User login
Antibody shows promise in eosinophilic gastritis/duodenitis
In a phase 2 trial in eosinophilic gastritis and duodenitis, an anti–Siglec-8 antibody greatly reduced populations of eosinophilic cells, and also led to improved symptoms. The sponsoring company, Alkalos, is currently conducting a phase 3 trial in eosinophilic gastritis or duodenitis and a phase 2-3 trial in eosinophilic esophagitis.
The news is welcome to clinicians who treat these rare conditions, since the only current treatment option is steroids. This is particularly challenging because most patients with these conditions present in their 30s and 40s, according to Carol Semrad, MD, professor of medicine at the University of Chicago, who was asked to comment on the study. She noted that the study’s results were impressive, but it will take a phase 3 trial to convince. It’s also unclear if the clinical benefit tracks with the impressive reduction seen in eosinophil count. “There’s somewhat of a disconnect between symptom reduction and reduction of the eosinophil counts. Sometimes just blocking the inflammation [isn’t enough]. Maybe there is other damage to the bowel.”
Still, “it looks like they have a proof of concept that it’s very effective at blocking the eosinophils and mast cells that are thought to be causing eosinophilic gastritis and duodenitis in human disease. That, along with improvement in symptoms, is highly promising, given that we have no other treatment beside steroids,” said Dr. Semrad.
The research, led by Evan S. Dellon, MD, MPH, of the University of North Carolina at Chapel Hill, and Ikuo Hirano, MD, of Northwestern University, Chicago, appeared in the New England Journal of Medicine.
The antibody (lirentelimab) targets sialic acid–binding immunoglobulin-like lectin 8 (Siglec-8), which is an inhibitory receptor found on mature eosinophils and mast cells, and expressed at low levels on basophils. The antibody reduces eosinophil population through natural killer cell-mediated cellular cytotoxicity and apoptosis, and other antibodies against the same target have been shown to inhibit activation of mast cells.
At 22 sites across the United States, researchers randomized 65 adults with active, uncontrolled eosinophilic gastritis or eosinophilic duodenitis, or both, to receive four monthly low doses (0.3, 1, 1, and 1 mg/kg) or high-dose lirentelimab (0.3, 1, 3, and 3 mg/kg), or placebo. A total of 10 patients had gastritis, 25 had duodenitis, and 30 had both.
In the intention-to-treat analysis, there was a mean 86% reduction in eosinophil count in patients in the treatment groups, compared with a 9% increase in controls (P < .001). In the per-protocol analysis, there was a 95% reduction versus a 10% increase (P < .001). Of treated patients, 95% had a gastrointestinal eosinophil count of 6 or fewer per high-powered field, compared with 0% in the placebo group.
In the intention-to-treat analysis, 63% of treated patients experienced a treatment response, defined as at least a 30% reduction in total symptom score and at least a 75% reduction in eosinophil count. About 5% of patients had a response in the placebo group (P < .001). The mean percentage change in total symptom score was –48 versus –22 in the placebo group (P = .004). In the per-protocol analysis, 69% responded versus 5% (P < .001). The mean percentage change in total symptom score was –53 versus –24 (P = .001).
91% of patients in the treatment groups experienced at least one adverse event, compared with 82% in the placebo group. About 60% in the treatment group had an infusion-related reaction versus 23% who received placebo; 93% of reactions were mild to moderate. Serious adverse events occurred in 9% of the treatment group and 14% of patients on placebo. The only serious adverse event thought to be related to the drug was a single grade 4 infusion-related event in the high-dose treatment group, which led to discontinuation of the drug and withdrawal from the trial. 86% of patients in the treatment group experienced transient lymphopenia, as did 47% of the placebo group, but there were no clinical consequences.
One potential concern with the therapy is the effect that long-term suppression of eosinophils and mast cells, and to a lesser extent basophils, could have on the immune system. Eosinophils are key to defenses against parasites, so that will need to be looked at in further studies, according to Dr. Semrad: “How is blocking those inflammatory cells going to impact gut function and the ability to fight off certain organisms? That’s going to be one of the real questions.”
The study was funded by Alkalos. Dr. Semrad has no relevant financial disclosures.
SOURCE: Dellon ES et al. N Engl J Med. 2020 Oct 22. doi: 10.1056/NEJMoa2012047.
In a phase 2 trial in eosinophilic gastritis and duodenitis, an anti–Siglec-8 antibody greatly reduced populations of eosinophilic cells, and also led to improved symptoms. The sponsoring company, Alkalos, is currently conducting a phase 3 trial in eosinophilic gastritis or duodenitis and a phase 2-3 trial in eosinophilic esophagitis.
The news is welcome to clinicians who treat these rare conditions, since the only current treatment option is steroids. This is particularly challenging because most patients with these conditions present in their 30s and 40s, according to Carol Semrad, MD, professor of medicine at the University of Chicago, who was asked to comment on the study. She noted that the study’s results were impressive, but it will take a phase 3 trial to convince. It’s also unclear if the clinical benefit tracks with the impressive reduction seen in eosinophil count. “There’s somewhat of a disconnect between symptom reduction and reduction of the eosinophil counts. Sometimes just blocking the inflammation [isn’t enough]. Maybe there is other damage to the bowel.”
Still, “it looks like they have a proof of concept that it’s very effective at blocking the eosinophils and mast cells that are thought to be causing eosinophilic gastritis and duodenitis in human disease. That, along with improvement in symptoms, is highly promising, given that we have no other treatment beside steroids,” said Dr. Semrad.
The research, led by Evan S. Dellon, MD, MPH, of the University of North Carolina at Chapel Hill, and Ikuo Hirano, MD, of Northwestern University, Chicago, appeared in the New England Journal of Medicine.
The antibody (lirentelimab) targets sialic acid–binding immunoglobulin-like lectin 8 (Siglec-8), which is an inhibitory receptor found on mature eosinophils and mast cells, and expressed at low levels on basophils. The antibody reduces eosinophil population through natural killer cell-mediated cellular cytotoxicity and apoptosis, and other antibodies against the same target have been shown to inhibit activation of mast cells.
At 22 sites across the United States, researchers randomized 65 adults with active, uncontrolled eosinophilic gastritis or eosinophilic duodenitis, or both, to receive four monthly low doses (0.3, 1, 1, and 1 mg/kg) or high-dose lirentelimab (0.3, 1, 3, and 3 mg/kg), or placebo. A total of 10 patients had gastritis, 25 had duodenitis, and 30 had both.
In the intention-to-treat analysis, there was a mean 86% reduction in eosinophil count in patients in the treatment groups, compared with a 9% increase in controls (P < .001). In the per-protocol analysis, there was a 95% reduction versus a 10% increase (P < .001). Of treated patients, 95% had a gastrointestinal eosinophil count of 6 or fewer per high-powered field, compared with 0% in the placebo group.
In the intention-to-treat analysis, 63% of treated patients experienced a treatment response, defined as at least a 30% reduction in total symptom score and at least a 75% reduction in eosinophil count. About 5% of patients had a response in the placebo group (P < .001). The mean percentage change in total symptom score was –48 versus –22 in the placebo group (P = .004). In the per-protocol analysis, 69% responded versus 5% (P < .001). The mean percentage change in total symptom score was –53 versus –24 (P = .001).
91% of patients in the treatment groups experienced at least one adverse event, compared with 82% in the placebo group. About 60% in the treatment group had an infusion-related reaction versus 23% who received placebo; 93% of reactions were mild to moderate. Serious adverse events occurred in 9% of the treatment group and 14% of patients on placebo. The only serious adverse event thought to be related to the drug was a single grade 4 infusion-related event in the high-dose treatment group, which led to discontinuation of the drug and withdrawal from the trial. 86% of patients in the treatment group experienced transient lymphopenia, as did 47% of the placebo group, but there were no clinical consequences.
One potential concern with the therapy is the effect that long-term suppression of eosinophils and mast cells, and to a lesser extent basophils, could have on the immune system. Eosinophils are key to defenses against parasites, so that will need to be looked at in further studies, according to Dr. Semrad: “How is blocking those inflammatory cells going to impact gut function and the ability to fight off certain organisms? That’s going to be one of the real questions.”
The study was funded by Alkalos. Dr. Semrad has no relevant financial disclosures.
SOURCE: Dellon ES et al. N Engl J Med. 2020 Oct 22. doi: 10.1056/NEJMoa2012047.
In a phase 2 trial in eosinophilic gastritis and duodenitis, an anti–Siglec-8 antibody greatly reduced populations of eosinophilic cells, and also led to improved symptoms. The sponsoring company, Alkalos, is currently conducting a phase 3 trial in eosinophilic gastritis or duodenitis and a phase 2-3 trial in eosinophilic esophagitis.
The news is welcome to clinicians who treat these rare conditions, since the only current treatment option is steroids. This is particularly challenging because most patients with these conditions present in their 30s and 40s, according to Carol Semrad, MD, professor of medicine at the University of Chicago, who was asked to comment on the study. She noted that the study’s results were impressive, but it will take a phase 3 trial to convince. It’s also unclear if the clinical benefit tracks with the impressive reduction seen in eosinophil count. “There’s somewhat of a disconnect between symptom reduction and reduction of the eosinophil counts. Sometimes just blocking the inflammation [isn’t enough]. Maybe there is other damage to the bowel.”
Still, “it looks like they have a proof of concept that it’s very effective at blocking the eosinophils and mast cells that are thought to be causing eosinophilic gastritis and duodenitis in human disease. That, along with improvement in symptoms, is highly promising, given that we have no other treatment beside steroids,” said Dr. Semrad.
The research, led by Evan S. Dellon, MD, MPH, of the University of North Carolina at Chapel Hill, and Ikuo Hirano, MD, of Northwestern University, Chicago, appeared in the New England Journal of Medicine.
The antibody (lirentelimab) targets sialic acid–binding immunoglobulin-like lectin 8 (Siglec-8), which is an inhibitory receptor found on mature eosinophils and mast cells, and expressed at low levels on basophils. The antibody reduces eosinophil population through natural killer cell-mediated cellular cytotoxicity and apoptosis, and other antibodies against the same target have been shown to inhibit activation of mast cells.
At 22 sites across the United States, researchers randomized 65 adults with active, uncontrolled eosinophilic gastritis or eosinophilic duodenitis, or both, to receive four monthly low doses (0.3, 1, 1, and 1 mg/kg) or high-dose lirentelimab (0.3, 1, 3, and 3 mg/kg), or placebo. A total of 10 patients had gastritis, 25 had duodenitis, and 30 had both.
In the intention-to-treat analysis, there was a mean 86% reduction in eosinophil count in patients in the treatment groups, compared with a 9% increase in controls (P < .001). In the per-protocol analysis, there was a 95% reduction versus a 10% increase (P < .001). Of treated patients, 95% had a gastrointestinal eosinophil count of 6 or fewer per high-powered field, compared with 0% in the placebo group.
In the intention-to-treat analysis, 63% of treated patients experienced a treatment response, defined as at least a 30% reduction in total symptom score and at least a 75% reduction in eosinophil count. About 5% of patients had a response in the placebo group (P < .001). The mean percentage change in total symptom score was –48 versus –22 in the placebo group (P = .004). In the per-protocol analysis, 69% responded versus 5% (P < .001). The mean percentage change in total symptom score was –53 versus –24 (P = .001).
91% of patients in the treatment groups experienced at least one adverse event, compared with 82% in the placebo group. About 60% in the treatment group had an infusion-related reaction versus 23% who received placebo; 93% of reactions were mild to moderate. Serious adverse events occurred in 9% of the treatment group and 14% of patients on placebo. The only serious adverse event thought to be related to the drug was a single grade 4 infusion-related event in the high-dose treatment group, which led to discontinuation of the drug and withdrawal from the trial. 86% of patients in the treatment group experienced transient lymphopenia, as did 47% of the placebo group, but there were no clinical consequences.
One potential concern with the therapy is the effect that long-term suppression of eosinophils and mast cells, and to a lesser extent basophils, could have on the immune system. Eosinophils are key to defenses against parasites, so that will need to be looked at in further studies, according to Dr. Semrad: “How is blocking those inflammatory cells going to impact gut function and the ability to fight off certain organisms? That’s going to be one of the real questions.”
The study was funded by Alkalos. Dr. Semrad has no relevant financial disclosures.
SOURCE: Dellon ES et al. N Engl J Med. 2020 Oct 22. doi: 10.1056/NEJMoa2012047.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Budesonide orodispersible tablets maintained remissions in EoE
Budesonide orodispersible tablets maintained remissions of eosinophilic esophagitis for 48 weeks in approximately 75% of patients and did not increase the risk for most adverse events, compared with placebo, according to the findings of a multicenter, randomized, double-blind trial.
While prior studies have shown that swallowed topical corticosteroids such as budesonide or fluticasone induce remission in EoE, this is the first multicenter phase 3 study of budesonide orodispersible tablets (BOTs) for maintaining remissions over the long term, wrote Alex Straumann, MD, of the Swiss EoE Research Group and University Hospital Zurich, and associates in Gastroenterology.
Eosinophilic esophagitis is the most common cause of esophageal dysphagia and food impaction. Swallowed topical corticosteroids improve symptoms and inflammation, but the off-label use of formulations developed for airway administration in asthma shows “suboptimal esophageal targeting and efficacy,” the researchers wrote. In the phase 3 EOS-2 trial, twice-daily treatment with 1.0 mg BOTs had induced clinicohistologic remissions in 58% of adults with EoE at 6 weeks and in 85% at 12 weeks.
To study long-term maintenance BOT therapy, the researchers randomly assigned 204 of the remitted patients to 48 weeks of twice-daily BOT 0.5 mg, BOT 1.0 mg, or placebo. There were 68 patients per group. A total of 141 patients completed this double-blind phase, but all 204 were evaluable for the primary analysis. The primary outcome was remission at week 48, defined as freedom from relapse (dysphagia or odynophagia rated as 4 or higher on a 10-point numeric rating scale), histologic relapse (≥48 eosinophils per mm2 high-power field), food impaction requiring endoscopic intervention, and dilation.
After 48 weeks, 51 patients in the 1-mg group (75%) and 50 patients in the 0.5-mg group (73.5%) remained in remission, compared with only three patients in the placebo group (4.4%; both P less than .0001). Patients in the placebo group relapsed after a median of 87 days off BOTs. Overall, BOT therapy was similarly efficacious regardless of factors such as history of allergic diseases, location of inflammation at the start of induction, or concomitant use of proton pump inhibitors. However, patients with inflammation of all three esophageal segments achieved “clinically relevant” greater rates of remission with twice-daily 1.0-mg BOT, compared with twice-daily 0.5-mg BOT (80% vs. 68%). In secondary analyses, rates of histologic relapse were 13.2% with 0.5-mg BOT twice daily, 10.3% with 1.0-mg BOT twice daily, and 90% with placebo, and rates of clinical relapse were 10.3%, 7.4%, and 60.3%, respectively. “Histological remission in the BOT 0.5 and 1.0mg twice daily group was independently maintained in all esophageal segments,” the researchers reported.
Rates of most adverse events were similar across treatment groups, and no serious treatment-emergent adverse events were reported. Average morning serum cortisol levels were similar among groups and did not change after treatment ended, but four patients on BOT therapy developed asymptomatic subnormal levels of morning cortisol. “Clinically manifested candidiasis was suspected in 16.2% of patients in the BOT 0.5mg group and in 11.8% of patients in the BOT 1.0mg group; all infections resolved with treatment,” the researchers wrote.
The study and editorial support were funded by Dr. Falk Pharma GmbH, a pharmaceutical company in Germany. Dr. Falk Pharma was involved in the study design and data collection, analysis, and interpretation. Dr. Straumann disclosed fees from several pharmaceutical companies, including Dr. Falk Pharma and AstraZeneca, which makes budesonide. Several other coinvestigators also disclosed ties to Dr. Falk Pharma, AstraZeneca, and other pharmaceutical companies.
SOURCE: Straumann A et al. Gastroenterology. 2020 July 25. doi: 10.1053/j.gastro.2020.07.039.
Eosinophilic esophagitis (EoE) continues to rise in prevalence and prescription steroid therapy is limited to off-label use leading to a call for action for directed therapy for EoE and understanding long-term remission rates. In this phase 3 study, Straumann and colleagues studied budesonide orodispersible tablets (BOTs) and their ability to maintain remission, compared with placebo, at two doses specifically designed for EoE in adults with proton pump inhibitor–refractory EoE. Regardless of dose, at either 1.0 mg twice a day or 0.5 mg twice a day, there was an improvement in maintaining remission (73.5% for low dose and 75% for high dose, compared with 4.4% with placebo) at 48 weeks of therapy. Common side effects studied include an increase in candidiasis (12%-16% of patients), but there was no statistical change in morning cortisol.
