User login
Official Newspaper of the American College of Surgeons
Surgeries account for almost half of hospital costs
according to the Agency for Healthcare Research and Quality (AHRQ).
Of the 35.4 million inpatient stays in 2014 – the last full year of ICD-9-CM coding – 10.1 million (28.6%) involved at least one any-listed surgical procedure, and 25.2 million (71.4%) did not. The total cost of all admissions was $386.2 billion, of which $187.1 billion (48.4%) went for stays with surgeries and $199.1 billion (51.6%) went for nonsurgical stays, the AHRQ reported in a statistical brief.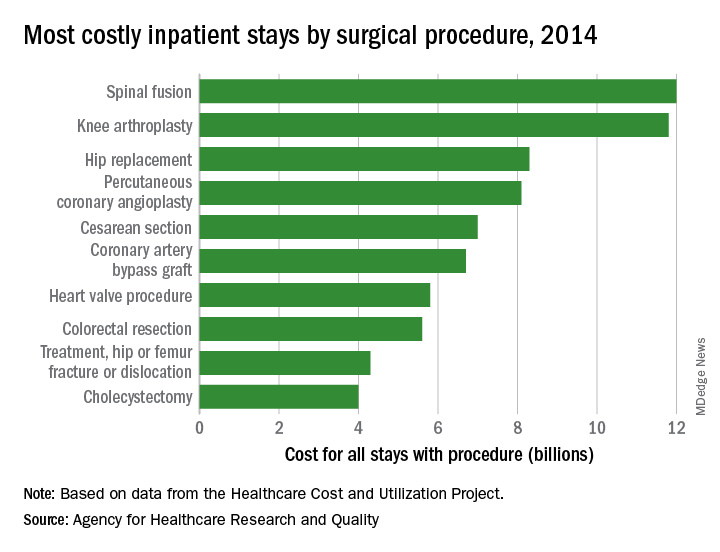
There were five other musculoskeletal procedures among the 20 most costly surgery-related admissions: knee arthroplasty was second at $11.8 billion, hip replacement was third at $8.3 billion, treatment of hip and femur fracture/dislocation was ninth at $4.3 billion, amputation of lower extremity was 13th at $2.5 billion, and treatment of lower extremity (other than hip or femur) fracture/dislocation was 14th at $2.4 billion. Those six procedures combined were $41.2 billion in hospital costs, which was a quarter of the total for all stays with a first-listed OR procedure, the AHRQ said.
The nonmusculoskeletal procedures in the top five were percutaneous coronary angioplasty in fourth, with an aggregate cost of $8.1 billion, and cesarean section in fifth at an even $7 billion. Coronary artery bypass graft, the most expensive procedure per stay ($52,000) among the top 20 procedures, was sixth in aggregate cost at $6.7 billion, according to the AHRQ researchers.
according to the Agency for Healthcare Research and Quality (AHRQ).
Of the 35.4 million inpatient stays in 2014 – the last full year of ICD-9-CM coding – 10.1 million (28.6%) involved at least one any-listed surgical procedure, and 25.2 million (71.4%) did not. The total cost of all admissions was $386.2 billion, of which $187.1 billion (48.4%) went for stays with surgeries and $199.1 billion (51.6%) went for nonsurgical stays, the AHRQ reported in a statistical brief.
There were five other musculoskeletal procedures among the 20 most costly surgery-related admissions: knee arthroplasty was second at $11.8 billion, hip replacement was third at $8.3 billion, treatment of hip and femur fracture/dislocation was ninth at $4.3 billion, amputation of lower extremity was 13th at $2.5 billion, and treatment of lower extremity (other than hip or femur) fracture/dislocation was 14th at $2.4 billion. Those six procedures combined were $41.2 billion in hospital costs, which was a quarter of the total for all stays with a first-listed OR procedure, the AHRQ said.
The nonmusculoskeletal procedures in the top five were percutaneous coronary angioplasty in fourth, with an aggregate cost of $8.1 billion, and cesarean section in fifth at an even $7 billion. Coronary artery bypass graft, the most expensive procedure per stay ($52,000) among the top 20 procedures, was sixth in aggregate cost at $6.7 billion, according to the AHRQ researchers.
according to the Agency for Healthcare Research and Quality (AHRQ).
Of the 35.4 million inpatient stays in 2014 – the last full year of ICD-9-CM coding – 10.1 million (28.6%) involved at least one any-listed surgical procedure, and 25.2 million (71.4%) did not. The total cost of all admissions was $386.2 billion, of which $187.1 billion (48.4%) went for stays with surgeries and $199.1 billion (51.6%) went for nonsurgical stays, the AHRQ reported in a statistical brief.
There were five other musculoskeletal procedures among the 20 most costly surgery-related admissions: knee arthroplasty was second at $11.8 billion, hip replacement was third at $8.3 billion, treatment of hip and femur fracture/dislocation was ninth at $4.3 billion, amputation of lower extremity was 13th at $2.5 billion, and treatment of lower extremity (other than hip or femur) fracture/dislocation was 14th at $2.4 billion. Those six procedures combined were $41.2 billion in hospital costs, which was a quarter of the total for all stays with a first-listed OR procedure, the AHRQ said.
The nonmusculoskeletal procedures in the top five were percutaneous coronary angioplasty in fourth, with an aggregate cost of $8.1 billion, and cesarean section in fifth at an even $7 billion. Coronary artery bypass graft, the most expensive procedure per stay ($52,000) among the top 20 procedures, was sixth in aggregate cost at $6.7 billion, according to the AHRQ researchers.
Musculoskeletal procedures predominate in top 20 surgeries
The represented almost 55% of the 14.2 million operating room procedures performed during inpatient stays in the United States, according to the Agency for Healthcare Research and Quality (AHRQ).
