User login
TAMIS for rectal cancer holds its own vs. TEM
JACKSONVILLE, FLA. – Over the past 30 years, transanal endoscopic microsurgery (TEM) has emerged as a technique for localized rectal cancer, but the need for expensive specialized equipment put it beyond the reach of most hospitals.
Now, early results with transanal minimally invasive surgery (TAMIS) may open the door to an option that achieves the benefits of TEM while using commonly available and less expensive equipment, according to a study presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
Dr. John Costello, general surgery resident at Georgetown University, Washington, presented a poster summarizing the findings of a systematic literature review of TEM and TAMIS studies. The experience with TAMIS is more limited since Dr. Sam Atallah of Sebring, Fla., first introduced it in 2010. The review included the only head-to-head study of the technical aspects of TAMIS and TEM to date.
“Overall the results are very similar between the two approaches,” Dr. Costello said. “In many ways there are, at least anecdotally, some benefits potentially toward the TAMIS technique aside from cost: The perioperative morbidity may be a little lower and, particularly, there seemed to be fewer early problems with continence after surgery.”
The review found similar outcomes between the two approaches: low recurrence rates for small tumors (up to 3 cm) of 4% for TAMIS and 5% for TEM, although the study found that the recurrence rate for TEM increased with larger tumors. Surgery-related deaths with TAMIS ranged from 7.4% to19% and TEM from 6% to 31% across the studies reviewed.
The challenge with the systematic review was that the population of patients who had TAMIS was fewer than 500.
Dr. Costello elucidated the reasons that rectal cancer surgery has proved so challenging to surgeons over the years. The choice of operation was either limited to transabdominal or transanal excision, but the transanal approach had limitations anatomically and was found to be oncologically inferior for early stage cancer. Even with the evolution of the TEM approach, its adoption has been slow.
Either TEM or TAMIS would be a good option for patients too frail for the radical resection that low anterior resection or abdominal perineal resection demand, and would offer an option for palliation for advanced disease, Dr. Costello said. “You could locally resect patients in a way that they go home the same day or at most stay one day in the hospital,” he said.
“The challenge with TEM is that, although the oncologic outcomes are quite good with early-stage disease, the adoption has been very poor over 3 decades mainly because it requires specialized equipment with a very large upfront cost that is limited to use in the rectum,” Dr. Costello said. He estimated the initial capital investment cost for TEM equipment at up to $60,000 on average.
The TAMIS approach, on the other hand, carries a per-procedure equipment cost of about $500 over traditional laparoscopic surgery, he said. It can utilize the single-incision laparoscopic port (SILS) for the transanal approach. TAMIS sacrifices the three-dimensional view of TEM for two-dimensional, but it does provide 360-degree visualization. The surgeon must also be facile with the laparoscopic technique. “In the past that was a big challenge, but now all trainees are very familiar with laparoscopic surgery,” Dr. Costello said.
While the paucity of data on the TAMIS approach makes it difficult to make a strong case for the procedure, the path forward is clear, Dr. Costello said.
“We feel, as do a number of authors of the most papers, that the time truly is now for an actual prospective randomized trial to compare these techniques head-to-head, because colorectal surgeons now have the skill set to be facile at both,” Dr. Costello said.
The investigators had no financial relationships to disclose.
JACKSONVILLE, FLA. – Over the past 30 years, transanal endoscopic microsurgery (TEM) has emerged as a technique for localized rectal cancer, but the need for expensive specialized equipment put it beyond the reach of most hospitals.
Now, early results with transanal minimally invasive surgery (TAMIS) may open the door to an option that achieves the benefits of TEM while using commonly available and less expensive equipment, according to a study presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
Dr. John Costello, general surgery resident at Georgetown University, Washington, presented a poster summarizing the findings of a systematic literature review of TEM and TAMIS studies. The experience with TAMIS is more limited since Dr. Sam Atallah of Sebring, Fla., first introduced it in 2010. The review included the only head-to-head study of the technical aspects of TAMIS and TEM to date.
“Overall the results are very similar between the two approaches,” Dr. Costello said. “In many ways there are, at least anecdotally, some benefits potentially toward the TAMIS technique aside from cost: The perioperative morbidity may be a little lower and, particularly, there seemed to be fewer early problems with continence after surgery.”
The review found similar outcomes between the two approaches: low recurrence rates for small tumors (up to 3 cm) of 4% for TAMIS and 5% for TEM, although the study found that the recurrence rate for TEM increased with larger tumors. Surgery-related deaths with TAMIS ranged from 7.4% to19% and TEM from 6% to 31% across the studies reviewed.
The challenge with the systematic review was that the population of patients who had TAMIS was fewer than 500.
Dr. Costello elucidated the reasons that rectal cancer surgery has proved so challenging to surgeons over the years. The choice of operation was either limited to transabdominal or transanal excision, but the transanal approach had limitations anatomically and was found to be oncologically inferior for early stage cancer. Even with the evolution of the TEM approach, its adoption has been slow.
Either TEM or TAMIS would be a good option for patients too frail for the radical resection that low anterior resection or abdominal perineal resection demand, and would offer an option for palliation for advanced disease, Dr. Costello said. “You could locally resect patients in a way that they go home the same day or at most stay one day in the hospital,” he said.
“The challenge with TEM is that, although the oncologic outcomes are quite good with early-stage disease, the adoption has been very poor over 3 decades mainly because it requires specialized equipment with a very large upfront cost that is limited to use in the rectum,” Dr. Costello said. He estimated the initial capital investment cost for TEM equipment at up to $60,000 on average.
The TAMIS approach, on the other hand, carries a per-procedure equipment cost of about $500 over traditional laparoscopic surgery, he said. It can utilize the single-incision laparoscopic port (SILS) for the transanal approach. TAMIS sacrifices the three-dimensional view of TEM for two-dimensional, but it does provide 360-degree visualization. The surgeon must also be facile with the laparoscopic technique. “In the past that was a big challenge, but now all trainees are very familiar with laparoscopic surgery,” Dr. Costello said.
While the paucity of data on the TAMIS approach makes it difficult to make a strong case for the procedure, the path forward is clear, Dr. Costello said.
“We feel, as do a number of authors of the most papers, that the time truly is now for an actual prospective randomized trial to compare these techniques head-to-head, because colorectal surgeons now have the skill set to be facile at both,” Dr. Costello said.
The investigators had no financial relationships to disclose.
JACKSONVILLE, FLA. – Over the past 30 years, transanal endoscopic microsurgery (TEM) has emerged as a technique for localized rectal cancer, but the need for expensive specialized equipment put it beyond the reach of most hospitals.
Now, early results with transanal minimally invasive surgery (TAMIS) may open the door to an option that achieves the benefits of TEM while using commonly available and less expensive equipment, according to a study presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
Dr. John Costello, general surgery resident at Georgetown University, Washington, presented a poster summarizing the findings of a systematic literature review of TEM and TAMIS studies. The experience with TAMIS is more limited since Dr. Sam Atallah of Sebring, Fla., first introduced it in 2010. The review included the only head-to-head study of the technical aspects of TAMIS and TEM to date.
“Overall the results are very similar between the two approaches,” Dr. Costello said. “In many ways there are, at least anecdotally, some benefits potentially toward the TAMIS technique aside from cost: The perioperative morbidity may be a little lower and, particularly, there seemed to be fewer early problems with continence after surgery.”
The review found similar outcomes between the two approaches: low recurrence rates for small tumors (up to 3 cm) of 4% for TAMIS and 5% for TEM, although the study found that the recurrence rate for TEM increased with larger tumors. Surgery-related deaths with TAMIS ranged from 7.4% to19% and TEM from 6% to 31% across the studies reviewed.
The challenge with the systematic review was that the population of patients who had TAMIS was fewer than 500.
Dr. Costello elucidated the reasons that rectal cancer surgery has proved so challenging to surgeons over the years. The choice of operation was either limited to transabdominal or transanal excision, but the transanal approach had limitations anatomically and was found to be oncologically inferior for early stage cancer. Even with the evolution of the TEM approach, its adoption has been slow.
Either TEM or TAMIS would be a good option for patients too frail for the radical resection that low anterior resection or abdominal perineal resection demand, and would offer an option for palliation for advanced disease, Dr. Costello said. “You could locally resect patients in a way that they go home the same day or at most stay one day in the hospital,” he said.
“The challenge with TEM is that, although the oncologic outcomes are quite good with early-stage disease, the adoption has been very poor over 3 decades mainly because it requires specialized equipment with a very large upfront cost that is limited to use in the rectum,” Dr. Costello said. He estimated the initial capital investment cost for TEM equipment at up to $60,000 on average.
The TAMIS approach, on the other hand, carries a per-procedure equipment cost of about $500 over traditional laparoscopic surgery, he said. It can utilize the single-incision laparoscopic port (SILS) for the transanal approach. TAMIS sacrifices the three-dimensional view of TEM for two-dimensional, but it does provide 360-degree visualization. The surgeon must also be facile with the laparoscopic technique. “In the past that was a big challenge, but now all trainees are very familiar with laparoscopic surgery,” Dr. Costello said.
While the paucity of data on the TAMIS approach makes it difficult to make a strong case for the procedure, the path forward is clear, Dr. Costello said.
“We feel, as do a number of authors of the most papers, that the time truly is now for an actual prospective randomized trial to compare these techniques head-to-head, because colorectal surgeons now have the skill set to be facile at both,” Dr. Costello said.
The investigators had no financial relationships to disclose.
AT THE ACADEMIC SURGICAL CONGRESS
Key clinical point: TAMIS for removal of rectal tumors achieved equal outcomes to TEM with measurable cost savings.
Major finding: The review found similar outcomes between the two procedures and low recurrence rates for small tumors (up to 3 cm) of 4% for TAMIS and 5% for TEM.
Data source: Systematic literature review of fewer than 500 cases of TAMIS, compared with results of TEM literature.
Disclosures: The study authors reported having no financial disclosures.
Cancer death rates show wide geographic variation
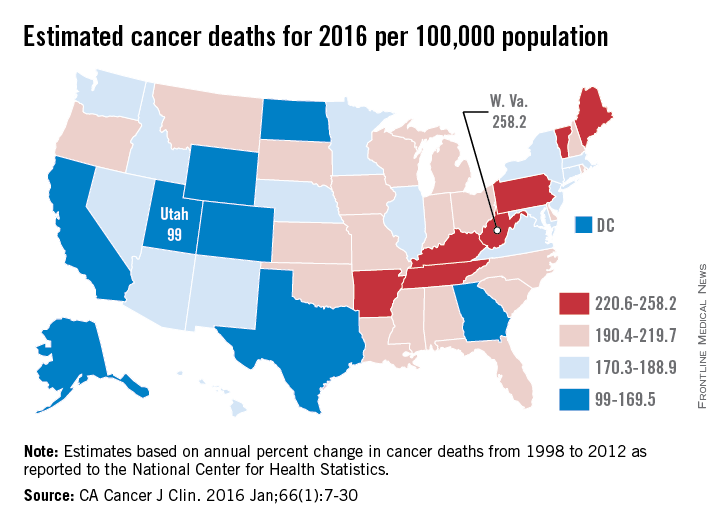
Over 595,000 cancer deaths – an average of about 1,600 each day – are expected in the United States in 2016, but those deaths are not evenly distributed among the states, according to investigators from the American Cancer Society.
Estimates from the ACS show that, in 2016, Utah will have a cancer death rate of 99 per 100,000 population, the lowest in the country. Those same estimates predict that West Virginia will have a highest-in-the-country death rate of 258.2 per 100,000 – 2.6 times higher than Utah’s. Other states with high estimated death rates include Maine, Kentucky, Arkansas, and Pennsylvania, noted Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).
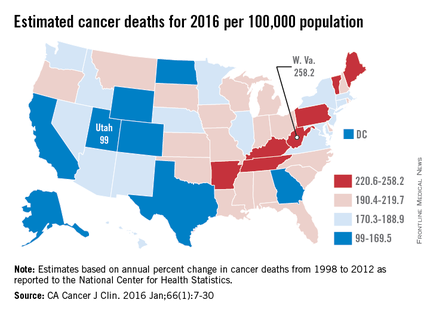
Besides Utah, the states with the lowest estimated cancer death rates in 2016 are Colorado, Texas, and Alaska, along with the District of Columbia. The national death rate for cancer has dropped 23% since 1991, the investigators said, but cancer is now the leading cause of death in 21 states. This good news/bad news situation comes about mainly as a result of “exceptionally large reductions in death from heart disease,” they added.
Ms. Siegel and her associates calculated the expected number of cancer deaths “based on the annual percent change in reported numbers of cancer deaths from 1998 through 2012 at the state and national levels as reported to the [National Center for Health Statistics].” Death rates were calculated here using estimated populations for 2015 from the U.S. Census Bureau.
Over 595,000 cancer deaths – an average of about 1,600 each day – are expected in the United States in 2016, but those deaths are not evenly distributed among the states, according to investigators from the American Cancer Society.
Estimates from the ACS show that, in 2016, Utah will have a cancer death rate of 99 per 100,000 population, the lowest in the country. Those same estimates predict that West Virginia will have a highest-in-the-country death rate of 258.2 per 100,000 – 2.6 times higher than Utah’s. Other states with high estimated death rates include Maine, Kentucky, Arkansas, and Pennsylvania, noted Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).

Besides Utah, the states with the lowest estimated cancer death rates in 2016 are Colorado, Texas, and Alaska, along with the District of Columbia. The national death rate for cancer has dropped 23% since 1991, the investigators said, but cancer is now the leading cause of death in 21 states. This good news/bad news situation comes about mainly as a result of “exceptionally large reductions in death from heart disease,” they added.
Ms. Siegel and her associates calculated the expected number of cancer deaths “based on the annual percent change in reported numbers of cancer deaths from 1998 through 2012 at the state and national levels as reported to the [National Center for Health Statistics].” Death rates were calculated here using estimated populations for 2015 from the U.S. Census Bureau.
Over 595,000 cancer deaths – an average of about 1,600 each day – are expected in the United States in 2016, but those deaths are not evenly distributed among the states, according to investigators from the American Cancer Society.
Estimates from the ACS show that, in 2016, Utah will have a cancer death rate of 99 per 100,000 population, the lowest in the country. Those same estimates predict that West Virginia will have a highest-in-the-country death rate of 258.2 per 100,000 – 2.6 times higher than Utah’s. Other states with high estimated death rates include Maine, Kentucky, Arkansas, and Pennsylvania, noted Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).

Besides Utah, the states with the lowest estimated cancer death rates in 2016 are Colorado, Texas, and Alaska, along with the District of Columbia. The national death rate for cancer has dropped 23% since 1991, the investigators said, but cancer is now the leading cause of death in 21 states. This good news/bad news situation comes about mainly as a result of “exceptionally large reductions in death from heart disease,” they added.
