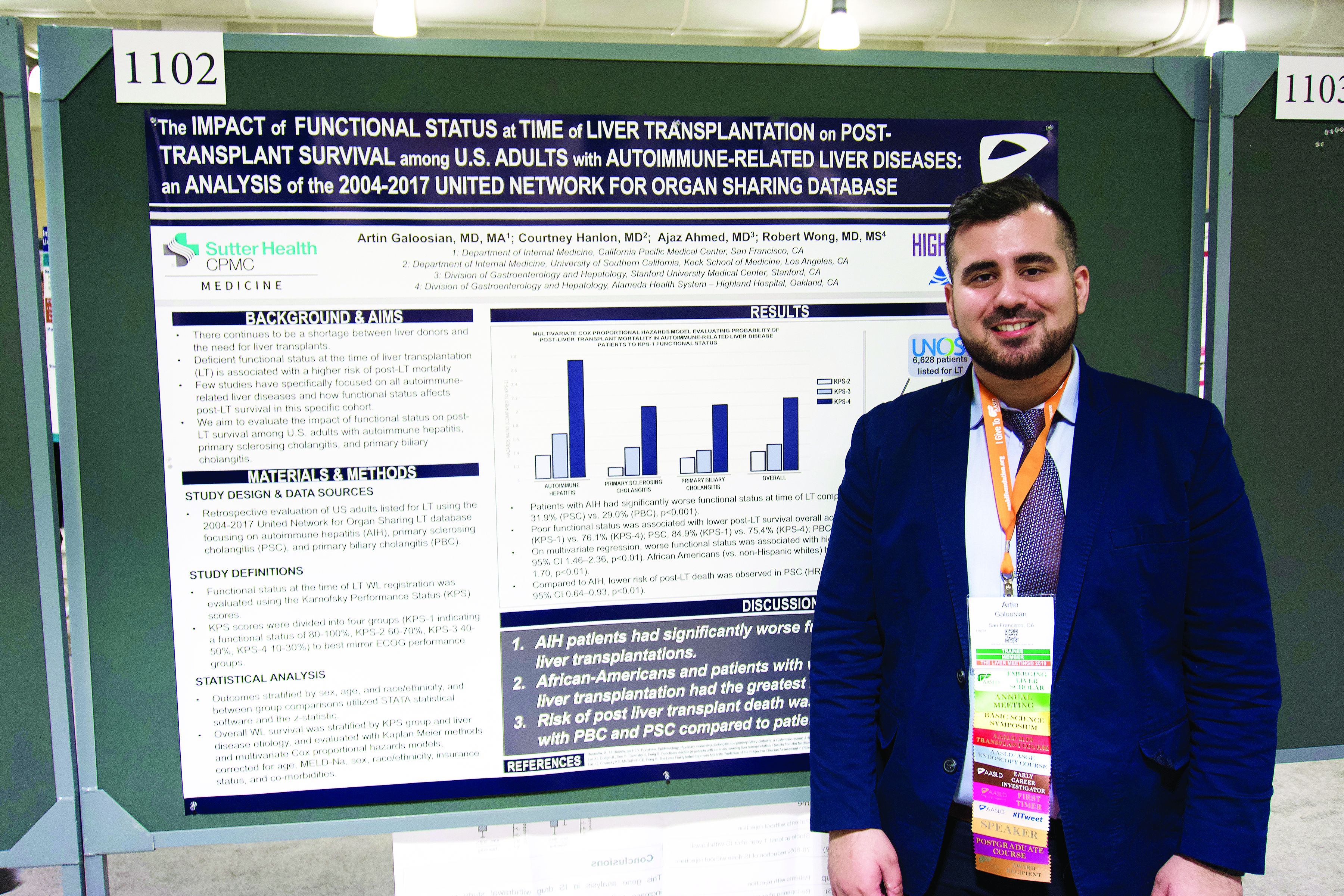
User login
HIV free 30 months after stem cell transplant, is the London patient cured?
A patient with HIV remission induced by stem cell transplantation continues to be disease free at the 30-month mark.
The individual, referred to as the London patient, received allogeneic hematopoietic stem cell transplantation (allo-HSCT) for stage IVB Hodgkin lymphoma. The transplant donor was homozygous for the CCR5 delta-32 mutation, which confers immunity to HIV because there’s no point of entry for the virus into immune cells.
After extensive sampling of various tissues, including gut, lymph node, blood, semen, and cerebrospinal fluid (CSF), Ravindra Kumar Gupta, MD, PhD, and colleagues found no detectable virus that was competent to replicate. However, they reported that the testing did detect some “fossilized” remnants of HIV DNA persisting in certain tissues.
The results were shared in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The London patient’s HIV status had been reported the previous year at CROI 2019, but only blood samples were used in that analysis.
In a commentary accompanying the simultaneously published study in the Lancet, Jennifer Zerbato, PhD, and Sharon Lewin, FRACP, PHD, FAAHMS, asked: “A key question now for the area of HIV cure is how soon can one know if someone has been cured of HIV?
“We will need more than a handful of patients cured of HIV to really understand the duration of follow-up needed and the likelihood of an unexpected late rebound in virus replication,” continued Dr. Zerbato, of the University of Melbourne, and Dr. Lewin, of the Royal Melbourne Hospital and Monash University, also in Melbourne.
In their ongoing analysis of data from the London patient, Dr. Gupta, a virologist at the University of Cambridge (England), and associates constructed a mathematical model that maps the probability for lifetime remission or cure of HIV against several factors, including the degree of chimerism achieved with the stem cell transplant.
In this model, when chimerism reaches 80% in total HIV target cells, the probability of remission for life is 98%; when donor chimerism reaches 90%, the probability of lifetime remission is greater than 99%. Peripheral T-cell chimerism in the London patient has held steady at 99%.
Dr. Gupta and associates obtained some testing opportunistically: A PET-CT scan revealed an axillary lymph node that was biopsied after it was found to have avid radiotracer uptake. Similarly, the CSF sample was obtained in the course of a work-up for some neurologic symptoms that the London patient was having.
In contrast to the first patient who achieved ongoing HIV remission from a pair of stem cell transplants received over 13 years ago – the Berlin patient – the London patient did not receive whole-body radiation, but rather underwent a reduced-intensity conditioning regimen. The London patient experienced a bout of gut graft-versus-host disease (GVHD) about 2 months after his transplant, but has been free of GVHD in the interval. He hasn’t taken cytotoxic agents or any GVHD prophylaxis since 6 months post transplant.
Though there’s no sign of HIV that’s competent to replicate, “the London patient has shown somewhat slow CD4 reconstitution,” said Dr. Gupta and coauthors in discussing the results.
The patient had a reactivation of Epstein-Barr virus (EBV) about 21 months after analytic treatment interruption (ATI) of antiretroviral therapy that was managed without any specific treatment, but he hasn’t experienced any opportunistic infections. However, his CD4 count didn’t rebound to pretransplant levels until 28 months after ATI. At that point, his CD4 count was 430 cells per mcL, or 23.5% of total T cells. The CD4:CD8 ratio was 0.86; normal range is 1.5-2.5.
The researchers used quantitative real-time polymerase chain reaction (rt-PCR) to look for packaging site and envelope (env) DNA fragments, and droplet digital PCR to quantify HIV-1 DNA.
The patient’s HIV-1 plasma load measured at 30 months post ATI on an ultrasensitive assay was below the lower limit of detection (less than 1 copy per mL). Semen viremia measured at 21 months was also below the lower limit of detection, as was CSF measured at 25 months.
Samples were taken from the patient’s rectum, cecum, sigmoid colon, and terminal ileum during a colonoscopy conducted 22 months post ATI; all tested negative for HIV DNA via droplet digital PCR.
The lymph node had large numbers of EBV-positive cells and was positive for HIV-1 env and long-terminal repeat by double-drop PCR, but no integrase DNA was detected. Additionally, no intact proviral DNA was found on assay.
Dr. Gupta and associates speculated that “EBV reactivation could have triggered EBV-specific CD4 and CD8 T-cell responses and proliferation, potentially including CD4 T cells containing HIV-1 DNA.” Supporting this hypothesis, EBV-specific CD8 T-cell responses in peripheral blood were “robust,” and the researchers also saw some CD4 response.
“Similar to the Berlin patient, highly sensitive tests showed very low levels of so-called fossilized HIV-1 DNA in some tissue samples from the London patient. Residual HIV-1 DNA and axillary lymph node tissue could represent a defective clone that expanded during hyperplasia within the lymph note sampled,” noted Dr. Gupta and coauthors.
Responses of CD4 and CD8 T cells to HIV have also remained below the limit of detection, though cytomegalovirus-specific responses persist in the London patient.
As with the Berlin patient, standard enzyme-linked immunosorbent assay (ELISA) testing has remained positive in the London patient. “Standard ELISA testing, therefore, cannot be used as a marker for cure, although more work needs to be done to assess the role of detuned low-avidity antibody assays in defining cure,” noted Dr. Gupta and associates.
The ongoing follow-up plan for the London patient is to obtain viral load testing twice yearly up to 5 years post ATI, and then obtain yearly tests for a total of 10 years. Ongoing testing will confirm the investigators’ belief that “these findings probably represent the second recorded HIV-1 cure after CCR5 delta-32/delta-32 allo-HSCT, with evidence of residual low-level HIV-1 DNA.”
Dr. Zerbato and Dr. Lewin advised cautious optimism and ongoing surveillance: “In view of the many cells sampled in this case, and the absence of any intact virus, is the London patient truly cured? The additional data provided in this follow-up case report is certainly exciting and encouraging but, in the end, only time will tell.”
Dr. Gupta reported being a consultant for ViiV Healthcare and Gilead Sciences; several coauthors also reported financial relationships with pharmaceutical companies. The work was funded by amfAR, the American Foundation for AIDS Research, and the Wellcome Trust. Dr. Lewin reported grants from the National Health and Medical Research Council of Australia, the National Institutes of Health, the American Foundation for AIDS Research, Gilead Sciences, Merck, ViiV Healthcare, Leidos, the Wellcome Trust, the Australian Centre for HIV and Hepatitis Virology Research, and the Melbourne HIV Cure Consortium. Dr. Zerbato reported grants from the Melbourne HIV Cure Consortium,
SOURCE: Gupta R et al. Lancet. 2020 Mar 10. doi: 10.1016/ S2352-3018(20)30069-2.
A patient with HIV remission induced by stem cell transplantation continues to be disease free at the 30-month mark.
The individual, referred to as the London patient, received allogeneic hematopoietic stem cell transplantation (allo-HSCT) for stage IVB Hodgkin lymphoma. The transplant donor was homozygous for the CCR5 delta-32 mutation, which confers immunity to HIV because there’s no point of entry for the virus into immune cells.
After extensive sampling of various tissues, including gut, lymph node, blood, semen, and cerebrospinal fluid (CSF), Ravindra Kumar Gupta, MD, PhD, and colleagues found no detectable virus that was competent to replicate. However, they reported that the testing did detect some “fossilized” remnants of HIV DNA persisting in certain tissues.
The results were shared in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The London patient’s HIV status had been reported the previous year at CROI 2019, but only blood samples were used in that analysis.
In a commentary accompanying the simultaneously published study in the Lancet, Jennifer Zerbato, PhD, and Sharon Lewin, FRACP, PHD, FAAHMS, asked: “A key question now for the area of HIV cure is how soon can one know if someone has been cured of HIV?
“We will need more than a handful of patients cured of HIV to really understand the duration of follow-up needed and the likelihood of an unexpected late rebound in virus replication,” continued Dr. Zerbato, of the University of Melbourne, and Dr. Lewin, of the Royal Melbourne Hospital and Monash University, also in Melbourne.
In their ongoing analysis of data from the London patient, Dr. Gupta, a virologist at the University of Cambridge (England), and associates constructed a mathematical model that maps the probability for lifetime remission or cure of HIV against several factors, including the degree of chimerism achieved with the stem cell transplant.
In this model, when chimerism reaches 80% in total HIV target cells, the probability of remission for life is 98%; when donor chimerism reaches 90%, the probability of lifetime remission is greater than 99%. Peripheral T-cell chimerism in the London patient has held steady at 99%.
Dr. Gupta and associates obtained some testing opportunistically: A PET-CT scan revealed an axillary lymph node that was biopsied after it was found to have avid radiotracer uptake. Similarly, the CSF sample was obtained in the course of a work-up for some neurologic symptoms that the London patient was having.
In contrast to the first patient who achieved ongoing HIV remission from a pair of stem cell transplants received over 13 years ago – the Berlin patient – the London patient did not receive whole-body radiation, but rather underwent a reduced-intensity conditioning regimen. The London patient experienced a bout of gut graft-versus-host disease (GVHD) about 2 months after his transplant, but has been free of GVHD in the interval. He hasn’t taken cytotoxic agents or any GVHD prophylaxis since 6 months post transplant.
Though there’s no sign of HIV that’s competent to replicate, “the London patient has shown somewhat slow CD4 reconstitution,” said Dr. Gupta and coauthors in discussing the results.
The patient had a reactivation of Epstein-Barr virus (EBV) about 21 months after analytic treatment interruption (ATI) of antiretroviral therapy that was managed without any specific treatment, but he hasn’t experienced any opportunistic infections. However, his CD4 count didn’t rebound to pretransplant levels until 28 months after ATI. At that point, his CD4 count was 430 cells per mcL, or 23.5% of total T cells. The CD4:CD8 ratio was 0.86; normal range is 1.5-2.5.
The researchers used quantitative real-time polymerase chain reaction (rt-PCR) to look for packaging site and envelope (env) DNA fragments, and droplet digital PCR to quantify HIV-1 DNA.
The patient’s HIV-1 plasma load measured at 30 months post ATI on an ultrasensitive assay was below the lower limit of detection (less than 1 copy per mL). Semen viremia measured at 21 months was also below the lower limit of detection, as was CSF measured at 25 months.
Samples were taken from the patient’s rectum, cecum, sigmoid colon, and terminal ileum during a colonoscopy conducted 22 months post ATI; all tested negative for HIV DNA via droplet digital PCR.
The lymph node had large numbers of EBV-positive cells and was positive for HIV-1 env and long-terminal repeat by double-drop PCR, but no integrase DNA was detected. Additionally, no intact proviral DNA was found on assay.
