User login
Late-week discharges to home after CRC surgery prone to readmission
BOSTON – The day of the week a patient is discharged from the hospital may have an impact the likelihood of readmission.
Patients discharged home from the hospital on a Thursday after colorectal cancer surgery are more likely to be readmitted within 30 days than those discharged on any other day of the week, investigators found.
In contrast, there were no significant day-dependent differences in readmission rates among patients discharged to a skilled nursing facility or acute rehabilitation program, although patients admitted to clinical facilities had higher overall readmission rates, reported Anna Gustin and coinvestigators at the Levine Cancer Institute at the Carolinas Medical Center in Charlotte, N.C.
“For a patient discharged on a Thursday, if you’re going to get an infection, it’s going to be probably during the weekend, when it’s difficult to contact your primary physician, and when other resources are not as readily available,” said Ms. Gustin, who conducts epidemiologic research at Levine Cancer Center and is also a pre-med student and Japanese major at Wake Forest University in Winston-Salem, N.C.
In a study presented in a poster session at the annual Society of Surgical Oncology Cancer Symposium, Ms. Gustin and her coauthors looked at factors influencing readmission rates among patients undergoing surgery for primary, nonmetastatic colorectal cancer resections.
They drew on the to evaluate outcomes for 93,04 SEER-(Surveillance, Epidemiology, and End Results) Medicare database seven patients aged 66 years and older treated for primary colorectal cancer from 1998 through 2009.
They looked at potential contributing factors such as patient demographics, socioeconomic status, length of stay, days of admission and discharge, and discharge setting (home or clinical facility).
They use multivariate logistic regression models to analyze readmission rates at 14 and 30 days after initial discharge.
Focusing on home discharges, they found that as the week progressed, there was a significant likelihood that a patient discharged home would be readmitted (P less then .001 by chi-square and Cochran-Armitage tests). As noted before, the highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with 10.1% for patients discharged on Sunday, the discharge day least likely to be associated with rehospitalization.
In multivariate analysis, factors significantly associated with risk for 30-day readmission included male vs. female (hazard ratio, 1.16), black vs. other race (HR, 1.22), length of stay 5, 6-7, or 8-10 vs. 12 or more days (HR, 0.48, 0.59, 0.77, respectively), Charlson comorbidity index score 0, 1 or 3 vs. 3 (HR, 0.59, 0.73, 0.82, respectively), and home discharge vs. other (HR, 0.66; all above comparisons significant as shown by 95% confidence intervals).
The authors concluded that although home discharge itself reduces the likelihood of readmission, “improvements in preparing patients for discharge to home are needed. Additional outpatient interventions could rescue patients from readmission.”
They also suggested reexamining staffing policies and weekend availability of resources for patients, and call for addressing disparities in readmissions based on race, sex, length of stay, and comorbidities.
The study was internally supported. The authors reported having no relevant disclosures.
BOSTON – The day of the week a patient is discharged from the hospital may have an impact the likelihood of readmission.
Patients discharged home from the hospital on a Thursday after colorectal cancer surgery are more likely to be readmitted within 30 days than those discharged on any other day of the week, investigators found.
In contrast, there were no significant day-dependent differences in readmission rates among patients discharged to a skilled nursing facility or acute rehabilitation program, although patients admitted to clinical facilities had higher overall readmission rates, reported Anna Gustin and coinvestigators at the Levine Cancer Institute at the Carolinas Medical Center in Charlotte, N.C.
“For a patient discharged on a Thursday, if you’re going to get an infection, it’s going to be probably during the weekend, when it’s difficult to contact your primary physician, and when other resources are not as readily available,” said Ms. Gustin, who conducts epidemiologic research at Levine Cancer Center and is also a pre-med student and Japanese major at Wake Forest University in Winston-Salem, N.C.
In a study presented in a poster session at the annual Society of Surgical Oncology Cancer Symposium, Ms. Gustin and her coauthors looked at factors influencing readmission rates among patients undergoing surgery for primary, nonmetastatic colorectal cancer resections.
They drew on the to evaluate outcomes for 93,04 SEER-(Surveillance, Epidemiology, and End Results) Medicare database seven patients aged 66 years and older treated for primary colorectal cancer from 1998 through 2009.
They looked at potential contributing factors such as patient demographics, socioeconomic status, length of stay, days of admission and discharge, and discharge setting (home or clinical facility).
They use multivariate logistic regression models to analyze readmission rates at 14 and 30 days after initial discharge.
Focusing on home discharges, they found that as the week progressed, there was a significant likelihood that a patient discharged home would be readmitted (P less then .001 by chi-square and Cochran-Armitage tests). As noted before, the highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with 10.1% for patients discharged on Sunday, the discharge day least likely to be associated with rehospitalization.
In multivariate analysis, factors significantly associated with risk for 30-day readmission included male vs. female (hazard ratio, 1.16), black vs. other race (HR, 1.22), length of stay 5, 6-7, or 8-10 vs. 12 or more days (HR, 0.48, 0.59, 0.77, respectively), Charlson comorbidity index score 0, 1 or 3 vs. 3 (HR, 0.59, 0.73, 0.82, respectively), and home discharge vs. other (HR, 0.66; all above comparisons significant as shown by 95% confidence intervals).
The authors concluded that although home discharge itself reduces the likelihood of readmission, “improvements in preparing patients for discharge to home are needed. Additional outpatient interventions could rescue patients from readmission.”
They also suggested reexamining staffing policies and weekend availability of resources for patients, and call for addressing disparities in readmissions based on race, sex, length of stay, and comorbidities.
The study was internally supported. The authors reported having no relevant disclosures.
BOSTON – The day of the week a patient is discharged from the hospital may have an impact the likelihood of readmission.
Patients discharged home from the hospital on a Thursday after colorectal cancer surgery are more likely to be readmitted within 30 days than those discharged on any other day of the week, investigators found.
In contrast, there were no significant day-dependent differences in readmission rates among patients discharged to a skilled nursing facility or acute rehabilitation program, although patients admitted to clinical facilities had higher overall readmission rates, reported Anna Gustin and coinvestigators at the Levine Cancer Institute at the Carolinas Medical Center in Charlotte, N.C.
“For a patient discharged on a Thursday, if you’re going to get an infection, it’s going to be probably during the weekend, when it’s difficult to contact your primary physician, and when other resources are not as readily available,” said Ms. Gustin, who conducts epidemiologic research at Levine Cancer Center and is also a pre-med student and Japanese major at Wake Forest University in Winston-Salem, N.C.
In a study presented in a poster session at the annual Society of Surgical Oncology Cancer Symposium, Ms. Gustin and her coauthors looked at factors influencing readmission rates among patients undergoing surgery for primary, nonmetastatic colorectal cancer resections.
They drew on the to evaluate outcomes for 93,04 SEER-(Surveillance, Epidemiology, and End Results) Medicare database seven patients aged 66 years and older treated for primary colorectal cancer from 1998 through 2009.
They looked at potential contributing factors such as patient demographics, socioeconomic status, length of stay, days of admission and discharge, and discharge setting (home or clinical facility).
They use multivariate logistic regression models to analyze readmission rates at 14 and 30 days after initial discharge.
Focusing on home discharges, they found that as the week progressed, there was a significant likelihood that a patient discharged home would be readmitted (P less then .001 by chi-square and Cochran-Armitage tests). As noted before, the highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with 10.1% for patients discharged on Sunday, the discharge day least likely to be associated with rehospitalization.
In multivariate analysis, factors significantly associated with risk for 30-day readmission included male vs. female (hazard ratio, 1.16), black vs. other race (HR, 1.22), length of stay 5, 6-7, or 8-10 vs. 12 or more days (HR, 0.48, 0.59, 0.77, respectively), Charlson comorbidity index score 0, 1 or 3 vs. 3 (HR, 0.59, 0.73, 0.82, respectively), and home discharge vs. other (HR, 0.66; all above comparisons significant as shown by 95% confidence intervals).
The authors concluded that although home discharge itself reduces the likelihood of readmission, “improvements in preparing patients for discharge to home are needed. Additional outpatient interventions could rescue patients from readmission.”
They also suggested reexamining staffing policies and weekend availability of resources for patients, and call for addressing disparities in readmissions based on race, sex, length of stay, and comorbidities.
The study was internally supported. The authors reported having no relevant disclosures.
Key clinical point: Patients discharged home on a Thursday following surgery for primary colorectal cancer are more likely to be readmitted with 30 days than are patients discharged home on any other day of the week.
Major finding: The highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with lowest rate of 10.1% for patients discharged on Sunday.
Data source: Retrospective SEER-Medicare database review of records on 93,047 patients treated for colorectal cancer.
Disclosures: The study was internally supported. The authors reported having no relevant disclosures.
VIDEO: Dr. Ann Partridge discusses counseling young breast cancer patients
MIAMI BEACH – Despite significant improvements in detection and treatment of contralateral breast cancer, there’s a “huge increase” in the number of women choosing to undergo bilateral mastectomy, Dr. Ann Partridge of Dana-Farber Cancer Institute in Boston said.
Physicians can counsel patients that the risk of cancer recurrence in the body elsewhere is more of a concern than a new breast cancer, Dr. Partridge said, and provide a realistic picture of the side effects and potential complications of bilateral versus unilateral surgery. Conversations between physicians and patients regarding the pros and cons of more aggressive therapy are essential, she said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
Some ethical considerations arise when counseling younger women with a genetic mutation that raises the risk of breast cancer (for example, BRCA1 or BRCA2), especially when they plan to undergo in vitro fertilization and pre-implantation embryo analysis. Dr. Partridge shares advice on how to help these women make the best decision for them.
Many women diagnosed with breast cancer before age 40 wonder if it’s safe to have a baby, Dr. Partridge said. Ask about intentions to get pregnant at the first visit, she advised, and share data from retrospective outcome comparisons when guiding these women on their options.
Dr. Partridge had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MIAMI BEACH – Despite significant improvements in detection and treatment of contralateral breast cancer, there’s a “huge increase” in the number of women choosing to undergo bilateral mastectomy, Dr. Ann Partridge of Dana-Farber Cancer Institute in Boston said.
Physicians can counsel patients that the risk of cancer recurrence in the body elsewhere is more of a concern than a new breast cancer, Dr. Partridge said, and provide a realistic picture of the side effects and potential complications of bilateral versus unilateral surgery. Conversations between physicians and patients regarding the pros and cons of more aggressive therapy are essential, she said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
Some ethical considerations arise when counseling younger women with a genetic mutation that raises the risk of breast cancer (for example, BRCA1 or BRCA2), especially when they plan to undergo in vitro fertilization and pre-implantation embryo analysis. Dr. Partridge shares advice on how to help these women make the best decision for them.
Many women diagnosed with breast cancer before age 40 wonder if it’s safe to have a baby, Dr. Partridge said. Ask about intentions to get pregnant at the first visit, she advised, and share data from retrospective outcome comparisons when guiding these women on their options.
Dr. Partridge had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MIAMI BEACH – Despite significant improvements in detection and treatment of contralateral breast cancer, there’s a “huge increase” in the number of women choosing to undergo bilateral mastectomy, Dr. Ann Partridge of Dana-Farber Cancer Institute in Boston said.
Physicians can counsel patients that the risk of cancer recurrence in the body elsewhere is more of a concern than a new breast cancer, Dr. Partridge said, and provide a realistic picture of the side effects and potential complications of bilateral versus unilateral surgery. Conversations between physicians and patients regarding the pros and cons of more aggressive therapy are essential, she said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
Some ethical considerations arise when counseling younger women with a genetic mutation that raises the risk of breast cancer (for example, BRCA1 or BRCA2), especially when they plan to undergo in vitro fertilization and pre-implantation embryo analysis. Dr. Partridge shares advice on how to help these women make the best decision for them.
Many women diagnosed with breast cancer before age 40 wonder if it’s safe to have a baby, Dr. Partridge said. Ask about intentions to get pregnant at the first visit, she advised, and share data from retrospective outcome comparisons when guiding these women on their options.
Dr. Partridge had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM MBCC
VIDEO: Physicians must counsel women on mastectomy misperceptions
MIAMI BEACH – Although many women with breast cancer who choose a mastectomy believe they will lower their risk for recurrence, compared with breast conservation therapy, physicians should counsel them about this misperception for most instances, Dr. Mike Dixon said in a video interview at the annual Miami Breast Cancer Conference.
Multiple factors suggest that the risk of cancer recurrence with breast conservation therapy have declined over time. When combined with advances in imaging and gains in systemic therapy and radiation therapy, offering women with early breast cancer a choice between mastectomy and breast conservation may no longer make sense, said Dr. Dixon, professor of surgery at the University of Edinburgh.
More favorable patient outcomes and lower overall costs also favor breast conservation therapy over mastectomy for most women, he explained.
