical differences between the patients who continued use of their first biologic for at least 2 years (“good response”), and those who discontinued two or more biologics of different classes, each used for at least 90 days, because of inadequate efficacy.
Of 1,039 evaluated patients, 490 (47.2%) had good clinical response to their first biologic and 65 (6.3%) had multiple biologic failure. All biologic classes were represented among those who failed multiple biologics. The first and second biologic classes used were attempted for a mean duration of 10 months – “an adequate trial” of each, Dr. Liao said.
In multivariable regression analysis, six variables were significantly associated with multiple biologic failure: female sex at birth, shorter disease duration, earlier year of biologic initiation, prior nonbiologic systemic therapy, having Medicaid insurance, and a history of hyperlipidemia. The latter is “interesting because other studies have shown that metabolic syndrome, of which hyperlipidemia is a component, can also relate to poor response to biologics,” Dr. Liao said.
The most common sequences of first-to-second biologics among those with multiple biologic failure were TNF inhibitor to IL-17 inhibitor (30.8%); IL-12/23 inhibitor to IL-17 inhibitor (21.5%); TNF inhibitor to IL-12/23 inhibitor (12.3%); and IL-17 inhibitor to IL-23 inhibitor (10.8%).
The vast majority of patients failed more than two biologics, however, and “more than 20% had five or more biologics tried over a relatively short period,” Dr. Liao said.
Comorbidities and biologic failure in psoriasis, PsA
In practice, it was said during a discussion period, biologic failures in psoriasis can be of two types: a primary inadequate response or initial failure, or a secondary failure with initial improvement followed by declining or no response. “I agree 100% that these probably represent two different endotypes,” Dr. Liao said. “There’s research emerging that psoriasis isn’t necessarily a clean phenotype.”
The option of focusing on comorbidities in the face of biologic failure was another point of discussion. “Maybe the next biologic is not the answer,” a meeting participant said. “Maybe we should focus on metabolic syndrome.”
“I agree,” Dr. Liao said. “In clinic, there are people who may not respond to therapies but have other comorbidities and factors that make it difficult to manage [their psoriasis] ... that may be causative for psoriasis. Maybe if we treat the comorbidities, it will make it easier to treat the psoriasis.”
Addressing comorbidities and “extra-articular traits” such as poorly controlled diabetes, centralized pain, anxiety and depression, and obesity is something Dr. Ritchlin advocates for PsA. “Centralized pain, I believe, is a major driver of nonresponse,” he said at the meeting. “We have to be careful about blaming nonresponse and lack of efficacy of biologics when it could be a wholly different mechanism the biologic won’t treat ... for example, centralized pain.”
As with psoriasis, the emergence of antidrug antibodies may be one reason for the secondary failure of biologic agents for PsA, Dr. Ritchlin and his coauthors wrote in their paper on management of PsA after failure of one TNF inhibitor. Other areas to consider in evaluating failure, they wrote, are compliance and time of dosing, and financial barriers.
Low long-term persistence of second-line biologics for patients with PsA was demonstrated in a national cohort study utilizing the French health insurance database, Dr. Ritchlin noted at the research meeting.
The French study covered almost 3,000 patients who started a second biologic after discontinuing a TNF inhibitor during 2015-2020. Overall, 1-year and 3-year persistence rates were 42% and 17%, respectively.
Dr. Liao disclosed research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and Trex Bio. Dr. Ritchlin reported no disclosures.
WASHINGTON – Multiple biologic failure in a minority of patients with psoriasis may have several causes, from genetic endotypes and immunologic factors to lower serum drug levels, the presence of anti-drug antibody levels, female sex, and certain comorbidities, Wilson Liao, MD, said at the annual research symposium of the National Psoriasis Foundation.
“Tough-to-treat psoriasis remains a challenge despite newer therapies ... Why do we still have this sub-population of patients who seem to be refractory?” said Dr. Liao, professor and associate vice chair of research in the department of dermatology at the University of California, San Francisco, who coauthored a 2015-2022 prospective cohort analysis that documented about 6% of patients failing two or more biologic agents of different mechanistic classes.
“These patients are really suffering,” he said. “We need to have better guidelines and treatment algorithms for these patients.”
A significant number of patients with psoriatic arthritis (PsA), meanwhile, are inadequate responders to tumor necrosis factor (TNF) inhibition, Christopher T. Ritchlin, MD, PhD, professor of medicine in the division of allergy/immunology and rheumatology and the Center of Musculoskeletal Research at the University of Rochester (N.Y.), said during another session at the meeting.
The long-term “persistence,” or usage, of first-line biologics in patients with PsA – and of second-line biologics in patients who failed one TNF-inhibitor – is low, but the literature offers little information on the reasons for TNF-inhibitor discontinuation, said Dr. Ritchlin, who coauthored a perspective piece in Arthritis & Rheumatology on managing the patient with PsA who fails one TNF inhibitor.
Dr. Ritchlin and his coauthors were asked to provide evidence-informed advice and algorithms, but the task was difficult. “It’s hard to know what to recommend for the next step if we don’t know why patients failed the first,” he said. “The point is, we need more data. [Clinical trials] are not recording the kind of information we need.”
Anti-drug antibodies, genetics, other factors in psoriasis
Research shows that in large cohorts, “all the biologics do seem to lose efficacy over time,” said Dr. Liao, who directs the UCSF Psoriasis and Skin Treatment Center. “Some are better than others, but we do see a loss of effectiveness over time.”
A cohort study published in 2022 in JAMA Dermatology, for instance, documented declining “drug survival” associated with ineffectiveness during 2 years of treatment for each of five biologics studied (adalimumab [Humira], ustekinumab [Stelara], secukinumab [Cosentyx], guselkumab [Tremfya], and ixekizumab [Taltz]).
“There have been a number of theories put forward” as to why that’s the case, including lower serum drug levels, “which of course can be related to anti-drug antibody production,” he said.
He pointed to two studies of ustekinumab: One prospective observational cohort study that reported an association of lower early drug levels of the IL-12/23 receptor antagonist with lower Psoriasis Area and Severity Index (PASI) scores, and another observational study that documented an association between anti-drug antibody positivity with lower ustekinumab levels and impaired clinical response.
“We also now know ... that there are genetic endotypes in psoriasis, and that patients who are [HLA-C*06:02]-positive tend to respond a little better to drugs like ustekinumab, and those who are [HLA-C*06:02]-negative tend to do a little better with the TNF inhibitors,” Dr. Liao said. The human leukocyte antigen (HLA) allele HLA-C*06:02 is associated with susceptibility to psoriasis.
In a study using a national psoriasis registry, HLA-C*06:02-negative patients were 3 times more likely to achieve PASI90 status in response to adalimumab, a TNF-alpha inhibitor, than with ustekinumab treatment. And in a meta-analysis covering eight studies with more than 1,000 patients with psoriasis, the median PASI75 response rate after 6 months of ustekinumab therapy was 92% in the HLA-C*06:02-positive group and 67% in HLA-C*06:02-negative patients.
The recently published cohort study showing a 6% rate of multiple biologic failure evaluated patients in the multicenter CorEvitas Psoriasis Registry who initiated their first biologic between 2015 and 2020 and were followed for 2 or more years. Investigators looked for sociodemographic and clinical differences between the patients who continued use of their first biologic for at least 2 years (“good response”), and those who discontinued two or more biologics of different classes, each used for at least 90 days, because of inadequate efficacy.
Of 1,039 evaluated patients, 490 (47.2%) had good clinical response to their first biologic and 65 (6.3%) had multiple biologic failure. All biologic classes were represented among those who failed multiple biologics. The first and second biologic classes used were attempted for a mean duration of 10 months – “an adequate trial” of each, Dr. Liao said.