Dr. Gupta and associates speculated that “EBV reactivation could have triggered EBV-specific CD4 and CD8 T-cell responses and proliferation, potentially including CD4 T cells containing HIV-1 DNA.” Supporting this hypothesis, EBV-specific CD8 T-cell responses in peripheral blood were “robust,” and the researchers also saw some CD4 response.
“Similar to the Berlin patient, highly sensitive tests showed very low levels of so-called fossilized HIV-1 DNA in some tissue samples from the London patient. Residual HIV-1 DNA and axillary lymph node tissue could represent a defective clone that expanded during hyperplasia within the lymph note sampled,” noted Dr. Gupta and coauthors.
Responses of CD4 and CD8 T cells to HIV have also remained below the limit of detection, though cytomegalovirus-specific responses persist in the London patient.
As with the Berlin patient, standard enzyme-linked immunosorbent assay (ELISA) testing has remained positive in the London patient. “Standard ELISA testing, therefore, cannot be used as a marker for cure, although more work needs to be done to assess the role of detuned low-avidity antibody assays in defining cure,” noted Dr. Gupta and associates.
The ongoing follow-up plan for the London patient is to obtain viral load testing twice yearly up to 5 years post ATI, and then obtain yearly tests for a total of 10 years. Ongoing testing will confirm the investigators’ belief that “these findings probably represent the second recorded HIV-1 cure after CCR5 delta-32/delta-32 allo-HSCT, with evidence of residual low-level HIV-1 DNA.”
Dr. Zerbato and Dr. Lewin advised cautious optimism and ongoing surveillance: “In view of the many cells sampled in this case, and the absence of any intact virus, is the London patient truly cured? The additional data provided in this follow-up case report is certainly exciting and encouraging but, in the end, only time will tell.”
Dr. Gupta reported being a consultant for ViiV Healthcare and Gilead Sciences; several coauthors also reported financial relationships with pharmaceutical companies. The work was funded by amfAR, the American Foundation for AIDS Research, and the Wellcome Trust. Dr. Lewin reported grants from the National Health and Medical Research Council of Australia, the National Institutes of Health, the American Foundation for AIDS Research, Gilead Sciences, Merck, ViiV Healthcare, Leidos, the Wellcome Trust, the Australian Centre for HIV and Hepatitis Virology Research, and the Melbourne HIV Cure Consortium. Dr. Zerbato reported grants from the Melbourne HIV Cure Consortium,
SOURCE: Gupta R et al. Lancet. 2020 Mar 10. doi: 10.1016/ S2352-3018(20)30069-2.
A patient with HIV remission induced by stem cell transplantation continues to be disease free at the 30-month mark.
The individual, referred to as the London patient, received allogeneic hematopoietic stem cell transplantation (allo-HSCT) for stage IVB Hodgkin lymphoma. The transplant donor was homozygous for the CCR5 delta-32 mutation, which confers immunity to HIV because there’s no point of entry for the virus into immune cells.
After extensive sampling of various tissues, including gut, lymph node, blood, semen, and cerebrospinal fluid (CSF), Ravindra Kumar Gupta, MD, PhD, and colleagues found no detectable virus that was competent to replicate. However, they reported that the testing did detect some “fossilized” remnants of HIV DNA persisting in certain tissues.
The results were shared in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The London patient’s HIV status had been reported the previous year at CROI 2019, but only blood samples were used in that analysis.
In a commentary accompanying the simultaneously published study in the Lancet, Jennifer Zerbato, PhD, and Sharon Lewin, FRACP, PHD, FAAHMS, asked: “A key question now for the area of HIV cure is how soon can one know if someone has been cured of HIV?
“We will need more than a handful of patients cured of HIV to really understand the duration of follow-up needed and the likelihood of an unexpected late rebound in virus replication,” continued Dr. Zerbato, of the University of Melbourne, and Dr. Lewin, of the Royal Melbourne Hospital and Monash University, also in Melbourne.
In their ongoing analysis of data from the London patient, Dr. Gupta, a virologist at the University of Cambridge (England), and associates constructed a mathematical model that maps the probability for lifetime remission or cure of HIV against several factors, including the degree of chimerism achieved with the stem cell transplant.
In this model, when chimerism reaches 80% in total HIV target cells, the probability of remission for life is 98%; when donor chimerism reaches 90%, the probability of lifetime remission is greater than 99%. Peripheral T-cell chimerism in the London patient has held steady at 99%.
Dr. Gupta and associates obtained some testing opportunistically: A PET-CT scan revealed an axillary lymph node that was biopsied after it was found to have avid radiotracer uptake. Similarly, the CSF sample was obtained in the course of a work-up for some neurologic symptoms that the London patient was having.
In contrast to the first patient who achieved ongoing HIV remission from a pair of stem cell transplants received over 13 years ago – the Berlin patient – the London patient did not receive whole-body radiation, but rather underwent a reduced-intensity conditioning regimen. The London patient experienced a bout of gut graft-versus-host disease (GVHD) about 2 months after his transplant, but has been free of GVHD in the interval. He hasn’t taken cytotoxic agents or any GVHD prophylaxis since 6 months post transplant.
Though there’s no sign of HIV that’s competent to replicate, “the London patient has shown somewhat slow CD4 reconstitution,” said Dr. Gupta and coauthors in discussing the results.
The patient had a reactivation of Epstein-Barr virus (EBV) about 21 months after analytic treatment interruption (ATI) of antiretroviral therapy that was managed without any specific treatment, but he hasn’t experienced any opportunistic infections. However, his CD4 count didn’t rebound to pretransplant levels until 28 months after ATI. At that point, his CD4 count was 430 cells per mcL, or 23.5% of total T cells. The CD4:CD8 ratio was 0.86; normal range is 1.5-2.5.
The researchers used quantitative real-time polymerase chain reaction (rt-PCR) to look for packaging site and envelope (env) DNA fragments, and droplet digital PCR to quantify HIV-1 DNA.
The patient’s HIV-1 plasma load measured at 30 months post ATI on an ultrasensitive assay was below the lower limit of detection (less than 1 copy per mL). Semen viremia measured at 21 months was also below the lower limit of detection, as was CSF measured at 25 months.
Samples were taken from the patient’s rectum, cecum, sigmoid colon, and terminal ileum during a colonoscopy conducted 22 months post ATI; all tested negative for HIV DNA via droplet digital PCR.
The lymph node had large numbers of EBV-positive cells and was positive for HIV-1 env and long-terminal repeat by double-drop PCR, but no integrase DNA was detected. Additionally, no intact proviral DNA was found on assay.
Dr. Gupta and associates speculated that “EBV reactivation could have triggered EBV-specific CD4 and CD8 T-cell responses and proliferation, potentially including CD4 T cells containing HIV-1 DNA.” Supporting this hypothesis, EBV-specific CD8 T-cell responses in peripheral blood were “robust,” and the researchers also saw some CD4 response.
“Similar to the Berlin patient, highly sensitive tests showed very low levels of so-called fossilized HIV-1 DNA in some tissue samples from the London patient. Residual HIV-1 DNA and axillary lymph node tissue could represent a defective clone that expanded during hyperplasia within the lymph note sampled,” noted Dr. Gupta and coauthors.
Responses of CD4 and CD8 T cells to HIV have also remained below the limit of detection, though cytomegalovirus-specific responses persist in the London patient.
As with the Berlin patient, standard enzyme-linked immunosorbent assay (ELISA) testing has remained positive in the London patient. “Standard ELISA testing, therefore, cannot be used as a marker for cure, although more work needs to be done to assess the role of detuned low-avidity antibody assays in defining cure,” noted Dr. Gupta and associates.
The ongoing follow-up plan for the London patient is to obtain viral load testing twice yearly up to 5 years post ATI, and then obtain yearly tests for a total of 10 years. Ongoing testing will confirm the investigators’ belief that “these findings probably represent the second recorded HIV-1 cure after CCR5 delta-32/delta-32 allo-HSCT, with evidence of residual low-level HIV-1 DNA.”
Dr. Zerbato and Dr. Lewin advised cautious optimism and ongoing surveillance: “In view of the many cells sampled in this case, and the absence of any intact virus, is the London patient truly cured? The additional data provided in this follow-up case report is certainly exciting and encouraging but, in the end, only time will tell.”
Dr. Gupta reported being a consultant for ViiV Healthcare and Gilead Sciences; several coauthors also reported financial relationships with pharmaceutical companies. The work was funded by amfAR, the American Foundation for AIDS Research, and the Wellcome Trust. Dr. Lewin reported grants from the National Health and Medical Research Council of Australia, the National Institutes of Health, the American Foundation for AIDS Research, Gilead Sciences, Merck, ViiV Healthcare, Leidos, the Wellcome Trust, the Australian Centre for HIV and Hepatitis Virology Research, and the Melbourne HIV Cure Consortium. Dr. Zerbato reported grants from the Melbourne HIV Cure Consortium,
SOURCE: Gupta R et al. Lancet. 2020 Mar 10. doi: 10.1016/ S2352-3018(20)30069-2.
FROM CROI 2020
Stored CD34 cells for multiple myeloma patients largely unused
ORLANDO – Collecting and storing extra stem cells on the off chance that a patient with multiple myeloma will need a salvage autologous stem cell transplant may not be worth the money or effort, investigators say.
Among patients with multiple myeloma who had adequate collection of mobilized and stored cells, only 3 of 146 eligible patients were given the stored cells in a second autologous stem cell transplant (ASCT), reported Nausheen Ahmed, MD, from the Case Western Reserve Cancer Center and University Hospitals Seidman Cancer Center, both in Cleveland.
“We found overall low utilization of salvage transplants and storage stem cells at our institution, which may not justify the strategy of early collection for all patients fit for transplant,” she said at the Transplantation and Cellular Therapy Meetings.
But Sergio Giralt, MD, a transplant specialist from Memorial Sloan Kettering Cancer Center, New York, who was not involved in the study, warned against changing practice “for the wrong reason, because it’s just a financial reason.”
Get them while they’re fresh
The rationale for collecting and storing extra cells is the risk that mobilization will fail in the future following prolonged maintenance with immunomodulatory agents such as lenalidomide (Revlimid), and the risk for genetic or epigenetic damage to cells from high-dose melphalan used in transplant-conditioning regimens, Dr. Ahmed noted at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“However, there are potential issues with early mobilization and storage, including cost, resources, apheresis scheduling, uncertainty of cell viability, and liability. There’s also risk of side effects with filgrastim and plerixafor use [for mobilization],” she said.
Dr. Ahmed and colleagues conducted a study to determine how stored stem cells for second ASCT were used, describe how second ASCTs are used in patients who meet the Mayo Consensus Stratification for Myeloma & Risk-Adapted Therapy (mSMART) criteria, and the costs of mobilizing and storing stem cells for a second ASCT.
They took a retrospective look at all adults aged 18 years and older with a diagnosis of multiple myeloma who received a first ASCT at their institution from 2009 to 2017. They excluded patients who had amyloidosis without myeloma or POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma proliferative disorder, skin changes) syndrome.
Patients were considered eligible for a second ASCT based on mSMART recommendations if they had a relapse either 18 or more months without maintenance therapy or after at least 36 months on maintenance. The investigators defined an extra day of collection as an additional day of apheresis to obtain 2 million or more CD34 cells/kg for storage only.
They estimated costs from the institution’s charge master as the sum of cell processing, leukapheresis costs, additional plerixafor costs, and storage costs, and calculated the total duration of storage as months from the date of collection until the last follow-up.
The median age of the total study population of 179 patients was 61 years, with a majority of male and white patients. Of this group, 98% had an Eastern Cooperative Oncology Group performance score of 0-1. In all, 63.7% of the patients had standard-risk cytogenetics, 22.4% had high-risk disease, and the remainder had unknown cytogenetic risk.
At a median follow-up of 56.5 months, 95 patients (53.1%) had experienced a relapse after transplant with a median time to progression of 47.5 months. The majority of patients (166; 92.7%) had received a single transplant, 10 (5.6%) had received tandem transplants, and only 3 (1.6%) had a second transplant at relapse.
Looking at the use of second transplant in patients who met the criteria for salvage transplant based on mSMART (excluding patients who had undergone tandem transplant) and whose maintenance status was known, they identified 61 patients on maintenance therapy and 24 with no maintenance. A total of 31 patients (18 in the maintenance group and 13 in the no-maintenance group) met mSMART criteria for salvage ASCT.
Dr. Ahmed and colleagues next looked at the 146 patients who had at least 2 million stored cells/kg, and found that the stored cells were used for only three patients. Of the 146 patients, 66 had 1 extra collection day, 17 had 2 extra days, and 4 had 3 extra days, for an average additional cost per patient of $16,859.
‘Woefully underutilized’
Discussing the study, Dr. Giralt asked: “How valid are the SMART criteria of 36 months? And the answer is there is no data to support it, and if we actually go back to our oncology, any patient who has had more than 18 months without exposure to a drug can continue to have sensitivity to that drug, and that’s why if we used the ASBMT criteria of greater than 18 months you’d have a larger population” of patients eligible for salvage transplant.
He stated that, “we know these patients exist, we know they have cells in the freezer, but we’re not using those cells. Second transplant is woefully underutilized in myeloma patients,” and he added that stored cells could also be used to support those patients who develop cytopenias following chimeric antigen receptor (CAR) T-cell therapy.
Yago Nieto, MD, from the University of Texas MD Anderson Cancer Center, Houston, who comoderated the session where the data were presented, agreed with Dr. Giralt that stored stem cells are underutilized in the treatment of patients with multiple myeloma.
“I don’t think that the experience from Case Western, where the percentage of patients who are eligible for salvage transplant and actually got it was less than 10%, can be extrapolated to many other centers. I think that in most centers the actual percentage is higher than that,” he said in an interview.
“There are going to be therapies like CAR T that will compete with salvage transplants, but I think more patients should be considered for this salvage procedure,” he added.
No funding source for the story was disclosed. Dr. Ahmed reported no financial disclosures. Dr. Giralt reported consulting/advisory activities and receiving research funding from multiple companies. Dr. Nieto disclosed research funding from, and consultancy for, several companies.