More than 1.2 million of those procedures involved cesarean sections, making it the most frequently performed in-hospital surgery in 2014 with a rate of 390 per 100,000 population, which works out to 8.8% of all procedures. Circumcision was second with 337 procedures per 100,000, followed by knee arthroplasty (236), total and partial hip replacement (164), and percutaneous coronary angioplasty (146), the AHRQ reported in a statistical brief.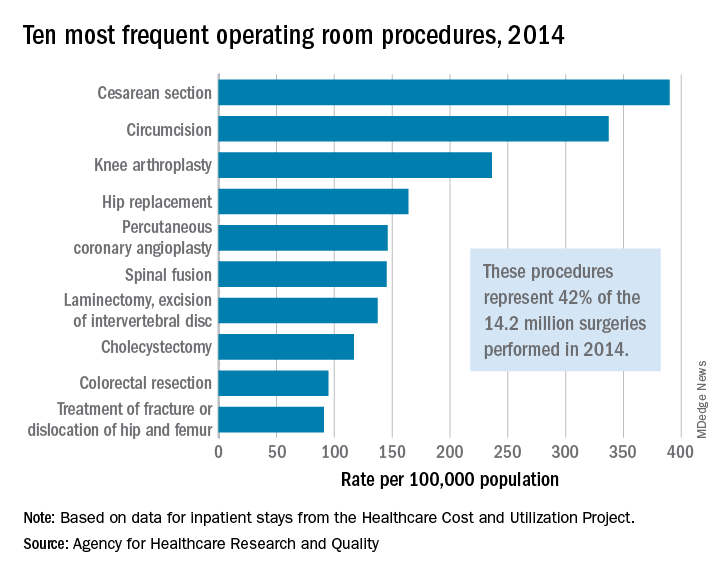
There were four obstetric/gynecologic procedures among the top 20 – namely, cesarean section, ligation of fallopian tubes (ranked 11th), hysterectomy (13th), and oophorectomy (15th) – and these together represented 13.5% of all surgeries that year. The three digestive procedures in the top 20 – cholecystectomy (ranked 8th), colorectal resection (9th), and appendectomy (12th) – made up 6.4% of all operating room procedures, they said based on data from the AHRQ’s Healthcare Cost and Utilization Project.
The represented almost 55% of the 14.2 million operating room procedures performed during inpatient stays in the United States, according to the Agency for Healthcare Research and Quality (AHRQ).
More than 1.2 million of those procedures involved cesarean sections, making it the most frequently performed in-hospital surgery in 2014 with a rate of 390 per 100,000 population, which works out to 8.8% of all procedures. Circumcision was second with 337 procedures per 100,000, followed by knee arthroplasty (236), total and partial hip replacement (164), and percutaneous coronary angioplasty (146), the AHRQ reported in a statistical brief.
There were four obstetric/gynecologic procedures among the top 20 – namely, cesarean section, ligation of fallopian tubes (ranked 11th), hysterectomy (13th), and oophorectomy (15th) – and these together represented 13.5% of all surgeries that year. The three digestive procedures in the top 20 – cholecystectomy (ranked 8th), colorectal resection (9th), and appendectomy (12th) – made up 6.4% of all operating room procedures, they said based on data from the AHRQ’s Healthcare Cost and Utilization Project.
The represented almost 55% of the 14.2 million operating room procedures performed during inpatient stays in the United States, according to the Agency for Healthcare Research and Quality (AHRQ).
More than 1.2 million of those procedures involved cesarean sections, making it the most frequently performed in-hospital surgery in 2014 with a rate of 390 per 100,000 population, which works out to 8.8% of all procedures. Circumcision was second with 337 procedures per 100,000, followed by knee arthroplasty (236), total and partial hip replacement (164), and percutaneous coronary angioplasty (146), the AHRQ reported in a statistical brief.
There were four obstetric/gynecologic procedures among the top 20 – namely, cesarean section, ligation of fallopian tubes (ranked 11th), hysterectomy (13th), and oophorectomy (15th) – and these together represented 13.5% of all surgeries that year. The three digestive procedures in the top 20 – cholecystectomy (ranked 8th), colorectal resection (9th), and appendectomy (12th) – made up 6.4% of all operating room procedures, they said based on data from the AHRQ’s Healthcare Cost and Utilization Project.
Dr. Pellegrini receives Seattle Business Leaders in Health Care Lifetime Achievement Award
Carlos A. Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), a Past-President of the American College of Surgeons, has received Seattle Business magazine’s 2018 Leaders in Health Care Lifetime Achievement Award for his committed service to improving the quality of patient care in the Seattle, WA, area.
Dr. Pellegrini has worked in the University of Washington (UW), Seattle, department of surgery since 1993, first as chair of the department and then in 1996 as the Henry N. Harkins Professor and Chair, until 2015, when he was appointed to serve as UW Medicine’s first chief medical officer (CMO).
According to the Seattle Business article on Dr. Pellegrini’s achievement, as CMO, Dr. Pellegrini oversees thousands of health care providers and has led a program that has visibly improved patient care quality, reduced costs, and “ensured that all of the health care system’s 270,000 patients have an assigned primary care provider across its primary care clinics.” He also integrated clinical services for key programs and created a training program to prepare young clinicians for leadership roles.
Dr. Pellegrini said that his motivation has always been to help people, as a surgeon, a mentor, or, as he notes about his role as CMO, by “advancing social issues and the care that we provide our patients.”
Read more about Dr. Pellegrini’s life and career in the Seattle Business article at http://seattlebusinessmag.com:8080/health-care/2018-leaders-health-care-lifetime-achievement-award-carlos-pellegrini-uw-medicine.
Carlos A. Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), a Past-President of the American College of Surgeons, has received Seattle Business magazine’s 2018 Leaders in Health Care Lifetime Achievement Award for his committed service to improving the quality of patient care in the Seattle, WA, area.
Dr. Pellegrini has worked in the University of Washington (UW), Seattle, department of surgery since 1993, first as chair of the department and then in 1996 as the Henry N. Harkins Professor and Chair, until 2015, when he was appointed to serve as UW Medicine’s first chief medical officer (CMO).
According to the Seattle Business article on Dr. Pellegrini’s achievement, as CMO, Dr. Pellegrini oversees thousands of health care providers and has led a program that has visibly improved patient care quality, reduced costs, and “ensured that all of the health care system’s 270,000 patients have an assigned primary care provider across its primary care clinics.” He also integrated clinical services for key programs and created a training program to prepare young clinicians for leadership roles.
Dr. Pellegrini said that his motivation has always been to help people, as a surgeon, a mentor, or, as he notes about his role as CMO, by “advancing social issues and the care that we provide our patients.”
Read more about Dr. Pellegrini’s life and career in the Seattle Business article at http://seattlebusinessmag.com:8080/health-care/2018-leaders-health-care-lifetime-achievement-award-carlos-pellegrini-uw-medicine.
Carlos A. Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), a Past-President of the American College of Surgeons, has received Seattle Business magazine’s 2018 Leaders in Health Care Lifetime Achievement Award for his committed service to improving the quality of patient care in the Seattle, WA, area.