In multivariable regression analysis, six variables were significantly associated with multiple biologic failure: female sex at birth, shorter disease duration, earlier year of biologic initiation, prior nonbiologic systemic therapy, having Medicaid insurance, and a history of hyperlipidemia. The latter is “interesting because other studies have shown that metabolic syndrome, of which hyperlipidemia is a component, can also relate to poor response to biologics,” Dr. Liao said.
The most common sequences of first-to-second biologics among those with multiple biologic failure were TNF inhibitor to IL-17 inhibitor (30.8%); IL-12/23 inhibitor to IL-17 inhibitor (21.5%); TNF inhibitor to IL-12/23 inhibitor (12.3%); and IL-17 inhibitor to IL-23 inhibitor (10.8%).
The vast majority of patients failed more than two biologics, however, and “more than 20% had five or more biologics tried over a relatively short period,” Dr. Liao said.
Comorbidities and biologic failure in psoriasis, PsA
In practice, it was said during a discussion period, biologic failures in psoriasis can be of two types: a primary inadequate response or initial failure, or a secondary failure with initial improvement followed by declining or no response. “I agree 100% that these probably represent two different endotypes,” Dr. Liao said. “There’s research emerging that psoriasis isn’t necessarily a clean phenotype.”
The option of focusing on comorbidities in the face of biologic failure was another point of discussion. “Maybe the next biologic is not the answer,” a meeting participant said. “Maybe we should focus on metabolic syndrome.”
“I agree,” Dr. Liao said. “In clinic, there are people who may not respond to therapies but have other comorbidities and factors that make it difficult to manage [their psoriasis] ... that may be causative for psoriasis. Maybe if we treat the comorbidities, it will make it easier to treat the psoriasis.”
Addressing comorbidities and “extra-articular traits” such as poorly controlled diabetes, centralized pain, anxiety and depression, and obesity is something Dr. Ritchlin advocates for PsA. “Centralized pain, I believe, is a major driver of nonresponse,” he said at the meeting. “We have to be careful about blaming nonresponse and lack of efficacy of biologics when it could be a wholly different mechanism the biologic won’t treat ... for example, centralized pain.”
As with psoriasis, the emergence of antidrug antibodies may be one reason for the secondary failure of biologic agents for PsA, Dr. Ritchlin and his coauthors wrote in their paper on management of PsA after failure of one TNF inhibitor. Other areas to consider in evaluating failure, they wrote, are compliance and time of dosing, and financial barriers.
Low long-term persistence of second-line biologics for patients with PsA was demonstrated in a national cohort study utilizing the French health insurance database, Dr. Ritchlin noted at the research meeting.
The French study covered almost 3,000 patients who started a second biologic after discontinuing a TNF inhibitor during 2015-2020. Overall, 1-year and 3-year persistence rates were 42% and 17%, respectively.
Dr. Liao disclosed research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and Trex Bio. Dr. Ritchlin reported no disclosures.
AT THE NPF RESEARCH SYMPOSIUM 2023
AI app can do biomechanical analysis in minutes
Stanford (Calif.) University’s human performance lab sits next to its physical therapy clinic, so orthopedic surgeons often stop by to request biomechanical analyses for their patients, such as athletes with repeat injuries.
“It would take us days to analyze the data, so we would only do it a handful of times per year,” said Scott Uhlrich, PhD, director of research at the lab.
Now an app can do the job in less than 10 minutes.
The motion-capture app, created by Dr. Uhlrich and fellow bioengineers at Stanford, could help clinicians design better interventions to ward off mobility problems and speed recovery. It could also help researchers fill huge knowledge gaps about human mobility.
It’s currently available free for research and educational use. Model Health, a startup affiliated with the Stanford researchers, provides licenses for commercial use and clinical practice.
Here’s how it works. Footage of human movement, recorded by two smartphones, gets uploaded to the cloud, where an algorithm identifies a set of points on the body. The app relies on computer vision algorithms, a form of AI that trains computers to “understand” visual data – in this case, a person’s pose.
Next, the app quantifies how the body is moving through three-dimensional space. Musculoskeletal system models reveal insights into that movement, such as the angle of a joint, the stretch in a tendon, or the force being transferred through the joints.
“These are the quantities that relate to injuries and disease,” said Dr. Uhlrich, co-author of a study introducing the app. “We need to get to those quantities to be able to inform medical research and eventually clinical practice.”
The conventional approach to getting this kind of analysis requires special expertise and costs $150,000. By contrast, the app is free and easy to use.
It “democratizes” human movement analysis, said senior study author Scott Delp, PhD, professor of bioengineering and mechanical engineering at Stanford. The researchers hope this will “improve outcomes for patients across the world.”
‘Endless opportunities’
A lot about human mobility remains mysterious.
In aging adults, researchers can’t say when balance starts to degrade or by how much every year. They’re also still unraveling how sports injuries occur and how degenerative joint diseases like arthritis progress.
“We don’t really understand the onset of a lot of things, because we’ve just never measured it,” Dr. Uhlrich said.
OpenCap could help change that in a big way. Although biomechanics studies tend to be small – just 14 participants, on average – the app could allow for much larger studies, thanks to its lower cost and ease of use. In the study, the app collected movement data on 100 participants in less than 10 hours and computed results in 31 hours – an effort that would otherwise have taken a year.
“Studies of hundreds will be common, and thousands will be feasible, especially if assessments are integrated into clinic visits,” Dr. Uhlrich said.
About 2,600 researchers around the world are already using the app, according to Dr. Uhlrich. Many had never created a dynamic simulation before.
“The opportunities here are endless,” said Eni Halilaj, PhD, an assistant professor of mechanical engineering at Carnegie Mellon, Pittsburgh, who was not involved in creating the app. That’s especially true for “highly heterogeneous conditions that we have not been able to fully characterize through traditional studies with limited patients.”
In one case, researcher Reed Gurchiek, a former Stanford postdoc and current professor at Clemson (S.C.) University, used the app to study hamstring strain injuries during sprinting and found that these muscles lengthen faster during acceleration, compared with running at a constant speed.
“This aligns with the higher observed injury rates when athletes are accelerating,” Dr. Uhlrich explained. “Varied-speed sprinting studies are not possible in the lab, so this was really enabled by OpenCap’s portability.”
Movement as a biomarker
The researchers are already using the app to build new tools, including metrics to identify risk for anterior cruciate ligament injury in young athletes and to measure balance.
Someday, the technology could augment annual physicals, establishing movement as a biomarker. By having patients perform a few movements, like walking or standing up, clinicians could assess their disease risk and progression or their risk of falling.
Excessive loading in the knee joint puts patients at higher risk of developing osteoarthritis, for instance, but clinicians can’t easily access this information. The disease is typically diagnosed after symptoms appear, even though intervention could happen much earlier.
“Prevention is still not as embraced as it should be,” said Pamela Toto, PhD, professor of occupational therapy at the University of Pittsburgh, who also was not involved in making the app. “If we could tie the technology to intervention down the road, that could be valuable.”
A version of this article first appeared on Medscape.com.
Stanford (Calif.) University’s human performance lab sits next to its physical therapy clinic, so orthopedic surgeons often stop by to request biomechanical analyses for their patients, such as athletes with repeat injuries.
“It would take us days to analyze the data, so we would only do it a handful of times per year,” said Scott Uhlrich, PhD, director of research at the lab.
Now an app can do the job in less than 10 minutes.
The motion-capture app, created by Dr. Uhlrich and fellow bioengineers at Stanford, could help clinicians design better interventions to ward off mobility problems and speed recovery. It could also help researchers fill huge knowledge gaps about human mobility.
It’s currently available free for research and educational use. Model Health, a startup affiliated with the Stanford researchers, provides licenses for commercial use and clinical practice.
Here’s how it works. Footage of human movement, recorded by two smartphones, gets uploaded to the cloud, where an algorithm identifies a set of points on the body. The app relies on computer vision algorithms, a form of AI that trains computers to “understand” visual data – in this case, a person’s pose.
