User login
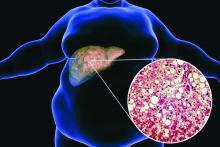
Balanced fat intake links with less type 2 diabetes
Researchers published the study covered in this summary on Preprints with The Lancet as a preprint that has not yet been peer reviewed.
Key takeaways
- Adults in China who consumed a “balanced,” moderate ratio (middle three quintiles) of animal-to-vegetable cooking oil had a lower rate of developing type 2 diabetes during a median follow-up of 8.6 years, compared with those who consumed the lowest ratio (first quintile), after multivariable adjustment using prospectively collected data.
- The results also indicate that increasing animal cooking oil (such as lard, tallow, or butter) and vegetable cooking oil (such as peanut or soybean oil) consumption were each positively associated with a higher rate of developing type 2 diabetes.
- Those who consumed the highest ratio (fifth quintile) of animal-to-vegetable cooking oil had a nonsignificant difference in their rate of developing type 2 diabetes, compared with those in the first quintile.
Why this matters
- The findings suggest that consuming a diet with a “balanced” moderate intake of animal and vegetable oil might lower the risk of type 2 diabetes, which would reduce disease burden and health care expenditures.
- The results imply that using a single source of cooking oil, either animal or vegetable, contributes to the incidence of type 2 diabetes.
- This is the first large epidemiological study showing a relationship between the ratio of animal- and vegetable-derived fats in people’s diets and their risk for incident type 2 diabetes.
Study design
- The researchers used data collected prospectively starting in 2010-2012 from 7,274 adult residents of Guizhou province, China, with follow-up assessment in 2020 after a median of 8.6 years.
- At baseline, participants underwent an oral glucose tolerance test and provided information on demographics, family medical history, and personal medical history, including whether they had been diagnosed with type 2 diabetes or were taking antihyperglycemic medications. The study did not include anyone with a history of diabetes.
- Data on intake of animal and vegetable cooking oil came from a dietary questionnaire.
- The authors calculated hazard ratios for development of type 2 diabetes after adjusting for multiple potential confounders.
Key results
- The study cohort averaged 44 years old, and 53% were women.
- During a median follow-up of 8.6 years, 747 people developed type 2 diabetes.
- Compared with those who had the lowest intake of animal cooking oil (first quintile), those with the highest intake (fifth quintile) had a significant 28% increased relative rate for developing type 2 diabetes after adjustment for several potential confounders.
- Compared with those with the lowest intake of vegetable cooking oil, those with the highest intake had a significant 56% increased rate of developing type 2 diabetes after adjustment.
- Compared with adults with the lowest animal-to-vegetable cooking oil ratio (first quintile), those in the second, third, and fourth quintiles for this ratio had significantly lower adjusted relative rates of developing type 2 diabetes, with adjusted hazard ratios of 0.79, 0.65, and 0.68, respectively. Those in the highest quintile (fifth quintile) did not have a significantly different risk, compared with the first quintile.
- The protective effect of a balanced ratio of animal-to-vegetable cooking oils was stronger in people who lived in rural districts and in those who had obesity.
Limitations
- The dietary information came from participants’ self-reports, which may have produced biased data.
- The study only included information about animal and vegetable cooking oil consumed at home.
- There may have been residual confounding from variables not included in the study.
- The time of diagnosis of type 2 diabetes may have been inaccurate because follow-up occurred only once.
- The study may have underestimated the incidence of type 2 diabetes because of a lack of information about hemoglobin A1c levels at follow-up.
Disclosures
- The study did not receive commercial funding.
- The authors reported no financial disclosures.
This is a summary of a preprint article “The consumption ratio of animal cooking oil to vegetable cooking oil and reduced risk of type 2 diabetes mellitus: A prospective cohort study in Southwest China” written by researchers primarily from Zunyi Medical University, China, on Preprints with The Lancet. This study has not yet been peer reviewed. The full text of the study can be found on papers.ssrn.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on Preprints with The Lancet as a preprint that has not yet been peer reviewed.
Key takeaways
- Adults in China who consumed a “balanced,” moderate ratio (middle three quintiles) of animal-to-vegetable cooking oil had a lower rate of developing type 2 diabetes during a median follow-up of 8.6 years, compared with those who consumed the lowest ratio (first quintile), after multivariable adjustment using prospectively collected data.
- The results also indicate that increasing animal cooking oil (such as lard, tallow, or butter) and vegetable cooking oil (such as peanut or soybean oil) consumption were each positively associated with a higher rate of developing type 2 diabetes.
- Those who consumed the highest ratio (fifth quintile) of animal-to-vegetable cooking oil had a nonsignificant difference in their rate of developing type 2 diabetes, compared with those in the first quintile.
Why this matters
- The findings suggest that consuming a diet with a “balanced” moderate intake of animal and vegetable oil might lower the risk of type 2 diabetes, which would reduce disease burden and health care expenditures.
- The results imply that using a single source of cooking oil, either animal or vegetable, contributes to the incidence of type 2 diabetes.
- This is the first large epidemiological study showing a relationship between the ratio of animal- and vegetable-derived fats in people’s diets and their risk for incident type 2 diabetes.
Study design
- The researchers used data collected prospectively starting in 2010-2012 from 7,274 adult residents of Guizhou province, China, with follow-up assessment in 2020 after a median of 8.6 years.
- At baseline, participants underwent an oral glucose tolerance test and provided information on demographics, family medical history, and personal medical history, including whether they had been diagnosed with type 2 diabetes or were taking antihyperglycemic medications. The study did not include anyone with a history of diabetes.
- Data on intake of animal and vegetable cooking oil came from a dietary questionnaire.
- The authors calculated hazard ratios for development of type 2 diabetes after adjusting for multiple potential confounders.
Key results
- The study cohort averaged 44 years old, and 53% were women.
- During a median follow-up of 8.6 years, 747 people developed type 2 diabetes.
- Compared with those who had the lowest intake of animal cooking oil (first quintile), those with the highest intake (fifth quintile) had a significant 28% increased relative rate for developing type 2 diabetes after adjustment for several potential confounders.
- Compared with those with the lowest intake of vegetable cooking oil, those with the highest intake had a significant 56% increased rate of developing type 2 diabetes after adjustment.
- Compared with adults with the lowest animal-to-vegetable cooking oil ratio (first quintile), those in the second, third, and fourth quintiles for this ratio had significantly lower adjusted relative rates of developing type 2 diabetes, with adjusted hazard ratios of 0.79, 0.65, and 0.68, respectively. Those in the highest quintile (fifth quintile) did not have a significantly different risk, compared with the first quintile.
- The protective effect of a balanced ratio of animal-to-vegetable cooking oils was stronger in people who lived in rural districts and in those who had obesity.
Limitations
- The dietary information came from participants’ self-reports, which may have produced biased data.
- The study only included information about animal and vegetable cooking oil consumed at home.
- There may have been residual confounding from variables not included in the study.
- The time of diagnosis of type 2 diabetes may have been inaccurate because follow-up occurred only once.
- The study may have underestimated the incidence of type 2 diabetes because of a lack of information about hemoglobin A1c levels at follow-up.
Disclosures
- The study did not receive commercial funding.
- The authors reported no financial disclosures.
This is a summary of a preprint article “The consumption ratio of animal cooking oil to vegetable cooking oil and reduced risk of type 2 diabetes mellitus: A prospective cohort study in Southwest China” written by researchers primarily from Zunyi Medical University, China, on Preprints with The Lancet. This study has not yet been peer reviewed. The full text of the study can be found on papers.ssrn.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on Preprints with The Lancet as a preprint that has not yet been peer reviewed.
Key takeaways
- Adults in China who consumed a “balanced,” moderate ratio (middle three quintiles) of animal-to-vegetable cooking oil had a lower rate of developing type 2 diabetes during a median follow-up of 8.6 years, compared with those who consumed the lowest ratio (first quintile), after multivariable adjustment using prospectively collected data.
- The results also indicate that increasing animal cooking oil (such as lard, tallow, or butter) and vegetable cooking oil (such as peanut or soybean oil) consumption were each positively associated with a higher rate of developing type 2 diabetes.
- Those who consumed the highest ratio (fifth quintile) of animal-to-vegetable cooking oil had a nonsignificant difference in their rate of developing type 2 diabetes, compared with those in the first quintile.
Why this matters
- The findings suggest that consuming a diet with a “balanced” moderate intake of animal and vegetable oil might lower the risk of type 2 diabetes, which would reduce disease burden and health care expenditures.
- The results imply that using a single source of cooking oil, either animal or vegetable, contributes to the incidence of type 2 diabetes.
- This is the first large epidemiological study showing a relationship between the ratio of animal- and vegetable-derived fats in people’s diets and their risk for incident type 2 diabetes.
Study design
- The researchers used data collected prospectively starting in 2010-2012 from 7,274 adult residents of Guizhou province, China, with follow-up assessment in 2020 after a median of 8.6 years.
- At baseline, participants underwent an oral glucose tolerance test and provided information on demographics, family medical history, and personal medical history, including whether they had been diagnosed with type 2 diabetes or were taking antihyperglycemic medications. The study did not include anyone with a history of diabetes.
- Data on intake of animal and vegetable cooking oil came from a dietary questionnaire.
- The authors calculated hazard ratios for development of type 2 diabetes after adjusting for multiple potential confounders.
Key results
- The study cohort averaged 44 years old, and 53% were women.
- During a median follow-up of 8.6 years, 747 people developed type 2 diabetes.
- Compared with those who had the lowest intake of animal cooking oil (first quintile), those with the highest intake (fifth quintile) had a significant 28% increased relative rate for developing type 2 diabetes after adjustment for several potential confounders.
- Compared with those with the lowest intake of vegetable cooking oil, those with the highest intake had a significant 56% increased rate of developing type 2 diabetes after adjustment.
- Compared with adults with the lowest animal-to-vegetable cooking oil ratio (first quintile), those in the second, third, and fourth quintiles for this ratio had significantly lower adjusted relative rates of developing type 2 diabetes, with adjusted hazard ratios of 0.79, 0.65, and 0.68, respectively. Those in the highest quintile (fifth quintile) did not have a significantly different risk, compared with the first quintile.
- The protective effect of a balanced ratio of animal-to-vegetable cooking oils was stronger in people who lived in rural districts and in those who had obesity.
Limitations
- The dietary information came from participants’ self-reports, which may have produced biased data.
- The study only included information about animal and vegetable cooking oil consumed at home.
- There may have been residual confounding from variables not included in the study.
- The time of diagnosis of type 2 diabetes may have been inaccurate because follow-up occurred only once.
- The study may have underestimated the incidence of type 2 diabetes because of a lack of information about hemoglobin A1c levels at follow-up.
Disclosures
- The study did not receive commercial funding.
- The authors reported no financial disclosures.
This is a summary of a preprint article “The consumption ratio of animal cooking oil to vegetable cooking oil and reduced risk of type 2 diabetes mellitus: A prospective cohort study in Southwest China” written by researchers primarily from Zunyi Medical University, China, on Preprints with The Lancet. This study has not yet been peer reviewed. The full text of the study can be found on papers.ssrn.com.
A version of this article first appeared on Medscape.com.
How to improve diagnosis of HFpEF, common in diabetes
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Once-weekly insulin promising in phase 3 trial in type 2 diabetes
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
AT EASD 2022
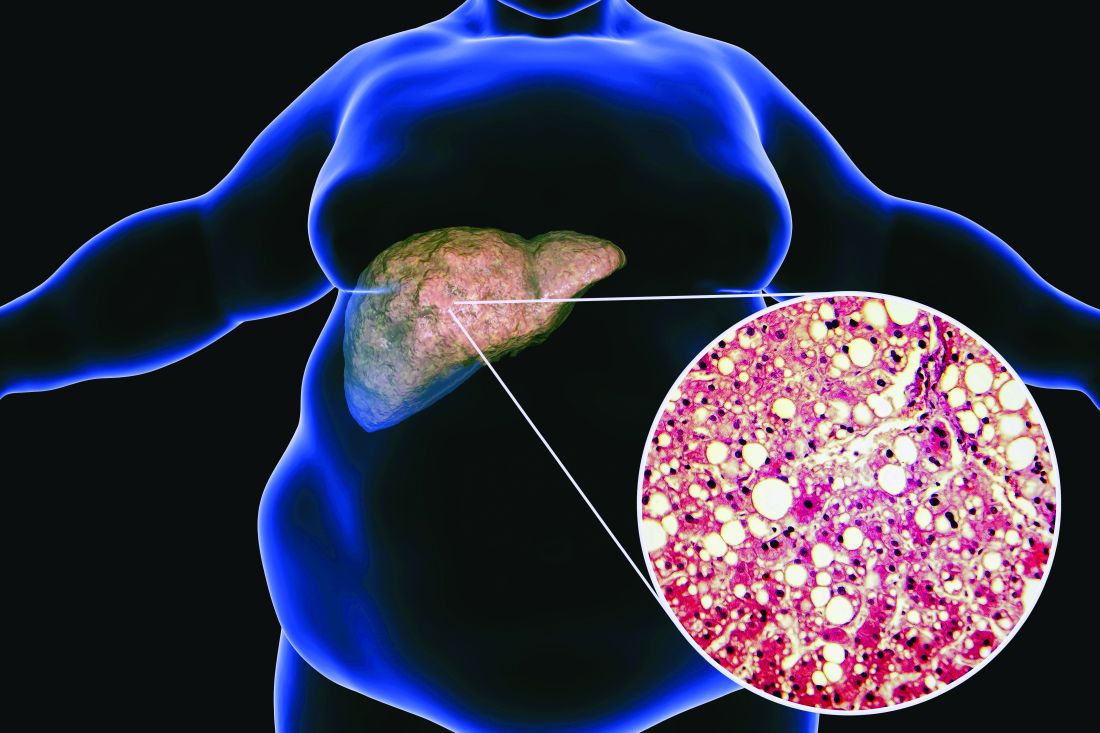
Ezetimibe-statin combo lowers liver fat in open-label trial
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
FROM EASD 2022
Cre8 EVO stent loses sweet spot in diabetes at 2 years: SUGAR
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
AT TCT 2022
Emphasis on weight loss in new type 2 diabetes guidance
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Commentary: Preventing and Predicting T2D Complications, October 2022
Diabetes guidelines recommend sodium-glucose transport protein 2 (SGLT2) inhibitors to reduce kidney disease progression in patients with type 2 diabetes (T2D) and moderate-to-severe albuminuric kidney disease on the basis of renal outcomes trials, such as CREDENCE and DAPA-CKD. However, these trials did not include patients who are at low risk for kidney disease progression.
Mozenson and colleagues published a post hoc analysis of the DECLARE-TIMI 58 trial and focused on patients with low kidney risk. They demonstated that dapagliflozin slowed the progression of kidney disease in patients with T2D at high cardiovascular risk, including those who are at low risk for kidney progression. The absolute benefit for slowing kidney progression was much lower in patients with low kidney risk compared with those who are at high or very high risk (number needed to treat 177 vs 13-23). Though dapagliflozin does provide kidney protection across a spectrum of patients with kidney risk, clinicians should consider the level of risk when starting an SGLT2 inhibitor for slowing kidney disease.
SGLT2 inhibitor outcome trials and meta-analyses have mainly shown neutral results for ischemic stroke, except for sotagliflozin vs placebo in the SCORED trial. In this trial, sotagliflozin was shown to reduce total stroke. Recently, in a retrospective longitudinal cohort study of patients with T2D in Taiwan, Lin and colleagues have shown a significant reduction in new onset stroke among those who use SGLT2 inhibitor compared with those who don't. A 15% relative risk reduction in stroke was shown in an analysis that adjusted for age, sex, and duration of T2D, with a similar reduction in a propensity score-matched analysis. Although limited by its observational design, this study suggests that further research should be continued regarding the impact of SGLT2 inhibitors on stroke outcomes.
Severe hypoglycemia is a serious complication of insulin and insulin secretagogue therapy. There have been few studies regarding the association between long-term glycemic variability of A1c and fasting plasma glucose (FPG) and the risk for severe hypoglycemia. Long and colleagues performed a post hoc analysis of the ACCORD study and found that both A1c and FPG variability were associated with a greater risk for severe hypoglycemia in T2D, with FPG being a more sensitive indicator than is A1c variability. Clinicians need to be aware that A1c and FPG variability in insulin- or insulin secretagogue–treated patients with T2D places them at greater risk for severe hypoglycemia and such variability should be considered a potential target of treatment.
Although a higher mean A1c has been linked to diabetes microvascular and macrovascular complications, there is a paucity of data comparing mean A1c and A1c variability and diabetes complications. In a prospective study from Taiwan, Wu and colleagues demonstrated that both mean A1c and A1c variability predicted most diabetes-related complications, with mean A1c being more effective at predicting retinopathy and A1c variability being more effective at predicting a decline in kidney function and cardiovascular and total mortality. Perhaps physicians need to pay more attention to A1c variability and not just the mean A1c over time when assessing an individual and their overall risk for diabetes complications.
Diabetes guidelines recommend sodium-glucose transport protein 2 (SGLT2) inhibitors to reduce kidney disease progression in patients with type 2 diabetes (T2D) and moderate-to-severe albuminuric kidney disease on the basis of renal outcomes trials, such as CREDENCE and DAPA-CKD. However, these trials did not include patients who are at low risk for kidney disease progression.
Mozenson and colleagues published a post hoc analysis of the DECLARE-TIMI 58 trial and focused on patients with low kidney risk. They demonstated that dapagliflozin slowed the progression of kidney disease in patients with T2D at high cardiovascular risk, including those who are at low risk for kidney progression. The absolute benefit for slowing kidney progression was much lower in patients with low kidney risk compared with those who are at high or very high risk (number needed to treat 177 vs 13-23). Though dapagliflozin does provide kidney protection across a spectrum of patients with kidney risk, clinicians should consider the level of risk when starting an SGLT2 inhibitor for slowing kidney disease.
SGLT2 inhibitor outcome trials and meta-analyses have mainly shown neutral results for ischemic stroke, except for sotagliflozin vs placebo in the SCORED trial. In this trial, sotagliflozin was shown to reduce total stroke. Recently, in a retrospective longitudinal cohort study of patients with T2D in Taiwan, Lin and colleagues have shown a significant reduction in new onset stroke among those who use SGLT2 inhibitor compared with those who don't. A 15% relative risk reduction in stroke was shown in an analysis that adjusted for age, sex, and duration of T2D, with a similar reduction in a propensity score-matched analysis. Although limited by its observational design, this study suggests that further research should be continued regarding the impact of SGLT2 inhibitors on stroke outcomes.
Severe hypoglycemia is a serious complication of insulin and insulin secretagogue therapy. There have been few studies regarding the association between long-term glycemic variability of A1c and fasting plasma glucose (FPG) and the risk for severe hypoglycemia. Long and colleagues performed a post hoc analysis of the ACCORD study and found that both A1c and FPG variability were associated with a greater risk for severe hypoglycemia in T2D, with FPG being a more sensitive indicator than is A1c variability. Clinicians need to be aware that A1c and FPG variability in insulin- or insulin secretagogue–treated patients with T2D places them at greater risk for severe hypoglycemia and such variability should be considered a potential target of treatment.
Although a higher mean A1c has been linked to diabetes microvascular and macrovascular complications, there is a paucity of data comparing mean A1c and A1c variability and diabetes complications. In a prospective study from Taiwan, Wu and colleagues demonstrated that both mean A1c and A1c variability predicted most diabetes-related complications, with mean A1c being more effective at predicting retinopathy and A1c variability being more effective at predicting a decline in kidney function and cardiovascular and total mortality. Perhaps physicians need to pay more attention to A1c variability and not just the mean A1c over time when assessing an individual and their overall risk for diabetes complications.
Diabetes guidelines recommend sodium-glucose transport protein 2 (SGLT2) inhibitors to reduce kidney disease progression in patients with type 2 diabetes (T2D) and moderate-to-severe albuminuric kidney disease on the basis of renal outcomes trials, such as CREDENCE and DAPA-CKD. However, these trials did not include patients who are at low risk for kidney disease progression.
Mozenson and colleagues published a post hoc analysis of the DECLARE-TIMI 58 trial and focused on patients with low kidney risk. They demonstated that dapagliflozin slowed the progression of kidney disease in patients with T2D at high cardiovascular risk, including those who are at low risk for kidney progression. The absolute benefit for slowing kidney progression was much lower in patients with low kidney risk compared with those who are at high or very high risk (number needed to treat 177 vs 13-23). Though dapagliflozin does provide kidney protection across a spectrum of patients with kidney risk, clinicians should consider the level of risk when starting an SGLT2 inhibitor for slowing kidney disease.
SGLT2 inhibitor outcome trials and meta-analyses have mainly shown neutral results for ischemic stroke, except for sotagliflozin vs placebo in the SCORED trial. In this trial, sotagliflozin was shown to reduce total stroke. Recently, in a retrospective longitudinal cohort study of patients with T2D in Taiwan, Lin and colleagues have shown a significant reduction in new onset stroke among those who use SGLT2 inhibitor compared with those who don't. A 15% relative risk reduction in stroke was shown in an analysis that adjusted for age, sex, and duration of T2D, with a similar reduction in a propensity score-matched analysis. Although limited by its observational design, this study suggests that further research should be continued regarding the impact of SGLT2 inhibitors on stroke outcomes.
Severe hypoglycemia is a serious complication of insulin and insulin secretagogue therapy. There have been few studies regarding the association between long-term glycemic variability of A1c and fasting plasma glucose (FPG) and the risk for severe hypoglycemia. Long and colleagues performed a post hoc analysis of the ACCORD study and found that both A1c and FPG variability were associated with a greater risk for severe hypoglycemia in T2D, with FPG being a more sensitive indicator than is A1c variability. Clinicians need to be aware that A1c and FPG variability in insulin- or insulin secretagogue–treated patients with T2D places them at greater risk for severe hypoglycemia and such variability should be considered a potential target of treatment.
Although a higher mean A1c has been linked to diabetes microvascular and macrovascular complications, there is a paucity of data comparing mean A1c and A1c variability and diabetes complications. In a prospective study from Taiwan, Wu and colleagues demonstrated that both mean A1c and A1c variability predicted most diabetes-related complications, with mean A1c being more effective at predicting retinopathy and A1c variability being more effective at predicting a decline in kidney function and cardiovascular and total mortality. Perhaps physicians need to pay more attention to A1c variability and not just the mean A1c over time when assessing an individual and their overall risk for diabetes complications.
Unsure on the best T2D drug choice? Let patients decide
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
AT EASD 2022
Early age at hysterectomy ups type 2 diabetes risk
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
FROM EASD 2022
Could cold exposure, especially shivering, combat type 2 diabetes?
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022