The conference was held by Physicians’ Education Resource. Dr. Dixon has no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MIAMI BEACH – Although many women with breast cancer who choose a mastectomy believe they will lower their risk for recurrence, compared with breast conservation therapy, physicians should counsel them about this misperception for most instances, Dr. Mike Dixon said in a video interview at the annual Miami Breast Cancer Conference.
Multiple factors suggest that the risk of cancer recurrence with breast conservation therapy have declined over time. When combined with advances in imaging and gains in systemic therapy and radiation therapy, offering women with early breast cancer a choice between mastectomy and breast conservation may no longer make sense, said Dr. Dixon, professor of surgery at the University of Edinburgh.
More favorable patient outcomes and lower overall costs also favor breast conservation therapy over mastectomy for most women, he explained.
The conference was held by Physicians’ Education Resource. Dr. Dixon has no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MIAMI BEACH – Although many women with breast cancer who choose a mastectomy believe they will lower their risk for recurrence, compared with breast conservation therapy, physicians should counsel them about this misperception for most instances, Dr. Mike Dixon said in a video interview at the annual Miami Breast Cancer Conference.
Multiple factors suggest that the risk of cancer recurrence with breast conservation therapy have declined over time. When combined with advances in imaging and gains in systemic therapy and radiation therapy, offering women with early breast cancer a choice between mastectomy and breast conservation may no longer make sense, said Dr. Dixon, professor of surgery at the University of Edinburgh.
More favorable patient outcomes and lower overall costs also favor breast conservation therapy over mastectomy for most women, he explained.
The conference was held by Physicians’ Education Resource. Dr. Dixon has no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM MBCC
Neoadjuvant chemo reduces extent of axillary dissection in some breast cancers
BOSTON – The majority of patients with hormone receptor–negative breast cancer in the United States get chemotherapy in the adjuvant setting, but neoadjuvant chemotherapy is gaining in use, and is associated with a higher likelihood of breast-conserving surgery for some women with advanced disease, as well as less extensive axillary node dissection.
A review of data on more than 130,000 patients with breast tumors negative for both estrogen and progesterone receptors (ER–/PR–) showed that among patients with clinical stage T3 disease, 26% of those who had received neoadjuvant chemotherapy were able to have breast-conserving surgery, compared with 20% of patients who had received adjuvant chemotherapy, reported Dr. Carlos A. Puig and his colleagues from the Mayo Clinic in Rochester, Minn.
“Patients treated with neoadjuvant chemotherapy have less extensive axillary surgery and a lower rate of nodal positivity,” he added at the annual Society of Surgical Oncology Cancer Symposium.
The data showed that nearly a third of all patients with clinically node-positive disease (cN1-3) who received neoadjuvant chemotherapy had pathologically node-negative disease at the end of therapy.
The aggressive biology of ER–/PR– tumors makes them suitable targets for chemotherapy in the neoadjuvant setting, Dr. Puig said. Overall survival among patients with ER–/PR– who receive neoadjuvant chemotherapy is comparable to that of patients who receive chemotherapy in the adjuvant setting. Neoadjuvant regimens can also downstage tumors before surgery, and a pathologic complete response to chemotherapy delivered prior to surgery is prognostic for outcomes.
Trends in chemotherapy
Dr. Puig and his colleagues combed through the National Cancer Data Base, looking for trends in national practice patterns of use of neoadjuvant chemotherapy in ER–/PR– breast cancer from 2004 through 2012.
They identified a total of 108,128 patients with invasive ER–/PR– breast cancer who received adjuvant chemotherapy, and 24,848 who received neoadjuvant chemo; an additional 43,969 patients who did not receive chemotherapy were excluded from the analysis.
Factors significantly associated with the choice to administer neoadjuvant chemotherapy included age younger than 50, no comorbidities vs. one or two comorbidities on the Charlson/Deyo index, academic/research center vs. community cancer program, higher clinical T stage, and higher clinical N stage.
There was a gradual increase in the use of neoadjuvant chemotherapy over time, from 14.2% in 2004 to 22.3% of all patients in 2012 (P less than .001).
The overall breast-conserving surgery rates were lower among patients who had chemotherapy in the neoadjuvant setting – 33.2% vs. 54.7% (P less than .001). The rates of conservative surgery increased over time, from 32% in 2004 to 36% in 2012 (P less than .001), but decreased over the same period in patients who had adjuvant chemotherapy, from 58% to 51%, respectively.
In a breakdown of surgery type by clinical T stage, mastectomy was more frequently performed in patients who had undergone neoadjuvant treatment, compared with adjuvant therapy, except for stage T3 disease. A higher percentage of those with T3 tumors who had neoadjuvant therapy had breast-conserving surgery in comparison with women who had T3 tumors and adjuvant therapy (26.2% vs. 20.2%, respectively; P less than .001).
To identify the extent of axillary surgery, the authors considered one to five nodes removed to be a surrogate for sentinel lymph node biopsy, and six or more nodes as a surrogate for axillary lymph node dissection.
They found that among patients with clinical stage N1 through N3 who received neoadjuvant chemotherapy, 31.8% converted to pathologically node-negative status.
Dr. Puig noted that the study was limited by the retrospective design and the lack of data on HER2 receptor status, making it impossible to distinguish between ER–/PR– and triple-negative tumors. In addition, they did not have data on genetic testing that could affect surgical choices, such as the presence of BRCA1 or BRCA2.
Following Dr. Puig’s presentation, Dr. Monica Morrow, chief of breast surgery at Memorial Sloan-Kettering Cancer Center, New York, commented that the study put together “patients in whom we would consider there to be an absolute indication for neoadjuvant chemotherapy – meaning T4, N2-N3 – and patients where it’s optional.” The study results suggested that not all patients who should receive neoadjuvant therapy were getting it “which is a little disturbing,” Dr. Morrow said.
She also noted that the decrease in the use of axillary lymph node dissection began prior to publication of studies suggesting that it might be safe to do so.
Dr. Puig and Dr. Morrow had no relevant disclosures.
BOSTON – The majority of patients with hormone receptor–negative breast cancer in the United States get chemotherapy in the adjuvant setting, but neoadjuvant chemotherapy is gaining in use, and is associated with a higher likelihood of breast-conserving surgery for some women with advanced disease, as well as less extensive axillary node dissection.
A review of data on more than 130,000 patients with breast tumors negative for both estrogen and progesterone receptors (ER–/PR–) showed that among patients with clinical stage T3 disease, 26% of those who had received neoadjuvant chemotherapy were able to have breast-conserving surgery, compared with 20% of patients who had received adjuvant chemotherapy, reported Dr. Carlos A. Puig and his colleagues from the Mayo Clinic in Rochester, Minn.
“Patients treated with neoadjuvant chemotherapy have less extensive axillary surgery and a lower rate of nodal positivity,” he added at the annual Society of Surgical Oncology Cancer Symposium.
The data showed that nearly a third of all patients with clinically node-positive disease (cN1-3) who received neoadjuvant chemotherapy had pathologically node-negative disease at the end of therapy.
The aggressive biology of ER–/PR– tumors makes them suitable targets for chemotherapy in the neoadjuvant setting, Dr. Puig said. Overall survival among patients with ER–/PR– who receive neoadjuvant chemotherapy is comparable to that of patients who receive chemotherapy in the adjuvant setting. Neoadjuvant regimens can also downstage tumors before surgery, and a pathologic complete response to chemotherapy delivered prior to surgery is prognostic for outcomes.
Trends in chemotherapy
Dr. Puig and his colleagues combed through the National Cancer Data Base, looking for trends in national practice patterns of use of neoadjuvant chemotherapy in ER–/PR– breast cancer from 2004 through 2012.
They identified a total of 108,128 patients with invasive ER–/PR– breast cancer who received adjuvant chemotherapy, and 24,848 who received neoadjuvant chemo; an additional 43,969 patients who did not receive chemotherapy were excluded from the analysis.
Factors significantly associated with the choice to administer neoadjuvant chemotherapy included age younger than 50, no comorbidities vs. one or two comorbidities on the Charlson/Deyo index, academic/research center vs. community cancer program, higher clinical T stage, and higher clinical N stage.
There was a gradual increase in the use of neoadjuvant chemotherapy over time, from 14.2% in 2004 to 22.3% of all patients in 2012 (P less than .001).
The overall breast-conserving surgery rates were lower among patients who had chemotherapy in the neoadjuvant setting – 33.2% vs. 54.7% (P less than .001). The rates of conservative surgery increased over time, from 32% in 2004 to 36% in 2012 (P less than .001), but decreased over the same period in patients who had adjuvant chemotherapy, from 58% to 51%, respectively.
In a breakdown of surgery type by clinical T stage, mastectomy was more frequently performed in patients who had undergone neoadjuvant treatment, compared with adjuvant therapy, except for stage T3 disease. A higher percentage of those with T3 tumors who had neoadjuvant therapy had breast-conserving surgery in comparison with women who had T3 tumors and adjuvant therapy (26.2% vs. 20.2%, respectively; P less than .001).
To identify the extent of axillary surgery, the authors considered one to five nodes removed to be a surrogate for sentinel lymph node biopsy, and six or more nodes as a surrogate for axillary lymph node dissection.
They found that among patients with clinical stage N1 through N3 who received neoadjuvant chemotherapy, 31.8% converted to pathologically node-negative status.
Dr. Puig noted that the study was limited by the retrospective design and the lack of data on HER2 receptor status, making it impossible to distinguish between ER–/PR– and triple-negative tumors. In addition, they did not have data on genetic testing that could affect surgical choices, such as the presence of BRCA1 or BRCA2.
Following Dr. Puig’s presentation, Dr. Monica Morrow, chief of breast surgery at Memorial Sloan-Kettering Cancer Center, New York, commented that the study put together “patients in whom we would consider there to be an absolute indication for neoadjuvant chemotherapy – meaning T4, N2-N3 – and patients where it’s optional.” The study results suggested that not all patients who should receive neoadjuvant therapy were getting it “which is a little disturbing,” Dr. Morrow said.
She also noted that the decrease in the use of axillary lymph node dissection began prior to publication of studies suggesting that it might be safe to do so.
Dr. Puig and Dr. Morrow had no relevant disclosures.
BOSTON – The majority of patients with hormone receptor–negative breast cancer in the United States get chemotherapy in the adjuvant setting, but neoadjuvant chemotherapy is gaining in use, and is associated with a higher likelihood of breast-conserving surgery for some women with advanced disease, as well as less extensive axillary node dissection.
A review of data on more than 130,000 patients with breast tumors negative for both estrogen and progesterone receptors (ER–/PR–) showed that among patients with clinical stage T3 disease, 26% of those who had received neoadjuvant chemotherapy were able to have breast-conserving surgery, compared with 20% of patients who had received adjuvant chemotherapy, reported Dr. Carlos A. Puig and his colleagues from the Mayo Clinic in Rochester, Minn.
“Patients treated with neoadjuvant chemotherapy have less extensive axillary surgery and a lower rate of nodal positivity,” he added at the annual Society of Surgical Oncology Cancer Symposium.
The data showed that nearly a third of all patients with clinically node-positive disease (cN1-3) who received neoadjuvant chemotherapy had pathologically node-negative disease at the end of therapy.
The aggressive biology of ER–/PR– tumors makes them suitable targets for chemotherapy in the neoadjuvant setting, Dr. Puig said. Overall survival among patients with ER–/PR– who receive neoadjuvant chemotherapy is comparable to that of patients who receive chemotherapy in the adjuvant setting. Neoadjuvant regimens can also downstage tumors before surgery, and a pathologic complete response to chemotherapy delivered prior to surgery is prognostic for outcomes.
Trends in chemotherapy
Dr. Puig and his colleagues combed through the National Cancer Data Base, looking for trends in national practice patterns of use of neoadjuvant chemotherapy in ER–/PR– breast cancer from 2004 through 2012.
They identified a total of 108,128 patients with invasive ER–/PR– breast cancer who received adjuvant chemotherapy, and 24,848 who received neoadjuvant chemo; an additional 43,969 patients who did not receive chemotherapy were excluded from the analysis.
Factors significantly associated with the choice to administer neoadjuvant chemotherapy included age younger than 50, no comorbidities vs. one or two comorbidities on the Charlson/Deyo index, academic/research center vs. community cancer program, higher clinical T stage, and higher clinical N stage.
There was a gradual increase in the use of neoadjuvant chemotherapy over time, from 14.2% in 2004 to 22.3% of all patients in 2012 (P less than .001).
The overall breast-conserving surgery rates were lower among patients who had chemotherapy in the neoadjuvant setting – 33.2% vs. 54.7% (P less than .001). The rates of conservative surgery increased over time, from 32% in 2004 to 36% in 2012 (P less than .001), but decreased over the same period in patients who had adjuvant chemotherapy, from 58% to 51%, respectively.