Given the need for maintenance therapy for EoE, this study proves long-term efficacy and safety for the treatment of this chronic condition with a targeted esophageal formulation. We now have evidence of maintaining remission for EoE with a safe side-effect profile. Future research will be needed to look at long-term steroid use on bone health and immune dysregulation, especially in the pediatric population, which was not studied in this cohort. Moreover, future studies are needed to determine a minimally effective dose to help prevent potential side effects that can maintain remission while allowing discontinuation of all stable proton pump inhibitor doses to ensure no confounding effect.
Rishi D. Naik, MD, MSCI, is an assistant professor, department of medicine, section of gastroenterology & hepatology, Esophageal Center at Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
Eosinophilic esophagitis (EoE) continues to rise in prevalence and prescription steroid therapy is limited to off-label use leading to a call for action for directed therapy for EoE and understanding long-term remission rates. In this phase 3 study, Straumann and colleagues studied budesonide orodispersible tablets (BOTs) and their ability to maintain remission, compared with placebo, at two doses specifically designed for EoE in adults with proton pump inhibitor–refractory EoE. Regardless of dose, at either 1.0 mg twice a day or 0.5 mg twice a day, there was an improvement in maintaining remission (73.5% for low dose and 75% for high dose, compared with 4.4% with placebo) at 48 weeks of therapy. Common side effects studied include an increase in candidiasis (12%-16% of patients), but there was no statistical change in morning cortisol.
Given the need for maintenance therapy for EoE, this study proves long-term efficacy and safety for the treatment of this chronic condition with a targeted esophageal formulation. We now have evidence of maintaining remission for EoE with a safe side-effect profile. Future research will be needed to look at long-term steroid use on bone health and immune dysregulation, especially in the pediatric population, which was not studied in this cohort. Moreover, future studies are needed to determine a minimally effective dose to help prevent potential side effects that can maintain remission while allowing discontinuation of all stable proton pump inhibitor doses to ensure no confounding effect.
Rishi D. Naik, MD, MSCI, is an assistant professor, department of medicine, section of gastroenterology & hepatology, Esophageal Center at Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
Eosinophilic esophagitis (EoE) continues to rise in prevalence and prescription steroid therapy is limited to off-label use leading to a call for action for directed therapy for EoE and understanding long-term remission rates. In this phase 3 study, Straumann and colleagues studied budesonide orodispersible tablets (BOTs) and their ability to maintain remission, compared with placebo, at two doses specifically designed for EoE in adults with proton pump inhibitor–refractory EoE. Regardless of dose, at either 1.0 mg twice a day or 0.5 mg twice a day, there was an improvement in maintaining remission (73.5% for low dose and 75% for high dose, compared with 4.4% with placebo) at 48 weeks of therapy. Common side effects studied include an increase in candidiasis (12%-16% of patients), but there was no statistical change in morning cortisol.
Given the need for maintenance therapy for EoE, this study proves long-term efficacy and safety for the treatment of this chronic condition with a targeted esophageal formulation. We now have evidence of maintaining remission for EoE with a safe side-effect profile. Future research will be needed to look at long-term steroid use on bone health and immune dysregulation, especially in the pediatric population, which was not studied in this cohort. Moreover, future studies are needed to determine a minimally effective dose to help prevent potential side effects that can maintain remission while allowing discontinuation of all stable proton pump inhibitor doses to ensure no confounding effect.
Rishi D. Naik, MD, MSCI, is an assistant professor, department of medicine, section of gastroenterology & hepatology, Esophageal Center at Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
Budesonide orodispersible tablets maintained remissions of eosinophilic esophagitis for 48 weeks in approximately 75% of patients and did not increase the risk for most adverse events, compared with placebo, according to the findings of a multicenter, randomized, double-blind trial.
While prior studies have shown that swallowed topical corticosteroids such as budesonide or fluticasone induce remission in EoE, this is the first multicenter phase 3 study of budesonide orodispersible tablets (BOTs) for maintaining remissions over the long term, wrote Alex Straumann, MD, of the Swiss EoE Research Group and University Hospital Zurich, and associates in Gastroenterology.
Eosinophilic esophagitis is the most common cause of esophageal dysphagia and food impaction. Swallowed topical corticosteroids improve symptoms and inflammation, but the off-label use of formulations developed for airway administration in asthma shows “suboptimal esophageal targeting and efficacy,” the researchers wrote. In the phase 3 EOS-2 trial, twice-daily treatment with 1.0 mg BOTs had induced clinicohistologic remissions in 58% of adults with EoE at 6 weeks and in 85% at 12 weeks.
To study long-term maintenance BOT therapy, the researchers randomly assigned 204 of the remitted patients to 48 weeks of twice-daily BOT 0.5 mg, BOT 1.0 mg, or placebo. There were 68 patients per group. A total of 141 patients completed this double-blind phase, but all 204 were evaluable for the primary analysis. The primary outcome was remission at week 48, defined as freedom from relapse (dysphagia or odynophagia rated as 4 or higher on a 10-point numeric rating scale), histologic relapse (≥48 eosinophils per mm2 high-power field), food impaction requiring endoscopic intervention, and dilation.
After 48 weeks, 51 patients in the 1-mg group (75%) and 50 patients in the 0.5-mg group (73.5%) remained in remission, compared with only three patients in the placebo group (4.4%; both P less than .0001). Patients in the placebo group relapsed after a median of 87 days off BOTs. Overall, BOT therapy was similarly efficacious regardless of factors such as history of allergic diseases, location of inflammation at the start of induction, or concomitant use of proton pump inhibitors. However, patients with inflammation of all three esophageal segments achieved “clinically relevant” greater rates of remission with twice-daily 1.0-mg BOT, compared with twice-daily 0.5-mg BOT (80% vs. 68%). In secondary analyses, rates of histologic relapse were 13.2% with 0.5-mg BOT twice daily, 10.3% with 1.0-mg BOT twice daily, and 90% with placebo, and rates of clinical relapse were 10.3%, 7.4%, and 60.3%, respectively. “Histological remission in the BOT 0.5 and 1.0mg twice daily group was independently maintained in all esophageal segments,” the researchers reported.
Rates of most adverse events were similar across treatment groups, and no serious treatment-emergent adverse events were reported. Average morning serum cortisol levels were similar among groups and did not change after treatment ended, but four patients on BOT therapy developed asymptomatic subnormal levels of morning cortisol. “Clinically manifested candidiasis was suspected in 16.2% of patients in the BOT 0.5mg group and in 11.8% of patients in the BOT 1.0mg group; all infections resolved with treatment,” the researchers wrote.
The study and editorial support were funded by Dr. Falk Pharma GmbH, a pharmaceutical company in Germany. Dr. Falk Pharma was involved in the study design and data collection, analysis, and interpretation. Dr. Straumann disclosed fees from several pharmaceutical companies, including Dr. Falk Pharma and AstraZeneca, which makes budesonide. Several other coinvestigators also disclosed ties to Dr. Falk Pharma, AstraZeneca, and other pharmaceutical companies.
SOURCE: Straumann A et al. Gastroenterology. 2020 July 25. doi: 10.1053/j.gastro.2020.07.039.
Budesonide orodispersible tablets maintained remissions of eosinophilic esophagitis for 48 weeks in approximately 75% of patients and did not increase the risk for most adverse events, compared with placebo, according to the findings of a multicenter, randomized, double-blind trial.
While prior studies have shown that swallowed topical corticosteroids such as budesonide or fluticasone induce remission in EoE, this is the first multicenter phase 3 study of budesonide orodispersible tablets (BOTs) for maintaining remissions over the long term, wrote Alex Straumann, MD, of the Swiss EoE Research Group and University Hospital Zurich, and associates in Gastroenterology.
Eosinophilic esophagitis is the most common cause of esophageal dysphagia and food impaction. Swallowed topical corticosteroids improve symptoms and inflammation, but the off-label use of formulations developed for airway administration in asthma shows “suboptimal esophageal targeting and efficacy,” the researchers wrote. In the phase 3 EOS-2 trial, twice-daily treatment with 1.0 mg BOTs had induced clinicohistologic remissions in 58% of adults with EoE at 6 weeks and in 85% at 12 weeks.
To study long-term maintenance BOT therapy, the researchers randomly assigned 204 of the remitted patients to 48 weeks of twice-daily BOT 0.5 mg, BOT 1.0 mg, or placebo. There were 68 patients per group. A total of 141 patients completed this double-blind phase, but all 204 were evaluable for the primary analysis. The primary outcome was remission at week 48, defined as freedom from relapse (dysphagia or odynophagia rated as 4 or higher on a 10-point numeric rating scale), histologic relapse (≥48 eosinophils per mm2 high-power field), food impaction requiring endoscopic intervention, and dilation.
After 48 weeks, 51 patients in the 1-mg group (75%) and 50 patients in the 0.5-mg group (73.5%) remained in remission, compared with only three patients in the placebo group (4.4%; both P less than .0001). Patients in the placebo group relapsed after a median of 87 days off BOTs. Overall, BOT therapy was similarly efficacious regardless of factors such as history of allergic diseases, location of inflammation at the start of induction, or concomitant use of proton pump inhibitors. However, patients with inflammation of all three esophageal segments achieved “clinically relevant” greater rates of remission with twice-daily 1.0-mg BOT, compared with twice-daily 0.5-mg BOT (80% vs. 68%). In secondary analyses, rates of histologic relapse were 13.2% with 0.5-mg BOT twice daily, 10.3% with 1.0-mg BOT twice daily, and 90% with placebo, and rates of clinical relapse were 10.3%, 7.4%, and 60.3%, respectively. “Histological remission in the BOT 0.5 and 1.0mg twice daily group was independently maintained in all esophageal segments,” the researchers reported.
Rates of most adverse events were similar across treatment groups, and no serious treatment-emergent adverse events were reported. Average morning serum cortisol levels were similar among groups and did not change after treatment ended, but four patients on BOT therapy developed asymptomatic subnormal levels of morning cortisol. “Clinically manifested candidiasis was suspected in 16.2% of patients in the BOT 0.5mg group and in 11.8% of patients in the BOT 1.0mg group; all infections resolved with treatment,” the researchers wrote.
The study and editorial support were funded by Dr. Falk Pharma GmbH, a pharmaceutical company in Germany. Dr. Falk Pharma was involved in the study design and data collection, analysis, and interpretation. Dr. Straumann disclosed fees from several pharmaceutical companies, including Dr. Falk Pharma and AstraZeneca, which makes budesonide. Several other coinvestigators also disclosed ties to Dr. Falk Pharma, AstraZeneca, and other pharmaceutical companies.
SOURCE: Straumann A et al. Gastroenterology. 2020 July 25. doi: 10.1053/j.gastro.2020.07.039.
FROM GASTROENTEROLOGY
PPIs associated with diabetes risk, but questions remain
Regular use of proton pump inhibitors (PPIs) is associated with an increased risk of type 2 diabetes, according to a large prospective analysis of the Nurses’ Health Study. The results follow on other studies suggesting other potential adverse effects of PPIs such as dementia, kidney damage, and micronutrient deficiencies.
The authors, led by Jinqiu Yuan and Changhua Zhang of Sun Yat-sen University (Guangdong, China), call for regular blood glucose testing and diabetes screening for patients on long-term PPIs. But not all are convinced. “I think that’s a strong recommendation from the available data and it’s unclear how oe who would implement that in practice. I think instead practitioners should adhere to best practices, which emphasize using the lowest effective dose of PPIs for patients with appropriate indications,” David Leiman, MD, MSHP, assistant professor of medicine at Duke University, Durham, N.C. said in an interview.
“Overall, the data from the study can be classified as provocative results that I think may warrant further study,” he added. Consistent and strongly positive findings from more observationsal studies would be required to establish causality between PPI use and diabetes risk, and in any case the findings of the surrent study don't warrant a change in practice, Dr. Leiman said, noting that the study’s design makes it likely that much or all of the observed associations were due to unmeasured confounding.
The study appeared online Sept. 28 in Gut.
The researchers analyzed data from 80,500 women from the Nurses’ Health Study, 95,550 women from the Nurses’ Health Study II, and 28,639 men from the Health Professionals Follow-up Study (HPFS), with a median follow-up time of 12 years in NHS and NHS2 and 9.8 years in HPFS.
The absolute risk of diabetes was 7.44 per 1,000 person-years in PPI users versus 4.32 among nonusers. After adjustment for lagging PPI use for 2 years and stratification by age and study period, PPI use was associated with a 74% increased risk of diabetes (hazard ratio , 1.74; 95% confidence interval, 1.37-2.20). Multivariable adjustment for demographic factors, lifestyle habits, comorbidities, and use of other medications and clinical indications for PPI use attenuated the association but did not eliminate it (HR, 1.24; 95% CI, 1.17-1.31).
There was no statistically significant association in the HPFS group (HR, 1.12; 95% CI, 0.91-1.38), possibly because of the smaller sample size.
At 1 year, the number needed to harm with PPIs was 318.9 (95% CI, 285.2-385.0). At 2 years it was 170.8 (95% CI, 150.8-209.7) and at 3 years it was 77.3 (95% CI, 66.8-97.0).
At 0-2 years, PPI use was associated with a 5% increase in diabetes risk (HR, 1.05; 95% CI, 0.93-1.19). More than 2 years of use was associated with higher risk (HR, 1.26; 95% CI, 1.18-1.35).
There was also an association between stopping PPI use and a decreased risk of diabetes: Compared with current PPI users, those who had stopped within the past 2 years had a 17% reduction in risk (HR, 0.83; 95% CI, 0.70-0.98), and those who had stopped more than 2 years previously had a 19% reduction (HR, 0.81; 95% CI, 0.76-0.86).
The researchers also examined diabetes risk associated with use of H2 receptor agonists (H2RAs), since the drugs share clinical indications with PPIs. H2RA use was also associated with a higher risk of diabetes (adjusted HR, 1.14; 95% CI, 1.07-1.23).
The researchers suggested that the fact that the less potent H2RA inhibitors had a less pronounced association with diabetes risk supports the idea that acid suppression may be related to diabetes pathogenesis.
The authors also suggest that changes to the gut microbiota may underlie increased risk. PPI use has been shown to reduce gut microbiome diversity and alter its phenotype. Such changes could lead to weight gain, metabolic syndrome, and chronic liver disease, which could in turn heighten risk.
The study is limited by its observational nature, and lacked detailed information on dosage, frequency, and indications for PPI use.
SOURCE: Yuan J et al. Gut. 2020 Sep 28. doi: 10.1136/gutjnl-2020-322557.
This story was updated on 11/24/2020.
Help your patients understand the risks and benefits of long-term PPI use by sharing the AGA Clinical Practice Update patient companion at http://ow.ly/N1
Regular use of proton pump inhibitors (PPIs) is associated with an increased risk of type 2 diabetes, according to a large prospective analysis of the Nurses’ Health Study. The results follow on other studies suggesting other potential adverse effects of PPIs such as dementia, kidney damage, and micronutrient deficiencies.
The authors, led by Jinqiu Yuan and Changhua Zhang of Sun Yat-sen University (Guangdong, China), call for regular blood glucose testing and diabetes screening for patients on long-term PPIs. But not all are convinced. “I think that’s a strong recommendation from the available data and it’s unclear how oe who would implement that in practice. I think instead practitioners should adhere to best practices, which emphasize using the lowest effective dose of PPIs for patients with appropriate indications,” David Leiman, MD, MSHP, assistant professor of medicine at Duke University, Durham, N.C. said in an interview.
“Overall, the data from the study can be classified as provocative results that I think may warrant further study,” he added. Consistent and strongly positive findings from more observationsal studies would be required to establish causality between PPI use and diabetes risk, and in any case the findings of the surrent study don't warrant a change in practice, Dr. Leiman said, noting that the study’s design makes it likely that much or all of the observed associations were due to unmeasured confounding.
The study appeared online Sept. 28 in Gut.
The researchers analyzed data from 80,500 women from the Nurses’ Health Study, 95,550 women from the Nurses’ Health Study II, and 28,639 men from the Health Professionals Follow-up Study (HPFS), with a median follow-up time of 12 years in NHS and NHS2 and 9.8 years in HPFS.
The absolute risk of diabetes was 7.44 per 1,000 person-years in PPI users versus 4.32 among nonusers. After adjustment for lagging PPI use for 2 years and stratification by age and study period, PPI use was associated with a 74% increased risk of diabetes (hazard ratio , 1.74; 95% confidence interval, 1.37-2.20). Multivariable adjustment for demographic factors, lifestyle habits, comorbidities, and use of other medications and clinical indications for PPI use attenuated the association but did not eliminate it (HR, 1.24; 95% CI, 1.17-1.31).
There was no statistically significant association in the HPFS group (HR, 1.12; 95% CI, 0.91-1.38), possibly because of the smaller sample size.
At 1 year, the number needed to harm with PPIs was 318.9 (95% CI, 285.2-385.0). At 2 years it was 170.8 (95% CI, 150.8-209.7) and at 3 years it was 77.3 (95% CI, 66.8-97.0).
At 0-2 years, PPI use was associated with a 5% increase in diabetes risk (HR, 1.05; 95% CI, 0.93-1.19). More than 2 years of use was associated with higher risk (HR, 1.26; 95% CI, 1.18-1.35).