Next, the app quantifies how the body is moving through three-dimensional space. Musculoskeletal system models reveal insights into that movement, such as the angle of a joint, the stretch in a tendon, or the force being transferred through the joints.
“These are the quantities that relate to injuries and disease,” said Dr. Uhlrich, co-author of a study introducing the app. “We need to get to those quantities to be able to inform medical research and eventually clinical practice.”
The conventional approach to getting this kind of analysis requires special expertise and costs $150,000. By contrast, the app is free and easy to use.
It “democratizes” human movement analysis, said senior study author Scott Delp, PhD, professor of bioengineering and mechanical engineering at Stanford. The researchers hope this will “improve outcomes for patients across the world.”
‘Endless opportunities’
A lot about human mobility remains mysterious.
In aging adults, researchers can’t say when balance starts to degrade or by how much every year. They’re also still unraveling how sports injuries occur and how degenerative joint diseases like arthritis progress.
“We don’t really understand the onset of a lot of things, because we’ve just never measured it,” Dr. Uhlrich said.
OpenCap could help change that in a big way. Although biomechanics studies tend to be small – just 14 participants, on average – the app could allow for much larger studies, thanks to its lower cost and ease of use. In the study, the app collected movement data on 100 participants in less than 10 hours and computed results in 31 hours – an effort that would otherwise have taken a year.
“Studies of hundreds will be common, and thousands will be feasible, especially if assessments are integrated into clinic visits,” Dr. Uhlrich said.
About 2,600 researchers around the world are already using the app, according to Dr. Uhlrich. Many had never created a dynamic simulation before.
“The opportunities here are endless,” said Eni Halilaj, PhD, an assistant professor of mechanical engineering at Carnegie Mellon, Pittsburgh, who was not involved in creating the app. That’s especially true for “highly heterogeneous conditions that we have not been able to fully characterize through traditional studies with limited patients.”
In one case, researcher Reed Gurchiek, a former Stanford postdoc and current professor at Clemson (S.C.) University, used the app to study hamstring strain injuries during sprinting and found that these muscles lengthen faster during acceleration, compared with running at a constant speed.
“This aligns with the higher observed injury rates when athletes are accelerating,” Dr. Uhlrich explained. “Varied-speed sprinting studies are not possible in the lab, so this was really enabled by OpenCap’s portability.”
Movement as a biomarker
The researchers are already using the app to build new tools, including metrics to identify risk for anterior cruciate ligament injury in young athletes and to measure balance.
Someday, the technology could augment annual physicals, establishing movement as a biomarker. By having patients perform a few movements, like walking or standing up, clinicians could assess their disease risk and progression or their risk of falling.
Excessive loading in the knee joint puts patients at higher risk of developing osteoarthritis, for instance, but clinicians can’t easily access this information. The disease is typically diagnosed after symptoms appear, even though intervention could happen much earlier.
“Prevention is still not as embraced as it should be,” said Pamela Toto, PhD, professor of occupational therapy at the University of Pittsburgh, who also was not involved in making the app. “If we could tie the technology to intervention down the road, that could be valuable.”
A version of this article first appeared on Medscape.com.
Stanford (Calif.) University’s human performance lab sits next to its physical therapy clinic, so orthopedic surgeons often stop by to request biomechanical analyses for their patients, such as athletes with repeat injuries.
“It would take us days to analyze the data, so we would only do it a handful of times per year,” said Scott Uhlrich, PhD, director of research at the lab.
Now an app can do the job in less than 10 minutes.
The motion-capture app, created by Dr. Uhlrich and fellow bioengineers at Stanford, could help clinicians design better interventions to ward off mobility problems and speed recovery. It could also help researchers fill huge knowledge gaps about human mobility.
It’s currently available free for research and educational use. Model Health, a startup affiliated with the Stanford researchers, provides licenses for commercial use and clinical practice.
Here’s how it works. Footage of human movement, recorded by two smartphones, gets uploaded to the cloud, where an algorithm identifies a set of points on the body. The app relies on computer vision algorithms, a form of AI that trains computers to “understand” visual data – in this case, a person’s pose.
Next, the app quantifies how the body is moving through three-dimensional space. Musculoskeletal system models reveal insights into that movement, such as the angle of a joint, the stretch in a tendon, or the force being transferred through the joints.
“These are the quantities that relate to injuries and disease,” said Dr. Uhlrich, co-author of a study introducing the app. “We need to get to those quantities to be able to inform medical research and eventually clinical practice.”
The conventional approach to getting this kind of analysis requires special expertise and costs $150,000. By contrast, the app is free and easy to use.
It “democratizes” human movement analysis, said senior study author Scott Delp, PhD, professor of bioengineering and mechanical engineering at Stanford. The researchers hope this will “improve outcomes for patients across the world.”
‘Endless opportunities’
A lot about human mobility remains mysterious.
In aging adults, researchers can’t say when balance starts to degrade or by how much every year. They’re also still unraveling how sports injuries occur and how degenerative joint diseases like arthritis progress.
“We don’t really understand the onset of a lot of things, because we’ve just never measured it,” Dr. Uhlrich said.
OpenCap could help change that in a big way. Although biomechanics studies tend to be small – just 14 participants, on average – the app could allow for much larger studies, thanks to its lower cost and ease of use. In the study, the app collected movement data on 100 participants in less than 10 hours and computed results in 31 hours – an effort that would otherwise have taken a year.
“Studies of hundreds will be common, and thousands will be feasible, especially if assessments are integrated into clinic visits,” Dr. Uhlrich said.
About 2,600 researchers around the world are already using the app, according to Dr. Uhlrich. Many had never created a dynamic simulation before.
“The opportunities here are endless,” said Eni Halilaj, PhD, an assistant professor of mechanical engineering at Carnegie Mellon, Pittsburgh, who was not involved in creating the app. That’s especially true for “highly heterogeneous conditions that we have not been able to fully characterize through traditional studies with limited patients.”
In one case, researcher Reed Gurchiek, a former Stanford postdoc and current professor at Clemson (S.C.) University, used the app to study hamstring strain injuries during sprinting and found that these muscles lengthen faster during acceleration, compared with running at a constant speed.
“This aligns with the higher observed injury rates when athletes are accelerating,” Dr. Uhlrich explained. “Varied-speed sprinting studies are not possible in the lab, so this was really enabled by OpenCap’s portability.”
Movement as a biomarker
The researchers are already using the app to build new tools, including metrics to identify risk for anterior cruciate ligament injury in young athletes and to measure balance.
Someday, the technology could augment annual physicals, establishing movement as a biomarker. By having patients perform a few movements, like walking or standing up, clinicians could assess their disease risk and progression or their risk of falling.
Excessive loading in the knee joint puts patients at higher risk of developing osteoarthritis, for instance, but clinicians can’t easily access this information. The disease is typically diagnosed after symptoms appear, even though intervention could happen much earlier.
“Prevention is still not as embraced as it should be,” said Pamela Toto, PhD, professor of occupational therapy at the University of Pittsburgh, who also was not involved in making the app. “If we could tie the technology to intervention down the road, that could be valuable.”
A version of this article first appeared on Medscape.com.
FDA OKs first ustekinumab biosimilar
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
FDA approves abatacept for pediatric patients with psoriatic arthritis
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
RA precision medicine using synovial biopsy ‘remains elusive’
Researchers have yet to crack how to analyze synovial tissue biopsy specimens to predict treatment responses for patients with rheumatoid arthritis.
According to new research, classifying patients by synovial fluid B-cell status was not reliable in predicting treatment response to etanercept, tocilizumab, or rituximab. More comprehensive molecular analyses may hold promise in developing models to predict treatment of a disease as heterogeneous as RA, wrote the authors, led by chief investigator Costantino Pitzalis, MD, head of the Centre for Experimental Medicine and Rheumatology at Queen Mary University of London.