Ms. Siegel and her associates calculated the expected number of cancer deaths “based on the annual percent change in reported numbers of cancer deaths from 1998 through 2012 at the state and national levels as reported to the [National Center for Health Statistics].” Death rates were calculated here using estimated populations for 2015 from the U.S. Census Bureau.
Survival of pancreatic cancer is better when adjuvant therapy is given in high-volume centers
SAN FRANCISCO – Receiving adjuvant therapy for pancreatic cancer at a center that treats a high volume of patients with the disease confers a survival advantage, according to results of a retrospective cohort study reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
The analysis of 245 patients found that those given adjuvant therapy at Virginia Mason Medical Center – a high-volume center seeing up to 300 patients with newly diagnosed pancreatic cancer each year and putting about a third of them in trials – had a 37% reduction in the adjusted risk of death when compared with peers referred to community clinics for this therapy, reported first author Margaret T. Mandelson, Ph.D., director of research and quality at the center’s cancer institute in Seattle.
“Our study does lend some support to the concept of using high-volume centers for all therapy components for pancreatic cancer that is treated with curative intent,” she commented. “Ongoing investigation of patterns of care and volume impact in medical oncology is certainly warranted.”
A variety of factors may be driving the observed survival difference, such as the regimens used, with some evidence suggesting, for example, that patients treated in the community are more likely to receive single-agent therapy, she noted.
“We know that we have a strong setting for supportive care [at the center] and that we try to maximize our patients’ tolerance to treatment,” she added. “We have a high rate of completion of treatment in this setting. And of course the impact of optimism and hope cannot be underestimated in this patient population.”
Giving the academic medical center perspective, Dr. James L. Abbruzzese of the Duke Cancer Institute, Duke University, Durham, N.C., speculated that volume is a proxy for processes of care: staffing, use of guidelines or treatment algorithms, staging practices, and especially a multidisciplinary approach with components such as tumor boards and use of clinical trials. And larger centers are in a better position to offer these processes.
“While the primary determinant of the long-term outcome of patients requires adequate volumes, I don’t think this is the whole answer,” he summarized. “I think it relies on and relates much more to the processes and the extent to which we can bring the multidisciplinary team to the patients.”
Giving the community oncology perspective, Dr. Michael V. Seiden, chief medical officer of the US Oncology Network, contended that instead of focusing solely on outcomes, the field should be focusing on the value of care, broadly defined as outcome divided by cost.
“I don’t really think this is a discussion about should your pancreatic cancer be treated in the community or in an academic center or a large regional health center. What we have to realize is that tens of thousands of patients with pancreatic cancer who will be diagnosed in the years ahead are going to receive care across the country in a lot of different venues,” he commented. “The questions we need to answer are how do we maximize value? What should be done in the ‘mouse’ hospitals? What should be done in the gigantic centers of excellence? What should be done in the well-organized health care systems? And what should be done in the community? Because delivering maximal value requires keeping an eye not only on best outcomes, but also on patient convenience and cost.”
Giving some background to the study, Dr. Mandelson noted that a volume-outcome relationship has been established when it comes to surgery for pancreatic cancer, but not when it comes to adjuvant therapy for the disease.
She and her colleagues used registry data to identify patients who received a pancreatic cancer diagnosis during 2003-2014 and underwent primary resection at Virginia Mason Medical Center. They compared outcomes between those who stayed at the center to receive their adjuvant therapy and those who were referred to a community oncology practice to receive this therapy.
Patients were excluded if they had received neoadjuvant therapy, had synchronous cancers, died or were lost to follow-up within 3 months of surgery, or had contraindications to receiving adjuvant therapy. Also excluded were any who declined this therapy and for whom a medical oncologist could not be identified.
Results showed that the patients treated in the high-volume center and in community clinics were similar with respect to sex, insurance status, travel distance to a high-volume center, performance status, and tumor size, nodal status, and margin status, Dr. Mandelson reported. Those treated in the community were, on average, 5 years older.
At the high-volume center, 96% of patients started chemotherapy, 81% received a multiagent regimen, and 53% underwent chemoradiation. Detailed data on therapies received were not available for the community group.
The patients treated in the high-volume center had a more than one-third reduction in the adjusted risk of death relative to peers treated in the community (hazard ratio, 0.63; P less than .01). Median overall survival was 43.6 months for the former, compared with 27.9 months for the latter (P less than .01). The corresponding 5-year rates of overall survival were 38.6% and 24.8% (P less than .01).
“We know from the literature that pancreas cancer is undertreated in the community as a whole, both from the surgical perspective and the medical perspective. So it wouldn’t be surprising if some of the patients with a referral to an outside oncologist in fact never received treatment,” Dr. Mandelson commented.
“The patient population that received surgery in the community setting and then came to Virginia Mason for adjuvant therapy has not yet been analyzed, which is essentially the inverse of this study,” she noted. “That will be very powerful evidence.”
Dr. Mandelson disclosed that she had no relevant conflicts of interest. Dr. Abbruzzese disclosed that he receives honoraria from Celgene and Halozyme, and that he has a consulting or advisory role with Acerta Pharma, Bessor, Celgene, Cornerstone Pharma, Daiichi Sankyo, EMD Serono, Halozyme, Progen, Merck Sharpe & Dohme, Sun BioPharma, and Viba Therapeutics. Dr. Seiden disclosed that he is an employee of McKesson Specialty Health and Texas Oncology; that he is chief medical officer of US Oncology; and that he owns stock in and receives travel expenses from McKesson Specialty Health.
SAN FRANCISCO – Receiving adjuvant therapy for pancreatic cancer at a center that treats a high volume of patients with the disease confers a survival advantage, according to results of a retrospective cohort study reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
The analysis of 245 patients found that those given adjuvant therapy at Virginia Mason Medical Center – a high-volume center seeing up to 300 patients with newly diagnosed pancreatic cancer each year and putting about a third of them in trials – had a 37% reduction in the adjusted risk of death when compared with peers referred to community clinics for this therapy, reported first author Margaret T. Mandelson, Ph.D., director of research and quality at the center’s cancer institute in Seattle.
“Our study does lend some support to the concept of using high-volume centers for all therapy components for pancreatic cancer that is treated with curative intent,” she commented. “Ongoing investigation of patterns of care and volume impact in medical oncology is certainly warranted.”
A variety of factors may be driving the observed survival difference, such as the regimens used, with some evidence suggesting, for example, that patients treated in the community are more likely to receive single-agent therapy, she noted.
“We know that we have a strong setting for supportive care [at the center] and that we try to maximize our patients’ tolerance to treatment,” she added. “We have a high rate of completion of treatment in this setting. And of course the impact of optimism and hope cannot be underestimated in this patient population.”
Giving the academic medical center perspective, Dr. James L. Abbruzzese of the Duke Cancer Institute, Duke University, Durham, N.C., speculated that volume is a proxy for processes of care: staffing, use of guidelines or treatment algorithms, staging practices, and especially a multidisciplinary approach with components such as tumor boards and use of clinical trials. And larger centers are in a better position to offer these processes.
“While the primary determinant of the long-term outcome of patients requires adequate volumes, I don’t think this is the whole answer,” he summarized. “I think it relies on and relates much more to the processes and the extent to which we can bring the multidisciplinary team to the patients.”
Giving the community oncology perspective, Dr. Michael V. Seiden, chief medical officer of the US Oncology Network, contended that instead of focusing solely on outcomes, the field should be focusing on the value of care, broadly defined as outcome divided by cost.
“I don’t really think this is a discussion about should your pancreatic cancer be treated in the community or in an academic center or a large regional health center. What we have to realize is that tens of thousands of patients with pancreatic cancer who will be diagnosed in the years ahead are going to receive care across the country in a lot of different venues,” he commented. “The questions we need to answer are how do we maximize value? What should be done in the ‘mouse’ hospitals? What should be done in the gigantic centers of excellence? What should be done in the well-organized health care systems? And what should be done in the community? Because delivering maximal value requires keeping an eye not only on best outcomes, but also on patient convenience and cost.”
Giving some background to the study, Dr. Mandelson noted that a volume-outcome relationship has been established when it comes to surgery for pancreatic cancer, but not when it comes to adjuvant therapy for the disease.
She and her colleagues used registry data to identify patients who received a pancreatic cancer diagnosis during 2003-2014 and underwent primary resection at Virginia Mason Medical Center. They compared outcomes between those who stayed at the center to receive their adjuvant therapy and those who were referred to a community oncology practice to receive this therapy.
Patients were excluded if they had received neoadjuvant therapy, had synchronous cancers, died or were lost to follow-up within 3 months of surgery, or had contraindications to receiving adjuvant therapy. Also excluded were any who declined this therapy and for whom a medical oncologist could not be identified.
Results showed that the patients treated in the high-volume center and in community clinics were similar with respect to sex, insurance status, travel distance to a high-volume center, performance status, and tumor size, nodal status, and margin status, Dr. Mandelson reported. Those treated in the community were, on average, 5 years older.
At the high-volume center, 96% of patients started chemotherapy, 81% received a multiagent regimen, and 53% underwent chemoradiation. Detailed data on therapies received were not available for the community group.
The patients treated in the high-volume center had a more than one-third reduction in the adjusted risk of death relative to peers treated in the community (hazard ratio, 0.63; P less than .01). Median overall survival was 43.6 months for the former, compared with 27.9 months for the latter (P less than .01). The corresponding 5-year rates of overall survival were 38.6% and 24.8% (P less than .01).
“We know from the literature that pancreas cancer is undertreated in the community as a whole, both from the surgical perspective and the medical perspective. So it wouldn’t be surprising if some of the patients with a referral to an outside oncologist in fact never received treatment,” Dr. Mandelson commented.
“The patient population that received surgery in the community setting and then came to Virginia Mason for adjuvant therapy has not yet been analyzed, which is essentially the inverse of this study,” she noted. “That will be very powerful evidence.”
Dr. Mandelson disclosed that she had no relevant conflicts of interest. Dr. Abbruzzese disclosed that he receives honoraria from Celgene and Halozyme, and that he has a consulting or advisory role with Acerta Pharma, Bessor, Celgene, Cornerstone Pharma, Daiichi Sankyo, EMD Serono, Halozyme, Progen, Merck Sharpe & Dohme, Sun BioPharma, and Viba Therapeutics. Dr. Seiden disclosed that he is an employee of McKesson Specialty Health and Texas Oncology; that he is chief medical officer of US Oncology; and that he owns stock in and receives travel expenses from McKesson Specialty Health.
SAN FRANCISCO – Receiving adjuvant therapy for pancreatic cancer at a center that treats a high volume of patients with the disease confers a survival advantage, according to results of a retrospective cohort study reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
The analysis of 245 patients found that those given adjuvant therapy at Virginia Mason Medical Center – a high-volume center seeing up to 300 patients with newly diagnosed pancreatic cancer each year and putting about a third of them in trials – had a 37% reduction in the adjusted risk of death when compared with peers referred to community clinics for this therapy, reported first author Margaret T. Mandelson, Ph.D., director of research and quality at the center’s cancer institute in Seattle.
“Our study does lend some support to the concept of using high-volume centers for all therapy components for pancreatic cancer that is treated with curative intent,” she commented. “Ongoing investigation of patterns of care and volume impact in medical oncology is certainly warranted.”
A variety of factors may be driving the observed survival difference, such as the regimens used, with some evidence suggesting, for example, that patients treated in the community are more likely to receive single-agent therapy, she noted.
“We know that we have a strong setting for supportive care [at the center] and that we try to maximize our patients’ tolerance to treatment,” she added. “We have a high rate of completion of treatment in this setting. And of course the impact of optimism and hope cannot be underestimated in this patient population.”
Giving the academic medical center perspective, Dr. James L. Abbruzzese of the Duke Cancer Institute, Duke University, Durham, N.C., speculated that volume is a proxy for processes of care: staffing, use of guidelines or treatment algorithms, staging practices, and especially a multidisciplinary approach with components such as tumor boards and use of clinical trials. And larger centers are in a better position to offer these processes.
“While the primary determinant of the long-term outcome of patients requires adequate volumes, I don’t think this is the whole answer,” he summarized. “I think it relies on and relates much more to the processes and the extent to which we can bring the multidisciplinary team to the patients.”
Giving the community oncology perspective, Dr. Michael V. Seiden, chief medical officer of the US Oncology Network, contended that instead of focusing solely on outcomes, the field should be focusing on the value of care, broadly defined as outcome divided by cost.
“I don’t really think this is a discussion about should your pancreatic cancer be treated in the community or in an academic center or a large regional health center. What we have to realize is that tens of thousands of patients with pancreatic cancer who will be diagnosed in the years ahead are going to receive care across the country in a lot of different venues,” he commented. “The questions we need to answer are how do we maximize value? What should be done in the ‘mouse’ hospitals? What should be done in the gigantic centers of excellence? What should be done in the well-organized health care systems? And what should be done in the community? Because delivering maximal value requires keeping an eye not only on best outcomes, but also on patient convenience and cost.”
Giving some background to the study, Dr. Mandelson noted that a volume-outcome relationship has been established when it comes to surgery for pancreatic cancer, but not when it comes to adjuvant therapy for the disease.
She and her colleagues used registry data to identify patients who received a pancreatic cancer diagnosis during 2003-2014 and underwent primary resection at Virginia Mason Medical Center. They compared outcomes between those who stayed at the center to receive their adjuvant therapy and those who were referred to a community oncology practice to receive this therapy.
Patients were excluded if they had received neoadjuvant therapy, had synchronous cancers, died or were lost to follow-up within 3 months of surgery, or had contraindications to receiving adjuvant therapy. Also excluded were any who declined this therapy and for whom a medical oncologist could not be identified.
Results showed that the patients treated in the high-volume center and in community clinics were similar with respect to sex, insurance status, travel distance to a high-volume center, performance status, and tumor size, nodal status, and margin status, Dr. Mandelson reported. Those treated in the community were, on average, 5 years older.
At the high-volume center, 96% of patients started chemotherapy, 81% received a multiagent regimen, and 53% underwent chemoradiation. Detailed data on therapies received were not available for the community group.
The patients treated in the high-volume center had a more than one-third reduction in the adjusted risk of death relative to peers treated in the community (hazard ratio, 0.63; P less than .01). Median overall survival was 43.6 months for the former, compared with 27.9 months for the latter (P less than .01). The corresponding 5-year rates of overall survival were 38.6% and 24.8% (P less than .01).