There was also an association between stopping PPI use and a decreased risk of diabetes: Compared with current PPI users, those who had stopped within the past 2 years had a 17% reduction in risk (HR, 0.83; 95% CI, 0.70-0.98), and those who had stopped more than 2 years previously had a 19% reduction (HR, 0.81; 95% CI, 0.76-0.86).
The researchers also examined diabetes risk associated with use of H2 receptor agonists (H2RAs), since the drugs share clinical indications with PPIs. H2RA use was also associated with a higher risk of diabetes (adjusted HR, 1.14; 95% CI, 1.07-1.23).
The researchers suggested that the fact that the less potent H2RA inhibitors had a less pronounced association with diabetes risk supports the idea that acid suppression may be related to diabetes pathogenesis.
The authors also suggest that changes to the gut microbiota may underlie increased risk. PPI use has been shown to reduce gut microbiome diversity and alter its phenotype. Such changes could lead to weight gain, metabolic syndrome, and chronic liver disease, which could in turn heighten risk.
The study is limited by its observational nature, and lacked detailed information on dosage, frequency, and indications for PPI use.
SOURCE: Yuan J et al. Gut. 2020 Sep 28. doi: 10.1136/gutjnl-2020-322557.
This story was updated on 11/24/2020.
Help your patients understand the risks and benefits of long-term PPI use by sharing the AGA Clinical Practice Update patient companion at http://ow.ly/N1
Regular use of proton pump inhibitors (PPIs) is associated with an increased risk of type 2 diabetes, according to a large prospective analysis of the Nurses’ Health Study. The results follow on other studies suggesting other potential adverse effects of PPIs such as dementia, kidney damage, and micronutrient deficiencies.
The authors, led by Jinqiu Yuan and Changhua Zhang of Sun Yat-sen University (Guangdong, China), call for regular blood glucose testing and diabetes screening for patients on long-term PPIs. But not all are convinced. “I think that’s a strong recommendation from the available data and it’s unclear how oe who would implement that in practice. I think instead practitioners should adhere to best practices, which emphasize using the lowest effective dose of PPIs for patients with appropriate indications,” David Leiman, MD, MSHP, assistant professor of medicine at Duke University, Durham, N.C. said in an interview.
“Overall, the data from the study can be classified as provocative results that I think may warrant further study,” he added. Consistent and strongly positive findings from more observationsal studies would be required to establish causality between PPI use and diabetes risk, and in any case the findings of the surrent study don't warrant a change in practice, Dr. Leiman said, noting that the study’s design makes it likely that much or all of the observed associations were due to unmeasured confounding.
The study appeared online Sept. 28 in Gut.
The researchers analyzed data from 80,500 women from the Nurses’ Health Study, 95,550 women from the Nurses’ Health Study II, and 28,639 men from the Health Professionals Follow-up Study (HPFS), with a median follow-up time of 12 years in NHS and NHS2 and 9.8 years in HPFS.
The absolute risk of diabetes was 7.44 per 1,000 person-years in PPI users versus 4.32 among nonusers. After adjustment for lagging PPI use for 2 years and stratification by age and study period, PPI use was associated with a 74% increased risk of diabetes (hazard ratio , 1.74; 95% confidence interval, 1.37-2.20). Multivariable adjustment for demographic factors, lifestyle habits, comorbidities, and use of other medications and clinical indications for PPI use attenuated the association but did not eliminate it (HR, 1.24; 95% CI, 1.17-1.31).
There was no statistically significant association in the HPFS group (HR, 1.12; 95% CI, 0.91-1.38), possibly because of the smaller sample size.
At 1 year, the number needed to harm with PPIs was 318.9 (95% CI, 285.2-385.0). At 2 years it was 170.8 (95% CI, 150.8-209.7) and at 3 years it was 77.3 (95% CI, 66.8-97.0).
At 0-2 years, PPI use was associated with a 5% increase in diabetes risk (HR, 1.05; 95% CI, 0.93-1.19). More than 2 years of use was associated with higher risk (HR, 1.26; 95% CI, 1.18-1.35).
There was also an association between stopping PPI use and a decreased risk of diabetes: Compared with current PPI users, those who had stopped within the past 2 years had a 17% reduction in risk (HR, 0.83; 95% CI, 0.70-0.98), and those who had stopped more than 2 years previously had a 19% reduction (HR, 0.81; 95% CI, 0.76-0.86).
The researchers also examined diabetes risk associated with use of H2 receptor agonists (H2RAs), since the drugs share clinical indications with PPIs. H2RA use was also associated with a higher risk of diabetes (adjusted HR, 1.14; 95% CI, 1.07-1.23).
The researchers suggested that the fact that the less potent H2RA inhibitors had a less pronounced association with diabetes risk supports the idea that acid suppression may be related to diabetes pathogenesis.
The authors also suggest that changes to the gut microbiota may underlie increased risk. PPI use has been shown to reduce gut microbiome diversity and alter its phenotype. Such changes could lead to weight gain, metabolic syndrome, and chronic liver disease, which could in turn heighten risk.
The study is limited by its observational nature, and lacked detailed information on dosage, frequency, and indications for PPI use.
SOURCE: Yuan J et al. Gut. 2020 Sep 28. doi: 10.1136/gutjnl-2020-322557.
This story was updated on 11/24/2020.
Help your patients understand the risks and benefits of long-term PPI use by sharing the AGA Clinical Practice Update patient companion at http://ow.ly/N1
FROM GUT
GERD: Endoscopic therapies may offer alternative to PPIs
For patients with gastroesophageal reflux disease (GERD), endoscopic and minimally invasive surgical techniques may be viable alternatives to proton pump inhibitor (PPI) therapy, according to investigators.
Still, their exact role in the treatment process remains undetermined, reported Michael F. Vaezi, MD, PhD, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues.
“The frequent incomplete response to PPI therapy, in addition to recent studies suggesting chronic complications with PPI therapy, have fueled discussion of alternative strategies for treating patients with GERD,” the investigators wrote in Gastroenterology. “For a substantial number of patients and providers with the above concerns who are unwilling to pursue the traditional surgical gastric fundoplication, endoscopic or less invasive surgical strategies have gained some traction.”
Dr. Vaezi and colleagues noted that they conducted the scoping review with intentions of being more descriptive than prescriptive.
“Our goal is not to recommend the utility of any of the discussed techniques in specific clinical scenarios,” they wrote. “Rather, it is to summarize the currently available evidence and identify where more research may be helpful.”
Across 22 randomized, controlled trials and observational studies, objective and symptomatic improvement varied between modalities. Measured outcomes also varied; most studies reported symptoms, health-related quality of life, and PPI use; fewer studies (but still a majority) reported intraesophageal acid exposure and/or lower esophageal sphincter (LES) pressure. Conclusions drawn by Dr. Vaezi and colleagues are summarized below.
Magnetic sphincter augmentation of the LES
In multiple trials, magnetic sphincter augmentation demonstrated a “high degree of efficacy” in the short or midterm, and a favorable safety profile. Dr. Vaezi and colleagues highlighted significant improvements in disease-related quality of life, with “a substantial proportion” of patients achieving normalization or at least 50% improvement in acid exposure. While some patients required esophageal dilation after the procedure, this was not needed any more frequently than after surgical fundoplication.
Radiofrequency ablation
Across five trials, radiofrequency ablation, which involves delivery of energy to the LES and gastric cardia, improved GERD-related quality of life, and reduced, but did not normalize, acid exposure. The technique lessened short-term need for PPIs, but long-term relief was not observed. Compared with observational studies, efficacy signals were weaker in randomized, controlled trials. The procedure was generally safe.
Surgical implantation of LES pacemaker
Limited data were available for LES sphincter stimulation among patients with GERD, and the most recent study, involving a comparison of device placement with or without stimulation, was terminated early. Still, available data suggest that the technique is generally well tolerated, with reduced need for PPIs, improved symptoms, and lessened acid exposure. Dr. Vaezi and colleagues noted that the manufacturing company, EndoStim, is in receivership, putting U.S. availability in question.
Full-thickness fundoplication
Endoscopic full-thickness fundoplication was associated with improvement of symptoms and quality of life, and a favorable safety profile. Although the procedure generally reduced PPI use, most patients still needed PPIs long-term. Reflux improved after the procedure, but not to the same degree as laparoscopic plication.
Transoral incisionless fundoplication
Based on a number of studies, including five randomized, controlled trials, transoral incisionless fundoplication appears safe and effective, with reduced need for PPIs up to 5 years. According to Dr. Vaezi and colleagues, variable results across studies are likely explained by variations in the technique over time and heterogeneous patient populations. Recent studies in which the “TIF 2.0 technique” has been performed on patients with hiatal hernias less than 2 cm have met objective efficacy outcomes.
Incisionless fundoplication with magnetic ultrasonic surgical endostapler
The magnetic ultrasonic surgical endostapler, which allows for incisionless fundoplication, had more limited data. Only two studies have been conducted, and neither had sham-controlled nor comparative-trial data. Furthermore, multiple safety signals have been encountered, with “substantial” complication rates and serious adverse events that were “noticeable and concerning,” according to Dr. Vaezi and colleagues.
Concluding their discussion, the investigators suggested that some endoscopic and minimally invasive approaches to GERD are “promising” alternatives to PPI therapy.
“However, their place in the treatment algorithm for GERD will be better defined when important clinical parameters, especially the durability of their effect, are understood,” they wrote.
The investigators reported no conflicts of interest.
SOURCE: Vaezi MF et al. Gastroenterology. 2020 Jul 1. doi: 10.1053/j.gastro.2020.05.097.
For patients with gastroesophageal reflux disease (GERD), endoscopic and minimally invasive surgical techniques may be viable alternatives to proton pump inhibitor (PPI) therapy, according to investigators.
Still, their exact role in the treatment process remains undetermined, reported Michael F. Vaezi, MD, PhD, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues.
“The frequent incomplete response to PPI therapy, in addition to recent studies suggesting chronic complications with PPI therapy, have fueled discussion of alternative strategies for treating patients with GERD,” the investigators wrote in Gastroenterology. “For a substantial number of patients and providers with the above concerns who are unwilling to pursue the traditional surgical gastric fundoplication, endoscopic or less invasive surgical strategies have gained some traction.”
Dr. Vaezi and colleagues noted that they conducted the scoping review with intentions of being more descriptive than prescriptive.
“Our goal is not to recommend the utility of any of the discussed techniques in specific clinical scenarios,” they wrote. “Rather, it is to summarize the currently available evidence and identify where more research may be helpful.”
Across 22 randomized, controlled trials and observational studies, objective and symptomatic improvement varied between modalities. Measured outcomes also varied; most studies reported symptoms, health-related quality of life, and PPI use; fewer studies (but still a majority) reported intraesophageal acid exposure and/or lower esophageal sphincter (LES) pressure. Conclusions drawn by Dr. Vaezi and colleagues are summarized below.
Magnetic sphincter augmentation of the LES
In multiple trials, magnetic sphincter augmentation demonstrated a “high degree of efficacy” in the short or midterm, and a favorable safety profile. Dr. Vaezi and colleagues highlighted significant improvements in disease-related quality of life, with “a substantial proportion” of patients achieving normalization or at least 50% improvement in acid exposure. While some patients required esophageal dilation after the procedure, this was not needed any more frequently than after surgical fundoplication.
Radiofrequency ablation
Across five trials, radiofrequency ablation, which involves delivery of energy to the LES and gastric cardia, improved GERD-related quality of life, and reduced, but did not normalize, acid exposure. The technique lessened short-term need for PPIs, but long-term relief was not observed. Compared with observational studies, efficacy signals were weaker in randomized, controlled trials. The procedure was generally safe.
Surgical implantation of LES pacemaker
Limited data were available for LES sphincter stimulation among patients with GERD, and the most recent study, involving a comparison of device placement with or without stimulation, was terminated early. Still, available data suggest that the technique is generally well tolerated, with reduced need for PPIs, improved symptoms, and lessened acid exposure. Dr. Vaezi and colleagues noted that the manufacturing company, EndoStim, is in receivership, putting U.S. availability in question.
Full-thickness fundoplication
Endoscopic full-thickness fundoplication was associated with improvement of symptoms and quality of life, and a favorable safety profile. Although the procedure generally reduced PPI use, most patients still needed PPIs long-term. Reflux improved after the procedure, but not to the same degree as laparoscopic plication.
Transoral incisionless fundoplication
Based on a number of studies, including five randomized, controlled trials, transoral incisionless fundoplication appears safe and effective, with reduced need for PPIs up to 5 years. According to Dr. Vaezi and colleagues, variable results across studies are likely explained by variations in the technique over time and heterogeneous patient populations. Recent studies in which the “TIF 2.0 technique” has been performed on patients with hiatal hernias less than 2 cm have met objective efficacy outcomes.
Incisionless fundoplication with magnetic ultrasonic surgical endostapler
The magnetic ultrasonic surgical endostapler, which allows for incisionless fundoplication, had more limited data. Only two studies have been conducted, and neither had sham-controlled nor comparative-trial data. Furthermore, multiple safety signals have been encountered, with “substantial” complication rates and serious adverse events that were “noticeable and concerning,” according to Dr. Vaezi and colleagues.
Concluding their discussion, the investigators suggested that some endoscopic and minimally invasive approaches to GERD are “promising” alternatives to PPI therapy.
“However, their place in the treatment algorithm for GERD will be better defined when important clinical parameters, especially the durability of their effect, are understood,” they wrote.
The investigators reported no conflicts of interest.
SOURCE: Vaezi MF et al. Gastroenterology. 2020 Jul 1. doi: 10.1053/j.gastro.2020.05.097.
For patients with gastroesophageal reflux disease (GERD), endoscopic and minimally invasive surgical techniques may be viable alternatives to proton pump inhibitor (PPI) therapy, according to investigators.
Still, their exact role in the treatment process remains undetermined, reported Michael F. Vaezi, MD, PhD, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues.
“The frequent incomplete response to PPI therapy, in addition to recent studies suggesting chronic complications with PPI therapy, have fueled discussion of alternative strategies for treating patients with GERD,” the investigators wrote in Gastroenterology. “For a substantial number of patients and providers with the above concerns who are unwilling to pursue the traditional surgical gastric fundoplication, endoscopic or less invasive surgical strategies have gained some traction.”
Dr. Vaezi and colleagues noted that they conducted the scoping review with intentions of being more descriptive than prescriptive.
“Our goal is not to recommend the utility of any of the discussed techniques in specific clinical scenarios,” they wrote. “Rather, it is to summarize the currently available evidence and identify where more research may be helpful.”
Across 22 randomized, controlled trials and observational studies, objective and symptomatic improvement varied between modalities. Measured outcomes also varied; most studies reported symptoms, health-related quality of life, and PPI use; fewer studies (but still a majority) reported intraesophageal acid exposure and/or lower esophageal sphincter (LES) pressure. Conclusions drawn by Dr. Vaezi and colleagues are summarized below.
Magnetic sphincter augmentation of the LES
In multiple trials, magnetic sphincter augmentation demonstrated a “high degree of efficacy” in the short or midterm, and a favorable safety profile. Dr. Vaezi and colleagues highlighted significant improvements in disease-related quality of life, with “a substantial proportion” of patients achieving normalization or at least 50% improvement in acid exposure. While some patients required esophageal dilation after the procedure, this was not needed any more frequently than after surgical fundoplication.
Radiofrequency ablation
Across five trials, radiofrequency ablation, which involves delivery of energy to the LES and gastric cardia, improved GERD-related quality of life, and reduced, but did not normalize, acid exposure. The technique lessened short-term need for PPIs, but long-term relief was not observed. Compared with observational studies, efficacy signals were weaker in randomized, controlled trials. The procedure was generally safe.
Surgical implantation of LES pacemaker
Limited data were available for LES sphincter stimulation among patients with GERD, and the most recent study, involving a comparison of device placement with or without stimulation, was terminated early. Still, available data suggest that the technique is generally well tolerated, with reduced need for PPIs, improved symptoms, and lessened acid exposure. Dr. Vaezi and colleagues noted that the manufacturing company, EndoStim, is in receivership, putting U.S. availability in question.
Full-thickness fundoplication
Endoscopic full-thickness fundoplication was associated with improvement of symptoms and quality of life, and a favorable safety profile. Although the procedure generally reduced PPI use, most patients still needed PPIs long-term. Reflux improved after the procedure, but not to the same degree as laparoscopic plication.
Transoral incisionless fundoplication
Based on a number of studies, including five randomized, controlled trials, transoral incisionless fundoplication appears safe and effective, with reduced need for PPIs up to 5 years. According to Dr. Vaezi and colleagues, variable results across studies are likely explained by variations in the technique over time and heterogeneous patient populations. Recent studies in which the “TIF 2.0 technique” has been performed on patients with hiatal hernias less than 2 cm have met objective efficacy outcomes.