The article was published in the November 2023 issue of The Lancet Rheumatology..
Trial-and-error treatment
Because clinicians do not have a way to reliably predict how patients may respond to certain medications, the current RA treatments involve trial and error.

“Both with regard to the first conventional synthetic disease-modifying antirheumatic drug (DMARD) for all patients and the first biologic DMARD for the subgroup of patients requiring these drugs, the choice of a drug is based on pragmatic arguments rather than on individual patient characteristics,” Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at the Leiden University Medical Center, the Netherlands, wrote in an accompanying editorial.
Most research on predicting treatment response has used clinical features and blood biomarkers, but neither approach is reliably predictive. “Precision medicine, therefore, remains elusive,” she said. Newer research has focused on synovial tissue biopsy to inform research decisions.
The STRAP studies
In this newest study, researchers combined data from two clinical studies with identical design: the Stratification of Biological Therapies by Pathobiology in Biologic-Naive Patients With Rheumatoid Arthritis (STRAP) trial, taking place in the United Kingdom, and STRAP-EU, which recruited patients from the European Union. The trials are the largest biopsy-driven trials to date, according to the researchers.
In total, researchers recruited 223 biologic-naive adult patients from 26 universities in the United Kingdom and Europe. Participants underwent ultrasound-guided synovial tissue biopsy and were then randomly assigned to receive etanercept (72 patients), tocilizumab (73 patients), or rituximab (78 patients). In a histologic analysis, 121 patients were characterized as B-cell poor (BCP), 100 patients were classified as B-cell rich (BCR), and two patients were classified as undetermined.
“We hypothesized that patients with a low synovial histological or molecular B-cell score would have a lower response to rituximab than to etanercept or tocilizumab,” the authors wrote.
However, among the BCP patients, there were no significant differences between responses to treatment at week 16 in the rituximab group compared with the etanercept and tocilizumab groups put together. In both groups, around 60% of patients achieved the primary endpoint of at least 20% improvement in American College of Rheumatology response criteria.
The results suggest that “a dichotomic classification into synovial B cell poor versus rich is unable to predict treatment response in patients treated with rituximab compared with etanercept or tocilizumab,” the authors wrote.
The findings echo work from the same group of researchers in 2021. This study – the R4RA trial – enrolled 164 patients who previously had an inadequate response to tumor necrosis factor blockers. Researchers then used a similar histologic approach to compare patients’ responses to tocilizumab or rituximab.
Among patients classified as BCP, there was not a significant difference in treatment response between the tocilizumab group and the rituximab group. However, classifying patients as BCP with RNA sequencing did make a difference. In this subgroup, patients given tocilizumab demonstrated a significantly higher response rate than those treated with rituximab.
Molecular-level analyses needed
This 2021 study showed – and this most recent study further confirmed – that “histology is not the way to understand what’s going on with or be predictive with tissue,” said Harris R. Perlman, PhD, chief of rheumatology at Northwestern University in Chicago. He was not involved with the research.
“Most people now believe that you really have to understand the tissue on a single-cell basis – using the gene expression of each individual cell – to really give you an idea of what’s happening in tissue,” he noted.
“RA continues to tell us that it is more complex than just something dichotomous,” added Elena Myasoedova, MD, PhD, director of the Inflammatory Arthritis Subspecialty Group at the Mayo Clinic in Rochester, Minn. She was not involved with the work.
“Understanding more about the heterogeneity using different ‘-omics’ approaches and introducing a two- or three-dimensional approach with spatial biology can be helpful,” she said.
Spatial transcriptomics, for example, allows scientists to measure all gene activity in a tissue sample and to map where that gene activity is occurring.
“It helps us to understand and visualize molecules and their unique context within individual cells and tissues,” Dr. Myasoedova explained.
With advanced molecular analyses already available, Dr. Perlman is adamant that synovial tissue remains the key to unlocking precision medicine.
“The tissue is the golden ticket,” he said, “but it’s how you analyze it.”
And it’s clear that older analytic methods – such as histology – are not enough, he said.
A larger study of a size similar to that of STRAP that incorporates multiple sources of patient information, from gene expression to clinical symptoms, to create a predictive model would be key to understanding how to move the field of precision treatment for RA forward, he added.
“Precision medicine for RA is close,” he said. “We still have to get the numbers.”
The STRAP and STRAP-EU trials were jointly funded by the UK Medical Research Council and Versus Arthritis. Pfizer and Roche donated the study drugs through an investigator-sponsored research grant. Many authors, including Dr. Pitzalis, have multiple financial relationships with pharmaceutical companies. Dr. Van der Helm-van Mil and Dr. Myasoedova have disclosed no relevant financial relationships. Dr. Perlman consults for AbbVie, AnaptysBio, Exagen, Janssen, and Kiniksa.
A version of this article first appeared on Medscape.com.
Researchers have yet to crack how to analyze synovial tissue biopsy specimens to predict treatment responses for patients with rheumatoid arthritis.
According to new research, classifying patients by synovial fluid B-cell status was not reliable in predicting treatment response to etanercept, tocilizumab, or rituximab. More comprehensive molecular analyses may hold promise in developing models to predict treatment of a disease as heterogeneous as RA, wrote the authors, led by chief investigator Costantino Pitzalis, MD, head of the Centre for Experimental Medicine and Rheumatology at Queen Mary University of London.
The article was published in the November 2023 issue of The Lancet Rheumatology..
Trial-and-error treatment
Because clinicians do not have a way to reliably predict how patients may respond to certain medications, the current RA treatments involve trial and error.

“Both with regard to the first conventional synthetic disease-modifying antirheumatic drug (DMARD) for all patients and the first biologic DMARD for the subgroup of patients requiring these drugs, the choice of a drug is based on pragmatic arguments rather than on individual patient characteristics,” Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at the Leiden University Medical Center, the Netherlands, wrote in an accompanying editorial.
Most research on predicting treatment response has used clinical features and blood biomarkers, but neither approach is reliably predictive. “Precision medicine, therefore, remains elusive,” she said. Newer research has focused on synovial tissue biopsy to inform research decisions.
The STRAP studies
In this newest study, researchers combined data from two clinical studies with identical design: the Stratification of Biological Therapies by Pathobiology in Biologic-Naive Patients With Rheumatoid Arthritis (STRAP) trial, taking place in the United Kingdom, and STRAP-EU, which recruited patients from the European Union. The trials are the largest biopsy-driven trials to date, according to the researchers.
In total, researchers recruited 223 biologic-naive adult patients from 26 universities in the United Kingdom and Europe. Participants underwent ultrasound-guided synovial tissue biopsy and were then randomly assigned to receive etanercept (72 patients), tocilizumab (73 patients), or rituximab (78 patients). In a histologic analysis, 121 patients were characterized as B-cell poor (BCP), 100 patients were classified as B-cell rich (BCR), and two patients were classified as undetermined.
“We hypothesized that patients with a low synovial histological or molecular B-cell score would have a lower response to rituximab than to etanercept or tocilizumab,” the authors wrote.
However, among the BCP patients, there were no significant differences between responses to treatment at week 16 in the rituximab group compared with the etanercept and tocilizumab groups put together. In both groups, around 60% of patients achieved the primary endpoint of at least 20% improvement in American College of Rheumatology response criteria.
The results suggest that “a dichotomic classification into synovial B cell poor versus rich is unable to predict treatment response in patients treated with rituximab compared with etanercept or tocilizumab,” the authors wrote.
The findings echo work from the same group of researchers in 2021. This study – the R4RA trial – enrolled 164 patients who previously had an inadequate response to tumor necrosis factor blockers. Researchers then used a similar histologic approach to compare patients’ responses to tocilizumab or rituximab.