In a breakdown of surgery type by clinical T stage, mastectomy was more frequently performed in patients who had undergone neoadjuvant treatment, compared with adjuvant therapy, except for stage T3 disease. A higher percentage of those with T3 tumors who had neoadjuvant therapy had breast-conserving surgery in comparison with women who had T3 tumors and adjuvant therapy (26.2% vs. 20.2%, respectively; P less than .001).
To identify the extent of axillary surgery, the authors considered one to five nodes removed to be a surrogate for sentinel lymph node biopsy, and six or more nodes as a surrogate for axillary lymph node dissection.
They found that among patients with clinical stage N1 through N3 who received neoadjuvant chemotherapy, 31.8% converted to pathologically node-negative status.
Dr. Puig noted that the study was limited by the retrospective design and the lack of data on HER2 receptor status, making it impossible to distinguish between ER–/PR– and triple-negative tumors. In addition, they did not have data on genetic testing that could affect surgical choices, such as the presence of BRCA1 or BRCA2.
Following Dr. Puig’s presentation, Dr. Monica Morrow, chief of breast surgery at Memorial Sloan-Kettering Cancer Center, New York, commented that the study put together “patients in whom we would consider there to be an absolute indication for neoadjuvant chemotherapy – meaning T4, N2-N3 – and patients where it’s optional.” The study results suggested that not all patients who should receive neoadjuvant therapy were getting it “which is a little disturbing,” Dr. Morrow said.
She also noted that the decrease in the use of axillary lymph node dissection began prior to publication of studies suggesting that it might be safe to do so.
Dr. Puig and Dr. Morrow had no relevant disclosures.
Key clinical point: Neoadjuvant chemotherapy can benefit some patients with advanced hormone receptor–negative breast cancer.
Major finding: Among patients with cT3 disease, 26% who had neoadjuvant chemotherapy were able to have breast-conserving surgery, compared with 20% of patients who had received adjuvant chemotherapy.
Data source: Retrospective review of data on 132,976 patients with invasive ER–/PR– breast cancer.
Disclosures: Dr. Puig and Dr. Morrow had no relevant disclosures.
Hold rectal surgery decision until neoadjuvant chemo is done
BOSTON – Good results come to those who wait, suggest the findings of a study of optimal timing of surgical decisions in patients with advanced stage cancers in the distal rectum.
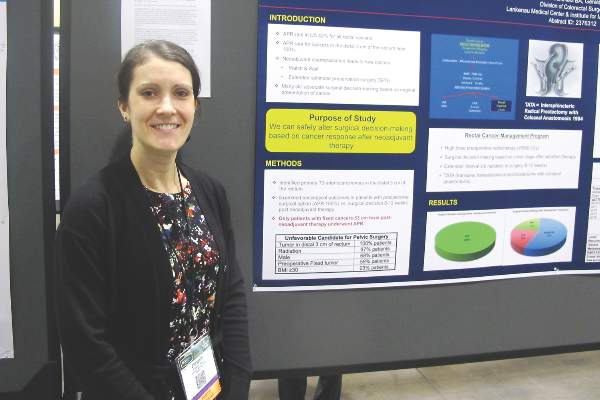
When surgeons waited 8 to 12 weeks after the completion of neoadjuvant chemoradiation to decide whether to proceed with radical sphincter preservation surgery (SPS) or abdominoperineal resection (APR) in patients with T3 cancers of the distal third of the rectum, they were able to avoid creating a colostomy with no adverse oncologic outcomes in 79% of patients, reported Dr. Elizabeth A. Myers and her colleagues from the Lankenau Medical Center and Institute for Medical Research in Wynnewood, Pennsylvania.
“An interesting thing, but still quite controversial, is the timing of when you do surgery,” Dr. Myers said in an interview during a poster session at the annual Society of Surgical Oncology Cancer Symposium.
“There is a large school of thought that still believes you should base your surgical plan on the tumor at its presentation, versus making the decision once the patient has undergone neoadjuvant chemoradiation treatment. The purpose of our study is to show with our data that you can safely alter your decision making based on what the cancer presents as following neoadjuvant chemoradiation,” she said.
The investigators looked at 192 consecutive patients with T3 cancers of the distal third of the rectum who were included in a prospectively maintained database. At the time of presentation, all of the patients would have met criteria for requiring APR and colostomy, due to unfavorable factors for pelvic surgery such as prior radiation (97%), male sex (68%), preoperative fixed tumor (59%), or obesity (23%).
The patients underwent neoadjuvant radiation given at a mean of dose of 5,580 cGy, consisting of a 4,500 cGy standard dose and a 1,080 cGy boost to the area of tumor. Most (87%) also received concurrent 5-fluorauracil-based chemotherapy.
“We have found that this helps to downgrade the tumor quite well,” Dr. Myers said.
Following the completion of therapy, they waited for 8 to 12 additional weeks before planning surgery to allow for the maximum benefit of radiation.
All patients, except those who at the end of neoadjuvant chemotherapy still had a fixed cancer at the 3 cm level or below, were offered SPS. The mean time from completion of chemotherapy to surgery was 11 weeks.
Of the 192 patients, 41 underwent APR, 109 had radical SPS, including 107 receiving transanal transabdominal proctocolectomy with coloanal anastomosis (TATA), and 2 receiving low anterior resection. The remaining patients had local excision with either a transanal technique (TAE; 15 patients) or transanal endoscopic microsurgery (TEM, 27 patients).
After a mean follow-up of 55.4 months (range, 1-242 months) the 5-year stoma-free survival rate was 79%. Kaplan-Meier 5-year actuarial survival rates were 98% for all patients who underwent radical SPS, 100% for those who had local excision with TEM, 82% for those who had local excision with TAE (combined SPS procedures, 95%), and 72% for patients who underwent APR.
Local recurrences occurred in 6.6% of all patients who underwent SPS, compared with 7.3% of those who underwent APR. Distant metastases occurred in 22.5% and 24.4%, respectively.
“Holding surgical decision-making until after completion of neoadjuvant therapy allows for increased sphincter preservation with good oncologic outcomes in rectal cancer patients,” the investigators concluded.
BOSTON – Good results come to those who wait, suggest the findings of a study of optimal timing of surgical decisions in patients with advanced stage cancers in the distal rectum.
When surgeons waited 8 to 12 weeks after the completion of neoadjuvant chemoradiation to decide whether to proceed with radical sphincter preservation surgery (SPS) or abdominoperineal resection (APR) in patients with T3 cancers of the distal third of the rectum, they were able to avoid creating a colostomy with no adverse oncologic outcomes in 79% of patients, reported Dr. Elizabeth A. Myers and her colleagues from the Lankenau Medical Center and Institute for Medical Research in Wynnewood, Pennsylvania.
“An interesting thing, but still quite controversial, is the timing of when you do surgery,” Dr. Myers said in an interview during a poster session at the annual Society of Surgical Oncology Cancer Symposium.
“There is a large school of thought that still believes you should base your surgical plan on the tumor at its presentation, versus making the decision once the patient has undergone neoadjuvant chemoradiation treatment. The purpose of our study is to show with our data that you can safely alter your decision making based on what the cancer presents as following neoadjuvant chemoradiation,” she said.
The investigators looked at 192 consecutive patients with T3 cancers of the distal third of the rectum who were included in a prospectively maintained database. At the time of presentation, all of the patients would have met criteria for requiring APR and colostomy, due to unfavorable factors for pelvic surgery such as prior radiation (97%), male sex (68%), preoperative fixed tumor (59%), or obesity (23%).
The patients underwent neoadjuvant radiation given at a mean of dose of 5,580 cGy, consisting of a 4,500 cGy standard dose and a 1,080 cGy boost to the area of tumor. Most (87%) also received concurrent 5-fluorauracil-based chemotherapy.
“We have found that this helps to downgrade the tumor quite well,” Dr. Myers said.
Following the completion of therapy, they waited for 8 to 12 additional weeks before planning surgery to allow for the maximum benefit of radiation.
All patients, except those who at the end of neoadjuvant chemotherapy still had a fixed cancer at the 3 cm level or below, were offered SPS. The mean time from completion of chemotherapy to surgery was 11 weeks.
Of the 192 patients, 41 underwent APR, 109 had radical SPS, including 107 receiving transanal transabdominal proctocolectomy with coloanal anastomosis (TATA), and 2 receiving low anterior resection. The remaining patients had local excision with either a transanal technique (TAE; 15 patients) or transanal endoscopic microsurgery (TEM, 27 patients).
After a mean follow-up of 55.4 months (range, 1-242 months) the 5-year stoma-free survival rate was 79%. Kaplan-Meier 5-year actuarial survival rates were 98% for all patients who underwent radical SPS, 100% for those who had local excision with TEM, 82% for those who had local excision with TAE (combined SPS procedures, 95%), and 72% for patients who underwent APR.
Local recurrences occurred in 6.6% of all patients who underwent SPS, compared with 7.3% of those who underwent APR. Distant metastases occurred in 22.5% and 24.4%, respectively.
“Holding surgical decision-making until after completion of neoadjuvant therapy allows for increased sphincter preservation with good oncologic outcomes in rectal cancer patients,” the investigators concluded.
BOSTON – Good results come to those who wait, suggest the findings of a study of optimal timing of surgical decisions in patients with advanced stage cancers in the distal rectum.
When surgeons waited 8 to 12 weeks after the completion of neoadjuvant chemoradiation to decide whether to proceed with radical sphincter preservation surgery (SPS) or abdominoperineal resection (APR) in patients with T3 cancers of the distal third of the rectum, they were able to avoid creating a colostomy with no adverse oncologic outcomes in 79% of patients, reported Dr. Elizabeth A. Myers and her colleagues from the Lankenau Medical Center and Institute for Medical Research in Wynnewood, Pennsylvania.
“An interesting thing, but still quite controversial, is the timing of when you do surgery,” Dr. Myers said in an interview during a poster session at the annual Society of Surgical Oncology Cancer Symposium.
“There is a large school of thought that still believes you should base your surgical plan on the tumor at its presentation, versus making the decision once the patient has undergone neoadjuvant chemoradiation treatment. The purpose of our study is to show with our data that you can safely alter your decision making based on what the cancer presents as following neoadjuvant chemoradiation,” she said.
The investigators looked at 192 consecutive patients with T3 cancers of the distal third of the rectum who were included in a prospectively maintained database. At the time of presentation, all of the patients would have met criteria for requiring APR and colostomy, due to unfavorable factors for pelvic surgery such as prior radiation (97%), male sex (68%), preoperative fixed tumor (59%), or obesity (23%).
The patients underwent neoadjuvant radiation given at a mean of dose of 5,580 cGy, consisting of a 4,500 cGy standard dose and a 1,080 cGy boost to the area of tumor. Most (87%) also received concurrent 5-fluorauracil-based chemotherapy.
“We have found that this helps to downgrade the tumor quite well,” Dr. Myers said.
Following the completion of therapy, they waited for 8 to 12 additional weeks before planning surgery to allow for the maximum benefit of radiation.
All patients, except those who at the end of neoadjuvant chemotherapy still had a fixed cancer at the 3 cm level or below, were offered SPS. The mean time from completion of chemotherapy to surgery was 11 weeks.
Of the 192 patients, 41 underwent APR, 109 had radical SPS, including 107 receiving transanal transabdominal proctocolectomy with coloanal anastomosis (TATA), and 2 receiving low anterior resection. The remaining patients had local excision with either a transanal technique (TAE; 15 patients) or transanal endoscopic microsurgery (TEM, 27 patients).
After a mean follow-up of 55.4 months (range, 1-242 months) the 5-year stoma-free survival rate was 79%. Kaplan-Meier 5-year actuarial survival rates were 98% for all patients who underwent radical SPS, 100% for those who had local excision with TEM, 82% for those who had local excision with TAE (combined SPS procedures, 95%), and 72% for patients who underwent APR.
Local recurrences occurred in 6.6% of all patients who underwent SPS, compared with 7.3% of those who underwent APR. Distant metastases occurred in 22.5% and 24.4%, respectively.
“Holding surgical decision-making until after completion of neoadjuvant therapy allows for increased sphincter preservation with good oncologic outcomes in rectal cancer patients,” the investigators concluded.
FROM SSO 2016
Key clinical point: Waiting 8-12 weeks following neoadjuvant chemoradiation in patients with T3 distal rectal cancers improves chances for sphincter preservation.
Major finding: The 5-year stoma-free survival rate was 79% in patients initially considered candidates for APR and colostomy.
Data source: Retrospective review of 192 patients in a prospectively maintained database.
Disclosures: The study was internally supported. Dr. Myers reported having no conflicts of interest.