SOURCE: Ahmed N et al. TCT 2020, Abstract 28.
ORLANDO – Collecting and storing extra stem cells on the off chance that a patient with multiple myeloma will need a salvage autologous stem cell transplant may not be worth the money or effort, investigators say.
Among patients with multiple myeloma who had adequate collection of mobilized and stored cells, only 3 of 146 eligible patients were given the stored cells in a second autologous stem cell transplant (ASCT), reported Nausheen Ahmed, MD, from the Case Western Reserve Cancer Center and University Hospitals Seidman Cancer Center, both in Cleveland.
“We found overall low utilization of salvage transplants and storage stem cells at our institution, which may not justify the strategy of early collection for all patients fit for transplant,” she said at the Transplantation and Cellular Therapy Meetings.
But Sergio Giralt, MD, a transplant specialist from Memorial Sloan Kettering Cancer Center, New York, who was not involved in the study, warned against changing practice “for the wrong reason, because it’s just a financial reason.”
Get them while they’re fresh
The rationale for collecting and storing extra cells is the risk that mobilization will fail in the future following prolonged maintenance with immunomodulatory agents such as lenalidomide (Revlimid), and the risk for genetic or epigenetic damage to cells from high-dose melphalan used in transplant-conditioning regimens, Dr. Ahmed noted at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“However, there are potential issues with early mobilization and storage, including cost, resources, apheresis scheduling, uncertainty of cell viability, and liability. There’s also risk of side effects with filgrastim and plerixafor use [for mobilization],” she said.
Dr. Ahmed and colleagues conducted a study to determine how stored stem cells for second ASCT were used, describe how second ASCTs are used in patients who meet the Mayo Consensus Stratification for Myeloma & Risk-Adapted Therapy (mSMART) criteria, and the costs of mobilizing and storing stem cells for a second ASCT.
They took a retrospective look at all adults aged 18 years and older with a diagnosis of multiple myeloma who received a first ASCT at their institution from 2009 to 2017. They excluded patients who had amyloidosis without myeloma or POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma proliferative disorder, skin changes) syndrome.
Patients were considered eligible for a second ASCT based on mSMART recommendations if they had a relapse either 18 or more months without maintenance therapy or after at least 36 months on maintenance. The investigators defined an extra day of collection as an additional day of apheresis to obtain 2 million or more CD34 cells/kg for storage only.
They estimated costs from the institution’s charge master as the sum of cell processing, leukapheresis costs, additional plerixafor costs, and storage costs, and calculated the total duration of storage as months from the date of collection until the last follow-up.
The median age of the total study population of 179 patients was 61 years, with a majority of male and white patients. Of this group, 98% had an Eastern Cooperative Oncology Group performance score of 0-1. In all, 63.7% of the patients had standard-risk cytogenetics, 22.4% had high-risk disease, and the remainder had unknown cytogenetic risk.
At a median follow-up of 56.5 months, 95 patients (53.1%) had experienced a relapse after transplant with a median time to progression of 47.5 months. The majority of patients (166; 92.7%) had received a single transplant, 10 (5.6%) had received tandem transplants, and only 3 (1.6%) had a second transplant at relapse.
Looking at the use of second transplant in patients who met the criteria for salvage transplant based on mSMART (excluding patients who had undergone tandem transplant) and whose maintenance status was known, they identified 61 patients on maintenance therapy and 24 with no maintenance. A total of 31 patients (18 in the maintenance group and 13 in the no-maintenance group) met mSMART criteria for salvage ASCT.
Dr. Ahmed and colleagues next looked at the 146 patients who had at least 2 million stored cells/kg, and found that the stored cells were used for only three patients. Of the 146 patients, 66 had 1 extra collection day, 17 had 2 extra days, and 4 had 3 extra days, for an average additional cost per patient of $16,859.
‘Woefully underutilized’
Discussing the study, Dr. Giralt asked: “How valid are the SMART criteria of 36 months? And the answer is there is no data to support it, and if we actually go back to our oncology, any patient who has had more than 18 months without exposure to a drug can continue to have sensitivity to that drug, and that’s why if we used the ASBMT criteria of greater than 18 months you’d have a larger population” of patients eligible for salvage transplant.
He stated that, “we know these patients exist, we know they have cells in the freezer, but we’re not using those cells. Second transplant is woefully underutilized in myeloma patients,” and he added that stored cells could also be used to support those patients who develop cytopenias following chimeric antigen receptor (CAR) T-cell therapy.
Yago Nieto, MD, from the University of Texas MD Anderson Cancer Center, Houston, who comoderated the session where the data were presented, agreed with Dr. Giralt that stored stem cells are underutilized in the treatment of patients with multiple myeloma.
“I don’t think that the experience from Case Western, where the percentage of patients who are eligible for salvage transplant and actually got it was less than 10%, can be extrapolated to many other centers. I think that in most centers the actual percentage is higher than that,” he said in an interview.
“There are going to be therapies like CAR T that will compete with salvage transplants, but I think more patients should be considered for this salvage procedure,” he added.
No funding source for the story was disclosed. Dr. Ahmed reported no financial disclosures. Dr. Giralt reported consulting/advisory activities and receiving research funding from multiple companies. Dr. Nieto disclosed research funding from, and consultancy for, several companies.
SOURCE: Ahmed N et al. TCT 2020, Abstract 28.
ORLANDO – Collecting and storing extra stem cells on the off chance that a patient with multiple myeloma will need a salvage autologous stem cell transplant may not be worth the money or effort, investigators say.
Among patients with multiple myeloma who had adequate collection of mobilized and stored cells, only 3 of 146 eligible patients were given the stored cells in a second autologous stem cell transplant (ASCT), reported Nausheen Ahmed, MD, from the Case Western Reserve Cancer Center and University Hospitals Seidman Cancer Center, both in Cleveland.
“We found overall low utilization of salvage transplants and storage stem cells at our institution, which may not justify the strategy of early collection for all patients fit for transplant,” she said at the Transplantation and Cellular Therapy Meetings.
But Sergio Giralt, MD, a transplant specialist from Memorial Sloan Kettering Cancer Center, New York, who was not involved in the study, warned against changing practice “for the wrong reason, because it’s just a financial reason.”
Get them while they’re fresh
The rationale for collecting and storing extra cells is the risk that mobilization will fail in the future following prolonged maintenance with immunomodulatory agents such as lenalidomide (Revlimid), and the risk for genetic or epigenetic damage to cells from high-dose melphalan used in transplant-conditioning regimens, Dr. Ahmed noted at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“However, there are potential issues with early mobilization and storage, including cost, resources, apheresis scheduling, uncertainty of cell viability, and liability. There’s also risk of side effects with filgrastim and plerixafor use [for mobilization],” she said.
Dr. Ahmed and colleagues conducted a study to determine how stored stem cells for second ASCT were used, describe how second ASCTs are used in patients who meet the Mayo Consensus Stratification for Myeloma & Risk-Adapted Therapy (mSMART) criteria, and the costs of mobilizing and storing stem cells for a second ASCT.
They took a retrospective look at all adults aged 18 years and older with a diagnosis of multiple myeloma who received a first ASCT at their institution from 2009 to 2017. They excluded patients who had amyloidosis without myeloma or POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma proliferative disorder, skin changes) syndrome.
Patients were considered eligible for a second ASCT based on mSMART recommendations if they had a relapse either 18 or more months without maintenance therapy or after at least 36 months on maintenance. The investigators defined an extra day of collection as an additional day of apheresis to obtain 2 million or more CD34 cells/kg for storage only.
They estimated costs from the institution’s charge master as the sum of cell processing, leukapheresis costs, additional plerixafor costs, and storage costs, and calculated the total duration of storage as months from the date of collection until the last follow-up.
The median age of the total study population of 179 patients was 61 years, with a majority of male and white patients. Of this group, 98% had an Eastern Cooperative Oncology Group performance score of 0-1. In all, 63.7% of the patients had standard-risk cytogenetics, 22.4% had high-risk disease, and the remainder had unknown cytogenetic risk.
At a median follow-up of 56.5 months, 95 patients (53.1%) had experienced a relapse after transplant with a median time to progression of 47.5 months. The majority of patients (166; 92.7%) had received a single transplant, 10 (5.6%) had received tandem transplants, and only 3 (1.6%) had a second transplant at relapse.
Looking at the use of second transplant in patients who met the criteria for salvage transplant based on mSMART (excluding patients who had undergone tandem transplant) and whose maintenance status was known, they identified 61 patients on maintenance therapy and 24 with no maintenance. A total of 31 patients (18 in the maintenance group and 13 in the no-maintenance group) met mSMART criteria for salvage ASCT.
Dr. Ahmed and colleagues next looked at the 146 patients who had at least 2 million stored cells/kg, and found that the stored cells were used for only three patients. Of the 146 patients, 66 had 1 extra collection day, 17 had 2 extra days, and 4 had 3 extra days, for an average additional cost per patient of $16,859.
‘Woefully underutilized’
Discussing the study, Dr. Giralt asked: “How valid are the SMART criteria of 36 months? And the answer is there is no data to support it, and if we actually go back to our oncology, any patient who has had more than 18 months without exposure to a drug can continue to have sensitivity to that drug, and that’s why if we used the ASBMT criteria of greater than 18 months you’d have a larger population” of patients eligible for salvage transplant.
He stated that, “we know these patients exist, we know they have cells in the freezer, but we’re not using those cells. Second transplant is woefully underutilized in myeloma patients,” and he added that stored cells could also be used to support those patients who develop cytopenias following chimeric antigen receptor (CAR) T-cell therapy.
Yago Nieto, MD, from the University of Texas MD Anderson Cancer Center, Houston, who comoderated the session where the data were presented, agreed with Dr. Giralt that stored stem cells are underutilized in the treatment of patients with multiple myeloma.
“I don’t think that the experience from Case Western, where the percentage of patients who are eligible for salvage transplant and actually got it was less than 10%, can be extrapolated to many other centers. I think that in most centers the actual percentage is higher than that,” he said in an interview.
“There are going to be therapies like CAR T that will compete with salvage transplants, but I think more patients should be considered for this salvage procedure,” he added.
No funding source for the story was disclosed. Dr. Ahmed reported no financial disclosures. Dr. Giralt reported consulting/advisory activities and receiving research funding from multiple companies. Dr. Nieto disclosed research funding from, and consultancy for, several companies.
SOURCE: Ahmed N et al. TCT 2020, Abstract 28.
REPORTING FROM TCT 2020
No reduction in oral mucositis with folinic acid post transplant
ORLANDO – Folinic acid does not prevent oral mucositis in patients who are receiving a calcineurin inhibitor and methotrexate for graft-vs.-host disease prophylaxis, results of a multicenter study from Israel suggest.
A randomized clinical trial to determine whether folinic acid rescue 24 hours after a methotrexate dose protects patients against severe oral mucositis was halted for futility after an interim analysis showed no advantage to adding folinic acid, reported Moshe Yeshrun, MD, of Rabin Medical Center at Tel Aviv University, Israel.
“Regarding the primary and secondary endpoints, we observed identical rates and duration of severe oral mucositis, as well as identical rates of oral mucositis of any grade in the folinic acid and placebo groups,” he said at the annual Transplantation and Cellular Therapy Meetings.
There were also no significant differences between the folinic acid and placebo control groups in time to neutrophil and platelet engraftment, rates of febrile neutropenia and bloodstream infections, veno-occlusive disease, need for opiates or total parenteral nutrition (TPN), or time from transplant to discharge, Dr. Yeshrun added at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Folinic acid therapy did not, however, appear to abrogate or interfere with the effects of methotrexate on prevention of either acute or chronic graft-vs-host disease (GVHD).
Severe adverse event
Oral mucositis can be a serious complication of therapy, associated with increased morbidity and mortality; significant pain; difficulty with eating or speaking; difficulty swallowing water, food, and medications; prolonged hospitalizations; and increased costs of care, Dr. Yeshrun noted.
The presence of oral mucositis sometimes leads clinicians to reduce or even skip methotrexate doses, thereby increasing risk for GVHD.
There are limited data from nonrandomized studies indicating that folinic acid (also called leucovorin) may reduce methotrexate-associated toxicities, and both the European Society for Blood and Marrow Transplantation and European LeukemiaNet working group recommend the use of folinic-acid rescue 24 hours following each methotrexate dose, Dr. Yeshrun said.
To see whether folinic acid rescue actually reduces the rate of methotrexate-induced toxicity and affects outcomes for patients who receive methotrexate post transplant for GVHD prophylaxis, Dr. Yeshrun and colleagues in three Israeli medical centers conducted a randomized, placebo-controlled trial.
The eligible study population included patients 18 and older with hematological malignancies in complete remission or minimal residual disease who underwent myeloablative conditioning and allogeneic transplant from HLA-matched or 1-antigen mismatched siblings or unrelated donors.
The patients were stratified by treatment center and intensity of the conditioning regimen, and then randomized on a 1:1 basis to receive folinic acid or placebo beginning 24 hours after each methotrexate dose, with the assigned medication given at a dose of 15 mg three times daily on the first day, and once daily on days 3 and 6.
Patients who received a transplant from an unrelated donor were also given anti-thymocyte globulin at a total dose of 15 mg/kg.
Supportive care included filgrastim (Neupogen) 5 mcg/kg from day 7 until neutrophil engraftment, infection prophylaxis, and ursodeoxycholic acid for prevention of veno-occlusive disease.
Trial stopped
Although the study was designed to enroll 116 patients to have a power of 80% to detect a 50% reduction in the rate of severe oral mucositis from an anticipated 50% in the placebo arm, a planned interim analysis conducted after approximately half of the target events occurred showed no difference in the rates of severe oral mucositis, and the trial was halted.
A total of 28 patients in the folinic acid group and 24 in the placebo group were available for the analysis.
The rate of grade 3 or 4 mucositis, the primary endpoint, was 46.6% in the folinic acid group, and 45.8% in the placebo group.
Respectively, the median duration of severe oral mucositis was 4 days in each group, days to neutrophil engraftment were a median of 12 and to platelet engraftment were a median of 13 days in each group, rates of febrile neutropenia were 57.1% and 58.3%, rates of bloodstream infections were 10.7% and 16.6%, rates of veno-occlusive disease were 7% and 12.5%, need for TPN occurred in 14.2% vs. 25%, need for opiates occurred in 78.5% vs. 66.6%, and median time to discharge was 18 and 19 days. As noted, none of the differences were statistically significant.