Among patients classified as BCP, there was not a significant difference in treatment response between the tocilizumab group and the rituximab group. However, classifying patients as BCP with RNA sequencing did make a difference. In this subgroup, patients given tocilizumab demonstrated a significantly higher response rate than those treated with rituximab.
Molecular-level analyses needed
This 2021 study showed – and this most recent study further confirmed – that “histology is not the way to understand what’s going on with or be predictive with tissue,” said Harris R. Perlman, PhD, chief of rheumatology at Northwestern University in Chicago. He was not involved with the research.
“Most people now believe that you really have to understand the tissue on a single-cell basis – using the gene expression of each individual cell – to really give you an idea of what’s happening in tissue,” he noted.
“RA continues to tell us that it is more complex than just something dichotomous,” added Elena Myasoedova, MD, PhD, director of the Inflammatory Arthritis Subspecialty Group at the Mayo Clinic in Rochester, Minn. She was not involved with the work.
“Understanding more about the heterogeneity using different ‘-omics’ approaches and introducing a two- or three-dimensional approach with spatial biology can be helpful,” she said.
Spatial transcriptomics, for example, allows scientists to measure all gene activity in a tissue sample and to map where that gene activity is occurring.
“It helps us to understand and visualize molecules and their unique context within individual cells and tissues,” Dr. Myasoedova explained.
With advanced molecular analyses already available, Dr. Perlman is adamant that synovial tissue remains the key to unlocking precision medicine.
“The tissue is the golden ticket,” he said, “but it’s how you analyze it.”
And it’s clear that older analytic methods – such as histology – are not enough, he said.
A larger study of a size similar to that of STRAP that incorporates multiple sources of patient information, from gene expression to clinical symptoms, to create a predictive model would be key to understanding how to move the field of precision treatment for RA forward, he added.
“Precision medicine for RA is close,” he said. “We still have to get the numbers.”
The STRAP and STRAP-EU trials were jointly funded by the UK Medical Research Council and Versus Arthritis. Pfizer and Roche donated the study drugs through an investigator-sponsored research grant. Many authors, including Dr. Pitzalis, have multiple financial relationships with pharmaceutical companies. Dr. Van der Helm-van Mil and Dr. Myasoedova have disclosed no relevant financial relationships. Dr. Perlman consults for AbbVie, AnaptysBio, Exagen, Janssen, and Kiniksa.
A version of this article first appeared on Medscape.com.
Researchers have yet to crack how to analyze synovial tissue biopsy specimens to predict treatment responses for patients with rheumatoid arthritis.
According to new research, classifying patients by synovial fluid B-cell status was not reliable in predicting treatment response to etanercept, tocilizumab, or rituximab. More comprehensive molecular analyses may hold promise in developing models to predict treatment of a disease as heterogeneous as RA, wrote the authors, led by chief investigator Costantino Pitzalis, MD, head of the Centre for Experimental Medicine and Rheumatology at Queen Mary University of London.
The article was published in the November 2023 issue of The Lancet Rheumatology..
Trial-and-error treatment
Because clinicians do not have a way to reliably predict how patients may respond to certain medications, the current RA treatments involve trial and error.

“Both with regard to the first conventional synthetic disease-modifying antirheumatic drug (DMARD) for all patients and the first biologic DMARD for the subgroup of patients requiring these drugs, the choice of a drug is based on pragmatic arguments rather than on individual patient characteristics,” Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at the Leiden University Medical Center, the Netherlands, wrote in an accompanying editorial.
Most research on predicting treatment response has used clinical features and blood biomarkers, but neither approach is reliably predictive. “Precision medicine, therefore, remains elusive,” she said. Newer research has focused on synovial tissue biopsy to inform research decisions.
The STRAP studies
In this newest study, researchers combined data from two clinical studies with identical design: the Stratification of Biological Therapies by Pathobiology in Biologic-Naive Patients With Rheumatoid Arthritis (STRAP) trial, taking place in the United Kingdom, and STRAP-EU, which recruited patients from the European Union. The trials are the largest biopsy-driven trials to date, according to the researchers.
In total, researchers recruited 223 biologic-naive adult patients from 26 universities in the United Kingdom and Europe. Participants underwent ultrasound-guided synovial tissue biopsy and were then randomly assigned to receive etanercept (72 patients), tocilizumab (73 patients), or rituximab (78 patients). In a histologic analysis, 121 patients were characterized as B-cell poor (BCP), 100 patients were classified as B-cell rich (BCR), and two patients were classified as undetermined.
“We hypothesized that patients with a low synovial histological or molecular B-cell score would have a lower response to rituximab than to etanercept or tocilizumab,” the authors wrote.
However, among the BCP patients, there were no significant differences between responses to treatment at week 16 in the rituximab group compared with the etanercept and tocilizumab groups put together. In both groups, around 60% of patients achieved the primary endpoint of at least 20% improvement in American College of Rheumatology response criteria.
The results suggest that “a dichotomic classification into synovial B cell poor versus rich is unable to predict treatment response in patients treated with rituximab compared with etanercept or tocilizumab,” the authors wrote.
The findings echo work from the same group of researchers in 2021. This study – the R4RA trial – enrolled 164 patients who previously had an inadequate response to tumor necrosis factor blockers. Researchers then used a similar histologic approach to compare patients’ responses to tocilizumab or rituximab.
Among patients classified as BCP, there was not a significant difference in treatment response between the tocilizumab group and the rituximab group. However, classifying patients as BCP with RNA sequencing did make a difference. In this subgroup, patients given tocilizumab demonstrated a significantly higher response rate than those treated with rituximab.
Molecular-level analyses needed
This 2021 study showed – and this most recent study further confirmed – that “histology is not the way to understand what’s going on with or be predictive with tissue,” said Harris R. Perlman, PhD, chief of rheumatology at Northwestern University in Chicago. He was not involved with the research.
“Most people now believe that you really have to understand the tissue on a single-cell basis – using the gene expression of each individual cell – to really give you an idea of what’s happening in tissue,” he noted.
“RA continues to tell us that it is more complex than just something dichotomous,” added Elena Myasoedova, MD, PhD, director of the Inflammatory Arthritis Subspecialty Group at the Mayo Clinic in Rochester, Minn. She was not involved with the work.
“Understanding more about the heterogeneity using different ‘-omics’ approaches and introducing a two- or three-dimensional approach with spatial biology can be helpful,” she said.
Spatial transcriptomics, for example, allows scientists to measure all gene activity in a tissue sample and to map where that gene activity is occurring.
“It helps us to understand and visualize molecules and their unique context within individual cells and tissues,” Dr. Myasoedova explained.
With advanced molecular analyses already available, Dr. Perlman is adamant that synovial tissue remains the key to unlocking precision medicine.
“The tissue is the golden ticket,” he said, “but it’s how you analyze it.”
And it’s clear that older analytic methods – such as histology – are not enough, he said.
A larger study of a size similar to that of STRAP that incorporates multiple sources of patient information, from gene expression to clinical symptoms, to create a predictive model would be key to understanding how to move the field of precision treatment for RA forward, he added.
“Precision medicine for RA is close,” he said. “We still have to get the numbers.”
The STRAP and STRAP-EU trials were jointly funded by the UK Medical Research Council and Versus Arthritis. Pfizer and Roche donated the study drugs through an investigator-sponsored research grant. Many authors, including Dr. Pitzalis, have multiple financial relationships with pharmaceutical companies. Dr. Van der Helm-van Mil and Dr. Myasoedova have disclosed no relevant financial relationships. Dr. Perlman consults for AbbVie, AnaptysBio, Exagen, Janssen, and Kiniksa.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Positive trial of methotrexate in hand OA has modest results
Patients with hand osteoarthritis (OA) and MRI-detected synovitis who took methotrexate (MTX) 20 mg weekly over a 6-month period had a significant and potentially clinically meaningful reduction in pain and stiffness over those who received placebo in the first randomized controlled trial of its kind to show positive results with the drug.