Incisionless fundoplication with magnetic ultrasonic surgical endostapler
The magnetic ultrasonic surgical endostapler, which allows for incisionless fundoplication, had more limited data. Only two studies have been conducted, and neither had sham-controlled nor comparative-trial data. Furthermore, multiple safety signals have been encountered, with “substantial” complication rates and serious adverse events that were “noticeable and concerning,” according to Dr. Vaezi and colleagues.
Concluding their discussion, the investigators suggested that some endoscopic and minimally invasive approaches to GERD are “promising” alternatives to PPI therapy.
“However, their place in the treatment algorithm for GERD will be better defined when important clinical parameters, especially the durability of their effect, are understood,” they wrote.
The investigators reported no conflicts of interest.
SOURCE: Vaezi MF et al. Gastroenterology. 2020 Jul 1. doi: 10.1053/j.gastro.2020.05.097.
FROM GASTROENTEROLOGY
CagA-positive H. pylori patients at higher risk of osteoporosis, fracture
A new study has found that older patients who test positive for the cytotoxin associated gene-A (CagA) strain of Helicobacter pylori may be more at risk of both osteoporosis and fractures.
“Further studies will be required to replicate these findings in other cohorts and to better clarify the underlying pathogenetic mechanisms leading to increased bone fragility in subjects infected by CagA-positive H. pylori strains,” wrote Luigi Gennari, MD, PhD, of the University of Siena (Italy), and coauthors. The study was published in the Journal of Bone and Mineral Research.
To determine the effects of H. pylori on bone health and potential fracture risk, the researchers launched a population-based cohort study of 1,149 adults between the ages of 50 and 80 in Siena. The cohort comprised 174 males with an average (SD) age of 65.9 (plus or minus 6 years) and 975 females with an average age of 62.5 (plus or minus 6 years). All subjects were examined for H. pylori antibodies, and those who were infected were also examined for anti-CagA serum antibodies. As blood was sampled, bone mineral density (BMD) of the lumbar spine, femoral neck, total hip, and total body was measured via dual-energy x-ray absorptiometry.
In total, 53% of male participants and 49% of female participants tested positive for H. pylori, with CagA-positive strains found in 27% of males and 26% of females. No differences in infection rates were discovered in regard to socioeconomic status, age, weight, or height. Patients with normal BMD (45%), osteoporosis (51%), or osteopenia (49%) had similar prevalence of H. pylori infection, but CagA-positive strains were more frequently found in osteoporotic (30%) and osteopenic (26%) patients, compared to patients with normal BMD (21%, P < .01). CagA-positive female patients also had lower lumbar (0.950 g/cm2) and femoral (0.795 g/cm2) BMD, compared to CagA-negative (0.987 and 0.813 g/cm2) or H. pylori-negative women (0.997 and 0.821 g/cm2), respectively.
After an average follow-up period of 11.8 years, 199 nontraumatic fractures (72 vertebral and 127 nonvertebral) had occurred in 158 participants. Patients with CagA-positive strains of H. pylori had significantly increased risk of a clinical vertebral fracture (hazard ratio [HR], 5.27; 95% confidence interval, 2.23-12.63; P < .0001) or a nonvertebral incident fracture (HR, 2.09; 95% CI, 1.27-2.46; P < .01), compared to patients without H. pylori. After adjustment for age, sex, and body mass index, the risk among CagA-positive patients remained similarly significantly elevated for both vertebral (aHR, 4.78; 95% CI, 1.99-11.47; P < .0001) and nonvertebral fractures (aHR, 2.04; 95% CI, 1.22-3.41; P < .01).
The authors acknowledged their study’s limitations, including a cohort that was notably low in male participants, an inability to assess the effects of eradicating H. pylori on bone, and uncertainty as to which specific effects of H. pylori infection increase the risk of osteoporosis or fracture. Along those lines, they noted that an association between serum CagA antibody titer and gastric mucosal inflammation could lead to malabsorption of calcium, hypothesizing that antibody titer rather than antibody positivity “might be a more relevant marker for assessing the risk of bone fragility in patients affected by H. pylori infection.”
The study was supported in part by a grant from the Italian Association for Osteoporosis. The authors reported no potential conflicts of interest.
SOURCE: Gennari L et al. J Bone Miner Res. 2020 Aug 13. doi: 10.1002/jbmr.4162.
A new study has found that older patients who test positive for the cytotoxin associated gene-A (CagA) strain of Helicobacter pylori may be more at risk of both osteoporosis and fractures.
“Further studies will be required to replicate these findings in other cohorts and to better clarify the underlying pathogenetic mechanisms leading to increased bone fragility in subjects infected by CagA-positive H. pylori strains,” wrote Luigi Gennari, MD, PhD, of the University of Siena (Italy), and coauthors. The study was published in the Journal of Bone and Mineral Research.
To determine the effects of H. pylori on bone health and potential fracture risk, the researchers launched a population-based cohort study of 1,149 adults between the ages of 50 and 80 in Siena. The cohort comprised 174 males with an average (SD) age of 65.9 (plus or minus 6 years) and 975 females with an average age of 62.5 (plus or minus 6 years). All subjects were examined for H. pylori antibodies, and those who were infected were also examined for anti-CagA serum antibodies. As blood was sampled, bone mineral density (BMD) of the lumbar spine, femoral neck, total hip, and total body was measured via dual-energy x-ray absorptiometry.
In total, 53% of male participants and 49% of female participants tested positive for H. pylori, with CagA-positive strains found in 27% of males and 26% of females. No differences in infection rates were discovered in regard to socioeconomic status, age, weight, or height. Patients with normal BMD (45%), osteoporosis (51%), or osteopenia (49%) had similar prevalence of H. pylori infection, but CagA-positive strains were more frequently found in osteoporotic (30%) and osteopenic (26%) patients, compared to patients with normal BMD (21%, P < .01). CagA-positive female patients also had lower lumbar (0.950 g/cm2) and femoral (0.795 g/cm2) BMD, compared to CagA-negative (0.987 and 0.813 g/cm2) or H. pylori-negative women (0.997 and 0.821 g/cm2), respectively.
After an average follow-up period of 11.8 years, 199 nontraumatic fractures (72 vertebral and 127 nonvertebral) had occurred in 158 participants. Patients with CagA-positive strains of H. pylori had significantly increased risk of a clinical vertebral fracture (hazard ratio [HR], 5.27; 95% confidence interval, 2.23-12.63; P < .0001) or a nonvertebral incident fracture (HR, 2.09; 95% CI, 1.27-2.46; P < .01), compared to patients without H. pylori. After adjustment for age, sex, and body mass index, the risk among CagA-positive patients remained similarly significantly elevated for both vertebral (aHR, 4.78; 95% CI, 1.99-11.47; P < .0001) and nonvertebral fractures (aHR, 2.04; 95% CI, 1.22-3.41; P < .01).
The authors acknowledged their study’s limitations, including a cohort that was notably low in male participants, an inability to assess the effects of eradicating H. pylori on bone, and uncertainty as to which specific effects of H. pylori infection increase the risk of osteoporosis or fracture. Along those lines, they noted that an association between serum CagA antibody titer and gastric mucosal inflammation could lead to malabsorption of calcium, hypothesizing that antibody titer rather than antibody positivity “might be a more relevant marker for assessing the risk of bone fragility in patients affected by H. pylori infection.”
The study was supported in part by a grant from the Italian Association for Osteoporosis. The authors reported no potential conflicts of interest.
SOURCE: Gennari L et al. J Bone Miner Res. 2020 Aug 13. doi: 10.1002/jbmr.4162.
A new study has found that older patients who test positive for the cytotoxin associated gene-A (CagA) strain of Helicobacter pylori may be more at risk of both osteoporosis and fractures.
“Further studies will be required to replicate these findings in other cohorts and to better clarify the underlying pathogenetic mechanisms leading to increased bone fragility in subjects infected by CagA-positive H. pylori strains,” wrote Luigi Gennari, MD, PhD, of the University of Siena (Italy), and coauthors. The study was published in the Journal of Bone and Mineral Research.
To determine the effects of H. pylori on bone health and potential fracture risk, the researchers launched a population-based cohort study of 1,149 adults between the ages of 50 and 80 in Siena. The cohort comprised 174 males with an average (SD) age of 65.9 (plus or minus 6 years) and 975 females with an average age of 62.5 (plus or minus 6 years). All subjects were examined for H. pylori antibodies, and those who were infected were also examined for anti-CagA serum antibodies. As blood was sampled, bone mineral density (BMD) of the lumbar spine, femoral neck, total hip, and total body was measured via dual-energy x-ray absorptiometry.
In total, 53% of male participants and 49% of female participants tested positive for H. pylori, with CagA-positive strains found in 27% of males and 26% of females. No differences in infection rates were discovered in regard to socioeconomic status, age, weight, or height. Patients with normal BMD (45%), osteoporosis (51%), or osteopenia (49%) had similar prevalence of H. pylori infection, but CagA-positive strains were more frequently found in osteoporotic (30%) and osteopenic (26%) patients, compared to patients with normal BMD (21%, P < .01). CagA-positive female patients also had lower lumbar (0.950 g/cm2) and femoral (0.795 g/cm2) BMD, compared to CagA-negative (0.987 and 0.813 g/cm2) or H. pylori-negative women (0.997 and 0.821 g/cm2), respectively.
After an average follow-up period of 11.8 years, 199 nontraumatic fractures (72 vertebral and 127 nonvertebral) had occurred in 158 participants. Patients with CagA-positive strains of H. pylori had significantly increased risk of a clinical vertebral fracture (hazard ratio [HR], 5.27; 95% confidence interval, 2.23-12.63; P < .0001) or a nonvertebral incident fracture (HR, 2.09; 95% CI, 1.27-2.46; P < .01), compared to patients without H. pylori. After adjustment for age, sex, and body mass index, the risk among CagA-positive patients remained similarly significantly elevated for both vertebral (aHR, 4.78; 95% CI, 1.99-11.47; P < .0001) and nonvertebral fractures (aHR, 2.04; 95% CI, 1.22-3.41; P < .01).
The authors acknowledged their study’s limitations, including a cohort that was notably low in male participants, an inability to assess the effects of eradicating H. pylori on bone, and uncertainty as to which specific effects of H. pylori infection increase the risk of osteoporosis or fracture. Along those lines, they noted that an association between serum CagA antibody titer and gastric mucosal inflammation could lead to malabsorption of calcium, hypothesizing that antibody titer rather than antibody positivity “might be a more relevant marker for assessing the risk of bone fragility in patients affected by H. pylori infection.”
The study was supported in part by a grant from the Italian Association for Osteoporosis. The authors reported no potential conflicts of interest.
SOURCE: Gennari L et al. J Bone Miner Res. 2020 Aug 13. doi: 10.1002/jbmr.4162.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
AGA updates endoscopic management of nonvariceal upper GI bleeding
The American Gastroenterological Association (AGA) has published a clinical practice update for endoscopic management of nonvariceal upper GI bleeding (NVUGIB).
The update includes 10 best practice recommendations based on clinical experience and a comprehensive literature review, reported lead author Daniel K. Mullady, MD, of Washington University in St. Louis.
“Numerous endoscopic devices have been developed over the past 30 years with demonstrated effectiveness in treating NVUGIB,” Dr. Mullady and colleagues wrote in Gastroenterology. “The purpose of this clinical practice update is to review the key concepts, new devices, and therapeutic strategies in endoscopically combating this age-old clinical dilemma.”
According to the investigators, endoscopy is central to management of NVUGIB, but only after patients are appropriately triaged and stabilized.
“[E]ndoscopy should be performed to determine the source of bleeding, to assess rebleeding risk, and to treat lesions at high risk for rebleeding,” they wrote. “Exactly when the endoscopy should be performed is a clinical judgment made by the gastroenterologist in consultation with the primary service.”
The investigators recommended that endoscopy be performed within 12 hours for emergent cases and within 24 hours for urgent cases, whereas elective cases could wait longer.
They noted that NVUGIB can range from mild and self-limiting, allowing for outpatient management, to severe and life-threatening, necessitating intensive care. Because of this broad range, the investigators recommended familiarity with triage scoring systems, including the Glasgow-Blatchford Score, the Rockall Score, and AIMS-65.
“A common decision is deciding whether or not to wait until the next morning to perform endoscopy on a patient presenting after hours with suspected NVUGIB,” the investigators wrote.
The investigators cautioned that emergent endoscopy may actually be associated with poorer outcomes because of “inadequate resuscitation,” and suggested that “[p]atients who are hemodynamically stable, do not have ongoing hematemesis, and have melena only can generally be deferred to the following morning.”
Concerning hemostatic technique, Dr. Mullady and colleagues recommended familiarity with conventional thermal therapy and placement of hemoclips. If these approaches are unsuccessful, or deemed unlikely to succeed, they recommended an over-the-scope clip.
For ulcers “with a rigid and fibrotic base,” or those that are hard to reach, the investigators recommended monopolar hemostatic forceps with low-voltage coagulation.
According to the update, hemostatic powder should be reserved for scenarios in which bleeding is diffuse and difficult to locate.
“In most instances, hemostatic powder should be preferentially used as a rescue therapy and not for primary hemostasis, except in cases of malignant bleeding or massive bleeding with inability to perform thermal therapy or hemoclip placement,” the investigators wrote.
They noted that hemostatic powder generally dissolves in less than 24 hours, so additional treatment approaches should be considered, particular when there is a high risk of rebleeding.
When deciding between transcatheter arterial embolization (TAE) and surgery after endoscopic failure, the update calls for a comprehensive clinical assessment that incorporates patient factors, such as coagulopathy, hemodynamic instability, and multiorgan failure; bleeding etiology; potential adverse effects; and rebleeding risk.
“An important point is that prophylactic TAE of high-risk ulcers after successful endoscopic therapy is not recommended,” the investigators wrote.
Beyond these recommendations, the update includes a comprehensive discussion of relevant literature and strategies for effective clinical decision making. The discussion concludes with global remarks about the evolving role of endoscopy in managing NVUGIB, including a note about cost-effectiveness despite up-front expenses associated with some methods.
“With this expanded endoscopic armamentarium, endoscopic therapy should achieve hemostasis in the majority of patients with NVUGIB,” the investigators wrote. “Despite the increased costs of newer devices or multimodal therapy, effective hemostasis to preventing rebleeding and the need for hospital readmission is likely to be a dominant cost-saving strategy.”
Dr. Mullady disclosed relationships with Boston Scientific, ConMed, and Cook Medical.
This story was updated on 9/9/2020.
SOURCE: Mullady DK et al. Gastro. 2020 Jun 20. doi: 10.1053/j.gastro.2020.05.095.
The American Gastroenterological Association (AGA) has published a clinical practice update for endoscopic management of nonvariceal upper GI bleeding (NVUGIB).
The update includes 10 best practice recommendations based on clinical experience and a comprehensive literature review, reported lead author Daniel K. Mullady, MD, of Washington University in St. Louis.
“Numerous endoscopic devices have been developed over the past 30 years with demonstrated effectiveness in treating NVUGIB,” Dr. Mullady and colleagues wrote in Gastroenterology. “The purpose of this clinical practice update is to review the key concepts, new devices, and therapeutic strategies in endoscopically combating this age-old clinical dilemma.”
According to the investigators, endoscopy is central to management of NVUGIB, but only after patients are appropriately triaged and stabilized.
“[E]ndoscopy should be performed to determine the source of bleeding, to assess rebleeding risk, and to treat lesions at high risk for rebleeding,” they wrote. “Exactly when the endoscopy should be performed is a clinical judgment made by the gastroenterologist in consultation with the primary service.”
The investigators recommended that endoscopy be performed within 12 hours for emergent cases and within 24 hours for urgent cases, whereas elective cases could wait longer.
They noted that NVUGIB can range from mild and self-limiting, allowing for outpatient management, to severe and life-threatening, necessitating intensive care. Because of this broad range, the investigators recommended familiarity with triage scoring systems, including the Glasgow-Blatchford Score, the Rockall Score, and AIMS-65.
“A common decision is deciding whether or not to wait until the next morning to perform endoscopy on a patient presenting after hours with suspected NVUGIB,” the investigators wrote.
The investigators cautioned that emergent endoscopy may actually be associated with poorer outcomes because of “inadequate resuscitation,” and suggested that “[p]atients who are hemodynamically stable, do not have ongoing hematemesis, and have melena only can generally be deferred to the following morning.”
Concerning hemostatic technique, Dr. Mullady and colleagues recommended familiarity with conventional thermal therapy and placement of hemoclips. If these approaches are unsuccessful, or deemed unlikely to succeed, they recommended an over-the-scope clip.
For ulcers “with a rigid and fibrotic base,” or those that are hard to reach, the investigators recommended monopolar hemostatic forceps with low-voltage coagulation.
According to the update, hemostatic powder should be reserved for scenarios in which bleeding is diffuse and difficult to locate.
“In most instances, hemostatic powder should be preferentially used as a rescue therapy and not for primary hemostasis, except in cases of malignant bleeding or massive bleeding with inability to perform thermal therapy or hemoclip placement,” the investigators wrote.
They noted that hemostatic powder generally dissolves in less than 24 hours, so additional treatment approaches should be considered, particular when there is a high risk of rebleeding.