“These unequivocal interim results led to our decision to discontinue the study,” Dr. Yeshrun said.
Arnon Nagler, MD, MSc, from Sheba Medical Center Tel HaShomer at Tel Aviv University in Israel, who was not involved in the study, said in an interview “I think this is a very practical and important study, because we need evidence-based data such as this.”
Dr. Nagler, who comoderated the session where the data were presented, noted that the study was limited by the small number of patients and the lack of a subgroup analysis, but emphasized that the findings were important nonetheless.
His comoderator, Maria Gilleece, MD, director of the Yorkshire (England) Blood and Marrow Transplant Program, noted in an interview that folinic acid is frequently used in the United Kingdom for patients receiving high-dose methotrexate, ”and certainly, this study suggests that may be inappropriate.”
Rabin Medical Center sponsored the trial. Dr. Yeshrun, Dr. Nagler, and Dr. Gilleece reported no relevant conflicts of interest.
SOURCE: Yeshrun M. et al. TCT 2020. Abstract 61.
ORLANDO – Folinic acid does not prevent oral mucositis in patients who are receiving a calcineurin inhibitor and methotrexate for graft-vs.-host disease prophylaxis, results of a multicenter study from Israel suggest.
A randomized clinical trial to determine whether folinic acid rescue 24 hours after a methotrexate dose protects patients against severe oral mucositis was halted for futility after an interim analysis showed no advantage to adding folinic acid, reported Moshe Yeshrun, MD, of Rabin Medical Center at Tel Aviv University, Israel.
“Regarding the primary and secondary endpoints, we observed identical rates and duration of severe oral mucositis, as well as identical rates of oral mucositis of any grade in the folinic acid and placebo groups,” he said at the annual Transplantation and Cellular Therapy Meetings.
There were also no significant differences between the folinic acid and placebo control groups in time to neutrophil and platelet engraftment, rates of febrile neutropenia and bloodstream infections, veno-occlusive disease, need for opiates or total parenteral nutrition (TPN), or time from transplant to discharge, Dr. Yeshrun added at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Folinic acid therapy did not, however, appear to abrogate or interfere with the effects of methotrexate on prevention of either acute or chronic graft-vs-host disease (GVHD).
Severe adverse event
Oral mucositis can be a serious complication of therapy, associated with increased morbidity and mortality; significant pain; difficulty with eating or speaking; difficulty swallowing water, food, and medications; prolonged hospitalizations; and increased costs of care, Dr. Yeshrun noted.
The presence of oral mucositis sometimes leads clinicians to reduce or even skip methotrexate doses, thereby increasing risk for GVHD.
There are limited data from nonrandomized studies indicating that folinic acid (also called leucovorin) may reduce methotrexate-associated toxicities, and both the European Society for Blood and Marrow Transplantation and European LeukemiaNet working group recommend the use of folinic-acid rescue 24 hours following each methotrexate dose, Dr. Yeshrun said.
To see whether folinic acid rescue actually reduces the rate of methotrexate-induced toxicity and affects outcomes for patients who receive methotrexate post transplant for GVHD prophylaxis, Dr. Yeshrun and colleagues in three Israeli medical centers conducted a randomized, placebo-controlled trial.
The eligible study population included patients 18 and older with hematological malignancies in complete remission or minimal residual disease who underwent myeloablative conditioning and allogeneic transplant from HLA-matched or 1-antigen mismatched siblings or unrelated donors.
The patients were stratified by treatment center and intensity of the conditioning regimen, and then randomized on a 1:1 basis to receive folinic acid or placebo beginning 24 hours after each methotrexate dose, with the assigned medication given at a dose of 15 mg three times daily on the first day, and once daily on days 3 and 6.
Patients who received a transplant from an unrelated donor were also given anti-thymocyte globulin at a total dose of 15 mg/kg.
Supportive care included filgrastim (Neupogen) 5 mcg/kg from day 7 until neutrophil engraftment, infection prophylaxis, and ursodeoxycholic acid for prevention of veno-occlusive disease.
Trial stopped
Although the study was designed to enroll 116 patients to have a power of 80% to detect a 50% reduction in the rate of severe oral mucositis from an anticipated 50% in the placebo arm, a planned interim analysis conducted after approximately half of the target events occurred showed no difference in the rates of severe oral mucositis, and the trial was halted.
A total of 28 patients in the folinic acid group and 24 in the placebo group were available for the analysis.
The rate of grade 3 or 4 mucositis, the primary endpoint, was 46.6% in the folinic acid group, and 45.8% in the placebo group.
Respectively, the median duration of severe oral mucositis was 4 days in each group, days to neutrophil engraftment were a median of 12 and to platelet engraftment were a median of 13 days in each group, rates of febrile neutropenia were 57.1% and 58.3%, rates of bloodstream infections were 10.7% and 16.6%, rates of veno-occlusive disease were 7% and 12.5%, need for TPN occurred in 14.2% vs. 25%, need for opiates occurred in 78.5% vs. 66.6%, and median time to discharge was 18 and 19 days. As noted, none of the differences were statistically significant.
“These unequivocal interim results led to our decision to discontinue the study,” Dr. Yeshrun said.
Arnon Nagler, MD, MSc, from Sheba Medical Center Tel HaShomer at Tel Aviv University in Israel, who was not involved in the study, said in an interview “I think this is a very practical and important study, because we need evidence-based data such as this.”
Dr. Nagler, who comoderated the session where the data were presented, noted that the study was limited by the small number of patients and the lack of a subgroup analysis, but emphasized that the findings were important nonetheless.
His comoderator, Maria Gilleece, MD, director of the Yorkshire (England) Blood and Marrow Transplant Program, noted in an interview that folinic acid is frequently used in the United Kingdom for patients receiving high-dose methotrexate, ”and certainly, this study suggests that may be inappropriate.”
Rabin Medical Center sponsored the trial. Dr. Yeshrun, Dr. Nagler, and Dr. Gilleece reported no relevant conflicts of interest.
SOURCE: Yeshrun M. et al. TCT 2020. Abstract 61.
ORLANDO – Folinic acid does not prevent oral mucositis in patients who are receiving a calcineurin inhibitor and methotrexate for graft-vs.-host disease prophylaxis, results of a multicenter study from Israel suggest.
A randomized clinical trial to determine whether folinic acid rescue 24 hours after a methotrexate dose protects patients against severe oral mucositis was halted for futility after an interim analysis showed no advantage to adding folinic acid, reported Moshe Yeshrun, MD, of Rabin Medical Center at Tel Aviv University, Israel.
“Regarding the primary and secondary endpoints, we observed identical rates and duration of severe oral mucositis, as well as identical rates of oral mucositis of any grade in the folinic acid and placebo groups,” he said at the annual Transplantation and Cellular Therapy Meetings.
There were also no significant differences between the folinic acid and placebo control groups in time to neutrophil and platelet engraftment, rates of febrile neutropenia and bloodstream infections, veno-occlusive disease, need for opiates or total parenteral nutrition (TPN), or time from transplant to discharge, Dr. Yeshrun added at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Folinic acid therapy did not, however, appear to abrogate or interfere with the effects of methotrexate on prevention of either acute or chronic graft-vs-host disease (GVHD).
Severe adverse event
Oral mucositis can be a serious complication of therapy, associated with increased morbidity and mortality; significant pain; difficulty with eating or speaking; difficulty swallowing water, food, and medications; prolonged hospitalizations; and increased costs of care, Dr. Yeshrun noted.
The presence of oral mucositis sometimes leads clinicians to reduce or even skip methotrexate doses, thereby increasing risk for GVHD.
There are limited data from nonrandomized studies indicating that folinic acid (also called leucovorin) may reduce methotrexate-associated toxicities, and both the European Society for Blood and Marrow Transplantation and European LeukemiaNet working group recommend the use of folinic-acid rescue 24 hours following each methotrexate dose, Dr. Yeshrun said.
To see whether folinic acid rescue actually reduces the rate of methotrexate-induced toxicity and affects outcomes for patients who receive methotrexate post transplant for GVHD prophylaxis, Dr. Yeshrun and colleagues in three Israeli medical centers conducted a randomized, placebo-controlled trial.
The eligible study population included patients 18 and older with hematological malignancies in complete remission or minimal residual disease who underwent myeloablative conditioning and allogeneic transplant from HLA-matched or 1-antigen mismatched siblings or unrelated donors.
The patients were stratified by treatment center and intensity of the conditioning regimen, and then randomized on a 1:1 basis to receive folinic acid or placebo beginning 24 hours after each methotrexate dose, with the assigned medication given at a dose of 15 mg three times daily on the first day, and once daily on days 3 and 6.
Patients who received a transplant from an unrelated donor were also given anti-thymocyte globulin at a total dose of 15 mg/kg.
Supportive care included filgrastim (Neupogen) 5 mcg/kg from day 7 until neutrophil engraftment, infection prophylaxis, and ursodeoxycholic acid for prevention of veno-occlusive disease.
Trial stopped
Although the study was designed to enroll 116 patients to have a power of 80% to detect a 50% reduction in the rate of severe oral mucositis from an anticipated 50% in the placebo arm, a planned interim analysis conducted after approximately half of the target events occurred showed no difference in the rates of severe oral mucositis, and the trial was halted.
A total of 28 patients in the folinic acid group and 24 in the placebo group were available for the analysis.
The rate of grade 3 or 4 mucositis, the primary endpoint, was 46.6% in the folinic acid group, and 45.8% in the placebo group.
Respectively, the median duration of severe oral mucositis was 4 days in each group, days to neutrophil engraftment were a median of 12 and to platelet engraftment were a median of 13 days in each group, rates of febrile neutropenia were 57.1% and 58.3%, rates of bloodstream infections were 10.7% and 16.6%, rates of veno-occlusive disease were 7% and 12.5%, need for TPN occurred in 14.2% vs. 25%, need for opiates occurred in 78.5% vs. 66.6%, and median time to discharge was 18 and 19 days. As noted, none of the differences were statistically significant.
“These unequivocal interim results led to our decision to discontinue the study,” Dr. Yeshrun said.
Arnon Nagler, MD, MSc, from Sheba Medical Center Tel HaShomer at Tel Aviv University in Israel, who was not involved in the study, said in an interview “I think this is a very practical and important study, because we need evidence-based data such as this.”
Dr. Nagler, who comoderated the session where the data were presented, noted that the study was limited by the small number of patients and the lack of a subgroup analysis, but emphasized that the findings were important nonetheless.
His comoderator, Maria Gilleece, MD, director of the Yorkshire (England) Blood and Marrow Transplant Program, noted in an interview that folinic acid is frequently used in the United Kingdom for patients receiving high-dose methotrexate, ”and certainly, this study suggests that may be inappropriate.”
Rabin Medical Center sponsored the trial. Dr. Yeshrun, Dr. Nagler, and Dr. Gilleece reported no relevant conflicts of interest.
SOURCE: Yeshrun M. et al. TCT 2020. Abstract 61.
REPORTING FROM TCT 2020
Safer CAR uses modified NK cells for advanced CLL, NHL
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Survival for older AML patients better with HSCT from unrelated donors
For adults aged 50 and older in first or second remission after induction therapy for acute myeloid leukemia, hematopoietic stem cell transplants (HSCT) from young matched unrelated donors was associated with better overall survival and lower risk for relapse than transplants from haploidentical donors, a retrospective study suggests,
Among 823 patients from the aged 50 to 75 with acute myeloid leukemia (AML) in a transplant registry, hazard ratios for both mortality and relapse were significantly higher for patients who received transplants from haploidentical siblings or offspring, compared with patients who received transplants from HLA-matched unrelated donors aged 40 or younger, reported Miguel-Angel Perales, MD, who is affiliated with Memorial Sloan Kettering Cancer Center in New York City, and colleagues.
“Our findings lend support to our hypothesis that a young [matched unrelated donor] should be the donor of choice when available. Furthermore, the data presented here suggest comparable times to transplantation in both treatment groups, confirming timely access to unrelated donors is no longer a barrier,” they wrote in Haematologica.Allogeneic transplants from matched unrelated donors have been performed for more than 30 years for treatment of patients with advanced myeloid and lymphoid malignancies. More recently, T-cell-replete bone marrow or peripheral blood transplants from haploidentical relatives, with post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil to lower risk for graft-versus-host disease (GvHD) have become commonplace worldwide, and are established treatment options for patients with myeloid and lymphoid malignancies. There are conflicting studies suggesting that outcomes with haploidentical transplants are equivalent or superior to those seen with matched unrelated donors, the authors noted, but pointed to a 2018 study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplant and the Center for International Blood and Marrow Transplant Research (CIBMTR). Those study results found that, among transplant recipients aged 55 through 76, graft failure, nonrelapse mortality, and overall mortality were higher when the donors were haploidentical offspring rather than HLA-matched siblings.
To see whether patients aged 50 and older with AML might benefit more with transplants from hapolidentical relatives or matched unrelated donors, the investigators used CIBMTR data to review outcomes for 823 adults with AML who received a transplant in first or second remission at one of 90 U.S. centers from 2008 through 2015.
Of this cohort, 192 patients received grafts from haploidentical donors (25% from siblings and 75% from offspring), and 631 received grafts from matched unrelated donors ranging from 18 to 40 years of age.
Although the two groups were generally similar in demographic and disease characteristics, patients in the matched unrelated donor group had significantly higher frequency of poor-risk cytogenetics (P = .03) and were significantly more likely to have received a myeloablative condition regimen than a reduced-intensity regimen (P less than .001).
In the haploidentical group, 76% of patients were in first complete remission, and the remaining 24% were in second complete remission. In the HLA-matched group the respective proportions were 83% and 17%.
The median follow-up was 42 months in the haploidentical group and 47 months in the HLA-matched group. Five-year overall survival rates were 32% and 42%, respectively.