Patients who were randomly assigned to MTX took 10 mg orally for the first 4 weeks then increased to 20 mg for the rest of the trial, with differences in the primary outcome of pain measured by visual analog scale (VAS) first becoming significant over placebo at 3 months.
Senior author of the METHODS study (Methotrexate to Treat Hand Osteoarthritis with Synovitis), Flavia Cicuttini, PhD, MSc, head of the musculoskeletal unit at Monash University and head of rheumatology at Alfred Hospital, Melbourne, Australia, noted that the effect of MTX was higher than effect sizes that have been reported for NSAIDs on pain in hip or knee OA.
The study was published online October 12 in The Lancet.
METHODS makes improvements on past studies
While OA is traditionally categorized as a noninflammatory process, it’s known that there are some patients who have a clinical phenotype characterized by joint swelling (synovitis) and others develop erosive disease. MTX is one of the most common therapies for inflammatory arthritis and standard of care for rheumatoid arthritis (RA) management. Previous studies of methotrexate showed lack of efficacy in hand OA but may have been because of the use of a low dose, poor power due to moderate sample size, and failure to target the specific inflammatory OA phenotype.
In an interview, Dr. Cicuttini noted the selection of methotrexate for this trial was intentional. “We considered the evidence and decided to test methotrexate because we know it is effective in inflammatory arthritis, and its mode of action is broader than the more selective anti-TNF [tumor necrosis factor] agents,” which she noted have failed in prior hand OA trials. She also noted that the only previous randomized controlled trial of MTX tested a dose of 10 mg/week, rather than the 20 mg/week dose used in METHODS.
Study details and results
METHODS was a randomized, double-blind, placebo-controlled trial at multiple sites within Australia. Patients were recruited from 2017 to 2022, with a temporary pause in 2020 during the COVID-19 pandemic because of safety concerns regarding MTX use. Participants included in this study were aged 40-75 years, had pain in hand joints for most days in the past 3 months, and a pain score of at least 40 mm on a 100-mm VAS in the past 7 days.
The participants’ hand OA fulfilled American College of Rheumatology criteria, radiographic osteoarthritis (Kellgren and Lawrence grade 2 or more) in at least one joint, and MRI-detected synovitis of grade 1 or more in at least one joint. They excluded patients with concomitant rheumatic disease, gout, psoriasis, positive rheumatoid factor or anti-cyclic citrullinated peptides, or elevated inflammatory markers (erythrocyte sedimentation rate or C-reactive protein), as well as those with contraindication to methotrexate or MRI.
The trials’ 97 participants were assigned 1:1 to MTX or placebo using block randomization. The MTX group started on oral MTX 10 mg weekly for the first 4 weeks, followed by 20 mg weekly for the remainder of the study. Participants took folic acid 5 mg once a day to reduce risk of MTX-related side effects.
The mean age of the participants was 61 years, with 70% female. Baseline characteristics were generally well-balanced, except for higher mean BMI in the MTX group. At 6 months, the MTX group had a greater reduction in mean VAS pain than the placebo group (–15.2 mm vs. –7.7 mm; adjusted between-group difference, –9.9 mm). The minimally clinically important difference for OA trials is a 15-mm change (out of 100) in VAS pain.
The MTX group also had greater reduction in mean Australian Canadian OA Hand Index (AUSCAN) score for pain and stiffness at 6 months, compared with placebo, but there were no differences in other secondary outcomes (mean AUSCAN, Functional Index for Hand Osteoarthritis, Health Assessment Questionnaire, Michigan Hand Outcomes Questionnaire, or grip strength).
MTX was well-tolerated with no serious adverse events related to treatment; only 5 of 50 participants in the MTX group and 4 of 47 in the placebo group discontinued study medication. Incidence of adverse events was similar in the two groups throughout the trial, including mild leukopenia, elevated liver enzymes, mild reduction of hemoglobin, and raised creatinine. None of the laboratory abnormalities required change in medication dosage or affected ability to continue in the study.
Qualifications and considerations for MTX use
Commenting on the study, OA researcher Amanda E. Nelson, MD, MSCR, associate professor of medicine at the University of North Carolina at Chapel Hill’s Thurston Arthritis Research Center, said that its overall design was “excellent,” including appropriate masking, controls, randomization, and power and sample size calculations, contributing to “the trial’s relatively positive, although still modest, results.” Dr. Nelson was not involved with the METHODS study.
Several factors may have contributed to the study’s success, including the broader mechanism of action of methotrexate and higher dose used, Ida K. Haugen, MD, PhD, senior researcher at Diakonhjemmet Hospital’s Center for Treatment of Rheumatic and Musculoskeletal Diseases in Oslo, Norway, told this news organization.
She noted that the MTX 20 mg/week dosage is similar to what is used in treatment of RA and may be key to better targeting inflammation.
“Furthermore, the study included individuals with hand OA and synovitis by MRI, and thus may have found the right patient population in comparison to prior studies,” Dr. Haugen said.
Dr. Cicuttini agreed, noting that “previous trials [of MTX] did not target the inflammatory phenotype of hand osteoarthritis that would be expected to respond.” In the previous randomized controlled trial of MTX in hand OA, only 29 of a total 1024 joints had synovitis, she explained. “Their inclusion criteria were individuals with severe erosive hand osteoarthritis, suggesting that this later-stage disease is less likely to respond.”
Dr. Cicuttini said that she saw no specific issues in regard to potential use of MTX in the OA population in the clinic. “The data we have, together with the large experience we have with using methotrexate and the fact that treatments for hand OA are not very effective, means that it would be reasonable to offer this to patients with hand OA and inflammation,” she said. “The level of evidence for an effect of methotrexate would need to be discussed with the patient. The discussion around the use of methotrexate would then need to proceed in the same way we discuss the use of methotrexate with patients when used for other inflammatory joint diseases, and the decision is then made with the patient.”
In contrast, Dr. Nelson expressed some concerns regarding the immediate use of MTX for this population. “Many individuals with hand OA have multiple medical comorbidities and polypharmacy, which are important when considering additional treatments, particularly those with modest benefit and potential adverse effects over the long term,” she said. “I do not think this single study provides enough evidence to suggest that all such patients should be treated with methotrexate, and more data, particularly about long-term use and optimal risk stratification, is needed.”
While MTX use in refractory inflammatory hand OA is not yet recommended in international guidelines, Dr. Haugen believes this study supports use of MTX in this patient population. Given that MRI and ultrasound may not always be available to identify synovitis, Dr. Nelson and Dr. Haugen suggested identifying patients who may benefit through careful history and clinical examination for evidence of swollen joints. In addition, Dr. Cicuttini explained that she would not necessarily use MTX in patients with erosive OA radiographically because it may be a later stage of disease that is less likely to respond.
The authors highlighted potential limitations of the METHODS trial. They initially planned to study whether MTX reduced pain and improved radiographic progression at 2 years, but since they paused the study for 7 months during the COVID-19 pandemic, they amended the trial protocol and focused on pain reduction at 6 months as the primary endpoint instead.
Tender and swollen joint counts were initially going to be included, but this was modified given the use of virtual telemedicine visits during the pandemic. Dr. Cicuttini said that further studies are underway to identify potential subpopulations who may benefit from immunosuppression, and others are needed to determine whether MTX reduces joint damage and slows disease progression in hand OA with inflammation.
Dr. Cicuttini said her research group is interested to see whether women who develop hand OA around the time of menopause (“menopausal OA”) are a group that could benefit. Dr. Haugen noted that she is involved in a study testing MTX in erosive hand OA (the MERINO trial).
The study was funded by a project grant from the National Health and Medical Research Council of Australia. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with hand osteoarthritis (OA) and MRI-detected synovitis who took methotrexate (MTX) 20 mg weekly over a 6-month period had a significant and potentially clinically meaningful reduction in pain and stiffness over those who received placebo in the first randomized controlled trial of its kind to show positive results with the drug.