Elective CRC resections increase with universal insurance
BOSTON – Expanding access to health insurance for low- and moderate-income families has apparently improved colorectal cancer care in Massachusetts, and may do the same for other states that participate in Medicaid expansion under the Affordable Care Act.
That assertion comes from investigators at Massachusetts General Hospital in Boston. They found that following the introduction in 2006 of a universal health insurance law in the Bay State – the law that would serve as a model for the Affordable Care Act – the rate of elective colorectal resections increased while the rate of emergent resections decreased.
In contrast, in three states used as controls, the opposite occurred.
“This could be due to a variety of different factors, including earlier diagnosis, presenting with disease more amenable to surgical resection. It could also be due to increased referrals from primary care providers or GI doctors,” said Dr. Andrew P. Loehrer from the Massachusetts General Hospital Department of Surgery, at the annual Society of Surgical Oncology Cancer Symposium.
He acknowledged, however, that the administrative dataset he and his colleagues used in the study lacks information about clinical staging or use of neoadjuvant therapy, making it difficult to determine whether insured patients actually present at an earlier, more readily treatable disease stage.
Nonetheless, “from a cancer standpoint, my study provides early, hopeful evidence. In order to definitively say that this improves care, we need to have some more of the cancer-specific variables, but with this study, combined with some other work that we and other groups have done, we see that patients in Massachusetts are presenting with earlier stage disease, whether it’s acute disease or cancer, and they’re getting more appropriate care in a more timely fashion,” he said in an interview.
Role model
Dr. Loehrer noted that disparities in access to health care have been shown in previous studies to be associated with the likelihood of unfavorable outcomes for patients with colorectal cancer. For example, a 2008 study (Lancet Oncol. 2008 Mar;9:222-31) showed that uninsured patients with colorectal cancer had a twofold greater risk for presenting with advanced disease than privately insured patients. Additionally, a 2004 study (Br J Surg. 91:605-9) showed that patients who presented with colorectal cancer requiring emergent resection had significantly lower 5-year overall survival than patients who underwent elective resection.
Massachusetts implemented its pioneering health insurance reform law in 2006. The law increased eligibility for persons with incomes up to 150% of the Federal Poverty Level, created government-subsidized insurance for those with incomes from 150% to 300% of the poverty line, mandated that all Bay State residents have some form of health insurance, and allowed young adults up to the age of 26 to remain on their parents’ plans.
To see whether insurance reform could have a salutary effect on cancer care, the investigators drew on Agency for Health Research and Quality (AHRQ) State Inpatient Databases for Massachusetts and for Florida, New Jersey, and New York as control states. They used ICD-9 diagnosis codes to identify patients with colorectal cancer, including those who underwent resection.
To establish procedure rates, they used U.S. Census Bureau data to establish the population of denominators, which included all adults 18-54 years of age who were insured either through Medicaid, Commonwealth Care (in Massachusetts), or were listed as uninsured or self-pay. Medicare-insured patients were not included, as they were not directly affected by the reform law.
They identified 18,598 patients admitted to Massachusetts hospitals for colorectal cancer from 2001 through 2011, and 147,482 admitted during the same period to hospitals in the control states.
The authors created Poisson difference-in-differences models which compare changes in the selected outcomes in Massachusetts with changes in the control states. The models were adjusted for age, sex, race, hospital type, and secular trends.
They found that admission rates for colorectal cancer increased over time in Massachusetts by 13.3 per 100,000 residents per quarter, compared with 8.3/100,000 in the control states, translating into an adjusted rate ratio (ARR) of 1.13. Resection rates for cancer, the primary study outcome, also grew by a significantly larger margin in Massachusetts, by 5.5/100,000, compared with 0.5/100,000 in control states, with an ARR of 1.37 (P less than .001 for both comparisons).
For the secondary outcome of changes in emergent and elective resections after admission, they found that emergent surgeries in Massachusetts declined by 2.7/100,000, but increased by 4.4/100,000 in the states without insurance reform. Similarly, elective resections after admission increased in the Bay State by 7.4/100,000, but decreased by 1.8/100,000 in control states.
Relative to controls, the adjusted probability that a patient with colorectal cancer in Massachusetts would have emergent surgery after admission declined by 6.1% (P = .014) and the probability that he or she would have elective resection increased by 7.8% (P = .005).
An analysis of the odds ratio of resection during admission, adjusted for age, race, presentation with metastatic disease, hospital type, and secular trends, showed that prior to reform uninsured patients in both Massachusetts and control states were significantly less likely than privately insured patients to have resections (odds ratio, 0.42 in Mass.; 0.45 in control states).
However, after the implementation of reform the gap between previously uninsured and privately insured in Massachusetts narrowed (OR, 0.63) but remained the same in control states (OR, 0.44).
Dr. Loehrer acknowledged in an interview that Massachusetts differs from other states in some regards, including in concentrations of health providers and in requirements for insurance coverage that were in place even before the 2006 reforms, but is optimistic that improvements in colorectal cancer care can occur in states that have embraced the Affordable Care Act.
“There are a lot of services that were available and we had high colonoscopy rates prior to all of this, but that said, the mechanism is exactly the same, there are still vulnerable populations, and at this point I think it’s hopeful and promising that we will see similar results in other states,” he said.
BOSTON – Expanding access to health insurance for low- and moderate-income families has apparently improved colorectal cancer care in Massachusetts, and may do the same for other states that participate in Medicaid expansion under the Affordable Care Act.
That assertion comes from investigators at Massachusetts General Hospital in Boston. They found that following the introduction in 2006 of a universal health insurance law in the Bay State – the law that would serve as a model for the Affordable Care Act – the rate of elective colorectal resections increased while the rate of emergent resections decreased.
In contrast, in three states used as controls, the opposite occurred.
“This could be due to a variety of different factors, including earlier diagnosis, presenting with disease more amenable to surgical resection. It could also be due to increased referrals from primary care providers or GI doctors,” said Dr. Andrew P. Loehrer from the Massachusetts General Hospital Department of Surgery, at the annual Society of Surgical Oncology Cancer Symposium.
He acknowledged, however, that the administrative dataset he and his colleagues used in the study lacks information about clinical staging or use of neoadjuvant therapy, making it difficult to determine whether insured patients actually present at an earlier, more readily treatable disease stage.
Nonetheless, “from a cancer standpoint, my study provides early, hopeful evidence. In order to definitively say that this improves care, we need to have some more of the cancer-specific variables, but with this study, combined with some other work that we and other groups have done, we see that patients in Massachusetts are presenting with earlier stage disease, whether it’s acute disease or cancer, and they’re getting more appropriate care in a more timely fashion,” he said in an interview.
Role model
Dr. Loehrer noted that disparities in access to health care have been shown in previous studies to be associated with the likelihood of unfavorable outcomes for patients with colorectal cancer. For example, a 2008 study (Lancet Oncol. 2008 Mar;9:222-31) showed that uninsured patients with colorectal cancer had a twofold greater risk for presenting with advanced disease than privately insured patients. Additionally, a 2004 study (Br J Surg. 91:605-9) showed that patients who presented with colorectal cancer requiring emergent resection had significantly lower 5-year overall survival than patients who underwent elective resection.
Massachusetts implemented its pioneering health insurance reform law in 2006. The law increased eligibility for persons with incomes up to 150% of the Federal Poverty Level, created government-subsidized insurance for those with incomes from 150% to 300% of the poverty line, mandated that all Bay State residents have some form of health insurance, and allowed young adults up to the age of 26 to remain on their parents’ plans.
To see whether insurance reform could have a salutary effect on cancer care, the investigators drew on Agency for Health Research and Quality (AHRQ) State Inpatient Databases for Massachusetts and for Florida, New Jersey, and New York as control states. They used ICD-9 diagnosis codes to identify patients with colorectal cancer, including those who underwent resection.
To establish procedure rates, they used U.S. Census Bureau data to establish the population of denominators, which included all adults 18-54 years of age who were insured either through Medicaid, Commonwealth Care (in Massachusetts), or were listed as uninsured or self-pay. Medicare-insured patients were not included, as they were not directly affected by the reform law.
They identified 18,598 patients admitted to Massachusetts hospitals for colorectal cancer from 2001 through 2011, and 147,482 admitted during the same period to hospitals in the control states.
The authors created Poisson difference-in-differences models which compare changes in the selected outcomes in Massachusetts with changes in the control states. The models were adjusted for age, sex, race, hospital type, and secular trends.
They found that admission rates for colorectal cancer increased over time in Massachusetts by 13.3 per 100,000 residents per quarter, compared with 8.3/100,000 in the control states, translating into an adjusted rate ratio (ARR) of 1.13. Resection rates for cancer, the primary study outcome, also grew by a significantly larger margin in Massachusetts, by 5.5/100,000, compared with 0.5/100,000 in control states, with an ARR of 1.37 (P less than .001 for both comparisons).
For the secondary outcome of changes in emergent and elective resections after admission, they found that emergent surgeries in Massachusetts declined by 2.7/100,000, but increased by 4.4/100,000 in the states without insurance reform. Similarly, elective resections after admission increased in the Bay State by 7.4/100,000, but decreased by 1.8/100,000 in control states.
Relative to controls, the adjusted probability that a patient with colorectal cancer in Massachusetts would have emergent surgery after admission declined by 6.1% (P = .014) and the probability that he or she would have elective resection increased by 7.8% (P = .005).
An analysis of the odds ratio of resection during admission, adjusted for age, race, presentation with metastatic disease, hospital type, and secular trends, showed that prior to reform uninsured patients in both Massachusetts and control states were significantly less likely than privately insured patients to have resections (odds ratio, 0.42 in Mass.; 0.45 in control states).
However, after the implementation of reform the gap between previously uninsured and privately insured in Massachusetts narrowed (OR, 0.63) but remained the same in control states (OR, 0.44).
Dr. Loehrer acknowledged in an interview that Massachusetts differs from other states in some regards, including in concentrations of health providers and in requirements for insurance coverage that were in place even before the 2006 reforms, but is optimistic that improvements in colorectal cancer care can occur in states that have embraced the Affordable Care Act.
“There are a lot of services that were available and we had high colonoscopy rates prior to all of this, but that said, the mechanism is exactly the same, there are still vulnerable populations, and at this point I think it’s hopeful and promising that we will see similar results in other states,” he said.
BOSTON – Expanding access to health insurance for low- and moderate-income families has apparently improved colorectal cancer care in Massachusetts, and may do the same for other states that participate in Medicaid expansion under the Affordable Care Act.
That assertion comes from investigators at Massachusetts General Hospital in Boston. They found that following the introduction in 2006 of a universal health insurance law in the Bay State – the law that would serve as a model for the Affordable Care Act – the rate of elective colorectal resections increased while the rate of emergent resections decreased.
In contrast, in three states used as controls, the opposite occurred.
“This could be due to a variety of different factors, including earlier diagnosis, presenting with disease more amenable to surgical resection. It could also be due to increased referrals from primary care providers or GI doctors,” said Dr. Andrew P. Loehrer from the Massachusetts General Hospital Department of Surgery, at the annual Society of Surgical Oncology Cancer Symposium.
He acknowledged, however, that the administrative dataset he and his colleagues used in the study lacks information about clinical staging or use of neoadjuvant therapy, making it difficult to determine whether insured patients actually present at an earlier, more readily treatable disease stage.
Nonetheless, “from a cancer standpoint, my study provides early, hopeful evidence. In order to definitively say that this improves care, we need to have some more of the cancer-specific variables, but with this study, combined with some other work that we and other groups have done, we see that patients in Massachusetts are presenting with earlier stage disease, whether it’s acute disease or cancer, and they’re getting more appropriate care in a more timely fashion,” he said in an interview.
Role model
Dr. Loehrer noted that disparities in access to health care have been shown in previous studies to be associated with the likelihood of unfavorable outcomes for patients with colorectal cancer. For example, a 2008 study (Lancet Oncol. 2008 Mar;9:222-31) showed that uninsured patients with colorectal cancer had a twofold greater risk for presenting with advanced disease than privately insured patients. Additionally, a 2004 study (Br J Surg. 91:605-9) showed that patients who presented with colorectal cancer requiring emergent resection had significantly lower 5-year overall survival than patients who underwent elective resection.
Massachusetts implemented its pioneering health insurance reform law in 2006. The law increased eligibility for persons with incomes up to 150% of the Federal Poverty Level, created government-subsidized insurance for those with incomes from 150% to 300% of the poverty line, mandated that all Bay State residents have some form of health insurance, and allowed young adults up to the age of 26 to remain on their parents’ plans.
