User login
Long-term mogamulizumab appears safe, effective in CTCL
LA JOLLA, CALIF. — Prolonged exposure to mogamulizumab can improve responses without compromising safety in patients with cutaneous T-cell lymphoma (CTCL), according to a post hoc analysis of the MAVORIC trial.
Investigators found that exposure to mogamulizumab correlated with response. The highest response rate — 75.6% — was observed in patients exposed to the drug for at least 351 days, and the lowest — 1.9% — was observed in patients exposed to mogamulizumab for less than 72 days.
On the other hand, rates of adverse events (AEs) were similar regardless of how long patients were treated with mogamulizumab.
Youn H. Kim, MD, of Stanford Cancer Institute at Stanford (Calif.) University, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The phase 3 MAVORIC trial (NCT01728805) included 372 adults with CTCL who had failed at least one systemic therapy. The patients were randomized to treatment with mogamulizumab or vorinostat.
Results from this comparison were previously reported at the 10th annual T-cell Lymphoma Forum.
At this year’s meeting, Dr. Kim and her colleagues reported results in 184 patients who were randomized to mogamulizumab — 105 of whom had mycosis fungoides (MF) and 79 of whom had Sézary syndrome (SS).
Patients were exposed to mogamulizumab for a mean of 275.2 days and a median of 170.0 days (range, 1-1,617 days).
The investigators divided patients into the following quartiles according to mogamulizumab exposure:
- Less than 72 days — 52 patients (28%)
- 72-170 days — 40 patients (22%)
- 171-351 days — 47 patients (26%)
- More than 351 days — 45 patients (24%).
Patients exposed to mogamulizumab for longer were more likely to have SS, stage III/IV disease, blood involvement, and a performance status of 0.
Dr. Kim said the SS patients “benefited a lot” from mogamulizumab and therefore remained on treatment longer.
Response
As expected, patients exposed to mogamulizumab for the longest period had the highest global response rates. Confirmed response rates according to drug exposure were as follows:
- Less than 72 days: 1.9% overall, 0% for SS, and 2.9% for MF
- 72-170 days: 10% overall, 18.8% for SS, and 4.2% for MF
- 171-351 days: 29.8% overall, 36.4% for SS, and 24% for MF
- More than 351 days: 75.6% overall, 83.3% for SS, and 66.7% for MF.
In addition, rates of complete response (CR) and partial response (PR) tended to increase with mogamulizumab exposure. Rates of CR, PR, and stable disease (SD) according to exposure time were as follows:
- Less than 72 days: 0% CR, 7.7% PR, and 38.5% SD
- 72-170 days: 2.5% CR, 20% PR, and 62.5% SD
- 171-351 days: 2.1% CR, 34% PR, and 57.4% SD
- More than 351 days: 6.7% CR, 71.1% PR, and 17.8% SD.
Safety
“The percentage of patients reporting adverse events was not different in the long-term treatment-exposure patients, compared to the short-term,” Dr. Kim said.
Percentages of treatment-emergent AEs (TEAEs) and serious AEs (SAEs) according to mogamulizumab exposure were as follows:
- Less than 72 days: 26.6% TEAEs and 6.5% SAEs
- 72-170 days: 18.5% TEAEs and 3.3% SAEs
- 171-351 days: 23.4% TEAEs and 6.0% SAEs
- More than 351 days: 21.7% TEAEs and 4.3% SAEs.
“The majority of the grade 3 events occurred in the first two quartiles, not later, which is important to show,” Dr. Kim said.
Most grade 3 AEs occurred within 170 days of treatment initiation, and the median time to a grade 3 or higher AE was 109 days.
The most common treatment-related AEs in the longest exposure cohort were drug eruption (20.0%), thrombocytopenia (11.1%), stomatitis (8.9%), and anemia (8.9%).
Of all patients in this analysis, 45 experienced drug eruption, which was defined as a skin rash possibly, probably, or definitely related to the study drug.
Nine drug eruption events were grade 3, and the rest were grade 1 or 2. The median time to drug eruption was 107 days.
While drug eruption “didn’t show up early,” there is no cumulative risk with longer exposure to mogamulizumab, Dr. Kim said. Likewise, she said, autoimmune AEs were not dose-cumulative events.
There were two patients with definite autoimmune disease — a 65-year-old man with Miller Fisher syndrome (occurring 199 days after mogamulizumab initiation) and a 40-year-old woman with myositis (151 days) and myocarditis (310 days).
The investigators also identified three patients with possible autoimmune disease, including:
- Pneumonitis (310 days) in a 74-year-old woman
- Polymyalgia rheumatica (209 days) and myopathy (not available) in an 84-year-old man
- Hepatitis (144 days), pneumonitis (about 174 days), and polymyositis (about 174 days) in a 73-year-old man.
Dr. Kim and her colleagues said these data suggest prolonged treatment with mogamulizumab is not associated with an increased safety risk in patients with MF or SS. And the manageable safety profile of mogamulizumab meant that patients who derived a clinical benefit could remain on the drug for an extended period of time.
The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Dr. Kim reported relationships with Merck, Portola Pharmaceuticals, Soligenix, Takeda, TetraLogic Pharmaceuticals, Kyowa Kirin, Seattle Genetics, Medivir, Neumedicines, Eisai, Innate Pharma, Galderma, Miragen Therapeutics, Forty Seven, and Horizon Pharma. Her coinvestigators reported relationships with several companies.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Prolonged exposure to mogamulizumab can improve responses without compromising safety in patients with cutaneous T-cell lymphoma (CTCL), according to a post hoc analysis of the MAVORIC trial.
Investigators found that exposure to mogamulizumab correlated with response. The highest response rate — 75.6% — was observed in patients exposed to the drug for at least 351 days, and the lowest — 1.9% — was observed in patients exposed to mogamulizumab for less than 72 days.
On the other hand, rates of adverse events (AEs) were similar regardless of how long patients were treated with mogamulizumab.
Youn H. Kim, MD, of Stanford Cancer Institute at Stanford (Calif.) University, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The phase 3 MAVORIC trial (NCT01728805) included 372 adults with CTCL who had failed at least one systemic therapy. The patients were randomized to treatment with mogamulizumab or vorinostat.
Results from this comparison were previously reported at the 10th annual T-cell Lymphoma Forum.
At this year’s meeting, Dr. Kim and her colleagues reported results in 184 patients who were randomized to mogamulizumab — 105 of whom had mycosis fungoides (MF) and 79 of whom had Sézary syndrome (SS).
Patients were exposed to mogamulizumab for a mean of 275.2 days and a median of 170.0 days (range, 1-1,617 days).
The investigators divided patients into the following quartiles according to mogamulizumab exposure:
- Less than 72 days — 52 patients (28%)
- 72-170 days — 40 patients (22%)
- 171-351 days — 47 patients (26%)
- More than 351 days — 45 patients (24%).
Patients exposed to mogamulizumab for longer were more likely to have SS, stage III/IV disease, blood involvement, and a performance status of 0.
Dr. Kim said the SS patients “benefited a lot” from mogamulizumab and therefore remained on treatment longer.
Response
As expected, patients exposed to mogamulizumab for the longest period had the highest global response rates. Confirmed response rates according to drug exposure were as follows:
- Less than 72 days: 1.9% overall, 0% for SS, and 2.9% for MF
- 72-170 days: 10% overall, 18.8% for SS, and 4.2% for MF
- 171-351 days: 29.8% overall, 36.4% for SS, and 24% for MF
- More than 351 days: 75.6% overall, 83.3% for SS, and 66.7% for MF.
In addition, rates of complete response (CR) and partial response (PR) tended to increase with mogamulizumab exposure. Rates of CR, PR, and stable disease (SD) according to exposure time were as follows:
- Less than 72 days: 0% CR, 7.7% PR, and 38.5% SD
- 72-170 days: 2.5% CR, 20% PR, and 62.5% SD
- 171-351 days: 2.1% CR, 34% PR, and 57.4% SD
- More than 351 days: 6.7% CR, 71.1% PR, and 17.8% SD.
Safety
“The percentage of patients reporting adverse events was not different in the long-term treatment-exposure patients, compared to the short-term,” Dr. Kim said.
Percentages of treatment-emergent AEs (TEAEs) and serious AEs (SAEs) according to mogamulizumab exposure were as follows:
- Less than 72 days: 26.6% TEAEs and 6.5% SAEs
- 72-170 days: 18.5% TEAEs and 3.3% SAEs
- 171-351 days: 23.4% TEAEs and 6.0% SAEs
- More than 351 days: 21.7% TEAEs and 4.3% SAEs.
“The majority of the grade 3 events occurred in the first two quartiles, not later, which is important to show,” Dr. Kim said.
Most grade 3 AEs occurred within 170 days of treatment initiation, and the median time to a grade 3 or higher AE was 109 days.
The most common treatment-related AEs in the longest exposure cohort were drug eruption (20.0%), thrombocytopenia (11.1%), stomatitis (8.9%), and anemia (8.9%).
Of all patients in this analysis, 45 experienced drug eruption, which was defined as a skin rash possibly, probably, or definitely related to the study drug.
Nine drug eruption events were grade 3, and the rest were grade 1 or 2. The median time to drug eruption was 107 days.
While drug eruption “didn’t show up early,” there is no cumulative risk with longer exposure to mogamulizumab, Dr. Kim said. Likewise, she said, autoimmune AEs were not dose-cumulative events.
There were two patients with definite autoimmune disease — a 65-year-old man with Miller Fisher syndrome (occurring 199 days after mogamulizumab initiation) and a 40-year-old woman with myositis (151 days) and myocarditis (310 days).
The investigators also identified three patients with possible autoimmune disease, including:
- Pneumonitis (310 days) in a 74-year-old woman
- Polymyalgia rheumatica (209 days) and myopathy (not available) in an 84-year-old man
- Hepatitis (144 days), pneumonitis (about 174 days), and polymyositis (about 174 days) in a 73-year-old man.
Dr. Kim and her colleagues said these data suggest prolonged treatment with mogamulizumab is not associated with an increased safety risk in patients with MF or SS. And the manageable safety profile of mogamulizumab meant that patients who derived a clinical benefit could remain on the drug for an extended period of time.
The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Dr. Kim reported relationships with Merck, Portola Pharmaceuticals, Soligenix, Takeda, TetraLogic Pharmaceuticals, Kyowa Kirin, Seattle Genetics, Medivir, Neumedicines, Eisai, Innate Pharma, Galderma, Miragen Therapeutics, Forty Seven, and Horizon Pharma. Her coinvestigators reported relationships with several companies.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Prolonged exposure to mogamulizumab can improve responses without compromising safety in patients with cutaneous T-cell lymphoma (CTCL), according to a post hoc analysis of the MAVORIC trial.