Patients who were randomly assigned to MTX took 10 mg orally for the first 4 weeks then increased to 20 mg for the rest of the trial, with differences in the primary outcome of pain measured by visual analog scale (VAS) first becoming significant over placebo at 3 months.
Senior author of the METHODS study (Methotrexate to Treat Hand Osteoarthritis with Synovitis), Flavia Cicuttini, PhD, MSc, head of the musculoskeletal unit at Monash University and head of rheumatology at Alfred Hospital, Melbourne, Australia, noted that the effect of MTX was higher than effect sizes that have been reported for NSAIDs on pain in hip or knee OA.
The study was published online October 12 in The Lancet.
METHODS makes improvements on past studies
While OA is traditionally categorized as a noninflammatory process, it’s known that there are some patients who have a clinical phenotype characterized by joint swelling (synovitis) and others develop erosive disease. MTX is one of the most common therapies for inflammatory arthritis and standard of care for rheumatoid arthritis (RA) management. Previous studies of methotrexate showed lack of efficacy in hand OA but may have been because of the use of a low dose, poor power due to moderate sample size, and failure to target the specific inflammatory OA phenotype.
In an interview, Dr. Cicuttini noted the selection of methotrexate for this trial was intentional. “We considered the evidence and decided to test methotrexate because we know it is effective in inflammatory arthritis, and its mode of action is broader than the more selective anti-TNF [tumor necrosis factor] agents,” which she noted have failed in prior hand OA trials. She also noted that the only previous randomized controlled trial of MTX tested a dose of 10 mg/week, rather than the 20 mg/week dose used in METHODS.
Study details and results
METHODS was a randomized, double-blind, placebo-controlled trial at multiple sites within Australia. Patients were recruited from 2017 to 2022, with a temporary pause in 2020 during the COVID-19 pandemic because of safety concerns regarding MTX use. Participants included in this study were aged 40-75 years, had pain in hand joints for most days in the past 3 months, and a pain score of at least 40 mm on a 100-mm VAS in the past 7 days.
The participants’ hand OA fulfilled American College of Rheumatology criteria, radiographic osteoarthritis (Kellgren and Lawrence grade 2 or more) in at least one joint, and MRI-detected synovitis of grade 1 or more in at least one joint. They excluded patients with concomitant rheumatic disease, gout, psoriasis, positive rheumatoid factor or anti-cyclic citrullinated peptides, or elevated inflammatory markers (erythrocyte sedimentation rate or C-reactive protein), as well as those with contraindication to methotrexate or MRI.
The trials’ 97 participants were assigned 1:1 to MTX or placebo using block randomization. The MTX group started on oral MTX 10 mg weekly for the first 4 weeks, followed by 20 mg weekly for the remainder of the study. Participants took folic acid 5 mg once a day to reduce risk of MTX-related side effects.
The mean age of the participants was 61 years, with 70% female. Baseline characteristics were generally well-balanced, except for higher mean BMI in the MTX group. At 6 months, the MTX group had a greater reduction in mean VAS pain than the placebo group (–15.2 mm vs. –7.7 mm; adjusted between-group difference, –9.9 mm). The minimally clinically important difference for OA trials is a 15-mm change (out of 100) in VAS pain.
The MTX group also had greater reduction in mean Australian Canadian OA Hand Index (AUSCAN) score for pain and stiffness at 6 months, compared with placebo, but there were no differences in other secondary outcomes (mean AUSCAN, Functional Index for Hand Osteoarthritis, Health Assessment Questionnaire, Michigan Hand Outcomes Questionnaire, or grip strength).
MTX was well-tolerated with no serious adverse events related to treatment; only 5 of 50 participants in the MTX group and 4 of 47 in the placebo group discontinued study medication. Incidence of adverse events was similar in the two groups throughout the trial, including mild leukopenia, elevated liver enzymes, mild reduction of hemoglobin, and raised creatinine. None of the laboratory abnormalities required change in medication dosage or affected ability to continue in the study.
Qualifications and considerations for MTX use
Commenting on the study, OA researcher Amanda E. Nelson, MD, MSCR, associate professor of medicine at the University of North Carolina at Chapel Hill’s Thurston Arthritis Research Center, said that its overall design was “excellent,” including appropriate masking, controls, randomization, and power and sample size calculations, contributing to “the trial’s relatively positive, although still modest, results.” Dr. Nelson was not involved with the METHODS study.
Several factors may have contributed to the study’s success, including the broader mechanism of action of methotrexate and higher dose used, Ida K. Haugen, MD, PhD, senior researcher at Diakonhjemmet Hospital’s Center for Treatment of Rheumatic and Musculoskeletal Diseases in Oslo, Norway, told this news organization.
She noted that the MTX 20 mg/week dosage is similar to what is used in treatment of RA and may be key to better targeting inflammation.
“Furthermore, the study included individuals with hand OA and synovitis by MRI, and thus may have found the right patient population in comparison to prior studies,” Dr. Haugen said.
Dr. Cicuttini agreed, noting that “previous trials [of MTX] did not target the inflammatory phenotype of hand osteoarthritis that would be expected to respond.” In the previous randomized controlled trial of MTX in hand OA, only 29 of a total 1024 joints had synovitis, she explained. “Their inclusion criteria were individuals with severe erosive hand osteoarthritis, suggesting that this later-stage disease is less likely to respond.”
Dr. Cicuttini said that she saw no specific issues in regard to potential use of MTX in the OA population in the clinic. “The data we have, together with the large experience we have with using methotrexate and the fact that treatments for hand OA are not very effective, means that it would be reasonable to offer this to patients with hand OA and inflammation,” she said. “The level of evidence for an effect of methotrexate would need to be discussed with the patient. The discussion around the use of methotrexate would then need to proceed in the same way we discuss the use of methotrexate with patients when used for other inflammatory joint diseases, and the decision is then made with the patient.”
In contrast, Dr. Nelson expressed some concerns regarding the immediate use of MTX for this population. “Many individuals with hand OA have multiple medical comorbidities and polypharmacy, which are important when considering additional treatments, particularly those with modest benefit and potential adverse effects over the long term,” she said. “I do not think this single study provides enough evidence to suggest that all such patients should be treated with methotrexate, and more data, particularly about long-term use and optimal risk stratification, is needed.”
While MTX use in refractory inflammatory hand OA is not yet recommended in international guidelines, Dr. Haugen believes this study supports use of MTX in this patient population. Given that MRI and ultrasound may not always be available to identify synovitis, Dr. Nelson and Dr. Haugen suggested identifying patients who may benefit through careful history and clinical examination for evidence of swollen joints. In addition, Dr. Cicuttini explained that she would not necessarily use MTX in patients with erosive OA radiographically because it may be a later stage of disease that is less likely to respond.
The authors highlighted potential limitations of the METHODS trial. They initially planned to study whether MTX reduced pain and improved radiographic progression at 2 years, but since they paused the study for 7 months during the COVID-19 pandemic, they amended the trial protocol and focused on pain reduction at 6 months as the primary endpoint instead.
Tender and swollen joint counts were initially going to be included, but this was modified given the use of virtual telemedicine visits during the pandemic. Dr. Cicuttini said that further studies are underway to identify potential subpopulations who may benefit from immunosuppression, and others are needed to determine whether MTX reduces joint damage and slows disease progression in hand OA with inflammation.
Dr. Cicuttini said her research group is interested to see whether women who develop hand OA around the time of menopause (“menopausal OA”) are a group that could benefit. Dr. Haugen noted that she is involved in a study testing MTX in erosive hand OA (the MERINO trial).