When deciding between transcatheter arterial embolization (TAE) and surgery after endoscopic failure, the update calls for a comprehensive clinical assessment that incorporates patient factors, such as coagulopathy, hemodynamic instability, and multiorgan failure; bleeding etiology; potential adverse effects; and rebleeding risk.
“An important point is that prophylactic TAE of high-risk ulcers after successful endoscopic therapy is not recommended,” the investigators wrote.
Beyond these recommendations, the update includes a comprehensive discussion of relevant literature and strategies for effective clinical decision making. The discussion concludes with global remarks about the evolving role of endoscopy in managing NVUGIB, including a note about cost-effectiveness despite up-front expenses associated with some methods.
“With this expanded endoscopic armamentarium, endoscopic therapy should achieve hemostasis in the majority of patients with NVUGIB,” the investigators wrote. “Despite the increased costs of newer devices or multimodal therapy, effective hemostasis to preventing rebleeding and the need for hospital readmission is likely to be a dominant cost-saving strategy.”
Dr. Mullady disclosed relationships with Boston Scientific, ConMed, and Cook Medical.
This story was updated on 9/9/2020.
SOURCE: Mullady DK et al. Gastro. 2020 Jun 20. doi: 10.1053/j.gastro.2020.05.095.
The American Gastroenterological Association (AGA) has published a clinical practice update for endoscopic management of nonvariceal upper GI bleeding (NVUGIB).
The update includes 10 best practice recommendations based on clinical experience and a comprehensive literature review, reported lead author Daniel K. Mullady, MD, of Washington University in St. Louis.
“Numerous endoscopic devices have been developed over the past 30 years with demonstrated effectiveness in treating NVUGIB,” Dr. Mullady and colleagues wrote in Gastroenterology. “The purpose of this clinical practice update is to review the key concepts, new devices, and therapeutic strategies in endoscopically combating this age-old clinical dilemma.”
According to the investigators, endoscopy is central to management of NVUGIB, but only after patients are appropriately triaged and stabilized.
“[E]ndoscopy should be performed to determine the source of bleeding, to assess rebleeding risk, and to treat lesions at high risk for rebleeding,” they wrote. “Exactly when the endoscopy should be performed is a clinical judgment made by the gastroenterologist in consultation with the primary service.”
The investigators recommended that endoscopy be performed within 12 hours for emergent cases and within 24 hours for urgent cases, whereas elective cases could wait longer.
They noted that NVUGIB can range from mild and self-limiting, allowing for outpatient management, to severe and life-threatening, necessitating intensive care. Because of this broad range, the investigators recommended familiarity with triage scoring systems, including the Glasgow-Blatchford Score, the Rockall Score, and AIMS-65.
“A common decision is deciding whether or not to wait until the next morning to perform endoscopy on a patient presenting after hours with suspected NVUGIB,” the investigators wrote.
The investigators cautioned that emergent endoscopy may actually be associated with poorer outcomes because of “inadequate resuscitation,” and suggested that “[p]atients who are hemodynamically stable, do not have ongoing hematemesis, and have melena only can generally be deferred to the following morning.”
Concerning hemostatic technique, Dr. Mullady and colleagues recommended familiarity with conventional thermal therapy and placement of hemoclips. If these approaches are unsuccessful, or deemed unlikely to succeed, they recommended an over-the-scope clip.
For ulcers “with a rigid and fibrotic base,” or those that are hard to reach, the investigators recommended monopolar hemostatic forceps with low-voltage coagulation.
According to the update, hemostatic powder should be reserved for scenarios in which bleeding is diffuse and difficult to locate.
“In most instances, hemostatic powder should be preferentially used as a rescue therapy and not for primary hemostasis, except in cases of malignant bleeding or massive bleeding with inability to perform thermal therapy or hemoclip placement,” the investigators wrote.
They noted that hemostatic powder generally dissolves in less than 24 hours, so additional treatment approaches should be considered, particular when there is a high risk of rebleeding.
When deciding between transcatheter arterial embolization (TAE) and surgery after endoscopic failure, the update calls for a comprehensive clinical assessment that incorporates patient factors, such as coagulopathy, hemodynamic instability, and multiorgan failure; bleeding etiology; potential adverse effects; and rebleeding risk.
“An important point is that prophylactic TAE of high-risk ulcers after successful endoscopic therapy is not recommended,” the investigators wrote.
Beyond these recommendations, the update includes a comprehensive discussion of relevant literature and strategies for effective clinical decision making. The discussion concludes with global remarks about the evolving role of endoscopy in managing NVUGIB, including a note about cost-effectiveness despite up-front expenses associated with some methods.
“With this expanded endoscopic armamentarium, endoscopic therapy should achieve hemostasis in the majority of patients with NVUGIB,” the investigators wrote. “Despite the increased costs of newer devices or multimodal therapy, effective hemostasis to preventing rebleeding and the need for hospital readmission is likely to be a dominant cost-saving strategy.”
Dr. Mullady disclosed relationships with Boston Scientific, ConMed, and Cook Medical.
This story was updated on 9/9/2020.
SOURCE: Mullady DK et al. Gastro. 2020 Jun 20. doi: 10.1053/j.gastro.2020.05.095.
FROM GASTROENTEROLOGY
Swallowable ‘sponge on string’ to diagnose esophageal cancer
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center at http://ow.ly/p9hU30r4oya.
This article first appeared on Medscape.com.
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center at http://ow.ly/p9hU30r4oya.
This article first appeared on Medscape.com.
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center at http://ow.ly/p9hU30r4oya.
This article first appeared on Medscape.com.
Eosinophilic esophagitis: Frequently asked questions (and answers) for the early-career gastroenterologist
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).
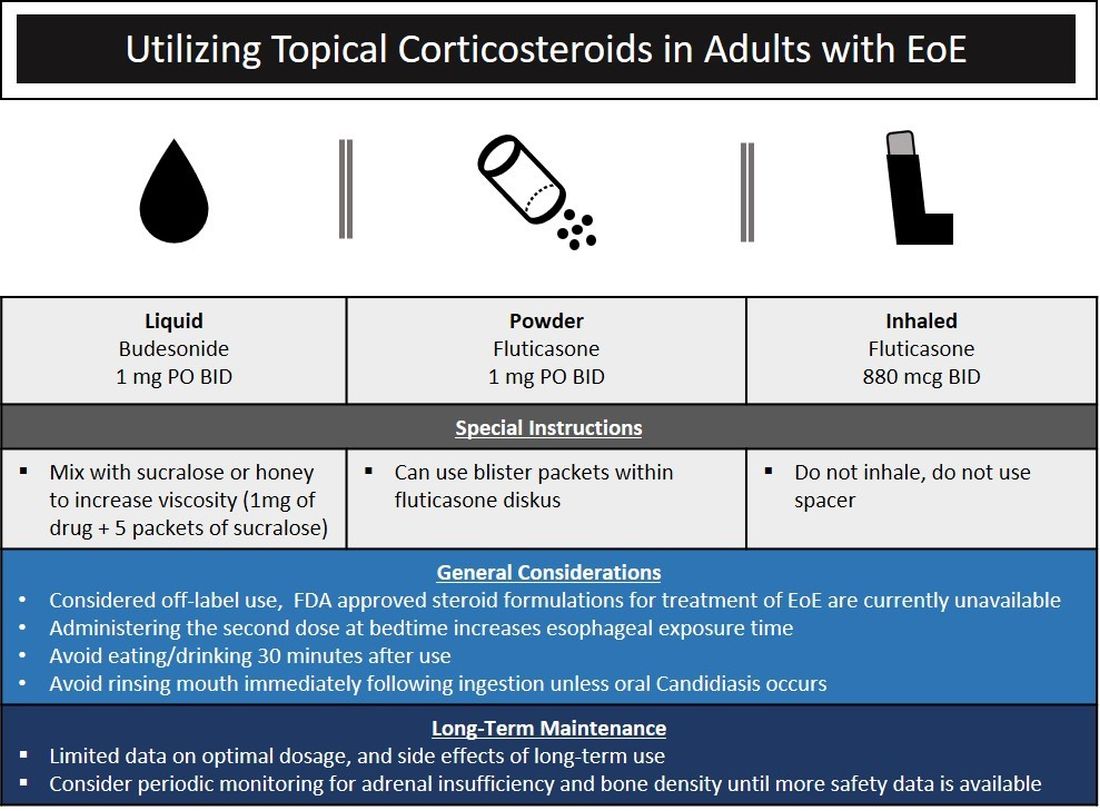
Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).
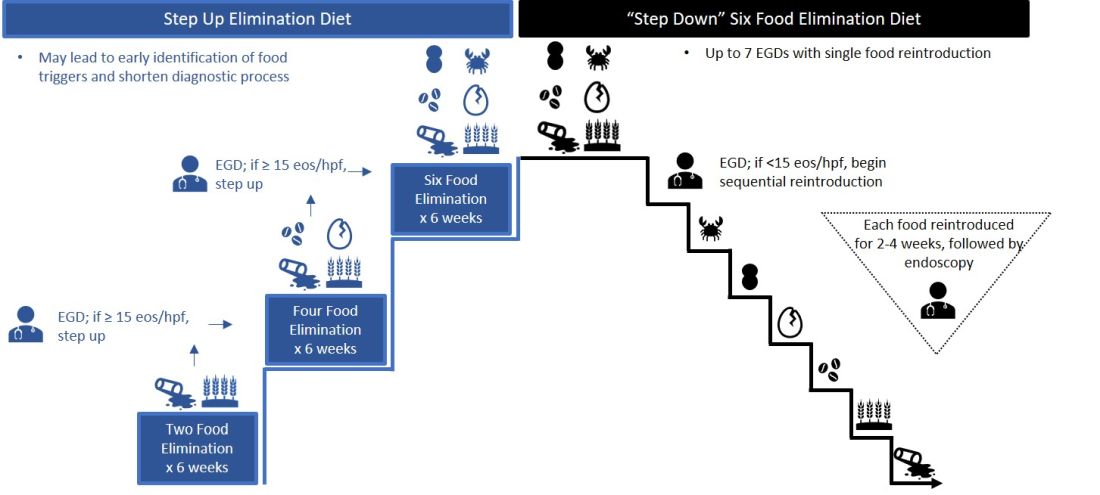
Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12
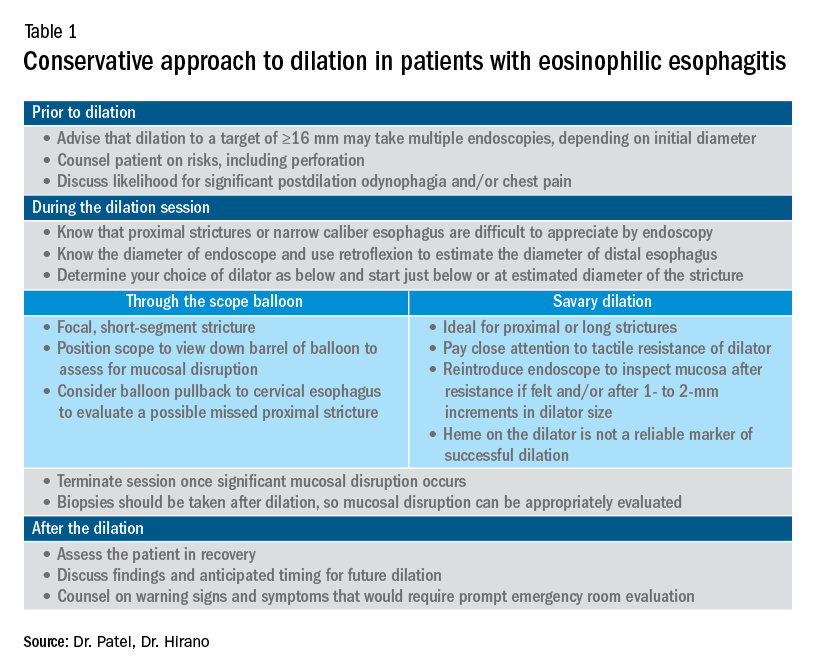
When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).

Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).

Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12

When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).

Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).

Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12

When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Proton pump inhibitors tied to COVID-19 risk
In light of this finding, physicians should consider which patients truly need these powerful acid-lowering drugs, said Brennan Spiegel, MD, MSHS, professor of medicine and public health at Cedars Sinai Medical Center in Los Angeles, Calif.
“All it means is that we’re going to have a conversation with our patients,” he said in an interview. “We don’t normally have that conversation because we don’t live in an environment with a high risk of enteric infection. But now we’re in a pandemic.”
The study by Dr. Spiegel and his colleagues was published online on July 7 in the American Journal of Gastroenterology.
Use of PPIs has skyrocketed over the past 2 decades. For ambulatory care visits, their use increased from 1.6% in 1998 to 7.6% in 2015. The increase raised questions about overprescription.
Although studies have not borne out many of the other concerns raised about adverse reactions, they have shown that the drugs increase the risk for enteric infections, including infections by SARS-CoV-1, a virus that is related to the COVID-19 virus, SARS-CoV-2, Dr. Spiegel said.
SARS-CoV-2 uses the angiotensin-converting enzyme–2 receptor to invade enterocytes. Dr. Spiegel theorized that an increase in stomach pH above 3 as a result of use of PPIs might allow the virus to enter the GI tract more easily, leading to enteritis, colitis, and systemic spread to other organs, including the lungs. “There is a reason we have acid in our stomachs,” Dr. Spiegel said.
To see how PPI use relates to COVID-19 infections, Dr. Spiegel and his colleagues surveyed online a nationally representative sample of Americans between May 3 and June 24, 2020, as part of a larger survey on gastroenterologic health.
Participants answered questions about gastrointestinal symptoms, current use of PPIs, and COVID-19 test results. They also answered questions about histamine-2 receptor agonists (H2RAs), also known as H2 blockers, which are used to treat some of the same conditions as PPIs but that do not reduce stomach acid as much.
The surveying firm, Cint, contacted 264,058 people. Of the 86,602 eligible participants who completed the survey, 53,130 said they had experienced abdominal discomfort, acid reflux, heartburn, or regurgitation. These survey participants were subsequently asked about PPI and H2RA use.
Of these, 6.4% reported testing positive for SARS-CoV-2. The researchers adjusted for age, sex, race/ethnicity, education, marital status, household income, body mass index, smoking, alcohol consumption, U.S. region, insurance status, and the presence of irritable bowel syndrome, celiac disease, gastroesophageal reflux disease, liver cirrhosis, Crohn’s disease, ulcerative colitis, diabetes, and HIV/AIDS.
After adjusting for these factors, the researchers found that those who took PPIs up to once a day were twice as likely to have had a positive COVID-19 test result than those who did not take the drugs (odds ratio, 2.15; 95% confidence interval, 1.90-2.44).
Those who took PPIs twice a day were almost four times as likely to have tested positive for the disease (OR, 3.67; 95% CI, 2.93-4.60).
By contrast, those taking H2RA drugs once daily were 15% less likely to report a positive COVID-19 test result (OR, 0.85; 95% CI, 0.74-0.99). Research is currently underway to determine whether H2RAs might protect against the disease for reasons unrelated to pH balance.
Dr. Spiegel cautioned that the current data show only an association between PPI use and COVID-19 positivity; it cannot prove cause and effect.
Nevertheless, Dr. Spiegel said the findings should encourage physicians to prescribe PPIs only when clearly indicated. “If somebody is not yet on a PPI and you’re considering whether to start them on a PPI, it’s a good idea to consider H2 blockers,” he said.
People who need a daily dose of a PPI to control a severe condition can safely continue doing so, but such patients should take care to follow standard public health recommendations for avoiding exposure to the virus. These recommendations include wearing a mask, maintaining social distance, and washing hands frequently.
“People who are older, comorbid, or smokers – if they get infected, it could be severe,” he said. “[For] someone like that, it’s reasonable to ask, do we really need to be on twice-daily PPIs? There is good evidence that they are no better off than if they are taking once-daily doses.”
Brian Lacy, MD, PhD, a professor of medicine at the Mayo Clinic in Jacksonville, Fla., agreed that the study should prompt physicians to take a second look at their patients’ PPI prescriptions. “My view is that PPIs are frequently overused, and maybe this is one more piece of data that, if someone is on PPIs, maybe they don’t need to be on this medication.”
On the other hand, the drugs are important for treating conditions such as erosive esophagitis and healing ulcers, he said. The overall risk of contracting COVID-19 is low, so even this finding of a 3.7-fold increased risk should not lead patients or providers to stop taking or prescribing PPIs.
The study also lends support to the idea that the gastrointestinal tract could be involved in SARS-CoV-2 transmission, and it supports warnings about aerosols emitted from flushing toilets and through exhalation, Dr. Spiegel said. There is less evidence of the virus being transmitted through food. “It may not be fecal-oral; it may be fecal-respiratory,” he said.
The study was part of a larger project funded by Ironwood Pharmaceuticals. Dr. Spiegel reported relationships with Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Shire Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals. Dr. Lacy has disclosed no relevant financial relationships.