In multivariable models controlling for donor and recipient age, sex, performance score, hematopoietic cell transplant comorbidity score, cytomegalovirus serostatus, disease status, cytogenetic risk, transplant conditioning regimen intensity and transplant period, the hazard ratio (HR) for the primary endpoint of overall mortality was 1.27 for haploidentical vs. HLA-matched grafts (P = .04). The HR for relapse risk with haploidentical transplants was 1.32 (P =.04). No significant differences in risk of nonrelapse mortality were found between the two study arms.
Bone marrow grafts from matched unrelated donors were associated with significantly higher risk for chronic GvHD than haploidentical grafts (HR, 3.12; P less than .001), but there was no difference in chronic graft-versus-host disease (GvHD) incidence between peripheral blood grafts from matched unrelated donors and haploidentical grafts.
“These data support the view that matched unrelated donor transplant with donors younger than 40 years is to be preferred,” the investigators wrote.
But in an interview, coauthor
“Even though there appears to be that clinical benefit for this older AML patient population, that benefit is not huge, and when you’re also accounting for the process of finding a donor and just getting someone into transplant, a lot of us weren’t sure if this was really going to be practice changing as the field does move into haploidentical transplants being more common,” he said.
He noted that the better outcomes among patients who received transplants from matched unrelated donors may be at least in part explained by the higher proportion of patients with unrelated donors who received myeloablative conditioning regimens. In this study, 65% of patients with haploidentical donors underwent reduced-intensity conditioning with total body irradiation, cyclophosphamide, and fludarabine.“If we do a comparison of equal conditioning regimens, are we really going to see the same outcomes in this setting? This might actually argue that, if you’re going to do a haploidentical transplant, you might start thinking about those newer, more ablative conditioning regimens,” he said.Dr. Tomlinson added that the data are reassuring, because of the modest size of the benefit, and because “many, many of our studies are showing that haploidentical transplants do almost as well as the matched ones. The big question mark will be what are the long-term outcomes? What happens after 3 years from those transplants? And that is going to take a lot more high quality, mature data.”In an editorial accompanying the study, Richard E. Champlin, MD, of the University of Texas MD Anderson Cancer Center in Houston, noted that the more frequent use of reduced-intensity conditioning used for most patients in the haploidentical group has been associated in other studies with higher relapse rates, compared with other, more intense reduced-intensity regimens.
While he agreed that the study by Dr. Perales and colleagues “should give pause for thought, however, for those considering jumping to haploidentical transplants as a preferred approach in general,” he also noted that the study’s conclusion might not apply to cases where time-to-transplant is critical, or when other conditioning and GvHD prophylaxis regimens are used.
“The ideal study would compare optimized versions of both haploidentical and unrelated donor transplants, and use “intention-to-treat” analysis, including all patients for whom a transplant is intended from the time of initial HLA typing,” he wrote.
The study was funded by grants from the National Institutes of Health and the Office of Naval Research. Dr. Tomlinson reported no relevant disclosures. Dr. Champlin did not report disclosures.
SOURCE: Perales M-A et al. Haematologica. 2020 Jan 31;105(2):407-13.
For adults aged 50 and older in first or second remission after induction therapy for acute myeloid leukemia, hematopoietic stem cell transplants (HSCT) from young matched unrelated donors was associated with better overall survival and lower risk for relapse than transplants from haploidentical donors, a retrospective study suggests,
Among 823 patients from the aged 50 to 75 with acute myeloid leukemia (AML) in a transplant registry, hazard ratios for both mortality and relapse were significantly higher for patients who received transplants from haploidentical siblings or offspring, compared with patients who received transplants from HLA-matched unrelated donors aged 40 or younger, reported Miguel-Angel Perales, MD, who is affiliated with Memorial Sloan Kettering Cancer Center in New York City, and colleagues.
“Our findings lend support to our hypothesis that a young [matched unrelated donor] should be the donor of choice when available. Furthermore, the data presented here suggest comparable times to transplantation in both treatment groups, confirming timely access to unrelated donors is no longer a barrier,” they wrote in Haematologica.Allogeneic transplants from matched unrelated donors have been performed for more than 30 years for treatment of patients with advanced myeloid and lymphoid malignancies. More recently, T-cell-replete bone marrow or peripheral blood transplants from haploidentical relatives, with post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil to lower risk for graft-versus-host disease (GvHD) have become commonplace worldwide, and are established treatment options for patients with myeloid and lymphoid malignancies. There are conflicting studies suggesting that outcomes with haploidentical transplants are equivalent or superior to those seen with matched unrelated donors, the authors noted, but pointed to a 2018 study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplant and the Center for International Blood and Marrow Transplant Research (CIBMTR). Those study results found that, among transplant recipients aged 55 through 76, graft failure, nonrelapse mortality, and overall mortality were higher when the donors were haploidentical offspring rather than HLA-matched siblings.
To see whether patients aged 50 and older with AML might benefit more with transplants from hapolidentical relatives or matched unrelated donors, the investigators used CIBMTR data to review outcomes for 823 adults with AML who received a transplant in first or second remission at one of 90 U.S. centers from 2008 through 2015.
Of this cohort, 192 patients received grafts from haploidentical donors (25% from siblings and 75% from offspring), and 631 received grafts from matched unrelated donors ranging from 18 to 40 years of age.
Although the two groups were generally similar in demographic and disease characteristics, patients in the matched unrelated donor group had significantly higher frequency of poor-risk cytogenetics (P = .03) and were significantly more likely to have received a myeloablative condition regimen than a reduced-intensity regimen (P less than .001).
In the haploidentical group, 76% of patients were in first complete remission, and the remaining 24% were in second complete remission. In the HLA-matched group the respective proportions were 83% and 17%.
The median follow-up was 42 months in the haploidentical group and 47 months in the HLA-matched group. Five-year overall survival rates were 32% and 42%, respectively.
In multivariable models controlling for donor and recipient age, sex, performance score, hematopoietic cell transplant comorbidity score, cytomegalovirus serostatus, disease status, cytogenetic risk, transplant conditioning regimen intensity and transplant period, the hazard ratio (HR) for the primary endpoint of overall mortality was 1.27 for haploidentical vs. HLA-matched grafts (P = .04). The HR for relapse risk with haploidentical transplants was 1.32 (P =.04). No significant differences in risk of nonrelapse mortality were found between the two study arms.
Bone marrow grafts from matched unrelated donors were associated with significantly higher risk for chronic GvHD than haploidentical grafts (HR, 3.12; P less than .001), but there was no difference in chronic graft-versus-host disease (GvHD) incidence between peripheral blood grafts from matched unrelated donors and haploidentical grafts.
“These data support the view that matched unrelated donor transplant with donors younger than 40 years is to be preferred,” the investigators wrote.
But in an interview, coauthor
“Even though there appears to be that clinical benefit for this older AML patient population, that benefit is not huge, and when you’re also accounting for the process of finding a donor and just getting someone into transplant, a lot of us weren’t sure if this was really going to be practice changing as the field does move into haploidentical transplants being more common,” he said.
He noted that the better outcomes among patients who received transplants from matched unrelated donors may be at least in part explained by the higher proportion of patients with unrelated donors who received myeloablative conditioning regimens. In this study, 65% of patients with haploidentical donors underwent reduced-intensity conditioning with total body irradiation, cyclophosphamide, and fludarabine.“If we do a comparison of equal conditioning regimens, are we really going to see the same outcomes in this setting? This might actually argue that, if you’re going to do a haploidentical transplant, you might start thinking about those newer, more ablative conditioning regimens,” he said.Dr. Tomlinson added that the data are reassuring, because of the modest size of the benefit, and because “many, many of our studies are showing that haploidentical transplants do almost as well as the matched ones. The big question mark will be what are the long-term outcomes? What happens after 3 years from those transplants? And that is going to take a lot more high quality, mature data.”In an editorial accompanying the study, Richard E. Champlin, MD, of the University of Texas MD Anderson Cancer Center in Houston, noted that the more frequent use of reduced-intensity conditioning used for most patients in the haploidentical group has been associated in other studies with higher relapse rates, compared with other, more intense reduced-intensity regimens.
While he agreed that the study by Dr. Perales and colleagues “should give pause for thought, however, for those considering jumping to haploidentical transplants as a preferred approach in general,” he also noted that the study’s conclusion might not apply to cases where time-to-transplant is critical, or when other conditioning and GvHD prophylaxis regimens are used.
“The ideal study would compare optimized versions of both haploidentical and unrelated donor transplants, and use “intention-to-treat” analysis, including all patients for whom a transplant is intended from the time of initial HLA typing,” he wrote.
The study was funded by grants from the National Institutes of Health and the Office of Naval Research. Dr. Tomlinson reported no relevant disclosures. Dr. Champlin did not report disclosures.
SOURCE: Perales M-A et al. Haematologica. 2020 Jan 31;105(2):407-13.
For adults aged 50 and older in first or second remission after induction therapy for acute myeloid leukemia, hematopoietic stem cell transplants (HSCT) from young matched unrelated donors was associated with better overall survival and lower risk for relapse than transplants from haploidentical donors, a retrospective study suggests,
Among 823 patients from the aged 50 to 75 with acute myeloid leukemia (AML) in a transplant registry, hazard ratios for both mortality and relapse were significantly higher for patients who received transplants from haploidentical siblings or offspring, compared with patients who received transplants from HLA-matched unrelated donors aged 40 or younger, reported Miguel-Angel Perales, MD, who is affiliated with Memorial Sloan Kettering Cancer Center in New York City, and colleagues.
“Our findings lend support to our hypothesis that a young [matched unrelated donor] should be the donor of choice when available. Furthermore, the data presented here suggest comparable times to transplantation in both treatment groups, confirming timely access to unrelated donors is no longer a barrier,” they wrote in Haematologica.Allogeneic transplants from matched unrelated donors have been performed for more than 30 years for treatment of patients with advanced myeloid and lymphoid malignancies. More recently, T-cell-replete bone marrow or peripheral blood transplants from haploidentical relatives, with post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil to lower risk for graft-versus-host disease (GvHD) have become commonplace worldwide, and are established treatment options for patients with myeloid and lymphoid malignancies. There are conflicting studies suggesting that outcomes with haploidentical transplants are equivalent or superior to those seen with matched unrelated donors, the authors noted, but pointed to a 2018 study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplant and the Center for International Blood and Marrow Transplant Research (CIBMTR). Those study results found that, among transplant recipients aged 55 through 76, graft failure, nonrelapse mortality, and overall mortality were higher when the donors were haploidentical offspring rather than HLA-matched siblings.
To see whether patients aged 50 and older with AML might benefit more with transplants from hapolidentical relatives or matched unrelated donors, the investigators used CIBMTR data to review outcomes for 823 adults with AML who received a transplant in first or second remission at one of 90 U.S. centers from 2008 through 2015.
Of this cohort, 192 patients received grafts from haploidentical donors (25% from siblings and 75% from offspring), and 631 received grafts from matched unrelated donors ranging from 18 to 40 years of age.
Although the two groups were generally similar in demographic and disease characteristics, patients in the matched unrelated donor group had significantly higher frequency of poor-risk cytogenetics (P = .03) and were significantly more likely to have received a myeloablative condition regimen than a reduced-intensity regimen (P less than .001).
In the haploidentical group, 76% of patients were in first complete remission, and the remaining 24% were in second complete remission. In the HLA-matched group the respective proportions were 83% and 17%.
The median follow-up was 42 months in the haploidentical group and 47 months in the HLA-matched group. Five-year overall survival rates were 32% and 42%, respectively.
In multivariable models controlling for donor and recipient age, sex, performance score, hematopoietic cell transplant comorbidity score, cytomegalovirus serostatus, disease status, cytogenetic risk, transplant conditioning regimen intensity and transplant period, the hazard ratio (HR) for the primary endpoint of overall mortality was 1.27 for haploidentical vs. HLA-matched grafts (P = .04). The HR for relapse risk with haploidentical transplants was 1.32 (P =.04). No significant differences in risk of nonrelapse mortality were found between the two study arms.
Bone marrow grafts from matched unrelated donors were associated with significantly higher risk for chronic GvHD than haploidentical grafts (HR, 3.12; P less than .001), but there was no difference in chronic graft-versus-host disease (GvHD) incidence between peripheral blood grafts from matched unrelated donors and haploidentical grafts.
“These data support the view that matched unrelated donor transplant with donors younger than 40 years is to be preferred,” the investigators wrote.
But in an interview, coauthor
“Even though there appears to be that clinical benefit for this older AML patient population, that benefit is not huge, and when you’re also accounting for the process of finding a donor and just getting someone into transplant, a lot of us weren’t sure if this was really going to be practice changing as the field does move into haploidentical transplants being more common,” he said.
He noted that the better outcomes among patients who received transplants from matched unrelated donors may be at least in part explained by the higher proportion of patients with unrelated donors who received myeloablative conditioning regimens. In this study, 65% of patients with haploidentical donors underwent reduced-intensity conditioning with total body irradiation, cyclophosphamide, and fludarabine.“If we do a comparison of equal conditioning regimens, are we really going to see the same outcomes in this setting? This might actually argue that, if you’re going to do a haploidentical transplant, you might start thinking about those newer, more ablative conditioning regimens,” he said.Dr. Tomlinson added that the data are reassuring, because of the modest size of the benefit, and because “many, many of our studies are showing that haploidentical transplants do almost as well as the matched ones. The big question mark will be what are the long-term outcomes? What happens after 3 years from those transplants? And that is going to take a lot more high quality, mature data.”In an editorial accompanying the study, Richard E. Champlin, MD, of the University of Texas MD Anderson Cancer Center in Houston, noted that the more frequent use of reduced-intensity conditioning used for most patients in the haploidentical group has been associated in other studies with higher relapse rates, compared with other, more intense reduced-intensity regimens.
While he agreed that the study by Dr. Perales and colleagues “should give pause for thought, however, for those considering jumping to haploidentical transplants as a preferred approach in general,” he also noted that the study’s conclusion might not apply to cases where time-to-transplant is critical, or when other conditioning and GvHD prophylaxis regimens are used.
“The ideal study would compare optimized versions of both haploidentical and unrelated donor transplants, and use “intention-to-treat” analysis, including all patients for whom a transplant is intended from the time of initial HLA typing,” he wrote.
The study was funded by grants from the National Institutes of Health and the Office of Naval Research. Dr. Tomlinson reported no relevant disclosures. Dr. Champlin did not report disclosures.