Dr. Pellegrini has worked in the University of Washington (UW), Seattle, department of surgery since 1993, first as chair of the department and then in 1996 as the Henry N. Harkins Professor and Chair, until 2015, when he was appointed to serve as UW Medicine’s first chief medical officer (CMO).
According to the Seattle Business article on Dr. Pellegrini’s achievement, as CMO, Dr. Pellegrini oversees thousands of health care providers and has led a program that has visibly improved patient care quality, reduced costs, and “ensured that all of the health care system’s 270,000 patients have an assigned primary care provider across its primary care clinics.” He also integrated clinical services for key programs and created a training program to prepare young clinicians for leadership roles.
Dr. Pellegrini said that his motivation has always been to help people, as a surgeon, a mentor, or, as he notes about his role as CMO, by “advancing social issues and the care that we provide our patients.”
Read more about Dr. Pellegrini’s life and career in the Seattle Business article at http://seattlebusinessmag.com:8080/health-care/2018-leaders-health-care-lifetime-achievement-award-carlos-pellegrini-uw-medicine.
Royal Australasian College of Surgeons partners with ACS for Annual Scientific Congress
The Royal Australasian College of Surgeons (RACS), along with the American College of Surgeons (ACS), will host the 87th Annual Scientific Congress, May 7–11 at the International Convention Centre in Sydney, Australia.
The theme of the 2018 Scientific Congress, Reflecting on What Really Matters, explores the challenges of providing quality patient care within complex health care systems—a universal situation familiar to U.S. surgeons.
The ACS has partnered in the planning of this program, and many U.S. surgeons will be featured as speakers throughout the week. Additionally, an ACS panel will take place the morning of Thursday, May 10. The ACS also will be involved in other Annual Scientific Congress activities, including the following:
• ACS Lecture, The Surgical Patient in the ICU—Insights into Survivorship, by Mayur B. Patel, MD, MPH, FACS, a neurosurgeon and surgical intensivist, from Nashville, TN
• Region 16 meeting for ACS Fellows from Australia and New Zealand, the U.S., and other Pacific countries
Among the ACS leaders attending the Congress are Barbara L. Bass, MD, FACS, FRCS(Hon),
ACS President; Clifford Y. Ko, MD, MS, MSHS, FACS, FASCRS, Director, ACS Division of
Research and Optimal Patient Care; Ronald V. Maier, MD, FACS, ACS President-Elect; M.
Margaret (Peggy) Knudson, MD, FACS, Medical Director, Military Health System Strategi
Partnership American College of Surgeons; and Tyler G. Hughes, MD, FACS, Co-Editor, ACS
Surgery News, and Editor-in-Chief, ACS Communities.
For more information on the conference and to register, visit the RACS 87th Annual Scientific Congress website at https://asc.surgeons.org/.
The Royal Australasian College of Surgeons (RACS), along with the American College of Surgeons (ACS), will host the 87th Annual Scientific Congress, May 7–11 at the International Convention Centre in Sydney, Australia.
The theme of the 2018 Scientific Congress, Reflecting on What Really Matters, explores the challenges of providing quality patient care within complex health care systems—a universal situation familiar to U.S. surgeons.
The ACS has partnered in the planning of this program, and many U.S. surgeons will be featured as speakers throughout the week. Additionally, an ACS panel will take place the morning of Thursday, May 10. The ACS also will be involved in other Annual Scientific Congress activities, including the following:
• ACS Lecture, The Surgical Patient in the ICU—Insights into Survivorship, by Mayur B. Patel, MD, MPH, FACS, a neurosurgeon and surgical intensivist, from Nashville, TN
• Region 16 meeting for ACS Fellows from Australia and New Zealand, the U.S., and other Pacific countries
Among the ACS leaders attending the Congress are Barbara L. Bass, MD, FACS, FRCS(Hon),
ACS President; Clifford Y. Ko, MD, MS, MSHS, FACS, FASCRS, Director, ACS Division of
Research and Optimal Patient Care; Ronald V. Maier, MD, FACS, ACS President-Elect; M.
Margaret (Peggy) Knudson, MD, FACS, Medical Director, Military Health System Strategi
Partnership American College of Surgeons; and Tyler G. Hughes, MD, FACS, Co-Editor, ACS
Surgery News, and Editor-in-Chief, ACS Communities.
For more information on the conference and to register, visit the RACS 87th Annual Scientific Congress website at https://asc.surgeons.org/.
The Royal Australasian College of Surgeons (RACS), along with the American College of Surgeons (ACS), will host the 87th Annual Scientific Congress, May 7–11 at the International Convention Centre in Sydney, Australia.
The theme of the 2018 Scientific Congress, Reflecting on What Really Matters, explores the challenges of providing quality patient care within complex health care systems—a universal situation familiar to U.S. surgeons.
The ACS has partnered in the planning of this program, and many U.S. surgeons will be featured as speakers throughout the week. Additionally, an ACS panel will take place the morning of Thursday, May 10. The ACS also will be involved in other Annual Scientific Congress activities, including the following:
• ACS Lecture, The Surgical Patient in the ICU—Insights into Survivorship, by Mayur B. Patel, MD, MPH, FACS, a neurosurgeon and surgical intensivist, from Nashville, TN
• Region 16 meeting for ACS Fellows from Australia and New Zealand, the U.S., and other Pacific countries
Among the ACS leaders attending the Congress are Barbara L. Bass, MD, FACS, FRCS(Hon),
ACS President; Clifford Y. Ko, MD, MS, MSHS, FACS, FASCRS, Director, ACS Division of
Research and Optimal Patient Care; Ronald V. Maier, MD, FACS, ACS President-Elect; M.
Margaret (Peggy) Knudson, MD, FACS, Medical Director, Military Health System Strategi
Partnership American College of Surgeons; and Tyler G. Hughes, MD, FACS, Co-Editor, ACS
Surgery News, and Editor-in-Chief, ACS Communities.
For more information on the conference and to register, visit the RACS 87th Annual Scientific Congress website at https://asc.surgeons.org/.
ACS releases 2018 update to the Physicians as Assistants at Surgery report
The American College of Surgeons (ACS), in collaboration with 15 other national specialty surgical organizations, has recently published the eighth edition of the Physicians as Assistants at Surgery report, a study first undertaken in 1994. The 2018 report reflects the most recent clinical practices and provides guidance on how often an operation might require a physician to assist at surgery. The report is available on the ACS website at www.facs.org/~/media/files/advocacy/pubs/2018_pas.ashx.
Using the American Medical Association’s Current Procedural Terminology (CPT) codes from the 2018 manual, each participating organization reviewed new or revised codes since 2016 and any other codes of interest that are applicable to their specialty and indicated whether the operation requires a physician as an assistant with the following frequency: almost always, almost never, or some of the time. The 2018 report adds 93 codes that the CPT Editorial Panel has approved since the last report was issued in 2016. In addition, the 2018 report updates 384 revised codes and deletes 48 codes that are no longer in CPT.
The ACS maintains that a physician who assists with an operation should be trained to participate in and actively assist the surgeon in safely completing the operation. When a surgeon is unavailable to serve as an assistant, a qualified surgical resident or other qualified health care professional, such as a nurse practitioner or physician assistant with experience in assisting, may participate in operations, according to the ACS Statements on Principles (available at www.facs.org/about-acs/statements/stonprin).
Organizations that collaborated with the ACS to conduct the study include the American Academy of Ophthalmology, the American Academy of Orthopaedic Surgeons, the American Academy of Otolaryngology–Head and Neck Surgery, the American Association of Neurological Surgeons, the American Pediatric Surgical Association, the American Society of Colon and Rectal Surgeons, the American Society of Plastic Surgeons, the American Society of Transplant Surgeons, the American Urological Association, the Congress of Neurological Surgeons, the Society for Surgical Oncology, the Society for Vascular Surgery, the Society of American Gastrointestinal Endoscopic Surgeons, the American College of Obstetricians and Gynecologists, and the Society of Thoracic Surgeons.
The American College of Surgeons (ACS), in collaboration with 15 other national specialty surgical organizations, has recently published the eighth edition of the Physicians as Assistants at Surgery report, a study first undertaken in 1994. The 2018 report reflects the most recent clinical practices and provides guidance on how often an operation might require a physician to assist at surgery. The report is available on the ACS website at www.facs.org/~/media/files/advocacy/pubs/2018_pas.ashx.
Using the American Medical Association’s Current Procedural Terminology (CPT) codes from the 2018 manual, each participating organization reviewed new or revised codes since 2016 and any other codes of interest that are applicable to their specialty and indicated whether the operation requires a physician as an assistant with the following frequency: almost always, almost never, or some of the time. The 2018 report adds 93 codes that the CPT Editorial Panel has approved since the last report was issued in 2016. In addition, the 2018 report updates 384 revised codes and deletes 48 codes that are no longer in CPT.
The ACS maintains that a physician who assists with an operation should be trained to participate in and actively assist the surgeon in safely completing the operation. When a surgeon is unavailable to serve as an assistant, a qualified surgical resident or other qualified health care professional, such as a nurse practitioner or physician assistant with experience in assisting, may participate in operations, according to the ACS Statements on Principles (available at www.facs.org/about-acs/statements/stonprin).
Organizations that collaborated with the ACS to conduct the study include the American Academy of Ophthalmology, the American Academy of Orthopaedic Surgeons, the American Academy of Otolaryngology–Head and Neck Surgery, the American Association of Neurological Surgeons, the American Pediatric Surgical Association, the American Society of Colon and Rectal Surgeons, the American Society of Plastic Surgeons, the American Society of Transplant Surgeons, the American Urological Association, the Congress of Neurological Surgeons, the Society for Surgical Oncology, the Society for Vascular Surgery, the Society of American Gastrointestinal Endoscopic Surgeons, the American College of Obstetricians and Gynecologists, and the Society of Thoracic Surgeons.
The American College of Surgeons (ACS), in collaboration with 15 other national specialty surgical organizations, has recently published the eighth edition of the Physicians as Assistants at Surgery report, a study first undertaken in 1994. The 2018 report reflects the most recent clinical practices and provides guidance on how often an operation might require a physician to assist at surgery. The report is available on the ACS website at www.facs.org/~/media/files/advocacy/pubs/2018_pas.ashx.
Using the American Medical Association’s Current Procedural Terminology (CPT) codes from the 2018 manual, each participating organization reviewed new or revised codes since 2016 and any other codes of interest that are applicable to their specialty and indicated whether the operation requires a physician as an assistant with the following frequency: almost always, almost never, or some of the time. The 2018 report adds 93 codes that the CPT Editorial Panel has approved since the last report was issued in 2016. In addition, the 2018 report updates 384 revised codes and deletes 48 codes that are no longer in CPT.
The ACS maintains that a physician who assists with an operation should be trained to participate in and actively assist the surgeon in safely completing the operation. When a surgeon is unavailable to serve as an assistant, a qualified surgical resident or other qualified health care professional, such as a nurse practitioner or physician assistant with experience in assisting, may participate in operations, according to the ACS Statements on Principles (available at www.facs.org/about-acs/statements/stonprin).
Organizations that collaborated with the ACS to conduct the study include the American Academy of Ophthalmology, the American Academy of Orthopaedic Surgeons, the American Academy of Otolaryngology–Head and Neck Surgery, the American Association of Neurological Surgeons, the American Pediatric Surgical Association, the American Society of Colon and Rectal Surgeons, the American Society of Plastic Surgeons, the American Society of Transplant Surgeons, the American Urological Association, the Congress of Neurological Surgeons, the Society for Surgical Oncology, the Society for Vascular Surgery, the Society of American Gastrointestinal Endoscopic Surgeons, the American College of Obstetricians and Gynecologists, and the Society of Thoracic Surgeons.
Applications for ACS Academy of Master Surgeon Educators are now being accepted –
The American College of Surgeons (ACS) Academy of Master Surgeon Educators, a new College enterprise that will advance the science and implementation of education across all surgical specialties, is now accepting applications for Membership and Associate Membership. Applications are due May 14, 2018.
You could be considered for membership in the Academy through two avenues:
• You may apply directly.
• You may be nominated by a colleague and then complete the application.
Background
In October 2014, the American College of Surgeons (ACS) Board of Regents approved a proposal from the ACS Division of Education to establish the ACS Academy of Master Surgeon Educators. A Steering Committee was appointed to create a model for the Academy, which articulated the desired outcomes, defined standards and criteria for membership, and developed the process for application. The ACS Steering Committee for the Academy of Master Surgeon Educators is co-chaired by ACS Past-President L.D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), and Ajit K. Sachdeva, MD, FACS, FRCSC, Director, ACS Division of Education. Other members include Sir Murray Brennan, MD, FACS, ACS Distinguished Service Award recipient; Haile Debas, MD, FACS, founding executive director, Global Health Sciences, University of California, San Francisco; David B. Hoyt, MD, FACS, ACS Executive Director; L. Scott Levin, MD, FACS, ACS Regent; Leigh Neumayer, MD, FACS, Chair, ACS Board of Regents; and Carlos Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), ACS Past-President.
The Academy formally launched at the ACS Clinical Congress 2017 in San Diego, CA, and was received enthusiastically.
Purposes of the Academy
The goals of this unique Academy are to define megatrends in surgical education, steer advances in this field, and underscore the critical importance of surgical education in the changing milieu of health care. The Academy will meet these goals by recognizing and assembling a cadre of master surgeon educators of national and international renown who will support cutting-edge surgical education and provide mentorship to the next generation of surgeon educators.
Members of the Academy will be selected through a rigorous peer-review process, and induction will be a high honor in the field of surgical education. Members of the Academy will be expected to engage in activities to address the aforementioned goals. Membership in the Academy will be open to Master Surgeon Educators from across the surgical specialties.
Three categories of membership will be available: Member, Associate Member, and Affiliate Member. Applications for Membership and Associate Membership in the Academy are now being accepted. You are invited to apply or nominate a colleague for membership via the ACS website at facs.org/acsacademy.
The ACS is truly excited about this seminal endeavor, which will impact the profession of surgery for generations to come.
The American College of Surgeons (ACS) Academy of Master Surgeon Educators, a new College enterprise that will advance the science and implementation of education across all surgical specialties, is now accepting applications for Membership and Associate Membership. Applications are due May 14, 2018.
You could be considered for membership in the Academy through two avenues:
• You may apply directly.
• You may be nominated by a colleague and then complete the application.
Background
In October 2014, the American College of Surgeons (ACS) Board of Regents approved a proposal from the ACS Division of Education to establish the ACS Academy of Master Surgeon Educators. A Steering Committee was appointed to create a model for the Academy, which articulated the desired outcomes, defined standards and criteria for membership, and developed the process for application. The ACS Steering Committee for the Academy of Master Surgeon Educators is co-chaired by ACS Past-President L.D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), and Ajit K. Sachdeva, MD, FACS, FRCSC, Director, ACS Division of Education. Other members include Sir Murray Brennan, MD, FACS, ACS Distinguished Service Award recipient; Haile Debas, MD, FACS, founding executive director, Global Health Sciences, University of California, San Francisco; David B. Hoyt, MD, FACS, ACS Executive Director; L. Scott Levin, MD, FACS, ACS Regent; Leigh Neumayer, MD, FACS, Chair, ACS Board of Regents; and Carlos Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), ACS Past-President.
The Academy formally launched at the ACS Clinical Congress 2017 in San Diego, CA, and was received enthusiastically.
Purposes of the Academy
The goals of this unique Academy are to define megatrends in surgical education, steer advances in this field, and underscore the critical importance of surgical education in the changing milieu of health care. The Academy will meet these goals by recognizing and assembling a cadre of master surgeon educators of national and international renown who will support cutting-edge surgical education and provide mentorship to the next generation of surgeon educators.
Members of the Academy will be selected through a rigorous peer-review process, and induction will be a high honor in the field of surgical education. Members of the Academy will be expected to engage in activities to address the aforementioned goals. Membership in the Academy will be open to Master Surgeon Educators from across the surgical specialties.
Three categories of membership will be available: Member, Associate Member, and Affiliate Member. Applications for Membership and Associate Membership in the Academy are now being accepted. You are invited to apply or nominate a colleague for membership via the ACS website at facs.org/acsacademy.
The ACS is truly excited about this seminal endeavor, which will impact the profession of surgery for generations to come.
The American College of Surgeons (ACS) Academy of Master Surgeon Educators, a new College enterprise that will advance the science and implementation of education across all surgical specialties, is now accepting applications for Membership and Associate Membership. Applications are due May 14, 2018.
You could be considered for membership in the Academy through two avenues:
• You may apply directly.
• You may be nominated by a colleague and then complete the application.
Background
In October 2014, the American College of Surgeons (ACS) Board of Regents approved a proposal from the ACS Division of Education to establish the ACS Academy of Master Surgeon Educators. A Steering Committee was appointed to create a model for the Academy, which articulated the desired outcomes, defined standards and criteria for membership, and developed the process for application. The ACS Steering Committee for the Academy of Master Surgeon Educators is co-chaired by ACS Past-President L.D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), and Ajit K. Sachdeva, MD, FACS, FRCSC, Director, ACS Division of Education. Other members include Sir Murray Brennan, MD, FACS, ACS Distinguished Service Award recipient; Haile Debas, MD, FACS, founding executive director, Global Health Sciences, University of California, San Francisco; David B. Hoyt, MD, FACS, ACS Executive Director; L. Scott Levin, MD, FACS, ACS Regent; Leigh Neumayer, MD, FACS, Chair, ACS Board of Regents; and Carlos Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), ACS Past-President.
The Academy formally launched at the ACS Clinical Congress 2017 in San Diego, CA, and was received enthusiastically.
Purposes of the Academy
The goals of this unique Academy are to define megatrends in surgical education, steer advances in this field, and underscore the critical importance of surgical education in the changing milieu of health care. The Academy will meet these goals by recognizing and assembling a cadre of master surgeon educators of national and international renown who will support cutting-edge surgical education and provide mentorship to the next generation of surgeon educators.
Members of the Academy will be selected through a rigorous peer-review process, and induction will be a high honor in the field of surgical education. Members of the Academy will be expected to engage in activities to address the aforementioned goals. Membership in the Academy will be open to Master Surgeon Educators from across the surgical specialties.
Three categories of membership will be available: Member, Associate Member, and Affiliate Member. Applications for Membership and Associate Membership in the Academy are now being accepted. You are invited to apply or nominate a colleague for membership via the ACS website at facs.org/acsacademy.
The ACS is truly excited about this seminal endeavor, which will impact the profession of surgery for generations to come.
ACS WiSC seeks ACS Fellows to serve as new members
The mission of the WiSC is to enable women surgeons of all ages, specialties, and practice types to develop their individual potential as professionals; promote an environment that fosters inclusion, respect, and success; develop, encourage, and advance women surgeons as leaders; and provide a forum and networking opportunities to enhance women’s surgical career satisfaction.
Surgeons interested in advancing the role of women in the ACS and encouraging and mentoring women in surgery should apply. Nominations are open to both men and women, and the committee encourages representation by individuals of diverse cultural, racial, and ethnic backgrounds.
Read the full eligibility requirements and how to apply on the ACS website at facs.org/about-acs/governance/acs-committees/women-in-surgery-committee/wisc-call. Eligible candidates will be selected and notified by the committee in June, and will be invited to attend the October 22 meeting of the WiSC, held in conjunction with Clinical Congress 2018 in Boston, MA. Travel reimbursement will not be provided.
Apply online at www.surveymonkey.com/r/2018WiSCMbrApp. Applications are due May 31, 2018. Questions can be directed to Connie Bura at [email protected].
The mission of the WiSC is to enable women surgeons of all ages, specialties, and practice types to develop their individual potential as professionals; promote an environment that fosters inclusion, respect, and success; develop, encourage, and advance women surgeons as leaders; and provide a forum and networking opportunities to enhance women’s surgical career satisfaction.
Surgeons interested in advancing the role of women in the ACS and encouraging and mentoring women in surgery should apply. Nominations are open to both men and women, and the committee encourages representation by individuals of diverse cultural, racial, and ethnic backgrounds.
Read the full eligibility requirements and how to apply on the ACS website at facs.org/about-acs/governance/acs-committees/women-in-surgery-committee/wisc-call. Eligible candidates will be selected and notified by the committee in June, and will be invited to attend the October 22 meeting of the WiSC, held in conjunction with Clinical Congress 2018 in Boston, MA. Travel reimbursement will not be provided.
Apply online at www.surveymonkey.com/r/2018WiSCMbrApp. Applications are due May 31, 2018. Questions can be directed to Connie Bura at [email protected].
The mission of the WiSC is to enable women surgeons of all ages, specialties, and practice types to develop their individual potential as professionals; promote an environment that fosters inclusion, respect, and success; develop, encourage, and advance women surgeons as leaders; and provide a forum and networking opportunities to enhance women’s surgical career satisfaction.
Surgeons interested in advancing the role of women in the ACS and encouraging and mentoring women in surgery should apply. Nominations are open to both men and women, and the committee encourages representation by individuals of diverse cultural, racial, and ethnic backgrounds.
Read the full eligibility requirements and how to apply on the ACS website at facs.org/about-acs/governance/acs-committees/women-in-surgery-committee/wisc-call. Eligible candidates will be selected and notified by the committee in June, and will be invited to attend the October 22 meeting of the WiSC, held in conjunction with Clinical Congress 2018 in Boston, MA. Travel reimbursement will not be provided.
Apply online at www.surveymonkey.com/r/2018WiSCMbrApp. Applications are due May 31, 2018. Questions can be directed to Connie Bura at [email protected].
ERAS reduced opioid use, improved same-day discharge after gyn surgery
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
REPORTING FROM SGS 2018
Key clinical point: ERAS pathways improve same-day discharge rates and reduce opioid use in gynecologic surgery.
Major finding: Same-day discharge rates before and after ERAS were 25.9% and 91.7%, respectively.
Study details: A retrospective review of 258 patients; a study of 161 patients.
Disclosures: Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
Sources: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
Dexamethasone lowered risk of urinary retention in laparoscopic hernia repair
SEATTLE – Intraoperative in 955 men at the NorthShore University HealthSystem, Chicago.
Urinary retention occurs in up to a third of hernia repair patients. Men are at far higher risk than women; benign prostatic hypertrophy (BPH) and older age also increase the risk.
Intraoperative dexamethasone is a common antiemetic. The investigators had a hunch that it also reduces urinary retention by calming overstimulation of the bladder neck and prostate during the operation. “We believe this overstimulation” causes urinary retention, lead investigator Merritt Denham, BS, said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
She and her team went back in the records about 8 years to compare 617 laparoscopic inguinal hernia repair patients who received intraoperative dexamethasone with 338 who did not. They were all men: women were excluded from the review. The men voided before surgery. Those who received dexamethasone received more fluids during the operation than those who did not, a mean of 973 mL versus 878 mL (P = .0019).
Even so, urinary retention was far less likely in the dexamethasone group (3.7% vs. 9.8%; P = .0001). Controlling for age and BPH, the corticosteroid was highly protective (odds ratio, 0.48; 95% confidence interval, 0.26-0.87; P = .0147). The benefit was the same regardless of the intraoperative dexamethasone dosage, which ranged from 4 mg to 8 mg.
Dexamethasone patients had a shorter length of stay, and, counterintuitively, fewer surgical site infections (0.2% vs. 1.7%; P = .0109). They were also less likely to have BPH (16.7% vs. 22.5%; P = 0.026). The urinary retention odds ratio controlled for the difference in BPH.
The results are “interesting, but I don’t think the conclusion is there yet; there are a lot of variables to consider. We need more data,” said moderator Eduardo Parra-Davila, MD, FACS, director of minimally invasive and colorectal surgery at Florida Hospital Celebration Health in Celebration, Fla.
In both groups, mean body mass index was about 26 kg/m2, mean age about 57 years, and mean operative time about 40 minutes. Four general surgeons performed the repairs.
There was no external funding for the work, and the investigators had no disclosures.
SOURCE: Denham M. et al. SAGES 2018, Abstract S006.
SEATTLE – Intraoperative in 955 men at the NorthShore University HealthSystem, Chicago.
Urinary retention occurs in up to a third of hernia repair patients. Men are at far higher risk than women; benign prostatic hypertrophy (BPH) and older age also increase the risk.
Intraoperative dexamethasone is a common antiemetic. The investigators had a hunch that it also reduces urinary retention by calming overstimulation of the bladder neck and prostate during the operation. “We believe this overstimulation” causes urinary retention, lead investigator Merritt Denham, BS, said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
She and her team went back in the records about 8 years to compare 617 laparoscopic inguinal hernia repair patients who received intraoperative dexamethasone with 338 who did not. They were all men: women were excluded from the review. The men voided before surgery. Those who received dexamethasone received more fluids during the operation than those who did not, a mean of 973 mL versus 878 mL (P = .0019).
Even so, urinary retention was far less likely in the dexamethasone group (3.7% vs. 9.8%; P = .0001). Controlling for age and BPH, the corticosteroid was highly protective (odds ratio, 0.48; 95% confidence interval, 0.26-0.87; P = .0147). The benefit was the same regardless of the intraoperative dexamethasone dosage, which ranged from 4 mg to 8 mg.