Help your patients better understand GERD and available treatment options by sharing the AGA patient education at http://ow.ly/ol2r30qYPpk.
A version of this article originally appeared on Medscape.com.
In light of this finding, physicians should consider which patients truly need these powerful acid-lowering drugs, said Brennan Spiegel, MD, MSHS, professor of medicine and public health at Cedars Sinai Medical Center in Los Angeles, Calif.
“All it means is that we’re going to have a conversation with our patients,” he said in an interview. “We don’t normally have that conversation because we don’t live in an environment with a high risk of enteric infection. But now we’re in a pandemic.”
The study by Dr. Spiegel and his colleagues was published online on July 7 in the American Journal of Gastroenterology.
Use of PPIs has skyrocketed over the past 2 decades. For ambulatory care visits, their use increased from 1.6% in 1998 to 7.6% in 2015. The increase raised questions about overprescription.
Although studies have not borne out many of the other concerns raised about adverse reactions, they have shown that the drugs increase the risk for enteric infections, including infections by SARS-CoV-1, a virus that is related to the COVID-19 virus, SARS-CoV-2, Dr. Spiegel said.
SARS-CoV-2 uses the angiotensin-converting enzyme–2 receptor to invade enterocytes. Dr. Spiegel theorized that an increase in stomach pH above 3 as a result of use of PPIs might allow the virus to enter the GI tract more easily, leading to enteritis, colitis, and systemic spread to other organs, including the lungs. “There is a reason we have acid in our stomachs,” Dr. Spiegel said.
To see how PPI use relates to COVID-19 infections, Dr. Spiegel and his colleagues surveyed online a nationally representative sample of Americans between May 3 and June 24, 2020, as part of a larger survey on gastroenterologic health.
Participants answered questions about gastrointestinal symptoms, current use of PPIs, and COVID-19 test results. They also answered questions about histamine-2 receptor agonists (H2RAs), also known as H2 blockers, which are used to treat some of the same conditions as PPIs but that do not reduce stomach acid as much.
The surveying firm, Cint, contacted 264,058 people. Of the 86,602 eligible participants who completed the survey, 53,130 said they had experienced abdominal discomfort, acid reflux, heartburn, or regurgitation. These survey participants were subsequently asked about PPI and H2RA use.
Of these, 6.4% reported testing positive for SARS-CoV-2. The researchers adjusted for age, sex, race/ethnicity, education, marital status, household income, body mass index, smoking, alcohol consumption, U.S. region, insurance status, and the presence of irritable bowel syndrome, celiac disease, gastroesophageal reflux disease, liver cirrhosis, Crohn’s disease, ulcerative colitis, diabetes, and HIV/AIDS.
After adjusting for these factors, the researchers found that those who took PPIs up to once a day were twice as likely to have had a positive COVID-19 test result than those who did not take the drugs (odds ratio, 2.15; 95% confidence interval, 1.90-2.44).
Those who took PPIs twice a day were almost four times as likely to have tested positive for the disease (OR, 3.67; 95% CI, 2.93-4.60).
By contrast, those taking H2RA drugs once daily were 15% less likely to report a positive COVID-19 test result (OR, 0.85; 95% CI, 0.74-0.99). Research is currently underway to determine whether H2RAs might protect against the disease for reasons unrelated to pH balance.
Dr. Spiegel cautioned that the current data show only an association between PPI use and COVID-19 positivity; it cannot prove cause and effect.
Nevertheless, Dr. Spiegel said the findings should encourage physicians to prescribe PPIs only when clearly indicated. “If somebody is not yet on a PPI and you’re considering whether to start them on a PPI, it’s a good idea to consider H2 blockers,” he said.
People who need a daily dose of a PPI to control a severe condition can safely continue doing so, but such patients should take care to follow standard public health recommendations for avoiding exposure to the virus. These recommendations include wearing a mask, maintaining social distance, and washing hands frequently.
“People who are older, comorbid, or smokers – if they get infected, it could be severe,” he said. “[For] someone like that, it’s reasonable to ask, do we really need to be on twice-daily PPIs? There is good evidence that they are no better off than if they are taking once-daily doses.”
Brian Lacy, MD, PhD, a professor of medicine at the Mayo Clinic in Jacksonville, Fla., agreed that the study should prompt physicians to take a second look at their patients’ PPI prescriptions. “My view is that PPIs are frequently overused, and maybe this is one more piece of data that, if someone is on PPIs, maybe they don’t need to be on this medication.”
On the other hand, the drugs are important for treating conditions such as erosive esophagitis and healing ulcers, he said. The overall risk of contracting COVID-19 is low, so even this finding of a 3.7-fold increased risk should not lead patients or providers to stop taking or prescribing PPIs.
The study also lends support to the idea that the gastrointestinal tract could be involved in SARS-CoV-2 transmission, and it supports warnings about aerosols emitted from flushing toilets and through exhalation, Dr. Spiegel said. There is less evidence of the virus being transmitted through food. “It may not be fecal-oral; it may be fecal-respiratory,” he said.
The study was part of a larger project funded by Ironwood Pharmaceuticals. Dr. Spiegel reported relationships with Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Shire Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals. Dr. Lacy has disclosed no relevant financial relationships.
Help your patients better understand GERD and available treatment options by sharing the AGA patient education at http://ow.ly/ol2r30qYPpk.
A version of this article originally appeared on Medscape.com.
In light of this finding, physicians should consider which patients truly need these powerful acid-lowering drugs, said Brennan Spiegel, MD, MSHS, professor of medicine and public health at Cedars Sinai Medical Center in Los Angeles, Calif.
“All it means is that we’re going to have a conversation with our patients,” he said in an interview. “We don’t normally have that conversation because we don’t live in an environment with a high risk of enteric infection. But now we’re in a pandemic.”
The study by Dr. Spiegel and his colleagues was published online on July 7 in the American Journal of Gastroenterology.
Use of PPIs has skyrocketed over the past 2 decades. For ambulatory care visits, their use increased from 1.6% in 1998 to 7.6% in 2015. The increase raised questions about overprescription.
Although studies have not borne out many of the other concerns raised about adverse reactions, they have shown that the drugs increase the risk for enteric infections, including infections by SARS-CoV-1, a virus that is related to the COVID-19 virus, SARS-CoV-2, Dr. Spiegel said.
SARS-CoV-2 uses the angiotensin-converting enzyme–2 receptor to invade enterocytes. Dr. Spiegel theorized that an increase in stomach pH above 3 as a result of use of PPIs might allow the virus to enter the GI tract more easily, leading to enteritis, colitis, and systemic spread to other organs, including the lungs. “There is a reason we have acid in our stomachs,” Dr. Spiegel said.
To see how PPI use relates to COVID-19 infections, Dr. Spiegel and his colleagues surveyed online a nationally representative sample of Americans between May 3 and June 24, 2020, as part of a larger survey on gastroenterologic health.
Participants answered questions about gastrointestinal symptoms, current use of PPIs, and COVID-19 test results. They also answered questions about histamine-2 receptor agonists (H2RAs), also known as H2 blockers, which are used to treat some of the same conditions as PPIs but that do not reduce stomach acid as much.
The surveying firm, Cint, contacted 264,058 people. Of the 86,602 eligible participants who completed the survey, 53,130 said they had experienced abdominal discomfort, acid reflux, heartburn, or regurgitation. These survey participants were subsequently asked about PPI and H2RA use.
Of these, 6.4% reported testing positive for SARS-CoV-2. The researchers adjusted for age, sex, race/ethnicity, education, marital status, household income, body mass index, smoking, alcohol consumption, U.S. region, insurance status, and the presence of irritable bowel syndrome, celiac disease, gastroesophageal reflux disease, liver cirrhosis, Crohn’s disease, ulcerative colitis, diabetes, and HIV/AIDS.
After adjusting for these factors, the researchers found that those who took PPIs up to once a day were twice as likely to have had a positive COVID-19 test result than those who did not take the drugs (odds ratio, 2.15; 95% confidence interval, 1.90-2.44).
Those who took PPIs twice a day were almost four times as likely to have tested positive for the disease (OR, 3.67; 95% CI, 2.93-4.60).
By contrast, those taking H2RA drugs once daily were 15% less likely to report a positive COVID-19 test result (OR, 0.85; 95% CI, 0.74-0.99). Research is currently underway to determine whether H2RAs might protect against the disease for reasons unrelated to pH balance.
Dr. Spiegel cautioned that the current data show only an association between PPI use and COVID-19 positivity; it cannot prove cause and effect.
Nevertheless, Dr. Spiegel said the findings should encourage physicians to prescribe PPIs only when clearly indicated. “If somebody is not yet on a PPI and you’re considering whether to start them on a PPI, it’s a good idea to consider H2 blockers,” he said.
People who need a daily dose of a PPI to control a severe condition can safely continue doing so, but such patients should take care to follow standard public health recommendations for avoiding exposure to the virus. These recommendations include wearing a mask, maintaining social distance, and washing hands frequently.
“People who are older, comorbid, or smokers – if they get infected, it could be severe,” he said. “[For] someone like that, it’s reasonable to ask, do we really need to be on twice-daily PPIs? There is good evidence that they are no better off than if they are taking once-daily doses.”
Brian Lacy, MD, PhD, a professor of medicine at the Mayo Clinic in Jacksonville, Fla., agreed that the study should prompt physicians to take a second look at their patients’ PPI prescriptions. “My view is that PPIs are frequently overused, and maybe this is one more piece of data that, if someone is on PPIs, maybe they don’t need to be on this medication.”
On the other hand, the drugs are important for treating conditions such as erosive esophagitis and healing ulcers, he said. The overall risk of contracting COVID-19 is low, so even this finding of a 3.7-fold increased risk should not lead patients or providers to stop taking or prescribing PPIs.
The study also lends support to the idea that the gastrointestinal tract could be involved in SARS-CoV-2 transmission, and it supports warnings about aerosols emitted from flushing toilets and through exhalation, Dr. Spiegel said. There is less evidence of the virus being transmitted through food. “It may not be fecal-oral; it may be fecal-respiratory,” he said.
The study was part of a larger project funded by Ironwood Pharmaceuticals. Dr. Spiegel reported relationships with Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Shire Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals. Dr. Lacy has disclosed no relevant financial relationships.
Help your patients better understand GERD and available treatment options by sharing the AGA patient education at http://ow.ly/ol2r30qYPpk.
A version of this article originally appeared on Medscape.com.
Endoscopic myotomy for achalasia
Dear colleagues and friends,
In this edition of Perspectives, Dr. Mouen Khashab and Dr. Robert Siwiec tackle an exciting and constantly evolving topic, which is the optimal approach to myotomy for patients with achalasia. Dr. Khashab makes the case for endoscopic myotomy, while Dr. Siwiec argues that surgical myotomy remains the gold standard. I hope that you will find this debate as useful and thought provoking as I did. As always, I welcome your comments and suggestions for future topics at [email protected].
Charles Kahi, MD, MS, AGAF, is a professor of medicine at Indiana University, Indianapolis. He is also an associate editor for GI & Hepatology News.
Endoscopic myotomy for achalasia is ready for prime time
BY MOUEN A. KHASHAB, MD
When I encounter a symptomatic patient with manometrically confirmed achalasia, I discuss three effective treatment modalities: pneumatic dilation (PD), peroral endoscopic myotomy (POEM), and laparoscopic Heller myotomy (LHM). I recommend against botulinum toxin injection and reserve it for patients who are not candidates for the aforementioned definitive therapies. I also present to the patient the current level I evidence from randomized, controlled trials (RCTs) comparing achalasia treatment modalities.
One landmark RCT reported comparative outcomes at 2 years following POEM and PD and found higher treatment success at the 2-year follow-up in the POEM group (92% vs. 54%; P < .001).1 Reflux esophagitis was observed significantly more frequently in patients treated with POEM (41% in the POEM group, of whom 35% were assigned Los Angeles grade A-B and 6% were assigned LA grade C versus 7% in the PD group, all of whom were assigned LA grade A; P = .002).1
Another milestone RCT included 221 patients and compared outcomes of POEM and LHM plus Dor fundoplication.2 Clinical success at the 2-year follow-up was observed in 83.0% of patients in the POEM group, and was noninferior to the LHM group (81.7%). Serious adverse events occurred in 2.7% of patients in the POEM group and in 7.3% of patients in the LHM group. Although 57% of patients in the POEM group and 20% of patients in the LHM group had reflux esophagitis as assessed by endoscopy at 3 months, the corresponding proportions were 44% and 29% at 24 months. Importantly, the rate of severe esophagitis was not different between both groups (6% vs. 3% at 3 months, and 5% vs. 6% at 24 months).2
I summarize these results by stating that POEM seems to be superior to PD and equivalent to LHM in terms of clinical success. Nonetheless, POEM also seems to be associated with increased risk of early gastroesophageal reflux disease.
POEM is now a ubiquitous procedure performed worldwide and is endorsed as a primary achalasia treatment by multiple society guidelines.3 It is a minimally invasive, effective, and safe therapeutic option for patients with all types of achalasia and is considered the treatment of choice for achalasia type III. POEM has also been shown to be effective in the treatment of spastic esophageal disorders (e.g. Jackhammer esophagus, diffuse esophageal spasm) and esophagogastric junction outflow obstruction. It can be performed in the endoscopy unit or operating theater either by experienced therapeutic endoscopists or surgical endoscopists in less than an hour. The procedure can be performed on an outpatient basis in appropriate individuals and allows tailoring the myotomy length to specific clinical scenarios. For example, patients with type III achalasia (and those with spastic esophageal disorders) typically require a long myotomy and that can be readily accomplished during POEM as opposed to LHM. POEM has also proven effective in children; octogenarians; and patients with sigmoid esophagus, epiphrenic diverticula, and those who had undergone prior interventions for achalasia, including LHM and PD. In experienced hands, the rate of adverse events is low and serious events are rare and occur in 0.5% of cases. Perforations/leakage are also uncommon and occur in 0.7% of patients. It is an incisionless procedure that eliminates the risk of wound infection and shortens postprocedural recovery. Patients are typically admitted for an overnight observation postprocedure, discharged home the following day, and back to activities of daily living (including work) within a few days. Postprocedural pain is minimal in most patients and narcotics are rarely needed. Resumption of a soft diet is carried out on the first postoperative day and normal diet 1 week later.
LHM is an established procedure with proven long-term efficacy in the treatment of achalasia. Nonetheless, it is invasive and requires placement of multiple trocars. The procedure is more time consuming than POEM and length of hospital stay can also be longer. This results in possibly higher cost than POEM. Importantly, recovery of dysphagia and resumption of normal diet is significantly delayed and is likely the result of the partial concomitant fundoplication procedure. Finally, LHM is not appropriate for the treatment of spastic esophageal disorders, including type III achalasia.
A major advantage of LHM plus partial fundoplication over POEM is the diminished risk of gastroesophageal reflux disease (GERD). However, this advantage seems short lived as the risk of GERD increases over time after surgery, likely because of the loosening of the wrap over time. From the New England Journal of Medicine paper mentioned earlier, it seems that the increased risk of GERD after POEM as compared with LHM diminishes over time.2 Importantly, it also appears that the rate of significant esophagitis (LA grade C-D) is similar between both procedures.2
In an effort to assess the long-term antireflux efficacy of surgical partial fundoplication, one study noted that 12% of 182 patients who had surgical myotomy with partial fundoplication continued to have occasional or continuous heartburn symptoms at a median of 18 years after surgery. Esophagitis and Barrett’s esophagus were found in 14.5% and 0.8% of patients, respectively. De novo esophageal adenocarcinoma has been reported after both POEM and LHM.4
Therefore, GERD and its complications can occur after any procedure that disrupts the lower esophageal sphincter (POEM, LHM, and PD) and postprocedural management of patients should include long-term testing and management of possible GERD. Different strategies have been proposed and include objective periodic testing for esophageal acid exposure, long-term and possible lifelong proton pump inhibitor use, and surveillance for long-term consequences of GER via periodic upper endoscopy.3
It is important to acknowledge that the lack of symptoms or the absence of endoscopic evidence of GER on initial endoscopy does not necessarily rule out GER. Approximately a third of post-POEM patients with clinically successful outcome and absence of reflux esophagitis on their first surveillance endoscopy eventually develop esophagitis at subsequent surveillance endoscopy.5
In summary, POEM has deservingly taken a prime time spot in the management of patients with achalasia. It is an efficient, efficacious and safe treatment modality that results in rapid resolution of achalasia symptoms in the majority of patients. Research should focus on technical modifications (e.g., short gastric myotomy; addition of endoscopic fundoplication) that reduce the incidence of postprocedural GERD.
References
1. Ponds FA et al. Effect of peroral endoscopic myotomy versus pneumatic dilation on symptom severity and treatment outcomes among treatment-naive patients with achalasia: A randomized clinical trial. JAMA. 2019;322:134-44.