SOURCE: Perales M-A et al. Haematologica. 2020 Jan 31;105(2):407-13.
FROM HAEMATOLOGICA
CAR T-cell therapy may worsen mental health in some patients
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Several factors may drive recent improvements in allo-HCT outcomes
A cancer center has seen improved outcomes of allogeneic transplant in recent years, despite increases in patient age and comorbidities.
Researchers compared patients who received allogeneic hematopoietic cell transplants (allo-HCTs) during two periods, 2003-2007 and 2013-2017.
Patients treated in the 2013-2017 period were older and had more HCT-specific comorbidities at baseline, but they had lower rates of mortality, relapse, and graft-versus-host disease (GVHD) post transplant. George B. McDonald, MD, an emeritus member at Fred Hutchinson Cancer Research Center in Seattle, and coauthors described these findings in Annals of Internal Medicine.
“The primary question being addressed by this study was whether the striking improvement in survival … from the 1990s to the early 2000s, that we and other transplant centers have reported, had reached a plateau or whether further improvements in survival were being seen,” Dr. McDonald said in an interview.
“We knew that older and sicker patients were now coming for transplant, compared to 10 years ago. Our transplant protocols have backed away from the highest doses of chemotherapy and irradiation used to prepare patients for transplant, toward less toxic therapies, including reduced-intensity conditioning,” he added. “Our investigators have sought to prevent and more effectively treat the myriad of complications of allogeneic transplant, based on research done at the Fred Hutchinson Cancer Research Center and at transplant centers throughout the world.”
Baseline characteristics and treatment
Dr. McDonald and his colleagues analyzed data on patients who received allo-HCTs at Seattle Cancer Care Alliance. There were 1,148 patients treated in the 2003-2007 period and 1,131 patients treated in the 2013-2017 period.
Indications for allo-HCT were similar between the time periods. Patients were diagnosed with aplastic anemia, acute and chronic leukemias, Hodgkin and non-Hodgkin lymphomas, myelodysplastic syndromes, and multiple myeloma.
Patients in the 2013-2017 group were older and had more HCT-specific comorbidities than did the patients in the 2003-2007 group. The median age was 50.0 years (range, 0.1-80.9 years) and 47.2 years (range, 0.4-78.9 years), respectively. The median score on the augmented HCT-specific comorbidity index was 4.0 and 3.0, respectively.
The 2013-2017 group was more likely to have intermediate-risk disease (73% vs. 54%) but less likely to have high-risk disease (14% vs. 31%). The 2013-2017 group was less likely to receive high-dose myeloablative conditioning (15% vs. 67%) but more likely to have an unrelated donor (70% vs. 59%) or receive a cord blood transplant (13% vs. 4%).
GVHD prophylaxis differed between the time periods, with patients in the 2013-2017 group being more likely to receive sirolimus, posttransplant cyclophosphamide, and abatacept.
Outcomes
Overall, outcomes were superior in the 2013-2017 group. The rate of nonrelapse mortality at day 200 was higher in the 2003-2007 group than in the 2013-2017 group – 16% and 11%, respectively (adjusted hazard ratio, 0.66; P = .008).
Relapse or progression was more common in the 2003-2007 group – 348 patients vs. 244 patients (aHR, 0.76; P = .011). More patients died from relapse in the 2003-2007 group – 307 patients vs. 186 patients (aHR, 0.69; P = .002). More patients died from any cause in the 2003-2007 group – 653 patients vs. 418 patients (aHR, 0.66; P less than .001). The rate of grade 2-4 acute GVHD was higher in the 2003-2007 group – 71% vs. 69% (aHR, 0.80) – and so was the rate of chronic GVHD – 44% vs. 29% (aHR, 0.40). The risk of developing gram-negative bacteremia was lower in the 2013-2017 group (aHR, 0.42), as was the risk of invasive mold infection (aHR, 0.55).
Patients in the 2013-2017 group had a higher risk of cytomegalovirus (CMV) infection (aHR = 1.15), but they were less likely to have high levels of CMV viremia (aHR, 0.78 for greater than 250 IU/mL; aHR, 0.46 for greater than 1,000 IU/mL). Having higher levels of CMV viremia was associated with an increased risk of non-relapse mortality.
Potential drivers of outcome
Dr. McDonald said this study’s design makes it difficult to determine the causes of improved outcomes in the 2013-2017 period. However, the researchers do have theories about which practice changes may have contributed to better allo-HCT outcomes.
Dr. McDonald said the decrease in GVHD over time was “likely owing to the introduction of newer preventive strategies and immune-suppressive drugs.”
The decrease in nonrelapse mortality may have been driven, in part, by a reduction in fatal infections. Dr. McDonald said these infections were less frequent in the 2013-2017 period because of “molecular methods of diagnosis (especially for herpesviruses) and newer treatments (especially for fungal infections).”
“Another reason for a lower frequency of serious infection was a change in practice for treating graft-versus-host disease,” Dr. McDonald added. “Based on a randomized trial comparing lower- versus higher-dose prednisone for less-severe GVHD … both initial doses of prednisone and total prednisone exposure were reduced.”
Another factor that may have improved allo-HCT outcomes is the center’s change in approach to conditioning therapy over time.
“The gradual shift from very-high-dose conditioning therapy to less-intense myeloablative therapy and to reduced-intensity conditioning was likely responsible for a reduction in damage to the liver, lungs, and kidneys over the last 10 years,” Dr. McDonald said. “We were able to identify patients who were at especially high risk for mortality during a screening process before transplant ... thus allowing patients at highest risk to receive less intense conditioning therapy.”
Dr. McDonald added that this study’s results are encouraging, particularly the reduction in nonrelapse mortality. However, there is still room for improvement when it comes to relapse and progression.
This research was funded by the National Institutes of Health, the American Cancer Society, and the Patient-Centered Outcomes Research Institute. Dr. McDonald reported relationships with Sangamo Therapeutics, Soligenix Therapeutics, and Lucent Medical Systems. His coauthors disclosed relationships with a range of companies.
SOURCE: McDonald GB et al. Ann Intern Med. 2020 Jan 20. doi: 10.7326/M19-2936.
A cancer center has seen improved outcomes of allogeneic transplant in recent years, despite increases in patient age and comorbidities.
Researchers compared patients who received allogeneic hematopoietic cell transplants (allo-HCTs) during two periods, 2003-2007 and 2013-2017.
Patients treated in the 2013-2017 period were older and had more HCT-specific comorbidities at baseline, but they had lower rates of mortality, relapse, and graft-versus-host disease (GVHD) post transplant. George B. McDonald, MD, an emeritus member at Fred Hutchinson Cancer Research Center in Seattle, and coauthors described these findings in Annals of Internal Medicine.
“The primary question being addressed by this study was whether the striking improvement in survival … from the 1990s to the early 2000s, that we and other transplant centers have reported, had reached a plateau or whether further improvements in survival were being seen,” Dr. McDonald said in an interview.
“We knew that older and sicker patients were now coming for transplant, compared to 10 years ago. Our transplant protocols have backed away from the highest doses of chemotherapy and irradiation used to prepare patients for transplant, toward less toxic therapies, including reduced-intensity conditioning,” he added. “Our investigators have sought to prevent and more effectively treat the myriad of complications of allogeneic transplant, based on research done at the Fred Hutchinson Cancer Research Center and at transplant centers throughout the world.”
Baseline characteristics and treatment
Dr. McDonald and his colleagues analyzed data on patients who received allo-HCTs at Seattle Cancer Care Alliance. There were 1,148 patients treated in the 2003-2007 period and 1,131 patients treated in the 2013-2017 period.
Indications for allo-HCT were similar between the time periods. Patients were diagnosed with aplastic anemia, acute and chronic leukemias, Hodgkin and non-Hodgkin lymphomas, myelodysplastic syndromes, and multiple myeloma.
Patients in the 2013-2017 group were older and had more HCT-specific comorbidities than did the patients in the 2003-2007 group. The median age was 50.0 years (range, 0.1-80.9 years) and 47.2 years (range, 0.4-78.9 years), respectively. The median score on the augmented HCT-specific comorbidity index was 4.0 and 3.0, respectively.
The 2013-2017 group was more likely to have intermediate-risk disease (73% vs. 54%) but less likely to have high-risk disease (14% vs. 31%). The 2013-2017 group was less likely to receive high-dose myeloablative conditioning (15% vs. 67%) but more likely to have an unrelated donor (70% vs. 59%) or receive a cord blood transplant (13% vs. 4%).
GVHD prophylaxis differed between the time periods, with patients in the 2013-2017 group being more likely to receive sirolimus, posttransplant cyclophosphamide, and abatacept.
Outcomes
Overall, outcomes were superior in the 2013-2017 group. The rate of nonrelapse mortality at day 200 was higher in the 2003-2007 group than in the 2013-2017 group – 16% and 11%, respectively (adjusted hazard ratio, 0.66; P = .008).
Relapse or progression was more common in the 2003-2007 group – 348 patients vs. 244 patients (aHR, 0.76; P = .011). More patients died from relapse in the 2003-2007 group – 307 patients vs. 186 patients (aHR, 0.69; P = .002). More patients died from any cause in the 2003-2007 group – 653 patients vs. 418 patients (aHR, 0.66; P less than .001). The rate of grade 2-4 acute GVHD was higher in the 2003-2007 group – 71% vs. 69% (aHR, 0.80) – and so was the rate of chronic GVHD – 44% vs. 29% (aHR, 0.40). The risk of developing gram-negative bacteremia was lower in the 2013-2017 group (aHR, 0.42), as was the risk of invasive mold infection (aHR, 0.55).
Patients in the 2013-2017 group had a higher risk of cytomegalovirus (CMV) infection (aHR = 1.15), but they were less likely to have high levels of CMV viremia (aHR, 0.78 for greater than 250 IU/mL; aHR, 0.46 for greater than 1,000 IU/mL). Having higher levels of CMV viremia was associated with an increased risk of non-relapse mortality.
Potential drivers of outcome
Dr. McDonald said this study’s design makes it difficult to determine the causes of improved outcomes in the 2013-2017 period. However, the researchers do have theories about which practice changes may have contributed to better allo-HCT outcomes.
Dr. McDonald said the decrease in GVHD over time was “likely owing to the introduction of newer preventive strategies and immune-suppressive drugs.”
The decrease in nonrelapse mortality may have been driven, in part, by a reduction in fatal infections. Dr. McDonald said these infections were less frequent in the 2013-2017 period because of “molecular methods of diagnosis (especially for herpesviruses) and newer treatments (especially for fungal infections).”
“Another reason for a lower frequency of serious infection was a change in practice for treating graft-versus-host disease,” Dr. McDonald added. “Based on a randomized trial comparing lower- versus higher-dose prednisone for less-severe GVHD … both initial doses of prednisone and total prednisone exposure were reduced.”
Another factor that may have improved allo-HCT outcomes is the center’s change in approach to conditioning therapy over time.
“The gradual shift from very-high-dose conditioning therapy to less-intense myeloablative therapy and to reduced-intensity conditioning was likely responsible for a reduction in damage to the liver, lungs, and kidneys over the last 10 years,” Dr. McDonald said. “We were able to identify patients who were at especially high risk for mortality during a screening process before transplant ... thus allowing patients at highest risk to receive less intense conditioning therapy.”
Dr. McDonald added that this study’s results are encouraging, particularly the reduction in nonrelapse mortality. However, there is still room for improvement when it comes to relapse and progression.
This research was funded by the National Institutes of Health, the American Cancer Society, and the Patient-Centered Outcomes Research Institute. Dr. McDonald reported relationships with Sangamo Therapeutics, Soligenix Therapeutics, and Lucent Medical Systems. His coauthors disclosed relationships with a range of companies.
SOURCE: McDonald GB et al. Ann Intern Med. 2020 Jan 20. doi: 10.7326/M19-2936.
A cancer center has seen improved outcomes of allogeneic transplant in recent years, despite increases in patient age and comorbidities.
Researchers compared patients who received allogeneic hematopoietic cell transplants (allo-HCTs) during two periods, 2003-2007 and 2013-2017.
Patients treated in the 2013-2017 period were older and had more HCT-specific comorbidities at baseline, but they had lower rates of mortality, relapse, and graft-versus-host disease (GVHD) post transplant. George B. McDonald, MD, an emeritus member at Fred Hutchinson Cancer Research Center in Seattle, and coauthors described these findings in Annals of Internal Medicine.
“The primary question being addressed by this study was whether the striking improvement in survival … from the 1990s to the early 2000s, that we and other transplant centers have reported, had reached a plateau or whether further improvements in survival were being seen,” Dr. McDonald said in an interview.
“We knew that older and sicker patients were now coming for transplant, compared to 10 years ago. Our transplant protocols have backed away from the highest doses of chemotherapy and irradiation used to prepare patients for transplant, toward less toxic therapies, including reduced-intensity conditioning,” he added. “Our investigators have sought to prevent and more effectively treat the myriad of complications of allogeneic transplant, based on research done at the Fred Hutchinson Cancer Research Center and at transplant centers throughout the world.”
Baseline characteristics and treatment
Dr. McDonald and his colleagues analyzed data on patients who received allo-HCTs at Seattle Cancer Care Alliance. There were 1,148 patients treated in the 2003-2007 period and 1,131 patients treated in the 2013-2017 period.
Indications for allo-HCT were similar between the time periods. Patients were diagnosed with aplastic anemia, acute and chronic leukemias, Hodgkin and non-Hodgkin lymphomas, myelodysplastic syndromes, and multiple myeloma.
Patients in the 2013-2017 group were older and had more HCT-specific comorbidities than did the patients in the 2003-2007 group. The median age was 50.0 years (range, 0.1-80.9 years) and 47.2 years (range, 0.4-78.9 years), respectively. The median score on the augmented HCT-specific comorbidity index was 4.0 and 3.0, respectively.
The 2013-2017 group was more likely to have intermediate-risk disease (73% vs. 54%) but less likely to have high-risk disease (14% vs. 31%). The 2013-2017 group was less likely to receive high-dose myeloablative conditioning (15% vs. 67%) but more likely to have an unrelated donor (70% vs. 59%) or receive a cord blood transplant (13% vs. 4%).