“We know from the literature that pancreas cancer is undertreated in the community as a whole, both from the surgical perspective and the medical perspective. So it wouldn’t be surprising if some of the patients with a referral to an outside oncologist in fact never received treatment,” Dr. Mandelson commented.
“The patient population that received surgery in the community setting and then came to Virginia Mason for adjuvant therapy has not yet been analyzed, which is essentially the inverse of this study,” she noted. “That will be very powerful evidence.”
Dr. Mandelson disclosed that she had no relevant conflicts of interest. Dr. Abbruzzese disclosed that he receives honoraria from Celgene and Halozyme, and that he has a consulting or advisory role with Acerta Pharma, Bessor, Celgene, Cornerstone Pharma, Daiichi Sankyo, EMD Serono, Halozyme, Progen, Merck Sharpe & Dohme, Sun BioPharma, and Viba Therapeutics. Dr. Seiden disclosed that he is an employee of McKesson Specialty Health and Texas Oncology; that he is chief medical officer of US Oncology; and that he owns stock in and receives travel expenses from McKesson Specialty Health.
AT THE ASCO GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: Patients with pancreatic cancer live longer if given adjuvant therapy in a center that treats a high volume of patients with this disease.
Major finding: The risk of death was lower for patients who received adjuvant therapy in a high-volume center, compared with peers receiving this therapy in community clinics (HR, 0.63).
Data source: A retrospective cohort study of 139 patients treated in a high-volume center and 106 patients treated in community clinics.
Disclosures: Dr. Mandelson disclosed that she had no relevant conflicts of interest. Dr. Abbruzzese disclosed that he receives honoraria from Celgene and Halozyme, and that he has a consulting or advisory role with Acerta Pharma, Bessor, Celgene, Cornerstone Pharma, Daiichi Sankyo, EMD Serono, Halozyme, Progen, Merck Sharpe & Dohme, Sun BioPharma, and Viba Therapeutics. Dr. Seiden disclosed that he is an employee of McKesson Specialty Health and Texas Oncology; that he is chief medical officer of US Oncology; and that he owns stock in and receives travel expenses from McKesson Specialty Health.
Poor adherence to quality indicators found for NSCLC surgery
PHOENIX – National adherence to quality indicators for surgery in stage I non–small cell lung cancer is suboptimal, results from a large analysis of national data suggest.
“Compliance with such guidelines is a strong predictor of long-term survival, and vigorous efforts should be instituted at the level of national societies to improve such adherence,” researchers led by Dr. Pamela P. Samson wrote in an abstract presented at the annual meeting of the Society of Thoracic Surgeons. “National organizations, including American College of Chest Physicians, the National Comprehensive Cancer Network, and the American College of Surgeons Commission on Cancer, have recommended quality standards for surgery in early-stage non–small cell lung cancer (NSCLC). The determinants and outcomes of adherence to these guidelines for early-stage lung cancer patients are largely unknown.”
Dr. Samson, a general surgery resident at Washington University in St. Louis, and her associates used the National Cancer Data Base to evaluate data from 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013. They selected the following four quality measures for evaluation: performing an anatomical pulmonary resection, surgery within 8 weeks of diagnosis, R0 resection, and evaluation of 10 or more lymph nodes. Next, the researchers fitted multivariate models to identify variables independently associated with adherence to quality measures, and created a Cox multivariate model to evaluate long-term overall survival.
Dr. Varun Puri, senior author of the study, presented the findings at the STS meeting on behalf of Dr. Samson, and discussed the findings in a video interview. The researchers found that between 2004 and 2013, nearly 100% of patients met at least one of the four recommended criteria, 95% met two, 69% met three, and 22% met all four. Sampling of 10 or more lymph nodes was the least frequently met measure, occurring in only 31% of surgical patients. Patient factors associated with a greater likelihood of receiving all four quality measures included average income in ZIP code of at least $38,000 (odds ratio, 1.20), private insurance (OR, 1.22), or having Medicare (OR, 1.16). Institutional factors associated with a greater likelihood of meeting all four quality measures included higher-volume centers, defined as treating at least 38 cases per year (OR, 1.18), or being an academic institution (OR, 1.31).
At the same time, factors associated with a lower likelihood of recommended surgical care included increasing age (per year increase, OR, 0.99) and a higher Charlson/Deyo comorbidity score (OR, 0.90 for a score of 1 and OR, 0.82 for a score of 2 or more). The strongest determinant of long-term overall survival included pathologic upstaging (HR 1.84) and meeting all four quality indicators (HR 0.39). Every additional quality met was associated with a significant reduction in overall mortality.
“We believe this study can be a starting point to draw attention to institution- and surgeon-specific practice patterns that may vary widely,” Dr. Samson said in an interview prior to the meeting. “At our own institution, we are working to decrease time to surgery, as well as implementing quality improvement measures to increase nodal sampling rates. Improving these trends nationally must start at the local level, with a tailored approach.”
Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – National adherence to quality indicators for surgery in stage I non–small cell lung cancer is suboptimal, results from a large analysis of national data suggest.
“Compliance with such guidelines is a strong predictor of long-term survival, and vigorous efforts should be instituted at the level of national societies to improve such adherence,” researchers led by Dr. Pamela P. Samson wrote in an abstract presented at the annual meeting of the Society of Thoracic Surgeons. “National organizations, including American College of Chest Physicians, the National Comprehensive Cancer Network, and the American College of Surgeons Commission on Cancer, have recommended quality standards for surgery in early-stage non–small cell lung cancer (NSCLC). The determinants and outcomes of adherence to these guidelines for early-stage lung cancer patients are largely unknown.”
Dr. Samson, a general surgery resident at Washington University in St. Louis, and her associates used the National Cancer Data Base to evaluate data from 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013. They selected the following four quality measures for evaluation: performing an anatomical pulmonary resection, surgery within 8 weeks of diagnosis, R0 resection, and evaluation of 10 or more lymph nodes. Next, the researchers fitted multivariate models to identify variables independently associated with adherence to quality measures, and created a Cox multivariate model to evaluate long-term overall survival.
Dr. Varun Puri, senior author of the study, presented the findings at the STS meeting on behalf of Dr. Samson, and discussed the findings in a video interview. The researchers found that between 2004 and 2013, nearly 100% of patients met at least one of the four recommended criteria, 95% met two, 69% met three, and 22% met all four. Sampling of 10 or more lymph nodes was the least frequently met measure, occurring in only 31% of surgical patients. Patient factors associated with a greater likelihood of receiving all four quality measures included average income in ZIP code of at least $38,000 (odds ratio, 1.20), private insurance (OR, 1.22), or having Medicare (OR, 1.16). Institutional factors associated with a greater likelihood of meeting all four quality measures included higher-volume centers, defined as treating at least 38 cases per year (OR, 1.18), or being an academic institution (OR, 1.31).
At the same time, factors associated with a lower likelihood of recommended surgical care included increasing age (per year increase, OR, 0.99) and a higher Charlson/Deyo comorbidity score (OR, 0.90 for a score of 1 and OR, 0.82 for a score of 2 or more). The strongest determinant of long-term overall survival included pathologic upstaging (HR 1.84) and meeting all four quality indicators (HR 0.39). Every additional quality met was associated with a significant reduction in overall mortality.
“We believe this study can be a starting point to draw attention to institution- and surgeon-specific practice patterns that may vary widely,” Dr. Samson said in an interview prior to the meeting. “At our own institution, we are working to decrease time to surgery, as well as implementing quality improvement measures to increase nodal sampling rates. Improving these trends nationally must start at the local level, with a tailored approach.”
Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – National adherence to quality indicators for surgery in stage I non–small cell lung cancer is suboptimal, results from a large analysis of national data suggest.
“Compliance with such guidelines is a strong predictor of long-term survival, and vigorous efforts should be instituted at the level of national societies to improve such adherence,” researchers led by Dr. Pamela P. Samson wrote in an abstract presented at the annual meeting of the Society of Thoracic Surgeons. “National organizations, including American College of Chest Physicians, the National Comprehensive Cancer Network, and the American College of Surgeons Commission on Cancer, have recommended quality standards for surgery in early-stage non–small cell lung cancer (NSCLC). The determinants and outcomes of adherence to these guidelines for early-stage lung cancer patients are largely unknown.”
Dr. Samson, a general surgery resident at Washington University in St. Louis, and her associates used the National Cancer Data Base to evaluate data from 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013. They selected the following four quality measures for evaluation: performing an anatomical pulmonary resection, surgery within 8 weeks of diagnosis, R0 resection, and evaluation of 10 or more lymph nodes. Next, the researchers fitted multivariate models to identify variables independently associated with adherence to quality measures, and created a Cox multivariate model to evaluate long-term overall survival.
Dr. Varun Puri, senior author of the study, presented the findings at the STS meeting on behalf of Dr. Samson, and discussed the findings in a video interview. The researchers found that between 2004 and 2013, nearly 100% of patients met at least one of the four recommended criteria, 95% met two, 69% met three, and 22% met all four. Sampling of 10 or more lymph nodes was the least frequently met measure, occurring in only 31% of surgical patients. Patient factors associated with a greater likelihood of receiving all four quality measures included average income in ZIP code of at least $38,000 (odds ratio, 1.20), private insurance (OR, 1.22), or having Medicare (OR, 1.16). Institutional factors associated with a greater likelihood of meeting all four quality measures included higher-volume centers, defined as treating at least 38 cases per year (OR, 1.18), or being an academic institution (OR, 1.31).
At the same time, factors associated with a lower likelihood of recommended surgical care included increasing age (per year increase, OR, 0.99) and a higher Charlson/Deyo comorbidity score (OR, 0.90 for a score of 1 and OR, 0.82 for a score of 2 or more). The strongest determinant of long-term overall survival included pathologic upstaging (HR 1.84) and meeting all four quality indicators (HR 0.39). Every additional quality met was associated with a significant reduction in overall mortality.
“We believe this study can be a starting point to draw attention to institution- and surgeon-specific practice patterns that may vary widely,” Dr. Samson said in an interview prior to the meeting. “At our own institution, we are working to decrease time to surgery, as well as implementing quality improvement measures to increase nodal sampling rates. Improving these trends nationally must start at the local level, with a tailored approach.”
Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE STS ANNUAL MEETING
Key clinical point: At the national level, compliance with core indicators for surgery in stage I NSCLC is poor.
Major finding: Between 2004 and 2013, nearly 100% of patients met at least one of four recommended criteria for evaluation of stage I NSCLC, 95% met two, 69% met three, and 22% met all four.
Data source: An analysis of 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013.
Disclosures: Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
STS: Hybrid thoracic suite leverages CT’s imaging sensitivity
PHOENIX – Using CT imaging to detect lung cancers in people at high risk for developing it has made it possible to find small tumors with substantially increased sensitivity than is possible with radiography, However, this approach has posed a new challenge to thoracic surgeons: How to visualize these nodules – subcentimeter and nonpalpable – for biopsy or for resection?
The answer may be the hybrid thoracic operating room developed by Dr. Kazuhiro Yasufuku and his associates at Toronto General Hospital, a novel surgical suite that he described at the annual meeting of the Society of Thoracic Surgeons.
Dr. Yasufuku and his team began using the hybrid operating room on an investigational basis in 2013 and have now done about 50 cases as part of several research protocols. The trials address the feasibility of resection, biopsy, and nodule localization, as well as whether the hybrid approach reduces the amount of radiation exposure to both patients and to the surgical team, he said. They plan to report some of their initial results later this year.
The Toronto group assembled the hybrid array of equipment into a single operating room that includes both a dual-source, dual-energy CT scanner and a robotic cone-beam CT scanner, equipment for minimally invasive procedures including video-assisted thoracoscopic and robotic surgery, and advanced endoscopic technology including endobronchial ultrasound and navigational bronchoscopy. “We use innovative methods that we already know about, but bring them all together” within a single space, Dr. Yasufuku explained. “Rather than having patients go to several locations, we can do everything at the same time in one room.”
Perhaps the most novel aspect of this operating room is inclusion of a robotic cone-beam CT scanner, which uses mobile, flat CT-imaging panels that overcome the limitations of a conventional, fixed CT scanner. “They scan the patient and then we can retract them and get them out of the way” to better facilitate surgery, he said in an interview.
“We do not have a culture in thoracic surgery of using imaging during surgery,” said Dr. Yasufuku, director of the interventional thoracic surgery program at the University of Toronto. Hybrid operating rooms using noninvasive or minimally invasive equipment and procedures have become commonplace for cardiovascular surgeons and cardiac interventionalists, but this approach has generally not yet been applied to thoracic surgery for cancer, in large part because of the imaging limitations, he said. “It is difficult to perform video-assisted thorascopic surgery using fixed CT.”
Bronchoscopic technologies provide additional, important tools for minimally invasive thoracic surgery. “We use the hybrid operating room to mark small [nonpalpable] lesions.” One approach to marking is to place a microcoil within the nodule with a percutaneous needle. Another approach is to tag the nodule with a fluorescent dye using navigational bronchoscopy.
Dr. Yasufuku also emphasized that the hybrid operating room will also be valuable when new, minimally invasive, nonsurgical therapeutic options for treatment of lung cancer become available in the near future.
Dr. Yasufuku said that he had no relevant disclosures.
On Twitter @mitchelzoler
PHOENIX – Using CT imaging to detect lung cancers in people at high risk for developing it has made it possible to find small tumors with substantially increased sensitivity than is possible with radiography, However, this approach has posed a new challenge to thoracic surgeons: How to visualize these nodules – subcentimeter and nonpalpable – for biopsy or for resection?
The answer may be the hybrid thoracic operating room developed by Dr. Kazuhiro Yasufuku and his associates at Toronto General Hospital, a novel surgical suite that he described at the annual meeting of the Society of Thoracic Surgeons.
Dr. Yasufuku and his team began using the hybrid operating room on an investigational basis in 2013 and have now done about 50 cases as part of several research protocols. The trials address the feasibility of resection, biopsy, and nodule localization, as well as whether the hybrid approach reduces the amount of radiation exposure to both patients and to the surgical team, he said. They plan to report some of their initial results later this year.
The Toronto group assembled the hybrid array of equipment into a single operating room that includes both a dual-source, dual-energy CT scanner and a robotic cone-beam CT scanner, equipment for minimally invasive procedures including video-assisted thoracoscopic and robotic surgery, and advanced endoscopic technology including endobronchial ultrasound and navigational bronchoscopy. “We use innovative methods that we already know about, but bring them all together” within a single space, Dr. Yasufuku explained. “Rather than having patients go to several locations, we can do everything at the same time in one room.”
Perhaps the most novel aspect of this operating room is inclusion of a robotic cone-beam CT scanner, which uses mobile, flat CT-imaging panels that overcome the limitations of a conventional, fixed CT scanner. “They scan the patient and then we can retract them and get them out of the way” to better facilitate surgery, he said in an interview.