Investigators found that exposure to mogamulizumab correlated with response. The highest response rate — 75.6% — was observed in patients exposed to the drug for at least 351 days, and the lowest — 1.9% — was observed in patients exposed to mogamulizumab for less than 72 days.
On the other hand, rates of adverse events (AEs) were similar regardless of how long patients were treated with mogamulizumab.
Youn H. Kim, MD, of Stanford Cancer Institute at Stanford (Calif.) University, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The phase 3 MAVORIC trial (NCT01728805) included 372 adults with CTCL who had failed at least one systemic therapy. The patients were randomized to treatment with mogamulizumab or vorinostat.
Results from this comparison were previously reported at the 10th annual T-cell Lymphoma Forum.
At this year’s meeting, Dr. Kim and her colleagues reported results in 184 patients who were randomized to mogamulizumab — 105 of whom had mycosis fungoides (MF) and 79 of whom had Sézary syndrome (SS).
Patients were exposed to mogamulizumab for a mean of 275.2 days and a median of 170.0 days (range, 1-1,617 days).
The investigators divided patients into the following quartiles according to mogamulizumab exposure:
- Less than 72 days — 52 patients (28%)
- 72-170 days — 40 patients (22%)
- 171-351 days — 47 patients (26%)
- More than 351 days — 45 patients (24%).
Patients exposed to mogamulizumab for longer were more likely to have SS, stage III/IV disease, blood involvement, and a performance status of 0.
Dr. Kim said the SS patients “benefited a lot” from mogamulizumab and therefore remained on treatment longer.
Response
As expected, patients exposed to mogamulizumab for the longest period had the highest global response rates. Confirmed response rates according to drug exposure were as follows:
- Less than 72 days: 1.9% overall, 0% for SS, and 2.9% for MF
- 72-170 days: 10% overall, 18.8% for SS, and 4.2% for MF
- 171-351 days: 29.8% overall, 36.4% for SS, and 24% for MF
- More than 351 days: 75.6% overall, 83.3% for SS, and 66.7% for MF.
In addition, rates of complete response (CR) and partial response (PR) tended to increase with mogamulizumab exposure. Rates of CR, PR, and stable disease (SD) according to exposure time were as follows:
- Less than 72 days: 0% CR, 7.7% PR, and 38.5% SD
- 72-170 days: 2.5% CR, 20% PR, and 62.5% SD
- 171-351 days: 2.1% CR, 34% PR, and 57.4% SD
- More than 351 days: 6.7% CR, 71.1% PR, and 17.8% SD.
Safety
“The percentage of patients reporting adverse events was not different in the long-term treatment-exposure patients, compared to the short-term,” Dr. Kim said.
Percentages of treatment-emergent AEs (TEAEs) and serious AEs (SAEs) according to mogamulizumab exposure were as follows:
- Less than 72 days: 26.6% TEAEs and 6.5% SAEs
- 72-170 days: 18.5% TEAEs and 3.3% SAEs
- 171-351 days: 23.4% TEAEs and 6.0% SAEs
- More than 351 days: 21.7% TEAEs and 4.3% SAEs.
“The majority of the grade 3 events occurred in the first two quartiles, not later, which is important to show,” Dr. Kim said.
Most grade 3 AEs occurred within 170 days of treatment initiation, and the median time to a grade 3 or higher AE was 109 days.
The most common treatment-related AEs in the longest exposure cohort were drug eruption (20.0%), thrombocytopenia (11.1%), stomatitis (8.9%), and anemia (8.9%).
Of all patients in this analysis, 45 experienced drug eruption, which was defined as a skin rash possibly, probably, or definitely related to the study drug.
Nine drug eruption events were grade 3, and the rest were grade 1 or 2. The median time to drug eruption was 107 days.
While drug eruption “didn’t show up early,” there is no cumulative risk with longer exposure to mogamulizumab, Dr. Kim said. Likewise, she said, autoimmune AEs were not dose-cumulative events.
There were two patients with definite autoimmune disease — a 65-year-old man with Miller Fisher syndrome (occurring 199 days after mogamulizumab initiation) and a 40-year-old woman with myositis (151 days) and myocarditis (310 days).
The investigators also identified three patients with possible autoimmune disease, including:
- Pneumonitis (310 days) in a 74-year-old woman
- Polymyalgia rheumatica (209 days) and myopathy (not available) in an 84-year-old man
- Hepatitis (144 days), pneumonitis (about 174 days), and polymyositis (about 174 days) in a 73-year-old man.
Dr. Kim and her colleagues said these data suggest prolonged treatment with mogamulizumab is not associated with an increased safety risk in patients with MF or SS. And the manageable safety profile of mogamulizumab meant that patients who derived a clinical benefit could remain on the drug for an extended period of time.
The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Dr. Kim reported relationships with Merck, Portola Pharmaceuticals, Soligenix, Takeda, TetraLogic Pharmaceuticals, Kyowa Kirin, Seattle Genetics, Medivir, Neumedicines, Eisai, Innate Pharma, Galderma, Miragen Therapeutics, Forty Seven, and Horizon Pharma. Her coinvestigators reported relationships with several companies.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: The highest response rate – 75.6% – was observed in patients exposed to mogamulizumab for at least 351 days.
Study details: A post hoc analysis of the MAVORIC trial, including 184 patients treated with mogamulizumab.
Disclosures: The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Investigators disclosed relationships with several companies.
Chidamide may be more effective in PTCL than previously thought
LA JOLLA, CALIF. – Real-world data suggest chidamide may be more effective against relapsed or refractory peripheral T-cell lymphoma (PTCL) than a pivotal study indicated.
Single-agent chidamide produced an overall response rate of 47.0% in a real-world study of more than 1,000 patients, compared with the 28.0% overall response rate that was observed in the phase 2 study of chidamide (Ann Oncol. 2015 Aug;26[8]:1766-71).
Yuqin Song, MD, PhD, of Peking University Cancer Hospital and Institute in Beijing, China, presented data from the real-world study at the annual T-cell Lymphoma Forum.
Dr. Song said this study is the largest cohort of real-world patients with relapsed or refractory PTCL. She and her colleagues analyzed data on 1,064 patients treated at 216 sites across China between February 2015 and December 2017.
The patients had a median age of 54 years, 63.9% were male, and 88.1% had stage III-IV disease.
Disease subtypes included PTCL not otherwise specified (NOS, 38.0%), angioimmunoblastic T-cell lymphoma (AITL, 29.1%), extranodal natural killer T-cell lymphoma (ENKTL, 13.4%), anaplastic large-cell lymphoma (ALCL, 9.1%), and others (10.3%), including cutaneous T-cell lymphoma (CTCL).
Fifty-two percent of patients (n = 553) received chidamide as a single agent, and 48% (n = 511) received the drug with other agents. The most common treatment regimens combined with chidamide were the following
- Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP, 20.7%).
- Gemcitabine, dexamethasone, and cisplatin (GDP, 11.8%).
- Etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH, 9.8%).
- Patients with ENKTL received chidamide with L-asparaginase (35.4%) or without it (64.5%).
The median follow-up was 4.9 months (range, 0-36.2 months). Across disease subtypes, the overall response rate was 47.0% with single-agent chidamide and 65.4% when chidamide was given in combination with other agents (P less than .01).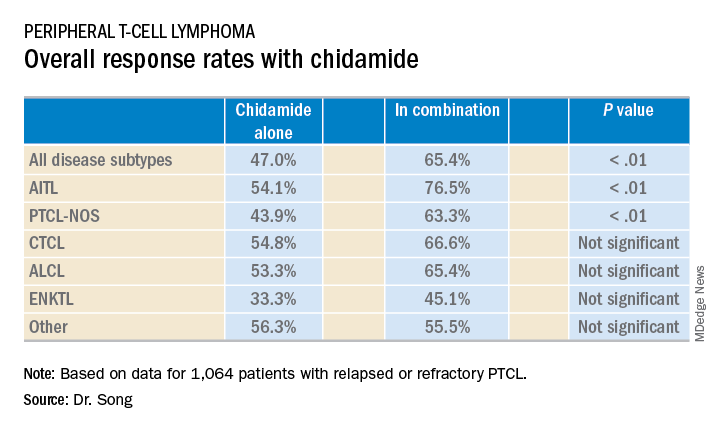
The median overall survival was 400 days for all patients, 342 days for patients treated with chidamide alone, and 457 days for patients who received combination therapy. The 1-year overall survival rates were 52%, 48%, and 56%, respectively.
Dr. Song said these data verify the efficacy of chidamide as a single agent and suggest chidamide might lead to improved survival in refractory or relapsed PTCLs.
Chidamide was generally well tolerated in this study, Dr. Song said. There were no unexpected adverse events (AEs) and most were grade 1 or 2.
The most common AEs (of any grade) observed with single-agent chidamide were neutropenia (42.9%), thrombocytopenia (40.5%), fatigue (38.3%), anemia (31.6%), and nausea/vomiting (21.0%).
The most common AEs observed with chidamide in combination were neutropenia (61.4%), thrombocytopenia (58.5%), fatigue (56.2%), anemia (54.2%), nausea/vomiting (30.7%), and fever (22.1%).
This study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Real-world data suggest chidamide may be more effective against relapsed or refractory peripheral T-cell lymphoma (PTCL) than a pivotal study indicated.
Single-agent chidamide produced an overall response rate of 47.0% in a real-world study of more than 1,000 patients, compared with the 28.0% overall response rate that was observed in the phase 2 study of chidamide (Ann Oncol. 2015 Aug;26[8]:1766-71).
Yuqin Song, MD, PhD, of Peking University Cancer Hospital and Institute in Beijing, China, presented data from the real-world study at the annual T-cell Lymphoma Forum.
Dr. Song said this study is the largest cohort of real-world patients with relapsed or refractory PTCL. She and her colleagues analyzed data on 1,064 patients treated at 216 sites across China between February 2015 and December 2017.
The patients had a median age of 54 years, 63.9% were male, and 88.1% had stage III-IV disease.
Disease subtypes included PTCL not otherwise specified (NOS, 38.0%), angioimmunoblastic T-cell lymphoma (AITL, 29.1%), extranodal natural killer T-cell lymphoma (ENKTL, 13.4%), anaplastic large-cell lymphoma (ALCL, 9.1%), and others (10.3%), including cutaneous T-cell lymphoma (CTCL).
Fifty-two percent of patients (n = 553) received chidamide as a single agent, and 48% (n = 511) received the drug with other agents. The most common treatment regimens combined with chidamide were the following
- Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP, 20.7%).
- Gemcitabine, dexamethasone, and cisplatin (GDP, 11.8%).
- Etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH, 9.8%).
- Patients with ENKTL received chidamide with L-asparaginase (35.4%) or without it (64.5%).
The median follow-up was 4.9 months (range, 0-36.2 months). Across disease subtypes, the overall response rate was 47.0% with single-agent chidamide and 65.4% when chidamide was given in combination with other agents (P less than .01).
The median overall survival was 400 days for all patients, 342 days for patients treated with chidamide alone, and 457 days for patients who received combination therapy. The 1-year overall survival rates were 52%, 48%, and 56%, respectively.
Dr. Song said these data verify the efficacy of chidamide as a single agent and suggest chidamide might lead to improved survival in refractory or relapsed PTCLs.
Chidamide was generally well tolerated in this study, Dr. Song said. There were no unexpected adverse events (AEs) and most were grade 1 or 2.
The most common AEs (of any grade) observed with single-agent chidamide were neutropenia (42.9%), thrombocytopenia (40.5%), fatigue (38.3%), anemia (31.6%), and nausea/vomiting (21.0%).
The most common AEs observed with chidamide in combination were neutropenia (61.4%), thrombocytopenia (58.5%), fatigue (56.2%), anemia (54.2%), nausea/vomiting (30.7%), and fever (22.1%).
This study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Real-world data suggest chidamide may be more effective against relapsed or refractory peripheral T-cell lymphoma (PTCL) than a pivotal study indicated.
Single-agent chidamide produced an overall response rate of 47.0% in a real-world study of more than 1,000 patients, compared with the 28.0% overall response rate that was observed in the phase 2 study of chidamide (Ann Oncol. 2015 Aug;26[8]:1766-71).
Yuqin Song, MD, PhD, of Peking University Cancer Hospital and Institute in Beijing, China, presented data from the real-world study at the annual T-cell Lymphoma Forum.
Dr. Song said this study is the largest cohort of real-world patients with relapsed or refractory PTCL. She and her colleagues analyzed data on 1,064 patients treated at 216 sites across China between February 2015 and December 2017.
The patients had a median age of 54 years, 63.9% were male, and 88.1% had stage III-IV disease.
Disease subtypes included PTCL not otherwise specified (NOS, 38.0%), angioimmunoblastic T-cell lymphoma (AITL, 29.1%), extranodal natural killer T-cell lymphoma (ENKTL, 13.4%), anaplastic large-cell lymphoma (ALCL, 9.1%), and others (10.3%), including cutaneous T-cell lymphoma (CTCL).
Fifty-two percent of patients (n = 553) received chidamide as a single agent, and 48% (n = 511) received the drug with other agents. The most common treatment regimens combined with chidamide were the following
- Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP, 20.7%).
- Gemcitabine, dexamethasone, and cisplatin (GDP, 11.8%).
- Etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH, 9.8%).
- Patients with ENKTL received chidamide with L-asparaginase (35.4%) or without it (64.5%).
The median follow-up was 4.9 months (range, 0-36.2 months). Across disease subtypes, the overall response rate was 47.0% with single-agent chidamide and 65.4% when chidamide was given in combination with other agents (P less than .01).
The median overall survival was 400 days for all patients, 342 days for patients treated with chidamide alone, and 457 days for patients who received combination therapy. The 1-year overall survival rates were 52%, 48%, and 56%, respectively.
Dr. Song said these data verify the efficacy of chidamide as a single agent and suggest chidamide might lead to improved survival in refractory or relapsed PTCLs.
Chidamide was generally well tolerated in this study, Dr. Song said. There were no unexpected adverse events (AEs) and most were grade 1 or 2.
The most common AEs (of any grade) observed with single-agent chidamide were neutropenia (42.9%), thrombocytopenia (40.5%), fatigue (38.3%), anemia (31.6%), and nausea/vomiting (21.0%).
The most common AEs observed with chidamide in combination were neutropenia (61.4%), thrombocytopenia (58.5%), fatigue (56.2%), anemia (54.2%), nausea/vomiting (30.7%), and fever (22.1%).
This study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Single-agent chidamide had an overall response rate of 47.0% among relapsed/refractory PTCL patients, compared with 65.4% when used in combination with other agents (P less than .01).
Study details: A real-world cohort of 1,064 relapsed/refractory PTCL patients treated at 216 sites across China between February 2015 and December 2017.
Disclosures: The study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.
Epigenetics is a hot topic at TCLF 2019
LA JOLLA, CALIF. –

In a video interview, meeting cochair Owen O’Connor, MD, PhD, of Columbia University Medical Center in New York, discussed a few presentations that addressed epigenetics in T-cell lymphomas.
Stephen Baylin, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, gave the meeting’s keynote address, which focused on the idea that epigenetic therapy can enhance immune checkpoint therapy.
Susan Bates, MD, of Columbia University Medical Center, presented data that suggest romidepsin and other histone deacetylase inhibitors fight cutaneous T-cell lymphoma via epigenetic effects on gene expression, as well as DNA damage.
And Enrica Marchi, MD, PhD, of Columbia University Medical Center, discussed the use of epigenetic-based combination therapies to improve responses in T-cell lymphomas.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. –

In a video interview, meeting cochair Owen O’Connor, MD, PhD, of Columbia University Medical Center in New York, discussed a few presentations that addressed epigenetics in T-cell lymphomas.
Stephen Baylin, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, gave the meeting’s keynote address, which focused on the idea that epigenetic therapy can enhance immune checkpoint therapy.
Susan Bates, MD, of Columbia University Medical Center, presented data that suggest romidepsin and other histone deacetylase inhibitors fight cutaneous T-cell lymphoma via epigenetic effects on gene expression, as well as DNA damage.
And Enrica Marchi, MD, PhD, of Columbia University Medical Center, discussed the use of epigenetic-based combination therapies to improve responses in T-cell lymphomas.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. –

In a video interview, meeting cochair Owen O’Connor, MD, PhD, of Columbia University Medical Center in New York, discussed a few presentations that addressed epigenetics in T-cell lymphomas.
Stephen Baylin, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, gave the meeting’s keynote address, which focused on the idea that epigenetic therapy can enhance immune checkpoint therapy.
Susan Bates, MD, of Columbia University Medical Center, presented data that suggest romidepsin and other histone deacetylase inhibitors fight cutaneous T-cell lymphoma via epigenetic effects on gene expression, as well as DNA damage.
And Enrica Marchi, MD, PhD, of Columbia University Medical Center, discussed the use of epigenetic-based combination therapies to improve responses in T-cell lymphomas.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Registry data favor CHOEP regimen for PTCL
LA JOLLA, CALIF. – Data from the Czech National Lymphoma Registry (NiHiL) suggest the CHOEP regimen (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone) prolongs survival in newly diagnosed patients with peripheral T-cell lymphoma (PTCL), but consolidation with autologous stem cell transplant (ASCT) does not.
“We failed to show that there is a real benefit for the patient to undergo autologous stem cell consolidation, so, nowadays, in the majority of the centers in our group, CHOEP is the chemotherapy of choice, and the patients are not consolidated,” said Marek Trneny, MD, of Charles University General Hospital in Prague.
Dr. Trneny presented results from NiHiL on behalf of the Czech Lymphoma Study Group at the annual T-cell Lymphoma Forum.
The NiHiL project is a prospective, observational study (NCT03199066) of patients newly diagnosed with non-Hodgkin lymphoma in the Czech Republic.
Dr. Trneny presented data on 838 PTCL patients who were diagnosed between 1999 and 2018, 462 of whom were included in a survival analysis (1999-2016).
The 462 patients had a median age of 61 years and more than half were men.
Patients had PTCL not otherwise specified (NOS, 43.9%), Anaplastic lymphoma kinase (ALK)–negative Anaplastic large-cell lymphoma (ALCL, 20.8%), ALK-positive ALCL (7.6%), unclassified ALCL (0.9%), angioimmunoblastic T-cell lymphoma (AITL, 10.6%), and other subtypes (16.2%).
Most patients (79.1%) had tumors measuring less than 7.5 cm, about half of patients (52.3%) had B symptoms, most (70.5%) had a performance status of 0-1, and most (68.5%) had stage III-IV disease. Half of patients were low or low/intermediate risk according to the International Prognostic Index.
For the entire cohort, the median progression-free survival (PFS) was 1.15 years, and the 5-year PFS rate was 34.2%. The median overall survival (OS) was 2.83 years, and the 5-year OS rate was 43.3%.
There was no significant difference in PFS or OS for patients diagnosed from 1999 to 2007 and those diagnosed from 2008 to 2016. The median PFS was 1.05 years and 1.23 years, respectively (P= .4487), and the median OS was 2.15 years and 3.39 years, respectively (P = .176).
Patients with ALK-positive ALCL had superior PFS and OS when compared to patients with the other disease subtypes (P = .0002 for PFS and P = .0009 for OS).
Treatment comparison
When Dr. Trneny and his colleagues compared the 223 patients who received CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and the 65 patients who received CHOEP, the researchers found that patients who received CHOEP had superior PFS and OS.
In the CHOP group, the median PFS was 1.18 years and the 5-year PFS rate was 34.2%. In the CHOEP group, the median PFS was 3.67 years and the 5-year PFS rate was 49.0% (hazard ratio, 0.6781; P = .0373).
In the CHOP group, the median OS was 3.74 years and the 5-year OS was 45.5%. In the CHOEP group, the median OS was 7.46 years and the 5-year OS was 56.4% (HR, 0.6475; P = .0381).
Dr. Trneny noted that patients who received CHOP were significantly younger (P less than .0001), more likely to have AITL or ALK-positive ALCL (P = .0152), and more likely to have a performance status of 2-4 (P = .0385).
Dr. Trneny and his colleagues also compared survival in 56 ASCT recipients and 189 patients who received chemotherapy alone (either CHOP or CHOEP).
Patients who underwent ASCT were significantly younger (P less than .0001) and more likely to have stage III or IV disease (P = .0345).
However, the researchers found no significant survival differences between patients who underwent ASCT and those who did not.
ASCT recipients had a median PFS of 7.50 years and a 5-year PFS rate of 56.0%, while patients who received chemotherapy alone had a median PFS of 4.69 years and a 5-year PFS rate of 47.3% (P = .6537).
In ASCT recipients, the median OS was not reached, and the 5-year OS rate was 63.3%. Among patients who received chemotherapy alone, the median OS was 6.78 years, and the 5-year OS was 63.0% (P = .6201).
“For those patients who are eligible for a CHOP-like regimen, CHOEP gives a higher chance for longer progression-free survival and overall survival, at least in our ... analysis,” Dr. Trneny said. “Autologous stem cell transplant, on the other hand, doesn’t seem to prolong PFS or overall survival.”
This research is sponsored by the Czech Lymphoma Study Group. Dr. Trneny did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Data from the Czech National Lymphoma Registry (NiHiL) suggest the CHOEP regimen (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone) prolongs survival in newly diagnosed patients with peripheral T-cell lymphoma (PTCL), but consolidation with autologous stem cell transplant (ASCT) does not.
“We failed to show that there is a real benefit for the patient to undergo autologous stem cell consolidation, so, nowadays, in the majority of the centers in our group, CHOEP is the chemotherapy of choice, and the patients are not consolidated,” said Marek Trneny, MD, of Charles University General Hospital in Prague.
Dr. Trneny presented results from NiHiL on behalf of the Czech Lymphoma Study Group at the annual T-cell Lymphoma Forum.
The NiHiL project is a prospective, observational study (NCT03199066) of patients newly diagnosed with non-Hodgkin lymphoma in the Czech Republic.
Dr. Trneny presented data on 838 PTCL patients who were diagnosed between 1999 and 2018, 462 of whom were included in a survival analysis (1999-2016).
The 462 patients had a median age of 61 years and more than half were men.
Patients had PTCL not otherwise specified (NOS, 43.9%), Anaplastic lymphoma kinase (ALK)–negative Anaplastic large-cell lymphoma (ALCL, 20.8%), ALK-positive ALCL (7.6%), unclassified ALCL (0.9%), angioimmunoblastic T-cell lymphoma (AITL, 10.6%), and other subtypes (16.2%).
Most patients (79.1%) had tumors measuring less than 7.5 cm, about half of patients (52.3%) had B symptoms, most (70.5%) had a performance status of 0-1, and most (68.5%) had stage III-IV disease. Half of patients were low or low/intermediate risk according to the International Prognostic Index.
For the entire cohort, the median progression-free survival (PFS) was 1.15 years, and the 5-year PFS rate was 34.2%. The median overall survival (OS) was 2.83 years, and the 5-year OS rate was 43.3%.
There was no significant difference in PFS or OS for patients diagnosed from 1999 to 2007 and those diagnosed from 2008 to 2016. The median PFS was 1.05 years and 1.23 years, respectively (P= .4487), and the median OS was 2.15 years and 3.39 years, respectively (P = .176).
Patients with ALK-positive ALCL had superior PFS and OS when compared to patients with the other disease subtypes (P = .0002 for PFS and P = .0009 for OS).
Treatment comparison
When Dr. Trneny and his colleagues compared the 223 patients who received CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and the 65 patients who received CHOEP, the researchers found that patients who received CHOEP had superior PFS and OS.
In the CHOP group, the median PFS was 1.18 years and the 5-year PFS rate was 34.2%. In the CHOEP group, the median PFS was 3.67 years and the 5-year PFS rate was 49.0% (hazard ratio, 0.6781; P = .0373).
In the CHOP group, the median OS was 3.74 years and the 5-year OS was 45.5%. In the CHOEP group, the median OS was 7.46 years and the 5-year OS was 56.4% (HR, 0.6475; P = .0381).
Dr. Trneny noted that patients who received CHOP were significantly younger (P less than .0001), more likely to have AITL or ALK-positive ALCL (P = .0152), and more likely to have a performance status of 2-4 (P = .0385).
Dr. Trneny and his colleagues also compared survival in 56 ASCT recipients and 189 patients who received chemotherapy alone (either CHOP or CHOEP).
Patients who underwent ASCT were significantly younger (P less than .0001) and more likely to have stage III or IV disease (P = .0345).
However, the researchers found no significant survival differences between patients who underwent ASCT and those who did not.
ASCT recipients had a median PFS of 7.50 years and a 5-year PFS rate of 56.0%, while patients who received chemotherapy alone had a median PFS of 4.69 years and a 5-year PFS rate of 47.3% (P = .6537).
In ASCT recipients, the median OS was not reached, and the 5-year OS rate was 63.3%. Among patients who received chemotherapy alone, the median OS was 6.78 years, and the 5-year OS was 63.0% (P = .6201).
“For those patients who are eligible for a CHOP-like regimen, CHOEP gives a higher chance for longer progression-free survival and overall survival, at least in our ... analysis,” Dr. Trneny said. “Autologous stem cell transplant, on the other hand, doesn’t seem to prolong PFS or overall survival.”
This research is sponsored by the Czech Lymphoma Study Group. Dr. Trneny did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Data from the Czech National Lymphoma Registry (NiHiL) suggest the CHOEP regimen (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone) prolongs survival in newly diagnosed patients with peripheral T-cell lymphoma (PTCL), but consolidation with autologous stem cell transplant (ASCT) does not.
“We failed to show that there is a real benefit for the patient to undergo autologous stem cell consolidation, so, nowadays, in the majority of the centers in our group, CHOEP is the chemotherapy of choice, and the patients are not consolidated,” said Marek Trneny, MD, of Charles University General Hospital in Prague.
Dr. Trneny presented results from NiHiL on behalf of the Czech Lymphoma Study Group at the annual T-cell Lymphoma Forum.
The NiHiL project is a prospective, observational study (NCT03199066) of patients newly diagnosed with non-Hodgkin lymphoma in the Czech Republic.
Dr. Trneny presented data on 838 PTCL patients who were diagnosed between 1999 and 2018, 462 of whom were included in a survival analysis (1999-2016).
The 462 patients had a median age of 61 years and more than half were men.
Patients had PTCL not otherwise specified (NOS, 43.9%), Anaplastic lymphoma kinase (ALK)–negative Anaplastic large-cell lymphoma (ALCL, 20.8%), ALK-positive ALCL (7.6%), unclassified ALCL (0.9%), angioimmunoblastic T-cell lymphoma (AITL, 10.6%), and other subtypes (16.2%).
Most patients (79.1%) had tumors measuring less than 7.5 cm, about half of patients (52.3%) had B symptoms, most (70.5%) had a performance status of 0-1, and most (68.5%) had stage III-IV disease. Half of patients were low or low/intermediate risk according to the International Prognostic Index.
For the entire cohort, the median progression-free survival (PFS) was 1.15 years, and the 5-year PFS rate was 34.2%. The median overall survival (OS) was 2.83 years, and the 5-year OS rate was 43.3%.
There was no significant difference in PFS or OS for patients diagnosed from 1999 to 2007 and those diagnosed from 2008 to 2016. The median PFS was 1.05 years and 1.23 years, respectively (P= .4487), and the median OS was 2.15 years and 3.39 years, respectively (P = .176).
Patients with ALK-positive ALCL had superior PFS and OS when compared to patients with the other disease subtypes (P = .0002 for PFS and P = .0009 for OS).
Treatment comparison
When Dr. Trneny and his colleagues compared the 223 patients who received CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and the 65 patients who received CHOEP, the researchers found that patients who received CHOEP had superior PFS and OS.
In the CHOP group, the median PFS was 1.18 years and the 5-year PFS rate was 34.2%. In the CHOEP group, the median PFS was 3.67 years and the 5-year PFS rate was 49.0% (hazard ratio, 0.6781; P = .0373).
In the CHOP group, the median OS was 3.74 years and the 5-year OS was 45.5%. In the CHOEP group, the median OS was 7.46 years and the 5-year OS was 56.4% (HR, 0.6475; P = .0381).
Dr. Trneny noted that patients who received CHOP were significantly younger (P less than .0001), more likely to have AITL or ALK-positive ALCL (P = .0152), and more likely to have a performance status of 2-4 (P = .0385).
Dr. Trneny and his colleagues also compared survival in 56 ASCT recipients and 189 patients who received chemotherapy alone (either CHOP or CHOEP).
Patients who underwent ASCT were significantly younger (P less than .0001) and more likely to have stage III or IV disease (P = .0345).
However, the researchers found no significant survival differences between patients who underwent ASCT and those who did not.
ASCT recipients had a median PFS of 7.50 years and a 5-year PFS rate of 56.0%, while patients who received chemotherapy alone had a median PFS of 4.69 years and a 5-year PFS rate of 47.3% (P = .6537).
In ASCT recipients, the median OS was not reached, and the 5-year OS rate was 63.3%. Among patients who received chemotherapy alone, the median OS was 6.78 years, and the 5-year OS was 63.0% (P = .6201).
“For those patients who are eligible for a CHOP-like regimen, CHOEP gives a higher chance for longer progression-free survival and overall survival, at least in our ... analysis,” Dr. Trneny said. “Autologous stem cell transplant, on the other hand, doesn’t seem to prolong PFS or overall survival.”
This research is sponsored by the Czech Lymphoma Study Group. Dr. Trneny did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: The 5-year progression free survival rate was 49.0% in the group receiving CHOEP, compared with 34.2% in the group receiving CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone).
Study details: A survival analysis of 462 patients with PTCL who were part of the Czech National Lymphoma Registry.
Disclosures: This research is sponsored by the Czech Lymphoma Study Group. Dr. Trneny did not disclose any conflicts of interest.
ECHELON-2: BV-CHP boosts survival in PTCL
SAN DIEGO – A newly approved treatment regimen provides a survival benefit over standard therapy for patients with CD30-positive peripheral T-cell lymphomas (PTCLs), according to new research presented at the annual meeting of the American Society of Hematology.
In the ECHELON-2 trial, patients who received brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, and prednisone (CHP) had superior progression-free survival (PFS) and overall survival (OS), compared with patients who received standard treatment with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results supported the recent U.S. approval of BV in combination with CHP for adults with previously untreated, systemic anaplastic large cell lymphoma or other CD30-expressing PTCLs.
“ECHELON-2 is the first prospective trial in peripheral T-cell lymphoma to show an overall survival benefit over CHOP,” said Steven M. Horwitz, MD, of Memorial Sloan Kettering Cancer Center, with locations in New York and New Jersey.
Dr. Horwitz presented data from this trial at the ASH meeting. Results were simultaneously published in the Lancet (2018 Dec 3. doi: 10.1016/S0140-6736[18]32984-2).
ECHELON-2 (NCT01777152) enrolled 452 patients with previously untreated, CD30-positive PTCL. Subtypes included ALK-positive or ALK-negative systemic anaplastic large-cell lymphoma, PTCL not otherwise specified, angioimmunoblastic T-cell lymphoma, enteropathy-associated T-cell lymphoma, and adult T-cell leukemia/lymphoma.
Patients were randomized to receive BV-CHP plus placebo (n = 226) or CHOP plus placebo (n = 226) every 3 weeks for six to eight cycles.
At baseline, the median age was 58 in the BV-CHP arm and the CHOP arm. The majority of patients were male – 59% in the BV-CHP arm and 67% in the CHOP arm – and most patients had stage III/IV disease, 81% and 80%, respectively.
In all, 89% of patients in the BV-CHP arm and 81% in the CHOP arm completed six or more cycles of their assigned treatment.
The overall response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P = .0032). The complete response rates were 68% and 56%, respectively (P = .0066).
At a median follow-up of 36.2 months, the median PFS was 48.2 months in the BV-CHP arm and 20.8 months in the CHOP arm. The rate of death or progression was 42% in the BV-CHP arm and 55% in the CHOP arm (hazard ratio = 0.71, P = .011).
At a median follow-up of 42.1 months, the median OS was not reached in either treatment arm. The rate of death was 23% in the BV-CHP arm and 32% in the CHOP arm (HR = 0.66, P = .0244).
Dr. Horwitz noted that this study was not powered to determine differences in PFS or OS by PTCL subtypes.
BV-CHP had a safety profile comparable with that of CHOP, Dr. Horwitz said.
The rate of adverse events (AEs) was 99% in the BV-CHP arm and 98% in the CHOP arm. Grade 3 or higher AEs occurred in 66% and 65% of patients, respectively. Serious AEs occurred in 39% and 38%, respectively.
Three percent of patients in the BV-CHP arm and 4% of those in the CHOP arm had fatal AEs.
The study was funded by Seattle Genetics, Millennium Pharmaceuticals, and the National Institutes of Health. Dr. Horwitz reported relationships with Seattle Genetics, Millennium Pharmaceuticals, and other companies.
SOURCE: Horwitz S et al. ASH 2018, Abstract 997.
SAN DIEGO – A newly approved treatment regimen provides a survival benefit over standard therapy for patients with CD30-positive peripheral T-cell lymphomas (PTCLs), according to new research presented at the annual meeting of the American Society of Hematology.
In the ECHELON-2 trial, patients who received brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, and prednisone (CHP) had superior progression-free survival (PFS) and overall survival (OS), compared with patients who received standard treatment with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results supported the recent U.S. approval of BV in combination with CHP for adults with previously untreated, systemic anaplastic large cell lymphoma or other CD30-expressing PTCLs.
“ECHELON-2 is the first prospective trial in peripheral T-cell lymphoma to show an overall survival benefit over CHOP,” said Steven M. Horwitz, MD, of Memorial Sloan Kettering Cancer Center, with locations in New York and New Jersey.
Dr. Horwitz presented data from this trial at the ASH meeting. Results were simultaneously published in the Lancet (2018 Dec 3. doi: 10.1016/S0140-6736[18]32984-2).
ECHELON-2 (NCT01777152) enrolled 452 patients with previously untreated, CD30-positive PTCL. Subtypes included ALK-positive or ALK-negative systemic anaplastic large-cell lymphoma, PTCL not otherwise specified, angioimmunoblastic T-cell lymphoma, enteropathy-associated T-cell lymphoma, and adult T-cell leukemia/lymphoma.
Patients were randomized to receive BV-CHP plus placebo (n = 226) or CHOP plus placebo (n = 226) every 3 weeks for six to eight cycles.
At baseline, the median age was 58 in the BV-CHP arm and the CHOP arm. The majority of patients were male – 59% in the BV-CHP arm and 67% in the CHOP arm – and most patients had stage III/IV disease, 81% and 80%, respectively.
In all, 89% of patients in the BV-CHP arm and 81% in the CHOP arm completed six or more cycles of their assigned treatment.
The overall response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P = .0032). The complete response rates were 68% and 56%, respectively (P = .0066).
At a median follow-up of 36.2 months, the median PFS was 48.2 months in the BV-CHP arm and 20.8 months in the CHOP arm. The rate of death or progression was 42% in the BV-CHP arm and 55% in the CHOP arm (hazard ratio = 0.71, P = .011).
At a median follow-up of 42.1 months, the median OS was not reached in either treatment arm. The rate of death was 23% in the BV-CHP arm and 32% in the CHOP arm (HR = 0.66, P = .0244).
Dr. Horwitz noted that this study was not powered to determine differences in PFS or OS by PTCL subtypes.
BV-CHP had a safety profile comparable with that of CHOP, Dr. Horwitz said.
The rate of adverse events (AEs) was 99% in the BV-CHP arm and 98% in the CHOP arm. Grade 3 or higher AEs occurred in 66% and 65% of patients, respectively. Serious AEs occurred in 39% and 38%, respectively.
Three percent of patients in the BV-CHP arm and 4% of those in the CHOP arm had fatal AEs.
The study was funded by Seattle Genetics, Millennium Pharmaceuticals, and the National Institutes of Health. Dr. Horwitz reported relationships with Seattle Genetics, Millennium Pharmaceuticals, and other companies.
SOURCE: Horwitz S et al. ASH 2018, Abstract 997.
SAN DIEGO – A newly approved treatment regimen provides a survival benefit over standard therapy for patients with CD30-positive peripheral T-cell lymphomas (PTCLs), according to new research presented at the annual meeting of the American Society of Hematology.
In the ECHELON-2 trial, patients who received brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, and prednisone (CHP) had superior progression-free survival (PFS) and overall survival (OS), compared with patients who received standard treatment with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results supported the recent U.S. approval of BV in combination with CHP for adults with previously untreated, systemic anaplastic large cell lymphoma or other CD30-expressing PTCLs.
“ECHELON-2 is the first prospective trial in peripheral T-cell lymphoma to show an overall survival benefit over CHOP,” said Steven M. Horwitz, MD, of Memorial Sloan Kettering Cancer Center, with locations in New York and New Jersey.
Dr. Horwitz presented data from this trial at the ASH meeting. Results were simultaneously published in the Lancet (2018 Dec 3. doi: 10.1016/S0140-6736[18]32984-2).
ECHELON-2 (NCT01777152) enrolled 452 patients with previously untreated, CD30-positive PTCL. Subtypes included ALK-positive or ALK-negative systemic anaplastic large-cell lymphoma, PTCL not otherwise specified, angioimmunoblastic T-cell lymphoma, enteropathy-associated T-cell lymphoma, and adult T-cell leukemia/lymphoma.
Patients were randomized to receive BV-CHP plus placebo (n = 226) or CHOP plus placebo (n = 226) every 3 weeks for six to eight cycles.
At baseline, the median age was 58 in the BV-CHP arm and the CHOP arm. The majority of patients were male – 59% in the BV-CHP arm and 67% in the CHOP arm – and most patients had stage III/IV disease, 81% and 80%, respectively.
In all, 89% of patients in the BV-CHP arm and 81% in the CHOP arm completed six or more cycles of their assigned treatment.
The overall response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P = .0032). The complete response rates were 68% and 56%, respectively (P = .0066).
At a median follow-up of 36.2 months, the median PFS was 48.2 months in the BV-CHP arm and 20.8 months in the CHOP arm. The rate of death or progression was 42% in the BV-CHP arm and 55% in the CHOP arm (hazard ratio = 0.71, P = .011).
At a median follow-up of 42.1 months, the median OS was not reached in either treatment arm. The rate of death was 23% in the BV-CHP arm and 32% in the CHOP arm (HR = 0.66, P = .0244).
Dr. Horwitz noted that this study was not powered to determine differences in PFS or OS by PTCL subtypes.
BV-CHP had a safety profile comparable with that of CHOP, Dr. Horwitz said.
The rate of adverse events (AEs) was 99% in the BV-CHP arm and 98% in the CHOP arm. Grade 3 or higher AEs occurred in 66% and 65% of patients, respectively. Serious AEs occurred in 39% and 38%, respectively.
Three percent of patients in the BV-CHP arm and 4% of those in the CHOP arm had fatal AEs.
The study was funded by Seattle Genetics, Millennium Pharmaceuticals, and the National Institutes of Health. Dr. Horwitz reported relationships with Seattle Genetics, Millennium Pharmaceuticals, and other companies.
SOURCE: Horwitz S et al. ASH 2018, Abstract 997.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: The rate of death or progression was 42% in the BV-CHP arm and 55% in the CHOP arm (hazard ratio = 0.71, P = .011), while the rate of death alone was 23% and 32%, respectively (HR = 0.66, P = .0244).
Study details: A phase 3 trial of 452 patients with peripheral T-cell lymphoma.
Disclosures: The study was funded by Seattle Genetics, Millennium Pharmaceuticals, and the National Institutes of Health. Dr. Horwitz reported relationships with Seattle Genetics, Millennium Pharmaceuticals, and other companies.
Source: Horwitz S et al. ASH 2018, Abstract 997.
FDA expands approval of brentuximab vedotin to PTCL
The , marking the first FDA approval of a treatment for newly-diagnosed PTCL.
The drug, which is marketed by Seattle Genetics as Adcetris, is a monoclonal antibody that binds to CD30 protein found on some cancer cells.
It was previously approved for adult patients with untreated stage III or IV classical Hodgkin lymphoma (cHL), cHL after relapse, cHL after stem cell transplant in patients at high risk for relapse or progression, systemic anaplastic large cell lymphoma (ALCL) after other treatments fail, and primary cutaneous ALCL or CD30-expressing mycosis fungoides after other treatments fail.
The expanded approval, which followed the granting of Priority Review and Breakthrough Therapy designations for the supplemental Biologic License Application, was made using the FDA’s new Real-Time Oncology Review pilot program (RTOR). This program allows for data review and communication with a sponsor prior to official application submission with the goal of speeding up the review process.
The brentuximab vedotin approval now extends to previously untreated systemic ALCL and other CD30-expressing PTCLs in combination with chemotherapy.
Approval was based on the ECHELON-2 clinical trial involving 452 patients, which demonstrated improved progression-free survival (PFS) in patients with certain types of PTCL who were treated first-line with either brentuximab vedotin plus chemotherapy with cyclophosphamide, doxorubicin, prednisone (CHP), or standard chemotherapy with CHP and vincristine (CHOP). Median PFS was 48 months vs. 21 months in the groups, respectively (hazard ratio, 0.71).
The FDA advises health care providers to “monitor patients for infusion reactions, life-threatening allergic reactions (anaphylaxis), neuropathy, fever, gastrointestinal complications, and infections,” according to a press release announcing the approval, which also states that patients should be monitored for tumor lysis syndrome, serious skin reactions, pulmonary toxicity, and hepatotoxicity.
The drug may cause harm to a developing fetus or newborn and should not be used in women who are pregnant or breastfeeding. A Boxed Warning regarding risk of progressive multifocal leukoencephalopathy is also included in the prescribing information.
The current standard of care for initial treatment of PTCL is multiagent chemotherapy – a treatment that “has not significantly changed in decades and is too often unsuccessful in leading to long-term remissions, underscoring the need for new treatments, ” Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a statement issued by Seattle Genetics.
“With this approval, clinicians have the opportunity to transform the way newly diagnosed CD30-expressing PTCL patients are treated,” Dr. Horwitz said.
The ECHELON-2 data will be presented at the American Society of Hematology annual meeting in San Diego on Monday, Dec. 3, 2018.
The , marking the first FDA approval of a treatment for newly-diagnosed PTCL.
The drug, which is marketed by Seattle Genetics as Adcetris, is a monoclonal antibody that binds to CD30 protein found on some cancer cells.
It was previously approved for adult patients with untreated stage III or IV classical Hodgkin lymphoma (cHL), cHL after relapse, cHL after stem cell transplant in patients at high risk for relapse or progression, systemic anaplastic large cell lymphoma (ALCL) after other treatments fail, and primary cutaneous ALCL or CD30-expressing mycosis fungoides after other treatments fail.
The expanded approval, which followed the granting of Priority Review and Breakthrough Therapy designations for the supplemental Biologic License Application, was made using the FDA’s new Real-Time Oncology Review pilot program (RTOR). This program allows for data review and communication with a sponsor prior to official application submission with the goal of speeding up the review process.
The brentuximab vedotin approval now extends to previously untreated systemic ALCL and other CD30-expressing PTCLs in combination with chemotherapy.
Approval was based on the ECHELON-2 clinical trial involving 452 patients, which demonstrated improved progression-free survival (PFS) in patients with certain types of PTCL who were treated first-line with either brentuximab vedotin plus chemotherapy with cyclophosphamide, doxorubicin, prednisone (CHP), or standard chemotherapy with CHP and vincristine (CHOP). Median PFS was 48 months vs. 21 months in the groups, respectively (hazard ratio, 0.71).
The FDA advises health care providers to “monitor patients for infusion reactions, life-threatening allergic reactions (anaphylaxis), neuropathy, fever, gastrointestinal complications, and infections,” according to a press release announcing the approval, which also states that patients should be monitored for tumor lysis syndrome, serious skin reactions, pulmonary toxicity, and hepatotoxicity.
The drug may cause harm to a developing fetus or newborn and should not be used in women who are pregnant or breastfeeding. A Boxed Warning regarding risk of progressive multifocal leukoencephalopathy is also included in the prescribing information.
The current standard of care for initial treatment of PTCL is multiagent chemotherapy – a treatment that “has not significantly changed in decades and is too often unsuccessful in leading to long-term remissions, underscoring the need for new treatments, ” Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a statement issued by Seattle Genetics.
“With this approval, clinicians have the opportunity to transform the way newly diagnosed CD30-expressing PTCL patients are treated,” Dr. Horwitz said.
The ECHELON-2 data will be presented at the American Society of Hematology annual meeting in San Diego on Monday, Dec. 3, 2018.
The , marking the first FDA approval of a treatment for newly-diagnosed PTCL.
The drug, which is marketed by Seattle Genetics as Adcetris, is a monoclonal antibody that binds to CD30 protein found on some cancer cells.
It was previously approved for adult patients with untreated stage III or IV classical Hodgkin lymphoma (cHL), cHL after relapse, cHL after stem cell transplant in patients at high risk for relapse or progression, systemic anaplastic large cell lymphoma (ALCL) after other treatments fail, and primary cutaneous ALCL or CD30-expressing mycosis fungoides after other treatments fail.
The expanded approval, which followed the granting of Priority Review and Breakthrough Therapy designations for the supplemental Biologic License Application, was made using the FDA’s new Real-Time Oncology Review pilot program (RTOR). This program allows for data review and communication with a sponsor prior to official application submission with the goal of speeding up the review process.
The brentuximab vedotin approval now extends to previously untreated systemic ALCL and other CD30-expressing PTCLs in combination with chemotherapy.
Approval was based on the ECHELON-2 clinical trial involving 452 patients, which demonstrated improved progression-free survival (PFS) in patients with certain types of PTCL who were treated first-line with either brentuximab vedotin plus chemotherapy with cyclophosphamide, doxorubicin, prednisone (CHP), or standard chemotherapy with CHP and vincristine (CHOP). Median PFS was 48 months vs. 21 months in the groups, respectively (hazard ratio, 0.71).
The FDA advises health care providers to “monitor patients for infusion reactions, life-threatening allergic reactions (anaphylaxis), neuropathy, fever, gastrointestinal complications, and infections,” according to a press release announcing the approval, which also states that patients should be monitored for tumor lysis syndrome, serious skin reactions, pulmonary toxicity, and hepatotoxicity.
The drug may cause harm to a developing fetus or newborn and should not be used in women who are pregnant or breastfeeding. A Boxed Warning regarding risk of progressive multifocal leukoencephalopathy is also included in the prescribing information.
The current standard of care for initial treatment of PTCL is multiagent chemotherapy – a treatment that “has not significantly changed in decades and is too often unsuccessful in leading to long-term remissions, underscoring the need for new treatments, ” Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a statement issued by Seattle Genetics.
“With this approval, clinicians have the opportunity to transform the way newly diagnosed CD30-expressing PTCL patients are treated,” Dr. Horwitz said.
The ECHELON-2 data will be presented at the American Society of Hematology annual meeting in San Diego on Monday, Dec. 3, 2018.
When to choose stem cell transplant in PTCL
DUBROVNIK, CROATIA – , according to one expert.
The success of HSCT varies according to the subtype of PTCL and the type of transplant, Ali Bazarbachi, MD, PhD, of the American University of Beirut, Lebanon, said at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
For example, autologous (auto) HSCT given as frontline consolidation can be considered the standard of care for PTCL–not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and certain patients with anaplastic large-cell lymphoma (ALCL), according to Dr. Bazarbachi.
On the other hand, auto-HSCT should never be used in patients with adult T-cell leukemia/lymphoma (ATLL).
Both auto-HSCT and allogeneic (allo) HSCT are options for patients with nonlocalized, extranodal natural killer T-cell lymphoma (ENKTL), nasal type, but only at certain times.
State of PTCL treatment
Patients with newly diagnosed PTCL are no longer treated like patients with B-cell lymphoma, but treatment outcomes in PTCL still leave a lot to be desired, Dr. Bazarbachi said.
He noted that, with any of the chemotherapy regimens used, typically, about a third of patients are primary refractory, a third relapse, and a quarter are cured. Only two forms of PTCL are frequently curable – localized ENKTL and anaplastic lymphoma kinase–positive (ALK-positive) ALCL.
Current treatment strategies for PTCL do include HSCT, but recommendations vary. Dr. Bazarbachi made the following recommendations, supported by evidence from clinical trials.
PTCL-NOS, AITL, and ALCL
For patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, auto-HSCT as frontline consolidation can be considered the standard of care in patients who responded to induction, Dr. Bazarbachi said.
In a study published in 2012, high-dose chemotherapy and auto-HSCT as consolidation improved 5-year overall survival – compared with previous results with CHOP – in patients with ALK-negative ALCL, AITL, PTCL-NOS, and enteropathy-associated T-cell lymphoma (J Clin Oncol. 2012 Sep 1;30[25]:3093-9; ISRN Hematol. 2011 Jun 16. doi: 10.5402/2011/623924).
Allo-HSCT may also be an option for frontline consolidation in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, according to Dr. Bazarbachi.
“Allo-transplant is not dead in this indication,” he said. “But it should be either part of a clinical trial or [given] to some selected patients – those with persistent bone marrow involvement, very young patients, or patients with primary refractory disease.”
Results from the COMPLETE study showed improved survival in patients who received consolidation with auto- or allo-HSCT, compared with patients who did not receive a transplant (Blood. 2017;130:342).
COMPLETE patients with AITL or PTCL-NOS had improvements in progression-free and overall survival with HSCT. The survival advantage was “less evident” in patients with ALCL, the researchers said, but this trial included both ALK-negative and ALK-positive patients.
Allo- and auto-HSCT can be options after relapse in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, Dr. Bazarbachi said.
However, chemosensitive patients who have relapsed should receive auto-HSCT only if they did not receive it frontline. Patients who have already undergone auto-HSCT can receive allo-HSCT, Dr. Bazarbachi said.
He added that refractory patients should not undergo auto-HSCT and should receive allo-HSCT only within the context of a clinical trial.
ATLL
ATLL has a dismal prognosis, but allo-HSCT as frontline consolidation is potentially curative, Dr. Bazarbachi said. It is most effective in patients who have achieved a complete or partial response to induction (Blood. 2012 Aug 23;120[8]:1734-41).
However, allo-HSCT should not be given as consolidation to ATLL patients who have received prior mogamulizumab. These patients have an increased risk of morbidity and mortality if they undergo allo-HSCT.
Also, allo-HSCT should not be given to refractory ATLL patients, although it may be an option for relapsed patients.
Dr. Bazarbachi stressed that ATLL patients should not receive auto-HSCT at any time, as frontline consolidation, after relapse, or if they have refractory disease.
Auto-HSCT “does not work in this disease,” he said. In a study published in 2014, all four ATLL patients who underwent auto-HSCT “rapidly” died (Bone Marrow Transplant. 2014 Oct;49[10]:1266-8).
ENKTL
Dr. Bazarbachi said frontline consolidation with auto-HSCT should be considered the standard of care for patients with non-localized ENKTL, nasal type.
Auto-HSCT has been shown to improve survival in these patients, and it is most effective when patients have achieved a complete response to induction (Biol Blood Marrow Transplant. 2008 Dec;14[12]:1356-64).
Allo-HSCT also is an option for frontline consolidation in patients with nonlocalized ENKTL, nasal type, Dr. Bazarbachi said.
He added that chemosensitive patients who have relapsed can receive allo-HSCT, but they should receive auto-HSCT only if they did not receive it in the frontline setting. Both types of transplant should take place when patients are in complete remission.
Patients with refractory, nonlocalized ENKTL, nasal type, should not receive auto-HSCT, but allo-HSCT is an option, Dr. Bazarbachi said.
Dr. Bazarbachi did not declare any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Associates, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – , according to one expert.
The success of HSCT varies according to the subtype of PTCL and the type of transplant, Ali Bazarbachi, MD, PhD, of the American University of Beirut, Lebanon, said at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
For example, autologous (auto) HSCT given as frontline consolidation can be considered the standard of care for PTCL–not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and certain patients with anaplastic large-cell lymphoma (ALCL), according to Dr. Bazarbachi.
On the other hand, auto-HSCT should never be used in patients with adult T-cell leukemia/lymphoma (ATLL).
Both auto-HSCT and allogeneic (allo) HSCT are options for patients with nonlocalized, extranodal natural killer T-cell lymphoma (ENKTL), nasal type, but only at certain times.
State of PTCL treatment
Patients with newly diagnosed PTCL are no longer treated like patients with B-cell lymphoma, but treatment outcomes in PTCL still leave a lot to be desired, Dr. Bazarbachi said.