GVHD prophylaxis differed between the time periods, with patients in the 2013-2017 group being more likely to receive sirolimus, posttransplant cyclophosphamide, and abatacept.
Outcomes
Overall, outcomes were superior in the 2013-2017 group. The rate of nonrelapse mortality at day 200 was higher in the 2003-2007 group than in the 2013-2017 group – 16% and 11%, respectively (adjusted hazard ratio, 0.66; P = .008).
Relapse or progression was more common in the 2003-2007 group – 348 patients vs. 244 patients (aHR, 0.76; P = .011). More patients died from relapse in the 2003-2007 group – 307 patients vs. 186 patients (aHR, 0.69; P = .002). More patients died from any cause in the 2003-2007 group – 653 patients vs. 418 patients (aHR, 0.66; P less than .001). The rate of grade 2-4 acute GVHD was higher in the 2003-2007 group – 71% vs. 69% (aHR, 0.80) – and so was the rate of chronic GVHD – 44% vs. 29% (aHR, 0.40). The risk of developing gram-negative bacteremia was lower in the 2013-2017 group (aHR, 0.42), as was the risk of invasive mold infection (aHR, 0.55).
Patients in the 2013-2017 group had a higher risk of cytomegalovirus (CMV) infection (aHR = 1.15), but they were less likely to have high levels of CMV viremia (aHR, 0.78 for greater than 250 IU/mL; aHR, 0.46 for greater than 1,000 IU/mL). Having higher levels of CMV viremia was associated with an increased risk of non-relapse mortality.
Potential drivers of outcome
Dr. McDonald said this study’s design makes it difficult to determine the causes of improved outcomes in the 2013-2017 period. However, the researchers do have theories about which practice changes may have contributed to better allo-HCT outcomes.
Dr. McDonald said the decrease in GVHD over time was “likely owing to the introduction of newer preventive strategies and immune-suppressive drugs.”
The decrease in nonrelapse mortality may have been driven, in part, by a reduction in fatal infections. Dr. McDonald said these infections were less frequent in the 2013-2017 period because of “molecular methods of diagnosis (especially for herpesviruses) and newer treatments (especially for fungal infections).”
“Another reason for a lower frequency of serious infection was a change in practice for treating graft-versus-host disease,” Dr. McDonald added. “Based on a randomized trial comparing lower- versus higher-dose prednisone for less-severe GVHD … both initial doses of prednisone and total prednisone exposure were reduced.”
Another factor that may have improved allo-HCT outcomes is the center’s change in approach to conditioning therapy over time.
“The gradual shift from very-high-dose conditioning therapy to less-intense myeloablative therapy and to reduced-intensity conditioning was likely responsible for a reduction in damage to the liver, lungs, and kidneys over the last 10 years,” Dr. McDonald said. “We were able to identify patients who were at especially high risk for mortality during a screening process before transplant ... thus allowing patients at highest risk to receive less intense conditioning therapy.”
Dr. McDonald added that this study’s results are encouraging, particularly the reduction in nonrelapse mortality. However, there is still room for improvement when it comes to relapse and progression.
This research was funded by the National Institutes of Health, the American Cancer Society, and the Patient-Centered Outcomes Research Institute. Dr. McDonald reported relationships with Sangamo Therapeutics, Soligenix Therapeutics, and Lucent Medical Systems. His coauthors disclosed relationships with a range of companies.
SOURCE: McDonald GB et al. Ann Intern Med. 2020 Jan 20. doi: 10.7326/M19-2936.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: At a single center, outcomes of allogeneic hematopoietic cell transplant improved for patients treated in 2013-2017, compared with patients treated in 2003-2007.
Major finding: Rates of nonrelapse mortality at day 200 were higher in the 2003-2007 group than in the 2013-2017 group – 16% and 11%, respectively (adjusted hazard ratio, 0.66; P = .008).
Study details: A single-center study of 1,148 patients treated in the 2003-2007 period and 1,131 patients treated in the 2013-2017 period.
Disclosures: The research was funded by the National Institutes of Health, the American Cancer Society, and the Patient-Centered Outcomes Research Institute. Dr. McDonald reported relationships with Sangamo Therapeutics, Soligenix Therapeutics, and Lucent Medical Systems. His coauthors disclosed relationships with a range of companies.
Source: McDonald GB et al. Ann Intern Med. 2020 Jan 20. doi: 10.7326/M19-2936.
Telehealth appears to help speed front end of liver transplant process
The incorporation of telehealth in the liver transplantation process is demonstrating the potential to expedite the evaluation of patients and get them listed on the transplant wait list.
New research shows “a ,” Binu V. John, MD, of McGuire VA Medical Center, Richmond, and colleagues wrote in a report published in Clinical Gastroenterology and Hepatology (2019 Dec 27. doi: 10.1016/j.cgh.2019.12.021).
Researchers looked at 465 patients who had evaluations for liver transplants at the Richmond Veterans Affairs Medical Center from 2005 through 2017. Nearly half (232 patients) were evaluated via telehealth, with the remaining 233 evaluated with traditional in-person evaluations.
“Patients in the telehealth group were evaluated significantly faster than patients in the usual care group (22 vs. 54 days, P less than .001),” Dr. John and colleagues wrote, adding that, after conducting a propensity-matched analysis, “telehealth was associated with an 85% reduction in time from referral to evaluation.”
Additionally, patients “who underwent the initial evaluation by telehealth were listed significantly earlier than the usual care group (95 vs. 149 days; P less than .001),” the authors stated, adding that “telehealth was associated with a 74% reduction in time to listing” after conducting a propensity-matched analysis.
However, while speeding up time to referral and listing, “the median time to transplant was not significantly different between the two groups on unadjusted (218 vs. 244 days; P = .084) or adjusted analysis (325 vs. 409 days; P = .08),” they added.
Additionally, “there was no difference in pretransplant mortality between [those] evaluated by telehealth or usual care in unadjusted analysis,” Dr. John and colleagues observed, noting that 169 of 465 patients (51 on the waiting list for a transplant and 118 who were not listed) who were referred died without receiving a liver transplant.
Researchers suggested that while evaluation times may have been shorter with the use of telehealth, they did not translate to shorter transplantation times “likely because the latter is a complex metric that is driven primarily by organ availability.”
Dr. John and colleagues cautioned that the centralized nature of the VA medical system could make the results of this study not generalizable across private care settings, particularly when care needs to cross state lines, which does not present an issue within the VA medical system.
That being said, the “ability to successfully evaluate and list patients via telehealth and obtain the same outcomes in terms of time to transplant and pretransplant mortality is significant because of the numerous advantages that telehealth offers to improve overall access to transplantation,” they stated, adding that more studies are needed, both in and out of the VA system, “to confirm that telehealth is a safe and effective way to expand access for patients undergoing evaluation for liver transplantation.”
Lead author Dr. Binu John serves on medical advisory boards for Gilead and Eisai and received research funding from a number of pharmaceutical manufacturers. No conflicts of interest were reported by the other authors.
SOURCE: John BV et al. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2019.12.021.
The incorporation of telehealth in the liver transplantation process is demonstrating the potential to expedite the evaluation of patients and get them listed on the transplant wait list.
New research shows “a ,” Binu V. John, MD, of McGuire VA Medical Center, Richmond, and colleagues wrote in a report published in Clinical Gastroenterology and Hepatology (2019 Dec 27. doi: 10.1016/j.cgh.2019.12.021).
Researchers looked at 465 patients who had evaluations for liver transplants at the Richmond Veterans Affairs Medical Center from 2005 through 2017. Nearly half (232 patients) were evaluated via telehealth, with the remaining 233 evaluated with traditional in-person evaluations.
“Patients in the telehealth group were evaluated significantly faster than patients in the usual care group (22 vs. 54 days, P less than .001),” Dr. John and colleagues wrote, adding that, after conducting a propensity-matched analysis, “telehealth was associated with an 85% reduction in time from referral to evaluation.”
Additionally, patients “who underwent the initial evaluation by telehealth were listed significantly earlier than the usual care group (95 vs. 149 days; P less than .001),” the authors stated, adding that “telehealth was associated with a 74% reduction in time to listing” after conducting a propensity-matched analysis.
However, while speeding up time to referral and listing, “the median time to transplant was not significantly different between the two groups on unadjusted (218 vs. 244 days; P = .084) or adjusted analysis (325 vs. 409 days; P = .08),” they added.
Additionally, “there was no difference in pretransplant mortality between [those] evaluated by telehealth or usual care in unadjusted analysis,” Dr. John and colleagues observed, noting that 169 of 465 patients (51 on the waiting list for a transplant and 118 who were not listed) who were referred died without receiving a liver transplant.
Researchers suggested that while evaluation times may have been shorter with the use of telehealth, they did not translate to shorter transplantation times “likely because the latter is a complex metric that is driven primarily by organ availability.”
Dr. John and colleagues cautioned that the centralized nature of the VA medical system could make the results of this study not generalizable across private care settings, particularly when care needs to cross state lines, which does not present an issue within the VA medical system.
That being said, the “ability to successfully evaluate and list patients via telehealth and obtain the same outcomes in terms of time to transplant and pretransplant mortality is significant because of the numerous advantages that telehealth offers to improve overall access to transplantation,” they stated, adding that more studies are needed, both in and out of the VA system, “to confirm that telehealth is a safe and effective way to expand access for patients undergoing evaluation for liver transplantation.”
Lead author Dr. Binu John serves on medical advisory boards for Gilead and Eisai and received research funding from a number of pharmaceutical manufacturers. No conflicts of interest were reported by the other authors.
SOURCE: John BV et al. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2019.12.021.
The incorporation of telehealth in the liver transplantation process is demonstrating the potential to expedite the evaluation of patients and get them listed on the transplant wait list.
New research shows “a ,” Binu V. John, MD, of McGuire VA Medical Center, Richmond, and colleagues wrote in a report published in Clinical Gastroenterology and Hepatology (2019 Dec 27. doi: 10.1016/j.cgh.2019.12.021).
Researchers looked at 465 patients who had evaluations for liver transplants at the Richmond Veterans Affairs Medical Center from 2005 through 2017. Nearly half (232 patients) were evaluated via telehealth, with the remaining 233 evaluated with traditional in-person evaluations.
“Patients in the telehealth group were evaluated significantly faster than patients in the usual care group (22 vs. 54 days, P less than .001),” Dr. John and colleagues wrote, adding that, after conducting a propensity-matched analysis, “telehealth was associated with an 85% reduction in time from referral to evaluation.”
Additionally, patients “who underwent the initial evaluation by telehealth were listed significantly earlier than the usual care group (95 vs. 149 days; P less than .001),” the authors stated, adding that “telehealth was associated with a 74% reduction in time to listing” after conducting a propensity-matched analysis.
However, while speeding up time to referral and listing, “the median time to transplant was not significantly different between the two groups on unadjusted (218 vs. 244 days; P = .084) or adjusted analysis (325 vs. 409 days; P = .08),” they added.
Additionally, “there was no difference in pretransplant mortality between [those] evaluated by telehealth or usual care in unadjusted analysis,” Dr. John and colleagues observed, noting that 169 of 465 patients (51 on the waiting list for a transplant and 118 who were not listed) who were referred died without receiving a liver transplant.
Researchers suggested that while evaluation times may have been shorter with the use of telehealth, they did not translate to shorter transplantation times “likely because the latter is a complex metric that is driven primarily by organ availability.”
Dr. John and colleagues cautioned that the centralized nature of the VA medical system could make the results of this study not generalizable across private care settings, particularly when care needs to cross state lines, which does not present an issue within the VA medical system.
That being said, the “ability to successfully evaluate and list patients via telehealth and obtain the same outcomes in terms of time to transplant and pretransplant mortality is significant because of the numerous advantages that telehealth offers to improve overall access to transplantation,” they stated, adding that more studies are needed, both in and out of the VA system, “to confirm that telehealth is a safe and effective way to expand access for patients undergoing evaluation for liver transplantation.”
Lead author Dr. Binu John serves on medical advisory boards for Gilead and Eisai and received research funding from a number of pharmaceutical manufacturers. No conflicts of interest were reported by the other authors.
SOURCE: John BV et al. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2019.12.021.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Myeloma patients over age 70 can benefit from auto-HC transplant
ORLANDO – Age 70 may be the new 60, at least when it comes to outcomes following autologous hematopoietic cell transplantation (auto-HCT) in multiple myeloma.
A large-scale study looking at transplant outcomes across age groups in multiple myeloma patients found similar rates of nonrelapse mortality, relapse/progression, progression-free survival, and overall survival between patients who were aged 70 years and older and those who were aged 60-69 years.
“Age has no implication in terms of the antimyeloma effect of transplant,” Anita D’Souza, MD, of the Medical College of Wisconsin, Milwaukee, said at the annual meeting of the American Society of Hematology.
The study analyzed outcomes from 15,999 multiple myeloma patients aged 20 years or older in the United States who received a single auto-HCT with melphalan conditioning within 12 months of diagnosis between 2013 and 2017. Within that dataset, the researchers compared outcomes from 7,032 patients aged 60-69 years and 2,092 patients aged 70 years and older.
This is the largest study of auto-HCT in older adults with multiple myeloma, the researchers said, and provides important data about the benefit of transplant at any age.
Univariate analysis showed that 100-day nonrelapse mortality was higher in patients aged 70 years and older – at 1% – compared with younger patients (P less than .01). Also, 2-year overall survival was lower in older adults – at 86% – compared with 60- to 69-year-olds (P less than .01).
However, on multivariate analysis with 60- to 69-year-olds as the reference group, patients older than age 70 years had similar nonrelapse mortality (hazard ratio [HR] 1.3, 95% confidence interval [CI] 1, 1.7, P = .06). The same trends were seen for relapse/progression (HR 1.0, 95% CI, 0.9-1, P = .6), progression-free survival (HR 1.1, 95% CI 1-1.2, P = .2), and overall survival (HR 1.2, 95% CI 1-1.4, P = .03). Given the large sample size, a P value of .01 was considered statistically significant.
Over the course of the study period, the percentage of patients aged 70 and older who received a transplant grew each year, rising to 28% by 2017. But Dr. D’Souza said that number is still too low given the safety and efficacy of auto-HCT in these patients.