“We do not have a culture in thoracic surgery of using imaging during surgery,” said Dr. Yasufuku, director of the interventional thoracic surgery program at the University of Toronto. Hybrid operating rooms using noninvasive or minimally invasive equipment and procedures have become commonplace for cardiovascular surgeons and cardiac interventionalists, but this approach has generally not yet been applied to thoracic surgery for cancer, in large part because of the imaging limitations, he said. “It is difficult to perform video-assisted thorascopic surgery using fixed CT.”
Bronchoscopic technologies provide additional, important tools for minimally invasive thoracic surgery. “We use the hybrid operating room to mark small [nonpalpable] lesions.” One approach to marking is to place a microcoil within the nodule with a percutaneous needle. Another approach is to tag the nodule with a fluorescent dye using navigational bronchoscopy.
Dr. Yasufuku also emphasized that the hybrid operating room will also be valuable when new, minimally invasive, nonsurgical therapeutic options for treatment of lung cancer become available in the near future.
Dr. Yasufuku said that he had no relevant disclosures.
On Twitter @mitchelzoler
PHOENIX – Using CT imaging to detect lung cancers in people at high risk for developing it has made it possible to find small tumors with substantially increased sensitivity than is possible with radiography, However, this approach has posed a new challenge to thoracic surgeons: How to visualize these nodules – subcentimeter and nonpalpable – for biopsy or for resection?
The answer may be the hybrid thoracic operating room developed by Dr. Kazuhiro Yasufuku and his associates at Toronto General Hospital, a novel surgical suite that he described at the annual meeting of the Society of Thoracic Surgeons.
Dr. Yasufuku and his team began using the hybrid operating room on an investigational basis in 2013 and have now done about 50 cases as part of several research protocols. The trials address the feasibility of resection, biopsy, and nodule localization, as well as whether the hybrid approach reduces the amount of radiation exposure to both patients and to the surgical team, he said. They plan to report some of their initial results later this year.
The Toronto group assembled the hybrid array of equipment into a single operating room that includes both a dual-source, dual-energy CT scanner and a robotic cone-beam CT scanner, equipment for minimally invasive procedures including video-assisted thoracoscopic and robotic surgery, and advanced endoscopic technology including endobronchial ultrasound and navigational bronchoscopy. “We use innovative methods that we already know about, but bring them all together” within a single space, Dr. Yasufuku explained. “Rather than having patients go to several locations, we can do everything at the same time in one room.”
Perhaps the most novel aspect of this operating room is inclusion of a robotic cone-beam CT scanner, which uses mobile, flat CT-imaging panels that overcome the limitations of a conventional, fixed CT scanner. “They scan the patient and then we can retract them and get them out of the way” to better facilitate surgery, he said in an interview.
“We do not have a culture in thoracic surgery of using imaging during surgery,” said Dr. Yasufuku, director of the interventional thoracic surgery program at the University of Toronto. Hybrid operating rooms using noninvasive or minimally invasive equipment and procedures have become commonplace for cardiovascular surgeons and cardiac interventionalists, but this approach has generally not yet been applied to thoracic surgery for cancer, in large part because of the imaging limitations, he said. “It is difficult to perform video-assisted thorascopic surgery using fixed CT.”
Bronchoscopic technologies provide additional, important tools for minimally invasive thoracic surgery. “We use the hybrid operating room to mark small [nonpalpable] lesions.” One approach to marking is to place a microcoil within the nodule with a percutaneous needle. Another approach is to tag the nodule with a fluorescent dye using navigational bronchoscopy.
Dr. Yasufuku also emphasized that the hybrid operating room will also be valuable when new, minimally invasive, nonsurgical therapeutic options for treatment of lung cancer become available in the near future.
Dr. Yasufuku said that he had no relevant disclosures.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE STS ANNUAL MEETING
Acupressure improves persistent fatigue in breast cancer survivors
SAN ANTONIO – Self-administered acupressure focused on enhancing relaxation significantly reduced persistent fatigue symptoms in breast cancer survivors, according to a randomized clinical trial presented at the San Antonio Breast Cancer Symposium.
“Self-administered relaxation acupressure offers an inexpensive, easy-to-learn method to manage fatigue and co-occurring poor sleep quality and overall quality of life in breast cancer survivors with persistent fatigue,” said Suzanna M. Zick, N.D., MPH, of the department of family medicine, and the complementary and alternative medicine research center at the University of Michigan, Ann Arbor.
She conducted the study because persistent fatigue is arguably the most common and debilitating symptom experienced by breast cancer survivors, affecting 30% of women for up to 10 years after they’ve completed their breast cancer therapy. Yet treatment options remain limited, she said.
Acupressure is a form of traditional Chinese medicine in which pressure is applied to a few specific acupoints on the body using the fingers, thumbs, or a device. Two forms were evaluated in the three-arm, single-blind clinical trial: relaxation acupressure, traditionally used to improve sleep, and stimulation acupressure, which targets pressure points that boost energy.
Dr. Zick presented a 10-week study in which 288 breast cancer survivors who had completed cancer therapy other than hormone treatment at least 12 months before and who still experienced persistent fatigue as defined by a score of 4 or more on the validated Brief Fatigue Inventory. Participants were randomized single-blind to usual care as directed by their physician or to 6 weeks of relaxation or stimulation acupressure, which they administered on their own after receiving instruction. After 6 weeks, women were instructed to stop the acupressure. They were reassessed at week 10 to determine whether acupressure had a sustained carryover effect.
At 6 weeks, 66% of the relaxation acupressure group and 61% of the stimulation acupressure cohort had achieved a normal Brief Fatigue Inventory score of less than 4, as did only 31% of the usual-care controls. Both acupressure groups showed maintenance of benefit at week 10, after 4 weeks of no acupressure, indicating the self-treatment isn’t something patients need to do continuously in order to derive the desired effect.
While both forms of acupressure were similarly effective at reducing complaints of fatigue, there was an important difference between the two. Only relaxation acupressure resulted in significant improvement in sleep quality as measured on the Pittsburgh Sleep Quality Index. Moreover, relaxation acupressure but not stimulation acupressure resulted in quality-of-life improvements on the somatic, fitness, and social support subscales of the Long-Term Quality of Life scale. However, neither form of acupressure had a significant on the spiritual subscale, the quality-of-life instrument’s fourth subscale.
“We really have to conclude that even though both forms of acupressure reduce fatigue to a similar extent, relaxation acupressure is the one we should think about as being more effective,” Dr. Zick said.
One might have predicted, incorrectly as it turns out, that breast cancer survivors complaining of persistent fatigue would find stimulation acupressure to be more beneficial than relaxation acupressure. Dr. Zick suspects the two techniques might reduce chronic fatigue via different mechanisms. She and her coinvestigators have conducted brain imaging studies that show patients with persistent cancer-related fatigue have three neurochemical markers: elevated brain levels of insular glutamate, which causes excitation, as well as high brain levels of creatine phosphokinase and proinflammatory cytokines. In their next round of imaging studies, the investigators plan to see whether the two forms of acupressure have differing effects on this markers.
Session moderator Dr. Norah Lynn Henry liked the concept of self-administered acupressure.
“The great thing about this is you don’t have to make appointments with an acupuncturist. You can do it at home. But is acupressure ready for prime time in clinical practice?” asked Dr. Henry, a medical oncologist at the University of Michigan.
“My answer is yes,” Dr. Zick replied, “because it’s got pretty much zero side effects, it’s inexpensive, and it’s easy to learn. If it doesn’t work for a person then they can just stop, but if it works, great.”
As the next step in this research, Dr. Zick and her coinvestigators hope to develop a smartphone app to deliver instruction in self-administered relaxation acupressure in a readily accessible way.
Her clinical trial was funded by the National Cancer Institute. She reported having no financial conflicts.
SAN ANTONIO – Self-administered acupressure focused on enhancing relaxation significantly reduced persistent fatigue symptoms in breast cancer survivors, according to a randomized clinical trial presented at the San Antonio Breast Cancer Symposium.
“Self-administered relaxation acupressure offers an inexpensive, easy-to-learn method to manage fatigue and co-occurring poor sleep quality and overall quality of life in breast cancer survivors with persistent fatigue,” said Suzanna M. Zick, N.D., MPH, of the department of family medicine, and the complementary and alternative medicine research center at the University of Michigan, Ann Arbor.
She conducted the study because persistent fatigue is arguably the most common and debilitating symptom experienced by breast cancer survivors, affecting 30% of women for up to 10 years after they’ve completed their breast cancer therapy. Yet treatment options remain limited, she said.
Acupressure is a form of traditional Chinese medicine in which pressure is applied to a few specific acupoints on the body using the fingers, thumbs, or a device. Two forms were evaluated in the three-arm, single-blind clinical trial: relaxation acupressure, traditionally used to improve sleep, and stimulation acupressure, which targets pressure points that boost energy.
Dr. Zick presented a 10-week study in which 288 breast cancer survivors who had completed cancer therapy other than hormone treatment at least 12 months before and who still experienced persistent fatigue as defined by a score of 4 or more on the validated Brief Fatigue Inventory. Participants were randomized single-blind to usual care as directed by their physician or to 6 weeks of relaxation or stimulation acupressure, which they administered on their own after receiving instruction. After 6 weeks, women were instructed to stop the acupressure. They were reassessed at week 10 to determine whether acupressure had a sustained carryover effect.
At 6 weeks, 66% of the relaxation acupressure group and 61% of the stimulation acupressure cohort had achieved a normal Brief Fatigue Inventory score of less than 4, as did only 31% of the usual-care controls. Both acupressure groups showed maintenance of benefit at week 10, after 4 weeks of no acupressure, indicating the self-treatment isn’t something patients need to do continuously in order to derive the desired effect.
While both forms of acupressure were similarly effective at reducing complaints of fatigue, there was an important difference between the two. Only relaxation acupressure resulted in significant improvement in sleep quality as measured on the Pittsburgh Sleep Quality Index. Moreover, relaxation acupressure but not stimulation acupressure resulted in quality-of-life improvements on the somatic, fitness, and social support subscales of the Long-Term Quality of Life scale. However, neither form of acupressure had a significant on the spiritual subscale, the quality-of-life instrument’s fourth subscale.
“We really have to conclude that even though both forms of acupressure reduce fatigue to a similar extent, relaxation acupressure is the one we should think about as being more effective,” Dr. Zick said.
One might have predicted, incorrectly as it turns out, that breast cancer survivors complaining of persistent fatigue would find stimulation acupressure to be more beneficial than relaxation acupressure. Dr. Zick suspects the two techniques might reduce chronic fatigue via different mechanisms. She and her coinvestigators have conducted brain imaging studies that show patients with persistent cancer-related fatigue have three neurochemical markers: elevated brain levels of insular glutamate, which causes excitation, as well as high brain levels of creatine phosphokinase and proinflammatory cytokines. In their next round of imaging studies, the investigators plan to see whether the two forms of acupressure have differing effects on this markers.
Session moderator Dr. Norah Lynn Henry liked the concept of self-administered acupressure.
“The great thing about this is you don’t have to make appointments with an acupuncturist. You can do it at home. But is acupressure ready for prime time in clinical practice?” asked Dr. Henry, a medical oncologist at the University of Michigan.
“My answer is yes,” Dr. Zick replied, “because it’s got pretty much zero side effects, it’s inexpensive, and it’s easy to learn. If it doesn’t work for a person then they can just stop, but if it works, great.”
As the next step in this research, Dr. Zick and her coinvestigators hope to develop a smartphone app to deliver instruction in self-administered relaxation acupressure in a readily accessible way.
Her clinical trial was funded by the National Cancer Institute. She reported having no financial conflicts.
SAN ANTONIO – Self-administered acupressure focused on enhancing relaxation significantly reduced persistent fatigue symptoms in breast cancer survivors, according to a randomized clinical trial presented at the San Antonio Breast Cancer Symposium.
“Self-administered relaxation acupressure offers an inexpensive, easy-to-learn method to manage fatigue and co-occurring poor sleep quality and overall quality of life in breast cancer survivors with persistent fatigue,” said Suzanna M. Zick, N.D., MPH, of the department of family medicine, and the complementary and alternative medicine research center at the University of Michigan, Ann Arbor.
She conducted the study because persistent fatigue is arguably the most common and debilitating symptom experienced by breast cancer survivors, affecting 30% of women for up to 10 years after they’ve completed their breast cancer therapy. Yet treatment options remain limited, she said.
Acupressure is a form of traditional Chinese medicine in which pressure is applied to a few specific acupoints on the body using the fingers, thumbs, or a device. Two forms were evaluated in the three-arm, single-blind clinical trial: relaxation acupressure, traditionally used to improve sleep, and stimulation acupressure, which targets pressure points that boost energy.
Dr. Zick presented a 10-week study in which 288 breast cancer survivors who had completed cancer therapy other than hormone treatment at least 12 months before and who still experienced persistent fatigue as defined by a score of 4 or more on the validated Brief Fatigue Inventory. Participants were randomized single-blind to usual care as directed by their physician or to 6 weeks of relaxation or stimulation acupressure, which they administered on their own after receiving instruction. After 6 weeks, women were instructed to stop the acupressure. They were reassessed at week 10 to determine whether acupressure had a sustained carryover effect.
At 6 weeks, 66% of the relaxation acupressure group and 61% of the stimulation acupressure cohort had achieved a normal Brief Fatigue Inventory score of less than 4, as did only 31% of the usual-care controls. Both acupressure groups showed maintenance of benefit at week 10, after 4 weeks of no acupressure, indicating the self-treatment isn’t something patients need to do continuously in order to derive the desired effect.
While both forms of acupressure were similarly effective at reducing complaints of fatigue, there was an important difference between the two. Only relaxation acupressure resulted in significant improvement in sleep quality as measured on the Pittsburgh Sleep Quality Index. Moreover, relaxation acupressure but not stimulation acupressure resulted in quality-of-life improvements on the somatic, fitness, and social support subscales of the Long-Term Quality of Life scale. However, neither form of acupressure had a significant on the spiritual subscale, the quality-of-life instrument’s fourth subscale.
“We really have to conclude that even though both forms of acupressure reduce fatigue to a similar extent, relaxation acupressure is the one we should think about as being more effective,” Dr. Zick said.
One might have predicted, incorrectly as it turns out, that breast cancer survivors complaining of persistent fatigue would find stimulation acupressure to be more beneficial than relaxation acupressure. Dr. Zick suspects the two techniques might reduce chronic fatigue via different mechanisms. She and her coinvestigators have conducted brain imaging studies that show patients with persistent cancer-related fatigue have three neurochemical markers: elevated brain levels of insular glutamate, which causes excitation, as well as high brain levels of creatine phosphokinase and proinflammatory cytokines. In their next round of imaging studies, the investigators plan to see whether the two forms of acupressure have differing effects on this markers.
Session moderator Dr. Norah Lynn Henry liked the concept of self-administered acupressure.
“The great thing about this is you don’t have to make appointments with an acupuncturist. You can do it at home. But is acupressure ready for prime time in clinical practice?” asked Dr. Henry, a medical oncologist at the University of Michigan.
“My answer is yes,” Dr. Zick replied, “because it’s got pretty much zero side effects, it’s inexpensive, and it’s easy to learn. If it doesn’t work for a person then they can just stop, but if it works, great.”
As the next step in this research, Dr. Zick and her coinvestigators hope to develop a smartphone app to deliver instruction in self-administered relaxation acupressure in a readily accessible way.
Her clinical trial was funded by the National Cancer Institute. She reported having no financial conflicts.
AT SABCS 2015
Key clinical point: Acupressure is an easily learned, effective method for self-treatment of persistent fatigue in breast cancer survivors.
Major finding: Two-thirds of breast cancer survivors with persistent fatigue experienced significant improvement in response to 6 weeks of self-administered relaxation acupressure, compared with 31% of usual-care controls.
Data source: This was a 10-week, single-blind study involving 288 breast cancer survivors with persistent fatigue who were randomized to relaxation acupressure, stimulation acupressure, or usual care.
Disclosures: The study was sponsored by the National Cancer Institute. Dr. Zick reported having no financial conflicts.
Fertility preservation in early cervical cancer
Historically, the standard of care for women diagnosed with early cervical cancer has been radical hysterectomy. Thus, young women are not only being confronted with a cancer diagnosis, but may also be forced to cope with the loss of their fertility.
As many young women with cervical cancer were not accepting of this treatment, Dr. Daniel Dargent pioneered the vaginal radical trachelectomy as a fertility-preserving treatment option for early cervical cancer in 1994. There have now been more than 900 vaginal radical trachelectomies performed and they have been shown to have oncologic outcomes similar to those of traditional radical hysterectomy, while sparing a woman’s fertility (Int J Gynecol Cancer. 2013 Jul;23[6]:982-9).
Obstetric outcomes following vaginal radical trachelectomy are acceptable with 17% miscarriage rate in the first trimester (compared to 10%-20% in the general population) and 8% in the second trimester (compared to 1%-5% in the general population) (Am Fam Physician. 2007 Nov 1;76[9]:1341-6). Following vaginal radical trachelectomy, 64% of pregnancies deliver at term.
The usual criteria required to undergo radical trachelectomy include:
1) Reproductive age with desire for fertility.
2) Stage IA1 with LVSI (lymphovascular space invasion), IA2, or IB1 with tumor less than 2 cm.
3) Limited endocervical involvement via preoperative MRI.
4) Negative pelvic lymph nodes.
Preoperative PET scan can be used to evaluate nodal status, but suspicious lymph nodes should be evaluated on frozen section at the time of surgery. The presence of LVSI alone is not a contraindication to trachelectomy.
A key limitation of vaginal radical trachelectomy is the specialized training required to perform this technically challenging procedure. Few surgeons in the United States are trained to perform vaginal radical trachelectomy. In response to this limitation, surgeons began to attempt radical trachelectomy via laparotomy (Gynecol Oncol. 2006 Dec;103[3]:807-13). Oncologic outcomes following fertility-sparing abdominal radical trachelectomy have been reported to be equivalent to radical hysterectomy. Concerns regarding the abdominal approach to radical trachelectomy include higher rates of second trimester loss (19%) when compared to the vaginal approach (8%), higher rate of loss of fertility (30%), and risk of postoperative adhesions.
The advent of minimally invasive surgery, particularly robotic surgery, now offers surgeons the ability to perform a procedure technically similar to radical hysterectomy using a minimally invasive approach. Given the similarity of procedural steps of radical trachelectomy to radical hysterectomy using the robotic platform, this procedure is gaining acceptance in the United States with an associated improved surgeon learning curve (Gynecol Oncol. 2008 Nov;111[2]:255-60). In addition, the use of minimally invasive surgery should result in less adhesion formation facilitating natural fertility options postoperatively.
Obstetric and fertility outcomes are limited following minimally invasive radical trachelectomy via laparoscopy or robotic surgery given the novelty of this procedure. Emerging obstetric outcomes appear reassuring, but further data are needed to fully understand the effects of this procedure on pregnancy outcomes and the need for assisted reproductive techniques to achieve pregnancy.
The management of pregnancies following radical trachelectomy is also an area with limited data, which presents a clinical challenge to obstetricians. Many gynecologic oncologists perform a permanent cerclage at the time of trachelectomy and recommend delivery via scheduled cesarean at term for all subsequent pregnancies prior to labor (usually 37-38 weeks).
At our institution, we recommend the use of progesterone from 16 to 36 weeks despite no clear evidence on the role of progesterone in this setting. Maternal-fetal medicine consultation should be considered to either follow these patients during their pregnancies or to perform a single consultative visit to guide antepartum care.
Some have advocated for less radical surgery, such as simple trachelectomy or large cold knife conization, as the risk of parametrial extension in these patients is low (Gynecol Oncol. 2011 Dec;123[3]:557-60). More data are needed to determine if this is a safe approach. Further, the use of neoadjuvant chemotherapy followed by cold knife conization for fertility preservation in women with larger tumors has been proposed. This may be a feasible option in women with chemo-sensitive tumors, but progression on chemotherapy and increased recurrences have been reported with this approach (Gynecol Oncol. 2008 Dec;111[3]:438-43).
Women of reproductive age diagnosed with early cervical cancer now have multiple options for fertility preservation. Ongoing research regarding obstetric and fertility outcomes is needed; however, oncologic outcomes appear to be equivalent.
Dr. Clark is a fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina, Chapel Hill. Dr. Boggess is an expert in robotic surgery in gynecologic oncology and is a professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no financial disclosures relevant to this column. Email them at [email protected].
Historically, the standard of care for women diagnosed with early cervical cancer has been radical hysterectomy. Thus, young women are not only being confronted with a cancer diagnosis, but may also be forced to cope with the loss of their fertility.
As many young women with cervical cancer were not accepting of this treatment, Dr. Daniel Dargent pioneered the vaginal radical trachelectomy as a fertility-preserving treatment option for early cervical cancer in 1994. There have now been more than 900 vaginal radical trachelectomies performed and they have been shown to have oncologic outcomes similar to those of traditional radical hysterectomy, while sparing a woman’s fertility (Int J Gynecol Cancer. 2013 Jul;23[6]:982-9).
Obstetric outcomes following vaginal radical trachelectomy are acceptable with 17% miscarriage rate in the first trimester (compared to 10%-20% in the general population) and 8% in the second trimester (compared to 1%-5% in the general population) (Am Fam Physician. 2007 Nov 1;76[9]:1341-6). Following vaginal radical trachelectomy, 64% of pregnancies deliver at term.
The usual criteria required to undergo radical trachelectomy include:
1) Reproductive age with desire for fertility.
2) Stage IA1 with LVSI (lymphovascular space invasion), IA2, or IB1 with tumor less than 2 cm.
3) Limited endocervical involvement via preoperative MRI.
4) Negative pelvic lymph nodes.
Preoperative PET scan can be used to evaluate nodal status, but suspicious lymph nodes should be evaluated on frozen section at the time of surgery. The presence of LVSI alone is not a contraindication to trachelectomy.
A key limitation of vaginal radical trachelectomy is the specialized training required to perform this technically challenging procedure. Few surgeons in the United States are trained to perform vaginal radical trachelectomy. In response to this limitation, surgeons began to attempt radical trachelectomy via laparotomy (Gynecol Oncol. 2006 Dec;103[3]:807-13). Oncologic outcomes following fertility-sparing abdominal radical trachelectomy have been reported to be equivalent to radical hysterectomy. Concerns regarding the abdominal approach to radical trachelectomy include higher rates of second trimester loss (19%) when compared to the vaginal approach (8%), higher rate of loss of fertility (30%), and risk of postoperative adhesions.
The advent of minimally invasive surgery, particularly robotic surgery, now offers surgeons the ability to perform a procedure technically similar to radical hysterectomy using a minimally invasive approach. Given the similarity of procedural steps of radical trachelectomy to radical hysterectomy using the robotic platform, this procedure is gaining acceptance in the United States with an associated improved surgeon learning curve (Gynecol Oncol. 2008 Nov;111[2]:255-60). In addition, the use of minimally invasive surgery should result in less adhesion formation facilitating natural fertility options postoperatively.
Obstetric and fertility outcomes are limited following minimally invasive radical trachelectomy via laparoscopy or robotic surgery given the novelty of this procedure. Emerging obstetric outcomes appear reassuring, but further data are needed to fully understand the effects of this procedure on pregnancy outcomes and the need for assisted reproductive techniques to achieve pregnancy.
The management of pregnancies following radical trachelectomy is also an area with limited data, which presents a clinical challenge to obstetricians. Many gynecologic oncologists perform a permanent cerclage at the time of trachelectomy and recommend delivery via scheduled cesarean at term for all subsequent pregnancies prior to labor (usually 37-38 weeks).
At our institution, we recommend the use of progesterone from 16 to 36 weeks despite no clear evidence on the role of progesterone in this setting. Maternal-fetal medicine consultation should be considered to either follow these patients during their pregnancies or to perform a single consultative visit to guide antepartum care.
Some have advocated for less radical surgery, such as simple trachelectomy or large cold knife conization, as the risk of parametrial extension in these patients is low (Gynecol Oncol. 2011 Dec;123[3]:557-60). More data are needed to determine if this is a safe approach. Further, the use of neoadjuvant chemotherapy followed by cold knife conization for fertility preservation in women with larger tumors has been proposed. This may be a feasible option in women with chemo-sensitive tumors, but progression on chemotherapy and increased recurrences have been reported with this approach (Gynecol Oncol. 2008 Dec;111[3]:438-43).
Women of reproductive age diagnosed with early cervical cancer now have multiple options for fertility preservation. Ongoing research regarding obstetric and fertility outcomes is needed; however, oncologic outcomes appear to be equivalent.
Dr. Clark is a fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina, Chapel Hill. Dr. Boggess is an expert in robotic surgery in gynecologic oncology and is a professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no financial disclosures relevant to this column. Email them at [email protected].
Historically, the standard of care for women diagnosed with early cervical cancer has been radical hysterectomy. Thus, young women are not only being confronted with a cancer diagnosis, but may also be forced to cope with the loss of their fertility.
As many young women with cervical cancer were not accepting of this treatment, Dr. Daniel Dargent pioneered the vaginal radical trachelectomy as a fertility-preserving treatment option for early cervical cancer in 1994. There have now been more than 900 vaginal radical trachelectomies performed and they have been shown to have oncologic outcomes similar to those of traditional radical hysterectomy, while sparing a woman’s fertility (Int J Gynecol Cancer. 2013 Jul;23[6]:982-9).
Obstetric outcomes following vaginal radical trachelectomy are acceptable with 17% miscarriage rate in the first trimester (compared to 10%-20% in the general population) and 8% in the second trimester (compared to 1%-5% in the general population) (Am Fam Physician. 2007 Nov 1;76[9]:1341-6). Following vaginal radical trachelectomy, 64% of pregnancies deliver at term.
The usual criteria required to undergo radical trachelectomy include:
1) Reproductive age with desire for fertility.
2) Stage IA1 with LVSI (lymphovascular space invasion), IA2, or IB1 with tumor less than 2 cm.
3) Limited endocervical involvement via preoperative MRI.
4) Negative pelvic lymph nodes.
Preoperative PET scan can be used to evaluate nodal status, but suspicious lymph nodes should be evaluated on frozen section at the time of surgery. The presence of LVSI alone is not a contraindication to trachelectomy.
A key limitation of vaginal radical trachelectomy is the specialized training required to perform this technically challenging procedure. Few surgeons in the United States are trained to perform vaginal radical trachelectomy. In response to this limitation, surgeons began to attempt radical trachelectomy via laparotomy (Gynecol Oncol. 2006 Dec;103[3]:807-13). Oncologic outcomes following fertility-sparing abdominal radical trachelectomy have been reported to be equivalent to radical hysterectomy. Concerns regarding the abdominal approach to radical trachelectomy include higher rates of second trimester loss (19%) when compared to the vaginal approach (8%), higher rate of loss of fertility (30%), and risk of postoperative adhesions.
The advent of minimally invasive surgery, particularly robotic surgery, now offers surgeons the ability to perform a procedure technically similar to radical hysterectomy using a minimally invasive approach. Given the similarity of procedural steps of radical trachelectomy to radical hysterectomy using the robotic platform, this procedure is gaining acceptance in the United States with an associated improved surgeon learning curve (Gynecol Oncol. 2008 Nov;111[2]:255-60). In addition, the use of minimally invasive surgery should result in less adhesion formation facilitating natural fertility options postoperatively.
Obstetric and fertility outcomes are limited following minimally invasive radical trachelectomy via laparoscopy or robotic surgery given the novelty of this procedure. Emerging obstetric outcomes appear reassuring, but further data are needed to fully understand the effects of this procedure on pregnancy outcomes and the need for assisted reproductive techniques to achieve pregnancy.
The management of pregnancies following radical trachelectomy is also an area with limited data, which presents a clinical challenge to obstetricians. Many gynecologic oncologists perform a permanent cerclage at the time of trachelectomy and recommend delivery via scheduled cesarean at term for all subsequent pregnancies prior to labor (usually 37-38 weeks).
At our institution, we recommend the use of progesterone from 16 to 36 weeks despite no clear evidence on the role of progesterone in this setting. Maternal-fetal medicine consultation should be considered to either follow these patients during their pregnancies or to perform a single consultative visit to guide antepartum care.
Some have advocated for less radical surgery, such as simple trachelectomy or large cold knife conization, as the risk of parametrial extension in these patients is low (Gynecol Oncol. 2011 Dec;123[3]:557-60). More data are needed to determine if this is a safe approach. Further, the use of neoadjuvant chemotherapy followed by cold knife conization for fertility preservation in women with larger tumors has been proposed. This may be a feasible option in women with chemo-sensitive tumors, but progression on chemotherapy and increased recurrences have been reported with this approach (Gynecol Oncol. 2008 Dec;111[3]:438-43).
Women of reproductive age diagnosed with early cervical cancer now have multiple options for fertility preservation. Ongoing research regarding obstetric and fertility outcomes is needed; however, oncologic outcomes appear to be equivalent.
Dr. Clark is a fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina, Chapel Hill. Dr. Boggess is an expert in robotic surgery in gynecologic oncology and is a professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no financial disclosures relevant to this column. Email them at [email protected].
Presurgery radiation shows benefit in lung cancer
The popularity of extrapleural pneumonectomy to treat asbestos-related thoracic mesothelioma has yielded to extended pleurectomy/decortication in recent years, but a recent study suggests that the extrapleural pneumonectomy procedure can achieve good results in a new protocol that involves administering radiation therapy before surgery as opposed the more conventional approach of radiation after surgery.
Researchers at the University of Toronto reported on their protocol that uses accelerated intensity modulated radiation therapy (IMRT) for malignant pleural mesothelioma (MPM) (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.09.129). They call the protocol SMART, for Surgery for Mesothelioma After Radiation Therapy.
“The rationale to develop this protocol was to optimize the delivery of radiation to the whole tumor bed, sterilize the edges of the tumor to limit the risk of spillage at the time of surgery, develop a shorter treatment plan and potentiate the activation of the immune system by using a hypofractionated regimen,” wrote Dr. Marc de Perrot and colleagues.
The protocol involves delivering 25 Gy of radiation in five daily fractions over a week to the entire side of the thorax with 5 Gy boosts based on imaging, followed by extrapleural pneumonectomy (EPP) 4-6 days later. Patients with three or more positive lymph notes (ypN2 disease) also are offered adjuvant chemotherapy.
The researchers performed the protocol on 62 patients from November 2008 to October 2014, which represents 24% of all patients with MPM seen at the institution in that period. Fifty-two patients were men and ages ranged from 41 to 75 years. Clinical stage of cancer ranged from T1N0 in 10 patients, to T2N0 in 35 and T3N0 in 13 (two had T4N0 and two had T3N2). Forty-five had right-side cancers. Six patients received an extended protocol for various reasons, including tumor extending to the chest wall.
All 62 patients completed IMRT and EPP. All but one had resection and reconstruction of the diaphragm, and all but four had resection and reconstruction of the pericardium.
Overall death rate was 4.8% (three patients). Results were better in patients with epithelioid tumors, with a median survival of 51 months and disease-free survival of 47 months. Those with biphasic subtypes had median survival of 10 months and disease-free survival of 8 months. Eight patients had ipsilateral chest recurrence. “This analysis demonstrates that the SMART approach is particularly encouraging for patients with epithelial subtype,” Dr. de Perrot and coauthors said. They no longer perform the SMART protocol on patients with biphasic subtype.
The protocol was not without complications. Twenty-four patients, about 38%, had serious complications that required intervention or worse. Twelve had atrial fibrillation, but none advanced to life-threatening disease. Among other complications, four had empyema – one resulting in death – and three had pulmonary emboli. One other patient in the complications group died from pneumonia, and another died from a heart attack at home.
This is the Toronto researchers’ second attempt at studying the three-modality approach. In their first attempt, only half the patients who started with preoperative chemotherapy went onto complete the radiation after surgery because of difficulties administering it (J Thorac Cardiovasc Surg. 2007;133:111-6; J Clin Oncol. 2009;27:1413-8). Also, about 25% of patients had disease progression during induction chemotherapy and could not go onto surgery.
They designed the most recent trial to deliver radiation before surgery because of the excellent local control of cancer along with evidence that MPM tumors were radio-sensitive. “Considering the risk of disease progression on induction chemotherapy, we felt that switching the order of therapy was potentially a better option for patients with surgically resectable disease,” Dr. de Perrott and colleagues said.
The researchers cited the study’s single-center nature with a single treatment arm, and the lack of longer-term follow-up, as limitations. “However, in our own experience, this approach has been very encouraging and has become our primary option for patients with surgically resectable MPM,” they noted.
The study authors had no conflicts to disclose.
Implementing the treatment regimen for malignant pleural mesothelioma (MPM) that the Toronto researchers studied poses “several high stakes challenges,” Dr. Valerie Rusch and coauthors at Memorial Sloan-Kettering Cancer Center, New York, said in their invited commentary (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.10.038).

|
Dr. Valerie W. Rusch |
But they noted challenges involved with conventional multi-modality treatment for MPM, namely the 6 months of intensive treatment. However, the experience of the Toronto researchers will be difficult to replicate, they said. “Such outstanding results reflect the expertise of Dr. de Perrot and colleagues in the surgical care of MPM and the excellence of their multidisciplinary program,” Dr. Rusch and coauthors said.
The study results are among the best reported for MPM to date, they added, but they asked why. “Are they solely related to patient selection or do they reflect the true impact of a novel approach to treatment?”
Patients selected for the treatment need to be able to undergo the extrapleural pneumonectomy (EPP) and the surgeon has to be able to predict the resectability of the tumor. But limitations in existing staging methods for MPM make it difficult to predict tumor resectability. “To avoid bronchial stump leaks and other serious complications after EPP requires experience along with meticulous surgical technique and postoperative care,” Dr. Rusch and colleagues said. “Only high-volume centers of excellence could potentially reproduce these results.”
Despite the waning in popularity of EPP, the study results underscore its effectiveness in carefully selected patients – “those with epithelioid tumor histology and no tumor metastases.” To corroborate the findings, reports on other centers’ experience along with human and animal studies rather than a randomized clinical trial are needed. “Dr. de Perrot and colleagues may have been not only bold but SMART,” Dr. Rusch and colleagues said.
Implementing the treatment regimen for malignant pleural mesothelioma (MPM) that the Toronto researchers studied poses “several high stakes challenges,” Dr. Valerie Rusch and coauthors at Memorial Sloan-Kettering Cancer Center, New York, said in their invited commentary (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.10.038).

|
Dr. Valerie W. Rusch |
But they noted challenges involved with conventional multi-modality treatment for MPM, namely the 6 months of intensive treatment. However, the experience of the Toronto researchers will be difficult to replicate, they said. “Such outstanding results reflect the expertise of Dr. de Perrot and colleagues in the surgical care of MPM and the excellence of their multidisciplinary program,” Dr. Rusch and coauthors said.
The study results are among the best reported for MPM to date, they added, but they asked why. “Are they solely related to patient selection or do they reflect the true impact of a novel approach to treatment?”
Patients selected for the treatment need to be able to undergo the extrapleural pneumonectomy (EPP) and the surgeon has to be able to predict the resectability of the tumor. But limitations in existing staging methods for MPM make it difficult to predict tumor resectability. “To avoid bronchial stump leaks and other serious complications after EPP requires experience along with meticulous surgical technique and postoperative care,” Dr. Rusch and colleagues said. “Only high-volume centers of excellence could potentially reproduce these results.”
Despite the waning in popularity of EPP, the study results underscore its effectiveness in carefully selected patients – “those with epithelioid tumor histology and no tumor metastases.” To corroborate the findings, reports on other centers’ experience along with human and animal studies rather than a randomized clinical trial are needed. “Dr. de Perrot and colleagues may have been not only bold but SMART,” Dr. Rusch and colleagues said.
Implementing the treatment regimen for malignant pleural mesothelioma (MPM) that the Toronto researchers studied poses “several high stakes challenges,” Dr. Valerie Rusch and coauthors at Memorial Sloan-Kettering Cancer Center, New York, said in their invited commentary (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.10.038).

|
Dr. Valerie W. Rusch |
But they noted challenges involved with conventional multi-modality treatment for MPM, namely the 6 months of intensive treatment. However, the experience of the Toronto researchers will be difficult to replicate, they said. “Such outstanding results reflect the expertise of Dr. de Perrot and colleagues in the surgical care of MPM and the excellence of their multidisciplinary program,” Dr. Rusch and coauthors said.
The study results are among the best reported for MPM to date, they added, but they asked why. “Are they solely related to patient selection or do they reflect the true impact of a novel approach to treatment?”
Patients selected for the treatment need to be able to undergo the extrapleural pneumonectomy (EPP) and the surgeon has to be able to predict the resectability of the tumor. But limitations in existing staging methods for MPM make it difficult to predict tumor resectability. “To avoid bronchial stump leaks and other serious complications after EPP requires experience along with meticulous surgical technique and postoperative care,” Dr. Rusch and colleagues said. “Only high-volume centers of excellence could potentially reproduce these results.”
Despite the waning in popularity of EPP, the study results underscore its effectiveness in carefully selected patients – “those with epithelioid tumor histology and no tumor metastases.” To corroborate the findings, reports on other centers’ experience along with human and animal studies rather than a randomized clinical trial are needed. “Dr. de Perrot and colleagues may have been not only bold but SMART,” Dr. Rusch and colleagues said.
The popularity of extrapleural pneumonectomy to treat asbestos-related thoracic mesothelioma has yielded to extended pleurectomy/decortication in recent years, but a recent study suggests that the extrapleural pneumonectomy procedure can achieve good results in a new protocol that involves administering radiation therapy before surgery as opposed the more conventional approach of radiation after surgery.
Researchers at the University of Toronto reported on their protocol that uses accelerated intensity modulated radiation therapy (IMRT) for malignant pleural mesothelioma (MPM) (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.09.129). They call the protocol SMART, for Surgery for Mesothelioma After Radiation Therapy.
“The rationale to develop this protocol was to optimize the delivery of radiation to the whole tumor bed, sterilize the edges of the tumor to limit the risk of spillage at the time of surgery, develop a shorter treatment plan and potentiate the activation of the immune system by using a hypofractionated regimen,” wrote Dr. Marc de Perrot and colleagues.
The protocol involves delivering 25 Gy of radiation in five daily fractions over a week to the entire side of the thorax with 5 Gy boosts based on imaging, followed by extrapleural pneumonectomy (EPP) 4-6 days later. Patients with three or more positive lymph notes (ypN2 disease) also are offered adjuvant chemotherapy.
The researchers performed the protocol on 62 patients from November 2008 to October 2014, which represents 24% of all patients with MPM seen at the institution in that period. Fifty-two patients were men and ages ranged from 41 to 75 years. Clinical stage of cancer ranged from T1N0 in 10 patients, to T2N0 in 35 and T3N0 in 13 (two had T4N0 and two had T3N2). Forty-five had right-side cancers. Six patients received an extended protocol for various reasons, including tumor extending to the chest wall.
All 62 patients completed IMRT and EPP. All but one had resection and reconstruction of the diaphragm, and all but four had resection and reconstruction of the pericardium.
Overall death rate was 4.8% (three patients). Results were better in patients with epithelioid tumors, with a median survival of 51 months and disease-free survival of 47 months. Those with biphasic subtypes had median survival of 10 months and disease-free survival of 8 months. Eight patients had ipsilateral chest recurrence. “This analysis demonstrates that the SMART approach is particularly encouraging for patients with epithelial subtype,” Dr. de Perrot and coauthors said. They no longer perform the SMART protocol on patients with biphasic subtype.
The protocol was not without complications. Twenty-four patients, about 38%, had serious complications that required intervention or worse. Twelve had atrial fibrillation, but none advanced to life-threatening disease. Among other complications, four had empyema – one resulting in death – and three had pulmonary emboli. One other patient in the complications group died from pneumonia, and another died from a heart attack at home.
This is the Toronto researchers’ second attempt at studying the three-modality approach. In their first attempt, only half the patients who started with preoperative chemotherapy went onto complete the radiation after surgery because of difficulties administering it (J Thorac Cardiovasc Surg. 2007;133:111-6; J Clin Oncol. 2009;27:1413-8). Also, about 25% of patients had disease progression during induction chemotherapy and could not go onto surgery.
They designed the most recent trial to deliver radiation before surgery because of the excellent local control of cancer along with evidence that MPM tumors were radio-sensitive. “Considering the risk of disease progression on induction chemotherapy, we felt that switching the order of therapy was potentially a better option for patients with surgically resectable disease,” Dr. de Perrott and colleagues said.
The researchers cited the study’s single-center nature with a single treatment arm, and the lack of longer-term follow-up, as limitations. “However, in our own experience, this approach has been very encouraging and has become our primary option for patients with surgically resectable MPM,” they noted.
The study authors had no conflicts to disclose.
The popularity of extrapleural pneumonectomy to treat asbestos-related thoracic mesothelioma has yielded to extended pleurectomy/decortication in recent years, but a recent study suggests that the extrapleural pneumonectomy procedure can achieve good results in a new protocol that involves administering radiation therapy before surgery as opposed the more conventional approach of radiation after surgery.
Researchers at the University of Toronto reported on their protocol that uses accelerated intensity modulated radiation therapy (IMRT) for malignant pleural mesothelioma (MPM) (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.09.129). They call the protocol SMART, for Surgery for Mesothelioma After Radiation Therapy.
“The rationale to develop this protocol was to optimize the delivery of radiation to the whole tumor bed, sterilize the edges of the tumor to limit the risk of spillage at the time of surgery, develop a shorter treatment plan and potentiate the activation of the immune system by using a hypofractionated regimen,” wrote Dr. Marc de Perrot and colleagues.
The protocol involves delivering 25 Gy of radiation in five daily fractions over a week to the entire side of the thorax with 5 Gy boosts based on imaging, followed by extrapleural pneumonectomy (EPP) 4-6 days later. Patients with three or more positive lymph notes (ypN2 disease) also are offered adjuvant chemotherapy.
The researchers performed the protocol on 62 patients from November 2008 to October 2014, which represents 24% of all patients with MPM seen at the institution in that period. Fifty-two patients were men and ages ranged from 41 to 75 years. Clinical stage of cancer ranged from T1N0 in 10 patients, to T2N0 in 35 and T3N0 in 13 (two had T4N0 and two had T3N2). Forty-five had right-side cancers. Six patients received an extended protocol for various reasons, including tumor extending to the chest wall.
All 62 patients completed IMRT and EPP. All but one had resection and reconstruction of the diaphragm, and all but four had resection and reconstruction of the pericardium.
Overall death rate was 4.8% (three patients). Results were better in patients with epithelioid tumors, with a median survival of 51 months and disease-free survival of 47 months. Those with biphasic subtypes had median survival of 10 months and disease-free survival of 8 months. Eight patients had ipsilateral chest recurrence. “This analysis demonstrates that the SMART approach is particularly encouraging for patients with epithelial subtype,” Dr. de Perrot and coauthors said. They no longer perform the SMART protocol on patients with biphasic subtype.
The protocol was not without complications. Twenty-four patients, about 38%, had serious complications that required intervention or worse. Twelve had atrial fibrillation, but none advanced to life-threatening disease. Among other complications, four had empyema – one resulting in death – and three had pulmonary emboli. One other patient in the complications group died from pneumonia, and another died from a heart attack at home.
This is the Toronto researchers’ second attempt at studying the three-modality approach. In their first attempt, only half the patients who started with preoperative chemotherapy went onto complete the radiation after surgery because of difficulties administering it (J Thorac Cardiovasc Surg. 2007;133:111-6; J Clin Oncol. 2009;27:1413-8). Also, about 25% of patients had disease progression during induction chemotherapy and could not go onto surgery.
They designed the most recent trial to deliver radiation before surgery because of the excellent local control of cancer along with evidence that MPM tumors were radio-sensitive. “Considering the risk of disease progression on induction chemotherapy, we felt that switching the order of therapy was potentially a better option for patients with surgically resectable disease,” Dr. de Perrott and colleagues said.
The researchers cited the study’s single-center nature with a single treatment arm, and the lack of longer-term follow-up, as limitations. “However, in our own experience, this approach has been very encouraging and has become our primary option for patients with surgically resectable MPM,” they noted.
The study authors had no conflicts to disclose.
Key clinical point: A new protocol that involves accelerated hemithoracic intensity modulated radiation before surgery for lung mesothelioma delivered encouraging results in patients with epithelioid tumors.
Major finding: Disease-free survival was 47 months in epithelial subtypes compared with 8 months in biphasic subtypes.
Data source: A single-center population of 62 patients with malignant pleural mesothelioma treated between November 2008 and October 2014.
Disclosures: The study authors had no relationships to disclose.
Significant risk of relapse remains for ER-positive breast cancer patients beyond 10 years
The risk of breast cancer relapse decreases consistently for 10 years, then remains stable through 25 years, with ER-positive disease carrying higher risk than ER-negative disease from years 5 to 25, according to researchers.
At a median follow up of 24 years, the study reported outcomes of 4,105 patients who were diagnosed from 1978 to 1985 and participated in the International Breast Cancer Study Group Trials I to V. During the first 5 years of follow-up, risk of recurrence was lower for ER-positive compared with ER-negative disease: 9.9% vs. 11.5%. Beyond 5 years, risk was higher: 5-10 years, 5.4% vs. 3.3%; 10-15 years, 2.9% vs. 1.3%, 15-20 years, 2.8% vs. 1.2%. At 20-25 years, risk was 1.3% vs. 1.4% (P less than .001).
“We identified a population (ER positive) that maintains a significant risk of relapse even after more than 10 years of follow-up. New targeted treatments and different modes of breast cancer surveillance for preventing late recurrences within this population should be studied,” wrote Dr. Marco Colleoni of the European Institute of Oncology and International Breast Cancer Study Group, and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.3504).
For the entire patient group, breast cancer recurrence reached a peak at years 1-2 (15.2%), and decreased consistently through year 10 (5-10 years, 4.5%), then remained stable (10-15 years, 2.2%; 15-20, 1.5%; 20-25, 0.7%). Cumulative incidence of distant recurrence for the ER-positive group occurred less frequently than for the ER-negative group during the first 5 years and more frequently from 5 to 25 years: at 5 years, 27.1% vs. 23.4%; at 10 years, 31.9% vs. 31.8%; at 15 years, 35% vs. 33.4%; at 20 years, 37.4% vs. 34.1%; at 25 years, 38.3% vs. 35.3% (P less than .001).
All patients in the trials had undergone mastectomy and axillary clearance with at least eight nodes removed, with no locoregional radiotherapy, as was standard at the time.
Within the ER-positive group, patients who had zero to three positive nodes had had a stable risk of recurrence beyond 10 years, whereas for patients with four or more involved nodes, risk decreased gradually from 10 to 24 years.
Recent studies have shown that 10 years of adjuvant tamoxifen further improves breast cancer survival compared with 5 years of adjuvant therapy, albeit at a cost of 0.4% due to mortality resulting from endometrial carcinoma or pulmonary embolism. The studies underline the critical importance of sufficiently large patient populations and longer follow-up to provide accurate outcome data.
The report by Colleoni et al. describes clinical trial results from a median 24-year follow up. Patients with ER-positive disease require long-term follow up, which should be fundamentally different from those with ER-negative and/or human epidermal growth factor receptor 2–positive disease, who require shorter follow-up. Given these differences, adjuvant clinical studies with shorter follow-up periods will underreport events for ER-positive patients.
The study also reports a secondary malignancy rate of 4.9%, a figure likely to be underreported in studies with shorter follow-up schedules.
Limitations of the study arise from the time period in which it began. Regimens used in the study (different schedules of cyclophosphamide, methotrexate, and fluorouracil, and 1 year of tamoxifen with or without prednisone) have been shown to be inferior to newer regimens. None of the patients received postoperative radiotherapy, in accordance with data available at the time. Today, women with node-positive disease receive locoregional radiotherapy to reduce the risk of locoregional recurrence.
Studies with long-term follow up periods offer insights into both efficacy and safety; however, such studies require extensive resources. The entities that decide which types of cancer care are made available, such as insurance companies, governments, and regional health care funders, must shoulder the responsibility to ensure the required long-term evaluation of these treatments is conducted. Joint projects between pharmaceutical companies and health care providers could identify long-term benefits and adverse effects. The process may be more readily implemented in countries with population-based cancer registries.
Dr. Jonas Bergh is professor in the department of oncology-pathology at Karolinska Institutet and University Hospital, Stockholm. Dr. Kathleen Pritchard is a medical oncologist at Sunnybrook Odette Cancer Centre and professor at the University of Toronto. Dr. David Cameron is clinical director and chair of oncology at the University of Edinburgh Cancer Research Centre, Scotland. These remarks were part of an editorial accompanying the report by Colleoni et al. (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.65.2255). Dr. Bergh reported financial ties to Amgen, AstraZeneca, Bayer HealthCare Pharmaceuticals, Merck, Pfizer, Roche, and Sanofi. Dr. Pritchard reported ties to AstraZeneca, Pfizer, Roche, Amgen, Novartis, GlaxoSmithKline, and Eisai. Dr. Cameron reported ties to Novartis.
Recent studies have shown that 10 years of adjuvant tamoxifen further improves breast cancer survival compared with 5 years of adjuvant therapy, albeit at a cost of 0.4% due to mortality resulting from endometrial carcinoma or pulmonary embolism. The studies underline the critical importance of sufficiently large patient populations and longer follow-up to provide accurate outcome data.
The report by Colleoni et al. describes clinical trial results from a median 24-year follow up. Patients with ER-positive disease require long-term follow up, which should be fundamentally different from those with ER-negative and/or human epidermal growth factor receptor 2–positive disease, who require shorter follow-up. Given these differences, adjuvant clinical studies with shorter follow-up periods will underreport events for ER-positive patients.
The study also reports a secondary malignancy rate of 4.9%, a figure likely to be underreported in studies with shorter follow-up schedules.
Limitations of the study arise from the time period in which it began. Regimens used in the study (different schedules of cyclophosphamide, methotrexate, and fluorouracil, and 1 year of tamoxifen with or without prednisone) have been shown to be inferior to newer regimens. None of the patients received postoperative radiotherapy, in accordance with data available at the time. Today, women with node-positive disease receive locoregional radiotherapy to reduce the risk of locoregional recurrence.
Studies with long-term follow up periods offer insights into both efficacy and safety; however, such studies require extensive resources. The entities that decide which types of cancer care are made available, such as insurance companies, governments, and regional health care funders, must shoulder the responsibility to ensure the required long-term evaluation of these treatments is conducted. Joint projects between pharmaceutical companies and health care providers could identify long-term benefits and adverse effects. The process may be more readily implemented in countries with population-based cancer registries.
Dr. Jonas Bergh is professor in the department of oncology-pathology at Karolinska Institutet and University Hospital, Stockholm. Dr. Kathleen Pritchard is a medical oncologist at Sunnybrook Odette Cancer Centre and professor at the University of Toronto. Dr. David Cameron is clinical director and chair of oncology at the University of Edinburgh Cancer Research Centre, Scotland. These remarks were part of an editorial accompanying the report by Colleoni et al. (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.65.2255). Dr. Bergh reported financial ties to Amgen, AstraZeneca, Bayer HealthCare Pharmaceuticals, Merck, Pfizer, Roche, and Sanofi. Dr. Pritchard reported ties to AstraZeneca, Pfizer, Roche, Amgen, Novartis, GlaxoSmithKline, and Eisai. Dr. Cameron reported ties to Novartis.
Recent studies have shown that 10 years of adjuvant tamoxifen further improves breast cancer survival compared with 5 years of adjuvant therapy, albeit at a cost of 0.4% due to mortality resulting from endometrial carcinoma or pulmonary embolism. The studies underline the critical importance of sufficiently large patient populations and longer follow-up to provide accurate outcome data.
The report by Colleoni et al. describes clinical trial results from a median 24-year follow up. Patients with ER-positive disease require long-term follow up, which should be fundamentally different from those with ER-negative and/or human epidermal growth factor receptor 2–positive disease, who require shorter follow-up. Given these differences, adjuvant clinical studies with shorter follow-up periods will underreport events for ER-positive patients.
The study also reports a secondary malignancy rate of 4.9%, a figure likely to be underreported in studies with shorter follow-up schedules.
Limitations of the study arise from the time period in which it began. Regimens used in the study (different schedules of cyclophosphamide, methotrexate, and fluorouracil, and 1 year of tamoxifen with or without prednisone) have been shown to be inferior to newer regimens. None of the patients received postoperative radiotherapy, in accordance with data available at the time. Today, women with node-positive disease receive locoregional radiotherapy to reduce the risk of locoregional recurrence.
Studies with long-term follow up periods offer insights into both efficacy and safety; however, such studies require extensive resources. The entities that decide which types of cancer care are made available, such as insurance companies, governments, and regional health care funders, must shoulder the responsibility to ensure the required long-term evaluation of these treatments is conducted. Joint projects between pharmaceutical companies and health care providers could identify long-term benefits and adverse effects. The process may be more readily implemented in countries with population-based cancer registries.
Dr. Jonas Bergh is professor in the department of oncology-pathology at Karolinska Institutet and University Hospital, Stockholm. Dr. Kathleen Pritchard is a medical oncologist at Sunnybrook Odette Cancer Centre and professor at the University of Toronto. Dr. David Cameron is clinical director and chair of oncology at the University of Edinburgh Cancer Research Centre, Scotland. These remarks were part of an editorial accompanying the report by Colleoni et al. (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.65.2255). Dr. Bergh reported financial ties to Amgen, AstraZeneca, Bayer HealthCare Pharmaceuticals, Merck, Pfizer, Roche, and Sanofi. Dr. Pritchard reported ties to AstraZeneca, Pfizer, Roche, Amgen, Novartis, GlaxoSmithKline, and Eisai. Dr. Cameron reported ties to Novartis.
The risk of breast cancer relapse decreases consistently for 10 years, then remains stable through 25 years, with ER-positive disease carrying higher risk than ER-negative disease from years 5 to 25, according to researchers.
At a median follow up of 24 years, the study reported outcomes of 4,105 patients who were diagnosed from 1978 to 1985 and participated in the International Breast Cancer Study Group Trials I to V. During the first 5 years of follow-up, risk of recurrence was lower for ER-positive compared with ER-negative disease: 9.9% vs. 11.5%. Beyond 5 years, risk was higher: 5-10 years, 5.4% vs. 3.3%; 10-15 years, 2.9% vs. 1.3%, 15-20 years, 2.8% vs. 1.2%. At 20-25 years, risk was 1.3% vs. 1.4% (P less than .001).
“We identified a population (ER positive) that maintains a significant risk of relapse even after more than 10 years of follow-up. New targeted treatments and different modes of breast cancer surveillance for preventing late recurrences within this population should be studied,” wrote Dr. Marco Colleoni of the European Institute of Oncology and International Breast Cancer Study Group, and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.3504).
For the entire patient group, breast cancer recurrence reached a peak at years 1-2 (15.2%), and decreased consistently through year 10 (5-10 years, 4.5%), then remained stable (10-15 years, 2.2%; 15-20, 1.5%; 20-25, 0.7%). Cumulative incidence of distant recurrence for the ER-positive group occurred less frequently than for the ER-negative group during the first 5 years and more frequently from 5 to 25 years: at 5 years, 27.1% vs. 23.4%; at 10 years, 31.9% vs. 31.8%; at 15 years, 35% vs. 33.4%; at 20 years, 37.4% vs. 34.1%; at 25 years, 38.3% vs. 35.3% (P less than .001).
All patients in the trials had undergone mastectomy and axillary clearance with at least eight nodes removed, with no locoregional radiotherapy, as was standard at the time.
Within the ER-positive group, patients who had zero to three positive nodes had had a stable risk of recurrence beyond 10 years, whereas for patients with four or more involved nodes, risk decreased gradually from 10 to 24 years.
The risk of breast cancer relapse decreases consistently for 10 years, then remains stable through 25 years, with ER-positive disease carrying higher risk than ER-negative disease from years 5 to 25, according to researchers.
At a median follow up of 24 years, the study reported outcomes of 4,105 patients who were diagnosed from 1978 to 1985 and participated in the International Breast Cancer Study Group Trials I to V. During the first 5 years of follow-up, risk of recurrence was lower for ER-positive compared with ER-negative disease: 9.9% vs. 11.5%. Beyond 5 years, risk was higher: 5-10 years, 5.4% vs. 3.3%; 10-15 years, 2.9% vs. 1.3%, 15-20 years, 2.8% vs. 1.2%. At 20-25 years, risk was 1.3% vs. 1.4% (P less than .001).
“We identified a population (ER positive) that maintains a significant risk of relapse even after more than 10 years of follow-up. New targeted treatments and different modes of breast cancer surveillance for preventing late recurrences within this population should be studied,” wrote Dr. Marco Colleoni of the European Institute of Oncology and International Breast Cancer Study Group, and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.3504).
For the entire patient group, breast cancer recurrence reached a peak at years 1-2 (15.2%), and decreased consistently through year 10 (5-10 years, 4.5%), then remained stable (10-15 years, 2.2%; 15-20, 1.5%; 20-25, 0.7%). Cumulative incidence of distant recurrence for the ER-positive group occurred less frequently than for the ER-negative group during the first 5 years and more frequently from 5 to 25 years: at 5 years, 27.1% vs. 23.4%; at 10 years, 31.9% vs. 31.8%; at 15 years, 35% vs. 33.4%; at 20 years, 37.4% vs. 34.1%; at 25 years, 38.3% vs. 35.3% (P less than .001).
All patients in the trials had undergone mastectomy and axillary clearance with at least eight nodes removed, with no locoregional radiotherapy, as was standard at the time.
Within the ER-positive group, patients who had zero to three positive nodes had had a stable risk of recurrence beyond 10 years, whereas for patients with four or more involved nodes, risk decreased gradually from 10 to 24 years.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The risk of breast cancer recurrence continues through 24 years after primary treatments, especially for estrogen receptor–positive disease.
Major finding: During the first 5 years, risk of recurrence was lower for ER-positive disease than for ER-negative disease (9.9% vs. 11.5%). Risk was higher 5-10 years later (5.4% vs. 3.3%), at 10-15 years (2.9% vs. 1.3%), and at 15-20 years (2.8% vs. 1.2%). From 20 to 25 years on, the risk was 1.3% vs. 1.4% (P less than .001).
Data sources: The International Breast Cancer Study Group Trials I to V, comprising 4,105 patients with breast cancer diagnosed from 1978 to 1985.
Disclosures: Dr. Colleoni reported financial ties to Novartis, Boehringer Ingelheim, Taiho Pharmaceutical, AbbVie, AstraZeneca, Pierre Fabre, and Pfizer. Several of his coauthors reported ties to industry.
RCTs vs. observational studies: mortality ‘strikingly’ different following RT
Analyses of mortality after breast cancer radiotherapy using randomized clinical trial data versus observational data produced strikingly different results, according to researchers.
Analyses of randomized data indicated radiotherapy reduced mortality after breast-conserving surgery and mastectomy in node-positive disease; by contrast, SEER data analyses showed radiotherapy was associated with a significantly larger reduction in breast cancer mortality after breast-conserving surgery but higher mortality after mastectomy. Among patients with node-positive breast cancer who underwent mastectomy and axillary dissection, radiotherapy was associated with lower mortality by clinical trial data (rate ratio, 0.84; 95% CI, 0.76-0.94) but was associated with higher mortality by observational data (1.34; 1.31-1.37).
Furthermore, analyses of randomized trial data indicated increased mortality from heart disease (1.27; 1.12-1.44) and lung cancer (1.78; 1.30-2.46) following radiotherapy, but analyses of SEER data indicated reduced mortality from heart disease (0.56; 0.53-0.60) and lung cancer (0.86; 0.75-0.99) associated with radiotherapy.
“It is not plausible that these negative associations in the SEER data are causal and, clearly, they are strongly influenced by factors other than the effect or radiotherapy,” wrote Dr. Katherine Henson of the University of Oxford (England), and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
“Randomized trials are needed wherever possible to investigate the effect of treatment on mortality from the original cancer,” according to the investigators, and, “selection biases can be problematic even when analyzing treatment-related toxicities using observational data.”
Analyses of randomized data demonstrated reduced breast cancer mortality associated with radiotherapy after breast-conserving surgery (0.82; 95% CI, 0.75-0.90). Compared with randomized data, analyses of observational data showed a much greater reduction in mortality associated with radiotherapy after breast-conserving surgery (0.64; 95% CI, 0.62-0.66).
Randomized evidence came from the Early Breast Cancer Trialists’ Collaborative Group, meta analyses of 17 trials (n = 10,801) of radiotherapy after breast-conserving surgery, 14 trials (n = 3,131) of radiotherapy after mastectomy, and 78 trials (n = 42,080) of mortality from causes other than breast cancer. Observational evidence came from the SEER data base (n = 393,840).
The researchers offered plausible explanations for selection bias that may have resulted in the divergent results calculated using observational data. Because radiotherapy following mastectomy is indicated only in the presence of adverse disease characteristics, patients who did not receive radiotherapy may have survived longer because of especially favorable characteristics, despite of lack of radiotherapy.
“When evaluating rare late effects for which sufficient randomized evidence cannot reasonably be obtained, analyses of observational data comparing treated and untreated patients may often be the only source of information, but they must always be interpreted with considerable caution,” wrote the investigators.
The hierarchy of evidence establishes that meta-analyses and randomized clinical trials (RCTs) offer the highest quality of evidence, and RCTs clearly offer the best assessment of effects of therapy. Observational studies, on the other hand, have considerable limitations, yet they have helped establish key causal relationships. Large prospective cohort studies, such as Framingham Heart Study, the National Child Development Study, and the Nurse’s Health Study, among others, continue to provide important data.
The study by Henson et al. reopens the debate over the value of RCTs versus observational studies (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
It is well known the nonrandomized studies can produce misleading results due to selection bias. Selection bias favoring treatment for healthier patients produces improved survival among treated patients. On the other hand, selection bias disfavoring treatment occurs in studies of patients with more aggressive tumors who receive more treatment, and despite interventions, have worse outcomes.
The observation that postmastectomy radiotherapy is associated with worse outcomes reflects a real phenomenon, likely explained by confounding by indication. Patients treated with radiotherapy likely have worse prognoses, and despite therapy, had worse outcomes.
In the Henson et al. study, detailed data on treatment, comorbid conditions, or other health determinants were not available. In recognizing the importance of observational data for comparative effectiveness research, we must also understand and account for the limitations.
There should be no battle between RCTs and observational data, as both can provide valid and important knowledge to help clinicians make decisions and deliver evidence-based compassionate care.
Dr. Mariana Chavez-MacGregor is assistant professor in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center, Houston. Dr. Sharon Giordano is associate professor in the department of breast medical oncology at the university. These remarks were part of an editorial (J Clin Oncol. 2016 Jan. 18. doi: 10.1200/JCO.2015.64.7487). Dr. Chavez-MacGregor reported financial ties with Roche, Novarits, InVitae, Pfizer, Genomic Health, and Genentech/Roche. Dr. Giordano reported having no disclosures.
The hierarchy of evidence establishes that meta-analyses and randomized clinical trials (RCTs) offer the highest quality of evidence, and RCTs clearly offer the best assessment of effects of therapy. Observational studies, on the other hand, have considerable limitations, yet they have helped establish key causal relationships. Large prospective cohort studies, such as Framingham Heart Study, the National Child Development Study, and the Nurse’s Health Study, among others, continue to provide important data.
The study by Henson et al. reopens the debate over the value of RCTs versus observational studies (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
It is well known the nonrandomized studies can produce misleading results due to selection bias. Selection bias favoring treatment for healthier patients produces improved survival among treated patients. On the other hand, selection bias disfavoring treatment occurs in studies of patients with more aggressive tumors who receive more treatment, and despite interventions, have worse outcomes.
The observation that postmastectomy radiotherapy is associated with worse outcomes reflects a real phenomenon, likely explained by confounding by indication. Patients treated with radiotherapy likely have worse prognoses, and despite therapy, had worse outcomes.
In the Henson et al. study, detailed data on treatment, comorbid conditions, or other health determinants were not available. In recognizing the importance of observational data for comparative effectiveness research, we must also understand and account for the limitations.
There should be no battle between RCTs and observational data, as both can provide valid and important knowledge to help clinicians make decisions and deliver evidence-based compassionate care.
Dr. Mariana Chavez-MacGregor is assistant professor in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center, Houston. Dr. Sharon Giordano is associate professor in the department of breast medical oncology at the university. These remarks were part of an editorial (J Clin Oncol. 2016 Jan. 18. doi: 10.1200/JCO.2015.64.7487). Dr. Chavez-MacGregor reported financial ties with Roche, Novarits, InVitae, Pfizer, Genomic Health, and Genentech/Roche. Dr. Giordano reported having no disclosures.
The hierarchy of evidence establishes that meta-analyses and randomized clinical trials (RCTs) offer the highest quality of evidence, and RCTs clearly offer the best assessment of effects of therapy. Observational studies, on the other hand, have considerable limitations, yet they have helped establish key causal relationships. Large prospective cohort studies, such as Framingham Heart Study, the National Child Development Study, and the Nurse’s Health Study, among others, continue to provide important data.
The study by Henson et al. reopens the debate over the value of RCTs versus observational studies (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
It is well known the nonrandomized studies can produce misleading results due to selection bias. Selection bias favoring treatment for healthier patients produces improved survival among treated patients. On the other hand, selection bias disfavoring treatment occurs in studies of patients with more aggressive tumors who receive more treatment, and despite interventions, have worse outcomes.
The observation that postmastectomy radiotherapy is associated with worse outcomes reflects a real phenomenon, likely explained by confounding by indication. Patients treated with radiotherapy likely have worse prognoses, and despite therapy, had worse outcomes.
In the Henson et al. study, detailed data on treatment, comorbid conditions, or other health determinants were not available. In recognizing the importance of observational data for comparative effectiveness research, we must also understand and account for the limitations.
There should be no battle between RCTs and observational data, as both can provide valid and important knowledge to help clinicians make decisions and deliver evidence-based compassionate care.
Dr. Mariana Chavez-MacGregor is assistant professor in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center, Houston. Dr. Sharon Giordano is associate professor in the department of breast medical oncology at the university. These remarks were part of an editorial (J Clin Oncol. 2016 Jan. 18. doi: 10.1200/JCO.2015.64.7487). Dr. Chavez-MacGregor reported financial ties with Roche, Novarits, InVitae, Pfizer, Genomic Health, and Genentech/Roche. Dr. Giordano reported having no disclosures.
Analyses of mortality after breast cancer radiotherapy using randomized clinical trial data versus observational data produced strikingly different results, according to researchers.
Analyses of randomized data indicated radiotherapy reduced mortality after breast-conserving surgery and mastectomy in node-positive disease; by contrast, SEER data analyses showed radiotherapy was associated with a significantly larger reduction in breast cancer mortality after breast-conserving surgery but higher mortality after mastectomy. Among patients with node-positive breast cancer who underwent mastectomy and axillary dissection, radiotherapy was associated with lower mortality by clinical trial data (rate ratio, 0.84; 95% CI, 0.76-0.94) but was associated with higher mortality by observational data (1.34; 1.31-1.37).
Furthermore, analyses of randomized trial data indicated increased mortality from heart disease (1.27; 1.12-1.44) and lung cancer (1.78; 1.30-2.46) following radiotherapy, but analyses of SEER data indicated reduced mortality from heart disease (0.56; 0.53-0.60) and lung cancer (0.86; 0.75-0.99) associated with radiotherapy.
“It is not plausible that these negative associations in the SEER data are causal and, clearly, they are strongly influenced by factors other than the effect or radiotherapy,” wrote Dr. Katherine Henson of the University of Oxford (England), and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
“Randomized trials are needed wherever possible to investigate the effect of treatment on mortality from the original cancer,” according to the investigators, and, “selection biases can be problematic even when analyzing treatment-related toxicities using observational data.”
Analyses of randomized data demonstrated reduced breast cancer mortality associated with radiotherapy after breast-conserving surgery (0.82; 95% CI, 0.75-0.90). Compared with randomized data, analyses of observational data showed a much greater reduction in mortality associated with radiotherapy after breast-conserving surgery (0.64; 95% CI, 0.62-0.66).
Randomized evidence came from the Early Breast Cancer Trialists’ Collaborative Group, meta analyses of 17 trials (n = 10,801) of radiotherapy after breast-conserving surgery, 14 trials (n = 3,131) of radiotherapy after mastectomy, and 78 trials (n = 42,080) of mortality from causes other than breast cancer. Observational evidence came from the SEER data base (n = 393,840).
The researchers offered plausible explanations for selection bias that may have resulted in the divergent results calculated using observational data. Because radiotherapy following mastectomy is indicated only in the presence of adverse disease characteristics, patients who did not receive radiotherapy may have survived longer because of especially favorable characteristics, despite of lack of radiotherapy.
“When evaluating rare late effects for which sufficient randomized evidence cannot reasonably be obtained, analyses of observational data comparing treated and untreated patients may often be the only source of information, but they must always be interpreted with considerable caution,” wrote the investigators.
Analyses of mortality after breast cancer radiotherapy using randomized clinical trial data versus observational data produced strikingly different results, according to researchers.
Analyses of randomized data indicated radiotherapy reduced mortality after breast-conserving surgery and mastectomy in node-positive disease; by contrast, SEER data analyses showed radiotherapy was associated with a significantly larger reduction in breast cancer mortality after breast-conserving surgery but higher mortality after mastectomy. Among patients with node-positive breast cancer who underwent mastectomy and axillary dissection, radiotherapy was associated with lower mortality by clinical trial data (rate ratio, 0.84; 95% CI, 0.76-0.94) but was associated with higher mortality by observational data (1.34; 1.31-1.37).
Furthermore, analyses of randomized trial data indicated increased mortality from heart disease (1.27; 1.12-1.44) and lung cancer (1.78; 1.30-2.46) following radiotherapy, but analyses of SEER data indicated reduced mortality from heart disease (0.56; 0.53-0.60) and lung cancer (0.86; 0.75-0.99) associated with radiotherapy.
“It is not plausible that these negative associations in the SEER data are causal and, clearly, they are strongly influenced by factors other than the effect or radiotherapy,” wrote Dr. Katherine Henson of the University of Oxford (England), and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
“Randomized trials are needed wherever possible to investigate the effect of treatment on mortality from the original cancer,” according to the investigators, and, “selection biases can be problematic even when analyzing treatment-related toxicities using observational data.”
Analyses of randomized data demonstrated reduced breast cancer mortality associated with radiotherapy after breast-conserving surgery (0.82; 95% CI, 0.75-0.90). Compared with randomized data, analyses of observational data showed a much greater reduction in mortality associated with radiotherapy after breast-conserving surgery (0.64; 95% CI, 0.62-0.66).
Randomized evidence came from the Early Breast Cancer Trialists’ Collaborative Group, meta analyses of 17 trials (n = 10,801) of radiotherapy after breast-conserving surgery, 14 trials (n = 3,131) of radiotherapy after mastectomy, and 78 trials (n = 42,080) of mortality from causes other than breast cancer. Observational evidence came from the SEER data base (n = 393,840).
The researchers offered plausible explanations for selection bias that may have resulted in the divergent results calculated using observational data. Because radiotherapy following mastectomy is indicated only in the presence of adverse disease characteristics, patients who did not receive radiotherapy may have survived longer because of especially favorable characteristics, despite of lack of radiotherapy.
“When evaluating rare late effects for which sufficient randomized evidence cannot reasonably be obtained, analyses of observational data comparing treated and untreated patients may often be the only source of information, but they must always be interpreted with considerable caution,” wrote the investigators.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Analysis of randomized trial data compared with observational data on mortality after breast cancer radiotherapy shows strikingly different results.
Major finding: Radiotherapy after mastectomy and axillary dissection was associated with lower mortality by clinical trial data (rate ratio, 0.84; 95% CI, 0.76-0.94) but was associated with higher mortality by observational data (1.34; 95% CI, 1.31-1.37).
Data sources: Randomized evidence came from the Early Breast Cancer Trialists’ Collaborative Group, meta-analyses of 31 trials (n = 13,932); observational evidence came from the SEER data base (n = 393,840).
Disclosures: Dr. Henson reported having no disclosures. One of her coauthors reported ties to industry.