The study was funded by a project grant from the National Health and Medical Research Council of Australia. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with hand osteoarthritis (OA) and MRI-detected synovitis who took methotrexate (MTX) 20 mg weekly over a 6-month period had a significant and potentially clinically meaningful reduction in pain and stiffness over those who received placebo in the first randomized controlled trial of its kind to show positive results with the drug.
Patients who were randomly assigned to MTX took 10 mg orally for the first 4 weeks then increased to 20 mg for the rest of the trial, with differences in the primary outcome of pain measured by visual analog scale (VAS) first becoming significant over placebo at 3 months.
Senior author of the METHODS study (Methotrexate to Treat Hand Osteoarthritis with Synovitis), Flavia Cicuttini, PhD, MSc, head of the musculoskeletal unit at Monash University and head of rheumatology at Alfred Hospital, Melbourne, Australia, noted that the effect of MTX was higher than effect sizes that have been reported for NSAIDs on pain in hip or knee OA.
The study was published online October 12 in The Lancet.
METHODS makes improvements on past studies
While OA is traditionally categorized as a noninflammatory process, it’s known that there are some patients who have a clinical phenotype characterized by joint swelling (synovitis) and others develop erosive disease. MTX is one of the most common therapies for inflammatory arthritis and standard of care for rheumatoid arthritis (RA) management. Previous studies of methotrexate showed lack of efficacy in hand OA but may have been because of the use of a low dose, poor power due to moderate sample size, and failure to target the specific inflammatory OA phenotype.
In an interview, Dr. Cicuttini noted the selection of methotrexate for this trial was intentional. “We considered the evidence and decided to test methotrexate because we know it is effective in inflammatory arthritis, and its mode of action is broader than the more selective anti-TNF [tumor necrosis factor] agents,” which she noted have failed in prior hand OA trials. She also noted that the only previous randomized controlled trial of MTX tested a dose of 10 mg/week, rather than the 20 mg/week dose used in METHODS.
Study details and results
METHODS was a randomized, double-blind, placebo-controlled trial at multiple sites within Australia. Patients were recruited from 2017 to 2022, with a temporary pause in 2020 during the COVID-19 pandemic because of safety concerns regarding MTX use. Participants included in this study were aged 40-75 years, had pain in hand joints for most days in the past 3 months, and a pain score of at least 40 mm on a 100-mm VAS in the past 7 days.
The participants’ hand OA fulfilled American College of Rheumatology criteria, radiographic osteoarthritis (Kellgren and Lawrence grade 2 or more) in at least one joint, and MRI-detected synovitis of grade 1 or more in at least one joint. They excluded patients with concomitant rheumatic disease, gout, psoriasis, positive rheumatoid factor or anti-cyclic citrullinated peptides, or elevated inflammatory markers (erythrocyte sedimentation rate or C-reactive protein), as well as those with contraindication to methotrexate or MRI.
The trials’ 97 participants were assigned 1:1 to MTX or placebo using block randomization. The MTX group started on oral MTX 10 mg weekly for the first 4 weeks, followed by 20 mg weekly for the remainder of the study. Participants took folic acid 5 mg once a day to reduce risk of MTX-related side effects.
The mean age of the participants was 61 years, with 70% female. Baseline characteristics were generally well-balanced, except for higher mean BMI in the MTX group. At 6 months, the MTX group had a greater reduction in mean VAS pain than the placebo group (–15.2 mm vs. –7.7 mm; adjusted between-group difference, –9.9 mm). The minimally clinically important difference for OA trials is a 15-mm change (out of 100) in VAS pain.
The MTX group also had greater reduction in mean Australian Canadian OA Hand Index (AUSCAN) score for pain and stiffness at 6 months, compared with placebo, but there were no differences in other secondary outcomes (mean AUSCAN, Functional Index for Hand Osteoarthritis, Health Assessment Questionnaire, Michigan Hand Outcomes Questionnaire, or grip strength).
MTX was well-tolerated with no serious adverse events related to treatment; only 5 of 50 participants in the MTX group and 4 of 47 in the placebo group discontinued study medication. Incidence of adverse events was similar in the two groups throughout the trial, including mild leukopenia, elevated liver enzymes, mild reduction of hemoglobin, and raised creatinine. None of the laboratory abnormalities required change in medication dosage or affected ability to continue in the study.
Qualifications and considerations for MTX use
Commenting on the study, OA researcher Amanda E. Nelson, MD, MSCR, associate professor of medicine at the University of North Carolina at Chapel Hill’s Thurston Arthritis Research Center, said that its overall design was “excellent,” including appropriate masking, controls, randomization, and power and sample size calculations, contributing to “the trial’s relatively positive, although still modest, results.” Dr. Nelson was not involved with the METHODS study.
Several factors may have contributed to the study’s success, including the broader mechanism of action of methotrexate and higher dose used, Ida K. Haugen, MD, PhD, senior researcher at Diakonhjemmet Hospital’s Center for Treatment of Rheumatic and Musculoskeletal Diseases in Oslo, Norway, told this news organization.
She noted that the MTX 20 mg/week dosage is similar to what is used in treatment of RA and may be key to better targeting inflammation.
“Furthermore, the study included individuals with hand OA and synovitis by MRI, and thus may have found the right patient population in comparison to prior studies,” Dr. Haugen said.
Dr. Cicuttini agreed, noting that “previous trials [of MTX] did not target the inflammatory phenotype of hand osteoarthritis that would be expected to respond.” In the previous randomized controlled trial of MTX in hand OA, only 29 of a total 1024 joints had synovitis, she explained. “Their inclusion criteria were individuals with severe erosive hand osteoarthritis, suggesting that this later-stage disease is less likely to respond.”
Dr. Cicuttini said that she saw no specific issues in regard to potential use of MTX in the OA population in the clinic. “The data we have, together with the large experience we have with using methotrexate and the fact that treatments for hand OA are not very effective, means that it would be reasonable to offer this to patients with hand OA and inflammation,” she said. “The level of evidence for an effect of methotrexate would need to be discussed with the patient. The discussion around the use of methotrexate would then need to proceed in the same way we discuss the use of methotrexate with patients when used for other inflammatory joint diseases, and the decision is then made with the patient.”
In contrast, Dr. Nelson expressed some concerns regarding the immediate use of MTX for this population. “Many individuals with hand OA have multiple medical comorbidities and polypharmacy, which are important when considering additional treatments, particularly those with modest benefit and potential adverse effects over the long term,” she said. “I do not think this single study provides enough evidence to suggest that all such patients should be treated with methotrexate, and more data, particularly about long-term use and optimal risk stratification, is needed.”
While MTX use in refractory inflammatory hand OA is not yet recommended in international guidelines, Dr. Haugen believes this study supports use of MTX in this patient population. Given that MRI and ultrasound may not always be available to identify synovitis, Dr. Nelson and Dr. Haugen suggested identifying patients who may benefit through careful history and clinical examination for evidence of swollen joints. In addition, Dr. Cicuttini explained that she would not necessarily use MTX in patients with erosive OA radiographically because it may be a later stage of disease that is less likely to respond.
The authors highlighted potential limitations of the METHODS trial. They initially planned to study whether MTX reduced pain and improved radiographic progression at 2 years, but since they paused the study for 7 months during the COVID-19 pandemic, they amended the trial protocol and focused on pain reduction at 6 months as the primary endpoint instead.
Tender and swollen joint counts were initially going to be included, but this was modified given the use of virtual telemedicine visits during the pandemic. Dr. Cicuttini said that further studies are underway to identify potential subpopulations who may benefit from immunosuppression, and others are needed to determine whether MTX reduces joint damage and slows disease progression in hand OA with inflammation.
Dr. Cicuttini said her research group is interested to see whether women who develop hand OA around the time of menopause (“menopausal OA”) are a group that could benefit. Dr. Haugen noted that she is involved in a study testing MTX in erosive hand OA (the MERINO trial).
The study was funded by a project grant from the National Health and Medical Research Council of Australia. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET