User login
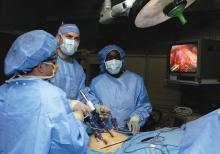
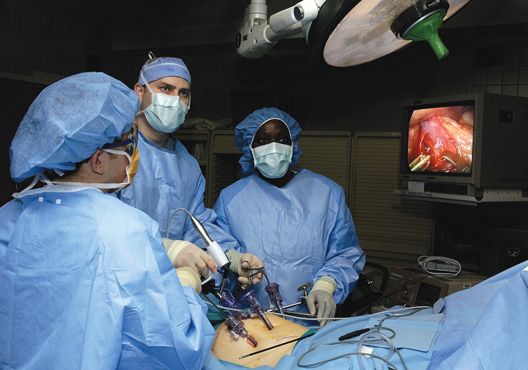
Stress incontinence surgery found to improve sexual dysfunction
An analysis of four commonly performed surgical procedures for stress urinary incontinence found that they all improved sexual dysfunction to a similar degree over the course of 24 months.
“There is a growing body of literature concerning female sexual function after treatment for urinary incontinence,” Stephanie M. Glass Clark, MD, of the University of Pittsburgh, and colleagues wrote in a study published in Obstetrics & Gynecology. “Pelvic floor muscle therapy has been shown to improve sexual function as well as urinary incontinence symptoms. Surgical treatment, on the other hand, has had unclear effects on sexual function.”
Dr. Glass Clark and colleagues conducted a combined secondary analysis of the SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) and TOMUS (Trial of Mid-Urethral Slings) studies. Women in the original trials were randomized to receive surgical treatment for stress urinary incontinence with an autologous fascial sling or Burch colposuspension (SISTEr), or a retropubic or transobturator midurethral sling (TOMUS). Sexual function as assessed by the short version of the Pelvic Organ Prolapse/ Urinary Incontinence Sexual Questionnaire (PISQ-12) was compared between groups at baseline, 12 months, and 24 months.
Of the 924 women included, 249 (27%) had an autologous fascial sling, 239 (26%) underwent Burch colposuspension, 216 (23%) had a retropubic midurethral sling placed, and 220 (24%) had a transobturator midurethral sling placed. The researchers observed no significant differences in mean PISQ-12 scores between the four treatment groups at the time of baseline (P = .07) or at the 12- and 24-month visits (P = .42 and P = .50, respectively). Patients in the two studies showed an overall improvement in sexual function over the 24-month study period.
Specifically, PISQ-12 scores at baseline were 32.6 in the transobturator sling group, 33.1 in the retropubic sling group, 31.9 in the Burch procedure group, and 31.4 in the fascial sling group. At 12 months, the PISQ-12 scores rose to 37.7 in the transobturator sling group, 37.8 in the retropubic sling group, 36.9 in the Burch procedure group, and 37.1 in the fascial sling group. These scores were generally maintained at 24 months (37.7 in the transobturator sling group, 37.1 in the retropubic sling group, 36.7 in the Burch procedure group, and 37.4 in the fascial sling group), and were not statistically different than the scores tabulated at the 12-month follow-up visit (P = .97).
“This study and others demonstrate that sexual function improves with surgical improvement of stress incontinence which may suggest a possible association of urinary incontinence and sexual dysfunction,” Dr. Glass Clark and colleagues concluded. “As we continue to explore the complex and multifaceted problem of sexual dysfunction, further evaluation of the effect of pelvic floor disorders – and their treatments – will be important and necessary research.”
The researchers acknowledged certain limitations of the study, including the fact that there was a low degree of diversity among women in the studied trials, which limits the generalizability of the findings. They also pointed out that the PISQ-12 does not address sexual stimulation or nonpenetrative vaginal intercourse. “Additionally, it limits partner-related problems to erectile dysfunction and premature ejaculation; some eligible participants may be excluded secondary to sexual preferences given the assumptions inherent to the questionnaire that the partner is male,” they wrote.
This secondary analysis had no outside sources of funding. Dr. Glass Clark reported that she received a travel stipend from the Society of Gynecologic Surgeons, sponsored by OB-STATS. Her coauthors reported having no financial conflicts.
SOURCE: Glass Clark SM et al. Obstet Gynecol 2020;135(2):352-60.
At face value, this is a retrospective analysis of sexual function after surgical correction for urinary incontinence. However, the researchers looked at two well-known and well-respected randomized, controlled trials comparing two types of incontinence procedures head to head, each. So the reader gets an opportunity to examine the influence of four different surgical procedures on sexual function.
Although I expected to see there would be an initial improvement with surgical correction, I did not expect that improvement would be so well maintained over time. There was sustained – and even continued – improvement in many cases, and this suggests a closer link to urinary incontinence that just embarrassment or worry about leakage during sex. I think the “take-home message” is that women who undergo anti-incontinence procedures can expect an improvement in sexual function from baseline, with the majority happening within the first year, and maintain this improvement between years 1 and 2.
I think this is the type of study that we all envisioned being able to do 25 years ago when female pelvic medicine and reconstructive surgery was in its infancy as an “official” subspecialty, and the National Institutes of Health had developed the Urinary Incontinence Network and the Pelvic Floor Disorders Network. It is gratifying that enough good research has been done to finally enjoy the fruits of their/our labor! The study had large numbers, used a widely known, validated questionnaire, and used data generated from randomized, controlled trials. Although the subjects may not represent all demographics, the study findings can be an aid to most practicing gynecologists to help counsel their patients.
The major limitations of any retrospective study are the inability to go back and ask questions not addressed in the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire Short Form. For instance, the authors discussed that it might be nice to have an “open-ended” question about why the nonresponders were not having sex.
Patrick Woodman, DO, MS , is a urogynecologist with the Michigan State University, East Lansing. He is also the program director for the obstetrics and gynecology residency for Ascension Macomb-Oakland Hospital, Warren (Michigan) Campus. Dr. Woodman is a member of the Ob.Gyn. News editorial advisory board.
At face value, this is a retrospective analysis of sexual function after surgical correction for urinary incontinence. However, the researchers looked at two well-known and well-respected randomized, controlled trials comparing two types of incontinence procedures head to head, each. So the reader gets an opportunity to examine the influence of four different surgical procedures on sexual function.
Although I expected to see there would be an initial improvement with surgical correction, I did not expect that improvement would be so well maintained over time. There was sustained – and even continued – improvement in many cases, and this suggests a closer link to urinary incontinence that just embarrassment or worry about leakage during sex. I think the “take-home message” is that women who undergo anti-incontinence procedures can expect an improvement in sexual function from baseline, with the majority happening within the first year, and maintain this improvement between years 1 and 2.
I think this is the type of study that we all envisioned being able to do 25 years ago when female pelvic medicine and reconstructive surgery was in its infancy as an “official” subspecialty, and the National Institutes of Health had developed the Urinary Incontinence Network and the Pelvic Floor Disorders Network. It is gratifying that enough good research has been done to finally enjoy the fruits of their/our labor! The study had large numbers, used a widely known, validated questionnaire, and used data generated from randomized, controlled trials. Although the subjects may not represent all demographics, the study findings can be an aid to most practicing gynecologists to help counsel their patients.
The major limitations of any retrospective study are the inability to go back and ask questions not addressed in the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire Short Form. For instance, the authors discussed that it might be nice to have an “open-ended” question about why the nonresponders were not having sex.
Patrick Woodman, DO, MS , is a urogynecologist with the Michigan State University, East Lansing. He is also the program director for the obstetrics and gynecology residency for Ascension Macomb-Oakland Hospital, Warren (Michigan) Campus. Dr. Woodman is a member of the Ob.Gyn. News editorial advisory board.
At face value, this is a retrospective analysis of sexual function after surgical correction for urinary incontinence. However, the researchers looked at two well-known and well-respected randomized, controlled trials comparing two types of incontinence procedures head to head, each. So the reader gets an opportunity to examine the influence of four different surgical procedures on sexual function.
Although I expected to see there would be an initial improvement with surgical correction, I did not expect that improvement would be so well maintained over time. There was sustained – and even continued – improvement in many cases, and this suggests a closer link to urinary incontinence that just embarrassment or worry about leakage during sex. I think the “take-home message” is that women who undergo anti-incontinence procedures can expect an improvement in sexual function from baseline, with the majority happening within the first year, and maintain this improvement between years 1 and 2.
I think this is the type of study that we all envisioned being able to do 25 years ago when female pelvic medicine and reconstructive surgery was in its infancy as an “official” subspecialty, and the National Institutes of Health had developed the Urinary Incontinence Network and the Pelvic Floor Disorders Network. It is gratifying that enough good research has been done to finally enjoy the fruits of their/our labor! The study had large numbers, used a widely known, validated questionnaire, and used data generated from randomized, controlled trials. Although the subjects may not represent all demographics, the study findings can be an aid to most practicing gynecologists to help counsel their patients.
The major limitations of any retrospective study are the inability to go back and ask questions not addressed in the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire Short Form. For instance, the authors discussed that it might be nice to have an “open-ended” question about why the nonresponders were not having sex.
Patrick Woodman, DO, MS , is a urogynecologist with the Michigan State University, East Lansing. He is also the program director for the obstetrics and gynecology residency for Ascension Macomb-Oakland Hospital, Warren (Michigan) Campus. Dr. Woodman is a member of the Ob.Gyn. News editorial advisory board.
An analysis of four commonly performed surgical procedures for stress urinary incontinence found that they all improved sexual dysfunction to a similar degree over the course of 24 months.
“There is a growing body of literature concerning female sexual function after treatment for urinary incontinence,” Stephanie M. Glass Clark, MD, of the University of Pittsburgh, and colleagues wrote in a study published in Obstetrics & Gynecology. “Pelvic floor muscle therapy has been shown to improve sexual function as well as urinary incontinence symptoms. Surgical treatment, on the other hand, has had unclear effects on sexual function.”
Dr. Glass Clark and colleagues conducted a combined secondary analysis of the SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) and TOMUS (Trial of Mid-Urethral Slings) studies. Women in the original trials were randomized to receive surgical treatment for stress urinary incontinence with an autologous fascial sling or Burch colposuspension (SISTEr), or a retropubic or transobturator midurethral sling (TOMUS). Sexual function as assessed by the short version of the Pelvic Organ Prolapse/ Urinary Incontinence Sexual Questionnaire (PISQ-12) was compared between groups at baseline, 12 months, and 24 months.
Of the 924 women included, 249 (27%) had an autologous fascial sling, 239 (26%) underwent Burch colposuspension, 216 (23%) had a retropubic midurethral sling placed, and 220 (24%) had a transobturator midurethral sling placed. The researchers observed no significant differences in mean PISQ-12 scores between the four treatment groups at the time of baseline (P = .07) or at the 12- and 24-month visits (P = .42 and P = .50, respectively). Patients in the two studies showed an overall improvement in sexual function over the 24-month study period.
Specifically, PISQ-12 scores at baseline were 32.6 in the transobturator sling group, 33.1 in the retropubic sling group, 31.9 in the Burch procedure group, and 31.4 in the fascial sling group. At 12 months, the PISQ-12 scores rose to 37.7 in the transobturator sling group, 37.8 in the retropubic sling group, 36.9 in the Burch procedure group, and 37.1 in the fascial sling group. These scores were generally maintained at 24 months (37.7 in the transobturator sling group, 37.1 in the retropubic sling group, 36.7 in the Burch procedure group, and 37.4 in the fascial sling group), and were not statistically different than the scores tabulated at the 12-month follow-up visit (P = .97).
“This study and others demonstrate that sexual function improves with surgical improvement of stress incontinence which may suggest a possible association of urinary incontinence and sexual dysfunction,” Dr. Glass Clark and colleagues concluded. “As we continue to explore the complex and multifaceted problem of sexual dysfunction, further evaluation of the effect of pelvic floor disorders – and their treatments – will be important and necessary research.”
The researchers acknowledged certain limitations of the study, including the fact that there was a low degree of diversity among women in the studied trials, which limits the generalizability of the findings. They also pointed out that the PISQ-12 does not address sexual stimulation or nonpenetrative vaginal intercourse. “Additionally, it limits partner-related problems to erectile dysfunction and premature ejaculation; some eligible participants may be excluded secondary to sexual preferences given the assumptions inherent to the questionnaire that the partner is male,” they wrote.
This secondary analysis had no outside sources of funding. Dr. Glass Clark reported that she received a travel stipend from the Society of Gynecologic Surgeons, sponsored by OB-STATS. Her coauthors reported having no financial conflicts.
SOURCE: Glass Clark SM et al. Obstet Gynecol 2020;135(2):352-60.
An analysis of four commonly performed surgical procedures for stress urinary incontinence found that they all improved sexual dysfunction to a similar degree over the course of 24 months.
“There is a growing body of literature concerning female sexual function after treatment for urinary incontinence,” Stephanie M. Glass Clark, MD, of the University of Pittsburgh, and colleagues wrote in a study published in Obstetrics & Gynecology. “Pelvic floor muscle therapy has been shown to improve sexual function as well as urinary incontinence symptoms. Surgical treatment, on the other hand, has had unclear effects on sexual function.”
Dr. Glass Clark and colleagues conducted a combined secondary analysis of the SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) and TOMUS (Trial of Mid-Urethral Slings) studies. Women in the original trials were randomized to receive surgical treatment for stress urinary incontinence with an autologous fascial sling or Burch colposuspension (SISTEr), or a retropubic or transobturator midurethral sling (TOMUS). Sexual function as assessed by the short version of the Pelvic Organ Prolapse/ Urinary Incontinence Sexual Questionnaire (PISQ-12) was compared between groups at baseline, 12 months, and 24 months.
Of the 924 women included, 249 (27%) had an autologous fascial sling, 239 (26%) underwent Burch colposuspension, 216 (23%) had a retropubic midurethral sling placed, and 220 (24%) had a transobturator midurethral sling placed. The researchers observed no significant differences in mean PISQ-12 scores between the four treatment groups at the time of baseline (P = .07) or at the 12- and 24-month visits (P = .42 and P = .50, respectively). Patients in the two studies showed an overall improvement in sexual function over the 24-month study period.
Specifically, PISQ-12 scores at baseline were 32.6 in the transobturator sling group, 33.1 in the retropubic sling group, 31.9 in the Burch procedure group, and 31.4 in the fascial sling group. At 12 months, the PISQ-12 scores rose to 37.7 in the transobturator sling group, 37.8 in the retropubic sling group, 36.9 in the Burch procedure group, and 37.1 in the fascial sling group. These scores were generally maintained at 24 months (37.7 in the transobturator sling group, 37.1 in the retropubic sling group, 36.7 in the Burch procedure group, and 37.4 in the fascial sling group), and were not statistically different than the scores tabulated at the 12-month follow-up visit (P = .97).
“This study and others demonstrate that sexual function improves with surgical improvement of stress incontinence which may suggest a possible association of urinary incontinence and sexual dysfunction,” Dr. Glass Clark and colleagues concluded. “As we continue to explore the complex and multifaceted problem of sexual dysfunction, further evaluation of the effect of pelvic floor disorders – and their treatments – will be important and necessary research.”
The researchers acknowledged certain limitations of the study, including the fact that there was a low degree of diversity among women in the studied trials, which limits the generalizability of the findings. They also pointed out that the PISQ-12 does not address sexual stimulation or nonpenetrative vaginal intercourse. “Additionally, it limits partner-related problems to erectile dysfunction and premature ejaculation; some eligible participants may be excluded secondary to sexual preferences given the assumptions inherent to the questionnaire that the partner is male,” they wrote.
This secondary analysis had no outside sources of funding. Dr. Glass Clark reported that she received a travel stipend from the Society of Gynecologic Surgeons, sponsored by OB-STATS. Her coauthors reported having no financial conflicts.
SOURCE: Glass Clark SM et al. Obstet Gynecol 2020;135(2):352-60.
FROM OBSTETRICS & GYNECOLOGY
Commentary: Should AVFs be ligated after kidney transplant?
Hemodynamic complications of arteriovenous (AV) access are uncommon but can be potentially life threatening. Fistulas and grafts can cause a decrease in systemic vascular resistance and secondary increase in cardiac output in patients who may already have myocardial dysfunction secondary to their end-stage renal disease.1 This increased cardiac output is usually insignificant but in rare cases can result in clinically significant cardiac failure. Patients with high-output fistulas with volume flow greater than 2 L/min may be at increased risk of heart failure but volume flow less than 2 L/min does not preclude this complication.2
In patients with AV access–related heart failure, optimal medical management and reduction of fistula flow or ligation of the dialysis access should be considered. If continued hemodialysis is necessary, loss of a functioning dialysis access is problematic and difficult management decisions must be made. Following successful renal transplantation, ligation of vascular access in the presence of symptomatic heart failure may represent a straightforward decision. Nonetheless, there is no clear consensus of how to manage patent fistulas or grafts in patients following renal transplantation in the absence of significant cardiac symptoms with particular concern to the important issues of transplant survival and long-term cardiac prognosis. Yaffe and Greenstein3 recommend preservation of almost all fistulas after transplantation in the absence of significant complications such as venous hypertension, pseudoaneurysm, significant high-output cardiac failure or hand ischemia. They recommend taking into account the 10-year adjusted renal transplantation graft survival rates and the relative paucity of donors, recognizing the possibility that the patient may have to return to dialysis at some point in the future. They also reference the lack of information regarding the beneficial impact of fistula ligation on cardiac morphology and function as a rationale for access preservation.
A recent presentation at the American Heart Association Scientific Sessions by Michael B. Stokes, MD,4 from the department of cardiology at Royal Adelaide Hospital in Australia, suggests that cardiovascular disease is responsible for 40% of deaths among kidney transplant recipients and that left ventricular (LV) mass is strongly associated with cardiovascular mortality.
He states that, although there is no guideline consensus on the management of an AV fistula following successful renal transplantation, the fistula continues to contribute adversely to cardiac remodeling and function. The lack of previous randomized controlled trials in this area led Dr. Stokes and his colleagues to randomly assign 64 patients at least 1 year following successful kidney transplantation with stable renal function and a functioning AV fistula to either fistula ligation or no intervention. All patients underwent cardiac MRI at baseline and 6 months.
The primary endpoint of decrease in LV mass at 6 months was significant in the ligation group but not in the control group. The ligation group also had significant decrease in LV end diastolic volume, LV end systolic volume, and multiple other parameters. In addition, NT-proBNP levels and left atrial volume were significantly reduced in the ligation group when compared with the control group. Complications in the ligation group included six patients with thrombosis of their fistula vein and two infections, all of which resolved with outpatient anti-inflammatory or antimicrobial therapy.
Dr. Stokes believes that control patients in his study face “persisting and substantial deleterious cardiac remodeling” and that “further investigation would clarify the impact of AV fistula ligation on clinical outcomes following kidney transplantation.”
I believe this is important information and represents the first randomized controlled data regarding the long-term adverse cardiac effects of a patent fistula after renal transplantation. Unfortunately, information regarding baseline fistula volume flow is not provided in this abstract. As discussed earlier, patients with high-flow fistulas may be at increased risk of heart failure and hemodynamic data can be critical in establishing an algorithm for managing these challenging patients.
Ligation of a functioning and asymptomatic access in a patient with a successful renal transplant should be recommended only after informed discussion with the patient weighing the ongoing potential negative effects on cardiac function of continued access patency versus the potential need for future hemodialysis. Dr. Stokes presents interesting data that must be considered in this controversy. I believe that, in the absence of a universally applicable algorithm, the clinical decision to recommend AV fistula ligation after successful kidney transplantation should be individualized and based on ongoing assessment of cardiac and renal function and fistula complications and hemodynamics.
References
1. Eur Heart J 2017;38:1913-23.
2. Nephrol Dial Transplant 2008;23:282-7.
3. J Vasc Access 2012;13:405-8.
4. Stokes MB, et al. LBS.05 – Late Breaking Clinical Trial: Hot News in HF. Presented at American Heart Association Scientific Sessions. 2018 Nov 10-12. Chicago.
Larry A. Scher, MD, is a vascular surgeon at the Montefiore Greene Medical Arts Pavilion, New York, and an associate medical editor for Vascular Specialist.
Hemodynamic complications of arteriovenous (AV) access are uncommon but can be potentially life threatening. Fistulas and grafts can cause a decrease in systemic vascular resistance and secondary increase in cardiac output in patients who may already have myocardial dysfunction secondary to their end-stage renal disease.1 This increased cardiac output is usually insignificant but in rare cases can result in clinically significant cardiac failure. Patients with high-output fistulas with volume flow greater than 2 L/min may be at increased risk of heart failure but volume flow less than 2 L/min does not preclude this complication.2
In patients with AV access–related heart failure, optimal medical management and reduction of fistula flow or ligation of the dialysis access should be considered. If continued hemodialysis is necessary, loss of a functioning dialysis access is problematic and difficult management decisions must be made. Following successful renal transplantation, ligation of vascular access in the presence of symptomatic heart failure may represent a straightforward decision. Nonetheless, there is no clear consensus of how to manage patent fistulas or grafts in patients following renal transplantation in the absence of significant cardiac symptoms with particular concern to the important issues of transplant survival and long-term cardiac prognosis. Yaffe and Greenstein3 recommend preservation of almost all fistulas after transplantation in the absence of significant complications such as venous hypertension, pseudoaneurysm, significant high-output cardiac failure or hand ischemia. They recommend taking into account the 10-year adjusted renal transplantation graft survival rates and the relative paucity of donors, recognizing the possibility that the patient may have to return to dialysis at some point in the future. They also reference the lack of information regarding the beneficial impact of fistula ligation on cardiac morphology and function as a rationale for access preservation.
A recent presentation at the American Heart Association Scientific Sessions by Michael B. Stokes, MD,4 from the department of cardiology at Royal Adelaide Hospital in Australia, suggests that cardiovascular disease is responsible for 40% of deaths among kidney transplant recipients and that left ventricular (LV) mass is strongly associated with cardiovascular mortality.
He states that, although there is no guideline consensus on the management of an AV fistula following successful renal transplantation, the fistula continues to contribute adversely to cardiac remodeling and function. The lack of previous randomized controlled trials in this area led Dr. Stokes and his colleagues to randomly assign 64 patients at least 1 year following successful kidney transplantation with stable renal function and a functioning AV fistula to either fistula ligation or no intervention. All patients underwent cardiac MRI at baseline and 6 months.
The primary endpoint of decrease in LV mass at 6 months was significant in the ligation group but not in the control group. The ligation group also had significant decrease in LV end diastolic volume, LV end systolic volume, and multiple other parameters. In addition, NT-proBNP levels and left atrial volume were significantly reduced in the ligation group when compared with the control group. Complications in the ligation group included six patients with thrombosis of their fistula vein and two infections, all of which resolved with outpatient anti-inflammatory or antimicrobial therapy.
Dr. Stokes believes that control patients in his study face “persisting and substantial deleterious cardiac remodeling” and that “further investigation would clarify the impact of AV fistula ligation on clinical outcomes following kidney transplantation.”
I believe this is important information and represents the first randomized controlled data regarding the long-term adverse cardiac effects of a patent fistula after renal transplantation. Unfortunately, information regarding baseline fistula volume flow is not provided in this abstract. As discussed earlier, patients with high-flow fistulas may be at increased risk of heart failure and hemodynamic data can be critical in establishing an algorithm for managing these challenging patients.
Ligation of a functioning and asymptomatic access in a patient with a successful renal transplant should be recommended only after informed discussion with the patient weighing the ongoing potential negative effects on cardiac function of continued access patency versus the potential need for future hemodialysis. Dr. Stokes presents interesting data that must be considered in this controversy. I believe that, in the absence of a universally applicable algorithm, the clinical decision to recommend AV fistula ligation after successful kidney transplantation should be individualized and based on ongoing assessment of cardiac and renal function and fistula complications and hemodynamics.
References
1. Eur Heart J 2017;38:1913-23.
2. Nephrol Dial Transplant 2008;23:282-7.
3. J Vasc Access 2012;13:405-8.
4. Stokes MB, et al. LBS.05 – Late Breaking Clinical Trial: Hot News in HF. Presented at American Heart Association Scientific Sessions. 2018 Nov 10-12. Chicago.
Larry A. Scher, MD, is a vascular surgeon at the Montefiore Greene Medical Arts Pavilion, New York, and an associate medical editor for Vascular Specialist.
Hemodynamic complications of arteriovenous (AV) access are uncommon but can be potentially life threatening. Fistulas and grafts can cause a decrease in systemic vascular resistance and secondary increase in cardiac output in patients who may already have myocardial dysfunction secondary to their end-stage renal disease.1 This increased cardiac output is usually insignificant but in rare cases can result in clinically significant cardiac failure. Patients with high-output fistulas with volume flow greater than 2 L/min may be at increased risk of heart failure but volume flow less than 2 L/min does not preclude this complication.2
In patients with AV access–related heart failure, optimal medical management and reduction of fistula flow or ligation of the dialysis access should be considered. If continued hemodialysis is necessary, loss of a functioning dialysis access is problematic and difficult management decisions must be made. Following successful renal transplantation, ligation of vascular access in the presence of symptomatic heart failure may represent a straightforward decision. Nonetheless, there is no clear consensus of how to manage patent fistulas or grafts in patients following renal transplantation in the absence of significant cardiac symptoms with particular concern to the important issues of transplant survival and long-term cardiac prognosis. Yaffe and Greenstein3 recommend preservation of almost all fistulas after transplantation in the absence of significant complications such as venous hypertension, pseudoaneurysm, significant high-output cardiac failure or hand ischemia. They recommend taking into account the 10-year adjusted renal transplantation graft survival rates and the relative paucity of donors, recognizing the possibility that the patient may have to return to dialysis at some point in the future. They also reference the lack of information regarding the beneficial impact of fistula ligation on cardiac morphology and function as a rationale for access preservation.
A recent presentation at the American Heart Association Scientific Sessions by Michael B. Stokes, MD,4 from the department of cardiology at Royal Adelaide Hospital in Australia, suggests that cardiovascular disease is responsible for 40% of deaths among kidney transplant recipients and that left ventricular (LV) mass is strongly associated with cardiovascular mortality.
He states that, although there is no guideline consensus on the management of an AV fistula following successful renal transplantation, the fistula continues to contribute adversely to cardiac remodeling and function. The lack of previous randomized controlled trials in this area led Dr. Stokes and his colleagues to randomly assign 64 patients at least 1 year following successful kidney transplantation with stable renal function and a functioning AV fistula to either fistula ligation or no intervention. All patients underwent cardiac MRI at baseline and 6 months.
The primary endpoint of decrease in LV mass at 6 months was significant in the ligation group but not in the control group. The ligation group also had significant decrease in LV end diastolic volume, LV end systolic volume, and multiple other parameters. In addition, NT-proBNP levels and left atrial volume were significantly reduced in the ligation group when compared with the control group. Complications in the ligation group included six patients with thrombosis of their fistula vein and two infections, all of which resolved with outpatient anti-inflammatory or antimicrobial therapy.
Dr. Stokes believes that control patients in his study face “persisting and substantial deleterious cardiac remodeling” and that “further investigation would clarify the impact of AV fistula ligation on clinical outcomes following kidney transplantation.”
I believe this is important information and represents the first randomized controlled data regarding the long-term adverse cardiac effects of a patent fistula after renal transplantation. Unfortunately, information regarding baseline fistula volume flow is not provided in this abstract. As discussed earlier, patients with high-flow fistulas may be at increased risk of heart failure and hemodynamic data can be critical in establishing an algorithm for managing these challenging patients.
Ligation of a functioning and asymptomatic access in a patient with a successful renal transplant should be recommended only after informed discussion with the patient weighing the ongoing potential negative effects on cardiac function of continued access patency versus the potential need for future hemodialysis. Dr. Stokes presents interesting data that must be considered in this controversy. I believe that, in the absence of a universally applicable algorithm, the clinical decision to recommend AV fistula ligation after successful kidney transplantation should be individualized and based on ongoing assessment of cardiac and renal function and fistula complications and hemodynamics.
References
1. Eur Heart J 2017;38:1913-23.
2. Nephrol Dial Transplant 2008;23:282-7.
3. J Vasc Access 2012;13:405-8.
4. Stokes MB, et al. LBS.05 – Late Breaking Clinical Trial: Hot News in HF. Presented at American Heart Association Scientific Sessions. 2018 Nov 10-12. Chicago.
Larry A. Scher, MD, is a vascular surgeon at the Montefiore Greene Medical Arts Pavilion, New York, and an associate medical editor for Vascular Specialist.
Clinical trial: Robotic or open radical cystectomy in treating patients with bladder cancer
Patients who are recruited will undergo either open or robotic radical cystectomy. In open radical cystectomy, the surgeon cuts into the lower abdomen to expose the urinary tract in order to remove the bladder. In robotic radical cystectomy, small cuts are made in the abdomen into which a scope is inserted; with robotic help, the surgeon removes the bladder. It is currently unknown which approach results in fewer complications, better quality of life, and faster recovery time.
Patients are eligible for the study if they are indicated for radical cystectomy, have Tis-T3 urothelial cancer, and meet surgical candidate criteria. Exclusion factors include having a bulky lymphadenopathy, prior pelvic radiation, or uncontrolled coagulopathy.
The primary outcome measure is change in patient-reported quality of life, as reported using the European Organization for Research and Treatment of Cancer (EORTC)-QLQ-C30, 1 month post surgery. The secondary outcome measure is change in erectile dysfunction, as measured by the Sexual Health Inventory For Men score, over a follow-up of up to 12 months.
Recruitment ends on Oct. 12, 2019, and the estimated study completion date is Oct. 12, 2020. About 208 participants are expected to be included.
Find more information on the study page at Clinicaltrials.gov.
Patients who are recruited will undergo either open or robotic radical cystectomy. In open radical cystectomy, the surgeon cuts into the lower abdomen to expose the urinary tract in order to remove the bladder. In robotic radical cystectomy, small cuts are made in the abdomen into which a scope is inserted; with robotic help, the surgeon removes the bladder. It is currently unknown which approach results in fewer complications, better quality of life, and faster recovery time.
Patients are eligible for the study if they are indicated for radical cystectomy, have Tis-T3 urothelial cancer, and meet surgical candidate criteria. Exclusion factors include having a bulky lymphadenopathy, prior pelvic radiation, or uncontrolled coagulopathy.
The primary outcome measure is change in patient-reported quality of life, as reported using the European Organization for Research and Treatment of Cancer (EORTC)-QLQ-C30, 1 month post surgery. The secondary outcome measure is change in erectile dysfunction, as measured by the Sexual Health Inventory For Men score, over a follow-up of up to 12 months.
Recruitment ends on Oct. 12, 2019, and the estimated study completion date is Oct. 12, 2020. About 208 participants are expected to be included.
Find more information on the study page at Clinicaltrials.gov.
Patients who are recruited will undergo either open or robotic radical cystectomy. In open radical cystectomy, the surgeon cuts into the lower abdomen to expose the urinary tract in order to remove the bladder. In robotic radical cystectomy, small cuts are made in the abdomen into which a scope is inserted; with robotic help, the surgeon removes the bladder. It is currently unknown which approach results in fewer complications, better quality of life, and faster recovery time.
Patients are eligible for the study if they are indicated for radical cystectomy, have Tis-T3 urothelial cancer, and meet surgical candidate criteria. Exclusion factors include having a bulky lymphadenopathy, prior pelvic radiation, or uncontrolled coagulopathy.
The primary outcome measure is change in patient-reported quality of life, as reported using the European Organization for Research and Treatment of Cancer (EORTC)-QLQ-C30, 1 month post surgery. The secondary outcome measure is change in erectile dysfunction, as measured by the Sexual Health Inventory For Men score, over a follow-up of up to 12 months.
Recruitment ends on Oct. 12, 2019, and the estimated study completion date is Oct. 12, 2020. About 208 participants are expected to be included.
Find more information on the study page at Clinicaltrials.gov.
Gap in care: Female patients with incontinence
LAS VEGAS – A pelvic surgeon brought a bold message to a gathering of gynecologists: There’s a great gap in American care for pelvic floor disorders such as urinary incontinence, and they’re the right physicians to make a difference by treating these common conditions.
“There are never going to be enough specialists to deal with these problems. This is a natural progression for many of you,” said urogynecologist and pelvic surgeon Mickey M. Karram, MD, in a joint presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium. In fact, he said, “there’s so much disease out there to fix that you may become more overwhelmed.”
Dr. Karram, who has offices in Cincinnati, Beverly Hills, and Orange County, Calif., spoke about female urinary incontinence with obstetrician-gynecologist Beri M. Ridgeway, MD, of Cleveland Clinic. They offered these tips:
Test for stress incontinence
Dr. Karram recommends using a “quick and easy” cystometrogram (CMG) test to “corroborate or refute what the patient thinks is going on” in regard to urinary function. “With this simple test, you’ll get a clear understanding of sensation [to urinate] and of what their fullness and capacity numbers are,” he said. And if you have the patient cough or strain during the test, “you should be able to duplicate a sign of stress incontinence 90% of the time.”
If patients don’t leak when they take this test, there may be another problem such as overactive bladder, a condition that can’t be duplicated via the test, he said.
Ask the right questions
When it comes to identifying when they have urinary difficulties, some patients “say yes to every question we ask,” said Dr. Ridgeway, and they may not be able to distinguish between urgency and leakage.
A better approach is to ask women to provide specific examples of when they have continence issues, she said. It’s also useful to ask patients about what bothers them the most if they have multiple symptoms: Is it urgency (“Gotta go; gotta go”)? Leakage during certain situations like coughing and laughing? “That helps me decide how to go about treating them first and foremost,” she said. “It doesn’t mean you won’t treat both [problems], but it really gives you a reference point of where to start.”
Research suggests that women tend to be more bothered by urge incontinence than stress incontinence, she said, because they can regulate their activities or avoid the stress form.
Beware of acute incontinence cases
“If a woman walks in and says ‘Everything was great until a week or two ago, but now I’m living in pads,’ it could be a fecal impaction or a pelvic mass,” Dr. Karram said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Discuss the many treatment options
In some cases of incontinence, Dr. Ridgeway said she’ll mention “the array of treatment options, such as pelvic floor physical therapy, bladder retraining, vaginal estrogen, medications, and Botox.”
She added: “I explain that we’ll work together, and sometimes it will take a couple tries, or we’ll try a couple things at once.”
Dr. Ridgeway disclosed consulting for Coloplast and serving as an independent contractor (legal) for Ethicon. Dr. Karram disclosed speaking for Allergan, Astellas Pharma, Coloplast, and Cynosure/Hologic; consulting for Coloplast and Cynosure/Hologic; and receiving royalties from BihlerMed.
LAS VEGAS – A pelvic surgeon brought a bold message to a gathering of gynecologists: There’s a great gap in American care for pelvic floor disorders such as urinary incontinence, and they’re the right physicians to make a difference by treating these common conditions.
“There are never going to be enough specialists to deal with these problems. This is a natural progression for many of you,” said urogynecologist and pelvic surgeon Mickey M. Karram, MD, in a joint presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium. In fact, he said, “there’s so much disease out there to fix that you may become more overwhelmed.”
Dr. Karram, who has offices in Cincinnati, Beverly Hills, and Orange County, Calif., spoke about female urinary incontinence with obstetrician-gynecologist Beri M. Ridgeway, MD, of Cleveland Clinic. They offered these tips:
Test for stress incontinence
Dr. Karram recommends using a “quick and easy” cystometrogram (CMG) test to “corroborate or refute what the patient thinks is going on” in regard to urinary function. “With this simple test, you’ll get a clear understanding of sensation [to urinate] and of what their fullness and capacity numbers are,” he said. And if you have the patient cough or strain during the test, “you should be able to duplicate a sign of stress incontinence 90% of the time.”
If patients don’t leak when they take this test, there may be another problem such as overactive bladder, a condition that can’t be duplicated via the test, he said.
Ask the right questions
When it comes to identifying when they have urinary difficulties, some patients “say yes to every question we ask,” said Dr. Ridgeway, and they may not be able to distinguish between urgency and leakage.
A better approach is to ask women to provide specific examples of when they have continence issues, she said. It’s also useful to ask patients about what bothers them the most if they have multiple symptoms: Is it urgency (“Gotta go; gotta go”)? Leakage during certain situations like coughing and laughing? “That helps me decide how to go about treating them first and foremost,” she said. “It doesn’t mean you won’t treat both [problems], but it really gives you a reference point of where to start.”
Research suggests that women tend to be more bothered by urge incontinence than stress incontinence, she said, because they can regulate their activities or avoid the stress form.
Beware of acute incontinence cases
“If a woman walks in and says ‘Everything was great until a week or two ago, but now I’m living in pads,’ it could be a fecal impaction or a pelvic mass,” Dr. Karram said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Discuss the many treatment options
In some cases of incontinence, Dr. Ridgeway said she’ll mention “the array of treatment options, such as pelvic floor physical therapy, bladder retraining, vaginal estrogen, medications, and Botox.”
She added: “I explain that we’ll work together, and sometimes it will take a couple tries, or we’ll try a couple things at once.”
Dr. Ridgeway disclosed consulting for Coloplast and serving as an independent contractor (legal) for Ethicon. Dr. Karram disclosed speaking for Allergan, Astellas Pharma, Coloplast, and Cynosure/Hologic; consulting for Coloplast and Cynosure/Hologic; and receiving royalties from BihlerMed.
LAS VEGAS – A pelvic surgeon brought a bold message to a gathering of gynecologists: There’s a great gap in American care for pelvic floor disorders such as urinary incontinence, and they’re the right physicians to make a difference by treating these common conditions.
“There are never going to be enough specialists to deal with these problems. This is a natural progression for many of you,” said urogynecologist and pelvic surgeon Mickey M. Karram, MD, in a joint presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium. In fact, he said, “there’s so much disease out there to fix that you may become more overwhelmed.”
Dr. Karram, who has offices in Cincinnati, Beverly Hills, and Orange County, Calif., spoke about female urinary incontinence with obstetrician-gynecologist Beri M. Ridgeway, MD, of Cleveland Clinic. They offered these tips:
Test for stress incontinence
Dr. Karram recommends using a “quick and easy” cystometrogram (CMG) test to “corroborate or refute what the patient thinks is going on” in regard to urinary function. “With this simple test, you’ll get a clear understanding of sensation [to urinate] and of what their fullness and capacity numbers are,” he said. And if you have the patient cough or strain during the test, “you should be able to duplicate a sign of stress incontinence 90% of the time.”
If patients don’t leak when they take this test, there may be another problem such as overactive bladder, a condition that can’t be duplicated via the test, he said.
Ask the right questions
When it comes to identifying when they have urinary difficulties, some patients “say yes to every question we ask,” said Dr. Ridgeway, and they may not be able to distinguish between urgency and leakage.
A better approach is to ask women to provide specific examples of when they have continence issues, she said. It’s also useful to ask patients about what bothers them the most if they have multiple symptoms: Is it urgency (“Gotta go; gotta go”)? Leakage during certain situations like coughing and laughing? “That helps me decide how to go about treating them first and foremost,” she said. “It doesn’t mean you won’t treat both [problems], but it really gives you a reference point of where to start.”
Research suggests that women tend to be more bothered by urge incontinence than stress incontinence, she said, because they can regulate their activities or avoid the stress form.
Beware of acute incontinence cases
“If a woman walks in and says ‘Everything was great until a week or two ago, but now I’m living in pads,’ it could be a fecal impaction or a pelvic mass,” Dr. Karram said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Discuss the many treatment options
In some cases of incontinence, Dr. Ridgeway said she’ll mention “the array of treatment options, such as pelvic floor physical therapy, bladder retraining, vaginal estrogen, medications, and Botox.”
She added: “I explain that we’ll work together, and sometimes it will take a couple tries, or we’ll try a couple things at once.”
Dr. Ridgeway disclosed consulting for Coloplast and serving as an independent contractor (legal) for Ethicon. Dr. Karram disclosed speaking for Allergan, Astellas Pharma, Coloplast, and Cynosure/Hologic; consulting for Coloplast and Cynosure/Hologic; and receiving royalties from BihlerMed.
EXPERT ANALYSIS FROM PAGS
Risk factors identified for urinary retention after lap hernia repair
was more likely in patients aged more than 60 years, patients with benign prostatic hyperplasia, and patients with a decreased body mass index, according to findings published in the Journal of Surgical Research.
The researchers note that, while laparoscopic TEP is growing in popularity for inguinal hernia repair, postop urinary retention (POUR) is estimated at 2%-30%, but for open procedures, it is estimated at 0.4%-3%. POUR is linked to the development of urinary tract infections and also hospital readmissions. Since TEP may eventually become the norm, the study authors suggest that identifying patients at higher risk for POUR would contribute to the safety and quality of care for this operation.
In a retrospective chart review of 578 patients who had the procedure between 2009 and 2016, patients over age 60 years, patients with benign prostatic hyperplasia, and those with a body mass index (BMI) of less than or equal to 25.8 kg/m2 were more likely to develop postoperative urinary retention (POUR). Patients with these risk factors were also more likely to develop a urinary tract infection within 30 days, reported Daniel Roadman, a medical student in the department of surgery at Medical College of Wisconsin in Milwaukee, and coauthors.
Investigators conducted a retrospective chart review of patients 18 years of age or older with a direct, indirect, and/or femoral inguinal hernia. POUR was defined as “inability to void spontaneously prior to hospital discharge, requiring straight or indwelling catheter placement,” the authors wrote.
Patients were required to void before being discharged. For patients unable to void, an indwelling catheter was placed and removed the following morning. Patients still unable to void at this point were discharged with an indwelling catheter and scheduled for follow-up within 1 week.
POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI. Patients with POUR had increased incidence of UTI (6.3%), compared with patients without POUR (0.6%; P less than .0001).
Among patients who developed POUR, 54 (84.3%) were admitted for overnight observation with a stay of approximately 1.5 days. Three of these patients (5.6%) had a straight catheterization, and 51 (94.4%) had an indwelling urinary catheter placed. Two patients developed a UTI.
Of the 10 patients discharged home, six (60%) returned to the emergency department for catheterization; two (33.3%) patients had straight catheterization, and four (66.7%) were discharged home with an indwelling catheter. Two of these patients developed a UTI. In both groups, all patients who developed a UTI had an indwelling catheter placed, Mr. Roadman and colleagues reported.
During the study period, institutional protocol changed from routine intraoperative urinary catheterization to catheterization per surgeon discretion, though this did not affect POUR incidence.
“This is the first study to show a significant increase in UTI within 30 days after POUR,” the authors wrote. “Urinary stasis within the bladder due to the inability to void could lead to increased bacterial load and risk of infection.”
“It is important for providers, especially surgeons, to understand the risk of POUR and therefore increased risk of UTI after laparoscopic TEP inguinal hernia repair,” they added. “Identifying patients at higher risk … can help with patient education and expectations.”
No disclosures were reported by the study authors.
SOURCE: Roadman D et al. J Surg Res. 2018;11(231):309-15.
was more likely in patients aged more than 60 years, patients with benign prostatic hyperplasia, and patients with a decreased body mass index, according to findings published in the Journal of Surgical Research.
The researchers note that, while laparoscopic TEP is growing in popularity for inguinal hernia repair, postop urinary retention (POUR) is estimated at 2%-30%, but for open procedures, it is estimated at 0.4%-3%. POUR is linked to the development of urinary tract infections and also hospital readmissions. Since TEP may eventually become the norm, the study authors suggest that identifying patients at higher risk for POUR would contribute to the safety and quality of care for this operation.
In a retrospective chart review of 578 patients who had the procedure between 2009 and 2016, patients over age 60 years, patients with benign prostatic hyperplasia, and those with a body mass index (BMI) of less than or equal to 25.8 kg/m2 were more likely to develop postoperative urinary retention (POUR). Patients with these risk factors were also more likely to develop a urinary tract infection within 30 days, reported Daniel Roadman, a medical student in the department of surgery at Medical College of Wisconsin in Milwaukee, and coauthors.
Investigators conducted a retrospective chart review of patients 18 years of age or older with a direct, indirect, and/or femoral inguinal hernia. POUR was defined as “inability to void spontaneously prior to hospital discharge, requiring straight or indwelling catheter placement,” the authors wrote.
Patients were required to void before being discharged. For patients unable to void, an indwelling catheter was placed and removed the following morning. Patients still unable to void at this point were discharged with an indwelling catheter and scheduled for follow-up within 1 week.
POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI. Patients with POUR had increased incidence of UTI (6.3%), compared with patients without POUR (0.6%; P less than .0001).
Among patients who developed POUR, 54 (84.3%) were admitted for overnight observation with a stay of approximately 1.5 days. Three of these patients (5.6%) had a straight catheterization, and 51 (94.4%) had an indwelling urinary catheter placed. Two patients developed a UTI.
Of the 10 patients discharged home, six (60%) returned to the emergency department for catheterization; two (33.3%) patients had straight catheterization, and four (66.7%) were discharged home with an indwelling catheter. Two of these patients developed a UTI. In both groups, all patients who developed a UTI had an indwelling catheter placed, Mr. Roadman and colleagues reported.
During the study period, institutional protocol changed from routine intraoperative urinary catheterization to catheterization per surgeon discretion, though this did not affect POUR incidence.
“This is the first study to show a significant increase in UTI within 30 days after POUR,” the authors wrote. “Urinary stasis within the bladder due to the inability to void could lead to increased bacterial load and risk of infection.”
“It is important for providers, especially surgeons, to understand the risk of POUR and therefore increased risk of UTI after laparoscopic TEP inguinal hernia repair,” they added. “Identifying patients at higher risk … can help with patient education and expectations.”
No disclosures were reported by the study authors.
SOURCE: Roadman D et al. J Surg Res. 2018;11(231):309-15.
was more likely in patients aged more than 60 years, patients with benign prostatic hyperplasia, and patients with a decreased body mass index, according to findings published in the Journal of Surgical Research.
The researchers note that, while laparoscopic TEP is growing in popularity for inguinal hernia repair, postop urinary retention (POUR) is estimated at 2%-30%, but for open procedures, it is estimated at 0.4%-3%. POUR is linked to the development of urinary tract infections and also hospital readmissions. Since TEP may eventually become the norm, the study authors suggest that identifying patients at higher risk for POUR would contribute to the safety and quality of care for this operation.
In a retrospective chart review of 578 patients who had the procedure between 2009 and 2016, patients over age 60 years, patients with benign prostatic hyperplasia, and those with a body mass index (BMI) of less than or equal to 25.8 kg/m2 were more likely to develop postoperative urinary retention (POUR). Patients with these risk factors were also more likely to develop a urinary tract infection within 30 days, reported Daniel Roadman, a medical student in the department of surgery at Medical College of Wisconsin in Milwaukee, and coauthors.
Investigators conducted a retrospective chart review of patients 18 years of age or older with a direct, indirect, and/or femoral inguinal hernia. POUR was defined as “inability to void spontaneously prior to hospital discharge, requiring straight or indwelling catheter placement,” the authors wrote.
Patients were required to void before being discharged. For patients unable to void, an indwelling catheter was placed and removed the following morning. Patients still unable to void at this point were discharged with an indwelling catheter and scheduled for follow-up within 1 week.
POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI. Patients with POUR had increased incidence of UTI (6.3%), compared with patients without POUR (0.6%; P less than .0001).
Among patients who developed POUR, 54 (84.3%) were admitted for overnight observation with a stay of approximately 1.5 days. Three of these patients (5.6%) had a straight catheterization, and 51 (94.4%) had an indwelling urinary catheter placed. Two patients developed a UTI.
Of the 10 patients discharged home, six (60%) returned to the emergency department for catheterization; two (33.3%) patients had straight catheterization, and four (66.7%) were discharged home with an indwelling catheter. Two of these patients developed a UTI. In both groups, all patients who developed a UTI had an indwelling catheter placed, Mr. Roadman and colleagues reported.
During the study period, institutional protocol changed from routine intraoperative urinary catheterization to catheterization per surgeon discretion, though this did not affect POUR incidence.
“This is the first study to show a significant increase in UTI within 30 days after POUR,” the authors wrote. “Urinary stasis within the bladder due to the inability to void could lead to increased bacterial load and risk of infection.”
“It is important for providers, especially surgeons, to understand the risk of POUR and therefore increased risk of UTI after laparoscopic TEP inguinal hernia repair,” they added. “Identifying patients at higher risk … can help with patient education and expectations.”
No disclosures were reported by the study authors.
SOURCE: Roadman D et al. J Surg Res. 2018;11(231):309-15.
FROM THE JOURNAL OF SURGICAL RESEARCH
Key clinical point: Postoperative urinary retention was more likely to occur in patients more than 60 years of age and patients with benign prostatic hyperplasia.
Major finding: POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI.
Study details: A retrospective chart review of 578 patients who had laparoscopic total extraperitoneal inguinal hernia repair between 2009 and 2016.
Disclosures: No disclosures were reported.
Source: Roadman D et al. J Surg Res. 2018;11(231):309-15.
Prostate cancer risk before age 55 higher for black men
according to a new study that drew subjects aged 40-54 years from three public and two private hospitals in the Chicago area.
Black race, rather than socioeconomic or clinical factors, appeared to be the strongest nonmodifiable predictor of prostate cancer risk in that age group, the researchers concluded, based on multivariate analyses that examined the association between prostate cancer risk and clinical setting, race, genetically determined West African ancestry, and clinical and socioeconomic risk factors.
The results suggest that screening practices should be altered, said study investigator Oluwarotimi S. Nettey, MD, of Northwestern University, Chicago. “You might want to think about screening black men who are younger than 55.”
“In the prebiopsy space, most studies have looked at race, age, PSA [level], and prostate volume, and they’ve said that the reason we see that black men have disparate prostate cancer risk on diagnosis is probably because of access to care issues, so that’s been the confounder. We tried to control for this by looking at socioeconomic status through income, marriage, and education, as well as hospital setting,” said Dr. Nettey, who presented the study at a poster session at the annual meeting of the American Urological Association.
Previous studies have examined populations and then conducted a secondary analysis on outcomes in black men. The current study has greater power and is more convincing because outcomes in black men was the primary outcome of the study, according to Robert L. Waterhouse Jr., MD, who is the public policy liaison for the R. Frank Jones Urological Society of the National Medical Association. Dr. Waterhouse, a urologist in Charlotte, N.C., attended the poster session and was not involved in the research.
“This study helps to provide some evidence that black heritage is indeed a significant risk factor in men who develop prostate cancer at an earlier age, and efforts at identifying prostate cancer at an earlier age [should consider] black race as a high-risk group,” said Dr. Waterhouse.
For patients of all ages, biopsies were positive in 63.1% of black men, compared with 41.5% of nonblack men (P less than .001). Cancers were also more advanced in black men: 47.5% were Gleason 3+4 in black men, compared with 40% in nonblack men (P less than .001), and 14.4% were Gleason 4+4 in black men, compared with 9.6% in nonblack men (P = .02).
After researchers controlled for other risk factors, black race was associated with heightened risk of prostate cancer diagnosis (OR, 5.66; P = .02), as was family history (OR, 4.98; P = .01).
There was no association between West African ancestry and prostate cancer risk either as a continuous variable or in quartiles.
Limitations of the study include the fact that race was self-reported and that this was a referred population.
The study received funding from the National Institutes of Health and the U.S. Department of Veterans Affairs. Dr. Nettey reported having no financial disclosures.
SOURCE: Nettey OS et al. AUA Annual Meeting. Abstract MP 21-17.
according to a new study that drew subjects aged 40-54 years from three public and two private hospitals in the Chicago area.
Black race, rather than socioeconomic or clinical factors, appeared to be the strongest nonmodifiable predictor of prostate cancer risk in that age group, the researchers concluded, based on multivariate analyses that examined the association between prostate cancer risk and clinical setting, race, genetically determined West African ancestry, and clinical and socioeconomic risk factors.
The results suggest that screening practices should be altered, said study investigator Oluwarotimi S. Nettey, MD, of Northwestern University, Chicago. “You might want to think about screening black men who are younger than 55.”
“In the prebiopsy space, most studies have looked at race, age, PSA [level], and prostate volume, and they’ve said that the reason we see that black men have disparate prostate cancer risk on diagnosis is probably because of access to care issues, so that’s been the confounder. We tried to control for this by looking at socioeconomic status through income, marriage, and education, as well as hospital setting,” said Dr. Nettey, who presented the study at a poster session at the annual meeting of the American Urological Association.
Previous studies have examined populations and then conducted a secondary analysis on outcomes in black men. The current study has greater power and is more convincing because outcomes in black men was the primary outcome of the study, according to Robert L. Waterhouse Jr., MD, who is the public policy liaison for the R. Frank Jones Urological Society of the National Medical Association. Dr. Waterhouse, a urologist in Charlotte, N.C., attended the poster session and was not involved in the research.
“This study helps to provide some evidence that black heritage is indeed a significant risk factor in men who develop prostate cancer at an earlier age, and efforts at identifying prostate cancer at an earlier age [should consider] black race as a high-risk group,” said Dr. Waterhouse.
For patients of all ages, biopsies were positive in 63.1% of black men, compared with 41.5% of nonblack men (P less than .001). Cancers were also more advanced in black men: 47.5% were Gleason 3+4 in black men, compared with 40% in nonblack men (P less than .001), and 14.4% were Gleason 4+4 in black men, compared with 9.6% in nonblack men (P = .02).
After researchers controlled for other risk factors, black race was associated with heightened risk of prostate cancer diagnosis (OR, 5.66; P = .02), as was family history (OR, 4.98; P = .01).
There was no association between West African ancestry and prostate cancer risk either as a continuous variable or in quartiles.
Limitations of the study include the fact that race was self-reported and that this was a referred population.
The study received funding from the National Institutes of Health and the U.S. Department of Veterans Affairs. Dr. Nettey reported having no financial disclosures.
SOURCE: Nettey OS et al. AUA Annual Meeting. Abstract MP 21-17.
according to a new study that drew subjects aged 40-54 years from three public and two private hospitals in the Chicago area.
Black race, rather than socioeconomic or clinical factors, appeared to be the strongest nonmodifiable predictor of prostate cancer risk in that age group, the researchers concluded, based on multivariate analyses that examined the association between prostate cancer risk and clinical setting, race, genetically determined West African ancestry, and clinical and socioeconomic risk factors.
The results suggest that screening practices should be altered, said study investigator Oluwarotimi S. Nettey, MD, of Northwestern University, Chicago. “You might want to think about screening black men who are younger than 55.”
“In the prebiopsy space, most studies have looked at race, age, PSA [level], and prostate volume, and they’ve said that the reason we see that black men have disparate prostate cancer risk on diagnosis is probably because of access to care issues, so that’s been the confounder. We tried to control for this by looking at socioeconomic status through income, marriage, and education, as well as hospital setting,” said Dr. Nettey, who presented the study at a poster session at the annual meeting of the American Urological Association.
Previous studies have examined populations and then conducted a secondary analysis on outcomes in black men. The current study has greater power and is more convincing because outcomes in black men was the primary outcome of the study, according to Robert L. Waterhouse Jr., MD, who is the public policy liaison for the R. Frank Jones Urological Society of the National Medical Association. Dr. Waterhouse, a urologist in Charlotte, N.C., attended the poster session and was not involved in the research.
“This study helps to provide some evidence that black heritage is indeed a significant risk factor in men who develop prostate cancer at an earlier age, and efforts at identifying prostate cancer at an earlier age [should consider] black race as a high-risk group,” said Dr. Waterhouse.
For patients of all ages, biopsies were positive in 63.1% of black men, compared with 41.5% of nonblack men (P less than .001). Cancers were also more advanced in black men: 47.5% were Gleason 3+4 in black men, compared with 40% in nonblack men (P less than .001), and 14.4% were Gleason 4+4 in black men, compared with 9.6% in nonblack men (P = .02).
After researchers controlled for other risk factors, black race was associated with heightened risk of prostate cancer diagnosis (OR, 5.66; P = .02), as was family history (OR, 4.98; P = .01).
There was no association between West African ancestry and prostate cancer risk either as a continuous variable or in quartiles.
Limitations of the study include the fact that race was self-reported and that this was a referred population.
The study received funding from the National Institutes of Health and the U.S. Department of Veterans Affairs. Dr. Nettey reported having no financial disclosures.
SOURCE: Nettey OS et al. AUA Annual Meeting. Abstract MP 21-17.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: Black race appears to be a key risk factor for prostate cancer in younger men.
Major finding: Black men younger than age 55 years undergoing prostate biopsies were 5.6 times more likely than other men to have a positive biopsy result.
Study details: Retrospective analysis of 564 men.
Disclosures: The study received funding from the National Institutes of Health and the U.S. Department of Veterans Affairs. Dr. Nettey reported having no financial disclosures.
Source: Nettey OS et al. AUA Annual Meeting. Abstract MP 21-17.
Complete MUS mesh removal not linked to incontinence
SAN FRANCISCO – Stress urinary incontinence (SUI) following removal of a mid-urethral sling (MUS) mesh is not necessarily associated with increased risk of postsurgical urinary incontinence, according to a retrospective study at a high-volume, tertiary medical center.
Follow-up procedures occurred more often in women with preoperative urodynamic SUI and less often in women who were stress continent.
Among women who were stress continent, obesity and postmenopausal status were linked to postsurgical SUI. There was no association between postsurgical SUI and the extent of mesh excision or prior revisions.
The study grew out of observations that SUI occurred less often than expected.
“There’s an increasing recognition of complications related to synthetic MUS,” said Janine Oliver, MD, who presented the study at the annual meeting of the American Urological Association. “As mesh removal procedures were being performed, we assumed that the majority of patients, if not all, would be incontinent afterward, since we were removing the sling that was put in to fix stress incontinence in most cases.”
In a patient who would benefit from a complete mesh removal, “the fear that it may lead to a higher risk of urinary incontinence is not a good justification to not do it,” Dr. Oliver said. She did note, however, that the procedures were done by specialists, so findings may not be applicable to general practitioners.
The study was performed while Dr. Oliver was a fellow at the University of California, Los Angeles. She is now with the division of urology at the University of Colorado, Anschutz, in Aurora.
Dr. Oliver and her colleagues analyzed data from 233 patients who underwent MUS excision at UCLA for MUS-related complications during 2013-2015, and who had at least 3 months of follow-up. The average patient age was 55.4 years; an average of 5.4 years passed between the placement and excision of MUS. The mean body mass index was 28.9, and mean follow-up was 23.5 months.
A total of 84% of patients underwent a total excision; 45% of MUS were retropubic, 35% were transobturator, 10% were single incision, and 10% were multiple incision.
Nearly half (49%) of patients required a second procedure for SUI, such as bulking agent injection, bladder neck suspension, or repeat sling procedure.
In the entire cohort, multivariate analyses found significant associations between heightened risk of postoperative SUI and increasing time to MUS excision (odds ratio, 1.16; 95% confidence interval, 1.03-1.30), total MUS excision (OR, 4.14; 95% CI, 1.38-12.37), and preoperative urodynamic SUI (OR, 4.66; 95% CI, 2.13-10.19).
Of 51 patients who had preoperative urodynamic SUI, 39 (76%) ultimately underwent another surgery. Although increased time to MUS excision and total mesh removal were associated with urinary incontinence in this group in univariate analyses, they were no longer significant following a multivariate analysis.
Of 140 patients with a negative preoperative urodynamic testing for SUI, 59 (42%) went on to have another SUI procedure. After multivariate analysis, the only risk factors for urinary incontinence were obesity (OR, 4.74; 95% CI, 1.73-13.02) and postmenopausal status (OR, 3.78; 95% CI, 1.16-12.33).
“I think there’s a lot of fear, even among urologists and specialists who see these problems, that complete mesh removal is associated with a higher risk of complications and a higher risk of incontinence,” said Dr. Oliver. “These data would suggest that, in certain subgroups, that’s not true. The risks factors that we identified in a multivariate analysis were being obese and being postmenopausal, but not complete mesh removal.”
The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
SOURCE: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
SAN FRANCISCO – Stress urinary incontinence (SUI) following removal of a mid-urethral sling (MUS) mesh is not necessarily associated with increased risk of postsurgical urinary incontinence, according to a retrospective study at a high-volume, tertiary medical center.
Follow-up procedures occurred more often in women with preoperative urodynamic SUI and less often in women who were stress continent.
Among women who were stress continent, obesity and postmenopausal status were linked to postsurgical SUI. There was no association between postsurgical SUI and the extent of mesh excision or prior revisions.
The study grew out of observations that SUI occurred less often than expected.
“There’s an increasing recognition of complications related to synthetic MUS,” said Janine Oliver, MD, who presented the study at the annual meeting of the American Urological Association. “As mesh removal procedures were being performed, we assumed that the majority of patients, if not all, would be incontinent afterward, since we were removing the sling that was put in to fix stress incontinence in most cases.”
In a patient who would benefit from a complete mesh removal, “the fear that it may lead to a higher risk of urinary incontinence is not a good justification to not do it,” Dr. Oliver said. She did note, however, that the procedures were done by specialists, so findings may not be applicable to general practitioners.
The study was performed while Dr. Oliver was a fellow at the University of California, Los Angeles. She is now with the division of urology at the University of Colorado, Anschutz, in Aurora.
Dr. Oliver and her colleagues analyzed data from 233 patients who underwent MUS excision at UCLA for MUS-related complications during 2013-2015, and who had at least 3 months of follow-up. The average patient age was 55.4 years; an average of 5.4 years passed between the placement and excision of MUS. The mean body mass index was 28.9, and mean follow-up was 23.5 months.
A total of 84% of patients underwent a total excision; 45% of MUS were retropubic, 35% were transobturator, 10% were single incision, and 10% were multiple incision.
Nearly half (49%) of patients required a second procedure for SUI, such as bulking agent injection, bladder neck suspension, or repeat sling procedure.
In the entire cohort, multivariate analyses found significant associations between heightened risk of postoperative SUI and increasing time to MUS excision (odds ratio, 1.16; 95% confidence interval, 1.03-1.30), total MUS excision (OR, 4.14; 95% CI, 1.38-12.37), and preoperative urodynamic SUI (OR, 4.66; 95% CI, 2.13-10.19).
Of 51 patients who had preoperative urodynamic SUI, 39 (76%) ultimately underwent another surgery. Although increased time to MUS excision and total mesh removal were associated with urinary incontinence in this group in univariate analyses, they were no longer significant following a multivariate analysis.
Of 140 patients with a negative preoperative urodynamic testing for SUI, 59 (42%) went on to have another SUI procedure. After multivariate analysis, the only risk factors for urinary incontinence were obesity (OR, 4.74; 95% CI, 1.73-13.02) and postmenopausal status (OR, 3.78; 95% CI, 1.16-12.33).
“I think there’s a lot of fear, even among urologists and specialists who see these problems, that complete mesh removal is associated with a higher risk of complications and a higher risk of incontinence,” said Dr. Oliver. “These data would suggest that, in certain subgroups, that’s not true. The risks factors that we identified in a multivariate analysis were being obese and being postmenopausal, but not complete mesh removal.”
The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
SOURCE: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
SAN FRANCISCO – Stress urinary incontinence (SUI) following removal of a mid-urethral sling (MUS) mesh is not necessarily associated with increased risk of postsurgical urinary incontinence, according to a retrospective study at a high-volume, tertiary medical center.
Follow-up procedures occurred more often in women with preoperative urodynamic SUI and less often in women who were stress continent.
Among women who were stress continent, obesity and postmenopausal status were linked to postsurgical SUI. There was no association between postsurgical SUI and the extent of mesh excision or prior revisions.
The study grew out of observations that SUI occurred less often than expected.
“There’s an increasing recognition of complications related to synthetic MUS,” said Janine Oliver, MD, who presented the study at the annual meeting of the American Urological Association. “As mesh removal procedures were being performed, we assumed that the majority of patients, if not all, would be incontinent afterward, since we were removing the sling that was put in to fix stress incontinence in most cases.”
In a patient who would benefit from a complete mesh removal, “the fear that it may lead to a higher risk of urinary incontinence is not a good justification to not do it,” Dr. Oliver said. She did note, however, that the procedures were done by specialists, so findings may not be applicable to general practitioners.
The study was performed while Dr. Oliver was a fellow at the University of California, Los Angeles. She is now with the division of urology at the University of Colorado, Anschutz, in Aurora.
Dr. Oliver and her colleagues analyzed data from 233 patients who underwent MUS excision at UCLA for MUS-related complications during 2013-2015, and who had at least 3 months of follow-up. The average patient age was 55.4 years; an average of 5.4 years passed between the placement and excision of MUS. The mean body mass index was 28.9, and mean follow-up was 23.5 months.
A total of 84% of patients underwent a total excision; 45% of MUS were retropubic, 35% were transobturator, 10% were single incision, and 10% were multiple incision.
Nearly half (49%) of patients required a second procedure for SUI, such as bulking agent injection, bladder neck suspension, or repeat sling procedure.
In the entire cohort, multivariate analyses found significant associations between heightened risk of postoperative SUI and increasing time to MUS excision (odds ratio, 1.16; 95% confidence interval, 1.03-1.30), total MUS excision (OR, 4.14; 95% CI, 1.38-12.37), and preoperative urodynamic SUI (OR, 4.66; 95% CI, 2.13-10.19).
Of 51 patients who had preoperative urodynamic SUI, 39 (76%) ultimately underwent another surgery. Although increased time to MUS excision and total mesh removal were associated with urinary incontinence in this group in univariate analyses, they were no longer significant following a multivariate analysis.
Of 140 patients with a negative preoperative urodynamic testing for SUI, 59 (42%) went on to have another SUI procedure. After multivariate analysis, the only risk factors for urinary incontinence were obesity (OR, 4.74; 95% CI, 1.73-13.02) and postmenopausal status (OR, 3.78; 95% CI, 1.16-12.33).
“I think there’s a lot of fear, even among urologists and specialists who see these problems, that complete mesh removal is associated with a higher risk of complications and a higher risk of incontinence,” said Dr. Oliver. “These data would suggest that, in certain subgroups, that’s not true. The risks factors that we identified in a multivariate analysis were being obese and being postmenopausal, but not complete mesh removal.”
The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
SOURCE: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: There was no association between postsurgical incontinence risk and complete mesh removal.
Major finding: In patients without presurgical urodynamic incontinence, only obesity (OR, 4.74) and postmenopausal status (OR, 3.78) were linked to incontinence risk.
Study details: A retrospective analysis of 233 patients.
Disclosures: The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
Source: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
Bladder cancer: Two chemoradiation therapy regimens on par for muscle-invasive disease
SAN FRANCISCO – Two concurrent chemoradiation induction regimens had similar safety and efficacy when used as part of a bladder-sparing strategy in patients with muscle-invasive bladder cancer, suggest primary results of the NRG/RTOG 0712 trial. But one offers better patient convenience.
The selective bladder-preservation paradigm entails maximal transurethral resection of the bladder tumor (TURBT) followed by induction radiation and concomitant chemotherapy, lead author John J. Coen, MD, a radiation oncologist with 21st Century Oncology, Providence, Rhode Island, noted at the 2018 Genitourinary Cancers Symposium. Patients then undergo cystoscopy to assess their response.
“A key component of this therapy is close urologic surveillance,” he noted. “This is trimodality therapy with close urologic surveillance, and cystectomy is prompted at the earliest time it’s indicated.”
The 70 patients enrolled in the multicenter randomized phase 2 trial had undergone TURBT and were randomized evenly to twice-daily radiation plus 5-flourouracil-cisplatin (the RTOG standard at the time of trial planning) or to daily radiation plus gemcitabine (a modification of a successful regimen developed at the University of Michigan). All were offered adjuvant chemotherapy regardless of whether they responded and whether they underwent consolidation therapy or cystectomy.
At a median follow-up of 5.1 years among the 52 evaluable patients, the 3-year rate of freedom from distant metastasis, the trial’s primary endpoint, was 78% with twice-daily radiation plus 5-flourouracil-cisplatin and 84% with daily radiation plus gemcitabine, according to results reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Both values were higher than the trial’s predefined benchmark of 75% for defining the regimen as promising. “This trial wasn’t necessarily powered to compare arms, so the conclusion would be that both arms exceeded the benchmark and it would be appropriate to evaluate both arms further in subsequent trials,” Dr. Coen commented.
In each trial arm, more than three-fourths of patients had a complete response, and about two-thirds of patients were alive and free of distant metastasis with their bladder intact at 3 years.
Toxicity, which was supposed to be the tie-breaker if efficacy was similar, was also essentially the same for the two regimens. Both were fairly well tolerated in the acute period; the primary grade 3 and 4 toxicities were hematologic ones.
“So where do we go from here? I think this trial does demonstrate concurrent gemcitabine is a reasonable alternative to cisplatin for patients undergoing selective bladder preservation,” Dr. Coen summarized. “This is especially important in patients with poor renal function or hearing loss. And bladder cancer is often a disease in the elderly, so tolerance of various regimens is a very important aspect to planning further trials.”
“This trial also demonstrates that daily radiation is a reasonable alternative to twice-a-day radiation, which had become the standard through the RTOG on more contemporary trials,” he added. “Daily radiation would allow wider adoption of selective bladder preservation by trimodality therapy.”
Clinical implications
“I would have to see more details about the toxicity data for the chemotherapy, the radiosensitizers,” session co-chair Yair Lotan, MD, a professor of urology at the UT Southwestern Medical Center, Dallas, Texas, commented in an interview. “But in general, if you have two equivalent protocols in terms of effectiveness, and one is less toxic or more convenient, that’s always preferable. From the standpoint of coming once a day rather than twice a day to get radiation, if I were a patient, I would prefer that regimen”
Current practice in this patient population likely hinges on where a patient is treated, he said. “Based on the new nonmetastatic muscle-invasive guidelines, many of them would be recommended surgery or possibly chemoradiation protocols, and then there are a lot of factors such as patient preference and outcomes that would probably impact the decision making.”
“Certainly, this study won’t prove standard of care because standard of care in the global picture for a patient with muscle-invasive disease really will depend on a randomized trial of surgery versus radiation or chemoradiation, multimodal therapies,” Dr. Lotan maintained. “Not every patient is even eligible; some patients with hydronephrosis or unresectable disease may not be the best candidates for multimodal therapy. But more information from these types of trials may help clinicians decide which multimodal therapy approach to use.”
Study details
Patients enrolled in NRG/RTOG 0712, a multicenter randomized phase 2 trial supported by the National Cancer Institute, had clinical T2 or T3-4a bladder cancer. Hydronephrosis and obvious lymph node involvement were exclusion criteria.
Like the rate of freedom from metastasis, the rate of complete response after induction therapy was similar for the two arms: 87.9% with twice-daily radiation plus 5-flourouracil-cisplatin and 75.8% with daily radiation plus gemcitabine.
“These rates exceed historical complete response rates, and this is likely a result of improved selection over time and more thorough TURBTs performed over time,” Dr. Coen proposed. “Over the course of multiple successive RTOG trials, our selection criteria have been refined. One excellent example would be that hydronephrosis has been excluded on more contemporary trials, but if you look at older trials, those patients were included.”
The 3-year rate of bladder-intact distant metastasis–free survival was 66.7% and 69%, respectively. “This is a very important endpoint. These results are excellent,” he commented. “And there is really no appreciable difference between the two arms on the actuarial analysis.”
As far as specific treatment failure events in the trial overall, three patients died, eight underwent cystectomy, and eight developed distant metastases. Although numbers were small, these events appeared fairly evenly distributed across arms.
The rate of grade 3 or 4 acute toxicity was 57.6% with twice-daily radiation plus 5-flourouracil-cisplatin and 54.6% with daily radiation plus gemcitabine. The large majority of events were blood and bone marrow toxicity. “It’s quite notable that the rates of GU and GI toxicity were very low,” commented Dr. Coen, who disclosed that he had no relevant conflicts of interest.
SOURCE: Coen, J. et al, 2018 Genitourinary Cancers Symposium, Abstract 408
SAN FRANCISCO – Two concurrent chemoradiation induction regimens had similar safety and efficacy when used as part of a bladder-sparing strategy in patients with muscle-invasive bladder cancer, suggest primary results of the NRG/RTOG 0712 trial. But one offers better patient convenience.
The selective bladder-preservation paradigm entails maximal transurethral resection of the bladder tumor (TURBT) followed by induction radiation and concomitant chemotherapy, lead author John J. Coen, MD, a radiation oncologist with 21st Century Oncology, Providence, Rhode Island, noted at the 2018 Genitourinary Cancers Symposium. Patients then undergo cystoscopy to assess their response.
“A key component of this therapy is close urologic surveillance,” he noted. “This is trimodality therapy with close urologic surveillance, and cystectomy is prompted at the earliest time it’s indicated.”
The 70 patients enrolled in the multicenter randomized phase 2 trial had undergone TURBT and were randomized evenly to twice-daily radiation plus 5-flourouracil-cisplatin (the RTOG standard at the time of trial planning) or to daily radiation plus gemcitabine (a modification of a successful regimen developed at the University of Michigan). All were offered adjuvant chemotherapy regardless of whether they responded and whether they underwent consolidation therapy or cystectomy.
At a median follow-up of 5.1 years among the 52 evaluable patients, the 3-year rate of freedom from distant metastasis, the trial’s primary endpoint, was 78% with twice-daily radiation plus 5-flourouracil-cisplatin and 84% with daily radiation plus gemcitabine, according to results reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Both values were higher than the trial’s predefined benchmark of 75% for defining the regimen as promising. “This trial wasn’t necessarily powered to compare arms, so the conclusion would be that both arms exceeded the benchmark and it would be appropriate to evaluate both arms further in subsequent trials,” Dr. Coen commented.
In each trial arm, more than three-fourths of patients had a complete response, and about two-thirds of patients were alive and free of distant metastasis with their bladder intact at 3 years.
Toxicity, which was supposed to be the tie-breaker if efficacy was similar, was also essentially the same for the two regimens. Both were fairly well tolerated in the acute period; the primary grade 3 and 4 toxicities were hematologic ones.
“So where do we go from here? I think this trial does demonstrate concurrent gemcitabine is a reasonable alternative to cisplatin for patients undergoing selective bladder preservation,” Dr. Coen summarized. “This is especially important in patients with poor renal function or hearing loss. And bladder cancer is often a disease in the elderly, so tolerance of various regimens is a very important aspect to planning further trials.”
“This trial also demonstrates that daily radiation is a reasonable alternative to twice-a-day radiation, which had become the standard through the RTOG on more contemporary trials,” he added. “Daily radiation would allow wider adoption of selective bladder preservation by trimodality therapy.”
Clinical implications
“I would have to see more details about the toxicity data for the chemotherapy, the radiosensitizers,” session co-chair Yair Lotan, MD, a professor of urology at the UT Southwestern Medical Center, Dallas, Texas, commented in an interview. “But in general, if you have two equivalent protocols in terms of effectiveness, and one is less toxic or more convenient, that’s always preferable. From the standpoint of coming once a day rather than twice a day to get radiation, if I were a patient, I would prefer that regimen”
Current practice in this patient population likely hinges on where a patient is treated, he said. “Based on the new nonmetastatic muscle-invasive guidelines, many of them would be recommended surgery or possibly chemoradiation protocols, and then there are a lot of factors such as patient preference and outcomes that would probably impact the decision making.”
“Certainly, this study won’t prove standard of care because standard of care in the global picture for a patient with muscle-invasive disease really will depend on a randomized trial of surgery versus radiation or chemoradiation, multimodal therapies,” Dr. Lotan maintained. “Not every patient is even eligible; some patients with hydronephrosis or unresectable disease may not be the best candidates for multimodal therapy. But more information from these types of trials may help clinicians decide which multimodal therapy approach to use.”
Study details
Patients enrolled in NRG/RTOG 0712, a multicenter randomized phase 2 trial supported by the National Cancer Institute, had clinical T2 or T3-4a bladder cancer. Hydronephrosis and obvious lymph node involvement were exclusion criteria.
Like the rate of freedom from metastasis, the rate of complete response after induction therapy was similar for the two arms: 87.9% with twice-daily radiation plus 5-flourouracil-cisplatin and 75.8% with daily radiation plus gemcitabine.
“These rates exceed historical complete response rates, and this is likely a result of improved selection over time and more thorough TURBTs performed over time,” Dr. Coen proposed. “Over the course of multiple successive RTOG trials, our selection criteria have been refined. One excellent example would be that hydronephrosis has been excluded on more contemporary trials, but if you look at older trials, those patients were included.”
The 3-year rate of bladder-intact distant metastasis–free survival was 66.7% and 69%, respectively. “This is a very important endpoint. These results are excellent,” he commented. “And there is really no appreciable difference between the two arms on the actuarial analysis.”
As far as specific treatment failure events in the trial overall, three patients died, eight underwent cystectomy, and eight developed distant metastases. Although numbers were small, these events appeared fairly evenly distributed across arms.
The rate of grade 3 or 4 acute toxicity was 57.6% with twice-daily radiation plus 5-flourouracil-cisplatin and 54.6% with daily radiation plus gemcitabine. The large majority of events were blood and bone marrow toxicity. “It’s quite notable that the rates of GU and GI toxicity were very low,” commented Dr. Coen, who disclosed that he had no relevant conflicts of interest.
SOURCE: Coen, J. et al, 2018 Genitourinary Cancers Symposium, Abstract 408
SAN FRANCISCO – Two concurrent chemoradiation induction regimens had similar safety and efficacy when used as part of a bladder-sparing strategy in patients with muscle-invasive bladder cancer, suggest primary results of the NRG/RTOG 0712 trial. But one offers better patient convenience.
The selective bladder-preservation paradigm entails maximal transurethral resection of the bladder tumor (TURBT) followed by induction radiation and concomitant chemotherapy, lead author John J. Coen, MD, a radiation oncologist with 21st Century Oncology, Providence, Rhode Island, noted at the 2018 Genitourinary Cancers Symposium. Patients then undergo cystoscopy to assess their response.
“A key component of this therapy is close urologic surveillance,” he noted. “This is trimodality therapy with close urologic surveillance, and cystectomy is prompted at the earliest time it’s indicated.”
The 70 patients enrolled in the multicenter randomized phase 2 trial had undergone TURBT and were randomized evenly to twice-daily radiation plus 5-flourouracil-cisplatin (the RTOG standard at the time of trial planning) or to daily radiation plus gemcitabine (a modification of a successful regimen developed at the University of Michigan). All were offered adjuvant chemotherapy regardless of whether they responded and whether they underwent consolidation therapy or cystectomy.
At a median follow-up of 5.1 years among the 52 evaluable patients, the 3-year rate of freedom from distant metastasis, the trial’s primary endpoint, was 78% with twice-daily radiation plus 5-flourouracil-cisplatin and 84% with daily radiation plus gemcitabine, according to results reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Both values were higher than the trial’s predefined benchmark of 75% for defining the regimen as promising. “This trial wasn’t necessarily powered to compare arms, so the conclusion would be that both arms exceeded the benchmark and it would be appropriate to evaluate both arms further in subsequent trials,” Dr. Coen commented.
In each trial arm, more than three-fourths of patients had a complete response, and about two-thirds of patients were alive and free of distant metastasis with their bladder intact at 3 years.
Toxicity, which was supposed to be the tie-breaker if efficacy was similar, was also essentially the same for the two regimens. Both were fairly well tolerated in the acute period; the primary grade 3 and 4 toxicities were hematologic ones.
“So where do we go from here? I think this trial does demonstrate concurrent gemcitabine is a reasonable alternative to cisplatin for patients undergoing selective bladder preservation,” Dr. Coen summarized. “This is especially important in patients with poor renal function or hearing loss. And bladder cancer is often a disease in the elderly, so tolerance of various regimens is a very important aspect to planning further trials.”
“This trial also demonstrates that daily radiation is a reasonable alternative to twice-a-day radiation, which had become the standard through the RTOG on more contemporary trials,” he added. “Daily radiation would allow wider adoption of selective bladder preservation by trimodality therapy.”
Clinical implications
“I would have to see more details about the toxicity data for the chemotherapy, the radiosensitizers,” session co-chair Yair Lotan, MD, a professor of urology at the UT Southwestern Medical Center, Dallas, Texas, commented in an interview. “But in general, if you have two equivalent protocols in terms of effectiveness, and one is less toxic or more convenient, that’s always preferable. From the standpoint of coming once a day rather than twice a day to get radiation, if I were a patient, I would prefer that regimen”
Current practice in this patient population likely hinges on where a patient is treated, he said. “Based on the new nonmetastatic muscle-invasive guidelines, many of them would be recommended surgery or possibly chemoradiation protocols, and then there are a lot of factors such as patient preference and outcomes that would probably impact the decision making.”
“Certainly, this study won’t prove standard of care because standard of care in the global picture for a patient with muscle-invasive disease really will depend on a randomized trial of surgery versus radiation or chemoradiation, multimodal therapies,” Dr. Lotan maintained. “Not every patient is even eligible; some patients with hydronephrosis or unresectable disease may not be the best candidates for multimodal therapy. But more information from these types of trials may help clinicians decide which multimodal therapy approach to use.”
Study details
Patients enrolled in NRG/RTOG 0712, a multicenter randomized phase 2 trial supported by the National Cancer Institute, had clinical T2 or T3-4a bladder cancer. Hydronephrosis and obvious lymph node involvement were exclusion criteria.
Like the rate of freedom from metastasis, the rate of complete response after induction therapy was similar for the two arms: 87.9% with twice-daily radiation plus 5-flourouracil-cisplatin and 75.8% with daily radiation plus gemcitabine.
“These rates exceed historical complete response rates, and this is likely a result of improved selection over time and more thorough TURBTs performed over time,” Dr. Coen proposed. “Over the course of multiple successive RTOG trials, our selection criteria have been refined. One excellent example would be that hydronephrosis has been excluded on more contemporary trials, but if you look at older trials, those patients were included.”
The 3-year rate of bladder-intact distant metastasis–free survival was 66.7% and 69%, respectively. “This is a very important endpoint. These results are excellent,” he commented. “And there is really no appreciable difference between the two arms on the actuarial analysis.”
As far as specific treatment failure events in the trial overall, three patients died, eight underwent cystectomy, and eight developed distant metastases. Although numbers were small, these events appeared fairly evenly distributed across arms.
The rate of grade 3 or 4 acute toxicity was 57.6% with twice-daily radiation plus 5-flourouracil-cisplatin and 54.6% with daily radiation plus gemcitabine. The large majority of events were blood and bone marrow toxicity. “It’s quite notable that the rates of GU and GI toxicity were very low,” commented Dr. Coen, who disclosed that he had no relevant conflicts of interest.
SOURCE: Coen, J. et al, 2018 Genitourinary Cancers Symposium, Abstract 408
AT THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point:
Major finding: The rate of freedom from distant metastasis at 3 years was 77.8% with twice-daily radiation plus 5-flourouracil-cisplatin and 84.0% with daily radiation plus gemcitabine.
Data source: A multicenter randomized phase 2 trial among 70 patients with muscle-invasive (cT2-4a) bladder cancer who had undergone TURBT (NRG/RTOG 0712 trial).
Disclosures: Dr. Coen disclosed that he had no relevant conflicts of interest. The trial was supported by the National Cancer Institute.
Source: Coen, J. et al, 2018 Genitourinary Cancers Symposium, Abstract 408.
Adjuvant chemo halves DFS events in upper-tract urothelial cancer
SAN FRANCISCO – Patients who have undergone nephro-ureterectomy for upper-tract urothelial cancer (UTUC) fare much better if they are given adjuvant chemotherapy, according to the first results of the POUT trial reported at the 2018 Genitourinary Cancers Symposium.
Standard treatment for this rare cancer is radical nephro-ureterectomy followed by surveillance, noted lead author Alison Jane Birtle, MD, MRCP, FRCR, a consultant clinical oncologist at the Rosemere Cancer Centre, Royal Preston Hospital, Preston, United Kingdom. Evidence has been insufficient to recommend adjuvant therapy.
“We know that UTUC shares a similar etiology with bladder cancer, where there is strong evidence for chemotherapy,” she said. “Trials of adjuvant chemotherapy in bladder cancer have been challenging because of cystectomy being a much more morbid operation. So adjuvant chemotherapy after nephro-ureterectomy should be much easier to give because of the less morbid procedure.”
The POUT (Peri-Operative Chemotherapy Versus sUrveillance in Upper Tract Urothelial Cancer, NCT0199397) investigators enrolled in the trial 261 patients from 57 UK centers who had undergone radical nephro-ureterectomy for urothelial cancer of the renal pelvis or ureter. Patients were randomized evenly to receive surveillance or adjuvant platinum-based combination chemotherapy, with specific platinum (cisplatin or carboplatin) based on glomerular filtration rate (GFR).
Trial enrollment was stopped early, after a median follow-up of 19.3 months, because of efficacy of chemotherapy, Dr. Birtle reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Main results showed that risks of both disease-free survival events and metastasis-free survival events were 51% lower for the chemotherapy group as compared with the surveillance group. Overall survival showed a trend toward benefit as well.
Not surprisingly, grade 3 or worse adverse events during treatment were about four times more common with chemotherapy, but the treatment was overall feasible and safe, even though the majority of patients were older than 60 years.
“Based on these results, adjuvant platinum-based chemotherapy should be considered a new standard of care in these patients,” Dr. Birtle maintained. “The next thing is how are we going to move this data forward? We are looking at the successor study at the moment, which is currently in development…[POUT] has been a triumph of UK urologists really getting behind it, but to do a further study, it would be great to look at international collaboration.”
Optimal timing of chemotherapy, before or after surgery, remains an open question, according session co-chair Jeanny B. Aragon-Ching, MD, a medical oncologist with the Inova Medical Group, Fairfax, Virginia.
“This trial offers high-level evidence for consideration of adjuvant chemotherapy in those who are unable to receive neoadjuvant chemotherapy in UTUC, although it is still important to evaluate what the prospective neoadjuvant chemotherapy data in UTUC would show,” she said in an interview. “Ongoing studies include the ECOG 8141 study (clinicaltrials.gov NCT02412670), but certainly, several retrospective trials showed benefit of neoadjuvant chemotherapy in UTUC.”
Other viewpoints
“It’s fantastic that we finally have data in upper-tract cancer. However, I worry about how this data is going to be interpreted, and I argue that it should not be considered the standard of care,” session attendee Matthew Campbell, MD, of the MD Anderson Cancer Center, Houston, commented during a question and answer period.
“I believe that this is showing that chemotherapy is very important in this disease, and if anything, it should be moved into the neoadjuvant setting, where more patients will be cisplatin candidates. Though there is challenge with staging upper-tract disease, at MD Anderson, we consider patients with high-grade disease or hydronephrosis to be at high enough risk to consider for neoadjuvant chemotherapy, and that’s been our approach.”
“When we looked at developing POUT, there was a big debate about whether it should be a neoadjuvant or adjuvant study,” Dr. Birtle replied. UK oncologists expressed concern that not all patients have histologic confirmation of UTUC preoperatively; therefore, chemotherapy could lead to overtreatment for some.
“We plan to go back and look at the CT urograms and the diagnostic imaging done prior to surgery just to see if we can be 100% confident in our diagnostic accuracy preoperatively, to see if we would be more confident with a neoadjuvant study,” she added. The investigators have also reviewed data from the British Association of Urological Surgeons on the postoperative dip in GFR. “It didn’t really seem from that 2015 data that there was a huge unmet need for patients postoperatively, where we would have missed them had we not treated them preoperatively,” she said.
On a related note, session attendee Surena Matin, MD, also of MD Anderson, asked “How many patients were potentially precluded because of their GFR, and do you have any data regarding response rates in the carboplatin arm?”
The main reason for exclusion was ineligible pathology and not GFR, according to Dr. Birtle. “This goes back to the previous question of can you be certain that a patient has locally advanced disease prior to nephro-ureterectomy? About 60% of patients who were thought to have muscle-invasive disease ultimately on nephro-ureterectomy didn’t.”
Confidence intervals for chemotherapy benefit in the subgroup given carboplatin were wide and overlapped unity, but still favoring benefit and falling within the overall treatment effect, she said.
Session attendee Joaquim Bellmunt, MD, Dana-Farber Cancer Institute, Boston, noted that the chemotherapy benefit was not significant in that subgroup and also in the subgroups with lymph node–positive disease and with positive margins. “I think that saying … chemotherapy is for everybody based on this trial” is incorrect, he asserted. “It may be good just to tone down the message that this is the new standard of care.” He further questioned the trial’s early stopping, noting that continuing would have provided more information in these patients.
Those subgroups were small, so analyses were underpowered to definitively rule out chemotherapy benefit, according to Dr. Birtle. The investigators had intensive discussion about the recommendation to stop early, because of a goal to determine overall survival impact. Ultimately, “when we saw the data in terms of disease-free survival and metastasis-free survival, the magnitude of the effect was so big that we felt it was uncomfortable and unethical not to offer patients treatment,” she said.
Study details
Patients in POUT’s chemotherapy arm received four cycles of chemotherapy—gemcitabine-cisplatin if their GFR was 50 mL/min or higher, or gemcitabine-carboplatin if their GFR was 30-49 mL/min—starting within 90 days of nephro-ureterectomy.
Of note, approximately 40% of all patients in the trial were aged 70 years or older, including the 5% who were aged 80 years or older. “This is very reassuring for a study of adjuvant platinum-based chemotherapy,” Dr. Birtle commented. Fully 71.2% of the chemotherapy patients received all four planned cycles.
In an intention-to-treat analysis, risk of disease-free survival events (death from any cause, metastasis, or any ureteric or renal bed recurrence) was sharply reduced with chemotherapy versus surveillance (hazard ratio, 0.49; P=.001). The proportion of patients event free at 2 years was 71% in the chemotherapy group and 54% in the surveillance group. Benefit was generally similar across subgroups, and findings were much the same after adjustment for nodal involvement, microscopic margin status, and planned chemotherapy regimen (hazard ratio, 0.47; P=.001).
Risk of metastasis-free survival events was also sharply lower with chemotherapy (hazard ratio, 0.49; P=.002), with a proportion event free at 2 years of 74% in the chemotherapy group and 60% in the surveillance group. These findings were also much the same after adjustment for the above factors (hazard ratio, 0.47; P=.002).
Overall survival tended to be better with chemotherapy than with surveillance (hazard ratio, 0.55), but data are still immature for this endpoint.
The rate of grade 3 or worse adverse events during the treatment period was 53.2% with chemotherapy and 13.5% with surveillance; events with chemotherapy were as expected, with neutropenia, thrombocytopenia, and gastrointestinal events predominating. The rate of febrile neutropenia was 5.7% with gemcitabine-cisplatin and 7.8% with gemcitabine-carboplatin, but there were no neutropenic deaths.
The rate of grade 3 or worse adverse events for the entire trial period was 62.1% with chemotherapy and 24.8% with surveillance.
Data on nephrotoxicity are still being evaluated, according to Dr. Birtle. Only seven patients who started on gemcitabine-cisplatin had to switch to gemcitabine-carboplatin because their GFR fell.
In a related translational study, the investigators are evaluating both the baseline CT urograms and the resected tumors to identify prognostic and predictive markers, she said.
Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
SOURCE: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407
SAN FRANCISCO – Patients who have undergone nephro-ureterectomy for upper-tract urothelial cancer (UTUC) fare much better if they are given adjuvant chemotherapy, according to the first results of the POUT trial reported at the 2018 Genitourinary Cancers Symposium.
Standard treatment for this rare cancer is radical nephro-ureterectomy followed by surveillance, noted lead author Alison Jane Birtle, MD, MRCP, FRCR, a consultant clinical oncologist at the Rosemere Cancer Centre, Royal Preston Hospital, Preston, United Kingdom. Evidence has been insufficient to recommend adjuvant therapy.
“We know that UTUC shares a similar etiology with bladder cancer, where there is strong evidence for chemotherapy,” she said. “Trials of adjuvant chemotherapy in bladder cancer have been challenging because of cystectomy being a much more morbid operation. So adjuvant chemotherapy after nephro-ureterectomy should be much easier to give because of the less morbid procedure.”
The POUT (Peri-Operative Chemotherapy Versus sUrveillance in Upper Tract Urothelial Cancer, NCT0199397) investigators enrolled in the trial 261 patients from 57 UK centers who had undergone radical nephro-ureterectomy for urothelial cancer of the renal pelvis or ureter. Patients were randomized evenly to receive surveillance or adjuvant platinum-based combination chemotherapy, with specific platinum (cisplatin or carboplatin) based on glomerular filtration rate (GFR).
Trial enrollment was stopped early, after a median follow-up of 19.3 months, because of efficacy of chemotherapy, Dr. Birtle reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Main results showed that risks of both disease-free survival events and metastasis-free survival events were 51% lower for the chemotherapy group as compared with the surveillance group. Overall survival showed a trend toward benefit as well.
Not surprisingly, grade 3 or worse adverse events during treatment were about four times more common with chemotherapy, but the treatment was overall feasible and safe, even though the majority of patients were older than 60 years.
“Based on these results, adjuvant platinum-based chemotherapy should be considered a new standard of care in these patients,” Dr. Birtle maintained. “The next thing is how are we going to move this data forward? We are looking at the successor study at the moment, which is currently in development…[POUT] has been a triumph of UK urologists really getting behind it, but to do a further study, it would be great to look at international collaboration.”
Optimal timing of chemotherapy, before or after surgery, remains an open question, according session co-chair Jeanny B. Aragon-Ching, MD, a medical oncologist with the Inova Medical Group, Fairfax, Virginia.
“This trial offers high-level evidence for consideration of adjuvant chemotherapy in those who are unable to receive neoadjuvant chemotherapy in UTUC, although it is still important to evaluate what the prospective neoadjuvant chemotherapy data in UTUC would show,” she said in an interview. “Ongoing studies include the ECOG 8141 study (clinicaltrials.gov NCT02412670), but certainly, several retrospective trials showed benefit of neoadjuvant chemotherapy in UTUC.”
Other viewpoints
“It’s fantastic that we finally have data in upper-tract cancer. However, I worry about how this data is going to be interpreted, and I argue that it should not be considered the standard of care,” session attendee Matthew Campbell, MD, of the MD Anderson Cancer Center, Houston, commented during a question and answer period.
“I believe that this is showing that chemotherapy is very important in this disease, and if anything, it should be moved into the neoadjuvant setting, where more patients will be cisplatin candidates. Though there is challenge with staging upper-tract disease, at MD Anderson, we consider patients with high-grade disease or hydronephrosis to be at high enough risk to consider for neoadjuvant chemotherapy, and that’s been our approach.”
“When we looked at developing POUT, there was a big debate about whether it should be a neoadjuvant or adjuvant study,” Dr. Birtle replied. UK oncologists expressed concern that not all patients have histologic confirmation of UTUC preoperatively; therefore, chemotherapy could lead to overtreatment for some.
“We plan to go back and look at the CT urograms and the diagnostic imaging done prior to surgery just to see if we can be 100% confident in our diagnostic accuracy preoperatively, to see if we would be more confident with a neoadjuvant study,” she added. The investigators have also reviewed data from the British Association of Urological Surgeons on the postoperative dip in GFR. “It didn’t really seem from that 2015 data that there was a huge unmet need for patients postoperatively, where we would have missed them had we not treated them preoperatively,” she said.
On a related note, session attendee Surena Matin, MD, also of MD Anderson, asked “How many patients were potentially precluded because of their GFR, and do you have any data regarding response rates in the carboplatin arm?”
The main reason for exclusion was ineligible pathology and not GFR, according to Dr. Birtle. “This goes back to the previous question of can you be certain that a patient has locally advanced disease prior to nephro-ureterectomy? About 60% of patients who were thought to have muscle-invasive disease ultimately on nephro-ureterectomy didn’t.”
Confidence intervals for chemotherapy benefit in the subgroup given carboplatin were wide and overlapped unity, but still favoring benefit and falling within the overall treatment effect, she said.
Session attendee Joaquim Bellmunt, MD, Dana-Farber Cancer Institute, Boston, noted that the chemotherapy benefit was not significant in that subgroup and also in the subgroups with lymph node–positive disease and with positive margins. “I think that saying … chemotherapy is for everybody based on this trial” is incorrect, he asserted. “It may be good just to tone down the message that this is the new standard of care.” He further questioned the trial’s early stopping, noting that continuing would have provided more information in these patients.
Those subgroups were small, so analyses were underpowered to definitively rule out chemotherapy benefit, according to Dr. Birtle. The investigators had intensive discussion about the recommendation to stop early, because of a goal to determine overall survival impact. Ultimately, “when we saw the data in terms of disease-free survival and metastasis-free survival, the magnitude of the effect was so big that we felt it was uncomfortable and unethical not to offer patients treatment,” she said.
Study details
Patients in POUT’s chemotherapy arm received four cycles of chemotherapy—gemcitabine-cisplatin if their GFR was 50 mL/min or higher, or gemcitabine-carboplatin if their GFR was 30-49 mL/min—starting within 90 days of nephro-ureterectomy.
Of note, approximately 40% of all patients in the trial were aged 70 years or older, including the 5% who were aged 80 years or older. “This is very reassuring for a study of adjuvant platinum-based chemotherapy,” Dr. Birtle commented. Fully 71.2% of the chemotherapy patients received all four planned cycles.
In an intention-to-treat analysis, risk of disease-free survival events (death from any cause, metastasis, or any ureteric or renal bed recurrence) was sharply reduced with chemotherapy versus surveillance (hazard ratio, 0.49; P=.001). The proportion of patients event free at 2 years was 71% in the chemotherapy group and 54% in the surveillance group. Benefit was generally similar across subgroups, and findings were much the same after adjustment for nodal involvement, microscopic margin status, and planned chemotherapy regimen (hazard ratio, 0.47; P=.001).
Risk of metastasis-free survival events was also sharply lower with chemotherapy (hazard ratio, 0.49; P=.002), with a proportion event free at 2 years of 74% in the chemotherapy group and 60% in the surveillance group. These findings were also much the same after adjustment for the above factors (hazard ratio, 0.47; P=.002).
Overall survival tended to be better with chemotherapy than with surveillance (hazard ratio, 0.55), but data are still immature for this endpoint.
The rate of grade 3 or worse adverse events during the treatment period was 53.2% with chemotherapy and 13.5% with surveillance; events with chemotherapy were as expected, with neutropenia, thrombocytopenia, and gastrointestinal events predominating. The rate of febrile neutropenia was 5.7% with gemcitabine-cisplatin and 7.8% with gemcitabine-carboplatin, but there were no neutropenic deaths.
The rate of grade 3 or worse adverse events for the entire trial period was 62.1% with chemotherapy and 24.8% with surveillance.
Data on nephrotoxicity are still being evaluated, according to Dr. Birtle. Only seven patients who started on gemcitabine-cisplatin had to switch to gemcitabine-carboplatin because their GFR fell.
In a related translational study, the investigators are evaluating both the baseline CT urograms and the resected tumors to identify prognostic and predictive markers, she said.
Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
SOURCE: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407
SAN FRANCISCO – Patients who have undergone nephro-ureterectomy for upper-tract urothelial cancer (UTUC) fare much better if they are given adjuvant chemotherapy, according to the first results of the POUT trial reported at the 2018 Genitourinary Cancers Symposium.
Standard treatment for this rare cancer is radical nephro-ureterectomy followed by surveillance, noted lead author Alison Jane Birtle, MD, MRCP, FRCR, a consultant clinical oncologist at the Rosemere Cancer Centre, Royal Preston Hospital, Preston, United Kingdom. Evidence has been insufficient to recommend adjuvant therapy.
“We know that UTUC shares a similar etiology with bladder cancer, where there is strong evidence for chemotherapy,” she said. “Trials of adjuvant chemotherapy in bladder cancer have been challenging because of cystectomy being a much more morbid operation. So adjuvant chemotherapy after nephro-ureterectomy should be much easier to give because of the less morbid procedure.”
The POUT (Peri-Operative Chemotherapy Versus sUrveillance in Upper Tract Urothelial Cancer, NCT0199397) investigators enrolled in the trial 261 patients from 57 UK centers who had undergone radical nephro-ureterectomy for urothelial cancer of the renal pelvis or ureter. Patients were randomized evenly to receive surveillance or adjuvant platinum-based combination chemotherapy, with specific platinum (cisplatin or carboplatin) based on glomerular filtration rate (GFR).
Trial enrollment was stopped early, after a median follow-up of 19.3 months, because of efficacy of chemotherapy, Dr. Birtle reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Main results showed that risks of both disease-free survival events and metastasis-free survival events were 51% lower for the chemotherapy group as compared with the surveillance group. Overall survival showed a trend toward benefit as well.
Not surprisingly, grade 3 or worse adverse events during treatment were about four times more common with chemotherapy, but the treatment was overall feasible and safe, even though the majority of patients were older than 60 years.
“Based on these results, adjuvant platinum-based chemotherapy should be considered a new standard of care in these patients,” Dr. Birtle maintained. “The next thing is how are we going to move this data forward? We are looking at the successor study at the moment, which is currently in development…[POUT] has been a triumph of UK urologists really getting behind it, but to do a further study, it would be great to look at international collaboration.”
Optimal timing of chemotherapy, before or after surgery, remains an open question, according session co-chair Jeanny B. Aragon-Ching, MD, a medical oncologist with the Inova Medical Group, Fairfax, Virginia.
“This trial offers high-level evidence for consideration of adjuvant chemotherapy in those who are unable to receive neoadjuvant chemotherapy in UTUC, although it is still important to evaluate what the prospective neoadjuvant chemotherapy data in UTUC would show,” she said in an interview. “Ongoing studies include the ECOG 8141 study (clinicaltrials.gov NCT02412670), but certainly, several retrospective trials showed benefit of neoadjuvant chemotherapy in UTUC.”
Other viewpoints
“It’s fantastic that we finally have data in upper-tract cancer. However, I worry about how this data is going to be interpreted, and I argue that it should not be considered the standard of care,” session attendee Matthew Campbell, MD, of the MD Anderson Cancer Center, Houston, commented during a question and answer period.
“I believe that this is showing that chemotherapy is very important in this disease, and if anything, it should be moved into the neoadjuvant setting, where more patients will be cisplatin candidates. Though there is challenge with staging upper-tract disease, at MD Anderson, we consider patients with high-grade disease or hydronephrosis to be at high enough risk to consider for neoadjuvant chemotherapy, and that’s been our approach.”
“When we looked at developing POUT, there was a big debate about whether it should be a neoadjuvant or adjuvant study,” Dr. Birtle replied. UK oncologists expressed concern that not all patients have histologic confirmation of UTUC preoperatively; therefore, chemotherapy could lead to overtreatment for some.
“We plan to go back and look at the CT urograms and the diagnostic imaging done prior to surgery just to see if we can be 100% confident in our diagnostic accuracy preoperatively, to see if we would be more confident with a neoadjuvant study,” she added. The investigators have also reviewed data from the British Association of Urological Surgeons on the postoperative dip in GFR. “It didn’t really seem from that 2015 data that there was a huge unmet need for patients postoperatively, where we would have missed them had we not treated them preoperatively,” she said.
On a related note, session attendee Surena Matin, MD, also of MD Anderson, asked “How many patients were potentially precluded because of their GFR, and do you have any data regarding response rates in the carboplatin arm?”
The main reason for exclusion was ineligible pathology and not GFR, according to Dr. Birtle. “This goes back to the previous question of can you be certain that a patient has locally advanced disease prior to nephro-ureterectomy? About 60% of patients who were thought to have muscle-invasive disease ultimately on nephro-ureterectomy didn’t.”
Confidence intervals for chemotherapy benefit in the subgroup given carboplatin were wide and overlapped unity, but still favoring benefit and falling within the overall treatment effect, she said.
Session attendee Joaquim Bellmunt, MD, Dana-Farber Cancer Institute, Boston, noted that the chemotherapy benefit was not significant in that subgroup and also in the subgroups with lymph node–positive disease and with positive margins. “I think that saying … chemotherapy is for everybody based on this trial” is incorrect, he asserted. “It may be good just to tone down the message that this is the new standard of care.” He further questioned the trial’s early stopping, noting that continuing would have provided more information in these patients.
Those subgroups were small, so analyses were underpowered to definitively rule out chemotherapy benefit, according to Dr. Birtle. The investigators had intensive discussion about the recommendation to stop early, because of a goal to determine overall survival impact. Ultimately, “when we saw the data in terms of disease-free survival and metastasis-free survival, the magnitude of the effect was so big that we felt it was uncomfortable and unethical not to offer patients treatment,” she said.
Study details
Patients in POUT’s chemotherapy arm received four cycles of chemotherapy—gemcitabine-cisplatin if their GFR was 50 mL/min or higher, or gemcitabine-carboplatin if their GFR was 30-49 mL/min—starting within 90 days of nephro-ureterectomy.
Of note, approximately 40% of all patients in the trial were aged 70 years or older, including the 5% who were aged 80 years or older. “This is very reassuring for a study of adjuvant platinum-based chemotherapy,” Dr. Birtle commented. Fully 71.2% of the chemotherapy patients received all four planned cycles.
In an intention-to-treat analysis, risk of disease-free survival events (death from any cause, metastasis, or any ureteric or renal bed recurrence) was sharply reduced with chemotherapy versus surveillance (hazard ratio, 0.49; P=.001). The proportion of patients event free at 2 years was 71% in the chemotherapy group and 54% in the surveillance group. Benefit was generally similar across subgroups, and findings were much the same after adjustment for nodal involvement, microscopic margin status, and planned chemotherapy regimen (hazard ratio, 0.47; P=.001).
Risk of metastasis-free survival events was also sharply lower with chemotherapy (hazard ratio, 0.49; P=.002), with a proportion event free at 2 years of 74% in the chemotherapy group and 60% in the surveillance group. These findings were also much the same after adjustment for the above factors (hazard ratio, 0.47; P=.002).
Overall survival tended to be better with chemotherapy than with surveillance (hazard ratio, 0.55), but data are still immature for this endpoint.
The rate of grade 3 or worse adverse events during the treatment period was 53.2% with chemotherapy and 13.5% with surveillance; events with chemotherapy were as expected, with neutropenia, thrombocytopenia, and gastrointestinal events predominating. The rate of febrile neutropenia was 5.7% with gemcitabine-cisplatin and 7.8% with gemcitabine-carboplatin, but there were no neutropenic deaths.
The rate of grade 3 or worse adverse events for the entire trial period was 62.1% with chemotherapy and 24.8% with surveillance.
Data on nephrotoxicity are still being evaluated, according to Dr. Birtle. Only seven patients who started on gemcitabine-cisplatin had to switch to gemcitabine-carboplatin because their GFR fell.
In a related translational study, the investigators are evaluating both the baseline CT urograms and the resected tumors to identify prognostic and predictive markers, she said.
Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
SOURCE: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407
AT THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point:
Major finding: Compared with surveillance, adjuvant chemotherapy halved risk of disease-free survival events (hazard ratio, 0.49; P=.001).
Data source: A phase 3 randomized trial of adjuvant platinum-based chemotherapy versus surveillance in 261 patients with upper-tract urothelial cancer (POUT trial).
Disclosures: Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
Source: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407.
For interstitial cystitis, restrictive diet pays off
ESTES PARK, CO. – When patients with interstitial cystitis (IC) learn that first-line therapy is a rigorous diet designed to eliminate common bladder irritants, they tend to react in one of two ways, according to Julie A. Chacko, MD, a urologist in private practice in Santa Barbara, Calif.
Some “are just so grateful that they’re not crazy, which is what they’ve been told after 15 negative urine cultures. (Others) “look at the diet and think I’m sentencing them to death,” she said.
The sole medication approved by the Food and Drug Administration for IC is pentosan polysulfate sodium (Elmiron), and it should be reserved for the minority of patients who don’t experience significant improvement after giving the diet a reasonable shot, Dr. Chako advised. “When Elmiron works it’s great, but it’s not usually my go-to agent because it’s very expensive, you have to take it for 3-6 months to know for sure if it’s efficacious, and it has to be taken on an empty stomach. It’s a difficult medication.”
She advises patients to work with the diet. “Over time, they’re going to be able to find what I call their island – a point where they know very well their limitations and become quite comfortable with them,” she said at a conference on internal medicine sponsored by the University of Colorado.
A poorly understood yet common disorder, IC has a prevalence estimated at 0.5%-4% in women, less in men. Although typically diagnosed in the fourth decade or later, IC occurs at all ages. In some studies, the delay from first appearance of symptoms to arrival at a diagnosis is up to 8 years.
Interstitial cystitis is increasingly being called bladder pain syndrome in the literature, said Dr. Chako, who added, “I personally don’t love bladder pain syndrome as a description for this process. This syndrome has variable symptoms, and patients can have no pain at all.”
The mechanisms that result in IC are a mystery. The leading theory is that a bladder permeability problem allows urinary irritants to reach the interstitium. Nearly 80% of patients with IC can, with coaxing, identify dietary triggers for their symptoms, thereby basically establishing the diagnosis.
Other proposed mechanisms include an infectious agent that’s yet to be identified, allergic reaction, and neuromodulatory dysfunction. Common triggers other than foods include menses, copulation, emotional distress, and bladder trauma, including transvaginal ultrasound.
Conditions commonly associated with IC include fibromyalgia, irritable bowel syndrome, chronic fatigue, vulvodynia, migraines, depression, and anxiety.
The most common symptoms of IC are urinary urgency and frequency. Many affected patients have dysuria. Some have pain, which is typically suprapubic. However, pain can be present anywhere in a band circumscribing the whole central section of the torso, including the lower back, lower abdomen, urethra, vagina, and vulva. Patients describe a range of pain – burning, aching, stabbing, itching, buzzing, or a feeling of pressure.
“Most women who come in with IC are married to the idea that they’re having recurrent UTIs. They’re going to get antibiotics any way they can for their UTIs: over the phone, at urgent care. You need to get them to buy into the idea that even though UTIs are common, maybe not all of their flares are infections. They ask, ‘Then why do I feel better when I’m on antibiotics for recurrent UTI even though the cultures are negative?’ I say, ‘You feel less stress and anxiety because you think you’re on effective treatment,” Dr. Chacko said.
The diagnosis of IC is one of exclusions. Diagnoses to rule out before arriving at IC include recurrent UTI; overactive bladder, which should present with pure urge frequency and respond to medications for that condition; kidney stone disease present at the end of the ureter where it enters the bladder; gastrointestinal pathology; bladder cancer; and ovarian or uterine pathology.
Referral to a urologist for cystoscopy and cytology is appropriate in patients with microscopic hematuria, a significant smoking history predisposing to bladder cancer, or severe pain with severe frequency, which raises the possibility of Hunner’s ulcers, considered pathognomic for IC, respond “beautifully” to fulguration, she said.
Otherwise, IC can readily be managed by interested primary care physicians. The IC diet initially calls for 2 weeks of strict avoidance of all high-risk foods, most of which are acidic foods. These include fruits and fruit juices, especially citrus and cranberry juices; tomatoes and tomato products, including ketchup; yogurt; chocolate; coffee and tea, including decaf; vinegar; spicy foods; and carbonated beverages, water included.
These foods can later be added back one at a time to the diet while watching for IC flares, which typically occur within hours to several days of re-introducing the food. The return to coffee consumption, if that’s something important to the patient, should be with low-acid coffee. If that triggers an IC flare, try decaf. In time, many patients find they can consume some trigger foods in modest amounts.
“I tell patients it will take 12-18 months to get a good handle on their IC,” Dr. Chacko noted.
The use of OTC alkalizing agents such as Prelief may diffuse dietary triggers. A teaspoon of baking soda in water is also effective.
Second-line treatments include oral hydroxyzine 10-20 mg at bedtime; amitriptyline 10-20 mg at bedtime, mainly for patients with predominant pain symptoms; cimeditine; and pentosan polysulfate at 100 mg TID.
For IC patients with pelvic muscle tightness on pelvic examination, referral to a physical therapist adept at pelvic floor trigger point release can work wonders, she added.
One second-line option is bladder instillations of dimethyl sulfoxide weekly for 6 weeks, cutting back to once monthly maintenance therapy if the more intensive regimen is effective. Instillation of “heparin with lidocaine is a rescue solution. If it’s going to work, it kicks in within a few hours and usually lasts for 24-72 hours. It gets patients through a weekend, a wedding, or a funeral. A response can help make the IC diagnosis, too,” Dr. Chacko said.
She reported having no financial conflicts of interest regarding her presentation.
ESTES PARK, CO. – When patients with interstitial cystitis (IC) learn that first-line therapy is a rigorous diet designed to eliminate common bladder irritants, they tend to react in one of two ways, according to Julie A. Chacko, MD, a urologist in private practice in Santa Barbara, Calif.
Some “are just so grateful that they’re not crazy, which is what they’ve been told after 15 negative urine cultures. (Others) “look at the diet and think I’m sentencing them to death,” she said.
The sole medication approved by the Food and Drug Administration for IC is pentosan polysulfate sodium (Elmiron), and it should be reserved for the minority of patients who don’t experience significant improvement after giving the diet a reasonable shot, Dr. Chako advised. “When Elmiron works it’s great, but it’s not usually my go-to agent because it’s very expensive, you have to take it for 3-6 months to know for sure if it’s efficacious, and it has to be taken on an empty stomach. It’s a difficult medication.”
She advises patients to work with the diet. “Over time, they’re going to be able to find what I call their island – a point where they know very well their limitations and become quite comfortable with them,” she said at a conference on internal medicine sponsored by the University of Colorado.
A poorly understood yet common disorder, IC has a prevalence estimated at 0.5%-4% in women, less in men. Although typically diagnosed in the fourth decade or later, IC occurs at all ages. In some studies, the delay from first appearance of symptoms to arrival at a diagnosis is up to 8 years.
Interstitial cystitis is increasingly being called bladder pain syndrome in the literature, said Dr. Chako, who added, “I personally don’t love bladder pain syndrome as a description for this process. This syndrome has variable symptoms, and patients can have no pain at all.”
The mechanisms that result in IC are a mystery. The leading theory is that a bladder permeability problem allows urinary irritants to reach the interstitium. Nearly 80% of patients with IC can, with coaxing, identify dietary triggers for their symptoms, thereby basically establishing the diagnosis.
Other proposed mechanisms include an infectious agent that’s yet to be identified, allergic reaction, and neuromodulatory dysfunction. Common triggers other than foods include menses, copulation, emotional distress, and bladder trauma, including transvaginal ultrasound.
Conditions commonly associated with IC include fibromyalgia, irritable bowel syndrome, chronic fatigue, vulvodynia, migraines, depression, and anxiety.
The most common symptoms of IC are urinary urgency and frequency. Many affected patients have dysuria. Some have pain, which is typically suprapubic. However, pain can be present anywhere in a band circumscribing the whole central section of the torso, including the lower back, lower abdomen, urethra, vagina, and vulva. Patients describe a range of pain – burning, aching, stabbing, itching, buzzing, or a feeling of pressure.
“Most women who come in with IC are married to the idea that they’re having recurrent UTIs. They’re going to get antibiotics any way they can for their UTIs: over the phone, at urgent care. You need to get them to buy into the idea that even though UTIs are common, maybe not all of their flares are infections. They ask, ‘Then why do I feel better when I’m on antibiotics for recurrent UTI even though the cultures are negative?’ I say, ‘You feel less stress and anxiety because you think you’re on effective treatment,” Dr. Chacko said.
The diagnosis of IC is one of exclusions. Diagnoses to rule out before arriving at IC include recurrent UTI; overactive bladder, which should present with pure urge frequency and respond to medications for that condition; kidney stone disease present at the end of the ureter where it enters the bladder; gastrointestinal pathology; bladder cancer; and ovarian or uterine pathology.
Referral to a urologist for cystoscopy and cytology is appropriate in patients with microscopic hematuria, a significant smoking history predisposing to bladder cancer, or severe pain with severe frequency, which raises the possibility of Hunner’s ulcers, considered pathognomic for IC, respond “beautifully” to fulguration, she said.
Otherwise, IC can readily be managed by interested primary care physicians. The IC diet initially calls for 2 weeks of strict avoidance of all high-risk foods, most of which are acidic foods. These include fruits and fruit juices, especially citrus and cranberry juices; tomatoes and tomato products, including ketchup; yogurt; chocolate; coffee and tea, including decaf; vinegar; spicy foods; and carbonated beverages, water included.
These foods can later be added back one at a time to the diet while watching for IC flares, which typically occur within hours to several days of re-introducing the food. The return to coffee consumption, if that’s something important to the patient, should be with low-acid coffee. If that triggers an IC flare, try decaf. In time, many patients find they can consume some trigger foods in modest amounts.
“I tell patients it will take 12-18 months to get a good handle on their IC,” Dr. Chacko noted.
The use of OTC alkalizing agents such as Prelief may diffuse dietary triggers. A teaspoon of baking soda in water is also effective.
Second-line treatments include oral hydroxyzine 10-20 mg at bedtime; amitriptyline 10-20 mg at bedtime, mainly for patients with predominant pain symptoms; cimeditine; and pentosan polysulfate at 100 mg TID.
For IC patients with pelvic muscle tightness on pelvic examination, referral to a physical therapist adept at pelvic floor trigger point release can work wonders, she added.
One second-line option is bladder instillations of dimethyl sulfoxide weekly for 6 weeks, cutting back to once monthly maintenance therapy if the more intensive regimen is effective. Instillation of “heparin with lidocaine is a rescue solution. If it’s going to work, it kicks in within a few hours and usually lasts for 24-72 hours. It gets patients through a weekend, a wedding, or a funeral. A response can help make the IC diagnosis, too,” Dr. Chacko said.
She reported having no financial conflicts of interest regarding her presentation.
ESTES PARK, CO. – When patients with interstitial cystitis (IC) learn that first-line therapy is a rigorous diet designed to eliminate common bladder irritants, they tend to react in one of two ways, according to Julie A. Chacko, MD, a urologist in private practice in Santa Barbara, Calif.
Some “are just so grateful that they’re not crazy, which is what they’ve been told after 15 negative urine cultures. (Others) “look at the diet and think I’m sentencing them to death,” she said.
The sole medication approved by the Food and Drug Administration for IC is pentosan polysulfate sodium (Elmiron), and it should be reserved for the minority of patients who don’t experience significant improvement after giving the diet a reasonable shot, Dr. Chako advised. “When Elmiron works it’s great, but it’s not usually my go-to agent because it’s very expensive, you have to take it for 3-6 months to know for sure if it’s efficacious, and it has to be taken on an empty stomach. It’s a difficult medication.”
She advises patients to work with the diet. “Over time, they’re going to be able to find what I call their island – a point where they know very well their limitations and become quite comfortable with them,” she said at a conference on internal medicine sponsored by the University of Colorado.
A poorly understood yet common disorder, IC has a prevalence estimated at 0.5%-4% in women, less in men. Although typically diagnosed in the fourth decade or later, IC occurs at all ages. In some studies, the delay from first appearance of symptoms to arrival at a diagnosis is up to 8 years.
Interstitial cystitis is increasingly being called bladder pain syndrome in the literature, said Dr. Chako, who added, “I personally don’t love bladder pain syndrome as a description for this process. This syndrome has variable symptoms, and patients can have no pain at all.”
The mechanisms that result in IC are a mystery. The leading theory is that a bladder permeability problem allows urinary irritants to reach the interstitium. Nearly 80% of patients with IC can, with coaxing, identify dietary triggers for their symptoms, thereby basically establishing the diagnosis.
Other proposed mechanisms include an infectious agent that’s yet to be identified, allergic reaction, and neuromodulatory dysfunction. Common triggers other than foods include menses, copulation, emotional distress, and bladder trauma, including transvaginal ultrasound.
Conditions commonly associated with IC include fibromyalgia, irritable bowel syndrome, chronic fatigue, vulvodynia, migraines, depression, and anxiety.
The most common symptoms of IC are urinary urgency and frequency. Many affected patients have dysuria. Some have pain, which is typically suprapubic. However, pain can be present anywhere in a band circumscribing the whole central section of the torso, including the lower back, lower abdomen, urethra, vagina, and vulva. Patients describe a range of pain – burning, aching, stabbing, itching, buzzing, or a feeling of pressure.
“Most women who come in with IC are married to the idea that they’re having recurrent UTIs. They’re going to get antibiotics any way they can for their UTIs: over the phone, at urgent care. You need to get them to buy into the idea that even though UTIs are common, maybe not all of their flares are infections. They ask, ‘Then why do I feel better when I’m on antibiotics for recurrent UTI even though the cultures are negative?’ I say, ‘You feel less stress and anxiety because you think you’re on effective treatment,” Dr. Chacko said.
The diagnosis of IC is one of exclusions. Diagnoses to rule out before arriving at IC include recurrent UTI; overactive bladder, which should present with pure urge frequency and respond to medications for that condition; kidney stone disease present at the end of the ureter where it enters the bladder; gastrointestinal pathology; bladder cancer; and ovarian or uterine pathology.
Referral to a urologist for cystoscopy and cytology is appropriate in patients with microscopic hematuria, a significant smoking history predisposing to bladder cancer, or severe pain with severe frequency, which raises the possibility of Hunner’s ulcers, considered pathognomic for IC, respond “beautifully” to fulguration, she said.
Otherwise, IC can readily be managed by interested primary care physicians. The IC diet initially calls for 2 weeks of strict avoidance of all high-risk foods, most of which are acidic foods. These include fruits and fruit juices, especially citrus and cranberry juices; tomatoes and tomato products, including ketchup; yogurt; chocolate; coffee and tea, including decaf; vinegar; spicy foods; and carbonated beverages, water included.
These foods can later be added back one at a time to the diet while watching for IC flares, which typically occur within hours to several days of re-introducing the food. The return to coffee consumption, if that’s something important to the patient, should be with low-acid coffee. If that triggers an IC flare, try decaf. In time, many patients find they can consume some trigger foods in modest amounts.
“I tell patients it will take 12-18 months to get a good handle on their IC,” Dr. Chacko noted.
The use of OTC alkalizing agents such as Prelief may diffuse dietary triggers. A teaspoon of baking soda in water is also effective.
Second-line treatments include oral hydroxyzine 10-20 mg at bedtime; amitriptyline 10-20 mg at bedtime, mainly for patients with predominant pain symptoms; cimeditine; and pentosan polysulfate at 100 mg TID.
For IC patients with pelvic muscle tightness on pelvic examination, referral to a physical therapist adept at pelvic floor trigger point release can work wonders, she added.
One second-line option is bladder instillations of dimethyl sulfoxide weekly for 6 weeks, cutting back to once monthly maintenance therapy if the more intensive regimen is effective. Instillation of “heparin with lidocaine is a rescue solution. If it’s going to work, it kicks in within a few hours and usually lasts for 24-72 hours. It gets patients through a weekend, a wedding, or a funeral. A response can help make the IC diagnosis, too,” Dr. Chacko said.
She reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM THE ANNUAL INTERNAL MEDICINE PROGRAM