2. Werner YB et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med. 2019;381:2219-29.
3. Khashab MA et al. ASGE guideline on the management of achalasia. Gastrointest Endosc. 2020;91:213-27 e6.
4. Ichkhanian Y et al. Case of early Barrett cancer following peroral endoscopic myotomy. Gut. 2019;68:2107-110.
5. Werner YB et al. Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years. Gut. 2016;65:899-906.
Dr. Khashab is associate professor of medicine, director of therapeutic endoscopy, division of gastroenterology and hepatology, Johns Hopkins Hospital, Baltimore. He is a consultant for BSCI, Olympus, and Medtronic.
Heller myotomy is still the gold standard
BY ROBERT M. SIWIEC, MD
Achalasia is a rare, primary esophageal motor disorder characterized by ineffective relaxation of the lower esophageal sphincter (LES) and concomitant loss of esophageal peristalsis. High-resolution esophageal manometry has allowed for the diagnosis and classification of achalasia into relevant clinical subtypes which become important when discussing and considering treatment options. Confirmatory studies (e.g., timed barium esophagram) and provocative manometric maneuvers (e.g., upright swallows, rapid swallow sequence, and/or rapid drink challenge) can be helpful when distinguishing between true achalasia versus achalasia variants and esophagogastric junction outflow obstruction.
Treatment options only provide palliation by eliminating outflow obstruction caused by a nonrelaxing and often times hypertensive LES. Pharmacotherapy (e.g., oral nitrates, 5-phosphodiesterase inhibitors, anticholinergics) is the least effective option because of medication side effects and short-acting duration. I only consider it for patients who are either unwilling or unable to tolerate invasive therapies. Botulinum toxin injection into the LES can be considered in patients who are not good candidates for more definitive therapy with PD or myotomy (endoscopic or surgical). Although the success rates with botulinum toxin are comparable with PD and surgical myotomy, patients treated with botulinum toxin require retreatment. Furthermore, continued botulinum toxin injections can compromise tissue planes making myotomy complex and challenging.
During the 1970s and 1980s, PD was the primary treatment modality for achalasia. Surgical myotomy was reserved for patients who suffered a perforation during PD or developed recurrent symptoms after multiple dilations. Minimally invasive surgery (left thoracoscopic approach) for achalasia was first introduced in the early 1990s and was shown to be a feasible, safe, and effective procedure, becoming the primary treatment approach in most centers. Patients fared well; however, it was soon discovered that >50% had pathological reflux based on pH monitoring. A few centers then began to perform a Heller myotomy through a laparoscopic approach with the addition of a fundoplication resulting in significant reductions in pathological reflux by pH monitoring. Eventually, a seminal RCT confirmed the importance of fundoplication with laparoscopic Heller myotomy (LHM) – resolution of dysphagia was unaffected and pathological reflux was avoided in most patients.1 Overall, clinical success rates for LHM with fundoplication are typically >90% and reflux incidence rates <10% with the overall complication rate being about 5% with reported mortality <0.1%.
PD remains appealing in that it is cost effective and less invasive, compared with POEM and LHM. Initial success rates and short-term efficacy are comparable with LHM but unfortunately PD’s efficacy significantly wanes over time. POEM, introduced by Inoue et al. in 2010, is a novel endoscopic technique with an excellent safety profile that provides good symptom relief while avoiding abdominal wall scars for patients. It has been shown to have a distinct advantage in patients with type III achalasia by nature of the longer myotomy not achievable by LHM.2 POEM has seen increasing enthusiasm and acceptance as a standard treatment option for achalasia largely because of the fact that its safety and efficacy have been shown to be comparable and in most cases equal with LHM. However, in 2020, direct comparison with LHM is challenging given that the follow-up in the majority of studies is either short or incomplete. The most recent multicenter, randomized trial comparing POEM with LHM plus Dor fundoplication showed POEM’s noninferiority in controlling symptoms of achalasia, but only after a 24 month follow-up.3 A recent report included one of the largest cohorts of post-POEM patients (500), but the 36-month data were based on the follow-up of only 61 patients (about 12%).4
Once the muscle fibers of the LES are disrupted, reflux will occur in the majority of patients. Unlike LHM, no concomitant fundoplication is performed during POEM and this increases the incidence of GERD and its long-term sequelae including peptic strictures, Barrett’s esophagus, and adenocarcinoma. A meta-analysis from 2018 looked at published series of POEM and LHM with fundoplication and found that GERD symptoms were present in 19% of POEM patients, compared with 8.8% of LHM patients. Worse yet, esophagitis was seen in 29.4% of the POEM group and 7.6% of the LHM group, with more individuals in the POEM group also having abnormal acid exposure based on ambulatory pH monitoring (39.0% vs. 16.8%).5
Proponents of POEM will argue that proton pump inhibitors (PPIs) are the panacea for post-POEM GERD. Unfortunately, this approach has its own problems. PPIs are very effective at reducing acid secretion by parietal cells, but do not block reflux through an iatrogenically incompetent LES. The drumbeat of publications on potential complications from chronic PPI use has greatly contributed to patients’ reluctance to commit to long-term PPI use. Lastly, the first case of early Barrett’s cancer was recently reported in a patient 4 years post-POEM despite adherence to an aggressive antisecretory regimen (b.i.d. PPI and H2 blocker at bedtime).6 LHM with fundoplication significantly reduces the risk of pathological GERD and spares patients from committing to lifelong PPI therapy and routine endoscopic surveillance (appropriate interval yet to be determined) and needing to consider additional procedures (i.e., endoscopic or surgical fundoplication).
Despite POEM’s well established efficacy and safety, the development of post-POEM GERD is a major concern that has yet to be adequately addressed. A significant number of post-POEM patients with pathological reflux have asymptomatic and unrecognized GERD and current management and monitoring strategies for post-POEM GERD are anemic and poorly established. Without question, there are individual patients who are clearly better served with POEM (type III achalasia and other spastic esophageal disorders). However, as we continue to learn more about post-POEM GERD and how to better prevent, manage, and monitor it, LHM with fundoplication for the time being remains the tried-and-tested treatment option for patients with non–type III achalasia.
References
1. Richards WO et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: A prospective randomized double-blind clinical trial. Ann Surg. 2004;240:405-12.
2. Podboy AJ et al. Long-term outcomes of peroral endoscopic myotomy compared to laparoscopic Heller myotomy for achalasia: A single-center experience. Surg Endosc. 2020. doi: 10.1007/s00464-020-07450-6.
3. Werner YB et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med. 2019;381(23):2219-29.
4. Inoue H et al. Peroral endoscopic myotomy: A series of 500 patients. J Am Coll Surg. 2015;221:256-64.
5. Repici A et al. GERD after peroral endoscopic myotomy as compared with Heller’s myotomy with fundoplication: A systematic review with meta-analysis. Gastrointest Endosc. 2018;87(4):934-43.
6. Ichkhanian Y et al. Case of early Barrett cancer following peroral endoscopic myotomy. Gut. 2019;68:2107-10.
Dr. Siwiec is assistant professor of clinical medicine, division of gastroenterology and hepatology, GI motility and neurogastroenterology unit, Indiana University, Indianapolis. He has no conflicts of interest.
Dear colleagues and friends,
In this edition of Perspectives, Dr. Mouen Khashab and Dr. Robert Siwiec tackle an exciting and constantly evolving topic, which is the optimal approach to myotomy for patients with achalasia. Dr. Khashab makes the case for endoscopic myotomy, while Dr. Siwiec argues that surgical myotomy remains the gold standard. I hope that you will find this debate as useful and thought provoking as I did. As always, I welcome your comments and suggestions for future topics at [email protected].
Charles Kahi, MD, MS, AGAF, is a professor of medicine at Indiana University, Indianapolis. He is also an associate editor for GI & Hepatology News.
Endoscopic myotomy for achalasia is ready for prime time
BY MOUEN A. KHASHAB, MD
When I encounter a symptomatic patient with manometrically confirmed achalasia, I discuss three effective treatment modalities: pneumatic dilation (PD), peroral endoscopic myotomy (POEM), and laparoscopic Heller myotomy (LHM). I recommend against botulinum toxin injection and reserve it for patients who are not candidates for the aforementioned definitive therapies. I also present to the patient the current level I evidence from randomized, controlled trials (RCTs) comparing achalasia treatment modalities.
One landmark RCT reported comparative outcomes at 2 years following POEM and PD and found higher treatment success at the 2-year follow-up in the POEM group (92% vs. 54%; P < .001).1 Reflux esophagitis was observed significantly more frequently in patients treated with POEM (41% in the POEM group, of whom 35% were assigned Los Angeles grade A-B and 6% were assigned LA grade C versus 7% in the PD group, all of whom were assigned LA grade A; P = .002).1
Another milestone RCT included 221 patients and compared outcomes of POEM and LHM plus Dor fundoplication.2 Clinical success at the 2-year follow-up was observed in 83.0% of patients in the POEM group, and was noninferior to the LHM group (81.7%). Serious adverse events occurred in 2.7% of patients in the POEM group and in 7.3% of patients in the LHM group. Although 57% of patients in the POEM group and 20% of patients in the LHM group had reflux esophagitis as assessed by endoscopy at 3 months, the corresponding proportions were 44% and 29% at 24 months. Importantly, the rate of severe esophagitis was not different between both groups (6% vs. 3% at 3 months, and 5% vs. 6% at 24 months).2
I summarize these results by stating that POEM seems to be superior to PD and equivalent to LHM in terms of clinical success. Nonetheless, POEM also seems to be associated with increased risk of early gastroesophageal reflux disease.
POEM is now a ubiquitous procedure performed worldwide and is endorsed as a primary achalasia treatment by multiple society guidelines.3 It is a minimally invasive, effective, and safe therapeutic option for patients with all types of achalasia and is considered the treatment of choice for achalasia type III. POEM has also been shown to be effective in the treatment of spastic esophageal disorders (e.g. Jackhammer esophagus, diffuse esophageal spasm) and esophagogastric junction outflow obstruction. It can be performed in the endoscopy unit or operating theater either by experienced therapeutic endoscopists or surgical endoscopists in less than an hour. The procedure can be performed on an outpatient basis in appropriate individuals and allows tailoring the myotomy length to specific clinical scenarios. For example, patients with type III achalasia (and those with spastic esophageal disorders) typically require a long myotomy and that can be readily accomplished during POEM as opposed to LHM. POEM has also proven effective in children; octogenarians; and patients with sigmoid esophagus, epiphrenic diverticula, and those who had undergone prior interventions for achalasia, including LHM and PD. In experienced hands, the rate of adverse events is low and serious events are rare and occur in 0.5% of cases. Perforations/leakage are also uncommon and occur in 0.7% of patients. It is an incisionless procedure that eliminates the risk of wound infection and shortens postprocedural recovery. Patients are typically admitted for an overnight observation postprocedure, discharged home the following day, and back to activities of daily living (including work) within a few days. Postprocedural pain is minimal in most patients and narcotics are rarely needed. Resumption of a soft diet is carried out on the first postoperative day and normal diet 1 week later.
LHM is an established procedure with proven long-term efficacy in the treatment of achalasia. Nonetheless, it is invasive and requires placement of multiple trocars. The procedure is more time consuming than POEM and length of hospital stay can also be longer. This results in possibly higher cost than POEM. Importantly, recovery of dysphagia and resumption of normal diet is significantly delayed and is likely the result of the partial concomitant fundoplication procedure. Finally, LHM is not appropriate for the treatment of spastic esophageal disorders, including type III achalasia.
A major advantage of LHM plus partial fundoplication over POEM is the diminished risk of gastroesophageal reflux disease (GERD). However, this advantage seems short lived as the risk of GERD increases over time after surgery, likely because of the loosening of the wrap over time. From the New England Journal of Medicine paper mentioned earlier, it seems that the increased risk of GERD after POEM as compared with LHM diminishes over time.2 Importantly, it also appears that the rate of significant esophagitis (LA grade C-D) is similar between both procedures.2
In an effort to assess the long-term antireflux efficacy of surgical partial fundoplication, one study noted that 12% of 182 patients who had surgical myotomy with partial fundoplication continued to have occasional or continuous heartburn symptoms at a median of 18 years after surgery. Esophagitis and Barrett’s esophagus were found in 14.5% and 0.8% of patients, respectively. De novo esophageal adenocarcinoma has been reported after both POEM and LHM.4
Therefore, GERD and its complications can occur after any procedure that disrupts the lower esophageal sphincter (POEM, LHM, and PD) and postprocedural management of patients should include long-term testing and management of possible GERD. Different strategies have been proposed and include objective periodic testing for esophageal acid exposure, long-term and possible lifelong proton pump inhibitor use, and surveillance for long-term consequences of GER via periodic upper endoscopy.3
It is important to acknowledge that the lack of symptoms or the absence of endoscopic evidence of GER on initial endoscopy does not necessarily rule out GER. Approximately a third of post-POEM patients with clinically successful outcome and absence of reflux esophagitis on their first surveillance endoscopy eventually develop esophagitis at subsequent surveillance endoscopy.5
In summary, POEM has deservingly taken a prime time spot in the management of patients with achalasia. It is an efficient, efficacious and safe treatment modality that results in rapid resolution of achalasia symptoms in the majority of patients. Research should focus on technical modifications (e.g., short gastric myotomy; addition of endoscopic fundoplication) that reduce the incidence of postprocedural GERD.
References
1. Ponds FA et al. Effect of peroral endoscopic myotomy versus pneumatic dilation on symptom severity and treatment outcomes among treatment-naive patients with achalasia: A randomized clinical trial. JAMA. 2019;322:134-44.
2. Werner YB et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med. 2019;381:2219-29.
3. Khashab MA et al. ASGE guideline on the management of achalasia. Gastrointest Endosc. 2020;91:213-27 e6.
4. Ichkhanian Y et al. Case of early Barrett cancer following peroral endoscopic myotomy. Gut. 2019;68:2107-110.
5. Werner YB et al. Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years. Gut. 2016;65:899-906.
Dr. Khashab is associate professor of medicine, director of therapeutic endoscopy, division of gastroenterology and hepatology, Johns Hopkins Hospital, Baltimore. He is a consultant for BSCI, Olympus, and Medtronic.
Heller myotomy is still the gold standard
BY ROBERT M. SIWIEC, MD
Achalasia is a rare, primary esophageal motor disorder characterized by ineffective relaxation of the lower esophageal sphincter (LES) and concomitant loss of esophageal peristalsis. High-resolution esophageal manometry has allowed for the diagnosis and classification of achalasia into relevant clinical subtypes which become important when discussing and considering treatment options. Confirmatory studies (e.g., timed barium esophagram) and provocative manometric maneuvers (e.g., upright swallows, rapid swallow sequence, and/or rapid drink challenge) can be helpful when distinguishing between true achalasia versus achalasia variants and esophagogastric junction outflow obstruction.
Treatment options only provide palliation by eliminating outflow obstruction caused by a nonrelaxing and often times hypertensive LES. Pharmacotherapy (e.g., oral nitrates, 5-phosphodiesterase inhibitors, anticholinergics) is the least effective option because of medication side effects and short-acting duration. I only consider it for patients who are either unwilling or unable to tolerate invasive therapies. Botulinum toxin injection into the LES can be considered in patients who are not good candidates for more definitive therapy with PD or myotomy (endoscopic or surgical). Although the success rates with botulinum toxin are comparable with PD and surgical myotomy, patients treated with botulinum toxin require retreatment. Furthermore, continued botulinum toxin injections can compromise tissue planes making myotomy complex and challenging.
During the 1970s and 1980s, PD was the primary treatment modality for achalasia. Surgical myotomy was reserved for patients who suffered a perforation during PD or developed recurrent symptoms after multiple dilations. Minimally invasive surgery (left thoracoscopic approach) for achalasia was first introduced in the early 1990s and was shown to be a feasible, safe, and effective procedure, becoming the primary treatment approach in most centers. Patients fared well; however, it was soon discovered that >50% had pathological reflux based on pH monitoring. A few centers then began to perform a Heller myotomy through a laparoscopic approach with the addition of a fundoplication resulting in significant reductions in pathological reflux by pH monitoring. Eventually, a seminal RCT confirmed the importance of fundoplication with laparoscopic Heller myotomy (LHM) – resolution of dysphagia was unaffected and pathological reflux was avoided in most patients.1 Overall, clinical success rates for LHM with fundoplication are typically >90% and reflux incidence rates <10% with the overall complication rate being about 5% with reported mortality <0.1%.