Dexamethasone patients had a shorter length of stay, and, counterintuitively, fewer surgical site infections (0.2% vs. 1.7%; P = .0109). They were also less likely to have BPH (16.7% vs. 22.5%; P = 0.026). The urinary retention odds ratio controlled for the difference in BPH.
The results are “interesting, but I don’t think the conclusion is there yet; there are a lot of variables to consider. We need more data,” said moderator Eduardo Parra-Davila, MD, FACS, director of minimally invasive and colorectal surgery at Florida Hospital Celebration Health in Celebration, Fla.
In both groups, mean body mass index was about 26 kg/m2, mean age about 57 years, and mean operative time about 40 minutes. Four general surgeons performed the repairs.
There was no external funding for the work, and the investigators had no disclosures.
SOURCE: Denham M. et al. SAGES 2018, Abstract S006.
SEATTLE – Intraoperative in 955 men at the NorthShore University HealthSystem, Chicago.
Urinary retention occurs in up to a third of hernia repair patients. Men are at far higher risk than women; benign prostatic hypertrophy (BPH) and older age also increase the risk.
Intraoperative dexamethasone is a common antiemetic. The investigators had a hunch that it also reduces urinary retention by calming overstimulation of the bladder neck and prostate during the operation. “We believe this overstimulation” causes urinary retention, lead investigator Merritt Denham, BS, said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
She and her team went back in the records about 8 years to compare 617 laparoscopic inguinal hernia repair patients who received intraoperative dexamethasone with 338 who did not. They were all men: women were excluded from the review. The men voided before surgery. Those who received dexamethasone received more fluids during the operation than those who did not, a mean of 973 mL versus 878 mL (P = .0019).
Even so, urinary retention was far less likely in the dexamethasone group (3.7% vs. 9.8%; P = .0001). Controlling for age and BPH, the corticosteroid was highly protective (odds ratio, 0.48; 95% confidence interval, 0.26-0.87; P = .0147). The benefit was the same regardless of the intraoperative dexamethasone dosage, which ranged from 4 mg to 8 mg.
Dexamethasone patients had a shorter length of stay, and, counterintuitively, fewer surgical site infections (0.2% vs. 1.7%; P = .0109). They were also less likely to have BPH (16.7% vs. 22.5%; P = 0.026). The urinary retention odds ratio controlled for the difference in BPH.
The results are “interesting, but I don’t think the conclusion is there yet; there are a lot of variables to consider. We need more data,” said moderator Eduardo Parra-Davila, MD, FACS, director of minimally invasive and colorectal surgery at Florida Hospital Celebration Health in Celebration, Fla.
In both groups, mean body mass index was about 26 kg/m2, mean age about 57 years, and mean operative time about 40 minutes. Four general surgeons performed the repairs.
There was no external funding for the work, and the investigators had no disclosures.
SOURCE: Denham M. et al. SAGES 2018, Abstract S006.
REPORTING FROM SAGES 2018
Key clinical point: Patients given dexamethasone intraoperatively were less likely to have urinary retention.
Major finding: The dexamethasone group was less likely to have urinary retention, 3.7% versus 9.8% (P = 0.0001).
Study details: A review of 955 laparoscopic inguinal hernia repairs.
Disclosures: There was no external funding for the work, and the investigators had no disclosures.
Source: Denham M et al. SAGES 2018, Abstract S006.
Robotic approach falls short for sleeve gastrectomy
SEATTLE – according to a review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database.
“Robotic sleeve gastrectomy increases [use of] hospital resources. ... These findings may explain the low utilization rate of the robotic approach to sleeve gastrectomy,” said lead investigator Reza Alizadeh, MD, a surgery resident at the University of California, Irvine.
Sleeve gastrectomy has eclipsed gastric bypass as the most common weight loss surgery in United States. While most are done laparoscopically, the use of robots is becoming more common, so the investigators wanted to compare outcomes in a large number of cases. They turned to the metabolic and bariatric surgery database, which is jointly maintained by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Emergent, converted, and revision cases were excluded from the analysis to avoid confounding.
Almost 94% of the cases were done laparoscopically, with the rest done robotically. Mean operative time was 101 min in the robotic arm, and 1.5% of patients developed anastomotic leaks. Mean operative time in the laparoscopic group was 74 minutes, and 0.5% of patients developed leaks. After adjustment for potential confounders, leaks were 3.4 times more likely with the robotic approach (95% confidence interval, 2.47-4.0; P less than .01). It wasn’t possible to determine whether there were any differences in the type of stapling done in the two groups.
Meanwhile, 0.8% of robotic surgery patients developed surgical site infections versus 0.6% of the laparoscopic cases. After adjustment, infections were 38% more likely with the robot (95% CI, 1.01-1.89; P = 0.03). Dr. Alizadeh noted that the database only goes out to 30 days, so “the true complication rates may be underestimated.”
The findings are consistent with previous investigations. It’s unclear whether there’s something inherently riskier about robotic sleeve gastrectomy itself or whether surgeons haven’t quite got the knack of it yet. The higher leak rate with robotic surgery, “I believe, is mostly related to the small number of [robotic] cases being done. We are still in the beginning stages of utilizing the robotic approach. Maybe there’s a learning curve, and we need more experience and more practice,” Dr. Alizadeh said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Indeed, others have reported that it takes more than two dozen cases to become proficient in another procedure, robotic esophagectomy.
The mean length of stay in the study was slightly, but not statistically significantly, longer in the robotic arm (1.8 vs. 1.7 days; P = 0.17). There was no statistically significant difference in in-hospital mortality.
The laparoscopic group had more men than did the robotic group (21.4% vs. 19.7%, respectively) and more chronic steroid use (1.7% vs. 1.3%), plus more patients were dependent on oxygen (0.7% vs. 0.3%). The robotic group had more obstructive sleep apnea than did the laparoscopic group (37.3% vs. 36% of cases) and a higher incidence of hypoalbuminemia (8.4% vs. 7%). The analysis adjusted for the differences.
The findings were pretty much the same when the team repeated their analysis with the 2016 database numbers, which were released while the SAGES presentation was being prepared. The only big difference was an increase in the number of robotic cases, up from 6.1% in 2015 to 6.6% of cases in 2016.
The was no external funding for the work, and the investigators had no relevant disclosures.
SOURCE: Alizadeh RF et al. SAGES 2018, Abstract S024.
SEATTLE – according to a review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database.
“Robotic sleeve gastrectomy increases [use of] hospital resources. ... These findings may explain the low utilization rate of the robotic approach to sleeve gastrectomy,” said lead investigator Reza Alizadeh, MD, a surgery resident at the University of California, Irvine.
Sleeve gastrectomy has eclipsed gastric bypass as the most common weight loss surgery in United States. While most are done laparoscopically, the use of robots is becoming more common, so the investigators wanted to compare outcomes in a large number of cases. They turned to the metabolic and bariatric surgery database, which is jointly maintained by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Emergent, converted, and revision cases were excluded from the analysis to avoid confounding.
Almost 94% of the cases were done laparoscopically, with the rest done robotically. Mean operative time was 101 min in the robotic arm, and 1.5% of patients developed anastomotic leaks. Mean operative time in the laparoscopic group was 74 minutes, and 0.5% of patients developed leaks. After adjustment for potential confounders, leaks were 3.4 times more likely with the robotic approach (95% confidence interval, 2.47-4.0; P less than .01). It wasn’t possible to determine whether there were any differences in the type of stapling done in the two groups.
Meanwhile, 0.8% of robotic surgery patients developed surgical site infections versus 0.6% of the laparoscopic cases. After adjustment, infections were 38% more likely with the robot (95% CI, 1.01-1.89; P = 0.03). Dr. Alizadeh noted that the database only goes out to 30 days, so “the true complication rates may be underestimated.”
The findings are consistent with previous investigations. It’s unclear whether there’s something inherently riskier about robotic sleeve gastrectomy itself or whether surgeons haven’t quite got the knack of it yet. The higher leak rate with robotic surgery, “I believe, is mostly related to the small number of [robotic] cases being done. We are still in the beginning stages of utilizing the robotic approach. Maybe there’s a learning curve, and we need more experience and more practice,” Dr. Alizadeh said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Indeed, others have reported that it takes more than two dozen cases to become proficient in another procedure, robotic esophagectomy.
The mean length of stay in the study was slightly, but not statistically significantly, longer in the robotic arm (1.8 vs. 1.7 days; P = 0.17). There was no statistically significant difference in in-hospital mortality.
The laparoscopic group had more men than did the robotic group (21.4% vs. 19.7%, respectively) and more chronic steroid use (1.7% vs. 1.3%), plus more patients were dependent on oxygen (0.7% vs. 0.3%). The robotic group had more obstructive sleep apnea than did the laparoscopic group (37.3% vs. 36% of cases) and a higher incidence of hypoalbuminemia (8.4% vs. 7%). The analysis adjusted for the differences.
The findings were pretty much the same when the team repeated their analysis with the 2016 database numbers, which were released while the SAGES presentation was being prepared. The only big difference was an increase in the number of robotic cases, up from 6.1% in 2015 to 6.6% of cases in 2016.
The was no external funding for the work, and the investigators had no relevant disclosures.
SOURCE: Alizadeh RF et al. SAGES 2018, Abstract S024.
SEATTLE – according to a review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database.
“Robotic sleeve gastrectomy increases [use of] hospital resources. ... These findings may explain the low utilization rate of the robotic approach to sleeve gastrectomy,” said lead investigator Reza Alizadeh, MD, a surgery resident at the University of California, Irvine.
Sleeve gastrectomy has eclipsed gastric bypass as the most common weight loss surgery in United States. While most are done laparoscopically, the use of robots is becoming more common, so the investigators wanted to compare outcomes in a large number of cases. They turned to the metabolic and bariatric surgery database, which is jointly maintained by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Emergent, converted, and revision cases were excluded from the analysis to avoid confounding.
Almost 94% of the cases were done laparoscopically, with the rest done robotically. Mean operative time was 101 min in the robotic arm, and 1.5% of patients developed anastomotic leaks. Mean operative time in the laparoscopic group was 74 minutes, and 0.5% of patients developed leaks. After adjustment for potential confounders, leaks were 3.4 times more likely with the robotic approach (95% confidence interval, 2.47-4.0; P less than .01). It wasn’t possible to determine whether there were any differences in the type of stapling done in the two groups.
Meanwhile, 0.8% of robotic surgery patients developed surgical site infections versus 0.6% of the laparoscopic cases. After adjustment, infections were 38% more likely with the robot (95% CI, 1.01-1.89; P = 0.03). Dr. Alizadeh noted that the database only goes out to 30 days, so “the true complication rates may be underestimated.”
The findings are consistent with previous investigations. It’s unclear whether there’s something inherently riskier about robotic sleeve gastrectomy itself or whether surgeons haven’t quite got the knack of it yet. The higher leak rate with robotic surgery, “I believe, is mostly related to the small number of [robotic] cases being done. We are still in the beginning stages of utilizing the robotic approach. Maybe there’s a learning curve, and we need more experience and more practice,” Dr. Alizadeh said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Indeed, others have reported that it takes more than two dozen cases to become proficient in another procedure, robotic esophagectomy.
The mean length of stay in the study was slightly, but not statistically significantly, longer in the robotic arm (1.8 vs. 1.7 days; P = 0.17). There was no statistically significant difference in in-hospital mortality.
The laparoscopic group had more men than did the robotic group (21.4% vs. 19.7%, respectively) and more chronic steroid use (1.7% vs. 1.3%), plus more patients were dependent on oxygen (0.7% vs. 0.3%). The robotic group had more obstructive sleep apnea than did the laparoscopic group (37.3% vs. 36% of cases) and a higher incidence of hypoalbuminemia (8.4% vs. 7%). The analysis adjusted for the differences.
The findings were pretty much the same when the team repeated their analysis with the 2016 database numbers, which were released while the SAGES presentation was being prepared. The only big difference was an increase in the number of robotic cases, up from 6.1% in 2015 to 6.6% of cases in 2016.
The was no external funding for the work, and the investigators had no relevant disclosures.
SOURCE: Alizadeh RF et al. SAGES 2018, Abstract S024.
REPORTING FROM SAGES 2018
Key clinical point: Operative times are longer, and leaks and surgical site infections more common, when surgeons opt for robotic instead of laparoscopic sleeve gastrectomy.
Major finding: Anastomotic leaks were 3.4 times more likely with the robotic approach (95% CI 2.47-4.0; P less than .01).
Study details: Review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database
Disclosures: The was no external funding for the project, and the investigators had no relevant disclosures.
Source: Alizadeh RF et al. SAGES 2018, Abstract S024