“Every patient with myeloma should be referred to a transplant center,” she said.
Dr. D’Souza reported financial disclosures related to EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio, Prothena, Pfizer, Imbrium, and Akcea. Other study authors reported financial relationships with multiple companies including Celgene, Takeda, BMS, and Janssen.
SOURCE: Munshi PN et al. ASH 2019, Abstract 782.
ORLANDO – Age 70 may be the new 60, at least when it comes to outcomes following autologous hematopoietic cell transplantation (auto-HCT) in multiple myeloma.
A large-scale study looking at transplant outcomes across age groups in multiple myeloma patients found similar rates of nonrelapse mortality, relapse/progression, progression-free survival, and overall survival between patients who were aged 70 years and older and those who were aged 60-69 years.
“Age has no implication in terms of the antimyeloma effect of transplant,” Anita D’Souza, MD, of the Medical College of Wisconsin, Milwaukee, said at the annual meeting of the American Society of Hematology.
The study analyzed outcomes from 15,999 multiple myeloma patients aged 20 years or older in the United States who received a single auto-HCT with melphalan conditioning within 12 months of diagnosis between 2013 and 2017. Within that dataset, the researchers compared outcomes from 7,032 patients aged 60-69 years and 2,092 patients aged 70 years and older.
This is the largest study of auto-HCT in older adults with multiple myeloma, the researchers said, and provides important data about the benefit of transplant at any age.
Univariate analysis showed that 100-day nonrelapse mortality was higher in patients aged 70 years and older – at 1% – compared with younger patients (P less than .01). Also, 2-year overall survival was lower in older adults – at 86% – compared with 60- to 69-year-olds (P less than .01).
However, on multivariate analysis with 60- to 69-year-olds as the reference group, patients older than age 70 years had similar nonrelapse mortality (hazard ratio [HR] 1.3, 95% confidence interval [CI] 1, 1.7, P = .06). The same trends were seen for relapse/progression (HR 1.0, 95% CI, 0.9-1, P = .6), progression-free survival (HR 1.1, 95% CI 1-1.2, P = .2), and overall survival (HR 1.2, 95% CI 1-1.4, P = .03). Given the large sample size, a P value of .01 was considered statistically significant.
Over the course of the study period, the percentage of patients aged 70 and older who received a transplant grew each year, rising to 28% by 2017. But Dr. D’Souza said that number is still too low given the safety and efficacy of auto-HCT in these patients.
“Every patient with myeloma should be referred to a transplant center,” she said.
Dr. D’Souza reported financial disclosures related to EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio, Prothena, Pfizer, Imbrium, and Akcea. Other study authors reported financial relationships with multiple companies including Celgene, Takeda, BMS, and Janssen.
SOURCE: Munshi PN et al. ASH 2019, Abstract 782.
ORLANDO – Age 70 may be the new 60, at least when it comes to outcomes following autologous hematopoietic cell transplantation (auto-HCT) in multiple myeloma.
A large-scale study looking at transplant outcomes across age groups in multiple myeloma patients found similar rates of nonrelapse mortality, relapse/progression, progression-free survival, and overall survival between patients who were aged 70 years and older and those who were aged 60-69 years.
“Age has no implication in terms of the antimyeloma effect of transplant,” Anita D’Souza, MD, of the Medical College of Wisconsin, Milwaukee, said at the annual meeting of the American Society of Hematology.
The study analyzed outcomes from 15,999 multiple myeloma patients aged 20 years or older in the United States who received a single auto-HCT with melphalan conditioning within 12 months of diagnosis between 2013 and 2017. Within that dataset, the researchers compared outcomes from 7,032 patients aged 60-69 years and 2,092 patients aged 70 years and older.
This is the largest study of auto-HCT in older adults with multiple myeloma, the researchers said, and provides important data about the benefit of transplant at any age.
Univariate analysis showed that 100-day nonrelapse mortality was higher in patients aged 70 years and older – at 1% – compared with younger patients (P less than .01). Also, 2-year overall survival was lower in older adults – at 86% – compared with 60- to 69-year-olds (P less than .01).
However, on multivariate analysis with 60- to 69-year-olds as the reference group, patients older than age 70 years had similar nonrelapse mortality (hazard ratio [HR] 1.3, 95% confidence interval [CI] 1, 1.7, P = .06). The same trends were seen for relapse/progression (HR 1.0, 95% CI, 0.9-1, P = .6), progression-free survival (HR 1.1, 95% CI 1-1.2, P = .2), and overall survival (HR 1.2, 95% CI 1-1.4, P = .03). Given the large sample size, a P value of .01 was considered statistically significant.
Over the course of the study period, the percentage of patients aged 70 and older who received a transplant grew each year, rising to 28% by 2017. But Dr. D’Souza said that number is still too low given the safety and efficacy of auto-HCT in these patients.
“Every patient with myeloma should be referred to a transplant center,” she said.
Dr. D’Souza reported financial disclosures related to EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio, Prothena, Pfizer, Imbrium, and Akcea. Other study authors reported financial relationships with multiple companies including Celgene, Takeda, BMS, and Janssen.
SOURCE: Munshi PN et al. ASH 2019, Abstract 782.
REPORTING FROM ASH 2019
Autoimmune liver disease: Karnofsky score predicts transplant survival
BOSTON – The Karnofsky Performance Status is predictive of 5-year survival among patients with autoimmune-related liver disease who undergo a transplant, based on a retrospective look at more than 6,500 patients.
The analysis also showed that African American patients had a 33% higher mortality risk than non-Hispanic white patients, reported lead author Artin Galoosian, MD, of California Pacific Medical Center in San Francisco, who presented findings at the annual meeting of the American Association for the Study of Liver Diseases.
According to Dr. Galoosian, previous research has shown that Karnofsky scores are a quick and reliable means of predicting survival with liver transplant, but minimal research has evaluated this clinical tool specifically for patients with autoimmune-related liver diseases, which prompted the present study.
Drawing data from the United Network for Organ Sharing (UNOS; 2004-2017), the investigators evaluated performance status and survival in 6,628 patients who underwent liver transplant for one of three diseases: autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), or primary biliary cholangitis (PBC). Karnofsky scores were divided into quartiles 1 through 4, from best to worst functional status. The investigators used Kaplan-Meier methods and multivariate Cox proportional hazard ratios to determine relationships between disease etiology, Karnofsky score, and survival; in addition, they evaluated the impact of demographic factors on outcomes.
The population was predominantly non-Hispanic white (73.0%) with smaller proportions of African American (13.4%) and Hispanic patients (11.5%). Of the three diseases, PBC was most common (38.2%), followed by PSC (32.1%), then AIH (29.7%).
Across all etiologies, Karnofsky status was significantly associated with survival; a score of 4 came with a 90% increased risk of posttransplant death, compared with a score of 1. Patients with AIH were most likely to have poor pretransplant functional status, as 39.1% of these patients had a Karnofsky score of 4, compared with 31.9% of patients with PSC and 29.0% of patients with PBC. AIH was also associated with a significantly higher risk of posttransplant death; relative risks for PSC and PBC were 20% and 17% lower, respectively.
Five years after surgery, 84.9% of AIH patients with a Karnofsky score of 1 were alive, compared with 76.1% of patients who had a score of 4. A similar association with functional status was found for PSC (84.9% vs. 75.4%), while PBC had a narrower survival margin (88.7% vs. 86.9%).
Analysis also revealed a wide survival gap between patients of different ethnic backgrounds. Compared with white patients, African American patients had a 33% higher risk of dying on the wait list or after transplant.
“[This gap] could reflect a multitude of issues, one being delayed referral to a hepatologist and being listed for transplant much later, so [patients] tend to be more sick,” Dr. Galoosian said.
He also offered some insight into clinical relevance.
“A broader implication of this research could be in the primary care setting,” Dr. Galoosian said. “[Clinicians need to be] aware that someone’s functional status has a broader impact on their health and be aware that ethnic minorities need to be more vigilantly up to date on their health care maintenance and more vigilantly connected to social workers if needed, in terms of getting the resources that they need to help break the [chain] of worse outcomes.”
The investigators disclosed relationships with Gilead, Salix, and AbbVie.
SOURCE: Galoosian A et al. The Liver Meeting 2019. Abstract 1102.
BOSTON – The Karnofsky Performance Status is predictive of 5-year survival among patients with autoimmune-related liver disease who undergo a transplant, based on a retrospective look at more than 6,500 patients.
The analysis also showed that African American patients had a 33% higher mortality risk than non-Hispanic white patients, reported lead author Artin Galoosian, MD, of California Pacific Medical Center in San Francisco, who presented findings at the annual meeting of the American Association for the Study of Liver Diseases.
According to Dr. Galoosian, previous research has shown that Karnofsky scores are a quick and reliable means of predicting survival with liver transplant, but minimal research has evaluated this clinical tool specifically for patients with autoimmune-related liver diseases, which prompted the present study.
Drawing data from the United Network for Organ Sharing (UNOS; 2004-2017), the investigators evaluated performance status and survival in 6,628 patients who underwent liver transplant for one of three diseases: autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), or primary biliary cholangitis (PBC). Karnofsky scores were divided into quartiles 1 through 4, from best to worst functional status. The investigators used Kaplan-Meier methods and multivariate Cox proportional hazard ratios to determine relationships between disease etiology, Karnofsky score, and survival; in addition, they evaluated the impact of demographic factors on outcomes.
The population was predominantly non-Hispanic white (73.0%) with smaller proportions of African American (13.4%) and Hispanic patients (11.5%). Of the three diseases, PBC was most common (38.2%), followed by PSC (32.1%), then AIH (29.7%).
Across all etiologies, Karnofsky status was significantly associated with survival; a score of 4 came with a 90% increased risk of posttransplant death, compared with a score of 1. Patients with AIH were most likely to have poor pretransplant functional status, as 39.1% of these patients had a Karnofsky score of 4, compared with 31.9% of patients with PSC and 29.0% of patients with PBC. AIH was also associated with a significantly higher risk of posttransplant death; relative risks for PSC and PBC were 20% and 17% lower, respectively.
Five years after surgery, 84.9% of AIH patients with a Karnofsky score of 1 were alive, compared with 76.1% of patients who had a score of 4. A similar association with functional status was found for PSC (84.9% vs. 75.4%), while PBC had a narrower survival margin (88.7% vs. 86.9%).
Analysis also revealed a wide survival gap between patients of different ethnic backgrounds. Compared with white patients, African American patients had a 33% higher risk of dying on the wait list or after transplant.
“[This gap] could reflect a multitude of issues, one being delayed referral to a hepatologist and being listed for transplant much later, so [patients] tend to be more sick,” Dr. Galoosian said.
He also offered some insight into clinical relevance.
“A broader implication of this research could be in the primary care setting,” Dr. Galoosian said. “[Clinicians need to be] aware that someone’s functional status has a broader impact on their health and be aware that ethnic minorities need to be more vigilantly up to date on their health care maintenance and more vigilantly connected to social workers if needed, in terms of getting the resources that they need to help break the [chain] of worse outcomes.”
The investigators disclosed relationships with Gilead, Salix, and AbbVie.
SOURCE: Galoosian A et al. The Liver Meeting 2019. Abstract 1102.
BOSTON – The Karnofsky Performance Status is predictive of 5-year survival among patients with autoimmune-related liver disease who undergo a transplant, based on a retrospective look at more than 6,500 patients.
The analysis also showed that African American patients had a 33% higher mortality risk than non-Hispanic white patients, reported lead author Artin Galoosian, MD, of California Pacific Medical Center in San Francisco, who presented findings at the annual meeting of the American Association for the Study of Liver Diseases.
According to Dr. Galoosian, previous research has shown that Karnofsky scores are a quick and reliable means of predicting survival with liver transplant, but minimal research has evaluated this clinical tool specifically for patients with autoimmune-related liver diseases, which prompted the present study.
Drawing data from the United Network for Organ Sharing (UNOS; 2004-2017), the investigators evaluated performance status and survival in 6,628 patients who underwent liver transplant for one of three diseases: autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), or primary biliary cholangitis (PBC). Karnofsky scores were divided into quartiles 1 through 4, from best to worst functional status. The investigators used Kaplan-Meier methods and multivariate Cox proportional hazard ratios to determine relationships between disease etiology, Karnofsky score, and survival; in addition, they evaluated the impact of demographic factors on outcomes.
The population was predominantly non-Hispanic white (73.0%) with smaller proportions of African American (13.4%) and Hispanic patients (11.5%). Of the three diseases, PBC was most common (38.2%), followed by PSC (32.1%), then AIH (29.7%).
Across all etiologies, Karnofsky status was significantly associated with survival; a score of 4 came with a 90% increased risk of posttransplant death, compared with a score of 1. Patients with AIH were most likely to have poor pretransplant functional status, as 39.1% of these patients had a Karnofsky score of 4, compared with 31.9% of patients with PSC and 29.0% of patients with PBC. AIH was also associated with a significantly higher risk of posttransplant death; relative risks for PSC and PBC were 20% and 17% lower, respectively.
Five years after surgery, 84.9% of AIH patients with a Karnofsky score of 1 were alive, compared with 76.1% of patients who had a score of 4. A similar association with functional status was found for PSC (84.9% vs. 75.4%), while PBC had a narrower survival margin (88.7% vs. 86.9%).
Analysis also revealed a wide survival gap between patients of different ethnic backgrounds. Compared with white patients, African American patients had a 33% higher risk of dying on the wait list or after transplant.
“[This gap] could reflect a multitude of issues, one being delayed referral to a hepatologist and being listed for transplant much later, so [patients] tend to be more sick,” Dr. Galoosian said.
He also offered some insight into clinical relevance.
“A broader implication of this research could be in the primary care setting,” Dr. Galoosian said. “[Clinicians need to be] aware that someone’s functional status has a broader impact on their health and be aware that ethnic minorities need to be more vigilantly up to date on their health care maintenance and more vigilantly connected to social workers if needed, in terms of getting the resources that they need to help break the [chain] of worse outcomes.”
The investigators disclosed relationships with Gilead, Salix, and AbbVie.
SOURCE: Galoosian A et al. The Liver Meeting 2019. Abstract 1102.
REPORTING FROM THE LIVER MEETING 2019