PD remains appealing in that it is cost effective and less invasive, compared with POEM and LHM. Initial success rates and short-term efficacy are comparable with LHM but unfortunately PD’s efficacy significantly wanes over time. POEM, introduced by Inoue et al. in 2010, is a novel endoscopic technique with an excellent safety profile that provides good symptom relief while avoiding abdominal wall scars for patients. It has been shown to have a distinct advantage in patients with type III achalasia by nature of the longer myotomy not achievable by LHM.2 POEM has seen increasing enthusiasm and acceptance as a standard treatment option for achalasia largely because of the fact that its safety and efficacy have been shown to be comparable and in most cases equal with LHM. However, in 2020, direct comparison with LHM is challenging given that the follow-up in the majority of studies is either short or incomplete. The most recent multicenter, randomized trial comparing POEM with LHM plus Dor fundoplication showed POEM’s noninferiority in controlling symptoms of achalasia, but only after a 24 month follow-up.3 A recent report included one of the largest cohorts of post-POEM patients (500), but the 36-month data were based on the follow-up of only 61 patients (about 12%).4
Once the muscle fibers of the LES are disrupted, reflux will occur in the majority of patients. Unlike LHM, no concomitant fundoplication is performed during POEM and this increases the incidence of GERD and its long-term sequelae including peptic strictures, Barrett’s esophagus, and adenocarcinoma. A meta-analysis from 2018 looked at published series of POEM and LHM with fundoplication and found that GERD symptoms were present in 19% of POEM patients, compared with 8.8% of LHM patients. Worse yet, esophagitis was seen in 29.4% of the POEM group and 7.6% of the LHM group, with more individuals in the POEM group also having abnormal acid exposure based on ambulatory pH monitoring (39.0% vs. 16.8%).5
Proponents of POEM will argue that proton pump inhibitors (PPIs) are the panacea for post-POEM GERD. Unfortunately, this approach has its own problems. PPIs are very effective at reducing acid secretion by parietal cells, but do not block reflux through an iatrogenically incompetent LES. The drumbeat of publications on potential complications from chronic PPI use has greatly contributed to patients’ reluctance to commit to long-term PPI use. Lastly, the first case of early Barrett’s cancer was recently reported in a patient 4 years post-POEM despite adherence to an aggressive antisecretory regimen (b.i.d. PPI and H2 blocker at bedtime).6 LHM with fundoplication significantly reduces the risk of pathological GERD and spares patients from committing to lifelong PPI therapy and routine endoscopic surveillance (appropriate interval yet to be determined) and needing to consider additional procedures (i.e., endoscopic or surgical fundoplication).
Despite POEM’s well established efficacy and safety, the development of post-POEM GERD is a major concern that has yet to be adequately addressed. A significant number of post-POEM patients with pathological reflux have asymptomatic and unrecognized GERD and current management and monitoring strategies for post-POEM GERD are anemic and poorly established. Without question, there are individual patients who are clearly better served with POEM (type III achalasia and other spastic esophageal disorders). However, as we continue to learn more about post-POEM GERD and how to better prevent, manage, and monitor it, LHM with fundoplication for the time being remains the tried-and-tested treatment option for patients with non–type III achalasia.
References
1. Richards WO et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: A prospective randomized double-blind clinical trial. Ann Surg. 2004;240:405-12.
2. Podboy AJ et al. Long-term outcomes of peroral endoscopic myotomy compared to laparoscopic Heller myotomy for achalasia: A single-center experience. Surg Endosc. 2020. doi: 10.1007/s00464-020-07450-6.
3. Werner YB et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med. 2019;381(23):2219-29.
4. Inoue H et al. Peroral endoscopic myotomy: A series of 500 patients. J Am Coll Surg. 2015;221:256-64.
5. Repici A et al. GERD after peroral endoscopic myotomy as compared with Heller’s myotomy with fundoplication: A systematic review with meta-analysis. Gastrointest Endosc. 2018;87(4):934-43.
6. Ichkhanian Y et al. Case of early Barrett cancer following peroral endoscopic myotomy. Gut. 2019;68:2107-10.
Dr. Siwiec is assistant professor of clinical medicine, division of gastroenterology and hepatology, GI motility and neurogastroenterology unit, Indiana University, Indianapolis. He has no conflicts of interest.
Dear colleagues and friends,
In this edition of Perspectives, Dr. Mouen Khashab and Dr. Robert Siwiec tackle an exciting and constantly evolving topic, which is the optimal approach to myotomy for patients with achalasia. Dr. Khashab makes the case for endoscopic myotomy, while Dr. Siwiec argues that surgical myotomy remains the gold standard. I hope that you will find this debate as useful and thought provoking as I did. As always, I welcome your comments and suggestions for future topics at [email protected].
Charles Kahi, MD, MS, AGAF, is a professor of medicine at Indiana University, Indianapolis. He is also an associate editor for GI & Hepatology News.
Endoscopic myotomy for achalasia is ready for prime time
BY MOUEN A. KHASHAB, MD
When I encounter a symptomatic patient with manometrically confirmed achalasia, I discuss three effective treatment modalities: pneumatic dilation (PD), peroral endoscopic myotomy (POEM), and laparoscopic Heller myotomy (LHM). I recommend against botulinum toxin injection and reserve it for patients who are not candidates for the aforementioned definitive therapies. I also present to the patient the current level I evidence from randomized, controlled trials (RCTs) comparing achalasia treatment modalities.
One landmark RCT reported comparative outcomes at 2 years following POEM and PD and found higher treatment success at the 2-year follow-up in the POEM group (92% vs. 54%; P < .001).1 Reflux esophagitis was observed significantly more frequently in patients treated with POEM (41% in the POEM group, of whom 35% were assigned Los Angeles grade A-B and 6% were assigned LA grade C versus 7% in the PD group, all of whom were assigned LA grade A; P = .002).1
Another milestone RCT included 221 patients and compared outcomes of POEM and LHM plus Dor fundoplication.2 Clinical success at the 2-year follow-up was observed in 83.0% of patients in the POEM group, and was noninferior to the LHM group (81.7%). Serious adverse events occurred in 2.7% of patients in the POEM group and in 7.3% of patients in the LHM group. Although 57% of patients in the POEM group and 20% of patients in the LHM group had reflux esophagitis as assessed by endoscopy at 3 months, the corresponding proportions were 44% and 29% at 24 months. Importantly, the rate of severe esophagitis was not different between both groups (6% vs. 3% at 3 months, and 5% vs. 6% at 24 months).2
I summarize these results by stating that POEM seems to be superior to PD and equivalent to LHM in terms of clinical success. Nonetheless, POEM also seems to be associated with increased risk of early gastroesophageal reflux disease.
POEM is now a ubiquitous procedure performed worldwide and is endorsed as a primary achalasia treatment by multiple society guidelines.3 It is a minimally invasive, effective, and safe therapeutic option for patients with all types of achalasia and is considered the treatment of choice for achalasia type III. POEM has also been shown to be effective in the treatment of spastic esophageal disorders (e.g. Jackhammer esophagus, diffuse esophageal spasm) and esophagogastric junction outflow obstruction. It can be performed in the endoscopy unit or operating theater either by experienced therapeutic endoscopists or surgical endoscopists in less than an hour. The procedure can be performed on an outpatient basis in appropriate individuals and allows tailoring the myotomy length to specific clinical scenarios. For example, patients with type III achalasia (and those with spastic esophageal disorders) typically require a long myotomy and that can be readily accomplished during POEM as opposed to LHM. POEM has also proven effective in children; octogenarians; and patients with sigmoid esophagus, epiphrenic diverticula, and those who had undergone prior interventions for achalasia, including LHM and PD. In experienced hands, the rate of adverse events is low and serious events are rare and occur in 0.5% of cases. Perforations/leakage are also uncommon and occur in 0.7% of patients. It is an incisionless procedure that eliminates the risk of wound infection and shortens postprocedural recovery. Patients are typically admitted for an overnight observation postprocedure, discharged home the following day, and back to activities of daily living (including work) within a few days. Postprocedural pain is minimal in most patients and narcotics are rarely needed. Resumption of a soft diet is carried out on the first postoperative day and normal diet 1 week later.
LHM is an established procedure with proven long-term efficacy in the treatment of achalasia. Nonetheless, it is invasive and requires placement of multiple trocars. The procedure is more time consuming than POEM and length of hospital stay can also be longer. This results in possibly higher cost than POEM. Importantly, recovery of dysphagia and resumption of normal diet is significantly delayed and is likely the result of the partial concomitant fundoplication procedure. Finally, LHM is not appropriate for the treatment of spastic esophageal disorders, including type III achalasia.
A major advantage of LHM plus partial fundoplication over POEM is the diminished risk of gastroesophageal reflux disease (GERD). However, this advantage seems short lived as the risk of GERD increases over time after surgery, likely because of the loosening of the wrap over time. From the New England Journal of Medicine paper mentioned earlier, it seems that the increased risk of GERD after POEM as compared with LHM diminishes over time.2 Importantly, it also appears that the rate of significant esophagitis (LA grade C-D) is similar between both procedures.2
In an effort to assess the long-term antireflux efficacy of surgical partial fundoplication, one study noted that 12% of 182 patients who had surgical myotomy with partial fundoplication continued to have occasional or continuous heartburn symptoms at a median of 18 years after surgery. Esophagitis and Barrett’s esophagus were found in 14.5% and 0.8% of patients, respectively. De novo esophageal adenocarcinoma has been reported after both POEM and LHM.4
Therefore, GERD and its complications can occur after any procedure that disrupts the lower esophageal sphincter (POEM, LHM, and PD) and postprocedural management of patients should include long-term testing and management of possible GERD. Different strategies have been proposed and include objective periodic testing for esophageal acid exposure, long-term and possible lifelong proton pump inhibitor use, and surveillance for long-term consequences of GER via periodic upper endoscopy.3
It is important to acknowledge that the lack of symptoms or the absence of endoscopic evidence of GER on initial endoscopy does not necessarily rule out GER. Approximately a third of post-POEM patients with clinically successful outcome and absence of reflux esophagitis on their first surveillance endoscopy eventually develop esophagitis at subsequent surveillance endoscopy.5
In summary, POEM has deservingly taken a prime time spot in the management of patients with achalasia. It is an efficient, efficacious and safe treatment modality that results in rapid resolution of achalasia symptoms in the majority of patients. Research should focus on technical modifications (e.g., short gastric myotomy; addition of endoscopic fundoplication) that reduce the incidence of postprocedural GERD.
References
1. Ponds FA et al. Effect of peroral endoscopic myotomy versus pneumatic dilation on symptom severity and treatment outcomes among treatment-naive patients with achalasia: A randomized clinical trial. JAMA. 2019;322:134-44.
2. Werner YB et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med. 2019;381:2219-29.
3. Khashab MA et al. ASGE guideline on the management of achalasia. Gastrointest Endosc. 2020;91:213-27 e6.
4. Ichkhanian Y et al. Case of early Barrett cancer following peroral endoscopic myotomy. Gut. 2019;68:2107-110.
5. Werner YB et al. Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years. Gut. 2016;65:899-906.
Dr. Khashab is associate professor of medicine, director of therapeutic endoscopy, division of gastroenterology and hepatology, Johns Hopkins Hospital, Baltimore. He is a consultant for BSCI, Olympus, and Medtronic.
Heller myotomy is still the gold standard
BY ROBERT M. SIWIEC, MD
Achalasia is a rare, primary esophageal motor disorder characterized by ineffective relaxation of the lower esophageal sphincter (LES) and concomitant loss of esophageal peristalsis. High-resolution esophageal manometry has allowed for the diagnosis and classification of achalasia into relevant clinical subtypes which become important when discussing and considering treatment options. Confirmatory studies (e.g., timed barium esophagram) and provocative manometric maneuvers (e.g., upright swallows, rapid swallow sequence, and/or rapid drink challenge) can be helpful when distinguishing between true achalasia versus achalasia variants and esophagogastric junction outflow obstruction.
Treatment options only provide palliation by eliminating outflow obstruction caused by a nonrelaxing and often times hypertensive LES. Pharmacotherapy (e.g., oral nitrates, 5-phosphodiesterase inhibitors, anticholinergics) is the least effective option because of medication side effects and short-acting duration. I only consider it for patients who are either unwilling or unable to tolerate invasive therapies. Botulinum toxin injection into the LES can be considered in patients who are not good candidates for more definitive therapy with PD or myotomy (endoscopic or surgical). Although the success rates with botulinum toxin are comparable with PD and surgical myotomy, patients treated with botulinum toxin require retreatment. Furthermore, continued botulinum toxin injections can compromise tissue planes making myotomy complex and challenging.
During the 1970s and 1980s, PD was the primary treatment modality for achalasia. Surgical myotomy was reserved for patients who suffered a perforation during PD or developed recurrent symptoms after multiple dilations. Minimally invasive surgery (left thoracoscopic approach) for achalasia was first introduced in the early 1990s and was shown to be a feasible, safe, and effective procedure, becoming the primary treatment approach in most centers. Patients fared well; however, it was soon discovered that >50% had pathological reflux based on pH monitoring. A few centers then began to perform a Heller myotomy through a laparoscopic approach with the addition of a fundoplication resulting in significant reductions in pathological reflux by pH monitoring. Eventually, a seminal RCT confirmed the importance of fundoplication with laparoscopic Heller myotomy (LHM) – resolution of dysphagia was unaffected and pathological reflux was avoided in most patients.1 Overall, clinical success rates for LHM with fundoplication are typically >90% and reflux incidence rates <10% with the overall complication rate being about 5% with reported mortality <0.1%.
PD remains appealing in that it is cost effective and less invasive, compared with POEM and LHM. Initial success rates and short-term efficacy are comparable with LHM but unfortunately PD’s efficacy significantly wanes over time. POEM, introduced by Inoue et al. in 2010, is a novel endoscopic technique with an excellent safety profile that provides good symptom relief while avoiding abdominal wall scars for patients. It has been shown to have a distinct advantage in patients with type III achalasia by nature of the longer myotomy not achievable by LHM.2 POEM has seen increasing enthusiasm and acceptance as a standard treatment option for achalasia largely because of the fact that its safety and efficacy have been shown to be comparable and in most cases equal with LHM. However, in 2020, direct comparison with LHM is challenging given that the follow-up in the majority of studies is either short or incomplete. The most recent multicenter, randomized trial comparing POEM with LHM plus Dor fundoplication showed POEM’s noninferiority in controlling symptoms of achalasia, but only after a 24 month follow-up.3 A recent report included one of the largest cohorts of post-POEM patients (500), but the 36-month data were based on the follow-up of only 61 patients (about 12%).4
Once the muscle fibers of the LES are disrupted, reflux will occur in the majority of patients. Unlike LHM, no concomitant fundoplication is performed during POEM and this increases the incidence of GERD and its long-term sequelae including peptic strictures, Barrett’s esophagus, and adenocarcinoma. A meta-analysis from 2018 looked at published series of POEM and LHM with fundoplication and found that GERD symptoms were present in 19% of POEM patients, compared with 8.8% of LHM patients. Worse yet, esophagitis was seen in 29.4% of the POEM group and 7.6% of the LHM group, with more individuals in the POEM group also having abnormal acid exposure based on ambulatory pH monitoring (39.0% vs. 16.8%).5
Proponents of POEM will argue that proton pump inhibitors (PPIs) are the panacea for post-POEM GERD. Unfortunately, this approach has its own problems. PPIs are very effective at reducing acid secretion by parietal cells, but do not block reflux through an iatrogenically incompetent LES. The drumbeat of publications on potential complications from chronic PPI use has greatly contributed to patients’ reluctance to commit to long-term PPI use. Lastly, the first case of early Barrett’s cancer was recently reported in a patient 4 years post-POEM despite adherence to an aggressive antisecretory regimen (b.i.d. PPI and H2 blocker at bedtime).6 LHM with fundoplication significantly reduces the risk of pathological GERD and spares patients from committing to lifelong PPI therapy and routine endoscopic surveillance (appropriate interval yet to be determined) and needing to consider additional procedures (i.e., endoscopic or surgical fundoplication).
Despite POEM’s well established efficacy and safety, the development of post-POEM GERD is a major concern that has yet to be adequately addressed. A significant number of post-POEM patients with pathological reflux have asymptomatic and unrecognized GERD and current management and monitoring strategies for post-POEM GERD are anemic and poorly established. Without question, there are individual patients who are clearly better served with POEM (type III achalasia and other spastic esophageal disorders). However, as we continue to learn more about post-POEM GERD and how to better prevent, manage, and monitor it, LHM with fundoplication for the time being remains the tried-and-tested treatment option for patients with non–type III achalasia.
References
1. Richards WO et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: A prospective randomized double-blind clinical trial. Ann Surg. 2004;240:405-12.
2. Podboy AJ et al. Long-term outcomes of peroral endoscopic myotomy compared to laparoscopic Heller myotomy for achalasia: A single-center experience. Surg Endosc. 2020. doi: 10.1007/s00464-020-07450-6.
3. Werner YB et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med. 2019;381(23):2219-29.
4. Inoue H et al. Peroral endoscopic myotomy: A series of 500 patients. J Am Coll Surg. 2015;221:256-64.
5. Repici A et al. GERD after peroral endoscopic myotomy as compared with Heller’s myotomy with fundoplication: A systematic review with meta-analysis. Gastrointest Endosc. 2018;87(4):934-43.
6. Ichkhanian Y et al. Case of early Barrett cancer following peroral endoscopic myotomy. Gut. 2019;68:2107-10.
Dr. Siwiec is assistant professor of clinical medicine, division of gastroenterology and hepatology, GI motility and neurogastroenterology unit, Indiana University, Indianapolis. He has no conflicts of interest.