He noted that, with any of the chemotherapy regimens used, typically, about a third of patients are primary refractory, a third relapse, and a quarter are cured. Only two forms of PTCL are frequently curable – localized ENKTL and anaplastic lymphoma kinase–positive (ALK-positive) ALCL.
Current treatment strategies for PTCL do include HSCT, but recommendations vary. Dr. Bazarbachi made the following recommendations, supported by evidence from clinical trials.
PTCL-NOS, AITL, and ALCL
For patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, auto-HSCT as frontline consolidation can be considered the standard of care in patients who responded to induction, Dr. Bazarbachi said.
In a study published in 2012, high-dose chemotherapy and auto-HSCT as consolidation improved 5-year overall survival – compared with previous results with CHOP – in patients with ALK-negative ALCL, AITL, PTCL-NOS, and enteropathy-associated T-cell lymphoma (J Clin Oncol. 2012 Sep 1;30[25]:3093-9; ISRN Hematol. 2011 Jun 16. doi: 10.5402/2011/623924).
Allo-HSCT may also be an option for frontline consolidation in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, according to Dr. Bazarbachi.
“Allo-transplant is not dead in this indication,” he said. “But it should be either part of a clinical trial or [given] to some selected patients – those with persistent bone marrow involvement, very young patients, or patients with primary refractory disease.”
Results from the COMPLETE study showed improved survival in patients who received consolidation with auto- or allo-HSCT, compared with patients who did not receive a transplant (Blood. 2017;130:342).
COMPLETE patients with AITL or PTCL-NOS had improvements in progression-free and overall survival with HSCT. The survival advantage was “less evident” in patients with ALCL, the researchers said, but this trial included both ALK-negative and ALK-positive patients.
Allo- and auto-HSCT can be options after relapse in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, Dr. Bazarbachi said.
However, chemosensitive patients who have relapsed should receive auto-HSCT only if they did not receive it frontline. Patients who have already undergone auto-HSCT can receive allo-HSCT, Dr. Bazarbachi said.
He added that refractory patients should not undergo auto-HSCT and should receive allo-HSCT only within the context of a clinical trial.
ATLL
ATLL has a dismal prognosis, but allo-HSCT as frontline consolidation is potentially curative, Dr. Bazarbachi said. It is most effective in patients who have achieved a complete or partial response to induction (Blood. 2012 Aug 23;120[8]:1734-41).
However, allo-HSCT should not be given as consolidation to ATLL patients who have received prior mogamulizumab. These patients have an increased risk of morbidity and mortality if they undergo allo-HSCT.
Also, allo-HSCT should not be given to refractory ATLL patients, although it may be an option for relapsed patients.
Dr. Bazarbachi stressed that ATLL patients should not receive auto-HSCT at any time, as frontline consolidation, after relapse, or if they have refractory disease.
Auto-HSCT “does not work in this disease,” he said. In a study published in 2014, all four ATLL patients who underwent auto-HSCT “rapidly” died (Bone Marrow Transplant. 2014 Oct;49[10]:1266-8).
ENKTL
Dr. Bazarbachi said frontline consolidation with auto-HSCT should be considered the standard of care for patients with non-localized ENKTL, nasal type.
Auto-HSCT has been shown to improve survival in these patients, and it is most effective when patients have achieved a complete response to induction (Biol Blood Marrow Transplant. 2008 Dec;14[12]:1356-64).
Allo-HSCT also is an option for frontline consolidation in patients with nonlocalized ENKTL, nasal type, Dr. Bazarbachi said.
He added that chemosensitive patients who have relapsed can receive allo-HSCT, but they should receive auto-HSCT only if they did not receive it in the frontline setting. Both types of transplant should take place when patients are in complete remission.
Patients with refractory, nonlocalized ENKTL, nasal type, should not receive auto-HSCT, but allo-HSCT is an option, Dr. Bazarbachi said.
Dr. Bazarbachi did not declare any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Associates, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – , according to one expert.
The success of HSCT varies according to the subtype of PTCL and the type of transplant, Ali Bazarbachi, MD, PhD, of the American University of Beirut, Lebanon, said at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
For example, autologous (auto) HSCT given as frontline consolidation can be considered the standard of care for PTCL–not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and certain patients with anaplastic large-cell lymphoma (ALCL), according to Dr. Bazarbachi.
On the other hand, auto-HSCT should never be used in patients with adult T-cell leukemia/lymphoma (ATLL).
Both auto-HSCT and allogeneic (allo) HSCT are options for patients with nonlocalized, extranodal natural killer T-cell lymphoma (ENKTL), nasal type, but only at certain times.
State of PTCL treatment
Patients with newly diagnosed PTCL are no longer treated like patients with B-cell lymphoma, but treatment outcomes in PTCL still leave a lot to be desired, Dr. Bazarbachi said.
He noted that, with any of the chemotherapy regimens used, typically, about a third of patients are primary refractory, a third relapse, and a quarter are cured. Only two forms of PTCL are frequently curable – localized ENKTL and anaplastic lymphoma kinase–positive (ALK-positive) ALCL.
Current treatment strategies for PTCL do include HSCT, but recommendations vary. Dr. Bazarbachi made the following recommendations, supported by evidence from clinical trials.
PTCL-NOS, AITL, and ALCL
For patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, auto-HSCT as frontline consolidation can be considered the standard of care in patients who responded to induction, Dr. Bazarbachi said.
In a study published in 2012, high-dose chemotherapy and auto-HSCT as consolidation improved 5-year overall survival – compared with previous results with CHOP – in patients with ALK-negative ALCL, AITL, PTCL-NOS, and enteropathy-associated T-cell lymphoma (J Clin Oncol. 2012 Sep 1;30[25]:3093-9; ISRN Hematol. 2011 Jun 16. doi: 10.5402/2011/623924).
Allo-HSCT may also be an option for frontline consolidation in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, according to Dr. Bazarbachi.
“Allo-transplant is not dead in this indication,” he said. “But it should be either part of a clinical trial or [given] to some selected patients – those with persistent bone marrow involvement, very young patients, or patients with primary refractory disease.”
Results from the COMPLETE study showed improved survival in patients who received consolidation with auto- or allo-HSCT, compared with patients who did not receive a transplant (Blood. 2017;130:342).
COMPLETE patients with AITL or PTCL-NOS had improvements in progression-free and overall survival with HSCT. The survival advantage was “less evident” in patients with ALCL, the researchers said, but this trial included both ALK-negative and ALK-positive patients.
Allo- and auto-HSCT can be options after relapse in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, Dr. Bazarbachi said.
However, chemosensitive patients who have relapsed should receive auto-HSCT only if they did not receive it frontline. Patients who have already undergone auto-HSCT can receive allo-HSCT, Dr. Bazarbachi said.
He added that refractory patients should not undergo auto-HSCT and should receive allo-HSCT only within the context of a clinical trial.
ATLL
ATLL has a dismal prognosis, but allo-HSCT as frontline consolidation is potentially curative, Dr. Bazarbachi said. It is most effective in patients who have achieved a complete or partial response to induction (Blood. 2012 Aug 23;120[8]:1734-41).
However, allo-HSCT should not be given as consolidation to ATLL patients who have received prior mogamulizumab. These patients have an increased risk of morbidity and mortality if they undergo allo-HSCT.
Also, allo-HSCT should not be given to refractory ATLL patients, although it may be an option for relapsed patients.
Dr. Bazarbachi stressed that ATLL patients should not receive auto-HSCT at any time, as frontline consolidation, after relapse, or if they have refractory disease.
Auto-HSCT “does not work in this disease,” he said. In a study published in 2014, all four ATLL patients who underwent auto-HSCT “rapidly” died (Bone Marrow Transplant. 2014 Oct;49[10]:1266-8).
ENKTL
Dr. Bazarbachi said frontline consolidation with auto-HSCT should be considered the standard of care for patients with non-localized ENKTL, nasal type.
Auto-HSCT has been shown to improve survival in these patients, and it is most effective when patients have achieved a complete response to induction (Biol Blood Marrow Transplant. 2008 Dec;14[12]:1356-64).
Allo-HSCT also is an option for frontline consolidation in patients with nonlocalized ENKTL, nasal type, Dr. Bazarbachi said.
He added that chemosensitive patients who have relapsed can receive allo-HSCT, but they should receive auto-HSCT only if they did not receive it in the frontline setting. Both types of transplant should take place when patients are in complete remission.
Patients with refractory, nonlocalized ENKTL, nasal type, should not receive auto-HSCT, but allo-HSCT is an option, Dr. Bazarbachi said.
Dr. Bazarbachi did not declare any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Associates, which is owned by the parent company of this news organization.
EXPERT ANALYSIS FROM LEUKEMIA AND LYMPHOMA 2018
AITL responds to 5-azacytidine in small series
Older patients with refractory angioimmunoblastic T cell lymphoma (AITL) appear to respond well to treatment with 5-azacytidine, regardless of mutations.
Francois Lemonnier, MD, of Henri Mondor University Hospitals in Créteil, France, and his colleagues, reported on a retrospective series of 12 AITL patients who received 5-azacytidine for concomitant myeloid neoplasm or as compassionate therapy for relapsed or refractory AITL. The findings were published in Blood.
Patients were given 5-azacytidine subcutaneously at a dose of 75 mg/m2 daily for 7 consecutive days. The treatment was given every 28 days until progression or unacceptable toxicity for a median of 5.5 cycles. Along with 5-azacytidine, half of the patients received rituximab due to the presence of EBV replication or EBV B-blasts in the lymph node biopsy.
The patients were assessed via CT scan and responses were evaluated by investigators following the Cheson criteria.
This was a heavily pretreated patient population. The median age was 70 years and 11 of the patients had relapsed or refractory disease and had received a median of two lines of therapy. There was only one treatment-naive patient in the series.
Treatment with 5-azacytidine produced an overall response rate of 75%, with six patients achieving a complete response and three patients achieving a partial response. The median progression-free survival was 15 months and median overall survival was 21 months at a median follow-up of 27 months.
The researchers noted that some elderly patients with poor performance status achieved a sustained response after treatment with an acceptable tolerance.
Treatment was well tolerated overall. There were no treatment-related deaths and no patients developed neutropenia. Three patients required transfusion and another had grade 3 diarrhea.
The researchers also performed molecular studies using targeted deep sequencing. They detected TET2 mutations in all 12 patients, with seven patients having two mutations. Four patients had DNMT3A mutations, five patients had RHOA mutations, and four patients had p.G17V substitution. One patient had an IDH2R172 mutation.
Since all patients had a TET2 mutation, the researchers were unable to assess its impact on treatment response. However, they saw no association between the number of TET2 mutations and treatment response, or mutations in DNMT3A, IDH2, and RHOA and treatment response.