To see whether insurance reform could have a salutary effect on cancer care, the investigators drew on Agency for Health Research and Quality (AHRQ) State Inpatient Databases for Massachusetts and for Florida, New Jersey, and New York as control states. They used ICD-9 diagnosis codes to identify patients with colorectal cancer, including those who underwent resection.
To establish procedure rates, they used U.S. Census Bureau data to establish the population of denominators, which included all adults 18-54 years of age who were insured either through Medicaid, Commonwealth Care (in Massachusetts), or were listed as uninsured or self-pay. Medicare-insured patients were not included, as they were not directly affected by the reform law.
They identified 18,598 patients admitted to Massachusetts hospitals for colorectal cancer from 2001 through 2011, and 147,482 admitted during the same period to hospitals in the control states.
The authors created Poisson difference-in-differences models which compare changes in the selected outcomes in Massachusetts with changes in the control states. The models were adjusted for age, sex, race, hospital type, and secular trends.
They found that admission rates for colorectal cancer increased over time in Massachusetts by 13.3 per 100,000 residents per quarter, compared with 8.3/100,000 in the control states, translating into an adjusted rate ratio (ARR) of 1.13. Resection rates for cancer, the primary study outcome, also grew by a significantly larger margin in Massachusetts, by 5.5/100,000, compared with 0.5/100,000 in control states, with an ARR of 1.37 (P less than .001 for both comparisons).
For the secondary outcome of changes in emergent and elective resections after admission, they found that emergent surgeries in Massachusetts declined by 2.7/100,000, but increased by 4.4/100,000 in the states without insurance reform. Similarly, elective resections after admission increased in the Bay State by 7.4/100,000, but decreased by 1.8/100,000 in control states.
Relative to controls, the adjusted probability that a patient with colorectal cancer in Massachusetts would have emergent surgery after admission declined by 6.1% (P = .014) and the probability that he or she would have elective resection increased by 7.8% (P = .005).
An analysis of the odds ratio of resection during admission, adjusted for age, race, presentation with metastatic disease, hospital type, and secular trends, showed that prior to reform uninsured patients in both Massachusetts and control states were significantly less likely than privately insured patients to have resections (odds ratio, 0.42 in Mass.; 0.45 in control states).
However, after the implementation of reform the gap between previously uninsured and privately insured in Massachusetts narrowed (OR, 0.63) but remained the same in control states (OR, 0.44).
Dr. Loehrer acknowledged in an interview that Massachusetts differs from other states in some regards, including in concentrations of health providers and in requirements for insurance coverage that were in place even before the 2006 reforms, but is optimistic that improvements in colorectal cancer care can occur in states that have embraced the Affordable Care Act.
“There are a lot of services that were available and we had high colonoscopy rates prior to all of this, but that said, the mechanism is exactly the same, there are still vulnerable populations, and at this point I think it’s hopeful and promising that we will see similar results in other states,” he said.
FROM SSO 2016
Key clinical point: Outcomes for patients with colorectal cancer (CRC) who undergo elective resection are better than for those who require emergent resections.
Major finding: Elective CRC resection rates increased and emergent resections decreased after universal insurance was instituted in Massachusetts in 2006.
Data source: Retrospective study comparing differences over time between CRC resection rates in Massachusetts vs. those in Florida, New Jersey, and New York.
Disclosures: The study was supported in part by a grant from the National Institute on Aging. Dr. Loehrer and his coauthors reported no conflicts of interest.
Ultrasound bested tomosynthesis for screening dense breast tissue
Ultrasound was about 1.8 times more sensitive than tomosynthesis for the incremental detection of breast cancer in women with radiologically dense breasts and negative two-dimensional mammography screening, according to interim results from the first prospective trial to directly compare the two modalities.
“However, future application of adjunct screening should consider that tomosynthesis detected more than 50% of the additional breast cancers in these women, and could potentially be [a] primary screening modality,” wrote Dr. Alberto Tagliafico of the University of Genoa (Italy) and his associates. The study was published online March 9 in the Journal of Clinical Oncology and presented simultaneously at the European Breast Cancer Conference.
Radiologically dense breast tissue undermines the sensitivity of mammography and is itself an independent risk factor for breast cancer. Recently, many states began requiring that women be informed of their breast density and adjunct screening measures, such as ultrasound. But estimates of the sensitivity of ultrasound have ranged from about 1.9 to 4.2 cancers for every 1,000 screens, said the researchers. This variance, combined with costs and concerns about false-positive recalls, have fueled debates about the value of adjunct measures in breast cancer screening, they added. To help clarify these issues, the multicenter ASTOUND (Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts) study compared physician-performed ultrasound and tomosynthesis results for 3,231 asymptomatic women aged 44 to 71 years, whose median age was 51 years (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.63.4147).
In all, the researchers detected 24 additional breast cancers, 23 of which were invasive. Thus, ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 screens for tomosynthesis (95% CI, 1.8-6.2; P = .006). Only one cancer was detected by tomosynthesis alone. The rate of false-positive recalls was similar for the two modalities – 53 cases for tomosynthesis, versus 63 for ultrasound (P = .26). Rates of false-positive recalls leading to biopsy also were similar. Needle biopsies usually sufficed in recalled cases, but two women underwent surgical biopsies, both of which revealed radial scars.
If the final results of ASTOUND confirm these interim data, “it could be argued that breast tomosynthesis has little value in a setting where adjunct ultrasound is frequently used for screening women with mammography-dense breasts,” said the researchers. But tomosynthesis may have a role as a primary screening modality in other setting, especially because tomosynthesis acquisitions that also provide reconstructed 2D mammography are now available, lessening concerns about unjustified radiation exposure, they added.
The “modest” number of cancers in the interim report led to relatively wide confidence intervals, the investigators noted. Biomarker data were not available for all cancers, and both prevalent and incident ultrasound data were compared with prevalent tomosynthesis data, which might bias false-positive recall results in favor of ultrasound, they added.
Currently, 24 American states have laws requiring that women receive some level of notification about breast density with their mammography results. Dense breast tissue can hide cancer on mammography, especially when the cancer lacks calcifications, resulting in delayed diagnosis and worse outcomes. Moreover, dense breast tissue is an independent risk factor for developing breast cancer.
Because the primary goal of screening is detection of early breast cancer, ultrasound would seem the clear choice, compared with tomosynthesis. Given comparable false-positive rates in ASTOUND, the estimated cost per cancer detected would be similar or more favorable for ultrasound than tomosynthesis. Ultrasound equipment is becoming much less expensive, requires no ionizing radiation, and it is easy to guide needle biopsy of lesions seen only on ultrasound.
Preliminary results from ASTOUND are extremely important in helping to inform personalized screening choices for women with dense breasts. Guidelines on these issues are planned, but often limit recommendations to those based on evidence from randomized trials with mortality as an end point. Our knowledge of the natural history of breast cancer and results from randomized trials of mammography should inform guidelines for supplemental screening.
Dr. Wendie A. Berg is at Magee-Womens Hospital of University of Pittsburgh Medical Center. She reported serving in a consulting or advisory role with SuperSonic Imagine. These comments were taken from her accompanying editorial (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.65.8674).
Currently, 24 American states have laws requiring that women receive some level of notification about breast density with their mammography results. Dense breast tissue can hide cancer on mammography, especially when the cancer lacks calcifications, resulting in delayed diagnosis and worse outcomes. Moreover, dense breast tissue is an independent risk factor for developing breast cancer.
Because the primary goal of screening is detection of early breast cancer, ultrasound would seem the clear choice, compared with tomosynthesis. Given comparable false-positive rates in ASTOUND, the estimated cost per cancer detected would be similar or more favorable for ultrasound than tomosynthesis. Ultrasound equipment is becoming much less expensive, requires no ionizing radiation, and it is easy to guide needle biopsy of lesions seen only on ultrasound.
Preliminary results from ASTOUND are extremely important in helping to inform personalized screening choices for women with dense breasts. Guidelines on these issues are planned, but often limit recommendations to those based on evidence from randomized trials with mortality as an end point. Our knowledge of the natural history of breast cancer and results from randomized trials of mammography should inform guidelines for supplemental screening.
Dr. Wendie A. Berg is at Magee-Womens Hospital of University of Pittsburgh Medical Center. She reported serving in a consulting or advisory role with SuperSonic Imagine. These comments were taken from her accompanying editorial (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.65.8674).
Currently, 24 American states have laws requiring that women receive some level of notification about breast density with their mammography results. Dense breast tissue can hide cancer on mammography, especially when the cancer lacks calcifications, resulting in delayed diagnosis and worse outcomes. Moreover, dense breast tissue is an independent risk factor for developing breast cancer.
Because the primary goal of screening is detection of early breast cancer, ultrasound would seem the clear choice, compared with tomosynthesis. Given comparable false-positive rates in ASTOUND, the estimated cost per cancer detected would be similar or more favorable for ultrasound than tomosynthesis. Ultrasound equipment is becoming much less expensive, requires no ionizing radiation, and it is easy to guide needle biopsy of lesions seen only on ultrasound.
Preliminary results from ASTOUND are extremely important in helping to inform personalized screening choices for women with dense breasts. Guidelines on these issues are planned, but often limit recommendations to those based on evidence from randomized trials with mortality as an end point. Our knowledge of the natural history of breast cancer and results from randomized trials of mammography should inform guidelines for supplemental screening.
Dr. Wendie A. Berg is at Magee-Womens Hospital of University of Pittsburgh Medical Center. She reported serving in a consulting or advisory role with SuperSonic Imagine. These comments were taken from her accompanying editorial (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.65.8674).
Ultrasound was about 1.8 times more sensitive than tomosynthesis for the incremental detection of breast cancer in women with radiologically dense breasts and negative two-dimensional mammography screening, according to interim results from the first prospective trial to directly compare the two modalities.
“However, future application of adjunct screening should consider that tomosynthesis detected more than 50% of the additional breast cancers in these women, and could potentially be [a] primary screening modality,” wrote Dr. Alberto Tagliafico of the University of Genoa (Italy) and his associates. The study was published online March 9 in the Journal of Clinical Oncology and presented simultaneously at the European Breast Cancer Conference.
Radiologically dense breast tissue undermines the sensitivity of mammography and is itself an independent risk factor for breast cancer. Recently, many states began requiring that women be informed of their breast density and adjunct screening measures, such as ultrasound. But estimates of the sensitivity of ultrasound have ranged from about 1.9 to 4.2 cancers for every 1,000 screens, said the researchers. This variance, combined with costs and concerns about false-positive recalls, have fueled debates about the value of adjunct measures in breast cancer screening, they added. To help clarify these issues, the multicenter ASTOUND (Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts) study compared physician-performed ultrasound and tomosynthesis results for 3,231 asymptomatic women aged 44 to 71 years, whose median age was 51 years (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.63.4147).
In all, the researchers detected 24 additional breast cancers, 23 of which were invasive. Thus, ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 screens for tomosynthesis (95% CI, 1.8-6.2; P = .006). Only one cancer was detected by tomosynthesis alone. The rate of false-positive recalls was similar for the two modalities – 53 cases for tomosynthesis, versus 63 for ultrasound (P = .26). Rates of false-positive recalls leading to biopsy also were similar. Needle biopsies usually sufficed in recalled cases, but two women underwent surgical biopsies, both of which revealed radial scars.
If the final results of ASTOUND confirm these interim data, “it could be argued that breast tomosynthesis has little value in a setting where adjunct ultrasound is frequently used for screening women with mammography-dense breasts,” said the researchers. But tomosynthesis may have a role as a primary screening modality in other setting, especially because tomosynthesis acquisitions that also provide reconstructed 2D mammography are now available, lessening concerns about unjustified radiation exposure, they added.
The “modest” number of cancers in the interim report led to relatively wide confidence intervals, the investigators noted. Biomarker data were not available for all cancers, and both prevalent and incident ultrasound data were compared with prevalent tomosynthesis data, which might bias false-positive recall results in favor of ultrasound, they added.
Ultrasound was about 1.8 times more sensitive than tomosynthesis for the incremental detection of breast cancer in women with radiologically dense breasts and negative two-dimensional mammography screening, according to interim results from the first prospective trial to directly compare the two modalities.
“However, future application of adjunct screening should consider that tomosynthesis detected more than 50% of the additional breast cancers in these women, and could potentially be [a] primary screening modality,” wrote Dr. Alberto Tagliafico of the University of Genoa (Italy) and his associates. The study was published online March 9 in the Journal of Clinical Oncology and presented simultaneously at the European Breast Cancer Conference.
Radiologically dense breast tissue undermines the sensitivity of mammography and is itself an independent risk factor for breast cancer. Recently, many states began requiring that women be informed of their breast density and adjunct screening measures, such as ultrasound. But estimates of the sensitivity of ultrasound have ranged from about 1.9 to 4.2 cancers for every 1,000 screens, said the researchers. This variance, combined with costs and concerns about false-positive recalls, have fueled debates about the value of adjunct measures in breast cancer screening, they added. To help clarify these issues, the multicenter ASTOUND (Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts) study compared physician-performed ultrasound and tomosynthesis results for 3,231 asymptomatic women aged 44 to 71 years, whose median age was 51 years (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.63.4147).