The study was funded by a grant from the Leukemia & Lymphoma Society. Three of the coauthors received honoraria from Celgene.
SOURCE: Lemonnier F et al. Blood. 2018 Oct 2. doi: 10.1182/blood-2018-04-840538.
Older patients with refractory angioimmunoblastic T cell lymphoma (AITL) appear to respond well to treatment with 5-azacytidine, regardless of mutations.
Francois Lemonnier, MD, of Henri Mondor University Hospitals in Créteil, France, and his colleagues, reported on a retrospective series of 12 AITL patients who received 5-azacytidine for concomitant myeloid neoplasm or as compassionate therapy for relapsed or refractory AITL. The findings were published in Blood.
Patients were given 5-azacytidine subcutaneously at a dose of 75 mg/m2 daily for 7 consecutive days. The treatment was given every 28 days until progression or unacceptable toxicity for a median of 5.5 cycles. Along with 5-azacytidine, half of the patients received rituximab due to the presence of EBV replication or EBV B-blasts in the lymph node biopsy.
The patients were assessed via CT scan and responses were evaluated by investigators following the Cheson criteria.
This was a heavily pretreated patient population. The median age was 70 years and 11 of the patients had relapsed or refractory disease and had received a median of two lines of therapy. There was only one treatment-naive patient in the series.
Treatment with 5-azacytidine produced an overall response rate of 75%, with six patients achieving a complete response and three patients achieving a partial response. The median progression-free survival was 15 months and median overall survival was 21 months at a median follow-up of 27 months.
The researchers noted that some elderly patients with poor performance status achieved a sustained response after treatment with an acceptable tolerance.
Treatment was well tolerated overall. There were no treatment-related deaths and no patients developed neutropenia. Three patients required transfusion and another had grade 3 diarrhea.
The researchers also performed molecular studies using targeted deep sequencing. They detected TET2 mutations in all 12 patients, with seven patients having two mutations. Four patients had DNMT3A mutations, five patients had RHOA mutations, and four patients had p.G17V substitution. One patient had an IDH2R172 mutation.
Since all patients had a TET2 mutation, the researchers were unable to assess its impact on treatment response. However, they saw no association between the number of TET2 mutations and treatment response, or mutations in DNMT3A, IDH2, and RHOA and treatment response.
The study was funded by a grant from the Leukemia & Lymphoma Society. Three of the coauthors received honoraria from Celgene.
SOURCE: Lemonnier F et al. Blood. 2018 Oct 2. doi: 10.1182/blood-2018-04-840538.
Older patients with refractory angioimmunoblastic T cell lymphoma (AITL) appear to respond well to treatment with 5-azacytidine, regardless of mutations.
Francois Lemonnier, MD, of Henri Mondor University Hospitals in Créteil, France, and his colleagues, reported on a retrospective series of 12 AITL patients who received 5-azacytidine for concomitant myeloid neoplasm or as compassionate therapy for relapsed or refractory AITL. The findings were published in Blood.
Patients were given 5-azacytidine subcutaneously at a dose of 75 mg/m2 daily for 7 consecutive days. The treatment was given every 28 days until progression or unacceptable toxicity for a median of 5.5 cycles. Along with 5-azacytidine, half of the patients received rituximab due to the presence of EBV replication or EBV B-blasts in the lymph node biopsy.
The patients were assessed via CT scan and responses were evaluated by investigators following the Cheson criteria.
This was a heavily pretreated patient population. The median age was 70 years and 11 of the patients had relapsed or refractory disease and had received a median of two lines of therapy. There was only one treatment-naive patient in the series.
Treatment with 5-azacytidine produced an overall response rate of 75%, with six patients achieving a complete response and three patients achieving a partial response. The median progression-free survival was 15 months and median overall survival was 21 months at a median follow-up of 27 months.
The researchers noted that some elderly patients with poor performance status achieved a sustained response after treatment with an acceptable tolerance.
Treatment was well tolerated overall. There were no treatment-related deaths and no patients developed neutropenia. Three patients required transfusion and another had grade 3 diarrhea.
The researchers also performed molecular studies using targeted deep sequencing. They detected TET2 mutations in all 12 patients, with seven patients having two mutations. Four patients had DNMT3A mutations, five patients had RHOA mutations, and four patients had p.G17V substitution. One patient had an IDH2R172 mutation.
Since all patients had a TET2 mutation, the researchers were unable to assess its impact on treatment response. However, they saw no association between the number of TET2 mutations and treatment response, or mutations in DNMT3A, IDH2, and RHOA and treatment response.
The study was funded by a grant from the Leukemia & Lymphoma Society. Three of the coauthors received honoraria from Celgene.
SOURCE: Lemonnier F et al. Blood. 2018 Oct 2. doi: 10.1182/blood-2018-04-840538.
FROM BLOOD
Key clinical point:
Major finding: The overall response rate was 75% among the 12 patients, with 6 patients achieving complete response.
Study details: A retrospective case series of 12 patients with angioimmunoblastic T cell lymphoma.
Disclosures: The study was funded by a grant from the Leukemia & Lymphoma Society. Three of the coauthors received honoraria from Celgene.
Source: Lemonnier F et al. Blood. 2018 Oct 2. doi: 10.1182/blood-2018-04-840538.
Brentuximab vendotin plus CHP meets PFS endpoint in ECHELON-2
Takeda Pharmaceuticals and Seattle Genetics announced top-line results in the ECHELON-2 phase 3 trial of brentuximab vedotin plus CHP (cyclophosphamide, doxorubicin, prednisone) in the frontline treatment of CD-30 expressing peripheral T-cell lymphoma (PTCL).
The combination achieved statistically significant improvement in progression-free survival (PFS), compared with the control arm of standard chemotherapy alone using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The PFS was assessed by an Independent Review Facility (hazard ratio, 0.71; P = .0110).
The combination of brentuximab vedotin plus CHP also outperformed CHOP in overall survival, a secondary endpoint of the trial (hazard ratio, 0.66, P = .0244), according to the drug sponsors.
Full results of ECHELON-2 will be presented in December 2018 at the annual meeting of the American Society of Hematology, according to the announcement from Seattle Genetics and Takeda.
Takeda Pharmaceuticals and Seattle Genetics announced top-line results in the ECHELON-2 phase 3 trial of brentuximab vedotin plus CHP (cyclophosphamide, doxorubicin, prednisone) in the frontline treatment of CD-30 expressing peripheral T-cell lymphoma (PTCL).
The combination achieved statistically significant improvement in progression-free survival (PFS), compared with the control arm of standard chemotherapy alone using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The PFS was assessed by an Independent Review Facility (hazard ratio, 0.71; P = .0110).
The combination of brentuximab vedotin plus CHP also outperformed CHOP in overall survival, a secondary endpoint of the trial (hazard ratio, 0.66, P = .0244), according to the drug sponsors.
Full results of ECHELON-2 will be presented in December 2018 at the annual meeting of the American Society of Hematology, according to the announcement from Seattle Genetics and Takeda.
Takeda Pharmaceuticals and Seattle Genetics announced top-line results in the ECHELON-2 phase 3 trial of brentuximab vedotin plus CHP (cyclophosphamide, doxorubicin, prednisone) in the frontline treatment of CD-30 expressing peripheral T-cell lymphoma (PTCL).
The combination achieved statistically significant improvement in progression-free survival (PFS), compared with the control arm of standard chemotherapy alone using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The PFS was assessed by an Independent Review Facility (hazard ratio, 0.71; P = .0110).
The combination of brentuximab vedotin plus CHP also outperformed CHOP in overall survival, a secondary endpoint of the trial (hazard ratio, 0.66, P = .0244), according to the drug sponsors.
Full results of ECHELON-2 will be presented in December 2018 at the annual meeting of the American Society of Hematology, according to the announcement from Seattle Genetics and Takeda.
FDA lifts partial hold on tazemetostat trials
The U.S. Food and Drug Administration has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas, according to a press release from the drug’s developer Epizyme.
The patient had been on study for approximately 15 months and had achieved a confirmed partial response. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” Robert Bazemore, president and chief executive officer of Epizyme, said in a webcast on Sept. 24.
Epizyme assessed the risk of secondary malignancies, including T-LBL, as well as the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials. A panel of external scientific and medical experts who reviewed the findings concluded that T-LBL risks appear to be confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
“The team at Epizyme has worked diligently in collaboration with external experts and the FDA over the past several months,” Mr. Bazemore said.
The company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials. However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies. Mr. Bazemore said Epizyme hopes to submit a New Drug Application for tazemetostat in the treatment of epithelioid sarcoma.
Tazemetostat is under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid-tumor malignancies. The drug is also being studied as part of combination therapy for non–small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Epizyme is now working to resolve partial clinical holds placed on tazemetostat in France and Germany in order to resume trial enrollment in those countries.
The U.S. Food and Drug Administration has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas, according to a press release from the drug’s developer Epizyme.
The patient had been on study for approximately 15 months and had achieved a confirmed partial response. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” Robert Bazemore, president and chief executive officer of Epizyme, said in a webcast on Sept. 24.
Epizyme assessed the risk of secondary malignancies, including T-LBL, as well as the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials. A panel of external scientific and medical experts who reviewed the findings concluded that T-LBL risks appear to be confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
“The team at Epizyme has worked diligently in collaboration with external experts and the FDA over the past several months,” Mr. Bazemore said.
The company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials. However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies. Mr. Bazemore said Epizyme hopes to submit a New Drug Application for tazemetostat in the treatment of epithelioid sarcoma.
Tazemetostat is under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid-tumor malignancies. The drug is also being studied as part of combination therapy for non–small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Epizyme is now working to resolve partial clinical holds placed on tazemetostat in France and Germany in order to resume trial enrollment in those countries.
The U.S. Food and Drug Administration has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas, according to a press release from the drug’s developer Epizyme.
The patient had been on study for approximately 15 months and had achieved a confirmed partial response. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” Robert Bazemore, president and chief executive officer of Epizyme, said in a webcast on Sept. 24.
Epizyme assessed the risk of secondary malignancies, including T-LBL, as well as the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials. A panel of external scientific and medical experts who reviewed the findings concluded that T-LBL risks appear to be confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
“The team at Epizyme has worked diligently in collaboration with external experts and the FDA over the past several months,” Mr. Bazemore said.
The company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials. However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies. Mr. Bazemore said Epizyme hopes to submit a New Drug Application for tazemetostat in the treatment of epithelioid sarcoma.
Tazemetostat is under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid-tumor malignancies. The drug is also being studied as part of combination therapy for non–small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Epizyme is now working to resolve partial clinical holds placed on tazemetostat in France and Germany in order to resume trial enrollment in those countries.