In all, the researchers detected 24 additional breast cancers, 23 of which were invasive. Thus, ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 screens for tomosynthesis (95% CI, 1.8-6.2; P = .006). Only one cancer was detected by tomosynthesis alone. The rate of false-positive recalls was similar for the two modalities – 53 cases for tomosynthesis, versus 63 for ultrasound (P = .26). Rates of false-positive recalls leading to biopsy also were similar. Needle biopsies usually sufficed in recalled cases, but two women underwent surgical biopsies, both of which revealed radial scars.
If the final results of ASTOUND confirm these interim data, “it could be argued that breast tomosynthesis has little value in a setting where adjunct ultrasound is frequently used for screening women with mammography-dense breasts,” said the researchers. But tomosynthesis may have a role as a primary screening modality in other setting, especially because tomosynthesis acquisitions that also provide reconstructed 2D mammography are now available, lessening concerns about unjustified radiation exposure, they added.
The “modest” number of cancers in the interim report led to relatively wide confidence intervals, the investigators noted. Biomarker data were not available for all cancers, and both prevalent and incident ultrasound data were compared with prevalent tomosynthesis data, which might bias false-positive recall results in favor of ultrasound, they added.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Ultrasound outperformed tomosynthesis for the incremental detection of breast cancer in women with negative mammograms and radiologically dense breast tissue.
Major finding: Ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 tomosynthesis screens (95% CI, 1.8-6.2; P = .006).
Data source: A multicenter, prospective trial comparing the two modalities in 3,231 women.
Disclosures: The University of Genoa sponsors the ASTOUND study. Dr. Tagliafico disclosed honoraria from Esaote-Philips, patents, royalties, or other intellectual property from Springer, and travel, accommodations, and expense support from Hologic and Technologic. Four coinvestigators reported financial relationships with several device and pharmaceutical companies. The senior author and the other seven coinvestigators had no disclosures.
Report: Heterogeneity of ovarian cancer should drive research, treatment
Ovarian cancer is not a single disease, but a heterogeneous constellation of cancers – an emerging idea that should shape the paths of research, prevention, diagnosis, and treatment.
The recent understanding that most ovarian cancers do not arise from ovarian tissue poses a unique set of challenges in all of these areas, according to a new, congressionally mandated report by the National Academies of Sciences. The 400-page report discusses research opportunities that could – if addressed – greatly affect the number of women who are diagnosed with or die from ovarian cancers.
“We are at an inflection point,” in this understanding, and how it will shape ovarian cancer research, Dr. Jerome F. Strauss III, chair of the writing committee, said during a press briefing. “This new knowledge will help us classify subtypes and have a profound impact, and help improve the lives of women who at risk of, or who have been diagnosed, with ovarian cancer.”
The report, “Ovarian Cancers: Evolving Paradigms in Research and Care,” was authored by a panel of 15 experts. It plumbed not only their own expertise, but a wealth of clinical, research, and policy data.
The committee made 11 recommendations in four key areas: preclinical research into ovarian cancer biology; prevention and early detection; diagnosis and treatment; and supportive care during survivorship.
One of the most important points, though, relates not to the acquisition of knowledge, but to its dissemination, said Mary Scroggins, an ovarian cancer survivor, patient advocate, and a member of the writing committee.
“Gathering evidence about risks and treatment and prevention strategies doesn’t ensure that the knowledge will be used,” she said. “We need to act on what we learn. We are calling for coordination among stakeholders to develop and implement rapid dissemination of new findings – and to find out why we are not adopting the current evidence-based practices.”
Recommendation 1
There is an urgent need to elucidate the biology and pathophysiology of the diverse subtypes of ovarian cancers. As these are more fully understood, appropriate research agendas may be constructed to determine each subtype’s unique biomarkers, which could be used as screening and diagnosis tools, and its unique vulnerabilities, which can be exploited in targeted treatments.
In addition to learning about the tumors themselves, Dr. Strauss said it’s important to understand why the ovary appears to be such a hospitable environment for the proliferation of premalignant and malignant seed cells shed from other reproductive tissues, including the Fallopian tube and endometrium.
Recommendation 2
As tumor subtypes are identified, they need to be described in a common language. Consensus must be reached on diagnostic criteria, nomenclature, and classification systems.
“This is terribly important,” Dr. Strauss said. “Without it, we cannot build a research agenda and move forward.”
Recommendation 3
Some tumor subtypes are genetically driven; others are not. Some genetically driven tumors are familial; others arise from spontaneous mutation. Understanding these genetic underpinnings will be crucial to developing targeted therapy. There is a need to increase both genetic testing and counseling, not just for the patient but for her immediate family. This will certainly extend beyond the BRCA 1 and 2 genes as knowledge of tumor subtypes unfolds.
“Our ability to identify women at high risk will have a significant impact on morbidity and mortality,” said committee member Dr. Beth Y. Karlan, a gynecologic oncologist from the University of California, Los Angeles. “We already know that family history and genetic mutations are all strong risk factors that call for genetic counseling, but the referral often doesn’t happen.”
Recommendation 4
Most of the now-known ovarian cancers are not genetically driven. Research should delve into the underlying mechanisms and risk factors for these tumors as well. Findings here may lead to more effective prevention strategies, as well as risk assessment tools.
Recommendation 5
Both surgical and nonsurgical treatment approaches should be tailored to individual tumor subtypes. These, as well as preventive measures, should be studied in light of their benefits and potential harms, both for diagnosed patients and those at high risk of developing the disease.
Recommendation 6
Ovarian cancers are highly lethal, in part, because they are often diagnosed at a late stage. Developing effective early-identification methods will be key to reducing morbidity and improving long-term survival.
“Our efforts at early detection have improved, but have not had a substantial impact on mortality,” Dr. Karlan said. “And, since many tumors don’t arise in the ovary, looking at the ovary may not really be much help in early detection. We need to expand the development of new techniques,” which may differ by tumor subtypes.
Recommendation 7
Studies consistently show that women who have access to expert care conducted according to evidence-based standards respond better to treatment and live significantly longer. But the distribution of this care is unequal; older women and those with comorbidities; women of color; lower socioeconomic status; and who live in rural areas are much less likely to receive such care. These disparities must be reduced.
“Our efforts here will require adopting innovative models of care, which may include telemedicine,” and other ways of bringing high-quality care to underserved women, Dr. Karlan said.
Recommendation 8
Little is known about why some ovarian tumors become treatment resistant. As subtypes become categorized, this knowledge gap may become even greater. A comprehensive biological categorization of tumor subtypes will help pave the way for targeted, personalized treatment strategies with both existing and yet-to-be developed agents.
“We also need more research on the optimal timing of surgeries and the impact of the multiple subsequent surgeries that most women undergo,” Dr. Karlan said.
Recommendation 9
In addition to more effective therapies, more varied therapies are also needed. These could be both pharmacologic and nonpharmacologic. Their development requires an organized and effective clinical trial system and an improved understanding of tumors’ basic biology.
Recommendation 10
Supportive care is not just necessary at the end of life. Physical and psychosocial factors are important determinants of treatment success as well as quality of life, from diagnosis through treatment and into terminal stages. Optimizing these factors and removing barriers to improving them can lead to benefits all along this spectrum, said Ms. Scroggins.
“We need research not only into improving how we can survive, but how well we survive,” she said. “It’s not an either-or proposition. Both are important.”
Recommendation 11
Knowledge gained is helpful only if that knowledge is shared. Learning how to disseminate new findings is the only way to ensure that patients benefit from them. “We need to examine what keeps us from fully implementing current standards of care, and to investigate multiple modalities and innovative pathways to communicate new understandings,” Ms. Scroggins said.
The document was created under the auspices of the National Academy of Sciences and supported by federal funds. None of the committee members declared any financial conflicts.
Ovarian cancer is not a single disease, but a heterogeneous constellation of cancers – an emerging idea that should shape the paths of research, prevention, diagnosis, and treatment.
The recent understanding that most ovarian cancers do not arise from ovarian tissue poses a unique set of challenges in all of these areas, according to a new, congressionally mandated report by the National Academies of Sciences. The 400-page report discusses research opportunities that could – if addressed – greatly affect the number of women who are diagnosed with or die from ovarian cancers.
“We are at an inflection point,” in this understanding, and how it will shape ovarian cancer research, Dr. Jerome F. Strauss III, chair of the writing committee, said during a press briefing. “This new knowledge will help us classify subtypes and have a profound impact, and help improve the lives of women who at risk of, or who have been diagnosed, with ovarian cancer.”
The report, “Ovarian Cancers: Evolving Paradigms in Research and Care,” was authored by a panel of 15 experts. It plumbed not only their own expertise, but a wealth of clinical, research, and policy data.
The committee made 11 recommendations in four key areas: preclinical research into ovarian cancer biology; prevention and early detection; diagnosis and treatment; and supportive care during survivorship.
One of the most important points, though, relates not to the acquisition of knowledge, but to its dissemination, said Mary Scroggins, an ovarian cancer survivor, patient advocate, and a member of the writing committee.
“Gathering evidence about risks and treatment and prevention strategies doesn’t ensure that the knowledge will be used,” she said. “We need to act on what we learn. We are calling for coordination among stakeholders to develop and implement rapid dissemination of new findings – and to find out why we are not adopting the current evidence-based practices.”
Recommendation 1
There is an urgent need to elucidate the biology and pathophysiology of the diverse subtypes of ovarian cancers. As these are more fully understood, appropriate research agendas may be constructed to determine each subtype’s unique biomarkers, which could be used as screening and diagnosis tools, and its unique vulnerabilities, which can be exploited in targeted treatments.
In addition to learning about the tumors themselves, Dr. Strauss said it’s important to understand why the ovary appears to be such a hospitable environment for the proliferation of premalignant and malignant seed cells shed from other reproductive tissues, including the Fallopian tube and endometrium.
Recommendation 2
As tumor subtypes are identified, they need to be described in a common language. Consensus must be reached on diagnostic criteria, nomenclature, and classification systems.
“This is terribly important,” Dr. Strauss said. “Without it, we cannot build a research agenda and move forward.”
Recommendation 3
Some tumor subtypes are genetically driven; others are not. Some genetically driven tumors are familial; others arise from spontaneous mutation. Understanding these genetic underpinnings will be crucial to developing targeted therapy. There is a need to increase both genetic testing and counseling, not just for the patient but for her immediate family. This will certainly extend beyond the BRCA 1 and 2 genes as knowledge of tumor subtypes unfolds.
“Our ability to identify women at high risk will have a significant impact on morbidity and mortality,” said committee member Dr. Beth Y. Karlan, a gynecologic oncologist from the University of California, Los Angeles. “We already know that family history and genetic mutations are all strong risk factors that call for genetic counseling, but the referral often doesn’t happen.”
Recommendation 4
Most of the now-known ovarian cancers are not genetically driven. Research should delve into the underlying mechanisms and risk factors for these tumors as well. Findings here may lead to more effective prevention strategies, as well as risk assessment tools.
Recommendation 5
Both surgical and nonsurgical treatment approaches should be tailored to individual tumor subtypes. These, as well as preventive measures, should be studied in light of their benefits and potential harms, both for diagnosed patients and those at high risk of developing the disease.
Recommendation 6
Ovarian cancers are highly lethal, in part, because they are often diagnosed at a late stage. Developing effective early-identification methods will be key to reducing morbidity and improving long-term survival.
“Our efforts at early detection have improved, but have not had a substantial impact on mortality,” Dr. Karlan said. “And, since many tumors don’t arise in the ovary, looking at the ovary may not really be much help in early detection. We need to expand the development of new techniques,” which may differ by tumor subtypes.
Recommendation 7
Studies consistently show that women who have access to expert care conducted according to evidence-based standards respond better to treatment and live significantly longer. But the distribution of this care is unequal; older women and those with comorbidities; women of color; lower socioeconomic status; and who live in rural areas are much less likely to receive such care. These disparities must be reduced.
“Our efforts here will require adopting innovative models of care, which may include telemedicine,” and other ways of bringing high-quality care to underserved women, Dr. Karlan said.
Recommendation 8
Little is known about why some ovarian tumors become treatment resistant. As subtypes become categorized, this knowledge gap may become even greater. A comprehensive biological categorization of tumor subtypes will help pave the way for targeted, personalized treatment strategies with both existing and yet-to-be developed agents.
“We also need more research on the optimal timing of surgeries and the impact of the multiple subsequent surgeries that most women undergo,” Dr. Karlan said.
Recommendation 9
In addition to more effective therapies, more varied therapies are also needed. These could be both pharmacologic and nonpharmacologic. Their development requires an organized and effective clinical trial system and an improved understanding of tumors’ basic biology.
Recommendation 10
Supportive care is not just necessary at the end of life. Physical and psychosocial factors are important determinants of treatment success as well as quality of life, from diagnosis through treatment and into terminal stages. Optimizing these factors and removing barriers to improving them can lead to benefits all along this spectrum, said Ms. Scroggins.
“We need research not only into improving how we can survive, but how well we survive,” she said. “It’s not an either-or proposition. Both are important.”
Recommendation 11
Knowledge gained is helpful only if that knowledge is shared. Learning how to disseminate new findings is the only way to ensure that patients benefit from them. “We need to examine what keeps us from fully implementing current standards of care, and to investigate multiple modalities and innovative pathways to communicate new understandings,” Ms. Scroggins said.
The document was created under the auspices of the National Academy of Sciences and supported by federal funds. None of the committee members declared any financial conflicts.
Ovarian cancer is not a single disease, but a heterogeneous constellation of cancers – an emerging idea that should shape the paths of research, prevention, diagnosis, and treatment.
The recent understanding that most ovarian cancers do not arise from ovarian tissue poses a unique set of challenges in all of these areas, according to a new, congressionally mandated report by the National Academies of Sciences. The 400-page report discusses research opportunities that could – if addressed – greatly affect the number of women who are diagnosed with or die from ovarian cancers.
“We are at an inflection point,” in this understanding, and how it will shape ovarian cancer research, Dr. Jerome F. Strauss III, chair of the writing committee, said during a press briefing. “This new knowledge will help us classify subtypes and have a profound impact, and help improve the lives of women who at risk of, or who have been diagnosed, with ovarian cancer.”
The report, “Ovarian Cancers: Evolving Paradigms in Research and Care,” was authored by a panel of 15 experts. It plumbed not only their own expertise, but a wealth of clinical, research, and policy data.
The committee made 11 recommendations in four key areas: preclinical research into ovarian cancer biology; prevention and early detection; diagnosis and treatment; and supportive care during survivorship.
One of the most important points, though, relates not to the acquisition of knowledge, but to its dissemination, said Mary Scroggins, an ovarian cancer survivor, patient advocate, and a member of the writing committee.
“Gathering evidence about risks and treatment and prevention strategies doesn’t ensure that the knowledge will be used,” she said. “We need to act on what we learn. We are calling for coordination among stakeholders to develop and implement rapid dissemination of new findings – and to find out why we are not adopting the current evidence-based practices.”
Recommendation 1
There is an urgent need to elucidate the biology and pathophysiology of the diverse subtypes of ovarian cancers. As these are more fully understood, appropriate research agendas may be constructed to determine each subtype’s unique biomarkers, which could be used as screening and diagnosis tools, and its unique vulnerabilities, which can be exploited in targeted treatments.
In addition to learning about the tumors themselves, Dr. Strauss said it’s important to understand why the ovary appears to be such a hospitable environment for the proliferation of premalignant and malignant seed cells shed from other reproductive tissues, including the Fallopian tube and endometrium.
Recommendation 2
As tumor subtypes are identified, they need to be described in a common language. Consensus must be reached on diagnostic criteria, nomenclature, and classification systems.
“This is terribly important,” Dr. Strauss said. “Without it, we cannot build a research agenda and move forward.”
Recommendation 3
Some tumor subtypes are genetically driven; others are not. Some genetically driven tumors are familial; others arise from spontaneous mutation. Understanding these genetic underpinnings will be crucial to developing targeted therapy. There is a need to increase both genetic testing and counseling, not just for the patient but for her immediate family. This will certainly extend beyond the BRCA 1 and 2 genes as knowledge of tumor subtypes unfolds.
“Our ability to identify women at high risk will have a significant impact on morbidity and mortality,” said committee member Dr. Beth Y. Karlan, a gynecologic oncologist from the University of California, Los Angeles. “We already know that family history and genetic mutations are all strong risk factors that call for genetic counseling, but the referral often doesn’t happen.”
Recommendation 4
Most of the now-known ovarian cancers are not genetically driven. Research should delve into the underlying mechanisms and risk factors for these tumors as well. Findings here may lead to more effective prevention strategies, as well as risk assessment tools.
Recommendation 5
Both surgical and nonsurgical treatment approaches should be tailored to individual tumor subtypes. These, as well as preventive measures, should be studied in light of their benefits and potential harms, both for diagnosed patients and those at high risk of developing the disease.
Recommendation 6
Ovarian cancers are highly lethal, in part, because they are often diagnosed at a late stage. Developing effective early-identification methods will be key to reducing morbidity and improving long-term survival.
“Our efforts at early detection have improved, but have not had a substantial impact on mortality,” Dr. Karlan said. “And, since many tumors don’t arise in the ovary, looking at the ovary may not really be much help in early detection. We need to expand the development of new techniques,” which may differ by tumor subtypes.
Recommendation 7
Studies consistently show that women who have access to expert care conducted according to evidence-based standards respond better to treatment and live significantly longer. But the distribution of this care is unequal; older women and those with comorbidities; women of color; lower socioeconomic status; and who live in rural areas are much less likely to receive such care. These disparities must be reduced.
“Our efforts here will require adopting innovative models of care, which may include telemedicine,” and other ways of bringing high-quality care to underserved women, Dr. Karlan said.
Recommendation 8
Little is known about why some ovarian tumors become treatment resistant. As subtypes become categorized, this knowledge gap may become even greater. A comprehensive biological categorization of tumor subtypes will help pave the way for targeted, personalized treatment strategies with both existing and yet-to-be developed agents.
“We also need more research on the optimal timing of surgeries and the impact of the multiple subsequent surgeries that most women undergo,” Dr. Karlan said.
Recommendation 9
In addition to more effective therapies, more varied therapies are also needed. These could be both pharmacologic and nonpharmacologic. Their development requires an organized and effective clinical trial system and an improved understanding of tumors’ basic biology.
Recommendation 10
Supportive care is not just necessary at the end of life. Physical and psychosocial factors are important determinants of treatment success as well as quality of life, from diagnosis through treatment and into terminal stages. Optimizing these factors and removing barriers to improving them can lead to benefits all along this spectrum, said Ms. Scroggins.
“We need research not only into improving how we can survive, but how well we survive,” she said. “It’s not an either-or proposition. Both are important.”
Recommendation 11
Knowledge gained is helpful only if that knowledge is shared. Learning how to disseminate new findings is the only way to ensure that patients benefit from them. “We need to examine what keeps us from fully implementing current standards of care, and to investigate multiple modalities and innovative pathways to communicate new understandings,” Ms. Scroggins said.
The document was created under the auspices of the National Academy of Sciences and supported by federal funds. None of the committee members declared any financial conflicts.
Smoking affects molecular profile of HPV-positive oropharyngeal cancer
SCOTTSDALE, ARIZ. – The human papillomavirus (HPV)–positive oropharyngeal cancers of heavy smokers and light smokers have distinctly different molecular profiles, which may have implications for treatment, according to a study presented at the Multidisciplinary Head and Neck Cancer Symposium.
The population-based cohort study of 66 patients found that mutations in certain genes associated with tobacco exposure and poorer survival – for example, NOTCH1, TP53, CDKN2A, and KRAS – were found almost exclusively in heavy smokers, investigators reported in a session and related press briefing. Also, the number of HPV reads detected in tumors was lower for heavy smokers as compared with light smokers.
Taken together, the findings suggest that although HPV-positive cancers in heavy smokers may be initiated through virus-related mutations, they go on to acquire tobacco-related mutations and become less dependent on the E6/E7 carcinogenesis mechanisms typically associated with the virus, said first author Dr. Jose P. Zevallos of the University of North Carolina, Chapel Hill.
“We think that this study and future studies based on this work will have important implications for personalizing treatment and decision making in HPV-positive oropharynx cancer, particularly in the era of less aggressive treatments for HPV-positive tumors because of their excellent prognosis,” he said. “As opposed to arbitrarily deciding that 10 pack-years [of smoking] is a number that we use to define more aggressive disease, we are trying to provide a molecular basis for more aggressive disease in order to decide who will benefit from less-aggressive versus more-aggressive treatment.”
Press briefing moderator Dr. Christine Gourin of Johns Hopkins University, Baltimore, said “This study is so important because we know that the molecular fingerprint of HPV-related oropharyngeal cancer is really different from anything that we have seen before – different patient population, different outcomes than when I was in training.”
“We don’t really understand fully why this fingerprint is so different and why tobacco affects the fingerprint,” she added. “The finding of differences in the molecular phenotypes of light smokers versus heavy smokers is something that we all appreciate clinically and we need to understand better to tailor treatment.”
Introducing the study, Dr. Zevallos noted that the HPV-positive cancers of smokers are known to have prognosis intermediate between those of the more favorable HPV-positive cancers of never smokers and the less favorable HPV-negative cancers. What remains unclear is the molecular basis for these differences.
Patients came from the population-based CHANCE (Carolina Head and Neck Cancer Epidemiology) study conducted during 2001-2006. The investigators performed targeted next-generation DNA sequencing in tumors with an assay for more than 700 genes associated with human cancers.
“We focused our attention on genes that overlap with those in COSMIC [the Catalogue of Somatic Mutations in Cancer] as well as on TCGA [The Cancer Genome Atlas] genes that were demonstrated to be significant in head and neck cancer,” Dr. Zevallos explained.
All 66 patients studied had HPV-positive tumors according to p16 expression or HPV polymerase chain reaction findings. Overall, 61% were heavy smokers, defined as having a greater than 10 pack-year history of smoking.
In terms of clinical outcome, the 5-year overall survival rate was 82% among the heavy smokers and 60% among the light or never smokers.
Mutations associated with tobacco use were found almost exclusively in the heavy smokers, Dr. Zevallos reported. For example, they had higher prevalences of mutations in NOTCH1 (18% vs. 0%), FAT1 (14% vs. 6%), and FGFR3 (10% vs. 0%), among others. On the other hand, the light and never smokers had a higher prevalence of mutations in PIK3CA (50% vs. 34%). Additionally, KRAS mutations were found only in the heavy smokers (4% vs. 0%), whereas HRAS mutations were found in the light and never smokers only (6% vs. 0%).
A pathway analysis incorporating the new information for HPV-positive heavy smokers confirmed that despite persistence of the HPV-related signature, these tumors also had signaling in several of the pathways typically associated with HPV-negative cancers, according to Dr. Zevallos.
HPV DNA was detected in all of the tumors, and in 95% of cases, the viral type was type 16. However, PCR for HPV was falsely negative in 9%. “This is a very important number as we rely on this as a surrogate for HPV status,” he commented. “p16 was the main inclusion criterion for this particular study, but this should be noted.”
Heavy smokers and patients who had died had a lower number of HPV reads per tumor. “This tells us that there are potentially subclones developing in these patients that are driven by tobacco-associated mutations, and this may explain worse outcomes in this patient population and warrants further exploration,” Dr. Zevallos elaborated.
SCOTTSDALE, ARIZ. – The human papillomavirus (HPV)–positive oropharyngeal cancers of heavy smokers and light smokers have distinctly different molecular profiles, which may have implications for treatment, according to a study presented at the Multidisciplinary Head and Neck Cancer Symposium.
The population-based cohort study of 66 patients found that mutations in certain genes associated with tobacco exposure and poorer survival – for example, NOTCH1, TP53, CDKN2A, and KRAS – were found almost exclusively in heavy smokers, investigators reported in a session and related press briefing. Also, the number of HPV reads detected in tumors was lower for heavy smokers as compared with light smokers.
Taken together, the findings suggest that although HPV-positive cancers in heavy smokers may be initiated through virus-related mutations, they go on to acquire tobacco-related mutations and become less dependent on the E6/E7 carcinogenesis mechanisms typically associated with the virus, said first author Dr. Jose P. Zevallos of the University of North Carolina, Chapel Hill.
“We think that this study and future studies based on this work will have important implications for personalizing treatment and decision making in HPV-positive oropharynx cancer, particularly in the era of less aggressive treatments for HPV-positive tumors because of their excellent prognosis,” he said. “As opposed to arbitrarily deciding that 10 pack-years [of smoking] is a number that we use to define more aggressive disease, we are trying to provide a molecular basis for more aggressive disease in order to decide who will benefit from less-aggressive versus more-aggressive treatment.”
Press briefing moderator Dr. Christine Gourin of Johns Hopkins University, Baltimore, said “This study is so important because we know that the molecular fingerprint of HPV-related oropharyngeal cancer is really different from anything that we have seen before – different patient population, different outcomes than when I was in training.”
“We don’t really understand fully why this fingerprint is so different and why tobacco affects the fingerprint,” she added. “The finding of differences in the molecular phenotypes of light smokers versus heavy smokers is something that we all appreciate clinically and we need to understand better to tailor treatment.”
Introducing the study, Dr. Zevallos noted that the HPV-positive cancers of smokers are known to have prognosis intermediate between those of the more favorable HPV-positive cancers of never smokers and the less favorable HPV-negative cancers. What remains unclear is the molecular basis for these differences.
Patients came from the population-based CHANCE (Carolina Head and Neck Cancer Epidemiology) study conducted during 2001-2006. The investigators performed targeted next-generation DNA sequencing in tumors with an assay for more than 700 genes associated with human cancers.
“We focused our attention on genes that overlap with those in COSMIC [the Catalogue of Somatic Mutations in Cancer] as well as on TCGA [The Cancer Genome Atlas] genes that were demonstrated to be significant in head and neck cancer,” Dr. Zevallos explained.
All 66 patients studied had HPV-positive tumors according to p16 expression or HPV polymerase chain reaction findings. Overall, 61% were heavy smokers, defined as having a greater than 10 pack-year history of smoking.
In terms of clinical outcome, the 5-year overall survival rate was 82% among the heavy smokers and 60% among the light or never smokers.
Mutations associated with tobacco use were found almost exclusively in the heavy smokers, Dr. Zevallos reported. For example, they had higher prevalences of mutations in NOTCH1 (18% vs. 0%), FAT1 (14% vs. 6%), and FGFR3 (10% vs. 0%), among others. On the other hand, the light and never smokers had a higher prevalence of mutations in PIK3CA (50% vs. 34%). Additionally, KRAS mutations were found only in the heavy smokers (4% vs. 0%), whereas HRAS mutations were found in the light and never smokers only (6% vs. 0%).
A pathway analysis incorporating the new information for HPV-positive heavy smokers confirmed that despite persistence of the HPV-related signature, these tumors also had signaling in several of the pathways typically associated with HPV-negative cancers, according to Dr. Zevallos.
HPV DNA was detected in all of the tumors, and in 95% of cases, the viral type was type 16. However, PCR for HPV was falsely negative in 9%. “This is a very important number as we rely on this as a surrogate for HPV status,” he commented. “p16 was the main inclusion criterion for this particular study, but this should be noted.”
Heavy smokers and patients who had died had a lower number of HPV reads per tumor. “This tells us that there are potentially subclones developing in these patients that are driven by tobacco-associated mutations, and this may explain worse outcomes in this patient population and warrants further exploration,” Dr. Zevallos elaborated.
SCOTTSDALE, ARIZ. – The human papillomavirus (HPV)–positive oropharyngeal cancers of heavy smokers and light smokers have distinctly different molecular profiles, which may have implications for treatment, according to a study presented at the Multidisciplinary Head and Neck Cancer Symposium.
The population-based cohort study of 66 patients found that mutations in certain genes associated with tobacco exposure and poorer survival – for example, NOTCH1, TP53, CDKN2A, and KRAS – were found almost exclusively in heavy smokers, investigators reported in a session and related press briefing. Also, the number of HPV reads detected in tumors was lower for heavy smokers as compared with light smokers.
Taken together, the findings suggest that although HPV-positive cancers in heavy smokers may be initiated through virus-related mutations, they go on to acquire tobacco-related mutations and become less dependent on the E6/E7 carcinogenesis mechanisms typically associated with the virus, said first author Dr. Jose P. Zevallos of the University of North Carolina, Chapel Hill.
“We think that this study and future studies based on this work will have important implications for personalizing treatment and decision making in HPV-positive oropharynx cancer, particularly in the era of less aggressive treatments for HPV-positive tumors because of their excellent prognosis,” he said. “As opposed to arbitrarily deciding that 10 pack-years [of smoking] is a number that we use to define more aggressive disease, we are trying to provide a molecular basis for more aggressive disease in order to decide who will benefit from less-aggressive versus more-aggressive treatment.”
Press briefing moderator Dr. Christine Gourin of Johns Hopkins University, Baltimore, said “This study is so important because we know that the molecular fingerprint of HPV-related oropharyngeal cancer is really different from anything that we have seen before – different patient population, different outcomes than when I was in training.”
“We don’t really understand fully why this fingerprint is so different and why tobacco affects the fingerprint,” she added. “The finding of differences in the molecular phenotypes of light smokers versus heavy smokers is something that we all appreciate clinically and we need to understand better to tailor treatment.”
Introducing the study, Dr. Zevallos noted that the HPV-positive cancers of smokers are known to have prognosis intermediate between those of the more favorable HPV-positive cancers of never smokers and the less favorable HPV-negative cancers. What remains unclear is the molecular basis for these differences.
Patients came from the population-based CHANCE (Carolina Head and Neck Cancer Epidemiology) study conducted during 2001-2006. The investigators performed targeted next-generation DNA sequencing in tumors with an assay for more than 700 genes associated with human cancers.
“We focused our attention on genes that overlap with those in COSMIC [the Catalogue of Somatic Mutations in Cancer] as well as on TCGA [The Cancer Genome Atlas] genes that were demonstrated to be significant in head and neck cancer,” Dr. Zevallos explained.
All 66 patients studied had HPV-positive tumors according to p16 expression or HPV polymerase chain reaction findings. Overall, 61% were heavy smokers, defined as having a greater than 10 pack-year history of smoking.
In terms of clinical outcome, the 5-year overall survival rate was 82% among the heavy smokers and 60% among the light or never smokers.
Mutations associated with tobacco use were found almost exclusively in the heavy smokers, Dr. Zevallos reported. For example, they had higher prevalences of mutations in NOTCH1 (18% vs. 0%), FAT1 (14% vs. 6%), and FGFR3 (10% vs. 0%), among others. On the other hand, the light and never smokers had a higher prevalence of mutations in PIK3CA (50% vs. 34%). Additionally, KRAS mutations were found only in the heavy smokers (4% vs. 0%), whereas HRAS mutations were found in the light and never smokers only (6% vs. 0%).
A pathway analysis incorporating the new information for HPV-positive heavy smokers confirmed that despite persistence of the HPV-related signature, these tumors also had signaling in several of the pathways typically associated with HPV-negative cancers, according to Dr. Zevallos.
HPV DNA was detected in all of the tumors, and in 95% of cases, the viral type was type 16. However, PCR for HPV was falsely negative in 9%. “This is a very important number as we rely on this as a surrogate for HPV status,” he commented. “p16 was the main inclusion criterion for this particular study, but this should be noted.”
Heavy smokers and patients who had died had a lower number of HPV reads per tumor. “This tells us that there are potentially subclones developing in these patients that are driven by tobacco-associated mutations, and this may explain worse outcomes in this patient population and warrants further exploration,” Dr. Zevallos elaborated.
AT THE MULTIDISCIPLINARY HEAD AND NECK CANCER SYMPOSIUM
Key clinical point: The molecular profile of HPV-positive oropharyngeal cancer differs distinctly between heavy and light smokers.
Major finding: Heavy smokers were more likely to have mutations of NOTCH1 (18% vs. 0%), TP53 (6% vs. 0%), and KRAS (4% vs. 0%), and they had fewer HPV reads in their tumors.
Data source: A population-based cohort study of 66 patients with HPV-positive oropharyngeal cancer.
Disclosures: Dr. Zevallos disclosed that he had no relevant conflicts of interest.
Prevention driving increase in mastectomies
The overall mastectomy rate rose 36% from 2005 to 2013, even though “the incidence of breast cancer overall remained stable,” according to the Agency for Healthcare Research and Quality (AHRQ).
The rate of bilateral mastectomies jumped 226% over that time period, going from 9.1 per 100,000 adult women in 2005 to 29.7 per 100,000 in 2013. The unilateral mastectomy rate, starting at 57.3 per 100,000 women in 2005, rose to 69.8 in 2009 and then fell to 60.6 in 2013, for an increase of 6% overall, the AHRQ reported.
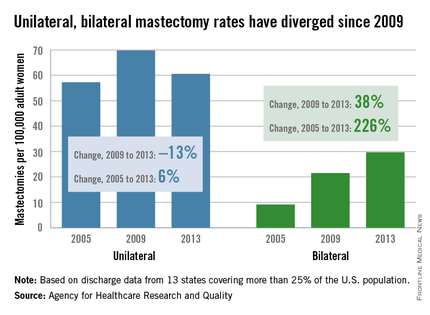
The rate of bilateral mastectomies without any cancer diagnosis increased from 2.0 to 4.4 per 100,000 from 2005 to 2013, and the unilateral rate rose from 2.7 to 3.7 per 100,000, the report noted.
Both unilateral and bilateral mastectomies are increasingly performed as outpatient procedures. The rate done in hospital-based ambulatory surgery settings nearly doubled from 16.1 to 31 per 100,000 women, and the overall proportion performed in ambulatory settings reached 45% in 2013, the AHRQ said.
The analysis shows that “more women are opting for mastectomies, particularly preventive double mastectomies, and more of those surgeries are being done as outpatient procedures,” AHRQ Director Rick Kronick, Ph.D., said in a written statement. These “changing patterns of care for breast cancer [highlight] the need for further evidence about the effects of choices women are making on their health, well-being, and safety.”
The analysis of the State Inpatient Databases and the State Ambulatory Surgery and Services Databases involved data from 13 states that had cases in both databases and used coding that allowed identification of unilateral versus bilateral mastectomies. Those 13 states represent more than 25% of the U.S. population.
The overall mastectomy rate rose 36% from 2005 to 2013, even though “the incidence of breast cancer overall remained stable,” according to the Agency for Healthcare Research and Quality (AHRQ).
The rate of bilateral mastectomies jumped 226% over that time period, going from 9.1 per 100,000 adult women in 2005 to 29.7 per 100,000 in 2013. The unilateral mastectomy rate, starting at 57.3 per 100,000 women in 2005, rose to 69.8 in 2009 and then fell to 60.6 in 2013, for an increase of 6% overall, the AHRQ reported.

The rate of bilateral mastectomies without any cancer diagnosis increased from 2.0 to 4.4 per 100,000 from 2005 to 2013, and the unilateral rate rose from 2.7 to 3.7 per 100,000, the report noted.
Both unilateral and bilateral mastectomies are increasingly performed as outpatient procedures. The rate done in hospital-based ambulatory surgery settings nearly doubled from 16.1 to 31 per 100,000 women, and the overall proportion performed in ambulatory settings reached 45% in 2013, the AHRQ said.
The analysis shows that “more women are opting for mastectomies, particularly preventive double mastectomies, and more of those surgeries are being done as outpatient procedures,” AHRQ Director Rick Kronick, Ph.D., said in a written statement. These “changing patterns of care for breast cancer [highlight] the need for further evidence about the effects of choices women are making on their health, well-being, and safety.”
The analysis of the State Inpatient Databases and the State Ambulatory Surgery and Services Databases involved data from 13 states that had cases in both databases and used coding that allowed identification of unilateral versus bilateral mastectomies. Those 13 states represent more than 25% of the U.S. population.
The overall mastectomy rate rose 36% from 2005 to 2013, even though “the incidence of breast cancer overall remained stable,” according to the Agency for Healthcare Research and Quality (AHRQ).
The rate of bilateral mastectomies jumped 226% over that time period, going from 9.1 per 100,000 adult women in 2005 to 29.7 per 100,000 in 2013. The unilateral mastectomy rate, starting at 57.3 per 100,000 women in 2005, rose to 69.8 in 2009 and then fell to 60.6 in 2013, for an increase of 6% overall, the AHRQ reported.

The rate of bilateral mastectomies without any cancer diagnosis increased from 2.0 to 4.4 per 100,000 from 2005 to 2013, and the unilateral rate rose from 2.7 to 3.7 per 100,000, the report noted.
Both unilateral and bilateral mastectomies are increasingly performed as outpatient procedures. The rate done in hospital-based ambulatory surgery settings nearly doubled from 16.1 to 31 per 100,000 women, and the overall proportion performed in ambulatory settings reached 45% in 2013, the AHRQ said.
The analysis shows that “more women are opting for mastectomies, particularly preventive double mastectomies, and more of those surgeries are being done as outpatient procedures,” AHRQ Director Rick Kronick, Ph.D., said in a written statement. These “changing patterns of care for breast cancer [highlight] the need for further evidence about the effects of choices women are making on their health, well-being, and safety.”
The analysis of the State Inpatient Databases and the State Ambulatory Surgery and Services Databases involved data from 13 states that had cases in both databases and used coding that allowed identification of unilateral versus bilateral mastectomies. Those 13 states represent more than 25% of the U.S. population.